- 1Affiliated Baotou Clinical College of Inner Mongolia Medical University, Baotou, China
- 2Hepatobiliary Surgery Department, Baotou Central Hospital, Baotou, China
Gallstone disease (GSD) is a prevalent digestive disorder traditionally believed to stem from disturbances in cholesterol metabolism and imbalances in bile composition. Recent evidence highlights a shift in understanding GSD from a primarily metabolic disorder to a microbial-mediated pathology. The biliary tract, rather than a sterile environment, may harbor a distinct microbial community that, under homeostatic conditions, may coexist with the host to maintain biliary health. Disruption of this equilibrium can initiate GSD. Gut microbiota contributes to GSD by modulating enterohepatic circulation via the FXR-FGF15 pathway and producing metabolites, including β-glucuronidase, that promote cholesterol precipitation. Biofilm formation by biliary microbes further enhances nucleation and gallstone formation. Recent studies have characterized biliary microbial communities but are limited by small sample sizes, methodological heterogeneity and scant mechanistic insight. These limitations impede translation into clinical practice. Despite these limitations, accumulating evidence underscores the potential of targeting biliary and intestinal microbiota in GSD prevention and therapy. This review integrates current evidence to elucidate microbiota-mediated mechanisms and translational opportunities, offering an innovative perspective for preventive and therapeutic strategies.
Introduction
Gallstone disease (GSD), also known as cholelithiasis, refers to the formation of stones within the biliary system, including the gallbladder (the major phenotype) as well as intra- and extrahepatic bile ducts. This frequently-occurring clinical condition is among the most prevalent disorders encountered in hepatobiliary surgery (Marschall and Einarsson, 2007; Unalp-Arida and Ruhl, 2024). Fundamentally, GSD may stem from the imbalanced proportions of organic solutes (e.g., bilirubin, bile salts, phospholipids, and cholesterol) in bile, leading to the precipitation of solid components (Reshetnyak, 2012; Sun et al., 2022). According to the specific composition, GSD is classified into cholesterol gallstones, and pigment stones (black and brown) (Kumar et al., 2021). The estimated prevalence of GSD in the general population reaches approximately 10–15% (Lysandra et al., 2022). The prevalence of GSD continues to rise due to rapid economic development, dietary changes, and population aging, making it an important public health concern. GSD can lead to various complications, such as biliary colic, cholecystitis, cholangitis, and pancreatitis (Costanzo et al., 2023), significantly compromising patient quality of life and increasing healthcare burdens.
Microorganisms are ubiquitous in the human body, with bacteria being the most abundant and forming stable symbiotic communities, particularly in the gastrointestinal (GI) tract (Hou et al., 2022). Bile was traditionally considered sterile due to conventional culture results and the presumed antimicrobial properties of bile and the sphincter of Oddi (Begley et al., 2005; Csendes et al., 1996). However, organisms such as Escherichia coli (E. coli), Klebsiella pneumoniae, and Enterococcus spp. have been detected subsequently in bile and gallstone specimens, through culture-based methods and polymerase chain reaction (PCR) techniques, indicating that the biliary tract may harbor a resident or transient microbiota (Pratt and Kolter, 1998; Swidsinski et al., 1998; Wang et al., 2023). However, culture-dependent methods exhibit limited sensitivity for bacterial identification, which may be explained primarily by the coexistence of diverse microbial species in the biliary tract that may constrain the comprehensive characterization of the biliary microbiota. With the advancement of research and the widespread adoption of high-throughput sequencing technologies in human microbiome studies, our understanding of the human microbiota has been enhanced significantly owing to non-culture-based molecular approaches such as 16S rRNA gene sequencing and metagenomics (Wensel et al., 2022; Zhang et al., 2024). In particular, these methods have enabled the identification of previously unrecognized biliary microorganisms when applied to biliary research, drawing increasing attention to the role of biliary microbiota in GSD and propelling research on GSD pathogenesis into a new microbially oriented paradigm (Hu et al., 2024; Tan et al., 2025).
Pathogenesis of GSD
Gallstone disease has traditionally been explained by a tripartite model: supersaturation of bile with cholesterol, impaired gallbladder motility, and enhanced nucleation (Carey, 1993; Di Ciaula et al., 2018). Supersaturation occurs when the balance among cholesterol, bile acids, and phospholipids is disrupted, leading to cholesterol crystal precipitation (Di Ciaula et al., 2018). Impaired gallbladder motility delays bile emptying, prolonging crystal retention and facilitating aggregation (Carey, 1993). Meanwhile, mucin hypersecretion provides a pronucleating matrix that accelerates crystal growth and stone formation (Di Ciaula et al., 2018). These mechanisms also underlie the classification of gallstones into cholesterol and pigment types, with the latter often associated with hemolysis, cirrhosis, or biliary infection (Di Ciaula et al., 2018).
Emerging evidence indicates that microbial factors, particularly dysbiosis of the gut and biliary microbiota, may interact with these classical lithogenic pathways (Banerjee et al., 2024; Hu et al., 2022; Hu et al., 2024; Tan et al., 2025). Microbial alterations may influence bile composition, cholesterol solubility, and mucin expression, thereby facilitating crystal nucleation and stone formation (Hu et al., 2022; Hu et al., 2024; Tan et al., 2025). While the precise mechanisms are complex and under active investigation, these findings highlight the importance of considering microbial contributions to GSD, setting the stage for a focused discussion of biliary microbiota dysbiosis.
Relationship between biliary microbiota dysbiosis and GSD
Recently, a distinct microbial community, termed the biliary microbiota, has been identified to reside in the biliary tract. Under physiological conditions, the biliary microbiota maintain a dynamic homeostasis with the host. Nevertheless, disruption of this balance, known as dysbiosis, is implicated in the development of biliary diseases and GSD. Zhang et al. (2024). Historically, obtaining viable biliary samples has been challenging, resulting in scarce and less comprehensive research compared with studies of the gut or skin microbiota, which has limited our understanding of the normal biliary microbial community. Recent large-scale clinical cultivation and detection analyses, however, have provided a more robust data foundation for investigating the biliary microbiota (Zheng et al., 2024).
In 2014, Jiménez et al. (2014) performed the first microbiota analysis of bile, gallbladder mucus, and gallbladder tissue biopsies from healthy pigs, identifying Proteobacteria, Firmicutes, and Bacteroidetes as the dominant bacterial phyla. These results suggested that the gallbladder harbors its own specific microbiota and raised the critical question of whether the biliary microbial communities originate from resident bacteria or from other anatomical sites.
This question has generated an ongoing debate regarding the origin of the biliary microbiota. One school of thought supports the residency hypothesis, proposing that the biliary tract harbors a relatively stable, self-sustained microbial community (Nicoletti et al., 2020). In contrast, the reflux origin hypothesis suggests that microbes detected in bile largely derive from retrograde translocation from the duodenum or oral cavity (Han et al., 2021). Current evidence is inconclusive: sequencing studies identifying consistent taxa across individuals favor the residency hypothesis, whereas clinical observations and experimental models showing overlap with duodenal microbiota support the reflux hypothesis. These conflicting findings underscore methodological heterogeneity and highlight the need for longitudinal studies with carefully selected control samples to distinguish true residents from transient colonizers.
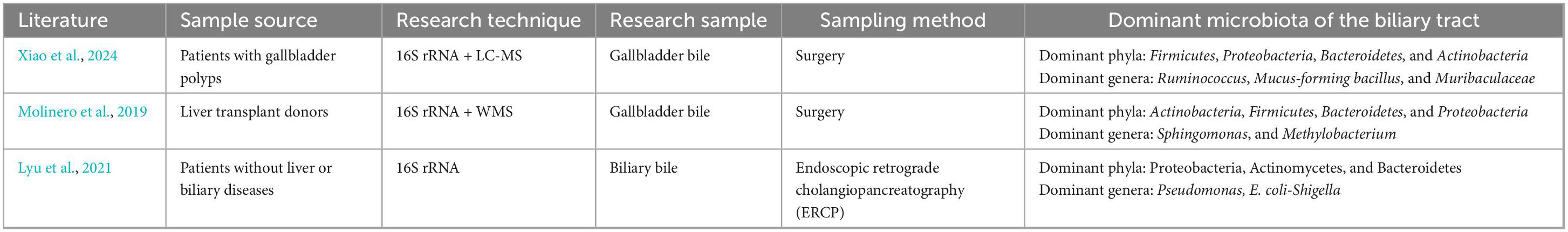
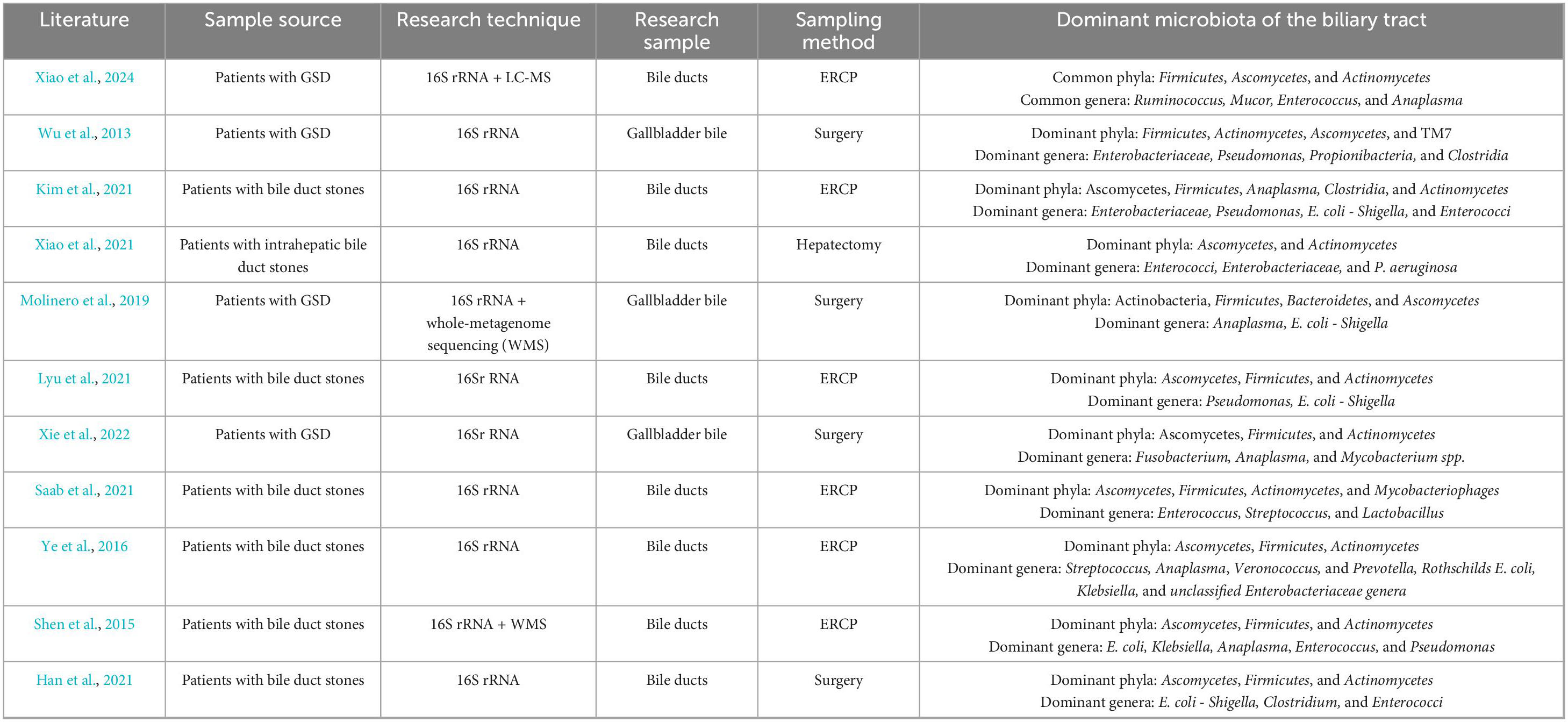
Given the ethical and practical limitations of sampling bile from healthy individuals, many studies have adopted alternative approaches. Specifically, bile samples from patients with non-inflammatory gallbladder polyps or from liver transplant donors without hepatobiliary disease are commonly utilized as surrogate controls, providing the closest possible representation of normal biliary microbiota and composition. Higashi et al. (2022) demonstrated the reflux of bile from the bile ducts into the gallbladder. With the use of cine-dynamic magnetic resonance cholangiopancreatography with a spatially selective inversion-recovery pulse, Ito et al. (2014) reported that retrograde bile movement occurred as a physiologic phenomenon in healthy controls, which was found in 74% (26 out of 35) of the nondilated group. Accordingly, we hypothesize that the biliary microbiota encompasses microbial communities residing in the gallbladder as well as in the intrahepatic and extrahepatic bile ducts. Phylum-level analysis using 16S rRNA gene sequencing or metagenomic provides a broad overview of microbial composition and trends. In contrast, genus-level composition and diversity typically indicate the specific microenvironmental conditions of individual anatomical niches (Gwak and Rho, 2020). Therefore, our research continued to carry out statistical analyses of bacterial community composition at both the phylum and genus levels across all included studies.
