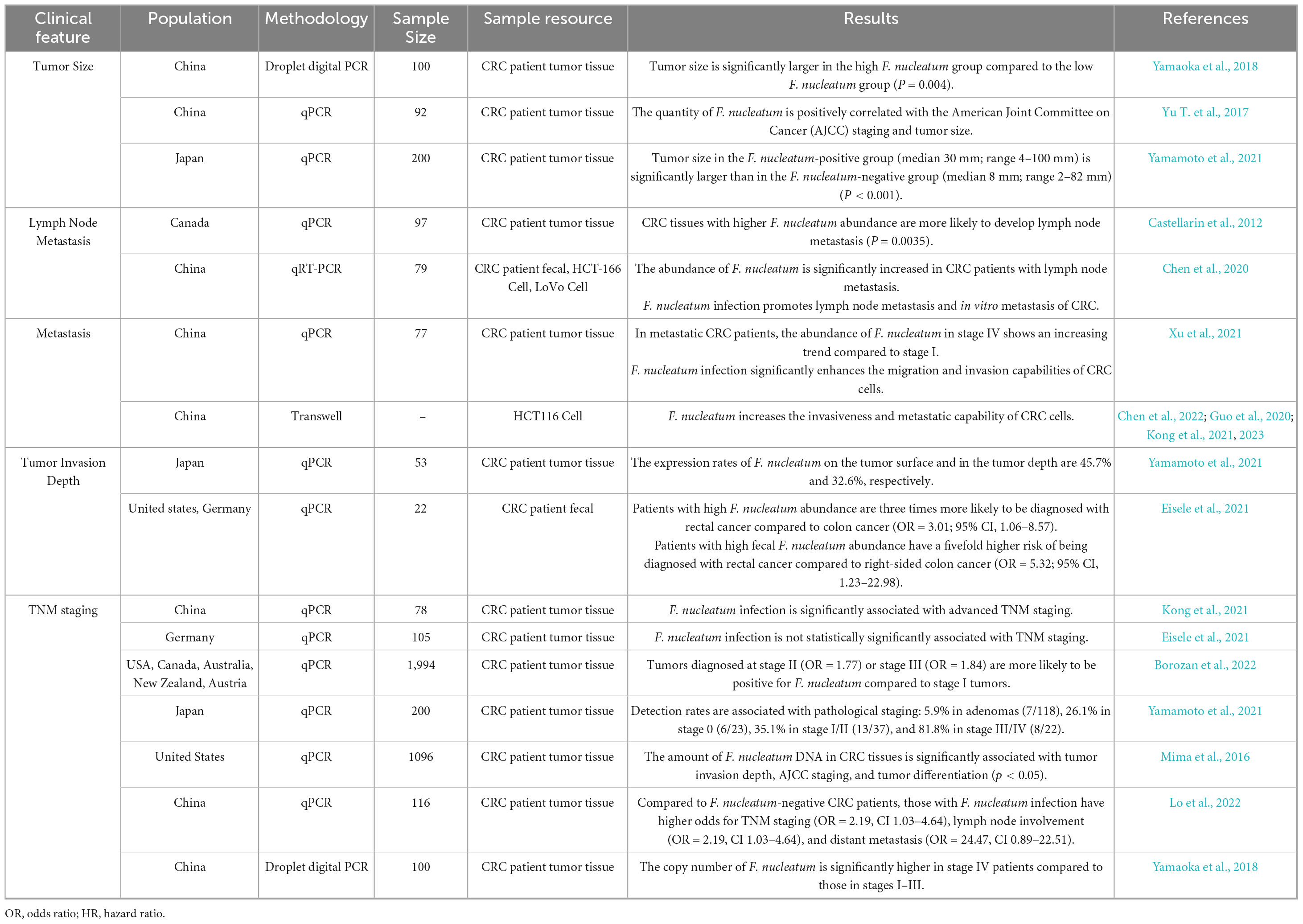
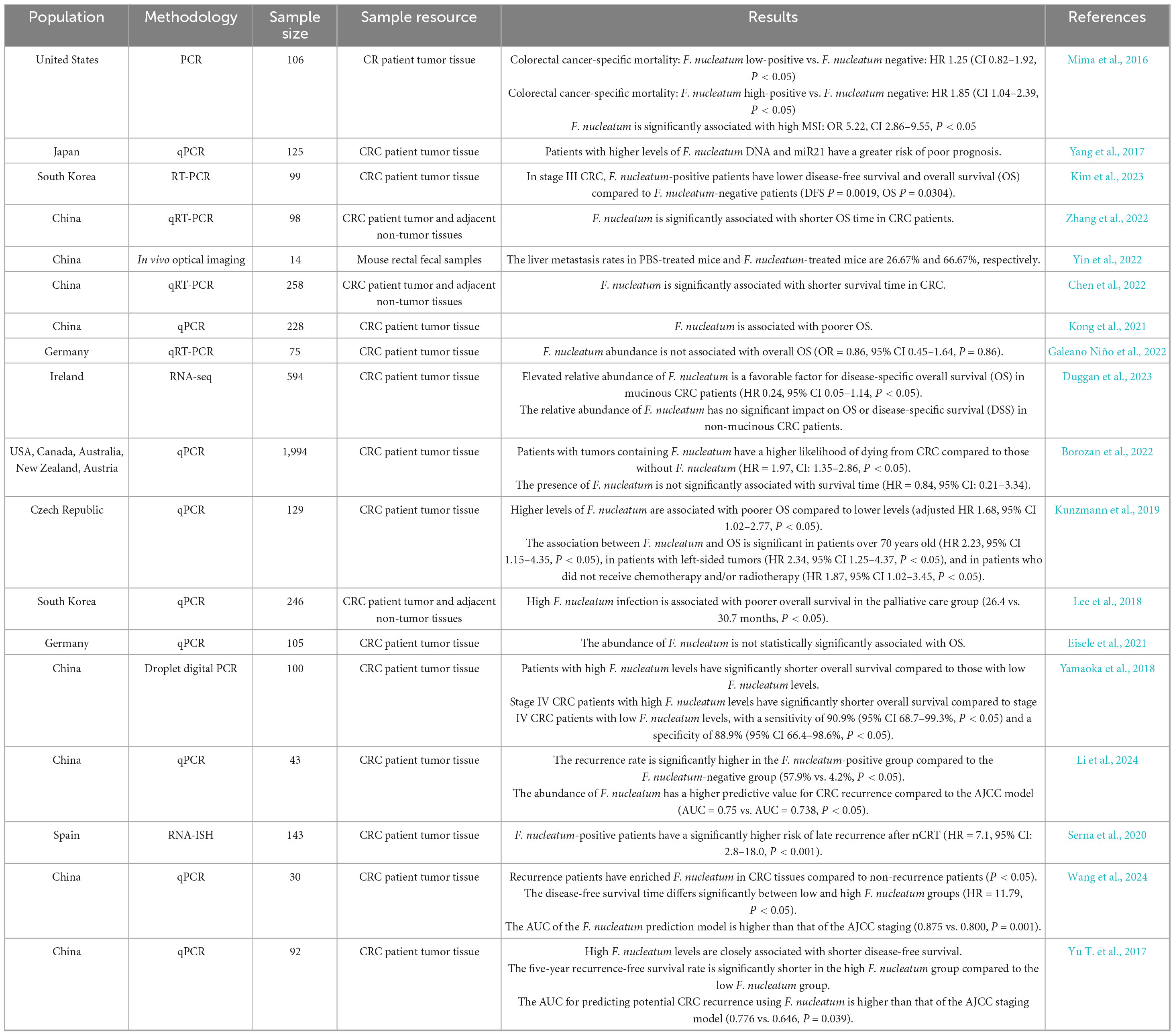
- 1Department of Clinical Laboratory, Chongqing University Jiangjin Hospital, Chongqing, China
- 2Department of Clinical Laboratory, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 3Department of Clinical Laboratory, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 4Chongqing Mental Health Center, Chongqing, China
Colorectal cancer (CRC), as a globally prevalent malignant tumor, relies on in-depth analysis of tumor microenvironment regulation mechanisms for precision diagnosis and treatment. Fusobacterium nucleatum (F. nucleatum), a key carcinogenic bacterium, has been revealed in recent studies to play multidimensional roles in CRC initiation, progression, and metastasis. This review systematically summarizes the progress of Fn applications in CRC full-cycle management: (1) In the diagnostic field, Fn detection technology based on fecal samples has developed into a new non-invasive screening strategy. Cohort studies show its diagnostic performance (AUC 0.82–0.89), with significant correlations to tumor stage (III/IV stage OR = 2.87), lymph node metastasis (HR = 1.94), and reduced 5-year survival rate (35% vs. 62%); (2) For therapeutic monitoring, dynamic Fn load changes can predict chemotherapy (OR = 0.63) and immunotherapy responses (PFS extended by 2.1 months); (3) In prognostic evaluation, metagenomic analysis shows that high Fn abundance is closely related to TNM staging (C-index 0.81 vs. 0.69) and recurrence risk (AUC = 0.88). Notably, a nomogram model integrating Fn biomarkers can improve the predictive accuracy of the traditional TNM staging system by 17.3%. Although existing evidence supports the clinical translational value of Fn, its standardized detection protocols, threshold setting, and targeted intervention strategies (such as antibiotic therapy and phage therapy) still require validation through multi-center prospective studies. This review provides evidence-based medical evidence for the application of Fn in CRC precision medicine by integrating multi-omics data.
1 Introduction
1.1 Epidemiology of colorectal cancer and disease background related to F. nucleatum
Colorectal cancer (CRC) is the third most common malignant tumor worldwide, with over one million newly diagnosed cases annually and a rising trend (Figure 1A). Its incidence is significantly higher in China, Europe, and North America compared to the global average (Figure 1D). Disease risk increases sharply with age, with a significant rise in incidence after 50–55 years and mortality after 45–50 years (Figure 1C). Due to improved living standards and lifestyle changes in China, CRC incidence and mortality continue to increase (Figure 1B). According to 2022 Chinese cancer statistics, CRC ranks second in incidence and fourth in mortality among malignant tumors, with approximately 517,100 new cases and 240,000 deaths annually (Han et al., 2024). F. nucleatum is a core microorganism in oral dental plaque (Bolstad et al., 1996; Borisy and Valm, 2021), widely distributed in the human digestive system, reproductive system, and other sites. It is associated with various diseases, including periodontitis, pancreatitis, CRC, pelvic inflammatory disease, and adverse pregnancy outcomes (Han, 2015; Swidsinski et al., 2011; Xu and Han, 2022). Multiple studies indicate significantly elevated F. nucleatum abundance in CRC tissue and fecal samples, suggesting its close association with CRC development (Castellarin et al., 2012; Wong and Yu, 2019). The detection rate of this bacterium in CRC tissues is much higher than in normal tissues, indicating its potential as a diagnostic biomarker or therapeutic target.
