- 1Department of Medical Microbiology and Infection Prevention, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
- 2Department of Medical Microbiology, Radboud University Medical Center, Nijmegen, Netherlands
- 3Centre for Infectious Disease Control, The National Institute for Public Health and the Environment (RIVM), Bilthoven, Netherlands
- 4Laboratory of Medical Microbiology and Infectious Diseases, Isala Academy, Isala Hospital, Zwolle, Netherlands
- 5Department of Pathology, University of Utah School of Medicine, Salt Lake City, UT, United States
Introduction: Understanding host factor-related mechanisms that drive variability in respiratory particle emission and virus presence in exhaled particles is essential to assess transmission risk and potentially identify individuals with elevated infectiousness.
Methods: We conducted a systematic review of human observational studies examining associations between host factors and either respiratory particle emission or virus presence in exhaled particles. Searches in PubMed, EMBASE, and Web of Science covered studies up to September 2024. Risk of bias was assessed using STROBE-based criteria. Findings were synthesized narratively, grouped by host factor and outcome type.
Results: Forty-four studies met inclusion criteria: 34 assessed host factors in relation to particle emission, and 11 examined viral presence in exhaled particles. Fine particle emission (<5 μm) was most consistently associated with older age (n = 16), physical exercise (n = 6), and active infection (n = 6). No consistent associations were found for sex (n = 21), body mass index (BMI; n = 10), or smoking (n = 6). Viral presence—mainly influenza and SARS-CoV-2—was more strongly associated with time since symptom onset (n = 8) and lower respiratory symptoms (n = 3), based largely on genomic detection. Associations with other factors, including upper respiratory symptoms (n = 6), swab viral load (n = 11), age (n = 6), sex (n = 6), and BMI (n = 2), were inconsistent or absent. Physical exercise was not evaluated in relation to viral presence.
Discussion: Fine respiratory particles (<5 μm) were the predominant size fraction detected and often contained higher concentrations of viral RNA. Age, physical exercise, and active infection were consistently associated with increased emission of these particles. The presence of respiratory viruses in exhaled air was more strongly linked to infection-related factors such as early symptom onset and lower respiratory involvement. These patterns suggest distinct mechanisms contributing to airborne transmission. Interpretation was limited by methodological heterogeneity and predominant reliance on PCR. Still, consistent associations with host factors suggest their potential as indicators for transmission risk. As evidence focused mainly on influenza and SARS-CoV-2, generalizability is limited. Standardized methods and further research are needed to strengthen outbreak preparedness.
1 Introduction
Respiratory viruses continue to cause a substantial global burden of disease, resulting in millions of hospitalizations and considerable mortality each year (Organization WH, 2024; Sirota et al., 2025; Bender et al., 2024). Among the recognized transmission routes, airborne spread has gained increasing attention as a key contributor to the dissemination of these pathogens, especially in enclosed or poorly ventilated environments. The airborne route of transmission involves small virus-laden particles generated during routine expiratory activities, which can remain suspended in the air, travel over long distances, and deposit within the lower airways (Wang et al., 2021; Pöhlker et al., 2023).
However, respiratory particle generation is not a single, uniform process, but rather a complex and dynamic one that can be modulated by various physiological factors, such as airway structure, airflow dynamics, surface tension, and fluid rheology (Pöhlker et al., 2023; Bagheri et al., 2023; Johnson and Morawska, 2009; Almstrand et al., 2010; Roth et al., 2023; Edwards et al., 2004). In addition, different respiratory activities engage distinct regions of the airway, resulting in varied particle formation mechanisms and size distributions (Wang et al., 2021; Pöhlker et al., 2023; Kutter et al., 2018) (Figure 1). Fine respiratory particles (<5 μm) are primarily produced in the distal lung, where cyclic closure and reopening of small airways disrupts the airway lining fluid in the bronchioles and alveoli. Variations in tidal volume and breathing rate can further modulate this mechanism (Pöhlker et al., 2023; Bagheri et al., 2023; Johnson and Morawska, 2009; Almstrand et al., 2010; Bake et al., 2017; Morawska et al., 2009; Tinglev et al., 2016; Oldham and Moss, 2019). In more proximal regions, such as the larynx and oral cavity, particle generation is mainly driven by shear-induced fragmentation and fluid-film rupture caused by vocal fold oscillation, oral articulation, and increased airflow, typically resulting in larger particles (Pöhlker et al., 2023; Bagheri et al., 2023). Expulsive events such as coughing and sneezing commonly intensify these mechanisms, generating even broader particle size distributions (Pöhlker et al., 2023; Morawska et al., 2009; Zayas et al., 2012; Xie et al., 2009).
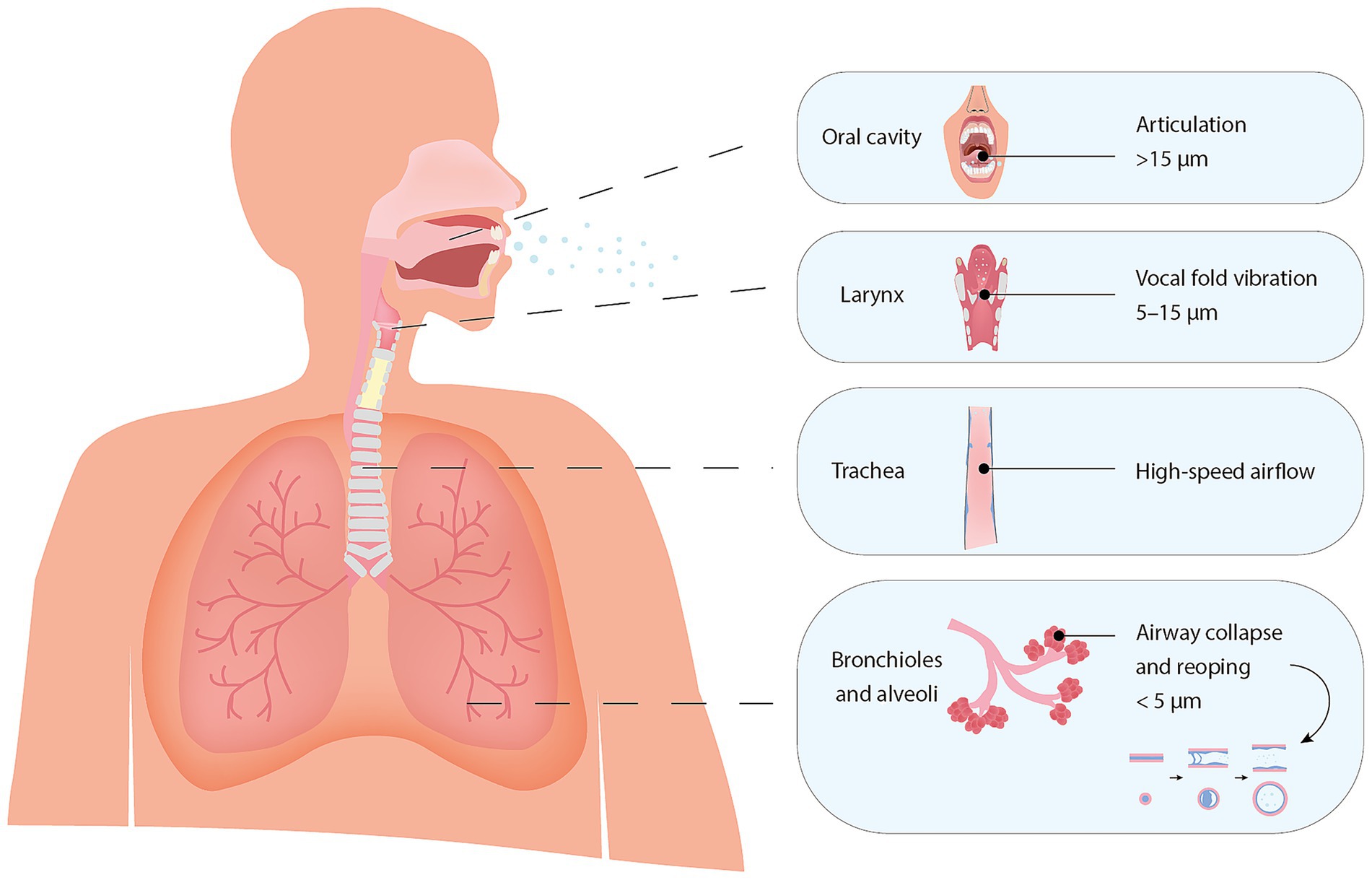
Figure 1. Anatomical sites of respiratory particle generation and associated particle size ranges at the moment of generation. The illustration highlights hypothesized generation sites based on Pöhlker et al. (2023) and Pan et al. (2023), with associated particle size ranges adapted from Bagheri et al. (2023), reflecting their estimated diameters at the point of generation.
Importantly, transmission risk also depends on whether these exhaled particles contain infectious virus. Active infection at sites of particle generation, as discussed above, may contribute to viral presence in exhaled particles, although this relationship remains unconfirmed. Virus-specific factors, such as cellular tropism and strain specificity, along with host-related factors including immune competence and infection severity, likely further modulate virus presence (Wang et al., 2021; Puhach et al., 2023; Flerlage et al., 2021).
Despite growing understanding of the physiological mechanisms underlying respiratory particle formation and infection kinetics, considerable inter-individual variability in respiratory virus transmission persists, and its underlying drivers remain poorly understood. Epidemiological findings indicate that a small proportion of individuals contribute disproportionately to secondary transmission, highlighting the need to investigate host-specific drivers of transmission risk (Guo et al., 2023; Wegehaupt et al., 2023). Demographic factors (e.g., age, sex, body mass index), lifestyle factors (e.g., smoking status, physical activity), and infection-related factors (e.g., symptom onset, disease severity) have been associated with differences in airway characteristics, mucus properties, and viral shedding profiles (Lee et al., 2017; Dixon and Peters, 2018; Molgat-Seon et al., 2018; Schneider et al., 2021; Hussain-Alkhateeb et al., 2021; Marshall et al., 2020; Zanin et al., 2016; Sun et al., 2024; Li and Tang, 2021; Roman et al., 2016; Rathnayake et al., 2023; LoMauro and Aliverti, 2018). Through these effects, they may shape both the volume of particles produced and the likelihood that those particles carry infectious virus.
This systematic review synthesizes current evidence on the associations between host-related factors and both respiratory particle emission and the detection of virus in exhaled respiratory particles. Understanding how host factors influence both the generation and infectiousness of airborne particles can provide valuable proxies for transmission risk and offer insights into underlying physiological processes. By identifying individual-level determinants of airborne transmission, these findings aim to support risk assessment and guide public health strategies in clinical, occupational, and community settings, especially in preparation for future respiratory virus outbreaks.
