- 1UR 3073: PHAVI: Groupe Borrelia, Institut de Bactériologie, University of Strasbourg, Strasbourg, France
- 2French National Reference Center for Borrelia, Hôpitaux Universitaires, Strasbourg, France
- 3Department of Wildlife Biology, Forestry and Forest Products Research Institute, Tsukuba, Japan
- 4Laboratory of Parasitology, Graduate School of Infectious Diseases, Faculty of Veterinary Medicine, Hokkaido University, Sapporo, Japan
- 5Division of Parasitology, Veterinary Research Unit, International Institute for Zoonosis Control, Hokkaido University, Sapporo, Japan
- 6One Health Research Center, Hokkaido University, Sapporo, Japan
- 7Department of Medical Sciences, Frank H. Netter, M. D., School of Medicine, Quinnipiac University, Hamden, CT, United States
Temperate zones of the northern hemisphere are increasingly impacted by human biting ticks and the human pathogens they transmit. The relationships among ticks, hosts, and pathogens are undergoing significant changes with consequences for human health. This northern hemisphere focused review examines human biting ticks and the disease causing agents they transmit as increasing public health threats due to geographic range expansion, increasing size of tick populations, emergence of newly recognized pathogens, introduction of invasive tick species that are resulting in part from changing weather patterns, land use modifications, biodiversity loss, and human activities/behaviors; all of which result in significant challenges for tick control and disease prevention. As a result of these evolving interactions and the resulting threats they pose, there exist critical needs to implement existing and develop novel tools and strategies to prevent tick bites, control tick populations, and reduce transmission of tick-borne pathogens. Timely, up to date knowledge of which ticks and tick-borne infectious agents are present within an area is foundational for physicians, public health authorities tasked with disease prevention, and the public. Achieving these objectives poses significant challenges. Here, we examine current medically important tick – host - pathogen relationships in Asia, Europe, and North America.
Ticks and tick-borne diseases situation
Emerging, re-emerging, and endemic infectious diseases are increasing threats in this era of global change in part due to climate change (Baker et al., 2022; Semenza et al., 2022). Vector-borne diseases are particularly susceptible to climate variations (Campbell-Lendrum et al., 2015; Thomson and Stanberry, 2022). Amongst disease vector arthropods, ticks transmit the greatest diversity of potentially pathogenic microorganisms such as bacteria, protozoa, and viruses (Colwell et al., 2011; Estrada-Peña and Jongejan, 1999; Jongejan and Uilenberg, 2004). Human biting ticks and the disease causing agents they transmit are increasingly challenging global public health threats due to their expanding geographic ranges; appearance and establishment of invasive tick species; emergence and resurgence of potential pathogens; changing vertebrate host populations; limited effective tick control tools and strategies; absence of anti-tick and anti-pathogen vaccines; and, changing biotic and abiotic factors that influence tick-host-pathogen relationships (Beard et al., 2019; Boulanger et al., 2024; Dantas-Torres, 2015; Eisen and Stafford, 2021; Sonenshine, 2018; Tsao et al., 2021; van Oosterwijk and Wikel, 2021; Wikel, 2022).
Climate impacts the seasonal dynamics of ticks. The ixodid tick life cycle involves free-living periods of months to years interspersed with brief periods for days of host attachment and blood feeding (Sonenshine, 2018). Free-living life cycle stages are susceptible to climate variations that influence tick population sizes, geographic ranges, pathogens transmitted, and their interactions with other tick species that are unique from those of other disease vector arthropods (Estrada-Peña, 2015; Ogden and Lindsay, 2016). Additional factors that influence tick communities are vegetation, land use, habitat modification, and changes in animal host communities. Environmental warming occurring in the northern hemisphere will drive tick geographic range expansions further northward and to higher altitudes, while concurrent range contractions could occur in subtropical and tropical regions. Data regarding geographic range changes are becoming available for some of the more significant human biting ixodid tick vectors in Asia, Europe, and North America. Range expansions of human biting ixodid ticks into new territories are introducing previously unencountered pathogens and creating potential changes in tick ecologies, balance among tick species, and tick-host associations that present new challenges for tick control, disease prevention, and interventions (Eisen, 2020a; Sonenshine, 2018; Wikel, 2022). Tick-transmitted infections are zoonotic diseases (Pfäffle et al., 2013). This diverse community of infectious agents is not static as evidenced by the increasing frequency of emergence of newly discovered zoonotic pathogens during this time of unprecedented global geoclimatic, demographic, socioeconomic, and technological changes (Rupasinghe et al., 2022; Skowron et al., 2023).
Knowing where ticks and tick-borne pathogens are present is foundational to understanding the health threats they represent. Ranges of human biting ticks and tick-borne pathogens are changing and thus creating further gaps in our already fragmented knowledge of where these vectors and pathogens are present (Eisen and Paddock, 2021). Surveillance of tick populations is needed on an ongoing basis combined with next generation sequencing of tick microbiomes (bacteria, viruses, eukaryotic parasites and fungi, commensal and pathogenic) to identify and link to specific geographic areas established, resurging, emerging, and potential human pathogens (Osikowicz et al., 2024). Concomitant with these surveillance strategies must be development of broadly searchable, accessible databases on tick and tick-borne pathogens. Discussions are occurring about development of national strategies relating to surveillance, control, knowledge gaps, interventions, and development of new management tools (Beard et al., 2019; Eisen and Stafford, 2021).
This review draws together in one document essential information on the current distributions of ticks, tick-borne pathogens, and pathogen reservoir species in the northern hemisphere that will further identify knowledge gaps regarding tick and pathogen biology and ecology, intervention measures and control strategies, and need for government support for such endeavors to support public health and the wellbeing of society at large.
Main tick species on each continent
Ticks are arachnids of the subclass Acari within the monotypic order Ixodida. There are three families of ticks, though only two are medically important: The Ixodidae (hard ticks) and the Argasidae (soft ticks) (Supplementary Table 1). The Nuttalliellidae is monotypic, only present in Africa, and not observed for decades; while the Argasidae are species rich with approximately 200 species, and distributed on all continents, except Antarctica (Guglielmone et al., 2010). The hard ticks have a worldwide distribution with 683 species, of which 283 species bite humans (69 Prostriata and 214 Metastriata) (Guglielmone and Robbins, 2018). The hard ticks are divided into two groups: Prostriata containing the genus Ixodes and Metastriata with 14 genera (Guglielmone and Robbins, 2018).
Ticks are strictly hematophagous, feeding on various vertebrate hosts: amphibians, reptiles, birds, and mammals. Hard ticks are characterized by long feeding periods of days, duration dependent upon life cycle stage (larva, nymph, adult), whereas soft ticks typically feed for short durations of minutes to hours and repeatedly (de La Fuente et al., 2008). Hard ticks have 4 life stages (eggs, larvae, nymphs and adults), of which 3 are active. Their complete development can occur within 3 weeks for Rhipicephalus microplus (Oyen and Poh, 2025), or more typically a year, or several years depending on the species and local abiotic (e.g., temperature and humidity) and biotic (e.g., host availability) factors. Species can be either nidicolous (living in nests, burrow or wall cracks), or non-nidicolous (on vegetation or leaf litter in the environment), which is more conducive to exposure to humans.
In the United States, 36 ixodid species and 13 argasid species have been recorded infesting humans (Eisen, 2022). The four main medically important hard tick genera are Ixodes, Amblyomma, Dermacentor, and Rhipicephalus with the invasive species Haemaphysalis longicornis an increasing threat as a human biter. In Europe, around 40 tick species potentially biting humans have been identified with Ixodes and Dermacentor being the most medically important (Estrada-Pena et al., 2017; Perez et al., 2023; Rubel et al., 2018). Asia has a significantly more species-rich tick community than both Europe and the United States combined with more than 100 species of which most belong to the genera Dermacentor, Ixodes, and Haemaphysalis (Zhao et al., 2021).
Ixodes, the largest tick genus, includes more than 250 species (Estrada-Pena et al., 2017; Guglielmone and Robbins, 2018; Keirans and Durden, 2014). The Ixodes ricinus complex consists of at least 15 species with some of the most notable being I. ricinus and I. persulcatus in Eurasia, and I. scapularis, and I. pacificus in North America. Human biting species in Asia include I. granulatus, I. nipponenis, I. pavlovskyi and I. sinensis.
Ixodes are three host ticks which often exhibit non-nidicolous behavior as adults. However, the immature stages of some Ixodes, such as I. ovatus, are nidicolous. Many medically important Ixodes species are non-nidicolous generalists. Their biology and ecology are strongly influenced by temperature, humidity, habitat change, and host availability, all of which influence tick range expansion and abundance (Cunze et al., 2022; Medlock et al., 2013). Nymphs are the most common stage infesting humans (Barbour and Fish, 1993). The I. ricinus complex is abundant in deciduous and coniferous forested areas with relatively high humidity and increasingly in urban and suburban areas (Diuk-Wasser et al., 2010; Kahl and Gray, 2023; Rizzoli et al., 2014; VanAcker et al., 2019).
The geographic ranges of I. ricinus and I. persulcatus are expanding with I. ricinus in Europe and I. persulcatus in Asia with overlap in some interfacing regions (Bugmyrin et al., 2022). The range of I. ricinus extends from the Ural Mountains to Ireland and from northern Africa to Scandinavia (Estrada-Peña et al., 2012). It has expanded in northern Sweden and become more abundant in central and southern Sweden during the past three decades (Jaenson et al., 2012a). Range extension of I. ricinus northward in Norway is attributed to increased mean temperatures over the past decade (Hvidsten et al., 2020). As a result, the vegetation growing season is longer. Over 170 days, this approximate index is associated with sites of permanent tick populations (Hvidsten et al., 2020). In addition to range extensions at the previously recognized limits of altitude and latitude, I. ricinus distribution is changing within its established range (Medlock et al., 2013).
Range expansions are also occurring for multiple medically important North American tick species (Wikel, 2022; Table 1). Ixodes scapularis, the most important vector of Lyme borreliosis in North America, doubled the number of counties in the United States in which it was established in the 20 years period from 1996 to 2016 (Eisen et al., 2016). Northward expansions of the geographic range of I. scapularis in the eastern and midwestern United States resulted in established populations in Ontario and Quebec, Canada (Robinson et al., 2022; Scott and Pesapane, 2021; Slatculescu et al., 2020). Ixodes scapularis is reclaiming its historic range across a broad area of eastern regions of midwestern North America (Eisen and Eisen, 2018).
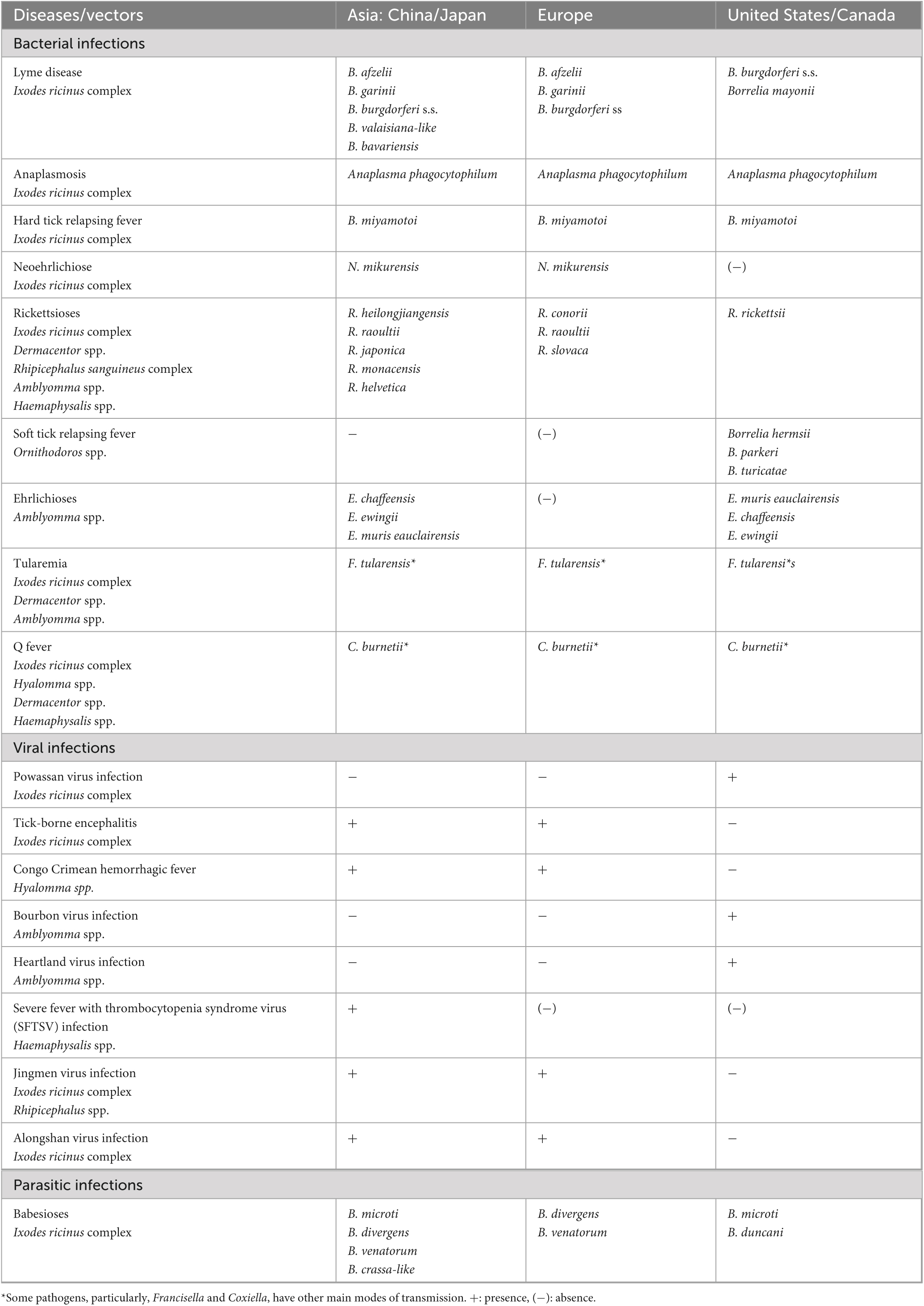
Table 1. Main tick-borne diseases and associated pathogens affecting human according to the continent (Zhao et al., 2021).
As the primary vector of Lyme borreliosis and tick-borne encephalitis in Eurasia (Table 1), the genus Ixodes receives the most attention. In addition, more recently, different pathogens have been associated with this genus such as Babesia spp., Anaplasma phagocytophilum, Neoehrlichia mikurensis, and Borrelia miyamotoi. Ixodes ticks and their associated pathogens’ distribution is likely shifting across much of the world due to climate change, ecosystem modifications, and socio-economic changes (Eisen, 2022; Kahl and Gray, 2023; Sprong et al., 2018).
The genus Dermacentor includes around 40 species in the Palearctic, Oriental, Afrotropical, and Nearctic zoogeographic regions with five species particularly relevant to human medicine in the Northern hemisphere: Dermacentor variabilis (American dog tick), Dermacentor andersoni (Rocky Mountain wood tick), Dermacentor occidentalis (Pacific Coast tick), Dermacentor marginatus (Eurasia) and Dermacentor reticulatus (Eurasia) (Guglielmone and Robbins, 2018). Most are three-host ticks, though some species engage in facultative two-host life cycles. Dermacentor species are predominantly mammalian ectoparasites, although immature stages are sometimes also recorded on birds. The immature stages are often endophilic, feeding on small mammals, adults predominantly obtain blood meals from large ruminants. Major medically important species are D. reticulatus and D. marginatus in Eurasia (Földvári et al., 2016; Garcia-Vozmediano et al., 2020), D. andersoni and D. variabilis in the United States and Canada (Eisen, 2022), and D. belullus and D. auratus in Asia (Apanaskevich and Apanaskevich, 2015; Kwak et al., 2021; Supplementary Table 1). They are important vectors of rickettsial infections worldwide. Dermacentor andersoni and D. variabilis are vectors of Rocky Mountain spotted fever (RMSF) in United States (Keirans and Durden, 2014), while D. reticulatus and D. marginatus are potential vectors of tick-borne lymphadenopathy in Europe (Chitimia-Dobler et al., 2019; Keirans and Durden, 2014). Tick-borne lymphadenopathy is caused by spotted fever group rickettsia, frequently Rickettsia slovaca (Silva-Pinto et al., 2014). In Asia, D. auratus has been implicated in the transmission of Kyasanur forest disease (Kwak et al., 2021). D. auratus is frequently encountered in both forests and urban parklands due to its close association with wild pigs (Sus scrofa), which act as the major reservoir host for the Kyasanur virus (Teo et al., 2024). D. reticulatus is an important vector of human pathogens that is significantly expanding its range in Europe (Cunze et al., 2022; Földvári et al., 2016). While climate change is a major driver, this highly adaptable species continues to establish populations in multiple new regions of Europe, likely in response to changing land use, and the widespread abandonment of previously approved tick control chemicals (Dautel et al., 2006; Piesman and Eisen, 2008; Randolph, 2010). Ecological modeling predicts range expansion of both D. reticulatus and D. marginatus toward northeastern Europe, more widely in central Europe, and the existence of areas where these species are sympatric (Cunze et al., 2022). In North America, the northern distribution limit in the prairie of southwestern Canada remains stable for D. andersoni, a significant medically important western North American tick, while expansion to the north and west by the closely related D. variabilis, an eastern North American species, results in overlapping ranges of these two species within an approximately 200 kilometer zone (Dergousoff et al., 2013).
The genus Rhipicephalus contains around 75 species; however, there exists uncertainty regarding the identity of certain species (Dantas-Torres and Otranto, 2015). We will focus on the R. sanguineus complex, which is the most widely distributed tick taxon. It is mainly associated with domestic dogs. Members of the complex are generally regarded to be three host ticks. Their development can be rapid, sometimes far less than a year (2–4 months) under warm climates and this species is commonly associated with dog shelters and human habitats where these ticks hide in wall crevices (Dantas-Torres, 2010). All stages can bite humans; and, they are reported to be more aggressive under warmer temperatures. Rhipicephalus sanguineus s.l. is considered to be an important vector of the rickettsial infections, Mediterranean Spotted fever (MSF) in Europe and Rocky Mountain spotted fever in Mexico and the southwestern United States (Dantas-Torres and Otranto, 2015).
The genus Amblyomma contains around 138 species mainly in the Neotropical, Afrotropical, Oriental, and Australasian zoogeographic regions (Guglielmone and Robbins, 2018). However, in North America, A. americanum, larvae, nymphs, and adults of the lone star tick, frequently bite humans (Eisen, 2022). It is a generalist species with all active stages (larva, nymph, and adult) feeding on all classes of terrestrial vertebrates. It is the vector of tularemia, RMSF, Q fever, and ehrlichiosis (E. chaffeensis) (Keirans and Durden, 2014). A. americanum is also known as the vector of STARI, Southern Tick Associated Rash Illness, Ehrlichia ewingii, Heartland virus and bourbon virus. Both viruses are emerging infectious diseases in North America (Dupuis et al., 2023). A. americanum is an aggressive human-biting tick that is expanding from its traditional range in the southeastern United States into the mid-Atlantic, northeastern, and midwestern United States and into southern Canada (Gasmi et al., 2018; Molaei et al., 2019; Sagurova et al., 2019; Sonenshine, 2018; Springer et al., 2015). A. maculatum, vector of the spotted fever rickettsia, R. parkeri, is also expanding from its historic range along the coastal southeastern United States northward into the mid-Atlantic, southern New England, and significantly further inland along its range (Bajwa et al., 2022; Paddock and Goddard, 2015; Teel et al., 2010). Isolated populations of A. maculatum occur at multiple locations in the midwestern and southwestern United States, well beyond the traditional range for this tick species (Paddock and Goddard, 2015). A. testudinarium, A. kappa, and A. helvolum are also known to bite humans in Asia. A. testudinarium in particular is a vector of the zoonotic Oz virus and R. tamurae. It is also suspected to be a vector of Severe Fever with Thrombocytopenia Syndrome (SFTS) virus and Hepatozoon spp. (Nakao et al., 2021). A. testudinarium is the main species responsible for human tick-bite cases in Japan, and the main Amblyomma species biting humans throughout East and Southeast Asia (Natsuaki, 2021). The study of tick bites in wild animals and humans showed that the expansion of wild boar was to blame, and that the majority of these cases were associated with A. testudinarium (Shimada et al., 2022). This tick is also linked to cases of alpha-gal syndrome, a hypersensitivity reaction to red meat mediated by IgE antibody (Hashizume et al., 2018).
The genus Haemaphysalis includes around 166 species with a cosmopolitan distribution (Guglielmone and Robbins, 2018). Its highest species diversity occurs in East and Southeast Asia, less so in Europe and the Americas (Durden and Beati, 2014; Guglielmone and Robbins, 2018). Haemaphysalis longicornis in China, Japan, and Korea is a vector of SFTS virus, a significant emerging infectious disease. Presently, this tick species is considered an invasive tick in many regions. Originally native to East Asia, it spread to Australia, New-Zeland and many Pacific Islands, and was recently detected in the eastern United States (Zhao et al., 2021), and Turkey (Keskin and Doi, 2025). The introduction and establishment of this tick, into North America was first confirmed in 2017 (Rainey et al., 2018). As of March 2024, H. longicornis populations are established in nineteen states in the eastern United States (APHIS-USDA, 2024). Examination of archival field collected specimens revealed that H. longicornis was already present in North America in 2010 (Beard et al., 2018). Genetic analyses established that at least three unrelated H. longicornis females were introduced into North America (Egizi et al., 2020). Habitat suitability modeling predicts the potential for geographic range expansion of a large part of North America (Namgyal et al., 2020) with major concerns focused on its capacity to transmit SFTS virus, spotted fever group rickettsiae (Rickettsia japonica) and A. phagocytophilum (Zhao et al., 2021). Although SFTS virus is not known to be present in North America, H. longicornis is a competent vector of Heartland virus, a close relative of SFTS virus, in the United States. Both the closely genetically related Heartland virus and SFTSV were discovered in 2009 (Brault et al., 2018). Significantly, H. longicornis in North America undergoes parthenogenetic reproduction producing large numbers of progeny (Beard et al., 2018). All parasitic stages bite humans (Guglielmone and Robbins, 2018). In addition, Turkey and surrounding countries, including parts of Europe and the Middle East, are likely to become the next countries to be invaded by H. longicornis. The other members of this genus are mostly exophilic three-host ticks. In Eurasia, H. concinna is endemic in wide areas from western Europe to far-east Asia including Japan. Many Haemaphysalis are present in mixed and deciduous forests in moist habitats. The adults often prefer to infest large mammals and domestic animals while the immature stages are often found on birds, small/medium sized mammals. Haemaphysalis species are also regarded as vectors of TBE virus, SFTS virus, and R. japonica (Rubel et al., 2018).