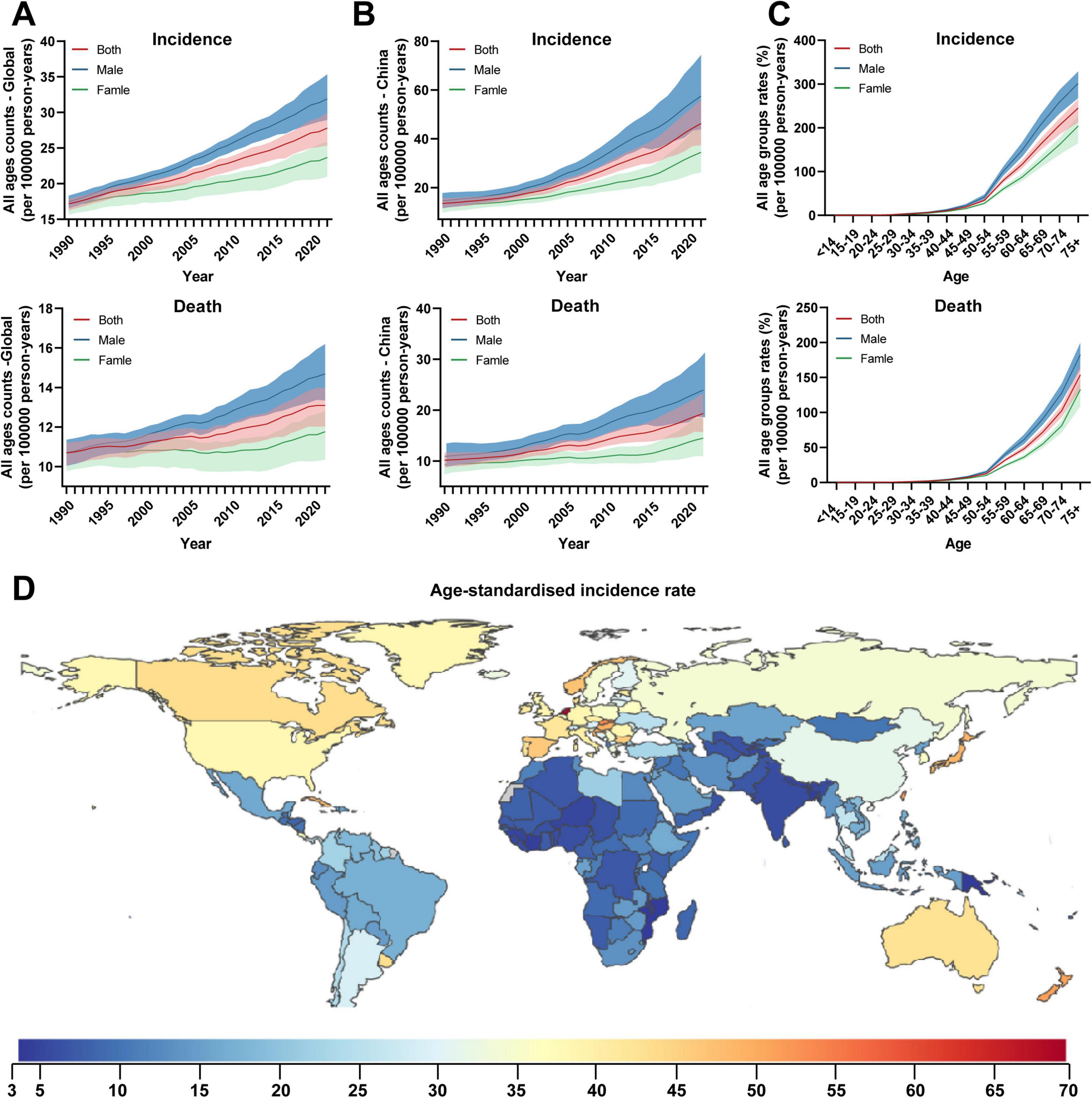
Figure 1. Global temporal patterns of colorectal cancer burden, 1990–2021. (A) All-age counts. The global annual number of newly diagnosed CRC cases exceeds one million and shows an upward trend; (B) Age-standardized rates. In China, the number of newly diagnosed cases reached 517,100 in 2022; (C) All age groups rates in 2021. (D) Geographical distribution of age-standardized rates of colorectal cancer in 2021. Data source: Global Burden of Diseases, Injuries, and Risk Factors Study 2024 (Institute for Health Metrics and Evaluation, 2024).
1.2 Core role and clinical significance of F. nucleatum in colorectal cancer
As a Gram-negative anaerobic bacterium, F. nucleatum plays a critical role in CRC initiation, recurrence, metastasis, and drug resistance. It participates in CRC progression through mechanisms such as activating inflammatory responses, promoting tumor cell proliferation and invasion, and inducing resistance to chemotherapy and immunotherapy. Systematic studies on F. nucleatum abundance in intestinal tissues and related molecular expressions are valuable for elucidating CRC pathogenesis, optimizing diagnostic and therapeutic strategies, and assessing prognosis. Existing reviews often focus on basic research and lack clinical guidance. This study integrates the association, pathogenic mechanisms, and clinical applications of F. nucleatum and CRC, constructing a review framework with both theoretical depth and clinical practicality to inform CRC precision medicine. The study emphasizes the following directions: (1) exploring interactions between F. nucleatum, intestinal microbiota, and host factors; (2) developing targeted therapeutic strategies against F. nucleatum; (3) promoting standardization of detection methods and normalization of data analysis to ensure scientific rigor and reproducibility. These efforts will provide new insights for personalized CRC treatment and ultimately improve patient survival and prognosis.
1.3 Literature search strategy
To systematically review research progress on F. nucleatum in colorectal cancer, this study employed a structured literature search approach. Databases searched included PubMed, Embase, Web of Science, and China National Knowledge Infrastructure (CNKI), with a search period from 1 January 2010, to 30 June 2024. A combined keyword strategy was used: (“Fusobacterium nucleatum” OR “F. nucleatum”) AND (“colorectal cancer” OR “CRC”) AND (“diagnosis” OR “therapy” OR “prognosis”). Inclusion criteria were as follows: (1) original research papers; (2) provision of explicit detection methods or clinical data; (3) sample size ≥ 50 cases. Exclusion criteria were as follows: (1) reviews, conference abstracts, or case reports; (2) non-human studies; (3) duplicate published data. Finally, the included literature underwent independent screening and cross-validation by two researchers.
2 Biological characteristics and functions of F. nucleatum in the gut
2.1 Classification and phylogeny of F. nucleatum
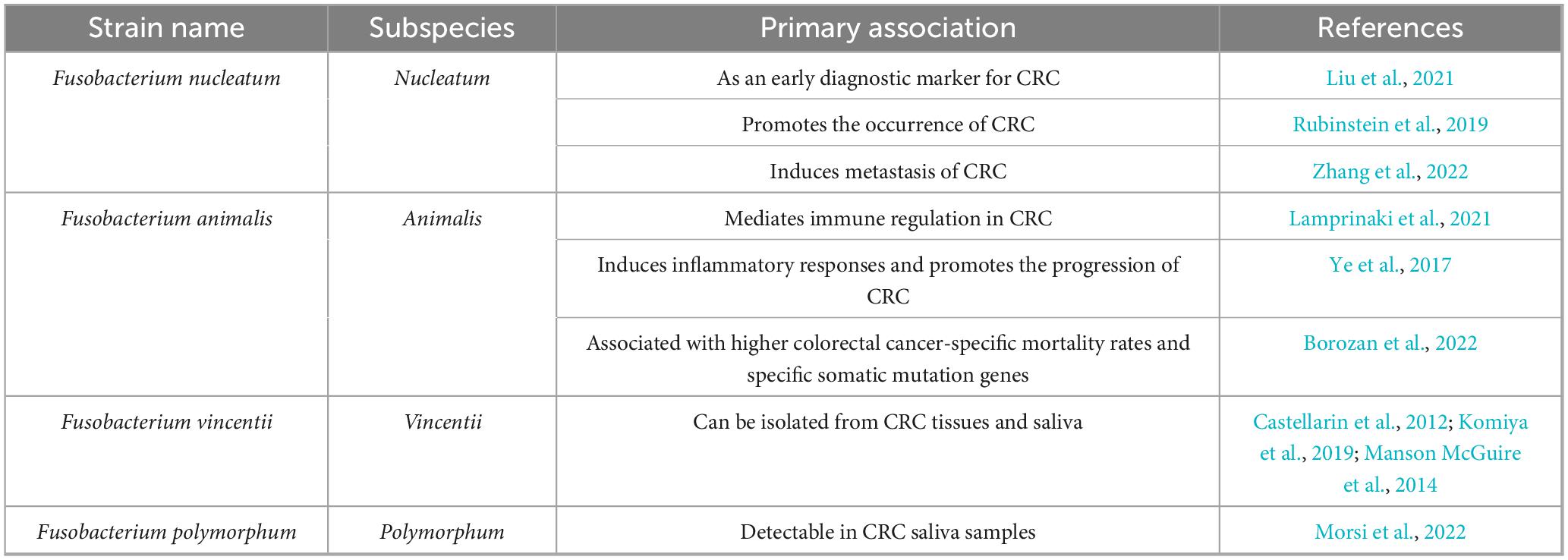
F. nucleatum belongs to the family Fusobacteriaceae. This bacterium is anaerobic but can still grow in environments with oxygen levels up to 6% (Bolstad et al., 1996). Early observations in the human oral cavity identified fusiform microorganisms, and the Fusobacterium genus was isolated based on sensitivity to dyes and antibiotics (Baird-Parker, 1957). Active strains that ferment amino acids, produce acetate and butyrate, and exhibit limited sugar-degrading activity are classified as F. nucleatum (Yu et al., 2022). Subsequent studies on 16S genomics have suggested that the common ancestor of Fusobacterium was Leptotrichia, which underwent adaptive radiation during evolution, diverging into three main lineages and five major clades (Manson McGuire et al., 2014). Based on 16S rRNA gene sequence analysis, it can be further classified into four subspecies: nucleatum, animalis, vincentii (including fusiforme), and polymorphum (Nie et al., 2015). These subspecies have been found in clinical tissues and fecal samples of patients with CRC, with a significant increase in F. nucleatum (Bi et al., 2022; Table 1).
