- People's Hospital of Xinjiang Uygur Autonomous Region, Urumqi, China
Objective: Metabolic syndrome is an important risk factor for calcium oxalate stone, yet the underlying mechanism remain unclear. Gut microbiota is involved in human metabolic processes and is associated with both metabolic syndrome and calcium oxalate stone formation.
Methods: In this study, 100 subjects were divided into four groups: calcium oxalate stone with metabolic syndrome (Group A), metabolic syndrome only (Group B), calcium oxalate stone only (Group C), and healthy controls (Group D), with 25 cases in each group. Gut microbiota composition and function were analyzed using 16S rRNA gene sequencing. Microbiota diversity, species differences, and metabolic function changes were assessed by combining clinical parameters and metabolic pathway (KEGG) annotation.
Results: The α diversity in Group A was significantly lower than in the other three groups (Shannon index, P < 0.05), and β diversity analysis revealed significant differences in bacterial community structure among all four groups (ANOSIM, P < 0.05). In Group A, short-chain fatty acid (SCFA)-producing probiotics (e.g., Faecalibacterium, Faecalibacillus, Prevotella) were reduced, while pro-inflammatory bacteria (e.g., Eggerthella and Anaerobacteriaceae) were enriched. RDA correlation analysis indicated that Faecalibacterium is negatively correlated with blood glucose levels, Faecalibacterium and Roseburia are positively correlated with urinary pH. KEGG analysis showed that the bisphenol degradation pathway was reduced (logFC = −1.45, P = 0.027) and the retinol metabolism pathway was enriched (logFC = 0.928, P = 0.006) in Group A compared to Group B.
Conclusion: Patients with calcium oxalate stone and metabolic syndrome exhibit a “double imbalance” in gut microbiota: on the one hand, the reduced diversity of the microbiota and the decrease of SCFAs-producing microbiota weakened the metabolic protective effect of the gut microbiota; on the other hand, the enrichment of pro-inflammatory and pathogenic bacteria exacerbated metabolic disorders and inflammatory reactions. The present study reveals that gut microbiota play a role in the mechanism of metabolic syndrome promoting calcium oxalate stone formation, and these findings provide a theoretical basis for the use of probiotics to prevent calcium oxalate stone.
Introduction
The prevalence and recurrence rate of urinary stone are extremely high, with a prevalence rate of about 5.6%−11.1% in China, and the incidence rate is increasing year by year. Most patients need repeated surgery, causing serious health and economic burden (Tan et al., 2023). The etiologic mechanism of primary calcium oxalate stone is still unclear, and their formation is regulated by multiple factors such as genetics, metabolism and environment (Shastri et al., 2023). Currently, calcium oxalate stone is considered to be a systemic metabolic disease, and Metabolic syndrome (MetS) is closely associated with it (Liu et al., 2024). The clustering of MetS traits is associated with greater kidney stone disease severity, with patients exhibiting all four characteristics facing an 1.8 fold higher risk of recurrent or multiple stones than those without MetS (Kohjimoto et al., 2013). However, there is no clear mechanism to explain the promotion of stone by MetS. Gut microbiota are involved in stone formation through oxalate metabolism and immune regulation. Oxalobacter formigenes (Oxf) degrades oxalate using key enzymes such as formyl-CoA transferase and oxalyl-CoA decarboxylase, but it is not the only stone - related bacterial species (Ticinesi et al., 2019). Patients with calcium oxalate stone exhibit abnormal microbiota diversity. Compared with healthy individuals, the relative abundances of harmful bacteria such as the genus Bacteroides and the genus Escherichia - Shigella increase, while those of probiotics such as Lactobacillus, Bifidobacterium, Prevotella, and Faecalibacterium decrease. These microbiota affect the progression of kidney stone through mechanisms such as oxalate metabolism, lipid regulation, and inflammatory responses (Yuan et al., 2023; Millán Rodríguez et al., 2020). Patients with MetS also exhibit reduced diversity and significant structural alterations in their gut microbiota. This dysbiosis impacts host metabolism, immunity, and inflammation, creating a vicious “microbiota-metabolism” cycle (Nie et al., 2024). Gut microbiota is involved in the development of stone and metabolic syndrome by influencing host metabolic status, immune function and inflammation, and metabolic syndrome is also closely related to stone and gut microbiota disorders. Based on this relationship, we analyzed the gut microbiota characteristics of calcium oxalate stone patients with metabolic syndrome by 16S rRNA sequencing, exploring the metabolic syndrome promoting oxalate calcium stone potential mechanisms.
Materials and methods
Study subjects
The study was approved by the Ethics Committee of Xinjiang Uygur Autonomous Region People's Hospital (Ethics No. KY20240130157). A total of 100 research subjects were enrolled in this study from February 2024 to December 2024, and all subjects signed an informed consent form. They were divided into four groups, calcium oxalate stone combined with metabolic syndrome group (Group A), metabolic syndrome alone group (Group B), calcium oxalate stone alone group (Group C), and healthy control group (Group D), with 25 people in each group.
Inclusion criteria
Patients should meet the following criteria: at least 18 years old; diagnosed with upper urinary tract stone by urinary ultrasound, KUB, or CT; underwent surgical stone removal and stone composition analysis confirmed that the calcium oxalate content was ≥80%; MetS group met ≥3 of the following criteria (Peterseim et al., 2024): abdominal obesity (male waist circumference ≥90 cm, female waist circumference ≥85 cm), fasting blood glucose ≥6.1 mmol/L or a history of diabetes, blood pressure ≥130/85 mmHg or a history of hypertension treatment, triglycerides ≥1.7 mmol/L, male high-density lipoprotein cholesterol (HDL-C) < 1.04 mmol/L.
Exclusion criteria
Exclusion criteria were as follows: pregnant women, patients with malignant tumors, patients with organic diseases of the digestive system (such as inflammatory bowel disease, irritable bowel syndrome, infections, tumors, or a history of bowel resection), patients who have used antibiotics or microecological agents in the last month, patients with secondary stone (due to anatomical anomalies of the urinary tract, metabolic disorders, or hereditary stone disease), and patients who have used calcium supplements, vitamins C or D, and metabolism-affecting drugs (such as corticosteroids, acetazolamide, etc.) for a long time.
Clinical data collection
Using our hospital's electronic medical record system, the following data were collected from the subjects: gender, age, ethnicity, height, weight, body mass index (BMI), waist circumference, history of diagnosed diabetes mellitus and fasting blood glucose, history of diagnosed hypertension, triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total cholesterol, serum total protein, albumin, serum sodium, potassium, calcium, magnesium, blood creatinine, urea nitrogen, uric acid, urine specific gravity, urine pH, urine leukocyte, urine protein, urine glucose, urine vitamin C, urine crystals, and stone composition analysis results.
