- 1College of Resources and Environmental Sciences, Nanjing Agricultural University, Nanjing, Jiangsu, China
- 2North China Power Engineering Co., Ltd. of China Power Engineering Consulting Group, Beijing, China
- 3Jiangsu Provincial Key Lab for Organic Solid Waste Utilization, Nanjing Agricultural University, Nanjing, China
Phosphite serves as an alternative phosphorus material in terrestrial ecosystems. Aspergillus niger (A. niger), a prominent phosphate-solubilizing fungus (PSF), facilitates phosphorus and leaches heavy metal ions via organic acids and enzymes. With the synergistic effect of phosphate materials, heavy metal ions can be effectively immobilized by A. niger to achieve remediation of contaminated soils. This study investigated the structural distinctions between phosphite and phosphate compounds by using ATR-IR and Raman spectroscopy, while concurrently assessing the physiological impact of phosphite on A. niger. After incubation with phosphite, the average fungal biomass and acid phosphatase activities were reduced by approximately 50% with respect to phosphate. These results demonstrated a significant inhibitory effect of phosphite on PSF functionality. This inhibition likely stems from fundamental differences in the molecular structures of phosphite and phosphate, which influence their biochemical interactions. The observed suppression underscores the limited evolutionary adaptation of organisms to phosphite detoxification or metabolic assimilation. Consequently, phosphate persists as the dominant bioavailable phosphorus form on Earth. Finally, this induces its geological abundance and the lower metabolic cost for assimilation.
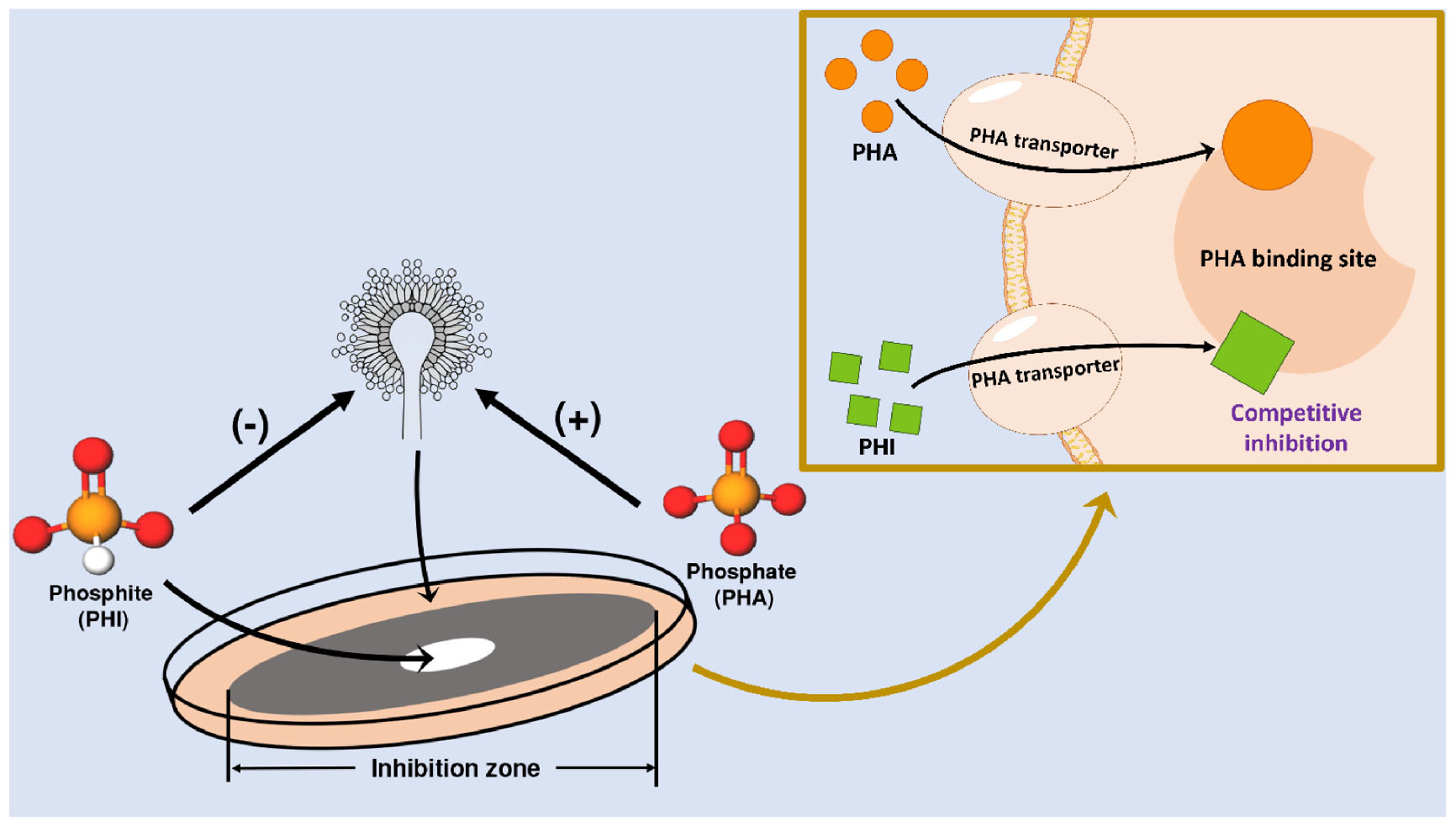
Graphical Abstract. Schematic representation of the distinct effects of phosphate versus phosphite on Aspergillus niger and the proposed mechanisms mediating these responses.
1 Introduction
Phosphorus (P) is a critical element for the metabolism of organisms (da Silva et al., 2023). While it predominantly exists as the +5 oxidation state in phosphate (PHA), P can also appear as other reduced forms, e.g., hypophosphite (+1), phosphite (PHI, +3) and hypophosphate (+4) (Morton and Edwards, 2005; Bindi et al., 2023). As a typical reduce P species, PHI is widely distributed in terrestrial and aquatic systems, including soils, freshwater, and marine environments (Hanrahan et al., 2005; Stone and White, 2012; Han et al., 2013; Pasek et al., 2014). Natural sources of PHI include meteorites, lightning strikes, volcanic eruption and microbial activity, whereas its anthropogenic sources are related to industrial production or use of relevant products (Morton and Edwards, 2005; Pasek et al., 2013; Liu et al., 2023). It is estimated that PHI concentration ranges from 0.1 to 1.3 μM in different environment, though levels may exceed 10 μM or even higher in some reduction conditions underground (Figueroa and Coates, 2017; Liu et al., 2023). PHI has been marketed for decades as the active ingredient released after hydrolysis of organic phosphonates and has emerged as an eco-friendly compound that bolsters plant resilience to abiotic and biotic stresses (Lambers et al., 2013; Koyukan et al., 2025).