2.2 Ecological niche and symbiotic relationships of F. nucleatum
In the intestinal tract, F. nucleatum forms complex interactions with host microbiota, influencing its colonization and pathogenicity, thereby affecting gastrointestinal immunity and metabolism. When microbial diversity is high and butyrate-producing bacteria (e.g., Faecalibacterium, Roseburia) are abundant, F. nucleatum struggles to obtain sufficient nutrients and adhesion sites. However, microbiota dysbiosis caused by antibiotics, high-fat diets, or inflammatory bowel disease (IBD) weakens the “colonization resistance” of commensal bacteria, providing a window for F. nucleatum colonization (Dewan et al., 2025). Evidence from the oral-intestinal axis indicates that F. nucleatum can form “corncob-like” co-aggregates with oral resident bacteria Streptococcus sanguinis, utilizing its filamentous structure to more easily penetrate the mucus layer and colonize colorectal mucosa (Sun et al., 2019). F. nucleatum can exploit ornithine (ArcD-dependent) excreted by Streptococcus gordonii as a nitrogen source, accelerating its own proliferation (Sun et al., 2019). F. nucleatum acts as a bridge between early and late colonizers in dental plaque by forming biofilms (Borisy and Valm, 2021). Signaling molecules from P. gingivalis can accelerate F. nucleatum biofilm formation (Yamaguchi-Kuroda et al., 2023). In biofilm form, F. nucleatum exhibits enhanced virulence and invasiveness, enabling it to invade multi-layered epithelial collagen matrices and survive under aerobic conditions (Gursoy et al., 2010), thereby disrupting gastrointestinal immune and metabolic homeostasis.
2.3 Pathogenic mechanisms of F. nucleatum in colorectal cancer
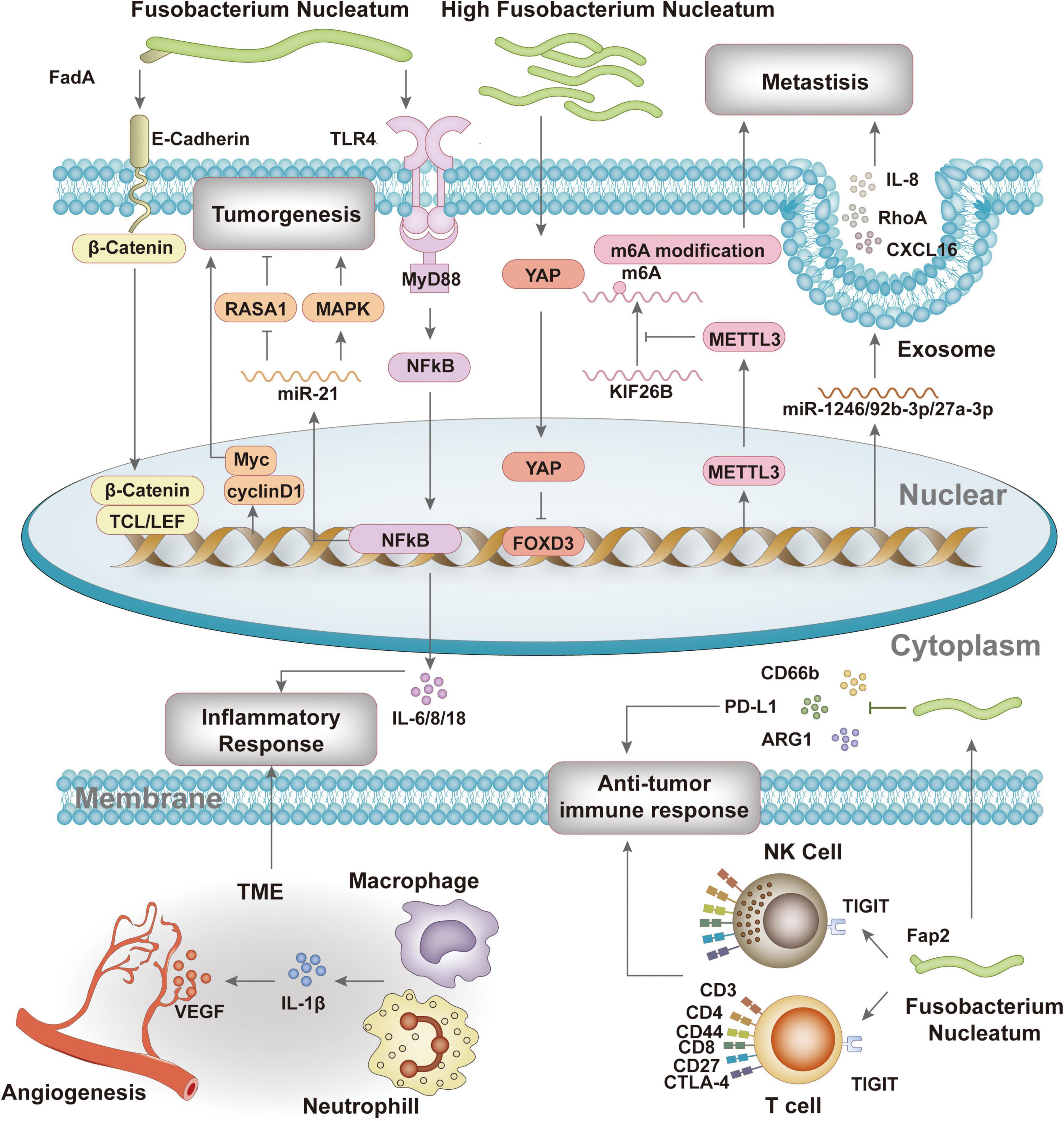
Studies have confirmed that F. nucleatum participates in the formation, progression, and treatment response of colorectal cancer through a series of complex mechanisms (Figure 2).
Firstly, F. nucleatum binds to colorectal epithelial cells via adhesin FadA and E-cadherin, promoting tumor cell proliferation and invasion (Manson McGuire et al., 2014; Rubinstein et al., 2013). Gal-Gal-NAc overexpression in colorectal cancer enables F. nucleatum recognition and binding, leading to its accumulation in tumor tissues (Abed et al., 2016). F. nucleatum abundance changes are significant in colorectal cancer patients, highlighting its potential as a biomarker for screening and diagnosis (Kwong et al., 2018; Liang et al., 2020; Lin et al., 2022; Yu J. et al., 2017). It also reduces m6A modification in CRC cells, enhancing invasiveness (Chen et al., 2022), shifts central carbon metabolism in tumor cells, and promotes CRC cell invasion (Ternes et al., 2022). F. nucleatum activates TLR4 signaling, leading to NF-κB activation and increased miR-21 expression, which promotes tumor metastasis (Yang et al., 2017).
Secondly, F. nucleatum activates inflammatory responses and immune evasion, inhibiting host immune responses and promoting tumor development. It induces pro-inflammatory factors such as NF-κB, IL-6, and IL-8 (Queen et al., 2021; Rubinstein et al., 2013), increases inflammation-related gene expression (Galeano Niño et al., 2022), and exists in immunosuppressive microecological niches, reducing CD4 and CD8 levels while upregulating CD66b+, ARG1, and CTLA4 (Galeano Niño et al., 2022). F. nucleatum’s Fap2 protein interacts with the inhibitory receptor TIGIT on NK and T cells (Gur et al., 2015), and upregulates PD-L1 expression in CRC cell lines, promoting immune evasion (Galeano Niño et al., 2022; Gao et al., 2023).
Additionally, F. nucleatum influences the tumor microenvironment by regulating angiogenesis and metastasis. Inflammatory responses induce IL-1β production, which activates endothelial cells to produce pro-angiogenic factors, promoting angiogenesis and tumor progression (Jagielska et al., 2012; Nakao et al., 2005). F. nucleatum alters miRNA and chemokine expression in host cells, delivered via exosomes, increasing cell migration and tumor metastasis (Guo et al., 2020). It also upregulates KRT7-AS, regulating CRC cell lymph node migration (Chen et al., 2020, 2022). Regarding treatment, F. nucleatum levels correlate with colorectal cancer treatment response. Increased F. nucleatum levels are associated with improved response to PD-L1 blockade therapy, possibly by activating STING signaling and increasing PD-L1 expression (Gao et al., 2021). However, F. nucleatum may also impair CD8+ T cell immunity, reducing sensitivity to anti-PD-1 mAb and increasing immunotherapy resistance (Jiang et al., 2023). It initiates protective autophagy via the TLR4 pathway, enhancing chemotherapy resistance (Zheng et al., 2019).
F. nucleatum promotes CRC progression through dual mechanisms: (1) F. nucleatum mediates signaling pathways, including the FadA/E-cadherin/β-catenin regulatory axis and METTL3/m6A modification, to regulate CRC proliferation and invasion capabilities, thereby directly influencing CRC progression; (2) F. nucleatum induces the production of pro-inflammatory factors such as NF-κB, IL-6, and IL-8 while modulating PD-L1 expression to affect inflammatory and anti-tumor immune responses, thereby indirectly regulating CRC progression.
2.4 Genetic research progress and tool development of F. nucleatum
Recent advances in genetic technologies have significantly deepened the understanding of F. nucleatum-CRC association mechanisms. At the genomic level, whole-genome sequencing and functional annotation revealed key differences among subspecies: for example, the F. animalis genome harbors a unique fnp gene cluster closely associated with its tumor metastasis-promoting capacity, while the nucleatum subspecies (F. nucleatum) carries more adhesin genes related to oral colonization. Comparative genomic analysis further identified differentially expressed genes among subspecies (e.g., fadA adhesin, gal-galNAc receptor) that directly influence their pathogenic potential in the intestine (Ma et al., 2023; Queen et al., 2021). At the transcriptomic level, RNA sequencing has constructed high-resolution global RNA profiles of Fn subspecies during early, mid-exponential, and early stationary growth phases, aiding in elucidating functional characteristics at different disease progression stages (Ponath et al., 2021).
In multi-omics integration, combining transcriptomics and metabolomics revealed that Fn promotes cell proliferation by modulating amino acid biosynthesis, central carbon metabolism, protein digestion/absorption, and other metabolic pathways in CRC cells (Wu et al., 2023). At the genetic engineering level, tools including CRISPR interference (CRISPRi) systems, suicide plasmid-based gene inactivation systems, replicative plasmid-based gene expression control systems, and transposon-based random mutagenesis systems have become critical strategies for studying Fn pathogenicity. These tools enabled targeted silencing of key virulence genes (e.g., fadA, gal-galNAc), facilitating validation of their roles in tumor cell adhesion and invasion (Guan et al., 2025; Zhou et al., 2024). The establishment of these genetic tools not only clarified subspecies-specific pathogenic mechanisms (e.g., F. animalis exhibits significantly stronger metastasis-promoting capacity than F. nucleatum) but also advanced the development of precision intervention strategies against F. nucleatum, such as subspecies-specific antigen-based vaccine design and inhibitors targeting key metabolic pathways. Integrating multi-omics technologies with organoid models holds promise for further dissecting the dynamic microbiota-host interaction network, providing novel targets for CRC precision medicine.
