- 1Department of Plant Science, MJP Rohilkhand University, Bareilly, India
- 2Department of Microbiology, MJP Rohilkhand University, Bareilly, India
- 3Department of Environmental Microbiology, Babasaheb Bhimrao Ambedkar University, Lucknow, India
- 4Faculty of Agriculture, Mahayogi Gorakhnath University, Gorakhpur, India
Hexavalent chromium [Cr(VI)] pollution is a serious environmental issue because it is highly toxic and persistent. We aimed to investigate the growth, Cr(VI) reducing capacity, and chemotaxis of Bacillus licheniformis strain KNP under the stress of Cr(VI) in the form of K2Cr2O7 (250–1,000 ppm). Bacterial growth decreased as Cr(VI) concentration increased, with a maximum at 250 ppm K2Cr2O7 and strong inhibition at 1,000 ppm K2Cr2O7. Significantly, strain KNP completely reduced Cr(VI) in the form of 500 ppm K2Cr2O7 within 48 h. Fourier Transform Infrared spectroscopy (FTIR) showed biochemical changes of functional groups present on bacterial cell wall due to interaction with chromium. A scanning electron microscope (SEM) and energy dispersive X-ray spectroscopy (EDX) analysis confirmed the accumulation of Cr on the surface of bacteria with morphological changes. Strain KNP also showed negative chemotaxis away from Cr(VI) and positive chemotaxis toward glucose, as indicated by drop plate and chemical plug assays. Genomic analysis revealed major chemotaxis-related genes that play a role in Cr(VI) sensing and avoidance, indicating a complex survival strategy. These results indicated that B. licheniformis strain KNP is a good candidate for bioremediation purposes, providing an effective combination of Cr(VI) detoxification and tactical bacterial migration in polluted environments.
Introduction
Chromium (Cr) is a widespread heavy metal in the environment, which primarily occurs in two oxidation states: trivalent chromium [Cr(III)] and hexavalent chromium [Cr(VI)] (Abbas et al., 2025; Kumari et al., 2025). Though Cr(III) is an essential trace element with negligible toxicity, Cr(VI) is very toxic, carcinogenic, and causes significant environmental and health risks due to its high solubility and mobility in aquatic systems (Xing et al., 2025). Industrial operations like stainless steel production, leather tanning, and mining are major contributors to Cr(VI) contamination, which underscores the necessity for efficient remediation strategies (Xiang et al., 2025; Xing et al., 2025).
The biotransformation of chromium is a crucial microbially mediated process wherein Cr(VI) is detoxified through reduction to the less toxic and insoluble Cr(III), which subsequently precipitates and becomes less bioavailable (Xing et al., 2025). Various bacterial, fungal, and archaeal strains have evolved enzymatic mechanisms to counteract Cr(VI) toxicity via intracellular and extracellular reduction mechanisms. These detoxification pathways generally include chromate reductases, electron transport chains, and oxidative stress response systems, allowing microorganisms to tolerate and metabolize Cr(VI) in a wide variety of environmental settings (Xing et al., 2025). A comprehensive understanding of these microbial metabolic pathways is fundamental to optimizing bioremediation strategies for chromium-contaminated environments (Saxena et al., 2025).
The comparative analysis of bacterial species involved in Cr(VI) reduction reveals a diverse array of efficiencies across genera. Bacillus licheniformis stands out for its broad reduction range, effectively reducing 20–1,500 ppm Cr(VI) within 24–72 h (Kavitha et al., 2011), whereas its thermotolerant strain B22 tolerates up to 100 ppm (Doganli and Dogan, 2014). Other Bacillus species also demonstrate significant reduction capabilities: B. cohnii SR2 and B. licheniformis SR3 achieved 94%−95% reduction of 100 ppm Cr(VI) within 25 h (Sarankumar et al., 2020). B. megaterium, B. carboniphilus, B. subtilis, and B. licheniformis showed tolerance and reduction across 125–500 ppm concentrations (Pinki et al., 2021). B. wiedmannii S1 showed a 70% reduction of Cr(VI) when the initial concentration was 200 μg/ml (Adhikary et al., 2025). Bacillus tropicus CRB14 reduced 86.57% Cr(VI) within 96 h at higher concentrations (Tuli et al., 2024). B. paramycoides S48 achieved a 90% reduction of chromium within 48 h at concentrations ranging from 25 to 50 mg/L. However, the reduction efficiency progressively declined as the concentration of K2Cr2O7 increased from 100 to 500 mg/L (Kalsoom et al., 2025). Notably, B. vallismortis and B. haynesii formed biofilms in response to chromium stress, which contributed 60%−99% Cr(VI) reduction (Maurya et al., 2022), suggesting biofilm-mediated detoxification as a potent strategy.
In contrast, Cellulosimicrobium species exhibit high tolerance and reduction efficiency, with SCRB10 tolerating up to 800 ppm and reducing 96.98% of 100 ppm Cr(VI) in 96 h (Bharagava and Mishra, 2018). Strain A8 reduces 98.6% of 900 μg/ml Cr(VI) (Naeem et al., 2013). However, other strains like C. cellulans KUCr3 and CrK16 showed moderate reduction efficiencies of 40% and 41%, respectively (Chatterjee et al., 2011; Rehman and Faisal, 2015). Microbacterium species also demonstrate promising capabilities: M. oleivorans A1 reduced 750 μM Cr(VI) in 85 h (Sarkar et al., 2015), whereas M. testaceum B-HS2 exhibited exceptional tolerance up to 48 mM (Elahi et al., 2019). Both Microbacterium sp. M5 and M. paraoxydans SCRB19 reduced 400 and 500 ppm Cr(VI), respectively, although with different efficiencies (Kumar and Saini, 2019; Mishra et al., 2021).
Other genera also contribute to Cr(VI) bioremediation. Brucella sp. showed complete reduction of 50 ppm Cr(VI) using cell-free extracts (Thacker et al., 2007), and B. intermedius TJ-5 demonstrated bioremediation potential (Chen et al., 2022). Exiguobacterium acetylicum demonstrated 95.4% Cr(VI) reduction at 50 mg/L concentration (Garcia da Cunha et al., 2025). Pseudomonas strains, such as CPSB21, exhibited high resistance (up to 700 ppm) and reduction efficiency (up to 90%; Gupta et al., 2018; Kumar et al., 2023), with MAI4 identified as a potent reducer (Wani et al., 2019). Kocuria species, particularly the radio-resistant ASB107, are known for effective biosorption of Cr(VI) (Akbarpour Nesheli et al., 2017), whereas Klebsiella pneumoniae MS15 and K. aerogenes MUM reduced Cr(VI) up to 80 and 600 ppm, respectively, indicating moderate to high tolerance (Kumar et al., 2023). Brevibacillus borstelensis SSAU-3T demonstrated 99% Cr(VI) removal of 400 ppm Cr(VI) at 55 °C (Agrawal et al., 2025).
Chemotaxis of chromium involves the ability of microorganisms to sense and move toward or away from chromium gradients in the environment (Pandey and Jain, 2002). Some bacterial species exhibit positive chemotaxis toward Cr(VI)-contaminated regions to increase their chances of colonization and, consequently, removal of Cr(VI) (Shamim et al., 2014; Barrionuevo and Vullo, 2012). However, some microorganisms exhibit negative chemotaxis, attempting to move away from high chromium concentrations due to its toxicity (Arora et al., 2015). Chemotactic behavior is crucial for microbial bioremediation, as it guides chromium-reducing bacteria (CRB) to contaminated areas, thus enhancing detoxification efficiency. This process is regulated by transmembrane receptors, intracellular signaling pathways, and flagellar motility, which collectively increase microbial adaptation and survival under metal stress conditions (Xu et al., 2024).
Biochemical synergy between biotransformation and chemotaxis is crucial in controlling the overall efficiency of microbial Cr(VI) remediation (Pal et al., 2022). By using microbial motility and metabolic equipment, researchers are trying to create a low-cost, eco-friendly, and sustainable approach to chromium detoxification in polluted environments.
While numerous studies have explored the enzymatic and molecular mechanisms of Cr(VI) reduction in bacteria (Agrawal et al., 2025; Adhikary et al., 2025; Saxena et al., 2025), the comprehensive role of chemotaxis in influencing microbial survival and spatial behavior under Cr(VI) stress remains largely underexplored. Chemotaxis, particularly negative chemotaxis, may serve as a critical early survival mechanism by allowing bacteria to evade lethal concentrations of Cr(VI). Although Pannonibacter phragmitetus BB has been reported to exhibit negative chemotaxis to Cr(VI) (Chai et al., 2019), such behavioral responses have not been systematically studied across diverse taxa. In particular, Bacillus spp., despite being well-documented for their high Cr(VI) tolerance and reduction capabilities, lack comprehensive characterization in terms of chemotactic behavior under chromium gradients. In our previous research, we sequenced the genome of Bacillus licheniformis KNP (Arora et al., 2020) and confirmed its potent Cr(VI)-reducing ability (Kumar et al., 2023). However, the molecular basis and phenotypic manifestation of chemotactic response to Cr(VI) in this strain remained unexplored. The present study addresses this critical gap by linking phenotypic evidence from soft agar chemotaxis assays with genomic annotation of chemotaxis-related genes in B. licheniformis KNP. To the best of our knowledge, this is the first comprehensive report showing that Cr(VI)-induced negative chemotaxis in B. licheniformis, along with genomic evidence supporting the underlying molecular machinery. This study not only broadens the understanding of adaptive bacterial behavior under metal stress but also provides novel mechanistic insight that may help in developing more effective bioremediation strategies for chromium-contaminated environments.
Materials and methods
Bacterial strain
Bacillus licheniformis KNP was previously isolated from a sample collected from the effluent of a tannery located at Jajmau (26.4670°°N, 80.3500 °E) area of Kanpur, India (Kumar et al., 2023; Arora et al., 2020). The effluent sample was aseptically collected in a sterilized screw-capped bottle. For selective enrichment of hexavalent chromium-resistant bacteria, 1 ml of the effluent sample was inoculated into 500 ml of nutrient broth supplemented with 500 ppm potassium dichromate and incubated for 72 h at 30 °C under shaking conditions (Kumar et al., 2023; Arora et al., 2020). After 72 h of enrichment, serial dilutions of the culture were prepared up to 10−6 using sterile distilled water. Aliquots (100 μl) from appropriate dilutions were spread onto nutrient agar plates containing 500 ppm potassium dichromate and incubated at 30 °C for 48 h. The average microbial load was found to be ~1.3 × 103 CFU/ml of enriched culture, indicating a substantial population of chromium-resistant bacteria in the tannery effluent sample. Colonies exhibiting distinct morphologies were selected; a total of 18 morphotypes were purified through repeated streaking and preserved in 10% glycerol stocks at −80 °C for subsequent analyses (Kumar et al., 2023; Arora et al., 2020). B. licheniformis KNP was able to transform hexavalent chromium at concentrations of 1,000 ppm K2Cr2O7.
