- 1Department of Laboratory Medicine, National Clinical Research Center for Laboratory Medicine, The First Hospital of China Medical University, Shenyang, China
- 2Research Unit of Medical Laboratory, Chinese Academy of Medical Sciences, Beijing, China
- 3Department of Radiology, Shengjing Hospital of China Medical University, Shenyang, China
Objective: This study aimed to investigate the clinical and microbiological characteristics of invasive and noninvasive Klebsiella pneumoniae liver abscesses (KPLAs) and elucidate the risk factors for invasive KPLA.
Methods: We conducted a case–control study involving 50 patients with invasive KPLA and 50 patients with noninvasive KPLA from two medical centers between 2019 and 2024. Demographic and clinical data were collected for all patients from the hospital medical records system. Univariate and multivariate analyses were then performed to compare the characteristics of invasive and noninvasive KPLAs. In addition, antimicrobial resistance testing and whole-genome sequencing were performed for 50 K. pneumoniae strains from one medical center.
Results: The comparison of patients with invasive and noninvasive KPLAs revealed that diabetes mellitus, smoking history, drinking history, smaller maximum diameter of abscess, neutrophil count, fasting blood glucose, blood urea nitrogen, and length of hospital stay were independent risk factors for invasive KPLA. Phylogenetic analysis revealed that K. pneumoniae strains from patients with invasive and noninvasive KPLAs were intermingled. ST23 with K1 serotype was the predominant sequence type (66.0%), followed by ST65 with K2 serotype. Multilocus sequence types, capsular serotypes, antimicrobial resistance patterns, virulence genes, and SNPs of K. pneumoniae strains isolated from patients with invasive and noninvasive KPLAs showed no significant differences between the two patient groups.
Conclusion: Overall, our results indicate that patients’ contaminant conditions, such as poor glycemic control, smoking history, and drinking history, rather than bacterial virulence, contribute to the development of invasive KPLA.
1 Introduction
Pyogenic liver abscess (PLA) is a life-threatening infectious disease caused by the invasion of bacteria into the liver, resulting in the formation of single or multiple purulent infection foci. Klebsiella pneumoniae liver abscess (KPLA) was first reported in 1986 in Taiwan, China (Liu et al., 1986). Recently, it has been increasingly reported in Asia and has been designated an emerging infectious disease globally during the past two decades (Siu et al., 2012). The most common clinical manifestations in patients with KPLA are fever, chills, and abdominal pain, with a mortality rate of approximately 3.8% (Lee et al., 2020). KPLA can also cause extrahepatic migratory infections, such as endophthalmitis, lung abscess, renal abscess, and even meningitis, which have been reported in approximately 10–16% of cases (Wang et al., 1998), with lungs and eyes being the common sites of invasive infections (Sun et al., 2021). KPLA complicated with extrahepatic metastatic infection is defined as invasive K. pneumoniae liver abscess syndrome (IKPLAS). Invasive KPLA is associated with increased rates of mortality and disability as well as higher lengths of hospital stay and cost of hospitalization compared with noninvasive KPLA, posing a serious threat to human health. For example, KPLA with endophthalmitis is often accompanied by decreased vision or eye pain, and it can also progress to permanent vision loss (Hussain et al., 2020).
Hypervirulent K. pneumoniae (hvKP), the major pathogen responsible for KPLA, differs significantly from classical K. pneumoniae (cKP) in terms of its biological characteristics and the clinical features of the diseases it causes. HvKP strains exhibit a hypermucoviscous phenotype and K1 or K2 serotype; moreover, they harbor multiple hypervirulence determinants related to capsular polysaccharides, lipopolysaccharides, and siderophores (e.g., peg-344, iroB, iucA, rmpA, and rmpA2) (Zhu et al., 2021). hvKP strains are usually susceptible to commonly used antibiotics (Kocsis, 2023); however, extended-spectrum β-lactamase-producing and carbapenem-resistant hvKP are increasingly reported since 2015 (Morales-Leon et al., 2021; Lei et al., 2024). The predominant sequence type that causes KPLA worldwide is ST23 (Sohrabi et al., 2022). Unlike cKP, which is a major nosocomial pathogen, hvKP usually causes infections in the community in healthy people or individuals with diabetes mellitus. HvKP has been associated with various clinical manifestations, such as liver and renal abscesses and bacteremia (Chen et al., 2023).
A few studies have investigated clinical characteristics of IKPLAS, which reported different risk factors for invasive KPLA, such as sequential organ failure assessment (SOFA) score ≥ 4, breathlessness, abscess size, diabetes mellitus, thrombocytopenia, etc. (Chen et al., 2006; Yoon et al., 2014; Chang et al., 2015; Mukherjee et al., 2022; Gu et al., 2025). Additionally, results obtained from different studies were also inconsistent. For example, smaller abscess size was reported to be an independent risk factor for invasive KPLA (Shin et al., 2013; Chang et al., 2015), whereas another study indicated that patients with larger abscess size was at a greater risk of metastatic KPLA (Mukherjee et al., 2022). This may be due to small sample size of patients with invasive KPLA included in the studies. Besides, the role of bacterial virulence in the development of IKPLAS remains poorly characterized. In the present multicenter study, we investigated the clinical characteristics of patients with invasive and noninvasive KPLAs. In addition, we compared the microbiological and genomic characteristics of K. pneumoniae isolates isolated from patients with invasive and noninvasive KPLAs. Our results may aid in the early identification of patients with KPLA at increased risk of invasiveness, thereby improving clinical management and enabling personalized treatment.
2 Materials and methods
2.1 Study participants
This retrospective case–control study was performed at two medical centers in Northeast China: The First Hospital of China Medical University and Shengjing Hospital of China Medical University. The inclusion criteria of KPLA were: (1) imaging showing one or more lesions in the liver; (2) Positive culture for K. pneumoniae from pus, fluid, or blood culture samples. The exclusion criteria was liver abscess secondary to liver surgery, liver tumors, and other liver diseases. Invasive KPLA was defined as KPLA accompanied with extrahepatic metastatic infections at other body sites indicated by imaging during the same admission. Whereas noninvasive KPLA was defined as KPLA without bloodstream infection or extrahepatic infections. The clinical groups were determined by two clinicians independently. In cases of disagreement, a third clinician was consulted.
Fifty patients were enrolled in the invasive KPLA group from January 2019 to December 2024. The specimen types with positive K. pneumoniae cultures were: blood (17), pus (13), fluid (9), both blood and pus (8), both blood and fluid (3). Another 50 patients with KLPA were randomly selected to form the noninvasive KPLA group. Among them, 26 and 24 patients had positive K. pneumoniae cultures in pus and fluid specimens, respectively. Patients with invasive KPLA had the following extrahepatic infections: lung abscess (22), endophthalmitis (16), endophthalmitis and lung abscess (5), lung abscess and renal abscess (3), renal abscess (2), endophthalmitis and osteomyelitis (1), endophthalmitis and brain abscess (1). Fifty patients were enrolled in each medical center respectively, including 25 patients with invasive KPLA and 25 patients with noninvasive KPLA.