The genus Hyalomma is a medically important species present in Eurasia and Africa with 27 species (Guglielmone and Robbins, 2018). They develop in open ecosystems with relatively hot and dry climates, and at low altitudes (Keirans and Durden, 2014). They are two or three-host ticks, with larvae and nymphs often being endophilic and feeding on small vertebrates while adults occur on large ungulates, including livestock. Adults can bite humans, and the most common human biting Hyalomma species are H. anatolicum, H. marginatum, H. aegyptium, and H. lusitanicum. They often exhibit hunting behavior, and will actively pursue potential hosts (Bernard et al., 2022). Due to climate change, this tick has expanded into south-western Europe, likely introduced into new geographic areas by migratory birds, and is considered an invasive species there. It is now established in the south of France and in Spain and it is responsible for human cases of Crimean Congo Hemorrhagic Fever (CCHF) (Bernard et al., 2024; Portillo et al., 2021). In a model of CCHF virus expansion in the Western Palearctic, Estrada-Peña et al. (2013) established that risk is associated with variations in temperature and host presence. These two factors have a direct impact on the development and survival of infected ticks. In Spain, the first human CCHF cases appeared in 2016, since then 17 cases have been reported with four cases in 2024. In Portugal, the first death associated to CCHF occurred during July 2024 (Zé-Zé et al., 2025).
Main hosts/reservoirs of ticks
Ticks are strictly hematophagous ectoparasites which rely on various vertebrate hosts for their bloodmeal including mammals, birds, and reptiles. Ticks rely on their hosts for dispersal due to their small size and inability to fly. This makes the host-tick relationship crucial for tick survival and spread. Some of these tick host vertebrates are reservoirs of pathogens and maintain enzootic cycles of tick-borne pathogens. Humans are generally considered as incidental hosts (Eisen and Eisen, 2018; Nepveu-Traversy et al., 2024; Rizzoli et al., 2014). Ticks exhibit varying degrees of dependence on individual hosts and can be separated into one-host ticks (those which spend their entire life on a single individual host without leaving it to shed their cuticle between instars) (e.g., R. microplus), two-host ticks (e.g., Hyalomma scupense) which often remain on an individual host through both the larval and nymphal stage, only leaving once the nymph has engorged, and three-host ticks (e.g., I. ricinus), which leave the host between each instar (Leal et al., 2020). Additionally, ticks exhibit varying degrees of specificity for their hosts. Tick species can be associated with a single host species (monoxenic) (e.g., Haemaphysalis pentalagi and its rabbit host Pentalagu funessi) (Kwak et al., 2024b), a small number of host species (oligoxenic) (e.g., Amblyomma nitidum and Laticauda spp. snakes) (Kwak et al., 2020), or a wide range of host species (polyxenic) (I. ricinus) (Humair and Gern, 2000). Throughout different stages of development, some ticks exhibit distinctive changes in host specificity. It is well-known that ticks have established moderate to strong associations with specific wildlife as their primary hosts.
Small mammals, especially rodents (order Rodentia), are known as major reservoirs of zoonotic pathogens due to their high diversity. Those involved in tick-borne disease maintenance live in close association with humans (Keesing and Ostfeld, 2024). They are important hosts for larval and nymphal ticks (Eisen, 2022; Földvári et al., 2016; Furuno et al., 2017). One of the best example is Lyme borreliosis where rodents play a role as a host for Ixodes larvae and as reservoir for B. burgdorferi s.l. (Keesing and Ostfeld, 2024). In the northeastern United States, the most important rodent is the white-footed mouse (Peromyscus leucopus); in Europe, small rodents such as Apodemus, Myodes, Microtus (Gern and Humair, 2002; Rizzoli et al., 2014) and in Asia Apodemus is also a genus well-represented (Hou et al., 2014; Margos et al., 2013; Taylor et al., 2013). These rodents are also reservoirs of anaplasmosis, neoerhichiosis, TBE, relapsing fever associated to Borrelia miyamotoi, and babesiosis to name a few (Eisen, 2023; Rizzoli et al., 2014; Zhao et al., 2021). The complex nature of multiple pathogen reservoirs, tick vectors, and enzootic cycles is evident in North America where groundhogs (Marmota monax) are the main reservoirs for Powassan virus followed by Peromyscus leucopus with Ixodes cookei and I. scapularis as the primary vector ticks (Lange et al., 2023). Furthermore, tick density on rodents may differ from year to year as well as by the abundance of other hosts. Nymphal tick density on rodents increased by the exclusion of deer (Perkins et al., 2006).
Circulation of tick-borne pathogens is linked to other small mammals, such as insectivores, erinaceomorpha, and lagomorpha that can serve as hosts for all tick stages and a wide variety of tick species. In Europe, Erinaceus (hedgehog) is a good host for Ixodes and Dermacentor and reservoir for Lyme borreliosis, anaplasmosis, and TBE virus (Rizzoli et al., 2014). Its role as reservoir of pathogens in urban and peri-urban areas has been clearly shown in Europe for N. mikurensis, A. phagocytophilum, and B. burgdorferi s.l. (Földvári et al., 2014; Rizzoli et al., 2014). Lagomorphs are also an important tick host as the reservoir of Francisella tularensis and CCHFV (Bernard et al., 2022; Sharma et al., 2023). In East Asia, I. ovatus, Ha. longicornis, Ha. flava, R. haemaphysaloides, and I. sinensis are common ticks collected from hares (Lepus spp.) (Zheng et al., 2011).
Large-sized mammals especially wild ungulates such as deer and boars represent about half of the combined biomass of terrestrial wild mammals (Greenspoon et al., 2023). Historically, ruminant populations have decreased with human expansion (Chen et al., 2019; Fish, 2022). However, the populations of some deer (Côté et al., 2004; Fish, 2022; Iijima et al., 2023) and wild boar (Massei et al., 2015) are recently increasing. Because of their large biomass and population increase, the increase of large mammals has been suggested to be related to tick increase. The higher abundance of white-tailed deer (Odocoileus virginianus) (Kilpatrick et al., 2014; Ostfeld et al., 2018), sika deer (Cervus Nippon) (Iijima et al., 2022), roe deer (Capreolus capreolus) (Jaenson et al., 2012b), and wild boar (Sus scrofa), (Doi et al., 2021; Ferroglio et al., 2024) are responsible for the greater abundance of tick species in the geographic regions where these large mammals occur. It is suggested that deer presence was more important on tick abundance than deer density. Indeed, in a study performed in dutch forests, the density of ticks did not increase with abundance of deer but experimental exclosure of deer significantly reduced tick population on a period of 2 years (Hofmeester et al., 2017). Wild boars contribute less to the maintenance of tick populations, especially for Ixodes ticks. In Europe, they harbor less ticks than cervids (Fabri et al., 2021; Hrazdilová et al., 2021). In contrast, Dermacentor on wild boar contribute to the circulation of Rickettsia in Europe (Sgroi et al., 2021). The expansion of wild boar in Japan resulted in an increase in the number of patients with tick-bites caused by A. testudinarium (Shimada et al., 2024). In United States, deer are critical as a blood meal source for adult I. scapularis to maintain the life cycle. They are also reservoir for Ehrlichia chaffeensis and E. ewingii (Eisen et al., 2017).
Birds play a role in the long distance dissemination of ticks and associated pathogens (Buczek et al., 2020; Estrada-Peña et al., 2021; Pitó et al., 2024). Ticks especially prefer to infest ground feeding birds such as Erithacus rubecula, Turdus merula, Fringilla coelebs, and Passer domesticus (Bacak et al., 2024; Wilhelmsson et al., 2020). Migratory birds coming from Africa with Hyalomma lusitanicum allowed the introduction of CCHV in new regions South of Europe, including Spain, Portugal and France (Bernard et al., 2022; Portillo et al., 2021; Zé-Zé et al., 2025). Ixodes uriae, I. pavlovskyi, I. philipi, I. lividus, I. turdus, H. concinna have a wide distribution within Eurasian, in part due to their association with birds. In Asia, the immature stage of I. ovatus, I. persulcatus, and Ha. wellingtoni are common examples of bird infestation ticks (Byun et al., 2024; Kwak et al., 2024a).
Migratory bird flyways that cross Asia, Europe, and North America converge in the arctic which is warming at a rate four times faster than the rest of the world (Rantanen et al., 2022). What impact might this warming trend have on ticks that are transported into the arctic on migratory birds in regard to survival, interactions with arctic fauna, and advancement to the next life cycle stage? Novel exchanges of tick species with their associated pathogens might occur that allows them to be introduced into different migratory bird populations and different migratory pathways, resulting in their introductions into new geographic areas. This is an area that should be investigated and further supports the importance of establishing tick and associated pathogen surveillance networks (Buczek et al., 2020; Pitó et al., 2024).
Multiple Amblyomma species are associated with reptiles. Common reptile ticks in Asia include A. geoemydae, A. nitidum, A. helvolum, and A. varanense which are also ticks reported with human infestations. In Turkey, Hy. aegyptium is the well-known tortoise tick which harbor CCHF virus. The tortoise could participate in the cryptic circulation of the virus with a potential transmission of CCHFV to humans (Kar et al., 2020). In Europe, some lizards including Lacerta agilis are host for I. ricinus nymphs and are reservoir for Borrelia lusitaniae but not for other Lyme associated Borrelia species (Tijsse-Klasen et al., 2010).
Invasive non-native hosts can also become novel hosts for local ticks as well as introduce “foreign” ticks into a new region. Doi et al. (2024) showed that introduced raccoons (Procyon lotor) and masked palm civets (Paguma larvata) are infested by Japanese tick species. Klink et al. (2024) also reported European Ixodid ticks were found on introduced raccoons and raccoon dogs (Nyctereutes procyonoides) in Germany (Byun et al., 2024). The eastern chipmunk (Tamias striatus) a rodent particularly present in United States has been introduced into France (Marchant et al., 2017) and Italy (Mori et al., 2018). It is an excellent host for ticks and reservoir of Lyme borreliosis (Keesing and Ostfeld, 2024), as such, it could modify the dynamic of transmission of tick-borne pathogens in Europe.
In recent years, the importance of host biodiversity in vector-borne diseases expansion has been suggested (Keesing et al., 2006; Keesing and Ostfeld, 2021). The complexity of the enzootic cycle of Lyme disease with different reservoir hosts, the major role of the white-footed mouse (Peromyscus leucopus), the deer population, and the concept of vector competence for optimal Borrelia circulation was suggested (Matuschka and Spielman, 1986). A diverse assemblage of vertebrates reduces the risk of Lyme disease in human (Ostfeld and Keesing, 2000). Based on these results, the concept of the dilution effect, i.e., that the presence of vertebrate hosts with a low capacity to infect food vectors (incompetent reservoirs) dilutes the effect of highly competent reservoirs, thus reducing the risk of disease, was suggested (Schmidt and Ostfeld, 2001; LoGiudice et al., 2003). The authors stressed the importance of biodiversity conservation in the face of anthropogenic activities affecting the host community in this type of zoonosis. However, this model has subsequently been questioned, and the effect of different host communities may in fact be a dilution or amplification of tick populations depending on competition between hosts, contact between hosts and ticks and host resistance to tick bites (Ogden and Tsao, 2009; Randolph and Dobson, 2012). The various models proposed show that the host community is not sufficient to predict risk, and that additional parameters, including temperature and vegetation, need to be included in the models (Kilpatrick et al., 2017).
Tick-borne diseases on each continent
Due to tick population expansions, tick-borne diseases (TBDs) are also more frequently being diagnosed in humans in areas where those infections were seldom, if ever previously encountered (Table 1). Better awareness by clinicians and patients and improvements in diagnostic tools also contribute to the increase in TBD detections. However, TBDs continue to be underdiagnosed worldwide. They sometimes are introduced into new geographic areas which can complicate our understanding of the epidemiology of these diseases. Ticks transmit viruses, bacteria, and parasites to vertebrate hosts, and although they can harbor different potentially pathogenic microorganisms, co-infections are less frequently observed in patients than might be anticipated (Boyer et al., 2022). TBDs are zoonoses and humans constitute incidental, typically dead-end, hosts.
Tick-borne diseases have been known since the second half of the 19th century; new ones are regularly identified and their medical importance is constantly growing (Figure 1). They are increasing in diversity and occurring in greater numbers due to climate change and socio-ecosystemic modifications (Madison-Antenucci et al., 2020; Pfäffle et al., 2013; Wikel, 2022). In North America, presently the United States is experiencing the greater burden of TBDs, and due to climate change Canada is becoming increasingly impacted by these diseases (Wikel, 2022). In Europe, expansion of TBDs to the North is observed especially for Lyme borreliosis, and TBE due to climate change (Lindgren et al., 2000). In south-western European countries such as Spain and France, the introduction of Hyalomma ticks, probably via migratory birds from Africa, has led to the appearance of human cases of CCHF in Spain (Portillo et al., 2021) and the detection of the virus in ticks in France (Bernard et al., 2024). At present, Asia seems to be more impacted by the emergence of new zoonotic tick-borne viruses, such as SFTS virus (Zhao et al., 2021). Ticks and tick-borne pathogens are increasingly transboundary threats. Lyme borreliosis is the most common vector-borne disease in the northern hemisphere and it has steadily increased in incidence and geographical range in many regions of Europe and North America (Eisen and Eisen, 2018; Eisen et al., 2016; Goren et al., 2023; Kugeler et al., 2021).
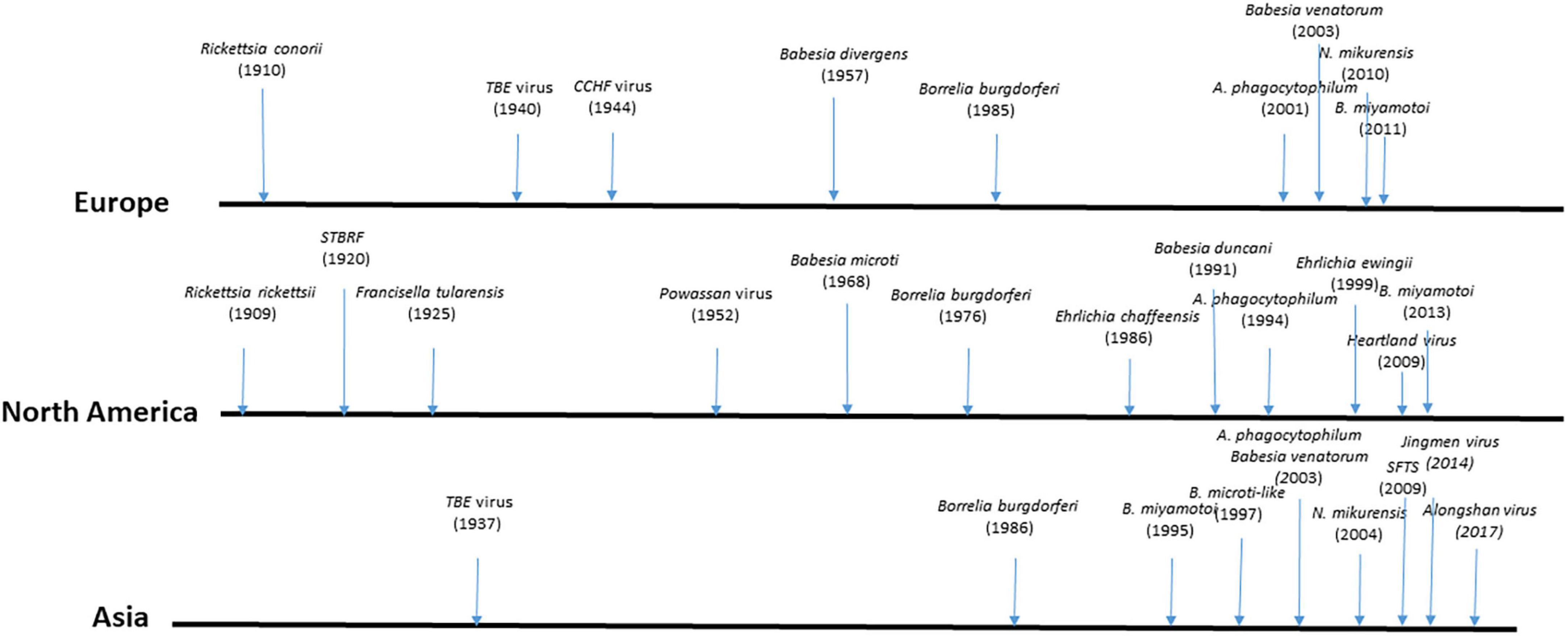
Figure 1. Main dates in the discovery of most important human tick-borne diseases. TBE, tick-borne encephalitis; CCHF, Crimean Congo Hemorrhagic Fever; STBRF, soft tick-borne relapsing fever; SFTS, Severe Fever Thrombocytopenia Syndrome.
We review the current status of the main TBDs of the northern hemisphere, bearing in mind that they do not all present the same risk in terms of exposure, transmission, and infection. Transmission is linked to the duration of attachment of the infected tick, which differs according to the pathogen: B. burgdorferi sensu lato optimal transmission at 24 h with many more at 48–72 h, Babesia microti (36 h or more), Francisella (48 h), Ehrlichia and A. phagocytophilum (starting at 24 h), almost immediately for viral infections (de La Fuente et al., 2007; Eisen, 2018; Richards et al., 2017). Treatments are available for tick-borne bacterial and parasitic infections that are currently free of antibiotic resistance, the situation is more critical for tick-borne viral infections (Nepveu-Traversy et al., 2024), while vaccines against TBD are significantly lacking. Therefore, raising awareness among healthcare professionals and the general public about changing tick-borne disease threats is essential. Currently, the only vaccine available for TBDs in humans targets the TBE virus and two vaccines were approved in 2000 in Europe (Jaenson et al., 2018; Süss, 2003). More recently, a TBE virus vaccine was approved in the United States in 2021 (Hills et al., 2023).
The prevalence of infection in ticks varies according to the pathogens, the local environment, the tick stage, reservoirs, and the enzootic cycles: it can be as high in the case of some bacteria with up to 60% for B. burgdorferi s.s. in the United States (Maggi et al., 2019) while in Europe, an average prevalence of 16% in ticks has been recorded (Strnad et al., 2017). Tick infection is generally relatively low for viruses, often less than 1% (Randolph et al., 2000) and for parasites (Kumar et al., 2021).
In recent years, with the development of metagenomics, the characterization of vector microbiota has complicated our understanding of vector-pathogen-host interactions (Wang et al., 2023). New micro-organisms have been identified, some of which are simple endosymbionts such as Coxiella-type endosymbionts, Rickettsia-type endosymbionts and Francisella-type endosymbionts (Bonnet et al., 2017; Duron et al., 2018), others are potential pathogenic microorganisms like spiroplasma (Eimer et al., 2022; Matet et al., 2020). Symbionts are essential to tick survival as nutritional factors, but their role in the development of pathogens within the tick and in the transmission of pathogenic microorganisms to the vertebrate host is increasingly being described (Boulanger et al., 2023; Narasimhan et al., 2021).
Another important point to keep in mind in vector-borne diseases is that the detection within a tick of the molecular signature of a pathogen does not establish vector competence of that tick species for that pathogen (Eisen, 2020b; Kahl et al., 2002).
Bacterial infections
Spirochetes include the genera Treponema, Leptospira and Borrelia. Within the genus Borrelia, two types of spirochete caused tick-borne diseases can be diagnosed in humans: Lyme borreliosis and relapsing fevers.
Lyme borreliosis (LB) is the most well-known, and an extensively well-studied TBD. It is the most commonly encountered vector-borne disease of the temperate zones of the Northern hemisphere. This bacterial infection is caused by six spirochete genospecies among the 20 validated genospecies (Table 1). The bacteria are maintained in a complex network of Ixodes vectors and different reservoir hosts, mainly rodents and birds (Wolcott et al., 2021). The vectors belong to the I. ricinus complex and the pathogens are extracellular spirochetes. Twenty-one species of B. burgdorferi sensu lato have been identified with three major pathogenic species: B. afzelii and B. garinii in Eurasia and B. burgdorferi sensu stricto mainly in North America (Stanek et al., 2012). First identified in Connecticut on the East coast of the United States in 1975, its presence was already suspected in Europe at the end of the 19th century (Lenormand et al., 2016).
