- 1Institute of Marine Sciences and Guangdong Provincial Key Laboratory of Marine Biology, Shantou University, Shantou, China
- 2Guangxi Key Laboratory of Aquatic Genetic Breeding and Healthy Aquaculture, Guangxi Academy of Fishery Sciences, Nanning, China
Aquaculture, a cornerstone of global food security, faces critical threats from disease outbreaks, antimicrobial resistance, and ecological disruption. Through a narrative analysis of over 160 studies, this review synthesizes advances in microbiome engineering—a sustainable approach to enhancing disease resistance in aquatic animals—addressing key gaps: the inconsistent efficacy of conventional probiotics and prebiotics under field conditions, and the need for climate-resilient solutions. Critically, we highlight the emergence of precision microbiome engineering as a transformative paradigm. We integrate findings from genomics, metabolomics, clustered regularly interspaced short palindromic repeats, and artificial intelligence to identify microbial strategies that enhance host resilience. Genomic and multi-omics methods reveal health-associated microbes and metabolites, such as Vibrio-dominated dysbiosis markers in shrimp and butyrate-mediated immunity. Guided by these biomarkers, we describe precision-tailored probiotics—host-derived or genome-edited Bacillus subtilis strains whose adhesion factors, metabolic outputs (e.g., butyrate, bacteriocins), and heat stress tolerance are matched to the target species’ gut niche. These are combined with complementary prebiotics (e.g., chitosan oligosaccharides) and synbiotics (e.g., Lactiplantibacillus plantarum plus king oyster mushroom extracts) that suppress pathogens through competitive exclusion and immune modulation. Ecologically rational innovations—interventions explicitly grounded in ecological theory (niche complementarity, K-selection) to stabilize resource-efficient microbiomes—such as fecal microbiota transplantation and synthetic consortia, demonstrate further disease control potential. Our synthesis reveals that translating microbiome engineering from laboratory to farm requires overcoming host-microbiome compatibility challenges and ecological risks. Policy alignment with the United Nations Sustainable Development Goals—Zero Hunger (Sustainable Development Goal 2), Climate Action (Sustainable Development Goal 13), and Life Below Water (Sustainable Development Goal 14)—is critical for sustainable adoption.
1 Introduction
Global aquaculture reached a historic milestone in 2022 by producing 94.4 million tonnes of aquatic animals, representing 51% of total production, and 130.9 million tonnes when including algae (FAO, 2024). This achievement highlights aquaculture’s crucial role in global food security, yet rapid expansion has intensified sustainability challenges such as disease outbreaks and antimicrobial resistance (AMR). Asia, responsible for 70% of aquatic animal production, faces severe disease-driven losses (Sampson, 2024). Despite vaccine development, pathogens like Vibrio parahaemolyticus (causing acute hepatopancreatic necrosis disease, AHPND) and Streptococcus agalactiae (causing streptococcosis) continue to cause global annual losses exceeding one billion dollars due to strain- and host-specific pathogenicity, inadequate infrastructure, and limited vaccine coverage in small-scale farms (Yen et al., 2021; Wang T. et al., 2024). These impacts are worsened in low- and middle-income countries (LMICs) by inadequate infrastructure and limited access to sustainable alternatives. The limitations of antibiotics and inconsistent probiotics spurred interest in holistic ‘microbial community management’ (Bossier et al., 2016), recognizing hosts as holobionts dependent on balanced microbiota. For example, a survey of 231 small-scale carp polyculture farms in Bangladesh revealed 46.8% report outbreaks of epizootic ulcerative syndrome (EUS) and columnaris, with an average mortality of 10.23% (Debnath et al., 2024). Similarly, a study of Lao PDR farms found 57.5% of fish farms rely on antibiotics against streptococcosis, with specialized operations exhibiting the highest antimicrobial dependence (Poupaud et al., 2022). Other threats include Edwardsiella tarda in Japanese eel and Aeromonas hydrophila in hybrid catfish, both causing 30–50% reductions in yield in intensive systems (Huang J. et al., 2024; Lin et al., 2024).
The reliance on antibiotics has fueled a global AMR crisis. In China, testing of 102 V. parahaemolyticus isolates from farmed shrimp found 46% resist multiple antibiotics, notably sulfamoxazole (56.9%) and erythromycin (33.3%) (Zhang F. et al., 2024). Meta-analyses reveal alarming trends: sulfonamide (sul1) and tetracycline (tetA) resistance genes dominate aquaculture systems, and groundwater antibiotic resistance gene (ARG) concentrations correlate with antibiotic use (Zainab et al., 2020). In Bangladesh, 97% of E. coli isolates from cultured fish were multidrug-resistant, carrying blaTEM/blaCTX genes (Rana et al., 2025), while 71.3% of foodborne E. coli strains in China showed tetracycline resistance (Liu C. et al., 2024). Chronic antibiotic exposure in aquatic environments amplifies environmental AMR risks by increasing resistance gene abundance (Chen Z. et al., 2025). Viral pathogens such as Decapod iridescent virus 1 (DIV1) exacerbate these problems by disrupting host microbiomes and risking spillover to wildlife (Wan et al., 2024).
Conventional alternatives like probiotics and prebiotics remain underutilized due to gaps in efficacy and knowledge. Probiotics often fail to consistently colonize the gut, while prebiotics lack pathogen-targeted precision (Guo et al., 2023). In Malaysia, 88.1% of shrimp farmers misunderstand AMR, and 50.5% use antibiotics prophylactically (Devadas et al., 2025), reflecting a critical need for effective alternatives. This necessitates a paradigm shift toward proactive ‘microbial education’ (Dantan et al., 2024) to establish resilient, health-promoting microbiomes early in development. The gut microbiome is a pivotal determinant of aquatic animal health. In grass carp, enrichment of SCFA-producing genera within Lactobacillaceae (e.g., Lactobacillus) and Bacteroidaceae (e.g., Bacteroides) correlated with upregulation of immune genes such as MHC2 and TNF-α; conversely, antibiotic-induced dysbiosis reduced microbial diversity and antioxidant capacity, triggering oxidative stress (Chen Z. et al., 2025).
Precision microbiome engineering addresses these limitations through targeted interventions that integrate ecological principles and functional enhancement. For example, CRISPR-edited Cetobacterium somerae XMX-1 knocks down viral receptors in zebrafish, reducing challenge-mortality by 75% (Liang et al., 2024). AI-designed synthetic communities (SynComs) that incorporate native Photobacterium spp. improve thermal-stress resilience (Toxqui-Rodríguez et al., 2025). Functional enhancement is evident in xylanase-expressing Bacillus, which elevates butyrate production in tilapia and activates immunity against Aeromonas hydrophila (Wang T. et al., 2024).
Despite these advances, key deployment barriers persist. These include (i) risks of horizontal gene transfer—such as temperature-amplified plasmid exchange in catfish systems (Li et al., 2025); (ii) host-specific microbiome variability that hampers generalizable formulations; (iii) regulatory inconsistencies across jurisdictions (Okoli et al., 2022; Rahayu et al., 2024); and (iv) socioeconomic constraints that limit access in low- and middle-income countries (LMICs) (Ghosh et al., 2022).
This review synthesizes advances in microbiome engineering—including next-generation probiotics, engineered synbiotics, fecal microbiota transplantation (FMT), and synthetic communities—to enhance disease resistance in aquaculture. Specifically, it explores CRISPR and AI-driven precision tools, identifies ecological, regulatory, and socioeconomic adoption barriers, and proposes a Sustainable Development Goals (SDG)-aligned roadmap targeting antimicrobial resistance reduction (SDG 3), food security (SDG 2), and marine biodiversity conservation (SDG 14). We emphasize that future advancements require tailored farm-specific probiotics, circular aquaculture systems, and global policy integration to ensure scalable, eco-safe aquaculture.
2 Literature search and study selection
We conducted a structured narrative review and searched Web of Science, Scopus, and PubMed for peer-reviewed studies published from January 2015 to August 2025 (last search: 11 August 2025). Search strings combined aquaculture terms with microbiome-engineering concepts using Boolean operators and truncation, for example: (aquaculture OR fish* OR shrimp OR prawn OR mollusc* OR mollusk*) AND (microbiome OR microbiota) AND (probiotic* OR prebiotic* OR synbiotic* OR postbiotic* OR FMT OR “fecal microbiota transplant” OR “faecal microbiota transplant” OR SynCom* OR “synthetic communit*” OR CRISPR OR gut-on-chip). Titles and abstracts, then full texts, were screened against inclusion criteria: (i) aquatic animals (finfish, crustaceans, mollusks); (ii) disease-resistance or immunity outcomes; and (iii) interventions involving probiotics, prebiotics/synbiotics, postbiotics, FMT, SynComs, or host/microbe engineering. We excluded non-primary studies (e.g., reviews, editorials, conference abstracts without data), terrestrial models, and interventions not targeting the microbiome from the primary synthesis. Relevant reviews and included studies’ reference lists were hand-searched to identify additional primary studies. Methods/tool papers (e.g., gut-on-chip) and non-aquatic models were cited for methodological context only and excluded from the primary synthesis. After deduplication, 162 primary intervention studies were included in the narrative synthesis.
3 Multi-omics insights guiding microbiome engineering
Recent multi-omics advances clarify host–microbiome–environment interactions by resolving microbial composition, function, and host responses at high resolution. These insights enable precision microbiome engineering, shifting from observation to targeted interventions in aquaculture. Integrating genomic, transcriptomic, metabolomic, and epigenetic findings with probiotic, prebiotic, and synbiotic applications illustrates how multi-omics accelerates microbiome engineering.
3.1 Genomic and metagenomic approaches
Genomic and metagenomic approaches decode host-microbiome-environment interactions by mapping microbial community dynamics under health, disease, or stress. These methods identify keystone taxa (e.g., opportunistic pathogens like Aeromonas) and functional shifts linked to dysbiosis, enabling targeted interventions. High-throughput sequencing (e.g., 16S rRNA gene profiling, shotgun metagenomics) enhances resolution of microbial profiles across host health and environmental gradients. These approaches identify key microbial players such as opportunistic pathogens (e.g., Aeromonas, Vibrio) and beneficial taxa (e.g., Cetobacterium, Weissella), with abundance shifts strongly correlating to host status. Critically, functionality is strain-specific: pathogenic potential varies within Aeromonas/Vibrio, and probiotic properties are not universal in Cetobacterium/Weissella (Wang T. et al., 2024; Li et al., 2025). Metagenomics facilitates pathogen discovery and dysbiosis-disease linkages, exemplified by metabarcoding identifying a novel Flavobacterium species causing peracute skin disease in rainbow trout, distinct from classical columnaris strains (Zamparo et al., 2025). Viral profiling through virome analyses detects pathogens like white spot syndrome virus in environmental reservoirs and novel caliciviruses linked to mass fish mortality (Mercer et al., 2024; Su et al., 2024). Advanced techniques such as Oxford Nanopore sequencing enhance resolution in complex matrices like fish mucus (Domingo-Bretón et al., 2024). However, these DNA-centred tools have well-recognized constraints: short-read assemblies can mask low-abundance taxa and hamper strain-level resolution; draft metagenomes rely on gene annotations that are predictive rather than experimental; and high host DNA backgrounds can dilute microbial signals, particularly in gut, gill, or skin biopsies. Stable isotope probing (DNA-SIP/RNA-SIP) and long-read sequencing are now being combined with metagenomics to assign functional genes to active taxa and partially alleviate these blind spots (Alcolombri et al., 2022; Han Y. et al., 2024). Spatial heterogeneity is critical, with distinct microbiomes inhabiting mucosal surfaces (gill, skin, gut, ovary). For instance, the gut of olive flounder harbors more antibiotic resistance genes and Vibrionaceae than functionally diverse gill/skin communities, underscoring the need for site-specific probiotics (Yu et al., 2025).
3.2 Metabolomic insights into host-microbiome crosstalk
Metabolomics reveals how microbial metabolites (e.g., SCFAs) mediate host-microbe crosstalk, influencing immune pathways and stress resilience. This mechanistic insight identifies therapeutic targets for precision engineering. For example, butyrate and other SCFAs serve dual roles as enterocyte energy sources and immunomodulators, acting via histone deacetylase (HDAC) inhibition or G-protein-coupled receptor signaling. Dietary interventions, such as supplementation with Clostridium butyricum in shrimp, elevate beneficial metabolites and enhance mucosal immunity and pathogen resistance (Li S. et al., 2022; Liao et al., 2023). Yet metabolite profiles alone seldom reveal which organism produced a given compound. Emerging compound-specific stable isotope labeling (e.g., 13C or 15N SIP) tracked by high-resolution MS, as well as spatial metabolomics coupled with fluorescence in situ hybridization (FISH-SIMS), now help connect metabolite fluxes to specific microbial producers (Uengwetwanit et al., 2020; Alcolombri et al., 2022). Beyond SCFAs, metabolomics detects broader shifts in bile acid and amino acid metabolism that contribute to immune resilience. Critically, metabolomics reveals pollutant-induced dysbiosis, where microplastics, pesticides (e.g., deltamethrin), and polychlorinated biphenyls disrupt gut-liver axes. These pollutants alter lipid metabolites like lysophosphatidylcholines, trigger oxidative stress, and dysregulate signaling pathways (e.g., PPAR, apoptosis genes), detectable through integrated metabolomic analyses (Song et al., 2025; Zhong et al., 2025).
3.3 Transcriptomic and epigenetic regulation
Transcriptomics and epigenetics uncover host response mechanisms to microbiome shifts, including immune gene regulation and epigenetic priming of ‘trained immunity’—even in invertebrates lacking adaptive immunity. Nevertheless, transcript counts rarely translate directly into protein function; low-expression genes may be missed, and host RNA often dominates libraries. Coupling metatranscriptomics with metaproteomics or ribosome profiling can overcome these bottlenecks by verifying actual protein synthesis (Zhao C. et al., 2023; Heyer et al., 2025). Probiotics such as Lactiplantibacillus plantarum and microbial metabolites like butyrate upregulate immune genes (e.g., proPO, lysozyme, IL-10, antimicrobial peptides) and pathways (Toll, Imd, NLRP3 inflammasome), enhancing defense mechanisms (Shan et al., 2021; Wang T. et al., 2024; Guzman et al., 2025). Under environmental stress, transcriptomics uncovers conserved response pathways: in Pacific white shrimp exposed to heat stress, it reveals energy repartitioning through glycolysis, immune modulation via C-type lectin and IL-17, and glutathione-mediated antioxidant defense (Liu et al., 2025). Similarly, ammonia stress in fish disrupts amino acid metabolism and activates apoptosis pathways (Peng et al., 2025). Epigenetic mechanisms mediate microbiome effects on immunity; butyrate, as an HDAC inhibitor, alters chromatin accessibility (e.g., enhancing IL-17D expression in tilapia for neutrophil recruitment) and influences DNA methylation. Microbiome-induced “trained immunity” occurs even in invertebrates lacking adaptive immunity through epigenetic priming (Liao et al., 2023; Wang T. et al., 2024; Guzman et al., 2025). Gnotobiotic models confirm that microbiome composition directly shapes host physiology, including immune modulation and spatial microbial distribution (Adade et al., 2023).
3.4 Integrated multi-omics for precision engineering
Integrated multi-omics bridges microbial composition, host physiology, and environmental interactions to identify biomarkers (e.g., microbial ratios signaling dysfunction) and refine precision strategies. This integration yields systems-level insights linking microbial dynamics, host physiology, and environmental interactions. It identifies robust biomarkers, such as genus-level microbial ratios (e.g., Vibrio/Photobacterium in shrimp) signaling stress-associated dysbiosis and immune disruption, and unravels complex mechanisms. For example, multi-omics links Vibrio proliferation to glutathione depletion and immune dysregulation specifically in shrimp under combined ammonia/salinity stress, and Vibrionaceae increase (with a shift in commensal genera) in seabream during parasitic infection (Li et al., 2025; Toxqui-Rodríguez et al., 2025). Because each single-omics layer is imperfect, integrated designs (e.g., genome-resolved metaproteomics, metabolite SIP linked to metagenome-assembled genomes [MAGs]) are indispensable for triangulating taxon→gene expression→metabolite relationships and thereby closing attribution gaps highlighted above (Rasmussen et al., 2022; Hansen et al., 2023). These insights directly inform precision strategies: optimizing probiotic formulations (e.g., Lactococcus lactis D1813 for specific salinity/dissolved oxygen conditions), designing targeted dietary supplements (e.g., antioxidants based on stress pathways), and developing novel therapies like antimicrobial peptides (e.g., Lvvibriocin-GK). Omics reveals that such peptides reduce pathogen loads, stimulate immunity (e.g., lysozyme activity), modulate microbiota, and suppress inflammation (Adil et al., 2025; Sun et al., 2025). Nutraceuticals like astaxanthin further mitigate tissue damage by regulating apoptosis and metabolism genes (He et al., 2025). Collectively, multi-omics underpins three synergistic pillars of microbiome engineering: identifying biomarkers to inform probiotic/synbiotic design; leveraging omics-derived targets (e.g., butyrate-producing taxa) for pathogen suppression; and using transplantation strategies (e.g., synthetic consortia) for ecological restoration. This closed-loop system—where omics guides intervention design and outcomes refine models—enables sustainable disease management in aquaculture.
