Abstract
Objectives:
This study reviews meta-analyses of perioperative supplementation with probiotics/synbiotics in colorectal cancer (CRC), systematically assessing the quality of meta-analyses and synthesizing study results to provide robust evidence.
Methods:
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The search was conducted by two authors in four databases, PubMed, CENTRAL, EMBASE and Web of Science, up until August 3rd, 2025, and conducted independent assessments of the methodological quality of the meta-analysis via A Measure Tool to Assess Systematic Reviews (AMSTAR) 2.
Results:
A total of 11 meta-analyses were included in this umbrella review. 3 meta-analyses rated “Critically low” shared ≥3 critical flaws and 2 high-rated reviews adhered to ≥80% AMSTAR 2 criteria. Compared with the control group, the probiotic/synbiotic group presented lower incidence rates of overall infections (OR 0.49, 95% CI: 0.43, 0.56; P < 0. 001, I2 = 6%), surgical site infections (OR 0.58, 95% CI: 0.50, 0.67; P < 0.00001, I2 = 0%), urinary tract infections (OR 0.39, 95% CI: 0.27, 0.54; P < 0.00001, I2 = 0%), and pneumonia infections (OR 0.34, 95% CI: 0.26, 0.45; P < 0.00001, I2 = 0%), and diarrhea incidence (OR 0.41, 95% CI: 0.34, 0.51; P < 0.00001, I2 = 0%).
Conclusion:
According to the results of our analyses, perioperative probiotic/synbiotic supplementation in CRC patients is associated with a reduced incidence of overall infections, surgical site infections, urinary tract infections, pneumonia infections, and diarrhea.
Systematic review registration:
https://www.crd.york.ac.uk/prospero/, identifier CRD42024619853.
Introduction
Colorectal cancer is the third most common cancer and the second leading cause of cancer death worldwide, and is a major contributor to morbidity and mortality in the global population (Siegel et al., 2023). Patients with CRC can experience certain intestinal microecological disturbances of their own, such as a significant enrichment of pathogenic bacteria such as Clostridium nucleatum, Streptococcus anaerogenes, Aeromonas riceii, and Staphylococcus stomatitis, and a decrease in the composition of Thermophilic Streptococcus, Lactobacillus fowleri, Streptococcus maltophagus, Clostridium butyricum and Streptococcus salivarius, which constitute a decreased proportion, etc., and this imbalance is exacerbated by preoperative mechanical bowel preparation and surgery (Jin et al., 2019; Weaver et al., 2024; Yu et al., 2017). After CRC surgery, the number of Bifidobacteria and the Shannon index decreased, whereas the proportions of Enterococci, staphylococci, and Pseudomonas increased. This change in the commensal-pathogenic bacterial balance after surgery may lead to complications such as surgical site infection (SSI) (Masheghati et al., 2024). Among these, SSI is significantly associated with recurrence and survival in CRC patients, and its incidence should be minimized to improve surgical and long-term oncological outcomes (Chen et al., 2021).
Recent studies have shown that the gut microbiome is often associated with cancer therapies (including surgical, chemical, radiation, and immune therapies), therapeutic effectiveness, and side effects (Dai et al., 2024; Zhou et al., 2024). Previous studies have confirmed that probiotic supplementation during the perioperative period of CRC produces significant clinical benefits, reducing the incidence of intestinal obstruction, peritoneal effusion, diarrhea, sepsis, pneumonia and SSI in CRC patients (Araújo et al., 2023). In addition, probiotics can improve the intestinal microenvironment and promote the repair and regeneration of the intestinal mucosa, thus accelerating the recovery of postoperative intestinal function, which can help reduce the incidence of postoperative intestinal paralysis, intestinal obstruction, and other complications (Chen et al., 2022; Liu et al., 2016). CRC patients may suffer from dyspepsia, nausea, diarrhea and other discomfort during the perioperative period, and probiotic supplementation can help alleviate these discomforts by adjusting the balance of the intestinal flora and improving the digestive function to alleviate these discomfort symptoms (Kasatpibal et al., 2017; Xu et al., 2019). The probiotic groups most widely used in the perioperative period of CRC are Lactobacillus and Bifidobacterium, which play important roles in promoting food absorption, enhancing host resistance to infection, strengthening the intestinal immune system, and regulating host metabolism (Eslami et al., 2019).
Although probiotic/synbiotic supplementation in the perioperative period of CRC has received increasing attention and numerous meta-analyses have been conducted to evaluate it, the results are inconsistent (Araújo et al., 2023; Kothari et al., 2019). Umbrella reviews represent the pinnacle of evidence-based medicine, systematically assessing the quality of meta-analyses and synthesizing relevant findings to provide reliable evidence (Tang et al., 2024). Therefore, to provide new perspectives for clinical practice, the present study is an umbrella review of meta-analyses on perioperative probiotic/synbiotic supplementation in CRC.
Methods
Our work has been reported in line with PRISMA (Page et al., 2021) and AMSTAR 2 (Shea et al., 2017) Guidelines.
Search strategy
Two authors conducted a comprehensive literature search of PubMed, Cochrane Central Register of Controlled Trials, EMBASE and Web of Science from inception to August 3rd, 2025 via a combination of Medical Subject Headings (MeSH) and free words. The keywords selected were “probiotics,” “symbiotic,” “meta” and “colorectal cancer,” etc. The search strategy was adapted to the characteristics of each database. (Supplementary Appendix 1 shows an example search strategy for the PubMed database). In addition, references to the included articles were manually searched for additional studies. All studies were imported into EndNote (version X9.2), where they were deduplicated. When differences of opinion were encountered, a third author was involved in the discussion until a consensus was reached.
Eligibility criteria
To identify relevant meta-analysis studies, we outlined inclusion criteria according to the reporting structure of populations, interventions, comparisons, outcomes and study designs (PICOs): (1) any meta-analysis comparing perioperative probiotic/synbiotic preparations with placebo or standard care in elective colorectal cancer surgery; and (2) a meta-analysis including only Randomized controlled trials (RCTs). The exclusion criteria were as follows (1) no clinical outcomes associated with the use of probiotics/synbiotics were reported, and (2) systematic reviews without meta-analyses.
