- 1Department of General Diagnostics, Istituto Zooprofilattico Sperimentale del Lazio e della Toscana “M. Aleandri”, Rome, Italy
- 2Veterinary Practitioner, Rome, Italy
- 3Azienda Sanitaria Roma 1, Servizi Veterinari, Rome, Italy
Introduction: Tuberculosis in humans is mainly caused by two closely related bacteria within the Mycobacterium tuberculosis complex (MTBC), which are Mycobacterium tuberculosis and Mycobacterium africanum. M. tuberculosis is widely spread, while M. africanum is more ecologically restricted to Africa.
Methods and results: In 2023, we examined a skin biopsy from a 3-year-old female domestic cat with multifocal nodular cutaneous lesions and respiratory problems. The animal was an indoor cat kept in Rome, reportedly taken in as a stray kitten from a village in southern Italy (Central Calabria Region). Skin histology with Ziehl–Neelsen staining was consistent with suspected mycobacteriosis. Bacterial cultures for Mycobacterium spp. yielded an isolate, identified by polymerase chain reaction (PCR) as a Mycobacterium tuberculosis complex (MTBC). Whole-genome sequencing and bioinformatics further identified the isolate as M. africanum lineage 6, and phylogeny with 634 other MTBC genomes placed it within a West African cluster (mainly from Gambia) of the L6.1.2 sublineage. Resistome analysis indicated the presence of resistance genes intrinsic in M. tuberculosis and point mutations not associated with resistance. The cat died roughly 1 year later, most probably from systemic tuberculosis, but the owner did not request a necropsy.
Discussion: This represents the first reported case of M. africanum infection in a carnivore and in a companion animal. The case history reports a stray kitten collected in an area of southern Italy, near the first migrant reception centers and croplands where workers coming from West Africa are often employed, consistent with our phylogenetic evidence.
1 Introduction
Mammalian tuberculosis (TB) is a chronic granulomatous disease that affects both animals and humans and is caused by bacteria within the Mycobacterium tuberculosis complex (MTBC). MTBC members belong to the family Mycobacteriaceae and are Gram-positive, acid-fast bacilli. The taxonomy of organisms in the MTBC is in a state of constant evolution (World Organisation for Animal Health, 2022). Recent genomic analyses suggest that all MTBC members belong to a single species, M. tuberculosis, with Mycobacterium africanum, Mycobacterium bovis, Mycobacterium caprae, Mycobacterium microti, and Mycobacterium pinnipedii considered heterotypic synonyms (variants) of M. tuberculosis, and Mycobacterium canettii, Mycobacterium mungi, and Mycobacterium orygis are recognized as strains of M. tuberculosis (World Organisation for Animal Health, 2022; Riojas et al., 2018). To retain linkage with historical nomenclature, the more widely recognized designations are commonly used instead of infra-subspecific designations (World Organisation for Animal Health, 2022). Hence, throughout the paper, we used the historical nomenclature (e.g., M. africanum) designations instead of M. tuberculosis var. africanum for ease of prior association.
All MTBC members share remarkable genomic similarity, with more than 99.95% nucleotide identity. Although all mammalian species are considered susceptible to tuberculosis, they vary considerably in their host tropism and ability to cause disease (Silva-Pereira et al., 2019). Single nucleotide polymorphisms (SNPs) and deletions of genomic regions ranging from 2 to 12.7 Kb, denominated “regions of difference (RDs),” allow for species differentiation (Brosch et al., 2002; Cousins et al., 2003); World lineage-wise classification of MTBC was also achieved using restriction fragment length polymorphism (RFLP) and PCR, such as mycobacterial interspersed repetitive units – variable number of tandem repeats (MIRU-VNTR) spoligotyping (Brosch et al., 2002).
M. tuberculosis and M. africanum are primarily human pathogens, but are also known to infect animals, and are considered pathogens of high Public-Health and One-Health relevance (Comín et al., 2021; World Organisation for Animal Health, 2022). M. tuberculosis is widely spread, while M. africanum, first described in 1968 from TB patients in Senegal (Castets et al., 1968), is more ecologically restricted to Africa and, in particular, West Africa, where it is responsible for almost half of all the TB cases (Comín et al., 2021; World Organisation for Animal Health, 2022; Silva et al., 2022). TB caused by M. africanum strains outside West Africa, although rare, has been described in several countries, mostly found in migrants from endemic areas (Yeboah-Manu et al., 2017; Comín et al., 2021; Silva et al., 2022).
Ten human-adapted lineages (L) belong to the MTBC. In particular, L1–L4 and L7 and L8 comprise M. tuberculosis sensu stricto (which majorly infects humans), while L5, L6, L9, and L10 consist of M. africanum (Blouin et al., 2012; Firdessa et al., 2013; Riojas et al., 2018; Gagneux, 2018; Ngabonziza et al., 2020; Coscolla et al., 2021; Balamurugan et al., 2022; Silva et al., 2022; Guyeux et al., 2024). L6 is known to be geographically restricted to West Africa, and L5 is known to have moved from West Africa to Central Africa (Coscolla et al., 2021; Balamurugan et al., 2022). In contrast, L9 belongs to a sister clade of L6, being placed between L6 and the animal-adapted lineages (Coscolla et al., 2021). Recently, a proposed L10 was also described, a sister lineage of L6 and L9 associated with Central Africa. Phylogenetic reconstruction suggests L10 could represent a missing link in the evolutionary and geographic migration histories of M. africanum (Guyeux et al., 2024).
Historically, M. africanum shows “intermediate” phenotypic characteristics between M. tuberculosis and M. bovis based on biochemical testing (Castets et al., 1968; Meyer and David, 1979; Thorel, 1980); however, lineage classification based on genotypic differences is more accurate than one based on phenotypic assays, also because phenotypic diversity among isolates of the same lineage or even sublineage cannot be excluded (Silva et al., 2022). Compared to M. tuberculosis, L5 and L6 are reported to have slower growth in culture, along with lower bacterial load and delayed disease progression (Cá et al., 2019; Baya et al., 2020; Balamurugan et al., 2022).
Phylogenetically, L5, L6, L9, L10, and the animal-adapted lineages share a common ancestor lacking the RD9, but L5 split from the common phylogenetic branch before the others. Some L5 genomes (L5.1.1) have also undergone RD711 deletion, while L6 has lost RD702 (Mostowy et al., 2004; De Jong et al., 2010; Ates et al., 2018; Coscolla et al., 2021; Comín et al., 2021). L6 has also undergone deletion of RD7, RD8, and RD10 regions (Gagneux, 2018; Comín et al., 2021; Balamurugan et al., 2022). L9 shares some genomic deletions with those of strains belonging to L6, such as RD702, but not with others, which are also present in the genomes of strains of animal-associated lineages, such as RD1 and RD5 (Coscolla et al., 2021; Silva et al., 2022). L10 lacks RD9, RD7, RD8, and RD10, and it harbors a specific large 9,134-no turning (nt) deletion (Rv0613c–Rv0622) in M. tuberculosis H37Rv (NC\_000962.3:706602–715,736) not observed in any other lineage (Guyeux et al., 2024).
