- 1Emerging Pathogens Institute, University of Florida, Gainesville, FL, United States
- 2Department of Animal Sciences, University of Florida, Gainesville, FL, United States
- 3School of Plant, Environmental and Soil Sciences, Louisiana State University, Baton Rouge, LA, United States
- 4Department of Agricultural and Biological Engineering, University of Florida, Gainesville, FL, United States
The rhizosphere microbiome plays an important role in plant growth, nutrient acquisition, and overall health. In this study, we investigated the relationship between the rhizosphere microbiome and the health status of strawberries (Fragaria × ananassa) under identical soil and environmental conditions. Strawberry plants were categorized into a healthy group (H) and an unhealthy group (UH) based on morphological characteristics, and the soil microbial community was analyzed using 16S rRNA gene sequencing. The H group exhibited significantly higher nitrogen concentrations, whereas the UH group showed excessive accumulation of iron, manganese, zinc, and copper. Microbiota analysis revealed distinct structural differences between the H and UH groups, with several bacterial taxa displaying significant differences in relative abundance. Notably, Microvirga and JG30-KF-CM45 emerged as key bacterial taxa associated with plant nutrient status. Microvirga was positively correlated with nitrogen levels but negatively associated with micronutrient accumulation, while JG30-KF-CM45 showed the opposite trend. Furthermore, co-occurrence network analysis indicated that microbial communities in the UH group were characterized by intensified competitive interactions, which may contribute to rhizosphere microbiome destabilization and impaired plant growth. These findings indicate that microbial interactions within the rhizosphere influence nutrient homeostasis and plant health. The observed microbial imbalances in UH plants suggest the importance of maintaining a stable microbial community for improved crop productivity. This study provides valuable insights into the role of rhizosphere microbiome in sustainable strawberry cultivation and underscores the potential of microbiome-based strategies to improve plant health and productivity.
1 Introduction
The soil microbiome particularly in the rhizosphere plays a fundamental role in plant growth, health, and productivity (Schnitzer et al., 2011). The rhizosphere is a dynamic area where plants interact with diverse microbial communities, including bacteria, fungi, archaea, and viruses, which influence nutrient cycling, pathogen suppression, and plant stress resistance (Wagg et al., 2011; Michl et al., 2023). These microbial communities can facilitate the exchange of essential nutrients, such as sodium, phosphorus, and potassium, between the soil and plant. In addition, they produce plant growth regulators, including auxins and cytokinins (Lazcano et al., 2021; Compant et al., 2025). Understanding the microbial composition and activities in the rhizosphere is therefore crucial for assessing plant performance under both optimal and stressful environmental conditions.
Strawberries (Fragaria × ananassa) are highly sensitive to soil quality and environmental stressors (Busby et al., 2017), both of which are influenced by the composition and diversity of the rhizosphere microbiome, thereby playing a critical role in modulating plant health and productivity (Yan et al., 2017). Specifically, plant growth-promoting rhizobacteria (PGPR) contribute to enhanced nutrient uptake, regulation of growth hormone production, and protection against pathogens (Yang et al., 2020). Disruptions in rhizosphere microbiome or dysbiosis can compromise plant defense mechanisms, leading to impaired growth, reduced yield, irregular plant maturation, and increased susceptibility to diseases (Siegieda et al., 2024). Consequently, the establishment and maintenance of a healthy microbiome are essential for ensuring sustainable strawberry productivity.
Certain bacterial taxa have been directly associated with improved nutrient assimilation and enhanced plant resistance to antibiotics and pathogens (Deng et al., 2019). Notably, Pseudomonas, Bosea, Microvirga, and Paenibacillus contribute to nutrient cycling and plant-microbe interactions (Xu et al., 2015; Marin et al., 2024; Wu et al., 2025). Additionally, studies have shown that plants grown in identical soil under the same environmental conditions exhibit varying growth patterns. This observation suggests that differences in the rhizosphere microbiome may play a crucial role in influencing nutrient uptakes and plant development. Therefore, we hypothesized that variations in rhizosphere microbial communities directly impact plant growth and nutrient acquisition. In this study, we cultivated strawberry plants in identical soil under uniform management conditions to investigate the relationship between the rhizosphere microbiome and plant growth performance. Furthermore, we compared plants grown differently to elucidate the role of key microbial taxa in plant health and nutrient levels. Understanding these microbial interactions is critical for enhancing crop yield, improving food safety and security by promoting healthier plant growth.
