- 1Key Laboratory of Shaanxi Administration of Traditional Chinese Medicine for TCM Compatibility, and State Key Laboratory of Research & Development of Characteristic Qin Medicine Resources (Cultivation), and Shaanxi Collaborative Innovation Center of Chinese Medicinal Resources Industrialization, and Shaanxi Traditional Chinese Medicine Processing Technology Heritage Base, Shaanxi University of Chinese Medicine, Xi’an, China
- 2College of Sports and Health, Nanjing Sport Institute, Nanjing, Jiangsu, China
- 3Hanlin College Nanjing University of Chinese Medicine, Taizhou, Jiangsu, China
Diarrhea is a common gastrointestinal disease and closely related to the balance of the gut microbiota (GM). In turn, dysregulation of the GM can affect the onset and progression of diarrhea through regulating the metabolism, intestinal immune function, intestinal barrier function and changes in the brain-gut axis of host. Although increasing evidence suggests that GM is associated with gastrointestinal homeostasis and disease, the underlying mechanisms are not fully understood. GM disorder was often accompanied by diarrhea patients and animals, and the diarrhea caused by GM imbalance mainly involved the effects on short chain fatty acids (SCFAs), bile acids (BAs), intestinal barrier, immune system, and brain-gut microbiota axis (BGMA). In addition, intervening in the GM (probiotics, fecal microbiota transplantation and bacteriophage therapy) has been shown to be an effective way to alleviate diarrhea. In this review, the mechanism of diarrhea occurrence, probiotics, fecal microbiota transplantation and bacteriophage therapy intervene in diarrhea by regulating GM from basic and clinical research were summarized and discussed. We aim to provide the latest reference for studying the mechanism of treating diarrhea from the perspective of GM, and provide data support for clinical treatment of diarrhea.
1 Introduction
Despite improvements in living conditions and widespread vaccination, diarrhea remains one of the most prevalent global health issues, resulting in approximately 1.3 million deaths annually (Collaborators G.D.D, 2017; Stockmann et al., 2017). Diarrhea is an intestinal disorder characterized by increased gastrointestinal motility, leading to elevated stool frequency and higher water content, often presenting as watery stools. It can be caused by a variety of pathogens and other factors (Brehm et al., 2020; Schiller et al., 2017). Common forms of diarrhea include infectious diarrhea (caused by bacteria, viruses, parasites, or fungi), organic-associated diarrhea, antibiotic-associated diarrhea (AAD), functional diarrhea, and diarrhea-predominant irritable bowel syndrome (IBS-D) (Wilkins and Sequoia, 2017). Although the current pharmaceuticals for diarrhea includes various agents from traditional Chinese (e.g., Shen-Ling-Bai-Zhu-San) and Western medicine (e.g., loperamide, diphenoxylate), the development of novel, safer, and more effective treatment strategies is urgently required (Ali et al., 2020; Khan et al., 2019; Wang et al., 2020).
The gut microbiota (GM) is increasingly recognized as a pivotal factor in human health, influencing nutrient absorption, immune regulation and gastrointestinal homeostasis (Paul et al., 2025). Alterations in the composition and function of the GM, often termed GM dysregulation, are closely linked to the development of gastrointestinal diseases (Quaglio et al., 2022). The onset of diarrhea is frequently accompanied by GM disturbances, aberrant metabolite levels, reduced immune function, and impaired intestinal barrier function (Anbazhagan et al., 2018; Wu et al., 2022).
For instance, patients with IBS-D exhibit an increased relative abundance of Shigella, Enterococcus, Streptococcus and Ruminococcus, alongside a decreased abundance of Faecalibacterium (Wei et al., 2020). Notably, Faecalibacterium is a dominant butyric acid-producing genus. Butyric acid serves as a crucial energy source for colonocytes and can exert anti-inflammatory, immunomodulatory, and intestinal barrier-protecting functions by inhibiting the activation of the toll-like receptor 4-myeloid differentiation factor 88-nuclear factor-κB (TLR4-MyD88-NF-κB) signaling pathway. However, a decline in Faecalibacterium abundance is frequently associated with diarrheal symptoms and intestinal inflammation (Anbazhagan et al., 2024; Karim et al., 2024). Moreover, it was found that the occurrence of IBS-D was closely related to the expression of 5-Hydroxytryptamine (5-HT) in the brain-gut microbiota axis (BGMA), and Ruminococcus can regulate the production of 5-HT through Trace Amine-Associated Receptor 1 (TAAR1) signaling mediated by phenethylamine and tryptamine, which can stimulate gastrointestinal transit and lead to diarrhea in patients with IBS-D (Shen et al., 2022; Zhai et al., 2023). Studies in AAD models have shown reduced GM richness and diversity, downregulation of the tight junction (TJ) protein zonula occluden 1 (ZO-1) in the colon, and elevated levels of pro-inflammatory cytokines including interleukin-2 (IL-2), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α) (Cui et al., 2021; Xu H. et al., 2023). Collectively, these findings highlight the pivotal role of GM in the pathogenesis of diarrhea (Gallardo et al., 2020; Mei et al., 2021).
Recently, probiotic interventions, fecal microbiota transplantation (FMT) techniques and bacteriophage therapy have demonstrated considerable potential in the treatment of diarrhea (Fujimoto and Uematsu, 2022; Lai et al., 2019; Liu M. et al., 2024). Lactiplantibacillus plantarum P9 has been shown to alleviate diarrhea by regulating the composition of GM and increasing the patient’s functional intestinal metabolites. The specific action mechanisms include increased the relative abundances of Butyricicoccus_A sp002395695 and Streptococcus thermophilus, reduced the relative abundances of Phascolarctobacterium faecium and Faecalibacterium sp., increased the content of acetic acid and butyric acid in short chain fatty acids (SCFAs), and decreased the level of deoxycholic acid (Yang et al., 2024). Additionally, studies have reported that transplantation of fecal fluid from healthy donors into AAD patients alleviated GM disorders, modulated GM composition and quantity, and lowered interleukin-8 (IL-8) and C-reactive protein (CRP). These changes thereby enhanced intestinal immune function and alleviated AAD symptoms (Wang L. et al., 2024). Furthermore, the study found that phage A221 effectively treated diarrhea caused by Escherichia coli (E. coli) GXXW-1103 in weaned piglets, increased their daily weight gain, and reduced the proportion of Enterobacteriaceae in the duodenum to 0.64%, thereby alleviating lesions in the cecum and duodenum (Mao et al., 2023). Thus, targeted modulation of the GM represents a promising therapeutic strategy for alleviating diarrhea.
Therefore, in this review, we summarize the regulatory mechanisms of GM and its metabolites in diarrhea. Specifically, we discuss the mechanisms of probiotics, FMT and bacteriophage in the treatment of diarrhea, aiming to provide insights for future research on targeted modulation of the GM as a therapeutic strategy for diarrheal diseases.
2 The pivotal role of GM in diarrhea related diseases
As a complex and diverse ecosystem, the GM colonizes the entire gastrointestinal tract in a symbiotic fashion, participating in the growth and development of the host organism while regulating the body’s immune system (Takiishi et al., 2017). The GM is crucial for maintaining host homeostasis and overall health. Its diversity and abundance have direct implications for disease pathogenesis and clinical treatment outcomes (Chen et al., 2021). Under normal physiological conditions, the species composition and proportional distribution of GM remain in a homeostatic balance, and it exerts multiple pivotal functions in the host, including modulating immune responses, mediating metabolic processes, and sustaining the homeostasis of the intestinal barrier (Jandhyala et al., 2015; Wu and Wu, 2012). In contrast, under pathological circumstances, perturbations to the intestinal microecosystem can disrupt this balance, ultimately resulting in GM dysbiosis. This dysbiosis subsequently impairs host health via a variety of mechanisms, including alterations in SCFAs metabolism (Morrison and Preston, 2016), BAs (Cai et al., 2022), intestinal barriers (Allam-Ndoul et al., 2020), the immune system (Donald and Finlay, 2023), and BGMA (Hillestad et al., 2022). Such alterations may contribute to the development of diseases such as diarrhea (Shao et al., 2020), inflammatory bowel disease (Quaglio et al., 2022), and cardiovascular diseases (Witkowski et al., 2020).
2.1 Effect on the composition of the GM
Under physiological conditions, a homeostatic GM supports key host functions including immune regulation, metabolic processes, and the maintenance of intestinal barrier integrity (Yue et al., 2019).
In healthy individuals, the GM is predominantly composed of the phyla Firmicutes and Bacteroidetes, followed by Actinobacteria and Verrucomicrobia (Hollister et al., 2014). Under normal physiological conditions, GM is in homeostasis and plays functions of immunity, metabolism and maintenance of intestinal barrier homeostasis in the body (Yue et al., 2019). However, the composition and diversity of GM is easily influenced by various factors (such as diet, drugs, pathogens, and environmental factors), further affecting human and animal health (Cryan et al., 2019; Lange et al., 2016; Zhang, 2022). Compelling evidence indicates that GM dysbiosis increases host vulnerability to a broad spectrum of pathogens and promotes the development of diverse diseases, including diarrhea, IBS, and allergies (Leong et al., 2018; Shchikota et al., 2021).
Despite improvements in living conditions and healthcare in recent years, diarrhea remains a globally prevalent issue (Wolf et al., 2022). A topic of growing interest currently is the interplay between diarrhea and the GM, which is featured by pathogen-dominated GM dysregulation, encompassing bacterial, fungal, and viral etiologies (Li et al., 2021). Invasive pathogens suppress the proliferation of commensal bacteria, thereby reducing beneficial gut microbiota and increasing pathogenic strains. This imbalance further induces intestinal dysfunction and activates immune responses, ultimately culminating in diarrhea (Czepiel et al., 2019; Jesser et al., 2023).
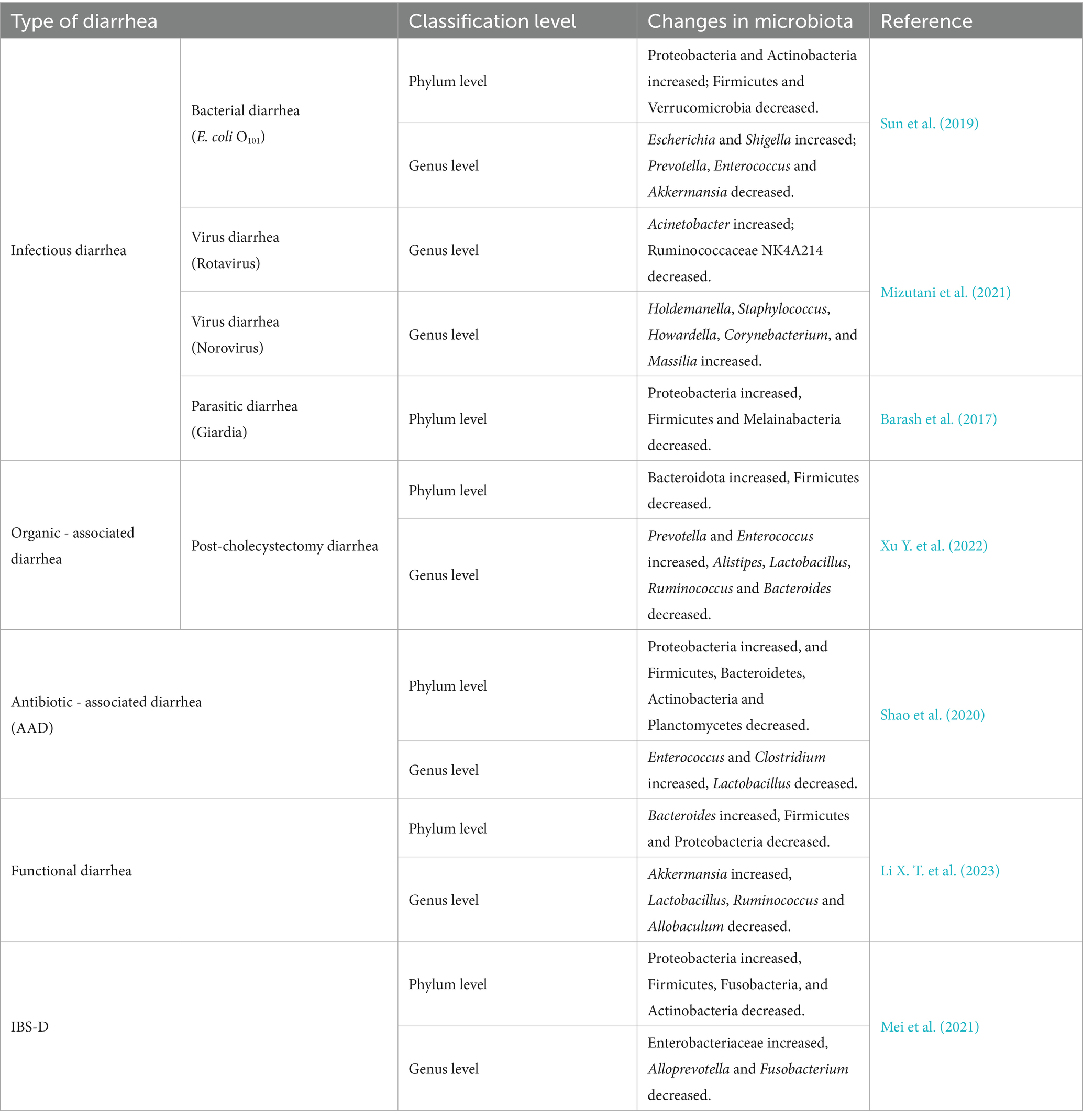
Furthermore, it has been observed that other types of diarrhea are also closely associated with alterations in the GM (Table 1). Thus, it is evident that dysbiosis of the GM exists across various forms of diarrhea.
2.2 Effect on metabolites of the GM
SCFAs, including acetate, propionate, and butyrate (Liu et al., 2021), are mainly produced by GM via anaerobic fermentation of indigestible carbohydrates and host-derived substrates (Zhang et al., 2023). SCFAs contribute to the enhancement of intestinal barrier function, exhibit anti-inflammatory effects, and participate in immunomodulation (Parada Venegas et al., 2019). Specifically, acetate and propionate serve as energy sources for peripheral tissues (den Besten et al., 2013). A recent study demonstrated that acetic acid mediates crosstalk between epithelial and immune cells and promotes T cell-dependent immunoglobulin A (IgA) production by stimulating CD4+ T cells (Takeuchi et al., 2021). Moreover, propionate has been shown to prevent the reduction of TJ proteins, such as ZO-1 and occludin, in colon tissue, and to suppress the mRNA expression of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α (Tong et al., 2016). Butyrate also exerts anti-inflammatory effects by inhibiting the secretion of IL-8, IL-6, IL-12, and TNF-α, while promoting the production of the anti-inflammatory cytokine IL-10, thereby contributing to the maintenance of the intestinal epithelial barrier (Lee et al., 2017). Additionally, they promote the growth of beneficial bacteria, improve GM composition, and help regulate host immune homeostasis (Fukuda et al., 2011; Mann et al., 2024). Moreover, a separate study demonstrated that propionic acid, secreted by Akkermansia muciniphila, binds to G-protein-coupled receptor 43 on the surface of intestinal epithelial cells. This interaction enhances histone acetylation, which in turn upregulates the expression of TJ proteins occludin and ZO-1 and increases mucin levels, ultimately improving the integrity of the intestinal epithelial barrier (He et al., 2023). In addition, another study demonstrated that, relative to healthy calves, calves with diarrhea induced by bovine rotavirus (BRV) exhibited significantly reduced concentrations of total SCFAs, acetic acid, propionic acid, and isocaproic acid; in contrast, only propionic acid concentrations were markedly decreased in calves with diarrhea caused by bovine coronavirus. Notably, the depletion of Parabacteroides and Ruminococcus was strongly associated with reduced acetic acid levels, while declines in isocaproic acid content were closely linked to the loss of Parabacteroides, Ruminococcus, Fournierella, and Rikenellaceae_RC9_gut_group. Furthermore, a significant reduction in propionic acid concentrations showed a positive correlation with the depletion of Collinsella (Cui et al., 2023). Both propionic and butyric acids are believed to enhance the integrity of epithelial cells, with butyric acid serving as the primary energy source for colonocytes (Furusawa et al., 2013; Tong et al., 2016). Reduced butyrate levels have been shown to elevate intestinal oxygenation, which not only drives gut microbial dysbiosis and promotes the expansion of aerobic pathogens but also disrupts intestinal homeostasis (Handa et al., 2023). SCFAs are absorbed by epithelial cells, which in turn stimulates Na+-dependent absorption of water and electrolytes, thereby mitigating diarrhea symptoms (Binder, 2010). Several studies have indicated that ADD-type mice exhibit reduced levels of SCFAs (Min et al., 2024; Yang L. et al., 2021; Zhan et al., 2023). Furthermore, piglets infected with E. coli developed diarrhea and exhibited decreased levels of SCFAs in their feces (Liu et al., 2019).
Bile acids (BAs) serve as essential signaling molecules that significantly regulate glucose homeostasis, lipid metabolism, and energy expenditure (Sah et al., 2022; Yu et al., 2023). They consist of primary bile acids (PBAs), which are synthesized by the liver, and secondary bile acids (SBAs), which are metabolized by the GM. Among them, PBAs include chenodeoxycholic acid (CDCA) and cholic acid (CA), while SBAs comprise lithocholic acid (LCA) and deoxycholic acid (DCA). Nearly 95% of luminal BAs are reabsorbed in the distal ileum, while the remainder undergoes microbial modification by the GM prior to excretion or passive absorption. In humans, the GM is instrumental in the generation of SBAs via a series of enzymatic reactions, including deconjugation, 7α-dehydroxylation, oxidation, epimerization, desulfation, and esterification. Of these, deconjugation and 7α-dehydroxylation are the most physiologically significant processes (Ridlon et al., 2016; Wahlström et al., 2017). When intestinal homeostasis is disrupted, dysbiosis of the GM affects BAs metabolism and ultimately alters the host response. In IBS-D, excessive fecal BAs are considered a contributing factor to pathogenesis, and there are higher levels of PBAs in fecal samples of IBS-D patients compared to healthy subjects (Duboc et al., 2012; Wei et al., 2021; Wei et al., 2020). However, research has indicated that a microbiota rich in Clostridia can promote BAs excretion in IBS-D patients (Zhao et al., 2020). It is well established that BAs modulate intestinal mucosal permeability and participate in inflammatory responses. Specifically, CDCA and DCA exert their effects by promoting epidermal growth factor receptor (EGFR) autophosphorylation and occludin dephosphorylation, leading to the reorganization of occludin within TJs and a consequent increase in paracellular permeability (Raimondi et al., 2008). Additionally, CDCA contributes to pro-inflammatory responses by stimulating the release of IL-8 and reactive oxygen species (ROS), as well as amplifying the effects of TNF-α and IL-1β on interferon-γ (IFN-γ) production (Sarathy et al., 2017). Ursodeoxycholic Acid (UDCA) has been shown to reduce the production of inflammatory cytokines by participating in the BA receptor Farnesoid X Receptor (FXR), while inhibiting NF-κB activation in macrophages (Pi et al., 2023). BAs metabolites were found to be excessive in the feces of Primary Sclerosing Cholangitis (PCD) patients and PCD mice, and SBAs [DCA, LCA and Hyodeoxycholic Acid (HDCA)] were found to be associated with the onset of diarrhea. These SBAs shortened the gastrointestinal transit time by 0.6-fold, increased the fecal water content by 1.3-fold and stimulated 5-HT levels in vitro and in vivo (Xu Y. et al., 2023). However, blocking BAs conjugated Takeda G protein-coupled receptor 5/Transient Receptor Potential Ankyrin 1 (TGR5/TRPA1) receptors significantly alleviated PCD GM-induced diarrhea. The present study demonstrates that GM and BA metabolism play a role in diarrhea. These results offer promising biomarkers for diagnosing and treating diarrhea and lay the groundwork for further investigation.
Branched chain amino acids (BCAAs), which include leucine (Leu), isoleucine (Ile), and valine (Val), are essential amino acids for the human body (Peng et al., 2020). These amino acids exert direct or indirect effects on diverse physiological functions, including energy metabolism, protein synthesis, and immune responses (Ma et al., 2018; Stipanuk, 2007). Similarly, BCAAs function as modulators that promote intestinal development and enhance gut health (Ren et al., 2016; Ren et al., 2015). Currently, the majority of research has concentrated on the function of Leu, with less attention paid to Val and Ile in the gut. Leu can maintain intestinal health by enhancing TJ in fish (Jiang et al., 2015). Additionally, it has been shown to improve intestinal epithelial cell proliferation, increase villus height, and promote growth in the small intestine of pigs. However, intestinal growth was inhibited when Leu levels were as high as 2.57% (Ren et al., 2015). Dietary Ile improves intestinal immune function and microbial population, and regulates gene expression of antioxidant enzymes, TJ, Nuclear factor erythroid 2-related factor 2 (Nrf2), Kelch-like ECH-associated protein 1 (Keap1), p38, and Extracellular Signal-regulated Kinase 1 (ERK1) in the intestine of Jian carp (Zhao et al., 2014). Additionally, BCAAs have been significantly linked to diarrhea; a decreasing trend in BCAA levels was observed in both the functional diarrhea group and the IBS-D group (James et al., 2023). Rotavirus infection induces diarrhea in weaned pigs via systemic protein metabolic disorders and jejunal mucosal dysfunction. However, dietary supplementation with 1% leucine alleviated rotavirus-induced diarrhea in weaned pigs, potentially due to leucine’s roles in enhancing protein metabolism, improving intestinal digestive and absorptive capacities, and reinforcing the non-specific barrier function of the intestinal mucosa (Mao et al., 2015). Furthermore, L-isoleucine supplementation has been found to significantly reduce stool output and fluid intake in children suffering from non-cholera acute watery diarrhea (Alam et al., 2011). More recently, a study demonstrated that supplementation with Ile increased the relative abundance of Prevotella and decreased the relative abundance of Rikenellaceae in the colon of diarrhea piglets infected with rotavirus, increased the secretion of interleukin-4 (IL-4), IL-10, and Secretory Immunoglobulin A (sIgA), and increased the expression of Claudin-3, Occludin, ZO-1 and mucin 1 (MUC-1), improved the immunity, colon barrier function and colon GM of piglets with diarrhea (Jiang C. et al., 2024; Jiang C. Y. et al., 2024). Nevertheless, the existing literature displays a striking imbalance toward Leu, leaving the mechanisms and efficacy of Val and Ile underexplored. Future studies should thus prioritize elucidating the individualized and synergistic contributions of all three BCAAs—particularly Val and Ile—ac different physiological and pathological contexts, to enable more precise and effective nutritional strategies for intestinal health.