Recently, whole-genome sequencing (WGS) analyses allowed the construction of detailed MTBC phylogenetic trees also based on several specific SNPs (Brites et al., 2018; Ates et al., 2018; Otchere et al., 2018; Sanoussi et al., 2021; Coscolla et al., 2021; Balamurugan et al., 2022). WGS has now become a fundamental tool for resolving epidemiological relationships, phylogeny, host adaptation, resistance and virulence determinants, etc. (Coscolla et al., 2021; Balamurugan et al., 2022). Phylogenetically, L5 is placed closer to the human-adapted MTBC and L6 closer to the animal-adapted strains (Gagneux, 2018). The proposed evolutionary scenario is that the L6 ancestor was a generalist pathogen that subsequently adapted to different host species, with the possible hypothesis that L6 strains may have originated from an animal reservoir (Silva et al., 2022). L6 also shows a more differentiated population structure than L5, with three distinct monophyletic main sublineages (L6.1, L6.2, and L6.3) that can be further subdivided into at least three other subgroups/sublineages each (Coscolla et al., 2021; Balamurugan et al., 2022).
The objectives of the present study were: to describe the tuberculosis case that occurred in a domestic cat and its etiology; to perform an in-depth genomics characterization by WGS and bioinformatic analysis of the isolate for identification at the lineage/subspecies level, and to compare it with other genomes available in public repositories, gaining an insight into phylogenetic aspects.
2 Materials and methods
2.1 Cat origin and clinical picture
In February 2023, a skin biopsy was taken from a 3-year-old spayed female domestic European Shorthair cat with multifocal nodular cutaneous lesions and respiratory problems, and was sent to our Institute for diagnostic purposes. The animal was an indoor cat kept in Rome, reportedly taken in as a stray kitten at a village located on the Ionian coast of southern Italy, Calabria region (Cropani municipality, Catanzaro province).
Reportedly, 7 months before sampling, the cat started coughing, and after 3 months, cutaneous nodules appeared on the end of the anterior paws. Simultaneously, palpable lymph nodes became enlarged, and the cat showed inappetence and depression. The cat was treated with prednisolone and antibiotics (fluoroquinolones and macrolides) for about 2 months, with improvement in the respiratory signs. In July 2023, the presence of cutaneous nodules increased and expanded to other sites, one on the tail became ulcerated. In April 2024, a computed tomography scan (CT scan) was performed, revealing several nodular neoformations at the head and muzzle level, tail, and limbs, with involvement of the afferent lymph nodes. The lung parenchyma presented a severe picture characterized by thickening of the bronchial network and widespread areas of hepatization.
The cat died in May 2024, but unfortunately, the owner did not request a necropsy, and the only sample received by our Institution was the skin biopsy collected in February 2023.
2.2 Skin biopsy, histopathological, and microbiological investigation
Histological sections of the biopsy were routinely processed and stained with hematoxylin and eosin (HE). Histochemical Ziehl–Neelsen (ZN) stain was also performed on five new different sections.
For bacteria isolation and identification, the tissue was cultured on Columbia Agar supplemented with 5% sheep blood (VWR, Belgium) and brain heart infusion broth; following incubation for up to a week under aerobic and microaerobic (10% CO2) conditions at 37 °C, growth colonies were subcultured and pure colonies screened using standard techniques including colony morphology, Gram staining, catalase test, oxidase test, and biochemically identified at species level with API test kits (bioMérieux, France). The biopsy was also cultured using specific solid commercial media for the isolation of Mycobacterium spp. (Stonebrink and Loewenstein-Jensen media, Microbiol S.n.c., Italy), following incubation under aerobic conditions at 37 °C and 42 °C. Mycobacterium solid media were periodically evaluated for bacterial growth for up to 3 months, following the guidelines outlined in the World Organization for Animal Health (WOAH) Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2022 (World Organisation for Animal Health, 2022).
2.3 Mycobacterium molecular identification and genomics
DNA from growth colonies referable to Mycobacterium spp. was extracted using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol and as previously described (Iurescia et al., 2021). The extracted DNA was subjected to real-time polymerase chain reaction (PCR) (Ferrari et al., 2024) and a multiplex end-point PCR (Kulski et al., 1995) for the identification at the genus level and to assess their belonging to MTBC.
The MTBC isolates retrieved were also investigated by WGS analysis. Libraries for short-read pair-end sequencing were prepared using the Nextera XT DNA library preparation kit (Illumina, Inc., San Diego, CA, USA) following the Nextera XT R Guide 150319425031942 and sequenced on an Illumina platform (MiSeq). Quality trimming of the raw reads was performed using Trimmomatic version 0.39 with the following parameters: LEADING:30, TRAILING:30, SLIDINGWINDOW:10:20, MINLEN:50 (Bolger et al., 2014). Assembly was performed using SPAdes version 3.13.0 (Prjibelski et al., 2020). The quality of the assembly was addressed using QUAST version 5.0.2 (Gurevich et al., 2013).
Multilocus sequence typing (MLST) was performed using the scheme published in the pubMLST.org database (Jolley et al., 2018) by uploading the complete assembly. TB Profiler version 5.0.1 (Phelan et al., 2019), with its own database, was used for “in silico” ribotyping, assigning a lineage, and identifying the resistance and virulence genes. In particular, point mutations in chromosomal genes that confer antimicrobial resistance in the MTB complex (M. tuberculosis) were interpreted in accordance with the World Health Organization (WHO) catalogue of mutations in M. tuberculosis complex and their association with drug resistance (World Health Organization, 2023).
Resistance and virulence genes were confirmed using AMRFinderPlus version 3.12.8 (Feldgarden et al., 2021), with the following cut-offs: minimum 80% coverage and 80% identity.
For the identification of the regions of difference (RDs), our complete genome was mapped against the reference M. tuberculosis H37Rv (NC_000962.3) using minimap2 version 2.24-r1122 (Li, 2018), samtools version 1.12 (Danecek et al., 2021) for sorting and indexing, and IGV version 2.5.3 (Thorvaldsdóttir et al., 2013) for visualization.
For a first genetic identification, a tree including the following publicly available raw reads from different MTBC strains was built: ERR150046 (chimpanzee bacillus), SRR3745458 (dassie bacillus), ERR234255 and SRR998578 (M. africanum), SRR6705904 (M. bovis), DRR120409 (M. caprae), ERR027298 (M. microti), SRR3500411 (M. mungi), SRR5642712 (M. orygis), ERR970409 (M. suricattae), SRR1239339 (M. pinnipedii). For a more in-depth identification, our isolate was then compared with raw reads from 675 publicly available M. africanum L5, L6, and L9 strains and 5 related genomes that could not be classified into any of the known human- or animal-associated MTBC lineages.
