- 1School of Heilongjiang River and Lake Management, Heilongjiang University, Harbin, China
- 2School of Energy & Environmental Engineering, Hebei University of Technology, Tianjin, China
- 3Department of Resources and Environment, Moutai Institute, Renhuai, Guizhou, China
Background: Polyhydroxybutyrate (PHB) production from food waste by photosynthetic bacteria (PSB) face the bottleneck of low production efficiency. Metal ions have the potential to enhance the PHB production by PSB. Thus, for the first time, this study explored the effect of Fe3+ and Mn2+ on the enhancement of PHB production from kitchen waste digestate by PSB and their enhancement mechanism.
Methods: FeCl3·6H2O and MnCl2·4H2O were the main sources of Fe3+ and Mn2+. Five Hundred milliliter sealed Schott bottles were used as the fermentation reactor. Kitchen waste digestate was diluted to soluble chemical oxygen demand (SCOD) of 2.5 g/L as substrate and inoculated with 20% (v/v) mixed PSB. Fe3+ concentrations in these reactors were 10, 20, 30, and 40 mg/L, respectively. Mn2+ concentrations in these reactors were 1, 2, 3, and 4 mg/L, respectively. The initial pH of these reactors was adjusted to 8.0 and was carried out at room temperature of 26–30°C. All reactors were placed in a light-proof experimental chamber with a light intensity of 4,000 lx.
Results: The optimal concentrations of 10 mg/L Fe3+ and 2 mg/L Mn2+ promoted PSB biomass and PHB accumulation, while excessive concentrations of metal ions inhibited them. Concentrations of PSB biomass reached 2366.3 and 2109.2 mg/L, respectively under the 10 mg/L Fe3+ and 2 mg/L Mn2+ concentrations, and PHB content reached 46.0 and 43.8%, respectively. Removal rate of SCOD and ammonia nitrogen in the kitchen waste digestate exceeded 90 and 70% under the 10 mg/L Fe3+ and 2 mg/L Mn2+ concentrations. The concentration of intracellular Fe3+ and Mn2+ that PSB adapts to growth was approximately 5.5 and 0.5 mg/L, respectively. The 10 mg/L Fe3+ and 2 mg/L Mn2+ concentrations decreased the diversity, altered the composition, and enhanced functional metabolism of microbial communities.
Conclusion: The concentration of 10 mg/L Fe3+ and 2 mg/L Mn2+ significantly enhanced PSB biomass and PHB accumulation (p < 0.05). Enhancement mechanism was to increase the relative abundance of key microorganisms, improve metabolic functions, and promote the expression of key functional genes. This study provides new ideas and insights for efficient production of PHB.
1 Introduction
Bioplastics are the preferred alternative to traditional petroleum-based plastics due to their easy degradability, renewability, and high safety (Patel et al., 2025). Polyhydroxybutyrate (PHB) are a class of biopolymers that are natural polyesters stored by microorganisms under stress conditions (Bhatia et al., 2024). PHB are widely recognized as a green and environmentally friendly polymer material, and can be applied in many fields such as bulk plastics, medical materials, biofuels, etc. and have been widely used in enhancement due to their diversity of types and properties (Liang et al., 2024b). However, the high cost of substrates hinders the PHB production with microbial methods (Zhang et al., 2025). Kitchen waste contains abundant organic matters, such as carbohydrates, proteins, etc. These organic matters can be converted into organic acids via anaerobic fermentation, which are high-quality substrates for PHB production (Amabile et al., 2024; Wang et al., 2024). Photosynthetic bacteria (PSB) are one of the potential microorganisms for PHB production, and can synthesize PHB under physiological stress conditions of nutrient deficiency or imbalance of redox (Zhang et al., 2024). Dan et al. (2023) reported that Rhodopseudomonas palustris could ferment kitchen waste digestate for PHB production, and PHB content reached 33% dry cell weight (DCW) during 35 d fermentation. Zhang et al. (2024) also reported that a mixed PSB microbial community achieved a PHB content of 41.6% DCW using kitchen waste digestate as a substrate. However, the production efficiency of PHB from kitchen waste is relatively low and needs further improvement.
Among the chemical, physical, and biological enhancements of PSB growth and product accumulation, trace elements as additives are simple, cheap, and effective (Jiang et al., 2023; Zhang et al., 2023). Essential metal ions (such as Fe2+/Fe3+, Zn2+, Cu2+, etc.) are key cofactors in various enzymes and electron transport chains, participating in key physiological processes such as microbial energy metabolism, DNA synthesis, and antioxidant defense (Verma et al., 2025; Wang et al., 2024). Some studies have been reported that metal ions can increase the utilization efficiency of organic matters and production of products in PSB (Montiel-Corona and Buitrón, 2025; Cao et al., 2020). Currently, metal ions such as Fe2+, Fe3+, Mg2+, etc. have been used to enhance PSB growth and product production. Zhang et al. (2024) found that Fe2+ and Mo2+ improved the productivity of H2 by PSB using wheat straw as a substrate, and H2 productivity was 48.1 mL/h/L. Montiel-Corona and Buitrón (2025) also reported that Fe3+ addition enhanced the simultaneous production of 5-ALA, coenzyme Q10, and pigments by PSB fermentation of residual wine lees. Thus, the addition of metal ions has the potential to enhance the PHB production by PSB. Currently, the effectiveness of metal ion enhanced PSB in producing PHB using kitchen waste digestate is not yet known. The enhancement mechanism of metal ions is also unclear.
Fe3+ and Mn2+ as essential metal ions play key cofactors and metabolic regulatory roles in bacteria, and have been widely used in enhancement of PSB growth and product accumulation (Liang et al., 2024b; Verma et al., 2025). Thus, Fe3+ and Mn2+ were selected to enhance PHB production by PSB in this study. The enhancement effect and mechanism of Fe3+ and Mn2+ on the PHB production by PSB using kitchen waste digestate were investigated for the first time. This study aims, specifically, (1) to explore the effects of different concentrations of Fe3+ and Mn2+ on the PSB biomass, PHB concentration, and PHB content, (2) to measure the soluble chemical oxygen demand (SCOD), lactate, and ammonia nitrogen concentrations in the kitchen waste digestate by PSB, (3) to clarify the diversity, community structure, and functional characteristics of PSB, and (4) to reveal the enhancement mechanism of Fe3+ and Mn2+ for PHB production by PSB. This study offers a new approach to enhance the PHB production by PSB using kitchen waste digestate and provides new insights of the enhancement mechanism.
2 Materials and methods
2.1 Material
To ensure the stability of kitchen waste, this study adopted manual configuration of kitchen waste, consisting of 40% rice, 10% noodles, 5% Mantou, 25% vegetables, 9% tofu, and 11% meat (Zhang et al., 2024). Kitchen waste was pulverized, then mixed with an appropriate amount of water, and finally placed in sealed bottles for natural anaerobic fermentation. Anaerobic fermentation was considered complete until the pH in the fermentation system dropped to about 4.0. The indices of kitchen waste before and after natural anaerobic fermentation were shown in Supplementary Table S1. Kitchen waste digestate was used as a substrate for subsequent PHB production. The basic properties of kitchen waste digestate were pH of 3.9, SCOD concentration of 56.4 g/L, ammonia nitrogen concentration of 0.21 g/L, and soluble protein concentration of 4.7 g/L. SCOD was mainly composed of soluble sugars, soluble proteins and small molecules of organic acids. Noted that lactate concentration, as the main organic acid, was 11.6 g/L. Thus, the type of acid-producing fermentation in natural anaerobic fermentation of kitchen waste was lactate type fermentation.
The inoculum used in this study was a mixed PSB community, purchased from Yancheng Bainuo Biotechnology Co., Ltd. Rhodopseudomona sp. was the dominant genus according to the 16S rRNA sequencing. PSB were activated in RCVBN medium, which contained sodium acetate (4.5 g/L), ammonium chloride (0.8 g/L), sodium bicarbonate (4 g/L), potassium dihydrogen phosphate (0.2 g/L), and yeast extract (0.5 g/L) (Liu et al., 2022). The inoculum was cultured at appropriate conditions and used for subsequent inoculation experiments when the PSB reached the logarithmic growth stage.
