- 1Graduate School, Guangxi University of Chinese Medicine, Nanning, Guangxi, China
- 2Ruikang Hospital, Guangxi University of Chinese Medicine, Nanning, Guangxi, China
The global prevalence of depression and anxiety continues to rise, with major depressive disorder and anxiety disorders estimated to affect approximately 3.1 and 4.8% of the world’s population. Yet current pharmacological treatments demonstrate limited efficacy. This limitation has spurred extensive research into alternative treatment methods. Emerging evidence highlights a complex correlation between gut microbiota (GM) imbalance and mental health disorders. Disruptions in GM may trigger or exacerbate symptoms of anxiety and depression by interfering with communication pathways between the gut and brain. These pathways include neural signaling through the vagus nerve, hormone regulation via the hypothalamic–pituitary–adrenal (HPA) axis, immune responses involving pro-inflammatory cytokines, and metabolic processes related to short-chain fatty acids (SCFAs). Preclinical studies and initial clinical trials indicate promising results for therapeutic interventions targeting gut microbiota. Given that current evidence remains constrained by insufficient depth of understanding regarding underlying mechanisms, this review explores the intricate interactions among the gut microbiota, and brain, highlighting opportunities for advanced therapeutic approaches, focusing on probiotics, prebiotics, postbiotics, synbiotics, dietary modifications, fecal microbiota transplantation (FMT), fecal virome transplantation (FVT), and traditional Chinese medicine (TCM). It elucidates the role of gut microbiota in depression/anxiety and advances therapeutic approaches.
1 Introduction
Depression and anxiety are widespread psychological conditions affecting people globally, regardless of age or background (Meier et al., 2016). In recent years, the incidence of mental health disorders, particularly depression, has risen significantly, attracting increasing social attention (Fukuda et al., 2019). In 2020, major depressive disorder and anxiety disorders imposed an extremely heavy global disease burden, with approximately 49.4 million and 44.5 million disability-adjusted life years (DALYs) attributable to depressive disorders and anxiety disorders, respectively. Notably, the COVID-19 pandemic directly contributed to a surge in this burden, adding an estimated 10.7 million and 9.05 million DALYs for these conditions. Approximately 3.15% of the global population suffers from major depressive disorder, while 4.80% experiences anxiety disorders (COVID-19 Mental Disorders Collaborators, 2021). According to a report released by the World Health Organization, the incidence of anxiety disorders and major depressive disorder increased by approximately 26 and 28%, respectively, within just 1 year following the onset of the pandemic (World Health Organization, 2025). These figures underscore the significant impact of mental disorders on population health and emphasize the imperative to strengthen mental health management. Although new pharmacological treatments and psychotherapeutic approaches continue to develop, considerable gaps persist in the treatment landscape (Medina-Rodríguez et al., 2024). Therefore, exploring novel treatment options is necessary to address these issues. Extensive research has highlighted the crucial impact of gut microbiota on the etiology and treatment of depression and anxiety disorders (Chen et al., 2023).
The gut microbiota comprises diverse microbial communities inhabiting the digestive system (Gagnon et al., 2023). As scientific investigation into gut microbiota advances, researchers have discovered that this vast ecosystem of trillions of microorganisms within our digestive tract plays roles extending far beyond digestion. Emerging research demonstrates that these microorganisms significantly influence physical health and mental state (Mayer et al., 2014). Numerous studies have associated gut microbiota composition with the occurrence of anxiety and depression (Ye et al., 2022). Preclinical evidence strongly supports the existence of bidirectional communication among the brain, gastrointestinal system, and gut microbiota (Mayer et al., 2022). Although the exact mechanisms linking the gut and brain in depression remain unclear, there is an increasing awareness of the urgent need to investigate interactions among microbiota, the digestive system, and neural pathways in patients with depression (Chang et al., 2022; Matin and Dadkhah, 2024). By advancing understanding of these interactions, researchers can better develop innovative microbiota-based treatments for depression and anxiety (Liu et al., 2023).
Although extensive research has established the association between the gut microbiota and anxiety disorders and depression, the current knowledge gap in the field is shifting from “whether there is a correlation” to “how it functions” and “how to intervene.” This review aims to synthesize key evidence demonstrating how gut microbiota influence mental health, with a particular emphasis on integrating mechanistic explorations from animal models to human studies. It assesses the existing clinical evidence and translational potential of microbiota-targeted therapeutic strategies, thereby providing a clear theoretical foundation for developing novel microbiome-based treatments for psychiatric disorders.
2 The gut microbiota
Microorganisms are abundant in our environment, and even daily activities can influence our microbial communities (Lach et al., 2018). The human gastrointestinal tract harbors a diverse microbial community, including bacteria and fungi, collectively known as the intestinal microbiota. Throughout human evolution, these microorganisms have developed mutually beneficial symbiotic relationships with their hosts. As the host grows, develops, and undergoes physiological changes, the gut microbiota also evolves gradually (Adak and Khan, 2019). The high species diversity of GM provides functional redundancy within the ecosystem, enabling resilience and adaptability to environmental perturbations (Claesson et al., 2012). Specifically, inflammasome activation in patients with depression can exacerbate neuroinflammation, subsequently leading to reduced diversity and imbalance in GM. Therefore, further exploration and confirmation of the interaction between GM composition and these diseases is necessary (Rutsch et al., 2020).
Research indicates an intricate relationship between the GM and its host (Liu et al., 2022), involving numerous key physiological functions (Wang H. et al., 2022). These functions include immune system development and function, food digestion and energy absorption, intestinal endocrine function and nerve signaling, medication metabolism, endotoxin removal, and effects on host mental health (Fan and Pedersen, 2021; Shanahan et al., 2021).
3 The microbiota–gut–brain axis
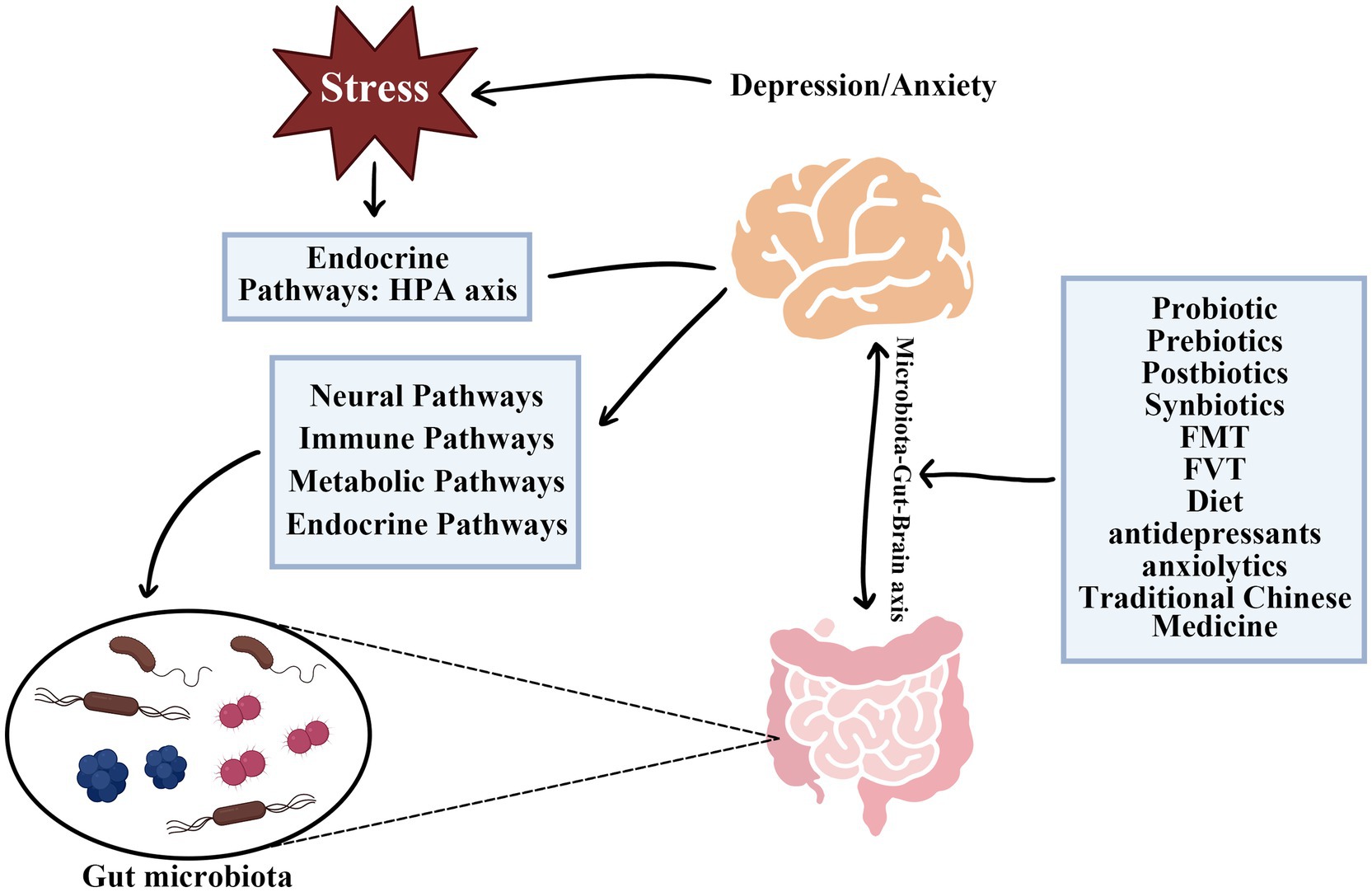
The Microbiota-Gut-Brain (MGB) axis is an intricate, bidirectional signaling system dynamically connecting the digestive tract and the brain. This connection significantly influences the functions and homeostasis of both systems (Thomaz et al., 2021). Interaction between intestinal microbiota and the nervous system occurs through multiple barriers, including the intestinal epithelial barrier (IEB), gut vascular barrier (GVB), blood–brain barrier (BBB), plexus vascular barrier (PVB), and blood-cerebrospinal fluid barrier (B-CSF) (Carloni and Rescigno, 2023). Communication within the MGB axis involves dynamic interactions among metabolic, endocrine, neural, and immune systems, highlighting the complexity of gut-brain connections (Góralczyk-Bińkowska et al., 2022). Specific mechanisms of the MGB axis include neural pathways, endocrine pathways (HPA axis regulation), immune pathway, and metabolic pathways (Góralczyk-Bińkowska et al., 2022; Alli et al., 2022; Borkent et al., 2022). Signal transmission in the MGB axis occurs mainly via these neural, endocrine, immune, and metabolic pathways (Dacaya et al., 2025; Figure 1).
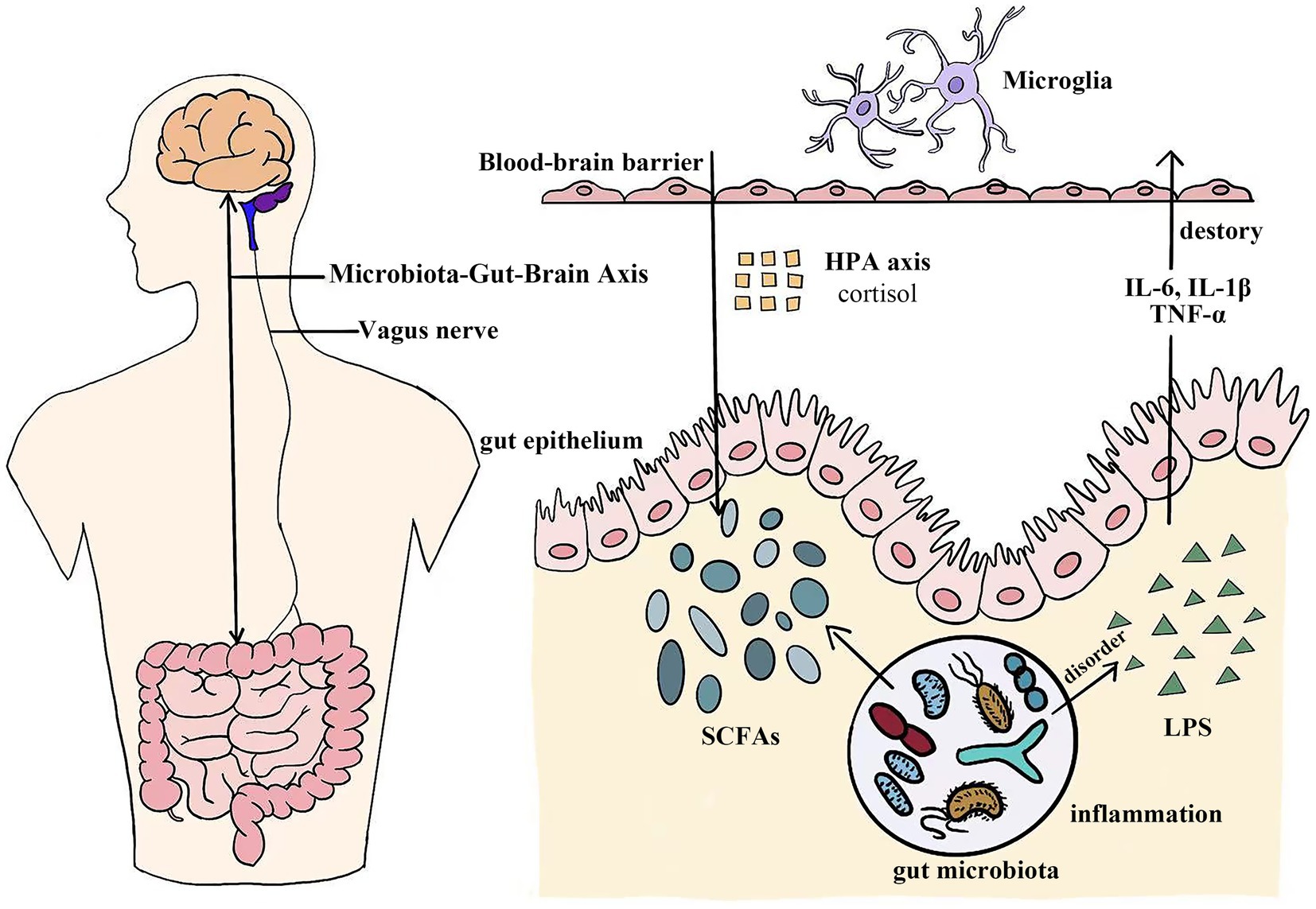
Figure 1. The microbiota-gut-brain axis functions as a sophisticated bidirectional channel connecting the digestive system and the central nervous system. Complex biological pathways, including neural, endocrine, immune, and metabolic pathways, facilitate this dynamic interaction. SCFAs, Short-chain fatty acids; LPS, Lipopolysaccharide; IL-6, Interleukin-6; IL-1β, Interleukin-1β; TNF-α, Tumor necrosis factor-α.
3.1 Key pathways connecting GM and the brain
3.1.1 Neural pathways
One critical neural pathway is the vagus nerve, which acts as a primary conduit for signal transmission between the gut and brain, influencing emotional regulation and stress responses (Bonaz et al., 2018). Animal studies indicate that vagus nerve stimulation (VNS) effectively alleviates depression, playing a pivotal role in stress modulation and mood regulation (Breit et al., 2018). Research has demonstrated that repetitive VNS (rVNS) produces anxiolytic effects through the noradrenergic pathway. This treatment is associated with α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)-mediated excitatory neurotransmission in the central amygdala (CeL), achieved via AMPAR transport activation (Zhang et al., 2022; Toader et al., 2024). Microglia, the primary immune cells of the central nervous system (CNS), initiate immune responses when recognizing neurotransmitter signals (Gao H. et al., 2022; Agirman et al., 2021). Recent studies suggest that gut microbiota in animals experiencing stress activate microglia in the dentate gyrus. This response may interfere with hippocampal neurogenesis and increase depression-related behaviors (He et al., 2024). GM significantly affect brain function through the synthesis of neurotransmitters such as serotonin and gamma-aminobutyric acid (GABA), which are essential for maintaining emotional balance (Dinan and Cryan, 2020). VNS therapy also reduces peripheral blood TNF-α levels, which are elevated in depressed patients (Toader et al., 2024; Koopman et al., 2016). Brain-derived neurotrophic factors (BDNF), as a growth factor associated with the gut microbiota, can be influenced by the gut microbiota through neuroendocrine pathways to regulate its expression, thereby modulates hippocampal neurogenesis and contributes to the onset and progression of depression (Han et al., 2025). Thus, neural modulation represents an innovative clinical approach to treating depression and anxiety.
3.1.2 Endocrine pathways
The HPA axis is a central mediator of the body’s stress response and is significantly influenced by the gut microbiota (Bear et al., 2021). The hippocampus plays a key role in regulating the HPA axis, primarily by controlling corticotropin-releasing hormone (CRH) secretion. This effect is mainly exerted by regulating the hypothalamic paraventricular nucleus (PVN), which prompts the anterior pituitary gland to secrete adrenocorticotropic hormone (ACTH). ACTH subsequently induces glucocorticoid (cortisol in humans) secretion from the adrenal cortex (Misiak et al., 2020; Ding et al., 2024). Cortisol, a glucocorticoid produced by the adrenal cortex (Rehman et al., 2022), increases in response to gut microbiota alterations affecting the HPA axis (Misiak et al., 2020). These hormones significantly influence anxiety and depression (Martin et al., 2019; Sun et al., 2022). Chronic stress negatively affects gut microbiota composition, elevating cortisol levels and exacerbating anxiety and depression. This cascade results from dysfunction of the HPA axis, disturbing gut bacterial balance (Hassamal, 2023). Additionally, gut microbiota modulates neuropeptide and hormone secretion, including mood-regulating hormones such as ghrelin and leptin (Zheng et al., 2016).