2.2 Microbiological methods
Fifty K. pneumoniae strains from 25 patients with invasive KLPA and 25 patients with noninvasive KPLA recovered from one medical center (The First Hospital of China Medical University) were subjected to microbiological testing and whole-genome sequencing. Suspicious K. pneumoniae strains growing on Columbia blood agar or MacConkey agar were identified by Vitek 2 or Matrix-Assisted Laser Desorption/ Ionization Time of Flight Mass Spectrometry (MALDI-TOF, bioMérieux, France). Additionally, antimicrobial resistance profiles of the strains were determined using Vitek 2 and interpreted according to the criteria of Clinical and Laboratory Standards Institute (CLSI) M100 (35th Edition). E. coli ATCC 25922 and P. aeruginosa ATCC 27853 were used for quality control of antimicrobial susceptibility testing. We also performed the string test, a well-known test to detect the mucoviscous phenotype of K. pneumoniae, with a viscous string of >5 mm as positive (Chen et al., 2021). K. pneumoniae NTUH-K2044 and ATCC 700603 were used as positive and negative controls, respectively.
2.3 Clinical data collection
We collected the following demographic and clinical data from the hospital medical records system using a standardized form: demographics (age and sex), history of cigarette smoking and alcohol drinking, underlying conditions (hypertension, diabetes mellitus, etc.), clinical symptoms (fever, chill, vomiting, and abdominal pain), laboratory test results (white blood cell count [WBC], neutrophil count, lymphocyte count, hemoglobin, platelet count [PLT], aspartate transaminase [AST], alanine aminotransferase [ALT], albumin, total bilirubin, blood glucose, HbA1c, blood urea nitrogen [BUN], creatinine [Cr], prothrombin time [PT], procalcitonin [PCT], C-reactive protein [CRP], etc.), and clinical outcomes (length of hospital stay, admission to intensive care unit [ICU], septic shock, and in-hospital deaths), shown in Supplementary Table S1.
2.4 Whole-genome sequencing and data analysis
Whole-genome sequencing was performed in Personalbio company (Shanghai, China). DNA was extracted from bacteria that were grown overnight with the CTAB extraction assay previously described (Wilson, 2001). Then TruSeq™ DNA Sample Prep Kit was used to construct the genomic library following the manufacturer’s operating procedures. Then resequencing was performed using an Illumina NovaSeq platform, with a sequencing mode of paired-end, 2 × 150 bp. Sequencing data were concatenated using SPAdes v3.15.4.1 In addition, pathogenwatch2 was used to determine the virulence gene score, resistance gene score, ST type, wzi type, and K/O type of bacterial strains. GATK v3.8 was used to detect single nucleotide polymorphisms (SNP), and a core-genome SNP-based phylogenetic tree was then constructed using the Maximum Likelihood algorithm implemented in the FastTree v2.1.11. The reliability of the phylogenetic tree was evaluated using bootstrap analysis with 1,000 replications. Finally, VFDB3 was used to analyze the virulence genes of the K. pneumoniae strains.
2.5 Statistical analysis
SPSS 20.0 was used for statistical analysis. Quantitative data were expressed as mean ± standard deviation or median (interquartile range) and compared using Student’s t-test or nonparametric test. In contrast, qualitative data were represented as rates and compared using chi-square test or Fisher’s test. After univariate analysis, variables with p < 0.1 rather than p < 0.05 were included in the multivariate analysis, as p < 0.1 reduced the risk of missing important variables (Sauerbrei et al., 2007). Multivariate binary logistic regression analysis was used to determine the risk factors associated with invasive KPLA after multiple imputations of the missing data. Statistical significance was determined at p < 0.05.
3 Results
3.1 Univariate analysis of the clinical characteristics of patients with invasive and noninvasive KPLAs
Univariate analysis revealed a high percentage of smoking history and diabetes mellitus among patients with invasive KPLA (Table 1). 68.0% of patients with invasive KPLA had a history of diabetes mellitus or were recently diagnosed with diabetes mellitus. Moreover, patients with invasive KPLA had higher neutrophil counts, fasting blood glucose levels, and BUN levels than those with noninvasive KPLA. Patients with invasive KPLA tended to have longer hospital stay than those with noninvasive KPLA.
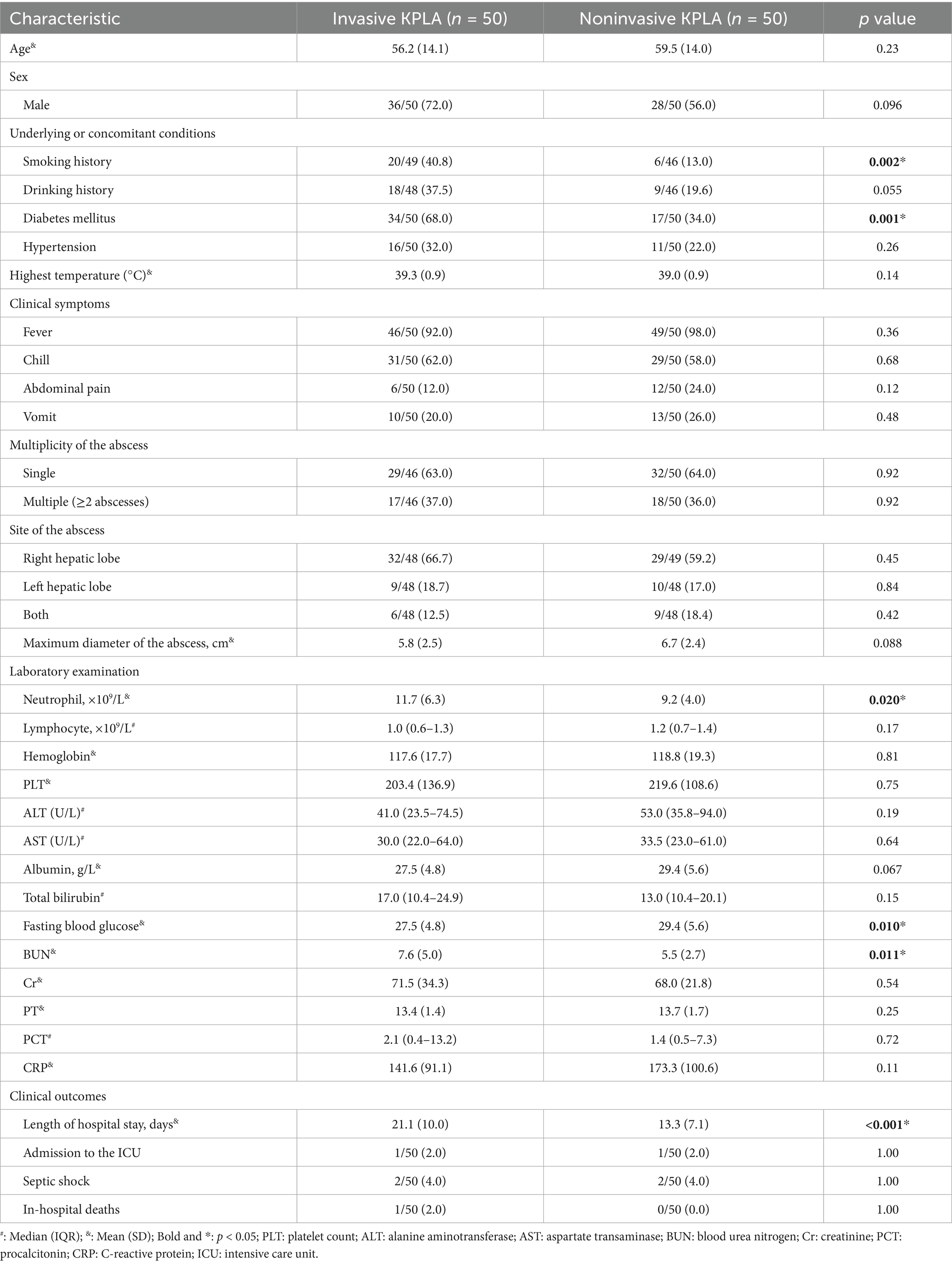
Table 1. Comparison of the clinical characteristics of patients with invasive and noninvasive KPLAs.