The three-dimensional structure of PHI molecule is a balanced tetrahedron like PHA molecule, differing by the substitution of one oxygen atom with hydrogen (Tapia-Torres et al., 2016). Structural distinctions between PHI and PHA lead to significant variations in their solubility and ionic properties. PHI is about 1000 times more soluble than PHA in water (Figueroa and Coates, 2017). This enhanced solubility facilitates its practical use in agriculture, where PHI is typically administered to plants via aqueous solutions. Unlike PHA, which serves as a primary P fertilizer to boost crop yields, PHI is predominantly utilized as a fungistatic agent (Gómez-Merino and Trejo-Téllez, 2015; Achary et al., 2017). PHI combats phytopathogens by directly inhibiting fungal hyphal development and indirectly triggering systemic inducible resistance pathways in plants (Thao and Yamakawa, 2009; Mohammadi et al., 2021; Hunter et al., 2024). For instance, PHI treatment increased survival rates of papaya plants infected by Phytophthora palmivora to 93%, compared to 24% in untreated controls (Vawdrey and Westerhuis, 2007). PHI can inhibit phytopathogens particularly those belonging to the oomycetes (Phytophthora spp., Pythium spp.) (McDonald et al., 2001; Achary et al., 2017; Manghi et al., 2021). However, in the absence of PHA, where PHI is provided as the sole P source for plants, PHI usually shows negative effects on plant growth (Schroetter et al., 2006; Thao et al., 2008; Ratjen and Gerendás, 2009).
Aspergillus niger (A. niger) is one sizeable genus belonging to Aspergillaceae family. Owing to superior environmental adaptability and stress tolerance, A. niger is widespread across diverse environments (Yu et al., 2021). This fungus can produce various secondary metabolites, especially low molecular weight organic acids (Schneider et al., 2010). These metabolites acidify microenvironments to solubilize PHA minerals while simultaneously functioning as chelating agents to improve PO43– bioavailability (Khan et al., 2007; Klaic et al., 2021; Tian et al., 2021). Previous studies have demonstrated that A. niger can effectively leach heavy metals from contaminated soils or enhance plant uptake of heavy metals (Ren et al., 2009; Khan et al., 2019; Niu et al., 2021; Meng et al., 2024), which means A. niger plays a significant role in remediation of the contaminated soils. However, the influences of PHI on A. niger remain unexplored.
This study was aimed to characterize the response of A. niger to PHI exposure and quantitatively assess its effects. To comprehensively evaluate the influence of PHI, A. niger was cultured in both solid and liquid system.
2 Materials and methods
2.1 Fungal strain and chemicals
Aspergillus niger applied in this study has an accession number in China Center for Type Culture Collection (CCTCC) of M 2023240. The fungi were cultured on potato dextrose agar (PDA) at 28°C for 5 d to induce sporulation. After drenching the medium with sterile water, spores were scrapped from the surface and filtered through sterile gauze. The spore concentration was then determined and adjusted to 107 cfu/mL with 0.85% sterile saline.
Sodium phosphite dibasic pentahydrate (Na2HPO3⋅5H2O, analytical reagent, Shanghai Macklin Biochemical, Ltd., China) was configured to different concentrations of solution. Sodium dihydrogen phosphate dihydrate (NaH2PO4⋅2H2O, analytical reagent, Nanjing Chemical Reagent, Ltd., China) was selected as PHA source for the disk-diffusion experiment and spectroscopical test.
2.2 Incubation experiment
The fungistatic effects of PHI and PHA were assessed using disk-diffusion, plate culture and liquid culture assays. In the disk-diffusion assay, fungal suspensions (100 μL) were inoculated onto PDA plates, followed by application of 6 mm sterile disks impregnated with 10 μL of 105 μg/mL PHI or PHA solutions.
Six treatments were tested in plate cultures: Control (no PHI) and the solutions amended with PHI at 200, 400, 600, 800, or 1000 μg/mL (denoted as PHA@200PHI to PHA@1000PHI). The phosphate source in these solutions were originated from PDA. All treatments were performed in triplicate and incubated at 28°C for 5 d.
For liquid culture evaluation, PHI treatments were set consistent with the plate experiment (without agar). Spore suspensions (1 mL) were inoculated into the media and incubated at 28°C for 7 d with 180 rpm shaking. All the experiments were conducted in triplicate and all media were sterilized at 121°C for 20 min before experiments.
2.3 Chemical and microbial analyses
After 7 d of incubation, the liquid culture systems were filtered. The filtrate was prepared for pH and enzyme activity analysis. For the determination of biomass, the precipitate was carefully collected and subjected to a drying process at 65°C for 24 h in a controlled-temperature drying oven to ensure complete removal of residual moisture. After drying, the samples were weighed using an analytical balance with high precision to minimize measurement errors. The ACP (acid phosphatase) activity was analyzed by detection kit (ACP-1-W, Suzhou Keming Biotechnology Inc., China).
2.4 Instrumentation and data analyses
The growth of hypha and spores on agar media was observed by light microscope (LM, Olympus BX53, Japan). The pH value of liquid culture system was measured with an SG98 pH meter with an InLab Expert Pro-ISM-IP67 probe (Mettler Toledo Inc., USA). The data of ACP activity was recorded by a SpectraMax i3x Multi-Mode Microplate Reader (Molecular Devices, LLC., Austria) at 405 nm.
The attenuated total reflection fourier-transform infrared (ATR-IR) measurements were performed with a Thermo Scientific Nicolet iS5 Spectrometer (ThermoFisher Scientific Inc., USA) from 400 to 4000 cm–1. The recording was performed with 16 times scans for each sample at a spectral resolution of 4 cm–1. Raman spectra were obtained using Alpha 300 confocal Raman microscope (WITech, Germany). The spectral region of 100–4000 cm–1 was recorded using 473 nm laser.
One-way ANOVAs were used to test the datasets. Statistical significance was set at p < 0.05.
3 Results
3.1 Morphological evaluation of A. niger
The growth of A. niger cultured with PHA formed a dense, dark brown mycelial network (Figure 1A). However, when PHI was added, A. niger exhibited restricted growth across the entire culture medium, with black spore production occurring exclusively at the colony periphery (Figure 1B). Notably, A. niger maintained growth despite the antifungal treatment, due to P availability in the PDA media.