3.5 Probiotics, prebiotics, and synbiotics as engineering tools
A key premise in probiotic development is that strain-specific traits (e.g., spore formation, bacteriocin production) must align with host physiology and environmental conditions for efficacy. Probiotics exert their protective effects in aquaculture through multifaceted mechanisms, including the production of antimicrobial compounds (e.g., bacteriocins, organic acids, enzymes), competitive exclusion of pathogens, and immunomodulation (Figure 1; Table 1). These mechanisms are harnessed in microbiome engineering to design targeted interventions, though their efficacy depends critically on strain selection, host compatibility, and environmental stability. For instance, Bacillus subtilis subsp. inaquosorum BSXE-2102 synthesizes 12 secondary metabolites with potent antagonistic activity against aquatic pathogens (Qin et al., 2025), while Lactobacillus plantarum strains from kefir produce bacteriocins that suppress Vibrio alginolyticus (Tseng et al., 2023). Strain-specific efficacy is evident in Bacillus velezensis FiA2, which produces broad-spectrum oxydifficidin (Khan et al., 2025), and Streptomyces sp. D-6, which delivers novel bioactive metabolites (Zhao et al., 2025). Meta-analyses confirm Bacillus spp. outperform Lactobacillus in pathogen inhibition due to spore-forming resilience, whereas Streptomyces strains show unique bioremediation potential (Giri et al., 2024; Hoseinifar et al., 2024). However, limitations persist: survivability under gastrointestinal stress varies (31–75% acid tolerance at pH 2–4), adhesion capacity to the GI tract (a critical determinant of colonization success) is often strain-specific, efficacy is host-dependent, and inconsistent trial results occur across environments or host genotypes. For example, Bacillus subtilis NTU-18 improves growth in Anguilla japonica glass eels but requires precise dosing for other species (Lin et al., 2024). These constraints highlight the need for engineered solutions, such as encapsulation or host-adapted consortia, to improve reliability.
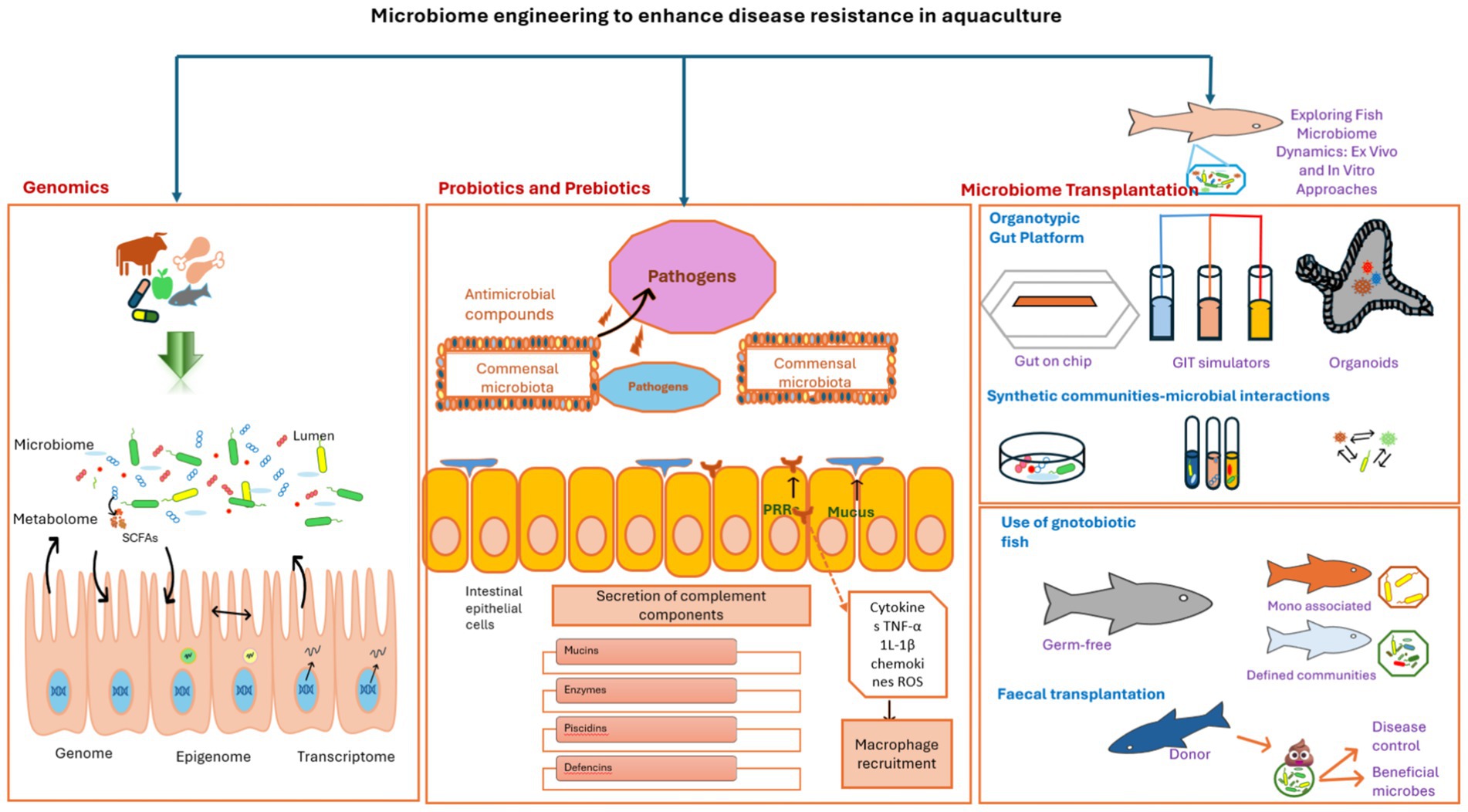
Figure 1. Integrated conceptual framework of microbiome engineering strategies for enhancing disease resistance in aquaculture. The framework unifies three synergistic pillars: Multi-omics approaches (left) dissect microbial community dynamics, host–microbe interactions, and functional pathways (e.g., immune modulation, stress response) through genomics, transcriptomics, metabolomics, and epigenomics. These insights identify therapeutic targets (e.g., butyrate-producing bacteria) and dysbiosis biomarkers. Probiotics and prebiotics (center) deploy antimicrobial compounds (e.g., bacteriocins), engineered microbial consortia (e.g., Bacillus spp.), and host-targeted strategies (e.g., barrier reinforcement) to exclude pathogens (e.g., Vibrio, Aeromonas) and enhance resilience. Microbiome transplantation (right) restores dysbiotic microbiomes via fecal microbiota transplantation (FMT), rationally designed synthetic communities (SynComs), or in vitro platforms (e.g., gut-on-chip), validated through gnotobiotic models (germ-free hosts colonized with defined microbial communities). Bidirectional arrows illustrate functional synergy: multi-omics guides probiotic and SynCom design; transplantation efficacy is monitored via omics; and probiotics/transplantation generate data to refine omics models. Together, these pillars enable pathogen suppression, immune enhancement, and ecological stability while reducing antibiotic dependence.
3.5.1 Probiotic mechanisms and strain selection
Host genetic background critically modulates probiotic interactions. This host-specificity underscores the need for precision engineering: Genetically selected gilthead sea bream (Sparus aurata) exhibit enhanced intestinal barrier function with Bacillus-based probiotics, while non-selected strains show reduced performance (Naya-Català et al., 2024). Similarly, hybrid grouper displays genotype-dependent immune responses to Exiguobacterium acetylicum G1-33, with optimal dosing (108 CFU/g) improving survival against Vibrio harveyi by 72% (Zhang M. et al., 2024). Variable colonization across species (e.g., Pediococcus acidilactici efficacy in salmonids but not non-salmonids) further necessitates host-adapted formulations (Soto-Dávila et al., 2024).
Probiotics competitively exclude pathogens by colonizing mucosal surfaces and consuming essential nutrients (Table 2). Ecological strategies based on promoting K-selected microbial communities—characterized by stability and resource efficiency (typical of slow-growing, competitive species)—over opportunistic r-strategists (fast-growing species adapted to unstable environments) enhance colonization success and pathogen exclusion (Vadstein et al., 2018). This principle is demonstrated in a study where dietary Bacillus subtilis BSXE-1601 enhanced disease resistance of Penaeus vannamei against Vibrio parahaemolyticus and significantly modulated the rearing water microbiota, reducing its overall diversity and ecological network complexity while increasing the relative abundance of the genus Marivita, a member of the often beneficial Rhodobacteraceae family (Luo et al., 2024a). Effective engineering prioritizes strains with niche competence (e.g., Bacillus aryabhattai CKNJh11 biofilm formation) and complementary functions (Dangsawat et al., 2025). Concurrently, probiotics prime immune responses: SYNLAC Prime upregulates serine protease (SP), prophenoloxidase (proPO), and peneidin genes in shrimp (Cheng et al., 2024), while Leuconostoc mesenteroides B4 with dextran activates Toll and Imd pathways (Huang M. Y. et al., 2024). Immunomodulatory secretion—decoupled from live-cell requirements via postbiotics (e.g., B. subtilis AAHM-BS2360 inducing lysozyme activity)—offers alternative engineering avenues (Wiratama et al., 2025).
3.5.2 Host-adapted and precision formulations
Prebiotics, such as chitosan oligosaccharide (COS), selectively stimulate beneficial gut bacteria. COS supplementation in Penaeus vannamei enriches beneficial bacteria such as Algorimicrobium and Roseibium while suppressing pathogenic Vibrio spp., including V. parahaemolyticus and V. rotiferianus (Fu C. et al., 2025), and fermented pomegranate peel polyphenols elevate beneficial genera including Lactobacillus and Bifidobacterium (Yu et al., 2024). These shifts enhance gut barrier function but are dose-sensitive; high resistant starch (3%) disrupts microbiota balance in Micropterus salmoides (Zhang X. et al., 2025). Prebiotics also enhance innate immunity: dietary piperine elevates superoxide dismutase (SOD) and glutathione peroxidase (GPx) in shrimp (Albaqami, 2024), while tea polyphenols mitigate enteritis in grass carp by suppressing NF-κB and activating Nrf2/Keap1 pathways (Ma et al., 2025). However, prebiotics alone often fail to sustain microbial shifts without probiotics, highlighting the need for integrated approaches.
3.5.3 Prebiotics and Postbiotics for targeted modulation
Postbiotics—“preparations of inanimate microorganisms and/or their components that confer a health benefit on the host” (Salminen et al., 2021)—are emerging as a practical alternative to live probiotics in aquaculture. Because the cells are non-viable, postbiotics tolerate pelleting temperatures, avoid horizontal gene-transfer risks, and typically face a lighter regulatory burden (Vinderola et al., 2022; Gervasoni et al., 2023). Their bioactivity arises from (i) immunomodulatory cell-wall fragments that up-regulate TLR-dependent NF-κB/MAPK signalling and boost plasma IgM (↑ 37% in Atlantic salmon; Table 2) (Kumar et al., 2025); (ii) secreted antimicrobial peptides that competitively exclude pathogens such as Vibrio spp. (↓ 60% colonisation in shrimp) (Sudhakaran et al., 2022); and (iii) metabolites that strengthen epithelial barriers by inducing mucin genes and antioxidant enzymes (Kavita et al., 2024). Comparative trials show postbiotics can out-perform their live counterparts, delivering 40–65% higher survival against WSSV and lowering production costs by ~30% (Abdel-Latif et al., 2022; Thorakkattu et al., 2022).
3.5.4 Synbiotic strategies and system-level optimization
Synbiotics—rational combinations of probiotics and prebiotics—are formally categorized as either complementary (independent mechanisms) or synergistic (prebiotics selectively enhancing co-administered probiotics) under the updated ISAPP framework (Swanson et al., 2020). In aquaculture, synergistic formulations are prioritized to overcome individual limitations of probiotics (e.g., survivability) and prebiotics (e.g., transient effects), representing a cornerstone of microbiome engineering. For example, Lactobacillus plantarum 7–40 with king oyster mushroom extract (KOME) enriches lactic acid bacteria, reduces Vibrio counts, and upregulates immune genes (proPO, lysozyme, crustin), achieving 72% survival against V. alginolyticus in shrimp (Prabawati et al., 2022). The synergy arises from prebiotics enhancing probiotic survival/metabolic activity (e.g., KOME stimulating L. plantarum growth) and probiotics metabolizing prebiotics into immunomodulatory compounds like short-chain fatty acids (Kewcharoen and Srisapoome, 2022). Commercial-scale trials confirm synbiotic efficacy: Bacillus subtilis and Lactococcus lactis PH3-05 improved survival of tropical gar larvae by 46% (Prabawati et al., 2022), while Bacillus spp. + BiOWISH Feedbuilder Syn3 (BiOWiSH Technologies, Cincinnati, OH, USA) boosted Nile tilapia survival by 20% in biofloc systems (Oliveira et al., 2025). Encapsulation technologies (e.g., alginate-microencapsulated B. licheniformis with >90% gastric viability) further stabilize synbiotics for targeted delivery (Cota-Gastélum et al., 2025). These studies underscore the potential of synbiotics to optimize microbiome engineering in aquaculture. Meta-analyses confirm that biofloc systems—0inherently synbiotic environments – consistently enhance key immune parameters (e.g., lysozyme, immunoglobulins, antioxidant enzymes) and disease resistance across aquatic species (Khanjani et al., 2023). The host-specificity and environmental constraints of conventional probiotics underscore the need for precision solutions. FMT and SynComs address these gaps by restoring or designing communities tailored to host ecology. When resident microbial communities are deeply disrupted, whole-community approaches—fecal microbiota transplantation and purpose-built synthetic consortia—offer a broader remedy.
4 Microbiome transplantation and synthetic communities
As scalable implementations of precision engineering, FMT and SynComs leverage multi-omics insights to reintroduce keystone taxa or synthetically assemble consortia that optimize host functions beyond restoration. Transplantation strategies are posited to restore dysbiotic ecosystems by reintroducing keystone taxa, with their success largely contingent on donor-recipient compatibility. Standardized protocols for FMT—such as 7-day antibiotic decontamination followed by 3 weekly transplant doses—significantly improve microbial engraftment and stability in aquatic models, as demonstrated in murine studies adapted for aquaculture applications (Amorim et al., 2022). However, optimal antibiotic pre-treatment durations (ranging from days to weeks) and dosing frequencies (single to weekly administrations) remain species-dependent and require further refinement (Marclay et al., 2022; Han Z. et al., 2024; Karimianghadim et al., 2025). Long-term engraftment viability depends on species-specific factors, microbiota composition, and environmental conditions (Han Z. et al., 2024).
4.1 Fecal microbiota transplantation
Microbiome transplantation strategies represent two distinct approaches for enhancing aquaculture sustainability. Fecal microbiota transplantation (FMT) leverages naturally evolved communities from healthy donors to restore dysbiotic hosts, functioning primarily as a restorative intervention (El-Son et al., 2025). In contrast, synthetic microbial communities (SynComs) employ rationally designed, precision-engineered consortia of defined beneficial strains to modulate host phenotypes beyond restoration (Guo et al., 2023; El-Son et al., 2025).
