- 1Interdisciplinary Doctoral School, Lodz University of Technology, Łódź, Poland
- 2Department of Environmental Biotechnology, Lodz University of Technology, Łódź, Poland
- 3Department of Biological Sciences and Technology, Beijing Forestry University, Beijing, China
The unconventional yeasts Metschnikowia spp. represent a valuable microorganisms with enormous yet untapped potential. Metschnikowia species are briefly reviewed, demonstrating that taxonomic and genomic analysis can open numerous opportunities to exploit their unique character and potential in the development of modern winemaking and brewing, probiotics and biocontrol, and the synthesis of single-cell proteins. These yeasts can be used in both bioprocesses and biorefineries, contributing to the production of biofuels and unique products recovered from agro-industrial wastes. This review, through a comprehensive bibliographic analysis, examines various green strategies for the production of alcohols, lipids, unsaturated fatty acids, and other valuable metabolites. Furthermore, the article discusses the challenges and barriers hindering the full implementation of Metschnikowia spp. in new approaches and technologies.
1 Introduction
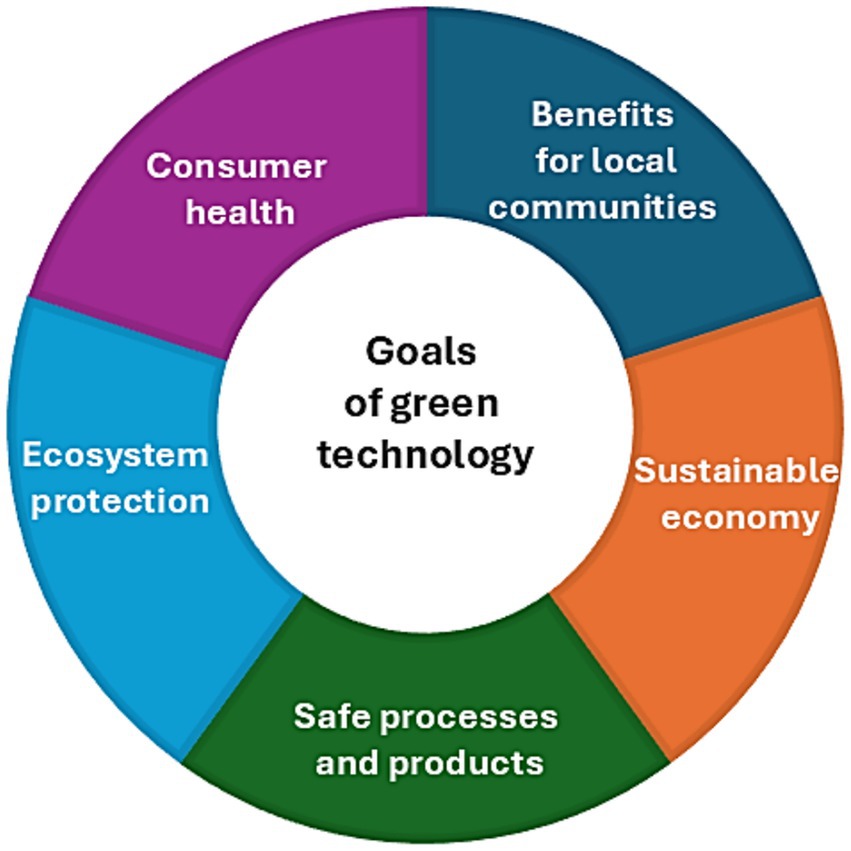
Green technology is commonly defined as the development and use of processes that minimize the negative impact of human activity on the environment and society (Figure 1).
It encompasses a diverse range of technologies and practices that address environmental issues, paving the way for sustainable development. It creates solutions and strategies to mitigate the effects of climate change, reduce environmental degradation, and promote the efficient use of natural resources. Green technology leverages both scientific knowledge and innovation to conserve natural resources, mitigate greenhouse gas emissions, and promote a circular economy. A key aspect of green technology is also its positive contribution to human health, both through the development of processes that do not negatively impact the health of workers involved in production and through the nature of manufactured consumer goods that support consumer health (Al-Emran and Griffy-Brown, 2023).
Among the main principles of green technology, including renewable energy and energy efficiency, transportation, water and wastewater treatment, and carbon capture technologies, agro-industrial waste management also plays a crucial role. Waste management technologies include advanced recycling plants that convert waste into valuable resources. Sustainable agricultural practices, on the other hand, aim to reduce the environmental impact of food production and maintain food security (Domínguez et al., 2024; Mishra et al., 2023).
The idea of a “circular economy,” which refers to the use of organic waste from one industry as a raw material for another, is based on the sustainability principle known as the “5Rs” (reduction, recycling, reuse, recovery, and regeneration) and replaces the traditional linear model (production, use, disposal). In recent decades, the growth of the food and agro-industrial sectors has dramatically increased food waste production. The amount of waste generated by agro-based industries has more than tripled (Bibi et al., 2023).
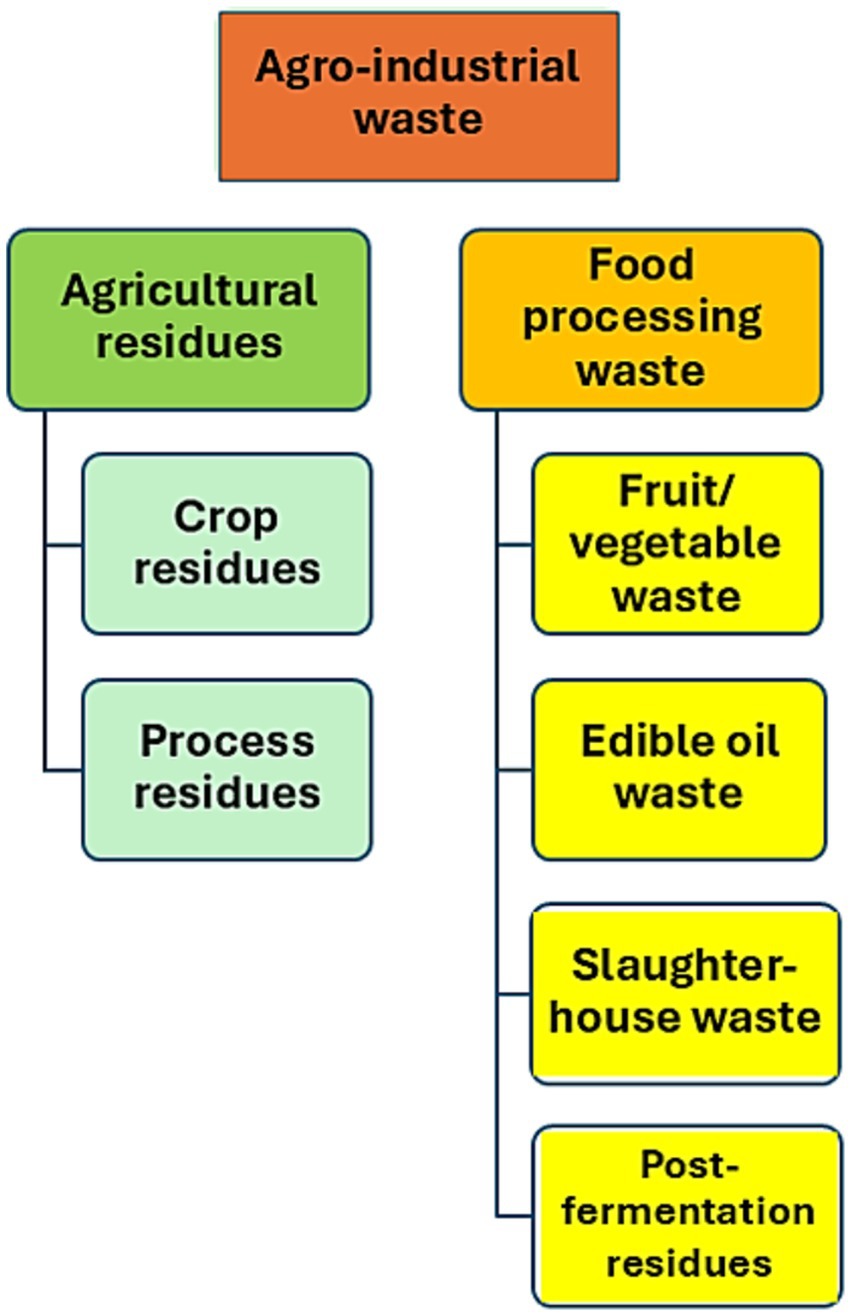
The Food and Agriculture Organization of the United Nations (FAO) estimates that approximately 1.3 billion tons of food are wasted each year, representing one-third of global production (Food and Agriculture Organization of the United Nations, 2022). Wastes pollute the environment. However, they serve as beneficial biomass resources. In addition to food waste, various types of agro-industrial residues are generated annually worldwide (Figure 2). Such organic wastes, a rich source of carbohydrates, proteins, lipids, organic acids, and other essential compounds, can be used in bioconversion processes (Sharma et al., 2022). They can serve as an inexpensive substrate for the manufacturing of various goods, including biogas, biofuel, single-cell biomass, as well as probiotics, biocontrol agents, fertilizers, enzymes, vitamin supplements, or antioxidants (Bibi et al., 2023).
The production of high-quality products represents one possibility for exploiting rich and valuable sources of organic molecules derived from agricultural waste and beneficial microorganisms (Phiri et al., 2024).
2 Unveiling Metschnikowia spp.: biology, physiology, and taxonomic studies
Several types of yeasts, which are eukaryotic microorganisms, have been widely used in various industries due to their potential applications, both for fermentation and the production of specific metabolites. Metschnikowia spp. are non-conventional yeasts with great biotechnological potential, and knowledge about them is still relatively limited, in comparison to conventional Saccharomyces cerevisiae.
The Metschnikowia genus was first identified approximately 130 years ago, and currently, the number of described Metschnikowia species exceeds 80 (Vicente et al., 2020). Species of the genus Metschnikowia form a monophyletic group within the family Metschnikowiaceae, which also includes the several other genera, such as Australozyma, Candidiozyma, Clavispora, Danielia, Gabaldonia, Gaillardinia, Helenozyma, Hermanozyma, Isabelozyma, Osmozyma, Soucietia, Sungouiella, Tanozyma, or Wilhelminamyces (Liu et al., 2024; Index Fungorum, 2025; NCBI Taxonomy Browser, n.d.).
The principal habitats where Metschnikowia species are encountered regularly include various parts of plants, namely flowers, fruits, barks, and leaves, or also the digestive tract or frass of some insects, as well as aquatic organisms (Kurtzman et al., 2018; Agarbati et al., 2024a). It is worth noting that some species of the Metschnikowia genus are globally distributed, while others exhibit extreme endemism. The nature of the association between Metschnikowia species and insects remains unclear; however, the relationship seems to be species-specific (Lachance, 2016).
The ecological diversity of Metschnikowia yeasts is not the only characteristic of these unique yeasts. Their uniqueness also extends to their morphology and physiology. Metschnikowia sp. reproduces by multilateral budding. The cells are spherical or ellipsoidal and may be pear-shaped, cylindrical, or lunate in shape. Pseudohyphae are weakly developed but often absent. In sexual reproduction, the ascospores are needle-shaped, tapered at one or both ends, sometimes swollen along one half, and the asci are elongated, club-shaped, spheroid, or ellipsoid-stalked. Depending on the species, one or two spores are produced per ascus. In some cases, spores can reach enormous sizes, exceeding 200 μm in length. In some strains, ascospore formation is preceded by the development of chlamydospores. Metschnikowia species also differ in the efficiency of forming these thick-walled cells. The adaptive properties of these features remain to be elucidated (Lachance, 2011; Lachance, 2016). Some species of this genus can produce pulcherrimin, a red pigment constituting a chelate of pulcherrimic acid and iron ions (Sipiczki, 2020). M. pulcherrima species can express different extracellular hydrolytic enzymes, namely amylase, cellulase, glucanase, β-glucosidase, β-lyase, lipase, lichenase, pectinase, protease, sulfite reductase, and xylanase, which makes them very interesting microorganisms in bioconversion processes (Mateo, 2023; Chaudhary and Karita, 2017).
