- 1Department of Biological and Environmental Sciences, Southeast Missouri State University, Cape Girardeau, MO, United States
- 2Biotechnology and Genetic Engineering Discipline, Khulna University, Khulna, Bangladesh
Staphylococcus aureus is a clinically significant pathogen known for its antibiotic resistance, immune evasion, and biofilm formation, making it a major contributor to persistent infections. Lactobacillus plantarum, a versatile probiotic bacterium, has emerged as a promising antagonist against S. aureus through multifaceted inhibitory mechanisms. This review synthesizes current evidence on the antagonistic interactions between L. plantarum and S. aureus, highlighting bacteriocin-mediated membrane disruption, quorum sensing interference, biofilm degradation, and metabolic competition. In addition, we explore how L. plantarum contributes to a less favorable inflammatory environment for S. aureus by modulating local immune responses at infection sites. Clinical relevance is explored across diverse anatomical sites such as the skin, vaginal tract, urinary system, and gastrointestinal tract, where L. plantarum demonstrates both direct and adjunctive therapeutic potential. We also consider environmental influences like pH and nutrient availability that modulate this antagonism. Together, the findings position L. plantarum as a compelling candidate for probiotic-based interventions against persistent and device-associated S. aureus infections.
Introduction
Staphylococcus aureus has long been recognized as a notorious Gram-positive pathogen that invades skin, mucous membranes, and deeper tissues, leading to a wide range of clinical infections that pose significant challenges in healthcare settings (Tong et al., 2015). Its ability to form biofilms, evade host immune responses, and develop resistance to multiple antibiotics renders it as a formidable adversary, particularly in cases of device-associated infections and chronic wounds (Ahn et al., 2018; Brunel and Benoit, 2017; Otto, 2018). Due to this, considerable attention has been directed toward understanding the interactions between S. aureus and its host environment, with the aim of uncovering novel therapeutic strategies to combat its pathogenicity.
One promising area of research involves the utilization of probiotics, especially Lactobacillus plantarum, a species of bacteria that is part of the healthy gastrointestinal microbiota and has demonstrated to exhibit potent antimicrobial as well as anti-inflammatory properties (Douillard and de Vos, 2014; Hu et al., 2019; van den Nieuwboer et al., 2016). Studies have shown that L. plantarum can exert significant antagonistic effects against S. aureus, a dynamic that is evidenced by its multimodal ability to suppress the growth of S. aureus through the production of antimicrobial compounds and modulation of inflammatory responses (Ren et al., 2017). This antagonistic interplay not only highlights the potential of L. plantarum as a natural inhibitor of critical pathogens, but also opens avenues for its application as an adjunct or alternative to conventional antibiotic therapies (Brunel and Benoit, 2017; Hu et al., 2019; Dolan et al., 2017; Kalliomäki et al., 2003; Kalliomäki et al., 2007; Ong et al., 2020).
The clinical relevance of L. plantarum is further underscored by its role in specific host environments where S. aureus poses a significant risk. In the vaginal tract, for instance, L. plantarum naturally acidifies the local milieu through the production of lactic acid, thereby maintaining an environment that is hostile to pathogenic invaders. Moreover, engineered strains of L. plantarum expressing lysostaphin have been explored as innovative approaches to target S. aureus infections by degrading its cell wall components, which is particularly important in preventing conditions such as menstrual toxic shock syndrome (Boskey et al., 2001; Boskey et al., 1999; Liu et al., 2011; Sodré et al., 2023; Arena et al., 2016; Li et al., 2023a; Turner et al., 2007). In addition to the vaginal ecosystem, the probiotic potential of L. plantarum has been implicated in enhancing wound healing and modulating immune responses at other anatomical sites, thereby contributing to a reduction in the incidence and severity of S. aureus infections. Recent studies have provided compelling evidence that the ability of L. plantarum to interfere with biofilm formation and disrupt established biofilms could serve as a crucial therapeutic strategy in overcoming persistent infections (Reid et al., 2001; Rönnqvist et al., 2006; Wang et al., 2018; Wang et al., 2021). This review seeks to synthesize the current understanding of the interactions between L. plantarum and S. aureus, focusing on the underlying mechanisms of antagonisms, its clinical implications, and the potential for developing probiotic-based interventions to address the challenges posed by S. aureus in diverse clinical scenarios.
By integrating findings from in vitro studies, mammalian models, and clinical investigations, this review aims to provide a comprehensive overview of the role of L. plantarum as a therapeutic agent in the management of S. aureus infections. This serves to lay the groundwork for future research and to inform clinical practice by highlighting both the benefits and limitations of leveraging probiotic strategies such as L. plantarum against S. aureus that continues to challenge conventional therapeutic paradigms.
Mechanisms of Lactobacillus plantarum antagonism against Staphylococcus aureus
L. plantarum counters S. aureus through several distinct yet complementary mechanisms. These include the secretion of bacteriocins that disrupt membrane integrity, interference with S. aureus quorum sensing pathways that regulate virulence, and modulation of host immune responses to reduce inflammation and promote pathogen clearance. This section outlines these strategies in detail, highlighting the multifaceted nature of L. plantarum’s antagonism.
One principal mechanism involves the production and secretion of bacteriocins, proteinaceous molecules that directly target S. aureus by compromising cell membrane integrity and inhibiting biofilm formation (Sodré et al., 2023; Arena et al., 2016). Although the exact mechanisms differ among bacteriocin subclasses, most plantaricins ultimately disrupt the target membrane, leading to ion leakage and cell death. For instance, class IIA plantaricins specifically target the mannose phosphotransferase system (Man-PTS) in S. aureus (Figure 1), with the IIC and IID subunits serving as docking sites for insertion into the lipid bilayer (Arief et al., 2015; Tymoszewska et al., 2018; Oppegård et al., 2016). Upon localization, they infiltrate the bacterial phospholipid bilayer and form oligomers, which disrupt the regularity of the structure and induce pore formation. This puncture leads to the loss of cytosolic components, most notably the electrolytic leakage of ions such as potassium and sodium, as well as amino acids and other solutes (Ennahar et al., 2000). In a similar vein, plantaricin YKX interferes with the ionic homeostasis of S. aureus by destabilizing cell signaling pathways that are critical for biofilm formation (Pei et al., 2022). Additionally, certain bacteriocins such as plantacyclin B21AG are characterized by a circular structure, which endows them with enhanced resistance to proteolytic enzymes, thereby ensuring sustained antimicrobial activity even in diverse and challenging environments (Golneshin et al., 2020). These bacteriocin-mediated effects not only curtail the proliferation of S. aureus but also contribute to reducing the likelihood of resistance development.
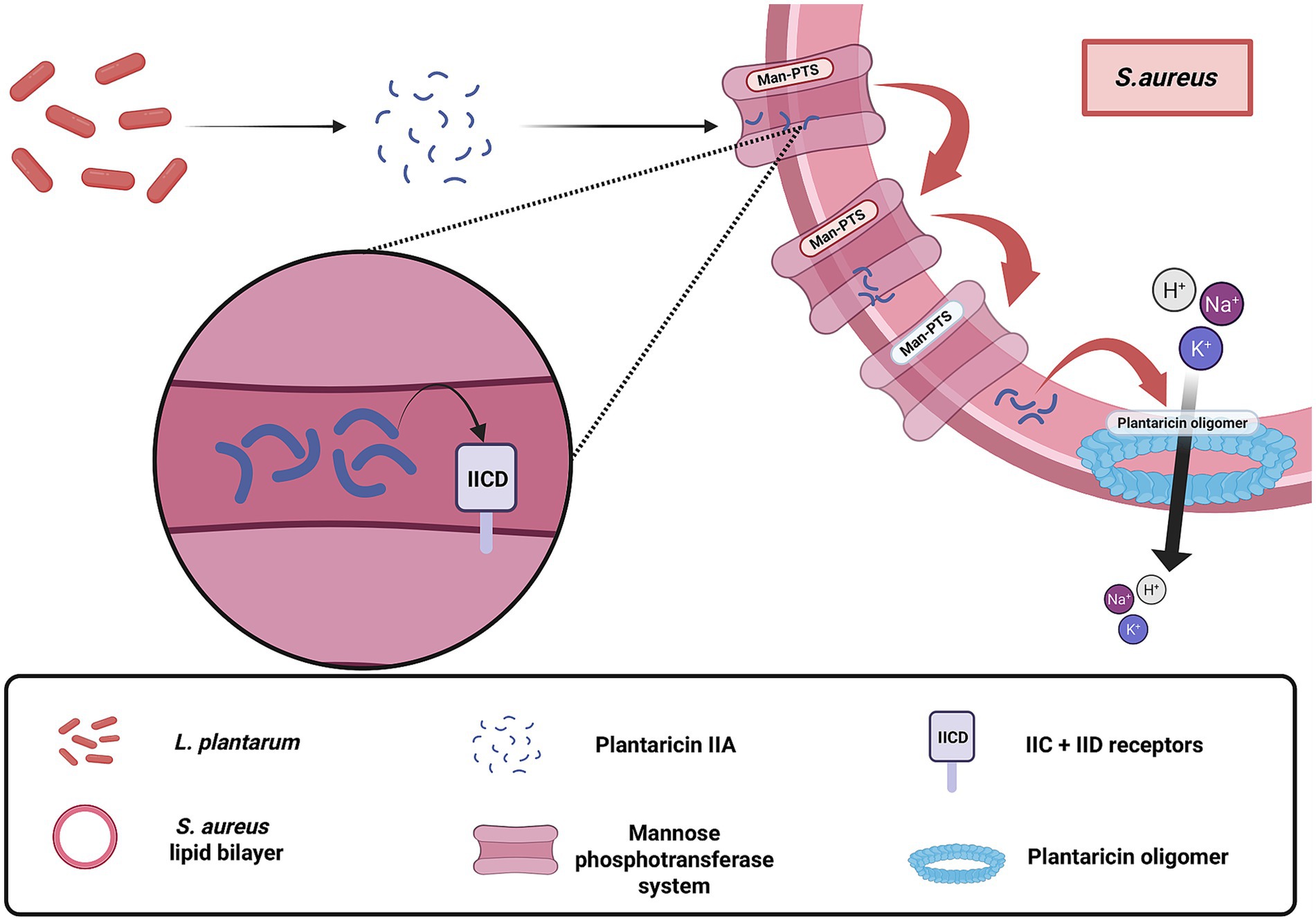
Figure 1. Membrane-disruptive mechanism of plantaricin-class bacteriocins produced by Lactobacillus plantarum targeting Staphylococcus aureus. These bacteriocins, primarily from the plantaricin family, exert direct antimicrobial activity by embedding into the bacterial phospholipid bilayer, oligomerizing, and forming pores that lead to ion leakage and cell death. The figure illustrates the receptor-dependent action of class IIA plantaricins, such as plantaricin IIA-1A5, which utilize the Mannose Phosphotransferase System, particularly the IIC and IID subunits, as docking sites for membrane localization and insertion. This mode of action contributes to the direct killing of S. aureus independent of host factors. Created with BioRender.
