- 1College of Animal Medicine, Shandong Agricultural University, Tai'an, Shandong, China
- 2Shandong Provincial Key Laboratory of Zoonotic Diseases, Jinan, Shandong, China
- 3Jinan Nursing Vocational College, Jinan, China
- 4Shandong Provincial Center for Animal Disease Provention and Control (Shandong Provincial Center for Zoonoses Epidemiology Investigation and Surveillance), Jinan, Shandong, China
- 5Beijing Animal Husbandry and Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Beijing, China
Brucellosis and tuberculosis are two zoonotic, chronic infectious diseases caused by bacteria of the genus Brucella and Mycobacterium, respectively, which pose significant hazards to both animal husbandry and human health. Currently, mixed infections of these two pathogens are prevalent in livestock production; thus, establishing a molecular diagnostic method for the simultaneous detection and analysis of brucellosis and tuberculosis is crucial for the prevention and control of these diseases. By utilizing conserved regions within the genomes of Brucella and Mycobacterium, we designed specific primers and probes. After optimizing the developed qPCR assay conditions, we determined the lower limit of detection to be ten copies/ μL. Cross-testing with other bovine-derived pathogens demonstrated no cross-reactivity. Repeatability tests indicated that the coefficient of variation for the developed qPCR assay was less than 4.10% both within and between batches. We employed both the developed qPCR assay and a commercial qPCR assay to analyze sixty mixed infection samples of Brucella and Mycobacterium from various regions. The results revealed positivity rates of 100% and 96.67% for Brucella, and 100% and 95.00% for Mycobacterium, respectively. These findings indicate that a highly sensitive, specific, reproducible, and versatile qPCR method has been developed for the simultaneous quantitative detection of Brucella and Mycobacterium, which can be applied in studying the pathogenesis and epidemiology of these pathogens.
1 Introduction
Brucellosis, caused by Brucella infection, is a zoonotic systemic infectious disease primarily responsible for abortion, retention of the fetal coat, stillbirths, and weak calves in ewes, as well as testicular and epididymitis and arthritis in bulls (Dawood et al., 2023; Yang et al., 2023). The bacterium was first identified and isolated by the Scottish pathologist and microbiologist (David, 1887). The disease often manifests as a chronic or latent infection that can be transmitted via aerosols and poses a potential biological threat (Wang et al., 2021). Tuberculosis is a chronic infectious disease affecting both humans and animals, caused by bacteria of the genus Mycobacterium, characterized by tuberculous nodular granulomas in tissues and organs, alongside necrotic foci of caseation and calcification (Ramos et al., 2020; Reis et al., 2021). This disease can occur year-round, with a higher incidence observed in housed cattle. Factors such as overcrowding, darkness, dampness, poor hygiene, excessive labor and milking, and inadequate nutrition can facilitate the occurrence and spread of the disease (Santos et al., 2020; Pozo et al., 2022). Dairy cows and buffaloes are particularly susceptible. Transmission primarily occurs through the respiratory and digestive tracts, but can also occur via the placenta or during mating (Santos et al., 2022). Tuberculosis-infected animals serve as the main source of infection, with the tuberculosis bacillus distributed throughout various organ foci (Jalili et al., 2020; Perez-Morote et al., 2020). Diseased animals can excrete pathogens through feces, milk, urine, and tracheal secretions, contaminating the surrounding environment and facilitating the spread of infection (Dorn-In et al., 2020; Barnes et al., 2023). Brucellosis and tuberculosis are critically important to public health concerning livestock products, as they not only jeopardize livestock production but also pose serious risks to human health (Martinez-Guijosa et al., 2020; Zai et al., 2021). Therefore, the detection of both pathogens is essential in the study of bovine brucellosis and Mycobacteria (Allen et al., 2021; Zhang et al., 2025).
Rapid detection methods are crucial for the effective control of brucellosis and Mycobacterium infections (Pereira et al., 2022; Qin et al., 2024). In recent years, a quantitative real-time PCR (qPCR) has been developed that facilitates the accurate and reproducible quantification of gene copies (Ginzinger, 2002). This method has been extensively utilized to quantify the genomic copies of pathogenic microorganisms. qPCR has emerged as a powerful alternative in microbiological diagnostics due to its simplicity, rapidity, reproducibility, and high sensitivity when compared to other diagnostic methods (Chen et al., 2020).
Various detection methods for Brucella and Mycobacterium have been documented in the literature, including PCR techniques targeting the Brucella Bcsp31 gene and the Mycobacterium pncA and RD1 genes (Abdel-Hamid et al., 2021). Mascarenhas et al. validated qPCR technology for detecting Mycobacterium bovis and Brucella abortus in raw cow's milk (Mascarenhas et al., 2020). While these methods can identify pathogenic factors associated with Brucella and Mycobacterium, they exhibit significant limitations due to the complex and chaotic infection dynamics of these pathogens (Pinto et al., 2024; Kurmanov et al., 2022). Specifically, existing detection methods are unable to simultaneously detect the pathogenic factors of both Brucella and Mycobacterium; they can only assess each pathogen individually (Gabbassov et al., 2021; Rerkyusuke et al., 2024). Consequently, designing a method for the simultaneous detection of pathogenic factors from both Brucella and Mycobacterium is of paramount importance for advancing research in this area.
This study aims to establish test methods for the rapid detection of brucellosis and tuberculosis in dairy cows, assess the contamination status of fresh milk, and trace transmission routes. To achieve this, we designed specific primers and probes targeting the conserved regions of the Brucella Omp25 and Mycobacterium Mpt83 genes, and developed a dual qPCR method utilizing TaqMan probes. This method will be widely used in the detection of Brucella and Mycobacterium, laying the foundation for disease diagnosis and epidemiological investigations.
