- 1Institute of Neuropsychiatry, The Affiliated Brain Hospital, Guangzhou Medical University, Guangzhou, Guangdong, China
- 2Key Laboratory of Neurogenetics and Channelopathies of Guangdong Province and the Ministry of Education of China, Guangzhou Medical University, Guangzhou, China
Prenatal polyinosinic-polycytidylic acid (Poly I:C) exposure-induced maternal immune activation (MIA) causes schizophrenia-like abnormal behaviors in offspring. Extensive evidence suggests that patients with schizophrenia exhibit gut microbiota dysbiosis and tryptophan (TRP) metabolism dysregulation, which is correlated with psychotic and cognitive symptoms. However, the role of gut microbiota and TRP metabolism in Poly I:C MIA-induced schizophrenia-like behaviors is unclear. In this study, pregnant C57/BL6 mice were injected with Poly I:C (20 mg/kg) or vehicle at gestational day (GD) 9. We found that prenatal Poly I:C exposure at GD 9 led to gut microbiota dysbiosis, thereby activating the TRP-kynurenine (KYN)-quinolinic acid (QA) pathway in the hippocampus, serum, and feces, inhibiting the hippocampal and serum TRP-KYN-kynurenic acid (KYNA) pathway and the hippocampal, serum, and fecal TRP-5-hydroxytryptamine (5-HT) pathway, thus leading to anxiety- and depression-like behaviors and impairments in prepulse inhibition (PPI) and recognition memory in female and/or male offspring during adolescence and/or adulthood. In addition, prenatal Poly I:C exposure caused sex-dependent changes in QA levels and gut microbiota composition in offspring. These results suggest that gut dysbiosis may contribute to prenatal Poly I:C exposure-induced schizophrenia-like behaviors by disturbing the TRP metabolism pathway in adolescent and adult offspring of both sexes. Our study indicates possible strategies for ameliorating prenatal Poly I:C exposure-induced schizophrenia-like behaviors. Our findings provide additional evidence that gut microbiota dysbiosis is an underlying mechanism for Poly I:C MIA-induced schizophrenia-like behaviors and behavioral impairments in schizophrenia. Given the sex-related differences in gut microbiota and QA levels, both sexes should be included in studies that explore the mechanisms of Poly I:C MIA-induced schizophrenia-like behaviors.
1 Introduction
Schizophrenia is a chronic and serious psychotic disease characterized by positive, negative, and cognitive symptoms during late adolescence or early adulthood (Abel et al., 2010). This disease exhibits clear sexual dimorphism in age of onset and progression (Abel et al., 2010). Male patients with schizophrenia show a higher rate of diagnosis and earlier age of onset than female patients (Abel et al., 2010). However, the underlying molecular mechanisms of this disorder remain unclear. Epidemiological studies have shown that maternal immune activation (MIA) induced by prenatal infection is a risk factor for the development of schizophrenia in offspring (Sekar et al., 2016). Polyinosinic-polycytidylic acid (Poly I:C), a synthetic analog of double-stranded RNA, binds to Toll-like receptor 3 (TLR3) to activate proinflammatory downstream signaling, leading to increased expression of proinflammatory cytokines and promoting glial cell maturation and differentiation (Bergdolt and Dunaevsky, 2019). Poly I:C exposure during gestation may enhance the risk of schizophrenia in the offspring by inducing MIA (Yildiz Taskiran et al., 2023a,b; Su et al., 2022). Recent studies have found that the offspring of pregnant dams injected with Poly I:C exhibit schizophrenia-like behavioral deficits, such as anxiety- and depression-like behaviors, and deficits in prepulse inhibition (PPI) and recognition memory (Yildiz Taskiran et al., 2023a,b; Su et al., 2022). Therefore, the Poly I:C-induced MIA model has been used to investigate the pathogenesis of schizophrenia (Yildiz Taskiran et al., 2023a,b). However, the molecular mechanism by which Poly I:C-induced MIA causes psychotic and cognitive symptoms in the offspring is not well understood.
The potential role of gut microbiota dysbiosis in schizophrenia has attracted extensive attention in recent years. Previous studies have shown that gut microbiota plays an important role in the development of schizophrenia by leading to abnormalities in nervous system development, metabolic dysfunction, and immune system disorders through the brain-gut axis (Zhu et al., 2020a,b; Guo et al., 2021; Cussotto et al., 2018). Mounting evidence suggests that schizophrenia patients exhibit abnormal composition of gut microbiota that is associated with negative and cognitive symptoms, such as social function, verbal learning, visual learning, working memory, and depression (Zhu et al., 2021; Zhu et al., 2020a,b). In a ketamine mouse model of schizophrenia, mice show an abnormal composition of gut microbiota, which is similar to the microbiome disorder in schizophrenia patients (Xu et al., 2025). In an MK-801 mouse model of schizophrenia, chronic MK-801 exposure for 14 days results in intestinal microbiota dysfunction, anxiety and depression behaviors, and spatial recognition memory impairments, indicating that gut microbiota is associated with these psychotic and cognitive symptoms occurring after chronic MK-801 exposure (Guo et al., 2021). Most importantly, two recent animal studies reported that transplantation of gut microbiota from schizophrenia patients into mice significantly elicits schizophrenia-like behaviors, including psychomotor hyperactivity, learning and memory impairments, and elevated startle responses (Zhu et al., 2020a,b; Zheng et al., 2019). These findings support a crucial role for gut microbiota in the psychotic and cognitive symptoms of schizophrenia.
Tryptophan (TRP) is a biosynthetic precursor of a large number of microbial and host metabolites. TRP is transformed by TRP hydroxylase 1/2 enzyme (TPH1/2) into 5-hydroxytryptophan (5-HTP), which is further metabolized into 5-hydroxytryptamine (5-HT). Another branch of TRP is converted to kynurenine (KYN) by indoleamine 2,3-dioxygenase (IDO). Then, KYN is catabolized by kynurenine aminotransferases (KATs) into kynurenic acid (KYNA), or converted by kynurenine monooxygenase (KMO) to 3-hydroxykynurenine (3-HK), which can be further converted into quinolinic acid (QA) (Agus et al., 2018). TRP metabolic pathways are largely mediated by gut microbiota (Agus et al., 2018). Two previous studies have shown that specific gut microbiota and microbiota metabolites, such as short-chain fatty acids (SCFAs) and secondary bile acids, can stimulate TPH1 expression, which in turn promotes 5-HT synthesis (Yano et al., 2015; Reigstad et al., 2015). As an important neurotransmitter, 5-HT plays a crucial role in the modulation of cognition, immune response, and physiological processes (Yano et al., 2015; Reigstad et al., 2015; Deng et al., 2021). Moreover, gut microbiota could transform TRP into several neuroactive molecules including KYN, KYNA, and QA via stimulating IDO1 (Agus et al., 2018). QA is an N-methyl-D-aspartate (NMDA) receptor agonist that causes excitotoxicity, neurodegeneration, neuroinflammation, and cell damage (Cao et al., 2021). KYNA is an NMDA receptor antagonist that protects against excitotoxic and apoptotic effects and modulates dopaminergic, glutamatergic, and γ-aminobutyric acid (GABA)ergic neurotransmission (Cao et al., 2021). An increase in QA and a decrease in KYNA would cause excessive NMDA receptor activation, leading to increased release of reactive oxygen species, resulting in neuronal damage and increased release of glutamate and dopamine, thus impairing brain function (Cao et al., 2021; Agus et al., 2018). In an MK-801 mouse model of schizophrenia, reduced 5-HT levels in the brain are found in mice exposed to MK-801 for 14 days, which are correlated with changes in gut microbiota (Guo et al., 2021). Clinical studies have shown that elevated abundance of gut microbiota in schizophrenia patients is negatively correlated with serum TRP levels and positively correlated with serum KYNA levels (Zhu et al., 2020a,b). A previous study found that schizophrenia patients show a decrease in 5-HT levels in full blood that is negatively correlated with depressive symptoms (Peitl et al., 2016). KYN and KYNA levels are reduced, while QA concentrations are elevated in the serum and plasma of schizophrenia patients; increased QA levels are positively related to cognitive deficits in attention, executive function, language function, and visual learning (Cathomas et al., 2021; Chen et al., 2024). Taken together, these findings suggest that TRP metabolism is a potential mediator between intestinal microbiota and schizophrenia and that gut microbiota may be implicated in schizophrenia by modulating TRP metabolism (Zhu et al., 2020a,b; Guo et al., 2021).
Despite a number of evidences for the involvement of gut microbiota and TRP metabolism in schizophrenia, few studies have investigated the important role of gut microbiota and TRP metabolism in Poly I:C MIA-induced schizophrenia-like psychotic and cognitive symptoms. Two studies have indicated that prenatal Poly I:C exposure at gestational days (GD) 9 and 15 causes abnormal changes in the composition of intestinal flora that may be involved in schizophrenia-like behaviors such as anxiety-like behaviors, recognition memory deficits, and PPI impairments in adult male offspring (Li et al., 2021; Romero-Miguel et al., 2023). Only one study has demonstrated that prenatal Poly I:C exposure increases 3-HK levels in the placenta and fetal brain, which are associated with impaired recognition memory in adult male offspring (Hasegawa et al., 2024). However, the correlations between gut microbiota and Poly I:C MIA-induced schizophrenia-like abnormal behaviors and TRP metabolism are not well explored. It is unclear whether gut microbiota is involved in prenatal Poly I:C exposure-induced schizophrenia-like behavioral alterations by modulating TRP metabolism. As we know, there have been no attempts to study the potential relationship between the gut microbiome and TRP metabolism in Poly I:C MIA-induced schizophrenia-like behaviors. In the present study, the molecular mechanisms underlying schizophrenia-like abnormal behaviors induced by prenatal Poly I:C exposure in offspring were investigated. The aim of this study was to determine whether prenatal Poly I:C exposure at GD 9 caused changes in gut microbiota, elicited TRP metabolism pathway abnormalities, and led to schizophrenia-like behavioral deficits, including anxiety-like behaviors, depression-like behaviors, and impairments in PPI and recognition memory in female and male offspring during adolescence and adulthood. We also determined the correlations between gut microbiota and behavioral parameters and the TRP metabolism pathway.
2 Methods and materials
2.1 Animals
Male and female C57/BL6 mice aged 6–9 weeks (Guangdong Medical Laboratory Animal Center, Guangzhou, China) were used in this study. Four animals per cage (of the same sex) were housed at room temperature (22–26 °C) under a 12 h light/dark cycle (lights on at 7:00 a.m.) with access to water and food ad libitum. After mating (1 male: 2 females), the females with the presence of vaginal plugs were identified as pregnant; this was considered GD 0. Pregnant mice were randomly divided into control and treatment groups. All experimental procedures involving animals were approved by the Animal Experimentation Ethics Committee of Guangzhou Medical University (Ethics approval number: N2025-29047) and were performed in accordance with the guidelines of the National Institutes of Health on the care and ethical treatment of animals.
2.2 Poly I:C administration
Previous studies have demonstrated that descendants whose mothers were injected with Poly I:C at GD 9 subsequently exhibit schizophrenia-like psychotic and cognitive impairments (Hao et al., 2019). In our study, pregnant dams received a single intraperitoneal (i.p.) injection of Poly I:C (20 mg/kg, Sodium salt, Sigma-Aldrich, P1530) or an equal volume of vehicle (Veh) at GD 9 (Juckel et al., 2021). After birth, all pups were kept with their respective dams (n = 10 litters for each treatment group) until weaning on postnatal day (PND) 21 and were housed 3–4 per cage according to sex and littermates. Behavioral assessments, sequencing analysis, and TRP metabolism detection were conducted in offspring of both sexes during PND 40 and 60. For each analysis, only one offspring per sex from each litter was employed. One male offspring and one female offspring were randomly selected from each litter for behavioral tests (10 males and 10 females per group), TRP metabolism analysis in serum (8 males and 8 females per group), hippocampus (6 males and 6 females per group), and feces (10 males and 10 females per group), as well as gut microbiota sequencing and analysis (10 males and 10 females per group).
2.3 Liquid chromatography tandem mass spectrometry
Blood samples were collected by removing eyeballs. Serum was obtained by centrifuging at 4,000 × g for 15 min at 4 °C, then aliquoted and stored at −80 °C before use. Hippocampal proteins were extracted as described in our previous study (Xu et al., 2025). The expressions of TRP, 5-HT, KYN, KYNA, and QA in serum, as well as the levels of TRP, 5-HT, KYN, and QA in the hippocampus, were detected by Metware Biotechnology Co., Ltd. (Wuhan, China) using LC-MS/MS. LC-MS/MS was conducted with the ExionLC™AD UPLC system and QTRAP® 6,500 + triple quadrupole mass spectrometer equipped with an electrospray ionization source (SCIEX, Inc., Framingham, MA, United States). The mobile phases were water containing 0.1% formic acid (component A) and acetonitrile (component B). The chromatography was performed under multistep gradient conditions at a flow rate of 0.35 mL/min. Multiple Reaction Monitoring was used for quantitative analysis. Data acquisition and analysis were performed using Analyst 1.6.3 and MultiQuant 3.0.3 software.
2.4 Enzyme-linked immunosorbent assay
Proteins were extracted from the hippocampus and feces as described in our previous study (Xu et al., 2025). Briefly, the hippocampus and feces were fully homogenized with the appropriate cell lysis buffer. Then, samples were centrifuged at 14,000 × g for 15 min at 4 °C, and the supernatants of homogenates were obtained for further measurement. Fecal TRP, 5-HT, KYN, KYNA, and QA levels and hippocampal KYNA concentrations were detected using commercial ELISA kits (Cloud Clone, Wuhan, China; Fine test, Wuhan, China) according to the manufacturers’ instructions. The sensitivities of TRP, 5-HT, KYN, KYNA, and QA were 0.55 μg/mL, 0.5 ng/mL, 4.688 pmol/mL, 0.89 ng/mL, and 0.6 ng/mL, respectively. All measurements were detected in duplicate and expressed as ng/mL. The absorbance was determined using a microtiter plate reader (iMark; Bio-Rad, Hercules, CA, United States) set at 450 nm.
2.5 Real-time quantitative PCR
For gene expression experiments, total RNA was extracted from hippocampal tissue using the Mini BEST Universal RNA Extraction Kit (Takara, Shiga, Japan) in accordance with the manufacturer’s protocols. Total RNA was reverse-transcribed into cDNA (37 °C for 15 min and 85 °C for 5 s) using PrimeScript™ RT Master Mix (Takara). RT-qPCR amplification was carried out using the Applied Biosystems ViiA 7 Real-time PCR System (Applied Biosystems, Carlsbad, CA, United States) with the TB Green® Premix Ex Taq™ II (Takara). Thermal cycling conditions were as follows: 50 °C for 2 min; 95 °C for 30 s; and 40 cycles of 95 °C for 5 s, 56 °C for 30 s, and 72 °C for 1 min.
2.6 Western blot analysis
Proteins were extracted from the hippocampus using the Tissue Protein Extraction Reagent (T-PER; Thermo Scientific) according to the methods described in our previous study (Luo et al., 2021). Proteins were run on 10% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Bio-Rad). The membranes were blocked with 3% bovine serum albumin in Tris-buffered saline for 1 h at room temperature and probed with primary antibodies and horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse secondary antibodies (CST, Boston, United States). Then, the membranes were visualized with SuperSignal West Pico chemiluminescence substrate (Thermo Scientific) and imaged using an imaging system (ChemiDoc XRS+; Bio-Rad).
2.7 Fecal microbiota 16S ribosomal RNA sequencing and analysis
Fresh feces were harvested from each mouse at PND 40 and 60, immediately frozen in dry ice individually, and then stored at −80 °C until DNA extraction. Microbial DNA was extracted using a QIAamp DNA Stool Mini Kit (Qiagen, Dusseldorf, Germany) as described by the manufacturer. 16S rRNA sequencing of fecal samples was carried out by Guangdong Magigene Biotechnology Co., Ltd. (Guangzhou, China) as described in our previous study (Xu et al., 2025).
2.8 PPI test
The acoustic startle reactivity (ASR) and PPI of the startle reflex were detected using the SR-LAB™ startle response system (San Diego Instruments, San Diego, CA, United States). The mice were allowed to acclimate to the behavioral room for 1 h before the experiment. They were habituated to the startle response chamber with background noise (69 dB) for 5 min. Thereafter, the mice were exposed to 10 presentations of a startling pulse (120 dB, 40 ms duration). The aim of this phase was to promote within-test habituation to startle stimuli. The protocol was consisted 80 trials pseudo-randomly divided into eight trial types as follows: 10 presentations of no pulse, 10 presentations of a startling pulse alone (120 dB, 40 ms duration), 10 presentations of each prepulse alone (76, 79, and 85 dB; 20 ms duration), and 10 presentations of each prepulse with a startling pulse (76 + 120 dB, 79 + 120 dB, and 85 + 120 dB; 100 ms interval). The interval between continuous presentations varied from 10 to 20 s. The percentage of PPI was calculated using the following formula: (ASR amplitude of startling pulse-ASR amplitude of a prepulse with a startling pulse)/(ASR amplitude of a startling pulse) × 100 (Luo et al., 2024). The startle response was assessed by measuring the average ASR amplitude of the startling pulses in every group.