Bacterial growth in the presence of chromium
The cells of B. licheniformis KNP were grown in nutrient media containing various concentrations of potassium dichromate (250, 500, 750, and 1,000 ppm) under shaking conditions (160 rpm) at 30 °C. The samples were collected at regular intervals, and the optical density of the sample was determined by spectrophotometry at 600 nm.
Chromium (VI) reduction using B. licheniformis strain KNP
Chromium (VI) reduction analysis was conducted in nutrient broth medium with 500 ppm K2Cr2O7. An overnight culture of B. licheniformis KNP was added to the nutrient broth medium containing 500 ppm K2Cr2O7. An uncultured nutrient broth enriched with 500 ppm K2Cr2O7 served as a control. The flasks were incubated on an orbital shaker at 160 rpm and 30 °C. At regular intervals, samples were collected, and the Cr(VI) reduction was measured using a spectrophotometric method with the 1,5-diphenylcarbazide assay (Thacker et al., 2007).
Diphenylcarbazide (DPC) assay
To find the amount of residual Cr(VI) in the culture supernatant, we analyzed the absorbance of the purple color complex formed by Cr(VI) and 1,5-diphenylcarbazide (DPC) at 540 nm using a UV-Vis spectrophotometer. We prepared the DPC reagent by dissolving 250 mg of DPC in 50 ml of acetone. The reaction mixture included 100 or 200 μl of culture supernatant, 330 μl of 6M H2SO4, and 400 μl of DPC reagent, with the final volume brought to 10 ml using distilled water. We kept the sample at room temperature for 20 min to develop the color complex and then measured the absorbance at 540 nm (Thacker et al., 2007). To calculate the Cr(VI) reduction (%), the following formula was used:
[Where Ci = initial Cr(VI) concentration (mg/L) and Cf = final Cr(VI) concentration (mg/L)]
Effects of temperature
To monitor the effects of temperature on growth and Cr(VI) reduction, strain KNP was grown on nutrient media containing 500 ppm K2Cr2O7 at various temperatures (20, 25, 30, and 42 °C), and samples were collected after 48 h. The growth was monitored by spectrophotometry at 600 nm, and total chromium Cr(VI) reduction was measured by the DPC assay as described above.
Effects of pH
To monitor the effects of pH on growth and Cr(VI) reduction, strain KNP was grown on nutrient media containing 500 ppm K2Cr2O7 at various pH levels (5, 6, 7, 8, 9, 10, and 11), and samples were collected after 48 h. The growth was monitored by spectrophotometry at 600 nm, and total chromium Cr(VI) reduction was measured by the DPC assay as described above.
SEM-EDX analysis
The scanning electron microscope-energy dispersive X-ray spectroscopy (SEM-EDX) analysis was performed to characterize the changes in cell morphology of bacteria in the presence of chromium. Chromium-unexposed cells were used as a control. Strain KNP was grown in nutrient broth amended with K2Cr2O7 as a source for Cr(VI) at a 500 ppm concentration and incubated for 48 h at 150 rpm. After incubation, the medium was centrifuged at 4 °C for 10 min at 8,000 rpm. Clear supernatant of broth was removed, and the bacterial cell pellets were washed with 0.5 M phosphate buffer saline (PBS). After washing, the cells were fixed with 2.5% glutaraldehyde for about 2 to 4 h and then washed with PBS. The cells were finally fixed with osmium tetroxide for at least 1 h. After completing these steps, cell pellets were dehydrated for 15 min with acetone at various concentrations, i.e., 30%, 50%, 70%, 90%, and 100%. Finally, the dehydrated sample was fixed or mounted with platinum by an ion sputter coater onto the carbon conductive adhesive tape before performing SEM, combined with EDX (Kumar et al., 2024).
Fourier transform infrared spectroscopy (FTIR)
To obtain Fourier Transform Infrared spectroscopy (FTIR) spectra, strain KNP was grown overnight at 30 °C in nutrient broth amended with 500 ppm K2Cr2O7, while in the control, K2Cr2O7 was not added. After incubation, cells were centrifuged at 10,000 rpm for 10 minutes. After centrifugation, the cell pellets at the bottom were washed with 0.85% saline water, followed by double-distilled water. Washed cell pellets were dried and mixed with potassium bromide (KBr) and analyzed by an FTIR spectrophotometer (Kumar et al., 2019). The absorbance of the IR spectrum was recorded in the range of 400–4,000 cm−1. The spectra were recorded with 32 scans per sample at a resolution of 4 cm−1 to ensure adequate spectral quality for biochemical interpretation.
Bacterial chemotaxis away from hexavalent chromium
Drop plate assay
The drop plate assay was performed following a method that had previously been described (Arora et al., 2015). Bacteria were cultured overnight in nutrient broth supplemented with 500 ppm potassium dichromate. The cells were centrifuged, washed twice with saline solution, and suspended in a minimal medium containing 0.3% agar. This suspension was poured onto a Petri dish and allowed to solidify. To assess bacterial attraction and repellence to chromium, a small quantity of potassium dichromate or glucose (positive control) was placed in the center of the Petri dish. The plates were incubated for a few hours. Bacterial attraction was indicated by visible rings forming around the crystals, while negative chemotaxis resulted in bacteria dispersing away from the compound.
Chemical-in-plug method
In the chemical-in-plug method, the bacterial solution was prepared using the same protocol as for the drop plate assay (Arora et al., 2015). This solution was then poured around hard agar plugs made of minimal media, 2% Bacto agar, and either potassium dichromate (500 ppm) or glucose (100 ppm) as a positive control. The plates were incubated at 30 °C for 6 h after the plugs had solidified, and then checked for chemotactic activity.
Identification of genes involved in chemotaxis
The draft genome of Bacillus licheniformis KNP was previously submitted to DDBJ/ENA/GenBank under the accession JACDXS000000000 (Arora et al., 2020). To identify the chemotactic genes, the KNP genome was annotated using the NCBI Prokaryotic Genome Annotation Pipeline (PGAP; Arora et al., 2020).
Statistical analysis
All the experiment setup and data analysis were performed in triplicate (n = 3) to reduce analytical errors, and the results were represented as mean ± standard deviation (SD).
Results and discussion
Growth and Cr(VI) reduction studies
The growth of Bacillus licheniformis strain KNP was monitored in nutrient broth containing various concentrations of K2Cr2O7 (250, 500, 750, and 1,000 ppm) as a source of Cr(VI). As the concentration of chromium increased, the bacterial growth was reduced. In the presence of chromium, the maximum bacterial growth was observed when the K2Cr2O7 concentration was 250 ppm (Figure 1A). When exposed to 1,000 ppm K2Cr2O7, Bacillus licheniformis strain KNP showed extremely reduced growth. In comparison, K2Cr2O7 concentrations from 250 and 500 ppm had only a mild impact on bacterial growth, whereas a concentration of 750 ppm resulted in a significant reduction in bacterial growth, indicating that higher concentrations of Cr(VI) are more toxic to bacterial growth. Previous studies also reported that bacterial growth was reduced at high concentrations of Cr(VI) (Kumar et al., 2023). Liu et al. (2006) reported that the cells of Bacillus sp. could not grow when exposed to 100 ppm chromium, whereas at 80 ppm, bacterial growth was reduced compared to concentrations of 5, 10, 20, and 40 ppm. Upadhyay et al. (2017) observed that Bacillus subtilis MNU16 grew more slowly at higher chromium concentrations (250 and 300 mg/L) compared to 50, 100, 150, and 200 mg/L. Wang et al. (2025) reported that Microbacterium oxydans strain S-1 exhibits robust growth (OD600 > 5.03) under Cr(VI) stress, highlighting its strong bioremediation potential.
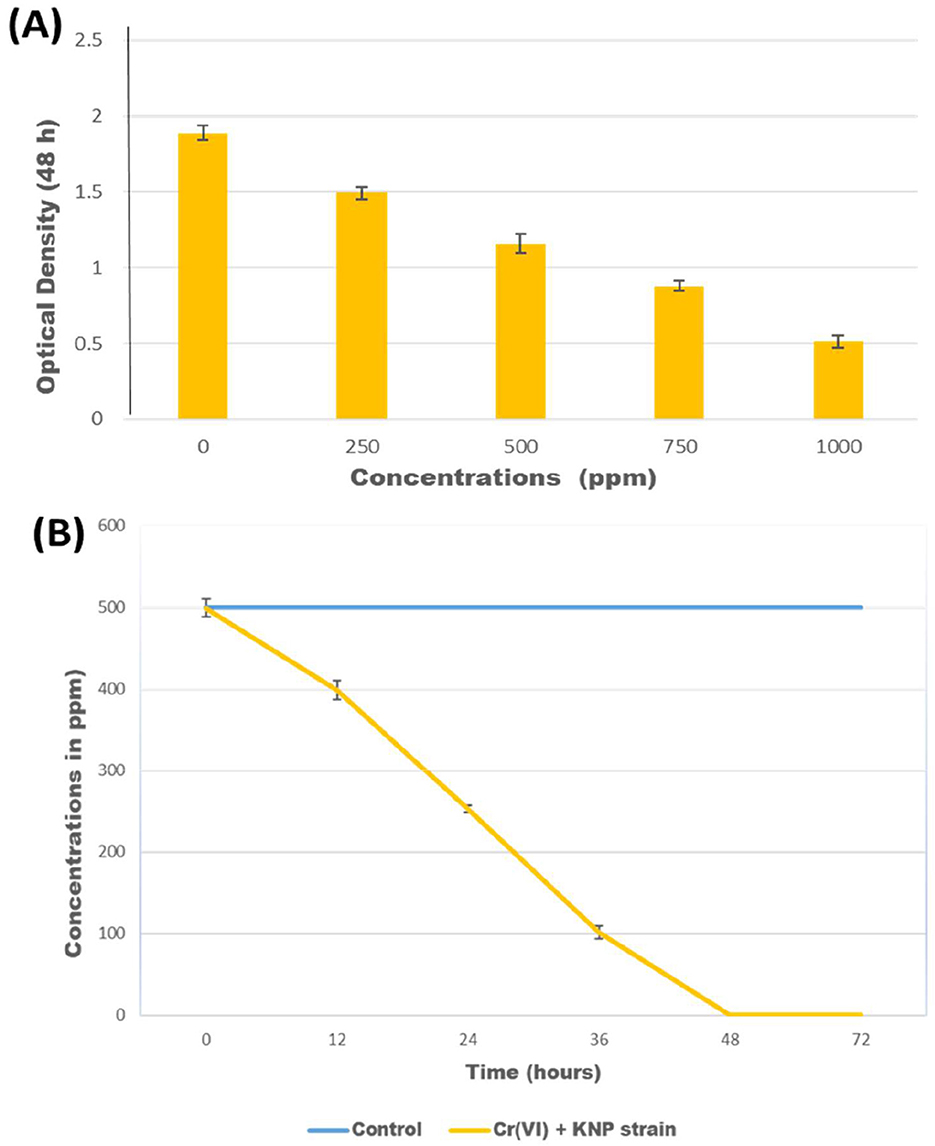
Figure 1. (A) Optical density of culture of Bacillus licheniformis KNP after growth for 48 h in media containing various concentrations of K2Cr2O7; (B) Reduction of Cr(VI) in concentrations of 500 ppm K2Cr2O7 by Bacillus licheniformis KNP.