2 Materials and methods
2.1 Strains and clinical samples
The strains of Brucella spp., Mycobacterium spp., Escherichia coli, Salmonella, Streptococcus, Staphylococcus, and Pasteurella multocida utilized in this study were preserved in the laboratories of the Centre for Prevention and Control of Animal Diseases in Shandong Province. Milk, blood, and vaginal swab samples from animals suspected of brucellosis and tuberculosis were collected at cattle farms in a specific region of China. Positive clinical samples were isolated, identified, and stored by the China Animal Health and Epidemiology Centre.
2.2 Specific primers and probes
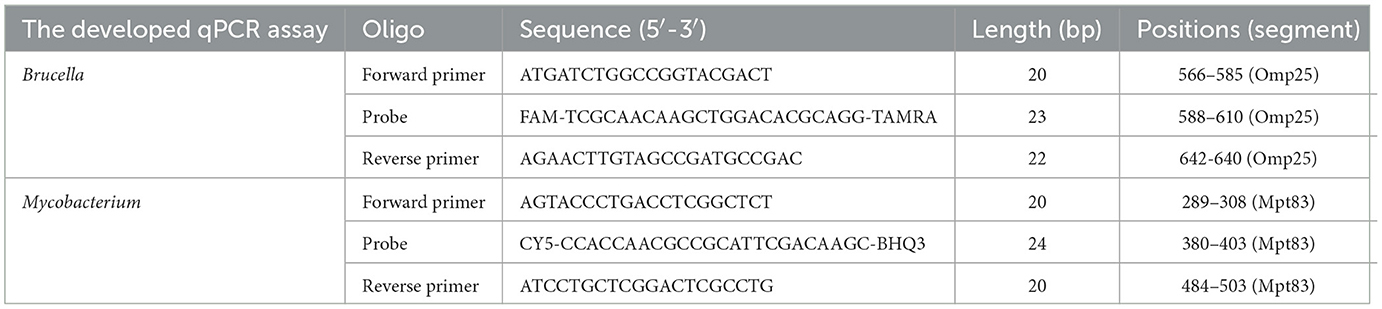
In this study, specific primers and probes were designed based on the conserved regions of the Brucella Omp25 gene and the Mycobacterium Mpt83 gene. The DNASTAR MegAlign software was used to align the Omp25 gene and the Mpt83 gene, respectively, to identify conserved regions and verify the specificity of the designed probes and primers.
2.3 DNA extraction and qPCR assay
Total DNA was extracted using the Bacterial Genomic DNA Extraction Kit (50 preps, Catalog DP302; TIANGEN, Beijing, China) following the manufacturer's instructions. All DNA templates were stored at −80°C until use. The developed qPCR assay was performed in a 25 μL reaction system (Gold Star Probe Mixture; CWBIO, CW0932M, Beijing, China), which included 12.5 μL of 2 × Gold Star Probe Mixture, 0.5 μL of Brucella forward primer (10 μM), 0.5 μL of Brucella reverse primer (10 μM), 0.5 μL of Mycobacterium forward primer (10 μM), 0.5 μL of Mycobacterium reverse primer (10 μM), 0.5 μL of Brucella probe (10 μM), 0.5 μL of Mycobacterium probe (10 μM), 7.5 μL of ddH2O, 1.0 μL of Brucella DNA template, and 1.0 μL of Mycobacterium DNA template. The developed qPCR assay was conducted using the Gentier 96 E/96 R Fully automated medical PCR analysis system. The reaction conditions included an initial denaturation at 95°C for 10 m, followed by forty cycles of denaturation at 95°C for 15 s and annealing/extension at 60°C for 1 m. During the extension step, fluorescent signals were collected.
2.4 Optimization of conditions for the developed qPCR assay
In this study, we optimized the concentrations of primers and TaqMan-probes, as well as the annealing temperature, to achieve the lowest Ct value with high fluorescence intensity. Six concentrations (0.1 M, 0.2 M, 0.4 M, 0.6 M, 0.8 M, and 1.0 M) suitable for primers and probes were tested, with three replicates for each concentration, while keeping other factors constant. After identifying the optimal concentrations of primers and TaqMan-probes, we evaluated five different annealing temperatures (56°C, 58°C, 60°C, 62°C, and 64 °C), again with three replicates for each temperature group. The optimal annealing temperature was selected based on the assay results.
2.5 Standard plasmid preparation, construct standard curves, and sensitivity
The Omp25 gene (85 bp) of Brucella was amplified using specific forward (Brucella-F) and reverse (Brucella-R) primers. Similarly, the Mpt83 gene (85 bp) of Mycobacterium was amplified using designated forward (Mycobacterium-F) and reverse (Mycobacterium-R) primers. The resulting PCR products were cloned into the plasmid vector pUC57 and subsequently verified through sequencing. The validated plasmids, pUC57-Omp25 and pUC57-Mpt83, were purified using the Rapid Plasmid Miniaturization Kit (TIANGEN, DP105, Beijing, China) and quantified with a De Novix DS-11 spectrophotometer. A 10-fold dilution of the plasmids, pUC57-Omp25 and pUC57-Mpt83, was prepared in 10 × Tris-EDTA buffer (pH 7.4) for the construction of a standard curve and to ascertain the detection limit of the developed qPCR assay.
2.6 Specificity analysis of the developed qPCR assay
In this study, the specificity of the developed qPCR assay was validated in triplicate using the following bovine bacterial species: B. abortus, M. bovis, E. coli, Salmonella, Streptococcus, Staphylococcus, and P. multocida.