2.9 Novel object recognition test
The recognition memory of mice was examined in an open field using a white Plexiglas chamber (50 × 50 × 50 cm) without a lid. Mice were allowed to acclimate to the behavioral room for 1 h before the experiment. They were then placed in the experimental chamber without objects to habituate for 5 min. The novel object recognition test comprised two phases: a 10 min acquisition trial and a 10 min recognition trial, with a 1 h interval between the two trials. During the acquisition trial phase, the mice were allowed to freely explore two identical objects (A, pink polypropylene cubes; length, 3 cm; width, 3 cm; height, 3 cm) placed in opposite corners 15 cm from the walls. During the recognition trial phase, the mice were allowed to freely interact with a familiar object (A) and a novel object (B, yellow polypropylene cylinders; diameter, 3 cm; height, 6 cm). Before the behavioral test, an independent group of mice showed no significant difference in the exploration time between objects A and B (Xu et al., 2025). Behaviors were recorded using Ethovision XT 11.0 video tracking software (Noldus, Wageningen, Netherlands). Exploratory behavior was defined as touching, licking, and sniffing an object. The percentage of exploratory preference was estimated as follows: (time exploring the novel object/total exploration time) × 100 (Luo et al., 2022).
2.10 Open field test
The locomotor activity and anxiety-like behavior were measured in an open field with a white Plexiglas chamber (50 × 50 × 50 cm) without a lid. The 20 × 20 cm central section inside the arena is designated as the center of the arena, while the others were designated as periphery. Mice were allowed to acclimate to the behavioral room for 1 h before the experiment. They were then gently placed in the center of the apparatus and allowed to move freely for 5 min. Behaviors were recorded by an EthoVision XT 11.0 video tracking system (Noldus). Time spent in the central area and the number of entries to the center zone were used to measure anxiety-like behavior (Su et al., 2022). Total distance moved was used to evaluate the locomotion of mice (Su et al., 2022).
2.11 Elevated plus maze test
The anxiety-like behavior was assessed in the EPM (Reisinger et al., 2016). The EPM was a white Plexiglas cross-shaped maze, elevated 70 cm above the floor, consisting of two open arms and two closed arms. Mice were allowed to acclimate to the behavioral room for 1 h before the experiment. They were then placed in the central platform of the apparatus facing an open arm and allowed to freely explore the maze for 5 min. Behaviors were analyzed by an EthoVision XT 11.0 video tracking system (Noldus). The percentage of time spent in open arms was calculated as: (time spent in open arms/total time spent in open and closed arms) × 100. The percentage of the number of entries to open arms was calculated as: (number of entries to open arms / total number of entries) × 100.
2.12 Forced swimming test
The depression-like behavior was evaluated in the FST (Su et al., 2022). Mice were allowed to acclimate to the behavioral room for 1 h before the experiment. They were then placed individually in Plexiglas cylinders (height: 30 cm, diameter: 20 cm) with 15 cm deep water, maintained at 23–25 °C. The test lasted 6 min, and only the data of from the last 4 min were analyzed using the EthoVision XT 11.0 video tracking system (Noldus). Total immobility time was recorded to assess the depression-like behavior of mice. A mouse was considered immobile when it remained in an upright floating position and made only the movements needed to keep its head above the water.
2.13 Statistical analysis
Data are shown as mean ± standard error of the mean (SEM). IBM SPSS Statistics for Windows version 25.0 was used for data analysis. The Shapiro-Wilk test and Levene’s test were used to test normality and equal variance between the group values, respectively. In OFT, NOR, EPM, FST, and PPI test (startle response data), differences in behavioral parameters were assessed by two-way analysis of variance (ANOVA) followed by Bonferroni’s post-hoc test. PPI% data from the PPI test were evaluated using repeated-measures three-way ANOVA followed by Bonferroni’s post-hoc test. LC-MS/MS, ELISA, RT-qPCR, Western blot, and microbiota data were analyzed by two-way ANOVA followed by Bonferroni’s post-hoc test. The Ln transformation was applied to microbiota data prior to performing ANOVA. Pearson’s correlations were applied to analyze the correlations between gut microbiota and behavioral parameters and the TRP metabolism pathway. A p-value <0.05 was defined as statistically significant.
3 Results
3.1 Poly I:C MIA induced schizophrenia-like behavioral alterations in adolescent and adult offspring of both sexes
We detected the schizophrenia-like behavioral deficits using PPI, NOR, OFT, EPM, and FST in female and male offspring of dams exposed to prenatal Poly I:C at GD 9. As shown in Figures 1A,J, prenatal Poly I:C exposure at GD 9 led to a reduction in PPI with prepulse intensities of 76, 79, and 85 dB in male and female offspring at PND 40 and 60, indicating deficits in sensorimotor gating. As differences in the startle reflex among groups could confound the PPI results, we also measured the startle response. We found no significant differences in startle amplitude in the PPI test between Poly I:C and Veh offspring of both sexes at PND 40 and 60 (Figures 1B,K). Prenatal Poly I:C exposure at GD 9 significantly decreased the novel object recognition index at PND 40 and 60 in offspring of both sexes, indicating impaired recognition memory (Figures 1C,L). The immobility time in the FST was significantly increased in Poly I:C MIA offspring of both sexes during PND 40 and 60, suggesting depression-like behaviors (Figures 1D,M). Prenatal Poly I:C administration at GD 9 reduced the time spent in open arms and the number of entries to open arms in the EPM test, indicating anxiety-like behaviors at PND 40 and 60 in offspring of both sexes (Figures 1E,F,N,O). By contrast, no significant differences in time spent in the center zone, number of entries to the center zone, and total distance moved in the OFT were observed between Poly I:C MIA and Veh offspring of both sexes at PND 40 and 60 (Figures 1G–IP–R).
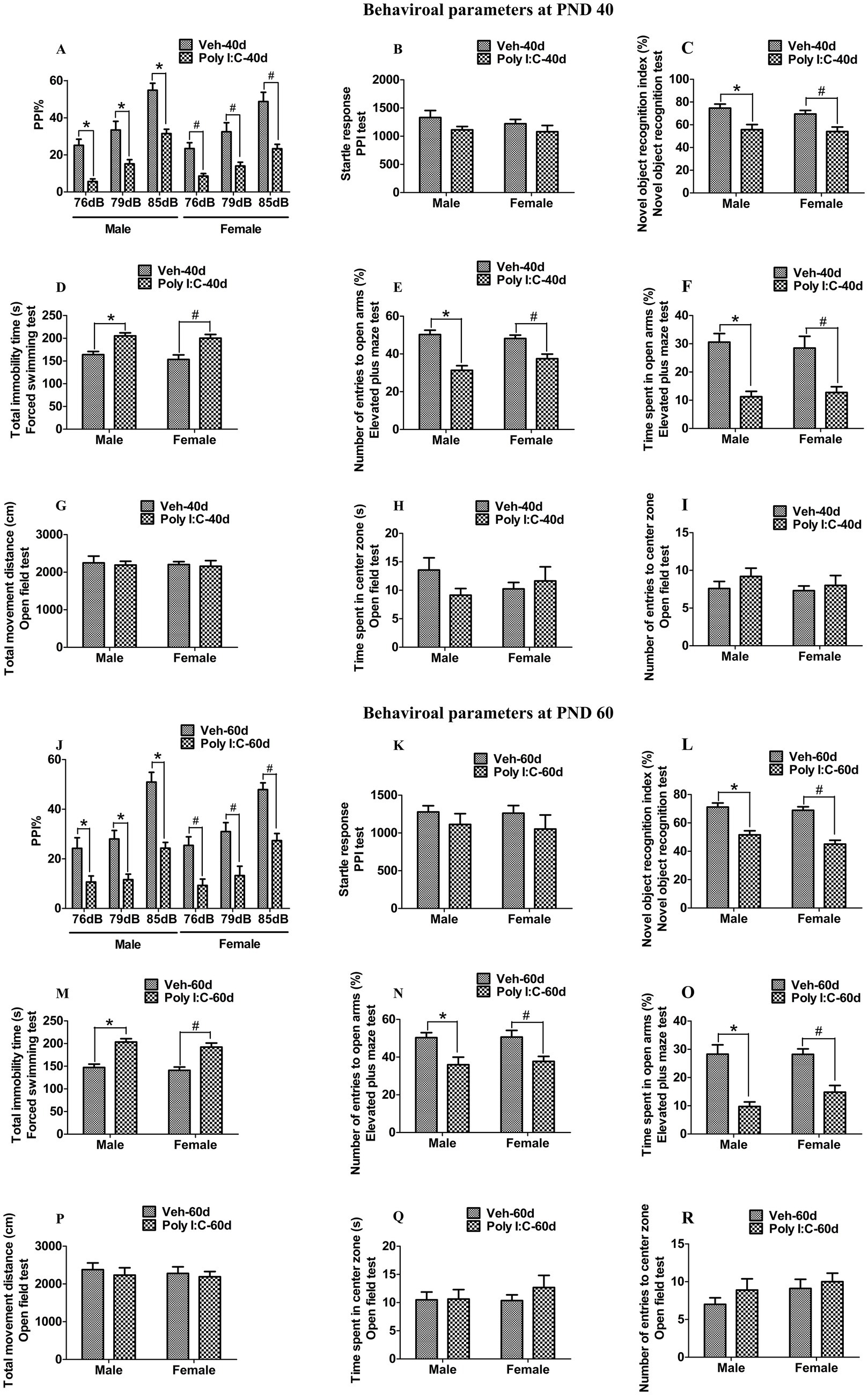
Figure 1. Poly I:C MIA caused schizophrenia-like behavioral changes in the offspring of both sexes during adolescence and adulthood. (A,J) A significant reduction in PPI with prepulse intensities of 76, 79, and 85 dB was found in MIA offspring of both sexes at PND 40 and PND 60 (n = 10). (B,K) There were no significant differences between MIA and Veh offspring of both sexes in terms of startle amplitude in the PPI test at PND 40 and PND 60 (n = 10). (C,L) A significant decrease in the novel object recognition index was observed in MIA offspring of both sexes at PND 40 and PND 60 (n = 10). (D,M) Total immobility time in the forced swimming test was significantly elevated in MIA male and female offspring at PND 40 and PND 60 (n = 10). (E,N) Number of entries to the open arm in the elevated plus maze test was significantly reduced in MIA offspring of both sexes during PND 40 and PND 60 (n = 10). (F,O) Time spent in the open arms in the elevated plus maze test was significantly decreased in MIA offspring of both sexes during PND 40 and PND 60 (n = 10). (G–I,P–R) There were no significant differences between MIA and Veh offspring of both sexes in time spent in the center zone, number of entries to the center zone, and total movement distance in the open field test at PND 40 and PND 60 (n = 10). Data are expressed as mean ± SEM; * p < 0.05 vs. the Veh male, # p < 0.05 vs. the Veh female. Veh, vehicle; MIA, maternal immune activation; PND 40, postnatal day 40; PND 60, postnatal day 60.
3.2 Poly I:C MIA activated the TRP-KYN-QA metabolism pathway in peripheral serum, hippocampus, and feces of male and female offspring during adolescence and adulthood
To verify whether prenatal Poly I:C exposure altered the TRP metabolism pathway in peripheral serum, hippocampus, and feces of offspring, we used LC-MS/MS and ELISA to detect the expression of TRP, 5-HT, KYN, KYNA, and QA.
At PND 40, TRP, 5-HT, KYN, and KYNA levels were significantly reduced in both peripheral serum and the hippocampus of male and female offspring of Poly I:C MIA mothers (Figures 2A–D, 3A–D). Serum QA levels were significantly elevated at PND 40 in MIA female offspring (Figure 2E). Hippocampal QA levels were significantly elevated at PND 40 in MIA male and female offspring, and the QA levels were significantly higher in female offspring than in male offspring (Figure 3E). The 5-HT/TRP, KYN/TRP, and KYNA/KYN ratios were significantly decreased, while the QA/KYNA ratio was significantly increased in both peripheral serum and the hippocampus of MIA offspring of both sexes at PND 40 (Figures 2F–I, 3F–I). In addition, the TRP, 5-HT, and KYN levels were significantly reduced, while QA levels were significantly elevated in the feces of MIA offspring of both sexes at PND 40 (Figures 4A–C,E). The KYNA levels were unchanged in the feces of Poly I:C MIA offspring of both sexes at PND 40 (Figure 4D). The fecal QA/KYNA ratio was significantly increased only in MIA female offspring at PND 40 (Figure 4I). There were no significant differences in the ratios of 5-HT/TRP, KYN/TRP, and KYNA/KYN in feces between Poly I:C and Veh offspring of both sexes at PND 40 (Figures 4F–H).
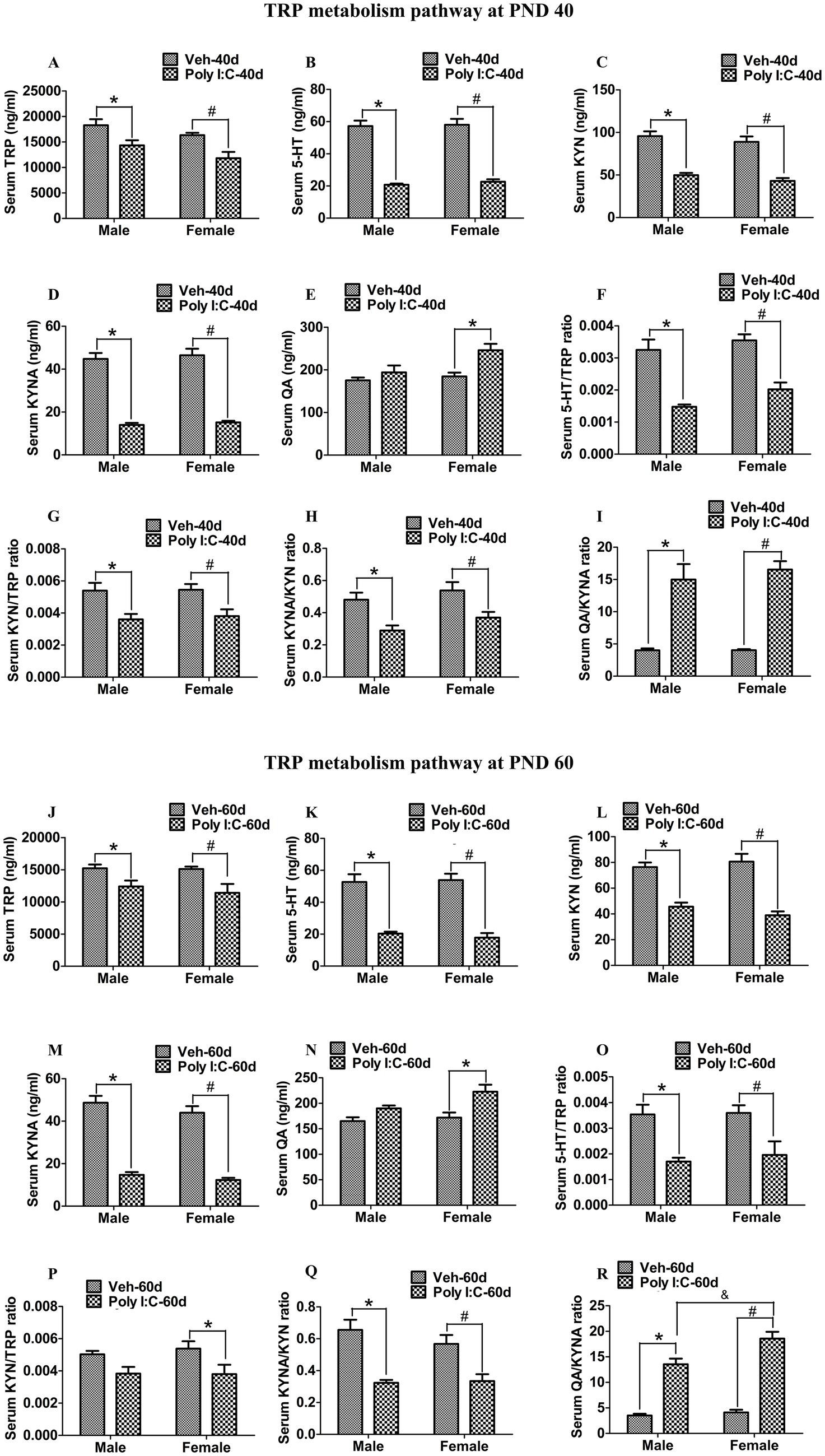
Figure 2. Poly I:C MIA activated the TRP-KYN-QA metabolism pathway in the serum of offspring during adolescence and adulthood. (A–E,J–N) Serum TRP, 5-HT, KYN, and KYNA levels were significantly decreased in MIA male and female offspring, while serum QA levels were significantly increased only in MIA female offspring at PND 40 and PND 60. Quantification was determined by LC-MS/MS (n = 8). (F–I) The ratios of 5-HT/TRP, KYN/TRP, and KYNA/KYN in serum were significantly decreased, while the serum QA/KYNA ratio was significantly increased in MIA male and female offspring at PND 40 (n = 8). (O–Q) The ratios of 5-HT/TRP and KYNA/KYN in serum were significantly decreased in MIA offspring of both sexes, while serum KYN/TRP ratio was significantly reduced only in MIA female offspring at PND 60 (n = 8). (R) Serum QA/KYNA ratio was significantly elevated in MIA offspring of both sexes and was higher in females than in males at PND 60 (n = 8). Data are expressed as mean ± SEM; * p < 0.05 vs. the Veh male, # p < 0.05 vs. the Veh female, & p < 0.05 vs. the Poly I:C male. Veh, vehicle; MIA, maternal immune activation; LC-MS/MS, liquid chromatography tandem mass spectrometry; PND 40, postnatal day 40; PND 60, postnatal day 60.
