- 1College of Clinical Medicine, Guizhou Medical University, Guiyang, China
- 2Department of Urology, Affiliated Hospital of Guizhou Medical University, Guiyang, China
Background: Kidney stones, particularly calcium oxalate stones, remain a significant health issue despite advances in treatment techniques. Residual stones that persist after treatment can lead to recurrent stone formation and further complications. The role of gut microbiota, specifically probiotics, in modulating oxalate metabolism has gained increasing attention as a potential therapeutic strategy to reduce residual stones and prevent recurrence.
Summary: This paper reviews the potential of probiotics, including recombinant strains, in the treatment of calcium oxalate kidney stones. Probiotics are thought to promote the degradation of oxalate in the gut, thereby reducing its absorption and preventing stone formation. Recent studies have highlighted the beneficial effects of probiotic interventions on gut microbiota composition, oxalate degradation pathways, and calcium oxalate stone formation. Moreover, recombinant probiotics, engineered to enhance oxalate-degrading capabilities, hold promise for improving treatment outcomes.
Key messages: This review summarizes recent advancements in the use of probiotics for the prevention and treatment of calcium oxalate kidney stones, with a focus on their role in oxalate degradation and the potential of recombinant probiotics to improve treatment outcomes.
1 Introduction
Kidney stone disease is a prevalent condition affecting the urinary system. The global morbidity and prevalence of nephrolithiasis are steadily increasing, with recent data indicating a prevalence of 12% in men and 10% in women (Singh et al., 2022). The formation of kidney stones is believed to be closely associated with various factors, including diet, genetics, and metabolism (Khan et al., 2016). Although the mechanisms underlying kidney stone formation remain unclear, urine supersaturation and crystallization are considered the primary drivers of renal crystal deposition (Wang Z. et al., 2021). Kidney stones are classified based on their composition, which includes calcium oxalate, uric acid, phosphate, and cystine stones, with calcium oxalate accounting for approximately 80% of cases (Coello et al., 2023). Calcium oxalate stones are characterized by high incidence and recurrence rates, leading to complications such as ureteral obstruction, frequent urinary tract infections, painful urination, and, if left untreated, kidney damage (Ziemba and Matlaga, 2017).
Current treatment options for calcium oxalate kidney stones primarily include surgery, medication, and lifestyle modifications. However, these treatments have limitations, including the invasive nature and high recurrence rates of surgical interventions, as well as significant side effects associated with medications (Zhang et al., 2023). Therefore, it is critical to explore safer and more effective methods for preventing and treating calcium oxalate nephrolithiasis. Recent advancements in technologies such as high-throughput sequencing have suggested that probiotics may influence the occurrence and recurrence of calcium oxalate stones, although the specific mechanisms remain unclear (Kelsey, 2016). Understanding the potential effects of probiotics on calcium oxalate nephrolithiasis could provide valuable insights into the pathogenesis of nephrolithiasis and open new avenues for its prevention and treatment.
2 Probiotics and the “gut-kidney axis”
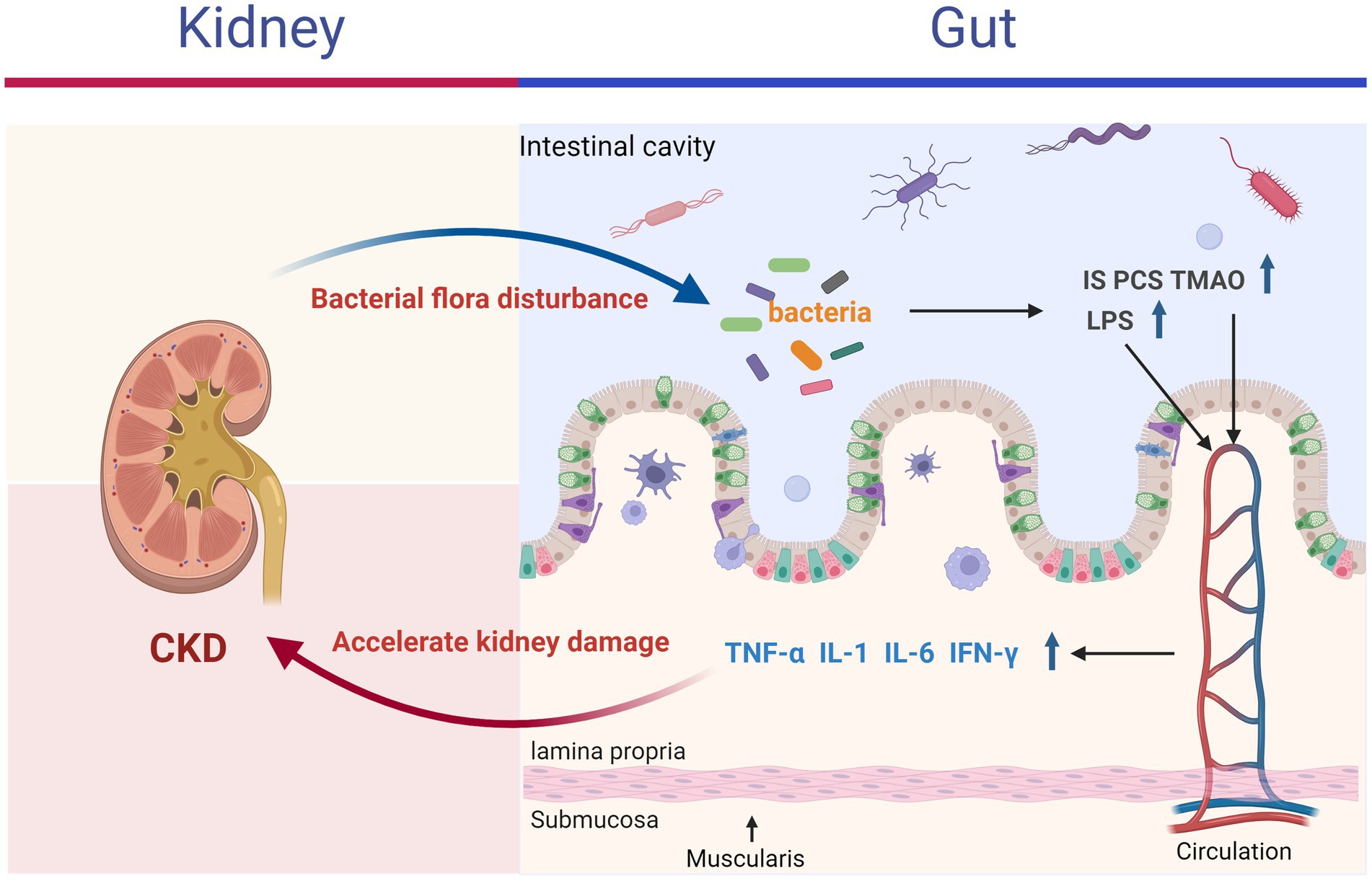
The “gut-kidney axis” theory posits that alterations in the gastrointestinal tract and kidneys can mutually influence each other, leading to adverse outcomes through mechanisms such as energy metabolism, immune inflammation, and interactions with gut microbiota (Ni et al., 2022). Increasing evidence indicates that conditions like inflammatory bowel disease, chronic kidney disease, kidney stones, and uremia are associated with the gut-kidney axis (Colella et al., 2023). According to this theory, kidney disease can disrupt the balance of intestinal microbiota, promoting the proliferation of enteric pathogens and the production of enterogenic urotoxins (Meijers and Evenepoel, 2011). These toxins may accumulate in the kidneys, exacerbating the decline in kidney function (Wing et al., 2016; Meijers et al., 2018). Conversely, disruptions in intestinal homeostasis can impair the gut barrier, allowing bacteria, bacterial components (e.g., endotoxins such as lipopolysaccharides), and urotoxins to enter the bloodstream, thereby triggering systemic inflammation through the release of inflammatory mediators, including IL-1, IL-6, TNF-α, and IFN-γ (shown in Figure 1) (Ghosh et al., 2020; Foresto-Neto et al., 2021).