2 Methods
2.1 Study design
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021).
2.2 Strategy and selection process
On February 23, 2024, a comprehensive search was performed across three databases: PubMed (via NCBI), EMBASE (via Elsevier), and Web of Science. To ensure the inclusion of more recent articles, an additional search was conducted on September 30, 2024.
The search strategy was developed in consultation with a librarian and structured using the PEO framework. It incorporated search terms related to aerosols as the study problem, respiratory activities or viruses as the exposure of interest, and emissions as the outcome of interest. The following search strategy was used: (aerosol OR droplet nuclei) AND (respirator* OR cough* OR sneez* OR speak* OR speech* OR breath* OR shout* OR SARS* OR COVID* OR corona* OR virus* OR influenza OR flu OR rhinovirus OR common cold OR RSV OR infect*) AND (expel* OR exhal* OR emiss* OR emit*). The strategy was further refined to include relevant Medical Subject Headings (MeSH) and Emtree terms specific to PubMed and EMBASE.
To refine the search and minimize the inclusion of irrelevant studies, three additional exclusion string blocks were applied: (1) “terms related to the dental field, medical procedures, drug delivery, and environmental aerosols” to exclude studies focused on these specific contexts, (2) “limitation to human studies,” excluding animal-based research, and (3) “exclusion of review papers.
The identified articles were deduplicated, and two authors (NH and KL) independently screened them for eligibility with the use of SR-Accelerator (Bond University) (Clark et al., 2020). Discrepancies in the title and abstract screening were discussed by the two authors to determine final inclusion. Any remaining disagreements were resolved through discussion. The following automation tools were used during the process: Deduplicator, Screenatron, and Disputatron (Forbes et al., 2024).
2.3 Eligibility criteria and exclusion criteria
Full-text articles written in English were included if they addressed: (1) the size and quantity of emissions from human subjects, (2) the presence of viral particles in emissions and (3) the correlation between emissions and host factors, such as demographic characteristics, lifestyle factors, and respiratory infection characteristics.
Studies were excluded if they focused on mitigation or leakage, toxicity, deposition, aerosol generation procedures, non-respiratory pathogens, animal studies, reviews, conference papers, opinion/editorial pieces, or publications in languages other than English.
2.4 Study categorization, data extraction and processing
To facilitate analysis, included studies were grouped into two main categories based on their primary outcome measure: (I) quantification of particles during respiratory emissions and (II) detection of viral particles in respiratory emissions. Categorization was determined through a detailed review of each study’s objectives and methodologies to ensure accurate classification.
For each included study, data were extracted on study design, sample size, participant characteristics (including age range, sex distribution, body mass index, and smoking status), type of virus investigated, respiratory activities assessed, particle size fractions analyzed, and sampling methods used. Data extraction was performed using Microsoft Excel (version 16.92).
Given the heterogeneity in study methodologies and reporting formats, a narrative synthesis approach was adopted. Associations between host factors and outcomes were categorized as positive, negative, null, or inconsistent, based on the reported direction and statistical significance. Where available, effect measures such as odds ratios, correlation coefficients, and p-values were recorded. No meta-analyses were performed due to variability in study designs and outcome measures.
The studies collectively examined a range of host factors, including age, sex, body mass index (BMI), smoking status, exercise intensity, infection status, symptom presence and severity, symptom onset timing, viral load in clinical swabs, and vaccination status, in relation to respiratory particle emissions, or presence of viral particles.
Data analysis and table generation were performed using R (version 4.3.2) and RStudio (version 2024.04.2 + 764), utilizing the Tidyverse (version 2.0.0) and gt (version 0.10.0) packages (Team RC, 2023; Wickham et al., 2019; Iannone et al., 2023).
2.5 Quality assessment
The quality of all included studies was assessed using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cohort, case–control, and cross-sectional studies (von Elm et al., 2007). An overview of the assessment results is provided in Supplementary Table 3.
2.6 Terminology statement
Following the latest WHO consultation report (Organization GWH, 2024), this review acknowledges the continuous size distribution of respiratory particles but adopts the commonly used classification distinguishing fine particles (< 5 μm) from coarse particles (≥ 5 μm).
3 Results
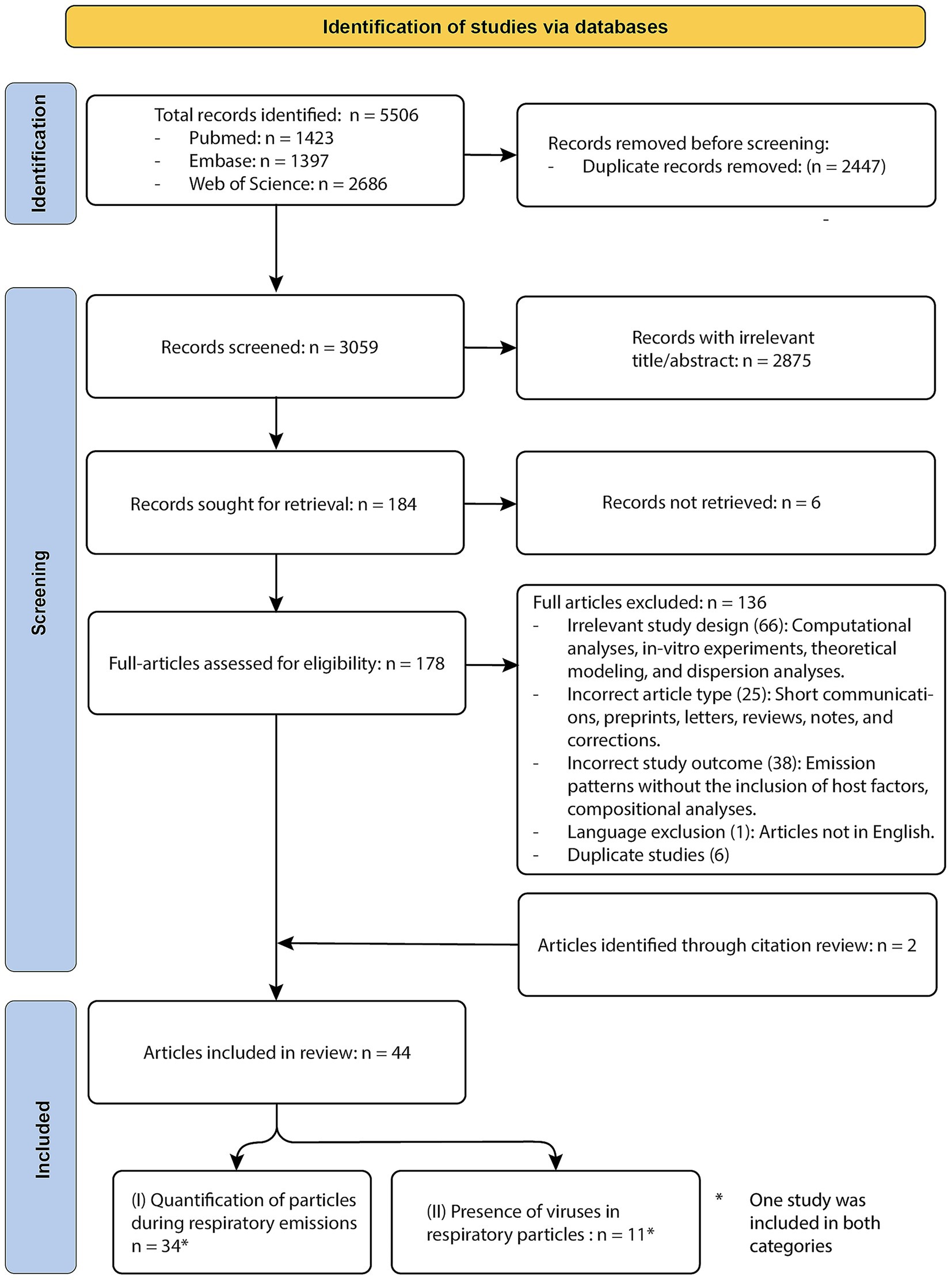
The database searches yielded a total of 5,506 articles. After deduplication, 3,059 unique citations remained and were screened by title and abstract. Of these, 2,875 were excluded for not meeting the inclusion criteria. An additional six articles were excluded due to retrieval issues, leaving 178 studies for full-text review. Following full-text screening, 136 articles were excluded due to ineligible study designs, outcomes, article types, language, or duplication. Two more studies were added through reference list screening, resulting in 44 studies meeting the eligibility criteria and included in this systematic review. Of these, host factor associations with respiratory particle emission were examined in 34 studies. An additional 11 studies assessed viral presence in exhaled particles in relation to host factors; all of these were limited to influenza virus or SARS-CoV-2. One study contributed to both outcome categories and is therefore included in both counts (Viklund et al., 2022). A detailed overview of the database-specific search terms and corresponding results is provided in Supplementary Tables 1, 2. Detailed reviewer comments and final decisions from both the title/abstract and full-text screening stages are provided in Supplementary File 1. A PRISMA flow diagram is provided in Figure 2.
