- 1Institute for Bioscience and Biotechnology Research, University of Maryland, Rockville, MD, United States
- 2Department of Cell Biology and Molecular Genetics, University of Maryland, College Park, MD, United States
- 3Department of Veterinary Medicine, University of Maryland, College Park, MD, United States
Streptococcus pneumoniae is responsible for causing a range of diseases, from self-limiting otitis media to more severe disease such as pneumonia, sepsis, and meningitis. Vaccines and antibiotics have successfully decreased this disease burden; however, serotype replacement due to vaccines and antibiotic resistance remain issues pointing to the need for further research into alternative methods of treatment. S. pneumoniae continues to pose a global threat to young children and the elderly. Endolysins, which function as peptidoglycan hydrolases, are an attractive alternative treatment given their rapid bactericidal activity, specificity, and lack of noted resistance. This review considers the uses of endolysins within the main niches of infection for S. pneumoniae: pulmonary, middle ear, and the bloodstream and central nervous system. Therapeutic hurdles, such as delivery and mechanical barrier challenges, are discussed and endolysin-focused solutions to circumvent these challenges are proposed. The ability to address niche-specific hurdles using endolysins will allow for an increase in effective therapies against S. pneumoniae.
1 Introduction
Streptococcus pneumoniae is a Gram-positive bacterium known for colonizing and infecting the mucosal membranes of the upper respiratory tract, particularly in elderly individuals and children under 5 years of age. Although both adults and children are often asymptomatic carriers, an increased bacterial load can lead to diseases such as pneumonia, otitis media, sepsis, and meningitis (Austrian, 1981; Shenoy and Orihuela, 2016; Weiser et al., 2018) (Figure 1). The Active Bacterial Core Surveillance system reported 3,297 cases of S. pneumoniae infection and 361 deaths per 100,000 people in the United States in 2018 (CDC, 2018). Additionally, the Centers for Disease Control and Prevention has identified S. pneumoniae as a top-priority threat pathogen due to its high disease burden, antibiotic resistance, serotype replacement, vaccine escape, transmission, and asymptomatic carriage (CDC, 2019).
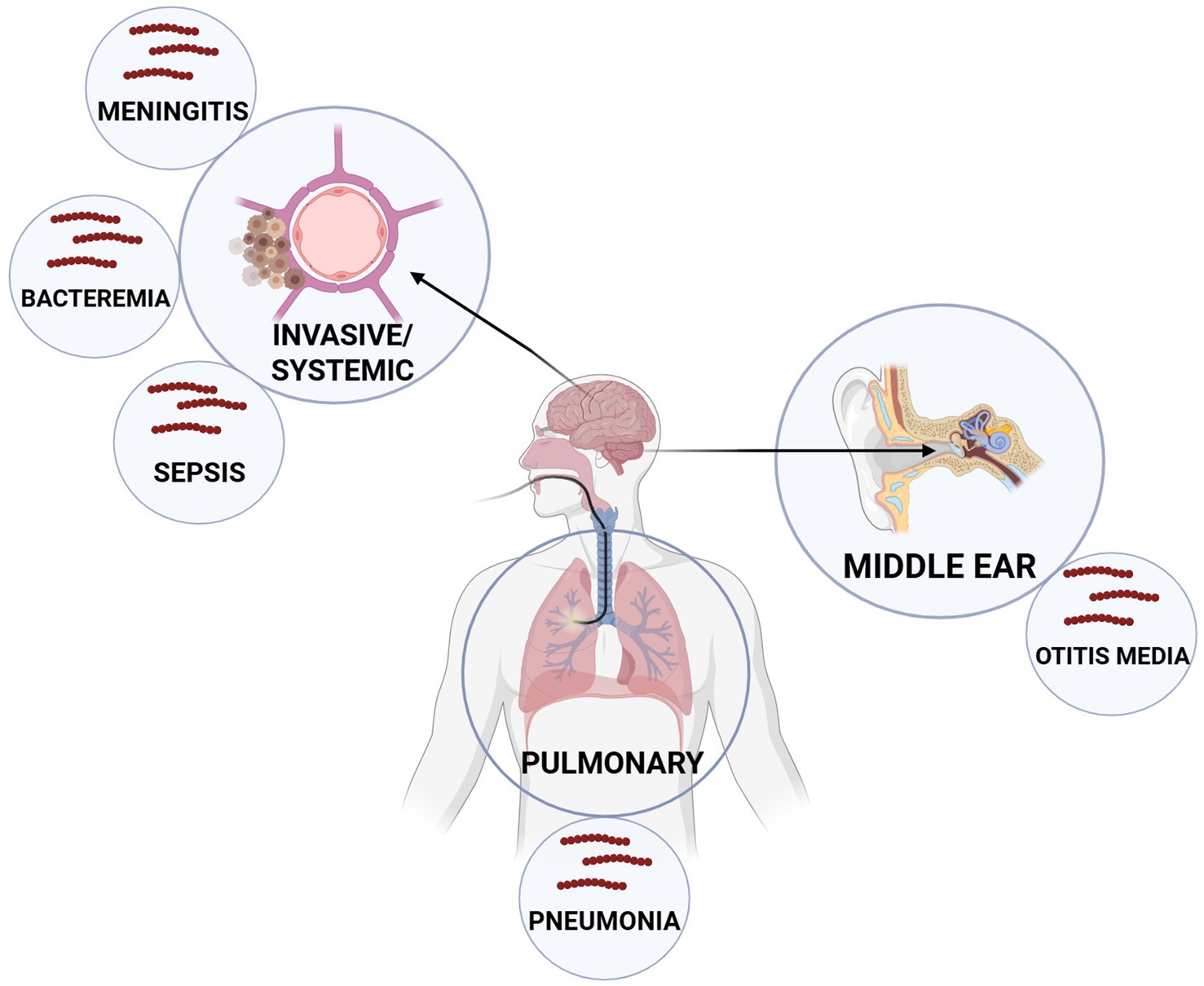
Figure 1. Major anatomical niches and associated diseases caused by Streptococcus pneumoniae. S. pneumoniae colonizes the nasopharynx, where it migrates, primarily infecting the pulmonary system (pneumonia) or the middle ear (otitis media). However, it can also disseminate systemically to cause invasive diseases such as sepsis, bacteremia, and meningitis. These distinct infection sites represent key targets for therapeutic intervention and highlight the diverse clinical manifestations of pneumococcal disease.
The disease mechanism requires shedding of the bacterium from mucosal membranes followed by either direct contact or airborne transmission to an uninfected person. This is facilitated by the ability of S. pneumoniae to release of pneumolysin, a pore-forming toxin, which causes host cell destruction, epithelial and endothelial barrier disruption, immune response modulation, and inflammation (Weiser et al., 2018). Viral co-infections, like influenza A, enhance the distribution of S. pneumoniae by increasing inflammation and triggering mucosal shedding. To protect itself from the immune system, S. pneumoniae uses multiple evasion strategies such as production of a polysaccharide capsule that inhibits phagocytosis, display of surface proteins that block complement activation, and the aforementioned pneumolysin-mediated cytotoxicity. S. pneumoniae also develops biofilms on mucosal surfaces that allow it to persist on biotic surfaces and leads to repeated chronic infections. The treatment of pneumococcal diseases relies on broad-spectrum antibiotics and preventative vaccines, yet their long-term effectiveness and sustainability remain limited (Kato, 2024).
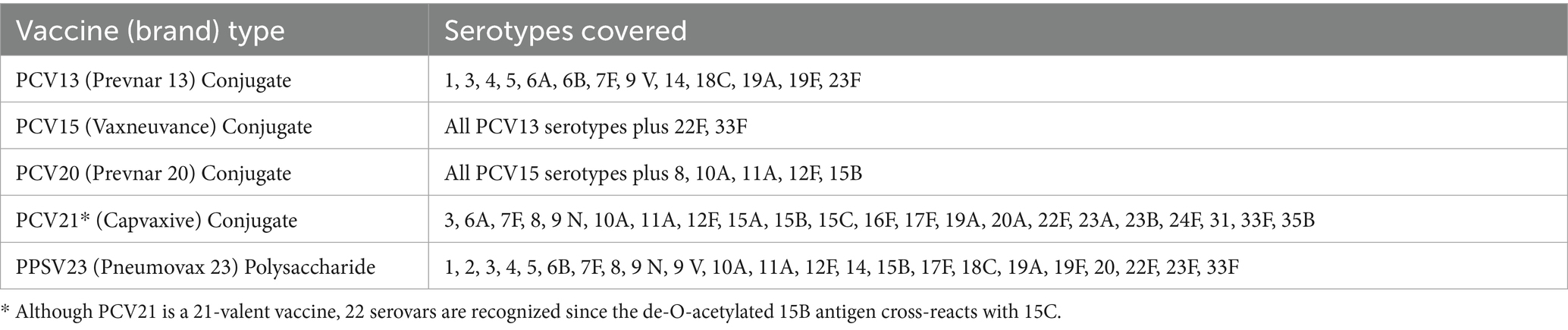
Vaccines are primarily designed to target the polysaccharide capsule of S. pneumoniae, but only a limited number of capsule serotypes are represented across the two main types of vaccines: polysaccharide vaccines and polysaccharide-conjugate vaccines (PCVs) (Table 1) (Owusu-Edusei et al., 2022). PCVs combine polysaccharide antigens with diphtheria-derived cross-reactive protein domains and an aluminum phosphate adjuvant to create conjugates that boost immunogenicity while generating stronger immune responses (Davies et al., 2022). The introduction of PCVs resulted in a 51% reduction in child mortality between 2000 and 2015 (Wahl et al., 2018). Nonetheless, vaccine efficacy is limited to only the serotypes included in the formulation: Polysaccharide vaccines include up to 24 serotypes, whereas currently approved conjugate vaccines protect against 13, 15, 20, or 21 serotypes depending on the vaccine, although more than 100 capsular serotypes have been identified for S. pneumoniae (CDC, 2010; Haber et al., 2016; Keller et al., 2016). The restricted coverage of serotypes by current vaccines can result in serotype replacement, whereby S. pneumoniae with non-vaccine capsule serotypes can become the main source of infection (Lo et al., 2019). Toward this end, Capvaxie (PCV21), which as approved by the FDA in July, 2024, excludes 11 serotypes that are present in prior PCV vaccines and includes 8 new serotypes not found in any previous PCV vaccines (Kobayashi et al., 2024). Furthermore, vaccine effectiveness may be reduced in carrier populations due to age-associated immune function. Children have the highest rates of S. pneumoniae carriage, ranging from 27 to 65%, whereas adults exhibit carriage rates of less than 10% (Westerink et al., 2012; Castelo-Branco and Soveral, 2014; Albright et al., 2016). Additionally, elderly individuals who received PCV vaccines targeting serotypes 14 and 23F exhibited no increases in IgM levels and had lower opsonophagocytic activity compared to younger adults (Leggat et al., 2013). Likewise, older individuals had lower IgG antibody titers against common S. pneumoniae serotypes (Simell et al., 2008). These findings suggest that the most frequent carriers of S. pneumoniae, i.e., children and elderly individuals, may remain susceptible to infection despite vaccination efforts. Compounding this issue, vaccine uptake remains suboptimal: in 2018, only 23% of adults aged 19–64 and 69% of adults over 65 years of age in the United States were vaccinated (Owusu-Edusei et al., 2022).
