- 1School of Medicine, Nankai University, Tianjin, China
- 2National Key Laboratory of Intelligent Tracking and Forecasting for Infectious Diseases, National Institute for Communicable Disease Control and Prevention, Beijing, China
- 3Research Unit for Unknown Microbe, Institute of Pathogen Biology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 4Department of Epidemiology and Biostatistics, School of Public Health, Peking University, Beijing, China
- 5Infection Management Office, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
- 6Research Center for Reverse Microbial Etiology, Workstation of Academician, Shanxi Medical University, Taiyuan, China
Introduction: This study investigates the immunoenhancing effects of the probiotic Lactobacillus brevis ZG2488 on an adenovirus-vectored SARS-CoV-2 vaccine (AdC68-Delta-S) in mice.
Methods: Mice were administered ZG2488 in combination with AdC68-Delta-S. Immune responses were evaluated by measuring SARS-CoV-2-specific IgG in bronchoalveolar lavage fluid (BALF), IFN-γ secretion by splenocytes, and transcriptomic and CIBERSORT analyses of splenic immune cells.
Results: ZG2488 intervention significantly enhanced local mucosal humoral immunity in the respiratory tract (increased SARS-CoV-2-specific IgG in BALF) and systemic Th1 cellular immunity (increased IFN-γ secretion by splenocytes). Transcriptomic analysis revealed upregulation of the JAK-STAT signaling pathway, antigen processing and presentation pathways, and pro-inflammatory pathways (IL-17/TNF/NLR/PI3K-Akt), alongside downregulation of hyperinflammation-associated pathways. CIBERSORT analysis showed that ZG2488 reshaped splenic immune cell composition, increasing memory CD4+ T cells, Th1 cells, and dendritic cells (DCs), while decreasing macrophages, follicular helper T (Tfh) cells, monocytes, and γδ T cells.
Discussion: These findings demonstrate that L. brevis ZG2488 enhances the immune response to the SARS-CoV-2 vaccine through synergistic and multi-mechanistic actions, supporting its potential as a probiotic adjuvant strategy.
1 Introduction
With the escalating threat of viral infections, the development of effective vaccine strategies has become critically important (Piot et al., 2019). Although a variety of vaccines are already in widespread use, their immunogenicity remains suboptimal, particularly in immunocompromised or high-risk populations (Meng et al., 2022). Therefore, improving vaccine efficacy, especially in these high-risk groups, is of paramount importance.
In recent years, research has shown that probiotics, when used as immunoadjuvants, can enhance vaccine-induced immune responses by modulating both gut and systemic immunity (Lin et al., 2021; Peroni and Morelli, 2021). Probiotics from the Lactobacillus genus, including Lactobacillus brevis, have been demonstrated to regulate immune responses through mechanisms such as cytokine production (Yin et al., 2023; Zielińska et al., 2019), T cell responses (Karimi et al., 2009; Maassen et al., 2000), and antigen presentation (Prado Acosta et al., 2021), thereby enhancing immune protection.
Lactobacillus brevis ZG2488 (hereafter referred to as L. brevis ZG2488) is a novel strain isolated by our research group from the feces of healthy humans (Cao et al., 2025). In preliminary studies, we found that ZG2488 exhibits significant probiotic characteristics, including resistance to artificial gastric juice and bile salts, high adhesion ability, and antimicrobial activity. Further research has shown that ZG2488 can enhance nitric oxide production and modulate the expression of pro-inflammatory cytokines (such as IL-1β, IL-6, and TNF-α) in macrophages, thereby positively influencing immune responses (Cao et al., 2025). Despite its demonstrated immunomodulatory effects, the role of ZG2488 in enhancing the immunogenicity of the SARS-CoV-2 vaccine remains understudied.
This study hypothesizes that Lactobacillus brevis ZG2488, through its immunomodulatory properties, can enhance the immune response to the SARS-CoV-2 vaccine, particularly in mucosal and cellular immunity, thus improving the overall vaccine efficacy. To test this hypothesis, we assessed the effects of ZG2488 on the immune response in a mouse model, specifically evaluating its ability to enhance the immunogenicity of the SARS-CoV-2 vaccine (AdC68-Delta-S). We focused on examining the impact of ZG2488 on local mucosal immune responses, systemic cellular immunity, and immune cell infiltration patterns, and further explored its potential molecular mechanisms through transcriptomic analysis.
2 Methods
2.1 Bacterial culture
Lactobacillus brevis ZG2488 isolated from healthy human feces was grown in De Man Rogosa and Sharpe (MRS) agar plate at 37 °C for 24 h under aerobic condition. The logarithmic phase of L. brevis ZG2488 was washed and resuspended in sterile phosphate-buffered saline (PBS) for oral inoculation of mice. Lactobacillus plantarum GUANKE (LPG) was used as a comparative strain owing to its boosting immune response to the vaccine (Xu et al., 2021). It was also isolated from the feces of healthy people and grown in MRS plate at 37 °C for 24 h under aerobic condition.
2.2 Animal experimental design
Institute of Cancer Research (ICR) mice (n = 5 per group) were primed and boosted via intramuscular injection with AdC68-Delta-S vaccine (5 × 1010 viral particles per dose) at weeks 0 and 4 (Xu et al., 2021). Beginning 3 days prior to the booster immunization, mice were provided with drinking water containing 1 g/L of ampicillin daily to deplete gut microbiota. Subsequently, mice were orally gavaged with ZG2488 (5 × 109 CFU/200 μL) or phosphate-buffered saline (PBS; control group) for 3 consecutive days; 7 days post-intervention, mice were sacrificed, and serum, bronchoalveolar lavage fluid (BALF), and spleens were collected for analysis. Schematic overview of experimental design is shown in Figure 1A.
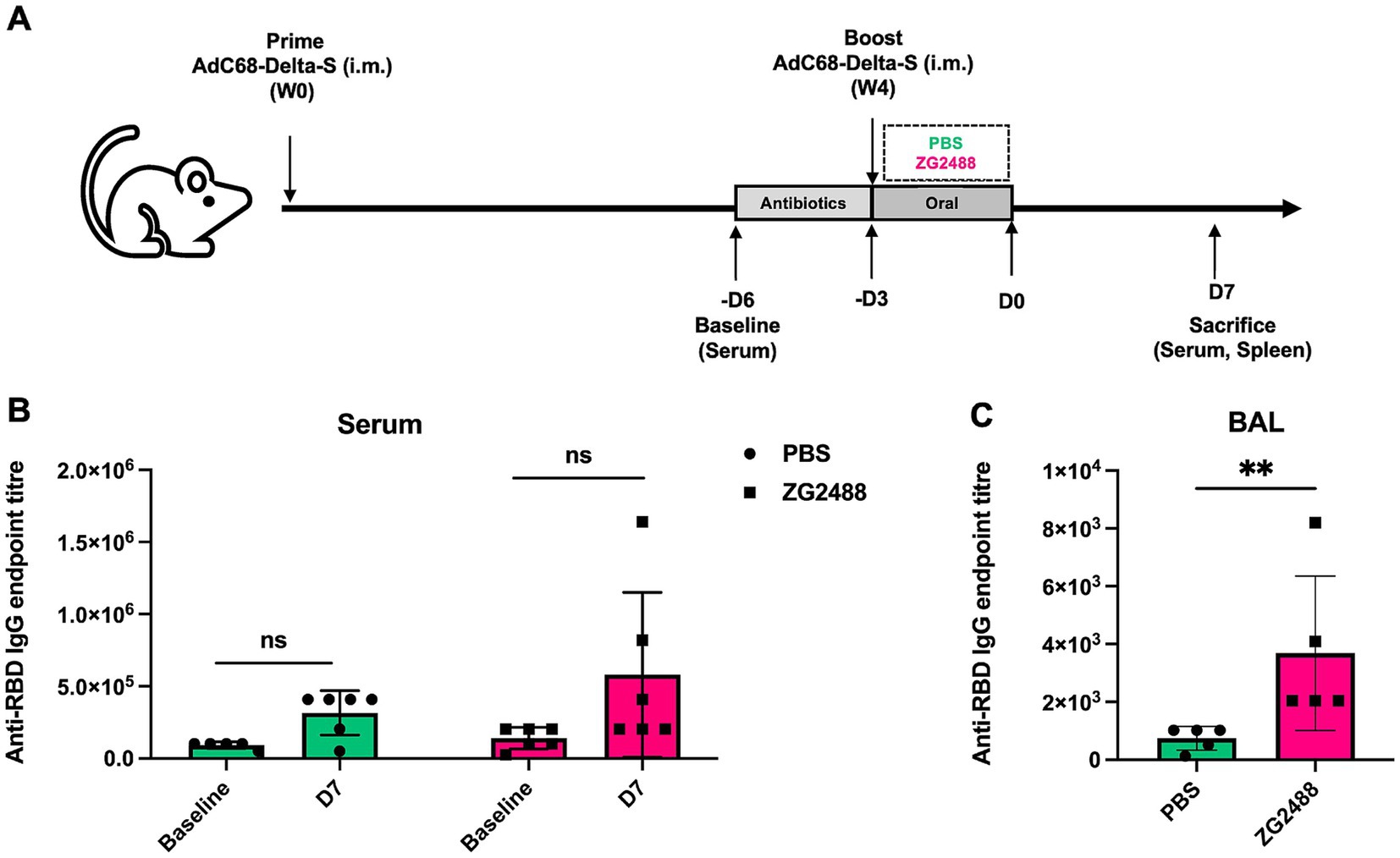
Figure 1. Effect of Lactobacillus brevis ZG2488 on SARS-CoV-2-specific IgG levels in serum and BALF of mice following AdC68-Delta-S vaccination. (A) Schematic overview of experimental design. (B) The concentration of anti-RBD IgG endpoint titer in serum at baseline and day 7 (D7) post-vaccination in PBS and ZG2488-treated mice. No significant difference was observed between groups. (C) Anti-RBD IgG endpoint titer in BALF at D7 post-vaccination. ZG2488 treatment significantly increased IgG levels compared to PBS (p < 0.01). Data are presented as median ± interquartile range. ns, not significant, **p < 0.01.