Beyond direct membrane disruption, Lactiplantibacillus species can interfere with intercellular communication systems, particularly the quorum sensing system in S. aureus, which regulates virulence and biofilm formation. Among these species, Lactiplantibacillus paraplantarum shows a strong ability to inhibit S. aureus quorum sensing by producing AIP-like peptides through its agr-like lamBDCA system. These peptides can suppress S. aureus agr activation, especially in agr groups I, II, and IV, leading to significant reductions in hemolysin production and virulence gene expression (Wang et al., 2025). The inhibitory effect is context dependent, with stronger suppression observed in co-culture conditions compared to cell-free supernatants. Disruption of the lamBDCA locus eliminates this effect, confirming its essential role. L. plantarum also contains a lamBDCA locus and produces an AIP peptide known as LamD558, but its inhibitory effects on S. aureus are modest and mainly limited to agr group I (Wang et al., 2025). Structural differences in the peptide, including its tailless thiolactone conformation, may influence its stability and activity. Additionally, other small molecules such as dipeptides like cyclo (L-Phe-L-Pro) may contribute to the limited quorum sensing interference observed in L. plantarum. Collectively, these disruptions weaken S. aureus coordination of adhesion, toxin production, and biofilm formation (Arena et al., 2016; Wang et al., 2025; Qazi et al., 2006; Spangler et al., 2019).
In addition to bacterial interactions, L. plantarum also influences the host immune response through interactions with their innate and adaptive systems (Ong et al., 2020), facilitating indirect counteraction against S. aureus pathogenesis (Figure 2). Recent studies have demonstrated that components of L. plantarum are recognized by host immune cells and mediate the outcomes of reduced site inflammation (Ong et al., 2020). Wound-healing is shown to be preserved in the host due to a mixture of bioactive components, which include both nanoparticles and small metabolites, derived from lysates of L. plantarum strain K8 (K8NPs) for both in vivo and in vitro murine models (Ong et al., 2020; Hong et al., 2023). In live cells, these molecules are exported through extracellular vesicles (Kim et al., 2018). One known element, lipoteichoic acid, binds with TLR2 and TLR6 downregulating the NF-kB pathway (Wells, 2011). This reduction in pro-inflammatory mediators not only diminishes tissue damage during S. aureus infections but also enhances the overall capacity of the host’s immune system to combat the pathogenesis (Hong et al., 2023). Together, these effects position L. plantarum as a potent antagonist capable of targeting both the pathogen and the inflammatory environment it exploits.
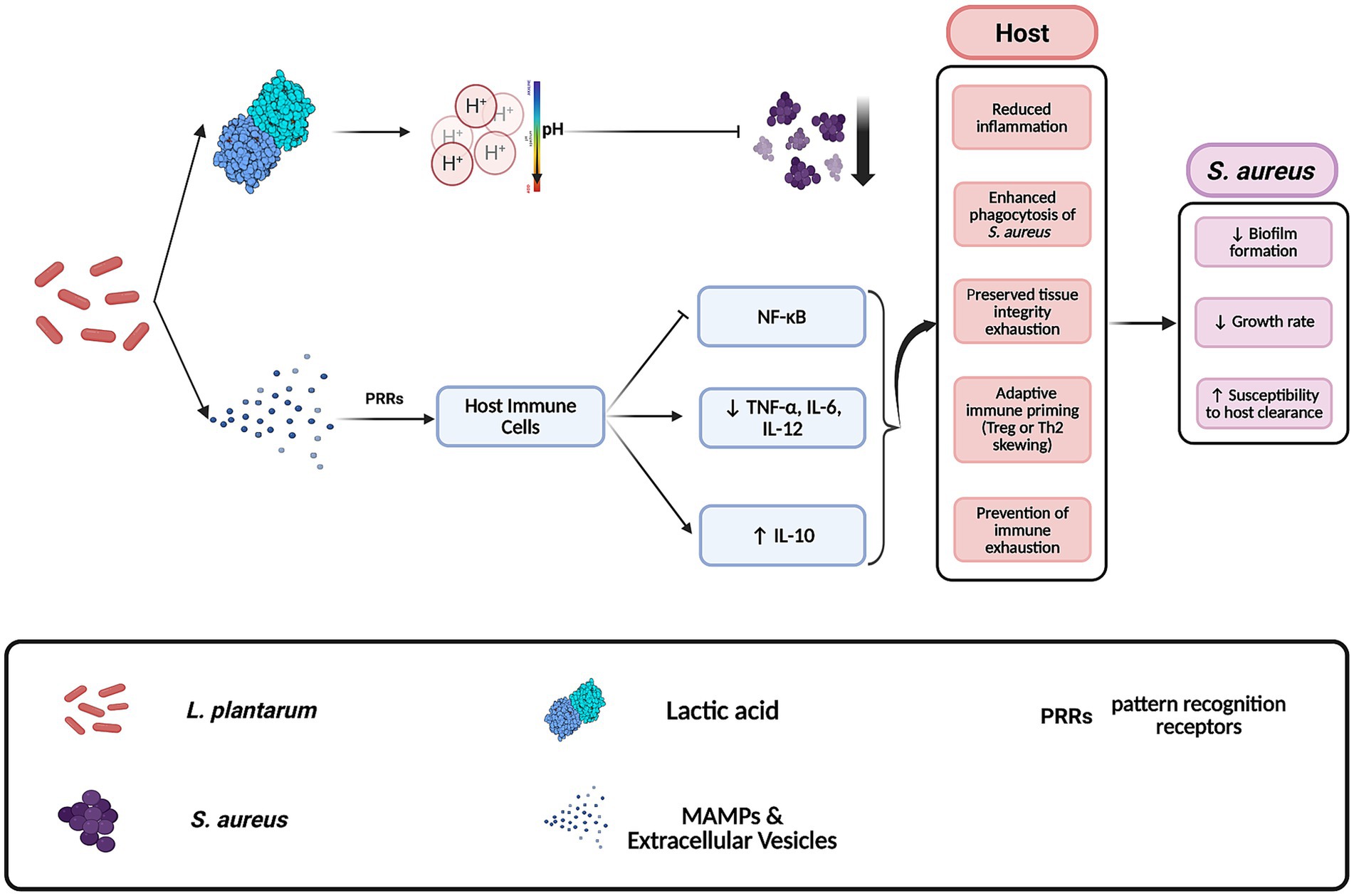
Figure 2. Mechanisms by which Lactobacillus plantarum antagonizes Staphylococcus aureus in host environments. L. plantarum inhibits S. aureus through the secretion of antimicrobial compounds such as lactic acid and by modulating host immune responses. This includes the release of extracellular vesicles that contribute to anti-inflammatory signaling in host tissues. These combined effects reduce S. aureus virulence and proliferation. Created with BioRender.
Staphylococcus aureus biofilm formation and Lactobacillus plantarum interventions
Staphylococcus aureus is well recognized for its capacity to form robust biofilms, which serve as a formidable defense mechanism against both antibiotic treatments and host immune responses. These biofilms are complex structures composed of exopolysaccharides, proteins, and extracellular DNA that envelop bacterial cells, thereby protecting them from environmental stressors and facilitating their persistence on medical devices and tissues (Otto, 2018; Jefferson, 2004). Biofilm development occurs in several distinct stages: initially, free-floating bacterial cells adhere to surfaces and aggregate to form microcolonies. These clusters then produce a sticky extracellular matrix (ECM) that binds the cells together into a three-dimensional structure. Finally, when the biofilm reaches a critical density, signaling prompt partial disintegration of the ECM, allowing some bacteria to disperse and colonize new niches (Moormeier and Bayles, 2017). This dynamic process not only contributes to the chronicity of S. aureus infections but also complicates their treatment, particularly in clinical settings where biofilm-associated infections are notoriously resistant to conventional antibiotics.
