- 1Division of Animal Science, ICAR-Central Island Agricultural Research Institute (I.C.A.R-C.I.A.R.I), Port Blair, India
- 2Department of Veterinary Microbiology, West Bengal University of Animal and Fishery Sciences (W.B.U.A.F.S), Kolkata, India
- 3Institute of Animal Hygiene and Veterinary Public Health, University of Leipzig, Leipzig, Germany
- 4Division of Biochemistry, ICMR-Regional Medical Research Centre (I.C.M.R-R.M.R.C), Port Blair, India
- 5Department of Veterinary Pathology, West Bengal University of Animal and Fishery Sciences (W.B.U.A.F.S), Kolkata, India
Antibiotic growth promoters (AGPs) are widely used as feed additives to enhance the immunity, and productivity in the poultry industries. But over usage of AGPs has led to multi drug-resistance among pathogens. Nonspecific immunomodulators like probiotics have emerged as competent replacements of AGPs. Probiotics plays a key role in gut microbial health by its mechanism of action and modulation of host immune system. No prior research has been conducted in the Andaman and Nicobar Islands, India to elucidate the direct influence of probiotics on health and immunity of backyard poultry. To explore an efficient alternative to AGP, a commercial multi strain probiotics (BifilacR) was evaluated in Vanaraja, a popular backyard poultry breed reared in the islands. 120 newly hatched Vanaraja chicks were chosen, 30 chicks were randomly allocated into 4 different groups for 60 days. For the negative control (NC), chicks were fed only basal diet. For the positive control (PC), chicks were fed with basal diet + AGP (Tetracycline). Test group (T1) was fed basal diet + 0.1% of BifilacR. The test group (T2) was fed basal diet + 0.3% of BifilacR. The results showed that the mean body weight of chicks supplemented with 0.1% (T1) and 0.3% (T2) of multi-strain probiotics was significantly higher (p ≤ 0.05) compared to the control groups. A significant increase (p ≤ 0.05) in FCR was also observed among T1 and T2 at different time intervals. Both T1 and T2 expressed significant changes (p ≤ 0.05) in biochemical parameters such as albumin, globulin, BUN, total bilirubin, SGOT and SGPT at different time intervals than the control groups. A significant decrease (p ≤ 0.05) was noticed in T1 and T2 groups in the levels of triglycerides, HDLc, LDLc, total cholesterol, superoxide production, lipid peroxidation at different time intervals. A significant increase (p ≤ 0.05) was observed in the levels of HSP70, IL4, IL2, and lymphocyte proliferation in T1 and T2 compared to the control groups. After histomorphological analysis, an increase (p ≤ 0.001) in villus height (μm) and crypt depth (μm) in duodenum and jejunum were noticed in T1 and T2. In short, multi-strain probiotics supplementation showed its potential as an overall growth promoter in terms of improved growth performance, favorable physiological functions, enhanced immunomodulatory effects and better intestinal morphology in a widely reared backyard poultry breed of Andaman and Nicobar Islands, India, hence can be nominated as a potential alternative to commercial antibiotics at ground level.
Introduction
The poultry sector is known for contributing remarkably in livelihood support and ensuring nutritional security amid growing global demands. In particular, rural poultry is vital for small and resource-limited rural communities and is typically reared in extensive and semi-intensive systems, making it one of the fastest-growing segments in agriculture (Nyoni and Masika, 2012). Backyard poultry breeds are always highly acclaimed for their superior adaptability in their habitat, ability to survive in harsh climate, disease resilience, high nutritional value, also the requirement of less infrastructure set-up and low management (Gondwe and Wollny, 2002).
To meet the increasing global demand, poultry flocks often endure significant stress. As a consequence, commercial antibiotics are frequently used to prevent various avian disease, to promote growth, and boost immunity in poultry flocks (Cheng et al., 2015). However, the widespread use of antibiotics has led to the emergence of antibiotic-resistant pathogens (Garcia-Migura et al., 2014; Roth et al., 2019). World Health Organization has identified antibiotic resistance as “a serious threat to public health worldwide that requires action across all government sectors and society” (WHO, 2015). In response, several countries such as Sweden, Denmark, South Korea, Germany, and Taiwan have banned the use of antibiotics in animal feed (Ziggers, 2011). Also, nations like Netherlands, France, Italy, Belgium, Norway, and Finland have established national programs to monitor antimicrobial resistance (Garcia-Migura et al., 2014).
Over an extended period, studies have highlighted the potentials of probiotics and their ability to curb antibiotic resistance in pathogenic bacteria within the poultry sector. In recent years, non-specific immunomodulators like prebiotics, probiotics, postbiotics, synbiotics, essential oils, polysaccharides, enzymes, and organic acids have emerged as remarkably effective substitute for marketed antibiotics, demonstrating safety, feasibility to strengthen the poultry microbiota (Callaway et al., 2017; Shi et al., 2019). The word “probiotics” originated from Greek language, which means “prolife” (Shokryazdan et al., 2017). FAO/WHO illustrated probiotics as “live organisms that when administered in adequate amount confer a health benefit on the host” (Food Agriculture Organization/World Health Organization, 2001). Probiotics are recognized for their capacity to enhancing the microflora and bolstering immunity (Chen et al., 2017). Integrating probiotics into animal diets has upgraded their growth and productivity outcomes, digestibility, immunity, fecal microflora etc. in livestock (Cavalheiro et al., 2015; Zhao and Kim, 2015; Lan et al., 2017). The action mechanism of probiotics primarily involves outcompeting harmful pathogens. This includes producing inhibitory substances, blocking pathogen adhesion, competing for available nutrients, reducing toxin bioavailability, and influencing the host’s immune response (Hernandez-Patlan et al., 2020).
Multi-strain probiotics are combinations of different strains from the same species or various bacterial genera, which support the host’s health and immune system (Kwoji et al., 2021). An increase in the chicken body weight was observed when enriched with a Lactobacillus-based probiotics at a concentration of 1 × 109CFU/g, either alone or in combination with prebiotics or synbiotics (Mookiah et al., 2014). Pasteurella multocida challenged broilers showed improved growth efficiency, feed consumption, and intestinal wellbeing when enriched with multi-strain probiotics blend containing L. fermentum, S. cerevisiae, E. faecium, L. plantarum, and P. acidilactici (Lambo et al., 2021). Earlier reports showed the positive effects of multi-strain probiotics supplementation on growth efficiency, lipid oxidation, intestinal structure, pathogen control, and gut microbiome development in chickens (Kazemi et al., 2019; Ramlucken et al., 2020; Fesseha et al., 2021).
The usage of probiotics in rural backyard poultry is still an unexplored area of study, especially in tropical islands. Hence, to assess the benefits and potentials of multi-strain probiotics on rural poultry, this study is conceptualized. The present study sheds light on the impact of multi-strain probiotics on the growth outcomes, biochemical serum profile, immunomodulation, and intestinal architecture of a rural poultry, viz. Vanaraja, a dual-purpose chicken variety, under the tropical climate of the Andaman and Nicobar Islands, India.
Materials and methods
Study area
The study was conducted in the livestock farm of I.C.A.R – Central Island Agricultural Research Institute (C.I.A.R.I), Port Blair, South Andaman, India (11.6060°N, 92.7058°E) (Figure 1). Open-source software QGIS 3.16.0v “Hannover” software was used to develop the map.
Figure 1. Map showing the area of the present study (C.I.A.R.I, Port Blair, Andaman and Nicobar Islands, India).
Ethical approval
Approval was obtained in accordance with F. No: AS/IAEC/22, from Institute Animal Ethical Committee, Animal House Facility of Establishment (247/GO/RBi/SL/2000/CPCSEA), ICAR-CIARI, Port Blair, Andaman and Nicobar Islands, India.
Recording environmental data
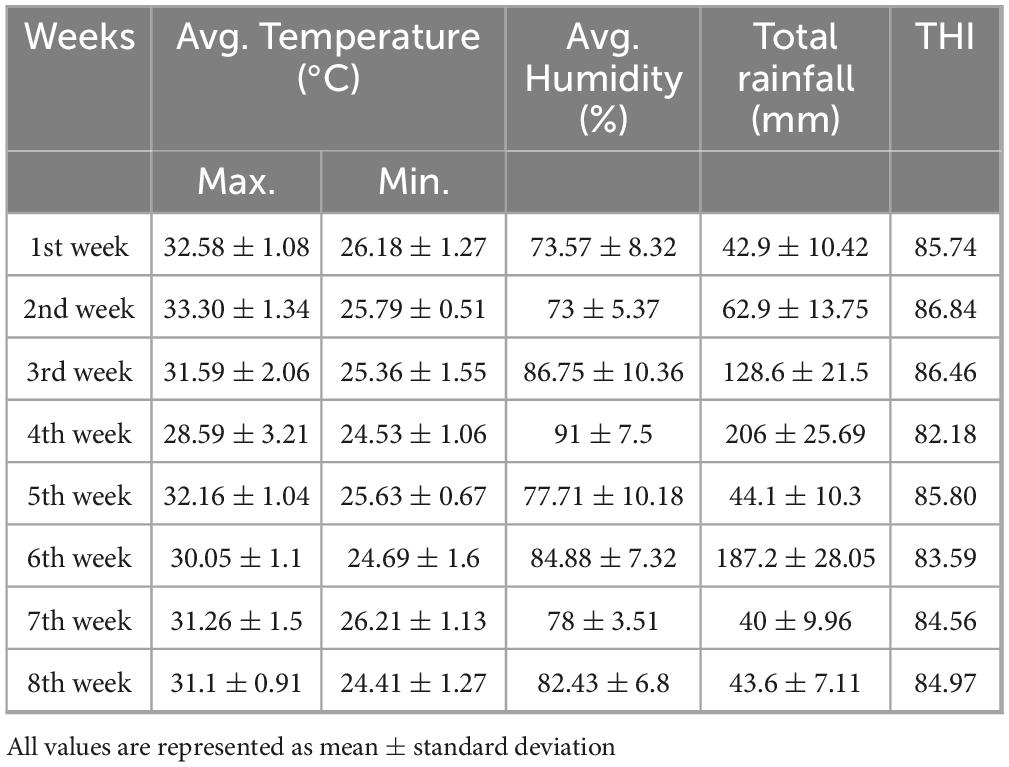
Key environmental parameters—temperature, rainfall, humidity, and temperature-humidity index (THI) were monitored daily throughout the experiment (Table 1). THI was calculated using the formula: THI = 0.8 *T + RH * (T-14.4) + 46.4, where T = dry-bulb temperature in°C and RH = relative humidity expressed as a proportion, i.e., 65% humidity is expressed as 0.65 (Habeeb et al., 2018).
Source of probiotics
The commercially available multi-strain probiotics – BifilacR (Tablets India Ltd., India) was procured from a commercial medical supplier. Composition of BifilacR: Lactobacillus sporogenes (50M), Streptococcus faecalis T-110 JPC (30M), Bacillus mesentericus TO-A JPC (1M), Clostridium butyricum TO-A (2M) and excipients (q.s.).
The viability of the microorganisms present in the multi-strain probiotic “BifilacR” and their content per gram of the product (CFU/g) were evaluated in the laboratory through standard procedures. The concentration (CFU/g) of all four microorganisms was found to be equivalent to the claims made by the commercial company. Moreover, after incorporation in the basal diet, viability of the microorganisms in “BifilacR” was checked to ensure similar experimental conditions throughout the trial period.
Feed preparation
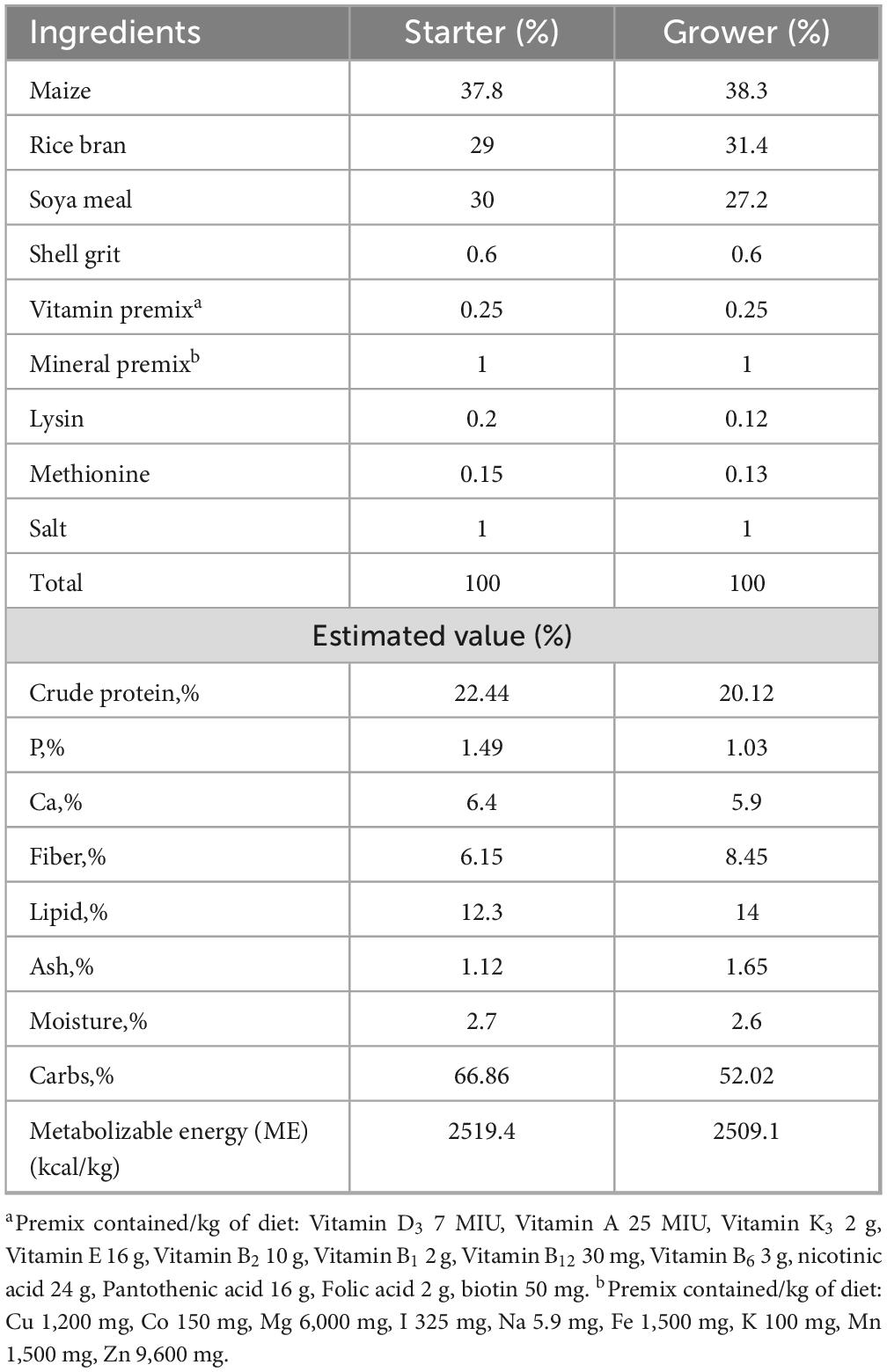
The basal diet was developed as per ICAR-2013 feed standard which provides the growing chicks with essential nutrients required for ideal growth and performance. The details of the diet composition and nutrient profile are presented in Table 2.