2.3 Ethics statement and animals
Specific pathogen-free (SPF) female ICR mice (6–8 weeks old) were procured from the Beijing Vital River Laboratory Animal Technology Co., Ltd. [Laboratory Animal Permit No. SYXK (Jing) 2022-0029] and housed in the Laboratory Animal Center of the Chinese Center for Disease Control and Prevention. All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee (IACUC) of the same center (Approval Code: 2022-023) and conducted in accordance with national and institutional guidelines for animal care and use.
2.4 ELISpot assay
Splenocytes were isolated from vaccinated mice and resuspended in complete RPMI-1640 medium. Cells were seeded at 5 × 105 cells per well in 96-well ELISpot plates pre-coated with anti-mouse IFN-γ capture antibody. The splenocytes were stimulated with RBD peptide pool (15-mer peptides overlapping by 11 amino acids, spanning the SARS-CoV-2 Delta variant spike RBD domain) at 2 μg/mL per peptide (Xu et al., 2021). Positive control wells received PMA (50 ng/mL) plus ionomycin (1 μg/mL), while negative control wells contained medium only. After 20 h in a 5% CO2 atmosphere, cells were removed and plates were processed using the mouse IFN-γ ELISpot kit (BD Bioscience, #551083) following manufacturer’s instructions: biotinylated detection antibody incubation (2 h, room temperature), streptavidin-HRP conjugate incubation (1 h, room temperature), and development with AEC substrate (15 min). IFN-γ-producing spot-forming cells (SFCs) were quantified using an iSpot Spectrum plate reader (AID Autoimmun Diagnostika GmbH). Results were expressed as IFN-γ SFCs per 106 splenocytes after subtracting negative control values.
2.5 Antibody detection
RBD-specific antibody titers in serum and BAL fluid were quantified via ELISA. Samples were heat-inactivated (56 °C for 30 min) prior to analysis. 96-well plates were coated with 100 μL/well recombinant SARS-CoV-2 RBD protein (1 mg/mL; Z03483-1, Genscript) overnight at 4 °C. After PBS-T (0.5% Tween-20) washes, plates were blocked with PBS-T/5% non-fat milk (200 μL/well, 2 h, RT). Serial two-fold dilutions (initial 1:100) of samples were added and incubated (3 h, RT). For IgG detection, HRP-conjugated goat anti-mouse IgG (1:5,000; ZB-5305, Zsbio) in PBS-T/5% milk was added (100 μL/well, 1 h, RT). Following PBS-T washes, OPD substrate (SIGMAFAST OPD tablet SLCC0308 in 20 mL H2O) was added. Reaction was stopped with 1 M H2SO4 after 5 min (RT), and absorbance was read at 492 nm (Synergy Microplate Reader, Bio-Tek). Endpoint titers were defined as the highest dilution with OD492 value greater than twice the background signal.
2.6 Transcriptomic and immune cell infiltration analysis
RNA sequencing was performed on splenocytes using the Illumina NovaSeq 6000 platform (Illumina, San Diego, CA). RNA libraries were prepared using the TruSeq Stranded mRNA Library Prep Kit (Illumina, San Diego, CA) according to the manufacturer’s instructions. Differentially expressed genes (DEGs) were analyzed using DESeq2 (version 1.30.0), and gene set enrichment analysis (GSEA) was performed with the GSEA software (version 4.1.0). The proportions of splenic immune cell subsets were inferred using the CIBERSORT (version 1.03) deconvolution algorithm. Statistical analysis was performed using GraphPad Prism (version 9.0.0), with p < 0.05 considered statistically significant.
2.7 Data analysis
Statistical analyses were conducted using GraphPad Prism version 10.0 (GraphPad Software, Inc., San Diego, CA, United States). Experimental values are expressed as mean ± standard deviation (SD). Intergroup differences were evaluated as follows: comparisons between two groups utilized two-tailed unpaired Student’s t-tests with Welch’s correction. For comparisons involving three or more groups, one-way analysis of variance (ANOVA) was applied, followed by Dunnett’s post hoc test for multiple comparisons. A p-value <0.05 was considered statistically significant, with significance levels denoted as *p < 0.05, **p < 0.01, and ***p < 0.001. For transcriptome data analysis, the Benjamini–Hochberg method was used to perform false discovery rate (FDR) correction on the p-values obtained from DESeq2 and GSEA analyses. All reported p-values for differentially expressed genes and enriched pathways are FDR-adjusted values, with the significance threshold set at FDR <0.1.
3 Results
3.1 Lactobacillus brevis ZG2488 enhances SARS-CoV-2 IgG antibody against SARS-CoV-2 in the respiratory tract
We first evaluated the levels of SARS-CoV-2-specific IgG antibodies in serum and bronchoalveolar lavage fluid (BALF). Results showed that the level of SARS-CoV-2-specific IgG antibodies in BALF was significantly higher in the L. brevis ZG2488 intervention group compared to the PBS control group (p < 0.05, Figure 1B). However, although the serum SARS-CoV-2 IgG antibody level showed an upward trend in the probiotic group, the difference between the two groups did not reach statistical significance (p > 0.05, Figure 1C). These results indicate that L. brevis ZG2488 preferentially enhances the respiratory tract’s local mucosal immune response, significantly promoting the production of SARS-CoV-2-specific antibodies in BALF, suggesting an important role for probiotics in local immunomodulation.
3.2 Lactobacillus brevis ZG2488 enhances antigen-specific Th1 cellular immune responses both in vivo and in vitro
To evaluate the effect of L. brevis ZG2488 on systemic cellular immunity in vivo, splenocytes were isolated from mice administered L. brevis ZG2488 or PBS after vaccination and subjected to an ELISpot assay. Compared to the PBS control group, the number of IFN-γ-producing spot-forming cells (SFCs) in the spleens of the L. brevis ZG2488 group was significantly increased (p < 0.05, Figure 2A), clearly indicating that in vivo L. brevis ZG2488 effectively enhanced the antigen-specific IFN-γ response of splenocytes to the SARS-CoV-2 vaccine, suggesting enhanced Th1-type cellular immunity.