3.1 Associations between host factors and respiratory particle emission
3.2 Study characteristics
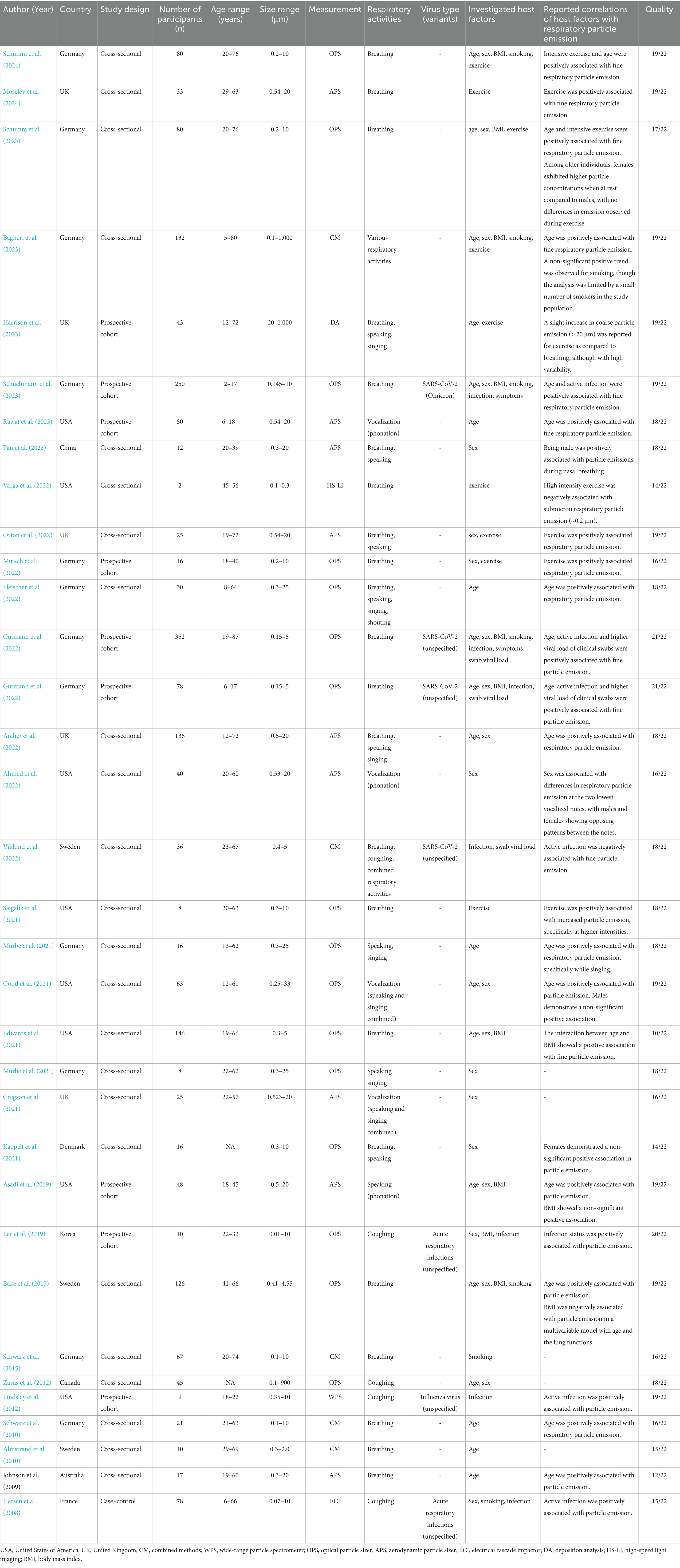
Thirty-four studies investigating respiratory particle emission in relation to host factors were published between 2008 and 2024 (Table 1). A majority originated from Germany, the United States, and the United Kingdom. Most employed cross-sectional designs, with sample sizes ranging from small pilot cohorts to larger observational groups. Participant age across studies spanned from 2 to 87 years. A variety of respiratory activities were assessed, including breathing, speaking, singing, sustained phonation, and coughing. Sampling approaches differed across studies, resulting in the inclusion of a broad particle size range. Overall, the sampling window extended from 0.01 to 1,000 μm, although most studies focused on a narrower range between 0.3 and 10 μm. Inter-individual variability in respiratory particle emission was reported in 22 studies (Bagheri et al., 2023; Johnson and Morawska, 2009; Almstrand et al., 2010; Bake et al., 2017; Zayas et al., 2012; Viklund et al., 2022; Ahmed et al., 2022; Archer et al., 2022; Good et al., 2021; Gregson et al., 2021; Harrison et al., 2023; Lee et al., 2019; Lindsley et al., 2012; Mürbe et al., 2021; Mürbe et al., 2021; Mutsch et al., 2022; Pan et al., 2023; Rawat et al., 2023; Schuchmann et al., 2023; Schwarz et al., 2010; Schwarz et al., 2015; Gutmann et al., 2022). In contrast, six studies observed relatively stable intra-individual emission patterns across multiple sampling sessions (Bagheri et al., 2023; Almstrand et al., 2010; Bake et al., 2017; Rawat et al., 2023; Schwarz et al., 2010; Schwarz et al., 2015).
3.3 Fine respiratory particle emission
Fine particle emissions were reported in most studies (n = 33), with seven studies focusing exclusively on this size fraction (Almstrand et al., 2010; Bake et al., 2017; Viklund et al., 2022; Gutmann et al., 2022; Gutmann et al., 2022; Edwards et al., 2021; Varga et al., 2022). Among the 26 studies that also included coarse particles, fine particles consistently represented the dominant mode of emission. A modal diameter of approximately 1 μm or smaller was reported in 21 studies (Bagheri et al., 2023; Johnson and Morawska, 2009; Almstrand et al., 2010; Zayas et al., 2012; Viklund et al., 2022; Ahmed et al., 2022; Archer et al., 2022; Good et al., 2021; Gregson et al., 2021; Mürbe et al., 2021; Mürbe et al., 2021; Mutsch et al., 2022; Rawat et al., 2023; Schuchmann et al., 2023; Schwarz et al., 2010; Schwarz et al., 2015; Gutmann et al., 2022; Asadi et al., 2019; Fleischer et al., 2022; Orton et al., 2022; Sajgalik et al., 2021; Schumm et al., 2023; Hersen et al., 2008). A slightly larger secondary mode, ranging from 1.3 to 2 μm, was identified in a subset of these studies (Archer et al., 2022; Good et al., 2021; Rawat et al., 2023; Orton et al., 2022). If coarse particles were detected, they were either infrequently observed or found at substantially lower concentrations (Archer et al., 2022; Gregson et al., 2021; Lindsley et al., 2012; Mürbe et al., 2021; Mürbe et al., 2021; Pan et al., 2023; Asadi et al., 2019; Fleischer et al., 2022; Sajgalik et al., 2021). Accordingly, the host-related associations described across studies were predominantly based on measurements within the fine particle fraction. Table 2 provides a summary of these findings, organized by host factor and reported direction of association.
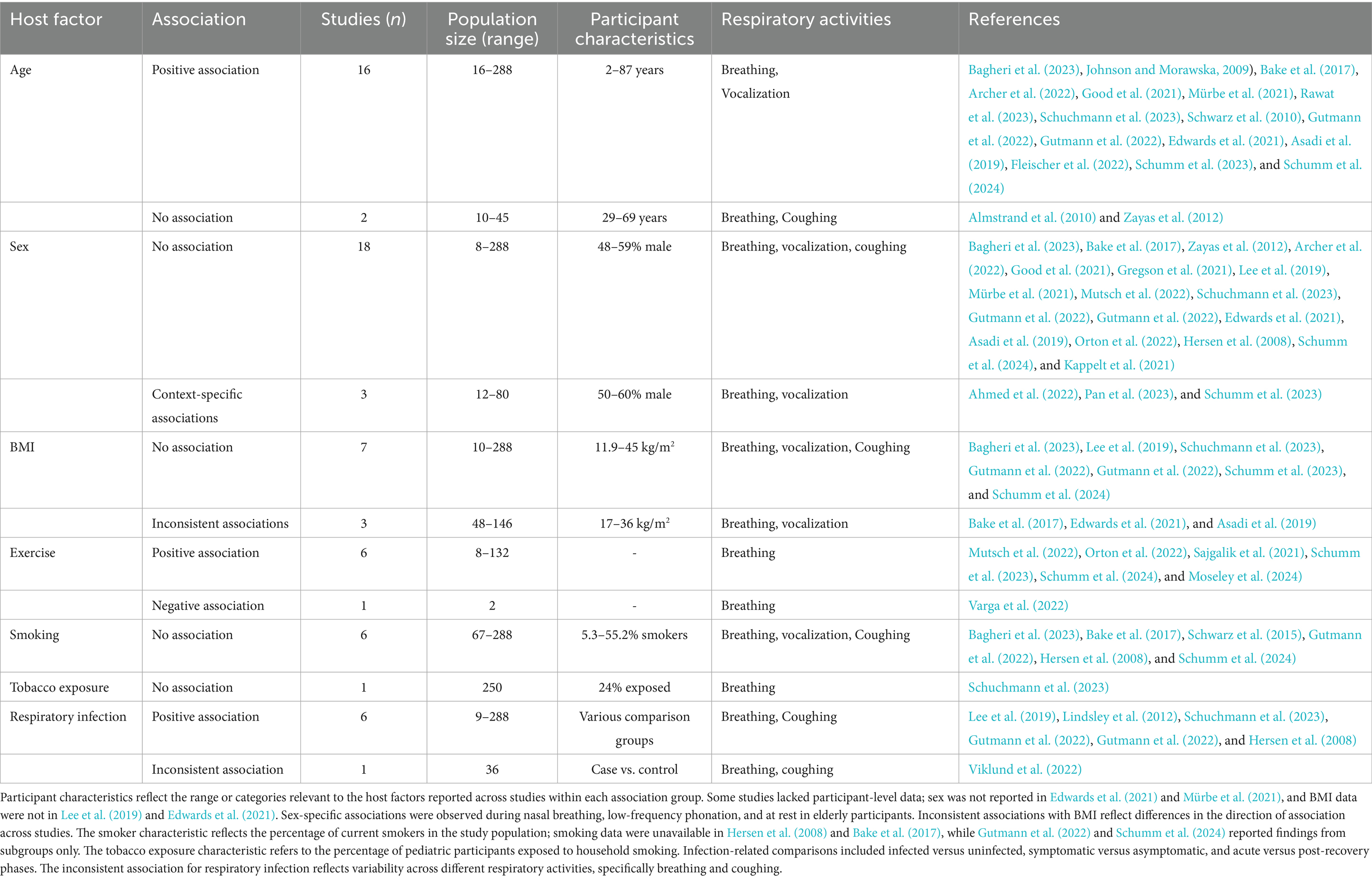
Table 2. Summary of reported associations between host factors and fine respiratory particle emission.
3.3.1 Demographic factors
Age was the demographic factor most consistently associated with fine respiratory particle emission. Of the 18 studies evaluating this relationship, 16 reported increased emission rates with advancing age (Bagheri et al., 2023; Johnson and Morawska, 2009; Bake et al., 2017; Archer et al., 2022; Good et al., 2021; Mürbe et al., 2021; Rawat et al., 2023; Schuchmann et al., 2023; Schwarz et al., 2010; Gutmann et al., 2022; Gutmann et al., 2022; Edwards et al., 2021; Asadi et al., 2019; Fleischer et al., 2022; Schumm et al., 2023; Schumm et al., 2024). This trend was generally gradual, with higher emission observed across progressively older age groups. Associations were identified across a range of expiratory activities and population age spans, including pediatric and older adult participants. The two studies that did not report an association included smaller sample sizes and narrower age distributions (Almstrand et al., 2010; Zayas et al., 2012).
Sex was not associated with fine particle emission in most studies. Among the 21 studies assessing sex-related differences, 18 reported no significant variation between male and female participants (Bagheri et al., 2023; Bake et al., 2017; Zayas et al., 2012; Archer et al., 2022; Good et al., 2021; Gregson et al., 2021; Lee et al., 2019; Mürbe et al., 2021; Mutsch et al., 2022; Schuchmann et al., 2023; Gutmann et al., 2022; Gutmann et al., 2022; Edwards et al., 2021; Asadi et al., 2019; Orton et al., 2022; Hersen et al., 2008; Schumm et al., 2024; Kappelt et al., 2021). Most included balanced sex distributions and assessed multiple expiratory activities. Three studies reported context-specific differences under select conditions, such as nasal breathing, low-pitch phonation, or elevated emission in elderly females at rest; however, these observations were limited to isolated analyses and were not systematically evaluated across studies (Ahmed et al., 2022; Pan et al., 2023; Schumm et al., 2023).