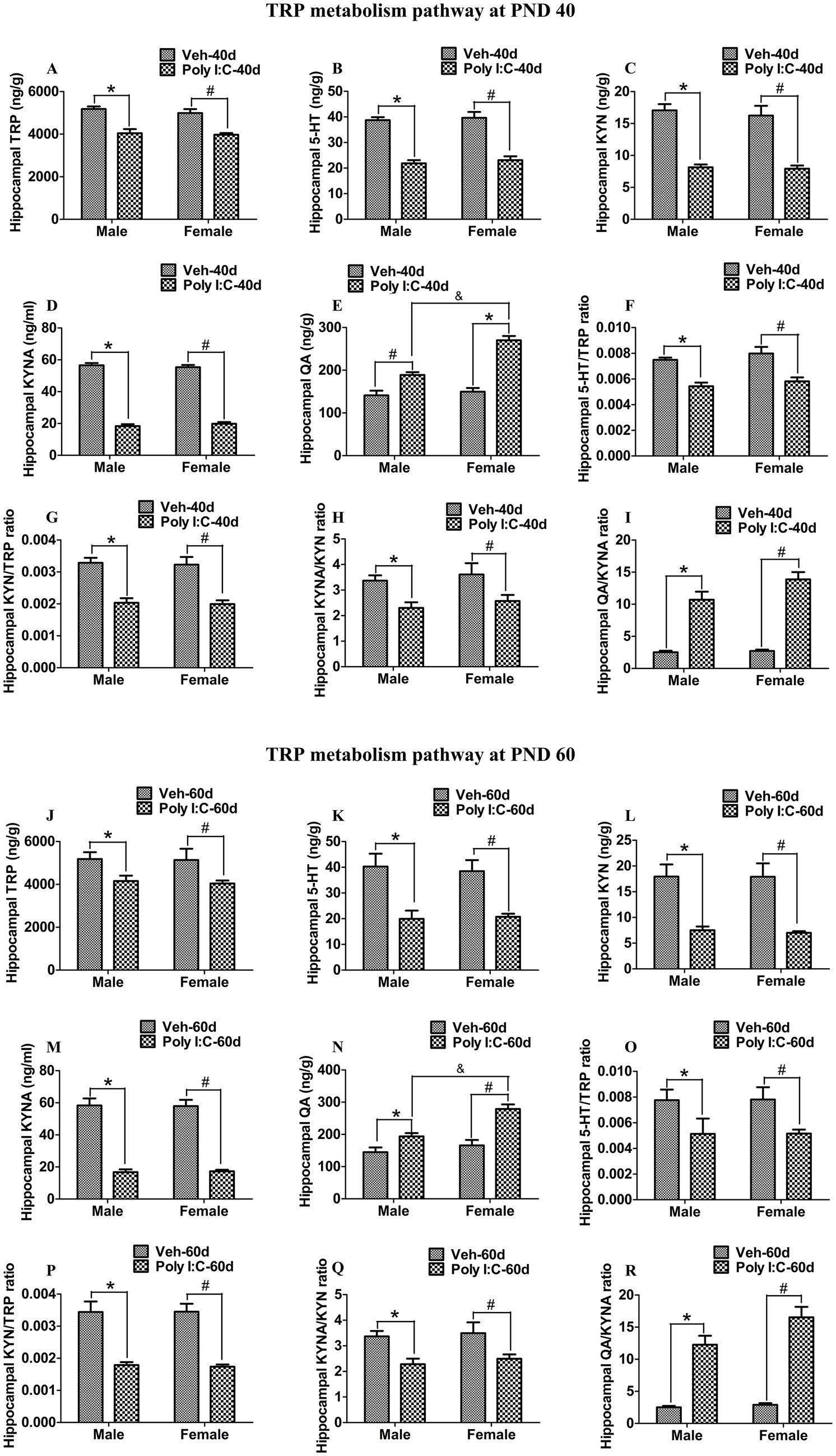
Figure 3. Poly I:C MIA activated the TRP-KYN-QA metabolism pathway in the hippocampus of offspring during adolescence and adulthood. (A–D,J–M) Hippocampal TRP, 5-HT, KYN, and KYNA levels were significantly reduced in MIA offspring of both sexes at PND 40 and PND 60. Quantification was determined by LC-MS/MS and ELISA (n = 6). (E,N) Hippocampal QA levels were significantly enhanced in MIA offspring of both sexes and were higher in females than in males at PND 40 and PND 60. Quantification was determined by LC-MS/MS (n = 6). (F–I,O–R) The ratios of 5-HT/TRP, KYN/TRP, and KYNA/KYN were significantly decreased, while the QA/KYNA ratio was significantly increased in the hippocampus of MIA male and female offspring at PND 40 and PND 60 (n = 6). Data are expressed as mean ± SEM; * p < 0.05 vs. the Veh male, # p < 0.05 vs. the Veh female, & p < 0.05 vs. the Poly I:C male. Veh, vehicle; MIA, maternal immune activation; LC-MS/MS, liquid chromatography tandem mass spectrometry; PND 40, postnatal day 40; PND 60, postnatal day 60.
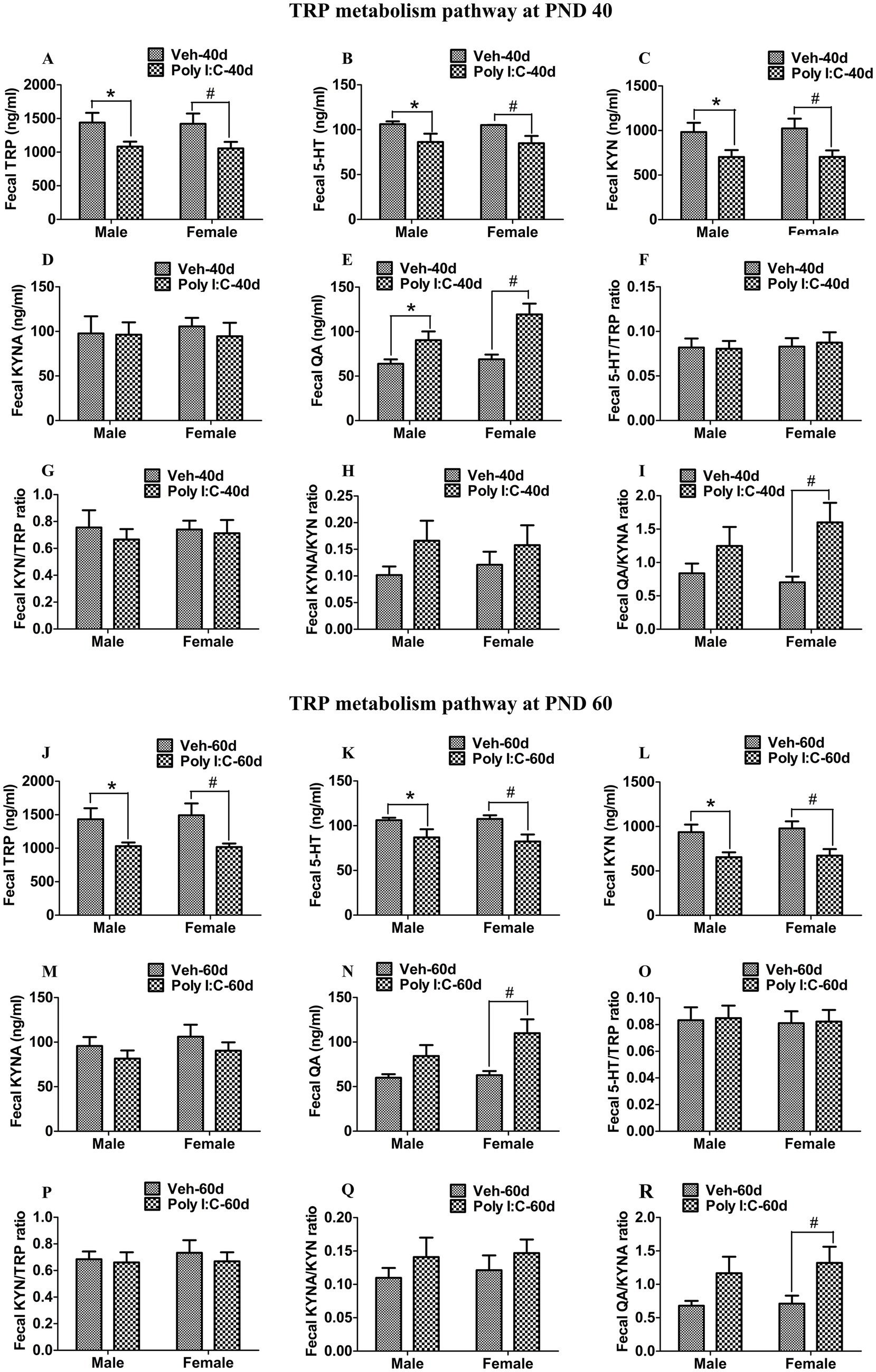
Figure 4. Poly I:C MIA activated the TRP-KYN-QA metabolism pathway in the feces of offspring during adolescence and adulthood. (A–D,J–M) Fecal TRP, 5-HT, and KYN levels were significantly reduced, while fecal KYNA levels were unchanged in MIA offspring of both sexes at PND 40 and PND 60. Quantification was determined by ELISA (n = 10). (E,N) Fecal QA levels were significantly increased in MIA offspring of both sexes at PND 40 and were increased only in MIA female offspring at PND 60. Quantification was determined by ELISA (n = 10). (F–I,O–R) Fecal 5-HT/TRP, KYN/TRP, and KYNA/KYN ratios were unaltered in MIA offspring of both sexes, while the fecal QA/KYNA ratio was significantly elevated in MIA female offspring at PND 40 and PND 60. (n = 10). Data are expressed as mean ± SEM; * p < 0.05 vs. the Veh male, # p < 0.05 vs. the Veh female, & p < 0.05 vs. the Poly I:C male. Veh, vehicle; MIA, maternal immune activation; PND 40, postnatal day 40; PND 60, postnatal day 60.
At PND 60, TRP, 5-HT, KYN, and KYNA levels were significantly reduced in both peripheral serum and the hippocampus of male and female offspring of dams administered Poly I:C at GD 9 (Figures 2J–M, 3J–M). Serum QA levels were significantly increased at PND 60 in MIA female offspring (Figure 2N). Hippocampal QA levels were significantly elevated at PND 60 in MIA male and female offspring, and the QA levels were significantly higher in female offspring than in male offspring (Figure 3N). The 5-HT/TRP and KYNA/KYN ratios were significantly reduced at PND 60 in both peripheral serum and the hippocampus of female and male offspring of prenatal Poly I:C-exposed mothers (Figures 2O,Q, 3O,Q). The serum KYN/TRP ratio was significantly decreased at PND 60 in MIA female offspring, while the hippocampal KYN/TRP ratio was significantly decreased at PND 60 in MIA female and male offspring (Figures 2P, 3P). The QA/KYNA ratio was significantly elevated at PND 60 in both peripheral serum and the hippocampus of MIA offspring of both sexes, and the serum QA/KYNA ratio was significantly higher in female offspring than in male offspring (Figures 2R, 3R). In addition, the TRP, 5-HT, and KYN levels were significantly decreased at PND 60 in the feces of MIA offspring of both sexes (Figures 4J–L). Fecal KYNA levels were unchanged in Poly I:C offspring of both sexes at PND 60 (Figure 4M). The QA levels and QA/KYNA ratio were significantly elevated in feces of MIA female offspring at PND 60 (Figures 4N,R). There were no significant differences in the ratios of 5-HT/TRP, KYN/TRP, and KYNA/KYN in the feces of Poly I:C offspring of both sexes at PND 60 (Figures 4O–Q).
3.3 Poly I:C MIA significantly altered the expression of TPH2, IDO1, KATII, and KMO in hippocampus of male and female offspring during adolescence and adulthood
The TRP metabolism pathway is largely controlled by several rate-limiting enzymes, including TPH2, IDO1, KATII, and KMO. TPH2 and IDO1 are rate-limiting enzymes responsible for the initiation of 5-HT and KYN metabolic pathways, respectively. On the one hand, TRP is metabolized by TPH2 to 5-HTP, which is further converted into 5-HT (Agus et al., 2018). On the other hand, TRP is catabolized by IDO1 into KYN, which is further transformed by KATII to KYNA or by KMO to 3-HK; 3-HK could be further converted into QA (Agus et al., 2018). Therefore, we used RT-qPCR to evaluate changes in the mRNA levels of these key enzymes. At PND 40 and 60, we found that the hippocampal mRNA levels of IDO1 and KMO were significantly elevated, while the hippocampal mRNA levels of TPH2 and KATII were significantly reduced in Poly I:C MIA offspring of both sexes (Figures 5B–EG–J).
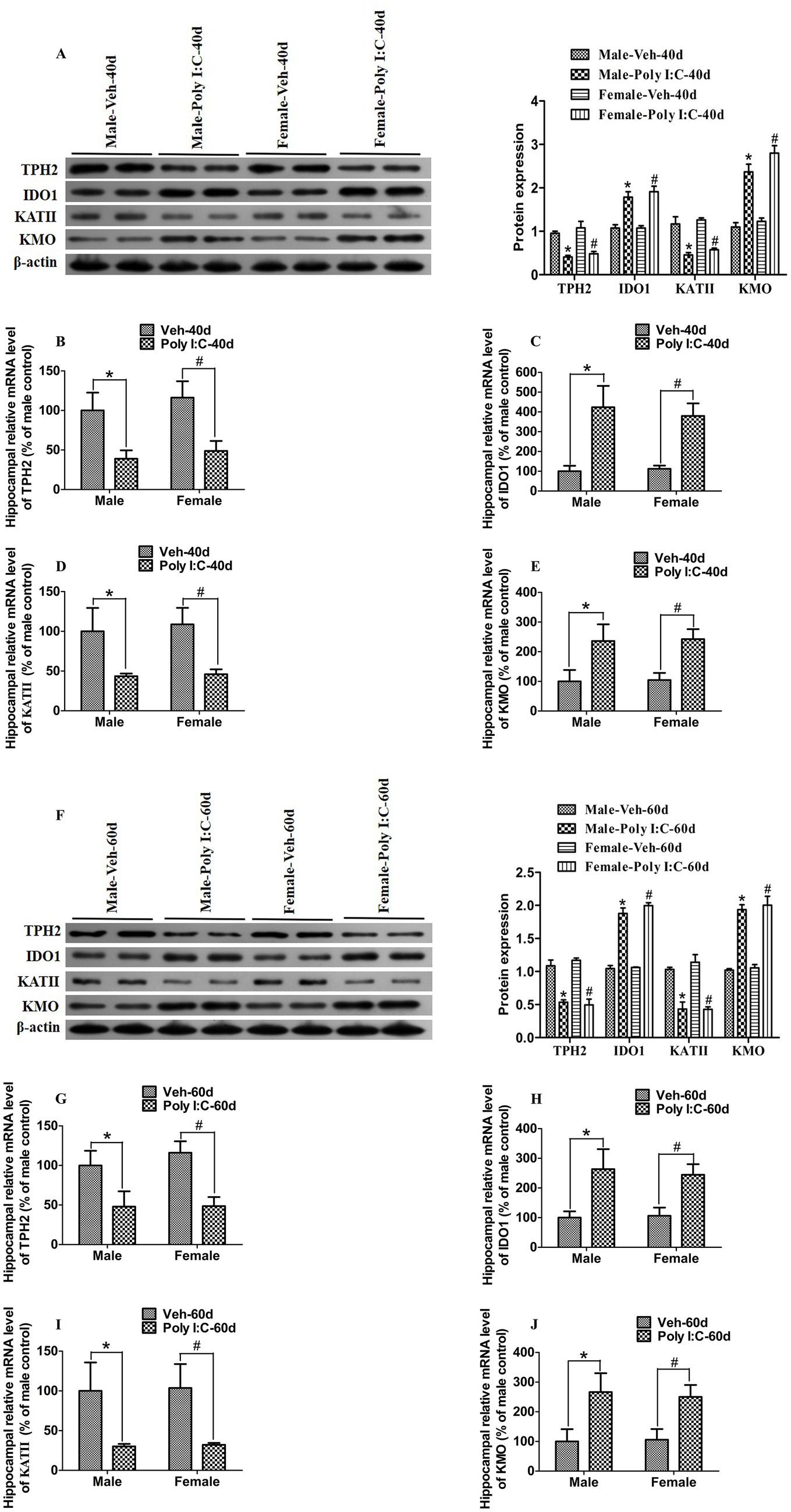
Figure 5. Poly I:C MIA significantly changed the expression of TPH2, IDO1, KATII, and KMO in the hippocampus of male and female offspring during adolescence and adulthood. (A,F) The hippocampal protein levels of TPH2 and KATII were significantly reduced, while the hippocampal protein levels of IDO1 and KMO were significantly increased in MIA male and female offspring during PND 40 and PND 60. Left: immunoblot analysis of TPH2, IDO1, KATII, and KMO. Right: quantification of TPH2, IDO1, KATII, and KMO (n = 6). (B–E,G–J) Hippocampal mRNA levels of TPH2 and KATII were significantly decreased, while hippocampal mRNA levels of IDO1 and KMO were significantly increased in MIA offspring of both sexes at PND 40 and PND 60. Quantification was determined by RT-qPCR (n = 6). Data are expressed as mean ± SEM; * p < 0.05 vs. the Veh male, # p < 0.05 vs. the Veh female. Veh, vehicle; MIA, maternal immune activation; PND 40, postnatal day 40; PND 60, postnatal day 60.