For both analyses, SNP identification was performed by using Snippy version 4.61 with the default parameters (minimum quality of the nucleotide set as 13; minimum coverage set as 10; minimum proportion of those reads that must differ from the reference set as 0.9), and using M. tuberculosis H37Rv as the reference strain. Duplicate genomes and isolates with >200.000 base pairs of the genome not aligned with the reference one were discarded.
The first MTBC phylogenetic tree was built using Randomized Axelerated Maximum Likelihood (RAxML) 8.2.12 (Stamatakis, 2014) and the maximum likelihood (ML) algorithm with the “gtrcat” model and 1,000 bootstrap inferences.
The second phylogenetic tree was constructed using FastTree version 2.1.11 (Price et al., 2010), using a general time-reversible model (gtr) (Supplementary Table S1). The visualization of the figures (trees) was created using iTol (Letunic and Bork, 2021).
The raw reads obtained were submitted to the European Nucleotide Archive (ENA) under the study accession number ERR14161356.
3 Results
3.1 Histopathological and microbiological investigation
Microscopic examination of the skin lesions showed non-capsulated multifocal to coalescent granulomas of variable size in the dermis (Figure 1). Most of the large granulomas were composed of macrophages mixed with a few degenerated neutrophils and scattered lymphocytes, sometimes with multiple foci of necrosis. Smaller granulomas were characterized by a necrotic center with aggregates of degenerated neutrophils, and numerous macrophages with lymphocytic infiltrates at the periphery of the lesion (Figure 2). Multifocal areas of superficial skin ulceration were seen, which were associated with intradermal granulomas. Ziehl–Neelsen staining highlighted the presence of rare and scattered acid-fast bacilli, morphologically resembling mycobacteria, both in macrophages and in necrosis foci.
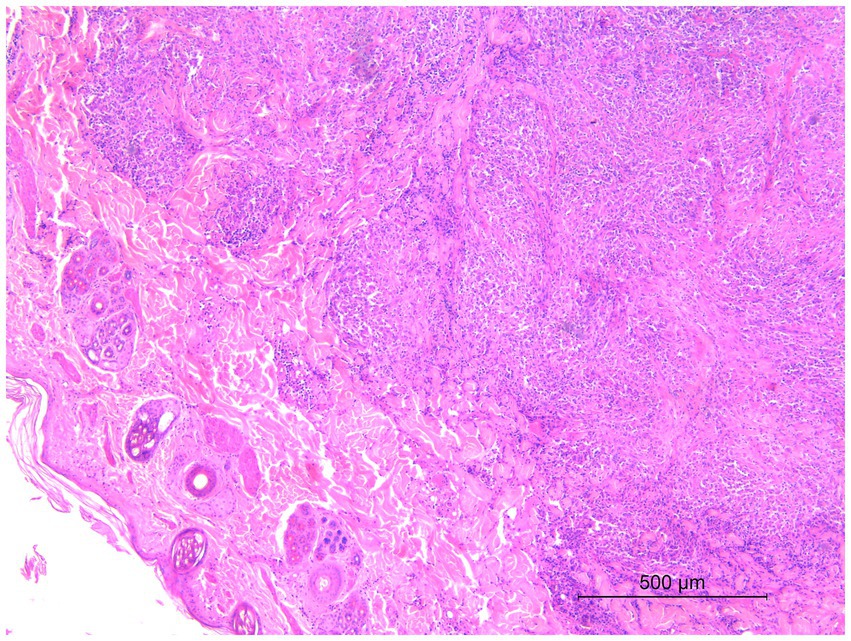
Figure 1. Histological section of a biopsy specimen collected from one of the cutaneous lesions. Non-capsulated multifocal to coalescent granulomas of variable size in the dermis. HE stain, 5×. Scale bar: 500 μm.
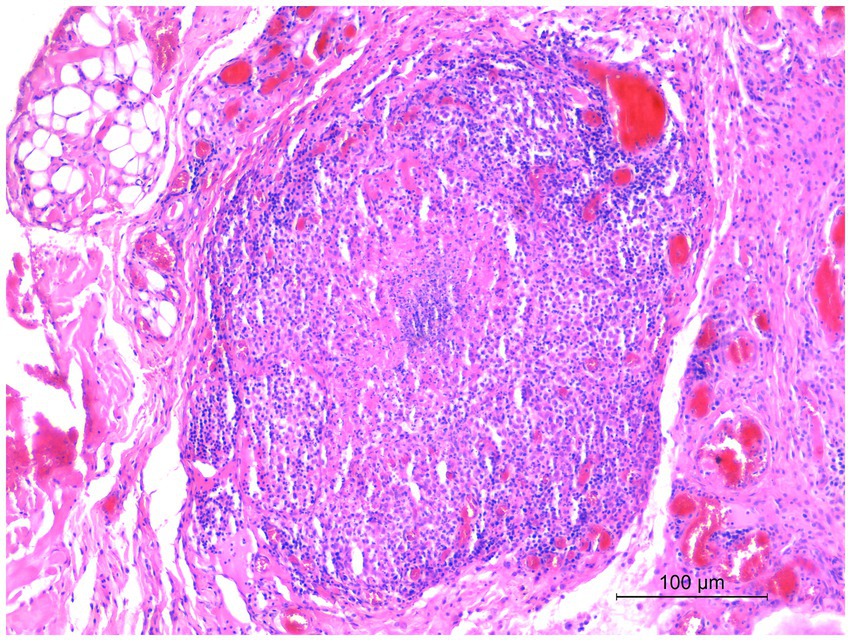
Figure 2. Histological section of a biopsy specimen collected from one of the cutaneous lesions. Smaller granulomas were characterized by a necrotic center with aggregates of degenerated neutrophils, and numerous macrophages with lymphocytic infiltrates at the periphery of the lesion. HE stain, 20×. Scale bar: 100 μm.
After approximately 12 weeks post-inoculation, suspect colonies referable to Mycobacterium spp. were detected on Loewenstein–Jensen media incubated at 37 °C. From the other bacterial standard cultures, Staphylococus aureus was isolated.
3.2 Mycobacterium molecular identification and genomics
Mycobacterium spp. suspected colonies from the skin biopsy were positive at the genus level and for MTBC by real-time PCR and multiplex end-point PCR, but negative for M. bovis, M. caprae, and M. tuberculosis. The WGS analysis using TBprofiler indicated that the isolate was M. africanum L6 (West-Africa 2). The octal spolygotype was 770777740000071. MLST analysis indicated that the isolate belonged to ST215 and to cgST-5847 (95% of loci matched). Moreover, the deletion of RD7, RD8, RD9, RD10, and RD702 was manually confirmed by mapping with the M. tuberculosis reference genome.
SNP-based phylogenomic approach confirmed the identification as M. africanum (Figure 3) and included the genome of our isolate (ERR14161356) in a large cluster together with isolates mainly from Gambia and Ivory Coast, belonging to the L6.1.2 sublineage (Figure 4). According to this analysis, the most similar genome (229 SNPs) was a L6 (not specified sublineage) M. africanum isolated in France in 2007 from a human TB case (ERR2704811) (Ates et al., 2018).