The ongoing challenge of suboptimal vaccine protection combined with rising antibiotic resistance creates a parallel threat. Serotype replacement has contributed to a steady rise in antibiotic resistance among replacement serovars (Lo et al., 2019). S. pneumoniae has demonstrated resistance to three major classes of antibiotics: Resistance to β-lactams occurs through modifications of penicillin-binding proteins; resistance to macrolides is due to ermB, which alters the ribosomal subunit structure; and fluoroquinolones encounter resistance through mutations in the quinolone resistance-determining regions of DNA gyrase and topoisomerase IV genes (Cilloniz et al., 2018). While vaccines have reduced the overall mortality associated with S. pneumoniae infection, the emergence of serotype replacement and antibiotic resistance reveal a need for further development of alternative treatment strategies.
1.1 Endolysin as an advantageous treatment for S. pneumoniae
Bacteriophages (or phages) are viruses that selectively infect and lyse bacteria and have re-emerged as promising alternatives to antibiotics. While phages offer specificity and the potential for self-amplification within bacterial hosts, their therapeutic use remains constrained by several limitations. These include rapid clearance by the host immune system, potential emergence of bacterial resistance via receptor modification or Clustered Regularly Interspaced Short Palindromic Repeats–CRISPR-associated systems, and the lack of standardized protocols for clinical-grade manufacturing and regulatory approval, all of which complicate clinical adoption (Skurnik et al., 2025).
To address these issues, attention has increasingly turned to a class of phage-derived enzymes, particularly a group of peptidoglycan hydrolases known as endolysins. These enzymes are produced during the lytic phase of the phage life cycle and act from the inside-out to release progeny phage, accessing the peptidoglycan only after phage produced holins permeabilize the bacterial membrane (Oliveira et al., 2018). However, when applied externally to Gram-positive bacteria as purified proteins, endolysins act from the outside-in, a phenomenon known as “lysis from without.” In this case, the peptidoglycan substrate is identical, but the enzyme has direct access. Once the peptidoglycan is sufficiently degraded, the underlying membrane ruptures from osmotic lysis, resulting in bacterial cell death. This outside-in activity allows endolysins to be harnessed for therapeutic use, all while retaining specificity for the peptidoglycan of the original phage host species (Rahman et al., 2021).
Endolysins derived from phage that infect Gram-positive hosts have evolved a modular structure. Typically, they are composed of an N-terminal enzymatically active domain (EAD) and a C-terminal cell wall binding domain (CBD) (Fischetti, 2010; Roach and Donovan, 2015; Ajuebor et al., 2016; Rahman et al., 2021). The modular architecture of pneumococcal endolysins and their mechanisms of lysis are illustrated in Figure 2. Endolysin EADs can be classified into five enzymatic categories based on the specific bonds they cleave within the bacterial peptidoglycan: acetylmuramidases, transglycosylases, glucosaminidases, amidases, and endopeptidases (Abdelrahman et al., 2021). In contrast, the CBD confers specificity by recognizing conserved epitopes, often carbohydrates or teichoic acids, on the bacterial cell wall that facilitate targeted binding. Depending on the epitope, the recognition of the CBD can be to the genus, species, sub-species, or even strain level. In the case of pneumococcal-specific endolysins, the CBDs typically recognize phosphorylcholine in the wall teichoic acids, a signature unique to S. pneumoniae. Upon binding to the bacterial surface, the EAD cleaves its designated bond, resulting in eventual osmotic cell lysis when enough bonds have been cleaved to destabilize the peptidoglycan superstructure.
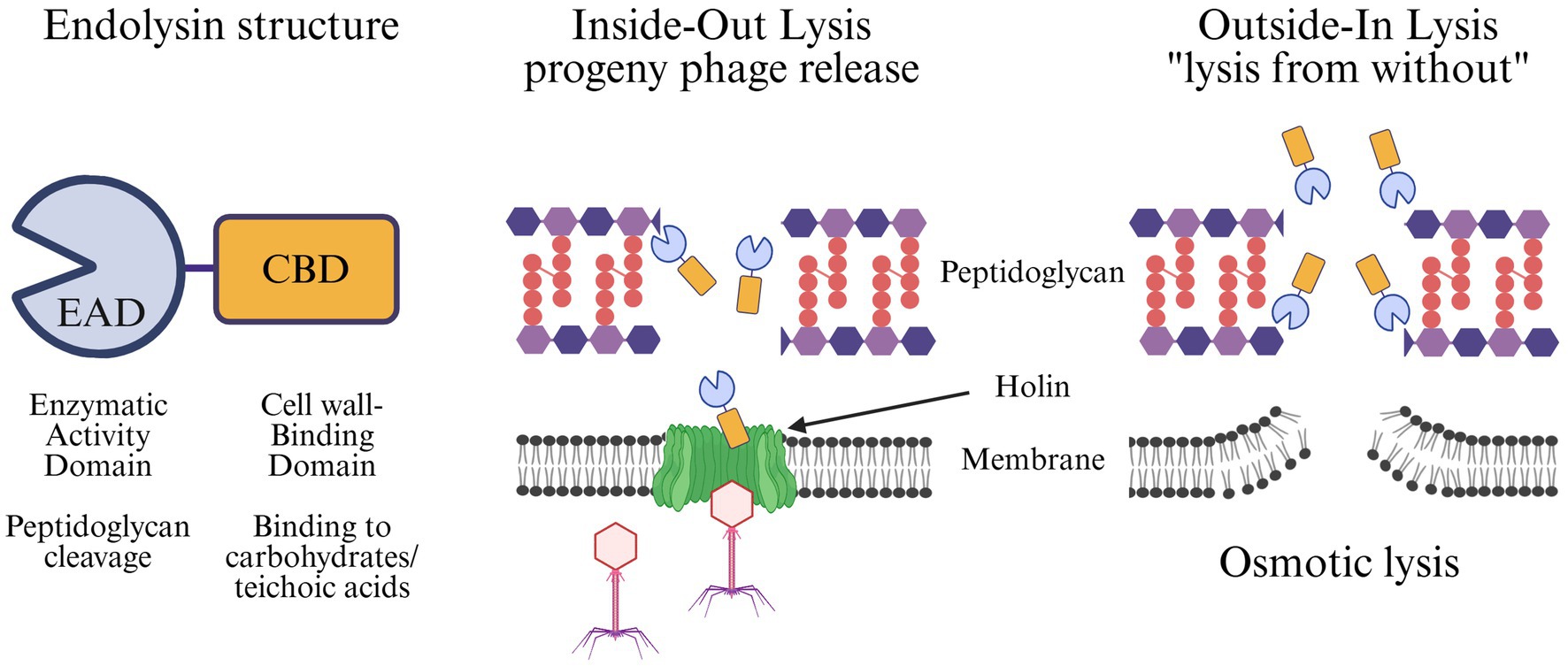
Figure 2. Structure and mechanisms of endolysin-mediated bacterial lysis. Endolysins are modular proteins composed of an N-terminal Enzymatic Activity Domain (EAD), which cleaves specific bonds in the peptidoglycan, and a C-terminal Cell wall-Binding Domain (CBD), which mediates recognition of cell wall carbohydrates or teichoic acids (left). Endolysins can act via two distinct modes: (middle) Inside-out lysis during the phage lytic cycle, where holins form pores in the bacterial membrane, allowing endolysins to degrade the peptidoglycan and release progeny phage, and (right) Outside-in lysis, also knowns as “lysis from without,” where externally applied endolysins directly access and cleave peptidoglycan, weakening the cell wall and leading to osmotic lysis of the bacterium.
Extensive in vitro and in vivo studies have shown that endolysins maintain potent lytic activity when applied to susceptible bacteria (Schmelcher and Loessner, 2021). Most notably, endolysins are unaffected by many resistance mechanisms common to traditional antibiotics, including efflux pumps, altered penicillin-binding proteins, and modifications of metabolic pathways (Nelson et al., 2012). Their high specificity, rapid bactericidal activity, and low rates of resistance development make endolysins attractive candidates for therapeutic use.