Specimen collection and preservation
Subjects took an appropriate amount of fecal specimen (5 ml) in a sterile collection tube using a sterile fecal sampler. All samples were collected at the beginning of the admission period (before antibiotic administration) and aseptic handling was used throughout. The specimens were stored in a refrigerator at −80°C immediately after collection.
Specimen processing and bioinformatics analysis
Frozen samples were thawed and 0.2–0.5 g of fecal sample was taken and mechanically lysed using a Tissuelyser-48 Tissue Mill (60 Hz). Genomic DNA was extracted using OMEGA Soil DNA Kit, 0.8% agarose electrophoresis was used to assess integrity, and Nanodrop NC2000 was used to detect purity. Then the V3-V4 region (338F/806R primer) was amplified using high-fidelity enzyme (NEB Q5). Reaction program: pre-denaturation at 98 °C for 5 min; 25 cycles (98 °C for 30 s/53 °C for 30 s/72 °C for 45 s); final extension at 72 °C for 5 min. Products were verified by 2% electrophoresis and then purified by magnetic beads. After quantification and standardization, the library was constructed by TruSeq kit: end repair → addition of A-tail → junction ligation → magnetic bead screening → PCR enrichment. Single-stranded templates were prepared by NaOH denaturation after passing Agilent 2100 QC. Bipartite sequencing was carried out on MiSeq/NovaSeq platforms (300/500 cycles), and the recommended length of the target fragment was controlled in the 200–450 bp range. The target fragment length was recommended to be in the range of 200–450 bp. Raw data were preprocessed by QIIME2 (Bolyen et al., 2019) and OTUs were clustered using DADA2 (Callahan et al., 2016). Alpha diversity was assessed based on OTU distribution using Chao1 index, Shannon index, and Simpson index. Analysis of dimensionality was performed with PCoA and NMDS downscaling, ANOSIM analysis of inter- (intra-) group differences, and LEfSe multivariate statistical analysis of species differing between groups. LEfSe analysis (Segata et al., 2011) was applied to identify between-group differing species and attempts were made to find marker species. Redundancy analysis (RDA) methods were used to analyze potential associations between gut microbiota and relevant clinical parameters. KEGG metabolic pathway annotation based on PICRUSt2 (Douglas et al., 2020) algorithm to predict the metabolic function of gut microbiota and identify differential functional pathways, and use RStudio (Huber et al., 2015) and Python (Cock et al., 2009) to Draw statistical figures.
Statistical methods
For the description of baseline characteristics, the median (quartiles) was used for continuous variables, while categorical variables were expressed as frequencies and percentages. For comparisons of continuous variables, the Student's t-test was used if the data conformed to a normal distribution, and the Mann-Whitney U-test was used if the data did not conform to a normal distribution. For comparisons of categorical variables, the χ2 test was used. All statistical analyses were performed using SPSS software version 26.0 and R language version 4.3.2, with a two-tailed P-value < 0.05 set as the threshold for statistical significance.
Result
Clinical characteristics of the four groups
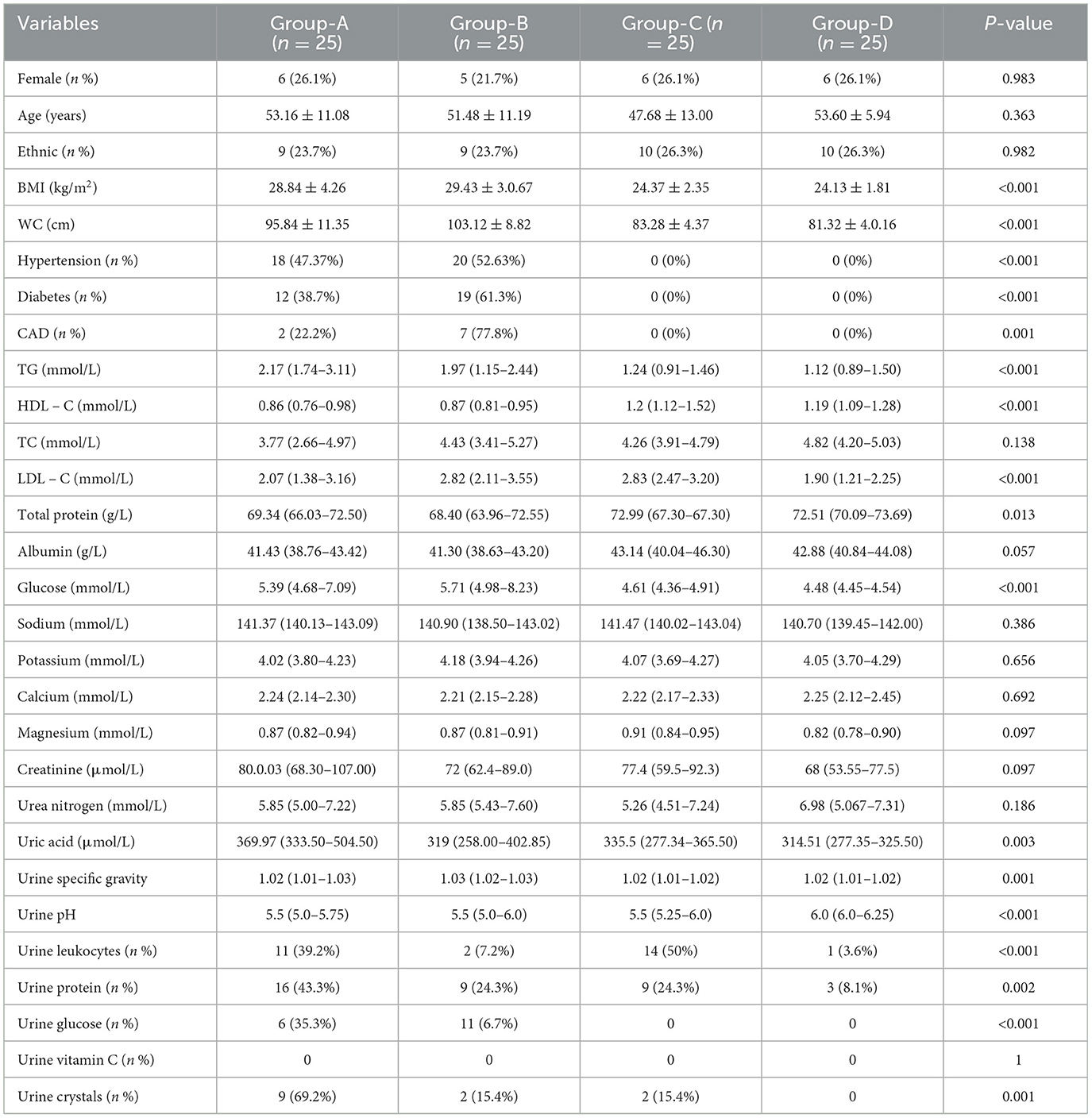
There were no statistically significant differences among the four groups in terms of gender, age, and ethnicity (P > 0.05). The weights, BMIs, waist circumferences, prevalences of diabetes mellitus, hypertension, hyperlipidemia, and coronary heart disease in Groups A and B were significantly higher than those in Groups C and D (P < 0.05). The mean value of total serum protein in Groups A and B was significantly lower than that in Groups C and D (P < 0.05). The serum uric acid level (369.97 μmol/L) in Groups A and B was significantly lower than that in Groups C and D (P < 0.05). The serum uric acid levels differed significantly among the four groups (P = 0.003), with a higher level in Group A. Regarding the routine urine examination, the urine pH value in Group D was 6 (6.0–6.25), which was higher than that in the other groups, and the urine specific gravity was 1.016 (1.012–1.019), which was lower than that in the other groups (P < 0.05). The numbers of positive urine leukocytes and erythrocytes in Groups A and C were higher than those in Groups B and D (P < 0.05). The proportion of urinary crystals (69.2%) in Group A was significantly higher than that in Groups B and D (P = 0.001) (Table 1).