2.3 Effect on intestinal barrier function
The intestinal barrier is a complex physiological structure that serves as a physical, biological, chemical, and immunological barrier. It interacts with the external environment and regulates host health (Zhou et al., 2024). The intestinal barrier, being semi-permeable, serves a dual function: it safeguards the internal milieu against the potential translocation of pathological molecules and microorganisms, while facilitating the absorption of nutrients and water (Martini et al., 2017). However, in pathological conditions, the integrity of the intestinal barrier can be compromised, leading to many local and systemic diseases (Aleman et al., 2023; Wang et al., 2022). TJs serve as a crucial form of connection between intestinal epithelial cells.
The proteins ZO-1 and occludin are key structural components of TJs. They are essential for maintaining cellular morphology and TJ structural integrity, and are widely used as indicators for assessing intestinal barrier function (Al-Sadi et al., 2011; Haas et al., 2022). Mucins are the primary glycoproteins that constitute the intestinal mucosal barrier. Among them, Mucin 2 (MUC2) is the most secreted mucin in the gastrointestinal tract and maintains the integrity of the mucus barrier, which is closely related to GM homeostasis (Liu et al., 2023; Yao et al., 2021). Tropini et al. demonstrated that diarrhea is closely related to the GM and the intestinal mucus barrier (Tropini et al., 2018). Diarrhea significantly disrupts the GM and is associated with thinning or loss of the intestinal mucus layer. This effect may be linked to dysregulated expression of the tight junction proteins ZO-1 and occludin, which compromises intestinal barrier integrity and increases permeability (Chen H. R. et al., 2024; Tropini et al., 2018).
Impaired intestinal mucosal barrier function serves as the primary pathological basis for the development of IBS-D (Shi et al., 2023). Upon the onset of IBS-D, patients exhibit a significant reduction in the expression of occludin, ZO-1, and other epithelial tight junction proteins, resulting in compromised intestinal epithelial barrier integrity and elevated intestinal permeability (Guo et al., 2023; Wang L. et al., 2023). Recent studies have found that Lactobacillus promotes occludin and ZO-1 expression and improves diarrheal symptoms (Hou et al., 2020). Related studies have further demonstrated that in diarrheic piglets infected with E. coli, increased abundances of Lactobacillus and Cyanobacterium are associated with reduced intestinal permeability and enhanced barrier repair, with Lactobacillus showing a particularly strong correlation with key intestinal barrier markers (Luo et al., 2022). Xu et al. found that MUC2 is a crucial protein in the prevention and treatment of rotavirus infections and diarrhea. It functions by safeguarding the epithelial barrier and enhancing intestinal permeability resistance (Xu et al., 2016). Wang et al. further observed that elevated MUC2 content in the ileum of diarrheal rats enhances intestinal barrier defense and confers intestinal protection (Wang et al., 2019). Furthermore, MUC2 concentration was significantly lower in AAD mice than in normal mice. A significant negative correlation was also identified between MUC2 and two gut microbial taxa, Prevotellaceae_NK3B31_group and Rothia (Li C. et al., 2023; Li X. T. et al., 2023). Collectively, these findings demonstrate a close association between diarrhea development, GM composition, and intestinal barrier function.
2.4 Effects on intestinal immune function
The GM intricately interacts with the host immune system. The crosstalk between the GM and enterocytes plays a crucial role in shaping the intestinal environment, thereby profoundly influencing intestinal immune homeostasis (Hold, 2016). Different types of diarrhea induce alterations in GM composition, which in turn modulates the expression of inflammatory factors. For example, in patients with diarrhea-predominant IBS-D, levels of IL-8 and TNF-α are elevated, while IL-10 is reduced; in mice with AAD, GM dysbiosis is observed, characterized by a marked increase in Proteobacteria and decreases in Bacteroidetes and Firmicutes, accompanied by upregulated IL-1 and IL-6 levels (Chen et al., 2022; Zhen et al., 2015; Zhu et al., 2022). It was observed that E. coli O1 caused diarrhea in calves with disturbances in the GM and an increased abundance of Proteobacteria and Clostridiales. This condition was accompanied by a decreased expression of CD4+ T and an elevated expression of Cluster of Differentiation 8 Positive T Lymphocyte (CD8+ T) and CD11c-positive T lymphocyte (CD11c+ T) in the ileum. Additionally, there were reduced serum levels of IgA and Immunoglobulin G (IgG), alongside heightened levels of IL-6 and TNF-α (Chen H. et al., 2023). T helper cell 17 (Th17) contribute to the maintenance of host intestinal immune homeostasis through interleukin-17A (IL-17A)-induced expression of the epithelial polymeric immunoglobulin receptor (Cao et al., 2012). In colonic tissues of IBS-D mice, the Th17/Tregs ratio was found to be significantly altered, characterized by reduced Tregs and IL-10+Foxp3+T cells alongside increased Th17 cells. Correlation analysis further revealed positive associations between Ruminococcus_gnavus with the Th17/Tregs ratio (Zhang M. M. et al., 2024; Zhang Y. et al., 2024; Zhang Z. et al., 2024).
It is thus clear that GM plays an important role in regulating intestinal immune homeostasis during diarrhea.
2.5 Effects on BGMA
In recent years, this concept has expanded to include BGMA, prompted by the growing recognition of the gut microbiota’s critical role in human health and disease (Lee et al., 2023). The BGMA acts as a bidirectional communication pathway between the central nervous system (CNS) and the gastrointestinal tract. It mediates interactions involving the CNS, enteric nervous system (ENS), neuroendocrine system, and immune system, with signals being transmitted either directly or indirectly between the CNS and ENS (Arneth, 2018; Grenham et al., 2011; Morais et al., 2021).
Notably, microbial metabolic activity profoundly shapes brain–gut signaling. For example, disruptions in tryptophan metabolism impact the synthesis of serotonin (5-HT), a key intermediate, which can activate brain–gut neural circuits and precipitate diarrheal responses (Morais et al., 2021; Spencer and Hu, 2020). Furthermore, central processes modulate gut function via the hypothalamic–pituitary–adrenal (HPA) axis: psychological stress triggers cortisol release, altering intestinal permeability and compounding gut dysfunction (Chen H. et al., 2023; Chen X. et al., 2023; Chen J. et al., 2023; Morais et al., 2021). Evidence underscores a robust association between functional diarrhea and impairment of the BGMA. Notably higher rates of this condition occur in patients with mental disorders, with GM-CNS crosstalk serving as a potential mediator of this comorbidity (Zhang et al., 2021). The pathogenesis of IBS-D involves multifaceted interactions among brain–gut peptides, immune activation, and microbial composition (Li et al., 2020). For instance, Gao et al. demonstrated that dampening HPA axis activity via CRHR1 downregulation alleviates diarrheal symptoms in IBS models, underscoring the therapeutic relevance of BGMA modulation (Gao et al., 2023). Wu et al. provided further mechanistic insight, identifying correlations between specific microbial genera and neuro-immune markers in IBS-D rats. The genus Paraprevotella was positively associated with elevated 5-HT, CRF, and NPY, suggesting its potential role in modulating the HPA axis via serotonergic pathways (Wu et al., 2022). Additionally, microbial metabolites such as SCFAs and 5-HT are implicated in bidirectional BGMA communication, and their aberrant levels have been consistently reported in IBS-D patients (Dinan and Cryan, 2017; Luo et al., 2021). Interventions including probiotic supplementation have shown promise in reducing 5-HT levels and ameliorating IBS-D symptoms, highlighting the translational potential of targeting microbial components (Gu et al., 2022; Wu et al., 2024). Another compelling example comes from Chen et al. reported that alkaline mineral complex (AMC) water improved diarrhea resistance in stressed piglets by rebalancing the HPA axis and enriching beneficial bacteria such as Lactobacillus helveticus and Ruminococcus gnavus. This reinforces the notion that BGMA-oriented interventions can restore gut homeostasis through multifactorial mechanisms (Chen H. et al., 2023; Chen X. et al., 2023; Chen J. et al., 2023).
Overall, research on the BGMA provides critical insights into the mechanisms underlying diarrhea and reveals promising therapeutic potential. Current evidence suggests that targeting the BGMA—through modulation of microbial metabolites, neuroendocrine pathways, and immune signaling—may alleviate both intestinal and psychiatric symptoms. However, most studies to date remain correlative or reliant on animal models, highlighting a need for causal validation and clinical translation. Future work should integrate multi-omics approaches to elucidate precise molecular targets within the BGMA, ultimately facilitating the development of personalized therapies and bridging the gap between mechanistic discovery and clinical application.
In summary, the occurrence of diarrhea can alter the composition of the gut microbiota and the levels of its metabolites, regulate immune function, affect the gut-brain axis, and impair intestinal barrier integrity. The potential mechanisms mediating these effects are illustrated in Figure 1.
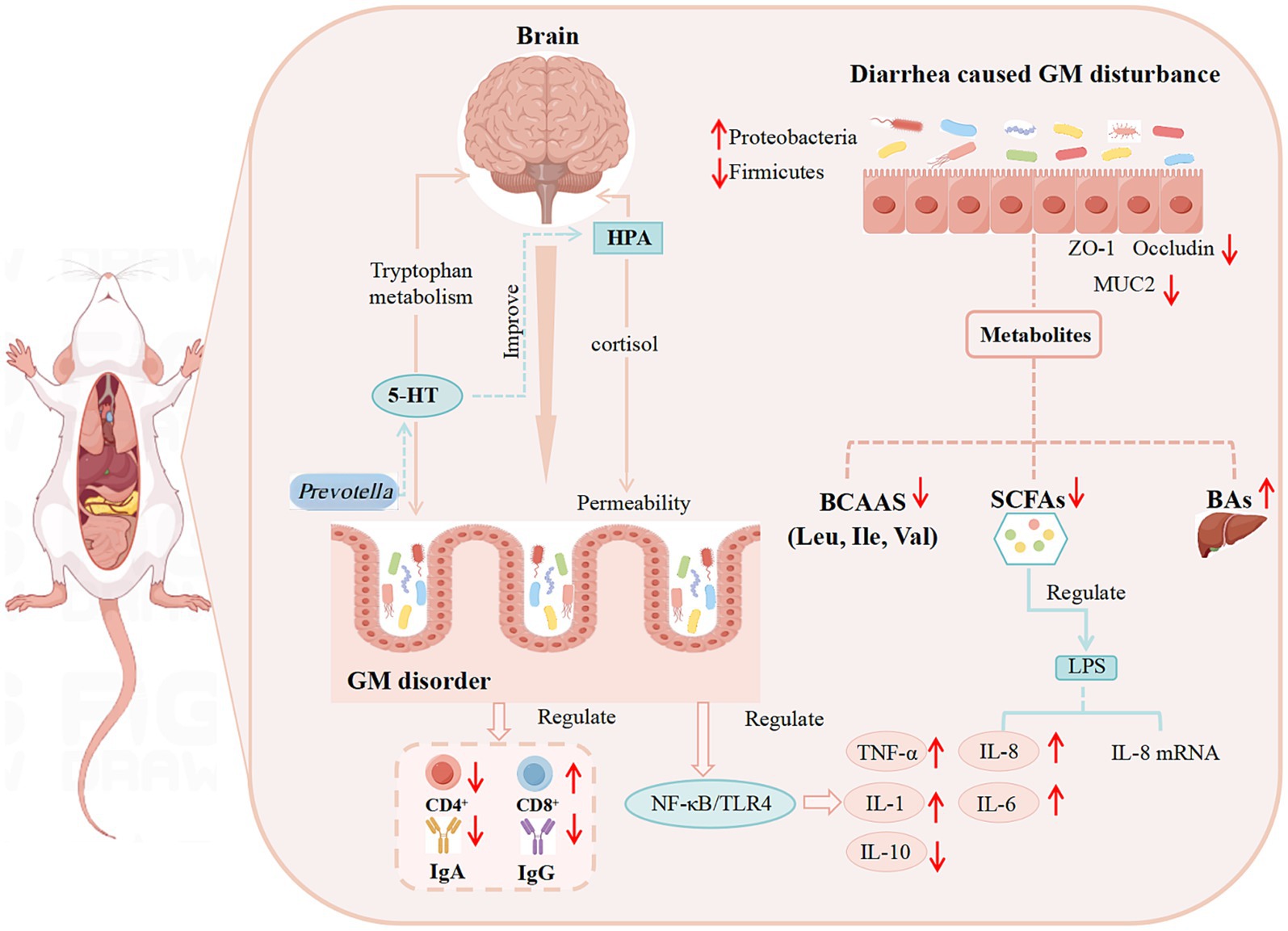
Figure 1. The potential mechanism of diarrhea development. (The relationship between diarrhea and GM, GM metabolites, the immune system, the intestinal barrier, and BGMA).
3 Impact of interventions targeting GM on diarrhea
3.1 Probiotic interventions
Probiotics are defined as beneficial, viable microorganisms, and a growing body of evidence has shown that numerous probiotic strains alleviate diarrhea by modulating the GM (Wieërs et al., 2019), regulation of inflammatory factor production (Wang F. et al., 2023), and enhancement of the intestinal mucosal barrier function (Camilleri, 2021; Su et al., 2022).
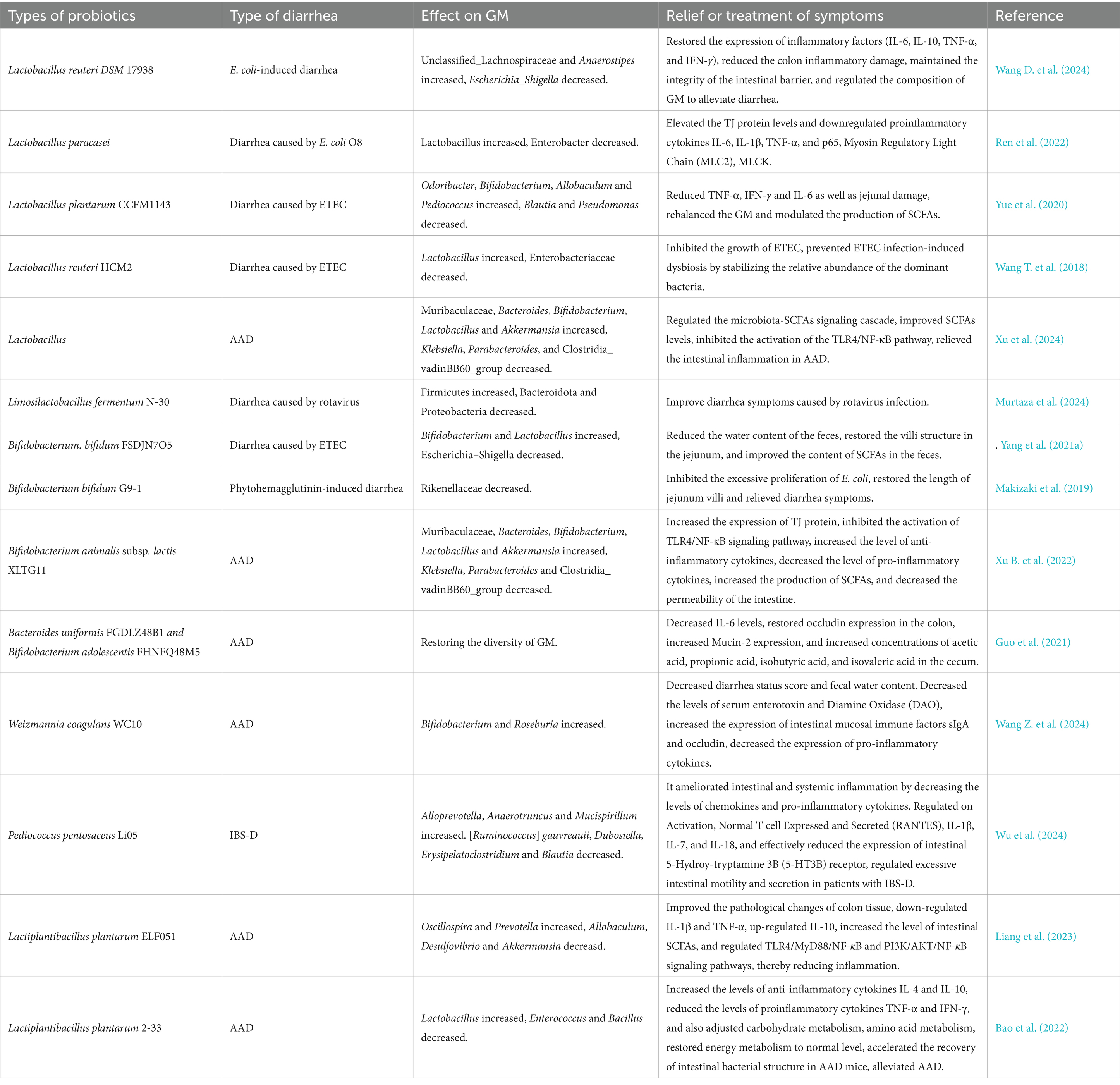
Lactobacillus and Bifidobacterium are widely employed as probiotics in the treatment of diarrhea, owing to their crucial functions in alleviating inflammation and promoting the balance of the intestinal microbiota. Lactoferricin produced by Lactobacillus reuteri CO21 was found to be able to modulate the intestinal physical barrier function by inhibiting the TLR4, Myd88 and Myosin light-chain kinase (MLCK) pathways and up-regulating the expression of the TJ proteins ZO-1 and claudin-2, thereby increasing piglets’ resistance to Enterotoxigenic E. coli and alleviating diarrhea (Xie et al., 2021). Lactobacillus and Saccharomyces boulardii have also shown effectiveness in the prevention or treatment of AAD (Doar and Samuthiram, 2023; Yang Y. et al., 2023; Yang Q. et al., 2023). Saccharomyces boulardii alleviates GM disorders and improves intestinal barrier function (Bustos Fernández et al., 2023). Saccharomyces boulardii mitigates mucosal injury by modulating intestinal mucin composition and secretion, strengthening the mucin barrier, and reducing SN-38 penetration into epithelial cells (Sezer et al., 2009).
In conclusion, probiotics exert beneficial effects on both the prevention and treatment of diarrhea, with such effects being strain-and dose-dependent. Thus, further studies are required to identify and optimize the selection and application of probiotics for managing different types of diarrhea.
3.1.1 Basic experiments
During animal development, exposure to various pathogenic bacteria and toxic compounds often leads to intestinal barrier dysfunction, thereby contributing to the onset of diarrhea and impaired growth (Kovanda et al., 2023; Satitsri et al., 2016). However, probiotics can modulate intestinal barrier function, alleviate intestinal injury, and mitigate diarrhea.
ETEC is a major pathogen of animal diarrhea (Xu C. et al., 2023; Xu Y. et al., 2023; Xu H. et al., 2023), which disrupts the intestinal epithelial barrier through adhesins and enterotoxins (Zhu et al., 2018). It has been found that Lactobacillus plantarum ZLP001 has antimicrobial activity, which prevents ETEC growth by producing certain antimicrobial substances and generating a relatively acidic environment (Wang et al., 2018b). Treatment with L. plantarum ZLP001 alleviated ETEC-induced intestinal damage by preserving the expression of TJ proteins (claudin-1, occludin, ZO-1), downregulating pro-inflammatory cytokines (IL-6, IL-8, TNF-α), and strengthening the intestinal barrier via enhancing epithelial defense and modulating the GM (Wang et al., 2018a). AAD triggered by GM dysbiosis post-antibiotic therapy, poses serious threat to human and animal health. However, Lactobacillus plantarum H-6 was found to modulate the colonic microbial composition in mice by increasing the abundance of Lactobacillus and Akkermansia, decreasing that of Bacteroides, downregulating the expression of pro-inflammatory factors (e.g., IL-1β, IL-6), and elevating the levels of L-tryptophan and LysoPC. These changes improve serum metabolism, thereby alleviating AAD (Yan et al., 2023). In addition, Akkermansia muciniphila was able to reduce the relative abundance of Citrobacter at the genus level, inhibit intestinal inflammation by up-regulating the expression of G protein-Coupled Receptor 109A (GPR109A) and Solute Carrier family 5 member 8 (SLC5A8) and down-regulating the expression of TNF-α, IFN-γ, IL-1β, and IL-6, and at the same time improve the down-regulation of ZO-1, Occludin, Claudin-4 (CLDN4), and Muc2 in AAD model mice, restore the intestinal barrier function and optimize intestinal health to prevent AAD (S. Liu et al., 2024). Saccharomyces boulardii can upregulate Serotonin Transporter (SERT) through activation of epidermal growth factor receptor and modulate GM to inhibit gut motility to alleviate IBS-D symptoms (Gu et al., 2022). The efficacy of other probiotics in modulating the GM for diarrhea treatment is summarized in Table 2, while the potential mechanisms underlying probiotic-mediated diarrhea management are illustrated in Figure 2.
