- 1School of Pharmacy, Binzhou Medical University, Yantai, Shandong, China
- 2School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai, China
Introduction: β-nicotinamide mononucleotide (NMN), a precursor of NAD+, holds promise as a functional food ingredient for mitigating age-related decline. This study enhanced NMN biosynthesis in probiotic Lactiplantibacillus plantarum.
Methods: A putative niacin transporter, lp2514, was identified via molecular docking and validated by CRISPR/Cas9. A dual-copy expression strategy was also employed to increase NMN production. In parallel, RNA-seq was used to analyze genome-wide transcriptional changes associated with enhanced NMN biosynthesis.
Results: Overexpression of lp2514 increased NMN production by 62.3%, and a dual-copy strategy raised NMN titers to 203 μmol L−1-269% increase compared to empty-vector control without NAM and the highest yield reported in lactic acid bacteria. Transcriptomic analysis revealed 598 differentially expressed genes, including upregulated ribosomal proteins (rpsJ, rplE) and NAD+ salvage enzymes (aspA), indicating enhanced translation and precursor flux. Deleting cinA, encoding a metabolic constraint, further boosted NMN levels, confirming transcriptomic predictions.
Discussion: This combined transporter engineering and transcriptome-guided strategy establishes a food-grade L. plantarum platform for efficient NMN production in functional fermented foods.
1 Introduction
β-nicotinamide mononucleotide (NMN), one of an important precursor of nicotinamide adenine dinucleotide (NAD+), is naturally present as a bioactive nucleotide (Cheng et al., 2024; He et al., 2022). Given the decline in NAD+ levels with increasing age, NAD+ supplementation could offer potential benefits in preventing age-related diseases, including metabolic disorders and cardiovascular dysfunctions (Nadeeshani et al., 2022; Poddar et al., 2019, Kuerec et al., 2024; Yang et al., 2021; Yoshino et al., 2018). Owing to its beneficial effects, NMN has gained attention as a functional food ingredient for supporting healthy aging and disease prevention (Luo et al., 2023). While chemical synthesis and enzymatic methods for NMN production exist, microbial fermentation offers a sustainable and cost-effective alternative (Bi et al., 2023; Wang et al., 2023).
Lactiplantibacillus plantarum, a generally recognized as safe (GRAS) lactic acid bacterium (LAB), has emerged as an attractive microbial chassis for functional compound biosynthesis, due to its probiotic attributes, food compatibility, and robust metabolic versatility (Liu et al., 2017; Oleksy and Klewicka, 2016; Kong et al., 2024). Recent studies in Escherichia coli have demonstrated the feasibility of NMN production from inexpensive substrates such as nicotinamide (NAM), primarily through enhancement of NAM uptake and its conversion via the NAD+ salvage pathway (Huang et al., 2023; Liu et al., 2021; Maharjan et al., 2021; Shoji et al., 2021) (Figure 1). Marinescu et al. (2018) proposed a strategy for cost-effective NMN production in the presence of NAM, resulting in the production of NMN at 15.42 mg L−1. The yield of NMN was 16.2 g L−1 with a molar conversion rate of 97.0% from NAM in E. coli (Huang et al., 2022). These studies suggest that the biosynthesis of NMN by inexpensive NAM uptake has the advantage of reducing production costs. NAM often remains at low concentrations within the host, and niacin transporters (NiaPs) restrict the entry of NAM into the host cell (Bao et al., 2025). However, E. coli is not suitable for direct application in food systems, and efficient NAM transporters for NMN biosynthesis have not been characterized in LAB.
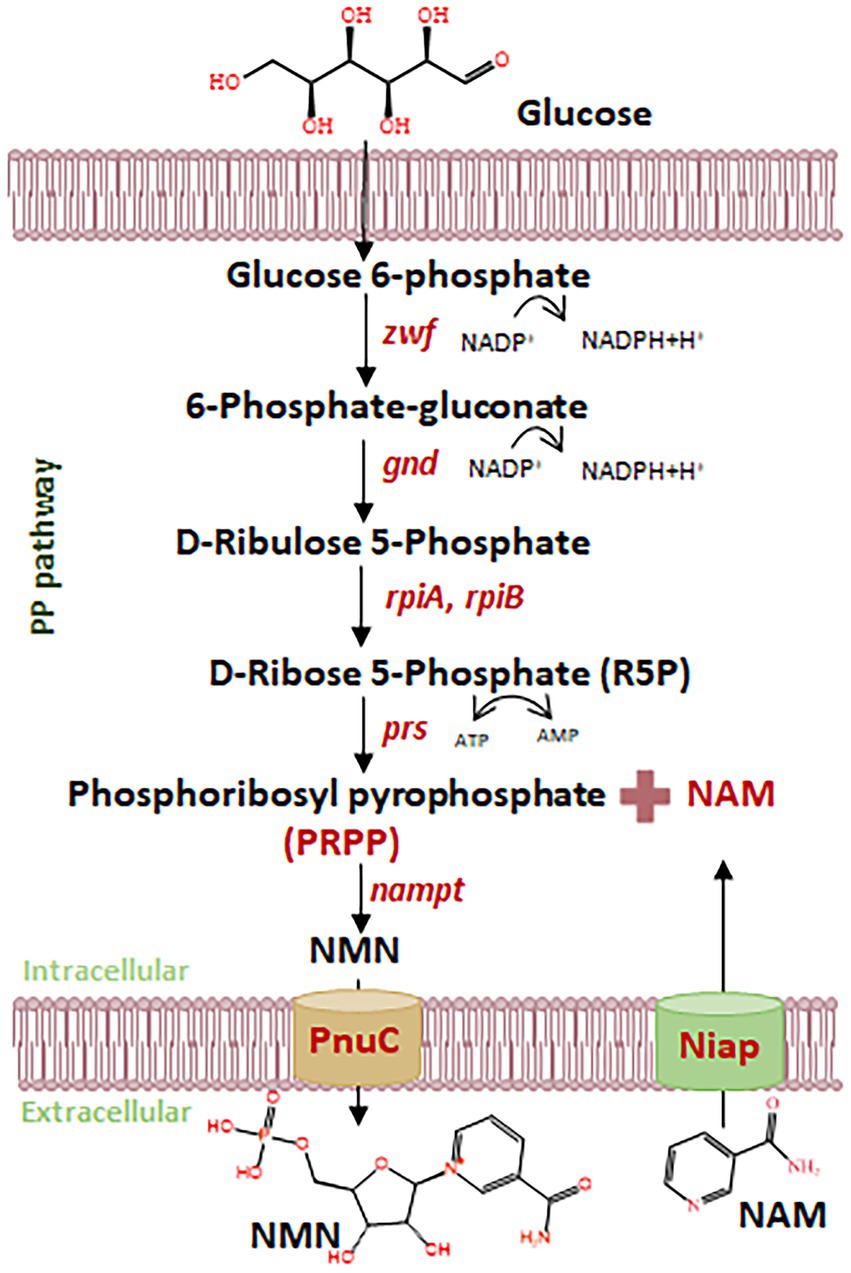
Figure 1. Schematic diagram of NMN biosynthesis using NAM and glucose. Nampt, NAM phosphoribosyltransferase; prs, ribose-phosphate diphosphokinase; zwf, glucose 6-phosphate dehydrogenase; gnd, 6-phosphogluconate dehydrogenase; rpiA, ribose 5-phosphate isomerase A; rpiB, ribose 5-phosphate isomerase B; NiaP, niacin transporter.
In this study, a native NiaP gene, lp2514, was identified and engineered in L. plantarum WCFS1-a well-characterized strain, with CRISPR-based tools to investigate its capacity for food-safe NMN production (Huang et al., 2022; Kleerebezem et al., 2003). We hypothesize that lp2514 overexpression, combined with multicopy chromosomal integration, enhances NAM uptake and metabolic flux toward NMN, while transcriptomic profiling elucidates the systemic adaptations underlying yield optimization. This integrated approach establishes a food-grade microbial platform for functional NMN-enriched fermentation and provides new insights into metabolic control points for probiotic strain optimization.
2 Materials and methods
2.1 Bacterial strains, plasmids, and culture conditions
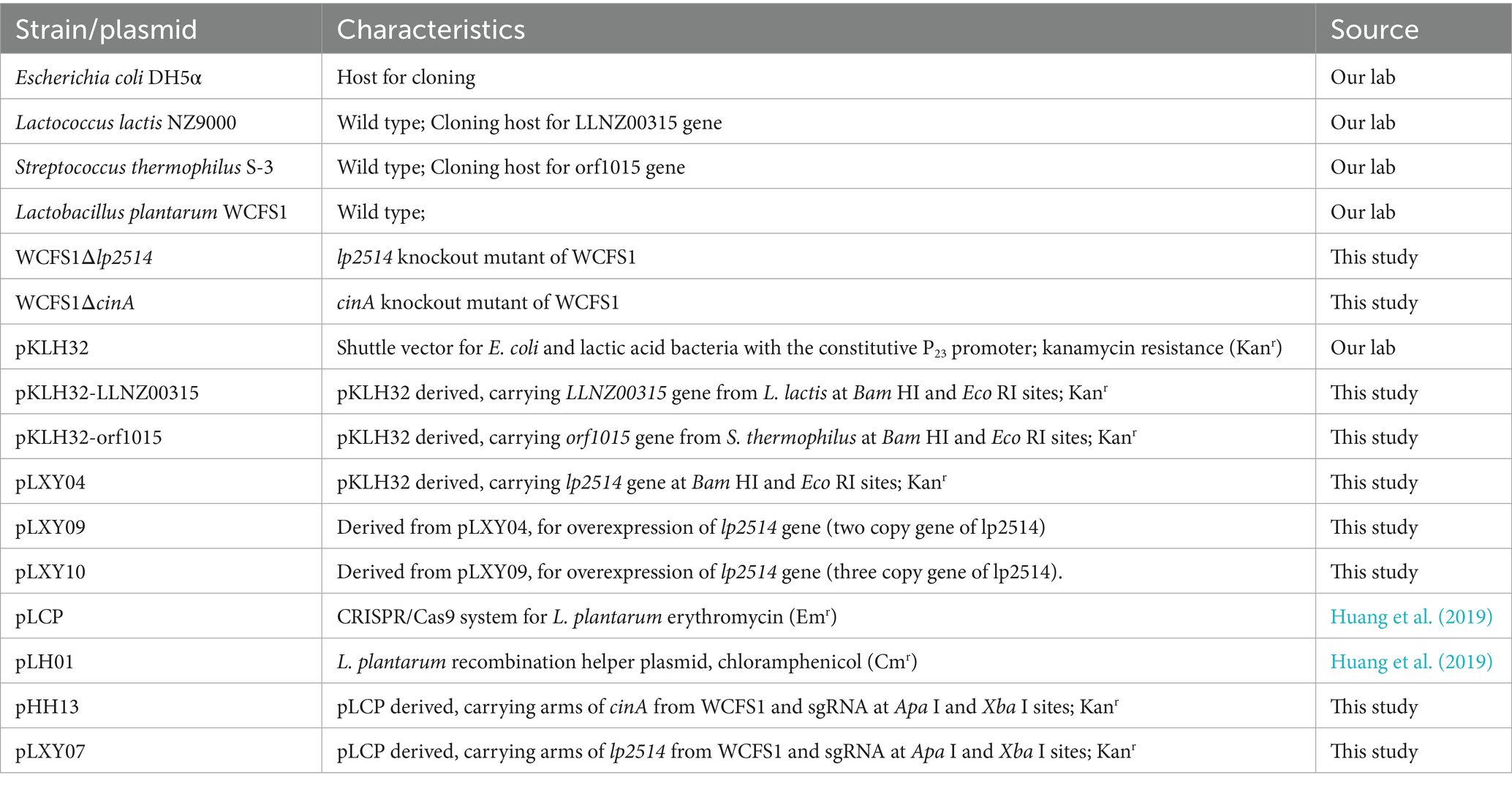
All strains and plasmids used in this study are listed in Table 1. E. coli DH5α strains were employed as the cloning hosts for plasmid construction. E. coli DH5α containing genome editing plasmid was cultivated in Luria-Bertani medium supplemented with 50 μg mL−1 kanamycin (Kan). L. plantarum WCFS1 was routinely cultured on MRS media without agitation. Then, 10 μg mL−1 erythromycin (Em) and 10 μg mL−1 chloramphenicol were added as needed. An appropriate quantity of NAM was introduced during the culture of L. plantarum WCFS1 to increase the biosynthesis of NMN.
