- 1Gynecology Department, The Second Affiliated Hospital of Hunan University of Chinese Medicine, Changsha, Hunan, China
- 2College of Integrative Medicine, Hunan University of Traditional Chinese Medicine, Changsha, Hunan, China
- 3The First Hospital of Hunan University of Chinese Medicine, Changsha, Hunan, China
Polycystic ovary syndrome (PCOS) is a complex endocrine and metabolic disorder, primarily characterized by symptoms such as ovulatory dysfunction, hyperandrogenism, and polycystic ovarian morphology. In recent years, research has revealed that gut microbiota dysbiosis plays a crucial role in the pathogenesis of PCOS. Diet, as an essential factor in regulating gut microbiota, significantly impacts the clinical presentation and metabolic status of PCOS patients. Although substantial research has explored the relationship between PCOS and gut microbiota, many controversies and gaps remain, including the unclear mechanisms by which dietary structure and nutritional interventions specifically influence PCOS. This review aims to summarize the interaction between PCOS and gut microbiota, explore the role of diet in modulating gut microbiota and improving the pathological state of PCOS, and evaluate the potential therapeutic effects of probiotics, high-fat diets, and ketogenic diets on PCOS. Ultimately, it looks forward to personalized nutritional treatment strategies based on gut microbiota and future research directions, providing new insights into the treatment of PCOS.
1 Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine disorder among reproductive-age women that significantly impacts fertility (Deans, 2019). Its primary characteristics include irregular menstrual cycles, increased body hair, ovulatory dysfunction (OD), hyperandrogenism (HA), and polycystic ovary morphology (PCOM) (Dewailly et al., 2016; Li et al., 2019). Currently, there are three definitions of PCOS, with the Rotterdam criteria being the most widely accepted and recognized standard. According to the Rotterdam criteria (Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, 2004), at least two of the following criteria must be met to diagnose PCOS: Clinical and/or biochemical hyperandrogenism (HA); Ovulatory dysfunction (OD); Polycystic ovarian morphology (PCOM). From 1990 to 2019, the incidence of PCOS increased from 1.4 million to 2.1 million, with the highest incidence globally observed in the 10–19 age group (Zhang et al., 2024). In recent years, with the deepening of research on PCOS, it has been closely related to metabolic disorders, obesity, insulin resistance, and other conditions, and these factors are commonly present in PCOS patients (Chen et al., 2022a; Luo et al., 2022, p. 2). PCOS increases the risk of further complications such as cardiovascular disease (Gao et al., 2023a), type 2 diabetes (Chen et al., 2022b), metabolic syndrome, depression, and anxiety (Damone et al., 2019). Therefore, a thorough understanding of the epidemiological characteristics and clinical manifestations of PCOS is crucial for developing effective prevention and treatment strategies.
The gut microbiota is often referred to as the “second organ” and plays a crucial role in maintaining metabolic and endocrine balance in the human body. The diversity and function of gut microbiota regulate steroid hormone levels through the enterohepatic circulation, including estrogen (Flores et al., 2012; Li et al., 2025c). Studies have found that the gut microbiota composition of PCOS patients differs significantly from that of healthy women, and gut microbiota dysbiosis may further exacerbate metabolic disorders and related complications in PCOS (Lindheim et al., 2017; Torres et al., 2018). Regulating the gut microbiota may represent a novel therapeutic strategy for managing PCOS. Diet, as a key modulator of the gut microbiota, has potential implications for PCOS that should not be overlooked. Studies have shown that a ketogenic diet improves the clinical phenotype and insulin resistance in PCOS rats, while also altering the composition of gut microbiota and metabolites associated with androgen metabolism (Wang et al., 2023). Conversely, a high-sugar, high-fat diet disrupts gut microbiota balance, increases the Firmicutes/Bacteroidetes ratio, and expands the Firmicutes phylum, thereby exacerbating PCOS symptoms (Zheng et al., 2021b). Therefore, optimizing dietary structure and enhancing gut microbiota diversity can help improve metabolic status and physiological function in patients with PCOS. The graphical abstract is shown in Figure 1.

Figure 1. Graphical abstract: PCOS is influenced by dietary structure, gut microbiota, and their metabolic products. Using probiotics, prebiotics, and traditional Chinese medicine to regulate the microbiota, as well as improving the dietary structure, can effectively alleviate PCOS.
2 The role of gut microbiota and their metabolic products in PCOS
2.1 Gut microbiota and sex hormones
The gut microbiota is a complex ecosystem composed of approximately 1014 biological species, performing functions such as metabolism and nutrition, antimicrobial protection, maintenance of intestinal mucosal integrity, and regulation of immune responses (Becattini et al., 2016; Sebastián Domingo and Sánchez Sánchez, 2018). Since estrogen levels influence the composition of the microbiota, the gut microbiota composition in women differs significantly from that in men. Studies have shown that the gut microbiota exhibits gender-dependent differences (Wu et al., 2022b; Brown et al., 2023). The gut microbiota plays a crucial role in the onset and progression of PCOS. Clinical studies have shown that compared to healthy women, PCOS patients exhibit lower fecal microbiota diversity, altered gut barrier function, and changes in specific markers of endotoxemia. Hyperandrogenism, total testosterone levels, and hirsutism are negatively correlated with α-diversity (Lindheim et al., 2017; Torres et al., 2018). Compared with non-obese PCOS patients and healthy controls, obese PCOS patients exhibit increased Enterobacteriaceae levels, reduced Lactobacillus and Bifidobacterium levels, and changes in the gut microbiota associated with inflammation and insulin resistance (Zhou et al., 2020). Animal studies have shown that in PCOS mice induced by dehydroepiandrosterone and a high-fat diet, there is a reduction in Bacteroides and Blautia, and a decrease in Ruminococcus, Clostridium, and Alistipes (Lin et al., 2021). PCOS mice with impaired glucose and lipid metabolism, hyperinsulinemia, and insulin resistance (IR) exhibited worsened hyperandrogenemia and lipid metabolism disorders following antibiotic mixture intervention (Wang et al., 2022). After fecal microbiota transplantation from PCOS individuals, mice showed insulin resistance, disrupted estrous cycles, increased numbers of cystic follicles, fewer corpus lutea, and elevated levels of testosterone and luteinizing hormone (Qi et al., 2019). In summary, the close relationship between gut microbiota, fecal metabolites, and serum sex hormones influences the progression of PCOS.
The complex interactions between gut microbiota and sex hormones play a significant role in gender differences and PCOS. PCOS leads to excessive production of androgens by the ovaries, resulting in hyperandrogenism, which typically manifests as menstrual irregularities, hirsutism, and acne (Rosenfield, 2024). Hyperandrogenism is associated with the gut microbiota. Studies have shown that PCOS patients commonly exhibit hyperandrogenism, and elevated testosterone levels can alter gut microbiota structure by regulating bile acid signaling pathways (such as FXR, CYP7A1, etc.), leading to a reduction in the abundance of metabolically protective microbiota such as Akkermansia (Duan et al., 2024). Dehydroepiandrosterone (DHEA) induces gut microbiota dysbiosis in PCOS rats. Eliminating the gut microbiota with an antibiotic cocktail does not prevent the development of PCOS phenotypes. Transplanting PCOS rat gut microbiota into germ-free rats disrupts hepatic glucose and lipid metabolism and causes reproductive hormone imbalances (Han et al., 2021). Hyperandrogenemia may lead to insulin resistance and PCOS metabolic abnormalities by causing intestinal bacterial enrichment. This microbiota dysbiosis exacerbates insulin resistance, forming a vicious cycle of “hyperandrogenism-microbiota dysbiosis-metabolic abnormalities” (Zeng et al., 2019). Androgens are produced by theca cells, while estrogens are produced by granulosa cells and can be interconverted within the body. Gut microbiota can interfere with the enterohepatic circulation of sex hormones by secreting enzymes, such as β-glucuronidase, which further increases serum free testosterone levels (Cross et al., 2024). Gut microbial β-glucuronidase is a key factor in regulating host estrogen metabolism, as it activates estrogen. Additionally, estrogen levels also influence the composition and diversity of the gut microbiota (Ervin et al., 2019). Studies have found that abnormalities in the estrogen receptor signaling pathway may weaken the microbiota's protective role in the intestinal barrier, allowing endotoxin to enter the bloodstream and induce chronic, low-grade inflammation (Kumari et al., 2024). In summary, gut microbiota dysbiosis not only affects the metabolism and levels of sex hormones but also exacerbates PCOS symptoms. Improving gut microbiota health may emerge as a new therapeutic strategy for PCOS, helping to regulate hormone levels, alleviate symptoms, and enhance patients' quality of life.
Insulin resistance (IR) and the resulting compensatory hyperinsulinemia are core metabolic abnormalities in polycystic ovary syndrome (PCOS), particularly in obese phenotypes. Alterations in the microbial metabolite profile disrupt bile acid pools and short-chain fatty acid levels, impair mucosal energy supply and intestinal barrier integrity, and modulate incretin hormone secretion, thereby altering FXR and GPBAR1 signaling in the intestine and liver, ultimately leading to IR (He and Li, 2020; Yang et al., 2021). In parallel, Gram-negative bacterial overgrowth elevates lipopolysaccharide (LPS) translocation, triggering low-grade endotoxemia and macrophage infiltration in adipose and ovarian tissue; this inflammatory milieu further amplifies insulin resistance and hyperandrogenism (Lindheim et al., 2017). Clinical microbiome studies confirm that the microbial composition of insulin-resistant PCOS patients differs significantly from that of non-IR individuals, underscoring a distinct microbial signature associated with metabolic impairment (Torres et al., 2018). Thus, IR occupies a central position at the intersection of metabolic, endocrine, and microbial axes in PCOS. Beyond androgens and insulin, other sex steroids—estrogens and progesterone—also exert bidirectional interactions with the gut microbiota. Dysbiosis, characterized by low microbial diversity or reduced estrobolome activity, decreases circulating free estrogens and alters the estrogen-to-androgen ratio, thereby affecting reproductive and metabolic homeostasis (Li et al., 2025c). Conversely, fluctuating estrogen and progesterone levels throughout the menstrual cycle, pregnancy, or menopause reshape the intestinal microbiota, influencing mucosal permeability, motility, and immune tone (Atoum and Padma, 2025; Li et al., 2025a). Restoration of a healthy gut ecosystem—through dietary modulation, probiotics, or targeted microbial therapies—may therefore offer a promising strategy to alleviate insulin resistance, rebalance sex hormones, and improve clinical outcomes in PCOS.