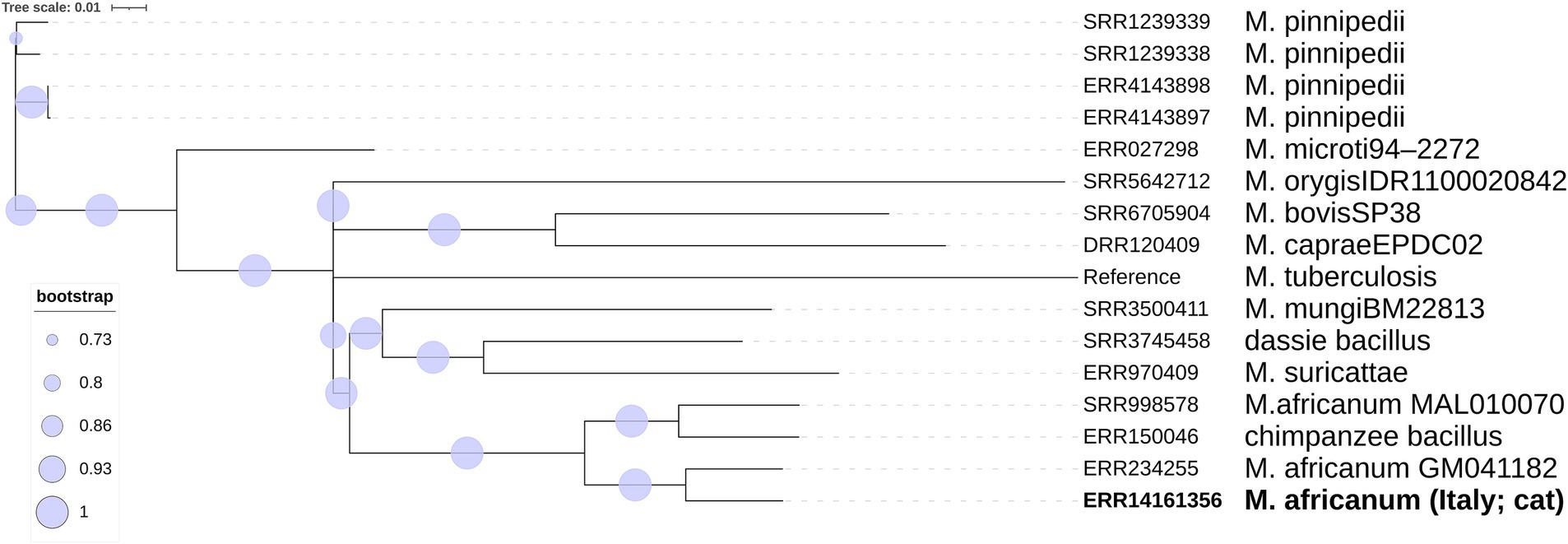
Figure 3. Phylogenetic SNP tree built with the ML algorithm of the main Mycobacterium tuberculosis variants.
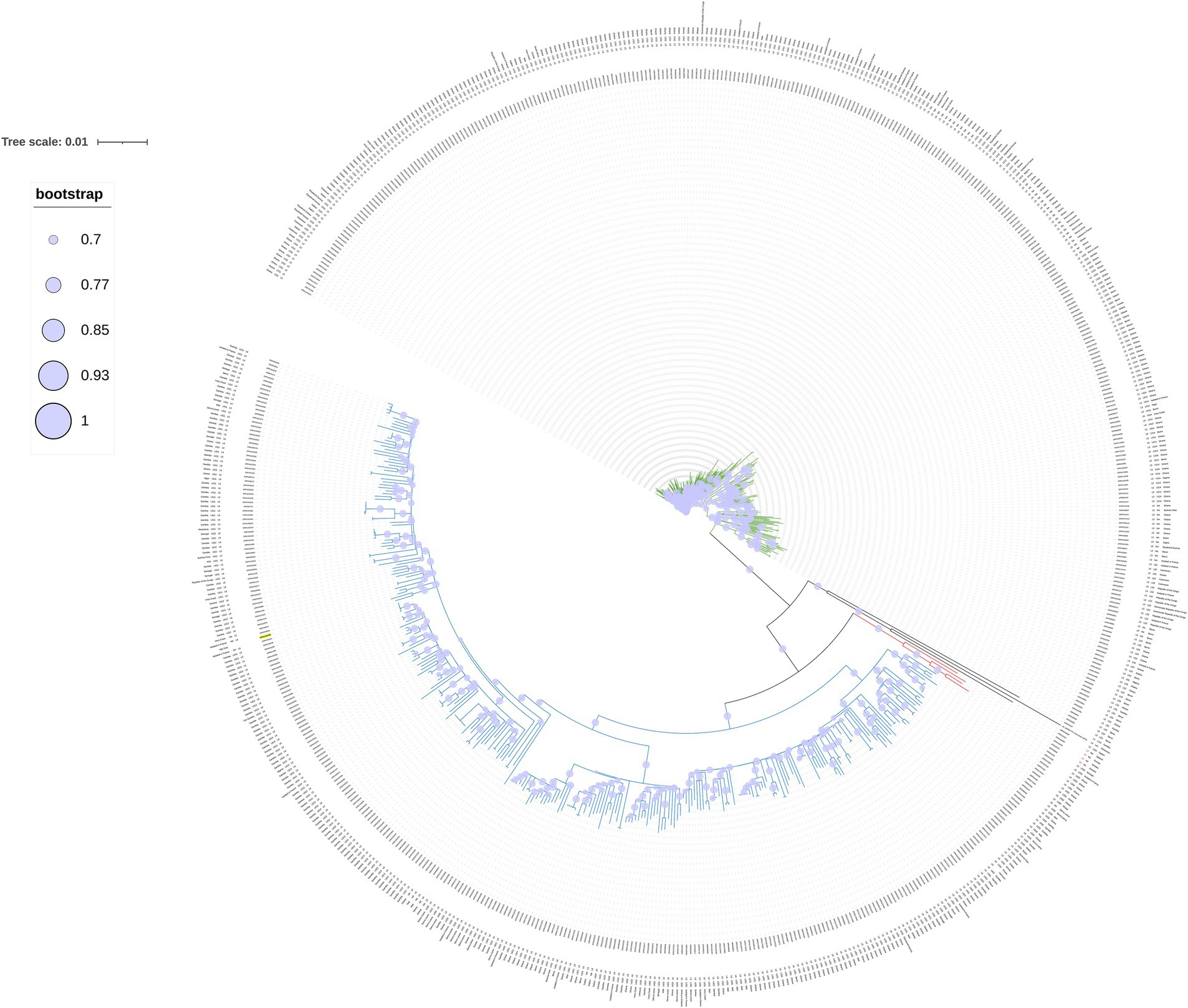
Figure 4. Circular phylogenetic SNPs tree built with 634 M. africanum genomes belonging to L5 (green), L6 (blue) and L9 (red), our cat isolate (ERR14161356, underlined in yellow) and the reference genome (NC_000962.3 M. tuberculosis H37Rv). Bootstrap values higher than 70% are represented with a circle within the clade.