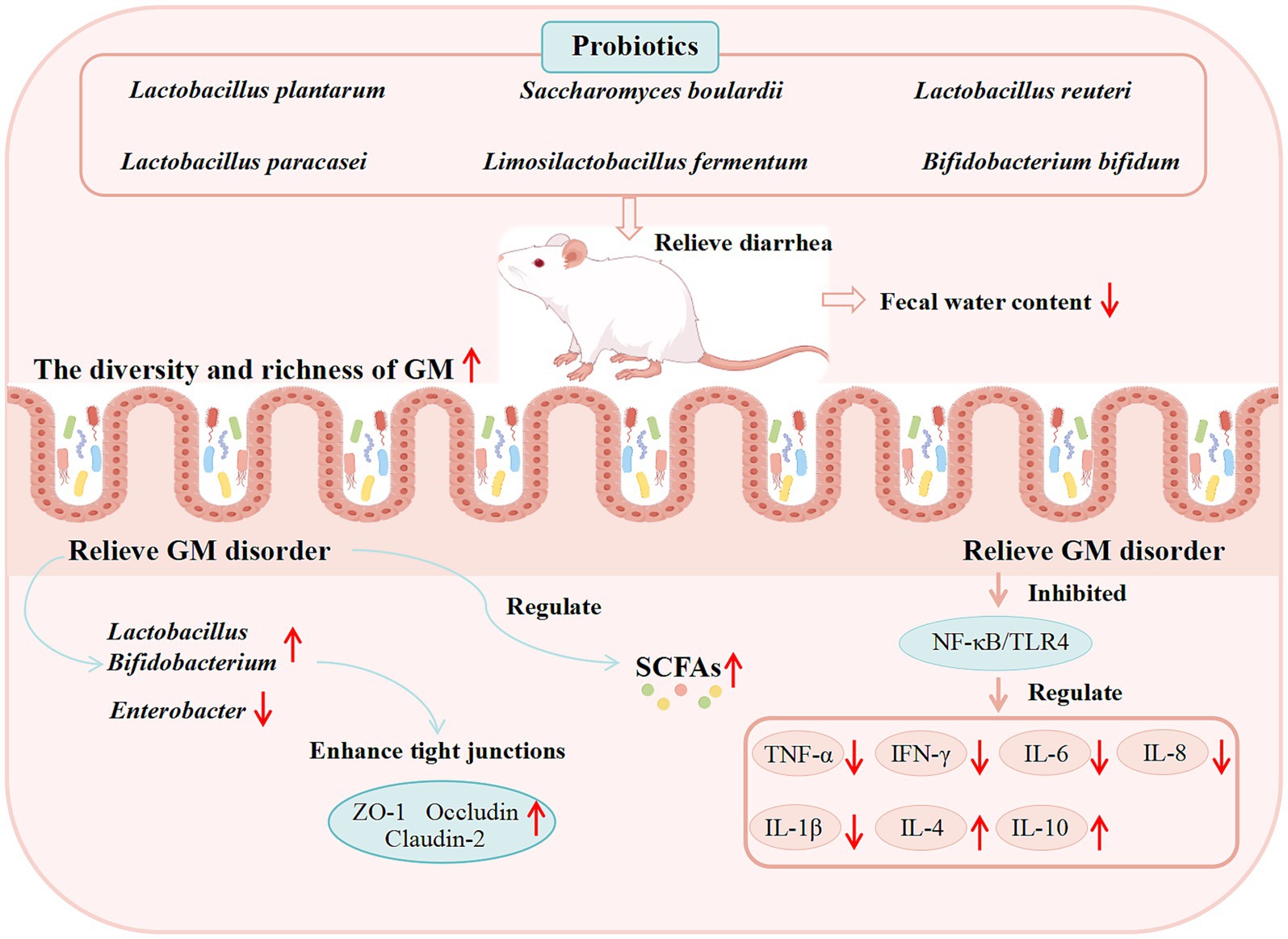
Figure 2. Proposed mechanisms of action of probiotics against diarrhea derived from basic studies. (These potential mechanisms mainly involve the GM, SCFAs, the immune system, and intestinal barrier function).
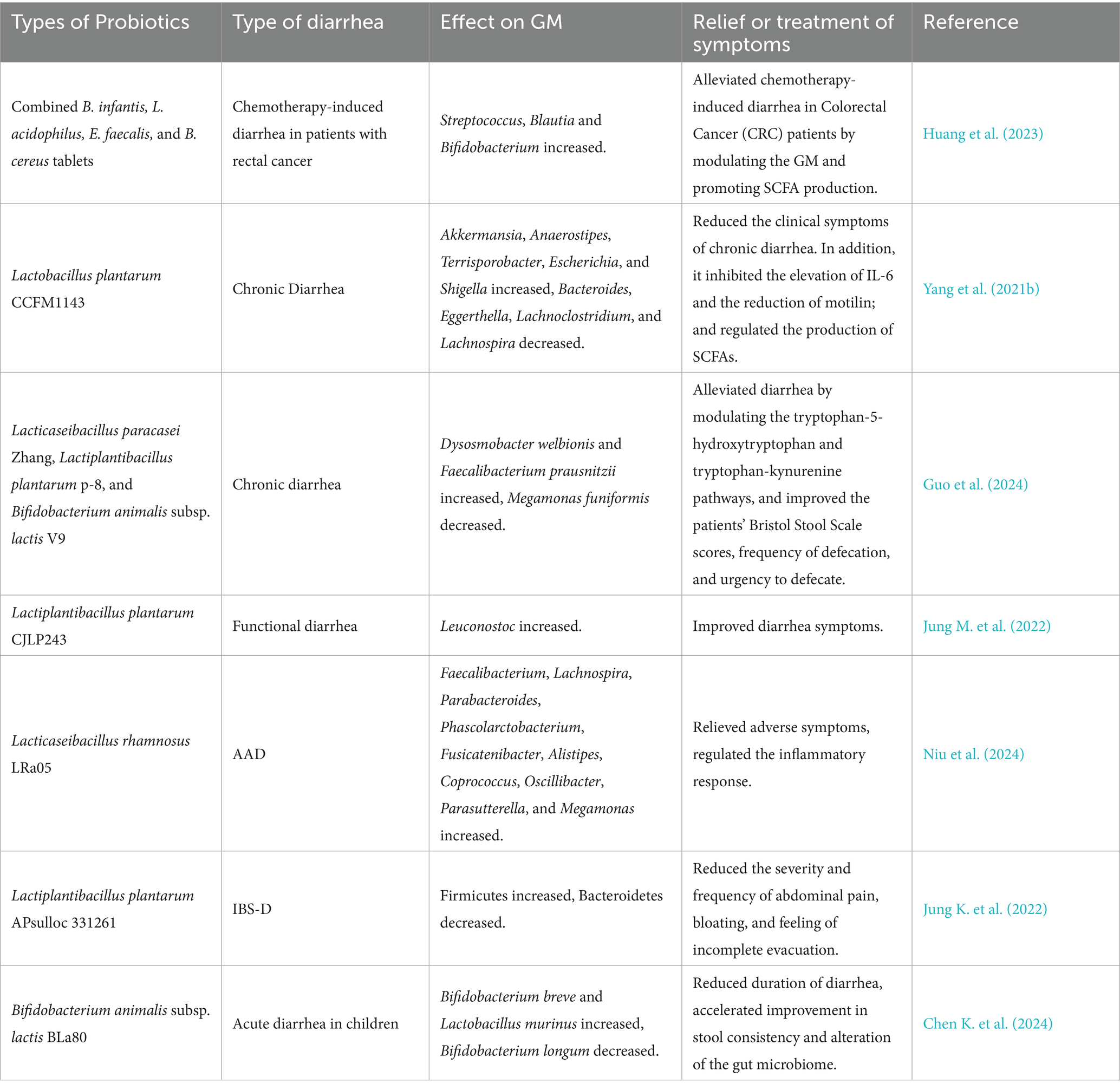
3.1.2 Clinical experiments
Clinical studies have extensively documented the efficacy of probiotics in managing diarrhea. Bifidobacterium bifidum G9-1 was reported to reduce serum pro-inflammatory cytokine (Monocyte Chemoattractant Protein-1, IL-8 and Macrophage Inflammatory Protein-1β) levels and increase the abundance of Bifidobacterium, alleviating diarrhea in IBS-D patients (Tomita et al., 2023). Similarly, longum ES1 significantly lowered serum IL-6 and TNF-α levels in IBS-D patients compared to baseline (Caviglia et al., 2020). Clostridium butyricum ameliorated diarrhea in IBS-D patients by decreasing stool frequency and modulating GM composition (Sun et al., 2018). Lactobacillus plantarum LRCC5310 improved diarrhea and Vesikari scores in rotavirus-infected children while suppressing viral proliferation (Shin et al., 2020). Alkalihalobacillus clausii 088AE exerts a therapeutic effect on diarrhea in children, adolescents, and adults. Specifically, in the treatment of AAD, it has been demonstrated to be safe and effective in reducing diarrhea episodes and alleviating associated severe symptoms, such as abdominal discomfort, pain, bloating, and flatulence (Maity and Gupta, 2021). However, supplementation with Bifidobacterium breve BB05 can partially restore the disrupted GM at both the phylum and genus levels, notably by elevating the abundances of Bifidobacterium and Roseburia. In the probiotic-supplemented group, fecal 5-HT concentration was increased, whereas levels of acetylcholine, epinephrine, and norepinephrine were reduced—suggesting that Bifidobacterium breve BB05 may alleviate anxiety and diarrhea by BGMA (Wang Y. et al., 2024).
Most existing literature has only summarized the therapeutic effects of probiotics on clinical diarrhea (focusing on diarrhea alleviation) and their safety profiles in patients, with relatively few studies investigating their specific efficacy and underlying mechanisms of action. Supplementary details regarding the clinical application of probiotics for diarrhea treatment and their corresponding mechanisms are provided in Table 3, while the potential mechanisms through which probiotics may exert anti-diarrheal effects in clinical settings are illustrated in Figure 3.
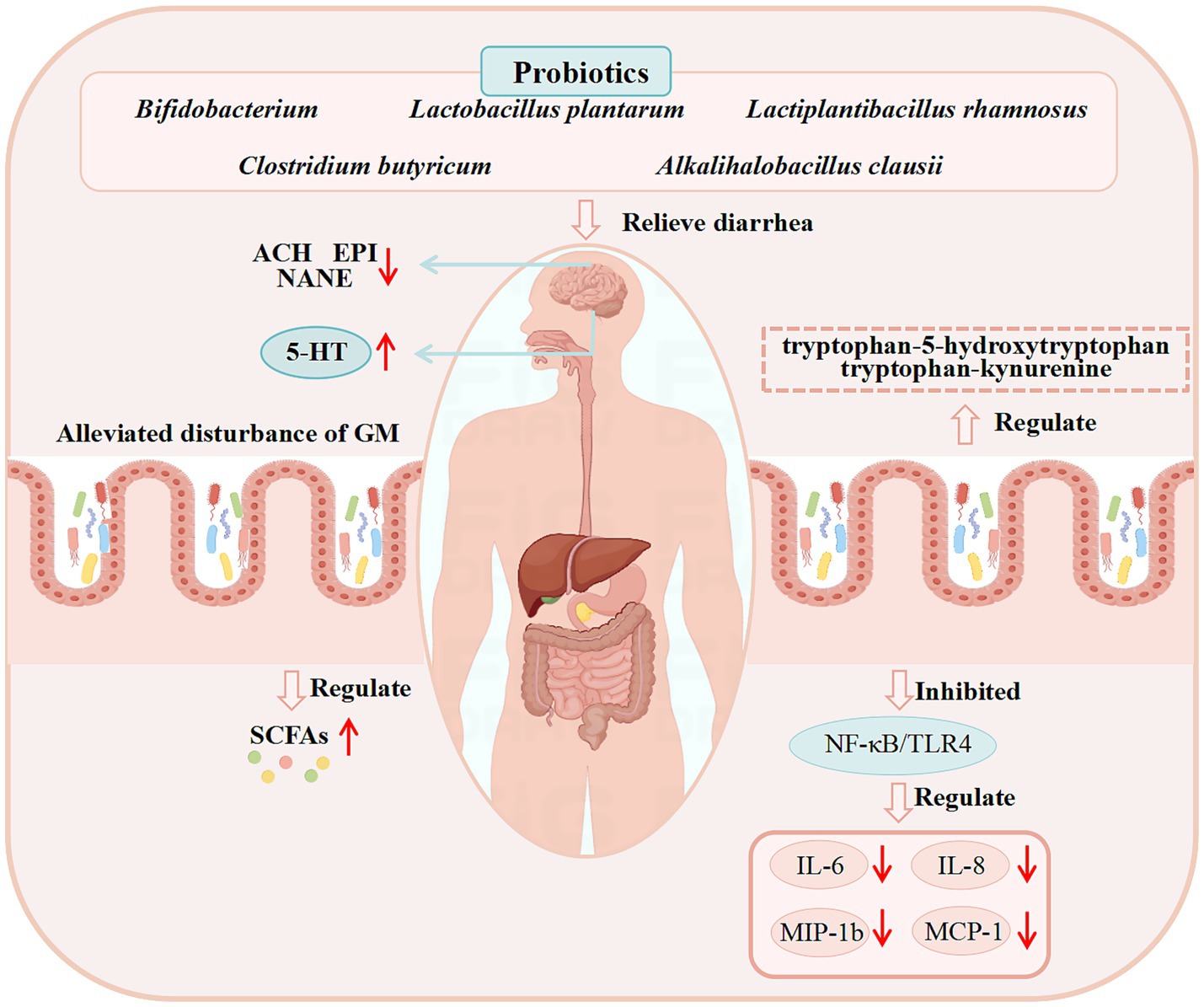
Figure 3. Demonstrated mechanisms of probiotics against diarrhea from clinical studies. (These potential mechanisms mainly involve GM, SCFAs, the immune system, and BGMA).
3.2 FMT
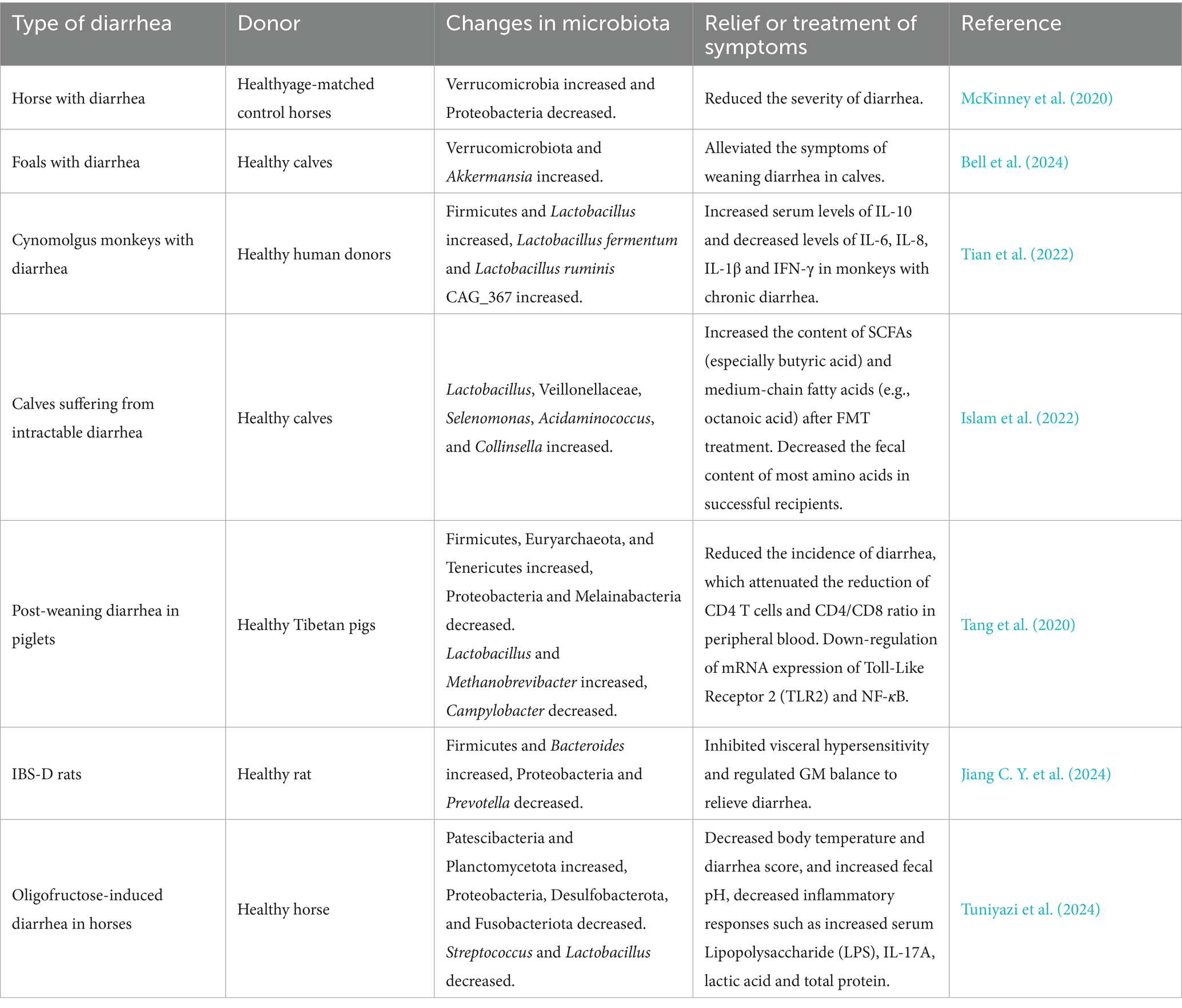
FMT is a procedure that involves transferring GM from healthy donors into the gastrointestinal tract of patients to restore a balanced microbial community and treat diseases, particularly those associated with gut dysbiosis such as diarrhea (Almeida et al., 2022; Li et al., 2022).
The therapeutic efficacy of FMT in diarrhea alleviation is closely linked to the modulation of GM composition. On one hand, FMT reintroduces a healthy microbial community that competes for ecological niches in the gastrointestinal tract, thereby suppressing pathogen colonization-this process further facilitates the restoration of immune function and mitigates host tissue damage. On the other hand, FMT aids in replenishing essential metabolites for host metabolism, such as SCFAs, antimicrobial peptides, bacteriocins, and BAs (Ademe, 2020). IBS-D is a common gastrointestinal disorder and is characterized by altered GM, especially involving Firmicutes and Bacteroidetes (Mei et al., 2021; Zhen et al., 2021). However, FMT can reduce intestinal permeability and alleviate the diarrheal effects of IBS-D by modulating GM disorders and affecting GM-produced metabolites such as increasing the production of SCFAs (Lin et al., 2021; Singh et al., 2022; Song et al., 2023). Clostridium difficile infection (CDI) is considered a common cause of AAD (Bosnjak et al., 2023; Tubau-Juni et al., 2023). One study demonstrated that FMT administration to children with recurrent CDI enhanced GM diversity while driving shifts in GM composition and function toward those of the donor (Fareed et al., 2018). During the weaning transition, piglets are prone to diarrhea, which is related to the damaged state of the microbiome and immature immune system (Han et al., 2024). In diarrheal piglets infected with E. coli K88, the application of FMT increased the number of beneficial bacteria in the gut and reduced the number of harmful bacteria, and further research found that FMT triggered intestinal mucosal autophagy and reduced the damage of E. coli K88 to the intestinal barrier (Cheng et al., 2018).
Currently, research on the underlying mechanisms of FMT remains limited. Available evidence suggests that alterations in the GM following FMT play a significant role in the pathogenesis of diarrhea. However, several studies indicate that the therapeutic efficacy of FMT may be constrained. Additionally, to date, FMT has been predominantly investigated for the treatment of CDI and diarrhea-predominant IBS-D, while its efficacy in diarrhea of other etiologies remains less established. Thus, further clinical trials are warranted to validate the potential benefits of FMT across diverse forms of diarrhea and to better define its role in managing diarrhea-related disorders. It is also important to note that the limited efficacy of FMT in some cases of diarrhea may be attributable to insufficient donor-recipient matching, among other factors, highlighting the need for more personalized approaches in future studies.
3.2.1 Basic experiment
Diarrhea in animals, triggered by multiple etiologies, is highly prevalent and remains a major challenge afflicting the animal husbandry industry. To address this issue, FMT-an emerging therapeutic technology-has been increasingly applied to the treatment of animal diarrhea in recent years.