In Europe, the incidence rate is ∼22 cases per 100,000 people, the actual number is most certainly higher, because LB is not notifiable in all European countries (Steinbrink et al., 2022). In the USA, it is a notifiable disease and based upon laboratory data is responsible for an estimated 476,000 human cases annually (Borchers et al., 2015; Nepveu-Traversy et al., 2024). In Asia, China reported its first case of LB in 1986. A meta-analysis of LB seroprevalence from 2005 to 2020 documented the presence of the disease in 29 provinces across China, with a core zone of endemicity in the northeastern provinces (Stark et al., 2022). In Japan, the disease was also identified for the first time in 1986. It is mainly reported from Hokkaido, the northernmost prefecture of Japan (Yamaji et al., 2018). Lyme borreliosis has been reported from other Asian countries including Korea, Taiwan, Nepal, Indonesia, and Malaysia. Human disease is characterized by a multisystemic disorder affecting the central nervous system with facial nerve palsy as a common clinical manifestation; the joints, heart, and/or skin can also be affected. The early manifestation may be a localized erythema migrans in around 80% of patients which occurs 3–30 days after the bite of an infected tick (Stanek et al., 2012). Some clinical manifestations such as lymphocytoma, late neuroborreliosis, and acrodermatitis chronica atrophicans are reported in Europe, due to different Borrelia species (Steinbrink et al., 2022). A vaccine candidate based on different OspA serotypes is currently in a phase 3 clinical trial (Bézay et al., 2024; Ghadge et al., 2024). It relies on a transmission-blocking process of the bacteria within the tick involving OspA (Wormser, 2022). OspA is a major antigen expressed by Borrelia in the Ixodes midgut (Pal et al., 2000).
Relapsing fever (RF) is caused by multiple Borrelia species and most commonly associated with transmission by Argasid, soft ticks, of the genus, Ornithodoros (Supplementary Table 1). These RFs are mainly present in Africa, the Middle-East and the Americas where different Borrelia species are associated to specific tick species (Jakab et al., 2022; Talagrand-Reboul et al., 2018). More recently, a new RF Borrelia transmitted by Ixodes was described: B. miyamotoi. First discovered in the hard tick I. persulcatus in Japan in 1995, it was thereafter identified in patients in Russia (2011), the United States (2013), and Europe (Madison-Antenucci et al., 2020). Its prevalence in hard ticks of the I. ricinus complex is not negligible but human cases are still limited. An extensive analysis determined the average incidence of B. miyamotoi infection of questing I. ricinus (1.0%), I. scapularis (1.1%), Ixodes pacificus (0.7%), and I. persulcatus (2.8%) with infection rate variations within a species (Burde et al., 2023). During 2022 and 2023, number of human B. miyamotoi infections reported to the Connecticut State Public Health Department were 7 and 4, respectively, which is likely fewer cases than actually occurred (Department of Public Health, 2025). Clinical symptoms are similar to A. phagocytophilum with fever and elevated liver enzymes (Madison-Antenucci et al., 2020). In immunocompromised individuals, the disease can be neuro-invasive (Sprong et al., 2018). Its reservoirs are mainly small rodents, Peromyscus, Apodemus, and Myodes species, and birds (Cleveland et al., 2023; Krause et al., 2015).
Bacteria of the Rickettsiales order are small obligate intracellular microorganisms transmitted by arthropod vectors (Londoño et al., 2023). As human pathogens, they possess two families, Rickettsiaceae responsible for rickettsiosis and Anaplasmataceae responsible for ehrlichiosis, anaplasmosis and neoehrlichiosis.
Rickettsioses have a worldwide distribution with a large variety of hosts and arthropods involved in their transmission. They can cause life-threatening diseases and are transmitted either by ticks (spotted fever group) or by non-tick vectors such as mites, fleas, and lice (Merhej et al., 2014). Rickettsiae are obligate intracellular bacteria infecting endothelial cells and macrophages and the pathogenesis of these infections was the subject of recent excellent reviews (Blanton, 2019; Helminiak et al., 2022; Sahni et al., 2019). In North America, R. rickettsii is transmitted by ticks (Dermacentor, Amblyomma and Haemaphysalis) and by R. sanguineus in Mexico and Brazil. Rickettsia rickettsii is responsible for RMSF, characterized by fever, headache, and rash. It can be deadly if not treated by appropriate antibiotic therapy. In Europe, R. conorii transmitted by R. sanguineus is responsible for Mediterranean spotted fever and R. slovaca and R. raoultii, responsible for TIBOLA (tick-borne lymph-adenopathy) is transmitted by D. marginatus and D. reticulatus (Oteo and Portillo, 2012). In China, R. heilongjiangensis and R. raoultii are the most widely distributed spotted fever group rickettsiae (Zhao et al., 2021) whereas in Japan, R. japonica, R. heilongjiangensis, and R. tamurae cause spotted fever (Imaoka et al., 2011); other human infectious rickettsial species are also present (R. monacensis and R. helvetica) (Thu et al., 2019). Rickettsial interactions with tick vectors and vertebrate hosts are being advanced by genome characterizations, functional genomics, and molecular studies (Kim, 2022).
Human granulocytic anaplasmosis (HGA) was first discovered in 1994 in the United States. It is caused by the Gram-negative bacterium, A. phagocytophilum, which displays an intracellular tropism for neutrophils. Vectors are members of the I. ricinus complex and the disease is present in Europe, North America, and Asia (Bakken and Dumler, 2015). This bacterial infection affects both domestic animals and humans. Infection can be asymptomatic or cause non-specific febrile illness with greater risk of morbidity and mortality in immunocompromised patients (Madison-Antenucci et al., 2020). A recent systematic literature review addressed clinical, diagnostic, therapeutic and epidemiologic aspects of infections with this increasingly important tick-borne pathogen (Schudel et al., 2024). Different A. phagocytophilum ecotypes have been identified that exhibit different host ranges. All human cases belong to ecotype I in Europe (Jahfari et al., 2014). There are two distinct variants of A. phagocytophilum in North America with the Ap-ha (human active) variant causing illness in humans and domestic animals and associated with a rodent reservoir (white footed mice, Eastern chipmunks) while the Ap-V1 variant is associated only with white tailed deer and considered to be non-pathogenic (Aardema, 2023; Keesing et al., 2014). North American A. phagocytophilum is genetically less diverse than those in Europe, suggesting an introduction of this organism from Europe (Aardema, 2023). The overall HGA prevalence worldwide is 8.13% with the highest incidence in humans at 11.07% occurring in North America, with occupationally exposed populations at greatest risk of infection (Karshima et al., 2023). There are several other Anaplasma species with zoonotic significance. In Cyprus, Anaplasma ova was detected in a human with fever after a tick bite (Chochlakis et al., 2010). In China, Anaplasma capra was detected in patients with febrile presentation associated with a history of tick bite (Li et al., 2015).
Neoehrlichiosis is caused by the Gram-negative bacterium, N. mikurensis (Portillo et al., 2018). The bacterium is intracellular, developing in the cytoplasm of endothelial cells. It was first discovered in brown rats, R. norvegicus, and the tick I. ovatus in Japan (Kawahara et al., 2004). The first clinical case was subsequently described in Sweden in an immunocompromised patient with thromboembolic complications (Welinder-Olsson et al., 2010). Presently, the disease is described as an infection with fever, rash, and thromboembolic complications in both immunocompromised and immunocompetent individuals (Portillo et al., 2018; Silaghi et al., 2016). In Europe, rodents (Apodemus and Myodes) are reservoirs and the level of Ixodes tick infection varies from 0.1% to 24.3% (Portillo et al., 2018). The disease has also been described in Asia (Zhao et al., 2021).
Ehrlichioses caused by obligate intracellular Gram-negative bacteria of the order Rickettsiales were considered veterinary pathogens until 1954 when human infection with Rickettsia sennetsu (later reclassified as Ehrlichia sennetsu, now Neorickettsia sennetsu) was identified in Japan (Gygax et al., 2024). Medical importance of Ehrlichia changed significantly with the identification of E. chaffeensis, causative agent of human monocytic ehrlichiosis (Paddock and Childs, 2003). Subsequently, E. ewingii, closely related to Ehrlichia canis, was found to infect humans (Adams et al., 2025; Gygax et al., 2024). Both E. chaffeensis and E. ewingii are transmitted by A. americanum, whose larvae, nymphs, and adults readily blood feed on humans and whited tailed deer, reservoir for these pathogens (Gygax et al., 2024; Paddock and Childs, 2003). Ehrlichia muris subsp. eauclairensis is a rarely encountered human pathogen whose primary reservoir is Peromyscus leucopus and vector is I. scapularis (Gygax et al., 2024; Xu et al., 2023). Human infections with other Ehrlichia species (E. muris, E, canis, and E, ruminantum) are rare (Gygax et al., 2024). Human monocytic ehrlichiosis almost entirely occurs within the United States; however, human ehrlichial infects have been rarely reported in Africa, Asia, and South America (Gygax et al., 2024). The clinical presentation, diagnostic, laboratory, and epidemiologic aspects of human monocytic ehrlichiosis are subjects of recent reviews (Gygax et al., 2024; Ismail and McBride, 2017) as is the molecular pathogenesis (Rikihisa, 2015).
Tularemia is a clinical entity caused by the Gram-negative, pleiomorphic, catalase-positive, non-motile coccobacillus Francisella tularensis, one of the most virulent bacteria known that is infectious at extremely low doses (Eickhoff and Blaylock, 2017; Sharma et al., 2023). Of the two important subspecies of this important pathogen, Francisella tularensis Type A (subspecies tularensis), the more virulent of the two, occurs exclusively in North America commonly associated with wild rabbits and hares. It can infect a diverse range of animal species (Keim et al., 2007). The second one, Type B (subspecies holartica) occurs in Europe and Russia is associated to rodents (Gray et al., 2024). Francisella tularensis is one of the most adaptable microbes to environmental conditions, hosts, and vectors with transmission cycles linked to differing ecologies of a Type A terrestrial cycle and Type B aquatic cycle (Carvalho et al., 2014). Francisella tularensis is resistant to environmental stresses and able to survive in soil, water, and carcasses for weeks (Carvalho et al., 2014).
Human infection occurs by direct contact with infected animal tissues or blood; indirectly by consumption of contaminated food, water, or inhalation of bacteria contaminated aerosols; and by the bites of infected ticks and tabanids (Carvalho et al., 2014; Sharma et al., 2023). Tularemia most frequently occurs during late spring, summer, and autumn with men more frequently infected than women (Ellis et al., 2002). Clinical presentation and course of tularemia depends upon virulence of bacterial strain, route of infection, infectious dose, host immune status (Nigrovic and Wingerter, 2008; Oyston, 2008). Incubation period post-infection is typically three to 5 days; however, it can range up to 20 days (Sharma et al., 2023). Clinical presentations include ulceroglandular, glandular, oculoglandular, orophyrangeal, and pneumonic with the ulceroglandular and glandular forms the most commonly occurring in North America with tick bite as the route of transmission (Eickhoff and Blaylock, 2017; Nigrovic and Wingerter, 2008; Sharma et al., 2023).
Francisella tularensis has been bioengineered and weaponized to be a significant agent of bioterrorism due to its high virulence, extremely low infectious dose and significant threat posed by aerosol delivery (Dennis et al., 2001; Oyston et al., 2004).
Q fever or coxiellosis is due to Coxiella burnetii, an obligate intracellular Gram-negative bacterium that infects a wide variety of mammals, birds, reptiles, and arthropods. It occurs over a wide geographic range with the exception of New Zealand and Antarctica as an environmentally stable microbe. It has a low infective dose of less than ten bacteria (Celina and Cerný, 2022). It is a zoonosis with the most common reservoirs being cattle, sheep, and goats (España et al., 2020). Infections in humans and farm animals occur predominantly due to inhalation of wind distributed spore-like (pseudospores) organisms (España et al., 2020). Davis et al. (1938) isolated a microorganism, later identified as Rickettsia burnetii and now known as C. burnetii, from the Rocky Mountain wood tick, Dermacentor andersoni, in western Montana. Although ticks are competent vectors of C. burnetii, they and other arthropods play minor roles in pathogen transmission to humans; however, ticks are likely more important in maintenance of the bacteria among other animal species in nature in a sylvatic cycle (Celina and Cerný, 2022; McQuiston et al., 2002). Coxiella burnetii is maintained in the infected tick population throughout the life cycle by both transovarial and transstadial transmission (Celina and Cerný, 2022). Additional arthropod hosts and sources of infection of vertebrate species are sucking lice and mites. Infection can also be acquired by consumption of unpasteurized milk and milk products as well as environmental exposure to infected placentas, aborted tissues, urine, and feces (Celina and Cerný, 2022; McQuiston et al., 2002).
Detection of serum antibodies in cattle, goats, and sheep, the main reservoirs, does not necessarily correlate with shedding of C. burnetii as indicated by fewer than 10 percent of sheep and 35 percent of bovines that were actively shedding C. burnetii being seropositive (Eldin et al., 2017). Multiple strategies have been employed to develop a C. burnetii vaccine, but no effective and fully safe vaccine has been developed (Sam et al., 2023).
The resistance and the possibility of airborne transmission have led to this bacterium being classified in list 3 in terms of biosafety. C. burnetii is a significant potential bioterrorism agent (Oyston and Davies, 2011).
Viral infections
More than 35 virus species from six different families are transmitted by ticks (Wu et al., 2024). These viruses are RNA viruses. Their incidence is increasing: some are expanding their geographic range such as CCHF, TBE, Powassan, Heartland, Bourbon, and SFTS viruses and new ones are regularly identified in China (Alongshan, Wetland, Xu-Cheng virus) and in Europe (Tacheng virus 1&2) (Ergunay et al., 2023; Madison-Antenucci et al., 2020; Shah et al., 2023; Zhang et al., 2025). Because ticks can also transmit viruses transovarially to their offspring, ticks also are reservoirs of viruses in ecosystems (Moming et al., 2024; Nuttall and Labuda, 2003; Shah et al., 2023).
Crimean-Congo hemorrhagic fever (CCHF) is caused by infection with CCHF virus, an enveloped single-stranded negative-sense RNA virus belonging to the Orthonairovirus genus in the Nairoviridae family of the Bunyavirales order (Hawman and Feldmann, 2023). Seven genotypes with different virulence and different geographic range have been characterized (Bernard et al., 2024; Shahhosseini et al., 2021). Because of the risk of human-human transmission and the severity of the disease, CCHF virus is placed in biohazard class IV. It is the most medically important and widespread tick-borne viral disease, characterized by sporadic cases and outbreaks of hemorrhagic fever that occur over a broad geographic range from western China across Asia to southern Europe, the Middle East, and a vast area of Africa (Bente et al., 2013; Ergönül, 2006; Fereidouni et al., 2023). The geographic range of CCHF virus extends into southern Europe, likely due to the movement of migratory birds coming from Africa (Bernard et al., 2022; Portillo et al., 2021). It is mainly transmitted to humans by Hyalomma tick bites, but also through contact with blood or tissue of viremic animals during slaughtering, including human-to-human nosocomial transmission (Hawman and Feldmann, 2023; Shahhosseini et al., 2021). Most infections are subclinical. After a short incubation, it initially presents as prehemorrhagic and hemorrhagic stage with severe bleeding and ecchymosis and occasionally multiorgan failure. The case fatality rate of 5 to 30%. Differential diagnosis from other hemorrhagic fevers is essential (Madison-Antenucci et al., 2020)
Tick-borne encephalitis (TBE) is caused by an Orthoflavivirus of the Flaviviridae family. This is a single-stranded RNA virus with a positive sense genome. Discovered in 1937 in the Far east of Russia, the virus is present only in Eurasia where three main virus clades occur: Far Eastern, Siberian, and Western European viruses. It is responsible for encephalitis with varying morbidity and mortality according to the virus type, the Far Eastern virus is the most lethal (30% case fatality) (Pustijanac et al., 2023). The main vector is I. ricinus in Western Europe and I. persulcatus in Asia; D. reticulatus and Haemaphysalis spp. are considered as secondary vectors of TBE virus (Chitimia-Dobler et al., 2019; Michelitsch et al., 2019). The infection rate of I. ricinus ranges from 0.1% to 5% and up to 40% for I. persulcatus in endemic areas of Siberia. Virus can also be transmitted by the consumption of unpasteurized milk or milk products derived primarily from goats exposed to infected tick bites (Pustijanac et al., 2023). Effective vaccines exist against TBE virus (Hills et al., 2023; Kunz, 2003).
Powassan virus is very closely related to TBE virus. It is classified into two genetically and ecologically distinct lineages: lineage I (POWV) mainly transmitted by I. cookei and lineage II (deer tick virus) mainly transmitted by I. scapularis (Shah et al., 2023). It spreads to people by the bite of an infected Ixodes tick. Although still rare, the number of reported cases of people ill from Powassan virus has increased in recent years. Most cases in the United States occur in the northeast and Great Lakes regions from late spring through mid-fall when ticks are most active. Increases in virus detection were recorded in Canada and Far East Asia (Madison-Antenucci et al., 2020). Initial symptoms can include fever, headache, vomiting, and weakness. Powassan virus can cause severe disease, including inflammation of the brain (encephalitis) or the membranes around the brain and spinal cord (meningitis). Symptoms of severe disease include confusion, loss of coordination, difficulty speaking, and seizures. The mortality rate is 15% (Shah et al., 2023).
Severe fever with thrombocytopenia syndrome (SFTS) is caused by a phlebovirus, named Dabie bandavirus, in the order Bunyavirales (Cui et al., 2024), isolated in central China in 2009 (Yu et al., 2011), and identified in Korea in 2012, Japan in 2013, and Vietnam in 2017 (Cui et al., 2024; Takahashi et al., 2014). The genome of the virus is a negative strand RNA. Spread of SFTS virus in East Asia, resulting in increased incidence of infections and the widespread distribution of the tick vector, indicates the expanding range of this tick-borne virus and its potential to become an increasing public health threat (Casel et al., 2021; Li et al., 2021; Liu et al., 2014; Seo et al., 2021; Yang et al., 2022). Haemaphysalis longicornis is the main vector of SFTS virus, with an infection rate of 8% (Casel et al., 2021; Cui et al., 2024). Clinically, SFTS is associated with fever, thrombocytopenia, leukocytopenia, multiorgan dysfunction, and a case fatality of 12-50% (Zhao et al., 2021). Human to human (Chen et al., 2013) and domestic cats (Felis silvestris catus) to human (Tsuru et al., 2021) direct transmissions have been reported. To date, SFTS virus has not been detected in H. longicornis populations introduced into North America. Animals with the highest positivity for the virus (infection rate and antibody detection) were mink (Mustela siberica) (91.11%) and pheasant (Phasianus colchicus) (42.86%). Better notification rate of SFTS, land use (Yasuo and Nishiura, 2019), favorable climate conditions (Iijima et al., 2025), and expansion of transmission with a potential role of migratory birds (Cui et al., 2024) might explain its higher rates of incidence in recent years.
Heartland virus disease was discovered in 2009 in the USA (Missouri) (McMullan et al., 2012). It belongs to the genus Bandavirus, family Phenuiviridae, order Bunyavirales. It is related to SFTS virus (Dembek et al., 2024). Pathogenesis of Heartland virus and SFTS virus infections share many features. Based upon serosurveys, Heartland virus occurs over a broad area of the eastern and midwestern United States. Heartland virus infection is likely under reported and expanding in geographic range (Dembek et al., 2024). Amblyomma americanum is a competent vector (Brault et al., 2018) and white-tailed deer a virus reservoir (Clarke et al., 2018). Significantly, H. longicornis is a competent vector for Heartland virus as well as transmit the virus transovarially to offspring (Raney et al., 2022). The current range of H. longicornis in North America is within a geographic area where Heartland virus transmission is established (Mantlo and Haley, 2023; Namgyal et al., 2020). Habitat range modeling for this species predicts a broad range in eastern North America from southern Canada to the coast of the Gulf of Mexico and an extensive region of the southern and midwestern United States that overlaps with Heartland virus range (Rochlin, 2019). This situation appears to be the confluence of all the factors needed to establish a robust enzootic cycle and spillover of human infections with a readily adapting and establishing invasive tick vector. Tick-borne disease implications of the invasion of H. longicornis into North America are unexpected and reveal the complexity of existing tick-host-pathogen ecologies and how those associations can be changed by introduction of an invasive tick species.
Bourbon virus is an emerging Thogotovirus also transmitted by A. americanum (Godsey et al., 2021; Lambert et al., 2015; Savage et al., 2017). Here as well, there are increasing concerns about the potential role of H. longicornis as a vector of Bourbon virus in North America due to discovery of Bourbon virus in field collected Hae. longicornis in Virginia (Cumbie et al., 2022).
Other viruses have been described as potential pathogens for humans but their occurrence is still rare and their impact on public health still limited. They belong to the families of Flaviviridae (Asian Omsk hemmorrhagic fever virus, Kyasanur Forest disease virus, Alkhurma virus, Louping Ill virus, Alongshan virus, Jingmen virus), Nairoviridae (Beiji Nairovirus, Tacheng Tick virus-1, Yezo virus, Songling virus, and Wetland virus), Phenuiviridae (Bhanja virus), Orthomixoviridae (Thogoto virus, Dhori virus, Oz virus and Bourbon virus), and Reoviridae (New World Colorado tick fever, Old World Eyach virus) (Ergunay et al., 2023; Moming et al., 2024; Wu et al., 2024). A novel member of the orthonairovirus genus of the Nairoviridae, Xue-Cheng virus, was identified from patients in northeastern China and detected in H. concinna and Haemaphysalis japonica ticks from the region (Zhang et al., 2025). Alongshan virus has been identified in different European countries (Ren et al., 2025).