Next, we used Western blot analysis to determine the expression of TPH2, IDO1, KATII, and KMO in the hippocampus. As shown in Figures 5A,F, the hippocampal expression levels of TPH2 and KATII were significantly decreased, while the hippocampal expression levels of IDO1 and KMO were significantly increased in Poly I:C MIA offspring of both sexes during PND 40 and 60.
3.4 Effect of Poly I:C MIA on the α-diversity and β-diversity of gut microbiota in offspring of both sexes during adolescence and adulthood
We further tested whether prenatal Poly I:C exposure altered the α-diversity and β-diversity of gut microbiota in offspring. At PND 40, we obtained 3, 512, 980 high-quality sequences across all the samples, which were clustered into 813 operational taxonomic units (OTUs) at 97% sequence similarity. A Venn diagram showed that 585 of the 813 OTUs were commonly identified in the four groups, while 16, 23, 29, and 27 were unique to Veh male, Veh female, Poly I:C male, and Poly I:C female at PND 40 in offspring, respectively (Supplementary Figure 1A). At PND 60, a total of 3, 565, 989 valid sequences were obtained from all samples and clustered into 2, 236 OTUs. A Venn diagram showed that 541 of the 2, 236 OTUs were commonly detected among groups, while 340, 155, 28, and 21 were unique to Veh male, Veh female, Poly I:C male, and Poly I:C female at PND 60 in offspring, respectively (Supplementary Figure 1C). The majority of rarefaction curves tended to approach the saturation plateau, suggesting that the sequencing depth was sufficient to cover the whole bacterial diversity at PND 40 and 60 (Supplementary Figures 1B,D). The α-diversity refers to the diversity of bacteria within a community or habitat, quantified by metrics that integrate bacterial richness and evenness (Juckel et al., 2021). Chao 1, Shannon, and Simpson indices are commonly used to estimate the α-diversity of gut microbiota (Xu et al., 2025). In addition, the β-diversity measures the differences in the composition of gut microbiota between groups (Yang P. et al., 2024; Yang Z. et al., 2024). Principal coordinates analysis (PCoA) is a widely utilized indicator for assessing the β-diversity of gut microbiota (Juckel et al., 2021; Yang P. et al., 2024; Yang Z. et al., 2024). In this study, there were no significant differences in the α-diversity of gut microbiota estimated by Chao 1, Shannon, and Simpson indices between MIA and Veh offspring of both sexes at PND 40 and 60 (Figures 6A–CE–G), indicating that prenatal Poly I:C MIA did not significantly change the richness and evenness of gut microbiota. Based on the principal coordinate analysis (PCoA), there were significant differences in the β-diversity of gut microbiota between the Poly I:C and Veh offspring of both sexes at PND 40 and 60 (Figures 6D,H), suggesting that prenatal Poly I:C MIA significantly altered the composition and structure of gut microbiota.
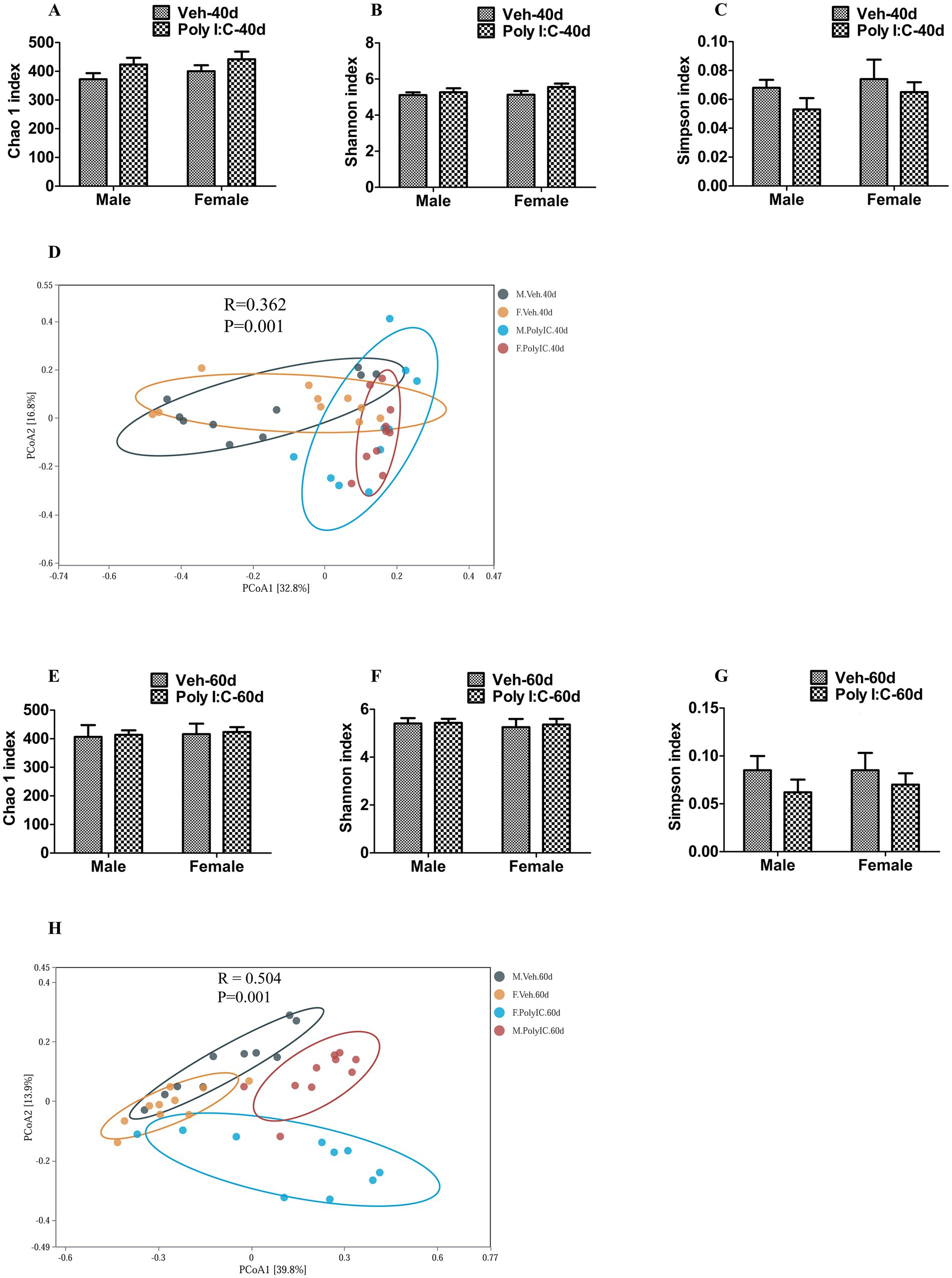
Figure 6. Effect of Poly I:C MIA on α- and β-diversity of gut microbiota in offspring of two sexes during adolescence and adulthood. (A–C,E–G) There were no significant differences in the α-diversity of gut microbiota evaluated by Chao 1, Shannon, and Simpson indices between MIA and Veh offspring of both sexes at PND 40 and PND 60 (n = 10). (D,H) There were significant differences in β-diversity of gut microbiota estimated by PCoA between the MIA and Veh offspring of both sexes at PND 40 and PND 60 (n = 10). Data are expressed as mean ± SEM. Veh, vehicle; MIA, maternal immune activation; PND 40, postnatal day 40; PND 60, postnatal day 60; PCoA, principal coordinate analysis.
3.5 Poly I:C MIA altered the composition of gut microbiota in adolescent and adult offspring of both sexes
We further analyzed whether prenatal Poly I:C exposure changed the composition of gut microbiota in offspring. With respect to the PND 40 time point, at the phylum level, Firmicutes was significantly increased, while Verrucomicrobiota was significantly decreased in Poly I:C offspring of both sexes (Figures 7A,B). At the class level, Clostridia was significantly increased, whereas Verrucomicrobiae was significantly reduced in Poly I:C offspring of both sexes (Figures 7C,D); Desulfovibrionia was significantly elevated only in Poly I:C male offspring (Figure 7E). At the order level, Lachnospirales and Oscillospirales were significantly enhanced, whereas Verrucomicrobiales was significantly decreased in Poly I:C male and female offspring (Figures 7F–H); Clostridia_UCG-014 was significantly elevated only in Poly I:C male offspring (Figure 7I). At the family level, Muribaculaceae and Akkermansiaceae were significantly reduced, but Lachnospiraceae and Ruminococcaceae were significantly elevated in MIA offspring of both sexes (Figures 7J–M); Desulfovibrionaceae, Oscillospiraceae, and Eggerthellaceae were significantly increased only in Poly I:C male offspring (Figures 7N–P). At the genus level, Alitipes, Colidextribacter, and Lachnoclostridium were significantly increased, while Akkermansia was significantly decreased in MIA offspring of both sexes (Figures 7Q–T); Lachnospiraceae_NK4A136_group and Helicobacter were significantly elevated only in Poly I:C female offspring (Figures 7U,V), and Parabacteroides was significantly reduced only in Poly I:C male offspring (Figure 7W).
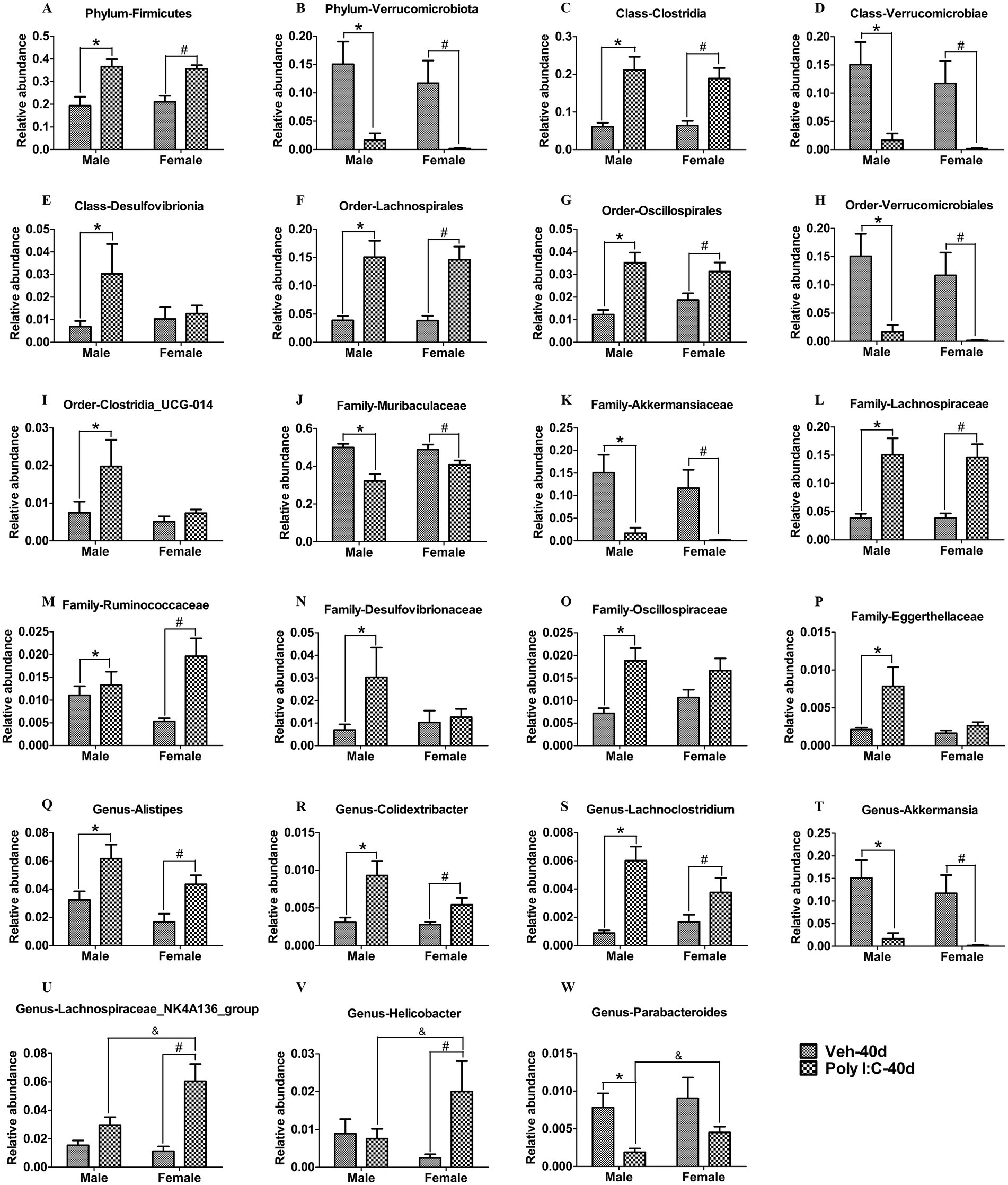
Figure 7. Poly I:C MIA led to changes in the composition of gut microbiota in male and female offspring during adolescence. (A,B) At the phylum levels, Firmicutes was significantly increased, but Verrucomicrobiota was significantly decreased in MIA offspring of both sexes (n = 10). (C,D) At the class levels, Clostridia was significantly elevated, while Verrucomicrobiae was significantly reduced in MIA offspring of both sexes (n = 10). (E) The abundance of Desulfovibrionia was significantly increased only in MIA male offspring (n = 10). (F–H) At the order levels, Lachnospirales and Oscillospirales were significantly enhanced, while Verrucomicrobiales was significantly decreased in MIA male and female offspring (n = 10). (I) The abundance of Clostridia_UCG-014 was significantly elevated only in MIA male offspring (n = 10). (J–M) At the family levels, Muribaculaceae and Akkermansiaceae were significantly reduced, while Lachnospiraceae and Ruminococcaceae were significantly enhanced in MIA offspring of both sexes (n = 10). (N–P) Desulfovibrionaceae, Oscillospiraceae, and Eggerthellaceae were significantly increased only in MIA male offspring (n = 10). (Q–T) At the genus levels, Alistipes, Colidextribacter, and Lachnoclostridium were significantly increased, but Akkermansia was significantly decreased in MIA male and female offspring (n = 10). (U,V) Lachnospiraceae_NK4A136_group and Helicobacter were significantly elevated only in MIA female offspring (n = 10). (W) Parabacteroides was significantly decreased only in MIA male offspring (n = 10). Data are expressed as mean ± SEM; * p < 0.05 vs. the Veh male, # p < 0.05 vs. the Veh female, & p < 0.05 vs. the Poly I:C male. Veh, vehicle; MIA, maternal immune activation.
With respect to the PND 60 time point, at the phylum level, Firmicutes and Patescibacteria were significantly decreased in Poly I:C offspring of both sexes (Figures 8A,B). At the class level, Bacilli and Saccharimonadia were significantly decreased in Poly I:C offspring of both sexes (Figures 8C,E), but Verrucomicrobiae was significantly reduced only in Poly I:C female offspring (Figure 8D). At the order level, Lactobacillales was significantly decreased in male and female offspring of Poly I:C (Figure 8F), but Verrucomicrobiales was significantly reduced only in Poly I:C female offspring (Figure 8G). At the family level, Lactobacillaceae was significantly decreased in Poly I:C offspring of both sexes (Figure 8H); Akkermansiaceae was significantly reduced, while Oscillospiraceae was significantly increased only in Poly I:C female offspring (Figures 8I,K); Prevotellaceae was significantly increased only in Poly I:C male offspring (Figure 8J), while Bacteroidaceae was significantly increased only in Poly I:C male offspring (Figure 8L). At the genus level, Lachnospiraceae_NK4A136_group and Ralstonia were significantly enhanced in Poly I:C offspring of both sexes (Figures 8M,P); Desulfovibrio was significantly decreased only in Poly I:C male offspring (Figure 8N); Bacteroides was significantly increased only in Poly I:C male offspring (Figure 8O); and Oscillibacter was significantly elevated only in Poly I:C female offspring (Figure 8Q).