3 Application of F. nucleatum in the diagnosis of colorectal cancer
3.1 Quantitative and localization analysis of F. nucleatum
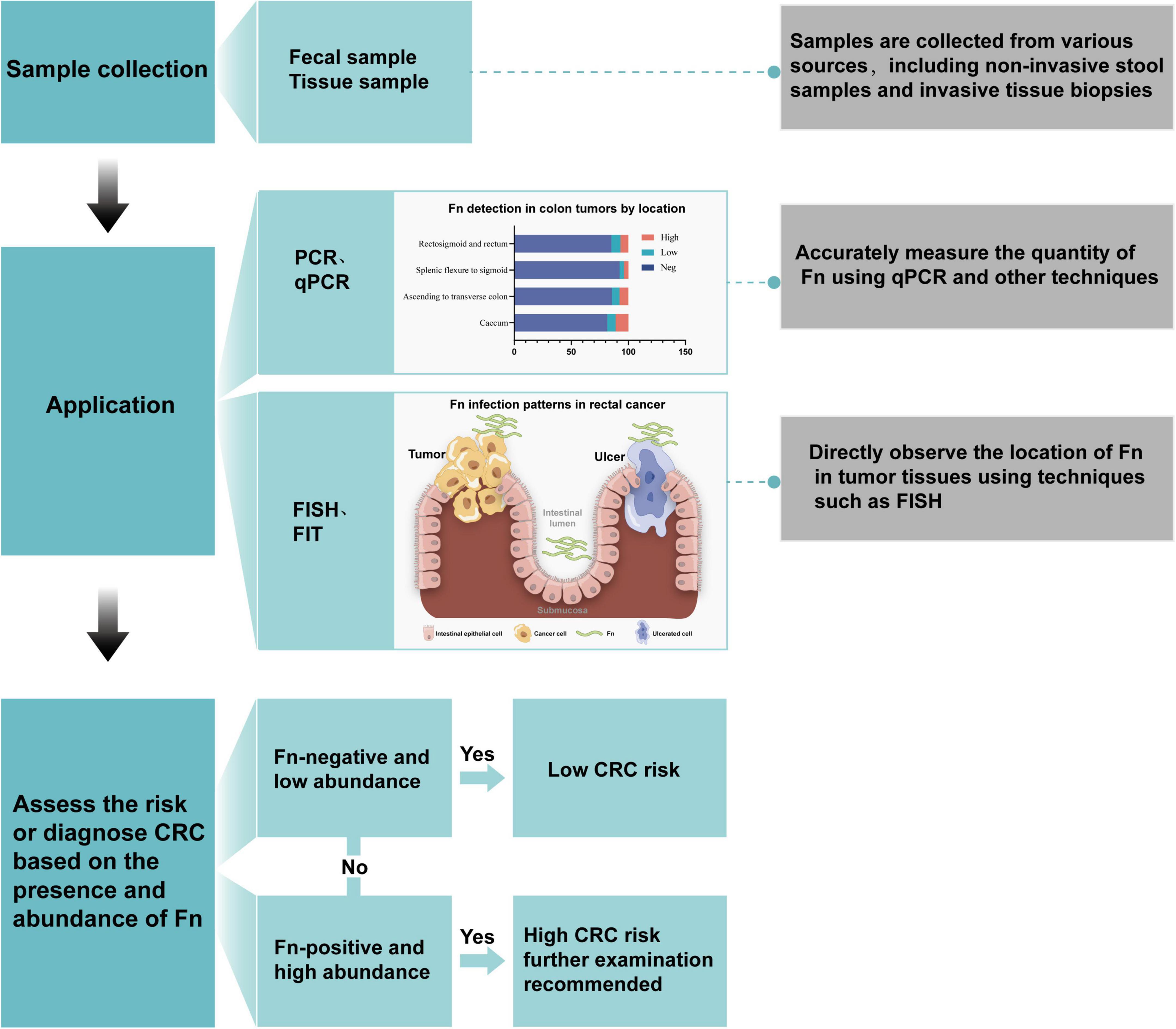
Quantitative and localization analysis of F. nucleatum is a significant direction in current colorectal cancer research. It aims to deepen the understanding of its role in the occurrence and development of colorectal cancer and may also provide new strategies for future diagnosis and treatment. Currently, commonly used methods for detecting F. nucleatum include culture, PCR, qPCR, FISH, FIT, etc. PCR can determine the presence and abundance of F. nucleatum by detecting its specific genes or 16S rRNA sequences. The diagnostic workflow consists of three stages: (1) sample preprocessing; (2) quantitative PCR (qPCR) using FadA gene-specific primers; and (3) bioinformatics analysis. The application process is shown in Figure 3.
3.1.1 Culture
Isolation and identification of F. nucleatum from clinical samples is a reliable diagnostic method that provides insight into the pathogen. However, routine detection through culture is challenging due to difficulties in sample transportation and culturing, as well as the low abundance of F. nucleatum in the intestinal tract and interference from other bacterial flora. We developed an IMB assay for direct isolation and culture of F. nucleatum from human feces, with a sensitivity of 103 CFU mL–1, but it is challenging and suitable only for experienced microbiologists. To address this, we further developed a selective chromogenic solid medium that promotes F. nucleatum growth while inhibiting other bacteria and facilitates identification through color differences. This method improves the positive rate of isolation and culture in clinical specimens.
3.1.2 Serological test
A serological test can detect F. nucleatum-specific antibodies in serum, saliva, and urine. It is inexpensive, non-invasive, and convenient to detect IgG antibodies using laboratory-based serology, and is especially suitable for large-scale epidemic research. The presence of specific antibodies in the blood can persist for several weeks following F. nucleatum infection. Hence, a positive serum test for antibodies cannot serve as the basis for an ongoing infection. In conclusion, serology is not recommended as a routine method for diagnosing F. nucleatum infection, but it can be helpful when combined with other methods.
3.1.3 Fecal immunochemical test
The fecal immunochemical test (FIT) is non-invasive, rapid, and convenient for sampling. The stability of the fecal bacterial composition can last up to 144 h, with low levels of bacterial contamination. Furthermore, bacterial biomarkers can be stably detected in FIT, making it suitable for CRC screening (Grobbee et al., 2020). FIT performs well in detecting colonic lesions in symptomatic patients but has limited overall diagnostic efficacy (Liang et al., 2021). Combining FIT with qPCR or sDNA for the detection of other biomarkers can significantly improve the sensitivity of F. nucleatum detection (Liang et al., 2021; Wong et al., 2017).
3.1.4 Molecular methods
Quantitative analysis, primarily using qPCR and high-throughput sequencing, measures the abundance of F. nucleatum in CRC tissues or fecal samples. These techniques have revealed that F. nucleatum levels are significantly higher in CRC patients compared to healthy individuals and correlate with tumor malignancy, staging, and prognosis (Guo et al., 2020; Yang et al., 2017). Quantitative analysis can also predict treatment response in CRC patients (Lee et al., 2021).
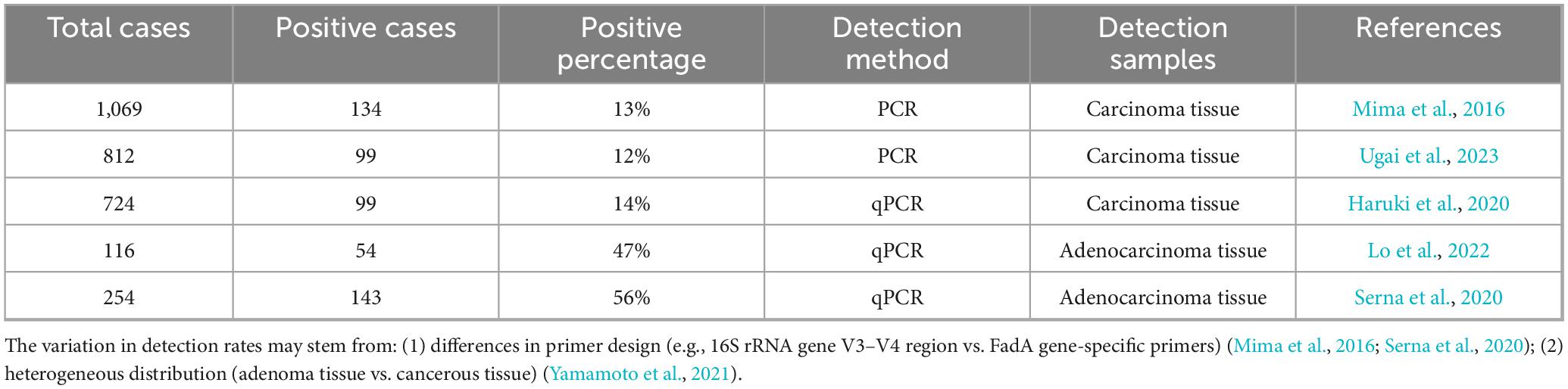
Detection rates of F. nucleatum in CRC tissues vary across studies (Table 2). The variability in detection rates of colonic adenomas and colorectal cancers may stem from differences in microbiota colonization sites and primer specificity. During colorectal cancer progression, F. nucleatum exhibits heterogeneous distribution, with significantly higher abundance in superficial regions compared to deep regions, leading to differences in detection rates between colonic adenomas and CRC tissues (Yamamoto et al., 2021). Additionally, primers targeting the FadA adhesin gene (e.g., those used in Yamamoto et al., 2021) demonstrate 2.3-fold higher sensitivity than universal 16S rRNA primers, explaining discrepancies among studies (OR = 3.82, 95% CI 1.25–11.7). qPCR can also detect F. nucleatum in fecal samples, enabling non-invasive detection (Tunsjø et al., 2019). Fecal metagenomic analysis has identified gene markers for CRC, including two validated by qPCR in an independent CRC patient cohort, highlighting the potential for early-stage CRC diagnosis (Yu J. et al., 2017). In a study on cancer-related fecal microbial markers, F. nucleatum showed a specificity of 76.9%, a sensitivity of 69.2%, and an ROC of 0.737 for predicting CRC (Eklöf et al., 2017).