Data extraction
We extracted the characteristics of all included meta-analysis studies via a predesigned custom Microsoft Excel spreadsheet, and the selection process is summarized in Figure 1. These data included the following details: (1) Study characteristics: author, publication date, number of studies and participants, outcome and AMSTAR 2 quality. (2) Primary outcomes: overall infection incidence and surgical site infections; secondary outcomes: urinary tract infection, pneumonia infection, diarrhea incidence, effect of perioperative administration of probiotics/synbiotics on surgical site infections, and effect of preoperative/preoperative and postoperative administration of probiotics on overall infection complications.
FIGURE 1
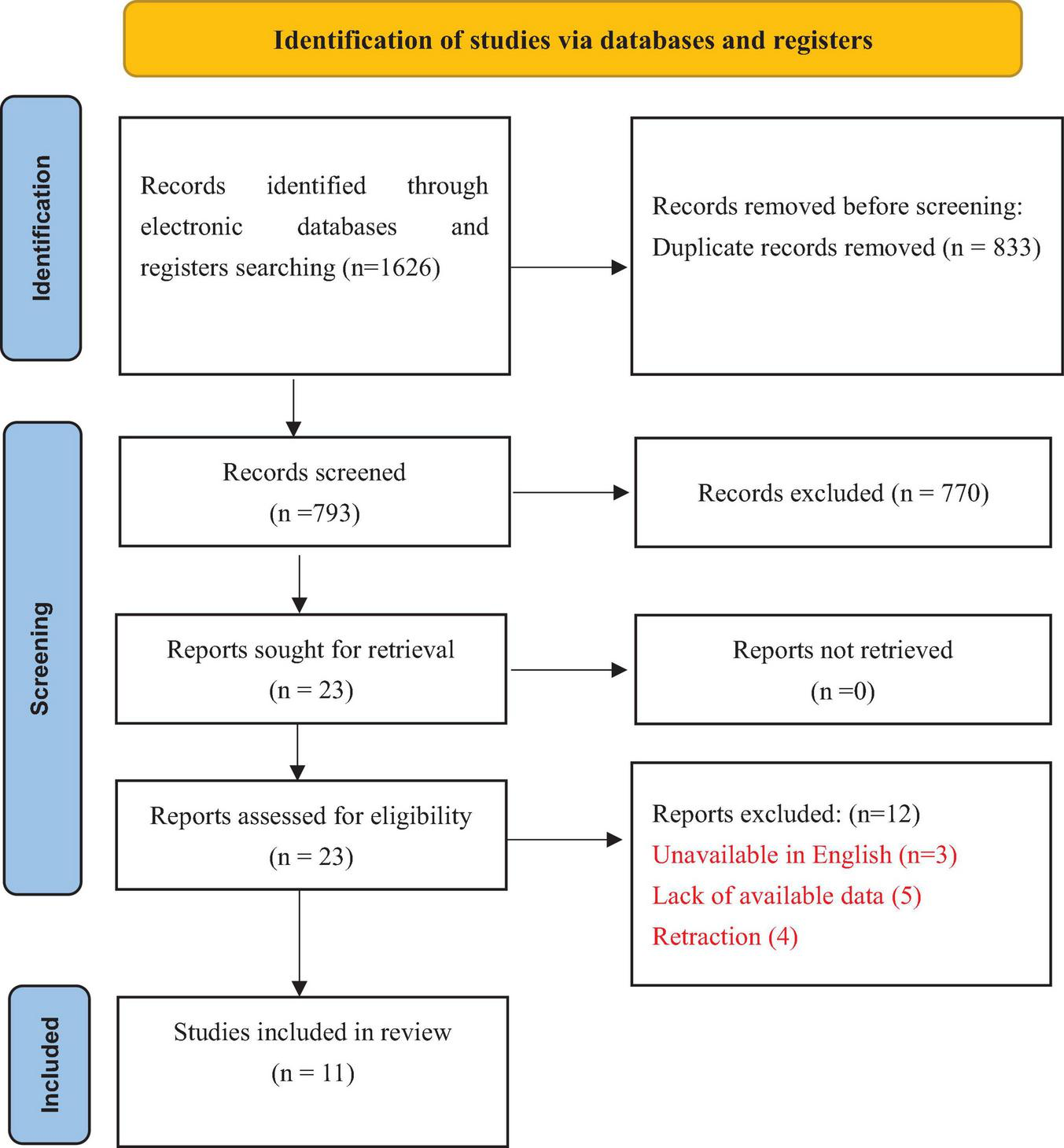
The PRISMA flow diagram to show study selection.
Quality assessment
Two reviewers independently assessed the quality of the meta-analysis methodology via AMSTAR 2, with results expressed as “high,” “medium,” “low” or “critically low.” When disagreements arose, they were discussed with a third author to reach a consensus.
Data analysis
Statistical analysis was performed via RevMan 5.4. The results of the meta-analyses were pooled and expressed as the mean difference (MD) or odds ratio (OR) with the corresponding 95% CIs. For dichotomous variables, we used the Mantel-Haenszel method to run fixed and random object models. The inverse variance method was used for continuous variables. If I2 was ≥50%, there was significant heterogeneity, and a random effects model was used to combine the effect sizes. If I2 is <50%, the heterogeneity is small, and the fixed-effects model can be used to combine the effect size. The test level was bilateral (α = 0.05).
Results
Search results
In accordance with the search strategy, the two authors initially retrieved a total of 1,626 articles, of which 833 were excluded because of duplication and 770 were excluded because the titles and abstracts were read. The study selection flow and the reasons for exclusion are summarized in Figure 1. Finally, 11 studies were included (Amitay et al., 2020; An et al., 2022; Chen et al., 2022, 2024; de Andrade Calaça et al., 2017; Jiang and Ren, 2024; Liu et al., 2016, 2017; Ouyang et al., 2019; Paterson et al., 2025; Persson et al., 2024; Veziant et al., 2022; Wu et al., 2018; Zeng et al., 2021). Figure 1 illustrates the literature screening process.