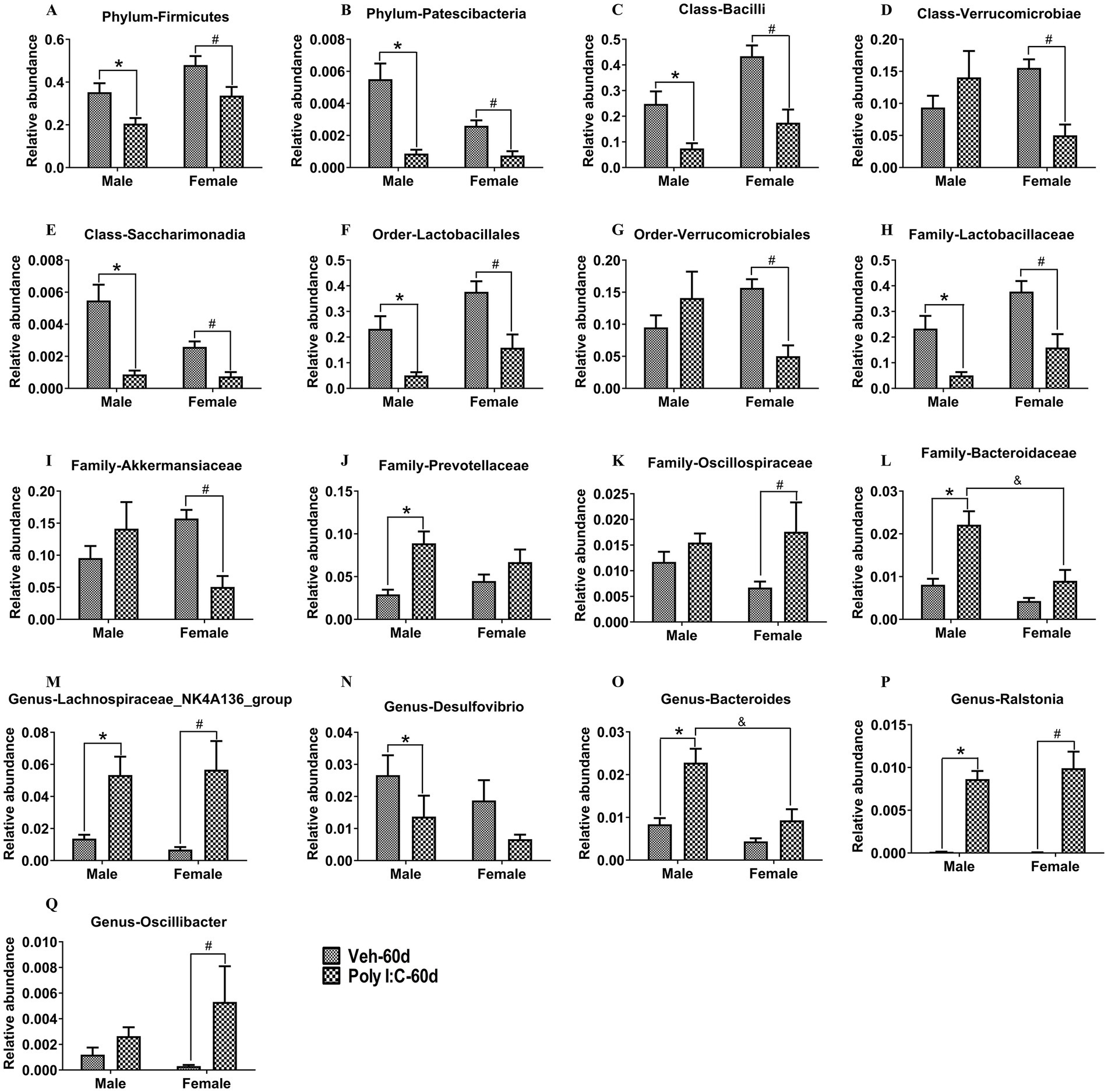
Figure 8. MIA caused alterations in the composition of gut microbiota in male and female offspring at adult period. (A,B) At the phylum levels, Firmicutes and Patescibacteria were significantly decreased in MIA offspring of both sexes (n = 10). (C,E) At the class levels, Bacilli and Saccharimonadia were significantly decreased in MIA offspring of both sexes (n = 10). (D) Verrucomicrobiae was significantly reduced only in MIA female offspring (n = 10). (F) At the order levels, Lactobacillales was significantly decreased in MIA offspring of both sexes (n = 10). (G) Verrucomicrobiales was significantly reduced only in MIA female offspring (n = 10). (H) At the family levels, Lactobacillaceae was significantly decreased in MIA offspring of both sexes (n = 10). (I,K) Akkermansiaceae was significantly reduced, while Oscillospiraceae was significantly increased only in MIA female offspring (n = 10). (J,L) Prevotellaceae and Bacteroidaceae were significantly increased only in MIA male offspring (n = 10). (M,P) At the genus levels, Lachnospiraceae_NK4A136_group and Ralstonia were significantly enhanced in MIA offspring of both sexes (n = 10). (N,O) Desulfovibrio was significantly decreased, while Bacteroides was significantly increased only in MIA male offspring (n = 10). (Q) Oscillibacter was significantly elevated only in MIA female offspring (n = 10). Data are expressed as mean ± SEM; *p < 0.05 vs. the Veh male, #p < 0.05 vs. the Veh female, &p < 0.05 vs. the Poly I:C male. Veh, vehicle; MIA, maternal immune activation.
3.6 Correlational analysis of gut microbiota with behavioral parameters and TRP metabolism pathway
Finally, we investigated whether changes in gut microbiota were correlated with behavioral parameters and the TRP metabolism pathway in Poly I:C offspring of both sexes during PND 40 and 60. Because prenatal Poly I:C exposure-induced schizophrenia-like abnormal behaviors were evident in both sexes, we focused on those bacterial taxa and TRP metabolites that were similarly altered in Poly I:C male and female offspring.
As shown in Figure 9A, at PND 40, Clostridia at the class level and Colidextribacter at the genus level were negatively correlated with PPI at 76 dB. Lachnoclostridium at the genus level was negatively correlated with PPI at 76 dB and positively correlated with hippocampal QA levels. Alistipes at the genus level was negatively associated with the novel object recognition index and hippocampal KYNA levels. Firmicutes at the phylum level was negatively associated with hippocampal 5-HT levels. Akkermansiaceae at the family level and Akkermansia at the genus level were positively correlated with hippocampal KYN levels. Muribaculaceae, at the family level showed a positive association with hippocampal 5-HT levels.
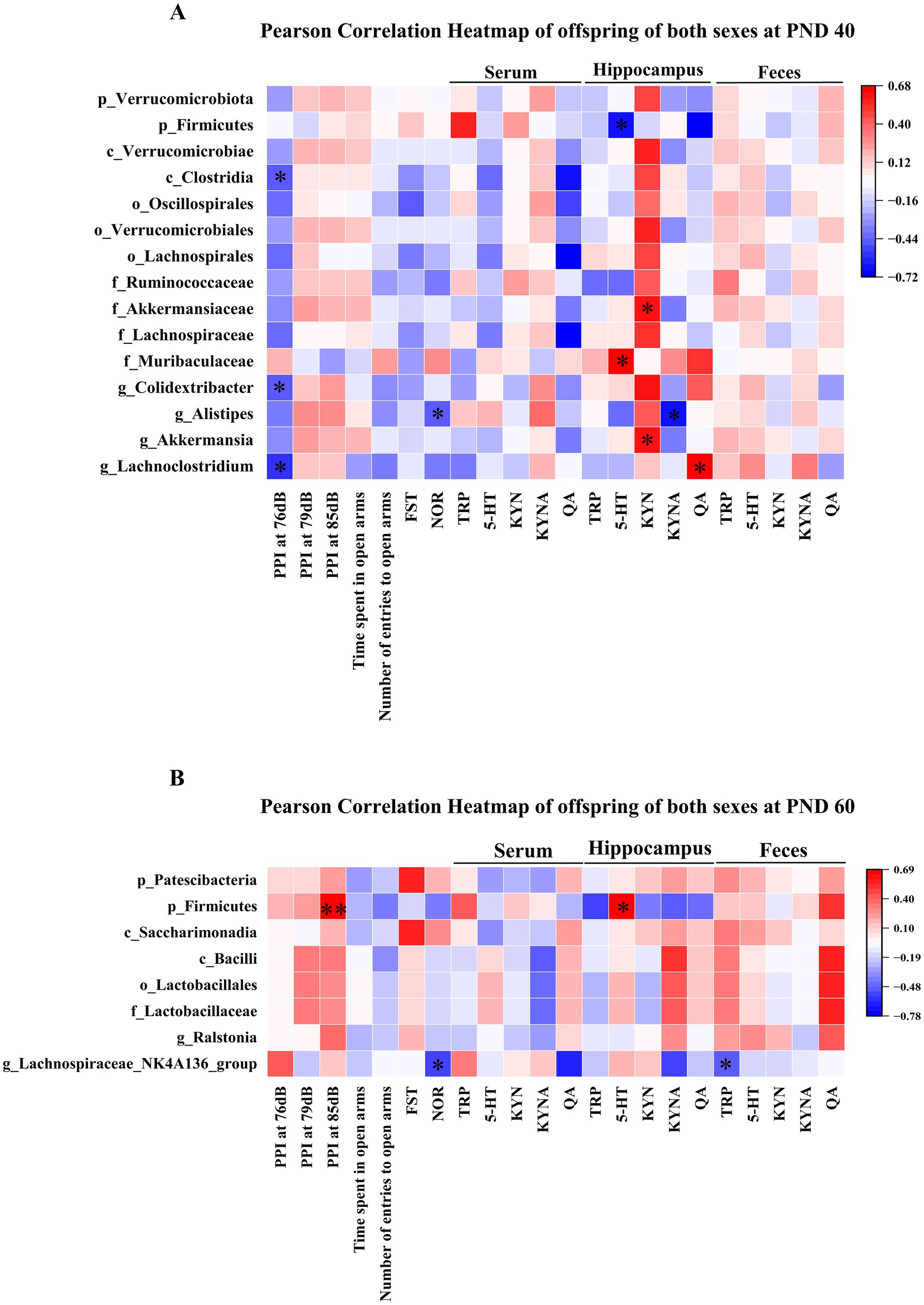
Figure 9. Correlational analysis of gut microbiota with behavioral parameters and the TRP metabolism pathway in MIA offspring of both sexes. (A) The heat map showed the correlation of gut microbiota with PPI at 76 dB, novel object recognition index, 5-HT, KYN, KYNA, and QA in MIA offspring of both sexes at PND 40. (B) The heat map showed the correlational analysis between gut microbiota and PPI at 85 dB, novel object recognition index, TRP, and 5-HT in MIA male and female offspring during PND 60. Colors ranged from red (positive association) to blue (negative association). * p < 0.05, ** p < 0.01. p, phylum; c, class; o, order; f, family; g, genus; PND 40, postnatal day 40; PND 60, postnatal day 60.
As shown in Figure 9B, at PND 60, Firmicutes at the phylum level was positively correlated with PPI at 85 dB and hippocampal 5-HT levels. The Lachnospiraceae_NK4A136_group at the genus level showed a negative association with the novel object recognition index and fecal TRP levels.
In addition, our results showed that prenatal Poly I:C exposure has different impacts on the composition of gut microbiota in offspring of two sexes, suggesting sex-related differences in gut microbiota composition. Thus, we investigated, separately in Poly I:C MIA male and female offspring, the correlations between gut microbiota alterations and behavioral parameters as well as the TRP metabolism pathway at PND 40 and 60.
At PND 40, Desulfovibrionia at the class level and Desulfovibrionaceae at the family level were negatively correlated with the number of entries to open arms in male offspring (Figure 10A). Oscillospiraceae at the family level was negatively correlated with serum TRP levels in male offspring (Figure 10A). Eggerthellaceae at the family level was positively associated with serum QA levels, while Parabacteroides at the genus level was negatively associated with serum QA levels in male offspring (Figure 10A). In addition, Helicobacter at the genus level was positively related to the immobility time of the FST and serum QA levels in female offspring (Figure 10B).
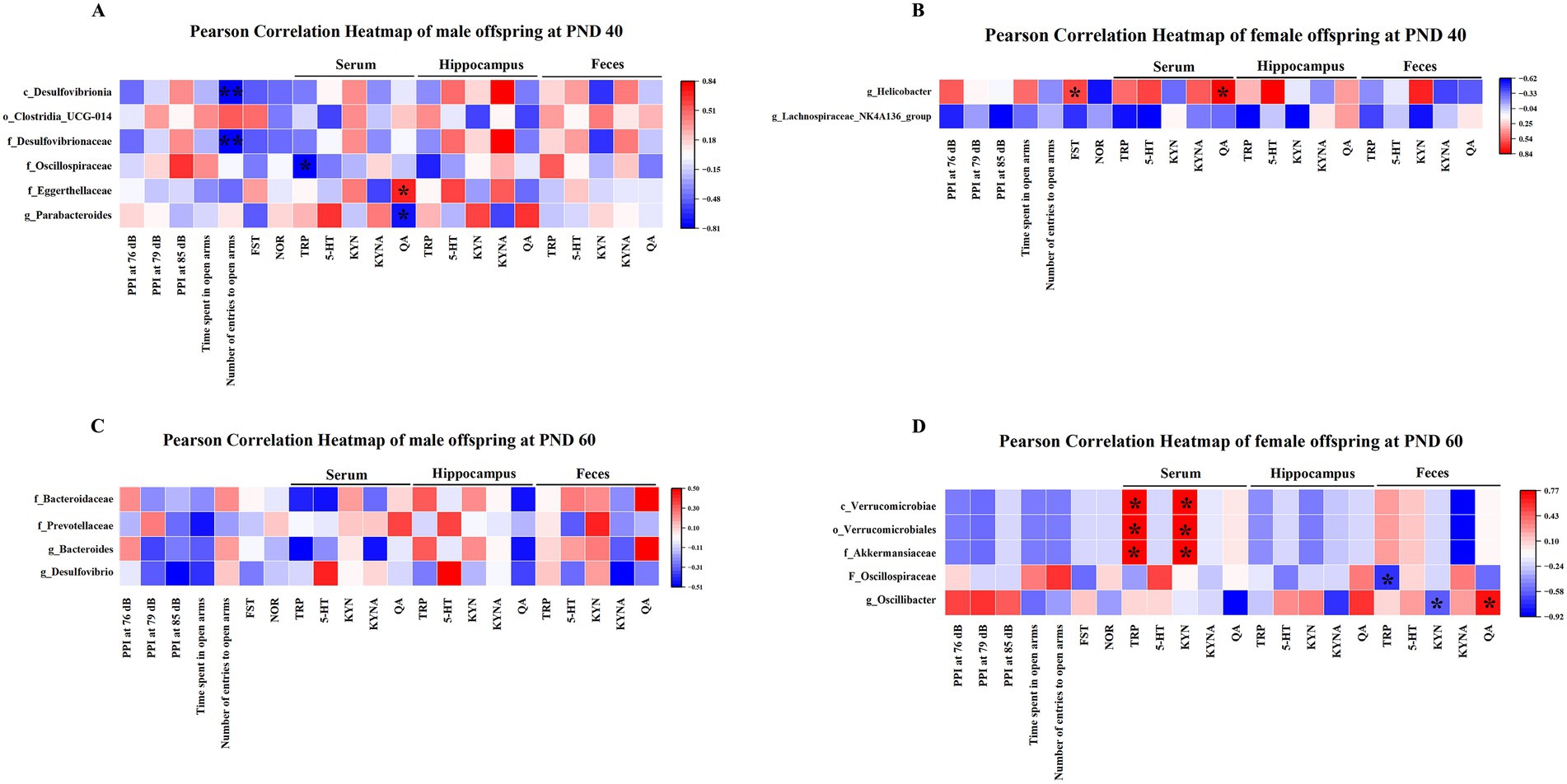
Figure 10. Correlational analysis of gut microbiota with behavioral parameters and the TRP metabolism pathway separately in MIA males and females. (A) The heat map revealed the correlation of the gut microbiota with the number of entries to open arms, TRP, and QA in MIA male offspring at PND 40. (B) The heat map revealed the association between the gut microbiota and immobility time of FST as well as QA in MIA female offspring at PND 40. (C) The heat map showed the associational analysis in MIA male offspring at PND 60. (D) The heat map showed the correlation of the gut microbiota with TRP, KYN, and QA in MIA female offspring at PND 60. Colors ranged from red (positive association) to blue (negative association). *p < 0.05, **p < 0.01. c, class; o, order; f, family; g, genus; PND 40, postnatal day 40; PND 60, postnatal day 60.