3.1.5 Fluorescence in situ hybridization
Localization analysis uses immunohistochemistry and FISH to determine the location of F. nucleatum in CRC tissues. FISH can detect F. nucleatum, visualize its interaction with tumor cells, and show if bacteria are adhered to or have invaded cells (Guo et al., 2021; Li et al., 2016). Studies found that F. nucleatum closely interacts with tumor cells and may invade them. Galeano Niño et al. (2022) used RNAscope-FISH to show F. nucleatum within CRC epithelial cells, associated with higher immune cell presence in F. nucleatum-positive samples. F. nucleatum is mainly in the tumor region, with clear contact with tumor cells, potentially affecting CRC cell transcription and gene expression, promoting proliferation and invasion.
However, quantitative and localization analysis results may be affected by sample collection, processing, and detection technique sensitivity/specificity. Researchers must control experimental conditions for accuracy and reliability. Technological advancements are yielding new methods, which will further reveal F. nucleatum’s role in CRC and provide new diagnostic and treatment strategies.
3.2 Correlation and sensitivity of F. nucleatum with colorectal cancer
The high enrichment of F. nucleatum in colorectal cancer tissues suggests its role in cancer development. Its abundance correlates with malignancy grade, clinical stage, and prognosis, with high levels indicating poorer prognosis and higher recurrence risk. F. nucleatum enhances cancer cell stemness, invasion, and metastasis, promoting tumor progression. It also affects the tumor microenvironment and regulates immune cell function and distribution. While the correlation is significant, the specific mechanism is not fully understood (see Table 3 for more information). Future research should explore F. nucleatum’s role in cancer development, its interactions with the tumor microenvironment and immune system, and develop targeted therapeutic strategies. A deeper understanding will provide new insights and methods for colorectal cancer diagnosis and treatment.
4 Application of F. nucleatum in monitoring the therapeutic effect of CRC
4.1 Association between F. nucleatum and chemotherapy and immunotherapy
F. nucleatum plays a pivotal role in CRC chemotherapy. Studies indicate that F. nucleatum significantly promotes the development of chemotherapy resistance in colorectal cancer. F. nucleatum facilitates CRC resistance by activating autophagy and inhibiting pyroptosis and ferroptosis (Li et al., 2024; Wang et al., 2024; Yu T. et al., 2017). Additionally, F. nucleatum promotes the secretion of hsa_circ_0004085 via exosomes, influencing endoplasmic reticulum stress and thereby enhancing chemotherapy resistance in CRC (Hui et al., 2024).
With the widespread application of immunotherapy in colorectal cancer, the association between F. nucleatum burden and treatment efficacy has become a research focus. Recent studies reveal a bidirectional regulatory relationship between F. nucleatum load and response to immune checkpoint inhibitor (ICI) therapy: on one hand, it upregulates PD-L1 expression through m6A modification of IFIT1 or activation of the STING pathway, while recruiting IFN-γ+CD8+ tumor-infiltrating lymphocytes (TILs), thereby enhancing tumor sensitivity to PD-L1 therapy (OR = 3.82, 95% CI 1.25–11.7) (Gao et al., 2021, 2023); on the other hand, succinate produced by the bacterium reduces levels of IFN-γ, TNF-α, and chemokines such as CCL5/CXCL10 in the tumor microenvironment, inhibiting CD8+ T cell infiltration and leading to resistance against anti-PD-1 monoclonal antibodies (HR = 2.14, 95% CI 1.07–4.28) (Jiang et al., 2023). This contradictory phenomenon may relate to differences in F. nucleatum colonization sites, which drive activation of distinct intracellular and extracellular signaling pathways in CRC, resulting in opposing immunotherapeutic regulatory effects.
Given the critical role of F. nucleatum in CRC chemotherapy and immunotherapy resistance, studies have begun exploring F. nucleatum-targeted therapeutic strategies to enhance treatment sensitivity. Research indicates that antibiotic treatment with metronidazole can reduce intestinal F. nucleatum and restore immunotherapy sensitivity (Jiang et al., 2023; Wang et al., 2023). Oral or intravenous administration of azide-modified phage covalently linked to dextran nanoparticles, which inhibit F. nucleatum growth, significantly improves the efficacy of first-line CRC chemotherapy (Zheng et al., 2019). The use of tubercidin I (TBI) simultaneously enhances dendritic cell (DC) vaccine efficacy and suppresses F. nucleatum infection, thereby improving immunotherapy outcomes (Tong et al., 2023). The development of F. nucleatum-targeted therapeutic approaches holds promise for reducing CRC resistance.
4.2 F. nucleatum as an indicator for assessing treatment effectiveness
Beyond its critical role in CRC drug resistance, F. nucleatum also demonstrates clinical potential in monitoring treatment efficacy, post-therapy recurrence rates, and mortality. F. nucleatum is significantly associated with poor response to chemotherapy/immunotherapy, increased post-treatment recurrence, and elevated patient mortality in CRC (Jiang et al., 2023; Wang et al., 2024; Yu T. et al., 2017). Studies indicate that F. nucleatum significantly elevates chemotherapy-specific mortality in colon cancer patients [hazard ratio (HR) = 1.92, 95% confidence interval (CI): 1.07–3.45] (Borozan et al., 2022); F. nucleatum positivity markedly increases recurrence risk in chemotherapy-treated CRC patients (HR = 7.5, 95% CI: 3.0–19.0; P < 0.001) (Serna et al., 2020).
5 Multidimensional application of F. nucleatum in CRC prognostic assessment
F. nucleatum is closely associated with prognostic evaluation in colorectal cancer (CRC). Research shows that F. nucleatum abundance increases progressively with tumor invasion depth (T1–T4) (P < 0.001) (Figure 4A); its levels correlate positively with AJCC staging (C-index = 0.81) (Figure 4B); among patients receiving neoadjuvant chemotherapy, those with high F. nucleatum levels exhibit the lowest relapse-free survival (RFS) (Figures 4C–D). Meta-analysis of multi-center data from China, the US, and Germany revealed that, except for Germany, all cohorts showed significant association between high F. nucleatum abundance and shortened overall survival (OS) (Table 4). The inconsistency in Germany may relate to cohort heterogeneity in demographic-molecular backgrounds and “negative confounding” from treatment modal differences across countries (Kunzmann et al., 2019; Lee et al., 2019). Multivariate analysis demonstrated a gradient elevation in CRC-specific mortality risk for F. nucleatum-low (HR = 1.25, 95% CI: 0.82–1.92) and F. nucleatum-high (HR = 1.58, 95% CI: 1.04–2.39) patients compared to negatives (Mima et al., 2016). Notably, F. nucleatum exhibits higher multivariate HR in left-sided colon cancer, suggesting anatomic site-specific prognostic value (Mouradov et al., 2023). Given its prognostic significance, researchers are exploring the integration of F. nucleatum into prognostic models. A proposed model combining F. nucleatum with four other bacterial species outperforms traditional markers like CEA and lymph node metastasis (baseline C-index = 0.69; C-index + M5 = 0.78) (Huh et al., 2022).
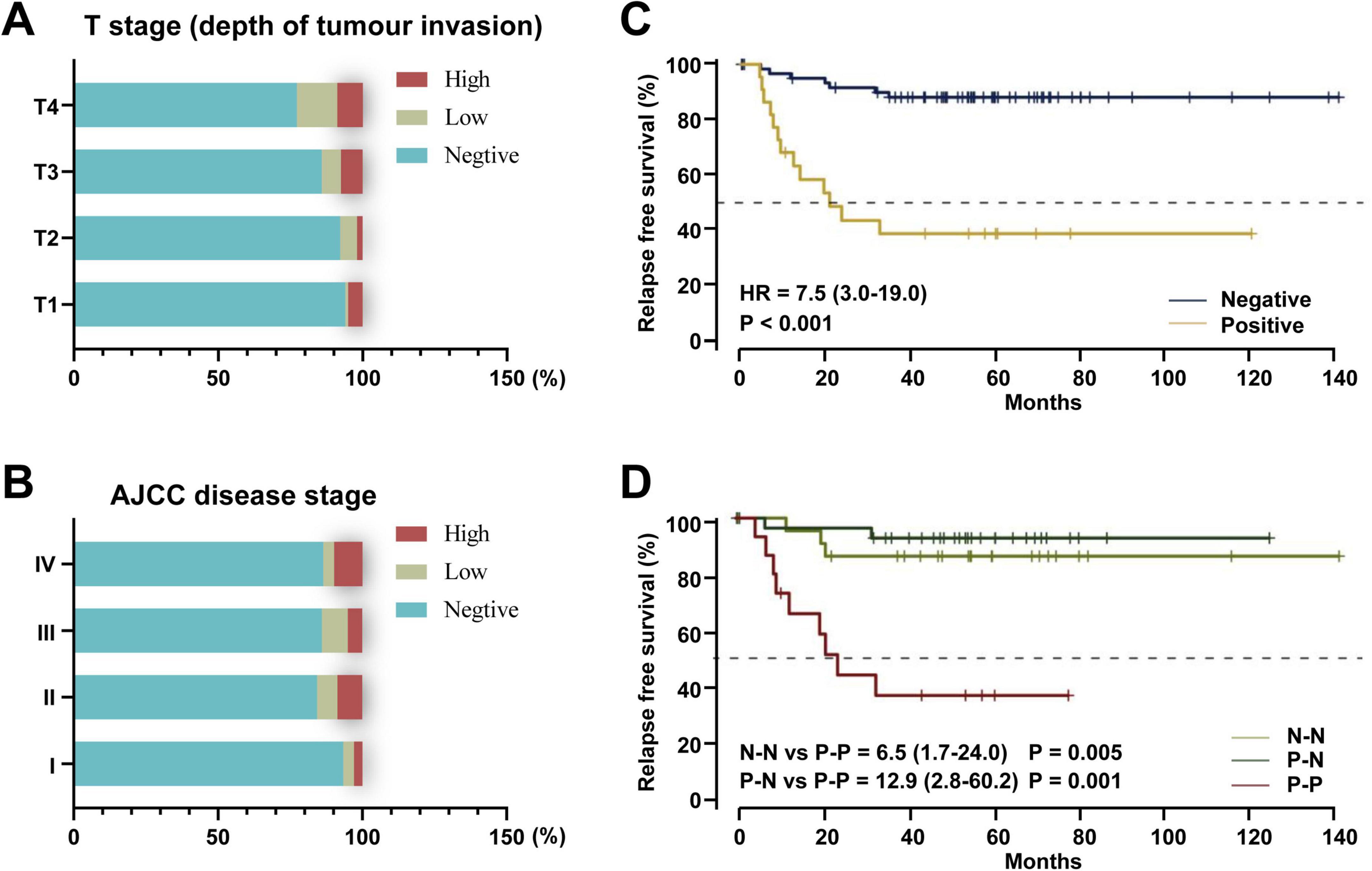
Figure 4. The role of F. nucleatum in the prognostic evaluation of colorectal cancer. (A) F. nucleatum detection by tumor infiltration depth (T1, submucosa; pT2, muscularis propria; T3, subserosa; T4, serosa or other organs). (B) F. nucleatum detection by AJCC disease stage (Mima et al., 2016). (C) Treated cohort by F. nucleatum status in post-nCRT tumor samples. (D) Paired treated cohort grouped according to the shift in F. nucleatum status between pre-nCRT and post-nCRT paired samples. N-N: patients who maintained negative F. nucleatum status before and after treatment. P-N: patients in whom F. nucleatum was negative after treatment. P-P: patients with a positive F. nucleatum status in both samples (Serna et al., 2020). HR: hazard ratio.