Bacillus licheniformis KNP completely reduced chromium (500 ppm K2Cr2O7) within 48 h, whereas in the control, no reduction of chromium was observed (Figure 1B). B. licheniformis KNP was found to be more effective than any other Bacillus species reported for chromium reduction due to its ability to reduce 100% chromium at high concentrations. Bacillus subtilis MNU16 reduces Cr(VI) by 75% when exposed to 50 mg/L (Upadhyay et al., 2017). Zhao et al. (2012) reported a 96.85% reduction of Cr(VI) by Bacillus cereus when the initial Cr(VI) concentration was < 50 mg/L. Another report showed that 96.7% and 72.1% of Cr(VI) could be reduced by Bacillus cereus after the initial addition of 60 and 70 mg/L of Cr(VI), respectively (Murugavelh and Mohanty, 2013). After an initial exposure to 2 mM of Cr(VI), the B. cereus D strain reduced 87.8% of the metal in 24 h (Li et al., 2020). Wang et al. (2025) reported that Microbacterium oxydans strain S-1 efficiently reduced Cr(VI), achieving complete detoxification within 48–72 h depending on the initial concentration.
Effects of temperature
The effect of temperature on the Cr(VI) reduction efficiency and growth of Bacillus licheniformis KNP was evaluated over a 48-h incubation period. The results showed a clear temperature-dependent pattern in both chromium detoxification and bacterial proliferation. Maximum Cr(VI) reduction (99.47%) was observed at 30 °C, accompanied by the highest optical density (OD = 1.153), indicating optimal growth and metabolic activity under mesophilic conditions (Figures 2A, B). At 25 °C, Cr(VI) reduction remained substantial (80.47%) with a moderate growth level (OD = 0.911), suggesting that enzymatic activity and biomass accumulation are still effectively maintained near the optimal range. A further increase in temperature to 42 °C resulted in a decline in both reduction efficiency (77.33%) and OD (0.883), indicating the onset of thermal stress, which likely impaired chromate reductase activity and membrane integrity. The lowest reduction efficiency (51.6%) and growth (OD = 0.593) occurred at 20 °C, indicating suboptimal enzymatic kinetics and reduced cellular metabolism under cold stress. Wang et al. (2025) reported that Microbacterium oxydans strain S-1 efficiently reduced over 94.8% of 100 mg·L−1 Cr(VI) within 48 h at 30 °C. Due to the extreme temperatures, bacterial growth is adversely affected, thereby negatively affecting Cr(VI) bacterial reduction. Specifically, low temperatures inhibited cell growth more strongly by decreasing the fluidity of the cell membrane and affecting the transport system, implying that the substrate cannot enter the cell quickly to promote cell growth. Increasing temperatures may alter membrane structure and inhibit reductase and protein synthesis mechanisms (Kathiravan et al., 2010). It is therefore important that the right temperature be maintained in order to facilitate Cr(VI) reduction.
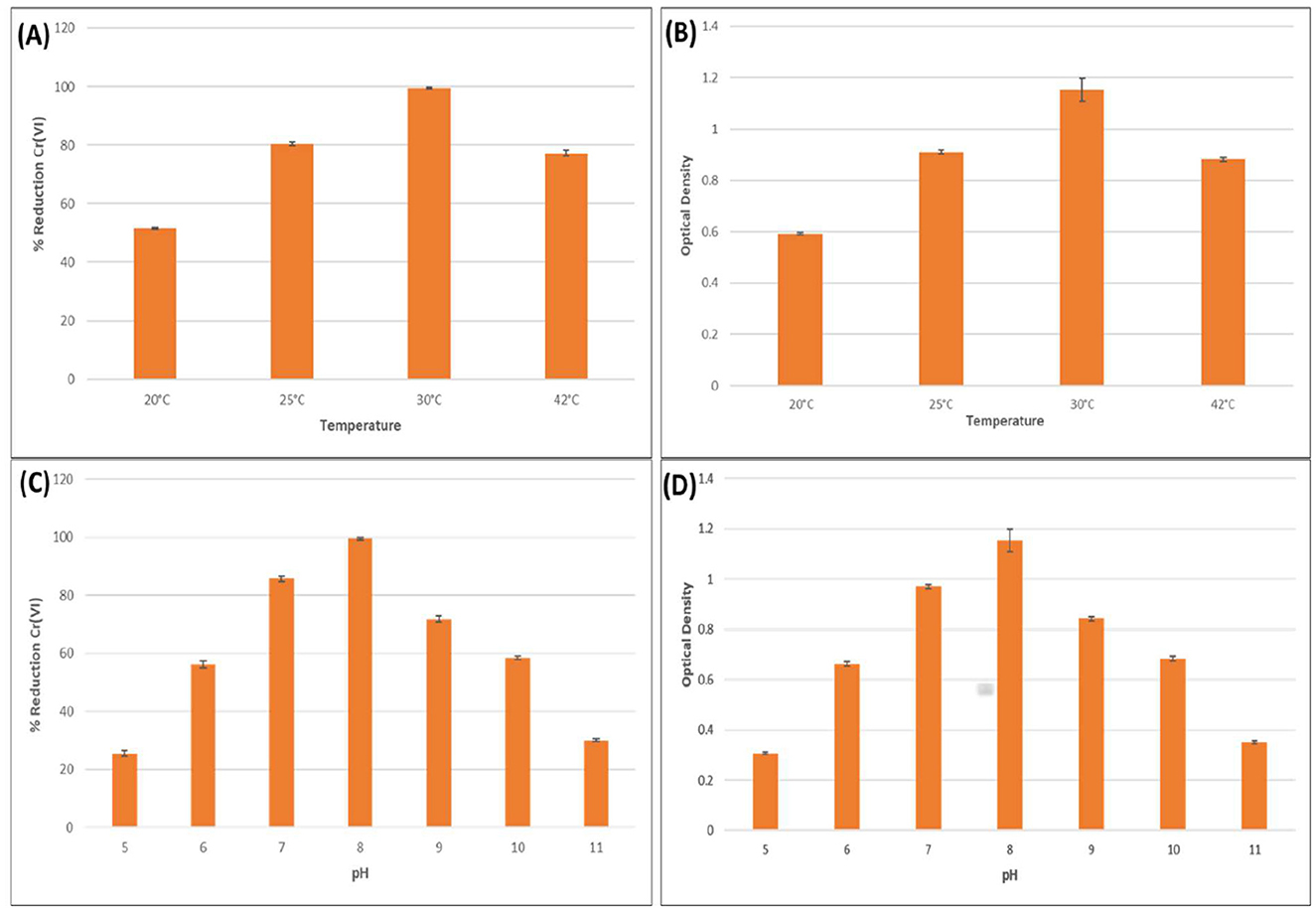
Figure 2. Effects of various temperatures and pH on bacterial growth and hexavalent chromium reduction. Effect of various temperatures (20, 25, 30, and 42 °C) on (A) hexavalent chromium reduction, (B) bacterial growth. Effects of various pH (5, 6, 7, 8, 9, 10, and 11) on (C) hexavalent chromium reduction (D) bacterial growth.
These findings align with other temperature-optimization studies in chromium bioremediation. For instance, Staphylococcus succinus subsp. succinus AMG-D1 demonstrated complete Cr(VI) removal at 200 mg/L within 120 h at 35 °C, reinforcing the importance of mesophilic conditions (Harboul et al., 2025). Similarly, Bacillus subtilis, when immobilized in alginate beads, showed effective Cr(VI) reduction from contaminated soil with optimal performance at pH 6 and 40 °C (Alsamhary, 2025). Although slightly higher than the optimal temperature observed for B. licheniformis KNP, this highlights the species-specific thermal tolerance ranges. The use of immobilization likely contributes to this enhanced thermal stability by providing physical protection to bacterial cells and supporting enzyme retention. Immobilization matrices such as alginate have been shown to buffer external temperature fluctuations, thus preserving enzymatic conformation and reducing potential damage at higher thermal conditions.
The effect of temperature on the chromium-reducing efficiency of various bacterial strains was evaluated to determine optimal conditions for Cr(VI) bioremediation. Bacillus subtilis MNU16 was tested for its Cr(VI) reduction capability under a range of incubation temperatures (20, 25, 30, 37, and 42 °C) over 72 h (Upadhyay et al., 2017). The results demonstrated a strong temperature dependence, with only 32.4% Cr(VI) reduction at 20 °C, which increased to 55.8% at 25 °C. A significant enhancement was observed at 30 °C, where the strain reduced nearly 75% of Cr(VI), and the maximum reduction (>90%) occurred at 37 °C, indicating this as the optimal temperature for enzymatic chromium reduction activity. However, a decline in efficiency (~68%) was recorded at 42 °C, suggesting the onset of thermal stress and possible enzyme denaturation at higher temperatures (Upadhyay et al., 2017). Similar trends were observed with other bacterial strains. Enterococcus italicus, immobilized in alginate beads, achieved a high reduction efficiency of 91% at 35 °C within just 2 h, which further improved to 94% with glucose supplementation, highlighting its rapid and thermally optimized detoxification potential (Srivastava et al., 2025a). Likewise, Klebsiella sp. (BH-A1), when immobilized in alginate beads containing 1000 mg/g biomass, showed maximum Cr(VI) reduction (87%) at 30 °C. Reduction declined slightly at 25 °C (62%) and 35 °C (78%), confirming 30 °C as the optimal range for chromate reductase activity in this strain as well (Srivastava et al., 2025b). Furthermore, Priestia megaterium strain BM.1 and its immobilized composite form (BM.1-Ca) were assessed over the 20 °C to 40 °C range. The BM.1-Ca composite consistently outperformed free cells, with peak reduction occurring at 35 °C. However, a decrease in activity was noted at 40 °C, possibly due to heat-induced inactivation of microbial enzymes, particularly chromate reductases. The immobilization matrix likely conferred additional thermal protection and stability, enhancing bioremediation performance across temperature ranges (Wu et al., 2025). Collectively, these results confirmed that mesophilic conditions, particularly in the 30–37 °C range, are most conducive to bacterial Cr(VI) reduction. They also underscore the role of immobilization techniques in improving thermal resilience and functional efficiency of bioremediating strains.