2.2 Plasmid constructions
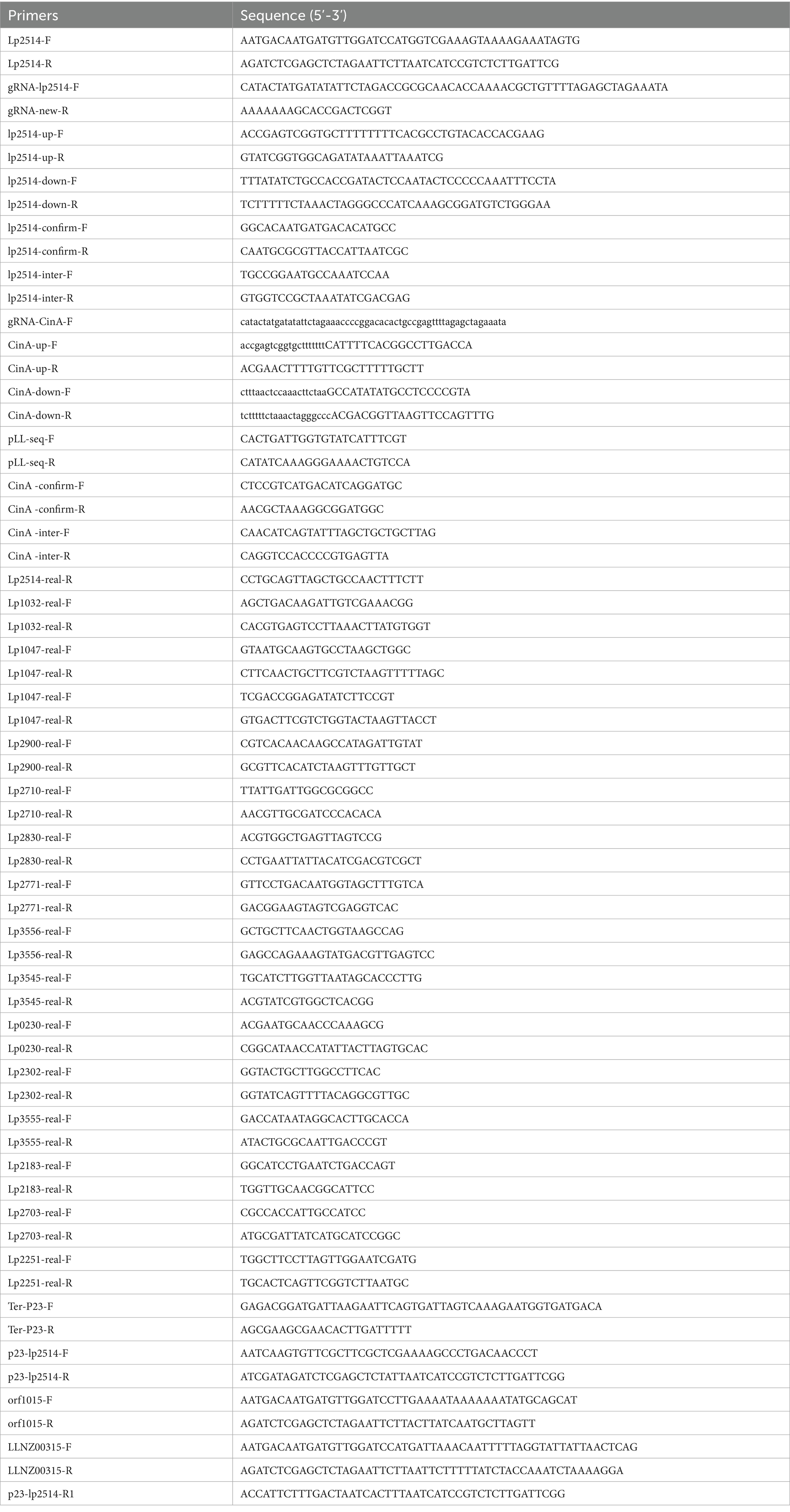
All primers used in this study are listed in Table 2, and the successful plasmids were validated by DNA sequencing. The pair of primers was used to amplify LLNZ00315, orf1015 and lp2514 genes from L. lactis NZ9000, Streptococcus thermophilus S-3 and WCFS1, respectively, and inserted into pKLH32 (digested with Bam HI/Eco RI) to create plasmids for NMN production. To further enhance lp2514 expression, multi-copy expression cassettes were constructed. A second copy of lp2514 was amplified and inserted into a separate expression region of the pKLH32 backbone, generating pLXY09 (dual-copy lp2514). To explore the effect of additional copies, pLXY10 was constructed by inserting three tandem copies of lp2514.
The homologous arms of lp2514 were amplified from WCFS1. The specific guide RNA (gRNA), composed of a 20 bp protospacer sequence, was cloned from pLCP using gRNA-lp2514-F/gRNA-lp2514-R primers (Huang et al., 2019). The plasmid pLCP (CRISPR/Cas9 system) was digested with the restriction enzymes Apa I and Xba I, and then ligated to the fragments using the ClonExpress one-step cloning kit, resulting in the creation of recombinant plasmid pLXY07 for lp2514 gene knockout. The pHH13 plasmid was constructed as described above.
2.3 Genome manipulation procedures using CRISPR/Cas9 in Lactiplantibacillus plantarum
The competent cells of L. plantarum WCFS1 were generated in accordance with previous report (Huang et al., 2019). The helper plasmid pLH01 was transformed into L. plantarum WCFS1, and the resulting positive transformants were screened on Cm plates for the preparation of the competent cells. The plasmid pLXY07 was subsequently transferred into WCFS1 via electroporation, and cultivated for 72 h at 37°C. To confirm positive recombinants, colony PCR analysis was performed using the primers (lp2514-confirm-F/lp2514-confirm-R; lp2514-inter -F/lp2514-inter -R) that targeted the homologous regions of the lp2514 gene. After successful genome editing, the recombinant strain was serially passaged without antibiotics for 2–3 generations to facilitate plasmid curing. Colonies were screened for the loss of antibiotic resistance, and plasmid loss was further confirmed by colony PCR.
2.4 Fermentation of engineered Lactiplantibacillus plantarum for lp2514 expression and NMN production
The plasmids pLXY04, pLXY09, pLXY10 and pKLH32 (as a control) were introduced into L. plantarum WCFS1. The engineered L. plantarum WCFS1 and WCFS1Δlp2514 strains were grown at 37°C. Cell growth was quantified by measuring optical density at 600 nm (OD 600 nm) with a UV–Vis spectrophotometer. The fermentation broths were collected by centrifugation (12,000 g, 10 min, 4°C), and the cells were resuspended in buffer solution for disruption using a low temperature ultrahigh-pressure continuous flow cell disrupter. The resulting cell lysate was then centrifuged (12,000 g, 3 min, 4°C), and the supernatants were decanted for the NMN assay. NMN was derived from L. plantarum and detected with reported fluorometric methods (Kong et al., 2023; Marinescu et al., 2018).
2.5 Real-time quantitative PCR (RT-qPCR) assay
Total RNA was extracted from L. plantarum WCFS1/pKLH32, WCFS1/pLXY04, WCFS1/pLXY09, and WCFS1/pLXY10 using the RNAiso Plus reagent (TaKaRa, Japan) following the manufacturer’s protocol. First-strand cDNA was synthesized from 1 μg of total RNA using a reverse transcription kit. GAPDH was used as the internal control, and relative transcript levels were calculated using the 2^−ΔΔCt method.
2.6 RNA-sequencing and transcriptomic analysis
Total RNA was extracted from L. plantarum strains WCFS1/pLXY04 (lp2514 overexpression with 0.1% NAM) and WCFS1/pKLH32 (empty vector control with 0.1% NAM) using RNAiso Plus reagent (TaKaRa, Japan) following the manufacturer’s instructions. RNA purity and concentration were assessed using a NanoDrop spectrophotometer (Thermo Fisher Scientific, United States), and RNA integrity was verified via agarose gel electrophoresis and an Agilent 2,100 Bioanalyzer (Agilent Technologies, United States). Sequencing was conducted on an Illumina HiSeq 2000 platform (Majorbio Bio-Pharm Technology Co., Ltd., Shanghai, China). Clean reads were mapped to the reference genome of L. plantarum WCFS1, and gene expression levels were quantified as fragments per kilobase of transcript per million mapped reads (FPKM). Differentially expressed genes (DEGs) between the two groups (N04 vs. N32) were identified using DESeq2 software with thresholds of |log₂Fold Change| ≥ 1 and adjusted p-value (FDR) < 0.05. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were performed to investigate functional categories and metabolic pathways significantly affected by lp2514 overexpression.
2.7 Statistical analysis
All the experimental data were independently repeated in triplicate. Statistical analyses of the data were performed with GraphPad Prism and are presented as the means ± standard deviations (*p < 0.05 and **p < 0.01).
3 Results and discussion
3.1 Screening NAM transporter for NMN biosynthesis from LAB
The efficient biosynthesis of NMN by LAB using NAM as the substrate was shown to be an effective synthesis strategy in our previous study (Kong et al., 2023). The translocation of the NAM substrate into the host cell to increase NMN production requires a robust NAM transporter (Figure 1). However, there have been no reports on the endogenous proteins responsible for transporting NAM in LAB. Native proteins are more favorable for expression within the host. NiaP from Burkholderia cenocepacia significantly improved NAM uptake leading to an enhance in the synthesis of NMN from 185 mg L−1 to 231 mg L−1 (Shoji et al., 2021). In silico analysis revealed the presence of putative NAM transporters in LAB, including L. plantarum, L. lactis and S. thermophilus. Using BCNiaP as a query sequence for BLAST analysis with the target genomes, the genes encoding orf1015 from S. thermophilus S-3, LLNZ00315 from L. lactis NZ9000 and lp2514 from L. plantarum WCFS1 were identified. Phylogenetic tree analysis showed that the NiaP from different sources clustered into distinct branches, and that lp2514 from L. plantarum WCFS1 was positioned in close proximity to BCNiaP (Figure 2A). The above genes were subsequently inserted into the pKLH32 plasmid, and the resulting plasmids were transferred to L. plantarum WCFS1 (Figure 2B). Among these strains, strain WCFS1/pLXY04, which expressed lp2514 could biosynthesize 89.6 μmol L−1 of NMN intracellularly, resulting in 62.3% increase in NMN levels, compared with those of strain WCFS1/pKLH32, which contained only the empty plasmid of pKLH32 (Figure 2C). These results clearly demonstrate that the expression of NiaP (lp2514) facilitates the production of NMN in L. plantarum, which is consistent with the phylogenetic tree. The lp2514 gene markedly affected the NMN yield and was applied for subsequent experiments.