2.2 Gut microbiota metabolites and PCOS
Gut microbiota metabolites such as bile acids (BA), short-chain fatty acids (SCFA), branched-chain amino acids (BCAA), and trimethylamine N-oxide (TMAO) can be produced from food and interact with gut bacteria in the body, and are associated with the onset and progression of PCOS (Chen and Pang, 2021). Clinical studies have found that lean women with PCOS have significantly increased levels of chenodeoxycholic acid (CDCA), which is positively correlated with total testosterone and free androgen index (Zhu et al., 2024). In PCOS patients, serum CDCA and LCA levels are significantly elevated, and DCA is associated with the sedimentation index, fasting, and postprandial insulin levels, and is influenced by changes in testosterone (Yu et al., 2023). PCOS patients exhibit significantly elevated levels of glycine- and taurine-conjugated primary bile acids, and the increase in circulating conjugated primary bile acids is positively correlated with hyperandrogenism in PCOS women (Zhang et al., 2019a). Alterations in the bile acid profile are a risk factor for hyperandrogenism in PCOS patients. Animal studies have confirmed that women with PCOS have reduced levels of glycocholic acid and taurocholic acid. Transplantation of fecal microbiota from PCOS women or Bacteroides vulgatus into mice resulted in increased ovarian dysfunction, insulin resistance, and altered bile acid metabolism (Qi et al., 2019). Bacteroides vulgatus also acts independently of bile acids via its metabolite guanidine, activating the farnesoid X receptor (FXR) pathway, which subsequently inhibits L-cell secretion of glucagon-like peptide-1 (GLP-1), leading to insulin resistance and ovarian dysfunction and thereby causing PCOS-like symptoms (Yun et al., 2024). In summary, elevated levels of Bacteroides vulgatus in the gut microbiota are associated with altered bile acid metabolism in PCOS.
SCFAs influence PCOS by regulating glucose metabolism or hormonal signaling, and specific gut microbiota, fecal fatty acids, and serum metabolites may mediate the onset and progression of PCOS. The Clostridiaceae, Erysipelotrichidae, Lachnospiraceae, Lactobacillaceae, and Ruminococcaceae can produce SCFAs [37]. Studies have found that PCOS patients exhibit reduced levels of Faecalibacterium, Bifidobacterium, and Blautia in their gut microbiota, suggesting an association between PCOS and the reduction of SCFA-producing bacteria (Zhang et al., 2019b). PCOS rats exhibit reduced β-diversity and decreased butyrate-producing bacteria. Regulating the butyrate-dependent gut-brain-ovary axis to improve PCOS is effective (Feng et al., 2022). Following sleeve gastrectomy, PCOS rats exhibited increased abundance of Bacteroides and Blautia, along with reduced levels of fecal SCFAs, particularly butyrate, which significantly improved PCOS-related symptoms such as hyperandrogenism, ovarian dysfunction, and impaired glucose tolerance (Lin et al., 2021). It was found that serum butyrate levels in obese PCOS patients were lower compared to other groups. Butyrate can alleviate inflammation in granulosa cells by regulating mRNA modifications through the METTL3-mediated N6-methyladenosine (m6A) pathway. Supplementing with butyrate improves ovarian function and reduces the expression of local inflammatory factors in the ovaries (Liu et al., 2023). The levels of acetate and propionate in the feces of PCOS patients were significantly higher than those in healthy controls (Li et al., 2022). Compared with other SCFAs such as acetate and propionate, butyrate plays a significant role in alleviating PCOS symptoms (He et al., 2022b). In summary, SCFAs, particularly butyrate, play a crucial role in regulating the gut microbiota composition, reducing inflammation, and alleviating PCOS symptoms.
2.3 Endotoxins and inflammatory responses
The metabolic pathways of the gut microbiota play a significant role in regulating systemic inflammation, particularly through short-chain fatty acids (SCFAs), which help maintain glucose and insulin homeostasis and alleviate systemic chronic inflammation. SCFAs can significantly improve insulin sensitivity, inhibit chronic inflammatory responses, and thereby promote overall health by regulating the composition and activity of the gut microbiota (Chen et al., 2023). Additionally, IL-22, a key cytokine, plays a crucial role in regulating insulin sensitivity and lipid metabolism. Reduced IL-22 levels disrupt the integrity of the intestinal barrier and the microbiota's hemostatic function, thereby exacerbating endotoxemia and chronic inflammatory states (Kriebs, 2019; Qi et al., 2020). Patients with PCOS typically exhibit chronic, low-grade inflammation, characterized by significantly elevated circulating C-reactive protein levels, indicating persistent inflammatory responses (Aboeldalyl et al., 2021). Reduced IL-22 levels in PCOS individuals, while IL-22 administration improves insulin resistance, menstrual cycle disorders, and ovarian morphological abnormalities, making it a potential treatment for PCOS with hyperandrogenism phenotypes (Qi et al., 2020). The possible mechanism by which IL-22 regulates insulin resistance and ovarian dysfunction associated with PCOS may be related to the suppression of inflammation following BA administration (Qi et al., 2019).
Regarding the potential mechanisms underlying PCOS, studies have shown that BA administration can improve insulin resistance and ovarian dysfunction by inhibiting inflammatory responses. This mechanism of action may be closely related to the repair of the intestinal barrier and the regulation of the microbiota. When the intestinal mucosal barrier is damaged, endotoxins, such as lipopolysaccharides, can enter the bloodstream, leading to chronic inflammation (Rooks and Garrett, 2016). Butyrate can increase the expression of tight junction proteins in intestinal epithelial cells and reduce epithelial cell death, thereby promoting intestinal mucosal immunity and barrier integrity (Mathewson et al., 2016). SCFAs may play a role in repairing intestinal mucosal damage and improving PCOS inflammation. Studies have found that PCOS rats have reduced SCFA absorption, increased fecal SCFA concentrations, and positive correlations with tumor necrosis factor and IL-6 levels. Enhancing SCFA absorption can improve intestinal mucosal barrier integrity and inhibit intestinal and extraintestinal inflammation (Lin et al., 2021). Therefore, the critical role of SCFAs and BAs in regulating PCOS-related inflammation and insulin resistance suggests that improving intestinal microbiota metabolic pathways and repairing intestinal barrier function may provide new directions for PCOS treatment.
3 The regulatory effects of diet on gut microbiota and PCOS
3.1 High-fat diet in PCOS
Dietary fat has been shown to alter gut microbiota composition, with high-fat diets reducing the abundance of the Bacteroidetes phylum while increasing that of the Firmicutes and Proteobacteria phyla (Hildebrandt et al., 2009; Zhang et al., 2012). Changes in the ratio of Firmicutes to Bacteroidetes may be associated with obesity and weight loss following dietary intervention (Ley et al., 2006). Obesity-related gut microbiota dysbiosis is directly linked to a high-fat diet (HFD), which promotes a microbiota similar to that found in obese male mice (de La Serre et al., 2010). The disrupted gut microbiota caused by the HFD is also considered a major mediator of the link between obesity-related diseases and the primary pathological conditions associated with their development (inflammation, hyperandrogenism, and lipid metabolism disorders) (Cani et al., 2009; Zheng et al., 2021a; Wang et al., 2022). Gut microbiota diversity is a crucial factor in maintaining health, and its reduction is often associated with the onset of various metabolic diseases. Studies have shown that HFD leads to significant changes in gut microbiota diversity and composition, characterized by a substantial reduction in diversity, a decrease in beneficial bacteria such as Lactobacillus, and an increase in inflammation-associated microbiota, including certain Firmicutes and Bacteroidetes (Tan et al., 2025). The decline in Lactobacillus may be associated with metabolic disorders and inflammatory responses induced by a high-fat, high-sugar diet. Lactobacillus is generally considered a beneficial gut microorganism that maintains a healthy gut environment by producing lactic acid and other short-chain fatty acids (SCFAs) (He et al., 2020). When its abundance decreases, it may lead to an imbalance in the gut environment, increasing the risk of conditions such as metabolic syndrome and insulin resistance (Liow et al., 2024).
