- 1Department of Microbiology, National Pirogov Memorial Medical University, Vinnytsia, Ukraine
- 2Center of Thermal Injury and Plastic Surgery, MNPE “Vinnytsia Regional Clinical Hospital Vinnytsia Regional Council”, Vinnytsia, Ukraine
- 3Clinical Microbiology, Department of Translational Medicine, Faculty of Medicine, Lund University, Malmö, Sweden
- 4Department of General and Clinical Epidemiology and Biosafety with a Course on Microbiology and Virology, Odesa National Medical University, Odesa, Ukraine
- 5Department of Microbiology, Virology and Immunology, Poltava State Medical University, Poltava, Ukraine
- 6Department of General Surgery, National Pirogov Memorial Medical University, Vinnytsya, Ukraine
- 7Department of Ophthalmology, National Pirogov Memorial Medical University, Vinnytsya, Ukraine
- 8Central Policlinic of Internal Affairs of Ukraine, Kyiv, Ukraine
- 9Department of Anesthesiology, Intensive Care and Emergency Medicine, National Pirogov Memorial Medical University, Vinnytsya, Ukraine
Susceptibility testing of clinical multidrug-resistant (MDR) and reference P. aeruginosa strains was performed using the standard twofold serial dilution method. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of antiseptics were determined. MIC and MBC values were also interpreted as the bacteriostatic index of antiseptic activity (BSIAA) and the bactericidal index of antiseptic activity (BCIAA). The ability of strains to form biofilms, the inhibition of biofilm formation, and the destruction of mature biofilms under the influence of bacteriostatic, bactericidal, and ½ of the initial antiseptic concentration were modeled using Christensen’s test. Antiseptics from the detergent group, decamethoxine (0.1 and 0.02%) and polyhexanide (0.1%), demonstrated the highest antimicrobial activity. Their bacteriostatic concentrations were 63.2 ± 5.2 μg/mL and 68.7 ± 4.2 μg/mL, respectively. The ranking of antiseptics by bacteriostatic efficacy was: decamethoxine > polyhexanide > octenidine > miramistin > chlorhexidine. The highest BSIAA values were observed for povidone-iodine 10%, decamethoxine 0.1%, octenidine 0.1%, and polyhexanide 0.1%. The highest bactericidal IAA values were found for povidone-iodine 10%, decamethoxine 0.1%, octenidine 0.1%, and polyhexanide 0.1%. Miramistin 0.01% was deemed insufficiently effective. Polyhexanide exhibited the highest bactericidal activity, with a BCIAA to BSIAA ratio of 0.88. For all other antiseptics, this ratio ranged from 0.5 to 0.6. All tested strains exhibited a high capacity for biofilm formation. All antiseptics significantly inhibited biofilm formation. Octenidine had the strongest effect on immature biofilms, reducing their formation by 28.5% (p < 0.0001). The MICs of most antiseptics stimulated mature biofilm development. The bacteriostatic concentration of octenidine led to the eradication of biofilm by 4.7% (p < 0.001) compared to the control. The MBC of most antiseptics (except chlorhexidine) eradicated mature biofilms by 4–30.6%, whereas chlorhexidine stimulated mature biofilm growth by 17.9%. All antiseptics, at half their initial concentration, partially eradicated MDR Pseudomonas biofilms by 11.3–42.4%. Analysing the effect of octenidine at different concentrations and stages of biofilm formation highlights its strong activity against P. aeruginosa biofilms. Our findings underscore the importance of carefully monitoring P. aeruginosa isolates for antiseptic susceptibility. This approach can help prevent the development of selective conditions that promote resistant microorganisms and limit their spread.
1 Introduction
Bacteria with multidrug resistance (MDR) have become a serious threat in the clinic. This is especially true for opportunistic pathogens, which have high natural (intrinsic) resistance, and isolates with rapidly acquired MDR. Pseudomonas aeruginosa, a gram-negative, rod-shaped microbe, is one of the predominant pathogens in healthcare-associated infections due to its biological flexibility and can be considered a prime example of adaptability among opportunistic pathogens (Moradali et al., 2017; Sathe et al., 2023; Sanya et al., 2023; Theuretzbacher et al., 2020; Oliver et al., 2015; Pang et al., 2019; Brüggemann et al., 2018; Murray et al., 2015; Ruffin and Brochiero, 2019).
P. aeruginosa is an unpretentious, versatile, and ubiquitous microbe. It can literally survive without food, not only persisting but also multiplying in distilled water due to minimal contamination. It is omnivorous, with even antimicrobial drugs serving as a source of nutrients. It can grow and multiply within a wide temperature range, from 4 to 42 °C. Being oxidase-positive, it uses oxygen as an electron acceptor but can also grow and reproduce in the absence of oxygen, where nitrate serves as the final electron acceptor, or it retains the ability to microaerobically respire (Moradali et al., 2017; Diggle and Whiteley, 2020; Favero et al., 1971; Liao et al., 2022; Nolan and Behrends, 2021).
Its “basic settings” are perfect and universal, but its initial strategy of existence and survival is not so aggressive toward humans: P. aeruginosa is a saprophyte that lives freely in water, soil, and can be part of the human and animal microbiome. This is true as long as we have the immune status of a healthy person. As soon as P. aeruginosa is able to colonize a niche, its adaptive base allows it to implement a huge range of virulence factors, even showing contact-dependent secretion of toxins directly into target cells through the type 3 secretion system (Moradali et al., 2017; Sanya et al., 2023; Diggle and Whiteley, 2020; Liao et al., 2022; Nolan and Behrends, 2021; Dasgupta et al., 2006; Goldberg et al., 2022; Balasubramanian et al., 2013).
As an opportunistic pathogen, it is also a universal pathogen, as it is pathogenic to humans, vertebrates and invertebrates, and phytopathogenic. The epidemiologically significant reservoir of hospital-acquired blue blood cell infection is medical and service personnel and patients themselves (Nolan and Behrends, 2021).
Infections caused by P. aeruginosa are very difficult to treat, as this microbe uses resistance mechanisms (intrinsic and acquired), forms and states of existence (planktonic and biofilm forms, persistent cell), which usually cause and lead to difficult-to-treat chronic infections (Sanya et al., 2023; Theuretzbacher et al., 2020; Driscoll et al., 2007; Lewenza et al., 2018).
A saprophytic bacterium has turned into a clinical nightmare. Its resistance and minimal nutritional requirements determine the nearly universal presence of P. aeruginosa in hospital environments, creating ample opportunities for the emergence of nosocomial strains. Biofilm formation is also a key factor in the success of P. aeruginosa as a healthcare-associated pathogen. This is especially relevant in infections of the skin and soft tissues (Thuenauer et al., 2020; Behzadi et al., 2021; Kaiser et al., 2017; Rossi Gonçalves et al., 2017; Liew et al., 2019; Ruffin and Brochiero, 2019).
The situation has become significantly more complicated in Ukraine since the start of the full-scale war. The prevalence of multidrug-resistant Gram-negative bacteria is high among those with war-related injuries (Loban’ et al., 2023). War causes special injuries, including complex fractures with bone fragmentation, traumatic limb amputations, extensive deep burns, and severe soft tissue lacerations from artillery shells and mines. The situation is complicated by the rapid infection of wounds with explosive metabolites, dirt, and dust (Loban’ et al., 2023; Melwani, 2022). It is obvious that under such conditions, the patient’s life is the main priority on the front line, which requires the immediate use of antibacterial drugs without any testing. Until the wounded arrive at specialized medical facilities, medical care is provided directly in the combat zone and at all stages of temporary evacuation, which sometimes takes days and even weeks. In addition, throughout the entire evacuation chain, additional colonization of wounds by microorganisms, very often resistant to antibiotics, occurs. This forms a special group of resistant strains, characteristic specifically of war wound infections (Loban’ et al., 2023).
Infections caused by P. aeruginosa require special empirical and targeted antibiotic regimens, given its innate resistance to many classes of drugs and the ability to rapidly acquire resistance to current treatments (Karruli et al., 2023). And the treatment of biofilm infections is a serious problem, as there is currently no targeted therapy that can completely destroy biofilms in vivo (Sathe et al., 2023; Sanya et al., 2023; Kaiser et al., 2017; Rossi Gonçalves et al., 2017).
To overcome the growing problem of resistance and taking into account the biofilm status of the pathogen, an approach involving the use of a combination of antibiotics with alternative therapies will be necessary. Combination therapy has significant advantages over conventional antibiotic therapy, as the former exerts minimal selective pressure on P. aeruginosa and is therefore less likely to cause drug resistance. In wound care, comprehensive topical treatment is extremely important, with antiseptic agents being a key element (Liao et al., 2022; Goldberg et al., 2022; Kramer et al., 2018; Babalska et al., 2021; Murray et al., 2015; Schultz et al., 2017; Alves et al., 2023).
This article focuses on the activity of antiseptics as important means of combating P. aeruginosa MDR, their ability to effectively counteract the formation of biofilm and promote its eradication.
2 Materials and methods
2.1 Study design and participants
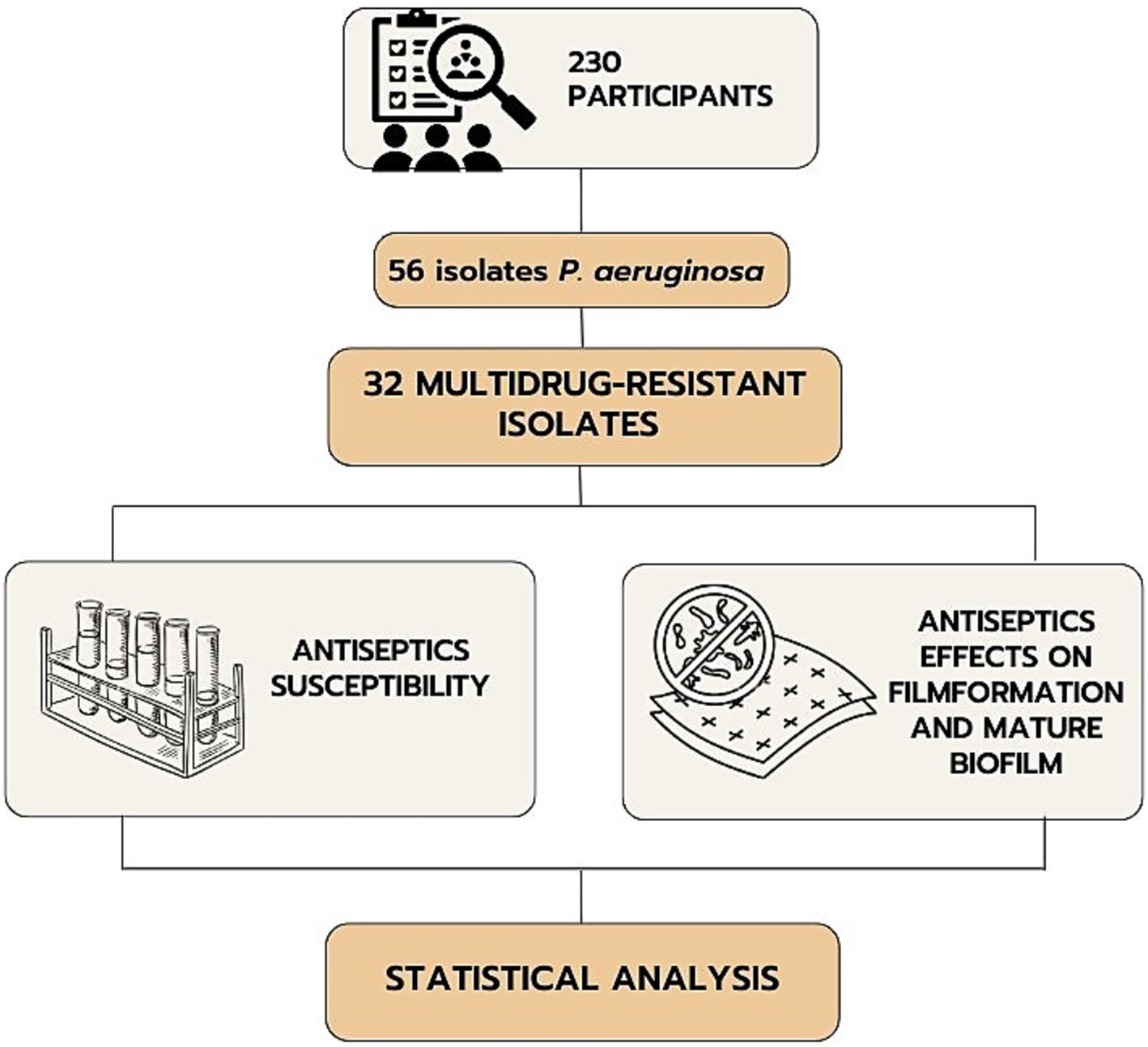
This cross-sectional study involved 230 patients with infected combat burns and shrapnel wounds of various localizations who were treated during 2022–2023. The inclusion criteria were the presence of infected combat wounds and the patient’s consent to participate in the study. The exclusion criteria were inconsistency of the diagnosis with the study objective, lack of consciousness in the patient, diabetes mellitus, congenital or acquired immunodeficiencies, mental disorders, and refusal to participate in the study. The study included the selection of multidrug-resistant P. aeruginosa among isolates from patients with the following determination of the sensitivity of their planktonic and film forms to antiseptics (Figure 1).