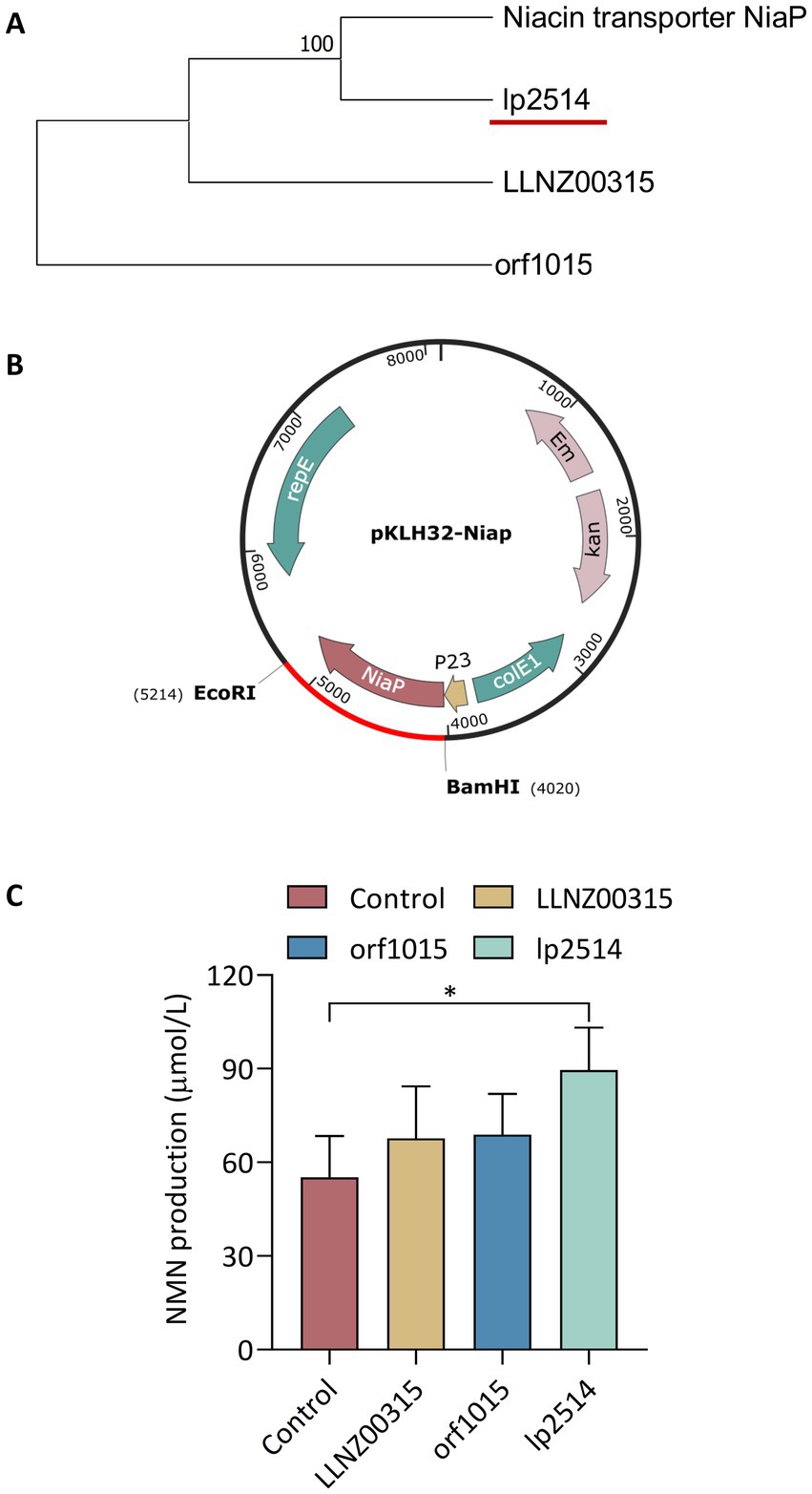
Figure 2. Screening of niacin transporters for NMN production in lactic acid bacteria. Phylogenetic analysis of NAM transporters in LAB (A). Schematic representation of recombinant expression plasmids containing orf1015 from S. thermophilus S-3, LLNZ00315 from L. lactis NZ9000 and lp2514 from WCFS1 (B). The ability of different recombinant strains expressing NiaP to synthesize NMN (C). *p < 0.05, **p < 0.01.
3.2 Identification of the lp2514 gene via in silico molecular docking and CRISPR/Cas9 technology
We performed domain analysis of the NAM transporter using the search tool for conserved domains from the NCBI, and reported that BCNiaP and lp2514 possessed the same domains as MFS_SV2_like, UhpC and MFS_1, suggesting that lp2514 has the potential to conjugate NAM to NMN. The MFS transporter (UniProt A0A2R3JML5; seq similarity 53%; coverage 98%) was selected as template for homology modeling of lp2514 (Figure 3A). The Ramachandran plot revealed that all the residues were predominantly in the most favored region (96.4%) and in an additional allowable area (3.6%), suggesting that the conformation of the generated model adheres to principles of stereochemistry (Supplementary Figure S1). The structural models of lp2514 could be applied to simulate molecular docking with the substrate NAM molecule being docked in the substrate binding pocket of lp2514 using a docking server. The key residues of ASP140, TRP19, TRP318, and ILE322 were located close to the substrate NAM (Figure 3B). These findings suggest that the lp2514 protein has strong binding affinity for the NAM substrate.
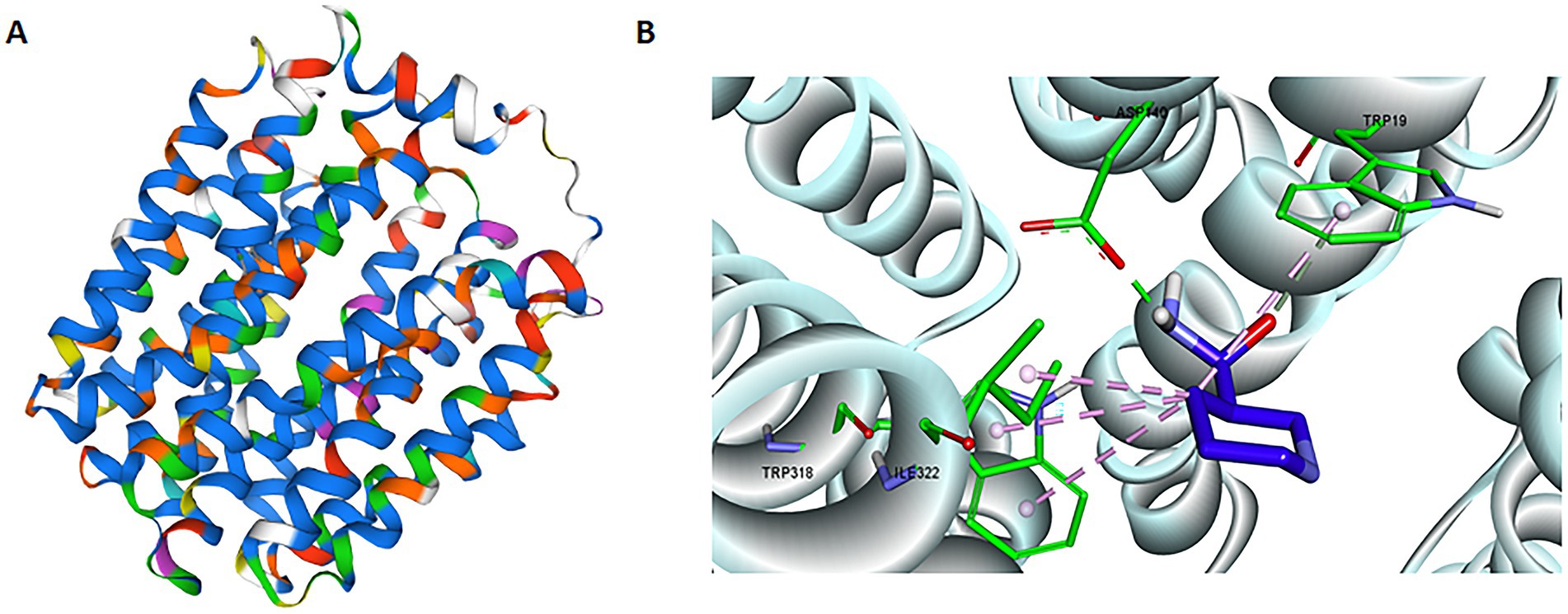
Figure 3. Structural model of lp2514 and the key residues close to NAM. In silico tertiary structure modeling results for the lp2514 protein using the SWISS-MODEL server (A). In silico molecular docking of NAM to lp2514 was simulated by docking server, and the residues (ASP140, TRP19, TRP318, and ILE322) are shown in stick format (B).
Considering the potential role of the lp2514 gene in promoting NMN biosynthesis via NAM uptake, it was worthwhile to verify its functionality by knockout of lp2514 via CRISPR/Cas9 technology in L. plantarum WCFS1 (Figure 4A). A pLXY07 plasmid was constructed to develop the gene deletion mutant of lp2514, which was subsequently delivered into WCFS1 (Supplementary Figure S2). One positive mutant (WCFS1Δlp2514) was successfully generated from eight transformants via colony PCR (Figure 4B). The acquisition of a deletion was verified by sequencing the PCR amplification product of the lp2514 mutant (Figure 4C). When 1 g L−1 NAM was added, the intracellular NMN production of WCFS1Δlp2514 was decreased by 17% compared with that of the wild-type WCFS1 (Figure 4D). Interestingly, when lp2514 was overexpressed in the WCFS1Δlp2514 strain, NMN production was restored. These findings revealed that the lp2514 gene plays a role as NAM transporter affecting the biosynthesis of NMN, which agrees well with our above results. This is the first report on newly developed NAM transporter screening for NMN biosynthesis in LAB.