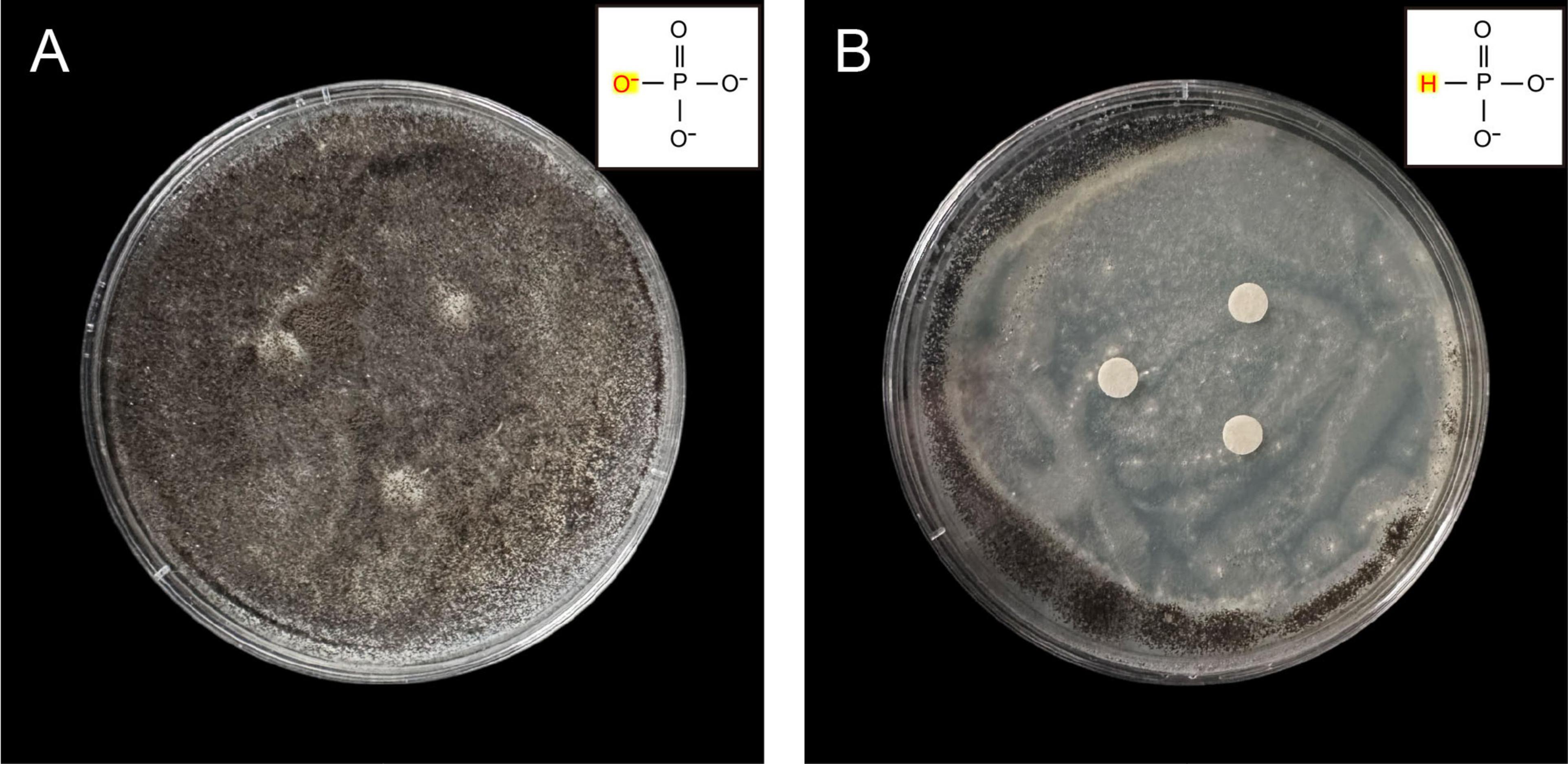
Figure 1. Antifungal activity by disk diffusion method of A. niger against PHI. The fungi suspension was inoculated onto plates using the spread plate method. (A) A. niger grown on PDA medium amended with 105 μg/mL PHA, (B) A. niger grown on PDA medium amended with 105 μg/mL PHI.
Under LM, six distinct A. niger colonies could be observed in the PHI-free environment (Figure 2A). After PHI addition, the colony count decreased to one (Figures 2B–D). With increasing PHI concentration, we detected enhanced spore aggregation (Figure 2E). Figure 2F revealed a distinct colonial growth pattern characterized by sparse mycelial networks and compact spore clusters.
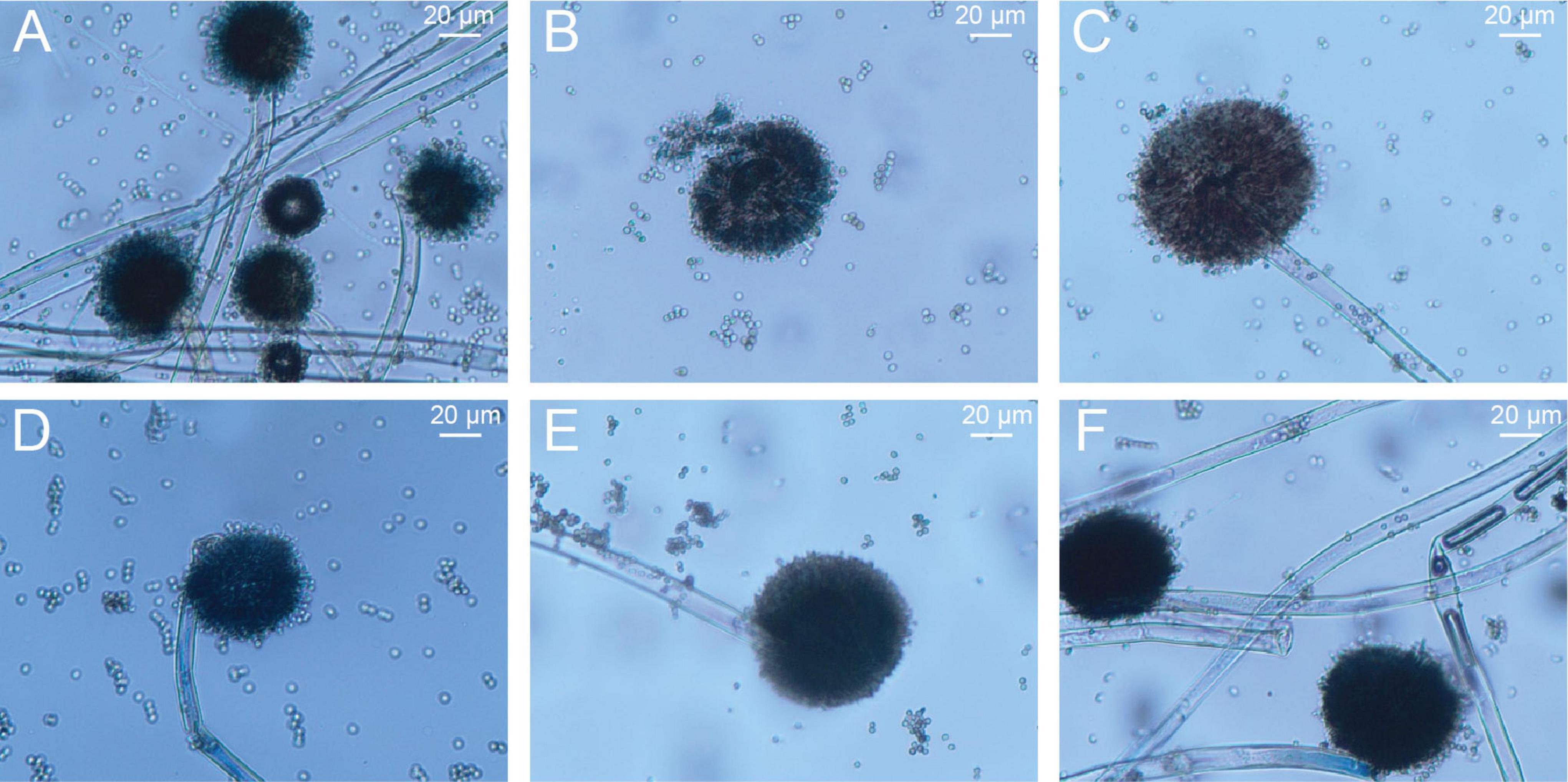
Figure 2. The growth of A. niger mycelia and spores on PDA solid culture media amended with different concentrations of PHI under light microscopy. (A–F) the corresponding concentrations of PHI were 0, 200, 400, 600, 800 and 1000 μg/mL.
3.2 ATR-IR and Raman analysis
PO43– ions can be mainly characterized by two types of vibrations, i.e., bending vibrations of O-P-O fragment (390-600 cm–1) and stretching vibrations of P-O (1000-1300 cm–1) (Ma et al., 2005; Jastrzêbski et al., 2011). In Figure 3, the ATR-IR