- 1School of Basic Medical Sciences, Henan University, Kaifeng, China
- 2Faculty of Pharmacy, Department of Microbiology and Immunology, Zagazig University, Zagazig, Egypt
- 3Henan Provincial Research Center of Engineering Technology for Nuclear Protein Medical Detection, Zhengzhou Health College, Zhengzhou, Henan, China
- 4Henan International Joint Laboratory for Nuclear Protein Regulation, Department of Nuclear Medicine, The First Affiliated Hospital, Henan University College of Medicine, Kaifeng, Henan, China
Phage therapy has emerged as a promising alternative to conventional antibiotics for combating intestinal bacterial infections, especially in the era of rising antimicrobial resistance. Despite its therapeutic potential, the clinical translation of phage therapy remains hindered by limited large-scale trial data and incomplete mechanistic understanding. This review systematically evaluates the efficacy of phage therapy in animal models of intestinal diseases, encompassing bacterial infection-induced diarrhea (e.g., cholera, typhoid fever), bacterial enteritis, and sepsis. By synthesizing evidence from bacterial colonization assays, histopathological analyses, and disease severity assessments, we highlight features such as phage-mediated pathogen clearance, changes in inflammatory factors, and intestinal pathology. Furthermore, challenges including phage selection difficulties, host specificity issues, and safety considerations are discussed, along with future research directions aimed at bridging the gap between experimental models and clinical applications.
1 Introduction
Despite the early discovery of bacteriophages' antimicrobial properties a century ago, phage therapy remained largely marginalized during the antibiotic era, overshadowed by the remarkable efficacy and convenience of conventional antibiotics (D'herelle, 1931). However, the escalating crisis of antibiotic misuse has reignited interest in phage-based interventions. Two major challenges underscore this urgency: (1) the accelerated emergence of multidrug-resistant (MDR) pathogens, which severely limits treatment options, and (2) the collateral damage to commensal microbiota, where antibiotic-induced dysbiosis compromises colonization resistance and exacerbates opportunistic infections (Ranjbar et al., 2022). Recent decades have witnessed transformative progress in phage research, with clinical evidence now robustly supporting its therapeutic potential (Wang et al., 2020; Zheng et al., 2023). In a 2008–2022 retrospective cohort of 100 consecutive refractory infections, personalized bacteriophage therapy achieved clinical improvement in 77.2 % (88/114) of episodes and eradicated the targeted pathogen in 61.3 % (65/106) (Pirnay et al., 2024). These findings not only validate historical observations but also offer a framework for integrating phage biology into modern antimicrobial stewardship.
The human gut microbiota constitutes an extraordinarily complex ecosystem, harboring a diverse array of symbiotic bacteria, viruses, and yet-to-be-identified microorganisms (Allaband et al., 2019). Metagenomic sequencing has revealed over 140,000 intestinal phage genomes and more than 1,000 pathogenic bacterial genomes in the human gut (Sunagawa et al., 2013; Camarillo-Guerrero et al., 2021). Concurrently, extensive research has established a link between gut microbiota dysbiosis and human diseases, with phages emerging as key modulators in these interactions (Khan Mirzaei et al., 2020; Hannigan et al., 2018; Nakatsu et al., 2018). While the exact mechanisms underlying phage-mediated disease modulation remain incompletely elucidated, accumulating evidence from in vitro studies, animal models, and clinical observations supports the therapeutic potential of phage therapy (Ott et al., 2017; Merabishvili et al., 2012).
Herein, we review common intestinal disease models and their corresponding phage therapy experiments, categorizing and discussing relevant studies to provide a theoretical basis for the application of phage therapy in treating human intestinal diseases (Figure 1).
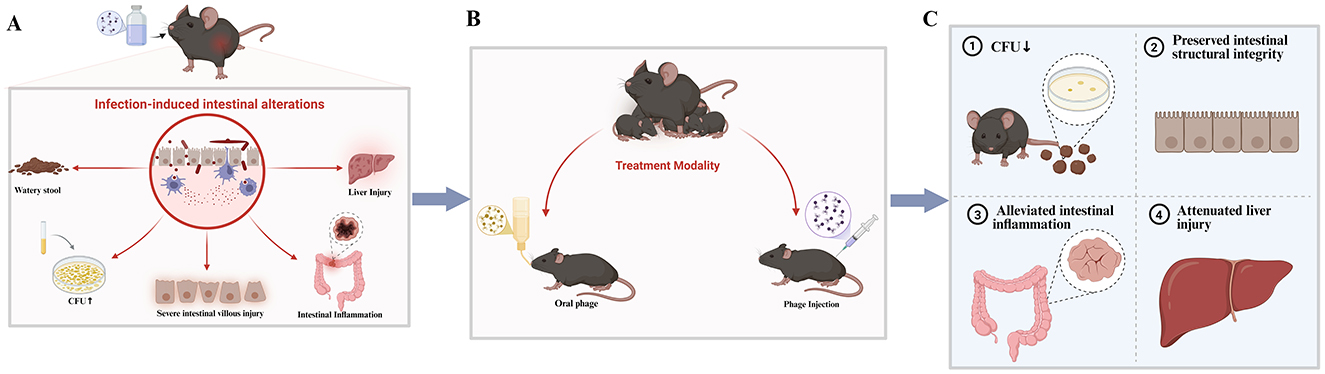
Figure 1. (A) Intestinal pathological changes and symptoms induced by pathogenic bacterial infection; (B) representative phage treatment regimens in animal models; (C) improvement of intestinal pathology and symptoms in mice following phage intervention. Created using Biorender, licensed under Academic License.
2 Phage-targeted therapy for diarrheal diseases
Diarrhea remains a leading cause of gastrointestinal morbidity worldwide, with bacterial etiologies representing a major public health concern (GBD 2021 Diarrhoeal Diseases Collaborators, 2025). Clinically, bacterial diarrhea manifests as acute-onset, high-frequency watery stools, often accompanied by fluid-electrolyte imbalances and compromised intestinal function (Shankar and Rosenbaum, 2020). Notable bacterial pathogens responsible include Shigella species, enteropathogenic Escherichia coli, Listeria monocytogenes and toxigenic Clostridioides difficile (Colston et al., 2025; Hensen et al., 2025).
2.1 Phage therapy for Shigella-associated diarrhea
Diarrheal disease caused by Shigella spp. manifests as a severe gastrointestinal infection, characterized by bloody or mucopurulent stools and high mortality in severe cases. Globally, Shigella is estimated to cause approximately 188 million infections and 164,000 deaths annually (Kotloff et al., 2018). In resource-limited settings, it represents the leading etiology of invasive (bloody) diarrhea among children under 5 years of age, with an incidence rate of 0.34 episodes per child-year reported in communities within the Peruvian Amazon (Kosek et al., 2008; Liu et al., 2016). Notably, the 2016 Shigella outbreak in Michigan, which resulted in 177 cases with an 18% hospitalization rate over 8 months across two counties, was the region's most severe in 30 years (Mcclung et al., 2020).