2 Materials and methods
2.1 Experimental design and sampling
Two strawberry cultivars, Brilliance and Medallion, were used in this study. They were chosen since they were most commonly grown by strawberry growers in Florida. They were planted in an experimental field located in the Plant Science Research and Education Unit (PSREU) at the University of Florida in Citra, Florida, USA on October 13th, 2023, using bare-root transplants grown in a nursery in California, USA. Standard fertilization and pest control practices were followed, with no microbial inoculants applied. The strawberries were grown in 10 raised beds, and two rows of strawberry plants were planted in each bed. The length of the bed was 61 m, and the width of each bed was 0.5 m. The plants in each row were 0.3 m apart. To minimize potential microenvironmental variability, healthy (H) and unhealthy (UH) plants were selected from adjacent locations within the same raised bed. From the field, a total of 10 healthy and 10 unhealthy plants were randomly chosen for collecting leaf and soil samples in March 2024. Most of the U and UH sample plants were grown nearby, exhibiting an interesting situation of growing status. Each group consisted of five Brilliance and five Medallion strawberries. The H plants were selected based on their bigger canopy sizes and dark green leaf conditions without any vivid signs of nutrient deficiencies, whereas the selected UH plants showed smaller canopy sizes and signs of nutrient deficiencies. Figure 1 shows an example of H and UH plants growing nearby.

Figure 1. Representative images of healthy (A) and unhealthy (B) strawberry plants growing in close proximity.
Several leaves of each plant were collected and put in a sterile plastic bag for later nutrient analyses. Approximately 5 g of soil samples were collected under each plant from the root area using a cotton swab directly from root surfaces (within ~2 mm) and put in a clean plastic bag for lab analyses. The soil type was Arredondo sand with 0 to 5 percent slopes (United States Department of Agriculture, 2025).
2.2 Nutrient profiling of leaves
The sampled leaves were dried at 70 °C for 48 h in an oven, sieved using a 250 μm sieve, and analyzed at the Extension Soil Testing Laboratory at the University of Florida, Gainesville, Florida, USA. Leaf tissue samples were analyzed by the total Kjeldahl nitrogen (TKN) for nitrogen contents and the inductively coupled plasma (ICP) metals procedure for other elements (Mylavarapu et al., 2024).
2.3 16S rRNA gene sequencing and bioinformatic analysis
Genomic DNA was extracted from 0.25 g of soil based on the protocols set by the manufacturer from QIAamp DNeasy PowerSoil Pro kit (Qiagen, United States). To study the bacterial diversity, the DNA library was prepared and sequenced as described by Fan et al. (2021) and Kwak et al. (2021). Briefly, the V4 region of the 16S rRNA gene was amplified by polymerase chain reaction (PCR) with dual-index primers with the following reaction mixture: 1 μL forward index primer (10 mM), 1 μL reverse index primer (10 mM), 1 μL 10 ng/μL DNA samples, and 17 μL Pfx AccuPrime master mix (Invitrogen, United States). The PCR conditions were denaturation for 5 min at 95 °C, followed by 30 cycles of 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C. The amplicons were then subsequently purified and normalized using the SequalPrep Plate Normalization Kit (Invitrogen, United States). The DNA library was then constructed by pooling the same amount of barcoded V4 amplicons from all 20 samples. The final DNA library was loaded into MiSeq v2, 2 × 250 cycle cartridge (Illumina, United States), and sequenced using the Illumina MiSeq platform.
Raw sequencing data were acquired from the Illumina BaseSpace platform and processed using the Quantitative Insights into Microbial Ecology (QIIME 2) software (version 2024.10.). Paired-end reads were imported, and the quality of the initial bases was assessed according to the Interactive Quality Plot. The sequence quality control was conducted with the Divisive Amplicon Denoising Algorithm (DADA2) pipeline implemented in QIIME 2, including steps for filtering low-quality reads, denoising reads, merging the paired-end reads, and removing chimeric reads. The phylogenetic tree was generated using the align-to-tree-mafft-fasttree pipeline from the q2-phylogeny plugin of QIIME 2. The sequencing depth was normalized to 8,190 sequences per sample. The Shannon index and Weighted UniFrac distance were calculated using the core-metrics-phylogenetic pipeline. All amplicon sequence variants were classified into the bacterial taxonomy using the q2-feature-classifier plugin of QIIME 2 and the SILVA v138 database (Quast et al., 2012).
2.4 Association between the core microbiome and leaf nutrients
The relationship between rhizosphere core microbiome and leaf micronutrients was analyzed using multiple linear regression models with leaf micronutrient concentrations (nitrogen, phosphorus, potassium, calcium, magnesium, iron, manganese, zinc, copper, boron, and sulfur) and the relative abundance of core microbiome in phylum, family, and genus levels. A p < 0.05 was considered to be significantly different, and 0.05 < p < 0.10 was considered as a tendency (Cha et al., 2023).
2.5 Co-occurrence network analysis
To predict bacteria–bacteria interactions in the gut microbial community, co-occurrence patterns of core bacterial families, and genera present in at least 50% of samples were evaluated in the network interface using pairwise Spearman’s rank correlations based on the relative bacterial abundance. The Spearman rank correlation was analyzed using Hmisc package (v. 5.2-2) within R Studio (version 1.1456). A significant rank correlation between two taxa (rs > 0.5 or rs < −0.5, FDR-adjusted p-value <0.05) was considered a co-occurrence event. The network was visualized using the Force Atlas algorithm in the interactive platform Gephi.1 In the network, nodes represent different taxa, and edges indicate correlations among nodes. The thickness of the edges indicates the strength of the correlation.
