- Institute of Environment and Sustainable Development, Banaras Hindu University, Varanasi, India
Salinity is a major environmental stressor affecting crop productivity worldwide and a substantial portion of the agricultural ecosystem supporting cultivation of mustard (Brassica juncea L.) in Indian subcontinent is salinity stricken. However, plant growth promoting rhizobacteria has been noted to modulate salinity stress in plants through numerous direct and indirect mechanisms. Therefore, the present study was intended to determine the potential of a salt tolerant plant growth promoting rhizobacteria Bacillus flexus on alleviating the negative effects of salinity stress in mustard. The evaluation of germination percentage, growth parameters, and pigment content (chlorophyll and carotenoids) along with biochemical properties and antioxidant enzyme activities of mustard was studied by biopriming the seeds with Bacillus flexus both in absence and presence of salinity (100 mM NaCl) stress. The obtained results revealed a significant improvement in germination percentage and growth parameters (shoot length, root length, biomass and leaf area) of bioprimed mustard seedling both in presence and absence of salt stress. The biochemical properties such as pigment content, proline, total soluble protein, and total soluble sugar were found improved in bacterial treated seeds in comparison to control both in presence and absence of salinity stress. The percentage of electrolyte leakage and malondialdehyde (MDA) content was found decreased in bacterial treated plants under salinity induced condition as compared with non-treated plants. The antioxidant enzymes such as catalase (CAT), peroxidise (POX) and ascorbate peroxidise (APX) activities were found elevated in bacterial treated seeds in comparison to control both in presence and absence of salinity stress. The results obtained from the study revealed the protective and growth promoting abilities of Bacillus flexus against salinity stress. The bacterial strain used in the present study proved to be a promising candidate for improving mustard growth in soils challenged with salinity stress.
1 Introduction
Among various environmental stressors, salinity is one of the major and deleterious abiotic stressor which affects plant productivity substantially. Approximately 5.2 billion hectares of fertile land globally is stricken by different kinds of soil degradation problem. Out of which around 50% of this land is solitarily affected by problem of salinity (Numan et al., 2018; Singh and Prasad, 2020; Kumawat et al., 2023) which not only affects the soil fertility and plant growth but conjointly the living organisms (Bharti et al., 2016; Egamberdieva et al., 2019; AbuQamar et al., 2024).
Mustard (Brassica juncea) is a popular multipurpose plant belonging to family Brassicaceae. Its popularity in India and other countries is because of its oil content, which is edible and also has some medicinal value such as anti-inflammatory, anti-microbial and potential anti-cancer properties. The residues of plant have been used as cattle feed, biofuels and as a fertilizer (Shanker et al., 2011; Sharma et al., 2013; Ahmad et al., 2015). India, in spite of being largest producer of edible oil faces shortage to satisfy the daily requirement of its people (Ahmad et al., 2015). Mustard serves as a good model plant, because it often experiences salinity stress in arid and semiarid regions of the world (Parti et al., 2003). Salinity imposes ionic and osmotic stress that interferes with the growth, biomass yield, and physio-biochemical attributes of mustard seedlings (Parti et al., 2003; Sarma et al., 2012). The most common salinity impact on mustard plant could be a general stunting of plant growth, hefty delay and reduction in seed germination and seedlings growth characteristics (Ahmad et al., 2015). A significant portion of the agricultural ecosystem supporting cultivation of mustard in Indian subcontinent is salinity affected; therefore, it is imperative to investigate performance of mustard under salinity stress.
Plant growth promoting rhizobacteria (PGPR) has been noted to modulate salinity stress in plants through numerous direct and indirect mechanisms such as production of growth hormone indole acetic acid (IAA), solubilization of phosphorus, production of 1-aminocyclopropane-1-carboxylate (ACC) deaminase enzyme, ammonia and siderophore (Bharti et al., 2016; Bokhari et al., 2019; Arora et al., 2021). PGPR are known for their role in improving plant-water relations, hormonal signaling, ion homeostasis, antioxidant enzyme regulation and photosynthetic efficiency in plants exposed to salinity stress (Abbas et al., 2019; Gupta and Pandey, 2019; Ansari et al., 2025). These mechanisms are regulated by a complex network of signaling owing to their interaction and consequently ensuing stress alleviation (Habib et al., 2016; Ilanumaran and Smith, 2017; Kumawat et al., 2022). Under saline conditions, high level of Na+ not only hampers the uptake of nutrients but also causes peculiar ion toxicity (Barnawal et al., 2012; Slimani et al., 2023). The PGPR strains have the potential to protect the plants from deleterious effects of high Na+ concentration in saline soils and they do so by their ability to produce ACC-deaminase enzyme, stimulation of growth hormone and accumulation of osmoprotectors which protect the plants from injurious effects of the salinity stress (Abbas et al., 2019; Gupta and Pandey, 2019; Kibret et al., 2024). The ability of PGPR to produce exopolysaccharides (EPS) is also reported to limit the Na+ uptake in plants by their binding with it and biofilms (Yang et al., 2009; Qurashi and Sabri, 2013). The lowered availability of Na+ ends up in its reduced uptake thereby balancing high K+/Na+ ratio that facilitate the plant to survive better under salinity stress condition (Nadeem et al., 2014; Egamberdieva et al., 2019; Mukhtar et al., 2020; Kumawat et al., 2023). An efficient survival of rhizobacteria under saline condition is due to excessive accumulation of secondary metabolites may help in better colonization and prove beneficial in prospering strategies to promote plant growth in saline soils (Qurashi and Sabri, 2013). Several of the rhizobacterial strains such as Bacillus, Pseudomonas, Klebsiella, Azotobacter, Enterobacter etc. have been reported to influence the growth and development of wheat, tomatoes, peppers, beans, and lettuce grown in saline environments (Nadeem et al., 2014; Ahmad et al., 2015; Orhan, 2016; Egamberdieva et al., 2019; Kumawat et al., 2023). It has been proved from various studies that rhizobacteria from salinity affected soil with multiple PGP traits along with salt tolerance properties are more likely to endure salinity stress and would be suitable for use in sustainable agricultural practices (Mukhtar et al., 2020; Joshi et al., 2021; Kumawat et al., 2023).
Considering these facts, the present study was intended to evaluate the effectiveness of a salt tolerant plant growth promoting rhizobacterial strain Bacillus flexus for alleviating the negative impacts of salinity stress on mustard seedlings.
2 Materials and methods
2.1 Sampling and isolation of rhizobacteria
Soil samples were collected from the rhizosphere of mustard (Brassica juncea) plant from different salt affected agricultural sites of Azamgarh, Uttar Pradesh, India (26°03′N 83°13′E). For the isolation, 1 g of rhizospheric soil was taken with 10 mL of normal saline and mixed properly by shaking. Serial dilution was performed up to 10−6 fold dilution and 100 μL of suspension from each dilution was spread on nutrient agar (NA) media plates. Plates were incubated at 30°C for 24 h. The visible growth of bacterial colonies was selected for further tests.
2.2 Screening for salt tolerance
All the selected bacterial colonies were tested for their salt tolerance potential. All cultures along with DH5α (an E. coli strain) taken as a standard were streaked on nutrient agar medium supplemented with different concentration of NaCl salt (100 mM, 500 mM, 1 M, 1.5 M, and 2 M) making it selective medium. Based on observations of visible growth of the bacterial isolates on these plates after 24 h of incubation at 30°C they were considered positive or negative for their tolerance against salinity. A salt tolerant rhizobacterial strain Bacillus flexus (accession number MK968766) was used in the present study. This strain was able to grow on nutrient agar solid media amended with NaCl up to 2 molars (M).