Recent research on the gut-kidney axis has spurred the development of probiotic-based strategies aimed at preventing and treating calcium oxalate kidney stones. Probiotics, defined as live microorganisms that confer health benefits to the host, can colonize the gastrointestinal and reproductive systems, enhancing immune function by maintaining a balanced gut microbiota. The diversity and abundance of the gastrointestinal microbiome are closely linked to overall human health (Wang X. et al., 2021; de Vos et al., 2022). Kim et al. (2022) collected fecal samples from 915 adults, dividing them into three groups, and performed 16S rRNA gene sequencing to examine the diversity and taxonomic characteristics of gut microbiota associated with kidney stone status. Their findings indicated that kidney stones were linked to alterations in gut microbiota compared to the control group. Probiotics, a crucial component of gut microbiota, have been suggested to encompass various species, including formic acid bacteria, Lactobacilli, Bifidobacteria, and Enterobacteria, which are believed to play roles in the prevention and treatment of calcium oxalate kidney stones (Wigner et al., 2022). Yuan et al. (2023) further demonstrated that oral probiotic preparations, whether administered alone or in combination, including oxalate-degrading bacteria such as Oxalobacter formigenes, Lactobacilli, Bifidobacteria, and Enterococci, can reduce urinary oxalate excretion in both humans and animal models. Therefore, probiotics for the treatment and prevention of renal calcium oxalate stones may represent a promising new approach.
3 Effect of oxalic acid probiotics on renal calcium oxalate stones
3.1 Oxalobacter formigenes
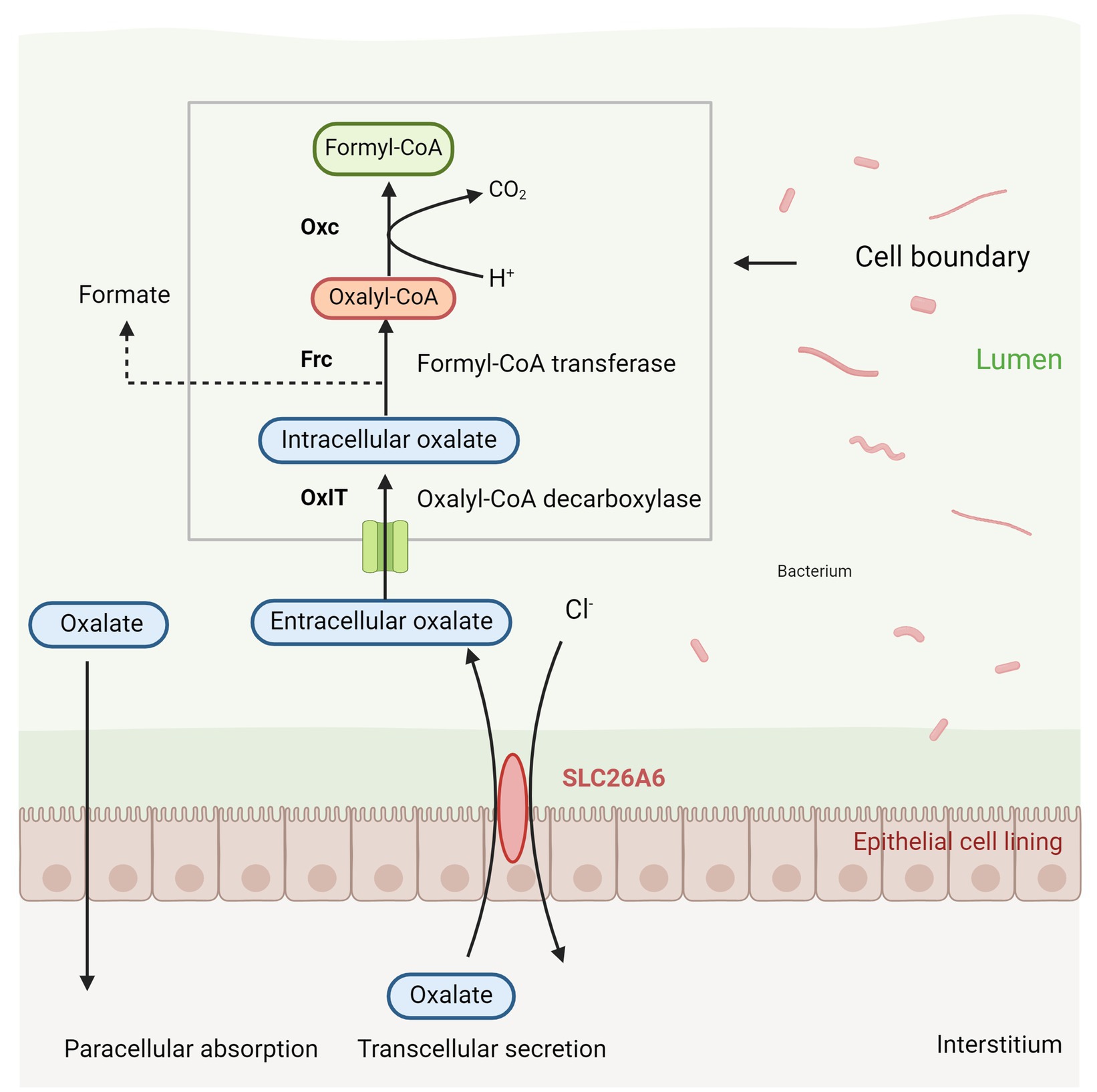
Oxalobacter formigenes is a Gram-negative, anaerobic bacterium that primarily degrades oxalic acid, serving as a major carbon source within the intestinal microbiota (Ellis M. E. et al., 2016). This bacterium plays a critical role in regulating the availability of oxalates in the intestinal lumen, thereby protecting against hyperoxaluria and preventing the formation of calcium oxalate stones (Daniel et al., 2021). Hyperoxaluria is a significant risk factor for the development of calcium oxalate stones, with approximately half of urinary oxalate originating from the gut. Consequently, limiting intestinal oxalate absorption is crucial for reducing the risk of stone formation or recurrence (Chen et al., 2023). Siener et al. (2013b) demonstrated that colonization by Oxalobacter formigenes reduces intestinal oxalate absorption, leading to decreased urinary oxalate excretion, as evidenced by fecal samples from patients with calcium oxalate stones. The bacterium’s mechanism for oxalate degradation involves the use of an oxalate-formate antiporter (OxlT) to transport extracellular oxalate into the cell, where it is converted into oxalyl-CoA by formyl-CoA transferase (Frc). Oxalyl-CoA is subsequently decarboxylated into formate and CO2 by oxalyl-CoA decarboxylase (Oxc), with formate being exported out of the cell via OxlT, thereby completing the degradation process (shown in Figure 2) (Youssef, 2024). Additionally, Oxalobacter formigenes may enhance the exudation of plasma oxalate into the gut by modulating the expression of the intestinal anion exchanger solute carrier family 26 member 6 gene (SLC26A6) (Stepanova, 2023).