The final assembly consisted of 153 contigs (≥1,000 bp) with an N50 of 77,339 bp and a total length of 4,606,140 bp, covering 98.21% of the genome with an average coverage depth of 58.65X. Using the AMRFinder tool, two natural (intrinsic) resistance genes encoding for macrolide and aminoglycoside, respectively, were found: erm-37 and aac(2′)-Ic. The blaC gene, the gene known to encode a beta-lactamase in M. tuberculosis, was also detected. Using TBprofiler, some point mutations in specific genes, associated with resistance to rifampicin and rifapentine (rpoB), isoniazid (inhA and katG), ethambutol (embB and embA), levofloxacin and moxifloxacin (gyrA and gyrB), bedaquiline and clofazimine (atpE), linezolid (rplC), delamanid and pretomanid (ddn, fbiC, fgd1, and Rv2983), amikacin (rrs), streptomycin (rrs and gid), ethionamide and prothionamide (ethA and inhA) were found, but were classified as “of uncertain significance” or “Not associated with R” (Supplementary Table S1), according to the WHO guidelines (World Health Organization, 2023).
Regarding virulence genes, 139 genes already described in MTBC were detected, including esxA (6 kDa early secretory antigenic target), esxB (10 kDa culture filtrate antigen), the mbtA, mbtB, mbtC, mbtD, mbtE, mbtF, and mbtG genes, involved in the biosynthesis of the siderophore mycobactin and plcA, plcB, and plcC, encoding the phospholipase C exotoxin.
4 Discussion
We report, for the first time, an infection due to M. africanum in a domestic cat. The histopathological skin lesions observed resembled those of other cutaneous mycobacteriosis in cats (Malik et al., 2000; Lloret et al., 2013; Gunn-Moore, 2014; Sykes, 2025). Unfortunately, no further samples in addition to the skin nodules were made available for our laboratory, and the owner declined to perform a necropsy on the deceased animal. The lack of postmortem examination prevented the possibility of testing additional tissue samples to fully characterize the disease progression and systemic involvement. In any case, the reported clinical history and the computed tomography (CT) scan, with respiratory/pulmonary and lymph nodes involvement, were indicative of a generalized form consistent with a systemic TB case. The isolation of a S. aureus from the biopsy is probably attributable to a secondary infection that might have contributed to the cutaneous lesions.
In animals, M. africanum has been seldom reported in monkeys (Thorel, 1980; Coscolla et al., 2013), cattle (Weber et al., 1998; Rahim et al., 2007), pigs (Alfredsen and Saxegaard, 1992), and in hyrax (Procavia capensis) (Gudan et al., 2008). However, no animal reservoir has ever been postulated (Silva et al., 2022), and it is accepted that the epidemiology of infection basically relies on inter-human transmission. To the best of our knowledge, this represents the first report of M. africanum infection in a carnivore and in a companion animal. Recently, we have already witnessed zoonotic exposure of a M. pinnipedii from a captive sea lion (Alba et al., 2023). Indeed, the zoonotic risk of disease caused by MTBC from domestic or captive-bred carnivores should be taken into consideration (World Organisation for Animal Health, 2022; Gunn-Moore, 2014; Sykes, 2025; Rüfenacht et al., 2011; O’Halloran et al., 2024; Commandeur et al., 2025), and our findings highlight the need for awareness of potential MTBC transmission between species, including the potential role of humans as a reservoir. To the best of our knowledge, at the time of paper submission, no human TB cases in the cat household were reported.
The genomic investigation demonstrated that the implicated isolate was a M. africanum L6, and our isolate, together with an L6 isolated from a wild Chimpanzee in Côte d’Ivoire in 2013 (Coscolla et al., 2013), is at present the only fully genetically characterized M. africanum isolated from an animal.
This identification was further confirmed by the high similarity with other L6 genomes in the SNP-based phylogenomic approach, conducted as in the study by Coscolla et al. (2021), which placed the cat Italian isolate (ERR14161356) in a cluster together with isolates belonging to the L6.1.2 sublineage, a sublineage representing genomes originating in West Africa, mostly The Gambia (Coscolla et al., 2021). In particular, the closest genome to our isolate is a historical isolate detected in France (Ates et al., 2018). Unfortunately, no further information on the patient’s geographical origin is available about this French isolate, although a likely association with a West African exposure or origin has been suggested (R. Brosch, personal communication). TB caused by M. africanum strains is prevalent in West Africa, while outside it has been found mostly—but not only—in migrants from endemic areas (Silva et al., 2022). Indeed, inter-human transmission has also been reported in TB low-incidence European countries (Eldholm et al., 2021), where immigration and travel patterns from endemic areas facilitate local exposure. However, most of the sublineages within L6 have been reported to originate from West Africa, and only a few L6 strains were found in Central Africa or outside Africa (Coscolla et al., 2021). The cat was taken as a stray kitten at a camping site located on the Ionian coast of southern Italy, where in the nearby area are present two first migrant reception centers and several croplands, where workers coming from West Africa are often employed. Although the exact source of the infection in our case remains unknown, it might be speculated that the cat was initially exposed to infected humans/animals/fomites originally from West Africa, possibly including The Gambia. M. africanum geographical restriction, which contrasts with the widespread distribution of M. tuberculosis strains, remains largely unexplained. Regarding this, there are basically three main hypotheses: M. africanum is not able to compete with modern M. tuberculosis lineages; it is adapted to the African population, perhaps mediated through differential modulation of the immune response; and there could be an animal reservoir, making it a zoonotic disease (Comín et al., 2021; Silva et al., 2022). Furthermore, the true incidence of M. africanum infection may be underestimated whenever accurate laboratory differentiation within the MTBC isolates is not performed routinely on human cases (Comín et al., 2021). Based on human infections and experimental models, several Authors suggested that M. africanum strains and L6 may be less transmissible or virulent than M. tuberculosis strains (Sharma et al., 2016; Cá et al., 2019; Baya et al., 2020; Coscolla et al., 2021; Silva et al., 2022). Still, in our case, the cat became infected and likely died from TB. Some studies showed a high proportion of extrapulmonary TB caused by M. africanum, suggesting that these strains might show a different ability to cause pulmonary disease than M. tuberculosis sensu stricto (Ates et al., 2018; Comín et al., 2021). In our animal case, the cat likely had a generalized type of disease, with involvement of the skin and a clinical picture resembling those of other systemic mycobacteriosis in cats (Gunn-Moore, 2014).