A murine model study investigating S. sonnei infection comprehensively validated the prophylactic and therapeutic potential of phage therapy (Mai et al., 2015). In this experiment, mice were orally challenged with 108 colony-forming units (CFU) of S. sonnei and administered phages (109 plaque-forming units, PFU) either pre-or post-infection. Notably, all phage treatment regimens drastically suppressed S. sonnei colonization in the gastrointestinal tract, including the feces, cecum, and ileum. Within 72 h, phage-treated mice (both pre- and post-infection) demonstrated complete bacterial clearance, whereas persistent colonization was maintained in untreated controls. Strikingly, phage therapy outperformed ampicillin, achieving rapid pathogen clearance within 24–48 h-a critical advantage in acute infections. Over the 28-day monitoring period, phage-treated mice maintained physiological stability, as evidenced by steady body weight, normal leukocyte counts, and absence of diarrheal symptoms. Furthermore, histopathological analysis confirmed no adverse effects in vital organs (heart, brain, liver, kidneys), underscoring the safety profile of this approach (Table 1).
2.2 Phage therapy for E. coli-associated diarrhea
Although E. coli typically colonizes the healthy human gut as a commensal microorganism (Kaper et al., 2004), pathogenic strains continue to contribute significantly to diarrheal morbidity and mortality, particularly in resource-limited regions such as Asia, Africa, and Latin America (Chua et al., 2021). The global burden of E. coli-associated enteric infections remains substantial, with recent analyses highlighting its continued contribution to diarrheal disease in low- and middle-income settings (2025).
Jamalludeen et al. demonstrated that prophylactic phage administration effectively mitigated E. coli-induced diarrhea in pigs, even at low fecal phage concentrations (< 103 PFU/g) (Jamalludeen et al., 2009). A three-phage cocktail exhibited pronounced efficacy, reinforcing phage therapy's utility in both preventive and therapeutic settings. Similarly, Vahedi et al. isolated a sewage-derived phage targeting enteropathogenic E. coli (EPEC) and confirmed its therapeutic potential in mice (Vahedi et al., 2018). Interestingly, phage monotherapy—whether administered preventively or curatively—outperformed both antibiotic treatment and combination therapies, resulting in complete pathogen clearance without compromising normal weight gain.
Clinical studies also have demonstrated the potential of phage therapy for E. coli-related diarrheal diseases. Bruttin et al. conducted a pioneering human trial involving 15 healthy male volunteers who received an oral dose of E. coli phage T4 (103 PFU/mL) (Bruttin and Brüssow, 2005). While fecal phage was detectable in 50% of participants at 24 h post-administration, complete clearance occurred within 9 days with no reported adverse effects, confirming the safety profile. Notably, in the German outbreak of E. coli O104:H4 infections, environmental phage isolates showed effective lytic activity against the outbreak strain, contributing to successful epidemic control (Merabishvili et al., 2012).
2.3 Phage-based treatment of L. monocytogenes-induced diarrhea
L. monocytogenes, a facultative anaerobic pathogen, is a prominent foodborne bacterium prominent in various food products, including meat, vegetables, fruits, and dairy (Gana et al., 2024). The rise of antibiotic-resistant L. monocytogenes strains has significantly complicated clinical management, with treatment failures potentially leading to life-threatening outcomes (Skrobas et al., 2024).
In a murine listeriosis model, mice were orally challenged with 105 CFU/mL of L. monocytogenes, followed by a 3-day course of six-phage cocktail therapy (105 PFU/day) initiated 72 h post-infection (Mai et al., 2010). Phage therapy achieved a significant reduction in L. monocytogenes load compared to controls, with about an 80 CFU/g decrease in fecal bacterial counts. Treated mice maintained stable body weight, while control mice experienced approximately 10% weight loss due to diarrhea. Notably, phage-treated animals showed none of the adverse effects observed in antibiotic-treated controls, including increased watery stool and cecal distension. The detection of approximately 102 CFU/g phage particles in cecal contents confirmed therapeutic phage amplification, demonstrating the efficacy of phage therapy in this diarrheal model.
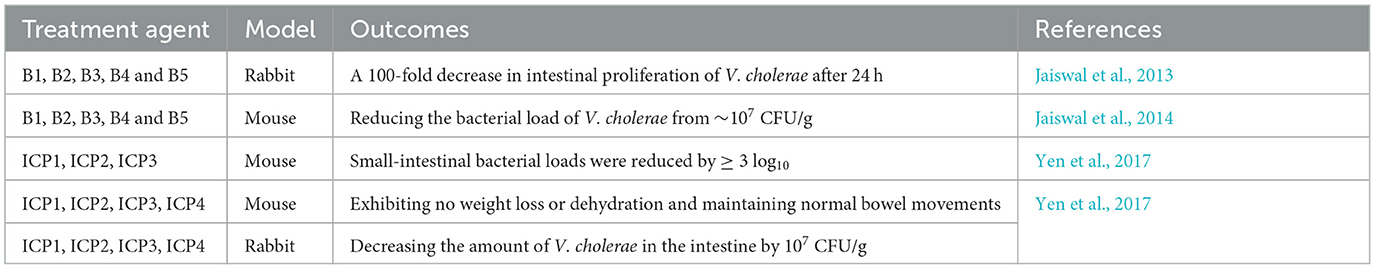
2.4 Phage-based treatment of cholera
Cholera is an acute intestinal infectious disease caused by Vibrio cholerae, with its pathogenicity relying on the synergistic action of the toxin-co-regulated pilus (TCP) and cholera toxin (CT) (Yoon and Waters, 2019). This disease holds landmark significance in the early development of phage therapy. In the early 20th century, microbiologist Félix d'Herelle isolated specific phages by studying fecal samples from recovered cholera patients, providing crucial evidence for phage-mediated pathogen clearance (Sabino et al., 2020). Subsequent clinical trials demonstrated that oral phage therapy could dramatically reduce the mortality rate in early-stage cholera patients from 63 to 8%, showcasing its therapeutic potential (Table 2) (Summers, 1993).
In a recent study by Bhandare et al. researchers utilized a rabbit model of V. cholerae infection to evaluate the efficacy of oral phage therapy administered both prophylactically (pre-infection) and therapeutically (6 h post-infection) at a dose of 109 PFU. Upon bacterial challenge, all experimental animals developed characteristic cholera-like symptoms, including significant cecal fluid accumulation and progressive diarrhea manifested through soft to watery stool consistency. Remarkably, phage-treated rabbits demonstrated complete restoration of normal fecal consistency following therapeutic intervention. Additionally, these treated animals maintained stable body temperatures and showed no observable behavioral abnormalities throughout the study period. Most notably, quantitative analysis revealed a substantial 100-fold reduction in intestinal V. cholerae colonization within 24 h of phage administration, accompanied by robust phage replication reaching titers of 107 PFU/g in intestinal tissues (Bhandare et al., 2019).