At PND 60, there was no significant correlation between gut microbiota and behavioral parameters and TRP metabolic pathways in male offspring (Figure 10C). In addition, Verrucomicrobiae at the class level, Verrucomicrobiales at the order level, and Akkermansiaceae at the family level were positively associated with serum TRP and KYN levels in female offspring (Figure 10D). Oscillospiraceae at the family level showed a negative correlation with fecal TRP levels in female offspring (Figure 10D). Moreover, Oscillibacter at the genus level was negatively related to fecal KYN levels and positively related to fecal QA levels in female offspring (Figure 10D).
4 Discussion
In the present study, we found that prenatal Poly I:C exposure at GD 9 induced gut microbiota dysbiosis, thereby activating the TRP-KYN-QA pathway in hippocampus, serum, and feces, suppressing the hippocampal and serum TRP-KYN-KYNA pathways and the hippocampal, serum, and fecal TRP-5-HT pathways, and thus resulting in anxiety- and depression-like behaviors and deficits in PPI and recognition memory in female and/or male offspring during adolescence and/or adulthood. In addition, prenatal Poly I:C exposure led to sex-dependent alterations in QA levels and gut microbiota composition.
PPI is used to measure impairments in sensorimotor gating. Deficits in sensorimotor gating are common psychopathological characteristics and core endophenotypic markers of schizophrenia (Abel et al., 2010). The NOR test is performed to assess recognition memory (Bevins and Besheer, 2006). The EPM test and FST are used to evaluate anxiety-like behavior and depression-like behavior, respectively (Su et al., 2022; Reisinger et al., 2016). Clinical studies have shown that patients with schizophrenia of both sexes exhibit anxiety- and depression-like behaviors as well as deficits in PPI and recognition memory (Belekou et al., 2023; Kanchanatawan et al., 2018a,b; Swerdlow et al., 2018). In a ketamine mouse model of schizophrenia, chronic ketamine exposure for 12 and 14 days elicits anxiety, depression, and impairments in PPI and recognition memory in mice (Fujikawa et al., 2021; Farkhakfar et al., 2023; Xu et al., 2025). In addition, prenatal Poly I:C exposure at GD 9, 12, and 15 has been reported to trigger anxiety- and depression-like behaviors as well as recognition memory and PPI deficits in male and female offspring during adolescence and adulthood (Yildiz Taskiran et al., 2023a,b; Su et al., 2022; Reisinger et al., 2016; Osborne et al., 2019; Arad et al., 2017; Kim et al., 2018; Yang et al., 2021). In line with these findings, we observed that prenatal Poly I:C exposure at GD 9 decreased the novel object recognition index, decreased PPI, increased the immobility time in the FST, and reduced the time spent in the open arms and the number of entries to the open arms in the EPM test in adolescent and adult offspring of both sexes, suggesting impairments in recognition memory, deficits in sensorimotor gating, anxiety-like behaviors, and depression-like behaviors, respectively. By contrast, no significant differences in time spent in the center zone, number of entries to the center zone, and total distance moved in the OFT were observed between Poly I:C MIA and Veh offspring of both sexes during adolescence and adulthood. On the other hand, a previous study has indicated that administration of Poly I:C at GD 12 does not affect locomotor activity in the OFT in adult female offspring (Reisinger et al., 2016). Similarly, our results showed that Poly I:C exposure at GD 9 did not change locomotor activity in female and male offspring at adolescence and adulthood, suggesting that the impairments in recognition memory are not due to deficits in locomotor activity.
There is extensive evidence that gut microbiota dysregulation contributes to schizophrenia pathogenesis and progression (Zhu et al., 2020a,b; Zheng et al., 2019). Patients with schizophrenia exhibit abnormalities in gut microbiota composition, and these altered microbes are associated with negative and cognitive symptoms, including social function, verbal learning, visual learning, working memory, and depression (Zhu et al., 2021; Zhu et al., 2020a,b). In MK-801 mouse model of schizophrenia, mice that received an injection of MK-801 for 14 days exhibit intestinal microbiota dysfunction, anxiety and depression behaviors, and spatial recognition memory impairments, indicating that gut microbiota may be involved in these psychotic and cognitive symptoms that occur after chronic MK-801 exposure (Guo et al., 2021). Notably, mice transplanted with gut flora from schizophrenia patients show schizophrenia-like behaviors, such as increased startle responses, impaired learning and memory, and psychomotor hyperactivity (Zheng et al., 2019; Zhu et al., 2020a,b). A recent study has found that prenatal Poly I:C administration at GD 9 causes gut microbiota dysregulation in offspring of both sexes during adolescence, similar to those observed in patients with schizophrenia (Juckel et al., 2021). Moreover, prenatal Poly I:C exposure at GD 9 and 15 leads to abnormal composition of intestinal flora that may be involved in anxiety-like behaviors and impairments in recognition memory and PPI in adult male offspring (Li et al., 2021; Romero-Miguel et al., 2023). However, no attempts have been made to investigate the potential relationship between gut microbiota and prenatal Poly I:C exposure-induced schizophrenia-like abnormal behaviors in adolescent male offspring and in adolescent and adult female offspring.
In this study, our results revealed that prenatal Poly I:C exposure causes gut microbiota dysfunction, similar to that found in patients with schizophrenia. Specifically, at PND 40, families Lachnospiraceae and genera Alistipes, Colidextribacter, and Lachnoclostridium were significantly increased, while families Muribaculaceae and genus Akkermansia were significantly decreased in Poly I:C MIA offspring of both sexes. Genus Lachnospiraceae_NK4A136_group was significantly elevated in MIA female offspring at PND 40, but genus Parabacteroides was significantly reduced in MIA male offspring at PND 40. At PND 60, family Prevotellaceae was significantly increased only in MIA male offspring. Genus Oscillibacter was significantly increased only in MIA female offspring at PND 60. Genera Lachnospiraceae_NK4A136_group and Ralstonia were significantly enhanced in MIA male and female offspring at PND 60. In addition, our Pearson’s correlation analysis showed that classes Clostridia and genera Colidextribacter and Lachnoclostridium were significantly negatively correlated with PPI at 76 dB in offspring of both sexes at PND 40, while genus Alistipes was significantly negatively correlated with the novel object recognition index in offspring of both sexes at PND 40. Phylum Firmicutes was significantly positively associated with PPI at 85 dB, while genus Lachnospiraceae_NK4A136_group was significantly correlated with the novel object recognition index at in offspring of both sexes at PND 60. Furthermore, Desulfovibrionia at the class level and Desulfovibrionaceae at the family level were negatively correlated with the number of entries to open arms in male offspring at PND 40. Helicobacter at the genus level was positively related to immobility time in the FST at PND 40. Consistent with our findings, several recent studies have shown that the Lachnospiraceae and Prevotellaceae families, along with genera Alistipes, Lachnospiraceae_NK4A136_group, and Lachnoclostridium, are significantly elevated, while the Muribaculaceae family and genera Akkermansia and Parabacteroides are significantly reduced in schizophrenia patients and the MK-801 mouse model of schizophrenia (Liu et al., 2024; Chen et al., 2021; Yang P. et al., 2024; Yang Z. et al., 2024; Yuan et al., 2021; Nguyen et al., 2021). The Oscillibacter genus is significantly increased and negatively associated with working memory, visual learning, and logical memory in patients with schizophrenia (Ma et al., 2022). Meanwhile, the Oscillibacter genus is positively related to neuroinflammation in a rodent model of Alzheimer’s disease, and the reduction of the Oscillibacter genus helps to improve cognitive function and elevate learning and memory abilities (Wang et al., 2021; Wang et al., 2022). The Alistipes genus is a GABA-producing flora that can metabolize TRP into indole and catabolize undigested proteins into harmful metabolites such as ammonia, H2S, and cresol, thereby causing or aggravating depression-like behaviors (Parker et al., 2020). The Parabacteroides genus, a core member of the gut microbiota in both mice and humans, has physiological characteristics of carbohydrate metabolism and SCFAs secretion (Cui et al., 2022). Meanwhile, the Parabacteroides genus may be viewed as a potential probiotic candidate due to its protective effects on inflammation and obesity, as well as its ability to partially ameliorate anxiety- and depression-like behaviors in mice (Cui et al., 2022; Deng et al., 2021). In addition, it has been reported that increased abundance of the Firmicutes phylum may be implicated in learning and memory impairments and mood disorders by enhancing proinflammatory cytokine levels and modulating immune cells (Porru et al., 2024). Therefore, gut microbiota dysbiosis caused by prenatal Poly I:C exposure at GD 9 may be responsible for anxiety- and depression-like behaviors, as well as deficits in recognition memory and PPI in female and male offspring of Poly I:C MIA mothers during adolescence and adulthood.
Increasing evidence suggests that TRP and its neuroactive catabolites in systemic circulation are largely regulated by gut microbiota (Clarke et al., 2014; Zhou et al., 2023; Guo et al., 2021; Zhu et al., 2020a,b). A recent clinical study has shown that the increased abundances of gut microbiota in schizophrenia patients are negatively associated with serum TRP levels and positively associated with serum KYNA levels (Zhu et al., 2020a,b). In the MK-801 mouse model of schizophrenia, the genera Parasutterella, Parabacteroides, and Eubacterium fissicatena are positively correlated with brain 5-HT levels, while the genera Lactobacillus, Muribaculum, Alistipes, and Lachnospiraceae_NK4A136_group are negatively correlated with brain 5-HT levels (Guo et al., 2021). In a mouse model of depression, recolonization of the genus Roseburia leads to increases in 5-HT levels and decreases in 3-HK and QA levels in brain and colon tissue (Zhou et al., 2023). Compared to microbiota-rich mice, germ-free mice exhibit elevated TRP levels and reduced 5-HT and KYN levels; these abnormal alterations are reverted to normal levels after bacterial recolonization (Clarke et al., 2014). It has been reported that the genera Alistipes and Lachnoclostridium could hydrolyze TRP to indole (Parker et al., 2020; Gao et al., 2019; Sun et al., 2021). In this study, at PND 40, we found that Firmicutes at the phylum level was negatively associated with hippocampal 5-HT levels, but Muribaculaceae at the family level was positively associated with hippocampal 5-HT levels in offspring of both sexes. Akkermansiaceae at the family level and Akkermansia at the genus level were positively correlated with hippocampal KYN levels in offspring of both sexes at PND 40. Alistipes at the genus level was negatively associated with hippocampal KYNA levels, and Lachnoclostridium at the genus level was positively correlated with hippocampal QA levels in offspring of both sexes at PND 40. Firmicutes at the phylum level was positively associated with hippocampal 5-HT levels, and Lachnospiraceae_NK4A136_group at the genus level was negatively associated with fecal TRP levels in offspring of both sexes at PND 60. In addition, Oscillospiraceae at the family level was negatively correlated with serum TRP levels in male offspring at PND 40. Eggerthellaceae at the family level was positively associated with serum QA levels, while Parabacteroides at the genus level was negatively associated with serum QA levels in male offspring at PND 40. Helicobacter at the genus level was positively related to serum QA levels in female offspring at PND 40. Verrucomicrobiae at the class level, Verrucomicrobiales at the order level, and Akkermansiaceae at the family level were positively associated with serum TRP and KYN levels in female offspring at PND 60. Oscillospiraceae at the family level showed a negative correlation with fecal TRP levels in female offspring at PND 60. Moreover, Oscillibacter at the genus level was negatively related to fecal KYN levels and positively related to fecal QA levels in female offspring at PND 60. Taken together, these findings reveal that gut microbiota plays an important role in regulating the TRP metabolism pathway.
It has been reported that the imbalance of the TRP metabolism pathway is associated with psychotic and cognitive symptoms in schizophrenia (Kanchanatawan et al., 2018a,b; Cathomas et al., 2021; Chen et al., 2024). As an excitotoxin, increased QA levels overactivate the NMDA receptor and increase the release of glutamate, leading to neurodegeneration, neuronal damage, and thus impairing brain function (Cao et al., 2021). Clinical studies have found that patients with schizophrenia show increased QA levels and reduced TRP, KYN, and KYNA levels in serum and plasma, and that elevated QA levels are correlated with cognitive deficits, including attention, executive function, language function, and visual learning (Cathomas et al., 2021; Chen et al., 2024). It has been reported that increased QA levels in plasma are involved in the pathogenesis of negative symptoms in schizophrenia (Kanchanatawan et al., 2018a,b). Brain injection of QA significantly elicits impairments in PPI and recognition memory in rats (Velloso et al., 2009; Shoemaker et al., 2003). Moreover, acute lipopolysaccharide (LPS) injection increases QA levels in the brain and plasma in mice, which may be correlated with LPS-induced depression- and anxiety-like behaviors (Verdonk et al., 2019). On the other hand, the lack of 5-HT in the central nervous system (CNS) affects postnatal CNS development and proper wiring of the brain, leading to brain dysfunction (Migliarini et al., 2013). A significant decrease in 5-HT levels in full blood is found in schizophrenia patients, and this decrease in 5-HT levels is negatively correlated with depressive symptoms in patients (Peitl et al., 2016). In the MK-801 rat model of schizophrenia, reduced frontal cortex levels of 5-HT are significantly associated with impairments in recognition memory in rats (Hereta et al., 2020). A previous animal study has indicated that reduced levels of median raphe nucleus 5-HT by a 5-HT neurotoxin will lead to deficits in PPI in rats (Kusljic et al., 2006). In a mouse model of constipation, decreased levels of serum 5-HT may be implicated in constipation-induced anxiety- and depression-like behaviors in mice (Zou et al., 2024). In addition, Poly I:C-induced MIA increases mRNA levels of KMO and the QA/KYNA ratio and decreases 5-HT levels in the frontal cortex in adult offspring of both sexes (MacDowell et al., 2021). Poly I:C MIA elevates QA levels and reduces KYN and KYNA levels in serum in adolescent male offspring (Zavitsanou et al., 2014). Increased brain serotonergic activity by selective 5-HT reuptake inhibitors improves impairments in PPI and recognition memory in adult male offspring of dams exposed to Poly I:C during gestation (Taskiran et al., 2024). Prenatal Poly I:C exposure increases 3-HK levels in the placenta and fetal brain, and this increase in 3-HK levels is correlated with impairment in recognition memory in adult male offspring (Hasegawa et al., 2024). In the present study, we found that prenatal Poly I:C exposure at GD 9 reduced serum and hippocampal levels of TRP, 5-HT, KYN, and KYNA, increased serum QA in female offspring at adolescence and adulthood, and increased hippocampal levels of QA in male and female offspring at adolescence and adulthood. In addition, we found that prenatal Poly I:C exposure at GD 9 increased hippocampal expression of IDO1 and KMO but decreased hippocampal expression of TPH2 and KATII in female and male offspring at adolescence and adulthood. Moreover, prenatal Poly I:C exposure at GD 9 decreased fecal levels of TRP, 5-HT, and KYN in female and male offspring at adolescence and adulthood and increased fecal QA levels in male and female offspring at adolescence but only in females at adulthood. Our results suggest that prenatal Poly I:C exposure at GD 9 facilitates the shift of TRP metabolism from the formation of KYNA and 5-HT toward the production of QA in offspring of both sexes at adolescence and adulthood. Together, these findings suggest that the upregulation of the TRP-KYN-QA pathway induced by gut dysbiosis may be responsible for anxiety- and depression-like behaviors as well as deficits in recognition memory and PPI during adolescence and adulthood in female and male offspring of Poly I:C MIA mothers.
It has been reported that Poly I:C binds to TLR3 to activate proinflammatory signaling, increasing proinflammatory cytokine levels that can cross the placental barrier and blood-brain barrier, thus leading to peripheral inflammation and neuroinflammation in offspring (Su et al., 2022). Proinflammatory cytokines are known inducers of IDO1 and KMO expression (Kindler et al., 2020), suggesting that an increased inflammatory response may activate the QA pathway. In addition, the Muribaculaceae family, a member of the Bacteroidetes order, has been reported to metabolize both endogenous and exogenous polysaccharides into SCFAs, thereby exerting anti-inflammatory effects (Zhu et al., 2024). Intestinal colonization of the Akkermansia genus has a close relationship with host health. The Akkermansia genus can effectively improve gut barrier function by increasing the thickness of the intestinal mucus layer and can a produce beneficial immune response by modulating immune cells (Cani et al., 2022). The Colidextribacter genus is involved in the modulation of inflammation markers, and the decrease in the abundance of the Colidextribacter genus may be beneficial in alleviating the severity of neuroinflammation (Yang P. et al., 2024; Yang Z. et al., 2024). It has been reported that the increased abundance of the Ralstonia genus leads to the overproduction of LPS and activation of the TLR4-NF-κB signaling pathway, which in turn triggers an inflammatory response (Zhang et al., 2024). Furthermore, the Lachnospiraceae family, a member of the Firmicutes phylum, could recruit macrophages and promote the translocation of LPS from the gastrointestinal tract into the systemic circulatory system, thereby elevating proinflammatory cytokine levels in the peripheral and central nervous system, and causing an immune inflammatory response (Sorbara et al., 2020; Yang et al., 2023). The Lachnospira ceae_NK4A136_group genus induces gastrointestinal inflammation by activating the TLR4-NF-κB pathway and is positively correlated with the pathological characteristics of colitis in mice (He et al., 2023). A recent animal study has found that the Prevotellaceae family, as Gram-positive bacteria, can induce a systemic inflammatory response by upregulating the sphingosine-1-phosphate receptor 2-NF-κB signaling pathway in mice (Chen et al., 2025). Meanwhile, the study also showed that the increased abundance of the Prevotellaceae family may be promote the colonization of pathogenic bacteria (Chen et al., 2025). In addition, the Clostridia class is an important producer of biotoxins and can secrete various toxins that induce apoptosis and interfere with nerve signal transmission (Philippe et al., 2006). Meanwhile, the Clostridia class could also lead to systemic infections (Morvan et al., 2021). These findings suggest that gut microbiota play a key role in the regulation of inflammatory responses. Thus, the upregulation of the QA pathway in our study may be partly caused by the enhanced inflammatory response mediated by gut microbiota dysbiosis that occurs after prenatal Poly I:C exposure.
