- 1School of Public Health, Shanghai Jiao Tong University, Shanghai, China
- 2School of Computer Science, Faculty of Engineering, The University of Sydney, Sydney, NSW, Australia
- 3Hainan Branch, Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, Sanya, China
- 4Shanghai Engineering Research Center of Intelligence Pediatrics (SERCIP), Shanghai Children’s Medical Center, affiliated with School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 5Child Health Advocacy Institute, National Children’s Medical Center, Shanghai Children’s Medical Center, affiliated with School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 6MOE-Shanghai Key Laboratory of Children’s Environmental Health, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Background: Asthma is a common chronic respiratory disease that usually originates in early childhood. Emerging evidence implicates the gut microbiota as a modulator in asthma development, with growing attention to the interplay between prenatal exposures, maternal/offspring gut microbiota, and subsequent asthma risk. However, no comprehensive review has systematically examined the relationships.
Objective: This review aimed to explore whether the gut microbiota acts as a mediating factor in the association between prenatal exposure and childhood asthma.
Results: A systematic search was performed in the PubMed, Scopus, and Web of Science databases up to March 1, 2025, employing keywords related to childhood asthma, gut microbiota, and prenatal exposure. Only population-based studies were considered. Eight studies met the inclusion criteria. Among which, two focused on pet exposure during pregnancy, three on delivery mode, two on the combined effects of delivery mode and antibiotic exposure, and one on maternal diet. Exposure to pets during pregnancy may result in distinct microbiota profiles in the offspring, which may potentially confer a protective effect against asthma. Antibiotic use and cesarean delivery were associated with increased asthma risk. Conversely, high maternal fecal short-chain fatty acid levels appeared protective against childhood asthma development. The gut microbiota may play a mediating role in these associations.
Conclusion: Prenatal factors significantly correlate with offspring gut microbiota and early immune development, thereby affecting asthma susceptibility. Further studies are needed to expand prenatal exposure assessments and elucidate the specific mechanisms by which the gut microbiota mediates the association between prenatal exposures and childhood asthma.
Introduction
Asthma is a chronic respiratory condition characterized by recurrent wheezing, shortness of breath, and chest tightness (Sockrider and Fussner, 2020). Epidemiological data showed that its prevalence ranged from 9.1 to 13.7% worldwide (Asher et al., 2020; García-Marcos et al., 2022), with the majority of countries continuing to demonstrate an upward trend (Stern et al., 2020). Asthma typically initiates during childhood and is triggered by allergens (Miller et al., 2021). T helper 2 cells (Th2 cells) responses play a central role in asthma pathogenesis, by activating cytokines like interleukin (IL)-4, IL-5, IL-9, and IL-13, which promote eosinophil infiltration into airway wall and excessive mucus production (Hammad and Lambrecht, 2021).
In recent years, evidence indicates that the abundance and diversity of gut microbiota correlate with childhood asthma susceptibility (Arrieta et al., 2015; Sordillo et al., 2019). Children with asthma exhibited decreased levels of beneficial bacteria such as Lactobacilli and Clostridium, alongside increased colonization by potential pathogens such as Bacteroides fragilis and Candida albicans (Kahhaleh et al., 2024; Budden et al., 2017; Chiu et al., 2023). Moreover, reduced abundance of microbiome-encoded carbohydrate-active enzyme genes was observed in the gut of asthma children, which may diminish butyrate production and negatively correlate with mite-specific IgE responses (Chiu et al., 2023). All these findings underscore the immunomodulatory potential of gut microbiota during childhood (Kahhaleh et al., 2024). Moreover, emerging findings have revealed that environmental exposures during pregnancy alter both the maternal gut microbiome and the establishment of the offspring’s gut microbiota (Xiao and Zhao, 2023). Robust association has been established that microbial alterations induced by prenatal exposures contribute to individuals’ immune-inflammatory responses and asthma pathogenesis (Budden et al., 2017). A 2021 review has synthesized evidence linking prenatal gut microbiota to offspring’s allergic diseases, suggesting that maternal gut microbiota plays a fundamental role in priming the developing immune system to acquire immune competence (Gao et al., 2021).
Of note is that no reviews have summarized whether prenatal environmental factors mediated maternal and/or offspring microbiome, thereby influencing childhood allergic outcomes. Given the context-dependent nature of gut microbiome alterations and the disease-specific effects of immune responses, there is a critical need to clarify the influence of prenatal exposures on microbiota composition and their subsequent effects on discrete allergic outcomes, such as asthma.
The purpose of this review is to investigate whether microbes act as an intermediate factor linking prenatal exposure to offspring asthma development, with particular focus on exposure-mediated alterations in maternal and offspring gut microbiota.
Methods
Data source and search strategy
This scoping review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) guidelines. We searched the PubMed, Scopus, and Web of Science databases up to March 1, 2025 using a combination of terms related to “asthma or respiratory conditions or wheezing,” “gut microbiota or intestinal flora,” “pregnancy or maternal exposure,” and “epidemiological or population-based studies.” No restrictions were applied regarding publication date or language. Reference lists of eligible studies were also reviewed for additional relevant studies.
Data management and study selection
All records were imported into EndNote (Clarivate Analytics). After removing duplicates, study selection was conducted in three stages: title screening, abstract review, and full-text evaluation. Two independent reviewers screened each study based on predefined eligibility criteria, with discrepancies resolved through discussion or by a third reviewer.
Inclusion and exclusion criteria
Studies were included if they met the following criteria: (1) Population-based studies with recruitment of both mothers and children. (2) Exposure assessment was restricted to the period spanning pregnancy through delivery. (3) Stool sample collection and microbial analysis from mothers and/or infants. (4) Documented childhood asthma outcomes or wheezing. (5) Included analysis of mediating role of the gut microbiota.
A total of 1,527 records were initially retrieved from PubMed (n = 147), Scopus (n = 548), and Web of Science (n = 832). After removing 414 duplicates, 1,113 records remained for screening. Following title and abstract screening, 789 were excluded. Subsequently, 324 full-text articles were sought for retrieval and further assessed. Of these, 316 were excluded due to not meeting the eligibility criteria upon full-text review. Ultimately, 8 articles met the inclusion criteria and were included in the present review. The study selection process is illustrated in the PRISMA flow diagram.
Data extraction
Two researchers extracted all the data independently. Each reviewer extracted the following from each study: name of the first authors, publication year, country of study, study aims, study design, study subjects, exposure factors, assessment of child outcomes, covariates and principal results. Disagreements were settled through consensus.