Differences in bacterial diversity between groups
The 16S rRNA sequencing data were clustered into OTUs (operational taxonomic units) according to 97% similarity, and the Venn diagrams showed the unique and shared status of each group: A (n = 7,941), B (n = 12,199), C (n = 12,241), and D (n = 15,767), the number of overlapping OTUs among the four groups is (n = 665) (Figure 1a).
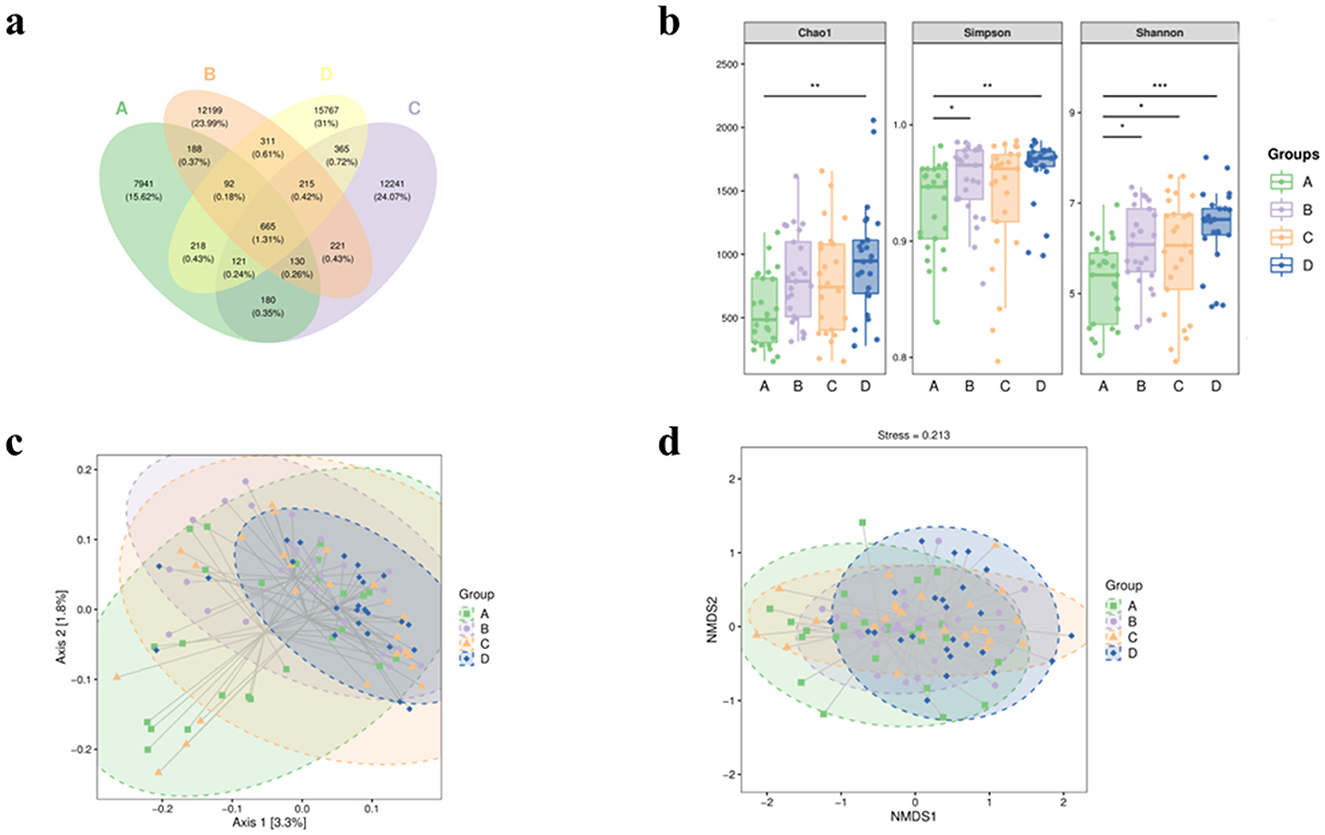
Figure 1. The α diversity and β diversity indices of fecal intestinal microbiota in four groups of patients. (a) Venn plot of OTU. (b) Block diagram of grouping based on Chao1, Shannon and Simpson's α diversity indices. Bacterial β-diversity map based on Jaccard distance (c) PCoA; (d) NMDS. A represents the group of calcium oxalate stone combined with metabolic syndrome, B the metabolic syndrome alone group, C the calcium oxalate stone alone group, and D the healthy control group. *p < 0.05, **p < 0.01, ***p < 0.001.
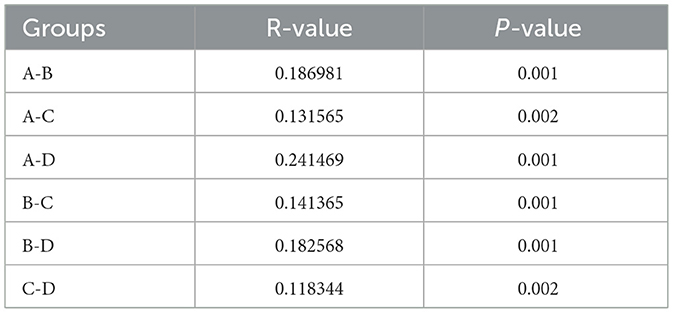
In terms of Chao1 index, which reflects species richness, statistically significant differences were found between Groups A and D (P = 0.0017), while there were no significant differences among the other groups. The Shannon index reflecting diversity was found to be significantly different between Groups A and B (P = 0.04), A and C (P = 0.048), and A and D (P = 0.00027). On the contrary, no significant differences were observed in the comparison between the other groups. Similarly, Simpson's index showed significant differences between Group A and Group B (P = 0.04) and Group A and Group D (P = 0.0016), but not between Group A and Group C (P = 0.24). Comparisons between the other groups remained insignificant (Figure 1b). β-diversity analysis was performed using the Jaccard distance matrix, visualized by PCoA and NMDS downscaling (Figure 1c), which showed that the composition of the microbiota in Group A was significantly different from that of the other groups. The ANOSIM test confirmed the differences between the groups (R > 0, P < 0.05) (Table 2).
