- 1Hebei Provincial Key Laboratory of Preventive Veterinary Medicine, Hebei Normal University of Science and Technology, Qinhuangdao, China
- 2Hebei Provincial Center for Livestock Breeding Improvement, Shijiazhuang, China
- 3Key Laboratory of Animal Biosafe Risk Prevention and Control (North), Ministry of Agriculture and Rural Affairs, Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing, China
- 4College of Animal Medicine, Huazhong Agricultural University, Wuhan, China
- 5The Second Hospital of Qinhuangdao, Qinhuangdao, China
Background: Salmonellosis caused by Salmonella sp. is a foodborne zoonotic disease that poses a significant threat to public health security. Vaccination is a safe and effective strategy for preventing and controlling Salmonella infections. PipC is a chaperone protein associated with Salmonella invasion proteins which is crucial for bacteria to invade host cells.
Methods: In this study, a ΔpipC mutant strain was generated. Subsequently, we examined the environmental stress tolerance of the mutant strain through in vitro simulation experiments. Moreover, its virulence by employing cell and mouse infection models was investigated. Furthermore, we utilized a mouse model to further explore its potential as an attenuated live vaccine against Salmonella enterica serovar Enteritidis infection.
Results: The Salmonella strain C50336 with a deletion of the pipC gene exhibits a significant reduction in its ability to resist environmental stress and virulence. Meanwhile, the expression levels of SPI-1-related genes (invH, sipA, sipB, sipC, sopB, and sopE2) and SPI-2-related genes (spvB, ssrA, orf245, ssaS, ssaT, ssaU, sseB, and sseD) encoding the Salmonella type III secretion system (T3SS) were found to be decreased, leading to a significant reduction in the bacteria’s invasion and intracellular survival abilities. The results of the mouse intraperitoneal challenge experiment showed that compared with the wild-type strain, the 50% lethal dose (LD50) of the ΔpipC strain increased by 47 times, and the bacterial loads in the liver, spleen, and cecum were significantly reduced. When mice were immunized with the ΔpipC mutant strain, the immunized mice showed a robust immune response, with significantly increased cytokine and antibody levels in their bodies. Mice vaccinated with the ΔpipC mutant strain had 100% immune protection against wild-type Salmonella infection.
Conclusion: This study demonstrates that lack of pipC affects SE pathogenicity by decreasing its virulence both in vitro and in vivo. Vaccination of mice with ΔpipC conferred development of an acquired immunity and efficacious protection against experimental systemic infection. These results indicated that the ΔpipC mutant strain can be used in the development of attenuated live vaccines.
Background
Salmonella is a facultative intracellular pathogen that belongs to the Gram-negative category and exhibits a remarkable ability to infect a diverse range of animals, including humans. This broad host range not only poses a severe threat to the healthy development of the global aquaculture industry but also undermines public health safety, resulting in substantial economic losses in various aspects (Ferrari et al., 2019). Among these, Salmonella enteritidis (SE) and Salmonella Typhimurium are the main serotypes that infect humans, accounting for approximately 40% of human salmonellosis cases (Cao et al., 2023). The transmission routes of SE to humans are diverse. It can be contracted through the consumption of contaminated food products such as pork, beef, poultry, and eggs. Additionally, in areas with poor sanitation where fecal matter exposure is more likely, the risk of human infection also increases significantly. Once infected, humans may experience a series of symptoms, including abdominal pain, diarrhea, nausea, vomiting, fever, and headaches, which can greatly affect their quality of life and overall health (Guard-Petter, 2001).
Antibiotics are commonly used to treat Salmonella infections, but their overuse leads to environmental pollution and accelerates the rise of multidrug-resistant strains. This not only reduces treatment effectiveness but also poses a significant threat to public health. In this context, vaccination has emerged as another crucial measure for the prevention and control of Salmonella infections, as emphasized by Ruvalcaba-Gómez et al. (2022) and Acevedo-Villanueva et al. (2021). Given the facultative intracellular nature of Salmonella, strong cellular immunity plays a vital role in clearing the pathogen. As a result, attenuated live vaccines are generally considered to offer more effective immune protection compared to other types of vaccines, as demonstrated by the research of Lin et al. (2017) and Jiang et al. (2022). Moreover, previous studies have shown that attenuated live vaccines of Salmonella have relatively low virulence to the host. They are capable of inducing a robust and long-lasting mucosal and humoral immune response, as pointed out by Tennant and Levine (2015). This immune response can effectively reduce bacterial adhesion and colonization within the host organism.
Currently, numerous Salmonella gene knockout strains have been utilized as live vaccines. For instance, Zhao et al. (2024) found in 2024 that immunizing mice with a Salmonella strain with the pal gene deleted could stimulate good immune protection. Zhang et al. (2024, 2025) found that an attenuated Salmonella enterica vaccine with mcpC and cheV gene knockouts was able to stimulate 100% immune protection in mice. In addition, Yin et al. (2022) and Kamble and Lee (2016) also demonstrated that attenuated vaccines prepared by deleting virulence genes such as cpxR, lon, and SPI2 were effective in reducing the colonization of wild-type strains in chickens and provided good immune protection. Overall, the exploration and improvement of Salmonella attenuated live vaccines based on gene deletion, are of great significance for safeguarding public health and promoting the sustainable development of the aquaculture industry. Salmonella Pathogenicity Island 5 (SPI-5) plays a critical role in the enteropathogenicity of Salmonella. It encodes five proteins, namely PipA, PipB, PipC, PipD, and SopB, that are involved in mucosal secretion and inflammatory responses in the intestine. These proteins are regulated by the type III secretion systems (T3SS) encoded by SPI-1 and SPI-2 (Wang et al., 2020). Devendra H. Shah et al. reported that sopB and pipB/C are co-regulated with SPI-1 and promote host cell invasion, suggesting that pipC may contribute to the invasive capacity of Salmonella (Darwin et al., 2001; Rodríguez-Escudero et al., 2011). Previous studies have shown that deletion of SPI-5 reduces the ability of SE to colonize the chicken intestine (Rychlik et al., 2009; Shah et al., 2012). Furthermore, pipC has been implicated in the folding of key virulence factors, including the T3SS effector protein SopB. Additionally, literature reports indicate that the expression level of pipC is significantly reduced in macrophages compared to bacteria in the early stationary phase (ESP) under in vitro conditions, suggesting a potential role of pipC in intracellular survival within macrophages. Taken together, these findings indicate that pipC may influence the virulence of SE, though further experimental validation is required.
To further elucidate the role of pipC in Salmonella infection and its contribution to immunoprotection, this study aims to construct a pipC gene deletion mutant of SE. The effects of this gene deletion on bacterial virulence will be evaluated through both in vitro and in vivo assays, and the immunoprotective efficacy of the deletion strain will be assessed in a mouse model.
Materials and methods
Bacterial strains, plasmids and cells
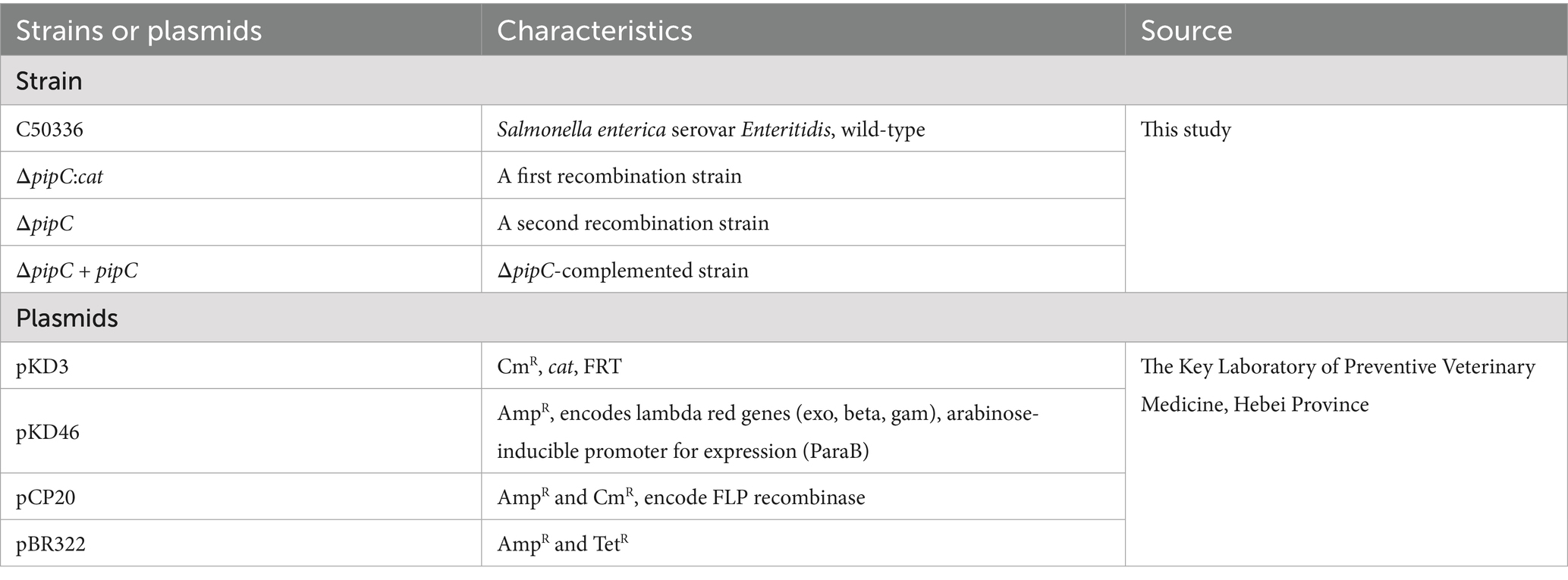
The bacterial strains and plasmids used in this study are shown in Table 1. Salmonella enterica serovar Enteritidis C50336 was the wild-type strain and used for constructing the ΔpipC mutant. The ΔpipC strain in this study was constructed following the λ-Red recombinase gene replacement method (Datsenko and Wanner, 2000). The primer sequences used for generating and confirming mutant strains are listed in Table 2. All bacterial strains were cultured on Luria-Bertani (LB) agar plates or in LB broth with necessary antibiotics at appropriate concentrations (for example, 100 μg/mL ampicillin and 34 μg/mL chloramphenicol) (Xiong et al., 2023).
Human epithelial Caco-2 BBE cells and mouse macrophage RAW264.7 cells used in this study were provided by BeNa Culture Collection (Shanghai, China). Both cell types were cultured in DMEM (Thermo Fisher Scientific Co., Ltd.) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific Co., Ltd.). Antibiotics were added as necessary, such as 50 μg/mL streptomycin and 50 U/mL penicillin, or 50 μg/mL gentamicin, in an incubator with 5% CO₂.
Experimental animals and ethical statement
Kunming (KM) mice were obtained from Beijing Speifu Biotechnology Co., Ltd. Throughout the experiment, the mice were maintained in a sterile environment under standard housing conditions with an ambient temperature consistently kept at 22.0 ± 0.5°C and relative humidity maintained at 60 ± 10%. A 12-h light/dark cycle was established for the housing conditions. All animal experiments were conducted in full compliance with international ethical standards and the Experimental Animal Regulation Ordinances (HPDST 2020-17) stipulated by the Hebei Provincial Department of Science and Technology. The study protocol was reviewed and approved by the Animal Care and Use Committee of Hebei Normal University of Science and Technology.