3.2 Multivariate analysis of the clinical characteristics of patients with invasive and noninvasive KPLAs
Variables with p-values <0.10 were included in the multivariate logistics analysis (Figure 1), which showed that diabetes mellitus (odds ratio [OR], 2.31; 95% CI, 1.36–3.91; p = 0.002), smoking history (OR, 2.99; 95% CI, 1.54–5.81; p = 0.001), and drinking history (OR, 2.21; 95% CI, 1.18–4.16; p = 0.014) were identified as statistically independent risk factors for invasive KPLA. Additionally, neutrophil count, fasting blood glucose, BUN, and length of hospital stay showed positive associations with invasive KPLA, whereas maximum diameter of abscess showed negative associations with invasive KPLA. From a clinical perspective, neutrophil count, BUN, and length of hospital stay are more likely to be the consequences of invasive KPLA, whereas diabetes mellitus, high fasting blood glucose, and smoking and drinking history are more likely to be risk factors for invasive KPLA. The data on neutrophil counts, BUN values, maximum diameter of the abscesses, lengths of hospital stay, and laboratory tests related to glycemic control (fasting blood glucose, random blood glucose, and HbA1c) were shown in Supplementary Figure S1.
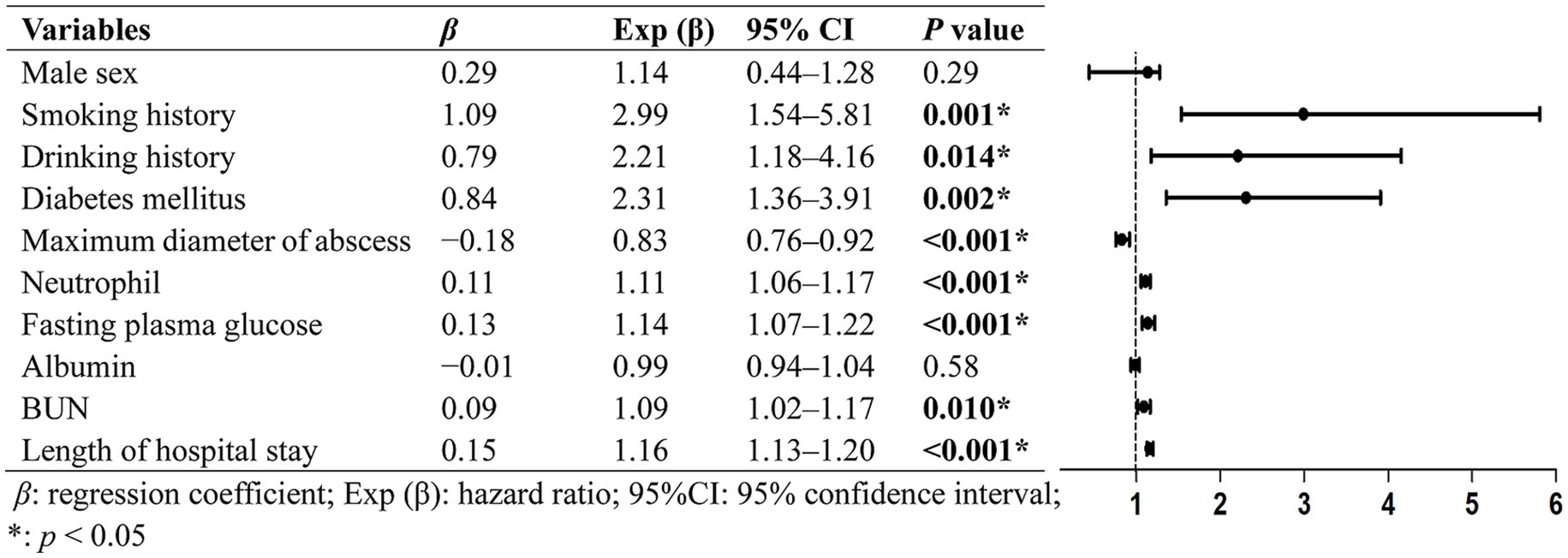
Figure 1. Multivariate analysis of the clinical characteristics associated with invasive KPLA. Variables with p-values <0.10 in univariate analysis were included in the multivariate logistics analysis. The results exhibited that diabetes mellitus, smoking history, drinking history, neutrophil count, fasting blood glucose, BUN, and length of hospital stay showed statistically significant positive associations with invasive KPLA. Whereas maximum diameter of abscess showed statistically significant negative associations with invasive KPLA.
3.3 Phylogenetic analysis of Klebsiella pneumoniae strains
The whole-genome sequences of 50 K. pneumoniae strains were used to construct a phylogenetic tree, as shown in Figure 2. It revealed that ST23 (33/50, 66.0%) was the predominant sequence type that was only found among K1 isolates. ST65 (8/50, 16.0%) was the second most prevalent sequence type that was only found among K2 isolates. K1 (36/50, 72.0%) and K2 (9/50, 18.0%) were the predominant capsular serotypes. Liver abscess was not caused by clonally related K. pneumoniae strains. Instead, these strains showed genetic differences as they contained different SNPs. All strains except for one (PLA11) carried multiple virulence genes and was classified as hvKP. Additionally, K. pneumoniae strains from patients with invasive and noninvasive KPLAs were intermingled as showed by the phylogenetic tree, without forming group-specific clusters.