As with sex, BMI was generally not associated with fine respiratory particle emission across studies. Ten studies assessed this relationship, most of which included participants with a broad range of BMI values. Seven studies reported no association (Bagheri et al., 2023; Lee et al., 2019; Schuchmann et al., 2023; Gutmann et al., 2022; Gutmann et al., 2022; Schumm et al., 2023; Schumm et al., 2024). The remaining studies described divergent patterns, including both positive and negative associations or interactions with other factors such as age, but these findings were limited to isolated analyses and were not replicated across the broader evidence base (Bake et al., 2017; Edwards et al., 2021; Asadi et al., 2019).
3.3.2 Lifestyle factors
Among lifestyle-related factors, physical exercise demonstrated the most consistent association with fine respiratory particle emission. Six of seven studies reported increased fine particle emission during exercise, particularly at peak intensity, based on measurements taken before and during exertion on a cycle ergometer (Mutsch et al., 2022; Orton et al., 2022; Sajgalik et al., 2021; Schumm et al., 2023; Schumm et al., 2024; Moseley et al., 2024). One pilot study, which focused on particles in the lower submicron range, observed a reduction in emission during peak exercise (Varga et al., 2022).
In contrast, studies reported no associations between smoking and fine particle emission. Six studies assessed this relationship across various respiratory activities and populations with differing proportions of smokers; none identified significant differences between smokers and non-smokers (Bagheri et al., 2023; Bake et al., 2017; Schwarz et al., 2015; Gutmann et al., 2022; Hersen et al., 2008; Schumm et al., 2024). Similarly, one study evaluating secondhand smoke exposure in a pediatric population found no association (Schuchmann et al., 2023).
3.3.3 Infection-related factors
The association between respiratory viral infections and fine particle emission was evaluated in seven studies. Of these, six studies observed increased emission during active infection, including investigations of SARS-CoV-2, influenza virus, and cases with confirmed viral respiratory infections lacking pathogen specification (Lee et al., 2019; Lindsley et al., 2012; Schuchmann et al., 2023; Gutmann et al., 2022; Gutmann et al., 2022; Hersen et al., 2008). Elevated emission was observed during both breathing and coughing in case–control comparisons, among symptomatic versus asymptomatic individuals, and during acute illness relative to post-recovery. In contrast to other findings, one study investigating SARS-CoV-2 reported reduced emission during breathing, while coughing showed no difference, indicating inconsistent associations across respiratory activities (Viklund et al., 2022).
Positive correlations between viral load in exhaled particles and clinical specimens were reported in two studies, though this relationship was not extensively investigated across the broader evidence base (Gutmann et al., 2022; Gutmann et al., 2022). Symptom-based comparisons were limited but did not yield statistically significant associations between fine particle emission and either upper or lower respiratory tract symptoms (Schuchmann et al., 2023; Gutmann et al., 2022).
3.3.4 Coarse respiratory particle emission
Associations between host factors and coarse respiratory particle emission were only assessed in two studies, as the predominance of fine particles in other studies limited evaluation of coarse particle-specific effects (Bagheri et al., 2023; Harrison et al., 2023). These two studies investigated a broader particle size range, extending up to the millimeter scale, and were able to distinguish between coarse and fine fractions, or did not include the fine particle fraction at all. Despite the overall inclusion of a broad age range (5–80 years), no association was observed between age and coarse particle emission (Bagheri et al., 2023; Harrison et al., 2023). One study reported age-related differences restricted to particles smaller than 5–8 μm, suggesting that observed associations did not extend into the coarse size range (Bagheri et al., 2023). A modest increase in coarse particle emission was observed during vigorous exercise in one study; however, emissions in this size range were generally low and highly variable (Harrison et al., 2023).
3.4 Associations between host factors on the presence of respiratory viruses in exhaled particles
3.4.1 Study characteristics
Eleven studies investigated associations between host factors and the presence of respiratory viruses in exhaled particles (Table 3). These studies, published between 2008 and 2024, were primarily conducted in the United States and employed cross-sectional or prospective cohort designs; one study was a single case report. Sample sizes ranged from individual cases to larger cohorts, with participant ages spanning 6 to 67 years.
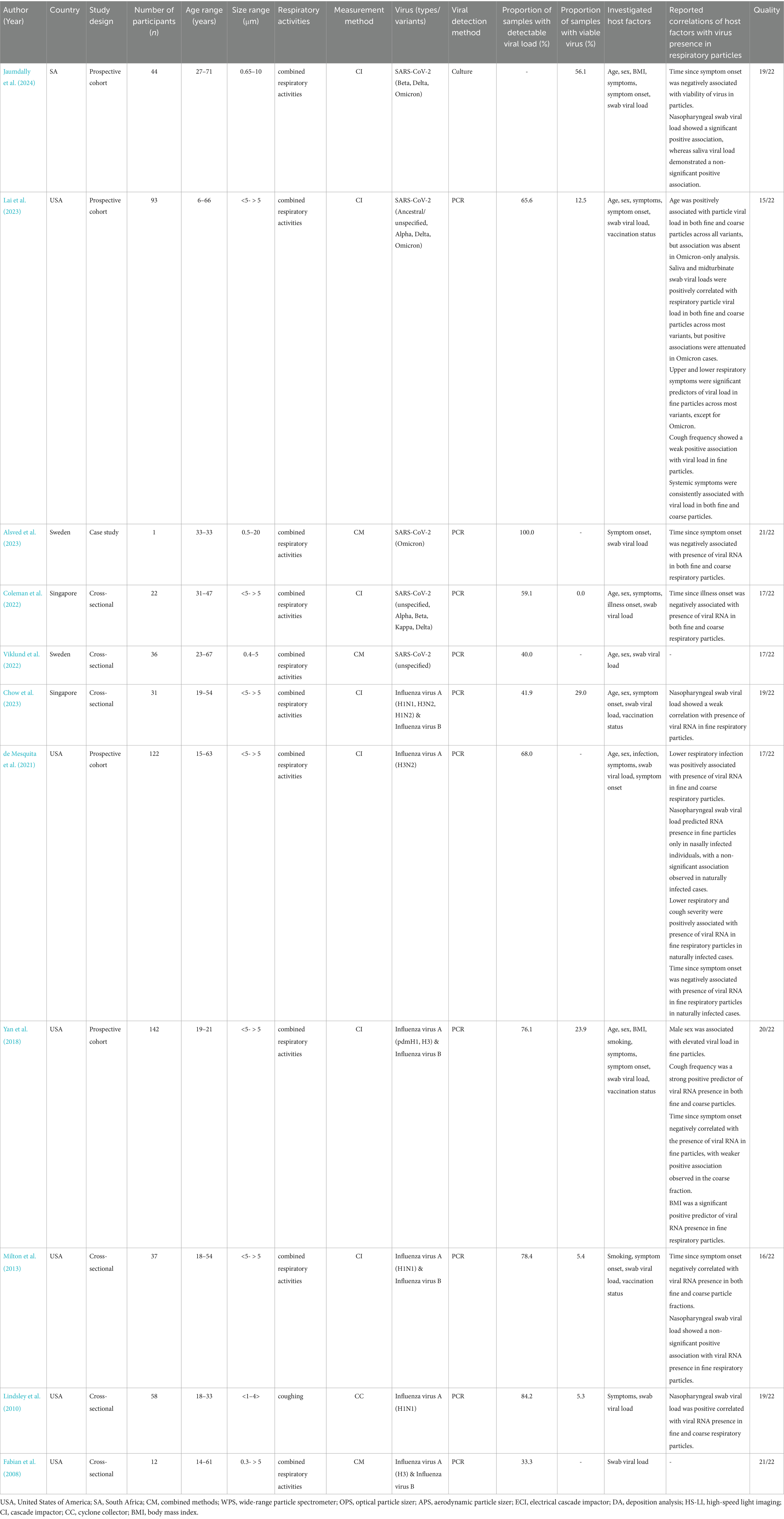
All studies involved outpatient populations with laboratory-confirmed Influenza virus or SARS-CoV-2 infection, though the viral variants and methods used to assess exhaled viral content varied. Most included multiple respiratory activities during sampling, precluding assessment of activity-specific associations with viral emission. An exception was one study that investigated associations during coughing alone (Lindsley et al., 2010). Particle size was commonly stratified into fine and coarse fractions using a single cut-off, although some studies applied multiple filter thresholds or combined physical sampling with optical sizing to enhance resolution. Viral presence was predominantly assessed using reverse transcription polymerase chain reaction (RT-PCR) to quantify viral RNA (hereafter referred to as viral load). Most infected individuals exhibited detectable viral load in exhaled particles, with consistently higher loads in fine compared to coarse fractions. Substantial inter-individual variability was reported in nine studies (Viklund et al., 2022; Lindsley et al., 2010; Chow et al., 2023; Coleman et al., 2022; de Mesquita et al., 2021; Fabian et al., 2008; Jaumdally et al., 2024; Lai et al., 2023; Milton et al., 2013). Although viral viability was assessed in six studies, only one investigated its relationship with host factors (Jaumdally et al., 2024). In general, viable virus was detected at much lower rates than viral RNA.
The inclusion of various influenza virus types and SARS-CoV-2 variants did not reveal any consistent strain- or subtype-specific patterns. A subset of studies explicitly reported the absence of such differences in their findings (Coleman et al., 2022; Milton et al., 2013; Yan et al., 2018). One study, however, observed some variability in a sub-analysis of SARS-CoV-2 variants, particularly when Omicron was analyzed separately from pooled SARS-CoV-2 variants (Lai et al., 2023). Although this observation was limited to a single study, it is considered alongside other findings related to host factor associations discussed below. Table 4 summarizes the identified associations for each host factor, stratified by virus type (influenza or SARS-CoV-2).