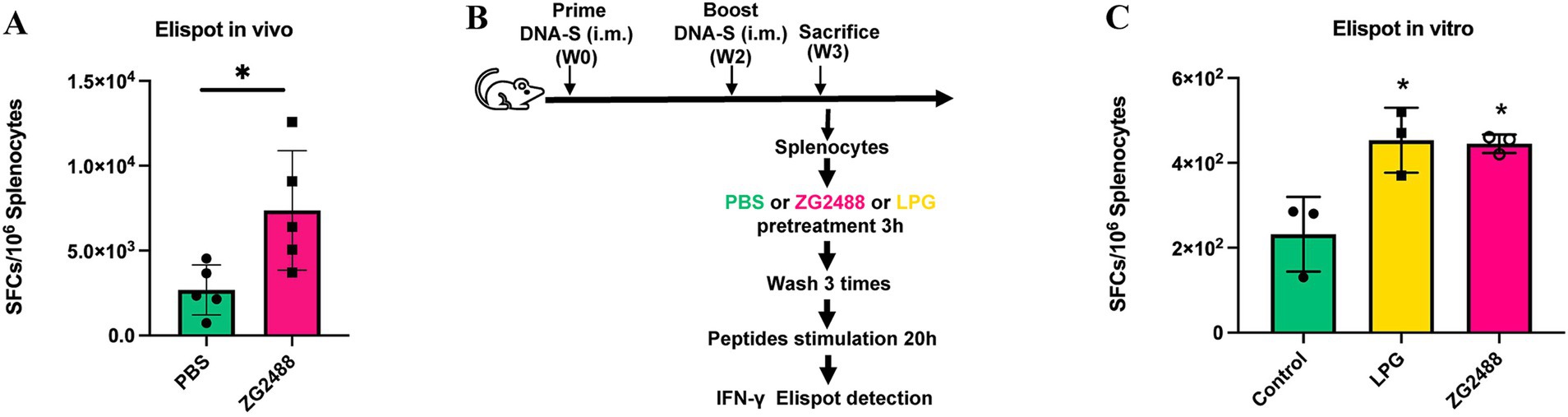
Figure 2. Lactobacillus brevis ZG2488 enhances Th1-type immune response to the AdC68-Delta-S vaccine. (A) In vivo ELISpot analysis showing increased IFN-γ producing splenocytes in the ZG2488-treated group compared to PBS controls (p < 0.05). (B) ELISpot analysis of splenocytes from in vitro culture with LPG, ZG2488, and PBS, showing significantly higher IFN-γ production in the ZG2488 group (p < 0.05). (C) Comparison with the positive control, LGP, confirmed ZG2488’s ability to stimulate IFN-γ production in vitro. Data are shown as mean ± SEM, *p < 0.05.
To assess the direct immunomodulatory effect of L. brevis ZG2488, splenocytes were harvested from vaccinated, non-probiotic-treated healthy mice, co-cultured in vitro with probiotics or PBS, and analyzed by ELISpot assay. Results found that compared to the PBS co-culture group, probiotic co-culture significantly increased the number of IFN-γ-producing SFCs in splenocytes (p < 0.05, Figures 2B,C), and the level of IFN-γ SFCs induced by L. brevis ZG2488 was not significantly different from that of the positive control strain co-culture group [L. plantarum GUANKE, previously confirmed by our laboratory to enhance IFN-γ production capability (Xu et al., 2021)] (p > 0.05). This in vitro result directly confirms that L. brevis ZG2488 possesses the immunomodulatory activity to directly stimulate splenocytes and promote IFN-γ production, with a stimulatory potency equivalent to that of the known effective positive control strain. In vivo intervention and in vitro co-culture experiments consistently demonstrate that L. brevis ZG2488 effectively promotes IFN-γ production by splenocytes in response to the SARS-CoV-2 vaccine antigen, strongly supporting the role of L. brevis ZG2488 in optimizing vaccine efficacy by enhancing Th1-type cellular immune responses.
3.3 Lactobacillus brevis ZG2488 specifically augments the splenocyte IFN-γ signaling axis
Transcriptomic analysis of splenocytes indicated that the immune regulatory effects of L. brevis ZG2488 intervention were significant. In the GO enrichment analysis, immune response-activating and regulating signaling pathways (such as “immune response-activating signaling pathway” and “immune response-regulating signaling pathway”) are significantly enriched, indicating the involvement of genes in key immune regulation processes related to the SARS-CoV-2 immune response. Additionally, the enrichment of terms related to transcription factors and immune cell activation in “cell cycle” and “immune response” processes suggests the immunoenhancing effect of the COVID-19 vaccine (Figure 3A).
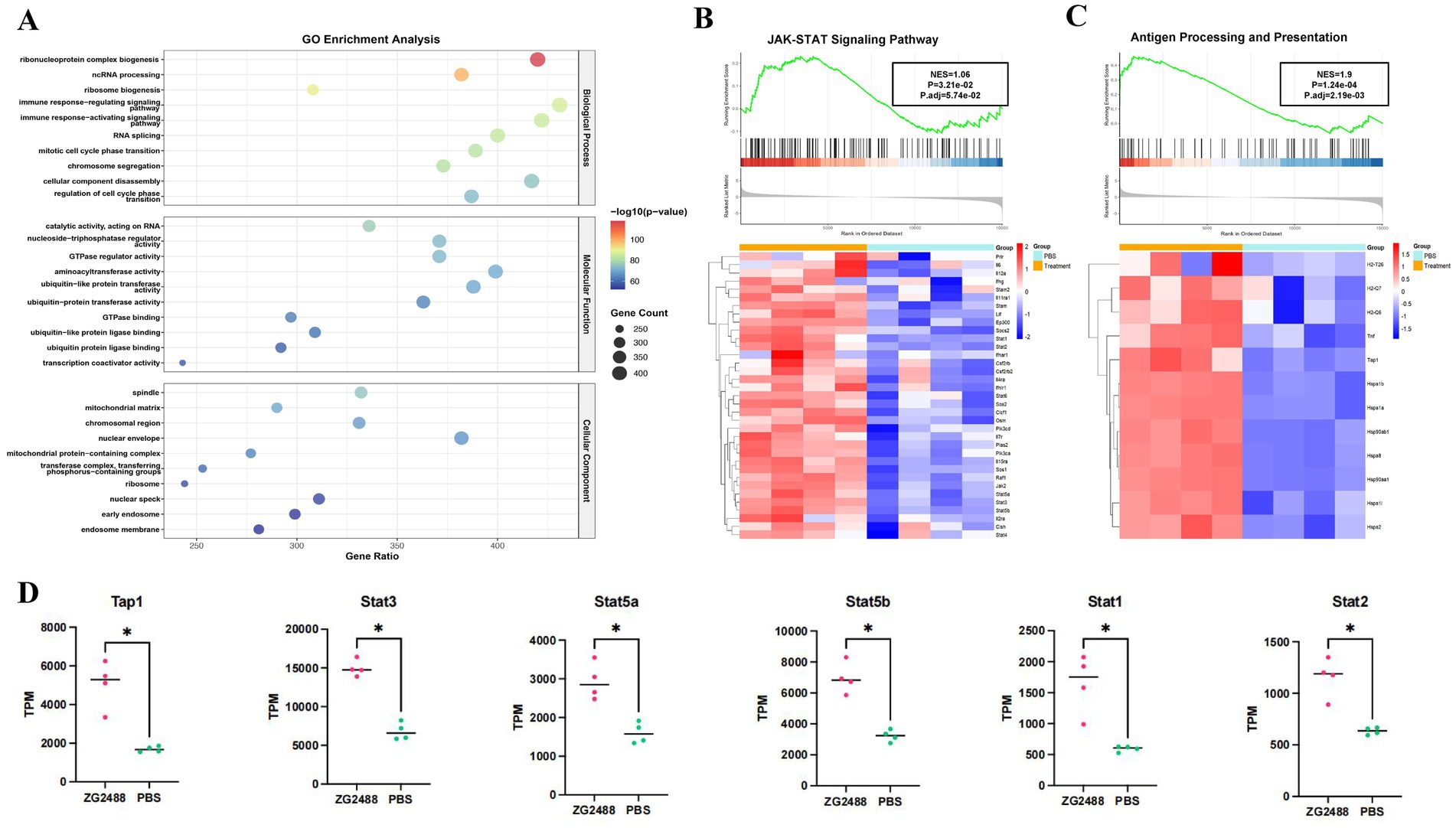
Figure 3. Transcriptomic analysis reveals activation of the JAK–STAT signaling pathway and antigen processing in ZG2488-treated mice. (A) GO enrichment analysis. (B,C) Gene set enrichment analysis (GSEA) of the JAK–STAT pathway and antigen processing and presentation pathway showing significant upregulations in ZG2488-treated mice. The heatmaps show the expression levels of key genes in these pathways. Statistical significance was determined using FDR adjusted p-values (Benjamini–Hochberg correction). (D) Expression levels of selected genes involved in JAK–STAT pathway in ZG2488-treated and PBS-treated groups. Data are presented as mean ± SD. *p < 0.05 and **p < 0.01.