3.1.3 Immune pathways
The gut microbiota in healthy individuals typically remains balanced and stable. When pathogens invade and disrupt host-microbial homeostasis, dysbiosis occurs, increasing intestinal barrier permeability (“leaky gut”). This increased permeability allows microbial metabolites, toxins, and pathogens to enter circulation, triggering systemic inflammation (Alhasson et al., 2017; Kociszewska and Vlajkovic, 2022). Intestinal permeability is intricately linked to systemic inflammation and psychiatric disorders (Ma et al., 2022). The enteric nervous system (ENS) plays a crucial role in modulating intestinal immune functions by mediating associated immune responses (Lu et al., 2024). Further research indicates that immune responses are closely associated with neuroinflammation, a major contributor to mood disorders (Sittipo et al., 2022). During inflammatory responses, the body produces numerous pro-inflammatory cytokines. These cytokines, upon entering the brain, modulate neural circuits and neurotransmitter systems related to mood, potentially causing depressive symptoms (Zhu R. et al., 2024). Disruption of gut microbiota also increases lipopolysaccharide (LPS) production (Li W. et al., 2020). LPS-induced inflammation can provoke depressive symptoms through the release of pro-inflammatory cytokines and inflammatory mediators (Shi et al., 2019). Consequently, LPS exacerbates inflammation and disrupts the blood–brain barrier (Logsdon et al., 2020). LPS and similar molecules activate immune cells to release pro-inflammatory cytokines (e.g., IL-6, IL-1β, TNF-α), which directly contribute to depression onset (Medina-Rodriguez et al., 2018; Simpson et al., 2021). Additionally, GM imbalance and HPA axis dysfunction mutually influence each other, jointly promoting inflammation (Warren et al., 2024). Microglia, the primary resident macrophages of the CNS, play a key role in brain immune defense (Thangaraj et al., 2018). The gut microbiota may impact microglia via inflammatory signaling along the MGB axis, affecting brain function and modulating depressive-like behaviors (Agirman et al., 2021; Donoso et al., 2023). Studies indicate an increased prevalence of depression and anxiety among patients with ulcerative colitis (UC). Preventive anti-inflammatory treatment effectively alleviates depressive behaviors and reduces systemic inflammatory cytokine levels in colitis models (Yuan et al., 2021).
3.1.4 Metabolic pathways
Key metabolites of the intestinal microbiota, SCFAs, including butyrate, acetate, and propionate (Dong et al., 2022). SCFAs can cross the BBB, providing neuroprotection by regulating neuroinflammation and enhancing barrier integrity (Dalile et al., 2019). SCFAs predominantly inhibit histone deacetylase (HDAC) activity, thereby dampening inflammation and mitigating overzealous immune reactions in microglia, or triggering free fatty acid receptors (e.g., FFAR2/3), to augment microglial activation (Cao et al., 2025). Human trials indicate that an almond-based low-carbohydrate diet (a-LCD) promotes the growth of SCFA-producing gut microbiota, thereby increasing SCFA production, activating the GPR43 receptor, and enhancing GLP-1 secretion to alleviate depressive symptoms (Ren et al., 2020). Butyrate is essential for maintaining intestinal barrier function in mammals. Reduced butyrate levels may trigger depressive symptoms by disrupting gut-derived metabolite balance and altering the expression of G protein-coupled receptors (GPCRs) and BDNF in the CNS (Nohesara et al., 2023). Butyrate stimulates BDNF secretion (Romo-Araiza et al., 2018), a gene potentially associated with anxiety and depression (Chu et al., 2022). Research indicates that sodium butyrate alters gene expression in hippocampal microglia, thereby eliminating lipopolysaccharide-induced depressive-like behavior (Yamawaki et al., 2018). Valeric acid, another gut microbial metabolite, influences MGB axis metabolism (Lin et al., 2021), has demonstrated potential to modulate anxiety and depression (Mann et al., 2014; Bhandage et al., 2019). This finding offers new insights into treating related neurological conditions. Moreover, GM significantly contributes to tryptophan metabolism (Zhao et al., 2022), a crucial precursor of serotonin (Emmerzaal et al., 2020), which profoundly affects mood and emotional regulation (Li Z. et al., 2020). During inflammatory states, tryptophan is metabolized by IDO1 (indoleamine 2,3-dioxygenase) into kynurenine, simultaneously producing neurotoxic metabolites that reduce 5-HT synthesis and promote depression and anxiety (Tahiri et al., 2024). Research indicates that patients who orally administered the probiotic B. breve CCFM1025 exhibited a significant increase in fecal concentrations of multiple tryptophan derivatives, such as 5-hydroxytryptophan (5-HTP). These changes in derivative concentrations were significantly correlated with improvements in patients’ depressive symptoms and gastrointestinal symptoms (Tian et al., 2022). Another metabolite, bile acids, showed in randomized controlled trials that elevated glycine-conjugated bile acids were significantly associated with decreased anxiety scores (HADA) (Martin et al., 2024). A study has demonstrated for the first time in humans that the probiotic strain LP299v can significantly reduce plasma homocysteine levels by modulating the tryptophan-homocysteine metabolic pathway. This reduction in homocysteine levels was significantly associated with improvements in cognitive function (Rudzki et al., 2019). Gut peptides are peptide hormones capable of regulating gastrointestinal motility through brain-gut axis interactions (Li et al., 2015; Młynarska et al., 2022). Certain intestinal peptides, including peptide YY (PYY), glucagon-like peptide-1 (GLP-1), cholecystokinin, CRH, growth inhibitory-releasing peptide, and oxytocin, are especially important, as they modulate depressive and anxiety-related behaviors (Młynarska et al., 2022; Dockray, 2014).
The MGB axis functions as a dynamic hub, through which gut microbial communities communicate via multiple signaling pathways (Figure 2), significantly influencing mental health. Clarifying these relationships is essential for developing novel therapeutic approaches to mental health disorders through GM modulation (Shoubridge et al., 2022).
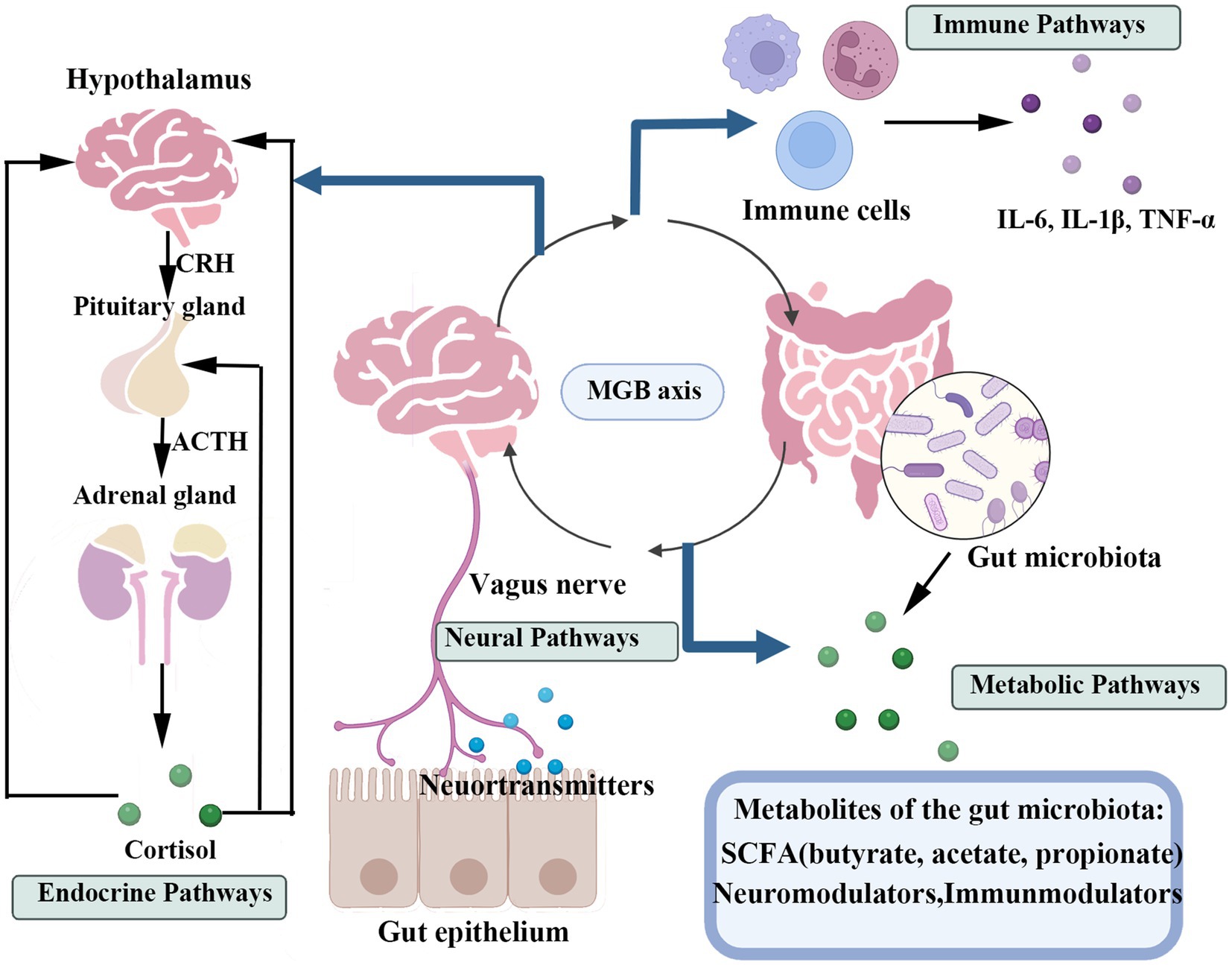
Figure 2. This figure illustrates the primary pathways of bidirectional communication between the gastrointestinal tract and the central nervous system, the GMB axis. The four key pathways include: The neural pathway, primarily mediated by the vagus nerve; The endocrine pathway, involving gut hormones and the HPA axis; The immune pathway, mediated by inflammatory responses and immune cells; And the metabolic pathway, driven by metabolites produced by the gut microbiota (such as SCFAs).
4 Composition and diversity of gut microbiota in anxiety and depression
The intestinal flora of healthy individuals predominantly includes Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteria, collectively comprising approximately 99% of the microbial community (Guo et al., 2022; Lee and Chang, 2021). These phyla are essential for maintaining the physiological and psychological balance of the host (Chen et al., 2022; Valdes et al., 2018). A diverse and stable gut microbiota is crucial for intestinal health, immune responses, nutrient assimilation, and mood modulation via the GMB axis (Cryan et al., 2020).
However, individuals with anxiety and depression frequently display significant shifts in GM composition (Barandouzi et al., 2020; Shen et al., 2021). Recent studies comparing intestinal microbial characteristics between patients with depression and healthy individuals revealed significant differences in microbial composition. Nikolova et al. identified a cross-diagnostic commonality present in psychiatric disorders such as major depressive disorder and anxiety disorders, characterized by a reduction in anti-inflammatory, butyrate-producing genera (e.g., Faecalibacterium and Coprococcus) and increased pro-inflammatory genera (e.g., Eggerthella) (Nikolova et al., 2021). This finding suggests a potential shared microbial. Extensive research has documented reduced microbial diversity, especially alpha diversity, associated with diminished stress resilience and increased susceptibility to mood disorders (Knight et al., 2023). Emerging research consistently identifies decreased abundance of beneficial bacteria, particularly Lactobacillus and Bifidobacterium, as characteristic features in individuals with mood disorders (Aizawa et al., 2018). Studies demonstrate that Lactobacillus and Bifidobacterium species produce GABA and serotonin (5-hydroxytryptamine), crucial neurotransmitters involved in mood regulation (Kaur et al., 2023). Further research indicates that depletion of these beneficial bacteria can disrupt neurotransmitter synthesis, influencing both gut and brain functions and significantly impacting mental health (Huang et al., 2020).
Specific microbial taxa have been associated with anxiety and depression. For example, elevated levels of pro-inflammatory bacteria from genera such as Escherichia and Enterobacter have been linked to increased systemic and neuroinflammatory responses. These inflammatory responses potentially contribute to the underlying pathophysiology of mood disorders (Zheng et al., 2016; Simpson et al., 2021). Individuals with depression often exhibit lower levels of SCFA-producing bacteria, indicating a potential protective role against depressive symptoms (Cheng et al., 2024).
Studies involving animal and human subjects suggest an association between gut microbial diversity and mental health, indicating that microbiota disturbances may contribute to depression onset (Shoubridge et al., 2022; Zhu et al., 2020). Yaoyong Lai’s study employed a bidirectional two-sample Mendelian randomization analysis, revealing that gut microbiota dysbiosis is a causative factor in depression and anxiety, rather than merely a consequence of these disorders (Lai and Xiong, 2025). Decreased gut microbial diversity may impair enteroendocrine cell (EEC) activity and disrupt peptide secretion. Such disturbances could affect emotional states and behavior via gut-brain interactions, increasing vulnerability to anxiety and depression (Lach et al., 2018). Dietary interventions represent promising strategies to modify diet components, alleviate depressive symptoms, promote healthier gut microbiota, and enhance beneficial microbial populations (Dai et al., 2025). Probiotics (e.g., Lactobacillus and Bifidobacterium) can reverse antibiotic-induced alterations in gut microbiota and improve cognitive function and memory through the MGB axis. Thus, probiotics may help restore gut microbial diversity and enhance mental health (Hao et al., 2020). These findings suggest that regulating gut microbiota through certain interventions could alleviate anxiety and depression.
5 Therapeutic interventions targeting gut microbiota
Recent studies conducted within the past 5 years have explored the correlation between anxiety, depression, and gut microbiota, as well as possible therapeutic approaches. For example, research suggests that Enterobacteriaceae bacteria may significantly influence mental health, although detailed investigations into the mechanisms remain ongoing. Preliminary experimental results indicate that colibactin-producing Escherichia coli (CoPEC), as part of the gut microbiota, may disrupt the microbiota-gut-brain (MGB) axis (Rondepierre et al., 2024). Experimental findings by Xu et al. (2024) suggest that NLRP3 inflammasomes are key components of the MGB axis, indicating that targeting the gut microbiota could become a novel treatment strategy for postpartum depression (PPD). Ongoing research involves numerous studies evaluating the potential effectiveness of gut microbiota regulation in treating depression.
Collectively, evidence elucidating the mechanisms by which gut microbiota influence mental disorders underscores their therapeutic potential in managing depression and anxiety, opening promising avenues for addressing mental health issues (Ancona et al., 2021). The therapeutic strategies outlined below(Table 1)—including probiotics, prebiotics, postbiotics, synbiotics, dietary interventions, FMT, FVT, and TCM all leverage this mechanistic link (Figure 3).
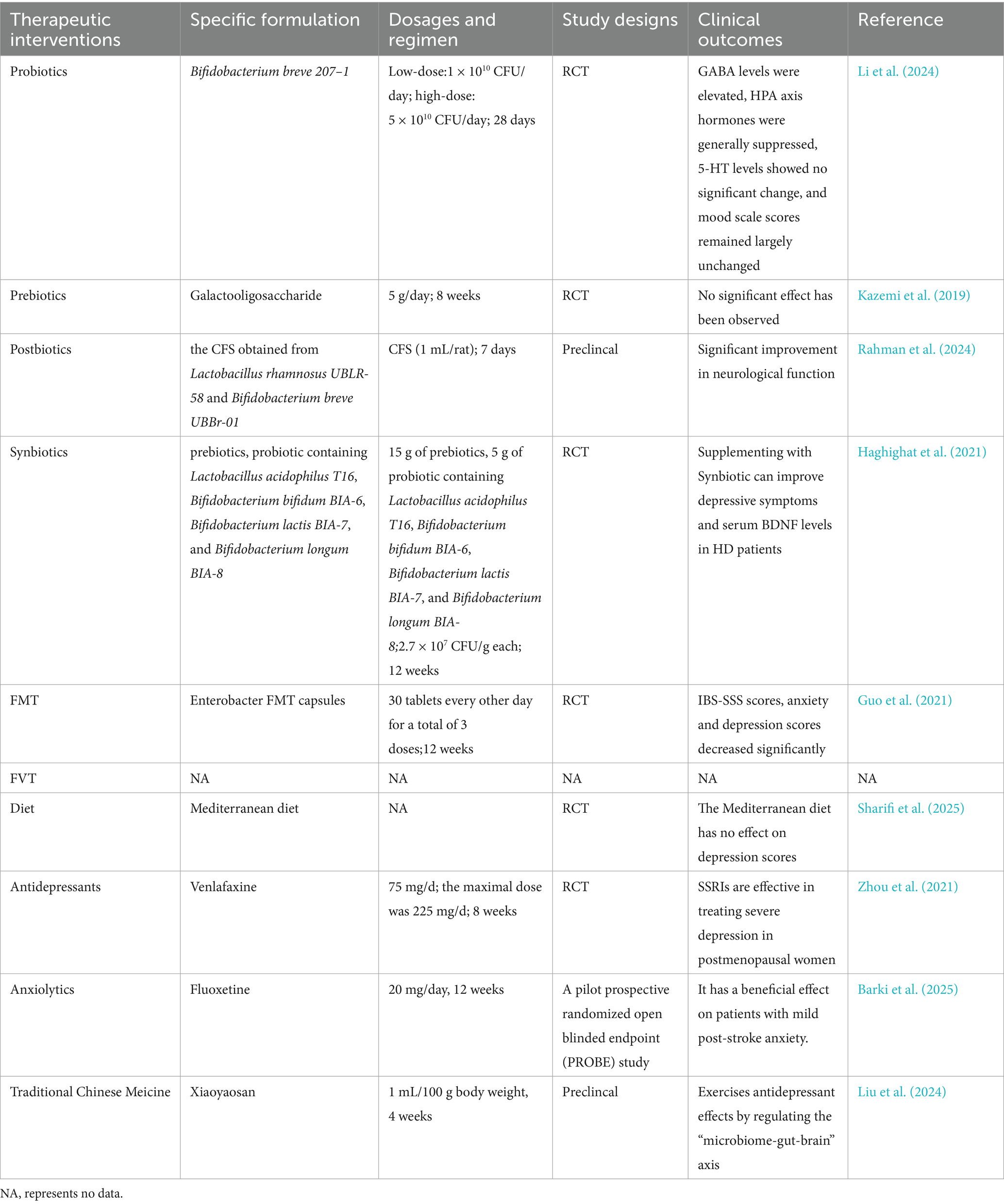
Table 1. Overview of gut microbiota-targeted interventions for anxiety and depression: clinical and preclincal studies.
