- 1Department of Gastroenterology, The First Hospital of Hunan University of Chinese Medicine, Changsha, Hunan, China
- 2Liuyang Hospital of Traditional Chinese Medicine, Changsha, Hunan, China
- 3Medical School, Hunan University of Chinese Medicine, Changsha, Hunan, China
Background: The pathogenesis of inflammatory bowel diseases (IBD) and their interaction with viruses have attracted growing scientific attention, as viral infections and gut virome dysregulation are increasingly recognized as key drivers of IBD onset, disease activity, and treatment responses. However, a systematic analysis of research trends in this field is lacking, leaving critical gaps in understanding how viral factors shape IBD pathogenesis and clinical management. This study conducted a bibliometric analysis to map trends, key areas, and emerging topics in the virus-IBD field from 2014 to 2024, with a focus on the pathogenic roles of viruses. The goal was to inform future research directions and bridge the gap between basic science and clinical practice.
Methods: Publications related to virus-IBD were retrieved from the Web of Science Core Collection. The analysis employed VOSviewer, CiteSpace, and HistCite software to explore bibliometric dimensions, including annual publication trends, contributions by countries/regions and institutions, collaboration networks, highly cited references, citation bursts, and keyword co-occurrence and evolution.
Results: Among 3,225 publications analyzed, three distinct phases were observed: fluctuating growth (2014–2019), a sharp rise (2020–2021), and a gradual decline (2022–2024). The United States (996 publications) and China (507 publications) were the dominant contributors to the field. The Mayo Clinic led in institutional publication output, while Jean-Frederic Colombel and Silvio Danese were among the most prolific and cited authors. Inflammatory Bowel Diseases and Frontiers in Immunology were leading journals. Keyword and reference analyses highlighted major research domains: gut virome mechanisms and modulation, clinical strategies for opportunistic viral infections, and IBD management during Coronavirus Disease 2019 (COVID-19).
Conclusion: This study establishes an integrated knowledge framework of virus-IBD research, highlighting three essential domains: gut virome regulation and therapy, clinical management of opportunistic viral infections, and IBD care in the COVID-19 era. It further clarified the pathophysiological interplay between viral factors and IBD. By synthesizing key contributors, core themes, and evolutionary trends, this work provides a practical foundation for guiding translational research and promoting clinical innovation.
1 Introduction
Inflammatory Bowel Diseases (IBD), which includes ulcerative colitis (UC) and Crohn disease (CD), is characterized by chronic intestinal inflammation and presents with symptoms such as bloody diarrhea, abdominal pain, and weight loss (Baumgart and Carding, 2007). The global burden of IBD has increased substantially, with an estimated 6.8 million prevalent cases worldwide in 2017 (GBD 2017 Inflammatory Bowel Disease Collaborators, 2020). From 1990 to 2021, the age-standardized incidence rate (ASIR) of IBD increased from 4.22 to 4.45 per 100,000 population, while age-standardized disability-adjusted life years declined from 21.55 to 18.07 per 100,000 population-suggesting improved disease management despite growing incidence. Geographically, East Asia experienced the steepest ASIR increase, particularly in China. Notably, the Netherlands recorded the highest age-standardized mortality rate (2.21 per 100,000) in 2021, underscoring regional disparities in IBD outcomes (Lin et al., 2024).
The pathogenesis of IBD involves the interplay of genetics, immune dysregulation, gut dysbiosis, and environmental factors, though precise mechanisms remain unclear. Genetic factors, particularly Nucleotide-Binding Oligomerization Domain 2 (NOD2) and Autophagy-Related 16-Like 1 (ATG16L1) mutations, are strongly linked to CD (Roda et al., 2020). Immune dysfunction presents as overactive mucosal responses with excessive pro-inflammatory cytokines causing intestinal damage (Friedrich et al., 2019), while gut dysbiosis features reduced beneficial bacteria like Faecalibacterium prausnitzii alongside increased pathogens (Cornuault et al., 2018). Environmental triggers include Westernized diets, smoking, and reduced early-life microbial exposure (Piovani et al., 2019). These genetic-environmental interactions disrupt host-microbe crosstalk, triggering IBD onset through intestinal barrier impairment and microbial translocation. Innate immune cells are then activated, releasing cytokines that recruit additional immune cells and initiate adaptive immunity. While regulatory T cells attempt to balance inflammation, chronic activation causes dysregulated cytokine release and immune resistance to anti-inflammatory signals, creating a self-sustaining inflammatory cycle (Neurath, 2019). The COVID-19 pandemic further highlighted critical gaps in IBD management, particularly regarding infection susceptibility, immunotherapy safety, and vaccination strategies in immunosuppressed patients (Ungaro et al., 2021). This global health event reshaped research priorities, intensifying the focus on infection risks and vaccine responses in this population. However, major knowledge gaps persist, limiting therapeutic efficacy and increasing the risks of adverse events (Kobayashi et al., 2020; Roda et al., 2020). Therefore, there is an urgent need for further research into IBD mechanisms and novel therapeutic strategies.
Current management strategies for IBD include 5-aminosalicylates, glucocorticoids, immunomodulators (e.g., azathioprine), and biologic agents targeting pro-inflammatory pathways. Diagnosis relies on clinical assessment, endoscopic evaluation of mucosal lesions, histopathological analysis of biopsies, and serological markers such as antineutrophil cytoplasmic antibody (ANCA) and anti-Saccharomyces cerevisiae antibody (ASCA) (Nakase et al., 2021; Khrom et al., 2024). Recent metagenomic advances underscore the gut microbiota’s influence on metabolism and immune function, with dysbiosis linked to IBD. Fecal microbiota transplantation helps correct this gut dysbiosis (Sartor and Wu, 2017). While bacterial communities are well-studied, the role of the gut virome in IBD pathogenesis is increasingly recognized (Wu et al., 2025). Virome imbalance, characterized by an increase in Caudovirales and a decrease in Microviridae, is associated with intestinal barrier disruption and aberrant immune activation. Certain viruses, such as Orthohepadnavirus, may worsen UC by damaging the epithelium (Tun et al., 2024). Various viruses, including bacteriophages, rotaviruses, and noroviruses, are involved in IBD (Tarris et al., 2021). Pediatric studies have revealed distinct virome patterns in CD and UC, indicating a significant role for viruses in the pathogenesis of IBD (Fernandes et al., 2019). These findings highlight the virome as a potential therapeutic target. However, virus-IBD research faces challenges in establishing causal inference, including unconfirmed causal links and the risk of secondary infections from immunosuppressive therapies (Rahier et al., 2014; Reddy et al., 2015).
Despite the growing body of research on viruses and IBD, a comprehensive bibliometric analysis of this field remains lacking. This gap is critical because bibliometric analysis helps identify emerging themes and underexplored topics amid the rapid growth of this field (Chen and Song, 2019). Unlike previous bibliometric analyses that focused broadly on gut microbiota or single viral taxa, our study is the first to perform a decade-long bibliometric analysis specifically targeting virus-IBD associations (Veisman et al., 2022; Zhang et al., 2022b,2023). By quantifying collaboration networks, tracking keyword evolution, and analyzing citation patterns, we aim to delineate research paradigms, highlight translational gaps, and inform future directions for both basic research and clinical applications.
2 Materials and methods
2.1 Data source and search strategy
A systematic literature search was conducted on August 30, 2025, at 20:38 Beijing Time (UTC + 8) using the Web of Science Core Collection (WoSCC) database, specifically the Science Citation Index Expanded (SCI-Expanded). WoSCC was selected for this bibliometric analysis due to its rigorous citation indexing, which ensures data integrity by excluding predatory and low-quality journals; its KeyWords Plus feature, which supplements author keywords and captures an additional 40%–60% of semantic linkages; and its native compatibility with analytical tools such as VOSviewer, which facilitates efficient data normalization.
The literature search encompassed publications from January 1, 2014, to December 31, 2024. This 10-year span was selected because it represents a recent and complete decade, which helps capture evolving research trends without including outdated studies. The timeframe is sufficiently long to identify meaningful patterns yet focused enough to maintain relevance and analytical consistency. The search strategy employed the following Boolean query: #1: “Inflammatory Bowel Disease*” OR IBD OR “Ulcerative Colitis” OR “Crohn* Disease” OR “Crohn’s Disease”; #2: Virus* OR Virology OR Virological OR Viral OR Virome OR Bacteriophage* OR Phage* OR Herpesvirus* OR Enterovirus* OR Retrovirus* OR Norovirus* OR Adenovirus* OR Rotavirus* OR “SARS-CoV-2” OR “COVID-19” OR “Epstein-Barr Virus” OR EBV OR “Cytomegalovirus” OR CMV OR “Human Immunodeficiency Virus” OR HIV OR “Hepatitis B Virus” OR HBV OR “Hepatitis C Virus” OR HCV OR “Varicella-Zoster Virus” OR VZV OR “JC Virus” OR JCV; #1 AND #2.
The search terms were informed by Medical Subject Headings (MeSH) descriptors, including [Inflammatory Bowel Diseases] (MeSH), and were supplemented with free-text variants. The TS (Topic Search) field encompasses titles, abstracts, author keywords, and KeyWords Plus, with wildcard truncation (e.g., “inflammatory bowel disease*”) employed to capture grammatical variants such as singular and plural forms.
This analysis included all retrieved literature relevant to viruses and IBD without preemptively excluding any virus type. Studies related to SARS-CoV-2 were retained even when IBD was not the central focus, as they represent a substantive thematic trend and contribute to the overall knowledge structure of the field, thereby aligning with the objective nature of bibliometric analysis.
2.2 Inclusion and exclusion criteria
Inclusion criteria: The study considered publications that met the following criteria: (1) Papers published in the English language; (2) Articles or reviews; (3) Studies examining the relationship between viruses and IBD; (4) Publication date between January 1, 2014, and December 31, 2024.
Exclusion criteria: Publications were excluded if they met any of the following conditions: (1) non-English language publications; (2) Document types other than articles and reviews (e.g., letters, meeting abstracts, and editorial materials); (3) Publications falling outside the specified timeframe; (4) Duplicate records; (5) Retracted publications; (6) Publications with irrelevant or incomplete content.
The collected data, including complete bibliometric records and citation information, were stored in plain text format. A three-tier quality control was implemented as follows: (1) Deduplication: When downloading raw data from WoSCC, Excel files containing “authors, titles, and sources” were saved simultaneously. Duplicates were removed based on author-title combinations using Microsoft Excel; (2) Resolution of data inconsistencies was achieved through verification against source metadata; (3) Misclassifications were addressed by re-evaluating publications against predefined criteria. To ensure accuracy, all publications underwent a dual-reviewer screening process: an initial assessment of titles and abstracts in accordance with the inclusion criteria, followed by a comprehensive evaluation of full texts. Any discrepancies were resolved through consensus with a third reviewer.
2.3 Data normalization and disambiguation
2.3.1 Keyword normalization
To ensure the accuracy and consistency of keyword co-occurrence analysis, a standardized workflow was applied to filter, clean, and normalize keywords. The synonym list and high-frequency keywords (both pre- and post-merger) are provided in Supplementary materials. The normalization procedure consisted of the following steps:
Keywords with a frequency of ≥15 were extracted using VOSviewer to focus on core research topics and minimize noise from infrequent terms. Each keyword was individually reviewed against the MeSH thesaurus to develop a synonym library. Synonyms with identical meanings were merged, while near-synonyms were retained as distinct entries to preserve conceptual nuance and avoid artificial conflation.
The keyword set underwent three normalization steps before being re-analyzed in VOSviewer: (1) Semantic unification: Variants with identical or highly similar meanings were mapped to standardized MeSH descriptors (e.g., “Crohn’s disease” → “Crohn disease”; “inflammatory-bowel-disease” → “inflammatory bowel diseases”). (2) Abbreviation standardization: Common medical abbreviations were expanded to full MeSH-preferred terms (e.g., “ibd” → “inflammatory bowel diseases”). Widely recognized abbreviations (e.g., “COVID-19”) were retained. (3) Grammatical and structural consistency: Singular/plural forms were unified based on MeSH conventions or prevailing usage (e.g., “infection” → “infections”). Incomplete terms were expanded into full semantic expressions (e.g., “short-chain fatty acids” from “chain fatty-acids”).
2.3.2 Author/institution disambiguation
Author information was extracted using VOSviewer, with a focus on 238 authors who had at least five publications. To resolve name variants (e.g., abbreviations or inconsistent name order), each author’s identity was cross-verified against Open Researcher and Contributor ID (ORCID) profiles and WoS records. VOSviewer-generated default formats (e.g., “farraye, francis a,” “ng, siew c”) were retained in visualizations, while full academic names (e.g., “Francis A. Farraye,” “Siew C. Ng”) were used in the text and tables based on WoS and ORCID records.
For institutional disambiguation, VOSviewer abbreviations were retained in figures but expanded to official full names elsewhere using the Research Organization Registry (ROR) and institutional websites (e.g., “univ coll cork” → “University College Cork”). Among the 97 institutions with at least 15 publications, no duplicates were identified. Four affiliated institutions–Brigham and Women’s Hospital, Massachusetts General Hospital, Harvard Medical School, and Harvard University–were treated as distinct entities due to their operational independence and distinct research profiles, thereby avoiding conflation of their contributions.
2.4 Bibliometric analysis
This study utilized a comprehensive array of domain-specific bibliometric tools for a multi-layered analysis. The tools and their functions included: (1) VOSviewer (version 1.6.20) for analyzing collaboration networks and keyword mapping, with Pajek for topology optimization; (2) CiteSpace (version 6.4.R1) for co-citation and the detection of emerging trends via Kleinberg’s burst detection algorithm; (3) HistCite Pro (version 2.1) for evaluating citation impact through both Local Citation Score (LCS), which measures citations received from within the curated dataset of 3,225 publications, and Global Citation Score (GCS), which reflects total citations from the entire WoSCC database. This distinction allows for separate interpretation of a publication’s influence within the specific research domain versus its broader academic impact; (4) Microsoft Excel 2016 for data preprocessing.
Moreover, for the analysis of productivity by country, institution, and author, we employed full counting methodology. This means that each publication was counted in full for every contributing country, institution, or author. Figure 1A illustrates this literature screening workflow, highlighting critical decision points from initial identification to final inclusion.