2.7 Repeatability analysis of the developed qPCR assay
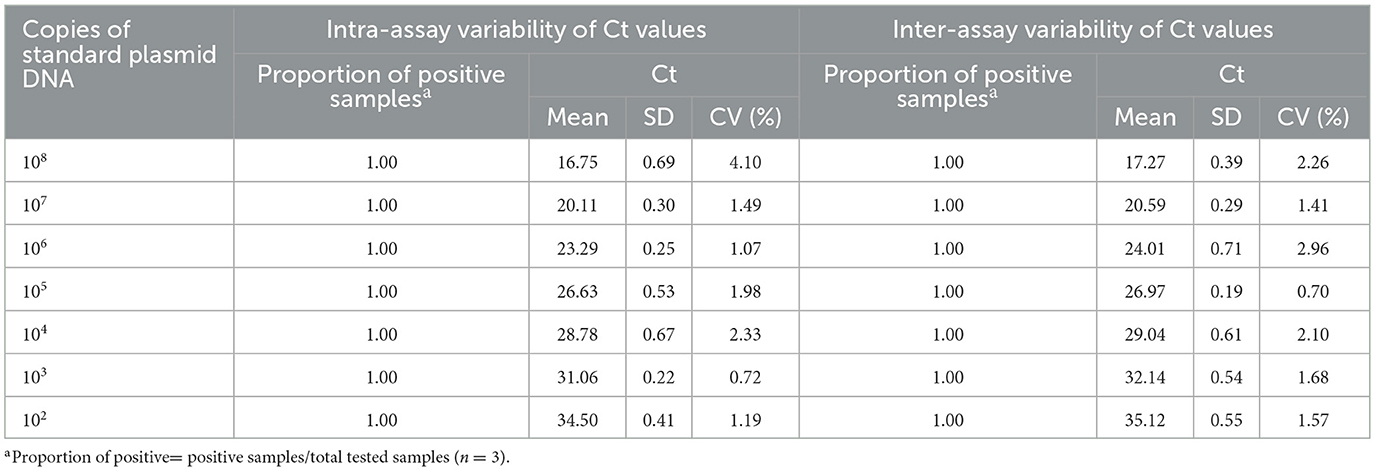
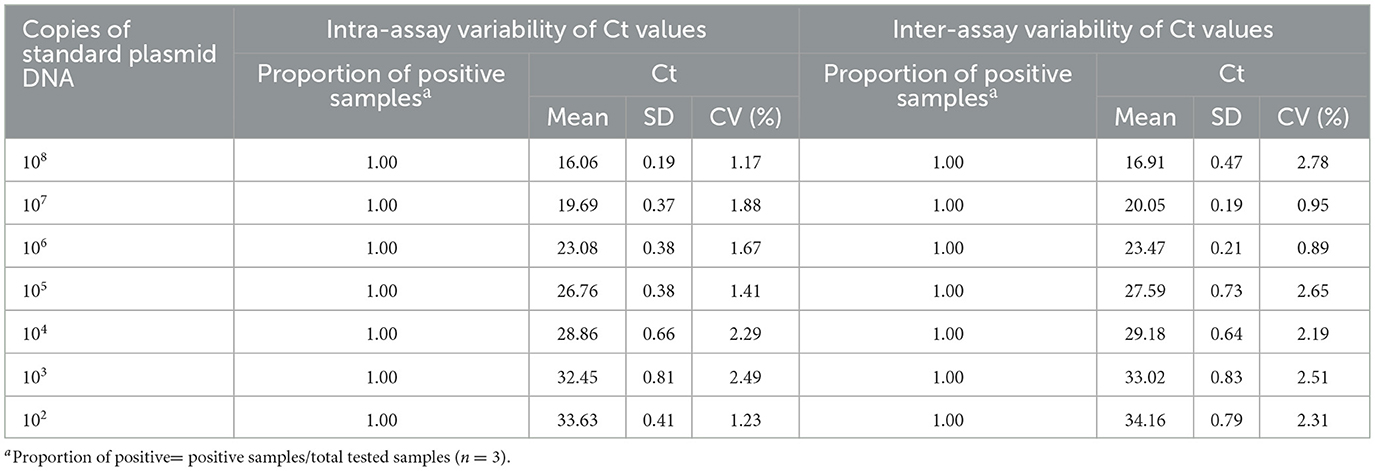
The dilutions of pUC57-Omp25 and pUC57-Mpt83, with concentrations ranging from 1.0 × 108 – 1.0 × 102 copies/μL, were assessed over three consecutive days, with each day's assay repeated three times. The reproducibility of the developed qPCR assay was evaluated by calculating the intra- and inter-batch Coefficients of Variation (CV) based on the assay results.
2.8 Clinical samples detection
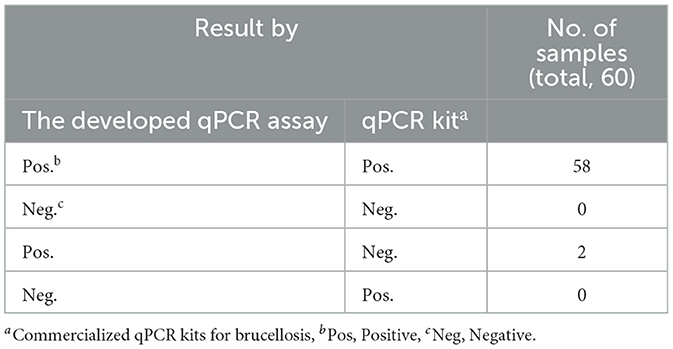
Sixty clinical samples suspected of Brucella and Mycobacterium infections were analyzed using the developed qPCR assay in this study and commercial kits. These samples were sourced from various regions, including Shandong, Inner Mongolia, and Henan. Sterile Phosphate-Buffered Saline (PBS) served as a control in the experiments.
2.9 Statistical analysis
Statistically significant differences in mean detection rates were determined using one-way ANOVA, conducted with Graph Pad Prism version 6. A p-value < 0.05 was considered significant, while a p-value < 0.01 was regarded as extremely significant.
3 Results
3.1 Primers and probes analysis
The probes and primers utilized in this study were specifically designed based on the Omp25 gene of Brucella and the Mpt83 gene of Mycobacterium, as detailed in Table 1. Sequence analysis confirmed that the primers and probes are situated within highly conserved regions of the Brucella Omp25 gene and the Mycobacterium Mpt83 gene, as illustrated in Figure 1. Notably, the primers and probes exhibit a perfect match with Brucella and Mycobacterium sequences, demonstrating 100% homology to these sequences.