Experimental animals
120 newly hatched Vanaraja chicks (sex ratio—1:1) were purchased from a commercial brooding facility (Amman Poultry and Feeds, Port Blair, A & N Islands, India). Each chick was individually weighed and randomly assigned to 4 groups of 30 chicks each.
• Group – I (Negative Control): This group was provided with only basal diet (n = 30).
• Group – II (Positive Control): This group was provided with basal diet supplemented with AGP (n = 30). AGP used was Tetracycline (200 g/ton)
• Group – III (T1): This group was provided with basal diet supplemented with 0.1% multi-strain probiotics (n = 30).
• Group – IV (T2): This group was provided with basal diet supplemented with 0.3% multi-strain probiotics (n = 30).
The farms were cleaned and disinfected thoroughly in advance. The light and dark periods were maintained according to the standard guidelines. The micro temperature of the farm was noted on daily basis. The chicks were raised in a deep litter system. Mortality was continuously monitored and recorded. Throughout the 60-day trial period, both feed and water were provided ad libitum.
Collection of samples
Blood was collected from individual chicken on Day 0 and 15-day intervals thereafter irrespective of the sex of the poultry birds up to the conclusion of the experiment. Following standard aseptic procedures, whole blood (1.5–2 mL) was drawn from the wing vein using syringe and transferred into vacutainers preloaded with clot activators (J.K. Diagnostics, Rajkot, India). The samples were left undisturbed at room temperature for 1 h before being centrifuged at 3,000 g for 10 min at 25°C.
Growth parameters
The body weight of each individual bird was measured on Day 0 and at 15-day intervals thereafter. Daily feed consumption of each group was recorded on regular basis. The daily feed intake for each group was determined by the subtracting the initial quantity of feed given from the final residual feed. For each trial group, the feed conversion ratio (FCR), average daily gain (ADG), and average daily feed intake (ADFI) were also calculated at 15-day intervals.
Estimation of serum biochemical parameters
The biochemical parameters such as albumin, total protein (TP) (ARKRAY Healthcare Pvt. Ltd), creatinine, lactate dehydrogenase (LDH), glucose, total bilirubin (TB), alkaline phosphatase (ALP), serum glutamic pyruvic transaminase (SGPT), serum glutamic oxaloacetic transaminase (SGOT) (OPTIMUS, Madurai, India), and blood urea nitrogen (BUN) (ERBA Diagnostics, Mannheim GmbH, Germany) were analyzed by commercially available kits with the manufacturer’s protocol. Globulin (G) was determined by subtracting the serum protein level from the serum albumin level (Total Protein –Albumin = Globulin) (Busher, 1990).
The lipid profile such as high-density lipoprotein cholesterol (HDLc), low-density lipoprotein cholesterol (LDLc), total cholesterol (TC), and triglycerides (TG), (OPTIMUS, Madurai, India) were analyzed using commercialized kits with the manufacturer’s protocol.
Antioxidant properties in serum were determined bycolorimetry (Antioxidant Activity Estimation kit, HiMedia Laboratories Pvt. Ltd., Nashik, India). Lipid peroxidation was estimated bya colorimetric kit (TBARS estimation kit for lipid peroxidation, HiMedia Laboratories Pvt. Ltd., Nashik, India) and nitric oxide was analyzed using commercial kit (HiMedia Pvt Ltd., Nashik, India), all performed as recommended by the manufacturer.
Estimation of immune parameters
IL6, IL4, IL2, HSP70, INF-γ, TLR4 responses were determined by using commercially available ELISA kits (Life Technologies Pvt Ltd., Delhi, India). In vitro oxidative radical production by neutrophils were measured by nitro blue tetrazolium (NBT) assay (Siwicki et al., 1998). Blood lymphocyte proliferation was assessed by in vitro lymphoproliferation assay (Daly et al., 1995).
Histomorphological analysis
A total of 12 poultry birds, three representative chicks from each trial group were humanely euthanized via cervical dislocation after the completion of experimental trials. Visceral samples were preserved in 10% Formalin till further processing. The samples were then processed for standard histomorphological analysis (Biswas et al., 2022). Respective slides of intestinal sections were prepared to measure villus height and crypt depth. The measurement for crypt depth and villi height were carried out using Olympus CX41RF Microscope (Olympus Corporation, Tokyo, Japan) at 40X magnification. Images of crypts and villus were captured via an industrial digital camera (Panasonic CMOS sensor) connected to a computer. The measurements were made using the imaging software –Image View.
Statistical analysis
Data are expressed as Mean ± Standard deviation (SD). Statistical significance was assessed by analysis of variance (ANOVA) following Bonferroni post hoc and t-test was performed with GraphPad Prism 9.3.1 software, considering the p ≤ 0.05 as significant.
Results
Growth performance
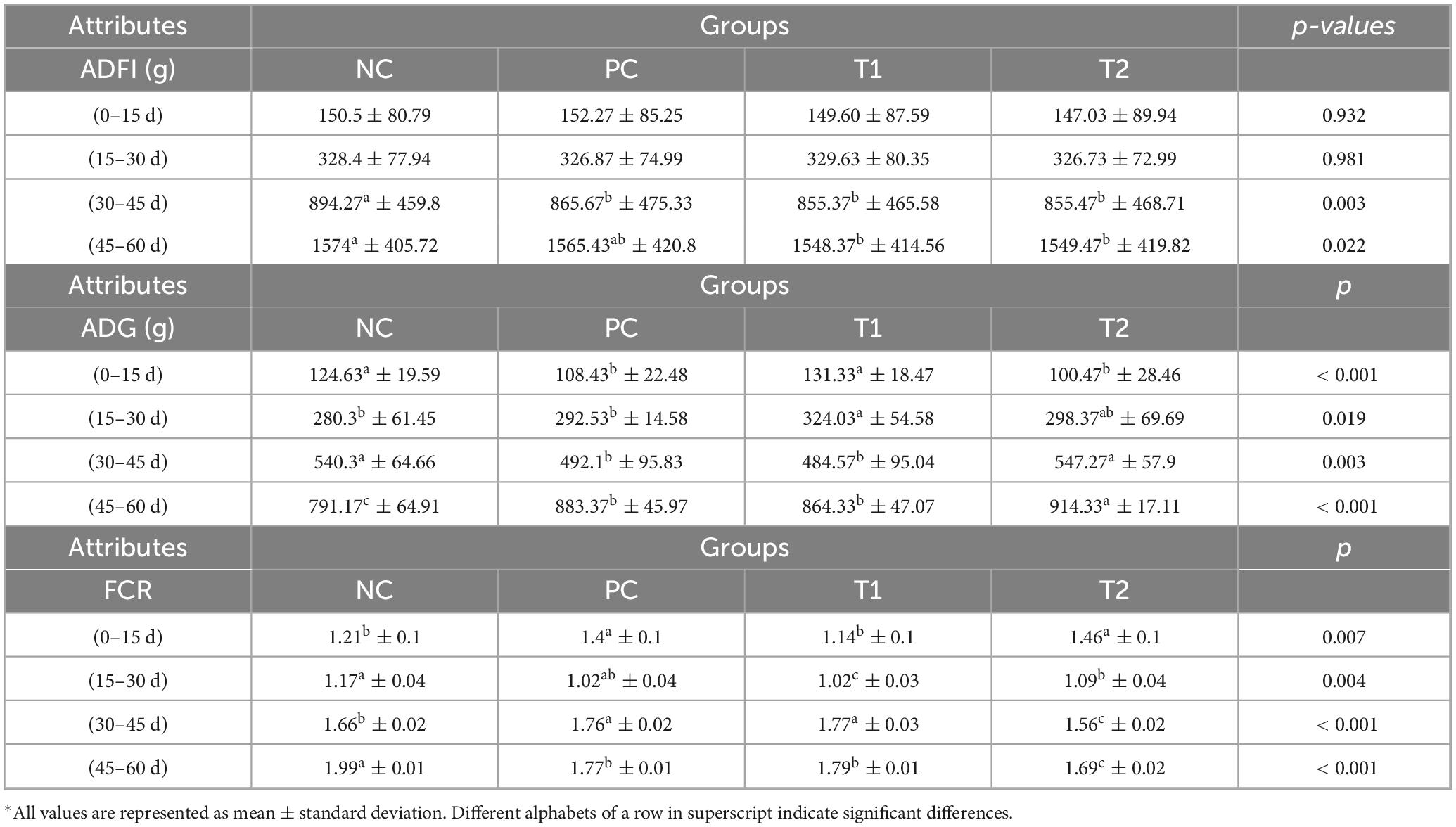
Multi-strain probiotics (BifilacR) supplementation significantly increased (p ≤ 0.05) the average daily weight gain (ADG) in groups T1 (0.1%) and T2 (0.3%) compared to the NC and PC groups during days 45–60 of the trial. A significant improvement (p ≤ 0.05) in the FCR was also observed in the T1 and T2 groups during days 30–45 and 45–60 of the experiment compared to the NC group. The PC group also showed a significant increase in FCR when compared to the NC and T2 groups during days 30–45 and 45–60 of the experiment. In summary, the addition of multi-strain probiotics to the diet positively influenced growth performance and feed utilization in growing chicks (Table 3).
Biochemical profile
Following multi-strain probiotics (BifilacR) supplementation, on the 15th and 30th day of the experiment, a significant decrease (p ≤ 0.05) in albumin (g/dL) level was observed in the T2 group relative to the control groups NC and PC. The level of globulin (g/dL) on the 45th day was significantly high in T1 (p ≤ 0.001) and T2 (p ≤ 0.05) groups when compared to NC. On the 60th day, the level of globulin (g/dL) was significantly reduced (p ≤ 0.01) in T2 than that of PC group. Blood urea nitrogen (BUN) (mg/dL) level was observed to be significantly decreased (p ≤ 0.05) in the T2 group than the PC group on the 45th day. BUN levels were significantly different (p ≤ 0.01) on 60th day among the T1 and T2 groups relative to the control groups NC and PC. A significant difference (p ≤ 0.05) was observed in ALP (U/L) level throughout the experimental trial among the treated groups and the control groups. Total bilirubin (mg/dL) levels were significantly different (p ≤ 0.001) on the 45th day of the trial among the treated groups T1 and T2 when compared to the control groups NC and PC. SGPT (U/L) level in T1 and T2 group showed significant decrease (p ≤ 0.05) on 15th, 30th, and 60th day of experiment when compared to the control groups NC and PC. SGOT (U/L) level has notably differed among the treated groups T1 and T2 relative to the control groups NC and PC throughout the experiment. Creatinine (mg/dL) level was significantly different (p ≤ 0.05) in the T1 group than the PC group on 45th day of the experiment. In brief, multi-strain probiotics (BifilacR) supplementation has considerably influenced the biochemical profile (Figure 2).
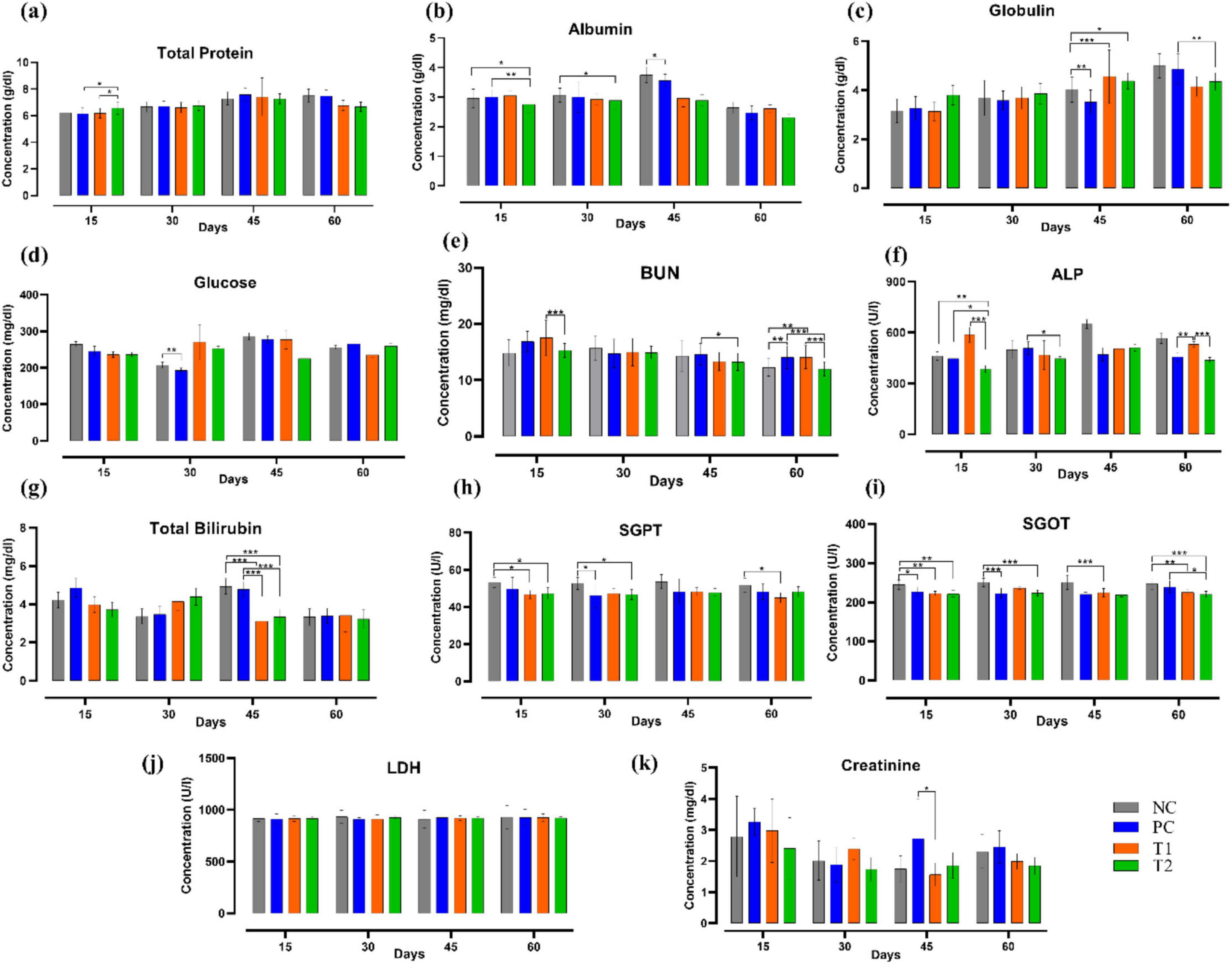
Figure 2. Effect of multi-strain probiotics supplementation on the biochemical profile. (a) Total protein, (b) albumin, (c) globulin, (d) glucose, (e) blood urea nitrogen (BUN), (f) alkaline phosphatase (ALP), (g) total bilirubin, (h) serum glutamate pyruvate transaminase (SGPT), (i) serum glutamate oxaloacetate transaminase (SGOT), (j) lactate dehydrogenase (LDH), and (k) creatinine. Significance levels are *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
Lipid profile
After multi-strain probiotics supplementation, LDLc (mg/dl) levels in the T1 and T2 groups were reduced significantly (p ≤ 0.001) on 30th, 45th day of the experiment when compared to the control groups NC and PC. Triglyceride (mg/dL) level was significantly decreased (p ≤ 0.001) in T1 and T2 groups relative to the control groups NC and PC on the 45th day of trial. On the 60th day, triglyceride (mg/dL) level was significantly reduced in T1 (p ≤ 0.001) and T2 (p ≤ 0.01) when compared to NC group. Total cholesterol (mg/dL) level was significantly reduced (p ≤ 0.001) in T1 and T2 groups when compared to the control groups NC and PC on the 45th day of the experiment. On the 60th day, total cholesterol (mg/dL) level in T1 was significantly decreased (p ≤ 0.05) relative to the PC group. In summary, multi-strain probiotics (BifilacR) supplementation has strongly affected the lipid profile (Figure 3).
