- 1Southern Medical University Hospital of Integrated Traditional Chinese and Western Medicine, Southern Medical University, Guangzhou, Guangdong, China
- 2School of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 3Third Level Research Laboratory of State Administration of Traditional Chinese Medicine, School of Traditional Chinese Medicine, Southern Medical University, Guangzhou, Guangdong, China
- 4Guangdong Provincial Key Laboratory of Chinese Medicine Pharmaceutics, School of Traditional Chinese Medicine, Southern Medical University, Guangzhou, Guangdong, China
- 5State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Health, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou Medical University, Guangzhou, Guangdong, China
Patients coinfected with influenza virus (IFV) and bacteria face significantly elevated risks of critical illness and mortality. This vulnerability stems primarily from IFV-induced immunosuppression and disruption of respiratory barrier integrity. Specifically, prior IFV infection compromises the airway epithelium and impairs immune cell function, creating a permissive environment for secondary bacterial infections that drive severe disease progression. Within the lung, resident immune cells are crucial for pathogen surveillance, antibacterial defense, and homeostasis maintenance. However, recruited neutrophils and macrophages paradoxically become key drivers of detrimental immunopathology during coinfection. The literatures involved in influenza bacterial infection, influenza bacterial superinfection, post-influenza bacterial infection and secondary bacterial infection, were included. In this review, we summarize the literatures about epidemiology, treatment options and two pivotal mechanisms: The primary mechanisms of IFV-mediated susceptibility to bacterial infection, focusing on epithelial barrier damage and immune cell dysfunction; the central roles of specific immune cells (notably neutrophils and macrophages) and their effector pathways in fueling hyperinflammatory responses that cause severe immunopathology. A comprehensive understanding of the interactions between the pathogens and the host will assist in the development of therapeutic modalities for the prevention and treatment of post-influenza bacterial infection.
1 Introduction
Lower respiratory infections ranked as the seventh-highest global cause of death in 2021, resulting in more than 2 million deaths in the world (Naghavi et al., 2024). Influenza virus (IFV) infection is one of the most common factors leading to lower respiratory infections (Troeger et al., 2019; Li et al., 2021). Patients with IFV infection are highly susceptible to developing bacterial infection. Flu patients coinfected with bacteria have a high risk of serious illness and fatality (Bartley et al., 2022; Lee et al., 2022; Nolan et al., 2018; Bal et al., 2020). Coinfection with bacteria in hospitalized flu patients is recognized as the major determinant of mortality (Arranz-Herrero et al., 2023).
The respiratory tract possesses a comprehensive defense system against bacterial infection. Once inhaled bacteria enter the respiratory tract, they can be easily captured by mucus and cleared through the mechanical action of ciliated cells in the upper respiratory tract. Epithelial cells can secrete large amounts of surfactant proteins and antimicrobial peptides against bacterial infection at the same time. When the bacteria break through physical and chemical barriers, resident leukocytes, such as dendritic cells (DCs), alveolar macrophages (AMs), γδ T cells and invariant natural killer T (iNKT) response to invaded bacteria. These resident leukocytes can directly remove inhaled bacteria or secrete inflammatory mediators to recruit neutrophils and monocyte-derived macrophages to kill bacteria. Therefore, bacterial infection can be immediately eliminated by epithelial barriers and pulmonary immune cells during homeostasis (Neupane et al., 2020). But the first week of IFV infection can create a favorable pulmonary environment for secondary bacterial infections, thereby causing severe illness and high mortality (Neupane et al., 2020; Chen et al., 2021; Jia et al., 2018; Nickol et al., 2020; Langouët-Astrié et al., 2022). The poor outcome of viral-bacterial infection depends on numerous factors, including the damage of epithelial barriers, the impairment of antibacterial immune response (decreased phagocytosis, impaired ROS production, inhibition of activation, etc.) and the overactivated inflammatory response (Chen et al., 2021; Herrera et al., 2023; Martínez-Colón et al., 2019; Deinhardt-Emmer et al., 2020; Nickol et al., 2019; Gu et al., 2025). This review discusses the recent advances in our understanding of mechanisms that drive the pathogenesis of secondary bacterial infection following IFV infection and how this might inform future treatment options for preventing and treating viral-bacterial infection.