Another complementary study, Jaiswal et al. demonstrated the therapeutic potential of a five-phage cocktail against V. cholerae infection in a rabbit model. When administered at 1 × 108 PFU 6 h post-infection, the phage treatment produced remarkable protective effects. Treated animals maintained normal hydration status and stable body temperatures, in contrast to control rabbits that developed severe clinical manifestations. Quantitative analysis revealed a dramatic 85% reduction in cecal fluid accumulation (from 0.39 to 0.06 mL) and near-complete suppression of V. cholerae proliferation. Of note, prophylactic administration of the phage cocktail (109 PFU) 6 h prior to bacterial challenge provided complete protection against cholera symptom development in juvenile rabbits (Jaiswal et al., 2013).
Furthermore, a five-phage cocktail demonstrated potent antibacterial efficacy in a V. cholerae mouse infection model. Phage therapy significantly suppressed bacterial proliferation, reducing intestinal V. cholerae loads from 7.1 × 106 CFU/g to 9.1 × 103 CFU/g on days 1 and 4 post-treatment. Concurrently, serum TNF-α levels decreased by 150 pg/mL after 4 days of therapy. Histopathological analysis revealed preserved intestinal architecture with minimal villous damage and no significant neutrophil infiltration in phage-treated mice, unlike the control group, which exhibited marked inflammatory and structural changes (Jaiswal et al., 2014). Prophylactic phage administration in both mouse and rabbit models substantially reduced intestinal V. cholerae colonization within 24 h post-infection (Yen et al., 2017). Control animals developed severe cholera-like symptoms, including cecal dilation, dehydration, and 10–12% weight loss, whereas phage-treated subjects maintained stable body weight, normal hydration, and regular bowel function, demonstrating the protective efficacy of phage therapy.
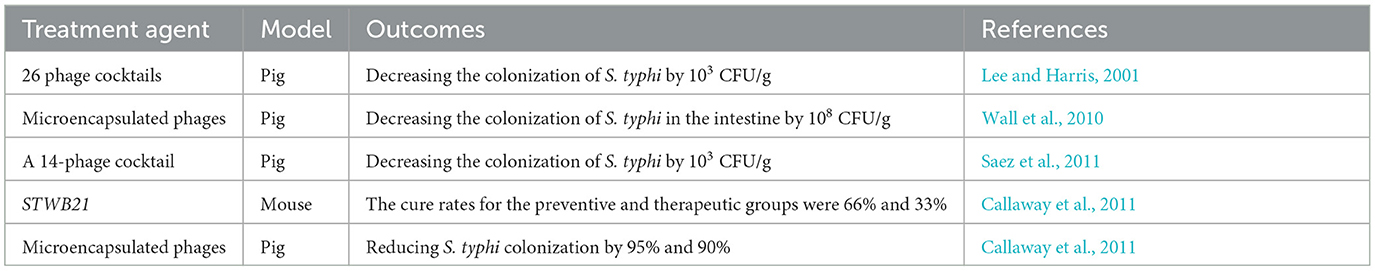
3 Targeted phage therapy against typhoid fever
Typhoid fever, caused by the invasive pathogen Salmonella enterica serovar Typhi, continues to pose a substantial global health challenge in endemic areas. Following intestinal colonization, S. typhi exhibits unique pathogenic capabilities to translocate across the intestinal epithelium, resulting in bloodstream invasion and systemic inflammatory responses. This predominantly waterborne/foodborne transmission cycle (Meiring et al., 2023) was first therapeutically targeted by Smith J. a century ago using intravenous phage administration (Smith, 1924), establishing an important proof-of-concept. Subsequent clinical advances by Knouf et al., demonstrated remarkable efficacy, reducing mortality rates from 14 to 5% in critically ill, comatose typhoid patients, highlighting phage therapy's potential against invasive salmonellosis (Table 3).
Mondal et al., recently identified STWB21, an environmentally stable lytic phage isolated from lake water, which demonstrated treatment-timing-dependent efficacy in a S. typhi-infected murine model. Prophylactic administration demonstrated superior efficacy with a 66% cure rate compared to therapeutic intervention's 33% success rate, likely attributable to STWB21's capacity to prevent initial S. typhi intestinal colonization (Mondal et al., 2022). Research indicated that prophylactic STWB21 administration was more effective than therapeutic application, likely attributable to its ability to suppress S. typhi intestinal colonization. Histopathological analysis demonstrated significantly lower S. typhi burdens in the liver and spleen of STWB21-treated mice compared to controls. Electron microscopy further revealed severe hepatic abscess formation and venous inflammation in control animals, whereas STWB21-treated mice maintained normal tissue architecture with only a moderate increase in mitochondrial and lysosomal activity (Mondal et al., 2023).
Three independent studies using S. typhi-infected porcine models demonstrated rapid and significant reductions in bacterial loads following phage therapy (Callaway et al., 2011; Lee and Harris, 2001; Saez et al., 2011). Saez et al., reported complete bacterial clearance within 6 h of oral administration of microencapsulated phages, with pathogenic bacteria detectable only in control animals upon analysis of cecal and colonic contents (Saez et al., 2011). Wall et al. observed 95 and 90% reductions in S. typhi colonization in the cecum and ileum, respectively, using similar microencapsulated phage formulations (Wall et al., 2010). In a parallel study by Callaway et al., pigs treated with a phage cocktail (3 × 109 PFU) at 24- and 48-h post-infection exhibited fecal S. typhi levels tenfold lower than controls by 48 h (Callaway et al., 2011).
4 Phage-based therapeutics for intestinal inflammation
Clinically significant enteropathogens; including E. coli, C. difficile, and Y. enterocolitica, employ distinct virulence mechanisms to establish persistent intestinal inflammation (Shuwen and Kefeng, 2022). In this context, phage therapy emerges as a transformative therapeutic strategy, offering targeted elimination of pathogenic bacteria while maintaining commensal microbiota homeostasis, thereby overcoming the limitations of conventional antibiotics that often aggravate microbial dysbiosis and providing a precision approach to interrupt this self-perpetuating pathogenic cycle (Arthur et al., 2012).