1.2 Known S. pneumoniae endolysins
The endolysins Cpl-1, Cpl-7, and Cpl-9 were among the first enzymes studied to exhibit the modular EAD/CBD architecture (Garcia et al., 1990). More recently, in vivo efficacy studies have shown effectiveness for several S. pneumoniae endolysins (Table 2). Pal, an endolysin derived from the Dp-1 phage, was the first to be evaluated for in vivo efficacy, demonstrating a protective effect in a mouse nasopharyngeal colonization model (Loeffler et al., 2001). However, Cpl-1 is the most extensively studied S. pneumoniae endolysin, exhibiting efficacy across multiple in vivo models, including intravenous bacteremia (Loeffler et al., 2003), mouse otitis media (McCullers et al., 2007), severe pneumonia (Witzenrath et al., 2009; Doehn et al., 2013), rat endocarditis (Entenza et al., 2005), and pneumococcal meningitis when administered intracisternally (Grandgirard et al., 2008). Recently, Alreja et al. (2024) identified a S. pneumoniae endolysin, SP-CHAP, which contains a cysteine, histidine-dependent amidohydrolase/peptidase (CHAP) domain within its EAD. SP-CHAP demonstrated superior activity compared to Cpl-1 across all major S. pneumoniae serotypes tested and was effective in eradicating pneumococcal biofilms at concentrations as low as 1.56 μg/mL.
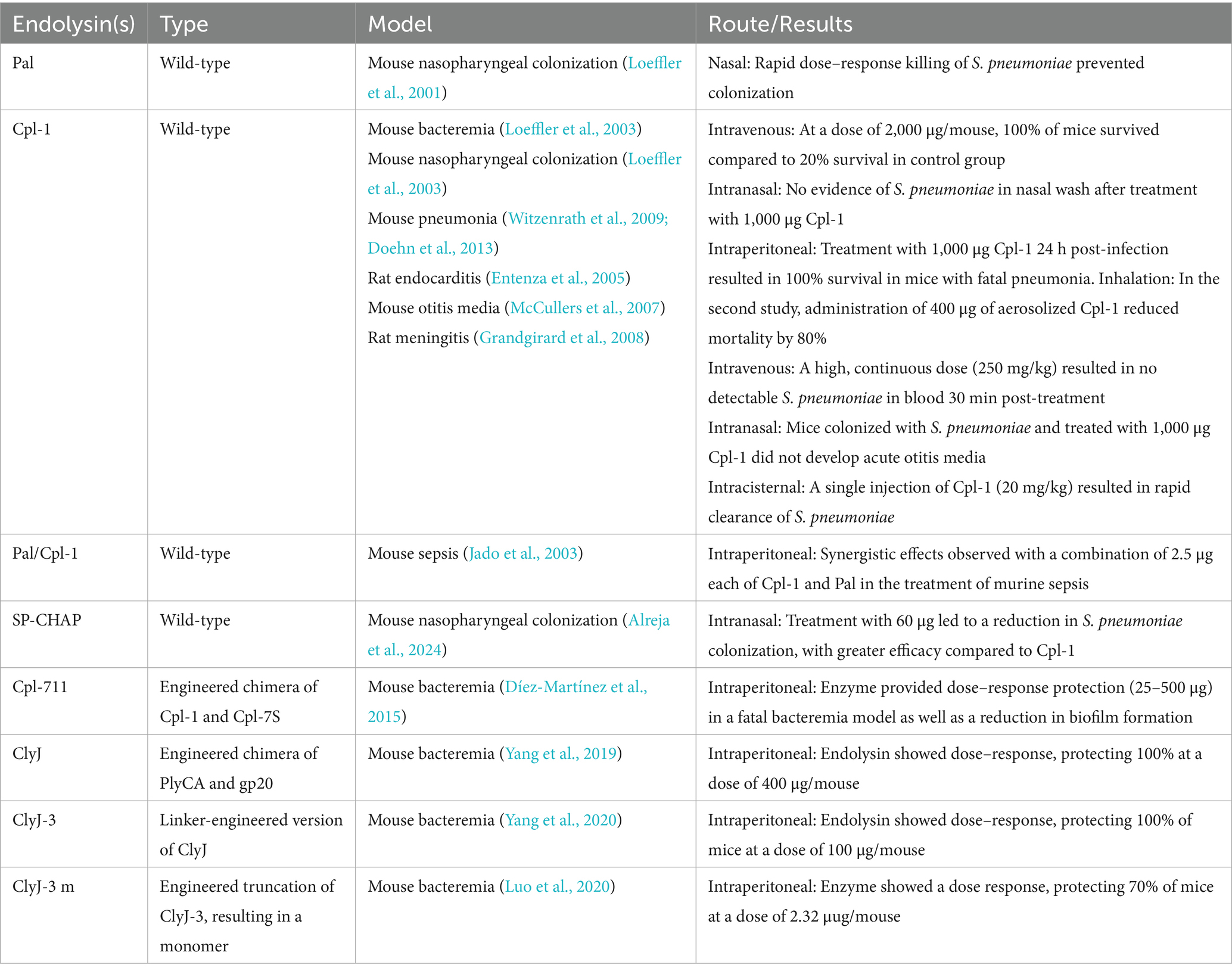
Table 2. Endolysins bacteriolytic against Streptococcus pneumoniae and their respective animal models.
Several other S. pneumoniae endolysins have been developed through chimeragenesis and protein engineering. One early example involved the construction of chimeric proteins by switching the EAD and CBD domains of Cpl-1 and LytA, and autolysin, which preserved enzymatic activity and choline-binding affinity common to S. pneumoniae endolysin CBDs (Diaz et al., 1990). Additional engineered variants include Cpl-7S, derived from Cpl-7 by inverting the charge of 15 amino acids within its CBD (Diez-Martinez et al., 2013). A further fusion of domains derived from Cpl-7S and Cpl-1 resulted in creation of Cpl-711, which demonstrated enhanced bactericidal activity both in vitro and in vivo when compared to the parental enzymes (Díez-Martínez et al., 2015). Similar, iterative rounds of engineering were also used to create ClyJ (Yang et al., 2019), its variant, ClyJ-3, featuring a shortened linker region between EAD and CBD (Yang et al., 2020), and its monomeric mutant, ClyJ-3 m (Luo et al., 2020). Each variant displayed greater antimicrobial activity than its predecessor.
The strategic combination of endolysins with EADs that target different bonds in the peptidoglycan matrix has also been shown to produce synergistic effects. These combinations can enhance antibacterial efficacy, reduce the required therapeutic dose, and mitigate the risk of resistance. For example, a synergistic interaction between Pal and Cpl-1 has been observed in vivo (Jado et al., 2003). Pal, with an EAD characterized as an N-acetylmuramoyl-L-alanine amidase, cleaves between the glycan strand and the stem peptide of the peptidoglycan, whereas Cpl-1 functions as an N-acetylmuramidase, cleaving directly within the glycan backbone. When administered at 2.5 μg each, the Pal/Cpl-1 combination conferred superior protection in mice challenged intraperitoneally with a lethal dose of S. pneumoniae compared to a 200 μg dose of either enzyme alone (Jado et al., 2003).
1.3 Site-targeted treatment for pneumococcal disease
The advantages of endolysins over conventional vaccines and antibiotics, and the availability to engineer and purify S. pneumoniae endolysins, make these agents promising candidates for development of new therapeutics. To date, however, only endolysins targeting Staphylococcus aureus and Clostridioides difficile have advanced to human clinical trials. These include exebacase (also known as CF-301), LSVT-1701 (formerly SAL200), and XZ.700 (Jun et al., 2017; Fowler et al., 2020; Kuiper et al., 2021), all of which target S. aureus, and LMN-201, which targets C. difficile. Notably, LSVT-1701 was granted fast-track designation by the U.S. Food and Drug Administration based on its efficacy against over 400 clinical isolates of S. aureus (Wire et al., 2022). As preclinical research continues to expand on endolysins, those targeting a wider range of pathogens, such as S. pneumoniae, are likely to progress toward clinical development.
Defining the anatomical site of infection to be targeted is essential for advancing pneumococcal endolysins into clinical use. Figure 3 provides an overview of therapeutic hurdles and potential solutions across all major niches. These will be described in greater detail in the following sections. All pneumococcal diseases begin with asymptomatic colonization of the upper respiratory tract mucosa, which is a necessary precursor for transmission to other tissues and anatomical sites (Bogaert et al., 2004). The principal anatomical niches affected by S. pneumoniae are: (1) the respiratory tract, where it causes pneumonia and other pulmonary diseases; (2) the middle ear, which results in otitis media; and (3) the bloodstream and central nervous system, which leads to bacteremia, sepsis, and meningitis after crossing the blood–brain barrier. Therapeutic intervention becomes complicated because each anatomical niche has its own distinct physiological and immunological barriers. This review focuses on identifying the unique treatment challenges of S. pneumoniae infection within different bodily niches and emphasizes ongoing research and potential future strategies to enhance endolysin effectiveness against pneumococcal diseases.