The diversity of Metschnikowia strains also extends to their molecular characteristics. The yeast identification is usually based on the assumption that differences in barcodes are more minor within a single species than between species. The most commonly used barcodes are chromosomal repeat segments encoding ribosomal RNA. Molecular analysis of such segments in strains of several species belonging to Metschnikowia, conducted by Sipiczki et al. (2024), showed that this is not possible for the species of this genus. In these studies, intragenomic diversity significantly exceeded the threshold gaps used to differentiate related yeast species. The genome structures of various Metschnikowia spp. isolates were compared using RAPD and RFLP of mitochondrial DNA, demonstrating their high heterogeneity. Also, the sequence analysis of the PUL4 gene (a component of the PUL cluster) involved in pulcherrimin production revealed substantial intragenomic differences, suggesting that the genomes may be chimerized. These features make Metschnikowia spp. unique among yeasts and indicate that these traits and features evolve in a non-standard manner. When the molecular differences were compared with the phenotypic differences, no clear correlation was observed between the examined genetic/genomic diversities and the phenotypic diversities. Thus, according to Sipiczki and co-workers, none of the molecular tests can be used for differentiating strains that exhibit different phenotypes (Sipiczki, 2022; Sipiczki et al., 2024).
Recent phylogenetic, genetic, and genomic studies at the molecular level have raised questions about the taxonomic classification of species within the Metschnikowia genus. These data, combined with the results obtained in many studies through comprehensive analysis of primary and secondary barcode sequences, physiological features, and hybridization experiments, prove that the species within Metschnikowia cannot be distinguished from each other based on any of the phenotypic, phylogenetic, or biological concepts. These taxonomic properties were further supported by Troiano et al. (2023), who conducted a comparative genomic analysis of seven strains belonging to the M. pulcherrima clade and revealed the absence of single-copy markers for species differentiation. Sipiczki proposed combining species belonging to the M. pulcherrima clade, characterized by the formation of pulcherrimin (M. andauensis, M. fructicola, M. leonuri, M. pulcherrima, M. rubicola, M shanxiensis, M. sinensis, and M. zizyphicola), into a single species under the oldest species name, M. pulcherrima (Sipiczki, 2022; Sipiczki and Czentye, 2024).
3 Safety aspects
Molecular biology and advanced genetic techniques have become essential tools in various fields of interest, including taxonomy, identification, classification, and metabolite production, as well as in potential applications. However, the safety of yeast used in biotechnological processes is also crucial to ensure benefits for humans and the environment (Tullio, 2022).
Knowledge about microorganisms involved in biotechnological processes has increased over the past decades, and the rapid development of molecular biology techniques has allowed for a deeper understanding of the genetic basis and specific metabolic pathways of microorganisms involved in these processes. However, the genetic refinement of microbial strains involved in processing remains controversial. Genetically modified microorganisms (GMOs) still encounter disapproval and are subject to extensive regulatory requirements. The use of GMOs as cell factories in closed systems that prevent their release into the environment is the least problematic aspect. Still, in the presented idea of green technologies, it seems to be wholly excluded (Plavec and Berlec, 2020).
Genome sequences are keystone data to explore the applicability of Metschnikowia spp. bioresources. They are also crucial for a comprehensive safety assessment, which is the principal regulatory concern, as requested by the European Food Safety Authority (EFSA) (EFSA, 2018; Binati et al., 2021; Miguel et al., 2022).
The safety analysis of yeasts is still under consideration because protocols are less developed than for bacterial assessments, and standardized methods have not yet been developed. According to EFSA, genome sequences should be searched to identify the presence/absence of metabolic pathways involved in toxigenicity or antifungal drug resistance. If detected, appropriate analysis is required to validate in silico evidence (EFSA, 2018). Genome studies are necessary because drug-resistant fungal infections pose a growing global health threat. However, no databases have been developed specifically for detecting antifungal drug resistance genes. In the studies of Larini et al. (2025), none of the Metschnikowia strains were capable of producing biogenic amines. In a recent study conducted by Rahmat et al. (2024), the toxicity assessment of extracts of M. pulcherrima (formerly M. persimmonsis) strains showed that they had no harmful effects on the liver and mitochondria of zebrafish, and no potential risk of cardiotoxicity was observed. Furthermore, other strains of M. pulcherrima (formerly M. ziziphicola), selected as potential probiotics, did not show any hemolytic activity (Staniszewski and Kordowska-Wiater, 2023).
However, the safety data are not consistent with previous publications that described a single strain of M. pulcherrima causing disease in immunocompromised patients (Mohl et al., 1998). A case report later described a non-pigment-producing strain of Metschnikowia isolated from the skin of a patient with dermatitis (Kuan et al., 2016). Another case involved an atypical strain of Metschnikowia isolated from a patient with leukemia (Savini et al., 2013).
In this context, the issue of the significant genetic diversity of Metschnikowia strains, which may reflect certain features typical of pathogenic microorganisms, is still the subject of intensive research. For example, the whole genome sequence of the pathogenic M. bicuspidata LNES0119 strain, responsible for disease effects in the crab Eriocheir sinensis, was sequenced by Jiang and co-authors (Jiang et al., 2022). Within the 16.13 Mb genome of M. bicuspidata LNES0119, encoding 5,567 genes, 1,467 genes were identified with significant homology to genes from the pathogen-host interaction database. Comparative genomic analyses of three M. bicuspidata strains and one nonpathogenic M. pulcherrima strain revealed 331 unique genes in M. bicuspidata LNES0119, 30 of which were putatively associated with its pathogenicity. Genomic and comparative analyses showed that the genome of M. bicuspidata LNES0119 contains a variety of putative pathogenic genes, primarily involved in cell wall assembly and construction. These genes may play a crucial role in adapting to the host environment, acting as virulence factors in pathogenicity, or triggering a cell-mediated host immune response.
In addition, special attention was paid to the genome analysis of Candida lusitaniae, a pathogenic yeast responsible for candidemia in humans and most phylogenetically related to Metschnikowia in the so-called “GTC clade.” Phenotypic echinocandin resistance in C. lusitaniae results from a missense mutation (S645F) in the FKS1 gene, which was not found in Metschnikowia strains. Specifically, sequenced M. pulcherrima strains exhibited more than one copy of the FKS1 protein-encoding gene, which contained either serine at position 645 (similar to the nonpathogenic wild-type C. lusitaniae) or proline (Larini et al., 2025).
Formally, M. pulcherrima was included on the list of microorganisms approved for food use developed in a joint project by the International Dairy Federation (IDF) and the European Food and Feed Cultures Association (EFFCA) in 2002. Later additions suggested by the IDF National Committees and EFFCA members, as well as additions found through a scientific literature search, were also included on the list (Bourdichon et al., 2012).
In the United States, two Metschnikowia species, M. pulcherrima and M. fructicola, have been Generally Recognized As Safe (GRAS) by the Food and Drug Administration (FDA) (FDA, 2022). Under Sections 201 and 409 of the Federal Food, Drug, and Cosmetic Act, any substance that is intentionally added to food is subject to premarket review and approval by FDA, unless the substance has been generally recognized to be safe by qualified experts under the conditions of its intended use, or unless the use of the substance has been otherwise excepted from the definition of a food additive. Therefore, GRAS is not simply a special regulatory marker. It represents a comprehensive approach to ensuring that food and dietary products are safe for their intended purposes.
In the European Union, EFSA has published a peer review risk assessment of the use of the active substance M. fructicola NRRL Y-27328 as a pesticide (EFSA, 2017). A completely natural origin characterizes this strain - it has been isolated from grapes grown in central Israel. The positive opinion was reached based on the evaluation of the representative uses of M. fructicola NRRL Y-27328 as a fungicide on stone fruits, strawberries, and grapes. Regulation (EU) 2018/1915 of 6 December 2018 approved the active substance M. fructicola strain NRRL Y-27328 under Regulation (EC) No 1107/2009 of the European Parliament and of the Council, concerning the placing of plant protection products on the market (European Union, 2018).
The results of molecular studies may provide a novel resource of knowledge for further analysis of the pathogenic mechanisms in Metschnikowia, as well as for the identification of potential targets for further research and therapeutic intervention.
According to the last EFSA statement, microorganisms used in the food chain, either as active agents, biomasses, or as production organisms of substances of interest, should be subject to a premarket authorization process. This procedure includes a complete molecular characterization of the organism under assessment. Data analysis can provide information on the unambiguous taxonomic identification of the strains, on the presence of genes of concern (e.g., those encoding virulence factors, resistance to antimicrobials of clinical relevance for humans and animals, production of harmful metabolites, or of clinically relevant antimicrobials) and on the characterization of genetic modifications (EFSA, 2024).
Therefore, the application potential of the strain must be verified through extensive studies to exclude the presence of any potential pathogenic features.
4 Application potential
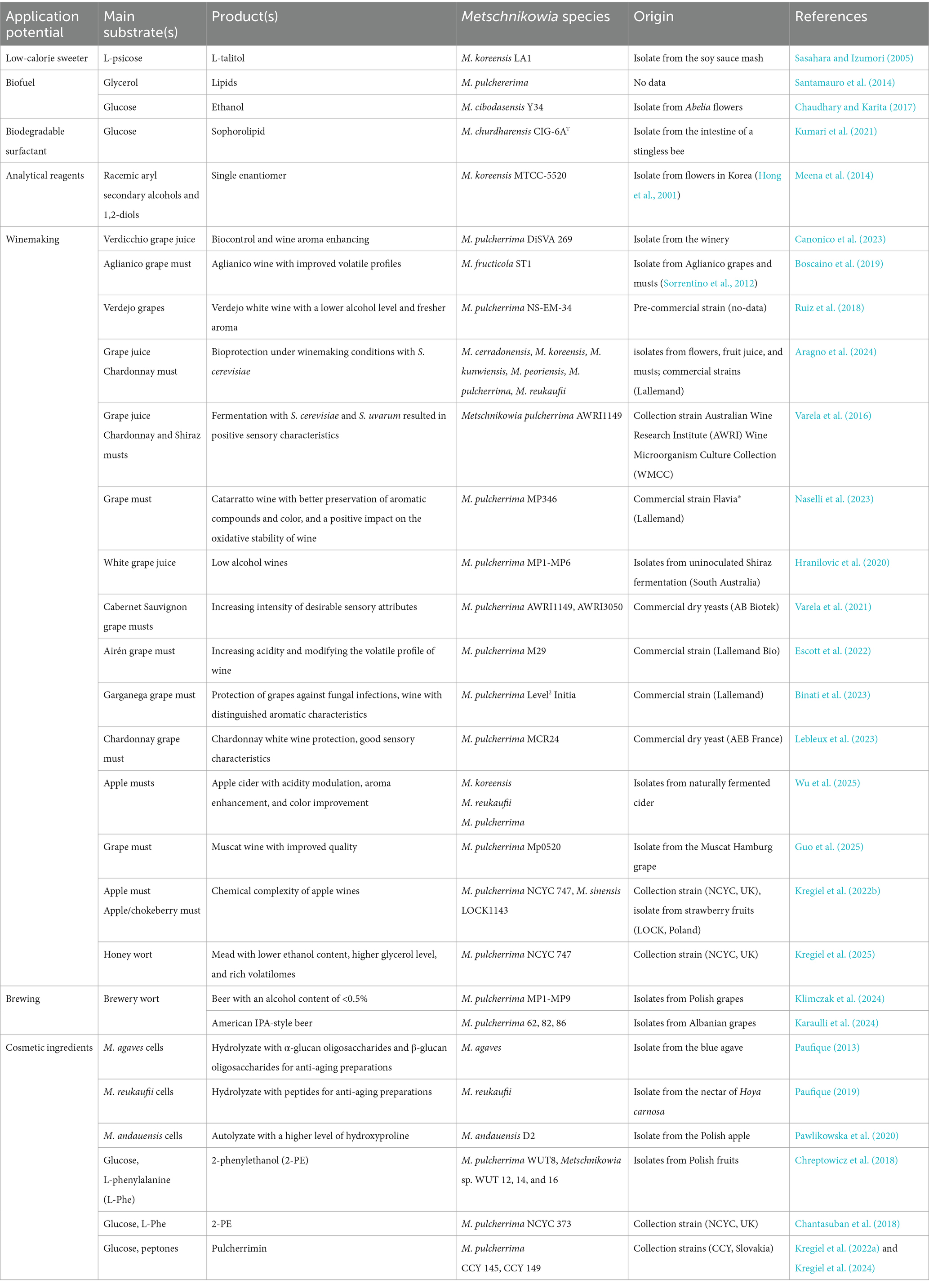
The unique features of yeasts of the genus Metschnikowia provide potential opportunities for their use in many biotechnological processes as well as in enology, agriculture, and the food and cosmetics industries (Haniffadli et al., 2024). The potential use of these yeasts in biocatalysis is particularly interesting. Research results are available on the production, from precisely defined substrates, various metabolites such as ethanol (Chaudhary and Karita, 2017) and lipids (Santamauro et al., 2014). What is more, Metschnikowia spp. demonstrate the potential to produce biodegradable surfactants (Kumari et al., 2021), low-calorie sweeteners (Sasahara and Izumori, 2005), as well as analytical reagents (Meena et al., 2014). The list of selected metabolites of the yeast Metschnikowia spp. from precisely defined substrates is presented in Table 1. It is complemented by the possible uses of these yeasts in the cosmetics industry, winemaking, and brewing.