Jafari et al. (2021) analyzed urine and fecal samples from 73 patients with calcium oxalate urolithiasis and found a significant negative correlation between the presence of Oxalobacter species and the formation of calcium oxalate stones. Earlier studies using rat models of primary hyperoxaluria demonstrated that Oxalobacter formigenes intervention reduced crystal formation in the kidneys (Verhulst et al., 2022). In a case–control study, Kim et al. (2021) found that colonization by formic acid-producing Oxalobacter reduced the risk of recurrent calcium oxalate stones by nearly 70% in 47 recurrent stone formers compared to 259 controls. Similarly, Hiremath and Viswanathan (2022) reported that 70% of kidney stone patients who were not colonized by Oxalobacter formigenes showed a noticeable increase in plasma oxalate after following a standardized diet. In addition, a phase III clinical trial conducted by Milliner et al. (2018) demonstrated that, compared with placebo, treatment with Oxalobacter formigenes did not result in a significant reduction in urinary oxalate levels. Other studies have reported no significant difference in urinary oxalate excretion between patients who tested positive or negative for Oxalobacter formigenes (Siener et al., 2013b). Such findings may be attributed to insufficient colonization, population heterogeneity, or inadequate treatment duration in these studies. Collectively, these findings suggest the potential use of Oxalobacter formigenes in preventing renal calcium oxalate stones.
Despite its promise, Oxalobacter formigenes has limitations, including its low tolerance to aerobic conditions and inhibition by bile salts and acidic environments, which complicate its colonization in the gut (Ellis M. L. et al., 2016). Studies have also indicated that Oxalobacter colonization is transient and may depend on the luminal concentrations of calcium, oxalate, and pH (Hatch et al., 2011). Furthermore, Nazzal et al. (2021) demonstrated that antibiotics, used in Helicobacter pylori eradication trials, significantly suppressed Oxalobacter colonization. The bacterium’s dependence on oxalate may also limit its efficacy in preventing other types of kidney stones. These factors pose challenges to its use as a probiotic for the prevention of calcium oxalate stones.
3.2 Lactobacillus species
Lactobacillus is a genus of Gram-positive, non-spore-forming bacilli abundant in the human gut, typically considered facultatively anaerobic with fermentative metabolism. Over fifty species of Lactobacillus are known to persistently colonize the gastrointestinal tract of healthy individuals (Rossi et al., 2016). Although present in smaller quantities, these bacteria are closely related to human health (Ren et al., 2020). Due to their generally safe profile, Lactobacilli are commonly used as probiotics.
The potential role of Lactobacilli in oxalate degradation and the prevention of calcium oxalate kidney stones has gained attention in recent years (Martín and Langella, 2019). Soliman et al. (2021) isolated 88 strains of Lactobacillus from various dairy products and identified five Lactobacillus fermentum and two Lactobacillus acidophilus strains with high oxalate-degrading capacity. Further analysis revealed that these strains were resistant to acid, bile salts, and tolerant to multiple antibiotics, suggesting lactobacilli as a promising strategy for preventing urinary stone formation. Mehra and Viswanathan (2021) and Mehra et al. (2022) confirmed this potential by applying two probiotic strains, Lactobacillus paragasseri UBLG-36 and Lactobacillus paracasei UBLPC-87, to a rat kidney stone model. Rats pretreated with these probiotics showed reduced urinary oxalate, lower serum urea nitrogen and creatinine levels, decreased stone formation, and reduced renal histological damage. These findings suggest that Lactobacillus paragasseri UBLG-36 and Lactobacillus paracasei UBLPC-87 can be incorporated into functional foods to reduce oxalate excretion, alleviate renal oxidative stress, and prevent calcium oxalate crystal formation.
Further studies indicate that the reduction in kidney crystals by Lactobacillus plantarum N-1 may occur through modulation of arginine metabolism, and supplementation with L. plantarum N-1 increases the abundance of beneficial bacteria, improves intestinal inflammation, and enhances intestinal barrier function (Liu et al., 2021b; Wei et al., 2021). Rats receiving prophylactic treatment with L. plantarum J-15 showed reduced urinary oxalate, endotoxins, pro-inflammatory factors, and prostaglandins, alongside improvements in enteritis and intestinal barrier function, attributed to reduced serum lipopolysaccharide levels and modulation of TLR4/NF-κB/COX-2 signaling (Tian et al., 2022). These findings suggest that Lactobacillus can prevent hyperoxaluria by modulating the gut microbiota and enhancing intestinal barrier function. However, some studies have found no reduction in urinary oxalate excretion or plasma oxalate concentrations with lactobacilli supplementation, possibly due to strain-specific effects or the limited impact of single bacterial strains compared to the gut microbiome as a whole (Siener et al., 2013a). Clinical studies have reported that treatment with high concentrations of freeze-dried lactic acid bacteria (L. acidophilus, L. plantarum, L. brevis, S. thermophilus, and B. infantis) markedly reduced urinary oxalate excretion (Campieri et al., 2001). Notably, most research on Lactobacillus has been limited to in vitro and animal studies, indicating a potential gap between in vitro and in vivo oxalate degradation capabilities. Further investigations in controlled human trials are needed to assess the efficacy of lactobacilli as oxalate-degrading probiotics.
3.3 Bifidobacteria
Bifidobacteria are anaerobic, Gram-positive bacilli often isolated from infant feces (Yao et al., 2021). Chen J. et al. (2021) reported that bifidobacteria gently modulate the gut microbiota while exhibiting antibacterial, antioxidative stress, and immunoregulatory properties, making them popular ingredients in functional foods. In addition to promoting gut health, Bifidobacteria influence various organs in the body through metabolic products of the gut microbiota (Zhang et al., 2024).
Recent studies have shown that Bifidobacteria can reduce urinary oxalate levels and prevent kidney stone formation. Klimesova et al. (2015) used a high-oxalate mouse model to show that Bifidobacteria can colonize in vivo and degrade dietary oxalates, reducing urinary oxalate excretion and limiting intestinal absorption, thus aiding in the prevention of kidney stone formation. Masoleh et al. (2023) studied a probiotic blend containing Bifidobacterium longum and found that it inhibited oxalate production in a rat kidney stone model, reducing urinary oxalate levels. Jung et al. (2023) reported that six idiopathic calcium oxalate stone patients with hyperoxaluria experienced a significant reduction in 24-h urinary oxalate excretion after 4 weeks of daily intake of a bifidobacteria-containing mixture. These studies support the role of bifidobacteria in oxalate degradation and the prevention of calcium oxalate stones.