2.6 Statistical analysis
All statistical analyses were performed with GraphPad Prism (version 10.2.1). Leaf nutrients and gut microbiota were analyzed by t-test and the linear correlation between nutrients and soil microbiota was estimated by Pearson’s correlation analysis. Significant differences between treatments were defined at p < 0.05.
3 Results
3.1 The growth and nutrient levels vary between strawberry plants
To understand if rhizosphere microbiome composition is associated with plant growth and nutrient levels, we cultivated strawberry plants in the same soil under identical management practices. We classified the plants into healthy (H) and unhealthy (UH) groups based on morphological differences, particularly leaf color and shape (Figure 1). Strawberries in the H group exhibited dark green leaves and typical fruit shapes (Figure 1A), whereas UH plants had light green or brown leaves and irregularly shaped fruits (Figure 1B).
We measured the leaf concentrations of macro- and micronutrients from the randomly selected plants to understand differences related to growth status (Table 1). The nitrogen concentration was significantly higher (p = 0.006) in the leaves of strawberry plants in the H group compared to the UH group. However, the concentrations of other macronutrients, including phosphorus, potassium, calcium, and magnesium did not differ significantly. In contrast, the levels of iron (p = 0.050), manganese (p = 0.011), zinc (p = 0.009), and copper (p = 0.004) were significantly higher in strawberry plants of the UH group than in the H group. In conclusion, there was considerable variation in plant growth and leaf nutrient concentrations, although plants were grown under the same conditions.
3.2 Soil microbiome related to the growth status of strawberries
To determine whether differences in growth and nutrient levels between the two types of strawberry leaves were associated with the rhizosphere microbiome, we analyzed soil microbiota. Their α-diversity indexes, including Shannon index (p = 0.386, measuring both species diversity and richness), Faith phylogenetic diversity (p = 0.371, reflecting the diversity of species), Pielou’s evenness (p = 0.315, assessing the uniformity of species distribution), and Chao1 index (p = 0.469, estimating species richness), were similar between the two groups (Figures 2A–D). However, microbiota structure differed significantly based on Bray–Curtis distances (p = 0.050; Figure 2E). The relative abundance of the phyla, Proteobacteria (27.64% vs. 25.11%), Actinobacteriota (13.95% vs. 17.53%), Chloroflexi (12.21% vs. 14.53%), Planctomycetota (9.24% vs. 9.86%), and Firmicutes (9.84% vs. 9.17%) were similar (Figure 2F). However, the relative abundance of specific genera varied according to growth performance (Figure 2G). The relative abundance of Bosea (p = 0.029), Chthoniobacter (p = 0.001), and JG30-KF-CM45 (p < 0.001) were significantly more abundant in the rhizosphere of UH group compared to H group (Figures 2H–J). In contrast, the relative abundance of Microbispora (p = 0.044), Microvirga (p = 0.005), and S0134 terrestrial group (p = 0.033) were significantly more abundant in the H group strawberries (Figures 2K–M). These findings collectively indicate that the rhizosphere microbiota associated with strawberry plants may contribute to regulating plant growth and nutrient levels.
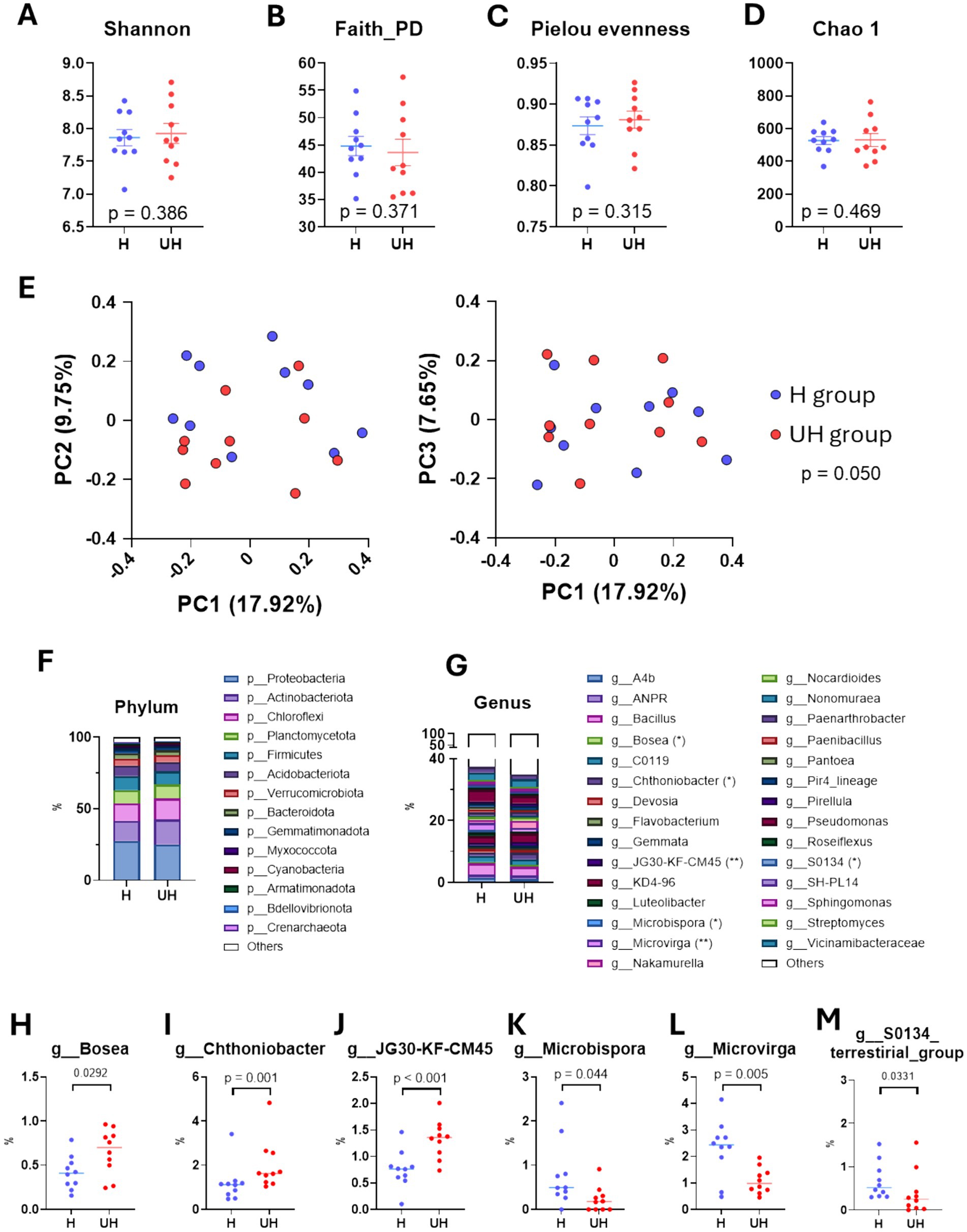