In response to the protective biofilm formation of S. aureus, L. plantarum employs a suite of targeted interventions aimed at both preventing biofilm development and disrupting established biofilms (Figure 3). One of the primary mechanisms involves the secretion of lipoteichoic acid (LTA) (Ahn et al., 2018). L. plantarum releases LTA that actively inhibits biofilm formation by suppressing the expression of the ica-operon, a critical genetic locus responsible for the synthesis of poly-N-acetylglucosamine (Figure 3B), a key component of the biofilm matrix (Ahn et al., 2018; Yasir et al., 2018).
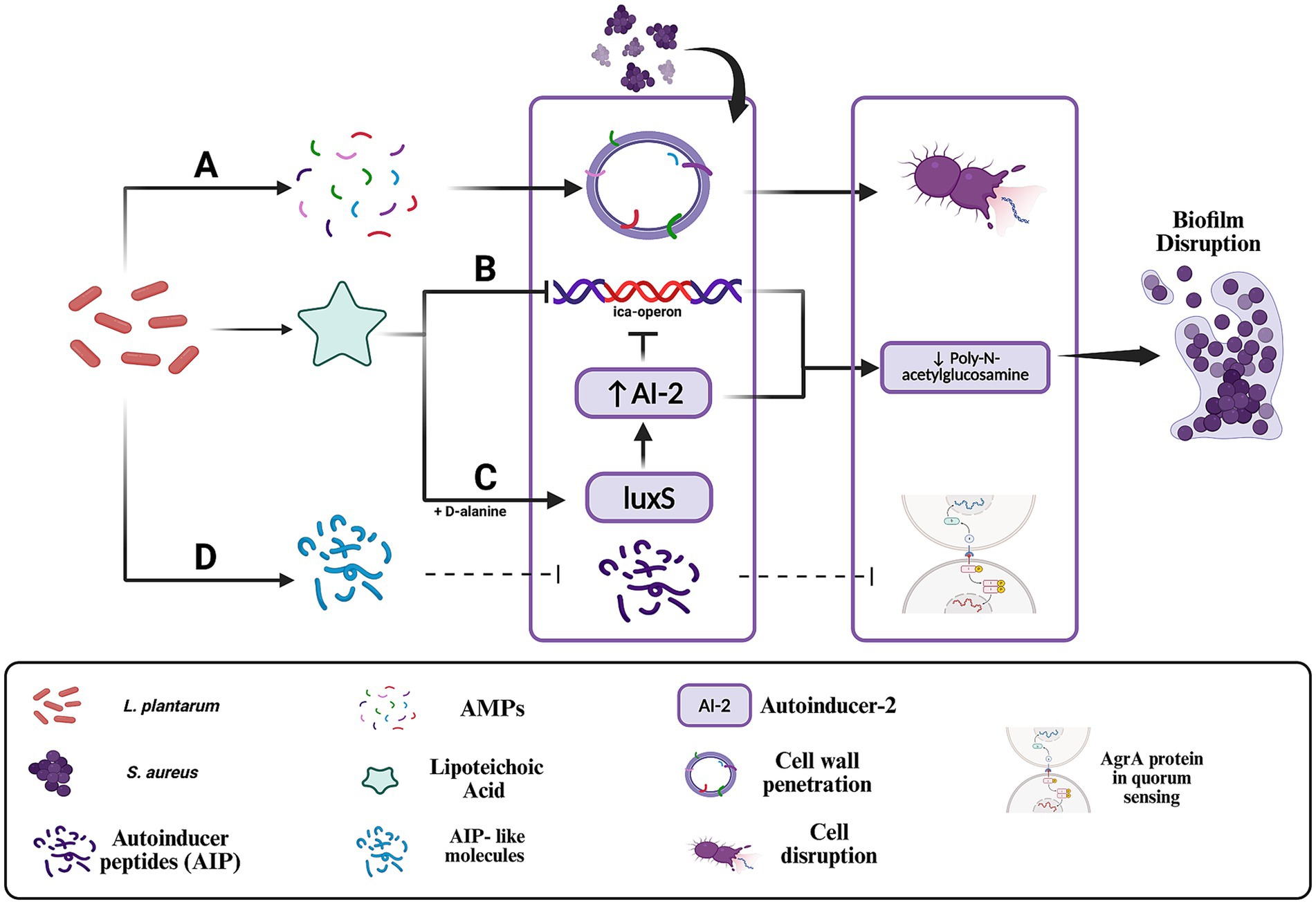
Figure 3. Mechanisms by which Lactobacillus plantarum disrupts Staphylococcus aureus quorum sensing and biofilm formation. (A) Antimicrobial peptides (AMPs) disrupt the bacterial membrane and inhibit the synthesis of essential biomolecules, leading to cell lysis. (B) Lipoteichoic acid (LTA) inhibits the ica operon, leading to reduced synthesis of poly-N-acetylglucosamine, a major structural component of the biofilm extracellular matrix. (C) D-alanine-modified LTA enhances luxS expression, increasing autoinducer-2 production and disrupting quorum-regulated gene expression critical for biofilm maturation. (D) AIP-like molecules produced by L. plantarum may competitively bind to the AgrC receptor, interfering with S. aureus quorum sensing. In engineered strains, secreted proteases such as lysostaphin may further degrade AIPs, amplifying quorum disruption. Created with BioRender.
This inhibitory effect is further enhanced by the LTA-induced release of autoinducer-2. However, LTA lacking D-alanine moieties is ineffective at influencing the luxS gene in S. aureus and fails to upregulate autoinducer-2 (Ahn et al., 2018). Through this mechanism, L. plantarum disrupts the ability of S. aureus to organize into a cohesive and protective biofilm structure (Figure 3C). In addition to LTA, L. plantarum produces antimicrobial peptides (AMPs) (Figure 3A) that contribute to the disintegration of biofilms. These AMPs interact with the lipoteichoic acid in the cell walls of Gram-positive bacteria, such as S. aureus, and penetrate the bacterial membrane, leading to cell lysis. The action of these peptides extends beyond mere membrane disruption; they also inhibit the synthesis of essential cellular components, including cell wall, DNA, RNA, and proteins, and can trigger autolytic enzyme activity, thereby promoting bacterial self-destruction (Yasir et al., 2018). This multifaceted approach not only reduces the viability of individual bacterial cells but also compromises the overall integrity of the biofilm matrix.
Another important strategy employed by L. plantarum is the interference with quorum-sensing mechanisms that S. aureus relies upon for biofilm maturation and maintenance. Quorum sensing is a critical communication system that coordinates the expression of virulence factors and biofilm-associated genes. L. plantarum disrupts this process by secreting organic acids and producing signaling molecules that interfere with S. aureus quorum sensing (Figure 3D) (Arena et al., 2016; Wang et al., 2025; Li et al., 2023b). Adapting from this approach, engineered strains of L. plantarum have been developed to sense S. aureus quorum-sensing signals and respond by producing lysostaphin, a potent bacteriolytic enzyme that specifically targets and degrades the cell wall of S. aureus, which prevent biofilm formation (Li et al., 2023a; Turner et al., 2007). This targeted interference with quorum sensing represents a promising avenue for mitigating the coordinated defense mechanisms of S. aureus. Beyond these molecular strategies, L. plantarum also secretes various matrix-degrading enzymes, including biosurfactants and proteases, which play a crucial role in dismantling the structural integrity of microorganism biofilms. One paper has investigated these substances in Pseudomonas aeruginosa where they act to degrade the extracellular matrix, thereby increasing the susceptibility of the embedded bacteria to both the host’s immune defenses and antimicrobial agents (Li et al., 2023b). Additionally, the production of lactic acid by L. plantarum not only contributes to a lower pH that destabilizes the biofilm but also directly inhibits microbial growth, further diminishing the survival prospects of S. aureus within its protective niche (Tejero-Sariñena et al., 2012). Research has also demonstrated that L. plantarum can secrete bacteriocins and exopolysaccharides which further disrupt biofilm production, and that the bacterium is capable of disintegrating existing biofilms through the action of proteolytic enzymes, effectively reducing biofilm viability when co-cultured with S. aureus (Arena et al., 2016; Vuotto et al., 2014).
Clinical applications in specific infection sites
The clinical utility of L. plantarum in countering S. aureus infections has been demonstrated across a variety of anatomical sites, underscoring its potential as both a standalone therapeutic and an adjunct to conventional antibiotic treatments (Ong et al., 2020; Mukherjee and Ramesh, 2015; Rizzo et al., 2015). In the context of skin infections, especially those involving wounds, the combination of L. plantarum with Lawsonia inermis (henna) has shown promising results. Studies indicate that this combination effectively reduces inflammatory cytokines such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α, thereby accelerating the healing process in S. aureus-infected wounds (Elebeedy et al., 2022). Excessive inflammation is a major barrier to efficient wound healing, and pro-inflammatory cytokines like IL-1, IL-6 and TNF-α play critical roles in this process (Figure 4) (Kagiwada et al., 2004). While these cytokines are essential for early immune responses, their prolonged activity inhibits fibroblast proliferation, delays collagen deposition, and impairs angiogenesis, ultimately slowing tissue repair (Barrientos et al., 2008; Eming et al., 2007). Inhibition of IL-6 and TNF-α promotes a faster transition from the inflammatory phase to the proliferative phase, reducing oxidative stress and enhancing re-epithelialization, which is particularly beneficial for chronic wounds (Eming et al., 2007). This synergistic effect not only enhances bacterial clearance but also mitigates local inflammation, which is a critical factor in managing infections that are often complicated by antibiotic resistance.