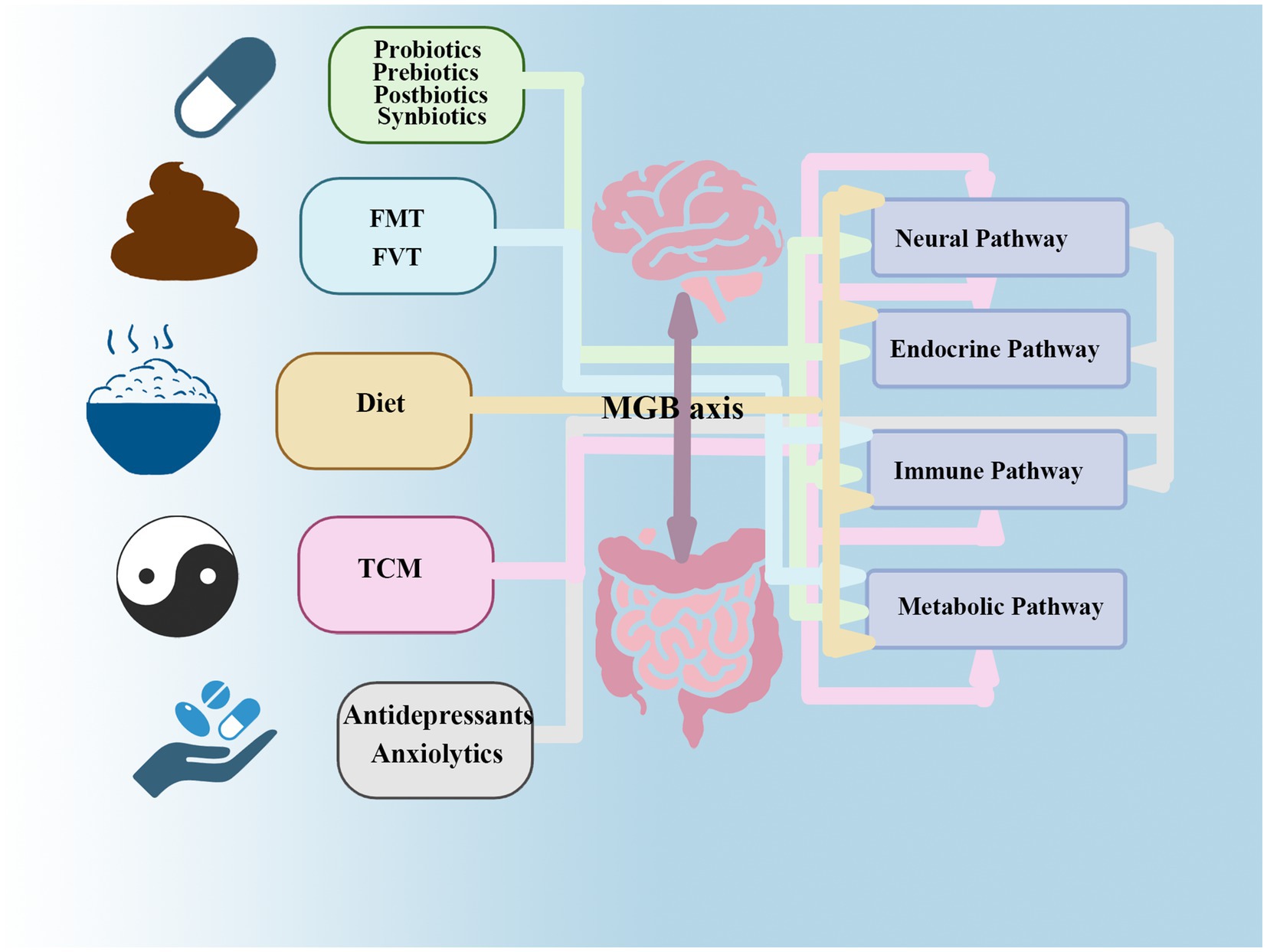
Figure 3. This figure reveals that gut microbiota interventions targeting anxiety and depression likely exert effects through multi-pathway mechanisms of the MGB axis. While certain therapies have primary sites of action (e.g., antidepressants acting on neural synapses), they also regulate endocrine (HPA axis) and immune (inflammatory) pathways. Interventions initiated in the intestinal lumen (e.g., dietary modifications) transmit signals upward through immune, endocrine, metabolic, and neural pathways, thereby achieving therapeutic effects at the neurological and psychological levels. FMT primarily acts by rapidly improving metabolic and immune pathways, subsequently triggering positive chain reactions in endocrine and neural pathways.
5.1 Microbiota-targeted biologics
5.1.1 Probiotics
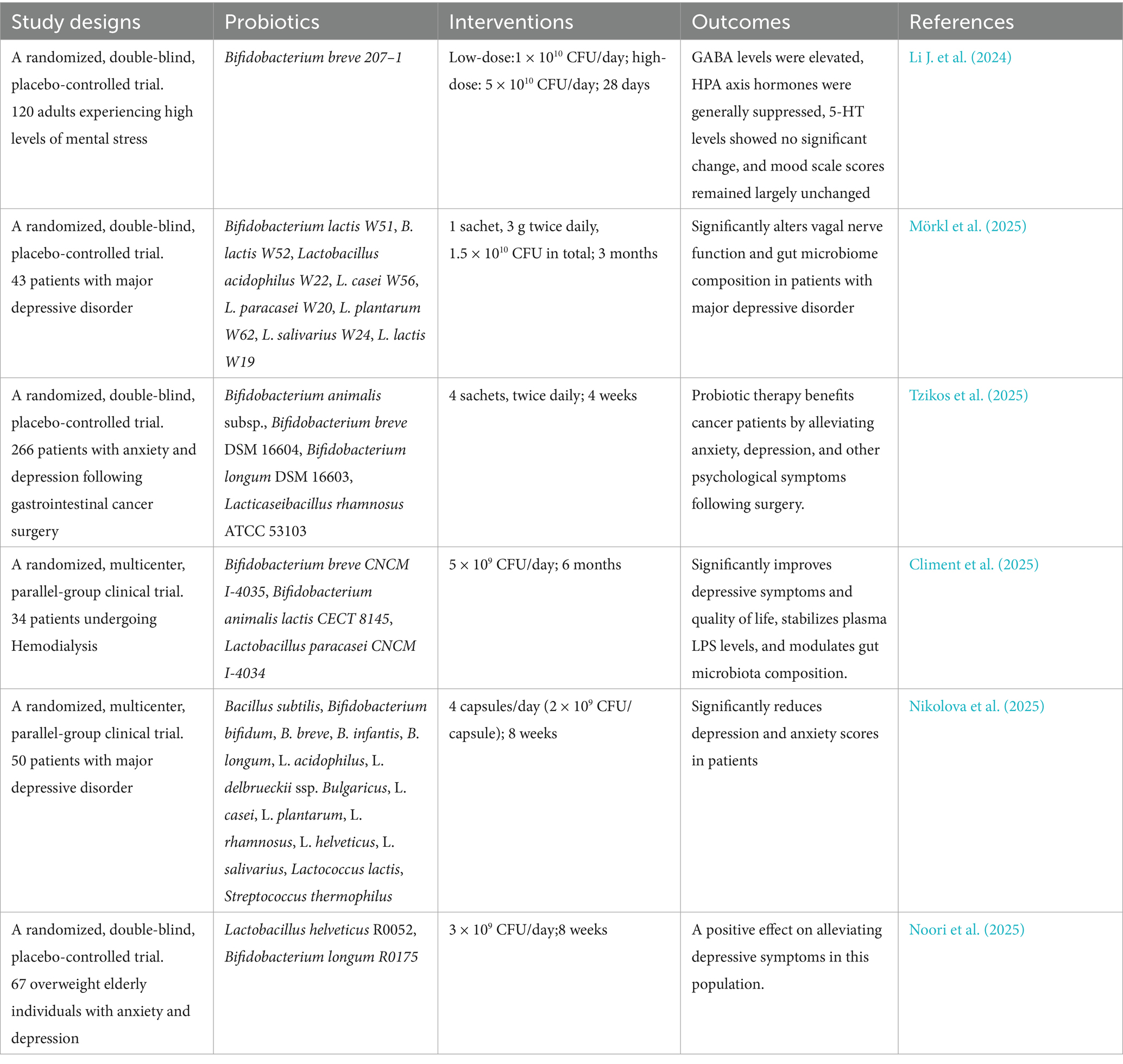
Given the correlation between gut microbiota and anxiety/depression, probiotics may alleviate symptoms by regulating dysbiosis (Jang et al., 2019). Probiotics, as a safe and well-tolerated non-pharmacological therapy, represent a promising therapeutic option for mood disorders (Merkouris et al., 2024). Research has shown that probiotics can enhance cognitive function and reduce depressive symptoms, suggesting their potential role in depression treatment (He et al., 2023). Timothy G et al. defined the concept of psycholbiotics as live microorganisms that, when ingested in sufficient quantities, confer health benefits to individuals with mental disorders. These probiotics belong to a class capable of producing and delivering neuroactive substances such as GABA, serotonin, and dopamine, thereby influencing brain function through the MGB axis (Dinan et al., 2013). Probiotic preparations commonly used in clinical practice include beneficial strains such as Lactobacillus casei, Lactobacillus acidophilus, and Bifidobacteria (Zhang et al., 2023). Research by Kirsten Tillisch et al. has demonstrated for the first time in humans that chronic consumption of fermented dairy products containing five probiotic strains—including Bifidobacterium lactis CNCM I-2494—significantly modulates the brain’s response to emotional stimuliation (Tillisch et al., 2013). In addition, Gu et al. (2020) confirmed that Lactobacillus casei exert significant antidepressant effects in rat models by regulating gut microbiota and the brain-derived neurotrophic factor-tyrosine kinase receptor (BDNF–TrkB) signaling pathway. Certain strains, such as Lactobacillus NK41, Bifidobacterium longum NK46, and their combinations, can alleviate stress-induced anxiety/depression-like behaviors. These effects involve reduced neuroinflammation (e.g., hippocampal NF-κB and microglial activation), increased neuroplasticity markers (e.g., BDNF), and improved peripheral stress/inflammation indicators (e.g., cortisol, TNF-α, and IL-6) (Han and Kim, 2019). Similarly, Sara Ferrari reported that probiotic combinations (e.g., Bifidobacterium longum novaBLG2) can mitigate glutamate-induced anxiety/depression symptoms and enhance cell survival by regulating gut-brain axis metabolites (Ferrari et al., 2024). Research also indicates that daily supplementation with Bifidobacterium breve Bif11 for 21 consecutive days can prevent LPS-induced depressive behavior in mice (Sushma et al., 2023). Bifidobacterium bifidum has demonstrated positive preventive and therapeutic effects on depression in animal models and humans (Li et al., 2023). Regarding dosage, studies have indicated that psychobiotics require a minimum daily dose of 1 billion CFU, with optimal results achieved using doses exceeding 10 billion CFU for at least 8 weeks (Jach et al., 2023). The following are partial experimental results from human studies (Table 2).
In recent years, multiple meta-analyses have attempted to comprehensively evaluate the efficacy of probiotics for mood disorders. For example, Liu’s analysis revealed no statistically significant difference between the probiotic group and the placebo group in alleviating anxiety symptoms (SMD = −0.12, 95% CI: −0.28 to 0.04, p = 0.14; Liu et al., 2018). However, Moshfeghinia et al. (2025) analysis indicates that probiotics, prebiotics, or synbiotics can significantly alleviate depressive and anxiety symptoms in individuals with depression, despite high heterogeneity among studies (I2: 96.29%). Notably, nearly all meta-analyses emphasize significant heterogeneity among included studies (I2 > 50%), underscoring the need for extreme caution in interpreting results.
Despite these promising findings, probiotic applications still face several limitations. Although evidence supports probiotics’ benefits for mood regulation, specific biological mechanisms remain unclear. Additionally, probiotic efficacy varies among individuals, likely due to differences in gut microbiota composition, lifestyle, and genetic background. Therefore, considering that the gut microbial composition of individuals with mental illness differs from that of healthy individuals, personalized intervention strategies can be designed to address specific microbial dysbiosis for therapeutic purposes (Vindegaard et al., 2021). Furthermore, probiotics used in different studies vary in strain, dosage, and administration protocols, reducing comparability across studies. Crucially, the effects of probiotics exhibit a high degree of strain specificity. This means that different strains-even of the same species-may have completely different effects on their hosts. For example, experiments have demonstrated that Lactobacillus johnsonii (NCC 533) or Lactobacillus paracasei (NCC 2461)—two strains of intestinal lactobacilli exhibiting similar characteristics in vitro—can elicit different immune responses (Ibnou-Zekri et al., 2003). Even the same bacterial strains exhibit differences in dosage and treatment duration across different experiments. Therefore, it is unscientific to summarize the results of probiotic research in general. Future research and clinical applications must operate accurately at the strain level. Although some studies have shown positive effects, research in this field is extremely heterogeneous, resulting in different studies often reaching conflicting conclusions. This heterogeneity mainly comes from inconsistent intervention plans, different study populations, diverse outcome measures, and lack of standardization.
5.1.2 Prebiotics
Prebiotics are substrates selectively utilized by host microorganisms to confer health benefits (Gibson et al., 2017). Non-digestible oligosaccharides, including fructan, galactan, and resistant starch, are the primary prebiotic components. Recently, the scope of the term has broadened to include dietary compounds such as polyphenols (Medina-Larqué et al., 2022). Prebiotics affect depression by modulating neurotransmitter production, SCFA production, and immune regulation (Yang Y. et al., 2023). SCFA production is closely linked to prebiotic intake and the composition of intestinal microbiota (Godínez-Méndez et al., 2021). SCFAs can cross the BBB, influencing brain function (Wang J. et al., 2022). Additionally, studies suggest that SCFAs regulate emotional states through the MGB axis and produce anti-inflammatory effects (Liu et al., 2021; Van de Wouw et al., 2018). Recently, Igor Henrique Rodrigues de Paiva succeeded in reversing depressive and anxiety symptoms in mice fed a high-fat diet through therapeutic intervention with fructooligosaccharides (FOS) and galactooligosaccharides (GOS) (Paiva et al., 2024). The administration of FOS and GOS not only demonstrated benefits in probiotic treatment for stress-related behaviors but also effectively reduced stress-induced increases in corticosterone and pro-inflammatory cytokines, attenuating depressive-like and anxiety-like behaviors (Burokas et al., 2017). Antonio Leo’s research confirmed that in the chronic unpredictable mild stress (CUMS) model, the combination of prebiotic α-lactalbumin (ALAC) and postbiotic sodium butyrate (NaB) effectively regulates the MGB axis. This synergy indicates potential for treating anxiety and depression (Leo et al., 2021). However, clinical data on prebiotics remain scarce. Thus, large-scale clinical trials are necessary to determine their sustained therapeutic effects, optimal dosage, and mechanisms of action against depression and anxiety (Asad et al., 2025).
5.1.3 Postbiotics
The International Scientific Association for Probiotics and Prebiotics (ISAPP) defines postbiotics as formulations containing non-viable microorganisms or their constituents that provide health benefits to the host (Salminen et al., 2021). Given the limitations associated with probiotic research, postbiotic compositions derived from probiotics may represent promising new microbial therapeutic approaches (Nataraj et al., 2020; Suez et al., 2019). Postbiotics comprise inactivated cell components (e.g., cell wall fragments and surface proteins) and bacterial cell metabolites (e.g., SCFAs and bacteriocins), collectively mediating physiological effects (Mehta et al., 2023). Postbiotics have been employed to regulate intestinal microbiota, showing effectiveness in resolving dysbiosis (Gao M. et al., 2022), which may positively impact mental health. A review by Icer et al. (2024) noted that GABA produced by lactic acid bacteria (LAB) may exert neuroprotective effects, improving depression. Another study reported that cell-free supernatant (CFS) obtained from Lactobacillus rhamnosus UBLR-58 and Bifidobacterium breve UBBr-01 can reduce nerve damage by inhibiting neuroinflammation and regulating the MGB axis pathway (Rahman et al., 2024). Additionally, SCFAs produced as postbiotics by probiotics play an important role in the MGB axis (Ragavan and Hemalatha, 2024). Increasing research into postbiotics and neurological dysfunction disorders suggests that modulation of the gut-brain axis offers promising future applications in mental health.
5.1.4 Synbiotics
Synbiotics, dietary supplements combining probiotics and prebiotics to deliver synergistic health benefits, demonstrate therapeutic potential in mood disorders (Ke et al., 2019). A 2020 double-blind randomized controlled trial (RCT) showed that Synbiotic 2000 supplements can improve emotional regulation in adults with attention deficit hyperactivity disorder (ADHD) (Skott et al., 2020; Arteaga-Henríquez et al., 2024). A multicenter, randomized, placebo-controlled “basket” trial by Arteaga-Henríquez et al. (2024) also demonstrated that synbiotics specifically improved mood symptoms and abnormalities in mood regulation, consistent with Scott’s findings. A preclinical mechanistic study by Lapmanee et al. (2024) revealed that synbiotics have neuroprotective and gut benefits in stressed rats. Sanjay Noonan et al. found that supplementation with Synbiotic produced statistically significant improvements in measures of depression (Noonan et al., 2020). Synthetic probiotics possess both probiotic and prebiotic properties, and combining these elements primarily enhances probiotic survival during gastrointestinal transit. When properly combined, they may produce better results than probiotics or prebiotics alone (Yoo et al., 2024; Markowiak and Śliżewska, 2018).