2.2 Experimental design
This study used 500 mL sealed Schott bottles as the photo-anaerobic fermentation reactors. Based on the result of preliminary experiment, 2.5 g/L of SCOD in kitchen waste digestate was the optimal concentration for PSB growth and PHB production. Thus, SCOD concentration of kitchen waste digestate was diluted to 2.5 g/L as substrate and inoculated with 20% (v/v) mixed PSB. Ferric chloride hexahydrate (FeCl3·6H2O) was added to the reactor to give Fe3+ concentrations of 10, 20, 30, and 40 mg/L, respectively. Similarly, manganese chloride tetrahydrate (MnCl2·4H2O) was added to the reactor to give Mn2+ concentrations of 1, 2, 3, and 4 mg/L, respectively. The initial pH of these reactors was adjusted to 8.0, magnetic stirrer rotation speed was 80 rpm, and photo-fermentation was carried out at room temperature of 26–30°C. The fermentation process was conducted without pH control in the reactor. To avoid the interference of natural light, all reactors were placed in a light-proof experimental chamber (0.6 m × 0.4 m × 0.4 m) with a light intensity of 4,000 lx (Supplementary Figure S1). For lighting, 12 W white LED strips with adjustable light intensity were implemented. Samples were taken from the reactor on days 1, 3, 5, 7, 9, 11, 13 and 15, respectively. Then, these samples were immediately centrifuged at 10,000 g for 10 min at 4°C after collection. The supernatant was used test for SCOD, ammonia nitrogen, and lactate concentrations. The precipitation after centrifugation were resuspended in an equal volume of deionized water and used to determine PSB biomass, PHB content, and intracellular ion concentration. Part of the precipitate was stored in −20°C refrigerator for subsequent microbial community analysis.
2.3 Analytical methods
The SCOD and ammonia nitrogen concentrations were determined according to standard methods (APHA, 2012). The concentration of lactate was measured using RQflex plus 10 kit (RQflex®plus 10, Germany) according to the manufacturer’s instructions. The intracellular Fe3+ and Mn2+ concentrations were measured by inductively coupled plasma-mass spectrometry (Agilent 8,800 ICP-MS/MS, Agilent Technologies, Tokyo, Japan). PSB biomass, PHB content, and PHB yield were determined based on the previous study (Zhang et al., 2025). Specifically, PSB biomass was tested using the optical density at 660 nm (OD660)-DCW method and the relationship between OD660 and DCW was biomass (mg/L, DCW) (Equation 1).
For the detection of PHB content, the PSB biomass was freeze-dried for 48 h, then put in a closed reaction vessel with 2 mL of chloroform, 1.7 mL of methanol, and 0.3 mL of sulfuric acid. The container was placed in a 100°C water bath for esterification reaction, and the lower organic solvent was taken for PHB determination. PHB standard was purchased from Shanghai Macklin Biochemical Technology Co., Ltd. China. PHB content was determined using an external standard method for gas chromatography (GC-2018, Simazu (China) Ltd., China). The gas chromatogram of PHB standard and the representative PSB sample was shown in Supplementary Figure S2, confirming the PHB production. PHB yield was calculated by Equation 2.
16S rRNA gene sequencing analysis was performed on the Shanghai Majorbio Technology Co., Ltd. and the specific sequencing process was described in detail in Supplementary Text S1 (Liang et al., 2024a,b; Zhang et al., 2025).
2.4 Statistical analysis
All experimental groups and indicators were tested three times in this study. Statistical significance was assessed by one-way ANOVA, with a threshold of p < 0.05 considered statistically significant.
3 Results and discussion
3.1 Effect of metal ions on PSB biomass and PHB accumulation
3.1.1 Fe3+
Figure 1 demonstrates the enhancement effect of Fe3+ on PSB biomass and PHB accumulation in kitchen waste digestate. The concentration of 10–30 mg/L Fe3+ improved the PSB biomass, PHB content, PHB concentration, and PHB yield. Among them, the optimal Fe3+ concentration was 10 mg/L, and PSB biomass, PHB content, PHB concentration, and PHB yield reached 2366.3 mg/L, 46.0%, 1,089 mg/L, and 0.47 g-PHB/g-COD-removal, respectively. These indicators were significantly improved by 21.8, 14.4, 39.3 and 27.7%, respectively compared to the control (p < 0.05). In this study, the increase of PSB biomass (21.8%) and PHB content (14.4%) at 10 mg/L Fe3+ concentration were higher than the low-intensity ultrasound enhanced PSB biomass (17.9%) and PHB content (12.4%) reported by Zhang et al. (2025). Meanwhile, the PHB concentration in this study was significantly higher than that of photo-fermentation of wine lees by PSB reported by Montiel-Corona and Buitrón (2025). Nur et al. (2023) also reported that Fe3+ addition increased PHB production of Synechocystis sp. in treating palm oil mill effluent, but the PHB concentration was significantly lower than that of this study. Fe3+, as the core component of key electron carriers such as cytochromes and ferritin, can participate in the electron transport chain of the photosystem, and promote the efficiency of photosynthesis of PSB, further improving PSB growth and biomass production (Chaiyarat and Saejung, 2022; Yang and Wang, 2018). Meanwhile, Fe3+ can enhance key metabolic pathways and alleviate oxidative stress responses, further enhancing PHB production (Lu et al., 2019). However, high concentrations of Fe3+ (40 mg/L) significantly inhibited the PSB biomass and PHB accumulation. Chen et al. (2020) and Wu et al. (2012) also reported that more than 20 mg/L of nano zero-valent iron and Fe2+ significantly inhibited PSB biomass. This might be due to the toxicity of excess iron ion to microorganisms and further reduces enzyme activity (Wu et al., 2012).
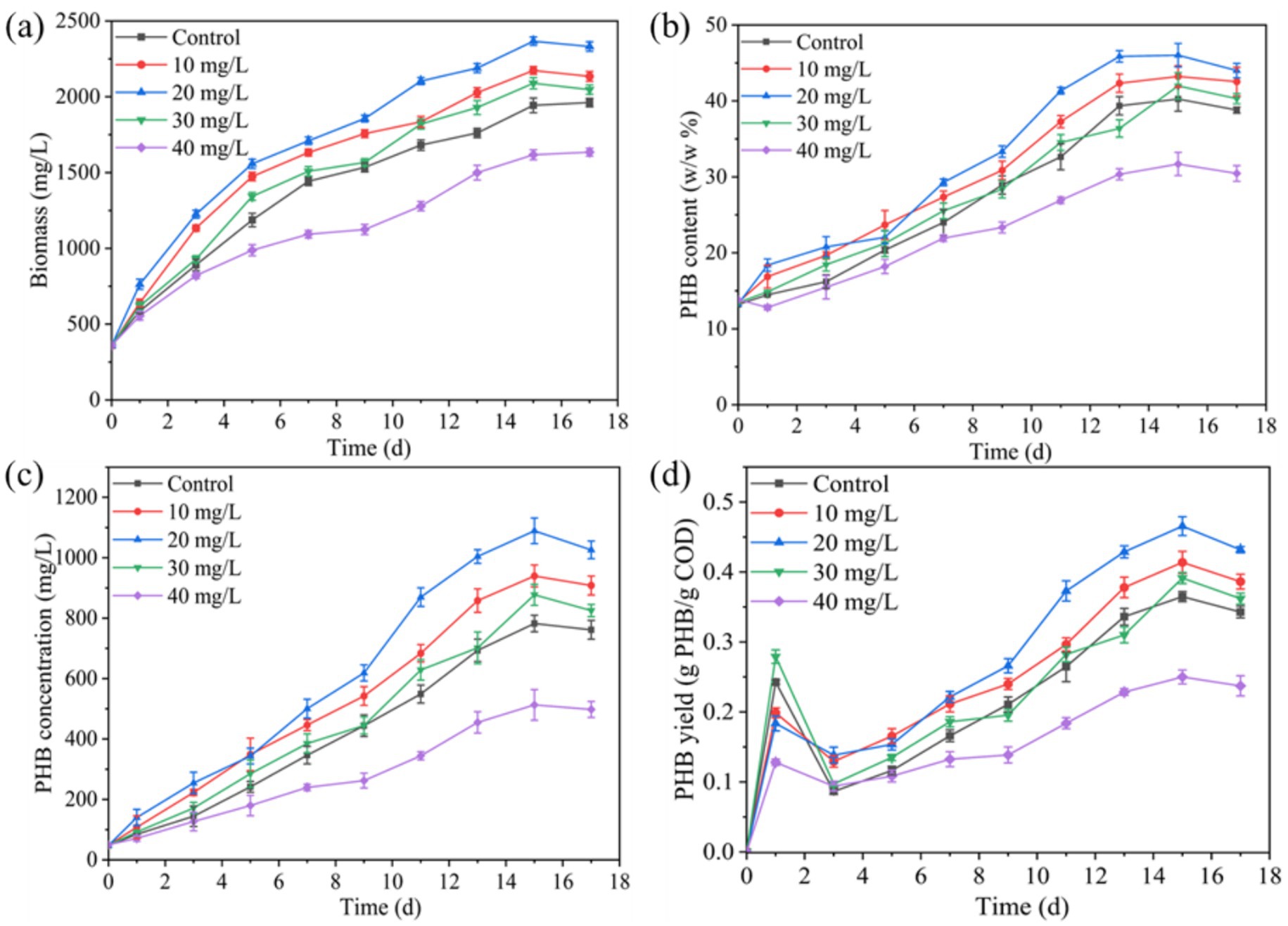
Figure 1. Effect of Fe3+ on changes in (a) PSB biomass, (b) PHB content, (c) PHB concentration, and (d) PHB yield.