Analysis of species differences
Species differences were analyzed by the LEfSe method with the LDA effect size threshold set at 3.0. Compared to Group A, Group B had a higher abundance of taxa primarily at the family Peptostreptococcaceae, Barnesiellaceae, etc., and genus Gemmiger_A, Faecalibacillus, etc. (Figure 2a).
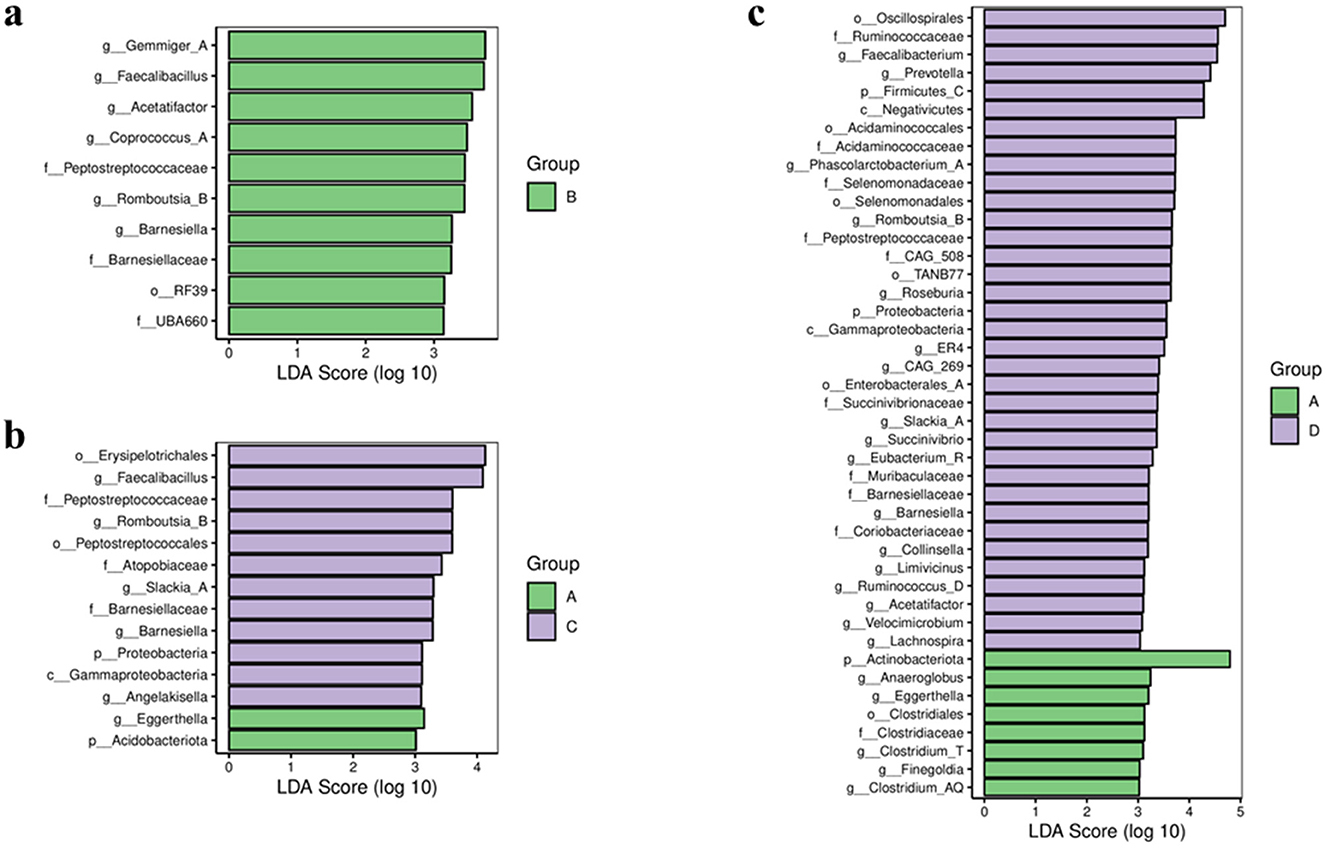
Figure 2. Bar chart of LDA effect sizes for differential species between groups. LDA scores (log 10) >3 and p < 0.05 are listed. (a) Group A vs. group B. (b) Group A vs. group C. (c) Group A vs. group D. A represents the group of calcium oxalate stone combined with metabolic syndrome, B the metabolic syndrome alone group, C the calcium oxalate stone alone group, and D the healthy control group.
Compared to Group A, Group C showed distinct taxonomic profiles. Group A was enriched in the Acidobacteriota phylum and Eggerthella genus. In contrast, Group C had higher relative abundances of the Proteobacteria phylum; Gammaproteobacteria class; the order Peptostreptococcales and Erysipelotrichales; family including Peptostreptococcaceae and Atopobiaceae, etc.; and genus such as Romboutsia_B, Barnesiella, Angelakisella, etc. (Figure 2b).
The comparison between Group A and Group D further revealed significant differences. Group A was characterized by a higher abundance of the Actinobacteria phylum, the order Clostridiales, and genus including Clostridium_T, Eggerthella, Anaerococcus, etc. Conversely, Group D was enriched across multiple taxonomic levels, comprising the Firmicutes_C and Proteobacteria phyla, among others; the Negativicutes and Gammaproteobacteria classes, etc.; the order Selenomonadales, Oscillospirales, Acidaminococcales, etc.; family such as Ruminococcaceae, Veillonellaceae, Selenomonadaceae, etc.; and genus including Faecalibacterium, Prevotella, Roseburia, etc. (Figure 2c).
Correlation analysis of microbiota alterations with clinical parameters
Redundancy Analysis (RDA) was used to examine the relationship between microbial communities and clinical parameters by constrained ordination. At the genus level, we screened the top 30 dominant genera in terms of relative abundance in all fecal samples for correlation analysis with the collected clinical data and plotted correlation heatmaps.
The results showed that Fusicatenibacter and Blautia-A are positively correlated with blood glucose, while Faecalibacterium is negatively correlated with blood glucose. Faecalibacillus is positively correlated with low-density lipoprotein and high-density lipoprotein. Bacteroides-H is negatively correlated with blood calcium ions and positively correlated with hypertension. Roseburia and Faecalibacterium are positively correlated with urine pH, while Escherichia is negatively correlated with urine pH. Collinsella is negatively correlated with urinary crystals. The genus Fusicatenibacter is positively correlated with magnesium ions and negatively correlated with urinary glucose (Figure 3).