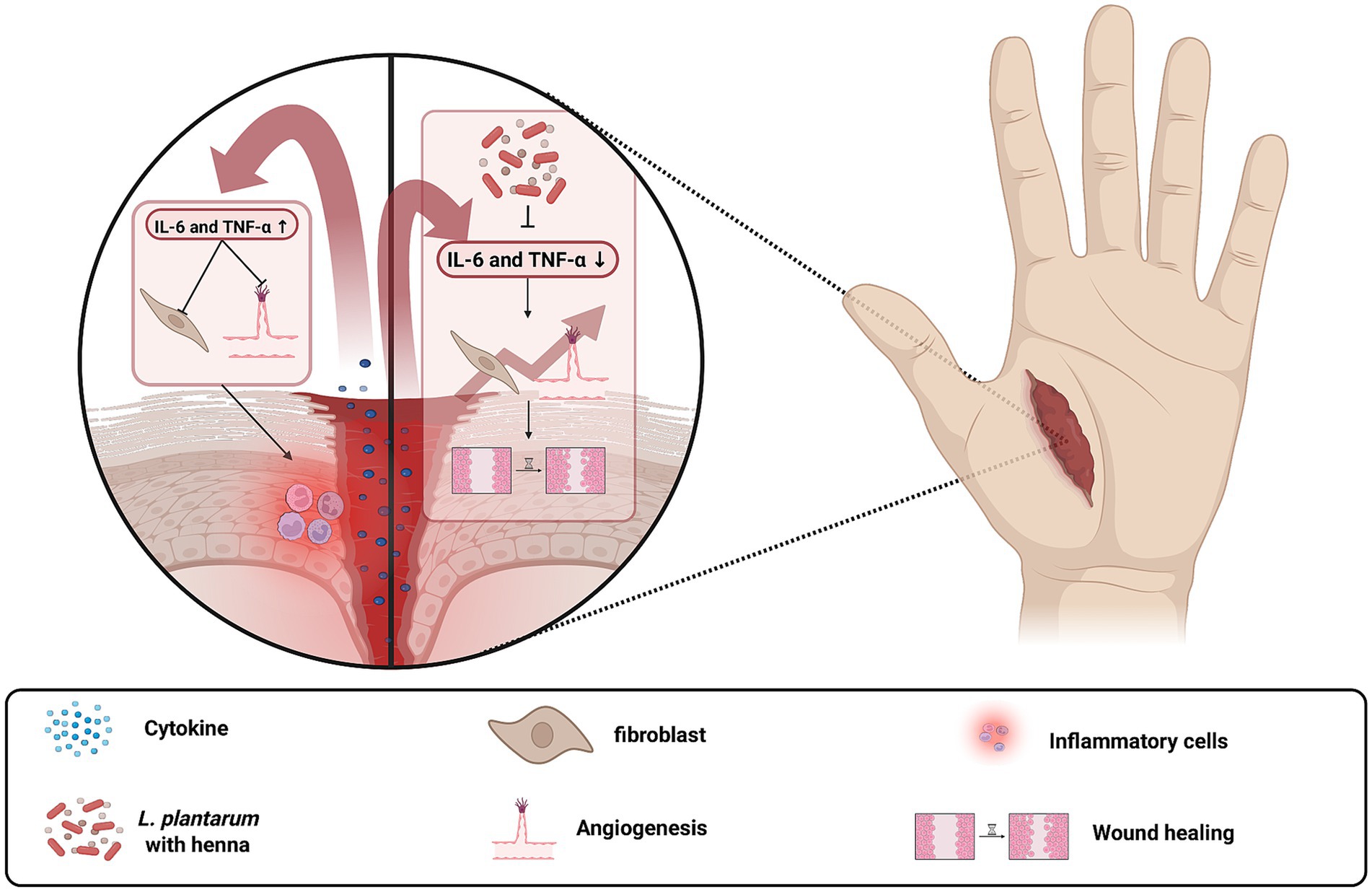
Figure 4. Inhibition of IL-6 and TNF-α promotes wound healing by reducing excessive inflammation and enhancing tissue regeneration. The left panel illustrates a condition with high IL-6 and TNF-α levels, leading to prolonged inflammation, impaired fibroblast activity, and delayed wound closure. The right panel shows the effects of IL-6 and TNF-α inhibition by L. plantarum with Lawsonia inermis, which reduces bacterial load, enhances fibroblast proliferation, promotes collagen deposition, and accelerates wound healing. Created with BioRender.
The vaginal tract represents another important site where L. plantarum exhibits significant clinical benefits (Reid et al., 2001; Rönnqvist et al., 2006; Spurbeck and Arvidson, 2011). Naturally, L. plantarum produces lactic acid, which maintains an acidic environment that suppresses the growth of S. aureus. Furthermore, the presence of L. plantarum prevent the adhesive proteins of S. aureus from attaching to the epithelial surfaces of the vaginal tract, thereby reducing the likelihood of colonization and subsequent infection (Christensen et al., 2021). Lysostaphin in the engineered strain also aids in this process by degrading cell walls and preventing biofilm formation (Liu et al., 2011; Turner et al., 2007; Christensen et al., 2021). This dual approach of environmental acidification and targeted enzymatic action is particularly valuable in preventing conditions such as toxic shock syndrome and other S. aureus-associated vaginal infections.
Urinary tract infections, particularly those related to indwelling medical devices, present another clinical challenge where L. plantarum has demonstrated potential benefits. Although S. aureus is responsible for only a small percentage of urinary tract infections, its capacity to form biofilms on urinary devices such as catheters and stents leads to persistent and often difficult-to-treat infections (Carvalho et al., 2021; Klein and Hultgren, 2020; Walker et al., 2017). L. plantarum can effectively compete with S. aureus by adhering to bladder epithelial cells, a process mediated by proteins such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Wang et al., 2018; Wang et al., 2021). While GAPDH is well known as a key enzyme in glycolysis, in L. plantarum it also “moonlights” as a cell surface adhesin. Once translocated to the bacterial surface via non-classical secretion pathways, GAPDH binds to glycoprotein receptors on bladder epithelial cells (Figure 5) through conserved amino acid motifs (Wang et al., 2018; Reid and Tieszer, 1994; Wang et al., 2022). Furthermore, its secretion of antimicrobial agents including plantaricin, lactic acid and hydrogen peroxide contributes to the disruption of S. aureus biofilms. This integrated mechanism, combining competitive adhesion with targeted antimicrobial production, reinforces L. plantarum’s capacity to prevent S. aureus colonization on urinary devices.
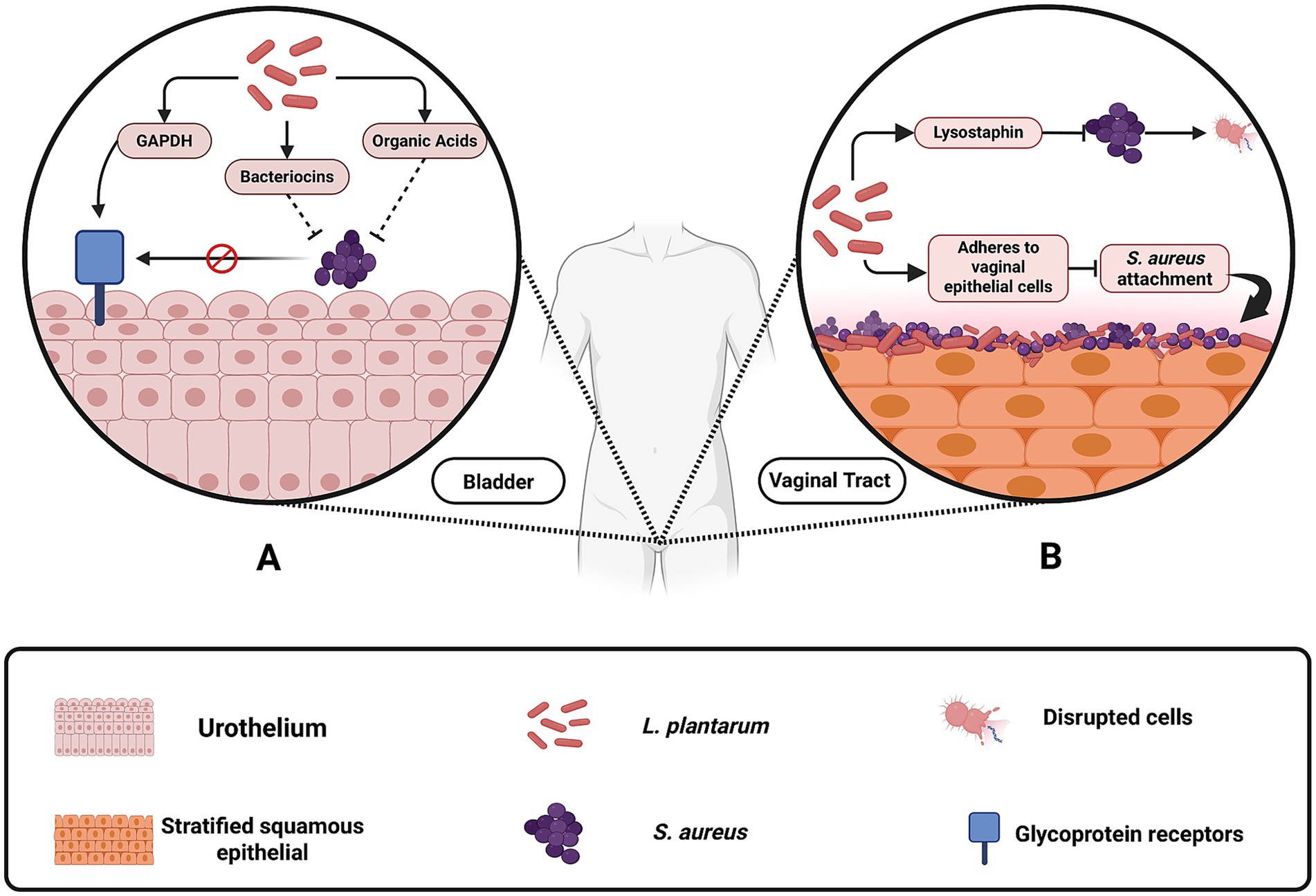
Figure 5. Mechanisms by which Lactobacillus plantarum inhibits Staphylococcus aureus colonization in the vaginal and urinary tracts. (A) In the urinary tract, L. plantarum produces glyceraldehyde-3-phosphate dehydrogenase (GAPDH), bacteriocins, and organic acids, which prevent S. aureus from adhering to bladder epithelial cells and forming biofilms on urinary devices. (B) In the vaginal tract, L. plantarum contributes to acidification by releasing organic acids, which lower pH and suppress S. aureus growth. Additionally, engineered L. plantarum strains produce lysostaphin, an enzyme that degrades the S. aureus cell wall, and adhere to vaginal epithelial cells, blocking S. aureus attachment and colonization. These protective mechanisms reduce the risk of infections such as toxic shock syndrome and catheter-associated urinary tract infections. Created with BioRender.