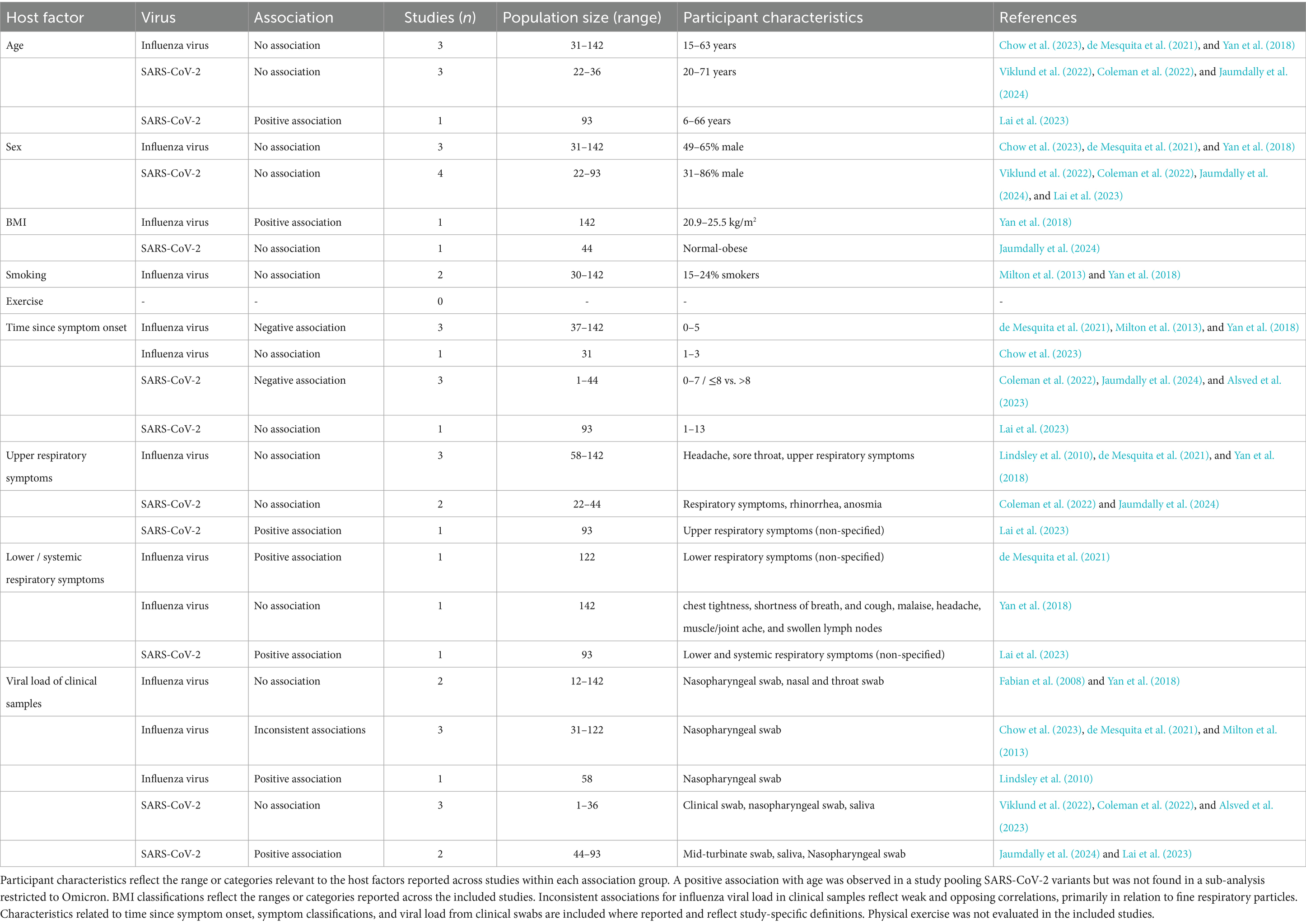
Table 4. Summary of reported associations between host factors and viral presence in exhaled respiratory particles, stratified by virus type.
3.4.2 Demographic and lifestyle factors
Demographic and lifestyle factors generally showed no significant associations with the presence of viruses in exhaled respiratory particles; however, some factors lack sufficient coverage across studies to make firm conclusions. Age and sex were each assessed in seven studies, most of which reported no significant association with viral load or virus viability within exhaled particles (Viklund et al., 2022; Chow et al., 2023; Coleman et al., 2022; de Mesquita et al., 2021; Jaumdally et al., 2024; Lai et al., 2023; Yan et al., 2018). Study populations were predominantly adults, although some also enrolled pediatric participants, and sex representation varied across cohorts. A single positive association between age and SARS-CoV-2 viral load was reported; however, this association did not persist in a sub-analysis limited to Omicron cases (Lai et al., 2023). Compared to age and sex, associations involving BMI and smoking status were examined less frequently. Findings for BMI were mixed: one study reported no association with SARS-CoV-2 viability within exhaled particles, while another study identified a positive association for influenza viral load (Jaumdally et al., 2024; Yan et al., 2018). Smoking status was assessed only in the context of influenza virus, with no significant associations observed (Milton et al., 2013; Yan et al., 2018). Physical exercise was not evaluated in relation to viral emission in any included studies.
3.4.3 Infection-related factors
Among infection-related factors, the time since symptom onset demonstrated the clearest overall association with virus detectability in exhaled respiratory particles. Of the eight studies evaluating this factor, Six studies consistently reported decreasing particle viral load or viability as time since symptom onset increased (Coleman et al., 2022; de Mesquita et al., 2021; Jaumdally et al., 2024; Milton et al., 2013; Yan et al., 2018; Alsved et al., 2023). However, one of the two studies that did not observe this association assessed a broader post-symptom onset interval than the other studies investigating this factor (Chow et al., 2023; Lai et al., 2023).
Associations with respiratory symptoms varied by anatomical site. Among six studies assessing upper respiratory tract symptoms, most found no significant association of symptom presence with viral load in exhaled particles (Lindsley et al., 2010; Coleman et al., 2022; de Mesquita et al., 2021; Jaumdally et al., 2024; Yan et al., 2018). One exception reported an association between upper respiratory symptoms and SARS-CoV-2 viral load, observed exclusively in the fine particle fraction and not seen in the Omicron-specific analyses (Lai et al., 2023). In contrast, the presence of lower respiratory symptoms, evaluated in three studies, was more frequently associated with elevated viral load in exhaled particles (de Mesquita et al., 2021; Lai et al., 2023). One study that did not report a significant association involved participants with predominantly mild symptoms (Yan et al., 2018). Additionally, statistical significance was not observed in analyses specifically restricted to the Omicron variant (Lai et al., 2023).
Correlations between viral load in clinical specimens (e.g., nasopharyngeal swabs or saliva) and exhaled particle viral load were assessed in eleven studies, yielding mixed findings. Five studies reported no significant correlation (Viklund et al., 2022; Coleman et al., 2022; Fabian et al., 2008; Yan et al., 2018; Alsved et al., 2023). Among the remaining studies, most reported positive correlations between swab viral load and viral load in both fine and coarse particle fractions, although the strength of these associations varied (Lindsley et al., 2010; de Mesquita et al., 2021; Jaumdally et al., 2024; Lai et al., 2023). Higher correlation estimates were reported for the Omicron variant compared to pooled SARS-CoV-2 variants, specifically for saliva samples (Lai et al., 2023). Two studies on Influenza reported correlations limited to the fine particle size fraction; however, the direction of these correlations was opposite (Chow et al., 2023; Milton et al., 2013).
4 Discussion
This systematic review synthesizes evidence from 44 studies to elucidate if host determinants are associated with two critical aspects of airborne transmission: the generation of exhaled aerosol particles and the incorporation of infectious virus into those particles. Most host factor associations were reported for fine particles (<5 μm), reflecting both their abundance in exhaled respiratory particles and the methodological challenges inherent in assessing coarse particle fractions. Several host factors, including age, physical exercise, and active infection, emerged as consistent predictors of increased fine particle emission, whereas sex, BMI, and smoking yielded limited or inconsistent associations. Viral presence within these particles was most strongly linked to infection-related characteristics, particularly time since symptom onset and lower respiratory tract involvement, rather than demographic or lifestyle factors.
The host factor associations identified in this review may be explained by several underlying biophysical processes governing respiratory particle generation. As previously discussed, the cyclic closure and reopening of small airways in the distal lung is a primary mechanism contributing to the formation of fine particles (Figure 1). This process may vary across the lifespan. In younger individuals, ongoing alveolar development into early adulthood may limit the surface area available for particle formation, contributing to lower emission levels. In contrast, older adults exhibit reduced elastic recoil and increased airway collapsibility, which may enhance airway reopening and shear forces, thereby promoting increased fine particle generation (Roman et al., 2016; Thomas et al., 2019; Martins Rodrigues et al., 2021; Nikolić et al., 2018). Age-related changes in mucus composition may further influence fluid rheology and reduce surface tension thresholds, facilitating droplet fragmentation (Edwards et al., 2004; Hussain-Alkhateeb et al., 2021). Physical exercise also acts along this pathway by increasing tidal volume and airflow velocities, thereby amplifying shear forces and the frequency of airway reopening events. These mechanical stresses promote fragmentation of the airway surface liquid and enhance fine particle emission (Johnson and Morawska, 2009; Morawska et al., 2009; Archer et al., 2022; Harrison et al., 2023). However, systemic dehydration during intense exercise may reduce airway hydration, potentially limiting submicron particle production despite elevated ventilation (Marshall et al., 2020; Varga et al., 2022). A respiratory infection may similarly enhance particle output by increasing mucus production and airway surface liquid, or indirectly through elevated respiratory rates associated with shortness of breath (Li and Tang, 2021; Weston and Frieman, 2019). Positive correlation reported between viral load in clinical swabs and the quantity of fine respiratory particles may reflect immune-mediated effects on airway physiology, although this hypothesis requires further validation. One contradictory finding was reported, suggesting that changes in viscosity may reduce particle emissions during infection. However, the study was limited by its small sample size as well as the duration and volume of sampling (Viklund et al., 2022).
Host factors such as sex, BMI, and smoking were not consistently associated with fine particle emission. While it is known that these factors can influence respiratory tract physiology, their effects on particle generation may be indirect or context dependent. For example, the lack of sex differences may reflect comparable alveolar structure and lung function across sexes in adulthood (Molgat-Seon et al., 2018; LoMauro and Aliverti, 2018). Occasional findings, such as increased emission during nasal breathing or low-frequency phonation, suggest a minor role for proximal production mechanisms, although supporting evidence remains limited. Variable associations with BMI may relate to comorbid conditions such as asthma or COPD, which can affect airway reopening dynamics (Sun et al., 2024). Similarly, although smoking is known to alter the bronchial mucus barrier and increase mucus production, it does not appear to enhance fine particle formation (Rathnayake et al., 2023). One possible explanation, also noted in the context of infection (Viklund et al., 2022), is that increased viscosity may limit the fragmentation of the airway lining fluid, thereby reducing fine particle emission and potentially masking any effect of smoking, although this remains speculative (Edwards et al., 2004).
By contrast, viral loading of respiratory particles appears to depend more on infection biology than on the mechanics of particle generation. Findings regarding host factor associations were largely consistent across influenza virus and SARS-CoV-2, indicating similar patterns in viral load dynamics despite differences in virus type and variant. However, the lack of studies on other respiratory viruses constrains the generalizability of these observations. This limitation may relate to differences in tissue tropism, as seen with Omicron, which shows altered anatomical targeting compared to earlier variants and may contribute to virus-specific patterns of respiratory particle emission (Lai et al., 2023; Chatterjee et al., 2023).
