- 1School of Laboratory Medicine, Jilin Medical University, Jilin, China
- 2General Surgery, Jilin People’s Hospital, Jilin, China
- 3Medical Image Center, Jilin Central Hospital, Jilin, China
- 4Department of Biomedical Sciences, Cedars-Sinai Medical Center, Los Angeles, CA, United States
- 5Center for Human Genetics and Genomics, New York University Grossman School of Medicine, New York, NY, United States
Legionella pneumophila, a Gram-negative bacillus, is the primary etiological agent of Legionnaires’ disease, a severe respiratory infection. The symbiotic relationship between L. pneumophila and free-living amoebae (FLAs), particularly Acanthamoeba spp., represents a critical intersection of microbial ecology and human pathogenesis. This symbiosis provides Legionella with a protective intracellular niche, enhancing its resistance to biocides, increasing its pathogenicity, and facilitating horizontal gene transfer. These interactions not only boost the environmental persistence and dissemination of L. pneumophila but also elevate the risk of human exposure through contaminated drinking water systems. This review delves into the sophisticated survival strategies employed by L. pneumophila within host cells, including evasion of endocytic pathways, inhibition of phagosome maturation and acidification, and prevention of phagosome-lysosome fusion. By elucidating these mechanisms, we underscore the critical need for in-depth research into the Legionella-amoebae symbiosis and its broader implications for public health. Additionally, we address the challenges and strategies for mitigating environmental risks, emphasizing the importance of innovative approaches to ensure water system safety and prevent pathogen transmission.
1 Introduction
Symbiosis exemplifies coexistence and coevolutionary trajectories across diverse life forms. These interactions, particularly between prokaryotes and environmental protists, are predominantly driven by nutritional exchanges with three main classes: mutualistic, commensalistic, and parasitic. However, the seminal work of Lynn Margulis in 1967 proposed the sophisticated endosymbiotic theory of organelle origins for mitochondria and chloroplasts (Sagan, 1967), which catalyzed a paradigm shift in understanding symbiotic relationships between unicellular eukaryotes and prokaryotes. Endosymbiosis, where one organism resides within another, is prevalent between protists and bacteria, underpinning adaptive evolution across multiple biological lineages (Nowack and Melkonian, 2010; Wernegreen, 2012). Such relationships facilitate niche adaptation and ecological diversification. Notably, free-living amoebae (FLAs) have emerged as critical hosts for a spectrum of microorganisms, including bacteria, viruses, and even other eukaryotes. These interactions confer advantages such as predation resistance, ecological resilience, and enhanced intracellular survival and proliferation.
The evolutionary interplay between protists and bacteria has been a cornerstone of research, with horizontal gene transfer from bacteria to protist hosts profoundly shaping protist genomes (López-García et al., 2017). While the evolutionary significance of prokaryote-eukaryote endosymbiosis is well-documented, its implications for human health remain underexplored. A quintessential example of such symbiosis is the relationship between Legionella pneumophila, a respiratory pathogen, and FLAs, a group of opportunistic pathogens. Their symbiosis is characterized by widespread environmental distribution from natural water sources to man-made water system with unique lifestyle adaptations befitting such extremes, which underscores the intricate dynamics of their unicellular eukaryote-bacteria interactions.
1.1 L. pneumophila
L. pneumophila belongs to the genus Legionella and is the primary pathogen responsible for severe pulmonary inflammation known as Legionnaires’ disease. It is also the most common pathogenic species within this genus. As a Gram-negative bacillus, L. pneumophila is characterized by its facultative intracellular parasitism (Cunha et al., 2016). This bacterium is widely distributed in nature and can be isolated from both soil and various aquatic environments. Notably, freshwater systems (Zhan and Zhu, 2018; Fliermans, 1996; van Heijnsbergen et al., 2015) and man-made water supply systems (van Heijnsbergen et al., 2015; Colbourne and Dennis, 1985; Wadowsky et al., 1982) often serve as its primary habitats. L. pneumophila exhibits remarkable adaptability, capable of surviving within a broad temperature range of 6°C to 63°C (Fliermans et al., 1981). Research has shown that the high-temperature environment of hot springs provides ideal conditions for its growth (Hsu et al., 2011; Ji et al., 2014; Rasch et al., 2016). This finding may explain why, compared to natural water systems, man-made water systems, with their higher average water temperatures (Ikedo and Yabuuchi, 1986; Fields et al., 2002; Lasheras et al., 2006), tend to support a greater abundance of these bacteria (Yamamoto et al., 1992).
L. pneumophila can exist as planktonic cells but predominantly colonizes biofilms under fluctuating and nutrient-poor conditions to acquire essential nutrients for survival and growth (Mampel et al., 2006; Singh and Coogan, 2005; Declerck et al., 2009; Declerck et al., 2007b). This pathogen integrates into existing biofilm structures (Lau and Ashbolt, 2009; Stewart et al., 2012) and establishes cooperative relationships with other biofilm-associated bacteria and cyanobacteria to enhance nutrient acquisition (Stewart et al., 2012; Tison et al., 1980; Bohach and Snyder, 1983; Stout et al., 1986; Koide et al., 2014; Pope et al., 1982). Remarkably, L. pneumophila employs a unique survival strategy by utilizing breakdown products from dead cell aggregates to sustain its metabolic activities in nutrient-scarce environments (Temmerman et al., 2006). While interactions with other bacteria facilitate survival in nutrient-deficient settings, proliferation within protozoan hosts remains a primary mode of its replication in natural habitats (Rowbotham, 1980).
The discovery of L. pneumophila traces back to the 1976 outbreak of Legionnaires’ disease among veterans attending a convention in Philadelphia, USA. Over 200 attendees fell ill, and 29 died, including bystanders outside the hotel (Fraser et al., 1977). Patients exhibited pneumonia-like symptoms, such as cough, headache, and high fever, with severe cases requiring prolonged hospitalization. This outbreak underscored the potential of water systems as reservoirs for respiratory pathogens (Fraser et al., 1977; McDade et al., 1977). Subsequent studies confirmed that infection occurs through inhalation of contaminated aerosols, with L. pneumophila invading lung macrophages and replicating intracellularly, leading to pneumonia (Steinert et al., 2007). Approximately 90% of Legionaries’ disease infections are attributed to L. pneumophila (Miyashita et al., 2020), with immunocompromised and elderly populations at heightened risk of severe outcomes, including respiratory failure (Fields et al., 2002; Gomez-Valero et al., 2009). In contrast, healthy individuals typically mount effective innate immune responses to control infection (Falcó et al., 1991; Shin, 2012).
Legionnaires’ disease exhibits seasonal peaks during summer and fall and is transmitted exclusively through airborne routes, with no evidence of human-to-human transmission (Burillo et al., 2017). Clinically, it presents in two forms: Pontiac fever, a mild flu-like illness that resolves spontaneously, and severe atypical pneumonia, characterized by acute respiratory symptoms, multi-organ damage, and high mortality if left untreated (Cunha et al., 2016). The incidence of Legionnaires’ disease has risen globally, with reported cases in the United States increasing ninefold from 2000 to 2018 (Yu et al., 2024), and doubling in Europe between 2010 and 2019 (Network, The European Centre for Disease Prevention and Control, 2021). Approximately 10–25% of cases result in fatalities, particularly among the elderly and immunocompromised individuals (World Health Organization, 2016). Healthcare-associated outbreaks are notably severe, with mortality rates reaching as high as 50% (Gomez-Valero et al., 2011; Lin et al., 2011; Marrie, 2008; Blatt et al., 1993; Sabria and Yu, 2002). Overall, mortality rates range from 10 to 25% (Yu et al., 2024), with higher rates observed in high-risk populations such as immunocompromised patients (Phin et al., 2014).
1.2 FLAs
FLAs are a diverse group of heterotrophic protists ubiquitously distributed in natural environments. Taxonomically, they span multiple phylogenetic lineages (Otero-Ruiz et al., 2022; Pazoki et al., 2020). Pathogenic genera affecting humans and animals include Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia pedata (Schuster and Visvesvara, 2004; Visvesvara et al., 2007), with Vermamoeba vermiformis also recognized as an emerging pathogenic free-living amoeba (FLA) capable of causing human infections (Scheid, 2019). Notably, Dictyostelium discoideum exhibits typical FLA characteristics (e.g., trophozoite morphology and phagocytic activity) while serving as a unique ‘social amoeba’ model. Its distinctive life cycle, which transitions from unicellular individuals to multicellular fruiting bodies under starvation, makes it invaluable for studying cell differentiation, signal transduction, and host-pathogen interactions (Bozzaro et al., 2019; Swart et al., 2018).
FLAs share a biphasic life cycle: (1) a metabolically active trophozoite stage that feeds, divides, and moves via pseudopodia; and (2) a dormant cyst stage resistant to environmental stressors such as nutrient deprivation, extreme pH, osmotic fluctuations, and temperature variations (Marciano-Cabral, 2009; Bellini et al., 2022). These organisms pose significant public health risks through both opportunistic and non-opportunistic infections. Central nervous system (CNS) infections include primary amoebic meningoencephalitis (PAM), an acute condition caused by N. fowleri (Güémez and García, 2021; Gharpure et al., 2021), and granulomatous amoebic encephalitis (GAE), a subacute-to-chronic disease induced by Acanthamoeba spp. and B. mandrillaris (Spottiswoode et al., 2024; Lee et al., 2020). The latter two pathogens are also implicated in cutaneous lesions and disseminated infections, primarily affecting immunocompromised individuals (Cabello-Vílchez and Ruiz-Ruiz, 2024; Maehara et al., 2022). Ocular infections include Acanthamoeba keratitis (AK), a progressive sight-threatening corneal disease (Garg and Daigavane, 2024; Petrillo et al., 2024), while S. pedata and V. vermiformis are rarely reported in encephalitis and keratitis cases, respectively (da Rocha-Azevedo et al., 2009; Qvarnstrom et al., 2009; Scheid, 2019).
Beyond direct pathogenicity, FLAs play critical ecological roles as microbial predators, shaping aquatic and soil microbiota through bacterivory (Fan et al., 2024; Avery et al., 1995; Santos, 2025). Their phagocytic activity facilitates the intracellular harboring of bacteria, fungi, viruses, and other microbes, earning them the designation “Trojan horses” for microbial transmission (Scheid, 2014; Rayamajhee et al., 2021; Barker and Brown, 1994). FLAs thrive in diverse habitats ranging from natural systems (soil, freshwater, marine environments) (Rodríguez-Zaragoza, 1994; Tawfeek et al., 2016) to artificial niches (biofilms, anthropogenic water systems including wastewater plants, cooling towers, and swimming pools) (Puzon et al., 2009; Üstüntürk-Onan and Walochnik, 2018; Shaheen et al., 2019; Pagnier et al., 2009; Potgieter et al., 2021; da Silva et al., 2024). Their prevalence in nutrient-rich sediments and biofilms (Potgieter et al., 2021; da Silva et al., 2024), coupled with expanding artificial water infrastructure, heightens human exposure to both FLAs and their symbiotic pathogens, escalating potential zoonotic risks.
1.3 Discovery of the symbiotic phenomenon between Legionella pneumophila and FLAs
The concept of bacteria as intracellular symbionts in amoebae was first recognized in 1975 (Proca-Ciobanu et al., 1975), with Acanthamoeba identified as a host for pathogenic microorganisms in 1978 (Krishna Prasad and Gupta, 1978). Early studies in 1967 (Jeon and Lorch, 1967) and 1975 (Proca-Ciobanu et al., 1975) observed bacteria within Acanthamoeba castellanii cells. Over the following decades, Jeon and Jeon (1976) published a series of studies detailing the infection of amoebae by an endogenous affinity bacterium (termed X-bacterium), later identified as L. jeonii (Park et al., 2004). Rowbotham (1980) first reported that L. pneumophila could survive and replicate within Acanthamoeba and Naegleria. Further studies revealed that L. pneumophila thrives in drinking water only in the presence of amoebae (Wadowsky et al., 1988) and can remain viable for up to 6 months in Acanthamoeba-containing cultures (Bouyer et al., 2007), whereas free-living Legionella in biofilms may lose viability within weeks (Declerck et al., 2009; Murga et al., 2001). These findings have spurred significant interest in the interactions between FLAs and pathogenic bacteria, particularly the intricate relationship between L. pneumophila and Acanthamoeba spp., which has emerged as a major research focus. The reported strains of L. pneumophila that are capable of engaging in symbiosis with amoebae are shown in Supplementary Table 1.
Earlier sequencing studies revealed that the genome of A. castellanii (Neff) contains approximately 15,450 intron-rich genes, many of which are thought to originate from extensive horizontal gene transfer between A. castellanii and bacteria, archaea, viruses, and other eukaryotes (Clarke et al., 2013). Notably, most of these genes are integrated into A. castellanii’s transcriptional program and are actively expressed (Clarke et al., 2013). Compared to other species, the genomes of several bacteria infecting Acanthamoeba are significantly larger (Moliner et al., 2010). Researchers have identified numerous horizontally transferred genes and mobile genetic elements, such as integrases and transposases, in the genomes of intracellular bacteria, including L. pneumophila, L. drancourtii, Candidatus “Protochlamydia amoebophila,” Rickettsia bellii, and Candidatus “Amoebophilus asiaticus.” A large number of insertion sequences have also been detected in the L. pneumophila genome (Moliner et al., 2010). Further studies reveal distinct gene expression patterns in L. pneumophila grown in human monocytes versus Acanthamoeba (Mou and Leung, 2018), suggesting host-specific adaptation. Certain adaptive changes may be essential for survival in Acanthamoeba but unnecessary in macrophages, and vice versa. In this review, we primarily examine the symbiotic mechanisms between Acanthamoeba spp. and L. pneumophila, while secondarily addressing interactions involving other FLAs and Legionella species. We evaluate how these relationships collectively impact human health, underscoring the critical role of symbiosis in microbial pathogenesis and its public health implications.
2 Symbiotic mechanisms of Legionella pneumophila and FLAs
2.1 The first step of symbiosis—adhesion
Adhesion of L. pneumophila to host cells is the primary step in establishing its ecological niche and a critical factor for successful symbiosis. This includes formation of biofilms that are highly resistant to environmental exposure and increase long term resilience. Key bacterial factors involved in L. pneumophila adhesion to Acanthamoeba surfaces include RtxA cytotoxin, Legionella collagen-like protein (Lcl), pillins such as PilE, the lipopolysaccharide (LPS), the putative L. pneumophila-specific adenylate cyclase (LadC) and the periplasmic protein, EnhC. The majority of these factors have evolved to target host cell membrane receptor proteins and sugars for binding, which enables mechanical attachment to facilitate invasion.
Although its precise mechanism remains incompletely understood, RtxA is thought to facilitate the adhesion and invasion of A. castellanii by binding to β2 integrin-like receptors (Ambagala et al., 1999; Lally et al., 1997). However, such receptors have not been identified in Acanthamoeba to date. Integrin-like receptors have been found in other amoebae, such as Hartmannella (Venkataraman et al., 1997) and Entamoeba (Adams et al., 1993; Vines et al., 1998), which share immunoreactive epitopes with human β2 integrins (Adams et al., 1993) and contain characteristic β2 integrin motifs in their cytoplasmic domains (Vines et al., 1998). This suggests that similar receptors may exist in macrophages and amoebic trophozoites, potentially mediating L. pneumophila binding via RtxA. Notably, the rtxA gene is not universally present in all Legionella species, and its presence correlates closely with their ability to cause human disease (Cirillo et al., 2002; Cirillo et al., 2001). Thus, further investigation into RTX protein-mediated adhesion and entry mechanisms could reveal novel therapeutic targets for Legionella infections.
Lcl is an extracellular membrane protein that recognizes sulfated glycosaminoglycans (GAGs) on eukaryotic cell surfaces and promotes bacterial aggregation in the presence of divalent cations (Rehman et al., 2024). GAGs are diverse linear carbohydrates composed of repeating disaccharide units of amino sugars (N-acetylglucosamine or N-acetylgalactosamine) and glucuronic acid or galactose (Gandhi and Mancera, 2008). These complex carbohydrates play crucial roles in various biological processes, including cell adhesion, cell signaling, and microbial pathogenesis (Gandhi and Mancera, 2008). The C-terminal domain of Lcl (Lcl-CTD) is highly conserved (>97%) across L. pneumophila strains and forms a unique trimer structure with a deep negatively charged cavity and a positively charged external surface (Rehman et al., 2024). The positively charged surface of Lcl-CTD is critical for interacting with the negatively charged sulfate groups of GAGs, enabling Lcl to mediate bacterial adhesion to host cells and biofilm formation (Rehman et al., 2024). Lcl plays a significant role in the biofilm formation of L. pneumophila. Biofilms are structures composed of a polysaccharide matrix secreted by bacteria, which protect the bacteria from external environmental stresses and promote bacterial colonization and infection in host tissues (Abdel-Nour et al., 2019). Studies demonstrate that Lcl expression is essential for L. pneumophila aggregation and biofilm stability and mutations in the lcl gene severely impair bacterial adhesion to biofilms (Chatfield et al., 2020; Abdel-Nour et al., 2019). Additionally, the N-terminal region of Lcl anchors the protein to the bacterial surface, localizing it to the outer membrane. This anchoring mechanism likely stabilizes Lcl’s surface display, enhancing its interaction with host cell GAGs (Rehman et al., 2024). Collectively, Lcl’s structural and functional properties establish it as a key virulence factor in L. pneumophila infections and a potential target for novel antibacterial strategies.