FMT has demonstrated efficacy in reversing antibiotic-induced dysbiosis by reintroducing keystone taxa and metabolites. Recent research confirms FMT rapidly reverses antibiotic-induced dysbiosis in fish by restoring aromatic amino acid metabolism and glutathione synthesis—critical for mucosal repair—while accelerating recovery 2.5-fold compared to natural restoration (Han Z. et al., 2024). For example, florfenicol-treated koi carp (Cyprinus carpio) exhibited reduced beneficial genera (e.g., Lactobacillus, Bifidobacterium) and mucosal damage, but FMT rapidly restored these populations and normalized critical metabolites like aromatic amino acids and glutathione, accelerating recovery compared to natural restoration (Han Z. et al., 2024). FMT also restores key metabolites, including short-chain fatty acids and lipid metabolism-related molecules, supporting a balanced metabolic profile (Xiao et al., 2020; Han Z. et al., 2024). Gut microbiota-derived metabolites like indole 3-propionic acid further demonstrate non-gut protective roles, such as radiation toxicity mitigation (Xiao et al., 2020). Similarly, FMT in large yellow croaker (Larimichthys crocea) larvae improved growth performance, digestive enzyme activity, and intestinal morphology by enhancing microbial diversity and introducing functional taxa linked to nutrient metabolism (Zhang et al., 2023). Notably, FMT can transfer phenotype-specific effects: Transplantation of microbiota from antipsychotic-exposed common carp induced behavioral abnormalities in recipient fish, confirming causal microbiota-host nervous system interactions (Chang et al., 2024). This supports evidence that FMT can transfer non-gut traits like behavior and stress tolerance in aquatic animals (Ouyang et al., 2023), and disease susceptibility/resistance between species (Ma et al., 2022). A meta-analysis revealed that higher donor strain engraftment significantly correlates with clinical success (p = 0.017) (Ianiro et al., 2022). The study found that species from the phyla Bacteroidota (e.g., genera like Bacteroides and Parabacteroides) and Actinobacteria (e.g., families like Bifidobacteriaceae and Coriobacteriaceae) demonstrated significantly higher engraftment success than most species from the Firmicutes phylum. This underscores the critical role of recruiting specific, highly engraftable taxa in achieving ecological resilience and clinical efficacy after FMT. Beyond restoration, early-life microbial interventions can induce durable and even intergenerational protection, as demonstrated in the Pacific oyster (Crassostrea gigas), where microbial exposure protected against Pacific Oyster Mortality Syndrome (POMS) across generations via epigenetic reprogramming and sustained immune gene expression (Fallet et al., 2022). Early-life FMT modulates immune programming and may enhance disease resistance, though epigenetic mechanisms and cross-generational evidence specifically from FMT remain emerging areas (Cao et al., 2023; Dong et al., 2024).
4.2 Synthetic microbial communities (SynComs)
SynComs transcend restoration by enabling targeted manipulation of host functions through ecological and functional strain selection. The advanced design incorporates AI-predicted strain interactions and CRISPR-enhanced traits (e.g., bile tolerance), validated within in vitro gut-on-chip systems—microfluidic devices that culture intestinal epithelial cells under controlled flow with co-cultured microbes to emulate gut physiology—before deployment (Guo et al., 2023). Gnotobiotic models have been pivotal in elucidating strain-specific roles, such as Cetobacterium dominance in probiotic-fed grey mullet correlating with upregulated immune genes (IL-1β, TNF-α) and improved survival against Nocardia seriolae (Chan et al., 2024). Rational design is exemplified by a shrimp SynCom (Paracoccus, Ruegeria, Microbacterium, Demequina, Tenacibaculum) that suppressed V. parahaemolyticus, improved growth, and restored immune parameters through competitive exclusion and metabolic synergy (Guo et al., 2023). This SynCom reduced pathogenic Vibrio by 89% and enhanced thermal resilience via Tenacibaculum-mediated metabolic adjustments (Guo et al., 2023). SynComs can also improve environmental resilience (e.g., thermal/salinity tolerance) via optimized resource utilization, member replacement, and microbial communication (Jiang et al., 2024; Dubey et al., 2025). Case studies highlight their precision: a Bacillus spp. and Lactobacillus plantarum mix reduced Streptococcus agalactiae mortality by 40% in Nile tilapia via gut barrier enhancement (Huang X. et al., 2024), while Streptomyces sp. D6 suppressed Aeromonas veronii in crucian carp using bacteriocin-like compounds (Zhao et al., 2025). Encapsulation technologies further optimize SynCom delivery by enhancing microbial persistence in hydrogels (Fu S. et al., 2025).
Both strategies require rigorous biosafety frameworks due to inherent risks. FMT may facilitate horizontal gene transfer (HGT) of antibiotic resistance or virulence factors, particularly if donors harbor dysbiotic or pro-inflammatory communities (e.g., carrageenan-induced microbiota exacerbating colitis) (Wu et al., 2021, 2022). Engraftment failure is common when recipient conditions (e.g., diet, water chemistry) mismatch donor niches, as shown in interspecific transplants where dietary alignment was critical for stability (Li W. J. et al., 2022; Ruiz et al., 2024). Multi-omics-guided keystone taxon identification (e.g., Akkermansia for mucus integrity) minimizes ecological disruption during transplantation (Huang et al., 2025). For SynComs, an incomplete understanding of strain interactions risks community collapse or dominance of opportunistic taxa. Furthermore, environmental persistence of introduced strains could disrupt native microbiomes or nutrient cycles (Zhao Z. et al., 2023). Standardized donor screening, multi-omics-guided keystone taxon identification, and ecological risk assessments are thus essential for responsible application.
5 Emerging tools and technologies
CRISPR and AI further refine precision by enabling targeted trait augmentation in probiotics and predictive optimization of SynComs, closing the loop between multi-omics discovery and intervention design. Together with gut-on-chip platforms and in vitro models, these tools form a unified technological framework enabling a cohesive closed-loop engineering cycle where experimental validation informs AI-driven SynCom design and CRISPR-based refinement. This synergy transforms fragmented approaches into a cohesive pipeline for precision interventions. The frontier of precision microbiome engineering is defined by an integrated technological framework where in vitro models, computational tools, genome editing, and synthetic ecology converge to enable iterative design-test-deploy cycles.
5.1 Gut-on-chip platforms
Gut-on-chip platforms—advanced microfluidic devices that emulate the structural complexity, cellular organization, and dynamic physiology of the intestinal tract—are revolutionizing the study of host–microbe interactions by simulating the intestinal microenvironment of aquatic species. These systems recreate critical features including mucus-secreting epithelia, vascular-like perfusion, mechanical peristalsis, and oxygen gradients, enabling physiologically relevant modeling of gut barrier integrity, immune responses, and probiotic colonization dynamics. By integrating living cells from target species (e.g., fish intestinal epithelium), these chips allow precise testing of probiotic efficacy, host-pathogen interactions, and metabolite exchange under controlled yet dynamic conditions. This technology bridges the gap between traditional in vitro models (oversimplified) and in vivo trials (ethically and logistically challenging), offering high-throughput screening of microbiome interventions. For example, a recent dual-sample microfluidic LAMP ‘gut-on-chip’ detects ten aquatic pathogens—including Vibrio parahaemolyticus—within 30 min (93% clinical sensitivity), providing a rapid pathogen-challenge module that can be coupled to probiotic screening workflows (Zhou et al., 2021). Integration with lateral flow assays (μLAMP-LFA) further enhances field applicability by enabling multiplexed diagnostics while preventing aerosol contamination (Zhu et al., 2025). Gnotobiotic models, such as germ-free zebrafish (Solis et al., 2020; Jia et al., 2024), have laid the groundwork for these platforms by demonstrating how specific bacterial communities influence host immunity and pathogen resistance. For instance, germ-free zebrafish colonized with synthetic microbial communities (SynComs) revealed that commensal bacteria like Cetobacterium somerae enhance glucose homeostasis via acetate production (Wang et al., 2021), insights critical for validating Gut-on-Chip responses. Recent studies using germ-free Atlantic salmon (Gómez de la Torre Canny, 2023) further highlight how mucosal barrier function and adipose tissue dynamics depend on microbiota composition, parameters that can be monitored in vitro using chip technology. By integrating multi-omics data from gnotobiotic models, Gut-on-Chip systems allow high-throughput screening of probiotics, reducing reliance on live animal tria0-9ls while accelerating the development of targeted microbial therapies. These platforms thus serve as physiologically relevant validation hubs within the engineering cycle.
5.2 Artificial intelligence and machine learning
Artificial intelligence (AI) and machine learning (ML) are transforming microbiome engineering by predicting host–microbe interactions and optimizing probiotic formulations through the integration of complex multi-omics datasets, environmental metadata, and phenotypic traits. For instance, support vector machines (SVM) and random ferns (RFerns) achieved 100% accuracy in detecting water quality parameters linked to aquaculture disease outbreaks (Çakir et al., 2023), while conditional forest and random forest algorithms accurately predicted Salmonella contamination in agricultural waters using microbiome signatures (Chung et al., 2023). Neural networks discriminated probiotics from non-probiotics with >90% accuracy by analyzing tRNA information content (Bergamini et al., 2022), and decision tree models predicted the in vivo immunomodulatory activity of lactic acid bacteria in snails with 88% accuracy by prioritizing phenotypic traits like hydrophobicity and autoaggregation (Charizani et al., 2024). Meta-analyses of zebrafish gut microbiota have identified stage-specific microbial taxa, data that can train ML algorithms to design age-specific probiotics (Garibay-Valdez et al., 2024). AI-driven tools like VirOncoTarget refine pathogen risk assessment by screening viral oncoproteins via adversarial networks (98% accuracy) (Beltrán et al., 2024), while ensemble spatial models predict methane emissions by correlating microbial taxa with environmental stressors (Chen C. C. et al., 2025). Bayesian networks (e.g., SAMBA tool) model how farming conditions alter gut microbiome diversity and predict responses to environmental shifts (Soriano et al., 2023). In shrimp aquaculture, SynComs enriched with Paracoccus and Ruegeria to suppress Vibrio parahaemolyticus, a process optimizable via ML-predicted synergistic combinations. Similarly, Stagaman et al. (2024) linked benzo[a]pyrene toxicity to microbiome diversity in zebrafish, demonstrating ML’s capacity to correlate pollutants with dysbiosis. ML also models climate impacts, such as warming-driven enterotype migration in Litopenaeus vannamei to forecast disease risk (Zeng et al., 2025). AI-driven resources like Microbiome Atlas (Lin et al., 2021) catalog bacterial growth patterns for colonization modeling, while foundation models with transfer learning adapt predictions to specific aquaculture contexts despite data sparsity (Han et al., 2025). These approaches are critical for aquaculture, where dynamic conditions demand adaptive strategies. Collectively, AI/ML provides the computational engine for in silico design and optimization of microbial consortia before empirical testing, enabling precision modulation of microbiomes for disease prevention, growth enhancement, and environmental resilience.
5.3 CRISPR-based genome editing
CRISPR-based genome editing is emerging as a powerful tool to engineer probiotics with enhanced pathogen-inhibiting capabilities. Recent research demonstrates how engineered riboregulators and CRISPR-based devices can enhance auxotrophic biocontainment in genetically modified probiotics. For instance, Cas9-assisted containment systems in Bacteroides thetaiotaomicron blocked transgene dissemination and prevented escape via thymidine auxotrophy, establishing a model for biosafety in aquaculture-engineered strains (Hayashi et al., 2024). Applications include CRISPR-Cas9 knockout of the hfq gene in Vibrio alginolyticus, reducing motility and mortality in scallops by disrupting biofilm formation (Ma et al., 2023), and Cas13a-mediated targeting of white spot syndrome virus (WSSV) in shrimp, extending survival by 33% post-infection (Pudgerd et al., 2024). Regulatory frameworks for CRISPR applications in aquaculture, particularly in the EU and Asia, are evolving to address biosafety and horizontal gene transfer risks, as highlighted in recent policy reviews (Gutási et al., 2023). Furthermore, phage-plasmid systems encoding toxin-antitoxin modules and CRISPR-Cas systems have been shown to limit horizontal gene transfer, enhancing host microbial immunity (Sayid et al., 2024). Direct host genome editing has also shown promise: CRISPR-modified tilapia exhibited enhanced resistance to Streptococcus agalactiae by targeting immune pathways like TLR2 (Yang et al., 2022), highlighting dual strategies for microbiome and host engineering. For instance, Aeromonas veronii secretes GlcNAc-binding protein (GbpA), which stimulates intestinal epithelial proliferation in zebrafish (Banse et al., 2023). CRISPR could augment GbpA expression or modify adhesion factors to improve probiotic persistence. Similarly, Cetobacterium somerae, which activates TLR2-mediated antiviral immunity (Liang et al., 2024), might be engineered to overexpress immunostimulatory exopolysaccharides. While CRISPR applications in aquaculture remain nascent, studies on Vibrio sp. and Aeromonas sp. (Xin et al., 2020) demonstrate how innate immune pathways can be targeted via genetically modified microbes. Challenges include ensuring horizontal gene transfer prevention and addressing regulatory concerns, but CRISPR’s precision offers unparalleled potential for tailored microbial solutions. This technology enables precise trait augmentation in probiotic chassis, feeding directly into AI-designed SynCom blueprints.
5.4 Climate-resilient strategies
Rising water temperatures threaten aquaculture productivity, necessitating probiotics that thrive under thermal stress. A case study by Guo et al. (2023) illustrates how SynComs designed for shrimp resilience can be adapted using ML. By analyzing microbiota from biofloc systems, their team identified heat-tolerant taxa like Tenacibaculum, which were incorporated into SynComs to enhance Vibrio resistance. Biopolymer encapsulation (e.g., alginate-microencapsulated Bacillus licheniformis) ensures >90% gastric viability and sustained function across temperature fluctuations (Cota-Gastélum et al., 2025), while directed evolution yields strains like Bacillus subtilis TLDK301120C24, validated in gnotobiotic zebrafish to displace pathogens via competitive biofilm exclusion (Nayak et al., 2023). Such climate-resilient strategies align with SDG 13 (Climate Action), as highlighted in Table 3, which maps microbiome engineering approaches to their contributions toward mitigating environmental stressors. ML algorithms can further optimize these communities by predicting strain interactions under simulated warming scenarios. For example, Cetobacterium, a dominant genus in zebrafish guts (Garibay-Valdez et al., 2024), shows temperature-dependent acetate production (Wang et al., 2021), a trait ML could exploit to design climate-resilient consortia. For cold-water species like olive flounder (Paralichthys olivaceus), AI-guided SynComs enriched with Pseudomonas and Comamonas restore PUFA synthesis at suboptimal temperatures, countering metabolic dysregulation (Chen C. C. et al., 2025). Integrating environmental metagenomics with host transcriptomic data (Andersen-Civil et al., 2023) will refine these models, enabling probiotics that buffer against both pathogen outbreaks and climate variability. Deploying these advances on working farms, however, brings regulatory, ecological, and socioeconomic hurdles into sharp relief.
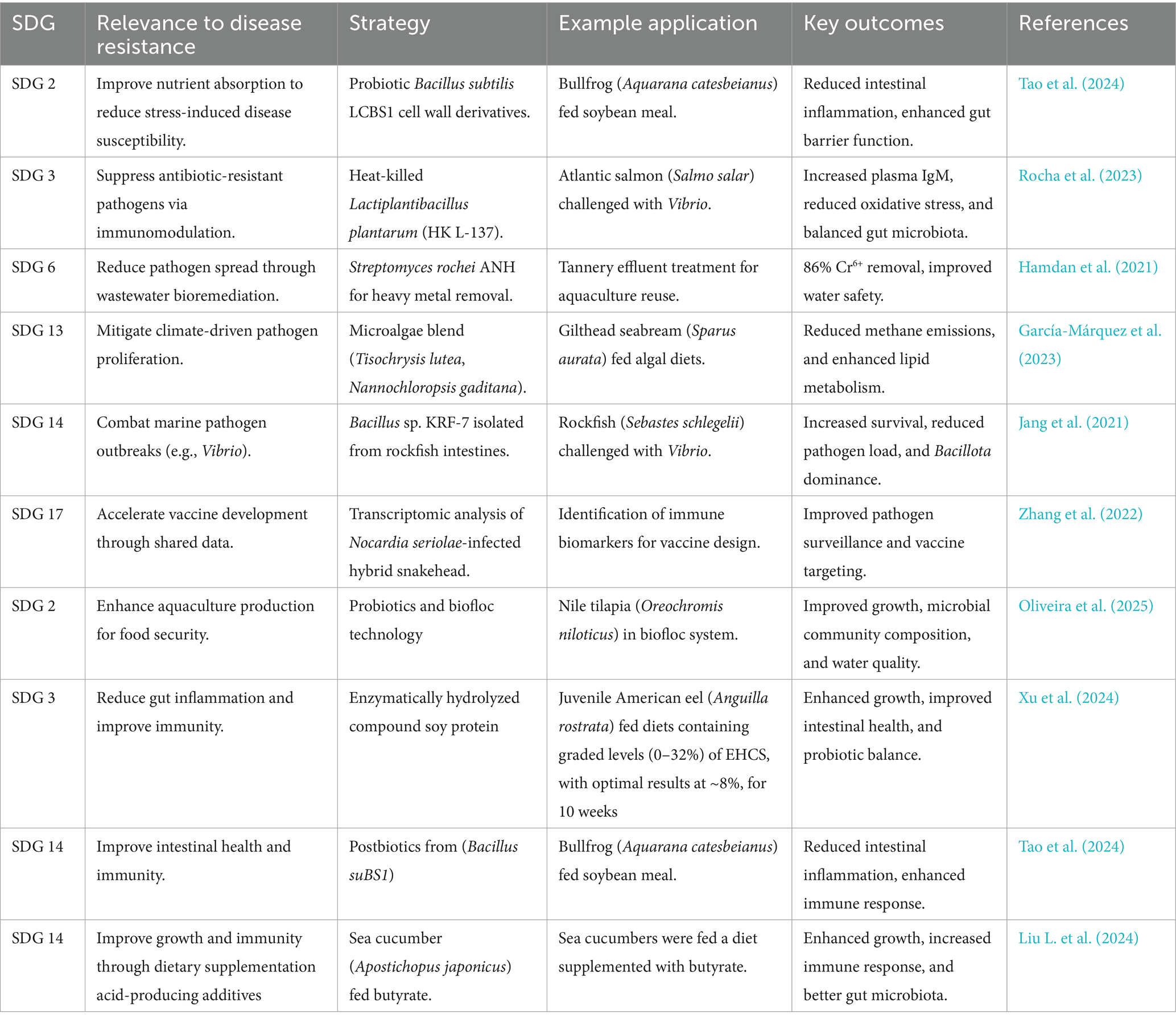
Table 3. Microbiome engineering strategies aligned with un sdgs to enhance disease resistance in aquaculture.