Parasitic infections
Babesia parasites are intraerythrocytic Apicomplexa, closely related to malaria parasites that are of vast veterinary and increasingly human medical importance with over 100 known species (Schnittger et al., 2012). Babesia microti is the most prevalent species in North America with Peromyscus leucopus reservoir and transmission by I. scapularis (Vannier and Krause, 2012). In Europe, Babesia divergens is transmitted by I. ricinus (Vannier and Krause, 2012). More recently, additional species have been described as human pathogens: B. venatorum in Europe and Asia, B. duncani in the USA, and a B. crassa-like species in China (Kumar et al., 2021). Typical symptoms include chills, fever, and fatigue with particular risks to immunocompromised patients. In the USA, awareness of babesiosis has increased in recent decades due to several factors including awareness by health workers and the public, expansion in deer population and I. scapularis tick population (Kumar et al., 2021). The co-infection of B. microti-B. burgdorferi s.s. in rodent reservoir provides a survival advantage to the parasites (Tufts et al., 2023). In Europe, the parasitic disease associated with B. divergens is less endemic with around 60 described cases but with a high mortality of 42% (Hildebrandt et al., 2021). In Asia, China is particularly affected by this parasitic infection with 4 species identified in human cases: B. microti, B. divergens, B. venatorum and a B. crassa-like (Zhao et al., 2021). The parasite is also present in India, Japan, Korea, and Mongolia (Kumar et al., 2021).
One health: an integrated concept for effective control of TBDs
Based on the recognition of the role of wildlife in the emergence of many infectious diseases (Daszak et al., 2000), the “One Health” approach has become important for managing these diseases (Zinsstag et al., 2011), including TBDs (Dantas-Torres et al., 2012). One Health is based on the idea of building a sustainable relationship between human, animals (wildlife, domestic, and companion), and the environment (ecosystems) based on an understanding of disease ecology and pathogen transmission dynamics (Destoumieux-Garzón et al., 2018; Mackenzie et al., 2013). One Health extends the conventional framework in which drugs and vaccines have been the primary tools used to deal with the health of humans and domestic animals, separately. By taking this broader perspective, One Health aims to address infectious disease issues in a more comprehensive manner, including through surveillance, diagnostic tool development, disease and vector control strategies, wildlife and ecosystem management, and maintaining an appropriate ecological distance from wildlife. At the same time, One Health has a strong focus on strengthening linkages among environmental sciences, ecology, biosciences, human medicine, and veterinary medicine (Leung et al., 2012). The One Health framework has not been fully utilized in many cases, including in the control of TBDs, due to a lack of understanding of the dynamics underlying pathogen ecology and the environmental factors driving spillover, which has led to insufficient organization of ideas, direction, and collaboration among disciplines (Cunningham et al., 2017). Relationships among tick species, tick-borne pathogens, humans, vertebrate reservoir host, and tick amplification hosts are changing, creating new balances in relationships of these factors that amplify the need for increased studies of changing vector and disease ecology, development of novel control interventions for disease threat reduction, and information networks to ensure timely delivery of information to public health officials, medical and veterinary clinicians, advocacy groups, and the public – a One Health strategy in action.
To overcome this situation, the involvement of ecology and environmental studies, which have always been concerned with humans, other animals, and ecosystems, will be key to promoting collaboration among various disciplines and stakeholders (Destoumieux-Garzón et al., 2018; Garcia-Vozmediano et al., 2020; Ogden et al., 2019). One of the challenges is the lack of basic knowledge on the characteristics of species involved in transmission cycles, which is necessary for zoonotic disease risk assessment and prediction. In such a situation, pathogeography which analyses the relationship between the occurrence of disease and all potential factors of disease transmission cycle like, wildlife distribution, climate, land use, geography, and human population (Murray et al., 2018) is useful. Although pathogeography cannot clarify the mechanism of pathogen transmission, it suggests the important component of One Health that should be surveyed intensively (Iijima et al., 2025).
Zoonotic pathogen transmission cycles involve wildlife, domestic, and/or companion animal species, and are shaped by biotic and abiotic ecological processes at various spatial scales in addition to the community structure and the home ranges of the host and vector species involved. If the characteristics of each animal species, for example, as a host and/or reservoir, were known in such cases, the framework of community ecology and landscape ecology that takes into account species interactions and spatial processes would improve the accuracy of assessing and predicting infectious disease risk. Such basic findings have been gradually obtained in recent years (Ostfeld et al., 2018; Tatemoto et al., 2022), and future progress in ecological approaches is expected. Furthermore, given the transmission cycles of zoonotic pathogens crosses the boundaries of the natural environment and human managed habitats, it is difficult to implement One Health approach for a single administrative sector, research field, or legal system to take action that requires collaboration across different organizations and disciplines (Johnson et al., 2022; Machtinger et al., 2024). This would be one of the biggest challenges in expanding the options for tick-borne zoonosis control.
In the following parts, we raise the important issues that should be studied to promote the prevention of TBDs based on the One Health approach (i.e., human-animal-ecosystem relationships).
Land use
Because forests harbor more ticks than other habitats (Bourdin et al., 2023), and are sites at higher risk of encountering emerging infectious diseases (Allen et al., 2017), deforestation or forest fragmentation affects the spillover of TBDs (Diuk-Wasser et al., 2020; Kilpatrick et al., 2017). Modification of ecosystems by humans sometimes increases populations of zoonotic pathogen reservoirs, such as rodents (Keesing and Ostfeld, 2021; Mendoza et al., 2020). Forest edges that are created by tree cutting provide a suitable habitat for deer (Miyashita et al., 2008), and can result in their increased abundance (Takarabe and Iijima, 2020). Forest fragmentation promotes tick abundance (Boulanger et al., 2024; Ehrmann et al., 2017; Ferrell and Brinkerhoff, 2018) and infection risk of TBDs (Iijima et al., 2025). The effect of ecosystem modification on the spillover of TBDs, however, is not simple. Mathematical models predicted that host community composition, not a single host abundance, may play a crucial role in explaining the variation in prevalence of tick-borne infections in hosts (O’Neill et al., 2023). The effect of land use on the spread of TBDs needs to be examined from several angles, including a multidisciplinary approach involving epidemiology, entomology, community ecology, and animal behavioral ecology.
Invasive non-native vertebrate hosts
The invasion of non-native species is considered a major cause of biodiversity and ecosystem service loss, but it is also sometimes associated with an increased risk of zoonotic diseases, including TBDs. There are two ways in which invasive alien mammals can be involved (Chalkowski et al., 2018; Strauss et al., 2012): the introduction of new pathogens and ticks from non-native hosts (i.e., spillover process) or through the amplification of pathogens and ticks originally present in the ecosystem by non-native mammals (i.e., spillback process). For example, On Niijima Island, Japan, the introduction of sika deer allowed the expansion of the non-native ticks, Haemaphysalis (Doi et al., 2020). Non-native hosts can also amplify the dynamic of pathogen transmission as shown in Japan with invasive raccoon (Procyon lotor) for SFTS (Doi et al., 2021; Tatemoto et al., 2022), in France with chipmunk (Tamias sibiricus) and Lyme borreliosis (Marsot et al., 2013) or in east of Europe with invasive raccoon (Procyon lotor) and A. phagocytophilum (Hildebrand et al., 2018). In United States, exotic game ranches have become a major business in Texas since the 1950s, resulting in what is now a $1.3 billion industry with over two million exotic animals representing 135 species, predominantly of African and Asian origin (Pitchstone Waters, 2020). The second largest antelope in the world, nilgai (Boselaphus tragocamelus), was introduced into South Texas in 1930 for meat production. Nilgai population in South Texas in 2020 was estimated at 30,000 with thousands of animals roaming freely outside of enclosed ranches and occupying diverse habitats (Pitchstone Waters, 2020). A significant concern is the threat of bovine babesiosis transmitted by R. microplus and R. annulatus and the dispersal of these ticks by increasing numbers of free ranging nilgai (Lohmeyer et al., 2018; Sliwa et al., 2023).
Ecosystem restoration
Currently, ecosystem restoration is a key issue in the conservation of biodiversity as the Kunming-Montreal Global Biodiversity Framework of the Convention on Biological Diversity ensures at least 30% of ecosystems are conserved or at least under restoration by 2030. However, careless conservation or restoration may cause the increase of TBDs risk by increasing host abundance. For example, density of infected ticks increased with the increase of landscape connectivity (Heylen et al., 2019). Same patterns have been observed in Europe and the United States due to the increase in host mammals and ticks in urban green areas and the associated increase in pathogens (Gassner et al., 2016; Rizzoli et al., 2014; VanAcker et al., 2019). Therefore, ecosystem restoration should be undertaken while also considering the impact of TBDs, and in some cases, reducing the abundance of important reservoir hosts like deer in close proximity to human habitat to reduce the risks of spillover, especially around urban areas (Millins et al., 2018).
Modifications of hunting practices and socio-economic changes
Socio-economic changes have been drastic the last century. Deforestation and deer hunting reduce tick populations (Fish, 2022; Godfrey and Randolph, 2011; Matuschka and Spielman, 1986). In the 1930’s for the United States and in the 1940’s for Europe, deer expansion and reforestation occurred (Fish, 2022; Matuschka and Spielman, 1986). In present times, the population of hunters is decreasing in many developed countries due to changing demographics, interests, and urbanization (Manfredo et al., 2020, 2017). Population control of deer and boar by game hunting sometimes fails (Massei et al., 2015; Simard et al., 2013). The decrease of hunting pressure on wildlife and other actions to protect and restore nature cause not only increased deer abundance, but also the expansion of wildlife in urban areas and promote tick favorable habitats (Honda et al., 2018; Sprong et al., 2018). Urban wildlife directly brings ticks into human dominated areas (e.g., parks) that increase tick-bite risk for pets and humans accompanied by TBDs infection risk. Multidisciplinary teams should be built, including biologists, veterinarians, local stakeholders, sociologists, and others, to deal with population management of tick reservoir hosts (Garcia-Vozmediano et al., 2022; Valente et al., 2020).
Medical aspect
It is reasonable to assume that several medical related factors contributed to the high incidence of emerging tick-borne diseases in recent years. Better awareness of the epidemiology, clinical manifestations, and diagnostic tools of TBDs have improved the detection and control of these diseases (Fang et al., 2015; Madison-Antenucci et al., 2020; Sprong et al., 2018). First, advances in sequencing technologies have made it feasible to obtain the sequences of previously unrecognized tick-borne pathogens (Tijsse-Klasen et al., 2014). Of note, the application of metagenomic approaches to clinical specimens has made it possible to detect pathogens without prior knowledge (Piantadosi et al., 2021). Furthermore, it is conceivable that improved therapeutic options and longer lifespan have increased the opportunities for immunosuppressed patients to be exposed to tick bites, resulting in clinical disease (Gynthersen et al., 2023). Finally, increased knowledge and awareness of TBDs among medical personnel could lead to the discovery of new tick-borne pathogens in patients with a history of tick bite (Eisen, 2022; Fang et al., 2015; Sprong et al., 2018).
Prevention of tick bites and transmission of tick-borne pathogens in the context of tick range expansion
As described in this review, significant geographic range expansions are occurring among human biting tick species across the Northern hemisphere. Tick range expansions into new regions have the potential to introduce previously unencountered pathogens into those areas (Paules et al., 2018). From a public health perspective, range expansion creates the challenges of tick and pathogen surveillance to inform physicians, veterinarians, public health workers, and the public about emerging disease threats. Introduction of a tick species into a new region raises questions regarding changes in tick ecologies; balance and interactions among established and newly arriving tick species; and, the need for revised and novel strategies to achieve tick and disease control prevention (Eisen, 2020a; Molaei et al., 2019; Sonenshine, 2018; Wikel, 2022).
Factors influencing geographic range and population size are dynamic and dependent upon the complex interplay among macro and micro climate variations, vegetation patterns, land use, land fragmentation, habitat modifications (agricultural, residential, recreational), host animal diversity (domestic, wildlife, exotic species), human behavior, commerce, economics, government policies, population growth, population movement, and evolutionary changes occurring in ticks and tick-borne pathogens (Alkishe et al., 2021; Baneth, 2014; Dantas-Torres, 2015; Gebreyes et al., 2014; Pfäffle et al., 2013; Sonenshine, 2018). Each of these continually evolving factors, to differing degrees, influence tick and pathogen ecology, enzootic cycles of tick transmitted infectious agents, disease incidence, epidemiology, and selection of appropriate control approaches (Ogden et al., 2021; Sonenshine, 2018; Wikel, 2018).
Changing biotic and abiotic factors influence the tick-host-pathogen triad (Dantas-Torres, 2015; Eisen and Stafford, 2021). Tick biology, ecology, geographic ranges, and population densities are influenced by variations in temperature, humidity, soil moisture, vegetation, leaf litter, shade, and availability of pathogen reservoir and tick population amplification vertebrate species (Alkishe et al., 2021; Sonenshine, 2018; Springer et al., 2020). Changing climate is resulting in a warmer environment, vegetation stress, and greater aridity (Overpeck and Udall, 2020). Increasing temperatures will continue to be a determinant of changing tick population sizes and geographic distributions (Alkishe et al., 2021; Dantas-Torres, 2015). Decreased rainfall and resultant drier environment could increase tick mortality (Ogden and Lindsay, 2016). However, the influence of rain events of increased intensity and flooding on tick ecology are essentially unknown. Warmer temperatures can increase the seasonal duration of tick and human activities that result in increased potential for exposure to ticks and pathogen transmission (Gilbert et al., 2014).
What are the interactions that can and do occur when a tick species expands its range into new territory? The fact that the newly introduced tick survives and eventually thrives indicates that climate and habit factors are favorable in consideration of the fact that the vast majority of the life cycle of nearly all ixodid tick species is spent free living from vertebrate hosts. An excellent review by Phillips and Sundaram examines factors impacting ticks introduced into an existing community of resident tick species and vertebrate hosts and the subsequent impacts on tick community ecology, infection dynamics, and human diseases. These are under studied areas that deserve increased attention (Phillips and Sundaram, 2025).
Ticks expanding into a new geographic area or invasive ticks may contribute to tick-borne disease by bringing infectious agents with them and introducing “new” infectious agents into the region or by becoming a competent vector of currently existing pathogens in the new habitat (Chalkowski et al., 2018; Phillips and Sundaram, 2025). As described previously, an example of the second scenario is the introduction of Ha. longicornis into North America and the subsequent demonstration of the competence of this species as a vector of the North American Heartland and Bourbon viruses.
Consequently, human biting ticks and the disease causing agents they transmit are increasing public health threats due to geographic range expansion, increasing size of tick populations, emergence of newly recognized pathogens, introduction of invasive tick species that are resulting in part from changing weather patterns, land use modifications, biodiversity loss, and human activities/behaviors; all of which result in significant challenges for tick control and disease prevention (Alkishe et al., 2021; Dantas-Torres, 2015; Eisen and Paddock, 2021; Köhler et al., 2023; Tsao et al., 2021; Wikel, 2022). As a result of these evolving interactions and the resulting public health threats there exist critical needs to implement existing and develop novel tools and strategies to prevent tick bites, control tick populations, and reduce transmission of tick-borne pathogens. Achieving these objectives poses significant challenges.
Control of mosquitoes and mosquito-borne diseases is commonly a publicly funded community activity while prevention of tick bites and TBD transmission is primarily the responsibility of individuals for themselves personally and for their property (Burtis et al., 2024). Prevention of tick bites and TBDs is achieved by use of personal protective measures that include application of skin repellents, wearing of protective clothing that is either untreated or treated, performing tick checks, showering to remove unattached ticks, and, when possible, placing removed outdoor clothing in a dryer at high heat to kill unattached ticks (Eisen, 2022). Protection measures may vary between Europe and the United States, particularly with regard to the use of pyrethroids in the environment or for impregnating clothing (Boulanger, 2022). In France, a recent report indicates the risk of these pesticides to human health, particularly for pregnant women (Pesticides et santé-Nouvelles données (2021)). So, permethrin-treated clothing is no more recommended by the French Ministry of Health. Environmental suppression of tick populations is another approach for achieving tick bite and pathogen control; this approach is particularly implemented in United States (Stafford et al., 2017). Methods used include application of acaricides, biopesticides, biological agents that target host seeking ticks, landscape management, rodent reservoir targeted bait boxes that can deliver an oral vaccine, antibiotic, or acaricide, and methods that exclude, eliminate, or deliver acaricides to deer (Burtis et al., 2024; Stafford et al., 2017).
Risk of tick exposure varies across populations by age groups, occupation, recreational activities, and human behaviors (Eisen, 2022; Stafford et al., 2017). The peridomestic environment is an increasingly common place for contact with ticks and for acquiring tick transmitted infections, particularly recognized for Lyme borreliosis (Hansford et al., 2022; Stafford et al., 2017). Personal protective measures are the most commonly used methods for prevention of tick bites, but there are significant variations among individuals in regard to consistency of use and how applied (Eisen, 2022). Area wide tick control measures can be increasingly challenging based upon considerations of effectiveness, cost, required scale of application, lack of organizational implementation structure, and the changing geographic ranges of ticks and the pathogens they transmit (Eisen and Stafford, 2021; Sprong et al., 2018). Effectiveness of these interventions individually and collectively remains a topic of controversy and requires ongoing study to improve outcomes (Keesing et al., 2022). In addition, novel tick control tools are needed that can facilitate advances in integrated tick management, highlighting the multiple challenges of support for basic research, industry investment and commitment for commercialization, navigating a complex regulatory environment, and an uncertain market (Eisen and Stafford, 2021; Stafford et al., 2017).
Fundamental studies of tick biology and ecology are needed for many species of human biting ticks across broad, expanding geographic ranges that result in newly recognized, unstudied, interactions and relationship changes among multiple tick and host species and their associated human pathogens. Until such studies are conducted there will be a lack of data to inform public health officials, health care providers, and the public about tick bite and disease threats and the development of optimal strategies for their control.
Tick-borne diseases are zoonotic diseases with wildlife species being pathogen reservoirs and amplifying hosts that increase tick populations (Pfäffle et al., 2013). These zoonotic diseases involve complex enzootic cycles that include complex interactions at the interface among vertebrate hosts, tick vectors, climate, ecosystems, and humans (Gebreyes et al., 2014). Control and prevention of zoonoses require robust surveillance networks at the wildlife-human interface, vector biologists, physicians, veterinarians, public health experts, laboratorians, laboratory capacity, skilled personnel whose expertise encompasses disciplines of human and veterinary medicine clinical and basic research and related sciences – in essence a One Health approach (Dantas-Torres et al., 2012; Destoumieux-Garzón et al., 2018; Gebreyes et al., 2014). A One Health approach promoting synergies among multiple disciplines, agencies, and governments is central to advancing global health security in a rapidly changing world (Garcia-Vozmediano et al., 2022; Sinclair, 2019).