Construction of pipC gene deletion and complementation strains of SE
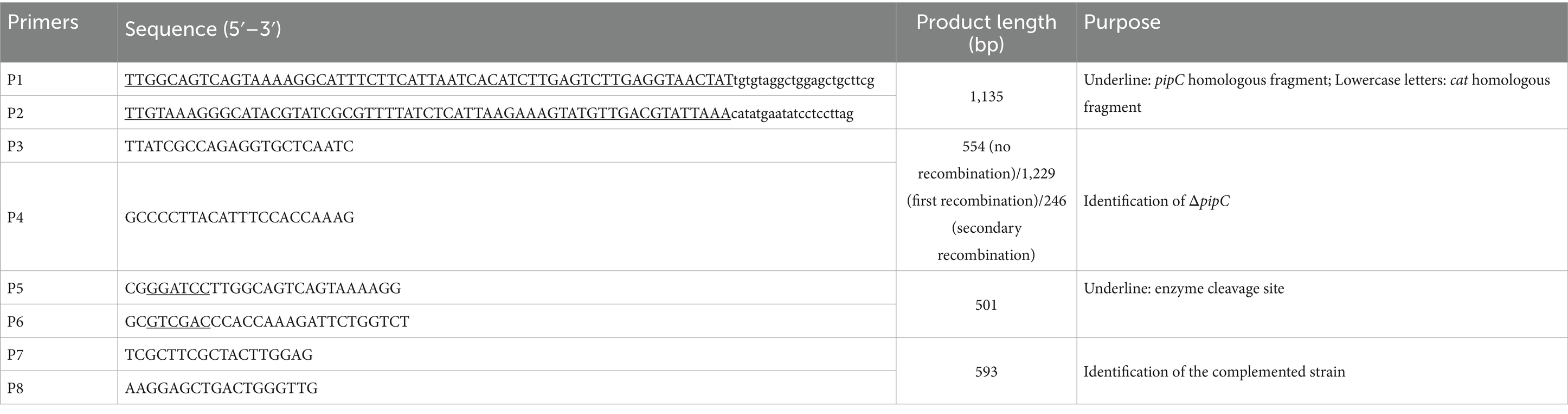
The pipC gene deletion strain in this study was constructed using the λ homologous recombination method (Supplementary Figure 1). Briefly, the auxiliary plasmid pKD46 was introduced into C50336 through electroporation, which encodes the Gam, Exo, and Beta proteins required for λ homologous recombination. Using pKD3 as a template, the knockout fragments were amplified by P1 and P2. The knockout fragment was then introduced into C50336 containing pKD46 via electroporation, resulting in a primary recombinant strain with chloramphenicol resistance. This strain was selected using LB agar plates containing chloramphenicol and verified using primers P3 and P4. The positive strain obtained was named ΔpipC:cat. The pCP20 plasmid, which encodes the Flp recombinase, was able to excise the cat gene from the knockout fragment. The pCP20 plasmid was introduced into ΔpipC:cat by electroporation, resulting in a secondary recombinant strain. This strain was verified using primers P3 and P4. The positive strain obtained was named ΔpipC.
To construct the complement strain, the nucleic acids of C50336 were used as a template, and the complement fragment was amplified using P5 and P6. The complement fragment and pBR322 vector plasmid were digested with restriction endonucleases BamH I and Sal I, respectively. The two digested fragments were ligated using T4 DNA ligase. The recombinant vector was introduced into ΔpipC by electroporation and verified with P7 and P8. The positive strain was named ΔpipC + pipC.
Genetic stability testing
To determine the genetic stability of the ΔpipC, it was serially passaged 40 times in LB medium, every 12 h. Nucleic acids from liquid cultures are extracted every other generation and PCR verification using P3 and P4.
Growth characteristics assay
The overnight cultures of C50336, ΔpipC, and ΔpipC + pipC were subcultured at a 1:100 ratio into 5 mL of LB liquid medium and incubated at 37°C in a shaking incubator. Growth was determined by monitoring the absorbance of bacterial cultures at 600 nm (OD600 values). Growth curves were plotted based on the growth of each strain at different time points.
In vitro stress simulation experiments
Overnight cultures of C50336, ΔpipC, and ΔpipC + pipC were washed three times with PBS and resuspended in the original volume. The bacterial counts before stress were determined using the traditional plate count method. The bacterial suspensions were exposed to acid stress (pH 3.5), alkaline stress (pH 10.0), and heat stress (42°C) for 1 h, as well as to oxidative stress (10 mmol/L H2O2) for 30 min. After stress exposure, the bacterial counts were determined. The survival rate of each strain under different conditions was calculated as follows: survival rate = (post-stress bacterial count)/(initial bacterial count).
Cell culture
The human epithelial cancer cell lines Caco-2 were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 20% fetal bovine serum (FBS) and 1% penicillin-streptomycin solution. The mouse macrophage RAW264.7 was cultured in DMEM containing 10% FBS and 1% penicillin-streptomycin solution. When the cells reached 80% confluence, the monolayers were washed three times with PBS. The cells were then seeded in 12-well tissue culture plates at a density of 1 × 106 cells/well. The plates were incubated at 37°C in an atmosphere containing 5% CO2.
Adherence and invasion assays
To investigate the impact of pipC gene deletion on the adhesion and invasion ability of SE, the overnight cultures of C50336, ΔpipC and ΔpipC + pipC were washed three times with PBS and subsequently resuspended. The number of bacteria per mL of bacterial suspension (number of infected bacteria) was measured. Following the washing of confluent cell monolayers with DMEM, C50336, ΔpipC and ΔpipC + pipC were inoculated, respectively, onto the Caco-2 cells at a multiplicity of infection (MOI) of 100:1 and were incubated for 1 h at 37°C under 5% CO2 (Xiong et al., 2023).
Adhesion assay
For bacterial adhesion, the cells were washed, and then incubated with PBS containing Triton X-100 (0.5%) at 37°C for 10 min. The cell lysates were serially diluted and inoculated onto LB agar for counting. The number of bacteria per mL of cell lysate (number of adherent bacteria) was measured. The adhesion rate was calculated using the formula: Adhesion rate = (number of adherent bacteria/number of infected bacteria) × 100%.
Invasion assay
For bacterial invasion, 1 h after bacterial colonization, the cells were incubated for an additional 1 h in DMEM with gentamicin (100 μg/mL), washed and incubated with PBS containing Triton X-100 (0.5%) at 37°C for 10 min. The cell lysates were serially diluted and inoculated onto LB agar for counting. The number of bacteria per mL of cell lysate (number of invading bacteria) was measured. Invasion rate = (number of invading bacteria/number of infected bacteria) × 100%.
Intracellular proliferation assay
To evaluate the survival rate of ΔpipC in phagocytic cells, after infecting the cells with bacteria for 2 h as described above, DMEM containing 100 μg/mL gentamicin was added and incubated for 1 h. One group of cells was then washed and lysed with 0.5% Triton X-100, and the intracellular bacteria were counted (intracellular bacteria at 3 h). Another group of cells was washed and incubated with DMEM containing 10 μg/mL gentamicin at 37°C for 20 h. These cells were also lysed with 0.5% Triton X-100, and the intracellular bacteria were counted (intracellular bacteria at 23 h). Intracellular survival rate = (intracellular bacteria at 23 h/intracellular bacteria at 3 h) × 100%.
Assessment of bacterial virulence
A total of 75 six-week-old KM mice were randomly divided into 15 groups (n = 5). These groups were categorized into three sets: five groups for the ΔpipC, five groups for the C50336, and the remaining five groups designated as the ΔpipC + pipC group. Mice in the ΔpipC groups were intraperitoneally (i.p.) inoculated with ΔpipC containing of 1.68 × 109, 1.68 × 108, 1.68 × 107, 1.68 × 106 or 1.68 × 105 CFU/mouse, respectively. Similarly, the C50336 groups were i.p. inoculated with C50336 containing of 2 × 107, 2 × 106, 2 × 105, 2 × 104 or 2 × 103 CFU/mouse, respectivly. The ΔpipC + pipC groups were i.p. inoculated with ΔpipC + pipC containing of 2 × 107, 2 × 106, 2 × 105, 2 × 104 or 2 × 103 CFU/mouse, respectively. Five additional mice were i.p. injected with the same volume of PBS as a negative control.
The number of dead mice was recorded for 14 days and LD50 was calculated, which was calculated using the formula of log10 [50% endpoint] = A + (B × C), where A = log10 [infectious dose showing a mortality next below 50%], B = difference of logarithms = [50% − (mortality at infectious dose next below 50%)]/[(mortality next above 50%) − (mortality next below 50%)], and C = log10 [difference between serial infectious doses used in challenge studies] (Park et al., 2022; Zhang et al., 2019).
RNA extraction and qPCR
In order to further investigate the effect of pipC gene deletion on the virulence of SE, qPCR was used to measure the expression levels of virulence genes in C50336, ΔpipC, and ΔpipC + pipC. In short, the bacteria were cultured to the logarithmic phase and total RNA was extracted using an RNA extraction kit (Aidlab, Beijing, China). Complementary DNA (cDNA) was synthesized using a reverse transcription kit (TOYOBO, Osaka, Japan). Primers were designed by referring to previous literature (Upadhyaya et al., 2013). The primer sequences used for qPCR are listed in Table 3. Using cDNA as a template, the relative gene expression was quantified using the comparative critical threshold (Ct) method through qPCR. Data were normalized to the endogenous control (16S rRNA), and the level of candidate gene expression between treated and control samples were determined. The qPCR thermal cycling conditions were as follows: initial denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min.
Bacterial colonization assay
Thirty mice were randomly divided into two groups (n = 15). One group was intraperitoneally injected with 1 × 104 CFU/mouse of ΔpipC, while the other group received an equal dose of C50336. Additionally, three mice were injected with 200 μL of PBS as a negative control. At predetermined time points (3 days, 7 days, and 14 days), five mice from each group were randomly selected and euthanized. Their spleens, livers, and cecums were collected, homogenized in PBS, and subsequently serially diluted before being evenly spread on XLT4 agar. The number of CFUs for each sample was determined 12 h later. For this animal study, all mice were anesthetized using a 20% urethane (ethyl carbamate) solution, and every effort was made to ensure humane treatment of the animals.
Vaccination schedules of oral attenuated live vaccine and sample collection
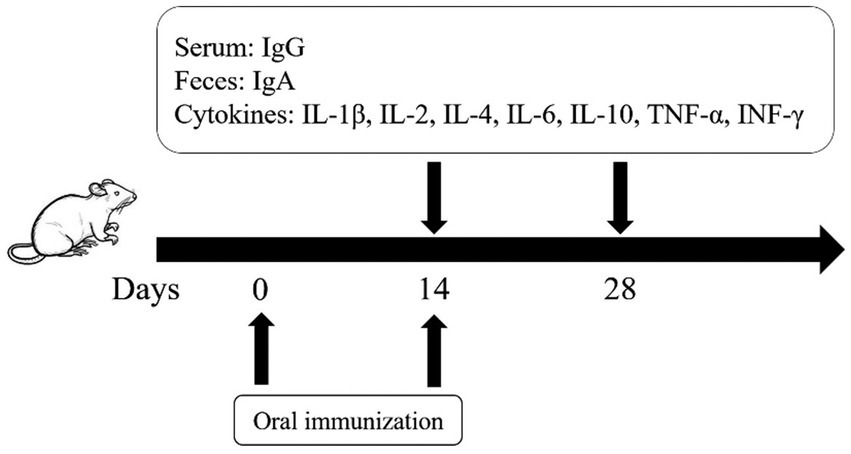
Twenty-four female KM mice, aged 8 weeks, were randomly divided into two groups (n = 12): an immunization group and a control group. On day 0, the immunization group received an oral administration of 1 × 106 CFU of ΔpipC, whereas the control group was orally administered 200 μL of PBS. A booster immunization with an identical dose was given to the immunization group on day 14 dpi, while the control group received an equivalent volume of PBS.