6 Conclusion and prospects
The geographic variability in the association between F. nucleatum and overall survival (OS) warrants in-depth investigation. Chinese cohort studies demonstrate a significant association between high F. nucleatum abundance and poor overall survival (OS), whereas German cohorts show no such correlation. This heterogeneity may stem from three factors: (1) microbiota interaction differences due to cohort heterogeneity (F. nucleatum in Chinese populations may form synergistic pathogenic networks with enterotype microbiota through specific subspecies, while protective bacteria like Faecalibacterium prausnitzii in German cohorts may antagonize its pathogenic effects); (2) therapeutic strategy impacts (F. nucleatum-positive patients in Chinese cohorts receive adjuvant chemotherapy at lower rates, while German patients more commonly use targeted therapies that may obscure its prognostic value); (3) methodological differences in detection (Chinese studies predominantly use ddPCR for quantification, while German studies employ qRT-PCR, potentially affecting absolute quantification accuracy). These conflicting findings underscore the need for globally standardized F. nucleatum detection protocols and multi-center prospective studies to validate subspecies-specific prognostic value.
Based on multi-omics evidence, we propose the “F. nucleatum subspecies-specific pathogenic model” hypothesis: different F. nucleatum subspecies exert stage-specific regulation of CRC progression through differentially expressed core virulence factors (e.g., fnp gene clusters and fadA adhesins). Specifically: (1) F. nucleatum subspecies activates β-catenin signaling via the FadA/E-cadherin pathway, participating in CRC initiation and late-stage metastasis; (2) F. animalis subspecies primarily remodels the CRC immune microenvironment by modulating inflammatory and immune responses; (3) F. vincentii and F. polymorphum subspecies, currently detectable mainly in CRC tissues and saliva, lack mechanistic exploration in CRC pathogenesis. This model explains observed heterogeneities in subspecies distribution and prognosis, providing a theoretical basis for developing subspecies-specific diagnostic markers (e.g., 16S–23S ITS sequences for F. animalis) and targeted interventions (e.g., fnp gene cluster inhibitors).
We systematically integrated, for the first time, the multi-dimensional roles of F. nucleatum across the entire CRC diagnostic and therapeutic continuum. F. nucleatum influences CRC development not only by promoting tumor progression, lymph node metastasis, and distant metastasis but also innovatively establishes a complete clinical application framework from early screening to prognostic assessment: its non-invasive fecal detection potential offers a new strategy for early diagnosis, while quantitative PCR/immunohistochemistry-based methods have achieved precise correlation with AJCC staging. By integrating therapeutic interventions (e.g., antibiotics, phages) with efficacy monitoring indicators, we propose a closed-loop “detection-intervention-assessment” management model, offering a novel perspective for clinical translation research.
Future studies require deepening in three dimensions: (1) mechanistic dissection using multi-omics technologies to reveal interaction networks between F. nucleatum, the tumor microenvironment, and immune escape; (2) technical optimization through development of ultra-sensitive detection methods like CRISPR or digital PCR to enhance clinical applicability; (3) clinical validation via multi-center randomized controlled trials to confirm intervention efficacy (Wang et al., 2023). Furthermore, the “microbiota-host-therapy” trinity framework proposed herein will provide theoretical support for developing F. nucleatum-targeted personalized precision medicine strategies.
Author contributions
XH: Data curation, Writing – original draft, Investigation, Methodology, Funding acquisition, Software, Formal analysis. QZ: Data curation, Formal analysis, Software, Writing – original draft, Investigation. JZ: Investigation, Writing – original draft, Software, Data curation, Methodology. JS: Software, Data curation, Writing – original draft, Formal analysis. NW: Software, Writing – original draft, Investigation, Validation. BTa: Resources, Visualization, Software, Project administration, Validation, Writing – review & editing. BTi: Resources, Visualization, Software, Formal analysis, Project administration, Validation, Writing – review & editing. PL: Resources, Funding acquisition, Writing – review & editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This study was supported by the Joint Key Project of the Chongqing Science and Technology Bureau and the Chongqing Municipal Health Commission (Grant No. 2025ZDXM001); the Key Project of Chongqing Municipal Education Commission (Grant No. KJZD-K202400103); the Chongqing Technology Innovation and Application Development, Chuan-Yu (Sichuan-Chongqing) Scientific and Technological Innovation Cooperation Program (Grant No. CSTB2024TIAD-CYKJCXX0031); the Chongqing Municipal Science and Technology Bureau, Natural Science Fund (Chongqing Science and Technology Development Foundation) Project (Grant No. CSTB2024NSCQ-KJFZMSX0018); the 2024 Hospital-level Cultivation Project of Chongqing University Jiangjin Hospital (Grant No. 2024YCXM010); and the Guangdong Medical Science and Technology Research Fund Project (Grant No. B2021181).
Acknowledgments
We sincerely thank all contributors who participated in this review. We also extend our gratitude to the three reviewers for their constructive comments and valuable suggestions, which have significantly improved this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CRC, colorectal cancer; F. nucleatum, Fusobacterium nucleatum; FIT, immunochemical test; qPCR, quantitative polymerase chain reaction; PCR, polymerase chain reaction; FISH, fluorescence in situ hybridization; AJCC, American Joint Committee on Cancer; OR, odds ratio; HR: hazard ratio.
References
Abed, J., Emgård, J. E. M., Zamir, G., Faroja, M., Almogy, G., Grenov, A., et al. (2016). Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed gal-GalNAc. Cell Host Microbe 20, 215–225. doi: 10.1016/j.chom.2016.07.006
Baird-Parker, A. C. (1957). Isolation of Leptotrichia buccalis and fusobacterium species from oral material. Nature 180, 1056–1057. doi: 10.1038/1801056b0
Bi, D., Zhu, Y., Gao, Y., Li, H., Zhu, X., Wei, R., et al. (2022). Profiling Fusobacterium infection at high taxonomic resolution reveals lineage-specific correlations in colorectal cancer. Nat. Commun. 13:3336. doi: 10.1038/s41467-022-30957-6
Bolstad, A. I., Jensen, H. B., and Bakken, V. (1996). Taxonomy, biology, and periodontal aspects of Fusobacterium nucleatum. Clin. Microbiol. Rev. 9, 55–71. doi: 10.1128/CMR.9.1.55
Borisy, G. G., and Valm, A. M. (2021). Spatial scale in analysis of the dental plaque microbiome. Periodontol. 2000 86, 97–112. doi: 10.1111/prd.12364
Borozan, I., Zaidi, S. H., Harrison, T. A., Phipps, A. I., Zheng, J., Lee, S., et al. (2022). Molecular and pathology features of colorectal tumors and patient outcomes are associated with Fusobacterium nucleatum and its subspecies animalis. Cancer Epidemiol. Biomark. Prev. 31, 210–220. doi: 10.1158/1055-9965.EPI-21-0463
Castellarin, M., Warren, R. L., Freeman, J. D., Dreolini, L., Krzywinski, M., Strauss, J., et al. (2012). Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 22, 299–306. doi: 10.1101/gr.126516.111
Chen, S., Su, T., Zhang, Y., Lee, A., He, J., Ge, Q., et al. (2020). Fusobacterium nucleatum promotes colorectal cancer metastasis by modulating KRT7-as/KRT7. Gut Microbes 11, 511–525. doi: 10.1080/19490976.2019.1695494
Chen, S., Zhang, L., Li, M., Zhang, Y., Sun, M., Wang, L., et al. (2022). Fusobacterium nucleatum reduces METTL3-mediated m(6)a modification and contributes to colorectal cancer metastasis. Nat. Commun. 13:1248. doi: 10.1038/s41467-022-28913-5
Dewan, A., Tattoli, I., and Mascellino, M. T. (2025). The impact of Fusobacterium nucleatum and the genotypic biomarker KRAS on colorectal cancer pathogenesis. Int. J. Mol. Sci. 26:6958. doi: 10.3390/ijms26146958
Duggan, W. P., Salvucci, M., Kisakol, B., Lindner, A. U., Reynolds, I. S., Dussmann, H., et al. (2023). Increased Fusobacterium tumoural abundance affects immunogenicity in mucinous colorectal cancer and may be associated with improved clinical outcome. J. Mol. Med. 101, 829–841. doi: 10.1007/s00109-023-02324-5
Eisele, Y., Mallea, P. M., Gigic, B., Stephens, W. Z., Warby, C. A., Buhrke, K., et al. (2021). Fusobacterium nucleatum and clinicopathologic features of colorectal cancer: Results from the ColoCare study. Clin. Colorectal Cancer 20, e165–e172. doi: 10.1016/j.clcc.2021.02.007
Eklöf, V., Löfgren-Burström, A., Zingmark, C., Edin, S., Larsson, P., Karling, P., et al. (2017). Cancer-associated fecal microbial markers in colorectal cancer detection. Int. J. Cancer 141, 2528–2536. doi: 10.1002/ijc.31011
Galeano Niño, J. L., Wu, H., LaCourse, K. D., Kempchinsky, A. G., Baryiames, A., Barber, B., et al. (2022). Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature 611, 810–817. doi: 10.1038/s41586-022-05435-0
Gao, Y., Bi, D., Xie, R., Li, M., Guo, J., Liu, H., et al. (2021). Fusobacterium nucleatum enhances the efficacy of PD-l1 blockade in colorectal cancer. Signal Transduct. Target. Ther. 6:398. doi: 10.1038/s41392-021-00795-x
Gao, Y., Zou, T., Xu, P., Wang, Y., Jiang, Y., Chen, Y., et al. (2023). Fusobacterium nucleatum stimulates cell proliferation and promotes PD-l1 expression via IFIT1-related signal in colorectal cancer. Neoplasia 35:100850. doi: 10.1016/j.neo.2022.100850
Grobbee, E. J., Lam, S. Y., Fuhler, G. M., Blakaj, B., Konstantinov, S. R., Bruno, M. J., et al. (2020). First steps towards combining faecal immunochemical testing with the gut microbiome in colorectal cancer screening. U. Eur. Gastroenterol. J. 8, 293–302. doi: 10.1177/2050640619890732
Guan, Z., Wang, H., and Feng, Q. (2025). Establishment and improvement of genetic manipulation tools for Fusobacterium nucleatum. Eng. Microbiol. 5:100192. doi: 10.1016/j.engmic.2025.100192
Guo, S., Chen, J., Chen, F., Zeng, Q., Liu, W., and Zhang, G. (2020). Exosomes derived from Fusobacterium nucleatum-infected colorectal cancer cells facilitate tumour metastasis by selectively carrying mir-1246/92b-3p/27a-3p and CXCL16. Gut 70, 1507–1519. doi: 10.1136/gutjnl-2020-321187
Guo, Y., Guo, Y., Chen, C., Fan, D., Wu, X., Zhao, L., et al. (2021). Circ3823 contributes to growth, metastasis and angiogenesis of colorectal cancer: Involvement of mir-30c-5p/TCF7 axis. Mol. Cancer 20:93. doi: 10.1186/s12943-021-01372-0
Gur, C., Ibrahim, Y., Isaacson, B., Yamin, R., Abed, J., Gamliel, M., et al. (2015). Binding of the fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 42, 344–355. doi: 10.1016/j.immuni.2015.01.010
Gursoy, U. K., Pöllänen, M., Könönen, E., and Uitto, V. (2010). Biofilm formation enhances the oxygen tolerance and invasiveness of Fusobacterium nucleatum in an oral mucosa culture model. J. Periodontol. 81, 1084–1091. doi: 10.1902/jop.2010.090664
Han, B., Zheng, R., Zeng, H., Wang, S., Sun, K., Chen, R., et al. (2024). Cancer incidence and mortality in China, 2022. J. Natl. Cancer Cent. 4, 47–53. doi: 10.1016/j.jncc.2024.01.006
Han, Y. W. (2015). Fusobacterium nucleatum: A commensal-turned pathogen. Curr. Opin. Microbiol. 23, 141–147. doi: 10.1016/j.mib.2014.11.013
Haruki, K., Kosumi, K., Hamada, T., Twombly, T. S., Väyrynen, J. P., Kim, S. A., et al. (2020). Association of autophagy status with amount of Fusobacterium nucleatum in colorectal cancer. J. Pathol. 250, 397–408. doi: 10.1002/path.5381
Huh, J., Kim, M. J., Kim, J., Lee, H. G., Ryoo, S., Ku, J., et al. (2022). Enterotypical prevotella and three novel bacterial biomarkers in preoperative stool predict the clinical outcome of colorectal cancer. Microbiome 10:203. doi: 10.1186/s40168-022-01388-8
Hui, B., Zhou, C., Xu, Y., Wang, R., Dong, Y., Zhou, Y., et al. (2024). Exosomes secreted by Fusobacterium nucleatum-infected colon cancer cells transmit resistance to oxaliplatin and 5-FU by delivering hsa_circ_0004085. J. Nanobiotechnol. 22:62. doi: 10.1186/s12951-024-02331-9
Institute for Health Metrics and Evaluation (2024). GBD compare-cancer. Seattle, WA: Institute for Health Metrics and Evaluation.