Author contributions
NB: Validation, Writing – review and editing, Supervision, Writing – original draft. HI: Writing – review and editing, Writing – original draft. KD: Writing – review and editing, Writing – original draft. YW: Writing – original draft, Writing – review and editing. MK: Writing – original draft, Writing – review and editing. RN: Writing – review and editing, Writing – original draft. SW: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1632832/full#supplementary-material
References
Aardema, M. L. (2023). Genomic analyses indicate the North American Ap-ha variant of the tick-vectored bacterium Anaplasma phagocytophilum was introduced from Europe. Parasit. Vectors 16:301. doi: 10.1186/s13071-023-05914-x
Adams, S. N., Bestul, N. C., Calloway, K. N., Kersh, G. J., and Salzer, J. S. (2025). National surveillance of human ehrlichiosis caused by Ehrlichia ewingii, United States, 2013–2021. Emerg. Infect. Dis. 31:279. doi: 10.3201/eid3102.240279
Alkishe, A., Raghavan, R. K., and Peterson, A. T. (2021). Likely geographic distributional shifts among medically important tick species and tick-associated diseases under climate change in north America: A review. Insects 12:225. doi: 10.3390/insects12030225
Allen, T., Murray, K. A., Zambrana-Torrelio, C., Morse, S. S., Rondinini, C., Di Marco, M., et al. (2017). Global hotspots and correlates of emerging zoonotic diseases. Nat. Commun. 8:1124. doi: 10.1038/s41467-017-00923-8
Apanaskevich, M. A., and Apanaskevich, D. A. (2015). Description of new Dermacentor (Acari: Ixodidae) species from malaysia and Vietnam. J. Med. Entomol. 52, 156–162. doi: 10.1093/jme/tjv001
Bacak, E., Ozsemir, A. C., Akyildiz, G., Gungor, U., Bente, D., Keles, A. G., et al. (2024). Bidirectional tick transport by migratory birds of the African-Western Palearctic flyway over Turkish Thrace: Observation of the current situation and future projection. Parasitol. Res. 123:37. doi: 10.1007/s00436-023-08069-x
Bajwa, W. I., Tsynman, L., Egizi, A. M., Tokarz, R., Maestas, L. P., and Fonseca, D. M. (2022). The gulf coast tick, Amblyomma maculatum (Ixodida: Ixodidae), and spotted fever group rickettsia in the highly urbanized Northeastern United States. J. Med. Entomol. 59, 1434–1442. doi: 10.1093/jme/tjac053
Baker, R. E., Mahmud, A. S., Miller, I. F., Rajeev, M., Rasambainarivo, F., Rice, B. L., et al. (2022). Infectious disease in an era of global change. Nat. Rev. Microbiol. 20, 193–205. doi: 10.1038/s41579-021-00639-z
Bakken, J., and Dumler, J. (2015). Human granulocytic anaplasmosis. Infect. Clin. North Am 29, 341–355. doi: 10.1016/j.idc.2015.02.007
Baneth, G. (2014). Tick-borne infections of animals and humans: A common ground. Int. J. Parasitol. 44, 591–596. doi: 10.1016/j.ijpara.2014.03.011
Barbour, A. G., and Fish, D. (1993). The biological and social phenomenon of lyme disease. Science 260, 1610–1616. doi: 10.1126/science.8503006
Beard, C. B., Occi, J., Bonilla, D. L., Egizi, A. M., Fonseca, D. M., Mertins, J. W., et al. (2018). Multistate Infestation with the exotic disease–vector tick Haemaphysalis longicornis — United States, August 2017–September 2018. MMWR Morb. Mortal. Wkly. Rep. 67, 1310–1313. doi: 10.15585/mmwr.mm6747a3
Beard, C. B., Visser, S. N., and Petersen, L. R. (2019). The need for a national strategy to address vector-borne disease threats in the United States. J. Med. Entomol. 56, 1199–1203. doi: 10.1093/jme/tjz074
Bente, D. A., Forrester, N. L., Watts, D. M., McAuley, A. J., Whitehouse, C. A., and Bray, M. (2013). Crimean-Congo hemorrhagic fever: History, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antivir. Res. 100, 159–189. doi: 10.1016/j.antiviral.2013.07.006
Bernard, C., Holzmuller, P., Bah, M. T., Bastien, M., Combes, B., Jori, F., et al. (2022). Systematic Review on crimean–congo hemorrhagic fever enzootic cycle and factors favoring virus transmission: Special focus on france, an apparently free-disease area in Europe. Front. Vet. Sci. 9:932304. doi: 10.3389/fvets.2022.932304
Bernard, C., Joly Kukla, C., Rakotoarivony, I., Duhayon, M., Stachurski, F., Huber, K., et al. (2024). Detection of Crimean–Congo haemorrhagic fever virus in Hyalomma marginatum ticks, southern France, May 2022 and April 2023. Eurosurveillance 29:23. doi: 10.2807/1560-7917.ES.2024.29.6.2400023
Bézay, N., Wagner, L., Kadlecek, V., Obersriebnig, M., Wressnigg, N., Hochreiter, R., et al. (2024). Optimisation of dose level and vaccination schedule for the VLA15 Lyme borreliosis vaccine candidate among healthy adults: Two randomised, observer-blind, placebo-controlled, multicentre, phase 2 studies. Lancet Infect. Dis. 24, 1045–1058. doi: 10.1016/S1473-3099(24)00175-0
Blanton, L. S. (2019). The rickettsioses. Infect. Dis. Clin. North Am. 33, 213–229. doi: 10.1016/j.idc.2018.10.010
Bonnet, S. I., Binetruy, F., Hernández-Jarguín, A. M., and and Duron, O. (2017). The tick microbiome: Why non-pathogenic microorganisms matter in tick biology and pathogen transmission. Front. Cell. Infect. Microbiol. 7:236–236. doi: 10.3389/fcimb.2017.00236
Borchers, A., Keen, C., Huntley, A., and Gershwin, M. (2015). Lyme disease: A rigorous review of diagnostic criteria and treatment. J. Autoimmun. 57, 82–115. doi: 10.1016/j.jaut.2014.09.004
Boulanger, N. (2022). “Prophylactic measures against lyme borreliosis including future perspectives,” in Lyme borreliosis, eds K.-P. Hunfeld and J. Gray (Cham: Springer International Publishing), 161–177. doi: 10.1007/978-3-030-93680-8_7
Boulanger, N., Aran, D., Maul, A., Camara, B. I., Barthel, C., Zaffino, M., et al. (2024). Multiple factors affecting Ixodes ricinus ticks and associated pathogens in European temperate ecosystems (northeastern France). Sci. Rep. 14:9391. doi: 10.1038/s41598-024-59867-x
Boulanger, N., Insonere, J.-L.-M., Van Blerk, S., Barthel, C., Serres, C., and Rais, O., et al. (2023). Cross-alteration of murine skin and tick microbiome concomitant with pathogen transmission after Ixodes ricinus bite. Microbiome 11:250. doi: 10.1186/s40168-023-01696-7
Bourdin, A., Dokhelar, T., Bord, S., Van Halder, I., Stemmelen, A., Scherer-Lorenzen, M., et al. (2023). Forests harbor more ticks than other habitats: A meta-analysis. For. Ecol. Manag. 541:121081. doi: 10.1016/j.foreco.2023.121081
Boyer, P. H., Lenormand, C., Jaulhac, B., and Talagrand-Reboul, E. (2022). Human Co-infections between Borrelia burgdorferi s.l. and other ixodes-borne microorganisms: A systematic review. Pathogens 11:282. doi: 10.3390/pathogens11030282
Brault, A. C., Savage, H. M., Duggal, N. K., Eisen, R. J., and Staples, J. E. (2018). Heartland virus epidemiology, vector association, and disease potential. Viruses 10:498. doi: 10.3390/v10090498
Buczek, A. M., Buczek, W., Buczek, A., and Bartosik, K. (2020). The potential role of migratory birds in the rapid spread of ticks and tick-borne pathogens in the changing climatic and environmental conditions in Europe. Int. J. Environ. Res. Public. Health 17:2117. doi: 10.3390/ijerph17062117
Bugmyrin, S. V., Romanova, L., Belova, O. A., Kholodilov, I. S., Bespyatova, L. A., Chernokhaeva, L. L., et al. (2022). Pathogens in Ixodes persulcatus and Ixodes ricinus ticks (Acari, Ixodidae) in Karelia (Russia). Ticks Tick Borne Dis. 13:102045. doi: 10.1016/j.ttbdis.2022.102045
Burde, J., Bloch, E. M., Kelly, J. R., and Krause, P. J. (2023). Human Borrelia miyamotoi infection in North America. Pathogens 12:553. doi: 10.3390/pathogens12040553
Burtis, J. C., Foster, E., Eisen, R. J., and Eisen, L. (2024). Willingness and capacity of publicly-funded vector control programs in the USA to engage in tick management. Parasit. Vectors 17:316. doi: 10.1186/s13071-024-06400-8
Byun, H.-R., Rieu, M.-S., Han, S.-W., Ji, S.-R., Nam, H.-Y., Seo, S., et al. (2024). Ixodid ticks from wild and domestic animals in East and Central Asian flyways. Acta Trop. 249:107091. doi: 10.1016/j.actatropica.2023.107091
Campbell-Lendrum, D., Manga, L., Bagayoko, M., and Sommerfeld, J. (2015). Climate change and vector-borne diseases: What are the implications for public health research and policy? Philos. Trans. R. Soc. B Biol. Sci. 370:20130552. doi: 10.1098/rstb.2013.0552
Carvalho, C. L., Lopes, De Carvalho, I., Zé-Zé, L., Núncio, M. S., and Duarte, E. L. (2014). Tularaemia: A challenging zoonosis. Comp. Immunol. Microbiol. Infect. Dis. 37, 85–96. doi: 10.1016/j.cimid.2014.01.002
Casel, M. A., Park, S. J., and Choi, Y. K. (2021). Severe fever with thrombocytopenia syndrome virus: Emerging novel phlebovirus and their control strategy. Exp. Mol. Med. 53, 713–722. doi: 10.1038/s12276-021-00610-1
Celina, S. S., and Cerný, J. (2022). Coxiella burnetii in ticks, livestock, pets and wildlife: A mini-review. Front. Vet. Sci. 9:1068129. doi: 10.3389/fvets.2022.1068129
Chalkowski, K., Lepczyk, C. A., and Zohdy, S. (2018). Parasite ecology of invasive species: Conceptual framework and new hypotheses. Trends Parasitol. 34, 655–663. doi: 10.1016/j.pt.2018.05.008
Chen, H., Hu, K., Zou, J., and Xiao, J. (2013). A cluster of cases of human-to-human transmission caused by severe fever with thrombocytopenia syndrome bunyavirus. Int. J. Infect. Dis. 17, e206–e208. doi: 10.1016/j.ijid.2012.11.006
Chen, L., Qiu, Q., Jiang, Y., Wang, K., Lin, Z., Li, Z., et al. (2019). Large-scale ruminant genome sequencing provides insights into their evolution and distinct traits. Science 364:eaav6202. doi: 10.1126/science.aav6202
Chitimia-Dobler, L., Lemhöfer, G., Król, N., Bestehorn, M., Dobler, G., and Pfeffer, M. (2019). Repeated isolation of tick-borne encephalitis virus from adult Dermacentor reticulatus ticks in an endemic area in Germany. Parasit. Vectors 12:90. doi: 10.1186/s13071-019-3346-6
Chochlakis, D., Ioannou, I., Tselentis, Y., and Psaroulaki, A. (2010). Human anaplasmosis and anaplasma ovis variant. Emerg. Infect. Dis. 16, 1031–1032. doi: 10.3201/eid1606.090175
Clarke, L. L., Ruder, M. G., Mead, D. G., and Howerth, E. W. (2018). Heartland virus exposure in white-tailed deer in the Southeastern United States, 2001–2015. Am. J. Trop. Med. Hyg. 99, 1346–1349. doi: 10.4269/ajtmh.18-0555
Cleveland, D. W., Anderson, C. C., and Brissette, C. A. (2023). Borrelia miyamotoi: A comprehensive review. Pathogens 12:267. doi: 10.3390/pathogens12020267
Colwell, D. D., Dantas-Torres, F., and Otranto, D. (2011). Vector-borne parasitic zoonoses: Emerging scenarios and new perspectives. Vet. Parasitol. 182, 14–21. doi: 10.1016/j.vetpar.2011.07.012
Côté, S. D., Rooney, T. P., Tremblay, J.-P., Dussault, C., and Waller, D. M. (2004). Ecological impacts of deer overabundance. Annu. Rev. Ecol. Evol. Syst. 35, 113–147. doi: 10.1146/annurev.ecolsys.35.021103.105725
Cui, H., Shen, S., Chen, L., Fan, Z., Wen, Q., Xing, Y., et al. (2024). Global epidemiology of severe fever with thrombocytopenia syndrome virus in human and animals: A systematic review and meta-analysis. Lancet Reg. Health West Pac. 48:101133. doi: 10.1016/j.lanwpc.2024.101133
Cumbie, A. N., Trimble, R. N., and Eastwood, G. (2022). Pathogen spillover to an invasive tick species: First detection of bourbon virus in Haemaphysalis longicornis in the United States. Pathogens 11:454. doi: 10.3390/pathogens11040454
Cunningham, A. A., Daszak, P., and Wood, J. L. N. (2017). One health, emerging infectious diseases and wildlife: Two decades of progress? Philos. Trans. R. Soc. B Biol. Sci. 372:20160167. doi: 10.1098/rstb.2016.0167
Cunze, S., Glock, G., Kochmann, J., and Klimpel, S. (2022). Ticks on the move—climate change-induced range shifts of three tick species in Europe: Current and future habitat suitability for Ixodes ricinus in comparison with Dermacentor reticulatus and Dermacentor marginatus. Parasitol. Res. 121, 2241–2252. doi: 10.1007/s00436-022-07556-x
Dantas-Torres, F. (2010). Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit. Vectors 3:26. doi: 10.1186/1756-3305-3-26
Dantas-Torres, F. (2015). Climate change, biodiversity, ticks and tick-borne diseases: The butterfly effect. Int. J. Parasitol. Parasites Wildl. 4, 452–461. doi: 10.1016/j.ijppaw.2015.07.001
Dantas-Torres, F., and Otranto, D. (2015). Further thoughts on the taxonomy and vector role of Rhipicephalus sanguineus group ticks. Vet. Parasitol. 208, 9–13. doi: 10.1016/j.vetpar.2014.12.014
Dantas-Torres, F., Chomel, B. B., and Otranto, D. (2012). Ticks and tick-borne diseases: A one health perspective. Trends Parasitol. 28, 437–446. doi: 10.1016/j.pt.2012.07.003
Daszak, P., Cunningham, A. A., and Hyatt, A. D. (2000). Emerging infectious diseases of wildlife– threats to biodiversity and human health. Science 287, 443–449. doi: 10.1126/science.287.5452.443
Dautel, H., Dippel, C., Oehme, R., Hartelt, K., and Schettler, E. (2006). Evidence for an increased geographical distribution of Dermacentor reticulatus in Germany and detection of Rickettsia sp. RpA4. Int. J. Med. Microbiol. 296, 149–156. doi: 10.1016/J.IJMM.2006.01.013
Davis, G. E., Cox, H. R., Parker, R. R., and Dyer, R. E. (1938). A filter-passing infectious agent isolated from ticks. Public Health Rep. 1970:2259. doi: 10.2307/4582746
de La Fuente, J., Kocan, K. M., and Blouin, E. F. (2007). Tick vaccines and the transmission of tick-borne pathogens. Vet. Res. Commun. 31, 85–90. doi: 10.1007/s11259-007-0069-5
de La Fuente, J., Kocan, K., Almazan, C., and Blouin, E. F. (2008). Targeting the tick-pathogen interface for novel control strategies. Front. Biosci. 13:6947–6956. doi: 10.2741/3201
Dembek, Z. F., Mothershead, J. L., Cirimotich, C. M., and Wu, A. (2024). Heartland virus disease—an underreported emerging infection. Microorganisms 12:286. doi: 10.3390/microorganisms12020286
Dennis, D. T., Inglesby, T. V., Henderson, D. A., Bartlett, J. G., Ascher, M. S., Eitzen, E., et al. (2001). Tularemia as a biological weapon: Medical and public health management. JAMA 285:2763. doi: 10.1001/jama.285.21.2763
Department of Public Health (2025). The Connecticut epidemiologist newsletter 2025. Available online at: https://portal.ct.gov/dph/epidemiology-and-emerging-infections/connecticut-epidemiologist-newsletter (accessed April 29, 2025).
Dergousoff, S. J., Galloway, T. D., Lindsay, L. R., Curry, P. S., and Chilton, N. B. (2013). Range expansion of Dermacentor variabilis and Dermacentor andersoni (Acari: Ixodidae) near their Northern Distributional limits. J. Med. Entomol. 50, 510–520. doi: 10.1603/ME12193
Destoumieux-Garzón, D., Mavingui, P., Boetsch, G., Boissier, J., Darriet, F., Duboz, P., et al. (2018). The one health concept: 10 years old and a long road ahead. Front. Vet. Sci. 5:14–14. doi: 10.3389/fvets.2018.00014
Diuk-Wasser, M. A., VanAcker, M. C., and Fernandez, M. P. (2020). Impact of land use changes and habitat fragmentation on the eco-epidemiology of tick-borne diseases. J. Med. Entomol. 58, 1546–1564. doi: 10.1093/jme/tjaa209
Diuk-Wasser, M. A., Vourch, G., Cislo, P., Hoen, A. G., Melton, F., Hamer, S. A., et al. (2010). Field and climate-based model for predicting the density of host-seeking nymphal Ixodes scapularis, an important vector of tick-borne disease agents in the eastern United States. Glob. Ecol. Biogeogr. 19, 504–514. doi: 10.1111/j.1466-8238.2010.00526.x
Doi, K., Kato, T., Kono, M., Yamasaki, F., and Hayama, S. (2024). Differences in tick infestation intensity by season, sex, age class, and body region of feral Raccoons (Procyon lotor) in the Miura Peninsula, Japan. Mammal Study 49:6. doi: 10.3106/ms2023-0006
Doi, K., Kono, M., Kato, T., and Hayama, S. (2021). Ecological traps and boosters of ixodid ticks: The differing ecological roles of two sympatric introduced mammals. Ticks Tick Borne Dis. 12:101687. doi: 10.1016/j.ttbdis.2021.101687
Doi, K., Nishida, K., Kato, T., and Hayama, S. (2020). Effects of introduced sika deer (Cervus nippon) and population control activity on the distribution of Haemaphysalis ticks in an island environment. Int. J. Parasitol. Parasites Wildl. 11, 302–307. doi: 10.1016/j.ijppaw.2020.03.001
Dupuis, A. P., Lange, R. E., and Ciota, A. T. (2023). Emerging tickborne viruses vectored by Amblyomma americanum (Ixodida: Ixodidae): Heartland and Bourbon viruses. J. Med. Entomol. 60, 1183–1196. doi: 10.1093/jme/tjad060
Durden, L., and Beati, L. (2014). “Modern tick systematics,” in Biology of ticks, eds D. Sonenshine and R. Roe (Oxford: Oxford University Press), 17–58.
Duron, O., Morel, O., Noël, V., Buysse, M., Binetruy, F., and Lancelot, R., et al. (2018). Tick-bacteria mutualism depends on B vitamin synthesis pathways. Curr. Biol. 28, 1896–1902.e5. doi: 10.1016/j.cub.2018.04.038
Eimer, J., Fernström, L., Rohlén, L., Grankvist, A., Loo, K., and Nyman, E., et al. (2022). Spiroplasma ixodetis infections in immunocompetent and immunosuppressed patients after tick exposure, Sweden. Emerg. Infect. Dis. 28, 1681–1685. doi: 10.3201/eid2808.212524
Egizi, A., Bulaga-Seraphin, L., Alt, E., Bajwa, W. I, Bernick, J., Bickerton, M., et al. (2020). First glimpse into the origin and spread of the Asian longhorned tick, Haemaphysalis longicornis, in the United States. Zoonoses Public Health 67, 637–650. doi: 10.1111/zph.12743
Ehrmann, S., Liira, J., Gärtner, S., Hansen, K., Brunet, J., Cousins, S. A. O., et al. (2017). Environmental drivers of Ixodes ricinus abundance in forest fragments of rural European landscapes. BMC Ecol. 17:31–31. doi: 10.1186/s12898-017-0141-0
Eickhoff, C., and Blaylock, J. (2017). Tickborne diseases other than Lyme in the United States. Cleve. Clin. J. Med. 84, 555–567. doi: 10.3949/ccjm.84a.16110
Eisen, L. (2018). Pathogen transmission in relation to duration of attachment by Ixodes scapularis ticks. Ticks Tick Borne Dis. 9, 535–542. doi: 10.1016/j.ttbdis.2018.01.002
Eisen, L. (2020a). Stemming the rising tide of human-biting ticks and Tickborne Diseases, United States. Emerg. Infect. Dis. J. 26, 641–641. doi: 10.3201/eid2604.191629
Eisen, L. (2020b). Vector competence studies with hard ticks and Borrelia burgdorferi sensu lato spirochetes: A review. Ticks Tick Borne Dis. 11:1359. doi: 10.1016/j.ttbdis.2019.101359
Eisen, L. (2022). Tick species infesting humans in the United States. Ticks Tick Borne Dis. 13:102025. doi: 10.1016/j.ttbdis.2022.102025
Eisen, L. (2023). Rodent-targeted approaches to reduce acarological risk of human exposure to pathogen-infected Ixodes ticks. Ticks Tick Borne Dis. 14:102119. doi: 10.1016/j.ttbdis.2023.102119
Eisen, L., and Stafford, K. C. (2021). Barriers to effective tick management and tick-bite prevention in the United States (Acari: Ixodidae). J. Med. Entomol. 58, 1588–1600. doi: 10.1093/jme/tjaa079
Eisen, R. J., and Paddock, C. D. (2021). Tick and tickborne pathogen surveillance as a public health tool in the United States. J. Med. Entomol. 58, 1490–1502. doi: 10.1093/jme/tjaa087
Eisen, R. J., Eisen, L., and Beard, C. B. (2016). County-Scale Distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J. Med. Entomol. 53, 349–386. doi: 10.1093/jme/tjv237
Eisen, R. J., Kugeler, K. J., Eisen, L., Beard, C. B., and Paddock, C. D. (2017). Tick-borne zoonoses in the United States: Persistent and emerging threats to human health. ILAR J. 58, 319–335. doi: 10.1093/ilar/ilx005
Eisen, R., and Eisen, L. (2018). The blacklegged tick, Ixodes scapularis: An increasing public health concern. Trends Parasitol. 34, 295–309. doi: 10.1016/j.pt.2017.12.006
Eldin, C., Mélenotte, C., Mediannikov, O., Ghigo, E., Million, M., Edouard, S., et al. (2017). From Q fever to Coxiella burnetii Infection: A paradigm change. Clin. Microbiol. Rev. 30, 115–190. doi: 10.1128/CMR.00045-16
Ellis, J., Oyston, P. C. F., Green, M., and Titball, R. W. (2002). Tularemia. Clin. Microbiol. Rev. 15, 631–646. doi: 10.1128/CMR.15.4.631-646.2002
Ergönül, Ö (2006). Crimean-Congo haemorrhagic fever. Lancet Infect. Dis. 6, 203–214. doi: 10.1016/S1473-3099(06)70435-2
Ergunay, K., Bourke, B. P., Reinbold-Wasson, D. D., Nikolich, M. P., Nelson, S. P., Caicedo-Quiroga, L., et al. (2023). The expanding range of emerging tick-borne viruses in Eastern Europe and the Black Sea region. Sci. Rep. 13:19824. doi: 10.1038/s41598-023-46879-2
España, P. P., Uranga, A., Cillóniz, C., and Torres, A. (2020). Q fever (Coxiella Burnetii). Semin. Respir. Crit. Care Med. 41, 509–521. doi: 10.1055/s-0040-1710594
Estrada-Peña, A. (2015). Ticks as vectors: Taxonomy, biology and ecology: -EN- -FR- Les tiques en tant que vecteurs: Taxonomie, biologie et écologie -ES- Las garrapatas como vectores: Taxonomía, biología y ecología. Rev. Sci. Tech. OIE 34, 53–65. doi: 10.20506/rst.34.1.2345
Estrada-Peña, A., and Jongejan, F. (1999). Ticks feeding on humans: A review of records on human-biting Ixodoidea with special reference to pathogen transmission. Exp. Appl. Acarol. 23, 685–715. doi: 10.1023/A:1006241108739
Estrada-Peña, A., Ayllón, N., and de la Fuente, J. (2012). Impact of climate trends on tick-borne pathogen transmission. Front. Physiol. 27:64. doi: 10.1016/j.pt.2017.12.006
Estrada-Peña, A., D’Amico, G., and Fernández-Ruiz, N. (2021). Modelling the potential spread of Hyalomma marginatum ticks in Europe by migratory birds. Int. J. Parasitol. 51, 1–11. doi: 10.1016/j.ijpara.2020.08.004
Estrada-Pena, A., Mihalca, A. D., and Petney, T. (2017). Ticks of Europe and North Africa: A guide to species identification, COST European cooperation in science and technology. Berlin: Springer.