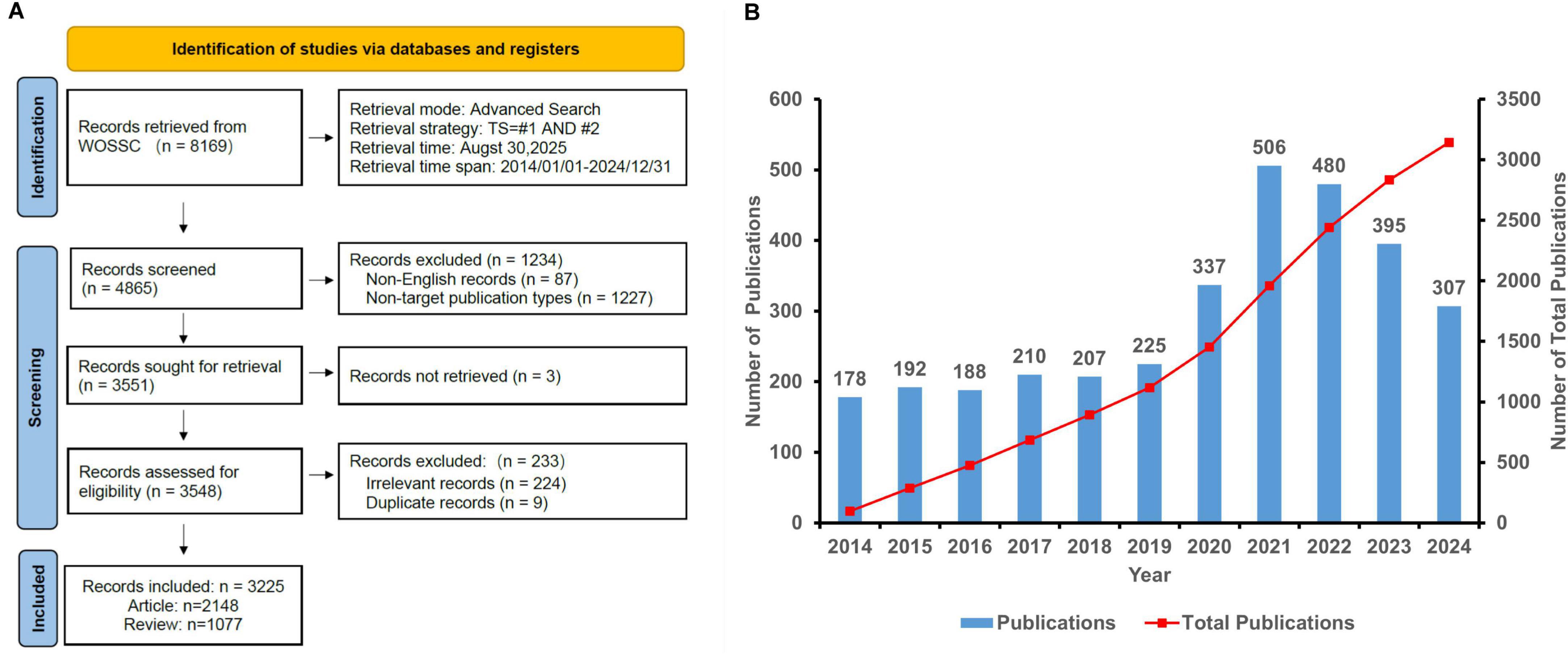
Figure 1. (A) Flow chart illustrating the literature screening process. (B) Annual publication trends in virus-IBD research (2014–2024).
3 Results
3.1 Analysis of publications
This study analyzed 3,225 publications (2,127 articles and 1,068 reviews) in the virus-IBD field. Figure 1B illustrates a three-phase publication trend from 2014 to 2024: fluctuating growth (2014–2019), a sharp surge (2020–2021), and a gradual decline (2022–2024).
3.2 Analysis of countries/regions
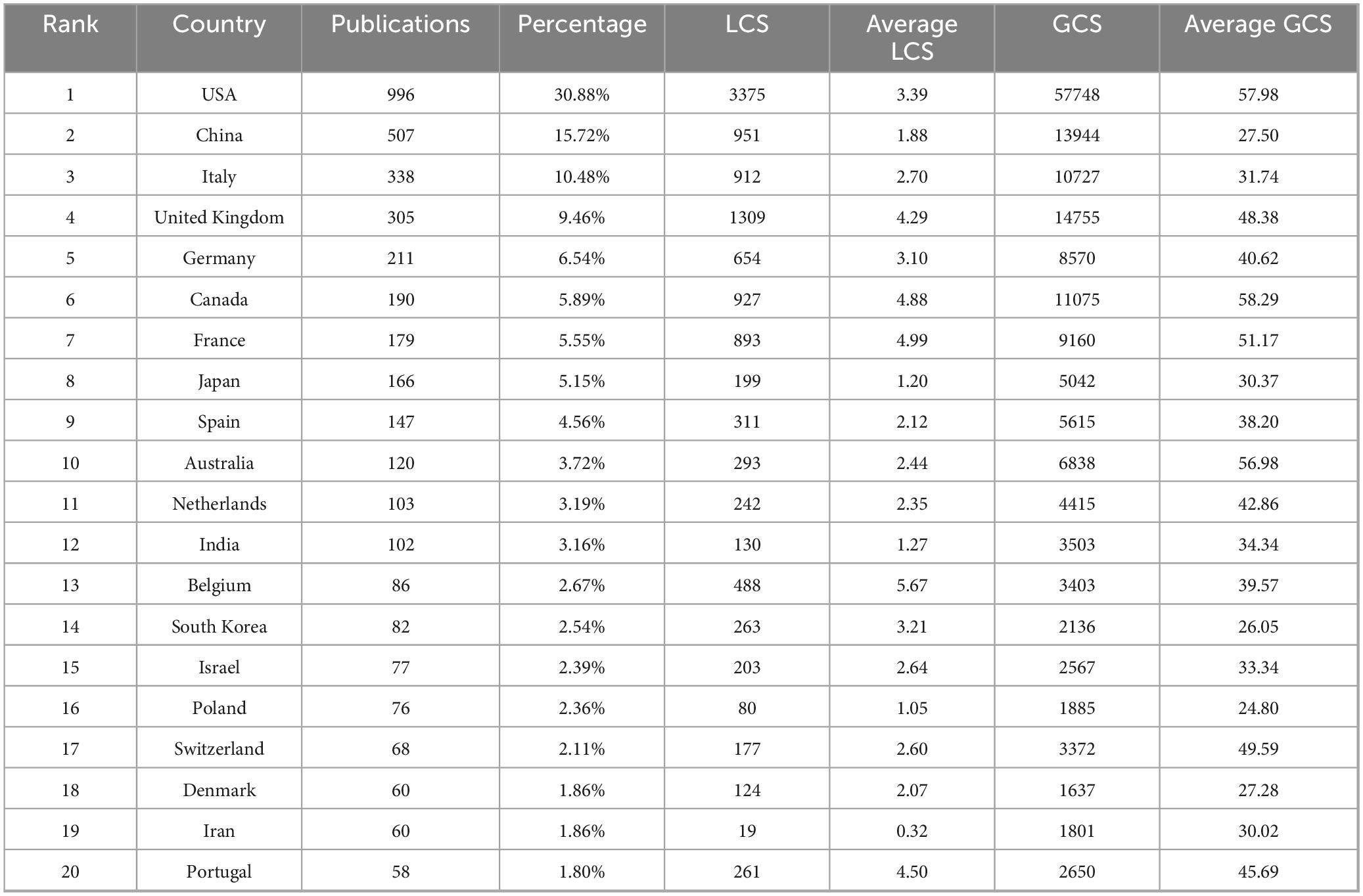
A total of 99 countries/regions contributed to the virus-IBD research field. The top 20 most productive countries are presented in Table 1, ranked by publication count. The United States of America (USA) led with 996 publications, accounting for 30.88% of the total publications, followed by China (507 publications, 15.72%), Italy (338 publications, 10.48%), the United Kingdom (UK, 305 publications, 9.46%), and Germany (211 publications, 6.54%). A positive correlation was observed between national publication output and total GCS, though considerable disparities existed in average GCS. The USA maintained a high average GCS (57.98), while Canada (58.29) and Australia (56.98) achieved comparable or even higher average GCS despite lower total publication output. In contrast, China (27.50) and South Korea (26.05) had relatively lower average GCS despite high productivity. In terms of LCS, average LCS varied more notably: Belgium led with 5.67, followed by France (4.99), Canada (4.88), and Portugal (4.50). Conversely, Iran (0.32), Poland (1.05), India (1.27), and Japan (1.20) showed the lowest average LCS, indicating regional differences in scholarly influence.
Figure 2 presents a geospatial map depicting a part of the top 30 countries ranked by publication output (≥27 publications each), where country size was scaled according to publication volume. The visualization highlights strong collaborative networks among developed regions–particularly the USA, Canada, and major European nations–which dominated both productivity and collaboration. The East Asian countries (e.g., China, Japan, South Korea) also contributed substantially, alongside emerging economies such as India, Iran, and Brazil.
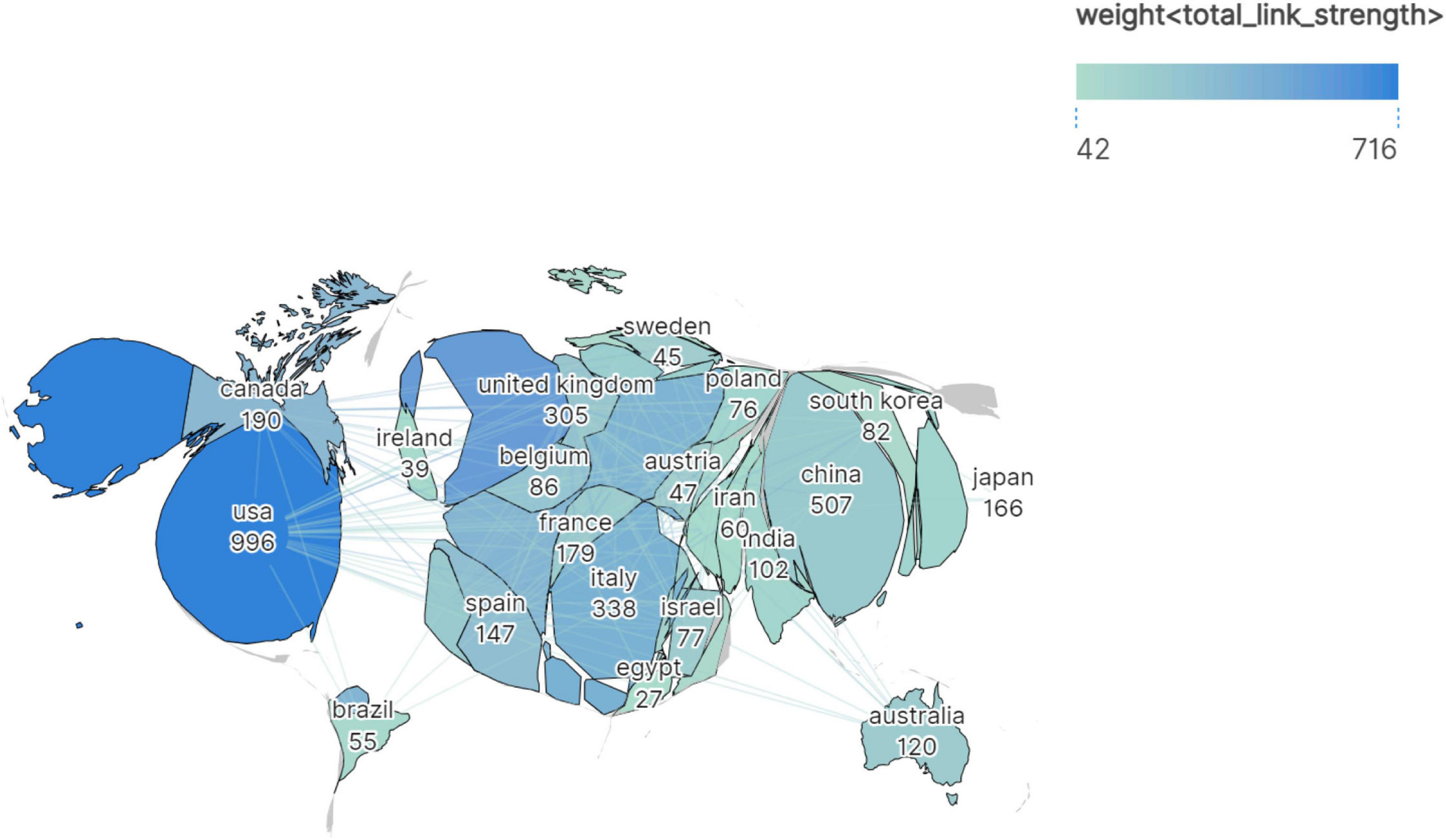
Figure 2. Geospatial collaboration map of publication output and international research networks in the virus-IBD field. Color intensity indicates link strength; geographic area is proportional to publication volume.