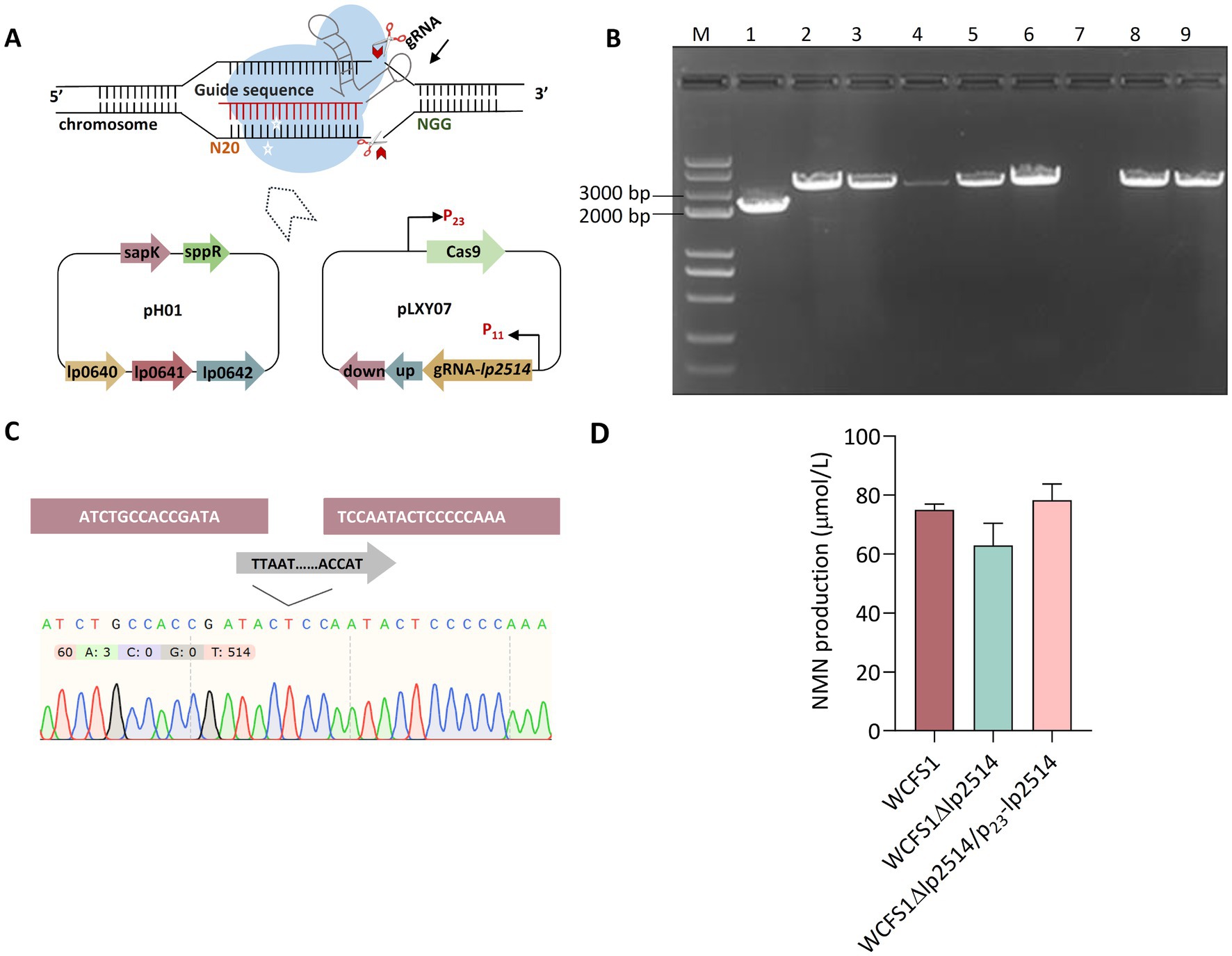
Figure 4. Characterization of the effects of lp2514 on NMN biosynthesis via CRISPR/Cas9 in L. plantarum WCFS1. Schematic representation of the pH01 and pLXY07 plasmids used for lp2514 gene knockout (A). Identification of lp2514 deletion using colony PCR (B). The wild-type WCFS1 was used as control. The 2.1 and 3.3-kb bands indicate the positive and negative mutants, respectively. 1–7: transformants; 8–9: Wild-type. Sequencing validation of the WCFS1Δlp2514 mutant (C). Intracellular NMN production of WCFS1Δlp2514 mutant and WCFS1Δlp2514/p23-lp2514 strains (D).
3.3 Effect of lp2514 on NMN biosynthesis via NAM uptake in Lactiplantibacillus plantarum
To evaluate whether WCFS1/pLXY04 could increase in NMN production, various substrate concentrations of NAM and fermentation durations were tested. Overexpression of lp2514 did not affect the growth of the recombinant strains WCFS1/pKLH32 and WCFS1/pLXY04 (Figure 5A). Kong et al. (2023) reported that various NAM concentrations had an impact on the growth of L. lactis NZ9000, under high concentration conditions (50 g L−1), NAM inhibited strain growth. The utilization of 50 g L−1 NAM exhibited toxicity toward recombinant E. coli (Marinescu et al., 2018). The growth of WCFS1/pKLH32 and WCFS1/pLXY04 was not limited by the addition of 1 g L−1 or 5 g L−1 NAM, although a slight decrease in the growth rate was observed with 10 g L−1 NAM. This finding is consistent with the findings of our previous study (Kong et al., 2023). Moreover, the growth rates are closely associated with the consumption of glucose (Figure 5B). NAM addition is a limiting factor for NMN biosynthesis, and various researchers have added different doses of NAM when is utilized NiaP (Jeanguenin et al., 2012; Shoji et al., 2021; Su et al., 2024). Compared with the control WCFS1/pKLH32, WCFS1/pLXY04 achieved significant NMN production, where varying amounts of NAM (1, 5 and 10 g L−1) were supplied to the MRS medium (Figure 5C). The maximum intracellular NMN titer of WCFS1/pLXY04 (155.5 μmol L−1; 1 g L−1 NAM) was synthesized after 12 h of fermentation, which was 182.7% greater than that of the control (55 μmol L−1) without NAM supplementation. Marinescu et al. (2018) reported that the addition of 1 g L−1 NAM resulted in the greatest NMN accumulation, which is consistent with our findings. These findings revealed that relatively high concentrations of NAM may not be conducive to NMN accumulation.
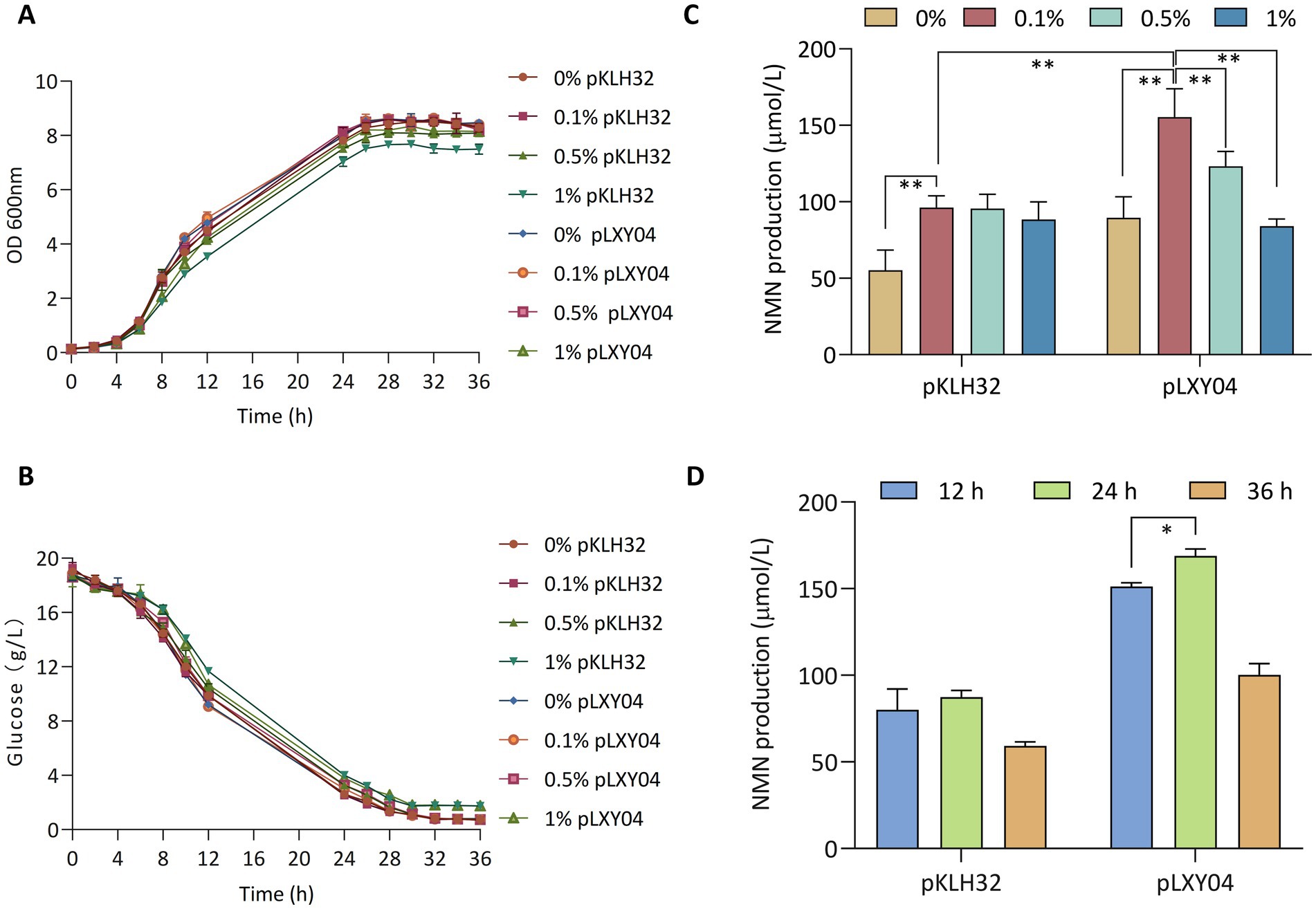
Figure 5. Effects of the NMN transporter lp2514 on NMN biosynthesis. The growth curves (OD 600 nm) (A) and remaining glucose (B) of lp2514 gene expression recombinants in MRS media supplemented with 0, 1, 5 and 10 g L−1 NAM. Effect of NAM concentration (0, 1, 5 and 10 g L−1) on NMN production by recombinant L. plantarum WCFS1/pLXY04 (C). Effects of the different fermentation time (12, 24, and 36 h) on NMN production in MRS media supplemented with 1 g L−1 NAM (D). *p < 0.05, **p < 0.01.
To determine the appropriate fermentation time for enhanced NMN production, the effects of fermentation for various time periods (12, 24 and 36 h) on NMN biosynthesis were evaluated, and the results indicated that fermentation times of 24 h (168.9 μmol L−1) or longer were was suitable for the biosynthesis of NMN (Figure 5D). Sugiyama et al. (2021) reported that Fructobacillus tropaeoli RD012354 presented the highest NMN production after 24 h cultivation. These findings revealed that the recombinant strain WCFS1/pLXY04, when cultured under optimized conditions (1 g L−1 NAM and 24 h cultivation), resulted in a 207% increase in the NMN yield, reaching 168.9 μmol L−1.