Subsections relevant for the subject
Description of studies
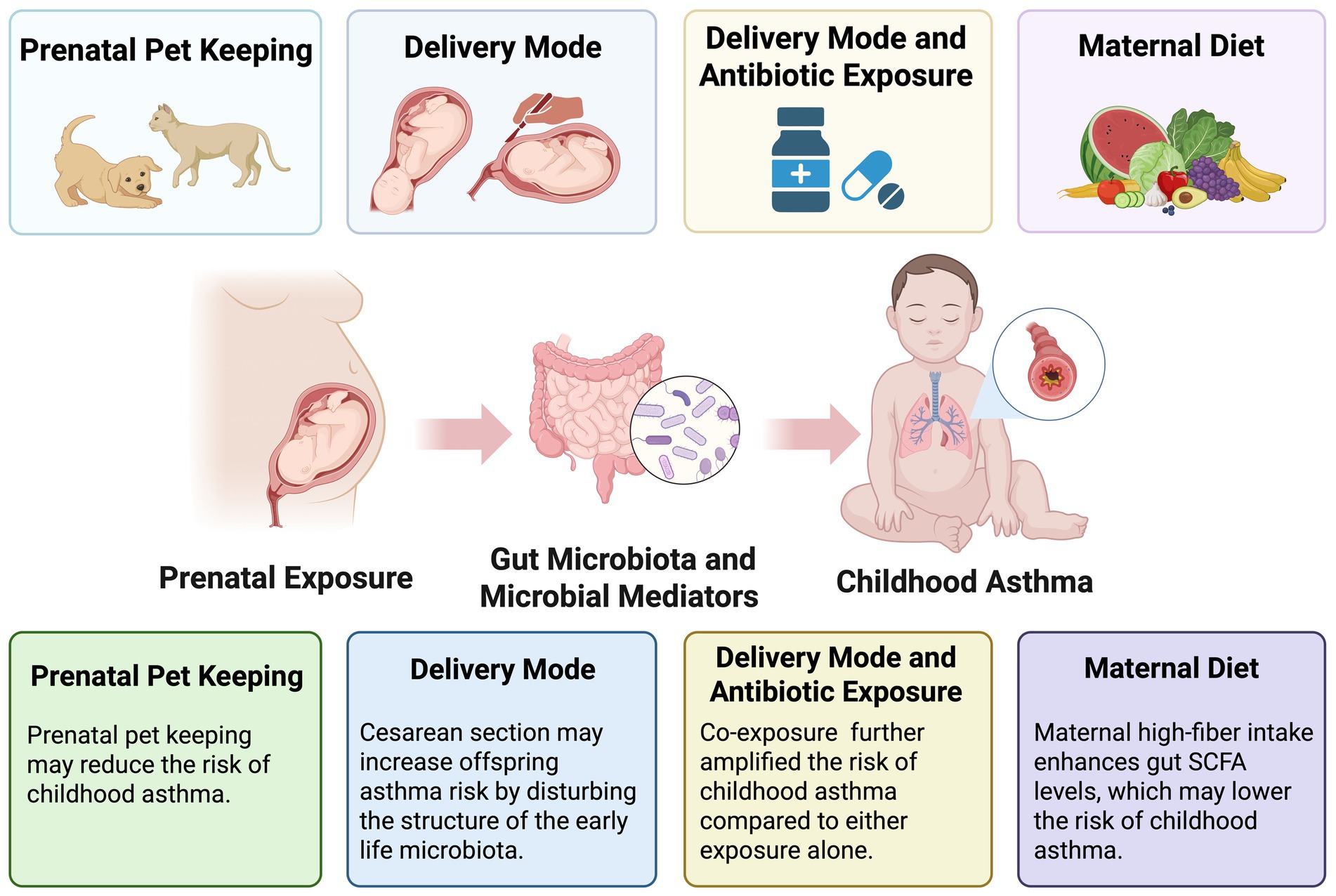
Table 1 presents the detailed data extracted from all included epidemiological studies (N = 8). Based on exposure during pregnancy, the studies were classified into four categories: pet exposure (n = 2), mode of delivery (n = 3), the combined effects of delivery mode and antibiotic exposure (n = 2), and maternal diet during pregnancy (n = 1). All included studies employed a cohort design. Geographically, these studies encompassed diverse populations across three continents. Four were conducted in Europe, specifically, one each in Finland and the Netherlands, and two in Denmark. Three studies originated from North America (United States), and one was conducted in Asia (South Korea). In terms of publication timelines, six were published within the past 5 years, while the remaining two were published in 2011 and 2013. Regarding fecal sample collection, four studies performed a single collection, whereas the other four utilized multiple time points. For outcome assessment, two evaluated wheeze, a common early clinical manifestation of asthma; one assessed early asthma at the age of 0–3 years and asthma at 6–7 years; and the remaining five focused exclusively on asthma aged 6–7 years. The information is depicted on sample collection timing and outcome measurement periods from eight studies (Figure 1), along with a summary of adjusted covariates (Table 2).
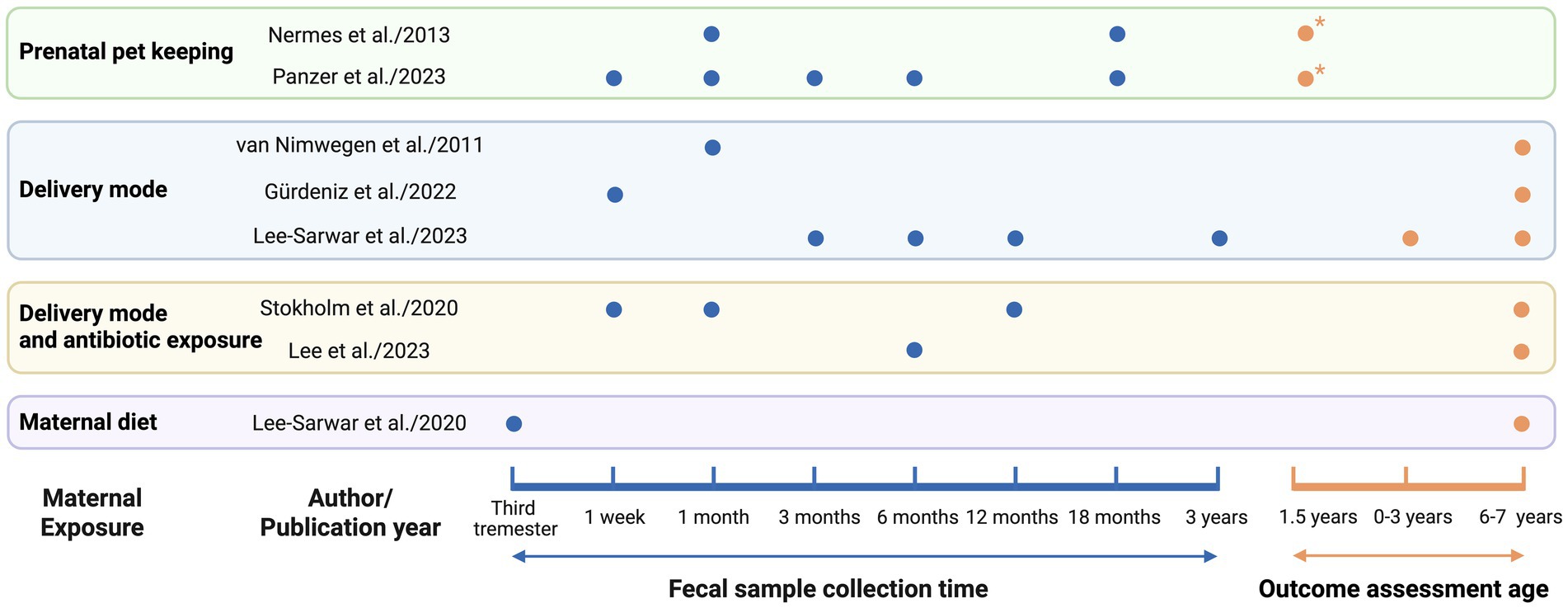
Figure 1. Timepoints of fecal sampling and asthma outcome assessment. Blue dots (●) indicate fecal sample collection for gut microbiota analysis. Orange dots (●) represent timepoints of asthma outcome assessment. Orange dots with an asterisk (●*) indicates wheeze assessment, which is commonly regarded as an early clinical manifestation of asthma.