2.3 Seed coating and germination experiment
Mustard seed (Brassica juncea cv. Varuna T59) were used for this study. Seeds were surface sterilized for 30 s with 70% ethyl alcohol followed by sterilization with 2% sodium hypochlorite for 2 min. Further seeds were subjected to 3 times washing with sterile distilled water. The seeds were sown in germination trays (7 columns and 5 rows, total 35 wells) with duplicate tray for each treatment and this experimental setup was kept for 21 days. Two seeds per well was sown, thus a total of 140 seeds were used for each treatment (Figure 1). Total 6 combinations of seed treatment were used in this study which was as follows;
1. Control without NaCl.
2. Control with NaCl (100 mM)
3. Talc without NaCl.
4. Talc with NaCl (100 mM)
5. Formulation of Bacillus flexus without NaCl.
6. Formulation of Bacillus flexus with NaCl (100 mM)
2.4 Inoculum preparation and growth condition
For making the bacterial formulation Bacillus flexus was grown in LB broth in a shaking incubator at 150 rpm for 18 h at 30°C. After incubation time the fully grown culture was taken and centrifuged at 3,000 rpm for 3 min. Following this the bacterial pellet was washed with sterile distilled water and re-suspended in normal saline (0.85%) solution and the culture suspension OD was maintained at A600nm = 1.0. Seeds were mixed with jaggery syrup followed by coating with bacterial formulation which was prepared by mixing 1.8 × 107 bacterial cells with 2 grams of talc. Talc was used as a carrier material in the bacterial formulation. The jaggery syrup was used to provide the adhesion of bacteria to the seed surface. Coated seeds were incubated overnight at 28 ± 2°C for proper adhesion of bacteria on seeds along with control and talc coated seeds. The control, talc and bacteria coated seeds were watered with distilled water in absence of salinity stress (no NaCl), while to induce artificial salinity stress in germinating mustard seedlings a solution of 100 mM NaCl (~ 10 dS/m) in distilled water was used for watering. Watering of seeds was done every alternate day.
The germination trays were kept outside the laboratory to simulate natural environmental condition. The experiment was performed from September–October 2024. The average temperature during the experiment ranged between 25.5°C–30.2°C and the relative humidity was approximately 79%.
2.5 Physico-chemical characteristics
The soil used in the experiment was garden soil of Institute of Environment and Sustainable Development, Banaras Hindu University, Varanasi, India with following physico-chemical characteristics as described in Table 1.
2.6 Germination and growth analysis of mustard seedlings
Germination was recorded on a daily basis for 21 days and at the end of experimental period total percentage of germination was calculated. The time (days) taken by seeds for 50% germination (T50) was calculated by following the formula of Farooq et al. (2018).
.
Where, N denotes final number of seeds germinated, ni and nj represents total number of seeds germinated by adjacent count at time ti and tj respectively, where ni < N/2 < nj.
2.7 Estimation of seedling biomass
Mustard seedlings were collected after 21 days of germination. Seedlings were randomly selected from each treatment for the estimation of growth-related parameters. The fresh weight (g) of plants (5 plants) from each treatment was taken immediately while dry weight (g) of plants was calculated after drying the seedling in oven at 60°C for 24 h (Forti et al., 2020). The root and shoot lengths of seedlings were measured on millimetric paper. Leaf area was calculated by using following formula (Mckee, 1964).
.
2.8 Estimation of electrolyte leakage
Electrolyte leakage (EL) was determined according to the method of Gonzalez and Gonzalez-Villar (2003). For the estimation of EL, 200 mg of fresh mustard leaves were cut into small pieces and placed into test tube, filled with 10 mL of deionized water and covered with plastic caps. Tubes were heated at a constant temperature of 32°C for 2 h in a water bath. The initial electrical conductivity (EC1) of the solution was recorded after 2 h by conductivity probe. Further the samples were autoclaved at 121°C for 20 min to properly kill/rupture the tissues facilitating in release of all the electrolytes. The final electrical conductivity (EC2) was recorded after cooling the samples at room temperature. The EL was measured using the following formula.
.
Where, EL represents electrolyte leakage and EC depict electrical conductivity.
2.9 Estimation of photosynthetic pigments
The pigments chlorophyll a, chlorophyll b, total chlorophyll and carotenoids were estimated from fresh leaves of mustard seedlings. One hundred milligrams leaf sample was placed in a test tube containing 10 mL of 80% acetone, kept for overnight in a refrigerator at 4°C. Later the sample was homogenized and centrifuged at 5,000 rpm for 7 min. The final volume of the supernatant was made up to 25 mL with 80% acetone. Absorbance of 80% acetone extracted samples were recorded at 480 nm and 510 nm for carotenoids and at 645 nm and 663 nm for chlorophyll estimation by using spectrophotometer (Thermo-Scientific, Evolution-201) (Arnon, 1949).
2.10 Biochemical properties and antioxidant enzymatic activity of mustard seedlings
2.10.1 Proline
For measurement of proline, method of Bates et al. (1973) was followed. For this, 100 mg of leaves were homogenized with 10 mL of 3% aqueous sulphosalicylic acid and centrifuged at 10,000 rpm for 15 min at 4°C. From this 1 mL of supernatant was taken and mixed with 1 mL of glacial acetic acid and 1 mL of ninhydrin. Mixture was incubated for 1 h at 90°C. After incubation time, samples were cooled immediately in ice water and subsequently 2 mL of toluene was added and vortexed for 2 min. The upper phase (wine red color) was collected and absorbance was recorded at 520 nm by using spectrophotometer (Thermo-Scientific, Evolution-201). Proline concentration was calculated by using the proline standard curve and reported as μg/g fresh weight.
2.10.2 Total soluble protein
The estimation of total protein content was done as per Bradford method (Bradford, 1976). For this 100 mg of plant material was taken with 1 mL of protein extraction buffer (10 mM of Tris HCL and EDTA (pH 8.0), 0.1 mg/mL phenylmethylsulfonyl fluoride (PMSF) and 5 mM β-mercaptoethanol) and homogenized in a chilled mortar and pestle. This solution was collected in a 1.5 mL microcentrifuge tube and centrifuged at 12,000 rpm for 20 min at 4°C. From this 100 μL of supernatant was taken in a test tube and 3 mL of Bradford reagent was added to it, mixed properly and incubated for 10 min at room temperature. After that OD was recorded at 595 nm. The standard curve was prepared by using different concentration of Bovine serum albumin (BSA) with a range of 5–100 μg protein.
2.10.3 Total soluble sugar
Total soluble sugar (mg/g) was estimated by following the PSA method of Dubois et al. (1956). For the analysis of total sugar in mustard seedlings 0.5 g fresh leaves were added to 10 mL of 80% acetone and heated at 80°C for 1 h in water bath. From this solution 0.5 mL aliquot was taken and mixed with 1 mL of phenol (5%) and 5 mL of 96% H2SO4. Solution was mixed properly and incubated for 1 h at room temperature. After 1 h reaction mixture was properly mixed by shaking and absorbance was recorded at 490 nm.
2.10.4 Lipid peroxidation
Lipid peroxidation (LPO) was estimated in terms of the production of malondialdehyde (MDA) due to the thiobarbituric acid (TBA) reaction (Heath and Packer, 1968). For the LPO measurement, 500 mg of fresh leaf were taken into 5 mL of ice-cold extracting solution (5% trichloroacetic acid, TCA), homogenized in a mortar and pestle and centrifuged at 10,000 rpm for 10 min. One milliliter of extract was collected and mixed with 4 mL of 0.5% thiobarbituric acid (prepared in 20% TCA), boiled for 30 min on water bath. The solution was cooled immediately in an ice bath and centrifuged at 1,000 rpm for 10 min. The absorbance of supernatant was recorded at 532 nm and 600 nm by using spectrophotometer (Thermo-Scientific, Evolution-201). MDA content was calculated in terms of its molar extinction coefficient (155 mM−1 cm−1).
.