GSEA enrichment analysis further elucidated the mechanistic basis for the enhanced immune response, specifically revealing the activation of core pathways involved in IFN-γ production and signaling following L. brevis ZG2488 intervention. First, the JAK–STAT signaling pathway was significantly enriched and upregulated in the L. brevis ZG2488 group (FDR < 0.05, normalized enrichment score NES = 1.06, Figure 3B), with increased expression of key genes (such as Ifng, Stat1, Stat2, Jak2, and Supplementary Figure S1 and Figure 3D). Since JAK–STAT is the core downstream cascade of IFN-γ receptor signaling (He et al., 2023; Lee and Benveniste, 1996; Xu et al., 2021), this upregulation provides the mechanistic basis for the enhanced IFN-γ secretion observed in the ELISpot assay (Figures 2A,C). Second, genes related to antigen processing and presentation were coordinately upregulated (FDR < 0.01, NES = 1.9, Figure 3C), involving increased expression of MHC-I genes (Tap1), MHC-II genes (H2-T26, H2-Q7, and H2-Q6), and antigen presentation-related genes (Hsp90ab1, Hsp90aa1, Hsp1b, and Hsp1a), indicating enhanced antigen-presenting cell (APC) function (Tesniere et al., 2008). This is consistent with the results of enhanced antigen-specific T cell activation and potent IFN-γ responses to the vaccine antigen (Figure 2A). These results demonstrate that L. brevis ZG2488 directly reinforces Th1-polarized cellular immunity by transcriptionally coordinating enhanced antigen presentation efficiency and amplification of the IFN-γ–JAK–STAT signaling program. The upregulation of IFN-γ, JAK–STAT pathway components (Stat1, Stat2, Jak2), and MHC-I/II genes, all key markers of Th1 immune responses (He et al., 2023), indicates the promotion of Th1 polarization, a hallmark of cellular immunity against intracellular pathogens. Although qPCR validation for key transcriptional changes was not performed due to sample limitations, the RNA-seq findings for these core pathway genes were supported by stringent statistical correction and, most importantly, are highly consistent with the observed functional immune outcomes.
3.4 Lactobacillus brevis ZG2488 pleiotropically modulates accessory immune and inflammatory pathways
Beyond the core IFN-γ axis, L. brevis ZG2488 differentially regulated multiple immune networks, reflecting its pleiotropic immunomodulatory effects. First, pro-inflammatory and innate immune pathways such as IL-17 signaling (FDR <0.05, NES = 1.446), TNF signaling (FDR <0.01, NES = 1.9), and NOD-like receptor signaling (FDR <0.05, NES = 1.31) were upregulated (Figure 4A), indicating synchronized enhancement of innate immune recognition, inflammatory responses, and cell survival signals.
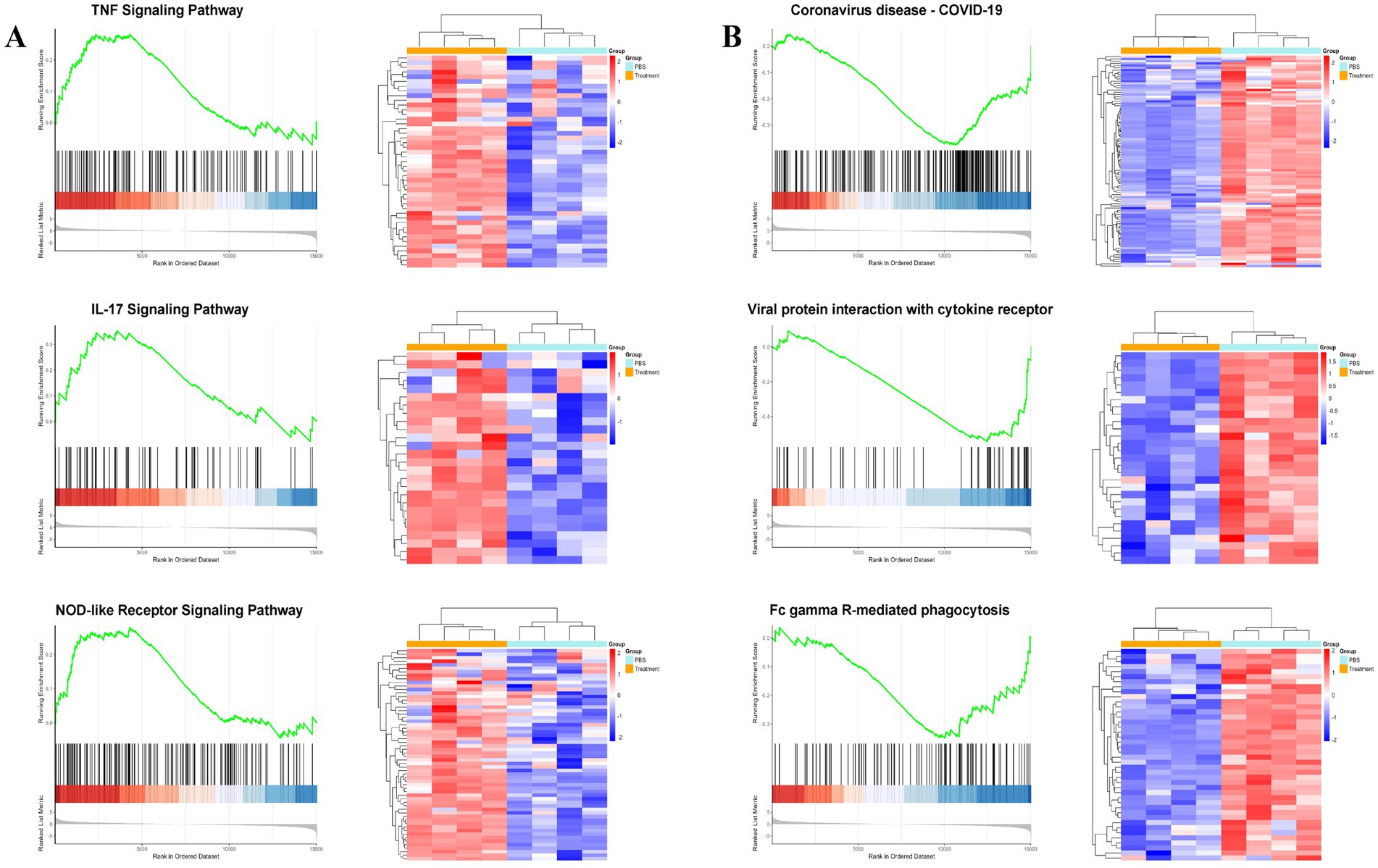
Figure 4. Lactobacillus brevis ZG2488 modulates immune and inflammatory pathways. (A) Upregulation of pro-inflammatory pathways, including IL-17, TNF, and NOD-like receptor signaling in ZG2488-treated mice. (B) Downregulation of immune-related pathways, such as viral protein-cytokine receptor interaction and coronavirus disease pathways. GSEA results for these pathways are shown with NES scores and adjusted p-values. Statistical significance was determined using FDR adjusted p-values (Benjamini–Hochberg correction). Data indicate modulation of inflammatory and antiviral responses. *p < 0.05 and **p < 0.01.
Second, genes expression in pathways such as viral protein-cytokine receptor interaction (FDR <0.05, NES = −1.927), coronavirus disease-COVID-19 (FDR <0.01, NES = −1.564), and FcγR-mediated phagocytosis (FDR <0.05, NES = −1.319) was suppressed (Figure 4B). This selective downregulation may prevent excessive inflammation or resource exhaustion during the immune priming phase induced by vaccination. L. brevis ZG2488 shapes a balanced immunotranscriptomic landscape, enhancing protective Th1 and innate immunity while actively suppressing hyperinflammation and virus susceptibility-related pathways.
3.5 Lactobacillus brevis ZG2488 alters the immune cell infiltration landscape in the spleen
According to CIBERSORT analysis results (Figure 5), there were significant differences in splenic immune cell composition between the L. brevis ZG2488 intervention group and the PBS group. The probiotic group showed higher proportions of memory CD4+ T cells, Th1 cells, and dendritic cells (DCs). These results are consistent with the upregulation of antigen processing and presentation-related genes (Figure 3B). Specifically, the increased infiltration of dendritic cells indicates an enhanced role for antigen presentation in the immune response. In contrast, the probiotic intervention group had lower infiltration of macrophages, monocytes, and γδ T cells. These changes in immune cell populations suggest that L. brevis ZG2488 may prevent excessive inflammatory responses by suppressing overactive immune cell populations (such as macrophages and monocytes). These alterations in immune cell subsets suggest that probiotic intervention reshapes the immune landscape by enhancing adaptive immune memory (e.g., memory CD4+ T cells), promoting Th1 responses, and increasing dendritic cell abundance, while concurrently reducing the proportions of pro-inflammatory cell populations (e.g., macrophages and monocytes). This shift is consistent with an immune profile associated with effective vaccine responses. Overall, the alteration in immune cell infiltration patterns induced by L. brevis ZG2488 helps explain the enhanced cellular immunity (e.g., IFN-γ production) and optimized immune response.