5.2 Microbiota transplantation therapies
5.2.1 Fecal microbiota transplantation
Fecal microbiota transplantation (FMT) is a therapeutic intervention involving the transfer of fecal material from a healthy individual to the intestinal tract of a recipient. This approach has recently gained attention as a potential treatment for depressive disorders by recalibrating intestinal microbiota (Joo et al., 2024). Multiple academic analyses indicate that FMT interventions reduce bacteria associated with psychiatric disorders (e.g., Lactobacillus acidophilus), thereby alleviating psychiatric symptoms (Yang C. et al., 2023). A recent RCT of patients with diarrhea-predominant irritable bowel syndrome (IBS-D) and comorbid anxiety/depression demonstrated that oral FMT capsules significantly alleviated both IBS-D and associated psychological symptoms (Guo et al., 2021). Critically, these clinical improvements were accompanied by the restoration of gut microbial communities, suggesting microbiota modulation as a potential underlying mechanism. A pilot RCT by Green et al. (2023) demonstrated the feasibility of a different FMT administration route (enema) for patients with major depressive disorder (MDD). Baske et al. (2024) reviewed evidence showing significantly elevated Escherichia-Shigella levels in patients with generalized anxiety disorder (GAD) and proposed that FMT might reduce these bacteria, positively affecting the gut-brain axis and providing a promising avenue for novel GAD therapies. Xu Qi et al. showed that FMT from healthy mice to postpartum depression (PPD) model mice improved depression/anxiety-like behaviors by regulating the NLRP-3/caspase-1 pathway in the gut and hippocampus (Campbell and Macqueen, 2004). Cai et al. (2022) confirmed that FMT improved CUMS-induced depressive behavior in rats, with mechanisms involving coordinated actions of the 5-HT system, BDNF, neurotransmitters (GABA/Glu), inflammatory factors (IL-6/LPS), and SCFAs. Rao et al. (2021) observed increased 5-HT and decreased IL-1β and TNF-α after FMT treatment, leading to improved depressive symptoms. An international consensus meeting convened by the American Gastroenterological Association (AGA) indicated that FMT demonstrates favorable short-term safety in treating Clostridium difficile infection, but long-term safety data remain insufficient and require ongoing follow-up studies (Cammarota et al., 2019). Moreover, evidence is lacking regarding its safety in extremely immunocompromised individuals (Yadegar et al., 2024). Mark Hofmeister’s recent meta-analysis ultimately included 62 studies, with only one evaluating the impact of FMT on depressive symptoms. The conclusion was that this intervention did not demonstrate a statistically significant benefit for depression (Hofmeister et al., 2021). However, the author has only included one relevant study thus far. Due to factors such as small sample size, it is currently not possible to draw definitive conclusions on this matter.
5.2.2 Fecal virome transplantation
The goal shared by FMT and FVT is to treat diseases by reshaping gut microbiota. Mao et al. (2024) suggested that the success of FMT may largely be attributed to the role played by its transferred viral components in reprogramming the host microbiota. Meanwhile, a double-blind randomized placebo-controlled trial demonstrated that FMT significantly alters the recipient’s intestinal phage community and promotes the long-term stable colonization of donor phages (Zuppi et al., 2024). Therefore, we have incorporated FVT as a novel transplant intervention into this chapter. FVT has emerged as an innovative therapeutic strategy focused specifically on transferring viral components of the donor’s gut microbiota (Castells-Nobau et al., 2024). FVT involves transplanting the viral components present in feces, specifically bacteriophages (Rasmussen et al., 2020). Experiments by Ritz et al. (2024) demonstrate that FVT can alleviate stress-induced behavioral, immunological, and neurobiological changes by modulating the MGB axis. FVT significantly reduces the risk of pathogen transmission associated with FMT through physical filtration and processing while maintaining therapeutic efficacy. Compared to FMT, it represents a safer potential treatment strategy capable of accommodating donor variability (Rasmussen et al., 2024). At present, few studies have been conducted in this area, and more research is required to clarify the underlying mechanisms.
Although FMT and FVT are promising therapeutic approaches, their clinical application poses significant safety concerns. Regarding FMT, reports of serious adverse events, including the transmission of multidrug-resistant infections resulting in fatalities—have prompted regulatory warnings (Kuijper et al., 2019). The long-term consequences of introducing intact foreign microbial communities remain unclear, and adverse reactions such as diarrhea, bloating, and abdominal pain may occur. For FVT, although the risk of bacterial infection is significantly reduced during preparation by eliminating bacteria, the possibility of eukaryotic virome transmission and potential microbial contamination cannot be completely ruled out. As invasive therapeutic procedures, FMT and FVT require strict screening of microbiota donors and necessitate long-term follow-up in clinical trials. Furthermore, these therapies face significant regulatory hurdles. Neither FMT nor FVT fits neatly into traditional drug or medical device classifications, creating regulatory ambiguity. The absence of standardized protocols for donor screening, material preparation, and efficacy assessment complicates quality control and approval processes. Establishing safe, high-quality donor banks involves complex ethical and legal challenges. Crucially, current evidence supporting the efficacy of FMT/FVT for treating mood disorders primarily stems from small-scale or open-label studies, which are highly susceptible to bias and placebo effects. Before considering any clinical application recommendations, large-scale randomized double-blind placebo-controlled trials are imperative.
5.3 Dietary interventions
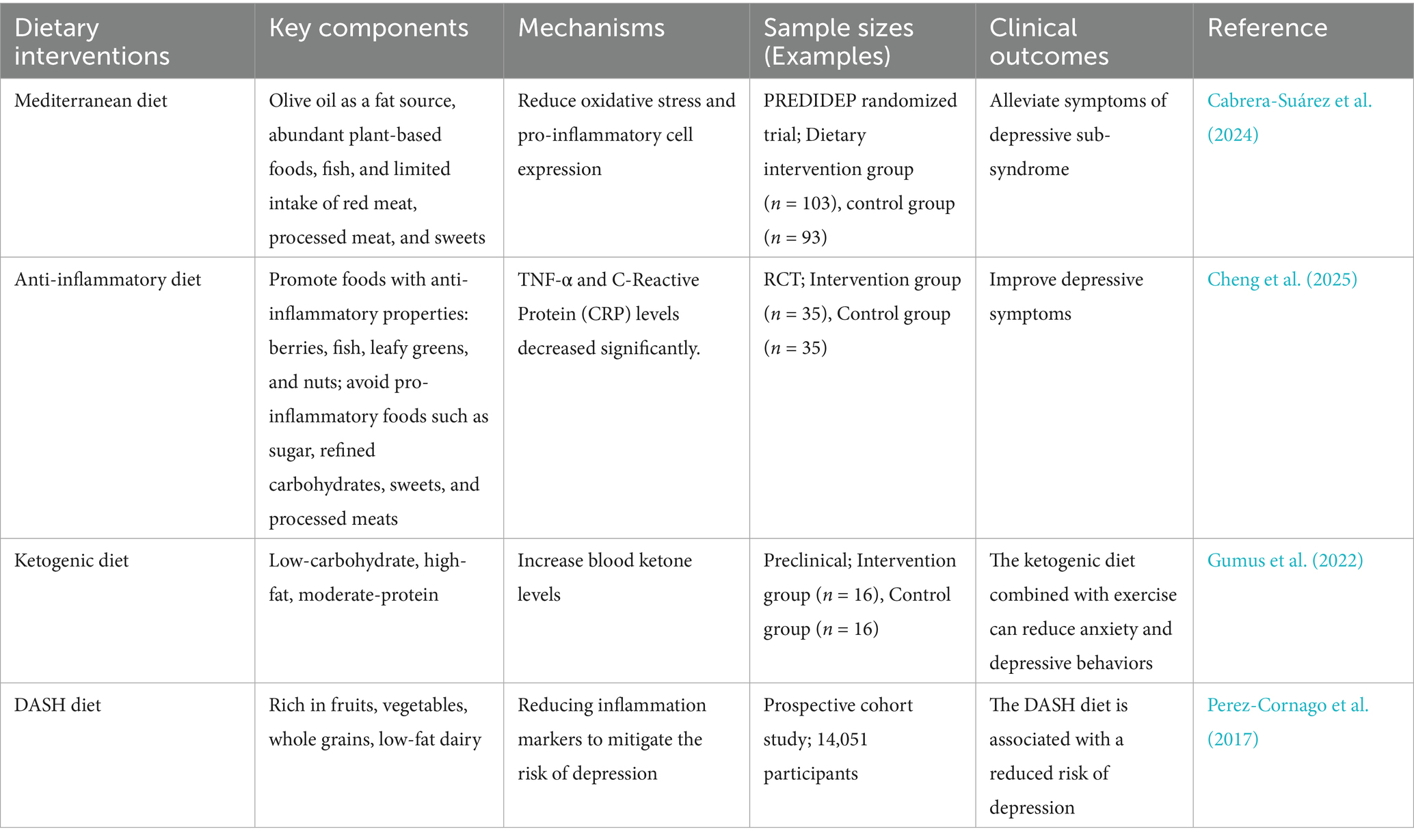
It is well known that diet significantly influences the composition of gut microbiota (Zou et al., 2021). Recent studies indicate that dietary factors can regulate depressive symptoms through both GM-dependent and -independent pathways (Maitiniyazi et al., 2022). Adherence to a healthy diet serves as an effective complementary strategy in managing depressive and anxiety disorders (Hamad et al., 2024; Enriquez-Martinez et al., 2021). Encouraging wholesome dietary practices should be integral to preventive and therapeutic approaches for depressive symptoms (Francis et al., 2022; Table 3). Some studies indicate that dietary diversity is inversely associated with symptoms of major depressive disorder (MDD) and generalized anxiety disorder (GAD), but reverse causality cannot be excluded (Pengpid and Peltzer, 2024). Diets associated with reduced anxiety and depression include “healthy” dietary patterns, the Mediterranean diet, anti-inflammatory diets, and diverse dietary practices (Lassale et al., 2019; Aucoin et al., 2021). In particular, the Mediterranean diet has demonstrated effectiveness for anxiety and depression (Johnson et al., 2022; Alnabulsi et al., 2024). This diet primarily includes olive oil as a fat source, abundant plant-based foods, fish, and limited intake of red meat, processed meat, and sweets (Bizzozero-Peroni et al., 2022; Altun et al., 2021). Such dietary patterns reduce oxidative stress and pro-inflammatory cell expression, both of which are key factors in depressive states (Bizzozero-Peroni et al., 2022; Fitó et al., 2007; Koelman et al., 2022; Huang et al., 2019). Multiple surveys examining populations from various countries, genders, and health conditions consistently support the beneficial role of the Mediterranean diet in anxiety and depression. Bizzozero-Peroni et al. (2025) recent meta-analysis indicates that the Mediterranean diet holds significant potential for alleviating depressive symptoms in individuals with depression. Tolkien et al. (2019) meta-analysis included 11 studies and concluded that adopting an anti-inflammatory diet may be an effective intervention or preventive measure for reducing the risk and symptoms of depression. Dong et al. (2024) team identified nonlinear bidirectional associations between dietary diversity and depressive symptoms through questionnaires and cross-lagged modeling analyses. Persistent inflammatory states are also linked to depression and anxiety (Flores Saiffe Farías et al., 2018). An analysis by Lv et al. (2022) further confirmed that anti-inflammatory dietary interventions could alleviate depressive symptoms in older adults. Additionally, the ketogenic diet may also improve anxiety or depression (Wu et al., 2022). Its neuroprotective and anti-inflammatory properties have been consistently demonstrated and may underline its antidepressant effects (Smolensky et al., 2023). Animal studies, such as those conducted by Gumus et al. (2022) showed that combining a ketogenic diet with voluntary exercise improves anxiety- and depression-like behaviors in mice, but larger-scale randomized clinical trials remain limited. Healthy dietary interventions can effectively reduce inflammation (Burrows et al., 2020). A recent observational study also showed that Dietary Approaches to Stop Hypertension (DASH) and the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diets have antidepressant potential (Cherian et al., 2021). Micronutrients, ω-3 polyunsaturated fatty acids, dietary fiber, polyphenols, and other bioactive dietary ingredients also show significant promise in improving depressive symptoms through different mechanisms (Wu et al., 2022).
5.4 Pharmacological and integrative approaches
5.4.1 Conventional antidepressants and anxiolytics
Oral antidepressants and anxiolytics continue to dominate the treatment of anxiety and depression. SSRIs and SNRIs serve as primary medications for managing depression and anxiety, while benzodiazepines are restricted to short-term anxiety management (Neil-Sztramko et al., 2025). Antidepressants are generally categorized into novel antidepressants, tricyclic antidepressants, traditional antidepressants, and SSRIs, collectively offering various therapeutic options for depression treatment (Hockenberry et al., 2019). Studies have shown that antidepressants affect gut microbiota composition and function (Tan et al., 2022). SSRIs modulate gut microbiota, potentially contributing to symptom relief in depressed individuals. Individuals treated with SSRIs showed significant alterations in gut microbiota composition, which might explain improvements in mood symptoms (Gao M. et al., 2023). Fluoxetine, an SSRI antidepressant, exerts effects beyond emotional stabilization, particularly in pregnant and nursing women. This drug alters gut microbiota balance and fundamentally modifies the metabolic functions of these microorganisms (Ramsteijn et al., 2020). Venlafaxine exerts antidepressant effects by inhibiting serotonin and norepinephrine reuptake, regulating serotonin and glutamate levels in mouse models of depression, and influencing gut microbiota diversity, particularly affecting key bacterial genera such as Blautia, Oscillibacter, Tyzzerella, Butyricoccus, and Enterorhabdus (Shen et al., 2023). Dexipramine, a tricyclic antidepressant, exhibited notable antimicrobial activity; notably, Mucinophilic Immobilized Bacillus and Escherichia coli were highly susceptible to this drug (Ait Chait et al., 2020). Evidence suggests antidepressants can regulate gut microbiota composition and function, which may be critical for their efficacy. Future research exploring this interaction could inform improved treatment strategies.
5.4.2 Traditional Chinese medicine
Currently, an increasing number of studies indicate that TCM treatments can alleviate psychological disorders, including depression and anxiety, by modulating the gut microbiota. The effectiveness of acupuncture (including electroacupuncture and manual acupuncture) in treating depression and anxiety has long been demonstrated (Zheng et al., 2019). Several studies show acupuncture can alleviate depression by modulating gut microbiota (Wang et al., 2024). Recent studies suggest acupuncture may ameliorate depressive-like behaviors in post-stroke depression (PSD) by modulating gut microbiota and inhibiting NLRP3 inflammasome overactivation in the colon (Cai et al., 2024). Electroacupuncture (EA) has minimal side effects, combining electrical stimulation and acupuncture. This innovative therapy has attracted attention in the field of neurological disorders, especially depression (Duan et al., 2024). EA may exert antidepressant effects by regulating gut microbiota (Duan et al., 2024), experimentally demonstrated by the upregulation of BDNF and increased abundance of SCFA-producing bacteria (Li S. et al., 2024). Umbilical moxibustion helps rebalance intestinal microbiota by promoting beneficial bacteria growth and inhibiting harmful strains. This microbial regulation plays an important role in alleviating subclinical depressive symptoms. The therapy creates a healthier intestinal environment, supporting mental health (Yu et al., 2024).
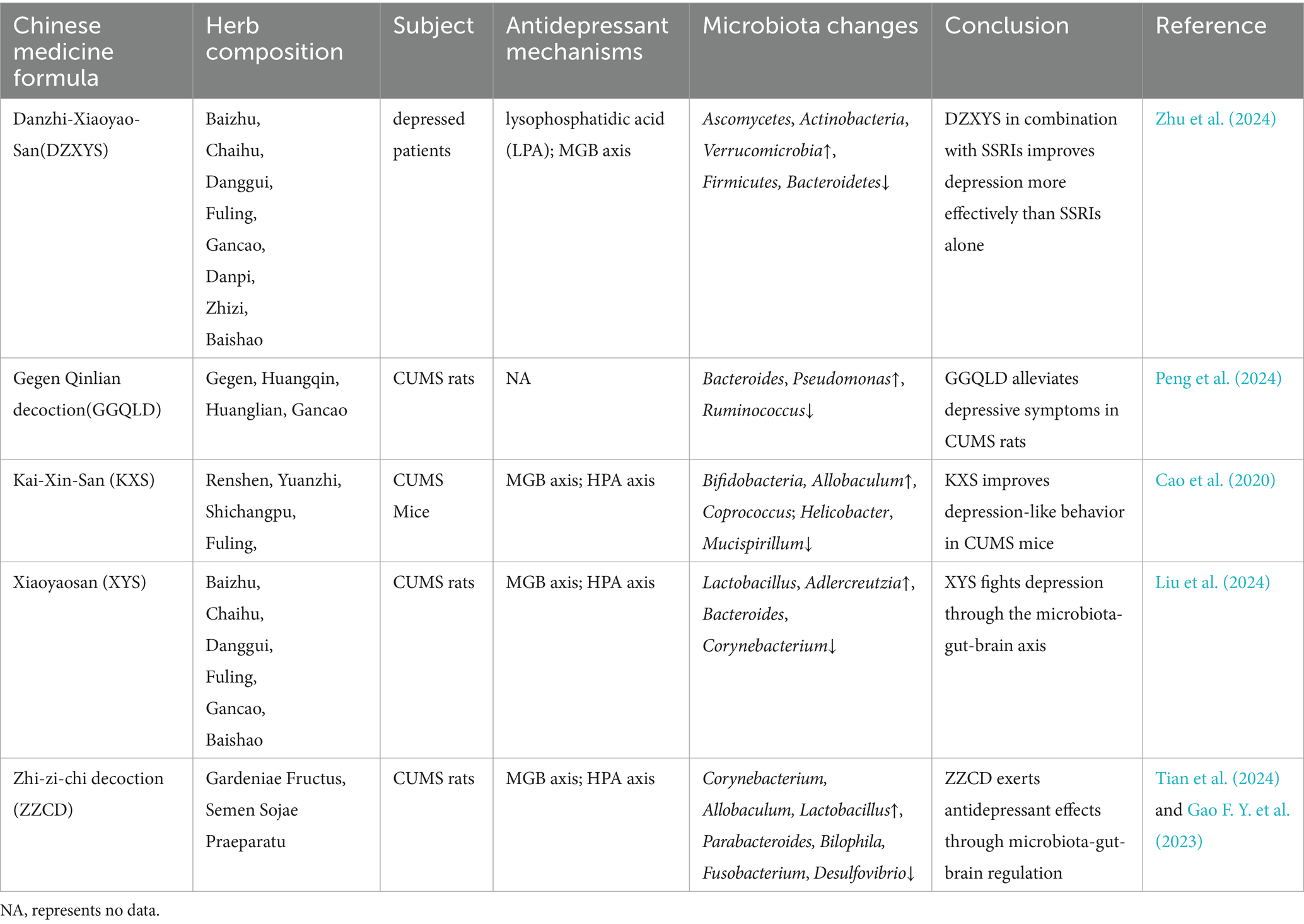
In addition, TCM herbal formulas have demonstrated efficacy in treating mental illnesses, including depression and anxiety, through alterations in gut microbiota (Table 4). Research indicates that Xiaoyao San alleviates depressive-like symptoms in rats subjected to chronic unpredictable mild stress (CUMS) by modulating gut microbiota, especially, it exerts antidepressant effects by inhibiting the TLR4/NLRP3 inflammatory pathway and enhancing intestinal barrier function (Liu et al., 2024). Similarly, data confirms that Gegen Qinlian decoction significantly alleviates depression-like behaviors in CUMS rats, also by regulating gut microbiota and metabolites such as oleanolic acid (Peng et al., 2024). Li J. L. et al. (2024) evaluated the efficacy and safety of Kaixin Powder in treating depression. The results showed that KXS was comparable to or superior to antidepressants in treating depression, with fewer side effects. KXS alleviates intestinal inflammation by modulating microbiota composition and reducing LPS levels, thereby decreasing pro-inflammatory cytokines and protecting both the intestinal barrier and blood–brain barrier. Simultaneously, it lowers HPA axis hormone levels to mitigate excessive HPA axis activation, thereby improving anxiety and depression (Cao et al., 2020). Dan Zhi Xiao Yao San exerts its antidepressant effects through a multi-component, multi-target, multi-pathway mechanism. Its primary active constituents are quercetin and luteolin, with key targets including AVPR2, EGFR, F2, and CDK6. Therapeutic effects are achieved by regulating LPA and the gut microbiota-brain axis (Zhu X. et al., 2024). Zhi-zi-chi decoction not only regulates intestinal microbiota but also delivers multiple bioactive compounds (such as oleanolic acid) that act on multi-target genes. This ultimately modulates the HPA axis (by reducing CRH, ACTH, etc.), elevates neurotransmitter levels (5-HT, GABA, etc.), reduce pro-inflammatory factors (IL-1β, TNF-α), and increase anti-inflammatory factors (IL-10) to alleviate anxiety and depression (Tian et al., 2024). In summary, robust evidence supports the efficacy of TCM interventions in alleviating depression and anxiety symptoms through gut microbiota regulation.