and Raman spectra of PHA both contained the main bands originating from the above vibrations. The characteristic P-O-H stretching vibration observed at 860 cm–1 was served as a feature band for PHA in comparison with PHI samples (Kolandaivel et al., 1993; Oliveira et al., 2021).
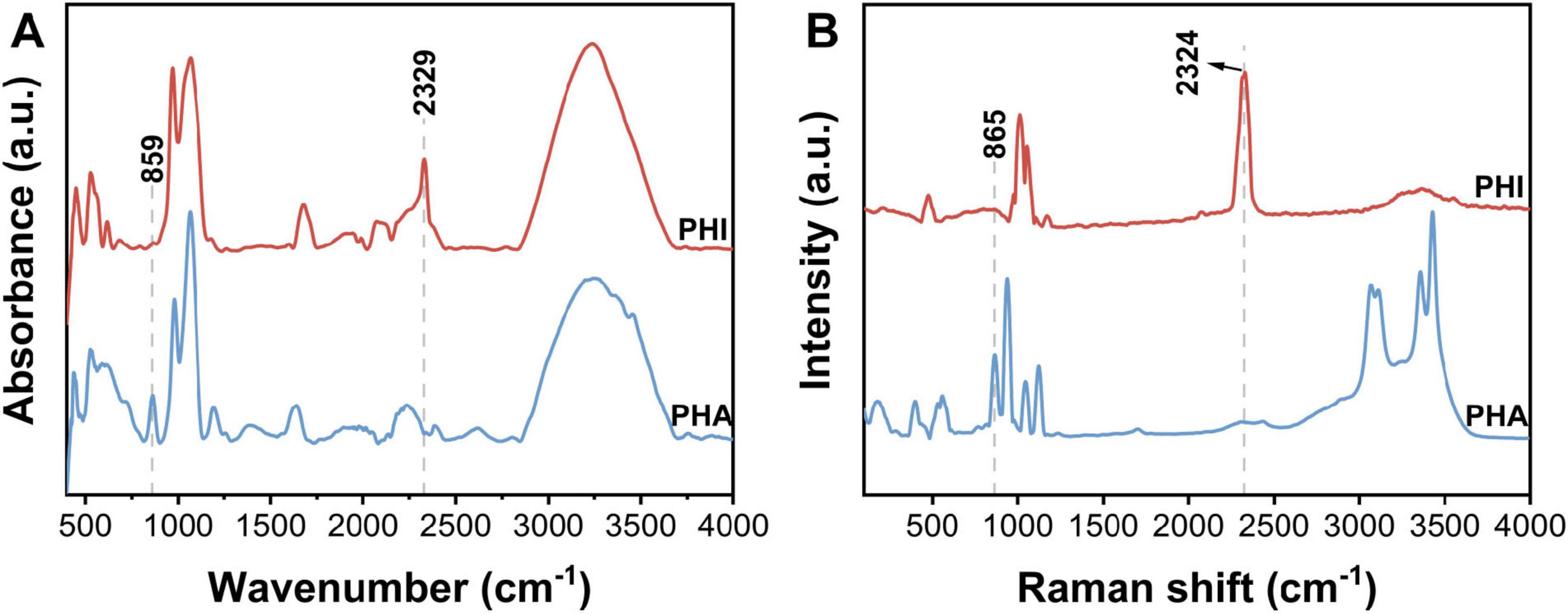
Figure 3. (A) ATR-IR and (B) Raman spectra of sodium phosphite dibasic pentahydrate (Na2HPO3⋅5H2O, PHI) and sodium dihydrogen phosphate dihydrate (NaH2PO4⋅2H2O, PHA). Data has been preprocessed with smoothing and baseline normalization.
Stretching vibrations and deformation modes of HPO32– anion are mainly divided to the following modes in IR spectrum, i.e., deformations of PO3 group (450-600 cm–1), stretching vibrations of PO3 group (967 cm–1), deformations of P-H (1031, 1063 cm–1), and stretching vibrations of P-H (2329 cm–1) (Chung et al., 2005; Ma et al., 2005; Kovrugin et al., 2017; Panicker, 2023). PHI exhibited coincident spectral bands in both Raman and IR across analogous wavenumber regions. Spectral analysis revealed that both PHA and PHI demonstrated overlapping vibrational features near 1000 cm–1, so the distinct band centered at ∼2300 cm–1 provided a diagnostic spectroscopic signature specific to PHI.