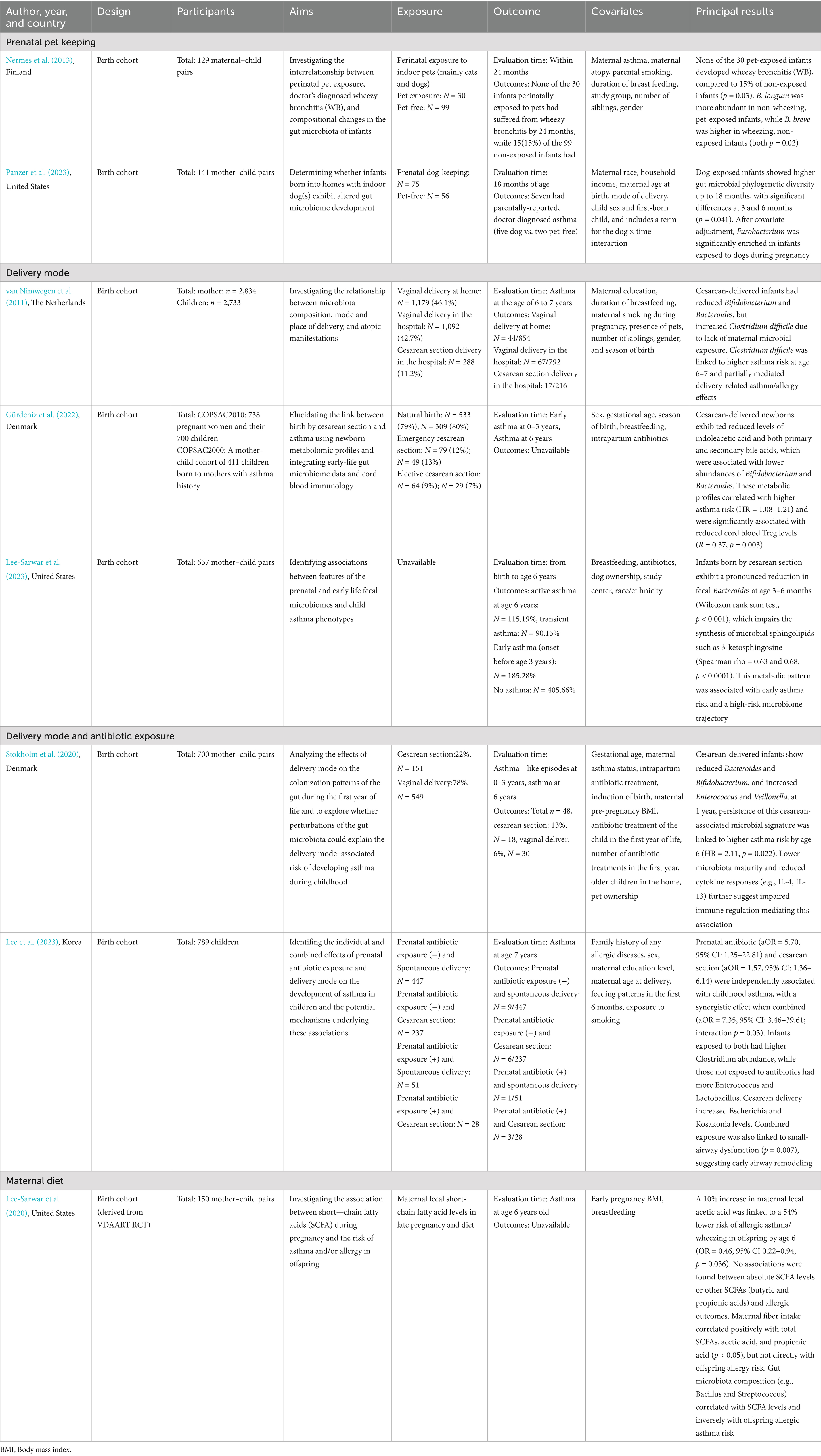
Table 1. Summary of epidemiological studies on prenatal exposures, gut microbiota, and childhood asthma.
Association of prenatal exposures, maternal/offspring gut microbiota, and childhood asthma risk
Prenatal pet keeping
Nermes et al. conducted a nested observational cohort study within a randomized controlled trial. In this study, 129 mothers and infants with indoor pet exposure data were analyzed to determine whether perinatal exposure to indoor pets influenced infant gut microbiota composition and the risk of developing wheezy bronchitis by 24 months of age (Nermes et al., 2013). Among 129 infants, 30 had been exposed to indoor pets (mainly cats and dogs), while 99 had not. None of the pet-exposed infants developed wheezy bronchitis, whereas 15 of the non-exposed infants did. Fecal samples collected at 1 month showed that Bifidobacterium longum was significantly enriched in non-wheezing, pet-exposed infants, while Bifidobacterium breve was more abundant in wheezing, non-exposed infants.
Panzer et al. conducted a longitudinal analysis observing the impact of living with indoor dogs during pregnancy on infant gut microbiota and the risk of allergic diseases including asthma at 18 months of age (Panzer et al., 2023). Among the participants, 81 mothers had been exposed to indoor dogs at least 12 h/day for over 6 months before pregnancy and throughout the whole pregnancy, and another 60 had no pets at home. Of their offspring, four and three were allergic to common inhalants, five and two were diagnosed with asthma, 24 and 11 reported eczema/atopic dermatitis, in the dog-exposed and non-exposed groups, respectively. Fusobacterium was found to be pronouncedly enriched in children whose mother underwent dog exposure during pregnancy.
The findings of the two studies support the broader argument that prenatal pet exposure may shape infant gut microbiota, potentially conferring protection against early-life wheezing and influencing long-term susceptibility to atopic, allergic, and asthmatic conditions.
Delivery mode
Three studies specifically focused on delivery mode. Van Nimwegen et al. used data from the Dutch KOALA birth cohort to investigate how delivery mode and setting influence infant gut microbiota composition and the subsequent asthma/allergy risk (van Nimwegen et al., 2011). The study included 2,733 children, of whom stool samples were available for 952 infants at 1 month, and asthma was assessed at ages 6–7. It was found that cesarean delivery reduced the colonization of beneficial genera including Bifidobacterium and Bacteroides and increased colonization by Clostridium difficile. Crucially, Clostridium difficile colonization was significantly associated with higher risks of asthma, eczema, and food allergy at age 6–7 years. C. difficile colonization partially mediated the effects of delivery mode/setting on allergic outcomes. Vaginal home delivery was associated with lower risks of asthma and food sensitization compared to vaginal hospital delivery, particularly in children with an atopic family history.
Gürdeniz et al. analyzed data from two cohorts of the Copenhagen Prospective Studies on Asthma in Childhood (COPSAC) established in 2010 and 2000, including 738 pregnant women and their 700 children from the COPSAC2010 cohort, as well as 411 full-term infants born to mothers with asthma from the COPSAC2000 cohort. Cord blood samples were collected from umbilical vein, dried blood spot (DBS) samples were, respectively, collected at age 2–3 days and 1–12 days in two cohorts, and fecal samples were both collected in infants 1 week after birth. The diagnoses of early asthma and asthma were made at ages 0–3 years and 6 years (Gürdeniz et al., 2022). DBS analysis revealed distinct metabolic profiles between cesarean-born and vaginally delivered infants that indoleacetic acid, an intermediate of tryptophan metabolism, as well as primary and secondary bile acids were lower in cesarean section newborns. The differences in DBS metabolic profile were correlated with varied abundance of Bifidobacterium and Bacteroides in infant gut. Moreover, the metabolic profiles of cesarean-born infants were positively associated with asthma risk up to ages 6. The frequency of cord blood regulatory T cells (Tregs) was also significantly linked to cesarean-associated metabolic profiles. Summing up all these results, it was suggested that cesarean section can lead to early microbiota-associated metabolic disruptions, thereby increasing asthma risk.