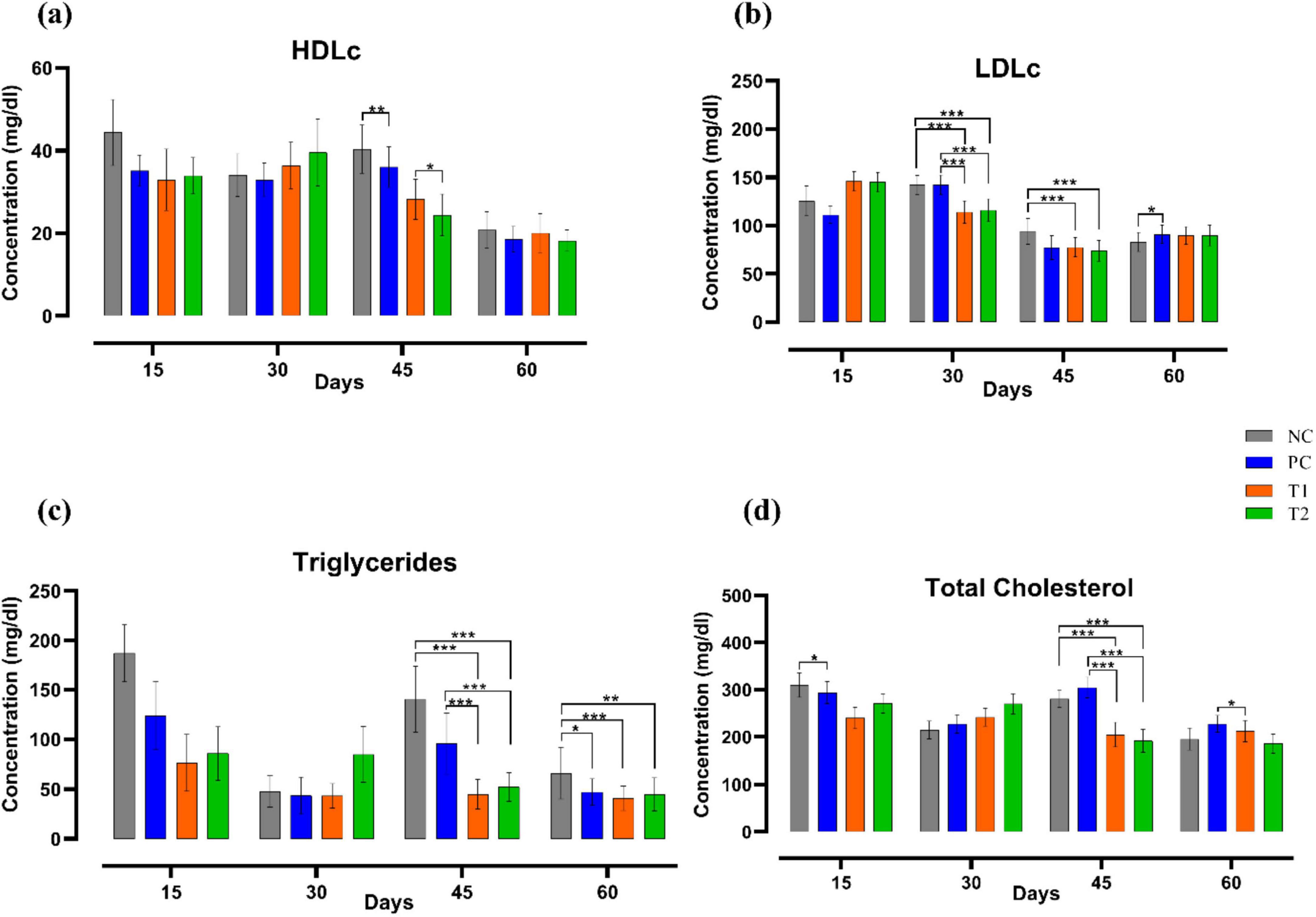
Figure 3. Effect of multi-strain probiotics supplementation on the lipid profile. (a) HDLc, (b) LDLc, (c) triglycerides, and (d) total cholesterol. Significance levels are *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
Antioxidant profile
Lipid peroxidation (μM) level was significantly decreased (p ≤ 0.001) in T1 and T2 when compared to the PC group on the 45th and 60th day of the experiment after multi-strain probiotics supplementation. The total antioxidant capacity (μM) after the supplementation was notably differed throughout the trial in the T1 and T2 groups relative to the control groups NC and PC. To summarize, multi-strain probiotics (BifilacR) supplementation has substantially impacted the antioxidant profile of Vanaraja breed (Figure 4).
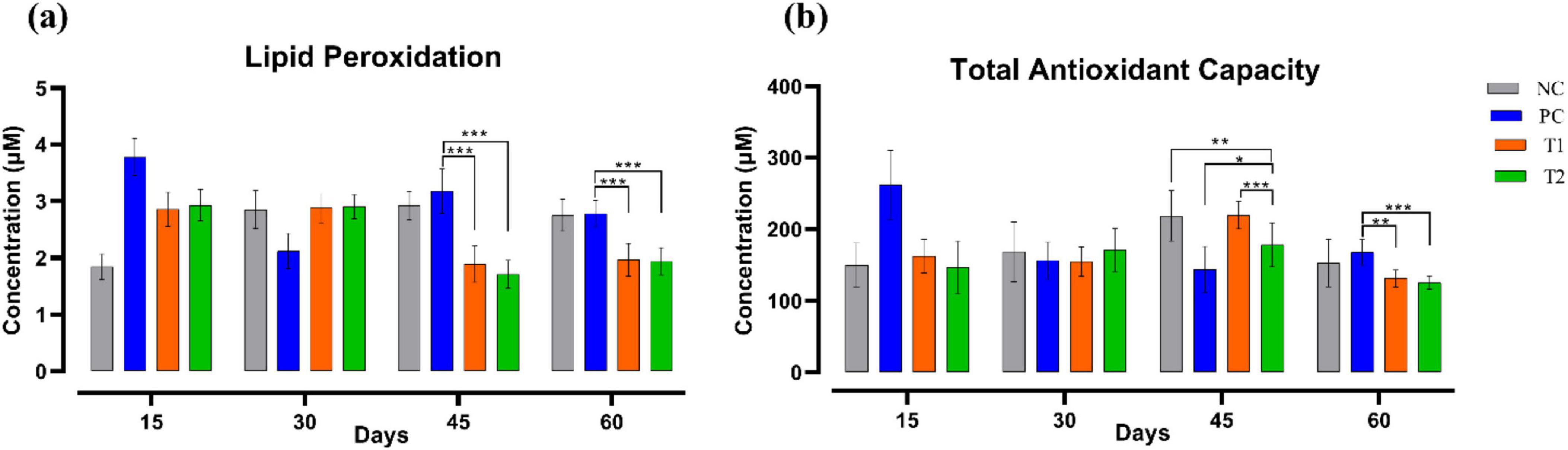
Figure 4. Effect of multi-strain probiotics supplementation on the antioxidant profile. (a) Lipid peroxidation, and (b) total antioxidant capacity. Significance levels are *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
Serum cytokine profile
After multi-strain probiotics supplementation, on the 15th day, the concentration of IL2 (pg/mL) in the T2 group showed significant upregulation (p ≤ 0.01) compared to the PC group. On the 30th day, the T2 group again showed significant upregulation (p ≤ 0.001) in the concentration of IL2 (pg/mL) than the NC group. On the 45th day, both T1 and T2 groups showed significant upregulation (p ≤ 0.001) in the concentration of IL2 (pg/mL) than the PC group. On the 60th day, the T1 group showed significant upregulation (p ≤ 0.001) in the concentration of IL2 (pg/mL) than the control groups NC and PC. After multi-strain probiotics supplementation, IL4 (pg/mL) concentrations in T1 and T2 were notably upregulated compared to the control groups throughout the experiment. Concentrations of IL6 (pg/mL), IFN-γ (pg/mL), and TLR4 (ng/L) were remarkably downregulated throughout the trial in the T1 and T2 groups relative to the control groups NC and PC. A significant increase (p ≤ 0.05) in HSP70 (pg/mL) concentration was observed after the supplementation in the T1 group when compared to the NC group. A significant increase (p ≤ 0.0) in NO (μM) concentration was also observed throughout the experiment in the T1 and T2 relative to the control groups NC and PC. In short, multi-strain probiotics (BifilacR) administration has highly influenced the serum cytokines (Figure 5).
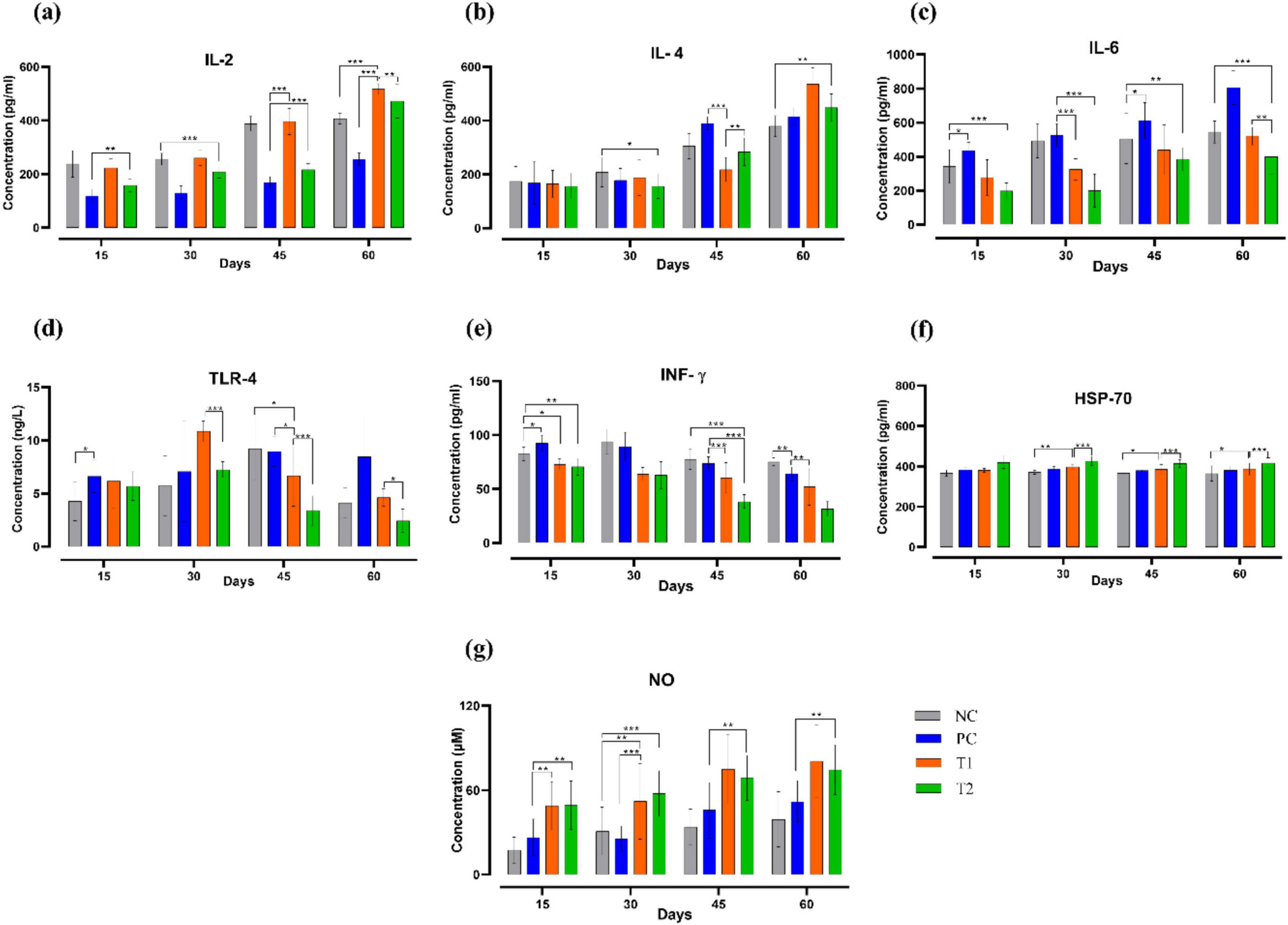
Figure 5. Effect of multi-strain probiotics supplementation on the serum cytokines. (a) IL2, (b) IL4, (c) IL6, (d) TLR4, (e) IFN-γ, (f) HSP70, and (g) Nitric Oxide (NO). Significance levels are *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
Immune parameters
Lymphoproliferation assay showed apparent elevation in the number of lymphocytes in different interval of time. On the 30th day, the T2 group showed significant increase (p ≤ 0.05) in the number of lymphocytes than the control groups NC and PC. On the 45th day, the T1 group showed significant surge in lymphocytes proliferation than the control groups NC (p ≤ 0.01) and PC (p ≤ 0.05). On the 45th day, the T2 group also showed significant increase (p ≤ 0.05) in lymphocytes proliferation than the NC group. On the 60th day, the T1 group showed significant increase (p ≤ 0.05) in lymphocytes proliferation than the NC group. NBT reduction assay showed considerable reduction in superoxide production in treated groups T1 and T2 than that of the control groups NC and PC across the trial. On 30th day, T1 group showed significant reduction (p ≤ 0.01) in superoxide production than the NC group. On 45th day, T2 group also showed significant decrease (p ≤ 0.001) in superoxide production when compared to both the control groups NC and PC. Similarly, on 60th day, T2 group showed significant decline (p ≤ 0.001) in superoxide production than the control groups NC and PC. To sum up, multi-strain probiotics (BifilacR) administration has noticeably modulated the immune parameters when given in various concentrations (Figure 6).