HFD not only alters the composition and abundance of the gut microbiota but also influences its metabolic functions by modifying the gut environment. HFD can also lead to dysbiosis of bacterial communities, which, in turn, weakens intestinal barrier function, allowing endogenous pathogenic bacteria and their metabolic products (such as endotoxins) to enter the bloodstream and trigger systemic inflammatory responses more readily (Crawford et al., 2019). Chronic low-grade inflammation is considered a key factor in the development of PCOS and is closely associated with insulin resistance (Barber et al., 2016; Cai et al., 2025). These bacteria may promote inflammation and metabolic disorders by producing active metabolites, further exacerbating the pathological state of PCOS (Gautam et al., 2024; Guan et al., 2024). Dysbiosis of the gut microbiota not only alters its composition but also disrupts its function. Studies have shown that a high-fat diet significantly reduces the abundance of SCFA-producing bacterial genera in the gut, including Roseburia and Faecalibacterium, which are the primary producers of butyrate and propionate (David et al., 2014). The reduction in SCFAs is closely associated with symptoms such as insulin resistance, obesity, and endocrine disorders in PCOS patients (Geng et al., 2025). Recent studies have shown that SCFAs, such as acetate and butyrate, can improve ovarian lipid metabolism and endocrine dysfunction in PCOS models (Olaniyi and Areloegbe, 2024; Olaniyi et al., 2025). Overall, the changes in gut microbiota diversity and composition induced by a high-fat diet are not only an important component of PCOS pathogenesis but also a potential target for future treatment. Through dietary intervention, probiotics, and other microbiota-modulating strategies, it is possible to improve metabolic and endocrine function in patients with PCOS, thereby enhancing their quality of life and fertility (Ju et al., 2025). Therefore, restoring the balance and diversity of the gut microbiota may provide new strategies and directions for managing and treating PCOS.
3.2 Ketogenic diet in PCOS
The ketogenic diet (KD) is a very-low-carbohydrate dietary pattern. Unlike an unrestricted high-fat diet, which typically promotes metabolic imbalance (as depicted in Figure 1), the KD couples controlled lipid intake with markedly reduced carbohydrate intake, thereby lowering circulating glucose and insulin levels and improving overall metabolic homeostasis (Balestra et al., 2025). The ketogenic diet enhances gut health by modifying the gut microbiota composition, promoting the growth of beneficial bacteria, and suppressing the activity of harmful bacteria (Palmas et al., 2025). KD is commonly used to treat neurological disorders. Still, research suggests that it may be an effective strategy for treating metabolic disorders, type 2 diabetes mellitus (T2DM), obesity, and non-alcoholic fatty liver disease, primarily by reducing fat accumulation and inhibiting postprandial insulin secretion (Cunha et al., 2020; Watanabe et al., 2020). The Italian Association of Dietetics and Clinical Nutrition, along with the Italian Society of Obesity, proposes the Very-low-calorie ketogenic diet (VLCKD) as a treatment option for NAFLD and obesity-related comorbidities (Caprio et al., 2019). Clinical trials have confirmed that overweight women with polycystic ovary syndrome (PCOS) who followed a high-protein, very-low-calorie diet for 4 weeks experienced significant reductions in fasting blood glucose levels and insulin levels, as well as a significant increase in insulin sensitivity (Andersen et al., 1995). The ketogenic diet can significantly improve weight management and insulin sensitivity, which is particularly important for PCOS patients, as PCOS is often associated with insulin resistance and obesity (Paoli et al., 2020). High-fat KD and VLCKD have shown beneficial effects on body weight, body composition, glucose metabolism parameters, and hormone profiles in women with PCOS. VLCKD has a better advantage in reducing fat mass and lowering triglyceride levels (Cannarella et al., 2025). The ketogenic diet also significantly affects hormone metabolism. A 12-week VLCKD intervention in obese, non-diabetic women with PCOS and regular menstrual cycles resulted in a significant decrease in serum anti-Müllerian hormone levels, a significant increase in progesterone and serum sex hormone-binding globulin levels, and VLCKD may also be beneficial for ovarian reserve and luteal function (Magagnini et al., 2022). Short-term KD treatment effectively regulates androgen levels in overweight/obese PCOS patients, reduces total testosterone levels, improves insulin resistance and glucose/lipid metabolism, and significantly reduces body weight (Li et al., 2025b). Compared with a carbohydrate-based diet model, KD demonstrates superior efficacy in improving BMI, weight, blood glucose, insulin, and free testosterone levels (Sharifi et al., 2024). Therefore, the ketogenic diet may offer a novel therapeutic approach for PCOS patients as an intervention strategy.
3.3 Mediterranean, DASH, and high-protein diets in PCOS
Beyond ketogenic or very-low-carbohydrate interventions, several other nutritional patterns, namely the Mediterranean diet (MedDiet), the Dietary Approaches to Stop Hypertension (DASH) diet, and high-protein diets (HPD), have emerged as critical dietary strategies for managing polycystic ovary syndrome (PCOS). These diets share overlapping mechanisms that target insulin resistance, oxidative stress, inflammation, and dysbiosis of the gut microbiota. The Mediterranean diet, characterized by abundant consumption of vegetables, fruits, legumes, whole grains, nuts, fish, and extra-virgin olive oil, with minimal processed or red meat, exerts multiple metabolic benefits through its high fiber and polyphenol content (Estruch et al., 2018; Rosato et al., 2019). Randomized controlled trials have demonstrated that an 8-week DASH intervention significantly improved HOMA-IR, hs-CRP, and abdominal adiposity in overweight and obese women with PCOS (Asemi and Esmaillzadeh, 2015). A 12-week trial further reported significant reductions in BMI, AMH, and oxidative stress markers, along with increased SHBG and decreased free androgen index (Foroozanfard et al., 2017).
The DASH diet, initially designed for blood-pressure control, is an isocaloric, nutrient-dense pattern rich in fruits, vegetables, low-fat dairy, legumes, nuts, and whole grains while limiting sodium, red meat, and added sugars (Sacks et al., 2001; Soltani et al., 2016). Randomized controlled trials have demonstrated that an 8-week DASH intervention significantly improved HOMA-IR, hs-CRP, and abdominal adiposity in overweight and obese women with PCOS (Asemi and Esmaillzadeh, 2015). A 12-week trial further reported significant reductions in BMI, AMH, and oxidative stress markers, along with increased SHBG and decreased free androgen index (Foroozanfard et al., 2017). Additional studies confirmed improvements in insulin metabolism, lipid profile, and body composition (Azadi-Yazdi et al., 2017). A recent network meta-analysis (2024) ranked the DASH diet as the most effective dietary pattern overall for metabolic and hormonal regulation in PCOS (Juhász et al., 2024). HPD providing approximately 25–35% of total energy from protein can improve satiety, preserve lean mass, and attenuate postprandial insulin secretion. In a controlled clinical trial, a hypocaloric high-protein/low-GI diet achieved greater improvements in insulin sensitivity and hs-CRP than a conventional low-protein diet (Homeira et al., 2012). A recent systematic review (2024) suggested that HPDs improve insulin resistance and body composition in PCOS patients, though outcomes depend on protein source, fiber content, and energy balance (Wang et al., 2024, 2025).
In summary, while the Mediterranean and DASH diets primarily exert their benefits through gut microbiota modulation, SCFA enhancement, bile acid signaling, and anti-inflammatory effects, high-protein diets mainly regulate satiety, weight, and insulin dynamics.
4 Advances in dietary intervention and gut microbiota regulation
4.1 Applications of probiotics and prebiotics
Gut microbiota has been shown to play a crucial role in the pathogenesis of PCOS. Therapies targeting the gut microbiota, such as probiotics, prebiotics, or fecal microbiota transplantation, offer promising treatment strategies for PCOS and its associated metabolic disorders (Duan et al., 2021). In terms of hormone regulation, probiotics influence sex hormone levels by modulating the gut microbiota and its metabolites. Lactiplantibacillus plantarum alleviates ovarian pathological changes, restores testosterone and luteinizing hormone levels, and increases the abundance of butyrate- and short-chain fatty acid-associated bacteria, such as Lachnospira and Ruminococcus, thereby alleviating PCOS (He et al., 2022a). Lactic acid bacteria improve gut dysbiosis in PCOS rat models by modulating the abundance of Akkermansia, Roseburia, Prevotella, Staphylococcus, and Lactobacillus species, which are associated with sex hormone levels, thereby helping to alleviate PCOS (He et al., 2020). Therefore, probiotics have significant potential to regulate gut microbiota, restore hormonal balance, and relieve symptoms of PCOS.
Probiotic supplements have been shown to improve the metabolic status of patients affected by PCOS. Clinical studies have found that Bifidobacterium lactis V9 significantly reduces LH and LH/FSH levels in PCOS patients, while also considerably increasing sex hormone and gut SCFA levels and regulating sex hormone levels by modulating the gut microbiome (Zhang et al., 2019b). Probiotic supplementation increased serum sex hormone-binding globulin (SHBG) levels by a considerable amount in PCOS patients, normalized menstrual cycles, and significantly decreased serum total testosterone, demonstrating the efficacy of multi-strain probiotics, along with dietary and lifestyle changes, in treating PCOS (Karamali et al., 2018; Kaur et al., 2022). Several clinical studies have confirmed that probiotics and synbiotic supplements improve insulin resistance and serum insulin levels in patients with PCOS [83], reduce HOMA-IR, fasting blood glucose, and blood lipid levels, and lower total testosterone; synbiotics show more pronounced effects than probiotics or prebiotics alone (Shamasbi et al., 2020; Martinez Guevara et al., 2024). Probiotic supplementation also significantly reduces weight, BMI, insulin levels, HOMA-IR, triglycerides, hirsutism, and total testosterone levels in PCOS patients, without affecting dehydroepiandrosterone sulfate (DHEAS) levels, as well as total cholesterol, LDL cholesterol, and HDL cholesterol (Tabrizi et al., 2022). In summary, probiotic supplementation not only improves metabolic disorders but also effectively regulates hormone levels, making it a promising adjunctive treatment for PCOS.