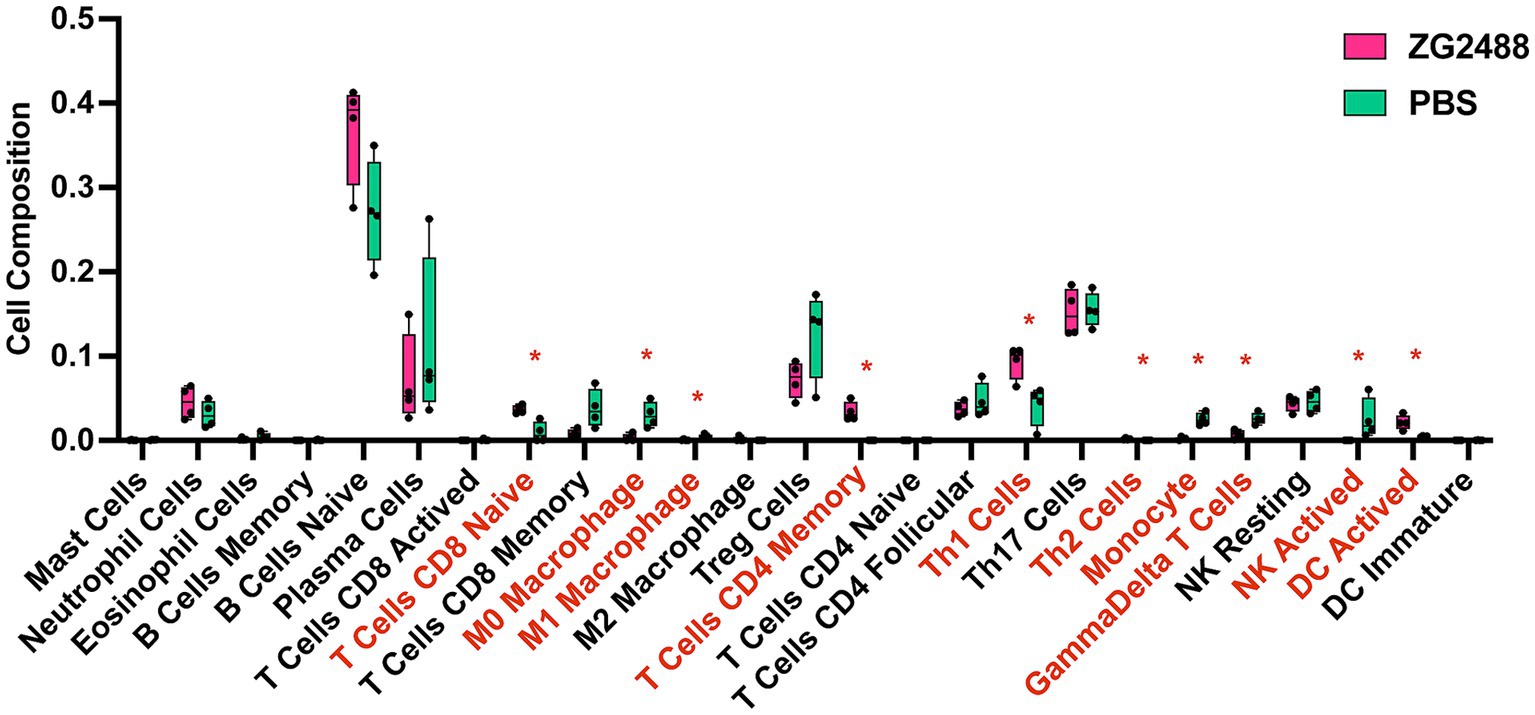
Figure 5. Lactobacillus brevis ZG2488 alters immune cell infiltration in the spleen. CIBERSORT analysis shows significant changes in the immune cell composition of spleens from ZG2488-treated versus PBS-treated mice. The ZG2488 treatment increased the proportions of memory CD4+ T cells, Th1 cells, and dendritic cells, while decreasing macrophages, monocytes, and γδ T cells. Data are shown as boxplots overlaid with individual data points. TPM, transcripts per million. Asterisks indicate significant differences. *p < 0.05.
4 Discussion
This study aimed to investigate the impact of L. brevis ZG2488 on the immune response to the SARS-CoV-2 vaccine in mice, focusing on various immune parameters such as SARS-CoV-2-specific IgG antibodies, IFN-γ secretion, immune cell infiltration, and transcriptomic changes. Our results indicate that L. brevis ZG2488 significantly enhanced antigen-specific antibody and T-cell responses, providing evidence for its immunomodulatory potential in improving vaccine efficacy (Redenti et al., 2024; Ryan et al., 2025; Zhu et al., 2025). Beyond mechanistic insights, our findings suggest the potential clinical relevance of ZG2488 as a candidate vaccine adjuvant. Orally administered ZG2488 was associated with enhanced mucosal humoral immunity (elevated BALF IgG) and systemic Th1-cell-mediated immunity (increased IFN-γ production). This dual activity may be advantageous against respiratory pathogens such as SARS-CoV-2, where immunity at the mucosal site of entry could contribute to overall protection. Further clinical studies are needed to evaluate the translational potential of ZG2488-adjuvanted vaccination in humans.
Lactobacillus brevis ZG2488 preferentially enhanced local mucosal immunity in the respiratory tract, as evidenced by a significant increase in SARS-CoV-2-specific IgG antibodies in bronchoalveolar lavage fluid (BALF), while serum IgG showed an upward trend but did not reach statistical significance. This result is consistent with previous studies suggesting that probiotics not only modulate systemic immunity but also enhance local immune responses, particularly in mucosal tissues (Jin et al., 2021; Ma et al., 2023; Tseng et al., 2025; Zhao et al., 2024). Lactobacilli, especially, are crucial in enhancing local immunity by promoting gut mucosal immune responses and boosting the efficacy of antigen-presenting cells, which are key for improving local vaccine immune responses (Rawling et al., 2023; van Baarlen et al., 2013; Zhang et al., 2023).
In addition to enhancing humoral immunity, L. brevis ZG2488 significantly improved the ability of splenocytes to produce IFN-γ in response to the SARS-CoV-2 vaccine, both in vivo and in vitro. IFN-γ, as a key effector cytokine in the Th1 immune response, is essential for antiviral defense and cellular immunity (Assaf et al., 2017; Lei et al., 2024; Rosloniec et al., 2002). The upregulation of IFN-γ production by L. brevis ZG2488 suggests that the probiotic enhances the ability to clear the virus, potentially improving the immune response to SARS-CoV-2. Transcriptomic analysis further revealed the molecular basis of this enhancement, highlighting the upregulation of genes involved in the JAK–STAT signaling pathway (Ifng, Stat1, Stat2, Jak2) and the antigen processing and presentation pathway (Tap1, MHC-II genes, Hsps). These findings align with other studies on Lactobacilli’s effects on immune regulation, confirming their role in enhancing immune function through activation of key immune signaling pathways (Ding et al., 2017; Ji et al., 2025). We believe that the existing data provides strong support for our mechanistic conclusions. However, due to current limitations in experimental conditions and resources, we were unable to conduct the relevant knockout validation experiments. Should the opportunity arise in the future, we would be eager to consider performing these knockout validations to further strengthen our conclusions.
Moreover, L. brevis ZG2488 displayed pleiotropic effects on innate immune signaling pathways, upregulating pro-inflammatory pathways such as IL-17, TNF, NOD-like receptor, and PI3K-Akt. Additionally, this study uncovered the pleiotropic effects of L. brevis ZG2488 on several immune signaling pathways. Interestingly, in contrast to earlier studies that found L. brevis ZG2488 downregulates TNF-α in macrophages, the current study observed an upregulation of the TNF pathway in splenocytes (Cao et al., 2025). Beyond the enrichment of the TNF signaling pathway by GSEA, we confirmed that the gene encoding the key ligand TNF-α itself was significantly upregulated in the spleens of ZG2488-treated mice compared to PBS controls (Supplementary Figure S2, p < 0.05 by t-test). This direct evidence at the gene expression level solidifies the conclusion that the TNF pathway activation is a hallmark of the immune response potentiated by L. brevis ZG2488. This apparent contradiction may be due to differences in the immune cell types involved and the specific context of the immune environment (Adami et al., 2020). While the downregulation of TNF-α in macrophages likely represents a protective effect against excessive innate inflammation, the upregulation of the TNF signaling pathway in vaccine-primed splenocytes suggests a potentiation of adaptive immunity. Many studies have demonstrated the crucial role of TNF-α in the early stages of vaccination (Maggioli et al., 2016). For example, research has shown that individuals receiving TNF inhibitors have a reduced response to the flu vaccine (Munusamy Ponnan et al., 2019). Furthermore, after COVID-19 vaccination, this pathway promotes local immune responses, helping to enhance antigen presentation, particularly in antigen processing and T cell activation (Kawakita et al., 2025). This is very important for the early immune response, aiding in the initiation of the immune response and the establishment of immune memory (Procaccini et al., 2021). Therefore, the upregulation of the TNF signaling pathway by L. brevis ZG2488 in this specific context is not necessarily contradictory but rather highlights its context-dependent immunomodulatory function. It may enhance vaccine responses under conditions that require robust T-cell activation—key processes for a strong cellular immune response (Geisen et al., 2022). This raises the possibility that L. brevis ZG2488 may act as an immunoadjuvant by upregulating TNF-α, thus stimulating a more robust inflammatory response, which in turn promotes effective antigen presentation and T-cell activation. This mechanism provides new insights into the potential of L. brevis ZG2488 as an adjuvant to enhance vaccine-induced immunity. Notably, while some probiotic strains modulate inflammation by downregulating TNF-α to prevent excessive immune activation, L. brevis ZG2488 may function differently by upregulating TNF-α to drive stronger immune responses, particularly in the early stages of immunity. These contrasting effects on TNF-α signaling reflect the strain-specific and context-dependent nature of probiotic-mediated immune modulation.