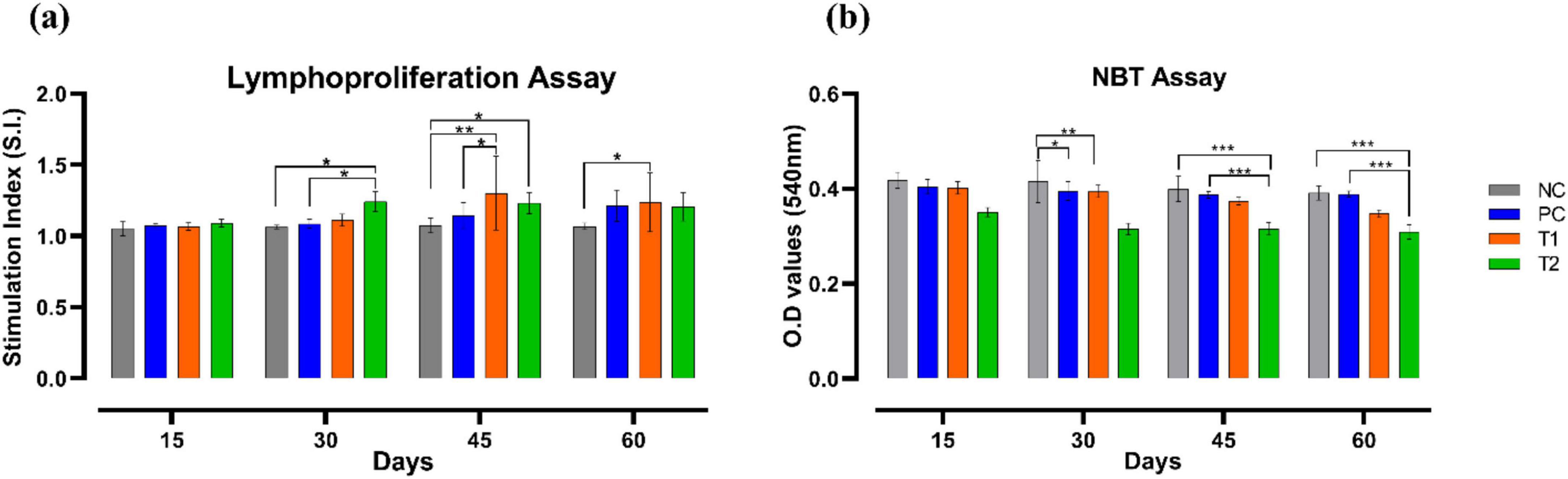
Figure 6. Effect of multi-strain probiotics supplementation on the immune parameters. (a) Lymphoproliferation assay, and (b) NBT reduction assay. Significance levels are *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
Histomorphological analysis
By assessing the influence of multi-strain probiotics on the histological parameters of intestine, it was observed that the villi height (μm) and crypt depth (μm) in duodenum was significantly greater (p ≤ 0.001) in the T2 group than the control groups NC and PC (Figure 7). In the jejunum, the height of villi (μm) and crypt depth (μm) was considerably more (p ≤ 0.05) in the T1 group than the other groups T2, NC and PC (Figure 7). In ileum, no significant changes were observed in villi height (μm) and crypt depth (μm) among any groups after the trial (Figure 7). In brief, multi-strain probiotics (BifilacR) showed clear evidences of positive effects on chicken intestinal morphology when given in different concentrations. Photomicrographic analysis was conducted to document and evaluate histomorphological alterations across all the experimental groups, assessing the villus height and crypt depth in duodenum (Figure 8), jejunum (Figure 9), and ileum (Figure 10).
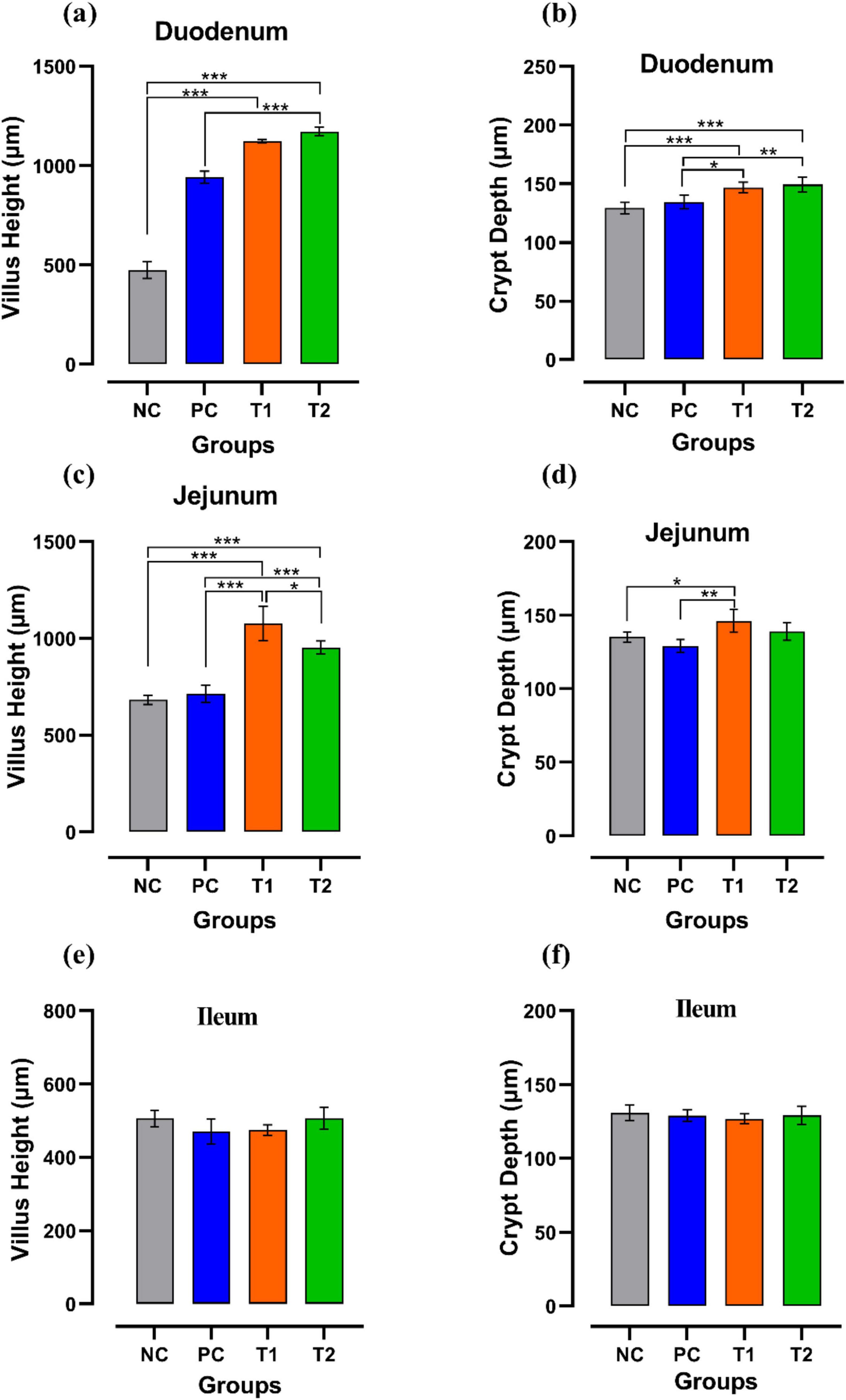
Figure 7. Effect of multi-strain probiotics supplementation: in duodenum. (a) Villus height (μm) (b) crypt depth (μm); in jejunum—(c) villus height (μm) (d) crypt depth (μm); and in ileum—(e) villus height (μm) (f) crypt depth (μm). Significance levels are *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
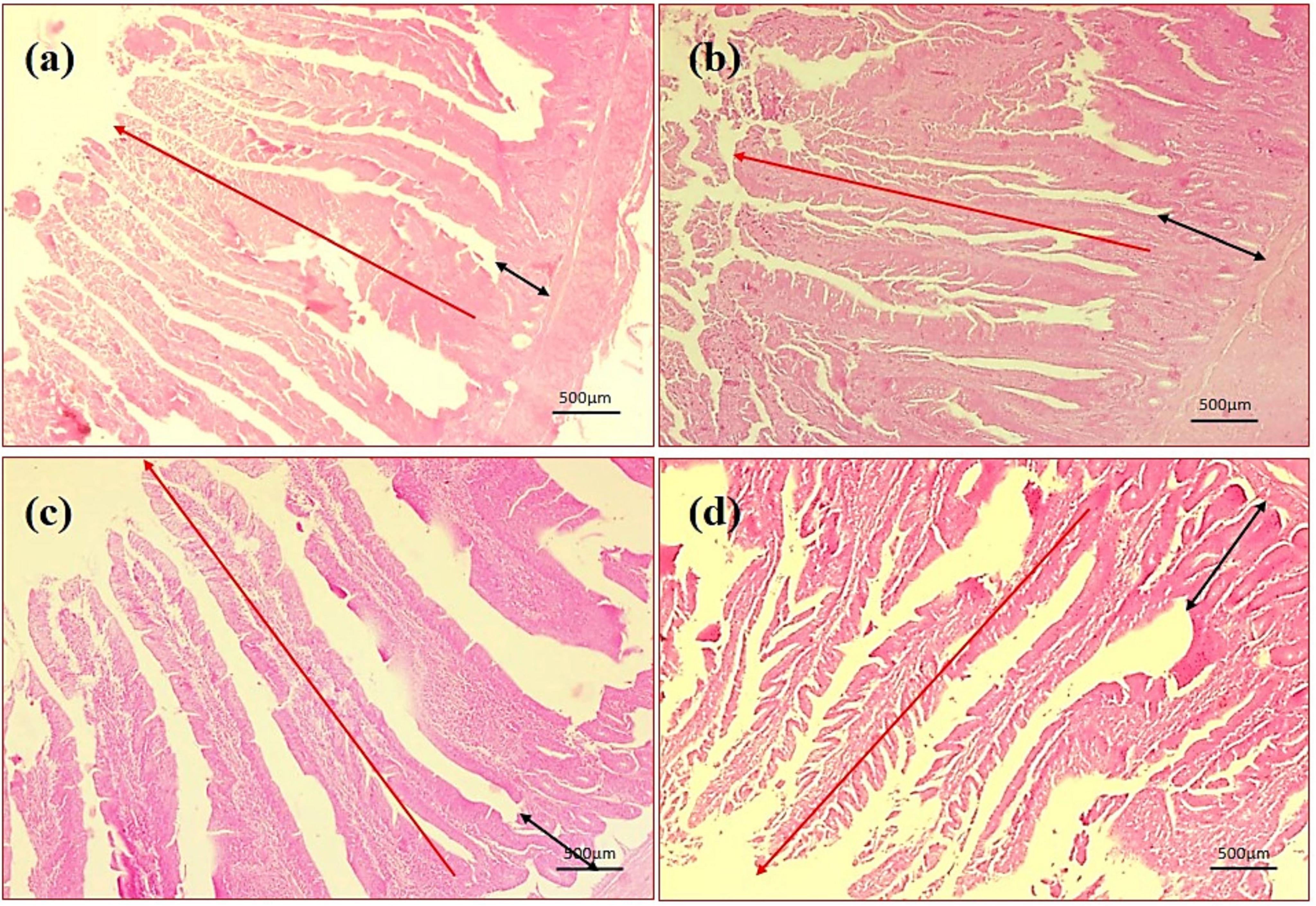
Figure 8. Photomicrography of duodenum (40X magnification). (a) Negative control (NC), (b) positive control (PC), (c) T1 supplemented with 0.1% of multi-strain probiotics, and (d) T2 supplemented with 0.3% of multi-strain probiotics.