2.10.5 Catalase
Catalase (CAT) activity was estimated according to the method of Chandlee and Scandalios (1984). For this, 100 mg of fresh plant material was taken and added to 10 mL of potassium phosphate buffer (0.1 M, pH 7). Plant material was crushed properly in to phosphate buffer and centrifuged at 10,000 rpm for 15 min. From this 0.4 mL of enzyme extract was taken and mixed with 2.6 mL of potassium phosphate buffer and 0.4 mL of hydrogen peroxide (H2O2). Solution was mixed properly and incubated for 1 min at 25°C. Catalase activity was determined in terms of reduction in absorbance of H2O2 at 240 nm.
2.10.6 Peroxidase
The estimation of peroxidase (POX) enzyme was done by following the method of Kar and Mishra (1976). For peroxidase analysis, 100 mg of fresh plant material was crushed with 10 mL of potassium phosphate buffer (0.1 M, pH 7) and centrifuged at 10,000 rpm for 15 min. From the solution 0.5 mL of enzyme extract was taken and mixed with 2 mL of phosphate buffer, 1 mL of pyrogallol and 1 mL of H2O2 and incubated for 5 min at 25°C. After 5 min, reaction was terminated by adding 1 mL of 200 mM H2O2. The quantity of purpurogallin produced was estimated by taking absorbance at 420 nm against blank and enzyme activity was expressed as change in U mg−1 protein. Here, 1 U defined as the change in the absorbance of 0.1 min−1 mg−1 protein.
2.10.7 Ascorbate peroxidase
The method of Nakano and Asada (1981) was followed for the estimation of ascorbate peroxidase (APOX) activity. For this, 100 mg of fresh plant material was taken and added to 10 mL of potassium phosphate buffer (0.1 M, pH 7) containing 2 mM ascorbate. Plant material was crushed properly in to phosphate buffer and centrifuged at 10,000 rpm for 15 min. The 3 mL reaction mixture contained 50 mM phosphate buffer (pH 7.0), 0.1 mM EDTA, 0.5 mM ascorbic acid; 0.1 mM H2O2 and 0.5 mL of enzyme extract were mixed properly. The reaction was carried out for 5 min at 25°C. Absorbance was recorded at 290 nm. All the enzyme activities were expressed as unit mg−1 protein.
2.11 Statistical analysis
One way analysis of variance (ANOVA) was performed to determine the significant differences between each treatment by using the software SPSS version 20, which was followed by Duncan’s multiple range test (DMRT) to compare the means. All the values were presented as mean ± standard error of the three replicates. Means with a significance value p ≤ 0.05 were considered statistically different. The significant differences among all samples were indicated by different alphabetic letters.
3 Results
3.1 Growth dynamics of rhizobacteria coated mustard seedling challenged with salt stress
3.1.1 Germination percentage
The results of seedling germination of treated and non-treated seeds are shown in Figure 2. The result of germination percentage showed that in absence of salinity stress talc coated seeds performed best (92 ± 1.43%) however, statistically no significant difference was observed in germination of seeds coated with bacterial formulation and control seeds without any salt treatment (87 ± 0.71% and 84 ± 1.23% respectively). In case of seeds having salinity exposure, minimum germination (75 ± 5.71%) was recorded in seeds which were subjected to salinity stress without any coating, while in seeds which were coated with bacteria 87 ± 5.71% germination was observed followed by talc coating (82 ± 2.85%) (Figure 2A). The time taken by seeds for all treatments to show 50% germination (T50) is represented in Figure 2B. There was no significant difference observed for T50 germination in between the treatment in absence of salinity stress. However, in presence of salt stress minimum time for 50% germination was observed for bacterial coated seeds while maximum was recorded for non-coated control seeds.
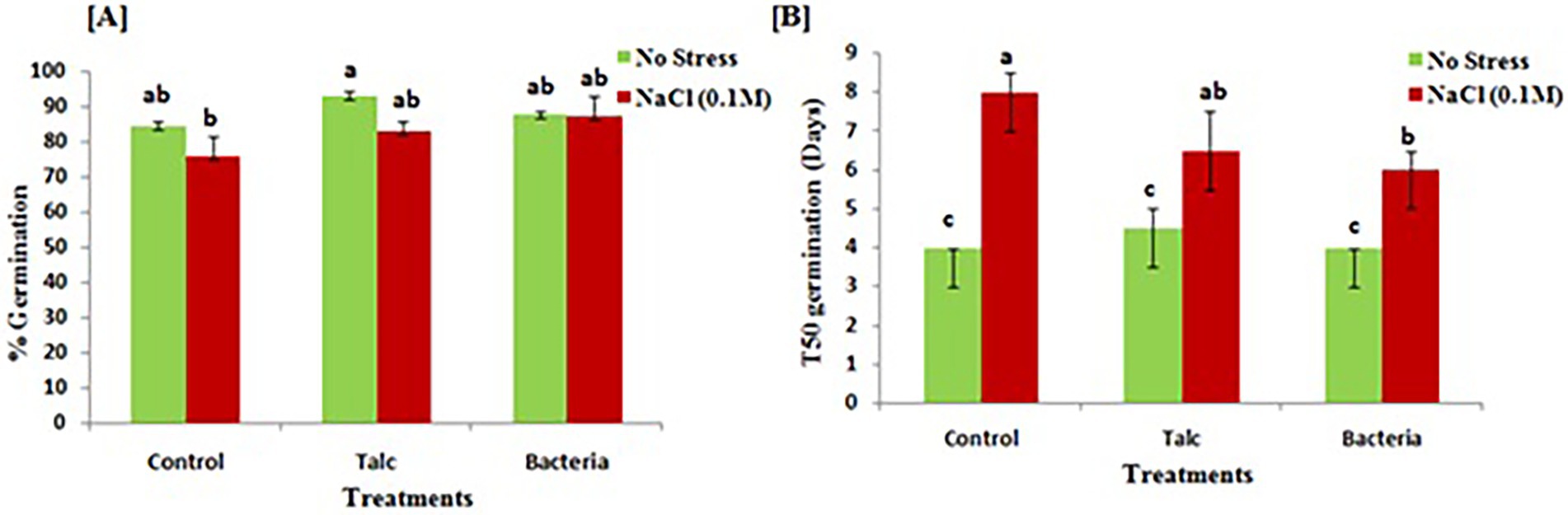
Figure 2. Effects of salinity stress on (A) germination and (B) time taken for 50% germination (T50) by mustard seeds both in presence and absence of bacterial treatment. Data presented are mean ± SE of three replicates. Different letters indicate statistically significant difference between each treatment (DMRT p < 0.05).
3.1.2 Seedling biomass
The results of physiological parameters of treated and non-treated seeds are shown in Figure 3. Plant height along with shoot and root length was recorded maximum in mustard seedlings which were coated with formulation of Bacillus flexus both in presence and absence of salt stress. However, there was no significant difference observed for root and shoot length in control and talc coated seedling both under presence or absence of salt stress (Figures 3A–C). The result for leaf area showed no significant difference between condition of presence and absence of salt stress (Figure 3D). However, a significant reduction in leaf area was observed in coated and non-coated seedlings under presence of salt stress. Mustard seedlings coated with Bacillus flexus were also recorded best for biomass (fresh weight and dry weight) under both conditions (presence and absence of salt). The control and talc coated seedlings exhibited similar results and did not show any significant difference with each other under both presence and absence of salt stress (Figures 3E,F).