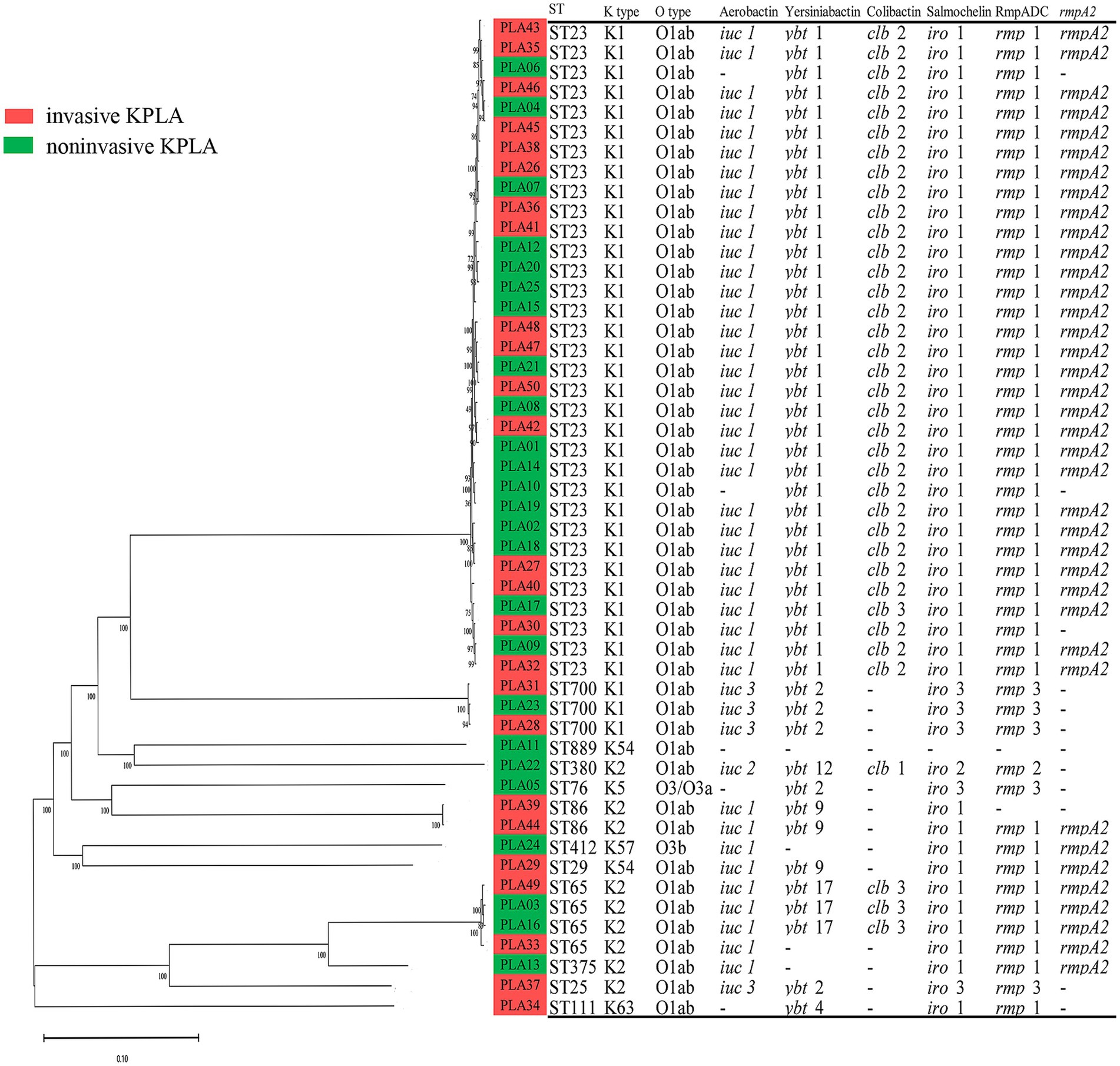
Figure 2. Phylogenetic analysis of 50 K. pneumoniae strains isolated from invasive KPLA (red) and noninvasive KPLA (green). The molecular characteristics (sequence type, K type, O type) and virulence determinants (Aerobactin, Yersiniabactin, Colibactin, Salmochelin, RmpADC, rmpA2) of the K. pneumoniae strains were also displayed. It showed that all strains except for one were hvKP, with ST23 and ST65 as the predominant sequence types. Besides, K. pneumoniae strains from invasive and noninvasive KPLAs were intermingled, without forming group-specific clusters.
3.4 Microbiological and molecular characteristics of Klebsiella pneumoniae strains from patients with invasive and noninvasive KPLAs
The multilocus sequence types, capsular serotypes, virulence determinants, string test results, and antimicrobial resistance patterns of K. pneumoniae strains isolated from patients with invasive and noninvasive KPLAs were shown in Table 2. There was no significant difference in these characteristics between the two groups. Approximately 94% of the K. pneumoniae strains showed a hypermucoviscous phenotype. In addition, 49 K. pneumoniae strains were susceptible to the antimicrobials tested, including piperacillin, cefazolin, cefepime, ceftriaxone, ceftazidime, ciprofloxacin, levofloxacin, imipenem, meropenem, gentamicin, amikacin, and aztreonam. PLA27 was resistant to cephalosporins and quinolones, which was consistent with the presence of the CTX-M-3, aac(3)-IId, and qnrS1 resistance genes in its genome.