2 The epidemiology and bacterial spectrum of coinfection
Influenza epidemics occur annually and cause about 1 billion infections worldwide (WHO, 2019). More than 20% of influenza patients are complicated with bacterial pneumonia (Klein et al., 2016; Qiao et al., 2023). Seasonal influenza epidemics can lead to 3,200,000 cases of hospitalization globally each year (Paget et al., 2023). The incidence of coinfection in hospitalizations and ICU patients accounts for 17 and 28%, respectively (Qiao et al., 2023). The incidence of coinfection is higher in infants under 2 years of age and the elderly, especially in people over 70 years old. Patients coinfected with IFV and bacteria increase the mortality risk by 2.6 to 3.4 times compared with influenza single-infection (Arranz-Herrero et al., 2023; Qiao et al., 2023). More than 50% of the influenza-related deaths were attributed to bacterial infections during pandemics (Morens et al., 2008; Centers for Disease Control and Prevention (CDC), 2009; Estenssoro et al., 2010; Shieh et al., 2010). Approximately 23.8% influenza-associated deaths are attributed to bacterial infection in patients with seasonal IFV infection (Qiao et al., 2023; MacIntyre et al., 2018). Streptococcus pneumoniae (S. pneumoniae), Staphylococccus aureus (S. aureus), Pseudomonas aeruginosa, Streptococcus pyogenes, Haemophilus influenzae, Klebsiella pneumoniae, Mycoplasma pneumoniae, Acinetobacter baumannii, Moraxella catarrhalis and Group A Streptococcus are common bacteria during coinfection with IFV and bacteria (Arranz-Herrero et al., 2023; Klein et al., 2016; Shi et al., 2025). Gram-positive bacteria are the most frequent microorganisms identified in patients coinfected with IFV (Arranz-Herrero et al., 2023; Klein et al., 2016; Qiao et al., 2023; MacIntyre et al., 2018). S. pneumoniae is the most frequent pathogens followed by S. aureus. Both of them account for over 30% among all of bacteria (Arranz-Herrero et al., 2023).
3 Mechanisms of pathogenesis during post-influenza bacterial infection
Post-influenza virus infection is correlated with a deterioration of clinical outcome and higher mortality rates. Bacteria are easily eradicated and fail to cause serious injury during mild bacterial infection alone (Neupane et al., 2020). Initial influenza virus infection plays a crucial role in the pathogenesis of coinfection. The occurrence of antibacterial immunosuppression caused by initial influenza virus infection was considered the primary cause of coinfection. The severity of post-influenza bacterial infection is also associated with dysregulated immune response.
3.1 IFV infection creates a favorable environment for bacterial infection
3.1.1 IFV infection disrupts the integrity of the epithelial barrier
The respiratory epithelium consists of multiple types of epithelial cells such as ciliated cells, secretory cells, basal cells, goblet cells and neuroendocrine cells, which cover the trachea and most of the proximal airways. It provides the first line of defense including physical barriers, secretory barriers and immune defense, against viral and bacterial infection. IFV infection causes multiple changes in the respiratory epithelial barrier, which weakens antibacterial defense and creates conditions for secondary bacterial infection that can be subverted by bacteria (LeMessurier et al., 2020). These changes can be divided into three major aspects, including damaging the integrity of respiratory barriers, suppressing antimicrobial immune responses and increasing bacterial colonization.
The respiratory mucosa serves as an initial barrier against invasive microorganisms and it can remove pathogens by mucin production and cilia activity (Chegini et al., 2023). The mucosa is constantly exposed to various bacteria. Invasive bacteria can be trapped and removed immediately by the secreted mucus. The physical barrier consists of multiple cell structures, including adherence junctions, gap junctions, tight junctions, desmosomes, and hemidesmosomes, which play an important role in defending against these invading pathogens (Gu et al., 2025). IFV infection can disrupt the integrity of barrier by reducing the expression of tight junction proteins and reorganizing zonula occludens-1 and occludin (Gu et al., 2025; Short et al., 2016; Golebiewski et al., 2011) (Figure 1). In addition, the epithelial cells can produce multiple antimicrobial peptides (AMPs) such as lipocalin 2, BPIFA1, LL-37, sPLA2-IIA, etc., which serve as a biochemical barrier to defense against bacterial penetration (Geitani et al., 2020). IFV infection can inhibit AMP production and facilitate secondary bacterial infection in vivo. Exogenous lipocalin 2 can assist in eliminating S. aureus in the lung during post-influenza S. aureus infection (Robinson et al., 2014; Lee et al., 2015). Chitinase-3-like 1 (CHI3L1) and IL-33 are protective factors associated with strengthening neutrophil-mediated immune response. IFV infection can inhibit the production of CHI3L1 and IL-33 in epithelial cells, thereby promoting secondary bacterial infection (Karwelat et al., 2020; Robinson et al., 2018b) (Figure 1).