The PilE gene encodes type IV pili in L. pneumophila, which are essential for adhesion to A. polyphaga and DNA transformation (Stone and Abu Kwaik, 1998; Stone and Kwaik, 1999). As a major structural component, PilE facilitates pili assembly, extension, and retraction (Nguyen et al., 2015), enabling attachment to both protozoan hosts and human cells, a dual role critical for environmental survival and pathogenicity (Stone and Abu Kwaik, 1998). PilE was initially identified through a genetic screen for adherence-deficient mutants, highlighting its importance in early infection (Stone and Abu Kwaik, 1998). While PilE is a key virulence factor, RtxA which was mentioned earlier, also contributes to adhesion. Notably, PilE- and RtxA-deficient mutants exhibit significantly reduced adhesion and invasion in epithelial cells and monocytes (Stone and Abu Kwaik, 1998; Cirillo et al., 2000), suggesting complementary roles via distinct host receptors. PilY1, a non-pilin protein associated with type IV pili, localizes at the pilus tip and functions as an adhesin for diverse substrates (Guo et al., 2024). It regulates pili dynamics, including calcium-dependent twitching motility and mechanosensing (Guo et al., 2024). During the stationary phase (a high-virulence state), PilY1 surface expression promotes L. pneumophila infection of human lung explants by enhancing adhesion, invasion, and motility (Hoppe et al., 2017). Specifically, PilY1 mediates adherence to alveolar epithelial cells (A549) and macrophages (THP-1), while its C-terminal PilY domain restores wild-type adherence, and the vWFa domain facilitates invasion into non-phagocytic cells (Hoppe et al., 2017). These findings implicate PilY1 in breaching epithelial barriers through multifunctional mechanisms. Given the conserved adhesion strategies between amoebae and macrophages, further studies should clarify the precise roles of PilE and PilY1 in L. pneumophila’s interaction with FLAs.
LPS composition of the bacterial outer membrane is a key determinant of L. pneumophila’s ability to adhere to host cells (Palusinska-Szysz et al., 2019). Studies have shown that LPS structure significantly influences the bacterium’s interaction with amoebal hosts such as A. castellanii. For instance, the wild-type Corby strain, possessing full-length LPS, exhibits highly efficient and rapid binding to the amoeba surface, followed by successful host cell invasion. In contrast, the TF3/1 mutant, which lacks high-molecular-weight LPS fractions, demonstrates reduced adhesion efficiency and impaired internalization, underscoring the essential role of LPS in mediating initial contact and subsequent host cell entry (Palusinska-Szysz et al., 2019). Among the 72 known Legionella species, L. pneumophila is the primary pathogen, responsible for 80–90% of legionellosis cases in Europe and the United States (European Centre for Disease Prevention and Control, 2021). This species can be classified into 15 serogroups based on conventional serotyping. Although serogroups 2–15 (Sg 2–15) are frequently detected in healthcare facilities, Sg 1 accounts for approximately 90% of clinical cases (Chahin and Opal, 2017; Alexandropoulou et al., 2015). Supplementary Table 2 summarizes the epidemiological characteristics, environmental distribution, and control strategies corresponding to each serogroup of L. pneumophila. The LPS of L. pneumophila Sg 1 consists of three major components: a surface-exposed O-specific chain, a core oligosaccharide, and lipid A. The core region contains sugars such as rhamnose, mannose, acetylquinovosamine, and acetylglucosamine, which facilitate interactions with eukaryotic cell surface receptors. These interactions are critical for the initial adhesion of L. pneumophila to A. castellanii (Kowalczyk et al., 2023). Interestingly, a mutant strain lacking O-acetyl groups on the rhamnose residues of the LPS core region exhibited enhanced adhesion to A. castellanii compared to the wild-type strain (Kowalczyk et al., 2023). This suggests that O-acetylation modulates host-pathogen interactions, possibly by altering bacterial surface properties and improving compatibility with amoebal receptors. Furthermore, the O-acetyl-deficient mutant displayed higher intracellular replication efficiency, indicating that LPS structure not only governs initial adhesion but also influences bacterial survival and proliferation within host cells (Kowalczyk et al., 2023). Overall, the LPS of L. pneumophila, particularly the core region and its O-acetylation status, plays a pivotal role in mediating adhesion to A. castellanii. This interaction is essential for the bacterium’s initial colonization and subsequent infection within the amoebal host.
The ladC gene, encoding a putative adenylate cyclase, is uniquely present in L. pneumophila but absent in other Legionella species, suggesting a specialized role in its pathogenesis (Newton et al., 2006). Studies indicate that ladC is upregulated during macrophage infection, implicating its importance in host-pathogen interactions (Newton et al., 2006). To investigate its function, researchers generated a ladC mutant and evaluated its infectivity in both mammalian cells and A. castellanii. Using differential immunofluorescence staining to distinguish adherent from intracellular bacteria, the study revealed that the ladC mutant exhibited significantly reduced adherence to A. castellanii compared to the wild-type strain. This defect was observed at early infection time points (2–12 h), indicating that ladC is critical for the initial contact between L. pneumophila and the amoebal host. Moreover, the mutant showed impaired recovery during early infection stages, suggesting that ladC influences both adhesion and subsequent intracellular survival (Newton et al., 2008). The adhesion and replication defects of the ladC mutant were fully rescued by transcomplementation with the wild-type ladC gene but not with a catalytically inactive variant (ladC N430A/R434A), demonstrating that LadC’s enzymatic activity is required for its role in infection (Newton et al., 2008). These findings establish LadC as a key mediator of L. pneumophila’s initial adhesion to A. castellanii, with its adenylate cyclase activity being indispensable for this process. The study highlights ladC as a virulence factor critical for early host colonization, though the precise mechanisms linking its signaling function to bacterial adhesion remain to be elucidated.
In addition to the aforementioned adhesion-related molecules in L. pneumophila, researchers employed ethyl methanesulfonate (EMS) mutagenesis to generate bacterial mutants with enhanced host cell entry capacity (Cirillo et al., 2000). These selected mutants demonstrated significantly increased invasion efficiency during co-culture with host cells. To identify the genetic determinants, the team constructed a genomic library from the mutant strains and introduced it into wild-type L. pneumophila. Through systematic transposon mutagenesis coupled with selective entry assays, two critical genetic loci (enh1 and enh2) were identified. Notably, enh1 harbored the known rtxA gene, while enh2 encoded a novel factor, EnhC. Heterologous expression of these genes conferred the enhanced-entry phenotype to wild-type bacteria. Functional validation via an EnhC deletion mutant revealed markedly impaired invasion capacity, confirming its essential role in host cell penetration (Cirillo et al., 2000). The EnhC protein may facilitate bacterial invasion by interacting with host cell surface receptors or modulating intracellular signaling pathways.
Host-specific factors also play a role in L. pneumophila adhesion, with the process influenced by the type of infected cell (e.g., amoebae vs. macrophages). Acanthamoeba approaches prey via chemotaxis and random movement (at ~0.4 μm/s), creating conditions for adhesion (Schuster and Levandowsky, 1996). A 170 kDa galactose/N-acetylgalactosamine-inhibitable lectin (Gal/GalNAc) has been identified as a receptor for L. pneumophila adhesion to Hartmannella vermiformis (Venkataraman et al., 1997). Interestingly, while Gal or GalNAc sugars completely block L. pneumophila adhesion to H. vermiformis, they only mildly affect adhesion to A. polyphaga (Harb et al., 1998). L. pneumophila exhibits high affinity for mannose receptors in A. castellanii, particularly the α1-3-d-mannose-binding fragment and d-mannose-binding receptor (Cao et al., 1998). However, this receptor may not be genus-specific, as d-mannose blocks uptake in A. castellanii but not in A. polyphaga (Harb et al., 1998; Declerck et al., 2007a). Additionally, cycloheximide (a protein synthesis inhibitor) and cytochalasin D (a microfilament disruptor) inhibit A. castellanii uptake of L. pneumophila but not A. polyphaga (Harb et al., 1998; Declerck et al., 2007a). These findings highlight significant differences in adhesion mechanisms between L. pneumophila and different Acanthamoeba hosts (e.g., A. castellanii vs. A. polyphaga) (Declerck et al., 2007b; Harb et al., 1998; Declerck et al., 2005), suggesting that L. pneumophila employs diverse strategies to adhere to different hosts. This diversity likely reflects host cell receptor specificity, offering new insights into the complexity of L. pneumophila–host interactions.
2.2 Phagocytosis and the dot/Icm system
The trophozoites of Acanthamoeba utilize surface pseudopodia to ingest bacteria, yeast, algae, and organic particles via both nonspecific pinocytosis and specific phagocytosis (Diesend et al., 2017; Khan, 2006). The Phagocytosis of L. pneumophila by A. castellanii involves curling pseudopodia, a mechanism similar to that in human macrophages (Bozue and Johnson, 1996). While host-mediated phagocytosis drives bacterial uptake, the Dot/Icm (defective in organelle trafficking/intracellular multiplication) type IVB secretion system (T4SS) of L. pneumophila significantly enhances this process (Hilbi et al., 2001; Khelef et al., 2001). The Dot/Icm system acts as a molecular syringe injecting effector proteins encoded by highly conserved dot/icm genes (Berger and Isberg, 1993; Brand et al., 1994). Remarkably, approximately 10% of the L. pneumophila genome encodes over 300 effector proteins, many of which contain eukaryotic domains that manipulate host pathways (e.g., preventing lysosome attachment, inhibiting phagosome maturation and acidification, and protecting bacteria from degradation) (Vincent and Vogel, 2006; Berk et al., 2008; Cazalet et al., 2004; de Felipe et al., 2008; Hubber and Roy, 2010; Nora et al., 2009; Rolando and Buchrieser, 2012). However, only a few effectors have defined functions, and their roles in amoebae remain largely unexplored due to functional redundancy and characterization challenges (Supplementary Table 3).
Phagocytosis of L. pneumophila is closely linked to actin dynamics (Lu and Clarke, 2005). In D. discoideum, this process is insensitive to cytochalasin-D but sensitive to cytochalasin-A, suggesting a role beyond simple actin polymerization (Lu and Clarke, 2005; Peracino et al., 2010; Weber et al., 2006). The effector VipA directly polymerizes actin filaments and alters host trafficking (Franco et al., 2012), though it is dispensable in A. castellanii, indicating host-specific functions. Coronins, actin-binding proteins conserved from amoebae to mammals (Yan et al., 2005), transiently localize to phagocytic cups during early infection before rapidly disassembling (Lu and Clarke, 2005; Hayashi et al., 2008). A coronin homolog in A. healyi (Ahcoronin) exhibits similar behavior, colocalizing with actin and disappearing during phagocytosis (Baldo et al., 2005). These findings suggest that actin remodeling, signaling, and phagosome formation are evolutionarily conserved mechanisms in Legionella-amoeba interactions.
Host predation behavior further influences these interactions. The L. pneumophila autoinducer LAI-1 disrupts protozoan chemotaxis, promoting bacterial uptake by A. castellanii (Tiaden et al., 2010; Simon et al., 2015). By restricting amoeboid motility, L. pneumophila concentrates host feeding in bacterial-rich areas, favoring its replication (Tiaden et al., 2010). Notably, LAI-1 is not conserved across all Legionella species (Burstein et al., 2016), suggesting diverse interaction strategies. Additionally, phagocytic efficiency varies among protozoa, e.g., A. castellanii uptakes L. pneumophila more efficiently than Naegleria lovaniensis (Declerck et al., 2005), likely due to differences in bacterial sensing, adhesion, and phagocytic machinery.
2.3 Inhibition of phagosome-lysosome fusion
After Acanthamoeba captures microorganisms, it internalizes them into acidic phagosomes filled with enzymes. Two outcomes are possible: (1) the bacteria follow the endosomal-lysosomal pathway, where internalized material is transported to early endosomes and gradually matures into phagolysosomes through fusion/fission processes, leading to the degradation of their contents; or (2) bacteria evade phago-lysosomal fusion or resist antimicrobial factors within the phagosome, thereby escaping destruction and establish a symbiotic relationship with Acanthamoeba (Shin and Roy, 2008). L. pneumophila falls into the latter category and employs a series of survival strategies to evade endocytic digestion by preventing phagolysosome formation (Horwitz, 1983b), and establish symbiosis to form a replication niche (Horwitz, 1983a). Notably, L. pneumophila is rapidly internalized and creates unique compartment, the Legionella-containing vacuole (LCV), which evades the endocytic pathway (Derré and Isberg, 2004). The LCV membrane is surrounded by vesicles derived from mitochondria and the endoplasmic reticulum (ER) (Horwitz, 1983a; Tilney et al., 2001). By utilizing ER-derived vesicles, host proteins, and mitochondria, L. pneumophila remodels the LCV, establishing an intracellular niche conducive to bacterial replication prior to ribosome recruitment (Tilney et al., 2001). These molecular events are highly conserved in both amoebae and macrophages.
Within 1 h of infection, the LCV is enveloped by smooth vesicles, with at least one mitochondrion closely associated with most vacuoles (Horwitz, 1983a). The mechanisms underlying mitochondrial recruitment and its benefits for L. pneumophila remain elusive. Studies suggest that translocated Dot/Icm effectors may mediate mitochondrial recruitment, as dot/icm mutants lacking a functional Type IVB Secretion System (T4BSS) fail to recruit mitochondria (Tilney et al., 2001; Berger et al., 1994; Chong et al., 2009). While certain Dot/Icm effectors [e.g., LncP (Horwitz, 1983b) and LegS2/Spl (Degtyar et al., 2009)] target mitochondria, no direct effectors involved in mitochondrial recruitment have been identified as ER-derived vesicles and mitochondria dissipate from the LCV, ribosome numbers increase significantly, promoting L. pneumophila replication in macrophages (Horwitz, 1983a; Tilney et al., 2001). This phenomenon extends to various hosts, including Acanthamoeba, H. vermiformis, N. fowleri, and ciliated protozoan Balanion (Fields et al., 1986; Newsome et al., 1985; Abu Kwaik, 1996). Additionally, in Acanthamoeba (e.g., A. castellanii and A. polyphaga), LCVs form ribosome-induced phagosomes (Harb et al., 1998; Bozue and Johnson, 1996; Horwitz and Maxfield, 1984; Horwitz, 1983b). Ribosome recruitment is a conserved feature in both macrophages and amoebae, with dot/icm mutants unable to recruit ribosomes (Tilney et al., 2001; Berger et al., 1994). Although the molecular mechanisms remain unclear, the LCV may mimic these organelles to avoid host defense systems.
A hallmark of phagosome maturation is acidification, mediated primarily by the ATP-dependent proton pump vacuolar H+-ATPase (v-ATPase) (Forgac, 2007). Mature phagolysosomes typically exhibit an acidic microenvironment enriched with hydrolytic enzymes capable of killing bacteria (Sturgill-Koszycki and Swanson, 2000). L. pneumophila targets the v-ATPase subunit VatA through its effector protein SidK, inhibiting ATP hydrolysis, proton transport, and subsequent vacuolar acidification (Xu et al., 2010). When translocated into macrophages, SidK inhibits vacuolar acidification and impairs the cell’s ability to digest non-pathogenic Escherichia coli (Xu et al., 2010). Studies have shown that recruitment of the v-ATPase transmembrane subunit VatM typically induces vacuolar acidification. However, in phagosomes containing wild-type L. pneumophila, VatM recruitment appears suppressed, preventing acidification of the LCV (Chen et al., 2004). Subsequent proteomic analyses revealed VatM in LCVs from wild-type L. pneumophila in PI(3)K-infected cells (Shevchuk et al., 2009; Urwyler et al., 2009). Despite these discrepancies, the absence of v-ATPase activity and its recruitment to LCVs during early infection are critical for avoiding acidification. Notably, phagosome acidification is inhibited only within the first 8 h post-infection (Horwitz and Maxfield, 1984; Horwitz, 1983a; Horwitz, 1983b). As L. pneumophila enters the replication phase, most infected phagosomes acquire lysosomal characteristics, and by ~18 h post-infection, vacuoles exhibit an acidic pH and endosomal markers such as lysosome-associated membrane protein 1 (LAMP-1) (Sturgill-Koszycki and Swanson, 2000). Furthermore, inhibition of LCV acidification and maturation by the v-ATPase inhibitor bafilomycin A1 effectively suppresses L. pneumophila replication (Sturgill-Koszycki and Swanson, 2000). The schematic diagram of the symbiotic interaction between host and L. pneumophila is shown in Figure 1.