Y. enterocolitica, a zoonotic enteropathogen, induces intestinal inflammation upon host colonization (Leon-Velarde et al., 2019). Xue et al., developed an intestinal-targeting lytic phage and evaluated its efficacy in a murine model (Xue et al., 2020). Following oral challenge with 2 × 108 CFU Y. enterocolitica, administration of a single phage dose (109 PFU/mL) at 6 h post-infection achieved complete bacterial clearance in 33% of mice, a 4-log reduction in colonic/cecal bacterial loads (from 107 to 103 CFU/g), and sustained suppression for 144 h. Phage-treated mice also showed significantly lower pro-inflammatory cytokine levels, demonstrating therapeutic potential against Yersinia-induced enteritis.
C. difficile has been widely recognized as a major pathogen driving intestinal inflammation (Dong et al., 2023), exerting its pathogenic effects through gut microbiota disruption and modulation of host immune responses. In a recent study, Shan et al. demonstrated the therapeutic potential of phage therapy using an in vitro colon cell model infected with C. difficile, where single-phage treatment successfully eliminated adherent bacteria without causing collateral damage to host cells (Shan et al., 2018), highlighting its safety profile for potential clinical applications. In vivo studies have yielded promising results. Ramesh et al. administered phage therapy (108 PFU/mL) by oral gavage in a hamster model of colitis. While all control animals succumbed within 72 h with severe cecal pathology (bleeding and swelling), the phage-treated group showed significantly improved survival, with 2 × 104 PFU of phages recovered from the cecum (Ramesh et al., 1999). Selle et al. employed an innovative approach by engineering a phage using the Type I CRISPR-Cas system to target C. difficile-induced intestinal inflammation. In their mouse model, a single dose of 109 PFU of the engineered phage administered on day 4 post-infection (initiated with 105 CFU of C. difficile) reduced tissue damage scores by 4 points compared to controls. Notably, high phage titers (108 PFU/g) persisted in feces 4 days post-treatment, accompanied by significant improvements in cecal inflammation and bacterial clearance (Selle et al., 2020).
5 Bacteriophage strategies to combat sepsis-associated infections
Sepsis, a life-threatening syndrome characterized by organ dysfunction, represents a critical global health challenge with persistently high mortality rates (Rudd et al., 2020). Of particular clinical relevance is gut-origin sepsis (Assimakopoulos et al., 2018), a distinct subtype that arises when intestinal pathogens compromise the mucosal barrier integrity, leading to both structural damage and functional impairment of the intestinal epithelium (Amornphimoltham et al., 2019). This reach initiates a pathogenic cascade involving bacterial translocation, which subsequently evokes a robust systemic inflammatory response that may culminate in intestinal failure and progressive multiple organ dysfunction syndrome (MODS) (Haussner et al., 2019). Mounting experimental and clinical evidence now underscores the pivotal contribution of gut microbiota dysbiosis to the pathogenesis of gut-derived sepsis, thereby establishing a compelling therapeutic rationale for investigating phage-based interventions as a precision antimicrobial strategy (Magnan et al., 2023).
Intestinal colonization by Pseudomonas aeruginosa can rapidly progress to life-threatening sepsis with high mortality rates (Tabarani and Baker, 2022). It is demonstrated that a single oral dose of lysogenic phage (1010 PFU/mL) significantly improved survival outcomes. Phage therapy increased survival rates by 66.7% compared to saline-treated controls while simultaneously reducing P. aeruginosa burden in the liver and spleen by 1 log CFU/g. Notably, phage-treated animals showed a 4–5-fold reduction in proinflammatory cytokine levels (Watanabe et al., 2007). In a complementary study, Prokopczuk et al., developed an engineered Pf phage that achieved >4-log CFU/g reduction in bacterial load in murine infection models. Most strikingly, while untreated controls exhibited near-complete (100%) mortality, all Pf-treated animals survived the entire observation period. Histopathological evaluation further confirmed the phage's ability to prevent bacterial dissemination to secondary organs (liver and spleen) (Prokopczuk et al., 2023).
Enterococcus faecium has evolved from a commensal organism to a leading nosocomial pathogen, with surveillance data demonstrating a striking increase in its association with life-threatening infections since the 1980s (García-Solache and Rice, 2019). This epidemiological shift is particularly concerning in cases of E. faecium-induced sepsis, where therapeutic options are severely constrained by both intrinsic high mortality rates and the expanding global prevalence of vancomycin-resistant (VREfm) and multidrug-resistant strains (Cattaneo et al., 2021; Torres et al., 2018). Stellfox et al., report the successful use of integrative phage therapy to treat recurrent E. faecium bacteremia from persistent gut colonization in an immunocompromised patient. The therapeutic protocol combined conventional antibiotics (vancomycin-daptomycin) with adjunctive phage therapy administered at 1 × 109 PFU via optimized dual-route delivery (oral and intravenous) (Stellfox et al., 2024). This therapeutic strategy achieved two essential clinical outcomes; complete sepsis resolution with blood culture sterilization within 27 days alongside durable prevention of recurrent bacteremia through sustained phage maintenance therapy.
6 Bridging the gap in gut phage therapy
Antibiotic misuse has fueled the rise of multidrug-resistant bacteria, posing a grave threat to public health worldwide. Equally concerning is the collateral damage from broad-spectrum antibiotics, which devastate the gut microbiota. This destruction triggers a chain of events: the ecological balance is disrupted, the gut barrier is compromised, and susceptibility to opportunistic infections rises (Singha et al., 2023). Unlike antibiotic treatment, which frequently causes gut microbiota dysbiosis in infected animals, phage therapy confers the added benefit of maintaining a relatively normal microbial community structure, a protective effect demonstrated in a C. difficile colitis model by improved survival, reduced intestinal damage, and minimal impact on the resident gut microbiota (Gundersen et al., 2023; Raeisi et al., 2023). Gut microbiota dysbiosis is a key driver of intestinal inflammation, and by precisely eliminating inflammatory pathogens such as Yersinia, Klebsiella pneumoniae, and C. difficile, bacteriophages reduce local and systemic pro-inflammatory cytokine (e.g., TNF-α) levels, thereby alleviating inflammation and promoting tissue repair (Wang et al., 2022; Federici et al., 2022). This causal strategy ultimately breaks the vicious cycle of pathogen-driven inflammation at its root.
Phage therapy has demonstrated potential for treating intestinal infections in animal models, yet its clinical translation faces four major challenges. First, as living biological entities, phages exhibit complex pharmacokinetics with significant inter-individual variability. Oral administration is susceptible to gastrointestinal environmental factors, and efficacy heavily depends on their replication capability at the infection site, making standardized dosing difficult (Benyamini, 2024). Second, the host immune system can generate neutralizing antibodies, particularly during systemic or repeated administration, which substantially compromises subsequent treatment efficacy (Van Nieuwenhuyse et al., 2022). Third, the production of “phage cocktails” faces challenges in achieving batch-to-batch consistency, and the absence of globally unified quality control standards hinders regulatory approval and large-scale application. Fourth, existing animal models have inherent limitations and cannot fully replicate the complex environment of human intestinal infections, thereby limiting the predictive value of preclinical data (Browne et al., 2016).