The material was obtained from the surface of burn or infected wounds using a sterile probe swab into transport tubes with Aimes medium, followed by cultivation under aerobic conditions at 37 °С. The final identification of clinical bacterial isolates was carried out by a standard bacteriological method, considering morphological, tentorial, cultural and biochemical properties of microorganisms using MIKRO-LA-Test kits (Erba Lachema, the Czech Republic).
The cohort included all patients with combined injuries of soft tissues, burns from who clinical isolates of gram-negative bacteria had been obtained. These patients were admitted from 12 different medical institutions of Ukraine (in 2022–2023) to provide them with specialized tertiary medical care. Pseudomonas aeruginosa (n = 56) was identified from patients with combined injuries.
In total 56 clinical isolates of P. aeruginosa were tested for antibiotic susceptibility to select MDR representatives. There were 9 antimicrobial agents from 6 antimicrobial categories used to characterize the resistance profile of P. aeruginosa isolates using the disk diffusion method (Kirby-Bauer test) according to EUCAST recommendations (Version 14.0, valid from 2024-01-01). MDR isolates were classified based on the criteria defined by Magiorakos et al. (2012). A total of 32 MDR strains were selected based on their resistance to one or more agents in three or more categories indicating the MDR category. The reference bacterial strain Pseudomonas aeruginosa ATCC 27853 from the American Type Culture Collection (Manassas, Virginia, USA) was used as a control.
2.2 Determination of susceptibility of MDR bacteria to antiseptics
The susceptibility tests of clinical MDR and reference P. aeruginosa strains were performed by the standard method of double serial dilutions in Mueller-Hinton broth (HiMedia Laboratories, India) according to the recommendations of ISO standard 20,776–1:2019 (CLSI, USA). Daily bacterial cultures were resuspended with a final concentration of 5 × 105 CFUs/ml (McFarland 0.5). Consecutive two-fold dilutions of the antiseptics were prepared. Then 0.1 mL of bacterial suspension was added to each tube and incubated for 24 h at 37 °C. The determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) by antiseptics was carried after inoculation of the contents of the tubes on Mueller-Hinton agar.
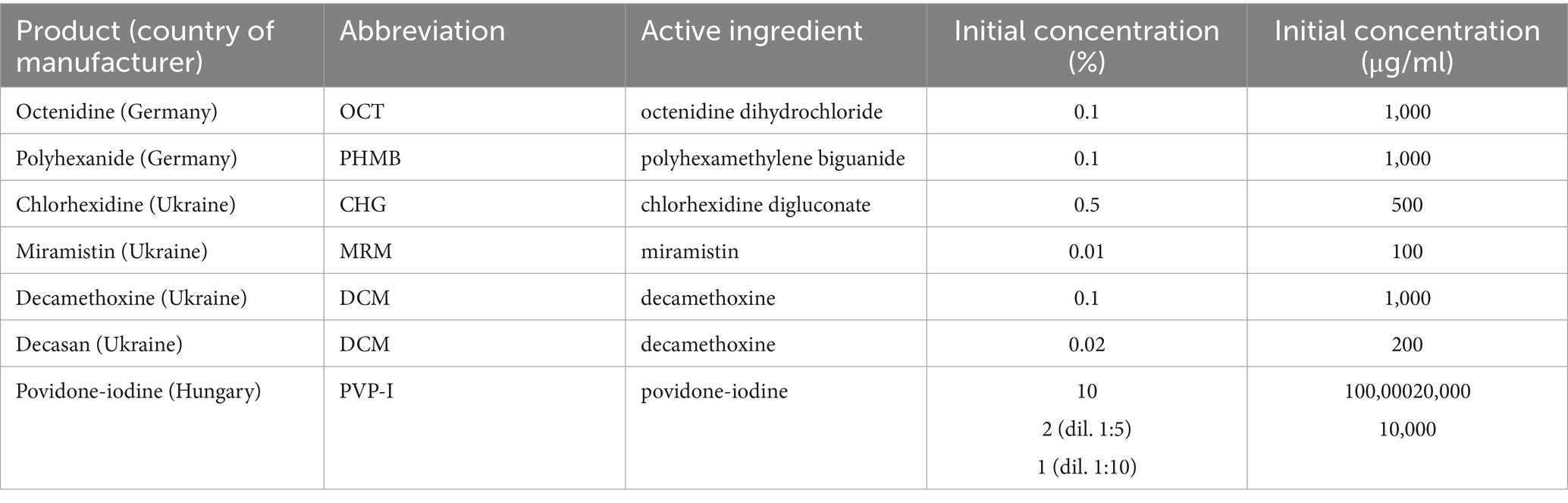
The obtained values of MIC and MBC were also recorded as the bacteriostatic index of antiseptic activity (BS IAA) as the ratio of the initial concentration of the antiseptic to its MIC, and the bactericidal index of antiseptic activity (BC IAA) as the ratio of the initial concentration of the antiseptic to its MBC, respectively. The antiseptic was considered active (bacteriostatic and bactericidal activity, respectively) if the IAA value was greater than four (IAA > 4), since under natural conditions the effectiveness of the antiseptic decreases 4-fold (Denysko et al., 2022). Information on the antiseptics included in the study is provided in Table 1.
2.3 Determination of the effect of antiseptics on immature and mature biofilms
The ability of the studied strains to form a biofilm was modeled using the microtiter plate method with sterile 96-well flat-bottomed polystyrene trays. The inhibition of biofilm formation was assessed by introducing an antiseptic in subbacteriostatic concentrations into the well along with the bacterial culture. The destruction of mature biofilm was studied under the influence of bacteriostatic, bactericidal, and half of the initial concentration of antiseptics.
A daily culture of bacteria in planktonic form, suspended in tryptic soy broth (TSB, EMD Millipore, Burlington, Massachusetts, USA) with 1% glucose, with a concentration of ~105 CFUs/ml, which corresponds to McFarland 0.5, was used. The negative control wells were inoculated with culture medium.
To simulate the inhibition of biofilm formation, 100 μL of the prepared suspension and 100 μL of the antiseptic solution at a concentration of 2 ˟ 1/2 MIC were added to a sterile 96-well flat-bottomed microtitration plate (USA Scientific), reaching a final antiseptic concentration of 1/2 MIC in the well. The plates were cultured in a humidified chamber in a thermostat at 37 °C for 24 h. After incubation, planktonic cells were removed from the wells by pipetting, the plate was washed three times with phosphate-buffered saline (PBS), pH 7.2 (Sigma, USA; cat. no. P-3813), fixed with Bouin solution, and stained with 150 μL of 2.0% crystal violet (Hucker formulation) for 15 min at room temperature. Thereafter, the optical density of the solution was measured at 620 nm. The intensity of staining of the well contents is directly proportional to the degree of biofilm formation; the quantitative expression of biofilm formation activity is the value of optical density, which was measured on a STAT FAX®4,300 spectrophotometer (the Netherlands) and expressed in optical density units (ODU). The value of ODU < 0.120 was evaluated as a low ability to form biofilms, 0.221–0.239 - as average, ODU > 0.240 - as a high indicator.
To determine the ability of antiseptics at MIC, MBC and ½ of the original concentration to destroy mature biofilm, planktonic cells were removed from the plate with the tested strain cultures after 72 h of incubation and 100 μL of antiseptic solution at concentrations of 2 ˟ MIC, 2 ˟ 1/2 MBC and at the original concentration were added to each well to achieve the tested concentrations. The incubation was then continued for 24 h in a humid chamber in a thermostat at 37 °C. The further procedure was similar to the one outlined above.
2.4 Statistical analysis
The mean, standard deviation, median, minimum, maximum frequency, and percentage were used for descriptive statistics. Student’s t-test was used to compare two normally distributed groups. One-way analysis of variance (ANOVA: one factor) was used to compare the results of three or more groups of data. The Bonferroni correction adjusted the significance level to control for the overall probability of errors (false positives) for testing multiple hypotheses. The result was considered reliable if the p-value was less than 0.05. Statistical data processing was performed using licensed Microsoft Office (365) Excel 2019, IBM SPSS Statistics version 22.0, and GraphPad Prism Software 10.1.0 (US, 2023).
3 Results
3.1 Antiseptic susceptibility testing
The MIC and MBC values of most antiseptics against P. aeruginosa strains were consistently lower than their initial commercial concentrations. Antiseptics from the detergent group as exemplified by decamethoxine (0.1 and 0.02%) and polyhexanide (0.1%) demonstrated the highest antimicrobial activity. As can be seen from the data in Table 2, the bacteriostatic concentrations of these antiseptics were 63.2 ± 5.2 μg/mL and 68.7 ± 4.2 μg/mL, respectively, and the bactericidal concentrations were 107.9 ± 5.8 μg/mL and 103.2 ± 12.9 μg/mL. The mean values of MIC for miramistin, chlorhexidine and octenidine were 94.2 ± 2.5 μg/mL, 95.8 ± 13.2 μg/mL and 84.7 ± 7.6 μg/mL, respectively. The MBC values for chlorhexidine and octenidine were 193.9 ± 22.9 μg/mL and 155.5 ± 16.4 μg/mL. As for the antiseptic Miramistin, the initial concentration of the active substance of this agent (100 μg/mL) was not sufficient to determine the bactericidal concentration, i.e., the MIC values against P. aeruginosa strains were > 100 μg/mL.
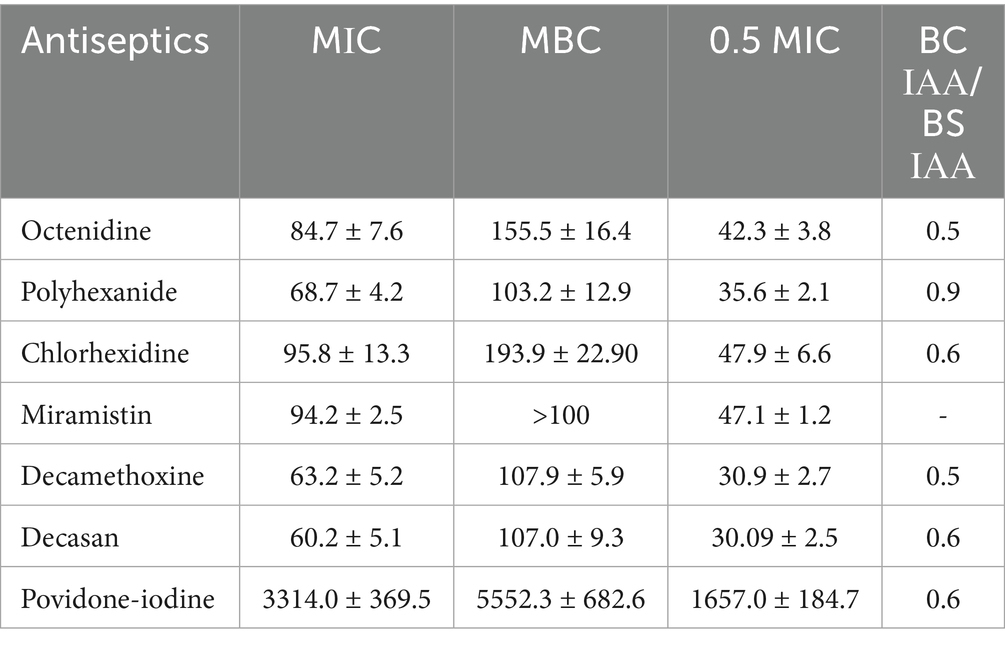
Table 2. Characteristics of the bacteriostatic and bactericidal effects of antiseptics on Pseudomonas aeruginosa strains (n = 33), in μg/ml (arithmetic mean ± arithmetic mean error: M ± m).