At 14 dpi and 28 dpi, 6 mice from each group were randomly euthanized, and spleens were collected for splenic lymphocyte stimulation test and detection of cytokine expression levels. Additionally, serum and feces were collected from 3 mice for IgG and SIgA detection. The vaccination regimen of this study is shown in Figure 1.
Salmonella soluble antigen preparation
C50336 was cultivated overnight at 37°C, 180 rpm with shaking until it reached the logarithmic phase. The bacteria were centrifuged at a speed of 13,000 × g at 4°C, washed three times with PBS, and resuspended in PBS to a concentration of 1 × 1010 CFU/mL. Use SCIENTZ ultrasonic homogenizer (SCIENTZ-IID, Ningbo, China) to sonicate the cells and centrifuge the 13,000 × g sample at 4°C to granulate the fragments. The sample was sterilized through a 0.22 μm PES (polyethersulfone) filter (Merck, Darmstadt, Germany) (Ji et al., 2022).
Splenic lymphocytes stimulation test
Lymphocytes were isolated from each group of three spleen samples at 14 and 28 dpi. After Trypan blue dye exclusion testing, suspensions of splenic mononuclear cells (1 × 107 cells/well) were cultured in Roswell Park Memorial Institute 1,640 medium (RPMI-1640) supplemented with 10% FBS and 100 μg/mL penicillin-streptomycin within 96-well tissue culture plates. These cultures were then incubated with 10 μg/mL soluble antigen or without any stimulant as a negative control, under conditions of 37°C and 5% CO₂ for a duration of 72 h. Lymphocyte proliferation was measured using an MTT kit (Beyotime, Shanghai, China). The cell proliferation was expressed as the stimulation index (SI), which was calculated using the equation: SI = (OD570 of the antigen-stimulated cells)/(OD570 of the unstimulated cells) (Kang et al., 2021).
The expression of cytokines in the spleen
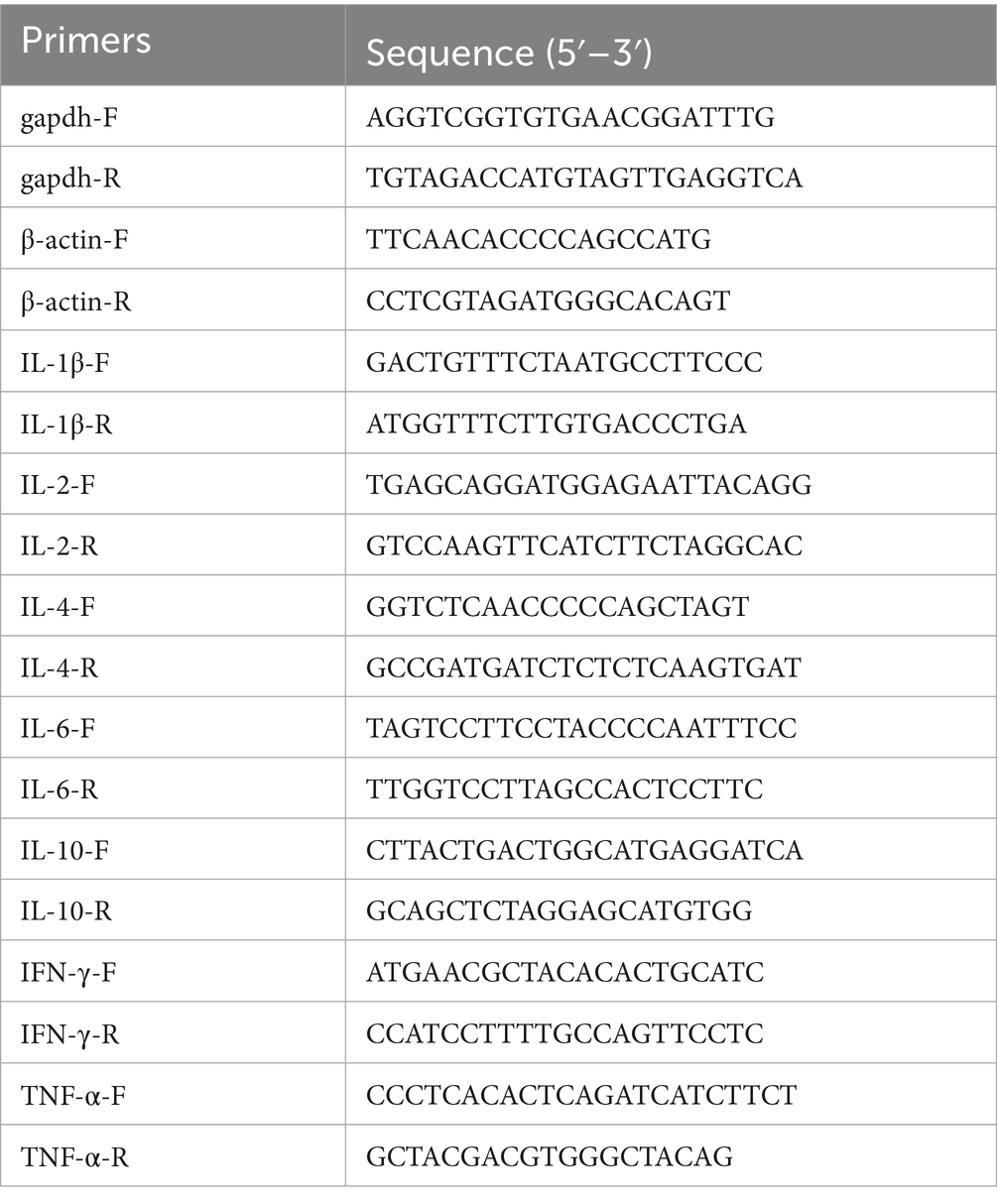
Quantitative real-time PCR (qPCR) was employed to assess the mRNA expression levels of splenic cytokines, including IL-1β, IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ, at 14 and 28 days post-immunization (dpi). Total RNA was extracted from spleen tissues using Triquick Reagent (Solarbio, Beijing, China), and first-strand cDNA was synthesized with a cDNA synthesis kit (TOYOBO, Osaka, Japan). Samples were stored at −80°C until further use. qPCR was performed using the Ultra SYBR Green Mixture (CWBio, Jiangsu, China) on a Lepgen-96 Real-Time PCR System (LEpu, Lepgen-96, China). The primer sequences used for qPCR are listed in Table 4. Cytokine expression levels were normalized to the internal reference genes gapdh and β-actin, and relative expression was calculated using the 2−ΔΔCt method (Kang et al., 2021). The thermal cycling conditions were as follows: initial denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s and annealing/extension at 60°C for 1 min.
Detection of IgG and SIgA
To examine the antibody responses, serum and feces samples were collected from 3 mice per group at 14 and 28 dpi after inoculation with the initial and booster doses of ΔpipC. Enzyme-linked immunosorbent assay (ELISA) was employed to quantify the serum IgG and fecal IgA (SIgA) responses against the soluble antigen derived from SE. For serum extraction, whole blood samples were centrifuged for 10 min at 10,000 × g at 4°C. The resultant supernatant, containing the serum, was carefully separated from the pellet and preserved at −20°C until use. To obtain the fecal supernatant, the feces samples were weighed, followed by the addition of a 25% weight/volume (w/v) solution of fecal slurry comprised of 0.01% sodium azide, 1% protease inhibitor in PBS. The samples were homogenized by vortexing for 15 min. After centrifugation, collect the supernatant and store it at −80° C for IgA detection.
The ELISA plates were coated with Salmonella soluble antigen (1 μg/well) and incubated overnight at 4°C. Wells were blocked with 5% skim milk at 37°C for 2 h (200 μL/well), followed by washing three times with PBS containing 0.05% Tween-20 (PBST) (280 μL/well). Dilute the serum sample at 1:200 and add it to the well, then incubate at 4°C for 1 h (100 μL/well), and wash three times with PBST. The secondary Ab goat anti-mouse IgG-HRP diluted 1:10,000 was added (100 μL/well) (Applygen, Beijing, China). The Ab was allowed to interact with the samples for 35 min at 37°C, followed by washing three times with PBST. Add 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (100 μL/well) and incubate at 37°C for 10 min. The reaction was terminated by adding 50 μL of 2 M H2SO4 to each well, and the absorbance was read at 450 nm in plate reader (Tecan, Shanghai, China).
For IgA, the ELISA plates were coated with 1 μg Salmonella soluble antigen per well and incubated overnight. Wells were blocked with 5% skim milk at 37°C for 2 h, followed by washing three times with PBST. The undiluted fecal supernatant was added to the well (100 μL/well), followed by incubated at 4°C for 1 h. The secondary Ab goat anti-mouse IgA-HRP diluted 1:10,000 was added, and the rest of the protocol was performed as described above (Emerson et al., 2022).
Immune protection assessment for the ΔpipC
To evaluate the immune protection of ΔpipC. Twenty 6-week-old female KM mice were randomly divided into 2 groups (n = 10), namely the vaccinated group and the unvaccinated group. The vaccinated group was orally immunized with 1 × 106 CFU/mouse of ΔpipC, while the unvaccinated group was orally immunized with 200 μL of PBS. In addition, another 10 mice without vaccination and challenge were used as the control group. At 14 dpi, the vaccinated mice received the same dose of ΔpipC for enhanced immunity. At 28 dpi, the vaccinated group and unvaccinated group were challenged with 2 × 107 CFU/mouse of C50336 by intraperitoneal injections. Deaths and clinical symptoms were recorded daily for 14 d post the challenge (dpc), and calculate the relative survival rate according to the formula: Relative survival rate = (mortality rate of the unvaccinated group − mortality rate of the vaccinated group)/mortality rate of the unvaccinated group × 100%.
Statistical analysis
Statistical analysis was conducted using GraphPad Prism 9 (GraphPad Software, CA, United States) and IBM SPSS (IBM Corporation, Armonk, NY, United States) software. Data are expressed as the mean ± standard error of the mean (SEM). All statistical analysis were two-way ANOVA and post-test. Differences between two samples were evaluated using Student’s t-test. Significant differences are indicated with an asterisk (*), where *: p < 0.05, **: 0.001 < p < 0.01, and ***: p < 0.001 are considered to represent statistically significant differences in mean values. ns means not significant (Kirthika et al., 2020).
Results
The ΔpipC and complementation strain were successfully constructed
We constructed a pipC gene deletion strain of SE by using the λ-Red homologous recombination method and constructed a complementation strain by using the pBR322 plasmid. The primers P3 and P4, P7 and P8 were used to identify ΔpipC and Δ pipC + pipC, respectively. As shown in Figures 2A,B, the pipC gene deletion strain and complementation strain have been successfully constructed.