Clinical studies have found that probiotic mixtures containing Lactobacillus and Bifidobacterium can reduce urinary oxalate levels and the prevalence of crystalluria in patients with kidney stones to some extent (Vittori et al., 2024), but the effect is less pronounced, possibly due to the complex interactions between the host diet, gut microbiota, and oxalate-degrading activity across different bacterial species (Tavasoli et al., 2021). Therefore, further research is needed to determine the optimal combinations of bifidobacteria and other probiotics for effective prevention of kidney stones.
4 Other probiotics and recombinant probiotics associated with oxalate metabolism
4.1 Enterococcus faecalis
Enterococcus faecalis is a Gram-positive, facultative anaerobic bacterium commonly found in the human intestinal microbiota. While it serves as a beneficial microorganism in the gut, it can also act as an opportunistic pathogen, contributing to biofilm-associated infections, particularly in the gastrointestinal and urinary tracts (Govindarajan et al., 2022). Hokama et al. (2005) demonstrated that E. faecalis degrades oxalic acid through three specific proteins, with molecular weights of 65, 48, and 40 kDa. These proteins were absent when the strain lost its oxalate-degrading capacity, highlighting their crucial role in the degradation process and suggesting they could be key targets for future research. The absence of these proteins under conditions with alternative energy sources further suggests that oxalate degradation may act as a compensatory mechanism in unfavorable conditions (García-Solache and Rice, 2019). Recent studies have also revealed interactions between E. faecalis and the host immune system, modulating inflammatory responses and immune cell activities, which may reduce inflammation associated with kidney stone formation (Wang et al., 2008). Further investigation of this interaction could lead to novel strategies for preventing and treating renal calculi. However, large-scale, randomized controlled trials have not yet validated the safety or efficacy of E. faecalis as a probiotic, and as such, enterococcus are not yet approved for clinical use in treating or improving human diseases (Wang et al., 2020).
4.2 Faecalibacterium
Faecalibacterium is a Gram-positive bacterium in the human gut, with its core member F. prausnitzii being an important butyrate-producing bacterium in the intestinal microbiota, playing a key role in maintaining gut health, metabolic regulation, and immune function (Martín et al., 2023). 16S rRNA sequencing has revealed that the relative abundance of Faecalibacterium is reduced in the gut microbiota of patients with calcium oxalate stones and is negatively correlated with stone formation (Ticinesi et al., 2018). This indicates that lower abundance of Faecalibacterium and F. prausnitzii is associated with a higher risk of kidney stone formation (Choy et al., 2024). Related studies administering Faecalibacterium prausnitzii to mice on a high-oxalate diet demonstrated that it alleviated renal calcium oxalate crystal deposition by reducing the production of deoxycholic acid and secondary bile acids (Liu et al., 2025). Although Faecalibacterium is not a specialized “oxalate-degrading bacterium,” it can improve the intestinal environment through butyrate, thereby indirectly enhancing the stability of intestinal oxalate metabolism (Chen F. et al., 2021).
4.3 Lachnospira
Lachnospira exhibits strict anaerobic metabolic characteristics. This genus can ferment complex carbohydrates such as pectin and polygalacturonic acid to produce short-chain fatty acids (SCFAs), including acetate and formate, making it a typical SCFA-producing bacterial group (Cornick and Stanton, 2015). In the aforementioned study by Suryavanshi, the proportion of oxalate-degrading species was higher in stone formers (Suryavanshi et al., 2016). Therefore, a high intake of oxalate-rich foods can lead to specific alterations in the gut microbiota composition, including increased relative abundance of Lachnospira, Roseburia, Dialister, Faecalibacterium, and Lactobacillus, accompanied by enhanced SCFA production (Ticinesi et al., 2020). To explore the relationship between gut microbiota and SCFAs in calcium oxalate nephrolithiasis, sequencing studies have shown that normal controls had higher levels of bacteria such as Lachnospiraceae, Ruminococcus, and Anaerostipes compared with kidney stone patients; these bacteria commonly produce SCFAs as metabolic products (Oliphant and Allen-Vercoe, 2019). SCFAs can also enter the host circulation and reach the kidneys, where they improve inflammation and fibrosis progression in chronic kidney disease (Li et al., 2017). Moreover, in vivo studies have demonstrated that SCFAs such as acetate, propionate, and butyrate can reduce renal calcium oxalate stone formation in model rats (Liu Y. et al., 2020).
4.4 Eubacterium genus
The genus Eubacterium consists of anaerobic, Gram-positive bacilli that play a significant role in oxalate metabolism and are key components of the gut microbiota. These species contribute to maintaining intestinal function, modulating inflammation, and regulating immune responses (Mukherjee et al., 2020). Ito et al. (1996) isolated Lentil WHY-1 from a male fecal sample and demonstrated its ability to degrade oxalate in artificial intestinal fluid. Its capacity to proliferate and metabolize oxalate in the presence of bile salts highlights the clinical potential of Eubacterium species. Additionally, studies have shown that SCFAs produced by various Eubacterium species significantly contribute to human health by providing nutrients and energy for the gut epithelium, maintaining the mucosal barrier, and alleviating inflammation (Louis and Flint, 2017; Zhou et al., 2023). Further research indicates that SCFAs can enhance the expression of the intestinal oxalate transporter SLC26A6, promoting oxalate secretion and reducing absorption, suggesting that SCFAs may influence kidney stone formation through multiple mechanisms (Liu et al., 2021a). Consequently, several Eubacterium species are being considered for trials as next-generation probiotics. However, there is currently no direct evidence confirming their oxalate-degrading abilities in vivo, and additional studies are needed to explore their potential in preventing and managing calcium oxalate kidney stones.
5 Recombinant probiotics for oxalate metabolism
As discussed earlier, probiotics hold significant promise for the prevention and treatment of renal calcium oxalate stones. However, not all probiotics are capable of degrading oxalate. Each probiotic strain has specific environmental requirements and functional properties, with those harboring one or more oxalate-degrading enzymes—such as oxalate decarboxylase (OxdC), Frc, or Oxc—demonstrating superior oxalate degradation capabilities. Given the vast diversity of probiotics, extensive screening is necessary to identify strains containing these enzymes. In addition to oxalate degradation, strategies aimed at inhibiting inflammatory factor production and alleviating oxidative stress have shown effectiveness in mitigating hyperoxaluria (Liu X. et al., 2020). Therefore, combining oxalate-degrading enzymes with Lactobacillus strains possessing anti-inflammatory and antioxidative properties may offer a novel therapeutic approach. Sasikumar et al. (2014b) successfully transferred the recombinant vector pLdhl0373OxdC into Lactobacillus WCFS1, resulting in the recombinant strain WCFS1-OxdC. OxdC activity was detected in the culture supernatant of the recombinant strain, leading to a 70% reduction in oxalate levels, while no reduction was observed in the supernatant of wild-type Lactobacillus WCFS1. Furthermore, when administered to rats fed a 5% potassium oxalate diet to induce calcium oxalate stones, WCFS1-OxdC significantly reduced urinary oxalate levels, while wild-type WCFS1 had no effect (Sasikumar et al., 2014a). Zhao et al. (2018) introduced genes encoding both oxalate decarboxylase and oxalate oxidase into Lactococcus lactis MG1363, resulting in the recombinant strain MG1363-OxdC. This strain exhibited the ability to degrade oxalate in vitro and effectively reduced oxalate levels in both the medium and rat urine when administered orally to a rat model of hyperoxaluria. Similarly, Paul et al. (2018) developed a food-grade recombinant Lactobacillus plantarum that secreted OxdC, which lowered urinary oxalate levels and reduced calcium deposits in rat kidneys. Collectively, these studies highlight the broad potential of recombinant lactobacilli as a next-generation therapeutic approach, offering alternative strategies for preventing calcium oxalate kidney stones.