Within the gastrointestinal tract, L. plantarum plays a vital role in maintaining gut homeostasis and preventing the transition of S. aureus from a commensal organism to a pathogen. Although S. aureus may be present as part of the normal gut flora, disruptions in intestinal homeostasis or immune function can trigger its pathogenic potential, leading to the release of exotoxins and degradation of secretory immunoglobulin A (SIgA) (Ren et al., 2017). L. plantarum contributes to intestinal health by producing lactic acid, which lowers the pH and inhibits S. aureus growth (Figure 2), while also modulating the immune response by enhancing interferon-gamma (IFN)-γ production and reducing IL-4 levels (Figure 6) (Ren et al., 2017; Rizzo et al., 2015; Lin et al., 2013). Additionally, it stimulates SIgA secretion, thereby strengthening the mucosal barrier and preventing pathogen invasion (Ren et al., 2017; Rizzo et al., 2015; Lin et al., 2013). One article also reported that Lactobacillus plantarum extracts inhibited HT-29 colon cancer cell apoptosis induced by Staphylococcus aureus and its alpha-toxin, demonstrating a potential role for probiotic-derived compounds in mitigating the harmful effects of pathogenic bacteria and their toxins on intestinal epithelial cells (Kim et al., 2015). This multifaceted probiotic activity helps to preserve the delicate balance of the gut microbiota and protects against inflammation and infection (Wang et al., 2024).
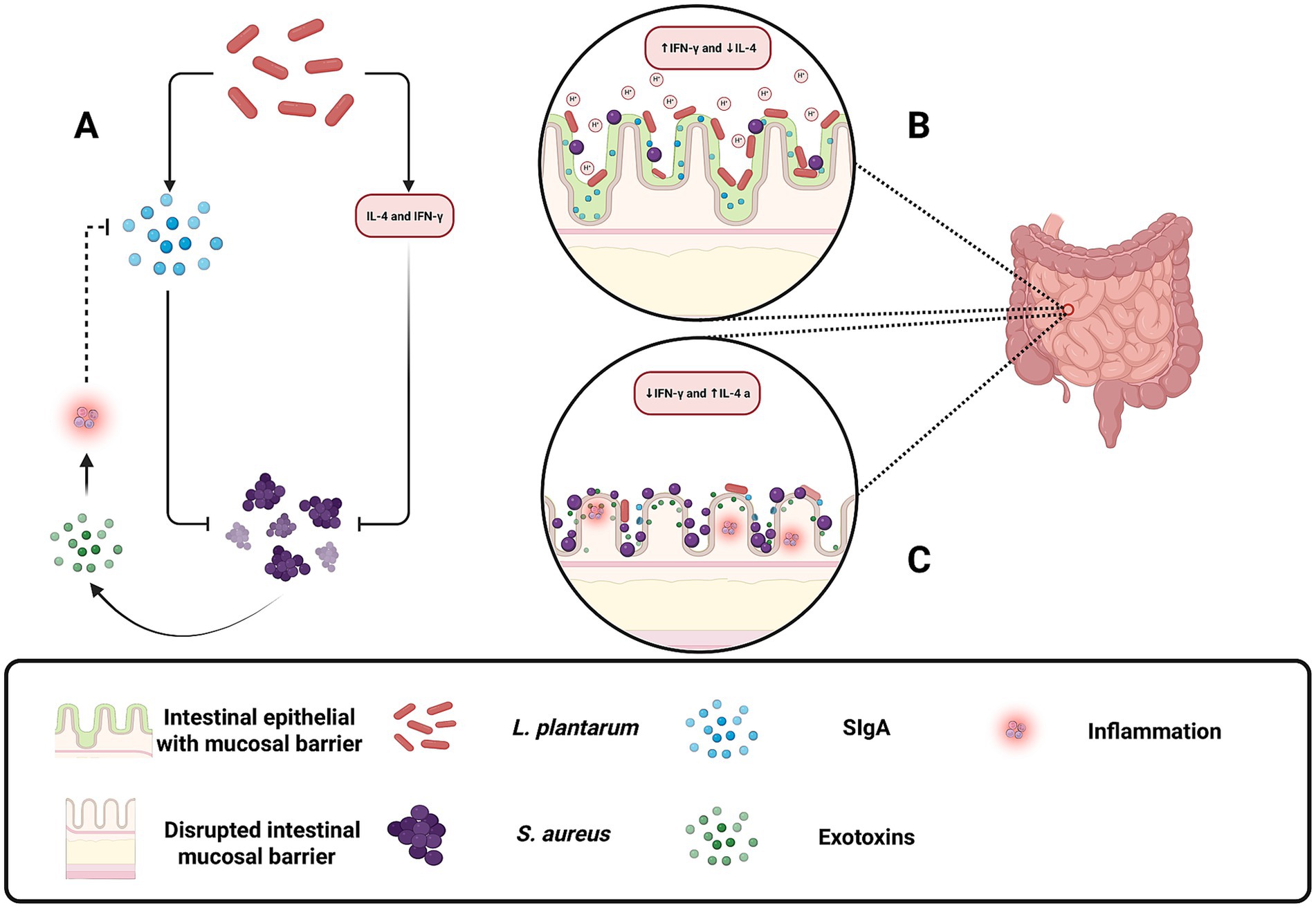
Figure 6. Protective role of Lactobacillus plantarum in maintaining gut homeostasis and preventing Staphylococcus aureus pathogenesis. (A) Illustrates how L. plantarum indirectly attenuates inflammation by promoting SIgA secretion and reducing S. aureus exotoxin-induced immune activation. (B) L. plantarum helps restore gut balance by producing lactic acid, lowering gut pH to inhibit S. aureus growth, and modulating the immune response by increasing IFN-γ and decreasing IL-4 levels. Additionally, L. plantarum enhances SIgA secretion, strengthening the mucosal barrier and preventing S. aureus adhesion and infection. (C) Under disrupted conditions, S. aureus overgrows, releases exotoxins, and degrades secretory immunoglobulin A (SIgA), leading to inflammation and epithelial damage. Increased IL-4 and reduced IFN-γ levels contribute to immune dysregulation and pathogen invasion. Created with BioRender.
Environmental influences on Lactobacillus plantarum and Staphylococcus aureus interactions
Environmental factors such as pH, temperature, and nutrient availability play a pivotal role in modulating the interactions between Lactobacillus plantarum and Staphylococcus aureus. Among these, temperature is a key determinant of L. plantarum’s metabolic activity, with optimal production of lactic acid and bacteriocins observed between 30°C and 37°C (Sodré et al., 2023; Arena et al., 2016; Golneshin et al., 2020). These antimicrobial products contribute to environmental acidification, which disrupts S. aureus metabolism, inhibits biofilm formation, and impairs expression of virulence genes sensitive to low pH conditions.
Another critical environmental pressure is oxidative stress, particularly in host-associated niches where reactive oxygen species (ROS) are abundant. While L. plantarum does not actively produce hydrogen peroxide in significant quantities, it demonstrates notable tolerance to oxidative environments. This resilience is partly attributed to its ability to utilize exogenous quinones for extracellular electron transport, a process that helps maintain redox balance and energy production under stress conditions (Stevens et al., 2023; Tian et al., 2022; Lebeer et al., 2008). Additionally, L. plantarum has been shown to attenuate host inflammation in S. aureus-infected tissues, likely reducing oxidative bursts through immune modulation (Xie et al., 2025). These findings suggest that L. plantarum may gain a competitive advantage not by generating oxidative stress, but by withstanding and adapting to it more effectively than S. aureus (Kondakala et al., 2024).