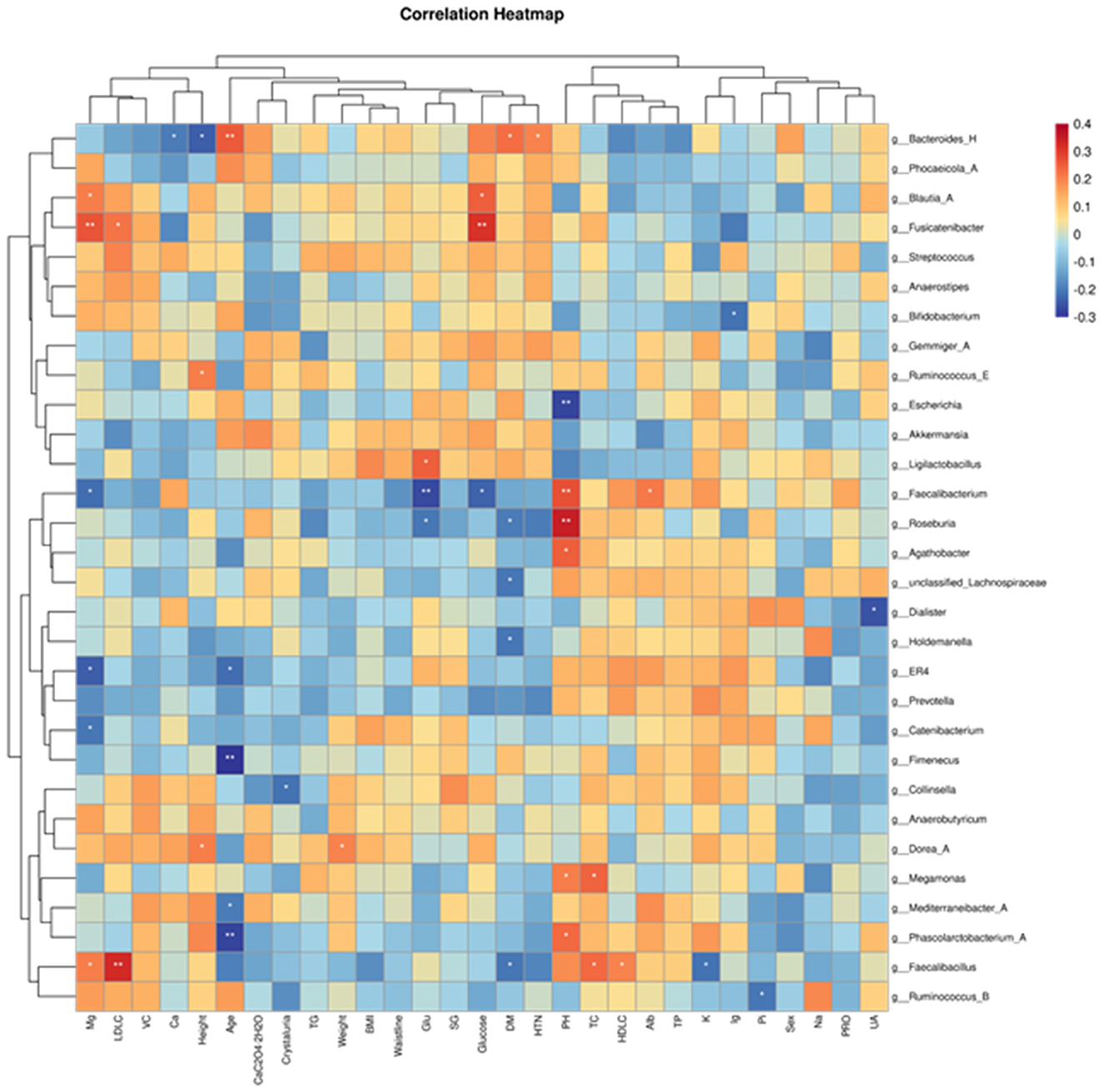
Figure 3. Heatmaps of correlations between gut microbiota abundance and relevant clinical characteristics. The horizontal coordinates are the clinical parameters and the vertical coordinates are the 30 most abundant genera.
Functional prediction of the gut microbiota based on the KEGG database
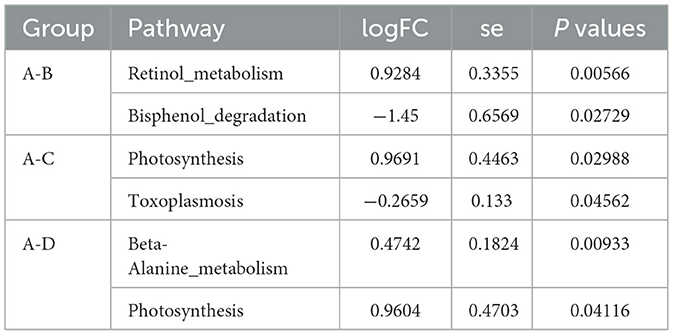
After obtaining the abundance data of metabolic pathways through KEGG functional annotation, we employed metagenomeSeq analysis to identify the functional pathways that were significantly different between groups. Compared with Group B, the retinol metabolism pathway is enriched in Group A, while the bisphenol degradation pathway is reduced. Compared with Group C, the photosynthesis - related pathways are enriched in Group A, and the toxoplasmosis-related pathways are reduced. Compared with Group D, the beta-alanine metabolism and photosynthesis pathways are enriched in Group A (Table 3).
Discussion
The formation of primary calcium oxalate stone is influenced by a variety of factors, including genes, metabolism, diet, lifestyle, and the environment, and there is a lack of an exact mechanism to explain the formation process (Shastri et al., 2023). Therefore, there are currently no effective preventive or pharmacological measures against the formation and recurrence of calcium oxalate stone. Several studies have shown that metabolic abnormalities play an important role in the formation of calcium oxalate stone, and metabolic syndrome, as a collection of metabolic abnormalities in human metabolism, is considered to be an important risk factor for the formation and recurrence of calcium oxalate stone (Kohjimoto et al., 2013). The gut, a critical metabolic organ, is predominantly regulated by the intestinal microbiota. Recent studies have established robust associations between gut microbiota composition and both metabolic syndrome and nephrolithiasis (Ezenabor et al., 2024; Hussain et al., 2024). While metabolic syndrome may enhance calcium oxalate stone formation via the gut microbiota, direct evidence is scarce. To address this, our study profiles the gut microbiota of patients who have both calcium oxalate stones and metabolic syndrome.