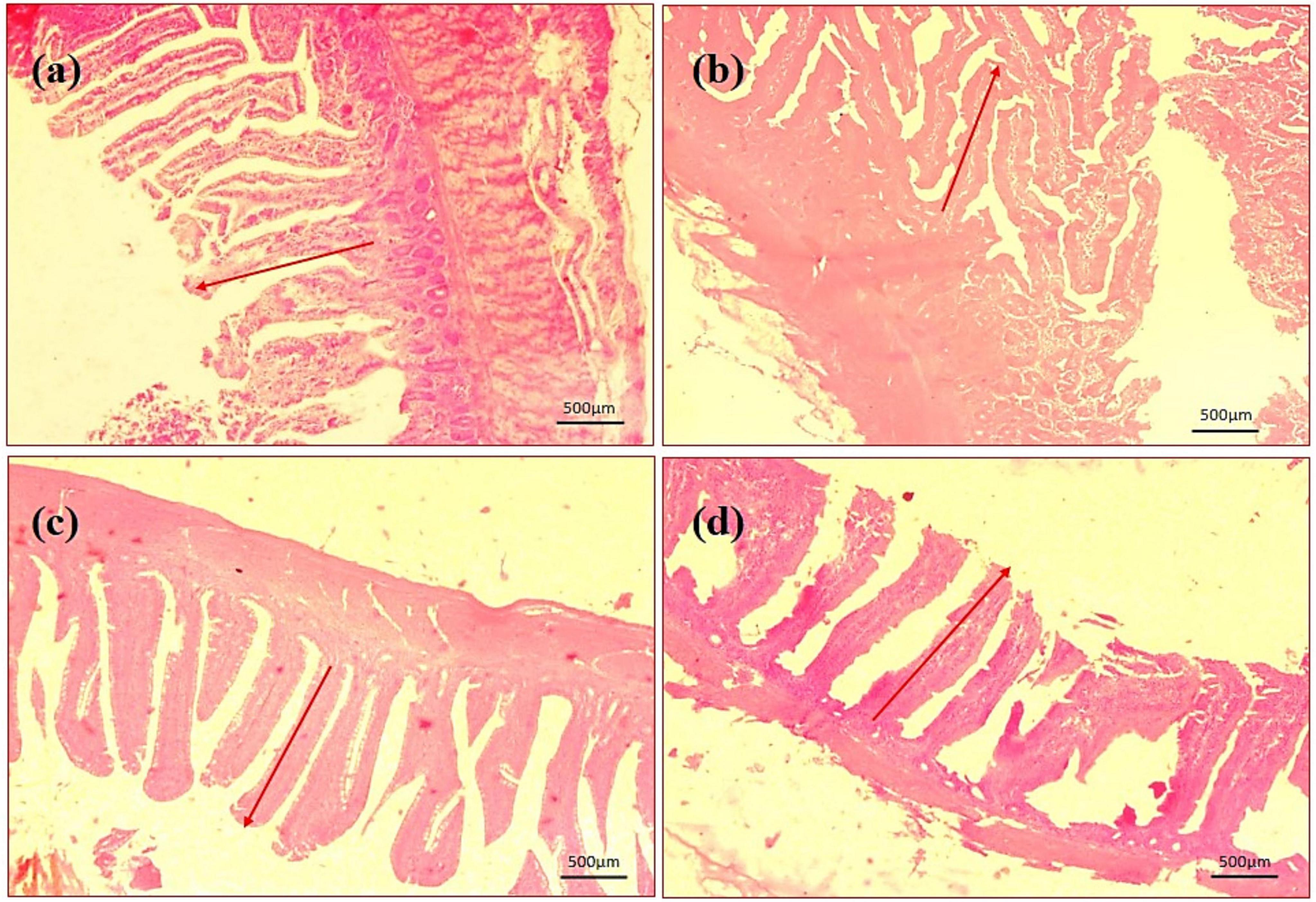
Figure 9. Photomicrography of jejunum (40X magnification). (a) Negative control (NC), (b) positive control (PC), (c) T1 supplemented with 0.1% of multi-strain probiotics, and (d) T2 supplemented with 0.3% of multi-strain probiotics.
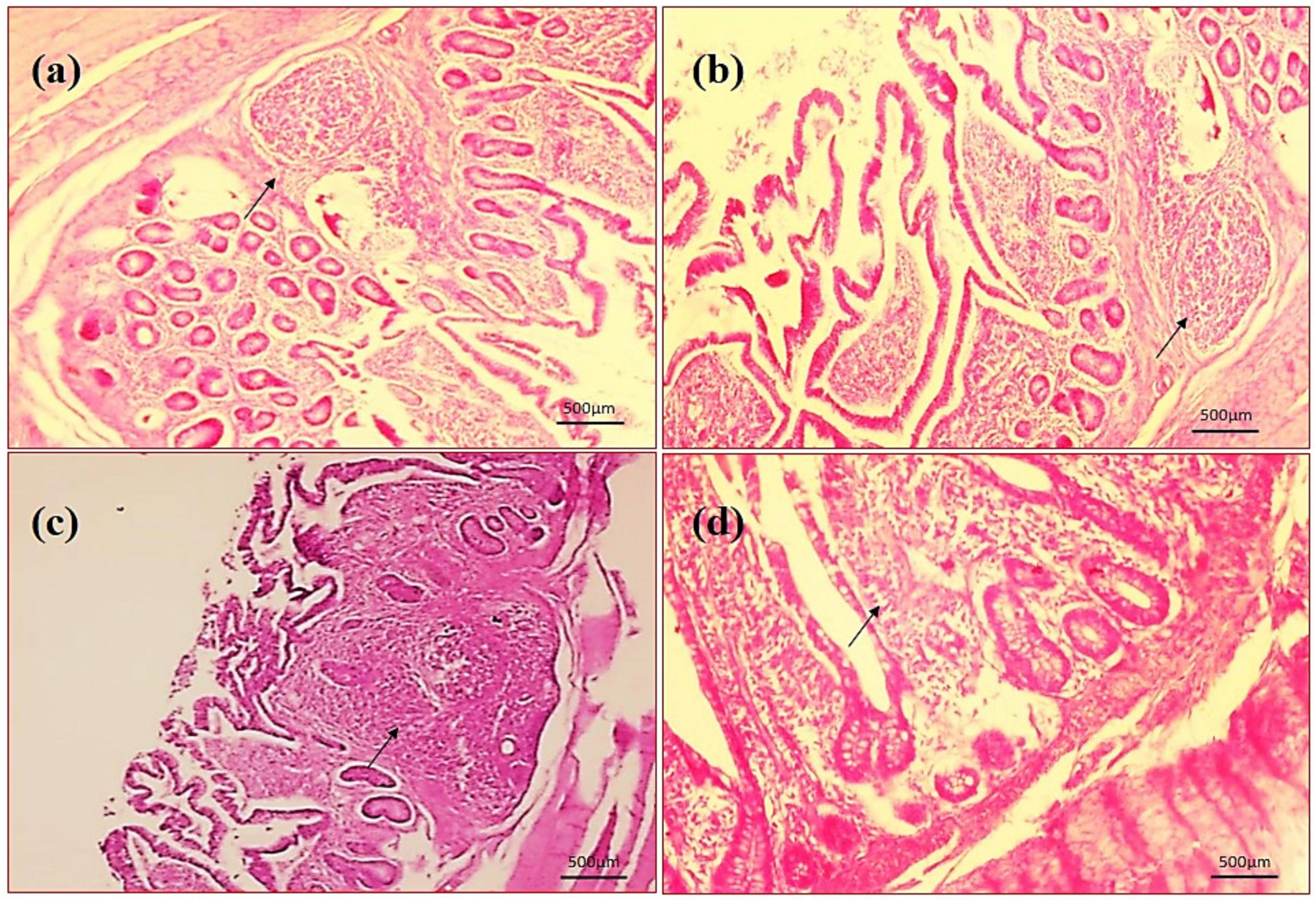
Figure 10. Photomicrography of ileum (40X magnification). (a) Negative control (NC), (b) positive control (PC), (c) T1 supplemented with 0.1% of multi-strain probiotics, and (d) T2 supplemented with 0.3% of multi-strain probiotics.
Discussion
Probiotics represents a promising substitute for the commercial AGPs. Their supplementation has demonstrated evident influence on animal health and production performance, particularly in poultry. Findings from this study indicate that multi-strain probiotics (BifilacR) supplementation has significantly improved feed intake, weight gain, and FCR in poultry birds. These results corroborate the findings of Li et al. (2011) and Hossain et al. (2015). Various factors can affect the feed consumption and FCR, such as type of probiotics, strain, dosage of supplementation, and the poultry breed (Zhang et al., 2021). The multi-strain probiotics intake has increased absorption of vitamins and minerals, leading to greater nutrient bioavailability and enhanced overall growth performance (Adil and Magray, 2012; Olukosi and Cowieson, 2007). The findings of this study corroborate with the results of some researchers who also observed a significant increase in BW, FI, and FCR after multi-strain probiotics supplementation in broilers (De França et al., 2023; Kalavathy et al., 2003; Neijat et al., 2019; Sobczak and Kozłowski, 2015). Multi-strain probiotics have been documented to enhance the enzymatic activity in the GI tract, which helps in the breakdown of complex materials in feed and makes them easy for absorption (Awad et al., 2009; Latorre et al., 2016). Factors affecting FCR and FI includes the feed type, feeding frequency, and host health (Musigwa et al., 2020). Several reports suggested that probiotics stimulates microbial conversion of non-digestible substances in the diet, leading to the production of short-chain fatty acids, which serve as an energy source for the host, resulting in better growth performance (Patterson and Burkholder, 2003). A nutrient-rich feed supplementation that contains the correct amount of energy and free amino acids mixed with the correct dosage of probiotics is influential for optimized feed utilization (Liang et al., 2024). Increased growth performance observed in the study after multi-strain probiotics supplementation may be due to an elevated population of good bacteria for better nutrient uptake and reduction of pathogenic microorganisms in the gut. A few contrary results indicate that multi-strain probiotics supplementation has no impact on feed intake or body weight gain in poultry birds (Anjum et al., 2005; Sirovnik et al., 2021).
Serum biochemical parameters are the indicators of the host body’s first line of defense. In this study, a noticeable surge in protein level was observed in the poultry group fed with multi-strain probiotics (BifilacR) when compared to the control groups. This result corroborates the observations of Silva et al. (2020) and Rehman et al. (2018), who stated significant changes in total protein, albumin, and globulin levels after multi-strain probiotics supplementation in broilers. The results of the current experiment are also supported by the results of Panda et al., who found apparent changes in albumin, globulin levels, and albumin/globulin ratio in serum after probiotics supplementation, indicating a positive influence on immune responses and disease resistance (Panda et al., 2006). This change can be attributed to the competition between probiotic gut bacteria and pathogenic microbes, which limits the protein breakdown into nitrogen, thereby increasing protein and amino acid utilization (Hossain et al., 2024; Mansoub, 2010). High total protein level generally shows high protein metabolism rate (Abdel-Moneim et al., 2020; Lee et al., 2013). A report showed a notable increase in glucose, globulin, and total protein in Salmonella-infected broilers following probiotics supplementation (Abudabos et al., 2019). Some researchers have reported an increase in protein intake as the beneficial bacteria prevent degradation of protein and utilize the nitrogen of pathogens by a competitive exclusion mechanism, which results in efficient protein absorption and total serum protein (Al-Khalaifa et al., 2019; Khattab et al., 2021; Yazhini et al., 2018). A substantial increase in glucose level was noticed in the current study, which aligns with the conclusions of Abudabos et al. (2019) and Hussein (2014), who reported high glucose levels in broilers administered with probiotics. A rise in glucose level can be related to the enhancement of nutrient utilization and glycogenolysis, which leads to increased glucose absorption (Das et al., 2005). SGOT and SGPT serve as primary indicators of liver function, and increased concentrations of these indicators can cause liver damage (De et al., 2023). Reduced levels of SGOT and SGPT were observed, supporting the results of Hussein et al. (2020), who reported that probiotics administration can significantly safeguard the treated birds from hepatocellular damage compared to the control groups. Decreased levels of SGOT and SGPT showed normal liver function in chickens fed with multi-strain probiotics. This may be a valuable point to include probiotics safely as a feed additive, as it may not exert any adverse effect on liver functions in poultry. A contrary study showed no significant changes in SGOT and SGPT activity in broilers after probiotics supplementation (Lee et al., 2010). ALP is a biomarker for renal functions and is primarily derived from the epithelial cells of the bile duct, kidneys, and gut lining (Żbikowski et al., 2020). ALP activity can be elevated in chicks during the initial starter phase, but can reduce with age. This study shows a downregulation of ALP activity in chicks fed with multi-strain probiotics, which aligns with the findings of Rashidi et al. (2020) and Sharma et al. (2014). A contrary report stated that there was no significance of ALP levels after probiotic treatment in broilers (Panda et al., 2006; Sharma et al., 2014). In this study, the BUN level was decreased in the multi-strain treated groups than the control groups, which agrees with the results of various researchers who observed a decrease in the level of BUN after probiotics supplementation (El-Baky, 2013; Chen et al., 2010; Harr, 2002; Quintavalla et al., 2001). Lower levels of the BUN can be due to increased protein utilization by probiotics bacteria, leading to balanced intestinal microflora (Tang et al., 2010). BUN level is negatively related to protein deposition, and a low BUN level suggests high protein production (Yao et al., 2009). Total bilirubin level was lower in the poultry groups fed with multi-strain probiotics than in the control groups, which corroborated the results of Oladipo et al. (2023) and Udeh et al. (2020). No noticeable changes were observed in LDH and creatinine levels in the current study after probiotics supplementation, which aligns with the observations of Wu et al. (2019).
In this study, the chickens treated with multi-strain probiotics had significantly lower total cholesterol levels relative to the control groups, which matches the results of Bidura et al. (2019), Pambuka et al. (2014). Significant reduction of lipid profile in probiotics-fed broiler chickens was observed this can be due to decreased absorption and synthesis of cholesterol in the GI tract by the action of probiotics (Mohan et al., 1996). Lower levels of HDLc and LDLc were observed in the probiotics-fed chickens in the current study, which is in line with the findings of Ahmed et al. (2019) and Sun and Kim (2021). Similar other reports were also found, which state that multi-strain probiotics reduced the HDLc and LDLc levels in broilers (Panda et al., 2006; Reuben et al., 2022). Triglyceride concentration was reduced in treated groups than compared to control group in the current experiment which corroborates with the reports of Wang and Zhou (2007). Lower triglyceride levels observed may be because of the effect of downregulated lipogenesis in the liver (Alimohamadi et al., 2014; Żbikowski et al., 2020). Triglyceride reduction may be due to upregulation of hydrolysis of bile salt, which leads to less lipid absorption in the small intestine (Alkhalf et al., 2010). Few researchers also observed decreased triglycerides and total cholesterol levels in broilers supplemented with Lactobacillus-based probiotics (Panda et al., 2006; Mansoub, 2010). Probiotics bacteria play a crucial role in reducing or terminating the cholesterol and triglycerides synthesis in the liver by upregulating the short-chain fatty acids which subsequently downregulate the blood metabolic products (Dev et al., 2020). Kalavathy et al., reported a reduction in levels of LDLc, total cholesterol, and triglycerides in broilers when supplemented with a mixture of 12 Lactobacillus strains together (Kalavathy et al., 2003). A conflicting report suggests that there was no notable change in total cholesterol and triglyceride levels after multi-strain probiotics supplementation in broilers (Dev et al., 2020).