Estrada-Peña, A., Ruiz-Fons, F., Acevedo, P., Gortazar, C., and de la Fuente, J. (2013). Factors driving the circulation and possible expansion of Crimean-Congo haemorrhagic fever virus in the western Palearctic. J. Appl. Microbiol. 114, 278–286. doi: 10.1111/jam.12039
Fabri, N. D., Sprong, H., Hofmeester, T. R., Heesterbeek, H., Donnars, B. F., Widemo, F., et al. (2021). Wild ungulate species differ in their contribution to the transmission of Ixodes ricinus-borne pathogens. Parasit. Vectors 14, 360–360. doi: 10.1186/s13071-021-04860-w
Fang, L., Liu, K., Li, X., Liang, S., Yang, Y., Yao, H., et al. (2015). Emerging tick-borne infections in mainland China: An increasing public health threat. Lancet Infect. Dis 15, 1467–1479. doi: 10.1016/S1473-3099(15)00177-2
Fereidouni, M., Apanaskevich, D. A., Pecor, D. B., Pshenichnaya, N., Abuova, G. N., Tishkova, F. H., et al. (2023). Crimean-Congo hemorrhagic fever virus in Central, Eastern, and South-eastern Asia. Virol. Sin. 38, 171–183. doi: 10.1016/j.virs.2023.01.001
Ferrell, A., and Brinkerhoff, R. (2018). Using landscape analysis to test hypotheses about drivers of tick abundance and infection prevalence with Borrelia burgdorferi. Int. J. Environ. Res. Public. Health 15:737. doi: 10.3390/ijerph15040737
Ferroglio, E., Vada, R., Occhibove, F., Fracchia, M., Cicco, F. D., Palencia, P., et al. (2024). An integrated approach to an emerging problem: Implementing a whole year of camera trap survey in evaluating the impact of wildlife on tick abundance. Transbound. Emerg. Dis. 2024:4064855. doi: 10.1155/2024/4064855
Fish, D. (2022). “Range expansion of Ixodes scapularis in the United States,” in Climate, ticks and disease, ed. P. Nuttall (Wallingford: CAB international).
Földvári, G., Jahfari, S., Rigó, K., Jablonszky, M., Szekeres, S., Majoros, G., et al. (2014). Candidatus Neoehrlichia mikurensis and Anaplasma phagocytophilum in Urban Hedgehogs. Emerg. Infect. Dis. 20:935. doi: 10.3201/eid2003.130935
Földvári, G., Široký, P., Szekeres, S., Majoros, G., and Sprong, H. (2016). Dermacentor reticulatus: A vector on the rise. Parasit. Vectors 9:314. doi: 10.1186/s13071-016-1599-x
Furuno, K., Lee, K., Itoh, Y., Suzuki, K., Yonemitsu, K., Kuwata, R., et al. (2017). Epidemiological study of relapsing fever borreliae detected in Haemaphysalis ticks and wild animals in the western part of Japan. PLoS One 12:e0174727. doi: 10.1371/journal.pone.0174727
Garcia-Vozmediano, A., De Meneghi, D., Sprong, H., Portillo, A., Oteo, J. A., and Tomassone, L. (2022). A one health evaluation of the surveillance systems on tick-borne diseases in the Netherlands, Spain and Italy. Vet. Sci. 9:504. doi: 10.3390/vetsci9090504
Garcia-Vozmediano, A., Giglio, G., Ramassa, E., Nobili, F., Rossi, L., and Tomassone, L. (2020). Dermacentor marginatus and Dermacentor reticulatus, and their infection by SFG Rickettsiae and Francisella-like endosymbionts, in mountain and Periurban habitats of Northwestern Italy. Vet. Sci. 7:157. doi: 10.3390/vetsci7040157
Gasmi, S., Bouchard, C., Ogden, N. H., Adam-Poupart, A., Pelcat, Y., Rees, E. E., et al. (2018). Evidence for increasing densities and geographic ranges of tick species of public health significance other than Ixodes scapularis in Québec, Canada. PLoS One 13:e0201924. doi: 10.1371/journal.pone.0201924
Gassner, F., Hansford, K., and Medlock, J. (2016). “Greener cities, a wild card for ticks?,” in Ecology and prevention of lyme borreliosis, eds M. Braks, W. Takken, and H. Spong (Wageningen: Academic Publishers), 187–203.
Gebreyes, W. A., Dupouy-Camet, J., Newport, M. J., Oliveira, C. J. B., Schlesinger, L. S., Saif, Y. M., et al. (2014). The global one health paradigm: Challenges and opportunities for tackling infectious diseases at the human, animal, and environment interface in low-resource settings. PLoS Negl. Trop. Dis. 8:e3257. doi: 10.1371/journal.pntd.0003257
Gern, L., and Humair, P. (2002). “Ecology of Borrelia burgdorferi sensu lato in Europe,” in Lyme borreliosis: Biology, epidemiology and control, eds J. Gray, R. S. Lane, and G. Stanek (Wallingford: CABI publishing), 347–347. doi: 10.1016/j.tpb.2019.10.004
Ghadge, S. K., Schneider, M., Dubischar, K., Wagner, L., Kadlecek, V., Obersriebnig, M., et al. (2024). Immunogenicity and safety of an 18-month booster dose of the VLA15 Lyme borreliosis vaccine candidate after primary immunisation in healthy adults in the USA: Results of the booster phase of a randomised, controlled, phase 2 trial. Lancet Infect. Dis. 24, 1275–1286. doi: 10.1016/S1473-3099(24)00372-4
Gilbert, L., Aungier, J., and Tomkins, J. L. (2014). Climate of origin affects tick (Ixodes ricinus) host-seeking behavior in response to temperature: Implications for resilience to climate change? Ecol. Evol. 4, 1186–1198. doi: 10.1002/ece3.1014
Godfrey, E. R., and Randolph, S. E. (2011). Economic downturn results in tick-borne disease upsurge. Parasit. Vectors 4:35. doi: 10.1186/1756-3305-4-35
Godsey, M. S., Rose, D., Burkhalter, K. L., Breuner, N., Bosco-Lauth, A. M., Kosoy, O. I., et al. (2021). Experimental infection of Amblyomma americanum (Acari: Ixodidae) with bourbon virus (Orthomyxoviridae: Thogotovirus). J. Med. Entomol. 58, 873–879. doi: 10.1093/jme/tjaa191
Goren, A., Viljugrein, H., Rivrud, I. M., Jore, S., Bakka, H., Vindenes, Y., et al. (2023). The emergence and shift in seasonality of Lyme borreliosis in Northern Europe. Proc. R. Soc. B Biol. Sci. 290:20222420. doi: 10.1098/rspb.2022.2420
Gray, J., Kahl, O., and Zintl, A. (2024). Pathogens transmitted by Ixodes ricinus. Ticks Tick Borne Dis. 15:102402. doi: 10.1016/j.ttbdis.2024.102402
Greenspoon, L., Krieger, E., Sender, R., Rosenberg, Y., Bar-On, Y. M., Moran, U., et al. (2023). The global biomass of wild mammals. Proc. Natl. Acad. Sci. U.S.A. 120:e2204892120. doi: 10.1073/pnas.2204892120
Guglielmone, A., and Robbins, R. (2018). Hard ticks (Acari: Ixodida: Ixodidae) parasitizing humans: A global overview. Berlin: Springer.
Guglielmone, A., Richad, R., Apanaskevich, D., Petney, T., Estrada-Pena, A., Horak, I. G., et al. (2010). The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: A list of valid species names. Zootaxa 2528, 1–28. doi: 10.11646/zootaxa.2528.1.1
Gygax, L., Schudel, S., Kositz, C., Kuenzli, E., and Neumayr, A. (2024). Human monocytotropic ehrlichiosis—A systematic review and analysis of the literature. PLoS Negl. Trop. Dis. 18:e0012377. doi: 10.1371/journal.pntd.0012377
Gynthersen, R. M. M., Stensvold, C. R., Nielsen, S. L., Møller, H. J., Nielsen, H. V., Lebech, A., et al. (2023). Neoehrlichia mikurensis —An emerging opportunistic tick-borne infection in immunosuppressed patients. J. Intern. Med. 293, 782–790. doi: 10.1111/joim.13638
Hansford, K. M., Wheeler, B. W., Tschirren, B., and Medlock, J. M. (2022). Questing Ixodes ricinus ticks and Borrelia spp. in urban green space across Europe: A review. Zoonoses Public Health 69, 153–166. doi: 10.1111/zph.12913
Hashizume, H., Fujiyama, T., Umayahara, T., Kageyama, R., Walls, A. F., and Satoh, T. (2018). Repeated Amblyomma testudinarium tick bites are associated with increased galactose-α-1,3-galactose carbohydrate IgE antibody levels: A retrospective cohort study in a single institution. J. Am. Acad. Dermatol. 78:1135–1141.e3. doi: 10.1016/j.jaad.2017.12.028
Hawman, D. W., and Feldmann, H. (2023). Crimean–Congo haemorrhagic fever virus. Nat. Rev. Microbiol. 21, 463–477. doi: 10.1038/s41579-023-00871-9
Helminiak, L., Mishra, S., and Kim, H. K. (2022). Pathogenicity and virulence of Rickettsia. Virulence 13, 1752–1771. doi: 10.1080/21505594.2022.2132047
Heylen, D., Lasters, R., Adriaensen, F., Fonville, M., Sprong, H., and Matthysen, E. (2019). Ticks and tick-borne diseases in the city: Role of landscape connectivity and green space characteristics in a metropolitan area. Sci. Total Environ. 670, 941–949. doi: 10.1016/J.SCITOTENV.2019.03.235
Hildebrand, J., Buñkowska-Gawlik, K., Adamczyk, M., Gajda, E., Merta, D., Popiołek, M., et al. (2018). The occurrence of Anaplasmataceae in European populations of invasive carnivores. Ticks Tick Borne Dis. 9, 934–937. doi: 10.1016/j.ttbdis.2018.03.018
Hildebrandt, A., Zintl, A., Montero, E., Hunfeld, K.-P., and Gray, J. (2021). Human Babesiosis in Europe. Pathogens 10:1165. doi: 10.3390/pathogens10091165
Hills, S. L., Poehling, K. A., Chen, W. H., and Staples, J. E. (2023). Tick-borne encephalitis vaccine: Recommendations of the advisory committee on immunization practices, United States, 2023. MMWR Recomm. Rep. 72, 1–29. doi: 10.15585/mmwr.rr7205a1
Hofmeester, T. R., Sprong, H., Jansen, P. A., Prins, H. H. T., and Van Wieren, S. E. (2017). Deer presence rather than abundance determines the population density of the sheep tick, Ixodes ricinus, in Dutch forests. Parasit. Vectors 10:433. doi: 10.1186/s13071-017-2370-7
Honda, T., Iijima, H., Tsuboi, J., and Uchida, K. (2018). A review of urban wildlife management from the animal personality perspective: The case of urban deer. Sci. Total Environ. 644, 576–582. doi: 10.1016/j.scitotenv.2018.06.335
Hou, X., Xu, J., Hao, Q., Xu, G., Geng, Z., and Zhang, L. (2014). Prevalence of Borrelia burgdorferi sensu lato in rodents from Jiangxi, southeastern China region. Int. J. Clin. Exp. Med. 7, 5563–5567.
Hrazdilová, K., Lesiczka, P. M., Bardoň, J., Vyroubalová, Š,Šimek, B., Zurek, L., et al. (2021). Wild boar as a potential reservoir of zoonotic tick-borne pathogens. Ticks Tick Borne Dis. 12:1558. doi: 10.1016/j.ttbdis.2020.101558
Humair, P.-F., and Gern, L. (2000). The wild hidden face of Lyme borreliosis in Europe. Microbes Infect. 2, 915–922. doi: 10.1016/S1286-4579(00)00393-2
Hvidsten, D., Frafjord, K., Gray, J. S., Henningsson, A. J., Jenkins, A., Kristiansen, B. E., et al. (2020). The distribution limit of the common tick, Ixodes ricinus, and some associated pathogens in north-western Europe. Ticks Tick Borne Dis. 11, 101388. doi: 10.1016/j.ttbdis.2020.101388
Iijima, H., Nagata, J., Izuno, A., Uchiyama, K., Akashi, N., Fujiki, D., et al. (2023). Current sika deer effective population size is near to reaching its historically highest level in the Japanese archipelago by release from hunting rather than climate change and top predator extinction. Holocene 33, 718–727. doi: 10.1177/09596836231157063
Iijima, H., Watari, Y., Doi, K., Yasuo, K., and Okabe, K. (2025). Forest fragmentation and warmer climate increase tick-borne disease infection. EcoHealth 22, 124–137. doi: 10.1007/s10393-025-01702-4
Iijima, H., Watari, Y., Furukawa, T., and Okabe, K. (2022). Importance of host abundance and microhabitat in tick abundance. J. Med. Entomol. 59, 2110–2119. doi: 10.1093/jme/tjac140
Imaoka, K., Kaneko, S., Tabara, K., Kusatake, K., and Morita, E. (2011). The first human case of Rickettsia tamurae infection in Japan. Case Rep. Dermatol. 3, 68–73. doi: 10.1159/000326941
Ismail, N., and McBride, J. W. (2017). Tick-borne emerging infections. Clin. Lab. Med. 37, 317–340. doi: 10.1016/j.cll.2017.01.006
Jaenson, T. G. T., Petersson, E. H., Jaenson, D. G. E., Kindberg, J., Pettersson, J. H.-O., Hjertqvist, M., et al. (2018). The importance of wildlife in the ecology and epidemiology of the TBE virus in Sweden: Incidence of human TBE correlates with abundance of deer and hares. Parasit. Vectors 11:477. doi: 10.1186/s13071-018-3057-4
Jaenson, T. G., Hjertqvist, M., Bergström, T., and Lundkvist, Å (2012a). Why is tick-borne encephalitis increasing? A review of the key factors causing the increasing incidence of human TBE in Sweden. Parasit. Vectors 5:184. doi: 10.1186/1756-3305-5-184
Jaenson, T. G., Jaenson, D. G. E., Eisen, L., Petersson, E., and Lindgren, E. (2012b). Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasit. Vectors 5:8. doi: 10.1186/1756-3305-5-8
Jahfari, S., Coipan, E. C., Fonville, M., Van Leeuwen, A. D., Hengeveld, P., Heylen, D., et al. (2014). Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasit. Vectors 7:365. doi: 10.1186/1756-3305-7-365
Jakab, Á,Kahlig, P., Kuenzli, E., and Neumayr, A. (2022). Tick borne relapsing fever - a systematic review and analysis of the literature. PLoS Negl. Trop. Dis. 16:e0010212. doi: 10.1371/journal.pntd.0010212
Johnson, N., Phipps, L. P., Hansford, K. M., Folly, A. J., Fooks, A. R., Medlock, J. M., et al. (2022). One health approach to tick and tick-borne disease surveillance in the United Kingdom. Int. J. Environ. Res. Public. Health 19:5833. doi: 10.3390/ijerph19105833
Jongejan, F., and Uilenberg, G. (2004). The global importance of ticks. Parasitology 129, S3–S14. doi: 10.1017/s0031182004005967
Kahl, O., and Gray, J. S. (2023). The biology of Ixodes ricinus with emphasis on its ecology. Ticks Tick Borne Dis. 14:102114. doi: 10.1016/j.ttbdis.2022.102114
Kahl, O., Gern, L., Eisen, L., and Lane, R. (2002). “Ecology research on Borrelia burgdorferi sensu lato: Ecology and some methodological pitfalls,” in Lyme borreliosis: Biology, epidemiology, and control, eds O. K. Gray, R. S. Lane, and G. Stanek (Wallingford: CABI publishing), 335–335.