Using data from the Vitamin D Antenatal Asthma Reduction Trial (VDAART), Lee-Sarwar et al. investigated the association of mode of delivery, fecal microbiome with childhood asthma phenotypes among 657 maternal–infant pairs (Lee-Sarwar et al., 2023). Fecal samples were collected at 3–6 months, 1 year, and 3 years. Childhood asthma was classified into three phenotypes: early asthma or recurrent wheeze by age 3, transient asthma (early asthma without symptoms at age 6), and active asthma at age 6. The fecal abundance of Bacteroides was observed to be decreased significantly in infants born by cesarean section at 3–6 months of age, which was further linked to higher risk of early asthma and transient asthma. Meanwhile, the reduction in Bacteroides was also linked to decreased levels of microbial sphingolipids at 3–6 months of age and then associated with a higher risk of early asthma. Additionally, fecal linoleic acid levels at 1 year were lower in children who developed active asthma by age 6. Although an association was not established between fecal metabolites at age 3 and asthma, the overall data support a potential pathway by which delivery mode influences childhood asthma susceptibility via alterations in the gut microbiome.
Delivery mode and antibiotic exposure
Two studies explored the combined effects of antibiotic exposure and delivery mode. Stokholm et al. used data from COPSAC 2010 cohort to investigate whether gut microbial perturbations associated with delivery mode were linked to asthma risk within the first 6 years of life (Stokholm et al., 2020). Fecal samples were collected at 1 week, 1 month, and 1 year of age. At 1 week and 1 month, cesarean-born infants exhibited lower abundance of Bacteroidetes and Actinobacteria and higher Firmicutes and Proteobacteria at the phylum level. By 1 year, cesarean-born infants still had higher Enterobacteriaceae and Escherichia/Shigella at the genus level. Moreover, children born via cesarean section whose intestinal microbiota retains the cesarean characteristics at the age of 1 year experienced significantly more asthma-like episodes at ages 2–3 compared to vaginally delivered children and cesarean section-born children with low cesarean microbiome scores. These children also show a lower overall immune mediator response during acute airway symptom onset and faced an increased risk of asthma by age 6. This study also considered the potential effect of antibiotic exposure since all sampled cesarean section mother received intrapartum antibiotics at delivery, while only 13% for vaginally birth. Although antibiotic use can shift the microbiome structure toward that of cesarean-delivered children, delivery mode remains the primary factor influencing gut microbes and higher childhood asthma risk.
Lee et al. conducted a prospective study based on 789 children from the COCOA birth cohort (Lee et al., 2023). Fecal samples were collected from 207 6-month-old infants, and asthma was diagnosed at age 7. Both prenatal antibiotic exposure and cesarean section were independently associated with an increased risk of asthma. Co-exposure showed an even greater effect, with a dose–response relationship found between the higher antibiotic exposure and childhood asthma in cesarean-born children. Moreover, children born by cesarean section with prenatal antibiotic exposure manifested greater airway dysfunction compared to those with spontaneous delivery without prenatal antibiotic exposure. In addition, an increased relative abundance of Enterococcus and Lactobacillus was observed in infants without prenatal antibiotics, regardless of delivery mode. Meanwhile, the abundance of Escherichia and Kosakonia was significantly higher in cesarean section born infants compared to those delivered vaginally, and cesarean-born infants with prenatal antibiotics exposure had the higher level of the relative abundance of Clostridium. Collectively, these findings suggest that prenatal antibiotic exposure and cesarean section may lead to childhood asthma development, potentially through changes in early-life gut microbiota.
Maternal diet
Lee-Sarwar et al. conducted a secondary cohort analysis within the VDAART trial to examine the association between maternal fecal short-chain fatty acids (SCFAs) in late pregnancy and offspring risk of asthma and allergic outcomes by age 6 (Lee-Sarwar et al., 2020). Among 150 mother–child pairs, the study found that higher relative concentrations of acetate cetic acid in maternal stool were significantly associated with a reduced risk of combined asthma/wheeze and allergic sensitization in children. In contrast, absolute SCFAs levels showed no significant association with individual allergic outcomes. Maternal dietary fiber intake was positively correlated with total SCFAs levels, particularly acetic and propionic acids, suggesting that microbial fermentation of fiber may underlie this association. Furthermore, maternal gut microbiota profiling revealed that specific taxa such as Eubacterium dolichum and Streptococcus anginosus were linked to SCFAs composition and directionally associated with asthma risk. These findings highlight the potential role of maternal diet–microbiota–metabolite interactions during pregnancy in shaping immune-related disease susceptibility in offspring.
Discussion
Prenatal pet keeping
Previous epidemiological studies have indicated a potential relationship between maternal pet exposure and the risk of childhood allergic diseases, such as eczema (Eapen et al., 2022), food allergy (Smejda et al., 2020), and asthma (Lodge et al., 2012). Meanwhile, it has been found that pet exposure during the preconception period can influence the human microbiome, thereby exerting a significant influence in programming offspring’s immune system development (Aichbhaumik et al., 2008).
Nermes et al. performed the first study demonstrating that prenatal pet exposure in urban infants was associated with a decreased risk of asthmatic bronchitis and concurrent alterations in gut microbiota composition (Nermes et al., 2013). The study identified two distinct bacterial patterns: Bifidobacterium longum was more abundant in non-asthmatic, pet-exposed infants, whereas Bifidobacterium breve was enriched in asthmatic infants without pet contact. Notably, a potentially important dichotomy in Bifidobacterial effects was identified. Although Bifidobacteria are generally recognized for their anti-inflammatory and immunoregulatory roles (Cukrowska et al., 2020), the study suggests that different species within this genus may exert opposing influences on asthma development.
Another study included in this review showed that prenatal pet exposure correlated with a higher diversity of infant intestinal microbiota, particularly enriched abundances of Collinsella stercoris, Ruminococcus, Lachnospiraceae, and Clostridiaceae (Panzer et al., 2023). Similarly, Ruminococcus levels were found to be elevated in 3–4-month-old infants exposed to furry pets (Tun et al., 2017). Ruminococcus and Clostridium are key gut microbes involved in fermenting complex carbohydrates to produce SCFAs, such as butyrate and propionate (Li et al., 2025; Adjele et al., 2024). These SCFAs support health by strengthening the intestinal barrier and serving as an energy source for epithelial cells (Rowland et al., 2025). Such microbiome–host interactions may regulate immune development and lower the risk of diseases like asthma (Alswat, 2024). However, due to the limited sample size and the relatively short follow-up period of less than 2 years, it remains challenging to determine whether the observed microbial changes associated with pet exposure are implicated in infant allergy outcomes. In addition, the mixed exposure of multiple pets may introduce confounding effects, further complicating the interpretation of results.
Notably, prenatal and postnatal pet exposures often co-occur. Tun et al. found that their co-exposure was associated with more profound microbial alterations compared to prenatal-only exposure (Tun et al., 2017). Specifically, prenatal-only exposure was associated with transient alterations in specific microbial taxa and abundance ratios, whereas sustained exposure was linked to broader and more stable modifications of the gut microbiota, including enrichment of key commensal genera such as Ruminococcus and Oscillospira, as well as the suppression of potentially pathogenic taxa. These findings underscore the necessity of accounting for persistent exposure throuthout prenatal and postnatal period in future research.
With pet ownership on the rise (Kretzler et al., 2022), further research is needed to clarify how different types and timings of pet exposure during pregnancy involve in infant microbiome and influence childhood asthma and allergy risks, and to disentangle the respective and combined effects of prenatal and postnatal exposures through longitudinal designs.
Delivery mode
Accumulating studies found that the mode of delivery influences the establishment of the neonatal gut microbiome (Dominguez-Bello et al., 2010). Notably, delivery mode not only alters microbial diversity but also modulates metabolite profiles, both of which exert a crucial role in early immune development (Sarkar et al., 2021). Vaginally delivered infants exhibit higher levels of Gram-negative bacteria and lipopolysaccharide (LPS), a bacterial component that activates immune response. Conversely, cesarean-born infants lack this LPS-mediated immune activation, which may potentially increasing lifelong susceptibility to chronic diseases (Wampach et al., 2018).