5.5 Synergistic closed-loop engineering cycle
These technologies form a closed-loop engineering cycle: AI/ML leverages multi-omics data to design optimized SynComs; CRISPR introduces precision traits into probiotic chassis; Gut-on-Chip platforms validate host–microbe interactions under simulated environmental conditions; and encapsulation ensures field resilience. For instance, AI-designed Vibrio-targeting consortia can be genome-edited for enhanced bile tolerance, functionally validated in microfluidic intestines, and deployed via temperature-stable encapsulates (Vijayaram et al., 2024; Ding et al., 2025). This iterative workflow bridges computational prediction with empirical delivery, establishing a scalable framework for precision microbiome engineering in aquaculture.
6 Current status of practical applications in aquaculture
Translating microbiome engineering innovations from laboratory concepts to commercial aquaculture practice reveals a continuum of adoption. Probiotics and synbiotics represent the most mature and widely implemented tools, while postbiotics, fecal microbiota transplantation (FMT), synthetic microbial communities (SynComs), artificial intelligence/machine learning (AI/ML) decision-support, and CRISPR-enabled interventions face distinct stages of field validation and regulatory hurdles. Success hinges on aligning strain functionality with host ecology, environmental parameters, and socioeconomic contexts (Guo et al., 2023; El-Son et al., 2025).
6.1 Commercial availability and on-farm performance
Probiotic formulations—particularly resilient Bacillus spp.—dominate commercial adoption in major aquaculture regions like Asia-Pacific, where they competitively exclude pathogens (Vibrio, Aeromonas, Edwardsiella) via quorum-quenching mechanisms and enhance digestive physiology (Muras et al., 2021; Santos et al., 2021). EU-approved probiotics (e.g., Pediococcus acidilactici) demonstrate immune modulation in salmonids, reducing mortality from pathogens like Yersinia ruckeri by 25–50% (Villumsen et al., 2020; Yousuf et al., 2023). Synbiotics show accelerated growth, with shrimp-specific formulations improving yields by >600 kg/pond through ammonia reduction and microbiome stabilization (Shinde et al., 2023; Wang Q. et al., 2024). Postbiotics gain traction for storage stability and reduced regulatory burdens; heat-inactivated Lactiplantibacillus plantarum and engineered Lactococcus lactis (expressing host cytokines) enhance immune responses and disease resistance in tilapia and salmon without live-cell risks (Muñoz et al., 2021; Ferro et al., 2024). Plant-derived supplements (e.g., fermented herbal blends, resveratrol) and encapsulated bioactives (e.g., nano-astaxanthin) further augment growth and stress tolerance, though adoption varies regionally (Fujaya et al., 2023; Elbahnaswy and Elshopakey, 2024; Kari et al., 2024; Lee et al., 2024).
6.2 Field trials and species-specific efficacy
Field validations confirm significant productivity gains: Bacillus velezensis supplementation in shrimp ponds increased survival by 23% and upregulated hepatopancreatic immune genes (Abdelsamad et al., 2024), while synbiotic strategies in giant freshwater prawns induced complete resistance to Aeromonas veronii (Chin et al., 2025). Postbiotics accelerated microbiome recovery post-antibiotics in shrimp, reducing pathogenic Vibrio by 40% (Luo et al., 2024b). Species-tailored blends (e.g., Pediococcus/Lactococcus/Weissella consortia in trout) enhanced intestinal morphology and cytokine expression (Mahmoodian et al., 2025). Performance gains are most consistent when formulations align with host ecology—e.g., temperature-adapted probiotics in olive flounder improved winter survival (Lee et al., 2024), and microalgae-phytase synergies boosted seabass growth without disrupting core microbiota (Peralta-Sánchez et al., 2024).
6.3 Emerging tools: SynComs and FMT
Rational SynComs designed from native taxa show promise in pathogen suppression (e.g., V. parahaemolyticus in shrimp) and stress resilience (e.g., salinity/thermal tolerance) but remain at the pilot stage due to challenges in donor-recipient compatibility and ecological instability (Wang Z. et al., 2023). FMT effectively restores antibiotic-disrupted microbiomes in controlled settings yet faces barriers in horizontal gene transfer (HGT) risk (e.g., antimicrobial resistance (AMR) propagation) and farm-scale workflow standardization (Behling et al., 2024; Tian et al., 2024). Encapsulation (e.g., pH-responsive microcapsules) is critical for SynCom viability and intestinal delivery, though ecological matching and gnotobiotic validation are prerequisites for field success (Dremova et al., 2023; Jiang et al., 2024).
6.4 AI/ML and CRISPR: readiness and constraints
AI/ML tools enable risk prediction and in silico consortium design but require integration into telemetry-linked farm trials to address domain shifts between lab and pond conditions (Bergamini et al., 2022; Çakir et al., 2023). CRISPR applications (e.g., containment-enhanced probiotics, host-immunity tuning) are constrained by regulatory ambiguity—particularly GMO classification in the EU—and unresolved technical hurdles like off-target effects and biocontainment validation (Okoli et al., 2022; Gutási et al., 2023; Sheng et al., 2025). Field-validated environmental DNA (eDNA) surveillance (Technology Readiness Level [TRL] 6) demonstrates utility in species detection but lacks standardized ecosystem integration (Kim et al., 2024).
7 Challenges and future directions
Technical barriers center on strain-specific complexities, where probiotic efficacy hinges critically on precise selection and dosing. For instance, while low-dose Bacillus subtilis enhances intestinal health in Chinese perch (Siniperca chuatsi), higher concentrations impair growth, and antimicrobial peptides exhibit variable efficacy across species due to divergent host physiologies and environmental conditions (Ji et al., 2023; Wang J. et al., 2023). Host-microbiome compatibility further complicates design, as evidenced by germ-free rainbow trout colonized with protective Flavobacterium spp. resisting Flavobacterium columnare infection, whereas mismatched consortia increase mortality (Perez-Pascual et al., 2021).
Ecological risks arise from introducing non-native strains, particularly HGT of antibiotic resistance genes (ARGs). Conjugative transfer rates in aquaculture settings range from 10−5 to 1%, amplified by environmental stressors like triclosan, which increases ARG transfer frequencies by 1.2–1.4-fold in Edwardsiella piscicida (Lu et al., 2022). Examples include aphA in sphingomonads and floR in Vibrio parahaemolyticus, both contributing to ARG dissemination (Fu et al., 2022; Qian et al., 2024). Engineered probiotics (e.g., CRISPR-modified Bacillus subtilis) may persist in sediments, disrupting nitrogen cycles or outcompeting keystone species (Yang et al., 2024; Lee et al., 2025). Mitigation requires CRISPR-based biocontainment (e.g., thymidine auxotrophy “kill switches”) (Lee et al., 2025), supplemented by a tiered ecological monitoring framework: (1) quantitative eDNA metabarcoding to track ARG dissemination (e.g., sul1, aadA1) at sensitivities of 106 copies/L across water/sediment matrices (Vandeputte et al., 2017; Zhang Y. et al., 2025); (2) stressor-responsive mesocosm trials evaluating HGT frequencies under heavy metal/pH fluctuations (Yue et al., 2023; Hazra et al., 2024); and (3) mandatory surveillance of mobile genetic elements (intI1) in effluents integrated with biocontrol alternatives like Traditional Chinese Medicine (40–60% ARG reduction) (Wang et al., 2018; Li et al., 2024).
Regulatory fragmentation impedes global deployment. The EU’s stringent pre-market assessments (Directive 2001/18/EC) contrast with ASEAN’s voluntary standards and China’s rapid but less-regulated adoption, resulting in trade disruptions—35% of genetically modified feed imports from China were rejected by the EU’s Rapid Alert System for unresolved ecological risks (Eissa et al., 2024). Harmonizing frameworks through FAO/WHO guidelines for strain-specific safety evaluations and incentivizing alternatives (e.g., chitooligosaccharides) (Mohan et al., 2023) are essential to bridge these disparities. These efforts must prioritize standardization of the ecological monitoring framework (eDNA, mesocosms, MGE surveillance) under One Health-aligned protocols to ensure global consistency in detecting unintended impacts (Arnold et al., 2024). Moreover, CRISPR/Cas-based genome editing introduces new uncertainties around off-target effects and trait predictability, which remain insufficiently addressed in current EU and international policies (Okoli et al., 2022).
Socioeconomic barriers include high production costs of synthetic probiotics and limited adoption in resource-poor regions. In the Indian Sundarbans, trained farmers using probiotics achieved a 66% higher benefit–cost ratio than traditional practices, yet adoption remains constrained by inadequate institutional support and financial access (Ghosh et al., 2022). Many operators also continue to prefer traditional antibiotics despite their ecological risks, indicating a need for improved training and awareness programs (He et al., 2022). Bridging this gap demands democratizing cost-effective solutions—such as integrating Rhodobacter sphaeroides with biofloc systems to recycle nitrogen waste (Cao et al., 2024)—and fostering ASEAN-China partnerships to align biocontainment standards (Noman et al., 2024).
Addressing these challenges necessitates integrated strategies grounded in ecological theory, including multi-omics-guided formulations (e.g., tannic acid-modulated PPAR pathways in turtles) (Ji et al., 2025), K-selection-based community management (Vadstein et al., 2018), circular aquaculture systems valorizing waste, and AI-optimized climate-resilient SynComs. By aligning with UN Sustainable Development Goals—particularly Zero Hunger (SDG 2), Climate Action (SDG 13), and Life Below Water (SDG 14)—microbiome engineering can mitigate antimicrobial resistance while securing ecologically balanced aquaculture.
8 Conclusion
Microbiome engineering represents a transformative approach anchored in precision tools. Multi-omics profiling identifies host-specific biomarkers, informing the design of probiotics, synbiotics, FMT, and SynComs. CRISPR-edited probiotics and AI-driven SynCom optimization then enable targeted, climate-resilient interventions by predictively manipulating host-microbe-environment interactions. Together, these strategies enhance disease resistance while reducing antibiotic reliance. Advanced tools such as CRISPR-engineered probiotics and AI-driven SynCom design further refine these interventions, enabling precise modulation of host–microbe interactions under climate stressors. For instance, Bacillus subtilis strains engineered to express pathogen-specific antigens and heat-tolerant SynComs incorporating Tenacibaculum exemplify the potential of these innovations to bolster resilience in dynamic environments. However, scaling these solutions requires overcoming critical challenges, including strain-host compatibility, horizontal gene transfer (HGT) risks, regulatory disparities, and socioeconomic barriers. Collaborative efforts to harmonize global policies—such as adopting FAO/WHO safety frameworks—and democratize access to cost-effective alternatives (e.g., chitooligosaccharides and biofloc systems) are essential. Future advancements require tailored farm-specific probiotics designed via multi-omics profiling, circular aquaculture systems integrating waste valorization, and climate-resilient SynComs optimized via machine learning. Global policies must align with the United Nations Sustainable Development Goals (SDGs)—Zero Hunger (SDG 2), Climate Action (SDG 13), and Life Below Water (SDG 14)—to ensure equitable adoption and ecological safety. Aligning with these SDGs can mitigate antimicrobial resistance (AMR), restore aquatic biodiversity, and secure food production for a growing population. To realize this vision, interdisciplinary collaboration among researchers, policymakers, and industry stakeholders is urgent. Only through coordinated innovation and equitable implementation can aquaculture transition from a sector burdened by ecological trade-offs to a model of sustainable food systems.