Effects of pH
The influence of pH on Bacillus licheniformis performance was assessed to determine its Cr(VI) reduction efficiency and growth at 500 ppm K2Cr2O7 over 48 h. The results showed a distinct pH-dependent trend, where both bacterial growth and Cr(VI) detoxification were significantly influenced by the pH of the medium. Maximum growth (OD600 = 1.153) and Cr(VI) reduction (99.47%) were observed at pH 8, suggesting that slightly alkaline conditions favored both cell proliferation and enzymatic activity essential for chromate reduction (Figures 2C, D). This could be attributed to enhanced membrane stability, optimal enzyme conformation, and improved electron transport under these conditions. In contrast, both growth and reduction efficiencies declined sharply at pH extremes. At pH values 5 and 11, the OD600 dropped to 0.306 and 0.350, respectively, while Cr(VI) reduction fell to 25.47% and 30.07%. These findings indicate that highly acidic or alkaline conditions disrupt cellular homeostasis and likely denature chromate reductase enzymes or hinder electron donor availability, thereby impairing reduction pathways. These observations are consistent with findings from other studies. For example, Enterococcus italicus immobilized in alginate beads exhibited optimal Cr(VI) reduction (91%) at neutral pH 7 within 2 h, highlighting the sensitivity of reduction efficiency to pH changes (Srivastava et al., 2025a). Similarly, Klebsiella sp. (BH-A1) demonstrated maximum Cr(VI) reduction (87%) at pH 7 and 30 °C, with reduced performance under both acidic and alkaline conditions (Srivastava et al., 2025b). While Bacillus wiedmannii showed the highest Cr(VI) removal efficiency of 70.27% at pH 8, indicating that slightly alkaline conditions are optimal for chromate reduction. Similarly, bacterial growth was also enhanced at this pH, suggesting a positive correlation between cell proliferation and Cr(VI) detoxification under favorable pH conditions (Adhikary et al., 2025). Bacillus paramycoides S48 chromate reductase exhibited 100% activity retention at pH 7 and high enzymatic stability across the range of pH 6.0–8.0, while activity sharply declined outside this window (Kalsoom et al., 2025). Conversely, for Cr(III) removal, Pseudomonas atacamensis M7D1 showed the maximum adsorption at pH 4, indicating a different biosorption mechanism through extracellular polymeric substances (EPS), which is more effective under acidic conditions (Tarahomi et al., 2025). These comparative results support the conclusion that a narrow pH range, typically near-neutral to slightly alkaline, is ideal for bacterial growth and efficient Cr(VI) detoxification in bioremediation systems.
SEM analysis and EDX analysis
In SEM-EDX analysis, Cr(VI) was shown to affect bacterial morphology and accumulate on cell surfaces. Cells of the strain were regular rod-shaped in the control, and there was no aggregation of cells (Figure 3aA). Furthermore, no Cr diffraction peaks were observed in the EDX spectrum. However, when stressed with Cr (VI), the cells showed imperfections, clusters, and aggregation (Figure 3aB), and the characteristic Cr peaks were found in the EDX spectrum (Figure 3b). Tan et al. (2020) observed the same result in Bacillus sp. CRB-B1 under Cr(VI) stress. In addition, Escherichia sp. TH-1 exhibited aggregation and rough surfaces after exposure to Cr(VI) (Wang et al., 2022). The aggregation and irregular cell morphology were also observed in Cr (VI)-treated cells of Sinorhizobium sp. SAR1 (Jobby et al., 2019). A part of bacteria, the fungus Trichoderma lixii CR700 also exhibited aggregation of mycelia during chromium stress (Kumar and Dwivedi, 2019). Wu et al. (2019) reported that cell deformation was caused by Cr adsorption.
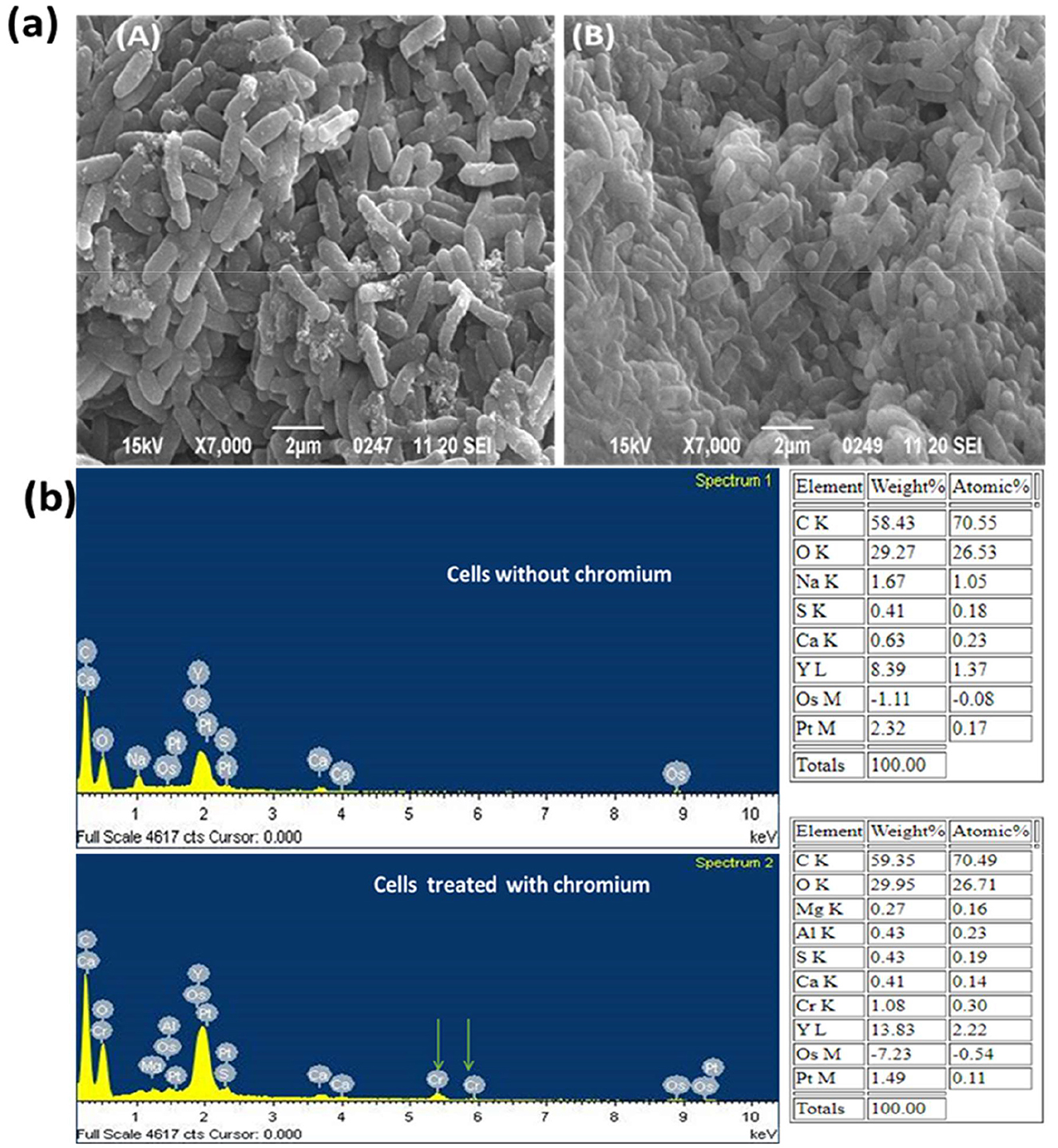
Figure 3. (a) SEM analysis of chromium untreated (A) and chromium treated cells (B) of B. licheniformis strain KNP; (b) Energy Dispersive X-ray (EDX) analysis of chromium untreated and chromium treated cells of B. licheniformis strain KNP.
The process of bacterial aggregation is considered a survival and detoxification mechanism under chromate stress. Additionally, bacteria secrete more extracellular materials under heavy metal stress (Karthik et al., 2017; Wu et al., 2019). Jobby et al. (2019) observed that Sinorhizobium sp. SAR1 secretes a large amount of capsular material in response to chromium exposure, resulting in increased cell size. An increase in size and the secretion of extra polysaccharide substances were also observed in a variety of bacteria under chromate stress. However, no increases in bacterial cell size were observed in this study.
Energy-dispersive X-ray spectroscopy (EDX) was used to assess the elemental profile of Bacillus licheniformis KNP following exposure to hexavalent chromium [Cr(VI)]. The resulting spectra indicated a chromium content of 1.08 % (by weight), suggesting limited surface binding of the metal. This low surface association implies that most of the chromium either penetrated the cells or was transformed intracellularly, rather than forming substantial extracellular precipitates.
This observation is supported by a related study by Ramli et al. (2025), who used EDX to examine Bacillus sp. S1 treated with chromium. Their findings showed a similarly low Cr peak (0.9% by weight) on the bacterial surface, suggesting minimal extracellular chromium accumulation. Based on these data, Ramli et al. (2025) proposed that the visible precipitate seen on the surface corresponds to untransformed Cr(VI). That chromium reduction does not occur extracellularly, implying that the bacterial cells likely reduce Cr(VI) internally.
Thus, in both cases, EDX confirmed only minimal Cr accumulation on the cell surface, supporting the conclusion that chromium reduction or detoxification processes likely occur within the bacterial cell, rather than outside it.