Study characteristics
All included studies were published between 2017 and 2025. Each meta-analysis included 7–28 RCTs; two studies did not report the population size (Amitay et al., 2020; Chen et al., 2024), and the remaining studies, Paterson et al. (2025) included a maximum of 2,686 participants. Notably, 3 meta-analyses rated “Critically low” (de Andrade Calaça et al., 2017; Liu et al., 2017; Ouyang et al., 2019) shared ≥3 critical flaws. Such as, inadequate gray literature retrieval (potentially missing negative results); Failure to assess RoB in primary RCTs (undermining heterogeneity interpretation); Lack of excluded study documentation (reducing reproducibility). These limitations collectively diminish confidence in their pooled effects. Conversely, high-rated reviews (Veziant et al., 2022; Wu et al., 2018) adhered to ≥80% AMSTAR 2 criteria. Table 1 shows the basic information of all included studies.
TABLE 1
| References | No. of studies/participants, n | Duration of administration | Outcome | AMSTAR 2 quality |
| Amitay et al., 2020 | 11/NR | During chemotherapy to 2 years after end of CRC treatment | Septicemia and sepsis, infection incidence, diarrhea incidence, LOS, return to normal gut function and first defecation, days of antibiotics use | Medium |
| An et al., 2022 | 19/1763 | 15 days before surgery to 30 days after surgery | Overall postoperative infection complication, probiotics-related adverse events, overall postoperative complications, LOS, quality of Life | Medium |
| Chen et al., 2022 | 14/1566 | 8 days before surgery to 15 days after surgery | Postoperative infection complications | Low |
| de Andrade Calaça et al., 2017 | 7/821 | 12 days before surgery to 12 days after surgery | Postoperative infection complications, detection of bacteria in blood | Critically low |
| Liu et al., 2017 | 9/1146 | 11 days before surgery to 15 days after surgery | SSI, urinary tract infection, pneumonia, bacteremia, bacterial translocation, anastomotic leakage | Critically low |
| Ouyang et al., 2019 | 13/1186 | 8 days before surgery to 15 days after surgery | Overall infection rate, pneumonia, the first flatus time, urinary tract infection, anastomotic leakage, and duration of postoperative pyrexia | Critically low |
| Persson et al., 2024 | 10/1276 | 1 days before surgery to 30 days after surgery | Diarrhea, SSI, urinary infection, pulmonary infection, abdominal distention, duration of postoperative pyrexia days, LOS, time to first defecation, and time to first solid diet | Medium |