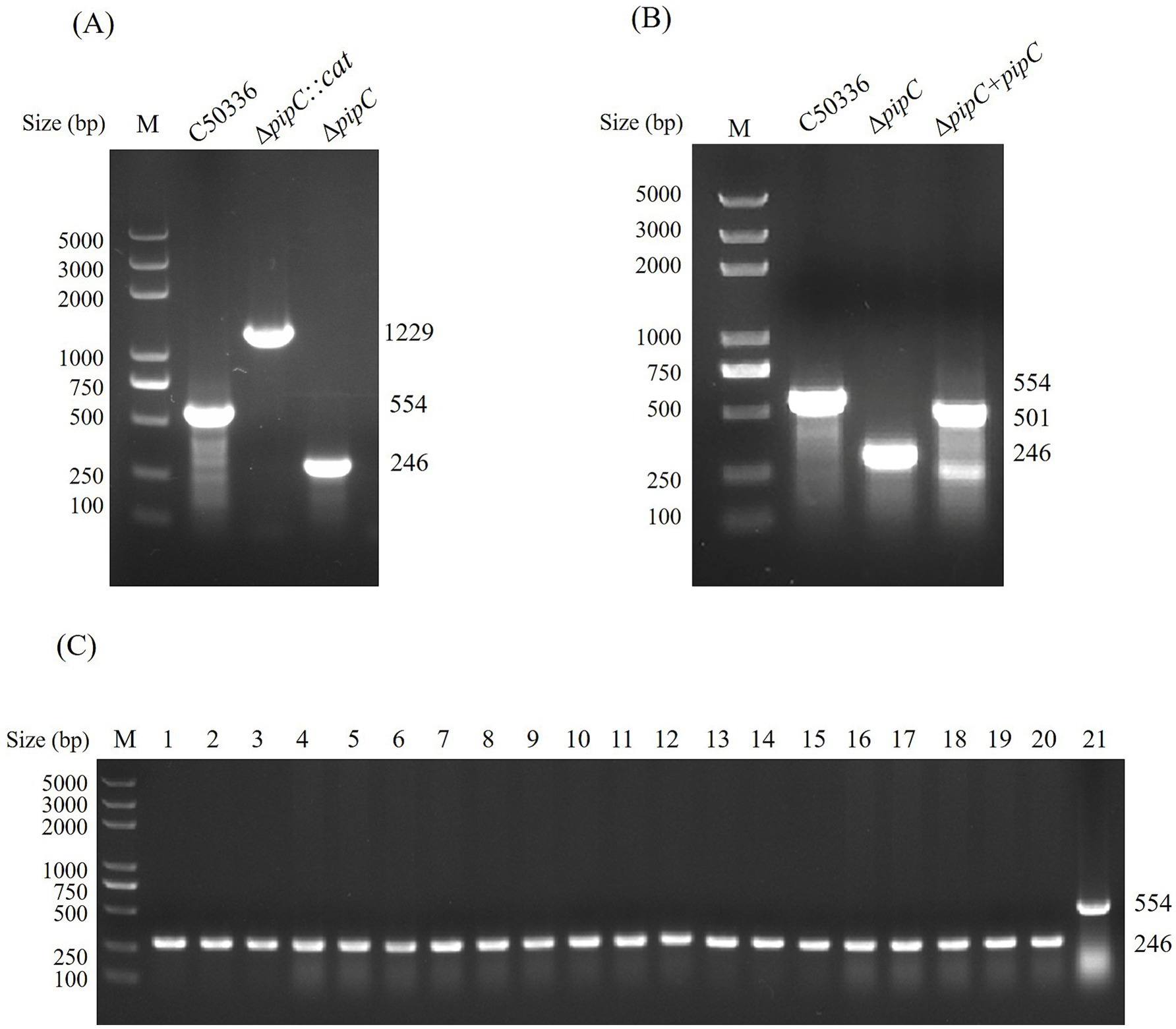
Figure 2. (A) PCR identification of ΔpipC and ΔpipC:cat mutants. The wild-type strain C50336 yields a 554 bp amplicon corresponding to the intact pipC gene. In contrast, the ΔpipC mutant produces a 246 bp fragment, while the ΔpipC:cat strain generates a 1,229 bp product. (B) PCR confirmation of the ΔpipC complemented strain. The ΔpipC + pipC strain yields a 594 bp PCR product, indicating successful complementation. (C) Assessment of ΔpipC genetic stability. Lanes 1–20 show 246 bp PCR products from sequential passages of the ΔpipC mutant, and lane 21 displays a 554 bp band from the wild-type C50336 strain.
ΔpipC has good genetic stability
The ΔpipC mutant was serially passaged 40 times in LB medium, and the presence of the pipC deletion was then assessed by PCR (Figure 2C). The pipC deletion was still detectable in the ΔpipC mutant strain, indicating that this strain has good genetic stability.
The pipC gene does not affect the growth ability of SE
As shown in Figure 3A, the growth characteristics of C50336, ΔpipC and ΔpipC + pipC in LB medium did not differ greatly. This indicated that pipC deletion did not influence the growth characteristics of SE.
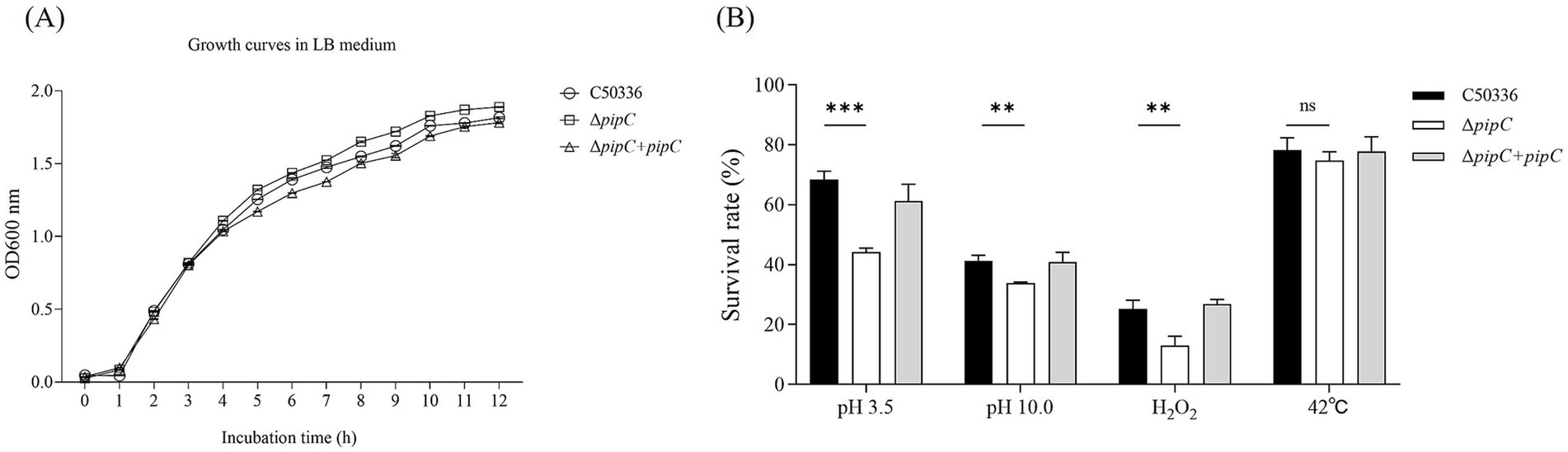
Figure 3. (A) Growth curves for the C50336, ΔpipC and ΔpipC + pipC. All strains were cultured in LB medium. (B) Survival rate of ΔpipC under different environmental stress. Data are presented as the mean ± SD of three independent replicates. Statistical significance was determined as p < 0.01 (**) and p < 0.001 (***).
The deletion of pipC gene weakens the resistance of SE to environmental stress
To investigate whether the deletion of the pipC gene affects the resistance of SE to environmental stress, the survival rates of C50336, ΔpipC, and ΔpipC + pipC were compared under acid stress (pH 3.5), alkaline stress (pH 10.0), oxidative stress (H2O2), and heat stress (42°C). The results shown in Figure 3B reveal that, compared to C50336, the survival rate of ΔpipC was significantly reduced under acidic, alkaline, and oxidative stress conditions, whereas no statistically significant difference was observed under heat stress conditions. Since pipC deletion did not affect the growth characteristics of SE, the reduced survival rate of the mutant strain can be attributed to the role of the pipC gene in enhancing resistance to acid, alkaline, and oxidative stress.
The ΔpipC mutant shows attenuated virulence in vitro
The adhesion rate and invasion rate in human epithelial Caco-2, and the intracellular survival rate in mouse macrophage RAW264.7 of ΔpipC and C50336 was determined. As shown in Figure 4A, the adhesion rate of bacteria was not different between C50336 and ΔpipC in Caco-2. However, the invasion rate in Caco-2 and the intracellular survival rate in RAW264.7 of ΔpipC, compared to of C50336, showed significantly reduced (Figures 4B,C). These results indicate that SE with pipC deletion shows attenuated virulence in vitro.
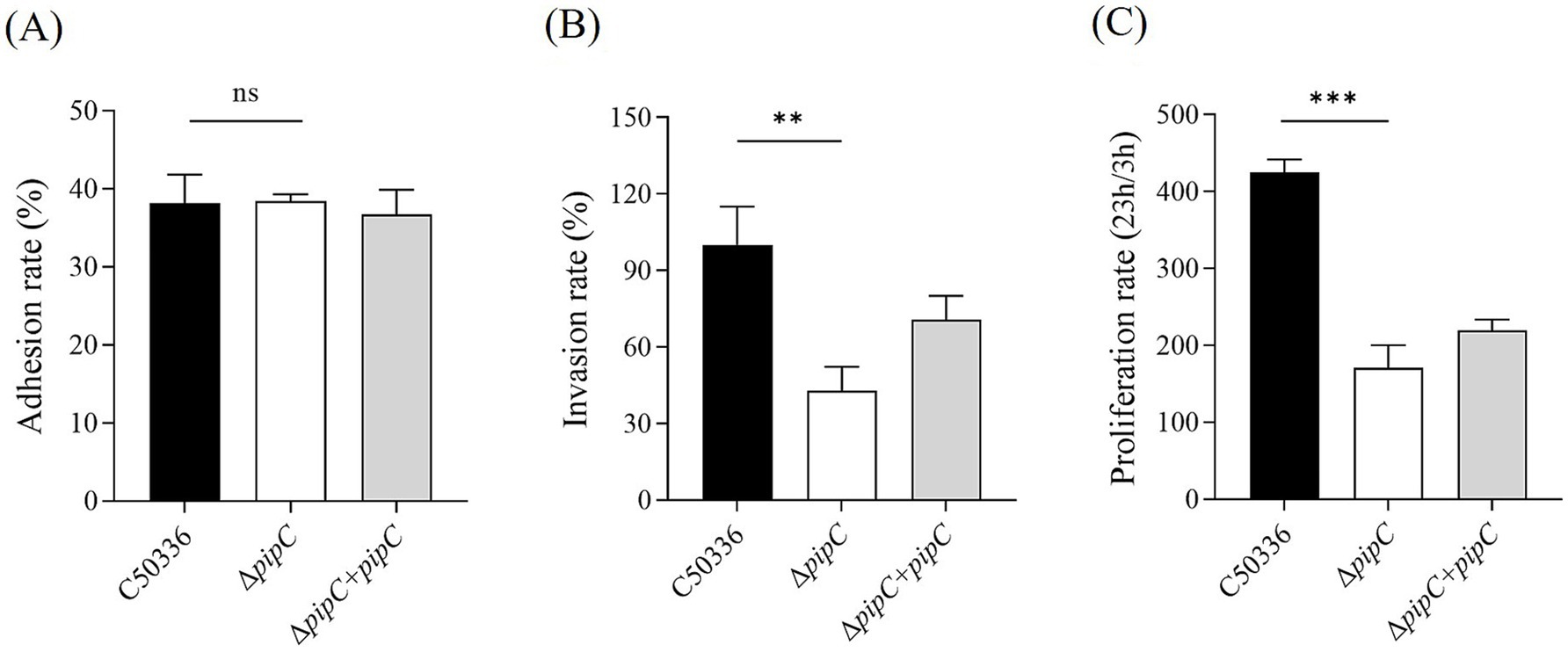
Figure 4. (A) Adherence and (B) invasion assays for C50336, ΔpipC and ΔpipC + pipC to Caco-2. The invasion rate of C50336 is considered to be 100%, and the invasiveness of ΔpipC or ΔpipC + pipC is calculated as its percentage. (C) Intracellular survival assay for C50336, ΔpipC and ΔpipC + pipC to RAW264.7. Data are presented as the mean ± SD of three independent replicates. Statistical significance was determined as p < 0.05 (*) and p < 0.01 (**).