Molinero et al. (2019) analyzed gallbladder bile samples from 13 liver transplant donors without hepatobiliary disease and 14 patients with GSD using 16S rRNA gene sequencing. They found that the dominant phyla in healthy donors were Actinobacteria, Firmicutes, Bacteroidetes, and Proteobacteria, while major genera included Sphingomonas and Methylobacterium (Molinero et al., 2019). This study provided baseline evidence that the biliary tract may harbor a resident microbiota in healthy controls, laying the groundwork for the identification of GSD-associated dysbiosis patterns (Molinero et al., 2019). Xiao et al. (2024) pioneered the integration of 16S rRNA gene sequencing and LC–MS–based metabolomics in comparing patients with asymptomatic gallbladder polyps and those with common bile duct (CBD) stones. Consequently, polyp patients exhibited a microbiota composition similar to healthy controls, while stone patients showed decreased Actinobacteria, increased Proteobacteria, and elevated Enterococcus abundance, implying a possible synergy between microbial and metabolic alterations in GSD pathogenesis (Xiao et al., 2024). Similarly, Wu et al. (2013) reported comparable findings in 29 Chinese patients with GSD, identifying six core bacterial phyla—primarily Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria—and noting Bacteroides as the most abundant genus. This study offered important population-based evidence and facilitated the clarification of conserved microbial signatures across individuals with GSD (Wu et al., 2013). In another study on biliary obstruction, Kim et al. (2021) documented significantly increased Enterococcus levels in the bile of patients with brown pigment stones compared to those with non-stone obstructions. Moreover, patients with GSD may also exhibit significantly reduced biliary microbial diversity compared to individuals without hepatobiliary disorders. Xiao et al. (2021) reported significantly reduced microbiota diversity in the bile of patients with intrahepatic bile duct stones. Similarly, Molinero et al. (2019) observed markedly lower microbiota diversity in patients with GSD compared to liver transplant donors without hepatobiliary disease. Consistently, Xiao et al. (2024) also observed significantly reduced microbial richness, diversity, and evenness in subjects with bile duct stones compared to the control (patients with gallbladder polyps).
Tables 1, 2 summarize the results of recent investigations into the human biliary microbiota, allowing us to derive the following principal insights:
(1) GSD patients and healthy controls exhibit markedly different taxonomic composition of the biliary microbiota at both the phylum and genus levels. At the phylum level, both healthy and GSD-affected biliary microbiota are dominated by Firmicutes, Proteobacteria, Bacteroidetes, and Actinobacteria. However, there are significant differences in their relative abundances. To be specific, the biliary microbiota of GSD patients exhibits a higher proportion of Firmicutes, Bacteroidetes, and Proteobacteria, while Actinobacteria and other phyla are comparatively underrepresented. At the genus level, dominant taxa in the normal biliary tract include Ruminococcus, Akkermansia, Sphingomonas, Pseudomonas, and Escherichia. As proposed by Lyu et al. (2021), Pseudomonas and Escherichia may be part of the normal bile microbiota. In contrast, the dominant genera in GSD patients differ significantly from those in healthy controls, with higher abundances of Escherichia, Enterococcus, Pseudomonas, Enterobacter, Klebsiella, Streptococcus, and Bacteroides.
(2) GSD is also characterized by markedly altered microbial diversity, in addition to compositional shifts. For instance, microbiota of GSD patients displays significantly reduced diversity, as compared to the normal biliary microbiota (as observed in patients with gallbladder polyps or liver transplant donors without hepatobiliary disease). The enrichment of single or few bacterial taxa may be associated with bile metabolic disturbances, bile stasis, or inflammatory responses. Indeed, patients with GSD are frequently observed with reduced microbial diversity, further longitudinal studies are needed to elucidate whether this alteration serves as a causal factor or a consequence of GSD.
Altogether, these findings raise an important question: how do specific biliary bacteria functionally contribute to GSD formation, beyond compositional shifts? An in-depth explanation of this issue will contribute to bridging the gap between correlation and causation.
Roles and mechanisms of specific biliary bacteria in the formation of GSD
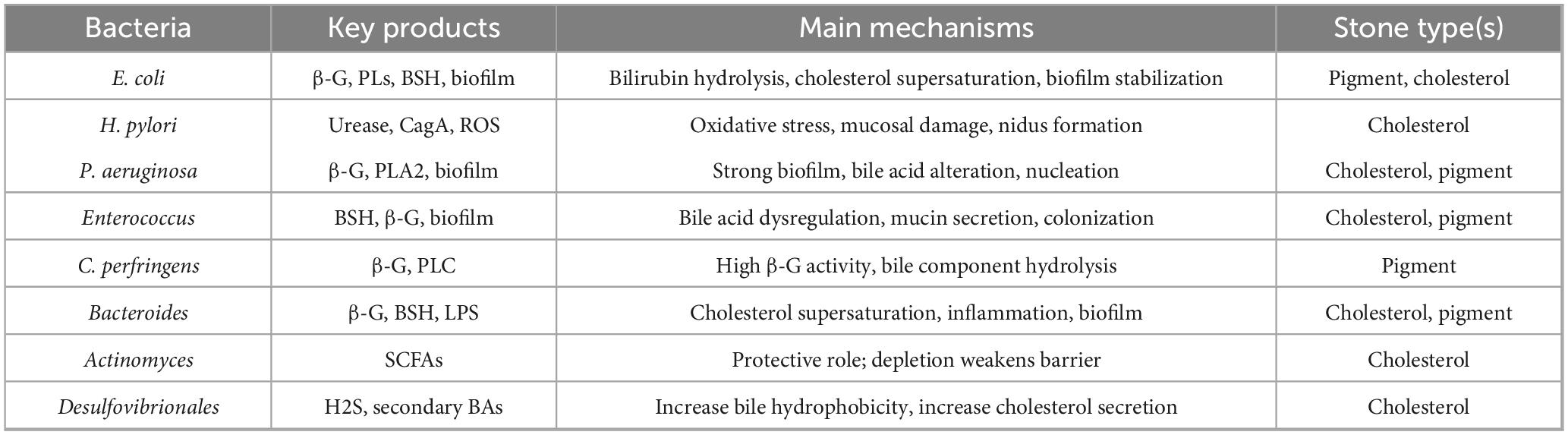
Gallstone disease can be classified into cholesterol gallstones and pigment stones, with the latter further divided into black pigment stones and brown pigment stones (Kumar et al., 2021). The formation of cholesterol gallstones is primarily attributed to bile composition imbalances—particularly cholesterol supersaturation—gallbladder dysmotility, and accelerated nucleation (Wang K. et al., 2025). In contrast, pigment stones are generally associated with hemolytic anemia, liver cirrhosis, and biliary tract infections (Lysandra et al., 2022). Traditionally, cholesterol gallstones were regarded as a consequence of metabolic disturbances. However, emerging evidence suggests that alterations in the biliary microbiota may also contribute to their development (Binda et al., 2022). Historically, the role of biliary bacteria has received little attention in GSD formation. Nevertheless, a potentially active role for microorganisms in GSD pathogenesis has been underscored by the frequent detection of bacteria in the biliary tract, with the rapid advancement of high-throughput sequencing and metagenomic technologies. Notably, specific bacterial taxa and their secreted products not only gain a colonization advantage within the biliary environment but also modulate bile composition, promote crystal nucleation, facilitate biofilm formation, and trigger local inflammation (Liu et al., 2025). Together, these mechanisms collectively drive gallstone formation. Our subsequent discussion will focus on the roles and mechanisms of major biliary bacterial phyla and representative genera in the pathogenesis of GSD. To provide a concise synthesis, we summarized the main bacteria, their key products, mechanisms, and related stone types in Table 3. This table complements the detailed descriptions in the text and improves readability.
Proteobacteria
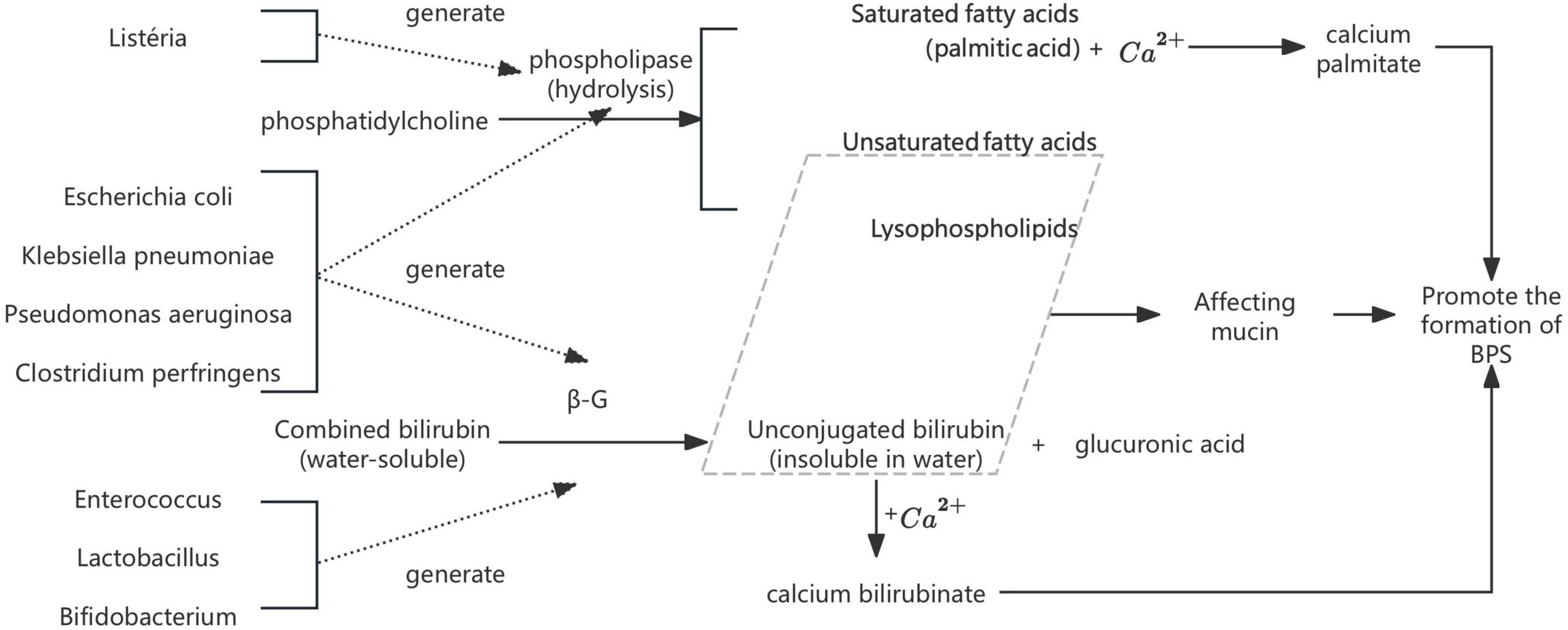
Escherichia coli (E. coli). Bacterial infection has long been recognized as a key contributor to pigment gallstone pathogenesis. As early as 1966, Japanese researcher Maki (Maki, 1966) proposed the seminal hypothesis that E. coli participates in calcium bilirubinate stones through the production of β-glucuronidase (β-G) and phospholipases (PLs). Subsequent studies confirmed that β-G hydrolyze conjugated bilirubin (CB) into unconjugated bilirubin (UCB), which readily binds to Ca2+ to form insoluble calcium bilirubinate (Nakano et al., 1988). Meanwhile, bacterial PLs degrade phosphatidylcholine (PC) into palmitic acid and lysolecithin: the former precipitates with Ca2+ to form calcium palmitate, while the latter interacts with mucin and bilirubin to promote calcium phosphate crystallization (Hattori et al., 2000). Both processes act synergistically to drive brown pigment stones development (Xiao et al., 2021; Figure 1). These findings collectively highlight the mechanistic role of bacterial enzymes in modulating bile composition and initiating stone nucleation, which has been validated in vitro by Higashijima et al. (1996) and Leung et al. (2001). Xiao et al. (2021) further proposed that other common biliary bacteria [e.g., Enterococcus, Clostridium perfringens (C. perfringens), and Pseudomonas aeruginosa (P. aeruginosa)] can also produce β-G, thereby contributing to GSD. These species will be listed in subsequent sections without further elaboration. Recent studies have corroborated these findings, identifying E. coli and other biliary bacteria as significant contributors to gallstone formation through their enzymatic activities and biofilm formation capabilities (Muturi et al., 2024; Tan et al., 2025; Zhang et al., 2025).