Figure 1. Result of sequence analysis. The results show that the primers and probes are situated within highly conserved regions of the Brucella Omp25 gene and the Mycobacterium Mpt83 gene. (A) The Brucella Omp25 gene. (B) The Mycobacterium Mpt83 gene.
3.2 The developed qPCR assay
The concentrations of primers and probes that resulted in high fluorescence were established (Figures 2A-D). The optimized volume for the developed qPCR assay reaction was set at 25 μL, comprising 12.5 μL of 2 × Gold Star Probe Mixture, 0.5 μL of the Omp25 forward primer, 0.5 μL of the Omp25 reverse primer, 0.5 μL of the Omp25 probe, 0.5 μL of the Mpt83 forward primer, 0.5 μL of the Mpt83 reverse primer, 0.5 μL of the Mpt83 probe, 1.0 μL of the Omp25 DNA template, 1.0 μL of the Mpt83 DNA template, and 7.5 μL of ddH2O. Although acceptable amplification was observed at temperatures ranging from 56°C to 64°C, the optimal conditions for the developed qPCR assay were determined to be at 60°C (Figure 2E).
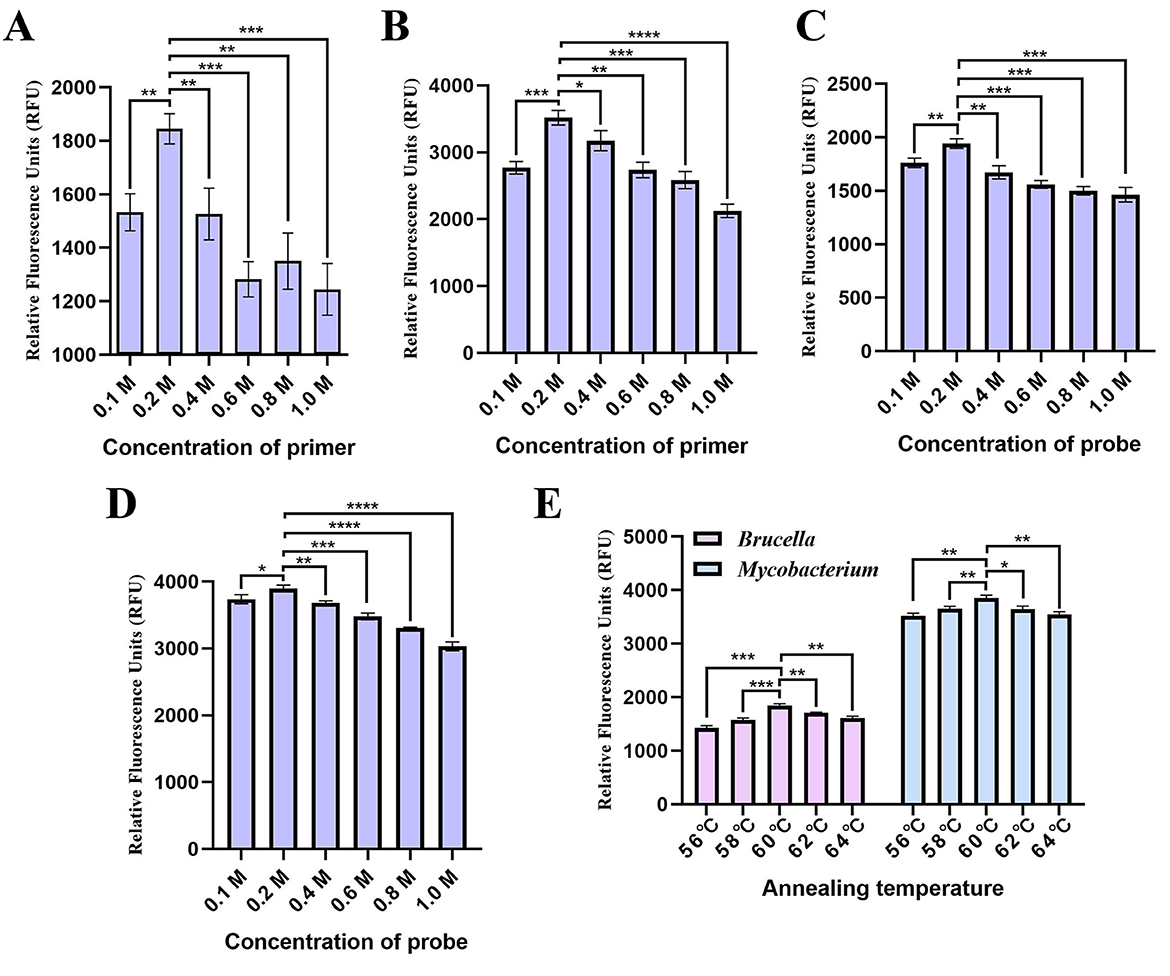
Figure 2. Optimization of the developed qPCR assay conditions. (A) Primer and (B) probe concentrations for the Brucella qPCR. (C) Primer and (D) probe concentrations for the Mycobacterium qPCR. (E) Annealing temperature.
3.3 Establishment of the standard curves
The triplicate standard curve plots demonstrate a linear correlation between the logarithm of the copy number and the cycle threshold (CT) values, as illustrated in Figure 3. The Brucella standard curve equation is given by Y = – 2.7405X + 39.531, where Y represents the threshold cycle and X denotes the logarithm of the standard. The linear correlation coefficient (R2) for this standard curve is 0.9941 (Figure 3A). Similarly, the Mycobacterium standard curve is represented by the equation Y = – 2.8543X + 39.642, with Y as the threshold cycle and X as the logarithm of the standard. The linear correlation coefficient (R2) for the Mycobacterium standard curve is 0.9915 (Figure 3B).