FTIR analysis
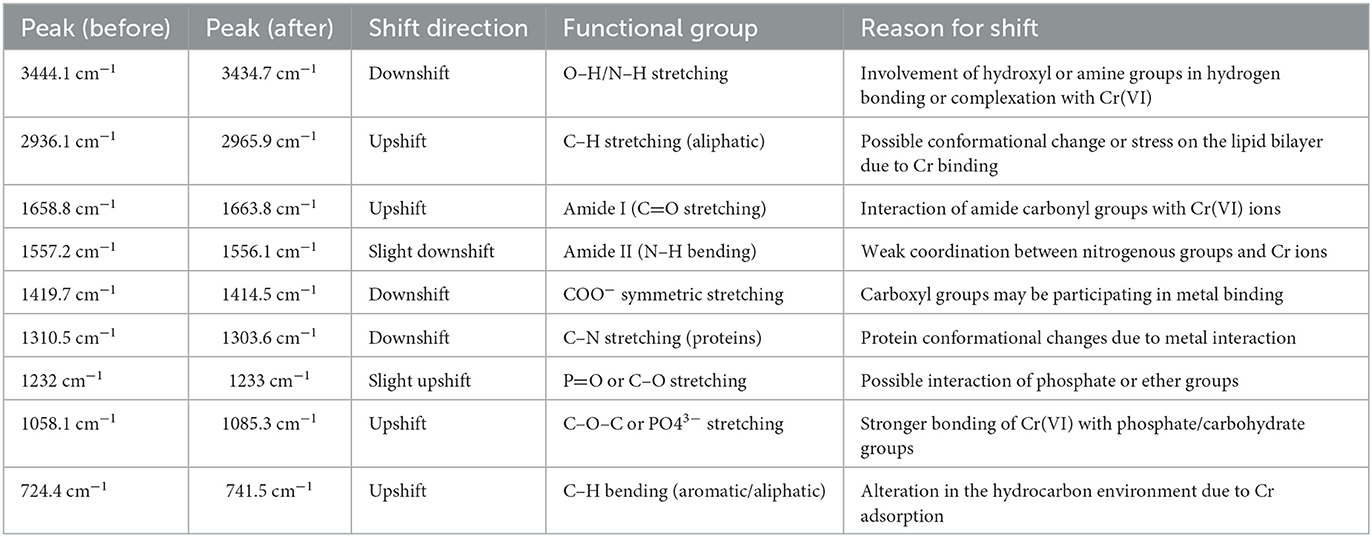
The FTIR spectroscopy analysis was performed to identify the functional groups and chemical bonds involved in the biosorption of hexavalent chromium. The FTIR analysis of Bacillus licheniformis KNP with and without chromium is shown in Figure 4. Infrared spectra of B. licheniformis KNP in the absence of chromium stress showed characteristic absorption peaks of an amine (–NH2), bonded and non-bonded hydroxyl (O–H) groups, amide (–CONH–), carboxyl (–COOH), aromatic (C = C) ring, alkenes (–C = C), aliphatic (–CH2) groups, etc., which confirmed the presence of corresponding groups on the cell surfaces (Figure 4a). The peak at 3444.1 cm−1 is attributed to the presence of amine N–H stretching or the presence of a hydroxyl group (O–H) in that region. The absorption peak at 2936.1 cm−1 was attributed to aliphatic (–CH2) groups, which indicates C–H asymmetric stretching. The FTIR spectra peaks at 1658.8, 1557.2, 1419.7, 1310.5, 1232, and 1058.1 cm−1 correspond to carboxyl group (C=O), amide (–CONH–), hydroxyl (–OH) groups, –NO, aromatic amine (C–N), and phosphate group, respectively. The peak at 724 cm−1 can be assigned to C–H out-of-plane bending on the disubstituted benzene ring. However, when the strain KNP was subjected to chromium stress, changes in peaks and peak intensities were observed. Under chromium stress, FTIR peaks of B. licheniformis KNP shifted from 3444.1 to 3434.7 cm−1, 2936.1 to 2965.9 cm−1, 1658.8 to 1663.8 cm−1, 1557.2 to 1556.1 cm−1, 1419.7 to 1414.5 cm−1, 1310.5 to 1303.6 cm−1, 1232 to 1233 cm−1, 1058.1 to 1085.3 cm−1, and 724.4 to 741.5 cm−1. In Cr (VI)-treated bacterial cells, these variations were primarily caused by carboxyl, amino, sulfonate, and hydroxyl functional groups, which are chemically reactive with Cr ions and form permanent bonds with Cr. Table 1 summarizes the observed FTIR spectral peak shifts in Bacillus licheniformis KNP before and after Cr(VI) exposure, indicating interactions between functional groups on the bacterial surface and chromium ions. Adhikary et al. (2025) also reported that, under Cr(VI) stress, spectral analysis showed changes in peak absorption and intensity due to interactions between Cr(VI) and functional groups on the bacterial cell surface. However, Wang et al. (2025) observed no change in intensity of the peaks in the spectra before and after treatment in cells of Microbacterium oxydans strain S-1, suggesting that there was no noticeable variation in the types of functional groups identified across the samples of strain S-1.
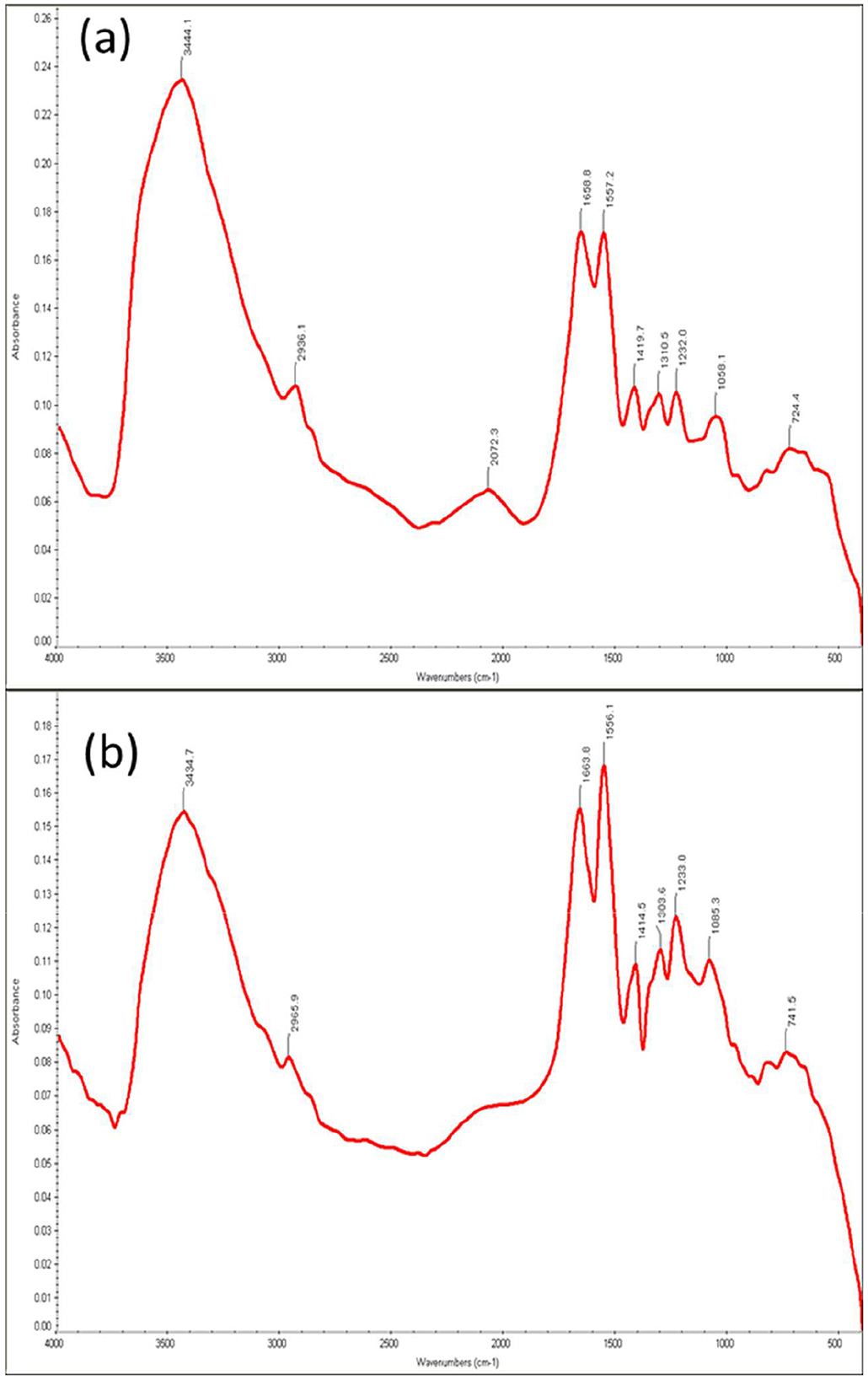
Figure 4. FTIR analysis of cells of Bacillus licheniformis KNP (a) without Cr(VI) treatment and (b) with Cr(VI) treatment.
Chemotaxis away from chromium
This research evaluated the chemotactic movement of Bacillus licheniformis strain KNP through the drop plate assay and chemical plug technique to assess its reactions to Cr(VI) and glucose. The two techniques presented information regarding the direction of movement of the strain toward or away from chemical stimuli.
Negative chemotaxis toward Cr(VI)
In the drop plate assay, Bacillus licheniformis strain KNP exhibited a clear avoidance response to Cr(VI), forming a well-defined zone of separation around the chromium crystal (Figure 5aB). This indicates that Cr(VI) acts as a repellent, triggering a negative chemotactic response in the strain. The same was observed under the chemical plug assay, where KNP cells migrated away from the Cr(VI) agar plug actively and formed a comparable clear zone (Figure 5bb). These results are consistent with previous studies that demonstrated that microorganisms react to several toxic compounds, such as heavy metals, hydrocarbons, and oxidizing agents like hydrogen peroxide, hypochlorite, and N-chlorotaurine, with negative chemotaxis (Tso and Adler, 1974; Young and Mitchell, 1973; Ohga et al., 1993; Benov and Fridovich, 1996). This avoidance response is considered a crucial survival strategy, where microbes avoid environments with poisonous levels of chemicals. The negative chemotaxis of B. licheniformis strain KNP toward Cr(VI) suggests its potential application in bioremediation. Although this avoidance behavior can initially be repulsive to microbial colonization of polluted sites, the ability of the strain to metabolize or immobilize chromium remains exploitable to reduce environmental pollution. A study of the metabolic pathways and control mechanisms governing this chemotactic response may further optimize the strategic use of B. licheniformis in bioremediation.
![Two-part image showing cell movement. In part (a), two petri dishes display cells moving towards glucose (left, cloudy) and away from Cr[VI] (right, yellowish). In part (b), an agar plate shows areas labeled a, b, and c. Cells move away from Cr[VI] and towards glucose, indicating chemotactic behavior.](https://www.frontiersin.org/files/Articles/1646518/fmicb-16-1646518-HTML/image_m/fmicb-16-1646518-g005.jpg)
Figure 5. Chemotaxis away from chromium. (a) Drop plate assay showing accumulation of KNP cells toward glucose (A) and movement of KNP cells away from hexavalent chromium (B). (b) Chemical in Plug assay showing accumulation of KNP cells toward glucose (a). Movement of KNP cells away from hexavalent chromium (b), where no chemotactic movement is observed toward agar plug without chromium or glucose.
Positive chemotaxis toward glucose
In contrast to its response to Cr(VI), strain KNP showed positive chemotaxis to glucose in both procedures. For the drop plate assay, cells migrated toward the source of glucose with a dense accumulation around it (Figure 5aA). The same was observed for the chemical plug assay, where the strain migrated toward the glucose agar plug to create an accumulation ring (Figure 5ba). This response highlights glucose as a strong attractant, likely due to its role as an energy source used in promoting bacterial growth and metabolism. Positive chemotaxis to glucose emphasizes the ability of the strain to detect and migrate toward nutrient-rich environments, which is essential for survival and colonization, particularly under conditions of nutrient limitation. This characteristic is beneficial in bioremediation strategies because organic nutrients can be supplemented to induce microbial growth and maximize detoxification efficiency. The glucose chemotactic response may also facilitate co-consumption of Cr(VI) and organic substrates, maximizing bioremediation efficiency in mixed-contaminant systems. These findings confirmed that Bacillus licheniformis strain KNP has special chemotactic activities: negative chemotaxis from Cr(VI), and positive chemotaxis toward glucose. These characteristics suggest that the strain KNP can be utilized for bioremediation, as its ability to repel toxic heavy metals while migrating toward nutrient-rich zones can be utilized to enhance remediation procedures. Further studies should focus on clarifying the molecular and genetic foundations of these chemotactic processes to further develop the environmental potential of the strain.