Recent evidence suggests that E. coli also contributes to cholesterol gallstone pathogenesis (Li et al., 2022). Cholesterol is a bile-excreted metabolic product of lipid metabolism in vivo. As a nonpolar molecule, cholesterol is poorly soluble in water, even though water constitutes approximately 85% of the bile (Tateyama and Matsushiro, 1981). Bile salts and phospholipids form bile salt–phospholipid–cholesterol complexes, also known as micelles, to maintain cholesterol solubility, eventually facilitating cholesterol solubilization (Yeo et al., 2023). Water-insoluble cholesterol crystals may be formed when these three key components are disrupted or distributed unevenly (Chatterjee and Irani, 2024). As discussed above, E. coli can survive and proliferate in infected bile. The phospholipase it produces can hydrolyze a substantial portion of bile phospholipids, which may increase the relative concentration of cholesterol, leading to cholesterol supersaturation and subsequent crystal precipitation, two key steps in triggering cholesterol gallstone development (Liu et al., 2025). Moreover, E. coli can also secrete bile salt hydrolases (BSH) and 7α-dehydroxylases, resulting in weakened capacity of emulsifying bile salts and boosting cholesterol precipitation (Berr et al., 1996). Furthermore, with the additional ability to form biofilms in the biliary system, it can also stabilize nascent cholesterol crystals and enhance nucleation (Swidsinski et al., 2005). Collectively, these findings support a multifactorial microbial contribution to lithogenesis, challenging the traditional notion that bacteria are irrelevant in cholesterol gallstone formation.
In addition to its enzymatic activity, E. coli can employ the AcrAB-TolC efflux pump [i.e., a resistance-nodulation-division (RND) family transporter] to expel toxic bile salts and fatty acids, thereby enhancing bacterial survival in the biliary system (Gaurav et al., 2023; Jang, 2023). Okusu et al. (1996) demonstrated that this efflux pump could also facilitate sustained colonization, in addition to strengthening bile salt tolerance. This persistent colonization, in turn, can exert functional roles in the formation of GSD. In addition to these effects, the AcrAB-TolC efflux pump is also involved in biofilm formation, enhanced virulence during infection, and bacterial adaptation to environmental stressors (Langevin and Dunlop, 2018; Pérez et al., 2012; Yamasaki et al., 2015), all of which may indirectly contribute to the pathogenesis of GSD. Therefore, colonization represents both a survival strategy and a mechanistic contributor to the formation of GSD. Recent studies have highlighted the significance of the AcrAB-TolC efflux pump in bacterial adaptation to the biliary environment and its potential as a therapeutic target (Jang, 2023; Tan et al., 2025).
Helicobacter pylori (H. pylori). H. pylori is a common bacterium that can live in the GI tract, which has been demonstrated to exhibit a potential association with GSD. Two prevailing hypotheses suggest that H. pylori may reach the biliary system via either duodeno-biliary reflux or the portal venous circulation (Helaly et al., 2014; Waluga et al., 2015). It is established that H. pylori can be present in the biliary tract, regardless of the pathway. Azimirad et al. (2023) identified H. pylori in bile via 16S rDNA sequencing. In another study examining gallbladder tissues from 94 symptomatic GSD patients, Attaallah et al. (2013) detected H. pylori in 35 patients (37%) by at least one method of urease testing, Giemsa staining, and immunohistochemistry. A large multi-center retrospective study by Yao et al. (2024), involving over 70,000 healthy controls, concluded a positive correlation between H. pylori infection and GSD. These findings were further supported by Wang et al. in a meta-analysis (Wang et al., 2021) that the prevalence of H. pylori infection in the gallbladder was significantly higher in patients with chronic cholecystitis and GSD compared to controls without biliary diseases (23.7% vs. 7.23%, P < 0.0001), suggesting a strong positive association between H. pylori colonization and the risk of GSD. Moreover, studies by Zhang et al. (2015) and Takahashi et al. (2014), involving 15,523 and 15,551 subjects respectively, assessed GSD prevalence following H. pylori eradication. Both studies reported significantly lower GSD prevalence in individuals following H. pylori eradication compared to those who remained H. pylori-positive (9.02% vs. 9.47%, p < 0.0001 (Zhang et al., 2015); 6.08% vs. 4.73%, p < 0.0001 (Takahashi et al., 2014), further supporting a potential causal relationship. A review of existing studies suggests that we can conclude several mechanisms by which H. pylori in the biliary tract may contribute to GSD pathogenesis as follows:
(1) H. pylori infection in the gallbladder can induce oxidative stress via reactive oxygen species (ROS) and reactive nitrogen species (RNS), along with the release of proinflammatory cytokines (e.g., IL-1, IL-6, and TNF-α), all of which are implicated in GSD pathogenesis (Binda et al., 2022; Sipos et al., 2001). Prior investigation has documented that oxidative stress can alter bile composition by promoting the biosynthesis of hepatic cholesterol, increasing hydrophobic bile acids, and enhancing phospholipase activity, leading to bile supersaturation and increased viscosity synergistically (Kasprzak et al., 2015). Administration of IL-1 and TNF-α in mice has been shown to elevate serum cholesterol levels and upregulate HMG-CoA reductase expression at both transcriptional and translational levels, thereby promoting cholesterol biosynthesis (Maurer et al., 2009). Exposure of cultured human gallbladder epithelial cells to TNF-α and IL-1 also increases susceptibility to GSD formation by disrupting sodium and chloride transport, and impairing absorptive function (Rege, 2000). CagA, a major virulence factor of H. pylori, can induce the formation of neutrophil extracellular traps (NETs) through ROS- and peptidylarginine deiminase 4 (PAD4)-dependent pathways, facilitating the release of chromatin networks. These NETs provide nucleation platforms for cholesterol crystal deposition, and, via the release of myeloperoxidase and other proinflammatory mediators, exacerbate biliary inflammation, increase bile viscosity, and promote cholesterol supersaturation, eventually triggering the formation of GSD (Muñoz et al., 2019). In addition, CagA can directly cause damage to the mechanical barrier of the gallbladder epithelium. Yu et al. (2024a) demonstrated that intra-gallbladder delivery of CagA in mice led to mucosal disruption, epithelial detachment, and necrosis. Further mechanistic insights suggest that CagA disrupted tight junctions and epithelial polarity, inducing inflammation and apoptosis (Yu et al., 2024a). This epithelial damage increases mucosal permeability, impair bile reabsorption, and promote bile stasis, thereby creating a stone-promoting microenvironment favorable to GSD development (Yu et al., 2024a).
(2) H. pylori may promote the formation of GSD through several additional mechanisms, beyond inducing oxidative stress and inflammatory responses. First, urease-positive H. pylori strains have been shown to hydrolyze urea into ammonia and bicarbonate, thereby increasing local pH and promoting the precipitation of insoluble calcium salts by interacting with carbonate and other anions (Belzer et al., 2006). These salts may serve as nucleation sites for facilitating the deposition of cholesterol and bilirubin (Belzer et al., 2006). However, this effect appears to present with individual variations, possibly due to differences in bile buffering capacity, bacterial load, or strain-specific urease activity. Moreover, some researchers hypothesized H. pylori itself might act as a nidus for lithogenesis, functioning as a foreign body around which bile components aggregate to initiate lithogenesis (Grigor’eva and Romanova, 2020). In addition, H. pylori infection has been found to be associated with increased endogenous β-G activity, further facilitating bilirubin precipitation and pigment stone formation—a mechanism detailed in the previous text (Mo, 2016). Together, H. pylori exerts multifaceted roles in GSD pathogenesis, beyond inflammation and oxidative injury.
P. aeruginosa. P. aeruginosa is a dominant species in the biliary tract of patients with GSD. In a 2024 multicenter study comparing bile microbiota in choledocholithiasis and gallbladder polyp patients, P. aeruginosa biofilm was observed to be significantly more metabolically active in the choledocholithiasis group and closely associated with stone formation (Xiao et al., 2024). P. aeruginosa can modulate the size and composition of the bile acid pool through multiple pathways, creating a favorable environment for GSD formation (Wang et al., 2024). Lyu et al. (2021) analyzed the biliary microbiota of 15 patients with primary CBD stones and 4 individuals without biliary disease using 16S rRNA gene sequencing. Their findings further revealed that P. aeruginosa was a dominant species in the bile of GSD patients, in contrast to its absence or very low abundance in healthy controls (Lyu et al., 2021). Peng et al. (2015) investigated the taxonomic composition and functional characteristics of bacteria present in cholesterol gallstones and bile. They identified 30 bacterial genera in the GSD and only 2 in the bile, among which P. aeruginosa was confirmed to be the predominant genus associated with cholesterol gallstones (Peng et al., 2015). Among all strains detected in the biliary tract, P. aeruginosa exhibited the highest β-G activity and the highest phospholipase A2 (PLA2) expression (Grigor’eva and Romanova, 2020).
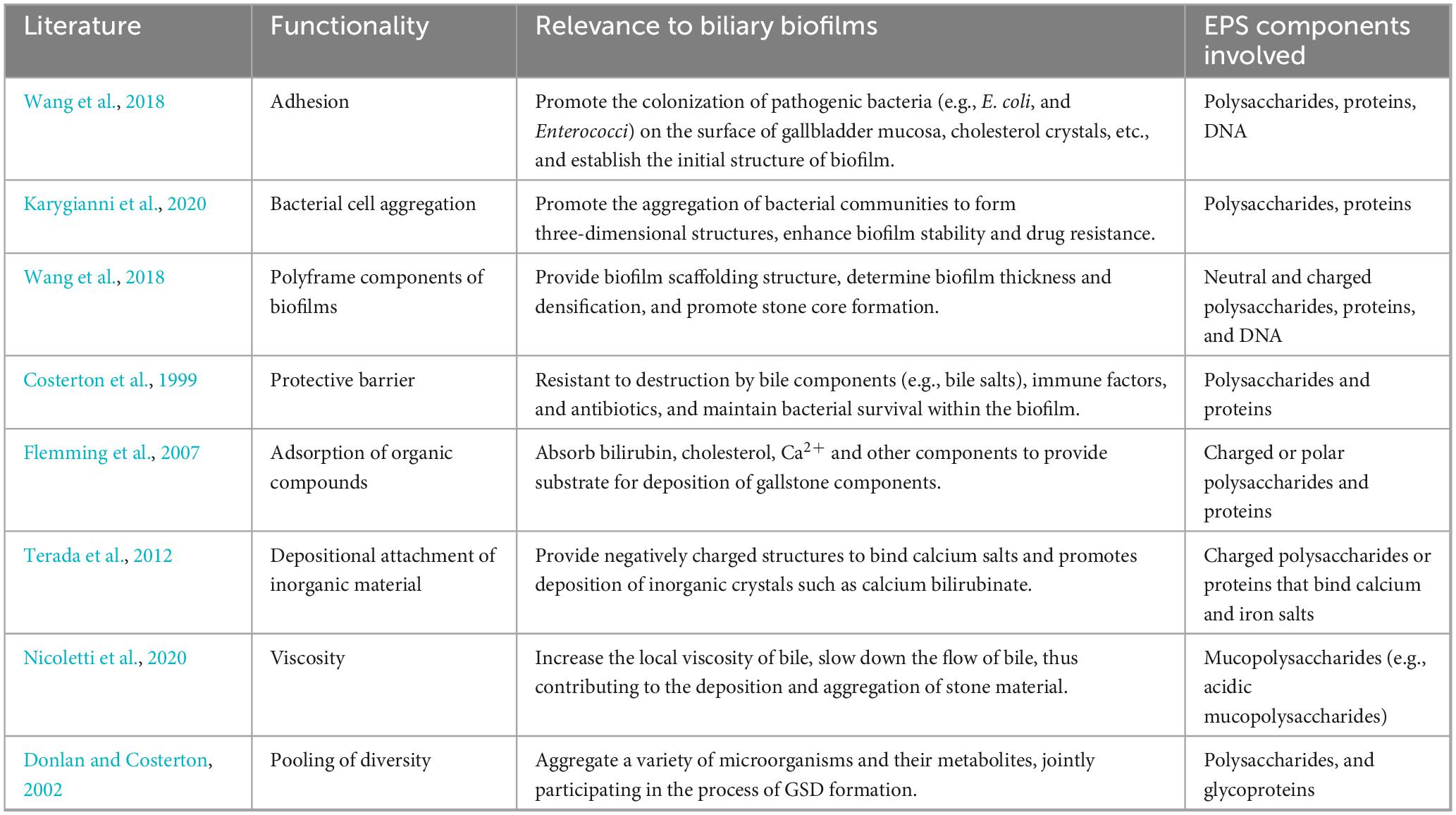
Typically, microorganisms aggregate at interfaces to form multispecies communities, rather than existing in isolation. Biofilms are a group or cluster (i.e., aggregates) of bacteria embedded in extracellular polymeric substances (EPS), which can form within the biliary tract (Montanari et al., 2025). EPS are composed mainly of polysaccharides, proteins, nucleic acids, and lipids, serving as the structural scaffold of the biofilm, which is pivotal for adhesion, protection, and metabolic activity (Flemming and Wingender, 2010). During the formation of GSD, bacteria can secrete EPS to establish biofilms that facilitate stable colonization of the biliary epithelium or bile. Under pathological conditions, these biofilms may transition from a benign to a lithogenic phenotype. By binding with Ca2+ and UCB, EPS can create a nucleation platform for compounds such as calcium bilirubinate, thereby promoting crystal aggregation and GSD initiation. Moreover, it may increase bile viscosity and obstruct bile flow, creating a microenvironment conducive to persistent bacterial colonization and biofilm maturation. In contrast, physiologically, the biliary biofilm, if present, is sparse, loosely adherent, and characterized by low-viscosity EPS with minimal polysaccharide content. Such biofilms exert negligible effects on bile dynamics or immune clearance, underscoring the importance of EPS composition and density in determining its pathological potential (Adcox et al., 2016; Prouty et al., 2002).