Addressing the clinical translation challenges of phage therapy necessitates an integrated multi-faceted approach. Key priorities include developing intelligent delivery systems, such as pH-responsive microcapsules, to safeguard phages during gastrointestinal transit and enable site-specific release, thereby augmenting their colonization and replication efficacy at infection sites (Vinner et al., 2019). Concurrently, phage engineering through gene editing and surface modifications like PEGylation can mitigate neutralization by host antibodies and extend systemic circulation (Gordillo Altamirano and Barr, 2019). Manufacturing standardization requires implementing Quality by Design principles and synthetic biology techniques to ensure consistent production of phage cocktails with reproducible therapeutic outcomes (Malik, 2021). Furthermore, establishing human-relevant models—including humanized intestinal organoids integrated with multi-omics platforms—will strengthen the predictive validity of translational studies. Collectively, these strategies will accelerate the transition of phage therapy from experimental research to clinical implementation (Shield et al., 2021).
7 Conclusion
This review systematically examines phage therapy against intestinal infections in animal models, including diseases such as diarrhea, enteritis, and systemic sepsis caused by pathogens like Shigella, pathogenic E. coli, L. monocytogenes, V. cholerae, S. typhi and C. difficile. Given the global rise of antibiotic resistance, developing such alternative therapies is critically important.
The results consistently demonstrate that phage therapy effectively clears targeted pathogens, alleviates intestinal inflammation, and preserves barrier integrity. It outperformed traditional antibiotics across multiple models without inducing the microbiota dysbiosis frequently associated with them. A key insight is that prophylactic administration generally affords stronger protection than therapeutic intervention. Furthermore, combining rationally engineered phages with polyvalent cocktail formulations produces synergistic effects, significantly boosting the therapeutic potential.
While persistent challenges—such as narrow host range, evolving bacterial resistance, and the complex gut environment—remain, advances in optimized delivery systems, precision phage engineering, and multi-omics integration are progressively addressing these limitations. These developments establish phage therapy as a key strategy against multidrug-resistant intestinal infections and provide a solid foundation for clinical translation. Future efforts should focus on developing novel engineering approaches to broaden host range and counter resistance, elucidating the multifaceted interactions between phages, host immunity, and gut microbiota, and optimizing clinical dosing regimens and formulations to facilitate well-controlled human trials.
Author contributions
XW: Writing – original draft, Writing – review & editing. JL: Writing – original draft. ZG: Writing – original draft. JF: Writing – original draft. DM: Writing – review & editing. HC: Writing – review & editing. JS: Writing – review & editing. YW: Supervision, Writing – review & editing. ZL: Investigation, Writing – review & editing. SG: Supervision, Writing – review & editing. XL: Conceptualization, Writing – review & editing. XJ: Conceptualization, Writing – review & editing. TT: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Key R&D and Promotion Projects of Henan Province (252102310322, 242102311172 and 242102310023); Henan University Laboratory Undergraduate Research Program (20243305115); Key Scientific Research Project of Higher Education of Henan Province (23A310009); the Key Scientific Research project of Henan Province, China (242102311182); National Natural Science Foundation of China (82401779) and an Open Project for College Students of Henan University (20243305198); the Program for Science and Technology Development in Kaifeng City (2053168); the Natural Science Foundation of Henan Province (242300420133).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
MDR, multidrug-resistant; CFU, colony-forming units; PFU, plaque-forming units; AMR, antimicrobial resistance; CDI, C. difficile infections; FFT, fecal filtrate transfer; EPEC, enteropathogenic E. coli; TCP, toxin-co-regulated pilus; MODS, multiple organ dysfunction syndrome; VREfm, vancomycin-resistant Enterococcus faecium.
References
Allaband, C., Mcdonald, D., Vázquez-Baeza, Y., Minich, J. J., Tripathi, A., Brenner, D. A., et al. (2019). Microbiome 101: studying, analyzing, and interpreting gut microbiome data for clinicians. Clin. Gastroenterol. Hepatol. 17, 218–230. doi: 10.1016/j.cgh.2018.09.017
Amornphimoltham, P., Yuen, P. S. T., Star, R. A., and Leelahavanichkul, A. (2019). Gut leakage of fungal-derived inflammatory mediators: part of a gut-liver-kidney axis in bacterial sepsis. Dig. Dis. Sci. 64, 2416–2428. doi: 10.1007/s10620-019-05581-y
Arthur, J. C., Perez-Chanona, E., Mühlbauer, M., Tomkovich, S., Uronis, J. M., Fan, T. J., et al. (2012). Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338, 120–123. doi: 10.1126/science.1224820
Assimakopoulos, S. F., Triantos, C., Thomopoulos, K., Fligou, F., Maroulis, I., Marangos, M., et al. (2018). Gut-origin sepsis in the critically ill patient: pathophysiology and treatment. Infection 46, 751–760. doi: 10.1007/s15010-018-1178-5
Benyamini, P. (2024). Beyond antibiotics: what the future holds. Antibiotics 13:919. doi: 10.3390/antibiotics13100919
Bhandare, S., Colom, J., Baig, A., Ritchie, J. M., Bukhari, H., Shah, M. A., et al. (2019). Reviving phage therapy for the treatment of Cholera. J. Infect. Dis. 219, 786–794. doi: 10.1093/infdis/jiy563
Browne, H. P., Forster, S. C., Anonye, B. O., Kumar, N., Neville, B. A., Stares, M. D., et al. (2016). Culturing of ‘unculturable' human microbiota reveals novel taxa and extensive sporulation. Nature 533, 543–546. doi: 10.1038/nature17645
Bruttin, A., and Brüssow, H. (2005). Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrob. Agents Chemother. 49, 2874–2878. doi: 10.1128/AAC.49.7.2874-2878.2005
Callaway, T. R., Edrington, T. S., Brabban, A., Kutter, B., Karriker, L., Stahl, C., et al. (2011). Evaluation of phage treatment as a strategy to reduce Salmonella populations in growing swine. Foodborne Pathog. Dis. 8, 261–266. doi: 10.1089/fpd.2010.0671
Camarillo-Guerrero, L. F., Almeida, A., Rangel-Pineros, G., Finn, R. D., and Lawley, T. D. (2021). Massive expansion of human gut bacteriophage diversity. Cell 184, 1098–1109.e9. doi: 10.1016/j.cell.2021.01.029
Cattaneo, C., Rieg, S., Schwarzer, G., Müller, M. C., Blümel, B., Kern, W. V., et al. (2021). Enterococcus faecalis bloodstream infection: does infectious disease specialist consultation make a difference? Infection 49, 1289–1297. doi: 10.1007/s15010-021-01717-3
Chua, P. L. C., Huber, V., Ng, C. F. S., Seposo, X. T., Madaniyazi, L., Hales, S., et al. (2021). Global projections of temperature-attributable mortality due to enteric infections: a modelling study. Lancet Planet Health 5, e436–e445. doi: 10.1016/S2542-5196(21)00152-2
Colston, J. M., Flynn, T. G., Denton, A. H., Schiaffino, F., Majowicz, S. E., Devleesschauwer, B., et al. (2025). Updating global estimates of pathogen-attributable diarrhoeal disease burden: a methodology and integrated protocol for a broad-scope systematic review of a syndrome with diverse infectious aetiologies. BMJ Open 15:e093018. doi: 10.1136/bmjopen-2024-093018
D'herelle, F. (1931). Bacteriophage as a treatment in acute medical and surgical infections. Bull. N. Y. Acad. Med. 7, 329–348.