3.3 Physiological analysis
The pH showed a significant increase with increasing PHI concentration (Figure 4B). The initial pH value of the media was 6.87, and initial pH of the system rose above 7 after adding PHI. After incubation, the pH of medium of control group was 1.49. In the treatment of PHA@200PHI, the pH slightly increased to 1.54. With increasing PHI concentration, PHA@800PHI and PHA@1000PHI, the pH value ultimately stabilized at approximately 1.65. These suggested that PHI inhibited the secretion of organic acids.
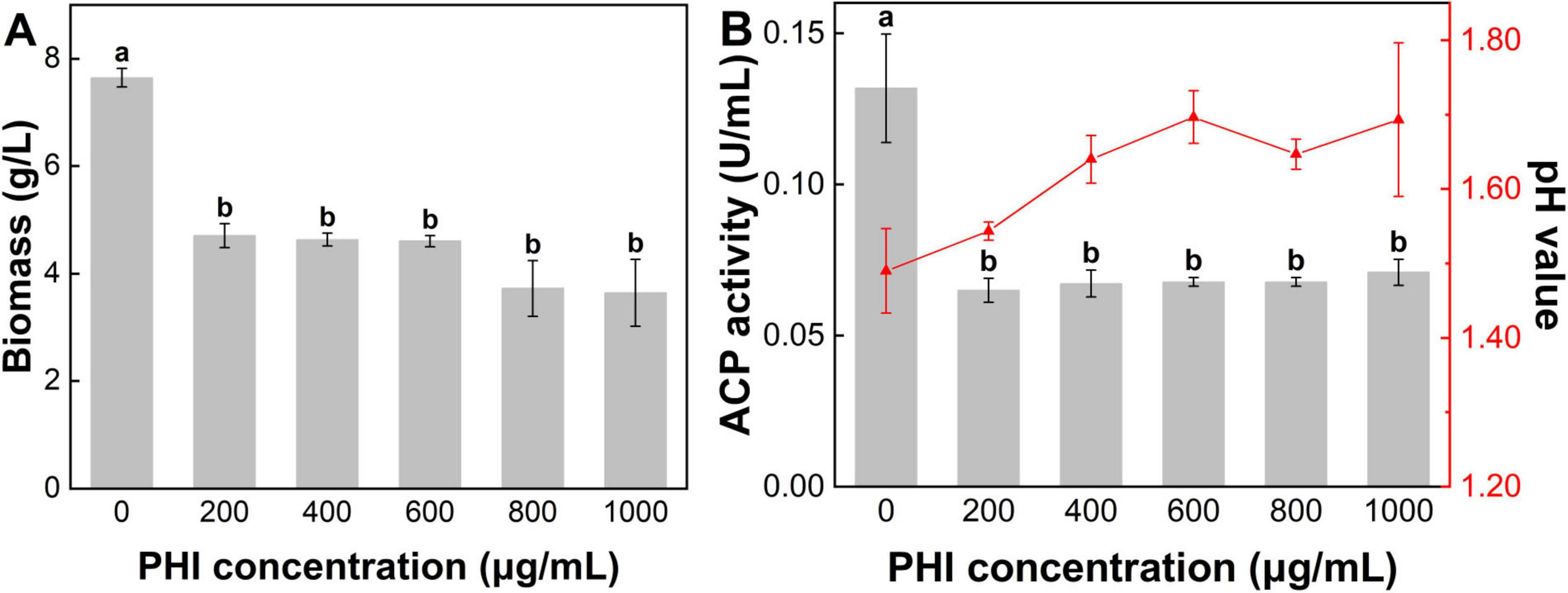
Figure 4. (A) The fungi biomass, (B) pH value and activity of acid phosphatase in the media after 7 d incubation. Lower-case letters above the bar indicate the significant differences among different PHI addition treatment (p < 0.05). Error bars represent the standard error.
The microbial biomass measurements revealed significant variations among the experimental groups (Figure 4A). PHA group exhibited the highest biomass value at 7.65 g/L. Increasing the PHI concentration led to a gradual reduction in biomass. The biomass value of PHA@200PHI recorded 4.70 g/L. Slightly lower biomass value was observed in PHA@400PHI (4.63 g/L) and PHA@600PHI (4.60 g/L). Moreover, a decline occurred in PHA@800PHI (3.73 g/L) and PHA@1000PHI (3.65 g/L). Notably, the biomass of control group was approximately 79.58% higher than the average of the remaining groups (4.26 g/L).
ACP is a class of diverse enzymes catalyzing the P metabolism, which reflects the activity level of A. niger. In the treatment of PHA, A. niger exhibited the highest ACP activity (0.13 U/mL). A sharp decline in ACP activity occurred with the addition of PHI, with values stabilizing around 0.06–0.07 U/mL (Figure 4B). However, elevated PHI concentration did not appear to significantly enhance its inhibitory effect.
4 Discussion
PHI can significantly suppress mycelial growth of A. niger. Even in PHA@200PHI group, significant inhibition can be observed. However, the growth of A. niger was inhibited but not completely suppressed. In addition, there were no significant differences in ACP activity between PHI treatment group. These findings collectively support the point that PHI exhibits fungistatic rather than fungicidal properties.
As a substitute material for PHA, PHI has significant differences in its effect on A. niger compared with PHA. The distinct effects of PHI and PHA can be fundamentally attributed to differences in their molecular structures. Spectroscopic analysis was performed to identify structural fingerprints of PHA and PHI that may underlie their differential effects on microbial activity (Figure 3). PHI can be easily absorbed by organisms through PHA transporter due to their similarity (Jost et al., 2015; Achary et al., 2017). Nevertheless, PHI cannot participate in P metabolism in cells but affect normal P metabolism. Phosphorylases have binding sites to PHA anion, while PHI anion can compete with other ligands for the sites (Martin et al., 1998). PHI has only one face of the tetrahedron, relatively similar to all the faces of the PHA tetrahedron. When PHI binds to the enzyme, it is the P-H that protrudes from the enzyme surface, rather than P-O. Therefore, PHI is biologically incompatible with the metabolic processes mediated by PHA (McDonald et al., 2001). Previous studies have generally indicated this process, i.e., the inhibitory site of PHI on microorganisms is related to relevant enzymes in the metabolic pathways (Barchietto et al., 1992; Stehmann and Grant, 2000). For example, PHI may act directly on phosphoribosyl diphosphate (PRPP) synthase, which is an important intermediate in adenylate synthesis (Griffith et al., 1990; Hove-Jensen et al., 2016). PHI occupies the position of PHA, and the P metabolism cannot proceed normally. Eventually, the levels of ATP and NAD are decreased and the growth of the organisms is inhibited (Niere et al., 1990). In addition, PHI might also interfere with the function of the cytoskeleton and cell wall synthesis (King et al., 2010).