However, translating these promising research findings into evidence-based medicine faces significant challenges, primarily due to the lack of standardization in traditional Chinese medicine formulations, which leads to substantial heterogeneity across studies. Future research must prioritize identifying key active components and developing standardized, quality-controlled TCM formulations to ensure reproducibility and reliability of results. Furthermore, experimental findings from animal models cannot be directly applied to human clinical settings due to species-specific differences in gut microbiota composition, host metabolism, and drug pharmacokinetics. Animal experiments can only partially replicate the complexity of human emotional disorders. Therefore, while our data provides strong mechanistic evidence, they cannot directly predict human efficacy. Future research should integrate systems biology approaches to elucidate the precise mechanisms of action and active components of TCM. In summary, rigorously designed human clinical trials using standardized TCM formulations are essential for validating their efficacy and safety in treating anxiety and depression.
6 Discussion
6.1 Limitations
Overall, the relationship between gut microbiota and anxiety and depression described in this review has been confirmed by numerous experiments, however, the study still has limitations. While preclinical models have been invaluable for elucidating mechanistic pathways along the gut-brain axis, current studies primarily involve animal models. Animal models cannot fully replicate the complexity of human mental disorders, social stressors, and dietary habits. Moreover, there are species differences between the animal gut microbiota and humans, and variations also exist among gut microbiota across different human populations. Although animal study results appear promising, translating these findings into human clinical applications remains challenging. Currently, there is a relative scarcity of high-quality, large-scale randomized controlled trials (RCTs) in humans, and animal studies currently dominate. Much current research in this field does not adequately consider subjects’ genetic predisposition, environmental factors, medication history, and dietary habits, all of which profoundly influence gut microbiota composition (Xie et al., 2024). Studies have shown substantial variability in gut microbiota composition among individuals with depression. This heterogeneity may result from differences in biological characteristics among study cohorts and methodological variations in sample processing (Averina et al., 2024). This likely explains the inconsistent efficacy observed in clinical trials involving probiotics, prebiotics, or fecal microbiota transplantation—where a particular intervention may prove effective in one group but ineffective in another. Significant methodological differences between studies constitute a key obstacle to progress in this field. Variations in bioinformatics analyses, intervention formulations (such as probiotic strains, dosages, and treatment durations), and the lack of standardized outcome measures for gastrointestinal and psychological symptoms hinder meta-analyses and cross-study comparisons. Furthermore, many studies are constrained by small sample sizes, resulting in insufficient analytical power and increased risk of false-positive findings. Each intervention type has unique risk–benefit characteristics and should thus be applied appropriately according to specific contexts. The main limitation of probiotics lies in their strain-specific effects; FMT has unknown long-term safety implications; and TCM faces challenges related to standardization. These factors pose significant challenges in clinical trials related to gut microbiota.
6.2 Future directions
Therefore, future studies should involve broader and more diverse populations to verify the generalizability and therapeutic potential of these treatments. Meanwhile, future research should explore the impact of specific microorganisms and their by-products on mental health, particularly their role in emotional regulation. Our findings underscore the imperative to prioritize rigorous human RCTs to validate efficacy and ensure safety for the target patient population. Future research must adopt consensus methodologies and standardize core outcome measures to enhance reproducibility and data integration capabilities. The field of psychiatry requires a shift toward personalized microbiome-targeted interventions, where treatment plans are tailored based on an individual’s baseline microbial composition, metabolome profile, and clinical characteristics. For instance, patients exhibiting specific inflammatory microbial signatures may benefit from distinct bacterial strains, while those characterized by insufficient SCFAs production require different strains. To achieve personalized treatment, integrating multi-omics approaches is crucial. By integrating metagenomics, metabolomics, and proteomics, we can comprehensively unravel disease mechanisms and the interactions between the microbiome and the host. This multi-omics approach also enables the discovery of biomarkers, advancing precision medicine and ultimately guiding the development of novel diagnostic methods and targeted therapeutic agents. Additionally, researchers need to clarify more precisely how probiotics, prebiotics, and other interventions influence specific neurobiological pathways. Current studies largely lack long-term follow-up, leaving treatments without sufficient experimental data to assess long-term efficacy and potential side effects. Addressing these gaps through comprehensive analysis could facilitate the development of more diverse and effective therapeutic strategies for anxiety and depression in the future.
Author contributions
ZRu: Writing – original draft. WR: Data curation, Writing – review & editing. WH: Data curation, Writing – review & editing. HZ: Data curation, Writing – review & editing. LJ: Data curation, Writing – review & editing. ZRo: Formal analysis, Writing – review & editing. JF: Funding acquisition, Writing – review & editing. SY: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82260901, 82560921); Guangxi Science and Technology Base and Talent Special Project (Gui Ke AD22035165); Guangxi University of Traditional Chinese Medicine: Gui Pai Apricot Grove Top-notch Talent Project [Gui TCM University Party (2022) No. 23]; Guangxi Key Research Laboratory of Traditional Chinese Medicine [Gui TCM Science and Education Development (2023) No. 9].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1664800/full#supplementary-material
References
Adak, A., and Khan, M. R. (2019). An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 76, 473–493. doi: 10.1007/s00018-018-2943-4
Agirman, G., Yu, K. B., and Hsiao, E. Y. (2021). Signaling inflammation across the gut-brain axis. Science 374, 1087–1092. doi: 10.1126/science.abi6087
Ait Chait, Y., Mottawea, W., Tompkins, T. A., and Hammami, R. (2020). Unravelling the antimicrobial action of antidepressants on gut commensal microbes. Sci. Rep. 10:17878. doi: 10.1038/s41598-020-74934-9
Aizawa, E., Tsuji, H., Asahara, T., Takahashi, T., Teraishi, T., Yoshida, S., et al. (2018). Bifidobacterium and Lactobacillus counts in the gut microbiota of patients with bipolar disorder and healthy controls. Front. Psych. 9:730. doi: 10.3389/fpsyt.2018.00730
Alhasson, F., Das, S., Seth, R., Dattaroy, D., Chandrashekaran, V., Ryan, C. N., et al. (2017). Altered gut microbiome in a mouse model of gulf war illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One 12:e0172914. doi: 10.1371/journal.pone.0172914
Alli, S. R., Gorbovskaya, I., Liu, J. C. W., Kolla, N. J., Brown, L., and Müller, D. J. (2022). The gut microbiome in depression and potential benefit of prebiotics, probiotics and Synbiotics: a systematic review of clinical trials and observational studies. Int. J. Mol. Sci. 23:494. doi: 10.3390/ijms23094494
Alnabulsi, M., Imam, A. A., Alawlaqi, A. A., Alhawaj, F. H., Jamjoom, G. F., Alsaeidi, L. D., et al. (2024). Adherence to the mediterranean diet in Saudi Arabia and its association with socioeconomic status and depression. Medicina (Kaunas) 60:642. doi: 10.3390/medicina60040642
Altun, E., Walther, C., Borof, K., Petersen, E., Lieske, B., Kasapoudis, D., et al. (2021). Association between dietary pattern and periodontitis-a cross-sectional study. Nutrients 13:167. doi: 10.3390/nu13114167
Ancona, A., Petito, C., Iavarone, I., Petito, V., Galasso, L., Leonetti, A., et al. (2021). The gut-brain axis in irritable bowel syndrome and inflammatory bowel disease. Dig. Liver Dis. 53, 298–305. doi: 10.1016/j.dld.2020.11.026
Arteaga-Henríquez, G., Ramos-Sayalero, C., Ibañez-Jimenez, P., Karina Rosales-Ortiz, S., Kilencz, T., Schiweck, C., et al. (2024). Efficacy of a synbiotic in the management of adults with attention-deficit and hyperactivity disorder and/or borderline personality disorder and high levels of irritability: results from a multicenter, randomized, placebo-controlled, “basket” trial. Brain Behav. Immun. 120, 360–371. doi: 10.1016/j.bbi.2024.06.012
Asad, A., Kirk, M., Zhu, S., Dong, X., and Gao, M. (2025). Effects of prebiotics and probiotics on symptoms of depression and anxiety in clinically diagnosed samples: systematic review and Meta-analysis of randomized controlled trials. Nutr. Rev. 83, e1504–e1520. doi: 10.1093/nutrit/nuae177
Aucoin, M., LaChance, L., Naidoo, U., Remy, D., Shekdar, T., Sayar, N., et al. (2021). Diet and anxiety: a scoping review. Nutrients 13:418. doi: 10.3390/nu13124418
Averina, O. V., Poluektova, E. U., Zorkina, Y. A., Kovtun, A. S., and Danilenko, V. N. (2024). Human gut microbiota for diagnosis and treatment of depression. Int. J. Mol. Sci. 25:782. doi: 10.3390/ijms25115782
Barandouzi, Z. A., Starkweather, A. R., Henderson, W. A., Gyamfi, A., and Cong, X. S. (2020). Altered composition of gut microbiota in depression: a systematic review. Front. Psych. 11:541. doi: 10.3389/fpsyt.2020.00541
Barki, S., Vibha, D., Pachipala, S., Tayade, K., Misra, S., Nath, M., et al. (2025). Safety and efficacy of fluoxetine in post-stroke anxiety: a pilot prospective randomized open blinded endpoint (PROBE) study. Int. J. Psychiatry Med. 60, 495–507. doi: 10.1177/00912174241296233
Baske, M. M., Timmerman, K. C., Garmo, L. G., Freitas, M. N., McCollum, K. A., and Ren, T. Y. (2024). Fecal microbiota transplant on Escherichia-Shigella gut composition and its potential role in the treatment of generalized anxiety disorder: a systematic review. J. Affect. Disord. 354, 309–317. doi: 10.1016/j.jad.2024.03.088
Bear, T., Dalziel, J., Coad, J., Roy, N., Butts, C., and Gopal, P. (2021). The microbiome-gut-brain Axis and resilience to developing anxiety or depression under stress. Microorganisms 9:723. doi: 10.3390/microorganisms9040723
Bhandage, A. K., Cunningham, J. L., Jin, Z., Shen, Q., Bongiovanni, S., Korol, S. V., et al. (2019). Depression, GABA, and age correlate with plasma levels of inflammatory markers. Int. J. Mol. Sci. 20:172. doi: 10.3390/ijms20246172
Bizzozero-Peroni, B., Godoy-Cumillaf, A., Fernández-Rodríguez, R., Rodríguez-Gutiérrez, E., Jiménez-López, E., Giakoni-Ramírez, F., et al. (2022). Mediterranean diet interventions for depressive symptoms in adults with depressive disorders: a protocol for a systematic review and Meta-analysis. Int. J. Environ. Res. Public Health 19:437. doi: 10.3390/ijerph192114437
Bizzozero-Peroni, B., Martínez-Vizcaíno, V., Fernández-Rodríguez, R., Jiménez-López, E., Núñez de Arenas-Arroyo, S., Saz-Lara, A., et al. (2025). The impact of the Mediterranean diet on alleviating depressive symptoms in adults: a systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 83, 29–39. doi: 10.1093/nutrit/nuad176
Bonaz, B., Bazin, T., and Pellissier, S. (2018). The Vagus nerve at the Interface of the microbiota-gut-brain Axis. Front. Neurosci. 12:49. doi: 10.3389/fnins.2018.00049
Borkent, J., Ioannou, M., Laman, J. D., Haarman, B. C. M., and Sommer, I. E. C. (2022). Role of the gut microbiome in three major psychiatric disorders. Psychol. Med. 52, 1222–1242. doi: 10.1017/S0033291722000897
Breit, S., Kupferberg, A., Rogler, G., and Hasler, G. (2018). Vagus nerve as modulator of the brain-gut Axis in psychiatric and inflammatory disorders. Front. Psych. 9:44. doi: 10.3389/fpsyt.2018.00044
Burokas, A., Arboleya, S., Moloney, R. D., Peterson, V. L., Murphy, K., Clarke, G., et al. (2017). Targeting the microbiota-gut-brain Axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol. Psychiatry 82, 472–487. doi: 10.1016/j.biopsych.2016.12.031
Burrows, K., Stewart, J. L., Antonacci, C., Kuplicki, R., Thompson, K., Taylor, A., et al. (2020). Association of poorer dietary quality and higher dietary inflammation with greater symptom severity in depressed individuals with appetite loss. J. Affect. Disord. 263, 99–106. doi: 10.1016/j.jad.2019.11.160
Cabrera-Suárez, B. M., Hernández-Fleta, J. L., Molero, P., González-Pinto, A., Lahortiga, F., Cabrera, C., et al. (2024). Mediterranean diet-based intervention to improve depressive symptoms: analysis of the PREDIDEP randomized trial. Nutr. Neurosci. 27, 951–961. doi: 10.1080/1028415X.2023.2283290
Cai, W., Wei, X. F., Zhang, J. R., Tao, L., Li, D., Zhang, K., et al. (2024). Acupuncture ameliorates depression-like behavior of poststroke depression model rats through the regulation of gut microbiota and NLRP3 inflammasome in the colon. Neuroreport 35, 883–894. doi: 10.1097/WNR.0000000000002076
Cai, T., Zheng, S. P., Shi, X., Yuan, L. Z., Hu, H., Zhou, B., et al. (2022). Therapeutic effect of fecal microbiota transplantation on chronic unpredictable mild stress-induced depression. Front. Cell. Infect. Microbiol. 12:900652. doi: 10.3389/fcimb.2022.900652
Cammarota, G., Ianiro, G., Kelly, C. R., Mullish, B. H., Allegretti, J. R., Kassam, Z., et al. (2019). International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut 68, 2111–2121. doi: 10.1136/gutjnl-2019-319548
Campbell, S., and Macqueen, G. (2004). The role of the hippocampus in the pathophysiology of major depression. J. Psychiatry Neurosci. 29, 417–426. doi: 10.1139/jpn.0441
Cao, C., Liu, M., Qu, S., Huang, R., Qi, M., Zhu, Z., et al. (2020). Chinese medicine formula Kai-Xin-San ameliorates depression-like behaviours in chronic unpredictable mild stressed mice by regulating gut microbiota-inflammation-stress system. J. Ethnopharmacol. 261:113055. doi: 10.1016/j.jep.2020.113055
Cao, Q., Shen, M., Li, R., Liu, Y., Zeng, Z., Zhou, J., et al. (2025). Elucidating the specific mechanisms of the gut-brain axis: the short-chain fatty acids-microglia pathway. J. Neuroinflammation 22:133. doi: 10.1186/s12974-025-03454-y
Carloni, S., and Rescigno, M. (2023). The gut-brain vascular axis in neuroinflammation. Semin. Immunol. 69:101802. doi: 10.1016/j.smim.2023.101802
Castells-Nobau, A., Mayneris-Perxachs, J., and Fernández-Real, J. M. (2024). Unlocking the mind-gut connection: impact of human microbiome on cognition. Cell Host Microbe 32, 1248–1263. doi: 10.1016/j.chom.2024.07.019
Chang, L., Wei, Y., and Hashimoto, K. (2022). Brain-gut-microbiota axis in depression: a historical overview and future directions. Brain Res. Bull. 182, 44–56. doi: 10.1016/j.brainresbull.2022.02.004
Chen, Q., Jia, T., Wu, X., Chen, X., Wang, J., and Ba, Y. (2023). Polygalae Radix oligosaccharide esters may relieve depressive-like behavior in rats with chronic unpredictable mild stress via modulation of gut microbiota. Int. J. Mol. Sci. 24:13877. doi: 10.3390/ijms241813877
Chen, Y., Lian, B., Li, P., Yao, S., and Hou, Z. (2022). Studies on irritable bowel syndrome associated with anxiety or depression in the last 20 years: a bibliometric analysis. Front. Public Health 10:947097. doi: 10.3389/fpubh.2022.947097