Beyond stress tolerance, nutrient availability is another crucial environmental factor influencing the competitive dynamics between these two microorganisms. L. plantarum is endowed with remarkable metabolic versatility, which enables it to utilize a wide array of sugars and amino acids efficiently. This versatility is facilitated by specialized sugar transport systems, such as sacPTS1 and sacPTS26, which allow L. plantarum to metabolize complex carbohydrates like fructooligosaccharides (FOS) and sucrose even under carbon-limited conditions (Cui et al., 2021). In contrast, S. aureus primarily depends on simpler carbon sources such as glucose and lactate. The relatively narrow metabolic repertoire of S. aureus renders it less competitive in nutrient-deprived environments compared to the adaptable L. plantarum (Halsey et al., 2017).
Furthermore, nitrogen acquisition represents a critical aspect of this competitive interplay. S. aureus relies on the catabolism of specific amino acids particularly those derived from proline, arginine, and histidine to fulfill its nitrogen requirements. This process is tightly regulated by systems such as CodY and Carbon Catabolite Repression (CCR), which prioritize amino acid utilization based on nutrient availability (Halsey et al., 2017; Somerville and Proctor, 2009). In contrast, L. plantarum employs proteolytic mechanisms, including the oligopeptide permease (Opp) system, to break down peptides into amino acids, ensuring a steady and efficient nitrogen supply (Halsey et al., 2017; Hu et al., 2024; Rezaei et al., 2021). Additionally, L. plantarum benefits from cross-feeding mechanisms in co-culture environments, utilizing metabolic byproducts released by other microorganisms. This strategy not only minimizes direct competition with S. aureus but also enhances the survival and persistence of L. plantarum in nitrogen-limited conditions (Hu et al., 2024; Rezaei et al., 2021).
Together, these environmental influences, including optimal temperature, fostering robust antimicrobial production and versatile nutrient utilization create conditions that favor the dominance of L. plantarum over S. aureus (Halsey et al., 2017; Somerville and Proctor, 2009; Hu et al., 2024; Rezaei et al., 2021). By thriving in environments that are less favorable to S. aureus, L. plantarum is better positioned to exert its probiotic and therapeutic effects, thereby contributing to the control of S. aureus infections in clinical settings.
Conclusion
Lactobacillus plantarum antagonism against Staphylococcus aureus has emerged as an area of growing interest, offering promising therapeutic strategies for managing biofilm-associated and antimicrobial-resistant infections. Importantly, the antimicrobial potential of L. plantarum is strain-dependent. Comparative genomic analyses have revealed substantial phylogenetic and functional diversity within the species, particularly in bacteriocin gene clusters, stress response elements, and quorum sensing systems (Choi et al., 2021). This strain-specific behavior is further supported by studies showing that plantaricin A requires precise peptide cooperation for activity (Nissen-Meyer et al., 1993), and that plantaricin gene activation is regulated in response to co-culture with specific Gram-positive bacteria (Maldonado et al., 2004). This diversity underscores the importance of targeted, strain-level evaluation when considering L. plantarum as a therapeutic agent.
This review further synthesizes current findings on the diverse inhibitory strategies used by L. plantarum, including bacteriocin production, metabolic acidification, quorum sensing interference, and immune modulation. These mechanisms collectively position L. plantarum as a compelling adjunct or alternative to conventional antibiotic therapies in the face of rising resistance (Marianelli et al., 2010). By interfering with cell wall synthesis, quorum sensing, and biofilm formation, L. plantarum effectively suppresses S. aureus growth and virulence (Ahn et al., 2018; Arena et al., 2016; Yasir et al., 2018; Wang et al., 2022), suggesting that probiotic–pathogen interactions could drive a paradigm shift in antimicrobial strategies beyond traditional antibiotics. Given the prevalence of recurrent S. aureus infections in clinical contexts, particularly in wound care, leveraging L. plantarum as a therapeutic probiotic merits further exploration. Its potential applications include prevention of medical-device-associated infections and modulation of mucosal microbiota (Ong et al., 2020). Nevertheless, significant challenges remain in translating laboratory findings into effective clinical interventions. Future directions should include comprehensive phylogenetic profiling, elucidation of molecular mechanisms underpinning probiotic–pathogen interactions, and well-controlled clinical trials with matched populations to rigorously assess the efficacy and safety of L. plantarum-based therapies (Aljohani et al., 2024; Parvez et al., 2006).
Author contributions
LB: Conceptualization, Software, Writing – review & editing, Writing – original draft. FB: Writing – original draft, Conceptualization, Writing – review & editing. PR: Writing – review & editing, Writing – original draft, Conceptualization. AR: Conceptualization, Software, Writing – review & editing, Writing – original draft. CL: Writing – review & editing, Writing – original draft. SMT: Conceptualization, Writing – original draft, Writing – review & editing. SAT: Writing – review & editing, Writing – original draft. IJ: Writing – review & editing, Writing – original draft. RD: Writing – review & editing, Writing – original draft. JS: Writing – review & editing. VP: Writing – review & editing. OO: Writing – review & editing. SK: Writing – review & editing. SI: Writing – review & editing, Project administration, Supervision, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding was provided by a startup grant to SI from the department of Biology, Southeast Missouri State University. SI was supported by a student mentorship research grant and a Summer Research and Creative Activities Grant.
Acknowledgments
The authors thank members of the Shariful Lab at Southeast Missouri State University for their feedback and critical review of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AMPs, Antimicrobial peptides; GAPDH, Glyceraldehyde-3-phosphate dehydrogenase; SIgA, Immunoglobulin A; IFN, Interferon-gamma; IL, Interleukin; LTA, Lipoteichoic acid.
References
Ahn, K. B., Baik, J. E., Yun, C.-H., and Han, S. H. (2018). Lipoteichoic acid inhibits Staphylococcus aureus biofilm formation. Front. Microbiol. 9:327. doi: 10.3389/fmicb.2018.00327
Aljohani, A., Rashwan, N., Vasani, S., Alkhawashki, A., Wu, T. T., Lu, X., et al. (2024). The health benefits of probiotic Lactobacillus plantarum: a systematic review and meta-analysis. Probiotics Antimicrob. Proteins. doi: 10.1007/s12602-024-10287-3
Arena, M. P., Silvain, A., Normanno, G., Grieco, F., Drider, D., Spano, G., et al. (2016). Use of Lactobacillus plantarum strains as a bio-control strategy against food-borne pathogenic microorganisms. Front. Microbiol. 7:464. doi: 10.3389/fmicb.2016.00464
Arief, I. I., Budiman, C., Jenie, B. S. L., Andreas, E., and Yuneni, A. (2015). Plantaricin IIA-1A5 from Lactobacillus plantarum IIA-1A5 displays bactericidal activity against Staphylococcus aureus. Benefic. Microbes 6, 603–614. doi: 10.3920/BM2014.0064
Barrientos, S., Stojadinovic, O., Golinko, M. S., Brem, H., and Tomic-Canic, M. (2008). Perspective article: growth factors and cytokines in wound healing. Wound Repair Regen. 16, 585–601. doi: 10.1111/j.1524-475X.2008.00410.x
Boskey, E. R., Cone, R. A., Whaley, K. J., and Moench, T. R. (2001). Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum. Reprod. 16, 1809–1813. doi: 10.1093/humrep/16.9.1809
Boskey, E. R., Telsch, K. M., Whaley, K. J., Moench, T. R., and Cone, R. A. (1999). Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect. Immun. 67, 5170–5175. doi: 10.1128/IAI.67.10.5170-5175.1999
Brunel, A.-S., and Benoit, G. (2017). Multidrug resistant (or antimicrobial-resistant) pathogens—alternatives to new antibiotics? Swiss Med. Wkly. 147:w14553. doi: 10.4414/smw.2017.14553
Carvalho, F. M., Teixeira-Santos, R., Mergulhão, F. J. M., and Gomes, L. C. (2021). Effect of Lactobacillus plantarum biofilms on the adhesion of Escherichia coli to urinary tract devices. Antibiotics 10:966. doi: 10.3390/antibiotics10080966
Choi, S., Baek, M., Chung, M.-J., Lim, S., and Yi, H. (2021). Distribution of bacteriocin genes in the lineages of Lactobacillus plantarum. Sci. Rep. 11:20063. doi: 10.1038/s41598-021-99683-1
Christensen, I. B., Vedel, C., Clausen, M.-L., Kjærulff, S., Agner, T., and Nielsen, D. S. (2021). Targeted screening of lactic acid Bacteria with antibacterial activity toward Staphylococcus aureus clonal complex type 1 associated with atopic dermatitis. Front. Microbiol. 12:733847. doi: 10.3389/fmicb.2021.733847
Cui, Y., Wang, M., Zheng, Y., Miao, K., and Qu, X. (2021). The carbohydrate metabolism of Lactobacillus plantarum. Int. J. Mol. Sci. 22:13452. doi: 10.3390/ijms222413452
Dolan, K. E., Pizano, J. M., Gossard, C. M., Williamson, C. B., Burns, C. M., Gasta, M. G., et al. (2017). Probiotics and disease: a comprehensive summary-part 6, skin health. Integr. Med. (Encinitas, Calif.) 16, 32–41.