4.1 Beverages
Metschnikowia is one of the most prevalent genera in grapevine phyllospheres, fruit flies, and grapes. This allows the use of some Metschnikowia species in winemaking (Table 1). Their ability to grow in association with other yeast species, such as S. cerevisiae or Lachancea thermotolerans, especially during the initial stages of wine fermentation, is also essential, modulating the synthesis of secondary metabolites. Metschnikowia yeasts have the potential to shape wine aromas and colors, and they can also be considered a tool for reducing ethanol content in wines (Hranilovic et al., 2020; Gonzalez et al., 2021). Reduction of both alcohol and acetic acid levels is achieved by controlling partial glucose respiration using a strain of M. pulcherrima (Guindal et al., 2023).
Metschnikowia spp. exhibit a moderate fermentative profile, but they show wide enzymatic activity, leading to the creation of flavor and color precursors. The combination of Metschnikowia and S. cerevisiae strains reduces alcohol production in wines, while imparting a fruitier and fresher aroma (Ruiz et al., 2018; Boscaino et al., 2019). Fermentations with L. thermotolerans and M. pulcherrima can increase acidity and modify the volatile profile of wines, imparting a fresher character. Escott et al. (2022) documented that the participation of these strains positively affected not only the volatile composition of wines but also color expression and consumer perception.
The key element here is the selection of yeast strains that may have a significant impact on wine quality (Torres-Díaz et al., 2025). M. pulcherrima strains are recommended in grape wine making for their contribution to the aromatic development of wine through their broad enzymatic activity (β-D-glucosidase, cysteine β-lyase) (Vicente et al., 2020) and the production of a wide range of esters and higher alcohols. This phenomenon was also observed in the case of fermentation of apple and apple-chokeberry musts (Kregiel et al., 2022b). The presence of Metschnikowia strains does not affect fermentation time, but reduces the fermentation rate of S. cerevisiae. Analysis of central carbon metabolism and volatile organic compounds reveals strain-dependent increases in metabolite production, including glycerol, acetate esters, medium-chain fatty acids, and ethyl esters (Aragno et al., 2024).
The results of numerous studies also suggest the potential of Metschnikowia species for bioprotection in wine production and quality (Kregiel et al., 2022b; Binati et al., 2023; Lebleux et al., 2023; Aragno et al., 2024). Metschnikowia spp. can be used as a stabilizer instead of SO₂ to obtain wines with low alcohol content and balanced color. It is possible to get wine stabilization with low SO₂ content and increase the content of aromatic substances such as ethyl butyrate and ethyl hexanoate (Canonico et al., 2023). The mixed cultures of Metschnikowia spp. and S. cerevisiae, and also Metschnikowia spp. and L. thermotolerans, act synergistically in the wine acidification process and may be used to improve sensory properties of grape and fruit beverages (Varela et al., 2021; Escott et al., 2022; Naselli et al., 2023; Kregiel et al., 2022b; Guo et al., 2025; Wu et al., 2025).
Recently, M. pulcherrima has also been used to ferment honey. The resulting beverages were characterized by lower ethanol content, higher glycerol level, and rich volatilomes. It is also worth noting that M. pulcherrima showed the highest tolerance to 30% w/v glucose (Kregiel et al., 2025).
Studies have shown that most Metschnikowia strains may be used in the brewery industry. These yeasts produce beer with an alcohol content of <0.5%. Higher β-glucosidase activity of Metschnikowia has a positive effect on beers (Klimczak et al., 2024).
Also other enzymes produced by Metschnikowia spp. may be particularly attractive in the beverage industry. De Souza et al. (2023) investigated the ability of M. australis to produce extracellular proteases at low temperatures. Yuivar et al. (2019) confirmed the yeast’s ability to produce extracellular gelatinase. Recent studies have demonstrated that M. koreensis can produce pectinases and proteases, which have numerous potential applications in the beverage industry (Theron et al., 2017; Snyman et al., 2019). These enzymes can regulate acidity, enhance aroma and clarity, and improve the color of fermented products (Wu et al., 2025). Pectinase has a significant impact on wine quality and clarity (Longhi et al., 2022). Furthermore, protease produced by M. pulcherrima can be used in American India Pale Ale (IPA) beer (Karaulli et al., 2024).
4.2 Cosmetics
The biological activity of Metschnikowia spp. also supports the use of their metabolites as active ingredients in cosmetic products (Table 1). Products derived from Metschnikowia species could reveal additional skin barrier-related attributes, because their secondary metabolites play a beneficial role in skin health (Mim et al., 2024).
The cosmetics industry mainly utilizes two species for skin care: M. agaves and M. reukaufii. M. reukaufii extracts contain various bioactive components, including peptides that have a beneficial effect on the skin microbiota (Paufique, 2019). In turn, M. agaves isolated from the blue agave of Mexico, and its hydrolase complex with α-glucan oligosaccharides and β-glucan oligosaccharides, naturally increases the production of hyaluronic acid, resulting in anti-aging, hydrating, and anti-wrinkle characteristics (Paufique, 2013).
Interestingly, Pawlikowska et al. (2020) studied the amino acid profiles of Metschnikowia spp. autolyzates. They produced biopreparations with a five-fold higher content of hydroxyproline, the main component of collagen. Therefore, such lysates could be used in the cosmetic industry as regenerating, revitalizing, smoothing, and moisturizing agents.
Metschnikowia spp. can produce 2-phenylethanol (2-PE). This compound is an aroma molecule primarily used in perfumes. Currently, the main method for producing this biobased compound is the extraction of trace amounts from rose petals, which is extremely expensive. However, this metabolite can be produced by Metschnikowia spp. and other yeasts using bioconversion processes. Saccharomyces, Kluyveromyces, Pichia, or Metschnikowia species can synthesize 2-PE by the biotransformation of L-phenylalanine (L-Phe) through the Ehrlich pathway. The productivity of 2-PE is associated with the type of yeast, carbon source, and media components (Mierzejewska et al., 2017). Chantasuban et al. (2018) developed a method for producing 2-PE using the M. pulcherrima strain in both batch and continuous modes. Other studies documented that 2-PE production varies depending on the kind of strain, medium composition, and fermentation conditions (Chreptowicz et al., 2018; Mitri et al., 2022).
Kregiel and co-workers found that M. pulcherrima strains can inhibit the growth of Candida- and Candida-related yeasts. This could be very useful for limiting the development of skin pathogens (Kregiel et al., 2023).
The interesting compound with great application potential in cosmetics is pulcherrimin—a red extracellular pigment formed by Metschnikowia spp. after growth in media enriched in iron (III). Freimoser and co-workers documented that pulcherrimin—an iron chelate of pulcherriminic acid—plays an essential environmental role in antagonistic microbial interactions, as well as in stress responses (Freimoser et al., 2019, 2024). Kregiel and co-workers investigated the biological activity of pulcherrimin produced by the M. pulcherrima clade. It was noted that this compound does not have antimicrobial properties. Still, its unique hydrophilic nature and Sun Protection Factor (SPF) may lead to interest in yeast pulcherrimin as an ingredient in moisturizing cosmetics with sun protection properties (Kregiel et al., 2022a; Kregiel et al., 2024).
4.3 Probiotics
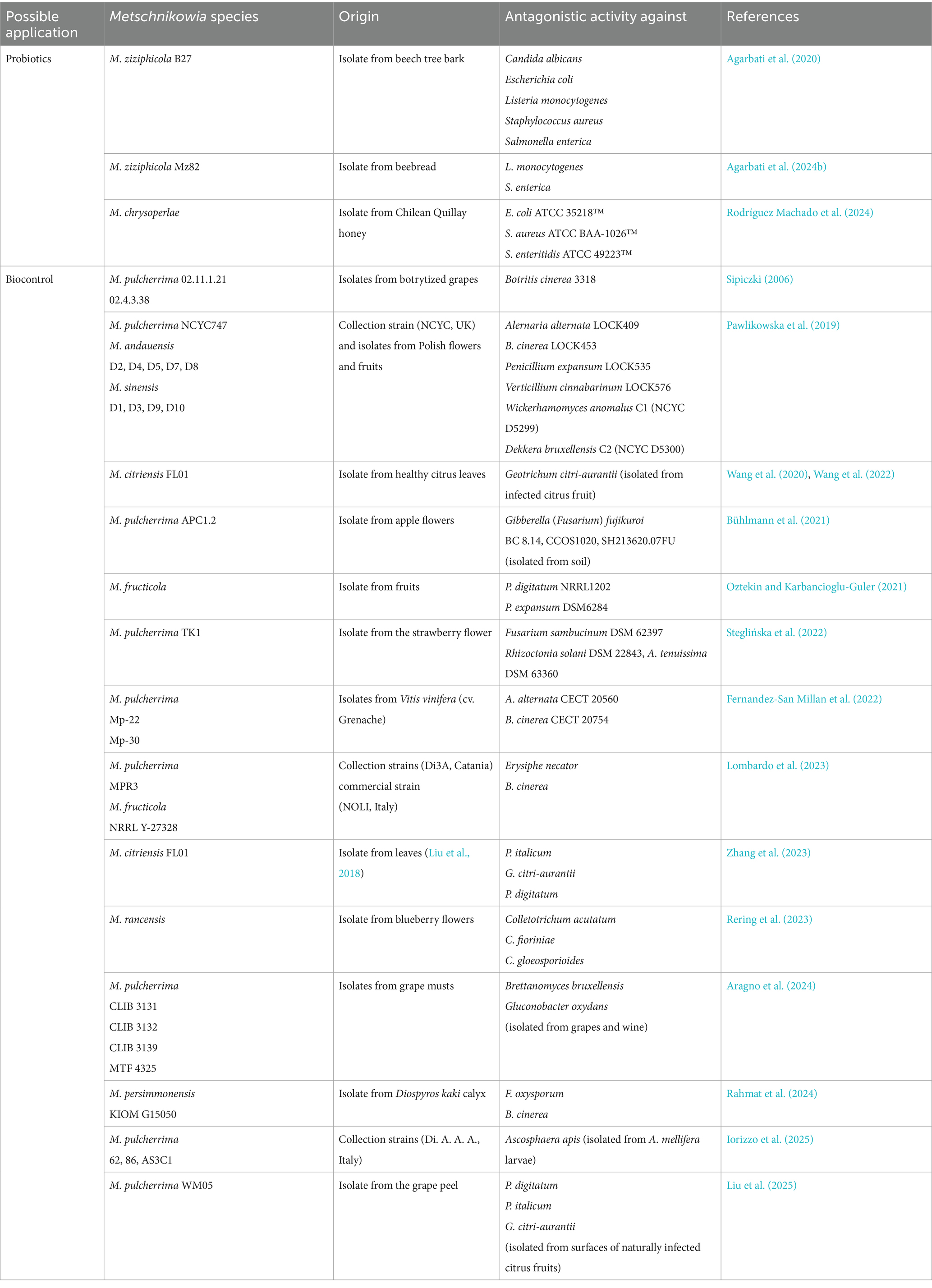
Metschnikowia species may be also considered probiotic microorganisms (Table 2). Probiotics are live microorganisms that, when consumed in sufficient amounts, provide health benefits to the host (Chen et al., 2025). They have traditionally been used in dairy product technologies. Probiotic microorganisms improve people’s health and wellness, and research on this topic is relevant and interesting. Unlike probiotic bacteria, probiotic yeasts are relatively understudied. Saccharomyces boulardii is a patented probiotic yeast with functionality demonstrated in many studies (O’Brien et al., 2024; Kaźmierczak-Siedlecka et al., 2020). Although S. boulardii is the most well-characterized yeast available on the market, improving probiotic function using other yeast species is an attractive future direction for research. Some yeast strains of M. ziziphicola show interesting probiotic characteristics (Agarbati et al., 2020, 2024a,b; Staniszewski and Kordowska-Wiater, 2023). However, it was documented that the probiotic abilities are strictly strain-dependent. Results obtained by Smith et al. (2015) demonstrated that M. gruessii can protect human epithelial cells from invasion by Salmonella enterica subsp. enterica serovar Typhimurium. In addition, a recent study conducted by Rodríguez Machado et al. (2024) showed that M. chrysoperlae strains may also be considered as probiotic agents. These yeast strains could be proposed for various probiotic applications, offering a valid alternative to or in combination with the probiotic yeast S. boulardii (Agarbati et al., 2020).