Advances in modern genetics and enhanced sow reproductive performance have facilitated the widespread implementation of artificial lactation systems in commercial swine production. However, these systems are linked to a high incidence of diarrhea in piglets. To tackle this challenge, researchers have utilized FMT as a therapeutic strategy to alleviate diarrhea induced by artificial feeding. Results demonstrated that FMT modulates the composition of colonic microbiota and its metabolites, promotes tryptophan metabolism and 5-hydroxyindoleacetic acid (5-HIAA) production, enhances intestinal mucosal barrier function, inhibits the activation of the Jun N-terminal kinase (JNK) pathway and the expression of matrix metalloproteinases (MMPs), reduces the secretion of proinflammatory cytokines and chemokines, and ultimately alleviates artificial feeding-induced diarrhea in piglets (Han, 2023). Bovine viral diarrhea virus (BVDV) is widespread throughout the world and has caused significant economic losses to animal husbandry (Pang et al., 2023). BVDV infection significantly decreased the diversity and changed the composition of GM in mice. However, after FMT, BVDV RNA and protein levels in duodenum, jejunum, spleen and liver were significantly inhibited, Interferon-α (IFN-α) and Interferon-β (IFN-β) mRNA levels were increased, and Interferon Regulatory Factor 1 (IRF1) and Interferon Regulatory Factor 7 (IRF7) mRNA levels were increased. The expression of Toll-Like Receptor 7 (TLR7) and Toll-Like Receptor 9 (TLR9) was restored, the proportion of Cluster of Differentiation 3 (CD3) and CD8 T cells was restored, the expression of ZO-1 protein was increased, and the proliferation of Peripheral Blood Leukocytes (PBL) was restored (Zhang Z. et al., 2024). FMT significantly alleviates symptoms of IBS-D, potentially through modulating the 5-HT signaling pathway within the BGMA. It was found that after FMT, the mental condition of IBS-D mice was improved, the diarrhea was improved, and the fecal water content was significantly reduced. Additionally, the expression levels of 5-HT and SP in brain tissue and serum were significantly decreased, the expression levels of SERT and 5-Hydroxytyryptamine Receptor 4 (5-HT4R) proteins in colon and brain tissues were increased, and the expression levels of Tryptophan Hydroxylase 1 (THP1) and (5-Hydroxytyryptamine Receptor 3) 5-HT3R proteins were significantly reduced (Ouyang et al., 2022).
Basic experimental data on the use of FMT for diarrhea treatment in other studies are presented in Table 4, while the potential mechanisms underlying FMT’s therapeutic effects on diarrhea are illustrated in Figure 4.
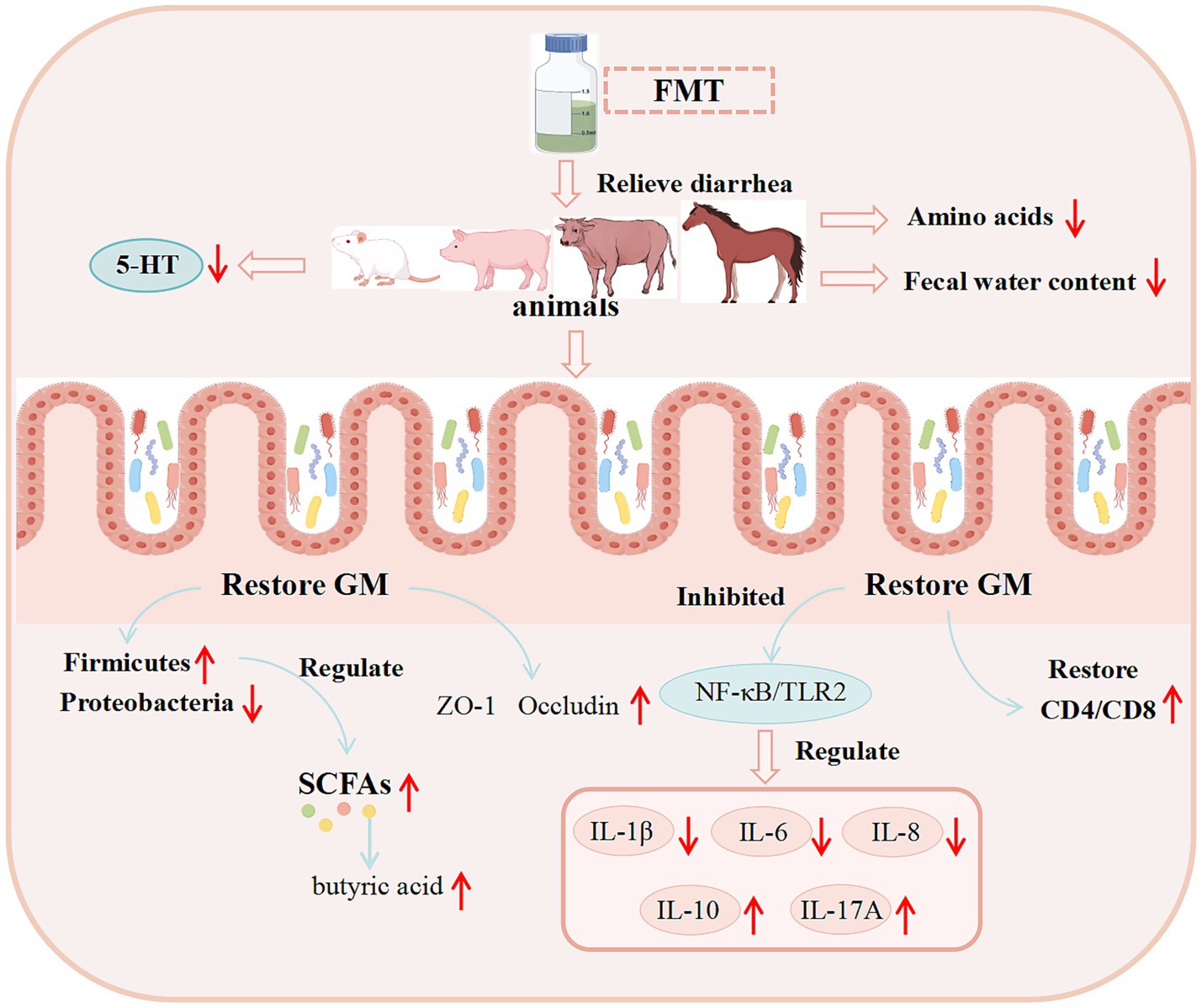
Figure 4. Potential mechanisms of FMT in treating diarrhea revealed by basic research. (These potential mechanisms mainly involve GM, SCFAs, the immune system, and intestinal barrier function).
3.2.2 Clinical experiments
FMT has emerged as a novel clinical strategy for treating diarrhea. As an intestinal microecological therapy with proven efficacy, FMT entails the transfer of GM from healthy donors to patients with diarrhea, which modulates GM composition, restores the intestinal mucosal immune barrier, and thereby exerts therapeutic effects.
CDI is the main cause of nosocomial infectious diarrhea and a high proportion of clinical cure rates have been achieved by restoring the GM with FMT in CDI therapy (Chen and Chiu, 2022; Roshan et al., 2020). Shao et al. found a significant increase in a diversity of GM in CDI patients after FMT, with GM composition more similar to that of healthy donors, increased the abundances of families Ruminococcaceae, Prevotellaceae, Coriobacteriaceae, Porphyromonadaceae, Bacteroidaceae, Bifidobacteriaceae, and Eubacteriaceae, and reduced the abundance of Enterobacteriaceae, Veillonellaceae, Enterococcaceae, and Peptostreptococcaceae (Wei et al., 2022). Clinically, FMT alleviates IBS-D symptoms and improves patients’ quality of life by restoring a balanced GM (Fu and Huang, 2022). Studies have shown that GM and SCFAs in patients with IBS-D differ from those of donors at baseline, such as decreased levels of Actinobacteria and Bifidobacterium and increased levels of Bacteroidetes and Proteobacteria, however these differences gradually return to normal after 3 weeks after FMT, while patients also have improved symptoms and quality of life of IBS-D during the same period (Mazzawi et al., 2019). However, some studies have also shown conflicting results. For example, studies in the treatment of IBS-D have shown that both FMT and placebo recipients showing improvements in irritable bowel syndrome-Severity Scoring System (IBS-SSS) and irritable bowel syndrome-Quality of Life (IBS-QOL) scores and reporting improvements in fecal morphology, however, no differences were found between the two groups (Aroniadis et al., 2019). Therefore, more research is needed to determine the efficacy of FMT for IBS-D.
Currently, FMT has shown expanding clinical applications across various diseases. However, clinical evidence supporting FMT for diarrhea remains limited, with existing studies reporting inconsistent therapeutic outcomes. Most available literature has documented improvements in clinical symptoms, FMT safety, and the efficacy and duration of single or multiple transplantation regimens in diarrhea patients, while studies investigating its specific mechanisms of action remain scarce. Additional information on FMT-induced symptomatic improvements and mechanisms in diarrhea treatment is presented in Table 5, and the potential mechanisms underlying FMT’s clinical efficacy in diarrhea are illustrated in Figure 5.
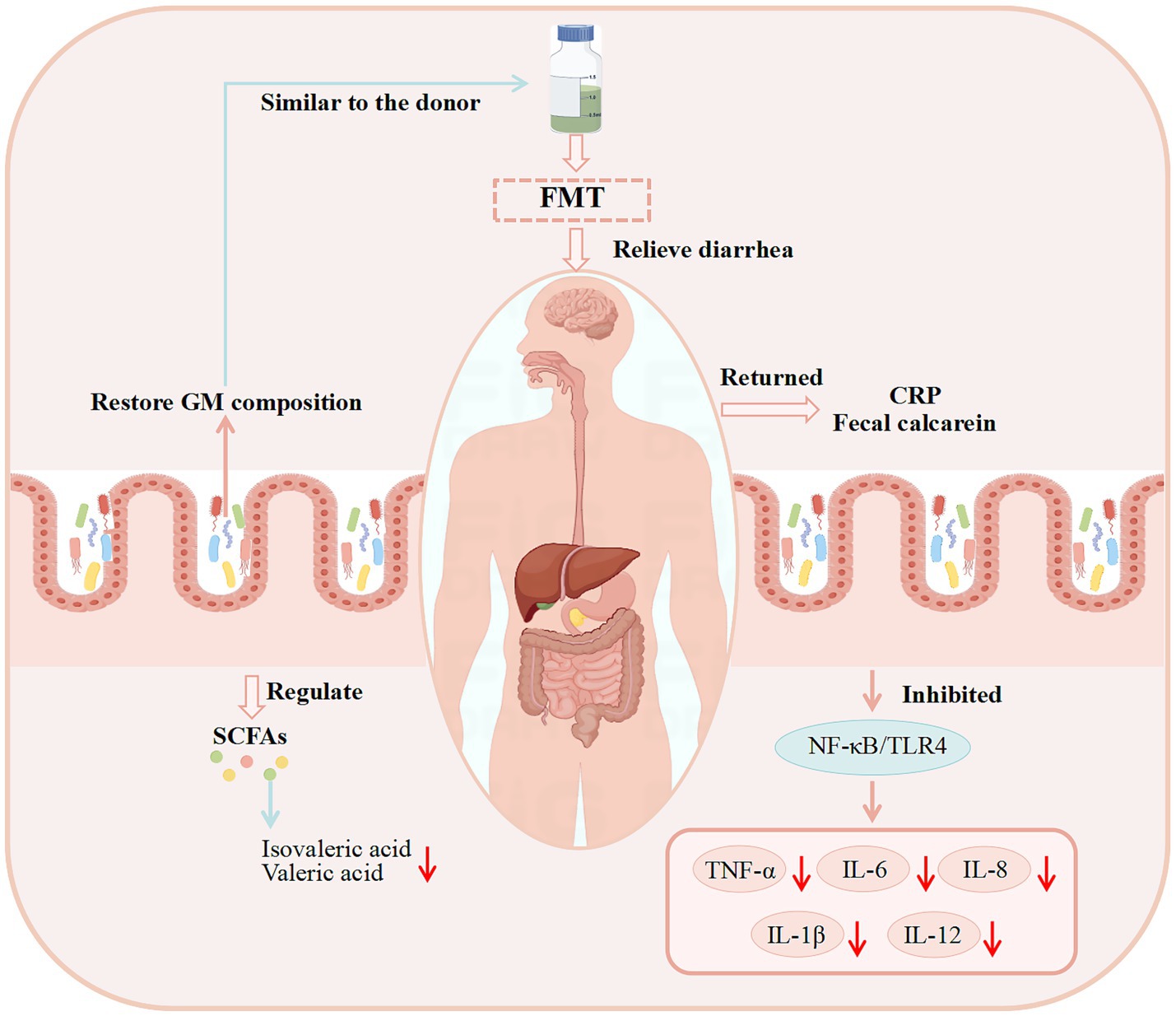
Figure 5. Observed outcomes and proposed mechanisms of FMT for diarrhea in clinical studies. (These potential mechanisms mainly involve GM, SCFAs, and immune system).
3.3 Bacteriophage therapy
Bacteriophages, viruses that specifically infect and lyse bacteria, offer a promising therapeutic strategy by precisely targeting pathogenic bacteria while preserving the commensal GM (Chen H. et al., 2023; Chen X. et al., 2023; Chen J. et al., 2023; Strathdee et al., 2023).
Antibiotics have always been the cornerstone of treating diarrhea, but rising antimicrobial resistance (AMR) has diminished their efficacy (Baran et al., 2023). Moreover, antibiotics disrupt the commensal GM, leading to dysbiosis and increased susceptibility to recurrent infections (Ramirez et al., 2020). Phage therapy, which uses viruses to specifically infect and kill bacteria, has re-emerged as a promising alternative due to its specificity, self-replicating nature, and ability to disrupt biofilms (Duan et al., 2022).
Multiple in vitro studies have demonstrated the effectiveness of phage therapy for treating diarrheal pathogens. Research has shown that a phage cocktail targeting E. coli, such as a combination of six bacteriophages, can reduce bacterial load by 3 log CFU/mL in vitro and effectively inhibit biofilm formation (Youssef et al., 2025). In addition, a 2023 study utilized a resource library termed the gut phage isolate collection (GPIC)—composed of bacteriophages isolated from healthy human guts—to demonstrate that a bacteriophage cocktail targeting Bacteroides fragilis significantly reduced the abundance of the target bacteria in in vitro fecal cultures, highlighting the potential of bacteriophages in modulating the GM (Shen et al., 2023). In animal model studies, phage therapy has also shown promising effects. Research has shown that the microencapsulated bacteriophage A221 is as effective as the antibiotic florfenicol in treating piglet diarrhea models (Youssef et al., 2025). In addition, phage cocktail therapy targeting Klebsiella pneumoniae associated with inflammatory bowel disease can alleviate intestinal inflammation and tissue damage in mouse models (Fuerte-Stone and Mimee, 2022). These studies indicate that bacteriophages can not only effectively reduce the load of pathogenic bacteria, but also alleviate the inflammatory response and tissue damage caused by it.
In recent years, clinical trials of phage therapy for diarrhea have also made some progress. A phase 1 clinical trial in 2022 tested two bacteriophages targeting Klebsiella pneumoniae associated with inflammatory bowel disease on 18 healthy volunteers. The results showed that when taken together with antacids such as CaCO₃, the bacteriophages not only survived at high levels but also remained active throughout the gastrointestinal tract without affecting the resident GM. All participants did not experience any serious treatment-related adverse events, laying the foundation for further research in patients (Federici et al., 2022).
Although these studies indicate that bacteriophages have great potential in treating diarrhea, a critical translational challenge involves phage instability in the harsh gastrointestinal environment. Gastric acidity and digestive enzymes rapidly denature phage particles, compromising therapeutic efficacy. Advanced encapsulation strategies using electrospun fibers, liposomes, or pH-responsive hydrogels are being developed to shield phages during transit and ensure targeted colonic release (Yang Y. et al., 2023; Yang Q. et al., 2023). Additionally, the field must address the complexity of phage ecology, particularly the potential for temperate phages to facilitate horizontal gene transfer of virulence or resistance genes. Careful selection of obligately lytic phages is therefore essential for clinical safety and efficacy (Gummalla et al., 2023).
3.3.1 Basic experiment
Diarrhea is now a significant public health concern. Consequently, bacteriophage therapy has emerged as a promising therapeutic strategy. Phages modulate the composition and abundance of the GM, which in turn alters the expression of intestinal proteins and inflammatory factors, ultimately alleviating various forms of diarrhea.
The mechanism of action of bacteriophages against diarrhea is multifaceted. It begins with the specific lysis of bacterial pathogens, which in turn drives the recovery of healthy GM. This rebalancing directly leads to a reduction in inflammation, an enhancement of the intestinal barrier, and a positive regulation of the immune response, collectively alleviating the symptoms and pathology of diarrhea. Specifically, bacteriophage vB_Ecos_ULIM2 effectively lysed F18 ETEC strain (Navez et al., 2023), ZC22 bacteriophage specifically targeted and reduced the load of Salmonella typhimurium in organs (Sun et al., 2025), and broad-spectrum cocktail reduced fecal E. coli count by 1.33 logarithmic units (Sun et al., 2025).
In addition to direct killing, bacteriophages also significantly regulate the GM to restore health: (1) Reduce pathogenic bacteria: Multiple studies have shown that bacteriophages effectively reduce the abundance of pathogenic families such as Enterobacteriaceae (Li et al., 2024; Mao et al., 2023) and specific genera such as Shigella, Clostridium, and Desulfovibrio (Chen et al., 2025; Choi et al., 2023). (2) Promotion of beneficial bacteria: A key finding is that targeted phage therapy can reduce or even promote the growth of beneficial bacteria. Research consistently reports an increase in the abundance of Lactobacillus (Canibe et al., 2022; Castro et al., 2022; Chen J. et al., 2025; Choi et al., 2023; Feng et al., 2025; Mao et al., 2023) and Bifidobacterium (Chen J. et al., 2025; Choi et al., 2023), which is crucial for gut health. (3) Increasing diversity: Phage cocktails can increase the richness and diversity of microorganisms that are infected and destroyed (such as Chao1 index) (Zeng et al., 2021).
Continuous treatment with bacteriophages can also lead to significant reductions in pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, and TNF-α (J. Chen et al., 2025; Choi et al., 2023; Dong et al., 2024; Kim et al., 2022; Sun et al., 2025; Zeng et al., 2021). On the contrary, they can increase the levels of anti-inflammatory cytokines such as IL-10 (Zeng et al., 2021). A reduction in inflammation often correlates with decreased intestinal damage. Bacteriophages contribute to the restoration of intestinal barrier integrity, they upregulated the expression of TJ proteins, including ZO-1, Occludin, and Claudin-1/3 (Choi et al., 2023; Dong et al., 2024; Feng et al., 2025; Kim et al., 2022). This will lead to a decrease in intestinal permeability (Kim et al., 2022). In addition, there will also be improvements in intestinal morphology, with studies showing an increase in villus height and a decrease in crypt depth (Chen J. et al., 2025; Choi et al., 2023; Zeng et al., 2021), indicating enhanced nutrient absorption and intestinal health.
In addition to the above, bacteriophages can regulate the host’s immune response, including enhancing specific immunity (increasing IgA and IgG levels) and non-specific immunity (such as increasing IFN-γ and lysozyme activity) (Alomari et al., 2021). Bacteriophages can also indirectly affect the intestinal environment, and some therapies lead to an increase in SCFAs (Dong et al., 2024), which are beneficial metabolites produced by intestinal bacteria, supporting barrier function and reducing inflammation.
The specific basic experiments of bacteriophages in the treatment of diarrhea were shown in Table 6, and the potential mechanism of bacteriophages in the treatment of diarrhea were shown in Figure 6.
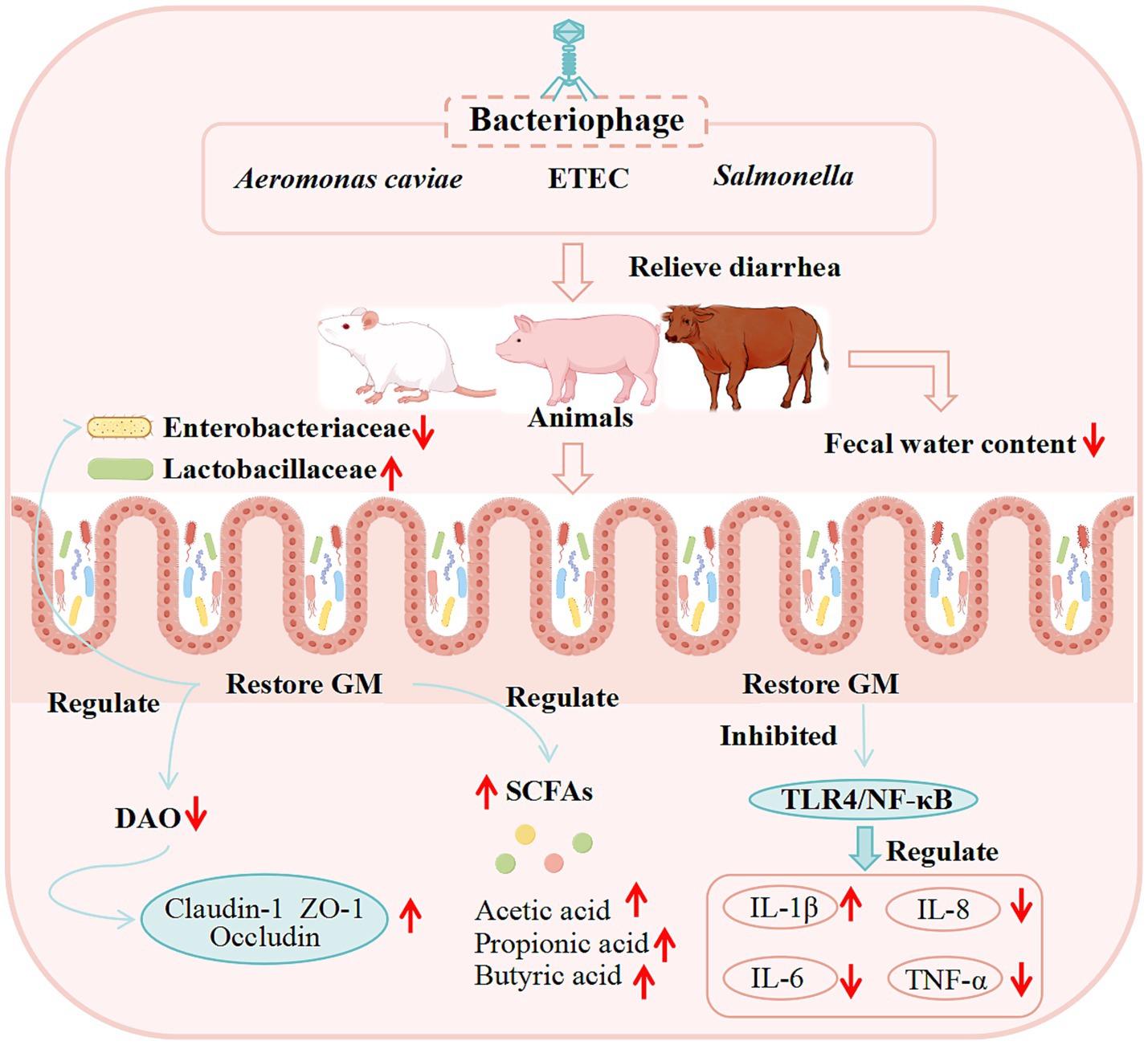
Figure 6. Potential mechanisms of bacteriophage therapy for diarrhea from basic studies. (These potential mechanisms mainly involve GM, SCFAs, the immune system, and intestinal barrier function).