3.3 Contribution of institutions
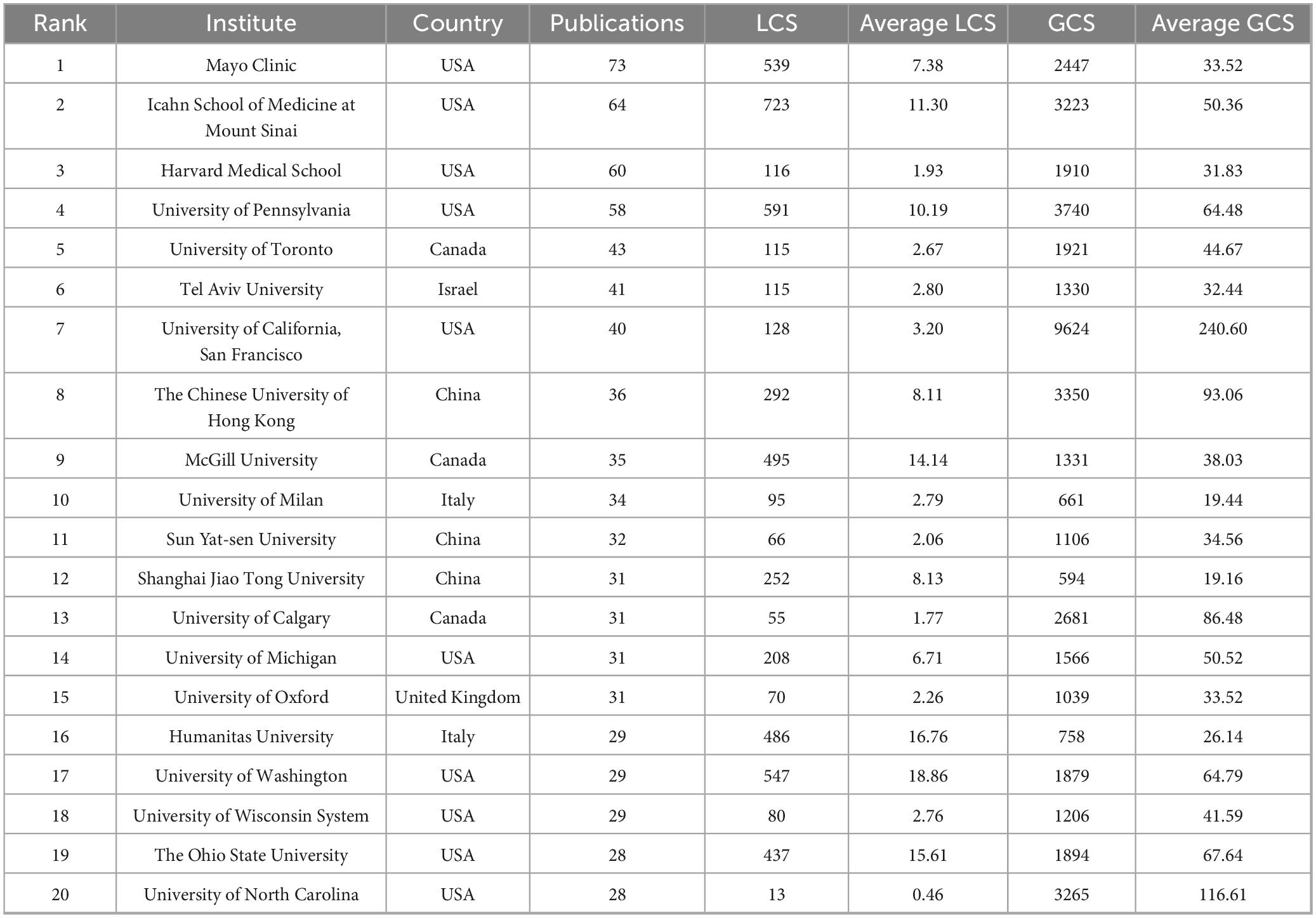
A total of 4,749 institutions contributed to this field. As listed in Table 2, the top 20 most productive institutions (each with ≥28 publications) were identified. Institutions from the USA predominated (13 out of 20), followed by China and Canada (3 each), Israel (1), Italy (2), and the UK (1).
Key observations include: (1) Publication output: The Mayo Clinic led with 73 publications, followed by the Icahn School of Medicine at Mount Sinai (64) and Harvard Medical School (60); (2) LCS: In terms of total LCS, the Icahn School of Medicine at Mount Sinai ranked highest with 723, followed by the University of Pennsylvania (591) and the University of Washington (547). For average LCS, the University of Washington led with 18.86, followed by Humanitas University (Italy, 16.76) and The Ohio State University (15.61); (3) GCS: In terms of total GCS, the University of California, San Francisco (UCSF) ranked first with 9,624, followed by the University of Pennsylvania (3,740) and The Chinese University of Hong Kong (3,350). For average GCS, UCSF still took the lead with 240.60, followed by the University of North Carolina (116.61) and The Chinese University of Hong Kong (93.06).
Several institutions-including UCSF, the University of North Carolina, and the Chinese University of Hong Kong–demonstrated high average citation metrics, reflecting strong scholarly impact relative to output.
Figure 3A displays the collaborative network among institutions. Major hubs-including the Mayo Clinic, Icahn School of Medicine, Harvard Medical School, and the University of Pennsylvania-show extensive collaborations, as indicated by large node size and dense linkage patterns.
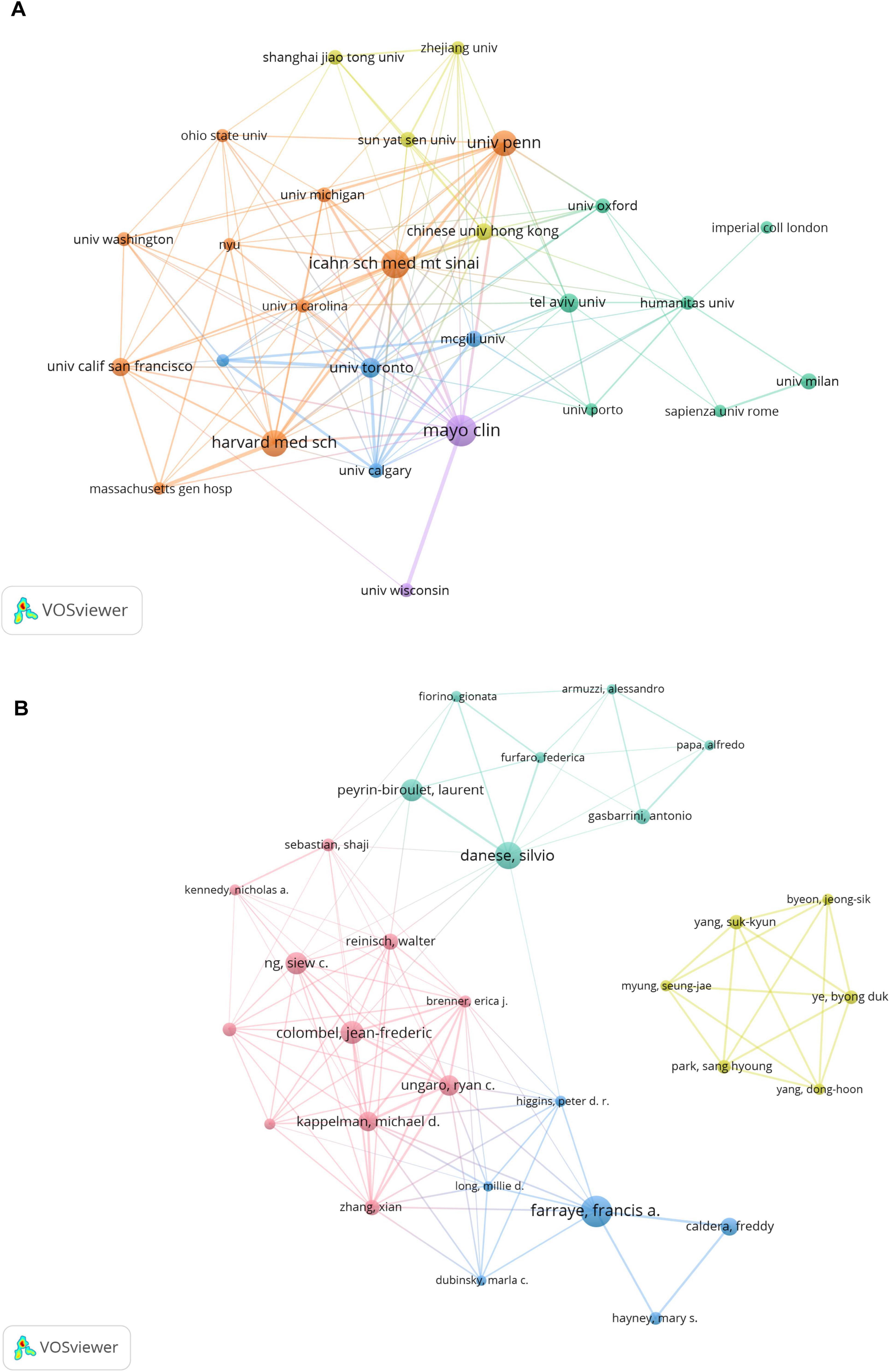
Figure 3. (A) Institutional and (B) author collaborative networks in virus-IBD research. Node size corresponds to publication count; line width denotes collaborative strength. The analysis included institutions with ≥28 publications (A) and authors with ≥10 publications (B). Normalization: association strength. Layout settings: (A) attraction = 3, repulsion = –3; (B) attraction = 2, repulsion = –1.
3.4 Analysis of authors
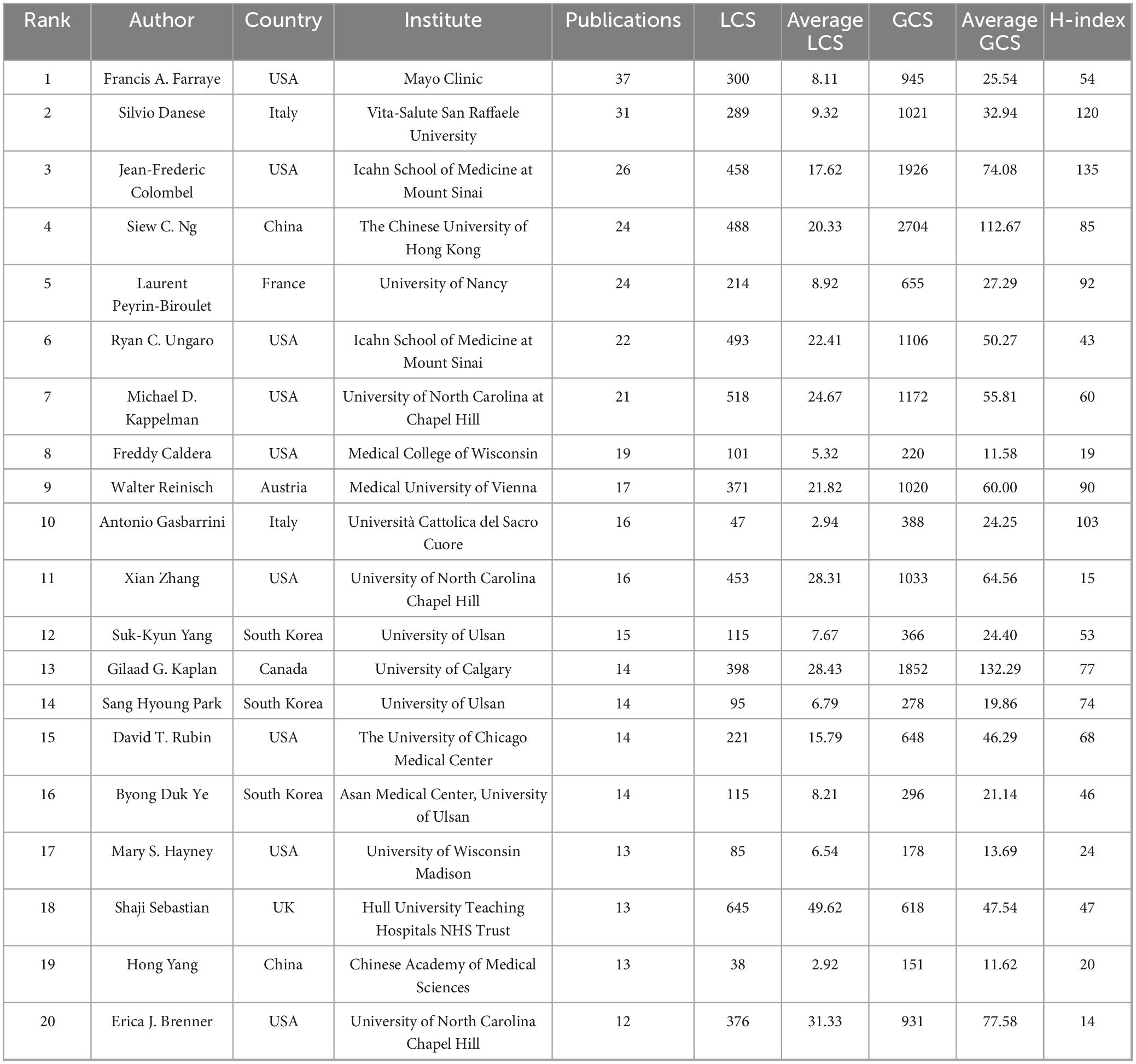
A total of 18,255 authors contributed to the 3,225 publications. Table 3 lists the top 20 most prolific authors (each with ≥12 publications). Francis A. Farraye (USA) led with 37 publications, followed by Silvio Danese (Italy) with 31, and Jean-Frederic Colombel (USA) with 26. In terms of total LCS, Shaji Sebastian (UK) ranked first with 645 points and also had the highest average LCS (49.62), followed by Michael D. Kappelman (USA, with a total LCS of 518 and an average LCS of 24.67) and Ryan C. Ungaro (USA, with a total LCS of 493 and an average LCS of 22.41). Regarding GCS, Siew C. Ng (China) took the lead with a total GCS of 2704, followed by Jean-Frederic Colombel (USA, with a total GCS of 1926 and an average GCS of 74.08) and Gilaad G. Kaplan (Canada, with a total GCS of 1852). For average GCS, Gilaad G. Kaplan topped the list with 132.29, followed by Siew C. Ng (112.67) and Erica J. Brenner (USA, 77.58) and Colombel (74.08). The H-index, a metric reflecting sustained scholarly influence, was highest for Jean-Frederic Colombel (135), followed by Silvio Danese (120) and Antonio Gasbarrini (103).
Figure 3B illustrates the co-authorship network among the top 30 authors, revealing four collaborative clusters. The cluster represented by Jean-Frederic Colombel, Silvio Danese, and Francis A. Farraye exhibits strong internal collaborations, whereas the cluster centered around Suk-Kyun Yang (South Korea) was relatively isolated, showing limited connections to researchers from other clusters.