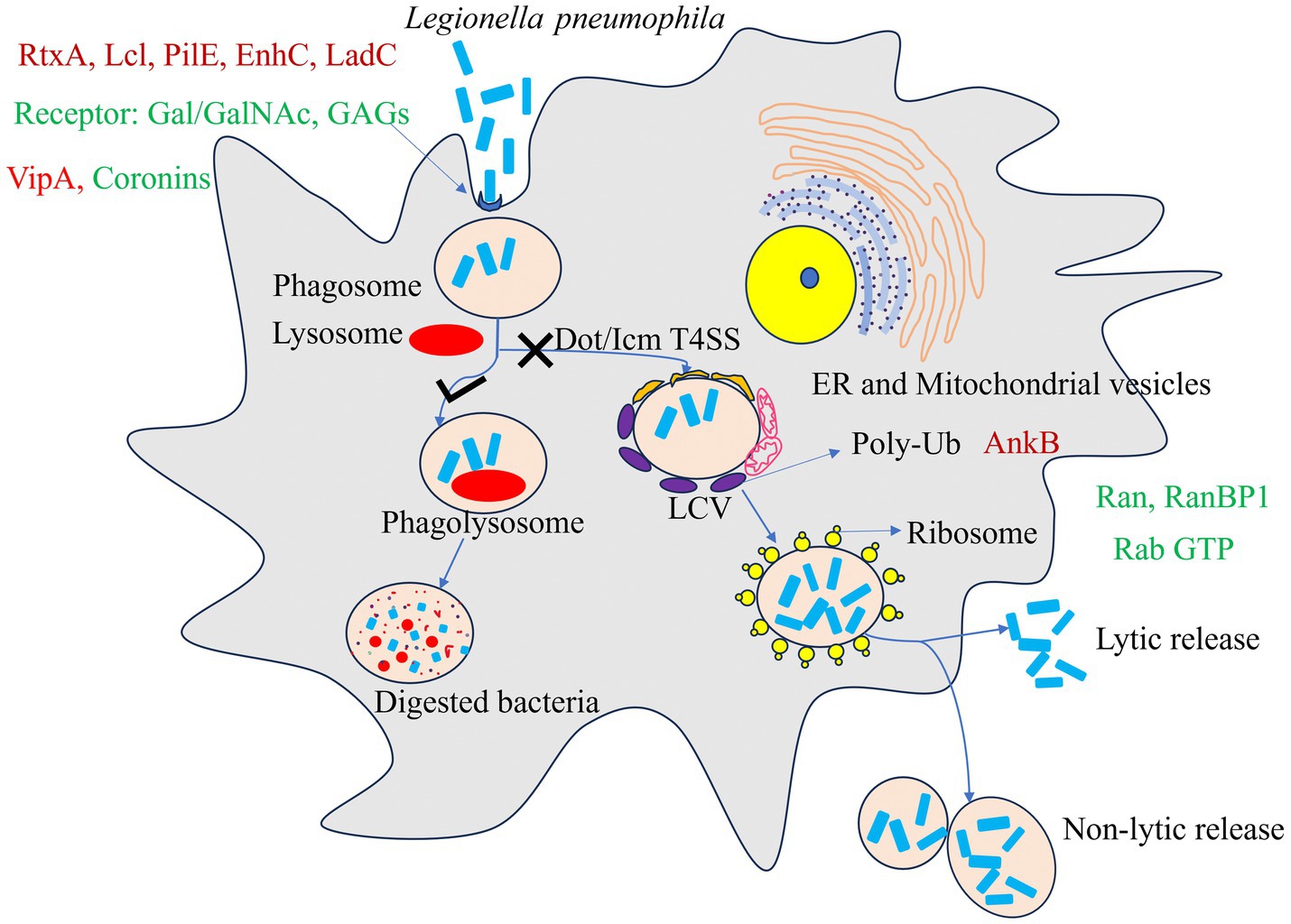
Figure 1. A model for Host and Legionella pneumophila symbiotic interactions. In non-permissive hosts, L. pneumophila is internalized into a phagosome, which typically fuses with lysosomes to form a degradative phagolysosome. In permissive amoebal hosts such as Acanthamoeba spp., L. pneumophila is internalized via phagocytosis and subsequently utilizes the Dot/Icm T4SS to manipulate the vacuole, forming a Legionella-containing vacuole (LCV) that recruits endoplasmic reticulum (ER)-derived vesicles, mitochondria, and host proteins while hijacking ribosomes to establish a replication-permissive niche. After replication, the bacteria exit via lytic and non-lytic release. The L. pneumophila effector AnkB activates the host ubiquitination machinery to degrade host proteins via the proteasome, releasing amino acids primarily utilized by the bacterium. Key bacterial (red) and host (green) proteins involved in this process are indicated in the figure.
2.4 Cellular signaling pathways
Bacterial invasion often triggers multiple host cell signaling pathways. Researchers have identified several biological pathways that may support L. pneumophila replication in amoebae and A. castellanii proliferation in natural environments. These pathways include Ran/Rab GTPase activity, tyrosine phosphorylation, mitogen-activated protein kinase (MAPK), ferrous iron transport (FeoB), and phosphoinositide (PI) signaling. Understanding these pathways may provide therapeutic targets for treatment of infection.
2.4.1 Ran/Rab GTPases
Proteomic analyses have identified up to 12 small Rab GTPases involved in endosomal and vesicular trafficking as components of the LCV (Hilbi et al., 2014). Rab GTPases are central regulators of eukaryotic membrane trafficking and major targets of bacterial effectors (Brumell and Scidmore, 2007; Cossart and Roy, 2010; Stein et al., 2012; Sherwood and Roy, 2013). They play critical roles in regulating the host endocytic cycle, which is essential for nutrient uptake, immune responses, cell division, migration, and adhesion (Allgood and Neunuebel, 2018). Rab35, a key regulator, drives endocytic recycling and phagosome maturation, providing pathogens access to host resources while evading degradation (Egami et al., 2011; Verma and Datta, 2017). Although the molecular mechanisms and biological significance of bacterial exploitation of Rab GTPases remain incompletely understood, existing research highlights their precise localization and selective functions in supporting bacterial replication (Allgood and Neunuebel, 2018).
Additionally, the small GTPase Ran and its effector protein RanBP1 localize to the pathogen vacuole. Ran, which typically regulates nucleocytoplasmic transport, spindle assembly, cytokinesis, and non-centrosomal microtubule organization, is activated by the Dot/Icm substrate LegG1 in L. pneumophila-infected Acanthamoeba. LegG1 promotes microtubule stabilization through Ran activation, enhancing intracellular vacuolar motility, bacterial growth, and the chemotactic and migratory capacity of infected cells (Hilbi et al., 2014). Further studies have revealed that LegG1, like other L. pneumophila effectors (PpgA and PieG), contains eukaryotic RCC1 (regulator of chromosome condensation 1) repeats, which are known to activate the small GTPase Ran. Although all three effectors, LegG1, PpgA, and PieG, promote Ran activation, they target distinct components of the Ran GTPase cycle, for instance, LegG1 binds RanBP10, PpgA interacts with RanGAP1, and PieG stabilizes Ran-GTP. This functional divergence suggests that L. pneumophila employs a spatiotemporally regulated strategy to modulate Ran signaling during infection. The split of ancestral pieG into lpg1975 and legG1 in certain strains further highlights how evolutionary diversification of RCC1 repeat effectors fine-tunes host-pathogen interactions by expanding target specificity within the Ran GTPase network (Swart et al., 2020).
2.4.2 Phosphoinositide metabolic pathway
Phosphoinositide lipid metabolism plays a pivotal role in membrane dynamics during phagocytosis, endocytosis, and exocytosis (Gillooly et al., 2001; De Matteis and Godi, 2004). Upon phagocytosis by host cells, L. pneumophila utilizes its Dot/Icm T4SS to deliver effector proteins that target the LCV by binding phosphoinositide lipids (Dolinsky et al., 2014). The hydrolysis of phosphoinositide (4,5)-bisphosphate (PtdIns(4,5)P2) by phosphoinositide phospholipase C (PI-PLC) generates diacylglycerol (DAG) and inositol-3-phosphate (IP3). DAG recruits C1 domain-containing proteins including protein kinase C (PKC), Rac GAP, and Rap1 to regulate phagosome maturaion (Botelho et al., 2000). Concurrently, the depletion of PtdIns(4,5)P2 is accompanied by the accumulation of DAG, PtdIns(3,4,5)P3, and PtdIns(3,4)P2, which promote actin depolymerization and facilitate phagosome remodeling (Botelho et al., 2000; Bozzaro et al., 2008; Dormann et al., 2004; Loovers et al., 2006; Scott et al., 2005; Vieira et al., 2001). These Phosphoinositides are dynamically interconverted by phosphoinositide kinases (PIKs) and phosphatases, serving as docking sites for small GTPases and proteins with specific lipid-binding domains (Lemmon, 2007; Vicinanza et al., 2008).
The class I phosphoinositide 3-kinase (PI3K) is critical for L. pneumophila infection, with its activity peaking during early infection stages and functioning independently of the actin cytoskeleton (Peracino et al., 2010). Interesting, while D. discoideum phagocytosis of L. pneumophila is PI3K-independent, PI3K activity inhibits bacterial replication and regulates LCV formation (Weber et al., 2006). Among the phosphoinositide-binding effectors, the SidC family plays a key role in ER recruitment to the LCV. L. longbeachae SidC (SidC(Llo)) exhibits a higher binding affinity for PtdIns(4)P with a Kd of 71 nM compared to its L. pneumophila SidC (SidC(Lpn)) (Dolinsky et al., 2014). Deletion of sidC impairs the recruitment of ER markers such as calnexin, Sec22b, and Rab1 to the LCV, but this defect can be functionally complemented by either SidCLlo or SidCLpn, despite their limited sequence similarity (40%) (Dolinsky et al., 2014). These findings demonstrate that Legionella exploits host phosphoinositide metabolism through Dot/Icm effectors to redirect ER-derived vesicles and establish a replication-permissive niche.
2.4.3 MAPK signaling pathway
MAPKs, a class of serine/threonine protein kinases, are key signaling proteins in eukaryotic cells, highly conserved from amoebae to mammals. MAPKs respond to diverse cellular stimuli, regulating gene expression, cell differentiation, proliferation, survival, and death (Shin, 2012). In mammals, the MAPK family comprises three major kinases: p38, c-Jun N-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK). These kinases are activated by upstream phosphorylation events, initiating kinase cascades that phosphorylate downstream proteins (Ma and Nicolet, 2023). In A. castellanii and D. discoideum, the ERK protein family has been identified as a core component of the MAPK signaling pathway (Clarke et al., 2013; Li et al., 2009; Hadwiger and Nguyen, 2011; Aranda et al., 2025). Upon contact with L. pneumophila, ERK1 undergoes rapid phosphorylation (Li et al., 2009). However, whether L. pneumophila modulates host MAPK signaling through Dot/Icm-dependent effectors and benefits from this regulation remains unclear. Some studies suggest that L. pneumophila effectors inhibiting host protein synthesis may trigger MAPK activation in mouse macrophages, potentially exerting adverse effects on the bacteria (Fontana et al., 2012).
2.4.4 Manipulation of host phosphorylation by bacterial phosphatases
L. pneumophila employs diverse effector phosphatases to hijack host tyrosine phosphorylation networks, targeting both systemic signaling pathways and localized cytoskeletal processes. The atypical HAD-like phosphotyrosine phosphatase Ceg4, translocated via the Dot/Icm system, exhibits broad phosphotyrosine dephosphorylation activity in vitro and suppresses MAPK signaling in yeast and human cells (Quaile et al., 2018). Through its haloacid dehalogenase (HAD) domain, Ceg4 precisely modulates the phosphorylation state of multiple MAPK cascade components (MAPKKK, MAPKK, MAPK, and MK), effectively dampening host immune responses to promote bacterial survival (Tegtmeyer et al., 2017; Krachler et al., 2011). In contrast, the classical protein tyrosine phosphatase WipA adopts a structurally distinct mechanism resembling serine/threonine phosphatases. WipA selectively dephosphorylates the Arp2/3 complex (e.g., p-N-WASP, p-ARP3) and related actin regulators (p-ACK1, p-NCK1), thereby inhibiting G-actin polymerization into F-actin and blocking phagosome maturation (Alonso and Pulido, 2016; Jia et al., 2018; Pinotsis and Waksman, 2017; He et al., 2019). This functional divergence highlights the strategy of L. pneumophila to concurrently disrupt global host defenses (via Ceg4-mediated MAPK inhibition) and localized barriers (via WipA-driven cytoskeletal paralysis). The evolutionary conservation of these phosphatases across bacterial strains further underscores their non-redundant roles in fine-tuning host-pathogen interactions.
2.4.5 Host genetic determinants in Legionella-amoebae Symbiosis
The genetic characteristics of amoebae significantly influence the infection process of L. pneumophila. When L. pneumophila interacts with H. vermiformis, it specifically induces host cell tyrosine dephosphorylation, with the 170-kD Gal/GalNAc lectin serving as a key receptor mediating bacterial adhesion and invasion (Venkataraman et al., 1997). This lectin is expressed on the amoebal cell surface and facilitates pathogen internalization through interactions with Legionella effector proteins. Studies demonstrate that this dephosphorylation process also involves multiple cytoskeletal proteins, suggesting that L. pneumophila may promote invasion by regulating host cytoskeletal remodeling (Venkataraman et al., 1997; Venkataraman et al., 1998). Furthermore, through activation of host signaling molecules such as tyrosine phosphatases, L. pneumophila reshapes the intracellular environment to create favorable conditions for its survival and replication within amoebae (Venkataraman et al., 1997). These findings reveal how pathogen hijacking of host genetic background provides molecular evidence supporting the hypothesis of eukaryotic cells serving as “evolutionary crucibles.”
2.4.6 Rhizoferrin transport pathway
L. pneumophila secretes rhizoferrin, a multicarboxylate iron carrier that facilitates growth in iron-limited environments and murine lungs. To investigate the role of the rhizoferrin biosynthesis gene (lbtA) in host cell infection and eliminate potential functional redundancy of the FeoB pathway, researchers constructed and analyzed lbtA-feoB double mutants. These mutants exhibited growth limitations in slightly iron-deficient media, confirming the central role of rhizoferrin-mediated trivalent iron uptake and FeoB-mediated ferrous iron acquisition (Lopez et al., 2023). Experiments demonstrated impaired growth of the lbtA-feoB mutant in A. castellanii, V. vermiformis, and human U937 macrophages, while the lbtA-complemented strain remained unaffected. This indicates that rhizoferrin not only regulates L. pneumophila extracellular survival but also plays a key role in intracellular infection. Purified rhizoferrin induced cytokine production in U937 cells, further highlighting its functional significance. Notably, rhizoferrin-related genes are highly conserved in most L. pneumophila strains but exhibit variability in other Legionella species, suggesting adaptive and survival strategy differences among strains (Lopez et al., 2023). The interplay between L. pneumophila and host signaling pathways is summarized in Table 1.
2.5 The biphasic life cycle of Legionella pneumophila and its molecular regulatory network
L. pneumophila is a facultative intracellular pathogen capable of proliferating within a diverse array of host cells, including free-living protozoa, mammalian macrophages, and epithelial cells (Fields, 1996; Isberg et al., 2009). The lifecycle of this bacterium is characterized by a typical biphasic nature, comprising two key stages: the replication phase and the transmission phase (Molofsky and Swanson, 2004). During the replication phase, the bacterium employs its Dot/Icm T4SS to translocate a vast array of effector proteins into the host cell. These effectors manipulate host cell signaling and metabolic pathways to establish a conductive environment for bacterial replication. For instance, the effector protein SidC specifically recognizes PtdIns(4)P to recruit the ER around the LCVs (Dolinsky et al., 2014), while LegG1 enhances the intracellular motility of LCVs by activating the Ran GTPase (Hilbi et al., 2014). Collectively, these mechanisms create an optimal intracellular niche for bacterial proliferation.
As nutrient conditions shift and the intracellular environment no longer supports sustained bacterial growth, L. pneumophila transitions into the transmission phase. Transcriptomic analyses reveal that this shift is accompanied by a reprogramming of nearly half of the bacterial genes, with a significant upregulation of virulence-associated and invasive genes, particularly those encoding T4SS effectors and motility-related proteins (Brüggemann et al., 2006). This pattern of gene expression has been confirmed in both in vitro culture systems and models of infection with A. castellanii (Brüggemann et al., 2006).
During the transmission phase, L. pneumophila is released from host cells via two primary mechanisms: lytic and non-lytic release (Figure 1). In the lytic release process, the bacterium expresses pore-forming proteins such as IcmT, which create nanoscale pores in the host cell membrane, leading to osmotic lysis (Kirby et al., 1998; Gao and Kwaik, 2000). This process relies not only on the inner membrane protein encoded by the dotA gene but also on other genetic loci involved in phagosome targeting and bacterial replication (Kirby et al., 1998). Concurrently, the bacterial toxin RtxA enhances bacterial adhesion and invasiveness by binding to β2 integrin-like receptors on the host cell surface, thereby setting the stage for the next round of infection (Cirillo et al., 2001; Cirillo et al., 2002).
Notably, in certain Acanthamoeba hosts, such as A. castellanii and A. polyphaga, L. pneumophila can also be released in a non-lytic manner (Rowbotham, 1983; Berk et al., 1998). In this mode, intact bacterial vacuoles (typically containing 2–3 bacteria) are expelled from the host cell, which remains viable and retains its basic life functions. This unique release mechanism not only provides a potential source for the spread of Legionnaires’ disease but is also likely a crucial strategy for the bacterium’s long-term survival in environmental reservoirs such as drinking water systems. Additionally, during the transmission phase, the bacterium synthesizes the siderophore rhizoferrin to cope with iron-limited environments (Lopez et al., 2023) and fine-tunes the expression of virulence genes through non-coding RNA (ncRNA)-mediated regulation, thereby maximizing its adaptability and transmission efficiency both inside and outside the host (Romby et al., 2006).
The complex and sophisticated regulatory mechanisms of L. pneumophila’s lifecycle enable it to flexibly switch survival strategies across different ecological niches and host systems. By coordinating the multifaceted actions of T4SS effectors, pore-forming factors, iron acquisition systems, and gene expression regulatory networks, this pathogen not only achieves efficient host infection and intracellular proliferation but also establishes a long-term persistence capability in artificial water systems, posing a continuous challenge to public health.
2.6 Host benefits from the symbiosis
While much research has focused on L. pneumophila’s exploitation of amoebae for intracellular replication and virulence enhancement, studies demonstrate that Acanthamoeba can also derive benefits from this interaction. Kunze et al. (2021) employed stable isotope labeling and GC–MS metabolomics to reveal that L. pneumophila utilizes host amino acids for protein synthesis during intracellular growth, while concurrently enhancing the amoeba’s capacity for nutrient uptake, including improved exogenous glucose utilization via glycolysis. The AnkB effector of L. pneumophila activates the amoebal proteasome, elevating intracellular free amino acid concentrations (Price et al., 2011; Al-Quadan et al., 2012). These amino acids can be imported into the LCV through transporters like SLC1A5 homologs or other unidentified channels (Wieland et al., 2005; Brüggemann et al., 2006; Sauer et al., 2005; Cazalet et al., 2004; Faucher et al., 2011), indicating a metabolic cross-feeding mechanism that provides nutrients to both partners.