3.3.2 Clinical experiments
At present, in addition to probiotics, FMT and other important treatments for diarrhea, phage therapy is also used in clinical diarrhea diseases.
Based on a randomized, double-blind, placebo-controlled clinical trial conducted in Bangladesh, oral bacteriophage therapy was evaluated as a method for treating acute bacterial diarrhea in children. These studies aim to evaluate the safety, in vivo kinetics, and clinical efficacy of a customized T4 like E. coli phage cocktail and a commercial Russian phage product (Microgen ColiProteus). A total of 120 male children aged 6–24 months hospitalized for acute diarrhea received phage or placebo treatment, as well as standard oral rehydration and zinc therapy. The results indicate that oral phage administration is safe, with no evidence of systemic phage exposure, endotoxin release, or immune response (such as anti phage or anti LPS antibodies) detected, and no serious adverse events or systemic inflammatory reactions observed. These studies emphasize the inherent instability of the gut microbiota in Bangladeshi children, which should be considered in future research on the association of microbiota diseases (Sarker et al., 2017; Sarker and Brüssow, 2016; Sarker et al., 2016). Another study conducted safety testing of phage therapy, which was designed as a single center, randomized, placebo-controlled study. Fifteen healthy volunteers received higher doses of bacteriophages (dose A, 105 PFU/ml), lower doses of bacteriophages (dose B, 103 PFU/ml), and placebo (dose C). The subjects were randomly assigned to one of the following treatment sequences: ABC, BCA, or CAB. During the study, participants provided all fecal samples produced daily. The incidence of adverse events in the high-dose phage group was comparable to that in the low-dose and placebo groups. Ultimately, no adverse events were found to be related to phage administration (Bruttin and Brüssow, 2005).
Although phage therapy is safe and has the potential to serve as an alternative to antibiotic treatment for drug-resistant infections, its efficacy in treating diarrhea has not been confirmed in controlled trials. In the future, we need to conduct pre-screening of phage susceptibility and pathogen dominance, and further fundamental research on phage bacterial dynamics in the human gut. The reason for poor efficacy in the diarrhea test described above may be that some patients have low abundance of the target pathogen (E. coli). There are other pathogens that bacteriophages do not target, such as streptococcus. Possible issues with phage stability, dosage, or delivery to the site of infection. Future research on phage therapy for diarrhea should incorporate more rigorous randomized controlled trials, improved phage characterization, comprehensive sensitivity testing, and optimized dosage regimens.
4 Discussion
Diarrhea, induced by diverse pathogens or contributing factors, is closely associated with alterations in the GM. As a complex and diverse ecosystem, the GM resides symbiotically within the gastrointestinal tract and plays critical roles in host immunity, metabolism, and the maintenance of intestinal barrier homeostasis (Riccio and Rossano, 2020). During episodes of diarrhea, however, disruption of this microbial ecosystem leads to GM dysbiosis, which impairs metabolite production and immune responses, thereby compromising intestinal barrier function (Rengarajan et al., 2020; Shi Z. et al., 2023). Deficiency of beneficial bacteria and overgrowth of certain pathogens (e.g., E. coli and Shigella) is one of the important pathogenic mechanisms of diarrhea (Baker and The, 2018; Khan et al., 2022).
Diarrhea can alter the composition of the GM, and in turn, the application of probiotics, FMT or bacteriophage can directly or indirectly influence the GM and the therapeutic outcome of diarrhea. Currently, probiotics, FMT and bacteriophage have demonstrated considerable anti-diarrheal potential, which can regulate the immune response and enhance intestinal barrier function by regulating the diversity and composition of GM and the content of metabolites, and effectively improving diarrhea symptoms (Pilla and Suchodolski, 2019; Sánchez et al., 2017). Notably, the use of probiotics for treating diarrhea is well-documented. These interventions broadly fall into three categories: single-strain preparations, multi-strain mixtures, or probiotics used in conjunction with conventional therapy. Probiotic supplementation not only helps prevent the occurrence of diarrhea but also enhances overall therapeutic efficacy and clinical cure rates, while shortening the duration of symptoms. Importantly, probiotic interventions are associated with a low incidence of adverse reactions (Liu et al., 2022; Steyer et al., 2022). Commonly used probiotics including Lactobacillus and Bifidobacterium, etc. Probiotics can gain a competitive advantage by altering the intestinal environment, e.g., inhibiting the growth of pathogenic bacteria through the competitive exclusion of intestinal binding sites; probiotics can up-regulate the synthesis of TJ proteins and then protect the intestinal barrier; and they can inhibit the production of pro-inflammatory cytokines to regulate intestinal immune function (Du et al., 2023). In addition, the application of FMT for the treatment of diarrhea has gained increasing attention in recent years owing to its favorable safety profile. The infusion of fecal material from healthy donors can help restore the GM of diarrhea patients to a state resembling that of the donor, thereby alleviating diarrheal symptoms (Li et al., 2019; Zheng et al., 2020). Currently, most studies have documented and summarized the phenomenon of healing in patients with diarrhea after treatment with FMT, such as clinical cure rate and duration of action (Lee et al., 2018; Pereira et al., 2018). Other investigations have explored alterations in the composition and structure of the GM post-FMT, as well as its effects on intestinal immune-inflammatory responses and barrier function, to elucidate the mechanisms underlying the alleviation of diarrhea (Li, 2020; Tian et al., 2022). Compared to probiotics and FMT, phage therapy demonstrates unique application value in the treatment of diarrhea due to its highly specific antibacterial effects (Chen X. et al., 2023). Phages can precisely recognize and lyse specific pathogenic bacteria (such as diarrheagenic E. coli and Salmonella), while preserving the stability of beneficial bacterial communities, thereby enabling precise modulation of the GM (Battistelli et al., 2024; Cui et al., 2022). Research has demonstrated that phage therapy can effectively alleviate symptoms of bacterial diarrhea, reduce levels of inflammatory cytokines, and promote the repair of the intestinal mucosal barrier (Dong et al., 2024). However, despite these results indicating the significant potential of phages in combating diarrhea, their clinical application still faces a critical translational challenge: the relatively poor stability of phages in the hostile gastrointestinal environment. Gastric acid and digestive enzymes can readily cause rapid denaturation of phage particles, thereby compromising therapeutic efficacy (Nobrega et al., 2016). Current research primarily focuses on the clearance of pathogens by phages and preliminary evaluation of clinical efficacy, while studies on post-treatment changes in gut microbiota diversity, metabolite profiles, and immune mechanisms remain relatively limited. Future efforts should involve more rigorous randomized controlled trials, along with optimization of phage characterization, sensitivity detection, and dosing regimens.
A review of the relationship between GM and disease reveals that GM dysbiosis is a critical factor in the pathogenesis of diarrhea. Alterations in the GM can lead to abnormal levels of microbial metabolites, such as SCFAs and BAs. These changes in the GM and its metabolites may further modulate immune cell functions and inflammatory factor levels, ultimately contributing to the onset of diarrhea. Although probiotics, FMT, and bacteriophage have been widely used in the treatment of diarrhea, and their efficacy and safety have encouraged the development of therapeutic approaches for gastrointestinal and other systemic diseases, there are still some issues that need to be addressed. Firstly, there is a scarcity of large-scale clinical trials, and secondly, the underlying mechanisms have not been sufficiently clarified through basic experimental research. Therefore, probiotic and bacteriophage interventions, as well as fecal microbiota transplantation, as safe and effective anti-diarrheal treatment strategies still need to go through a long journey.
5 Conclusion
Overall, GM alterations represent a crucial factor in diarrhea pathogenesis and a key target for its treatment. Diarrhea incidence has been closely linked to elevated levels of Proteobacteria and reduced Firmicutes; thus, targeted GM modulation aids in alleviating diarrhea symptoms. Currently, based on the principle of alleviating gut microbiota disorders, the use of probiotics (such as Lactobacillus and Bifidobacterium), FMT, and bacteriophages has been demonstrated to have definite effects on diarrhea. However, substantial clinical and basic research is still required to elucidate the optimal selection of these interventions, such as screening probiotic strains, FMT donors, and bacteriophages with superior pathogen-targeting advantages, as well as to investigate their long-term safety and efficacy in the treatment of diarrhea. Encouragingly, advances in multi-omics technologies have greatly facilitated investigations into diarrhea treatment mechanisms. Future studies should actively employ diverse research approaches to explore the potential mechanisms of different interventions in various diarrheal diseases and other related conditions, thereby providing data support for clinical diarrhea management and a foundation for the development of novel anti-diarrheal agents.
Author contributions
RT: Writing – original draft, Data curation, Investigation. C-JC: Data curation, Writing – original draft. Y-YB: Data curation, Writing – original draft. NC: Investigation, Writing – original draft. R-RQ: Writing – original draft, Investigation. KW: Writing – review & editing. Y-WW: Writing – review & editing. PZ: Writing – review & editing. C-BZ: Writing – review & editing. Y-PT: Writing – review & editing. LZ: Writing – review & editing. QZ: Writing – review & editing, Formal Analysis, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Key Research and Development plan project of Shaanxi Province (2025SF-YBXM-257), The Natural Science Foundation of the Jiangsu Higher Education Institutions of China (Numbers: 21KJB360014), Key Disciplines of High-level Traditional Chinese Medicine in Shaanxi Province for Science of Chinese Medicinal Preparation, Key Research and Development plan project of Shaanxi Province (2024SF2-GJHX-67), and Key Disciplines of High-level Traditional Chinese Medicine in Shaanxi Province for Science of Chinese Medicinal Preparation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ademe, M. (2020). Benefits of fecal microbiota transplantation: a comprehensive review. J. Infect. Dev. Ctries. 14, 1074–1080. doi: 10.3855/jidc.12780
Alam, N. H., Raqib, R., Ashraf, H., Qadri, F., Ahmed, S., Zasloff, M., et al. (2011). L-isoleucine-supplemented oral rehydration solution in the treatment of acute diarrhoea in children: a randomized controlled trial. J. Health Popul. Nutr. 29, 183–190. doi: 10.3329/jhpn.v29i3.7864
Aleman, R. S., Moncada, M., and Aryana, K. J. (2023). Leaky gut and the ingredients that help treat it: a review. Molecules 28:619. doi: 10.3390/molecules28020619
Ali, M., Mujahid, A., Bulathsinghala, C. P., and Surani, S. (2020). Cardiac arrhythmia secondary to Loperamide abuse and toxicity. Cureus 12:e6936. doi: 10.7759/cureus.6936
Allam-Ndoul, B., Castonguay-Paradis, S., and Veilleux, A. (2020). Gut microbiota and intestinal trans-epithelial permeability. Int. J. Mol. Sci. 21:6402. doi: 10.3390/ijms21176402
Almeida, C., Oliveira, R., Baylina, P., Fernandes, R., Teixeira, F. G., and Barata, P. (2022). Current trends and challenges of fecal microbiota transplantation-An easy method that works for all? Biomedicine 10:2742. doi: 10.3390/biomedicines10112742
Alomari, M. M. M., Dec, M., Nowaczek, A., Puchalski, A., Wernicki, A., Kowalski, C., et al. (2021). Therapeutic and prophylactic effect of the experimental bacteriophage treatment to control diarrhea caused by E. coli in newborn calves. ACS Infect. Dis. 7, 2093–2101. doi: 10.1021/acsinfecdis.1c00010
Al-Sadi, R., Khatib, K., Guo, S., Ye, D., Youssef, M., and Ma, T. (2011). Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G1054–G1064. doi: 10.1152/ajpgi.00055.2011
Anbazhagan, A. N., Ge, Y., Priyamvada, S., Kumar, A., Jayawardena, D., Palani, A. R. V., et al. (2024). A direct link implicating loss of SLC26A6 to gut microbial Dysbiosis, compromised barrier integrity, and inflammation. Gastroenterology 167, 704–717.e3. doi: 10.1053/j.gastro.2024.05.002
Anbazhagan, A. N., Priyamvada, S., Alrefai, W. A., and Dudeja, P. K. (2018). Pathophysiology of IBD associated diarrhea. Tissue Barriers. 6:e1463897. doi: 10.1080/21688370.2018.1463897
Arneth, B. M. (2018). Gut-brain axis biochemical signalling from the gastrointestinal tract to the central nervous system: gut dysbiosis and altered brain function. Postgrad. Med. J. 94, 446–452. doi: 10.1136/postgradmedj-2017-135424
Aroniadis, O. C., Brandt, L. J., Oneto, C., Feuerstadt, P., Sherman, A., Wolkoff, A. W., et al. (2019). Faecal microbiota transplantation for diarrhoea-predominant irritable bowel syndrome: a double-blind, randomised, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 4, 675–685. doi: 10.1016/s2468-1253(19)30198-0
Baker, S., and The, H. C. (2018). Recent insights into Shigella. Curr. Opin. Infect. Dis. 31, 449–454. doi: 10.1097/qco.0000000000000475
Bao, W., He, Y., Yu, J., Liu, M., Yang, X., Ta, N., et al. (2022). Regulatory effect of Lactiplantibacillus plantarum 2-33 on intestinal microbiota of mice with antibiotic-associated diarrhea. Front. Nutr. 9:921875. doi: 10.3389/fnut.2022.921875
Baran, A., Kwiatkowska, A., and Potocki, L. (2023). Antibiotics and bacterial resistance-a short story of an endless arms race. Int. J. Mol. Sci. 24:5777. doi: 10.3390/ijms24065777
Barash, N. R., Maloney, J. G., Singer, S. M., and Dawson, S. C. (2017). Giardia alters commensal microbial diversity throughout the murine gut. Infect. Immun. 85:e00137-18. doi: 10.1128/iai.00948-16
Battistelli, N., Tittarelli, F., Ruffini, F., Gavazzi, L., Scattolini, S., Acciari, V. A., et al. (2024). In vitro characterization and genome sequencing of two novel lytic phages against Salmonella Infantis isolated from poultry feces. Front. Microbiol. 15:1479700. doi: 10.3389/fmicb.2024.1479700
Bell, J., Radial, S. L., Cuming, R. S., Trope, G., and Hughes, K. J. (2024). Effects of fecal microbiota transplantation on clinical outcomes and fecal microbiota of foals with diarrhea. J. Vet. Intern. Med. 38, 2718–2728. doi: 10.1111/jvim.17185
Binder, H. J. (2010). Role of colonic short-chain fatty acid transport in diarrhea. Annu. Rev. Physiol. 72, 297–313. doi: 10.1146/annurev-physiol-021909-135817
Bosnjak, M., Karpe, A. V., Van, T. T. H., Kotsanas, D., Jenkin, G. A., Costello, S. P., et al. (2023). Multi-omics analysis of hospital-acquired diarrhoeal patients reveals biomarkers of enterococcal proliferation and Clostridioides difficile infection. Nat. Commun. 14:7737. doi: 10.1038/s41467-023-43671-8
Brehm, T. T., Lütgehetmann, M., Tannich, E., Addo, M. M., Lohse, A. W., Rolling, T., et al. (2020). Risk factors for different intestinal pathogens among patients with traveler's diarrhea: a retrospective analysis at a German travel clinic (2009-2017). Travel Med. Infect. Dis. 37:101706. doi: 10.1016/j.tmaid.2020.101706
Bruttin, A., and Brüssow, H. (2005). Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrob. Agents Chemother. 49, 2874–2878. doi: 10.1128/AAC.49.7.2874-2878
Bustos Fernández, L. M., Man, F., and Lasa, J. S. (2023). Impact of Saccharomyces boulardii CNCM I-745 on bacterial overgrowth and composition of intestinal microbiota in diarrhea-predominant irritable bowel syndrome patients: results of a randomized pilot study. Dig. Dis. 41, 798–809. doi: 10.1159/000528954
Cai, J., Sun, L., and Gonzalez, F. J. (2022). Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 30, 289–300. doi: 10.1016/j.chom.2022.02.004
Camilleri, M. (2021). Human intestinal barrier: effects of stressors, diet, prebiotics, and probiotics. Clin. Transl. Gastroenterol. 12:e00308. doi: 10.14309/ctg.0000000000000308
Canibe, N., Højberg, O., Kongsted, H., Vodolazska, D., Lauridsen, C., Nielsen, T. S., et al. (2022). Review on preventive measures to reduce post-weaning Diarrhoea in piglets. Animals (Basel). 12:2585. doi: 10.3390/ani12192585
Cao, A. T., Yao, S., Gong, B., Elson, C. O., and Cong, Y. (2012). Th17 cells upregulate polymeric Ig receptor and intestinal IgA and contribute to intestinal homeostasis. J. Immunol. 189, 4666–4673. doi: 10.4049/jimmunol.1200955
Castro, J., Barros, M. M., Araújo, D., Campos, A. M., Oliveira, R., Silva, S., et al. (2022). Swine enteric colibacillosis: current treatment avenues and future directions. Front Vet Sci. 9:981207. doi: 10.3389/fvets.2022.981207
Caviglia, G. P., Tucci, A., Pellicano, R., Fagoonee, S., Rosso, C., Abate, M. L., et al. (2020). Clinical response and changes of cytokines and Zonulin levels in patients with Diarrhoea-predominant irritable bowel syndrome treated with Bifidobacterium longum ES1 for 8 or 12 Weeks: a preliminary report. J. Clin. Med. 9:2353. doi: 10.3390/jcm9082353