Cheng, L., Chen, Y., He, J., Cheng, X., Wang, Y., Lin, X., et al. (2025). Effects of 12-week anti-inflammatory dietary education on depressive symptoms among depressed patients with breast Cancer undergoing adjuvant chemotherapy: a randomized controlled trial. Nutrients 17:957. doi: 10.3390/nu17060957
Cheng, J., Hu, H., Ju, Y., Liu, J., Wang, M., Liu, B., et al. (2024). Gut microbiota-derived short-chain fatty acids and depression: deep insight into biological mechanisms and potential applications. Gen. Psychiatr. 37:e101374. doi: 10.1136/gpsych-2023-101374
Cherian, L., Wang, Y., Holland, T., Agarwal, P., Aggarwal, N., and Morris, M. C. (2021). DASH and Mediterranean-Dash intervention for neurodegenerative delay (MIND) diets are associated with fewer depressive symptoms over time. J. Gerontol. A Biol. Sci. Med. Sci. 76, 151–156. doi: 10.1093/gerona/glaa044
Chu, L., Sun, X., Jia, X., Li, D., Gao, P., Zhang, Y., et al. (2022). The relationship among BDNF Val66Met polymorphism, plasma BDNF level, and trait anxiety in Chinese patients with panic disorder. Front. Psych. 13:932235. doi: 10.3389/fpsyt.2022.932235
Claesson, M. J., Jeffery, I. B., Conde, S., Power, S. E., O’Connor, E. M., Cusack, S., et al. (2012). Gut microbiota composition correlates with diet and health in the elderly. Nature 488, 178–184. doi: 10.1038/nature11319
Climent, E., Hevilla, F., Padial, M., Barril-Cuadrado, G., Blanca, M., Jiménez-Salcedo, T., et al. (2025). Psychobiotic protection of nutritional supplements and probiotics in patients undergoing Hemodialysis: a randomized trial. Nutrients 17:652. doi: 10.3390/nu17040652
COVID-19 Mental Disorders Collaborators (2021). Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 398, 1700–1712. doi: 10.1016/S0140-6736(21)02143-7
Cryan, J. F., O’Riordan, K. J., Sandhu, K., Peterson, V., and Dinan, T. G. (2020). The gut microbiome in neurological disorders. Lancet Neurol. 19, 179–194. doi: 10.1016/S1474-4422(19)30356-4
Dacaya, P., Sarapis, K., and Moschonis, G. (2025). The role and mechanisms of probiotic supplementation on depressive symptoms: a narrative review. Curr. Nutr. Rep. 14:53. doi: 10.1007/s13668-025-00644-1
Dai, H., Yang, H., Wang, R., Wang, X., and Zhang, X. (2025). Modulating gut microbiota with dietary components: a novel strategy for Cancer-depression comorbidity management. Nutrients 17:505. doi: 10.3390/nu17091505
Dalile, B., Van Oudenhove, L., Vervliet, B., and Verbeke, K. (2019). The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 16, 461–478. doi: 10.1038/s41575-019-0157-3
Dinan, T. G., and Cryan, J. F. (2020). Gut microbiota: a missing link in psychiatry. World Psychiatry 19, 111–112. doi: 10.1002/wps.20726
Dinan, T. G., Stanton, C., and Cryan, J. F. (2013). Psychobiotics: a novel class of psychotropic. Biol. Psychiatry 74, 720–726. doi: 10.1016/j.biopsych.2013.05.001
Ding, W., Wang, L., Li, L., Li, H., Wu, J., Zhang, J., et al. (2024). Pathogenesis of depression and the potential for traditional Chinese medicine treatment. Front. Pharmacol. 15:1407869. doi: 10.3389/fphar.2024.1407869
Dockray, G. J. (2014). Gastrointestinal hormones and the dialogue between gut and brain. J. Physiol. 592, 2927–2941. doi: 10.1113/jphysiol.2014.270850
Dong, X., Li, Y., Wang, X., Duan, Y., Liu, M., Wang, S., et al. (2024). Bidirectional associations between dietary diversity and depressive symptoms in Chinese adult women: a retrospective cohort study. J. Affect. Disord. 351, 683–693. doi: 10.1016/j.jad.2024.01.258
Dong, L., Zheng, Q., Cheng, Y., Zhou, M., Wang, M., Xu, J., et al. (2022). Gut microbial characteristics of adult patients with epilepsy. Front. Neurosci. 16:803538. doi: 10.3389/fnins.2022.803538
Donoso, F., Cryan, J. F., Olavarría-Ramírez, L., Nolan, Y. M., and Clarke, G. (2023). Inflammation, lifestyle factors, and the microbiome-gut-brain Axis: relevance to depression and antidepressant action. Clin. Pharmacol. Ther. 113, 246–259. doi: 10.1002/cpt.2581
Duan, D. M., Wang, Y. C., Hu, X., Wang, Y. B., Wang, Y. Q., Hu, Y., et al. (2024). Effects of regulating gut microbiota by electroacupuncture in the chronic unpredictable mild stress rat model. Neuroscience 557, 24–36. doi: 10.1016/j.neuroscience.2024.08.005
Emmerzaal, T. L., Preston, G., Geenen, B., Verweij, V., Wiesmann, M., Vasileiou, E., et al. (2020). Impaired mitochondrial complex I function as a candidate driver in the biological stress response and a concomitant stress-induced brain metabolic reprogramming in male mice. Transl. Psychiatry 10:176. doi: 10.1038/s41398-020-0858-y
Enriquez-Martinez, O. G., Martins, M. C. T., Pereira, T. S. S., Pacheco, S. O. S., Pacheco, F. J., Lopez, K. V., et al. (2021). Diet and lifestyle changes during the COVID-19 pandemic in Ibero-American countries: Argentina, Brazil, Mexico, Peru, and Spain. Front. Nutr. 8:671004. doi: 10.3389/fnut.2021.671004
Fan, Y., and Pedersen, O. (2021). Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19, 55–71. doi: 10.1038/s41579-020-0433-9
Ferrari, S., Mulè, S., Rosso, G., Parini, F., Galla, R., Molinari, C., et al. (2024). An innovative probiotic-based supplement to mitigate molecular factors connected to depression and anxiety: an in vitro study. Int. J. Mol. Sci. 25:774. doi: 10.3390/ijms25094774
Fitó, M., Guxens, M., Corella, D., Sáez, G., Estruch, R., de la Torre, R., et al. (2007). Effect of a traditional Mediterranean diet on lipoprotein oxidation: a randomized controlled trial. Arch. Intern. Med. 167, 1195–1203. doi: 10.1001/archinte.167.11.1195
Flores Saiffe Farías, A., Mendizabal, A. P., and Morales, J. A. (2018). An ontology systems approach on human brain expression and Metaproteomics. Front. Microbiol. 9:406. doi: 10.3389/fmicb.2018.00406
Francis, H. M., Stevenson, R. J., Tan, L. S. Y., Ehrenfeld, L., Byeon, S., Attuquayefio, T., et al. (2022). Kynurenic acid as a biochemical factor underlying the association between Western-style diet and depression: a cross-sectional study. Front. Nutr. 9:945538. doi: 10.3389/fnut.2022.945538
Fukuda, T., Ohya, R., Kobayashi, K., and Ano, Y. (2019). Matured hop Bitter acids in beer improve lipopolysaccharide-induced depression-like behavior. Front. Neurosci. 13:41. doi: 10.3389/fnins.2019.00041
Gagnon, E., Mitchell, P. L., Manikpurage, H. D., Abner, E., Taba, N., Esko, T., et al. (2023). Impact of the gut microbiota and associated metabolites on cardiometabolic traits, chronic diseases and human longevity: a mendelian randomization study. J. Transl. Med. 21:60. doi: 10.1186/s12967-022-03799-5
Gao, F. Y., Chen, X. F., Cui, L. X., Zhai, Y. J., Liu, J. L., Gao, C. C., et al. (2023). Gut microbiota mediates the pharmacokinetics of Zhi-zi-chi decoction for the personalized treatment of depression. J. Ethnopharmacol. 302:115934. doi: 10.1016/j.jep.2022.115934
Gao, M., Duan, X., Liu, X. R., Luo, S., Tang, S., Nie, H., et al. (2022). Modulatory effects of Huoxiang Zhengqi Oral liquid on gut microbiome homeostasis based on healthy adults and antibiotic-induced gut microbial dysbiosis mice model. Front. Pharmacol. 13:841990. doi: 10.3389/fphar.2022.841990
Gao, H., Ju, F., Ti, R., Zhang, Y., and Zhang, S. (2022). Differential regulation of microglial activation in response to different degree of ischemia. Front. Immunol. 13:792638. doi: 10.3389/fimmu.2022.792638
Gao, M., Tu, H., Liu, P., Zhang, Y., Zhang, R., Jing, L., et al. (2023). Association analysis of gut microbiota and efficacy of SSRIs antidepressants in patients with major depressive disorder. J. Affect. Disord. 330, 40–47. doi: 10.1016/j.jad.2023.02.143
Gibson, G. R., Hutkins, R., Sanders, M. E., Prescott, S. L., Reimer, R. A., Salminen, S. J., et al. (2017). Expert consensus document: the international scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14, 491–502. doi: 10.1038/nrgastro.2017.75
Godínez-Méndez, L. A., Gurrola-Díaz, C. M., Zepeda-Nuño, J. S., Vega-Magaña, N., Lopez-Roa, R. I., Íñiguez-Gutiérrez, L., et al. (2021). In vivo healthy benefits of Galacto-oligosaccharides from Lupinus albus (LA-GOS) in butyrate production through intestinal microbiota. Biomolecules 11:658. doi: 10.3390/biom11111658
Góralczyk-Bińkowska, A., Szmajda-Krygier, D., and Kozłowska, E. (2022). The microbiota-gut-brain Axis in psychiatric disorders. Int. J. Mol. Sci. 23:1245. doi: 10.3390/ijms231911245
Green, J. E., Berk, M., Mohebbi, M., Loughman, A., McGuinness, A. J., Castle, D., et al. (2023). Feasibility, acceptability, and safety of faecal microbiota transplantation in the treatment of major depressive disorder: a pilot randomized controlled trial. Can. J. Psychiatr. 68, 315–326. doi: 10.1177/07067437221150508
Gu, F., Wu, Y., Liu, Y., Dou, M., Jiang, Y., and Liang, H. (2020). Lactobacillus casei improves depression-like behavior in chronic unpredictable mild stress-induced rats by the BDNF-TrkB signal pathway and the intestinal microbiota. Food Funct. 11, 6148–6157. doi: 10.1039/D0FO00373E
Gumus, H., Ilgin, R., Koc, B., Yuksel, O., Kizildag, S., Guvendi, G., et al. (2022). A combination of ketogenic diet and voluntary exercise ameliorates anxiety and depression-like behaviors in Balb/c mice. Neurosci. Lett. 770:136443. doi: 10.1016/j.neulet.2021.136443
Guo, Q., Lin, H., Chen, P., Tan, S., Wen, Z., Lin, L., et al. (2021). Dynamic changes of intestinal flora in patients with irritable bowel syndrome combined with anxiety and depression after oral administration of enterobacteria capsules. Bioengineered 12, 11885–11897. doi: 10.1080/21655979.2021.1999374
Guo, J., Ma, B., Wang, Z., Chen, Y., Tian, W., and Dong, Y. (2022). Royal Jelly Protected against dextran-Sulfate-sodium-induced colitis by improving the colonic mucosal barrier and gut microbiota. Nutrients 14:69. doi: 10.3390/nu14102069
Haghighat, N., Rajabi, S., and Mohammadshahi, M. (2021). Effect of synbiotic and probiotic supplementation on serum brain-derived neurotrophic factor level, depression and anxiety symptoms in hemodialysis patients: a randomized, double-blinded, clinical trial. Nutr. Neurosci. 24, 490–499. doi: 10.1080/1028415X.2019.1646975
Hamad, N. A., Rahim, H. F. A., and Shi, Z. (2024). Association between dietary patterns and depression symptoms among adults with or without diabetes in Qatar: a population-based study. BMC Public Health 24:2260. doi: 10.1186/s12889-024-19716-y
Han, S. K., and Kim, D. H. (2019). Lactobacillus mucosae and Bifidobacterium longum synergistically alleviate immobilization stress-induced anxiety/depression in mice by suppressing gut dysbiosis. J. Microbiol. Biotechnol. 29, 1369–1374. doi: 10.4014/jmb.1907.07044
Han, H., Yao, J., Wu, J., Mao, S., Pan, H., Qv, L., et al. (2025). Implications of neurogenesis in depression through BDNF: rodent models, regulatory pathways, gut microbiota, and potential therapy. Mol. Psychiatry 30, 4409–4421. doi: 10.1038/s41380-025-03044-7
Hao, W. Z., Li, X. J., Zhang, P. W., and Chen, J. X. (2020). A review of antibiotics, depression, and the gut microbiome. Psychiatry Res. 284:112691. doi: 10.1016/j.psychres.2019.112691
Hassamal, S. (2023). Chronic stress, neuroinflammation, and depression: an overview of pathophysiological mechanisms and emerging anti-inflammatories. Front. Psych. 14:1130989. doi: 10.3389/fpsyt.2023.1130989
He, J., Chang, L., Zhang, L., Wu, W., and Zhuo, D. (2023). Effect of probiotic supplementation on cognition and depressive symptoms in patients with depression: a systematic review and meta-analysis. Medicine (Baltimore) 102:e36005. doi: 10.1097/MD.0000000000036005
He, H., He, H., Mo, L., You, Z., and Zhang, J. (2024). Priming of microglia with dysfunctional gut microbiota impairs hippocampal neurogenesis and fosters stress vulnerability of mice. Brain Behav. Immun. 115, 280–294. doi: 10.1016/j.bbi.2023.10.031
Hockenberry, J. M., Joski, P., Yarbrough, C., and Druss, B. G. (2019). Trends in treatment and spending for patients receiving outpatient treatment of depression in the United States, 1998-2015. JAMA Psychiatry 76, 810–817. doi: 10.1001/jamapsychiatry.2019.0633
Hofmeister, M., Clement, F., Patten, S., Li, J., Dowsett, L. E., Farkas, B., et al. (2021). The effect of interventions targeting gut microbiota on depressive symptoms: a systematic review and meta-analysis. CMAJ Open 9, E1195–e1204. doi: 10.9778/cmajo.20200283
Huang, Z., Heringa, J., Feenstra, K. A., and Liu, T. (2020). Influence of gut microbiota on mental health via neurotransmitters: a review. J. Artif. Intell. Med. Sci. 1, 1–14. doi: 10.2991/jaims.d.200420.001
Huang, Q., Liu, H., Suzuki, K., Ma, S., and Liu, C. (2019). Linking what we eat to our mood: a review of diet, dietary antioxidants, and depression. Antioxidants (Basel). 8:376. doi: 10.3390/antiox8090376
Ibnou-Zekri, N., Blum, S., Schiffrin, E. J., and von der Weid, T. (2003). Divergent patterns of colonization and immune response elicited from two intestinal Lactobacillus strains that display similar properties in vitro. Infect. Immun. 71, 428–436. doi: 10.1128/IAI.71.1.428-436.2003
Icer, M. A., Sarikaya, B., Kocyigit, E., Atabilen, B., Çelik, M. N., Capasso, R., et al. (2024). Contributions of gamma-aminobutyric acid (GABA) produced by lactic acid Bacteria on food quality and human health: current applications and future prospects. Foods 13:437. doi: 10.3390/foods13152437
Jach, M. E., Serefko, A., Szopa, A., Sajnaga, E., Golczyk, H., Santos, L. S., et al. (2023). The role of probiotics and their metabolites in the treatment of depression. Molecules 28:213. doi: 10.3390/molecules28073213
Jang, H. M., Lee, K. E., and Kim, D. H. (2019). The preventive and curative effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on immobilization stress-induced anxiety/depression and colitis in mice. Nutrients 11:819. doi: 10.3390/nu11040819
Johnson, C. S. C., Frye, B. M., Register, T. C., Snyder-Mackler, N., and Shively, C. A. (2022). Mediterranean diet reduces social isolation and anxiety in adult female nonhuman Primates. Nutrients 14:2852. doi: 10.3390/nu14142852
Joo, M. K., Lee, J. W., Woo, J. H., Kim, H. J., Kim, D. H., and Choi, J. H. (2024). Regulation of colonic neuropeptide Y expression by the gut microbiome in patients with ulcerative colitis and its association with anxiety- and depression-like behavior in mice. Gut Microbes 16:2319844. doi: 10.1080/19490976.2024.2319844
Kaur, S., Sharma, P., Mayer, M. J., Neuert, S., Narbad, A., and Kaur, S. (2023). Beneficial effects of GABA-producing potential probiotic Limosilactobacillus fermentum L18 of human origin on intestinal permeability and human gut microbiota. Microb. Cell Factories 22:256. doi: 10.1186/s12934-023-02264-2
Kazemi, A., Noorbala, A. A., Azam, K., Eskandari, M. H., and Djafarian, K. (2019). Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: a randomized clinical trial. Clin. Nutr. 38, 522–528. doi: 10.1016/j.clnu.2018.04.010
Ke, X., Walker, A., Haange, S. B., Lagkouvardos, I., Liu, Y., Schmitt-Kopplin, P., et al. (2019). Synbiotic-driven improvement of metabolic disturbances is associated with changes in the gut microbiome in diet-induced obese mice. Mol. Metab. 22, 96–109. doi: 10.1016/j.molmet.2019.01.012
Knight, R., Cedillo, Y., Judd, S., Baker, E., Fruge, A., and Moellering, D. (2023). A cross-sectional study observing the association of psychosocial stress and dietary intake with gut microbiota genera and alpha diversity among a young adult cohort of black and white women in Birmingham, Alabama. Res. Sq. 3:3146763. doi: 10.21203/rs.3.rs-3146763/v1
Kociszewska, D., and Vlajkovic, S. M. (2022). The Association of Inflammatory gut Diseases with neuroinflammatory and auditory disorders. Front. Biosci. (Elite Ed.) 14:8. doi: 10.31083/j.fbe1402008
Koelman, L., Egea Rodrigues, C., and Aleksandrova, K. (2022). Effects of dietary patterns on biomarkers of inflammation and immune responses: a systematic review and Meta-analysis of randomized controlled trials. Adv. Nutr. 13, 101–115. doi: 10.1093/advances/nmab086