A comparison of the data and an assessment of the reliability of their differences showed that polyhexanide was 1.37-fold more effective than miramistin in inhibiting the growth of P. aeruginosa (p < 0.001). Decamethoxine inhibited the growth of MDR P. aeruginosa strains 1.49-fold more effectively than miramistin (p < 0.001), 1.51-fold more effectively than chlorhexidine (p < 0.05), and 1.34-fold more effectively than octenidine (p < 0.05). The bactericidal activity of polyhexanide was 1.59-fold higher than that of chlorhexidine (p < 0.01) and 1.51-fold higher than that of octenidine (p < 0.05). The bactericidal concentrations of decamethoxine were 1.8-fold lower than those of chlorhexidine (p < 0.001) and 1.44-fold lower than those of octenidine (p < 0.01).
Thus, the ranking of the effectiveness of antiseptic drugs by bacteriostatic properties was (from the most effective drug):
decamethoxine > polyhexanide > octenidine > miramistin > chlorhexidine.
The scale of bactericidal activity of drugs will be as follows (from the most active):
decamethoxine > polyhexanide > octenidine > chlorhexidine > miramistin.
Povidone-iodine, as an active substance from the halogen group, acts at significantly higher concentrations than antiseptics from the detergent group. Therefore, we cannot compare their bacteriostatic and bactericidal concentration values, but we can compare their activity. The MIC of povidone-iodine was 3313.95 ± 369.45 μg/mL, and the MIC of MBC was 5552.33 ± 682.63 μg/mL.
The interpretation of the results was also presented in the calculations of the bacteriostatic and bactericidal index of antiseptic activity (BS IAA and BC IAA) and their ratio (Figure 2; Table 2).
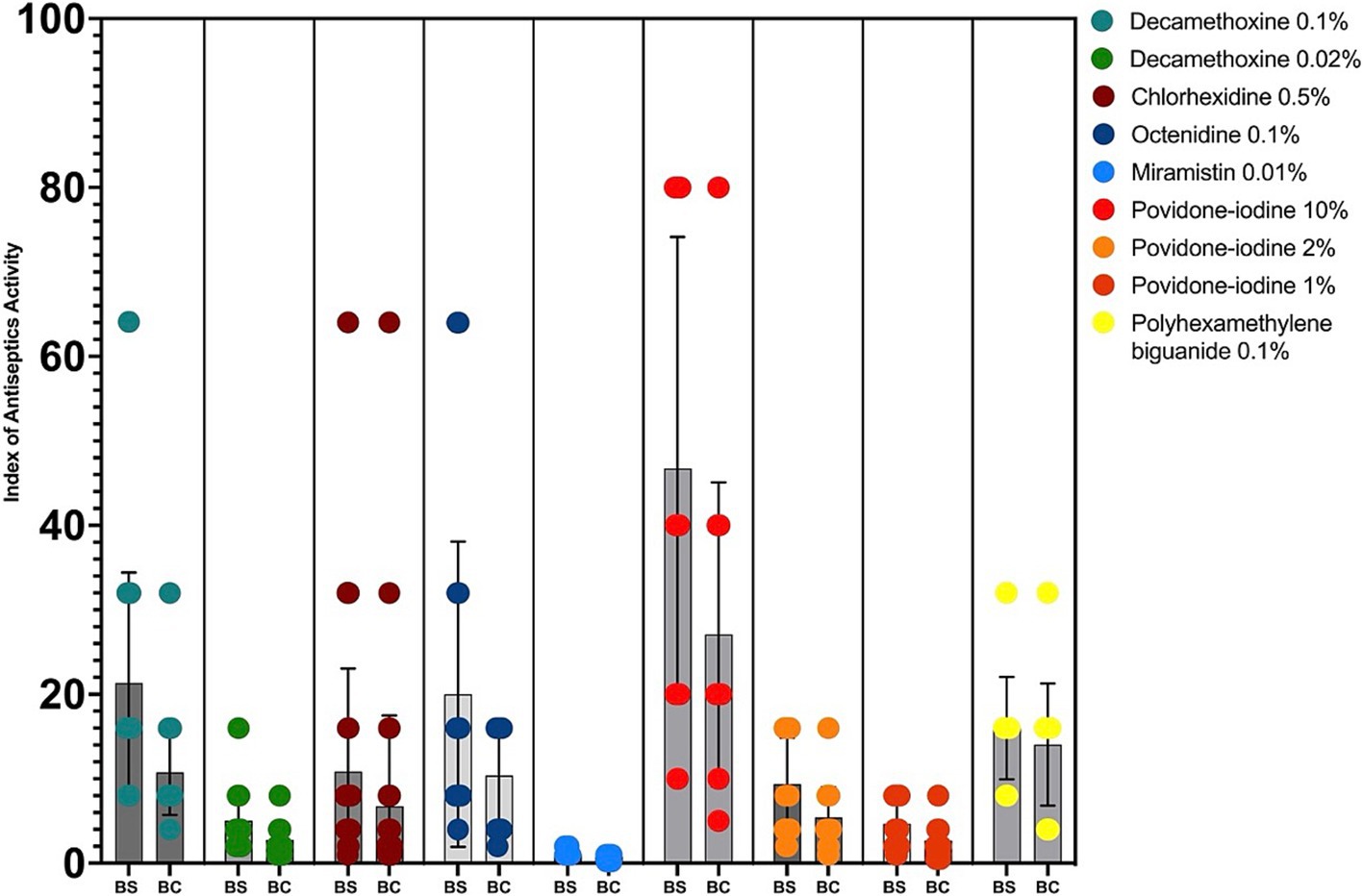
Figure 2. Bacteriostatic and bactericidal indexes of antiseptics activity against MDR P. aeruginosa (n = 32). BS – bacteriostatic, BC – bactericidal.
The antiseptic activity indices allow comparing drugs with different initial concentrations of active substance in the product, drugs of different chemical groups with different mechanisms of action, which makes it possible to assess the feasibility of using this drug and this particular concentration of active substance against a particular microorganism. “Active antiseptic” is characterized by an index of ≥4.
Since the Betadine® drug instruction (initial concentration of povidone-iodine - 10%) also recommends using a dilution of 1:5 and 1:10, the indices of antiseptic activity were additionally calculated for concentrations of povidone-iodine 2 and 1%.
The highest values of bacteriostatic IAA for clinical strains of P. aeruginosa with multidrug resistance were calculated for povidone iodine 10% (BS IAA = 46.7), decamethoxine 0.1% (BS IAA = 21.34), octenidine 0.1% (BS IAA = 20.0), polyhexanide 0.1% (BS IAA = 16.0). For decamethoxin 0.02%, the BS IAA was 5.02, for chlorhexidine 0.5% - 10.86, for povidone iodine 2% - 9.35, and for povidone iodine 1% - 4.67. The BS IAA of miramistin 0.01% was below the limit value and amounted to - 1.12. This concentration of the agent is not sufficient for use against MDR strains of P. aeruginosa.
The bactericidal activity indices took the highest values for povidone-iodine 10% (BC IAA = 27.09), decamethoxine 0.1% (BC IAA = 10.76), octenidine 0.1% (BC IAA = 10.37), polyhexanide 0.1% (BC IAA = 14.05). BC IAA of chlorhexidine 0.5% was 6.74, povidone-iodine 2% - 5.42. The bactericidal activity of decamethoxin 0.02% and povidone-iodine 1% was characterized by indices 2.77 and 2.71. The BC IAA of miramistin was not calculated, since the initial concentration of the agent was not sufficient to determine the bactericidal concentrations.
The highest cidal activity was found for polyhexanide: the ratio of BC IAA to BS IAA was 0.88. The values of the ratio BC IAA / BS IAA for all other antiseptics ranged from 0.5 to 0.6.
3.2 The effect of antiseptics on immature and formed biofilm of MDR strains of P. aeruginosa
The next step was to determine the sensitivity of biofilm forms of wound isolates of P. aeruginosa to antiseptics active against planktonic forms of these strains. The study showed that miramistin at its initial concentration of 0.01% had no activity against the planktonic forms of the studied bacteria (IAA ≤ 4), so this antiseptic was excluded from the biofilm testing.
All strains tested had the ability to form biofilms. Moreover, this property was interpreted as high, since the optical density values exceeded >0.240 units (average ODU = 0.415 ± 0.017).
3.2.1 The effect of antiseptics on immature biofilm: efficiency of inhibition of biofilm formation
All antiseptics significantly inhibited biofilm formation (p < 0.0001). The percentage of inhibitory effect was 79.3 ± 2.6% for polyhexanide, 71.5 ± 2.8% for OCT, 79.8 ± 2.7% for chlorhexidine, 77.6 ± 2.4% for decamethoxine and 79.9 ± 2.6% for povidone-iodine compared to the control (100.0%). Octenidine in sub-MIC concentrations demonstrated the strongest effect on immature biofilm and inhibited its formation by 28.5% (p < 0.0001). Next on the scale of effectiveness were decamethoxin and polyhexanide, which significantly inhibited biofilm formation by 22.4 and 20.7% compared to the control (p < 0.0001). Chlorhexidine and povidone-iodine inhibited biofilm formation by 20.2 and 20.1%, respectively (Figure 3; Supplementary Table 1).
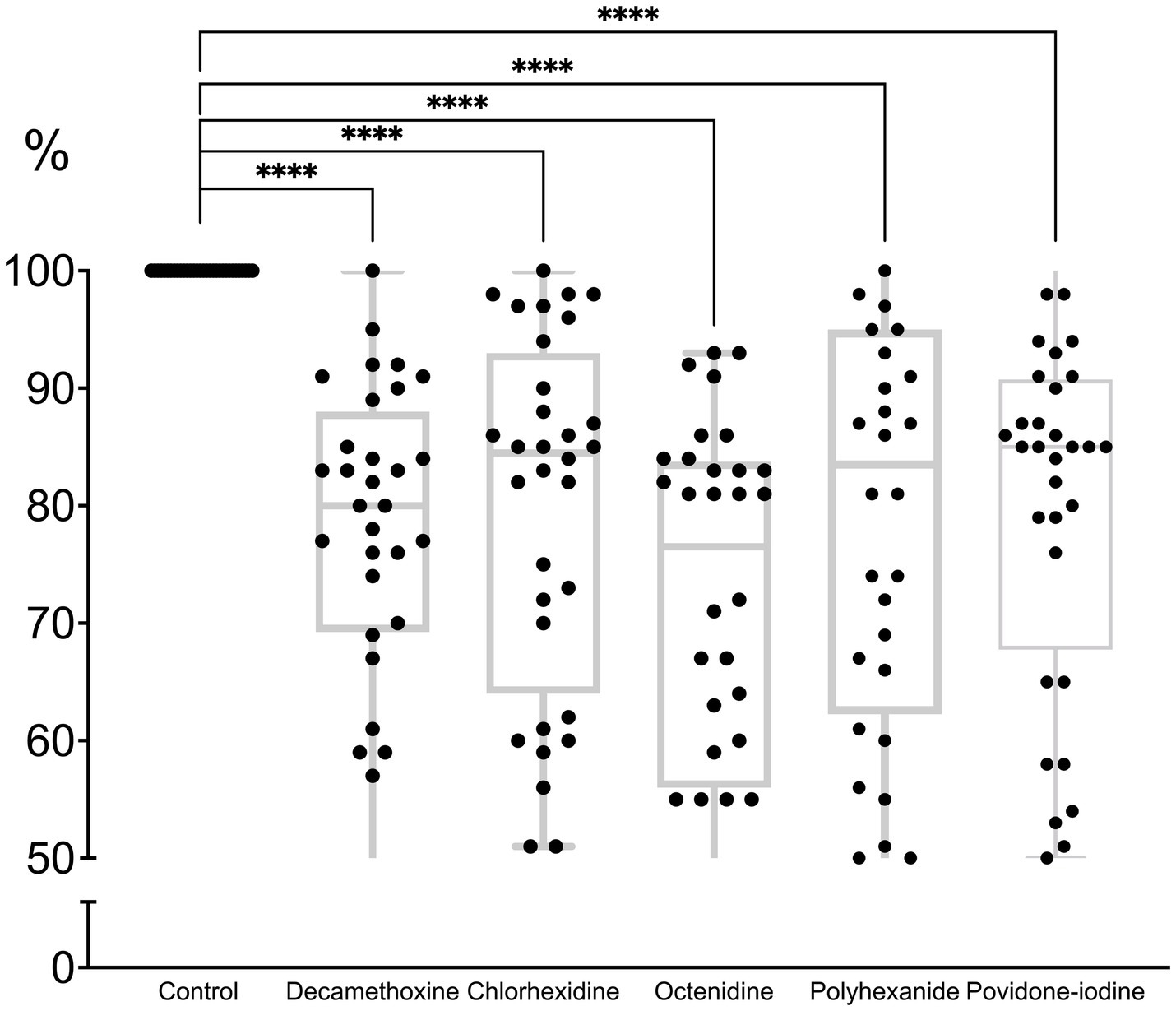
Figure 3. The effect of subbacteriostatic (1/2 MIC) antiseptic concentrations on biofilm formation by MDR P. aeruginosa strains (n = 32).