| Veziant et al., 2022 | 21/1961 | 5 days before surgery to 1 year after surgery | SSI, pulmonary infections, urinary infections, anastomotic leaks, wound infections, postoperative infections | High |
| Wu et al., 2018 | 34/2634 | 9 days before surgery to 6 months after surgery | SSI, postoperative infections, safety, cost-effectiveness, quality of life, intensive care unit stay and hospital stay and promoted earlier first defecation and first bowel movement | High |
| Zeng et al., 2021 | 19/1975 | 9 days before surgery to 15 days after surgery | SSI, inflammatory factors, intestinal dysbiosis, infection complications, and systemic symptoms, systemic inflammatory response syndrome incidence, celiac infections | Medium |
| Paterson et al., 2025 | 28/2686 | 9 days before surgery to 30 days after surgery | Overall postoperative infection, SSI, urinary infection, pulmonary infection | Low |
Basic information of the included studies.
NR, not reported; LOS, length of stay; SSI, surgical site infection.
Analysis via a fixed effects model revealed significant lower in the incidence of overall infection incidence between the intervention and control groups (OR 0.49, 95% CI: 0.43, 0.56; P < 0.001, I2 = 6%), surgical site infections (OR 0.58, 95% CI: 0.50, 0.67; P < 0.00001, I2 = 0%), urinary tract infections (OR 0.39, 95% CI: 0.27, 0.54; P < 0.00001, I2 = 0%), and pneumonia infections (OR 0.34, 95% CI: 0.26, 0.45; P < 0.00001, I2 = 0%) (Figure 2).
FIGURE 2
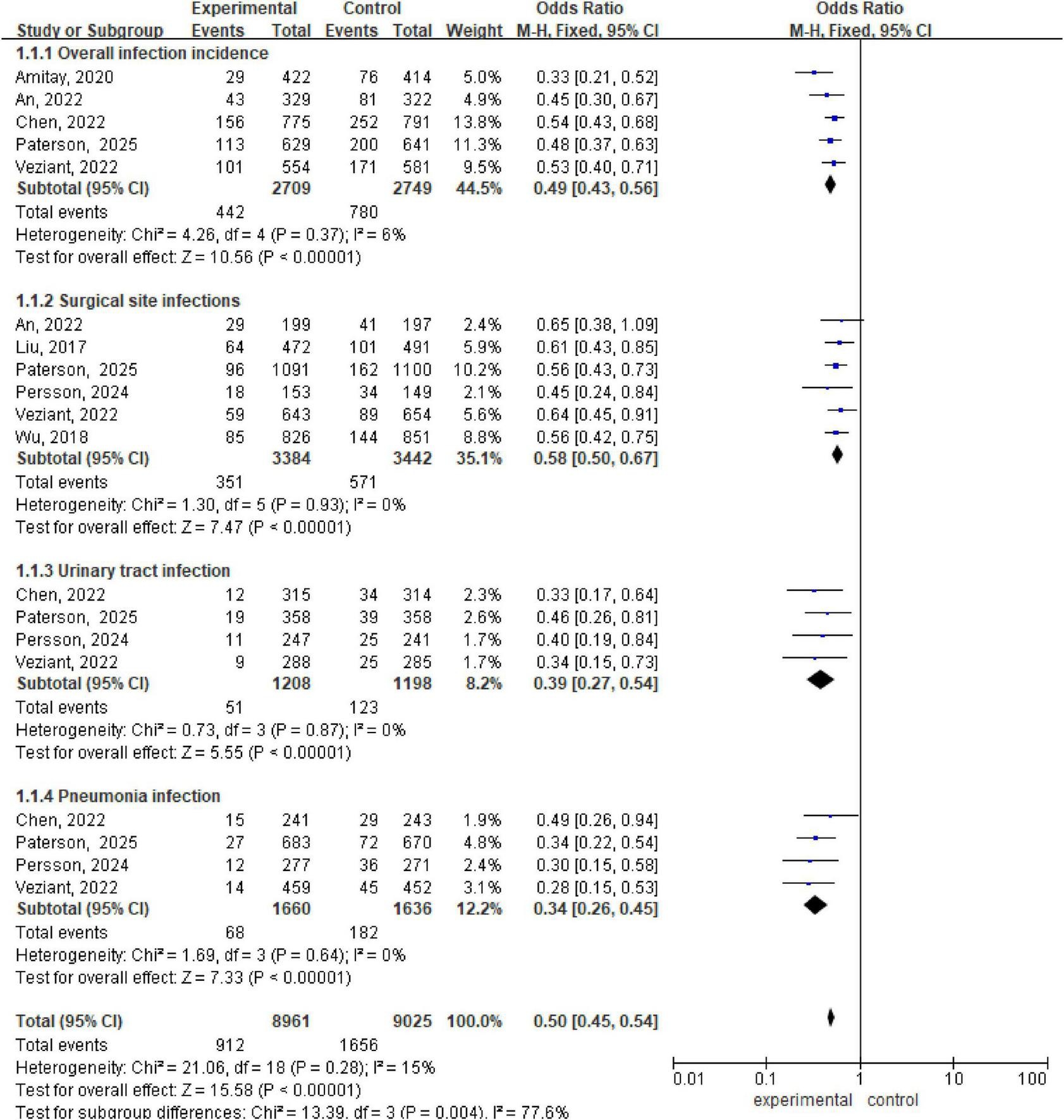
Forest plot of overall infection incidence, surgical site infections, urinary tract infection, and pneumonia infection.
There are 2 meta-analyses, with a total of 2537 patients included (Chen et al., 2022; Veziant et al., 2022). Analysis via a fixed effects model revealed statistically significant differences within the preoperative, pre and postoperative probiotic/synbiotic and control groups (OR 0.30, 95% CI: 0.18, 0.48; P < 0.00001, I2 = 0%), (OR 0.59, 95% CI: 0.49, 0.73; P < 0.00001, I2 = 0%). The subgroup difference was significant (OR 0.53, 95% CI: 0.44, 0.64, P = 0.01, I2 = 56%) (Figure 3).
FIGURE 3
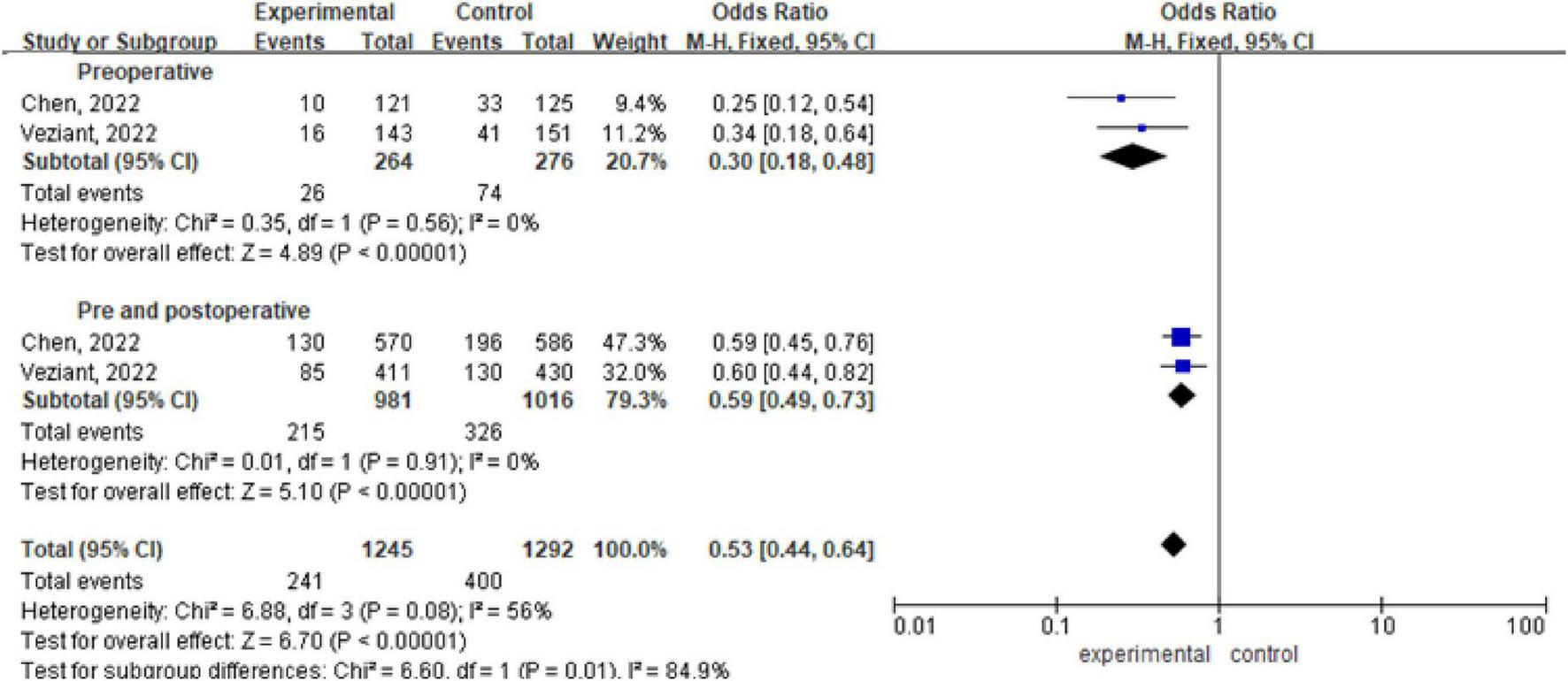
Forest plot of overall infection incidence with preoperative/preoperative and postoperative administration of probiotics/synbiotics.