Studies included in this review revealed that cesarean delivered infants exhibited reduced levels of Bacteroides and its metabolite sphingosine (Lee-Sarwar et al., 2023). They also showed decreased Bifidobacteria, leading to reduced production of tryptophan metabolites and bile acids (Gürdeniz et al., 2022). And these alterations were associated with an elevated risk of childhood asthma. An earlier study also linked lower fecal Bacteroides levels in infancy to the onset of atopy and wheezing by age five (Arrieta et al., 2018). Sphingosine can be converted into sphingosine-1-phosphate, which has been shown to regulate airway smooth muscle hyperreactivity and proliferation in asthma models (Maguire et al., 2023). In murine studies, Bifidobacterium, as a probiotic, has been shown to modulate lung granulocytes by altering the metabolism of short-chain fatty acids such as tryptophan in the mice intestines (Bezemer et al., 2024), which concurrently alleviated allergic asthma symptoms (Wang et al., 2024). In addition, bile acid metabolites regulate host immune responses by modulating Th17/Tregs balance (Hang et al., 2019). In the study by Gürdeniz et al. (2022), cesarean-born offspring exhibited altered bile acid metabolism and elevated Treg levels. Furthermore, specific components of gut microbiota can modulate immune function, potentially disrupting Th17/Tregs and Th1/Th2 balance (Di Gangi et al., 2020; Irvin et al., 2014), ultimately predisposing individuals to allergic inflammation and asthma (Kotrba et al., 2024).
In terms of multiparous women and delivery methods, previous delivery history holds predictive value for the current delivery mode. Evidence indicates that women with a previous cesarean delivery are more likely to opt for repeat cesarean section. Conversely, multiparous women without a history of cesarean section generally prefer natural childbirth (Ayachi et al., 2016; ACOG, 2019). Moreover, siblings also play a significant role in influcing the development of the infant gut and respiratory tract microbiota. Their influence has been shown to surpass other early-life exposures including mode of delivery, breastfeeding, or antibiotic use. This early microbial enrichment leads to a distinctive “sibling-associated microbiome signature” by age one, which is associated with a reduced risk of asthma by age six (Christensen et al., 2022). Furthermore, birth order has been consistently found to be inversely correlated with allergic disease risk, indicating later-born children tend to have progressively lower risks of developing allergies (Bernsen et al., 2003; Luukkonen et al., 2024). Therefore, whether the mother is a multiparous woman, especially her previous delivery method, and sibling factors should be taken into full account in examing the impact of delivery methods on the infant gut microbiota or the risk of asthma.
Importantly, the impact of delivery mode on early-life microbiota appears to be time-sensitive (Rutayisire et al., 2016) and may also impose effects on subsequent feeding patterns (Prior et al., 2012). Previous studies have shown that cesarean delivery was associated with delayed initiation of breastfeeding, lower rates of exclusive breastfeeding, and an earlier transition to formula feeding (Prior et al., 2012; Alrasheedi, 2023). These changes in early feeding practices may alter the infant gut microbiome, as dietary exposures become the predominant determinants of microbial composition by around 13 weeks of age, with breastfeeding cessation associated with further compositional shifts (Galazzo et al., 2020). Taken together, these interaction between delivery mode, feeding patterns and their temporal dynamics suggest that there are potential critical windows during which delivery mode shapes microbial colonization. Future studies should prioritize longitudinal tracking of delivery mode–related microbial changes, particularly during the first three months of life and the weaning transition.
Antibiotic
Epidemiological studies worldwide estimate that 20–30% of women are exposure to antibiotics during pregnancy (Loewen et al., 2018; Chandrakumar et al., 2018). The American College of Obstetricians and Gynecologists recommends routine prophylactic antibiotics before cesarean sections and selective use for vaginal births in high-risk cases (Committee on Practice Bulletins-Obstetrics, 2018). A study indicated limited impact of antibiotic administration during cesarean section on neonatal gut microbiome establishment (Sinha et al., 2024). These findings highlight the need for further investigation into the potential impacts of prenatal/pre-delivery antibiotic use on early microbial programming and the potential health outcomes.
According to Lee et al.’s study, prenatal antibiotic exposure was associated with small airway dysfunction and an increased asthma risk in offspring delivered by cesarean section (Lee et al., 2023). Animal models suggest that imbalanced maternal gut microbiota and reduced SCFAs concentrations may be key mechanisms linking prenatal antibiotic exposure to increased asthma severity in offspring (Alhasan et al., 2020; Alhasan et al., 2023). Butyrate deficiency downregulated type I interferon (IFN-I) signaling in neonatal type 2 innate lymphoid cells (ILC2s), increasing their responsiveness and promoting eosinophil infiltration and ILC2 numbers in the lungs. Changes in breast milk microbiota induced by antibiotics also influenced neonatal ILC2 phenotypes, contributing to offspring asthma development.
While prenatal antibiotics may affect offspring asthma via microbiota alterations, confounding factors especially delivery methods complicate epidemiologic interpretations. Future research should further explore whether antibiotic use at different stages of pregnancy exerts differential effects and emphasize rigorous adjustment for confoundere in study design.
Maternal diet
As a key determinant of intestinal flora, maternal diet during pregnancy can influence the composition, diversity, and metabolic activity of the gut microflora (Barrientos et al., 2024). Dietary factors affecting gut microbiota can be categorized into four groups: dietary pattern, food components, vitamins and trace elements, and prebiotics. Changes in any group can profoundly impact gut microbiota and subsequently affect the immune system of both maternal and offspring generations (Hebert et al., 2021; Drall et al., 2020; Sindi et al., 2021). Existing review indicates that a high-fat diet during pregnancy may reduce gut microbial diversity, whereas higher fiber intake appears to be positively associated with it (Maher et al., 2023). In animal models, it has been further demonstrated that a low-fiber diet during pregnancy delayed plasmacytoid dendritic cell (pDC) recruitment and lung Tregs expansion, leading to an increase in lower respiratory tract infections in offspring (Rago et al., 2019). Lee-Sarwar et al. highlighted the pivotal role of SCFAs, particularly acetic acid (Lee-Sarwar et al., 2020), which are primarily produced by the fermentation of dietary fibers in the gut and serve as key mediators linking maternal diet to immune regulation and respiratory health outcomes in offspring (Deleu et al., 2021). In a mouse study, acetate supplementation reduced allergic airway inflammation, airway hyperresponsiveness, and inflammatory cell infiltration, likely by modulating immune responses via G protein-coupled receptors (Thorburn et al., 2015).
Given the long-term implications of maternal diet during pregnancy, future research should further explore differences in dietary patterns and food components, as well as whether the supplementation of vitamins, trace elements, and prebiotics will have more specific effects on the microbiota of both the mother and the offspring, and how microbiota differences affect offspring susceptibility to allergic diseases.
Strengths and limitations
This review has several limitations to consider when interpreting the significance. First, despite a standardized search strategy, the potential omission of relevant studies cannot be excluded. Second, the included studies were categorized into four exposure groups, which could not be unified, limiting comparability. Third, although asthma was uniformly defined as the primary outcome, variations in follow-up duration led to inconsistencies in outcome evaluations. Moreover, substantial heterogeneity in microbial diversity measurements across studies complicated the identification of specific microbiome patterns associated with asthma development. Methodological variations also precluded both a formal quality assessment using standardized tools and a quantitative synthesis of results. In addition, most study populations were composed primarily of individuals of European descent. Only one study explicitly included race or ethnicity as an analytic covariate. These limitations underscore the importance of future studies employing standardized protocols for exposure assessment, outcome measurement, microbiome analysis among diverse populations.