3.5 Analysis of journals
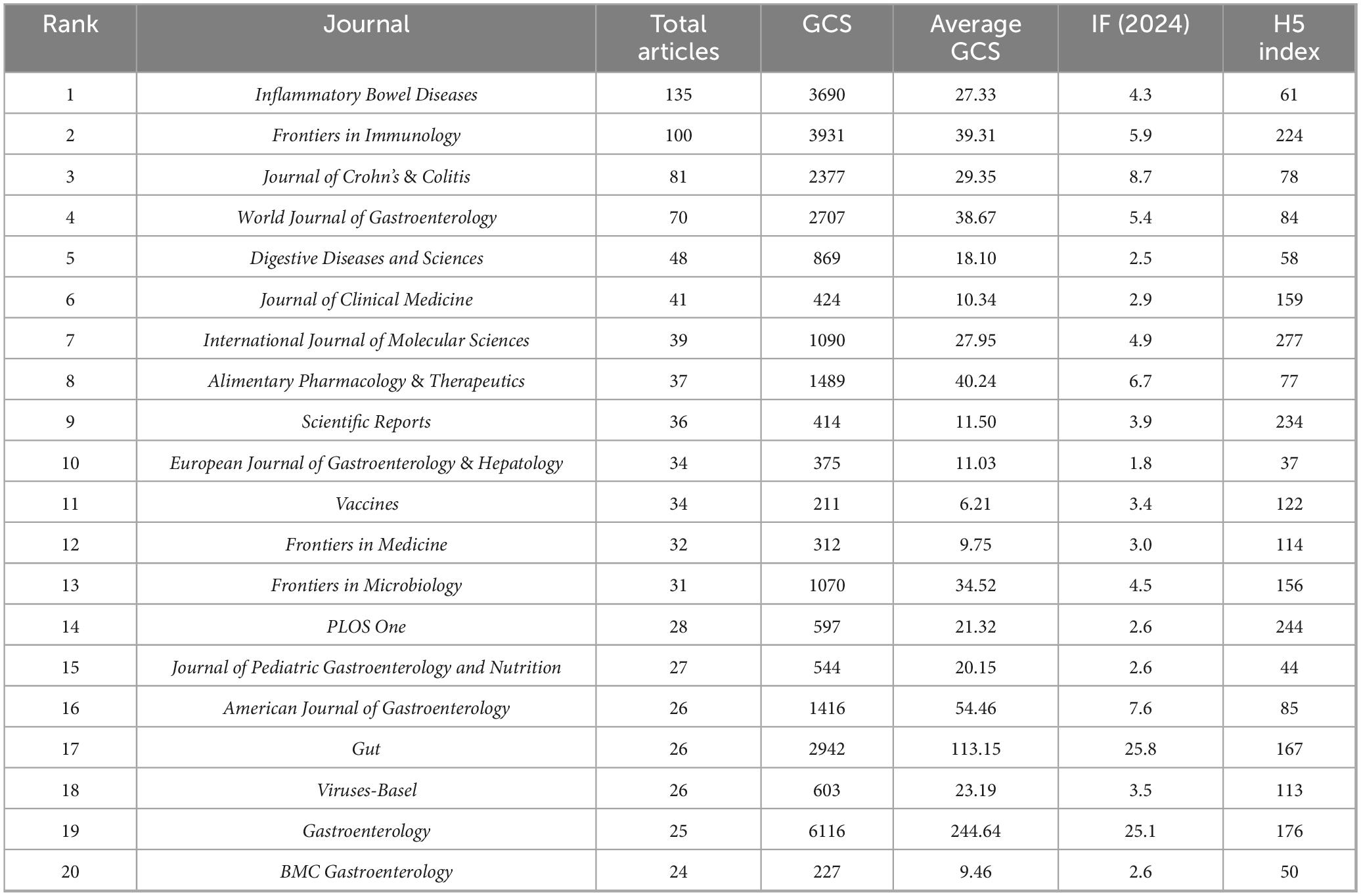
A total of 966 journals were represented in the virus-IBD research. Table 4 presents the top 20 journals by publication volume. Inflammatory Bowel Diseases led with 135 articles, followed by Frontiers in Immunology (100) and Journal of Crohn’s & Colitis (81). In terms of GCS, Gastroenterology received the highest total GCS (6,116), far exceeding other journals, and achieved the highest average GCS (244.64). Frontiers in Immunology ranked second in total GCS (3,931), while Gut recorded the second-highest average GCS (113.15). Regarding journal influence metrics, Gut and Gastroenterology led with the highest Impact Factors (25.8 and 25.1, respectively). Among the H5 index values, which reflect recent scholarly impact, International Journal of Molecular Sciences scored highest (277), followed by PLOS One (244) and Scientific Reports (234). These metrics collectively underscore the significant role of these journals in disseminating high-impact virus-IBD research.
Figure 4 presents a journal citation network based on an analysis of 27 journals (each with ≥20 publications). The visualization highlights several core journals in this field, including Inflammatory Bowel Diseases, Frontiers in Immunology, World Journal of Gastroenterology, and Journal of Crohn’s & Colitis.
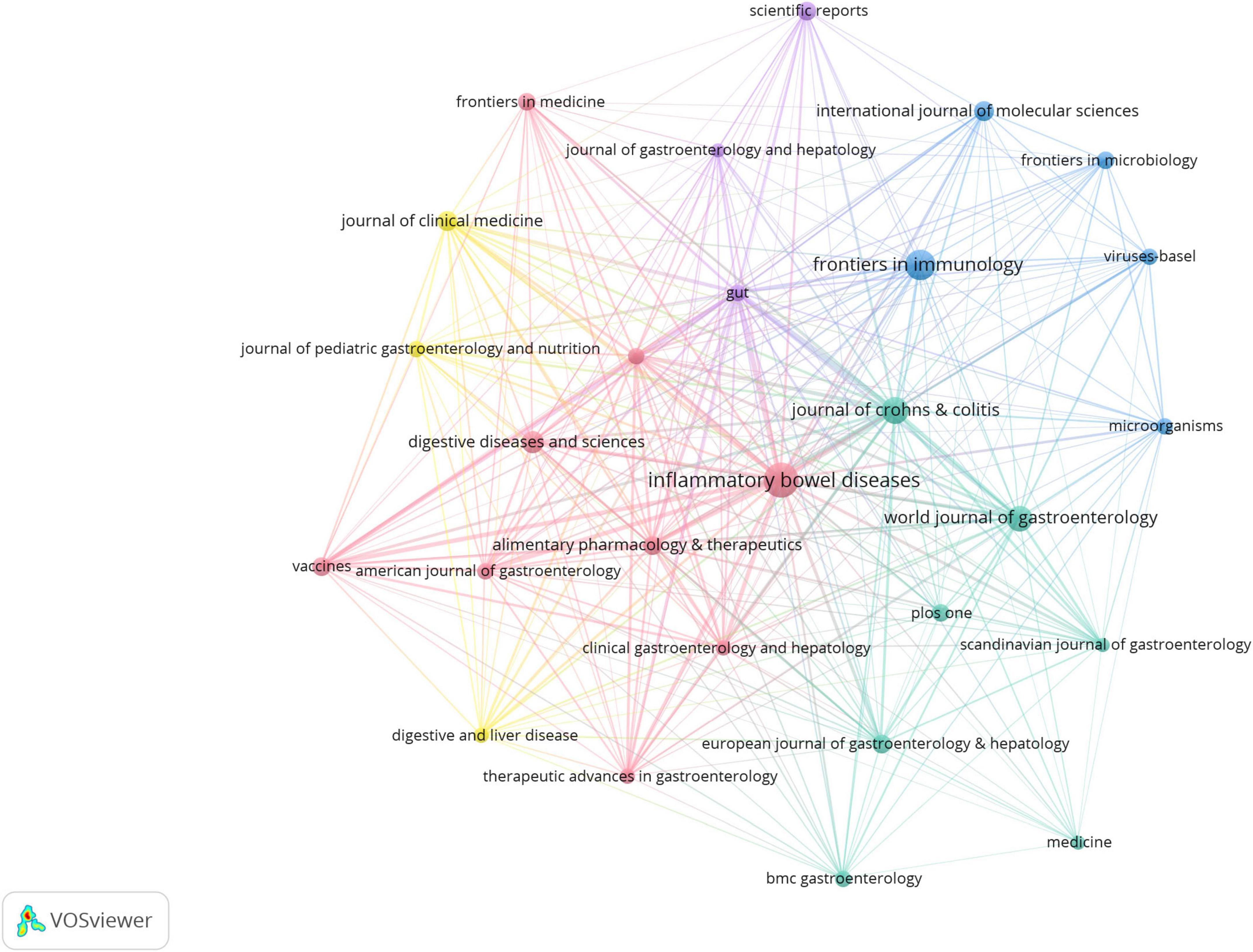
Figure 4. Journal citation network in virus-IBD research. Node size corresponds to the publication output; line width indicates citation strength between journals. Journals with ≥20 publications were included. Normalization: association strength. Layout settings: attraction = 2; repulsion = –1.
3.6 Analysis of highly cited references and burst
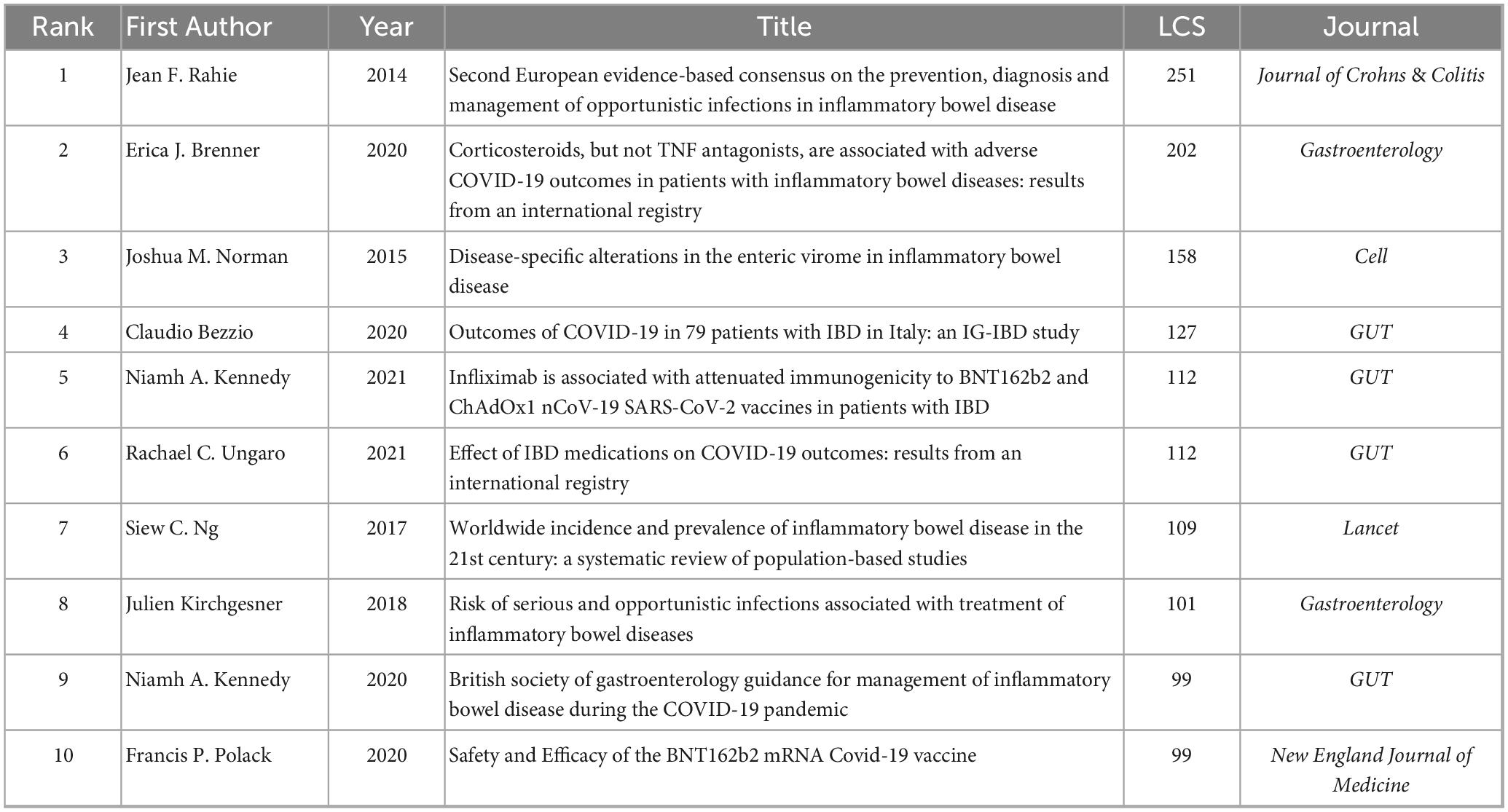
To pinpoint core foundational studies in virus-IBD research, we extracted the top 10 LCS using HistCite, detailed in Table 5. These publications reflect several prominent research themes: (1) Clinical management and guidelines for opportunistic infections and COVID-19 in patients with IBD (Rahier et al., 2014; Bezzio et al., 2020; Brenner et al., 2020; Kennedy et al., 2020; Ungaro et al., 2021); (2) Vaccine immunogenicity and outcomes in IBD (Polack et al., 2020; Kennedy et al., 2021); (3) Viral ecology in IBD, like enteric virome changes (Norman et al., 2015); (4) Epidemiology and infection risks associated with IBD treatments (Ng et al., 2017; Kirchgesner et al., 2018). The most cited reference was the second European consensus statement on opportunistic infections in IBD (LCS = 251) (Rahier et al., 2014), followed by a study on COVID-19 outcomes under corticosteroid therapy (LCS = 202) (Brenner et al., 2020), and a study on enteric virome changes (LCS = 158) (Norman et al., 2015). Notably, five of the top ten publications focused on COVID-19, highlighting its significant impact on recent IBD research.
Citation burst analysis was used to capture surges in citation activity exceeding base levels within a specific period, offering insight into evolving research trends and influential publications. As shown in Figure 5A, the top 25 references with the strongest citation bursts between 2014 and 2024 reveal two prominent thematic shifts: The early phase (2014–2019) was dominated by studies on opportunistic viral infections and microbiome interactions in IBD. Key contributions included cytomegalovirus (CMV) reactivation and management (strength = 17.3) (Delvincourt et al., 2014), disease-specific alterations of the enteric virome (strength = 31) (Norman et al., 2015), and the therapeutic potential of fecal microbiota transplantation (strength = 12.17) (Moayyedi et al., 2015). The second European Consensus on opportunistic infections remained highly influential throughout this period (strength = 54.79) (Rahier et al., 2014). The recent phase (2020–2024) is overwhelmingly marked by research related to COVID-19 in patients with IBD, particularly focusing on vaccination immunogenicity, clinical outcomes, and medication safety. Noteworthy bursts include studies on attenuated antibody responses under anti-tumor necrosis factor (TNF) therapy (strength = 27.01) (Kennedy et al., 2021), vaccine safety (strength = 15.23) (Polack et al., 2020), and international registry-based outcome studies (strength = 12.71) (Ungaro et al., 2021). This shift underscores how the pandemic has redirected research toward the intersection of COVID-19 and IBD.