3.4 Multi-copy expression of lp2514 unlock high-efficiency NMN biosynthesis in Lactiplantibacillus plantarum
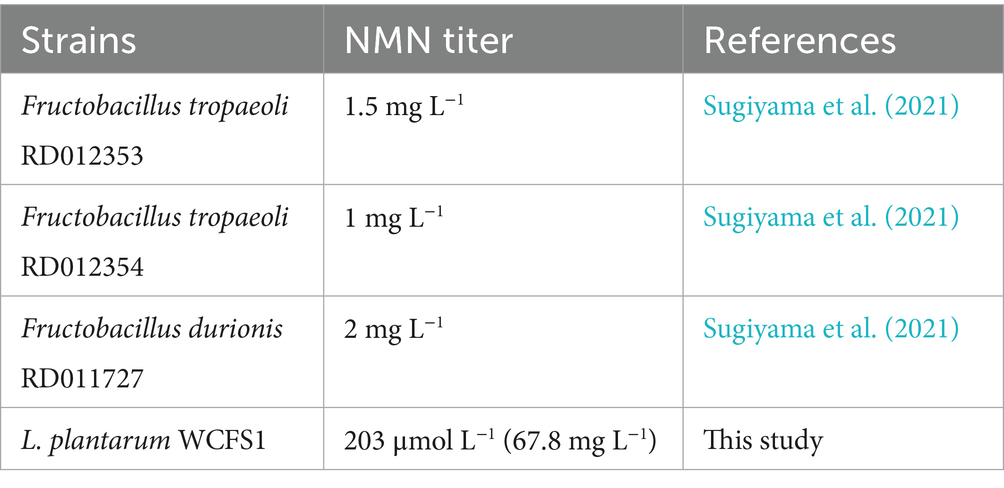
The multicopy expression cassette strategy represents a robust approach to increase natural product yields by increasing recombinant protein abundance, albeit with potential metabolic trade-offs. Cheng et al. (2023) employed a multicopy integration strategy to increase the production of germacrene A, which resulted in a 7.7-fold increase over the control. An optimal number of gene copies should be carefully studied because of the metabolic pressure associated with protein overexpression. To enhance the expression of lp2514, multicopy expression plasmids were constructed based on the pKLH32 vector. The lp2514 gene was amplified using overlap extension PCR and inserted sequentially into the Bam HI/Eco RI sites of pKLH32 to generate plasmids harboring one (pLXY04), two (pLXY09), and three (pLXY10) tandem copies of lp2514 under the control of the same promoter (Figure 6). Compared with that of WCFS1/pKLH32, the OD600 value of WCFS1/pLXY09 significantly decreased during 36 h cultivation, accompanied by a simultaneous reduction in the rate of glucose consumption (Figures 6A,B). The expression of multiple lp2514 imposed a certain burden on the growth of the strain. Notably, an obviously greater quantity of NMN (203 μmol L−1) was substantiated by WCFS1/pLXY09 expressing two copies of lp2514, which increased by 37 and 85% compared with those of WCFS1/pKLXY04 and WCFS1/pKLH32, respectively (Figure 6C). Kong et al. (2020) reported that dual-copy families of the SOD, not four-copy families, presented the greatest activity, with a similar conclusion. The NMN production of WCFS1/pLXY10 also increased by 42% compared with that of the control strain (WCFS1/pKLH32), but slightly decreased compared with that of WCFS1/pLXY09. It is possible that lp2514 overexpression in multiple copies may cause metabolic stress, which subsequently affects NMN biosynthesis. Compared with that of the control WCFS1/pKLH32 without NAM, the final NMN titer was increased by 269% via a series of regulatory mechanisms. The accumulation of NMN in WCFS1/pLXY09 was approximately 33.9-fold greater than that in Fructobacillus durionis RD011727 (Table 3). Moreover, the transcriptional analysis revealed a significant increase in the expression of lp2514 in multicopy recombinants, exceeding that of the control strain by more than 1,000-fold (Figure 6D). The gene copy number is strongly correlated with the transcriptional level of lp2514, rather than with NMN production. This achievement represents the highest reported yield of NMN using NAM as substrate in L. plantarum, marking a significant advancement in the field of NMN biosynthesis by LAB. Although the yield of NMN synthesized has reached g L−1 levels (17.2 g L−1) by E. coli, which are much greater than those of LAB (Maharjan et al., 2023), our L. plantarum platform retains distinct benefits for functional-food applications. Specifically, L. plantarum has GRAS status, an intrinsic probiotic effect (supporting gut health and immune modulation), and the ability to perform food-grade fermentations without extensive downstream purification. These properties make LAB particularly well suited for developing NMN – enriched fermented products that can be marketed directly to health – conscious consumers. This study establishes lp2514 multicopy engineering as a transformative strategy for LAB-based NMN bioproduction, bridging synthetic biology with functional food innovation.
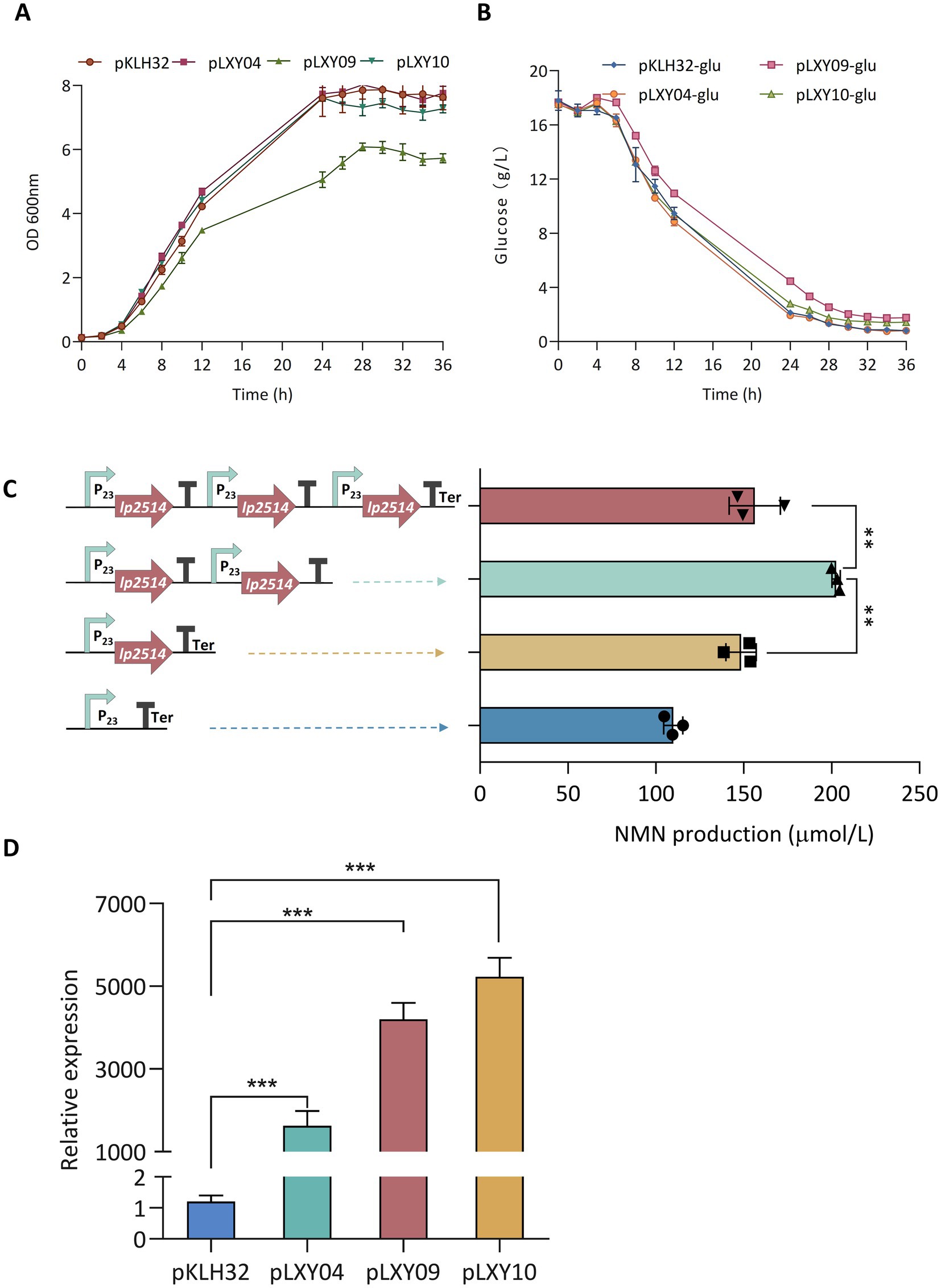
Figure 6. Expression of multicopy lp2514 in L. plantarum WCFS1. The growth curves (OD600 nm) (A) and remaining glucose (B) of multicopy-lp2514 gene expression recombinants supplemented with 1 g L−1 NAM. Construction of plasmids with increasing copy numbers of the lp2514 expression cassette, and analysis of the production of NMN in recombinants (C). Analysis of the expression level by RT-qPCR in L. plantarum WCFS1 recombinants (D). **p < 0.01, ***p < 0.0001.
3.5 Transcriptomic analysis of NMN biosynthesis pathways in lp2514-overexpressing Lactiplantibacillus plantarum
3.5.1 Screening of DEGs
To elucidate the transcriptional response of L. plantarum to lp2514 overexpression in the presence of NAM, we conducted RNA-seq analysis comparing strain N04 (overexpressing lp2514) with N32 (empty vector control) (Figure 7). The Venn diagram highlights 29 unique DEGs in N04, suggesting specific regulatory targets of lp2514 (Figure 7A). The DEGs are likely directly related to lp2514 overexpression, further supporting its pivotal role in NMN biosynthesis. Volcano plot analysis revealed that 332 genes were significantly upregulated and that 266 genes were downregulated (|log2FC| > 1, FDR < 0.05) in N04 (Figure 7B). Each point represents a gene, with red indicating upregulation, blue indicating downregulation, and gray denoting no significant expression change. The upregulated genes are likely associated with NMN biosynthesis pathways, whereas the downregulated genes may pertain to competing metabolic pathways.
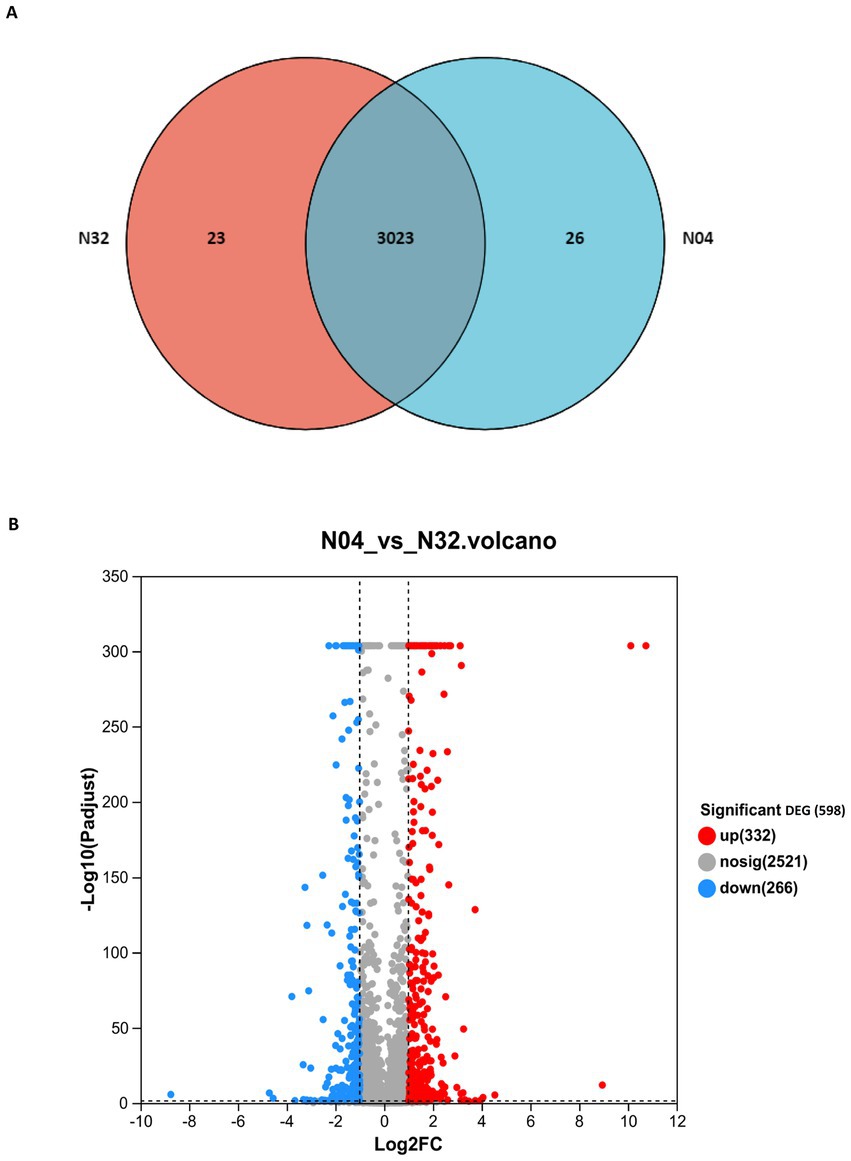
Figure 7. Transcriptomic profiling of lp2514-overexpressing L. plantarum (N04) versus control (N32). Venn diagram illustrating unique and shared genes between N04 and N32 (A). Volcano plot of differentially expressed genes (DEGs). Significantly upregulated (332 genes, red; |log2FC| >1, FDR <0.05) and downregulated (266 genes, blue) genes are highlighted (B).