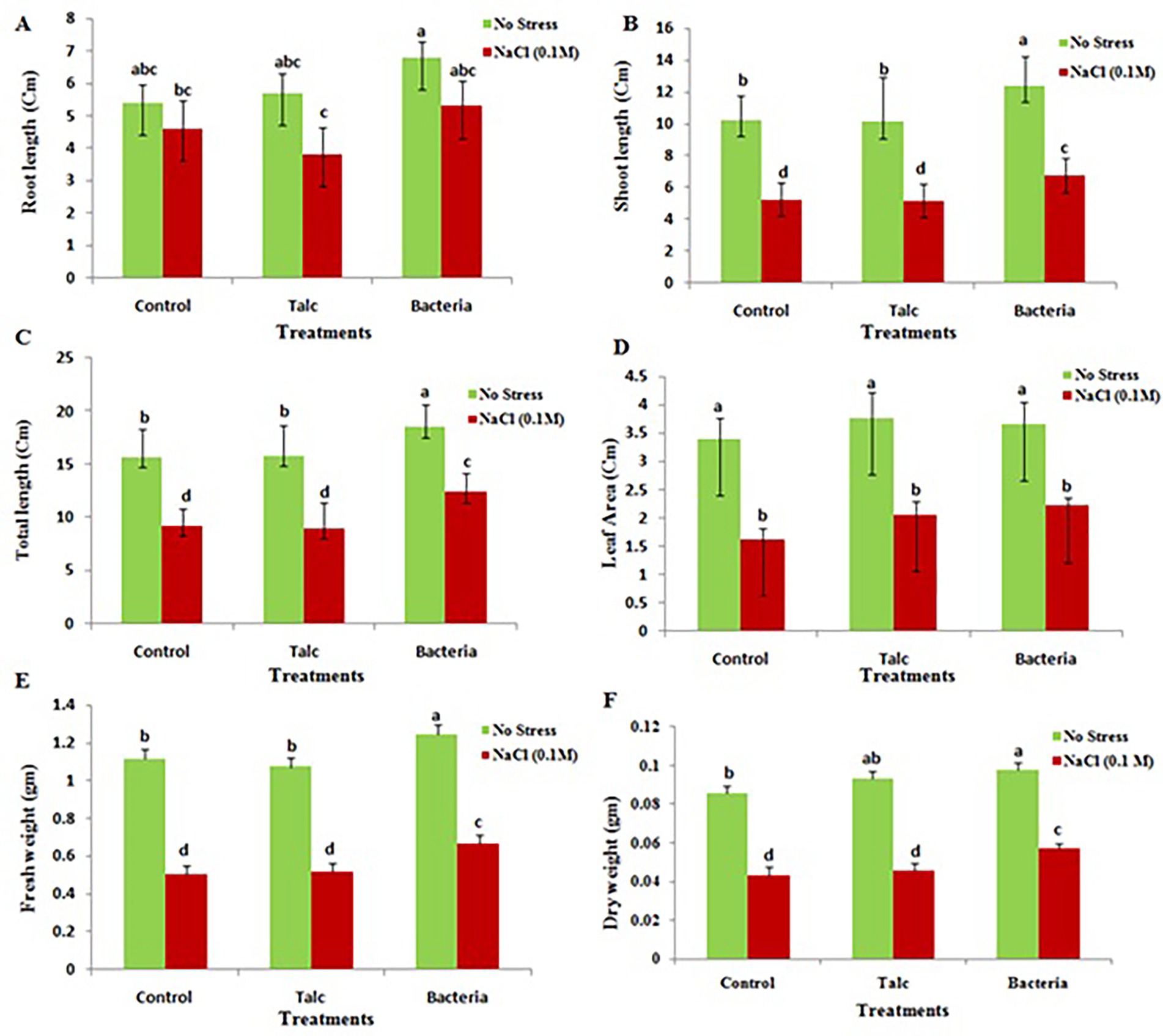
Figure 3. Effect of salinity stress on various growth parameters of differently coated mustard seedlings. (A) Root length, (B) shoot length, (C) total length, (D) leaf area, (E) fresh weight, and (F) dry weight. Data presented are mean ± SE of three replicates. Different letters indicate statistically significant difference between each treatment (DMRT p < 0.05).
3.2 Biochemical properties and antioxidant status of mustard seedlings
3.2.1 Photosynthetic pigments
The photosynthetic pigments (chlorophyll a, chlorophyll b, total chlorophyll and carotenoids) were found noticeably reduced in plants subjected to salt stress. However, seedlings with Bacillus flexus coating showed higher pigment contents both in presence and absence of salt. Somewhat similar results were observed for control and talc coated seedlings exposed to salt or without any salt exposure (Figure 4).
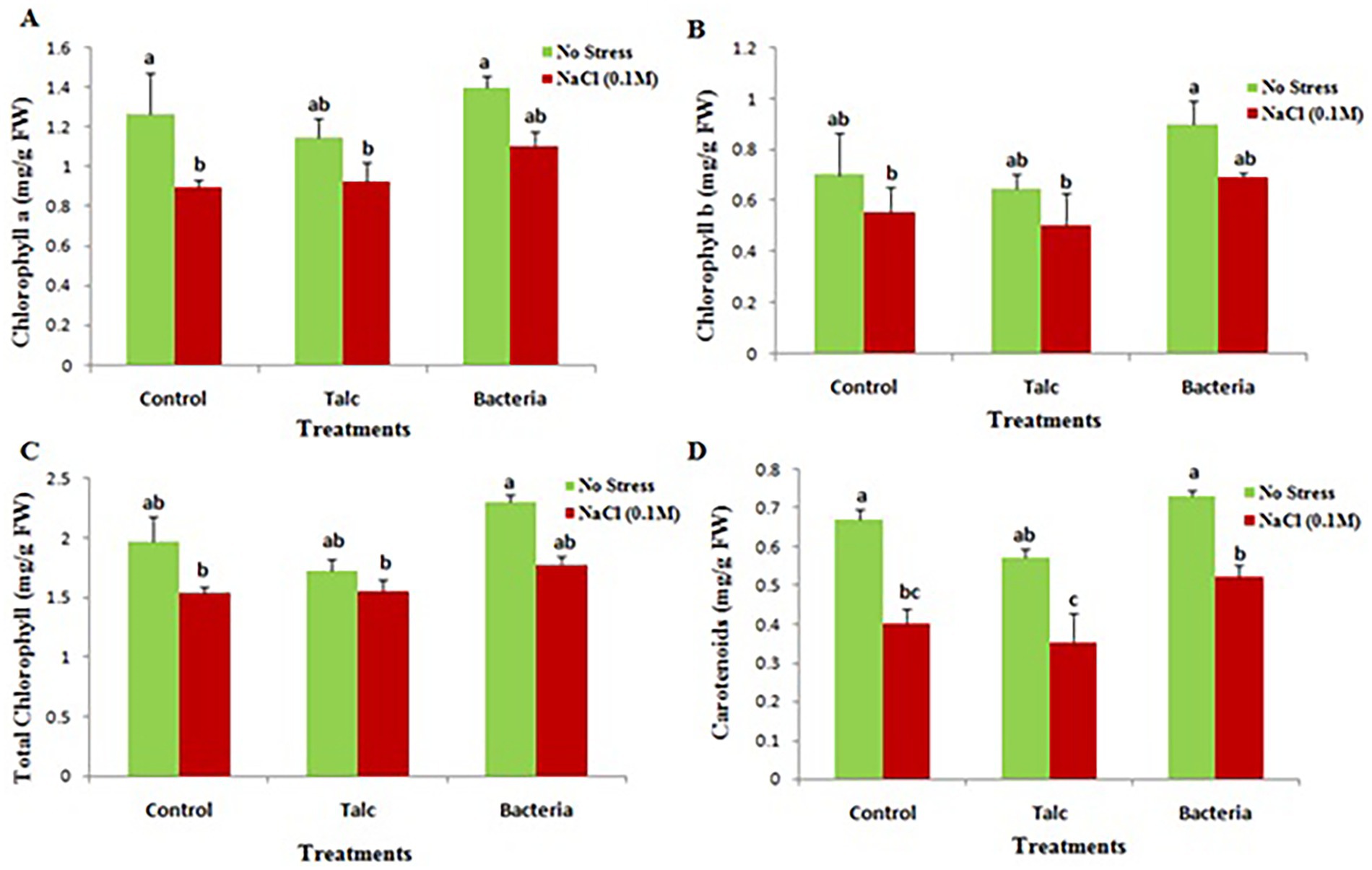
Figure 4. Effect of salinity stress on photosynthetic pigment content of differently coated mustard seedlings (A) Chlorophyll a, (B) chlorophyll b, (C) total chlorophyll, and (D) carotenoids. Data presented are mean ± SE of three replicates. Different letters indicate statistically significant difference between each treatment (DMRT p < 0.05).