At the molecular level, proteomic studies have further demonstrated that P. aeruginosa biofilms in bile are enriched with outer membrane proteins such as OmpA and chaperone proteins such as DnaK, which enhance adhesion and bile tolerance, providing novel insights into the persistence of infection in the biliary tract (Yang et al., 2023). Xiao et al. (2024) found the enrichment of six pathways in CBD stones among ten significantly different Kyoto Encyclopedia of Genes and Genomes pathways, especially the biofilm formation pathway associated with P. aeruginosa. Beyond clinical associations, mechanistic insights suggest that modulation of the purine–c-di-GMP signaling system represents a potential strategy to inhibit P. aeruginosa biofilm maturation, thereby attenuating its lithogenic potential (Kennelly et al., 2024).
As summarized in Table 4, biofilms and their extracellular matrix play multifaceted roles in GSD pathogenesis, manifesting in the initiation of stone nucleation, and sustenance of bacterial survival within an inflamed biliary environment. Importantly, it is not merely the presence of biofilms but the pathological remodeling of their EPS components, particularly under dysbiotic or inflammatory stimuli, ultimately driving the transition from colonization to lithogenesis. The accumulation of charged polysaccharides, proteins, and DNA within the biofilm matrix may enhance bacterial adhesion, resist bile salt-mediated degradation, and enhance the aggregation and retention of lithogenic substrates (Costerton et al., 1999; Donlan and Costerton, 2002; Flemming et al., 2007; Karygianni et al., 2020; Nicoletti et al., 2020; Terada et al., 2012; Wang et al., 2018). These structural and biochemical features of EPS-enriched biofilms may contribute to the formation of GSD, persistence, and recurrence, thus implicating biofilm modulation as a potential target for therapeutic intervention. In addition to P. aeruginosa, bacterial genera such as E. coli, Klebsiella pneumoniae, Enterococcus, C. perfringens, Bacteroides, and Acinetobacter are also contributors of biofilm formation in the biliary tract, exhibiting intimate associations with GSD development (Grigor’eva and Romanova, 2020).
Similar to E. coli, P. aeruginosa can harbor multiple efflux pump systems on its surface, among which the MexAB-OprM pump from the RND family plays a particularly important role (Ahmadian et al., 2023). This bacterium can also produce b-G, the mechanisms of which are detailed in the previous text. In addition, P. aeruginosa may also shorten the time required for cholesterol crystallization in bile models, although the exact mechanism remains poorly understood (Zhu et al., 2009).
Desulfovibrionales. In the context of cholesterol GSD, Hu et al. demonstrated that gut microbiota, particularly bacteria of the Desulfovibrionales order, play a critical role in gallstone formation (Hu et al., 2022). The authors reported that Desulfovibrionales were enriched in the feces of patients with cholesterol GSD as well as in gallstone-susceptible mice (Hu et al., 2022). Notably, fecal microbiota transplantation from these patients to gallstone-resistant mice resulted in gallstone formation, indicating a causal relationship (Hu et al., 2022). Mechanistically, this effect was associated with increased production of secondary bile acids in the cecum, which increased bile hydrophobicity and enhanced intestinal cholesterol absorption. Furthermore, H2S produced by Desulfovibrionales was shown to activate hepatic FXR, mechanistically associated with suppression of cholesterol 7α-hydroxylase (CYP7A1) expression and bile acid synthesis (Huang et al., 2024; Ye et al., 2022). As a consequence, hepatic cholesterol transporters Abcg5 and Abcg8 were upregulated, promoting biliary cholesterol secretion and facilitating gallstone formation (Huang et al., 2024; Ye et al., 2022). These findings underscore the significant role of specific gut bacterial taxa in modulating bile acid metabolism and biliary cholesterol homeostasis, thereby contributing to GSD pathogenesis.
Firmicutes
Enterococcus. Enterococcus in the biliary tract has been unveiled to be closely associated with the formation of GSD, as has been identified by Xiao et al. (2024) via 16S rRNA gene sequencing. Similarly, Enterococcus has likewise been detected in the bile of GSD patients (Han et al., 2021; Kim et al., 2021; Saab et al., 2021; Shen et al., 2015; Xiao et al., 2021). Enterococcus is an opportunistic pathogen that is typically absent in the healthy biliary tract and primarily resides in the intestine, where it contributes to digestion and vitamin synthesis (Krawczyk et al., 2021). Intestinal Enterococcus may retrogradely translocate into the bile ducts and proliferate in cases of biliary obstruction, impaired bile flow, or immunosuppression, which is consistent with the duodeno-biliary reflux hypothesis proposed by several researchers (Han et al., 2021; Kim et al., 2021; Lyu et al., 2021). Enterococcus is frequently implicated in acute cholangitis and malignant biliary obstruction (Karasawa et al., 2021). It can tolerate a wide range of pH values (4.5–10.0) and high sodium chloride concentrations, giving it strong adaptive capacity and pathogenic potential in new environments (Karasawa et al., 2021). Enterococcus can express quorum-sensing-regulated surface proteins that mediate adhesion to the extracellular matrix and confer resistance to phagocytosis, enhancing its persistence and pathogenicity within the biliary tract (Coburn and Gilmore, 2003; Ike and Clewell, 1992). Microbial surface components recognizing adhesive matrix molecules on Enterococcus can bind to host collagen, facilitating the colonization of biological surfaces and evading immune detection (Kao and Kline, 2019). Enterococcus can also secrete BSH (Franz et al., 2001), which disrupts bile acid homeostasis and promotes inflammation, contributing to the formation of GSD. Other bacteria such as Listeria (Dussurget et al., 2002), Clostridium (Gopal-Srivastava and Hylemon, 1988), Bacteroides (Kawamoto et al., 1989), and Bifidobacterium (Grill et al., 1995) can also produce BSH and participate in lithogenesis. In addition, Enterococcus in the biliary tract may also exert a GSD-promoting role through the production of β-G that facilitates brown pigment stones formation, and biofilm formation as well (Krawczyk et al., 2021; Shankar et al., 1999).
Clostridium perfringens (C. perfringens). C. perfringens in bile exhibits PLC activity, which can hydrolyze PC into fatty acids, indirectly contributing to the formation of GSD (Cetta, 1986; Nakano et al., 1988). Moreover, there are significant interspecies differences in enzyme activity, although many bacterial species are capable of producing β-G. Notably, the β-G activity of C. perfringens is reported to be 34 times higher than that of E. coli (Leung et al., 2001). In addition, C. perfringens may also participate in the development of GSD through the production of cell-associated mucus that contributes to biofilm formation.
Bacteroidetes
Bacteroides. GSD patients have been found to exhibit a significantly elevated relative abundance of Bacteroidetes in their bile or intestinal microbiota. The metabolic activities of this phylum are closely associated with bile component imbalance, local inflammation, and eventually the formation of GSD (He et al., 2025). Bacteroides can produce β-G to stimulate the formation of pigment stones (Ye et al., 2016). With BSH activity, it can also induce cholesterol supersaturation in bile and facilitate cholesterol crystal formation (Kawamoto et al., 1989). Bacteroides may also induce the formation of biofilms in the biliary tract (Grigor’eva and Romanova, 2020), providing a structural scaffold for stone development. Its lipopolysaccharides (LPS) can induce mucosal inflammation and stimulate biliary epithelial cells to secrete large amounts of mucus, thereby accelerating the nucleation and growth of GSD.
Actinobacteria
Actinomyces. Chronic inflammation is a well-established contributor to the development of bile duct stones (Lyons et al., 2010). By inducing regulatory T cells, Actinomyces has been suggested to act as a potential regulator of inflammation and immune responses. Xiao et al. (2024) documented a markedly reduced abundance of Actinobacteria in patients with CBD stones, suggesting a weakened biliary immune barrier and impaired inflammatory regulation, potentially creating a microenvironment favorable to stone formation. In addition, Actinomyces can ferment carbohydrates to produce short-chain fatty acids (SCFAs) (e.g., acetate, propionate, and butyrate). These SCFAs serve as important energy sources for epithelial cell renewal, with butyrate standing out for its critical role in defending against bacterial toxins (Song et al., 2006). As previously discussed, the depletion of these “beneficial bacteria” may facilitate the progression of GSD.
The relationship between gut microbiota and GSD
The GI tract, harboring both commensal and pathogenic bacteria (Binda et al., 2022), is regarded as one of the largest microbial reservoirs in humans. From an embryological perspective, the biliary system and the duodenum both originate from the primitive foregut, and the anatomical connection between the CBD and duodenum (i.e., the bile drainage pathway) is established early in development, highlighting their close relationship in both structure and function (Yeo et al., 2023). Therefore, in order to elucidate their potential roles in the pathogenesis of GSD, it is rational to investigate the relationship between the biliary microbiota and the duodenal—and even the entire intestinal—microbiome. Some researchers argue that the sphincter of Oddi, as a physiological barrier, can minimize the influence of gut microbiota on the biliary tract. However, human microbial ecosystems actually constitute a dynamic, interconnected network of microbial communities, instead of existing in isolation (Costello et al., 2009). Therefore, the biliary and intestinal microbiota, rather than being accepted as entirely separate systems, should be considered to be intrinsically linked. Numerous diseases, including Crohn’s disease, colorectal cancer, and metabolic disorders, have established a strong association with gut microbiota dysbiosis (Kanehisa, 2019). Wu et al. (2013) analyzed microbial communities in the intestines, bile, and gallstones of 29 GSD patients, along with fecal microbiota from 38 healthy controls. For the first time, these authors reported a dysbiotic gut microbiota profile in GSD patients, supporting a contributing role of microbiome imbalance in the GI tract, particularly in cholesterol gallstone formation (Wu et al., 2013). With the establishment of a murine model of cholesterol gallstones, Wang et al. (2017) observed reduced gut microbial richness and diversity in animals fed by a lithogenic diet. Specifically, there was a notable decline in the abundance of Firmicutes and a significant reduction in the Firmicutes-to-Bacteroidetes ratio; moreover, compositional differences were observed at the phylum, family, and genus levels, suggesting a strong link between gut dysbiosis and cholesterol gallstone pathogenesis in mice (Wang et al., 2017). Both Lyu et al. (2021) and Han et al. (2021) reported high similarity between the biliary microbiota of patients with CBD stones and that of duodenal fluid, with all detected biliary bacteria also found in the upper GI tract. These findings support the duodeno-biliary reflux theory as a significant mechanism in the formation of GSD (Han et al., 2021; Lyu et al., 2021). It is hypothesized that the sphincter of Oddi functions as an anatomical barrier, which can normally prevent retrograde bacterial migration from the intestine into the bile ducts. This barrier may become compromised in patients with biliary disease, allowing intestinal bacteria to reflux into the biliary tract, alter the local microenvironment, and eventually promote the formation of GSD. This concept was further validated by Kamada et al. (2013). Together, the gut microbiota can trigger the development of GSD through several key mechanisms, as outlined below.