Our analysis revealed distinct gut microbiota characteristics in patients with calcium oxalate stone comorbid with metabolic syndrome (CaOx-MetS). Specifically, the CaOx-MetS group exhibited significantly lower α diversity compared to the metabolic syndrome-only (MetS-only), stone-only, and healthy control groups. β diversity analysis further demonstrated a marked divergence in microbial community structure between the CaOx-MetS group and the other three cohorts. This reduction in microbial diversity was accompanied by a diminished abundance of beneficial bacteria, which may compromise critical metabolic functions of the gut microbiota. Such dysbiosis could impair systemic resistance to pathogenic stimuli, thereby elevating the overall risk of disease pathogenesis (Pickard et al., 2017). In the context of nephrolithiasis, impaired metabolic functions—particularly in oxalate degradation and short-chain fatty acid (SCFA) production—may directly exacerbate stone formation. SCFAs exert anti-lithogenic effects through multiple mechanisms, such as modulating intestinal pH, inhibiting oxalate absorption, and alleviating inflammatory responses (Liu et al., 2021).
LEfSe analysis revealed significant reductions in the relative abundances of Gemmiger_A, Faecalibacillus, Acetatifactor, and Coprococcus_A in the calcium oxalate stone with metabolic syndrome (CaOx-MetS) group compared to the metabolic syndrome-only group. Duan et al. (2021) observed that obese patients exhibit decreased gut microbiota diversity, with the genus Gemmiger showing significantly lower abundance in obese individuals than in healthy controls. Faecalibacillus is a genus of exclusively anaerobic bacteria, and less research has been done on Faecalibacillus. Faecalibacillus has good probiotic potential, producing SCFA, such as butyric acid, through fermentation of dietary fibers, a metabolite that is essential for maintaining intestinal barrier integrity, modulating immunity, and suppressing inflammation. Experiments in mice have found that Faecalibacillus isolates provide better relief from constipation (Huang et al., 2024). Another study found a relative decrease in the abundance of Faecalibacillus in a variety of malignant tumors such as gastrointestinal tumors, lung cancer, and breast cancer (Kulecka et al., 2024). The genus Acetatifactor exhibits bile salt hydrolase (BSH) activity, converting deoxycholic acid to lithocholic acid. This metabolite activates the TGR5 signaling pathway, stimulating intestinal L-cells to secrete glucagon-like peptide-1 (GLP-1), which promotes adipose tissue browning and enhances hepatic insulin signaling and glucose metabolism (Pathak et al., 2018). Additionally, Coprococcus_A generates butyrate, a key SCFA member essential for maintaining gut homeostasis, regulating inflammation, and reinforcing intestinal barrier integrity (Wang et al., 2024). It can be observed that in patients with calcium oxalate stone complicated by metabolic syndrome, the abundance of probiotics is reduced. These diminished probiotics exacerbate metabolic disorders, thereby promoting the development of kidney stone in patients with metabolic syndrome.
Compared to the stone-only group, the calcium oxalate stone with metabolic syndrome (CaOx-MetS) group exhibited significant enrichment of Acidobacteriota and Eggerthella. In contrast, the stone-only group demonstrated higher abundances of Erysipelotrichales, Faecalibacillus, and Peptostreptococcaceae. To date, no definitive evidence links Acidobacteriota directly to oxalate or short-chain fatty acid (SCFA) metabolism or urolithiasis. However, studies have reported elevated Acidobacteriota abundance in patients with neurodegenerative disorders such as Alzheimer's disease and amyotrophic lateral sclerosis (ALS). This enrichment of potentially pathogenic taxa may impair intestinal barrier integrity and trigger pro-inflammatory responses, thereby contributing to disease progression (Fontdevila et al., 2024; Heravi et al., 2023). Eggerthella, a Gram-positive bacillus, is frequently overrepresented in conditions including gastrointestinal inflammation and neurological diseases (Nikolova et al., 2021). Its pathogenic potential may arise from mechanisms such as increased intestinal permeability and systemic oxidative stress, which could indirectly exacerbate both metabolic syndrome and stone formation. Notably, Peptostreptococcaceae, a SCFA-producing family, has been associated with reduced diabetes risk and confers protective effects against metabolic syndrome (Chen et al., 2021). The observed reduction in SCFA-producing taxa (e.g., Faecalibacillus, Peptostreptococcaceae) in the CaOx-MetS group compared to the stone-only group underscores a dual depletion of beneficial microbiota and exacerbated microbial dysbiosis in comorbid patients.
When compared with healthy controls, the calcium oxalate stone combined with MetS group exhibited a greater enrichment of harmful bacteria and a decrease in health-related microbiota. Specifically, the abundance of Anaeroglobus, Eggerthella, and Finegoldia increased, whereas that of Faecalibacterium, Prevotella, Roseburia, and Acetatifactor relatively decreased. Previous studies indicate that Faecalibacterium abundance is markedly decreased in stone patients and is inversely correlated with stone formation, likely via its roles in oxalate metabolism and SCFA production (Ticinesi et al., 2018). Meta-analysis showed that Prevotella was significantly less abundant in patients with stone than in the healthy population, important protective bacteria (Yuan et al., 2023). In addition Roseburia and Acetatifactor, both SCFA-producing bacteria, calcium oxalate stone have a protective effect on the gut microbiota (Liu et al., 2022).
In this study, Fusicatenaibacter and Blautia_A abundance was negatively correlated with blood glucose levels, Faecalibacterium and Roseburia abundance was positively correlated with urinary PH levels, in addition, Collinsella was negatively correlated with urinary crystallization. It can be seen that the dysbiosis occurring in the group of calcium oxalate stone combined with metabolic syndrome may contribute to the development of calcium oxalate stone by affecting the metabolism and the physicochemical properties of the urine of the patients and, consequently, to the development of calcium oxalate stone. The exact mechanism needs to be confirmed by further research.
Furthermore, comparative analysis of metabolic pathway activity across groups revealed that the calcium oxalate stone with metabolic syndrome (CaOx-MetS) group exhibited significantly reduced bisphenol degradation pathway activity compared to the metabolic syndrome-only group (P = 0.0273), alongside elevated retinol metabolism pathway activity (P = 0.0057). Bisphenol A (BPA), a prevalent industrial chemical, is extensively utilized in the production of polycarbonate plastics and epoxy resins. As an endocrine-disrupting compound, BPA interferes with multisystem physiological functions, triggering diverse pathological effects. Its lipophilic nature facilitates accumulation in adipose tissue, where it disrupts adipokine secretion and exacerbates obesity and insulin resistance. Additionally, BPA induces cellular apoptosis via caspase-dependent mitochondrial damage pathways and impairs insulin signaling by modulating ion channel activity, particularly potassium channels (Akash et al., 2020). Enhanced bisphenol degradation capacity is hypothesized to mitigate BPA-driven metabolic dysregulation. Critically, BPA exposure may provoke renal tubular injury, impairing reabsorption and secretory functions. BPA-induced oxidative stress further damages tubular epithelial cells, amplifying lithogenic susceptibility (Jacobson et al., 2020). The progressive decline in bisphenol degradation capacity observed in comorbid patients suggests heightened sensitivity to environmental xenobiotics. Retinol (vitamin A), a fat-soluble vitamin essential for human health, is absorbed in the small intestine and metabolized in the liver to yield retinoic acid (RA), a pivotal signaling molecule (Rastinejad, 2022). All-trans retinoic acid (ATRA), a specific RA isomer, serves as a renal protective factor. It attenuates inflammatory cytokine expression, protects proximal tubular epithelial cells from hypoxic injury, and promotes tubular epithelial differentiation, thereby exerting renoprotective and reparative effects in chronic kidney disease (Nakamura et al., 2019). Our findings align with a pediatric nephrolithiasis metabolomics study (Wen et al., 2021) that identified upregulated retinol metabolism as a compensatory mechanism against crystal-induced tubular damage. Collectively, these results suggest that the depletion of protective metabolic pathways (e.g., bisphenol degradation) and compensatory activation of retinol metabolism may synergistically contribute to the heightened risk of stone formation in metabolic syndrome patients.