Bacillus subtilis is a versatile bacterium capable of taking up extracellular DNA, facilitating genetic modifications (Li et al., 2021). The oxalate decarboxylase derived from B. subtilis has shown potential as a therapeutic agent for calcium oxalate urolithiasis (Albert et al., 2017). The integration of the B. subtilis-derived oxalate decarboxylase gene into bacterial strains through targeted chromosomal mutagenesis, using the mobile genetic element Ll.LtrB, has demonstrated the ability to degrade extracellular oxalate (Paul et al., 2019). Pfau et al. (2021) conducted a clinical study using a recombinant oxalate decarboxylase, reloxaliase, derived from B. subtilis, and found that reloxaliase effectively reduced plasma oxalate concentrations in patients with enteric hyperoxaluria, thereby decreasing the risk of oxalate-related diseases. Furthermore, Lingeman et al. (2019) developed an oral enzyme formulation, ALLN-177, for the treatment of severe hyperoxaluria by expressing, purifying, and encapsulating oxalate decarboxylase from Bacillus subtilis. In a clinical trial involving 16 subjects with hyperoxaluria and a history of kidney stones, ALLN-177 significantly reduced 24-h urinary oxalate excretion and exhibited good tolerability. Collectively, these studies suggest that genetically engineered B. subtilis strains capable of degrading oxalate could serve as a promising adjunctive method for preventing and treating calcium oxalate nephrolithiasis.
6 Discussion
Probiotics are integral components of the human gut microbiome, closely linked to the development and progression of kidney stones. Significant advancements have been made in investigating the prophylactic and therapeutic roles of probiotics in the formation of renal calcium oxalate stones. Several oxalate-degrading probiotics that influence stone formation have been identified. However, most research has focused on Oxalobacter formigenes, a bacterium that is susceptible to antibiotics, which limits its clinical utility. To develop more effective probiotic interventions for preventing and treating calcium oxalate stones, gene recombination to introduce oxalate-degrading enzymes into well-colonizing Lactobacillus strains in the gut may represent a promising new approach. It has been reported that the intestinal microbiota collectively influences the development of renal calcium oxalate stones (Ticinesi et al., 2019). Therefore, probiotic interventions aimed at modulating intestinal oxalate absorption must account for the complexity of the gut microbial community.
Future studies must address the limitations of previous research. Historically, animal experiments have primarily involved probiotic supplementation and dietary modifications to modulate the gut microbiota in rodents. However, these approaches fail to fully replicate human intestinal conditions, necessitating the use of more representative animal models to validate efficacy. Additionally, experiments linking microbial populations, functional oxalate degradation, molecular analyses of related gene regulation, and bacterial survival in the gut are crucial for identifying probiotics suitable for treating renal stone disease. The safety and long-term efficacy of these probiotics for preventing and treating renal oxalate stones must be confirmed in both healthy subjects and patients with kidney stones. An increasing number of clinical trials are providing strong scientific evidence for the application of probiotics in medical practice. With continued exploration and research, probiotics hold the potential to become an ideal novel strategy for the prevention and treatment of calcium oxalate nephrolithiasis.
7 Conclusion
Probiotics, particularly recombinant strains, show considerable potential in the prevention and treatment of calcium oxalate kidney stones. By modulating gut microbiota and enhancing oxalate degradation, probiotics can reduce the absorption of oxalate, thereby preventing stone formation. Despite promising results, further clinical studies and in vivo research are needed to validate their efficacy and optimize therapeutic strategies. Probiotics offer an innovative, non-invasive approach for the management and recurrence prevention of kidney stones.
Author contributions
JY: Conceptualization, Data curation, Investigation, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. DL: Funding acquisition, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. TL: Supervision, Validation, Writing – review & editing. BJ: Data curation, Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Science and Technology Fund of Guizhou Provincial Health Planning Commission (No. gzwjkj2018-1-036).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Albert, A., Tiwari, V., Paul, E., Ganesan, D., Ayyavu, M., Kujur, R., et al. (2017). Expression of heterologous oxalate decarboxylase in HEK293 cells confers protection against oxalate induced oxidative stress as a therapeutic approach for calcium oxalate stone disease. J. Enzyme Inhib. Med. Chem. 32, 426–433. doi: 10.1080/14756366.2016.1256884
Campieri, C., Campieri, M., Bertuzzi, V., Swennen, E., Matteuzzi, D., Stefoni, S., et al. (2001). Reduction of oxaluria after an oral course of lactic acid bacteria at high concentration. Kidney Int. 60, 1097–1105. doi: 10.1046/j.1523-1755.2001.0600031097.x
Chen, F., Bao, X., Liu, S., Ye, K., Xiang, S., Yu, L., et al. (2021). Gut microbiota affect the formation of calcium oxalate renal calculi caused by high daily tea consumption. Appl. Microbiol. Biotechnol. 105, 789–802. doi: 10.1007/s00253-020-11086-w
Chen, J., Chen, X., and Ho, C. L. (2021). Recent development of probiotic Bifidobacteria for treating human diseases. Front. Bioeng. Biotechnol. 9:770248. doi: 10.3389/fbioe.2021.770248
Chen, T., Qian, B., Zou, J., Luo, P., Zou, J., Li, W., et al. (2023). Oxalate as a potent promoter of kidney stone formation. Front. Med. 10:1159616. doi: 10.3389/fmed.2023.1159616