Author contributions
MT: Writing – original draft, Writing – review & editing. YoZ: Writing – original draft, Writing – review & editing. YuZ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was primarily supported by the National Natural Science Foundation of China (No. U22A20536) and the Guangxi Science and Technology Major Special Project (No. AA23062046).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Latif, H. M. R., Yilmaz, E., Dawood, M. A. O., Ringø, E., Ahmadifar, E., and Yilmaz, S. (2022). Shrimp vibriosis and possible control measures using probiotics, postbiotics, prebiotics, and synbiotics: a review. Aquaculture 551:737951. doi: 10.1016/j.aquaculture.2022.737951
Abdelsamad, A. E. M., Said, R. E. M., Assas, M., Gaafar, A. Y., Hamouda, A. H., and Mahdy, A. (2024). Effects of dietary supplementation with Bacillus velezensis on the growth performance, body composition, antioxidant, immune-related gene expression, and histology of Pacific white shrimp, Litopenaeus vannamei. BMC Vet. Res. 20:368. doi: 10.1186/s12917-024-04207-4
Abomughaid, M. M. (2020). Isolation and identification of some probiotic Bacteria and their potential role in improving immune response and resistance of Nile Tilapia (Oreochromis niloticus) in comparison with a commercial product. Int. J. Microbiol. 2020:8865456. doi: 10.1155/2020/8865456
Adade, E. E., Stevick, R. J., Pérez-Pascual, D., Ghigo, J.-M., and Valm, A. M. (2023) Gnotobiotic zebrafish microbiota display inter-individual variability affecting host physiology Biorxiv [Preprint] doi: 10.1101/2023.02.01.526612
Adil, M., Xinbo, G., Cai, J., Waseem, M., Manzoor, M. F., and Tutu, C. O. (2025). Investigating the role of Lactococcus lactis D1813, salinity, and dissolved oxygen on the nutritional, chromatic, and textural profile of Litopenaeus vannamei. Food Chem. X 27:102404. doi: 10.1016/j.fochx.2025.102404
Albaqami, N. M. (2024). Effects of dietary piperine on growth, hemolymph chemistry, body composition, antioxidant state, immune response, and resistance against Vibrio parahemolyticus in whiteleg shrimp (Litopenaeus vannamei). J. Adv. Vet. Anim. Res. 11, 996–1006. doi: 10.5455/javar.2024.k850
Alcolombri, U., Pioli, R., Stocker, R., and Berry, D. (2022). Single-cell stable isotope probing in microbial ecology. ISME Commun. 2:55. doi: 10.1038/s43705-022-00142-3
Amorim, N., McGovern, E., Raposo, A., Khatiwada, S., Shen, S., Koentgen, S., et al. (2022). Refining a protocol for faecal microbiota engraftment in animal models after successful antibiotic-induced gut decontamination. Front. Med. 9:770017. doi: 10.3389/fmed.2022.770017
Andersen-Civil, A. I. S., Sawale, R. A., and Vanwalleghem, G. C. (2023). Zebrafish (Danio rerio) as a translational model for neuro-immune interactions in the enteric nervous system in autism spectrum disorders. Brain Behav. Immun. 112, 254–266. doi: 10.1016/j.bbi.2023.06.001
Arnold, K. E., Laing, G., McMahon, B. J., Fanning, S., Stekel, D. J., Pahl, O., et al. (2024). The need for one health systems-thinking approaches to understand multiscale dissemination of antimicrobial resistance. Lancet Planet. Health 8, e124–e133. doi: 10.1016/s2542-5196(23)00278-4
Bakky, M. A. H., Tran, N. T., Zhang, M., Wang, S., Zhang, Y., and Li, S. (2025). Synergistic effects of butyrate-producing bacteria (Clostridium senegalense I5 or Paraclostridium benzoelyticum G5) and Gracilaria lemaneiformis-originated polysaccharides on the growth and immunity of rabbitfish. Int. J. Biol. Macromol. 291:138683. doi: 10.1016/j.ijbiomac.2024.138683
Banse, A. V., VanBeuge, S., Smith, T. J., Logan, S. L., and Guillemin, K. (2023). Secreted Aeromonas GlcNAc binding protein GbpA stimulates epithelial cell proliferation in the zebrafish intestine. Gut Microbes 15:2183686. doi: 10.1080/19490976.2023.2183686
Behling, A. H., Wilson, B. C., Ho, D., Cutfield, W. S., Vatanen, T., and O’Sullivan, J. M. (2024). Horizontal gene transfer after faecal microbiota transplantation in adolescents with obesity. Microbiome 12:26. doi: 10.1186/s40168-024-01748-6
Beltrán, J. F., Herrera-Belén, L., Yáñez, A. J., and Jimenez, L. (2024). Prediction of viral oncoproteins through the combination of generative adversarial networks and machine learning techniques. Sci. Rep. 14:27108. doi: 10.1038/s41598-024-77028-y
Bergamini, C. M., Bianchi, N., Giaccone, V., Catellani, P., Alberghini, L., Stella, A., et al. (2022). Machine learning algorithms highlight tRNA information content and Chargaff’s second parity rule score as important features in discriminating probiotics from non-probiotics. Biology 11:1024. doi: 10.3390/biology11071024
Bossier, P., De Schrijver, P., Defoirdt, T., Ruwandeepika, H. A. D., Natrah, F., Ekasari, J., et al. (2016). Microbial Community Management in Aquaculture. Proc. Food Sci. 6, 37–39. doi: 10.1016/j.profoo.2016.02.007
Butt, U. D., Khan, S., Liu, X., Sharma, A., Zhang, X., and Wu, B. (2024). Present status, limitations, and prospects of using Streptomyces Bacteria as a potential probiotic agent in aquaculture. Probiotics Antimicrob. Proteins 16, 426–442. doi: 10.1007/s12602-023-10053-x
Çakir, M., Yilmaz, M., Oral, M. A., Kazanci, H. Ö., and Oral, O. (2023). Accuracy assessment of RFerns, NB, SVM, and kNN machine learning classifiers in aquaculture. J. King Saud Univ. 35:102754. doi: 10.1016/j.jksus.2023.102754
Cao, S., Guo, D., Yin, H., Ding, X., Bai, S., Zeng, Q., et al. (2023). Improvement in ovarian function following fecal microbiota transplantation from high-laying rate breeders. Poult. Sci. 102:102467. doi: 10.1016/j.psj.2022.102467
Cao, H., Zheng, X., Teng, C., Xu, L., Wang, Y., Gai, C., et al. (2024). Rhodobacter sphaeroides supplementation improves defense ability of Chinese mitten crab Eriocheir sinensis against Shewanella putrefaciens infection via intestinal flora and metabolism regulation. J. Invertebr. Pathol. 204:108120. doi: 10.1016/j.jip.2024.108120
Chan, C. H., Chen, L. H., Chen, K. Y., Chen, I. H., Lee, K. T., Lai, L. C., et al. (2024). Single-strain probiotics enhance growth, anti-pathogen immunity, and resistance to Nocardia seriolae in grey mullet (Mugil cephalus) via gut microbiota modulation. Anim. Microb. 6:67. doi: 10.1186/s42523-024-00353-0
Chang, X., Shen, Y., Yang, M., Yun, L., Liu, Z., Feng, S., et al. (2024). Antipsychotic drug-induced behavioral abnormalities in common carp: the potential involvement of the gut microbiota-brain axis. J. Hazard. Mater. 472:134444. doi: 10.1016/j.jhazmat.2024.134444
Charizani, E., Dushku, E., Kyritsi, M., Metallinou, E. T., Karathodorou, A., Amanetidou, E., et al. (2024). Predicting the immunomodulatory activity of probiotic lactic acid bacteria using supervised machine learning in a Cornu aspersum snail model. Fish Shellfish Immunol. 152:109788. doi: 10.1016/j.fsi.2024.109788
Chen, C. C., Chen, Y.-P., Yang, H.-T., Chen, Y.-L., Wu, C.-W., Gong, H.-Y., et al. (2025). Temperature-dependent shifts in gut microbiota and metabolome of olive flounder (Paralichthys olivaceus): implications for cold-water aquaculture expansion and probiotic applications. Anim. Microb. 7:49. doi: 10.1186/s42523-025-00417-9
Chen, Z., Ma, L., Chen, S., Huang, Y., Qin, Z., Lin, L., et al. (2025). Effects of enrofloxacin and povidone-iodine on immunity, the intestinal microbiome and transcriptome of juvenile grass carp (Ctenopharyngodon idella). J. Fish Biol. 107, 201–214. doi: 10.1111/jfb.70018
Cheng, A. C., Chang, H. T., Lee, T. Y., Lin, J. S., and Liu, C. H. (2024). SYNLAC prime probiotics enhances growth performance, and resistance of white shrimp, Penaeus vannamei to Enterocytozoon hepatopenaei and Vibrio alginollyticus: insights into immune and metabolic pathway modulations. Fish Shellfish Immunol. 155:110016. doi: 10.1016/j.fsi.2024.110016
Chin, Y. K., Azzam-Sayuti, M., Mohammad, A., Nazarudin, M. F., Salleh, A., Radin, M. A. A. B., et al. (2025). Synergistic of Lactobacillus plantarum L20 and Sargassum polycystum hydrolysate enhances growth, immunity, and disease resistance against necrotizing hepatopancreatitis-like diseases-causing Aeromonas veronii in giant freshwater prawn (Macrobrachium rosenbergii). Dev. Comp. Immunol. :105412. doi: 10.1016/j.dci.2025.105412
Chung, T., Yan, R., Weller, D. L., and Kovac, J. (2023). Conditional Forest models built using metagenomic data accurately predicted Salmonella contamination in northeastern streams. Microbiol. Spectr. 11, e00381–e00323. doi: 10.1128/spectrum.00381-23
Ci, Y., Ku, T., Su, Y., He, Z., Zhang, Y., Ji, J., et al. (2024). Response signatures of intestinal microbiota and gene transcription of yellow catfish (Pelteobagrus fulvidraco) to Aeromonas hydrophila infection. Fish Shellfish Immunol. 152:109797. doi: 10.1016/j.fsi.2024.109797
Cota-Gastélum, L. A., Reyes-López, M. Á., Escamilla-Montes, R., Luna-González, A., Calderón-Vázquez, C. L., and Diarte-Plata, G. (2025). In vitro controlled release of the probiotic strain Bacillus licheniformis PPL2016 microencapsulated: simulating the digestive system by age class and sex in the blue swimming crab Callinectes arcuatus. Braz. J. Microbiol. 56, 2007–2026. doi: 10.1007/s42770-025-01674-1
Dangsawat, O., Rattanawut, J., Srisawat, T., Sowanpreecha, R., Tang Phuc Khang, L., Srinual, O., et al. (2025). Bacillus aryabhattai CKNJh11 as a promising probiotic improves growth performance and egg quality in laying hens. Sci. Rep. 15:13659. doi: 10.1038/s41598-025-97553-8
Dantan, L., Toulza, E., Petton, B., Montagnani, C., Degremont, L., Morga, B., et al. (2024). Microbial education for marine invertebrate disease prevention in aquaculture. Rev. Aquac. 16, 1229–1243. doi: 10.1111/raq.12893
Debnath, P. P., Prukbenjakul, P., Bondad-Reantaso, M. G., Tyler, C. R., and Rodkhum, C. (2024). Factors influencing disease dynamics in small-scale carp polyculture in Bangladesh. Animals 14:966. doi: 10.3390/ani14060966
Devadas, S., Zakaria, Z., Din, M. S. M., Bhassu, S., Karim, M. M. A., Ikhsan, N., et al. (2025). Knowledge, attitudes and practices on antimicrobial use and antimicrobial resistance among shrimp aquaculturists in peninsular Malaysia. Prev. Vet. Med. 239:106513. doi: 10.1016/j.prevetmed.2025.106513
Ding, P., Li, X., Huang, X., Yu, Y., Zhao, Z., Wang, H., et al. (2025). Dietary Bacillus improves behavior, intestinal health, and growth of juvenile sea cucumbers Apostichopus japonicus at low temperature. J. Therm. Biol. 127:104053. doi: 10.1016/j.jtherbio.2025.104053
Domingo-Bretón, R., Moroni, F., Toxqui-Rodríguez, S., Belenguer, Á., Piazzon, M. C., Pérez-Sánchez, J., et al. (2024). Moving beyond Oxford Nanopore standard procedures: new insights from water and multiple fish microbiomes. Int. J. Mol. Sci. 25:12603. doi: 10.3390/ijms252312603
Dong, L., Tang, Y., Wen, S., He, Y., Li, F., Deng, Y., et al. (2024). Fecal microbiota transplantation alleviates allergic rhinitis via CD4+ T cell modulation through gut microbiota restoration. Inflammation 47, 1278–1297. doi: 10.1007/s10753-024-01975-x
Dremova, O., Mimmler, M., Paeslack, N., Khuu, M. P., Gao, Z., Bosmann, M., et al. (2023). Sterility testing of germ-free mouse colonies. Front. Immunol. 14:1275109. doi: 10.3389/fimmu.2023.1275109
Dubey, S., Bhattacharjee, A., Oza, Y., Saxena, S. S., Pradhan, S., Sharma, A., et al. (2025). Harnessing SynComs for rhizosphere engineering to alleviate salt stress in Vigna radiata: from lab experiments to the field. Plant Physiol. Biochem. 229:110304. doi: 10.1016/j.plaphy.2025.110304
Eissa, F., Zidan, N. E. H., and El-Banna, A. (2024). Analysis of EU RASFF notifications on genetically modified food and feed from 2002 to 2023. J. Food Compos. Anal. 136:106801. doi: 10.1016/j.jfca.2024.106801
Elbahnaswy, S., and Elshopakey, G. E. (2024). Recent progress in practical applications of a potential carotenoid astaxanthin in aquaculture industry: a review. Fish Physiol. Biochem. 50, 97–126. doi: 10.1007/s10695-022-01167-0
El-Son, M. A. M., Elbahnaswy, S., Khormi, M. A., Aborasain, A. M., Abdelhaffez, H. H., and Zahran, E. (2025). Harnessing the fish gut microbiome and immune system to enhance disease resistance in aquaculture. Fish Shellfish Immunol. 163:110394. doi: 10.1016/j.fsi.2025.110394
Fallet, M., Montagnani, C., Petton, B., Dantan, L., de Lorgeril, J., Comarmond, S., et al. (2022). Early life microbial exposures shape the Crassostrea gigas immune system for lifelong and intergenerational disease protection. Microbiome 10:85. doi: 10.1186/s40168-022-01280-5
FAO (2024) FAO report: global fisheries and aquaculture production reaches a new record high. Newsroom
Ferro, P. H. S., Ribeiro, G. C., Borba, L. E., Batista, R. O., da Rosa Farias, D., Fracalossi, D. M., et al. (2024). Effects of dietary supplementation with inactivated Lactobacillus plantarum on growth performance, haemato-biochemical parameters, liver fatty acids profile and intestinal microbiome of Nile tilapia. Vet. Res. Commun. 48, 2397–2406. doi: 10.1007/s11259-024-10425-w
Fu, C., Fu, X., Li, Z., Xu, C., Li, F., Wang, L., et al. (2025). Identification of intestinal microbial population structure of Penaeus vannamei supplemented with chitosan oligosaccharide using 2bRAD-M. Microbiol. Spectr. 13:e0314824. doi: 10.1128/spectrum.03148-24
Fu, S., Ma, K., Song, X., Sun, T., Chen, L., and Zhang, W. (2025). Synthetic biology strategies and tools to modulate photosynthesis in microbes. Int. J. Mol. Sci. 26:3116. doi: 10.3390/ijms26073116
Fu, S., Wang, Q., Wang, R., Zhang, Y., Lan, R., He, F., et al. (2022). Horizontal transfer of antibiotic resistance genes within the bacterial communities in aquacultural environment. Sci. Total Environ. 820:153286. doi: 10.1016/j.scitotenv.2022.153286
Fujaya, Y., Hidayani, A. A., Sari, D. K., Aslamyah, S., Rukminasari, N., Muthalib, A., et al. (2023). The optimal dosage of fermented herbal extract on growth and feed efficiency of Nile Tilapia (Oreochromis niloticus). Trop. Life Sci. Res. 34, 39–56. doi: 10.21315/tlsr2023.34.2.3
García-Márquez, J., Rico, R. M., Acién, F. G., Mancera, J. M., Figueroa, F. L., Vizcaíno, A. J., et al. (2023). Dietary effects of a short-term Administration of Microalgae Blend on growth performance, tissue fatty acids, and predominant intestinal microbiota in Sparus aurata. Microorganisms 11:463. doi: 10.3390/microorganisms11020463
Garibay-Valdez, E., Olivas-Bernal, C. A., Vargas-Albores, F., Martínez-Porchas, M., García-Godínez, D. M., Medina-Félix, D., et al. (2024). Deciphering the gut microbiota of zebrafish, the most used fish as a biological model: a meta-analytic approach. Comp. Biochem. Physiol. -Part A Mol. Integr. Physiol. 297:111713. doi: 10.1016/j.cbpa.2024.111713
Gervasoni, L. F., Gervasoni, K., de Oliveira Silva, K., Ferraz Mendes, M. E., Maddela, N. R., Prasad, R., et al. (2023). Postbiotics in active food packaging: the contribution of cellulose nanocomposites. Sustain. Chem. Pharm. 36:101280. doi: 10.1016/j.scp.2023.101280
Ghosh, S., Bhattacharya, M., and Rahaman, F. H. (2022). “Socioeconomic study of prospective of probiotics, prebiotics, and Synbiotics for sustainable development of aquaculture in Indian Sundarbans” in Prebiotics, probiotics and nutraceuticals, eds. Behera, K. K., Bist, R., Mohanty, S., and Bhattacharya, M. (Singapore: Springer Nature Singapore), 253–273.