4.2 Traditional Chinese medicine and gut microbiota regulation
Traditional Chinese medicine (TCM) plays an increasingly important role in managing PCOS, not only by modulating endocrine and metabolic abnormalities but also by restoring gut-microbiota balance and reducing chronic inflammation. Recent studies integrating network pharmacology, metabolomics, and gut-microbiota analysis have elucidated the multi-target mechanisms of several classical and emerging TCM prescriptions (Kwon et al., 2020).
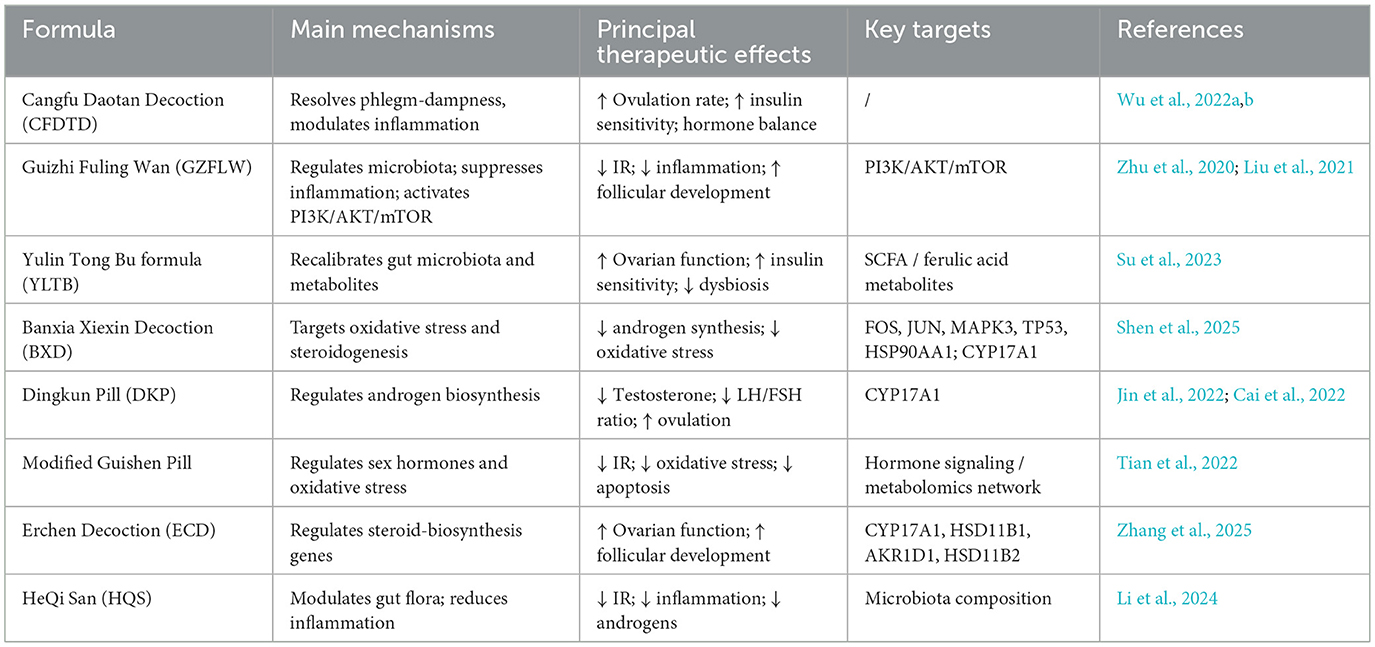
Cangfu Daotan Decoction (CFDTD), a representative formula for resolving phlegm and dampness, has been shown to improve ovulation rate, insulin sensitivity, and hormonal balance. A meta-analysis confirmed its efficacy and safety in the treatment of PCOS (Wu et al., 2022a). Guizhi Fuling Wan (GZFLW), traditionally used to promote blood circulation and remove stasis, enhances insulin sensitivity and reduces systemic inflammation by regulating gut-microbiota composition and suppressing inflammatory cytokines (Zhu et al., 2020). Mechanistically, GZFLW inhibits granulosa-cell autophagy and promotes follicular development via activation of the PI3K/AKT/mTOR signaling pathway (Liu et al., 2021). The Yulin Tong Bu formula (YLTB) restores metabolic and endocrine homeostasis by recalibrating gut-microbiota structure and fecal metabolites. In animal models, YLTB improved ovarian morphology, enhanced insulin sensitivity, and corrected glucose–lipid metabolism; metabolomic analysis identified ferulic acid as a key mediator of these effects (Su et al., 2023). The herb pair Banxia-Chenpi in the Banxia Xiexin Decoction formula reduces weight gain, normalizes sex hormone levels, and regulates the estrous cycle by activating the CYP17A1-centered steroid biosynthesis pathway (Shen et al., 2025). Dingkun Pill (DKP) significantly improves reproductive and metabolic function in PCOS, lowering testosterone and the LH/FSH ratio, and normalizing folliculogenesis through CYP17A1-Mediated modulation of androgen synthesis (Cai et al., 2022). A systematic review and meta-analysis further verified its clinical efficacy and safety (Jin et al., 2022). The modified Guishen pill significantly improved insulin resistance, apoptosis, and oxidative stress in PCOS rats by regulating sex hormone levels and alleviating ovarian pathological changes in rats (Tian et al., 2022). Traditional Chinese medicine has demonstrated unique advantages in the treatment of PCOS and holds promise as a potential resource for developing novel therapeutic agents.
Traditional Chinese medicine can also exert therapeutic effects on PCOS by improving the structure of the intestinal microbiota and regulating the intestinal microecological environment. Erchen decoction (ECD) enhances ovarian function by regulating the expression of CYP17A1, HSD11B1, AKR1D1, and HSD11B2 in the steroid hormone biosynthetic pathway, thereby improving hormone levels and follicle development (Zhang et al., 2025). HeQi San (HQS) reverses abnormal hormone elevation, improves insulin resistance, and reduces histopathological changes in ovarian tissue, thereby modulating the abundance of gut microbiota in PCOS mice, and may be a potential candidate for PCOS treatment (Li et al., 2024). Angelica sinensis regulates hormonal and lipid metabolism disorders by modulating PI3K/AKT, PPAR, MAPK, AMPK, and insulin signaling pathways in ovarian tissue, as well as maintaining gut microbiota homeostasis, thereby alleviating PCOS (Gao et al., 2023b). The Bushen Huatan formula may improve PCOS by regulating the gut microbiota to increase short-chain fatty acid levels, thereby activating the intestinal PPARγ pathway and improving intestinal barrier function (Cui et al., 2023). The Yulin Tong Bu formula reduces insulin resistance, improves ovarian dysfunction, lipid and glucose metabolism, and hormonal imbalances, and significantly alleviates PCOS-related gut microbiota dysbiosis (Su et al., 2023). In summary, traditional Chinese medicine demonstrates its potential in PCOS treatment by regulating the gut microbiota and improving endocrine imbalance through multiple pathways (Table 1). Although current research findings provide strong evidence, the specific mechanisms require further experimental validation and clinical studies to ensure safety and efficacy.
4.3 TCM monomers, mechanisms, and therapeutic effects in PCOS
In addition to multi-herb prescriptions, several TCM monomers/extracts demonstrate targeted benefits in PCOS. In rodent models and a pilot human study, artemisinin derivatives lowered testosterone, improved cycles and PCOS features by promoting LONP1-mediated degradation of CYP11A1, thereby suppressing ovarian androgen synthesis (Liu et al., 2024; Tysoe, 2024). Meta-analysis of animal/early human data shows quercetin reduces insulin, glucose, cholesterol and testosterone, improving histology and endocrine indices (Su et al., 2024). Mechanistic work indicates modulation of PI3K/AKT, steroidogenesis genes, and improved folliculogenesis (Shah et al., 2023). In PCOS RCTs and clinical studies, berberine improved insulin resistance, lipid profile, body composition and hormone status, in some endpoints comparable to or exceeding metformin (Wei et al., 2012; Mishra et al., 2022). A double-blind RCT showed resveratrol reduced ovarian/adrenal androgens with concurrent improvement in insulin sensitivity (Banaszewska et al., 2016). In RCTs, 12-week curcumin improved fasting glucose/insulin, HOMA-IR, lipids and modulated PPAR-γ/LDLR expression; a double-blind trial also reported favorable glucose/insulin and androgen endpoints (Jamilian et al., 2020; Heshmati et al., 2021). Collectively, these findings highlight that bioactive TCM monomers can simultaneously modulate metabolic, endocrine, and inflammatory networks, representing promising adjuncts or lead compounds for developing multi-targeted therapies against PCOS.
5 Future research directions and clinical application challenges in dietary intervention and gut microbiota regulation
Individual differences significantly affect the effectiveness of dietary interventions in the management of PCOS. Differences in gut microbiota composition among individuals can lead to varying responses to the same nutritional intervention. When developing dietary intervention protocols, it is essential to consider individual specificity to enhance their efficacy. Current traditional Chinese medicine research on PCOS primarily focuses on animal experiments. The conduct of long-term follow-up studies and large-scale clinical trials is significant, as this not only helps validate the long-term efficacy and safety of dietary interventions and microbiota regulation but also reveals differences in intervention responses among different PCOS subtypes. The development of novel microbiome-based formulations represents a cutting-edge area for enhancing gut microbiota in patients with PCOS. These formulations include probiotics and prebiotics, which can effectively regulate gut microbiome balance. However, current regulatory frameworks for probiotic formulations remain inadequate, potentially impacting their clinical safety and efficacy. Therefore, establishing standardized management protocols for novel microbiome-based formulations will be a critical measure for enhancing the effectiveness of PCOS management in the future.