The probiotic nature of yeasts is usually defined by resistance to low pH, survival and growth capacity at high temperatures (37 °C), tolerance to gastric acidity, resistance to bile salts, auto-aggregation capability, and resistance to the gastrointestinal tract. These are the most commonly used criteria for selecting probiotic strains to balance the intestinal microbiome. Rodríguez Machado et al. (2024) demonstrated that it is possible to isolate yeasts with potential probiotic characteristics from honey. The yeast M. chrysoperlae was shown to be able to tolerate low pH, bile salts, and temperatures of 37 °C in a simulated in vitro digestion system, maintaining cell concentrations above 106 CFU/mL. The yeasts can also auto-aggregate and show capabilities for controlling enteric pathogenic bacteria. Findings obtained by Agarbati et al. (2020) showed interesting probiotic characteristics for some non-conventional yeast isolates belonging to M. ziziphicola that inhibited the growth of both bacterial and fungal pathogens. Subsequent studies by Agarbati et al. (2024b) confirmed the suitability of M. ziziphicola, originating from honeybee ecosystems, to inhibit both Listeria monocytogenes and S. enterica. These findings encouraged future efforts aimed at confirming the observed effects in vivo and driving further strain development toward novel yeast probiotics.
4.4 Biocontrol
Diverse antagonistic properties of Metschnikowia strains provide a basis for the use of active strains as biocontrol agents. The antimicrobial nature of Metschnikowia species has been confirmed mainly for filamentous fungi and some yeasts (Table 2). Growth inhibition by Metschnikowia spp. was demonstrated against prevalent pathogens, including Alternaria alternata (Pawlikowska et al., 2019; Fernandez-San Millan et al., 2022), Botritis cinerera (Sipiczki, 2006; Fernandez-San Millan et al., 2022), Penicillium spp. (Pawlikowska et al., 2019; Oztekin and Karbancioglu-Guler, 2021; Liu et al., 2025), Geotrichum citri-auranti (Wang et al., 2020, 2022; Zhang et al., 2023) and Colleotrichum spp. (Rering et al., 2023). Also noteworthy is the activity of Metschnikowia spp. against Fusarium oxysporum and Gibberella (Fusarium) fujikuroi (Rahmat et al., 2024; Bühlmann et al., 2021), which are widespread worldwide and cause diseases called fusariosis. Antimicrobial activity of Metschnikowia species was also reported against more specific pathogens, e.g., Erysiphe nectator (Lombardo et al., 2023), which causes powdery mildew of grapevines, and Ascosphera apis (Iorizzo et al., 2025), which exclusively infects honeybee larvae.
For the biocontrol yeasts, multiple mechanisms such as competition for nutrients and space, secretion of enzymes, toxin production, formation of volatile organic compounds (VOCs), mycoparasitism, and induction of resistance in plants are likely to be involved in the antagonistic function. However, in most cases, the mechanisms outlined and discussed below have not been fully proven by molecular analyses (e.g., by gene deletion and complementation, heterologous expression), but instead proposed based on analogies with other biological systems (Freimoser et al., 2019).
Pulcherrimin formation and iron depletion are the main mechanisms by which Metschnikowia exerts biocontrol effects (Rahmat et al., 2024; Sipiczki, 2006, 2020; Pawlikowska et al., 2019). As the reaction of pulcherriminic acid with ferric ions is irreversible and pulcherrimin is insoluble in water, the process is unlikely to play a role in iron acquisition. The chelated iron in pulcherrimin is inaccessible to the biochemical processes of microorganisms. Since iron is required for the activity of many proteins and cellular processes, its immobilization by pulcherriminic acid adversely affects the propagation of many microorganisms. Due to antimicrobial properties, pulcherrimin-producing Metschnikowia strains can be utilized as biological agents to protect agricultural commodities and food products against pathogenic and destructive microorganisms (He et al., 2024).
Pulcherrimin formation may be variable and reversible (Sipiczki et al., 2024). Interestingly, pulcherrimin production also varied within species. Intraclonal changes (segregation) in the intensity of pulcherrimin production in M. pulcherrima were reported. The cultures formed sectors differing in color intensity and mixtures of differently colored colonies. It was assumed that these changes may be due to different processes, e.g., silencing and reactivation of regulators, as well as mutations and backmutations, but this was not clearly explained.
The pulcherrimin biosynthesis was studied by Larini et al. (2025), who extracted gene sequences responsible for proteins related to pulcherriminic acid production and transport (PUL1, PUL2, PUL3, and PUL4). Then, the analysis of the flanking regions was conducted to understand the genetic configuration and explain the reversible character of pulcherrimin formation. In almost all strains tested, at least two copies of each PUL gene were found per strain, but an exception was M. pulcherrima KIOM G15050 (formerly M. persimmonensis). Most genomes exhibited PUL genes arranged in the order PUL1-PUL2-PUL4-PUL3; however, this was not a universal rule. M. pulcherrima strain NRRL Y-7111 T had syntenic PUL1, PUL2, and PUL4, but another copy of PUL4 and PUL3 was localized to a different contig. In another strain, M. pulcherrima 277, the PUL3 gene was located between the PUL2 and PUL4 genes. The prediction of protein localization revealed that proteins PUL1 and PUL4 appear to be located in the cytoplasm or nucleus, PUL2 is in the endoplasmic reticulum, PUL3 is in the cell membrane, and SNF2, a transcriptional regulator involved in pulcherrimin production, is in the nucleus for all strains. The predicted localizations of these proteins reflected their functions, as PUL1 and PUL2 are involved in pulcherriminic acid synthesis, PUL3 appears to be a transporter, and PUL4 and SNF2 are transcription factors that regulate the biosynthesis process.
In the context of biocontrol, carbohydrate-active enzymes (CAZymes) are also of particular interest. Their activity reflects the strain’s ability to colonize plant surfaces and its potential as a biocontrol agent. Strains are specific to plant surfaces, especially for the glycoside hydrolases. Enzymes can also participate in fungal cell wall degradation. Larini et al. (2025) studied CAZymes for which a signal peptide was predicted, indicating a putative extracellular location. Signal peptides play a key role in protein secretion, making them particularly interesting for the development of biological control agents (Thak et al., 2020). For example, Jones and Prusky (2002) demonstrated the potential for expressing antifungal peptides in yeast, which represents a novel approach to post-harvest disease control.
Chitinase activity may also contribute to the antagonistic effect of M. pulcherrima (Minguet-Lobato et al., 2024). Banani et al. (2015) observed that the M. fructicola AP47 strain showed higher transcription intensity of the chitinase gene in the presence of the Monilinia fructicola fungus. Antagonistic testing studies have shown that Metschnikowia spp. exhibits chitinase activity, while other strains exhibit protease, pectinase, and cellulase activity. These enzymatic actions can destroy the surface structures of fungal pathogens, enhancing their inhibitory effects (Acar et al., 2024).
Volatile organic compounds (VOCs) produced by Metschnikowia can also act on the pathogen directly and exert resistance. Studies conducted by Vepštaitė-Monstavičė et al. (2025) and Steglińska et al. (2023) confirmed the important correlation between antimicrobial action and VOCs produced by Metschnikowia, highlighting their role in ensuring antagonistic efficacy. The most abundant VOCs produced by Metschnikowia are esters and alcohols. Among these, the most abundant esters are ethyl acetate and 3-methylbutyl acetate. In turn, the most abundant alcohols are 2-phenylethanol and ethanol.
Switching from the planktonic yeast growth to the formation of chains of non-separated cells (pseudohyphae) may play an essential role in the protection of the plant surface because the pseudomycelium formed by the invasive pseudohyphae can form biofilms on the lesions, which are also gateways for the invasion by destructive microorganisms (Sipiczki et al., 2024). The efficiency of the yeast-to-pseudomycelium transition and the morphology of substrate invasion may greatly vary among the strains (Lachance, 2011). Laboratory studies examined the ability of Metschnikowia planktonic cells to adhere to non-plant-defined surfaces, including glass (hydrophilic) and polypropylene (hydrophobic) (Pawlikowska et al., 2019). Sipiczki et al. (2024) examined the ability of the tested strains to form pseudohyphae. Because a pseudomycelium is a stronger structure than a layer of planktonic yeast cells, especially if its pseudohyphae establish a strong bond with the damaged plant tissue by penetrating it. The efficiency of the yeast-to-pseudohyphal transition and the morphology of substrate invasion by the pseudomycelium also varied significantly among the isolates.
According to the FDA, strains of M. pulcherrima and M. fructicola may be used post-harvest, individually or in combination, at a maximum level of 1 g yeast/kg fresh coffee cherries, providing up to 2 × 107 CFU/g coffee (FDA, 2022). Some strains of Metschnikowia spp. are active against P. expansum and can be used for apple protection (Acar et al., 2024; Settier-Ramírez et al., 2021). Others are active against B. cinerea and can be used for protection against grape or apple diseases (Altieri et al., 2023). Initial experiments have demonstrated the ability of M. pulcherrima to inhibit the growth of undesired microorganisms in horticultural plants (potato seeds and strawberries) during post-harvest processing (Steglińska et al., 2022; Pawlikowska et al., 2019). Steglińska et al. (2023) reported a favorable interaction between Metschnikowia spp. and garlic for biological control. In other studies, Metschnikowia strains have been used as a potential natural biocontrol agent for stored fruits, including lemons, apples, grapes, sweet cherries, strawberries, and mangoes (Oztekin and Karbancioglu-Guler, 2021; Tian et al., 2017; Fernandez-San Millan et al., 2022).
5 Bioconversion as a primary tool in green technology
Currently, Metschnikowia spp. are being explored as promising agents for environmentally friendly and cost-effective green technologies. Biotransformation using waste is mainly used to produce lipids, proteins, enzymes, and biofuels. Table 3 shows many potential bioconversions of various by-products by Metschnikowia species.
Possible raw materials for bioconversion processes involving Metschnikowia spp. included fruit wastes (Chaudhary et al., 2021a, 2021b, 2022, 2024; Tatay-Núñez et al., 2024) and various lignocellulosic biomass (Vong and Liu, 2017; Zhou et al., 2017; Abomohra et al., 2021; Dygas et al., 2023a, 2023b). Potential substrates also involved whey (Chreptowicz et al., 2018; Zhang et al., 2022; Chua et al., 2018), and even post-production animal fats and glycerol (Němcová et al., 2021; Diamantopoulou et al., 2020).
In this context, lipid production seems to be particularly interesting. The interest of biotechnologists is closely linked to the rising prices of fossil fuels. Their negative impact on the environment is undeniable. The composition of microbial lipids is very similar to that of vegetable oils, which creates significant potential for their use in biodiesel production. This biotechnological strategy could also be interesting for the food industry (Abeln and Chuck, 2019; Abeln et al., 2019; Abeln et al., 2020).