3.1.2 Mn2+
Figure 2 demonstrates the enhancement effect of Mn2+ on PSB biomass and PHB accumulation in kitchen waste digestate. The concentration of 1 and 2 mg/L Mn2+ improved the PSB biomass, PHB content, PHB concentration, and PHB yield. Under the Mn2+ concentration of 2 mg/L, PSB biomass, PHB content, PHB concentration, and PHB yield reached 2109.2 mg/L, 43.8%, 920.2 mg/L, and 0.39 g PHB/g-COD-removal, respectively, which increased by 8.5, 4.4, 37.2, and 8.3% respectively, compared to the control. Mn2+ exhibited enhancement effect for PSB biomass similar to that of magnesium, iron, zinc, and cobalt ions (Liu et al., 2015; Liu et al., 2016). Thus, Fe3+ and Mn2+ significantly enhance the production of PHB from PSB. PSB biomass and PHB accumulation at 2 mg/L Mn2+ concentration were significantly lower than that of 10 mg/L Fe3+ concentration (p < 0.05). Strategies for PHB accumulation by Fe3+ and Mn2+-enhanced PSB was higher than that of Calothrix scytonemicola under specific inorganic salt regulation strategies (Kaewbai-ngam et al., 2016). Similar to Fe3+ group, Mn2+ can also activate the antioxidant defense system, enhance the activity of key metabolic enzymes, and improve photosynthetic efficiency (Lu et al., 2019), thereby increasing PSB biomass and PHB accumulation. Similar to the Fe3+ group, PHB yield also showed an initial increase followed by a decrease in the Mn2+ group during the early stages of fermentation. This might be due to the rapid consumption of SCOD in the early stages of fermentation for PSB biomass growth, and the slower consumption of SCOD in subsequent fermentation for PHB synthesis.
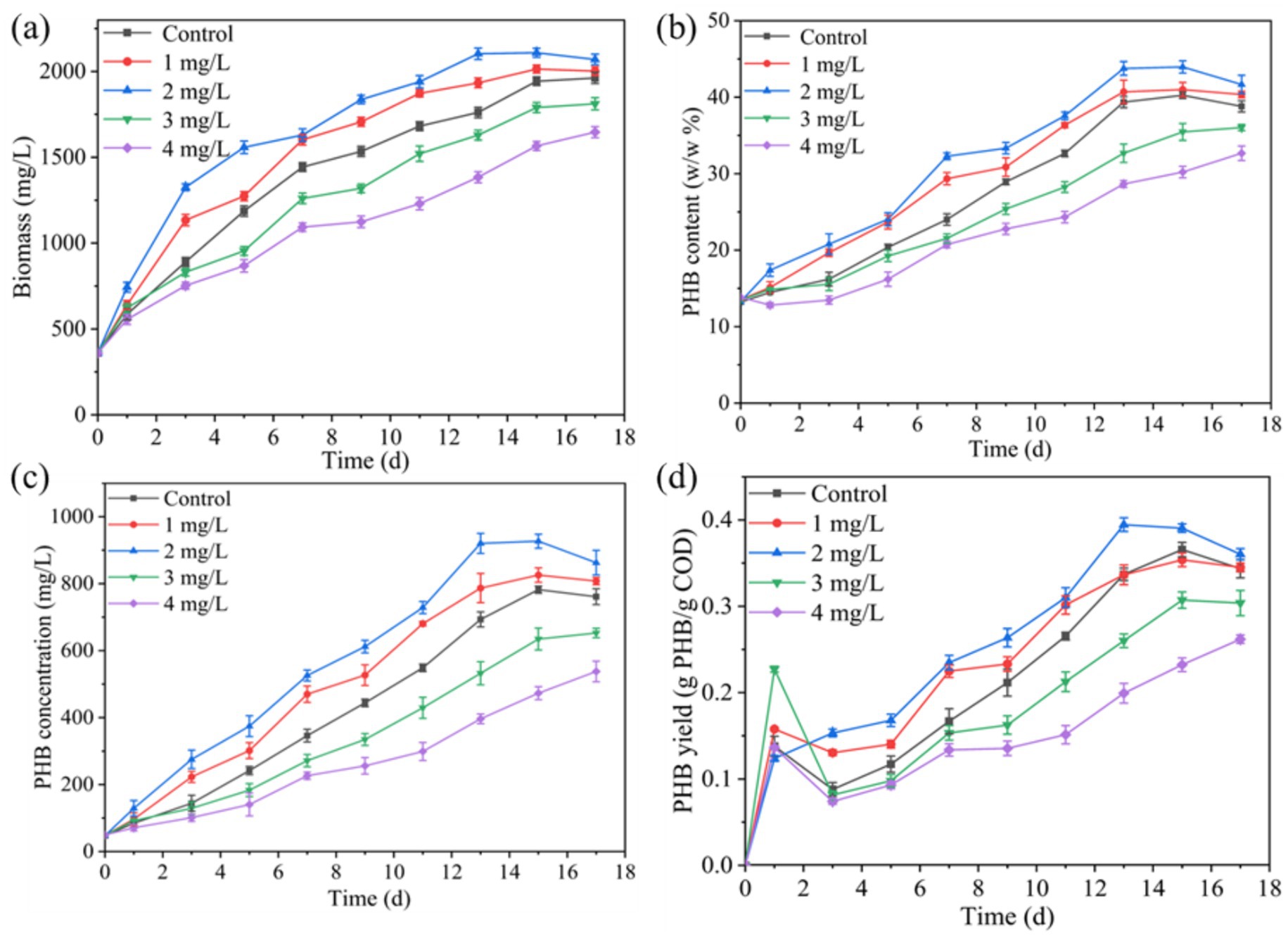
Figure 2. Effect of Mn2+ on changes in (a) PSB biomass, (b) PHB content, (c) PHB concentration, and (d) PHB yield.
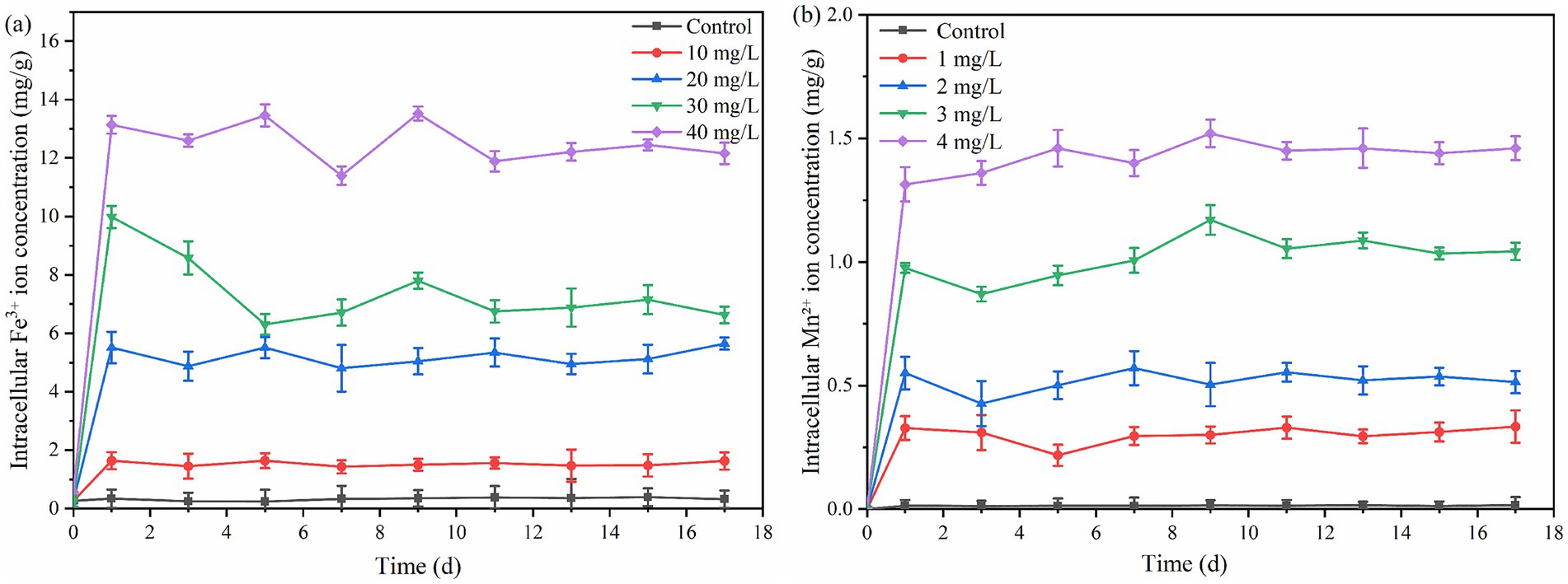
Mn2+ concentration exceeding 3 mg/L significantly inhibited the PSB biomass and PHB accumulation (p < 0.05). This phenomenon was consistent with excessive Fe3+ (Figure 1). As shown in Figure 3, the concentration of intracellular Fe3+ and Mn2+ that PSB adapted growth was approximately 5.5 and 0.5 mg/L, respectively. Excessive intracellular metal ions can deteriorate microorganisms, affect the metabolic activity, and reduce the enzyme activity (Chen et al., 2020). Wu et al. (2012) also reported that the biomass of PSB was greatly affected when the intracellular Fe2+ concentration was above 16 mg/L. Therefore, the suitable ion concentrations are required to promote PSB growth and product accumulation.