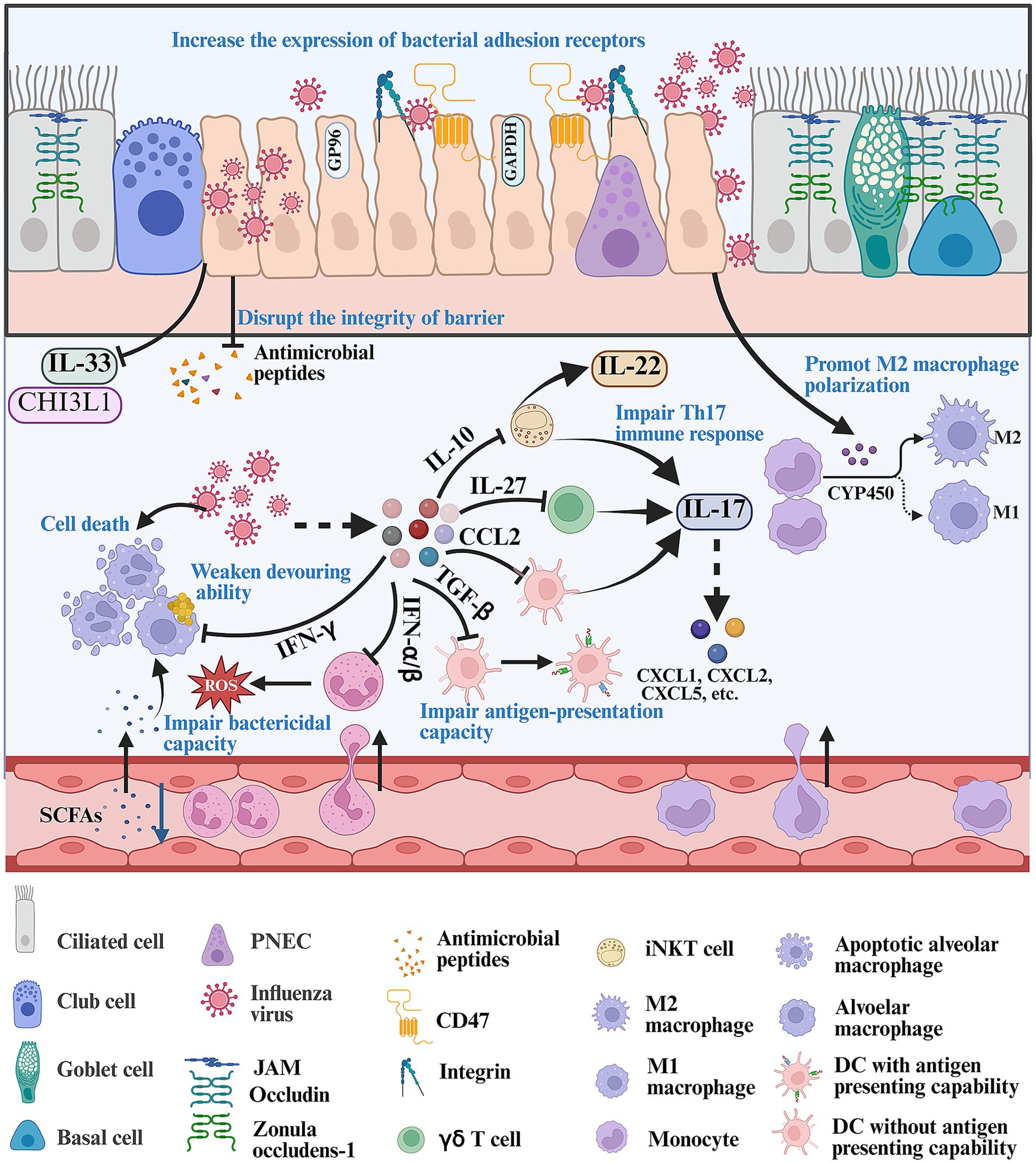
Figure 1. Mechanism about how IFV infection creates a favorable environment for bacterial infection. Firstly, IFV infection disrupts the integrity of respiratory barriers including reducing the expression of tight junction, IL-33, CHI3L1, etc., and decreasing the production of antimicrobial peptides. In addition, IFV infection can increase the expression of bacterial receptors such as CD47 and integrins, promoting bacterial infection. Once IFV breaks through the respiratory barrier, it can release various signals such as IL-10, IL-27, CCL2, TGF-β, IFN-α/β/γ and CYP450 metabolites, which impair the antibacterial ability of resident immune cells and recirculating innate immune cells. The figure was created via https://app.biorender.com/.
Attachment of invasive bacteria to the respiratory tract is the first step during respiratory bacterial infection. Invasive bacteria utilize various surface proteins to bind to epithelial tissues. IFV infection can promote the expression of bacterial adhesion receptors. Integrins and CD47 are exploited as important receptors for bacterial infections. IFV infection can increase the expression of integrins and promotes group A Streptococcus (GAS) coinfection by inducing the expression of cyclophilin A or activating TGF-β signaling pathway (Li et al., 2015; Bai et al., 2022) (Figure 1). IFV infection can induce the expression of CD47 in nasal and bronchial epithelial cells in an NF-κB-dependent manner, with which gram-positive bacteria utilize fibronectin-binding protein to interact (Moon et al., 2024) (Figure 1). IFV infection can also increase the expression of fibronectin, platelet activating factor receptor (PAFr), intracellular adhesion molecule-1 (ICAM-1) and cell adhesion molecule 1 (CEACAM-1), which can serve as receptors for attaching to the host by nontypeable Haemophilus influenzae (NTHi), S. aureus and S. pneumoniae (Prystopiuk et al., 2018; Shukla et al., 2020; Novotny and Bakaletz, 2016; Wu et al., 2021; Vimalanathan et al., 2017). GP96 is a receptor for various bacterial pathogens such as Escherichia coli, Listeria monocytogenes, S. pneumoniae and Neisseria gonorrhoeae. IFV infection can induce the ectopic localization of GP96 in epithelial cells, which can be easily bound by AliA and AliB proteins of S. pneumoniae (Sumitomo et al., 2021). Another virulence factor, pneumococcal surface protein A (PspA), can interact with host-cell-derived GAPDH by PspA’s α-helical domain in dying cells to increase bacterial colonization during post-influenza S. pneumoniae infection (Park et al., 2021). IFV infection can destruct respiratory tract by recruiting inflammatory cells, which also provides binding sites for bacterial adhesion and promotes the proliferation of pathogens. IFV infection can recruit large numbers of Ly6Chi inflammatory monocytes to the lung, which highly express tumor necrosis factor-related apoptosis-inducing ligand. Ly6Chi inflammatory monocytes can induce apoptosis in epithelial cells, which causes damage to the lung barrier and increases bacterial colonization in the lung (Ellis et al., 2015). Post-influenza bacterial infection can induce the PINK1/Parkin-mediated mitophagy and facilitate the proliferation of IFV and S. aureus in pulmonary epithelial cells (Huo et al., 2025).