Existing evidences indicate that prebiotic P geochemistry was dominated by reduced oxidation-state compounds, particularly PHI (Gulick, 1957; Li et al., 2025; Pasek, 2008). Some organisms from anoxic marine mud can perform DPO (dissimilatory phosphite oxidation) pathway, which means that they can utilize HPO32– as the sole electron donor and energy source, coupling its oxidation to cellular growth and replication (Walton et al., 2023). However, PHA exhibits higher thermodynamic stability under oxidizing conditions (Pasek and Lauretta, 2005). Following planetary oxygenation and biological diversification, PHI underwent progressive oxidation to PHA. Thus, this redox transition establishes the contemporary dominance of PHA in terrestrial P pools. This preference is also reflected in biomineralized tissues such as bones and teeth, which incorporate P exclusively in its +5 oxidation state. It is notable that PHI assimilation pathways are absent in most extant organisms (Loera-Quezada et al., 2015), potentially explaining its cytotoxic effects when it serves as the only P source for organisms. Many reduced P cycling genes are only expressed in microbial communities when more bioavailable forms of P limited (Boden et al., 2024). Microbial utilization of PHA as a P source is favored by their environmental abundance, chemical stability, and the lower energy demand. This evolutionary selection reflects an optimization for P metabolic efficiency, leaving PHI as a marginal relic in modern ecosystems.
5 Conclusion
The results demonstrate that PHI exerts significant inhibitory effects on the growth and sporulation of A. niger, a model phosphorus-solubilizing fungus. This finding suggests potential applications of alternative phosphate materials in fungal control strategies. However, the potential for efficiently utilization of PHI requires further investigation. Subsequent research should evaluate the dynamic responses to better understand the long-term efficacy of PHI as an inhibitory agent to functional fungi.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YW: Formal analysis, Investigation, Visualization, Writing – original draft. KD: Data curation, Investigation, Writing – original draft. JJ: Methodology, Visualization, Writing – original draft. MX: Data curation, Investigation, Writing – review & editing. SY: Methodology, Supervision, Writing – review & editing. YF: Formal analysis, Validation, Writing – review & editing. DY: Project administration, Writing – review & editing. ZL: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Key R&D Program of China (2023YFC3707600).
Acknowledgments
We thank Research Institute of Petroleum Exploration and Development for the technical assistance with Raman spectroscopy test.
Conflict of interest
DY was employed by North China Power Engineering Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Achary, V. M. M., Ram, B., Manna, M., Datta, D., Bhatt, A., Reddy, M. K., et al. (2017). Phosphite: A novel P fertilizer for weed management and pathogen control. Plant Biotechnol. J. 15, 1493–1508. doi: 10.1111/pbi.12803
Barchietto, T., Saindrenan, P., and Bompeix, G. (1992). Physiological responses of Phytophthora citrophthora to a subinhibitory concentration of phosphonate. Pesticide Biochem. Physiol. 42, 151–166. doi: 10.1016/0048-3575(92)90062-5
Bindi, L., Feng, T., and Pasek, M. A. (2023). Routes to reduction of phosphate by high-energy events. Commun. Earth Environ. 4:70. doi: 10.1038/s43247-023-00736-2
Boden, J. S., Zhong, J., Anderson, R. E., and Stüeken, E. E. (2024). Timing the evolution of phosphorus-cycling enzymes through geological time using phylogenomics. Nat. Commun. 15:3703. doi: 10.1038/s41467-024-47914-0
Chung, U. C., Mesa, J. L., Pizarro, J. L., Jubera, V., Lezama, L., Arriortua, M. I., et al. (2005). Mn(HPO3): A new manganese (II) phosphite with a condensed structure. J. Solid State Chem. 178, 2913–2921. doi: 10.1016/j.jssc.2005.06.038
da Silva, L. I., Pereira, M. C., de Carvalho, A. M. X., Buttrós, V. H., Pasqual, M., and Dória, J. (2023). Phosphorus-solubilizing microorganisms: A key to sustainable agriculture. Agric. Basel 13:30. doi: 10.3390/agriculture13020462
Figueroa, I. A., and Coates, J. D. (2017). Microbial phosphite oxidation and its potential role in the global phosphorus and carbon cycles. Adv. Appl. Microbiol. 98, 93–117. doi: 10.1016/bs.aambs.2016.09.004
Gómez-Merino, F. C., and Trejo-Téllez, L. I. (2015). Biostimulant activity of phosphite in horticulture. Sci. Hortic. 196, 82–90. doi: 10.1016/j.scienta.2015.09.035
Griffith, J. M., Smillie, R. H., and Grant, B. R. (1990). Alterations in nucleotide and pyrophosphate levels in Phytophthora palmivora following exposure to the antifungal agent potassium phosphonate (phosphite). J. General Microbiol. 136, 1285–1291. doi: 10.1099/00221287-136-7-1285
Gulick, A. (1957). Phosphorus and the origin of life. Ann. N. Y. Acad. Sci. 69, 309–313. doi: 10.1111/j.1749-6632.1957.tb49666.x
Han, C., Geng, J., Ren, H., Gao, S., Xie, X., and Wang, X. (2013). Phosphite in sedimentary interstitial water of Lake Taihu, a Large eutrophic shallow lake in China. Environ. Sci. Technol. 47, 5679–5685. doi: 10.1021/es305297y
Hanrahan, G., Salmassi, T. M., Khachikian, C. S., and Foster, K. L. (2005). Reduced inorganic phosphorus in the natural environment: Significance, speciation and determination. Talanta 66, 435–444. doi: 10.1016/j.talanta.2004.10.004