Dong, D., Su, T., Chen, W., Wang, D., Xue, Y., Lu, Q., et al. (2023). Clostridioides difficile aggravates dextran sulfate solution (DSS)-induced colitis by shaping the gut microbiota and promoting neutrophil recruitment. Gut Microbes. 15:2192478. doi: 10.1080/19490976.2023.2192478
Federici, S., Kredo-Russo, S., Valdés-Mas, R., Kviatcovsky, D., Weinstock, E., Matiuhin, Y., et al. (2022). Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell 185, 2879–2898.e24. doi: 10.1016/j.cell.2022.07.003
Gana, J., Gcebe, N., Moerane, R., Ngoshe, Y., Tshuma, T., Moabelo, K., et al. (2024). Antimicrobial resistance profiles of listeria species recovered from retail outlets in Gauteng Province, South Africa. J. Food Prot. 87:100322. doi: 10.1016/j.jfp.2024.100322
García-Solache, M., and Rice, L. B. (2019). The Enterococcus: a model of adaptability to its environment. Clin. Microbiol. Rev. 32, e00058–18. doi: 10.1128/CMR.00058-18
GBD 2021 Diarrhoeal Diseases Collaborators (2025). Global, regional, and national age-sex-specific burden of diarrhoeal diseases, their risk factors, and aetiologies, 1990-2021, for 204 countries and territories: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Infect. Dis. 25, 519–536.
Gordillo Altamirano, F. L., and Barr, J. J. (2019). Phage therapy in the postantibiotic era. Clin. Microbiol. Rev. 32, e00066–18. doi: 10.1128/CMR.00066-18
Gundersen, M. S., Fiedler, A. W., Bakke, I., and Vadstein, O. (2023). The impact of phage treatment on bacterial community structure is minor compared to antibiotics. Sci. Rep. 13:21032. doi: 10.1038/s41598-023-48434-5
Hannigan, G. D., Duhaime, M. B., Ruffin, M. T. T., Koumpouras, C. C., and Schloss, P. D. (2018). Diagnostic potential and interactive dynamics of the colorectal cancer virome. mBio 9, e02248–18. doi: 10.1128/mBio.02248-18
Haussner, F., Chakraborty, S., Halbgebauer, R., and Huber-Lang, M. (2019). Challenge to the intestinal mucosa during sepsis. Front. Immunol. 10:891. doi: 10.3389/fimmu.2019.00891
Hensen, A. D. O., Vehreschild, M., Gerding, D. N., Krut, O., Chen, W., Young, V. B., et al. (2025). How to develop a controlled human infection model for Clostridioides difficile. Clin. Microbiol. Infect. 31, 373–379. doi: 10.1016/j.cmi.2024.08.025
Jaiswal, A., Koley, H., Ghosh, A., Palit, A., and Sarkar, B. (2013). Efficacy Of Cocktail phage therapy in treating vibrio cholerae infection in rabbit model. Microbes Infect. 15, 152–156. doi: 10.1016/j.micinf.2012.11.002
Jaiswal, A., Koley, H., Mitra, S., Saha, D. R., and Sarkar, B. (2014). Comparative analysis of different oral approaches to treat Vibrio cholerae infection in adult mice. Int. J. Med. Microbiol. 304, 422–430. doi: 10.1016/j.ijmm.2014.02.007
Jamalludeen, N., Johnson, R. P., Shewen, P. E., and Gyles, C. L. (2009). Evaluation of bacteriophages for prevention and treatment of diarrhea due to experimental enterotoxigenic Escherichia coli O149 infection of pigs. Vet. Microbiol. 136, 135–141. doi: 10.1016/j.vetmic.2008.10.021
Kaper, J. B., Nataro, J. P., and Mobley, H. L. (2004). Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2, 123–140. doi: 10.1038/nrmicro818
Khan Mirzaei, M., Khan, M. A. A., Ghosh, P., Taranu, Z. E., Taguer, M., Ru, J., et al. (2020). Bacteriophages isolated from stunted children can regulate gut bacterial communities in an age-specific manner. Cell Host Microbe 27, 199–212.e5. doi: 10.1016/j.chom.2020.01.004
Kosek, M., Yori, P. P., Pan, W. K., Olortegui, M. P., Gilman, R. H., Perez, J., et al. (2008). Epidemiology of highly endemic multiply antibiotic-resistant shigellosis in children in the Peruvian Amazon. Pediatrics 122, e541–e549. doi: 10.1542/peds.2008-0458
Kotloff, K. L., Riddle, M. S., Platts-Mills, J. A., Pavlinac, P., and Zaidi, A. K. M. (2018). Shigellosis. Lancet 391, 801–812. doi: 10.1016/S0140-6736(17)33296-8
Lee, N., and Harris, D. L. H. (2001). The Effect of Bacteriophage Treatment to Reduce the Rapid Dissemination of Salmonella typhimurium in Pigs. IA, United States: Iowa State University.