Kar, S., Rodriguez, S. E., Akyildiz, G., Cajimat, M. N. B., Bircan, R., Mears, M. C., et al. (2020). Crimean-Congo hemorrhagic fever virus in tortoises and Hyalomma aegyptium ticks in East Thrace, Turkey: Potential of a cryptic transmission cycle. Parasit. Vectors 13:201. doi: 10.1186/s13071-020-04074-6
Karshima, S., Ahmed, M., Mohammed, K., and Pam, V. (2023). Global status of Anaplasma phagocytophilum infections in human population: A 50-year (1970–2020) meta-analysis. J. Vector Borne Dis. 60:265. doi: 10.4103/0972-9062.364756
Kawahara, M., Rikihisa, Y., Isogai, E., Takahashi, M., Misumi, H., Suto, C., et al. (2004). Ultrastructure and phylogenetic analysis of “Candidatus Neoehrlichia mikurensis” in the family Anaplasmataceae, isolated from wild rats and found in Ixodes ovatus ticks. Int. J. Syst. Evol. Microbiol. 54, 1837–1843. doi: 10.1099/ijs.0.63260-0
Keesing, F., and Ostfeld, R. S. (2021). Impacts of biodiversity and biodiversity loss on zoonotic diseases. Proc. Natl. Acad. Sci. U.S.A. 118:e2023540118. doi: 10.1073/pnas.2023540118
Keesing, F., and Ostfeld, R. S. (2024). Emerging patterns in rodent-borne zoonotic diseases. Science 385, 1305–1310. doi: 10.1126/science.adq7993
Keesing, F., Holt, R. D., and Ostfeld, R. S. (2006). Effects of species diversity on disease risk. Ecol. Lett. 9, 485–498. doi: 10.1111/j.1461-0248.2006.00885.x
Keesing, F., McHenry, D. J., Hersh, M., Tibbetts, M., Brunner, J. L., Killilea, M., et al. (2014). Prevalence of human-active and variant 1 strains of the tick-borne pathogen Anaplasma phagocytophilum in hosts and forests of Eastern North America. Am. Soc. Trop. Med. Hyg. 91, 302–309. doi: 10.4269/ajtmh.13-0525
Keesing, F., Mowry, S., Bremer, W., Duerr, S., Evans, A. S., Fischhoff, I. R., et al. (2022). Effects of tick-control interventions on tick abundance, human encounters with ticks, and incidence of tickborne diseases in residential neighborhoods, New York, USA. Emerg. Infect. Dis. 28, 957–966. doi: 10.3201/eid2805.211146
Keim, P., Johansson, A., and Wagner, D. M. (2007). Molecular Epidemiology, evolution, and ecology of Francisella. Ann. N. Y. Acad. Sci. U.S.A. 1105, 30–66. doi: 10.1196/annals.1409.011
Keirans, J. E., and Durden, L. A. (2014). “Tick systematics and identification,” in Tick-borne diseases of humans, eds J. L. Goodman, D. T. Dennis, and D. E. Sonenshine (Washington, DC: ASM Press), 123–140. doi: 10.1128/9781555816490.ch7
Keskin, A., and Doi, K. (2025). Discovery of the potentially invasive Asian longhorned tick, Haemaphysalis longicornis Neumann (Acari: Ixodidae) in Türkiye: An unexpected finding through citizen science. Exp. Appl. Acarol. 94:47. doi: 10.1007/s10493-025-01015-9
Kilpatrick, A. M., Dobson, A. D. M., Levi, T., Salkeld, D. J., Swei, A., Ginsberg, H. S., et al. (2017). Lyme disease ecology in a changing world: Consensus, uncertainty and critical gaps for improving control. Philos. Trans. R. Soc. B Biol. Sci. 372:20160117. doi: 10.1098/rstb.2016.0117
Kilpatrick, H., LaBonte, A., and Stafford, K. (2014). The relationship between deer density, tick abundance, and human cases of Lyme disease in a residential community. J. Med. Entomol. 51, 777–784. doi: 10.1603/me13232
Kim, H. K. (2022). Rickettsia -host-tick interactions: Knowledge advances and gaps. Infect. Immun. 90, e621–e621. doi: 10.1128/iai.00621-21
Klink, J. C., Rieger, A., Wohlsein, P., Siebert, U., and Obiegala, A. (2024). Vector-borne and zoonotic pathogens in Raccoon Dogs (Nyctereutes procyonoides) and Raccoons (Procyon lotor) from Schleswig-Holstein, Germany. Pathogens 13:270. doi: 10.3390/pathogens13030270
Köhler, C. F., Holding, M. L., Sprong, H., Jansen, P. A., and Esser, H. J. (2023). Biodiversity in the Lyme-light: Ecological restoration and tick-borne diseases in Europe. Trends Parasitol. 39, 373–385. doi: 10.1016/j.pt.2023.02.005
Krause, P. J., Fish, D., Narasimhan, S., and Barbour, A. G. (2015). Borrelia miyamotoi infection in nature and in humans. Clin. Microbiol. Infect. 21, 631–639. doi: 10.1016/j.cmi.2015.02.006
Kugeler, K. J., Schwartz, A. M., Delorey, M. J., Mead, P. S., and Hinckley, A. F. (2021). Estimating the frequency of lyme disease diagnoses, United States, 2010–2018. Emerg. Infect. Dis. 27, 616–619. doi: 10.3201/eid2702.202731
Kumar, A., O’Bryan, J., and Krause, P. (2021). The global emergence of human babesiosis. Pathogens 10:1447. doi: 10.3390/pathogens10111447
Kunz, C. (2003). TBE vaccination and the Austrian experience. Vaccine 21, S50–S55. doi: 10.1016/S0264-410X(02)00813-7
Kwak, M. L., Chavatte, J.-M., Chew, K. L., and Lee, B. P. Y.-H. (2021). Emergence of the zoonotic tick Dermacentor (Indocentor) auratus Supino, 1897 (Acari: Ixodidae) in Singapore. Ticks Tick Borne Dis. 12:101574. doi: 10.1016/j.ttbdis.2020.101574
Kwak, M. L., Kuo, C.-C., and Chu, H.-T. (2020). First record of the sea snake tick Amblyomma nitidum Hirst and Hirst, 1910 (Acari: Ixodidae) from Taiwan. Ticks Tick Borne Dis. 11:101383. doi: 10.1016/j.ttbdis.2020.101383
Kwak, M. L., Taya, Y., Suzuki, M., Matsuno, K., and Nakao, R. (2024b). Saving the Ryukyu rabbit tick Haemaphysalis pentalagi. Oryx 58, 556–557. doi: 10.1017/S003060532400098X
Kwak, M. L., Lee, L., Tan, D. J. X., Rheindt, F. E., and Nakao, R. (2024a). Nation-wide surveillance of ticks (Acari: Ixodidae) on birds in Singapore. Acta Trop. 260:107411. doi: 10.1016/j.actatropica.2024.107411
Lambert, A. J., Velez, J. O., Brault, A. C., Calvert, A. E., Bell-Sakyi, L., Bosco-Lauth, A. M., et al. (2015). Molecular, serological and in vitro culture-based characterization of Bourbon virus, a newly described human pathogen of the genus Thogotovirus. J. Clin. Virol. 73, 127–132. doi: 10.1016/j.jcv.2015.10.021
Lange, R. E., Dupuis, Ii, A. P., Prusinski, M. A., Maffei, J. G., Koetzner, C. A., et al. (2023). Identification and characterization of novel lineage 1 Powassan virus strains in New York State. Emerg. Microbes Infect. 12:2155585. doi: 10.1080/22221751.2022.2155585
Leal, B., Zamora, E., Fuentes, A., Thomas, D. B., and Dearth, R. K. (2020). Questing by tick larvae (Acari: Ixodidae): A review of the influences that affect off-host survival. Ann. Entomol. Soc. Am. 113, 425–438. doi: 10.1093/aesa/saaa013
Lenormand, C., Jaulhac, B., and Lipsker, D. (2016). Manifestations cutanées de la borréliose de Lyme. EMC Dermatol. 18, 1–12. doi: 10.1016/S0246-0319(16)63597-5
Leung, Z., Middleton, D., and Morrison, K. (2012). One Health and EcoHealth in Ontario: A qualitative study exploring how holistic and integrative approaches are shaping public health practice in Ontario. BMC Public Health 12:358. doi: 10.1186/1471-2458-12-358
Li, H., Zheng, Y.-C., Ma, L., Jia, N., Jiang, B.-G., Jiang, R.-R., et al. (2015). Human infection with a novel tick-borne Anaplasma species in China: A surveillance study. Lancet Infect. Dis. 15, 663–670. doi: 10.1016/S1473-3099(15)70051-4
Li, J., Li, S., Yang, L., Cao, P., and Lu, J. (2021). Severe fever with thrombocytopenia syndrome virus: A highly lethal bunyavirus. Crit. Rev. Microbiol. 47, 112–125. doi: 10.1080/1040841X.2020.1847037
Lindgren, E., Tälleklint, L., and Polfeldt, T. (2000). Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environ. Health Perspect. 108, 119–123. doi: 10.1289/ehp.00108119
Liu, Q., He, B., Huang, S.-Y., Wei, F., and Zhu, X.-Q. (2014). Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect. Dis. 14, 763–772. doi: 10.1016/S1473-3099(14)70718-2
LoGiudice, K., Ostfeld, R. S., Schmidt, K., and Keesing, F. (2003). The ecology of infectious disease: Effects of host diversity and community composition on Lyme disease risk. Proc. Natl. Acad. Sci. U.S.A. 100, 567–571. doi: 10.1073/pnas.0233733100
Lohmeyer, K. H., May, M. A., Thomas, D. B., and Pérez De León, A. A. (2018). Implication of Nilgai Antelope (Artiodactyla: Bovidae) in Reinfestations of Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) in South Texas: A review and update. J. Med. Entomol. 55, 515–522. doi: 10.1093/jme/tjy004
Londoño, A. F., Scorpio, D. G., and Dumler, J. S. (2023). Innate immunity in rickettsial infections. Front. Cell. Infect. Microbiol. 13:1187267. doi: 10.3389/fcimb.2023.1187267
Machtinger, E. T., Poh, K. C., Pesapane, R., and Tufts, D. M. (2024). An integrative framework for tick management: The need to connect wildlife science, One Health, and interdisciplinary perspectives. Curr. Opin. Insect Sci. 61:101131. doi: 10.1016/j.cois.2023.101131
Mackenzie, J. S., Jeggo, M., Daszak, P., and Richt, J. A. (eds) (2013). One health: The human-animal-environment interfaces in emerging infectious diseases: The concept and examples of a one health approach, current topics in microbiology and immunology. Berlin: Springer Berlin Heidelberg, doi: 10.1007/978-3-642-36889-9
Madison-Antenucci, S., Kramer, L. D., Gebhardt, L. L., and Kauffman, E. (2020). Emerging tick-borne diseases. Clin. Microbiol. Rev. 33:e0083–18. doi: 10.1128/CMR.00083-18
Maggi, R. G., Toliver, M., Richardson, T., Mather, T., and Breitschwerdt, E. B. (2019). Regional prevalences of Borrelia burgdorferi, Borrelia bissettiae, and Bartonella henselae in Ixodes affinis, Ixodes pacificus and Ixodes scapularis in the USA. Ticks Tick Borne Dis. 10, 360–364. doi: 10.1016/j.ttbdis.2018.11.015
Manfredo, M. J., Teel, T. L., Don Carlos, A. W., Sullivan, L., Bright, A. D., Dietsch, A. M., et al. (2020). The changing sociocultural context of wildlife conservation. Conserv. Biol. 34, 1549–1559. doi: 10.1111/cobi.13493
Manfredo, M. J., Teel, T. L., Sullivan, L., and Dietsch, A. M. (2017). Values, trust, and cultural backlash in conservation governance: The case of wildlife management in the United States. Biol. Conserv. 214, 303–311. doi: 10.1016/j.biocon.2017.07.032
Mantlo, E. K., and Haley, N. J. (2023). Heartland virus: An evolving story of an emerging zoonotic and vector-borne disease. Zoonotic Dis. 3, 188–202. doi: 10.3390/zoonoticdis3030016
Marchant, A., Le Coupanec, A., Joly, C., Perthame, E., Sertour, N., Garnier, M., et al. (2017). Infection of Ixodes ricinus by Borrelia burgdorferi sensu lato in peri-urban forests of France. PLoS One 12:e0183543. doi: 10.1371/journal.pone.0183543
Margos, G., Wilske, B., Sing, A., Hizo-Teufel, C., Cao, W.-C., Chu, C., et al. (2013). Borrelia bavariensis sp. nov. is widely distributed in Europe and Asia. Int. J. Syst. Evol. Microbiol. 63, 4284–4288. doi: 10.1099/ijs.0.052001-0
Marsot, M., Chapuis, JL., Gasqui, P., Dozières, A., and Masséglia, S. (2013). Introduced Siberian chipmunks (Tamias sibiricus barberi) contribute more to lyme borreliosis risk than native reservoir rodents. PLoS One 2013:e55377. doi: 10.1371/journal.pone.0055377
Massei, G., Kindberg, J., Licoppe, A., Gačić, D., Šprem, N., Kamler, J., et al. (2015). Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Manag. Sci. 71, 492–500. doi: 10.1002/ps.3965
Matet, A., Le Flèche-Matéos, A., Doz, F., Dureau, P., and Cassoux, N. (2020). Ocular Spiroplasma ixodetis in Newborns, France. Emerg. Infect. Dis. 26, 340–344. doi: 10.3201/eid2602.191097
Matuschka, F. R., and Spielman, A. (1986). The emergence of Lyme disease in a changing environment in North America and Central Europe. Exp. Appl. Acarol. 2, 337–353. doi: 10.1007/BF01193900
McMullan, L. K., Folk, S. M., Kelly, A. J., MacNeil, A., Goldsmith, C. S., Metcalfe, M. G., et al. (2012). A New Phlebovirus Associated with Severe Febrile Illness in Missouri. N. Engl. J. Med. 367, 834–841. doi: 10.1056/NEJMoa1203378
McQuiston, J. H., Childs, J. E., and Thompson, H. A. (2002). Q fever. J. Am. Vet. Med. Assoc. 221, 796–799. doi: 10.2460/javma.2002.221.796
Medlock, J. M., Hansford, K. M., Bormane, A., Derdakova, M., Estrada-Peña, A., George, J.-C., et al. (2013). Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit. Vectors 6:1. doi: 10.1186/1756-3305-6-1
Mendoza, H., Rubio, A. V., García-Peña, G. E., Suzán, G., and Simonetti, J. A. (2020). Does land-use change increase the abundance of zoonotic reservoirs? Rodents say yes. Eur. J. Wildl. Res. 66:6. doi: 10.1007/s10344-019-1344-9
Merhej, V., Angelakis, E., Socolovschi, C., and Raoult, D. (2014). Genotyping, evolution and epidemiological findings of Rickettsia species. Infect. Genet. Evol. 25, 122–137. doi: 10.1016/j.meegid.2014.03.014
Michelitsch, A., Wernike, K., Klaus, C., Dobler, G., and Beer, M. (2019). Exploring the reservoir hosts of tick-borne encephalitis virus. Viruses 11:669. doi: 10.3390/v11070669
Millins, C., Dickinson, E. R., Isakovic, P., Gilbert, L., Wojciechowska, A., Paterson, V., et al. (2018). Landscape structure affects the prevalence and distribution of a tick-borne zoonotic pathogen. Parasit. Vectors 11:621. doi: 10.1186/s13071-018-3200-2
Miyashita, T., Suzuki, M., Ando, D., Fujita, G., Ochiai, K., and Asada, M. (2008). Forest edge creates small-scale variation in reproductive rate of sika deer. Popul. Ecol. 50, 111–120. doi: 10.1007/s10144-007-0068-y
Molaei, G., Little, E. A. H., Williams, S. C., and Stafford, K. C. (2019). Bracing for the worst — range expansion of the lone star tick in the Northeastern United States. N. Engl. J. Med. 381, 2189–2192. doi: 10.1056/NEJMp1911661
Moming, A., Bai, Y., Wang, J., Zhang, Y., Tang, S., Fan, Z., et al. (2024). The known and unknown of global tick-borne viruses. Viruses 16:1807. doi: 10.3390/v16121807
Mori, E., Pisanu, B., Zozzoli, R., Solano, E., Olivieri, E., Sassera, D., et al. (2018). Arthropods and associated pathogens from native and introduced rodents in Northeastern Italy. Parasitol. Res. 117, 3237–3243. doi: 10.1007/s00436-018-6022-4
Murray, K. A., Olivero, J., Roche, B., Tiedt, S., and Guégan, J. (2018). Pathogeography: Leveraging the biogeography of human infectious diseases for global health management. Ecography 41, 1411–1427. doi: 10.1111/ecog.03625
Nakao, R., Shinjo, K., Sakiyama, T., Ogata, S., Kusakisako, K., Kinoshita, G., et al. (2021). Amblyomma testudinarium infestation on a brown bear (Ursus arctos yesoensis) captured in Hokkaido, a northern island of Japan. Parasitol. Int. 80:102209. doi: 10.1016/j.parint.2020.102209
Namgyal, J., Couloigner, I., Lysyk, T. J., Dergousoff, S. J., and Cork, S. C. (2020). Comparison of habitat suitability models for Haemaphysalis longicornis neumann in North America to determine its potential geographic range. Int. J. Environ. Res. Public. Health 17:8285. doi: 10.3390/ijerph17218285
Narasimhan, S., Swei, A., Abouneameh, S., Pal, U., Pedra, J. H. F., and Fikrig, E. (2021). Grappling with the tick microbiome. Trends Parasitol. 37, 722–733. doi: 10.1016/J.PT.2021.04.004
Nepveu-Traversy, M.-E., Fausther-Bovendo, H., and Babuadze, G. (2024). Human tick-borne diseases and advances in anti-tick vaccine approaches: A comprehensive review. Vaccines 12:141. doi: 10.3390/vaccines12020141
Nigrovic, L. E., and Wingerter, S. L. (2008). Tularemia. Infect. Dis. Clin. North Am. 22, 489–504. doi: 10.1016/j.idc.2008.03.004
Nuttall, P. A., and Labuda, M. (2003). Dynamics of infection in tick vectors and at the tick–host interface, in: Advances in virus research. Amsterdam: Elsevier, 233–272. doi: 10.1016/S0065-3527(03)60007-2
O’Neill, X., White, A., Gortázar, C., and Ruiz-Fons, F. (2023). The impact of host abundance on the epidemiology of tick-borne infection. Bull. Math. Biol. 85:30. doi: 10.1007/s11538-023-01133-8
Ogden, N. H., and Lindsay, L. R. (2016). Effects of climate and climate change on vectors and vector-borne diseases: Ticks are different. Trends Parasitol. 32, 646–656. doi: 10.1016/j.pt.2016.04.015
Ogden, N. H., and Tsao, J. I. (2009). Biodiversity and Lyme disease: Dilution or amplification? Epidemics 1, 196–206. doi: 10.1016/j.epidem.2009.06.002
Ogden, N. H., Ben Beard, C., Ginsberg, H. S., and Tsao, J. I. (2021). Possible effects of climate change on Ixodid ticks and the pathogens they transmit: Predictions and observations. J. Med. Entomol. 58, 1536–1545. doi: 10.1093/JME/TJAA220
Ogden, N. H., Wilson, J. R. U., Richardson, D. M., Hui, C., Davies, S. J., Kumschick, S., et al. (2019). Emerging infectious diseases and biological invasions: A call for a One Health collaboration in science and management. R. Soc. Open Sci. 6:181577. doi: 10.1098/rsos.181577
Osikowicz, L. M., Maes, S. E., Eisen, R. J., and Hojgaard, A. (2024). A next generation sequencing assay combining Ixodes species identification with pathogen detection to support tick surveillance efforts in the United States. Ticks Tick Borne Dis. 15:102343. doi: 10.1016/j.ttbdis.2024.102343
Ostfeld, R. S., and Keesing, F. (2000). Biodiversity series: The function of biodiversity in the ecology of vector-borne zoonotic diseases. Can. J. Zool. 78, 2061–2078. doi: 10.1139/z00-172
Ostfeld, R. S., Levi, T., Keesing, F., Oggenfuss, K., and Canham, C. D. (2018). Tick-borne disease risk in a forest food web. Ecology 99, 1562–1573. doi: 10.1002/ecy.2386
Oteo, J., and Portillo, A. (2012). Tick-borne rickettsioses in Europe. Ticks Tick Borne Dis. 3, 271–278. doi: 10.1016/j.ttbdis.2012.10.035
Overpeck, J. T., and Udall, B. (2020). Climate change and the aridification of North America. Proc. Natl. Acad. Sci.U.S.A. 117, 11856–11858. doi: 10.1073/pnas.2006323117
Oyen, K., and Poh, K. C. (2025). Rhipicephalus microplus (Southern cattle tick; Asian blue tick). Trends Parasitol. 41, 68–69. doi: 10.1016/j.pt.2024.11.004
Oyston, P. C. F. (2008). Francisella tularensis: Unravelling the secrets of an intracellular pathogen. J. Med. Microbiol. 57, 921–930. doi: 10.1099/jmm.0.2008/000653-0
Oyston, P. C. F., and Davies, C. (2011). Q fever: The neglected biothreat agent. J. Med. Microbiol. 60, 9–21. doi: 10.1099/jmm.0.024778-0
Oyston, P. C. F., Sjöstedt, A., and Titball, R. W. (2004). Tularaemia: Bioterrorism defence renews interest in Francisella tularensis. Nat. Rev. Microbiol. 2, 967–978. doi: 10.1038/nrmicro1045
Paddock, C. D., and Childs, J. E. (2003). Ehrlichia chaffeensis: A prototypical emerging pathogen. Clin. Microbiol. Rev. 16, 37–64. doi: 10.1128/CMR.16.1.37-64.2003
Paddock, C. D., and Goddard, J. (2015). The evolving medical and veterinary importance of the gulf coast tick (Acari: Ixodidae). J. Med. Entomol. 52, 230–252. doi: 10.1093/jme/tju022
Pal, U., de Silva, A., Montgomery, R., Fish, D., Anguita, J., Anderson, J., et al. (2000). Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J. Clin. Invest. 106, 561–569. doi: 10.1172/JCI9427
Paules, C. I., Marston, H. D., Bloom, M. E., and Fauci, A. S. (2018). Tickborne diseases — confronting a growing threat. N. Engl. J. Med. 379, 701–703. doi: 10.1056/nejmp1807870
Perez, G., Bournez, L., Boulanger, N., Fite, J., Livoreil, B., McCoy, K. D., et al. (2023). The distribution, phenology, host range and pathogen prevalence of Ixodes ricinus in France: A systematic map and narrative review. Peer Commun. J. 3:e81. doi: 10.24072/pcjournal.291
Perkins, S. E., Cattadori, I. M., Tagliapietra, V., Rizzoli, A. P., and Hudson, P. J. (2006). Localized deer absence leads to tick amplification. Ecology 87, 1981–1986. doi: 10.1890/0012-9658200687[1981:LDALTT]2.0.CO;2
Pesticides et santé-Nouvelles données (2021). Inserm, La science pour la santé. Available online at: https://www.inserm.fr/expertise-collective/pesticides-et-sante-nouvelles-donnees-2021/ (accessed February 18, 2025).
Pfäffle, M., Littwin, N., Muders, S. V., and Petney, T. N. (2013). The ecology of tick-borne diseases. Int. J. Parasitol. 43, 1059–1077. doi: 10.1016/j.ijpara.2013.06.009
Phillips, P., and Sundaram, M. (2025). From invasion to outbreak: Tick introductions and disease. Trends Parasitol. 41, 24–27. doi: 10.1016/j.pt.2024.11.005
Piantadosi, A., Mukerji, S. S., Ye, S., Leone, M. J., Freimark, L. M., Park, D., et al. (2021). Enhanced virus detection and metagenomic sequencing in patients with meningitis and encephalitis. mBio 12:e01143–21. doi: 10.1128/mBio.01143-21
Piesman, J., and Eisen, L. (2008). Prevention of tick-borne diseases. Annu. Rev. Entomol. 2008, 323–343. doi: 10.1146/annurev.ento.53.103106.093429
Pitchstone Waters (2020). Escaped exotics animals are changing the Texas landscape 2020. San Antonio, TX: Pitchstone Waters.