Choy, W. H., Adler, A., Morgan-Lang, C., Gough, E. K., Hallam, S. J., Manges, A. R., et al. (2024). Deficient butyrate metabolism in the intestinal microbiome is a potential risk factor for recurrent kidney stone disease. Urolithiasis 52:38. doi: 10.1007/s00240-024-01534-x
Coello, I., Sanchis, P., Pieras, E. C., and Grases, F. (2023). Diet in different calcium oxalate kidney stones. Nutrients 15:2607. doi: 10.3390/nu15112607
Colella, M., Topi, S., Palmirotta, R., D’Agostino, D., Charitos, I. A., Lovero, R., et al. (2023). An overview of the microbiota of the human urinary tract in health and disease: current issues and perspectives. Life (Basel) 13:1486. doi: 10.3390/life13071486
Cornick, N. A., and Stanton, T. B. (2015). Lachnospira. Bergey’s Manual of Systematics of Archaea and Bacteria, 1–6. doi: 10.1002/9781118960608.gbm00647
Daniel, S. L., Moradi, L., Paiste, H., Wood, K. D., Assimos, D. G., Holmes, R. P., et al. (2021). Forty years of Oxalobacter formigenes, a gutsy oxalate-degrading specialist. Appl. Environ. Microbiol. 87:e00544-21. doi: 10.1128/AEM.00544-21
de Vos, W. M., Tilg, H., Van Hul, M., and Cani, P. D. (2022). Gut microbiome and health: mechanistic insights. Gut 71, 1020–1032. doi: 10.1136/gutjnl-2021-326789
Ellis, M. L., Dowell, A. E., Li, X., and Knight, J. (2016). Probiotic properties of Oxalobacter formigenes: an in vitro examination. Arch. Microbiol. 198, 1019–1026. doi: 10.1007/s00203-016-1272-y
Ellis, M. E., Mobley, J. A., Holmes, R. P., and Knight, J. (2016). Proteome dynamics of the specialist oxalate degrader Oxalobacter formigenes. J. Proteomics Bioinform. 9, 19–24. doi: 10.4172/jpb.1000384
Foresto-Neto, O., Ghirotto, B., and Câmara, N. O. S. (2021). Renal sensing of bacterial metabolites in the gut-kidney Axis. Kidney360 2, 1501–1509. doi: 10.34067/KID.0000292021
García-Solache, M., and Rice, L. B. (2019). The enterococcus: a model of adaptability to its environment. Clin. Microbiol. Rev. 32:e00058-18. doi: 10.1128/CMR.00058-18
Ghosh, S. S., Wang, J., Yannie, P. J., and Ghosh, S. (2020). Intestinal barrier dysfunction, LPS translocation, and disease development. J. Endocr. Soc. 4:bvz039. doi: 10.1210/jendso/bvz039
Govindarajan, D. K., Meghanathan, Y., Sivaramakrishnan, M., Kothandan, R., Muthusamy, A., Seviour, T. W., et al. (2022). Enterococcus faecalis thrives in dual-species biofilm models under iron-rich conditions. Arch. Microbiol. 204:710. doi: 10.1007/s00203-022-03309-7
Hatch, M., Gjymishka, A., Salido, E. C., Allison, M. J., and Freel, R. W. (2011). Enteric oxalate elimination is induced and oxalate is normalized in a mouse model of primary hyperoxaluria following intestinal colonization with Oxalobacter. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G461–G469. doi: 10.1152/ajpgi.00434.2010
Hiremath, S., and Viswanathan, P. (2022). Oxalobacter formigenes: a new hope as a live biotherapeutic agent in the management of calcium oxalate renal stones. Anaerobe 75:102572. doi: 10.1016/j.anaerobe.2022.102572
Hokama, S., Toma, C., Iwanaga, M., Morozumi, M., Sugaya, K., and Ogawa, Y. (2005). Oxalate-degrading Providencia rettgeri isolated from human stools. Int. J. Urol. 12, 533–538. doi: 10.1111/j.1442-2042.2005.01083.x
Ito, H., Miura, N., Masai, M., Yamamoto, K., and Hara, T. (1996). Reduction of oxalate content of foods by the oxalate degrading bacterium, Eubacterium Lentum WYH-1. Int. J. Urol. 3, 31–34. doi: 10.1111/j.1442-2042.1996.tb00626.x
Jafari, G. A., Ardakani, R. F., Sepahi, M. A., Nowroozi, J., and Soltanpour, M. S. (2021). Development of an innovative method by optimizing qPCR technique for isolating and determining Oxalobacter formigenes microbial load in the stool of patients with urolithiasis. Iran. J. Kidney Dis. 15, 190–198.
Jung, H. D., Cho, S., and Lee, J. Y. (2023). Update on the effect of the urinary microbiome on urolithiasis. Diagnostics (Basel) 13:951. doi: 10.3390/diagnostics13050951
Kelsey, R. (2016). Gut microbiome is unique in kidney stone disease. Nat. Rev. Urol. 13:368. doi: 10.1038/nrurol.2016.93
Khan, S. R., Pearle, M. S., Robertson, W. G., Gambaro, G., Canales, B. K., Doizi, S., et al. (2016). Kidney stones. Nat. Rev. Dis. Primers 2:16008. doi: 10.1038/nrdp.2016.8
Kim, H.-N., Kim, J. H., Chang, Y., Yang, D., Joo, K. J., Cho, Y.-S., et al. (2022). Gut microbiota and the prevalence and incidence of renal stones. Sci. Rep. 12:3732. doi: 10.1038/s41598-022-07796-y
Kim, M.-G., Yang, J., and Jo, S.-K. (2021). Intestinal microbiota and kidney diseases. Kidney Res. Clin. Pract. 40, 335–343. doi: 10.23876/j.krcp.21.053
Klimesova, K., Whittamore, J. M., and Hatch, M. (2015). Bifidobacterium animalis subsp. lactis decreases urinary oxalate excretion in a mouse model of primary hyperoxaluria. Urolithiasis 43, 107–117. doi: 10.1007/s00240-014-0728-2
Li, L., Ma, L., and Fu, P. (2017). Gut microbiota-derived short-chain fatty acids and kidney diseases. Drug Des. Devel. Ther. 11, 3531–3542. doi: 10.2147/DDDT.S150825
Li, J.-Y., Wang, L., Liu, Y.-F., Zhou, L., Gang, H.-Z., Liu, J.-F., et al. (2021). Microbial Lipopeptide-producing strains and their metabolic roles under anaerobic conditions. Microorganisms 9:2030. doi: 10.3390/microorganisms9102030
Lingeman, J. E., Pareek, G., Easter, L., Pease, R., Grujic, D., Brettman, L., et al. (2019). ALLN-177, oral enzyme therapy for hyperoxaluria. Int. Urol. Nephrol. 51, 601–608. doi: 10.1007/s11255-019-02098-1
Liu, Y., Jin, X., Hong, H. G., Xiang, L., Jiang, Q., Ma, Y., et al. (2020). The relationship between gut microbiota and short chain fatty acids in the renal calcium oxalate stones disease. FASEB J. 34, 11200–11214. doi: 10.1096/fj.202000786R
Liu, Y., Jin, X., Ma, Y., Jian, Z., Wei, Z., Xiang, L., et al. (2021a). Short-chain fatty acids reduced renal calcium oxalate stones by regulating the expression of intestinal oxalate transporter SLC26A6. mSystems 6:e01045-21. doi: 10.1128/mSystems.01045-21
Liu, Y., Jin, X., Tian, L., Jian, Z., Ma, Y., Cheng, L., et al. (2021b). Lactiplantibacillus plantarum reduced renal calcium oxalate stones by regulating arginine metabolism in gut microbiota. Front. Microbiol. 12:743097. doi: 10.3389/fmicb.2021.743097