If we rank the effectiveness of drugs by the effect of their subbacteriostatic concentrations on the immature biofilm of multidrug-resistant pseudomonas, the scale of effectiveness will be as follows (from the most effective): octenidine > decamethoxine > polyhexanide > chlorhexidine > povidone iodine.
Octenidine showed the greatest activity against biofilm formation. As can be seen from Table 2, the bacteriostatic concentrations of octenidine are quite high, exceeding those of, for example, polyhexanide and decamethoxine. Thus, for the tested antiseptics from the group of detergents and halogen-containing compounds, the ability to inhibit biofilm formation depended on the concentration of the antiseptic, and not on the sensitivity of P. aeruginosa isolates to them.
3.2.2 The effectiveness of antiseptics on preformed P. aeruginosa biofilm: evaluation of the effect of MIC, MBC and ½ of the initial concentration of antiseptics on the formed biofilm
As can be seen in Figure 4, the minimum bacteriostatic concentration of most antiseptics stimulated the protective forces of the biofilm as a form of organization approaching the tissue level. “Quorum sensing” ensured the reaction of the structure to a greater extent in the form of production of a protective matrix (Shree et al., 2023).
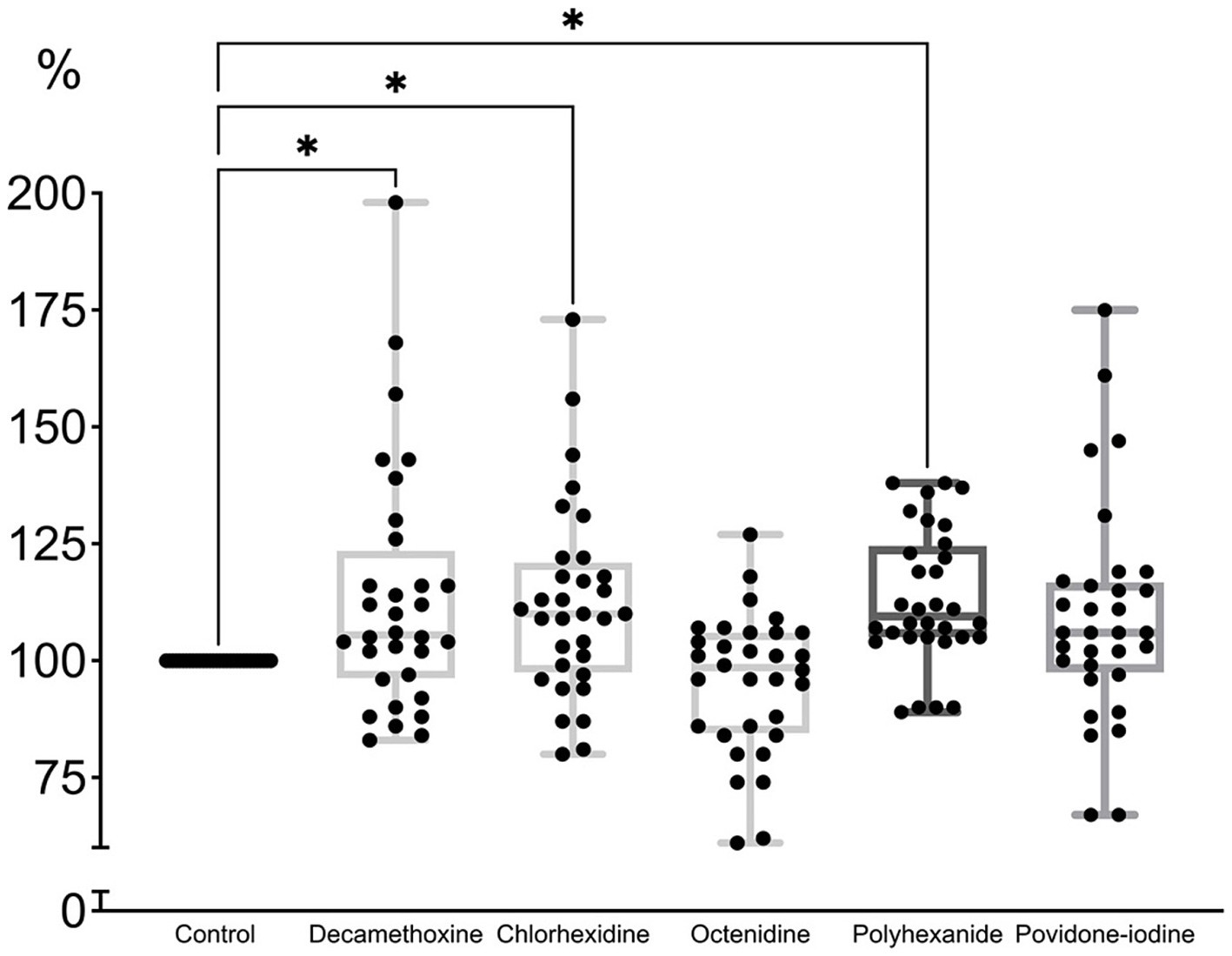
Figure 4. The effect of minimal inhibitory concentration (MIC) of antiseptics on the mature biofilm of P. aeruginosa (n = 32) in comparison with untreated control culture (in %).
MIC of povidone-iodine stimulated the development of biofilm by 9.4%, bacteriostatic concentration of polyhexanide - by 13.2%, chlorhexidine - by 12.2%, decamethoxin - by 13.7% (p < 0.001). The bacteriostatic concentration of octenidine (average 84.67 ± 7.63 μg/mL) led to the eradication of biofilm by 4.7% (p < 0.001) compared to the control (Figure 4; Supplementary Table 2).
When most MBCs of antiseptics were applied to the formed MDR biofilm of P. aeruginosa strains (Figure 5; Supplementary Table 3), the latter was eradicated by 4% with decamethoxin (p < 0.001), by 4.8% with polyhexanide (p < 0.001), by 6.2% with povidone iodine (p < 0.001) and by 30.6% with octenidine (p < 0.001). Chlorhexidine stimulated the biofilm by 17.9% (p < 0.001).
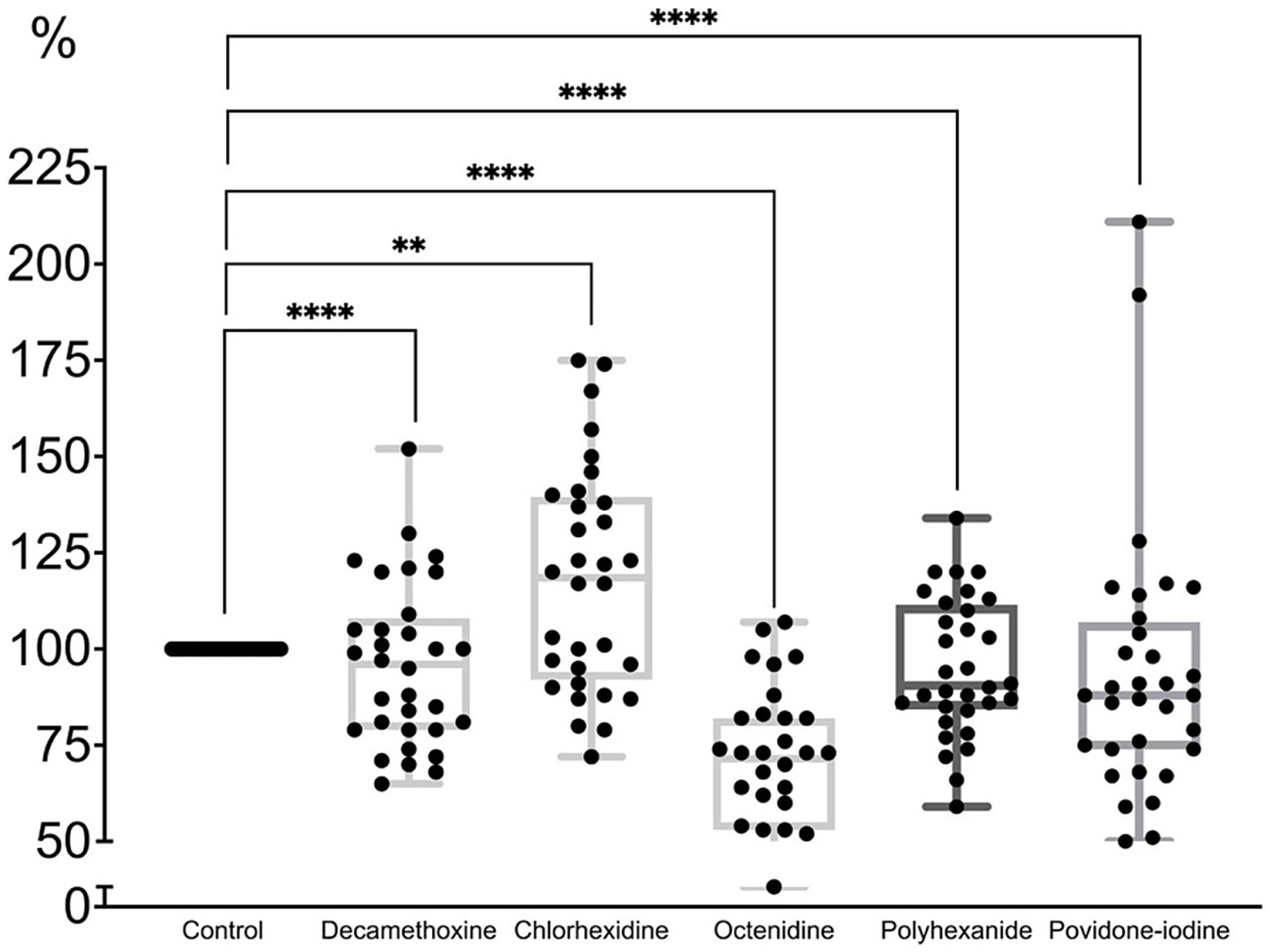
Figure 5. The effect of minimal bactericidal concentration of antiseptics on the mature biofilm of P. aeruginosa (n = 32) in comparison with untreated control culture (in %).
Thus, the percentage of biofilm in comparison with the control was 93.8% under povidone-iodine, 95.2% under polyhexanide, 69.4% under octenidine, 117.9% under chlorhexidine, 96.0% under decamethoxine (p < 0.001).
All antiseptics in a concentration equal to half the initial concentration of the active substance led to partial eradication of the MDR biofilm of pseudomonas strains by 11.3–42.4% (Figure 6; Supplementary Table 4).