Probiotics also play an important role in influencing the oxidation state of the gut by directly showcasing the antioxidant qualities and modulating the host’s antioxidant defense signaling (Amaretti et al., 2013; Zolotukhin et al., 2018). To eliminate the free radicals in the host, the levels of in vivo anti-oxidant enzymes are elevated, which reflects the intensity of stress by oxidation (Rehman et al., 2018). In the current study, the level of T-AOC differed significantly among the trial groups but didn’t show much difference in groups treated with multi-strain probiotics. These results corroborate with the findings of Attia et al. (2013, 2018). This is probably caused due to the probiotic dosage and individual bird health. Multi-strain probiotics, especially a mixture of lactobacillus strains, can affect antioxidant enzyme activity in the host body and help in the reduction of oxidative stress damage to the intestines (Bai et al., 2018; Inatomi and Otomaru, 2018; Yu et al., 2019). Malondialdehyde (MDA) is a by-product that shows the level of lipid peroxidation. Overproduction of free radicals contributes to detrimental oxidative stress, which leads to decreased growth performances, immunosuppression, and meat quality deterioration in broilers (Salami et al., 2015; Surai, 2016). Results of this experiment showed a substantial decrease in the MDA concentration, hence a reduction in lipid peroxidation and downregulation of oxidative stress after supplementation of multi-strain probiotics to the poultry birds. These findings are consistent with the results of Zhang et al. (2021), who observed a significant reduction in MDA concentration, which led to down-regulation of lipid peroxidation in broilers administered with probiotics. Equivalent results were also described by Elbaz et al. (2023), who observed a reduction in MDA concentration in heat-stressed broilers after probiotics supplementation.
Incorporating probiotics into the broiler diet showed massive improvement of immune functions and responses (Zhen et al., 2020). In this study, up-regulation of HSP70, IL4, IL2, and down-regulation of IL6, TLR4, and INF-γ in multi-strain probiotic-fed chickens was observed. These results resemble the outcomes of Zhang et al. (2021) and Gadde et al. (2017), who also noticed a surge in IL2 concentration in broilers fed with probiotics. IL2 regulation reflects elevated humoral immunity which is observed to be changed in age-dependent manner (Wang et al., 2015; Zhao et al., 2022). Xu et al. (2014) observed up-regulation of IL4 levels in heat-stressed chickens fed with feed additives. Reports of Yu et al. (2022) and Zhen et al. (2018) echo with the outcomes of the current study, which states that a noticeable down-regulation of pro-inflammatory factors (IL6, INF-γ) was observed in broilers fed with Bacillus strain-based probiotics than the control. TLR4 acts as the receptor for lipopolysaccharides, which are the primary component of the gram-negative bacteria’s membrane (Kannaki et al., 2010). TLRs recognize particular microbial substances and induce Th1 cytokine production through the NF-κB pathway (Murch, 2001). This study found reduced TLR4 levels in chickens treated with multi-strain probiotics, which aligns with the findings of Yitbarek et al. (2013). Probiotics can elevate TLR’s signaling, regulate mucosal cell-mediated immune responses and enhance epithelial barrier integrity in poultry birds (Gao et al., 2008). HSP70 is a heat shock protein, which is expressed when the host encounters an unfavorable environmental or pathogenic condition (Gu et al., 2012; Zhang et al., 2015). In this study, HSP70 level was elevated in the test groups supplemented with multi-strain probiotics, which corroborates the results of Hu et al. (2021). The down-regulation and up-regulation of HSP70 are the result of host’s response to various biotic and abiotic stress (Siddiqui et al., 2020). Increased HSP70 concentration also shows high heat stress tolerance because of improved cytoprotective effects (Chauhan et al., 2014). HSP70 also plays a crucial role in helping cells recover from stress. It is involved in repairing cellular damage, refolding of impaired proteins, preventing oxidative stress and programmed cell death (Jiang et al., 2020).
Probiotics enhance humoral responses of broilers (Huang et al., 2004). This study shows supplementation of multi-strain probiotics enhanced lymphocyte proliferation in the chickens compared to the control groups. These findings are consistent with the observations of Tollba and Mahmoud (2009), who reported a significant increase in lymphocyte count in probiotics-fed broilers. Muthusamy et al., (2013, 2020) and Paul et al. (2012) demonstrated that dietary supplementation ofβ-glucan in poultry enhanced lymphocyte production, reduced superoxide anion production by blood neutrophils, and exhibited notable immunostimulatory effects. However, a contrary report shows no impact on systemic humoral responses of broilers after probiotics supplementation (Mountzouris et al., 2010).
In this present experiment, it was observed that supplementation of multi-strain probiotics has shown an apparent elevation in the height of villi and depth of the crypts in the small intestines of poultry. The results reflected the conclusions of Ramlucken et al. (2020), Salim et al. (2013), and Sen et al. (2012), who also reported significantly taller villi in chickens after inclusion of multi-strain probiotics in their diet. The present results indicate enhanced gut morphology, which helped in improved overall growth performances and is most likely the major factor for improved FCR in treated chickens. Chang et al. (2020) and Samanya and Yamauchi (2002) also observed improvement in intestinal microflora in broilers administered with multi-strain probiotics. This shows that the enhancement of the villi heights and crypt depths expands the epithelial surface area, promoting more efficient absorption and maximum bioavailability of beneficial nutrients.
Mixed-strain and mixed-species probiotics function in various areas of the gut and perform different action pathway that together generates a combined beneficial effect (Timmerman et al., 2004; Kazemi et al., 2019). Several reports also stated that administration of the mixed-strain probiotics in the diet of poultry, like Lactobacillus spp., can elevate the population of favorable microflora and create a healthy gut environment (Khaksefidi and Rahimi, 2005; Swain et al., 2014; Hossain et al., 2015). Reports showed that broilers fed with 2 commercially available multi-strain probiotics displayed improved overall growth performance, enhanced intestinal microflora, and reduced lipid peroxidation (Kazemi et al., 2019). A study investigated that the probiotics mixtures enhanced feed conversion, immune responses, intestinal morphology, and inhibited the pathogenic bacteria such as E. coli, C. jejuni from colonizing the GI tract (Teo and Tan, 2007).
Conclusion
The present investigation revealed that inclusion of multi-strain probiotics (BifilacR) in poultry diet improved their growth performance, physiological functions, and immunological functions. These findings can help reducing the dependency on AGPs and furthermore probiotics can be suggested as an influential alternative benefiting poultry birds and indirectly humans. Further research is recommended to establish standardized dosages and to identify the beneficial strains of probiotics bacteria, ensuring their optimal production and extensive applicability on a larger scale.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Institute Animal Ethical Committee, Animal House Facility of Establishment (247/GO/RBi/SL/2000/CPCSEA), ICAR-CIARI, Port Blair, Andaman and Nicobar Islands, India. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
NH: Data curation, Formal Analysis, Investigation, Visualization, Writing – original draft. SJ: Conceptualization, Methodology, Supervision, Writing – review & editing. JS: Conceptualization, Methodology, Resources, Supervision, Writing – review & editing. AD: Conceptualization, Methodology, Resources, Supervision, Writing – review & editing. DB: Conceptualization, Methodology, Resources, Supervision, Writing – review & editing. AA: Writing – review & editing. RK: Writing – review & editing. MM: Resources, Writing – review & editing. SM: Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Supported by the Open Access Publishing Fund of Leipzig University.
Acknowledgments
The author would like to thank the Vice Chancellor, West Bengal University of Animal and Fishery Sciences (W.B.U.A.F.S), Kolkata, West Bengal and the Director, ICAR – Central Island Agricultural Research Institute (C.I.A.R.I), Port Blair, Andaman and Nicobar Islands for providing the necessary facilities required for the research work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
FAO, Food and Agriculture Organization; WHO, World Health Organization; A & N Islands, Andaman and Nicobar Islands; ICAR, Indian Council of Agricultural Research; CIARI, Central Island Agricultural Research Institute; AGP, Antibiotics growth promoters; THI, Temperature-Humidity Index; RH, Relative Humidity; FCR, Feed conversion ratio; ADG, Average daily gain; ADFI, Average daily feed intake; FI, Feed intake; CFU, Colony forming unit; NC, Negative control; PC, Positive Control; T1, Test group-1; T2, Test group-2; IL, Interleukins; TLR, Toll-like receptor; NF-κb, Nuclear factor-kappa B; INF-γ, Interferon-gamma; HSP70, Heat shock protein-70; NO, Nitric oxide; LPA, Lymphoproliferation assay; NBT, Nitroblue tetrazolium assay; TP, Total protein; TC, Total cholesterol; LDLc, Low-density lipoprotein cholesterol; HDLc, High-density lipoprotein cholesterol; ALP, Alkaline phosphatase; TG, Triglycerides; LDH, Lactate dehydrogenase; BUN, Blood urea nitrogen; TB, Total bilirubin; SGOT, Serum glutamic oxaloacetic transaminase; SGPT, Serum glutamic pyruvic transaminase; T-AOC, Total antioxidant capacity; MDA, Malondialdehyde.
References
Abdel-Moneim, A., Selim, D., Basuony, H., Sabic, E., Saleh, A., and Ebeid, T. (2020). Effect of dietary supplementation of Bacillus subtilis spores on growth performance, oxidative status, and digestive enzyme activities in Japanese quail birds. Trop. Anim. Health Prod. 52, 671–680. doi: 10.1007/s11250-019-02055-1
Abudabos, A., Alhouri, H., Alhidary, I., Nassan, M., and Swelum, A. (2019). Ameliorative effect of Bacillus subtilis, Saccharomyces boulardii, oregano, and calcium montmorillonite on growth, intestinal histology, and blood metabolites on Salmonella-infected broiler chicken. Env. Sci. Pollut. Res. Int. 26, 16274–16278. doi: 10.1007/s11356-019-05105-1
Adil, S., and Magray, S. (2012). Impact and manipulation of gut microflora in poultry: A review. J. Anim. Vet. Adv. 11, 873–877. doi: 10.3923/javaa.2012.873.877
Ahmed, E., Abdelrahman, M., and Gahreeb, K. (2019). Effect of probiotic on growth performance, carcass traits, and clinical health parameters of broilers reared under heat stress in upper Egypt. SVU-Int. J. Vet. Sci. 2, 27–44. doi: 10.21608/svu.2019.11221.1012
Alimohamadi, K., Taherpour, K., Ghasemi, H., and Fatahnia, F. (2014). Comparative effects of using black seed. (Nigella sativa), cumin seed. (Cuminum cyminum), probiotic or prebiotic on growth performance, blood haematology and serum biochemistry of broiler chicks. J. Anim. Physiol. Anim. Nutr. 98, 538–546. doi: 10.1111/jpn.12115
Al-Khalaifa, H., Al-Nasser, A., Al-Surayee, T., Al-Kandari, S., Al-EnziN, Al-Sharrah, T., et al. (2019). Effect of dietary probiotics and prebiotics on the performance of broiler chickens. Poult. Sci. 98, 4465–4479. doi: 10.3382/ps/pez282
Alkhalf, A., Alhaj, M., and Al-homidan, I. (2010). Influence of probiotic supplementation on blood parameters and growth performance in broiler chickens. Saudi J. Biol. Sci. 17, 219–225. doi: 10.1016/j.sjbs.2010.04.005
Amaretti, A., Di, N., Pompei, A., Raimondi, S., Rossi, M., and Bordoni, A. (2013). Antioxidant properties of potentially probiotic bacteria: In vitro and in vivo activities. Appl. Microbiol. Biotech. 97, 809–817. doi: 10.1007/s00253-012-4241-7
Anjum, M., Khan, A., Azim, A., and Afzal, M. (2005). Effect of dietary supplementation of multi-strain probiotic on broiler growth performance. Pakistan Vet. J. 25, 25–29.
Attia, Y., Allakany, H., Abd Al-Hamid, A., Al-Saffar, A., Hassan, R., and Mohamed, N. (2013). Capability of different non-nutritive feed additives on improving productive and physiological traits of broiler chicks fed diets with or without aflatoxin during the first 3 weeks of life. J. Anim. Physiol. Anim. Nutr. 97, 754–772. doi: 10.1111/j.1439-0396.2012.01317.x
Attia, Y., Hamid, A., Ismaiel, A., Oliveira, M., and Simon, G. (2018). Nitrate detoxification using antioxidants and probiotics in the water for rabbits. Rev. Colombiana Ciencias Pecuarias 31, 130–138. doi: 10.17533/udea.rccp.v31n2a06
Awad, W. A., Ghareeb, K., Abdel-Raheem, S., and Böhm, J. (2009). Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 88, 49–55. doi: 10.3382/ps.2008-00244
Bai, K., Feng, C., Jiang, L., Zhang, L., Zhang, J., Zhang, L., et al. (2018). Dietary effects of Bacillus subtilis fmbj on growth performance, small intestinal morphology, and its antioxidant capacity of broilers. Poult. Sci. 97, 2312–2321. doi: 10.3382/ps/pey116
Bidura, I., Siti, N., and Partama, I. (2019). Effect of probiotics, Saccharomyces spp. Kb-5 and Kb-8, in diets on growth performance and cholesterol levels in ducks. South African J. Anim. Sci. 49, 220–226. doi: 10.4314/sajas.v49i2.2
Biswas, A., Dev, K., Tyagi, P., and Mandal, A. (2022). The effect of multi-strain probiotics as feed additives on performance, immunity, expression of nutrient transporter genes and gut morphometry in broiler chickens. Anim. Biosci. 35, 64–74. doi: 10.5713/ab.20.0749
Busher, J. (1990). Serum Albumin and Globulin. Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd Edn. London: Butterworths.
Callaway, T., Edrington, T., Byrd, J., and Nisbet, D. (2017). “Use of direct-fed microbials in layer hen production-performance response and Salmonella control, producing safe eggs: The microbial ecology of Salmonella,” in Producing Safe Eggs, eds S. C. Ricke and R. K. Gast (Cambridge, MA: Academic Press), 301–322.
Cavalheiro, C., Ruiz-Capillas, C., Herrero, A., Jiménez-Colmenero, F., Ragagnin, M., and Martins, F. (2015). Application of probiotic delivery systems in meat products. Trends Food Sci. Technol. 46, 120–131. doi: 10.1016/j.tifs.2015.09.004
Chang, C., Teng, P., Lee, T., and Yu, B. (2020). Effects of multi-strain probiotic supplementation on intestinal microbiota, tight junctions, and inflammation in young broiler chickens challenged with Salmonella enterica subsp. enterica. Asian-Australasian J. Anim. Sci. 33, 1797–1808. doi: 10.5713/ajas.19.0427
Chauhan, S., Celi, P., Fahri, F., Leury, B., and Dunshea, F. (2014). Dietary antioxidants at supranutritional doses modulate skeletal muscle heat shock protein and inflammatory gene expression in sheep exposed to heat stress. J. Anim. Sci. 92, 4897–4908. doi: 10.2527/jas.2014-8047
Chen, J., Zhang, R., Wang, Q., Fu, Z., Zheng, Q., and Wang, C. (2010). Effects of Bacillus licheniformis on growth performance, antioxidant indices and blood biochemical parameters of broiler chickens. Chinese J. Anim. Nutr. 22, 1019–1023. doi: 10.1016/j.psj.2023.103210
Chen, T., Wu, Q., Zhou, H., Deng, K., Wang, X., Meng, F., et al. (2017). Assessment of commercial probiotic products in China for labelling accuracy and probiotic characterisation of selected isolates. Int. J. Dairy Tech. 70, 119–126. doi: 10.1111/1471-0307.12331
Cheng, C., Tu, W., Wang, S., Tang, P., Chen, C., Chen, H., et al. (2015). Annotation of differential gene expression in small yellow follicles of a broiler type strain of Taiwan country chickens in response to acute heat stress. PLoS One 10:e0143418. doi: 10.1371/journal.pone.0143418
Daly, J., Olivier, G., and Moore, A. (1995). A calorimetric assay for the quantification of brook trout. (Salvelinus fontinalis). lymphocyte mitogenesis. Fish Shellfish Immunol 5 266–273. doi: 10.1006/fsim.1995.0026
Das, H., Medhi, A., and Islam, M. (2005). Effect of probiotics on certain blood parameter and carcass characteristics of broiler chicken. Indian J. Poult. Sci. 40, 83–86.