Figure 2. Rhizosphere microbiome of strawberry plants based on growth status. (A–D) Dot plot showing Shannon, Faith PD, Pielou evenness, and Chao1 index results. (E) PCoA plots of Bray–Curtis distances comparing soil microbiome between treatments (left; PC1 vs. PC2, right; PC1 vs. PC3). (F) Bar plot showing microbial abundance at the phylum level. (G) Bar plot showing microbial abundance at the genus level. (H–M) Dot plots showing significantly different genera (Chthoniobacter, JG30-KF-CM45, Microbispora, and Microvirga) according to the treatment groups.
3.3 Correlation analysis between leaf nutrient and soil microbiome
As we observed significant differences in the microbial communities within the rhizosphere of strawberries based on their growth status, we sought to determine whether the rhizosphere microbiome was associated with strawberry nutrient levels. To investigate this, we analyzed associations between key bacterial taxa and plant macro- and micronutrient levels using multiple linear regressions. Consequently, we identified three key bacterial taxa, phylum Actinobacteriota and genera Microvirga and JG30-KF-CM45 that were associated with plant nutrition levels. The relative abundance of Actinobacteriota was negatively correlated with nitrogen concentration (p = 0.031) but positively correlated with iron (p = 0.006) and zinc (p = 0.015) levels (Figure 3A). Similarly, JG30-KF-CM45 showed a positive correlation with micronutrient concentrations, including iron (p = 0.023), manganese (p = 0.001), zinc (p = 0.037), and copper (p = 0.006) (Figures 3B–E). In contrast, Microvirga showed an opposite pattern to the other two key bacteria, showing a positive relationship with nitrogen (p = 0.048) but negative correlations with manganese (p = 0.001), zinc (p = 0.012), and copper (p = 0.003) (Figures 3F–I). Notably, Microvirga in the well-grown group and JG30-KF-CM45 in the poorly grown group were identified as key bacteria. Their consistent correlations suggest that rhizosphere bacteria may be associated with plant nutrition levels.

Figure 3. The relationship between leaf nutrients and core bacteria analyzed using linear correlation analysis. (A) Correlation between leaf nutrients (nitrogen, phosphorus, potassium, calcium, magnesium, iron, manganese, zinc, copper, boron, and sulfur) and the abundances of core bacteria analyzed using multiple linear regression models. Colors indicate significance levels, with red and blue representing negative and positive correlations, respectively. (B–E) Correlation between Microvirga abundance and leaf nutrient concentrations (sodium, manganese, zinc, and copper). (F–I) Correlation between JG30-KF-CM45 abundance and leaf nutrient concentrations (iron, manganese, zinc, and copper).
3.4 Co-occurrence of microbial networks based on the growth status of strawberries
To elucidate the role of microbe–microbe interactions in shaping microbial community structure and function, we performed a co-occurrence network analysis. In the network, nodes represent distinct bacterial genera, while edges denote significant interactions, with blue edges indicating co-occurrence and red edges indicating co-exclusion relationships. Node size is proportional to the number of connections between each genus, highlighting dominant taxa within the community. Additionally, the analysis revealed modular structures within the network, where nodes sharing the same color represent taxa grouped into the same module. These modules indicate that bacterial groups that potentially co-exist within shared ecological or functional niches.