Viral replication is reported to peak during the early phase of infection, likely contributing to a higher probability that exhaled particles contain infectious virus (Puhach et al., 2023; Chen et al., 2021). This aligns with consistent findings of a temporal decline in exhaled viral load as time since symptom onset increases. However, more severe infections can result in prolonged viral loads in the lower respiratory tract, potentially explaining the absence of a temporal decline in some studies (Puhach et al., 2023; Lim et al., 2021). The anatomical focus of infection appears to also influence the likelihood of detecting virus in exhaled particles. Specifically, associations between viral load in particles and lower respiratory tract symptoms were more frequently observed than with upper respiratory symptoms, which suggest independent mechanisms. This is consistent with studies reporting that especially involvement of the lower respiratory tract increases the likelihood of virus presence in exhaled particles (de Mesquita et al., 2021; Yan et al., 2018; Lindsley et al., 2016). Inconsistent correlations between swab-based viral load and exhaled viral particles underscore this anatomical disconnect. Notably, studies focusing specifically on coughing, which generates high-velocity shear-driven emissions, have demonstrated stronger correlations with upper airway viral load, suggesting that these clinical parameters may be more closely linked to proximal particle generation mechanisms (Lindsley et al., 2010; de Mesquita et al., 2021; Yan et al., 2018).
Demographic and lifestyle host factors did not consistently account for the presence of virus in exhaled particles. Although age was frequently associated with increased fine particle emission, it was less informative in identifying individuals likely to shed infectious virus. This may be attributed to the predominance of studies involving mildly symptomatic or outpatient populations, in whom minimal lower respiratory tract involvement may limit the generation of virus-laden particles from distal airways. However, a few studies did report positive associations for age and BMI, which may suggest potential links. Other physiological processes, particularly differences in immune response such as impaired immune clearance associated with advancing age or elevated BMI, could contribute to these associations (Flerlage et al., 2021; Schneider et al., 2021).
It is important to note that most reported associations between host factors and the presence of respiratory viruses are based on detection of genomic material rather than replication-competent virus. This detection shows that viral material can be present even in small particles, but infectious virus is less often recovered in the current evidence base. Therefore, RNA-based findings should be regarded as indicators of viral shedding rather than direct proof of infectiousness.
A limited number of studies assessed host factor associations with coarse particle emission. However, the findings suggest that the effects of age and physical exercise were weaker for coarse particles than for fine particles. The limited evidence likely reflects methodological constraints: sampling interfaces such as masks and tubing may reduce capture of larger particles due to impaction losses and flow restrictions, while dehydration and shrinkage may further bias size classification (Pöhlker et al., 2023; Bagheri et al., 2023; Edwards et al., 2004). Beyond particle size bias, other methodological constraints were common across studies. The majority were cross-sectional and conducted at a single time point, limiting the ability to capture dynamic changes in respiratory particle output or viral shedding over time. Few studies accounted for individual-level factors such as hydration status, which may influence particle generation (Gregson et al., 2021; Rawat et al., 2023; Oh et al., 2024). Heterogeneity in instrumentation and study protocols introduced variability across findings, making it difficult to isolate the absolute effect of host factors (Hu et al., 2024; Verreault et al., 2008). Symptom classification often relied on self-reported data, introducing potential misclassification.
Despite methodological variability, the inclusion of diverse study designs strengthens the overall conclusions of this review. Studies maintained internal consistency in sampling and analysis, allowing meaningful comparisons within cohorts. By evaluating both respiratory particle emission and viral presence, this review provides an integrated perspective on host-related determinants of airborne transmission. The findings suggest that certain host characteristics, particularly when considered in combination, may serve as practical indicators of infectiousness. Individuals with increased particle emission and a greater likelihood of virus presence, such as older adults in the early phase of infection or those with lower respiratory involvement, may benefit from targeted mitigation strategies. Future studies should examine the combined impact of host factors on viable viruses in respiratory particles, explore less-studied variables such as the effect of physical exercise, and include a broader range of respiratory pathogens. In parallel, investigating the underlying generation mechanisms that drive these associations may clarify causal pathways and strengthen mechanistic understanding. The adoption of standardized measurement approaches that include the coarse particle range, along with expanded clinical characterization in longitudinal studies, will be essential for improving data comparability and strengthening outbreak preparedness.
5 Conclusion
This systematic review highlights that host factors influence airborne transmission through two distinct mechanisms: airway biomechanics driving particle generation and infection biology determining viral presence in exhaled particles. Older age, physical exercise, and active respiratory infections were consistently associated with increased fine respiratory particle emission, whereas other variables, including sex, BMI, and smoking, showed no consistent associations or were insufficiently studied. Viral presence in respiratory particles was more closely linked to time since symptom onset and anatomical site rather than individual demographic or lifestyle factors. No single host factor explained both increased particle emission and viral presence, but combinations such as older age with lower respiratory involvement may better indicate transmission potential. Current evidence remains constrained by methodological heterogeneity, a predominant focus on SARS-CoV-2 and influenza, and reliance on genomic detection rather than viral viability. Closing these gaps will be essential not only for refining risk assessment and guiding targeted interventions, but also for strengthening outbreak preparedness and improving infection control strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
NH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Validation. KL: Investigation, Methodology, Writing – review & editing, Validation. MK: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing. LV: Conceptualization, Methodology, Supervision, Writing – review & editing. JR: Conceptualization, Methodology, Supervision, Writing – review & editing. AV: Conceptualization, Methodology, Supervision, Writing – review & editing. ML: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Dutch Research Council (NWO) under the project MItigation STrategies for Airborne Infection Control (MIST).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. The authors used OpenAI’s ChatGPT model 4o to assist with language editing and sentence refinement during the preparation of this manuscript. All content was critically reviewed and finalized by the authors to ensure accuracy and integrity.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1652124/fulll#supplementary-material
References
Ahmed, T., Rawat, M. S., Ferro, A. R., Mofakham, A. A., Helenbrook, B. T., Ahmadi, G., et al. (2022). Characterizing respiratory aerosol emissions during sustained phonation. J. Expo. Sci. Environ. Epidemiol. 32, 689–696. doi: 10.1038/s41370-022-00430-z
Almstrand, A.-C., Bake, B., Ljungström, E., Larsson, P., Bredberg, A., Mirgorodskaya, E., et al. (2010). Effect of airway opening on production of exhaled particles. J. Appl. Physiol. 108, 584–588. doi: 10.1152/japplphysiol.00873.2009
Alsved, M., Nygren, D., Thuresson, S., Fraenkel, C. J., Medstrand, P., and Löndahl, J. (2023). Size distribution of exhaled aerosol particles containing SARS-CoV-2 RNA. Infect. Dis. 55, 158–163. doi: 10.1080/23744235.2022.2140822
Archer, J., McCarthy, L. P., Symons, H. E., Watson, N. A., Orton, C. M., Browne, W. J., et al. (2022). Comparing aerosol number and mass exhalation rates from children and adults during breathing, speaking and singing. Interface Focus 12:20210078. doi: 10.1098/rsfs.2021.0078
Asadi, S., Wexler, A. S., Cappa, C. D., Barreda, S., Bouvier, N. M., and Ristenpart, W. D. (2019). Aerosol emission and superemission during human speech increase with voice loudness. Sci. Rep. 9:2348. doi: 10.1038/s41598-019-38808-z
Bagheri, G., Schlenczek, O., Turco, L., Thiede, B., Stieger, K., Kosub, J. M., et al. (2023). Size, concentration, and origin of human exhaled particles and their dependence on human factors with implications on infection transmission. J. Aerosol Sci. 168:106102. doi: 10.1016/j.jaerosci.2022.106102
Bake, B., Ljungström, E., Claesson, A., Carlsen, H. K., Holm, M., and Olin, A. C. (2017). Exhaled particles after a standardized breathing maneuver. J. Aerosol Med. Pulm. Drug Deliv. 30, 267–273. doi: 10.1089/jamp.2016.1330
Bender, R. G., Sirota, S. B., Swetschinski, L. R., Dominguez, R.-M. V., Novotney, A., Wool, E. E., et al. (2024). Global, regional, and national incidence and mortality burden of non-COVID-19 lower respiratory infections and aetiologies, 1990-2021: a systematic analysis from the global burden of disease study 2021. Lancet Infect. Dis. 24, 974–1002. doi: 10.1016/S1473-3099(24)00176-2
Chatterjee, S., Bhattacharya, M., Nag, S. A.-O., Dhama, K. A.-O., and Chakraborty, C. A.-O. X. (2023). A detailed overview of SARS-CoV-2 omicron: its sub-variants, mutations and pathophysiology, clinical characteristics, immunological landscape, immune escape, and therapies. Viruses 15:167. doi: 10.3390/v15010167
Chen, P. Z., Bobrovitz, N., Premji, Z., Koopmans, M., Fisman, D. N., and Gu, F. X. (2021). Heterogeneity in transmissibility and shedding SARS-CoV-2 via droplets and aerosols. eLife 10:65774. doi: 10.7554/eLife.65774
Chow, V. T. K., Tay, D. J. W., Chen, M. I. C., Tang, J. W., Milton, D. K., and Tham, K. W. (2023). Influenza A and B viruses in fine aerosols of exhaled breath samples from patients in tropical Singapore. Viruses 15:2033. doi: 10.3390/v15102033