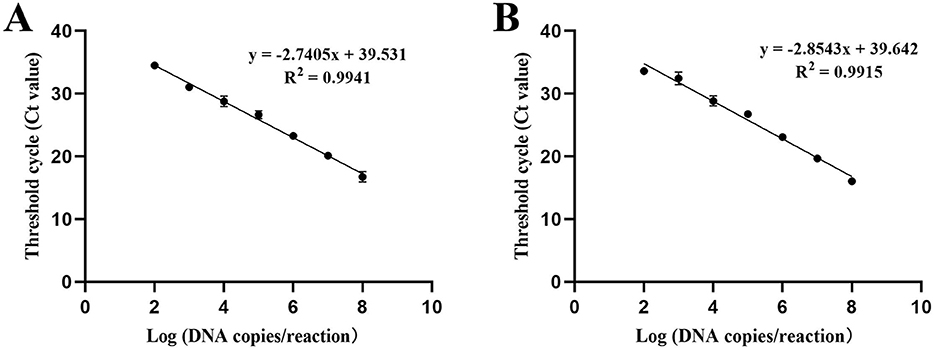
Figure 3. Standard curve of the the developed qPCR assay. The triplicate standard curve plots indicate a linear correlation between the Log of the copy number and the Cycle Threshold Value (CT). The logarithm values of the detected concentrations of the DNA standards (X axis) ranged from 1.0 × 108 to 1.0 × 102 copies/μ L, and used the corresponding Threshold cycle (CT value) of each reaction tube fluorescent signal approaching the set threshold (Y axis) of the amplification to perform linear regression. Three replicates were tested for each dilution. (A) Standard curve of Brucella. (B) Standard curve of Mycobacterium.
3.4 Sensitivity of the developed qPCR assay reaction
The sensitivity of the developed qPCR assay was evaluated by diluting the DNA standard plasmid from 1.0 × 109 copies/ μL to 1.0 × 100 copies/ μL. The results indicated that the assay's lowest limit of detection was 1.0 × 10 copies/ μL (Figure 4).
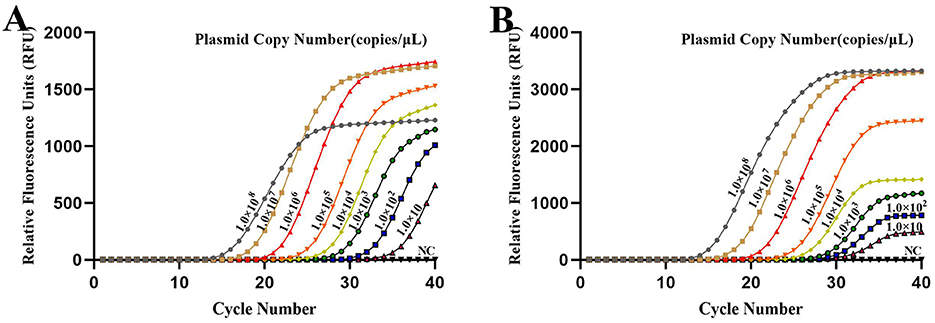
Figure 4. Sensitivity of the developed qPCR assay targeting (A) Brucella and (B) Mycobacterium. Sensitivity was assessed using serial dilutions of DNA standard plasmids, with a limit of detection of 1.0 × 10 copies/μL. NC, Nuclease-free water.
3.5 Specificity of the developed qPCR assay reaction
In this study, five distinct bovine pathogens were utilized to evaluate the specificity of the developed qPCR assay detection. PBS served as the negative control. Strong fluorescent signals were observed in reactions involving Brucella and Mycobacterium, while the signals from the other three pathogen samples and the PBS control remained at baseline levels under the optimized reaction conditions. Consequently, Brucella and Mycobacterium were effectively distinguished from the other pathogens based on the variation in signal intensity (Figure 5).
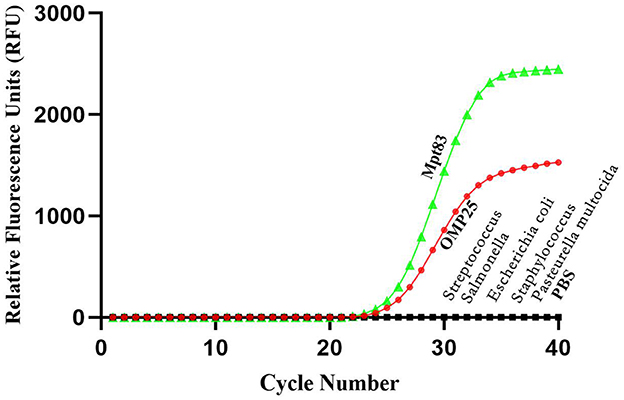
Figure 5. Specificity test results of real-time PCR assay using different strains. The results showed that this method can effectively distinguish Brucella and Mycobacterium from other pathogens.
3.6 Repeatability of the developed qPCR assay
The intra- and inter-assay reproducibility was assessed in triplicate over three different days using 10-fold serial dilutions of standard plasmid DNA, ranging from 1.0 × 108-1.0 × 102 copies. The results indicated that the intra-assay and inter-assay Coefficients of Variation (CV) for the Brucella qPCR method were 0.72 to 4.10 and 0.70 to 2.96, respectively (Table 2). Similarly, the intra-assay and inter-assay CVs for the Mycobacterium qPCR method were 1.17 to 2.49 and 0.89 to 2.78, respectively (Table 3). These findings suggest that the developed qPCR assay exhibits high reproducibility.
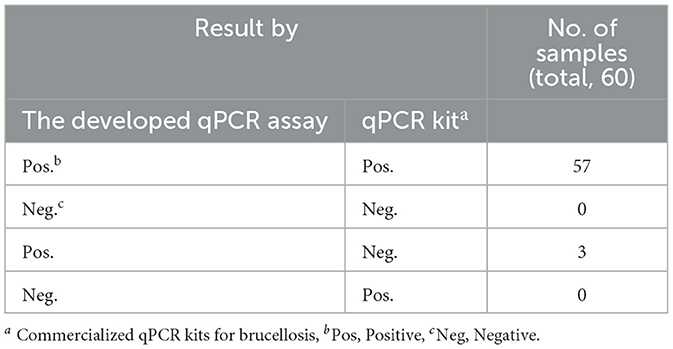
3.7 Clinical sample application
Sixty clinical samples (milk, blood, vaginal swabs) from animals co infected with Brucella and Mycobacterium from various regions of China, were evaluated using both the developed qPCR assay and commercial qPCR kits. The prevalence rates of brucellosis and Mycobacterium were found to be 96.67% and 95%, respectively, according to the commercial qPCR kits. In contrast, the prevalence rates determined by the developed qPCR assay were 100% for both brucellosis and Mycobacterium (Tables 4, 5). These findings suggest that the developed qPCR assay exhibits superior detection accuracy.