Beyond metabolic advantages, this symbiosis confers ecological benefits to Acanthamoeba. For instance, studies demonstrate that L. pneumophila-infected A. polyphaga exhibits higher resistance to certain disinfectants (e.g., NaOCl) compared to uninfected amoebae (García et al., 2007). Such reciprocal interactions establish a complex mutualism, wherein both partners gain metabolic and survival advantages, while simultaneously enhancing L. pneumophila’s ability to exploit host adaptations, a mechanism that further amplifies its pathogenicity, as elaborated in the subsequent section.
2.7 The role of Acanthamoeba in Legionella pneumophila pathogenicity
Beyond providing an intracellular niche, Acanthamoeba significantly enhances L. pneumophila’s resistance to biocides. This may result from two synergistic mechanisms: (1) Acanthamoeba trophozoites and cysts provide a physical barrier, reducing direct biocide contact; and (2) intracellular growth induces phenotypic changes that enhance bacterial resistance. These mechanisms are often intertwined and challenging to distinguish experimentally. Research reveals that L. pneumophila grown within Acanthamoeba exhibits distinct characteristics compared to broth-cultured bacteria. Barker et al. (1993) reported that L. pneumophila passaged in A. polyphaga displayed a surface antigen composed of a 15 kDa outer membrane protein and mono-unsaturated straight-chain fatty acids. Notably, mixing L. pneumophila with Acanthamoeba lysate did not replicate this effect. The same team found that biocides polyhexamethylene biguanide and benzisothiazolone, which severely damage the membrane integrity of broth-cultured L. pneumophila, showed reduced efficacy against L. pneumophila grown within A. polyphaga (Barker et al., 1992). This suggests that Acanthamoeba-derived proteins coating L. pneumophila confer biocide resistance. Additionally, L. pneumophila suspended in water is sensitive to 2 mg/L free chlorine (sodium hypochlorite), with viable cell numbers significantly declining after 3 min of exposure (Miyamoto et al., 2000). However, when encapsulated in A. polyphaga cysts, L. pneumophila survives for 18 h even under 50 mg/L free chlorine (Kilvington and Price, 1990), highlighting the protective role of Acanthamoeba cysts against biocides.
Within FLA, L. pneumophila forms a unique mature infectious form (MIF), morphologically distinct from bacteria grown on artificial media. MIFs are short rods with an electron-dense, layered outer membrane, containing poly-β-hydroxybutyrate granules and intramembranous layers derived from the plasma membrane (Garduño et al., 2002). Compared to fixed-phase cultures, MIFs exhibit reduced respiration rates, increased resistance to detergent-induced lysis, and enhanced survival under extreme pH conditions (Garduño et al., 2002). Furthermore, gene expression linked to intracellular infection and virulence is upregulated, bolstering L. pneumophila’s resistance to cationic antimicrobial peptides (Robey et al., 2001).
Legionella pneumophila also forms membrane-bound microporous structures within Acanthamoeba to evade host defenses while acquiring essential nutrients through interactions with the ER, membrane transport proteins, and cytoplasmic vesicles (Michard et al., 2015). Studies reveal that L. pneumophila escaping from A. castellanii phagosomes generates a persistent subpopulation characterized by high toxicity and antibiotic resistance (Personnic et al., 2019). These subpopulations undergo significant morphological and transcriptional changes during infection, further enhancing their adaptability and survival. Post-infection, L. pneumophila expresses key virulence factors, such as SdhA and LegK2, which inhibit phagosome-lysosome fusion and facilitate phagosome remodeling into replication compartments, creating favorable conditions for bacterial replication and dissemination (Gomes et al., 2018).
Legionella pneumophila grown within Acanthamoeba exhibits significantly enhanced invasiveness and pathogenicity toward human hosts. Studies demonstrate that L. pneumophila cultured in A. castellanii displays increased toxicity in a murine pneumonia model and a heightened ability to invade various cell lines, including human acute monocytic leukemia cells (THP-1), human peripheral blood mononuclear cells (hPBM), human epithelial carcinoma cells (HEp-2), and mouse leukemia monocytic macrophage cells (RAW 264.7) (Cirillo et al., 1994; Cirillo et al., 1999). Furthermore, several Legionella species, such as L. gormanii, L. micdadei, Legionella steigerwaltii, L. longbeachae, and L. dumoffii, show significantly enhanced proliferative capacity within Mono Mac 6 cells (MM6) following interaction with A. castellanii in vitro (Neumeister et al., 2000). These findings suggest that free-living protozoa, particularly Acanthamoeba, promote the expression of invasive phenotypes in L. pneumophila and other pathogenic microbes.
2.8 Potential impact on human health
Legionella bacteria are ubiquitous in natural water sources (e.g., rivers and lakes) and man-made water systems (e.g., cooling towers, tap water, and hot springs), with L. pneumophila being the most common species responsible for human disease (Kanarek et al., 2022; Carducci et al., 2010). Globally, over 10,000 cases of Legionnaires’ disease are reported annually, and L. pneumophila is the leading cause of waterborne disease outbreaks in the United States (van der Kooij et al., 2017; Yoder et al., 2008). A survey of 52 hot and cold water samples from urban and rural areas revealed that over 50% tested positive for Legionella, with detection rates of 55.88% in hot water and 55.56% in cold water (Sawczyn-Domańska, 2021). Sequencing confirmed the presence of L. pneumophila, and 55.17% (16/29) of positive environmental samples contained at least one virulence gene, highlighting the potential of L. pneumophila in water systems to cause human disease and posing a significant public health risk (Sawczyn-Domańska, 2021).
Legionella pneumophila can exist as free-living planktonic cells or within biofilms, adhering to filters and pipe surfaces, causing blockages, and increasing the risk of hospital-acquired infections (Mampel et al., 2006; Yoder et al., 2008; Rogers and Keevil, 1992). Lehtola et al. (2007) demonstrated that under high shear and turbulent conditions, pathogenic microorganisms such as Mycobacterium avium, L. pneumophila, E. coli, and cup-shaped viruses remain viable or infectious in artificial biofilms for weeks. Additionally, L. pneumophila can survive and grow on dead biofilm-associated microbial cells, such as those of A. castellanii and Saccharomyces boulardii (Temmerman et al., 2006). In drinking water distribution systems, biofilms may serve as persistent reservoirs for pathogenic L. pneumophila, exacerbating risks to human health (Shen et al., 2015).
To determine whether L. pneumophila in biofilms was actively growing or merely surviving in a “viable but nonculturable” state through endogenous metabolism, Murga et al. (2001) used plasmid loss as an indicator of cell division. The L. pneumophila strain carried a plasmid encoding kanamycin resistance and GFP, with loss of fluorescence signaling plasmid loss, and thus replication in the absence of selective pressure. When L. pneumophila-GFP was inoculated into a medium containing H. vermiformis (without a biofilm), bacterial counts increased exponentially alongside a steady decline in fluorescence. In contrast, L. pneumophila suspended in water without H. vermiformis (and without a biofilm) showed neither growth nor fluorescence loss. Biofilm reactor studies confirmed these findings, demonstrating that nearly all L. pneumophila cells in biofilms lacking H. vermiformis retained fluorescence, while those with H. vermiformis lost fluorescence and proliferated (Murga et al., 2001). These results suggest that while H. vermiformis is not essential for L. pneumophila survival in this system, it is required for growth.
Similar findings were reported at a international meeting (Szewzyk et al., 2000). Using a continuous flow chamber described elsewhere (Szewzyk et al., 1994), researchers tracked a L. pneumophila serogroup 1 strain (LP1) in biofilms and outflow water over 98 days. LP1 did not multiply in defined mixed biofilms with a natural water bacterium, and its numbers declined rapidly in outflow water. After 40 days, no LP1 cells were detected in the biofilm via Legionella-specific fluorescent in situ hybridization (FISH). In a parallel chamber where A. castellanii was added alongside LP1, bacterial counts in the outflow water increased by several logs and remained stable for 98 days. FISH with eukaryotic and Legionella-specific probes revealed colonized amoebae in the biofilm, though only half contained LP1 cells, and just 10% were heavily colonized. Some Legionella cells were found outside amoebae but always in close proximity. While this model system does not perfectly replicate natural drinking water biofilms, the stark contrast in Legionella survival and growth between biofilms with and without amoebae underscores the critical role of protozoa in sustaining Legionella in environmental niches.
Legionella pneumophila can be isolated from various environmental protozoa, a critical step in its life cycle. By parasitizing amoebae, L. pneumophila persists and replicates within biofilms, an adaptive survival mechanism with serious implications for human health. However, our understanding of Legionella concentrations in drinking water systems and its infectious dose in humans remains limited (Armstrong and Haas, 2008). Similarly, the diversity and density of protozoa that transmit Legionella are poorly characterized, increasing uncertainty in assessing the spread and risks of these microorganisms in water systems (Thomas et al., 2008).
Drinking water systems serve not only as potential reservoirs for pathogens but also as critical environments where biofilm-mediated growth of adapted strains occurs. Research demonstrates that Acanthamoeba spp. can significantly enhance the survival of various waterborne pathogens through symbiotic relationships, including L. pneumophila, Mycobacterium tuberculosis, Helicobacter pylori, E. coli, and Mycobacterium avium (Hojo et al., 2020; Hennebique et al., 2021; Samba-Louaka et al., 2018). These interactions not only facilitate pathogen persistence in water supply systems but may also confer chlorine resistance to bacteria (Thomas et al., 2010).
Notably, Acanthamoeba cysts can withstand chlorine treatment at 100 mg/L for 10 min, far exceeding conventional drinking water treatment concentrations (1–2 mg/L) (Shi et al., 2021). Significant variations exist in disinfectant susceptibility among amoebae. Acanthamoeba requires 3,500 mg·min /L chlorine for 4-log reduction (Loret et al., 2008), while 856 mg·min/L achieves only 2-log reduction (Dupuy et al., 2014). Hartmannella cysts show just 2-log reduction at 156 mg·min/L chlorine, whereas Vermamoeba cysts are completely inactivated by 15 mg/L chlorine for 10 min (Fouque et al., 2015). In contrast, Naegleria demonstrates greater sensitivity, with trophozoites inactivated by 0.79 mg/L chlorine in 30 min (Cursons et al., 1980) and cysts completely inactivated by 1.5 mg/L chlorine after 1 h (De Jonckheere and van de Voorde, 1976). However, biofilm-associated N. fowleri can tolerate 20 mg/L chlorine for up to 3 h (Miller et al., 2015).
Ozone treatment proves effective for water disinfection. A dose of 6.75 mg/L ozone achieves 99.9% inactivation of Acanthamoeba and Naegleria trophozoites within 30 min (Cursons et al., 1980), but shows limited efficacy against biofilm-associated Acanthamoeba, Hartmannella, and Vahlkampfia (Thomas et al., 2004). For UV disinfection, while WHO recommends 10 mJ/cm2 for 99.9% reduction of Giardia and Cryptosporidium (World Health Organization, 2004), Acanthamoeba trophozoites require 72.2 mJ/cm2 (Cervero-Aragó et al., 2014), with cysts surviving even after 800 mJ/cm2 exposure (Aksozek et al., 2002). N. fowleri needs 24 mJ/cm2 (trophozoites) and 121 mJ/cm2 (cysts) for 4-log reduction (Sarkar and Gerba, 2012). Notably, UV irradiation shows poor efficacy against biofilm-associated amoebae (Långmark et al., 2007).
These findings demonstrate the limited effectiveness of conventional disinfection methods against FLAs, particularly in cyst form. Combined treatment technologies (e.g., UV-photocatalytic oxidation) may offer more effective solutions (Ahlawat et al., 2023), though practical implementation requires careful consideration of treatment efficacy and operational feasibility.
3 Conclusion and future perspectives
FLAs, widely distributed protists in natural environments, serve dual ecological roles as both microbial community regulators and pathogen reservoirs. As a clinically significant FLA genus, Acanthamoeba spp. exhibit unique biological features: direct pathogenicity in humans (e.g., AK) and intracellular harboring of pathogens like L. pneumophila through endosymbiosis. This system provides an ideal model for studying microbial ecology and host-pathogen coevolution. Medically, Acanthamoeba functions dually as: (1) an opportunistic human pathogen, and (2) a microbial host enhancing pathogen virulence, particularly by providing protected intracellular niches (e.g., via lysosomal fusion inhibition) that increase L. pneumophila’s environmental resistance and pathogenic potential (Rayamajhee et al., 2021). Its environmental ubiquity makes it a critical transmission vector. Remarkably, Acanthamoeba’s structural and functional parallels with human macrophages establish it as a valuable surrogate model for studying pathogen-macrophage interactions (Brüssow, 2007).
The symbiotic interaction between Acanthamoeba and Legionella significantly enhances Legionella’s pathogenicity through three key mechanisms. First, Acanthamoeba provides an optimal intracellular niche for Legionella replication, offering abundant nutrients that facilitate high-density bacterial proliferation, thereby augmenting its pathogenic potential prior to human infection. Epidemiological evidence confirms the ubiquity of this relationship: among clinical Acanthamoeba strains, 61.5% (8 out of 13 strains) harbor intracellular bacteria, as demonstrated by Rayamajhee et al. (2022), a finding consistent with the 59.4% carriage rate reported in earlier studies (Iovieno et al., 2010). These bacterial communities include clinically relevant pathogens like Pseudomonas and Legionella, with co-infection shown to exacerbate keratitis severity (Rayamajhee et al., 2024). This interaction drives reciprocal evolutionary adaptations. Legionella acquires virulence genes associated with host adhesion and biofilm formation through horizontal gene transfer during its intracellular residence, while Acanthamoeba may derive metabolic benefits through enhanced nutrient utilization efficiency (Kunze et al., 2021; Price et al., 2011; Al-Quadan et al., 2012). Through this co-evolutionary process, Acanthamoeba effectively becomes a microbial “Trojan horse,” serving not only as a natural reservoir for Legionella but also providing a protected environment that shields pathogens from disinfectants (Shi et al., 2021) and significantly extends their environmental persistence (Bouyer et al., 2007). These combined effects ultimately amplify both the transmission risk and pathogenic potential of the harbored microorganisms.
The ecological coexistence of amoebae and Legionella in water systems not only reinforces their host-pathogen interaction but also poses a critical challenge to drinking water safety. Acanthamoeba cysts exhibit extraordinary resistance to chlorine, surviving exposures to 100 mg/L for 10 min, a concentration fifty times higher than standard water treatment doses (1–2 mg/L) (Shi et al., 2021). Current water quality assessment systems remain disproportionately focused on traditional pathogens like Giardia and Cryptosporidium (LeChevallier et al., 2024), while largely neglecting Acanthamoeba’s dual ecological role as both a microbial predator and potential pathogen vector. This fundamental oversight in risk assessment underscores the urgent need for a paradigm shift that integrates advances in mechanistic understanding of amoeba-pathogen interactions with the development of targeted disinfection technologies and corresponding policy reforms.
While current research has established Acanthamoeba as a critical driver of pathogen evolution, fundamental gaps persist across molecular, ecological, and evolutionary dimensions. Key unresolved questions include: (1) the precise mechanisms by which L. pneumophila hijacks host organelles (e.g., ER-mitochondria contact sites) through its effector repertoire to maintain intracellular survival while evading amoebal defenses; (2) how Acanthamoeba’s complex interactions with biofilm-associated microorganisms modulate Legionella transmission dynamics; and (3) the extent to which Acanthamoeba serves as a “genetic melting pot” facilitating pathogen genome innovation through horizontal gene transfer or environmental selection pressures, a process requiring validation through natural metagenomic datasets.
To comprehensively address these challenges, future research must develop integrated strategies targeting the Acanthamoeba-Legionella interaction across multiple fronts. At the molecular level, priority should be given to designing targeted inhibitors against key virulence determinants such as Dot/Icm T4SS effector proteins including SidC, alongside compounds disrupting cyst formation through chitin synthase inhibition. Concurrently, advancing monitoring capabilities will require the synergistic integration of qPCR for pathogen quantification, nanopore sequencing for strain characterization, and microfluidic platforms for single-cell behavioral analysis, collectively enabling real-time surveillance of this pathogenic partnership in water systems. For water treatment optimization, research should focus on evaluating combined disinfection approaches that leverage ultraviolet irradiation with photocatalytic oxidation technologies, while systematically assessing chlorine dosage regimens capable of overcoming the exceptional resistance exhibited by amoebal cysts. These technological advances must be paralleled by public health policy innovations, particularly the development of internationally recognized water safety standards that explicitly incorporate risk metrics for amoeba-associated pathogens, with special consideration for regions most vulnerable to climate-mediated disease transmission patterns.
Based on the aforementioned research challenges and countermeasures, the future holds promise for the establishment of a precision prevention and control system for the amoeba-pathogen symbiotic ecosystem through interdisciplinary innovation. This will provide a new solution for ensuring water safety. Breakthroughs in this field will not only deepen the understanding of host–microbe interactions but also drive the full-chain transformation from basic research to public health practice, ultimately achieving proactive defense and effective control of waterborne infectious diseases.