The scientific interest in understanding the variation of M. africanum with respect to epidemiology and virulence is high (Yeboah-Manu et al., 2017; Gagneux, 2018; Ates et al., 2018; Coscolla et al., 2021; Balamurugan et al., 2022). Since the resistome and virulome found in our isolate are considered intrinsic/common features of M. tuberculosis, their presence in our cat M. africanum isolate could also be considered intrinsic and indicators of its zoonotic potential.
5 Conclusion
In conclusion, we report for the first time an infection due to M. africanum in a cat that most likely died from TB. Our molecular data show that the isolated strain belongs to L6, a lineage that has been considered closer (Silva et al., 2022) to certain animal-adapted lineages.
Although MTBC comprises host-adapted taxa, inter-species transmission between wild, captive, and domesticated animals and humans has been frequently reported, thanks to the plasticity of many of the MTBC taxa, posing a worldwide threat to both human and animal health (Vågene et al., 2022).
Indeed, the finding of tuberculosis caused by M. africanum in a carnivore further raises the possibility of the existence of non-human reservoirs, which may contribute to the main pattern of inter-human transmission in endemic areas, at least for some sublineages. TB-infected people who live in close contact with pets should be aware of the possibility of human-to-animal transmission, as proposed in our study. Conversely, citizens should be informed about the hazards posed by some well-known zoonotic lineages within the MTBC. In this regard, rapid and reliable etiological identification and characterization are of paramount importance, not only for an accurate diagnosis, but also for a correct approach to treatment and management options. Indeed, nowadays, the accuracy has greatly improved by genomics, which also allowed phylogenetic and phylogeographic insights.
In any case, suspected or confirmed tuberculosis cases in companion animals, in addition to an appropriate clinical case management, should also rapidly prompt prevention and control action within the household settings.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found inside the article.
Ethics statement
Ethical approval was not required for the study, since data were collected as part of routine diagnostic activities. No laboratory animals were used during the research and no human samples/sampling were involved. No persons are identifiable in photographs or in the case descriptions. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
PA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Writing – original draft, Writing – review & editing, Visualization. AC: Conceptualization, Data curation, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing, Visualization. CC: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. CE: Data curation, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing, Formal analysis. VG: Data curation, Methodology, Visualization, Writing – original draft, Writing – review & editing, Formal analysis. AG: Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. LS: Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. FS: Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. FF: Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. RA: Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. AI: Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. FC: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing. MF: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing. VC: Data curation, Validation, Visualization, Writing – original draft, Writing – review & editing. AF: Data curation, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing. AB: Conceptualization, Data curation, Funding acquisition, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Istituto Zooprofilattico Sperimentale del Lazio e della Toscana M. Aleandri (IZSLT), Italy, was supported by the European Union funding from the NextGeneration EU-MUR PNRR Extended Partnership Initiative on Emerging Infectious Diseases (Project no. PE00000007, INF-ACT, PE13 INF-ACT, Nodes 3 and 4).
Acknowledgments
We thank Dr. Letizia Rillo for her support in the final editing of the article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that between the authors there was an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1633110/full#supplementary-material
Footnotes
References
Alba, P., Caprioli, A., Cocumelli, C., Eleni, C., Diaconu, E. L., Donati, V., et al. (2023). Genomics insights into a Mycobacterium pinnipedii isolate causing tuberculosis in a captive south American sea lion (Otaria flavescens) from Italy. Front. Microbiol. 14:1303682. doi: 10.3389/fmicb.2023.1303682
Alfredsen, S., and Saxegaard, F. (1992). An outbreak of tuberculosis in pigs and cattle caused by Mycobacterium africanum. Vet. Rec. 131, 51–53. doi: 10.1136/vr.131.3.51
Ates, L. S., Dippenaar, A., Sayes, F., Pawlik, A., Bouchier, C., Ma, L., et al. (2018). Unexpected genomic and phenotypic diversity of Mycobacterium africanum lineage 5 affects drug resistance, protein secretion, and immunogenicity. Genome Biol. Evol. 10, 1858–1874. doi: 10.1093/gbe/evy145
Balamurugan, M., Banerjee, R., Kasibhatla, S. M., Achalere, A., and Joshi, R. (2022). Understanding the genetic diversity of Mycobacterium africanum using Phylogenetics and population genomics approaches. Front. Genet. 13:800083. doi: 10.3389/fgene.2022.800083
Baya, B., Diarra, B., Diabate, S., Kone, B., Goita, D., Cohen, K., et al. (2020). Association of Mycobacterium africanum infection with slower disease progression compared with Mycobacterium tuberculosis in Malian patients with tuberculosis. Am. J. Trop. Med. Hyg. 102, 36–41. doi: 10.4269/ajtmh.19-0264
Blouin, Y., Hauck, Y., Soler, C., Fabre, M., Vong, R., Dehan, C., et al. (2012). Significance of the identification in the horn of Africa of an exceptionally deep branching Mycobacterium tuberculosis clade. PLoS One 7:e52841. doi: 10.1371/journal.pone.0052841
Bolger, A. M., Lohse, M., and Usadel, B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. doi: 10.1093/bioinformatics/btu170
Brites, D., Loiseau, C., Menardo, F., Borrell, S., Boniotti, M. B., Warren, R., et al. (2018). A new phylogenetic framework for the animal-adapted Mycobacterium tuberculosis complex. Front. Microbiol. 9:2820. doi: 10.3389/fmicb.2018.02820
Brosch, R., Gordon, S. V., Marmiesse, M., Brodin, P., Buchrieser, C., Eiglmeier, K., et al. (2002). A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc. Natl. Acad. Sci. USA 99, 3684–3689. doi: 10.1073/pnas.052548299
Cá, B., Fonseca, K. L., Sousa, J., Maceiras, A. R., Machado, D., Sanca, L., et al. (2019). Experimental evidence for limited in vivo virulence of Mycobacterium africanum. Front. Microbiol. 10:2102. doi: 10.3389/fmicb.2019.02102
Castets, M., Boisvert, H., Grumbach, F., Brunel, M., and Rist, N. (1968). Les bacilles tuberculeux de type Africain: Note préliminaire. Rev. Tuberc. Pneumol. 32, 179–184.