Leon-Velarde, C. G., Jun, J. W., and Skurnik, M. (2019). Yersinia phages and food safety. Viruses 11:1105. doi: 10.3390/v11121105
Liu, J., Platts-Mills, J. A., Juma, J., Kabir, F., Nkeze, J., Okoi, C., et al. (2016). Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet 388, 1291–1301. doi: 10.1016/S0140-6736(16)31529-X
Magnan, C., Lancry, T., Salipante, F., Trusson, R., Dunyach-Remy, C., Roger, C., et al. (2023). Role of gut microbiota and bacterial translocation in acute intestinal injury and mortality in patients admitted in ICU for septic shock. Front. Cell Infect. Microbiol. 13:1330900. doi: 10.3389/fcimb.2023.1330900
Mai, V., Ukhanova, M., Reinhard, M. K., Li, M., and Sulakvelidze, A. (2015). Bacteriophage administration significantly reduces Shigella colonization and shedding by Shigella-challenged mice without deleterious side effects and distortions in the gut microbiota. Bacteriophage 5:e1088124. doi: 10.1080/21597081.2015.1088124
Mai, V., Ukhanova, M., Visone, L., Abuladze, T., and Sulakvelidze, A. (2010). Bacteriophage administration reduces the concentration of listeria monocytogenes in the gastrointestinal tract and its translocation to spleen and liver in experimentally infected mice. Int. J. Microbiol. 2010:624234. doi: 10.1155/2010/624234
Malik, D. J. (2021). Bacteriophage encapsulation using spray drying for phage therapy. Curr. Issues Mol. Biol. 40, 303–316. doi: 10.21775/cimb.040.303
Mcclung, R. P., Karwowski, M., Castillo, C., Mcfadden, J., Collier, S., Collins, J., et al. (2020). Shigella sonnei outbreak investigation during a municipal water crisis-genesee and Saginaw Counties, Michigan, 2016. Am. J. Public Health 110, 842–849. doi: 10.2105/AJPH.2020.305577
Meiring, J. E., Khanam, F., Basnyat, B., Charles, R. C., Crump, J. A., Debellut, F., et al. (2023). Typhoid fever. Nat. Rev. Dis Primers 9:71. doi: 10.1038/s41572-023-00480-z
Merabishvili, M., De Vos, D., Verbeken, G., Kropinski, A. M., Vandenheuvel, D., Lavigne, R., et al. (2012). Selection and characterization of a candidate therapeutic bacteriophage that lyses the Escherichia coli O104:H4 strain from the 2011 outbreak in Germany. PLoS One 7:e52709. doi: 10.1371/journal.pone.0052709
Mondal, P., Halder, P., Mallick, B., Bhaumik, S., Koley, H., Dutta, S., et al. (2023). Controlling the bacterial load of Salmonella typhi in an experimental mouse model by a lytic Salmonella phage STWB21: a phage therapy approach. BMC Microbiol. 23:324. doi: 10.1186/s12866-023-03040-3
Mondal, P., Mallick, B., Dutta, M., and Dutta, S. (2022). Isolation, characterization, and application of a novel polyvalent lytic phage STWB21 against typhoidal and nontyphoidal Salmonella spp. Front. Microbiol. 13:980025. doi: 10.3389/fmicb.2022.980025
Nakatsu, G., Zhou, H., Wu, W. K. K., Wong, S. H., Coker, O. O., Dai, Z., et al. (2018). Alterations in enteric virome are associated with colorectal cancer and survival outcomes. Gastroenterology 155, 529–541.e5. doi: 10.1053/j.gastro.2018.04.018
Ott, S. J., Waetzig, G. H., Rehman, A., Moltzau-Anderson, J., Bharti, R., Grasis, J. A., et al. (2017). Efficacy of sterile fecal filtrate transfer for treating patients with clostridium difficile infection. Gastroenterology 152, 799–811.e7. doi: 10.1053/j.gastro.2016.11.010
Pirnay, J. P., Djebara, S., Steurs, G., Griselain, J., Cochez, C., De Soir, S., et al. (2024). Personalized bacteriophage therapy outcomes for 100 consecutive cases: a multicentre, multinational, retrospective observational study. Nat. Microbiol. 9, 1434–1453. doi: 10.1038/s41564-024-01705-x
Prokopczuk, F. I., Im, H., Campos-Gomez, J., Orihuela, C. J., and Martínez, E. (2023). Engineered superinfective Pf phage prevents dissemination of Pseudomonas aeruginosa in a mouse burn model. mBio 14:e0047223. doi: 10.1128/mbio.00472-23
Raeisi, H., Noori, M., Azimirad, M., Mohebbi, S. R., Asadzadeh Aghdaei, H., Yadegar, A., et al. (2023). Emerging applications of phage therapy and fecal virome transplantation for treatment of Clostridioides difficile infection: challenges and perspectives. Gut Pathog. 15:21. doi: 10.1186/s13099-023-00550-3
Ramesh, V., Fralick, J. A., and Rolfe, R. D. (1999). Prevention of Clostridium difficile -induced ileocecitis with bacteriophage. Anaerobe 5, 69–78. doi: 10.1006/anae.1999.0192
Ranjbar, R., Alam, M., and Antimicrobial Resistance Collaborators (2022). Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Evid. Based Nurs. doi: 10.1136/ebnurs-2022-103540
Rudd, K. E., Johnson, S. C., Agesa, K. M., Shackelford, K. A., Tsoi, D., Kievlan, D. R., et al. (2020). Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of disease study. Lancet 395, 200–211. doi: 10.1016/S0140-6736(19)32989-7
Sabino, J., Hirten, R. P., and Colombel, J. F. (2020). Review article: bacteriophages in gastroenterology-from biology to clinical applications. Aliment Pharmacol. Ther. 51, 53–63. doi: 10.1111/apt.15557
Saez, A. C., Zhang, J., Rostagno, M. H., and Ebner, P. D. (2011). Direct feeding of microencapsulated bacteriophages to reduce Salmonella colonization in pigs. Foodborne Pathog. Dis. 8, 1269–1274. doi: 10.1089/fpd.2011.0905
Selle, K., Fletcher, J. R., Tuson, H., Schmitt, D. S., Mcmillan, L., Vridhambal, G. S., et al. (2020). In vivo targeting of clostridioides difficile using phage-delivered CRISPR-Cas3 antimicrobials. mBio 11, e00019–20. doi: 10.1128/mBio.00019-20
Shan, J., Ramachandran, A., Thanki, A. M., Vukusic, F. B. I., Barylski, J., Clokie, M. R. J., et al. (2018). Bacteriophages are more virulent to bacteria with human cells than they are in bacterial culture; insights from HT-29 cells. Sci. Rep. 8:5091. doi: 10.1038/s41598-018-23418-y
Shankar, S., and Rosenbaum, J. (2020). Chronic diarrhoea in children: a practical algorithm-based approach. J. Paediatr. Child Health 56, 1029–1038. doi: 10.1111/jpc.14986