3.1.2 IFV infection induces the dysfunction of lung-resident immune cells
There are various resident immune cells in the lung including unconventional T cells, resident innate immune cells and memory adaptive immune cells, which play an important role in defending against inhaled viruses, bacteria and other pathogens. Some resident pulmonary immune cells such as AMs, γδ T cells, iNKT cells and DCs, can respond to inhaled bacteria immediately and provide local protection against bacterial infection before recruitment of recirculating neutrophils and monocyte-derived macrophages (Braverman et al., 2022; Barker et al., 2021; Shenoy et al., 2020; Tavares et al., 2022). These resident immune cells can directly eliminate inhaled bacteria or affect multiple types of antibacterial immune responses by producing diverse cytokines such as IL-17, IFN-γ, IL-22, etc. IFV infection can suppress their antibacterial capacity and alter their response to bacterial infection.
AMs are resident pulmonary macrophages and they are the main immune cells present in the alveoli during homeostasis (Neupane et al., 2020). They patrol the alveoli, clean the alveolar spaces and crawl to kill inhaled bacteria to maintain homeostasis, thereby preventing severe bacterial infection during homeostasis (Neupane et al., 2020; Tam et al., 2020). But IFV infection compromises the antibacterial ability of AMs including impairing the ability to crawl, decreasing the expression of phagocytosis receptor, weakening devouring ability and inducing cell death of AM, thus enabling noninvasive bacteria to cause fatal pneumonia in influenza (Verma et al., 2020; Sencio et al., 2020). NK, NKT and T cells can rapidly secrete IFN-γ during IFV infection. IFN-γ can impair the function of AM including impairing the ability to crawl and inhibiting the expression of phagocytic receptor during post-influenza bacterial infection (Casanova et al., 2024; Schmolke et al., 2021) (Figure 1). The class A scavenger receptor macrophage receptor with collagenous structure (MARCO) is an important scavenger receptor that recognizes and binds gram-positive and gram-negative bacteria, and is the main receptor for phagocytosis of particles and exogenous bacteria by macrophages. Blocking IFN-γ signaling can promote the expression of MARCO by inhibiting Akt activation and restore the ability of AM migration (Neupane et al., 2020; Wu et al., 2017). IL-6 also promotes macrophage phagocytosis by increasing the expression of MARCO during post-influenza S. pneumoniae infection (Gou et al., 2019). Microbiota-derived metabolites such as rhamnose, indole 3-propionic acid and short chain fatty acids (SCFAs) are recognized as important players promoting macrophage phagocytosis and protecting against bacterial infection (Sencio et al., 2020; Li et al., 2024; Huang et al., 2022; Schuijt et al., 2016). SCFAs are known to promote phagocytosis of AMs by interacting with the GPR43 receptor, and protect mice from bacterial infection during S. pneumoniae or Klebsiella pneumoniae infection (Schuijt et al., 2016; Le Guern et al., 2023; Machado et al., 2022; Galvão et al., 2018). Gut dysbiosis and related metabolic dysfunction, especially reduction in SCFAs, can increase susceptibility to secondary bacterial infection following IFV infection (Chen Q. et al., 2025) (Figure 1). Blautia faecis DSM33383 can produce large amounts of acetic acid and the intragastrical administration of Blautia faecis DSM33383 can protect mice from post-influenza bacterial infection (Verstraeten et al., 2022). In addition, IFV infection can induce cell death of resident AMs and the majority of AMs would be lost in a week (Ghoneim et al., 2013). GM-CSF is an important factor promoting the maturation, differentiation and activation of AMs (Chen Y. et al., 2023). GM-CSF treatment increases the number of AMs and promotes bacterial clearance in the lung during post-influenza S. aureus infection (Ghoneim et al., 2013). Deficiency in GM-CSF’s receptor (Csf2rb−/−) abrogates bacterial clearance during post-influenza S. pneumoniae infection (Verma et al., 2020). IL-1 signaling can also contribute to the maintenance of the number of AMs and promote bacterial clearance in the lung (Bansal et al., 2018).