3.2 Effect of metal ion-enhanced substrate utilization
Figure 4 shows the changes in substrate concentration for PHB production with PSB. As the fermentation time increased, the concentrations of SCOD, ammonia nitrogen, and lactate showed a significant decrease, indicating that the substrates were effectively utilized by PSB in all groups. Among them, SCOD, ammonia nitrogen, and lactate removal rate under 10–30 mg/L Fe3+ concentration were improved, corresponding to PSB biomass and PHB accumulation. Under the 10 mg/L Fe3+ concentration, the removal rate of SCOD, ammonia nitrogen, and lactate reached 90.5, 73.6, and 87.8%, respectively, which was increased by 7.3, 11.2, and 7.7% respectively, compared to the control (p < 0.05). Similarly, 1 and 2 mg/L Mn2+ concentration improved the removal rate of SCOD, ammonia nitrogen, and lactate. Under the 2 mg/L Mn2+ concentration, the removal rate of SCOD, ammonia nitrogen, and lactate reached 90.1, 67.3, and 87.6%, respectively, which was increased by 6.9, 4.9, and 7.6% respectively, compared to the control. These results were consistent with those reported by Chen et al. (2020). There was almost no difference in the removal rate of SCOD, ammonia nitrogen, and lactate under the optimal concentrations of Fe3+ and Mn2+. Meanwhile, excessive concentrations of Fe3+ and Mn2+ also inhibited the utilization of organic matter by PSB, further suppressing the PHB accumulation. This may be due to that the appropriate Fe3+ and Mn2+ concentrations can enhance the activity of key enzymes, improve photosynthetic efficiency, and further promote PSB growth (Chen et al., 2020), thereby increasing the utilization of substrates by PSB.
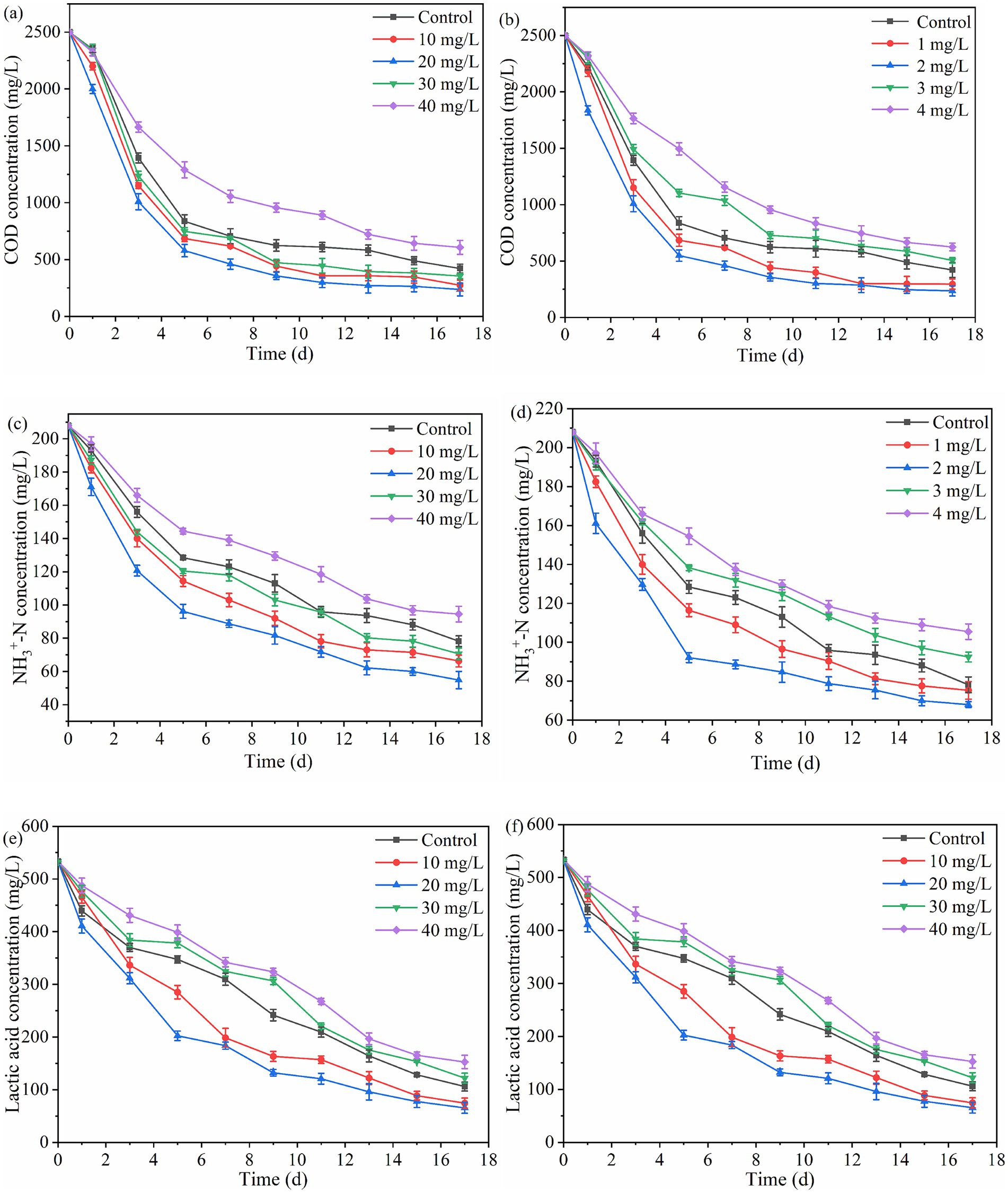
Figure 4. Effect of different Fe3+ and Mn2+ concentrations on the removal rate of organic matter. (a,b) SCOD concentration, (c,d) ammonia nitrogen concentration, and (e,f) lactate concentration.
3.3 Microbiological analysis
3.3.1 Diversity and richness
Typically, the Chao and Shannon indices represent the richness and diversity of microbial communities (Song et al., 2025). The effects of Fe3+ and Mn2+ on the Chao and Shannon indices of microbial communities were consistent (Figure 5). Compared to the inoculum, appropriate Fe3+ and Mn2+ reduced the Chao and Shannon indices of microorganisms, while excessive Fe3+ and Mn2+ reduced the Chao and increased the Shannon indices of microorganisms. Meanwhile, the Chao index of 10 mg/L Fe3+ was higher than that of 2 mg/L Mn2+, while the Shannon index was lower than that of Mn2+ (Figure 5). These results indicate that appropriate metal ions can reduce microbial diversity, while excessively high metal ion concentrations increase microbial diversity. This may be because appropriate metal ions are more conducive to the growth of dominant microorganisms, while excessive metal ions breed the growth of miscellaneous bacteria, further increasing the diversity of microorganisms.