Traditionally, economical and environmentally friendly production is achieved by metabolizing nutrients produced by algae. This method, which recycles solid waste such as macroalgae sugars and proteins, holds great potential for sustainable economic development. Li et al. (2021a) achieved a significant reduction in harmful substrate emissions from organic waste, alongside the production of Metschnikowia oil. It is worth noting that Metschnikowia spp. can utilize acetic acid from organic waste to enhance lipid accumulation (Li et al., 2021b).
Němcová et al. (2021) demonstrated the ability of yeast to produce significant amounts of unsaturated fatty acids from crude waste animal fat, with the accumulated lipids in yeast cells reaching 36% of cell dry weight. It was documented that controlling nitrogen content can increase lipid content compared to phosphorus-limited conditions.
Diamantopoulou et al. (2020) cultivated yeasts of the genus Metschnikowia, among others, under nitrogen-limited conditions using crude glycerol as a substrate. Lipids produced by yeasts contained mainly oleic and palmitic acids. However, Metschnikowia strain produced lower amounts of lipids than the best strain, Rhodosporidium toruloides, which was able to form oil from glycerol in an amount of 12.5 g/L.
In a study conducted by Tatay-Núñez et al. (2024), M. pulcherrima grew well in sugarcane and sugar beet molasses, as well as persimmon hydrolysate, and produced intracellular lipids. Their cells demonstrated increased tolerance to desiccation. However, the lipid yield was lower in comparison to other tested oleaginous yeasts.
Metschnikowia strains are also able to grow on lignocellulosic biomass for lipid production (Zhou et al., 2017; Abomohra et al., 2021). In the work of Zhou et al. (2017), rapid, microwave-assisted acidolysis of lignocellulosic biomass led to the production of fermentable saccharides. Under these conditions, 1.5 g/L of mono- and di saccharides were available for fermentation, and less than 0.5 g/L of acids and furfurals were produced, which prevented fermentation by S. cerevisiae and other ethanol-producing yeasts. M. pulcherrima could grow in this broth, producing small amounts of lipids with a composition similar to palm oil.
The valorization of food and lignocellulosic wastes into biodiesel using M. pulcherrima strain isolated from rotten food wastes was evaluated by Abomohra et al. (2021). Food waste hydrolysate was supplemented by rice straw and softwood sawdust as additional carbon sources to increase the C: N ratio. M. pulcherrima showed the ability to produce lipids with a maximum productivity of 2.49 g per liter per day, and good biodiesel characteristics.
Intensive research into fermentation technology for waste biomass revalorization resulted in a study of M. pulcherrima as a source of SCP production. In a study by Dygas et al. (2023b), M. pulcherrima was successfully grown on rapeseed meal. A slight increase in protein was obtained, measured by an increase in nitrogen content from 0.6 to 1.6%. However, simultaneous saccharification and fermentation led to the conversion of isoflavones into forms with fewer adverse effects and lower estrogenic activity. In other work, the same authors investigated the possible use of M. pulcherrima to enrich sugar beet pulp pretreated by enzymatic hydrolysis. In these conditions, Metschnikowia yeast can grow at the level 2 × 107–1 × 108 CFU/mL, and the protein increase measured by the increase in nitrogen content ranged from 0.9 to 1.7% (Dygas et al., 2023a). The results showed that sugar beet pulp provides a good matrix for SCP and feed production. However, when using lignocellulosic biomass, pre-treatment is necessary.
The production of bioethanol and other alcohols from agricultural byproducts offers a good solution for waste management. Metschnikowia strains were used for this purpose, even though they are moderate fermenting yeasts. Research on the use of Metschnikowia spp. in fermentation processes was conducted on various lignocellulosic wastes. Despite wide yeast enzymatic activity, lignocellulosic biomass pretreatment was necessary (Chaudhary et al., 2021a,b; Chaudhary et al., 2022; Chaudhary et al., 2024). However, the drastic thermal and chemical treatment of lignocellulosic biomass means these bioconversions cannot be classified as pure green technologies.
Chreptowicz et al. (2018) assessed the activity of M. chrysoperlae in the production of 2-phenylethanol from whey or molasses, but the results also were not satisfactory. Metschnikowia strain produced 2-PE at 1 g per liter in whey and sugar beet juice medium; however, the best producers were strains from S. cerevisiae, which produced about 3 g/L of this metabolite.
Mitri et al. (2022) described different weak points for 2-PE formation. Firstly, high concentrations of 2-PE inhibit the yeast cell growth. Of course, there are several ways to overcome the inhibiting restraint. For example, strain mutagenesis and culture medium composition, or the optimization of the fermentation conditions, are widely used strategies for the improvement of yield. Secondly, the nitrogen sources, including L-Phe, carbon sources, vitamins, and minerals, supplemented to the media affect the fermentation process. For this reason, these media are not cost-effective, and it is necessary to search not only for efficient strains but also for various alternative, cheap cultivation media. In addition, temperature, initial medium pH, and fermentation time are all factors that could affect the amount of product.
Dairy industry wastes can be explored as a cheap and attractive raw material also for producing various alcohols. Zhang et al. (2022) converted cheese whey powder into D-arabitol and L-galactitol in a two-step process. Firstly, the simultaneous lactose hydrolysis and isomerization of lactose-derived D-galactose was performed by an engineered E. coli strain. Subsequently, the mixture containing lactose-derived D-glucose and residual D-galactose was subjected to fermentation by M. pulcherrima E1 strain, which produced 60 g/L D-arabitol and 28 g/L galactitol, low-calorie sweeteners.
Metschnikowia spp. are a promising microorganism with huge biotechnological potential. However, so far, these biotechnological strategies are still not economically competitive with chemical synthesis. To sum up the examples of the use of waste materials by the Metschnikowia spp., it should be stated that the first ambitious goal for developing biotechnological production should be to identify highly productive yeast strains and substrates that can be revalorized. The task will also be to use appropriately selected mixed cultures, proper pre-treatment methods, and cultivation conditions.
6 Conclusion
The main standards of green technology focus on reducing environmental pollution and reusing waste to produce new products (Nandy et al., 2022). Green technologies offer numerous opportunities, but they also face significant challenges (Figure 3). Green innovations can leverage new ideas to develop new processes and products, as well as improve existing production processes through environmentally friendly practices. Therefore, implementing closed-loop green technologies is essential for a sustainable future on a global scale. Current research is focused on developing new industrial methods and technologies that not only reduce waste and dependence on raw materials but also promote ecosystem conservation.
The use of Metschnikowia spp. in green processes offers several advantages, such as utilizing their broad enzymatic capabilities, non-toxicity to humans and the environment, low cost, and long-lasting protection against pathogens. With the continuous advancement of green technologies, scientists are increasingly exploring the use of waste materials for culturing Metschnikowia cells, with broader applications in sectors such as food/feed, energy, cosmetics, and biocontrol. However, it is noteworthy that the long-term use of Metschnikowia in various natural environments can impact other beneficial microorganisms and disrupt microbial ecological systems (Nowak et al., 2025).
Several regulations govern the use of Metschnikowia spp. in the food/feed industry. According to these guidelines, particular attention should be paid to the safety of the yeast strain, human- and environmentally friendly process conditions, and the economic viability of such processes in large-scale industrial production. Therefore, to enable the full commercialization of Metschnikowia-based products, it is essential to establish quality standards and specifications that ensure safety, efficacy, and consistency. First, standards for raw materials should specify the required purity for yeast cell production. Additionally, process standards should be developed, covering fermentation conditions, hygiene protocols, and methods for detecting all potentially toxic substances. Both the stability of the innovative products and other key parameters must be clearly defined and implemented.
Author contributions
JL: Writing – original draft. AR: Conceptualization, Writing – review & editing. BZ: Formal analysis, Project administration, Writing – review & editing. DK: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abeln, F., and Chuck, C. J. (2019). Achieving a high-density oleaginous yeast culture: comparison of four processing strategies using Metschnikowia pulcherrima. Biotechnol. Bioeng. 116, 3200–3214. doi: 10.1002/bit.27141
Abeln, F., Fan, J., Budarin, V. L., Briers, H., Parsons, S., Allen, M. J., et al. (2019). Lipid production through the single-step microwave hydrolysis of macroalgae using the oleaginous yeast Metschnikowia pulcherrima. Algal Res. 38:101411. doi: 10.1016/j.algal.2019.101411
Abeln, F., Hicks, R. H., Auta, H., Moreno-Beltrán, M., Longanesi, L., Henk, D. A., et al. (2020). Semi-continuous pilot-scale microbial oil production with Metschnikowia pulcherrima on starch hydrolysate. Biotechnol. Biofuels 13:127. doi: 10.1186/s13068-020-01756-2
Abomohra, A. E. F., Wang, Q., Huang, J., and Saad-Allah, K. M. (2021). A sustainable approach for bioconversion of food and lignocellulosic wastes into liquid biofuel using a new Metschnikowia pulcherrima isolate. Int. J. Energy Res. 45, 3430–3441. doi: 10.1002/er.6028
Acar, E. G., Dikmetas, D. N., Devecioglu, D., Ozer, E. M., Sarikece, H., and Karbancioglu-Guler, F. (2024). Antagonistic activities of Metschnikowia pulcherrima isolates against Penicillium expansum on Amasya apples. Curr. Microbiol. 81:180. doi: 10.1007/s00284-024-03700-1
Agarbati, A., Canonico, L., Marini, E., Zannini, E., Ciani, M., and Comitini, F. (2020). Potential probiotic yeasts sourced from natural environmental and spontaneous processed foods. Foods 9:287. doi: 10.3390/foods9030287