Annotation of chemotactic genes
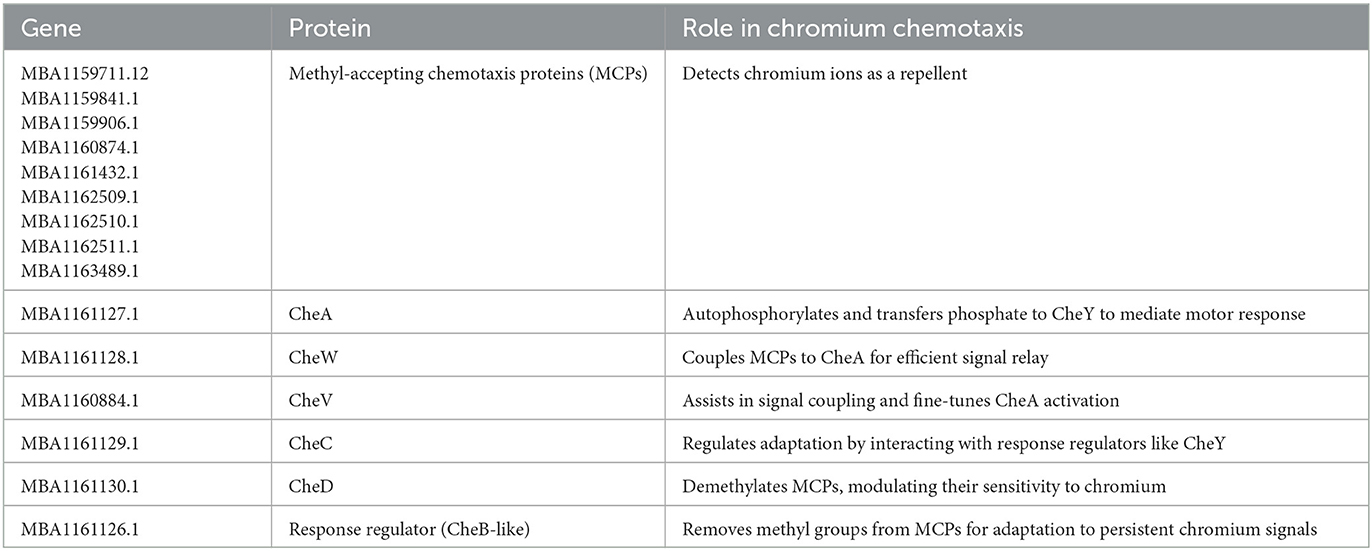
The identification of chemotaxis-specific genes in Bacillus licheniformis strain KNP provides us with insight into the mechanisms of its chemotactic responses to glucose and Cr(VI) at the molecular level. The genes encode proteins that participate in sensing chemical gradients, transduction, and regulation of motility. Bacterial chemotaxis is a highly regulated process by particular proteins like methyl-accepting chemotaxis proteins (MCPs) and chemotaxis (Che) proteins that facilitate signal perception, transduction, and movement based on environmental signals (Bi and Sourjik, 2018). MCPs are transmembrane receptors and are the primary sensors for chemical gradients. MCPs in Escherichia coli detect attractants or repellents and convey signals to downstream components of the chemotaxis signaling pathway (Rollins and Dahlquist, 1981; Parkinson et al., 2015). Specific MCPs have been identified in various bacteria that respond to particular compounds, such as NtdY and NbaY, which function as MCPs for 2-nitrotoluene and 2-nitrobenzoate in Acidovorax strain JS42 (Rabinovitch-Deere and Parales, 2012) and Pseudomonas fluorescens strain KU-7, respectively (Iwaki et al., 2007). Table 2 summarizes a list of annotated chemotactic genes in Bacillus licheniformis KNP.
Based on identified chemotactic genes, we proposed a mechanism of negative chemotaxis of chromium in the Bacillus licheniformis strain KNP (Figure 6).
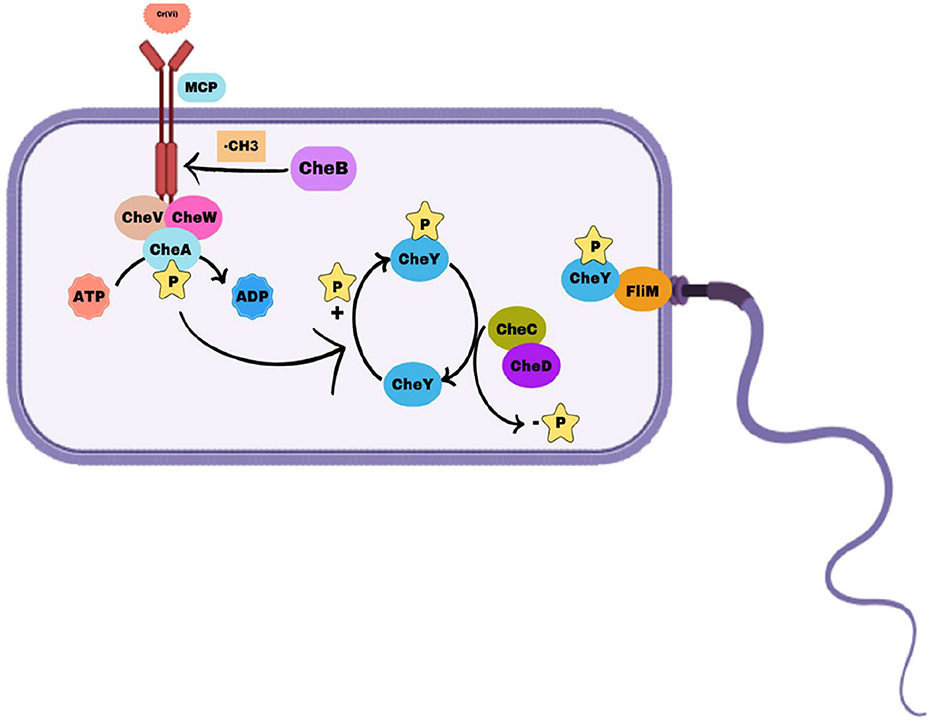
Figure 6. Mechanism of chemotaxis in Bacillus licheniformis strain KNP. Methyl-accepting chemotaxis proteins (MCPs) detect environmental signals and interact with CheW and the histidine kinase CheA to form a signaling complex. Upon activation, CheA autophosphorylates and transfers the phosphoryl group to the response regulator CheY. Phosphorylated CheY (CheY-P) binds to the flagellar motor protein FliM, inducing clockwise (CW) rotation and tumbling, whereas dephosphorylated CheY allows counterclockwise (CCW) rotation for smooth swimming. CheC and CheD modulate the dephosphorylation of CheY-P, influencing motor control, whereas CheV plays a role in signal adaptation by linking MCP signaling with the phosphorylation system. Methyl esterase dynamically regulates MCP activity, ensuring efficient bacterial navigation in response to environmental changes.
Sensing chromium as a repellent
Methyl-Accepting Chemotaxis Proteins (MCPs) like MBA1159711.1, MBA1159841.1, MBA1159906.1, and others detect chromium ions [e.g., Cr(VI)] as a repellent through their extracellular ligand-binding domains. The binding of chromium induces a conformational change in the MCPs, activating their intracellular signaling domains.
Signal relay and activation of CheA
The activated MCPs interact with CheW (MBA1161128.1) to form a complex that connects MCPs to the histidine kinase CheA (MBA1161127.1). The conformational change in MCPs stimulates the autophosphorylation of CheA on a conserved histidine residue. CheV (MBA1160884.1) assists in coupling the MCP activity to CheA, fine-tuning the response.
Phosphotransfer and flagellar control
Phosphorylated CheA transfers its phosphate group to the response regulator protein CheY (not listed but assumed present in strain KNP). Phosphorylated CheY interacts with the flagellar motor, inducing clockwise rotation. Clockwise rotation causes the strain to tumble, disrupting its forward motion and reorienting the cell away from the chromium source.
Adaptation mechanisms for persistent chromium signals
The methylation status of MCPs is dynamically regulated to enable adaptation: CheB (MBA1161126.1) removes methyl groups from MCPs, reducing their sensitivity to chromium over time. CheD (MBA1161130.1) also participates in MCP demethylation, supporting signal adaptation. CheC (MBA1161129.1) acts as a regulator, ensuring proper adaptation and preventing overstimulation of the pathway.
Resetting the chemotaxis machinery
As the bacterium moves away from the chromium gradient, MCPs return to their baseline state. CheB and CheD restore receptor sensitivity by demethylating MCPs, preparing the cell for future responses to chromium or other stimuli.
Movement away from chromium
The continuous phosphorylation and dephosphorylation cycle of CheA and CheY leads to controlled reorientation and swimming in new directions. The strain eventually develops a net movement away from the chromium source, avoiding toxicity.
Conclusion
Bacillus licheniformis KNP grew well in the presence of the hexavalent chromium and completely reduced Cr(VI) within 48 h when supplemented with 500 ppm K2Cr2O7. Compared to the control, Cr (VI)-treated KNP cells showed a decrease in cell size, cell aggregation, and slight deformity in cell shape. Furthermore, the Cr treated cells of strain KNP accumulated a small amount of Cr on their surface. A Cr(VI) molecule interacts with functional groups and chemical bonds in KNP cells.
The strain KNP uses MCPs to detect chromium ions as a repellent. The signal is transmitted through CheW and CheA to CheY, which alters flagellar rotation and promotes reorientation. Adaptation proteins such as CheB and CheD modulate receptor sensitivity, ensuring the strain maintains effective negative chemotaxis by moving away from chromium-contaminated environments to avoid its toxic effects.
Data availability statement
The genome sequence of the bacterial strain KNP is publicly available. This data can be found here: https://www.ncbi.nlm.nih.gov/nuccore/JACDXS000000000.1/. All other data are available on request to corresponding authors.
Author contributions
AG: Writing – review & editing, Formal analysis. MA: Writing – review & editing, Formal analysis. AA: Writing – review & editing, Formal analysis. PC: Writing – review & editing, Formal analysis. AK: Formal analysis, Writing – review & editing. RM: Formal analysis, Writing – review & editing. VD: Writing – review & editing, Formal analysis. AS: Writing – review & editing, Validation. SG: Writing – review & editing, Investigation. PA: Project administration, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. PA acknowledges Department of Biotechnology, India for financial support the work. AS acknowledges Department of Science and Technology, India for providing her INSPIRE Fellowship.