Chen, H. R., An, J. R., Yang, Y. F., Liu, J. T., and Shi, Y. (2024). Discussion on the repairing effect and mechanism of traditional Chinese medicine compound Yitangkang on intestinal barrier damage in small intestine of db/db mice. J. Liaoning Univ. Tradit. Chin. Med. 26, 31–36. doi: 10.13194/j.issn.1673-842x.2024.07.007
Chen, C. C., and Chiu, C. H. (2022). Current and future applications of fecal microbiota transplantation for children. Biom. J. 45, 11–18. doi: 10.1016/j.bj.2021.11.004
Chen, J., Han, J., Yang, Z., Zhou, W., He, Y., Chen, X., et al. (2025). Bacteriophages as potential anti-pathogenic agents for intestinal health of weaned piglets in the post-antibiotic era: an updated review. Animals (Basel) 15:1713. doi: 10.3390/ani15121713
Chen, H., Jia, Z., He, M., Chen, A., Zhang, X., Xu, J., et al. (2023). Arula-7 powder improves diarrhea and intestinal epithelial tight junction function associated with its regulation of intestinal flora in calves infected with pathogenic Escherichia coli O(1). Microbiome 11:172. doi: 10.1186/s40168-023-01616-9
Chen, K., Jin, S., Ma, Y., Cai, L., Xu, P., Nie, Y., et al. (2024). Adjudicative efficacy of Bifidobacterium animalis subsp. lactis BLa80 in treating acute diarrhea in children: a randomized, double-blinded, placebo-controlled study. Eur. J. Clin. Nutr. 78, 501–508. doi: 10.1038/s41430-024-01428-6
Chen, Y. M., Limaye, A., Chang, H. W., and Liu, J. R. (2022). Screening of lactic acid bacterial strains with antiviral activity against porcine epidemic diarrhea. Probiotics Antimicrob Proteins 14, 546–559. doi: 10.1007/s12602-021-09829-w
Chen, X., Mendes, B. G., Alves, B. S., and Duan, Y. (2023). Phage therapy in gut microbiome. Prog Mol Biol Transl Sci. 201, 93–118. doi: 10.1016/bs.pmbts.2023.04.005
Chen, J., Zhao, B. C., Dai, X. Y., Xu, Y. R., Kang, J. X., and Li, J. L. (2023). Drinking alkaline mineral water confers diarrhea resistance in maternally separated piglets by maintaining intestinal epithelial regeneration via the brain-microbe-gut axis. J. Adv. Res. 52, 29–43. doi: 10.1016/j.jare.2022.12.008
Chen, Y., Zhou, J., and Wang, L. (2021). Role and mechanism of gut microbiota in human disease. Front. Cell. Infect. Microbiol. 11:625913. doi: 10.3389/fcimb.2021.625913
Cheng, S., Ma, X., Geng, S., Jiang, X., Li, Y., Hu, L., et al. (2018). Fecal microbiota transplantation beneficially regulates intestinal mucosal autophagy and alleviates. Gut Barrier Injury 3:e00137-18. doi: 10.1128/mSystems.00137-18
Choi, Y., Hosseindoust, A., Ha, S. H., Kim, J., Min, Y., Jeong, Y., et al. (2023). Effects of dietary supplementation of bacteriophage cocktail on health status of weanling pigs in a non-sanitary environment. J Anim Sci Biotechnol. 14:64. doi: 10.1186/s40104-023-00869-6
Collaborators G.D.D (2017). Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the global burden of disease study 2015. Lancet Infect. Dis. 17, 909–948. doi: 10.1016/s1473-3099(17)30276-1
Cryan, J. F., O'Riordan, K. J., Cowan, C. S. M., Sandhu, K. V., Bastiaanssen, T. F. S., Boehme, M., et al. (2019). The microbiota-gut-brain Axis. Physiol. Rev. 99, 1877–2013. doi: 10.1152/physrev.00018.2018
Cui, S., Guo, S., Zhao, Q., Li, Y., Ma, Y., and Yu, Y. (2023). Alterations of microbiota and metabolites in the feces of calves with diarrhea associated with rotavirus and coronavirus infections. Front. Microbiol. 14:1159637. doi: 10.3389/fmicb.2023.1159637
Cui, J. Q., Liu, W. H., Zang, Y. X., Zhang, C., Zou, L., Sun, H. Z., et al. (2022). Characterization and complete genome analysis of a bacteriophage vB_EcoM_DE7 infecting donkey-derived Escherichia coli. Virus Res. 321:198913. doi: 10.1016/j.virusres.2022.198913
Cui, M., Wang, Y., Elango, J., Wu, J., Liu, K., and Jin, Y. (2021). Cereus sinensis polysaccharide alleviates antibiotic-associated diarrhea based on modulating the gut microbiota in C57BL/6 mice. Front. Nutr. 8:751992. doi: 10.3389/fnut.2021.751992
Czepiel, J., Dróżdż, M., Pituch, H., Kuijper, E. J., Perucki, W., Mielimonka, A., et al. (2019). Clostridium difficile infection: review. Eur. J. Clin. Microbiol. Infect. Dis. 38, 1211–1221. doi: 10.1007/s10096-019-03539-6
den Besten, G., van Eunen, K., Groen, A. K., Venema, K., Reijngoud, D. J., and Bakker, B. M. (2013). The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54, 2325–2340. doi: 10.1194/jlr.R036012
Dinan, T. G., and Cryan, J. F. (2017). The microbiome-gut-brain Axis in health and disease. Gastroenterol. Clin. N. Am. 46, 77–89. doi: 10.1016/j.gtc.2016.09.007
Doar, N. W., and Samuthiram, S. D. (2023). Qualitative analysis of the efficacy of probiotic strains in the prevention of antibiotic-associated diarrhea. Cureus 15:e40261. doi: 10.7759/cureus.40261
Donald, K., and Finlay, B. B. (2023). Early-life interactions between the microbiota and immune system: impact on immune system development and atopic disease. Nat. Rev. Immunol. 23, 735–748. doi: 10.1038/s41577-023-00874-w
Dong, C., Chen, Y., Ding, M., Liu, Y., Chen, X., He, Y., et al. (2024). Dietary bacteriophage administration alleviates Enterotoxigenic Escherichia coli-induced diarrhea and intestinal impairment through regulating intestinal inflammation and gut microbiota in a newly weaned mouse model. Int. J. Mol. Sci. 25:10736. doi: 10.3390/ijms251910736
Du, W., Wang, X., Hu, M., Hou, J., Du, Y., Si, W., et al. (2023). Modulating gastrointestinal microbiota to alleviate diarrhea in calves. Front. Microbiol. 14:1181545. doi: 10.3389/fmicb.2023.1181545
Duan, Y., Young, R., and Schnabl, B. (2022). Bacteriophages and their potential for treatment of gastrointestinal diseases. Nat. Rev. Gastroenterol. Hepatol. 19, 135–144. doi: 10.1038/s41575-021-00536-z
Duboc, H., Rainteau, D., Rajca, S., Humbert, L., Farabos, D., Maubert, M., et al. (2012). Increase in fecal primary bile acids and dysbiosis in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol. Motil. 24, 513–520. doi: 10.1111/j.1365-2982.2012.01893.x
Fareed, S., Sarode, N., Stewart, F. J., Malik, A., Laghaie, E., Khizer, S., et al. (2018). Applying fecal microbiota transplantation (FMT) to treat recurrent Clostridium difficile infections (rCDI) in children. PeerJ 6:e4663. doi: 10.7717/peerj.4663
Federici, S., Kredo-Russo, S., Valdés-Mas, R., Kviatcovsky, D., Weinstock, E., Matiuhin, Y., et al. (2022). Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell 185, 2879–2898.e24. doi: 10.1016/j.cell.2022.07.003
Feng, C., Wang, L., Bai, H., Huang, Q., Liang, S., Liang, R., et al. (2025). The high efficiency protective effectiveness of a newly isolated myoviruses bacteriophage vB_AceP_PAc in protecting mice from Aeromonas caviae infection in mice. BMC Microbiol. 25:112. doi: 10.1186/s12866-025-03796-w
Fu, W. Q., and Huang, C. B. (2022). Progress in the study of fecal microbiota transplantation for the treatment of diarrhea-type irritable bowel syndrome. Pract. Clin. Med. 23, 122–127. doi: 10.13764/j.cnki.lcsy.2022.03.035
Fuerte-Stone, J., and Mimee, M. (2022). Host happy hour: phage cocktail targets IBD-associated microbes. Cell Host Microbe 30, 1352–1353. doi: 10.1016/j.chom.2022.09.010
Fujimoto, K., and Uematsu, S. (2022). Phage therapy for Clostridioides difficile infection. Front. Immunol. 13:1057892. doi: 10.3389/fimmu.2022.1057892
Fukuda, S., Toh, H., Hase, K., Oshima, K., Nakanishi, Y., Yoshimura, K., et al. (2011). Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469, 543–547. doi: 10.1038/nature09646
Furusawa, Y., Obata, Y., Fukuda, S., Endo, T. A., Nakato, G., Takahashi, D., et al. (2013). Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450. doi: 10.1038/nature12721
Gallardo, P., Izquierdo, M., Vidal, R. M., Soto, F., Ossa, J. C., and Farfan, M. J. (2020). Gut microbiota-metabolome changes in children with diarrhea by Diarrheagenic E. coli. Front. Cell. Infect. Microbiol. 10:485. doi: 10.3389/fcimb.2020.00485
Gao, F., Yuan, W. H., Wu, S. B., Wang, Z. B., Zhu, G. Q., and Zhou, M. Q. (2023). Electroacupuncture in the treatment of IBS in rats: investigation of the mechanisms of CRH(+) neurons in the paraventricular nucleus. J. Neurophysiol. 130, 380–391. doi: 10.1152/jn.00156.2023
Grenham, S., Clarke, G., Cryan, J. F., and Dinan, T. G. (2011). Brain-gut-microbe communication in health and disease. Front. Physiol. 2:94. doi: 10.3389/fphys.2011.00094
Gu, Y., Wang, C., Qin, X., Zhou, B., Liu, X., Liu, T., et al. (2022). Saccharomyces boulardii, a yeast probiotic, inhibits gut motility through upregulating intestinal serotonin transporter and modulating gut microbiota. Pharmacol. Res. 181:106291. doi: 10.1016/j.phrs.2022.106291
Gummalla, V. S., Zhang, Y., Liao, Y. T., and Wu, V. C. H. (2023). The role of temperate phages in bacterial pathogenicity. Microorganisms 11:541. doi: 10.3390/microorganisms11030541
Guo, S., Ma, T., Kwok, L. Y., Quan, K., Li, B., Wang, H., et al. (2024). Effects of postbiotics on chronic diarrhea in young adults: a randomized, double-blind, placebo-controlled crossover trial assessing clinical symptoms, gut microbiota, and metabolite profiles. Gut Microbes 16:2395092. doi: 10.1080/19490976.2024.2395092
Guo, J. G., Rao, Y. F., Jiang, J., Li, X., and Zhu, S. M. (2023). MicroRNA-155-5p inhibition alleviates irritable bowel syndrome by increasing claudin-1 and ZO-1 expression. Ann Transl Med. 11:34. doi: 10.21037/atm-22-4859
Guo, H., Yu, L., Tian, F., Zhao, J., Zhang, H., Chen, W., et al. (2021). Effects of Bacteroides-based Microecologics against antibiotic-associated diarrhea in mice. Microorganisms 9:2492. doi: 10.3390/microorganisms9122492
Haas, A. J., Zihni, C., Krug, S. M., Maraspini, R., Otani, T., Furuse, M., et al. (2022). ZO-1 guides tight junction assembly and epithelial morphogenesis via cytoskeletal tension-dependent and -independent functions. Cells 11:3775. doi: 10.3390/cells11233775
Han, Q. (2023) Studies on the mechanism of action of fecal microbiota transplantation in alleviating diarrhea in artificially lactating piglets. Heilongjiang china: Northeast Agricultural University.
Han, X., Hu, X., Jin, W., and Liu, G. (2024). Dietary nutrition, intestinal microbiota dysbiosis and post-weaning diarrhea in piglets. Anim Nutr. 17, 188–207. doi: 10.1016/j.aninu.2023.12.010
Handa, O., Miura, H., Gu, T., Osawa, M., Matsumoto, H., Umegaki, E., et al. (2023). Reduction of butyric acid-producing bacteria in the ileal mucosa-associated microbiota is associated with the history of abdominal surgery in patients with Crohn's disease. Redox Rep. 28:2241615. doi: 10.1080/13510002.2023.2241615
He, K. Y., Lei, X. Y., Wu, D. H., Zhang, L., Li, J. Q., Li, Q. T., et al. (2023). Akkermansia muciniphila protects the intestine from irradiation-induced injury by secretion of propionic acid. Gut Microbes 15:2293312. doi: 10.1080/19490976.2023.2293312
Hillestad, E. M. R., van der Meeren, A., Nagaraja, B. H., Bjørsvik, B. R., Haleem, N., Benitez-Paez, A., et al. (2022). Gut bless you: The microbiota-gut-brain axis in irritable bowel syndrome. World J. Gastroenterol. 28, 412–431. doi: 10.3748/wjg.v28.i4.412
Hold, G. L. (2016). Gastrointestinal microbiota and Colon Cancer. Dig. Dis. 34, 244–250. doi: 10.1159/000443358
Hollister, E. B., Gao, C., and Versalovic, V. (2014). Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterology. 146, 1449–58. doi: 10.1053/j.gastro.2014.01.052
Hou, Q., Huang, Y., Wang, Y., Liao, L., Zhu, Z., Zhang, W., et al. (2020). Lactobacillus casei LC01 regulates intestinal epithelial permeability through miR-144 targeting of OCLN and ZO1. J. Microbiol. Biotechnol. 30, 1480–1487. doi: 10.4014/jmb.2002.02059
Huang, F., Li, S., Chen, W., Han, Y., Yao, Y., Yang, L., et al. (2023). Postoperative probiotics administration attenuates gastrointestinal complications and gut microbiota Dysbiosis caused by chemotherapy in colorectal Cancer patients. Nutrients 15:356. doi: 10.3390/nu15020356
Huang, H. L., Zhu, J. Q., Yang, L. S., Wu, Q., Shou, D. W., Chen, H. T., et al. (2022). Fecal microbiota transplantation combined with a low FODMAP diet for the treatment of irritable bowel syndrome with predominant diarrhea. Oxidative Med. Cell. Longev. 2022:5121496. doi: 10.1155/2022/5121496
Islam, J., Tanimizu, M., Shimizu, Y., Goto, Y., Ohtani, N., Sugiyama, K., et al. (2022). Development of a rational framework for the therapeutic efficacy of fecal microbiota transplantation for calf diarrhea treatment. Microbiome. 10:31. doi: 10.1186/s40168-021-01217-4
James, S. C., Fraser, K., Cooney, J., Günther, C. S., Young, W., Gearry, R. B., et al. (2023). Concentrations of plasma amino acids and neurotransmitters in participants with functional gut disorders and healthy controls. Meta 13:313. doi: 10.3390/metabo13020313
Jandhyala, S. M., Talukdar, R., Subramanyam, C., Vuyyuru, H., Sasikala, M., and Nageshwar Reddy, D. (2015). Role of the normal gut microbiota. World J. Gastroenterol. 21, 8787–8803. doi: 10.3748/wjg.v21.i29.8787
Jesser, K. J., Trueba, G., Konstantinidis, K. T., and Levy, K. (2023). Why are so many enteric pathogen infections asymptomatic? Pathogen and gut microbiome characteristics associated with diarrhea symptoms and carriage of diarrheagenic E. coli in northern Ecuador. Gut Microbes 15:2281010. doi: 10.1080/19490976.2023.2281010
Jiang, C., Chen, W., Yang, Y., Li, X., Jin, M., Ghonaim, A. H., et al. (2024). Regulation of isoleucine on colonic barrier function in rotavirus-infected weanling piglets and analysis of gut microbiota and metabolomics. Microorganisms. 12:2396. doi: 10.3390/microorganisms12122396
Jiang, W. D., Deng, Y. P., Liu, Y., Qu, B., Jiang, J., Kuang, S. Y., et al. (2015). Dietary leucine regulates the intestinal immune status, immune-related signalling molecules and tight junction transcript abundance in grass carp (Ctenopharyngodon idella). Aquaculture 444, 134–142. doi: 10.1016/j.aquaculture.2015.04.005
Jiang, C. Y., Wang, X. X., Zhang, S. S., Wang, Y., Wen, D., and Fei, S. J. (2024). Effect of fecal microbiota transplantation modulating PAR2-TRPV1 pathway on visceral hypersensitivity, mast cell activation and intestinal flora in an irritable bowel syndrome model rat. Prog. Mod. Biomed. 24, 2417–2422+2407. doi: 10.13241/j.cnki.pmb.2024.13.003
Jung, M., Jung, S., Kim, N., Ahn, H., Yun, H., and Kim, K. N. (2022). A randomized, double-blind, placebo-controlled trial to assess the efficacy and safety of Lactiplantibacillus plantarum CJLP243 in patients with functional diarrhea and high fecal calprotectin levels. Nutrients 14:389. doi: 10.3390/nu14020389
Jung, K., Kim, A., Lee, J. H., Cho, D., Seo, J., Jung, E. S., et al. (2022). Effect of oral intake of Lactiplantibacillus plantarum APsulloc 331261 (GTB1(TM)) on diarrhea-predominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled study. Nutrients 14:2015. doi: 10.3390/nu14102015
Karim, M. R., Iqbal, S., Mohammad, S., Morshed, M. N., Haque, M. A., Mathiyalagan, R., et al. (2024). Butyrate's (a short-chain fatty acid) microbial synthesis, absorption, and preventive roles against colorectal and lung cancer. Arch. Microbiol. 206:137. doi: 10.1007/s00203-024-03834-7
Khan, H. R., Ali Asghar, S., Kanwal, S., Qadar, L. T., and Qadri, K. H. (2019). Diphenoxylate-atropine (Lomotil) toxicity in infantile diarrhea: a case report of therapeutic failure. Cureus 11:e5875. doi: 10.7759/cureus.5875
Khan, J. R., Hossain, M. B., Chakraborty, P. A., and Mistry, S. K. (2022). Household drinking water E. coli contamination and its associated risk with childhood diarrhea in Bangladesh. Environ. Sci. Pollut. Res. Int. 29, 32180–32189. doi: 10.1007/s11356-021-18460-9
Kim, N., Gu, M. J., Kye, Y. C., Ju, Y. J., Hong, R., Ju, D. B., et al. (2022). Bacteriophage EK99P-1 alleviates enterotoxigenic Escherichia coli K99-induced barrier dysfunction and inflammation. Sci. Rep. 12:941. doi: 10.1038/s41598-022-04861-4
Konturek, P. C., Koziel, J., Dieterich, W., Haziri, D., Wirtz, S., Glowczyk, I., et al. (2016). Successful therapy of Clostridium difficile infection with fecal microbiota transplantation. J. Physiol. Pharmacol. 67, 859–866. Available online at: https://www.jpp.krakow.pl/
Kovanda, L., Park, J., Park, S., Kim, K., Li, X., and Liu, Y. (2023). Dietary butyrate and valerate glycerides impact diarrhea severity and immune response of weaned piglets under ETEC F4-ETEC F18 coinfection conditions. J. Anim. Sci. 101:skad401. doi: 10.1093/jas/skad401
Kreis, V., and Soutourina, O. (2022). Clostridioides difficile - phage relationship the RNA way. Curr. Opin. Microbiol. 66, 1–10. doi: 10.1016/j.mib.2021.11.012
Lai, H. H., Chiu, C. H., Kong, M. S., Chang, C. J., and Chen, C. C. (2019). Probiotic Lactobacillus casei: effective for managing childhood diarrhea by altering gut microbiota and attenuating fecal inflammatory markers. Nutrients 11:1150. doi: 10.3390/nu11051150
Lange, K., Buerger, M., Stallmach, A., and Bruns, T. (2016). Effects of antibiotics on gut microbiota. Dig. Dis. 34, 260–268. doi: 10.1159/000443360
Lee, S., Drennan, K., Simons, G., Hepple, A., Karlsson, K., Lowman, W., et al. (2018). The 'ins and outs' of faecal microbiota transplant for recurrent Clostridium difficile diarrhoea at Wits Donald Gordon medical Centre, Johannesburg, South Africa. S. Afr. Med. J. 108, 403–407. doi: 10.7196/SAMJ.2018.v108i5.12367
Lee, C., Kim, B. G., Kim, J. H., Chun, J., Im, J. P., and Kim, J. S. (2017). Sodium butyrate inhibits the NF-kappa B signaling pathway and histone deacetylation, and attenuates experimental colitis in an IL-10 independent manner. Int. Immunopharmacol. 51, 47–56. doi: 10.1016/j.intimp.2017.07.023
Lee, A., Lee, J. Y., Jung, S. W., Shin, S. Y., Ryu, H. S., Jang, S. H., et al. (2023). Brain-Gut-Microbiota Axis. Korean J. Gastroenterol. 81, 145–153. doi: 10.4166/kjg.2023.028
Leong, K. S. W., Derraik, J. G. B., Hofman, P. L., and Cutfield, W. S. (2018). Antibiotics, gut microbiome and obesity. Clin. Endocrinol. 88, 185–200. doi: 10.1111/cen.13495
Li, X. L. (2020). Multi-omics study on the effects of fecal microbiota transplantation on piglets with diarrhea after weaning. Chongqing, 9–25.