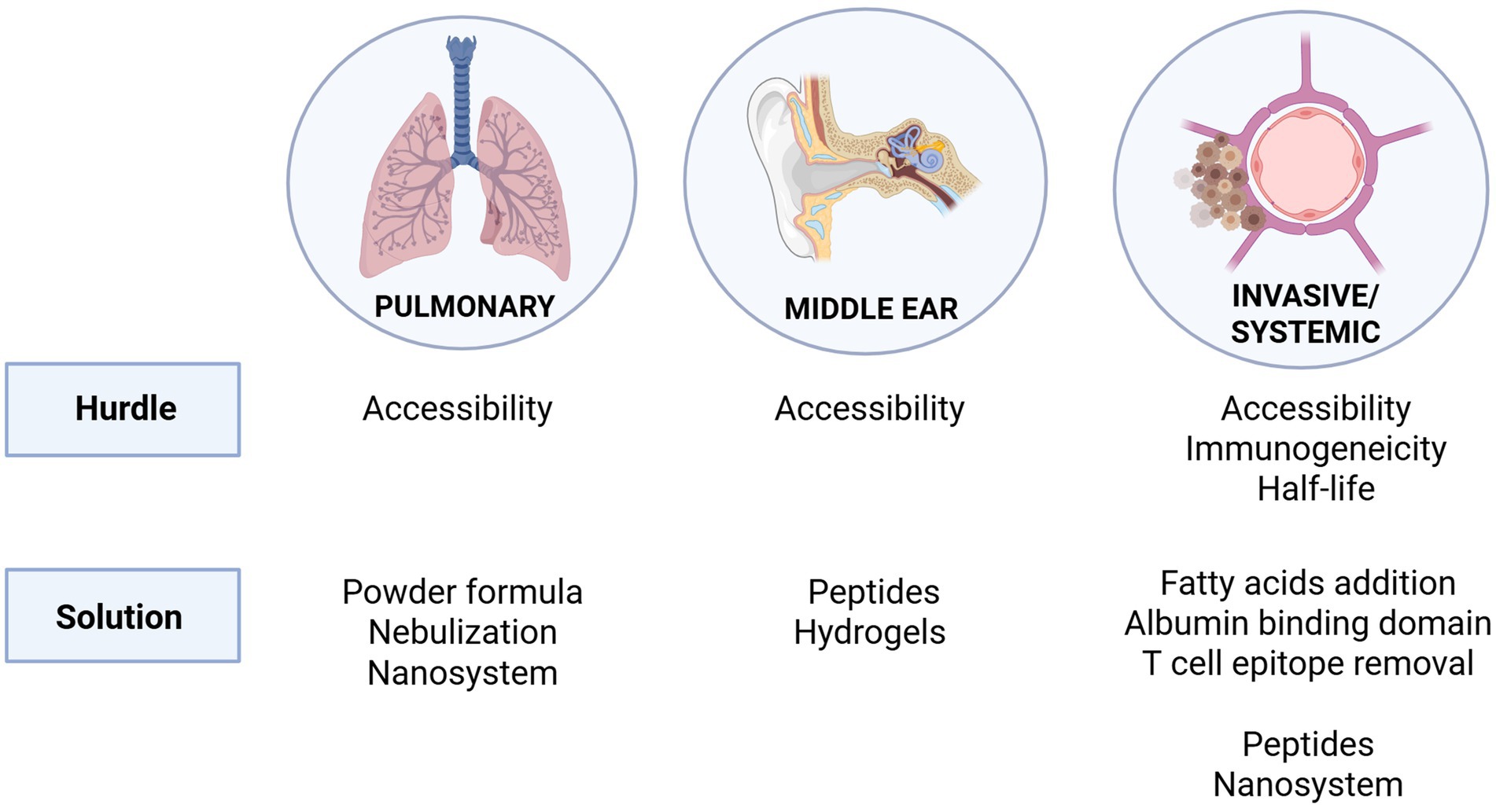
Figure 3. Therapeutic hurdles and potential solutions for targeting S. pneumoniae in its major niches of infection. The pulmonary and middle ear environments pose accessibility challenges for endolysin delivery, addressed through strategies such as powder formulations, nebulization, nanosystems, peptides, and hydrogels. In invasive/systemic infections, additional hurdles include immunogenicity and limited half-life. Proposed solutions include fatty acid conjugation, incorporation of albumin-binding domains, removal of T cell epitopes, as well as peptide-based and nanosystem delivery approaches.
2 Anatomical niche: pulmonary infection hurdles and solutions
Respiratory tract infections remain one of the most common causes of death in third world countries, with symptoms including cough, fever, shortness of breath, pain when breathing, and nausea (Ashrafi-Asgarabad et al., 2023). Upon colonization of the nasopharynx, changes in the host immune response or co-infection with a viral agent, such as influenza or respiratory syncytial virus, can enhance S. pneumoniae migration to the lungs. The expression of phosphorylcholine on the S. pneumoniae surface facilitates bacterial adherence to platelet-activating factor, while choline-binding protein A binds to human secretory components (e.g., epithelial glycoprotein, polymeric immunoglobulin receptor, etc.) on the host, contributing to the adherence and colonization of S. pneumoniae (Kadioglu et al., 2008). Once in the lungs, S. pneumoniae adheres to the epithelial cells through surface proteins, activating inflammatory factors. These, in turn, upregulate host receptors that enable internalization of S. pneumoniae in the epithelial cells leading to invasion and pneumonia disease (Bogaert et al., 2004). Community-acquired pneumonia is the most common form of pneumonia where S. pneumoniae is spread via personal contact with aerosol droplets within the community (Brooks and Mias, 2018).
Aside from the previously mentioned problems with the current treatments of antibiotics and vaccines, another important hurdle for treatment of pulmonary infections is that of the delivery method. Oral or intravenous injections have been proven suboptimal given the indirect route. Such indirect approaches result in lower drug concentrations in the lungs due to low half-life, measured in vivo at ~20 min for the endolysin Cpl-1 (Loeffler et al., 2003). Increasing drug concentrations to deliver adequate enzyme to the lungs raises concerns regarding toxicity. This underscores the need to further investigate methods for direct lung delivery of endolysins through aerosolization (Doehn et al., 2013), nebulization (Wang et al., 2020), powders (Wang et al., 2023), and nanotechnology (Falciani et al., 2020).
2.1 Delivery methods explored
2.1.1 Aerosolization
Doehn et al. (2013) showed that Cpl-1 could be delivered via aerosol inhalation 24 h post-lethal S. pneumoniae infection to successfully rescue mice. Upon infection, it was found that there were significant increases in inflammatory cytokines, IL-1β and IL-6, measured from bronchoalveolar lavage fluid of mice. A single dose Cpl-1 aerosol treatment led to a 4-log reduction in bacterial load in the lungs, prevented bacteremia and sepsis, without significant increases in inflammatory cytokine levels in lavage fluid of Cpl-1 treated mice. Delivery via aerosol is in clinical use for other biologics so the translational readiness would be relatively high, provided that the stability and dosing of endolysins can be established.
2.1.2 Nebulization
The use of commercial jet or mesh nebulizers has been studied using native Cpl-1 and a chimeric endolysin, ClyJ (Wang et al., 2020). Although ClyJ lost activity with either jet or mesh nebulization, mesh nebulization did not change the activity in Cpl-1 under any condition and only prolonged jet nebulization led to conformational changes of the enzyme. Mesh nebulized Cpl-1 at doses of 39.1–50.2 μg/mL showed a significant log reduction in S. pneumoniae in vitro when compared to the negative control without endolysin treatment, and a similar log reduction when compared to non-nebulized Cpl-1. Nebulizers are FDA-approved medical devices, although compatibility would need to be validated for each endolysin individually.
2.1.3 Dry powder formulation
A dry powder formulation of Cpl-1 was shown to be effective while also having better stability at room temperature, storage capabilities, and transportation compared to liquid formulations required for nebulization (Wang et al., 2023). Similar to the nebulization studies, Cpl-1 maintained its activity against S. pneumoniae after drying into powder form. Regulatory precedent for inhaled drugs exists for powder formulations, however the inhalation of proteins in powder form needs to be further evaluated for particle deposition, immunogenicity, and long-term stability.
2.1.4 Nanotechnology-based delivery
The use of nanotechnology approaches to stabilize endolysins has shown recent success. In general, nanotechnology in drug delivery systems utilizes nanostructures and/or nanoparticles to deliver drugs targeted to specific areas in a controlled manner (Patra et al., 2018). As an example, the SET-M33 peptide, a non-natural antimicrobial peptide synthesized in branched form, was electrostatically attached to dextran nanoparticles and shown to be efficacious in vivo against Pseudomonas aeruginosa in the lungs (Falciani et al., 2020). Adapting a similar nanosystem to be more specific to S. pneumoniae, for instance by incorporating an endolysin, could be a promising strategy. In the case of Cpl-1, nanoparticle delivery systems have already been explored, specifically through the use of chitosan nanoparticles to evaluate effects on Cpl-1 bioavailability (Gondil et al., 2020). Chitosan nanoparticles were chosen given their biodegradability, low immunogenicity, low toxicity, and the fact that they are already well-studied as a delivery platform (Ragelle et al., 2014; Mohammed et al., 2017). At 1 mg/mL, the highest concentration tested, in vitro epithelial cell viability after 24 h of incubation was 78 ± 2.2% for Cpl-1-loaded chitosan nanoparticles compared to 79 ± 3.6% for chitosan nanoparticles alone. This study also demonstrated the ability of chitosan nanoparticles to slowly release Cpl-1, thus marking it as a possible candidate for in vivo testing against S. pneumoniae (Gondil et al., 2020). Currently, nanoparticle systems for endolysin delivery remain preclinical and would require safety and biodistribution studies, in addition to further in vivo efficacy validation.
Collectively, the studies described above have shown the efficacy of Cpl-1 to treat S. pneumoniae through inhalation, nebulization, powder formulation, and nanosystem delivery, although the use of these technologies for other endolysins active against S. pneumoniae remains to be defined. Perhaps better alternatives exist through combinatorial solutions, such as using both nanoparticle technology and dry powder formulation (Sabuj et al., 2021). Multiple methods have been developed for drug delivery to the lungs that have positively impacted treatment outcomes; however, there remains a need for further testing of different endolysins within these delivery systems to fully explore treatment effectiveness. Continued innovation in delivery methods will be critical to realizing the therapeutic potential of endolysins for treating pneumococcal pulmonary infections.
3 Anatomical niche: middle ear hurdles and solutions
The S. pneumoniae from the nasopharynx reaches the ear via the Eustachian tube (ET) where it establishes infection leading to otitis media (OM) (Loughran et al., 2019). Nasopharyngeal viral infections worsen the condition by activating cytokines, which increase inflammation, thereby aiding S. pneumoniae adherence and invasion. Constriction of the ET due to inflammation generates negative pressure, allowing S. pneumoniae to ascend into the middle ear to cause disease (Silva, 2022). OM is one of the most common bacterial infections in children and a leading cause of pediatrician appointments and antibiotic prescriptions (Rovers et al., 2004). The short, wide structure of children’s ET makes them more vulnerable to OM (Silva, 2022). Age, alongside genetic influences and environmental factors, work together with sibling presence and daycare attendance to contribute to OM pathogenesis (Rovers, 2008). Acute OM is characterized by effusion in the middle ear paired with one or more of the following symptoms: otalgia, otorrhea, fever, or irritability. OM patients who receive antibiotic treatment may still face recurrent infections that can trigger permanent hearing loss including cholesteatoma development. Surgical procedures including tympanostomy tube placement and adenoidectomy serve as alternative treatments to antibiotics and prophylactic vaccination (Qureishi et al., 2014).