(1) Influence on enterohepatic circulation and bile acid metabolism
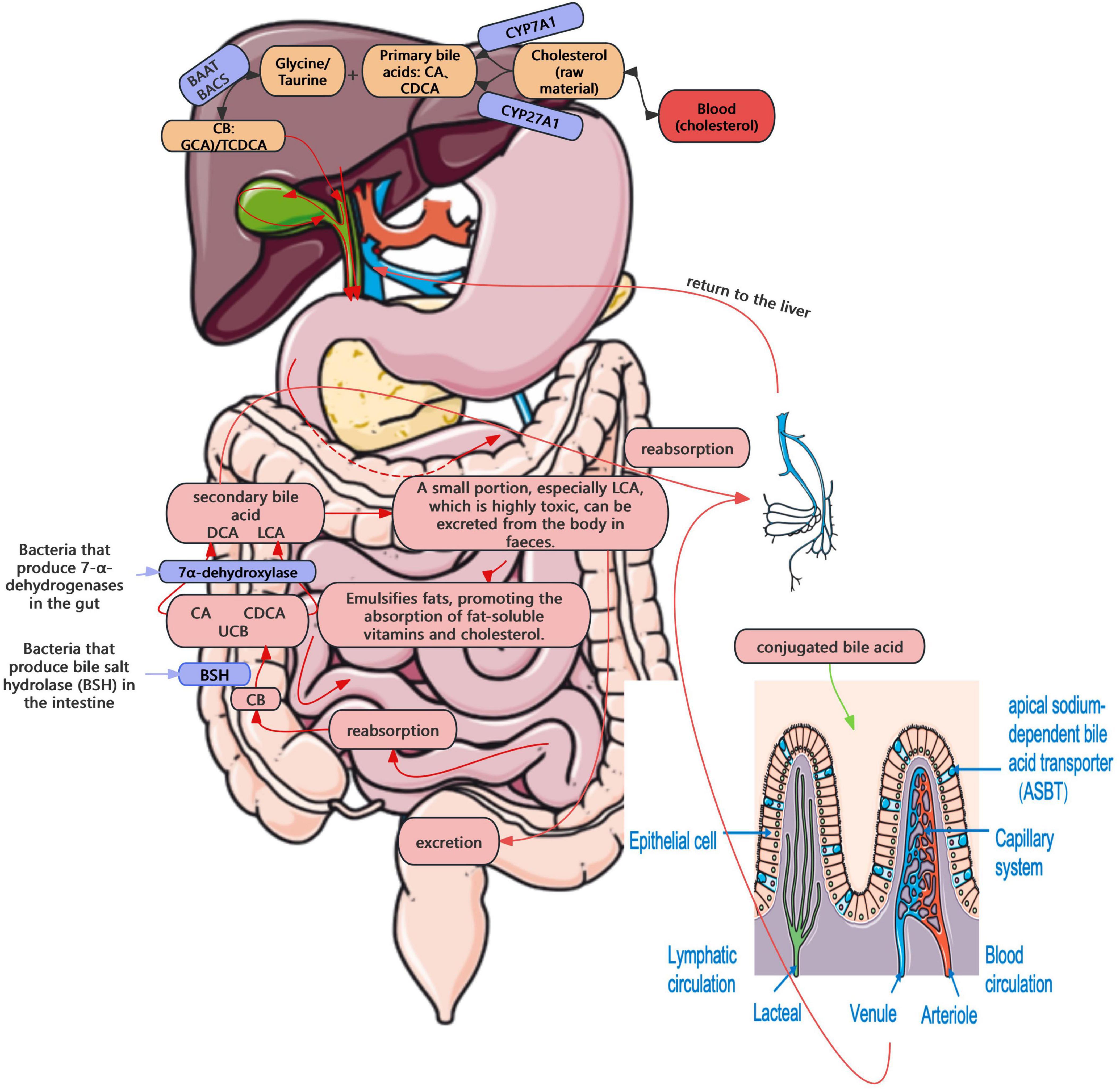
The enterohepatic circulation of bile acids plays a central role in cholesterol homeostasis and GSD pathogenesis (Cai and Chen, 2014). In this process, cholesterol is converted into primary bile acids, e.g., and chenodeoxycholic acid (CDCA), conjugated, secreted into bile, and recycled via intestinal reabsorption and hepatic uptake—functions tightly regulated by enzymes, including CYP7A1 and sterol 27-hydroxylase (CYP27A1), and transporters, including bile salt export pump (BSEP), apical sodium-dependent bile acid transporter (ASBT), and sodium-taurocholate cotransporting polypeptide (NTCP) (Cai and Chen, 2014). A small portion of bile acids that are not reabsorbed reach the colon, where they are deconjugated by gut bacteria expressing BSH, producing free bile acids (Wise and Cummings, 2022). Subsequently, they are converted by 7α-dehydroxylase into secondary bile acids, such as deoxycholic acid (DCA) and lithocholic acid (LCA) (Wise and Cummings, 2022). While some of these are reabsorbed into the portal system, others, particularly the more toxic LCA, are excreted in feces (Wise and Cummings, 2022). This enterohepatic circulation occurs 6–10 times per day, with a recycling efficiency of over 95% (Roberts et al., 2002; Figure 2). Enterohepatic circulation becomes disrupted in the presence of gut microbiota dysbiosis. A recent study also demonstrated that intestinal flora imbalance significantly affects bile acid metabolism, contributing to gallstone formation (Wang et al., 2020; Zhao et al., 2023). Elevated BSH activity may interfere with bile acid reabsorption, resulting in decreased bile salt concentration, reduced cholesterol solubility, and ultimately, the precipitation of cholesterol crystals, leading to the formation of GSD.
The farnesoid X receptor (FXR)–fibroblast growth factor 15 (FGF15) signaling axis is also pivotal in gut microbiota–mediated GSD pathogenesis, functioning as a classic negative feedback loop. Within the enterohepatic cycle, CYP7A1 is a rate-limiting enzyme in bile acid synthesis. In the intestine, bile acids bind and activate the FXR, further inducing the expression of small heterodimer partner (SHP) and fibroblast growth factor 15/19 (FGF15/19), in turn inhibiting intrahepatic CYP7A1 expression, thereby reducing bile acid synthesis (Song et al., 2022). During biliary microbiota dysbiosis, increased BSH activity has been shown to elevate the level of free bile acids in the intestine, further triggering the FXR–FGF15 feedback loop, and suppressing bile acid synthesis. The resultant cholesterol supersaturation, in conjunction with persistent dysbiosis, might create a vicious cycle that significantly increases the risk of cholesterol gallstone pathogenesis.
In addition, bacterially derived secondary bile acids (e.g., DCA and LCA) may activate the G protein-coupled bile acid receptor Takeda G-protein-coupled receptor 5 (TGR5), and increase the intracellular cAMP levels, eventually enhancing gallbladder relaxation and delaying bile emptying. Prolonged bile stasis may facilitate cholesterol precipitation, and consequently increase the risk of cholesterol gallstone formation. Simultaneously, TGR5 can modulate both gallbladder motility and local inflammatory responses, thus influencing stone formation (Keitel et al., 2009). TGR5 can also synergize with FXR to regulate the cholesterol efflux pump BSEP. Dysfunction in this pathway may impair cholesterol secretion, result in cholesterol supersaturation in bile, and accelerate stone formation (Li and Chiang, 2014). In addition, TGR5 can regulate anti-inflammatory effects; while its impairment may reduce these defenses, allowing inflammation to alter bile composition and promote cholesterol crystallization (Ye et al., 2024).
(2) Effects of microbial metabolites on bile composition and cholesterol saturation
Trimethylamine N-oxide (TMAO) is a microbial metabolite derived from dietary choline, L-carnitine, and PC, which are converted into trimethylamine by gut microbes and subsequently oxidized to TMAO in the liver by flavin-containing monooxygenase 3 (Janeiro et al., 2018). Existing clinical data reveals significantly elevated serum TMAO levels in individuals with GSD (Chen et al., 2019). TMAO enables the activation of the FXR–FGF15/SHP–FGFR4 signaling axis, thereby suppressing bile acid synthesis and promoting cholesterol supersaturation in bile (Ding et al., 2018). In addition, fermentation of carbohydrates by the gut microbiota can produce SCFAs such as acetate, propionate, and butyrate. Conversely, gut microbiota dysbiosis reduces SCFA production, thereby increasing the risk of GSD.
(3) Duodeno-biliary reflux theory
Intestinal bacteria may retrogradely translocate into the biliary tract via the sphincter of Oddi, thereby contributing to the formation of GSD. This mechanism has been discussed in detail in the previous text.
(4) Regulation of lipid and host energy metabolism
The gut microbiota can also modulate hepatic expression of lipid metabolism–related genes, such as SREBP-1c and LXRα, thereby regulating cholesterol synthesis and excretion (Wang W. et al., 2025), and ultimately promoting cholesterol gallstone pathogenesis.
Other bacterial mechanisms in the formation of GSD
In addition to gut and biliary microbiota, oral and parasitic-associated microbiota as well as other bacterial sources have also been implicated in GSD pathogenesis, suggesting a broader microbial network beyond the hepatobiliary axis. Oral bacteria may interfere with the biliary environment either directly via hematogenous spread, or indirectly by altering gut microbiota composition (Velsko et al., 2014). For example, Synergistetes, a phylum commonly associated with periodontal disease, was detected in high abundance in the bile of GSD patients, but was absent in individuals without hepatobiliary disease, as reported by Lyu et al. (2021). These bacteria are known to produce proteolytic enzymes that can degrade epithelial junctions and induce mucin overproduction, both of which may promote crystal aggregation and bile stasis, two hallmark features of GSD formation (Saltykova et al., 2016). Additionally, bacteria symbiotically associated with parasitic organisms can modulate host bile acid profiles or trigger localized immune responses in the biliary tract, thereby disturbing the physicochemical stability of bile and favoring lithogenesis (Yu et al., 2016). These alternative microbial pathways underscore the multifactorial and trans-compartmental nature of GSD.
Moreover, LPS, an essential structural component of the outer membrane of Gram-negative bacteria, has recently been implicated in GSD pathogenesis. Through activation of the TLR4/NF-κB signaling cascade, LPS induces local inflammation and mucin hypersecretion, thereby facilitating cholesterol crystal nucleation and biliary stasis (Yu et al., 2024b). These findings highlight LPS as an additional microbial factor linking Gram-negative bacterial infection to gallstone formation (Yu et al., 2024b).
Conclusion and future perspectives
Gallstone disease is currently viewed as the outcome of multifactorial interactions between host factors and complex microbial ecosystems, rather than a mere physicochemical imbalance in bile. This review highlights the biliary and intestinal microbiota as interconnected components of a broader biliary–intestinal microbial axis, whose dysregulation synergistically drives lithogenesis. In the biliary tract, specific phyla such as Proteobacteria (e.g., E. coli, P. aeruginosa) and Firmicutes (e.g., Enterococcus, C. perfringens) can promote stone formation via β-G secretion, phospholipid hydrolysis, efflux pump activation, and biofilm development. These mechanisms may disrupt bile composition, impair flow, and trigger inflammation. Bacteroidetes (e.g., Bacteroides) further participate in this process by producing BSH, LPS, and PLs that destabilize bile acid homeostasis and induce mucin secretion. Conversely, Actinobacteria (e.g., Actinomyces), through PLs production and barrier maintenance, may exert protective roles; while their depletion may favor stone formation. Meanwhile, the gut microbiota can also regulate bile composition and gallbladder motility via the enterohepatic circulation and signaling pathways such as FXR–FGF15 and TGR5. Intestinal dysbiosis can reduce bile acid recycling, increase cholesterol supersaturation, and impair gallbladder emptying—systemic disturbances that complement biliary microbial shifts. Therefore, GSD pathogenesis should be interpreted preferably as the result of dual-site dysbiosis.
This integrated view in our study holds clinical promise. Specifically, microbial profiling of bile and feces may aid risk stratification, while identifying specific bacterial signatures (e.g., β-G producers, and biofilm-forming taxa) can help predict recurrence or complications. Targeted microbial interventions (e.g., probiotics, bacteriophage therapy, fecal microbiota transplantation, etc.) may offer adjunctive strategies. Nevertheless, these applications remain exploratory, limited by methodological variability and a lack of clinical validation.
With regard to microbial interventions, although clinical evidence in GSD remains limited, it is nonetheless informative. A small randomized pilot trial evaluated probiotic supplementation, showing modest improvements in bile acid composition but no significant effect on gallstone recurrence (Prete et al., 2020; Sivamaruthi et al., 2020). Isolated observational reports have described altered biliary microbiota following fecal microbiota transplantation (FMT) in patients with recurrent biliary tract infections, although direct benefits for GSD prevention or treatment remain unproven (Hu et al., 2022). Moreover, to date, no controlled clinical trial has assessed phage therapy in GSD, leaving its application largely theoretical. Collectively, these small-sample trials and observational studies highlight both the promise and limitations of microbial interventions, thereby underscoring the need for larger, well-designed clinical studies in GSD populations.
Looking forward, future research should prioritize longitudinal, multi-center studies that integrate multiple methods, including multi-omics, spatial microbiota mapping, and metabolite profiling. Microbial contributions can be further clarified by employing gnotobiotic models and bile duct-mimicking organoids. Ultimately, such approaches may enable a deeper understanding of the biliary–intestinal microbiota axis and facilitate the development of precise, microbiota-informed strategies to prevent and treat GSD clinically.