Author contributions
YW: Writing – original draft. LJ: Writing – review & editing, Investigation, Data curation. FZ: Writing – review & editing, Data curation. YZ: Writing – review & editing. RF: Writing – review & editing. ML: Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Natural Science Foundation of the Jilin Province (no. YDZJ202501ZYTS782).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1634806/full#supplementary-material
References
Abdel-Nour, M., Su, H., Duncan, C., Li, S., Raju, D., Shamoun, F., et al. (2019). Polymorphisms of a collagen-like adhesin contributes to Legionella pneumophila adhesion, biofilm formation capacity and clinical prevalence. Front. Microbiol. 10:604. doi: 10.3389/fmicb.2019.00604
Abu Kwaik, Y. (1996). The phagosome containing Legionella pneumophila within the protozoan Hartmannella vermiformis is surrounded by the rough endoplasmic reticulum. Appl. Environ. Microbiol. 62, 2022–2028. doi: 10.1128/aem.62.6.2022-2028.1996
Adams, S. A., Robson, S. C., Gathiram, V., Jackson, T. F., Pillay, T. S., Kirsch, R. E., et al. (1993). Immunological similarity between the 170 kD amoebic adherence glycoprotein and human beta 2 integrins. Lancet 341, 17–19. doi: 10.1016/0140-6736(93)92483-a
Ahlawat, K., Jangra, R., Ish, A., Dixit, A., Fulwani, D., Jain, N., et al. (2023). Analysis of a Uv photocatalytic oxidation-based disinfection system for hydroxyl radicals, negative air ions generation and their impact on inactivation of pathogenic micro-organisms. Rev. Sci. Instrum. 94:619. doi: 10.1063/5.0151619
Aksozek, A., Mcclellan, K., Howard, K., Niederkorn, J. Y., and Alizadeh, H. (2002). Resistance of Acanthamoeba castellanii cysts to physical, chemical, and radiological conditions. J. Parasitol. 88, 621–623. doi: 10.1645/0022-3395(2002)088[0621:ROACCT]2.0.CO;2
Alexandropoulou, I. G., Ntougias, S., Konstantinidis, T. G., Parasidis, T. A., Panopoulou, M., and Constantinidis, T. C. (2015). Environmental surveillance and molecular epidemiology of waterborne pathogen Legionella pneumophila in health-care facilities of northeastern Greece: a 4-year survey. Environ. Sci. Pollut. Res. Int. 22, 7628–7640. doi: 10.1007/s11356-014-3740-8
Allgood, S. C., and Neunuebel, M. R. (2018). The recycling endosome and bacterial pathogens. Cell. Microbiol. 20:e12857. doi: 10.1111/cmi.12857
Alonso, A., and Pulido, R. (2016). The extended human Ptpome: a growing tyrosine phosphatase family. FEBS J. 283, 1404–1429. doi: 10.1111/febs.13600
Al-Quadan, T., Price, C. T., and Abu Kwaik, Y. (2012). Exploitation of evolutionarily conserved amoeba and mammalian processes by Legionella. Trends Microbiol. 20, 299–306. doi: 10.1016/j.tim.2012.03.005
Ambagala, T. C., Ambagala, A. P., and Srikumaran, S. (1999). The leukotoxin of Pasteurella haemolytica binds to beta(2) integrins on bovine leukocytes. FEMS Microbiol. Lett. 179, 161–167
Aranda, R. G., Fatima, S., Rafid, M. I., Mcgill, I., and Hadwiger, J. A. (2025). Regulatory differences between atypical and typical map kinases in Dictyostelium discoideum. Cell. Signal. 130:111701. doi: 10.1016/j.cellsig.2025.111701
Armstrong, T. W., and Haas, C. N. (2008). Legionnaires' disease: evaluation of a quantitative microbial risk assessment model. J. Water Health 6, 149–166. doi: 10.2166/wh.2008.026
Avery, S. V., Harwood, J. L., and Lloyd, D. (1995). Quantification and characterization of phagocytosis in the soil Amoeba Acanthamoeba castellanii by flow cytometry. Appl. Environ. Microbiol. 61, 1124–1132. doi: 10.1128/aem.61.3.1124-1132.1995
Baldo, E. T., Moon, E. K., Kong, H. H., and Chung, D. I. (2005). Acanthamoeba healyi: molecular cloning and characterization of a coronin homologue, an actin-related protein. Exp. Parasitol. 110, 114–122. doi: 10.1016/j.exppara.2005.02.007
Barker, J., and Brown, M. R. (1994). Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiology 140, 1253–1259.
Barker, J., Brown, M. R., Collier, P. J., Farrell, I., and Gilbert, P. (1992). Relationship between Legionella pneumophila and Acanthamoeba polyphaga: physiological status and susceptibility to chemical inactivation. Appl. Environ. Microbiol. 58, 2420–2425. doi: 10.1128/aem.58.8.2420-2425.1992
Barker, J., Lambert, P. A., and Brown, M. R. (1993). Influence of intra-amoebic and other growth conditions on the surface properties of Legionella pneumophila. Infect. Immun. 61, 3503–3510. doi: 10.1128/iai.61.8.3503-3510.1993
Bellini, N. K., Thiemann, O. H., Reyes-Batlle, M., Lorenzo-Morales, J., and Costa, A. O. (2022). A history of over 40 years of potentially pathogenic free-living amoeba studies in Brazil—a systematic review. Mem. Inst. Oswaldo Cruz 117:e210373. doi: 10.1590/0074-02760210373
Berger, K. H., and Isberg, R. R. (1993). Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol. Microbiol. 7, 7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x
Berger, K. H., Merriam, J. J., and Isberg, R. R. (1994). Altered intracellular targeting properties associated with mutations in the Legionella pneumophila dotA gene. Mol. Microbiol. 14, 809–822. doi: 10.1111/j.1365-2958.1994.tb01317.x
Berk, S. G., Faulkner, G., Garduño, E., Joy, M. C., Ortiz-Jimenez, M. A., and Garduño, R. A. (2008). Packaging of live Legionella pneumophila into pellets expelled by Tetrahymena spp. does not require bacterial replication and depends on a dot/Icm-mediated survival mechanism. Appl. Environ. Microbiol. 74, 2187–2199. doi: 10.1128/AEM.01214-07
Berk, S. G., Ting, R. S., Turner, G. W., and Ashburn, R. J. (1998). Production of respirable vesicles containing live Legionella pneumophila cells by two Acanthamoeba spp. Appl. Environ. Microbiol. 64, 279–286. doi: 10.1128/AEM.64.1.279-286.1998
Blatt, S. P., Parkinson, M. D., Pace, E., Hoffman, P., Dolan, D., Lauderdale, P., et al. (1993). Nosocomial legionnaires' disease: aspiration as a primary mode of disease acquisition. Am. J. Med. 95, 16–22. doi: 10.1016/0002-9343(93)90227-G
Bohach, G. A., and Snyder, I. S. (1983). Characterization of surfaces involved in adherence of Legionella pneumophila to Fischerella species. Infect. Immun. 42, 318–325. doi: 10.1128/iai.42.1.318-325.1983
Botelho, R. J., Teruel, M., Dierckman, R., Anderson, R., Wells, A., York, J. D., et al. (2000). Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J. Cell Biol. 151, 1353–1368. doi: 10.1083/jcb.151.7.1353
Bouyer, S., Imbert, C., Rodier, M. H., and Héchard, Y. (2007). Long-term survival of Legionella pneumophila associated with Acanthamoeba castellanii vesicles. Environ. Microbiol. 9, 1341–1344. doi: 10.1111/j.1462-2920.2006.01229.x
Bozue, J. A., and Johnson, W. (1996). Interaction of Legionella pneumophila with Acanthamoeba castellanii: uptake by coiling phagocytosis and inhibition of phagosome-lysosome fusion. Infect. Immun. 64, 668–673. doi: 10.1128/iai.64.2.668-673.1996
Bozzaro, S., Bucci, C., and Steinert, M. (2008). Phagocytosis and host-pathogen interactions in Dictyostelium with a look at macrophages. Int. Rev. Cell Mol. Biol. 271, 253–300. doi: 10.1016/S1937-6448(08)01206-9
Bozzaro, S., Buracco, S., Peracino, B., and Eichinger, L. (2019). Dictyostelium host response to Legionella infection: strategies and assays. Methods Mol. Biol. 1921, 347–370. doi: 10.1007/978-1-4939-9048-1_23
Brand, B. C., Sadosky, A. B., and Shuman, H. A. (1994). The Legionella pneumophila icm locus: a set of genes required for intracellular multiplication in human macrophages. Mol. Microbiol. 14, 797–808. doi: 10.1111/j.1365-2958.1994.tb01316.x
Brüggemann, H., Hagman, A., Jules, M., Sismeiro, O., Dillies, M. A., Gouyette, C., et al. (2006). Virulence strategies for infecting phagocytes deduced from the in vivo transcriptional program of Legionella pneumophila. Cell. Microbiol. 8, 1228–1240. doi: 10.1111/j.1462-5822.2006.00703.x
Brumell, J. H., and Scidmore, M. A. (2007). Manipulation of Rab Gtpase function by intracellular bacterial pathogens. Microbiol. Mol. Biol. Rev. 71, 636–652. doi: 10.1128/MMBR.00023-07
Brüssow, H. (2007). Bacteria between protists and phages: from antipredation strategies to the evolution of pathogenicity. Mol. Microbiol. 65, 583–589. doi: 10.1111/j.1365-2958.2007.05826.x
Burillo, A., Pedro-Botet, M. L., and Bouza, E. (2017). Microbiology and epidemiology of legionnaire's disease. Infect. Dis. Clin. N. Am. 31, 7–27. doi: 10.1016/j.idc.2016.10.002
Burstein, D., Amaro, F., Zusman, T., Lifshitz, Z., Cohen, O., Gilbert, J. A., et al. (2016). Genomic analysis of 38 Legionella species identifies large and diverse effector repertoires. Nat. Genet. 48, 167–175. doi: 10.1038/ng.3481
Cabello-Vílchez, A. M., and Ruiz-Ruiz, M. I. (2024). Molecular analysis unmasking a Balamuthia mandrillaris: skin lesion and granulomatous amebic encephalitis by Acanthamoeba sp close to genotype T4 with fatal outcome. Clin. Infect. Pract. 21:100246. doi: 10.1016/j.clinpr.2023.100246
Cao, Z., Jefferson, D. M., and Panjwani, N. (1998). Role of carbohydrate-mediated adherence in cytopathogenic mechanisms of Acanthamoeba. J. Biol. Chem. 273, 15838–15845. doi: 10.1074/jbc.273.25.15838
Carducci, A., Verani, M., and Battistini, R. (2010). Legionella in industrial cooling towers: monitoring and control strategies. Lett. Appl. Microbiol. 50, 24–29. doi: 10.1111/j.1472-765X.2009.02750.x
Cazalet, C., Rusniok, C., Brüggemann, H., Zidane, N., Magnier, A., Ma, L., et al. (2004). Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nat. Genet. 36, 1165–1173. doi: 10.1038/ng1447
Cervero-Aragó, S., Sommer, R., and Araujo, R. M. (2014). Effect of Uv irradiation (253.7 nm) on free Legionella and Legionella associated with its amoebae hosts. Water Res. 67, 299–309. doi: 10.1016/j.watres.2014.09.023
Chahin, A., and Opal, S. M. (2017). Severe pneumonia caused by Legionella pneumophila: differential diagnosis and therapeutic considerations. Infect. Dis. Clin. N. Am. 31, 111–121. doi: 10.1016/j.idc.2016.10.009
Chatfield, C. H., Zaia, J., and Sauer, C. (2020). Legionella pneumophila attachment to biofilms of an Acidovorax isolate from a drinking water-consortium requires the Lcl-Adhesin protein. Int. Microbiol. 23, 597–605. doi: 10.1007/s10123-020-00126-0
Chen, J., De Felipe, K. S., Clarke, M., Lu, H., Anderson, O. R., Segal, G., et al. (2004). Legionella effectors that promote nonlytic release from protozoa. Science 303, 1358–1361. doi: 10.1126/science.1094226
Chong, A., Lima, C. A., Allan, D. S., Nasrallah, G. K., and Garduño, R. A. (2009). The purified and recombinant Legionella pneumophila chaperonin alters mitochondrial trafficking and microfilament organization. Infect. Immun. 77, 4724–4739. doi: 10.1128/IAI.00150-09
Cirillo, S. L., Bermudez, L. E., El-Etr, S. H., Duhamel, G. E., and Cirillo, J. D. (2001). Legionella pneumophila entry gene rtxA is involved in virulence. Infect. Immun. 69, 508–517. doi: 10.1128/IAI.69.1.508-517.2001
Cirillo, J. D., Cirillo, S. L., Yan, L., Bermudez, L. E., Falkow, S., and Tompkins, L. S. (1999). Intracellular growth in Acanthamoeba castellanii affects monocyte entry mechanisms and enhances virulence of Legionella pneumophila. Infect. Immun. 67, 4427–4434. doi: 10.1128/IAI.67.9.4427-4434.1999
Cirillo, J. D., Falkow, S., and Tompkins, L. S. (1994). Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infect. Immun. 62, 3254–3261. doi: 10.1128/iai.62.8.3254-3261.1994
Cirillo, S. L. G., Lum, J., and Cirillo, J. D. (2000). Identification of novel loci involved in entry by Legionella pneumophila. Microbiology 146, 1345–1359. doi: 10.1099/00221287-146-6-1345
Cirillo, S. L. G., Yan, L., Littman, M., Samrakandi, M. M., and Cirillo, J. D. (2002). Role of the Legionella pneumophila rtxA gene in amoebae. Microbiology 148, 1667–1677. doi: 10.1099/00221287-148-6-1667
Clarke, M., Lohan, A. J., Liu, B., Lagkouvardos, I., Roy, S., Zafar, N., et al. (2013). Genome of Acanthamoeba castellanii highlights extensive lateral gene transfer and early evolution of tyrosine kinase signaling. Genome Biol. 14:R11. doi: 10.1186/gb-2013-14-2-r11
Colbourne, J. S., and Dennis, P. J. (1985). Distribution and persistence of Legionella in water systems. Microbiol. Sci. 2, 40–43.
Cossart, P., and Roy, C. R. (2010). Manipulation of host membrane machinery by bacterial pathogens. Curr. Opin. Cell Biol. 22, 547–554. doi: 10.1016/j.ceb.2010.05.006
Cunha, B. A., Burillo, A., and Bouza, E. (2016). Legionnaires' disease. Lancet 387, 376–385. doi: 10.1016/S0140-6736(15)60078-2
Cursons, R. T., Brown, T. J., and Keys, E. A. (1980). Effect of disinfectants on pathogenic free-living amoebae: in axenic conditions. Appl. Environ. Microbiol. 40, 62–66. doi: 10.1128/aem.40.1.62-66.1980
Da Rocha-Azevedo, B., Tanowitz, H. B., and Marciano-Cabral, F. (2009). Diagnosis of infections caused by pathogenic free-living amoebae. Interdiscip. Perspect. Infect. Dis. 2009:251406. doi: 10.1155/2009/251406
Da Silva, T. C. B., Chaúque, B. J. M., Benitez, G. B., and Rott, M. B. (2024). Global prevalence of potentially pathogenic free-living amoebae in sewage and sewage-related environments-systematic review with meta-analysis. Parasitol. Res. 123:148. doi: 10.1007/s00436-024-08164-7
De Felipe, K. S., Glover, R. T., Charpentier, X., Anderson, O. R., Reyes, M., Pericone, C. D., et al. (2008). Legionella eukaryotic-like type iv substrates interfere with organelle trafficking. PLoS Pathog. 4:e1000117. doi: 10.1371/journal.ppat.1000117
De Jonckheere, J., and Van De Voorde, H. (1976). Differences in destruction of cysts of pathogenic and nonpathogenic Naegleria and Acanthamoeba by chlorine. Appl. Environ. Microbiol. 31, 294–297. doi: 10.1128/aem.31.2.294-297.1976
De Matteis, M. A., and Godi, A. (2004). Pi-loting membrane traffic. Nat. Cell Biol. 6, 487–492. doi: 10.1038/ncb0604-487
Declerck, P., Behets, J., De Keersmaecker, B., and Ollevier, F. (2007a). Receptor-mediated uptake of Legionella pneumophila by Acanthamoeba castellanii and Naegleria lovaniensis. J. Appl. Microbiol. 103, 2697–2703. doi: 10.1111/j.1365-2672.2007.03530.x
Declerck, P., Behets, J., Delaedt, Y., Margineanu, A., Lammertyn, E., and Ollevier, F. (2005). Impact of non-Legionella bacteria on the uptake and intracellular replication of Legionella pneumophila in Acanthamoeba castellanii and Naegleria lovaniensis. Microb. Ecol. 50, 536–549. doi: 10.1007/s00248-005-0258-0
Declerck, P., Behets, J., Margineanu, A., Van Hoef, V., De Keersmaecker, B., and Ollevier, F. (2009). Replication of Legionella pneumophila in biofilms of water distribution pipes. Microbiol. Res. 164, 593–603. doi: 10.1016/j.micres.2007.06.001
Declerck, P., Behets, J., Van Hoef, V., and Ollevier, F. (2007b). Detection of Legionella spp. and some of their amoeba hosts in floating biofilms from anthropogenic and natural aquatic environments. Water Res. 41, 3159–3167. doi: 10.1016/j.watres.2007.04.011
Degtyar, E., Zusman, T., Ehrlich, M., and Segal, G. (2009). A Legionella effector acquired from protozoa is involved in sphingolipids metabolism and is targeted to the host cell mitochondria. Cell. Microbiol. 11, 1219–1235. doi: 10.1111/j.1462-5822.2009.01328.x
Derré, I., and Isberg, R. R. (2004). Legionella pneumophila replication vacuole formation involves rapid recruitment of proteins of the early secretory system. Infect. Immun. 72, 3048–3053. doi: 10.1128/IAI.72.5.3048-3053.2004
Diesend, J., Kruse, J., Hagedorn, M., and Hammann, C. (2017). Amoebae, giant viruses, and virophages make up a complex, multilayered threesome. Front. Cell. Infect. Microbiol. 7:527. doi: 10.3389/fcimb.2017.00527
Dolinsky, S., Haneburger, I., Cichy, A., Hannemann, M., Itzen, A., and Hilbi, H. (2014). The Legionella longbeachae Icm/dot substrate SidC selectively binds phosphatidylinositol 4-phosphate with nanomolar affinity and promotes pathogen vacuole-endoplasmic reticulum interactions. Infect. Immun. 82, 4021–4033. doi: 10.1128/IAI.01685-14
Dormann, D., Weijer, G., Dowler, S., and Weijer, C. J. (2004). In vivo analysis of 3-phosphoinositide dynamics during Dictyostelium phagocytosis and chemotaxis. J. Cell Sci. 117, 6497–6509. doi: 10.1242/jcs.01579
Dupuy, M., Berne, F., Herbelin, P., Binet, M., Berthelot, N., Rodier, M. H., et al. (2014). Sensitivity of free-living amoeba trophozoites and cysts to water disinfectants. Int. J. Hyg. Environ. Health 217, 335–339. doi: 10.1016/j.ijheh.2013.07.007
Egami, Y., Fukuda, M., and Araki, N. (2011). Rab35 regulates phagosome formation through recruitment of Acap2 in macrophages during FcγR-mediated phagocytosis. J. Cell Sci. 124, 3557–3567. doi: 10.1242/jcs.083881
European Centre for Disease Prevention and Control. (2021). Legionnaires’ disease. In Ecdc Annual Epidemiological Report for 2019, Ecdc: Stockholm, Sweden, 1–10.