3.5.2 GO analysis reveals functional rewiring toward NMN biosynthesis
GO annotation revealed that the overexpression of lp2514 in the N04 strain significantly alters the representation of gene categories compared to the N32 strain (Figure 8A). Notably, there is an increase in the number of DEGs associated with “transporter activity” and “catalytic activity” in N04, suggesting an enhanced capacity for the absorption of NAM and its conversion into NMN. Furthermore, elevated counts in categories “translation” and “structural constituent of ribosome” suggest a system-wide enhancement of the protein synthesis machinery, which is consistent with an increased demand for NMN-biosynthetic enzymes. GO enrichment analysis (represented as a bubble plot) identifies specific processes that were most strongly upregulated in N04 (Figure 8B). “Transporter activity” had the lowest adjusted p-value and a moderately rich factor, underscoring lp2514’s direct role in NAM import. There was a marked overrepresentation of biological processes related to peptide biosynthesis (rich factor = 0.65) and ribosome assembly (rich factor = 0.7) in N04 compared with N32. This is consistent with the 332 upregulated genes identified in the volcano plot, many of which encode ribosomal proteins, such as rpsJ and rplE. The enrichment of ribosome-associated terms indicates a potential connection to the efficient expression of enzymes required for NMN biosynthesis, while the increased activity of structural molecules may offer metabolic support to the cell.
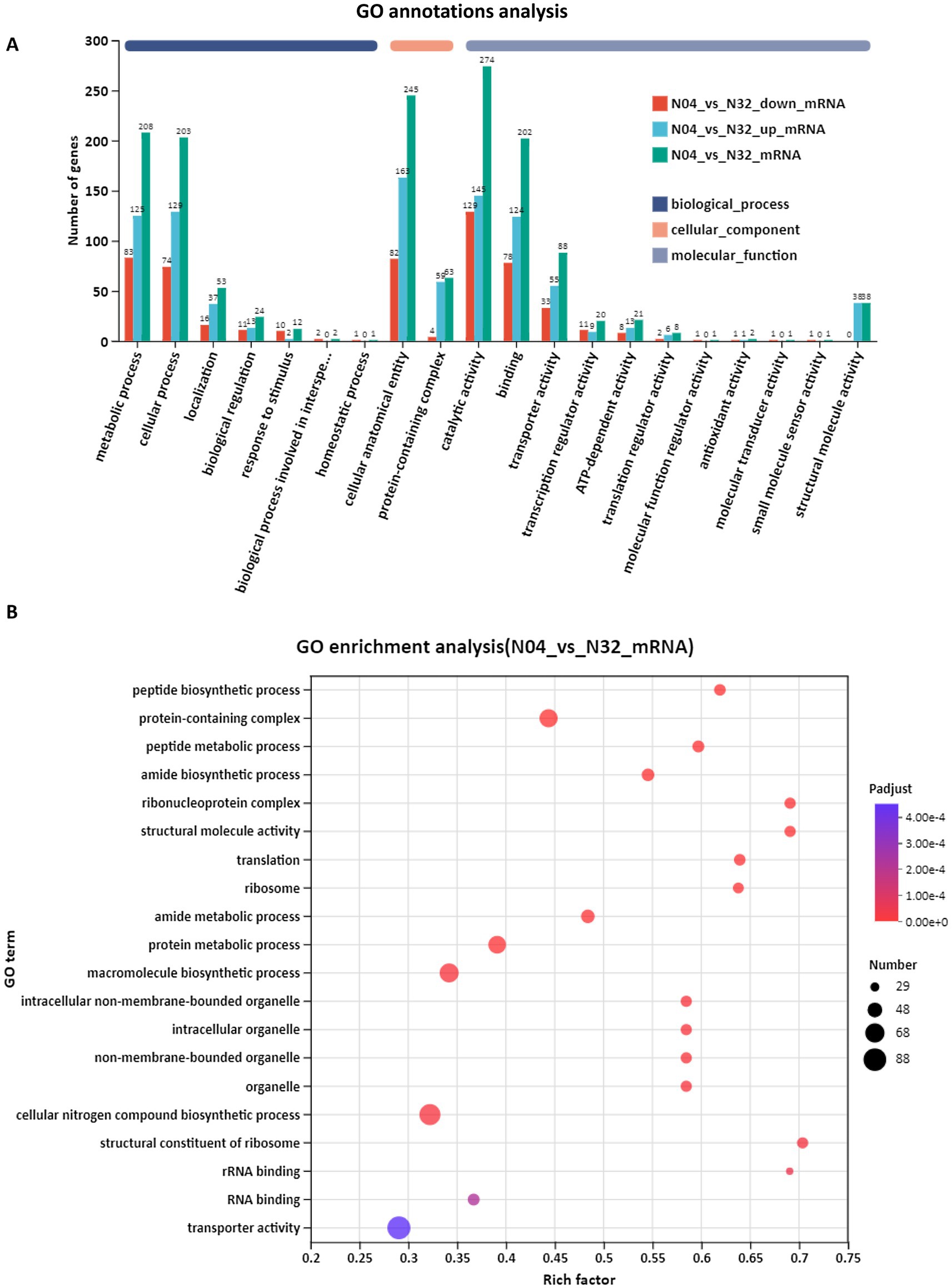
Figure 8. Gene Ontology (GO) annotation and enrichment analysis of N04 vs. N32. GO annotation summary of differentially expressed genes (DEGs) between N04 and N32 (A). GO enrichment bubble plot showing significantly overrepresented GO terms in N04 vs. N32 (B).
3.5.3 KEGG analysis reveals functional rewiring toward NMN biosynthesis
The KEGG annotation bar chart supports this metabolic transition, demonstrating that the majority of DEGs are associated with metabolism-related categories, such as “carbohydrate metabolism” (46 genes) and “amino acid metabolism” (31 genes), which dominate the profile, whereas non-metabolic categories are considerably smaller (Figure 9A). KEGG pathway enrichment analysis of the N04 vs. N32 transcriptomes revealed a coordinated reprogramming of core metabolic and translational pathways, which was consistent with increased NMN production (Figure 9B). The KEGG enrichment analysis further uncovered significant metabolic pathways in N04, including those related to ribosomes, fatty acid biosynthesis, and alanine, aspartate, and glutamate metabolism. The notable enrichment of ribosome pathways is in an agreement with the results of the GO analysis, highlighting the essential role of ribosomes in NMN biosynthesis. The upregulation of fatty acid biosynthesis may reflect increased synthesis of NADPH, a key cofactor for NMN assembly, which is facilitated by the reductive power generated through acetyl-CoA carboxylase activity. Xiong et al. (2025) demonstrated that engineering the NMN biosynthesis-related pathway led to a 73% increase in NADPH levels and redirected carbon flux toward NMN biosynthesis, thereby enhancing precursor availability for nucleotide assembly. Furthermore, the enrichment of alanine, aspartate, and glutamate metabolism suggests a metabolic shift toward the production of aspartate-derived precursors for the NAD+ salvage pathway, directly linking amino acid flux to NMN yield. Collectively, these findings demonstrate that lp2514 overexpression significantly alters the transcriptome of L. plantarum, optimizing metabolic pathways associated with NMN biosynthesis. This research provides a scientific basis for employing L. plantarum as a host for NMN production and lays the groundwork for future industrial applications.
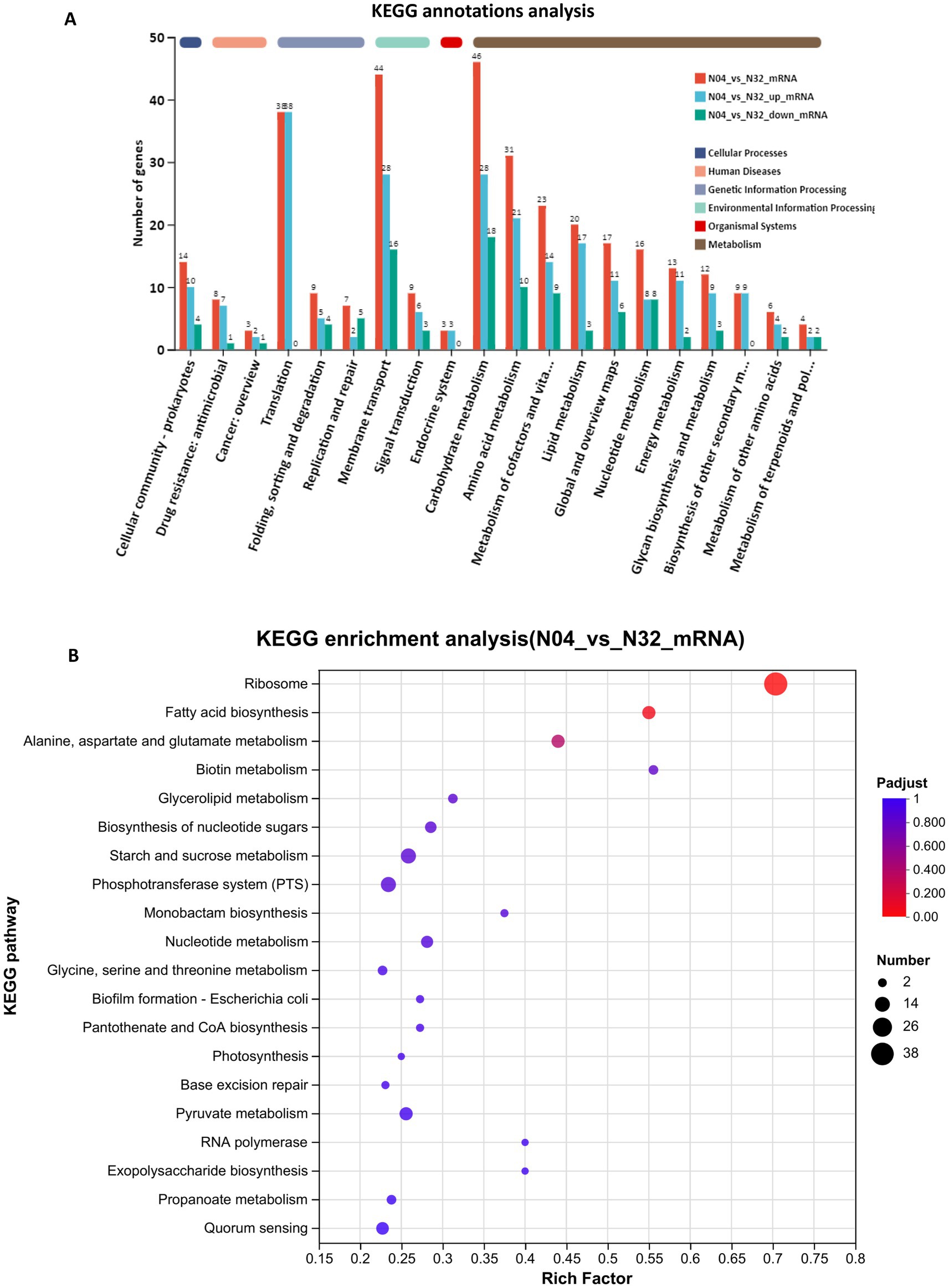
Figure 9. KEGG classification and enrichment of differentially expressed genes in N04 vs. N32. KEGG pathway annotation of DEGs between N04 and N32 (A). KEGG pathway enrichment analysis of DEGs between N04 and N32 (B). Each bubble represents a significantly enriched pathway, with the size corresponding to the number of DEGs and the color indicating the adjusted p-value.