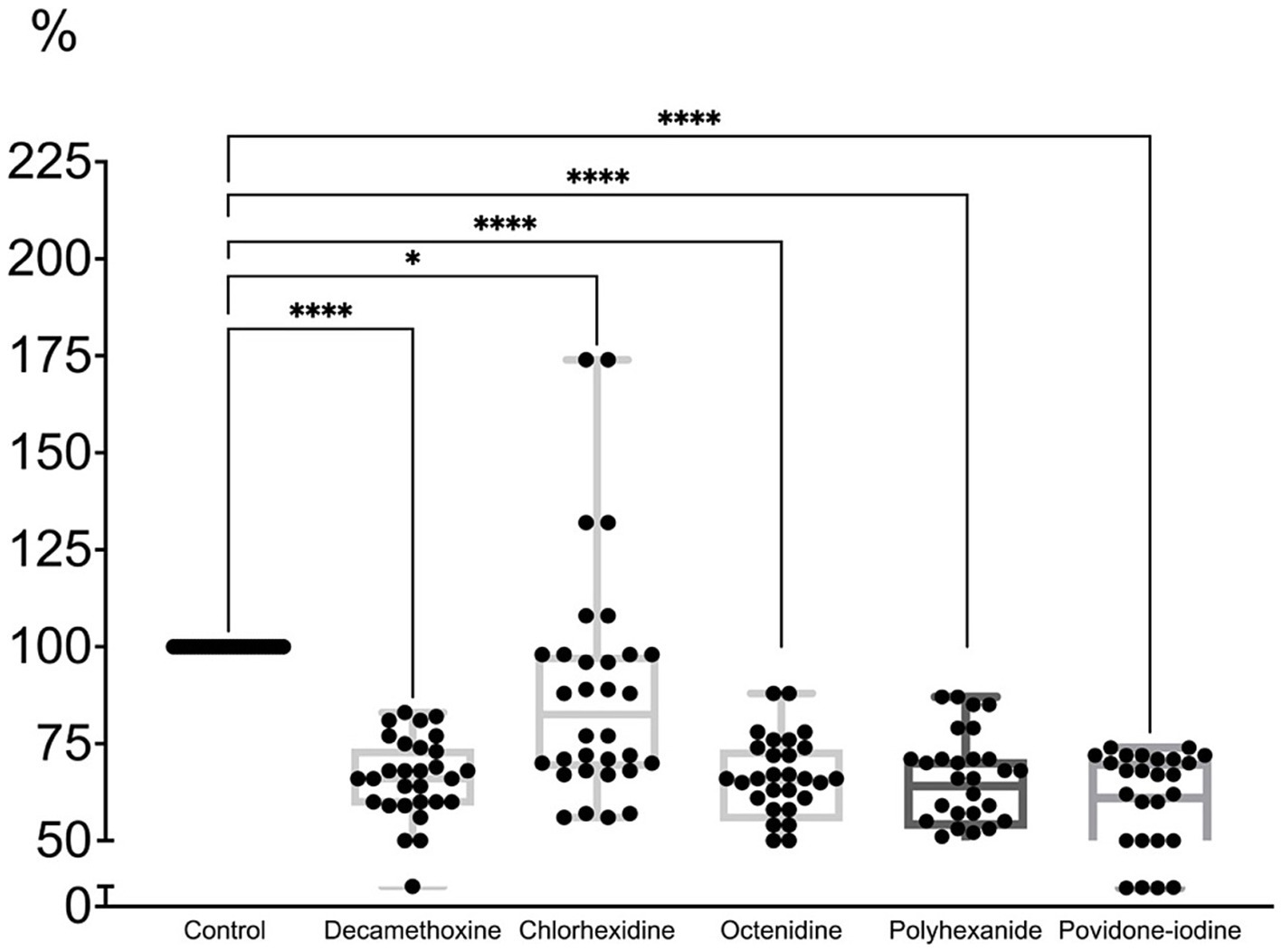
Figure 6. The effect of half of the initial concentration of antiseptics on the mature biofilm of P. aeruginosa (n = 32) compared to the untreated control (in %).
The percentage of biofilm compared to the control under the action of decamethoxine was lower by 35.4% (p < 0.001) and amounted to 64.6%, under the action of chlorhexidine - by 11.3% (p < 0.001) and amounted to 88. 7%, under the influence of octenidine - by 35.8% (p < 0.001) and amounted to 64.2%, under the influence of polyhexanide - by 36.5% (p < 0.001) and amounted to 63.5%, under the influence of povidone-iodine - by 42.4% (p < 0.001) and amounted to 57.6%. Chlorhexidine showed the lowest activity against the formed biofilm at a concentration of half the initial concentration. However, its initial concentration is half that of other detergents.
Thus, the sensitivity to antiseptics of cultures in mature biofilms is much lower. An effective effect on the formed biofilm requires much higher concentrations of antiseptics. It is much easier to inhibit or prevent its formation. The tested concentrations of antiseptics do not destroy the formed biofilm by more than 42.4%. The ability of the antiseptics to eradicate the biofilm depended on the concentration: the highest tested concentrations were the most effective, equal to half the concentration of the finished commercial product.
Tracing the trend of octenidine action at different concentrations at different stages of biofilm formation, it should be noted that it is most active against P. aeruginosa biofilm (Supplementary Table 5).
4 Discussion
The emergence of multidrug resistance in bacteria has become one of the most dauntingchallenges of this century: the prevalence of infections that are difficult to treat is increasing, and there are no appropriate therapeutic alternatives. The scale of the problem has been identified by the political leaders of the G7 countries, who have expressed strong support for the first World Health Organization (WHO) Global Action Plan on Antimicrobial Resistance (AMR) [Rossi Gonçalves et al., 2017; Global Antibiotic Research and Development Partnership (GARDP), n.d.]. The global collaborative organization Joint Programming Initiative on Antimicrobial Resistance (JPIAMR) has engaged 29 countries in the fight against antimicrobial resistance, based on the One Health approach (JPIAMR, n.d.; CDC, 2019).
Infections caused by antibiotic-resistant P. aeruginosa are spreading steadily around the world due to its high internal resistance and ability to rapidly acquire resistance to all classes of antibiotics. The emergence of a specific resistance type of P. aeruginosa, namely the emergence of carbapenem-resistant (CRPA) strains, has attracted considerable attention from clinical microbiologists and infection control specialists (Moradali et al., 2017; Nolan and Behrends, 2021; Rossi Gonçalves et al., 2017; Rossolini et al., 2014; Oliver et al., 2015; Kovalchuk et al., 2024). The WHO recognizes carbapenem-resistant P. aeruginosa (CRPA) as a high priority pathogen for which antibiotic development is urgently needed (World Health Organization, 2024; Gergova et al., 2024). The emergence, spread, and persistence of multidrug-resistant bacteria, or “superbugs,” threatens human, animal, and environmental health as interconnected components of a single ecosystem (Davies and Davies, 2010; Aslam et al., 2021). P. aeruginosa MDR exists in a triangle-reservoir of animals, humans, and the environment, and there is interconnected coexistence of these pathogens within this triad. Numerous causes of “global resistance” contribute to the pressure of genetic selection and the emergence of bacterial MDR infections in society (CDC, 2019; Gergova et al., 2024; Van Boeckel et al., 2015; Aslam et al., 2018; Ahmad I et al., 2021; Crone et al., 2019; Balcázar et al., 2015; Abd El-Ghany, 2021).
P. aeruginosa is striking in the variety of pathology it causes, being the cause of a wide range of diseases - from intoxication to extensive purulent inflammatory processes and septic shock. Purulent complications of wound processes are very significant. In the general structure of wound infections, the P. aeruginosa bacterium occupies a significant place, being one of the most common bacteria (Ruffin and Brochiero, 2019; Wolcott et al., 2016; Phan et al., 2023). Pseudomonas aeruginosa infections in the epithelium of the skin, cornea and respiratory tract are the main cause of hospitalizations, disability and deaths worldwide (Ruffin and Brochiero, 2019). Along with S. aureus, K. pneumoniae, E. coli, and Acinetobacter spp., P. aeruginosa is among the leading superbugs that complicate the course of combat trauma (Mende et al., 2022; Weintrob et al., 2018; Petersen et al., 2007; Ford et al., 2017; Kvasnevska et al., 2024). One of the predictors of high mortality P. aeruginosa infections is multidrug resistance of the causative strain (Ruffin and Brochiero, 2019; Oliver et al., 2015), and infections caused by antibiotic-resistant P. aeruginosa are increasing worldwide (Nolan and Behrends, 2021; Rossolini et al., 2014; Oliver et al., 2015; Galdino et al., 2019; Pogue et al., 2020; Buehrle et al., 2016; Rubio et al., 2021; Kyaw et al., 2015). For example, Mareș, C. and colleagues reported an increase in antibiotic resistance in opportunistic pathogens due to the COVID-19 pandemic, and some of the highest rates of increase were observed for P. aeruginosa (Mareș et al., 2022).
The problem of P. aeruginosa infections requires a joint international interdisciplinary effort to translate current knowledge into strategies to prevent and treat P. aeruginosa infections, while reducing antibiotic resistance and avoiding the spread of resistant strains in nature, as patient sanitation is one of the key measures in efforts to break the epidemic chain by acting on the source of infection, thus preventing the spread of MDR strains (Moradali et al., 2017; Sanya et al., 2023; Theuretzbacher et al., 2020; Pang et al., 2019; Brüggemann et al., 2018; Rossi Gonçalves et al., 2017; Loban’ et al., 2023; Babalska et al., 2021). As correctly summarized by Kramer, A. and colleagues, wound antisepsis has experienced a renaissance due to the development of effective wound-compatible antiseptic agents, their bactericidal effect instead of bacteriostatic, the relatively high level of sensitization to topically applied antibiotics, also due to the pandemic spread of multidrug-resistant microorganisms, and, to the advantage, the absence (rarely) of resistance to those antiseptics that irreversibly damage pathogens (Kramer et al., 2018).
There are no generally accepted recommendations for the use of antiseptics for wounds. Regular monitoring (control) of sensitivity, correction of initial antiseptic concentrations with adjustment for multidrug-resistant strains of pathogens, and especially given the potential presence of such a widespread and resistant pathogen as pseudomonas are important and necessary. Suppression of the associated microflora with prolonged use of antibiotics sometimes leads to the fact that P. aeruginosa remains the only bacterial species in the infection site, impeding wound healing (Betchen et al., 2022; Kawamura et al., 2019). The results of our study indicate the high efficiency of modern antiseptics against MDR strains of P. aeruginosa. The MIC values of antiseptics (except for miramistin) against P. aeruginosa strains were always lower than the initial commercial concentrations. Certainly, the MBC for all microbicides were higher than their respective MICs, but the ratio of MBC/ MBS was less than 4, indicating that the products exhibit predominantly bactericidal properties (Betchen et al., 2022; Levison, 2004).
In recent studies by Barrigah-Benissan and colleagues, the MIC values for polyhexanide, povidone-iodine, and octenidine were also always lower than the original commercial concentrations (Barrigah-Benissan et al., 2022). Grzegorz Krasowski and colleagues determined the bactericidal concentrations of polyhexanide and octenidine at dilutions several tens of times below the threshold of the initial solution (Krasowski et al., 2021). Similarly, Rafael López-Rojas and colleagues previously found high activity of polyhexanide against clinical isolates of P. aeruginosa with the MDR phenotype at much lower concentrations than the initial ones (López-Rojas et al., 2017). Studies by Tomasz M. Karpiński characterized octenidine as a very effective drug against clinical wound isolates and reference strains of P. aeruginosa. However, for their sample of isolates, the MIC values for octenidine and polyhexanide did not differ from previous studies (López-Rojas et al., 2017; Karpiński, 2019; Koburger et al., 2010; Murray et al., 2015). We selected strains with the MDR phenotype, and the MIC and MIC values for the antiseptics studied were higher than they were in previous studies. The same trend, for example, was observed by Gupta, P. et al. for MDR of P. aeruginosa and povidone-iodine (Gupta et al., 2018). Vásquez, Daniel and colleagues also found high MICs and MBCs of chlohexidine in many home and hospital isolates of P. aeruginosa (Vásquez et al., 2017). In our previous similar studies concerning other MDR opportunistic pathogens (Ljungquist et al., 2023; Kovalchuk et al., 2024; Nazarchuk et al., 2024), we referred to the review by Jean-Yves Maillard and colleagues, whose analysis convincingly confirmed the decrease in the sensitivity of wound pathogens, including P. aeruginosa, to all biocides, which is associated with the spread of resistance (Maillard et al., 2021). At the same time, antibiotics have more resistance determinants than antiseptics and disinfectants, and gene expression under the influence of antimicrobial agents is not a good predictor of these resistance determinants (Murray et al., 2015; Schultz et al., 2017). Antiseptics act on multiple targets, inside and on the surface of the bacterial cell, unlike antibiotics (Krasowski et al., 2021; Assadian, 2016). The antiseptic activity index allows you to assess the effectiveness and appropriateness of the drug, and compare antiseptics with each other. The antiseptic concentration should be at least 4 MIC. We interpreted the results using the differential IAA index, focusing on the cidal activity of the antiseptic (Kramer et al., 2018). According to the results of the evaluation of the activity of drugs based on bacteriostatic IAA, povidone-iodine 10%, decamethoxine 0.1%, octenidine 0.1%, polyhexanide 0.1%, decamethoxine 0.02%, chlorhexidine 0.5%, povidone-iodine 2%, povidone-iodine 1% are effective. The concentration of miramistin 0.01% is not sufficient for use against MDR strains of P. aeruginosa. The concentration of the active ingredient of this drug is the lowest among those studied here. According to the cidal activity index, the most effective are povidone-iodine 10%, decamethoxine 0.1%, octenidine 0.1%, polyhexanide 0.1% (BC IAA = 14.05), chlorhexidine 0.5%, povidone-iodine 2%. The highest values of the indices were taken for povidone-iodine 10%. The ratio of BC IAA to BS IAA in favor of cidal action was the highest for polyhexanide. Our research and that of colleagues from other countries shows that antiseptics, including those tested in this study, are effective against planktonic bacteria. However, pathogenic bacteria are mostly found in biofilms, as this is their natural state (Rossi Gonçalves et al., 2017; Günther et al., 2021; Nazarchuk et al., 2019).