Douillard, F. P., and de Vos, W. M. (2014). Functional genomics of lactic acid bacteria: from food to health. Microb. Cell Factories 13:S8. doi: 10.1186/1475-2859-13-S1-S8
Elebeedy, D., Ghanem, A., El-Sayed, M., Fayad, E., Abu Ali, O. A., Alyamani, A., et al. (2022). Synergistic antimicrobial effect of Lactobacillus plantarum and Lawsonia inermis against Staphylococcus aureus. Infect. Drug Resist. 15, 545–554. doi: 10.2147/IDR.S342976
Eming, S. A., Krieg, T., and Davidson, J. M. (2007). Inflammation in wound repair: molecular and cellular mechanisms. J. Invest. Dermatol. 127, 514–525. doi: 10.1038/sj.jid.5700701
Ennahar, S., Sashihara, T., Sonomoto, K., and Ishizaki, A. (2000). Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol. Rev. 24, 85–106. doi: 10.1111/j.1574-6976.2000.tb00534.x
Golneshin, A., Gor, M.-C., Williamson, N., Vezina, B., Van, T. T. H., May, B. K., et al. (2020). Discovery and characterisation of circular bacteriocin plantacyclin B21AG from Lactobacillus plantarum B21. Heliyon 6:e04715. doi: 10.1016/j.heliyon.2020.e04715
Halsey, C. R., Lei, S., Wax, J. K., Lehman, M. K., Nuxoll, A. S., Steinke, L., et al. (2017). Amino acid catabolism in Staphylococcus aureus and the function of carbon catabolite repression. mBio 8:e01434-16. doi: 10.1128/mBio.01434-16
Hong, J., Son, M., Sin, J., Kim, H., and Chung, D.-K. (2023). Nanoparticles of Lactobacillus plantarum K8 reduce Staphylococcus aureus respiratory infection and tumor necrosis factor alpha- and interferon gamma-induced lung inflammation. Nutrients 15:4728. doi: 10.3390/nu15224728
Hu, C., Ren, L., Zhou, Y., and Ye, B. (2019). Characterization of antimicrobial activity of three Lactobacillus plantarum strains isolated from Chinese traditional dairy food. Food Sci. Nutr. 7, 1997–2005. doi: 10.1002/fsn3.1025
Hu, M., Wang, D., Tang, X., Zhang, Q., Zhao, J., Mao, B., et al. (2024). Improving the utilization efficiency of nitrogen source through co-culture of Lactobacillus strains with different nitrogen source metabolisms. LWT 191:115701. doi: 10.1016/j.lwt.2023.115701
Jefferson, K. K. (2004). What drives bacteria to produce a biofilm? FEMS Microbiol. Lett. 236, 163–173. doi: 10.1111/j.1574-6968.2004.tb09643.x
Kagiwada, K., Chida, D., Sakatani, T., Asano, M., Nambu, A., Kakuta, S., et al. (2004). Interleukin (IL)-6, but not IL-1, induction in the brain downstream of Cyclooxygenase-2 is essential for the induction of febrile response against peripheral IL-1α. Endocrinology 145, 5044–5048. doi: 10.1210/en.2004-0054
Kalliomäki, M., Salminen, S., Poussa, T., Arvilommi, H., and Isolauri, E. (2003). Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet (London, England) 361, 1869–1871. doi: 10.1016/S0140-6736(03)13490-3
Kalliomäki, M., Salminen, S., Poussa, T., and Isolauri, E. (2007). Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 119, 1019–1021. doi: 10.1016/j.jaci.2006.12.608
Kim, M.-H., Choi, S. J., Choi, H.-I., Choi, J.-P., Park, H.-K., Kim, E. K., et al. (2018). Lactobacillus plantarum-derived extracellular vesicles protect atopic dermatitis induced by Staphylococcus aureus-derived extracellular vesicles. Allergy, Asthma Immunol. Res. 10, 516–532. doi: 10.4168/aair.2018.10.5.516
Kim, H., Kim, H. S., Park, W. J., and Chung, D. K. (2015). Inhibitory effect of Lactobacillus plantarum extracts on HT-29 Colon Cancer cell apoptosis induced by Staphylococcus aureus and its alpha-toxin. J. Microbiol. Biotechnol. 25, 1849–1855. doi: 10.4014/jmb.1504.04047
Klein, R. D., and Hultgren, S. J. (2020). Urinary tract infections: microbial pathogenesis, host–pathogen interactions and new treatment strategies. Nat. Rev. Microbiol. 18, 211–226. doi: 10.1038/s41579-020-0324-0
Kondakala, S., Yoon, S., Daddy Gaoh, S., Kweon, O., Kim, S.-J., and Hart, M. E. (2024). Directional and strain-specific interaction between Lactobacillus plantarum and Staphylococcus aureus. Microorganisms 12:2432. doi: 10.3390/microorganisms12122432
Lebeer, S., Vanderleyden, J., and De Keersmaecker, S. C. J. (2008). Genes and molecules of lactobacilli supporting probiotic action. Microbiol. Mol. Biol. Rev. 72, 728–764. doi: 10.1128/MMBR.00017-08
Li, H., Jia, M., Qi, Q., and Wang, Q. (2023a). Engineered probiotic Lactobacillus plantarum WCSF I for monitoring and treatment of Staphylococcus aureus infection. Microbiol. Spectr. 11, e01829–e01823. doi: 10.1128/spectrum.01829-23
Li, M., Xiao, H., Su, Y., Cheng, D., Jia, Y., Li, Y., et al. (2023b). Synergistic inhibitory effect of honey and Lactobacillus plantarum on pathogenic Bacteria and their promotion of healing in infected wounds. Pathogens 12:501. doi: 10.3390/pathogens12030501
Lin, W.-H., Wu, C.-R., Lee, H.-Z., Kuo, Y.-H., Wen, H.-S., Lin, T.-Y., et al. (2013). Induced apoptosis of Th2 lymphocytes and inhibition of airway hyperresponsiveness and inflammation by combined lactic acid bacteria treatment. Int. Immunopharmacol. 15, 703–711. doi: 10.1016/j.intimp.2012.10.025
Liu, H., Gao, Y., Yu, L.-R., Jones, R. C., Elkins, C. A., and Hart, M. E. (2011). Inhibition of Staphylococcus aureus by Lysostaphin-expressing Lactobacillus plantarum WCFS1 in a modified genital tract secretion medium. Appl. Environ. Microbiol. 77, 8500–8508. doi: 10.1128/AEM.06755-11
Maldonado, A., Jiménez-Díaz, R., and Ruiz-Barba, J. L. (2004). Induction of plantaricin production in Lactobacillus plantarum NC8 after coculture with specific gram-positive bacteria is mediated by an autoinduction mechanism. J. Bacteriol. 186, 1556–1564. doi: 10.1128/jb.186.5.1556-1564.2004
Marianelli, C., Cifani, N., and Pasquali, P. (2010). Evaluation of antimicrobial activity of probiotic bacteria against Salmonella enterica subsp. Enterica serovar typhimurium 1344 in a common medium under different environmental conditions. Res. Microbiol. 161, 673–680. doi: 10.1016/j.resmic.2010.06.007
Moormeier, D. E., and Bayles, K. W. (2017). Staphylococcus aureus biofilm: a complex developmental organism. Mol. Microbiol. 104, 365–376. doi: 10.1111/mmi.13634
Mukherjee, S., and Ramesh, A. (2015). Bacteriocin-producing strains of Lactobacillus plantarum inhibit adhesion of Staphylococcus aureus to extracellular matrix: quantitative insight and implications in antibacterial therapy. J. Med. Microbiol. 64, 1514–1526. doi: 10.1099/jmm.0.000181
Nissen-Meyer, J., Larsen, A. G., Sletten, K., Daeschel, M., and Nes, I. F. (1993). Purification and characterization of plantaricin a, a Lactobacillus plantarum bacteriocin whose activity depends on the action of two peptides. Microbiology 139, 1973–1978. doi: 10.1099/00221287-139-9-1973
Ong, J. S., Taylor, T. D., Yong, C. C., Khoo, B. Y., Sasidharan, S., Choi, S. B., et al. (2020). Lactobacillus plantarum USM8613 aids in wound healing and suppresses Staphylococcus aureus infection at wound sites. Probiot. Antimicrob. Proteins 12, 125–137. doi: 10.1007/s12602-018-9505-9
Oppegård, C., Kjos, M., Veening, J.-W., Nissen-Meyer, J., and Kristensen, T. (2016). A putative amino acid transporter determines sensitivity to the two-peptide bacteriocin plantaricin JK. MicrobiologyOpen 5, 700–708. doi: 10.1002/mbo3.363
Otto, M. (2018). Staphylococcal biofilms. Microbiol. Spectr. 6:10.1128/microbiolspec.gpp3-0023-2018. doi: 10.1128/microbiolspec.gpp3-0023-2018
Parvez, S., Malik, K. A., Ah Kang, S., and Kim, H.-Y. (2006). Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 100, 1171–1185. doi: 10.1111/j.1365-2672.2006.02963.x
Pei, J., Huang, Y., Ren, T., Guo, Y., Dang, J., Tao, Y., et al. (2022). The antibacterial activity mode of action of Plantaricin YKX against Staphylococcus aureus. Molecules 27:Article 13. doi: 10.3390/molecules27134280
Qazi, S., Middleton, B., Muharram, S. H., Cockayne, A., Hill, P., O’Shea, P., et al. (2006). N-acylhomoserine lactones antagonize virulence gene expression and quorum sensing in Staphylococcus aureus. Infect. Immun. 74, 910–919. doi: 10.1128/IAI.74.2.910-919.2006