The ΔpipC exhibits reduced virulence in a mouse model
The virulence of the ΔpipC and C50336 strains was evaluated in 6-week-old KM mouse after i.p. challenge. As shown in Table 5, the LD50 of ΔpipC was 2.99 × 107 CFU, which was 47-fold higher than that of the wild-type C50336 (2.99 × 107/6.32 × 105 ≈ 47). The LD50 of ΔpipC+pipC is 1 × 106 CFU/mouse. The result indicated that the virulence of the ΔpipC was attenuated compared to the C50336.
The deletion of pipC results in the downregulation of multiple virulence gene expression levels in SE
To investigate the potential mechanism underlying the attenuation of SE virulence due to the deletion of the pipC gene, qPCR was employed to assess the expression levels of virulence factors. The results are presented in Figure 5. Compared to C50336, the expression levels of SPI-1 associated genes (invH, sipA, sipB, sipC, sopB, and sopE2) and SPI-2 associated genes (spvB, ssrA, orf245, ssaS, ssaT, ssaU, sseB, and sseD) in ΔpipC were significantly decreased. These findings suggest that pipC may influence the virulence of SE by modulating the expression levels of multiple virulence genes.
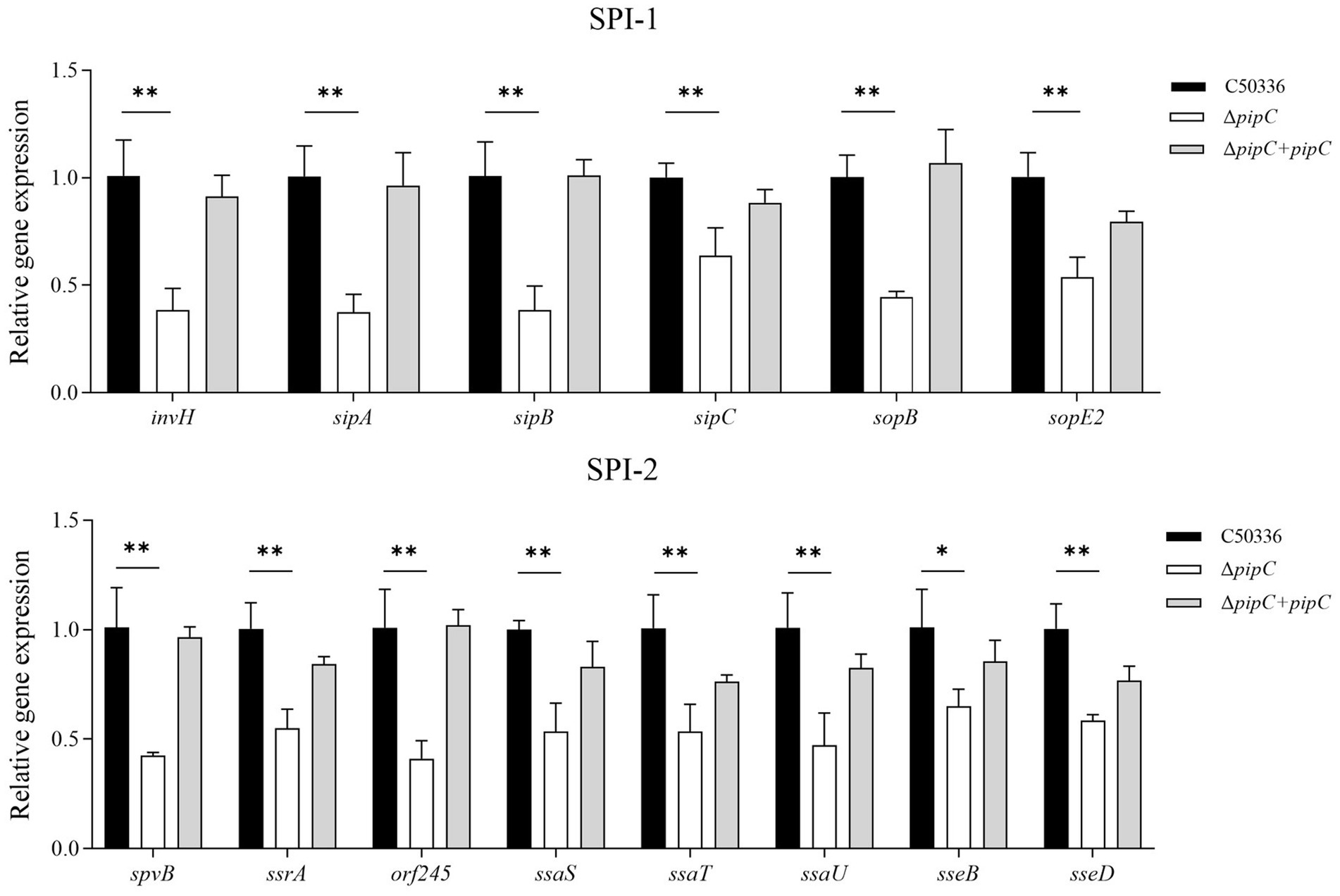
Figure 5. The expression level of virulence genes in C50336, ΔpipC and ΔpipC + pipC were detected by using qPCR, with 16S rRNA as the internal reference gene. Data are presented as the mean ± SD of three independent replicates. Statistical significance was determined as p < 0.05 (*) and p < 0.01 (**).
The deletion of pipC reduces the colonization of SE in organs
The results of bacteria colonization in the liver, spleen, and cecum are shown in Figure 6. All the liver, spleen and cecum samples from the blank control group were negative for Salmonella recovery. Bacteria could be isolated from the liver, spleen, and cecum at 3, 7, and 14 dpc. Compared to C50336, the counts of ΔpipC in the liver, spleen, and cecum were significantly lower at 3, 7, and 14 dpc, indicating that the colonization ability of ΔpipC in these organs was notably inferior to that of C50336. This suggests that the pipC gene can influence the colonization ability and virulence of SE.
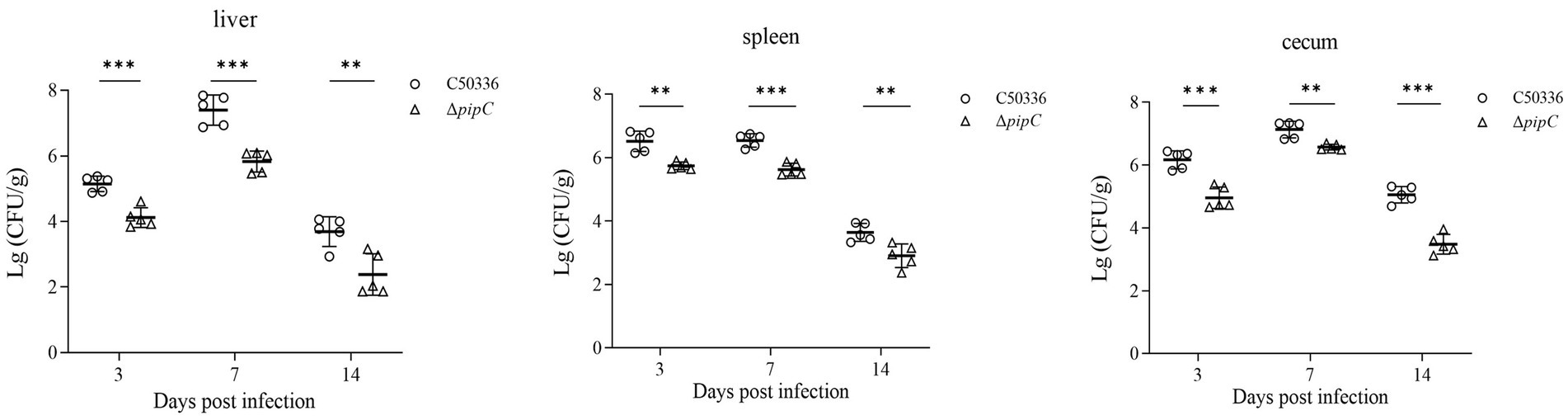
Figure 6. Bacterial colonization of SE in the liver, spleen, and cecum following challenge. Bacterial loads in these organs were quantified at the 3 dpc, 7 dpc, and 14 dpc, and results were expressed as log₁₀(CFU/g). Data represents the mean ± SD from five mice. Statistical significance is indicated as p < 0.01 (**) and p < 0.001 (***).
ΔpipC can induce immune responses
To elucidate the specific immune responses to SE antigens following ΔpipC immunization, a splenic lymphocyte proliferation assay was performed using soluble antigens at 14 and 28 dpi. As shown in Figure 7A, the stimulation indices against the SE antigens for immunized group was 8.60 ± 0.34 at 14 dpi. Likewise, the stimulation indices against SE antigens for immunized group was 14.07 ± 0.51 at 28 dpi. The SI value of splenic lymphocytes in the immunized group was significantly higher than that in the control group, and the lymphocyte proliferation level further increased after enhanced immunization. These findings indicate that ΔpipC can induce strong specific immune responses.