Therefore, biofilm elimination is important from a therapeutic point of view and for infection control (Rossi Gonçalves et al., 2017; Maurice et al., 2018). An effective antiseptic used for the treatment of colonized/infected chronic wounds should exhibit biofilm control properties (Krasowski et al., 2021; Schultz et al., 2017). The data are not yet clear on whether the MDR phenotype correlates with biofilm-forming properties (Rossi Gonçalves et al., 2017). Some researchers have noted an increased ability to form biofilm by P. aeruginosa strains (Magiorakos et al., 2012; Kaiser et al., 2017; Karballaei Mirzahosseini et al., 2020; Gurung et al., 2013). In any case, the biofilm is an important factor in the virulence of P. aeruginosa, the main form of its existence, which protects against the harmful effects of environmental factors, including biocides, and also contributes to the persistence and spread of MDR strains (Yin et al., 2019; Uddin et al., 2021). This was not the aim of our study, but it should be noted that the P. aeruginosa strains tested by MDR were characterized by high biofilm-forming capacity. The same was observed by Rossi Gonçalves I. et al., Bakht, M. et al., Sanchez, C et al. Behzadi, P. et al., Cepas, V. et al. point out that, indeed, strong biofilm producers are more common among clinical isolates, but MDR status or resistance to individual antibiotics does not imply an increased ability to form biofilms (Rossi Gonçalves et al., 2017; Bakht et al., 2022). This selection is logical, since biofilm-forming strains survive better and have a better chance of acquiring the determinants of acquired resistance. However, these are most likely not genetically linked traits.
Our studies of the effect of antiseptics on immature biofilm, i.e., their effectiveness in inhibiting biofilm formation, showed that all antiseptics have a high level of inhibitory capacity. Octenidine in sub-MIC concentrations showed the strongest effect on immature biofilm. Decamethoxine, polyhexanide, chlorhexidine, and povidone-iodine were next on the scale of effectiveness. A negative correlation was found between the ability of MDR strains of P. aeruginosa to form biofilms in the presence of subbacteriostatic concentrations of antiseptics and the susceptibility of these isolates to antiseptics. Thus, for the tested antiseptics from the group of detergents and halogen-containing compounds, the ability to inhibit biofilm formation depended on the concentration of the antiseptic, not on the sensitivity of P. aeruginosa isolates to them.
The sensitivity to antiseptics of cultures in mature biofilms was much lower. An effective effect on the formed biofilm requires much higher concentrations of antiseptics. It is much easier to inhibit or prevent its formation. The tested concentrations of antiseptics do not destroy the formed biofilm by more than 42.4%. The ability of the antiseptics to eradicate the biofilm depended on the concentration: the highest tested concentrations were the most effective, equal to half the concentration of the finished commercial product. Tracing the trend of octenidine action at different concentrations at different stages of biofilm formation, it should be noted that it is most active against P. aeruginosa biofilm. But, in general, it should be noted that all tested antiseptics are effective against P. aeruginosa biofilm. Junka A et al. also noted the high activity of octenidine and povidone iodine against biofilms of nosocomial P. aeruginosa strains (Sanchez Jr et al., 2013; Cepas et al., 2019; Junka et al., 2014). The results obtained by Grzegorz Krasowski et al. also indicate a high anti-biofilm activity of antiseptics based on polyhexanide and octenidine. The researchers note that antiseptics based on polyhexanide or octenidine are very useful for treating biofilm (Levison, 2004). Gryson, L et al. recently studied the anti-biofilm activity of povidone iodine and polyhexnide and reported that PVP-I and PHMB demonstrated sustained activity against biofilms in vitro, and PVP-I led to complete eradication of 3- and 5-day-old Pseudomonas aeruginosa biofilms (in ≤0.5 h) (Gryson et al., 2023).
There have also been important advances in the development of strategies for treating infections caused by P. aeruginosa and the use of combination therapy. Elodie Lefebvre et al. used a combination of polyhexanide, EDTA, and proteases in low concentrations, which had a synergistic effect that led to the complete eradication of dense P. aeruginosa biofilms (Lefebvre et al., 2016). Ciecholewska-Juśko D investigated the phenomenon of increasing the activity of an octenidine dihydrochloride-based antiseptic against Pseudomonas aeruginosa biofilms in the presence of a rotating magnetic field of two frequencies of 5 and 50 Hz. The authors noted that the combination of a rotating magnetic field and OCT may be particularly promising for the destruction of biofilms located in areas such as wound pockets, where physical obstacles limit antiseptic activity (Ciecholewska-Juśko et al., 2022).
P. aeruginosa has an innate resistance to many classes of drugs, the ability to form biofilms and, most importantly, the ability to quickly acquire resistance after treatment. One of the obvious unfortunate consequences of increased resistance to antimicrobial drugs is that bacteria are often treated at concentrations below their minimum inhibitory concentration (Nolan and Behrends, 2021). For example, in terms of biocides in general, Daniel Vásquez and colleagues note that hospitals with highly resistant strains of P. aeruginosa and A. baumannii with high drug resistance, it is necessary to review new formulations in cleaning and disinfection protocols (Vásquez et al., 2017). Also, Rasha Gharieb and colleagues report that carbapenem-resistant P. aeruginosa (CRPA) on intensive livestock farms is a serious problem that threatens animal and human health and increases the risk of P. aeruginosa infection in the community, so it is vital to control the spread of CRPA by limiting the use of antibiotics and applying proper cleaning and disinfection protocols on livestock farms (Ciecholewska-Juśko et al., 2022; Rasha Gharieb et al., 2021).
Regular monitoring of susceptibility, development of new therapeutic strategies against multidrug-resistant P. aeruginosa, correction of initial antiseptic concentrations with adjustment for multidrug-resistant strains and bacterial bloom status are relevant and important. The potential presence of such a resistant pathogen as P. aeruginosa should always be taken into account. It should be treated and prevented by following the “One Health” strategy.
5 Conclusion
The most active antiseptics against P. aeruginosa MDR are decamethoxin 0.1%, polyhexanide 0.1%, octenidine 0.1%, povidone-iodine 10%. The efficacy of miramistin 0.01% was found to be insufficient, as the IAA was below the threshold value (<4). Octenidine in sub-MIC concentrations demonstrated the strongest effect on immature biofilm (on its formation). The minimum bacteriostatic concentration of most antiseptics stimulated the development of mature biofilm. The bacteriostatic concentration of octenidine led to the eradication of mature biofilm by 4.7%. The MBC of most antiseptics (except chlorhexidine) led to eradication of mature biofilm by 4–30.6%. Chlorhexidine stimulated mature biofilm by 17.9%. Chlorhexidine showed the lowest activity against the formed biofilm at a concentration of half the initial concentration. But its initial concentration is half that of other detergents. The tested concentrations of antiseptics do not destroy the formed biofilm by more than 42.4%.
Tracing the trend of octenidine action at different concentrations at different stages of biofilm formation, its highest activity against P. aeruginosa biofilm should be emphasized. The results indicate the possibility of wider use of octenidine and decamethoxin for treatment of surgery wounds in patients with infection caused by MDR P. aeruginosa with possible recommendation for inclusion in wound infection treatment protocols.