Liu, L., Ma, Y., Jian, Z., Liao, B., Li, Y., Lin, L., et al. (2025). Gut microbiota-bile acid crosstalk contributes to calcium oxalate nephropathy through Hsp90α-mediated ferroptosis. Cell Rep. 44:115936. doi: 10.1016/j.celrep.2025.115936
Liu, X., Yuan, P., Sun, X., and Chen, Z. (2020). Hydroxycitric acid inhibits renal calcium oxalate deposition by reducing oxidative stress and inflammation. Curr. Mol. Med. 20, 527–535. doi: 10.2174/1566524020666200103141116
Louis, P., and Flint, H. J. (2017). Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 19, 29–41. doi: 10.1111/1462-2920.13589
Martín, R., and Langella, P. (2019). Emerging health concepts in the probiotics field: streamlining the definitions. Front. Microbiol. 10:1047. doi: 10.3389/fmicb.2019.01047
Martín, R., Rios-Covian, D., Huillet, E., Auger, S., Khazaal, S., Bermúdez-Humarán, L. G., et al. (2023). Faecalibacterium: a bacterial genus with promising human health applications. FEMS Microbiol. Rev. 47:fuad039. doi: 10.1093/femsre/fuad039
Masoleh, A. M., Khoshnood, S., Haddadi, M. H., Rostamzad, A., Narki, M., Hoshmandfar, R., et al. (2023). Potential role of Lactobacillus and Bifidobacterium for preventing kidney stones. Clin. Lab. 69:889. doi: 10.7754/Clin.Lab.2022.220645
Mehra, Y., Rajesh, N. G., and Viswanathan, P. (2022). Analysis and characterization of Lactobacillus paragasseri and Lacticaseibacillus paracasei: two probiotic Bacteria that can degrade intestinal oxalate in Hyperoxaluric rats. Probiotics Antimicrob. Proteins 14, 854–872. doi: 10.1007/s12602-022-09958-w
Mehra, Y., and Viswanathan, P. (2021). High-quality whole-genome sequence analysis of Lactobacillus paragasseri UBLG-36 reveals oxalate-degrading potential of the strain. PLoS One 16:e0260116. doi: 10.1371/journal.pone.0260116
Meijers, B. K. I., and Evenepoel, P. (2011). The gut-kidney axis: indoxyl sulfate, p-cresyl sulfate and CKD progression. Nephrol. Dial. Transplant. 26, 759–761. doi: 10.1093/ndt/gfq818
Meijers, B., Farré, R., Dejongh, S., Vicario, M., and Evenepoel, P. (2018). Intestinal barrier function in chronic kidney disease. Toxins (Basel) 10:298. doi: 10.3390/toxins10070298
Milliner, D., Hoppe, B., and Groothoff, J. (2018). A randomised phase II/III study to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Urolithiasis 46, 313–323. doi: 10.1007/s00240-017-0998-6
Mukherjee, A., Lordan, C., Ross, R. P., and Cotter, P. D. (2020). Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 12:1802866. doi: 10.1080/19490976.2020.1802866
Nazzal, L., Francois, F., Henderson, N., Liu, M., Li, H., Koh, H., et al. (2021). Effect of antibiotic treatment on Oxalobacter formigenes colonization of the gut microbiome and urinary oxalate excretion. Sci. Rep. 11:16428. doi: 10.1038/s41598-021-95992-7
Ni, Y., Zheng, L., Nan, S., Ke, L., Fu, Z., and Jin, J. (2022). Enterorenal crosstalks in diabetic nephropathy and novel therapeutics targeting the gut microbiota. Acta Biochim. Biophys. Sin. Shanghai 54, 1406–1420. doi: 10.3724/abbs.2022140
Oliphant, K., and Allen-Vercoe, E. (2019). Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome 7:91. doi: 10.1186/s40168-019-0704-8
Paul, E., Albert, A., Ponnusamy, S., Mishra, S. R., Vignesh, A. G., Sivakumar, S. M., et al. (2018). Designer probiotic Lactobacillus plantarum expressing oxalate decarboxylase developed using group II intron degrades intestinal oxalate in hyperoxaluric rats. Microbiol. Res. 215, 65–75. doi: 10.1016/j.micres.2018.06.009
Paul, E., Albert, A., Ponnusamy, S., Venkatesan, S., and Govindan Sadasivam, S. (2019). Chromosomal integration of heterologous oxalate decarboxylase in Lactobacillus plantarum WCFS1 using mobile genetic element Ll.LtrB. Arch. Microbiol. 201, 467–476. doi: 10.1007/s00203-018-1585-0
Pfau, A., Grujic, D., Keddis, M. T., Kausz, A. T., Lieske, J. C., and Knauf, F. (2021). Pilot study of reloxaliase in patients with severe enteric hyperoxaluria and hyperoxalemia. Nephrol. Dial. Transplant. 36, 945–948. doi: 10.1093/ndt/gfaa379
Ren, C., Faas, M. M., and de Vos, P. (2020). Disease managing capacities and mechanisms of host effects of lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 61, 1365–1393. doi: 10.1080/10408398.2020.1758625
Rossi, M., Martínez-Martínez, D., Amaretti, A., Ulrici, A., Raimondi, S., and Moya, A. (2016). Mining metagenomic whole genome sequences revealed subdominant but constant Lactobacillus population in the human gut microbiota. Environ. Microbiol. Rep. 8, 399–406. doi: 10.1111/1758-2229.12405
Sasikumar, P., Gomathi, S., Anbazhagan, K., Abhishek, A., Paul, E., Vasudevan, V., et al. (2014a). Recombinant Lactobacillus plantarum expressing and secreting heterologous oxalate decarboxylase prevents renal calcium oxalate stone deposition in experimental rats. J. Biomed. Sci. 21:86. doi: 10.1186/s12929-014-0086-y
Sasikumar, P., Gomathi, S., Anbazhagan, K., Baby, A. E., Sangeetha, J., and Selvam, G. S. (2014b). Genetically engineered Lactobacillus plantarum WCFS1 constitutively secreting heterologous oxalate decarboxylase and degrading oxalate under in vitro. Curr. Microbiol. 69, 708–715. doi: 10.1007/s00284-014-0644-2
Siener, R., Bade, D. J., Hesse, A., and Hoppe, B. (2013a). Dietary hyperoxaluria is not reduced by treatment with lactic acid bacteria. J. Transl. Med. 11:306. doi: 10.1186/1479-5876-11-306
Siener, R., Bangen, U., Sidhu, H., Hönow, R., Von Unruh, G., and Hesse, A. (2013b). The role of Oxalobacter formigenes colonization in calcium oxalate stone disease. Kidney Int. 83:1144. doi: 10.1038/ki.2013.104
Singh, P., Harris, P. C., Sas, D. J., and Lieske, J. C. (2022). The genetics of kidney stone disease and nephrocalcinosis. Nat. Rev. Nephrol. 18, 224–240. doi: 10.1038/s41581-021-00513-4
Soliman, N. R., Effat, B. A. M., Mehanna, N. S., Tawfik, N. F., and Ibrahim, M. K. (2021). Activity of probiotics from food origin for oxalate degradation. Arch. Microbiol. 203, 5017–5028. doi: 10.1007/s00203-021-02484-3