Lung-resident γδ T cells account for 8–20% of resident pulmonary lymphocytes and aid in eliminating bacteria (Min and Shilian, 2017; Cheng et al., 2012). Mice are susceptible to bacterial infections and develop deadly pneumonia after IFV infection in a week (Herrera et al., 2023; Yi et al., 2022). γδ T cells are a major source of IL-17A within these time points, because Th17 immune response develops slowly (Cao et al., 2014). IL-17A can enhance antimicrobial capacity by contributing to MIP-2-driven neutrophil recruitment, anti-microbial peptide secretion and enhancement of the mucosal barrier function (Mills, 2023). IFV infection induces the secretion of IL-27 that inhibits the Streptococcus-induced IL-17A expression via suppressing the activation of STAT1 signaling pathway in γδ T cells and promotes the development of secondary pneumococcal pneumonia (Cao et al., 2014; Robinson et al., 2015; Lee et al., 2017) (Figure 1). But dysregulated, chronic IL-17 signaling promotes excessive inflammation in lungs by sustaining neutrophil infiltration and triggering other pro-inflammatory pathways.
Invariant natural killer T (iNKT) cells are tissue-resident lymphocytes and account for approximately 5% of lymphocytes in mouse lung, which can response to S. pneumoniae infection and secrete IFN-γ and IL-17A within 13 h (Crosby and Kronenberg, 2018). IFN-γ is involved in activating NK cells that can assist in protecting against secondary bacterial infection following IFV infection (Casanova et al., 2024; Small et al., 2010). IFV infection dampens the activation of iNKT cells by inducing the production of IL-10, accompanied by a decrease in the production of IFN-γ and IL-17A (Barthelemy et al., 2017) (Figure 1). The activation of iNKT cells can prevent pneumococcal outgrowth during post-influenza bacterial infection by increasing the production of IFN-γ and IL-17A (Barthelemy et al., 2016). Thus, IFN-γ acts as a double-edged sword: it is a vital activating signal for mobilizing NK cells against post-influenza bacterial infection, yet it can simultaneously exacerbate the immunosuppressive state by further inhibiting the antibacterial functions of AMs, thereby worsening the outcome of influenza-bacterial coinfection. Additionally, iNKT cells can secrete IL-22 that is beneficial in limiting lung inflammation and alleviating ALI during post-influenza bacterial infection (Paget et al., 2012; Ivanov et al., 2013).
There are three types of resident DCs in the lung during homeostasis, including conventional DC1 (cDC1), conventional DC2 (cDC2) and pDC. DCs exhibit antigen-presentation capacity and secrete immunogenic cytokines such as IL-17 and IFN-γ, which strengthen both innate and adaptive immunity to promote bacterial clearance (Ardain et al., 2020). Prior IFV infection can impair its antigen-presentation capacity, affect self-renewal and influence the activation of antimicrobial immune responses. DCs can produce TGF-β and induce the accumulation of Treg cells during a primary IFV infection, which induces the differentiation of DCs into paralyzed DCs. These paralyzed DCs exhibit high amounts of Blimp1 that can induce tolerogenic functions in DCs, and low levels of IRF4 that can promote antigen presentation to CD4+ T cells. For these paralyzed DCs, MHC II-mediated T cell priming is defective for at least three weeks and antigen-presentation capacity is defective in vitro (Roquilly et al., 2017) (Figure 1). DCs must be constantly replenished by newly recruited cells from the bone marrow. Fms-like tyrosine kinase 3 ligand (Flt3-L) is a critical cDC differentiation factor. IFV infection can decrease the production of Flt3-L in the bone marrow and blood, which results in lower generation of cDC progenitors in the bone marrow, accompanied by a decrease of cDCs (cDC1 and cDC2) in the lung. Overexpression of Flt3-L promotes the cDC progenitors’ production in the bone marrow, replenishes cDCs in the lung and protects against post-influenza pneumococcal infection (Beshara et al., 2018). CC chemokine receptor 2 (CCR2) is an important receptor recruiting DCs and macrophages by interacting with CCL2. IFV infection can secrete high levels of CCL2. CCR2−/− mice exhibit decreased accumulation of cDC2 and increased accumulation of cDC1, accompanied by increased release of IL-17 in the lung during post-influenza S. aureus infection. Antagonizing IL-17 partially abrogated the protection seen in CCR2−/− mice (Gurczynski et al., 2019) (Figure 1).
3.1.3 IFV infection impairs the antimicrobial capacity of recirculating innate immune cells
Recirculating neutrophils and monocyte-derived macrophages are primary innate immune cells against bacterial infection. They can respond to inhaled bacteria and be recruited to the lung immediately. Inhaled bacteria can be attacked and eliminated by recirculating neutrophils and monocyte-derived macrophages with their weapons such as ROS, proteases, etc. IFV infection can alter their response to inhaled bacteria and their ability to kill bacteria (Martínez-Colón et al., 2019; Ho et al., 2018; Berg et al., 2017).