Similarly, IL-17 signaling contributes to mucosal immunity and defense against extracellular pathogens. Although dysregulated IL-17 can drive autoimmunity, its induction following vaccination supports neutrophil recruitment, barrier integrity, and antibody-mediated protection. In the context of a respiratory-targeted vaccine, this Th17-skewed response may enhance mucosal immunity in the airways (Schnoeller et al., 2014; Wu et al., 2021). The NOD-like receptor signaling pathway is involved in inflammasome activation and innate immune sensing. While aberrant NLR signaling can cause excessive inflammation, its role in vaccine adjuvancy is well-established. NLR activation promotes cytokine production and co-stimulatory molecule expression, thereby bridging innate and adaptive immunity (Higgins and Mills, 2010; Maisonneuve et al., 2014). Moreover, the PI3K-Akt signaling pathway, crucial for cell proliferation, survival, and immune responses, was also upregulated (Glaviano et al., 2023; Manning and Cantley, 2007). By activating this pathway, L. brevis ZG2488 may enhance immune cell proliferation, particularly T cells and B cells, further promoting a stronger immune defense. Importantly, the potential for immunopathology appears to be balanced by the concurrent downregulation of hyperinflammation-related pathways, such as viral protein-cytokine interactions and FcγR-mediated phagocytosis, as shown in our GSEA results (Figure 4B). This coordinated regulation—enhancing vaccine-relevant inflammation while suppressing nonspecific hyperactivation—suggests that ZG2488 promotes a focused and adaptive immune response rather than indiscriminate inflammation. Thus, the upregulation of these pathways is likely a hallmark of effective immune stimulation rather than uncontrolled inflammation. Future studies involving cytokine blockade or challenge models could further confirm the functional balance of these responses.
Conversely, L. brevis ZG2488 also selectively downregulated pathways like viral protein-cytokine receptor interaction, coronavirus disease-COVID-19, and FcγR-mediated phagocytosis. This selective downregulation could prevent excessive inflammation or resource depletion during the immune priming phase, helping to maintain a balanced immune response. These findings illustrate the complex and bidirectional nature of probiotic-mediated immune modulation, where probiotics like L. brevis ZG2488 may enhance immune responses while preventing potential harmful overactivation of certain immune pathways.
CIBERSORT analysis revealed that L. brevis ZG2488 modulates immune cell infiltration in the spleen, increasing the proportion of memory CD4+ T cells, Th1 cells, and DCs, while decreasing macrophages, CD4+ Tfh cells, monocytes, and γδ T cells. The increased DCs correlate with upregulated antigen presentation genes, which support the initiation of adaptive immune responses (Hilligan and Ronchese, 2020). The increased presence of memory CD4+ T and Th1 cells is indicative of a strengthened Th1 response, suggesting potential for long-term protection. The reduction in macrophages, monocytes, and Tfh cells may reflect an optimization of the immune response, suppressing overactive or potentially pro-inflammatory cell populations, thereby preventing excessive inflammation or adverse immune reactions. Lactobacilli are known to modulate immune cell infiltration in similar ways, enhancing the activity of memory T cells and Th1 cells while suppressing the overactivation of macrophages and γδ T cells to avoid excessive immune activation (Finamore et al., 2019; Liu et al., 2014; Min et al., 2023).
In summary, Lactobacillus brevis ZG2488 significantly enhanced the immune response to the SARS-CoV-2 vaccine in mice. This effect is achieved through several mechanisms: enhancing antigen presentation, modulating immune cell activity, promoting the secretion of protective cytokines (particularly IFN-γ), regulating immune signaling networks, and reshaping immune cell composition (e.g., increasing protective cells like DCs, Th1 cells, and memory CD4+ T cells). These findings underscore the important role of probiotics in enhancing vaccine-induced immunity, particularly through their immunomodulatory effects. We would like to emphasize that this study focused on the peak effector phase at 7 days post-boost to optimally capture the immunomodulatory effects of the probiotic, while future investigations incorporating additional timepoints will be essential to delineate the kinetics of immune response initiation, peak, and memory formation following ZG2488-adjuvanted vaccination.
We acknowledge that the sample size (n = 5 per group) is a limitation, although it meets the standard for preliminary mouse studies. However, the consistent and robust effects observed across bodily fluid, cellular, and transcriptome analyses, coupled with rigorous statistical corrections, strengthen our confidence in the conclusions. Future studies utilizing larger cohorts will be valuable for further validating these findings and exploring more subtle effects. Despite these promising results, one limitation of this study is the absence of a control group that received neither antibiotic nor probiotic treatment but was only vaccinated. Consequently, our findings specifically define the immune-enhancing effects of L. brevis ZG2488 within an antibiotic-perturbed microbiome background. It should also be noted that all mice in this study underwent antibiotic pretreatment to facilitate probiotic intervention. While this approach ensured that the observed immunomodulatory effects could be attributed to the action of L. brevis ZG2488 under controlled conditions, it prevented us from determining the independent impact of microbiota depletion itself on the vaccine response, as well as the potential influence of the probiotic mediated through gut microbiota. Future studies will include a non-antibiotic-treated control group to comprehensively dissect the respective contributions of the microbiome, antibiotic pretreatment, and probiotic intervention to vaccine-induced immune responses, and to clarify the specific roles of the microbiome and the probiotic in vaccine efficacy. Furthermore, while this study provides a foundational characterization of the novel strain L. brevis ZG2488, future research would benefit from direct comparisons with well-established probiotic adjuvants, such as LPG. Our group has previously demonstrated the efficacy of LPG in enhancing vaccine-induced immune responses in vivo (Xu et al., 2021). A dedicated comparative study, designed specifically to benchmark the efficacy of ZG2488 against LPG and other documented strains, would offer valuable insights into their relative potency and potential synergistic effects. Such research would significantly advance our understanding of the structure–activity relationships and precise mechanisms of action of different probiotic strains within the immune system. Also, while our study demonstrates enhanced immunity through antibody and IFN-γ ELISpot assays supported by transcriptomic mechanistic insights, it does not fully explore all immune functional outcomes. Future studies should include additional assays such as flow cytometry or viral neutralization to further define L. brevis ZG2488’s immunomodulatory scope. Finally, it is important to note that while our transcriptomic and immune deconvolution analyses provide a comprehensive and multi-faceted view of the immunomodulatory effects of L. brevis ZG2488, they are inherently correlative and descriptive. The causal mechanisms underlying these observations remain to be formally established. However, the strong coherence between the gene expression signatures, pathway activities, and functional immune outcomes (e.g., IFN-γ production) lends strong support to our model. Future studies employing mechanistic approaches, such as adoptive cell transfer or antibody-mediated neutralization of key cytokines like IFN-γ will be essential to definitively prove causal relationships and elucidate the precise molecular mechanisms by which L. brevis ZG2488 enhances vaccine immunity. These considerations will help optimize the therapeutic use of probiotics in vaccine adjuvant strategies.
5 Conclusion
This study demonstrates that supplementation with Lactobacillus brevis ZG2488 significantly potentiates the immunogenicity of the SARS-CoV-2 AdC68-Delta-S vaccine in mice through multifaceted immunomodulatory mechanisms. Mechanistically, ZG2488 orchestrated a dual enhancement of localized and systemic immunity by (1) augmenting mucosal humoral defenses in the respiratory tract, evidenced by elevated SARS-CoV-2-specific IgG in BALF; (2) amplifying systemic cellular immunity via robust IFN-γ production by splenocytes; (3) globally reprogramming immune signaling networks, with transcriptomic profiling revealing targeted upregulation of the IFN-γ-JAK–STAT axis and antigen presentation pathways, synergistic activation of pro-inflammatory pathways (IL-17, TNF, NLR, PI3K-Akt), and selective downregulation of antiviral/immune exhaustion pathways (viral protein–cytokine interactions, COVID-19-related genes, FcγR-mediated phagocytosis); and (4) remodeling splenic immune architecture through expansion of memory CD4+ T cells, Th1 subsets, and dendritic cells, coupled with contraction of macrophages, Tfh cells, monocytes, and γδ T cells. Although ZG2488 shows promise in enhancing vaccine-induced immune responses, this study did not assess its effects on clinically relevant outcomes such as protection from viral challenge, longevity of immune memory, or neutralizing antibody activity. Thus, while ZG2488 exhibits immunomodulatory properties supportive of adjuvant function, future studies are necessary to confirm its clinical utility in improving protective efficacy, sustaining immunological memory, and eliciting potent neutralizing antibody responses.