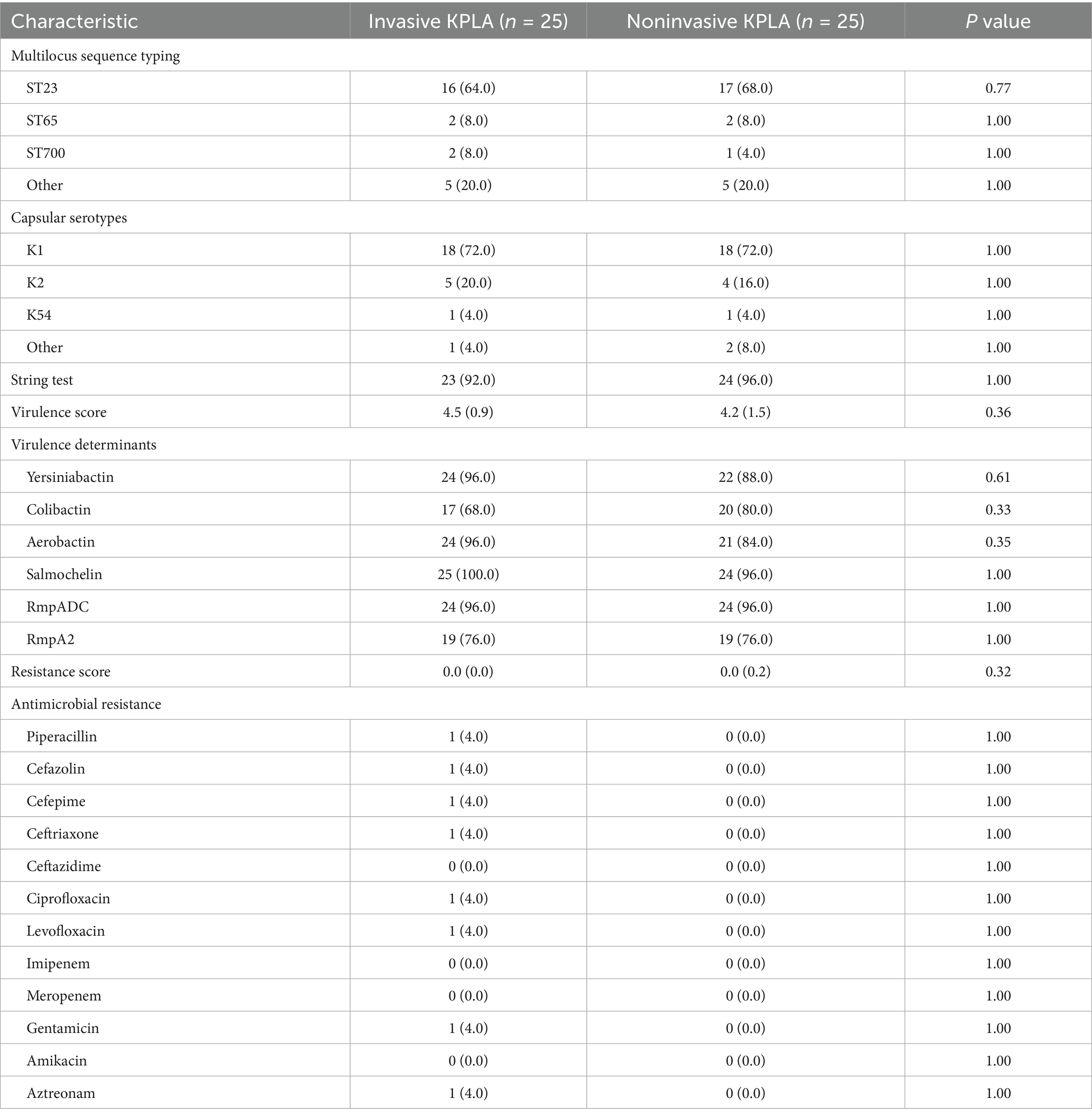
Table 2. Microbiological and molecular characteristics of K. pneumoniae strains isolated from patients with invasive and noninvasive KPLAs.
3.5 Virulence genes and SNPs of Klebsiella pneumoniae strains isolated from patients with invasive and noninvasive KPLAs
Analysis of the virulence genes of K. pneumoniae strains isolated from patients with invasive and noninvasive KPLAs (Table 3) revealed no significant differences in the frequencies of genes related to adherence (type 3 fimbriae, type I fimbriae, and type IV pili), iron uptake (aerobactin, siderophore, salmochelin, yersiniabactin, and iron transport), nutritional factors (allantoin utilization), regulation (RcsAB and RmpA), secretion system (T6SS I, T6SS II, and T6SS III), and toxin (colibactin) between the two groups. In addition, analysis of the SNPs of K. pneumoniae strains isolated from patients with invasive and noninvasive KPLAs (Supplementary Table S2) revealed no group-specific SNPs. Besides, analysis of SNP frequencies showed no significant differences between the two groups.
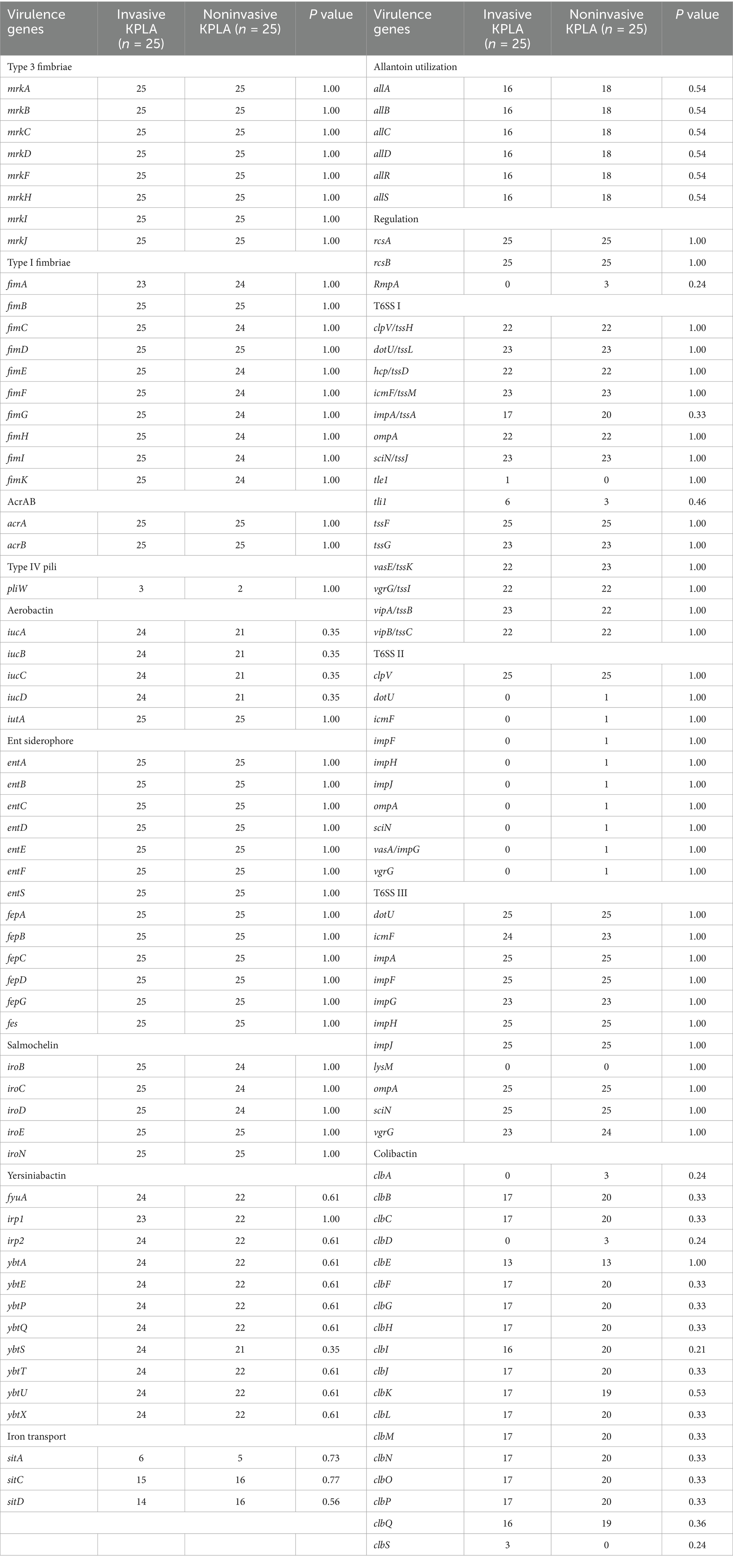
Table 3. Virulence genes of K. pneumoniae strains isolated from patients with invasive and noninvasive KPLAs.