Acknowledgments
PA acknowledges the Department of Biotechnology, India, for providing him with the Ramalingaswami Re-entry Fellowship.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author declares that Gen AI was used in the creation of this manuscript. The authors also acknowledge the use of various AI tools, including ChatGPT and Microsoft Copilot, for their assistance in improving the manuscript writing.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbas, A., Hameed, R., Balooch, S., Khattak, W. A., Raza, M. M., Zulfiqar, U., et al. (2025). “Evaluation and monitoring of chromium and beneficial elements,” in Beneficial Elements for Remediation of Heavy Metals in Polluted Soil, eds. S. Saud, S. Fahad, and D. Wang (Elsevier), 141–160. doi: 10.1016/B978-0-443-26522-8.00005-2
Adhikary, S., Saha, B. K., Roy, V., Saha, J., and Pal, A. (2025). Deciphering chromate tolerance and reduction ability of an indigenous Bacillus strain isolated from polluted pond sludge for chromium bioremediation. Sci. Rep. 15:23323. doi: 10.1038/s41598-025-07031-4
Agrawal, M., Sharma, A., Singh, A., and Sundaram, S. (2025). Bioremediation of Cr(VI) using novel thermophilic bacteria Brevibacillus borstelensis SSAU-3T: optimization, mechanism and phytotoxicity study. Biodegradation 36:52. doi: 10.1007/s10532-025-10145-1
Akbarpour Nesheli, M., Asgarani, E., and Dabbagh, R. (2017). Biosorption potential of Cr(VI) by Kocuria sp. ASB107, a radio-resistant bacterium isolated from Ramsar, Iran. Chem. Ecol. 34, 163–176. doi: 10.1080/02757540.2017.1399126
Alsamhary, K. E. (2025). Optimizing the process conditions for the biosorption of chromium (VI) by Bacillus subtilis in artificial wastewater. Electron. J. Biotechnol. 76, 22–38. doi: 10.1016/j.ejbt.2025.03.005
Arora, P. K., Jeong, M.-J., and Bae, H. (2015). Chemotaxis away from 4-chloro-2-nitrophenol, 4-nitrophenol, and 2,6-dichloro-4-nitrophenol by Bacillus subtilis PA-2. J. Chem. 2015:296231. doi: 10.1155/2015/296231
Arora, P. K., Mishra, R., Omar, R. A., Saroj, R. S., Srivastava, A., Garg, S. K., et al. (2020). Draft genome sequence data of a chromium-reducing bacterium, Bacillus licheniformis strain KNP. Data Brief 34:106640. doi: 10.1016/j.dib.2020.106640
Barrionuevo, M. R., and Vullo, D. L. (2012). Bacterial swimming, swarming and chemotactic response to heavy metal presence: which could be the influence on wastewater biotreatment efficiency? World J. Microbiol. Biotechnol. 28, 2813–2825. doi: 10.1007/s11274-012-1091-5
Benov, L., and Fridovich, I. (1996). Escherichia coli exhibits negative chemotaxis in gradients of hydrogen peroxide, hypochlorite, and N-chlorotaurine: products of the respiratory burst of phagocytic cells. Proc. Natl. Acad. Sci. U.S.A. 93, 4999–5002. doi: 10.1073/pnas.93.10.4999
Bharagava, R. N., and Mishra, S. (2018). Hexavalent chromium reduction potential of Cellulosimicrobium sp. isolated from common effluent treatment plant of tannery industries. Ecotoxicol. Environ. Saf. 147, 102–109. doi: 10.1016/j.ecoenv.2017.08.040
Bi, S., and Sourjik, V. (2018). Stimulus sensing and signal processing in bacterial chemotaxis. Curr. Opin. Microbiol. 45, 22–29. doi: 10.1016/j.mib.2018.02.002
Chai, L., Ding, C., Li, J., Yang, Z., and Shi, Y. (2019). Multi-omics response of Pannonibacter phragmitetus BB to hexavalent chromium. Environ. Pollut. 249, 63–73. doi: 10.1016/j.envpol.2019.03.005
Chatterjee, S., Sau, G. B., and Mukherjee, S. K. (2011). Bioremediation of Cr(VI) from chromium-contaminated wastewater by free and immobilized cells of Cellulosimicrobium cellulans KUCr3. Bioremediat. J. 15, 173–180. doi: 10.1080/10889868.2011.598488
Chen, W., Li, W., Wang, T., Wen, Y., Shi, W., Zhang, W., et al. (2022). Isolation of functional bacterial strains from chromium-contaminated site and bioremediation potentials. J. Environ. Manage. 307:114557. doi: 10.1016/j.jenvman.2022.114557
Doganli, G. A., and Dogan, N. M. (2014). Reduction of Cr(VI) to Cr(III) by thermal Bacillus licheniformis B22 under different temperatures using binary and ternary combinations of organic acids. Desalin. Water Treat. 52, 7163–7171. doi: 10.1080/19443994.2013.823117
Elahi, A., Ajaz, M., Rehman, A., Vuilleumier, S., Khan, Z., and Hussain, S. Z. (2019). Isolation, characterization, and multiple heavy metal-resistant and hexavalent chromium-reducing Microbacterium testaceum B-HS2 from tannery effluent. J. King Saud Univ. Sci. 31, 1437–1444. doi: 10.1016/j.jksus.2019.02.007
Garcia da Cunha, A., Braun Bunde, D. A., Gamboa Araújo Morselli, L. B., Rodrigues da Silva Júnior, F. M., Silveira Quadro, M., Andreazza, R., et al. (2025). Bioremediation of chromium by Exiguobacterium acetylicum isolated from the macrophyte Hydrocotyle ranunculoides present in a stream in Southern Brazil. Bioremediat. J. 1–15. doi: 10.1080/10889868.2025.2505155
Gupta, P., Rani, R., Chandra, A., and Kumar, V. (2018). Potential applications of Pseudomonas sp. (strain CPSB21) to ameliorate Cr6+ stress and phytoremediation of tannery effluent-contaminated agricultural soils. Sci. Rep. 8:4860. doi: 10.1038/s41598-018-23322-5
Harboul, K., Chtibi, H., and Amakdouf, H. others (2025). Bioreduction of hexavalent chromium and removal mechanisms using Staphylococcus succinus. World J. Microbiol. Biotechnol. 41:147. doi: 10.1007/s11274-025-04347-1
Iwaki, H., Muraki, T., Ishihara, S., Hasegawa, Y., Rankin, K. N., Sulea, T., et al. (2007). Characterization of a pseudomonad 2-nitrobenzoate nitroreductase and its catabolic pathway-associated 2-hydroxylaminobenzoate mutase and a chemoreceptor involved in 2-nitrobenzoate chemotaxis. J. Bacteriol. 189, 3502–3514. doi: 10.1128/JB.01098-06
Jobby, R., Jha, P., Gupta, A., Gupte, A., and Desai, N. (2019). Biotransformation of chromium by root nodule bacteria Sinorhizobium sp. SAR1. PLoS One 14:e0219387. doi: 10.1371/journal.pone.0219387
Kalsoom, K., Din, S. U., Ceylan, E., Hasan, F., Khan, S., Badshah, M., et al. (2025). Cloning and expression of chromate reductase from Bacillus paramycoides S48 for chromium remediation. Sci. Rep. 15:18796. doi: 10.1038/s41598-025-03412-x
Karthik, C., Barathi, S., Pugazhendhi, A., Ramkumar, V. S., Thi, N. B. D., and Arulselvi, P. I. (2017). Evaluation of Cr(VI) reduction mechanism and removal by Cellulosimicrobium funkei strain AR8, a novel haloalkaliphilic bacterium. J. Hazard. Mater. 333, 42–53. doi: 10.1016/j.jhazmat.2017.03.037
Kathiravan, M. N., Karthick, R., Muthu, N., Muthukumar, K., and Velan, M. (2010). Sonoassisted microbial reduction of chromium. Appl. Biochem. Biotechnol. 160, 2000–2013. doi: 10.1007/s12010-009-8716-7
Kavitha, V., Radhakrishnan, N., Gnanamani, A., and Mandal, A. B. (2011). Management of chromium-induced oxidative stress by marine Bacillus licheniformis. Biol. Med. 3, 16–26.