6 Conclusion
Dysbiosis of the gut microbiota plays a crucial role in the pathogenesis of PCOS. Changes in dietary patterns can effectively regulate the endocrine and metabolic status of patients with PCOS. This finding offers a new perspective on the complexity of PCOS and provides novel approaches for future therapeutic strategies. Dietary patterns, combined with microecological interventions using probiotics and prebiotics, show promising potential in improving clinical symptoms and metabolic abnormalities in PCOS. These interventions not only restore the balance of the gut microbiota but also improve insulin sensitivity, reduce inflammation, and subsequently influence hormone levels and ovarian function.
Author contributions
SZ: Conceptualization, Methodology, Resources, Supervision, Writing – original draft. HC: Methodology, Writing – review & editing. BH: Writing – review & editing. YZ: Writing – review & editing. PL: Writing – review & editing. JK: Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was supported by the Hunan Provincial Health Commission's 2025 Health Research Project (20258047), the Hunan Provincial Health Commission's 2025 Health Research Project (20257503), and the Hunan University of Traditional Chinese Medicine Joint Fund Project (2025XYLH053).
Acknowledgments
We thank the editors and reviewers for their valuable comments and suggestions on the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aboeldalyl, S., James, C., Seyam, E., Ibrahim, E. M., Shawki, H. E.-D., and Amer, S. (2021). The role of chronic inflammation in polycystic ovarian syndrome-a systematic review and meta-analysis. Int. J. Mol. Sci. 22:2734. doi: 10.3390/ijms22052734
Andersen, P., Seljeflot, I., Abdelnoor, M., Arnesen, H., Dale, P. O., Løvik, A., et al. (1995). Increased insulin sensitivity and fibrinolytic capacity after dietary intervention in obese women with polycystic ovary syndrome. Metabolism 44, 611–616. doi: 10.1016/0026-0495(95)90118-3
Asemi, Z., and Esmaillzadeh, A. (2015). DASH diet, insulin resistance, and serum hs-CRP in polycystic ovary syndrome: a randomized controlled clinical trial. Horm. Metab. Res. Horm. Stoffwechselforschung Horm. Metab. 47, 232–238. doi: 10.1055/s-0034-1376990
Atoum, M., and Padma, K. (2025). Gut microbiota-estrogen axis: its influence on female health outcomes - a narrative review. Acta Biomed. Atenei Parm. 96, 15980–15980. doi: 10.23750/abm.v96i1.15980
Azadi-Yazdi, M., Karimi-Zarchi, M., Salehi-Abargouei, A., Fallahzadeh, H., and Nadjarzadeh, A. (2017). Effects of Dietary Approach to Stop Hypertension diet on androgens, antioxidant status and body composition in overweight and obese women with polycystic ovary syndrome: a randomised controlled trial. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 30, 275–283. doi: 10.1111/jhn.12433
Balestra, F., Luca, M. D., Panzetta, G., Palieri, R., Shahini, E., Giannelli, G., et al. (2025). Advancing obesity management: the very low-energy ketogenic therapy (VLEKT) as an evolution of the “traditional” ketogenic diet. Curr. Obes. Rep. 14:30. doi: 10.1007/s13679-025-00622-2
Banaszewska, B., Wrotyńska-Barczyńska, J., Spaczynski, R. Z., Pawelczyk, L., and Duleba, A. J. (2016). Effects of resveratrol on polycystic ovary syndrome: a double-blind, randomized, placebo-controlled trial. J. Clin. Endocrinol. Metab. 101, 4322–4328. doi: 10.1210/jc.2016-1858
Barber, T. M., Dimitriadis, G. K., Andreou, A., and Franks, S. (2016). Polycystic ovary syndrome: insight into pathogenesis and a common association with insulin resistance. Clin. Med. 16, 262–266. doi: 10.7861/clinmedicine.16-3-262
Becattini, S., Taur, Y., and Pamer, E. G. (2016). Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol. Med. 22, 458–478. doi: 10.1016/j.molmed.2016.04.003
Brown, K., Thomson, C. A., Wacker, S., Drikic, M., Groves, R., Fan, V., et al. (2023). Microbiota alters the metabolome in an age- and sex- dependent manner in mice. Nat. Commun. 14:1348. doi: 10.1038/s41467-023-37055-1
Cai, J., Zhu, Q., Xiang, Y., Weng, L., Liang, N., Hong, X., et al. (2025). Hyperandrogenism triggers mtDNA release to participate in ovarian inflammation via mPTP/cGAS/STING in PCOS. iScience 28:112391. doi: 10.1016/j.isci.2025.112391
Cai, Y.-L., Zhang, F., Dou, X.-X., Zeng, H.-W., Wu, G.-S., Liang, Y.-L., et al. (2022). Integrated metabolomics and network pharmacology to reveal the therapeutic mechanism of Dingkun Pill on polycystic ovary syndrome. J. Ethnopharmacol. 295:115442. doi: 10.1016/j.jep.2022.115442
Cani, P. D., Possemiers, S., Van de Wiele, T., Guiot, Y., Everard, A., Rottier, O., et al. (2009). Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58, 1091–1103. doi: 10.1136/gut.2008.165886
Cannarella, R., Rubulotta, M., Leonardi, A., Crafa, A., Calvo, A., Barbagallo, F., et al. (2025). Effects of ketogenic diets on polycystic ovary syndrome: a systematic review and meta-analysis. Reprod. Biol. Endocrinol. RBE 23:74. doi: 10.1186/s12958-025-01411-1
Caprio, M., Infante, M., Moriconi, E., Armani, A., Fabbri, A., Mantovani, G., et al. (2019). Very-low-calorie ketogenic diet (VLCKD) in the management of metabolic diseases: systematic review and consensus statement from the Italian Society of Endocrinology (SIE). J. Endocrinol. Invest. 42, 1365–1386. doi: 10.1007/s40618-019-01061-2
Chen, J., Xiao, Y., Li, D., Zhang, S., Wu, Y., Zhang, Q., et al. (2023). New insights into the mechanisms of high-fat diet mediated gut microbiota in chronic diseases. iMeta 2:e69. doi: 10.1002/imt2.69
Chen, T., Yu, Y., Jia, F., Luan, P., and Liu, X. (2022a). The relationship between polycystic ovary syndrome and insulin resistance from 1983 to 2022: a bibliometric analysis. Front. Public Health 10:960965. doi: 10.3389/fpubh.2022.960965
Chen, W., and Pang, Y. (2021). Metabolic syndrome and PCOS: pathogenesis and the role of metabolites. Metabolites 11:869. doi: 10.3390/metabo11120869
Chen, X., Gissler, M., and Lavebratt, C. (2022b). Association of maternal polycystic ovary syndrome and diabetes with preterm birth and offspring birth size: a population-based cohort study. Hum. Reprod. Oxf. Engl. 37, 1311–1323. doi: 10.1093/humrep/deac050
Crawford, M., Whisner, C., Al-Nakkash, L., and Sweazea, K. L. (2019). Six-week high-fat diet alters the gut microbiome and promotes cecal inflammation, endotoxin production, and simple steatosis without obesity in male rats. Lipids 54, 119–131. doi: 10.1002/lipd.12131
Cross, T.-W. L., Simpson, A. M. R., Lin, C.-Y., Hottmann, N. M., Bhatt, A. P., Pellock, S. J., et al. (2024). Gut microbiome responds to alteration in female sex hormone status and exacerbates metabolic dysfunction. Gut Microbes 16:2295429. doi: 10.1080/19490976.2023.2295429
Cui, M., Hong, Y., Huang, J., Liu, K., Chen, J., Tan, Y., et al. (2023). Efficiency of Chinese medicine Bushen Huatan formula for treatment of polycystic ovary syndrome in mice via regulating gut microbiota and PPARγ pathway. Zhejiang Xue Xue Bao Yi Xue Ban J. Zhejiang Univ. Med. Sci. 52, 33–45. doi: 10.3724/zdxbyxb-2022-0456
Cunha, G. M., Guzman, G., Correa De Mello, L. L., Trein, B., Spina, L., Bussade, I., et al. (2020). Efficacy of a 2-month very low-calorie ketogenic diet (VLCKD) compared to a standard low-calorie diet in reducing visceral and liver fat accumulation in patients with obesity. Front. Endocrinol. 11:607. doi: 10.3389/fendo.2020.00607