Data availability statement
The datasets presented in this study are publicly available. This data can be found here: https://doi.org/10.6084/m9.figshare.30252403.
Ethics statement
All animal care and experimental procedures were reviewed and approved by the Laboratory Animal Welfare and Ethics Committee of the National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Prevention and Control (Approval Code: 2022-023). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MC: Writing – review & editing, Writing – original draft, Conceptualization, Validation, Project administration, Methodology, Formal analysis, Investigation, Software, Data curation, Visualization. ZC: Data curation, Supervision, Writing – original draft, Conceptualization, Methodology, Writing – review & editing, Funding acquisition. YC: Methodology, Data curation, Writing – review & editing. ZR: Conceptualization, Funding acquisition, Writing – review & editing, Visualization. SL: Methodology, Writing – review & editing. DP: Formal analysis, Methodology, Writing – review & editing. SZ: Writing – review & editing, Methodology. GZ: Writing – review & editing, Resources. JY: Writing – review & editing, Resources. JP: Software, Writing – review & editing, Formal analysis, Resources. JX: Supervision, Resources, Funding acquisition, Conceptualization, Writing – review & editing, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was funded by Technical Capacity Improvement of Infectious Disease Surveillance and Prevention, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Grant Number 102393240020020000003.
Acknowledgments
The authors thank the members of the JX and ZR laboratory for advice and assistance during the study. The authors also thank Prof. Jianqing Xu and Dr. Kangli Cao from Zhongshan Hospital, Institutes of Biomedical Sciences, Shanghai Medical College, Fudan University, for the SARS-CoV-2 vaccine.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1655383/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Expression of IFN-γ in response to ZG2488 and PBS treatments. The graph shows the transcript per million (TPM) levels of Ifng in samples treated with ZG2488 (pink) and PBS (green). Data points represent individual samples, and the horizontal bars indicate the mean value for each group. No significant difference (ns) in Ifng expression was observed between the two groups.
SUPPLEMENTARY FIGURE S2 | Expression of TNF-α in response to ZG2488 and PBS treatments. The graph shows the TPM levels of Ifng in samples treated with ZG2488 (pink) and PBS (green). Data points represent individual samples, and the horizontal bars indicate the mean value for each group. A significant difference in TNF-α expression was observed between the two groups (*p < 0.05).
References
Adami, G., Fassio, A., Orsolini, G., Giollo, A., Gatti, D., Rossini, M., et al. (2020). Effectiveness of influenza vaccine in TNF inhibitors treated patients: comment on the article by Burmester et al. Ann. Rheum. Dis. 79:e166. doi: 10.1136/annrheumdis-2019-215174
Assaf, A. M., Al-Abbassi, R., and Al-Binni, M. (2017). Academic stress-induced changes in Th1- and TH2-cytokine response. Saudi Pharm. J. 25, 1237–1247. doi: 10.1016/j.jsps.2017.09.009
Cao, Z., Chen, M., Chen, Y., and Sun, H. (2025). The probiotic potential, safety, and immunomodulatory properties of Levilactobacillus brevis ZG2488: a novel strain isolated from healthy human feces. Fermentation 11:287. doi: 10.3390/fermentation11050287
Ding, Y.-H., Qian, L.-Y., Pang, J., Lin, J.-Y., Xu, Q., Wang, L.-H., et al. (2017). The regulation of immune cells by Lactobacilli: a potential therapeutic target for anti-atherosclerosis therapy. Oncotarget 8, 59915–59928. doi: 10.18632/oncotarget.18346
Finamore, A., Roselli, M., Donini, L., Brasili, D. E., Rami, R., Carnevali, P., et al. (2019). Supplementation with Bifidobacterium longum Bar33 and Lactobacillus helveticus Bar13 mixture improves immunity in elderly humans (over 75 years) and aged mice. Nutrition 63-64, 184–192. doi: 10.1016/j.nut.2019.02.005
Geisen, U. M., Rose, R., Neumann, F., Ciripoi, M., Vullriede, L., Reid, H. M., et al. (2022). The long term vaccine-induced anti-SARS-CoV-2 immune response is impaired in quantity and quality under TNFα blockade. J. Med. Virol. 94, 5780–5789. doi: 10.1002/jmv.28063
Glaviano, A., Foo, A. S. C., Lam, H. Y., Yap, K. C. H., Jacot, W., Jones, R. H., et al. (2023). PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 22:138. doi: 10.1186/s12943-023-01827-6
He, Z., Tian, H., Xing, J., Tang, X., Sheng, X., Chi, H., et al. (2023). Full-length transcriptome sequencing of lymphocytes respond to IFN-γ reveals a Th1-skewed immune response in flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 134:108636. doi: 10.1016/j.fsi.2023.108636
Higgins, S. C., and Mills, K. H. G. (2010). TLR, NLR agonists, and other immune modulators as infectious disease vaccine adjuvants. Curr. Infect. Dis. Rep. 12, 4–12. doi: 10.1007/s11908-009-0080-9
Hilligan, K. L., and Ronchese, F. (2020). Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses. Cell. Mol. Immunol. 17, 587–599. doi: 10.1038/s41423-020-0465-0
Ji, H.-F., Li, M., Han, X., Fan, Y.-T., Yang, J.-J., Long, Y., et al. (2025). Lactobacilli-mediated regulation of the microbial-immune axis: a review of key mechanisms, influencing factors, and application prospects. Foods 14:1763. doi: 10.3390/foods14101763
Jin, Y.-B., Cao, X., Shi, C.-W., Feng, B., Huang, H.-B., Jiang, Y.-L., et al. (2021). Lactobacillus rhamnosus GG promotes early B lineage development and IgA production in the lamina propria in piglets. J. Immunol. 207, 2179–2191. doi: 10.4049/jimmunol.2100102
Karimi, K., Inman, M. D., Bienenstock, J., and Forsythe, P. (2009). Lactobacillus reuteri-induced regulatory T cells protect against an allergic airway response in mice. Am. J. Respir. Crit. Care Med. 179, 186–193. doi: 10.1164/rccm.200806-951OC
Kawakita, T., Sekiya, T., Kameda, Y., Nomura, N., Ohno, M., Handabile, C., et al. (2025). ARNAX is an ideal adjuvant for COVID-19 vaccines to enhance antigen-specific CD4+ and CD8+ T-cell responses and neutralizing antibody induction. J. Virol. 99:e0229024. doi: 10.1128/jvi.02290-24
Lee, Y. J., and Benveniste, E. N. (1996). Stat1 α expression is involved in IFN-γ induction of the class II transactivator and class II MHC genes. J. Immunol. 157, 1559–1568. doi: 10.4049/jimmunol.157.4.1559
Lei, X., Xiao, R., Chen, Z., Ren, J., Zhao, W., Tang, W., et al. (2024). Augmenting antitumor efficacy of Th17-derived Th1 cells through IFN-γ-induced type I interferon response network via IRF7. Proc. Natl. Acad. Sci. U.S.A. 121:e2412120121. doi: 10.1073/pnas.2412120121
Lin, S., Mukherjee, S., Li, J., Hou, W., Pan, C., and Liu, J. (2021). Mucosal immunity–mediated modulation of the gut microbiome by oral delivery of probiotics into Peyer’s patches. Sci. Adv. 7:eabf0677. doi: 10.1126/sciadv.abf0677
Liu, Y., Tran, D. Q., Fatheree, N. Y., and Marc Rhoads, J. (2014). Lactobacillus reuteri DSM 17938 differentially modulates effector memory T cells and Foxp3+ regulatory T cells in a mouse model of necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 307, G177–G186. doi: 10.1152/ajpgi.00038.2014