De França, T., Ferreira, R., and Leo, R. (2023). Effects of carbohydrase and phytase enzymes supplementation within low energy diets on performance and energy utilization of broiler chickens. Liv. Sci. 274:105271. doi: 10.1016/j.livsci.2023.105271
De, A. K., Chakraborty, D., and Ponraj, P. (2023). Supplementing turmeric rhizome powder in growing Andaman local pigs: a conflated approach for therapy evaluation. Trop. Anim. Health Prod. 55:45. doi: 10.1007/s11250-023-03459-w
Dev, K., Mir, N., Biswas, A., Kannoujia, J., Begum, J., Kant, R., et al. (2020). Dietary synbiotic supplementation improves the growth performance, body antioxidant pool, serum biochemistry, meat quality, and lipid oxidative stability in broiler chickens. Anim. Nutr. 6, 325–332. doi: 10.1016/j.aninu.2020.03.002
El-Baky, A. (2013). Clinico-pathological and immunological effects of multistrain probiotic on broiler chicken vaccinated against avian influenza virus. Global Vet. 10, 534–541. doi: 10.5829/idosi.gv.2013.10.5.7350
Elbaz, A., Ashmawy, E., Ali, S., Mourad, D., El-Samahy, H., Badri, F., et al. (2023). Effectiveness of probiotics and clove essential oils in improving growth performance, immuno-antioxidant status, ileum morphometric, and microbial community structure for heat-stressed broilers. Sci Rep 13:18846. doi: 10.1038/s41598-023-45868-9
Fesseha, H., Demlie, T., Mathewos, M., and Eshetu, E. (2021). Effect of Lactobacillus species probiotics on growth performance of dual-purpose chicken. Vet. Med. Res. Rep. 12, 75–83. doi: 10.2147/VMRR.S300881
Food and Agriculture Organization/World Health Organization. (2001). Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. FAO: Cordoba.
Gadde, U., Oh, S., Lee, Y., Davis, E., Zimmerman, N., Rehberger, T., et al. (2017). The effects of direct-fed microbial supplementation, as an alternative to antibiotics, on growth performance, intestinal immune status, and epithelial barrier gene expression in broiler chickens. Probiotics Antimicrob. Proteins 9, 397–405. doi: 10.1007/s12602-017-9275-9
Gao, J., Zhang, H., Yu, S., Wu, S., Yoon, I., Quigley, J., et al. (2008). Effects ofyeast culture in broiler diets on performance and immunomodulatory functions. Poult. Sci. 87, 1377–1384. doi: 10.3382/ps.2007-00418
Garcia-Migura, L., Hendriksen, R., Fraile, L., and Aarestrup, F. (2014). Antimicrobial resistance of zoonotic and commensal bacteria in Europe: The missing link between consumption and resistance in veterinary medicine. Vet. Microbiol. 170, 1–9. doi: 10.1016/j.vetmic.2014.01.013
Gondwe, T., and Wollny, C. (2002). “Traditional breeding systems in smallholder rural poultry in Malawi,” in Proceedings of the 7th World Congress on Genetics Applied to Livestock Production, Session 25, Communication no 25–26, Montpellier, France, 19-23 August 2002, (Montpellier: Institut National de la Recherche Agronomique (INRA)).
Gu, X., Hao, Y., and Wang, X. (2012). Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 2. Intestinal oxidative stress. Poult. Sci. 91, 790–799. doi: 10.3382/ps.2011-01628
Habeeb, A., Gad, A., and Atta, M. (2018). Temperature-Humidity Indices as Indicators to Heat Stress of Climatic Conditions with Relation to Production and Reproduction of Farm Animals. Int. J. Biotechnol. Recent. Adv. 1, 35–50. doi: 10.18689/ijbr-1000107
Harr, K. (2002). Clinical chemistry of companion avian species: A review. Vet. Clin. Pathol. 31, 140–151. doi: 10.1111/j.1939-165x.2002.tb00295.x
Hernandez-Patlan, D., Solis-Cruz, B., Hargis, B., and Tellez, G. (2020). “The Use of probiotics in poultry production for the control of bacterial infections and aflatoxins,” in Prebiotics and Probiotics - Potential Benefits in Nutrition and Health, eds E. Franco-Robles and J. Ramírez-Emiliano (London: IntechOpen), 1–21.
Hossain, M., Begum, M., and Kim, I. (2015). Effect of Bacillus subtilis, Clostridium butyricum and Lactobacillus acidophilus endospores on growth performance, nutrient digestibility, meat quality, relative organ weight, microbial shedding and excreta noxious gas emission in broilers. Vet. Med. 60, 77–86. doi: 10.17221/7981-VETMED
Hossain, M., Sardar, D., Afsana, S., Datta, M., and Habib, M. (2024). Comparative analysis between multi-strain probiotics and antibiotic as starter feed supplement of poultry on growth performance, serum metabolites and meat quality. Vet. Anim. Sci. 24:100346. doi: 10.1016/j.vas.2024.100346
Hu, H., Bai, X., Xu, K., Zhang, C., and Chen, L. (2021). Effect of phloretin on growth performance, serum biochemical parameters and antioxidant profile in heat-stressed broilers. Poult. Sci. 100:101217. doi: 10.1016/j.psj.2021.101217
Huang, M., Choi, Y., Houde, R., Lee, J., Lee, B., and Zhao, X. (2004). Effects of Lactobacilli and an acidophilic fungus on the production performance and immune responses in broiler chickens. Poult. Sci. 83, 788–795. doi: 10.1093/ps/83.5.788
Hussein, A. (2014). Effect of biological additives on growth indices and physiological responses of weaned najdi ram lambs. J. Exp. Biol. Agric. Sci. 2, 597–607.
Hussein, E., Ahmed, S., Abudabos, A., Aljumaah, M., Alkhlulaifi, M., Nassan, M., et al. (2020). Effect of antibiotic, phytobiotic and probiotic supplementation on growth, blood indices and intestine health in broiler chicks challenged with Clostridium perfringens. Animals 10:507. doi: 10.3390/ani10030507
Inatomi, T., and Otomaru, K. (2018). Effect of dietary probiotics on the semen traits and antioxidative activity of male broiler breeders. Sci. Rep. 8:5874. doi: 10.1038/s41598-018-24345-8
Jiang, S., Mohammed, A., Jacobs, J., Cramer, T., and Cheng, H. (2020). Effect of synbiotics on thyroid hormones, intestinal histomorphology, and heat shock protein 70 expression in broiler chickens reared under cyclic heat stress. Poultry Sci. 99, 142–150. doi: 10.3382/ps/pez571
Kalavathy, R., Abdullah, N., Jalaludin, S., and Ho, Y. (2003). Effects of Lactobacillus cultures ongrowth performance, abdominal fat deposition, serum lipids and weight of organs of broiler chickens. Br. Poult. Sci. 44, 139–144. doi: 10.1080/0007166031000085445
Kannaki, T., Reddy, M., Shanmugam, M., Verma, P., and Sharma, R. (2010). Chicken toll-like receptors and their role in immunity. Worlds Poult. Sci. J. 66, 727–738. doi: 10.1017/S0043933910000693
Kazemi, S., Ahmadi, H., and Torshizi, M. (2019). Evaluating two multi-strain probiotics on growth performance, intestinal morphology, lipid oxidation and ileal microflora in chickens. J. Anim. Physiol. Animl. Nutr. 103, 1399–1407. doi: 10.1111/jpn.13124
Khaksefidi, A., and Rahimi, S. (2005). Effect of probiotic inclusion in the diet of broiler chickens on performance, feed efficiency and carcass quality. Asian-Australasian J. Anim. Sci. 18, 1153–1156. doi: 10.5713/ajas.2005.1153
Khattab, A., El Basuini, M., El-Ratel, I., and Fouda, S. (2021). Dietary probiotics as a strategy for improving growth performance, intestinal efficacy, immunity, and antioxidant capacity of white Pekin ducks fed with different levels of CP. Poult. Sci. 100:100898. doi: 10.1016/j.psj.2020.11.067
Kwoji, I., Aiyegoro, O., Okpeku, M., and Adeleke, M. (2021). Multi-strain probiotics: Synergy among isolates enhances biological activities. Biology 10:322. doi: 10.3390/biology10040322
Lambo, M., Chang, X., and Liu, D. (2021). The recent trend in the use of multistrain probiotics in livestock production: An overview. Animals 11:2805. doi: 10.3390/ani11102805
Lan, R., Tran, H., and Kim, I. (2017). Effects of probiotic supplementation in different nutrient density diets on growth performance, nutrient digestibility, blood profiles, faecal microflora and noxious gas emission in weaning pig. J. Sci. Food Agric. 97, 1335–1341. doi: 10.1002/jsfa.7871
Latorre, J., Hernandez-Velasco, X., Wolfenden, R., Vicente, J., Wolfenden, A., Menconi, A., et al. (2016). Evaluation and selection of bacillus species based on enzyme production, antimicrobial activity, and biofilm synthesis as direct-fed microbial candidates for poultry. Front. Vet. Sci. 3:95. doi: 10.3389/fvets.2016.00095
Lee, K., Lillehoj, H., Jang, S., Lee, S., Bautista, D., and Siragusa, G. (2013). Effect of Bacillus subtilis-based direct-fed microbials on immune status in broiler chickens raised on fresh or used litter. Asian-Australas J. Anim. Sci. 26, 1592–1597. doi: 10.5713/ajas.2013.13178
Lee, S., Kim, J., Kim, J., An, B., and Kang, C. (2010). Effects of multiple enzyme. (ROVABIO® Max). containing carbohydrolases and phytase on growth performance and intestinal viscosity in broiler chicks fed corn-wheat-soybean meal based diets. Asian-Australas J. Animal Sci. 23, 1198–1204. doi: 10.5713/ajas.2010.90592
Li, W., Rajput, I., Xu, X., Li, Y., Lei, J., Huang, Q., et al. (2011). Effects of Probiotic. (Bacillus subtilis). on laying performance, blood biochemical properties and intestinal microflora of Shaoxing duck. Int. J. Poult. Sci. 10, 583–589. doi: 10.3923/ijps.2011.583.589
Liang, X., Yu, Y., Mei, J., Feng, J., Li, P., Bai, Y., et al. (2024). Effects of feed protein levels on Chinese mitten crabs. (Eriocheir sinensis). under the rice-crab co-culture model: Performance, nutrient composition, antioxidant capacity and immunity. Aquacult. Rep. 35:101963. doi: 10.1016/j.aqrep.2024.101963
Mansoub, N. (2010). Effect of probiotic bacteria utilization on serum cholesterol and triglycerides contents and performance of broiler chickens. Global Vet. 5, 184–186.
Mohan, B., Kadirvel, R., Natarajan, M., and Bhaskaran, M. (1996). Effect of probiotic supplementation on growth, nitrogen utilization and serum cholesterol in broilers. Br. Poult. Sci. 37, 395–401. doi: 10.1080/00071669608417870
Mookiah, S., Sieo, C., Ramasamy, K., Abdullah, N., and Ho, Y. (2014). Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations, and caecal fermentation concentrations of broiler chickens. J. Sci. Food Agric. 94, 341–348. doi: 10.1002/jsfa.6365
Mountzouris, K., Tsirtsikos, P., Palamidi, E., Arvaniti, A., Mohnl, M., Schatzmayr, G., et al. (2010). Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins and caecal microflora composition. Poult. Sci. 89, 58–67. doi: 10.3382/ps.2009-00308
Murch, S. (2001). Toll of allergy reduced by probiotics. Lancet 357, 1057–1059. doi: 10.1016/S0140-6736(00)04305-1
Musigwa, S., Morgan, N., Swick, R., Cozannet, P., and Wu, S. (2020). Energy dynamics, nitrogen balance, and performance in broilers fed high- and reduced-CP diets. J. Appl. Poult. Res. 29, 830–841. doi: 10.1016/j.japr.2020.08.001
Muthusamy, G., Joardar, S., and Samanta, I. (2020). Dietary administered purified β-glucan of edible mushroom. (Pleurotus florida). provides immunostimulation and protection in broiler experimentally challenged with virulent Newcastle disease virus. J. Basic Appl. Zoo. 81:55. doi: 10.1186/s41936-020-00180-0
Muthusamy, G., Joardar, S., Samanta, I., Isore, D., Roy, B., and Maiti, T. (2013). β–Glucan from Edible Mushroom. (Pleurotus florida). Enhances Mucosal Immunity in Poultry. Adv. Anim. Vet. Sci. 4, 116–119.
Neijat, M., Shirley, R., Barton, J., Thiery, P., Welsher, A., and Kiarie, E. (2019). Effect of dietary supplementation of Bacillus subtilis DSM29784 on hen performance, egg quality indices, and apparent retention of dietary components in laying hens from 19 to 48 weeks of age. Poult. Sci. 98, 5622–5635. doi: 10.3382/ps/pez324
Nyoni, N., and Masika, P. (2012). Village chicken production practices in the Amatola Basin of the Eastern Cape Province, South Africa. Afr. J. Agric. Res. 7, 2647–2652. doi: 10.5897/AJAR11.1689
Oladipo, M., Dagwi, N., Emmnna, P., Sati, N., and Obamedo, T. (2023). Effect of multi-enzymes and probiotics on growth and health status of cockerel chickens. Nigerian J. Anim. Sci. 25, 101–108.