Damone, A. L., Joham, A. E., Loxton, D., Earnest, A., Teede, H. J., and Moran, L. J. (2019). Depression, anxiety and perceived stress in women with and without PCOS: a community-based study. Psychol. Med. 49, 1510–1520. doi: 10.1017/S0033291718002076
David, L. A., Maurice, C. F., Carmody, R. N., Gootenberg, D. B., Button, J. E., Wolfe, B. E., et al. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563. doi: 10.1038/nature12820
de La Serre, C. B., Ellis, C. L., Lee, J., Hartman, A. L., Rutledge, J. C., and Raybould, H. E. (2010). Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G440–448. doi: 10.1152/ajpgi.00098.2010
Deans, R. (2019). Polycystic ovary syndrome in adolescence. Med. Sci. Basel Switz. 7:101. doi: 10.3390/medsci7100101
Dewailly, D., Robin, G., Peigne, M., Decanter, C., Pigny, P., and Catteau-Jonard, S. (2016). Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum. Reprod. Update 22, 709–724. doi: 10.1093/humupd/dmw027
Duan, L., An, X., Zhang, Y., Jin, D., Zhao, S., Zhou, R., et al. (2021). Gut microbiota as the critical correlation of polycystic ovary syndrome and type 2 diabetes mellitus. Biomed. Pharmacother. Biomedecine Pharmacother. 142:112094. doi: 10.1016/j.biopha.2021.112094
Duan, X., Nie, Y., Xie, X., Zhang, Q., Zhu, C., Zhu, H., et al. (2024). Sex differences and testosterone interfere with the structure of the gut microbiota through the bile acid signaling pathway. Front. Microbiol. 15:1421608. doi: 10.3389/fmicb.2024.1421608
Ervin, S. M., Li, H., Lim, L., Roberts, L. R., Liang, X., Mani, S., et al. (2019). Gut microbial β-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J. Biol. Chem. 294, 18586–18599. doi: 10.1074/jbc.RA119.010950
Estruch, R., Ros, E., Salas-Salvadó, J., Covas, M.-I., Corella, D., Arós, F., et al. (2018). Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 378:e34. doi: 10.1056/NEJMoa1800389
Feng, X., Wang, D., Hu, L., Lu, H., Ling, B., Huang, Y., et al. (2022). Dendrobium officinale polysaccharide ameliorates polycystic ovary syndrome via regulating butyrate dependent gut-brain-ovary axis mechanism. Front. Endocrinol. 13:962775. doi: 10.3389/fendo.2022.962775
Flores, R., Shi, J., Fuhrman, B., Xu, X., Veenstra, T. D., Gail, M. H., et al. (2012). Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J. Transl. Med. 10:253. doi: 10.1186/1479-5876-10-253
Foroozanfard, F., Rafiei, H., Samimi, M., Gilasi, H. R., Gorjizadeh, R., Heidar, Z., et al. (2017). The effects of dietary approaches to stop hypertension diet on weight loss, anti-Müllerian hormone and metabolic profiles in women with polycystic ovary syndrome: a randomized clinical trial. Clin. Endocrinol. 87, 51–58. doi: 10.1111/cen.13333
Gao, L., Zhao, Y., Wu, H., Lin, X., Guo, F., Li, J., et al. (2023a). Polycystic ovary syndrome fuels cardiovascular inflammation and aggravates ischemic cardiac injury. Circulation 148, 1958–1973. doi: 10.1161/CIRCULATIONAHA.123.065827
Gao, Y., Mo, S., Cao, H., Zhi, Y., Ma, X., Huang, Z., et al. (2023b). The efficacy and mechanism of Angelica sinensis (Oliv.) Diels root aqueous extract based on RNA sequencing and 16S rDNA sequencing in alleviating polycystic ovary syndrome. Phytomed. Int. J. Phytother. Phytopharm. 120:155013. doi: 10.1016/j.phymed.2023.155013
Gautam, R., Maan, P., Patel, A. K., Vasudevan, S., and Arora, T. (2024). Unveiling the complex interplay between gut microbiota and polycystic ovary syndrome: a narrative review. Clin. Nutr. Edinb. Scotl. 43, 199–208. doi: 10.1016/j.clnu.2024.10.028
Geng, L., Yang, X., Sun, J., Ran, X., Zhou, D., Ye, M., et al. (2025). Gut microbiota modulation by inulin improves metabolism and ovarian function in polycystic ovary syndrome. Adv. Sci. Weinh. Baden-Wurtt. Ger. 12:e2412558. doi: 10.1002/advs.202412558
Guan, H.-R., Li, B., Zhang, Z.-H., Wu, H.-S., Wang, N., Chen, X.-F., et al. (2024). Exploring the efficacy and mechanism of Bailing capsule to improve polycystic ovary syndrome in mice based on intestinal-derived LPS-TLR4 pathway. J. Ethnopharmacol. 331:118274. doi: 10.1016/j.jep.2024.118274
Han, Q., Wang, J., Li, W., Chen, Z.-J., and Du, Y. (2021). Androgen-induced gut dysbiosis disrupts glucolipid metabolism and endocrinal functions in polycystic ovary syndrome. Microbiome 9:101. doi: 10.1186/s40168-021-01046-5
He, F.-F., and Li, Y.-M. (2020). Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: a review. J. Ovarian Res. 13:73. doi: 10.1186/s13048-020-00670-3
He, Y., Mei, L., Wang, L., Li, X., Zhao, J., Zhang, H., et al. (2022a). Lactiplantibacillus plantarum CCFM1019 attenuate polycystic ovary syndrome through butyrate dependent gut-brain mechanism. Food Funct. 13, 1380–1392. doi: 10.1039/D1FO01744F
He, Y., Shi, L., Qi, Y., Wang, Q., Zhao, J., Zhang, H., et al. (2022b). Butylated starch alleviates polycystic ovary syndrome by stimulating the secretion of peptide tyrosine-tyrosine and regulating faecal microbiota. Carbohydr. Polym. 287:119304. doi: 10.1016/j.carbpol.2022.119304
He, Y., Wang, Q., Li, X., Wang, G., Zhao, J., Zhang, H., et al. (2020). Lactic acid bacteria alleviate polycystic ovarian syndrome by regulating sex hormone related gut microbiota. Food Funct. 11, 5192–5204. doi: 10.1039/C9FO02554E
Heshmati, J., Moini, A., Sepidarkish, M., Morvaridzadeh, M., Salehi, M., Palmowski, A., et al. (2021). Effects of curcumin supplementation on blood glucose, insulin resistance and androgens in patients with polycystic ovary syndrome: a randomized double-blind placebo-controlled clinical trial. Phytomed. Int. J. Phytother. Phytopharm. 80:153395. doi: 10.1016/j.phymed.2020.153395
Hildebrandt, M. A., Hoffman, C., Sherrill-Mix, S. A., Keilbaugh, S. A., Hamady, M., Chen, Y.-Y., et al. (2009). High fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 137, 1716–24.e1–2. doi: 10.1053/j.gastro.2009.08.042
Homeira, H. M., Saghar, S., Zohreh, A., Sara, J. F., Barbara, J. M., and Farideh, T. (2012). Beneficial effects of a high-protein, low-glycemic-load hypocaloric diet in overweight and obese women with polycystic ovary syndrome: a randomized controlled intervention study. J. Am. Coll. Nutr. 31, 117–125. doi: 10.1080/07315724.2012.10720017
Jamilian, M., Foroozanfard, F., Kavossian, E., Aghadavod, E., Shafabakhsh, R., Hoseini, A., et al. (2020). Effects of curcumin on body weight, glycemic control and serum lipids in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin. Nutr. ESPEN 36, 128–133. doi: 10.1016/j.clnesp.2020.01.005
Jin, B., Zhang, Y., Zhang, Z., Yang, G., Pan, Y., Xie, L., et al. (2022). The efficacy and safety of dingkun pill in women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Evid.-Based Complement. Altern. Med. ECAM 2022:8698755. doi: 10.1155/2022/8698755
Ju, S., Kang, Z. Y., Yang, L. Y., Xia, Y. J., Guo, Y. M., Li, S., et al. (2025). Gut microbiota and ovarian diseases: a new therapeutic perspective. J. Ovarian Res. 18:105. doi: 10.1186/s13048-025-01684-5
Juhász, A. E., Stubnya, M. P., Teutsch, B., Gede, N., Hegyi, P., Nyirády, P., et al. (2024). Ranking the dietary interventions by their effectiveness in the management of polycystic ovary syndrome: a systematic review and network meta-analysis. Reprod. Health 21:28. doi: 10.1186/s12978-024-01758-5
Karamali, M., Eghbalpour, S., Rajabi, S., Jamilian, M., Bahmani, F., Tajabadi-Ebrahimi, M., et al. (2018). Effects of probiotic supplementation on hormonal profiles, biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Arch. Iran. Med.21, 1–7.