4 Discussion
KPLA is caused by hvKP that initially colonizes the gastrointestinal tract of the host. From there, it translocates across the intestinal barrier via the hepatic portal vein into the liver, where it causes liver abscesses. Liver Kupffer cells and macrophages have been shown to kill hvKp in vitro (Hoh et al., 2019). However, the capsule of K. pneumoniae protects it from being captured by liver Kupffer cells, as evidenced by the ability of certain high-virulence capsular serotypes to completely block Kupffer cell capture, whereas the low-virulence counterparts confer only partial protection against Kupffer cell capture (Huang et al., 2022). Risk factor analysis for PLA indicates that men are at a higher risk of developing PLA than women. Besides, patients with diabetes, especially those with poor glycemic control and high BMI had a significantly higher risk of developing PLA (Wang et al., 2023). Certain comorbidities, such as liver transplantation and malignancy, increase the risk of developing PLA (Kaplan et al., 2004).
Serious cases of KPLA may be accompanied with metastatic infections, such as endophthalmitis and lung abscess. Despite its low prevalence, invasive KPLA is a severe damaging and difficult-to-treat disease. Such invasive infections may result in significant disability and high mortality in patients with KPLA. Therefore, the present study aimed to determine the risk factors associated with invasive KPLA. Our results showed that diabetes mellitus, drinking history, and small diameter of abscess were significant risk factors for invasive KPLA. This is consistent with previous studies which revealed that diabetes mellitus, alcoholism, and maximal diameter of abscess < 55 mm were independent risk factors for metastatic infection among patients with KPLA (Chen et al., 2006; Yoon et al., 2014; Chang et al., 2015). These three factors have been reported in more than one study, suggesting that they are important risk factors for invasive KPLA. However, other risk factors, such as smoking history, high fasting blood glucose level, high neutrophil count, high BUN level, long length of hospital stay, were first reported as independent risk factors invasive KPLA, which need to be confirmed by clinical studies in the future.
Diabetes mellitus and high fasting blood glucose levels, which indicate poor glycemic control, were risk factors for invasive KPLA. Moreover, patients with invasive KPLA showed higher random blood glucose and HbA1c levels than those with noninvasive KLPA. These results were consistent with those of previous studies showing that diabetes is a significant risk factor for the development of endogenous endophthalmitis (Lin et al., 2006; Sheu et al., 2011). Besides, another study showed that fasting blood glucose is related with invasion syndrome in patients with diabetes complicated with KPLA (Feng et al., 2024). On one hand, high glucose may up-regulate the levels of rmpA and ompA in hvKP and enhance its resistance to serum killing, thus increasing its virulence (Tang et al., 2023). On the other hand, high glucose levels may impair neutrophil phagocytosis of K1 and K2 K. pneumoniae (Lin et al., 2006), providing a reasonable explanation for the high incidences of invasive KPLA in patients with poor glycemic control.
To the best of our knowledge, this is the first study to report that cigarette smoking is an independent risk factor for invasive KPLA. Similar associations have been reported in certain respiratory infections. For example, cigarette smoking prolongs the inflammation caused by influenza A infection and delays the clearance of the infection in mice (Vlasma et al., 2024). Cigarette smoking also attenuates mice’s nasal response to S. pneumoniae and predisposes them to invasive pneumococcal disease (Shen et al., 2016). Another study reported that smoking increases the virulence of P. aeruginosa and induces resistance to neutrophil killing, resulting in a greater capacity to cause invasive disease (Chien et al., 2020). Cigarette smoking can modify neutrophil chemotaxis, and induce neutrophil extracellular trap (NET) formation (White et al., 2018; Wang et al., 2025); whereas, how cigarette smoking causes aggressive KPLA remains elusive and needs further research.
In addition, alcohol drinking was found to be a significant predictor of invasive KPLA. Previous research found that alcohol consumption was associated with abnormal neutrophil traps and reduced lipopolysaccharide-induced NET formation, which contribute to liver injury and sepsis severity (Bukong et al., 2018). Additionally, alcohol can induce decreased neutrophil phagocytosis and oxidative burst to E. coli (Khan et al., 2023). Therefore, we speculate that alcohol drinking impairs host defense functions by decreasing the phagocytic activity of neutrophils, thereby making patients more susceptible to invasive KPLA. Further experimental studies are needed to validate the hypothesis.
Previous studies have demonstrated that the hypervirulent capsular serotypes K1 and K2 of K. pneumoniae are the major serotypes responsible for KPLA (Chuang et al., 2016). These serotypes are hypermucoviscous and resistant to serum bactericidal activity and neutrophil phagocytosis (Al-Busaidi et al., 2024). These results are consistent with those of our study, which identified hvKP as the major pathogen responsible for both invasive and noninvasive KPLAs, with K1 serotype ST23 being the most prominent. However, we found no significant differences in the microbiological, molecular, and virulence gene characteristics as well as the SNPs of K. pneumoniae strains isolated from patients with invasive and noninvasive KPLAs. These results indicate that both invasive and noninvasive KPLAs are caused by hvKP, and bacterial virulence characteristics are not a critical factor for the development of invasive KPLA.
This present study has significant impact on the clinical management of KPLA. Firstly, results of the study may aid clinicians in early identification of KPLA patients with extrahepatic metastasis infection, so as to initiate optimized therapy as soon as possible to reduce the rate of mortality and adverse events. Secondly, poor glycemic control, smoking history, and drinking history were demonstrated to be significantly associated with increased risk of developing invasive KPLA, which indicates that effective glycemic control, smoking and alcohol quit should be recommended for patients with high risk of developing KPLA. However, this study has several limitations. Small sample size due to the relative low prevalence of invasive KPLA, may result in bias of the study. Additionally, accuracy of the findings may be affected due to missing data problems. Moreover, in-depth investigation into the molecular mechanisms underlying the findings in this study are warranted in the future.
5 Conclusion
Physicians should be vigilant for the possibility of invasive KPLA when patients with PLA are accompanied with conditions such as diabetes mellitus, smoking history, drinking history, high fasting blood glucose level, high neutrophil count, high BUN level, long length of hospital stay, and small diameter of abscess. In the present study, the bacterial molecular and virulence characteristics did not differ significantly between the two groups. Overall, our results indicate that patients’ contaminant conditions, such as poor glycemic control, smoking history, and drinking history, rather than bacterial virulence, contribute to the development of invasive KPLA. This study is of great significance for the early identification and clinical management of invasive KPLA.
Data availability statement
The raw sequence data reported in this paper have been deposited in China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: CRA026012) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa.