Giri, S. S., Kim, H. J., Jung, W. J., Bin Lee, S., Joo, S. J., Gupta, S. K., et al. (2024). Probiotics in addressing heavy metal toxicities in fish farming: current progress and perspective. Ecotoxicol. Environ. Saf. 282:116755. doi: 10.1016/j.ecoenv.2024.116755
Gómez de la Torre Canny, S. (2023). A novel gnotobiotic experimental system for Atlantic salmon (Salmo salar L.) reveals a microbial influence on mucosal barrier function and adipose tissue accumulation during the yolk sac stage. Front. Cell. Infect. Microbiol. 12:1068302. doi: 10.3389/fcimb.2022.1068302
Guo, H., Fu, X., He, J., Wang, R., Yan, M., Wang, J., et al. (2023). Gut bacterial consortium enriched in a biofloc system protects shrimp against Vibrio parahaemolyticus infection. Microbiome 11:230. doi: 10.1186/s40168-023-01663-2
Gutási, A., Hammer, S. E., El-Matbouli, M., and Saleh, M. (2023). Review: recent applications of gene editing in fish species and aquatic medicine. Animals 13:250. doi: 10.3390/ani13071250
Guzman, J. P. M. D., Nozaki, R., Aoki, M., Kuwahara, H., Mikata, K., Koiwai, K., et al. (2025). Transcriptome analyses of mRNA and circular RNA reveal dietary supplementation with freeze-dried Lactiplantibacillus plantarum primes immune memory of Whiteleg shrimp (Penaeus vannamei) against pathogens. Fish Shellfish Immunol. 157:110091. doi: 10.1016/j.fsi.2024.110091
Hamdan, A. M., Abd-El-Mageed, H., and Ghanem, N. (2021). Biological treatment of hazardous heavy metals by Streptomyces rochei ANH for sustainable water management in agriculture. Sci. Rep. 11:9314. doi: 10.1038/s41598-021-88843-y
Han, Y., He, J., Li, M., Peng, Y., Jiang, H., Zhao, J., et al. (2024). Unlocking the potential of metagenomics with the PacBio high-Fidelity sequencing technology. Microorganisms 12:482. doi: 10.3390/microorganisms12122482
Han, C., Song, S., Cui, C., Cai, Y., Zhou, Y., Wang, J., et al. (2024). Strain-specific benefits of Bacillus probiotics in hybrid grouper: growth enhancement, metabolic health, immune modulation, and Vibrio harveyi resistance. Animals 14:1062. doi: 10.3390/ani14071062
Han, Z., Sun, J., Jiang, B., Chen, K., Ge, L., Sun, Z., et al. (2024). Fecal microbiota transplantation accelerates restoration of florfenicol-disturbed intestinal microbiota in a fish model. Commun. Biol. 7:1006. doi: 10.1038/s42003-024-06727-z
Han, J., Zhang, H., and Ning, K. (2025). Techniques for learning and transferring knowledge for microbiome-based classification and prediction: review and assessment. Brief. Bioinform. 26:bbaf015. doi: 10.1093/bib/bbaf015
Hansen, S. B., Bozzi, D., Mak, S. S. T., Clausen, C. G., Nielsen, T. K., Kodama, M., et al. (2023). Intestinal epigenotype of Atlantic salmon (Salmo salar) associates with tenacibaculosis and gut microbiota composition. Genomics 115:110629. doi: 10.1016/j.ygeno.2023.110629
Hayashi, N., Lai, Y., Fuerte-Stone, J., Mimee, M., and Lu, T. K. (2024). Cas9-assisted biological containment of a genetically engineered human commensal bacterium and genetic elements. Nat. Commun. 15:2096. doi: 10.1038/s41467-024-45893-w
Hazra, M., Watts, J. E. M., Williams, J. B., and Joshi, H. (2024). An evaluation of conventional and nature-based technologies for controlling antibiotic-resistant bacteria and antibiotic-resistant genes in wastewater treatment plants. Sci. Total Environ. 917:170433. doi: 10.1016/j.scitotenv.2024.170433
He, L. X., He, L. Y., Gao, F. Z., Wu, D. L., Ye, P., Cheng, Y. X., et al. (2022). Antibiotics, antibiotic resistance genes and microbial community in grouper mariculture. Sci. Total Environ. 808:152042. doi: 10.1016/j.scitotenv.2021.152042
He, J., Ju, J., Jiang, Q., Zhao, H., Zhang, Y., and Yang, H. (2025). Protective effects of Astaxanthin on Thioacetamide-induced Hepatopancreatic damage in Procambarus clarkii: insights from biochemical, histological, and Metabolomic analyses. Animals 15:1537. doi: 10.3390/ani15111537
Heyer, R., Wolf, M., Benndorf, D., Uzzau, S., Seifert, J., Grenga, L., et al. (2025). Metaproteomics in the one health framework for unraveling microbial effectors in microbiomes. Microbiome 13:134. doi: 10.1186/s40168-025-02119-5
Hoseinifar, S. H., Faheem, M., Liaqat, I., Van Doan, H., Ghosh, K., and Ringø, E. (2024). Promising probiotic candidates for sustainable aquaculture: An updated review. Animals 14:3644. doi: 10.3390/ani14243644
Hou, X., Li, W., Yang, S., Huang, Y., Jian, J., and Cai, S. (2025). Effects of oral immunization with Bacillus subtilis displaying Vibrio harveyi FlgE protein on the intestinal structure and gut microbiota of grouper. Fish Shellfish Immunol. 160:110234. doi: 10.1016/j.fsi.2025.110234
Huang, J.-N., Gao, C.-C., Ren, H.-Y., Wen, B., Wang, Z.-N., Gao, J.-Z., et al. (2025). Multi-omics association pattern between gut microbiota and host metabolism of a filter-feeding fish in situ exposed to microplastics. Environ. Int. 197:109360. doi: 10.1016/j.envint.2025.109360
Huang, X., He, H., Li, Z., Liu, C., Jiang, B., Huang, Y., et al. (2024). Screening and effects of intestinal probiotics on growth performance, gut health, immunity, and disease resistance of Nile tilapia (Oreochromis niloticus) against Streptococcus agalactiae. Fish Shellfish Immunol. 151:109668. doi: 10.1016/j.fsi.2024.109668
Huang, J., Jordan, H. R., Older, C. E., Griffin, M. J., Allen, P. J., Wise, D. J., et al. (2024). Lactococcus lactis MA5 is a potential autochthonous probiotic for nutrient digestibility enhancement and bacterial pathogen inhibition in hybrid catfish (Ictalurus punctatus × I. furcatus). J. Fish Dis. 47:13997. doi: 10.1111/jfd.13997
Huang, M. Y., Lo, C. Y., Lai, C. Y., Yu, J. D., and Lee, P. T. (2023). Dietary supplementation of synbiotic Leuconostoc mesenteroide B4 and dextran improves immune regulation and disease resistance of Penaeus vannamei against Vibrio parahaemolyticus. Fish Shellfish Immunol. 132:108498. doi: 10.1016/j.fsi.2022.108498
Huang, M. Y., Truong, B. N., Nguyen, T. P., Ju, H. J., and Lee, P. T. (2024). Synergistic effects of combined probiotics Bacillus pumilis D5 and Leuconostoc mesenteroide B4 on immune enhancement and disease resistance in Litopenaeus vannamei. Dev. Comp. Immunol. 155:105158. doi: 10.1016/j.dci.2024.105158
Ianiro, G., Punčochář, M., Karcher, N., Porcari, S., Armanini, F., Asnicar, F., et al. (2022). Variability of strain engraftment and predictability of microbiome composition after fecal microbiota transplantation across different diseases. Nat. Med. 28, 1913–1923. doi: 10.1038/s41591-022-01964-3
Iorizzo, M., Albanese, G., Letizia, F., Testa, B., Tremonte, P., Vergalito, F., et al. (2022). Probiotic potentiality from versatile Lactiplantibacillus plantarum strains as resource to enhance freshwater fish health. Microorganisms 10:463. doi: 10.3390/microorganisms10020463
Jang, W. J., Lee, S. J., Jeon, M. H., Kim, T. Y., Lee, J. M., Hasan, M. T., et al. (2021). Characterization of a Bacillus sp. KRF-7 isolated from the intestine of rockfish and effects of dietary supplementation with mannan oligosaccharide in rockfish aquaculture. Fish Shellfish Immunol. 119, 182–192. doi: 10.1016/j.fsi.2021.09.039
Ji, L., Shangguan, Y., Chen, C., Wei, C., Zhu, J., Hong, X., et al. (2025). Dietary tannic acid promotes growth performance and resistance against Aeromonas hydrophila infection by improving the Antioxidative capacity and intestinal health in the Chinese soft-shelled turtle (Pelodiscus sinensis). Antioxidants 14:112. doi: 10.3390/antiox14010112
Ji, Z., Zhu, C., Zhu, X., Ban, S., Yu, L., Tian, J., et al. (2023). Dietary host-associated Bacillus subtilis supplementation improves intestinal microbiota, health and disease resistance in Chinese perch (Siniperca chuatsi). Anim. Nutr. 13, 197–205. doi: 10.1016/j.aninu.2023.01.001
Jia, P. P., Liu, X. R., Wu, M. F., Li, Y., Zhang, L. C., and Pei, D. S. (2024). Generation, maintenance, and identification of germ-free zebrafish models from larvae to juvenile stages. J. Vis. Exp. 2024:512. doi: 10.3791/66512
Jiang, X., Peng, Z., and Zhang, J. (2024). Starting with screening strains to construct synthetic microbial communities (SynComs) for traditional food fermentation. Food Res. Int. 190:114557. doi: 10.1016/j.foodres.2024.114557
Kari, Z. A., Téllez-Isaías, G., Khoo, M. I., Wee, W., Kabir, M. A., Cheadoloh, R., et al. (2024). Resveratrol impacts on aquatic animals: a review. Fish Physiol. Biochem. 50, 307–318. doi: 10.1007/s10695-024-01319-4
Karimianghadim, R., Satokari, R., Yeo, S., Arkkila, P., Kao, D., and Pakpour, S. (2025). Prolonged effect of antibiotic therapy on the gut microbiota composition, functionality, and antibiotic resistance genes’ profiles in healthy stool donors. Front. Microbiol. 16:1589704. doi: 10.3389/fmicb.2025.1589704
Kavita, O. H., Chand, U., and Kushawaha, P. K. (2024). Postbiotics: An alternative and innovative intervention for the therapy of inflammatory bowel disease. Microbiol. Res. 279:127550. doi: 10.1016/j.micres.2023.127550
Kewcharoen, W., and Srisapoome, P. (2022). Potential synbiotic effects of a Bacillus mixture and chitosan on growth, immune responses and VP (AHPND) resistance in Pacific white shrimp (Litopenaeus vannamei, Boone, 1931). Fish Shellfish Immunol. 127, 715–729. doi: 10.1016/j.fsi.2022.07.017
Khan, T. A., Liangliang, H., Attia, K. A., Bashir, S., Ziyuan, X., Alsubki, R. A., et al. (2025). Bacillus velezensis FiA2 as an oxydifficidin-producing strain and its effects on the growth performance, immunity, intestinal microbiota, and resistance to Aeromonas salmonicida infection in Carassius carassius. Probiotics Antimicrob. Proteins. 1–7. doi: 10.1007/s12602-025-10485-7
Khanjani, M. H., Sharifinia, M., and Emerenciano, M. G. C. (2023). A detailed look at the impacts of biofloc on immunological and hematological parameters and improving resistance to diseases. Fish Shellfish Immunol. 137:108796. doi: 10.1016/j.fsi.2023.108796
Kim, K., Maji, U. J., Shim, K.-Y., Yeo, I.-C., and Jeong, C.-B. (2024). Detection of the jellyfish Chrysaora pacifica by RPA-CRISPR-Cas12a environmental DNA (eDNA) assay and its evaluation through field validation and comparative eDNA analyses. Sci. Total Environ. 955:176945. doi: 10.1016/j.scitotenv.2024.176945
Kumar, M., Devi, W. M., Choudhury, T. G., Kamilya, D., Monsang, S. J., Irungbam, S., et al. (2025). Unraveling the bioactivities and immunomodulatory potential of postbiotics derived from Bacillus subtilis and B. amyloliquefaciens for aquaculture. Probiotics Antimicrob. Proteins. doi: 10.1007/s12602-025-10528-z
Lee, D., Muir, P., Lundberg, S., Lundholm, A., Sandegren, L., and Koskiniemi, S. (2025). A CRISPR-Cas9 system protecting E. coli against acquisition of antibiotic resistance genes. Sci. Rep. 15:1545. doi: 10.1038/s41598-025-85334-2
Lee, S.-J., Noh, D.-I., Lee, Y.-S., Hasan, M. T., Hur, S. W., Lee, S., et al. (2024). Effects of host-associated low-temperature probiotics in olive flounder (Paralichthys olivaceus) aquaculture. Sci. Rep. 14:2134. doi: 10.1038/s41598-024-52491-9
Li, S., Heng, X., Guo, L., Lessing, D. J., and Chu, W. (2022). SCFAs improve disease resistance via modulate gut microbiota, enhance immune response and increase antioxidative capacity in the host. Fish Shellfish Immunol. 120, 560–568. doi: 10.1016/j.fsi.2021.12.035
Li, Y., Huang, S., Jiang, S., Yang, L., Huang, J., Yang, Q., et al. (2025). Multi-omics insights into antioxidant and immune responses in Penaeus monodon under ammonia-N, low salinity, and combined stress. Ecotoxicol. Environ. Saf. 295:118156. doi: 10.1016/j.ecoenv.2025.118156
Li, W., Zeng, J., Li, Y., Ge, C., Su, J., and Yao, H. (2024). A fascinating finding: the application of traditional Chinese medicine in aquaculture prevents the spread and diffusion of antibiotic resistance genes among gut microbes. Aquaculture 583:740573. doi: 10.1016/j.aquaculture.2024.740573
Li, W.-J., Zhang, L., Wu, H.-X., Li, M., Wang, T., Zhang, W.-B., et al. (2022). Intestinal microbiota mediates gossypol-induced intestinal inflammation, oxidative stress, and apoptosis in fish. J. Agric. Food Chem. 70, 6688–6697. doi: 10.1021/acs.jafc.2c01263
Liang, H., Li, M., Chen, J., Zhou, W., Xia, D., Ding, Q., et al. (2024). The intestinal microbiome and Cetobacterium somerae inhibit viral infection through TLR2-type I IFN signaling axis in zebrafish. Microbiome 12:244. doi: 10.1186/s40168-024-01958-y
Liao, X., Lan, Y., Wang, W., Zhang, J., Shao, R., Yin, Z., et al. (2023). Vitamin D influences gut microbiota and acetate production in zebrafish (Danio rerio) to promote intestinal immunity against invading pathogens. Gut Microbes 15:2187575. doi: 10.1080/19490976.2023.2187575
Lin, Y. T., Hung, Y. C., Chen, L. H., Lee, K. T., and Han, Y. S. (2024). Effects of adding Bacillus subtilis natto NTU-18 in paste feed on growth, intestinal morphology, gastrointestinal microbiota diversity, immunity, and disease resistance of Anguilla japonica glass eels. Fish Shellfish Immunol. 149:109556. doi: 10.1016/j.fsi.2024.109556
Lin, L., Song, J., Li, J., Zuo, X., Wei, H., Yang, C., et al. (2021). Imaging the in vivo growth patterns of bacteria in human gut microbiota. Gut Microbes 13:134. doi: 10.1080/19490976.2021.1960134
Liu, C., Sun, S., Sun, Y., Li, X., Gu, W., Luo, Y., et al. (2024). Antibiotic resistance of Escherichia coli isolated from food and clinical environment in China from 2001 to 2020. Sci. Total Environ. 939:173498. doi: 10.1016/j.scitotenv.2024.173498
Liu, L., Wei, C., Li, Y., Wang, M., Mao, Y., and Tian, X. (2024). A comparative study on effects of three butyric acid-producing additives on the growth performance, non-specific immunity, and intestinal microbiota of the sea cucumber Apostichopus japonicus. Aquac. Nutr. 2024:246. doi: 10.1155/2024/6973951
Liu, L., Zhao, P., Yang, L., Li, Y., Huang, Z., Yang, Q., et al. (2025). Integration of transcriptomics and metabolomics reveals mechanisms of high-temperature stress tolerance in the hepatopancreas of Penaeus monodon. Biology 14:591. doi: 10.3390/biology14060591
Lu, J., Zhang, H., Pan, L., Guan, W., and Lou, Y. (2022). Environmentally relevant concentrations of triclosan exposure promote the horizontal transfer of antibiotic resistance genes mediated by Edwardsiella piscicida. Environ. Sci. Pollut. Res. 29, 64622–64632. doi: 10.1007/s11356-022-20082-8
Luo, K., Guo, Z., Liu, Y., Li, C., Ma, Z., and Tian, X. (2024a). Responses of growth performance, immunity, disease resistance of shrimp and microbiota in Penaeus vannamei culture system to Bacillus subtilis BSXE-1601 administration: dietary supplementation versus water addition. Microbiol. Res. 283:127693. doi: 10.1016/j.micres.2024.127693