4 Discussion
Brucellosis is a zoonotic infection caused by the gram-negative, partially intracellular bacterium Brucella (Okafor et al., 2022). This pathogen infects a wide range of hosts, including cattle, sheep, pigs, and other mammals (Khairullah et al., 2024). The primary manifestations of brucellosis in livestock include undulant fever, infertility, abortion, arthritis, and orchitis (Selim et al., 2019). In contrast, tuberculosis is a chronic zoonosis caused by Mycobacterium, characterized by progressive wasting, the formation of tuberculous nodules, and caseous necrotic foci in various tissues and organs (Lu et al., 2022; Rossi et al., 2020). Epidemiological investigations have revealed a cross-infection between Brucella and Mycobacterium, resulting in decreased milk production, lower annual calving rates, and reduced meat production (Loiseau et al., 2019; Bonilla-Aldana et al., 2023; Pellegrini et al., 2022). These factors lead to significant economic losses in the cattle industry and severely impact the development of the animal husbandry sector.
Currently, there is no efficient method for the simultaneous detection of Brucella and Mycobacterium; therefore, there is a significant need for a rapid, highly sensitive, and specific method for their simultaneous detection in both the bovine industry and the research community (Triguero-Ocana et al., 2020; Khoshnood et al., 2022; Zeineldin et al., 2023; Zheng et al., 2024). In this study, we established a dual TaqMan-based real-time PCR assay targeting the Omp25 gene of Brucella and the Mpt83 gene of Mycobacterium. Verified through a series of experiments, the developed qPCR assay demonstrates high sensitivity, specificity, and reproducibility. The limit of detection for both Brucella and Mycobacterium was determined to be 1.0 × 10 copies/ μL. The developed qPCR assay yielded a strong fluorescent signal exclusively for Brucella and Mycobacterium, with intra-assay and inter-assay variability measured at less than 4.10% and 2.78%, respectively. Sixty clinical samples (milk, blood, vaginal swabs) from animals co infected with Brucella and Mycobacterium from various regions of China were tested using the developed qPCR assay and commercial qPCR assays. The positivity rate for the developed qPCR assay test for infection was 100%, while the positivity rates for the commercial qPCR tests were 96.67% for Brucella and 95.00% for Mycobacterium, indicating that the sensitivity of the former is superior. The high sensitivity demonstrated by the developed qPCR assay in this study is significant in low-load infections, where there is a risk of false negative results. However, the use of the developed qPCR assay requires certain experimental conditions, which limits their applicability for on-site detection of pathogens. In addition, this study did not set up an Internal Amplification Control (IAC), so it cannot be ensured that the master mix was in an optimal amplification state and that there were no inhibitors in the reaction, which is a limitation.
In conclusion, we have clearly established and validated the developed qPCR assay for the quantification of Brucella and Mycobacterium due to its remarkable sensitivity, reproducibility, rapidity, versatility, and high-throughput potential compared to other diagnostic methods. This detection method has low intra- and inter-assay variability and does not show cross-reactivity with other animal bacteria. Furthermore, its sensitivity surpassed that of commercial qPCR assays. These findings suggest that the assay can be utilized to simultaneously quantify Brucella and Mycobacterium DNA from various regions, thereby contributing to epidemiological investigations of animals infected with Brucella and Mycobacterium.
5 Conclusion
The developed qPCR assay in this study serves as a reliable tool for the rapid and simultaneous detection of Brucella and Mycobacterium in clinical samples. This method will be beneficial for epidemiological investigations and outbreak surveillance of both Brucella and Mycobacterium.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was approved by Shandong Agricultural University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SZ: Writing – original draft, Visualization, Investigation. HZ: Writing – original draft, Investigation. QG: Formal analysis, Writing – original draft. RX: Investigation, Writing – original draft. ZJ: Investigation, Writing – original draft. WJ: Writing – original draft, Investigation. LX: Investigation, Writing – original draft. XW: Writing – original draft, Investigation. YD: Writing – review & editing. YT: Writing – review & editing, Investigation. ZL: Writing – review & editing, Investigation. YZ: Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Shandong Province Key R& D Programme (2022CXGC020711-1, 2022CXGC020711-3), Shandong Postdoctoral Innovation Project (SDCX-ZG-202400115), and National Key Research and Development Program of China (2025YFD1800062).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Hamid, N. H., Beleta, E. I. M., Kelany, M. A., Ismail, R. I., and Shalaby, N. A. (2021). Validation of real-time polymerase chain reaction versus conventional polymerase chain reaction for diagnosis of brucellosis in cattle sera. Vet World. 14, 144–154. doi: 10.14202/vetworld.2021.144-154
Allen, A. R., Ford, T., and Skuce, R. A. (2021). Does Mycobacterium spp. Tuberculosis var. bovis survival in the environment confound bovine tuberculosis control and eradication? A literature review. Vet. Med. Int. 2021:8812898. doi: 10.1155/2021/8812898
Barnes, A. P., Moxey, A., Brocklehurst, S., Barratt, A., McKendrick, I. J., Innocent, G., et al. (2023). The consequential costs of bovine tuberculosis (btb) breakdowns in England and Wales. Prev. Vet. Med. 211:105808. doi: 10.1016/j.prevetmed.2022.105808
Bonilla-Aldana, D. K., Trejos-Mendoza, A. E., Perez-Vargas, S., Rivera-Casas, E., Munoz-Lara, F., Zambrano, L. I., et al. (2023). A systematic review and meta-analysis of bovine brucellosis seroprevalence in Latin America and the Caribbean. New Microbes New Infect. 54:101168. doi: 10.1016/j.nmni.2023.101168
Chen, G., Seukep, A. J., and Guo, M. (2020). Recent advances in molecular docking for the research and discovery of potential marine drugs. Mar. Drugs 18:545. doi: 10.3390/md18110545
David, B. (1887). On the epidemic of Cholera in Malta during 1887. Trans. Epidemiol. Soc. Lond. 1889, 8,1–19.