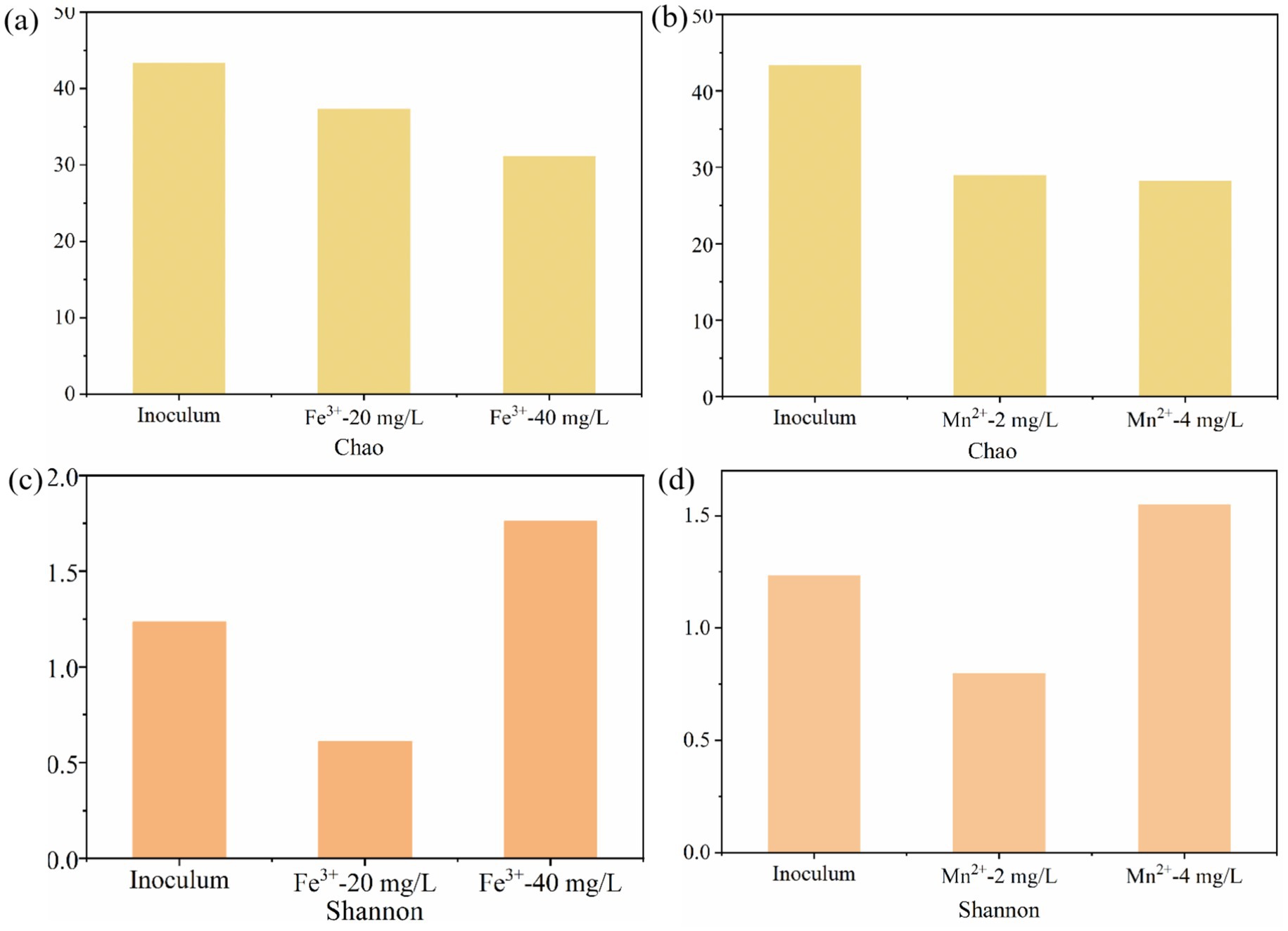
Figure 5. The effect of Fe3+ and Mn2+ on the microbial richness and diversity in photo-fermentation systems. (a,c) Chao index and (b,d) Shannon index.
3.3.2 Community composition
To investigate the effect of metal ions on microbial community composition, the community composition of PSB at the phylum and genus levels was analyzed. As shown in Figure 6, Proteobacteria, Firmicutes, and Bacteroidota were the main phyla in Fe3+ and Mn2+ photo-fermentation systems. Appropriate Fe3+ and Mn2+ increased the relative abundance of Proteobacteria, reaching 90.2 and 93.2%, respectively. Moreover, excessive Fe3+ and Mn2+ reduced the relative abundance of Proteobacteria, and greatly increased the relative abundance of Firmicutes.
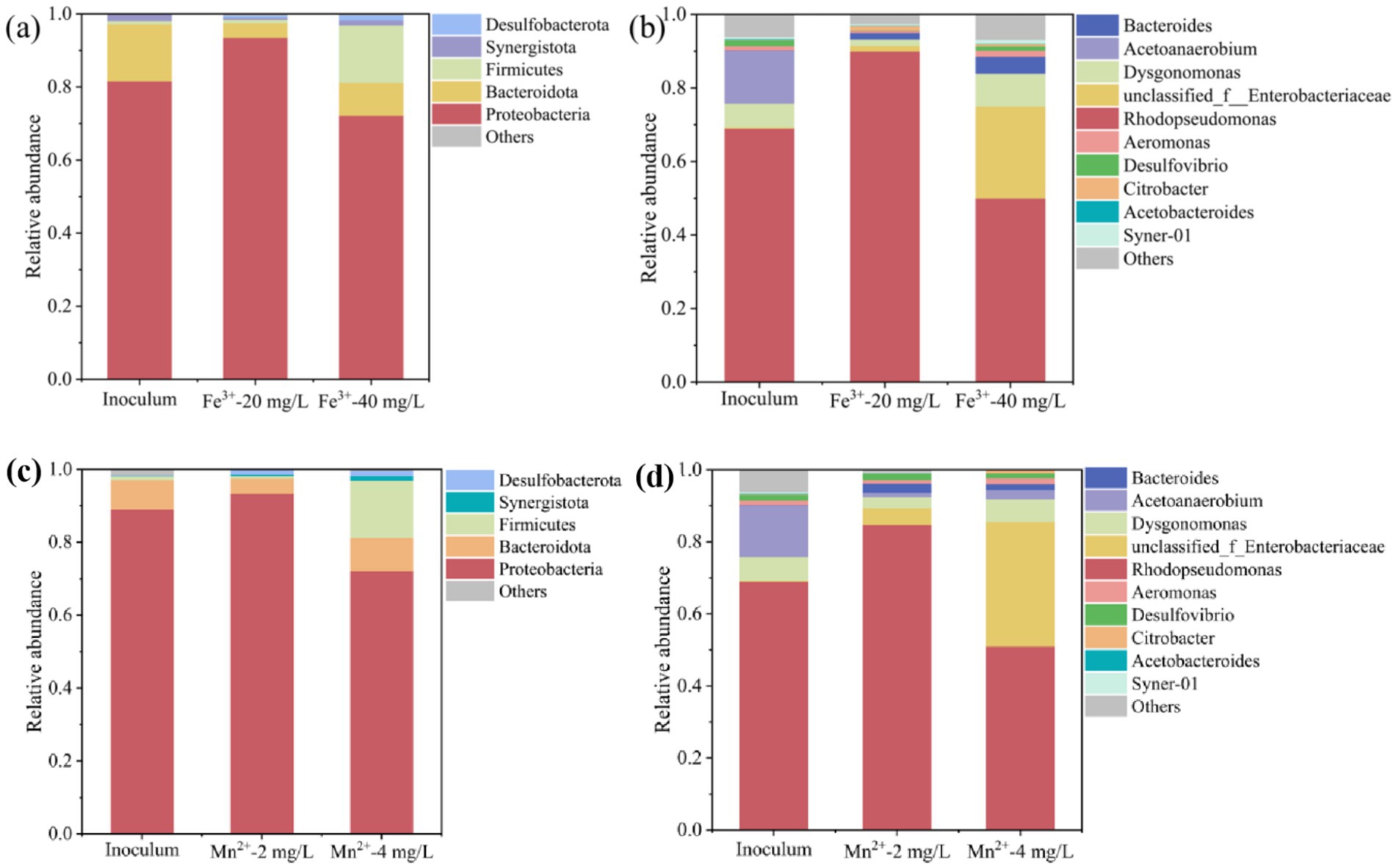
Figure 6. Effect of Fe3+ and Mn2+ on the microbial community composition of PSB at (a,c) phylum level and (b,d) genus level.
As shown in Figure 6, Rhodopseudomonas, Dysgonomonas, and Acetanaerobium were the main genera in photofermentation systems. Rhodopseudomonas is one of the most typical PSB genera with strong environmental adaptability and shock resistance, and has been widely used to treat different types of organic wastewater to produce high value-added products, such as proteins, PHB, and coenzyme Q10, etc. (Carlozzi et al., 2019; Sun et al., 2023). In this study, the appropriate Fe3+ and Mn2+ significantly increased the relative abundance of Rhodopseudomonas, reaching 93.4 and 84.7%, and dominated the microbial community, corresponding to a higher PSB biomass and PHB accumulation under this condition. Zhang et al. (2025) reported that the higher relative abundance of Rhodopseudomonas corresponds to higher PHB production. Montiel-Corona and Buitrón, (2025) also found that suitable conditions promoted the relative abundance of Rhodopseudomonas, corresponding to PSB biomass and PHB accumulation. The excessive concentration of Fe3+ and Mn2+ significantly reduced the relative abundance of Rhodopseudomonas, which was also the main reason for inhibiting PSB biomass and PHB accumulation. In addition, the relative abundance of some genera significantly increased at the excessive concentration of Fe3+ and Mn2+, such as unclassified_f_Enterobacteriaceae, Bacteroides, etc. These genera may compete with Rhodopseudomonas for ecological niches, further inhibiting PSB growth and PHB accumulation.