3.6 Validation of DEG expression levels via RT-qPCR
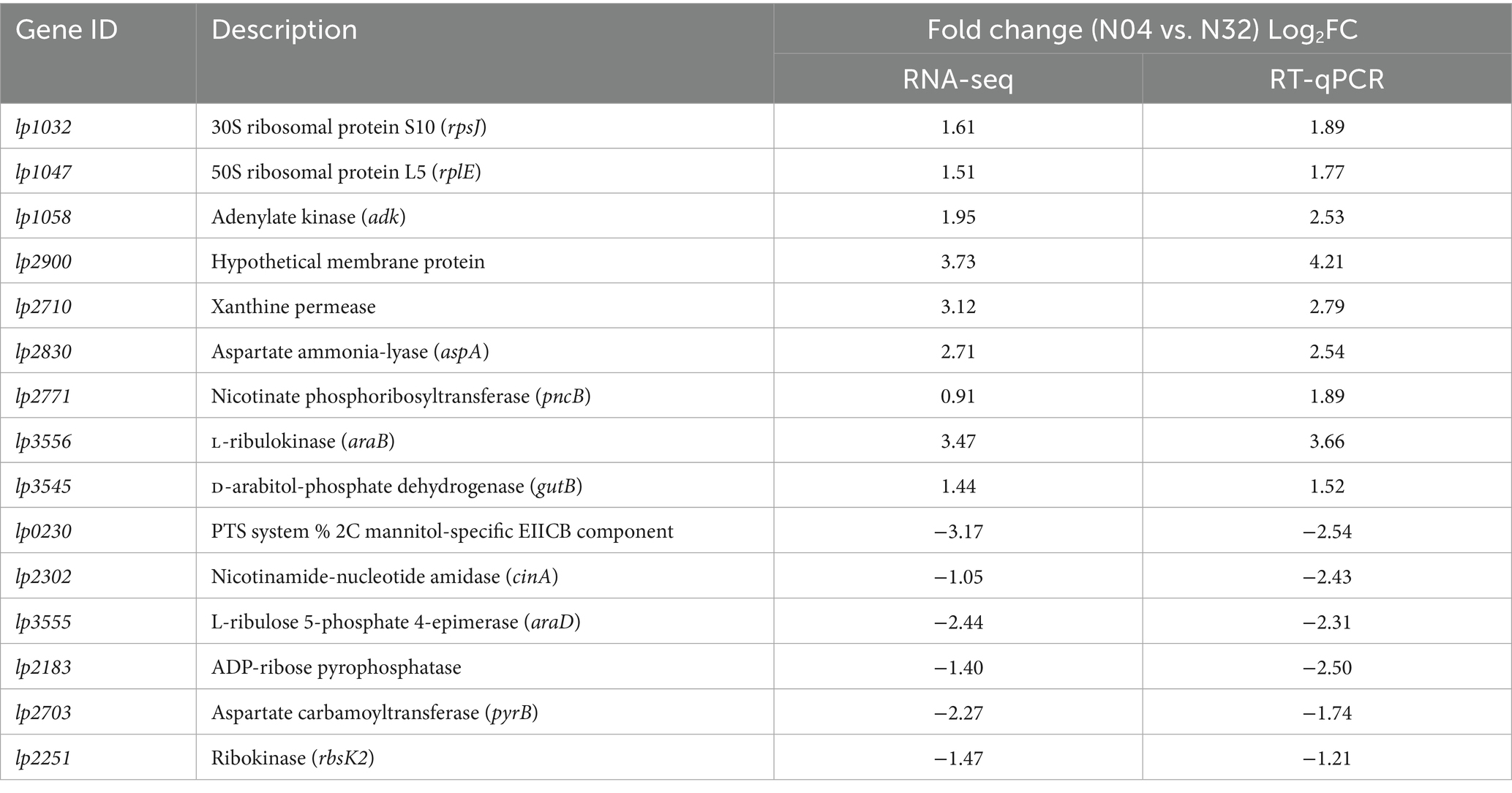
To validate the reliability of the RNA-seq data, a subset of DEGs (upregulated, downregulated) identified between N04 and N32 was selected for RT-qPCR analysis (Table 2). Genes were chosen on the basis of their fold change values, involvement in NAD+ metabolism, and relevance to membrane transport processes as indicated by GO and KEGG enrichment analyses. The RT-qPCR results were strongly concordant with the RNA-Seq data (Spearman’s r = 0.93, p < 0.0001), confirming the upregulation of key transport-related genes and NAD+ biosynthesis genes in the N04 strain (Table 4). Notably, lp3556 (encoding L-ribulokinase) exhibited a substantial upregulation (RNA-seq = 3.47; RT-qPCR = 3.66), suggesting a shift toward ribulose metabolism potentially linked to enhanced precursor availability for NMN biosynthesis. The key upregulated genes included ribosomal subunits (lp1032, RNA-seq = 1.61; RT-qPCR = 1.89) and NAD+ salvage enzymes (lp2830 = 2.71; RT-qPCR = 2.54), which directly support enhanced translational capacity and aspartate-derived precursor supply for NMN biosynthesis. Strikingly, lp2703, which is involved in aspartate carbamoyltransferase activity, was markedly downregulated (RNA-seq = −2.27; RT-qPCR = −1.74), possibly reflecting alterations in pyrimidine biosynthesis under lp2514-mediated conditions. Together, the RT-qPCR results were consistent with the RNA-seq data, confirming the transcriptional regulation of key metabolic nodes.
3.7 Characterization of cinA in NMN biosynthesis via CRISPR/Cas9
To further validate the influence of DEGs on NMN biosynthesis, we targeted cinA, encoding nicotinamide-nucleotide amidase, which was downregulated in the lp2514-overexpressing strain and implicated in NMN turnover (Table 4). A cinA deletion mutant (WCFS1ΔcinA) was constructed (Supplementary Figures S3, S4) and assayed alongside wild-type WCFS1 for NMN production. As shown in Figure 10A, WCFS1ΔcinA accumulated significantly greater amounts of NMN than did the parental strain 29%, which was consistent with the removal of the enzyme responsible for NMN degradation. Zhang et al. (2024) similarly showed that deletion of the cinA in Bacillus subtilis enhanced NMN synthesis, consistent with our findings. RT-qPCR analysis (Figure 10B) revealed the absence of the cinA transcript in WCFS1ΔcinA and the upregulation of key salvage pathway genes (e.g., aspA) and ribosomal components (rpsJ), mirroring the global enhancements in precursor flux and translational capacity identified by RNA-seq. These data substantiate a dual mechanism-enhanced NAM uptake via lp2514 and prevention of NMN hydrolysis through cinA deletion-underscoring the value of combining transporter engineering with targeted gene knockouts to maximize NMN biosynthesis. Together, these findings highlight the robustness of the sequencing data and suggest that lp2514 overexpression, in conjunction with NAM supplementation, modulates central carbon and nucleotide metabolism in L. plantarum. Such regulatory shifts are likely instrumental in enhancing NMN biosynthetic potential, laying a molecular foundation for further metabolic engineering aimed at boosting NAD+ cofactor synthesis in Lactobacillus.
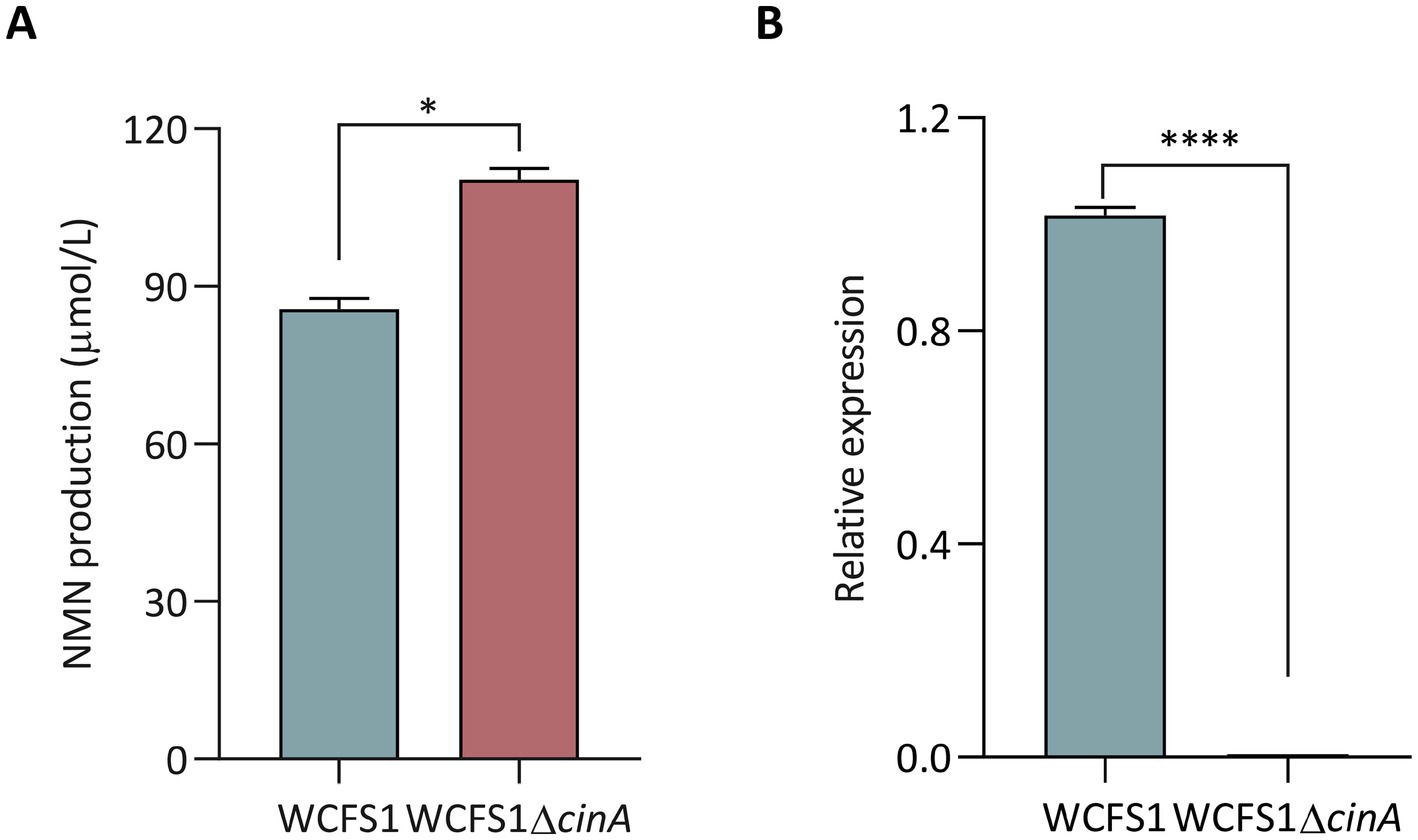
Figure 10. Characterization of the effect of cinA on NMN biosynthesis via CRISPR/Cas9 in L. plantarum WCFS1. Intracellular NMN production of the WCFS1ΔcinA mutant (A). Analysis of the expression level of the WCFS1ΔcinA mutant by RT-qPCR (B). *p < 0.05, ****p < 0.00001.