3.2.2 Electrolyte leakage
The minimum electrolyte leakage (64 ± 7.4%) was recorded in control seedlings which were not subjected to any stress, while the salt treated control seedlings were observed for maximum electrolyte leakage (96 ± 0.72%). In presence of salt the Bacillus flexus coated seedlings showed minimum electrolyte leakage (85.4 ± 2.9%) however no significant difference was observed between talc and bacterial coated seedlings in absence of salt stress (Figure 5A).
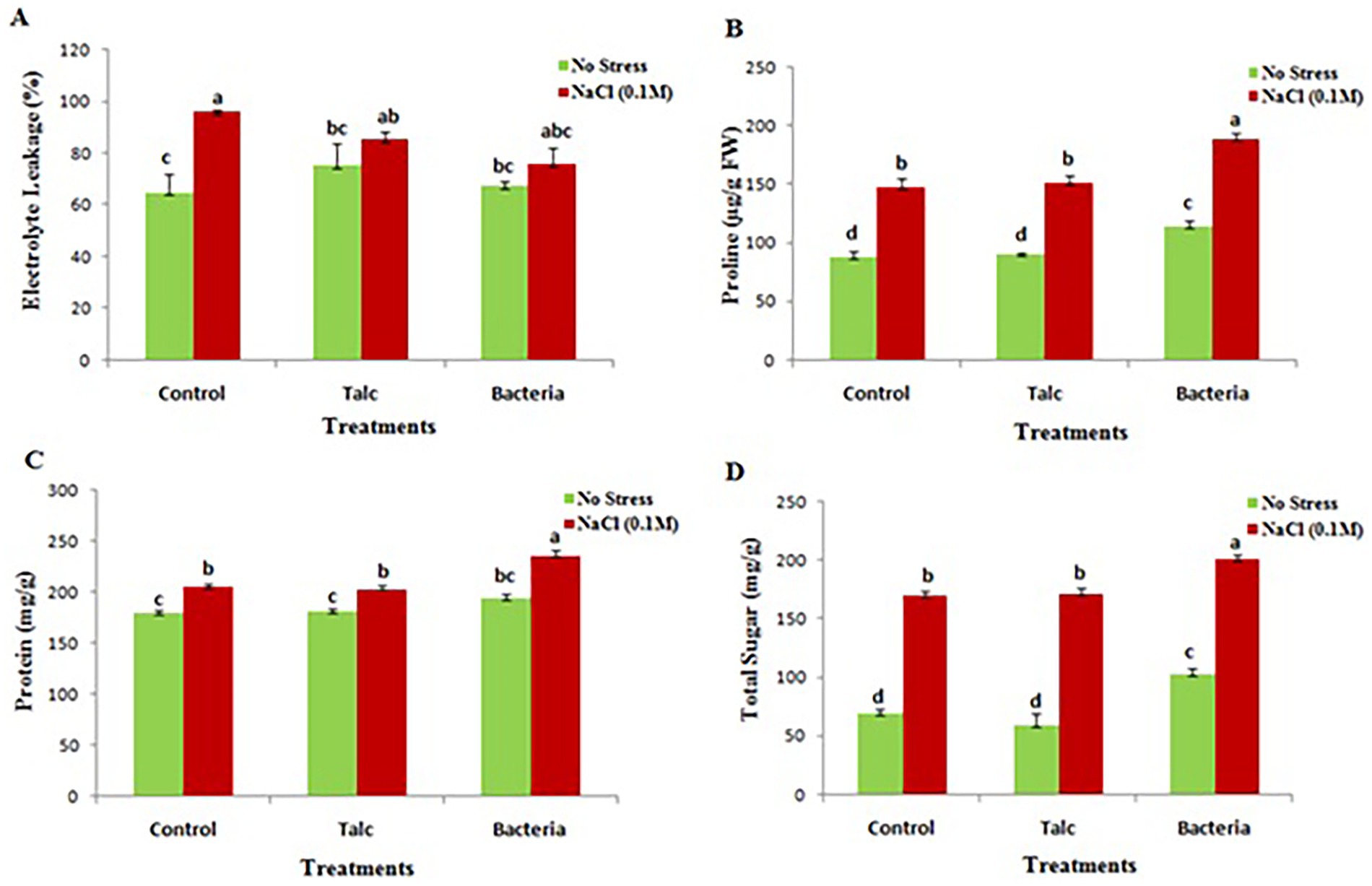
Figure 5. Effect of different seed coating on (A) electrolyte leakage, (B) proline, (C) total soluble protein and (D) total soluble sugar content of mustard seedlings under conditions of no stress and with salt stress. Data presented are mean ± SE of three replicates. Different letters indicate significant difference between each treatment (DMRT p < 0.05).
3.2.3 Proline
The accumulation of proline was elevated in seedlings coated with Bacillus flexus under conditions of no stress or with salt stress (113 ± 4.9 μg/g and 188 ± 8.05 μg/g respectively). In case of control and talc coating no significant difference was observed under conditions of no stress or with salt stress (Figure 5B).
3.2.4 Total soluble protein and sugar
Protein and sugar content were also observed high in those seedlings which were coated with Bacillus flexus both in presence and absence of salt stress while no significant difference was observed in control and talc coated seedlings when subjected to stress or under condition of no stress (Figures 5C,D).
3.2.5 Malondialdehyde
The MDA content was found minimum (0.304 μg/g) in Bacillus flexus coated seedlings under exposure to salinity stress while in absence of salt stress no significant difference was observed in any treatment (Figure 6A).