Shield, C. G., Swift, B. M. C., Mchugh, T. D., Dedrick, R. M., Hatfull, G. F., Satta, G., et al. (2021). Application of bacteriophages for mycobacterial infections, from diagnosis to treatment. Microorganisms 9:2366. doi: 10.3390/microorganisms9112366
Shuwen, H., and Kefeng, D. (2022). Intestinal phages interact with bacteria and are involved in human diseases. Gut Microbes. 14:2113717. doi: 10.1080/19490976.2022.2113717
Singha, B., Rawat, B. S., Venkataraman, R., Nair, T., Rosenn, E. H., Soni, V., et al. (2023). Gut microbiome associated dysbiosis: limited regimens and expanding horizons of phage therapy. Aspects Mol. Med. 2:100029. doi: 10.1016/j.amolm.2023.100029
Skrobas, U., Zie, W. S., Bielewicz, J., and Rejdak, K. (2024). The rapidly progressing and fatal outcome of rhombencephalitis by listeriosis in a 61-year-old male. Ann. Agric. Environ. Med. 31, 311–314. doi: 10.26444/aaem/178178
Smith, J. (1924). The bacteriophage in the treatment of typhoid fever. Br. Med. J. 2, 47–49. doi: 10.1136/bmj.2.3315.47
Stellfox, M. E., Fernandes, C., Shields, R. K., Haidar, G., Hughes Kramer, K., Dembinski, E., et al. (2024). Bacteriophage and antibiotic combination therapy for recurrent Enterococcus faecium bacteremia. mBio 15:e0339623. doi: 10.1128/mbio.03396-23
Summers, W. C. (1993). Cholera and plague in India: the bacteriophage inquiry of 1927-1936. J. Hist. Med. Allied Sci. 48, 275–301. doi: 10.1093/jhmas/48.3.275
Sunagawa, S., Mende, D. R., Zeller, G., Izquierdo-Carrasco, F., Berger, S. A., Kultima, J. R., et al. (2013). Metagenomic species profiling using universal phylogenetic marker genes. Nat. Methods 10, 1196–1199. doi: 10.1038/nmeth.2693
Tabarani, C., and Baker, C. J. (2022). Pseudomonas aeruginosa early-onset neonatal sepsis: could maternal healthcare occupation be a risk factor? Pediatr. Infect. Dis. J. 41, 854–856. doi: 10.1097/INF.0000000000003636
Torres, C., Alonso, C. A., Ruiz-Ripa, L., León-Sampedro, R., Del Campo, R., and Coque, T. M. (2018). Antimicrobial resistance in Enterococcus spp. of animal origin. Microbiol. Spectr. 6. doi: 10.1128/microbiolspec.ARBA-0032-2018
Vahedi, A., Soltan Dallal, M. M., Douraghi, M., Nikkhahi, F., Rajabi, Z., Yousefi, M., et al. (2018). Isolation and identification of specific bacteriophage against enteropathogenic Escherichia coli (EPEC) and in vitro and in vivo characterization of bacteriophage. FEMS Microbiol. Lett. 365:fny136. doi: 10.1093/femsle/fny136
Van Nieuwenhuyse, B., Van Der Linden, D., Chatzis, O., Lood, C., Wagemans, J., Lavigne, R., et al. (2022). Bacteriophage-antibiotic combination therapy against extensively drug-resistant Pseudomonas aeruginosa infection to allow liver transplantation in a toddler. Nat. Commun. 13:5725. doi: 10.1038/s41467-022-33294-w
Vinner, G. K., Richards, K., Leppanen, M., Sagona, A. P., and Malik, D. J. (2019). Microencapsulation of enteric bacteriophages in a pH-responsive solid oral dosage formulation using a scalable membrane emulsification process. Pharmaceutics 11:475. doi: 10.3390/pharmaceutics11090475
Wall, S. K., Zhang, J., Rostagno, M. H., and Ebner, P. D. (2010). Phage therapy to reduce preprocessing Salmonella infections in market-weight swine. Appl. Environ. Microbiol. 76, 48–53. doi: 10.1128/AEM.00785-09
Wang, C. H., Hsieh, Y. H., Powers, Z. M., and Kao, C. Y. (2020). Defeating antibiotic-resistant bacteria: exploring alternative therapies for a post-antibiotic era. Int. J. Mol. Sci. 21:1061. doi: 10.3390/ijms21031061
Wang, T., Yu, R., Zhu, L., Wang, X., and Yang, B. (2022). Differences in the intestinal flora of patients with inflammatory bowel disease in southwest China. Indian J. Microbiol. 62, 384–392. doi: 10.1007/s12088-022-01014-z
Watanabe, R., Matsumoto, T., Sano, G., Ishii, Y., Tateda, K., Sumiyama, Y., et al. (2007). Efficacy of bacteriophage therapy against gut-derived sepsis caused by Pseudomonas aeruginosa in mice. Antimicrob. Agents Chemother. 51, 446–452. doi: 10.1128/AAC.00635-06
Xue, Y., Zhai, S., Wang, Z., Ji, Y., Wang, G., Wang, T., et al. (2020). The Yersinia phage x1 administered orally efficiently protects a murine chronic enteritis model against yersinia enterocolitica infection. Front. Microbiol. 11:351. doi: 10.3389/fmicb.2020.00351
Yen, M., Cairns, L. S., and Camilli, A. (2017). A cocktail of three virulent bacteriophages prevents Vibrio cholerae infection in animal models. Nat. Commun. 8:14187. doi: 10.1038/ncomms14187
Yoon, S. H., and Waters, C. M. (2019). Vibrio cholerae. Trends Microbiol. 27, 806–807. doi: 10.1016/j.tim.2019.03.005
Keywords: phage therapy, intestinal infections, antimicrobial resistance, microbiome modulation, translational medicine
Citation: Wang X, Li J, Ge Z, Fan J, Ma D, Cao H, Shen J, Wang Y, Liu Z, Gomaa SE, Li X, Ji X and Teng T (2025) Phage therapy for intestinal infections: efficacy, challenges, and future directions in translational research. Front. Microbiol. 16:1672198. doi: 10.3389/fmicb.2025.1672198
Received: 24 July 2025; Revised: 10 November 2025;
Accepted: 11 November 2025; Published: 03 December 2025.
Edited by:
Callum Cooper, Wellbeing University of Sunderland, United KingdomReviewed by:
Biplab Singha, Cedars Sinai Medical Center, United StatesAudrey Addablah, Laval University, Canada
Copyright © 2025 Wang, Li, Ge, Fan, Ma, Cao, Shen, Wang, Liu, Gomaa, Li, Ji and Teng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianghui Li, MTAxOTAxNThAdmlwLmhlbnUuZWR1LmNu; Xinying Ji, MTAxOTAwOTZAdmlwLmhlbnUuZWR1LmNu; Tieshan Teng, MTAxOTAxMzZAdmlwLmhlbnUuZWR1LmNu
†These authors have contributed equally to this work
 Xiaoqing Wang1†
Xiaoqing Wang1† Jingjing Li
Jingjing Li Zimo Ge
Zimo Ge Junhao Fan
Junhao Fan Dongdao Ma
Dongdao Ma Huiru Cao
Huiru Cao Jiawen Shen
Jiawen Shen Yange Wang
Yange Wang Salwa E. Gomaa
Salwa E. Gomaa Xianghui Li
Xianghui Li Xinying Ji
Xinying Ji