Comín, J., Monforte, M. L., Samper, S., and Aragonese Working Group on Molecular Epidemiology of Tuberculosis (EPIMOLA) Otal, I. (2021). Analysis of Mycobacterium africanum in the last 17 years in Aragon identifies a specific location of IS6110 in lineage 6. Sci. Rep. 11:10359. doi: 10.1038/s41598-021-89511-x
Commandeur, S., van der Most, M., Koomen, J., van Keulen, L., Dinkla, A., Luinenburg, X., et al. (2025). Mycobacterium bovis infected domestic cats in an officially bovine tuberculosis free country resulting in human infection. One Health 20:101048. doi: 10.1016/j.onehlt.2025.101048
Coscolla, M., Gagneux, S., Menardo, F., Loiseau, C., Ruiz-Rodriguez, P., Borrell, S., et al. (2021). Phylogenomics of Mycobacterium africanum reveals a new lineage and a complex evolutionary history. Microb. Genom. 7:000477. doi: 10.1099/mgen.0.000477
Coscolla, M., Lewin, A., Metzger, S., Maetz-Rennsing, K., Calvignac-Spencer, S., Nitsche, A., et al. (2013). Novel Mycobacterium tuberculosis complex isolate from a wild chimpanzee. Emerg. Infect. Dis. 19, 969–976. doi: 10.3201/eid1906.121012
Cousins, D. V., Bastida, R., Cataldi, A., Quse, V., Redrobe, S., Dow, S., et al. (2003). Tuberculosis in seals caused by a novel member of the Mycobacterium tuberculosis complex: Mycobacterium pinnipedii sp. nov. Int. J. Syst. Evol. Microbiol. 53, 1305–1314. doi: 10.1099/ijs.0.02401-0
Danecek, P., Bonfield, J. K., Liddle, J., Marshall, J., Ohan, V., Pollard, M. O., et al. (2021). Twelve years of SAMtools and BCFtools. Gigascience 10:giab008. doi: 10.1093/gigascience/giab008
De Jong, B. C., Antonio, M., and Gagneux, S. (2010). Mycobacterium africanum--review of an important cause of human tuberculosis in West Africa. PLoS Negl. Trop. Dis. 4:e744. doi: 10.1371/journal.pntd.0000744
Eldholm, V., Rønning, J. O., Mengshoel, A. T., and Arnesen, T. (2021). Import and transmission of Mycobacterium orygis and Mycobacterium africanum, Norway. BMC Infect. Dis. 21:562. doi: 10.1186/s12879-021-06269-3
Feldgarden, M., Brover, V., Gonzalez-Escalona, N., Frye, J. G., Haendiges, J., Haft, D. H., et al. (2021). AMRFinderPlus and the reference gene catalog facilitate examination of the genomic links among antimicrobial resistance, stress response, and virulence. Sci. Rep. 11:12728. doi: 10.1038/s41598-021-91456-0
Ferrari, S., Zanoni, M., Mangeli, A., Pigoli, C., D’Incau, M., Alborali, G. L., et al. (2024). Bacteriological culture and direct PCR for detecting the Mycobacterium tuberculosis complex in the Italian eradication campaign: a decade of experience at the National Reference Laboratory. J. Appl. Microbiol. 135:lxae064. doi: 10.1093/jambio/lxae064
Firdessa, R., Berg, S., Hailu, E., Schelling, E., Gumi, B., Erenso, G., et al. (2013). Mycobacterial lineages causing pulmonary and extrapulmonary tuberculosis, Ethiopia. Emerg. Infect. Dis. 19, 460–463. doi: 10.3201/eid1903.120256
Gagneux, S. (2018). Ecology and evolution of Mycobacterium tuberculosis. Nat. Rev. Microbiol. 16, 202–213. doi: 10.1038/nrmicro.2018.8
Gudan, A., Artuković, B., Cvetnić, Z., Spicić, S., Beck, A., Hohsteter, M., et al. (2008). Disseminated tuberculosis in hyrax (Procavia capensis) caused by Mycobacterium africanum. J. Zoo Wildl. Med. 39, 386–391. doi: 10.1638/06-041.1
Gunn-Moore, D. A. (2014). Feline mycobacterial infections. Vet. J. 201, 230–238. doi: 10.1016/j.tvjl.2014.02.014
Gurevich, A., Saveliev, V., Vyahhi, N., and Tesler, G. (2013). QUAST: quality assessment tool for genome assemblies. Bioinformatics 29, 1072–1075. doi: 10.1093/bioinformatics/btt086
Guyeux, C., Senelle, G., Le Meur, A., Supply, P., Gaudin, C., Phelan, J. E., et al. (2024). Newly identified Mycobacterium africanum lineage 10, Central Africa. Emerg. Infect. Dis. 30, 560–563. doi: 10.3201/eid3003.231466
Iurescia, M., Romiti, F., Cocumelli, C., Diaconu, E. L., Stravino, F., Onorati, R., et al. (2021). Plasmodium matutinum transmitted by Culex pipiens as a cause of avian malaria in captive African penguins (Spheniscus demersus) in Italy. Front. Vet. Sci. 8:621974. doi: 10.3389/fvets.2021.621974
Jolley, K. A., Bray, J. E., and Maiden, M. C. J. (2018). Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 3:124. doi: 10.12688/wellcomeopenres.14826.1
Kulski, J. K., Khinsoe, C., Pryce, T., and Christiansen, K. (1995). Use of multiplex PCR to detect and identify Mycobacterium avium end M. intracellulare in blood culture fluids of AIDS patients. J. Clin. Microbiol. 33, 668–674.
Letunic, I., and Bork, P. (2021). Interactive tree of life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 49, W293–W296. doi: 10.1093/nar/gkab301
Li, H. (2018). Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34, 3094–3100. doi: 10.1093/bioinformatics/bty191
Lloret, A., Hartmann, K., Pennisi, M. G., Gruffydd-Jones, T., Addie, D., Belák, S., et al. (2013). Mycobacterioses in cats: ABCD guidelines on prevention and management. J. Feline Med. Surg. 15, 591–597. doi: 10.1177/1098612X13489221
Malik, R., Wigney, D. I., Dawson, D., Martin, P., Hunt, G. B., and Love, D. N. (2000). Infection of the subcutis and skin of cats with rapidly growing mycobacteria: a review of microbiological and clinical findings. J. Feline Med. Surg. 2, 35–48. doi: 10.1053/jfms.2000.0051
Meyer, L., and David, H. L. (1979). Evaluation de l’activité uréase et de l’activité beta-glucosidase pour l’identification pratique des mycobactéries [evaluation of urease and beta-glucosidase activity for the practical identification of mycobacteria (author’s transl)]. Ann. Microbiol. 130B, 323–332.