Author contributions
YL: Conceptualization, Writing – original draft, Writing – review & editing. BY: Data curation, Visualization, Writing – review & editing. XiY: Data curation, Investigation, Writing – original draft. QS: Investigation, Methodology, Writing – review & editing. XuY: Conceptualization, Funding acquisition, Investigation, Project administration, Writing – review & editing, LL: Conceptualization, Funding acquisition, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to sincerely thank teachers for their invaluable contributions to the development of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
GSD, gallstone disease; GI, gastrointestinal; PCR, polymerase chain reaction; CBD, common bile duct; β-G, β-glucuronidase; PLs, phospholipase; CB, conjugated bilirubin; UCB, unconjugated bilirubin; PC, phosphatidylcholine; ROS, reactive oxygen species; RNS, reactive nitrogen species; NETs, neutrophil extracellular traps; ERCP, Endoscopic retrograde cholangiopancreatography; FXR, farnesoid X receptor; SHP, small heterodimer partner; TGR5, Takeda G-protein-coupled receptor 5; LPS, lipopolysaccharides; BSH, bile salt hydrolase; DCA, deoxycholic acid; EPS, extracellular polymeric substances; FGF15/19, fibroblast growth factor 15/19; LCA, lithocholic acid; PLA2, phospholipase A2; RND, resistance-nodulation-division; SCFAs, short-chain fatty acids; TMAO, trimethylamine N-oxidel; CYP7A1, cholesterol 7α-hydroxylase; CDCA, chenodeoxycholic acid; CYP27A1, sterol 27-hydroxylase; BSEP, bile salt export pump; ASBT, apical sodium-dependent bile acid transporter; NTCP, sodium-taurocholate cotransporting polypeptide.
References
Adcox, H. E., Vasicek, E. M., Dwivedi, V., Hoang, K. V., Turner, J., and Gunn, J. S. (2016). Salmonella extracellular matrix components influence biofilm formation and gallbladder colonization. Infect. Immun. 84, 3243–3251. doi: 10.1128/iai.00532-16
Ahmadian, L., Haghshenas, M. R., Mirzaei, B., Khalili, Y., and Goli, H. R. (2023). Role of MexAB-OprM efflux pump in the emergence of multidrug-resistant clinical isolates of Pseudomonas aeruginosa in Mazandaran province of Iran. Mol. Biol. Rep. 50, 2603–2609. doi: 10.1007/s11033-022-08230-2
Attaallah, W., Yener, N., Ugurlu, M. U., Manukyan, M., Asmaz, E., and Aktan, A. O. (2013). Gallstones and concomitant gastric Helicobacter pylori infection. Gastroenterol. Res. Pract. 2013:643109. doi: 10.1155/2013/643109
Azimirad, M., Sadeghi, A., Hosseinkhan, N., Mirbagheri, S. Z., and Alebouyeh, M. (2023). Microbiome analysis of bile samples in patients with choledocholithiasis and hepatobiliary disorders. Germs 13, 238–253. doi: 10.18683/germs.2023.1390
Banerjee, T., Goswami, A. G., and Basu, S. (2024). Biliary microbiome and gallstones: A silent friendship. World J. Gastrointest. Surg. 16, 3395–3399. doi: 10.4240/wjgs.v16.i11.3395
Begley, M., Gahan, C. G., and Hill, C. (2005). The interaction between bacteria and bile. FEMS Microbiol. Rev. 29, 625–651. doi: 10.1016/j.femsre.2004.09.003
Belzer, C., Kusters, J. G., Kuipers, E. J., and van Vliet, A. H. (2006). Urease induced calcium precipitation by Helicobacter species may initiate gallstone formation. Gut 55, 1678–1679. doi: 10.1136/gut.2006.098319
Berr, F., Kullak-Ublick, G. A., Paumgartner, G., Münzing, W., and Hylemon, P. B. (1996). 7 alpha-dehydroxylating bacteria enhance deoxycholic acid input and cholesterol saturation of bile in patients with gallstones. Gastroenterology 111, 1611–1620. doi: 10.1016/s0016-5085(96)70024-0
Binda, C., Gibiino, G., Coluccio, C., Sbrancia, M., Dajti, E., Sinagra, E., et al. (2022). Biliary diseases from the microbiome perspective: How microorganisms could change the approach to benign and malignant diseases. Microorganisms 10:312. doi: 10.3390/microorganisms10020312
Cai, J. S., and Chen, J. H. (2014). The mechanism of enterohepatic circulation in the formation of gallstone disease. J. Membr. Biol. 247, 1067–1082. doi: 10.1007/s00232-014-9715-3
Carey, M. C. (1993). Pathogenesis of gallstones. Am. J. Surg. 165, 410–419. doi: 10.1016/s0002-9610(05)80932-8
Cetta, F. M. (1986). Bile infection documented as initial event in the pathogenesis of brown pigment biliary stones. Hepatology 6, 482–489. doi: 10.1002/hep.1840060327
Chatterjee, A., and Irani, R. (2024). “Chapter 6 - Molecular aspect of gallstone formation: A systematic review,” in Gallstone formation, diagnosis, treatment and prevention, eds R. Sharma, S. R. Sharma, and R. Prasad (New York, NY: Academic Press), 71–82.
Chen, Y., Weng, Z., Liu, Q., Shao, W., Guo, W., Chen, C., et al. (2019). FMO3 and its metabolite TMAO contribute to the formation of gallstones. Biochim. Biophys. Acta Mol. Basis Dis. 1865, 2576–2585. doi: 10.1016/j.bbadis.2019.06.016
Coburn, P. S., and Gilmore, M. S. (2003). The Enterococcus faecalis cytolysin: A novel toxin active against eukaryotic and prokaryotic cells. Cell Microbiol. 5, 661–669. doi: 10.1046/j.1462-5822.2003.00310.x
Costanzo, M. L., D’Andrea, V., Lauro, A., and Bellini, M. I. (2023). Acute cholecystitis from biliary lithiasis: Diagnosis, management and treatment. Antibiotics 12:482. doi: 10.3390/antibiotics12030482
Costello, E. K., Lauber, C. L., Hamady, M., Fierer, N., Gordon, J. I., and Knight, R. (2009). Bacterial community variation in human body habitats across space and time. Science 326, 1694–1697. doi: 10.1126/science.1177486
Costerton, J. W., Stewart, P. S., and Greenberg, E. P. (1999). Bacterial biofilms: A common cause of persistent infections. Science 284, 1318–1322. doi: 10.1126/science.284.5418.1318
Csendes, A., Burdiles, P., Maluenda, F., Diaz, J. C., Csendes, P., and Mitru, N. (1996). Simultaneous bacteriologic assessment of bile from gallbladder and common bile duct in control subjects and patients with gallstones and common duct stones. Arch. Surg. 131, 389–394. doi: 10.1001/archsurg.1996.01430160047008
Di Ciaula, A., Wang, D. Q., and Portincasa, P. (2018). An update on the pathogenesis of cholesterol gallstone disease. Curr. Opin. Gastroenterol. 34, 71–80. doi: 10.1097/mog.0000000000000423
Ding, L., Chang, M., Guo, Y., Zhang, L., Xue, C., Yanagita, T., et al. (2018). Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism. Lipids Health Dis. 17:286. doi: 10.1186/s12944-018-0939-6
Donlan, R. M., and Costerton, J. W. (2002). Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15, 167–193. doi: 10.1128/cmr.15.2.167-193.2002
Dussurget, O., Cabanes, D., Dehoux, P., Lecuit, M., Buchrieser, C., Glaser, P., et al. (2002). Listeria monocytogenes bile salt hydrolase is a PrfA-regulated virulence factor involved in the intestinal and hepatic phases of listeriosis. Mol. Microbiol. 45, 1095–1106. doi: 10.1046/j.1365-2958.2002.03080.x
Flemming, H. C., Neu, T. R., and Wozniak, D. J. (2007). The EPS matrix: The “house of biofilm cells”. J. Bacteriol. 189, 7945–7947. doi: 10.1128/jb.00858-07
Flemming, H. C., and Wingender, J. (2010). The biofilm matrix. Nat. Rev. Microbiol. 8, 623–633. doi: 10.1038/nrmicro2415
Franz, C. M., Specht, I., Haberer, P., and Holzapfel, W. H. (2001). Bile salt hydrolase activity of Enterococci isolated from food: Screening and quantitative determination. J. Food Prot. 64, 725–729. doi: 10.4315/0362-028x-64.5.725
Gaurav, A., Bakht, P., Saini, M., Pandey, S., and Pathania, R. (2023). Role of bacterial efflux pumps in antibiotic resistance, virulence, and strategies to discover novel efflux pump inhibitors. Microbiology 169:001333. doi: 10.1099/mic.0.001333
Gopal-Srivastava, R., and Hylemon, P. B. (1988). Purification and characterization of bile salt hydrolase from Clostridium perfringens. J. Lipid Res. 29, 1079–1085. doi: 10.1016/S0022-2275(20)38464-9
Grigor’eva, I. N., and Romanova, T. I. (2020). Gallstone disease and microbiome. Microorganisms 8:835. doi: 10.3390/microorganisms8060835
Grill, J., Schneider, F., Crociani, J., and Ballongue, J. (1995). Purification and characterization of conjugated bile salt hydrolase from Bifidobacterium longum BB536. Appl. Environ. Microbiol. 61, 2577–2582. doi: 10.1128/aem.61.7.2577-2582.1995
Gwak, H. J., and Rho, M. (2020). Data-driven modeling for species-level taxonomic assignment from 16S rRNA: Application to human microbiomes. Front. Microbiol. 11:570825. doi: 10.3389/fmicb.2020.570825
Han, J., Wu, S., Fan, Y., Tian, Y., and Kong, J. (2021). Biliary microbiota in choledocholithiasis and correlation with duodenal microbiota. Front. Cell Infect. Microbiol. 11:625589. doi: 10.3389/fcimb.2021.625589
Hattori, Y., Tazuma, S., Yamashita, G., Ochi, H., Sunami, Y., Nishioka, T., et al. (2000). Role of phospholipase A2 in cholesterol gallstone formation is associated with biliary phospholipid species selection at the site of hepatic excretion: Indirect evidence. Dig. Dis. Sci. 45, 1413–1421. doi: 10.1023/a:1005524624411
He, S., Lu, S., Yang, T., Ma, H., He, Y., Mi, J., et al. (2025). Bacteroides dorei RX2020-derived bile acid alleviates influenza virus infection through TGR5 signaling. Cell Commun. Signal. 23:382. doi: 10.1186/s12964-025-02384-9
Helaly, G. F., El-Ghazzawi, E. F., Kazem, A. H., Dowidar, N. L., Anwar, M. M., and Attia, N. M. (2014). Detection of Helicobacter pylori infection in Egyptian patients with chronic calcular cholecystitis. Br. J. Biomed. Sci. 71, 13–18. doi: 10.1080/09674845.2014.11669957
Higashi, M., Tanabe, M., Ihara, K., Iida, E., Furukawa, M., and Ito, K. (2022). Bile flow dynamics in patients with cholelithiasis: An evaluation with cine-dynamic magnetic resonance cholangiopancreatography using a spatially selective inversion-recovery pulse. Tomography 8, 815–823. doi: 10.3390/tomography8020067
Higashijima, H., Ichimiya, H., Nakano, T., Yamashita, H., Kuroki, S., Satoh, H., et al. (1996). Deconjugation of bilirubin accelerates coprecipitation of cholesterol, fatty acids, and mucin in human bile–in vitro study. J. Gastroenterol. 31, 828–835. doi: 10.1007/bf02358610
Hou, K., Wu, Z. X., Chen, X. Y., Wang, J. Q., Zhang, D., Xiao, C., et al. (2022). Microbiota in health and diseases. Signal Transduct. Target Ther. 7:135. doi: 10.1038/s41392-022-00974-4
Hu, H., Shao, W., Liu, Q., Liu, N., Wang, Q., Xu, J., et al. (2022). Gut microbiota promotes cholesterol gallstone formation by modulating bile acid composition and biliary cholesterol secretion. Nat. Commun. 13:252. doi: 10.1038/s41467-021-27758-8
Hu, X., Binxu, Q., Shao, G. Z., Huang, Y., and Qiu, W. (2024). Gut microbiota, circulating metabolites, and gallstone disease: A Mendelian randomization study. Front. Microbiol. 15:1336673. doi: 10.3389/fmicb.2024.1336673
Huang, D., Shen, S., Zhuang, Q., Ye, X., Qian, Y., Dong, Z., et al. (2024). Ganoderma lucidum polysaccharide ameliorates cholesterol gallstone formation by modulating cholesterol and bile acid metabolism in an FXR-dependent manner. Chin. Med. 19:16. doi: 10.1186/s13020-024-00889-y