Kumar, A., Mishra, R., Srivastava, A., Garg, S. K., Singh, V. P., and Arora, P. K. (2023). Diversity of hexavalent chromium-reducing bacteria and physicochemical properties of the Kanpur tannery wastewater. Environ. Chem. Ecotoxicol. 5, 205–212. doi: 10.1016/j.enceco.2023.11.001
Kumar, A., Singh, A. K., Singh, P. K., Singh, A. L., Saikia, B. K., and Kumar, A. (2019). Desulfurization of Giral lignite of Rajasthan (Western India) using Burkholderia sp. GR 8–02. Int. J. Coal Prep. Util. 42, 735–751. doi: 10.1080/19392699.2019.1651721
Kumar, A., Singh, A. L., Rajak, P. K., Kumar, A., and Singh, P. K. (2024). Beneficiation of high sulfur Tertiary coal of Assam with Burkholderia sp. GR 8-02: an eco-friendly approach toward clean coal production. Geomicrobiol. J. 41, 996–1007. doi: 10.1080/01490451.2024.2412005
Kumar, M., and Saini, H. S. (2019). Reduction of hexavalent chromium (VI) by indigenous alkaliphilic and halotolerant Microbacterium sp. M5: comparative studies under growth and nongrowth conditions. J. Appl. Microbiol. 127, 1057–1068. doi: 10.1111/jam.14366
Kumar, V., and Dwivedi, S. K. (2019). Hexavalent chromium stress response, reduction capability and bioremediation potential of Trichoderma sp. isolated from electroplating wastewater. Ecotoxicol. Environ. Saf. 185:109734. doi: 10.1016/j.ecoenv.2019.109734
Kumari, A., Kamaraj, N., Selvaraj, R., and Nanoth, R. (2025). Emerging trends and future outlook on chromium removal in the lab, pilot scale, and industrial wastewater system: an updated review exploring 10 years of research. Environ. Monit. Assess. 197:547. doi: 10.1007/s10661-025-13904-y
Li, M. H., Gao, X. Y., Li, C., Yang, C. L., Fu, C. A., Liu, J., et al. (2020). Isolation and identification of chromium-reducing Bacillus cereus species from chromium-contaminated soil for the biological detoxification of chromium. Int. J. Environ. Res. Public Health 17:2118. doi: 10.3390/ijerph17062118
Liu, Y. G., Xu, W. H., Zeng, G. M., Li, X., and Gao, H. (2006). Cr(VI) reduction by Bacillus sp. isolated from chromium landfill. Process Biochem. 41, 1981–1986. doi: 10.1016/j.procbio.2006.04.020
Maurya, A., Kumar, P. S., and Raj, A. (2022). Characterization of biofilm formation and reduction of hexavalent chromium by bacteria isolated from tannery sludge. Chemosphere 286:131795. doi: 10.1016/j.chemosphere.2021.131795
Mishra, S., Chen, S., Saratale, G. D., Saratale, R. G., Ferreira, L. F. R., Bilal, M., et al. (2021). Reduction of hexavalent chromium by Microbacterium paraoxydans isolated from tannery wastewater and characterization of its reduced products. J. Water Process Eng. 39:101748. doi: 10.1016/j.jwpe.2020.101748
Murugavelh, S., and Mohanty, K. (2013). Isolation, identification and characterization of Cr(VI) reducing Bacillus cereus from chromium-contaminated soil. Chem. Eng. J. 230, 1–9. doi: 10.1016/j.cej.2013.06.049
Naeem, A., Batool, R., and Jamil, N. (2013). Cr(VI) reduction by Cellulosimicrobium sp. isolated from tannery effluent. Turk. J. Biol. 37, 315–322. doi: 10.3906/biy-1209-18
Ohga, T., Masduki, A., Kato, J., and Ohtake, H. (1993). Chemotaxis away from thiocyanic and isothiocyanic esters in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 113, 63–66. doi: 10.1111/j.1574-6968.1993.tb06488.x
Pal, A., Bhattacharjee, S., Saha, J., Sarkar, M., and Mandal, P. (2022). Bacterial survival strategies and responses under heavy metal stress: a comprehensive overview. Crit. Rev. Microbiol. 48, 327–355. doi: 10.1080/1040841X.2021.1970512
Pandey, G., and Jain, R. K. (2002). Bacterial chemotaxis toward environmental pollutants: role in bioremediation. Appl. Environ. Microbiol. 68, 5789–5795. doi: 10.1128/AEM.68.12.5789-5795.2002
Parkinson, J. S., Hazelbauer, G. L., and Falke, J. J. (2015). Signaling and sensory adaptation in Escherichia coli chemoreceptors: 2015 update. Trends Microbiol. 23, 257–266. doi: 10.1016/j.tim.2015.03.003
Pinki, S. A., Karim, M. R., Dewanjee, D., Bhuiyan, H. R., Abdullah, H. M., Masud, A., et al. (2021). Microbial reduction and detoxification of chromium from tannery effluent by natural inhabitants. Nat. Environ. Pollut. Technol. 20, 1369–1380. doi: 10.46488/NEPT.2021.v20i03.051
Rabinovitch-Deere, C. A., and Parales, R. E. (2012). Three types of taxis used in the response of Acidovorax sp. strain JS42 to 2-nitrotoluene. Appl. Environ. Microbiol. 78, 2306–2315. doi: 10.1128/AEM.07183-11
Ramli, N. N., Othman, A. R., Said, N. S. M., Alias, J., Abdullah, S. R. S., Hasan, H. A., et al. (2025). Chromium reduction by indigenous Bacillus sp. isolate S1: optimal performance, characterization, and pathway. Int. J. Environ. Sci. Technol. doi: 10.1007/s13762-025-06606-y
Rehman, F., and Faisal, M. (2015). Toxic hexavalent chromium reduction by Bacillus pumilus, Cellulosimicrobium cellulans, and Exiguobacterium. Chin. J. Oceanol. Limnol. 33, 585–589. doi: 10.1007/s00343-015-4155-1
Rollins, C., and Dahlquist, F. W. (1981). The methyl-accepting chemotaxis proteins of Escherichia coli: a repellent-stimulated, covalent modification, distinct from methylation. Cell 25, 333–340. doi: 10.1016/0092-8674(81)90051-9
Sarankumar, R. K., Arulprakash, A., Devanesan, S., Selvi, A., AlSalhi, M. S., Rajasekar, A., et al. (2020). Bioreduction of hexavalent chromium by chromium-resistant alkalophilic bacteria isolated from tannery effluent. J. King Saud Univ. Sci. 32, 1969–1977. doi: 10.1016/j.jksus.2020.02.010
Sarkar, A., Sar, P., and Islam, E. (2015). Hexavalent chromium reduction by Microbacterium oleivorans A1: a possible mechanism of chromate detoxification and bioremediation. Recent Pat. Biotechnol. 9, 116–129. doi: 10.2174/187220830902160308192126
Saxena, S., Vatwani, S., Singh, S., Singh, A., Singh, Y., Sharma, R. K., et al. (2025). Exploring indigenous bacteria in wastewater for sustainable bioremediation for red and blue azo dyes decolouration, and chromium (VI) degradation in industrial effluents. Water Pract. Technol. 20, 814–825. doi: 10.2166/wpt.2025.036
Shamim, S., Rehman, A., and Qazi, M. H. (2014). Swimming, swarming, twitching, and chemotactic responses of Cupriavidus metallidurans CH34 and Pseudomonas putida mt2 in the presence of cadmium. Arch. Environ. Contam. Toxicol. 66, 407–414. doi: 10.1007/s00244-013-9966-5
Srivastava, A., Singh, A. L., Kumar, A., Tiwari, S., and Gupta, S. (2025b). Bioremediation of chromium (VI) from mining-contaminated soil using Klebsiella sp. (BH-A1): environmental implications. Environ. Geochem. Health 47:140. doi: 10.1007/s10653-025-02448-2
Srivastava, A., Singh, A. L., Yadav, M., Pandey, A., Yadav, A. N., and Chauhan, R. (2025a). Bioremediation of chromium-contaminated agricultural soil using alginate-encapsulated bacterial beads. Water Air Soil Pollut. 236:541. doi: 10.1007/s11270-025-08208-3
Tan, H., Wang, C., Zeng, G., Luo, Y., Li, H., and Xu, H. (2020). Bioreduction and biosorption of Cr(VI) by a novel Bacillus sp. CRB-B1 strain. J. Hazard. Mater. 386:121628. doi: 10.1016/j.jhazmat.2019.121628
Tarahomi, D. S., Hosseini, S. P., Abbaspour, A., Jafari, A., and Mousavi, S. M. (2025). Characterization and application of polysaccharides produced by Pseudomonas atacamensis M7D1 for chromium (III) removal from tannery effluent. Int. J. Biol. Macromol. 298:139944. doi: 10.1016/j.ijbiomac.2025.139944
Thacker, U., Parikh, R., Shouche, Y., and Madamwar, D. (2007). Reduction of chromate by cell-free extract of Brucella sp. isolated from Cr(VI)-contaminated sites. Bioresour. Technol. 98, 1541–1547. doi: 10.1016/j.biortech.2006.06.011
Tso, W. W., and Adler, J. (1974). Negative chemotaxis in Escherichia coli. J. Bacteriol. 118, 560–576. doi: 10.1128/jb.118.2.560-576.1974
Tuli, S. R., Ali, M. F., Jamal, T. B., Khan, M. A. S., Fatima, N., Ahmed, I., et al. (2024). Characterization and molecular insights of a chromium-reducing bacterium Bacillus tropicus. Microorganisms 12:2633. doi: 10.3390/microorganisms12122633
Upadhyay, N., Vishwakarma, K., Singh, J., Mishra, M., Kumar, V., Rani, R., et al. (2017). Tolerance and reduction of chromium(VI) by Bacillus sp. MNU16 isolated from contaminated coal mining soil. Front. Plant Sci. 8:778. doi: 10.3389/fpls.2017.00778
Wang, X., Li, H., Huang, H., Luo, H., Luo, S., Jiang, L., et al. (2022). Investigation on mechanism of hexavalent chromium bioreduction by Escherichia sp. TH-1 and the stability of reduction products. J. Environ. Chem. Eng. 10:107231. doi: 10.1016/j.jece.2022.107231
Wang, Y., Mo, T., Luo, Y., Luo, L., Li, C., He, J., et al. (2025). Cr(VI) bioremediation mechanism of a novel Microbacterium oxydans S-1 strain. J. Environ. Chem. Eng. 13:117200. doi: 10.1016/j.jece.2025.117200
Wani, P. A., Wahid, S., Khan, M. S. A., Rafi, N., and Wahid, N. (2019). Investigation of the role of chromium reductase for Cr(VI) reduction by Pseudomonas species isolated from Cr(VI)-contaminated effluent. Biotechnol. Res. Innov. 3, 38–46. doi: 10.1016/j.biori.2019.04.001
Wu, M., Li, Y., Li, J., Wang, Y., Xu, H., and Zhao, Y. (2019). Bioreduction of hexavalent chromium using a novel strain CRB-7 immobilized on multiple materials. J. Hazard. Mater. 368, 412–420. doi: 10.1016/j.jhazmat.2019.01.059
Wu, M., Ouyang, X., Li, Y., Zhang, J., Liu, J., and Yin, H. (2025). Mechanisms in hexavalent chromium removal from aquatic environment by the modified hydrochar-loaded bacterium Priestia megaterium strain BM.1. Sustainability 17:5172. doi: 10.3390/su17115172
Xiang, Y., Li, X., Liu, W., Luo, F., and Wang, M. (2025). Enhancing chromium supply chain security through resilience strategies: decision support based on system dynamics simulations. J. Clean. Prod. 493:144981. doi: 10.1016/j.jclepro.2025.144981
Xing, Y., Zheng, Y., and Wang, X. (2025). Integrated strategies for effective remediation of chromium-contaminated soils: advancements, challenges, and sustainability implications. Environ. Adv. 19:100614. doi: 10.1016/j.envadv.2025.100614
Xu, Q., Ali, S., Afzal, M., Nizami, A. S., Han, S., Dar, M. A., et al. (2024). Advancements in bacterial chemotaxis: utilizing the navigational intelligence of bacteria and its practical applications. Sci. Total Environ. 931:172967. doi: 10.1016/j.scitotenv.2024.172967
Young, L. Y., and Mitchell, R. (1973). Negative chemotaxis of marine bacteria to toxic chemicals. Appl. Microbiol. 25, 972–975. doi: 10.1128/am.25.6.972-975.1973
Keywords: chemotaxis, detoxification, chromium, heavy metal, Bacillus
Citation: Garg A, Ali M, Ahmad A, Chauhan P, Kumar A, Mishra R, Dubey VK, Srivastava A, Garg SK and Arora PK (2025) Survival tactics of Bacillus licheniformis KNP under hexavalent chromium stress: a study of detoxification and chemotactic responses. Front. Microbiol. 16:1646518. doi: 10.3389/fmicb.2025.1646518
Received: 13 June 2025; Accepted: 11 August 2025;
Published: 05 September 2025.
Edited by:
Kunal R. Jain, Sardar Patel University, IndiaReviewed by:
Eliseo Cristiani-Urbina, National Polytechnic Institute, MexicoAlok Kumar, Banaras Hindu University, India
Copyright © 2025 Garg, Ali, Ahmad, Chauhan, Kumar, Mishra, Dubey, Srivastava, Garg and Arora. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pankaj Kumar Arora, YXJvcmE0ODRAZ21haWwuY29t; Alok Srivastava, YWxvay5zcml2YXN0YXZhQG1qcHJ1LmFjLmlu
†These authors have contributed equally to this work
 Akansha Garg
Akansha Garg Mushtaq Ali
Mushtaq Ali Aiman Ahmad
Aiman Ahmad Prerna Chauhan1
Prerna Chauhan1 Ashish Kumar
Ashish Kumar Pankaj Kumar Arora
Pankaj Kumar Arora