Hove-Jensen, B., Andersen Kasper, R., Kilstrup, M., Martinussen, J., Switzer Robert, L., and Willemoës, M. (2016). Phosphoribosyl diphosphate (PRPP): Biosynthesis, enzymology, utilization, and metabolic significance. Microbiol. Mol. Biol. Rev. 81:e00040-16. doi: 10.1128/mmbr.00040-16
Hunter, S., Waipara, N., Burns, B., Scott, P., and Williams, N. (2024). Impacts of phosphite treatment on Phytophthora community assemblages and inoculum abundances in Phytophthora-infected forest soil. Trees For. People 18:100687. doi: 10.1016/j.tfp.2024.100687
Jastrzêbski, W., Sitarz, M., Rokita, M., and Bułat, K. (2011). Infrared spectroscopy of different phosphates structures. Spectrochim Acta A Mol. Biomol. Spectroscopy 79, 722–727. doi: 10.1016/j.saa.2010.08.044
Jost, R., Pharmawati, M., Lapis-Gaza, H. R., Rossig, C., Berkowitz, O., Lambers, H., et al. (2015). Differentiating phosphate-dependent and phosphate-independent systemic phosphate-starvation response networks in Arabidopsis thaliana through the application of phosphite. J. Exp. Bot. 66, 2501–2514. doi: 10.1093/jxb/erv025
Khan, I., Aftab, M., Shakir, S., Ali, M., Qayyum, S., Rehman, M. U., et al. (2019). Mycoremediation of heavy metal (Cd and Cr)–polluted soil through indigenous metallotolerant fungal isolates. Environ. Monit. Assess. 191:585. doi: 10.1007/s10661-019-7769-5
Khan, M. S., Zaidi, A., and Wani, P. A. (2007). Role of phosphate-solubilizing microorganisms in sustainable agriculture — A review. Agron. Sustainable Dev. 27, 29–43. doi: 10.1051/agro:2006011
King, M., Reeve, W., Van der Hoek, M. B., Williams, N., McComb, J., O’Brien, P. A., et al. (2010). Defining the phosphite-regulated transcriptome of the plant pathogen Phytophthora cinnamomi. Mol. Genet. Genomics 284, 425–435. doi: 10.1007/s00438-010-0579-7
Klaic, R., Guimarães, G. G. F., Giroto, A. S., Bernardi, A. C. C., Zangirolami, T. C., Ribeiro, C., et al. (2021). Synergy of Aspergillus niger and components in biofertilizer composites increases the availability of nutrients to plants. Curr. Microbiol. 78, 1529–1542. doi: 10.1007/s00284-021-02406-y
Kolandaivel, P., Parthipan, R., and Selvasekarapandian, S. (1993). Vibrational studies of magnesium hydrogen phosphate- and barium hydrogen phosphate crystals. Crystal Res. Technol. 28, 1139–1145. doi: 10.1002/crat.2170280818
Kovrugin, V. M., Colmont, M., Siidra, O. I., Krivovichev, S. V., Colis, S., and Mentré, O. (2017). The first lead cobalt phosphite, PbCo2(HPO3)3. Dalton Trans. 46, 12655–12662. doi: 10.1039/c7dt02279d
Koyukan, B., Ekim-Leventoglu, R., Turgut, A., Arikan-Abdulveli, B., Yildiztugay, E., and Ozfidan-Konakci, C. (2025). New insights into the responses of phosphite, as a plant biostimulator, on PSII photochemistry, gas exchange, redox state and antioxidant system in maize plants under boron toxicity. Plant Physiol. Biochem. 221:109605. doi: 10.1016/j.plaphy.2025.109605
Lambers, H., Ahmedi, I., Berkowitz, O., Dunne, C., Finnegan, P. M., Hardy, G. E., et al. (2013). Phosphorus nutrition of phosphorus-sensitive Australian native plants: Threats to plant communities in a global biodiversity hotspot. Conserv. Physiol. 1:cot010. doi: 10.1093/conphys/cot010
Li, Z., Kong, X., Zhang, Z., Tang, F., Wang, M., Zhao, Y., et al. (2025). The functional mechanisms of phosphite and its applications in crop plants. Front. Plant Sci. 16:1538596. doi: 10.3389/fpls.2025.1538596
Liu, W., Zhang, Y., Yu, M., Xu, J., Du, H., Zhang, R., et al. (2023). Role of phosphite in the environmental phosphorus cycle. Sci. Total Environ. 881:163463. doi: 10.1016/j.scitotenv.2023.163463
Loera-Quezada, M. M., Leyva-González, M. A., López-Arredondo, D., and Herrera-Estrella, L. (2015). Phosphite cannot be used as a phosphorus source but is non-toxic for microalgae. Plant Sci. 231, 124–130. doi: 10.1016/j.plantsci.2014.11.015
Ma, Y., Li, N., and Xiang, S. (2005). Synthesizing pure AlPO4-41 phase from the gels containing H3PO3 as the phosphorous source: A new reproducible route. Microporous Mesoporous Mater. 86, 329–334. doi: 10.1016/j.micromeso.2005.08.001
Manghi, M. C., Masiol, M., Calzavara, R., Graziano, P. L., Peruzzi, E., and Pavoni, B. (2021). The use of phosphonates in agriculture. Chemical, biological properties and legislative issues. Chemosphere 283:131187. doi: 10.1016/j.chemosphere.2021.131187
Martin, H., Grant, B. R., and Stehmann, C. (1998). Inhibition of inorganic pyrophosphatase by phosphonate—A site of action in Phytophthora spp.? Pesticide Biochem. Physiol. 61, 65–77. doi: 10.1006/pest.1998.2353
McDonald, A. E., Grant, B. R., and Plaxton, W. C. (2001). Phosphite (phosphorous acid): Its relevance in the environment and agriculture and influence on plant phosphate starvation response. J. Plant Nutr. 24, 1505–1519. doi: 10.1081/pln-100106017
Meng, L., Ding, K., Qiu, Y., Chen, Y., Huo, H., Yu, D., et al. (2024). Application of phosphogypsum and phosphate-solubilizing fungi to Pb remediation: From simulation to in vivo incubation. Sci. Total Environ. 933:173171. doi: 10.1016/j.scitotenv.2024.173171
Mohammadi, M. A., Cheng, Y., Aslam, M., Jakada, B. H., Wai, M. H., Ye, K. Z., et al. (2021). ROS and oxidative response systems in plants under biotic and abiotic stresses: Revisiting the crucial role of phosphite triggered plants defense response. Front. Microbiol. 12:21. doi: 10.3389/fmicb.2021.631318