4 Conclusion
In this study, lp2514 was identified as a novel endogenous NAM transporter in L. plantarum through molecular docking and CRISPR/Cas9 validation. Overexpressing lp2514, particularly using multicopy expression strategy, significantly enhanced NAM uptake and activated NMN biosynthesis. Transcriptomic analysis revealed broad metabolic reprogramming, including upregulation of ribosomal and NAD+ salvage pathway genes, supporting increased biosynthetic capacity. Furthermore, deletion of cinA further improved NMN yields and validated transcriptome-derived insights. These findings elucidate a new mechanism of NMN biosynthesis in LAB and establish L. plantarum as a promising food-grade chassis for sustainable, cost-effective NMN production. This work provides a foundation for engineering probiotics to develop functional foods enriched with bioactive NAD+ precursors.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
LK: Software, Writing – review & editing, Funding acquisition, Writing – original draft, Validation. XL: Formal analysis, Data curation, Writing – original draft. QH: Data curation, Writing – original draft. QY: Investigation, Writing – review & editing. LA: Writing – review & editing. JQ: Writing – original draft, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by National Natural Science Foundation of China (Grant no. 32302050) and Natural Science Foundation of Shandong Province (Grant no. ZR2022QC004).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1637666/full#supplementary-material
References
Bao, T., Weng, P., Wang, J., Cui, J., Tao, Y., Huang, J., et al. (2025). Systematic engineering for high-level production of β-nicotinamide mononucleotide from NAM and ribose. Food Biosci. 63:105725. doi: 10.1016/j.fbio.2024.105725
Bi, T., Wu, T., Yang, L., Xu, Y., and Mu, X. (2023). Comprehensive transformation of Escherichia coli for nicotinamide mononucleotide production. Catalysts 13:815. doi: 10.3390/catal13050815
Cheng, F., Li, K. X., Wu, S. S., Liu, H. Y., Li, H., Shen, Q., et al. (2024). Biosynthesis of nicotinamide mononucleotide: synthesis method, enzyme, and biocatalytic system. J. Agric. Food Chem. 72, 3302–3313. doi: 10.1021/acs.jafc.3c09217
Cheng, J., Zuo, Y., Liu, G., Li, D., Gao, J., Xiao, F., et al. (2023). Development of a Pichia pastoris cell factory for efficient production of germacrene a: a precursor of beta-elemene. Bioresour. Bioprocess. 10:38. doi: 10.1186/s40643-023-00657-0
He, Z., Yang, X., Tian, X., Li, L., and Liu, M. (2022). Yeast cell surface engineering of a nicotinamide riboside kinase for the production of beta-nicotinamide mononucleotide via whole-cell catalysis. ACS Synth. Biol. 11, 3451–3459. doi: 10.1021/acssynbio.2c00350
Huang, Z., Li, N., Yu, S., Zhang, W., Zhang, T., and Zhou, J. (2022). Systematic engineering of Escherichia coli for efficient production of nicotinamide mononucleotide from nicotinamide. ACS Synth. Biol. 11, 2979–2988. doi: 10.1021/acssynbio.2c00100
Huang, H., Song, X., and Yang, S. (2019). Development of a RecE/T-assisted CRISPR-Cas9 toolbox for Lactobacillus. Biotechnol. J. 14:e1800690. doi: 10.1002/biot.201800690
Huang, Z., Wang, X., Li, N., Song, F., and Zhou, J. (2023). Systematic engineering of Escherichia coli for efficient production of nicotinamide riboside from nicotinamide and 3-cyanopyridine. Bioresour. Technol. 377:128953. doi: 10.1016/j.biortech.2023.128953
Jeanguenin, L., Lara-Nunez, A., Rodionov, D. A., Osterman, A. L., Komarova, N. Y., Rentsch, D., et al. (2012). Comparative genomics and functional analysis of the NiaP family uncover nicotinate transporters from bacteria, plants, and mammals. Funct. Integr. Genomics 12, 25–34. doi: 10.1007/s10142-011-0255-y
Kleerebezem, M., Boekhorst, J., Van Kranenburg, R., Molenaar, D., Kuipers, O. P., Leer, R., et al. (2003). Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA 100, 1990–1995. doi: 10.1073/pnas.0337704100
Kong, L. H., Li, X. Y., Liu, T. Y., Yao, Q. S., and Qin, J. Y. (2024). Harnessing lactic acid bacteria for nicotinamide mononucleotide biosynthesis: a review of strategies and future directions. Front. Microbiol. 15:1492179. doi: 10.3389/fmicb.2024.1492179
Kong, L. H., Liu, T. Y., Yao, Q. S., Zhang, X. H., Xu, W. N., and Qin, J. Y. (2023). Enhancing the biosynthesis of nicotinamide mononucleotide in Lactococcus lactis by heterologous expression of FtnadE. J. Sci. Food Agric. 103, 450–456. doi: 10.1002/jsfa.12253
Kong, L., Xiong, Z., Song, X., Xia, Y., Zhang, H., Yang, Y., et al. (2020). Enhanced antioxidant activity in Streptococcus thermophilus by high-level expression of superoxide dismutase. Front. Microbiol. 11:579804. doi: 10.3389/fmicb.2020.579804
Kuerec, A. H., Wang, W., Yi, L., Tao, R., Lin, Z., Vaidya, A., et al. (2024). Towards personalized nicotinamide mononucleotide (NMN) supplementation: Nicotinamide adenine dinucleotide (NAD) concentration. Mechanisms of Ageing and Development, 218:111917. doi: 10.1016/j.mad.2024.111917
Liu, Y., Yasawong, M., and Yu, B. (2021). Metabolic engineering of Escherichia coli for biosynthesis of beta-nicotinamide mononucleotide from nicotinamide. Microb. Biotechnol. 14, 2581–2591. doi: 10.1111/1751-7915.13901
Liu, Z., Zhang, Z., Qiu, L., Zhang, F., Xu, X., Wei, H., et al. (2017). Characterization and bioactivities of the exopolysaccharide from a probiotic strain of Lactobacillus plantarum WLPL04. J. Dairy Sci. 100, 6895–6905. doi: 10.3168/jds.2016-11944
Luo, S. Q., Zhao, J. T., Zheng, Y. Y., Chen, T., and Wang, Z. W. (2023). Biosynthesis of nicotinamide mononucleotide: current metabolic engineering strategies, challenges, and prospects. Fermentation 9:594. doi: 10.3390/fermentation9070594
Maharjan, A., Singhvi, M., Kafle, S. R., and Kim, B. S. (2023). Enhanced production of nicotinamide mononucleotide by high cell density culture of engineered Escherichia coli. Process Biochem. 131, 264–271. doi: 10.1016/j.procbio.2023.07.002
Maharjan, A., Singhvi, M., and Kim, B. S. (2021). Biosynthesis of a therapeutically important nicotinamide mononucleotide through a phosphoribosyl pyrophosphate synthetase 1 and 2 engineered strain of Escherichia coli. ACS Synth. Biol. 10, 3055–3065. doi: 10.1021/acssynbio.1c00333
Marinescu, G. C., Popescu, R. G., Stoian, G., and Dinischiotu, A. (2018). beta-nicotinamide mononucleotide (NMN) production in Escherichia coli. Sci. Rep. 8:12278. doi: 10.1038/s41598-018-30792-0
Nadeeshani, H., Li, J. Y., Ying, T. L., Zhang, B. H., and Lu, J. (2022). Nicotinamide mononucleotide (NMN) as an anti-aging health product-promises and safety concerns. J. Adv. Res. 37, 267–278. doi: 10.1016/j.jare.2021.08.003
Oleksy, M., and Klewicka, E. (2016). Exopolysaccharides produced by Lactobacillus sp.: biosynthesis and applications. Crit. Rev. Food Sci. Nutr. 58, 1–13. doi: 10.1080/10408398.2016.1187112
Poddar, S. K., Sifat, A. E., Haque, S., Nahid, N. A., Chowdhury, S., and Mehedi, I. (2019). Nicotinamide mononucleotide: exploration of diverse therapeutic applications of a potential molecule. Biomolecules 9:34. doi: 10.3390/biom9010034
Shoji, S., Yamaji, T., Makino, H., Ishii, J., and Kondo, A. (2021). Metabolic design for selective production of nicotinamide mononucleotide from glucose and nicotinamide. Metab. Eng. 65, 167–177. doi: 10.1016/j.ymben.2020.11.008
Su, C., Cheng, L., Gong, J. S., Li, H., Xu, Z. H., and Shi, J. S. (2024). Systematic engineering for efficient production of nicotinamide mononucleotide from d-xylose and nicotinamide in Escherichia coli. Food Biosci. 59:103859. doi: 10.1016/j.fbio.2024.103859
Sugiyama, K., Iijima, K., Yoshino, M., Dohra, H., Tokimoto, Y., Nishikawa, K., et al. (2021). Nicotinamide mononucleotide production by fructophilic lactic acid bacteria. Sci. Rep. 11:7662. doi: 10.1038/s41598-021-87361-1
Wang, L., Li, N., Yu, S., and Zhou, J. (2023). Enhancing caffeic acid production in Escherichia coli by engineering the biosynthesis pathway and transporter. Bioresour. Technol. 368:128320. doi: 10.1016/j.biortech.2022.128320
Xiong, B., Yang, T., Zhang, Z., Li, X., Yu, H., Wang, L., et al. (2025). Metabolic reprogramming and machine learning-guided cofactor engineering to boost nicotinamide mononucleotide production in Escherichia coli. Bioresour. Technol. 426:132350. doi: 10.1016/j.biortech.2025.132350
Yang, L. Y., Mu, X. Q., Nie, Y., and Xu, Y. (2021). Improving the production of NAD(+) via multi-strategy metabolic engineering in Escherichia coli. Metab. Eng. 64, 122–133. doi: 10.1016/j.ymben.2021.01.012
Yoshino, J., Baur, J. A., and Imai, S. I. (2018). NAD(+) intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab. 27, 513–528. doi: 10.1016/j.cmet.2017.11.002
Keywords: nicotinamide mononucleotide, niacin transporter, multicopy engineering, transcriptomic analysis, CRISPR/Cas9, Lactiplantibacillus plantarum
Citation: Kong L, Li X, He Q, Yao Q, Ai L and Qin J (2025) Transcriptome-guided engineering of a native niacin transporter in Lactiplantibacillus plantarum unveils metabolic rewiring for NMN biosynthesis. Front. Microbiol. 16:1637666. doi: 10.3389/fmicb.2025.1637666
Edited by:
Bo Yang, Jiangnan University, ChinaCopyright © 2025 Kong, Li, He, Yao, Ai and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linghui Kong, bGluZ2h1aWtvbmdfY2huQDE2My5jb20=; Jiayang Qin, cWluanlAYnptYy5lZHUuY24=
†These authors have contributed equally to this work
 Linghui Kong
Linghui Kong Xinyu Li
Xinyu Li Qing He1
Qing He1 Qingshou Yao
Qingshou Yao Jiayang Qin
Jiayang Qin