Ma, N., Guo, P., Chen, J., Qi, Z., Liu, C., Shen, J., et al. (2023). Poly-β-hydroxybutyrate alleviated diarrhea and colitis via Lactobacillus johnsonii biofilm-mediated maturation of sulfomucin. Sci. China Life Sci. 66, 1569–1588. doi: 10.1007/s11427-022-2213-6
Maassen, C. B. M., van Holten-Neelen, C., Balk, F., den Bak-Glashouwer, M.-J., Leer, R. J., Laman, J. D., et al. (2000). Strain-dependent induction of cytokine profiles in the gut by orally administered Lactobacillus strains. Vaccine 18, 2613–2623. doi: 10.1016/S0264-410x(99)00378-3
Maggioli, M. F., Palmer, M. V., Thacker, T. C., Vordermeier, H. M., McGill, J. L., Whelan, A. O., et al. (2016). Increased TNF-α/IFN-γ/IL-2 and decreased TNF-α/IFN-γ production by central memory T cells are associated with protective responses against bovine tuberculosis following BCG vaccination. Front. Immunol. 7:421. doi: 10.3389/fimmu.2016.00421
Maisonneuve, C., Bertholet, S., Philpott, D. J., and De Gregorio, E. (2014). Unleashing the potential of NOD- and toll-like agonists as vaccine adjuvants. Proc. Natl. Acad. Sci. U.S.A. 111, 12294–12299. doi: 10.1073/pnas.1400478111
Manning, B. D., and Cantley, L. C. (2007). AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274. doi: 10.1016/j.cell.2007.06.009
Meng, H., Mao, J., and Ye, Q. (2022). Booster vaccination strategy: necessity, immunization objectives, immunization strategy, and safety. J. Med. Virol. 94, 2369–2375. doi: 10.1002/jmv.27590
Min, F., Hu, J., Huang, T., Huang, Y., Nie, S., Xiong, T., et al. (2023). Effects of Lactobacillus casei NCU011054 on immune response and gut microbiota of cyclophosphamide induced immunosuppression mice. Food Chem. Toxicol. 174:113662. doi: 10.1016/j.fct.2023.113662
Munusamy Ponnan, S., Pattabiram, S., Thiruvengadam, K., Goyal, R., Singla, N., Mukherjee, J., et al. (2019). Induction and maintenance of bi-functional (IFN-γ+ IL-2+ and IL-2+ TNF-α+) T cell responses by DNA prime MVA boosted subtype C prophylactic vaccine tested in a phase I trial in India. PLoS One 14:e0213911. doi: 10.1371/journal.pone.0213911
Peroni, D. G., and Morelli, L. (2021). Probiotics as adjuvants in vaccine strategy: is there more room for improvement? Vaccine 9:811. doi: 10.3390/vaccines9080811
Piot, P., Larson, H. J., O’Brien, K. L., N’kengasong, J., Ng, E., Sow, S., et al. (2019). Immunization: vital progress, unfinished agenda. Nature 575, 119–129. doi: 10.1038/s41586-019-1656-7
Prado Acosta, M., Goyette-Desjardins, G., Scheffel, J., Dudeck, A., Ruland, J., and Lepenies, B. (2021). S-layer from Lactobacillus brevis modulates antigen-presenting cell functions via the Mincle-Syk-Card9 axis. Front. Immunol. 12:602067. doi: 10.3389/fimmu.2021.602067
Procaccini, C., Garavelli, S., Carbone, F., Di Silvestre, D., La Rocca, C., Greco, D., et al. (2021). Signals of pseudo-starvation unveil the amino acid transporter SLC7A11 as key determinant in the control of Treg cell proliferative potential. Immunity 54, 1543–1560.e6. doi: 10.1016/j.immuni.2021.04.014
Rawling, M., Schiavone, M., Mugnier, A., Leclercq, E., Merrifield, D., Foey, A., et al. (2023). Modulation of zebrafish (Danio rerio) intestinal mucosal barrier function fed different postbiotics and a probiotic from Lactobacilli. Microorganisms 11:2900. doi: 10.3390/microorganisms11122900
Redenti, A., Im, J., Redenti, B., Li, F., Rouanne, M., Sheng, Z., et al. (2024). Probiotic neoantigen delivery vectors for precision cancer immunotherapy. Nature 635, 453–461. doi: 10.1038/s41586-024-08033-4
Rosloniec, E. F., Latham, K., and Guedez, Y. B. (2002). Paradoxical roles of IFN-γ in models of Th1-mediated autoimmunity. Arthritis Res. 4, 333–336. doi: 10.1186/ar432
Ryan, F. J., Clarke, M., Lynn, M. A., Benson, S. C., McAlister, S., Giles, L. C., et al. (2025). Bifidobacteria support optimal infant vaccine responses. Nature 641, 456–464. doi: 10.1038/s41586-025-08796-4
Schnoeller, C., Roux, X., Sawant, D., Raze, D., Olszewska, W., Locht, C., et al. (2014). Attenuated Bordetella pertussis vaccine protects against respiratory syncytial virus disease via an IL-17–dependent mechanism. Am. J. Respir. Crit. Care Med. 189, 194–202. doi: 10.1164/rccm.201307-1227OC
Tesniere, A., Apetoh, L., Ghiringhelli, F., Joza, N., Panaretakis, T., Kepp, O., et al. (2008). Immunogenic cancer cell death: a key-lock paradigm. Curr. Opin. Immunol. 20, 504–511. doi: 10.1016/j.coi.2008.05.007
Tseng, Y.-C., Liao, K.-S., Lin, W., Li, C., Chang, C.-B., Hsu, J.-W., et al. (2025). A human oral commensal-mediated protection against Sjögren’s syndrome with maintenance of T cell immune homeostasis and improved oral microbiota. npj Biofilms Microbiomes 11:18. doi: 10.1038/s41522-025-00654-5
van Baarlen, P., Wells, J. M., and Kleerebezem, M. (2013). Regulation of intestinal homeostasis and immunity with probiotic Lactobacilli. Trends Immunol. 34, 208–215. doi: 10.1016/j.it.2013.01.005
Wu, D., Poholek, C. H., Majumder, S., Liu, Q., Revu, S. K., Mohib, K., et al. (2021). IL-17–dependent fibroblastic reticular cell training boosts tissue protective mucosal immunity through IL-10–producing B cells. Sci. Immunol. 6:eaao3669. doi: 10.1126/sciimmunol.aao3669
Xu, J., Ren, Z., Cao, K., Li, X., Yang, J., Luo, X., et al. (2021). Boosting vaccine-elicited respiratory mucosal and systemic COVID-19 immunity in mice with the oral Lactobacillus plantarum. Front. Nutr. 8:789242. doi: 10.3389/fnut.2021.789242
Yin, T., Zhang, X., Iwatani, S., Miyanaga, K., and Yamamoto, N. (2023). Uptake of Levilactobacillus brevis JCM 1059 by THP-1 cells via interaction between SlpB and CAP-1 promotes cytokine production. Microorganisms 11:247. doi: 10.3390/microorganisms11020247
Zhang, Q., Zhao, Q., Li, T., Lu, L., Wang, F., Zhang, H., et al. (2023). Lactobacillus plantarum-derived indole-3-lactic acid ameliorates colorectal tumorigenesis via epigenetic regulation of CD8+ T cell immunity. Cell Metab. 35, 943–960.e9. doi: 10.1016/j.cmet.2023.04.015
Zhao, M., Liang, X., Meng, Y., Lu, H., Lin, K., Gong, P., et al. (2024). Probiotics induce intestinal IgA secretion in weanling mice potentially through promoting intestinal APRIL expression and modulating the gut microbiota composition. Food Funct. 15, 4862–4873. doi: 10.1039/d4fO00962b
Zhu, J., Sun, Y., Qian, X., Li, L., Liu, F., Wang, X., et al. (2025). Intranodal injection of neoantigen-bearing engineered Lactococcus lactis triggers epitope spreading and systemic tumor regressions. Acta Pharm. Sin. B 15, 2217–2236. doi: 10.1016/j.apsb.2025.02.041
Keywords: Lactobacillus brevis , SARS-CoV-2 vaccine, probiotics, transcriptomic analysis, immune cell reshaping
Citation: Chen M, Cao Z, Chen Y, Ren Z, Lu S, Pu D, Zhang S, Zhang G, Yang J, Pu J and Xu J (2025) Immunomodulatory effects of probiotic Lactobacillus brevis ZG2488 on SARS-CoV-2 vaccine responses in mice. Front. Microbiol. 16:1655383. doi: 10.3389/fmicb.2025.1655383
Edited by:
Eugenia Bezirtzoglou, Democritus University of Thrace, GreeceReviewed by:
Mousumi Ray, Meridian Biotech, United StatesGulbeena Saleem, University of Veterinary and Animal Sciences, Pakistan
Copyright © 2025 Chen, Cao, Chen, Ren, Lu, Pu, Zhang, Zhang, Yang, Pu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianguo Xu, eHVqaWFuZ3VvQGljZGMuY24=
 Mengshan Chen
Mengshan Chen Zhijie Cao2
Zhijie Cao2 Zhihong Ren
Zhihong Ren Simin Lu
Simin Lu Sihui Zhang
Sihui Zhang Jing Yang
Jing Yang Ji Pu
Ji Pu Jianguo Xu
Jianguo Xu