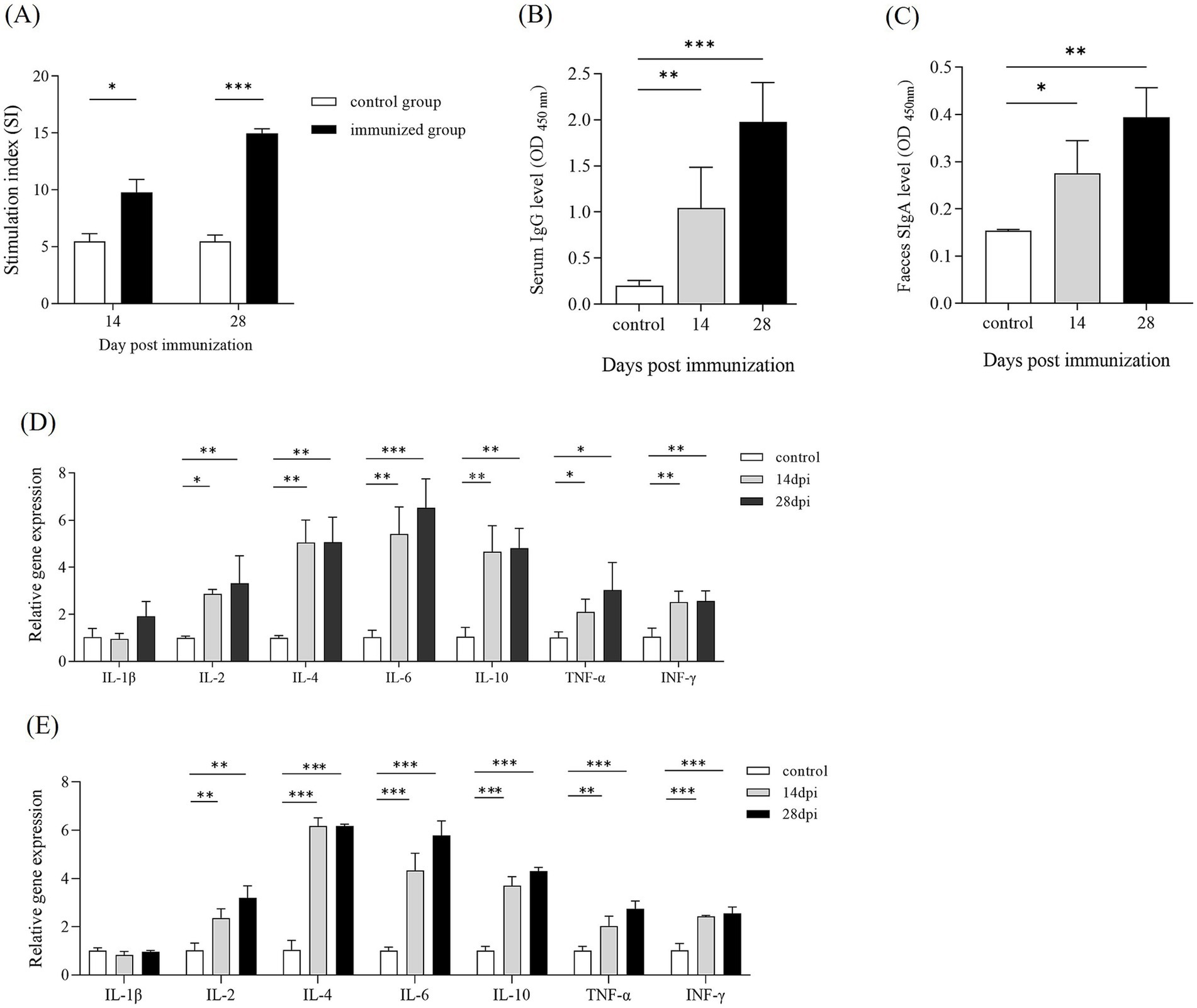
Figure 7. (A) The stimulation index (SI) of the splenic lymphocytes proliferation assay. Lymphocyte proliferation was measured with a MTT kit at 14 and 28 dpi. The SI was calculated using the following equation: SI = (OD570 of the antigen-stimulated cells)/(OD570 of the unstimulated cells). Data represent the mean ± SD from five mice. (B,C) Antibody levels in serum and feces of KM mice following immunization. Serum and fecal samples were collected from KM mice at 14 and 28 dpi. The levels of IgG in serum and IgA in feces were determined by ELISA. Data are presented as the mean ±SD from three independent replicates. (D,E) Cytokine expression levels in the spleen following immunization. Quantitative PCR analysis was performed to assess the mRNA expression levels of IL-1β, IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ at 14 and 28 dpi. The internal control gene were gapdh and β-actin, respectively. Data are presented as the mean ± SD of three independent replicates. Statistical significance was determined as p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***).
To evaluate the humoral and mucosal immune responses in mice immunized with ΔpipC, the serum IgG and mucosal IgA responses against SE soluble antigens were measured by ELISA. At 14 dpi and 28 dpi, mice immunized with ΔpipC exhibited significantly enhanced serum IgG levels (Figure 7B) and secretory IgA (SIgA) levels (Figure 7C) compared to the control group, with further increases observed after booster immunization. These findings indicate that ΔpipC effectively induces robust specific humoral and mucosal immune responses, which are augmented with booster immunization.
We detected the expression of cytokines in spleen cells of immunized mice at 14 and 28 dpi. The results, shown in Figures 7D,E, revealed that at 14 dpi, the expression levels of IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ in the ΔpipC group were significantly higher than those in the control group. At 28 dpi, the expression levels of IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ in the ΔpipC group were notably higher than those in the control group. Additionally, the expression levels of IL-6 and TNF-α after the booster immunization were significantly higher than those after the primary immunization. The increased expression levels of cytokines highlight that ΔpipC can effectively induce a strong immune response in mice.
Immune protection by the ΔpipC vaccination against virulent C50336 challenge
The survival percentages in the mice vaccinated orally with the ΔpipC followed by the challenge with the virulent SE are shown in Figure 8. The vaccinated group showed no mouse deaths and a 100% survival rate; among the 10 mice in the unvaccinated group, 8 mice died after the challenge, showing a survival rate of 20%. The clinical symptoms including anorexia, chills, diarrhea, emaciation, and depression in the vaccinated group were slight and temporary after challenged compared to the unvaccinated group. Therefore, immunization with 106 CFU of ΔpipC provided full protection against SE challenge (see Figure 9).
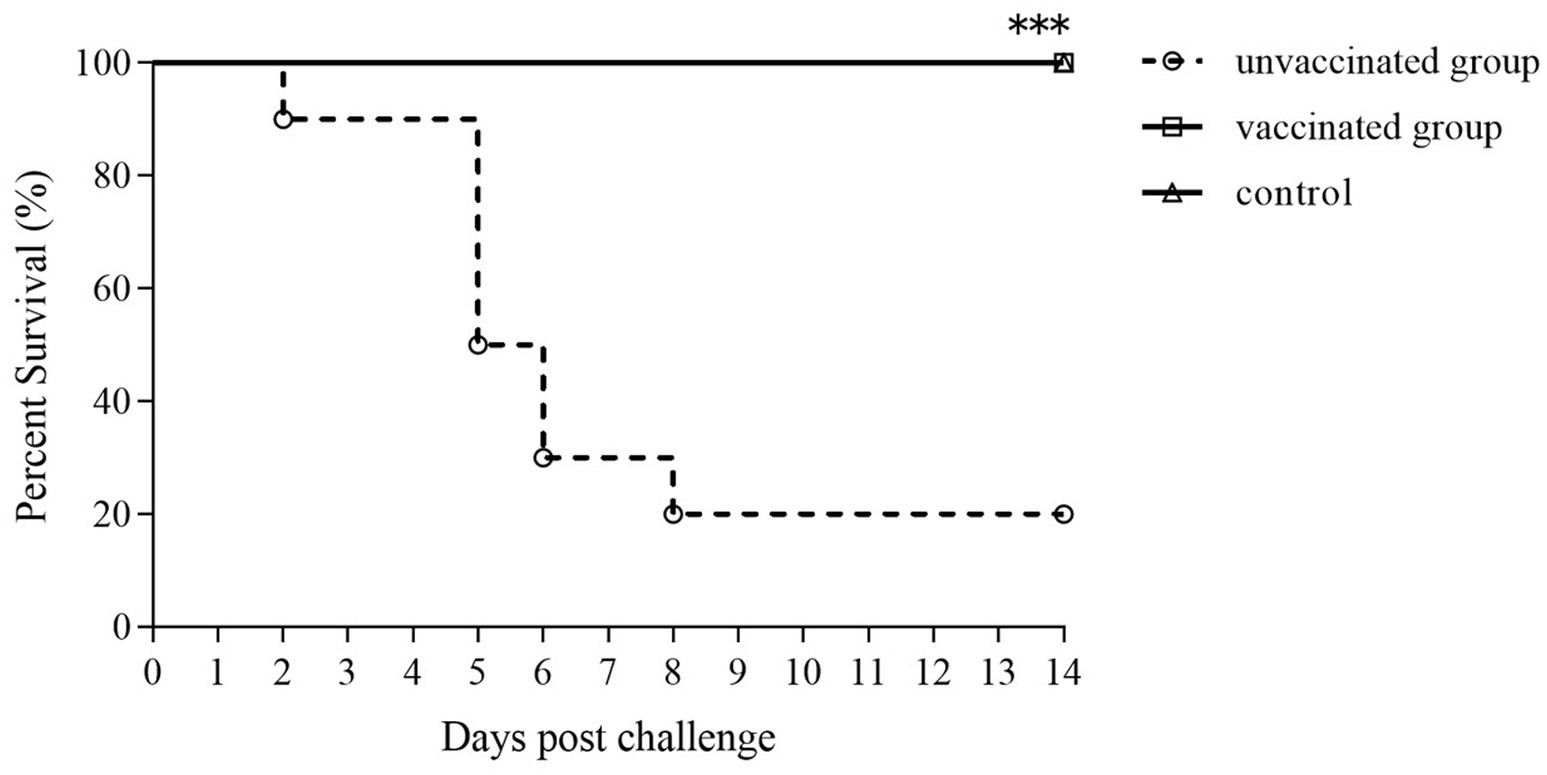
Figure 8. Protective efficacy of the ΔpipC following oral vaccination. KM mice were orally immunized with the ΔpipC and challenged with a lethal dose of C50336 at 28 dpi. Survival was monitored daily for 14 days after the challenge. The vaccinated group showed significantly improved survival compared to the unvaccinated group. Statistical significance was determined as p < 0.001 (***).
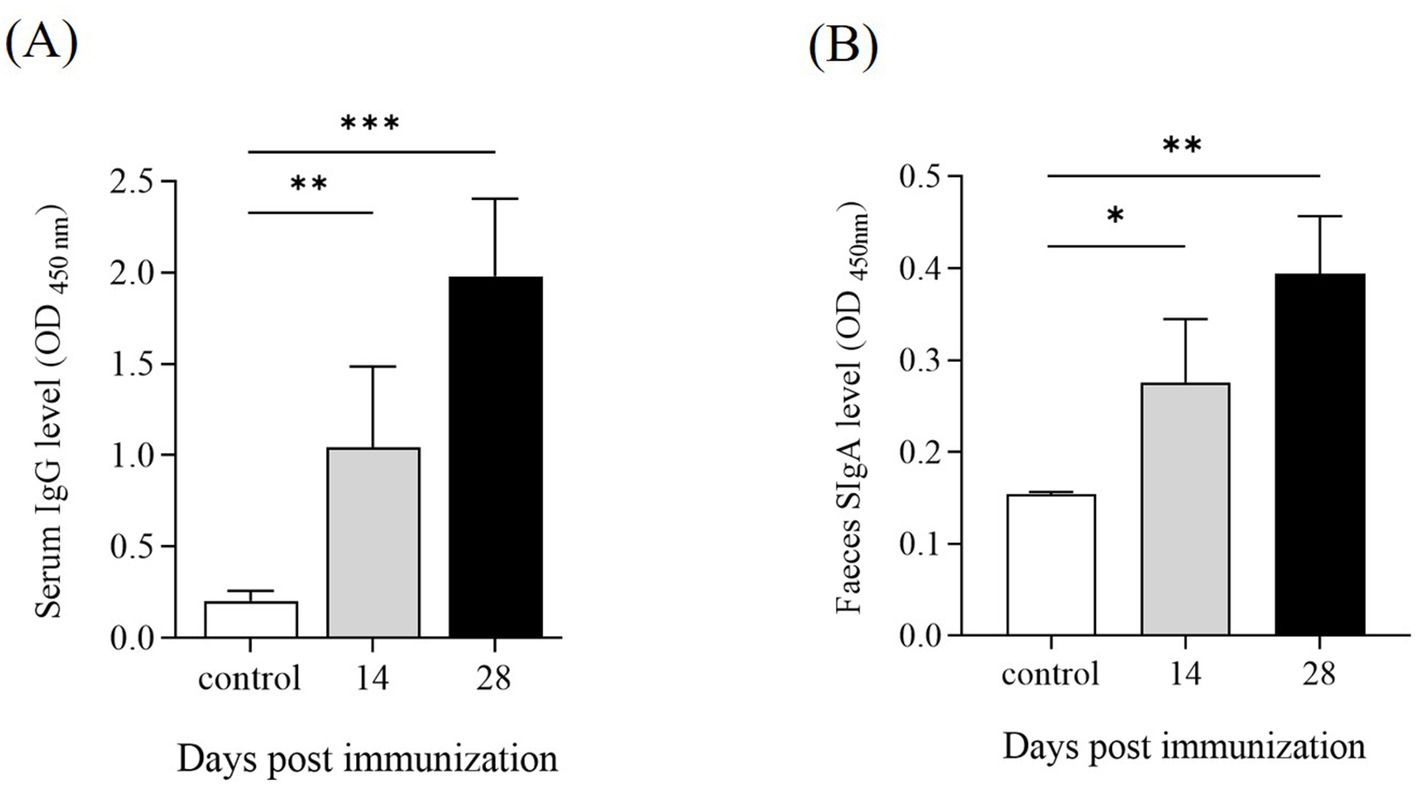
Figure 9. Antibody levels in serum and feces of KM mice following immunization. Serum and fecal samples were collected from KM mice at 14 and 28 days post-immunization (dpi). The levels of (A) IgG in serum and (B) IgA in feces were determined by ELISA. Data are presented as the mean ±SD from three independent replicates. Statistical significance was assessed as p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***).