In the microbial co-occurrence network of the H group, three primary modules were identified. The blue module dominated by Microvirga, the orange module dominated by Vicinamibacteraceae, and the green module dominated by Paenarthrobacter and Chthoniobacter. These taxa functioned as key hubs within their respective modules. Strong positive associations were observed between the blue and green modules, suggesting cooperative interactions that may contribute to community stability. In contrast, the orange module displayed competitive correlations with other modules, indicating potential antagonistic interactions that influence microbial community structure (Figure 4A). In the UH group, the purple module emerged as the dominant module, with Paenarthrobacter and Pseudomonas as hub taxa. This module exhibited strong negative correlations with other nodes, indicating a high degree of competitive interactions within the microbial network. Such competitive dynamics may account for the lower M-value (0.46) observed in the UH group compared to the H group (0.71), implying reduced community stability in the UH group (Figure 4B). Collectively, these findings underscore the pivotal role of bacterial interactions in modulating rhizosphere microbial community stability, thereby resulting in alterations in plant–microbiome interactions, even within the same soil environment. These changes can significantly impact plant health by modifying the nutrient dynamics within the rhizosphere.

Figure 4. Co-occurrence of bacterial networks based on the growth status of strawberries (modularity value: 0.71 vs. 0.46). Co-occurrence networks predicting the bacteria-bacteria interactions among core bacterial genera in the H group (A) and the UN group (B). Connections were detected using Spearman’s rank correlations (rs > 0.5 or rs < −0.5, FDR-adjusted p < 0.05). Dot sizes represent the number of connections per genus, and dot colors represent bacterial community clusters within the same module. Edge color represents either positive or negative associations between taxa.
4 Discussion
This study demonstrates that the rhizosphere microbiome is strongly associated with plant growth and nutrient uptake. Significant differences in microbial community structure were observed between the H and UH plant groups, with specific taxa, including Microvirga and JG30-KF-CM45, playing key roles in shaping microbiome composition. Moreover, specific taxa exhibited correlations with leaf nutrient concentrations, suggesting a potential role of rhizosphere-associated microbes in facilitating nutrient uptake and bioavailability. These findings underscore the critical importance of microbial interactions in maintaining plant health and productivity.
Significant differences in soil microbiota composition were observed based on the health status of strawberry plants (Figure 2). This microbial dysbiosis within the soil may disrupt essential physiological processes, thereby impairing the optimal growth and development of strawberries. Our results indicate that Microvirga is positively associated with nitrogen concentrations, suggesting its involvement in nitrogen metabolism within strawberry roots. The genus Microvirga comprises 17 validated species isolated from diverse environments, including human, aquatic, and terrestrial ecosystems. Notably, Microvirga is known for its ability to produce pigments and amylolytic enzymes via arsenic oxidation (Kanso and Patel, 2003; Caputo et al., 2016; Zhang et al., 2019). Han et al. (2024) reported a positive correlation between Microvirga and metabolites such as 0-oxononanoic acid, poly-D-glutamate, arginine, and calcium-phosphate (Ca–P), suggesting a pivotal role in carbon metabolism through nitrogen fixation. This metabolic function may contribute to plant growth promotion and enhanced resistance to environmental stress (Wang et al., 2022). Additionally, co-occurrence network analysis revealed significant interactions between Microvirga and other bacterial taxa in both H and UH strawberry rhizospheres. In soils from H strawberry plants, Microvirga exhibited a positive correlation with Paenibacillus, a known plant growth-promoting bacterium recognized for auxin production (Singh et al., 2024). In contrast, Microvirga was negatively correlated with Pseudomonas and Micrococcaceae in the rhizosphere of UH strawberry plants. In accordance with this study, Deng et al. (2019) also found that plant growth-promoting microbial modifications significantly altered the rhizosphere microbial community structure of strawberries, and the changes in the endogenous microbial community are closely related to plant nutritional status and health even under uniform environmental conditions. Because healthy plants are known to actively develop rhizosphere microbial communities, the enrichment of beneficial bacteria such as Microvirga in H plants may be partially explained by their introduction via root exudates. Conversely, abiotic stress or nutrient dysregulation in UH plants can disrupt these selection pressures, resulting in microbial imbalances characterized by increased competition and loss of functional redundancy. Collectively, these findings highlight the critical role of Microvirga in promoting plant growth not only through nitrogen fixation but also by fostering beneficial microbial interactions within the rhizosphere microbiome.