There are three meta-analyses (Chen et al., 2022; Veziant et al., 2022; Wu et al., 2018) including a total of 4540 patients. Analysis via a fixed effects model revealed statistically significant differences between the probiotic/synbiotic group and the control group during the perioperative period (OR 0.56, 95% CI: 0.45, 0.68; P < 0.00001, I2 = 0%) (OR 0.56, 95% CI: 0.43, 0.73; P < 0. 00001, I2 = 0%). The subgroup difference between probiotics and synbiotics was not significant (OR 0.56, 95% CI: 0.47, 0.65, P = 0.97, I2 = 0%) (Figure 4).
FIGURE 4
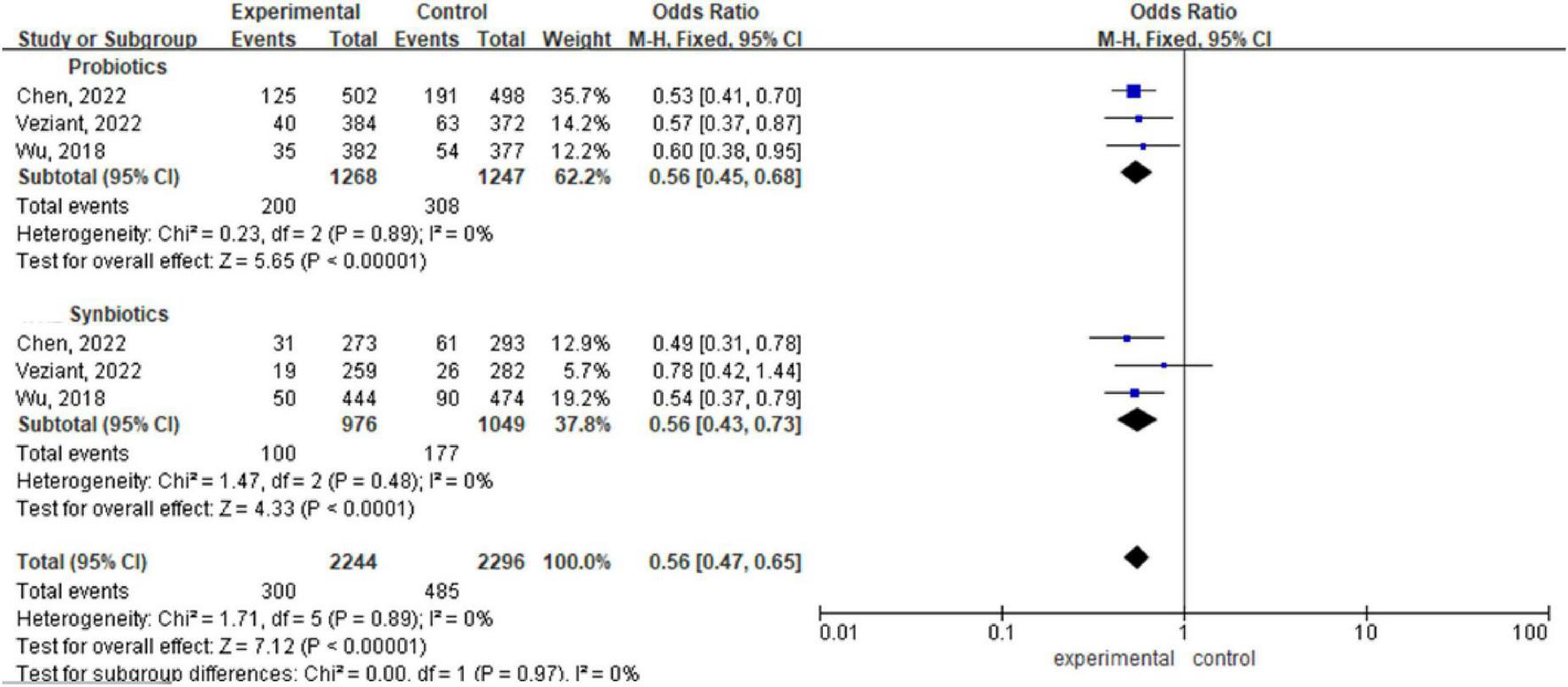
Forest plot comparing the effects of perioperative supplementation with probiotics or synbiotics on surgical site infections.
There are three meta-analyses (Amitay et al., 2020; Chen et al., 2022; Persson et al., 2024) with a total of 1786 patients included. Analysis via a fixed effects model revealed statistically significant differences between the probiotic/synbiotic group and the control group (OR 0.41, 95% CI: 0.34, 0.51; P < 0.00001, I2 = 0%) (Figure 5).
FIGURE 5
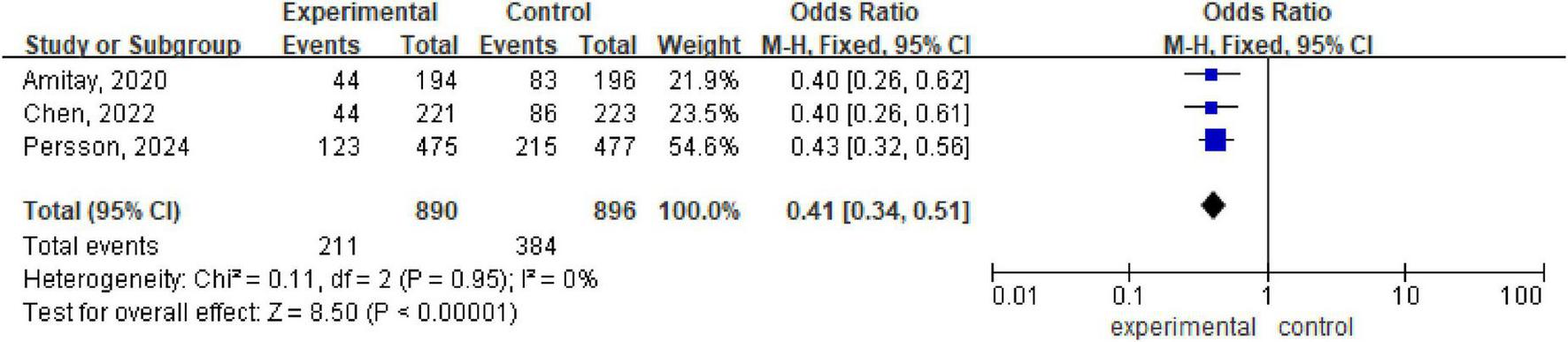
Forest plot of diarrhea incidence.