Agarbati, A., Gattucci, S., Canonico, L., Ciani, M., and Comitini, F. (2024a). Yeast communities related to honeybees: occurrence and distribution in flowers, gut mycobiota, and bee products. Appl. Microbiol. Biotechnol. 108:175. doi: 10.1007/s00253-023-12942-1
Agarbati, A., Moretti, L., Canonico, L., Ciani, M., and Comitini, F. (2024b). Agro-ecosystem of honeybees as source for native probiotic yeasts. World J. Microbiol. Biotechnol. 40:147. doi: 10.1007/s11274-024-03941-z
Al-Emran, M., and Griffy-Brown, C. (2023). The role of technology adoption in sustainable development: overview, opportunities, challenges, and future research agendas. Technol. Soc. 73:102240. doi: 10.1016/j.techsoc.2023.102240
Altieri, V., Rossi, V., and Fedele, G. (2023). Efficacy of preharvest application of biocontrol agents against gray mold in grapevine. Front. Plant Sci. 14:1154370. doi: 10.3389/fpls.2023.1154370
Aragno, J., Fernandez-Valle, P., Thiriet, A., Grondin, C., Legras, J. L., Camarasa, C., et al. (2024). Two-stage screening of Metschnikowia spp. bioprotective properties: from grape juice to fermented must by Saccharomyces cerevisiae. Microorganisms 12:1659. doi: 10.3390/microorganisms12081659
Banani, H., Spadaro, D., Zhang, D., Matic, S., Garibaldi, A., and Gullino, M. L. (2015). Postharvest application of a novel chitinase cloned from Metschnikowia fructicola and overexpressed in Pichia pastoris to control brown rot of peaches. Int. J. Food Microbiol. 199, 54–61. doi: 10.1016/j.ijfoodmicro.2015.01.002
Bibi, F., Ilyas, N., Saeed, M., Shabir, S., Shati, A. A., Alfaifi, M. Y., et al. (2023). Innovative production of value-added products using agro-industrial wastes via solid-state fermentation. Environ. Sci. Pollut. Res. Int. 30, 125197–125213. doi: 10.1007/s11356-023-28765-6
Binati, R. L., Maule, M., Luzzini, G., Martelli, F., Felis, G. E., Ugliano, M., et al. (2023). From bioprotective effects to diversification of wine aroma: expanding the knowledge on Metschnikowia pulcherrima oenological potential. Food Res. Int. 174:113550. doi: 10.1016/j.foodres.2023.113550
Binati, R. L., Salvetti, E., Bzducha-Wrobel, A., Basinskiene, L., Cizeikiene, D., Bolzonella, D., et al. (2021). Non-conventional yeasts for food and additives production in a circular economy perspective. FEMS Yeast Res. 21, 1–18. doi: 10.1093/femsyr/foab052
Boscaino, F., Ionata, E., La Cara, F., Guerriero, S., Marcolongo, L., and Sorrentino, A. (2019). Impact of Saccharomyces cerevisiae and Metschnikowia fructicola autochthonous mixed starter on Aglianico wine volatile compounds. J. Food Sci. Technol. 56, 4982–4991. doi: 10.1007/s13197-019-03970-9
Bourdichon, F., Casaregola, S., Farrokh, C., Frisvad, J. C., Gerds, M. L., Hammes, W. P., et al. (2012). Food fermentations: microorganisms with technological beneficial use. Int. J. Food Microbiol. 154, 87–97. doi: 10.1016/j.ijfoodmicro.2011.12.030
Bühlmann, A., Kammerecker, S., Müller, L., Hilber-Bodmer, M., and Perren, S. (2021). Stability of dry and liquid Metschnikowia pulcherrima formulations for biocontrol applications against apple postharvest diseases. Horticulturae 7:459. doi: 10.3390/horticulturae7110459
Canonico, L., Agarbati, A., Galli, E., Comitini, F., and Ciani, M. (2023). Metschnikowia pulcherrima as biocontrol agent and wine aroma enhancer in combination with a native Saccharomyces cerevisiae. LWT 181:114758. doi: 10.1016/j.lwt.2023.114758
Chantasuban, T., Santomauro, F., Gore-Lloyd, D., Parsons, S., Henk, D., Scott, R. J., et al. (2018). Elevated production of the aromatic fragrance molecule, 2-phenylethanol, using Metschnikowia pulcherrima through both de novo and ex novo conversion in batch and continuous modes. J. Chem. Technol. Biotechnol. 93, 2118–2130. doi: 10.1002/jctb.5597
Chaudhary, A., Akram, A. M., Aihetasham, A., Hussain, Z., Abbas, A. S., Rehman, R. A., et al. (2021a). Punica granatum waste to ethanol valorisation employing optimized levels of saccharification and fermentation. Saudi J. Biol. Sci. 28, 3710–3719. doi: 10.1016/j.sjbs.2021.04.049
Chaudhary, A., Hussain, I., Ahmad, Q. A., Hussain, Z., Akram, A. M., and Hussain, A. (2022). Efficient utilization of melon peels to produce ethanol: a step toward sustainable waste management. Biomass Convers. Biorefinery 14, 3463–3475. doi: 10.1007/s13399-022-02687-8
Chaudhary, A., Hussain, Z., Aihetasham, A., El-Sharnouby, M., Abdul Rehman, R., Khan, M. A. U., et al. (2021b). Pomegranate peels waste hydrolyzate optimization by response surface methodology for bioethanol production. Saudi J. Biol. Sci. 28, 4867–4875. doi: 10.1016/j.sjbs.2021.06.081
Chaudhary, A., Hussain, Z., Ajmal, H., Abdul Rehman, R., Abbas, G., Aihetasham, A., et al. (2024). Efficient bioconversion of mango waste into ethanol employing Plackett-Burman and central composite models. ACS Omega 9, 39652–39662. doi: 10.1021/acsomega.4c04374
Chaudhary, A., and Karita, S. (2017). Screening of yeast isolates from flowers for effective ethanol production. Turk. J. Biol. 41, 890–900. doi: 10.3906/biy-1704-7
Chen, J., Yu, Y., Sun, S., Yu, W., Lei, Y., Lu, C., et al. (2025). Probiotics and prebiotics: meeting dietary requirements for optimal health and planetary sustainability. J. Nutr. 155, 2519–2533. doi: 10.1016/j.tjnut.2025.03.027
Chreptowicz, K., Sternicka, M. K., Kowalska, P. D., and Mierzejewska, J. (2018). Screening of yeasts for the production of 2-phenylethanol (rose aroma) in organic waste-based media. Lett. Appl. Microbiol. 66, 153–160. doi: 10.1111/lam.12835
Chua, J. Y., Lu, Y., and Liu, S. Q. (2018). Evaluation of five commercial non-Saccharomyces yeasts in fermentation of soy (tofu) whey into an alcoholic beverage. Food Microbiol. 76, 533–542. doi: 10.1016/j.fm.2018.07.016
De Souza, L. M. D., Ogaki, M. B., Teixeira, E. A. A., De Menezes, G. C. A., Convey, P., Rosa, C. A., et al. (2023). Communities of culturable freshwater fungi present in Antarctic lakes and detection of their low-temperature-active enzymes. Braz. J. Microbiol. 54, 1923–1933. doi: 10.1007/s42770-022-00834-x
Diamantopoulou, P., Filippousi, R., Antoniou, D., Varfi, E., Xenopoulos, E., Sarris, D., et al. (2020). Production of added-value microbial metabolites during growth of yeast strains on media composed of biodiesel-derived crude glycerol and glycerol/xylose blends. FEMS Microbiol. Lett. 367:fnaa063. doi: 10.1093/femsle/fnaa063
Domínguez, J. R., Nuñez-Delgado, A., Gomes, H. T., Augusto, P. A., Varjani, S., García, J., et al. (2024). 6th international congress on water, waste and energy management (WWEM-22). 5th international conference on green chemistry and sustainable engineering (GreenChem-22). 2022 international conference on green energy and environmental technology (GEET-22). Environ. Sci. Pollut. Res. Int. 31, 36079–36082. doi: 10.1007/s11356-023-27445-9
Dygas, D., Kręgiel, D., and Berlowska, J. (2023a). Sugar beet pulp as a biorefinery substrate for designing feed. Molecules 28:2064. doi: 10.3390/molecules28052064
Dygas, D., Liszkowska, W., Steglińska, A., Sulyok, M., Kręgiel, D., and Berłowska, J. (2023b). Rapeseed meal waste biomass as a single-cell protein substrate for nutritionally-enhanced feed components. PRO 11:1556. doi: 10.3390/pr11051556
EFSA (2017). Peer review of the pesticide risk assessment of the active substance Metschnikowia fructicola NRRL Y-27328. EFSA J. 15:e05084. doi: 10.2903/j.efsa.2017.5084
EFSA (2018). Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA J. 16:5206. doi: 10.2903/j.efsa.2018.5206
EFSA (2024). EFSA statement on the requirements for whole genome sequence analysis of microorganisms intentionally used in the food chain. EFSA J. 22:e8912. doi: 10.2903/j.efsa.2024.8912
Escott, C., Vaquero, C., Loira, I., López, C., González, C., and Morata, A. (2022). Synergetic effect of Metschnikowia pulcherrima and Lachancea thermotolerans in acidification and aroma compounds in Airén wines. Foods 11:3734. doi: 10.3390/foods11223734
European Union (2018). Document 32018R1915. Available online at: http://data.europa.eu/eli/reg_impl/2018/1915/oj (Accessed June 12, 2025).
FDA (2022). GRN no. 1028 Metschnikowia pulcherrima DANMET-A and M. fructicola DANMET-B. Available online at: https://www.hfpappexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=1028 (Accessed May 12, 2025).
Fernandez-San Millan, A., Gamir, J., Farran, I., Larraya, L., and Veramendi, J. (2022). Identification of new antifungal metabolites produced by the yeast Metschnikowia pulcherrima involved in the biocontrol of postharvest plant pathogenic fungi. Postharvest Biol. Technol. 192:111995. doi: 10.1016/j.postharvbio.2022.111995
Food and Agriculture Organization of the United Nations. (2022) Food loss reduction CoP. Available online at: https://www.fao.org/platform-food-loss-waste/resources/detail/en/c/1378978/ (Accessed August 7, 2025).
Freimoser, F. M., Mahler, M., McCullough, M., Brachmann, A. O., Nägeli, L., Hilber-Bodmer, M., et al. (2024). Heterologous pulcherrimin production in Saccharomyces cerevisiae confers inhibitory activity on Botrytis conidiation. FEMS Yeast Res. 24:foad053. doi: 10.1093/femsyr/foad053
Freimoser, F. M., Rueda-Mejia, M. P., Tilocca, B., and Migheli, Q. (2019). Biocontrol yeasts: mechanisms and applications. World J. Microbiol. Biotechnol. 35:154. doi: 10.1007/s11274-019-2728-4
Gonzalez, R., Guindal, A. M., Tronchoni, J., and Morales, P. (2021). Biotechnological approaches to lowering the ethanol yield during wine fermentation. Biomolecules 11:1569. doi: 10.3390/biom11111569
Guindal, A. M., Morales, P., Tronchoni, J., and Gonzalez, R. (2023). Reduction of ethanol content in wine with an improved combination of yeast strains and process conditions. Food Microbiol. 115:104344. doi: 10.1016/j.fm.2023.104344
Guo, X., Zhu, X., Qian, Y., Yang, Y., Zhu, F., Zhao, Y., et al. (2025). Enhancing variety aromatic characteristics of Muscat wine through cold maceration with indigenous cryotolerant Metschnikowia pulcherrima Mp0520. Food Chem. 463:141097. doi: 10.1016/j.foodchem.2024.141097
Haniffadli, A., Ban, Y., Rahmat, E., Kang, C. H., and Kang, Y. (2024). Unforeseen current and future benefits of nncommon yeast: the Metschnikowia genus. AMB 108:534. doi: 10.1007/s00253-024-13369-y
He, Y., Degraeve, P., and Oulahal, N. (2024). Bioprotective yeasts: potential to limit postharvest spoilage and to extend shelf life or improve microbial safety of processed foods. Heliyon 10:e24929. doi: 10.1016/j.heliyon.2024.e24929
Hong, S. G., Chun, J., Oh, H. W., and Bae, K. S. (2001). Metschnikowia koreensis sp. nov., a novel yeast species isolated from flowers in Korea. Int. J. Syst. Evol. Microbiol. 51, 1927–1931. doi: 10.1099/00207713-51-5-1927
Hranilovic, A., Gambetta, J. M., Jeffery, D. W., Grbin, P. R., and Jiranek, V. (2020). Lower-alcohol wines produced by Metschnikowia pulcherrima and Saccharomyces cerevisiae co-fermentations: the effect of sequential inoculation timing. Int. J. Food Microbiol. 329:108651. doi: 10.1016/j.ijfoodmicro.2020.108651
Index Fungorum. (2025). Available online at: https://indexfungorum.org/Names/Names.asp (Accessed May 15, 2025).