Optimal nutrient management is essential for successful strawberry cultivation, as a balanced supply of macro- and micronutrients directly influences plant growth, yield, and fruit quality (Tagliavini et al., 2005). Osvalde et al. (2023) established baseline standards for optimal nutrient concentrations in commercially grown strawberry leaves during the period 2014–2022. Our findings confirm that the UH group exceeded these optimal concentrations especially in micronutrients (iron, manganese, and zinc). Ruano et al. (1988) demonstrated that excessive zinc levels in strawberries result in growth inhibition, yield reduction, and impaired translocation of photoassimilates from leaves to roots. In addition, excessive zinc accumulation disrupts photosynthesis by reducing pigment levels, damaging photosystem II, and increasing reactive oxygen species (Paunov et al., 2018). Similarly, copper toxicity negatively impacts photosynthesis, nutrient uptake, and oxidative stress management, leading to chlorosis and growth impairment (Mir et al., 2021). Chlorosis, characterized by yellowing of leaves, results from disrupted chlorophyll biosynthesis or degradation of existing chlorophyll. Furthermore, excess copper induces reactive oxygen species production, causing oxidative damage to the chloroplasts and ultimately leading to necrosis of leaf tissues, which compromises plant health and productivity (Kaleem et al., 2024). Consistently, we observed yellowing of strawberry leaves, which may be attributed to the downregulation of photosynthesis caused by micronutrient dysbiosis.
The co-occurrence network, constructed using Spearman’s rank correlation analysis, is recognized as a powerful tool for elucidating the non-random distribution patterns of microbial communities (Barberán et al., 2012). In this study, co-occurrence network analysis of the rhizosphere microbiome from UH strawberry plants revealed a higher proportion of negative interactions compared to H plants, indicating intensified competition among bacterial communities. Such negative interactions are known to destabilize microbial communities, ultimately impairing plant growth (Lee et al., 2022). The UH group exhibited a comparatively higher number of positive interactions, with the microbial interaction network displaying an increased number of nodes and edges relative to the healthy group. Consistent with these observations, Zhang et al. (2023) reported that lower modularity is associated with reduced structural and functional stability in microbial communities. The low modularity observed in the UH group reflects a decrease in ecological compartmentalization, suggesting a breakdown in ecological niche partitioning and functional redundancy. This shift from cooperative to competitive microbial interactions may lead to reduced nutrient exchange efficiency and reduced plant resilience to environmental stress. Their study on microbial networks in diseased strawberry soils similarly revealed a higher number of nodes and edges, accompanied by lower modularity.
Although co-occurrence network analysis provides valuable insights into cooperative relationships between microbial taxa, it is important to note that such associations do not always imply direct interactions. Microbial members can influence host performance indirectly, even in the absence of direct contact (van der Heijden and Hartmann, 2016). In addition, despite the insights gained from this study, we acknowledge certain limitations. Specifically, the observational and correlative nature of the microbiome analyses does not allow for definitive conclusions regarding the causal role of Microvirga in promoting plant growth and shaping rhizosphere microbial communities. To address this limitation, future research should incorporate gnotobiotic systems or synthetic microbial community assays under controlled environmental conditions. These experimental approaches would enable precise functional validation of Microvirga’s contributions, both individually and in interaction with other microbial taxa, to plant physiological responses, nutrient assimilation, and microbiome assembly.
5 Conclusion
In conclusion, this study highlights the critical role of soil microbiome in regulating the growth, nutrient uptake, and overall health of strawberry plants. The findings emphasize the significant influence of microbial community composition, particularly the role of Microvirga and its associations with macro- and micronutrients, on plant development. Nutrient dysbiosis and altered microbial interactions, particularly in UH strawberry plants, were linked to impaired photosynthesis, chlorosis, and reduced plant productivity. Further studies to elucidate the specific mechanisms through which rhizosphere microbiota mediate nutrient cycling and plant-microbe interactions will provide insights into develop microbial-based strategies to enhance nutrient use efficiency and promote sustainable strawberry cultivation practices.
Data availability statement
The 16S rRNA gene amplicon sequencing data generated during the current study were submitted to NCBI under BioProject PRJNA1230227.