The results of our study emphasize the importance of careful monitoring of P. aeruginosa isolates for antiseptic susceptibility. This will ultimately help prevent the creation of selective conditions for the emergence of resistant microorganisms and prevent their spread.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Bioethics Committee of National Pirogov Memorial Medical University, Vinnytsya (protocol No. 11, November 10, 2022). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ON: Project administration, Writing – original draft, Conceptualization, Validation, Supervision. KR: Supervision, Investigation, Writing – original draft, Validation, Data curation, Project administration, Conceptualization. VK: Data curation, Validation, Formal analysis, Writing – original draft, Supervision. TD: Software, Writing – original draft, Formal analysis, Methodology, Resources, Investigation. MF: Methodology, Resources, Formal analysis, Software, Writing – original draft. RC: Data curation, Investigation, Writing – review & editing. HN: Methodology, Writing – review & editing, Software, Resources. OP: Methodology, Writing – review & editing, Data curation, Investigation. NB: Writing – review & editing, Investigation, Data curation. DD: Conceptualization, Supervision, Methodology, Resources, Writing – review & editing, Formal analysis. VN: Writing – review & editing, Data curation, Investigation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1656270/full#supplementary-material
References
Abd El-Ghany, W. A. (2021). Pseudomonas aeruginosa infection of avian origin: zoonosis and one health implications. Vet. World 14, 2155–2159. doi: 10.14202/vetworld.2021.2155-2159
Ahmad, S. I, Malak, H. A., and Abulreesh, H. H. (2021). Environmental antimicrobial resistance and its drivers: a potential threat to public health. J. Global Antimicrob. Resistance 27, 101–111. doi: 10.1016/j.jgar.2021.08.001
Alves, P. J., Gryson, L., Hajjar, J., Lepelletier, D., Reners, M., Rodríguez Salazar, J., et al. (2023). Role of antiseptics in the prevention and treatment of infections in nursing homes. J. Hosp. Infect. 131, 58–69. doi: 10.1016/j.jhin.2022.09.021
Aslam, B., Khurshid, M., Arshad, M. I., Muzammil, S., Rasool, M., Yasmeen, N., et al. (2021). Antibiotic resistance: one health one world outlook. Front. Cell. Infect. Microbiol. 11:771510. doi: 10.3389/fcimb.2021.771510
Aslam, B., Wang, W., Arshad, M. I., Khurshid, M., Muzammil, S., Rasool, M. H., et al. (2018). Antibiotic resistance: a rundown of a global crisis. Infection Drug Resis. 11, 1645–1658. doi: 10.2147/IDR.S173867
Assadian, O. (2016). Octenidine dihydrochloride: chemical characteristics and antimicrobial properties. J. Wound Care 25, S3–S6. doi: 10.12968/jowc.2016.25.sup3.s3
Babalska, Z. Ł., Korbecka-Paczkowska, M., and Karpiński, T. M. (2021). Wound antiseptics and European guidelines for antiseptic application in wound treatment. Pharmaceuticals 14:1253. doi: 10.3390/ph14121253
Bakht, M., Alizadeh, S. A., Rahimi, S., Kazemzadeh Anari, R., Rostamani, M., Javadi, A., et al. (2022). Phenotype and genetic determination of resistance to common disinfectants among biofilm-producing and non-producing Pseudomonas aeruginosa strains from clinical specimens in Iran. BMC Microbiol. 22:124. doi: 10.1186/s12866-022-02524-y
Balasubramanian, D., Schneper, L., Kumari, H., and Mathee, K. (2013). A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucleic Acids Res. 41, 1–20. doi: 10.1093/nar/gks1039
Balcázar, J. L., Subirats, J., and Borrego, C. M. (2015). The role of biofilms as environmental reservoirs of antibiotic resistance. Front. Microbiol. 6:1216. doi: 10.3389/fmicb.2015.01216
Barrigah-Benissan, K., Ory, J., Dunyach-Remy, C., Pouget, C., Lavigne, J. P., and Sotto, A. (2022). Antibiofilm properties of antiseptic agents used on Pseudomonas aeruginosa isolated from diabetic foot ulcers. Int. J. Mol. Sci. 23:11270. doi: 10.3390/ijms231911270
Behzadi, P., Baráth, Z., and Gajdács, M. (2021). It's not easy being green: a narrative review on the microbiology, virulence and therapeutic prospects of multidrug-resistant Pseudomonas aeruginosa. Antibiotics (Basel, Switzerland) 10:42. doi: 10.3390/antibiotics10010042
Betchen, M., Giovinco, H. M., Curry, M., Luu, J., Fraimow, H., Carabetta, V. J., et al. (2022). Evaluating the effectiveness of hospital antiseptics on multidrug-resistant Acinetobacter baumannii: understanding the relationship between microbicide and antibiotic resistance. Antibiotics (Basel, Switzerland) 11:614. doi: 10.3390/antibiotics11050614
Brüggemann, H., Migliorini, L. B., Sales, R. O., Koga, P. C. M., Souza, A. V., Jensen, A., et al. (2018). Comparative genomics of nonoutbreak Pseudomonas aeruginosa strains underlines genome plasticity and geographic relatedness of the global clone ST235. Genome Biol. Evol. 10, 1852–1857. doi: 10.1093/gbe/evy139
Buehrle, D. J., Shields, R. K., Chen, L., Hao, B., Press, E. G., Alkrouk, A., et al. (2016). Evaluation of the in vitro activity of ceftazidime-avibactam and ceftolozane-tazobactam against meropenem-resistant Pseudomonas aeruginosa isolates. Antimicrob. Agents Chemother. 60, 3227–3231. doi: 10.1128/AAC.02969-15
CDC (2019) One health. Centers for Disease Control and Prevention Available online at: https://www.cdc.gov/onehealth/index.html
Cepas, V., López, Y., Muñoz, E., Rolo, D., Ardanuy, C., Martí, S., et al. (2019). Relationship between biofilm formation and antimicrobial resistance in gram-negative bacteria. Microb. Drug Resist. 25, 72–79. doi: 10.1089/mdr.2018.0027
Ciecholewska-Juśko, D., Żywicka, A., Junka, A. F., Woroszyło, M., Wardach, M., Chodaczek, G., et al. (2022). The effects of rotating magnetic field and antiseptic on in vitro pathogenic biofilm and its milieu. Sci. Rep. 12:8836. doi: 10.1038/s41598-022-12840-y
Crone, S., Vives-Flórez, M., Kvich, L., Saunders, A. M., Malone, M., Nicolaisen, M. H., et al. (2019). The environmental occurrence of Pseudomonas aeruginosa. APMIS 128, 220–231. doi: 10.1111/apm.13010
Dasgupta, N., Ashare, A., Hunninghake, G. W., and Yahr, T. L. (2006). Transcriptional induction of the Pseudomonas aeruginosa type III secretion system by low Ca2+ and host cell contact proceeds through two distinct signaling pathways. Infect. Immun. 74, 3334–3341. doi: 10.1128/IAI.00090-06
Davies, J., and Davies, D. (2010). Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 74, 417–433. doi: 10.1128/MMBR.00016-10
Denysko, T. V., Nazarchuk, O. A., Gruzevskyi, O., Bahniuk, N. À., Dmytriiev, D. V., Chornopyschuk, R. M., et al. (2022). In vitro evaluation of the antimicrobial activity of antiseptics against clinical Acinetobacter baumannii strains isolated from combat wounds. Front. Microbiol. 13:932467. doi: 10.3389/fmicb.2022.932467
Diggle, S. P., and Whiteley, M. (2020). Microbe profile: Pseudomonas aeruginosa: opportunistic pathogen and lab rat. Microbiology 166, 30–33. doi: 10.1099/mic.0.000860
Driscoll, J. A., Brody, S. L., and Kollef, M. H. (2007). The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs 67, 351–368. doi: 10.2165/00003495-200767030-00003
Favero, M. S., Carson, L. A., Bond, W. W., and Petersen, N. J. (1971). Pseudomonas aeruginosa: growth in distilled water from hospitals. Science 173, 836–838. doi: 10.1126/science.173.3999.836
Ford, M., Mende, K., Kaiser, S. J., et al. (2017). Clinical characteristics and resistance patterns of Pseudomonas aeruginosa isolated from operations enduring freedom and Iraqi freedom trauma patients. Open Forum Infect. Dis. 4, S155–S156.
Galdino, A. C. M., de Oliveira, M. P., Ramalho, T. C., de Castro, A. A., Branquinha, M. H., and Santos, A. L. S. (2019). Anti-virulence strategy against the multidrug-resistant bacterial pathogen Pseudomonas aeruginosa: Pseudolysin (elastase B) as a potential Druggable target. Curr. Protein Pept. Sci. 20, 471–487. doi: 10.2174/1389203720666190207100415
Gergova, R., Boyanov, V., Muhtarova, A., and Alexandrova, A. (2024). A review of the impact of streptococcal infections and antimicrobial resistance on human health. Antibiotics. 13:360. doi: 10.3390/antibiotics13040360
Global Antibiotic Research and Development Partnership (GARDP): GARDP and CARB-X welcome renewed commitment by G7 leaders to address antimicrobial resistance. [(accessed on 12 July 2022)]. Available online at: http://www.gardp.org/news-resources/gardp-and-carb-x-welcome-renewed-commitment-by-g7-leaders-to-address-antimicrobial-resistance/
Goldberg, J. B., Crisan, C. V., and Luu, J. M. (2022). Pseudomonas aeruginosa Antivirulence strategies: targeting the type III secretion system. Adv. Exp. Med. Biol. 1386, 257–280. doi: 10.1007/978-3-031-08491-1_9
Gryson, L., Meaume, S., Feldkaemper, I., and Favalli, F. (2023). Anti-biofilm activity of povidone-iodine and Polyhexamethylene Biguanide: evidence from in vitro tests. Curr. Microbiol. 80:161. doi: 10.1007/s00284-023-03257-5
Günther, F., Blessing, B., Dapunt, U., Mischnik, A., and Mutters, N. T. (2021). Ability of chlorhexidine, octenidine, polyhexanide and chloroxylenol to inhibit metabolism of biofilm-forming clinical multidrug-resistant organisms. J. Infect. Prev. 22, 12–18. doi: 10.1177/1757177420963829
Gupta, P., Bhatia, M., Gupta, P., and Omar, B. J. (2018). Emerging biocide resistance among multidrug-resistant Bacteria: myth or reality? A pilot study. J. Pharm. Bioallied Sci. 10, 96–101. doi: 10.4103/JPBS.JPBS_24_18
Gurung, J., Khyriem, A. B., Banik, A., Lyngdoh, W. V., Choudhury, B., and Bhattacharyya, P. (2013). Association of biofilm production with multidrug resistance among clinical isolates of Acinetobacter baumannii and Pseudomonas aeruginosa from intensive care unit. Indian J. Crit. Care Med. 17, 214–218. doi: 10.4103/0972-5229.118416
JPIAMR – Joint programming initiative on antimicrobial resistance. (n.d.). Available online at: https://www.jpiamr.eu
Junka, A., Bartoszewicz, M., Smutnicka, D., Secewicz, A., and Szymczyk, P. (2014). Efficacy of antiseptics containing povidone-iodine, octenidine dihydrochloride and ethacridine lactate against biofilm formed by Pseudomonas aeruginosa and Staphylococcus aureus measured with the novel biofilm-oriented antiseptics test. Int. Wound J. 11, 730–734. doi: 10.1111/iwj.12057
Kaiser, S. J., Mutters, N. T., DeRosa, A., Ewers, C., Frank, U., and Günther, F. (2017). Determinants for persistence of Pseudomonas aeruginosa in hospitals: interplay between resistance, virulence and biofilm formation. Eur. J. Clin. Microbiol. Infect. Dis. 36, 243–253. doi: 10.1007/s10096-016-2792-8
Karballaei Mirzahosseini, H., Hadadi-Fishani, M., Morshedi, K., and Khaledi, A. (2020). Meta-analysis of biofilm formation, antibiotic resistance pattern, and biofilm-related genes in Pseudomonas aeruginosa isolated from clinical samples. Microb. Drug Resist. 26, 815–824. doi: 10.1089/mdr.2019.0274
Karpiński, T. (2019). Efficacy of Octenidine against Pseudomonas Aeruginosa strains. Eur. J. Biol. Res. 9, 135–140.