Thus, an appropriate concentration of metal ions promoted the growth of Rhodopseudomonas, further increasing PSB biomass and PHB accumulation. While the excessive concentration of metal ions increased the growth of microorganisms other than PSB. These microorganisms competed with PSB and hindered the growth of Rhodopseudomonas, thereby inhibiting PSB biomass and PHB accumulation.
3.3.3 Expression of functional genes
To further analyze the enhancement mechanism of metal ions, the metabolic characteristics and functional gene expression of microorganisms were explored. From the first level, the appropriate concentration of metal ions significantly promoted metabolism, while excessive concentration of metal ions significantly inhibited almost all functional characteristics of microorganisms (Figures 7a,b). Carbohydrate metabolism and amino acid metabolism, which belong to metabolism, were the main metabolisms at secondary level metabolic pathway (Figures 7a,b), indicating that the appropriate concentration of metal ions significantly improved the C and N metabolism of PSB that utilize kitchen waste digestate to produce PHB. Meanwhile, metal ions increased the abundance of the TCA cycle, fatty acid metabolism, photosynthesis, pyruvate metabolism, etc. at third level metabolic pathway (Figures 7a,b). Therefore, metal ions promoted relevant metabolic functions at different levels, further promoting PHB accumulation.
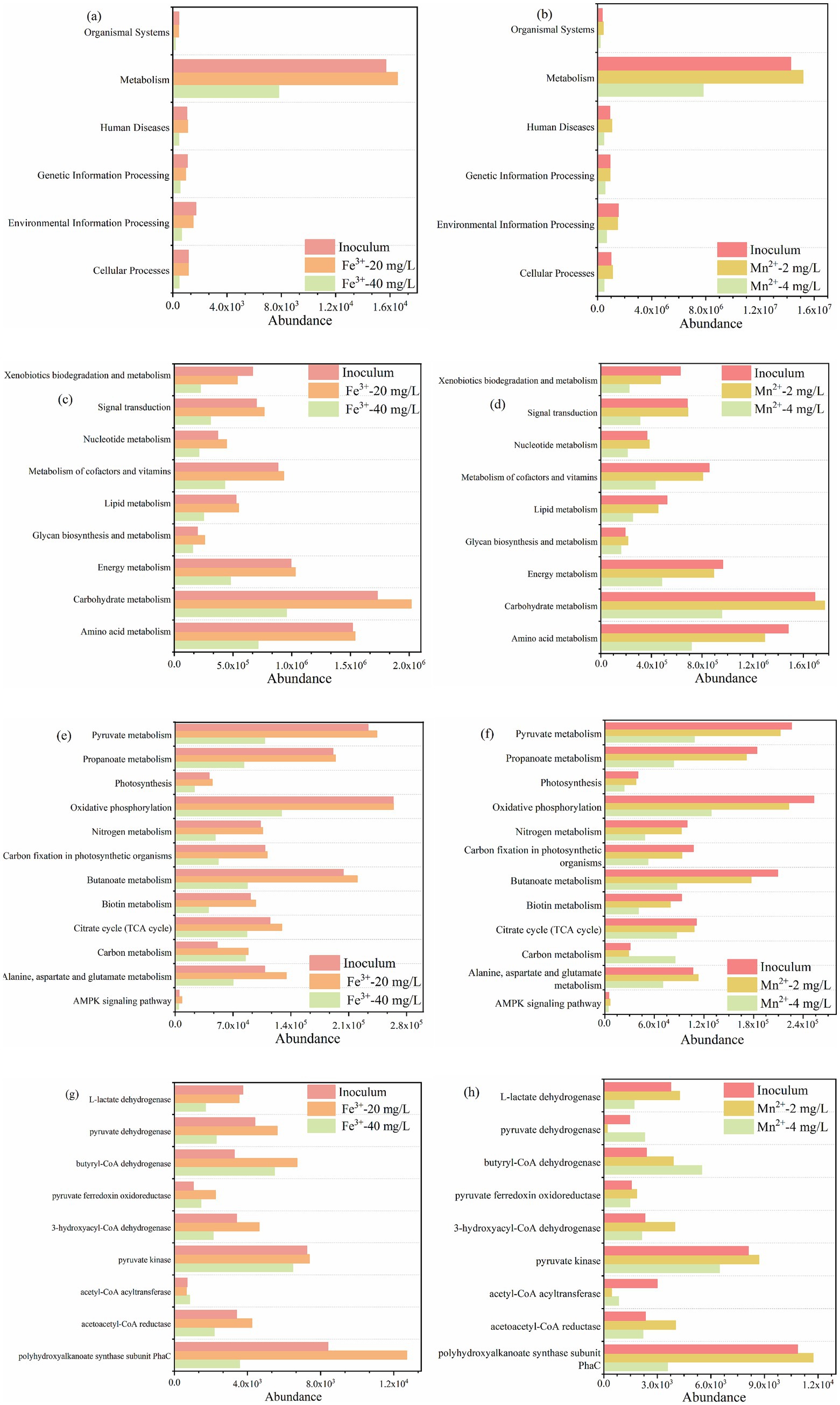
Figure 7. Changes in abundance of metabolic characteristics of PSB for PHB production with Fe3+ and Mn2+. (a,b) Level 1, (c,d) level 2, (e,f) level 3, and (g,h) functional genes.
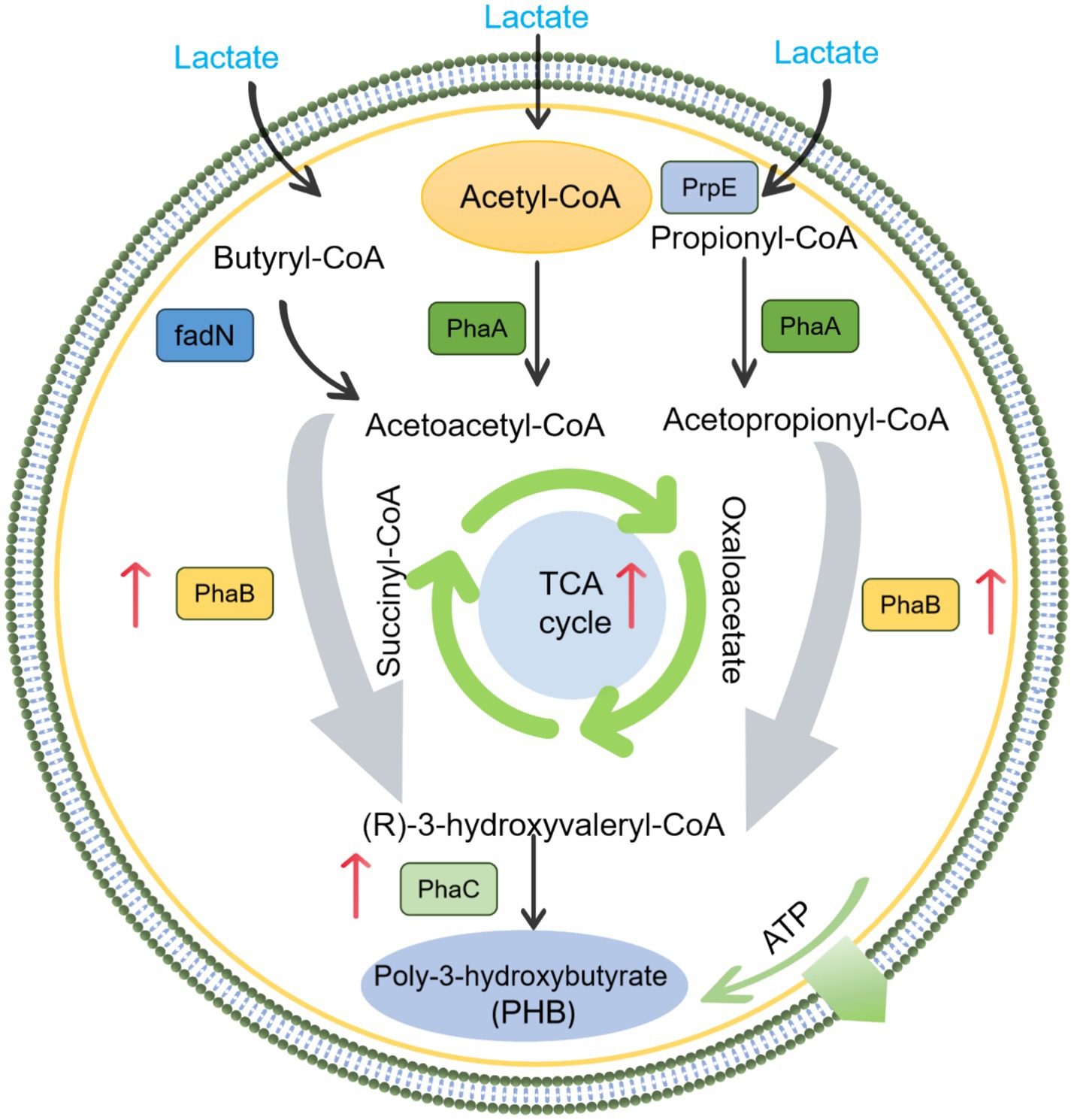
Based on published literatures, the expression of some key genes related to PHB production were selected to further explore the enhancement mechanism of metal ions (Zhang et al., 2025; Wang et al., 2025). As shown in Figure 7, the appropriate concentration of metal ions significantly promoted PhaC, acetoacetyl-CoA reductase, 3-hydroxyacyl-CoA dehydrogenase, butyryl-CoA dehydrogenase, pyruvate dehydrogenase, and L-lactate dehydrogenase. While the excessive metal ions significantly inhibited the expression of these functional genes. Among these functional genes, PhaC and acetoacetyl-CoA reductase are key enzymes in the metabolic pathway for PHB production. The abundance of PhaC for appropriate Fe3+ and Mn2+ were 2.6 and 3.3 times higher than that of excess metal ions, respectively, which also corresponded to PSB biomass and PHB accumulation. Zhang et al. (2025) reported that the expression of PhaC was significantly upregulated by 2.7 times during the improvement of PSB biomass and PHB accumulation by low-intensity ultrasound. Meanwhile, the abundance of acetoacetyl-CoA reductase for appropriate metal ions were higher than that of excess metal ions. In addition, L-lactate dehydrogenase can catalyze the conversion of lactate to pyruvate. The abundance of L-lactate dehydrogenase for appropriate metal ions were higher than that of excess metal ions, which also corresponded to the lactate utilization (Figure 8).