Author contributions
M-JK: Writing – review & editing, Methodology, Writing – original draft, Conceptualization, Investigation, Formal analysis, Data curation, Visualization, Validation. SP: Formal analysis, Writing – original draft, Writing – review & editing, Methodology, Data curation. YZ: Data curation, Formal analysis, Validation, Methodology, Writing – review & editing, Writing – original draft. BC: Formal analysis, Writing – review & editing, Methodology, Writing – original draft, Data curation. TS: Formal analysis, Data curation, Writing – review & editing, Writing – original draft. WL: Funding acquisition, Resources, Writing – original draft, Conceptualization, Supervision, Methodology, Writing – review & editing, Data curation. KJ: Resources, Funding acquisition, Writing – original draft, Project administration, Validation, Conceptualization, Writing – review & editing, Supervision, Investigation, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture (Award Nos. 2020-51181-32157 to KJ and 2023-67021-40618 to WL). Any opinions, findings, conclusions, or recommendations expressed in this presentation are those of the author(s) and do not necessarily reflect the view of the USDA.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Barberán, A., Bates, S. T., Casamayor, E. O., and Fierer, N. (2012). Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 6, 343–351. doi: 10.1038/ismej.2011.119
Busby, P. E., Soman, C., Wagner, M. R., Friesen, M. L., Kremer, J., Bennett, A., et al. (2017). Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol. 15:e2001793. doi: 10.1371/journal.pbio.2001793
Caputo, A., Lagier, J. C., Azza, S., Robert, C., Mouelhi, D., Fournier, P. E., et al. (2016). Microvirga massiliensis sp. nov., the human commensal with the largest genome. Microbiology 5, 307–322. doi: 10.1002/mbo3.329
Cha, T., Kim, H. H., Keum, J., Kwak, M. J., Park, J. Y., Hoh, J. K., et al. (2023). Gut microbiome profiling of neonates using Nanopore MinION and Illumina MiSeq sequencing. Front. Microbiol. 14:1148466. doi: 10.3389/fmicb.2023.1148466
Compant, S., Cassan, F., Kostic, T., Johnson, L., Brader, G., Trognitz, F., et al. (2025). Harnessing the plant microbiome for sustainable crop production. Nat. Rev. Microbiol. 23, 9–23. doi: 10.1038/s41579-024-01079-1
Deng, S., Wipf, H. M., Pierroz, G., Raab, T. K., Khanna, R., and Coleman-Derr, D. (2019). A plant growth-promoting microbial soil amendment dynamically alters the strawberry root bacterial microbiome. Sci. Rep. 9:17677. doi: 10.1038/s41598-019-53623-2
Fan, P., Kim, M., Liu, G., Zhai, Y., Liu, T., Driver, J. D., et al. (2021). The gut microbiota of newborn calves and influence of potential probiotics on reducing diarrheic disease by inhibition of pathogen colonization. Front. Microbiol. 12:772863. doi: 10.3389/fmicb.2021.772863
Han, H., Kan, D., Tian, M., and Ruan, Y. (2024). Phosphate-solubilizing bacteria reshaped the rhizosphere microbiome and metabolic profile of wheat to inhibit Cd absorption. Environ. Exp. Bot. 226:105929. doi: 10.1016/j.envexpbot.2024.105929
Kaleem, Z., Xu, W., Ulhassan, Z., Shahbaz, H., He, D., Naeem, S., et al. (2024). Harnessing the potential of copper-based nanoparticles in mitigating abiotic and biotic stresses in crops. Environ. Sci. Pollut. Res. 31, 59727–59748. doi: 10.1007/s11356-024-35174-w
Kanso, S., and Patel, B. K. (2003). Microvirga subterranea gen. nov., sp. nov., a moderate thermophile from a deep subsurface Australian thermal aquifer. Int. J. Syst. Evol. Microbiol. 53, 401–406. doi: 10.1099/ijs.0.02348-0
Kwak, M. J., Park, M. Y., Choi, Y. S., Cho, J., Pathiraja, D., Kim, J., et al. (2021). Dietary sophorolipid accelerates growth by modulation of gut microbiota population and intestinal environments in broiler chickens. J. Anim. Sci. Biotechnol. 12:81. doi: 10.1186/s40104-021-00606-x
Lazcano, C., Boyd, E., Holmes, G., Hewavitharana, S., Pasulka, A., and Ivors, K. (2021). The rhizosphere microbiome plays a role in the resistance to soil-borne pathogens and nutrient uptake of strawberry cultivars under field conditions. Sci. Rep. 11:3188. doi: 10.1038/s41598-021-82768-2
Lee, K. K., Kim, H., and Lee, Y.-H. (2022). Cross-kingdom co-occurrence networks in the plant microbiome: importance and ecological interpretations. Front. Microbiol. 13:953300. doi: 10.3389/fmicb.2022.953300
Marin, M. V., Carvalho, R., Paret, M. L., Jones, J. B., and Peres, N. A. (2024). Pseudomonas fragariae sp. nov., a novel bacterial species causing leaf spots on strawberry (Fragaria × ananassa). Int. J. Syst. Evol. Microbiol. 74:006476. doi: 10.1099/ijsem.0.006476
Michl, K., Berg, G., and Cernava, T. (2023). The microbiome of cereal plants: the current state of knowledge and the potential for future applications. Environ. Microbiome 18:28. doi: 10.1186/s40793-023-00484-y
Mir, A. R., Pichtel, J., and Hayat, S. (2021). Copper: uptake, toxicity and tolerance in plants and management of cu-contaminated soil. Biometals 34, 737–759. doi: 10.1007/s10534-021-00306-z