The following limitations exist in this study: The sample size of this study is limited. The cross-sectional design makes it difficult to establish a causal relationship, and longitudinal cohort studies, probiotic interventions, and fecal microbial transplantation (FMT) animal experiments are needed to verify the clinical value of the differential microbiota described above. The difference between the numbers of diabetes and obesity in the calcium oxalate stone combined with metabolic syndrome group and the metabolic syndrome group in this study may lead to bias, and further studies need to be conducted in the later stage by matching each metabolic syndrome composition one to one and expanding the sample size. Future studies could combine gut microbiota and urinary microbiota to analyze alterations in the microbiome at multiple sites. Gut microbiota analysis combined with metabolomics will allow in-depth analysis of the molecular mechanisms of key strains and explore therapeutic options such as probiotics, or microbiota transplantation.
Conclusion
This study reveals a “dual imbalance” in the gut microbiota of patients with calcium oxalate stone combined with metabolic syndrome, characterized by an enrichment of pro-inflammatory bacteria and a relative decrease in SCFA-producing bacteria. This dysbiosis may contribute to metabolic abnormalities by exacerbating oxidative stress and inflammation, which may contribute to stone formation. These findings provide a rationale for the development of interventional strategies targeted at the microbiota.
Data availability statement
The data presented in this study are publicly available. This data can be found here: https://www.ncbi.nlm.nih.gov/sra, accession PRJNA1273845.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Xinjiang Uygur Autonomous Region People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MB: Visualization, Data curation, Software, Conceptualization, Writing – original draft, Methodology, Investigation. XL: Resources, Project administration, Validation, Writing – review & editing, Conceptualization, Funding acquisition, Supervision. BL: Resources, Project administration, Methodology, Supervision, Validation, Software, Data curation, Writing – review & editing, Conceptualization. SW: Software, Writing – review & editing, Formal analysis, Resources, Methodology. QD: Validation, Conceptualization, Investigation, Software, Writing – review & editing, Resources. NA: Writing – original draft, Software, Data curation, Conceptualization, Investigation. ZL: Data curation, Methodology, Formal analysis, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to express our sincere gratitude to all individuals who supported and assisted us throughout this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akash, M. S. H., Sabir, S., and Rehman, K. (2020). Bisphenol A-induced metabolic disorders: from exposure to mechanism of action. Environ. Toxicol. Pharmacol. 77:103373. doi: 10.1016/j.etap.2020.103373
Bolyen, E., Rideout, J. R., Dillon, M. R., Bokulich, N. A., Abnet, C. C., Al-Ghalith, G. A., et al. (2019). Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857. doi: 10.1038/s41587-019-0209-9
Callahan, B. J., McMurdie, P. J., Rosen, M. J., Han, A. W., Johnson, A. J. A., and Holmes, S. P. (2016). DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. doi: 10.1038/nmeth.3869
Chen, Z., Radjabzadeh, D., Chen, L., Kurilshikov, A., Kavousi, M., Ahmadizar, F., et al. (2021). Association of insulin resistance and type 2 diabetes with gut microbial diversity. JAMA Netw. Open 4:e2118811. doi: 10.1001/jamanetworkopen.2021.18811
Cock, P. J. A., Antao, T., Chang, J. T., Chapman, B. A., Cox, C. J., Dalke, A., et al. (2009). Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 25, 1422–1423. doi: 10.1093/bioinformatics/btp163
Douglas, G. M., Maffei, V. J., Zaneveld, J. R., Yurgel, S. N., Brown, J. R., Taylor, C. M., et al. (2020). PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 38, 685–688. doi: 10.1038/s41587-020-0548-6
Duan, M., Wang, Y., Zhang, Q., Zou, R., Guo, M., and Zheng, H. (2021). Characteristics of gut microbiota in people with obesity. PLoS ONE 16:e0255446. doi: 10.1371/journal.pone.0255446
Ezenabor, E. H., Adeyemi, A. A., and Adeyemi, O. S. (2024). Gut microbiota and metabolic syndrome: relationships and opportunities for new therapeutic strategies. Scientifica 2024:4222083. doi: 10.1155/2024/4222083
Fontdevila, L., Povedano, M., Domínguez, R., Boada, J., Serrano, J. C., Pamplona, R., et al. (2024). Examining the complex Interplay between gut microbiota abundance and short-chain fatty acid production in amyotrophic lateral sclerosis patients shortly after onset of disease. Sci. Rep. 14:23497. doi: 10.1038/s41598-024-75083-z
Heravi, F. S., Naseri, K., and Hu, H. (2023). Gut microbiota composition in patients with neurodegenerative disorders (Parkinson's and Alzheimer's) and healthy controls: a systematic review. Nutrients 15:4365. doi: 10.3390/nu15204365
Huang, P., Dong, Q., Wang, Y., Tian, Y., Wang, S., Zhang, C., et al. (2024). Gut microbial genomes with paired isolates from China illustrate probiotic and cardiometabolic effects. Cell Genomics 4:100559. doi: 10.1016/j.xgen.2024.100559
Huber, W., Carey, V. J., Gentleman, R., Anders, S., Carlson, M., Carvalho, B. S., et al. (2015). Orchestrating high-throughput genomic analysis with Bioconductor. Nat. Methods 12, 115–121. doi: 10.1038/nmeth.3252
Hussain, B., Wu, C.-C., Tsai, H.-C., Chen, J.-S., Asif, A., Cheng, M.-C., et al. (2024). Species-level characterization of gut microbiota and their metabolic role in kidney stone formation using full-length 16S rRNA sequencing. Urolithiasis 52:115. doi: 10.1007/s00240-024-01610-2