Iorizzo, M., Coppola, F., Pannella, G., Ganassi, S., Matarazzo, C., Albanese, G., et al. (2025). First report on antifungal activity of Metschnikowia pulcherrima against Ascosphaera apis, the causative agent of chalkbrood disease in honeybee (Apis mellifera L.) colonies. J. Fungi 11:336. doi: 10.3390/jof11050336
Jiang, H., Bao, J., Xing, Y., Li, X., and Chen, Q. (2022). Comparative genomic analyses provide insight into the pathogenicity of Metschnikowia bicuspidata LNES0119. Front. Microbiol. 13:939141. doi: 10.3389/fmicb.2022.939141
Jones, R. W., and Prusky, D. (2002). Expression of an antifungal peptide in Saccharomyces: a new approach for biological control of the postharvest disease caused by Colletotrichum coccodes. Phytopathology 92, 33–37. doi: 10.1094/PHYTO.2002.92.1.33
Karaulli, J., Xhaferaj, N., Coppola, F., Testa, B., Letizia, F., Kyçyk, O., et al. (2024). Bioprospecting of Metschnikowia pulcherrima strains, isolated from a vineyard ecosystem, as novel starter cultures for craft beer production. Fermentation 10:513. doi: 10.3390/fermentation10100513
Kaźmierczak-Siedlecka, K., Ruszkowski, J., Fic, M., Folwarski, M., and Makarewicz, W. (2020). Saccharomyces boulardii CNCM I-745: a non-bacterial microorganism used as probiotic agent in supporting treatment of selected diseases. Curr. Microbiol. 77, 1987–1996. doi: 10.1007/s00284-020-02053-9
Klimczak, K., Cioch-Skoneczny, M., and Poreda, A. (2024). Evaluation of non-Saccharomyces yeast for low-alcohol beer production. Appl. Sci. 14:6755. doi: 10.3390/app14156755
Kregiel, D., Czarnecka-Chrebelska, K. H., Schusterová, H., Vadkertiová, R., and Nowak, A. (2023). The Metschnikowia pulcherrima clade as a model for assessing inhibition of Candida spp. and the toxicity of its metabolite, pulcherrimin. Molecules 28:13. doi: 10.3390/molecules28135064
Kregiel, D., Dziekonska-Kubczak, U., Czarnecka-Chrebelska, K. H., and Pielech-Przybylska, K. (2025). Chemical fingerprints of honey fermented by conventional and non-conventional yeasts. Molecules 30:2319. doi: 10.3390/molecules30112319
Kregiel, D., Krajewska, A., Kowalska-Baron, A., Czarnecka-Chrebelska, K. H., and Nowak, A. (2024). Photoprotective effects of yeast pulcherrimin. Molecules 29:4873. doi: 10.3390/molecules29204873
Kregiel, D., Nowacka, M., Rygala, A., and Vadkertiova, R. (2022a). Biological activity of pulcherrimin from the Meschnikowia pulcherrima clade. Molecules 27:1855. doi: 10.3390/molecules27061855
Kregiel, D., Pawlikowska, E., Antolak, H., Dziekońska-Kubczak, U., and Pielech-Przybylska, K. (2022b). Exploring use of the Metschnikowia pulcherrima clade to improve properties of fruit wines. Fermentation 8:247. doi: 10.3390/fermentation8060247
Kuan, C. S., Ismail, R., Kwan, Z., Yew, S. M., Yeo, S. K., Chan, C. L., et al. (2016). Isolation and characterization of an atypical Metschnikowia sp. strain from the skin scraping of a dermatitis patient. PLoS One 11:e0156119. doi: 10.1371/journal.pone.0156119
Kumari, A., Kumari, S., Prasad, G. S., and Pinnaka, A. K. (2021). Production of sophorolipid biosurfactant by insect derived novel yeast Metschnikowia churdharensis fa, sp. nov., and its antifungal activity against plant and human pathogens. Front. Microbiol. 12:678668. doi: 10.3389/fmicb.2021.678668
Kurtzman, C. P., Robnett, C. J., Basehoar, E., and Ward, T. J. (2018). Four new species of Metschnikowia and the transfer of seven Candida species to Metschnikowia and Clavispora as new combinations. Antonie Leeuwenhoek 111, 2017–2035. doi: 10.1007/s10482-018-1095-8
Lachance, M.-A. (2011). “Chapter 46 – Metschnikowia Kamienski (1899)” in The yeasts. eds. C. P. Kurtzman, J. W. Fell, and T. Boekhout. 5th ed (Amsterdam: Elsevier), 575–620.
Lachance, M.-A. (2016). Metschnikowia: half tetrads, a regicide and the fountain of youth. Yeast 33, 563–574. doi: 10.1002/yea.3208
Larini, I., Ferrara, M., Troiano, E., Gatto, V., Mulè, G., Vitulo, N., et al. (2025). Unlocking the potential of Metschnikowia pulcherrima: a dive into the genomic and safety characterization of four plant-associated strains. Appl. Microbiol. Biotechnol. 109:128. doi: 10.1007/s00253-025-13515-0
Lebleux, M., Alexandre, H., Romanet, R., Ballester, J., David-Vaizant, V., Adrian, M., et al. (2023). Must protection, sulfites versus bioprotection: a metabolomic study. Food Res. Int. 173:113383. doi: 10.1016/j.foodres.2023.113383
Li, Q., Wang, D., Li, A., and Gu, J. (2021a). Microbial lipids production from wastes by Metschnikowia pulcherrima: a review. Sheng Wu Gong Cheng Xue Bao 37, 2753–2764. doi: 10.13345/j.cjb.200599
Li, Q., Wang, D., Liu, X., Li, A., and Chandran, K. (2021b). Enhanced lipid accumulation in Metschnikowia pulcherrima using volatile fatty acids under non-sterile repeated batch cultivation. Int. Biodeterior. Biodegrad. 163:105256. doi: 10.1016/j.ibiod.2021.105256
Liu, F., Hu, Z. D., Zhao, X. M., Zhao, W. N., Feng, Z. X., Yurkov, A., et al. (2024). Phylogenomic analysis of the Candida auris-Candida haemuli clade and related taxa in the Metschnikowiaceae, and proposal of thirteen new genera, fifty-five new combinations and nine new species. Persoonia 52, 22–43. doi: 10.3767/persoonia.2024.52.02
Liu, W., Liu, C., Zeren, D., Wang, S., Tan, Z., Hang, F., et al. (2025). Biocontrol ability and possible mechanism of Metschnikowia pulcherrima against major diseases of postharvest citrus fruit and its biopreservative application. Int. J. Food Microbiol. 438:111230. doi: 10.1016/j.ijfoodmicro.2025.111230
Liu, Y., Yao, S., Deng, L., Ming, J., and Zeng, K. (2018). Metschnikowia citriensis sp. nov., a novel yeast species isolated from leaves with potential for biocontrol of postharvest fruit rot. Biol. Control 125, 15–19. doi: 10.1016/j.biocontrol.2018.05.018
Lombardo, M. F., Panebianco, S., Restuccia, C., and Cirvilleri, G. (2023). Biocontrol efficacy of Metschnikowia spp. yeasts in organic vineyards against major airborne diseases of table grapes in the field and in postharvest. Foods 12:3508. doi: 10.3390/foods12183508
Longhi, S. J., Martín, M. C., Merín, M. G., and Morata de Ambrosini, V. I. (2022). Yeast multi-enzymatic systems for improving colour extraction, technological parameters and antioxidant activity of wine. Food Technol. Biotechnol. 60, 556–570. doi: 10.17113/ftb.60.04.22.7777
Mateo, J. J. (2023). Physico-chemical characterization of an exocellular sugars tolerant β-glucosidase from grape Metschnikowia pulcherrima isolates. Microorganisms 11:964. doi: 10.3390/microorganisms11040964
Meena, V. S., Banoth, L., and Banerjee, U. C. (2014). Demonstration of redox potential of Metschnikowia koreensis for stereoinversion of secondary alcohols/1, 2-diols. Biomed. Res. Int. 2014:410530. doi: 10.1155/2014/410530
Mierzejewska, J., Tymoszewska, A., Chreptowicz, K., and Król, K. (2017). Mating of 2 laboratory Saccharomyces cerevisiae strains resulted in enhanced production of 2-phenylethanol by biotransformation of L-phenylalanine. J. Mol. Microbiol. Biotechnol. 27, 81–90. doi: 10.1159/000455169
Miguel, G. A., Carlsen, S., Arneborg, N., Saerens, S. M. G., Laulund, S., and Knudsen, G. M. (2022). Non-Saccharomyces yeasts for beer production: insights into safety aspects and considerations. Int. J. Food Microbiol. 383:109951. doi: 10.1016/j.ijfoodmicro.2022.109951
Mim, M. F., Sikder, M. H., Chowdhury, M. Z. H., Bhuiyan, A. U. A., Zinan, N., and Islam, S. M. N. (2024). The dynamic relationship between skin microbiomes and personal care products: a comprehensive review. Heliyon 10:e34549. doi: 10.1016/j.heliyon.2024.e34549
Minguet-Lobato, M., Cervantes, F. V., Míguez, N., Plou, F. J., and Fernández-Lobato, M. (2024). Chitinous material bioconversion by three new chitinases from the yeast Mestchnikowia pulcherrima. Microb. Cell Factories 23:31. doi: 10.1186/s12934-024-02300-9
Mishra, K., Siwal, S. S., Nayaka, S. C., Guan, Z., and Thakur, V. K. (2023). Waste-to-chemicals: green solutions for bioeconomy markets. Sci. Total Environ. 887:164006. doi: 10.1016/j.scitotenv.2023.164006
Mitri, S., Koubaa, M., Maroun, R. G., Rossignol, T., Nicaud, J. M., and Louka, N. (2022). Bioproduction of 2-phenylethanol through yeast fermentation on synthetic media and on agro-industrial waste and by-products: a review. Foods 11:109. doi: 10.3390/foods11010109
Mohl, W., Lerch, M. M., Klotz, M., Freidank, H., and Zeitz, M. (1998). Infection of an intravenous port system with Metschnikowia pulcherrima Pitt et miller. Mycoses 41, 425–426. doi: 10.1111/j.1439-0507.1998.tb00365.x
Nandy, S., Fortunato, E., and Martins, R. (2022). Green economy and waste management: an inevitable plan for materials science. Prog. Nat. Sci.: Mater. Int. 32, 1–9. doi: 10.1016/j.pnsc.2022.01.001
Naselli, V., Prestianni, R., Badalamenti, N., Matraxia, M., Maggio, A., Alfonzo, A., et al. (2023). Improving the aromatic profiles of Catarratto wines: impact of Metschnikowia pulcherrima and glutathione-rich inactivated yeasts. Antioxidants 12:439. doi: 10.3390/antiox12020439
NCBI Taxonomy Browser. Available online at: https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi (Accessed August 23, 2025).
Němcová, A., Szotkowski, M., Samek, O., Cagáňová, L., Sipiczki, M., and Márová, I. (2021). Use of waste substrates for the lipid production by yeasts of the genus Metschnikowia - screening study. Microorganisms 9:2295. doi: 10.3390/microorganisms9112295
Nowak, A., Steglińska, A., Gutarowska, B., and Kregiel, D. (2025). Cyto- and genotoxicity of selected plant extracts and microbial metabolites with confirmed activity against phytopathogens of potato seed (Solanum tuberosum L.). Molecules 30:701. doi: 10.3390/molecules30030701
O’Brien, E. S., Verleye, M., and Le Guern, M. E. (2024). Saccharomyces boulardii for the treatment of mood disorders. U.S. patent no 11.872.257 B2.
Oztekin, S., and Karbancioglu-Guler, F. (2021). Bioprospection of Metschnikowia sp. isolates as biocontrol agents against postharvest fungal decays on lemons with their potential modes of action. Postharvest Biol. Technol. 181:111634. doi: 10.1016/j.postharvbio.2021.111634
Paufique, J. (2013). Active ingredient with cutaneous application obtained from Metschnikowia agaves and uses for improving the state of the skin. Republic of France U.S. patent no 9.023.827 B2.
Paufique, J. (2019). Metschnikowia reukaufii extract and use in cosmetics. Republic of France U.S. patent no 11.564.880 B2.