The tympanic membrane (TM) is the tissue separating the outer ear from the middle ear, preventing bacteria, water, and other outside elements from entering the deeper parts of the ear (Kim et al., 2024). The TM stands as a significant barrier to treatment, as passage through it would be required for direct drug targeting of middle ear infections. While oral antibiotics are often prescribed for OM, the limited vasculature of the middle ear often reduces their effectiveness, signifying a need for treatment methods that offer a more direct route. This raises the critical question: how can one effectively pass the barrier between the outer and middle ear to enable direct drug targeting?
Endolysins that can externally cross the tympanic membrane would enable more direct and efficient treatment of middle ear infections such as OM. Recent studies have identified specific peptides capable of crossing the tympanic membrane. These peptides were first discovered using phage display technology, and through continued studies, three peptides demonstrated the most success in vivo in rat models of OM (Kurabi et al., 2018a). Prior to optimization from 12-mer to 18-mer peptides, one specific peptide, TM3, was demonstrated to be able to cross an intact human TM discarded during otologic surgery, providing validation in an ex vivo model (Kurabi et al., 2018b). Moreover, these peptides were shown to be able to transport large cargos (e.g., whole phage) across the TM, as well as smaller molecules, such as covalently bonded amoxicillin or neomycin (Kurabi et al., 2025). Unfortunately, covalently linking chemical antibiotics to the peptides resulted in a loss of their antimicrobial activity. However, following a similar concept, endolysins active against S. pneumoniae could be engineered onto these trans-TM peptides to enable penetration of the membrane for therapeutic delivery. Other groups have also utilized these peptides for delivery into the TM to treat OM; however, rather than delivering antibiotics, they used the peptides to transport V2O5 nanowires (Liu et al., 2024). The nanowires were able to successfully penetrate the TM, further demonstrating the potential of these peptides to deliver a variety of therapeutic cargoes across the TM for treatment.
Another avenue to explore in terms of engineering endolysins to improve accessibility to the middle ear would be the use of hydrogels for treatment. In the context of wound infections, hydrogels loaded with the endolysin LysP53 against Acinetobacter baumannii were able to kill the pathogen and promote wound healing; similar results were observed with the endolysin ClyC against S. aureus (Yang et al., 2025). The combination of a peptide-endolysin fusion loaded onto a hydrogel could be a promising strategy for treating OM due to its ability to maintain proximity and release to the TM. Sustained release is particularly important because, as stated above, limited vascularization in the middle ear restricts the effectiveness of systemically delivered antibiotics. In addition, repeated topical dosing can cause irritation, which lowers compliance (Patel et al., 2019). Notably, the use of hydrogel-based delivery vehicles has already been shown to be effective in vivo in other ear disease models, including animal models of OM, where they have been shown to prolong local exposure to drugs and enhance their therapeutic effects (Bruk et al., 2020). Additional work has also been conducted utilizing the S. pneumoniae endolysin MSlys loaded onto a PEGylated liposome (Silva et al., 2022). Ex vivo studies using human TMs showed the PEGylated liposomes improved transport by 6- to 7-fold compared to free endolysin at both 2 h and 24-h time points. Further, the liposomes retained MSlys anti-pneumococcal activity at 2 h post-transport. PEGylated liposomes are commonly used for systemic protein and peptide delivery in vivo, however, their use for middle ear delivery has only been tested in ex vivo models. Overall, these studies demonstrate the promise of engineering endolysin-based strategies that can overcome the anatomical barrier of the TM and enable more effective treatment of OM.
4 Anatomical niche: invasive/systemic infection hurdles and solutions
The third niche involves scenarios where S. pneumoniae can migrate into the bloodstream and cause systemic infections, such as bacteremia or sepsis. Once S. pneumoniae enters the bloodstream, it must evade innate immune defenses to survive and disseminate to secondary sites. The ability of S. pneumoniae to persist in the blood is maintained by several virulence factors, including the polysaccharide capsule, pneumolysin, and surface proteins that inhibit complement activation. When the infection becomes severe, S. pneumoniae crosses the blood–brain barrier (BBB), ultimately reaching the cerebrospinal fluid (CSF) and causing meningitis. This translocation of the BBB occurs either through tissue damage or via internalization of S. pneumoniae within recruited leukocytes at the membrane (Marra and Brigham, 2001). Within the CSF, S. pneumoniae triggers a strong inflammatory response, ultimately leading to neuronal damage and high mortality rates if left untreated.
With respect to bloodstream infections, previous studies have demonstrated that endolysins can provide protection in a bacteremia model when administered via intraperitoneal injection. One specific study combined Cpl-1 and Pal to treat mice challenged with 5 × 107 CFU of S. pneumoniae. A single dose of 200 μg of either endolysin administered 1 h post-infection protected mice and reduced bacteremia (Jado et al., 2003). Moreover, as previously noted in this same manuscript, synergistic effects were observed with the combination of Cpl-1 and Pal. However, treatment via the bloodstream raises important questions regarding both half-life and immunogenicity.
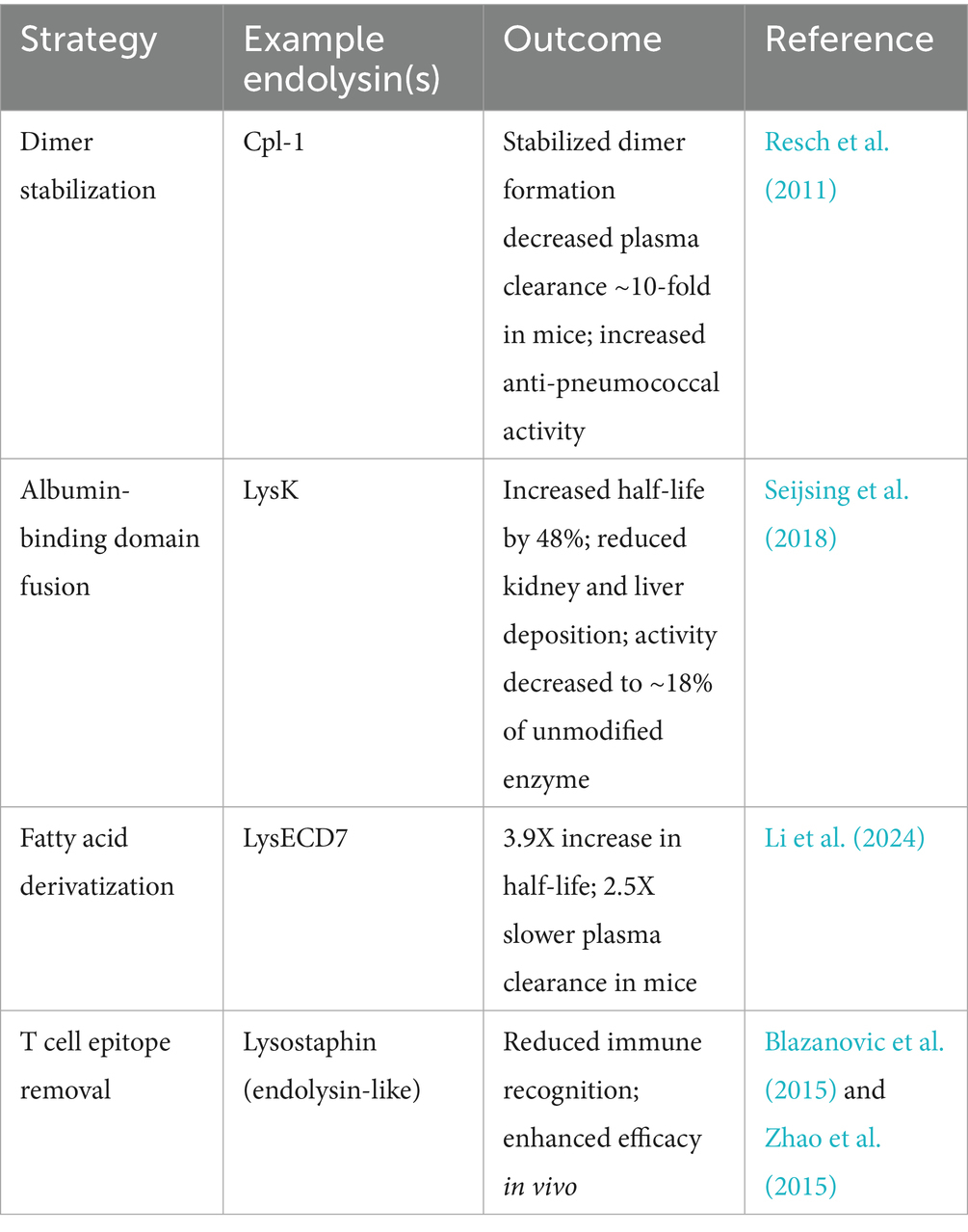
Regarding half-life, Cpl-1 is known to form dimers in the presence of choline, and stabilization of the Cpl-1 dimer was shown to increase its half-life by ten-fold in murine plasma (Resch et al., 2011). However, this effect has not been studied in other endolysins, and not all endolysins possess the ability to dimerize. In another approach, it was found that fusing LysK, a S. aureus-targeting endolysin, to an albumin-binding domain increased the half-life of LysK by 48% and decreased kidney and liver deposition. However, the bacteriolytic activity of this chimera was only ~18% of the unmodified LysK (Seijsing et al., 2018). Lastly, recent studies have utilized fatty acid derivatization technology to create an endolysin mutant with increased half-life and decreased clearance rates in the blood. In this work, endolysin LysECD7 was fused with lipopolysaccharide-interacting peptides and a valine to cysteine mutation allowed for conjugation with a C16 fatty acid side chain via reaction with acetyl bromide (Li et al., 2024). In vitro and in vivo studies testing efficacy and pharmacokinetics of this variant against A. baumannii showed that the mutant was removed from plasma at a 2.5-fold lower rate and exhibited a 3.9-fold increase in half-life compared to the unmodified enzyme.