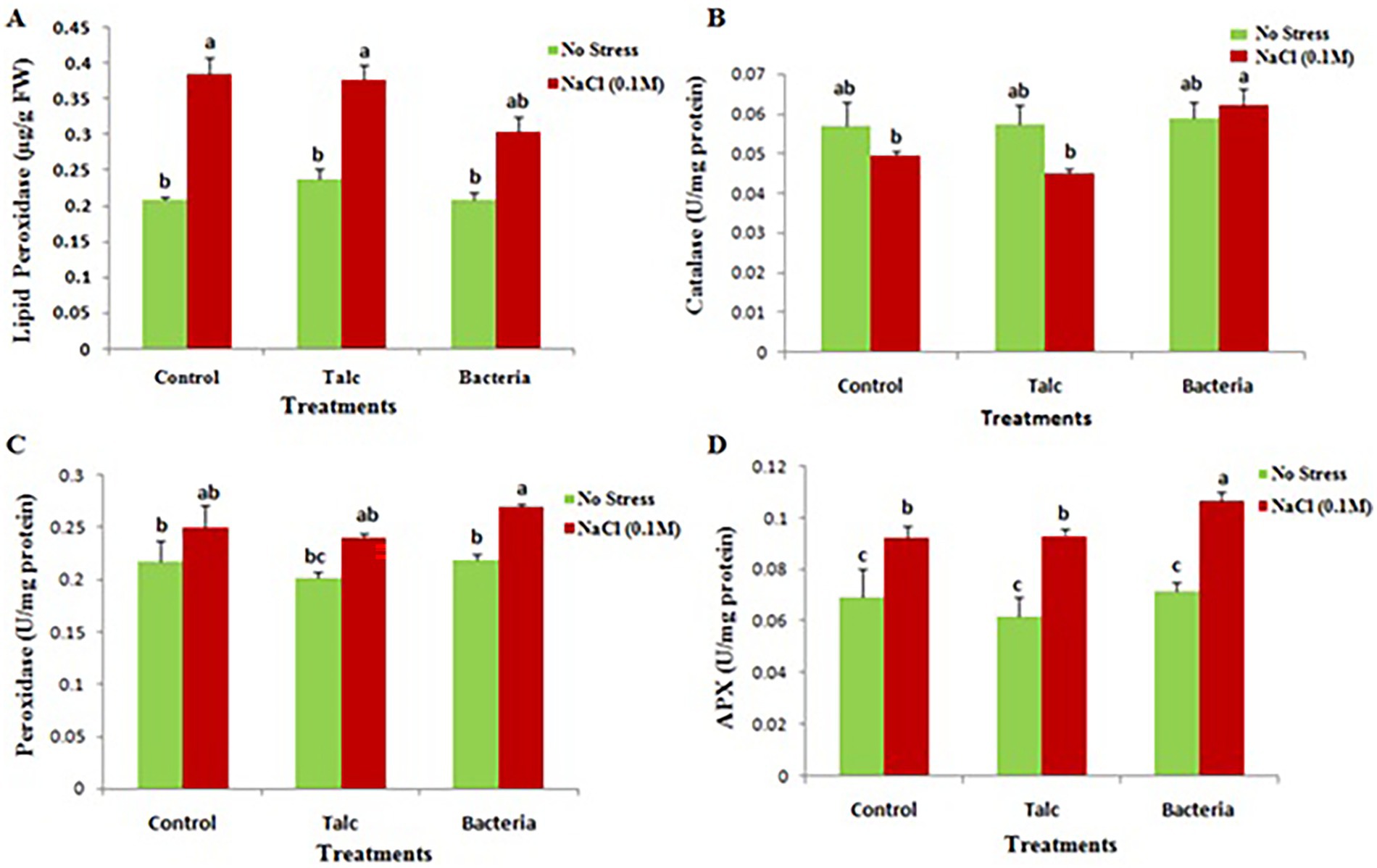
Figure 6. Effect of salinity stress on (A) malondialdehyde (MDA), (B) catalase, (C) peroxidase and (D) ascorbate peroxidase activity of differently coated mustard seedlings. Data presented are mean ± SE of three replicates. Different letters indicate significant difference between each treatment (DMRT p < 0.05).
3.2.6 Antioxidant enzymatic activity
An elevation in catalase activity was observed (0.058 U mg−1 protein) in Bacillus flexus coated seedlings under presence of salt stress while in absence of salt stress no difference was observed in any treatment (Figure 6B). The peroxidase and ascorbate peroxidase activity were found maximum (0.27 U mg−1 protein and 0.106 U mg−1 protein respectively) in Bacillus flexus coated seedlings subjected to salt stress. However, no significant difference was recorded in between treatments under absence of salt stress (Figures 6C,D).
4 Discussion
Salinity and its generation are a dynamic process and have been considered as a major limiting factor affecting productivity of agricultural soil, plant health and the living organisms within. In this regard, plant growth promoting rhizobacteria has been well known for their contribution in enhancing plant growth as well as for their ability to mitigate the effects of multifarious abiotic stresses including soil salinity in plants (Kumawat et al., 2022; Slimani et al., 2023; AbuQamar et al., 2024; Ansari et al., 2025). The present study intended to evaluate the effectiveness of a salt tolerant rhizobacteria Bacillus flexus for enhancing seed germination and growth of mustard seedlings against salinity stress. Mustard plant was chosen for this study because salinity is known to impose a hyper ionic and hyper osmotic stress that interferes with germination, growth and physio-biochemical properties of mustard seedlings (Parti et al., 2003; Ahmad et al., 2015; Arora et al., 2021).
During initial developmental stage, salinity caused noticeable delay and reduction in seed germination as well as seedling growth characteristics. The roots were observed to be affected more adversely as compared to the upper shoot part under conditions of exposed salinity (≥ EC 4.0dS m−1, i.e., ~40 mM). Toxic level of Na+ and Cl− ions create an outside osmotic potential that avoids water uptake and increased dormancy of seeds under salinity stress (Dimkpa et al., 2009; Nadeem et al., 2014). According to Singh and Panda (2012), the salinity (ECs) from about 2.1 to 3.5 dS m−1 of the upper soil profile decreased grain yields in mustard by 12–14% and straw yields by 18–19%. Sarma et al. (2012) reported in their study with mustard that germination time was considerably increased with a rise in salt concentration. Salinity influences germination time more than the germination percentage. This implies that increase in salt concentration results in prolongation of germination time as well as reduce plant growth, seed yield and total lipids of seeds (Parti et al., 2003). In the present study, significant reduction in seed germination, shoot and root length and biomass were found in mustard seedlings when exposed to NaCl induced salt stress, however seed coating with Bacillus flexus was observed to minimize the negative impacts of salinity on germination and seedling growth which is in conformity with earlier studies (Arvin et al., 2012; Ahmad et al., 2015).
Photosynthesis is one of the primary plant processes related to its growth and productivity. Slimani et al. (2023) reported disturbance in photosynthetic processes under salinity stress because of accumulation of toxic ions and decrease in water and osmotic potential. Here in the present study, it was observed that the application of NaCl induced salinity stress negatively affected the synthesis of chlorophyll and carotenoids. The reduction in pigment content under salt stress might be due to the impairment in delivery of crucial ions such as Fe2+, Mg2+, Mn2+, and Zn2+ that take part in chlorophyll synthesis. Carotenoids possess an excellent antioxidant property and scavenge ROS production thus giving photo protection to chlorophyll. Therefore, decrease in carotenoids content under NaCl stress overpowers the production of ROS that consequently induce oxidative injury to DNA, RNA and proteins thus affecting overall plant growth. The application of Bacillus flexus was observed to keep the chlorophyll and carotenoids content to an appreciable level in the present study and the results corroborates with the findings of Ahmad et al. (2015) and Bharti et al. (2016). The reason behind the increased pigment content might be the production of phytohormones that contributes for the stimulation of chlorophyll content and maintain nutrient balance hence the chlorophyll synthesis increases in bacteria inoculated/coated plants (Abbas et al., 2019; Mukhtar et al., 2020; Slimani et al., 2023).
Abiotic stresses are responsible for increased electrolyte leakage because such stresses cause displacement of membrane associated calcium from plasma lemma, thus damaging the membrane permeability consequently leading to higher efflux of electrolytes from plant cell or tissues (Garg and Manchanda, 2009; Kang et al., 2014). The findings of present study showed that Bacillus flexus coated mustard seedlings exhibited less electrolyte oozing as compared to non-coated seedlings under conditions of salinity stress. These findings indicated Bacillus flexus coating maintained the integrity and stability of cells and tissues of mustard seedlings while non-coated seedlings suffered more due to higher membrane injury leading to higher electrolyte leakage (Garg and Manchanda, 2009).
The increased protein content plays an important role in stress tolerance by preventing from various cellular damages (Campbell and Close, 1997) while soluble sugars are considered as important osmolytes and their increased concentration imparts osmotic adjustment for alleviating salinity stress (Naseem and Bano, 2014). In the present study protein and sugar content were also observed to be high in seedlings coated with Bacillus flexus. An elevated accumulation of proline was also observed in seedlings coated with Bacillus flexus under absence and presence of salinity stress. An increased concentration of proline in mustard seedlings coated with Bacillus flexus might be due to upregulation of proline biosynthesis pathway thus keeping the higher concentration of proline in presence of salinity stress, which further helps in maintaining the cell membranes under salt exposure. Similar results have also been reported by several researchers (Yoshiba et al., 1997; Qurashi and Sabri, 2013; Bharti et al., 2016).