Koopman, F. A., Chavan, S. S., Miljko, S., Grazio, S., Sokolovic, S., Schuurman, P. R., et al. (2016). Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc. Natl. Acad. Sci. U. S. A. 113, 8284–8289. doi: 10.1073/pnas.1605635113
Kuijper, E. J., Allegretii, J., Hawkey, P., Sokol, H., Goldenberg, S., Ianiro, G., et al. (2019). A necessary discussion after transmission of multidrug-resistant organisms through faecal microbiota transplantations. Lancet Infect. Dis. 19, 1161–1162. doi: 10.1016/S1473-3099(19)30545-6
Lach, G., Schellekens, H., Dinan, T. G., and Cryan, J. F. (2018). Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics 15, 36–59. doi: 10.1007/s13311-017-0585-0
Lai, Y., and Xiong, P. (2025). Analysis of gut microbiota and depression and anxiety: mendelian randomization from three datasets. Gen. Hosp. Psychiatry 94, 206–218. doi: 10.1016/j.genhosppsych.2025.03.012
Lapmanee, S., Supkamonseni, N., Bhubhanil, S., Treesaksrisakul, N., Sirithanakorn, C., Khongkow, M., et al. (2024). Stress-induced changes in cognitive function and intestinal barrier integrity can be ameliorated by venlafaxine and synbiotic supplementations. PeerJ 12:e17033. doi: 10.7717/peerj.17033
Lassale, C., Batty, G. D., Baghdadli, A., Jacka, F., Sánchez-Villegas, A., Kivimäki, M., et al. (2019). Healthy dietary indices and risk of depressive outcomes: a systematic review and meta-analysis of observational studies. Mol. Psychiatry 24, 965–986. doi: 10.1038/s41380-018-0237-8
Lee, M., and Chang, E. B. (2021). Inflammatory bowel diseases (IBD) and the microbiome-searching the crime scene for clues. Gastroenterology 160, 524–537. doi: 10.1053/j.gastro.2020.09.056
Leo, A., De Caro, C., Mainardi, P., Tallarico, M., Nesci, V., Marascio, N., et al. (2021). Increased efficacy of combining prebiotic and postbiotic in mouse models relevant to autism and depression. Neuropharmacology 198:108782. doi: 10.1016/j.neuropharm.2021.108782
Li, S., Huang, J., Luo, D., Fu, W., and Liu, J. (2024). Electro-acupuncture inhibits HDAC2 via modulating gut microbiota to ameliorate SNI-induced pain and depression-like behavior in rats. J. Affect. Disord. 360, 305–313. doi: 10.1016/j.jad.2024.02.069
Li, Z., Li, Z., Lv, X., Li, Z., Xiong, L., Hu, X., et al. (2020). Intracerebroventricular Administration of Interferon-Alpha Induced Depressive-like Behaviors and Neurotransmitter Changes in Rhesus monkeys. Front. Neurosci. 14:585604. doi: 10.3389/fnins.2020.585604
Li, J., Li, Y., Zhao, J., Li, L., Wang, Y., Chen, F., et al. (2024). Effects of Bifidobacterium breve 207-1 on regulating lifestyle behaviors and mental wellness in healthy adults based on the microbiome-gut-brain axis: a randomized, double-blind, placebo-controlled trial. Eur. J. Nutr. 63, 2567–2585. doi: 10.1007/s00394-024-03447-2
Li, J. L., Lin, L., Wu, M. M., Zhang, J. Y., Zhang, Y. X., Cao, M. R., et al. (2024). A meta-analysis of the efficacy and safety of the traditional Chinese medicine formula Kaixinsan decoction for depression. Medicine (Baltimore) 103:e36719. doi: 10.1097/MD.0000000000036719
Li, W., Lu, L., Liu, B., and Qin, S. (2020). Effects of phycocyanin on pulmonary and gut microbiota in a radiation-induced pulmonary fibrosis model. Biomed. Pharmacother. 132:110826. doi: 10.1016/j.biopha.2020.110826
Li, P., Tang, X. D., Cai, Z. X., Qiu, J. J., Lin, X. L., Zhu, T., et al. (2015). CNP signal pathway up-regulated in rectum of depressed rats and the interventional effect of Xiaoyaosan. World J. Gastroenterol. 21, 1518–1530. doi: 10.3748/wjg.v21.i5.1518
Li, J., Wang, J., Wang, M., Zheng, L., Cen, Q., Wang, F., et al. (2023). Bifidobacterium: a probiotic for the prevention and treatment of depression. Front. Microbiol. 14:1174800. doi: 10.3389/fmicb.2023.1174800
Lin, H., Guo, Q., Wen, Z., Tan, S., Chen, J., Lin, L., et al. (2021). The multiple effects of fecal microbiota transplantation on diarrhea-predominant irritable bowel syndrome (IBS-D) patients with anxiety and depression behaviors. Microb. Cell Factories 20:233. doi: 10.1186/s12934-021-01720-1
Liu, B., He, Y., Wang, M., Liu, J., Ju, Y., Zhang, Y., et al. (2018). Efficacy of probiotics on anxiety—a meta-analysis of randomized controlled trials. Depress. Anxiety 35, 935–945. doi: 10.1002/da.22811
Liu, Y., Ju, Y., Cui, L., Liu, T., Hou, Y., Wu, Q., et al. (2021). Association between dietary Fiber intake and incidence of depression and anxiety in patients with essential hypertension. Nutrients 13:159. doi: 10.3390/nu13114159
Liu, X., Liu, H., Wu, X., Zhao, Z., Wang, S., Wang, H., et al. (2024). Xiaoyaosan against depression through suppressing LPS mediated TLR4/NLRP3 signaling pathway in “microbiota-gut-brain” axis. J. Ethnopharmacol. 335:118683. doi: 10.1016/j.jep.2024.118683
Liu, G., Lu, J., Sun, W., Jia, G., Zhao, H., Chen, X., et al. (2022). Tryptophan supplementation enhances intestinal health by improving gut barrier function, alleviating inflammation, and modulating intestinal microbiome in lipopolysaccharide-challenged piglets. Front. Microbiol. 13:919431. doi: 10.3389/fmicb.2022.919431
Liu, L., Wang, H., Chen, X., Zhang, Y., Zhang, H., and Xie, P. (2023). Gut microbiota and its metabolites in depression: from pathogenesis to treatment. EBioMedicine 90:104527. doi: 10.1016/j.ebiom.2023.104527
Logsdon, A. F., Erickson, M. A., Chen, X., Qiu, J., Lim, Y. P., Stonestreet, B. S., et al. (2020). Inter-alpha inhibitor proteins attenuate lipopolysaccharide-induced blood-brain barrier disruption and downregulate circulating interleukin 6 in mice. J. Cereb. Blood Flow Metab. 40, 1090–1102. doi: 10.1177/0271678X19859465
Lu, S., Zhao, Q., Guan, Y., Sun, Z., Li, W., Guo, S., et al. (2024). The communication mechanism of the gut-brain axis and its effect on central nervous system diseases: a systematic review. Biomed. Pharmacother. 178:117207. doi: 10.1016/j.biopha.2024.117207
Lv, X., Sun, S., Wang, J., Chen, H., Li, S., Hu, Y., et al. (2022). Anti-inflammatory dietary diversity and depressive symptoms among older adults: a Nationwide cross-sectional analysis. Nutrients 14:5062. doi: 10.3390/nu14235062
Ma, X. Y., Son, Y. H., Yoo, J. W., Joo, M. K., and Kim, D. H. (2022). Citation: tight junction protein expression-inducing probiotics alleviate TNBS-induced cognitive impairment with colitis in mice. Nutrients 14:975. doi: 10.3390/nu14142975
Maitiniyazi, G., Cao, X., Chen, Y., Zhang, R., Liu, Y., Li, Z., et al. (2022). Impact of gut microbiota on the association between diet and depressive symptoms in breast Cancer. Nutrients 14:1186. doi: 10.3390/nu14061186
Mann, J. J., Oquendo, M. A., Watson, K. T., Boldrini, M., Malone, K. M., Ellis, S. P., et al. (2014). Anxiety in major depression and cerebrospinal fluid free gamma-aminobutyric acid. Depress. Anxiety 31, 814–821. doi: 10.1002/da.22278
Mao, X., Larsen, S. B., Zachariassen, L. S. F., Brunse, A., Adamberg, S., Mejia, J. L. C., et al. (2024). Transfer of modified gut viromes improves symptoms associated with metabolic syndrome in obese male mice. Nat. Commun. 15:4704. doi: 10.1038/s41467-024-49152-w
Markowiak, P., and Śliżewska, K. (2018). The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut. Pathog. 10:21. doi: 10.1186/s13099-018-0250-0
Martin, K., Anna, U., Michal, M., Ingrid, T., and Juraj, M. (2019). Simultaneous determination of Total cortisol and cortisone in human plasma by liquid chromatography-tandem mass spectrometry: method development, validation and preliminary clinical application. Curr. Pharm. Anal. 14:811. doi: 10.2174/1573412914666180427094811
Martin, F. P., Cominetti, O., Berger, B., Combremont, S., Marquis, J., Xie, G., et al. (2024). Metabolome-associated psychological comorbidities improvement in irritable bowel syndrome patients receiving a probiotic. Gut Microbes 16:2347715. doi: 10.1080/19490976.2024.2347715
Matin, S., and Dadkhah, M. (2024). BDNF/CREB signaling pathway contribution in depression pathogenesis: a survey on the non-pharmacological therapeutic opportunities for gut microbiota dysbiosis. Brain Res. Bull. 207:110882. doi: 10.1016/j.brainresbull.2024.110882
Mayer, E. A., Knight, R., Mazmanian, S. K., Cryan, J. F., and Tillisch, K. (2014). Gut microbes and the brain: paradigm shift in neuroscience. J. Neurosci. 34, 15490–15496. doi: 10.1523/JNEUROSCI.3299-14.2014
Mayer, E. A., Nance, K., and Chen, S. (2022). The gut-brain Axis. Annu. Rev. Med. 73, 439–453. doi: 10.1146/annurev-med-042320-014032
Medina-Larqué, A. S., Rodríguez-Daza, M. C., Roquim, M., Dudonné, S., Pilon, G., Levy, É., et al. (2022). Cranberry polyphenols and agave agavins impact gut immune response and microbiota composition while improving gut barrier function, inflammation, and glucose metabolism in mice fed an obesogenic diet. Front. Immunol. 13:871080. doi: 10.3389/fimmu.2022.871080
Medina-Rodriguez, E. M., Lowell, J. A., Worthen, R. J., Syed, S. A., and Beurel, E. (2018). Involvement of innate and adaptive immune systems alterations in the pathophysiology and treatment of depression. Front. Neurosci. 12:547. doi: 10.3389/fnins.2018.00547
Medina-Rodríguez, E. M., Martínez-Raga, J., and Sanz, Y. (2024). Intestinal barrier, immunity and microbiome: Partners in the Depression Crime. Pharmacol. Rev. 76, 956–969. doi: 10.1124/pharmrev.124.001202
Mehta, J. P., Ayakar, S., and Singhal, R. S. (2023). The potential of paraprobiotics and postbiotics to modulate the immune system: a review. Microbiol. Res. 275:127449. doi: 10.1016/j.micres.2023.127449
Meier, S. M., Mattheisen, M., Mors, O., Mortensen, P. B., Laursen, T. M., and Penninx, B. W. (2016). Increased mortality among people with anxiety disorders: total population study. Br. J. Psychiatry 209, 216–221. doi: 10.1192/bjp.bp.115.171975
Merkouris, E., Mavroudi, T., Miliotas, D., Tsiptsios, D., Serdari, A., Christidi, F., et al. (2024). Probiotics’ effects in the treatment of anxiety and depression: a comprehensive review of 2014–2023 clinical trials. Microorganisms 12:411. doi: 10.3390/microorganisms12020411
Misiak, B., Łoniewski, I., Marlicz, W., Frydecka, D., Szulc, A., Rudzki, L., et al. (2020). The HPA axis dysregulation in severe mental illness: can we shift the blame to gut microbiota? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 102:109951. doi: 10.1016/j.pnpbp.2020.109951
Młynarska, E., Gadzinowska, J., Tokarek, J., Forycka, J., Szuman, A., Franczyk, B., et al. (2022). The role of the microbiome-brain-gut Axis in the pathogenesis of depressive disorder. Nutrients 14:1921. doi: 10.3390/nu14091921
Mörkl, S., Narrath, M., Schlotmann, D., Sallmutter, M. T., Putz, J., Lang, J., et al. (2025). Multi-species probiotic supplement enhances vagal nerve function - results of a randomized controlled trial in patients with depression and healthy controls. Gut Microbes 17:2492377. doi: 10.1080/19490976.2025.2492377
Moshfeghinia, R., Nemati, H., Ebrahimi, A., Shekouh, D., Karami, S., Eraghi, M. M., et al. (2025). The impact of probiotics, prebiotics, and synbiotics on depression and anxiety symptoms of patients with depression: a systematic review and meta-analysis. J. Psychiatr. Res. 188, 104–116. doi: 10.1016/j.jpsychires.2025.05.053
Nataraj, B. H., Ali, S. A., Behare, P. V., and Yadav, H. (2020). Postbiotics-parabiotics: the new horizons in microbial biotherapy and functional foods. Microb. Cell Factories 19:168. doi: 10.1186/s12934-020-01426-w
Neil-Sztramko, S. E., Levy, A., Flint, A. J., Goodarzi, Z., Gough, A., Trenaman, S. C., et al. (2025). Pharmacological treatment of anxiety in older adults: a systematic review and meta-analysis. Lancet Psychiatry 12, 421–432. doi: 10.1016/S2215-0366(25)00100-2
Nikolova, V. L., Cleare, A. J., Young, A. H., and Stone, J. M. (2025). Exploring the mechanisms of action of probiotics in depression: results from a randomized controlled pilot trial. J. Affect. Disord. 376, 241–250. doi: 10.1016/j.jad.2025.01.153
Nikolova, V. L., Smith, M. R. B., Hall, L. J., Cleare, A. J., Stone, J. M., and Young, A. H. (2021). Perturbations in gut microbiota composition in psychiatric disorders: a review and Meta-analysis. JAMA Psychiatry 78, 1343–1354. doi: 10.1001/jamapsychiatry.2021.2573
Nohesara, S., Abdolmaleky, H. M., Zhou, J. R., and Thiagalingam, S. (2023). Microbiota-induced epigenetic alterations in depressive disorders are targets for nutritional and probiotic therapies. Genes (Basel) 14:2217. doi: 10.3390/genes14122217
Noonan, S., Zaveri, M., Macaninch, E., and Martyn, K. (2020). Food & mood: a review of supplementary prebiotic and probiotic interventions in the treatment of anxiety and depression in adults. BMJ Nutr. Prev. Health. 3, 351–362. doi: 10.1136/bmjnph-2019-000053
Noori, M., Shateri, Z., Babajafari, S., Eskandari, M. H., Parastouei, K., Ghasemi, M., et al. (2025). The effect of probiotic-fortified kefir on depression, appetite, oxidative stress, and inflammatory parameters in Iranian overweight and obese elderly: a randomized, double-blind, placebo-controlled clinical trial. J. Health Popul. Nutr. 44:30. doi: 10.1186/s41043-025-00773-x
Paiva, I. H. R., Maciel, L. M., Silva, R. S. D., Mendonça, I. P., Souza, J. R. B., and Peixoto, C. A. (2024). Prebiotics modulate the microbiota-gut-brain axis and ameliorate anxiety and depression-like behavior in HFD-fed mice. Food Res. Int. 182:114153. doi: 10.1016/j.foodres.2024.114153
Peng, Y., Du, Y., Zhang, Y., Wang, Z., Hu, T., Mai, Y., et al. (2024). Gegen Qinlian decoction alleviates depression-like behavior by modulating the gut microenvironment in CUMS rats. BMC Complement. Med. Ther. 24:339. doi: 10.1186/s12906-024-04638-4
Pengpid, S., and Peltzer, K. (2024). Dietary diversity among women with depressive and generalized anxiety symptoms in Nepal. Sci. Rep. 14:17688. doi: 10.1038/s41598-024-68346-2
Perez-Cornago, A., Sanchez-Villegas, A., Bes-Rastrollo, M., Gea, A., Molero, P., Lahortiga-Ramos, F., et al. (2017). Relationship between adherence to dietary approaches to stop hypertension (DASH) diet indices and incidence of depression during up to 8 years of follow-up. Public Health Nutr. 20, 2383–2392. doi: 10.1017/S1368980016001531
Ragavan, M. L., and Hemalatha, S. (2024). The functional roles of short chain fatty acids as postbiotics in human gut: future perspectives. Food Sci. Biotechnol. 33, 275–285. doi: 10.1007/s10068-023-01414-x
Rahman, Z., Padhy, H. P., and Dandekar, M. P. (2024). Cell-free supernatant of Lactobacillus rhamnosus and Bifidobacterium breve ameliorates ischemic stroke-generated neurological deficits in rats. Probiotics Antimicrob. Proteins 1:256. doi: 10.1007/s12602-024-10256-w
Ramsteijn, A. S., Jašarević, E., Houwing, D. J., Bale, T. L., and Olivier, J. D. (2020). Antidepressant treatment with fluoxetine during pregnancy and lactation modulates the gut microbiome and metabolome in a rat model relevant to depression. Gut Microbes 11, 735–753. doi: 10.1080/19490976.2019.1705728
Rao, J., Qiao, Y., Xie, R., Lin, L., Jiang, J., Wang, C., et al. (2021). Fecal microbiota transplantation ameliorates stress-induced depression-like behaviors associated with the inhibition of glial and NLRP3 inflammasome in rat brain. J. Psychiatr. Res. 137, 147–157. doi: 10.1016/j.jpsychires.2021.02.057
Rasmussen, T. S., Koefoed, A. K., Jakobsen, R. R., Deng, L., Castro-Mejía, J. L., Brunse, A., et al. (2020). Bacteriophage-mediated manipulation of the gut microbiome - promises and presents limitations. FEMS Microbiol. Rev. 44, 507–521. doi: 10.1093/femsre/fuaa020
Rasmussen, T. S., Mao, X., Forster, S., Larsen, S. B., Von Münchow, A., Tranæs, K. D., et al. (2024). Overcoming donor variability and risks associated with fecal microbiota transplants through bacteriophage-mediated treatments. Microbiome 12:119. doi: 10.1186/s40168-024-01820-1