Immunogenicity is another potential hurdle for systemic use of endolysins. Computational methods, such as the EpiSweep program, have been used to remove T cell epitopes of lysostaphin, an endolysin-like cell wall hydrolase that targets S. aureus, allowing lysostaphin to evade immune recognition (Blazanovic et al., 2015). Furthermore, additional studies on lysostaphin demonstrated greater treatment efficacy with T cell epitope-optimized variants compared to the wild-type enzyme (Zhao et al., 2015). Additionally, the EndoScan technology, a high-throughput linear B cell epitope mapping approach, was recently used to identify immunogenic regions in the S. pneumoniae-specific Cpl-1 and Pal endolysins (Harhala et al., 2023). Using this method, amino acids contributing to antibody binding were pinpointed, and engineered Pal variants were created. One Pal variant (280–282: DKP → GGA) demonstrated significantly enhanced antibacterial activity while successfully evading neutralization by antibodies raised against the wild-type enzyme. An overview of representative approaches to enhance the pharmacokinetics of endolysins for systemic administration, such as dimerization, fusion with albumin-binding domain, fatty acid derivatization, and removal of T cell epitopes, is summarized in Table 3.
For S. pneumoniae meningitis, therapeutic treatment faces the hurdle of crossing the BBB. The BBB is critical for protecting the central nervous system from pathogens and other harmful agents, but its structure and selectivity make it difficult for treatments to penetrate (Jiao et al., 2024). This challenge is underscored by the study by Valente et al., which showed that the endolysin PlyAZ3a was unable to cross the BBB in pharmacokinetic experiments using an infant rat model of S. pneumoniae meningitis (Valente et al., 2022). Notably, intracisternal injection of Cpl-1 against S. pneumoniae meningitis in a similar infant rat model demonstrated efficacy, decreasing S. pneumoniae counts by three logs with a single injection of 20 mg/kg, suggesting that if transit of the BBB can be achieved, S. pneumoniae endolysins are likely to be effective in the CSF.
Currently, several physical, biological, and chemical methods are being explored to optimize drug delivery across the BBB, including nanoparticle-based delivery systems (Jiao et al., 2024). Much like the strategies described for crossing the tympanic membrane, cell-penetrating peptides (CPPs) have been utilized to facilitate crossing of the BBB. Peptides such as TAT and xB3 have been discovered to cross the membrane in other therapeutic contexts (Bolhassani et al., 2017; Eyford et al., 2021) and could similarly be applied to deliver endolysins. To date, this application has only been proposed theoretically for endolysins, however, it is worth noting that TAT and other CPPs have been tested as fusions with endolysins for intracellular delivery into epithelial cells, where they demonstrated successful cellular uptake (Becker et al., 2016). Additionally, research into the use of exosomes as antimicrobial delivery systems has been expanding (Jiao et al., 2024). This “Trojan horse” approach could also be adapted for endolysins by exploiting tissue with low immunogenicity to enable entry into the central nervous system. Although promising, the application of CPP- and exosome-based strategies for delivery of endolysins across the BBB remains to be experimentally validated in vivo.
5 Conclusion
Research has demonstrated the effectiveness of endolysins against S. pneumoniae, but significant hurdles remain that must be overcome to achieve optimal therapeutic efficacy. This review explored specific challenges within the context of the major niches of infection: pulmonary, middle ear, and invasive/systemic. All niches present accessibility issues for endolysin treatment, whether due to limited delivery to the lungs, the need to cross the TM to reach the middle ear, or the formidable obstacle of the BBB. Within the pulmonary niche, work has been conducted on aerosolization, nebulization, and powder formulations for Cpl-1, with opportunities for future research into additional endolysins and nanosystem delivery methods. The middle ear and invasive/systemic niches, by contrast, remain unexplored areas, particularly in the context of endolysin-peptide fusions. The success of endolysins to combat S. pneumoniae infections relies on developing engineered adaptations that overcome the distinct barriers of different anatomical areas.
Developing new delivery methods remains essential for maximizing the therapeutic potential of endolysins as targeted treatments. To translate these promising agents into clinical practice, rigorous pharmacokinetic, immunogenicity, and toxicity studies are urgently needed, alongside well-designed efficacy trials in relevant preclinical models and eventual human studies. As most applications of endolysins and engineered variants will be classified as biologics, early consideration of regulatory pathways, including requirements for manufacturing quality, safety of novel excipients, and precedent from other endolysin clinical programs, will be key to optimize development and approval.
Author contributions
GC: Writing – original draft, Conceptualization. NG-J: Funding acquisition, Writing – review & editing. DN: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. All authors are supported by the National Institutes of Health award R01AI168313.
Acknowledgments
Graphics were created with BioRender.com.
Conflict of interest
DN is listed as inventor on several issued and pending patents related to pneumococcal endolysins.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelrahman, F., Easwaran, M., Daramola, O. I., Ragab, S., Lynch, S., Oduselu, T. J., et al. (2021). Phage-encoded endolysins. Antibiotics 10:124. doi: 10.3390/antibiotics10020124
Ajuebor, J., McAuliffe, O., O'Mahony, J., Ross, R. P., Hill, C., and Coffey, A. (2016). Bacteriophage endolysins and their applications. Sci. Prog. 99, 183–199. doi: 10.3184/003685016X14627913637705
Albright, J. M., Dunn, R. C., Shults, J. A., Boe, D. M., Afshar, M., and Kovacs, E. J. (2016). Advanced age alters monocyte and macrophage responses. Antioxid. Redox Signal. 25, 805–815. doi: 10.1089/ars.2016.6691
Alreja, A. B., Appel, A. E., Zhu, J. C., Riley, S. P., Gonzalez-Juarbe, N., and Nelson, D. C. (2024). SP-CHAP, an endolysin with enhanced activity against biofilm pneumococci and nasopharyngeal colonization. MBio 15:e0006924. doi: 10.1128/mbio.00069-24
Ashrafi-Asgarabad, A., Bokaie, S., Razmyar, J., Akbarein, H., Nejadghaderi, S. A., Carson-Chahhoud, K., et al. (2023). The burden of lower respiratory infections and their underlying etiologies in the Middle East and North Africa region, 1990-2019: results from the global burden of disease study 2019. BMC Pulm. Med. 23:2. doi: 10.1186/s12890-022-02301-7
Austrian, R. (1981). Pneumococcus: the first one hundred years. Rev. Infect. Dis. 3, 183–189. doi: 10.1093/clinids/3.2.183
Becker, S. C., Roach, D. R., Chauhan, V. S., Shen, Y., Foster-Frey, J., Powell, A. M., et al. (2016). Triple-acting lytic enzyme treatment of drug-resistant and intracellular Staphylococcus aureus. Sci. Rep. 6:25063. doi: 10.1038/srep25063
Blazanovic, K., Zhao, H., Choi, Y., Li, W., Salvat, R. S., Osipovitch, D. C., et al. (2015). Structure-based redesign of lysostaphin yields potent antistaphylococcal enzymes that evade immune cell surveillance. Mol. The.r Methods Clin. Dev. 2:15021. doi: 10.1038/mtm.2015.21
Bogaert, D., De Groot, R., and Hermans, P. W. (2004). Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4, 144–154. doi: 10.1016/S1473-3099(04)00938-7
Bolhassani, A., Jafarzade, B. S., and Mardani, G. (2017). In vitro and in vivo delivery of therapeutic proteins using cell penetrating peptides. Peptides 87, 50–63. doi: 10.1016/j.peptides.2016.11.011
Brooks, L. R. K., and Mias, G. I. (2018). Streptococcus pneumoniae's virulence and host immunity: aging, diagnostics, and prevention. Front. Immunol. 9:1366. doi: 10.3389/fimmu.2018.01366
Bruk, L. A., Dunkelberger, K. E., Khampang, P., Hong, W., Sadagopan, S., Alper, C. M., et al. (2020). Controlled release of ciprofloxacin and ceftriaxone from a single ototopical administration of antibiotic-loaded polymer microspheres and thermoresponsive gel. PLoS One 15:e0240535. doi: 10.1371/journal.pone.0240535
Castelo-Branco, C., and Soveral, I. (2014). The immune system and aging: a review. Gynecol. Endocrinol. 30, 16–22. doi: 10.3109/09513590.2013.852531
CDC (2010). Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children - advisory committee on immunization practices (ACIP). MMWR Morb. Mortal Wkly. Rep. 59, 258–261.
CDC (2018). Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Streptococcus pneumoniae, 2018. Atlanta, Georgia, USA: Centers for Disease Control and Prevention.
CDC (2019). Antibiotic resistance threats in the United States, 2019. Atlanta, GA: U.S. Department of Health and Human Resources.
Cilloniz, C., Garcia-Vidal, C., Ceccato, A., and Torres, A. (2018). “Antimicrobial resistance among Streptocccus pneumoniae” in Antimicrobial resistance in the 21st century. eds. I. Fong, D. Shlaes, and K. Drlica (Cham: Springer), 13–38.