Previous studies have found that there are significant differences in estrogen levels between female and male mice, with estrogen levels being higher in female mice than in male mice (Gegenhuber et al., 2022; Du et al., 2024). Estrogen has been reported to enhance the expression of IDO1 and inhibit KATII activity (Jayawickrama et al., 2017; Li et al., 2020), which may activate the TRP-KYN-3-HK pathway, leading to increases in QA levels. Young women who take oral contraceptives (generally containing both estrogen and progesterone analogues) exhibit increased excretion of KYN pathway metabolites, including 3-HK, 3-hydroxyanthranilic acid, and QA (de Bie et al., 2016). A previous study indicates sex-dependent differences in the neuroinflammatory responses of offspring induced by prenatal Poly I:C exposure (Posillico et al., 2021). Type I interferons are important to the anti-viral response and respond to viral stimulants such as Poly I:C (Posillico et al., 2021). It has been reported that type I interferons can facilitate IDO1 expression, thereby activating the TRP-KYN metabolism pathway (Zhu et al., 2020a,b). More importantly, the expression of type I interferons is significantly different in male and female offspring of pregnant dams injected with Poly I:C, suggesting a specific sex-related difference in the anti-viral response (Posillico et al., 2021). In this study, we revealed that prenatal Poly I:C exposure increased hippocampal QA levels in offspring of both sexes at PND 40 and 60, with hippocampal QA levels being higher in female offspring than in male offspring. Meanwhile, serum QA levels were elevated only in female MIA offspring at PND 40 and 60. Furthermore, fecal QA levels were significantly elevated only in MIA female offspring at PND 60. Our results revealed that the effect of prenatal Poly I:C exposure on the QA metabolism pathway is more pronounced in female offspring, suggesting sex-related differences in the TRP-KYN-QA pathway. In addition, estrogen has been shown to have a profound impact on gut microbiota composition (Dominianni et al., 2015; Juckel et al., 2021). Previous studies have found that microbiome composition differs significantly between males and females in humans as well as rodents (Dominianni et al., 2015; Juckel et al., 2021). In this study, we found that prenatal Poly I:C exposure increased the abundance of classes Desulfovibrionia, orders Clostridia_UCG-014, and families Desulfovibrionaceae, Oscillospiraceae, and Eggerthellaceae, decreased the abundance of genera Parabacteroides in male MIA offspring, and increased the abundance of genera Lachnospiraceae_NK4A136_group and Helicobacter in female MIA offspring at PND 40. Furthermore, prenatal Poly I:C exposure increased the abundance of families Prevotellaceae and Bacteroidaceae and genera Bacteroides, decreased the abundance of genera Desulfovibrio in male MIA offspring, elevated the abundance of Oscillospiraceae and genera Oscillibacter, and reduced the abundance of classes Verrucomicrobiae, orders Verrucomicrobiales, and families Akkermansiaceae in female MIA offspring at PND 60. Our results showed that prenatal Poly I:C exposure has different effects on the composition of gut microbiota in female and male offspring, suggesting sex-dependent differences in gut microbiota composition. Together, our study provides a foundation for further research to clarify whether sex-related TRP-KYN-QA metabolism pathways and gut microbiota changes caused by MIA could contribute to behavioral domains not detected here, or might confer susceptibility to – or protect against – double-hit effects.
Increasing evidence suggests that gut microbiota disorder is implicated in psychotic symptoms and cognitive impairments in patients with schizophrenia (Zhu et al., 2021; Zhu et al., 2020a,b). It has been reported that gut microbiota dysregulation impairs neurodevelopment, neurogenesis, and synaptic structure and function, affects neurotransmitter metabolism, and changes the levels of microbial metabolites, thereby causing psychotic symptoms and cognitive deficits in schizophrenia (Zhu et al., 2020a,b; Guo et al., 2021; Cussotto et al., 2018). Further studies are necessary to explore whether gut microbiota dysbiosis leads to Poly I:C MIA-induced anxiety- and depression-like behaviors, as well as PPI and recognition memory deficits by affecting nervous system development, synaptic plasticity, neurotransmitter metabolism, and microbial metabolite levels. In addition, given the intricate composition and structure of gut microbiota, the connection between aberrant abundance of specific bacterial genera and psychotic and cognitive symptoms remains unclear. Further studies, such as fecal microbiota transplant (FMT) experiments or probiotics/prebiotics interventions, are warranted to elucidate the potential mechanisms by which certain bacterial alterations contribute to Poly I:C MIA-induced psychotic symptoms and cognitive impairments. Furthermore, estrogen has been reported to affect the TRP-KYN metabolism pathway and the composition and structure of gut microbiota (Jayawickrama et al., 2017; Dominianni et al., 2015). Further studies are necessary to investigate whether estrogen is involved in Poly I:C MIA-caused psychotic and cognitive symptoms by mediating the TRP-KYN metabolism pathway and gut microbiota.
5 Conclusion
In summary, our results provide the first molecular and behavioral evidence that gut dysbiosis induced by prenatal Poly I:C exposure is likely responsible for prenatal Poly I:C exposure-induced anxiety- and depression-like behaviors and impairments in PPI and recognition memory by disrupting the TRP metabolism pathway in adolescent and adult offspring of both sexes. Our study suggests possible strategies to alleviate prenatal Poly I:C exposure-associated schizophrenia-like behaviors. Our findings provide additional evidence that gut microbiota dysbiosis is the mechanism underlying Poly I:C MIA-associated schizophrenia-like behaviors and behavioral deficits in schizophrenia. Considering the sex-related differences in the gut microbiota and TRP-KYN-QA pathway, both sexes should be included in the studies that investigate the mechanisms underlying Poly I:C MIA-associated schizophrenia-like behaviors.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Animal Experimentation Ethics Committee of Guangzhou Medical University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
ZX: Visualization, Data curation, Formal analysis, Writing – original draft, Investigation, Validation, Methodology. CH: Methodology, Investigation, Writing – original draft, Formal analysis. YL: Supervision, Conceptualization, Project administration, Writing – review & editing, Funding acquisition, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (grant number 82371509), the Guangdong Natural Science Funds (grant number 2024A1515010792), the Municipal School (Institute) Joint Funding Project from Guangzhou Municipal Science and Technology Bureau (grant number 2024A03J0217), the Plan on Enhancing Scientific Research in GMU (2023.01–2024.12), the Guangzhou Key Laboratory of Psychosomatic Medicine (grant number SL2023A03J00421), and the Guangzhou Municipal Key Discipline in Medicine (2025–2027).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1667164/full#supplementary-material
Abbreviations
PPI, prepulse inhibition; NOR, novel object recognition; OFT, open field test; FST, forced swimming test; EPM, elevated plus maze; Poly I:C, polyinosinic-polycytidylic acid; MIA, maternal immune activation; PND, postnatal day; GD, gestational day; TRP, tryptophan; KYN, kynurenine; KYNA, kynurenic acid; QA, quinolinic acid; 5-HT, 5-hydroxytryptamine; IDO, indoleamine 2,3-dioxygenases; KATs, kynurenine aminotransferases; KMO, kynurenine monooxygenase; TPH, tryptophan hydroxylase.
References
Abel, K. M., Drake, R., and Goldstein, J. M. (2010). Sex differences in schizophrenia. Int. Rev. Psychiatry 22, 417–428. doi: 10.3109/09540261.2010.515205
Agus, A., Planchais, J., and Sokol, H. (2018). Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23, 716–724. doi: 10.1016/j.chom.2018.05.003
Arad, M., Piontkewitz, Y., Albelda, N., Shaashua, L., and Weiner, I. (2017). Immune activation in lactating dams alters sucklings' brain cytokines and produces non-overlapping behavioral deficits in adult female and male offspring: a novel neurodevelopmental model of sex-specific psychopathology. Brain Behav. Immun. 63, 35–49. doi: 10.1016/j.bbi.2017.01.015
Belekou, A., Katshu, M. Z. U. H., Dundon, N. M., d'Avossa, G., and Smyrnis, N. (2023). Spatial and non-spatial feature binding impairments in visual working memory in schizophrenia. Schizophr. Res. Cogn. 32:100281. doi: 10.1016/j.scog.2023.100281
Bergdolt, L., and Dunaevsky, A. (2019). Brain changes in a maternal immune activation model of neurodevelopmental brain disorders. Prog. Neurobiol. 175, 1–19. doi: 10.1016/j.pneurobio.2018.12.002
Bevins, R. A., and Besheer, J. (2006). Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study 'recognition memory'. Nat. Protoc. 1, 1306–1311. doi: 10.1038/nprot.2006.205
Cani, P. D., Depommier, C., Derrien, M., Everard, A., and de Vos, W. M. (2022). Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 19, 625–637. doi: 10.1038/s41575-022-00631-9
Cao, B., Chen, Y., Ren, Z., Pan, Z., McIntyre, R. S., and Wang, D. (2021). Dysregulation of kynurenine pathway and potential dynamic changes of kynurenine in schizophrenia: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 123, 203–214. doi: 10.1016/j.neubiorev.2021.01.018
Cathomas, F., Guetter, K., Seifritz, E., Klaus, F., and Kaiser, S. (2021). Quinolinic acid is associated with cognitive deficits in schizophrenia but not major depressive disorder. Sci. Rep. 11:9992. doi: 10.1038/s41598-021-89335-9
Chen, W., Tian, Y., Gou, M., Wang, L., Tong, J., Zhou, Y., et al. (2024). Role of the immune-kynurenine pathway in treatment-resistant schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 130:110926. doi: 10.1016/j.pnpbp.2023.110926
Chen, X., Xu, J., Wang, H., Luo, J., Wang, Z., Chen, G., et al. (2021). Profiling the differences of gut microbial structure between schizophrenia patients with and without violent behaviors based on 16S rRNA gene sequencing. Int. J. Legal Med. 135, 131–141. doi: 10.1007/s00414-020-02439-1
Chen, L., Ye, Z., Li, J., Wang, L., Chen, Y., Yu, M., et al. (2025). Gut bacteria Prevotellaceae related lithocholic acid metabolism promotes colonic inflammation. J. Transl. Med. 23:55. doi: 10.1186/s12967-024-05873-6
Clarke, G., Stilling, R. M., Kennedy, P. J., Stanton, C., Cryan, J. F., and Dinan, T. G. (2014). Minireview: gut microbiota: the neglected endocrine organ. Mol. Endocrinol. 28, 1221–1238. doi: 10.1210/me.2014-1108
Cui, Y., Zhang, L., Wang, X., Yi, Y., Shan, Y., Liu, B., et al. (2022). Roles of intestinal Parabacteroides in human health and diseases. FEMS Microbiol. Lett. 369:fnac072. doi: 10.1093/femsle/fnac072
Cussotto, S., Sandhu, K. V., Dinan, T. G., and Cryan, J. F. (2018). The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. Front. Neuroendocrinol. 51, 80–101. doi: 10.1016/j.yfrne.2018.04.002
de Bie, J., Lim, C. K., and Guillemin, G. J. (2016). Kynurenines, gender and neuroinflammation; showcase schizophrenia. Neurotox. Res. 30, 285–294. doi: 10.1007/s12640-016-9641-5
Deng, Y., Zhou, M., Wang, J., Yao, J., Yu, J., Liu, W., et al. (2021). Involvement of the microbiota-gut-brain axis in chronic restraint stress: disturbances of the kynurenine metabolic pathway in both the gut and brain. Gut Microbes 13, 1–16. doi: 10.1080/19490976.2020.1869501
Dominianni, C., Sinha, R., Goedert, J. J., Pei, Z., Yang, L., Hayes, R. B., et al. (2015). Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS One 10:e124599. doi: 10.1371/journal.pone.0124599
Du, R., Lu, G., Luo, W., He, T., Li, C., Yu, Y., et al. (2024). Dyadic social interaction paradigm reveals selective role of ovarian estrogen in the caring behavior and socially transferred pain in female mice. Neuropharmacology 261:110138. doi: 10.1016/j.neuropharm.2024.110138
Farkhakfar, A., Hassanpour, S., and Zendehdel, M. (2023). Resveratrol plays neuroprotective role on ketamine-induced schizophrenia-like behaviors and oxidative damage in mice. Neurosci. Lett. 813:137436. doi: 10.1016/j.neulet.2023.137436
Fujikawa, R., Yamada, J., and Jinno, S. (2021). Subclass imbalance of parvalbumin-expressing GABAergic neurons in the hippocampus of a mouse ketamine model for schizophrenia, with reference to perineuronal nets. Schizophr. Res. 229, 80–93. doi: 10.1016/j.schres.2020.11.016
Gao, H., Jiang, Q., Ji, H., Ning, J., Li, C., and Zheng, H. (2019). Type 1 diabetes induces cognitive dysfunction in rats associated with alterations of the gut microbiome and metabolomes in serum and hippocampus. Biochim. Biophys. Acta Mol. Basis Dis. 1865:165541. doi: 10.1016/j.bbadis.2019.165541
Gegenhuber, B., Wu, M. V., Bronstein, R., and Tollkuhn, J. (2022). Gene regulation by gonadal hormone receptors underlies brain sex differences. Nature 606, 153–159. doi: 10.1038/s41586-022-04686-1
Guo, L., Xiao, P., Zhang, X., Yang, Y., Yang, M., Wang, T., et al. (2021). Inulin ameliorates schizophrenia via modulation of the gut microbiota and anti-inflammation in mice. Food Funct. 12, 1156–1175. doi: 10.1039/d0fo02778b
Hao, K., Su, X., Luo, B., Cai, Y., Chen, T., Yang, Y., et al. (2019). Prenatal immune activation induces age-related alterations in rat offspring: effects upon NMDA receptors and behaviors. Behav. Brain Res. 370:111946. doi: 10.1016/j.bbr.2019.111946
Hasegawa, M., Niijima, M., Kunisawa, K., Teshigawara, T., Kubota, H., Fujigaki, S., et al. (2024). Maternal immune activation induces neurodevelopmental impairments of adult offspring through alterations in tryptophane-kynurenine pathway in the placenta. Biochem. Biophys. Res. Commun. 737:150922. doi: 10.1016/j.bbrc.2024.150922
He, P., Zhang, Y., Chen, R., Tong, Z., Zhang, M., and Wu, H. (2023). The maca protein ameliorates DSS-induced colitis in mice by modulating the gut microbiota and production of SCFAs. Food Funct. 14, 10329–10346. doi: 10.1039/d3fo03654e
Hereta, M., Kamińska, K., Białoń, M., Wąsik, A., Lorenc-Koci, E., and Rogóż, Z. (2020). Effect of combined treatment with aripiprazole and antidepressants on the MK-801-induced deficits in recognition memory in novel recognition test and on the release of monoamines in the rat frontal cortex. Behav. Brain Res. 393:112769. doi: 10.1016/j.bbr.2020.112769
Jayawickrama, G. S., Nematollahi, A., Sun, G., Gorrell, M. D., and Church, W. B. (2017). Inhibition of human kynurenine aminotransferase isozymes by estrogen and its derivatives. Sci. Rep. 7:17559. doi: 10.1038/s41598-017-17979-7