Among other results, the administration of probiotics/synbiotics in perioperative CRC patients improves gastrointestinal discomfort (Persson et al., 2024), shortens the time to first postoperative bowel movement (Amitay et al., 2020), and improves postoperative quality of life (An et al., 2022; Wu et al., 2018). The administration of single versus multiple probiotic regimens had no significant effect on surgical site infections or overall infections complications (Chen et al., 2024; Veziant et al., 2022). An et al. (2022) reported that the use of probiotics/synbiotics was not associated with any probiotic-related adverse events; certain probiotic strains may exploit the impaired immune system function of cancer patients, transforming into opportunistic pathogens and causing life-threatening pneumonia, endocarditis, and sepsis. Furthermore, the uncontrolled overuse of probiotics may lead to the transfer of plasmid-mediated antibiotic resistance to intestinal pathogens. These factors may hinder the target population from deriving benefits from probiotics/synbiotics administration (Kothari et al., 2019).
We calculated and assessed the overlap in the original studies using corrected coverage area (CCA) calculations based on current guidelines for overlap issues (Hennessy and Johnson, 2020). Our analysis showed that CCA = 0.0123 (<0.15), indicating that the overlap between the original RCTs in different meta-analyses was negligible. Details of the calculation are provided in the Supplementary Appendix 2.
Discussion
Our review included 11 meta-analyses that evaluated the effects of perioperative probiotic/synbiotic supplementation in patients undergoing resection for CRC. In order to further evaluate the efficacy, we did an umbrella review in this study.
In our review, we found that perioperative CRC probiotic/synbiotic supplementation was beneficial in reducing the incidence of overall infection incidence, surgical site infection, urinary tract infection and pneumonia, and we also found that there was no difference in the reduction in surgical site infection between preoperative or preoperative and postoperative probiotic/synbiotic supplementation, and that there were no differences in the reduction in overall infection incidence with probiotics or synbiotics. Fortunately, there was little heterogeneity in our results, which may be because we were limited by the number of included trials and did not perform subgroup analyses of probiotic/symbiotic strains, dosage, time of administration, route of administration, and duration of administration to explore the effects on CRC patients. In particular, we combined CRC patients who used probiotics/synbiotic into one experimental group, which may have biased the results. Other potential sources of heterogeneity could be the demographic and clinical characteristics of CRC patients in different trials, such as age, treatment stage and surgical approach. These difficulties make it difficult to develop optimal protocols, which may be important factors affecting clinical practice and uptake.
An interesting finding of our study was that probiotic/synbiotic supplementation was better for “non-surgical” infection complications [i.e., pneumonia infection (OR = 0.34) and urinary tract infection (OR = 0.35)] than for SSI (OR = 0.60) and overall infection (OR = 0.50). This finding was first proposed by Liu et al. (2017). This leads us to hypothesize that this difference in effect size can prove that the gut microbiota has some association with lung and urinary tract infections, which will provide new ideas for the clinical prevention and treatment of lung and urinary tract infections (Neugent et al., 2020).
Previous studies have shown that probiotic/synbiotic supplementation has great potential to correct intestinal microbial dysbiosis, which may have significant clinical benefits for CRC patients, such as inhibiting the colonization of intestinal microbial pathogens (Richards et al., 2024), improving intestinal barrier integrity (Macharia et al., 2023), modulating host immune responses (Li et al., 2024), reducing therapeutic toxicity (Alam et al., 2022), and even inducing tumor cell apoptosis (Salek et al., 2024), thereby enhancing therapeutic efficacy (Xu et al., 2023). The metabolic metabolites of Lactobacillus, such as antioxidants such as glutathione, superoxide dismutase and catalase, have unique efficacy in alleviating intestinal inflammation and inhibiting the expression of tumor-specific proteins and polyamine components (Colbert et al., 2023; Garbacz, 2022; Wu et al., 2021). Bifidobacteria can activate immune cells such as macrophages, NK cells and B lymphocytes and promote their release of IL-1, IL-6 and TNF-α, thereby exerting indirect antitumor effects (Sivan et al., 2015). Probiotics or synbiotics, with their ability to reverse microbiota imbalances and modulate intestinal immune responses, are promising interventions for the comprehensive management of CRC. However, the American Society of Health-System Pharmacists does not recommend the use of probiotics in surgical patients (Bratzler et al., 2013) owing to limited data and poor implementation. More clinical trials and evidence are needed to support the promotion of the administration of probiotics or synbiotics in perioperative CRC patients (Yao et al., 2023).
Limitations
(1) The included meta-analyses differed in their inclusion/exclusion criteria, which may have affected the synthesis of the results. (2) Generally low quality of the included reviews and potential overlap bias. (3) Due to limitations in the number of included studies, this prevented us from determining the specific use of probiotics/synbiotics.
Conclusion
According to the results of our analyses, perioperative probiotic/synbiotic supplementation in CRC patients is associated with a reduced incidence of overall infections, surgical site infections, urinary tract infections, pneumonia infections, and diarrhea.