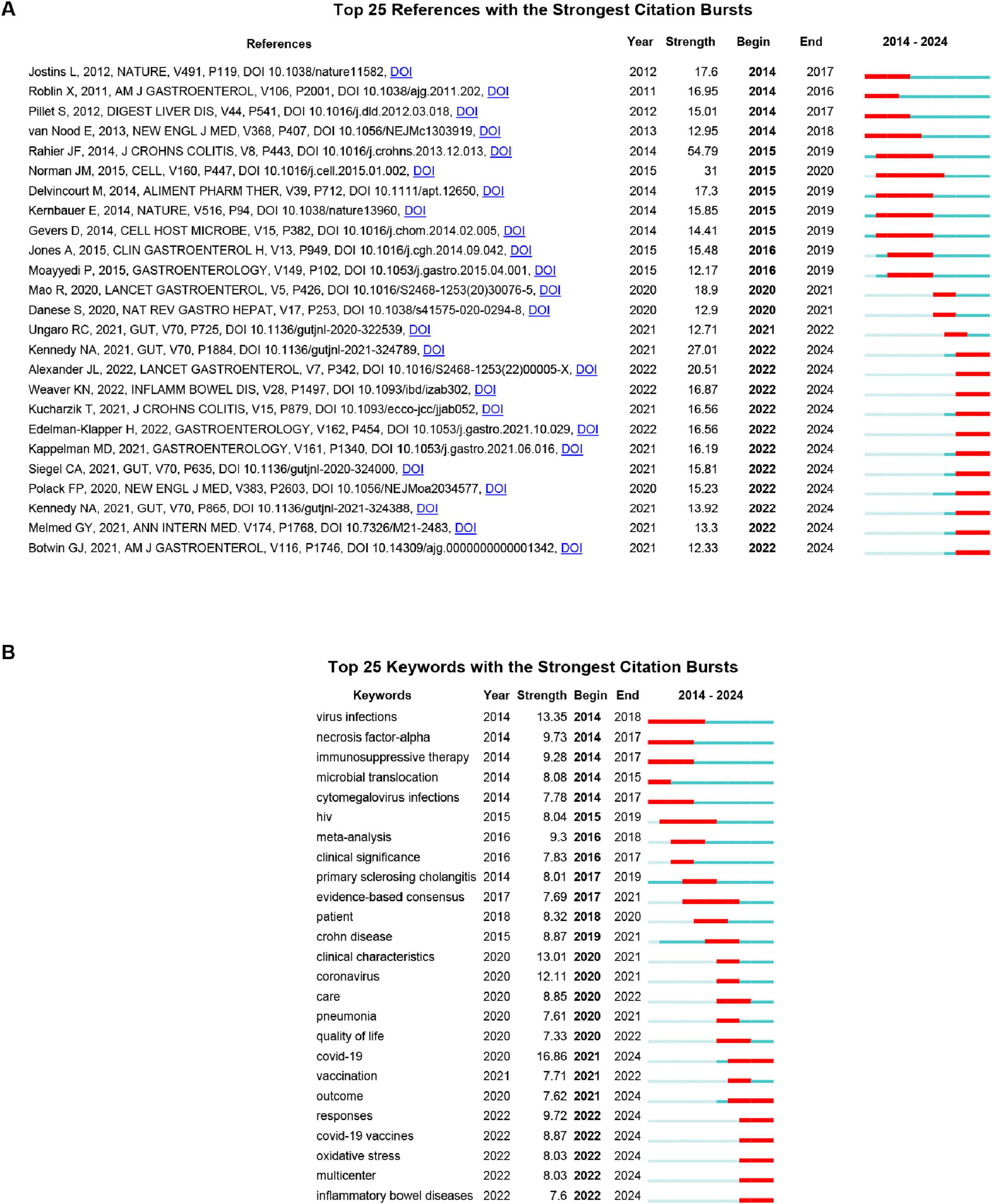
Figure 5. (A) Top 25 references with the strongest citation bursts. (B) Top 25 keywords with the strongest citation bursts. Blue bar represents the timeline; red bar indicates the burst period. “Strength” reflects the intensity of the citation surge relative to the baseline. “Begin” and “End” denote the start and end years of the burst duration. The burst detection was performed using the Kleinberg algorithm with the following parameters: γ = 1.0, minimum burst duration = 2 years, and a time window of 1 year.
3.7 Analysis of keywords co-occurrence and cluster
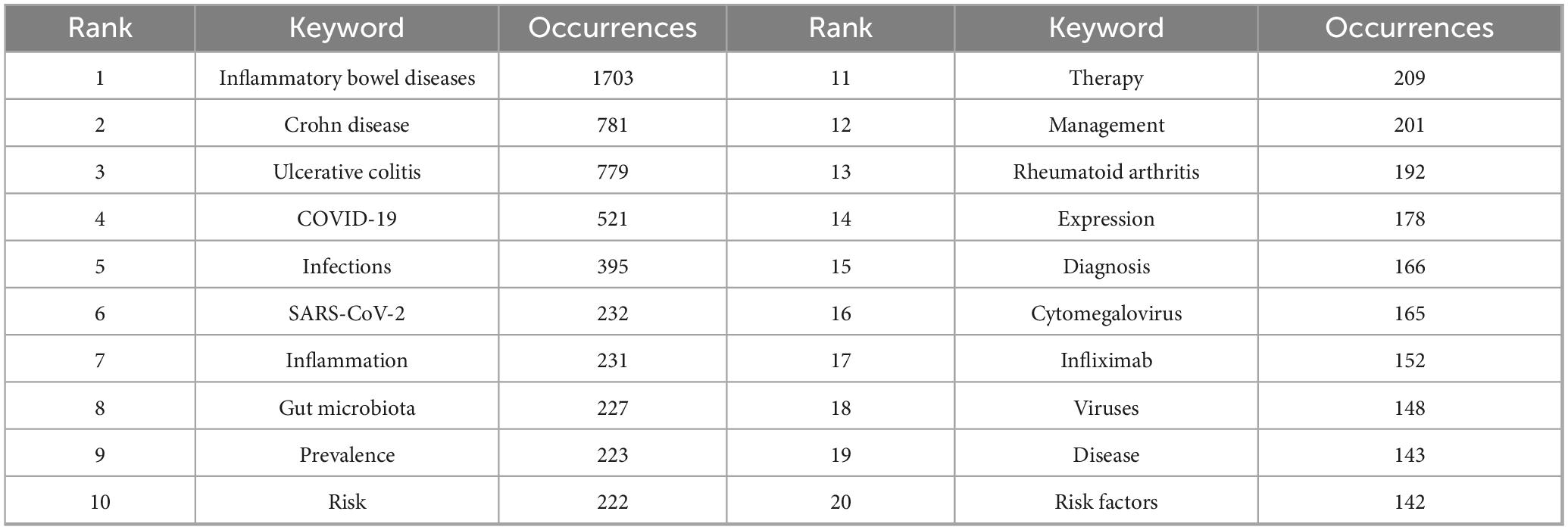
Keywords represent core research themes, and bibliometric analysis of their co-occurrence helps identify hotspots and trends. This study extracted 11,348 keywords, with the top 20 listed in Table 6. A keyword co-occurrence network was created using VOSviewer (minimum occurrence = 74), identifying 55 core keywords. Five distinct clusters were identified in Figure 6A: (1) The pink cluster focused on immune-inflammatory mechanisms and regulatory signaling pathways, featuring terms like inflammatory bowel diseases, inflammation, tumor necrosis factor-alpha, expression, nf-kappa b, and regulatory T-cells. (2) The yellow cluster addressed epidemiology, risk factors, and clinical management of infections in immunocompromised patients, featuring terms like viral infection, prevention, opportunistic infections, immunosuppression, prevalence, infliximab, vaccine, and hepatitis B. (3) The cyan cluster emphasized microbial communities, dysbiosis in IBD pathogenesis, and microbiome-targeted therapies, featuring terms like gut microbiota, bacteriophages, virome, dysbiosis, probiotics, and pathogenesis. (4) The blue cluster focused on disease-specific risk profiles and clinical management, particularly for viral infections like CMV, featuring terms like ulcerative colitis, Crohn disease, risk factors, cytomegalovirus, infections, management, azathioprine. (5) The purple cluster underscored COVID-19-era challenges in the management of patients with IBD, featuring terms like COVID-19, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), quality of life, and impact.
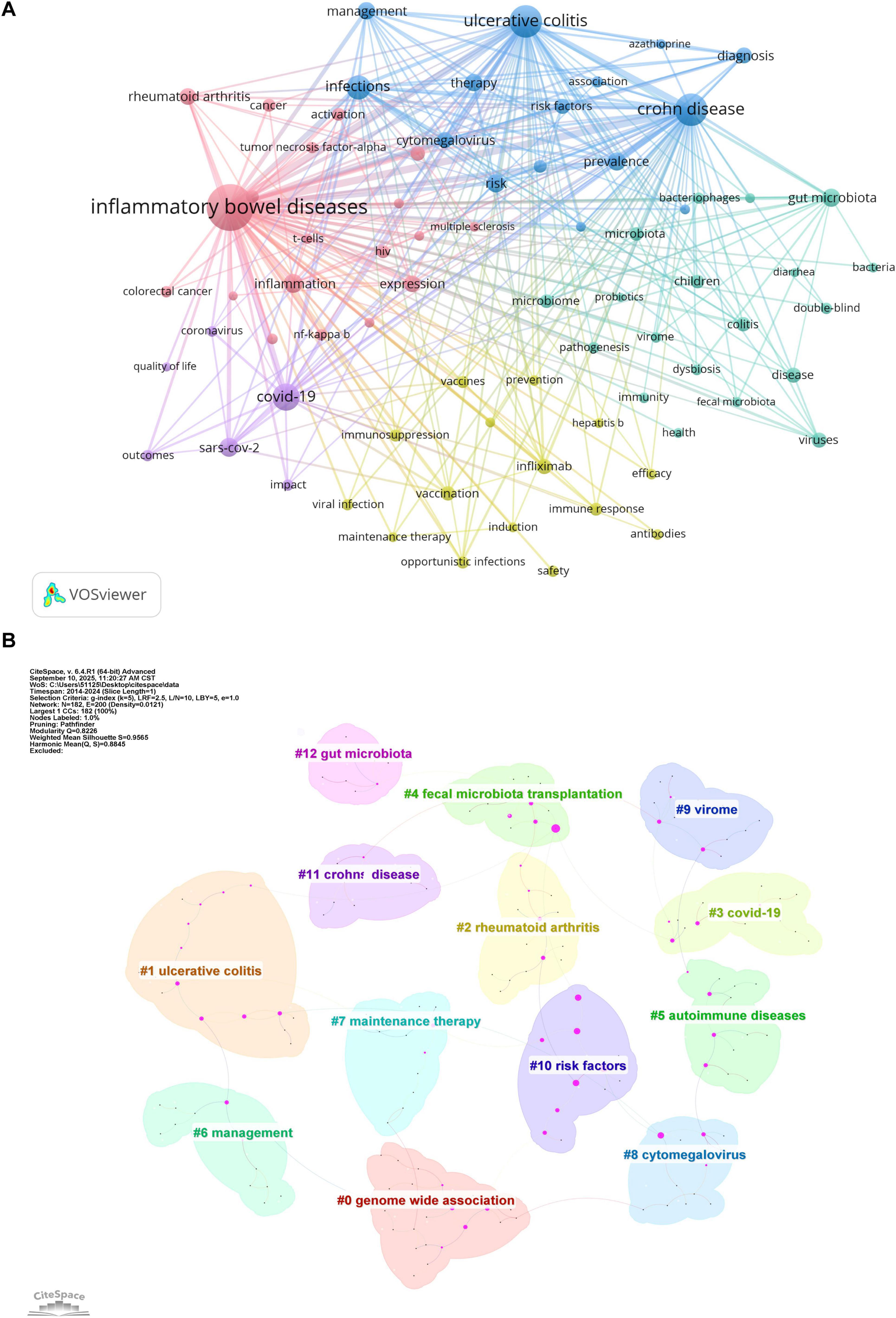
Figure 6. (A) Keyword co-occurrence network generated by VOSviewer. Node size indicates keyword frequency; line width represents connection strength. Minimum occurrence threshold = 74. Normalization: association strength. Layout settings: attraction = 2, repulsion = −1. (B) Keyword clustering map generated by CiteSpace. Time slice = 1; selection criteria: g-index (k = 5), LRF = 2.5, L/N = 10; pruning: pathfinder and sliced networks; Q = 0.8226; S = 0.9565; labels from LLR.
CiteSpace was used to generate a keyword cluster timeline to explore temporal evolution, identifying 13 major thematic groups (Figure 6B) genome-wide association, ulcerative colitis, rheumatoid arthritis, COVID-19, fecal microbiota transplantation, autoimmune diseases, management, maintenance therapy, cytomegalovirus, virome, risk factors, Crohn disease, and gut microbiota. The timeline illustrates inter-cluster relationships and chronological distribution, with keywords in the same cluster aligned horizontally (Figure 7). Clusters like risk factors, virome, management, fecal microbiota transplantation, and maintenance therapy were active initially but gradually declined. In contrast, the COVID-19 cluster emerged around 2020, peaked briefly, then reduced in activity. Notably, genome-wide association, cytomegalovirus, and gut microbiota remained active throughout the period, confirming their core status. The fecal microbiota transplantation cluster showed strong links to other groups, indicating interdisciplinary relevance. Recent keywords (2023–2024) point to emerging directions: follow-up studies in genome-wide association, fecal microbiota transplantation resistance, biological therapy for autoimmune diseases, DNA and immunosuppression in Crohn disease, and gut microbiota metabolism.