A high level of cellular ROS in the presence of salt stress is responsible for lipid peroxidation of membranes (Bharti et al., 2016; Mukhtar et al., 2020; Ansari et al., 2025). Plants possess various antioxidants enzymes and other antioxidant molecules which help in lowering the ROS level and combating oxidative stress. Results observed in the present study showed a reduction in MDA level in seedlings coated with Bacillus flexus under salt stress condition. The present finding is supported with findings of Ahmad et al. (2015), which reported that rhizobacteria improved lipid component of mustard seed under salt stress condition. Under NaCl induced salt stress condition MDA concentration was found to be lowered, however activities of the enzyme’s catalase, peroxidase and ascorbate peroxidase were observed to be increased in seedlings coated with Bacillus flexus in seedlings exposed to salinity stress. Catalase an important antioxidant enzyme plays a very essential role in detoxification of ROS under stressful conditions by dismutating hydrogen peroxide directly. POX induces the conversion of hydrogen peroxide into water and oxygen and protects plants from damages induced by salt stress. APX in comparison to CAT and POX enzymes has more affinity for hydrogen peroxide therefore plays an important role in detoxification of ROS under stressful conditions (Khan et al., 2010; Egamberdieva et al., 2019; Mukhtar et al., 2020; Arora et al., 2021; Ansari et al., 2025).
In the present study, the application of Bacillus flexus was observed to restore and improve percent germination, biomass, root shoot length, leaf area, osmolytes, chlorophyll and carotenoid contents to an appreciable level. The antioxidant enzyme activity in bacteria coated mustard seedlings was also found best in comparison to control or talc coated seedlings. The results revealed that, under NaCl imposed salinity stress MDA concentration was found to be reduced however catalase, peroxidase and ascorbate peroxidase enzyme activity were enhanced in bacteria coated seedling in comparison to non-coated NaCl stressed seedlings. These findings clearly indicated the protective role of rhizobacteria Bacillus flexus for mustard seedlings against adversities imposed by salinity stress.
5 Conclusion
Our findings indicates that the selection and use of salt tolerant rhizobacteria possessing multifarious PGP attributes along with osmolyte producing capability could be an effective strategy for the enhancement of plant growth in saline environment. The obtained results related to improved germination, physiological parameters, biochemical properties and antioxidant status of mustard plant treated with rhizobacteria in presence and absence of NaCl clearly indicating the protective responsibility of rhizobacteria B. flexus for mustard plant against salinity stress condition. There are very limited studies were reported regarding mustard plant growth promotion by plant growth promoting rhizobacteria under salinity stress condition. Therefore, the results obtained from the present study will definitely contribute in expanding the available information. The use of salt tolerant PGPR might be proved beneficial in prospering strategies to promote plant growth in saline soils because they not only have tolerance to high salinity but also improved crop productivity but much is yet to be explored at biochemical and molecular level that how these PGPR support them as well as associated plant under salinity stress condition. The current finding opens an opportunity to measure the major role of PGPR in reducing the problem of salinity in plants in field conditions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AS: Formal analysis, Methodology, Writing – review & editing, Software, Writing – original draft, Investigation, Validation. VP: Writing – original draft, Visualization, Resources, Supervision, Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbas, R., Rasul, S., Aslam, K., Baber, M., Shahid, M., Mubeen, F., et al. (2019). Halotolerant PGPR: A hope for cultivation of saline soils. J. King. Saud. Univ. Sci. 31, 1195–1201. doi: 10.1016/j.jksus.2019.02.019
AbuQamar, S. F., El-Saadony, M. T., Saad, A. M., El-Sayed, M. D., Elrys, A. S., Abd El-Mageed, T. A., et al. (2024). Halotolerant plant growth-promoting rhizobacteria improve soil fertility and plant salinity tolerance for sustainable agriculture—a review. Plant Stress 12:100482. doi: 10.1016/J.stress.2024.100482
Ahmad, P., Hashem, A., Abd-Allah, E. F., Alqarawi, A. A., John, R., Egamberdieva, D., et al. (2015). Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L) through antioxidative defense system. Front. Plant Sci. 6:868. doi: 10.3389/fpls.2015.00868
Ansari, W. A., Krishna, R., Kashyap, S. P., Al-Anazi, K. M., Abul Farah, M., Jaiswal, D. K., et al. (2025). Relevance of plant growth-promoting bacteria in reducing the severity of tomato wilt caused by fusarium oxysporum f. sp. lycopersici by altering metabolites and related genes. Front. Microbiol. 15:1534761. doi: 10.3389/fmicb.2024.1534761
Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts: polyphenol oxidase in Beta vulgaris. Plant Physiol. 24, 1–15. doi: 10.1104/pp.24.1.1
Arora, N. K., Egamberdieva, D., Mehnaz, S., Li, W.-J., and Mishra, I. (2021). Salt tolerant rhizobacteria: for better productivity and remediation of saline soils. Front. Microbiol. 12:660075. doi: 10.3389/fmicb.2021.660075
Arvin, P., Vafabakhsh, J., Mazaheri, D., Noormohamadi, G., and Azizi, M. (2012). Study of drought stress and plant growth promoting rhizobacteria (PGPR) on yield, yield components and seed oil content of different cultivars and species of Brassica oilseed rape. Ann. Biol. Res. 3, 4444–4451. doi: 10.3390/su13063562
Barnawal, D., Bharti, N., Maji, D., Chanotiya, C. S., and Kalra, A. (2012). 1-Aminocyclopropane-1-carboxylic acid (ACC) deaminase-containing rhizobacteria protect Ocimum sanctum plants during waterlogging stress via reduced ethylene generation. Plant Physiol. Biochem. 58, 227–235. doi: 10.1016/j.plaphy.2012.07.008
Bates, L. S., Waldern, R. O., and Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207.
Bharti, N., Pandey, S. S., Barnawal, D., Patel, V. K., and Kalra, A. (2016). Plant growth promoting rhizobacteria Dietzianatrono limnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 6:34768. doi: 10.1038/srep34768
Bokhari, A., Essack, M., Lafi, F. F., Andres-Barrao, C., Jalal, R., Alamoudi, S., et al. (2019). Bioprospecting desert plant Bacillus endophytic strains for their potential to enhance plant stress tolerance. Sci. Rep. 9:18154. doi: 10.1038/s41598-019-54685-y
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Campbell, S. A., and Close, T. J. (1997). Dehydrins: genes, proteins and association with phenotypic traits. New Phytol. 137, 61–74.
Chandlee, J. M., and Scandalios, J. G. (1984). Analysis of variants affecting the catalase developmental program in maize scutellum. Theor. Appl. Genet. 69, 71–77. doi: 10.1007/bf00262543
Dimkpa, C., Weinand, T., and Asch, F. (2009). Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 32, 1682–1694. doi: 10.1111/j.1365-3040.2009.02028.x
Dubois, M., Gilles, K. A., Hamilton, J. K., and Rebers, P. T. (1956). Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356. doi: 10.1021/ac60111a017
Egamberdieva, D., Wirth, S., Kimura, S. D. B., Mishra, J., and Arora, N. K. (2019). Salt-tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 10:2791. doi: 10.3389/fmicb.2019.02791
Farooq, M., Hussain, M., Usman, M., Farooq, S., Alghamdi, S. S., and Siddique, K. H. M. (2018). Impact of abiotic stresses on grain composition and quality in food legumes. J. Agric. Food Chem. 66, 8887–8897. doi: 10.1021/acs.jafc.8b02924
Forti, C., Shankar, A., Singh, A., Balestrazzi, A., Prasad, V., and Macovei, A. (2020). Hydropriming and biopriming improve Medicago truncatula seed germination and upregulate DNA repair and antioxidant genes. Genes 11, 1–15. doi: 10.3390/genes11030242
Garg, N., and Manchanda, G. (2009). Role of arbuscular mycorrhizae in the alleviation of ionic, osmotic and oxidative stresses induced by salinity in Cajanus cajan (L.) Millsp. (pigeon-pea). J. Agron. Crop Sci. 195, 110–123. doi: 10.1111/j.1439-037X.2008.00349.x
Gonzalez, L., and Gonzalez-Villar, M. (2003). “Determination of relative water content” in Handbook of plant ecophysiology techniques. ed. M. J. Reigosu (Dordrecht: Kluwer Academic), 207–212.