Olukosi, O., and Cowieson, A. (2007). Age-related influence of a cocktail of xylanase, amylase, and protease or phytase individually or in combination in broilers. Poult. Sci. 86, 77–86. doi: 10.1093/ps/86.1.77
Pambuka, R., Sjofjan, S., and Radiati, E. (2014). Effect of liquid probiotics mixed culture supplements through drinking water on laying hens performance and yolk cholesterol. J. World’s Poult. Res. 4, 5–09.
Panda, A., Ramarao, S., Raju, M., and Sharma, S. (2006). Dietary supplementation of probiotic Lactobacillus sporogenes on performance and serum biochemico-lipid profile of broiler chickens. J. Poult. Sci. 43, 235–240. doi: 10.2141/jpsa.43.235
Patterson, J., and Burkholder, K. (2003). Application of prebiotics and probiotics in poultry production. Poult. Sci. 82, 627–631. doi: 10.1093/ps/82.4.627
Paul, I., Isore, D., Joardar, S., Samanta, I., Biswas, U., Maiti, T., et al. (2012). Orally administered β-glucan of edible mushroom. (Pleuratus florida). origin upregulates innate immune response in broiler. Indian J. Anim. Sci. 82:745748.
Quintavalla, F., Bigliardi, E., and Bertoni, P. (2001). Blood biochemical baseline values in the ostrich. (Struthio camelus). Universita degli studi di Pharma Annali della Facolta di. Med. Vet. 21, 61–71.
Ramlucken, U., Ramchuran, S., Moonsamy, G., Lalloo, R., Thantsha, M., and Jansen, et al. (2020). A novel Bacillus based multi-strain probiotic improves growth performance and intestinal properties of Clostridium perfringens challenged broilers. Poult. Sci. 99, 331–341. doi: 10.3382/ps/pez496
Rashidi, N., Khatibjoo, A., Taherpour, K., Akbari-Gharaei, M., and Shirzadi, H. (2020). Effects of licorice extract, probiotic, toxin binder and poultry litter biochar on performance, immune function, blood indices and liver histopathology of broilers exposed to aflatoxin-B1. Poult. Sci. 99, 5896–5906. doi: 10.1016/j.psj.2020.08.034
Rehman, Z., Meng, C., Sun, Y., Safdar, A., Pasha, R., Munir, M., et al. (2018). Oxidative stress in poultry: lessons from the viral infections. Oxid. Med. Cell. Long. 2018:5123147. doi: 10.1155/2018/5123147
Reuben, R., Sarkar, S., Ibnat, H., Roy, P., and Jahid, I. (2022). Novel mono- and multi-strain probiotics supplementation modulates growth, intestinal microflora composition and haemato-biochemical parameters in broiler chickens. Vet. Med. Sci. 8, 668–680. doi: 10.1002/vms3.709
Roth, N., Käsbohrer, A., Mayrhofer, S., Zitz, U., Hofacre, C., and Domig, K. (2019). The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poult. Sci. 98, 1791–1804. doi: 10.3382/ps/pey539
Salami, S., Majoka, M., Saha, S., Garber, A., and Gabarrou, J. (2015). Efficacy of dietary antioxidants on broiler oxidative stress, performance and meat quality: science and market. Avian Biol. Res. 8, 65–78. doi: 10.3184/175815515X14291701859483
Salim, H., Kang, K., Akter, N., and Kim, D. (2013). Supplementation of direct-fed microbial as an alternative to antibiotic on growth performance, immune response, cecal microbial population, and ileal morphology of broiler chickens. J. Poult. Sci. 92, 2084–2090. doi: 10.3382/ps.2012-02947
Samanya, M., and Yamauchi, K. (2002). Histological alterations of intestinal villi in chickens fed dried Bacillus subtilis var. natto. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 133, 95–104. doi: 10.1016/s1095-6433(02)00121-6
Sen, S., Ingale, S., and Kim, Y. (2012). Effect of supplementation of Bacillus subtilis LS 1-2 to broiler diets on growth performance, nutrient retention, caecal microbiology and small intestinal morphology. Res. Vet. Sci. 93, 264–268. doi: 10.1016/j.rvsc.2011.05.021
Sharma, U., Pal, D., and Prasad, R. (2014). Alkaline phosphatase: An overview. Indian J. Clin. Biochem. 29, 269–278. doi: 10.1007/s12291-013-0408-y
Shi, Z., Rothrock, M., and Ricke, S. (2019). Applications of microbiome analyses in alternative poultry broiler production systems. Front. Vet. Sci. 6:157. doi: 10.3389/fvets.2019.00157
Shokryazdan, P., Jahromi, F., Liang, J., and Ho, Y. (2017). Probiotics: From Isolation to Application. J. Am. Coll. Nutr. 36, 666–676. doi: 10.1080/07315724.2017.1337529
Siddiqui, S., Kang, D., Park, J., et al. (2020). Chronic heat stress regulates the relation between heat shock protein and immunity in broiler small intestine. Sci. Rep. 10:18872. doi: 10.1038/s41598-020-75885-x
Silva, D., Sardi, J., Pitangui, N., Roque, S., Silva, A., and Rosalen, P. (2020). Probiotics as an alternative antimicrobial therapy: Current reality and future directions. J. Funct. Foods 73:104080. doi: 10.1016/j.jff.2020.104080
Sirovnik, J., Euteneuer, P., and Borstel, U. (2021). An attempt to use sound-imprinting to attract broilers onto elevated platforms for night-time roosting. Appl. Anim. Behav. Sci. 243:105448. doi: 10.1016/j.applanim.2021.105448
Siwicki, A., Kiczaka, W., Moranda, M., and Studnicka, M. (1998). Immunostimulatory effects of dimerized lysozyme. (KLP-602). on the non-specific defence mechanisms and protection against furunculosis in salmonids. Vet. Immunol. Immunopathol. 61, 369–378. doi: 10.1016/s0165-2427(97)00140-2
Sobczak, A., and Kozłowski, K. (2015). The effect of a probiotic preparation containing Bacillus subtilis ATCC PTA-6737 on egg production and physiological parameters of laying hens. Ann. Anim. Sci. 15, 711–723. doi: 10.1515/aoas-2015-0040
Sun, H., and Kim, I. (2021). Effects of microbial phytase supplementation on egg production and egg quality in hy-line brown hens during the late laying period. J. Poult. Sci. 58, 171–176. doi: 10.2141/jpsa.0200001
Surai, P. (2016). Antioxidant systems in poultry biology: Superoxide dismutase. J. Anim. Res. Nutr. 1:8. doi: 10.21767/2572-5459.100008
Swain, B., Naik, P., and Sing, N. (2014). Performance, carcass characteristics and economics of production of broiler chickens fed diet supplemented with multi-enzyme. Intl. J. Poult. Sci. 49, 163–166.
Tang, Z., Wang, J., Wen, C., Wang, T., and Zhou, Y. (2010). The effect of probiotics on broiler performance, immune organ index and serum index. Jiangsu Agric. Sci. 4, 208–210.
Teo, A., and Tan, H. (2007). Evaluation of the Performance and Intestinal Gut Microflora of Broilers Fed on Corn-Soy Diets Supplemented with Bacillus subtilis PB6. (CloSTAT). J. App. Poult. Res. 16, 296–303. doi: 10.1093/japr/16.3.296
Timmerman, H., Koning, C., Mulder, L., Rombouts, F., and Beynen, A. (2004). Monostrain, multistrain and multispecies probiotics—A comparison of functionality and efficacy. Int. J. Food Microbiol. 96, 219–233. doi: 10.1016/j.ijfoodmicro.2004.05.012
Tollba, A., and Mahmoud, R. (2009). How to control the broiler pathogenic intestinal flora under normal or heat stress conditions. 1-Medical plant-probiotics-sand as a litter. Egypt Poult Sci J 29, 565–587. doi: 10.1007/s00484-024-02779-2
Udeh, F., Udeh, V., and Chukwudi, P. (2020). Serum biochemistry profile and liver function indices of broiler chickens served dietary inclusion of probiotics. (Saccharomyces cerevisiae). and enzyme. Nigerian J. Anim. Prod. 47, 120–128. doi: 10.51791/njap.v47i6.2917
Wang, J., and Zhou, H. (2007). Comparison of the effects of Chinese herbs, probiotics and prebiotics with those of antibiotics in diets on the performance of meat ducks. J. Anim. Feed Sci. 16, 96–103.1. doi: 10.22358/jafs/66730/2007
Wang, L., Liu, C., Chen, M., Ya, T., Huang, W., Gao, P., et al. (2015). A novel Lactobacillus plantarum strain P-8 activates beneficial immune response of broiler chickens. Int. Immunopharmacol. 29, 901–907. doi: 10.1016/j.intimp.2015.07.024
Wu, Y., Wang, B., Zeng, Z., Liu, R., Tang, L., Gong, L., et al. (2019). Effects of probiotics Lactobacillus plantarum 16 and Paenibacillus polymyxa 10 on intestinal barrier function, antioxidative capacity, apoptosis, immune response, and biochemical parameters in broilers. Poult. Sci. 98, 5028–5039. doi: 10.3382/ps/pez226
Xu, D., Li, W., Huang, Y., He, J., and Tian, Y. (2014). The effect of selenium and polysaccharide of Atractylodes macrocephala Koidz. (PAMK). on immune response in chicken spleen under heat stress. Biol. Trace Element. Res. 160, 232–237. doi: 10.1007/s12011-014-0056-y
Yao, S., Zhang, A., Jiang, N., and Song, Y. (2009). Effects of probiotics on nutrient utilization, serum biochemical indexes and intestinal microflora of royal concubine chickens in the rearing period. Heilongjiang Anim. Husband Vet. 9, 113–115. doi: 10.21203/rs.3.rs-1475536/v1
Yazhini, P., Visha, P., Selvaraj, P., Vasanthakumar, P., and Chandran, V. (2018). Dietary encapsulated probiotic effect on broiler serum biochemical parameters. Vet. World 11, 1344–1348. doi: 10.14202/vetworld.2018.1344-1348
Yitbarek, A., Rodriguez-Lecompte, J., Echeverry, H., Munyaka, P., Barjesteh, N., Sharif, S., et al. (2013). Performance, histomorphology, and toll-like receptor, chemokine, and cytokine profile locally and systemically in broiler chickens fed diets supplemented with yeast-derived macromolecules. Poult. Sci. 92, 2299–2310. doi: 10.3382/ps.2013-03141
Yu, L., Peng, Z., Dong, L., Wang, H., and Shi, S. (2019). Enterococcus faecium NCIMB 10415 supplementation improves the meat quality and antioxidant capacity of muscle of broilers. J. Anim. Physiol. Anim. Nutr. 103, 1099–1106. doi: 10.1111/jpn.13097
Yu, Y., Li, Q., Zeng, X., Xu, Y., Jin, K., Liu, J., et al. (2022). Effects of Probiotics on the Growth Performance, Antioxidant Functions, Immune Responses, and Caecal Microbiota of Broilers Challenged by Lipopolysaccharide. Front. Vet. Sci. 9:846649. doi: 10.3389/fvets.2022.846649
Żbikowski, A., Pawłowski, K., Śliżewska, K., Dolka, B., Nerc, J., and Szeleszczuk, P. (2020). Comparative Effects of Using New Multi-Strain Synbiotics on Chicken Growth Performance, Hematology, Serum Biochemistry and Immunity. Animals 10:1555. doi: 10.3390/ani10091555
Zhang, J., Hu, Z., Lu, C., Bai, K., Zhang, L., and Wang, T. (2015). Effect of various levels of dietary curcumin on meat quality and antioxidant profile of breast muscle in broilers. J. Agric. Food Chem. 63, 3880–3886. doi: 10.1021/jf505889b
Zhang, L., Zhang, R., Jia, H., Zhu, Z., Li, H., and Ma, Y. (2021). Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens. Open Life Sci. 16, 311–322. doi: 10.1515/biol-2021-0031
Zhao, P., and Kim, I. (2015). Effect of direct-fed microbial on growth performance, nutrient digestibility, faecal noxious gas emission, faecal microbial flora and diarrhoea score in weanling pigs. Anim. Feed Sci. Technol. 200, 86–92. doi: 10.1016/j.anifeedsci.2014.12.010
Zhao, S., Zhang, N., Lu, B., He, Y., Liang, J., Zhou, Y., et al. (2022). Effect of probiotics on growth performance, immune function, serum indices and intestinal flora of broilers. Res. Square doi: 10.21203/rs.3.rs-1475536/v1
Zhen, W., Shao, Y., Gong, X., Wu, Y., Geng, Y., Wang, Z., et al. (2018). Effect of dietary Bacillus coagulans supplementation on growth performance and immune responses of broiler chickens challenged by Salmonella enteritidis. Poult. Sci. 97, 2654–2666. doi: 10.3382/ps/pey119
Zhen, Y., Chen, X., Xiao, J., Wang, T., Yi, X., and Zhang, X. (2020). Effect of mixture of yeast culture and lactobacillus culture on cecal microbiome and immune function of broilers. J. Jilin Agric. Univ. 42, 343–347. doi: 10.13327/j.jilau.2020.4252
Ziggers, D. (2011). Animal feed news. EU 12-point antibiotic action plan released. Available online at: https://www.allaboutfeed.net/animal-feed/feed-additives/eu-12-point-antibiotic-action-plan-released/
Keywords: Andaman and Nicobar Islands, multi-strain probiotics, backyard poultry, growth promoter, immunomodulation, serum biochemistry
Citation: Halder N, Joardar SN, Sunder J, De AK, Bhattacharya D, Abd El Wahed A, Kobialka RM, Madanan MG and Mondal S (2025) Impact of multi-strain probiotics supplementation on growth, immune responses and physiological traits in backyard poultry of Andaman and Nicobar Islands, India. Front. Microbiol. 16:1625167. doi: 10.3389/fmicb.2025.1625167
Received: 08 May 2025; Accepted: 09 July 2025;
Published: 12 August 2025.
Edited by:
Mahadevakumar Shivannegowda, Botanical Survey of India, IndiaReviewed by:
M. Y. Sreenivasa, University of Mysore, IndiaKandikere Ramaiah Sridhar, Mangalore University, India
Copyright © 2025 Halder, Joardar, Sunder, De, Bhattacharya, Abd El Wahed, Kobialka, Madanan and Mondal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rea Maja Kobialka, cmVhX21hamEua29iaWFsa2FAdmV0bWVkLnVuaS1sZWlwemlnLmRl
 Neha Halder1,2
Neha Halder1,2 Siddhartha Narayan Joardar
Siddhartha Narayan Joardar Arun Kumar De
Arun Kumar De Rea Maja Kobialka
Rea Maja Kobialka