Reid, G., Beuerman, D., Heinemann, C., and Bruce, A. W. (2001). Probiotic Lactobacillus dose required to restore and maintain a normal vaginal flora. FEMS Immunol. Med. Microbiol. 32, 37–41. doi: 10.1111/j.1574-695X.2001.tb00531.x
Reid, G., and Tieszer, C. (1994). Use of lactobacilli to reduce the adhesion of Staphylococcus aureus to catheters. Int. Biodeterior. Biodegrad. 34, 73–83. doi: 10.1016/0964-8305(95)00011-9
Ren, D., Gong, S., Shu, J., Zhu, J., Rong, F., Zhang, Z., et al. (2017). Mixed Lactobacillus plantarum strains inhibit Staphylococcus aureus induced inflammation and ameliorate intestinal microflora in mice. Biomed. Res. Int. 2017:7476467. doi: 10.1155/2017/7476467
Rezaei, Z., Khanzadi, S., and Salari, A. (2021). Biofilm formation and antagonistic activity of Lacticaseibacillus rhamnosus (PTCC1712) and Lactobacillus plantarum (PTCC1745). AMB Express 11:156. doi: 10.1186/s13568-021-01320-7
Rizzo, A., Fiorentino, M., Buommino, E., Donnarumma, G., Losacco, A., and Bevilacqua, N. (2015). Lactobacillus crispatus mediates anti-inflammatory cytokine interleukin-10 induction in response to Chlamydia trachomatis infection in vitro. Int. J. Med. Microbiol. 305, 815–827. doi: 10.1016/j.ijmm.2015.07.005
Rönnqvist, P. D. J., Forsgren-Brusk, U. B., and Grahn-Håkansson, E. E. (2006). Lactobacilli in the female genital tract in relation to other genital microbes and vaginal pH. Acta Obstet. Gynecol. Scand. 85, 726–735. doi: 10.1080/00016340600578357
Sodré, M. T. C., Ferraz, F. A., Alencar, A. K. V., Silva, K. F., Silva, D. H. D. S., Silva, L. D. S., et al. (2023). The potential of Lactiplantibacillus plantarum ATCC 14917 in the development of alginate-based gel formulations with anti-Staphylococcus aureus properties. Pharmaceuticals 16:1112. doi: 10.3390/ph16081112
Somerville, G. A., and Proctor, R. A. (2009). At the crossroads of bacterial metabolism and virulence factor synthesis in staphylococci. Microbiol. Mol. Biol. Rev. 73, 233–248. doi: 10.1128/MMBR.00005-09
Spangler, J. R., Dean, S. N., Leary, D. H., and Walper, S. A. (2019). Response of Lactobacillus plantarum WCFS1 to the gram-negative pathogen-associated quorum sensing molecule N-3-Oxododecanoyl Homoserine lactone. Front. Microbiol. 10:715. doi: 10.3389/fmicb.2019.00715
Spurbeck, R. R., & and Arvidson, C. G. (2011). Lactobacilli at the front line of defense against vaginally acquired infections. Future Microbiol., 6, 567–582. doi: 10.2217/fmb.11.36
Stevens, E. T., Van Beeck, W., Blackburn, B., Tejedor-Sanz, S., Rasmussen, A. R. M., Carter, M. E., et al. (2023). Lactobacillus plantarum uses ecologically relevant, exogenous quinones for extracellular electron transfer. MBio 14, e02234–e02223. doi: 10.1128/mbio.02234-23
Tejero-Sariñena, S., Barlow, J., Costabile, A., Gibson, G. R., and Rowland, I. (2012). In vitro evaluation of the antimicrobial activity of a range of probiotics against pathogens: evidence for the effects of organic acids. Anaerobe 18, 530–538. doi: 10.1016/j.anaerobe.2012.08.004
Tian, Y., Wang, Y., Zhang, N., Xiao, M., Zhang, J., Xing, X., et al. (2022). Antioxidant mechanism of Lactobacillus plantarum KM1 under H2O2 stress by proteomics analysis. Front. Microbiol. 13, 4–7. doi: 10.3389/fmicb.2022.897387
Tong, S. Y. C., Davis, J. S., Eichenberger, E., Holland, T. L., and Fowler, V. G. (2015). Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 28, 603–661. doi: 10.1128/CMR.00134-14
Turner, M. S., Waldherr, F., Loessner, M. J., and Giffard, P. M. (2007). Antimicrobial activity of lysostaphin and a Listeria monocytogenes bacteriophage endolysin produced and secreted by lactic acid bacteria. Syst. Appl. Microbiol. 30, 58–67. doi: 10.1016/j.syapm.2006.01.013
Tymoszewska, A., Diep, D. B., and Aleksandrzak-Piekarczyk, T. (2018). The extracellular loop of man-PTS subunit IID is responsible for the sensitivity of Lactococcus garvieae to garvicins a, B and C. Sci. Rep. 8:15790. doi: 10.1038/s41598-018-34087-2
van den Nieuwboer, M., van Hemert, S., Claassen, E., and de Vos, W. M. (2016). Lactobacillus plantarum WCFS1 and its host interaction: a dozen years after the genome. Microb. Biotechnol. 9, 452–465. doi: 10.1111/1751-7915.12368
Vuotto, C., Longo, F., and Donelli, G. (2014). Probiotics to counteract biofilm-associated infections: promising and conflicting data. Int. J. Oral Sci. 6, 189–194. doi: 10.1038/ijos.2014.52
Walker, J. N., Flores-Mireles, A. L., Pinkner, C. L., Schreiber, H. L., Joens, M. S., Park, A. M., et al. (2017). Catheterization alters bladder ecology to potentiate Staphylococcus aureus infection of the urinary tract. Proc. Natl. Acad. Sci. 114, E8721–E8730. doi: 10.1073/pnas.1707572114
Wang, K., Duan, F., Sun, T., Zhang, Y., and Lu, L. (2024). Galactooligosaccharides: synthesis, metabolism, bioactivities and food applications. Crit. Rev. Food Sci. Nutr. 64, 6160–6176. doi: 10.1080/10408398.2022.2164244
Wang, W., Kyrkou, I., Bojer, M. S., Kalloubi, D., Kali, A. J., Alena-Rodriguez, M., et al. (2025). Characterization of agr-like loci in Lactobacillus plantarum and L. paraplantarum and their role in quorum sensing and virulence inhibition of Staphylococcus aureus. Probiot. Antimicrob. Proteins. doi: 10.1007/s12602-025-10476-8
Wang, J., Li, Y., Pan, L., Li, J., Yu, Y., Liu, B., et al. (2021). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) moonlights as an adhesin in Mycoplasma hyorhinis adhesion to epithelial cells as well as a plasminogen receptor mediating extracellular matrix degradation. Vet. Res. 52:80. doi: 10.1186/s13567-021-00952-8
Wang, Z., Wu, J., Tian, Z., Si, Y., Chen, H., and Gan, J. (2022). The mechanisms of the potential probiotic Lactobacillus plantarum against cardiovascular disease and the recent developments in its fermented foods. Food Secur. 11:2549. doi: 10.3390/foods11172549
Wang, G., Zhang, M., Zhao, J., Xia, Y., Lai, P. F.-H., and Ai, L. (2018). A surface protein from Lactobacillus plantarum increases the adhesion of Lactobacillus strains to human epithelial cells. Front. Microbiol. 9:2858. doi: 10.3389/fmicb.2018.02858
Wells, J. M. (2011). Immunomodulatory mechanisms of lactobacilli. Microb. Cell Factories 10:S17. doi: 10.1186/1475-2859-10-S1-S17
Xie, X., Cao, M., Yan, S., Gao, H., Yang, Y., Zeng, J., et al. (2025). The preventive effect of probiotic Lactobacillus plantarum X86 isolated from raw milk on Staphylococcus aureus-induced mastitis in rats. Frontiers in Veterinary Science 12:1476232. doi: 10.3389/fvets.2025.1476232
Keywords: Staphylococcus aureus , Lactobacillus plantarum , antagonism, probiotics, antimicrobial, microbiota, metabolite, antibiotic resistance
Citation: Bui LTK, Bushra FA, Rattananon P, Rimi AA, Lee C, Tahmid SM, Tisha SA, Jisan IF, Das R, Sneha JI, Pitts V, Owasanoye OU, Khadka S and Islam S (2025) Strategic antagonism: how Lactobacillus plantarum counters Staphylococcus aureus pathogenicity. Front. Microbiol. 16:1635123. doi: 10.3389/fmicb.2025.1635123
Edited by:
Valério Monteiro-Neto, Federal University of Maranhão, BrazilReviewed by:
Qian Liu, Shanghai Jiao Tong University, ChinaFlavia Costa, University of Colorado Anschutz Medical Campus, United States
Copyright © 2025 Bui, Bushra, Rattananon, Rimi, Lee, Tahmid, Tisha, Jisan, Das, Sneha, Pitts, Owasanoye, Khadka and Islam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shariful Islam, c2lzbGFtQHNlbW8uZWR1
†These authors have contributed equally to this work and share second authorship
 Ly Tuan Kiet Bui
Ly Tuan Kiet Bui Fariha Alam Bushra
Fariha Alam Bushra Pirachat Rattananon
Pirachat Rattananon Arifa Afrose Rimi
Arifa Afrose Rimi Carmen Lee
Carmen Lee S. M. Tahmid1
S. M. Tahmid1 Sumaiya Akter Tisha
Sumaiya Akter Tisha Jahin Ibnat Sneha
Jahin Ibnat Sneha Victoria Pitts
Victoria Pitts Olalekan Uchechukwu Owasanoye
Olalekan Uchechukwu Owasanoye Saphal Khadka
Saphal Khadka Shariful Islam
Shariful Islam