Neutrophils, one of the most numerous circulating leukocytes, are required for eliminating bacteria in BALB/c mice coinfected with IFV and S. pneumoniae (Palani et al., 2023). They devour bacteria and kill them by producing high levels of ROS. A clinical study indicates that neutrophils isolated from airway fluid of patients coinfected with influenza and bacteria exhibit high levels of activation markers (HNE and MPO), but their ability to kill H. influenzae and S. aureus is dampened in vitro (Grunwell et al., 2019). Neutrophils isolated from mice also exhibit low levels of ROS and neutrophil elastase during post-influenza bacterial infection (Jie et al., 2023; Ishikawa et al., 2016; Sun and Metzger, 2014). Furthermore, IFV infection impairs their ability to devour and kill invasive bacteria during post-influenza P. aeruginosa infection in vivo (Jie et al., 2023). Type I IFN signaling can weaken the capacity of neutrophils to kill S. aureus during post-influenza S. aureus infection on day 7 after IFV infection (Shepardson et al., 2016) (Figure 1).
Recirculating macrophages are divided into proinflammatory M1 macrophages characterized by high levels of ROS and NO production, and immunosuppressive M2 macrophages (Chen S. et al., 2023). M1 macrophages play a vital role in resisting bacterial invasion. CYP450 metabolites are ligands for peroxisome proliferator-activated receptor α (PPARα). IFV infection induces the production of CYP450 metabolites (5,6-diHETrE, 8,9-diHETrE, 11,12-diHETrE and 14,15-diHETrE) and triggers the activation of the PPARα signaling pathway, which promotes polarization of M2 macrophages and dampens bacterial clearance during post-influenza S. aureus infection (Tam et al., 2020; Lucarelli et al., 2022) (Figure 1). STAT2 signaling is also linked to inhibiting macrophage (M1 and M2) accumulation and impairing bacterial clearance during post-influenza bacterial infection. Neutralizing IFN-γ (M1) and/or Arginase 1 (M2) can reduce bacterial clearance in Stat2−/− mice during post-influenza bacterial infection (Gopal et al., 2018). SHP2-deficient macrophages exhibit enhanced polarization towards an M2 phenotype and a decreased antibacterial capacity during post-influenza S. aureus infection (Ouyang et al., 2019).
3.2 Dual infection causes severe immunopathological damage
3.2.1 The overactivation of the inflammatory response
The immune system is in a hyperactive state during post-influenza bacterial infection, which can be activated by recognizing the components of IFV following bacteria. The overactivation of the inflammatory response is a key pathophysiological factor causing acute lung injury (ALI) and a high mortality rate (Klemm et al., 2017; Damjanovic et al., 2013; Jia et al., 2023). Post-influenza bacterial infection occurs in the early stage of IFV infection and causes the mortality of mice within days (Jia et al., 2018; Herrera et al., 2023; Li-Juan et al., 2022). The innate immune system plays a more important role in driving the development of post-influenza bacterial infection during the early stage of infection. This study mainly discusses the overactivation of inflammatory response during coinfection with IFV and bacteria.