Stepanova, N. (2023). Oxalate homeostasis in non-stone-forming chronic kidney disease: a review of key findings and perspectives. Biomedicine 11:1654. doi: 10.3390/biomedicines11061654
Suryavanshi, M. V., Bhute, S. S., Jadhav, S. D., Bhatia, M. S., Gune, R. P., and Shouche, Y. S. (2016). Hyperoxaluria leads to dysbiosis and drives selective enrichment of oxalate metabolizing bacterial species in recurrent kidney stone endures. Sci. Rep. 6:34712. doi: 10.1038/srep34712
Tavasoli, S., Jalali, S., Naji, M., Borumandnia, N., Shakiba Majd, G., Basiri, A., et al. (2021). Effect of a probiotic supplement containing Lactobacillus acidophilus and Bifidobacterium Animalis Lactis on urine oxalate in calcium stone formers with Hyperoxaluria: a randomized, placebo-controlled, double-blind and in-vitro trial. Urol. J. 19, 179–188. doi: 10.22037/uj.v18i.6789
Tian, L., Liu, Y., Xu, X., Jiao, P., Hu, G., Cui, Y., et al. (2022). Lactiplantibacillus plantarum J-15 reduced calcium oxalate kidney stones by regulating intestinal microbiota, metabolism, and inflammation in rats. FASEB J. 36:e22340. doi: 10.1096/fj.202101972RR
Ticinesi, A., Milani, C., Guerra, A., Allegri, F., Lauretani, F., Nouvenne, A., et al. (2018). Understanding the gut-kidney axis in nephrolithiasis: an analysis of the gut microbiota composition and functionality of stone formers. Gut 67, 2097–2106. doi: 10.1136/gutjnl-2017-315734
Ticinesi, A., Nouvenne, A., Chiussi, G., Castaldo, G., Guerra, A., and Meschi, T. (2020). Calcium oxalate nephrolithiasis and gut microbiota: not just a gut-kidney axis. A nutritional perspective. Nutrients 12:548. doi: 10.3390/nu12020548
Ticinesi, A., Nouvenne, A., and Meschi, T. (2019). Gut microbiome and kidney stone disease: not just an Oxalobacter story. Kidney Int. 96, 25–27. doi: 10.1016/j.kint.2019.03.020
Verhulst, A., Dehmel, B., Lindner, E., Akerman, M. E., and D’Haese, P. C. (2022). Oxalobacter formigenes treatment confers protective effects in a rat model of primary hyperoxaluria by preventing renal calcium oxalate deposition. Urolithiasis 50, 119–130. doi: 10.1007/s00240-022-01310-9
Vittori, M., Bove, P., Signoretti, M., Cipriani, C., Gasparoli, C., Antonucci, M., et al. (2024). Oral supplementation with probiotics, potassium citrate, and magnesium in reducing crystalluria in stone formers: a phase II study. Urologia 91, 681–686. doi: 10.1177/03915603241272146
Wang, S., Ng, L. H. M., Chow, W. L., and Lee, Y. K. (2008). Infant intestinal Enterococcus faecalis down-regulates inflammatory responses in human intestinal cell lines. World J. Gastroenterol. 14, 1067–1076. doi: 10.3748/wjg.14.1067
Wang, X., Yang, Y., and Huycke, M. M. (2020). Risks associated with enterococci as probiotics. Food Res. Int. 129:108788. doi: 10.1016/j.foodres.2019.108788
Wang, X., Zhang, P., and Zhang, X. (2021). Probiotics regulate gut microbiota: an effective method to improve immunity. Molecules 26:6076. doi: 10.3390/molecules26196076
Wang, Z., Zhang, Y., Zhang, J., Deng, Q., and Liang, H. (2021). Recent advances on the mechanisms of kidney stone formation (review). Int. J. Mol. Med. 48:149. doi: 10.3892/ijmm.2021.4982
Wei, Z., Cui, Y., Tian, L., Liu, Y., Yu, Y., Jin, X., et al. (2021). Probiotic Lactiplantibacillus plantarum N-1 could prevent ethylene glycol-induced kidney stones by regulating gut microbiota and enhancing intestinal barrier function. FASEB J. 35:e21937. doi: 10.1096/fj.202100887RR
Wigner, P., Bijak, M., and Saluk-Bijak, J. (2022). Probiotics in the prevention of the calcium oxalate urolithiasis. Cells 11:284. doi: 10.3390/cells11020284
Wing, M. R., Patel, S. S., Ramezani, A., and Raj, D. S. (2016). Gut microbiome in chronic kidney disease. Exp. Physiol. 101, 471–477. doi: 10.1113/EP085283
Yao, Y., Cai, X., Ye, Y., Wang, F., Chen, F., and Zheng, C. (2021). The role of microbiota in infant health: from early life to adulthood. Front. Immunol. 12:708472. doi: 10.3389/fimmu.2021.708472
Youssef, H. I. A. (2024). Detection of oxalyl-CoA decarboxylase (oxc) and formyl-CoA transferase (frc) genes in novel probiotic isolates capable of oxalate degradation in vitro. Folia Microbiol. (Praha) 69, 423–432. doi: 10.1007/s12223-024-01128-5
Yuan, T., Xia, Y., Li, B., Yu, W., Rao, T., Ye, Z., et al. (2023). Gut microbiota in patients with kidney stones: a systematic review and meta-analysis. BMC Microbiol. 23:143. doi: 10.1186/s12866-023-02891-0
Zhang, W., Jia, Q., Han, M., Zhang, X., Guo, L., Sun, S., et al. (2024). Bifidobacteria in disease: from head to toe. Folia Microbiol. 69, 1–15. doi: 10.1007/s12223-023-01087-3
Zhang, X., Lei, X., Jiang, Y., Zhao, L., Zou, C., Bai, Y., et al. (2023). Application of metabolomics in urolithiasis: the discovery and usage of succinate. Signal Transduct. Target. Ther. 8:41. doi: 10.1038/s41392-023-01311-z
Zhao, C., Yang, H., Zhu, X., Li, Y., Wang, N., Han, S., et al. (2018). Oxalate-degrading enzyme recombined lactic acid Bacteria strains reduce Hyperoxaluria. Urology 113, 253.e1–253.e7. doi: 10.1016/j.urology.2017.11.038
Zhou, X., Lian, P., Liu, H., Wang, Y., Zhou, M., and Feng, Z. (2023). Causal associations between gut microbiota and different types of dyslipidemia: a two-sample Mendelian randomization study. Nutrients 15:4445. doi: 10.3390/nu15204445
Keywords: probiotics, kidney stones, calcium oxalate stones, gut microbiota, oxalate degradation, recombinant probiotics
Citation: Yang J, Li D, Li T and Jia B (2025) Probiotics in the prevention and treatment of calcium oxalate kidney stones: mechanisms and therapeutic potential. Front. Microbiol. 16:1663138. doi: 10.3389/fmicb.2025.1663138
Edited by:
Shanshan Hu, Anhui Agricultural University, ChinaReviewed by:
Matteo Vittori, Policlinico Tor Vergata, ItalyCheng Cao, The Changshu Hospital Affiliated to Soochow University, China
Copyright © 2025 Yang, Li, Li and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benzhong Jia, amlhYnoxMjNAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Jinshan Yang
Jinshan Yang Dengbao Li2†
Dengbao Li2† Tao Li
Tao Li Benzhong Jia
Benzhong Jia