Conclusion
This review synthesizes emerging epidemiological evidence on the associations between prenatal exposures and offspring asthma, with a focus on the mediating role of the gut microbiota. By examining changes in infant gut microbial and immune development in response to prenatal factors, we clarify potential causal pathways. Specifically, prenatal pet exposure was associated with increased gut microbiota diversity in offspring, potentially decreasing the risk of asthma. In contrast, cesarean section was linked to reduced diversity of gut microbiota in infants, which could raise the risk of childhood asthma. Prenatal antibiotic exposure can disrupt gut microbiota balance in both mothers and offspring, thereby exacerbating asthma susceptibility. Maternal diet also played a role; high-fiber diets during pregnancy increased fecal short-chain fatty acids, especially acetate, which was shown to alleviate asthma in offspring.
Although heterogeneity in exposure definitions, microbial targets, and outcome assessments across studies limits the ability to draw unified conclusions, this review is the first to explicitly establish the link between prenatal exposures, offspring asthma, and the gut microbiome. Based on our findings, large-scale, multi-center longitudinal studies with extended follow-up are needed to systematically evaluate long-term asthma outcomes and early microbial alterations. In addition to environmental exposures, behavioral and psychological factors should be incorporated into exposure assessments. More cutting-edge methods are needed to identify microbial taxa, metabolites, and immune pathways linked to asthma development.
Author contributions
ZN: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. YZ: Data curation, Methodology, Writing – review & editing. RL: Data curation, Investigation, Writing – review & editing. AZ: Data curation, Investigation, Writing – review & editing. ZW: Writing – review & editing. JY: Funding acquisition, Project administration, Writing – review & editing. SL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (Nos. 82273651, 82204060 and 81874266) and the Hainan Province Science and Technology Special Fund (No. ZDYF2025SHFZ052) awarded to Shenghui Li. Additional support was provided by the National Natural Science Foundation of China (No. 82404292), the Hainan Province Science and Technology Special Fund (No. ZDYF2024SHFZ059), the Research Program of the Hainan Provincial Health Commission (No. 22A200147), and the Sanya Science and Technology Special Fund (No. 2022KJCX41), awarded to Jiajun Yuan.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
Th2 cells, T helper 2 cells; IL, Interleukin; IgE, Immunoglobulin E; KOALA, Child, parents and health: focus on lifestyle and genetic constitution; COPSAC, Copenhagen prospective studies on asthma in childhood; DBS, Dried blood spot; Tregs, Regulatory T cells; VDAART, Vitamin D antenatal asthma reduction trial; SCFAs, Short-chain fatty acids; COCOA, Cohort for childhood origin of asthma and allergic diseases; LPS, Lipopolysaccharide; IFN-I, Type I interferon; ILC2s, Type 2 innate lymphoid cells; pDC, Plasmacytoid dendritic cell.
References
ACOG (2019). ACOG Practice Bulletin No. 205: vaginal birth after cesarean delivery. Obstet. Gynecol. 133, e110–e127. doi: 10.1097/AOG.0000000000003078
Adjele, J. J. B., Devi, P., Kumari, P., Yadav, A., Tchuenchieu Kamgain, A. D., Mouafo, H. T., et al. (2024). Exploring the influence of age and diet on gut microbiota development in children during the first 5 years: a study from Yaoundé, Cameroon. Front. Microbiol. 15:1512111. doi: 10.3389/fmicb.2024.1512111
Aichbhaumik, N., Zoratti, E. M., Strickler, R., Wegienka, G., Ownby, D. R., Havstad, S., et al. (2008). Prenatal exposure to household pets influences fetal immunoglobulin E production. Clin. Exp. Allergy 38, 1787–1794. doi: 10.1111/j.1365-2222.2008.03079.x
Alhasan, M. M., Cait, A. M., Heimesaat, M. M., Blaut, M., Klopfleisch, R., Wedel, A., et al. (2020). Antibiotic use during pregnancy increases offspring asthma severity in a dose-dependent manner. Allergy 75, 1979–1990. doi: 10.1111/all.14234
Alhasan, M. M., Hölsken, O., Duerr, C., Helfrich, S., Branzk, N., Philipp, A., et al. (2023). Antibiotic use during pregnancy is linked to offspring gut microbial dysbiosis, barrier disruption, and altered immunity along the gut-lung axis. Eur. J. Immunol. 53:e2350394. doi: 10.1002/eji.202350394
Alrasheedi, A. T. (2023). Factors associated with early initiation of breastfeeding in Central Saudi Arabia: a hospital-based survey. Int. Breastfeed. J. 18:62. doi: 10.1186/s13006-023-00598-6
Alswat, A. S. (2024). The influence of the gut microbiota on host health: a focus on the gut-lung axis and therapeutic approaches. Life (Basel) 14:1279. doi: 10.3390/life14101279
Arrieta, M. C., Arévalo, A., Stiemsma, L., Dimitriu, P., Chico, M. E., Loor, S., et al. (2018). Associations between infant fungal and bacterial dysbiosis and childhood atopic wheeze in a nonindustrialized setting. J. Allergy Clin. Immunol. 142, 424–34.e10. doi: 10.1016/j.jaci.2017.08.041
Arrieta, M. C., Stiemsma, L. T., Dimitriu, P. A., Thorson, L., Russell, S., Yurist-Doutsch, S., et al. (2015). Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 7:307ra152. doi: 10.1126/scitranslmed.aab2271
Asher, M. I., García-Marcos, L., Pearce, N. E., and Strachan, D. P. (2020). Trends in worldwide asthma prevalence. Eur. Respir. J. 56:2002094. doi: 10.1183/13993003.02094-2020
Ayachi, A., Derouich, S., Morjene, I., Mkaouer, L., Mnaser, D., and Mourali, M. (2016). Predictors of birth outcomes related to women with a previous caesarean section: experience of a motherhood center, Bizerte. Pan Afr. Med. J. 25:76. doi: 10.11604/pamj.2016.25.76.9164
Barrientos, G., Ronchi, F., and Conrad, M. L. (2024). Nutrition during pregnancy: influence on the gut microbiome and fetal development. Am. J. Reprod. Immunol. 91:e13802. doi: 10.1111/aji.13802
Bernsen, R. M., de Jongste, J. C., and van der Wouden, J. C. (2003). Birth order and sibship size as independent risk factors for asthma, allergy, and eczema. Pediatr. Allergy Immunol. 14, 464–469. doi: 10.1046/j.0905-6157.2003.00108.x
Bezemer, G. F. G., Diks, M. A. P., Mortaz, E., van Ark, I., van Bergenhenegouwen, J., Kraneveld, A. D., et al. (2024). A synbiotic mixture of Bifidobacterium breve M16-V, oligosaccharides and pectin, enhances short chain fatty acid production and improves lung health in a preclinical model for pulmonary neutrophilia. Front. Nutr. 11:1371064. doi: 10.3389/fnut.2024.1371064