Davies, L. R. L., Cizmeci, D., Guo, W., Luedemann, C., Alexander-Parrish, R., Grant, L., et al. (2022). Polysaccharide and conjugate vaccines to Streptococcus pneumoniae generate distinct humoral responses. Sci. Transl. Med. 14:eabm4065. doi: 10.1126/scitranslmed.abm4065
Diaz, E., Lopez, R., and Garcia, J. L. (1990). Chimeric phage-bacterial enzymes: a clue to the modular evolution of genes. Proc. Natl. Acad. Sci. USA 87, 8125–8129. doi: 10.1073/pnas.87.20.8125
Diez-Martinez, R., de Paz, H. D., Bustamante, N., Garcia, E., Menendez, M., and Garcia, P. (2013). Improving the lethal effect of cpl-7, a pneumococcal phage lysozyme with broad bactericidal activity, by inverting the net charge of its cell wall-binding module. Antimicrob. Agents Chemother. 57, 5355–5365. doi: 10.1128/AAC.01372-13
Díez-Martínez, R., De Paz, H. D., García-Fernández, E., Bustamante, N., Euler, C. W., Fischetti, V. A., et al. (2015). A novel chimeric phage lysin with high in vitro and in vivo bactericidal activity against Streptococcus pneumoniae. J. Antimicrob. Chemother. 70, 1763–1773. doi: 10.1093/jac/dkv038
Doehn, J. M., Fischer, K., Reppe, K., Gutbier, B., Tschernig, T., Hocke, A. C., et al. (2013). Delivery of the endolysin Cpl-1 by inhalation rescues mice with fatal pneumococcal pneumonia. J. Antimicrob. Chemother. 68, 2111–2117. doi: 10.1093/jac/dkt131
Entenza, J. M., Loeffler, J. M., Grandgirard, D., Fischetti, V. A., and Moreillon, P. (2005). Therapeutic effects of bacteriophage Cpl-1 lysin against Streptococcus pneumoniae endocarditis in rats. Antimicrob. Agents Chemother. 49, 4789–4792. doi: 10.1128/AAC.49.11.4789-4792.2005
Eyford, B. A., Singh, C. S. B., Abraham, T., Munro, L., Choi, K. B., Hill, T., et al. (2021). A nanomule peptide carrier delivers siRNA across the intact blood-brain barrier to attenuate ischemic stroke. Front. Mol. Biosci. 8:611367. doi: 10.3389/fmolb.2021.611367
Falciani, C., Zevolini, F., Brunetti, J., Riolo, G., Gracia, R., Marradi, M., et al. (2020). Antimicrobial peptide-loaded nanoparticles as inhalation therapy for Pseudomonas aeruginosa infections. Int. J. Nanomedicine 15, 1117–1128. doi: 10.2147/IJN.S218966
Fischetti, V. A. (2010). Bacteriophage endolysins: a novel anti-infective to control gram-positive pathogens. Int. J. Med. Microbiol. 300, 357–362. doi: 10.1016/j.ijmm.2010.04.002
Fowler, V. G. Jr., Das, A. F., Lipka-Diamond, J., Schuch, R., Pomerantz, R., Jauregui-Peredo, L., et al. (2020). Exebacase for patients with Staphylococcus aureus bloodstream infection and endocarditis. J. Clin. Invest. 130, 3750–3760. doi: 10.1172/JCI136577
Garcia, P., Garcia, J. L., Garcia, E., Sanchez-Puelles, J. M., and Lopez, R. (1990). Modular organization of the lytic enzymes of Streptococcus pneumoniae and its bacteriophages. Gene 86, 81–88. doi: 10.1016/0378-1119(90)90116-9
Gondil, V. S., Dube, T., Panda, J. J., Yennamalli, R. M., Harjai, K., and Chhibber, S. (2020). Comprehensive evaluation of chitosan nanoparticle based phage lysin delivery system; a novel approach to counter S. pneumoniae infections. Int. J. Pharm. 573:118850. doi: 10.1016/j.ijpharm.2019.118850
Grandgirard, D., Loeffler, J. M., Fischetti, V. A., and Leib, S. L. (2008). Phage lytic enzyme Cpl-1 for antibacterial therapy in experimental pneumococcal meningitis. J. Infect. Dis. 197, 1519–1522. doi: 10.1086/587942
Haber, P., Arana, J., Pilishvili, T., Lewis, P., Moro, P. L., and Cano, M. (2016). Post-licensure surveillance of 13-valent pneumococcal conjugate vaccine (PCV13) in adults aged ⩾19years old in the United States, vaccine adverse event reporting system (VAERS), June 1, 2012-December 31, 2015. Vaccine 34, 6330–6334. doi: 10.1016/j.vaccine.2016.10.052
Harhala, M. A., Gembara, K., Rybicka, I., Kaźmierczak, Z. M., Miernikiewicz, P., Majewska, J. M., et al. (2023). Immunogenic epitope scanning in bacteriolytic enzymes pal and Cpl-1 and engineering pal to escape antibody responses. Front. Immunol. 14:1075774. doi: 10.3389/fimmu.2023.1075774
Jado, I., López, R., García, E., Fenoll, A., Casal, J., García, P., et al. (2003). Phage lytic enzymes as therapy for antibiotic-resistant Streptococcus pneumoniae infection in a murine sepsis model. J. Antimicrob. Chemother. 52, 967–973. doi: 10.1093/jac/dkg485
Jiao, Y., Yang, L., Wang, R., Song, G., Fu, J., Wang, J., et al. (2024). Drug delivery across the blood-brain barrier: a new strategy for the treatment of neurological diseases. Pharmaceutics 16:611. doi: 10.3390/pharmaceutics16121611
Jun, S. Y., Jang, I. J., Yoon, S., Jang, K., Yu, K. S., Cho, J. Y., et al. (2017). Pharmacokinetics and tolerance of the phage endolysin-based candidate drug SAL200 after a single intravenous administration among healthy volunteers. Antimicrob. Agents Chemother. 61:16. doi: 10.1128/AAC.02629-16
Kadioglu, A., Weiser, J. N., Paton, J. C., and Andrew, P. W. (2008). The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 6, 288–301. doi: 10.1038/nrmicro1871
Kato, H. (2024). Antibiotic therapy for bacterial pneumonia. J. Pharm Health Care Sci. 10:45. doi: 10.1186/s40780-024-00367-5
Keller, L. E., Robinson, D. A., and McDaniel, L. S. (2016). Nonencapsulated Streptococcus pneumoniae: emergence and pathogenesis. MBio 7:e01792. doi: 10.1128/mBio.01792-15
Kim, S., Goo, W., Karima, G., Lee, J. H., and Kim, H. D. (2024). Polyacrylamide/gel-based self-healing artificial tympanic membrane for drug delivery of otitis treatment. Biomater Res. 28:0049. doi: 10.34133/bmr.0049
Kobayashi, M., Leidner, A. J., Gierke, R., Farrar, J. L., Morgan, R. L., Campos-Outcalt, D., et al. (2024). Use of 21-valent pneumococcal conjugate vaccine among U.S. adults: recommendations of the advisory committee on immunization practices - United States, 2024. MMWR Morb. Mortal Wkly. Rep. 73, 793–798. doi: 10.15585/mmwr.mm7336a3
Kuiper, J. W. P., Hogervorst, J. M. A., Herpers, B. L., Bakker, A. D., Klein-Nulend, J., Nolte, P. A., et al. (2021). The novel endolysin XZ.700 effectively treats MRSA biofilms in two biofilm models without showing toxicity on human bone cells in vitro. Biofouling 37, 184–193. doi: 10.1080/08927014.2021.1887151
Kurabi, A., Schaerer, D., Chang, L., Pak, K., and Ryan, A. F. (2018a). Optimisation of peptides that actively cross the tympanic membrane by random amino acid extension: a phage display study. J. Drug Target. 26, 127–134. doi: 10.1080/1061186X.2017.1347791
Kurabi, A., Schaerer, D., Noack, V., Bernhardt, M., Pak, K., Alexander, T., et al. (2018b). Active transport of peptides across the intact human tympanic membrane. Sci. Rep. 8:11815. doi: 10.1038/s41598-018-30031-6
Kurabi, A., Sereno, E., and Ryan, A. F. (2025). Peptides rapidly transport antibiotic across the intact tympanic membrane to treat a middle ear infection. Drug Deliv. 32:2463427. doi: 10.1080/10717544.2025.2463427
Leggat, D. J., Thompson, R. S., Khaskhely, N. M., Iyer, A. S., and Westerink, M. A. (2013). The immune response to pneumococcal polysaccharides 14 and 23F among elderly individuals consists predominantly of switched memory B cells. J. Infect. Dis. 208, 101–108. doi: 10.1093/infdis/jit139
Li, X., Shangguan, W., Yang, X., Hu, X., Li, Y., Zhao, W., et al. (2024). Influence of lipopolysaccharide-lnteracting peptides fusion with endolysin LysECD7 and fatty acid derivatization on the efficacy against Acinetobacter baumannii infection in vitro and in vivo. Viruses 16:760. doi: 10.3390/v16050760
Liu, S. S., Lang, J., Wen, S., Chen, P., Shu, H., Shindler, S., et al. (2024). Transtympanic delivery of V2O5 nanowires with a tympanic-membrane penetrating peptide. Biomater. Sci. 12, 6310–6324. doi: 10.1039/d4bm00983e
Lo, S. W., Gladstone, R. A., van Tonder, A. J., Lees, J. A., du Plessis, M., Benisty, R., et al. (2019). Pneumococcal lineages associated with serotype replacement and antibiotic resistance in childhood invasive pneumococcal disease in the post-PCV13 era: an international whole-genome sequencing study. Lancet Infect. Dis. 19, 759–769. doi: 10.1016/S1473-3099(19)30297-X
Loeffler, J. M., Djurkovic, S., and Fischetti, V. A. (2003). Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia. Infect. Immun. 71, 6199–6204. doi: 10.1128/IAI.71.11.6199-6204.2003
Loeffler, J. M., Nelson, D., and Fischetti, V. A. (2001). Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294, 2170–2172. doi: 10.1126/science.1066869
Loughran, A. J., Orihuela, C. J., and Tuomanen, E. I. (2019). Streptococcus pneumoniae: invasion and inflammation. Microbiol. Spectr. 7:18. doi: 10.1128/microbiolspec.GPP1123-0004-2018