Ethics statement
The studies involving humans were approved by this study was approved by the Ethics Committee of the First Hospital of China Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because The ethics committee waived the need for informed consent due to the following reasons: (1) this study was retrospective; (2) the patient information in this study was anonymous; (3) the clinical strains were collected and stored in a routine diagnostic laboratory; and (4) this study had no impact on the patients. The authors have affirmed the confidentiality of patient data.
Author contributions
JC: Investigation, Conceptualization, Project administration, Writing – original draft, Formal analysis. XW: Methodology, Writing – original draft, Data curation. QW: Investigation, Software, Methodology, Writing – original draft. ST: Supervision, Writing – original draft, Investigation. FL: Data curation, Project administration, Investigation, Writing – review & editing, Supervision. RW: Data curation, Project administration, Writing – review & editing, Supervision. ZC: Project administration, Conceptualization, Writing – review & editing, Supervision. YC: Conceptualization, Supervision, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Noncommunicable Chronic Diseases-National Science and Technology Major Project (no. 2024ZD0532800).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1650703/full#supplementary-material
Footnotes
References
Al-Busaidi, B., Al-Muzahmi, M., Al-Shabibi, Z., Rizvi, M., Al-Rashdi, A., Al-Jardani, A., et al. (2024). Hypervirulent capsular serotypes K1 and K2 Klebsiella pneumoniae strains demonstrate resistance to serum bactericidal activity and galleria mellonella lethality. Int. J. Mol. Sci. 25:1944. doi: 10.3390/ijms25031944
Bukong, T. N., Cho, Y., Iracheta-Vellve, A., Saha, B., Lowe, P., Adejumo, A., et al. (2018). Abnormal neutrophil traps and impaired efferocytosis contribute to liver injury and sepsis severity after binge alcohol use. J. Hepatol. 69, 1145–1154. doi: 10.1016/j.jhep.2018.07.005
Chang, Z., Zheng, J., Ma, Y., and Liu, Z. (2015). Analysis of clinical and CT characteristics of patients with Klebsiella pneumoniae liver abscesses: an insight into risk factors of metastatic infection. Int. J. Infect. Dis. 33, 50–54. doi: 10.1016/j.ijid.2014.12.041
Chen, J., Hu, C., Wang, R., Li, F., Sun, G., Yang, M., et al. (2021). Shift in the dominant sequence type of Carbapenem-resistant Klebsiella pneumoniae bloodstream Infection from ST11 to ST15 at a medical Center in Northeast China, 2015-2020. Infect Drug Resist 14, 1855–1863. doi: 10.2147/IDR.S311968
Chen, S. C., Lee, Y. T., Lai, K. C., Cheng, K. S., Jeng, L. B., Wu, W. Y., et al. (2006). Risk factors for developing metastatic infection from pyogenic liver abscesses. Swiss Med. Wkly. 136, 119–126. doi: 10.4414/smw.2006.11341
Chen, J., Zhang, H., and Liao, X. (2023). Hypervirulent Klebsiella pneumoniae. Infect. Drug Resist. 16, 5243–5249. doi: 10.2147/IDR.S418523
Chien, J., Hwang, J. H., Nilaad, S., Masso-Silva, J. A., Jeong Ahn, S., McEachern, E. K., et al. (2020). Cigarette smoke exposure promotes virulence of Pseudomonas aeruginosa and induces resistance to neutrophil killing. Infect. Immun. 88:e00527–20. doi: 10.1128/IAI.00527-20
Chuang, C., Fan, W. C., Lin, Y. T., and Wang, F. D. (2016). The emergence of Klebsiella pneumoniae liver abscess in non-diabetic patients and the distribution of capsular types. Gut Pathog 8:46. doi: 10.1186/s13099-016-0128-y
Feng, C. Y., Zhang, L. W., Liu, T., Jiang, S. F., Li, X. M., and Di, J. (2024). Establishment and verification of invasion syndrome prediction model in patients with diabetes complicated with Klebsiella pneumoniae liver abscess. Zhonghua Yi Xue Za Zhi 104, 956–962. doi: 10.3760/cma.j.cn112137-20231019-00813
Gu, L., Wang, Y., Wang, H., and Xu, D. (2025). Analysis of clinical and microbiological characteristics of invasive Klebsiella pneumoniae liver abscess syndrome. BMC Infect. Dis. 25:626. doi: 10.1186/s12879-025-10981-9
Hoh, C. H., Tan, Y. H., and Gan, Y. H. (2019). Protective role of Kupffer cells and macrophages in Klebsiella pneumoniae-induced liver abscess disease. Infect. Immun. 87:e00369–19. doi: 10.1128/IAI.00369-19
Huang, X., Li, X., An, H., Wang, J., Ding, M., Wang, L., et al. (2022). Capsule type defines the capability of Klebsiella pneumoniae in evading Kupffer cell capture in the liver. PLoS Pathog. 18:e1010693. doi: 10.1371/journal.ppat.1010693
Hussain, I., Ishrat, S., Ho, D. C. W., Khan, S. R., Veeraraghavan, M. A., Palraj, B. R., et al. (2020). Endogenous endophthalmitis in Klebsiella pneumoniae pyogenic liver abscess: systematic review and meta-analysis. Int. J. Infect. Dis. 101, 259–268. doi: 10.1016/j.ijid.2020.09.1485
Kaplan, G. G., Gregson, D. B., and Laupland, K. B. (2004). Population-based study of the epidemiology of and the risk factors for pyogenic liver abscess. Clin. Gastroenterol. Hepatol. 2, 1032–1038. doi: 10.1016/s1542-3565(04)00459-8
Khan, R. S., Lalor, P. F., Thursz, M., and Newsome, P. N. (2023). The role of neutrophils in alcohol-related hepatitis. J. Hepatol. 79, 1037–1048. doi: 10.1016/j.jhep.2023.05.017
Kocsis, B. (2023). Hypervirulent Klebsiella pneumoniae: An update on epidemiology, detection and antibiotic resistance. Acta Microbiol. Immunol. Hung. 70, 278–287. doi: 10.1556/030.2023.02186
Lee, J. H., Jang, Y. R., Ahn, S. J., Choi, S. J., and Kim, H. S. (2020). A retrospective study of pyogenic liver abscess caused primarily by Klebsiella pneumoniae vs. non-Klebsiella pneumoniae: CT and clinical differentiation. Abdom. Radiol. (N. Y.). 45, 2669–2679. doi: 10.1007/s00261-019-02389-2
Lei, T. Y., Liao, B. B., Yang, L. R., Wang, Y., and Chen, X. B. (2024). Hypervirulent and carbapenem-resistant Klebsiella pneumoniae: a global public health threat. Microbiol. Res. 288:127839. doi: 10.1016/j.micres.2024.127839
Lin, J. C., Siu, L. K., Fung, C. P., Tsou, H. H., Wang, J. J., Chen, C. T., et al. (2006). Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J. Clin. Endocrinol. Metab. 91, 3084–3087. doi: 10.1210/jc.2005-2749
Liu, Y. C., Cheng, D. L., and Lin, C. L. (1986). Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Arch. Intern. Med. 146, 1913–1916.