3.4 Enhancement mechanisms
Appropriate Fe3+ and Mn2+ promoted the PSB biomass and PHB accumulation, while excessive iron and manganese ions inhibited them. Fe3+ altered the permeability of cell membranes, making it easier for nutrients and metabolites to pass through the cell membrane, potentially enhancing the metabolic activity of microorganisms (Worms et al., 2006; Wang, 2024). Fe3+ also promoted the capture and conversion of photosynthetic energy, drive the synthesis of PHB precursors, and directly activated key enzyme systems involved in PHB synthesis (Karimirad et al., 2024). Fe3+ is important coenzyme factors for catalase, which may enhance the antioxidant defense system of PSB, reduce reactive oxygen species (ROS), and prevent PHB depolymerase activation (Wu et al., 2012). Meanwhile, Mn2+ could ensure the electron transfer chain flux, provide sustained reducing power (NADPH) for PHB synthesis, and selectively regulated the flow of carbon metabolism toward PHB synthesis (Yang et al., 2021). As cofactor for pyruvate carboxylase, Mn2+ can replenish oxaloacetate for succinate synthesis, and can maintain redox homeostasis critical for PHB synthase activity (Lin et al., 2025). In this study, the appropriate concentration of Fe3+ and Mn2+ significantly promoted the relative abundance of Rhodopseudomonas, corresponding to higher PSB biomass production. Meanwhile, the appropriate concentration of Fe3+ and Mn2+ enhanced the metabolic characteristics of PSB, such as carbon metabolism, carbon fixation, photosynthesis, pyruvate metabolism, etc., further enhancing the PSB metabolic activity and PHB accumulation. In addition, the appropriate concentration of Fe3+ and Mn2+ upregulated the expression of key functional genes (PhaC and acetoacetyl CoA reductase), which further corresponded to the accumulation of PHB.
4 Conclusion
This study aims to investigate the enhancement effect of Fe3+ and Mn2+ on the PHB production from kitchen waste digestate with PSB, and to reveal their enhancement mechanism. The main conclusions are as follows:
(1) The optimal concentrations of Fe3+ and Mn2+ enhanced PSB biomass and PHB accumulation. Biomass of PSB reached 2366.3 and 2109.2 mg/L, respectively under the optimal Fe3+ and Mn2+ concentrations, and PHB content reached 46.0 and 43.8%, respectively.
(2) PSB showed good removal for lactate, SCOD, and ammonia nitrogen in the kitchen waste digestate, exceeding 90, 90, and 70% under optimal conditions for Fe3+ and Mn2+ concentrations.
(3) Addition of Fe3+ and Mn2+ reduced the diversity of PSB microbial communities and altered their composition. Rhodopseudomonas dominated the microbial community in the photofermentation system and was the main contributor to SCOD removal and PHB accumulation.
(4) Fe3+ and Mn2+ optimized the metabolic pathways of PSB, such as carbon metabolism, carbon fixation, photosynthesis, pyruvate metabolism, etc. The expression upregulation of PhaC and acetoacetyl CoA reductase enhanced the synthesis of PHB.
(5) The mechanism of metal ion enhanced PHB production by PSB was to increase the relative abundance of key microorganisms, improve metabolic functions, and promote the expression of key functional genes.
However, the mechanism of enhancement in this study was only based on 16S rRNA sequencing, and the enhancement mechanism of Fe3+ and Mn2+ in PHB production by PSB still needs to be further revealed. Combined with multi-omics techniques will be conducive to reveal the enhancement mechanism in depth. Meanwhile, metal ion-enhanced PHB production with PSB using kitchen waste digestate is still at the lab level, and the scale-up experiments need to be further carried out. In addition, the monomer composition of PHB should be explored, which will be more conducive to reveal the mechanism.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found in here: https://www.ncbi.nlm.nih.gov/, accession number PRJNA1295749.
Author contributions
WZ: Conceptualization, Investigation, Visualization, Writing – original draft. HL: Writing – review & editing, Data curation, Methodology. JYL: Writing – review & editing. SL: Writing – review & editing. XT: Writing – review & editing. LS: Funding acquisition, Writing – review & editing. LL: Writing – review & editing. JSL: Conceptualization, Funding acquisition, Investigation, Writing – review & editing, Supervision. GZ: Conceptualization, Funding acquisition, Investigation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (52360020), Natural Science Foundation of Hebei Province (E2024202058), and Hebei Province Postdoctoral Fund (B2024005006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1645573/full#supplementary-material
References
Amabile, C., Abate, T., Muñoz, R., Chianese, S., and Musmarra, D. (2024). Production of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from methane and volatile fatty acids: properties, metabolic routes and current trend. Sci. Total Environ. 927:172138. doi: 10.1016/j.scitotenv.2024.172138
APHA (2012). Standard methods for the examination of water and wastewater : American Public Health Association.
Bhatia, S. K., Patel, A. K., and Yang, Y. H. (2024). The green revolution of food waste upcycling to produce polyhydroxyalkanoates. Trends Biotechnol. 42, 1273–1287. doi: 10.1016/j.tibtech.2024.03.002
Cao, K., Zhi, R., and Zhang, G. (2020). Photosynthetic bacteria wastewater treatment with the production of value-added products: a review. Bioresour. Technol. 299:122648. doi: 10.1016/j.biortech.2019.122648
Carlozzi, P., Giovannelli, A., Traversi, M. L., Touloupakis, E., and Di Lorenzo, T. (2019). Poly-3-hydroxybutyrate and H2 production by Rhodopseudomonas sp. S16-VOGS3 grown in a new generation photobioreactor under single or combined nutrient deficiency. Int. J. Biol. Macromol. 135, 821–828. doi: 10.1016/j.ijbiomac.2019.05.220
Chaiyarat, A., and Saejung, C. (2022). Photosynthetic bacteria with iron oxide nanoparticles as catalyst for cooking oil removal and valuable products recovery with heavy metal co-contamination. Waste Manag. 140, 81–89. doi: 10.1016/j.wasman.2022.01.005
Chen, Y., Zhang, G., and Wang, H. (2020). Enhancement of photosynthetic bacteria biomass production and wastewater treatment efficiency by zero-valent iron nanoparticles. J. Biosci. Bioeng. 130, 306–310. doi: 10.1016/j.jbiosc.2020.04.004
Dan, T., Jing, H., Shen, T., Zhu, J., and Liu, Y. (2023). Performance of production of polyhydroxyalkanoates from food waste fermentation with Rhodopseudomonas palustris. Bioresource Technology. 385:129165.