Pawlikowska, E., James, S. A., Breierova, E., Antolak, H., and Kregiel, D. (2019). Biocontrol capability of local Metschnikowia sp. isolates. Antonie Leeuwenhoek 112, 1425–1445. doi: 10.1007/s10482-019-01272-w
Pawlikowska, E., Szymanska, M., Berlowska, J., and Kregiel, D. (2020). Lysates of Metschnikowia yeast with higher content of hydroxyproline. Bioresources 15, 3228–3236. doi: 10.15376/biores.15.2.3228-3236
Phiri, R., Rangappa, S. M., and Siengchin, S. (2024). Agro-waste for renewable and sustainable green production: a review. J. Clean. Prod. 434:139989. doi: 10.1016/j.jclepro.2023.139989
Plavec, T. V., and Berlec, A. (2020). Safety aspects of genetically modified lactic acid bacteria. Microorganisms 8:297. doi: 10.3390/microorganisms8020297
Rahmat, E., Yu, J. S., Lee, B. S., Lee, J., Ban, Y., Yim, N. H., et al. (2024). Secondary metabolites and transcriptomic analysis of novel pulcherrimin producer Metschnikowia persimmonesis KIOM G15050: a potent and safe food biocontrol agent. Heliyon. 10:e28464. doi: 10.1016/j.heliyon.2024.e28464
Rering, C. C., Lanier, A. M., and Peres, N. A. (2023). Blueberry floral probiotics: nectar microbes inhibit the growth of Colletotrichum pathogens. J. Appl. Microbiol. 134:lxad300. doi: 10.1093/jambio/lxad300
Rodríguez Machado, A., Caro, C. M., and Hurtado-Murillo, J. J. (2024). Unconventional yeasts isolated from Chilean honey: a probiotic and phenotypic characterization. Foods 13:1582. doi: 10.3390/foods13101582
Ruiz, J., Belda, I., and Beisert, B. (2018). Analytical impact of Metschnikowia pulcherrima in the volatile profile of Verdejo white wines. Appl. Microbiol. Biotechnol. 102, 8501–8509. doi: 10.1007/s00253-018-9255-3
Santamauro, F., Whiffin, F. M., Scott, R. J., and Chuck, C. J. (2014). Low-cost lipid production by an oleaginous yeast cultured in non-sterile conditions using model waste resources. Biotechnol. Biofuels 7, 1–11. doi: 10.1186/1754-6834-7-34
Sasahara, H., and Izumori, K. (2005). Production of L-talitol from L-psicose by Metschnikowia koreensis LA1 isolated from soy sauce mash. JBB 100, 335–338. doi: 10.1263/jbb.100.335
Savini, V., Hendrickx, M., Sisti, M., Masciarelli, G., Favaro, M., Fontana, C., et al. (2013). An atypical, pigment-producing Metschnikowia strain from a leukaemia patient. Med. Mycol. 51, 438–443. doi: 10.3109/13693786.2012.733429
Settier-Ramírez, L., López-Carballo, G., Hernández-Muñoz, P., Fontana, A., Strub, C., and Schorr-Galindo, S. (2021). New isolated Metschnikowia pulcherrima strains from apples for postharvest biocontrol of Penicillium expansum and patulin accumulation. Toxins 13:397. doi: 10.3390/toxins13060397
Sharma, V., Tsai, M.-L., Nargotra, P., Chen, C.-W., Kuo, C.-H., Sun, P.-P., et al. (2022). Agro-industrial food waste as a low-cost substrate for sustainable production of industrial enzymes: a critical review. Catalysts 12:1373. doi: 10.3390/catal12111373
Sipiczki, M. (2006). Metschnikowia strains isolated from botrytized grapes antagonize fungal and bacterial growth by iron depletion. Appl. Environ. Microbiol. 72, 6716–6724. doi: 10.1128/AEM.01275-06
Sipiczki, M. (2020). Metschnikowia pulcherrima and related pulcherrimin-producing yeasts: fuzzy species boundaries and complex antimicrobial antagonism. Microorganisms 8:1029. doi: 10.3390/microorganisms8071029
Sipiczki, M. (2022). Taxonomic revision of the pulcherrima clade of Metschnikowia (Fungi): merger of species. Taxon 2, 107–123. doi: 10.3390/taxonomy2010009
Sipiczki, M., and Czentye, K. (2024). Reversible stochastic epigenetic-like silencing of the production of pulcherriminic acid in the antimicrobial antagonist Metschnikowia pulcherrima. Sci. Rep. 14:29677. doi: 10.1038/s41598-024-80436-9
Sipiczki, M., Czentye, K., and Kállai, Z. (2024). High intragenomic, intergenomic, and phenotypic diversity in pulcherrimin-producing Metschnikowia yeasts indicates a special mode of genome evolution. Sci. Rep. 14:10521. doi: 10.1038/s41598-024-61335-5
Smith, I. M., Baker, A., Arneborg, N., and Jespersen, L. (2015). Non-Saccharomyces yeasts protect against epithelial cell barrier disruption induced by Salmonella enterica subsp. enterica serovar typhimurium. Lett. Appl. Microbiol. 61, 491–497. doi: 10.1111/lam.12481
Snyman, C., Theron, L. W., and Divol, B. (2019). The expression, secretion and activity of the aspartic protease MpAPr1 in Metschnikowia pulcherrima IWBT Y1123. J. Ind. Microbiol. Biotechnol. 46, 1733–1743. doi: 10.1007/s10295-019-02227-w
Sorrentino, A., Boscaino, F., Cozzolino, R., Volpe, M. G., Ionata, E., and La Cara, F. (2012). Autochthonous fermentation starters for the production of Aglianico wines. CET 27, 211–216. doi: 10.3303/CET1227036
Staniszewski, A., and Kordowska-Wiater, M. (2023). Probiotic yeasts and how to find them polish wines of spontaneous fermentation as source for potentially probiotic yeasts. Foods 12:3392. doi: 10.3390/foods12183392
Steglińska, A., Kołtuniak, A., Berłowska, J., Czyżowska, A., Szulc, J., Cieciura-Włoch, W., et al. (2022). Metschnikowia pulcherrima as a biocontrol agent against potato (Solanum tuberosum) pathogens. Agronomy 12:2546. doi: 10.3390/agronomy12102546
Steglińska, A., Sulyok, M., Janas, R., Grzesik, M., Liszkowska, W., Kręgiel, D., et al. (2023). Metabolite formation by fungal pathogens of potatoes (Solanum tuberosum L.) in the presence of bioprotective agents. Int. J. Environ. Res. Public Health 20:5221. doi: 10.3390/ijerph20065221
Tatay-Núñez, J., Albi-Puig, J., Garrigós, V., Orejas-Suáre, z. M., Matallana, E., and Aranda, A. (2024). Isolation of local strains of the yeast Metschnikowia for biocontrol and lipid production purposes. World J. Microbiol. Biotechnol. 40:88. doi: 10.1007/s11274-024-03918-y
Thak, E. J., Yoo, S. J., Moon, H. Y., and Hyun, H. A. (2020). Yeast synthetic biology for designed cell factories producing secretory recombinant proteins. FEMS Yeast Res. 20, 1–17. doi: 10.1093/femsyr/foaa009
Theron, L. W., Bely, M., and Divol, B. (2017). Characterisation of the enzymatic properties of MpAPr1, an aspartic protease secreted by the wine yeast Metschnikowia pulcherrima. J. Sci. Food Agric. 97, 3584–3593. doi: 10.1002/jsfa.8217
Tian, Y. Q., Li, W., Jiang, Z. T., Jing, M. M., and Shao, Y. Z. (2017). The preservation effect of Metschnikowia pulcherrima yeast on anthracnose of postharvest mango fruits and the possible mechanism. Food Sci. Biotechnol. 27, 95–105. doi: 10.1007/s10068-017-0213-0
Torres-Díaz, L. L., Sáenz de Urturi, I., Iribarren, M., Murillo-Peña, R., Marín-San Román, S., González-Lázaro, M., et al. (2025). Evaluation of the potential of Metschnikowia pulcherrima to reduce SO2 in winemaking: impact on wine phenolic compounds and their bottle evolution. Eur. Food Res. Technol. 251, 705–718. doi: 10.1007/s00217-025-04660-x
Troiano, E., Larini, I., Binati, R. L., Gatto, V., Torriani, S., Buzzini, P., et al. (2023). Finding a correct species assignment for a Metschnikowia strain: insights from the genome sequencing of strain DBT012. FEMS Yeast Res. 23:foad024. doi: 10.1093/femsyr/foad024
Tullio, V. (2022). Yeast genomics and its applications in biotechnological processes: what is our present and near future? J. Fungi (Basel) 8:752. doi: 10.3390/jof8070752
Varela, C., Bartel, C., Espinase Nandorfy, D. D., Bilogrevic, E., Tran, T., Heinrich, A., et al. (2021). Volatile aroma composition and sensory profile of shiraz and cabernet sauvignon wines produced with novel Metschnikowia pulcherrima yeast starter cultures. Aust. J. Grape Wine Res. 27, 406–418. doi: 10.1111/ajgw.12484
Varela, C., Sengler, F., Solomon, M., and Curtin, C. (2016). Volatile flavour profile of reduced alcohol wines fermented with the non-conventional yeast species Metschnikowia pulcherrima and Saccharomyces uvarum. Food Chem. 209, 57–64. doi: 10.1016/j.foodchem.2016.04.024
Vepštaitė-Monstavičė, I., Lukša-Žebelovič, J., Apšegaitė, V., Mozūraitis, R., Lisicinas, R., Stanevičienė, R., et al. (2025). Profiles of killer systems and volatile organic compounds of rowanberry and rosehip-inhabiting yeasts substantiate implications for biocontrol. Foods 14:288. doi: 10.3390/foods14020288
Vicente, J., Ruiz, J., Belda, I., Benito-Vázquez, I., Marquina, D., Calderón, F., et al. (2020). The genus Metschnikowia in enology. Microorganisms 8:1038. doi: 10.3390/microorganisms8071038
Vong, W. C., and Liu, S. Q. (2017). Changes in volatile profile of soybean residue (okara) upon solid-state fermentation by yeasts. J. Sci. Food Agric. 97, 135–143. doi: 10.1002/jsfa.7700
Wang, S., Ruan, C., Yi, L., Deng, L., Yao, S., and Zeng, K. (2020). Biocontrol ability and action mechanism of Metschnikowia citriensis against Geotrichum citri-aurantii causing sour rot of postharvest citrus fruit. Food Microbiol. 87:103375. doi: 10.1016/j.fm.2019.103375
Wang, S., Zhang, H., Qi, T., Deng, L., Yi, L., and Zeng, K. (2022). Influence of arginine on the biocontrol efficiency of Metschnikowia citriensis against Geotrichum citri-aurantii causing sour rot of postharvest citrus fruit. Food Microbiol. 101:103888. doi: 10.1016/j.fm.2021.103888
Wu, Y., Li, Y., Liang, H., Zhang, S., Lin, X., and Ji, C. (2025). Enhancing cider quality through co-fermentation with acid protease and esterase-producing Metschnikowia species and Saccharomyces cerevisiae. J. Sci. Food Agric. 105, 1003–1011. doi: 10.1002/jsfa.13891
Yuivar, Y., Alcaino, J., Cifuentes, V., and Baeza, M. (2019). Characterization of gelatinase produced by antarctic Mrakia sp. J. Basic Microbiol. 59, 846–852. doi: 10.1002/jobm.201900126
Zhang, H., Wang, S., Yi, L., and Zeng, K. (2023). Tryptophan enhances biocontrol efficacy of Metschnikowia citriensis FL01 against postharvest fungal diseases of citrus fruit by increasing pulcherriminic acid production. Int. J. Food Microbiol. 386:110013. doi: 10.1016/j.ijfoodmicro.2022.110013
Zhang, G., Zabed, H. M., An, Y., Yun, J., Huang, J., Zhang, Y., et al. (2022). Biocatalytic conversion of a lactose-rich dairy waste into D-tagatose, D-arabitol and galactitol using sequential whole cell and fermentation technologies. Bioresour. Technol. 358:127422. doi: 10.1016/j.biortech.2022.127422
Zhou, L., Santomauro, F., Fan, J., Macquarrie, D., Clark, J., Chuck, C. J., et al. (2017). Fast microwave-assisted acidolysis: a new biorefinery approach for the zero-waste utilisation of lignocellulosic biomass to produce high quality lignin and fermentable saccharides. Faraday Discuss. 202, 351–370. doi: 10.1039/c7fd00102a
Keywords: Metschnikowia, green technology, agro-industrial waste, benefits, limitations
Citation: Liu J, Rygała A, Zhang B and Kręgiel D (2025) Potential of Metschnikowia yeasts in green applications: a review. Front. Microbiol. 16:1652494. doi: 10.3389/fmicb.2025.1652494
Edited by:
Laurent Dufossé, Université de la Réunion, FranceReviewed by:
Andrea Trochine, CONICET Patagonia Norte, ArgentinaCopyright © 2025 Liu, Rygała, Zhang and Kręgiel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiayue Liu, amlheXVlLmxpdUBkb2t0LnAubG9kei5wbA==
†ORCID: Jiayue Liu, orcid.org/0000-0002-3046-6501
Anna Rygała, orcid.org/0009-0001-8525-0185
Bolin Zhang, orcid.org/0000-0002-6746-493X
Dorota Kregiel, orcid.org/0000-0002-4006-6464
 Jiayue Liu
Jiayue Liu Anna Rygała
Anna Rygała Bolin Zhang
Bolin Zhang Dorota Kręgiel
Dorota Kręgiel