Dawood, A. S., Elrashedy, A., Nayel, M., Salama, A., Guo, A., Zhao, G., et al. (2023). Brucella spp. as resilient intracellular pathogens: epidemiology, host-pathogen interaction, recent genomics and proteomics approaches, and future perspectives. Front. Vet. Sci. 10:1255239. doi: 10.3389/fvets.2023.1255239
Dorn-In, S., Korner, T., Buttner, M., Hafner-Marx, A., Muller, M., Heurich, M., et al. (2020). Shedding of Mycobacterium spp. caprae by wild red deer (Cervus elaphus) in the bavarian alpine regions, Germany. Transbound. Emerg. Dis. 67, 308–317. doi: 10.1111/tbed.13353
Gabbassov, E., Moreno-Molina, M., Comas, I., Libbrecht, M., and Chindelevitch, L. (2021). Splitstrains, a tool to identify and separate mixed Mycobacterium spp. tuberculosis infections from wgs data. Microb. Genom. 7:000607. doi: 10.1099/mgen.0.000607
Ginzinger, D. G. (2002). Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp. Hematol. 30, 503–512. doi: 10.1016/S0301-472X(02)00806-8
Jalili, V., Afgan, E., Gu, Q., Clements, D., Blankenberg, D., Goecks, J., et al. (2020). The galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2020 update. Nucleic Acids Res. 48, W395–W402. doi: 10.1093/nar/gkaa554
Khairullah, A. R., Kurniawan, S. C., Puspitasari, Y., Aryaloka, S., Silaen, O., Yanestria, S. M., et al. (2024). Brucellosis: unveiling the complexities of a pervasive zoonotic disease and its global impacts. Open Vet. J. 14, 1081–1097. doi: 10.5455/OVJ.2024.v14.i5.1
Khoshnood, S., Pakzad, R., Koupaei, M., Shirani, M., Araghi, A., Irani, G. M., et al. (2022). Prevalence, diagnosis, and manifestations of brucellosis: a systematic review and meta-analysis. Front. Vet. Sci. 9:976215. doi: 10.3389/fvets.2022.976215
Kurmanov, B., Zincke, D., Su, W., Hadfield, T. L., Aikimbayev, A., Karibayev, T., et al. (2022). Assays for identification and differentiation of Brucella spp. : a review. Microorganisms 10:1584. doi: 10.3390/microorganisms10081584
Loiseau, C., Brites, D., Moser, I., Coll, F., Pourcel, C., Robbe-Austerman, S., et al. (2019). Revised interpretation of the hain lifescience genotype mtbc to differentiate Mycobacterium spp. canettii and members of the Mycobacterium spp. tuberculosis complex. Antimicrob. Agents Chemother. 63, e00159–19. doi: 10.1128/AAC.00159-19
Lu, J., Rincon, N., Wood, D. E., Breitwieser, F. P., Pockrandt, C., Langmead, B., et al. (2022). Metagenome analysis using the kraken software suite. Nat. Protoc. 17, 2815–2839. doi: 10.1038/s41596-022-00738-y
Martinez-Guijosa, J., Romero, B., Infantes-Lorenzo, J. A., Diez, E., Boadella, M., Balseiro, A., et al. (2020). Environmental DNA: a promising factor for tuberculosis risk assessment in multi-host settings. PLoS ONE 15:e233837. doi: 10.1371/journal.pone.0233837
Mascarenhas, D. R., Schwarz, D. G. G., Fonseca, J. A. A., Oliveira, T. F. P., and Moreira, M. A. S. (2020).Validation of real-time PCR technique for detection of Mycobacterium spp. bovis and Brucella spp. abortus in bovine raw milk. Braz. J. Microbiol. 51, 2095–2100. doi: 10.1007/s42770-020-00319-9
Okafor, S. C., Ogugua, A. J., Ihedioha, J. I., Onunkwo, J. I., Ezenduka, E. V., Okafor, U. C., et al. (2022). Seroprevalence, hematological and biochemical alterations in Brucella spp.-seropositive muturu cattle in nigeria. Vet. Res. Commun. 46, 517–526. doi: 10.1007/s11259-021-09879-z
Pellegrini, J. M., Gorvel, J. P., and Memet, S. (2022). Immunosuppressive mechanisms in brucellosis in light of chronic bacterial diseases. Microorganisms 10:1260. doi: 10.3390/microorganisms10071260
Pereira, A. C., Tenreiro, A., Tenreiro, R., and Cunha, M. V. (2022). Stalking Mycobacterium spp. bovis in the total environment: flow-fish and facs to detect, quantify, and sort metabolically active and quiescent cells in complex matrices. J. Hazard. Mater. 432:128687. doi: 10.1016/j.jhazmat.2022.128687
Perez-Morote, R., Pontones-Rosa, C., Gortazar-Schmidt, C., and Munoz-Cardona, A. I. (2020). Quantifying the economic impact of bovine tuberculosis on livestock farms in south-western Spain. Animals 10:2433. doi: 10.3390/ani10122433
Pinto, D., Themudo, G., Pereira, A. C., Botelho, A., and Cunha, M. V. (2024). Rescue of Mycobacterium spp. bovis DNA obtained from cultured samples during official surveillance of animal tb: key steps for robust whole genome sequence data generation. Int. J. Mol. Sci. 25:3869. doi: 10.3390/ijms25073869
Pozo, P., Lorente-Leal, V., Robbe-Austerman, S., Hicks, J., Stuber, T., Bezos, J., et al. (2022). Use of whole-genome sequencing to unravel the genetic diversity of a prevalent Mycobacterium spp. bovis spoligotype in a multi-host scenario in Spain. Front. Microbiol. 13:915843. doi: 10.3389/fmicb.2022.915843