Jagielska, J., Kapopara, P. R., Salguero, G., Scherr, M., Schütt, H., Grote, K., et al. (2012). Interleukin-1 assembles a proangiogenic signaling module consisting of caveolin-1, tumor necrosis factor receptor-associated factor 6, p38-mitogen-activated protein kinase (MAPK), and MAPK-activated protein kinase 2 in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 32, 1280–1288. doi: 10.1161/ATVBAHA.111.243477
Jiang, S., Xie, Y., Xiao, X., Kang, Z., Lin, X., Zhang, L., et al. (2023). Fusobacterium nucleatum-derived succinic acid induces tumor resistance to immunotherapy in colorectal cancer. Cell Host Microbe 31, 781–797. doi: 10.1016/j.chom.2023.04.010
Kim, H. S., Kim, C. G., Kim, W. K., Kim, K., Yoo, J., Min, B. S., et al. (2023). Fusobacterium nucleatum induces a tumor microenvironment with diminished adaptive immunity against colorectal cancers. Front. Cell Infect. Microbiol. 13:1101291. doi: 10.3389/fcimb.2023.1101291
Komiya, Y., Shimomura, Y., Higurashi, T., Sugi, Y., Arimoto, J., Umezawa, S., et al. (2019). Patients with colorectal cancer have identical strains of Fusobacterium nucleatum in their colorectal cancer and oral cavity. Gut 68, 1335–1337. doi: 10.1136/gutjnl-2018-316661
Kong, C., Yan, X., Zhu, Y., Zhu, H., Luo, Y., Liu, P., et al. (2021). Fusobacterium nucleatum promotes the development of colorectal cancer by activating a cytochrome p450/epoxyoctadecenoic acid axis via TLR4/keap1/NRF2 signaling. Cancer Res. 81, 4485–4498. doi: 10.1158/0008-5472.CAN-21-0453
Kong, X., Zhang, Y., Xiang, L., You, Y., Duan, Y., Zhao, Y., et al. (2023). Fusobacterium nucleatum-triggered neutrophil extracellular traps facilitate colorectal carcinoma progression. J. Exp. Clin. Cancer Res. 42:236. doi: 10.1186/s13046-023-02817-8
Kunzmann, A. T., Proença, M. A., Jordao, H. W., Jiraskova, K., Schneiderova, M., Levy, M., et al. (2019). Fusobacterium nucleatum tumor DNA levels are associated with survival in colorectal cancer patients. Eur. J. Clin. Microbiol. Infect. Dis. 38, 1891–1899. doi: 10.1007/s10096-019-03649-1
Kwong, T. N. Y., Wang, X., Nakatsu, G., Chow, T. C., Tipoe, T., Dai, R. Z. W., et al. (2018). Association between bacteremia from specific microbes and subsequent diagnosis of colorectal cancer. Gastroenterology 155, 383–390. doi: 10.1053/j.gastro.2018.04.028
Lamprinaki, D., Garcia-Vello, P., Marchetti, R., Hellmich, C., McCord, K. A., Bowles, K. M., et al. (2021). Siglec-7 mediates immunomodulation by colorectal cancer-associated Fusobacterium nucleatum ssp. animalis. Front. Immunol. 12:744184. doi: 10.3389/fimmu.2021.744184
Lee, D., Han, S., Kang, J., Bae, J. M., Kim, H., Won, J., et al. (2018). Association between Fusobacterium nucleatum, pathway mutation, and patient prognosis in colorectal cancer. Ann. Surg. Oncol. 25, 3389–3395. doi: 10.1245/s10434-018-6681-5
Lee, J. B., Kim, K., Cho, H. Y., Kim, D., Kim, W. K., Yong, D., et al. (2021). Association between Fusobacterium nucleatum and patient prognosis in metastatic colon cancer. Sci. Rep. 11:20263. doi: 10.1038/s41598-021-98941-6
Lee, S. A., Liu, F., Riordan, S. M., Lee, C. S., and Zhang, L. (2019). Global investigations of Fusobacterium nucleatum in human colorectal cancer. Front. Oncol. 9:566. doi: 10.3389/fonc.2019.00566
Li, B., Wei, Z., Wang, Z., Xu, F., Yang, J., Lin, B., et al. (2024). Fusobacterium nucleatum induces oxaliplatin resistance by inhibiting ferroptosis through e-cadherin/β-catenin/GPX4 axis in colorectal cancer. Free. Radic. Biol. Med. 220, 125–138. doi: 10.1016/j.freeradbiomed.2024.04.226
Li, Y., Ge, Q., Cao, J., Zhou, Y., Du, Y., Shen, B., et al. (2016). Association of fusobacterium nucleatum infection with colorectal cancer in Chinese patients. World J. Gastroenterol. 22, 3227–3233. doi: 10.3748/wjg.v22.i11.3227
Liang, J. Q., Li, T., Nakatsu, G., Chen, Y., Yau, T. O., Chu, E., et al. (2020). A novel faecal lachnoclostridium marker for the non-invasive diagnosis of colorectal adenoma and cancer. Gut 69, 1248–1257. doi: 10.1136/gutjnl-2019-318532
Liang, J. Q., Wong, S. H., Szeto, C. H., Chu, E. S., Lau, H. C., Chen, Y., et al. (2021). Fecal microbial DNA markers serve for screening colorectal neoplasm in asymptomatic subjects. J. Gastroenterol. Hepatol. 36, 1035–1043. doi: 10.1111/jgh.15171
Lin, Y., Lau, H. C., Liu, Y., Kang, X., Wang, Y., Ting, N. L., et al. (2022). Altered mycobiota signatures and enriched pathogenic Aspergillus rambellii are associated with colorectal cancer based on multicohort fecal metagenomic analyses. Gastroenterology 163, 908–921. doi: 10.1053/j.gastro.2022.06.038
Liu, K., Yang, X., Zeng, M., Yuan, Y., Sun, J., He, P., et al. (2021). The role of fecal Fusobacterium nucleatum and pks(+) Escherichia coli as early diagnostic markers of colorectal cancer. Dis. Mark. 2021:1171239. doi: 10.1155/2021/1171239
Lo, C., Wu, D., Jao, S., Wu, C., Lin, C., Chuang, C., et al. (2022). Enrichment of prevotella intermedia in human colorectal cancer and its additive effects with Fusobacterium nucleatum on the malignant transformation of colorectal adenomas. J. Biomed. Sci. 29:88. doi: 10.1186/s12929-022-00869-0
Ma, X., Sun, T., Zhou, J., Zhi, M., Shen, S., Wang, Y., et al. (2023). Pangenomic study of Fusobacterium nucleatum reveals the distribution of pathogenic genes and functional clusters at the subspecies and strain levels. Microbiol. Spectr. 11:e0518422. doi: 10.1128/spectrum.05184-22
Manson McGuire, A., Cochrane, K., Griggs, A. D., Haas, B. J., and Abeel, T. (2014). Evolution of invasion in a diverse set of Fusobacterium species. Mbio 5:e01864. doi: 10.1128/mBio.01864-14
Mima, K., Nishihara, R., Qian, Z. R., Cao, Y., Sukawa, Y., Nowak, J. A., et al. (2016). Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 65, 1973–1980. doi: 10.1136/gutjnl-2015-310101
Morsi, H., Golizeh, M., Brosseau, N., Janati, A. I., Emami, E., Ndao, M., et al. (2022). Detection of Fusobacterium nucleatum subspecies in the saliva of pre-colorectal cancer patients, using tandem mass spectrometry. Arch. Oral Biol. 134:105337. doi: 10.1016/j.archoralbio.2021.105337
Mouradov, D., Greenfield, P., Li, S., In, E., Storey, C., Sakthianandeswaren, A., et al. (2023). Oncomicrobial community profiling identifies clinicomolecular and prognostic subtypes of colorectal cancer. Gastroenterology 165, 104–120. doi: 10.1053/j.gastro.2023.03.205
Nakao, S., Kuwano, T., Tsutsumi-Miyahara, C., Ueda, S., Kimura, Y. N., Hamano, S., et al. (2005). Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1 beta-induced neovascularization and tumor growth. J. Clin. Invest. 115, 2979–2991. doi: 10.1172/JCI23298
Nie, S., Tian, B., Wang, X., Pincus, D. H., Welker, M., Gilhuley, K., et al. (2015). Fusobacterium nucleatum subspecies identification by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 53, 1399–1402. doi: 10.1128/JCM.00239-15
Ponath, F., Tawk, C., Zhu, Y., Barquist, L., Faber, F., and Vogel, J. (2021). RNA landscape of the emerging cancer-associated microbe Fusobacterium nucleatum. Nat. Microbiol. 6, 1007–1020. doi: 10.1038/s41564-021-00927-7
Queen, J., Domingue, J. C., White, J. R., Stevens, C., Udayasuryan, B., Nguyen, T. T. D., et al. (2021). Comparative analysis of colon cancer-derived Fusobacterium nucleatum subspecies: Inflammation and colon tumorigenesis in murine models. Mbio 13:e0299121. doi: 10.1128/mbio.02991-21