Morales-Leon, F., Opazo-Capurro, A., Caro, C., Lincopan, N., Cardenas-Arias, A., Esposito, F., et al. (2021). Hypervirulent and hypermucoviscous extended-spectrum beta-lactamase-producing Klebsiella pneumoniae and Klebsiella variicola in Chile. Virulence 12, 35–44. doi: 10.1080/21505594.2020.1859274
Mukherjee, S., Archuleta, S., and Pang, J. (2022). Risk factors of septic metastatic infection among patients with Klebsiella pneumoniae liver abscess in Singapore: a case-control study. Am. J. Trop. Med. Hyg. 106, 805–808. doi: 10.4269/ajtmh.21-0623
Sauerbrei, W., Royston, P., and Binder, H. (2007). Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat. Med. 26, 5512–5528. doi: 10.1002/sim.3148
Shen, P., Morissette, M. C., Vanderstocken, G., Gao, Y., Hassan, M., Roos, A., et al. (2016). Cigarette smoke attenuates the nasal host response to Streptococcus pneumoniae and predisposes to invasive pneumococcal disease in mice. Infect. Immun. 84, 1536–1547. doi: 10.1128/IAI.01504-15
Sheu, S. J., Kung, Y. H., Wu, T. T., Chang, F. P., and Horng, Y. H. (2011). Risk factors for endogenous endophthalmitis secondary to klebsiella pneumoniae liver abscess: 20-year experience in southern Taiwan. Retina 31, 2026–2031. doi: 10.1097/IAE.0b013e31820d3f9e
Shin, S. U., Park, C. M., Lee, Y., Kim, E. C., Kim, S. J., and Goo, J. M. (2013). Clinical and radiological features of invasive Klebsiella pneumoniae liver abscess syndrome. Acta Radiol. 54, 557–563. doi: 10.1177/0284185113477400
Siu, L. K., Yeh, K. M., Lin, J. C., Fung, C. P., and Chang, F. Y. (2012). Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect. Dis. 12, 881–887. doi: 10.1016/S1473-3099(12)70205-0
Sohrabi, M., Alizade Naini, M., Rasekhi, A., Oloomi, M., Moradhaseli, F., Ayoub, A., et al. (2022). Emergence of K1 ST23 and K2 ST65 hypervirulent klebsiella pneumoniae as true pathogens with specific virulence genes in cryptogenic pyogenic liver abscesses shiraz Iran. Front. Cell. Infect. Microbiol. 12:964290. doi: 10.3389/fcimb.2022.964290
Sun, R., Zhang, H., Xu, Y., Zhu, H., Yu, X., and Xu, J. (2021). Klebsiella pneumoniae-related invasive liver abscess syndrome complicated by purulent meningitis: a review of the literature and description of three cases. BMC Infect. Dis. 21:15. doi: 10.1186/s12879-020-05702-3
Tang, L., Wang, H., Cao, K., Li, Y., Li, T., Huang, Y., et al. (2023). Epidemiological features and impact of high glucose level on virulence gene expression and serum resistance of Klebsiella pneumoniae causing liver abscess in diabetic patients. Infect Drug Resist 16, 1221–1230. doi: 10.2147/IDR.S391349
Vlasma, J. R., van der Veen, T. A., de Jager, M. H., Nawijn, M. C., Brandsma, C. A., and Melgert, B. N. (2024). Cigarette smoking prolongs inflammation associated with influenza infection and delays its clearance in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 327, L634–L645. doi: 10.1152/ajplung.00369.2023
Wang, J. L., Hsu, C. R., Wu, C. Y., and Lin, H. H. (2023). Diabetes and obesity and risk of pyogenic liver abscess. Sci. Rep. 13:7922. doi: 10.1038/s41598-023-34889-z
Wang, J. H., Liu, Y. C., Lee, S. S., Yen, M. Y., Chen, Y. S., Wang, J. H., et al. (1998). Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin. Infect. Dis. 26, 1434–1438. doi: 10.1086/516369
Wang, L., Zhu, L., Tang, Y., Wen, Z., Peng, L., and Ci, X. (2025). Long-term cigarette smoke exposure promotes neutrophil Ferroptosis resistance, inducing neutrophil extracellular trap formation and driving glucocorticoid resistance in chronic obstructive pulmonary disease. Research 8:0751. doi: 10.34133/research.0751
White, P. C., Hirschfeld, J., Milward, M. R., Cooper, P. R., Wright, H. J., Matthews, J. B., et al. (2018). Cigarette smoke modifies neutrophil chemotaxis, neutrophil extracellular trap formation and inflammatory response-related gene expression. J. Periodontal Res. 53, 525–535. doi: 10.1111/jre.12542
Wilson, K. (2001). Preparation of genomic DNA from bacteria. Curr. Protoc. Mol. Biol. Chapter 2:Unit 24. doi: 10.1002/0471142727.mb0204s56
Yoon, J. H., Kim, Y. J., Jun, Y. H., Kim, S. I., Kang, J. Y., Suk, K. T., et al. (2014). Liver abscess due to Klebsiella pneumoniae: risk factors for metastatic infection. Scand. J. Infect. Dis. 46, 21–26. doi: 10.3109/00365548.2013.851414
Keywords: liver abscess, Klebsiella pneumoniae , invasive, virulence, risk factor, fasting blood glucose
Citation: Chen J, Wang X, Wang Q, Tian S, Li F, Wang R, Chang Z and Chu Y (2025) Poor glycemic control and smoking and drinking history rather than bacterial virulence contribute to the development of invasive Klebsiella pneumoniae liver abscess: a case–control study in Northeast China. Front. Microbiol. 16:1650703. doi: 10.3389/fmicb.2025.1650703
Edited by:
Juan Xicohtencatl-Cortes, Hospital Infantil de México Federico Gómez, MexicoReviewed by:
Jetsi Mancilla Rojano, National Autonomous University of Mexico, MexicoYan Cheng, Army Medical University, China
Ricardo Ernesto Ahumada-Cota, Federico Gómez Children's Hospital, Mexico
Copyright © 2025 Chen, Wang, Wang, Tian, Li, Wang, Chang and Chu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fushun Li, Y211bGVvQDE2My5jb20=; Ruixuan Wang, cmV5bmFfd2FuZ0B5ZWFoLm5ldA==; Zhihui Chang, Y2hhbmd6aEBzai1ob3NwaXRhbC5vcmc=; Yunzhuo Chu, Y3l6NjYzMEAxNjMuY29t
 Jingjing Chen
Jingjing Chen Xinyi Wang
Xinyi Wang Qihui Wang
Qihui Wang Sufei Tian
Sufei Tian Fushun Li
Fushun Li Ruixuan Wang
Ruixuan Wang Zhihui Chang
Zhihui Chang Yunzhuo Chu
Yunzhuo Chu