Qin, Y., Zhou, G., Jiao, F., Cheng, C., Meng, C., Wang, L., et al. (2024). Brucella spp. mediates autophagy, inflammation, and apoptosis to escape host killing. Front. Cell Infect. Microbiol. 14:1408407. doi: 10.3389/fcimb.2024.1408407
Ramos, B., Pereira, A. C., Reis, A. C., and Cunha, M. V. (2020). Estimates of the global and continental burden of animal tuberculosis in key livestock species worldwide: a meta-analysis study. One Health 10:100169. doi: 10.1016/j.onehlt.2020.100169
Reis, A. C., Ramos, B., Pereira, A. C., and Cunha, M. V. (2021). The hard numbers of tuberculosis epidemiology in wildlife: a meta-regression and systematic review. Transbound. Emerg. Dis. 68, 3257–3276. doi: 10.1111/tbed.13948
Rerkyusuke, S., Lerk-U-Suke, S., Sukon, P., and Phuektes, P. (2024). Serological and molecular prevalence and associated risk factors in caprine brucellosis, northeastern Thailand. Vet. Med. Int. 2024:9966352. doi: 10.1155/2024/9966352
Rossi, G., Crispell, J., Balaz, D., Lycett, S. J., Benton, C. H., Delahay, R. J., et al. (2020). Identifying likely transmissions in Mycobacterium spp. bovis infected populations of cattle and badgers using the kolmogorov forward equations. Sci. Rep. 10:21980. doi: 10.1038/s41598-020-78900-3
Santos, N., Colino, E. F., Arnal, M. C., de Luco, D. F., Sevilla, I., Garrido, J. M., et al. (2022). Complementary roles of wild boar and red deer to animal tuberculosis maintenance in multi-host communities. Epidemics Neth. 41:100633. doi: 10.1016/j.epidem.2022.100633
Santos, N., Richomme, C., Nunes, T., Vicente, J., Alves, P. C., de la Fuente, J., et al. (2020). Quantification of the animal tuberculosis multi-host community offers insights for control. Pathogens 9:421. doi: 10.3390/pathogens9060421
Selim, A., Attia, K., Ramadan, E., Hafez, Y. M., and Salman, A. (2019). Seroprevalence and molecular characterization of Brucella spp. in naturally infected cattle and sheep. Prev. Vet. Med. 171:104756. doi: 10.1016/j.prevetmed.2019.104756
Triguero-Ocana, R., Martinez-Lopez, B., Vicente, J., Barasona, J. A., Martinez-Guijosa, J., and Acevedo, P. (2020). Dynamic network of interactions in the wildlife-livestock interface in mediterranean spain: an epidemiological point of view. Pathogens 9:120. doi: 10.3390/pathogens9020120
Wang, Y., Wang, Y., Zhang, L., Wang, A., Yan, Y., Chen, Y., et al. (2021). An epidemiological study of brucellosis on mainland china during 2004-2018. Transbound. Emerg. Dis. 68, 2353–2363. doi: 10.1111/tbed.13896
Yang, Y., Qiao, K., Yu, Y., Zong, Y., Liu, C., and Li, Y. (2023). Unravelling potential biomarkers for acute and chronic brucellosis through proteomic and bioinformatic approaches. Front. Cell Infect. Microbiol. 13:1216176. doi: 10.3389/fcimb.2023.1216176
Zai, X., Yin, Y., Guo, F., Yang, Q., Li, R., Li, Y., et al. (2021). Screening of potential vaccine candidates against pathogenic Brucella spp. Using compositive reverse vaccinology. Vet. Res. 52:75. doi: 10.1186/s13567-021-00939-5
Zeineldin, M., Camp, P., Farrell, D., Lehman, K., and Thacker, T. (2023). Whole genome sequencing of Mycobacterium spp. bovis directly from clinical tissue samples without culture. Front. Microbiol. 14:1141651. doi: 10.3389/fmicb.2023.1141651
Zhang, M., Qi, L., Li, J., Yuan, N., Zhai, Y., Hao, M., et al. (2025). Sirt2 inhibition enhances mitochondrial apoptosis in Brucella spp.-infected bovine placental trophoblast cells. Vet. Res. 56:97. doi: 10.1186/s13567-025-01518-8
Keywords: Brucella, Mycobacterium, real-time PCR, TaqMan probe, simultaneous detection
Citation: Zhang S, Zhao H, Guo Q, Xue R, Jiang Z, Jiang W, Xing L, Wei X, Diao Y, Tang Y, Lan Z and Zhang Y (2025) Establishment and application of a TaqMan-based quantitative PCR assay for simultaneous detection of bovine Brucella spp. and Mycobacterium spp. Front. Microbiol. 16:1633809. doi: 10.3389/fmicb.2025.1633809
Received: 26 May 2025; Accepted: 31 July 2025;
Published: 12 September 2025.
Edited by:
Xiaoli Qin, Hunan Agricultural University, ChinaReviewed by:
Lucia Herrán Ramírez, Federal Rural University of Rio de Janeiro, BrazilLerato Mabe, Council for Scientific and Industrial Research (CSIR), South Africa
Copyright © 2025 Zhang, Zhao, Guo, Xue, Jiang, Jiang, Xing, Wei, Diao, Tang, Lan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Tang, dHljazI4OEAxNjMuY29t; Zouran Lan, TGFuenJqbkAxNjMuY29t; Yue Zhang, c2RjYWRjX3p5QDE2My5jb20=
 Shuai Zhang
Shuai Zhang Hui Zhao1,2,4
Hui Zhao1,2,4 Ruixue Xue
Ruixue Xue Youxiang Diao
Youxiang Diao Yi Tang
Yi Tang