Jacobson, M. H., Wu, Y., Liu, M., Attina, T. M., Naidu, M., Karthikraj, R., et al. (2020). Serially assessed bisphenol A and phthalate exposure and association with kidney function in children with chronic kidney disease in the US and Canada: a longitudinal cohort study. PLoS Med. 17:e1003384. doi: 10.1371/journal.pmed.1003384
Kohjimoto, Y., Sasaki, Y., Iguchi, M., Matsumura, N., Inagaki, T., and Hara, I. (2013). Association of metabolic syndrome traits and severity of kidney stones: results from a nationwide survey on urolithiasis in Japan. Am. J. Kidney Dis. 61, 923–929. doi: 10.1053/j.ajkd.2012.12.028
Kulecka, M., Czarnowski, P., Bałabas, A., Turkot, M., Kruczkowska-Tarantowicz, K., Żeber-Lubecka, N., et al. (2024). Microbial and metabolic gut profiling across seven malignancies identifies fecal faecalibacillus intestinalis and formic acid as commonly altered in cancer patients. Int. J. Mol. Sci. 25:8026. doi: 10.3390/ijms25158026
Liu, J., Tan, Y., Cheng, H., Zhang, D., Feng, W., and Peng, C. (2022). Functions of gut microbiota metabolites, current status and future perspectives. Aging Dis. 13:1106. doi: 10.14336/AD.2022.0104
Liu, M., Gao, M., Wu, J., Zhu, Z., Hu, J., Chen, H., et al. (2024). Metabolic syndrome and the risk of kidney stones: evidence from 487 860 UK biobank participants. J. Clin. Endocrinol. Metab. 110, e1211–e1219. doi: 10.1210/clinem/dgae295
Liu, Y., Jin, X., Ma, Y., Jian, Z., Wei, Z., Xiang, L., et al. (2021). Short-chain fatty acids reduced renal calcium oxalate stones by regulating the expression of intestinal oxalate transporter SLC26A6. MSystems 6:e01045-21. doi: 10.1128/mSystems.01045-21
Millán Rodríguez, F., Sabiote Rubio, L., Girón Nanne, I., Sánchez Martín, F., Emiliani, E., and Angerri Feu, O. (2020). The relationship between calcium oxalate lithiasis and chronic proinflammatory intestinal dysbiosis pattern: a prospective study. Urolithiasis 48, 321–328. doi: 10.1007/s00240-020-01181-y
Nakamura, J., Sato, Y., Kitai, Y., Wajima, S., Yamamoto, S., Oguchi, A., et al. (2019). Myofibroblasts acquire retinoic acid–producing ability during fibroblast-to-myofibroblast transition following kidney injury. Kidney Int. 95, 526–539. doi: 10.1016/j.kint.2018.10.017
Nie, P., Hu, L., Feng, X., and Xu, H. (2024). Gut microbiota disorders and metabolic syndrome: tales of a crosstalk process. Nutr. Rev. 83, 908–924. doi: 10.1093/nutrit/nuae157
Nikolova, V. L., Smith, M. R. B., Hall, L. J., Cleare, A. J., Stone, J. M., and Young, A. H. (2021). Perturbations in gut microbiota composition in psychiatric disorders. JAMA Psychiatry 78:1343. doi: 10.1001/jamapsychiatry.2021.2573
Pathak, P., Xie, C., Nichols, R. G., Ferrell, J. M., Boehme, S., Krausz, K. W., et al. (2018). Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 68, 1574–1588. doi: 10.1002/hep.29857
Peterseim, C. M., Jabbour, K., and Kamath Mulki, A. (2024). Metabolic syndrome: an updated review on diagnosis and treatment for primary care clinicians. J. Prim. Care Commun. Health 15:21501319241309168. doi: 10.1177/21501319241309168
Pickard, J. M., Zeng, M. Y., Caruso, R., and Núñez, G. (2017). Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 279, 70–89. doi: 10.1111/imr.12567
Rastinejad, F. (2022). Retinoic acid receptor structures: the journey from single domains to full-length complex. J. Mol. Endocrinol. 69, T25–T36. doi: 10.1530/JME-22-0113
Segata, N., Izard, J., Waldron, L., Gevers, D., Miropolsky, L., Garrett, W. S., et al. (2011). Metagenomic biomarker discovery and explanation. Genome Biol. 12:R60. doi: 10.1186/gb-2011-12-6-r60
Shastri, S., Patel, J., Sambandam, K. K., and Lederer, E. D. (2023). Kidney stone pathophysiology, evaluation and management: core curriculum 2023. Am. J. Kidney Dis. 82, 617–634. doi: 10.1053/j.ajkd.2023.03.017
Tan, S., Yuan, D., Su, H., Chen, W., Zhu, S., Yan, B., et al. (2023). Prevalence of urolithiasis in China: a systematic review and meta-analysis. BJU Int. 133, 34–43. doi: 10.1111/bju.16179
Ticinesi, A., Milani, C., Guerra, A., Allegri, F., Lauretani, F., Nouvenne, A., et al. (2018). Understanding the gut–kidney axis in nephrolithiasis: an analysis of the gut microbiota composition and functionality of stone formers. Gut 67, 2097–2106. doi: 10.1136/gutjnl-2017-315734
Ticinesi, A., Nouvenne, A., and Meschi, T. (2019). Gut microbiome and kidney stone disease: not just an Oxalobacter story. Kidney Int. 96, 25–27. doi: 10.1016/j.kint.2019.03.020
Wang, W., Wang, F., Li, Y., Shi, Y., Wang, X., Chen, X., et al. (2024). Distinct gut microbiota profiles in normal weight obesity and their association with cardiometabolic diseases: results from two independent cohort studies. J. Cachexia Sarcopenia Muscle 16:e13644. doi: 10.1002/jcsm.13644
Wen, J., Cao, Y., Li, Y., Zhu, F., Yuan, M., Xu, J., et al. (2021). Metabolomics analysis of the serum from children with urolithiasis using UPLC-MS. Clin. Transl. Sci. 14, 1327–1337. doi: 10.1111/cts.12984
Keywords: calcium oxalate stone, metabolic syndrome, gut microbiome, renal calculi, etiology
Citation: Batur M, Li X, Liu B, Wang S, Dong Q, Abudurexiti N and Liu Z (2025) Characterization of gut microbiome dysbiosis in calcium oxalate stone patients with comorbid metabolic syndrome. Front. Microbiol. 16:1644416. doi: 10.3389/fmicb.2025.1644416
Received: 10 June 2025; Accepted: 28 October 2025;
Published: 25 November 2025.
Edited by:
Hoh Boon-Peng, International Medical University, MalaysiaReviewed by:
Vijay Nema, National AIDS Research Institute (ICMR), IndiaVidya V. Jadhav (Patil), North Carolina Agricultural and Technical State University, United States
Copyright © 2025 Batur, Li, Liu, Wang, Dong, Abudurexiti and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xun Li, MzY5MTgwNDc0QHFxLmNvbQ==
 Maimaitiaili Batur
Maimaitiaili Batur Xun Li*
Xun Li* Nueraili Abudurexiti
Nueraili Abudurexiti Zewei Liu
Zewei Liu