Falcó, V., Fernández De Sevilla, T., Alegre, J., Ferrer, A., and Martínez Vázquez, J. M. (1991). Legionella pneumophila. A cause of severe community-acquired pneumonia. Chest 100, 1007–1011. doi: 10.1378/chest.100.4.1007
Fan, S., Shen, Y., and Qian, L. (2024). Social life of free-living amoebae in aquatic environment-comprehensive insights into interactions of free-living amoebae with neighboring microorganisms. Front. Microbiol. 15:1382075. doi: 10.3389/fmicb.2024.1382075
Faucher, S. P., Mueller, C. A., and Shuman, H. A. (2011). Legionella pneumophila transcriptome during intracellular multiplication in human macrophages. Front. Microbiol. 2:60. doi: 10.3389/fmicb.2011.00060
Fields, B. S. (1996). The molecular ecology of legionellae. Trends Microbiol. 4, 286–290. doi: 10.1016/0966-842X(96)10041-X
Fields, B. S., Barbaree, J. M., Shotts, E. B. Jr., Feeley, J. C., Morrill, W. E., Sanden, G. N., et al. (1986). Comparison of guinea pig and protozoan models for determining virulence of Legionella species. Infect. Immun. 53, 553–559. doi: 10.1128/iai.53.3.553-559.1986
Fields, B. S., Benson, R. F., and Besser, R. E. (2002). Legionella and legionnaires' disease: 25 years of investigation. Clin. Microbiol. Rev. 15, 506–526. doi: 10.1128/CMR.15.3.506-526.2002
Fliermans, C. B. (1996). Ecology of Legionella: from data to knowledge with a little wisdom. Microb. Ecol. 32, 203–228. doi: 10.1007/BF00185888
Fliermans, C. B., Cherry, W. B., Orrison, L. H., Smith, S. J., Tison, D. L., and Pope, D. H. (1981). Ecological distribution of Legionella pneumophila. Appl. Environ. Microbiol. 41, 9–16. doi: 10.1128/aem.41.1.9-16.1981
Fontana, M. F., Shin, S., and Vance, R. E. (2012). Activation of host mitogen-activated protein kinases by secreted Legionella pneumophila effectors that inhibit host protein translation. Infect. Immun. 80, 3570–3575. doi: 10.1128/IAI.00557-12
Forgac, M. (2007). Vacuolar Atpases: rotary proton pumps in physiology and pathophysiology. Nat. Rev. Mol. Cell Biol. 8, 917–929. doi: 10.1038/nrm2272
Fouque, E., Héchard, Y., Hartemann, P., Humeau, P., and Trouilhé, M. C. (2015). Sensitivity of Vermamoeba (Hartmannella) vermiformis cysts to conventional disinfectants and protease. J. Water Health 13, 302–310. doi: 10.2166/wh.2014.154
Franco, I. S., Shohdy, N., and Shuman, H. A. (2012). The Legionella pneumophila effector VipA is an actin nucleator that alters host cell organelle trafficking. PLoS Pathog. 8:e1002546. doi: 10.1371/journal.ppat.1002546
Fraser, D. W., Tsai, T. R., Orenstein, W., Parkin, W. E., Beecham, H. J., Sharrar, R. G., et al. (1977). Legionnaires' disease: description of an epidemic of pneumonia. N. Engl. J. Med. 297, 1189–1197. doi: 10.1056/NEJM197712012972201
Gandhi, N. S., and Mancera, R. L. (2008). The structure of glycosaminoglycans and their interactions with proteins. Chem. Biol. Drug Des. 72, 455–482. doi: 10.1111/j.1747-0285.2008.00741.x
Gao, L. Y., and Kwaik, Y. A. (2000). The mechanism of killing and exiting the protozoan host Acanthamoeba polyphaga by Legionella pneumophila. Environ. Microbiol. 2, 79–90. doi: 10.1046/j.1462-2920.2000.00076.x
García, M. T., Jones, S., Pelaz, C., Millar, R. D., and Abu Kwaik, Y. (2007). Acanthamoeba polyphaga resuscitates viable non-culturable Legionella pneumophila after disinfection. Environ. Microbiol. 9, 1267–1277. doi: 10.1111/j.1462-2920.2007.01245.x
Garduño, R. A., Garduño, E., Hiltz, M., and Hoffman, P. S. (2002). Intracellular growth of Legionella pneumophila gives rise to a differentiated form dissimilar to stationary-phase forms. Infect. Immun. 70, 6273–6283. doi: 10.1128/IAI.70.11.6273-6283.2002
Garg, D., and Daigavane, S. (2024). A comprehensive review on Acanthamoeba keratitis: an overview of epidemiology, risk factors, and therapeutic strategies. Cureus 16:e67803. doi: 10.7759/cureus.67803
Gharpure, R., Bliton, J., Goodman, A., Ali, I. K. M., Yoder, J., and Cope, J. R. (2021). Epidemiology and clinical characteristics of primary amebic meningoencephalitis caused by Naegleria fowleri: a global review. Clin. Infect. Dis. 73, e19–e27. doi: 10.1093/cid/ciaa520
Gillooly, D. J., Simonsen, A., and Stenmark, H. (2001). Phosphoinositides and phagocytosis. J. Cell Biol. 155, 15–17. doi: 10.1083/jcb.200109001
Gomes, T. S., Gjiknuri, J., Magnet, A., Vaccaro, L., Ollero, D., Izquierdo, F., et al. (2018). The influence of Acanthamoeba-Legionella interaction in the virulence of two different Legionella species. Front. Microbiol. 9:2962. doi: 10.3389/fmicb.2018.02962
Gomez-Valero, L., Rusniok, C., and Buchrieser, C. (2009). Legionella pneumophila: population genetics, phylogeny and genomics. Infect. Genet. Evol. 9, 727–739. doi: 10.1016/j.meegid.2009.05.004
Gomez-Valero, L., Rusniok, C., Cazalet, C., and Buchrieser, C. (2011). Comparative and functional genomics of legionella identified eukaryotic like proteins as key players in host-pathogen interactions. Front. Microbiol. 2:208. doi: 10.3389/fmicb.2011.00208
Güémez, A., and García, E. (2021). Primary amoebic meningoencephalitis by Naegleria fowleri: pathogenesis and treatments. Biomolecules 11:1320. doi: 10.3390/biom11091320
Guo, S., Chang, Y., Brun, Y. V., Howell, P. L., Burrows, L. L., and Liu, J. (2024). PilY1 regulates the dynamic architecture of the type iv pilus machine in Pseudomonas aeruginosa. Nat. Commun. 15:9382. doi: 10.1038/s41467-024-53638-y
Hadwiger, J. A., and Nguyen, H. N. (2011). Mapks in development: insights from Dictyostelium signaling pathways. Biomol. Concepts 2, 39–46. doi: 10.1515/bmc.2011.004
Harb, O. S., Venkataraman, C., Haack, B. J., Gao, L. Y., and Kwaik, Y. A. (1998). Heterogeneity in the attachment and uptake mechanisms of the legionnaires' disease bacterium, Legionella pneumophila, by protozoan hosts. Appl. Environ. Microbiol. 64, 126–132. doi: 10.1128/AEM.64.1.126-132.1998
Hayashi, T., Miyake, M., Fukui, T., Sugaya, N., Daimon, T., Itoh, S., et al. (2008). Exclusion of actin-binding protein p57/coronin-1 from bacteria-containing phagosomes in macrophages infected with Legionella. Biol. Pharm. Bull. 31, 861–865. doi: 10.1248/bpb.31.861
He, L., Lin, Y., Ge, Z. H., He, S. Y., Zhao, B. B., Shen, D., et al. (2019). The Legionella pneumophila effector WipA disrupts host F-actin polymerisation by hijacking phosphotyrosine signalling. Cell. Microbiol. 21:e13014. doi: 10.1111/cmi.13014
Hennebique, A., Peyroux, J., Brunet, C., Martin, A., Henry, T., Knezevic, M., et al. (2021). Amoebae can promote the survival of Francisella species in the aquatic environment. Emerg Microbes Infect 10, 277–290. doi: 10.1080/22221751.2021.1885999
Hilbi, H., Rothmeier, E., Hoffmann, C., and Harrison, C. F. (2014). Beyond Rab Gtpases Legionella activates the small Gtpase ran to promote microtubule polymerization, pathogen vacuole motility, and infection. Small GTpases 5, 1–6. doi: 10.4161/21541248.2014.972859
Hilbi, H., Segal, G., and Shuman, H. A. (2001). Icm/dot-dependent upregulation of phagocytosis by Legionella pneumophila. Mol. Microbiol. 42, 603–617. doi: 10.1046/j.1365-2958.2001.02645.x
Hojo, F., Osaki, T., Yonezawa, H., Hanawa, T., Kurata, S., and Kamiya, S. (2020). Acanthamoeba castellanii supports extracellular survival of Helicobacter pylori in co-culture. J. Infect. Chemother. 26, 946–954. doi: 10.1016/j.jiac.2020.04.016
Hoppe, J., Ünal, C. M., Thiem, S., Grimpe, L., Goldmann, T., Gaßler, N., et al. (2017). PilY1 promotes Legionella pneumophila infection of human lung tissue explants and contributes to bacterial adhesion, host cell invasion, and twitching motility. Front. Cell. Infect. Microbiol. 7:63. doi: 10.3389/fcimb.2017.00063
Horwitz, M. A. (1983a). Formation of a novel phagosome by the legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J. Exp. Med. 158, 1319–1331. doi: 10.1084/jem.158.4.1319
Horwitz, M. A. (1983b). The legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J. Exp. Med. 158, 2108–2126. doi: 10.1084/jem.158.6.2108
Horwitz, M. A., and Maxfield, F. R. (1984). Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J. Cell Biol. 99, 1936–1943
Hsu, B. M., Huang, C. C., Chen, J. S., Chen, N. H., and Huang, J. T. (2011). Comparison of potentially pathogenic free-living amoeba hosts by Legionella spp. in substrate-associated biofilms and floating biofilms from spring environments. Water Res. 45, 5171–5183. doi: 10.1016/j.watres.2011.07.019
Hubber, A., and Roy, C. R. (2010). Modulation of host cell function by Legionella pneumophila type iv effectors. Annu. Rev. Cell Dev. Biol. 26, 261–283. doi: 10.1146/annurev-cellbio-100109-104034
Ikedo, M., and Yabuuchi, E. (1986). Ecological studies of Legionella species. I. Viable counts of Legionella pneumophila in cooling tower water. Microbiol. Immunol. 30, 413–423. doi: 10.1111/j.1348-0421.1986.tb02967.x
Iovieno, A., Ledee, D. R., Miller, D., and Alfonso, E. C. (2010). Detection of bacterial endosymbionts in clinical Acanthamoeba isolates. Ophthalmology 117, 445–452. doi: 10.1016/j.ophtha.2009.08.03
Isberg, R. R., O'connor, T. J., and Heidtman, M. (2009). The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat. Rev. Microbiol. 7, 13–24. doi: 10.1038/nrmicro1967
Jeon, K. W., and Jeon, M. S. (1976). Endosymbiosis in amoebae: recently established endosymbionts have become required cytoplasmic components. J. Cell. Physiol. 89, 337–344. doi: 10.1002/jcp.1040890216
Jeon, K. W., and Lorch, I. J. (1967). Unusual intra-cellular bacterial infection in large, free-living amoebae. Exp. Cell Res. 48, 236–240
Ji, W. T., Hsu, B. M., Chang, T. Y., Hsu, T. K., Kao, P. M., Huang, K. H., et al. (2014). Surveillance and evaluation of the infection risk of free-living amoebae and Legionella in different aquatic environments. Sci. Total Environ. 499, 212–219. doi: 10.1016/j.scitotenv.2014.07.116
Jia, Q., Lin, Y., Gou, X., He, L., Shen, D., Chen, D., et al. (2018). Legionella pneumophila effector WipA, a bacterial Ppp protein phosphatase with Ptp activity. Acta Biochim. Biophys. Sin. Shanghai 50, 547–554. doi: 10.1093/abbs/gmy042
Kanarek, P., Bogiel, T., and Breza-Boruta, B. (2022). Legionellosis risk-an overview of Legionella spp. habitats in Europe. Environ. Sci. Pollut. Res. Int. 29, 76532–76542. doi: 10.1007/s11356-022-22950-9
Khan, N. A. (2006). Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol. Rev. 30, 564–595. doi: 10.1111/j.1574-6976.2006.00023.x
Khelef, N., Shuman, H. A., and Maxfield, F. R. (2001). Phagocytosis of wild-type Legionella pneumophila occurs through a wortmannin-insensitive pathway. Infect. Immun. 69, 5157–5161. doi: 10.1128/IAI.69.8.5157-5161.2001
Kilvington, S., and Price, J. (1990). Survival of Legionella pneumophila within cysts of Acanthamoeba polyphaga following chlorine exposure. J. Appl. Bacteriol. 68, 519–525. doi: 10.1111/j.1365-2672.1990.tb02904.x
Kirby, J. E., Vogel, J. P., Andrews, H. L., and Isberg, R. R. (1998). Evidence for pore-forming ability by Legionella pneumophila. Mol. Microbiol. 27, 323–336. doi: 10.1046/j.1365-2958.1998.00680.x
Koide, M., Higa, F., Tateyama, M., Cash, H. L., Hokama, A., and Fujita, J. (2014). Role of Brevundimonas vesicularis in supporting the growth of Legionella in nutrient-poor environments. New Microbiol. 37, 33–39
Kowalczyk, B., Petzold, M., Kaczyński, Z., Szuster-Ciesielska, A., Luchowski, R., Gruszecki, W. I., et al. (2023). Lipopolysaccharide of Legionella pneumophila Serogroup 1 facilitates interaction with host cells. Int. J. Mol. Sci. 24:4602. doi: 10.3390/ijms241914602
Krachler, A. M., Woolery, A. R., and Orth, K. (2011). Manipulation of kinase signaling by bacterial pathogens. J. Cell Biol. 195, 1083–1092. doi: 10.1083/jcb.201107132
Krishna Prasad, B. N., and Gupta, S. K. (1978). Preliminary report on the engulement and retention of mycobacteria by trophozoites of exenically grown Acanthamoeba castellanii Douglas, 1930. Curr. Sci. 47, 245–247.