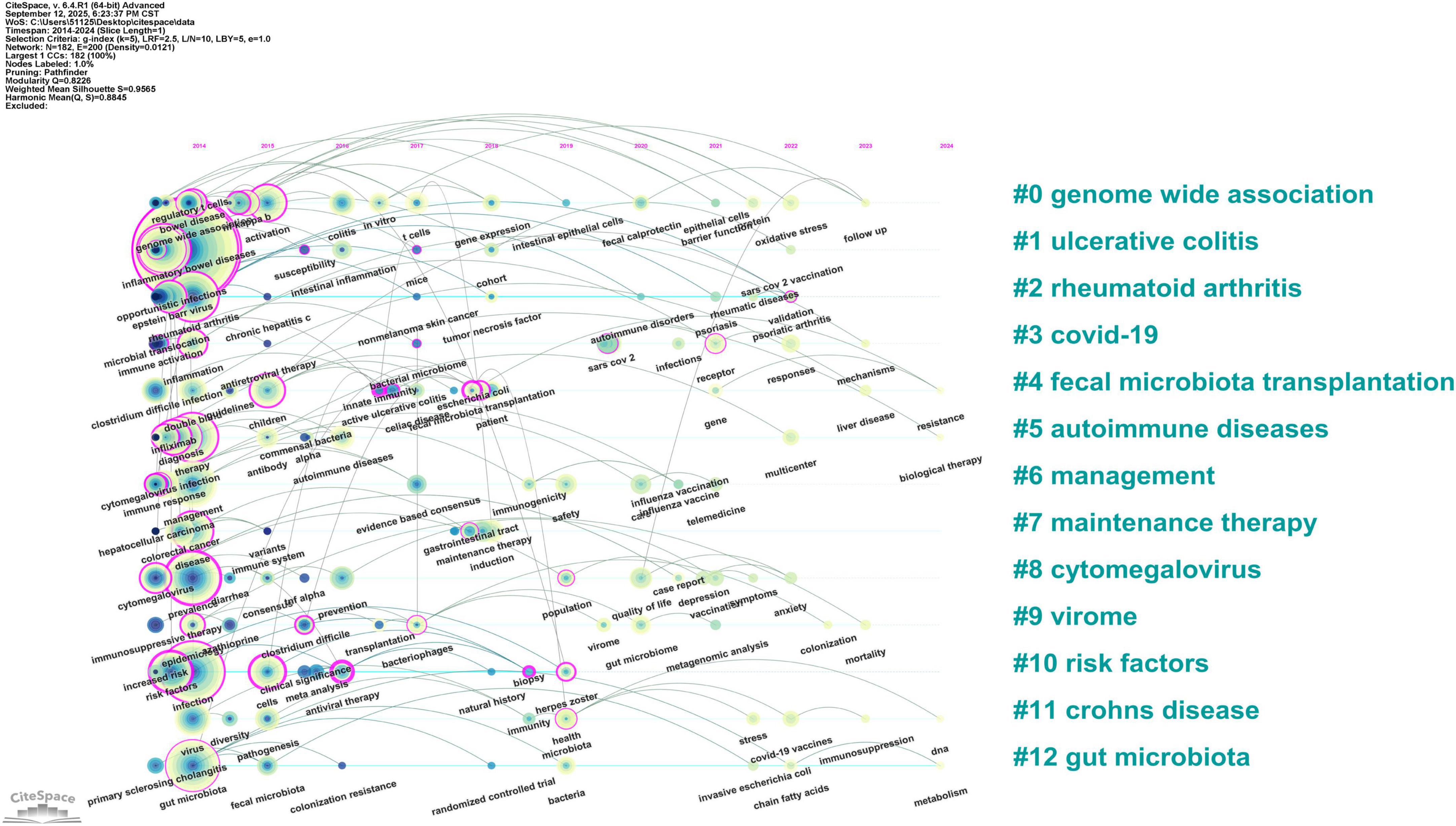
Figure 7. Timeline visualization of keyword clusters. The horizontal axis represents the timeline. Node size reflects keyword frequency, and connecting lines indicate thematic relationships between topics.
Keyword burst analysis (Figure 5B) identifies abrupt frequency surges, reflecting research focus shifts. The top 25 bursting keywords (2014–2024) align with reference citation burst trends, dividing into two phases: 2014–2019 (opportunistic viral infections and clinical management) saw core bursts of virus infections (strength = 13.35, 2014–2018), cytomegalovirus infections (7.78, 2014–2017), and immunosuppressive therapy (9.28, 2014–2017)–mirroring citation bursts on CMV reactivation (reference strength = 17.3). Human immunodeficiency virus (HIV) (8.04, 2015–2019) and primary sclerosing cholangitis (8.01, 2017–2019) expanded to viral comorbidities, while meta-analysis (9.3, 2016–2018) and evidence-based consensus (7.69, 2017–2021) aligned with Rahier et al.’s European Consensus (reference strength = 54.79). The 2020–2024 phase pivoted to COVID-19-IBD intersections: COVID-19 (16.86, 2021–2024, strongest burst) and coronavirus (12.11, 2020–2021) echoed COVID-19-focused references; COVID-19 vaccines (8.87, 2022–2024) and vaccination (7.71, 2021–2022) aligned with Kennedy et al. (27.01) and Polack et al. (15.23) on vaccine safety/efficacy; outcome (7.62, 2021–2024) and multicenter (8.03, 2022–2024) mirrored registry studies (Brenner et al. 18.9; Ungaro et al. 12.71); and care (8.85, 2020–2022) and quality of life (7.33, 2020–2022) addressed pandemic-era IBD care continuity.
4 Discussion
This bibliometric analysis of 3,225 publications from WoSCC establishes a comprehensive framework to map the research landscape of the virus-IBD field.
4.1 General information
The annual publication trends reveal a three-phase evolution: initial fluctuating growth (2014–2019), a sharp surge (2020–2021), and a subsequent decline (2022–2024). These shifts reflect the adoption trajectory of virome research in IBD, influenced by methodological advances in metaviromics, the accelerated integration of high-throughput sequencing, and the transient focus on virology during the COVID-19 pandemic. The recent decline may indicate a post-pandemic refocusing toward mechanistic studies of chronic virus-host interactions and microbiota-virome ecology, alongside persistent challenges in clinical translation. Despite these fluctuations, annual output remained within a stable range, underscoring the maturation of virus-IBD research as a distinct subspecialty.
The USA and China emerged as the dominant contributors, collectively accounting for 46.6% of publications, reflecting their substantial investments and research infrastructure. The USA led in total output (996 publications, 30.88%) and maintained a high average GCS (57.98), indicative of strong scholarly influence. China ranked second in output (507, 15.72%) but had a lower average GCS (27.50), suggesting room for greater international impact. Other high-output countries included Italy, the UK, and Germany. Notably, Canada (avg. GCS 58.29) and Australia (56.98) achieved high per-paper citation rates despite moderate productivity, reflecting focused research excellence. Regional disparities were further evident in LCS. Belgium, France, and Canada led in average LCS, whereas several East Asian nations and emerging economies (e.g., China, South Korea, Iran) showed lower regional citation visibility. Collaboration networks were most intensive among North American and Western European countries, highlighting the role of established research ecosystems in fostering high-impact partnerships.
Institutional analysis underlined the dominance of the institutions from the USA, which constituted 13 of the top 20 producers. The Mayo Clinic led in publication volume (73), the Icahn School of Medicine at Mount Sinai had the highest total LCS (723), and UCSF achieved the highest total and average GCS (9,624 and 240.60, respectively). Institutions such as Humanitas University (Italy) also demonstrated notable regional impact, with an exceptional average LCS (16.76). Network visualization confirmed that leading institutions–including the Mayo Clinic, Icahn School of Medicine, and Harvard Medical School–serve as central hubs within collaborative frameworks, bridging basic and clinical research domains.
Author analysis identified key contributors: Francis A. Farraye, Silvio Danese, and Jean-Frederic Colombel were among the most prolific, while Shaji Sebastian and Siew C. Ng led in LCS and GCS, respectively. Jean-Frederic Colombel had the highest H-index (135), reflecting sustained academic influence. The co-authorship network revealed four major clusters, with tightly integrated collaborations around Jean-Frederic Colombel, Silvio Danese, and Francis A. Farraye, while the cluster led by Suk-Kyun Yang (South Korea) was relatively isolated, suggesting regionally concentrated activity.
Journals such as Inflammatory Bowel Diseases (135 articles), Frontiers in Immunology (100), and Journal of Crohn’s & Colitis (81) were the most prolific. Gastroenterology had the highest average GCS (244.64) and, with Gut, the highest Impact Factors, affirming the role of leading clinical journals in high-impact virus-IBD research. Frontiers in Immunology ranked second in total GCS (3,931), highlighting its niche focus on immunovirological mechanisms. Journals including International Journal of Molecular Sciences and Scientific Reports exhibited strong recent impact based on H5 index, illustrating the multidisciplinary nature of the field.
4.2 Hotspots and Frontiers
Keyword co-occurrence and reference analysis reveal the conceptual evolution and emerging priorities within virus-IBD research. Two major thematic shifts were identified: an initial focus on opportunistic infections, virome ecology, and clinical management (2014–2019), followed by a pronounced pivot toward COVID-19-related outcomes, vaccination, and pandemic-era care (2020–2024). Concurrently, keyword clustering highlights persistent interest in immune mechanisms, viral pathogenesis, and microbiome interactions. These dynamics frame three current research hotspots: gut virome mechanisms and therapy, clinical strategies against opportunistic viral infections and IBD management during COVID-19-each supported by robust citation activity and keyword emergence, as further discussed below.
4.2.1 Mechanisms and therapeutic strategies of gut virome regulation in IBD pathogenesis
The gut virome plays a critical role in the pathogenesis of IBD through bacteriophage-mediated modulation of bacterial communities and immune interactions (Liang et al., 2021; Tun et al., 2024). Metagenomic analyses show consistent structural changes in IBD, including reduced viral α-diversity, increased Caudovirales bacteriophages, and disease-specific markers such as mucosal Hepadnaviridae in UC and Hepeviridae in CD (Wagner et al., 2013; Clooney et al., 2019; Ungaro et al., 2019; Zuo et al., 2019; Liang et al., 2020). Active CD is further characterized by a reduced ratio of lytic to temperate phages (Cao et al., 2024). These virome perturbations correlate directly with dysbiosis severity and show diagnostic potential (Gulyaeva et al., 2022; Tian et al., 2024), indicating their active role in pathogenesis (Norman et al., 2015; Shkoporov et al., 2019).
The interaction between the virome and the immune system occurs through distinct pathways. Pro-inflammatory processes are characterized by phage-mediated release of pathogen-associated molecular patterns (PAMPs), which activate Toll-like receptor 9 (TLR9)-dependent secretion of interferon gamma (IFN-γ), thereby intensifying mucosal inflammation (Gogokhia et al., 2019; Buttimer et al., 2023). Additionally, host genetic factors, such as mutations in ATG16L1 associated with CD, can interact with viral infections like norovirus to exacerbate pathological conditions (Nakase et al., 2021; Hamade et al., 2024; Zhang et al., 2024). Conversely, protective mechanisms mediated by enteric eukaryotic viruses enhance intestinal barrier integrity through the suppression of nuclear factor kappa B (NF-κB) via TLR3 and TLR7 pathways, particularly in UC (Yang et al., 2016; Nishio et al., 2021; Adiliaghdam et al., 2022).
These insights inform emerging therapeutic strategies. Phage-based precision therapy can target and eliminate specific pathogens (e.g., Streptococcus gallolyticus), reducing intestinal inflammation through selective bacterial lysis (Li et al., 2024; Zhao et al., 2025). Fecal virome transplantation has shown efficacy in resolving refractory infections in patients with IBD (Rasmussen et al., 2024). However, translational challenges related to delivery efficiency and safety require validation through large-scale clinical trials.
4.2.2 Clinical challenges and management strategies of opportunistic viral infections
Bibliometric analysis identifies opportunistic viral infections-particularly Epstein-Barr virus (EBV), CMV, Hepatitis B virus (HBV)/Hepatitis C virus (HCV), and HIV–as a major research cluster. Patients with IBD are at elevated risk due to immunosuppressive therapies (e.g., corticosteroids, thiopurines), malnutrition, and comorbidities, underscoring the importance of guideline-driven management (Dave et al., 2014; Rahier et al., 2014).
EBV seroprevalence in patients with IBD reaches 79.4%–100%, and CMV prevalence is 34.5%, markedly higher than those in healthy populations (Linton et al., 2013; Wang et al., 2022). Thiopurine use and chronic inflammation increase the risk of EBV- or CMV-related complications, including hemophagocytic lymphohistiocytosis and lymphoproliferative disorders (Deneau et al., 2010; Wu et al., 2019). EBV co-infection may mimic or exacerbate CD (Zhang et al., 2022a), while CMV is frequently detected in steroid-refractory UC and contributes to glucocorticoid resistance and histopathological severity (Siegmund, 2017; Kwon et al., 2022).
Immunosuppression also elevates HBV/HCV reactivation risk, particularly under anti-TNF therapy, necessitating preemptive screening, vaccination, and antiviral prophylaxis when indicated (Singh et al., 2022). Double-dose HBV vaccination improves seroconversion in these patients (Gisbert et al., 2012). HIV-IBD interplay presents a paradox: HIV-induced CD4+ depletion may attenuate intestinal inflammation in some cases (“immune exhaustion remission”), though meta-evidence remains conflicting (Guillo et al., 2022; Huang et al., 2024). Nevertheless, opportunistic infections remain more common in HIV-positive patients with IBD (Calafat et al., 2025).
Preventive strategies emphasize systematic screening (HBV, HCV, HIV, Varicella-Zoster virus) before immunosuppression, alongside inactivated vaccination where applicable (Farraye et al., 2017; Lamb et al., 2019; Caldera et al., 2025). Live vaccines are contraindicated during active immunosuppression (Kucharzik et al., 2021). During active viral infection, immunosuppressants should be withheld and antiviral therapy initiated. While antivirals are effective in CMV colitis, their role in EBV-related disease is less well-defined, highlighting a need for randomized trials (Ciccocioppo et al., 2015).