Mylavarapu, R. S., Wilkinson, N., and Jean, Y. (2024). UF/IFAS Analytical Services Laboratories (ANSERV Labs): Analytical Procedures and Training Manual: Circular 1248/SS312, 1/2024. EDIS. doi: 10.32473/edis-ss312-2002
Osvalde, A., Karlsons, A., Cekstere, G., and Āboliņa, L. (2023). Leaf nutrient status of commercially grown strawberries in Latvia, 2014–2022: a possible yield-limiting factor. Plants 12:945. doi: 10.3390/plants12040945
Paunov, M., Koleva, L., Vassilev, A., Vangronsveld, J., and Goltsev, V. (2018). Effects of different metals on photosynthesis: cadmium and zinc affect chlorophyll fluorescence in durum wheat. Int. J. Mol. Sci. 19:787. doi: 10.3390/ijms19030787
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., et al. (2012). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596. doi: 10.1093/nar/gks1219
Ruano, A., Poschenrieder, C., and Barcelo, J. (1988). Growth and biomass partitioning in zinc-toxic bush beans. J. Plant Nutr. 11, 577–588. doi: 10.1080/01904168809363824
Schnitzer, S. A., Klironomos, J. N., Hillerislambers, J., Kinkel, L. L., Reich, P. B., Xiao, K., et al. (2011). Soil microbes drive the classic plant diversity-productivity pattern. Ecology 92, 296–303. doi: 10.1890/10-0773.1
Siegieda, D., Panek, J., and Frac, M. (2024). Ecological processes of bacterial microbiome assembly in healthy and dysbiotic strawberry farms. BMC Plant Biol. 24:692. doi: 10.1186/s12870-024-05415-8
Singh, T., Bisht, N., Ansari, M. M., Mishra, S. K., and Chauhan, P. S. (2024). Paenibacillus lentimorbus alleviates nutrient deficiency-induced stress in Zea mays by modulating root system architecture, auxin signaling, and metabolic pathways. Plant Cell Rep. 43:49. doi: 10.1007/s00299-023-03133-3
Tagliavini, M., Baldi, E., Lucchi, P., Antonelli, M., Sorrenti, G., Baruzzi, G., et al. (2005). Dynamics of nutrients uptake by strawberry plants (Fragaria × ananassa Dutch.) grown in soil and soilless culture. Eur. J. Agron. 23, 15–25. doi: 10.1016/j.eja.2004.09.002
United States Department of Agriculture. (2025). Web soil survey. Available online at: https://www.nrcs.usda.gov/resources/data-and-reports/web-soil-survey (Accessed June 11, 2025).
van der Heijden, M. G., and Hartmann, M. (2016). Networking in the plant microbiome. PLoS Biol. 14:e1002378. doi: 10.1371/journal.pbio.1002378
Wagg, C., Jansa, J., Schmid, B., and van der Heijden, M. G. (2011). Belowground biodiversity effects of plant symbionts support aboveground productivity. Ecol. Lett. 14, 1001–1009. doi: 10.1111/j.1461-0248.2011.01666.x
Wang, X., Chen, S., Fan, L., Liu, H., Chen, S., Ye, X., et al. (2022). Characterization of a halotolerant GH2 family β-galactosidase GalM from Microvirga sp. strain MC18. Protein Expr. Purif. 194:106074. doi: 10.1016/j.pep.2022.106074
Wu, R., Li, Y., Meng, J., and Han, J. (2025). Effects of dazomet fumigation combined with Trichoderma harzianum on soil microbial community structure of continuously cropped strawberry. Horticulturae 11:35. doi: 10.3390/horticulturae11010035
Xu, X., Passey, T., Wei, F., Saville, R., and Harrison, R. J. (2015). Amplicon-based metagenomics identified candidate organisms in soils that caused yield decline in strawberry. Hortic. Res. 2:15022. doi: 10.1038/hortres.2015.22
Yan, Y., Kuramae, E. E., de Hollander, M., Klinkhamer, P. G., and van Veen, J. A. (2017). Functional traits dominate the diversity-related selection of bacterial communities in the rhizosphere. ISME J. 11, 56–66. doi: 10.1038/ismej.2016.108
Yang, J., Wei, S., Su, D., Zhang, Z., Chen, S., Luo, Z., et al. (2020). Comparison of the rhizosphere soil microbial community structure and diversity between powdery mildew-infected and noninfected strawberry plants in a greenhouse by high-throughput sequencing technology. Curr. Microbiol. 77, 1724–1736. doi: 10.1007/s00284-020-01948-x
Zhang, M., Kong, Z., Fu, H., Shu, X., Xue, Q., Lai, H., et al. (2023). Rhizosphere microbial ecological characteristics of strawberry root rot. Front. Microbiol. 14:1286740. doi: 10.3389/fmicb.2023.1286740
Keywords: rhizosphere microbiome, strawberry, nutrient uptake, food security, food safety
Citation: Kwak M-J, Pandey S, Zhai Y, Choi B, Sutthanonkul T, Lee WS and Jeong KC (2025) Understanding the impact of soil microbiome on strawberry growth and nutritional profiles. Front. Microbiol. 16:1654776. doi: 10.3389/fmicb.2025.1654776
Edited by:
Xiancan Zhu, Anhui Normal University, ChinaCopyright © 2025 Kwak, Pandey, Zhai, Choi, Sutthanonkul, Lee and Jeong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Won Suk Lee, d3NsZWVAdWZsLmVkdQ==; Kwangcheol C. Jeong, a2NqZW9uZ0B1ZmwuZWR1
 Min-Jin Kwak
Min-Jin Kwak Sriniwas Pandey
Sriniwas Pandey Yuting Zhai
Yuting Zhai Bugil Choi1,2
Bugil Choi1,2 Thanyachanok Sutthanonkul
Thanyachanok Sutthanonkul Won Suk Lee
Won Suk Lee Kwangcheol C. Jeong
Kwangcheol C. Jeong