Pitó, A., Fedorov, D., Brlík, V., Kontschán, J., Keve, G., Sándor, A. D., et al. (2024). East-to-west dispersal of bird-associated ixodid ticks in the northern Palaearctic: Review of already reported tick species according to longitudinal migratory avian hosts and first evidence on the genetic connectedness of Ixodes apronophorus between Siberia and Europe. Curr. Res. Parasitol. Vector Borne Dis. 6:100201. doi: 10.1016/j.crpvbd.2024.100201
Portillo, A., Palomar, A. M., Santibáñez, P., and Oteo, J. A. (2021). Epidemiological aspects of crimean-Congo hemorrhagic fever in Western Europe: What about the future? Microorganisms 9:649. doi: 10.3390/microorganisms9030649
Portillo, A., Santibáñez, P., Palomar, A. M., Santibáñez, S., and Oteo, J. A. (2018). ‘ Candidatus Neoehrlichia mikurensis’ in Europe. New Microbes New Infect. 22, 30–36. doi: 10.1016/j.nmni.2017.12.011
Pustijanac, E., Buršić, M., Talapko, J., Škrlec, I., Meštrović, T., and Lišnjić, D. (2023). Tick-borne encephalitis virus: A comprehensive review of transmission, pathogenesis, epidemiology, clinical manifestations, diagnosis, and prevention. Microorganisms 11:1634. doi: 10.3390/microorganisms11071634
Rainey, T., Occi, J. L., Robbins, R. G., and Egizi, A. (2018). Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) parasitizing a sheep in New Jersey, United States. J. Med. Entomol. 55, 757–759. doi: 10.1093/jme/tjy006
Randolph, S. E. (2010). To what extent has climate change contributed to the recent epidemiology of tick-borne diseases? Vet. Parasitol. 167, 92–94. doi: 10.1016/j.vetpar.2009.09.011
Randolph, S. E., Green, R. M., Peacey, M. F., and Rogers, D. J. (2000). Seasonal synchrony: The key to tick-borne encephalitis foci identified by satellite data. Parasitology 121, 15–23. doi: 10.1017/S0031182099006083
Randolph, S., and Dobson, A. (2012). Pangloss revisited: A critique of the dilution effect and the biodiversity-buffers-disease paradigm. Parasitology 139, 847–863. doi: 10.1017/S0031182012000200
Raney, W. R., Perry, J. B., and Hermance, M. E. (2022). Transovarial transmission of heartland virus by invasive asian longhorned ticks under laboratory conditions. Emerg. Infect. Dis. 28, 726–729. doi: 10.3201/eid2803.210973
Rantanen, M., Karpechko, A. Y., and Lipponen, A. (2022). The Arctic has warmed nearly four times faster than the globe since 1979. Commun. Earth Environ. 3:168. doi: 10.1038/s43247-022-00498-3
Ren, M., Pang, Z., Tu, Y., Wang, A., Xu, T., Yu, X., et al. (2025). Alongshan virus: An emerging arboviral challenge in regional health security. Virulence 16:2492360. doi: 10.1080/21505594.2025.2492360
Richards, S., Langley, R., Apperson, C., and Watson, E. (2017). Do tick attachment times vary between different tick-pathogen systems? Environments 4:37. doi: 10.3390/environments4020037
Rikihisa, Y. (2015). Molecular pathogenesis of Ehrlichia chaffeensis infection. Annu. Rev. Microbiol. 69, 283–304. doi: 10.1146/annurev-micro-091014-104411
Rizzoli, A., Silaghi, C., Obiegala, A., Rudolf, I., Hubálek, Z., Földvári, G., et al. (2014). Ixodes ricinus and its transmitted pathogens in urban and Peri-urban areas in Europe: New hazards and relevance for public health. Front. Public Health 2:251–251. doi: 10.3389/fpubh.2014.00251
Robinson, E. L., Jardine, C. M., Koffi, J. K., Russell, C., Lindsay, L. R., Dibernardo, A., et al. (2022). Range Expansion of Ixodes scapularis and Borrelia burgdorferi in Ontario, Canada, from 2017 to 2019. Vector Borne Zoonotic Dis. 22, 361–369. doi: 10.1089/vbz.2022.0015
Rochlin, I. (2019). Modeling the Asian Longhorned tick (Acari: Ixodidae) suitable habitat in North America. J. Med. Entomol. 56, 384–391. doi: 10.1093/jme/tjy210
Rubel, F., Brugger, K., Walter, M., Vogelgesang, J. R., Didyk, Y. M., Fu, S., et al. (2018). Geographical distribution, climate adaptation and vector competence of the Eurasian hard tick Haemaphysalis concinna. Ticks Tick Borne Dis. 9, 1080–1089. doi: 10.1016/J.TTBDIS.2018.04.002
Rupasinghe, R., Chomel, B. B., and Martínez-López, B. (2022). Climate change and zoonoses: A review of the current status, knowledge gaps, and future trends. Acta Trop. 226:06225. doi: 10.1016/j.actatropica.2021.106225
Sagurova, I., Ludwig, A., Ogden, N. H., Pelcat, Y., Dueymes, G., and Gachon, P. (2019). Predicted northward expansion of the geographic range of the tick vector Amblyomma americanum in North America under future climate conditions. Environ. Health Perspect. 127:107014. doi: 10.1289/EHP5668
Sahni, A., Fang, R., Sahni, S. K., and Walker, D. H. (2019). Pathogenesis of rickettsial diseases: Pathogenic and immune mechanisms of an endotheliotropic infection. Annu. Rev. Pathol. Mech. Dis. 14, 127–152. doi: 10.1146/annurev-pathmechdis-012418-012800
Sam, G., Stenos, J., Graves, S. R., and Rehm, B. H. A. (2023). Q fever immunology: The quest for a safe and effective vaccine. NPJ Vaccines 8:133. doi: 10.1038/s41541-023-00727-6
Savage, H. M., Burkhalter, K. L., Godsey, M. S., Panella, N. A., Ashley, D. C., Nicholson, W. L., et al. (2017). Bourbon virus in field-collected ticks, Missouri, USA. Emerg. Infect. Dis. 23, 2017–2022. doi: 10.3201/eid2312.170532
Schmidt, K. A., and Ostfeld, R. S. (2001). Biodiversity and the dilution effect in disease ecology. Ecology 82, 609–619. doi: 10.1890/0012-9658(2001)082[0609:BATDEI]2.0.CO;2
Schnittger, L., Rodriguez, A. E., Florin-Christensen, M., and Morrison, D. A. (2012). Babesia: A world emerging. Infect. Genet. Evol. 12, 1788–1809. doi: 10.1016/j.meegid.2012.07.004
Schudel, S., Gygax, L., Kositz, C., Kuenzli, E., and Neumayr, A. (2024). Human granulocytotropic anaplasmosis—A systematic review and analysis of the literature. PLoS Negl. Trop. Dis. 18:e0012313. doi: 10.1371/journal.pntd.0012313
Scott, J. D., and Pesapane, R. R. (2021). Detection of Anaplasma phagocytophilum, Babesia odocoilei, Babesia sp., Borrelia burgdorferi Sensu Lato, and Hepatozoon canis in Ixodes scapularis ticks collected in Eastern Canada. Pathogens 10:1265. doi: 10.3390/pathogens10101265
Semenza, J. C., Rocklöv, J., and Ebi, K. L. (2022). Climate change and cascading risks from infectious disease. Infect. Dis. Ther. 11, 1371–1390. doi: 10.1007/s40121-022-00647-3
Seo, J.-W., Kim, D., Yun, N., and Kim, D.-M. (2021). Clinical update of severe fever with thrombocytopenia syndrome. Viruses 13:1213. doi: 10.3390/v13071213
Sgroi, G., Iatta, R., Lia, R. P., D’Alessio, N., Manoj, R. R. S., Veneziano, V., et al. (2021). Spotted fever group rickettsiae in Dermacentor marginatus from wild boars in Italy. Transbound. Emerg. Dis. 68, 2111–2120. doi: 10.1111/tbed.13859
Shah, T., Li, Q., Wang, B., Baloch, Z., and Xia, X. (2023). Geographical distribution and pathogenesis of ticks and tick-borne viral diseases. Front. Microbiol. 14:1185829. doi: 10.3389/fmicb.2023.1185829
Shahhosseini, N., Wong, G., Babuadze, G., Camp, J. V., Ergonul, O., Kobinger, G. P., et al. (2021). Crimean-Congo hemorrhagic fever virus in Asia, Africa and Europe. Microorganisms 9:1907. doi: 10.3390/microorganisms9091907
Sharma, R., Patil, R. D., Singh, B., Chakraborty, S., Chandran, D., Dhama, K., et al. (2023). Tularemia – a re-emerging disease with growing concern. Vet. Q. 43, 1–16. doi: 10.1080/01652176.2023.2277753
Shimada, M., Doi, K., Yamauchi, T., Kawabata, H., Ando, S., Abe, T., et al. (2022). Preliminary report on the relationship between recent tick bite cases caused by Amblyomma testudinarium and ticks collected from wild boar and deer in Ashikaga City, Tochigi Prefecture, Japan. J. Acarol. Soc. Jpn. 31, 75–83. doi: 10.2300/acari.31.75
Shimada, M., Doi, K., Yamauchi, T., Kawabata, H., Ando, S., Abe, T., et al. (2024). Seasonality of tick species collected from wild boars and deer and by flagging in 2021 in Ashikaga City, Tochigi Prefecture, Japan: Reasons for tick-bite cases predominantly caused by Amblyomma testudinarium Koch in the region. J. Acarol. Soc. Jpn. 33, 85–97. doi: 10.2300/acari.33.85
Silaghi, C., Beck, R., Oteo, J. A., Pfeffer, M., and Sprong, H. (2016). Neoehrlichiosis: An emerging tick-borne zoonosis caused by Candidatus Neoehrlichia mikurensis. Exp. Appl. Acarol. 68, 279–297. doi: 10.1007/s10493-015-9935-y
Silva-Pinto, A., Santos, M. D. L., and Sarmento, A. (2014). Tick-borne lymphadenopathy, an emerging disease. Ticks Tick Borne Dis. 5, 656–659. doi: 10.1016/j.ttbdis.2014.04.016
Simard, M. A., Dussault, C., Huot, J., and Côté, S. D. (2013). Is hunting an effective tool to control overabundant deer? A test using an experimental approach. J. Wildl. Manag. 77, 254–269. doi: 10.1002/jwmg.477
Sinclair, J. R. (2019). Importance of a One Health approach in advancing global health security and the sustainable development goals: -EN- -FR- importance de l’approche Une seule santé pour améliorer la sécurité sanitaire mondiale et atteindre les objectifs de développement durable -ES- Importancia de la noción de Una sola salud para promover la seguridad sanitaria mundial y los Objetivos de Desarrollo Sostenible. Rev. Sci. Tech. OIE 38, 145–154. doi: 10.20506/rst.38.1.2949
Skowron, K., Grudlewska-Buda, K., and Khamesipour, F. (2023). Zoonoses and emerging pathogens. BMC Microbiol. 23:232. doi: 10.1186/s12866-023-02984-w
Slatculescu, A. M., Clow, K. M., McKay, R., Talbot, B., Logan, J. J., Thickstun, C. R., et al. (2020). Species distribution models for the eastern blacklegged tick, Ixodes scapularis, and the Lyme disease pathogen, Borrelia burgdorferi, in Ontario, Canada. PLoS One 15:e0238126. doi: 10.1371/journal.pone.0238126
Sliwa, K. M., Baumgardt, J. A., DeYoung, R. W., Ortega, S., Hewitt, D. G., Goolsby, J. A., et al. (2023). Movement ecology of exotic nilgai antelope: A threat to the re-emergence of cattle fever ticks in the southern USA. Ecosphere 14:e4401. doi: 10.1002/ecs2.4401
Sonenshine, D. (2018). Range expansion of tick disease vectors in North America: Implications for spread of tick-borne disease. Int. J. Environ. Res. Public. Health 15:478. doi: 10.3390/ijerph15030478
Springer, A., Glass, A., Topp, A.-K., and Strube, C. (2020). Zoonotic tick-borne pathogens in temperate and cold regions of Europe—a review on the prevalence in domestic animals. Front. Vet. Sci. 7:604910. doi: 10.3389/fvets.2020.604910
Springer, Y. P., Jarnevich, C. S., Barnett, D. T., Monaghan, A. J., and Eisen, R. J. (2015). Modeling the present and future geographic distribution of the lone star tick, Amblyomma americanum (Ixodida: Ixodidae), in the continental United States. Am. Soc. Trop. Med. Hyg. 93, 875–890. doi: 10.4269/ajtmh.15-0330
Sprong, H., Azagi, T., Hoornstra, D., Nijhof, A., Knorr, S., Baarsma, M., et al. (2018). Control of lyme borreliosis and other Ixodes ricinus-borne diseases. Parasit. Vectors 11, 145–145. doi: 10.1186/s13071-018-2744-5
Stafford, K. C., Williams, S. C., and Molaei, G. (2017). Integrated pest management in controlling ticks and tick-associated diseases. J. Integr. Pest Manag. 8:28. doi: 10.1093/jipm/pmx018
Stanek, G., Wormser, G. P., Gray, J., and Strle, F. (2012). Lyme borreliosis. Lancet 379, 461–473. doi: 10.1016/S0140-6736(11)60103-7
Stark, J. H., Li, X., Zhang, J. C., Burn, L., Valluri, S. R., Liang, J., et al. (2022). Systematic review and meta-analysis of lyme disease data and seropositivity for Borrelia burgdorferi, China, 2005-2020. Emerg. Infect. Dis. 28, 2389–2397. doi: 10.3201/eid2812.212612
Steinbrink, A., Brugger, K., Margos, G., Kraiczy, P., and Klimpel, S. (2022). The evolving story of Borrelia burgdorferi sensu lato transmission in Europe. Parasitol. Res. 121, 781–803. doi: 10.1007/s00436-022-07445-3
Strauss, A., White, A., and Boots, M. (2012). Invading with biological weapons: The importance of disease-mediated invasions. Funct. Ecol. 26, 1249–1261. doi: 10.1111/1365-2435.12011
Strnad, M., Hönig, V., Růžek, D., Grubhoffer, L., and Rego, R. O. M. (2017). Europe-Wide Meta-Analysis of Borrelia burgdorferi Sensu Lato Prevalence in Questing Ixodes ricinus Ticks. Appl. Environ. Microbiol. 83, doi: 10.1128/AEM.00609-17
Süss, J. (2003). Epidemiology and ecology of TBE relevant to the production of effective vaccines. Vaccine 21, S19–S35. doi: 10.1016/S0264-410X(02)00812-5
Takahashi, T., Maeda, K., Suzuki, T., Ishido, A., Shigeoka, T., Tominaga, T., et al. (2014). the first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. J. Infect. Dis. 209, 816–827. doi: 10.1093/infdis/jit603
Takarabe, K., and Iijima, H. (2020). Abundant artificial grasslands around forests increase the deer impact on forest vegetation. Eur. J. For. Res. 139, 473–482. doi: 10.1007/s10342-020-01262-y
Talagrand-Reboul, E., Boyer, P. H., Bergström, S., Vial, L., and Boulanger, N. (2018). Relapsing fevers: Neglected tick-borne diseases. Front. Cell. Infect. Microbiol. 8:98. doi: 10.3389/fcimb.2018.00098
Tatemoto, K., Ishijima, K., Kuroda, Y., Mendoza, M. V., Inoue, Y., Park, E., et al. (2022). Roles of raccoons in the transmission cycle of severe fever with thrombocytopenia syndrome virus. J. Vet. Med. Sci. 84, 982–991. doi: 10.1292/jvms.22-0236
Taylor, K. R., Takano, A., Konnai, S., Shimozuru, M., Kawabata, H., and Tsubota, T. (2013). Differential tick burdens may explain differential Borrelia afzelii and Borrelia garinii infection rates among four, wild, rodent species in Hokkaido, Japan. J. Vet. Med. Sci. 75, 785–790. doi: 10.1292/jvms.12-0439
Teel, P. D., Ketchum, H. R., Mock, D. E., Wright, R. E., and Strey, O. F. (2010). The gulf coast tick: A review of the life history, ecology, distribution, and emergence as an arthropod of medical and veterinary importance. J. Med. Entomol. 47, 707–722. doi: 10.1093/jmedent/47.5.707
Teo, E. J. M., Apanaskevich, D. A., Barker, S. C., and Nakao, R. (2024). Dermacentor (Indocentor) auratus Supino 1897: Potential geographic range, and medical and veterinary significance. Acta Trop. 254:107197. doi: 10.1016/j.actatropica.2024.107197
Thomson, M. C., and Stanberry, L. R. (2022). Climate change and vectorborne diseases. N. Engl. J. Med. 387, 1969–1978. doi: 10.1056/NEJMra2200092
Thu, M. J., Qiu, Y., Matsuno, K., Kajihara, M., Mori-Kajihara, A., Omori, R., et al. (2019). Diversity of spotted fever group rickettsiae and their association with host ticks in Japan. Sci. Rep. 9:1500. doi: 10.1038/s41598-018-37836-5
Tijsse-Klasen, E., Fonville, M., Reimerink, J. H., Spitzen, A., and Sprong, H. (2010). Role of sand lizards in the ecology of Lyme and other tick-borne diseases in the Netherlands. Parasit. Vectors 3:42. doi: 10.1186/1756-3305-3-42
Tijsse-Klasen, E., Koopmans, M. P. G., and Sprong, H. (2014). Tick-borne pathogen - reversed and conventional discovery of disease. Front. Public Health 2:73. doi: 10.3389/fpubh.2014.00073
Tsao, J. I., Hamer, S. A., Han, S., Sidge, J. L., and Hickling, G. J. (2021). The contribution of wildlife hosts to the rise of ticks and tick-borne diseases in North America. J. Med. Entomol. 58, 1565–1587. doi: 10.1093/jme/tjab047
Tsuru, M., Suzuki, T., Murakami, T., Matsui, K., Maeda, Y., Yoshikawa, T., et al. (2021). Pathological characteristics of a patient with severe fever with thrombocytopenia syndrome (SFTS) infected with SFTS virus through a Sick Cat’s Bite. Viruses 13:204. doi: 10.3390/v13020204
Tufts, D. M., Adams, B., and Diuk-Wasser, M. A. (2023). Ecological interactions driving population dynamics of two tick-borne pathogens, Borrelia burgdorferi and Babesia microti. Proc. R. Soc. B Biol. Sci. 290:20230642. doi: 10.1098/rspb.2023.0642
Valente, A. M., Acevedo, P., Figueiredo, A. M., Fonseca, C., and Torres, R. T. (2020). Overabundant wild ungulate populations in Europe: Management with consideration of socio-ecological consequences. Mammal Rev. 50, 353–366. doi: 10.1111/mam.12202
van Oosterwijk, J. G., and Wikel, S. K. (2021). Resistance to ticks and the path to anti-tick and transmission blocking vaccines. Vaccines 9, 725–725. doi: 10.3390/vaccines9070725
VanAcker, M. C., Little, E. A. H., Molaei, G., Bajwa, W. I., and Diuk-Wasser, M. A. (2019). Enhancement of risk for lyme disease by landscape connectivity, New York, New York, USA. Emerg. Infect. Dis. 25, 1136–1143. doi: 10.3201/eid2506.181741
Vannier, E., and Krause, P. J. (2012). Human babesiosis. N. Engl. J. Med. 366, 2397–2407. doi: 10.1056/NEJMra1202018
Wang, J., Gao, L., and Aksoy, S. (2023). Microbiota in disease-transmitting vectors. Nat. Rev. Microbiol. 21, 604–618. doi: 10.1038/s41579-023-00901-6
Welinder-Olsson, C., Kjellin, E., Vaht, K., Jacobsson, S., and Wennerås, C. (2010). First case of human “Candidatus Neoehrlichia mikurensis” infection in a febrile patient with chronic lymphocytic leukemia. J. Clin. Microbiol. 48, 1956–1959. doi: 10.1128/JCM.02423-09
Wikel, S. (2018). Ticks and tick-borne infections: Complex ecology, agents, and host interactions. Vet. Sci. 5, 60. doi: 10.3390/vetsci5020060
Wikel, S. (2022). Changing geographic ranges of human biting ticks and implications for tick-borne zoonoses in North America. Zoonotic Dis. 2, 126–146. doi: 10.3390/zoonoticdis2030013
Wilhelmsson, P., Jaenson, T. G. T., Olsen, B., Waldenström, J., and Lindgren, P.-E. (2020). Migratory birds as disseminators of ticks and the tick-borne pathogens Borrelia bacteria and tick-borne encephalitis (TBE) virus: A seasonal study at Ottenby Bird Observatory in South-eastern Sweden. Parasit. Vectors 13:607. doi: 10.1186/s13071-020-04493-5
Wolcott, K. A., Margos, G., Fingerle, V., and Becker, N. S. (2021). Host association of Borrelia burgdorferi sensu lato: A review. Ticks Tick Borne Dis. 12, 101766. doi: 10.1016/j.ttbdis.2021.101766
Wormser, G. P. (2022). A brief history of OspA vaccines including their impact on diagnostic testing for Lyme disease. Diagn. Microbiol. Infect. Dis. 102:115572. doi: 10.1016/j.diagmicrobio.2021.115572
Wu, Y., Zhou, Q., Mao, M., Chen, H., and Qi, R. (2024). Diversity of species and geographic distribution of tick-borne viruses in China. Front. Microbiol. 15:1309698. doi: 10.3389/fmicb.2024.1309698
Xu, G., Foster, E., Ribbe, F., Hojgaard, A., Eisen, R. J., Paull, S., et al. (2023). Detection of Ehrlichia muris eauclairensis in Blacklegged Ticks (Ixodes scapularis) and white-footed mice (Peromyscus leucopus) in Massachusetts. Vector Borne Zoonotic Dis. 23, 311–315. doi: 10.1089/vbz.2022.0098
Yamaji, K., Aonuma, H., and Kanuka, H. (2018). Distribution of tick-borne diseases in Japan: Past patterns and implications for the future. J. Infect. Chemother. 24, 499–504. doi: 10.1016/j.jiac.2018.03.012
Yang, T., Huang, H., Jiang, L., and Li, J. (2022). Overview of the immunological mechanism underlying severe fever with thrombocytopenia syndrome (Review). Int. J. Mol. Med. 50:118. doi: 10.3892/ijmm.2022.5174
Yasuo, K., and Nishiura, H. (2019). Spatial epidemiological determinants of severe fever with thrombocytopenia syndrome in Miyazaki, Japan: A GWLR modeling study. BMC Infect. Dis. 19:498. doi: 10.1186/s12879-019-4111-3
Yu, X.-J., Liang, M.-F., Zhang, S.-Y., Liu, Y., Li, J.-D., Sun, Y.-L., et al. (2011). Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 364, 1523–1532. doi: 10.1056/NEJMoa1010095
Zé-Zé, L., Nunes, C., Sousa, M., De Sousa, R., Gomes, C., Santos, A. S., et al. (2025). Fatal case of crimean-congo hemorrhagic fever, Portugal, 2024. Emerg. Infect. Dis. 31:1264. doi: 10.3201/eid3101.241264
Zhang, M.-Z., Bian, C., Ye, R.-Z., Cui, X.-M., Chu, Y.-L., Yao, N.-N., et al. (2025). Human infection with a novel tickborne orthonairovirus species in China. N. Engl. J. Med. 392, 200–202. doi: 10.1056/NEJMc2410853
Zhao, G.-P., Wang, Y.-X., Fan, Z.-W., Ji, Y., Liu, M., Zhang, W.-H., et al. (2021). Mapping ticks and tick-borne pathogens in China. Nat. Commun. 12:1075. doi: 10.1038/s41467-021-21375-1
Zheng, W., Chen, H., Liu, X., Guo, X., and Fu, R. (2011). Severe tick infestation in a hare and potential risk for transmitting pathogens to humans. Korean J. Parasitol. 49:419. doi: 10.3347/kjp.2011.49.4.419
Keywords: ticks, tick-borne diseases, climate change, invasive ticks, socio-ecosystems, prevention, management, One Health
Citation: Boulanger N, Iijima H, Doi K, Watari Y, Kwak M, Nakao R and Wikel S (2025) Ticks and tick-borne diseases in the northern hemisphere affecting humans. Front. Microbiol. 16:1632832. doi: 10.3389/fmicb.2025.1632832
Received: 21 May 2025; Accepted: 21 July 2025;
Published: 08 August 2025.
Edited by:
Leonard Peruski, Wadsworth Center, United StatesReviewed by:
Vipin Rana, University of Maryland, College Park, United StatesUday Turaga, Koneru Lakshmaiah Education Foundation, India
Copyright © 2025 Boulanger, Iijima, Doi, Watari, Kwak, Nakao and Wikel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nathalie Boulanger, bmJvdWxhbmdlckB1bmlzdHJhLmZy; Stephen Wikel, c3RlcGhlbi53aWtlbEBxdWlubmlwaWFjLmVkdQ==
 Nathalie Boulanger
Nathalie Boulanger Hayato Iijima
Hayato Iijima Kandai Doi3
Kandai Doi3 Ryo Nakao
Ryo Nakao Stephen Wikel
Stephen Wikel