Statements
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SG: Writing – original draft, Methodology, Writing – review & editing. XL: Writing – review & editing, Formal analysis. YH: Software, Writing – review & editing, Methodology. JY: Writing – review & editing, Methodology, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1635409/full#supplementary-material
References
1
Alam Z. Shang X. Effat K. Kanwal F. He X. Li Y. et al (2022). The potential role of prebiotics, probiotics, and synbiotics in adjuvant cancer therapy especially colorectal cancer.J. Food Biochem.46:e14302. 10.1111/jfbc.14302
2
Amitay E. Carr P. Gies A. Laetsch D. Brenner H. (2020). Probiotic/synbiotic treatment and postoperative complications in colorectal cancer patients: Systematic review and meta-analysis of randomized controlled trials.Clin. Transl. Gastroenterol.11:e00268. 10.14309/ctg.0000000000000268
3
An S. Kim K. Kim M. Jung J. Kim Y. (2022). Perioperative probiotics application for preventing postoperative complications in patients with colorectal cancer: A systematic review and meta-analysis.Medicina58:1644. 10.3390/medicina58111644
4
Araújo M. Montalvão-Sousa T. Teixeira P. Figueiredo A. Botelho P. (2023). The effect of probiotics on postsurgical complications in patients with colorectal cancer: A systematic review and meta-analysis.Nutr. Rev.81493–510. 10.1093/nutrit/nuac069
5
Bratzler D. Dellinger E. Olsen K. Perl T. Auwaerter P. Bolon M. et al (2013). Clinical practice guidelines for antimicrobial prophylaxis in surgery.Am. J. Health Syst. Pharm.70195–283. 10.2146/ajhp120568
6
Chen G. Wang J. Chen K. Kang M. Zhang H. Jin X. et al (2021). Relationship between postoperative complications and the prognosis of gastric carcinoma patients who underwent surgical resection: A systematic review and meta-analysis.Cancer Control28:10732748211011955. 10.1177/10732748211011955
7
Chen J. Zhao J. Wu H. Wang T. Gao C. (2024). Efficacy and safety of oral probiotic supplementation in mitigating postoperative surgical site infections in patients undergoing colorectal cancer surgery: A systematic review and meta-analysis.Int. Wound J.21:e14603. 10.1111/iwj.14603
8
Chen Y. Qi A. Teng D. Li S. Yan Y. Hu S. et al (2022). Probiotics and synbiotics for preventing postoperative infectious complications in colorectal cancer patients: A systematic review and meta-analysis.Tech. Coloproctol.26425–436. 10.1007/s10151-022-02585-1
9
Colbert L. El Alam M. Wang R. Karpinets T. Lo D. Lynn E. et al (2023). Tumor-resident Lactobacillus iners confer chemoradiation resistance through lactate-induced metabolic rewiring.Cancer Cell.411945–1962.e11. 10.1016/j.ccell.2023.09.012
10
Dai J. Tan X. Qiao H. Liu N. (2024). Emerging clinical relevance of microbiome in cancer: Promising biomarkers and therapeutic targets.Protein Cell.15239–260. 10.1093/procel/pwad052
11
de Andrade Calaça P. Bezerra R. Albuquerque W. Porto A. Cavalcanti M. (2017). Probiotics as a preventive strategy for surgical infection in colorectal cancer patients: A systematic review and meta-analysis of randomized trials.Transl. Gastroenterol. Hepatol.2:67. 10.21037/tgh.2017.08.01
12
Eslami M. Yousefi B. Kokhaei P. Hemati M. Nejad Z. Arabkari V. et al (2019). Importance of probiotics in the prevention and treatment of colorectal cancer.J. Cell. Physiol.23417127–17143. 10.1002/jcp.28473
13
Garbacz K. (2022). Anticancer activity of lactic acid bacteria.Semin. Cancer Biol.86356–366. 10.1016/j.semcancer.2021.12.013
14
Hennessy E. Johnson B. (2020). Examining overlap of included studies in meta-reviews: Guidance for using the corrected covered area index.Res. Synth. Methods11134–145. 10.1002/jrsm.1390
15
Jiang J. Ren F. (2024). Effect of probiotics and synbiotics on complications of wound infection after colorectal surgery: A meta-analysis.Int. Wound J.21:e14838. 10.1111/iwj.14838
16
Jin Y. Liu Y. Zhao L. Zhao F. Feng J. Li S. et al (2019). Gut microbiota in patients after surgical treatment for colorectal cancer.Environ. Microbiol.21772–783. 10.1111/1462-2920.14498
17
Kasatpibal N. Whitney J. Saokaew S. Kengkla K. Heitkemper M. Apisarnthanarak A. (2017). Effectiveness of probiotic, prebiotic, and synbiotic therapies in reducing postoperative complications: A systematic review and network meta-analysis.Clin. Infect. Dis.64S153–S160. 10.1093/cid/cix114
18
Kothari D. Patel S. Kim S. (2019). Probiotic supplements might not be universally-effective and safe: A review.Biomed. Pharmacother.111537–547. 10.1016/j.biopha.2018.12.104
19
Li N. Niu L. Liu Y. Wang Y. Su X. Xu C. et al (2024). Taking SCFAs produced by Lactobacillus reuteri orally reshapes gut microbiota and elicits antitumor responses.J. Nanobiotechnol.22:241. 10.1186/s12951-024-02506-4
20
Liu D. Jiang X. Zhou L. Song J. Zhang X. (2016). Effects of probiotics on intestinal mucosa barrier in patients with colorectal cancer after operation: Meta-analysis of randomized controlled trials.Medicine.95:e3342. 10.1097/MD.0000000000003342
21
Liu P. Yan Y. Ma Y. Wang X. Geng J. Wang M. et al (2017). Probiotics reduce postoperative infections in patients undergoing colorectal surgery: A systematic review and meta-analysis.Gastroenterol. Res. Pract.2017:6029075. 10.1155/2017/6029075
22
Macharia J. Kaposztas Z. Varjas T. Budán F. Zand A. Bodnar I. et al (2023). Targeted lactate dehydrogenase genes silencing in probiotic lactic acid bacteria: A possible paradigm shift in colorectal cancer treatment?Biomed. Pharmacother.160:114371. 10.1016/j.biopha.2023.114371
23
Masheghati F. Asgharzadeh M. Jafari A. Masoudi N. Maleki-Kakelar H. (2024). The role of gut microbiota and probiotics in preventing, treating, and boosting the immune system in colorectal cancer.Life Sci.344:122529. 10.1016/j.lfs.2024.122529