Kaur, I., Suri, V., Sachdeva, N., Rana, S. V., Medhi, B., Sahni, N., et al. (2022). Efficacy of multi-strain probiotic along with dietary and lifestyle modifications on polycystic ovary syndrome: a randomised, double-blind placebo-controlled study. Eur. J. Nutr. 61, 4145–4154. doi: 10.1007/s00394-022-02959-z
Kriebs, A. (2019). IL-22 links gut microbiota to PCOS. Nat. Rev. Endocrinol. 15:565. doi: 10.1038/s41574-019-0255-x
Kumari, N., Kumari, R., Dua, A., Singh, M., Kumar, R., Singh, P., et al. (2024). From gut to hormones: unraveling the role of gut microbiota in (Phyto)estrogen modulation in health and disease. Mol. Nutr. Food Res. 68:e2300688. doi: 10.1002/mnfr.202300688
Kwon, C.-Y., Cho, I.-H., and Park, K. S. (2020). Therapeutic effects and mechanisms of herbal medicines for treating polycystic ovary syndrome: a review. Front. Pharmacol. 11:1192. doi: 10.3389/fphar.2020.01192
Ley, R. E., Turnbaugh, P. J., Klein, S., and Gordon, J. I. (2006). Microbial ecology: human gut microbes associated with obesity. Nature 444, 1022–1023. doi: 10.1038/4441022a
Li, G., Liu, Z., Ren, F., Shi, H., Zhao, Q., Song, Y., et al. (2022). Alterations of gut microbiome and fecal fatty acids in patients with polycystic ovary syndrome in central China. Front. Microbiol. 13:911992. doi: 10.3389/fmicb.2022.911992
Li, J., Liu, D., Zhao, H., Zhang, P., Cai, F., Li, H., et al. (2024). Chinese medicine compound prescription HeQi San ameliorates chronic inflammatory states and modulates gut flora in dehydroepiandrosterone-induced polycystic ovary syndrome mouse model. Int. Immunopharmacol. 137:112491. doi: 10.1016/j.intimp.2024.112491
Li, J., Qiao, J., Li, Y., Qin, G., Xu, Y., Lao, K., et al. (2025a). Metabolic disorders in polycystic ovary syndrome: from gut microbiota biodiversity to clinical intervention. Front. Endocrinol. 16:1526468. doi: 10.3389/fendo.2025.1526468
Li, M., Zhang, L., Li, X., and Zhao, Y. (2025b). Impact of short-term ketogenic diet on sex hormones and glucose-lipid metabolism in overweight or obese patients with polycystic ovary syndrome. J. Obstet. Gynaecol. Res. 51:e16178. doi: 10.1111/jog.16178
Li, V. W., Dong, T. S., Funes, D., Hernandez, L., Kushnir, N. R., Nair, D., et al. (2025c). Mass spectrometric profiling of primary estrogens and estrogen metabolites in human stool and plasma partially elucidates the role of the gut microbiome in estrogen recycling. Mol. Cell. Endocrinol. 603:112534. doi: 10.1016/j.mce.2025.112534
Li, Y., Chen, C., Ma, Y., Xiao, J., Luo, G., Li, Y., et al. (2019). Multi-system reproductive metabolic disorder: significance for the pathogenesis and therapy of polycystic ovary syndrome (PCOS). Life Sci. 228, 167–175. doi: 10.1016/j.lfs.2019.04.046
Lin, W., Wen, L., Wen, J., and Xiang, G. (2021). Effects of sleeve gastrectomy on fecal gut microbiota and short-chain fatty acid content in a rat model of polycystic ovary syndrome. Front. Endocrinol. 12:747888. doi: 10.3389/fendo.2021.747888
Lindheim, L., Bashir, M., Münzker, J., Trummer, C., Zachhuber, V., Leber, B., et al. (2017). Alterations in gut microbiome composition and barrier function are associated with reproductive and metabolic defects in women with polycystic ovary syndrome (PCOS): a pilot study. PLoS ONE 12:e0168390. doi: 10.1371/journal.pone.0168390
Liow, Y. J., Kamimura, I., Umezaki, M., Suda, W., and Takayasu, L. (2024). Dietary fiber induces a fat preference associated with the gut microbiota. PLoS One 19:e0305849. doi: 10.1371/journal.pone.0305849
Liu, K., He, X., Huang, J., Yu, S., Cui, M., Gao, M., et al. (2023). Short-chain fatty acid-butyric acid ameliorates granulosa cells inflammation through regulating METTL3-mediated N6-methyladenosine modification of FOSL2 in polycystic ovarian syndrome. Clin. Epigenet. 15:86. doi: 10.1186/s13148-023-01487-9
Liu, M., Zhu, H., Zhu, Y., and Hu, X. (2021). Guizhi Fuling Wan reduces autophagy of granulosa cell in rats with polycystic ovary syndrome via restoring the PI3K/AKT/mTOR signaling pathway. J. Ethnopharmacol. 270:113821. doi: 10.1016/j.jep.2021.113821
Liu, Y., Jiang, J.-J., Du, S.-Y., Mu, L.-S., Fan, J.-J., Hu, J.-C., et al. (2024). Artemisinins ameliorate polycystic ovarian syndrome by mediating LONP1-CYP11A1 interaction. Science 384:eadk5382. doi: 10.1126/science.adk5382
Luo, P., Li, J., Li, P., Wang, G., Li, W., Song, Z., et al. (2022). A bibliometric and visual analysis of obesity and polycystic ovary syndrome from 2012 to 2022. Front. Endocrinol. 13:1011105. doi: 10.3389/fendo.2022.1011105
Magagnini, M. C., Condorelli, R. A., Cimino, L., Cannarella, R., Aversa, A., Calogero, A. E., et al. (2022). Does the ketogenic diet improve the quality of ovarian function in obese women? Nutrients 14:4147. doi: 10.3390/nu14194147
Martinez Guevara, D., Vidal Cañas, S., Palacios, I., Gómez, A., Estrada, M., Gallego, J., et al. (2024). Effectiveness of probiotics, prebiotics, and synbiotics in managing insulin resistance and hormonal imbalance in women with polycystic ovary syndrome (PCOS): a systematic review of randomized clinical trials. Nutrients 16:3916. doi: 10.3390/nu16223916
Mathewson, N. D., Jenq, R., Mathew, A. V., Koenigsknecht, M., Hanash, A., Toubai, T., et al. (2016). Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol. 17, 505–513. doi: 10.1038/ni.3400
Mishra, N., Verma, R., and Jadaun, P. (2022). Study on the effect of berberine, myoinositol, and metformin in women with polycystic ovary syndrome: a prospective randomised study. Cureus 14:e21781. doi: 10.7759/cureus.21781
Olaniyi, K. S., and Areloegbe, S. E. (2024). Acetate ameliorates ovarian mitochondrial dysfunction in letrozole-induced polycystic ovarian syndrome rat model by improving mitofusin-2. J. Physiol. Sci. JPS 74:22. doi: 10.1186/s12576-024-00908-5
Olaniyi, K. S., Areloegbe, S. E., and ul haq Shah, M. Z. (2025). Acetate abates adipose-ovarian endocrinometabolic disturbance in experimentally induced polycystic ovarian syndrome. Steroids 214:109554. doi: 10.1016/j.steroids.2024.109554
Palmas, V., Deledda, A., Heidrich, V., Sanna, G., Cambarau, G., Fosci, M., et al. (2025). Impact of ketogenic and mediterranean diets on gut microbiota profile and clinical outcomes in drug-naïve patients with diabesity: a 12-month pilot study. Metabolites 15:22. doi: 10.3390/metabo15010022
Paoli, A., Mancin, L., Giacona, M. C., Bianco, A., and Caprio, M. (2020). Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J. Transl. Med. 18:104. doi: 10.1186/s12967-020-02277-0
Qi, X., Yun, C., Liao, B., Qiao, J., and Pang, Y. (2020). The therapeutic effect of interleukin-22 in high androgen-induced polycystic ovary syndrome. J. Endocrinol. 245, 281–289. doi: 10.1530/JOE-19-0589
Qi, X., Yun, C., Sun, L., Xia, J., Wu, Q., Wang, Y., et al. (2019). Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat. Med. 25, 1225–1233. doi: 10.1038/s41591-019-0509-0
Rooks, M. G., and Garrett, W. S. (2016). Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 16, 341–352. doi: 10.1038/nri.2016.42
Rosato, V., Temple, N. J., La Vecchia, C., Castellan, G., Tavani, A., and Guercio, V. (2019). Mediterranean diet and cardiovascular disease: a systematic review and meta-analysis of observational studies. Eur. J. Nutr. 58, 173–191. doi: 10.1007/s00394-017-1582-0
Rosenfield, R. L. (2024). The search for the causes of common hyperandrogenism, 1965 to circa 2015. Endocr. Rev. 45, 553–592. doi: 10.1210/endrev/bnae007
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. (2004). Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. Oxf. Engl. 19, 41–47. doi: 10.1093/humrep/deh098
Sacks, F. M., Svetkey, L. P., Vollmer, W. M., Appel, L. J., Bray, G. A., Harsha, D., et al. (2001). Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N. Engl. J. Med. 344, 3–10. doi: 10.1056/NEJM200101043440101
Sebastián Domingo, J. J., and Sánchez Sánchez, C. (2018). From the intestinal flora to the microbiome. Rev. Esp. Enferm. Dig. 110, 51–56. doi: 10.17235/reed.2017.4947/2017
Shah, M. Z. U. H., Shrivastva, V. K., Mir, M. A., Sheikh, W. M., Ganie, M. A., Rather, G. A., et al. (2023). Effect of quercetin on steroidogenesis and folliculogenesis in ovary of mice with experimentally-induced polycystic ovarian syndrome. Front. Endocrinol. 14:1153289. doi: 10.3389/fendo.2023.1153289
Shamasbi, S. G., Ghanbari-Homayi, S., and Mirghafourvand, M. (2020). The effect of probiotics, prebiotics, and synbiotics on hormonal and inflammatory indices in women with polycystic ovary syndrome: a systematic review and meta-analysis. Eur. J. Nutr. 59, 433–450. doi: 10.1007/s00394-019-02033-1
Sharifi, M., Saber, A., Moludi, J., Salimi, Y., and Jahan-Mihan, A. (2024). The effects of portfolio moderate-carbohydrate and ketogenic diets on anthropometric indices, metabolic status, and hormonal levels in overweight or obese women with polycystic ovary syndrome: a randomized controlled trial. Nutr. J. 23:152. doi: 10.1186/s12937-024-01056-7