Rehman, M. U., Ghazanfar, S., Ul Haq, R., Ullah, S., Khan, S., Wu, J., et al. (2022). Probiotics (Bacillus clausii and Lactobacillus fermentum NMCC-14) ameliorate stress behavior in mice by increasing monoamine levels and mRNA expression of dopamine receptors (D(1) and D(2)) and synaptophysin. Front. Pharmacol. 13:915595. doi: 10.3389/fphar.2022.915595
Ren, M., Zhang, H., Qi, J., Hu, A., Jiang, Q., Hou, Y., et al. (2020). An almond-based low carbohydrate diet improves depression and Glycometabolism in patients with type 2 diabetes through modulating gut microbiota and GLP-1: a randomized controlled trial. Nutrients 12:36. doi: 10.3390/nu12103036
Ritz, N. L., Draper, L. A., Bastiaanssen, T. F. S., Turkington, C. J. R., Peterson, V. L., van de Wouw, M., et al. (2024). The gut virome is associated with stress-induced changes in behaviour and immune responses in mice. Nat. Microbiol. 9, 359–376. doi: 10.1038/s41564-023-01564-y
Romo-Araiza, A., Gutiérrez-Salmeán, G., Galván, E. J., Hernández-Frausto, M., Herrera-López, G., Romo-Parra, H., et al. (2018). Probiotics and prebiotics as a therapeutic strategy to improve memory in a model of middle-aged rats. Front. Aging Neurosci. 10:416. doi: 10.3389/fnagi.2018.00416
Rondepierre, F., Meynier, M., Gagniere, J., Deneuvy, V., Deneuvy, A., Roche, G., et al. (2024). Preclinical and clinical evidence of the association of colibactin-producing Escherichia coli with anxiety and depression in colon cancer. World J. Gastroenterol. 30, 2817–2826. doi: 10.3748/wjg.v30.i21.2817
Rudzki, L., Ostrowska, L., Pawlak, D., Małus, A., Pawlak, K., Waszkiewicz, N., et al. (2019). Probiotic Lactobacillus plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: a double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 100, 213–222. doi: 10.1016/j.psyneuen.2018.10.010
Rutsch, A., Kantsjö, J. B., and Ronchi, F. (2020). The gut-brain Axis: how microbiota and host inflammasome influence brain physiology and pathology. Front. Immunol. 11:604179. doi: 10.3389/fimmu.2020.604179
Salminen, S., Collado, M. C., Endo, A., Hill, C., Lebeer, S., Quigley, E. M. M., et al. (2021). The international scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 18, 649–667. doi: 10.1038/s41575-021-00440-6
Shanahan, E. R., McMaster, J. J., and Staudacher, H. M. (2021). Conducting research on diet-microbiome interactions: a review of current challenges, essential methodological principles, and recommendations for best practice in study design. J. Hum. Nutr. Diet. 34, 631–644. doi: 10.1111/jhn.12868
Sharifi, M. H., Poursadeghfard, M., Afshari, M., Alizadeh, Z., Vatanpour, M., Soltani, M., et al. (2025). The effectiveness of modified Mediterranean and traditional Persian diets in fatigue and depressive severity in people with multiple sclerosis: a randomized controlled clinical trial. Iran. J. Med. Sci. 50, 146–158. doi: 10.30476/ijms.2024.101961.3472
Shen, Y. C., Chang, C. E., Lin, M. N., and Lin, C. L. (2021). Vegetarian diet is associated with lower risk of depression in Taiwan. Nutrients 13:1059. doi: 10.3390/nu13041059
Shen, W., Tao, Y., Zheng, F., Zhou, H., Wu, H., Shi, H., et al. (2023). The alteration of gut microbiota in venlafaxine-ameliorated chronic unpredictable mild stress-induced depression in mice. Behav. Brain Res. 446:114399. doi: 10.1016/j.bbr.2023.114399
Shi, B., Luo, J., Fang, Y., Liu, X., Rao, Z., Liu, R., et al. (2019). Xiaoyao pills prevent lipopolysaccharide-induced depression by inhibiting inflammation and protecting nerves. Front. Pharmacol. 10:1324. doi: 10.3389/fphar.2019.01324
Shoubridge, A. P., Choo, J. M., Martin, A. M., Keating, D. J., Wong, M.-L., Licinio, J., et al. (2022). The gut microbiome and mental health: advances in research and emerging priorities. Mol. Psychiatry 27, 1908–1919. doi: 10.1038/s41380-022-01479-w
Simpson, C. A., Diaz-Arteche, C., Eliby, D., Schwartz, O. S., Simmons, J. G., and Cowan, C. S. M. (2021). The gut microbiota in anxiety and depression - a systematic review. Clin. Psychol. Rev. 83:101943. doi: 10.1016/j.cpr.2020.101943
Sittipo, P., Choi, J., Lee, S., and Lee, Y. K. (2022). The function of gut microbiota in immune-related neurological disorders: a review. J. Neuroinflammation 19:154. doi: 10.1186/s12974-022-02510-1
Skott, E., Yang, L. L., Stiernborg, M., Söderström, Å., Rȕegg, J., Schalling, M., et al. (2020). Effects of a synbiotic on symptoms, and daily functioning in attention deficit hyperactivity disorder – a double-blind randomized controlled trial. Brain Behav. Immun. 89, 9–19. doi: 10.1016/j.bbi.2020.05.056
Smolensky, I. V., Zajac-Bakri, K., Gass, P., and Inta, D. (2023). Ketogenic diet for mood disorders from animal models to clinical application. J. Neural Transm. (Vienna) 130, 1195–1205. doi: 10.1007/s00702-023-02620-x
Suez, J., Zmora, N., Segal, E., and Elinav, E. (2019). The pros, cons, and many unknowns of probiotics. Nat. Med. 25, 716–729. doi: 10.1038/s41591-019-0439-x
Sun, B., Ma, T., Li, Y., Yang, N., Li, B., Zhou, X., et al. (2022). Bifidobacterium lactis Probio-M8 adjuvant treatment confers added benefits to patients with coronary artery disease via target modulation of the gut-heart/-brain axes. mSystems 7:e0010022. doi: 10.1128/msystems.00100-22
Sushma, G., Vaidya, B., Sharma, S., Devabattula, G., Bishnoi, M., Kondepudi, K. K., et al. (2023). Bifidobacterium breve Bif11 supplementation improves depression-related neurobehavioural and neuroinflammatory changes in the mouse. Neuropharmacology 229:109480. doi: 10.1016/j.neuropharm.2023.109480
Tahiri, J., Mian, M., Aftan, F., Habbal, S., Salehi, F., Reddy, P. H., et al. (2024). Serotonin in depression and Alzheimer’s disease: focus on SSRI’S beneficial effects. Ageing Res. Rev. 101:102537. doi: 10.1016/j.arr.2024.102537
Tan, J., Li, X., Zhu, Y., Sullivan, M. A., Deng, B., Zhai, X., et al. (2022). Antidepressant Shugan Jieyu capsule alters gut microbiota and intestinal microbiome function in rats with chronic unpredictable mild stress -induced depression. Front. Pharmacol. 13:828595. doi: 10.3389/fphar.2022.828595
Thangaraj, A., Periyasamy, P., Liao, K., Bendi, V. S., Callen, S., Pendyala, G., et al. (2018). HIV-1 TAT-mediated microglial activation: role of mitochondrial dysfunction and defective mitophagy. Autophagy 14, 1596–1619. doi: 10.1080/15548627.2018.1476810
Thomaz, A. C., Iyer, V., Woodward, T. J., and Hohmann, A. G. (2021). Fecal microbiota transplantation and antibiotic treatment attenuate naloxone-precipitated opioid withdrawal in morphine-dependent mice. Exp. Neurol. 343:113787. doi: 10.1016/j.expneurol.2021.113787
Tian, P., Chen, Y., Zhu, H., Wang, L., Qian, X., Zou, R., et al. (2022). Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: a randomized clinical trial. Brain Behav. Immun. 100, 233–241. doi: 10.1016/j.bbi.2021.11.023
Tian, X., Wang, G., Teng, F., Xue, X., Pan, J., Mao, Q., et al. (2024). Zhi zi chi decoction (Gardeniae fructus and semen Sojae Praeparatum) attenuates anxious depression via modulating microbiota-gut-brain axis in corticosterone combined with chronic restraint stress-induced mice. CNS Neurosci. Ther. 30:e14519. doi: 10.1111/cns.14519
Tillisch, K., Labus, J., Kilpatrick, L., Jiang, Z., Stains, J., Ebrat, B., et al. (2013). Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 144:1401.e1391. doi: 10.1053/j.gastro.2013.02.043
Toader, C., Dobrin, N., Costea, D., Glavan, L. A., Covache-Busuioc, R. A., Dumitrascu, D. I., et al. (2024). Mind, mood and microbiota-gut-brain Axis in psychiatric disorders. Int. J. Mol. Sci. 25:340. doi: 10.3390/ijms25063340
Tolkien, K., Bradburn, S., and Murgatroyd, C. (2019). An anti-inflammatory diet as a potential intervention for depressive disorders: a systematic review and meta-analysis. Clin. Nutr. 38, 2045–2052. doi: 10.1016/j.clnu.2018.11.007
Tzikos, G., Chamalidou, E., Christopoulou, D., Apostolopoulou, A., Gkarmiri, S., Pertsikapa, M., et al. (2025). Psychobiotics ameliorate depression and anxiety status in surgical oncology patients: results from the ProDeCa study. Nutrients 17:857. doi: 10.3390/nu17050857
Valdes, A. M., Walter, J., Segal, E., and Spector, T. D. (2018). Role of the gut microbiota in nutrition and health. BMJ 361:k2179. doi: 10.1136/bmj.k2179
Van de Wouw, M., Boehme, M., Lyte, J. M., Wiley, N., Strain, C., O’Sullivan, O., et al. (2018). Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J. Physiol. 596, 4923–4944. doi: 10.1113/JP276431
Vindegaard, N., Speyer, H., Nordentoft, M., Rasmussen, S., and Benros, M. E. (2021). Gut microbial changes of patients with psychotic and affective disorders: a systematic review. Schizophr. Res. 234, 1–10. doi: 10.1016/j.schres.2019.12.014
Wang, J., Hu, J. Q., Song, Y. J., Yin, J., Wang, Y. Y., Peng, B., et al. (2022). 2’-Fucosyllactose ameliorates oxidative stress damage in d-galactose-induced aging mice by regulating gut microbiota and AMPK/SIRT1/FOXO1 pathway. Foods 11:151. doi: 10.3390/foods11020151
Wang, H., Wang, Q., Yang, C., Guo, M., Cui, X., Jing, Z., et al. (2022). Bacteroides acidifaciens in the gut plays a protective role against CD95-mediated liver injury. Gut Microbes 14:2027853. doi: 10.1080/19490976.2022.2027853
Wang, J., Zhu, H., Song, X., Zhao, J., Zhang, J., Zhang, J., et al. (2024). Electroacupuncture regulates gut microbiota to reduce depressive-like behavior in rats. Front. Microbiol. 15:1327630. doi: 10.3389/fmicb.2024.1327630
Warren, A., Nyavor, Y., Zarabian, N., Mahoney, A., and Frame, L. A. (2024). The microbiota-gut-brain-immune interface in the pathogenesis of neuroinflammatory diseases: a narrative review of the emerging literature. Front. Immunol. 15:1365673. doi: 10.3389/fimmu.2024.1365673
World Health Organization. (2025). Available online at: https://www.who.int/news-room/fact-sheets/detail/mental-disorders (Accessed August 2025).
Wu, T., Liu, R., Zhang, L., Rifky, M., Sui, W., Zhu, Q., et al. (2022). Dietary intervention in depression - a review. Food Funct. 13, 12475–12486. doi: 10.1039/D2FO02795J
Xie, Z., Huang, J., Sun, G., He, S., Luo, Z., Zhang, L., et al. (2024). Integrated multi-omics analysis reveals gut microbiota dysbiosis and systemic disturbance in major depressive disorder. Psychiatry Res. 334:115804. doi: 10.1016/j.psychres.2024.115804
Xu, Q., Sun, L., Chen, Q., Jiao, C., Wang, Y., Li, H., et al. (2024). Gut microbiota dysbiosis contributes to depression-like behaviors via hippocampal NLRP3-mediated neuroinflammation in a postpartum depression mouse model. Brain Behav. Immun. 119, 220–235. doi: 10.1016/j.bbi.2024.04.002
Yadegar, A., Bar-Yoseph, H., Monaghan, T. M., Pakpour, S., Severino, A., Kuijper, E. J., et al. (2024). Fecal microbiota transplantation: current challenges and future landscapes. Clin. Microbiol. Rev. 37, e00060–e00022. doi: 10.1128/cmr.00060-22
Yamawaki, Y., Yoshioka, N., Nozaki, K., Ito, H., Oda, K., Harada, K., et al. (2018). Sodium butyrate abolishes lipopolysaccharide-induced depression-like behaviors and hippocampal microglial activation in mice. Brain Res. 1680, 13–38. doi: 10.1016/j.brainres.2017.12.004
Yang, C., Hu, T., Xue, X., Su, X., Zhang, X., Fan, Y., et al. (2023). Multi-omics analysis of fecal microbiota transplantation’s impact on functional constipation and comorbid depression and anxiety. BMC Microbiol. 23:389. doi: 10.1186/s12866-023-03123-1
Yang, Y., Zhou, B., Zhang, S., Si, L., Liu, X., and Li, F. (2023). Prebiotics for depression: how does the gut microbiota play a role? Front. Nutr. 10:1206468. doi: 10.3389/fnut.2023.1206468
Ye, Z., Zhang, Y., Du, M., Lu, S., Zhao, Q., and Yang, S. (2022). The correlation between probiotics and anxiety and depression levels in Cancer patients: a retrospective cohort study. Front. Psych. 13:830081. doi: 10.3389/fpsyt.2022.830081
Yoo, S., Jung, S. C., Kwak, K., and Kim, J. S. (2024). The role of prebiotics in modulating gut microbiota: implications for human health. Int. J. Mol. Sci. 25:834. doi: 10.3390/ijms25094834
Yu, Z., Xian, J., Wang, L., and Yu, H. (2024). Effect of umbilical moxibustion on intestinal flora in patients with subthreshold depression. Zhongguo Zhen Jiu 44, 881–888. doi: 10.13703/j.0255-2930.20231019-0003
Yuan, X., Chen, B., Duan, Z., Xia, Z., Ding, Y., Chen, T., et al. (2021). Depression and anxiety in patients with active ulcerative colitis: crosstalk of gut microbiota, metabolomics and proteomics. Gut Microbes 13:1987779. doi: 10.1080/19490976.2021.1987779
Zhang, Q., Chen, B., Zhang, J., Dong, J., Ma, J., Zhang, Y., et al. (2023). Effect of prebiotics, probiotics, synbiotics on depression: results from a meta-analysis. BMC Psychiatry 23:477. doi: 10.1186/s12888-023-04963-x
Zhang, S. Q., Xia, Z. X., Deng, Q., Yang, P. F., Long, L. H., Wang, F., et al. (2022). Repeated vagus nerve stimulation produces anxiolytic effects via upregulation of AMPAR function in centrolateral amygdala of male rats. Neurobiol. Stress 18:100453. doi: 10.1016/j.ynstr.2022.100453
Zhao, W., Guo, M., Feng, J., Gu, Z., Zhao, J., Zhang, H., et al. (2022). Myristica fragrans extract regulates gut microbes and metabolites to attenuate hepatic inflammation and lipid metabolism disorders via the AhR-FAS and NF-κB Signaling pathways in mice with non-alcoholic fatty liver disease. Nutrients 14:1699. doi: 10.3390/nu14091699
Zheng, Y., He, J., Guo, L., Yao, L., Zheng, X., Yang, Z., et al. (2019). Transcriptome analysis on maternal separation rats with depression-related manifestations ameliorated by electroacupuncture. Front. Neurosci. 13:314. doi: 10.3389/fnins.2019.00314
Zheng, P., Zeng, B., Zhou, C., Liu, M., Fang, Z., Xu, X., et al. (2016). Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 21, 786–796. doi: 10.1038/mp.2016.44
Zhou, J., Wang, X., Feng, L., Xiao, L., Yang, R., Zhu, X., et al. (2021). Venlafaxine vs. fluoxetine in postmenopausal women with major depressive disorder: an 8-week, randomized, single-blind, active-controlled study. BMC Psychiatry 21:260. doi: 10.1186/s12888-021-03253-8
Zhu, F., Ju, Y., Wang, W., Wang, Q., Guo, R., Ma, Q., et al. (2020). Metagenome-wide association of gut microbiome features for schizophrenia. Nat. Commun. 11:1612. doi: 10.1038/s41467-020-15457-9
Zhu, X., Wu, S., Zhou, Y., Xiao, T., Xia, L., Wang, Y., et al. (2024). The pharmacological actions of Danzhi-xiaoyao-San on depression involve lysophosphatidic acid and microbiota-gut-brain axis: novel insights from a systems pharmacology analysis of a double-blind, randomized, placebo-controlled clinical trial. J. Biomol. Struct. Dyn. 42, 9309–9324. doi: 10.1080/07391102.2023.2251067
Zhu, R., Zhao, X., Wu, H., Zeng, X., Wei, J., and Chen, T. (2024). Psychobiotics Lactiplantibacillus plantarum JYLP-326: antidepressant-like effects on CUMS-induced depressed mouse model and alleviation of gut microbiota dysbiosis. J. Affect. Disord. 354, 752–764. doi: 10.1016/j.jad.2024.03.136
Zou, Y., Yu, H., Zhang, L., and Ruan, Z. (2021). Dietary vegetable powders modulate immune homeostasis and intestinal microbiota in mice. Foods 11:27. doi: 10.3390/foods11010027
Keywords: gut microbiota, depression, anxiety, microbiota-gut-brain axis, intervention
Citation: Ruohan Z, Ruting W, Hongxi W, Zhenjin H, Jiale L, Rongxin Z, Feng J and Yuanbo S (2025) Gut microbiota as a novel target for treating anxiety and depression: from mechanisms to multimodal interventions. Front. Microbiol. 16:1664800. doi: 10.3389/fmicb.2025.1664800
Edited by:
Shanshan Hu, Anhui Agricultural University, ChinaReviewed by:
Tingting Bi, Nanjing University of Chinese Medicine, ChinaNikhilesh Anand, The University of Texas Rio Grande Valley, United States
Copyright © 2025 Ruohan, Ruting, Hongxi, Zhenjin, Jiale, Rongxin, Feng and Yuanbo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiang Feng, amlhbmdmZW5nZG9jMjAyM0AxNjMuY29t; Song Yuanbo, Mjc1Nzg3MzQ5QHFxLmNvbQ==
 Zhang Ruohan
Zhang Ruohan Wang Ruting1
Wang Ruting1 Wu Hongxi
Wu Hongxi Liang Jiale
Liang Jiale