Luo, D., Huang, L., Gondil, V. S., Zhou, W., Yang, W., Jia, M., et al. (2020). A choline-recognizing monomeric lysin, ClyJ-3m, shows elevated activity against Streptococcus pneumoniae. Antimicrob. Agents Chemother. 64:20. doi: 10.1128/AAC.00311-20
Marra, A., and Brigham, D. (2001). Streptococcus pneumoniae causes experimental meningitis following intranasal and otitis media infections via a nonhematogenous route. Infect. Immun. 69, 7318–7325. doi: 10.1128/IAI.69.12.7318-7325.2001
McCullers, J. A., Karlström, A., Iverson, A. R., Loeffler, J. M., and Fischetti, V. A. (2007). Novel strategy to prevent otitis media caused by colonizing Streptococcus pneumoniae. PLoS Pathog. 3:e28. doi: 10.1371/journal.ppat.0030028
Mohammed, M. A., Syeda, J. T. M., Wasan, K. M., and Wasan, E. K. (2017). An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 9:53. doi: 10.3390/pharmaceutics9040053
Nelson, D. C., Schmelcher, M., Rodriguez-Rubio, L., Klumpp, J., Pritchard, D. G., Dong, S., et al. (2012). Endolysins as antimicrobials. Adv. Virus Res. 83, 299–365. doi: 10.1016/B978-0-12-394438-2.00007-4
Oliveira, H., Sao-Jose, C., and Azeredo, J. (2018). Phage-derived peptidoglycan degrading enzymes: challenges and future prospects for in vivo therapy. Viruses 10:292. doi: 10.3390/v10060292
Owusu-Edusei, K., Deb, A., and Johnson, K. D. (2022). Estimates of the health and economic burden of pneumococcal infections attributable to the 15-valent pneumococcal conjugate vaccine serotypes in the USA. Infect. Dis. Ther. 11, 987–999. doi: 10.1007/s40121-022-00588-x
Patel, J., Szczupak, M., Rajguru, S., Balaban, C., and Hoffer, M. E. (2019). Inner ear therapeutics: an overview of middle ear delivery. Front. Cell. Neurosci. 13:261. doi: 10.3389/fncel.2019.00261
Patra, J. K., Das, G., Fraceto, L. F., Campos, E. V. R., Rodriguez-Torres, M. D. P., Acosta-Torres, L. S., et al. (2018). Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 16:71. doi: 10.1186/s12951-018-0392-8
Qureishi, A., Lee, Y., Belfield, K., Birchall, J. P., and Daniel, M. (2014). Update on otitis media - prevention and treatment. Infect. Drug Resist. 7, 15–24. doi: 10.2147/IDR.S39637
Ragelle, H., Riva, R., Vandermeulen, G., Naeye, B., Pourcelle, V., Le Duff, C. S., et al. (2014). Chitosan nanoparticles for siRNA delivery: optimizing formulation to increase stability and efficiency. J. Control. Release 176, 54–63. doi: 10.1016/j.jconrel.2013.12.026
Rahman, M. U., Wang, W., Sun, Q., Shah, J. A., Li, C., Sun, Y., et al. (2021). Endolysin, a promising solution against antimicrobial resistance. Antibiotics 10:277. doi: 10.3390/antibiotics10111277
Resch, G., Moreillon, P., and Fischetti, V. A. (2011). A stable phage lysin (Cpl-1) dimer with increased antipneumococcal activity and decreased plasma clearance. Int. J. Antimicrob. Agents 38, 516–521. doi: 10.1016/j.ijantimicag.2011.08.009
Roach, D. R., and Donovan, D. M. (2015). Antimicrobial bacteriophage-derived proteins and therapeutic applications. Bacteriophage 5:e1062590. doi: 10.1080/21597081.2015.1062590
Rovers, M. M. (2008). The burden of otitis media. Vaccine 26, G2–G4. doi: 10.1016/j.vaccine.2008.11.005
Rovers, M. M., Schilder, A. G., Zielhuis, G. A., and Rosenfeld, R. M. (2004). Otitis media. Lancet 363, 465–473. doi: 10.1016/S0140-6736(04)15495-0
Sabuj, M. Z. R., Dargaville, T. R., Nissen, L., and Islam, N. (2021). Inhaled ciprofloxacin-loaded poly(2-ethyl-2-oxazoline) nanoparticles from dry powder inhaler formulation for the potential treatment of lower respiratory tract infections. PLoS One 16:e0261720. doi: 10.1371/journal.pone.0261720
Schmelcher, M., and Loessner, M. J. (2021). Bacteriophage endolysins - extending their application to tissues and the bloodstream. Curr. Opin. Biotechnol. 68, 51–59. doi: 10.1016/j.copbio.2020.09.012
Seijsing, J., Sobieraj, A. M., Keller, N., Shen, Y., Zinkernagel, A. S., Loessner, M. J., et al. (2018). Improved biodistribution and extended serum half-life of a bacteriophage endolysin by albumin binding domain fusion. Front. Microbiol. 9:2927. doi: 10.3389/fmicb.2018.02927
Shenoy, A. T., and Orihuela, C. J. (2016). Anatomical site-specific contributions of pneumococcal virulence determinants. Pneumonia 8:9. doi: 10.1186/s41479-016-0007-9
Silva, M. D. F. (2022). Development of a new approach to control otitis media pathogens PhD in Biomedical Engineering. Braga, Portugal: Universidade do Minho.
Silva, M. D., Ray, K., Gama, M., Remenschneider, A. K., and Sillankorva, S. (2022). Ex vivo transtympanic permeation of the liposome encapsulated S. pneumoniae endolysin MSlys. Int. J. Pharm. 620:121752. doi: 10.1016/j.ijpharm.2022.121752
Simell, B., Lahdenkari, M., Reunanen, A., Kayhty, H., and Vakevainen, M. (2008). Effects of ageing and gender on naturally acquired antibodies to pneumococcal capsular polysaccharides and virulence-associated proteins. Clin. Vaccine Immunol. 15, 1391–1397. doi: 10.1128/CVI.00110-08
Skurnik, M., Alkalay-Oren, S., Boon, M., Clokie, M., Sicheritz-Ponten, T., Dabrowska, K., et al. (2025). Phage therapy. Nat. Rev. Methods Primers 5:377. doi: 10.1038/s43586-024-00377-5
Valente, L. G., Le, N. D., Pitton, M., Chiffi, G., Grandgirard, D., Jakob, S. M., et al. (2022). Efficacy assessment of a novel endolysin PlyAZ3aT for the treatment of ceftriaxone-resistant pneumococcal meningitis in an infant rat model. PLoS One 17:e0266928. doi: 10.1371/journal.pone.0266928
Wahl, B., O'Brien, K. L., Greenbaum, A., Majumder, A., Liu, L., Chu, Y., et al. (2018). Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000-15. Lancet Glob. Health 6, e744–e757. doi: 10.1016/S2214-109X(18)30247-X
Wang, Y., Khanal, D., Alreja, A. B., Yang, H., Yk Chang, R., Tai, W., et al. (2023). Bacteriophage endolysin powders for inhaled delivery against pulmonary infections. Int. J. Pharm. 635:122679. doi: 10.1016/j.ijpharm.2023.122679
Wang, Y., Khanal, D., Chang, R. Y. K., Shang, X., Yang, H., Britton, W. J., et al. (2020). Can bacteriophage endolysins be nebulised for inhalation delivery against Streptococcus pneumoniae? Int. J. Pharm. 591:119982. doi: 10.1016/j.ijpharm.2020.119982
Weiser, J. N., Ferreira, D. M., and Paton, J. C. (2018). Streptococcus pneumoniae: transmission, colonization and invasion. Nat. Rev. Microbiol. 16, 355–367. doi: 10.1038/s41579-018-0001-8
Westerink, M. A., Schroeder, H. W., and Nahm, M. H. (2012). Immune responses to pneumococcal vaccines in children and adults: rationale for age-specific vaccination. Aging Dis. 3, 51–67.
Wire, M. B., Jun, S. Y., Jang, I. J., Lee, S. H., Hwang, J. G., and Huang, D. B. (2022). A phase 1 study to evaluate safety and pharmacokinetics following administration of single and multiple doses of the antistaphylococcal lysin LSVT-1701 in healthy adult subjects. Antimicrob. Agents Chemother. 66:e0184221. doi: 10.1128/AAC.01842-21
Witzenrath, M., Schmeck, B., Doehn, J. M., Tschernig, T., Zahlten, J., Loeffler, J. M., et al. (2009). Systemic use of the endolysin Cpl-1 rescues mice with fatal pneumococcal pneumonia. Crit. Care Med. 37, 642–649. doi: 10.1097/CCM.0b013e31819586a6
Yang, H., Gong, Y., Zhang, H., Etobayeva, I., Miernikiewicz, P., Luo, D., et al. (2019). ClyJ is a novel pneumococcal chimeric lysin with a cysteine- and histidine-dependent amidohydrolase/peptidase catalytic domain. Antimicrob. Agents Chemother. 63, e02043–e02018. doi: 10.1128/AAC.02043-18
Yang, P., Li, J., Ma, X., Hu, N., Song, Z., Chen, B., et al. (2025). Novel delivery systems for phages and lysins in the topical management of wound infections: a narrative review. Front. Microbiol. 16:1526096. doi: 10.3389/fmicb.2025.1526096
Yang, H., Luo, D., Etobayeva, I., Li, X., Gong, Y., Wang, S., et al. (2020). Linker editing of pneumococcal lysin ClyJ conveys improved bactericidal activity. Antimicrob. Agents Chemother. 64, e01610–e01619. doi: 10.1128/AAC.01610-19
Keywords: endolysin, peptidoglycan hydrolase, bacteriophage, Streptococcus pneumoniae, pneumonia, otitis media, meningitis, sepsis
Citation: Castallanos G, Gonzalez-Juarbe N and Nelson DC (2025) Endolysins: targeting Streptococcus pneumoniae in its major anatomical niches of infection and addressing the hurdles. Front. Microbiol. 16:1660791. doi: 10.3389/fmicb.2025.1660791
Edited by:
Silvère Baron, U1103 Génétique Reproduction et Développement (GReD) (INSERM), FranceCopyright © 2025 Castallanos, Gonzalez-Juarbe and Nelson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel C. Nelson, bmVsc29uZEB1bWQuZWR1; Norberto Gonzalez-Juarbe, bmdqQHVtZC5lZHU=
 Giovanna Castallanos1
Giovanna Castallanos1 Norberto Gonzalez-Juarbe
Norberto Gonzalez-Juarbe Daniel C. Nelson
Daniel C. Nelson