Kunze, M., Steiner, T., Chen, F., Huber, C., Rydzewski, K., Stämmler, M., et al. (2021). Metabolic adaption of Legionella pneumophila during intracellular growth in Acanthamoeba castellanii. Int. J. Med. Microbiol. 311:151504. doi: 10.1016/j.ijmm.2021.151504
Lally, E. T., Kieba, I. R., Sato, A., Green, C. L., Rosenbloom, J., Korostoff, J., et al. (1997). Rtx toxins recognize a beta2 integrin on the surface of human target cells. J. Biol. Chem. 272, 30463–30469
Långmark, J., Storey, M. V., Ashbolt, N. J., and Stenström, T. A. (2007). The effects of Uv disinfection on distribution pipe biofilm growth and pathogen incidence within the greater Stockholm area, Sweden. Water Res. 41, 3327–3336. doi: 10.1016/j.watres.2007.04.024
Lasheras, A., Boulestreau, H., Rogues, A. M., Ohayon-Courtes, C., Labadie, J. C., and Gachie, J. P. (2006). Influence of amoebae and physical and chemical characteristics of water on presence and proliferation of Legionella species in hospital water systems. Am. J. Infect. Control 34, 520–525. doi: 10.1016/j.ajic.2006.03.007
Lau, H. Y., and Ashbolt, N. J. (2009). The role of biofilms and protozoa in Legionella pathogenesis: implications for drinking water. J. Appl. Microbiol. 107, 368–378. doi: 10.1111/j.1365-2672.2009.04208.x
Lechevallier, M. W., Prosser, T., and Stevens, M. (2024). Opportunistic pathogens in drinking water distribution systems-a review. Microorganisms 12:916. doi: 10.3390/microorganisms12050916
Lee, D. C., Fiester, S. E., Madeline, L. A., Fulcher, J. W., Ward, M. E., Schammel, C. M., et al. (2020). Acanthamoeba spp. and Balamuthia mandrillaris leading to fatal granulomatous amebic encephalitis. Forensic Sci. Med. Pathol. 16, 171–176. doi: 10.1007/s12024-019-00202-6
Lehtola, M. J., Torvinen, E., Kusnetsov, J., Pitkänen, T., Maunula, L., Von Bonsdorff, C. H., et al. (2007). Survival of Mycobacterium avium, Legionella pneumophila, Escherichia coli, and caliciviruses in drinking water-associated biofilms grown under high-shear turbulent flow. Appl. Environ. Microbiol. 73, 2854–2859. doi: 10.1128/AEM.02916-06
Lemmon, M. A. (2007). Pleckstrin homology (Ph) domains and phosphoinositides. Biochem. Soc. Symp. 2007, 81–93. doi: 10.1042/BSS0740081
Li, Z., Dugan, A. S., Bloomfield, G., Skelton, J., Ivens, A., Losick, V., et al. (2009). The amoebal map kinase response to Legionella pneumophila is regulated by DupA. Cell Host Microbe 6, 253–267. doi: 10.1016/j.chom.2009.08.005
Lin, Y. E., Stout, J. E., and Yu, V. L. (2011). Prevention of hospital-acquired legionellosis. Curr. Opin. Infect. Dis. 24, 350–356. doi: 10.1097/QCO.0b013e3283486c6e
Loovers, H. M., Postma, M., Keizer-Gunnink, I., Huang, Y. E., Devreotes, P. N., and Van Haastert, P. J. (2006). Distinct roles of pi(3,4,5)P3 during chemoattractant signaling in Dictyostelium: a quantitative in vivo analysis by inhibition of Pi3-kinase. Mol. Biol. Cell 17, 1503–1513. doi: 10.1091/mbc.e05-09-0825
Lopez, A. E., Grigoryeva, L. S., Barajas, A., and Cianciotto, N. P. (2023). Legionella pneumophila Rhizoferrin promotes bacterial biofilm formation and growth within amoebae and macrophages. Infect. Immun. 91:e0007223. doi: 10.1128/iai.00072-23
López-García, P., Eme, L., and Moreira, D. (2017). Symbiosis in eukaryotic evolution. J. Theor. Biol. 434, 20–33. doi: 10.1016/j.jtbi.2017.02.031
Loret, J., Jousset, M., Robert, S., Anselme, C., Saucedo, G., Ribas, F., et al. (2008). Elimination of free-living amoebae by drinking water treatment processes. Eur. J. Water Qual. 39, 37–50.
Lu, H., and Clarke, M. (2005). Dynamic properties of Legionella-containing phagosomes in Dictyostelium amoebae. Cell. Microbiol. 7, 995–1007. doi: 10.1111/j.1462-5822.2005.00528.x
Ma, Y., and Nicolet, J. (2023). Specificity models in Mapk cascade signaling. Febs Open Bio 13, 1177–1192. doi: 10.1002/2211-5463.13619
Maehara, T., Mizuno, T., Tokoro, M., Hara, T., Tomita, Y., Makioka, K., et al. (2022). An autopsy case of granulomatous amebic encephalitis caused by Balamuthia mandrillaris involving prior amebic dermatitis. Neuropathology 42, 190–196. doi: 10.1111/neup.12798
Mampel, J., Spirig, T., Weber, S. S., Haagensen, J. A., Molin, S., and Hilbi, H. (2006). Planktonic replication is essential for biofilm formation by Legionella pneumophila in a complex medium under static and dynamic flow conditions. Appl. Environ. Microbiol. 72, 2885–2895. doi: 10.1128/AEM.72.4.2885-2895.2006
Marciano-Cabral, F. (2009). Free-living amoebae as agents of human infection. J. Infect. Dis. 199, 1104–1106. doi: 10.1086/597474
Marrie, T. J. (2008). “Legionnaires’ disease: clinical picture” in eds. Heuner, K and Swanson, M. Legionella pneumophila (New York: Springer-Verlag), 133–150.
Mcdade, J. E., Shepard, C. C., Fraser, D. W., Tsai, T. R., Redus, M. A., and Dowdle, W. R. (1977). Legionnaires' disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N. Engl. J. Med. 297, 1197–1203.
Michard, C., Sperandio, D., Baïlo, N., Pizarro-Cerdá, J., Leclaire, L., Chadeau-Argaud, E., et al. (2015). The Legionella kinase LegK2 targets the Arp2/3 complex to inhibit actin nucleation on phagosomes and allow bacterial evasion of the late endocytic pathway. MBio 6:e00354. doi: 10.1128/mBio.00354-15
Miller, H. C., Wylie, J., Dejean, G., Kaksonen, A. H., Sutton, D., Braun, K., et al. (2015). Reduced efficiency of chlorine disinfection of Naegleria fowleri in a drinking water distribution biofilm. Environ. Sci. Technol. 49, 11125–11131. doi: 10.1021/acs.est.5b02947
Miyamoto, M., Yamaguchi, Y., and Sasatsu, M. (2000). Disinfectant effects of hot water, ultraviolet light, silver ions and chlorine on strains of Legionella and nontuberculous mycobacteria. Microbios 101, 7–13
Miyashita, N., Higa, F., Aoki, Y., Kikuchi, T., Seki, M., Tateda, K., et al. (2020). Distribution of Legionella species and serogroups in patients with culture-confirmed Legionella pneumonia. J. Infect. Chemother. 26, 411–417. doi: 10.1016/j.jiac.2019.12.016
Moliner, C., Fournier, P. E., and Raoult, D. (2010). Genome analysis of microorganisms living in amoebae reveals a melting pot of evolution. FEMS Microbiol. Rev. 34, 281–294. doi: 10.1111/j.1574-6976.2009.00209.x
Molofsky, A. B., and Swanson, M. S. (2004). Differentiate to thrive: lessons from the Legionella pneumophila life cycle. Mol. Microbiol. 53, 29–40. doi: 10.1111/j.1365-2958.2004.04129.x
Mou, Q., and Leung, P. H. M. (2018). Differential expression of virulence genes in Legionella pneumophila growing in Acanthamoeba and human monocytes. Virulence 9, 185–196. doi: 10.1080/21505594.2017.1373925
Murga, R., Forster, T. S., Brown, E., Pruckler, J. M., Fields, B. S., and Donlan, R. M. (2001). Role of biofilms in the survival of Legionella pneumophila in a model potable-water system. Microbiology 147, 3121–3126. doi: 10.1099/00221287-147-11-3121
Network, The European Centre for Disease Prevention and Control. (2021). Surveillance Atlas of Infectious Diseases. Available online at: https://atlas.ecdc.europa.eu/public/index.aspx (Accessed October 3, 2021).
Neumeister, B., Reiff, G., Faigle, M., Dietz, K., Northoff, H., and Lang, F. (2000). Influence of Acanthamoeba castellanii on intracellular growth of different Legionella species in human monocytes. Appl. Environ. Microbiol. 66, 914–919. doi: 10.1128/AEM.66.3.914-919.2000
Newsome, A. L., Baker, R. L., Miller, R. D., and Arnold, R. R. (1985). Interactions between Naegleria fowleri and Legionella pneumophila. Infect. Immun. 50, 449–452. doi: 10.1128/iai.50.2.449-452.1985
Newton, H. J., Sansom, F. M., Bennett-Wood, V., and Hartland, E. L. (2006). Identification of Legionella pneumophila-specific genes by genomic subtractive hybridization with Legionella micdadei and identification of lpnE, a gene required for efficient host cell entry. Infect. Immun. 74, 1683–1691. doi: 10.1128/IAI.74.3.1683-1691.2006
Newton, H. J., Sansom, F. M., Dao, J., Cazalet, C., Bruggemann, H., Albert-Weissenberger, C., et al. (2008). Significant role for ladC in initiation of Legionella pneumophila infection. Infect. Immun. 76, 3075–3085. doi: 10.1128/IAI.00209-08
Nguyen, Y., Sugiman-Marangos, S., Harvey, H., Bell, S. D., Charlton, C. L., Junop, M. S., et al. (2015). Pseudomonas aeruginosa minor pilins prime type Iva pilus assembly and promote surface display of the PilY1 adhesin. J. Biol. Chem. 290, 601–611. doi: 10.1074/jbc.M114.616904
Nora, T., Lomma, M., Gomez-Valero, L., and Buchrieser, C. (2009). Molecular mimicry: an important virulence strategy employed by Legionella pneumophila to subvert host functions. Future Microbiol. 4, 691–701. doi: 10.2217/fmb.09.47
Nowack, E. C., and Melkonian, M. (2010). Endosymbiotic associations within protists. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 365, 699–712. doi: 10.1098/rstb.2009.0188
Otero-Ruiz, A., Gonzalez-Zuñiga, L. D., Rodriguez-Anaya, L. Z., Lares-Jiménez, L. F., Gonzalez-Galaviz, J. R., and Lares-Villa, F. (2022). Distribution and current state of molecular genetic characterization in pathogenic free-living amoebae. Pathogens 11:199. doi: 10.3390/pathogens11101199
Pagnier, I., Merchat, M., and La Scola, B. (2009). Potentially pathogenic amoeba-associated microorganisms in cooling towers and their control. Future Microbiol. 4, 615–629. doi: 10.2217/fmb.09.25
Palusinska-Szysz, M., Luchowski, R., Gruszecki, W. I., Choma, A., Szuster-Ciesielska, A., Lück, C., et al. (2019). The role of Legionella pneumophila Serogroup 1 lipopolysaccharide in host-pathogen interaction. Front. Microbiol. 10:2890. doi: 10.3389/fmicb.2019.02890
Park, M., Yun, S. T., Kim, M. S., Chun, J., and Ahn, T. I. (2004). Phylogenetic characterization of Legionella-like endosymbiotic X-bacteria in Amoeba proteus: a proposal for 'Candidatus Legionella jeonii' sp. nov. Environ. Microbiol. 6, 1252–1263. doi: 10.1111/j.1462-2920.2004.00659.x
Pazoki, H., Niyyati, M., Javanmard, E., Lasjerdi, Z., Spotin, A., Mirjalali, H., et al. (2020). Isolation and phylogenetic analysis of free-living amoebae (Acanthamoeba, Naegleria, and Vermamoeba) in the farmland soils and recreational places in Iran. Acta Parasitol. 65, 36–43. doi: 10.2478/s11686-019-00126-9
Peracino, B., Balest, A., and Bozzaro, S. (2010). Phosphoinositides differentially regulate bacterial uptake and Nramp1-induced resistance to Legionella infection in Dictyostelium. J. Cell Sci. 123, 4039–4051. doi: 10.1242/jcs.072124
Personnic, N., Striednig, B., Lezan, E., Manske, C., Welin, A., Schmidt, A., et al. (2019). Quorum sensing modulates the formation of virulent Legionella persisters within infected cells. Nat. Commun. 10:5216. doi: 10.1038/s41467-019-13021-8
Petrillo, F., Tortori, A., Vallino, V., Galdiero, M., Fea, A. M., De Sanctis, U., et al. (2024). Understanding Acanthamoeba keratitis: an in-depth review of a sight-threatening eye infection. Microorganisms 12:758. doi: 10.3390/microorganisms12040758
Phin, N., Parry-Ford, F., Harrison, T., Stagg, H. R., Zhang, N., Kumar, K., et al. (2014). Epidemiology and clinical management of legionnaires' disease. Lancet Infect. Dis. 14, 1011–1021. doi: 10.1016/S1473-3099(14)70713-3
Pinotsis, N., and Waksman, G. (2017). Structure of the WipA protein reveals a novel tyrosine protein phosphatase effector from Legionella pneumophila. J. Biol. Chem. 292, 9240–9251. doi: 10.1074/jbc.M117.781948
Pope, D. H., Soracco, R. J., Gill, H. K., and Fliermans, C. B. (1982). Growth of Legionella pneumophila in two-membered cultures with green algae and cyanobacteria. Curr. Microbiol. 7, 319–321.
Potgieter, N., Van Der Loo, C., and Barnard, T. G. (2021). Co-existence of free-living amoebae and potential human pathogenic bacteria isolated from rural household water storage containers. Biology 10:1228. doi: 10.3390/biology10121228
Price, C. T., Al-Quadan, T., Santic, M., Rosenshine, I., and Abu Kwaik, Y. (2011). Host proteasomal degradation generates amino acids essential for intracellular bacterial growth. Science 334, 1553–1557. doi: 10.1126/science.1212868
Proca-Ciobanu, M., Lupascu, G. H., Petrovici, A., and Ionescu, M. D. (1975). Electron microscopic study of a pathogenic Acanthamoeba castellani strain: the presence of bacterial endosymbionts. Int. J. Parasitol. 5, 49–56. doi: 10.1016/0020-7519(75)90097-1
Puzon, G. J., Lancaster, J. A., Wylie, J. T., and Plumb, I. J. (2009). Rapid detection of Naegleria fowleri in water distribution pipeline biofilms and drinking water samples. Environ. Sci. Technol. 43, 6691–6696. doi: 10.1021/es900432m
Quaile, A. T., Stogios, P. J., Egorova, O., Evdokimova, E., Valleau, D., Nocek, B., et al. (2018). The Legionella pneumophila effector Ceg4 is a phosphotyrosine phosphatase that attenuates activation of eukaryotic Mapk pathways. J. Biol. Chem. 293, 3307–3320. doi: 10.1074/jbc.M117.812727
Qvarnstrom, Y., Da Silva, A. J., Schuster, F. L., Gelman, B. B., and Visvesvara, G. S. (2009). Molecular confirmation of Sappinia pedata as a causative agent of amoebic encephalitis. J. Infect. Dis. 199, 1139–1142. doi: 10.1086/597473
Rasch, J., Krüger, S., Fontvieille, D., Ünal, C. M., Michel, R., Labrosse, A., et al. (2016). Legionella-protozoa-nematode interactions in aquatic biofilms and influence of Mip on Caenorhabditis elegans colonization. Int. J. Med. Microbiol. 306, 443–451. doi: 10.1016/j.ijmm.2016.05.012
Rayamajhee, B., Sharma, S., Willcox, M., Henriquez, F. L., Rajagopal, R. N., Shrestha, G. S., et al. (2022). Assessment of genotypes, endosymbionts and clinical characteristics of Acanthamoeba recovered from ocular infection. BMC Infect. Dis. 22:757. doi: 10.1186/s12879-022-07741-4
Rayamajhee, B., Subedi, D., Peguda, H. K., Willcox, M. D., Henriquez, F. L., and Carnt, N. (2021). A systematic review of intracellular microorganisms within Acanthamoeba to understand potential impact for infection. Pathogens 10:225. doi: 10.3390/pathogens10020225
Rayamajhee, B., Willcox, M., Henriquez, F. L., Vijay, A. K., Petsoglou, C., Shrestha, G. S., et al. (2024). The role of naturally acquired intracellular Pseudomonas aeruginosa in the development of Acanthamoeba keratitis in an animal model. PLoS Negl. Trop. Dis. 18:e0011878. doi: 10.1371/journal.pntd.0011878
Rehman, S., Antonovic, A. K., Mcintire, I. E., Zheng, H., Cleaver, L., Baczynska, M., et al. (2024). The Legionella collagen-like protein employs a distinct binding mechanism for the recognition of host glycosaminoglycans. Nat. Commun. 15:4912. doi: 10.1038/s41467-024-49255-4
Robey, M., O'connell, W., and Cianciotto, N. P. (2001). Identification of Legionella pneumophila rcp, a pagP-like gene that confers resistance to cationic antimicrobial peptides and promotes intracellular infection. Infect. Immun. 69, 4276–4286. doi: 10.1128/IAI.69.7.4276-4286.2001
Rogers, J., and Keevil, C. W. (1992). Immunogold and fluorescein immunolabelling of Legionella pneumophila within an aquatic biofilm visualized by using episcopic differential interference contrast microscopy. Appl. Environ. Microbiol. 58, 2326–2330. doi: 10.1128/aem.58.7.2326-2330.1992
Rolando, M., and Buchrieser, C. (2012). Post-translational modifications of host proteins by Legionella pneumophila: a sophisticated survival strategy. Future Microbiol. 7, 369–381. doi: 10.2217/fmb.12.9
Romby, P., Vandenesch, F., and Wagner, E. G. (2006). The role of RNAs in the regulation of virulence-gene expression. Curr. Opin. Microbiol. 9, 229–236. doi: 10.1016/j.mib.2006.02.005
Rowbotham, T. J. (1980). Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 33, 1179–1183. doi: 10.1136/jcp.33.12.1179
Rowbotham, T. J. (1983). Isolation of Legionella pneumophila from clinical specimens via amoebae, and the interaction of those and other isolates with amoebae. J. Clin. Pathol. 36, 978–986. doi: 10.1136/jcp.36.9.978
Sabria, M., and Yu, V. L. (2002). Hospital-acquired legionellosis: solutions for a preventable infection. Lancet Infect. Dis. 2, 368–373. doi: 10.1016/S1473-3099(02)00291-8
Samba-Louaka, A., Robino, E., Cochard, T., Branger, M., Delafont, V., Aucher, W., et al. (2018). Environmental Mycobacterium avium subsp. paratuberculosis hosted by free-living amoebae. Front. Cell. Infect. Microbiol. 8:28. doi: 10.3389/fcimb.2018.00028
Santos, H. L. C. (2025). Free-living amoebae: a journey into historical aspects and to current discoveries. Mem. Inst. Oswaldo Cruz 120:e240246. doi: 10.1590/0074-02760240246
Sarkar, P., and Gerba, C. P. (2012). Inactivation of Naegleria fowleri by chlorine and ultraviolet light. J. Am. Water Works Assoc. 104, E173–E180.