Different complement factors can bind to the surface of IFV or bacteria to trigger the complement system. During the early stage of infection, the components of IFV and bacteria can induce the release of C3a and C5a by activating the lectin pathway and the alternative pathway (Santos et al., 2021; Syed et al., 2020). C3a and C5a are potent chemoattractants recruiting neutrophils, macrophages, etc., to infectious sites. In addition, C3a and C5a can activate neutrophils, mast cells and basophils to release histamine, leukotriene, ROS, etc., increasing vascular permeability and destroying the epithelial-endothelial barrier (Figure 2A). Post-influenza bacterial infection can induce an overactivated inflammatory response by activating the complement system. The overactivation of complement is associated with ALI and high mortality in mice coinfected with IFV and S. aureus (Jia et al., 2023).
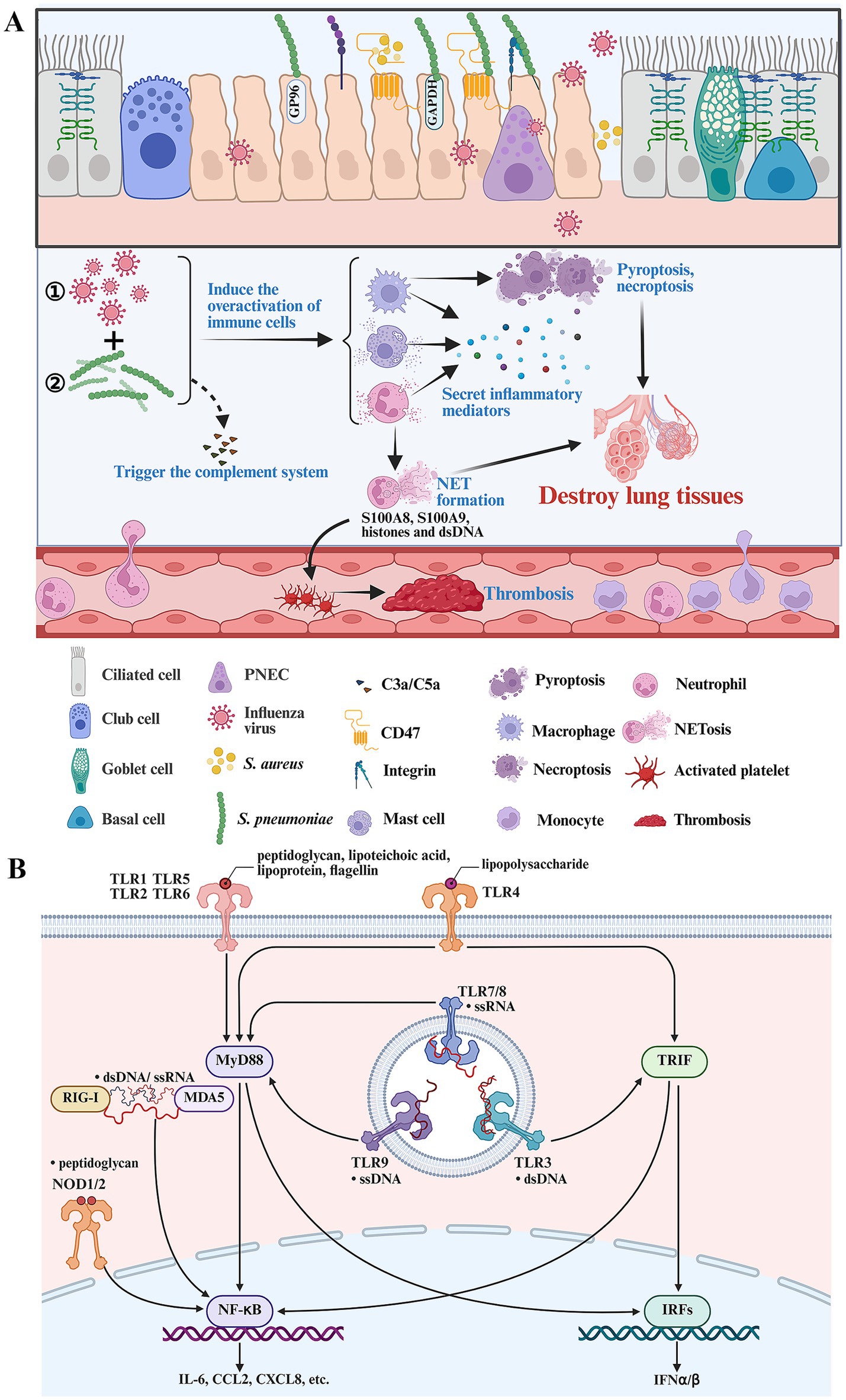
Figure 2. Key immune cells and signaling pathways in driving disease progression during secondary bacterial infection following IFV infection. (A) Post-influenza bacterial infection activates the complement system and immune cells. Activated macrophages, neutrophils and mast cells play an important role in driving the formation of overactivated inflammatory response during post-influenza bacterial infection. Activated neutrophils lead to NET formation and immune thrombosis by releasing S100A8, S100A9 and histones. Postinfluenza bacterial infection can result in pyroptosis and necroptosis. (B) The components of IFV and bacteria can be recognized by various PRRs and induce overactivated inflammatory response by activating multiple inflammatory signaling pathways. The figure was created via https://app.biorender.com/.
The components of IFV and bacteria can be sensed by pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), nucleotide binding oligomerization domain (NOD)-like receptors (NLRs), retinoic acid-inducible gene (RIG)-I-like receptors (RLRs) or DNA-sensing molecules. Post-influenza bacterial infection induces a dual inflammatory response in epithelial or immune cells (neutrophils, macrophages, etc.) by sensing the components of IFV and bacteria, respectively (Yuki and Koutsogiannaki, 2021; Verma et al., 2022). Viral single-stranded RNA (ssRNA) can be sensed by RIG-I, MDA5, TLR7 and TLR8 (Figure 2B). In addition, IFV can produce double-stranded RNA (dsRNA) during viral replication, which can be sensed by RIG-I, MDA5 and TLR3 (Figure 2B). The components of gram-positive bacteria such as peptidoglycan, lipoteichoic acid, lipoproteins and bacterial DNA, can be sensed by TLR2, NOD1/NOD2 and TLR9 (Figure 2B). The components of gram-negative bacteria such as peptidoglycan, lipopolysaccharide, flagellin and bacterial DNA, can be sensed by TLR2, NOD1/NOD2, TLR4, TLR5 and TLR9 (Figure 2B). Therefore, post-influenza bacterial infection induces a more aggressive inflammatory response by activating more inflammatory signaling pathways compared with influenza or bacterial infection alone (Jie et al., 2023; Chen B. et al., 2025). A recent longitudinal transcriptional study indicated that the top upregulated differentially expressed genes during post-influenza S. pneumonia infection are involved in inflammatory response (Cohn et al., 2024). Neutrophils and macrophages are the predominant drivers of overactivated inflammatory response during severe bacterial lung infection (Xiao et al., 2025). Recently, a single-cell RNA sequence study indicated that neutrophils, interstitial macrophages and classical monocytes are key drivers of cytokine storm during post-influenza S. aureus infection (Lei et al., 2025). But the comprehensive immune response within bronchoalveolar lavage fluid (BALF) samples from flu patients with bacterial pneumonia and healthy controls needs to be verified using single-cell RNA sequencing technology in the future.