Juckel, G., Manitz, M., Freund, N., and Gatermann, S. (2021). Impact of poly I:C induced maternal immune activation on offspring's gut microbiome diversity - implications for schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 110:110306. doi: 10.1016/j.pnpbp.2021.110306
Kanchanatawan, B., Sirivichayakul, S., Ruxrungtham, K., Carvalho, A. F., Geffard, M., Ormstad, H., et al. (2018a). Deficit, but not nondeficit, schizophrenia is characterized by mucosa-associated activation of the tryptophan catabolite (TRYCAT) pathway with highly specific increases in IgA responses directed to picolinic, xanthurenic, and quinolinic acid. Mol. Neurobiol. 55, 1524–1536. doi: 10.1007/s12035-017-0417-6
Kanchanatawan, B., Thika, S., Anderson, G., Galecki, P., and Maes, M. (2018b). Affective symptoms in schizophrenia are strongly associated with neurocognitive deficits indicating disorders in executive functions, visual memory, attention and social cognition. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 80, 168–176. doi: 10.1016/j.pnpbp.2017.06.031
Kim, H., Won, H., Im, J., Lee, H., Park, J., Lee, S., et al. (2018). Effects of Panax ginseng C.A. Meyer extract on the offspring of adult mice with maternal immune activation. Mol. Med. Rep. 18, 3834–3842. doi: 10.3892/mmr.2018.9417
Kindler, J., Lim, C. K., Weickert, C. S., Boerrigter, D., Galletly, C., Liu, D., et al. (2020). Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol. Psychiatry 25, 2860–2872. doi: 10.1038/s41380-019-0401-9
Kusljic, S., Brosda, J., and van den Buuse, M. (2006). Effects of haloperidol and clozapine on sensorimotor gating deficits induced by 5-hydroxytryptamine depletion in the brain. Br. J. Pharmacol. 147, 800–807. doi: 10.1038/sj.bjp.0706641
Li, W., Chen, M., Feng, X., Song, M., Shao, M., Yang, Y., et al. (2021). Maternal immune activation alters adult behavior, intestinal integrity, gut microbiota and the gut inflammation. Brain Behav. 11:e2133. doi: 10.1002/brb3.2133
Li, S., Zhao, S., Luo, J., Zhou, H., Xiao, Z., Weng, Y., et al. (2020). Estrogen induces indoleamine 2,3-dioxygenase expression via suppressors of cytokine signaling 3 in the chorionic villi and decidua of women in early pregnancy. Am. J. Reprod. Immunol. 83:e13197. doi: 10.1111/aji.13197
Liu, Y., Wu, H., Liu, B., Chen, S., Huang, L., Liu, Z., et al. (2024). Multi-omics analysis reveals the impact of gut microbiota on antipsychotic-induced weight gain in schizophrenia. Schizophr. Res. 270, 325–338. doi: 10.1016/j.schres.2024.06.040
Luo, Y., Yu, Y., He, H., and Fan, N. (2024). Acute ketamine induces neuronal hyperexcitability and deficits in prepulse inhibition by upregulating IL-6. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 130:110913. doi: 10.1016/j.pnpbp.2023.110913
Luo, Y., Yu, Y., Zhang, M., and Fan, N. (2022). GluN1 antibody causes behavioral deficits in prepulse inhibition and memory through CaMKIIβ signaling. J. Neuroimmunol. 373:577998. doi: 10.1016/j.jneuroim.2022.577998
Luo, Y., Yu, Y., Zhang, M., He, H., and Fan, N. (2021). Chronic administration of ketamine induces cognitive deterioration by restraining synaptic signaling. Mol. Psychiatry 26, 4702–4718. doi: 10.1038/s41380-020-0793-6
Ma, Q., Gao, F., Zhou, L., Fan, Y., Zhao, B., Xi, W., et al. (2022). Characterizing serum amino acids in schizophrenic patients: correlations with gut microbes. J. Psychiatr. Res. 153, 125–133. doi: 10.1016/j.jpsychires.2022.07.006
MacDowell, K. S., Munarriz-Cuezva, E., Meana, J. J., Leza, J. C., and Ortega, J. E. (2021). Paliperidone reversion of maternal immune activation-induced changes on brain serotonin and kynurenine pathways. Front. Pharmacol. 12:682602. doi: 10.3389/fphar.2021.682602
Migliarini, S., Pacini, G., Pelosi, B., Lunardi, G., and Pasqualetti, M. (2013). Lack of brain serotonin affects postnatal development and serotonergic neuronal circuitry formation. Mol. Psychiatry 18, 1106–1118. doi: 10.1038/mp.2012.128
Morvan, C., Folgosa, F., Kint, N., Teixeira, M., and Martin-Verstraete, I. (2021). Responses of Clostridia to oxygen: from detoxification to adaptive strategies. Environ. Microbiol. 23, 4112–4125. doi: 10.1111/1462-2920.15665
Nguyen, T. T., Kosciolek, T., Daly, R. E., Vázquez-Baeza, Y., Swafford, A., Knight, R., et al. (2021). Gut microbiome in schizophrenia: altered functional pathways related to immune modulation and atherosclerotic risk. Brain Behav. Immun. 91, 245–256. doi: 10.1016/j.bbi.2020.10.003
Osborne, A. L., Solowij, N., Babic, I., Lum, J. S., Huang, X., Newell, K. A., et al. (2019). Cannabidiol improves behavioural and neurochemical deficits in adult female offspring of the maternal immune activation (poly I:C) model of neurodevelopmental disorders. Brain Behav. Immun. 81, 574–587. doi: 10.1016/j.bbi.2019.07.018
Parker, B. J., Wearsch, P. A., Veloo, A. C. M., and Rodriguez-Palacios, A. (2020). The genus Alistipes: gut Bacteria with emerging implications to inflammation, Cancer, and mental health. Front. Immunol. 11:906. doi: 10.3389/fimmu.2020.00906
Peitl, V., Vidrih, B., Karlović, Z., Getaldić, B., Peitl, M., and Karlović, D. (2016). Platelet serotonin concentration and depressive symptoms in patients with schizophrenia. Psychiatry Res. 239, 105–110. doi: 10.1016/j.psychres.2016.03.006
Philippe, V. A., Méndez, M. B., Huang, I., Orsaria, L. M., Sarker, M. R., and Grau, R. R. (2006). Inorganic phosphate induces spore morphogenesis and enterotoxin production in the intestinal pathogen Clostridium perfringens. Infect. Immun. 74, 3651–3656. doi: 10.1128/IAI.02090-05
Porru, S., Esplugues, A., Llop, S., and Delgado-Saborit, J. M. (2024). The effects of heavy metal exposure on brain and gut microbiota: a systematic review of animal studies. Environ. Pollut. 348:123732. doi: 10.1016/j.envpol.2024.123732
Posillico, C. K., Garcia-Hernandez, R. E., and Tronson, N. C. (2021). Sex differences and similarities in the neuroimmune response to central administration of poly I:C. J. Neuroinflammation 18:193. doi: 10.1186/s12974-021-02235-7
Reigstad, C. S., Salmonson, C. E., Rainey, J. F. R., Szurszewski, J. H., Linden, D. R., Sonnenburg, J. L., et al. (2015). Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 29, 1395–1403. doi: 10.1096/fj.14-259598
Reisinger, S. N., Kong, E., Khan, D., Schulz, S., Ronovsky, M., Berger, S., et al. (2016). Maternal immune activation epigenetically regulates hippocampal serotonin transporter levels. Neurobiol. Stress 4, 34–43. doi: 10.1016/j.ynstr.2016.02.007
Romero-Miguel, D., Casquero-Veiga, M., Fernández, J., Lamanna-Rama, N., Gómez-Rangel, V., Gálvez-Robleño, C., et al. (2023). Maternal supplementation with N-acetylcysteine modulates the microbiota-gut-brain Axis in offspring of the poly I:C rat model of schizophrenia. Antioxidants (Basel) 12:970. doi: 10.3390/antiox12040970
Sekar, A., Bialas, A. R., de Rivera, H., Davis, A., Hammond, T. R., Kamitaki, N., et al. (2016). Schizophrenia risk from complex variation of complement component 4. Nature 530, 177–183. doi: 10.1038/nature16549
Shoemaker, J. M., Pitcher, L., Noh, H. R., and Swerdlow, N. R. (2003). Quetiapine produces a prolonged reversal of the sensorimotor gating-disruptive effects of basolateral amygdala lesions in rats. Behav. Neurosci. 117, 136–143. doi: 10.1037//0735-7044.117.1.136
Sorbara, M. T., Littmann, E. R., Fontana, E., Moody, T. U., Kohout, C. E., Gjonbalaj, M., et al. (2020). Functional and genomic variation between human-derived isolates of Lachnospi-raceae reveals inter- and intra-species diversity. Cell Host Microbe 28, 134–146.e4. doi: 10.1016/j.chom.2020.05.005
Su, Y., Lian, J., Hodgson, J., Zhang, W., and Deng, C. (2022). Prenatal poly I:C challenge affects Behaviors and neurotransmission via elevated neuroinflammation responses in female juvenile rats. Int. J. Neuropsychopharmacol. 25, 160–171. doi: 10.1093/ijnp/pyab087
Sun, Y., Wu, D., Zeng, W., Chen, Y., Guo, M., Lu, B., et al. (2021). The role of intestinal dysbacteriosis induced arachidonic acid metabolism disorder in inflammaging in atherosclerosis. Front. Cell. Infect. Microbiol. 11:618265. doi: 10.3389/fcimb.2021.618265
Swerdlow, N. R., Light, G. A., Thomas, M. L., Sprock, J., Calkins, M. E., Green, M. F., et al. (2018). Deficient prepulse inhibition in schizophrenia in a multi-site cohort: internal replication and extension. Schizophr. Res. 198, 6–15. doi: 10.1016/j.schres.2017.05.013
Taskiran, M., Yildiz Taskiran, S., Unal, G., Bozkurt, N. M., and Golgeli, A. (2024). Vortioxetine improved schizophrenia-like behavioral deficits in a poly I:C-induced maternal immune activation model of schizophrenia in rats. Fundam. Clin. Pharmacol. 38, 1069–1079. doi: 10.1111/fcp.13028
Velloso, N. A., Dalmolin, G. D., Gomes, G. M., Rubin, M. A., Canas, P. M., Cunha, R. A., et al. (2009). Spermine improves recognition memory deficit in a rodent model of Huntington's disease. Neurobiol. Learn. Mem. 92, 574–580. doi: 10.1016/j.nlm.2009.07.006
Verdonk, F., Petit, A., Abdel-Ahad, P., Vinckier, F., Jouvion, G., de Maricourt, P., et al. (2019). Microglial production of quinolinic acid as a target and a biomarker of the antidepressant effect of ketamine. Brain Behav. Immun. 81, 361–373. doi: 10.1016/j.bbi.2019.06.033
Wang, G., Zhou, H., Luo, L., Qin, L., Yin, J., Yu, Z., et al. (2021). Voluntary wheel running is capable of improving cognitive function only in the young but not the middle-aged male APPSwe/PS1De9 mice. Neurochem. Int. 145:105010. doi: 10.1016/j.neuint.2021.105010
Wang, J., Zhu, X., Li, Y., Guo, W., and Li, M. (2022). Jiedu-Yizhi formula alleviates neuroinflammation in AD rats by modulating the gut microbiota. Evid. Based Complement. Alternat. Med. 2022, 1–19. doi: 10.1155/2022/4023006
Xu, Z., Lu, H., Hu, C., Wen, Y., Shang, D., Gan, T., et al. (2025). Inulin alleviates chronic ketamine-induced impairments in memory and prepulse inhibition by regulating the gut microbiota, inflammation, and kynurenine pathway. Int. J. Biol. Macromol. 294:139503. doi: 10.1016/j.ijbiomac.2025.139503
Yang, P., Huang, S., Luo, Z., Zhou, S., Zhang, C., Zhu, Y., et al. (2024). Radix Bupleuri aqueous extract attenuates MK801-induced schizophrenia-like symptoms in mice: participation of intestinal flora. Biomed. Pharmacother. 172:116267. doi: 10.1016/j.biopha.2024.116267
Yang, Z., Liu, S., Wei, F., and Hu, J. (2024). The effects of Qingchang ligan formula on hepatic encephalopathy in mouse model: results from gut microbiome-metabolomics analysis. Front. Cell. Infect. Microbiol. 14:1381209. doi: 10.3389/fcimb.2024.1381209
Yang, J., Su, T., Zhang, Y., Jia, M., Yin, X., Lang, Y., et al. (2023). A bidirectional mendelian ran-domization study investigating the causal role between gut microbiota and insomnia. Front. Neurol. 14:1277996. doi: 10.3389/fneur.2023.1277996
Yang, Y., Wang, B., Zhong, Z., Chen, H., Ding, W., and Hoi, M. P. M. (2021). Clonazepam attenuates neurobehavioral abnormalities in offspring exposed to maternal immune activation by enhancing GABAergic neurotransmission. Biochem. Pharmacol. 192:114711. doi: 10.1016/j.bcp.2021.114711
Yano, J. M., Yu, K., Donaldson, G. P., Shastri, G. G., Ann, P., Ma, L., et al. (2015). Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276. doi: 10.1016/j.cell.2015.02.047
Yildiz Taskiran, S., Taskiran, M., Unal, G., Bozkurt, N. M., and Golgeli, A. (2023b). The long-lasting effects of aceclofenac, a COX-2 inhibitor, in a poly I:C-induced maternal immune activation model of schizophrenia in rats. Behav. Brain Res. 452:114565. doi: 10.1016/j.bbr.2023.114565
Yildiz Taskiran, S., Taskiran, M., Unal, G., and Golgeli, A. (2023a). Group I mGluRs positive allosteric modulators improved schizophrenia-related behavioral and molecular deficits in the poly I:C rat model. Pharmacol. Biochem. Behav. 229:173593. doi: 10.1016/j.pbb.2023.173593
Yuan, X., Wang, Y., Li, X., Jiang, J., Kang, Y., Pang, L., et al. (2021). Gut microbial biomarkers for the treatment response in first-episode, drug-naïve schizophrenia: a 24-week follow-up study. Transl. Psychiatry 11:422. doi: 10.1038/s41398-021-01531-3
Zavitsanou, K., Lim, C. K., Purves-Tyson, T., Karl, T., Kassiou, M., Banister, S. D., et al. (2014). Effect of maternal immune activation on the kynurenine pathway in preadolescent rat offspring and on MK801-induced hyperlocomotion in adulthood: amelioration by COX-2 inhibition. Brain Behav. Immun. 41, 173–181. doi: 10.1016/j.bbi.2014.05.011
Zhang, Z., Chen, H., and Li, Q. (2024). High-fat diet led to testicular inflammation and ferroptosis via dysbiosis of gut microbes. Int. Immunopharmacol. 142:113235. doi: 10.1016/j.intimp.2024.113235
Zheng, P., Zeng, B., Liu, M., Chen, J., Pan, J., Han, Y., et al. (2019). The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci. Adv. 5:u8317. doi: 10.1126/sciadv.aau8317
Zhou, M., Fan, Y., Xu, L., Yu, Z., Wang, S., Xu, H., et al. (2023). Microbiome and tryptophan metabolomics analysis in adolescent depression: roles of the gut microbiota in the regulation of tryptophan-derived neurotransmitters and behaviors in human and mice. Microbiome 11:145. doi: 10.1186/s40168-023-01589-9
Zhu, Y., Chen, B., Zhang, X., Akbar, M. T., Wu, T., Zhang, Y., et al. (2024). Exploration of the Muribaculaceae family in the gut microbiota: diversity, metabolism, and function. Nutrients 16:2660. doi: 10.3390/nu16162660
Zhu, F., Guo, R., Wang, W., Ju, Y., Wang, Q., Ma, Q., et al. (2020a). Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol. Psychiatry 25, 2905–2918. doi: 10.1038/s41380-019-0475-4
Zhu, F., Ju, Y., Wang, W., Wang, Q., Guo, R., Ma, Q., et al. (2020b). Metagenome-wide association of gut microbiome features for schizophrenia. Nat. Commun. 11:1612. doi: 10.1038/s41467-020-15457-9
Zhu, C., Zheng, M., Ali, U., Xia, Q., Wang, Z., Chenlong,, et al. (2021). Association between abundance of Haemophilus in the gut microbiota and negative symptoms of schizophrenia. Front. Psych. 12:685910. doi: 10.3389/fpsyt.2021.685910
Zou, H., Gao, H., Liu, Y., Zhang, Z., Zhao, J., Wang, W., et al. (2024). Dietary inulin alleviated constipation induced depression and anxiety-like behaviors: involvement of gut microbiota and microbial metabolite short-chain fatty acid. Int. J. Biol. Macromol. 259:129420. doi: 10.1016/j.ijbiomac.2024.129420
Keywords: Poly I:C, schizophrenia, maternal immune activation, gut microbiota, schizophrenia-like behaviors, tryptophan pathway
Citation: Xu Z, Hu C and Luo Y (2025) Poly I:C-induced maternal immune activation causes schizophrenia-like behaviors in the offspring of both sexes by regulating gut microbiota and tryptophan metabolism pathway. Front. Microbiol. 16:1667164. doi: 10.3389/fmicb.2025.1667164
Edited by:
Laura Mitrea, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaReviewed by:
German Gornalusse, University of Washington, United StatesAleksandra Góralczyk-Bińkowska, Medical University of Lodz, Poland
Copyright © 2025 Xu, Hu and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yayan Luo, eWF5YW5sdW9AMTYzLmNvbQ==
 Zhilong Xu1,2
Zhilong Xu1,2 Yayan Luo
Yayan Luo