24
Neugent M. Hulyalkar N. Nguyen V. Zimmern P. De Nisco N. (2020). Advances in understanding the human urinary microbiome and its potential role in urinary tract infection.mBio11:e00218-20. 10.1128/mBio.00218-20
25
Ouyang X. Li Q. Shi M. Niu D. Song W. Nian Q. et al (2019). Probiotics for preventing postoperative infection in colorectal cancer patients: A systematic review and meta-analysis.Int. J. Colorectal. Dis.34459–469. 10.1007/s00384-018-3214-4
26
Page M. McKenzie J. Bossuyt P. Boutron I. Hoffmann T. Mulrow C. et al (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews.Syst. Rev.10:89. 10.1186/s13643-021-01626-4
27
Paterson C. Nikolic A. Glyn T. Eglinton T. Singh P. Hill A. (2025). Do perioperative probiotics/synbiotics reduce postoperative infection rates following elective colorectal surgery? A systematic review and meta-analysis.J. Surg. Res.312163–176. 10.1016/j.jss.2025.05.026
28
Persson J. Viana P. Persson M. Relvas J. Danielski L. (2024). Perioperative or postoperative probiotics reduce treatment-related complications in adult colorectal cancer patients undergoing surgery: A systematic review and meta-analysis.J. Gastrointest. Cancer55740–748. 10.1007/s12029-024-01016-8
29
Richards P. Almutrafy A. Liang L. Flaujac Lafontaine G. King E. Fish N. et al (2024). Prebiotic galactooligosaccharide feed modifies the chicken gut microbiota to efficiently clear Salmonella.mSystems9:e0075424. 10.1128/msystems.00754-24
30
Salek S. Moazamian E. Mohammadi Bardbori A. Shamsdin S. (2024). The anticancer effect of potential probiotic L. fermentum and L. plantarum in combination with 5-fluorouracil on colorectal cancer cells.World J. Microbiol. Biotechnol.40:139. 10.1007/s11274-024-03929-9
31
Shea B. J. Reeves B. C. Wells G. Thuku M. Hamel C. Moran J. et al (2017). AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both.Bmj358:j4008. 10.1136/bmj.j4008
32
Siegel R. Wagle N. Cercek A. Smith R. Jemal A. (2023). Colorectal cancer statistics, 2023.CA Cancer J. Clin.73233–254. 10.3322/caac.21772
33
Sivan A. Corrales L. Hubert N. Williams J. Aquino-Michaels K. Earley Z. et al (2015). Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy.Science3501084–1089. 10.1126/science.aac4255
34
Tang P. Wen T. Lu W. Jin H. Pan L. Li H. et al (2024). The efficacy of extracorporeal shock wave therapy for knee osteoarthritis: An umbrella review.Int. J. Surg.1102389–2395. 10.1097/JS9.0000000000001116
35
Veziant J. Bonnet M. Occean B. Dziri C. Pereira B. Slim K. (2022). Probiotics/synbiotics to reduce infectious complications after colorectal surgery: A systematic review and meta-analysis of randomised controlled trials.Nutrients14:3066. 10.3390/nu14153066
36
Weaver L. Troester A. Jahansouz C. (2024). The impact of surgical bowel preparation on the microbiome in colon and rectal surgery.Antibiotics13:580. 10.3390/antibiotics13070580
37
Wu J. Zhang Y. Ye L. Wang C. (2021). The anti-cancer effects and mechanisms of lactic acid bacteria exopolysaccharides in vitro: A review.Carbohydr. Polym.253:117308. 10.1016/j.carbpol.2020.117308
38
Wu X. Liu M. Liang X. Hu N. Huang W. (2018). Effects of perioperative supplementation with pro-/synbiotics on clinical outcomes in surgical patients: A meta-analysis with trial sequential analysis of randomized controlled trials.Clin. Nutr.37505–515. 10.1016/j.clnu.2016.10.015
39
Xu H. Luo H. Zhang J. Li K. Lee M. (2023). Therapeutic potential of Clostridium butyricum anticancer effects in colorectal cancer.Gut Microbes15:2186114. 10.1080/19490976.2023.2186114
40
Xu Q. Xu P. Cen Y. Li W. (2019). Effects of preoperative oral administration of glucose solution combined with postoperative probiotics on inflammation and intestinal barrier function in patients after colorectal cancer surgery.Oncol. Lett.18694–698. 10.3892/ol.2019.10336
41
Yao D. He W. Hu Y. Yuan Y. Xu H. Wang J. et al (2023). Prevalence and influencing factors of probiotic usage among colorectal cancer patients in China: A national database study.PLoS One18:e0291864. 10.1371/journal.pone.0291864
42
Yu J. Feng Q. Wong S. Zhang D. Liang Q. Qin Y. et al (2017). Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer.Gut6670–78. 10.1136/gutjnl-2015-309800
43
Zeng J. Ji Y. Liang B. Zhang G. Chen D. Zhu M. et al (2021). The effect of pro/synbiotics on postoperative infections in colorectal cancer patients: A systematic review and meta-analysis.Complement. Ther. Clin. Pract.43:101370. 10.1016/j.ctcp.2021.101370
44
Zhou Y. Tao L. Qiu J. Xu J. Yang X. Zhang Y. et al (2024). Tumor biomarkers for diagnosis, prognosis and targeted therapy.Signal. Transduct. Target. Ther.9:132. 10.1038/s41392-024-01823-2
Summary
Keywords
probiotic, synbiotic, prebiotic, colorectal cancer, gut microbiota, umbrella review
Citation
Gao S, Liao X, He Y and Yang J (2025) Probiotics/synbiotics supplementation reduce the infection incidence in patients undergoing resection for colorectal cancer: an umbrella review. Front. Microbiol. 16:1635409. doi: 10.3389/fmicb.2025.1635409
Received
26 May 2025
Accepted
29 August 2025
Published
11 September 2025
Volume
16 - 2025
Edited by
Bharathi Muruganantham, Karpagam Academy of Higher Education, India
Reviewed by
Pugazhendhi Srinivasan, University of Kansas Medical Center, United States
Melhem Bilen, Stanford University, United States
Honghua Hu, Macquarie University, Australia
Updates
Copyright
© 2025 Gao, Liao, He and Yang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Yang, myjamie@126.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.