Shen, C., Li, H., Xiao, M., Jiang, X., Jin, J., Zhou, J., et al. (2025). Study on the mechanism of the Chinese herbal pair Banxia-Chenpi in ameliorating polycystic ovary syndrome based on the CYP17A1 gene. J. Ethnopharmacol. 344:119503. doi: 10.1016/j.jep.2025.119503
Soltani, S., Shirani, F., Chitsazi, M. J., and Salehi-Abargouei, A. (2016). The effect of dietary approaches to stop hypertension (DASH) diet on weight and body composition in adults: a systematic review and meta-analysis of randomized controlled clinical trials. Obes. Rev. 17, 442–454. doi: 10.1111/obr.12391
Su, P., Chen, C., Pang, L., Wu, K., and Sun, Y. (2024). Effects of quercetin on polycystic ovary syndrome in animal models: a systematic review and meta-analysis. Reprod. Biol. Endocrinol. RBE 22:46. doi: 10.1186/s12958-024-01220-y
Su, Y.-N., Wang, M.-J., Yang, J.-P., Wu, X.-L., Xia, M., Bao, M.-H., et al. (2023). Effects of Yulin Tong Bu formula on modulating gut microbiota and fecal metabolite interactions in mice with polycystic ovary syndrome. Front. Endocrinol. 14:1122709. doi: 10.3389/fendo.2023.1122709
Tabrizi, R., Ostadmohammadi, V., Akbari, M., Lankarani, K. B., Vakili, S., Peymani, P., et al. (2022). The effects of probiotic supplementation on clinical symptom, weight loss, glycemic control, lipid and hormonal profiles, biomarkers of inflammation, and oxidative stress in women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Probiotics Antimicrob. Proteins 14, 1–14. doi: 10.1007/s12602-019-09559-0
Tan, F., Zheng, Y., Wang, C., Huang, J., Liu, X., Su, W., et al. (2025). Effects of Chenpi Jiaosu on serum metabolites and intestinal microflora in a dyslipidemia population: a randomized controlled pilot trial. Front. Endocrinol. 16:1552117. doi: 10.3389/fendo.2025.1552117
Tian, J., Xu, Y., Xiong, Y., Zuo, L., Zhou, M., Cao, C., et al. (2022). Metabolomics combined with network pharmacology to explore the mechanisms of modified Guishen pill to ameliorate polycystic ovary syndrome. Comput. Biol. Med. 148:105790. doi: 10.1016/j.compbiomed.2022.105790
Torres, P. J., Siakowska, M., Banaszewska, B., Pawelczyk, L., Duleba, A. J., Kelley, S. T., et al. (2018). Gut microbial diversity in women with polycystic ovary syndrome correlates with hyperandrogenism. J. Clin. Endocrinol. Metab. 103, 1502–1511. doi: 10.1210/jc.2017-02153
Tysoe, O. (2024). Artemisinins as a promising treatment for polycystic ovary syndrome. Nat. Rev. Endocrinol. 20, 508–508. doi: 10.1038/s41574-024-01019-2
Wang, F., Dou, P., Wei, W., and Liu, P. J. (2024). Effects of high-protein diets on the cardiometabolic factors and reproductive hormones of women with polycystic ovary syndrome: a systematic review and meta-analysis. Nutr. Diabetes 14:6. doi: 10.1038/s41387-024-00263-9
Wang, F., Wei, W., and Liu, P. J. (2025). Evaluation of effects of a high-protein hypocaloric diet on body composition and cardio-metabolic factors in women with polycystic ovary syndrome and overweight or obesity. Diabetes Metab. Syndr. Obes. 18, 931–939. doi: 10.2147/DMSO.S501972
Wang, R., Zhao, Y., Fang, X., Miao, C., Ren, N., Chen, Y., et al. (2023). Effect of the ketogenic diet on gut microbiome composition and metabolomics in polycystic ovarian syndrome rats induced by letrozole and a high-fat diet. Nutr. Burbank Los Angel. Cty. Calif 114:112127. doi: 10.1016/j.nut.2023.112127
Wang, X., Gu, L., Zhang, Y., Xiong, C., Peng, Y., and Ding, X. (2022). Effects of dehydroepiandrosterone alone or in combination with a high-fat diet and antibiotic cocktail on the heterogeneous phenotypes of PCOS mouse models by regulating gut microbiota. Front. Endocrinol. 13:1030151. doi: 10.3389/fendo.2022.1030151
Watanabe, M., Risi, R., Camajani, E., Contini, S., Persichetti, A., Tuccinardi, D., et al. (2020). Baseline HOMA IR and circulating FGF21 levels predict NAFLD improvement in patients undergoing a low carbohydrate dietary intervention for weight loss: a prospective observational pilot study. Nutrients 12:2141. doi: 10.3390/nu12072141
Wei, W., Zhao, H., Wang, A., Sui, M., Liang, K., Deng, H., et al. (2012). A clinical study on the short-term effect of berberine in comparison to metformin on the metabolic characteristics of women with polycystic ovary syndrome. Eur. J. Endocrinol. 166, 99–105. doi: 10.1530/EJE-11-0616
Wu, L., Zhang, H., Fan, M., and Yan, Y. (2022a). Efficacy and safety of cangfu daotan decoction in patients with polycystic ovary syndrome: a systematic review and meta-analysis. Evid.-Based Complement. Altern. Med. ECAM 2022:4395612. doi: 10.1155/2022/4395612
Wu, Y., Peng, X., Li, X., Li, D., Tan, Z., and Yu, R. (2022b). Sex hormones influence the intestinal microbiota composition in mice. Front. Microbiol. 13:964847. doi: 10.3389/fmicb.2022.964847
Yang, Y.-L., Zhou, W.-W. S, Tang, W.-L., Wang, Z.-W., Zhou, Z.-Y., et al. (2021). Intestinal flora is a key factor in insulin resistance and contributes to the development of polycystic ovary syndrome. Endocrinology 162:bqab118. doi: 10.1210/endocr/bqab118
Yu, J., Zhang, Y., Zhu, Y., Li, Y., Lin, S., Liu, W., et al. (2023). Circulating bile acid profile characteristics in PCOS patients and the role of bile acids in predicting the pathogenesis of PCOS. Front. Endocrinol. 14:1239276. doi: 10.3389/fendo.2023.1239276
Yun, C., Yan, S., Liao, B., Ding, Y., Qi, X., Zhao, M., et al. (2024). The microbial metabolite agmatine acts as an FXR agonist to promote polycystic ovary syndrome in female mice. Nat. Metab. 6, 947–962. doi: 10.1038/s42255-024-01041-8
Zeng, B., Lai, Z., Sun, L., Zhang, Z., Yang, J., Li, Z., et al. (2019). Structural and functional profiles of the gut microbial community in polycystic ovary syndrome with insulin resistance (IR-PCOS): a pilot study. Res. Microbiol. 170, 43–52. doi: 10.1016/j.resmic.2018.09.002
Zhang, B., Shen, S., Gu, T., Hong, T., Liu, J., Sun, J., et al. (2019a). Increased circulating conjugated primary bile acids are associated with hyperandrogenism in women with polycystic ovary syndrome. J. Steroid Biochem. Mol. Biol. 189, 171–175. doi: 10.1016/j.jsbmb.2019.03.005
Zhang, C., Zhang, M., Pang, X., Zhao, Y., Wang, L., and Zhao, L. (2012). Structural resilience of the gut microbiota in adult mice under high-fat dietary perturbations. ISME J. 6, 1848–1857. doi: 10.1038/ismej.2012.27
Zhang, J., Huang, H., Xiao, M., Jiang, X., Yang, Y., Huang, M., et al. (2025). Erchen Decoction ameliorates the rat model of polycystic ovary syndrome by regulating the steroid biosynthesis pathway. Phytomedicine Int. J. Phytother. Phytopharm. 143:156852. doi: 10.1016/j.phymed.2025.156852
Zhang, J., Sun, Z., Jiang, S., Bai, X., Ma, C., Peng, Q., et al. (2019b). Probiotic Bifidobacterium lactis V9 regulates the secretion of sex hormones in polycystic ovary syndrome patients through the gut-brain axis. mSystems 4, e00017–19. doi: 10.1128/mSystems.00017-19
Zhang, J., Zhu, Y., Wang, J., Hu, H., Jin, Y., Mao, X., et al. (2024). Global burden and epidemiological prediction of polycystic ovary syndrome from 1990 to 2019: a systematic analysis from the Global Burden of Disease Study 2019. PLoS One 19:e0306991. doi: 10.1371/journal.pone.0306991
Zheng, Y., Yu, J., Liang, C., Li, S., Wen, X., and Li, Y. (2021a). Characterization on gut microbiome of PCOS rats and its further design by shifts in high-fat diet and dihydrotestosterone induction in PCOS rats. Bioprocess Biosyst. Eng. 44, 953–964. doi: 10.1007/s00449-020-02320-w
Zheng, Y.-H., Xu, Y., Ma, H.-X., Liang, C.-J., and Yang, T. (2021b). Effect of high-fat diet on the intestinal flora in letrozole-induced polycystic ovary syndrome rats. Evid.-Based Complement. Altern. Med. ECAM 2021:6674965. doi: 10.1155/2021/6674965
Zhou, L., Ni, Z., Cheng, W., Yu, J., Sun, S., Zhai, D., et al. (2020). Characteristic gut microbiota and predicted metabolic functions in women with PCOS. Endocr. Connect. 9, 63–73. doi: 10.1530/EC-19-0522
Zhu, Y., Li, Y., Liu, M., Hu, X., and Zhu, H. (2020). Guizhi Fuling Wan, Chinese herbal medicine, ameliorates insulin sensitivity in pcos model rats with insulin resistance via remodeling intestinal homeostasis. Front. Endocrinol. 11:575. doi: 10.3389/fendo.2020.00575
Keywords: polycystic ovary syndrome, gut microbiota, high-fat diets, ketogenic diets, traditional Chinese medicine
Citation: Zhu S, Chen H, He B, Zhang Y, Li P and Kuang J (2025) Gut microbiota dysbiosis in polycystic ovary syndrome: focus on diet, probiotics, and traditional Chinese medicine. Front. Microbiol. 16:1659783. doi: 10.3389/fmicb.2025.1659783
Received: 07 July 2025; Revised: 03 November 2025; Accepted: 10 November 2025;
Published: 25 November 2025.
Edited by:
Diogo Alpuim Costa, Hospital de Cascais Dr. José de Almeida, PortugalReviewed by:
Valentyn Oksenych, University of Bergen, NorwayLei Han, Binzhou Medical University Hospital, China
Copyright © 2025 Zhu, Chen, He, Zhang, Li and Kuang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jilin Kuang, a3VhbmdqbGFiY0BzaW5hLmNvbQ==
 Shuangquan Zhu
Shuangquan Zhu Hao Chen3
Hao Chen3 Jilin Kuang
Jilin Kuang