Sauer, J. D., Bachman, M. A., and Swanson, M. S. (2005). The phagosomal transporter a couples threonine acquisition to differentiation and replication of Legionella pneumophila in macrophages. Proc. Natl. Acad. Sci. USA 102, 9924–9929. doi: 10.1073/pnas.0502767102
Sawczyn-Domańska, A. (2021). Detection of Legionella spp. and occurrence of virulence genes: lvh, rtxA and enhC in water samples from artificial water systems. Ann. Agric. Environ. Med. 28, 617–620. doi: 10.26444/aaem/143745
Scheid, P. (2014). Relevance of free-living amoebae as hosts for phylogenetically diverse microorganisms. Parasitol. Res. 113, 2407–2414. doi: 10.1007/s00436-014-3932-7
Scheid, P. L. (2019). Vermamoeba vermiformis—a free-living amoeba with public health and environmental health significance. Open Parasitol J 7, 40–47. doi: 10.2174/1874421401907010040
Schuster, F. L., and Levandowsky, M. (1996). Chemosensory responses of Acanthamoeba castellanii: visual analysis of random movement and responses to chemical signals. J. Eukaryot. Microbiol. 43, 150–158. doi: 10.1111/j.1550-7408.1996.tb04496.x
Schuster, F. L., and Visvesvara, G. S. (2004). Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int. J. Parasitol. 34, 1001–1027. doi: 10.1016/j.ijpara.2004.06.004
Scott, C. C., Dobson, W., Botelho, R. J., Coady-Osberg, N., Chavrier, P., Knecht, D. A., et al. (2005). Phosphatidylinositol-4,5-bisphosphate hydrolysis directs actin remodeling during phagocytosis. J. Cell Biol. 169, 139–149. doi: 10.1083/jcb.200412162
Shaheen, M., Scott, C., and Ashbolt, N. J. (2019). Long-term persistence of infectious Legionella with free-living amoebae in drinking water biofilms. Int. J. Hyg. Environ. Health 222, 678–686. doi: 10.1016/j.ijheh.2019.04.007
Shen, Y., Monroy, G. L., Derlon, N., Janjaroen, D., Huang, C., Morgenroth, E., et al. (2015). Role of biofilm roughness and hydrodynamic conditions in Legionella pneumophila adhesion to and detachment from simulated drinking water biofilms. Environ. Sci. Technol. 49, 4274–4282. doi: 10.1021/es505842v
Sherwood, R. K., and Roy, C. R. (2013). A Rab-centric perspective of bacterial pathogen-occupied vacuoles. Cell Host Microbe 14, 256–268. doi: 10.1016/j.chom.2013.08.010
Shevchuk, O., Batzilla, C., Hägele, S., Kusch, H., Engelmann, S., Hecker, M., et al. (2009). Proteomic analysis of Legionella-containing phagosomes isolated from Dictyostelium. Int. J. Med. Microbiol. 299, 489–508. doi: 10.1016/j.ijmm.2009.03.006
Shi, Y., Queller, D. C., Tian, Y., Zhang, S., Yan, Q., He, Z., et al. (2021). The ecology and evolution of Amoeba-bacterium interactions. Appl. Environ. Microbiol. 87:20. doi: 10.1128/AEM.01866-20
Shin, S. (2012). Innate immunity to intracellular pathogens: lessons learned from Legionella pneumophila. Adv. Appl. Microbiol. 79, 43–71. doi: 10.1016/B978-0-12-394318-7.00003-6
Shin, S., and Roy, C. R. (2008). Host cell processes that influence the intracellular survival of Legionella pneumophila. Cell. Microbiol. 10, 1209–1220. doi: 10.1111/j.1462-5822.2008.01145.x
Simon, S., Schell, U., Heuer, N., Hager, D., Albers, M. F., Matthias, J., et al. (2015). Inter-kingdom signaling by the Legionella quorum sensing molecule Lai-1 modulates cell migration through an Iqgap1-Cdc42-Arhgef9-dependent pathway. PLoS Pathog. 11:e1005307. doi: 10.1371/journal.ppat.1005307
Singh, T., and Coogan, M. M. (2005). Isolation of pathogenic Legionella species and legionella-laden amoebae in dental unit waterlines. J. Hosp. Infect. 61, 257–262. doi: 10.1016/j.jhin.2005.05.001
Spottiswoode, N., Haston, J. C., Hanners, N. W., Gruenberg, K., Kim, A., Derisi, J. L., et al. (2024). Challenges and advances in the medical treatment of granulomatous amebic encephalitis. Ther. Adv. Infect. Dis. 11:20499361241228340. doi: 10.1177/20499361241228340
Stein, M. P., Müller, M. P., and Wandinger-Ness, A. (2012). Bacterial pathogens commandeer Rab Gtpases to establish intracellular niches. Traffic 13, 1565–1588. doi: 10.1111/tra.12000
Steinert, M., Heuner, K., Buchrieser, C., Albert-Weissenberger, C., and Glöckner, G. (2007). Legionella pathogenicity: genome structure, regulatory networks and the host cell response. Int. J. Med. Microbiol. 297, 577–587. doi: 10.1016/j.ijmm.2007.03.009
Stewart, C. R., Muthye, V., and Cianciotto, N. P. (2012). Legionella pneumophila persists within biofilms formed by Klebsiella pneumoniae, Flavobacterium sp., and Pseudomonas fluorescens under dynamic flow conditions. PLoS One 7:e50560. doi: 10.1371/journal.pone.0050560
Stone, B. J., and Abu Kwaik, Y. (1998). Expression of multiple pili by Legionella pneumophila: identification and characterization of a type IV pilin gene and its role in adherence to mammalian and protozoan cells. Infect. Immun. 66, 1768–1775. doi: 10.1128/IAI.66.4.1768-1775.1998
Stone, B. J., and Kwaik, Y. A. (1999). Natural competence for Dna transformation by Legionella pneumophila and its association with expression of type iv pili. J. Bacteriol. 181, 1395–1402. doi: 10.1128/JB.181.5.1395-1402.1999
Stout, J. E., Best, M. G., Yu, V. L., and Rihs, J. D. (1986). A note on symbiosis of Legionella pneumophila and Tatlockia micdadei with human respiratory flora. J. Appl. Bacteriol. 60, 297–299. doi: 10.1111/j.1365-2672.1986.tb01736.x
Sturgill-Koszycki, S., and Swanson, M. S. (2000). Legionella pneumophila replication vacuoles mature into acidic, endocytic organelles. J. Exp. Med. 192, 1261–1272. doi: 10.1084/jem.192.9.1261
Swart, A. L., Harrison, C. F., Eichinger, L., Steinert, M., and Hilbi, H. (2018). Acanthamoeba and Dictyostelium as cellular models for Legionella infection. Front. Cell. Infect. Microbiol. 8:61. doi: 10.3389/fcimb.2018.00061
Swart, A. L., Steiner, B., Gomez-Valero, L., Schütz, S., Hannemann, M., Janning, P., et al. (2020). Divergent evolution of Legionella Rcc1 repeat effectors defines the range of ran GTPase cycle targets. MBio 11:20. doi: 10.1128/mBio.00405-20
Szewzyk, R., Baz, G., and Bartocha, W. (2000). “Survival and growth of Legionella pneumophila in biofilms of drinking water bacteria and amoebae” in American Society for Microbiology conference on biofilms 2000 (Washington, DC: American Society for Microbiology).
Szewzyk, U., Manz, W., Amann, R., Schleifer, K. H., and Stenstrom, T. A. (1994). Growth and in-situ detection of a pathogenic Escherichia coli in biofilms of a heterotrophic water-bacterium by use of 16S-ribosomal-RNA-directed and 23S-ribosomal-RNA-directed fluorescent oligonucleotid. FEMS Microbiol. Ecol. 13, 169–175.
Tawfeek, G. M., Bishara, S. A., Sarhan, R. M., Elshabrawi Taher, E., and Elsaady Khayyal, A. (2016). Genotypic, physiological, and biochemical characterization of potentially pathogenic Acanthamoeba isolated from the environment in Cairo, Egypt. Parasitol. Res. 115, 1871–1881. doi: 10.1007/s00436-016-4927-3
Tegtmeyer, N., Neddermann, M., Asche, C. I., and Backert, S. (2017). Subversion of host kinases: a key network in cellular signaling hijacked by Helicobacter pylori CagA. Mol. Microbiol. 105, 358–372. doi: 10.1111/mmi.13707
Temmerman, R., Vervaeren, H., Noseda, B., Boon, N., and Verstraete, W. (2006). Necrotrophic growth of Legionella pneumophila. Appl. Environ. Microbiol. 72, 4323–4328. doi: 10.1128/AEM.00070-06
Thomas, V., Bouchez, T., Nicolas, V., Robert, S., Loret, J. F., and Lévi, Y. (2004). Amoebae in domestic water systems: resistance to disinfection treatments and implication in Legionella persistence. J. Appl. Microbiol. 97, 950–963. doi: 10.1111/j.1365-2672.2004.02391.x
Thomas, V., Loret, J. F., Jousset, M., and Greub, G. (2008). Biodiversity of amoebae and amoebae-resisting bacteria in a drinking water treatment plant. Environ. Microbiol. 10, 2728–2745. doi: 10.1111/j.1462-2920.2008.01693.x
Thomas, V., Mcdonnell, G., Denyer, S. P., and Maillard, J. Y. (2010). Free-living amoebae and their intracellular pathogenic microorganisms: risks for water quality. FEMS Microbiol. Rev. 34, 231–259. doi: 10.1111/j.1574-6976.2009.00190.x
Tiaden, A., Spirig, T., Sahr, T., Wälti, M. A., Boucke, K., Buchrieser, C., et al. (2010). The autoinducer synthase LqsA and putative sensor kinase LqsS regulate phagocyte interactions, extracellular filaments and a genomic island of Legionella pneumophila. Environ. Microbiol. 12, 1243–1259. doi: 10.1111/j.1462-2920.2010.02167.x
Tilney, L. G., Harb, O. S., Connelly, P. S., Robinson, C. G., and Roy, C. R. (2001). How the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER membrane. J. Cell Sci. 114, 4637–4650. doi: 10.1242/jcs.114.24.4637
Tison, D. L., Pope, D. H., Cherry, W. B., and Fliermans, C. B. (1980). Growth of Legionella pneumophila in association with blue-green algae (cyanobacteria). Appl. Environ. Microbiol. 39, 456–459. doi: 10.1128/aem.39.2.456-459.1980
Urwyler, S., Nyfeler, Y., Ragaz, C., Lee, H., Mueller, L. N., Aebersold, R., et al. (2009). Proteome analysis of Legionella vacuoles purified by magnetic immunoseparation reveals secretory and endosomal GTPases. Traffic 10, 76–87. doi: 10.1111/j.1600-0854.2008.00851.x
Üstüntürk-Onan, M., and Walochnik, J. (2018). Identification of free-living amoebae isolated from tap water in Istanbul, Turkey. Exp. Parasitol. 195, 34–37. doi: 10.1016/j.exppara.2018.10.002
Van Der Kooij, D., Bakker, G. L., Italiaander, R., Veenendaal, H. R., and Wullings, B. A. (2017). Biofilm composition and threshold concentration for growth of Legionella pneumophila on surfaces exposed to flowing warm tap water without disinfectant. Appl. Environ. Microbiol. 83:16. doi: 10.1128/AEM.02737-16
Van Heijnsbergen, E., Schalk, J. A., Euser, S. M., Brandsema, P. S., Den Boer, J. W., and De Roda Husman, A. M. (2015). Confirmed and potential sources of Legionella reviewed. Environ. Sci. Technol. 49, 4797–4815. doi: 10.1021/acs.est.5b00142
Venkataraman, C., Gao, L. Y., Bondada, S., and Kwaik, Y. A. (1998). Identification of putative cytoskeletal protein homologues in the protozoan host Hartmannella vermiformis as substrates for induced tyrosine phosphatase activity upon attachment to the legionnaires' disease bacterium, Legionella pneumophila. J. Exp. Med. 188, 505–514. doi: 10.1084/jem.188.3.505
Venkataraman, C., Haack, B. J., Bondada, S., and Abu Kwaik, Y. (1997). Identification of a gal/Galnac lectin in the protozoan Hartmannella vermiformis as a potential receptor for attachment and invasion by the legionnaires' disease bacterium. J. Exp. Med. 186, 537–547. doi: 10.1084/jem.186.4.537
Verma, K., and Datta, S. (2017). The monomeric Gtpase Rab35 regulates phagocytic cup formation and Phagosomal maturation in Entamoeba histolytica. J. Biol. Chem. 292, 4960–4975. doi: 10.1074/jbc.M117.775007
Vicinanza, M., D'angelo, G., Di Campli, A., and De Matteis, M. A. (2008). Function and dysfunction of the pi system in membrane trafficking. EMBO J. 27, 2457–2470. doi: 10.1038/emboj.2008.169
Vieira, O. V., Botelho, R. J., Rameh, L., Brachmann, S. M., Matsuo, T., Davidson, H. W., et al. (2001). Distinct roles of class I and class iii phosphatidylinositol 3-kinases in phagosome formation and maturation. J. Cell Biol. 155, 19–26. doi: 10.1083/jcb.200107069
Vincent, C. D., and Vogel, J. P. (2006). The Legionella pneumophila IcmS-LvgA protein complex is important for dot/Icm-dependent intracellular growth. Mol. Microbiol. 61, 596–613. doi: 10.1111/j.1365-2958.2006.05243.x
Vines, R. R., Ramakrishnan, G., Rogers, J. B., Lockhart, L. A., Mann, B. J., and Petri, W. A. Jr. (1998). Regulation of adherence and virulence by the Entamoeba histolytica lectin cytoplasmic domain, which contains a beta2 integrin motif. Mol. Biol. Cell 9, 2069–2079. doi: 10.1091/mbc.9.8.2069
Visvesvara, G. S., Moura, H., and Schuster, F. L. (2007). Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 50, 1–26. doi: 10.1111/j.1574-695X.2007.00232.x
Wadowsky, R. M., Butler, L. J., Cook, M. K., Verma, S. M., Paul, M. A., Fields, B. S., et al. (1988). Growth-supporting activity for Legionella pneumophila in tap water cultures and implication of hartmannellid amoebae as growth factors. Appl. Environ. Microbiol. 54, 2677–2682. doi: 10.1128/aem.54.11.2677-2682.1988
Wadowsky, R. M., Yee, R. B., Mezmar, L., Wing, E. J., and Dowling, J. N. (1982). Hot water systems as sources of Legionella pneumophila in hospital and nonhospital plumbing fixtures. Appl. Environ. Microbiol. 43, 1104–1110. doi: 10.1128/aem.43.5.1104-1110.1982
Weber, S. S., Ragaz, C., Reus, K., Nyfeler, Y., and Hilbi, H. (2006). Legionella pneumophila exploits pi(4)P to anchor secreted effector proteins to the replicative vacuole. PLoS Pathog. 2:e46. doi: 10.1371/journal.ppat.0020046
Wieland, H., Ullrich, S., Lang, F., and Neumeister, B. (2005). Intracellular multiplication of Legionella pneumophila depends on host cell amino acid transporter Slc1A5. Mol. Microbiol. 55, 1528–1537. doi: 10.1111/j.1365-2958.2005.04490.x
World Health Organization (2016). “Fact Sheet 285” in Legionellosis. June 2016 Edition (Geneva, Switzerland: WHO).
Xu, L., Shen, X., Bryan, A., Banga, S., Swanson, M. S., and Luo, Z. Q. (2010). Inhibition of host vacuolar H+-Atpase activity by a Legionella pneumophila effector. PLoS Pathog. 6:e1000822. doi: 10.1371/journal.ppat.1000822
Yamamoto, H., Sugiura, M., Kusunoki, S., Ezaki, T., Ikedo, M., and Yabuuchi, E. (1992). Factors stimulating propagation of legionellae in cooling tower water. Appl. Environ. Microbiol. 58, 1394–1397. doi: 10.1128/aem.58.4.1394-1397.1992
Yan, M., Collins, R. F., Grinstein, S., and Trimble, W. S. (2005). Coronin-1 function is required for phagosome formation. Mol. Biol. Cell 16, 3077–3087. doi: 10.1091/mbc.e04-11-0989
Yoder, J. S., Hlavsa, M. C., Craun, G. F., Hill, V., Roberts, V., Yu, P. A., et al. (2008). Surveillance for waterborne disease and outbreaks associated with recreational water use and other aquatic facility-associated health events--United States, 2005-2006. MMWR Surveill. Summ. 57, 1–29.
Yu, F., Nair, A. A., Lauper, U., Luo, G., Herb, J., Morse, M., et al. (2024). Mysteriously rapid rise in legionnaires' disease incidence correlates with declining atmospheric sulfur dioxide. PNAS Nexus 3:pgae085. doi: 10.1093/pnasnexus/pgae085
Keywords: Legionella pneumophila , free-living amoebae, symbiotic relationship, pathogenicity, public health
Citation: Wang Y, Jiang L, Zhou F, Zhang Y, Fine RD and Li M (2025) The hidden dancers in water: the symbiotic mystery of Legionella pneumophila and free-living amoebae. Front. Microbiol. 16:1634806. doi: 10.3389/fmicb.2025.1634806
Edited by:
James A. Garnett, King’s College London, United KingdomReviewed by:
Binod Rayamajhee, University of New South Wales, AustraliaLaura Cerqueira, University of Porto, Portugal
Copyright © 2025 Wang, Jiang, Zhou, Zhang, Fine and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingguang Li, bWdsMTgwMEBqbG11LmVkdS5jbg==
 Yuehua Wang
Yuehua Wang Linzhe Jiang
Linzhe Jiang Fei Zhou
Fei Zhou Yi Zhang
Yi Zhang Ryan D. Fine
Ryan D. Fine Mingguang Li
Mingguang Li