Jiang, W., Song, Y., Guo, T., Zhang, D., Lu, C., Hou, Y., et al. (2023). Ligands regulate the application of Fe(III)-ligands on se(IV) bioreduction: electron transfer, metabolism activity and EPS secretion. J. Environ. Chem. Eng. 11:111340. doi: 10.1016/j.jece.2023.111340
Kaewbai-Ngam, A., Incharoensakdi, A., and Monshupanee, T. (2016). Increased accumulation of polyhydroxybutyrate in divergent cyanobacteria under nutrient-deprived photoautotrophy: an efficient conversion of solar energy and carbon dioxide to polyhydroxybutyrate by Calothrix scytonemicola TISTR 8095. Bioresour. Technol. 212, 342–347. doi: 10.1016/j.biortech.2016.04.035
Karimirad, R., Luo, L., and Wong, J. W. C. (2024). Enhancing methane production in anaerobic co-digestion of food wastes and sewage sludge: roles of different types of iron amendments. Waste Dispos. Sustain. Energy. 6, 553–564. doi: 10.1007/s42768-024-00207-0
Liang, J., Liu, S., Zhang, R., Chang, J., Lv, L., Nabi, M., et al. (2024a). Yeast culture enhances long-term fermentation of corn straw by ruminal microbes for volatile fatty acid production: performance and mechanism. J. Environ. Manag. 370:122736. doi: 10.1016/j.jenvman.2024.122736
Liang, J., Zhang, P., Zhang, R., Chang, J., Chen, L., Zhang, G., et al. (2024b). Bioconversion of volatile fatty acids from organic wastes to produce high-value products by photosynthetic bacteria: a review. Environ. Res. 242:117796. doi: 10.1016/j.envres.2023.117796
Lin, X., Ge, Q., Zhou, X., Wang, Y., Zhu, C., Liu, K., et al. (2025). Enhancement of electron transfer between Fe/Mn promotes efficient activation of Peroxomonosulfate by FeMn-NBC. Water 17:1700. doi: 10.3390/w17111700
Liu, S., Li, H., Daigger, G. T., Huang, J., and Song, G. (2022). Material biosynthesis, mechanism regulation and resource recycling of biomass and high-value substances from wastewater treatment by photosynthetic bacteria: a review. Sci. Total Environ. 820:153200. doi: 10.1016/j.scitotenv.2022.153200
Liu, S., Li, X., and Zhang, G. (2015). Effect of magnesium ion on crt gene expression in improving carotenoid yield of Rhodobacter sphaeroides. Arch. Microbiol. 197, 1101–1108. doi: 10.1007/s00203-015-1150-z
Liu, S., Zhang, G., Li, J., Li, X., and Zhang, J. (2016). Effects of metal ions on biomass and 5-aminolevulinic acid production in Rhodopseudomonas palustris wastewater treatment. Water Sci. Technol. 73, 382–388. doi: 10.2166/wst.2015.479
Lu, H., Zhang, G., Zheng, Z., Meng, F., Du, T., and He, S. (2019). Bio-conversion of photosynthetic bacteria from non-toxic wastewater to realize wastewater treatment and bioresource recovery: a review. Bioresour. Technol. 278, 383–399. doi: 10.1016/j.biortech.2019.01.070
Montiel-Corona, V., and Buitrón, G. (2025). Light/dark cycles and iron supplementation to enhance the simultaneous production of polyhydroxyalkanoates, 5-aminolevulinic acid, coenzyme Q10, and pigments through photofermentation. Bioresour. Technol. 429:132513. doi: 10.1016/j.biortech.2025.132513
Nur, M. M. A., Achmad, Z., Jaya, D., Muji, S. T., Wahyu, W. T., Diyar, K. S., et al. (2023). Screening and optimization of cyanobacteria cultivated on palm oil mill effluent (POME) to produce polyhydroxybutyrate. J. Appl. Phycol. 35, 1213–1221. doi: 10.1007/s10811-023-02954-9
Patel, A., Murali, R., Choudhary, H., Purnima, D., and Pal, R. K. (2025). Vegetative and microbial proteins for bioplastics applications – a review in the Indian context. RSC Adv. 15, 16392–16432. doi: 10.1039/d4ra08544b
Song, C. S., Wang, C., Ma, C., Meng, Q. W., and Li, J. P. (2025). Low concentrations of acetic acid and its sodium salt enhance the anaerobic fermentation and aerobic preservation of Chinese cabbage waste. Environ. Technol. Innov. 38:104207. doi: 10.1016/j.eti.2025.104207
Sun, Y., Sun, Y., and Li, X. (2023). Removal of pollutants and accumulation of high-value cell inclusions in a batch reactor containing Rhodopseudomonas for treating real heavy oil refinery wastewater. J. Environ. Manag. 345:118834. doi: 10.1016/j.jenvman.2023.118834
Verma, Y., Iqbal, J., Naushad, M., Bhaskaralingam, A., Kumar, A., Dhiman, P., et al. (2025). Recent developments in photo-fermentative hydrogen evolution: fundamental biochemistry and influencing factors a review. J. Environ. Manag. 374:123976. doi: 10.1016/j.jenvman.2024.123976
Wang, J. (2024). Composition heterogeneity of metal ions bound at the oxygen-evolving center of photosystem II in living cells. Biochemistry 63, 1963–1968. doi: 10.1021/acs.biochem.4c00261
Wang, Z., Du, C., Yan, R., Li, S., Zheng, G., and Ding, D. (2025). Sustainable polyhydroxybutyrate (PHB) production from biowastes by Halomonas sp. WZQ-1 under non-sterile conditions. Int. J. Biol. Macromol. 311:143643. doi: 10.1016/j.ijbiomac.2025.143643
Wang, J., Huang, J., and Liu, S. (2024). The production, recovery, and valorization of polyhydroxybutyrate (PHB) based on circular bioeconomy. Biotechnol. Adv. 72:108340. doi: 10.1016/j.biotechadv.2024.108340
Worms, I., Simon, D. F., Hassler, C. S., and Wilkinson, K. J. (2006). Bioavailability of trace metals to aquatic microorganisms: importance of chemical, biological and physical processes on biouptake. Biochimie 88, 1721–1731. doi: 10.1016/j.biochi.2006.09.008
Wu, P., Zhang, G., Li, J., Lu, H., and Zhao, W. (2012). Effects of Fe2+ concentration on biomass accumulation and energy metabolism in photosynthetic bacteria wastewater treatment. Bioresour. Technol. 119, 55–59. doi: 10.1016/j.biortech.2012.05.133
Yang, X., Liu, H., Zhang, Y., and Shen, X. (2021). Roles of type VI secretion system in transport of metal ions. Front. Microbiol. 12:756136. doi: 10.3389/fmicb.2021.756136
Yang, G., and Wang, J. (2018). Improving mechanisms of biohydrogen production from grass using zero-valent iron nanoparticles. Bioresour. Technol. 266, 413–420. doi: 10.1016/j.biortech.2018.07.004
Zhang, G., Liu, H., Du, Z., Liu, S., Zhang, J., Lu, H., et al. (2024). Production of polyhydroxybutyrate with photosynthetic bacteria treating artificial kitchen waste: effect of light intensity. J. Environ. Chem. Eng. 12:114774. doi: 10.1016/j.jece.2024.114774
Zhang, G., Liu, H., and Liang, J. (2025). A novel strategy for promoting polyhydroxybutyrate production with low-intensity ultrasound. Waste Biomass Valor. doi: 10.1007/s12649-025-03107-4
Keywords: kitchen waste digestate, lactate, polyhydroxybutyrate, PhaC, metabolic characteristics
Citation: Zhao W, Liu H, Li J, Liu S, Tao X, Sun L, Lv L, Liang J and Zhang G (2025) Metal ion-enhanced photosynthetic bacteria for bioplastic production from kitchen waste digestate: performance and mechanism. Front. Microbiol. 16:1645573. doi: 10.3389/fmicb.2025.1645573
Edited by:
Bin Ji, Wuhan University of Science and Technology, ChinaReviewed by:
Venkata Giridhar Poosarla, Gandhi Institute of Technology and Management (GITAM), IndiaMuhamad Maulana Azimatun Nur, Universitas Pembangunan Nasional Veteran Yogyakarta, Indonesia
Jey-R. Ventura, University of the Philippines Los Baños, Philippines
Copyright © 2025 Zhao, Liu, Li, Liu, Tao, Sun, Lv, Liang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinsong Liang, MjAyMzkyMkBoZWJ1dC5lZHUuY24=; Guangming Zhang, MjAyMDAxN0BoZWJ1dC5lZHUuY24=
 Wei Zhao1
Wei Zhao1 Jinsong Liang
Jinsong Liang