Clark, J., Glasziou, P., Del Mar, C., Bannach-Brown, A., Stehlik, P., and Scott, A. M. (2020). A full systematic review was completed in 2 weeks using automation tools: a case study. J. Clin. Epidemiol. 121, 81–90. doi: 10.1016/j.jclinepi.2020.01.008
Coleman, K. K., Tay, D. J. W., Tan, K. S., Ong, S. W. X., Than, T. S., Koh, M. H., et al. (2022). Viral load of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in respiratory aerosols emitted by patients with coronavirus disease 2019 (COVID-19) while breathing, talking, and singing. Clin. Infect. Dis. 74, 1722–1728. doi: 10.1093/cid/ciab691
de Mesquita, P. J. B., Nguyen-Van-Tam, J., Killingley, B., Enstone, J., Lambkin-Williams, R., Gilbert, A. S., et al. (2021). Influenza A (H3) illness and viral aerosol shedding from symptomatic naturally infected and experimentally infected cases. Influenza Other Respir. Viruses 15, 154–163. doi: 10.1111/irv.12790
Dixon, A. E., and Peters, U. (2018). The effect of obesity on lung function. Expert Rev. Respir. Med. 12, 755–767. doi: 10.1080/17476348.2018.1506331
Edwards, D. A., Ausiello, D., Salzman, J., Devlin, T., Langer, R., Beddingfield, B. J., et al. (2021). Exhaled aerosol increases with COVID-19 infection, age, and obesity. Proc. Natl. Acad. Sci. USA 22:118. doi: 10.1073/pnas.2021830118
Edwards, D. A., Man, J. C., Brand, P., Katstra, J. P., Sommerer, K., Stone, H. A., et al. (2004). Inhaling to mitigate exhaled bioaerosols. Proc. Natl. Acad. Sci. USA 101, 17383–17388. doi: 10.1073/pnas.0408159101
Fabian, P., McDevitt, J. J., DeHaan, W. H., Fung, R. O., Cowling, B. J., Chan, K. H., et al. (2008). Influenza virus in human exhaled breath: an observational study. PLoS One 3:e2691. doi: 10.1371/journal.pone.0002691
Fleischer, M., Schumann, L., Hartmann, A., Walker, R. S., Ifrim, L., von Zadow, D., et al. (2022). Pre-adolescent children exhibit lower aerosol particle volume emissions than adults for breathing, speaking, singing and shouting. J. R. Soc. Interface 19:20210833. doi: 10.1098/rsif.2021.0833
Flerlage, T., Boyd, D. F., Meliopoulos, V., Thomas, P. G., and Schultz-Cherry, S. (2021). Influenza virus and SARS-CoV-2: pathogenesis and host responses in the respiratory tract. Nat. Rev. Microbiol. 19, 425–441. doi: 10.1038/s41579-021-00542-7
Forbes, C., Greenwood, H., Carter, M., and Clark, J. (2024). Automation of duplicate record detection for systematic reviews: Deduplicator. Syst. Rev. 13:206. doi: 10.1186/s13643-024-02619-9
Good, N., Fedak, K. M., Goble, D., Keisling, A., L'Orange, C., Morton, E., et al. (2021). Respiratory aerosol emissions from vocalization: age and sex differences are explained by volume and exhaled CO2. Environ. Sci. Technol. Lett. 8, 1071–1076. doi: 10.1021/acs.estlett.1c00760
Gregson, F. K. A., Watson, N. A., Orton, C. M., Haddrell, A. E., McCarthy, L. P., Finnie, T. J. R., et al. (2021). Comparing aerosol concentrations and particle size distributions generated by singing, speaking and breathing. Aerosol Sci. Technol. 55, 681–691. doi: 10.1080/02786826.2021.1883544
Guo, Z., Zhao, S., Lee, S. S., Hung, C. T., Wong, N. S., Chow, T. Y., et al. (2023). A statistical framework for tracking the time-varying superspreading potential of COVID-19 epidemic. Epidemics 42:100670. doi: 10.1016/j.epidem.2023.100670
Gutmann, D., Donath, H., Herrlich, L., Lehmkuehler, T., Landeis, A., Ume, E. R., et al. (2022). Exhaled aerosols in SARS-CoV-2 polymerase chain reaction-positive children and age-matched-negative controls. Front. Pediatr. 10:941785. doi: 10.3389/fped.2022.941785
Gutmann, D., Scheuch, G., Lehmkühler, T., Herrlich, L. S., Hutter, M., Stephan, C., et al. (2022). Aerosol measurement identifies SARS-CoV 2 PCR positive adults compared with healthy controls. Environ. Res. 216:114417. doi: 10.1101/2022.01.21.22269423
Harrison, J., Saccente-Kennedy, B., Orton, C. M., McCarthy, L. P., Archer, J., Symons, H. E., et al. (2023). Emission rates, size distributions, and generation mechanism of oral respiratory droplets. Aerosol Sci. Technol. 57, 187–199. doi: 10.1080/02786826.2022.2158778
Hersen, G., Moularat, S., Robine, E., Géhin, E., Corbet, S., Vabret, A., et al. (2008). Impact of health on particle size of exhaled respiratory aerosols: case-control study. Clean (Weinh) 36, 572–577. doi: 10.1002/clen.200700189
Hu, N., Yuan, F., Gram, A., Yao, R., and Sadrizadeh, S. (2024). Review of experimental measurements on particle size distribution and airflow behaviors during human respiration. Build. Environ. 247:110994. doi: 10.1016/j.buildenv.2023.110994
Hussain-Alkhateeb, L., Bake, B., Holm, M., Emilsson, Ö., Mirgorodskaya, E., and Olin, A. C. (2021). Novel non-invasive particles in exhaled air method to explore the lining fluid of small airways-a European population-based cohort study. BMJ Open Respir. Res. 8:804. doi: 10.1136/bmjresp-2020-000804
Iannone, R., Cheng, J., Schloerke, B., Hughes, E., Lauer, A., and Seo, J. (2023). Gt: easily create presentation-ready display tables. Cham: Springer.
Jaumdally, S., Tomasicchio, M., Pooran, A., Esmail, A., Kotze, A., Meier, S., et al. (2024). Frequency, kinetics and determinants of viable SARS-CoV-2 in bioaerosols from ambulatory COVID-19 patients infected with the beta, delta or omicron variants. Nat. Commun. 15:2003. doi: 10.1038/s41467-024-45400-1
Johnson, G. R., and Morawska, L. (2009). The mechanism of breath aerosol formation. J. Aerosol Med. Pulm. Drug Deliv. 22, 229–237. doi: 10.1089/jamp.2008.0720
Kappelt, N., Russell, H. S., Kwiatkowski, S., Afshari, A., and Johnson, M. S. (2021). Correlation of respiratory aerosols and metabolic carbon dioxide. Sustainability 13:2203. doi: 10.3390/su132112203
Kutter, J. S., Spronken, M. I., Fraaij, P. L., Fouchier, R. A., and Herfst, S. (2018). Transmission routes of respiratory viruses among humans. Curr. Opin. Virol. 28, 142–151. doi: 10.1016/j.coviro.2018.01.001
Lai, J., Coleman, K. K., Tai, S. H. S., German, J., Hong, F., Albert, B., et al. (2023). Exhaled breath aerosol shedding of highly transmissible versus prior severe acute respiratory syndrome coronavirus 2 variants. Clin. Infect. Dis. 76, 786–794. doi: 10.1093/cid/ciac846
Lee, S.-Y., Shih, S.-C., Leu, Y.-S., Chang, W.-H., Lin, H.-C., and Ku, H.-C. (2017). Implications of age-related changes in anatomy for geriatric-focused difficult airways. Int. J. Gerontol. 11, 130–133. doi: 10.1016/j.ijge.2016.11.003
Lee, J., Yoo, D., Ryu, S., Ham, S., Lee, K., Yeo, M., et al. (2019). Quantity, size distribution, and characteristics of cough-generated aerosol produced by patients with an upper respiratory tract infection. Aerosol Air Qual. Res. 19, 840–853. doi: 10.4209/aaqr.2018.01.0031
Li, Y., and Tang, X. X. (2021). Abnormal airway mucus secretion induced by virus infection. Front. Immunol. 12:701443. doi: 10.3389/fimmu.2021.701443
Lim, A.-Y., Cheong, H.-K., Oh, Y. J., Lee, J. K., So, J. B., Kim, H. J., et al. (2021). Modeling the early temporal dynamics of viral load in respiratory tract specimens of COVID-19 patients in Incheon, the Republic of Korea. Int. J. Infect. Dis. 108, 428–434. doi: 10.1016/j.ijid.2021.05.062
Lindsley, W. G., Blachere, F. M., Beezhold, D. H., Thewlis, R. E., Noorbakhsh, B., Othumpangat, S., et al. (2016). Viable influenza A virus in airborne particles expelled during coughs versus exhalations. Influenza Other Respir. Viruses 10, 404–413. doi: 10.1111/irv.12390
Lindsley, W. G., Blachere, F. M., Thewlis, R. E., Vishnu, A., Davis, K. A., Cao, G., et al. (2010). Measurements of airborne influenza virus in aerosol particles from human coughs. PLoS One 5:e15100. doi: 10.1371/journal.pone.0015100
Lindsley, W. G., Pearce, T. A., Hudnall, J. B., Davis, K. A., Davis, S. M., Fisher, M. A., et al. (2012). Quantity and size distribution of cough-generated aerosol particles produced by influenza patients during and after illness. J. Occup. Environ. Hyg. 9, 443–449. doi: 10.1080/15459624.2012.684582
LoMauro, A. A.-O., and Aliverti, A. (2018). Sex differences in respiratory function. Breathe 14, 131–140. doi: 10.1183/20734735.000318
Marshall, H., Gibson, O. R., Romer, L. M., Illidi, C., Hull, J. H., and Kippelen, P. (2020). Systemic but not local rehydration restores dehydration-induced changes in pulmonary function in healthy adults. J. Appl. Physiol. 130, 517–527. doi: 10.1152/japplphysiol.00311.2020
Martins Rodrigues, I., Torres Pereira, E., de Castro Lopes, A. L., Massaroni, C., Baroni, G., Cerveri, P., et al. (2021). Is age rating enough to investigate changes in breathing motion pattern associated with aging of physically active women? J. Biomech. 125:110582. doi: 10.1016/j.jbiomech.2021.110582
Milton, D. K., Fabian, M. P., Cowling, B. J., Grantham, M. L., and McDevitt, J. J. (2013). Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog. 9:e1003205. doi: 10.1371/journal.ppat.1003205
Molgat-Seon, Y., Peters, C. M., and Sheel, A. W. (2018). Sex-differences in the human respiratory system and their impact on resting pulmonary function and the integrative response to exercise. Curr. Opin. Physio. 6, 21–27. doi: 10.1016/j.cophys.2018.03.007
Morawska, L., Johnson, G. R., Ristovski, Z. D., Hargreaves, M., Mengersen, K., Corbett, S., et al. (2009). Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J. Aerosol Sci. 40, 256–269. doi: 10.1016/j.jaerosci.2008.11.002
Moseley, B., Archer, J., Orton, C. M., Symons, H. E., Watson, N. A., Saccente-Kennedy, B., et al. (2024). Relationship between exhaled aerosol and carbon dioxide emission across respiratory activities. Environ. Sci. Technol. 58, 15120–15126. doi: 10.1021/acs.est.4c01717
Mürbe, D., Kriegel, M., Lange, J., Rotheudt, H., and Fleischer, M. (2021). Aerosol emission in professional singing of classical music. Sci. Rep. 11:14861. doi: 10.1038/s41598-021-93281-x
Mürbe, D., Kriegel, M., Lange, J., Schumann, L., Hartmann, A., and Fleischer, M. (2021). Aerosol emission of adolescents voices during speaking, singing and shouting. PLoS One 16:e0246819. doi: 10.1371/journal.pone.0246819
Mutsch, B., Heiber, M., Grätz, F., Hain, R., Schönfelder, M., Kaps, S., et al. (2022). Aerosol particle emission increases exponentially above moderate exercise intensity resulting in superemission during maximal exercise. Proc. Natl. Acad. Sci. USA 119:e2202521119. doi: 10.1073/pnas.2202521119
Nikolić, M. Z., Sun, D., and Rawlins, E. L. (2018). Human lung development: recent progress and new challenges. Development 145:163485. doi: 10.1242/dev.163485
Oh, W., Bu, Y., Kikumoto, H., and Ooka, R. (2024). Correlation between beverage consumption and droplet production during respiratory activity using interferometric Mie imaging experiment. J. Aerosol Sci. 182:106458. doi: 10.1016/j.jaerosci.2024.106458
Oldham, M. J., and Moss, O. R. (2019). Pores of Kohn: forgotten alveolar structures and potential source of aerosols in exhaled breath. J. Breath Res. 13:021003. doi: 10.1088/1752-7163/ab0524
Organization GWH (2024). Global technical consultation report on proposed terminology for pathogens that transmit through the air;. Licence: CC BY-NC-SA 3.0 IGO. Geneva: WHO.