Budden, K. F., Gellatly, S. L., Wood, D. L., Cooper, M. A., Morrison, M., Hugenholtz, P., et al. (2017). Emerging pathogenic links between microbiota and the gut-lung axis. Nat. Rev. Microbiol. 15, 55–63. doi: 10.1038/nrmicro.2016.142
Chandrakumar, A., Bhardwaj, A., and Jong, G. W. (2018). Maternal antibiotic exposure during pregnancy and hospitalization with infection in offspring. Int. J. Epidemiol. 47:1723. doi: 10.1093/ije/dyy096
Chiu, C. Y., Chang, K. C., Chang, L. C., Wang, C. J., Chung, W. H., Hsieh, W. P., et al. (2023). Phenotype-specific signatures of systems-level gut microbiome associated with childhood airway allergies. Pediatr. Allergy Immunol. 34:e13905. doi: 10.1111/pai.13905
Christensen, E. D., Hjelmsø, M. H., Thorsen, J., Shah, S., Redgwell, T., Poulsen, C. E., et al. (2022). The developing airway and gut microbiota in early life is influenced by age of older siblings. Microbiome. 10:106. doi: 10.1186/s40168-022-01305-z
Committee on Practice Bulletins-Obstetrics (2018). ACOG practice bulletin No. 199: Use of prophylactic antibiotics in labor and delivery. Obstet. Gynecol. 132, e103–e119. doi: 10.1097/AOG.0000000000002833
Cukrowska, B., Bierła, J. B., Zakrzewska, M., Klukowski, M., and Maciorkowska, E. (2020). The relationship between the infant gut microbiota and allergy. The role of Bifidobacterium breve and prebiotic oligosaccharides in the activation of anti-allergic mechanisms in early life. Nutrients 12:946. doi: 10.3390/nu12040946
Deleu, S., Machiels, K., Raes, J., Verbeke, K., and Vermeire, S. (2021). Short chain fatty acids and its producing organisms: an overlooked therapy for IBD? EBioMedicine 66:103293. doi: 10.1016/j.ebiom.2021.103293
Di Gangi, A., Di Cicco, M. E., Comberiati, P., and Peroni, D. G. (2020). Go with your gut: the shaping of T-cell response by gut microbiota in allergic asthma. Front. Immunol. 11:1485. doi: 10.3389/fimmu.2020.01485
Dominguez-Bello, M. G., Costello, E. K., Contreras, M., Magris, M., Hidalgo, G., Fierer, N., et al. (2010). Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 107, 11971–11975. doi: 10.1073/pnas.1002601107
Drall, K. M., Field, C. J., Haqq, A. M., de Souza, R. J., Tun, H. M., Morales-Lizcano, N. P., et al. (2020). Vitamin D supplementation in pregnancy and early infancy in relation to gut microbiota composition and C. difficile colonization: implications for viral respiratory infections. Gut Microbes 12:1799734. doi: 10.1080/19490976.2020.1799734
Eapen, A. A., Sitarik, A. R., Cheema, G., Kim, H., Ownby, D., Johnson, C. C., et al. (2022). Effect of prenatal dog exposure on eczema development in early and late childhood. J Allergy Clin Immunol Pract 10, 3312–4.e1. doi: 10.1016/j.jaip.2022.09.007
Galazzo, G., van Best, N., Bervoets, L., Dapaah, I. O., Savelkoul, P. H., Hornef, M. W., et al. (2020). Development of the microbiota and associations with birth mode, diet, and atopic disorders in a longitudinal analysis of stool samples, collected from infancy through early childhood. Gastroenterology 158, 1584–1596. doi: 10.1053/j.gastro.2020.01.024
Gao, Y., Nanan, R., Macia, L., Tan, J., Sominsky, L., Quinn, T. P., et al. (2021). The maternal gut microbiome during pregnancy and offspring allergy and asthma. J. Allergy Clin. Immunol. 148, 669–678. doi: 10.1016/j.jaci.2021.07.011
García-Marcos, L., Asher, M. I., Pearce, N., Ellwood, E., Bissell, K., Chiang, C. Y., et al. (2022). The burden of asthma, hay fever and eczema in children in 25 countries: GAN phase i study. Eur. Respir. J. 60:2102866. doi: 10.1183/13993003.02866-2021
Gürdeniz, G., Ernst, M., Rago, D., Kim, M., Courraud, J., Stokholm, J., et al. (2022). Neonatal metabolome of caesarean section and risk of childhood asthma. Eur. Respir. J. 59:2102406. doi: 10.1183/13993003.02406-2021
Hammad, H., and Lambrecht, B. N. (2021). The basic immunology of asthma. Cell 184, 2521–2522. doi: 10.1016/j.cell.2021.04.019
Hang, S., Paik, D., Yao, L., Kim, E., Trinath, J., Lu, J., et al. (2019). Bile acid metabolites control T(H)17 and T(reg) cell differentiation. Nature 576, 143–148. doi: 10.1038/s41586-019-1785-z
Hebert, J. C., Radford-Smith, D. E., Probert, F., Ilott, N., Chan, K. W., Anthony, D. C., et al. (2021). Mom's diet matters: maternal prebiotic intake in mice reduces anxiety and alters brain gene expression and the fecal microbiome in offspring. Brain Behav. Immun. 91, 230–244. doi: 10.1016/j.bbi.2020.09.034
Irvin, C., Zafar, I., Good, J., Rollins, D., Christianson, C., Gorska, M. M., et al. (2014). Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J. Allergy Clin. Immunol. 134, 1175–86.e7. doi: 10.1016/j.jaci.2014.05.038
Kahhaleh, F. G., Barrientos, G., and Conrad, M. L. (2024). The gut-lung axis and asthma susceptibility in early life. Acta Physiol (Oxf.) 240:e14092. doi: 10.1111/apha.14092
Kotrba, J., Müller, I., Pausder, A., Hoffmann, A., Camp, B., Boehme, J. D., et al. (2024). Innate players in Th2 and non-Th2 asthma: emerging roles for the epithelial cell, mast cell, and monocyte/macrophage network. Am. J. Physiol. Cell Physiol. 327, C1373–C1383. doi: 10.1152/ajpcell.00488.2024
Kretzler, B., König, H. H., and Hajek, A. (2022). Pet ownership, loneliness, and social isolation: a systematic review. Soc. Psychiatry Psychiatr. Epidemiol. 57, 1935–1957. doi: 10.1007/s00127-022-02332-9
Lee, E., Park, Y. M., Lee, S. Y., Lee, S. H., Park, M. J., Ahn, K., et al. (2023). Associations of prenatal antibiotic exposure and delivery mode on childhood asthma inception. Ann. Allergy Asthma Immunol. 131, 52–58.e1. doi: 10.1016/j.anai.2023.03.020
Lee-Sarwar, K. A., Chen, Y. C., Chen, Y. Y., Kozyrskyj, A. L., Mandhane, P. J., Turvey, S. E., et al. (2023). The maternal prenatal and offspring early-life gut microbiome of childhood asthma phenotypes. Allergy 78, 418–428. doi: 10.1111/all.15516
Lee-Sarwar, K. A., Kelly, R. S., Lasky-Su, J., Zeiger, R. S., O'Connor, G. T., Sandel, M. T., et al. (2020). Fecal short-chain fatty acids in pregnancy and offspring asthma and allergic outcomes. J Allergy Clin Immunol Pract 8, 1100–2.e13. doi: 10.1016/j.jaip.2019.08.036
Li, K., Ran, X., Han, J., Ding, H., Wang, X., Li, Y., et al. (2025). Astragalus polysaccharide alleviates mastitis disrupted by Staphylococcus aureus infection by regulating gut microbiota and SCFAs metabolism. Int. J. Biol. Macromol. 286:138422. doi: 10.1016/j.ijbiomac.2024.138422
Lodge, C. J., Allen, K. J., Lowe, A. J., Hill, D. J., Hosking, C. S., Abramson, M. J., et al. (2012). Perinatal cat and dog exposure and the risk of asthma and allergy in the urban environment: a systematic review of longitudinal studies. Clin. Dev. Immunol. 2012:176484. doi: 10.1155/2012/176484