Li, L., Cui, H., Li, T., Qi, J., Chen, H., Gao, F., et al. (2020). Synergistic effect of Berberine-based Chinese medicine assembled nanostructures on diarrhea-predominant irritable bowel syndrome in vivo. Front. Pharmacol. 11:1210. doi: 10.3389/fphar.2020.01210
Li, L., Han, K., Mao, X., Wang, L., Cao, Y., Li, Z., et al. (2024). Oral phages prophylaxis against mixed Escherichia coli O157:H7 and Salmonella Typhimurium infections in weaned piglets. Vet. Microbiol. 288:109923. doi: 10.1016/j.vetmic.2023.109923
Li, X. T., Li, J. L., Cao, Z. Q., Kang, N., and Kong, W. Z. (2023). Based on 16S rRNA sequencing technology, the effect of Shenling Atractylodes medicinal diet paste on the intestinal microbiota of rats with functional diarrhea and spleen deficiency syndrome was explored. Lishizhen Med. Mater. Med. Res. 34, 3046–3051. doi: 10.3969/j.issn.1008-0805.2023.12.59
Li, N., Tian, H. L., Chen, Q. Y., Yang, B., Ma, C. L., Lin, Z. L., et al. (2019). Efficacy analysis of fecal microbiota transplantation in the treatment of 2010 patients with intestinal disorders. Zhonghua Wei Chang Wai Ke Za Zhi 22, 861–868. doi: 10.3760/cma.j.issn.1671-0274.2019.09.011
Li, Y., Xia, S., Jiang, X., Feng, C., Gong, S., Ma, J., et al. (2021). Gut microbiota and diarrhea: An updated review. Front. Cell. Infect. Microbiol. 11:625210. doi: 10.3389/fcimb.2021.625210
Li, C., Xiao, N., Deng, N., Li, D., Tan, Z., and Peng, M. (2023). Dose of sucrose affects the efficacy of Qiweibaizhu powder on antibiotic-associated diarrhea: association with intestinal mucosal microbiota, short-chain fatty acids, IL-17, and MUC2. Front. Microbiol. 14:1108398. doi: 10.3389/fmicb.2023.1108398
Li, K., Yang, J., Zhou, X., Wang, H., Ren, Y., Huang, Y., et al. (2022). The mechanism of important components in canine fecal microbiota transplantation. Vet Sci. 9:695. doi: 10.3390/vetsci9120695
Liang, W., Gao, Y., Zhao, Y., Gao, L., Zhao, Z., He, Z., et al. (2023). Lactiplantibacillus plantarum ELF051 alleviates antibiotic-associated diarrhea by regulating intestinal inflammation and gut microbiota. Probiotics Antimicrob. Proteins 16, 1996–2006. doi: 10.1007/s12602-023-10150-x
Lin, H., Guo, Q., Wen, Z., Tan, S., Chen, J., Lin, L., et al. (2021). The multiple effects of fecal microbiota transplantation on diarrhea-predominant irritable bowel syndrome (IBS-D) patients with anxiety and depression behaviors. Microb. Cell Factories 20:233. doi: 10.1186/s12934-021-01720-1
Liu, C. S., Liang, X., Wei, X. H., Jin, Z., Chen, F. L., Tang, Q. F., et al. (2019). Gegen Qinlian decoction treats diarrhea in piglets by modulating gut microbiota and short-chain fatty acids. Front. Microbiol. 10:825. doi: 10.3389/fmicb.2019.00825
Liu, M., Ma, J., Xu, J., Huangfu, W., Zhang, Y., Ali, Q., et al. (2024). Fecal microbiota transplantation alleviates intestinal inflammatory diarrhea caused by oxidative stress and pyroptosis via reducing gut microbiota-derived lipopolysaccharides. Int. J. Biol. Macromol. 261:129696. doi: 10.1016/j.ijbiomac.2024.129696
Liu, B., Wang, C., Huasai, S., Han, A., Zhang, J., He, L., et al. (2022). Compound probiotics improve the diarrhea rate and intestinal microbiota of newborn calves. Animals (Basel). 12:322. doi: 10.3390/ani12030322
Liu, P., Wang, Y., Yang, G., Zhang, Q., Meng, L., Xin, Y., et al. (2021). The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 165:105420. doi: 10.1016/j.phrs.2021.105420
Liu, Y., Yu, Z., Zhu, L., Ma, S., Luo, Y., Liang, H., et al. (2023). Orchestration of MUC2 - the key regulatory target of gut barrier and homeostasis: a review. Int. J. Biol. Macromol. 236:123862. doi: 10.1016/j.ijbiomac.2023.123862
Liu, S., Zhao, S., Cheng, Z., Ren, Y., Shi, X., Mu, J., et al. (2024). Akkermansia muciniphila protects against antibiotic-associated diarrhea in mice. Probiotics Antimicrob Proteins. 16, 1190–1204. doi: 10.1007/s12602-023-10101-6
Luo, Z., Liu, C., Hu, Y., Xia, T., Zhang, B., Chen, F., et al. (2022). Gegen Qinlian decoction restores the intestinal barrier in bacterial diarrhea piglets by promoting Lactobacillus growth and inhibiting the TLR2/MyD88/NF-κB pathway. Biomed. Pharmacother. 155:113719. doi: 10.1016/j.biopha.2022.113719
Luo, M., Zhuang, X., Tian, Z., and Xiong, L. (2021). Alterations in short-chain fatty acids and serotonin in irritable bowel syndrome: a systematic review and meta-analysis. BMC Gastroenterol. 21:14. doi: 10.1186/s12876-020-01577-5
Ma, N., Guo, P., Zhang, J., He, T., Kim, S. W., Zhang, G., et al. (2018). Nutrients mediate intestinal Bacteria-mucosal immune crosstalk. Front. Immunol. 9:5. doi: 10.3389/fimmu.2018.00005
Maity, C., and Gupta, A. K. (2021). Therapeutic efficacy of probiotic Alkalihalobacillus clausii 088AE in antibiotic-associated diarrhea: a randomized controlled trial. Heliyon 7:e07993. doi: 10.1016/j.heliyon.2021.e07993
Makizaki, Y., Maeda, A., Oikawa, Y., Tamura, S., Tanaka, Y., Nakajima, S., et al. (2019). Probiotic Bifidobacterium bifidum G9-1 ameliorates phytohemagglutinin-induced diarrhea caused by intestinal dysbiosis. Microbiol. Immunol. 63, 481–486. doi: 10.1111/1348-0421.12743
Mann, E. R., Lam, Y. K., and Uhlig, H. H. (2024). Short-chain fatty acids: linking diet, the microbiome and immunity. Nat. Rev. Immunol. 24, 577–595. doi: 10.1038/s41577-024-01014-8
Mao, X., Liu, M., Tang, J., Chen, H., Chen, D., Yu, B., et al. (2015). Dietary leucine supplementation improves the mucin production in the Jejunal mucosa of the weaned pigs challenged by porcine rotavirus. PLoS One 10:e0137380. doi: 10.1371/journal.pone.0137380
Mao, X., Wu, Y., Ma, R., Li, L., Wang, L., Tan, Y., et al. (2023). Oral phage therapy with microencapsulated phage A221 against Escherichia coli infections in weaned piglets. BMC Vet. Res. 19:165. doi: 10.1186/s12917-023-03724-y
Martini, E., Krug, S. M., Siegmund, B., Neurath, M. F., and Becker, C. (2017). Mend your fences: The epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell. Mol. Gastroenterol. Hepatol. 4, 33–46. doi: 10.1016/j.jcmgh.2017.03.007
Mazzawi, T., Hausken, T., Hov, J. R., Valeur, J., Sangnes, D. A., El-Salhy, M., et al. (2019). Clinical response to fecal microbiota transplantation in patients with diarrhea-predominant irritable bowel syndrome is associated with normalization of fecal microbiota composition and short-chain fatty acid levels. Scand. J. Gastroenterol. 54, 690–699. doi: 10.1080/00365521.2019.1624815
McKinney, C. A., Oliveira, B. C. M., Bedenice, D., Paradis, M. R., Mazan, M., Sage, S., et al. (2020). The fecal microbiota of healthy donor horses and geriatric recipients undergoing fecal microbial transplantation for the treatment of diarrhea. PLoS One 15:e0230148. doi: 10.1371/journal.pone.0230148
Mei, L., Zhou, J., Su, Y., Mao, K., Wu, J., Zhu, C., et al. (2021). Gut microbiota composition and functional prediction in diarrhea-predominant irritable bowel syndrome. BMC Gastroenterol. 21:105. doi: 10.1186/s12876-021-01693-w
Min, S. J., Kim, H., Yambe, N., and Shin, M. S. (2024). Ameliorative effects of Korean-red-ginseng-derived polysaccharide on antibiotic-associated diarrhea. Polymers (Basel). 16:231. doi: 10.3390/polym16020231
Mizutani, T., Aboagye, S. Y., Ishizaka, A., Afum, T., Mensah, G. I., Asante-Poku, A., et al. (2021). Gut microbiota signature of pathogen-dependent dysbiosis in viral gastroenteritis. Sci. Rep. 11:13945. doi: 10.1038/s41598-021-93345-y
Morais, L. H., Schreiber, H. L. T., and Mazmanian, S. K. (2021). The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 19, 241–255. doi: 10.1038/s41579-020-00460-0
Morrison, D. J., and Preston, T. (2016). Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 7, 189–200. doi: 10.1080/19490976.2015.1134082
Murtaza, N., Nawaz, M., Yaqub, T., and Mehmood, A. K. (2024). Impact of Limosilactobacillus fermentum probiotic treatment on gut microbiota composition in sahiwal calves with rotavirus diarrhea: a 16S metagenomic analysis study. BMC Microbiol. 24:114. doi: 10.1186/s12866-024-03254-z
Navez, M., Antoine, C., Laforêt, F., Goya-Jorge, E., Douny, C., Scippo, M. L., et al. (2023). In vitro effect on piglet gut microbiota and in vivo assessment of newly isolated bacteriophages against F18 enterotoxigenic Escherichia coli (ETEC). Viruses 15:1053. doi: 10.3390/v15051053
Niu, Y., Li, J., Qian, H., Liang, C., Shi, X., and Bu, S. (2024). Evaluation of efficacy and safety of Lacticaseibacillus rhamnosus LRa05 in the eradication of Helicobacter pylori: a randomized, double-blind, placebo-controlled trial. Front. Immunol. 15:1450414. doi: 10.3389/fimmu.2024.1450414
Nobrega, F. L., Costa, A. R., Santos, J. F., Siliakus, M. F., van Lent, J. W., Kengen, S. W., et al. (2016). Genetically manipulated phages with improved pH resistance for oral administration in veterinary medicine. Sci. Rep. 6:39235. doi: 10.1038/srep39235
Ouyang, Y. W., Li, Z. T., Lv, J., Zhao, G. H., Wang, Y. L., and Cui, X. Y. (2022). Effect of fecal microbiota transplantation on 5-HT signaling pathway in the brain-gut axis of rats with diarrhea-type irritable bowel syndrome model. Int. J. Lab. Med. 43, 1994–1999. doi: 10.3969/j.issn.1673-4130.2022.16.01
Pang, F., Long, Q., and Wei, M. (2023). Immune evasion strategies of bovine viral diarrhea virus. Front. Cell. Infect. Microbiol. 13:1282526. doi: 10.3389/fcimb.2023.1282526
Parada Venegas, D., De la Fuente, M. K., Landskron, G., González, M. J., Quera, R., Dijkstra, G., et al. (2019). Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 10:277. doi: 10.3389/fimmu.2019.00277
Paul, J. K., Azmal, M., Haque, A., Meem, M., Talukder, O. F., and Ghosh, A. (2025). Unlocking the secrets of the human gut microbiota: comprehensive review on its role in different diseases. World J. Gastroenterol. 31:99913. doi: 10.3748/wjg.v31.i5.99913
Peng, H., Wang, Y., and Luo, W. (2020). Multifaceted role of branched-chain amino acid metabolism in cancer. Oncogene 39, 6747–6756. doi: 10.1038/s41388-020-01480-z
Pereira, G. Q., Gomes, L. A., Santos, I. S., Alfieri, A. F., Weese, J. S., and Costa, M. C. (2018). Fecal microbiota transplantation in puppies with canine parvovirus infection. J. Vet. Intern. Med. 32, 707–711. doi: 10.1111/jvim.15072
Pi, Y., Wu, Y., Zhang, X., Lu, D., Han, D., Zhao, J., et al. (2023). Gut microbiota-derived ursodeoxycholic acid alleviates low birth weight-induced colonic inflammation by enhancing M2 macrophage polarization. Microbiome 11:19. doi: 10.1186/s40168-022-01458-x
Pilla, R., and Suchodolski, J. S. (2019). The role of the canine gut microbiome and metabolome in health and gastrointestinal disease. Front. Vet. Sci. 6:498. doi: 10.3389/fvets.2019.00498
Quaglio, A. E. V., Grillo, T. G., De Oliveira, E. C. S., Di Stasi, L. C., and Sassaki, L. Y. (2022). Gut microbiota, inflammatory bowel disease and colorectal cancer. World J. Gastroenterol. 28, 4053–4060. doi: 10.3748/wjg.v28.i30.4053
Raimondi, F., Santoro, P., Barone, M. V., Pappacoda, S., Barretta, M. L., Nanayakkara, M., et al. (2008). Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G906–G913. doi: 10.1152/ajpgi.00043.2007
Ramirez, J., Guarner, F., Bustos Fernandez, L., Maruy, A., Sdepanian, V. L., and Cohen, H. (2020). Antibiotics as major disruptors of gut microbiota. Front. Cell. Infect. Microbiol. 10:572912. doi: 10.3389/fcimb.2020.572912
Ren, S., Wang, C., Chen, A., Lv, W., and Gao, R. (2022). The probiotic Lactobacillus paracasei ameliorates diarrhea cause by Escherichia coli O(8) via gut microbiota modulation(1). Front. Nutr. 9:878808. doi: 10.3389/fnut.2022.878808
Ren, M., Zhang, S., Liu, X., Li, S., Mao, X., Zeng, X., et al. (2016). Different lipopolysaccharide branched-chain amino acids modulate porcine intestinal endogenous β-defensin expression through the Sirt1/ERK/90RSK pathway. J. Agric. Food Chem. 64, 3371–3379. doi: 10.1021/acs.jafc.6b00968
Ren, M., Zhang, S. H., Zeng, X. F., Liu, H., and Qiao, S. Y. (2015). Branched-chain amino acids are beneficial to maintain growth performance and intestinal immune-related function in weaned piglets fed protein restricted diet. Asian Australas. J. Anim. Sci. 28, 1742–1750. doi: 10.5713/ajas.14.0131
Rengarajan, S., Knoop, K. A., Rengarajan, A., Chai, J. N., Grajales-Reyes, J. G., Samineni, V. K., et al. (2020). A potential role for stress-induced microbial alterations in IgA-associated irritable bowel syndrome with diarrhea. Cell Rep Med. 1:100124. doi: 10.1016/j.xcrm.2020.100124
Riccio, P., and Rossano, R. (2020). The human gut microbiota is neither an organ nor a commensal. FEBS Lett. 594, 3262–3271. doi: 10.1002/1873-3468.13946
Ridlon, J. M., Harris, S. C., Bhowmik, S., Kang, D. J., and Hylemon, P. B. (2016). Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 7, 22–39. doi: 10.1080/19490976.2015.1127483
Roshan, N., Clancy, A. K., and Borody, T. J. (2020). Faecal microbiota transplantation is effective for the initial treatment of Clostridium difficile infection: a retrospective clinical review. Infect. Dis. Ther. 9, 935–942. doi: 10.1007/s40121-020-00339-w
Sah, D. K., Arjunan, A., Park, S. Y., and Jung, Y. D. (2022). Bile acids and microbes in metabolic disease. World J. Gastroenterol. 28, 6846–6866. doi: 10.3748/wjg.v28.i48.6846
Sánchez, B., Delgado, S., Blanco-Míguez, A., Lourenço, A., Gueimonde, M., and Margolles, A. (2017). Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 61:10. doi: 10.1002/mnfr.201600240
Sarathy, J., Detloff, S. J., Ao, M., Khan, N., French, S., Sirajuddin, H., et al. (2017). The Yin and Yang of bile acid action on tight junctions in a model colonic epithelium. Physiol. Rep. 5:e13294. doi: 10.14814/phy2.13294
Sarker, S. A., Berger, B., Deng, Y., Kieser, S., Foata, F., Moine, D., et al. (2017). Oral application of Escherichia coli bacteriophage: safety tests in healthy and diarrheal children from Bangladesh. Environ. Microbiol. 19, 237–250. doi: 10.1111/1462-2920.13574
Sarker, S. A., and Brüssow, H. (2016). From bench to bed and back again: phage therapy of childhood Escherichia coli diarrhea. Ann. N. Y. Acad. Sci. 1372, 42–52. doi: 10.1111/nyas.13087
Sarker, S. A., Sultana, S., Reuteler, G., Moine, D., Descombes, P., Charton, F., et al. (2016). Oral phage therapy of acute bacterial diarrhea with two Coliphage preparations: a randomized trial in children from Bangladesh. EBioMedicine 4, 124–137. doi: 10.1016/j.ebiom.2015.12.023
Satitsri, S., Pongkorpsakol, P., Srimanote, P., Chatsudthipong, V., and Muanprasat, C. (2016). Pathophysiological mechanisms of diarrhea caused by the Vibrio cholerae O1 El Tor variant: an in vivo study in mice. Virulence 7, 789–805. doi: 10.1080/21505594.2016.1192743
Schiller, L. R., Pardi, D. S., and Sellin, J. H. (2017). Chronic diarrhea: diagnosis and management. Clin. Gastroenterol. Hepatol. 15, 182–193.e3. doi: 10.1016/j.cgh.2016.07.028
Sezer, A., Usta, U., and Cicin, I. (2009). The effect of Saccharomyces boulardii on reducing irinotecan-induced intestinal mucositis and diarrhea. Med. Oncol. 26, 350–357. doi: 10.1007/s12032-008-9128-1
Shao, H., Zhang, C., Xiao, N., and Tan, Z. (2020). Gut microbiota characteristics in mice with antibiotic-associated diarrhea. BMC Microbiol. 20:313. doi: 10.1186/s12866-020-01999-x
Shchikota, A. M., Pogonchenkova, I. V., Turova, E. A., Starodubova, A. V., and Nosova, N. V. (2021). COVID-19-associated diarrhea. Vopr. Pitan. 90, 18–30. doi: 10.33029/0042-8833-2021-90-6-18-30
Shen, J., Zhang, B., Chen, J., Cheng, J., Wang, J., Zheng, X., et al. (2022). SAHA alleviates diarrhea-predominant irritable bowel syndrome through regulation of the p-STAT3/SERT/5-HT signaling pathway. J. Inflamm. Res. 15, 1745–1756. doi: 10.2147/jir.S331303
Shen, J., Zhang, J., Mo, L., Li, Y., Li, Y., Li, C., et al. (2023). Large-scale phage cultivation for commensal human gut bacteria. Cell Host Microbe 31, 665–677.e7. doi: 10.1016/j.chom.2023.03.013
Shi, L. T., Peng, Z. Y., Tan, Q., Yin, J. L., Chen, C. Q., and Li, G. X. (2023). Progress of the study on the addition and subtraction of SiniSan to mediate the intestinal mucosal barrier against diarrhea-type irritable bowel syndrome. Asia-Pacific Traditional Medicine. 19, 218–222. doi: 10.11954/ytctyy.202304048
Shi, Z., Wang, Y., Yan, X., Ma, X., Duan, A., Hassan, F.u., et al. (2023). Metagenomic and metabolomic analyses reveal the role of gut microbiome-associated metabolites in diarrhea calves. mSystems 8:e0058223. doi: 10.1128/msystems.00582-23
Shin, D. Y., Yi, D. Y., Jo, S., Lee, Y. M., Kim, J. H., Kim, W., et al. (2020). Effect of a new Lactobacillus plantarum product, LRCC5310, on clinical symptoms and virus reduction in children with rotaviral enteritis. Medicine (Baltimore) 99:e22192. doi: 10.1097/md.0000000000022192
Singh, V., Lee, G., Son, H., Koh, H., Kim, E. S., Unno, T., et al. (2022). Butyrate producers, "The sentinel of gut": their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 13:1103836. doi: 10.3389/fmicb.2022.1103836
Song, M., Zhang, Z., Li, Y., Xiang, Y., and Li, C. (2023). Midgut microbiota affects the intestinal barrier by producing short-chain fatty acids in Apostichopus japonicus. Front. Microbiol. 14:1263731. doi: 10.3389/fmicb.2023.1263731
Spencer, N. J., and Hu, H. (2020). Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol. 17, 338–351. doi: 10.1038/s41575-020-0271-2
Spiegelhauer, M. R., Offersen, S. M., Mao, X., Gambino, M., Sandris Nielsen, D., Nguyen, D. N., et al. (2025). Protection against experimental necrotizing enterocolitis by fecal filtrate transfer requires an active donor virome. Gut Microbes 17:2486517. doi: 10.1080/19490976.2025.2486517
Steyer, A., Mičetić-Turk, D., and Fijan, S. (2022). The efficacy of probiotics as antiviral agents for the treatment of rotavirus gastrointestinal infections in children: An updated overview of literature. Microorganisms 10:2392. doi: 10.3390/microorganisms10122392
Stipanuk, M. H. (2007). Leucine and protein synthesis: mTOR and beyond. Nutr. Rev. 65, 122–129. doi: 10.1111/j.1753-4887.2007.tb00289.x
Stockmann, C., Pavia, A. T., Graham, B., Vaughn, M., Crisp, R., Poritz, M. A., et al. (2017). Detection of 23 gastrointestinal pathogens among children who present with diarrhea. J. Pediatr. Infect. Dis. Soc. 6, 231–238. doi: 10.1093/jpids/piw020
Strathdee, S. A., Hatfull, G. F., Mutalik, V. K., and Schooley, R. T. (2023). Phage therapy: from biological mechanisms to future directions. Cell 186, 17–31. doi: 10.1016/j.cell.2022.11.017
Su, W., Gong, T., Jiang, Z., Lu, Z., and Wang, Y. (2022). The role of probiotics in alleviating Postweaning diarrhea in piglets from the perspective of intestinal barriers. Front. Cell. Infect. Microbiol. 12:883107. doi: 10.3389/fcimb.2022.883107
Sun, X., Gao, Y., Wang, X., Hu, G., Wang, Y., Feng, B., et al. (2019). Escherichia coli O(101)-induced diarrhea develops gut microbial dysbiosis in rats. Exp. Ther. Med. 17, 824–834. doi: 10.3892/etm.2018.6997
Sun, Y. Y., Li, M., Li, Y. Y., Li, L. X., Zhai, W. Z., Wang, P., et al. (2018). The effect of Clostridium butyricum on symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. Sci. Rep. 8:2964. doi: 10.1038/s41598-018-21241-z