Karruli, A., Catalini, C., D'Amore, C., Foglia, F., Mari, F., Harxhi, A., et al. (2023). Evidence-based treatment of Pseudomonas aeruginosa infections: a critical reappraisal. Antibiotics (Basel, Switzerland) 12:399. doi: 10.3390/antibiotics12020399
Kawamura, M., Fujimura, S., Tokuda, K., Aoyagi, T., Endo, S., Kanamori, H., et al. (2019). Mutant selection window of disinfectants for Staphylococcus aureus and Pseudomonas aeruginosa. J. Global Antimicrob. Resist. 17, 316–320. doi: 10.1016/j.jgar.2019.01.015
Koburger, T., Hübner, N. O., Braun, M., Siebert, J., and Kramer, A. (2010). Standardized comparison of antiseptic efficacy of triclosan, PVP-iodine, octenidine dihydrochloride, polyhexanide and chlorhexidine digluconate. J. Antimicrob. Chemother. 65, 1712–1719. doi: 10.1093/jac/dkq212
Kovalchuk, V., Riesbeck, K., Nazarchuk, O., Faustova, M., Dmytriiev, D., Nazarchuk, H., et al. (2024). A current view on the phenotypic antibiotic resistance of leading pathogens in wounded patients during the war in Ukraine. Acta Biomed 95:e2024030. doi: 10.23750/abm.v95i2.15395
Kramer, A., Dissemond, J., Kim, S., Willy, C., Mayer, D., Papke, R., et al. (2018). Consensus on wound antisepsis: update 2018. Skin Pharmacol. Physiol. 31, 28–58. doi: 10.1159/000481545
Krasowski, G., Junka, A., Paleczny, J., Czajkowska, J., Makomaska-Szaroszyk, E., Chodaczek, G., et al. (2021). In vitro evaluation of polihexanide, octenidine and NaClO/HClO-based antiseptics against biofilm formed by wound pathogens. Membranes 11:62. doi: 10.3390/membranes11010062
Kvasnevska, Y., Faustova, M., Voronova, K., Basarab, Y., and Lopatina, Y. (2024). Impact of war-associated factors on spread of sexually transmitted infections: a systemic review. Front. Public Health 12:1366600. doi: 10.3389/fpubh.2024.1366600
Kyaw, M. H., Kern, D. M., Zhou, S., Tunceli, O., Jafri, H. S., and Falloon, J. (2015). Healthcare utilization and costs associated with S. aureus and P. aeruginosa pneumonia in the intensive care unit: a retrospective observational cohort study in a US claims database. BMC Health Serv. Res. 15:241. doi: 10.1186/s12913-015-0917-x
Lefebvre, E., Vighetto, C., Di Martino, P., Larreta Garde, V., and Seyer, D. (2016). Synergistic antibiofilm efficacy of various commercial antiseptics, enzymes and EDTA: a study of Pseudomonas aeruginosa and Staphylococcus aureus biofilms. Int. J. Antimicrob. Agents 48, 181–188. doi: 10.1016/j.ijantimicag.2016.05.008
Levison, M. E. (2004). Pharmacodynamics of antimicrobial drugs. Infect. Dis. Clin. N. Am. 18, 451–465. doi: 10.1016/j.idc.2004.04.012
Lewenza, S., Abboud, J., Poon, K., Kobryn, M., Humplik, I., Bell, J. R., et al. (2018). Pseudomonas aeruginosa displays a dormancy phenotype during long-term survival in water. PLoS One 13:e0198384. doi: 10.1371/journal.pone.0198384
Liao, C., Huang, X., Wang, Q., Yao, D., and Lu, W. (2022). Virulence factors of Pseudomonas aeruginosa and Antivirulence strategies to combat its drug resistance. Front. Cell. Infect. Microbiol. 12:926758. doi: 10.3389/fcimb.2022.926758
Liew, S. M., Rajasekaram, G., Puthucheary, S. A., and Chua, K. H. (2019). Antimicrobial susceptibility and virulence genes of clinical and environmental isolates of Pseudomonas aeruginosa. PeerJ 7:e6217. doi: 10.7717/peerj.6217
Ljungquist, O., Nazarchuk, O., Kahlmeter, G., Andrews, V., Koithan, T., Wasserstrom, L., et al. (2023). Highly multidrug-resistant gram-negative bacterial infections in war victims in Ukraine, 2022. Lancet Infect. Dis. 23, 784–786. doi: 10.1016/S1473-3099(23)00291-8
Loban’, G., Faustova, M., Dobrovolska, O., and Tkachenko, P. (2023). War in Ukraine: incursion of antimicrobial resistance. Ir. J. Med. Sci. 192, 2905–2907. doi: 10.1007/s11845-023-03401-x
López-Rojas, R., Fernández-Cuenca, F., Serrano-Rocha, L., and Pascual, Á. (2017). In vitro activity of a polyhexanide-betaine solution against high-risk clones of multidrug-resistant nosocomial pathogens. Enferm. Infecc. Microbiol. Clin. 35, 12–19. doi: 10.1016/j.eimc.2016.02.008
Magiorakos, A. P., Srinivasan, A., Carey, R. B., Carmeli, Y., Falagas, M. E., Giske, C. G., et al. (2012). Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18, 268–281. doi: 10.1111/j.1469-0691.2011.03570.x
Maillard, J. Y., Kampf, G., and Cooper, R. (2021). Antimicrobial stewardship of antiseptics that are pertinent to wounds: the need for a united approach. JAC-antimicrobial resistance 3:dlab027. doi: 10.1093/jacamr/dlab027
Mareș, C., Petca, R. C., Petca, A., Popescu, R. I., and Jinga, V. (2022). Does the COVID pandemic modify the antibiotic resistance of uropathogens in female patients? A new storm? Antibiotics 11:376. doi: 10.3390/antibiotics11030376
Maurice, N. M., Bedi, B., and Sadikot, R. T. (2018). Pseudomonas aeruginosa biofilms: host response and clinical implications in lung infections. Am. J. Respir. Cell Mol. Biol. 58, 428–439. doi: 10.1165/rcmb.2017-0321tr
Melwani, M. (2022). How war is spreading drug resistant superbugs across Ukraine and beyond. BMJ 379:o2731. doi: 10.1136/bmj.o2731
Mende, K., Akers, K. S., Tyner, S. D., Bennett, J. W., Simons, M. P., Blyth, D. M., et al. (2022). Multidrug-resistant and virulent organisms trauma infections: trauma infectious disease outcomes study initiative. Mil. Med. 187, 42–51. doi: 10.1093/milmed/usab131
Moradali, M. F., Ghods, S., and Rehm, B. H. (2017). Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front. Cell. Infect. Microbiol. 7:39. doi: 10.3389/fcimb.2017.00039
Murray, J. L., Kwon, T., Marcotte, E. M., and Whiteley, M. (2015). Intrinsic antimicrobial resistance determinants in the superbug Pseudomonas aeruginosa. MBio 6:e01603–e1615. doi: 10.1128/mBio.01603-15
Nazarchuk, O., Faustova, M., and Kolodii, S. (2019). Microbiological characteristics of infectious complications, actual aspects of their prevention and treatment in surgical patients. Nov. Khir. 27, 318–327. doi: 10.18484/2305-0047.2019.3.318
Nazarchuk, O. A., Rusak, P. S., Chornopyshchuk, R. M., Denysko, T. V., Vovk, I. M., Grebeniuk, D. I., et al. (2024). Properties of the antimicrobial activity of polyhexanide drugs against the dominant pathogens of wound infection in Ukraine: modern realities. Paediatr. Surg. Ukraine 3, 12–21. doi: 10.15574/ps.2024.3(84).1221
Nolan, C., and Behrends, V. (2021). Sub-inhibitory antibiotic exposure and virulence in Pseudomonas aeruginosa. Antibiotics (Basel, Switzerland) 10:1393. doi: 10.3390/antibiotics10111393
Oliver, A., Mulet, X., López-Causapé, C., and Juan, C. (2015). The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist. Updat. 21-22, 41–59. doi: 10.1016/j.drup.2015.08.002
Pang, Z., Raudonis, R., Glick, B. R., Lin, T. J., and Cheng, Z. (2019). Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 37, 177–192. doi: 10.1016/j.biotechadv.2018.11.013
Petersen, K., Riddle, M. S., Danko, J. R., Blazes, D. L., Hayden, R., Tasker, S. A., et al. (2007). Trauma-related infections in battlefield casualties from Iraq. Ann. Surg. 245, 803–811. doi: 10.1097/01.sla.0000251707.32332.c1
Phan, S., Feng, C. H., Huang, R., Lee, Z. X., Moua, Y., Phung, O. J., et al. (2023). Relative abundance and detection of Pseudomonas aeruginosa from chronic wound infections globally. Microorganisms 11:1210. doi: 10.3390/microorganisms11051210
Pogue, J. M., Kaye, K. S., Veve, M. P., Patel, T. S., Gerlach, A. T., Davis, S. L., et al. (2020). Ceftolozane/tazobactam vs polymyxin or aminoglycoside-based regimens for the treatment of drug-resistant Pseudomonas aeruginosa. Clin. Infect. Dis. 71, 304–310. doi: 10.1093/cid/ciz816
Rasha Gharieb,, Saad, M., Khedr, M., Amany El Gohary,, and Ibrahim, H. (2021). Occurrence, virulence, carbapenem resistance, susceptibility to disinfectants and public health hazard of Pseudomonas aeruginosa isolated from animals, humans and environment in intensive farms. J. Appl. Microbiol. 132, 256–267. doi: 10.1111/jam.15191
Rossi Gonçalves, I., Dantas, R. C. C., Ferreira, M. L., Batistão, D. W. D. F., Gontijo-Filho, P. P., and Ribas, R. M. (2017). Carbapenem-resistant Pseudomonas aeruginosa: association with virulence genes and biofilm formation. Braz. J. Microbiol. 48, 211–217. doi: 10.1016/j.bjm.2016.11.004
Rossolini, G. M., Arena, F., Pecile, P., and Pollini, S. (2014). Update on the antibiotic resistance crisis. Curr. Opin. Pharmacol. 18, 56–60. doi: 10.1016/j.coph.2014.09.006
Rubio, A. M., Kline, E. G., Jones, C. E., Chen, L., Kreiswirth, B. N., Nguyen, M. H., et al. (2021). In vitro susceptibility of multidrug-resistant Pseudomonas aeruginosa following treatment-emergent resistance to ceftolozane-tazobactam. Antimicrob. Agents Chemother. 65:e00084-21. doi: 10.1128/AAC.00084-21
Ruffin, M., and Brochiero, E. (2019). Repair process impairment by Pseudomonas aeruginosa in epithelial tissues: major features and potential therapeutic avenues. Front. Cell. Infect. Microbiol. 9:182. doi: 10.3389/fcimb.2019.00182
Sanchez, C. J. Jr., Mende, K., Beckius, M. L., Akers, K. S., Romano, D. R., Wenke, J. C., et al. (2013). Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect. Dis. 13:47. doi: 10.1186/1471-2334-13-47
Sanya, D. R. A., Onésime, D., Vizzarro, G., and Jacquier, N. (2023). Recent advances in therapeutic targets identification and development of treatment strategies towards Pseudomonas aeruginosa infections. BMC Microbiol. 23:86. doi: 10.1186/s12866-023-02832-x
Sathe, N., Beech, P., Croft, L., Suphioglu, C., Kapat, A., and Athan, E. (2023). Pseudomonas aeruginosa: infections and novel approaches to treatment “knowing the enemy” the threat of Pseudomonas aeruginosa and exploring novel approaches to treatment. Inf. Med. 2, 178–194. doi: 10.1016/j.imj.2023.05.003
Schultz, G., Bjarnsholt, T., James, G. A., Leaper, D. J., McBain, A. J., Malone, M., et al. (2017). Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen. 25, 744–757. doi: 10.1111/wrr.12590
Shree, P., Singh, C. K., Sodhi, K. K., Surya, J. N., and Singh, D. K. (2023). Biofilms: understanding the structure and contribution towards bacterial resistance in antibiotics. Med. Microecol. 16:100084. doi: 10.1016/j.medmic.2023.100084
Theuretzbacher, U., Bush, K., Harbarth, S., Paul, M., Rex, J. H., Tacconelli, E., et al. (2020). Critical analysis of antibacterial agents in clinical development. Nat. Rev. Microbiol. 18, 286–298. doi: 10.1038/s41579-020-0340-0
Thuenauer, R., Landi, A., Trefzer, A., Altmann, S., Wehrum, S., Elerhoff, T., et al. (2020). The Pseudomonas aeruginosa lectin LecB causes integrin internalization and inhibits epithelial wound healing. MBio 11:e03260-19. doi: 10.1128/mBio.03260-19
Uddin, T. M., Chakraborty, A. J., Khusro, A., Zidan, B. R. M., Mitra, S., Emran, T. B., et al. (2021). Antibiotic resistance in microbes: history, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 14, 1750–1766. doi: 10.1016/j.jiph.2021.10.020
Van Boeckel, T. P., Brower, C., Gilbert, M., Grenfell, B. T., Levin, S. A., Robinson, T. P., et al. (2015). Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 112, 5649–5654. doi: 10.1073/pnas.1503141112
Vásquez, D., Libreros-Zúñiga, G., and Crespo, M. (2017). Effects of biocide exposure on P. aeruginosa, E. coli and A. baumannii complex isolates from hospital and household environments. Infection 21, 243–250. doi: 10.22354/in.v21i4.687
Weintrob, A. C., Murray, C. K., Xu, J., Krauss, M., Bradley, W., Warkentien, T. E., et al. (2018). Early infections complicating the care of combat casualties from Iraq and Afghanistan. Surg. Infect. 19, 286–297. doi: 10.1089/sur.2017.240
Wolcott, R. D., Hanson, J. D., Rees, E. J., Koenig, L. D., Phillips, C. D., Wolcott, R. A., et al. (2016). Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound. Repair. Regen. 24, 163–174. doi: 10.1111/wrr.12370
World Health Organization (2024). WHO bacterial priority pathogens list, 2024 : World Health Organization.
Keywords: multidrug-resistant bacteria, Pseudomonas aeruginosa , healthcare-associated infections, antiseptics, susceptibility to antiseptics, quaternary ammonium compounds, biguanide compounds, anti-biofilm activity
Citation: Nazarchuk O, Riesbeck K, Kovalchuk V, Denysko T, Faustova M, Chornopyshchuk R, Nazarchuk H, Parkhomenko O, Bahniuk N, Dmytriiev D and Nagaichuk V (2025) Modern antiseptics against multidrug-resistant Pseudomonas aeruginosa, emerging from war-related injuries in Ukraine. Front. Microbiol. 16:1656270. doi: 10.3389/fmicb.2025.1656270
Edited by:
Vinothkannan Ravichandran, Amity University, IndiaReviewed by:
Maria José Saavedra, University of Trás-os-Montes and Alto Douro, PortugalKurt Henry Piepenbrink, University of Nebraska-Lincoln, United States
Prakash Shankaran, SASTRA University, India
Copyright © 2025 Nazarchuk, Riesbeck, Kovalchuk, Denysko, Faustova, Chornopyshchuk, Nazarchuk, Parkhomenko, Bahniuk, Dmytriiev and Nagaichuk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mariia Faustova, bS5mYXVzdG92YUBwZG11LmVkdS51YQ==
 Oleksandr Nazarchuk
Oleksandr Nazarchuk Kristian Riesbeck
Kristian Riesbeck Valentyn Kovalchuk1
Valentyn Kovalchuk1 Tetiana Denysko
Tetiana Denysko Mariia Faustova
Mariia Faustova Roman Chornopyshchuk
Roman Chornopyshchuk Halyna Nazarchuk
Halyna Nazarchuk Nataliia Bahniuk
Nataliia Bahniuk Dmytro Dmytriiev
Dmytro Dmytriiev