4.2.3 Management of IBD during the COVID-19 pandemic
Keyword burst analysis revealed that pre-COVID-19 research focused on virome profiling, opportunistic infections, and virus-mediated immune-inflammatory pathways. The pandemic significantly reshaped research priorities, consistent with earlier bibliometric studies (Veisman et al., 2022; Wang et al., 2023). Research emphasis shifted toward COVID-19 susceptibility and outcomes in patients with IBD, as well as vaccination challenges during immunotherapy. Post-pandemic, attention has increasingly turned to the interaction networks between the gut microbiome, virome, and IBD pathogenesis.
Large-scale epidemiological studies indicate that patients with IBD have infection rates comparable to the general population (Monteleone and Ardizzone, 2020). However, the risk of severe COVID-19 is heightened in those with active disease, advanced age, comorbidities, or corticosteroid treatment (Brenner et al., 2020; Ungaro et al., 2021; Ricciuto et al., 2022). Immunotherapy management and vaccination optimization became critical priorities, leading to guidelines from the British Society of Gastroenterology and the American College of Gastroenterology (Kennedy et al., 2020; Rubin et al., 2020), later refined through international consensus on evidence-based vaccination strategies (Lin et al., 2022). Cohort studies confirm vaccine safety and efficacy without IBD exacerbation (Lev-Tzion et al., 2022; Summa and Hanauer, 2023; Viazis et al., 2023). This evidence base provides a framework for managing future infectious disease outbreaks in IBD, guiding immunotherapy and vaccine response optimization.
4.3 Limitations
This study offers a systematic bibliometric perspective on virus-IBD interactions but has several limitations. First, only English-language publications were included, which may overlook geographically distinctive virome patterns reported in non-English literature. This introduces potential bias in regional representation and keyword analysis, overrepresenting outputs from English-speaking regions. Second, the analysis relied solely on WoSCC. Although WoSCC was chosen for its well-curated metadata and compatibility with standard bibliometric tools, it underrepresents certain regions and journals compared to broader databases such as Scopus or PubMed, which may affect the geographic and thematic comprehensiveness of the results. Third, keyword co-occurrence analysis is constrained by author-defined terminology, which often includes inconsistent nomenclature leading to fragmented conceptual clusters. Despite applied normalization procedures, infrequent terms remain susceptible to semantic fragmentation. Furthermore, co-occurrence networks simplify complex semantic relationships, and algorithmic pruning may emphasize specific citation patterns. Finally, the inclusion of a considerable number of review articles may inflate citation counts, as they are typically cited more frequently than original articles. This could confound the identification of seminal original research. To address these issues, future studies should: (1) incorporate multiple databases to improve geographic and thematic coverage; (2) employ natural language processing for more consistent term standardization; and (3) test robustness using varied analytical parameters and document-type stratification. Nonetheless, multidimensional validation supports the stability of the core conclusions presented here.
5 Conclusion
This bibliometric analysis systematically delineates the knowledge structure and evolving trends in virus-IBD research, identifying three predominant research areas. The findings reflect an ongoing evolution from pathogen-specific studies toward a more integrative understanding of virus-host-microbiota interactions. The results provide actionable insights for both clinical practice and research strategy: they highlight promising translational domains such as virome-based biomarkers and phage therapy, while also revealing underexplored topics-such as cross-regional virome disparities-that warrant targeted funding. Future research should prioritize: (1) mechanistic investigations using multi-omics approaches to identify key regulatory nodes through which viruses influence gut immune homeostasis, with emphasis on innate immune receptor-virome interactions; (2) developing innovative virome-targeted therapies, including phage-based interventions; (3) advancing clinical translation by integrating virome-derived biomarkers into personalized therapeutic frameworks, such as virome-guided subtyping of IBD. This evidence-based framework helps identify interdisciplinary research gaps, supports resource allocation, and informs public health policy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
WZ: Writing – original draft, Conceptualization, Writing – review & editing. MZ: Methodology, Writing – review & editing, Supervision. YZhou: Data curation, Writing – review & editing, Supervision. QT: Writing – review & editing. YZhu: Methodology, Funding acquisition, Conceptualization, Writing – review & editing. YX: Writing – review & editing, Conceptualization, Funding acquisition, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the National Natural Science Foundation of China (grant numbers 82374426, 81874466, and 81904176), the Natural Science Foundation of Hunan Province (grant numbers 2021JJ30531 and 2023JJ60044), the Research Program Project of the Hunan Health Commission (grant number C202303036268), the Clinical Medical Technology Innovation Guide Project of Hunan Province (grant number 2021SK51413), the Postgraduate Scientific Research Innovation Project of Hunan Province (grant number CX20240748), the Hunan Province Administration of Traditional Chinese Medicine (grant number B2023079), the Research Program of Hunan University of Chinese Medicine (grant number 2023XYLH032), and the domestic first-class construction discipline of Chinese Medicine at Hunan University of Chinese Medicine.
Acknowledgments
We appreciate the involvement of all authors in this study.
Conflict of interest
The authors declare that the research was conducted without any commercial or financial relationships that could potentially create a conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://github.com/vigour7059/Data-for-bibliometric-analysis.git
References
Adiliaghdam, F., Amatullah, H., Digumarthi, S., Saunders, T. L., Rahman, R.-U., Wong, L. P., et al. (2022). Human enteric viruses autonomously shape inflammatory bowel disease phenotype through divergent innate immunomodulation. Sci. Immunol. 7:eabn6660. doi: 10.1126/sciimmunol.abn6660
Baumgart, D. C., and Carding, S. R. (2007). Inflammatory bowel disease: Cause and immunobiology. Lancet 369, 1627–1640. doi: 10.1016/S0140-6736(07)60750-8
Bezzio, C., Saibeni, S., Variola, A., Allocca, M., Massari, A., Gerardi, V., et al. (2020). Outcomes of COVID-19 in 79 patients with IBD in Italy: An IG-IBD study. Gut 69, 1213–1217. doi: 10.1136/gutjnl-2020-321411
Brenner, E. J., Ungaro, R. C., Gearry, R. B., Kaplan, G. G., Kissous-Hunt, M., Lewis, J. D., et al. (2020). Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: Results from an international registry. Gastroenterology 159, 481–491.e3. doi: 10.1053/j.gastro.2020.05.032
Buttimer, C., Khokhlova, E. V., Stein, L., Hueston, C. M., Govi, B., Draper, L. A., et al. (2023). Temperate bacteriophages infecting the mucin-degrading bacterium Ruminococcus gnavus from the human gut. Gut Microbes 15:2194794. doi: 10.1080/19490976.2023.2194794
Cadwell, K., Patel, K. K., Maloney, N. S., Liu, T.-C., Ng, A. C. Y., Storer, C. E., et al. (2010). Virus-plus-susceptibility gene interaction determines Crohn’s disease gene Atg16L1 phenotypes in intestine. Cell 141, 1135–1145. doi: 10.1016/j.cell.2010.05.009
Calafat, M., Suria, C., Mesonero, F., de Francisco, R., Yagüe Caballero, C., de la Peña, L., et al. (2025). HIV infection is associated with a less aggressive phenotype of inflammatory bowel disease: A multicenter study of the ENEIDA registry. Am. J. Gastroenterol. 120, 431–439. doi: 10.14309/ajg.0000000000002965
Caldera, F., Kane, S., Long, M., and Hashash, J. G. (2025). AGA clinical practice update on noncolorectal cancer screening and vaccinations in patients with inflammatory bowel disease: Expert review. Clin. Gastroenterol. Hepatol. 23, 695–706. doi: 10.1016/j.cgh.2024.12.011
Cao, Z., Fan, D., Sun, Y., Huang, Z., Li, Y., Su, R., et al. (2024). The gut ileal mucosal virome is disturbed in patients with Crohn’s disease and exacerbates intestinal inflammation in mice. Nat. Commun. 15:1638. doi: 10.1038/s41467-024-45794-y
Chen, C., and Song, M. (2019). Visualizing a field of research: A methodology of systematic scientometric reviews. PLoS One 14:e0223994. doi: 10.1371/journal.pone.0223994
Ciccocioppo, R., Racca, F., Paolucci, S., Campanini, G., Pozzi, L., Betti, E., et al. (2015). Human cytomegalovirus and Epstein-Barr virus infection in inflammatory bowel disease: Need for mucosal viral load measurement. World J. Gastroenterol. 21, 1915–1926. doi: 10.3748/wjg.v21.i6.1915
Clooney, A. G., Sutton, T. D. S., Shkoporov, A. N., Holohan, R. K., Daly, K. M., O’Regan, O., et al. (2019). Whole-Virome analysis sheds light on viral dark matter in inflammatory bowel disease. Cell Host Microbe 26, 764–778.e5. doi: 10.1016/j.chom.2019.10.009
Cornuault, J. K., Petit, M.-A., Mariadassou, M., Benevides, L., Moncaut, E., Langella, P., et al. (2018). Phages infecting Faecalibacterium prausnitzii belong to novel viral genera that help to decipher intestinal viromes. Microbiome 6:65. doi: 10.1186/s40168-018-0452-1
Dave, M., Purohit, T., Razonable, R., and Loftus, E. V. (2014). Opportunistic infections due to inflammatory bowel disease therapy. Inflamm. Bowel Dis. 20, 196–212. doi: 10.1097/MIB.0b013e3182a827d2
Delvincourt, M., Lopez, A., Pillet, S., Bourrier, A., Seksik, P., Cosnes, J., et al. (2014). The impact of cytomegalovirus reactivation and its treatment on the course of inflammatory bowel disease. Aliment Pharmacol. Ther. 39, 712–720. doi: 10.1111/apt.12650
Deneau, M., Wallentine, J., Guthery, S., O’Gorman, M., Bohnsack, J., Fluchel, M., et al. (2010). Natural killer cell lymphoma in a pediatric patient with inflammatory bowel disease. Pediatrics 126, e977–e981. doi: 10.1542/peds.2010-0486
Farraye, F. A., Melmed, G. Y., Lichtenstein, G. R., and Kane, S. V. (2017). ACG clinical guideline: Preventive care in inflammatory bowel disease. Am. J. Gastroenterol. 112, 241–258. doi: 10.1038/ajg.2016.537
Fernandes, M. A., Verstraete, S. G., Phan, T. G., Deng, X., Stekol, E., LaMere, B., et al. (2019). Enteric virome and bacterial microbiota in children with ulcerative colitis and crohn disease. J. Pediatr. Gastroenterol. Nutr. 68, 30–36. doi: 10.1097/MPG.0000000000002140
Friedrich, M., Pohin, M., and Powrie, F. (2019). Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity 50, 992–1006. doi: 10.1016/j.immuni.2019.03.017
GBD 2017 Inflammatory Bowel Disease Collaborators (2020). The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 5, 17–30. doi: 10.1016/S2468-1253(19)30333-4
Gisbert, J. P., Menchén, L., García-Sánchez, V., Marín, I., Villagrasa, J. R., and Chaparro, M. (2012). Comparison of the effectiveness of two protocols for vaccination (standard and double dosage) against hepatitis B virus in patients with inflammatory bowel disease. Aliment Pharmacol. Ther. 35, 1379–1385. doi: 10.1111/j.1365-2036.2012.05110.x
Gogokhia, L., Buhrke, K., Bell, R., Hoffman, B., Brown, D. G., Hanke-Gogokhia, C., et al. (2019). Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host Microbe 25, 285–299.e8. doi: 10.1016/j.chom.2019.01.008
Guillo, L., Uzzan, M., Beaugerie, L., Gornet, J.-M., Amiot, A., Pelletier, A.-L., et al. (2022). Impact of HIV infection on the course of inflammatory bowel disease and drug safety profile: A multicenter GETAID study. Clin. Gastroenterol. Hepatol. 20, 787–797.e2. doi: 10.1016/j.cgh.2020.12.023
Gulyaeva, A., Garmaeva, S., Ruigrok, R. A. A. A., Wang, D., Riksen, N. P., Netea, M. G., et al. (2022). Discovery, diversity, and functional associations of crAss-like phages in human gut metagenomes from four Dutch cohorts. Cell Rep. 38:110204. doi: 10.1016/j.celrep.2021.110204
Hamade, H., Tsuda, M., Oshima, N., Stamps, D. T., Wong, M. H., Stamps, J. T., et al. (2024). Toll-like receptor 7 protects against intestinal inflammation and restricts the development of colonic tissue-resident memory CD8+ T cells. Front. Immunol. 15:1465175. doi: 10.3389/fimmu.2024.1465175
Huang, W., Zhang, Y.-D., Wang, P., Song, C.-Y., Lu, X., Feng, M.-X., et al. (2024). HIV infection increases the risk of inflammatory bowel disease: A systematic review and meta-analysis. BMC Infect. Dis. 24:1030. doi: 10.1186/s12879-024-09964-z