Luo, K., Yang, Z., Wen, X., Wang, D., Liu, J., Wang, L., et al. (2024b). Recovery of intestinal microbial community in Penaeus vannamei after florfenicol perturbation. J. Hazard. Mater. 480:136158. doi: 10.1016/j.jhazmat.2024.136158
Ma, X., Xu, T., Qian, M., Zhang, Y., Yang, Z., and Han, X. (2022). Faecal microbiota transplantation alleviates early-life antibiotic-induced gut microbiota dysbiosis and mucosa injuries in a neonatal piglet model. Microbiol. Res. 255:126942. doi: 10.1016/j.micres.2021.126942
Ma, J., Zhang, P., Zheng, M., Wang, B., Gao, P., Qu, L., et al. (2023). A strain of Vibrio alginolyticus isolated from Azumapecten farreri and its pathogenic mechanism using CRISPR-Cas9 technology. Biotechnol. Lett. 45, 1279–1291. doi: 10.1007/s10529-023-03394-8
Ma, Y.-B., Zhou, X.-Q., Jiang, W.-D., Wu, P., Liu, Y., Ren, H.-M., et al. (2025). Tea polyphenols: a promising alternative to antibiotics for preventing bacterial enteritis in grass carp (Ctenopharyngodon idella). Food Res. Int. 213:116575. doi: 10.1016/j.foodres.2025.116575
Mahmoodian, S., Meimandipour, A., Faeed, M., Shamsara, M., Fatemi, S. S.-A., Ghasemi, M., et al. (2025). Dietary probiotic prototypes and their effects on growth performance, immune function, and gut microbiota of rainbow trout (Oncorhynchus mykiss). Res. Vet. Sci. 193:105808. doi: 10.1016/j.rvsc.2025.105808
Marclay, M., Dwyer, E., Suchodolski, J. S., Lidbury, J. A., Steiner, J. M., and Gaschen, F. P. (2022). Recovery of fecal microbiome and bile acids in healthy dogs after Tylosin administration with and without fecal microbiota transplantation. Vet. Sci. 9:324. doi: 10.3390/vetsci9070324
Mercer, L. K., Harding, E. F., Sridhar, T., and White, P. A. (2024). Novel viruses discovered in metatranscriptomic analysis of farmed barramundi in Asia and Australia. Virology 599:110208. doi: 10.1016/j.virol.2024.110208
Mohammadi, G., Hafezieh, M., Karimi, A. A., Azra, M. N., Van Doan, H., Tapingkae, W., et al. (2022). The synergistic effects of plant polysaccharide and Pediococcus acidilactici as a synbiotic additive on growth, antioxidant status, immune response, and resistance of Nile tilapia (Oreochromis niloticus) against Aeromonas hydrophila. Fish Shellfish Immunol. 120, 304–313. doi: 10.1016/j.fsi.2021.11.028
Mohan, K., Rajan, D. K., Ganesan, A. R., Divya, D., Johansen, J., and Zhang, S. (2023). Chitin, chitosan and chitooligosaccharides as potential growth promoters and immunostimulants in aquaculture: a comprehensive review. Int. J. Biol. Macromol. 251:126285. doi: 10.1016/j.ijbiomac.2023.126285
Muñoz, C., González-Lorca, J., Parra, M., Soto, S., Valdes, N., Sandino, A. M., et al. (2021). Lactococcus lactis expressing type I interferon from Atlantic Salmon enhances the innate antiviral immune response in vivo and in vitro. Front. Immunol. 12:696781. doi: 10.3389/fimmu.2021.696781
Muras, A., Romero, M., Mayer, C., and Otero, A. (2021). Biotechnological applications of Bacillus licheniformis. Crit. Rev. Biotechnol. 41, 609–627. doi: 10.1080/07388551.2021.1873239
Nababan, Y. I., Yuhana, M., Penataseputro, T., Nasrullah, H., Alimuddin, A., and Widanarni, W. (2022). Dietary supplementation of Pseudoalteromonas piscicida 1UB and fructooligosaccharide enhance growth performance and protect the whiteleg shrimp (Litopenaeus vannamei) against WSSV and Vibrio harveyi coinfection. Fish Shellfish Immunol. 131, 746–756. doi: 10.1016/j.fsi.2022.10.047
Naya-Català, F., Torrecillas, S., Piazzon, M. C., Sarih, S., Calduch-Giner, J., Fontanillas, R., et al. (2024). Can the genetic background modulate the effects of feed additives? Answers from gut microbiome and transcriptome interactions in farmed gilthead sea bream (Sparus aurata) fed with a mix of phytogenics, organic acids or probiotics. Aquaculture 586:770. doi: 10.1016/j.aquaculture.2024.740770
Nayak, A., Harshitha, M., Disha, S., Dubey, S., Munang’andu, H. M., Evensen, Ø., et al. (2023). In vitro determination of probiotic efficacy of Bacillus subtilis TLDK301120C24 isolated from tilapia against warm water fish pathogens and in vivo validation using gnotobiotic zebrafish model. Microb. Pathog. 185:106429. doi: 10.1016/j.micpath.2023.106429
Noman, M., Kazmi, S. S. U. H., Saqib, H. S. A., Fiaz, U., Pastorino, P., Barcelò, D., et al. (2024). Harnessing probiotics and prebiotics as eco-friendly solution for cleaner shrimp aquaculture production: a state of the art scientific consensus. Sci. Total Environ. 915:169921. doi: 10.1016/j.scitotenv.2024.169921
Okoli, A. S., Blix, T., Myhr, A. I., Xu, W., and Xu, X. (2022). Sustainable use of CRISPR/Cas in fish aquaculture: the biosafety perspective. Transgenic Res. 31, 1–21. doi: 10.1007/s11248-021-00274-7
Oliveira, B. P. N., Padeniya, U., Bledsoe, J. W., Davis, D. A., Liles, M. R., Hussain, A. S., et al. (2025). Evaluation of probiotic effects on the growth performance and microbiome of Nile Tilapia (Oreochromis niloticus) in a high-density biofloc system. Aquac. Nutr. 2025:806. doi: 10.1155/anu/5868806
Ouyang, S., Wang, X., Chen, Y., Deng, L., Yang, X., Hu, S., et al. (2023). Swimming training combined with fecal microbial transplantation protects motor functions in rats with spinal cord injury by improving the intestinal system. Neurosci. Lett. 799:137104. doi: 10.1016/j.neulet.2023.137104
Paul, S. I., and Rahman, M. M. (2022). Draft genome sequence of Bacillus subtilis YBS29, a potential fish probiotic that prevents motile Aeromonas septicemia in Labeo rohita. Microbiol. Resour. Announc. 11, 00915–00922. doi: 10.1128/mra.00915-22
Peng, L., Rahman, Z., Tian, Y., Yin, T., Xiong, S., You, J., et al. (2025). Comprehensive molecular biology and metabolomics analysis reveal the changes on muscle quality of Megalobrama amblycephala exposure to ammonia nitrogen during transportation. Food Res. Int. 212:116372. doi: 10.1016/j.foodres.2025.116372
Peralta-Sánchez, J. M., Rabelo-Ruiz, M., Martín-Platero, A. M., Vizcaíno, A. J., Flores-Moreno, S., Macías-Vidal, J., et al. (2024). Microalgae and phytase dietary supplementation improved growth and gut microbiota in juvenile European seabass (Dicentrarchus labrax). BMC Genomics 25:838. doi: 10.1186/s12864-024-10760-x
Perez-Pascual, D., Vendrell-Fernandez, S., Audrain, B., Bernal-Bayard, J., Patiño-Navarrete, R., Petit, V., et al. (2021). Gnotobiotic rainbow trout (Oncorhynchus mykiss) model reveals endogenous bacteria that protect against Flavobacterium columnare infection. PLoS Pathog. 17:e1009302. doi: 10.1371/JOURNAL.PPAT.1009302
Phan, T. C. T., Nguyen, T. K. L., Pham, T. T. N., Truong, Q. P., Huynh, T. G., and Tran, T. T. H. (2025). Synbiotic effects of Lactobacillus plantarum CMT1 and Morinda citrifolia on the growth performance and disease resistance of whiteleg shrimp. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 275:111037. doi: 10.1016/j.cbpb.2024.111037
Poupaud, M., Goutard, F. L., Phouthana, V., Muñoz Viera, F., Caro, D., Patriarchi, A., et al. (2022). Different kettles of fish: varying patterns of antibiotic use on pig, chicken and fish farms in Lao PDR and implications for antimicrobial resistance strategies. Transbound. Emerg. Dis. 69, 3940–3951. doi: 10.1111/tbed.14766
Prabawati, E., Hu, S. Y., Chiu, S. T., Balantyne, R., Risjani, Y., and Liu, C. H. (2022). A synbiotic containing prebiotic prepared from a by-product of king oyster mushroom, Pleurotus eryngii and probiotic, Lactobacillus plantarum incorporated in diet to improve the growth performance and health status of white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 120, 155–165. doi: 10.1016/j.fsi.2021.11.031
Pudgerd, A., Saedan, S., Santimanawong, W., Weerachatyanukul, W., Jariyapong, P., Chaijarasphong, T., et al. (2024). Genome editing of WSSV CRISPR/Cas9 and immune activation extends the survival of infected Penaeus vannamei. Sci. Rep. 14:26306. doi: 10.1038/s41598-024-78277-7
Qian, Y., Lai, L., Cheng, M., Fang, H., Fan, D., Zylstra, G. J., et al. (2024). Identification, characterization, and distribution of novel amidase gene aphA in sphingomonads conferring resistance to amphenicol antibiotics. Appl. Environ. Microbiol. 90:e0151224. doi: 10.1128/aem.01512-24
Qin, G., Wang, D., Luo, K., Liu, Y., Xie, Y., Wang, M., et al. (2025). Evaluation of probiotic properties and safety of a Bacillus strain for shrimp farming: integrating in vitro testing, genomic analysis and in vivo validation. Microbiol. Res. 297:128179. doi: 10.1016/j.micres.2025.128179
Radwan, M., Moussa, M. A., Manaa, E. A., El-Sharkawy, M. A., Darweesh, K. F., Elraey, S. M. A., et al. (2024). Synergistic effect of green synthesis magnesium oxide nanoparticles and seaweed extract on improving water quality, health benefits, and disease resistance in Nile tilapia. Ecotoxicol. Environ. Saf. 280:116522. doi: 10.1016/j.ecoenv.2024.116522
Rahayu, S., Amoah, K., Huang, Y., Cai, J., Wang, B., Shija, V. M., et al. (2024). Probiotics application in aquaculture: its potential effects, current status in China and future prospects. Front. Mar. Sci. 11:1455905. doi: 10.3389/fmars.2024.1455905
Rana, M. L., Ullah, M. A., Hoque, M. N., Hassan, J., Siddique, M. P., and Rahman, M. T. (2025). Preliminary survey of biofilm forming, antibiotic resistant Escherichia coli in fishes from land based aquaculture systems and open water bodies in Bangladesh. Sci. Rep. 15:7811. doi: 10.1038/s41598-024-80536-6
Rasmussen, J. A., Villumsen, K. R., Ernst, M., Hansen, M., Forberg, T., Gopalakrishnan, S., et al. (2022). A multi-omics approach unravels metagenomic and metabolic alterations of a probiotic and synbiotic additive in rainbow trout (Oncorhynchus mykiss). Microbiome 10:21. doi: 10.1186/s40168-021-01221-8
Rocha, S. D. C., Lei, P., Morales-Lange, B., Mydland, L. T., and Øverland, M. (2023). From a cell model to a fish trial: immunomodulatory effects of heat-killed Lactiplantibacillus plantarum as a functional ingredient in aquafeeds for salmonids. Front. Immunol. 14:1125702. doi: 10.3389/fimmu.2023.1125702
Ruiz, A., Gisbert, E., and Andree, K. B. (2024). Impact of the diet in the gut microbiota after an inter-species microbial transplantation in fish. Sci. Rep. 14:4007. doi: 10.1038/s41598-024-54519-6
Salminen, S., Collado, M. C., Endo, A., Hill, C., Lebeer, S., Quigley, E. M. M., et al. (2021). The international scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 18, 649–667. doi: 10.1038/s41575-021-00440-6
Sampson, S. (2024) FAO report global fisheries and aquaculture production reaches a new record high. Available online at: https://www.fao.org/newsroom/detail/fao-report-global-fisheries-and-aquaculture-production-reaches-a-new-record-high/en
Santos, R. A., Monteiro, M., Rangel, F., Jerusik, R., Saavedra, M. J., Carvalho, A. P., et al. (2021). Bacillus spp. inhibit edwardsiella tarda quorum-sensing and fish infection. Mar. Drugs 19:602. doi: 10.3390/md19110602
Sayid, R., van den Hurk, A. W. M., Rothschild-Rodriguez, D., Herrema, H., de Jonge, P. A., and Nobrega, F. L. (2024). Characteristics of phage-plasmids and their impact on microbial communities. Essays Biochem. 68, 583–592. doi: 10.1042/EBC20240014
Shan, C., Li, M., Liu, Z., Xu, R., Qiao, F., Du, Z. Y., et al. (2021). Pediococcus pentosaceus enhances host resistance against pathogen by increasing IL-1β production: understanding probiotic effectiveness and administration duration. Front. Immunol. 12:766401. doi: 10.3389/fimmu.2021.766401
Shang, X., Wang, B., Sun, Q., Zhang, Y., Lu, Y., Liu, S., et al. (2022). Selenium-enriched Bacillus subtilis reduces the effects of mercury-induced on inflammation and intestinal microbes in carp (Cyprinus carpio var. specularis). Fish Physiol. Biochem. 48, 215–226. doi: 10.1007/s10695-022-01046-8
Sheng, T., Meng, X.-Z., Yu, Q., Lv, W., Chen, Y., Cong, Q., et al. (2025). Transcriptome analysis and CRISPR-Cas9-mediated mutagenesis identify gpr116 as a candidate gene for growth promotion in grass carp (Ctenopharyngodon idella). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 305:111850. doi: 10.1016/j.cbpa.2025.111850
Shinde, A. H., Sonpal, V., Maiti, P., and Haldar, S. (2023). Evaluation of a synbiotic formulation for water remediation in a shrimp pond. Environ. Sci. Pollut. Res. 30, 65990–66001. doi: 10.1007/s11356-023-27006-0
Solis, C. J., Hamilton, M. K., Caruffo, M., Garcia-Lopez, J. P., Navarrete, P., Guillemin, K., et al. (2020). Intestinal inflammation induced by soybean meal ingestion increases intestinal permeability and neutrophil turnover independently of microbiota in zebrafish. Front. Immunol. 11:1330. doi: 10.3389/fimmu.2020.01330
Song, Y., Huang, H., Jia, K., Zou, S., Yang, Y., and Yi, M. (2025). Multi-omics analysis reveals toxicity and gut-liver axis disruption induced by polychlorinated biphenyls exposure in yellowfin seabream (Acanthopagrus latus). J. Hazard. Mater. 487:137296. doi: 10.1016/j.jhazmat.2025.137296
Soriano, B., Hafez, A. I., Naya-Català, F., Moroni, F., Moldovan, R. A., Toxqui-Rodríguez, S., et al. (2023). SAMBA: structure-learning of aquaculture microbiomes using a Bayesian approach. Genes 14:1650. doi: 10.3390/genes14081650
Soto-Dávila, M., Langlois Fiorotto, L., Heath, J. W., Lumsden, J. S., Reid, G., and Dixon, B. (2024). The effects of Pediococcus acidilactici MA18/5M on growth performance, gut integrity, and immune response using in vitro and in vivo Pacific salmonid models. Front. Immunol. 15:1306458. doi: 10.3389/fimmu.2024.1306458
Stagaman, K., Alexiev, A., Sieler, M. J., Hammer, A., Kasschau, K. D., Truong, L., et al. (2024). The zebrafish gut microbiome influences benzo[a]pyrene developmental neurobehavioral toxicity. Sci. Rep. 14:14618. doi: 10.1038/s41598-024-65610-3
Su, Y., Yu, H., Gao, C., Sun, S., Liang, Y., Liu, G., et al. (2024). Effects of vegetation cover and aquaculture pollution on viral assemblages in mangroves sediments. J. Hazard. Mater. 476:135147. doi: 10.1016/j.jhazmat.2024.135147
Sudhakaran, G., Guru, A., Haridevamuthu, B., Murugan, R., Arshad, A., and Arockiaraj, J. (2022). Molecular properties of postbiotics and their role in controlling aquaculture diseases. Aquac. Res. 53, 3257–3273. doi: 10.1111/are.15846
Sukul, T., Kari, Z. A., Téllez-Isaías, G., and Ghosh, K. (2023). Autochthonous Bacilli and Fructooligosaccharide as functional feed additives improve growth, feed utilisation, Haemato-immunological parameters and disease resistance in Rohu, Labeo rohita (Hamilton). Animals 13:2631. doi: 10.3390/ani13162631