Discussion
SE is a major foodborne zoonotic pathogen that poses significant economic burdens on the livestock industry and presents a serious global public health threat. The continued emergence of antimicrobial resistance (AMR), particularly multidrug resistance (MDR), in Salmonella has become a growing challenge worldwide. Vaccination has been recognized as an effective targeted strategy to control antibiotic-resistant Salmonella infections. Studies have demonstrated that vaccination significantly contributes to reducing infections caused by resistant strains (Bian et al., 2024).
Over the years, a variety of vaccines-including inactivated and live attenuated vaccines-have been developed to combat salmonellosis. Inactivated vaccines are considered safe but generally induce weak immunogenicity, often requiring adjuvants and booster immunizations, and mainly stimulate humoral rather than cellular immunity (Pan et al., 2024). In contrast, live attenuated vaccines represent a major advancement in vaccine technology by providing efficient and long-lasting protection. These vaccines use weakened but viable pathogens that are capable of eliciting strong immune responses without causing disease. A key advantage of live attenuated vaccines lies in their ability to closely mimic natural infections and engage both humoral and cell-mediated immune responses, thereby establishing robust and sustained protection (Nazir et al., 2025).
In recent years, the use of targeted gene deletion to attenuate virulence in Salmonella has become an increasingly common strategy in vaccine development (Hewawaduge et al., 2023). Given the association of pipC with host cell invasion and intracellular survival in macrophages, we constructed a pipC-deleted SE mutant using homologous recombination. Compared to the wild-type strain C50336, the ΔpipC mutant exhibited significantly reduced virulence. Immunization with the ΔpipC strain induced strong humoral and cellular immune responses and conferred full protection against subsequent SE challenge in mice.
Identification of virulence genes is essential for the rational design of live attenuated vaccines. During infection, SE faces various stress conditions--such as acid, alkali, and oxidative stress—both in the gastrointestinal tract and within Salmonella-containing vacuoles (SCVs) of epithelial and macrophage cells. Genes involved in stress responses are often linked to virulence (Pasqua et al., 2022). Our study demonstrated that deletion of pipC had no significant effect on the growth characteristics of SE under normal conditions, indicating that this gene is not essential for basic bacterial proliferation. However, the pipC mutant strain exhibited markedly reduced survival under acidic, alkaline, and oxidative stress conditions, suggesting that pipC is involved in the bacterium’s ability to withstand environmental stress. These findings underscore the important role of pipC in stress resistance, which may, in turn, contribute to the overall virulence and persistence of SE in hostile environments.
Upon entering the small intestine, Salmonella first adheres to epithelial cells and invades host tissues via M cells located over Peyer’s patches. Although macrophages within lymphoid tissues phagocytose Salmonella, the bacterium can evade destruction by interfering with phagosome-lysosome fusion, allowing it to survive and replicate intracellularly. Hence, adhesion to epithelial cells and intracellular survival in macrophages are crucial virulence determinants (Eakley et al., 2011). In this study, we found that the ΔpipC strain exhibited significantly reduced invasion of epithelial cells, consistent with findings by Shah et al. (2011). Additionally, intracellular proliferation was markedly impaired. A mouse intraperitoneal infection model showed that the LD₅₀ of the ΔpipC strain increased 47-fold compared to the wild-type strain, indicating substantial attenuation. The SPI-1 and SPI-2 pathogenicity islands encode the Type III Secretion System (T3SS), which delivers effector proteins into host cells to facilitate cytoskeletal rearrangement and invasion. Gene expression analysis further revealed that the ΔpipC strain had significantly downregulated expression of several virulence-associated genes. SPI-1 genes (invH, sipA, sipB, sipC, sopB, and sopE2) and SPI-2 genes (spvB, ssrA, orf245, ssaS, ssaT, ssaU, sseB, and sseD) were all suppressed. The downregulation of these genes in the ΔpipC strain implies impaired T3SS function and effector protein expression, ultimately leading to reduced invasion and virulence.
Live attenuated Salmonella vaccines must balance attenuation, immunogenicity, and protective efficacy. An ideal vaccine strain should be non-toxic or minimally toxic. In this study, mice infected with the ΔpipC strain had significantly higher LD₅₀ values and reduced colonization in the intestine, spleen, and liver compared to wild-type infections, confirming the attenuation of the mutant strain.
Upon entry, SE activates innate immunity, recruiting macrophages and monocytes, which secrete pro-inflammatory cytokines and promote inflammation. In parallel, B cells differentiate into effector cells, producing anti-inflammatory cytokines such as IL-10, which suppress inflammation and stimulate antibody production. Cytokines such as IFN-γ enhance macrophage activation and help regulate immune responses (Jan et al., 2022; Kung et al., 2022). In our study, mice immunized with the ΔpipC strain showed significantly higher splenic lymphocyte proliferation indices and serum antibody levels at both 14 dpi and 28 dpi compared to controls. Cytokine analysis revealed significantly increased expression of IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ, indicating robust activation of both humoral and cellular immune pathways. The ΔpipC strain provided complete protection (100%) against secondary wild-type challenge when administered orally at a dose of 1 × 106 CFU. It is important to note that we used only a single antigen concentration in our lymphocyte proliferation experiments, limiting the understanding of antigen sensitivity and cellular response thresholds. Assessment of lymphocyte response at multiple antigen concentrations should be considered in future further studies.
One of the main advantages of live attenuated vaccines over inactivated ones is their ability to stimulate both humoral and cellular immunity. Systemic dissemination of Salmonella promotes antigen presentation and induces specific immune responses that prevent secondary infections. Previous studies have validated the potential of gene-engineered live attenuated vaccines against Salmonella. Zhao et al. (2024) showed that deletion of the pal gene attenuated SE virulence, and oral immunization with 1 × 108 CFU of Δpal conferred 100% protection against a 5 × 106 CFU wild-type challenge. Zhou et al. (2023) demonstrated that deletion of rfbG increased the LD₅₀ by 56-fold. Immunizing chickens with 5 × 107 or 5 × 106 CFU of ΔrfbG achieved 100% survival after wild-type challenge, and significant antibody responses were observed, confirming its vaccine potential. Similarly, Pan et al. (2020) reported that the LD₅₀ of Salmonella Paratyphi A ΔsptP was 1.43 × 104-fold higher than the wild-type, and oral immunization with 2 × 105 CFU conferred complete protection against challenge with 1 × 103 CFU.
In summary, our present work demonstrates that lack of pipC affects SE pathogenicity by decreasing its virulence both in vitro and in vivo. Vaccination of mice with ΔpipC conferred development of acquired immunity and efficacious protection against experimental systemic infection. The pipC mutant possesses the safety and efficacy required for use as a live attenuated vaccine. Given the potential for field applications, a more comprehensive long-term assessment of safety indicators such as fecal-shedding duration, risk of virulence re-escalation, and potential for environmental transmission is needed (Pan et al., 2020). Although the ΔpipC strain exhibited attenuated virulence and genetic stability under laboratory conditions, its environmental safety and stability should still be verified by field experiments before practical application, and the applicability of target animals and the safe window of vaccine dose should be systematically evaluated.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Experimental Animal Regulation Ordinances defined by Hebei Provincial Department of Science and Technology HPDST2020-17. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
LZ: Formal analysis, Writing – original draft, Methodology, Data curation. YuC: Writing – original draft, Formal analysis, Data curation, Methodology. ZY: Writing – review & editing, Funding acquisition. YuL: Methodology, Writing – original draft. XY: Methodology, Writing – original draft. LC: Writing – original draft, Software. YZ: Writing – review & editing, Funding acquisition. YiC: Writing – original draft, Software. YoL: Writing – original draft, Software. QS: Funding acquisition, Writing – review & editing. TW: Validation, Supervision, Methodology, Writing – review & editing, Investigation, Writing – original draft, Conceptualization, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National Key Research and Development Program (2023YFD1800701); Hebei Agriculture Research System (HBCT2024280205 and HBCT2024280406), and Shijiazhuang “Open Challenge” Science and Technology Project (2417908002A).
Acknowledgments
The authors thank Prof. Zhen Wang from Beijing University of Agriculture for their valuable help in our experiment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1631008/full#supplementary-material
SUPPLEMENTARY FIGURE 1 | (A) Schematic representation of a gene knockout strategy adopted from Ranallo et al. (2006). (a) Linear DNA substrates containing chloramphenicol cassettes are generated using PCR primers with 59 bp homology (A and B) to the gene of interest (pipC). Priming from pKD3 produces linear DNA substrates. (b) These substrates introduced into bacteria made transiently hyper-recombinogenic using Gam, Beta, Exo expressed from pKD46. (c) The chloramphenicol cassette is eliminated via plasmid-based expression of a yeast derived recombinase (FLP) leaving behind an ~80 bp “scar” consisting of a single FRT site. (B) Schematic representation of pipC-complemented strain construction of SE.