Kennedy, N. A., Jones, G.-R., Lamb, C. A., Appleby, R., Arnott, I., Beattie, R. M., et al. (2020). British society of gastroenterology guidance for management of inflammatory bowel disease during the COVID-19 pandemic. Gut 69, 984–990. doi: 10.1136/gutjnl-2020-321244
Kennedy, N. A., Lin, S., Goodhand, J. R., Chanchlani, N., Hamilton, B., Bewshea, C., et al. (2021). Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut 70, 1884–1893. doi: 10.1136/gutjnl-2021-324789
Khrom, M., Long, M., Dube, S., Robbins, L., Botwin, G. J., Yang, S., et al. (2024). Comprehensive association analyses of extraintestinal manifestations in inflammatory bowel disease. Gastroenterology 167, 315–332. doi: 10.1053/j.gastro.2024.02.026
Kirchgesner, J., Lemaitre, M., Carrat, F., Zureik, M., Carbonnel, F., and Dray-Spira, R. (2018). Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology 155, 337–346.e10. doi: 10.1053/j.gastro.2018.04.012
Kobayashi, T., Siegmund, B., Le Berre, C., Wei, S. C., Ferrante, M., Shen, B., et al. (2020). Ulcerative colitis. Nat. Rev. Primer 6:74. doi: 10.1038/s41572-020-0205-x
Kucharzik, T., Ellul, P., Greuter, T., Rahier, J. F., Verstockt, B., Abreu, C., et al. (2021). ECCO guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J. Crohns Colitis 15, 879–913. doi: 10.1093/ecco-jcc/jjab052
Kwon, J., Fluxá, D., Farraye, F. A., and Kröner, P. T. (2022). Cytomegalovirus-related colitis in patients with inflammatory bowel disease. Int. J. Colorectal Dis. 37, 685–691. doi: 10.1007/s00384-022-04099-6
Lamb, C. A., Kennedy, N. A., Raine, T., Hendy, P. A., Smith, P. J., Limdi, J. K., et al. (2019). British society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 68, s1–s106. doi: 10.1136/gutjnl-2019-318484
Lev-Tzion, R., Focht, G., Lujan, R., Mendelovici, A., Friss, C., Greenfeld, S., et al. (2022). COVID-19 vaccine is effective in inflammatory bowel disease patients and is not associated with disease exacerbation. Clin. Gastroenterol. Hepatol. 20, e1263–e1282. doi: 10.1016/j.cgh.2021.12.026
Li, Y., Li, X.-M., Duan, H.-Y., Yang, K., and Ye, J.-F. (2024). Advances and optimization strategies in bacteriophage therapy for treating inflammatory bowel disease. Front. Immunol. 15:1398652. doi: 10.3389/fimmu.2024.1398652
Liang, G., Cobián-Güemes, A. G., Albenberg, L., and Bushman, F. (2021). The gut virome in inflammatory bowel diseases. Curr. Opin. Virol. 51, 190–198. doi: 10.1016/j.coviro.2021.10.005
Liang, G., Conrad, M. A., Kelsen, J. R., Kessler, L. R., Breton, J., Albenberg, L. G., et al. (2020). Dynamics of the stool virome in very early-onset inflammatory bowel disease. J. Crohns Colitis 14, 1600–1610. doi: 10.1093/ecco-jcc/jjaa094
Lin, D., Jin, Y., Shao, X., Xu, Y., Ma, G., Jiang, Y., et al. (2024). Global, regional, and national burden of inflammatory bowel disease, 1990-2021: Insights from the global burden of disease 2021. Int. J. Colorectal. Dis. 39:139. doi: 10.1007/s00384-024-04711-x
Lin, S., Lau, L. H., Chanchlani, N., Kennedy, N. A., and Ng, S. C. (2022). Recent advances in clinical practice: Management of inflammatory bowel disease during the COVID-19 pandemic. Gut 71, 1426–1439. doi: 10.1136/gutjnl-2021-326784
Linton, M. S., Kroeker, K., Fedorak, D., Dieleman, L., and Fedorak, R. N. (2013). Prevalence of epstein-barr virus in a population of patients with inflammatory bowel disease: A prospective cohort study. Aliment Pharmacol. Ther. 38, 1248–1254. doi: 10.1111/apt.12503
Moayyedi, P., Surette, M. G., Kim, P. T., Libertucci, J., Wolfe, M., Onischi, C., et al. (2015). Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 149, 102–109.e6. doi: 10.1053/j.gastro.2015.04.001
Monteleone, G., and Ardizzone, S. (2020). Are patients with inflammatory bowel disease at increased risk for Covid-19 infection? J. Crohns Colitis 14, 1334–1336. doi: 10.1093/ecco-jcc/jjaa061
Nakase, H., Uchino, M., Shinzaki, S., Matsuura, M., Matsuoka, K., Kobayashi, T., et al. (2021). Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J. Gastroenterol. 56, 489–526. doi: 10.1007/s00535-021-01784-1
Neurath, M. F. (2019). Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat. Immunol. 20, 970–979. doi: 10.1038/s41590-019-0415-0
Ng, S. C., Shi, H. Y., Hamidi, N., Underwood, F. E., Tang, W., Benchimol, E. I., et al. (2017). Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 390, 2769–2778. doi: 10.1016/S0140-6736(17)32448-0
Nishio, J., Negishi, H., Yasui-Kato, M., Miki, S., Miyanaga, K., Aoki, K., et al. (2021). Identification and characterization of a novel Enterococcus bacteriophage with potential to ameliorate murine colitis. Sci. Rep. 11:20231. doi: 10.1038/s41598-021-99602-4
Norman, J. M., Handley, S. A., Baldridge, M. T., Droit, L., Liu, C. Y., Keller, B. C., et al. (2015). Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell 160, 447–460. doi: 10.1016/j.cell.2015.01.002
Piovani, D., Danese, S., Peyrin-Biroulet, L., Nikolopoulos, G. K., Lytras, T., and Bonovas, S. (2019). Environmental risk factors for inflammatory bowel diseases: An umbrella review of meta-analyses. Gastroenterology 157, 647–659.e4. doi: 10.1053/j.gastro.2019.04.016
Polack, F. P., Thomas, S. J., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., et al. (2020). Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615. doi: 10.1056/NEJMoa2034577
Rahier, J. F., Magro, F., Abreu, C., Armuzzi, A., Ben-Horin, S., Chowers, Y., et al. (2014). Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J. Crohns Colitis 8, 443–468. doi: 10.1016/j.crohns.2013.12.013
Rasmussen, T. S., Mao, X., Forster, S., Larsen, S. B., Von Münchow, A., Tranæs, K. D., et al. (2024). Overcoming donor variability and risks associated with fecal microbiota transplants through bacteriophage-mediated treatments. Microbiome 12:119. doi: 10.1186/s40168-024-01820-1
Reddy, K. R., Beavers, K. L., Hammond, S. P., Lim, J. K., and Falck-Ytter, Y. T., and American Gastroenterological Association Institute. (2015). American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 148, 215–219; quiz e16-17. doi: 10.1053/j.gastro.2014.10.039
Ricciuto, A., Lamb, C. A., Benchimol, E. I., Walker, G. J., Kennedy, N. A., Kuenzig, M. E., et al. (2022). Inflammatory bowel disease clinical activity is associated with COVID-19 severity especially in younger patients. J. Crohns Colitis 16, 591–600. doi: 10.1093/ecco-jcc/jjab172
Roda, G., Chien, Ng, S., Kotze, P. G., Argollo, M., Panaccione, R., et al. (2020). Crohn’s disease. Nat. Rev. Primer 6:22. doi: 10.1038/s41572-020-0156-2
Rubin, D. T., Feuerstein, J. D., Wang, A. Y., and Cohen, R. D. (2020). AGA clinical practice update on management of inflammatory bowel disease during the COVID-19 pandemic: Expert commentary. Gastroenterology 159, 350–357. doi: 10.1053/j.gastro.2020.04.012
Sartor, R. B., and Wu, G. D. (2017). Roles for intestinal bacteria, viruses, and fungi in pathogenesis of inflammatory bowel diseases and therapeutic approaches. Gastroenterology 152, 327–339.e4. doi: 10.1053/j.gastro.2016.10.012
Shkoporov, A. N., Clooney, A. G., Sutton, T. D. S., Ryan, F. J., Daly, K. M., Nolan, J. A., et al. (2019). The human gut virome is highly diverse, stable, and individual specific. Cell Host Microbe 26, 527–541.e5. doi: 10.1016/j.chom.2019.09.009
Siegmund, B. (2017). Cytomegalovirus infection associated with inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2, 369–376. doi: 10.1016/S2468-1253(16)30159-5
Singh, A. K., Jena, A., Mahajan, G., Mohindra, R., Suri, V., and Sharma, V. (2022). Meta-analysis: Hepatitis B vaccination in inflammatory bowel disease. Aliment. Pharmacol. Ther. 55, 908–920. doi: 10.1111/apt.16880
Summa, K. C., and Hanauer, S. B. (2023). COVID-19 and Inflammatory Bowel Disease. Gastroenterol. Clin. North Am. 52, 103–113. doi: 10.1016/j.gtc.2022.10.005
Tarris, G., de Rougemont, A., Charkaoui, M., Michiels, C., Martin, L., and Belliot, G. (2021). Enteric viruses and inflammatory bowel disease. Viruses 13:104. doi: 10.3390/v13010104
Tian, X., Li, S., Wang, C., Zhang, Y., Feng, X., Yan, Q., et al. (2024). Gut virome-wide association analysis identifies cross-population viral signatures for inflammatory bowel disease. Microbiome 12:130. doi: 10.1186/s40168-024-01832-x
Tun, H. M., Peng, Y., Massimino, L., Sin, Z. Y., Parigi, T. L., Facoetti, A., et al. (2024). Gut virome in inflammatory bowel disease and beyond. Gut 73, 350–360. doi: 10.1136/gutjnl-2023-330001
Ungaro, F., Massimino, L., Furfaro, F., Rimoldi, V., Peyrin-Biroulet, L., D’Alessio, S., et al. (2019). Metagenomic analysis of intestinal mucosa revealed a specific eukaryotic gut virome signature in early-diagnosed inflammatory bowel disease. Gut Microbes 10, 149–158. doi: 10.1080/19490976.2018.1511664
Ungaro, R. C., Brenner, E. J., Gearry, R. B., Kaplan, G. G., Kissous-Hunt, M., Lewis, J. D., et al. (2021). Effect of IBD medications on COVID-19 outcomes: Results from an international registry. Gut 70, 725–732. doi: 10.1136/gutjnl-2020-322539
Veisman, I., Lederer, N. B., Ukashi, O., Kopylov, U., and Klang, E. (2022). Top 25 cited articles on Covid-19 and IBD: A bibliometric analysis. Clin. Res. Hepatol. Gastroenterol. 46:101959. doi: 10.1016/j.clinre.2022.101959
Viazis, N., Drygiannakis, I., Karmiris, K., Theodoropoulou, A., Zampeli, E., Tzouvala, M., et al. (2023). The natural history of COVID-19 in vaccinated inflammatory bowel disease patients. Dig. Liver Dis. 55, 305–309. doi: 10.1016/j.dld.2022.12.012
Wagner, J., Maksimovic, J., Farries, G., Sim, W. H., Bishop, R. F., Cameron, D. J., et al. (2013). Bacteriophages in gut samples from pediatric Crohn’s disease patients: Metagenomic analysis using 454 pyrosequencing. Inflamm. Bowel Dis. 19, 1598–1608. doi: 10.1097/MIB.0b013e318292477c
Wang, F., Xie, J., Xiong, H., and Xie, Y. (2023). A bibliometric analysis of inflammatory bowel disease and COVID-19 researches. Front. Public Health 11:1039782. doi: 10.3389/fpubh.2023.1039782
Wang, W., Chen, X., Pan, J., Zhang, X., and Zhang, L. (2022). Epstein-Barr virus and human cytomegalovirus infection in intestinal mucosa of chinese patients with inflammatory bowel disease. Front. Microbiol. 13:915453. doi: 10.3389/fmicb.2022.915453
Wu, S., He, C., Tang, T.-Y., and Li, Y.-Q. (2019). A review on co-existent Epstein-Barr virus-induced complications in inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 31, 1085–1091. doi: 10.1097/MEG.0000000000001474
Wu, Y., Cheng, R., Lin, H., Li, L., Jia, Y., Philips, A., et al. (2025). Gut virome and its implications in the pathogenesis and therapeutics of inflammatory bowel disease. BMC Med. 23:183. doi: 10.1186/s12916-025-04016-y
Yang, J.-Y., Kim, M.-S., Kim, E., Cheon, J. H., Lee, Y.-S., Kim, Y., et al. (2016). Enteric viruses ameliorate gut inflammation via toll-like receptor 3 and toll-like receptor 7-mediated Interferon-β production. Immunity 44, 889–900. doi: 10.1016/j.immuni.2016.03.009
Zhang, A., Wang, F., Li, D., Wang, C.-Z., Yao, H., Wan, J.-Y., et al. (2023). Emerging insights into inflammatory bowel disease from the intestinal microbiota perspective: A bibliometric analysis. Front. Immunol. 14:1264705. doi: 10.3389/fimmu.2023.1264705
Zhang, H., Zhao, S., and Cao, Z. (2022a). Impact of epstein-barr virus infection in patients with inflammatory bowel disease. Front. Immunol. 13:1001055. doi: 10.3389/fimmu.2022.1001055
Zhang, L., Xiong, S., Jin, F., Zhou, F., Zhou, H., Guo, J., et al. (2022b). Global trends in intestinal flora and ulcerative colitis research during the past 10 years: A bibliometric analysis. Front Microbiol. 13:1003905. doi: 10.3389/fmicb.2022.1003905
Zhang, W., Zou, M., Fu, J., Xu, Y., and Zhu, Y. (2024). Autophagy: A potential target for natural products in the treatment of ulcerative colitis. Biomed. Pharmacother. 176:116891. doi: 10.1016/j.biopha.2024.116891
Zhao, Y., Zhu, M., Ling, Y., Zhao, Y., Lu, X., Chu, B., et al. (2025). A DNA nanopatch-bacteriophage system targeting Streptococcus gallolyticus for inflammatory bowel disease treatment and colorectal cancer prevention. Adv. Mater. 37:e2417334. doi: 10.1002/adma.202417334
Keywords: inflammatory bowel diseases, Crohn disease, ulcerative colitis, virus, gut virome, opportunistic infections, COVID-19, bibliometric analysis
Citation: Zhang W, Zou M, Zhou Y, Tang Q, Zhu Y and Xu Y (2025) Potential value and research frontiers of viruses in inflammatory bowel diseases: a bibliometric analysis. Front. Microbiol. 16:1624508. doi: 10.3389/fmicb.2025.1624508
Received: 07 May 2025; Accepted: 23 September 2025;
Published: 15 October 2025.
Edited by:
Ashok Kumar Jangid, Dongguk University Seoul, Republic of KoreaReviewed by:
Yun Xia, Wuhan University, ChinaAbdullah Al Marzan, Dhaka Medical College and Hospital, Bangladesh
Suleiman Adeiza Shuaibu, Ahmadu Bello University, Nigeria
Mizbahul Karim Hemo, Primeasia University, Bangladesh
Copyright © 2025 Zhang, Zou, Zhou, Tang, Zhu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Zhu, emh1eWluZzA4OUAxMjYuY29t; Yin Xu, NjczMDE4ODcyQHFxLmNvbQ==
 Wei Zhang
Wei Zhang Menglong Zou
Menglong Zou Yao Zhou2
Yao Zhou2 Qiwei Tang
Qiwei Tang