Ike, Y., and Clewell, D. B. (1992). Evidence that the hemolysin/bacteriocin phenotype of Enterococcus faecalis subsp. zymogenes can be determined by plasmids in different incompatibility groups as well as by the chromosome. J. Bacteriol. 174, 8172–8177. doi: 10.1128/jb.174.24.8172-8177.1992
Ito, K., Kanki, A., Yamamoto, A., Tamada, T., Yasokawa, K., Tanimoto, D., et al. (2014). Assessment of physiologic bile flow in the extrahepatic bile duct with cine-dynamic MR cholangiopancreatography and a spatially selective inversion-recovery pulse. Radiology 270, 777–783. doi: 10.1148/radiol.13131046
Janeiro, M. H., Ramírez, M. J., Milagro, F. I., Martínez, J. A., and Solas, M. (2018). Implication of trimethylamine n-oxide (TMAO) in disease: Potential biomarker or new therapeutic target. Nutrients 10:1398. doi: 10.3390/nu10101398
Jang, S. (2023). AcrAB-TolC, a major efflux pump in Gram negative bacteria: Toward understanding its operation mechanism. BMB Rep. 56, 326–334. doi: 10.5483/BMBRep.2023-0070
Jiménez, E., Sánchez, B., Farina, A., Margolles, A., and Rodríguez, J. M. (2014). Characterization of the bile and gall bladder microbiota of healthy pigs. Microbiologyopen 3, 937–949. doi: 10.1002/mbo3.218
Kamada, N., Chen, G. Y., Inohara, N., and Núñez, G. (2013). Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 14, 685–690. doi: 10.1038/ni.2608
Kanehisa, M. (2019). Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951. doi: 10.1002/pro.3715
Kao, P. H. N., and Kline, K. A. (2019). Dr. Jekyll and Mr. Hide: How Enterococcus faecalis subverts the host immune response to cause infection. J. Mol. Biol. 431, 2932–2945. doi: 10.1016/j.jmb.2019.05.030
Karasawa, Y., Kato, J., Kawamura, S., Kojima, K., Ohki, T., Seki, M., et al. (2021). Risk factors for acute cholangitis caused by Enterococcus faecalis and Enterococcus faecium. Gut Liver 15, 616–624. doi: 10.5009/gnl20214
Karygianni, L., Ren, Z., Koo, H., and Thurnheer, T. (2020). Biofilm matrixome: Extracellular components in structured microbial communities. Trends Microbiol. 28, 668–681. doi: 10.1016/j.tim.2020.03.016
Kasprzak, A., Szmyt, M., Malkowski, W., Przybyszewska, W., Helak-Łapaj, C., Seraszek-Jaros, A., et al. (2015). Analysis of immunohistochemical expression of proinflammatory cytokines (IL-1α, IL-6, and TNF-α) in gallbladder mucosa: Comparative study in acute and chronic Calculous cholecystitis. Folia Morphol. 74, 65–72. doi: 10.5603/fm.2015.0011
Kawamoto, K., Horibe, I., and Uchida, K. (1989). Purification and characterization of a new hydrolase for conjugated bile acids, chenodeoxycholyltaurine hydrolase, from Bacteroides vulgatus. J. Biochem. 106, 1049–1053. doi: 10.1093/oxfordjournals.jbchem.a122962
Keitel, V., Cupisti, K., Ullmer, C., Knoefel, W. T., Kubitz, R., and Häussinger, D. (2009). The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders. Hepatology 50, 861–870. doi: 10.1002/hep.23032
Kennelly, C., Tran, P., and Prindle, A. (2024). Environmental purines decrease Pseudomonas aeruginosa biofilm formation by disrupting c-di-GMP metabolism. Cell Rep. 43:114154. doi: 10.1016/j.celrep.2024.114154
Kim, B., Park, J. S., Bae, J., and Hwang, N. (2021). Bile microbiota in patients with pigment common bile duct stones. J. Korean Med. Sci. 36:e94. doi: 10.3346/jkms.2021.36.e94
Krawczyk, B., Wityk, P., Gałęcka, M., and Michalik, M. (2021). The many faces of Enterococcus spp.-commensal, probiotic and opportunistic pathogen. Microorganisms 9:1900. doi: 10.3390/microorganisms9091900
Kumar, I. A., Ud Din, F. M., Raina, A. H., Attri, M. R., and Attri, A. (2021). “Pathophysiology of gallstones,” in Gallstones - Review and recent progress, eds Q. Yan, H. Shen, and Rijeka (London: IntechOpen).
Langevin, A. M., and Dunlop, M. J. (2018). Stress introduction rate alters the benefit of AcrAB-TolC Efflux pumps. J. Bacteriol. 200:e00525-17. doi: 10.1128/jb.00525-17
Leung, J. W., Liu, Y. L., Leung, P. S., Chan, R. C., Inciardi, J. F., and Cheng, A. F. (2001). Expression of bacterial beta-glucuronidase in human bile: An in vitro study. Gastrointest. Endosc. 54, 346–350. doi: 10.1067/mge.2001.117546
Li, T., and Chiang, J. Y. (2014). Bile acid signaling in metabolic disease and drug therapy. Pharmacol. Rev. 66, 948–983. doi: 10.1124/pr.113.008201
Li, Y., Tan, W. H., Wu, J. C., Huang, Z. X., Shang, Y. Y., Liang, B., et al. (2022). Microbiologic risk factors of recurrent choledocholithiasis post-endoscopic sphincterotomy. World J. Gastroenterol. 28, 1257–1271. doi: 10.3748/wjg.v28.i12.1257
Liu, Y., Li, H., Sun, T., Sun, G., Jiang, B., Liu, M., et al. (2025). Gut microbiome and metabolome characteristics of patients with cholesterol gallstones suggest the preventive potential of prebiotics. Imeta 4:e70000. doi: 10.1002/imt2.70000
Lyons, A., O’Mahony, D., O’Brien, F., MacSharry, J., Sheil, B., Ceddia, M., et al. (2010). Bacterial strain-specific induction of Foxp3+ T regulatory cells is protective in murine allergy models. Clin. Exp. Allergy 40, 811–819. doi: 10.1111/j.1365-2222.2009.03437.x
Lysandra, A. Z., Putri Wairooy, N. A., Ifadha, R. T., Ramainaldo, A. A. S., Albright, I. A., Izzah, A. F., et al. (2022). Risk factor of dietary habit with cholelithiasis. J. Commun. Med. Public Health Res. 3, 1–11. doi: 10.20473/jcmphr.v3i1.27931
Lyu, Z., Yu, T., Zhang, L., Xu, X., Zhang, Y., Li, J., et al. (2021). Analysis of the relationship between bile duct and duodenal microbiota reveals that potential dysbacteriosis is the main cause of primary common bile duct stones. Synth. Syst. Biotechnol. 6, 414–428. doi: 10.1016/j.synbio.2021.11.002
Maki, T. (1966). Pathogenesis of calcium bilirubinate gallstone: Role of E. coli, beta-glucuronidase and coagulation by inorganic ions, polyelectrolytes and agitation. Ann. Surg. 164, 90–100. doi: 10.1097/00000658-196607000-00010
Marschall, H. U., and Einarsson, C. (2007). Gallstone disease. J. Intern. Med. 261, 529–542. doi: 10.1111/j.1365-2796.2007.01783.x
Maurer, K. J., Carey, M. C., and Fox, J. G. (2009). Roles of infection, inflammation, and the immune system in cholesterol gallstone formation. Gastroenterology 136, 425–440. doi: 10.1053/j.gastro.2008.12.031
Mo, Y. A. N. G. (2016). Involvement of gut microbiota in the association between macrophage and gastrointestinal motility. J. Digest. Dis. 17, 13–112. doi: 10.1111/1751-2980.12389
Molinero, N., Ruiz, L., Milani, C., Gutiérrez-Díaz, I., Sánchez, B., Mangifesta, M., et al. (2019). The human gallbladder microbiome is related to the physiological state and the biliary metabolic profile. Microbiome 7:100. doi: 10.1186/s40168-019-0712-8
Montanari, E., Bernardo, G., Le Noci, V., Anselmi, M., Pupa, S. M., Tagliabue, E., et al. (2025). Biofilm formation by the host microbiota: A protective shield against immunity and its implication in cancer. Mol. Cancer 24:148. doi: 10.1186/s12943-025-02348-0
Muñoz, L. E., Boeltz, S., Bilyy, R., Schauer, C., Mahajan, A., Widulin, N., et al. (2019). Neutrophil extracellular traps initiate gallstone formation. Immunity 51, 443–450.e4. doi: 10.1016/j.immuni.2019.07.002.
Muturi, P., Wachira, P., Wagacha, M., Mbae, C., Kavai, S. M., Mugo, M. M., et al. (2024). Salmonella Typhi Haplotype 58 biofilm formation and genetic variation in isolates from typhoid fever patients with gallstones in an endemic setting in Kenya. Front. Cell Infect. Microbiol. 14:1468866. doi: 10.3389/fcimb.2024.1468866
Nakano, T., Yanagisawa, J., and Nakayama, F. (1988). Phospholipase activity in human bile. Hepatology 8, 1560–1564. doi: 10.1002/hep.1840080615
Nicoletti, A., Ponziani, F. R., Nardella, E., Ianiro, G., Gasbarrini, A., and Zileri Dal, et al. (2020). Biliary tract microbiota: A new kid on the block of liver diseases? Eur. Rev. Med. Pharmacol. Sci. 24, 2750–2775. doi: 10.26355/eurrev_202003_20548
Okusu, H., Ma, D., and Nikaido, H. (1996). AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J. Bacteriol. 178, 306–308. doi: 10.1128/jb.178.1.306-308.1996
Peng, Y., Yang, Y., Liu, Y., Nie, Y., Xu, P., Xia, B., et al. (2015). Cholesterol gallstones and bile host diverse bacterial communities with potential to promote the formation of gallstones. Microb. Pathog 83-84, 57–63. doi: 10.1016/j.micpath.2015.05.002
Pérez, A., Poza, M., Fernández, A., Fernández Mdel, C., Mallo, S., Merino, M., et al. (2012). Involvement of the AcrAB-TolC efflux pump in the resistance, fitness, and virulence of Enterobacter cloacae. Antimicrob. Agents Chemother. 56, 2084–2090. doi: 10.1128/aac.05509-11
Pratt, L. A., and Kolter, R. (1998). Genetic analysis of Escherichia coli biofilm formation: Roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 30, 285–293. doi: 10.1046/j.1365-2958.1998.01061.x
Prete, R., Long, S. L., Gallardo, A. L., Gahan, C. G., Corsetti, A., and Joyce, S. A. (2020). Beneficial bile acid metabolism from Lactobacillus plantarum of food origin. Sci. Rep. 10:1165. doi: 10.1038/s41598-020-58069-5
Prouty, A. M., Schwesinger, W. H., and Gunn, J. S. (2002). Biofilm formation and interaction with the surfaces of gallstones by Salmonella spp. Infect. Immun. 70, 2640–2649. doi: 10.1128/iai.70.5.2640-2649.2002
Rege, R. V. (2000). Inflammatory cytokines alter human gallbladder epithelial cell absorption/secretion. J. Gastrointest. Surg. 4, 185–192. doi: 10.1016/s1091-255x(00)80055-4
Reshetnyak, V. I. (2012). Concept of the pathogenesis and treatment of cholelithiasis. World J. Hepatol. 4, 18–34. doi: 10.4254/wjh.v4.i2.18
Roberts, M. S., Magnusson, B. M., Burczynski, F. J., and Weiss, M. (2002). Enterohepatic circulation: Physiological, Pharmacokinetic and clinical implications. Clin. Pharmacokinet. 41, 751–790. doi: 10.2165/00003088-200241100-00005
Saab, M., Mestivier, D., Sohrabi, M., Rodriguez, C., Khonsari, M. R., Faraji, A., et al. (2021). Characterization of biliary microbiota dysbiosis in extrahepatic cholangiocarcinoma. PLoS One 16:e0247798. doi: 10.1371/journal.pone.0247798
Saltykova, I. V., Petrov, V. A., Logacheva, M. D., Ivanova, P. G., Merzlikin, N. V., Sazonov, A. E., et al. (2016). Biliary microbiota, gallstone disease and infection with Opisthorchis felineus. PLoS Negl. Trop. Dis. 10:e0004809. doi: 10.1371/journal.pntd.0004809
Shankar, V., Baghdayan, A. S., Huycke, M. M., Lindahl, G., and Gilmore, M. S. (1999). Infection-derived Enterococcus faecalis strains are enriched in esp, a gene encoding a novel surface protein. Infect. Immun. 67, 193–200. doi: 10.1128/iai.67.1.193-200.1999
Shen, H., Ye, F., Xie, L., Yang, J., Li, Z., Xu, P., et al. (2015). Metagenomic sequencing of bile from gallstone patients to identify different microbial community patterns and novel biliary bacteria. Sci. Rep. 5:17450. doi: 10.1038/srep17450
Sipos, P., Krisztina, H., Blázovics, A., and Fehér, J. (2001). Cholecystitis, gallstones and free radical reactions in human gallbladder. Med. Sci. Monit. 7, 84–88.