Rubinstein, M. R., Baik, J. E., Lagana, S. M., Han, R. P., Raab, W. J., Sahoo, D., et al. (2019). Fusobacterium nucleatum promotes colorectal cancer by inducing wnt/β-catenin modulator annexin a1. EMBO Rep. 20:7638. doi: 10.15252/embr.201847638
Rubinstein, M. R., Wang, X., Liu, W., Hao, Y., Cai, G., and Han, Y. W. (2013). Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating e-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 14, 195–206. doi: 10.1016/j.chom.2013.07.012
Serna, G., Ruiz-Pace, F., Hernando, J., Alonso, L., Fasani, R., Landolfi, S., et al. (2020). Fusobacterium nucleatum persistence and risk of recurrence after preoperative treatment in locally advanced rectal cancer. Ann. Oncol. 31, 1366–1375. doi: 10.1016/j.annonc.2020.06.003
Sun, C., Li, B., Wang, B., Zhao, J., Zhang, X., Li, T., et al. (2019). The role of Fusobacterium nucleatum in colorectal cancer: From carcinogenesis to clinical management. Chron. Dis. Transl. Med. 5, 178–187. doi: 10.1016/j.cdtm.2019.09.001
Swidsinski, A., Dörffel, Y., Loening-Baucke, V., Theissig, F., Rückert, J. C., Ismail, M., et al. (2011). Acute appendicitis is characterised by local invasion with Fusobacterium nucleatum/necrophorum. Gut 60, 34–40. doi: 10.1136/gut.2009.191320
Ternes, D., Tsenkova, M., Pozdeev, V. I., Meyers, M., Koncina, E., Atatri, S., et al. (2022). The gut microbial metabolite formate exacerbates colorectal cancer progression. Nat. Metab. 4, 458–475. doi: 10.1038/s42255-022-00558-0
Tong, Y., Lu, G., Wang, Z., Hao, S., Zhang, G., and Sun, H. (2023). Tubeimuside i improves the efficacy of a therapeutic Fusobacterium nucleatum dendritic cell-based vaccine against colorectal cancer. Front. Immunol. 14:1154818. doi: 10.3389/fimmu.2023.1154818
Tunsjø, H. S., Gundersen, G., Rangnes, F., Noone, J. C., Endres, A., and Bemanian, V. (2019). Detection of Fusobacterium nucleatum in stool and colonic tissues from Norwegian colorectal cancer patients. Eur. J. Clin. Microbiol. Infect. Dis. 38, 1367–1376. doi: 10.1007/s10096-019-03562-7
Ugai, T., Shimizu, T., Kawamura, H., Ugai, S., Takashima, Y., Usui, G., et al. (2023). Inverse relationship between Fusobacterium nucleatum amount and tumor CD274 (PD-l1) expression in colorectal carcinoma. Clin. Transl. Immunol. 12:e1453. doi: 10.1002/cti2.1453
Wang, M., Li, Y., Yang, X., Liu, Z., Wang, K., Gong, D., et al. (2023). Effects of metronidazole on colorectal cancer occurrence and colorectal cancer liver metastases by regulating fusobacterium nucleatum in mice. Immun. Inflamm. Dis. 11:e1067. doi: 10.1002/iid3.1067
Wang, N., Zhang, L., Leng, X., Xie, Y., Kang, Z., Zhao, L., et al. (2024). Fusobacterium nucleatum induces chemoresistance in colorectal cancer by inhibiting pyroptosis via the hippo pathway. Gut Microbes 16:333790. doi: 10.1080/19490976.2024.2333790
Wong, S. H., and Yu, J. (2019). Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 16, 690–704. doi: 10.1038/s41575-019-0209-8
Wong, S. H., Kwong, T. N. Y., Chow, T., Luk, A. K. C., Dai, R. Z. W., Nakatsu, G., et al. (2017). Quantitation of faecal fusobacterium improves faecal immunochemical test in detecting advanced colorectal neoplasia. Gut 66, 1441–1448. doi: 10.1136/gutjnl-2016-312766
Wu, X., Xu, J., Yang, X., Wang, D., and Xu, X. (2023). Integrating transcriptomics and metabolomics to explore the novel pathway of Fusobacterium nucleatum invading colon cancer cells. Pathogens 12:201. doi: 10.3390/pathogens12020201
Xu, B., and Han, Y. W. (2022). Oral bacteria, oral health, and adverse pregnancy outcomes. Periodontol. 2000 89, 181–189. doi: 10.1111/prd.12436
Xu, C., Fan, L., Lin, Y., Shen, W., Qi, Y., Zhang, Y., et al. (2021). Fusobacterium nucleatum promotes colorectal cancer metastasis through mir-1322/CCL20 axis and m2 polarization. Gut Microbes 13:1980347. doi: 10.1080/19490976.2021.1980347
Yamaguchi-Kuroda, Y., Kikuchi, Y., Kokubu, E., and Ishihara, K. (2023). Porphyromonas gingivalis diffusible signaling molecules enhance Fusobacterium nucleatum biofilm formation via gene expression modulation. J. Oral Microbiol. 15:2165001. doi: 10.1080/20002297.2023.2165001
Yamamoto, S., Kinugasa, H., Hirai, M., Terasawa, H., Yasutomi, E., Oka, S., et al. (2021). Heterogeneous distribution of Fusobacterium nucleatum in the progression of colorectal cancer. J. Gastroenterol. Hepatol. 36, 1869–1876. doi: 10.1111/jgh.15361
Yamaoka, Y., Suehiro, Y., Hashimoto, S., Hoshida, T., Fujimoto, M., Watanabe, M., et al. (2018). Fusobacterium nucleatum as a prognostic marker of colorectal cancer in a Japanese population. J. Gastroenterol. 53, 517–524. doi: 10.1007/s00535-017-1382-6
Yang, Y., Weng, W., Peng, J., Hong, L., Yang, L., Toiyama, Y., et al. (2017). Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor-κb, and up-regulating expression of MicroRNA-21. Gastroenterology 152, 851–866. doi: 10.1053/j.gastro.2016.11.018
Ye, X., Wang, R., Bhattacharya, R., Boulbes, D. R., Fan, F., Xia, L., et al. (2017). Fusobacterium nucleatum subspecies animalis influences proinflammatory cytokine expression and monocyte activation in human colorectal tumors. Cancer Prev. Res. 10, 398–409. doi: 10.1158/1940-6207.CAPR-16-0178
Yin, H., Miao, Z., Wang, L., Su, B., Liu, C., Jin, Y., et al. (2022). Fusobacterium nucleatum promotes liver metastasis in colorectal cancer by regulating the hepatic immune niche and altering gut microbiota. Aging 14, 1941–1958. doi: 10.18632/aging.203914
Yu, J., Feng, Q., Wong, S. H., Zhang, D., Liang, Q. Y., Qin, Y., et al. (2017). Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut 66, 70–78. doi: 10.1136/gutjnl-2015-309800
Yu, T., Guo, F., Yu, Y., Sun, T., Ma, D., Han, J., et al. (2017). Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 170, 548–563. doi: 10.1016/j.cell.2017.07.008
Yu, T., Ji, L., Lou, L., Ye, S., Fang, X., Li, C., et al. (2022). Fusobacterium nucleatum affects cell apoptosis by regulating intestinal flora and metabolites to promote the development of colorectal cancer. Front. Microbiol. 13:841157. doi: 10.3389/fmicb.2022.841157
Zhang, Y., Zhang, L., Zheng, S., Li, M., Xu, C., Jia, D., et al. (2022). Fusobacterium nucleatum promotes colorectal cancer cells adhesion to endothelial cells and facilitates extravasation and metastasis by inducing ALPK1/NF-κb/ICAM1 axis. Gut Microbes 14:2038852. doi: 10.1080/19490976.2022.2038852
Zheng, D., Dong, X., Pan, P., Chen, K., Fan, J., Cheng, S., et al. (2019). Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy. Nat. Biomed. Eng. 3, 717–728. doi: 10.1038/s41551-019-0423-2
Keywords: F. nucleatum, colorectal cancer, therapeutic monitoring, prognostic evaluation, biomarker
Citation: He X, Zhao Q, Zhang J, Shi J, Wan N, Tang B, Tian B and Li P (2025) Potential and application of Fusobacterium nucleatum in the diagnosis and treatment of colorectal cancer. Front. Microbiol. 16:1652702. doi: 10.3389/fmicb.2025.1652702
Received: 11 July 2025; Accepted: 12 September 2025;
Published: 30 September 2025.
Edited by:
Bryan Swingle, Agricultural Research Service (USDA), United StatesReviewed by:
Sarbjeet Makkar, University of Michigan, United StatesPeng Zhou, University of Texas Health Science Center at Houston, United States
Toshimitsu Miyasaka, Nippon Medical School, Japan
Copyright © 2025 He, Zhao, Zhang, Shi, Wan, Tang, Tian and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Tian, MTM5OTk0MjQ3OEBxcS5jb20=; Pu Li, bGlwdS5jcXVAY3F1LmVkdS5jbg==
†These authors have contributed equally to this work
 Xin He1†
Xin He1† Bo Tian4*
Bo Tian4* Pu Li
Pu Li