Mostowy, S., Onipede, A., Gagneux, S., Niemann, S., Kremer, K., Desmond, E. P., et al. (2004). Genomic analysis distinguishes Mycobacterium africanum. J. Clin. Microbiol. 42, 3594–3599. doi: 10.1128/JCM.42.8.3594-3599.2004
Ngabonziza, J. C. S., Loiseau, C., Marceau, M., Jouet, A., Menardo, F., Tzfadia, O., et al. (2020). A sister lineage of the Mycobacterium tuberculosis complex discovered in the African Great Lakes region. Nat. Commun. 11:2917. doi: 10.1038/s41467-020-16626-6
O’Halloran, C., Barker, E. N., Hope, J. C., and Gunn-Moore, D. A. (2024). Canine tuberculosis: a review of 18 new and 565 previously reported confirmed cases. Vet. J. 304:106089. doi: 10.1016/j.tvjl.2024.106089
Otchere, I. D., Coscollá, M., Sánchez-Busó, L., Asante-Poku, A., Brites, D., Loiseau, C., et al. (2018). Comparative genomics of Mycobacterium africanum lineage 5 and lineage 6 from Ghana suggests distinct ecological niches. Sci. Rep. 8:11269. doi: 10.1038/s41598-018-29620-2
Phelan, J. E., O’Sullivan, D. M., Machado, D., Ramos, J., Oppong, Y. E., Campino, S., et al. (2019). Integrating informatics tools and portable sequencing technology for rapid detection of resistance to anti-tuberculous drugs. Genome Med. 11:41. doi: 10.1186/s13073-019-0650-x
Price, M. N., Dehal, P. S., and Arkin, A. P. (2010). Fasttree 2--approximately maximum-likelihood trees for large alignments. PLoS One 5:e9490. doi: 10.1371/journal.pone.0009490
Prjibelski, A., Antipov, D., Meleshko, D., Lapidus, A., and Korobeynikov, A. (2020). Using SPAdes De novo assembler. Curr. Protoc. Bioinformatics 70:e102. doi: 10.1002/cpbi.102
Rahim, Z., Möllers, M., te Koppele-Vije, A., de Beer, J., Zaman, K., Matin, M. A., et al. (2007). Characterization of Mycobacterium africanum subtype I among cows in a dairy farm in Bangladesh using spoligotyping. Southeast Asian J. Trop. Med. Public Health 38, 706–713.
Riojas, M. A., McGough, K. J., Rider-Riojas, C. J., Rastogi, N., and Hazbón, M. H. (2018). Phylogenomic analysis of the species of the Mycobacterium tuberculosis complex demonstrates that Mycobacterium africanum, Mycobacterium bovis, Mycobacterium caprae, Mycobacterium microti and Mycobacterium pinnipedii are later heterotypic synonyms of Mycobacterium tuberculosis. Int. J. Syst. Evol. Microbiol. 68, 324–332. doi: 10.1099/ijsem.0.002507
Rüfenacht, S., Bögli-Stuber, K., Bodmer, T., Jaunin, V. F., Jmaa, D. C., and Gunn-Moore, D. A. (2011). Mycobacterium microti infection in the cat: a case report, literature review and recent clinical experience. J. Feline Med. Surg. 13, 195–204. doi: 10.1016/j.jfms.2011.01.012
Sanoussi, C. N., Coscolla, M., Ofori-Anyinam, B., Otchere, I. D., Antonio, M., Niemann, S., et al. (2021). Mycobacterium tuberculosis complex lineage 5 exhibits high levels of within-lineage genomic diversity and differing gene content compared to the type strain H37Rv. Microb. Genom. 7:000437. doi: 10.1099/mgen.0.000437
Sharma, A., Bloss, E., Heilig, C. M., and Click, E. S. (2016). Tuberculosis caused by Mycobacterium Africanum, United States, 2004–2013. Emerg. Infect. Dis. 22, 396–403. doi: 10.3201/eid2203.151505
Silva, M. L., Cá, B., Osório, N. S., Rodrigues, P. N. S., Maceiras, A. R., and Saraiva, M. (2022). Tuberculosis caused by Mycobacterium africanum: knowns and unknowns. PLoS Pathog. 18:e1010490. doi: 10.1371/journal.ppat.1010490
Silva-Pereira, T. T., Ikuta, C. Y., Zimpel, C. K., Camargo, N. C. S., de Souza Filho, A. F., Ferreira Neto, J. S., et al. (2019). Genome sequencing of Mycobacterium pinnipedii strains: genetic characterization and evidence of superinfection in a south American sea lion (Otaria flavescens). BMC Genomics 20:1030. doi: 10.1186/s12864-019-6407-5
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Sykes, J. E. (2025). Cutaneous Mycobacterioses of cats and dogs. Vet. Clin. North Am. Small Anim. Pract. 55, 237–249. doi: 10.1016/j.cvsm.2024.11.009
Thorel, M. F. (1980). Isolation of Mycobacterium africanum from monkeys. Tubercle 61, 101–104. doi: 10.1016/0041-3879(80)90018-5
Thorvaldsdóttir, H., Robinson, J. T., and Mesirov, J. P. (2013). Integrative genomics viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 14, 178–192. doi: 10.1093/bib/bbs017
Vågene, Å. J., Honap, T. P., Harkins, K. M., Rosenberg, M. S., Giffin, K., Cárdenas-Arroyo, F., et al. (2022). Geographically dispersed zoonotic tuberculosis in pre-contact south American human populations. Nat. Commun. 13:1195. doi: 10.1038/s41467-022-28562-8
Weber, A., Reischl, U., and Naumann, L. (1998). Nachweis von Mycobacterium africanum bei einem rind in Nordbayern [demonstration of Mycobacterium africanum in a bull from North Bavaria]. Berl. Munch. Tierarztl. Wochenschr. 111, 6–8. German
World Health Organization (2023). Catalogue of mutations in Mycobacterium tuberculosis complex and their association with drug resistance. 2nd Edn. Geneva: World Health Organization.
World Organisation for Animal Health (2022). “Chapter 3.1.13. Mammalian tuberculosis (infection with Mycobacterium tuberculosis complex)” in WOAH manual of diagnostic tests and vaccines for terrestrial animals (version adopted in May 2022). Available at: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.01.13_Mammalian_tuberculosis.pdf
Keywords: Mycobacterium africanum, tuberculosis, genomics, antimicrobial resistance, zoonosis, cat, Italy, whole-genome sequencing (WGS)
Citation: Alba P, Caprioli A, Cocumelli C, Eleni C, Galietta V, Giacomi A, Sorbara L, Stravino F, Feltrin F, Amoruso R, Ianzano A, Ceccaroni F, Frega M, Carfora V, Franco A and Battisti A (2025) First report of tuberculosis in a cat from Italy caused by Mycobacterium africanum, lineage 6: genomic characterization and phylogenetic analysis. Front. Microbiol. 16:1633110. doi: 10.3389/fmicb.2025.1633110
Edited by:
Svetlana Khaiboullina, University of Nevada, Reno, United StatesReviewed by:
Álvaro Chiner-Oms, Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana (FISABIO), SpainAndrea Monserrat Negrete Paz, Michoacán University of San Nicolás de Hidalgo, Mexico
Copyright © 2025 Alba, Caprioli, Cocumelli, Eleni, Galietta, Giacomi, Sorbara, Stravino, Feltrin, Amoruso, Ianzano, Ceccaroni, Frega, Carfora, Franco and Battisti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Caprioli, YW5kcmVhLmNhcHJpb2xpQGl6c2x0Lml0
 Patricia Alba
Patricia Alba Andrea Caprioli
Andrea Caprioli Cristiano Cocumelli
Cristiano Cocumelli Claudia Eleni
Claudia Eleni Valentina Galietta
Valentina Galietta Angelo Giacomi1
Angelo Giacomi1 Fiorentino Stravino
Fiorentino Stravino Fabiola Feltrin
Fabiola Feltrin Virginia Carfora
Virginia Carfora Alessia Franco
Alessia Franco Antonio Battisti
Antonio Battisti