Sun, Y., Qu, Q., Huang, Y., Zhou, S., Xiang, H., and Wang, W. (2025). Isolation, characterization and therapeutic efficacy of lytic bacteriophage ZK22 against Salmonella Typhimurium in mice. BMC Microbiol. 25:39. doi: 10.1186/s12866-025-03772-4
Takeuchi, T., Miyauchi, E., Kanaya, T., Kato, T., Nakanishi, Y., Watanabe, T., et al. (2021). Acetate differentially regulates IgA reactivity to commensal bacteria. Nature 595, 560–564. doi: 10.1038/s41586-021-03727-5
Takiishi, T., Fenero, C. I. M., and Câmara, N. O. S. (2017). Intestinal barrier and gut microbiota: shaping our immune responses throughout life. Tissue Barriers. 5:e1373208. doi: 10.1080/21688370.2017.1373208
Tang, W., Chen, D., Yu, B., He, J., Huang, Z., Zheng, P., et al. (2020). Capsulized faecal microbiota transplantation ameliorates post-weaning diarrhoea by modulating the gut microbiota in piglets. Vet. Res. 51:55. doi: 10.1186/s13567-020-00779-9
Tian, P., Gao, J., Liang, L., Cui, B., Hu, Q., Zhou, W., et al. (2022). Fecal microbiota transplantation could improve chronic diarrhea in Cynomolgus monkey by alleviating inflammation and modulating gut microbiota. Biomedicine 10:3016. doi: 10.3390/biomedicines10123016
Tomita, T., Fukui, H., Okugawa, T., Nakanishi, T., Mieno, M., Nakai, K., et al. (2023). Effect of Bifidobacterium bifidum G9-1 on the intestinal environment and diarrhea-predominant irritable bowel syndrome (IBS-D)-like symptoms in patients with quiescent Crohn's disease: a prospective pilot study. J. Clin. Med. 12:3368. doi: 10.3390/jcm12103368
Tong, L. C., Wang, Y., Wang, Z. B., Liu, W. Y., Sun, S., Li, L., et al. (2016). Propionate ameliorates dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress. Front. Pharmacol. 7:253. doi: 10.3389/fphar.2016.00253
Tropini, C., Moss, E. L., Merrill, B. D., Ng, K. M., Higginbottom, S. K., Casavant, E. P., et al. (2018). Transient osmotic perturbation causes Long-term alteration to the gut microbiota. Cell 173, 1742–1754.e17. doi: 10.1016/j.cell.2018.05.008
Tubau-Juni, N., Bassaganya-Riera, J., Leber, A. J., Alva, S. S., Baker, R., and Hontecillas, R. (2023). Modulation of colonic immunometabolic responses during Clostridioides difficile infection ameliorates disease severity and inflammation. Sci. Rep. 13:14708. doi: 10.1038/s41598-023-41847-2
Tuniyazi, M., Tang, R., Hu, X., Fu, Y., and Zhang, N. (2024). Carbonate buffer mixture and fecal microbiota transplantation hold promising therapeutic effects on oligofructose-induced diarrhea in horses. Front Vet Sci. 11:1388227. doi: 10.3389/fvets.2024.1388227
Wahlström, A., Kovatcheva-Datchary, P., Ståhlman, M., Bäckhed, F., and Marschall, H. U. (2017). Crosstalk between bile acids and gut microbiota and its impact on Farnesoid X receptor Signalling. Dig. Dis. 35, 246–250. doi: 10.1159/000450982
Wang, Z., Guo, Z., Liu, L., Ren, D., Zu, H., Li, B., et al. (2024). Potential probiotic Weizmannia coagulans WC10 improved antibiotic-associated diarrhea in mice by regulating the gut microbiota and metabolic homeostasis. Probiotics Antimicrob Proteins. doi: 10.1007/s12602-024-10308-1
Wang, L., Guo, G., Xu, Y., Li, L., Yang, B., Zhao, D., et al. (2024). The effect of fecal microbiota transplantation on antibiotic-associated diarrhea and its impact on gut microbiota. BMC Microbiol. 24:160. doi: 10.1186/s12866-024-03261-0
Wang, J., Ji, H., Wang, S., Liu, H., Zhang, W., Zhang, D., et al. (2018a). Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front. Microbiol. 9:1953. doi: 10.3389/fmicb.2018.01953
Wang, L., Lei, J., Zhao, Z., Jia, J., and Wang, L. (2023). Therapeutic effects of paeoniflorin on irritable bowel syndrome in rats. J. Vet. Sci. 24:e23. doi: 10.4142/jvs.22083
Wang, Y. F., Liang, F. M., Liu, M., Ding, L. C., Hui, J. J., Xu, H. Y., et al. (2022). Is compromised intestinal barrier integrity responsible for the poor prognosis in critically ill patients with pre-existing hyperglycemia? Diabetol. Metab. Syndr. 14:172. doi: 10.1186/s13098-022-00943-5
Wang, F., Mei, X., Wang, Q., Zhao, P., Zhou, Y., Tang, L., et al. (2023). Compound Bacillus alleviates diarrhea by regulating gut microbes, metabolites, and inflammatory responses in pet cats. Anim Microbiome. 5:49. doi: 10.1186/s42523-023-00270-8
Wang, Y. Y., Tang, L. J., Li, J., Wang, J., Yan, X., Han, D. P., et al. (2019). Effect of Lactobacillus casei on the structure of ileal mucosa and MUC2 content in developmental diarrhea model rats. J. China Agric. Univ. 24, 94–101. doi: 10.11841/j.issn.1007-4333.2019.08.11
Wang, T., Teng, K., Liu, G., Liu, Y., Zhang, J., Zhang, X., et al. (2018). Lactobacillus reuteri HCM2 protects mice against Enterotoxigenic Escherichia coli through modulation of gut microbiota. Sci. Rep. 8:17485. doi: 10.1038/s41598-018-35702-y
Wang, Y., Wang, Y., Ding, K., Liu, Y., Liu, D., Chen, W., et al. (2024). Effectiveness of psychobiotic Bifidobacterium breve BB05 in managing psychosomatic diarrhea in college students by regulating gut microbiota: a randomized, double-blind, placebo-controlled trial. Nutrients 16:106541. doi: 10.3390/nu16131989
Wang, J., Zeng, Y., Wang, S., Liu, H., Zhang, D., Zhang, W., et al. (2018b). Swine-derived probiotic Lactobacillus plantarum inhibits growth and adhesion of Enterotoxigenic Escherichia coli and mediates host defense. Front. Microbiol. 9:1364. doi: 10.3389/fmicb.2018.01364
Wang, D., Zeng, J., Wujin, C., Ullah, Q., and Su, Z. (2024). Lactobacillus reuteri derived from horse alleviates Escherichia coli-induced diarrhea by modulating gut microbiota. Microb. Pathog. 188:106541. doi: 10.1016/j.micpath.2024.106541
Wang, Y., Zhang, S., Zhou, Q., Meng, M., and Chen, W. (2020). Efficacy of Shenlingbaizhu formula on irritable bowel syndrome: a systematic review. J. Tradit. Chin. Med. 40, 897–907. doi: 10.19852/j.cnki.jtcm.2020.06.001
Wei, S., Bahl, M. I., Baunwall, S. M. D., Dahlerup, J. F., Hvas, C. L., and Licht, T. R. (2022). Gut microbiota differs between treatment outcomes early after fecal microbiota transplantation against recurrent Clostridioides difficile infection. Gut Microbes 14:2084306. doi: 10.1080/19490976.2022.2084306
Wei, W., Wang, H., Zhang, Y., Zhang, Y., Niu, B., Chen, S., et al. (2021). Faecal bile acids and colonic bile acid membrane receptor correlate with symptom severity of diarrhoea-predominant irritable bowel syndrome: a pilot study. Dig. Liver Dis. 53, 1120–1127. doi: 10.1016/j.dld.2021.04.022
Wei, W., Wang, H. F., Zhang, Y., Zhang, Y. L., Niu, B. Y., and Yao, S. K. (2020). Altered metabolism of bile acids correlates with clinical parameters and the gut microbiota in patients with diarrhea-predominant irritable bowel syndrome. World J. Gastroenterol. 26, 7153–7172. doi: 10.3748/wjg.v26.i45.7153
Wieërs, G., Belkhir, L., Enaud, R., Leclercq, S., Philippart de Foy, J. M., Dequenne, I., et al. (2019). How probiotics affect the microbiota. Front. Cell. Infect. Microbiol. 9:454. doi: 10.3389/fcimb.2019.00454
Wilkins, T., and Sequoia, J. (2017). Probiotics for gastrointestinal conditions: a summary of the evidence. Am. Fam. Physician 96, 170–178. Available online at: http://www.aafp.org/afp
Witkowski, M., Weeks, T. L., and Hazen, S. L. (2020). Gut microbiota and cardiovascular disease. Circ. Res. 127, 553–570. doi: 10.1161/circresaha.120.316242
Wolf, J., Hubbard, S., Brauer, M., Ambelu, A., Arnold, B. F., Bain, R., et al. (2022). Effectiveness of interventions to improve drinking water, sanitation, and handwashing with soap on risk of diarrhoeal disease in children in low-income and middle-income settings: a systematic review and meta-analysis. Lancet 400, 48–59. doi: 10.1016/s0140-6736(22)00937-0
Wu, Y., Li, S., Lv, L., Jiang, S., Xu, L., Chen, H., et al. (2024). Protective effect of Pediococcus pentosaceus Li05 on diarrhea-predominant irritable bowel syndrome in rats. Food Funct. 15, 3692–3708. doi: 10.1039/d3fo04904c
Wu, H. J., and Wu, E. (2012). The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 3, 4–14. doi: 10.4161/gmic.19320
Wu, H., Zhan, K., Rao, K., Zheng, H., Qin, S., Tang, X., et al. (2022). Comparison of five diarrhea-predominant irritable bowel syndrome (IBS-D) rat models in the brain-gut-microbiota axis. Biomed. Pharmacother. 149:112811. doi: 10.1016/j.biopha.2022.112811
Xie, W., Song, L., Wang, X., Xu, Y., Liu, Z., Zhao, D., et al. (2021). A bovine lactoferricin-lactoferrampin-encoding Lactobacillus reuteri CO21 regulates the intestinal mucosal immunity and enhances the protection of piglets against enterotoxigenic Escherichia coli K88 challenge. Gut Microbes 13:1956281. doi: 10.1080/19490976.2021.1956281
Xu, Y., Jing, H., Wang, J., Zhang, S., Chang, Q., Li, Z., et al. (2022). Disordered gut microbiota correlates with altered fecal bile acid metabolism and post-cholecystectomy diarrhea. Front. Microbiol. 13:800604. doi: 10.3389/fmicb.2022.800604
Xu, R., Lei, Y. H., Shi, J., Zhou, Y. J., Chen, Y. W., and He, Z. J. (2016). Effects of lactadherin on plasma D-lactic acid and small intestinal MUC2 and claudin-1 expression levels in rats with rotavirus-induced diarrhea. Exp. Ther. Med. 11, 943–950. doi: 10.3892/etm.2016.3015
Xu, B., Liang, S., Zhao, J., Li, X., Guo, J., Xin, B., et al. (2022). Bifidobacterium animalis subsp. lactis XLTG11 improves antibiotic-related diarrhea by alleviating inflammation, enhancing intestinal barrier function and regulating intestinal flora. Food Funct. 13, 6404–6418. doi: 10.1039/d1fo04305f
Xu, C., Peng, K., She, Y., Fu, F., Shi, Q., Lin, Y., et al. (2023). Preparation of novel trivalent vaccine against enterotoxigenic Escherichia coli for preventing newborn piglet diarrhea. Am. J. Vet. Res. 84, 1–9. doi: 10.2460/ajvr.22.10.0183
Xu, H., Wang, S., Jiang, Y., Wu, J., Chen, L., Ding, Y., et al. (2023). Poria cocos polysaccharide ameliorated antibiotic-associated diarrhea in mice via regulating the homeostasis of the gut microbiota and intestinal mucosal barrier. Int. J. Mol. Sci. 24:ajvr.22.10.0183. doi: 10.3390/ijms24021423
Xu, B., Wang, Z., Wang, Y., Zhang, K., Li, J., Zhou, L., et al. (2024). Milk-derived Lactobacillus with high production of short-chain fatty acids relieves antibiotic-induced diarrhea in mice. Food Funct. 15, 5329–5342. doi: 10.1039/d3fo04706g
Xu, Y., Wang, J., Wu, X., Jing, H., Zhang, S., Hu, Z., et al. (2023). Gut microbiota alteration after cholecystectomy contributes to post-cholecystectomy diarrhea via bile acids stimulating colonic serotonin. Gut Microbes 15:2168101. doi: 10.1080/19490976.2023.2168101
Yan, Z., Liu, Z., Ma, Y., Yang, Z., Liu, G., and Fang, J. (2023). Effects of Lactobacillus plantarum and Weissella viridescens on the gut microbiota and serum metabolites of mice with antibiotic-associated diarrhea. Nutrients 15:4603. doi: 10.3390/nu15214603
Yang, Y., Du, H., Zou, G., Song, Z., Zhou, Y., Li, H., et al. (2023). Encapsulation and delivery of phage as a novel method for gut flora manipulation in situ: a review. J. Control. Release 353, 634–649. doi: 10.1016/j.jconrel.2022.11.048
Yang, Q., Hu, Z., Lei, Y., Li, X., Xu, C., Zhang, J., et al. (2023). Overview of systematic reviews of probiotics in the prevention and treatment of antibiotic-associated diarrhea in children. Front. Pharmacol. 14:1153070. doi: 10.3389/fphar.2023.1153070
Yang, B., Huang, Z., He, Z., Yue, Y., Zhou, Y., Ross, R. P., et al. (2021a). Protective effect of Bifidobacterium bifidum FSDJN7O5 and Bifidobacterium breve FHNFQ23M3 on diarrhea caused by enterotoxigenic Escherichia coli. Food Funct. 12, 7271–7282. doi: 10.1039/d1fo00504a
Yang, N., Ma, T., Xie, Y., Li, Q., Li, Y., Zheng, L., et al. (2024). Lactiplantibacillus plantarum P9 for chronic diarrhea in young adults: a large double-blind, randomized, placebo-controlled trial. Nat. Commun. 15:6823. doi: 10.1038/s41467-024-51094-2
Yang, B., Yue, Y., Chen, Y., Ding, M., Li, B., Wang, L., et al. (2021b). Lactobacillus plantarum CCFM1143 alleviates chronic diarrhea via inflammation regulation and gut microbiota modulation: a double-blind, randomized, placebo-controlled study. Front. Immunol. 12:746585. doi: 10.3389/fimmu.2021.746585
Yang, L., Zhang, Q., Huang, J., Liu, D., Lan, Y., Yuan, L., et al. (2021). Xianglian pill ameliorates antibiotic-associated diarrhea by restoring intestinal microbiota and attenuating mucosal damage. J. Ethnopharmacol. 264:113377. doi: 10.1016/j.jep.2020.113377
Yao, D., Dai, W., Dong, M., Dai, C., and Wu, S. (2021). MUC2 and related bacterial factors: therapeutic targets for ulcerative colitis. EBioMedicine 74:103751. doi: 10.1016/j.ebiom.2021.103751
Yau, Y. K., Su, Q., Xu, Z., Tang, W., Ching, J. Y. L., Mak, J. W. Y., et al. (2023). Randomised clinical trial: Faecal microbiota transplantation for irritable bowel syndrome with diarrhoea. Aliment. Pharmacol. Ther. 58, 795–804. doi: 10.1111/apt.17703
Youssef, R. A., Sakr, M. M., Shebl, R. I., and Aboshanab, K. M. (2025). Recent insights on challenges encountered with phage therapy against gastrointestinal-associated infections. Gut Pathog. 17:60. doi: 10.1186/s13099-025-00735-y
Yu, H., Nie, R., and Shen, C. (2023). The role of bile acids in regulating glucose and lipid metabolism. Endocr. J. 70, 359–374. doi: 10.1507/endocrj.EJ22-0544
Yue, Y., He, Z., Zhou, Y., Ross, R. P., Stanton, C., Zhao, J., et al. (2020). Lactobacillus plantarum relieves diarrhea caused by enterotoxin-producing Escherichia coli through inflammation modulation and gut microbiota regulation. Food Funct. 11, 10362–10374. doi: 10.1039/d0fo02670k
Yue, S. J., Wang, W. X., Yu, J. G., Chen, Y. Y., Shi, X. Q., Yan, D., et al. (2019). Gut microbiota modulation with traditional Chinese medicine: a system biology-driven approach. Pharmacol. Res. 148:104453. doi: 10.1016/j.phrs.2019.104453
Zeng, Y., Wang, Z., Zou, T., Chen, J., Li, G., Zheng, L., et al. (2021). Bacteriophage as an alternative to antibiotics promotes growth performance by regulating intestinal inflammation, intestinal barrier function and gut microbiota in weaned piglets. Front Vet Sci. 8:623899. doi: 10.3389/fvets.2021.623899
Zhai, L., Huang, C., Ning, Z., Zhang, Y., Zhuang, M., Yang, W., et al. (2023). Ruminococcus gnavus plays a pathogenic role in diarrhea-predominant irritable bowel syndrome by increasing serotonin biosynthesis. Cell Host Microbe 31, 33–44.e5. doi: 10.1016/j.chom.2022.11.006
Zhan, M., Liang, X., Chen, J., Yang, X., Han, Y., Zhao, C., et al. (2023). Dietary 5-demethylnobiletin prevents antibiotic-associated dysbiosis of gut microbiota and damage to the colonic barrier. Food Funct. 14, 4414–4429. doi: 10.1039/d3fo00516j
Zhang, P. (2022). Influence of foods and nutrition on the gut microbiome and implications for intestinal health. Int. J. Mol. Sci. 23:9588. doi: 10.3390/ijms23179588
Zhang, M. M., Dang, M., Wu, X., Ou, L., Li, M., Zhao, C. B., et al. (2024). Da-Jian-Zhong decoction alleviates diarrhea-predominant irritable bowel syndrome via modulation of gut microbiota and Th17/Treg balance. J. Ethnopharmacol. 331:118275. doi: 10.1016/j.jep.2024.118275
Zhang, Z., Huang, J., Li, C., Zhao, Z., Cui, Y., Yuan, X., et al. (2024). The gut microbiota contributes to the infection of bovine viral diarrhea virus in mice. J. Virol. 98:e0203523. doi: 10.1128/jvi.02035-23
Zhang, D., Jian, Y. P., Zhang, Y. N., Li, Y., Gu, L. T., Sun, H. H., et al. (2023). Short-chain fatty acids in diseases. Cell Commun. Signal 21:212. doi: 10.1186/s12964-023-01219-9
Zhang, C. Y., Peng, X. X., Shao, H. Q., Li, X. Y., Wu, Y., and Tan, Z. J. (2021). Gut microbiota comparison between intestinal contents and mucosa in mice with repeated stress-related diarrhea provides novel insight. Front. Microbiol. 12:626691. doi: 10.3389/fmicb.2021.626691
Zhang, Y., Wang, S., Wang, H., Cao, M., Wang, M., Zhang, B., et al. (2024). Efficacy of donor-recipient-matched faecal microbiota transplantation in patients with IBS-D: a single-Centre, randomized, double-blind placebo-controlled study. Digestion 105, 457–467. doi: 10.1159/000540420
Zhao, J., Feng, L., Liu, Y., Jiang, W., Wu, P., Jiang, J., et al. (2014). Effect of dietary isoleucine on the immunity, antioxidant status, tight junctions and microflora in the intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol. 41, 663–673. doi: 10.1016/j.fsi.2014.10.002
Zhao, L., Yang, W., Chen, Y., Huang, F., Lu, L., Lin, C., et al. (2020). A Clostridia-rich microbiota enhances bile acid excretion in diarrhea-predominant irritable bowel syndrome. J. Clin. Invest. 130, 438–450. doi: 10.1172/jci130976
Zhen, Y., Chu, C., Zhou, S., Qi, M., and Shu, R. (2015). Imbalance of tumor necrosis factor-α, interleukin-8 and interleukin-10 production evokes barrier dysfunction, severe abdominal symptoms and psychological disorders in patients with irritable bowel syndrome-associated diarrhea. Mol. Med. Rep. 12, 5239–5245. doi: 10.3892/mmr.2015.4079
Zhen, Z., Xia, L., You, H., Jingwei, Z., Shasha, Y., Xinyi, W., et al. (2021). An integrated gut microbiota and network pharmacology study on Fuzi-Lizhong pill for treating diarrhea-predominant irritable bowel syndrome. Front. Pharmacol. 12:746923. doi: 10.3389/fphar.2021.746923
Zheng, Y. M., He, X. X., Xia, H. H., Yuan, Y., Xie, W. R., Cai, J. Y., et al. (2020). Multi-donor multi-course faecal microbiota transplantation relieves the symptoms of chronic hemorrhagic radiation proctitis: a case report. Medicine (Baltimore) 99:e22298. doi: 10.1097/md.0000000000022298
Zhou, Y., Zhang, D., Cheng, H., Wu, J., Liu, J., Feng, W., et al. (2024). Repairing gut barrier by traditional Chinese medicine: roles of gut microbiota. Front. Cell. Infect. Microbiol. 14:1389925. doi: 10.3389/fcimb.2024.1389925
Zhu, Y., Luo, Q., Davis, S. M., Westra, C., Vickers, T. J., and Fleckenstein, J. M. (2018). Molecular determinants of Enterotoxigenic Escherichia coli heat-stable toxin secretion and delivery. Infect. Immun. 86:e00526-18. doi: 10.1128/iai.00526-18
Zhu, S. S., Wang, J. J., Zou, L., Chen, J. Y., Li, K. W., Liao, L. M., et al. (2022). Anti-inflammation effect of moxibustion for rats with diarrhea-predominant irritable bowel syndrome based on multiple miRNAs regulating NF-κB signal pathway. Zhongguo Zhen Jiu 42, 654–662. doi: 10.13703/j.0255-2930.20210521-k0002
Keywords: diarrhea, gut microbiota, metabolites, probiotics, fecal microbiota transplantation, bacteriophage therapy
Citation: Tian R, Chong C-J, Bai Y-Y, Chen N, Qiao R-R, Wang K, Wang Y-W, Zhao P, Zhao C-B, Tang Y-P, Zhang L and Zhang Q (2025) The role of gut microbiota in diarrhea and its alleviation through microbiota-targeted interventions. Front. Microbiol. 16:1630823. doi: 10.3389/fmicb.2025.1630823
Edited by:
Junling Shi, Northwestern Polytechnical University, ChinaReviewed by:
Lie Zheng, Shaanxi Provincial Hospital of Traditional Chinese Medicine, ChinaYongpeng Shi, University of Science and Technology of China, China
Copyright © 2025 Tian, Chong, Bai, Chen, Qiao, Wang, Wang, Zhao, Zhao, Tang, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiao Zhang, MTg3MDAwODExODRAMTYzLmNvbQ==
 Rui Tian
Rui Tian Chu-Jun Chong
Chu-Jun Chong Ya-Ya Bai
Ya-Ya Bai Ni Chen
Ni Chen Rui-Rui Qiao
Rui-Rui Qiao Kan Wang
Kan Wang Yu-Wei Wang
Yu-Wei Wang Peng Zhao
Peng Zhao Chong-Bo Zhao
Chong-Bo Zhao Yu-Ping Tang
Yu-Ping Tang Li Zhang
Li Zhang Qiao Zhang
Qiao Zhang