Orton, C. M., Symons, H. E., Moseley, B., Archer, J., Watson, N. A., Philip, K. E. J., et al. (2022). A comparison of respiratory particle emission rates at rest and while speaking or exercising. Commun Med 2:44. doi: 10.1038/s43856-022-00103-w
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. doi: 10.1136/bmj.n71
Pan, S., Xu, C., Francis Yu, C. W., and Liu, L. (2023). Characterization and size distribution of initial droplet concentration discharged from human breathing and speaking. Indoor and Built Environment. 32, 2020–2033. doi: 10.1177/1420326X221110975
Pöhlker, M. L., Pöhlker, C., Krüger, O. O., Förster, J.-D., Berkemeier, T., Elbert, W., et al. (2023). Respiratory aerosols and droplets in the transmission of infectious diseases. Rev. Mod. Phys. 95:045001. doi: 10.1103/RevModPhys.95.045001
Puhach, O., Meyer, B., and Eckerle, I. (2023). SARS-CoV-2 viral load and shedding kinetics. Nat. Rev. Microbiol. 21, 147–161. doi: 10.1038/s41579-022-00822-w
Rathnayake, S. A.-O., Ditz, B., van Nijnatten, J. A.-O., Sadaf, T., Hansbro, P. A.-O., Brandsma, C. A., et al. (2023). Smoking induces shifts in cellular composition and transcriptome within the bronchial mucus barrier. Respirology 28, 132–142. doi: 10.1111/resp.14401
Rawat, M. S., Agirsoy, M., Senarathna, D., Erath, B. D., Ahmed, T., Mondal, S., et al. (2023). Comparing respiratory aerosol emissions between children and adults during sustained phonation. Aerosol Sci. Technol. 57, 1186–1204. doi: 10.1080/02786826.2023.2261715
Roman, M. A., Rossiter, H. B., and Casaburi, R. (2016). Exercise, ageing and the lung. Eur. Respir. J. 48, 1471–1486. doi: 10.1183/13993003.00347-2016
Roth, A., Stiti, M., Frantz, D., Corber, A., and Berrocal, E. (2023). Exhaled aerosols and saliva dropletsmeasured in time and 3D space: quantification of pathogens flow rate applied to SARS-CoV-2. Nat. Sci. 3:7. doi: 10.1002/ntls.20230007
Sajgalik, P., Garzona-Navas, A., Csécs, I., Askew, J. W., Lopez-Jimenez, F., Niven, A. S., et al. (2021). Characterization of aerosol generation during various intensities of exercise. Chest 160, 1377–1387. doi: 10.1016/j.chest.2021.04.041
Schneider, J. L., Rowe, J. H., Garcia-de-Alba, C., Kim, C. F., Sharpe, A. H., and Haigis, M. C. (2021). The aging lung: physiology, disease, and immunity. Cell 184, 1990–2019. doi: 10.1016/j.cell.2021.03.005
Schuchmann, P., Scheuch, G., Naumann, R., Keute, M., Lücke, T., Zielen, S., et al. (2023). Exhaled aerosols among PCR-confirmed SARS-CoV-2-infected children. Front. Pediatr. 11:1156366. doi: 10.3389/fped.2023.1156366
Schumm, B., Bremer, S., Knödlseder, K., Schönfelder, M., Hain, R., Semmler, L., et al. (2023). Lung aerosol particle emission increases with age at rest and during exercise. Proc. Natl. Acad. Sci. USA 120:e2301145120. doi: 10.1073/pnas.2301145120
Schumm, B., Bremer, S., Knoedlseder, K., Schoenfelder, M., Hain, R., Semmler, L., et al. (2024). Indices of airway resistance and reactance from impulse oscillometry correlate with aerosol particle emission in different age groups. Sci. Rep. 14:4644. doi: 10.1038/s41598-024-55117-2
Schwarz, K., Biller, H., Windt, H., Koch, W., and Hohlfeld, J. M. (2010). Characterization of exhaled particles from the healthy human lung--a systematic analysis in relation to pulmonary function variables. J. Aerosol Med. Pulm. Drug Deliv. 23, 371–379. doi: 10.1089/jamp.2009.0809
Schwarz, K., Biller, H., Windt, H., Koch, W., and Hohlfeld, J. M. (2015). Characterization of exhaled particles from the human lungs in airway obstruction. J. Aerosol Med. Pulm. Drug Deliv. 28, 52–58. doi: 10.1089/jamp.2013.1104
Sirota, S. B., Doxey, M. C., Dominguez, R.-M. V., Bender, R. G., Vongpradith, A., Albertson, S. B., et al. (2025). Global, regional, and national burden of upper respiratory infections and otitis media, 1990–2021: a systematic analysis from the global burden of disease study 2021. Lancet Infect. Dis. 25, 36–51. doi: 10.1016/S1473-3099(24)00430-4
Sun, Y., Zhang, Y., Liu, X., Liu, Y., Wu, F., and Liu, X. (2024). Association between body mass index and respiratory symptoms in US adults: a national cross-sectional study. Sci. Rep. 14:940. doi: 10.1038/s41598-024-51637-z
Team RC (2023). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Thomas, E. T., Guppy, M., Straus, S. E., Bell, K. J. L., and Glasziou, P. (2019). Rate of normal lung function decline in ageing adults: a systematic review of prospective cohort studies. BMJ Open 9:e028150. doi: 10.1136/bmjopen-2018-028150
Tinglev, Å., Ullah, S., Ljungkvist, G., Viklund, E., Olin, A. C., and Beck, O. (2016). Characterization of exhaled breath particles collected by an electret filter technique. J. Breath Res. 10:026001. doi: 10.1088/1752-7155/10/2/026001
Varga, C. M., Kwiatkowski, K. J., Pedro, M. J., Groepenhoff, H., Rose, E. A., Gray, C., et al. (2022). Observation of aerosol generation by human subjects during cardiopulmonary exercise testing using a high-powered laser technique: A pilot project. J. Med. Biol. Eng. 42, 1–10. doi: 10.1007/s40846-021-00675-3
Verreault, D., Moineau, S., and Duchaine, C. (2008). Methods for sampling of airborne viruses. Microbiol. Mol. Biol. Rev. 72, 413–444. doi: 10.1128/mmbr.00002-08
Viklund, E., Kokelj, S., Larsson, P., Nordén, R., Andersson, M., Beck, O., et al. (2022). Severe acute respiratory syndrome coronavirus 2 can be detected in exhaled aerosol sampled during a few minutes of breathing or coughing. Influenza Other Respir. Viruses 16, 402–410. doi: 10.1111/irv.12964
von Elm, E., Altman, D. G., Egger, M., Pocock, S. J., Gøtzsche, P. C., and Vandenbroucke, J. P. (2007). The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370, 1453–1457. doi: 10.1016/S0140-6736(07)61602-X
Wang, C. C., Prather, K. A., Sznitman, J., Jimenez, J. L., Lakdawala, S. S., Tufekci, Z., et al. (2021). Airborne transmission of respiratory viruses. Science 373:eabd9149. doi: 10.1126/science.abd9149
Wegehaupt, O., Endo, A., and Vassall, A. (2023). Superspreading, overdispersion and their implications in the SARS-CoV-2 (COVID-19) pandemic: a systematic review and meta-analysis of the literature. BMC Public Health 23:1003. doi: 10.1186/s12889-023-15915-1
Weston, S., and Frieman, M. B. (2019). “Respiratory viruses” in Encyclopedia of microbiology. ed. T. M. Schmidt. Fourth ed (New York, NY: Academic Press), 85–101.
Wickham, H., Averick, M., Bryan, J., Chang, W., McGowan, L., François, R., et al. (2019). Welcome to the tidyverse. J. Open Source Softw. 4:1686. doi: 10.21105/joss.01686
Xie, X., Li, Y., Sun, H., and Liu, L. (2009). Exhaled droplets due to talking and coughing. J. R. Soc. Interface 6, S703–S714. doi: 10.1098/rsif.2009.0388.focus
Yan, J., Grantham, M., Pantelic, J., Bueno de Mesquita, P. J., Albert, B., Liu, F., et al. (2018). Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc. Natl. Acad. Sci. USA 115, 1081–1086. doi: 10.1073/pnas.1716561115
Zanin, M., Baviskar, P., Webster, R., and Webby, R. (2016). The interaction between respiratory pathogens and mucus. Cell Host Microbe 19, 159–168. doi: 10.1016/j.chom.2016.01.001
Keywords: airborne transmission, infectious, respiratory particles, respiratory pathogen transmission, SARS-CoV-2, influenza, disease transmission, human
Citation: Horstink N, Lassing K, Knoester M, Vermeulen LC, Rossen JWA, Voss A and Lokate M (2025) Host factors associated with respiratory particle emission and virus presence within respiratory particles: a systematic review. Front. Microbiol. 16:1652124. doi: 10.3389/fmicb.2025.1652124
Edited by:
Luminita Andronic, Transilvania University of Brașov, RomaniaReviewed by:
Israel Parra-Ortega, Federico Gómez Children's Hospital, MexicoHimadri Nath, Lovelace Respiratory Research Institute, United States
Copyright © 2025 Horstink, Lassing, Knoester, Vermeulen, Rossen, Voss and Lokate. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mariëtte Lokate, bS5sb2thdGVAdW1jZy5ubA==
 Nils Horstink
Nils Horstink Kirsten Lassing
Kirsten Lassing Marjolein Knoester
Marjolein Knoester Lucie C. Vermeulen3
Lucie C. Vermeulen3 John W. A. Rossen
John W. A. Rossen Mariëtte Lokate
Mariëtte Lokate