Morton, S. C., and Edwards, M. (2005). Reduced phosphorus compounds in the environment. Crit. Rev. Environ. Sci. Technol. 35, 333–364. doi: 10.1080/10643380590944978
Niere, J. O., Griffith, J. M., and Grant, B. R. (1990). 31P NMR studies on the effect of phosphite on Phytophthora palmivora. J. General Microbiol. 136, 147–156. doi: 10.1099/00221287-136-1-147
Niu, X., Wang, S., Zhou, J., Di, D., Sun, P., and Huang, D. (2021). Inoculation with indigenous rhizosphere microbes enhances aboveground accumulation of lead in salix integra thunb. by improving transport coefficients. Front. Microbiol. 12:686812. doi: 10.3389/fmicb.2021.686812
Oliveira, E. M., Rogero, M., Ferreira, E. C., and Gomes Neto, J. A. (2021). Simultaneous determination of phosphite and phosphate in fertilizers by Raman spectroscopy. Spectrochimica Acta A Mol. Biomol. Spectrosc. 246:119025. doi: 10.1016/j.saa.2020.119025
Panicker, L. (2023). 4-carboxyanilinium phosphite: A structural, vibrational, thermal and computational study. J. Mol. Struct. 1288:135773. doi: 10.1016/j.molstruc.2023.135773
Pasek, M. A. (2008). Rethinking early earth phosphorus geochemistry. Proc. Natl. Acad. Sci. U S A. 105, 853–858. doi: 10.1073/pnas.0708205105
Pasek, M. A., and Lauretta, D. S. (2005). Aqueous corrosion of phosphide minerals from iron meteorites: A highly reactive source of prebiotic phosphorus on the surface of the early Earth. Astrobiology 5, 515–535. doi: 10.1089/ast.2005.5.515
Pasek, M. A., Harnmeijer, J. P., Buick, R., Gull, M., and Atlas, Z. (2013). Evidence for reactive reduced phosphorus species in the early Archean ocean. Proc. Natl. Acad. Sci. U S A. 110, 10089–10094. doi: 10.1073/pnas.1303904110
Pasek, M. A., Sampson, J. M., and Atlas, Z. (2014). Redox chemistry in the phosphorus biogeochemical cycle. Proc. Natl. Acad. Sci. U S A. 111, 15468–15473. doi: 10.1073/pnas.1408134111
Ratjen, A. M., and Gerendás, J. (2009). A critical assessment of the suitability of phosphite as a source of phosphorus. J. Plant Nutr. Soil Sci. 172, 821–828. doi: 10.1002/jpln.200800287
Ren, W.-X., Li, P.-J., Geng, Y., and Li, X.-J. (2009). Biological leaching of heavy metals from a contaminated soil by Aspergillus niger. J. Hazardous Materials 167, 164–169. doi: 10.1016/j.jhazmat.2008.12.104
Schneider, K. D., van Straaten, P., de Orduna, R. M., Glasauer, S., Trevors, J., Fallow, D., et al. (2010). Comparing phosphorus mobilization strategies using Aspergillus niger for the mineral dissolution of three phosphate rocks. J. Appl. Microbiol. 108, 366–374. doi: 10.1111/j.1365-2672.2009.04489.x
Schroetter, S., Angeles-Wedlel, D., Kreuzig, R., and Schnug, E. (2006). Effects of phosphite on phosphorus supply and growth of corn (Zea mays). Landbauforschung Volkenrode 56, 87–99.
Stehmann, C., and Grant, B. R. (2000). Inhibition of enzymes of the glycolytic pathway and hexose monophosphate bypass by phosphonate. Pesticide Biochem. Physiol. 67, 13–24. doi: 10.1006/pest.1999.2465
Stone, B. L., and White, A. K. (2012). Most probable number quantification of hypophosphite and phosphite oxidizing bacteria in natural aquatic and terrestrial environments. Arch. Microbiol. 194, 223–228. doi: 10.1007/s00203-011-0775-9
Tapia-Torres, Y., Rodriguez-Torres, M. D., Elser, J. J., Islas, A., Souza, V., Garcia-Oliva, F., et al. (2016). How to live with phosphorus scarcity in soil and sediment: Lessons from bacteria. Appl. Environ. Microbiol. 82, 4652–4662. doi: 10.1128/AEM.00160-16
Thao, H. T. B., and Yamakawa, T. (2009). Phosphite (phosphorous acid): Fungicide, fertilizer or bio-stimulator? Soil Sci. Plant Nutr. 55, 228–234. doi: 10.1111/j.1747-0765.2009.00365.x
Thao, H. T. B., Yamakawa, T., Myint, A. K., and Sarr, P. S. (2008). Effects of phosphite, a reduced form of phosphate, on the growth and phosphorus nutrition of spinach (Spinacia oleracea L.). Soil Sci. Plant Nutr. 54, 761–768. doi: 10.1111/j.1747-0765.2008.00290.x
Tian, J., Ge, F., Zhang, D., Deng, S., and Liu, X. (2021). Roles of phosphate solubilizing microorganisms from managing soil phosphorus deficiency to mediating biogeochemical P Cycle. Biology 10:158. doi: 10.3390/biology10020158
Vawdrey, L. L., and Westerhuis, D. (2007). Field and glasshouse evaluations of metalaxyl, potassium phosphonate, acibenzolar and tea tree oil in managing Phytophthora root rot of papaya in far northern Queensland, Australia. Australasian Plant Pathol. 36, 270–276. doi: 10.1071/ap07016
Walton, C. R., Ewens, S., Coates, J. D., Blake, R. E., Planavsky, N. J., Reinhard, C., et al. (2023). Phosphorus availability on the early Earth and the impacts of life. Nat. Geosci. 16, 399–409. doi: 10.1038/s41561-023-01167-6
Keywords: phosphite, phosphate, reduced phosphorus material, Aspergillus niger, inhibitory effect
Citation: Wang Y, Ding K, Ji J, Xu M, Yan S, Fan Y, Yu D and Li Z (2025) Contrasting the influences of phosphate and phosphite on growth of Aspergillus niger. Front. Microbiol. 16:1664730. doi: 10.3389/fmicb.2025.1664730
Received: 12 July 2025; Accepted: 31 July 2025;
Published: 14 August 2025.
Edited by:
Teng Zedong, University of Science and Technology Beijing, ChinaReviewed by:
Liangliang Zhang, Anhui Agricultural University, ChinaXin Zhao, Henan Normal University, China
Copyright © 2025 Wang, Ding, Ji, Xu, Yan, Fan, Yu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Yu, eXVkQG5jcGUuY29tLmNu; Zhen Li, bGl6aGVuQG5qYXUuZWR1LmNu
†These authors have contributed equally to this work
 Ying Wang1†
Ying Wang1† Dan Yu
Dan Yu Zhen Li
Zhen Li