References
Acevedo-Villanueva, K. Y., Renu, S., Shanmugasundaram, R., Akerele, G. O., Gourapura, R. J., and Selvaraj, R. K. (2021). Salmonella chitosan nanoparticle vaccine administration is protective against Salmonella Enteritidis in broiler birds. PLoS One 16:e0259334. doi: 10.1371/journal.pone.0259334
Bian, X., Liu, Q., Chen, Y., Zhang, W., Li, M., Zhang, X., et al. (2024). Immunogenicity and cross-protective efficacy induced by delayed attenuated Salmonella with regulated length of lipopolysaccharide in mice. Gut Microbes 16:2424983. doi: 10.1080/19490976.2024.2424983
Cao, G., Zhao, S., Kuang, D., Hsu, C.-H., Yin, L., Luo, Y., et al. (2023). Geography shapes the genomics and antimicrobial resistance of Salmonella enterica serovar Enteritidis isolated from humans. Sci. Rep. 13:1331. doi: 10.1038/s41598-022-24150-4
Darwin, K. H., Robinson, L. S., and Miller, V. L. (2001). SigE is a chaperone for the Salmonella enterica serovar Typhimurium invasion protein SigD. J. Bacteriol. 183, 1452–1454. doi: 10.1128/JB.183.4.1452-1454.2001
Datsenko, K. A., and Wanner, B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645. doi: 10.1073/pnas.120163297
Eakley, N. M., Bochsler, P. N., Gopal Reddy, P., and Fadl, A. A. (2011). Biological and virulence characteristics of the YqhC mutant of Salmonella. Microbiol. Immunol. 55, 830–840. doi: 10.1111/j.1348-0421.2011.00387.x
Emerson, L. E., Barker, H., Tran, T., Barker, S., Enslow, S., Ou, M., et al. (2022). Extracellular vesicles elicit protective immune responses against Salmonella infection. J. Extracell. Vesicles 11:e12267. doi: 10.1002/jev2.12267
Ferrari, R. G., Rosario, D. K. A., Cunha-Neto, A., Mano, S. B., Figueiredo, E. E. S., and Conte-Junior, C. A. (2019). Worldwide epidemiology of Salmonella serovars in animal-based foods: a meta-analysis. Appl. Environ. Microbiol. 85:e00591-19. doi: 10.1128/AEM.00591-19
Guard-Petter, J. (2001). The chicken, the egg and Salmonella enteritidis. Environ. Microbiol. 3, 421–430. doi: 10.1046/j.1462-2920.2001.00213.x
Hewawaduge, C., Senevirathne, A., Sivasankar, C., and Lee, J. H. (2023). The impact of lipid A modification on biofilm and related pathophysiological phenotypes, endotoxicity, immunogenicity, and protection of Salmonella Typhimurium. Vet. Microbiol. 282:109759. doi: 10.1016/j.vetmic.2023.109759
Jan, T.-R., Lin, C.-S., Wang, S.-Y., and Yang, W.-Y. (2022). Cytokines and cecal microbiome modulations conferred by a dual vaccine in Salmonella-infected layers. Poult. Sci. 102:102373. doi: 10.1016/j.psj.2022.102373
Ji, H. J., Jang, A.-Y., Song, J. Y., Ahn, K. B., Han, S. H., Bang, S. J., et al. (2022). Development of live attenuated Salmonella Typhimurium vaccine strain using radiation mutation enhancement technology (R-MET). Front. Immunol. 13:931052. doi: 10.3389/fimmu.2022.931052
Jiang, X., Chu, C., Wang, Z., Gu, J., Hong, Y., Li, Q., et al. (2022). Preclinical evaluation of OMVs as potential vaccine candidates against Salmonella enterica serovar Enteritidis infection. Front. Cell. Infect. Microbiol. 12:1037607. doi: 10.3389/fcimb.2022.1037607
Kamble, N. M., and Lee, J. H. (2016). Characterization and evaluation of a Salmonella enterica serotype Senftenberg mutant created by deletion of virulence-related genes for use as a live attenuated vaccine. Clin. Vaccine Immunol. 23, 802–812. doi: 10.1128/CVI.00233-16
Kang, X., Yang, Y., Meng, C., Wang, X., Liu, B., Geng, S., et al. (2021). Safety and protective efficacy of Salmonella Pullorum spiC and rfaH deletion rough mutant as a live attenuated DIVA vaccine candidate. Poult. Sci. 101:101655. doi: 10.1016/j.psj.2021.101655
Kirthika, P., Senevirathne, A., Jawalagatti, V., Park, S., and Lee, J. H. (2020). Deletion of the lon gene augments expression of Salmonella Pathogenicity Island (SPI)-1 and metal ion uptake genes leading to the accumulation of bactericidal hydroxyl radicals and host pro-inflammatory cytokine-mediated rapid intracellular clearance. Gut Microbes 11, 1695–1712. doi: 10.1080/19490976.2020.1777923
Kung, Y.-J., Lam, B., Tseng, S.-H., MacDonald, A., Tu, H.-F., Wang, S., et al. (2022). Localization of Salmonella and albumin-IL-2 to the tumor microenvironment augments anticancer T cell immunity. J. Biomed. Sci. 29:57. doi: 10.1186/s12929-022-00841-y
Lin, Z., Tang, P., Jiao, Y., Kang, X., Li, Q., Xu, X., et al. (2017). Immunogenicity and protective efficacy of a Salmonella Enteritidis sptP mutant as a live attenuated vaccine candidate. BMC Vet. Res. 13:194. doi: 10.1186/s12917-017-1115-3
Nazir, J., Manzoor, T., Saleem, A., Gani, U., Bhat, S. S., Khan, S., et al. (2025). Combatting Salmonella: a focus on antimicrobial resistance and the need for effective vaccination. BMC Infect. Dis. 25:84. doi: 10.1186/s12879-025-10478-5
Pan, J., Wei, R., Xu, P., Liu, Y., Li, C., Ding, G., et al. (2024). Progress in the application of Salmonella vaccines in poultry: a mini review. Vet. Immunol. Immunopathol. 278:110855. doi: 10.1016/j.vetimm.2024.110855
Pan, P., Zou, F., He, C., He, Q., and Yin, J. (2020). Characterization and protective efficacy of a sptP mutant of Salmonella Paratyphi A. Immun. Inflamm. Dis. 8, 774–781. doi: 10.1002/iid3.369
Park, S., Jung, B., Kim, E., Yoon, H., and Hahn, T.-W. (2022). Evaluation of Salmonella Typhimurium lacking fruR, ssrAB, or hfq as a prophylactic vaccine against Salmonella lethal infection. Vaccine 10:1413. doi: 10.3390/vaccines10091413
Pasqua, M., Coluccia, M., Eguchi, Y., Okajima, T., Grossi, M., Prosseda, G., et al. (2022). Roles of two-component signal transduction systems in Shigella virulence. Biomol. Ther. 12:1321. doi: 10.3390/biom12091321
Ranallo, R. T., Barnoy, S., Thakkar, S., Urick, T., and Venkatesan, M. M. (2006). Developing live Shigella vaccines using λ red recombineering. FEMS Immunol. Med. Microbiol. 47, 462–469. doi: 10.1111/j.1574-695X.2006.00118.x
Rodríguez-Escudero, I., Ferrer, N. L., Rotger, R., Cid, V. J., and Molina, M. (2011). Interaction of the Salmonella Typhimurium effector protein SopB with host cell Cdc42 is involved in intracellular replication. Mol. Microbiol. 80, 1220–1240. doi: 10.1111/j.1365-2958.2011.07639.x
Ruvalcaba-Gómez, J. M., Villagrán, Z., Valdez-Alarcón, J. J., Martínez-Núñez, M., Gomez-Godínez, L. J., Ruesga-Gutiérrez, E., et al. (2022). Non-antibiotics strategies to control Salmonella infection in poultry. Animals 12:102. doi: 10.3390/ani12010102
Rychlik, I., Karasova, D., Sebkova, A., Volf, J., Sisak, F., Havlickova, H., et al. (2009). Virulence potential of five major pathogenicity islands (SPI-1 to SPI-5) of Salmonella enterica serovar Enteritidis for chickens. BMC Microbiol. 9:268. doi: 10.1186/1471-2180-9-268
Shah, D. H., Zhou, X., Addwebi, T., Davis, M. A., Orfe, L., Call, D. R., et al. (2011). Cell invasion of poultry-associated Salmonella enterica serovar Enteritidis isolates is associated with pathogenicity, motility and proteins secreted by the type III secretion system. Microbiology 157, 1428–1445. doi: 10.1099/mic.0.044461-0
Shah, D. H., Zhou, X., Kim, H.-Y., Call, D. R., and Guard, J. (2012). Transposon mutagenesis of Salmonella enterica serovar Enteritidis identifies genes that contribute to invasiveness in human and chicken cells and survival in egg albumen. Infect. Immun. 80, 4203–4215. doi: 10.1128/IAI.00790-12
Tennant, S. M., and Levine, M. M. (2015). Live attenuated vaccines for invasive Salmonella infections. Vaccine 33, C36–C41. doi: 10.1016/j.vaccine.2015.04.029
Upadhyaya, I., Upadhyay, A., Kollanoor-Johny, A., Darre, M. J., and Venkitanarayanan, K. (2013). Effect of plant derived antimicrobials on Salmonella Enteritidis adhesion to and invasion of primary chicken oviduct epithelial cells in vitro and virulence gene expression. Int. J. Mol. Sci. 14, 10608–10625. doi: 10.3390/ijms140510608
Wang, M., Qazi, I. H., Wang, L., Zhou, G., and Han, H. (2020). Salmonella virulence and immune escape. Microorganisms 8:407. doi: 10.3390/microorganisms8030407
Xiong, D., Song, L., Chen, Y., Jiao, X., and Pan, Z. (2023). Salmonella Enteritidis activates inflammatory storm via SPI-1 and SPI-2 to promote intracellular proliferation and bacterial virulence. Front. Cell. Infect. Microbiol. 13:1158888. doi: 10.3389/fcimb.2023.1158888
Yin, C., Gu, J., Gu, D., Wang, Z., Ji, R., Jiao, X., et al. (2022). The Salmonella T3SS1 effector IpaJ is regulated by ItrA and inhibits the MAPK signaling pathway. PLoS Pathog. 18:e1011005. doi: 10.1371/journal.ppat.1011005
Zhang, L., Chen, L., Zhang, X., Li, Y., Zheng, Q., Li, Y., et al. (2025). The mcpC mutant of Salmonella enteritidis exhibits attenuation and confers both immunogenicity and protective efficacy in mice. Front. Microbiol. 16:1548920. doi: 10.3389/fmicb.2025.1548920
Zhang, X., He, L., Zhang, C., Yu, C., Yang, Y., Jia, Y., et al. (2019). The impact of sseK2 deletion on Salmonella enterica serovar Typhimurium virulence in vivo and in vitro. BMC Microbiol. 19:182. doi: 10.1186/s12866-019-1543-2
Zhang, L., Wu, T., Wang, F., Liu, W., Zhao, G., Zhang, Y., et al. (2024). CheV enhances the virulence of Salmonella Enteritidis, and the Chev-deleted Salmonella vaccine provides immunity in mice. BMC Vet. Res. 20:100. doi: 10.1186/s12917-024-03951-x
Zhao, G., Duan, W., Zhang, L., Sun, W., Liu, W., Zhang, X., et al. (2024). The peptidoglycan-associated lipoprotein gene mutant elicits robust immunological defense in mice against Salmonella enteritidis. Front. Microbiol. 15:1422202. doi: 10.3389/fmicb.2024.1422202
Keywords: Salmonella enterica serovar Enteritidis, PipC, virulence, immune protection, vaccine
Citation: Zhang L, Chen Y, Yan Z, Li Y, Yang X, Chen L, Zhang Y, Chen Y, Li Y, Shi Q and Wu T (2025) PipC affects the virulence of Salmonella enterica serovar Enteritidis and its deletion strain provides effective immune protection in mice. Front. Microbiol. 16:1631008. doi: 10.3389/fmicb.2025.1631008
Edited by:
Lei Deng, Chinese Academy of Agricultural Sciences, ChinaReviewed by:
Yuwen Dong, University of Pennsylvania, United StatesQiangde Duan, Yangzhou University, China
Alaa A. Ghazy, National Research Centre (Egypt), Egypt
Chen Yuan, Hebei Agricultural University, China
Copyright © 2025 Zhang, Chen, Yan, Li, Yang, Chen, Zhang, Chen, Li, Shi and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tonglei Wu, NTMyOTY2OTUyQDE2My5jb20=
 Lu Zhang
Lu Zhang Yubin Chen1
Yubin Chen1 Yingyu Chen
Yingyu Chen Tonglei Wu
Tonglei Wu