Sun, H., Peng, H., Hong, X., Chen, F., Zheng, W., Gao, Y., et al. (2025). Lvvibriocin-GK effectively reduced skin ulcer syndrome of Apostichopus japonicus by eliminating surface bacteria, modulating gut microbiota, and enhancing host immune responses. Fish Shellfish Immunol. 165:110494. doi: 10.1016/j.fsi.2025.110494
Swanson, K. S., Gibson, G. R., Hutkins, R., Reimer, R. A., Reid, G., Verbeke, K., et al. (2020). The international scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 17, 687–701. doi: 10.1038/s41575-020-0344-2
Tao, B., Li, X., Li, X., Lu, K., Song, K., Mohsen, M., et al. (2024). Derivatives of postbiotics (cell wall constituents) from Bacillus subtilis (LCBS1) relieve soybean meal-induced enteritis in bullfrog (Aquarana catesbeianus). Int. J. Biol. Macromol. 279:135359. doi: 10.1016/j.ijbiomac.2024.135359
Thorakkattu, P., Khanashyam, A. C., Shah, K., Babu, K. S., Mundanat, A. S., Deliephan, A., et al. (2022). Postbiotics: current trends in food and pharmaceutical industry. Foods. 11:94. doi: 10.3390/foods11193094
Tian, H., Wang, X., Fang, Z., Li, L., Wu, C., Bi, D., et al. (2024). Fecal microbiota transplantation in clinical practice: present controversies and future prospects. hLife 2, 269–283. doi: 10.1016/j.hlife.2024.01.006
Toxqui-Rodríguez, S., Estensoro, I., Domingo-Bretón, R., Del Pozo, R., Pérez-Sánchez, J., Sipkema, D., et al. (2025). Interactions between gilthead seabream intestinal transcriptome and microbiota upon Enteromyxum leei infection: a multi–omic approach. Anim. Microbiome 7:22. doi: 10.1186/s42523-025-00388-x
Tseng, K. C., Huang, H. T., Huang, S. N., Yang, F. Y., Li, W. H., Nan, F. H., et al. (2023). Lactobacillus plantarum isolated from kefir enhances immune responses and survival of white shrimp (Penaeus vannamei) challenged with Vibrio alginolyticus. Fish Shellfish Immunol. 135:108661. doi: 10.1016/j.fsi.2023.108661
Uengwetwanit, T., Uawisetwathana, U., Arayamethakorn, S., Khudet, J., Chaiyapechara, S., Karoonuthaisiri, N., et al. (2020). Multi-omics analysis to examine microbiota, host gene expression and metabolites in the intestine of black tiger shrimp (Penaeus monodon) with different growth performance. PeerJ 8:e9646. doi: 10.7717/peerj.9646
Vadstein, O., Attramadal, K. J. K., Bakke, I., and Olsen, Y. (2018). K-selection as microbial community management strategy: a method for improved viability of larvae in aquaculture. Front. Microbiol. 9:2730. doi: 10.3389/fmicb.2018.02730
Van Doan, H., Hoseinifar, S. H., Naraballobh, W., Paolucci, M., Wongmaneeprateep, S., Charoenwattanasak, S., et al. (2021). Dietary inclusion of watermelon rind powder and Lactobacillus plantarum: effects on Nile tilapia’s growth, skin mucus and serum immunities, and disease resistance. Fish Shellfish Immunol. 116, 107–114. doi: 10.1016/j.fsi.2021.07.003
Vandeputte, D., Kathagen, G., D’hoe, K., Vieira-Silva, S., Valles-Colomer, M., Sabino, J., et al. (2017). Quantitative microbiome profiling links gut community variation to microbial load. Nature 551, 507–511. doi: 10.1038/nature24460
Vijayaram, S., Sinha, R., Faggio, C., Ringø, E., and Chou, C.-C. (2024). Biopolymer encapsulation for improved probiotic delivery: advancements and challenges. AIMS Microbiol. 10, 986–1023. doi: 10.3934/microbiol.2024043
Villumsen, K. R., Ohtani, M., Forberg, T., Aasum, E., Tinsley, J., and Bojesen, A. M. (2020). Synbiotic feed supplementation significantly improves lipid utilization and shows discrete effects on disease resistance in rainbow trout (Oncorhynchus mykiss). Sci. Rep. 10:16993. doi: 10.1038/s41598-020-73812-8
Vinderola, G., Sanders, M. E., Salminen, S., and Szajewska, H. (2022). Postbiotics: the concept and their use in healthy populations. Front. Nutr. 9:102213. doi: 10.3389/fnut.2022.1002213
Wan, B., Lei, Y., Yuan, Z., and Wang, W. (2024). Metagenomic dissection of the intestinal microbiome in the giant river prawn Macrobrachium rosenbergii infected with decapod iridescent virus 1. Fish Shellfish Immunol. 149:109617. doi: 10.1016/j.fsi.2024.109617
Wang, Q., Fan, D., Hu, Y., Liu, H., Tan, B., Xie, S., et al. (2024). Effects of supplementation with freeze-dried Clostridium butyricum powder after replacement of fishmeal with cottonseed protein concentrate on growth performance, immune response, and intestinal microbiota of Litopenaeus vannamei. BMC Vet. Res. 20:102533. doi: 10.1186/s12917-024-04372-6
Wang, X., Gu, J., Gao, H., Qian, X., and Li, H. (2018). Abundances of clinically relevant antibiotic resistance genes and bacterial community diversity in the Weihe River, China. Int. J. Environ. Res. Public Health 15:708. doi: 10.3390/ijerph15040708
Wang, Z., Hu, X., Solanki, M. K., and Pang, F. (2023). A synthetic microbial Community of Plant Core Microbiome can be a potential biocontrol tool. J. Agric. Food Chem. 71, 5030–5041. doi: 10.1021/acs.jafc.2c08017
Wang, J., Wilson, A. E., Su, B., and Dunham, R. A. (2023). Functionality of dietary antimicrobial peptides in aquatic animal health: multiple meta-analyses. Anim. Nutr. 12, 200–214. doi: 10.1016/j.aninu.2022.10.001
Wang, F., Xu, J., Wang, Z., Cao, J., and Lu, Y. (2024). Response signatures of intestinal microbiota and gene transcription of the pearl gentian grouper to Vibrio harveyi infection. Fish Shellfish Immunol. 149:109590. doi: 10.1016/j.fsi.2024.109590
Wang, A., Zhang, Z., Ding, Q., Yang, Y., Bindelle, J., Ran, C., et al. (2021). Intestinal Cetobacterium and acetate modify glucose homeostasis via parasympathetic activation in zebrafish. Gut Microbes 13, 1–15. doi: 10.1080/19490976.2021.1900996
Wang, T., Zhou, N., Ding, F., Hao, Z., Galindo-Villegas, J., Du, Z., et al. (2024). Xylanase enhances gut microbiota-derived butyrate to exert immune-protective effects in a histone deacetylase-dependent manner. Microbiome 12:212. doi: 10.1186/s40168-024-01934-6
Wiratama, N., Meachasompop, P., Kumwan, B., Adisornprasert, Y., Srisapoome, P., Phrompanya, P., et al. (2025). Dietary probiotic Bacillus subtilis AAHM-BS2360 and its Postbiotic metabolites enhance growth, immunity, and resistance to Edwardsiellosis in Pangasianodon hypophthalmus. Antioxidants 14:629. doi: 10.3390/antiox14060629
Wu, W., Zhou, J., Xuan, R., Chen, J., Han, H., Liu, J., et al. (2022). Dietary κ-carrageenan facilitates gut microbiota-mediated intestinal inflammation. Carbohydr. Polym. 277:118830. doi: 10.1016/j.carbpol.2021.118830
Wu, W., Zhou, D., Xuan, R., Zhou, J., Liu, J., Chen, J., et al. (2021). λ-Carrageenan exacerbates Citrobacter rodentium-induced infectious colitis in mice by targeting gut microbiota and intestinal barrier integrity. Pharmacol. Res. 174:105940. doi: 10.1016/j.phrs.2021.105940
Xiao, H., Cui, M., Li, Y., Dong, J., Zhang, S., Zhu, C., et al. (2020). Gut microbiota-derived indole 3-propionic acid protects against radiation toxicity via retaining acyl-CoA-binding protein. Microbiome 8:69. doi: 10.1186/s40168-020-00845-6
Xin, G. Y., Li, W. G., Suman, T. Y., Jia, P. P., Ma, Y. B., and Pei, D. S. (2020). Gut bacteria Vibrio sp. and Aeromonas sp. trigger the expression levels of proinflammatory cytokine: first evidence from the germ-free zebrafish. Fish Shellfish Immunol. 106, 518–525. doi: 10.1016/j.fsi.2020.08.018
Xu, Y., Wu, C., Wang, P., Han, X., Yang, J., and Zhai, S. (2024). Effects of dietary inclusion of enzymatically hydrolyzed compound soy protein on the growth performance and intestinal health of juvenile American eels (Anguilla rostrata). Animals 14:1. doi: 10.3390/ani14213096
Yang, W., Liang, H., Chen, R., Du, Z., Deng, T., Zheng, Y., et al. (2024). Effects of dietary probiotic (Clostridium butyricum I9, C. butyricum G15, or Paraclostridium bifermentans X13) on growth, digestive enzyme activities, immunity, and intestinal microbiota of Pacific white shrimp (Penaeus vannamei). Front. Microbiol. 15:1479446. doi: 10.3389/fmicb.2024.1479446
Yang, Z., Yu, Y., Tay, Y. X., and Yue, G. H. (2022). Genome editing and its applications in genetic improvement in aquaculture. Rev. Aquac. 14, 178–191. doi: 10.1111/raq.12591
Yen, S. C., Mao, J. Y., Lin, H. Y., Huang, H. T., Harroun, S. G., Nain, A., et al. (2021). Multifunctional carbonized nanogels to treat lethal acute hepatopancreatic necrosis disease. J. Nanobiotechnol. 19:448. doi: 10.1186/s12951-021-01194-8
Yousuf, S., Tyagi, A., and Singh, R. (2023). Probiotic supplementation as an emerging alternative to chemical therapeutics in finfish aquaculture: a review. Probiotics Antimicrob. Proteins 15, 1151–1168. doi: 10.1007/s12602-022-09971-z
Yu, J., Kang, M. J., Park, M.-J., Han, G. H., Kim, Y. J., Noh, C. H., et al. (2025). Microbial community structure and functional characteristics across the mucosal surfaces of olive flounder (Paralichthys olivaceus). Front. Microbiol. 16:1587288. doi: 10.3389/fmicb.2025.1587288
Yu, Z., Liu, G., Li, S., Hong, Y., Zhao, S., Zhou, M., et al. (2024). Effects of fermented pomegranate Peel polyphenols on the growth performance, immune response, Hepatopancreatic health, and disease resistance in White shrimp (Litopenaeus vannamei). Aquac. Nutr. 2024:9966772. doi: 10.1155/anu/9966772
Yue, Z., Zhang, J., Zhang, J., Wang, X., Li, L., Yu, H., et al. (2023). Combined virome analysis and metagenomic sequencing to reveal the viral communities and risk of virus–associated antibiotic resistance genes during composting. J. Hazard. Mater. 459:132088. doi: 10.1016/j.jhazmat.2023.132088
Zainab, S. M., Junaid, M., Xu, N., and Malik, R. N. (2020). Antibiotics and antibiotic resistant genes (ARGs) in groundwater: a global review on dissemination, sources, interactions, environmental and human health risks. Water Res. 187:116455. doi: 10.1016/j.watres.2020.116455
Zamparo, S., Brocca, G., Marroni, F., Radovic, S., Castellano, C., Torge, D., et al. (2025). Metabarcoding reveals a potentially undescribed columnaris-causing bacterium in peracute skin disease of rainbow trout (Oncorhynchus mykiss, Walbaum). J. Fish Dis. :e70004. doi: 10.1111/jfd.70004
Zeng, S., Huang, Z., Kriengkrai, S., Zhou, R., Yuan, D., Tuấn, N. V., et al. (2025). Warming-driven migration of enterotypes mediates host health and disease statuses in ectotherm Litopenaeus vannamei. Commun. Biol. 8:126. doi: 10.1038/s42003-025-07558-2
Zhang, M., Feng, Y., Zhong, Z., Du, Q., Yu, W., Wu, J., et al. (2024). Host gut-derived probiotic, Exiguobacterium acetylicum G1-33, improves growth, immunity, and resistance to Vibrio harveyi in hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂). Microorganisms 12:688. doi: 10.3390/microorganisms12081688
Zhang, X., Jiang, A., An, S., Guo, C., You, F., Huang, Z., et al. (2025). Dietary resistant starch supplementation improves the fish growth, lipid metabolism and intestinal barrier in largemouth bass (Micropterus salmoides) fed high-fat diets. Int. J. Biol. Macromol. 306:141356. doi: 10.1016/j.ijbiomac.2025.141356
Zhang, J., Wang, Y., Liu, J., Xu, W., Yin, Z., Liu, Y., et al. (2023). Effects of fecal bacteria on growth, digestive capacity, antioxidant capacity, intestinal health of large yellow croaker (Larimichthys crocea) larvae. Aquaculture 562:738796. doi: 10.1016/j.aquaculture.2022.738796
Zhang, D. F., Xiong, X. L., Wang, Y. J., Gao, Y. X., Ren, Y., Wang, Q., et al. (2021). Bacillus velezensis WLYS23 strain possesses antagonistic activity against hybrid snakehead bacterial pathogens. J. Appl. Microbiol. 131, 3056–3068. doi: 10.1111/jam.15162
Zhang, Y., Zhang, B., Ahmed, I., Zhang, H., and He, Y. (2025). Profiles and natural drivers of antibiotic resistome in multiple environmental media in penguin-colonized area in Antarctica. Fundam. Res. 5, 269–281. doi: 10.1016/j.fmre.2022.11.011
Zhang, N., Zhang, H., Dong, Z., and Wang, W. (2022). Molecular identification of Nocardia seriolae and comparative analysis of spleen transcriptomes of hybrid snakehead (Channa maculata female × Channa argus male) with Nocardiosis disease. Front. Immunol. 13:778915. doi: 10.3389/fimmu.2022.778915
Zhang, F., Zhang, J., Lin, G., Chen, X., Huang, H., Xu, C., et al. (2024). Antibiotic resistance and genetic profiles of Vibrio parahaemolyticus isolated from farmed Pacific White shrimp (Litopenaeus vannamei) in Ningde regions. Microorganisms 12:152. doi: 10.3390/microorganisms12010152
Zhao, W., Dai, Z., Qiu, Y., Wang, J., He, L., Khan, T. A., et al. (2025). Isolation and identification of Streptomyces sp. D-6 and its protective effect on Crucian auratus. Microb. Pathog. 203:107491. doi: 10.1016/j.micpath.2025.107491
Zhao, C., Men, X., Dang, Y., Zhou, Y., and Ren, Y. (2023). Probiotics mediate intestinal microbiome and microbiota-derived metabolites regulating the growth and immunity of rainbow trout (Oncorhynchus mykiss). Microbiol. Spectr. 11:22. doi: 10.1128/spectrum.03980-22
Zhao, Z., Zhang, L., Zhang, G., Gao, H., Chen, X., Li, L., et al. (2023). Hydrodynamic and anthropogenic disturbances co-shape microbiota rhythmicity and community assembly within intertidal groundwater-surface water continuum. Water Res. 242:120236. doi: 10.1016/j.watres.2023.120236
Zhong, C., Du, J., Zhu, H., Gao, J., Xu, G., and Xu, P. (2025). Intestinal microbiota and gene expression alterations in Chinese mitten crab (Eriocheir sinensis) under Deltamethrin exposure. Antioxidants 14:510. doi: 10.3390/antiox14050510
Zhou, Q. J., Lu, J. F., Su, X. R., Jin, J. L., Li, S. Y., Zhou, Y., et al. (2021). Simultaneous detection of multiple bacterial and viral aquatic pathogens using a fluorogenic loop-mediated isothermal amplification-based dual-sample microfluidic chip. J. Fish Dis. 44, 401–413. doi: 10.1111/jfd.13325
Keywords: aquaculture microbiome, climate resilience, CRISPR engineering, disease resistance, fecal microbiota transplantation (FMT), multi-omics, probiotics, sustainable aquaculture
Citation: Tayyab M, Zhao Y and Zhang Y (2025) Microbiome engineering to enhance disease resistance in aquaculture: current strategies and future directions. Front. Microbiol. 16:1625265. doi: 10.3389/fmicb.2025.1625265
Edited by:
Linh Nguyen Vu, Chiang Mai University, ThailandReviewed by:
Ioannis A. Giantsis, Aristotle University of Thessaloniki, GreeceCeline Cosseau, Université de Perpignan Via Domitia, France
Sanjit Chandra Debnath, University of Exeter, United Kingdom
Kevin Mok, Kasetsart University, Thailand
Copyright © 2025 Tayyab, Zhao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yueling Zhang, emhhbmd5bEBzdHUuZWR1LmNu
 Muhammad Tayyab
Muhammad Tayyab Yongzhen Zhao2
Yongzhen Zhao2