Gupta, S., and Pandey, S. (2019). ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean (Phaseolus vulgaris) plants. Front. Microbiol. 10:1506. doi: 10.3389/fmicb.2019.01506
Habib, S. H., Kausar, H., and Saud, H. M. (2016). Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. Biomed. Res. Int. 2016, 1–10. doi: 10.1155/2016/6284547
Heath, R. L., and Packer, L. (1968). Photoperoxidation in isolated chloroplast I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125, 189–198. doi: 10.1016/0003-9861(68)90654-1
Ilanumaran, G., and Smith, D. L. (2017). Plant growth promoting rhizobacteria in amelioration of salinity stress: a systems biology perspective. Front. Plant Sci. 8:1768. doi: 10.3389/fpls.2017.01768
Joshi, S., Jaggi, V., Gangola, S., Singh, A., Sah, V. K., and Sahgal, M. (2021). Contrasting rhizospher bacterial communities of healthy and wilted Dalbergia sissoo Roxb forests. Rhizosphere 17:100295. doi: 10.1016/j.rhisph.2020.100295
Kang, S. M., Khan, A. L., Waqas, M., You, Y. H., Kim, J. H., Kim, J. G., et al. (2014). Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant Interact. 9, 673–682. doi: 10.1080/17429145.2014.894587
Kar, M., and Mishra, D. (1976). Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 57, 315–319. doi: 10.1104/pp.57.2.315
Khan, N. A., Shabina, S., Masood, A., Nazar, R., and Iqbal, N. (2010). Application of salicylic acid increases contents of nutrients and antioxidative metabolism in mungbean and alleviates adverse effects of salinity stress. Int. J. Plant Biol. 1, 1–8. doi: 10.4081/pb.2010.e1
Kibret, M., Devkota, K., Bakrim, W. B., Ezzariai, A., Terefe, H., Karouach, F., et al. (2024). Plant growth promoting rhizobacteria mitigate drought and salinity stresses, and improve the physiological and agronomic performances in crops: A systematic review. CABI Rev. 19:1. doi: 10.1079/cabireviews.2024.0025
Kumawat, K. C., Nagpal, S., and Sharma, P. (2022). Potential of plant growth-promoting rhizobacteria-plant interactions in mitigating salt stress for sustainable agriculture: a review. Pedosphere 32, 223–245. doi: 10.1016/S1002-0160(21)60070-X
Kumawat, K. C., Sharma, B., Nagpal, S., Kumar, A., Tiwari, S., and Nair, R. M. (2023). Plant growth-promoting rhizobacteria: salt stress alleviators to improve crop productivity for sustainable agriculture development. Front. Plant Sci. 13:1101862. doi: 10.3389/fpls.2022.1101862
Mckee, G. W. (1964). A coefficient for computing leaf area in hybrid corn. J. Agron. 56, 240–241. doi: 10.2134/agronj1964.00021962005600020038x
Mukhtar, T., Rehman, S., Smith, D., Sultan, T., Seleiman, M. F., Alsadon, A. A., et al. (2020). Mitigation of heat stress in Solanum lycopersicum L. by ACC-deaminase and exopolysaccharide producing Bacillus cereus: effects on biochemical profiling. Sustain. For. 12:2159. doi: 10.3390/su12062159
Nadeem, S. M., Ahmad, M., Zahir, Z. A., Javaid, A., and Ashraf, M. (2014). The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 32, 429–448. doi: 10.1016/j.biotechadv.2013.12.005
Nakano, Y., and Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22, 867–880. doi: 10.1093/oxfordjournals.pcp.a076232
Naseem, H., and Bano, A. (2014). Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J. Plant Interact. 9, 689–701. doi: 10.1080/17429145.2014.902125
Numan, M., Bashir, S., Khan, Y., Mumtaz, R., Shinwari, Z. K., Khan, A. L., et al. (2018). Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: a review. Microbiol. Res. 209, 21–32. doi: 10.1016/j.micres.2018.02.003
Orhan, F. (2016). Alleviation of salt stress by halotolerant and halophillic plant growth promoting bacteria in wheat (Triticum aestivum). Braz. J. Microbiol. 47, 621–627. doi: 10.1016/j.bjm.2016.04.001
Parti, R. S., Deep, V., and Gupta, S. K. (2003). Effect of salinity on lipid components of mustard seeds (Brassica juncea L.). Plant Foods Hum. Nutr. 58, 1–10. doi: 10.1023/B:QUAL.0000041141.94233.b9
Qurashi, A. W., and Sabri, A. N. (2013). Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. Braz. J. Microbiol. 43, 1183–1191. doi: 10.1590/S1517-838220120003000046
Sarma, B. K., Yadav, S. K., Singh, D. P., and Singh, H. B. (2012). “Rhizobacteria mediated induced systemic tolerance in plants: prospects for abiotic stress management” in Bacteria in agrobiology: Stress management (Berlin, Heidelberg: Springer), 225–238.
Shanker, K., Parihar, S. K. S., Kuldeep,, Dubey, K. K., and Kumar, A. (2011). Relative salt tolerance of Indian mustard (Brassica juncea) genotypes in relation to germination, growth and seed yield. J. Oilseed Brassica 2, 76–82.
Sharma, P., Sardana, V., and Banga, S. S. (2013). Salt tolerance of Indian mustard (Brassica juncea) at germination and early seedling growth. Environ. Exp. Biol. 11, 39–46.
Singh, A., and Panda, S. N. (2012). Effect of saline irrigation water on mustard (Brassica juncea) crop yield and soil salinity in a semi-arid area of North India. Expl. Agricult. 48, 99–110. doi: 10.1017/S0014479711000780
Singh, A., and Prasad, V. (2020). Assessment of salinity in the field soils of Azamgarh and its relation to their physico-chemical properties. Int. J. Plant Environ. 6, 137–145. doi: 10.18811/ijpen.v6i02.07
Slimani, A., Raklami, A., Benmrid, B., Oufdou, K., and Meddich, A. (2023). Salt tolerant plant growth promoting rhizobacteria mitigate salinity in barley by improving photosynthetic capacity, antioxidant activity and soil fertility. Biologia 78, 3367–3379. doi: 10.1007/s11756-023-01541-0
Yang, J., Kloepper, J. W., and Ryu, C. M. (2009). Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 14, 1–4. doi: 10.1016/j.tplants.2008.10.004
Keywords: Bacillus flexus , biopriming, mustard, plant growth promoting bacteria, rhizosphere, salinity stress
Citation: Singh A and Prasad V (2025) Mitigation of salinity induced negative impacts by salt tolerant plant growth promoting rhizobacteria Bacillus flexus in mustard (Brassica juncea L.). Front. Microbiol. 16:1638366. doi: 10.3389/fmicb.2025.1638366
Edited by:
Durgesh K. Jaiswal, Graphic Era University, IndiaReviewed by:
Vivek Kumar, Udit Narayan Post Graduate College, IndiaGarima Gupta, Shri Ramswaroop Memorial University, India
Copyright © 2025 Singh and Prasad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anjali Singh, c2luZ2guYW5qYWxpMjQxQGdtYWlsLmNvbQ==
 Anjali Singh
Anjali Singh Vishal Prasad
Vishal Prasad