Loewen, K., Monchka, B., Mahmud, S. M., 't Jong, G., and Azad, M. B. (2018). Prenatal antibiotic exposure and childhood asthma: a population-based study. Eur. Respir. J. 52:1702070. doi: 10.1183/13993003.02070-2017
Luukkonen, J., Moustgaard, H., Martikainen, P., and Remes, H. (2024). Does having siblings really protect against childhood atopic diseases? A total population and within-family analysis. Eur. J. Epidemiol. 39, 289–298. doi: 10.1007/s10654-024-01104-w
Maguire, T. J. A., Yung, S., Ortiz-Zapater, E., Kayode, O. S., Till, S., Corrigan, C., et al. (2023). Sphingosine-1-phosphate induces airway smooth muscle hyperresponsiveness and proliferation. J. Allergy Clin. Immunol. 152, 1131–40.e6. doi: 10.1016/j.jaci.2023.05.028
Maher, S. E., O'Brien, E. C., Moore, R. L., Byrne, D. F., Geraghty, A. A., Saldova, R., et al. (2023). The association between the maternal diet and the maternal and infant gut microbiome: a systematic review. Br. J. Nutr. 129, 1491–1499. doi: 10.1017/S0007114520000847
Miller, R. L., Grayson, M. H., and Strothman, K. (2021). Advances in asthma: new understandings of asthma's natural history, risk factors, underlying mechanisms, and clinical management. J. Allergy Clin. Immunol. 148, 1430–1441. doi: 10.1016/j.jaci.2021.10.001
Nermes, M., Niinivirta, K., Nylund, L., Laitinen, K., Matomäki, J., Salminen, S., et al. (2013). Perinatal pet exposure, faecal microbiota, and wheezy bronchitis: is there a connection? ISRN Allergy 2013:827934. doi: 10.1155/2013/827934
Panzer, A. R., Sitarik, A. R., Fadrosh, D., Havstad, S. L., Jones, K., Davidson, B., et al. (2023). The impact of prenatal dog keeping on infant gut microbiota development. Clin. Exp. Allergy 53, 833–845. doi: 10.1111/cea.14303
Prior, E., Santhakumaran, S., Gale, C., Philipps, L. H., Modi, N., and Hyde, M. J. (2012). Breastfeeding after cesarean delivery: a systematic review and meta-analysis of world literature. Am. J. Clin. Nutr. 95, 1113–1135. doi: 10.3945/ajcn.111.030254
Rago, D., Rasmussen, M. A., Lee-Sarwar, K. A., Weiss, S. T., Lasky-Su, J., Stokholm, J., et al. (2019). Fish-oil supplementation in pregnancy, child metabolomics and asthma risk. EBioMedicine 46, 399–410. doi: 10.1016/j.ebiom.2019.07.057
Rowland, S. N., Green, C. G., Halliwill, J. R., Singanayagam, A., and Heaney, L. M. (2025). Gut feelings on short-chain fatty acids to regulate respiratory health. Trends Endocrinol. Metab. doi: 10.1016/j.tem.2024.12.007
Rutayisire, E., Huang, K., Liu, Y., and Tao, F. (2016). The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants' life: a systematic review. BMC Gastroenterol. 16:86. doi: 10.1186/s12876-016-0498-0
Sarkar, A., Yoo, J. Y., Valeria Ozorio Dutra, S., Morgan, K. H., and Groer, M. (2021). The association between early-life gut microbiota and long-term health and diseases. J. Clin. Med. 10:459. doi: 10.3390/jcm10030459
Sindi, A. S., Geddes, D. T., Wlodek, M. E., Muhlhausler, B. S., Payne, M. S., and Stinson, L. F. (2021). Can we modulate the breastfed infant gut microbiota through maternal diet? FEMS Microbiol. Rev. 45:fuab011. doi: 10.1093/femsre/fuab011
Sinha, T., Prins, J. R., Fernández-Pato, A., Kruk, M., Dierikx, T., de Meij, T., et al. (2024). Maternal antibiotic prophylaxis during cesarean section has a limited impact on the infant gut microbiome. Cell Host Microbe 32, 1444–54.e6. doi: 10.1016/j.chom.2024.07.010
Smejda, K., Polanska, K., Stelmach, W., Majak, P., and Stelmach, I. (2020). Dog keeping at home before and during pregnancy decreased the risk of food allergy in 1-year-old children. Postepy Dermatol. Alergol. 37, 255–261. doi: 10.5114/ada.2018.80584
Sockrider, M., and Fussner, L. (2020). What is asthma? Am. J. Respir. Crit. Care Med. 202, P25–P26. doi: 10.1164/rccm.2029P25
Sordillo, J. E., Korrick, S., Laranjo, N., Carey, V., Weinstock, G. M., Gold, D. R., et al. (2019). Association of the Infant gut Microbiome with Early Childhood Neurodevelopmental Outcomes: an ancillary study to the VDAART randomized clinical trial. JAMA Netw. Open 2:e190905. doi: 10.1001/jamanetworkopen.2019.0905
Stern, J., Pier, J., and Litonjua, A. A. (2020). Asthma epidemiology and risk factors. Semin. Immunopathol. 42, 5–15. doi: 10.1007/s00281-020-00785-1
Stokholm, J., Thorsen, J., Blaser, M. J., Rasmussen, M. A., Hjelmsø, M., Shah, S., et al. (2020). Delivery mode and gut microbial changes correlate with an increased risk of childhood asthma. Sci. Transl. Med. 12:eaax9929. doi: 10.1126/scitranslmed.aax9929
Thorburn, A. N., McKenzie, C. I., Shen, S., Stanley, D., Macia, L., Mason, L. J., et al. (2015). Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 6:7320. doi: 10.1038/ncomms8320
Tun, H. M., Konya, T., Takaro, T. K., Brook, J. R., Chari, R., Field, C. J., et al. (2017). Exposure to household furry pets influences the gut microbiota of infant at 3-4 months following various birth scenarios. Microbiome 5:40. doi: 10.1186/s40168-017-0254-x
van Nimwegen, F. A., Penders, J., Stobberingh, E. E., Postma, D. S., Koppelman, G. H., Kerkhof, M., et al. (2011). Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J. Allergy Clin. Immunol. 128, 948–955.e1-3. doi: 10.1016/j.jaci.2011.07.027
Wampach, L., Heintz-Buschart, A., Fritz, J. V., Ramiro-Garcia, J., Habier, J., Herold, M., et al. (2018). Birth mode is associated with earliest strain-conferred gut microbiome functions and immunostimulatory potential. Nat. Commun. 9:5091. doi: 10.1038/s41467-018-07631-x
Wang, H., He, Y., Dang, D., Feng, L., Huang, L., Zhao, J., et al. (2024). Bifidobacterium animalis subsp. lactis CCFM1274 relieved allergic asthma symptoms by modifying intestinal tryptophan metabolism in mice. Food Funct. 15, 8810–8822. doi: 10.1039/D4FO01079E
Keywords: childhood asthma, gut microbiota, prenatal exposure, maternal exposure during pregnancy, wheeze
Citation: Ning Z, Zhang Y, Lu R, Zhao A, Wang Z, Yuan J and Li S (2025) The association between prenatal exposure and childhood asthma: the mediating role of gut microbiota. Front. Microbiol. 16:1664708. doi: 10.3389/fmicb.2025.1664708
Edited by:
Merih Cetinkaya, University of Health Sciences, TürkiyeReviewed by:
Ye Peng, The Chinese University of Hong Kong, ChinaCopyright © 2025 Ning, Zhang, Lu, Zhao, Wang, Yuan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shenghui Li, bHNoOTkwN0AxNjMuY29t; c3VibWlzc2lvbjk5MDdAMTYzLmNvbQ==
 Zidi Ning1
Zidi Ning1 Anda Zhao
Anda Zhao Shenghui Li
Shenghui Li