Sivamaruthi, B. S., Fern, L. A., Rashidah, Pg, Hj Ismail, D. S. N., and Chaiyasut, C. (2020). The influence of probiotics on bile acids in diseases and aging. Biomed. Pharmacother. 128:110310. doi: 10.1016/j.biopha.2020.110310
Song, M., Xia, B., and Li, J. (2006). Effects of topical treatment of sodium butyrate and 5-aminosalicylic acid on expression of trefoil factor 3, interleukin 1beta, and nuclear factor kappaB in trinitrobenzene sulphonic acid induced colitis in rats. Postgrad. Med. J. 82, 130–135. doi: 10.1136/pgmj.2005.037945
Song, S. T., Cai, L. Y., Zeng, X., and Xie, W. F. (2022). Gut microbial profile in asymptomatic gallstones. Front. Microbiol. 13:882265. doi: 10.3389/fmicb.2022.882265
Sun, H., Warren, J., Yip, J., Ji, Y., Hao, S., Han, W., et al. (2022). Factors influencing gallstone formation: A review of the literature. Biomolecules 12:550. doi: 10.3390/biom12040550
Swidsinski, A., Khilkin, M., Pahlig, H., Swidsinski, S., and Priem, F. (1998). Time dependent changes in the concentration and type of bacterial sequences found in cholesterol gallstones. Hepatology 27, 662–665. doi: 10.1002/hep.510270304
Swidsinski, A., Schlien, P., Pernthaler, A., Gottschalk, U., Bärlehner, E., Decker, G., et al. (2005). Bacterial biofilm within diseased pancreatic and biliary tracts. Gut 54, 388–395. doi: 10.1136/gut.2004.043059
Takahashi, Y., Yamamichi, N., Shimamoto, T., Mochizuki, S., Fujishiro, M., Takeuchi, C., et al. (2014). Helicobacter pylori infection is positively associated with gallstones: A large-scale cross-sectional study in Japan. J. Gastroenterol. 49, 882–889. doi: 10.1007/s00535-013-0832-z
Tan, L., Jia, F., and Liu, Y. (2025). Advances in research on the role of gut microbiota in the pathogenesis and precision management of gallstone disease. Front. Med. 12:1535355. doi: 10.3389/fmed.2025.1535355
Tateyama, T., and Matsushiro, T. (1981). Bile acid composition affecting cholesterol dissolution rate: A use of multiple regression analysis. Tohoku J. Exp. Med. 133, 467–475. doi: 10.1620/tjem.133.467
Terada, A., Okuyama, K., Nishikawa, M., Tsuneda, S., and Hosomi, M. (2012). The effect of surface charge property on Escherichia coli initial adhesion and subsequent biofilm formation. Biotechnol. Bioeng. 109, 1745–1754. doi: 10.1002/bit.24429
Unalp-Arida, A., and Ruhl, C. E. (2024). Burden of gallstone disease in the United States population: Prepandemic rates and trends. World J. Gastrointest. Surg. 16, 1130–1148. doi: 10.4240/wjgs.v16.i4.1130
Velsko, I. M., Chukkapalli, S. S., Rivera, M. F., Lee, J. Y., Chen, H., Zheng, D., et al. (2014). Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PLoS One 9:e97811. doi: 10.1371/journal.pone.0097811
Waluga, M., Kukla, M., Żorniak, M., Bacik, A., and Kotulski, R. (2015). From the stomach to other organs: Helicobacter pylori and the liver. World J. Hepatol. 7, 2136–2146. doi: 10.4254/wjh.v7.i18.2136
Wang, D., Ye, A., and Jiang, N. (2024). The role of bacteria in gallstone formation. Folia Microbiol. 69, 33–40. doi: 10.1007/s12223-024-01131-w
Wang, K., Liu, Z., Tang, R., Sha, Y., Wang, Z., Chen, Y., et al. (2025). Gallstones in the era of metabolic syndrome: Pathophysiology, risk prediction, and management. Cureus 17:e80541. doi: 10.7759/cureus.80541
Wang, L., Chen, J., Jiang, W., Cen, L., Pan, J., Yu, C., et al. (2021). The relationship between Helicobacter pylori infection of the gallbladder and chronic cholecystitis and cholelithiasis: A systematic review and meta-analysis. Can. J. Gastroenterol. Hepatol. 2021:8886085. doi: 10.1155/2021/8886085
Wang, Q., Hao, C., Yao, W., Zhu, D., Lu, H., Li, L., et al. (2020). Intestinal flora imbalance affects bile acid metabolism and is associated with gallstone formation. BMC Gastroenterol. 20:59. doi: 10.1186/s12876-020-01195-1
Wang, Q., Jiao, L., He, C., Sun, H., Cai, Q., Han, T., et al. (2017). Alteration of gut microbiota in association with cholesterol gallstone formation in mice. BMC Gastroenterol. 17:74. doi: 10.1186/s12876-017-0629-2
Wang, W., Zhang, K., Liu, B., Zhou, T., Tang, Y., and Li, Y. (2023). Chaihu Shugan prevents cholesterol gallstone formation by ameliorating the microbiota dysbiosis and metabolic disturbance in mice. Front. Pharmacol. 14:1291236. doi: 10.3389/fphar.2023.1291236
Wang, W., Zhang, K., Zhang, K., Wu, R., Tang, Y., and Li, Y. (2025). Gut microbiota promotes cholesterol gallstone formation through the gut-metabolism-gene axis. Microb. Pathog 203:107446. doi: 10.1016/j.micpath.2025.107446
Wang, Y., Qi, M., Qin, C., and Hong, J. (2018). Role of the biliary microbiome in gallstone disease. Exp. Rev. Gastroenterol. Hepatol. 12, 1193–1205. doi: 10.1080/17474124.2018.1533812
Wensel, C. R., Pluznick, J. L., Salzberg, S. L., and Sears, C. L. (2022). Next-generation sequencing: Insights to advance clinical investigations of the microbiome. J. Clin. Invest. 132:e154944. doi: 10.1172/jci154944
Wise, J. L., and Cummings, B. P. (2022). The 7-α-dehydroxylation pathway: An integral component of gut bacterial bile acid metabolism and potential therapeutic target. Front. Microbiol. 13:1093420. doi: 10.3389/fmicb.2022.1093420
Wu, T., Zhang, Z., Liu, B., Hou, D., Liang, Y., Zhang, J., et al. (2013). Gut microbiota dysbiosis and bacterial community assembly associated with cholesterol gallstones in large-scale study. BMC Genom. 14:669. doi: 10.1186/1471-2164-14-669
Xiao, M., Zhou, Y., Wang, Z., Dai, W., Wang, D., Wan, Z., et al. (2024). The dysregulation of biliary tract microflora is closely related to primary choledocholithiasis: A multicenter study. Sci. Rep. 14:9004. doi: 10.1038/s41598-024-59737-6
Xiao, Z., Huang, Z., Gao, J., Wang, J., Lei, J., Zhou, F., et al. (2021). The imbalance of biliary microflora in hepatolithiasis. Microb. Pathog 157:104966. doi: 10.1016/j.micpath.2021.104966
Xie, M., Zhang, X., Wu, Y., Sun, J., Yu, W., and Cui, P. (2022). Study on the correlation between biliary tract and intestinal flora and the formation of gallstones. medRxiv [Preprint] doi: 10.1101/2022.06.28.22277035
Yamasaki, S., Wang, L. Y., Hirata, T., Hayashi-Nishino, M., and Nishino, K. (2015). Multidrug efflux pumps contribute to Escherichia coli biofilm maintenance. Int. J. Antimicrob Agents 45, 439–441. doi: 10.1016/j.ijantimicag.2014.12.005
Yang, X. T., Wang, J., Jiang, Y. H., Zhang, L., Du, L., Li, J., et al. (2023). Insight into the mechanism of gallstone disease by proteomic and metaproteomic characterization of human bile. Front. Microbiol. 14:1276951. doi: 10.3389/fmicb.2023.1276951
Yao, S. Y., Li, X. M., Cai, T., Li, Y., Liang, L. X., Liu, X. M., et al. (2024). Helicobacter pylori infection is associated with the risk and phenotypes of cholelithiasis: A multi-center study and meta-analysis. World J. Gastroenterol. 30, 4991–5006. doi: 10.3748/wjg.v30.i47.4991
Ye, D., He, J., and He, X. (2024). The role of bile acid receptor TGR5 in regulating inflammatory signalling. Scand. J. Immunol. 99:e13361. doi: 10.1111/sji.13361
Ye, F., Shen, H., Li, Z., Meng, F., Li, L., Yang, J., et al. (2016). Influence of the biliary system on biliary bacteria revealed by bacterial communities of the human biliary and upper digestive tracts. PLoS One 11:e0150519. doi: 10.1371/journal.pone.0150519
Ye, X., Huang, D., Dong, Z., Wang, X., Ning, M., Xia, J., et al. (2022). FXR signaling-mediated bile acid metabolism is critical for alleviation of cholesterol gallstones by Lactobacillus strains. Microbiol. Spectr. 10:e0051822. doi: 10.1128/spectrum.00518-22
Yeo, C. J., DeMeester, S. R., McFadden, D. W., Matthews, J. B., and Fleshman, J. W. (2023). Shackelford’s surgery of the alimentary tract: Hepatobiliary, pancreatic and splenic surgery, 8th Edn. Beijing: China Science and Technology Press.
Yu, J., He, Y., Yao, W., Liu, T., Liu, X., Zheng, Y., et al. (2024a). Helicobacter pylori CagA promotes the formation of gallstones by increasing the permeability of gallbladder epithelial cells. Helicobacter 29:e13100. doi: 10.1111/hel.13100
Yu, J., Meng, Z., Liu, X., Zheng, Y., Xue, D., Hao, C., et al. (2024b). Lipopolysaccharide in bile promotes the neutrophil extracellular traps-induced gallstone formation by activating the gallbladder immune barrier. Immunotargets Ther. 13, 789–803. doi: 10.2147/itt.S495095
Yu, X. L., Chan, Y., Zhuang, L. F., Lai, H. C., Lang, N. P., Lacap-Bugler, D. C., et al. (2016). Distributions of synergistetes in clinically-healthy and diseased periodontal and peri-implant niches. Microb. Pathog 94, 90–103. doi: 10.1016/j.micpath.2015.11.029
Zhang, F. M., Yu, C. H., Chen, H. T., Shen, Z., Hu, F. L., Yuan, X. P., et al. (2015). Helicobacter pylori infection is associated with gallstones: Epidemiological survey in China. World J. Gastroenterol. 21, 8912–8919. doi: 10.3748/wjg.v21.i29.8912
Zhang, R., Chen, C., Zheng, S., Zhang, J., Chen, W., and Chen, Z. (2025). Preliminary study of biliary microbiota and identification of bacterial species associated with pigmented gallstone formation. Front. Cell Infect. Microbiol. 15:1532512. doi: 10.3389/fcimb.2025.1532512
Zhang, X., Hu, J., Li, Y., Tang, J., Yang, K., Zhong, A., et al. (2024). Gallbladder microbial species and host bile acids biosynthesis linked to cholesterol gallstone comparing to pigment individuals. Front. Cell Infect. Microbiol. 14:1283737. doi: 10.3389/fcimb.2024.1283737
Zhao, Q., Wu, J., Ding, Y., Pang, Y., and Jiang, C. (2023). Gut microbiota, immunity, and bile acid metabolism: Decoding metabolic disease interactions. Life Metab. 2:load032. doi: 10.1093/lifemeta/load032
Zheng, X., Yan, Y., Li, X., Liu, M., Zhao, X., He, J., et al. (2024). Microbial characteristics of bile in gallstone patients: A comprehensive analysis of 9,939 cases. Front. Microbiol. 15:1481112. doi: 10.3389/fmicb.2024.1481112
Keywords: gallstone disease, biliary microbiota, gut microbiota, β-glucuronidase, biofilm, bile acid dysmetabolism
Citation: Liu Y, Yao B, Yang X, Sun Q, Yang X and Liang L (2025) Research progress on the role of microbiota in the pathogenesis of gallstone disease. Front. Microbiol. 16:1672767. doi: 10.3389/fmicb.2025.1672767
Received: 24 July 2025; Accepted: 22 September 2025;
Published: 08 October 2025.
Edited by:
Axel Cloeckaert, Institut National de recherche pour l’agriculture, l’alimentation et l’environnement (INRAE), FranceReviewed by:
Elena K Perry, Berkeley Lab (DOE), United StatesQixiang Zhao, Peking University, China
Priyanka Bhoj, Temple University, United States
Copyright © 2025 Liu, Yao, Yang, Sun, Yang and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu Liang, MTI5MTIwMTM2NEBxcS5jb20=
†These authors have contributed equally to this work
 Yuntian Liu
Yuntian Liu Bihui Yao2†
Bihui Yao2†