Neutrophils play a crucial role in clearing bacteria during the early stages of bacterial invasion following influenza infection. The capability of neutrophils to eliminate bacteria can be impaired by initial influenza virus infection. However, these neutrophils still possess pro-inflammatory properties. In addition, both initial influenza virus infection and secondary bacterial infection recruit large numbers of neutrophils to the lungs (Lei et al., 2025). Therefore, neutrophils inevitably cause tissue damage during post-influenza bacterial infection. Neutrophils can directly destroy normal lung tissues and release damage-associated molecular patterns (DAMPs) to exacerbate inflammatory response (Lei et al., 2025; Burn et al., 2021). Oxidative burst can induce the formation of neutrophil extracellular traps (NETs) accompanied by releasing high levels of ROS, histones and proteases, which contribute to lung damage. NET formation in mice coinfected with IFV and bacteria contributes to endothelial injury and lung damage (Yi et al., 2022; Narayana Moorthy et al., 2013). Activated neutrophils and NET formation can also release high levels of proinflammatory mediators including S100A8, S100A9, histones and dsDNA, which can induce overactivated inflammatory responses by activating TLR4, TLR2 or cGAS-STING signaling pathways (Xiao et al., 2025; Pruenster et al., 2016; Ramasubramanian et al., 2022; Huang et al., 2013; Wilson et al., 2022; Katsoulis et al., 2024). Patients coinfected with IFV and bacteria increase the risk of coagulation disorders (Brown et al., 2024). Neutrophil activation is associated with widespread pulmonary thrombosis and alveolar oedema during post-influenza S. pneumonia infection (Walters et al., 2015). The release of S100A8/A9 and histones in neutrophils binds to GPIbα or TLR2/4 receptors of platelets, which subsequently drive the formation of immune thrombosis (Colicchia et al., 2022; Semeraro et al., 2011) (Figure 2A). Most of recirculating inflammatory monocytes are recruited into the lung and differentiate into macrophages during infection. Activated macrophages can generate reactive nitrogen species (RNS), TNF-α, etc., to augment lung damage and exacerbate disease progression. Activated macrophages can also produce matrix metalloproteinases such as MMP-9 and MMP-12, to damage the alveoli during post-influenza bacterial infection (Rojas-Quintero et al., 2018; Villeret et al., 2020; Lee et al., 2018).
Mast cells are an important part of the mucosal immune system in the lung and can secrete high levels of cytokines and chemokines, responding to IFV and bacterial infection (Hu et al., 2012; Malaviya et al., 1996). Post-influenza S. aureus infection can inhibit autophagy and facilitate the secretion of inflammatory mediators in mast cells by activating the PI3K/Akt signaling pathway (Tang et al., 2023) (Figure 2A). Suppressing the PI3K/Akt signaling pathway can inhibit the production of inflammatory mediators and alleviate ALI caused by secondary S. pneumoniae or S. aureus infection following IFV infection (Tang et al., 2023; Yang et al., 2019).
3.2.2 Pyroptosis and necroptosis
Pyroptosis is an immunogenic form of cell death and can assist in eliminating pathogens. But it can also drive inflammatory damage by releasing inflammatory mediators (Corry et al., 2022; Zheng et al., 2023) (Figure 2A). Pyroptosis is characterized by inflammasome and caspase activation. Inflammasome activation including NLRP3/ASC, Pyrin/ASC, AIM2/ASC, NLRC4 and NLRP1, can contribute to caspase-1 activation that cleaves gasdermin D (GSDMD), exposes the N domain of GSDMD and results in pore formation (Zhao et al., 2011; Bai et al., 2025). In addition to classic inflammasome-dependent pyroptosis, caspases-4/5/8/11 can also directly cleave GSDMD through non-canonical pathways and initiate pyroptosis (Shi et al., 2015; Zanoni et al., 2016; Broz et al., 2019). TLR2-MYD88-NLRP3 axis mediates IL-1β production during post-influenza S. pneumoniae infection (Rodriguez et al., 2019). NLRP3 activation can increase bacterial burden and inflammatory response, which is associated with poor outcome in mice coinfected with IFV and bacteria (Shi et al., 2020). While ASC activation results in increased inflammation and mortality, but contributes to bacteria clearance during post-influenza S. aureus infection (Robinson et al., 2018a). The E3 ubiquitin ligase NEDD4 can promote GSDMD-mediated pyroptosis and result in poor outcome during post-influenza S. pneumoniae infection (You et al., 2023). IL-4 exerts protective effects against post-influenza S. pneumoniae infection by suppressing GSDMD-induced pyroptosis (Peng et al., 2021).
Necroptosis is a form of inflammatory cell death that aggregates tissue damage by releasing DAMPs and amplifying inflammation (Pasparakis and Vandenabeele, 2015) (Figure 2A). Necroptosis is initiated by death receptors (e.g., TNFR1) or pathogen sensors (e.g., ZBP1), which subsequently triggers RIPK1-RIPK3-MLKL cascade. MLKL forms pore-like structures, therefore disrupting membrane integrity and resulting in osmotic cell lysis, accompanied by the release of DAMPs (Yuan and Ofengeim, 2023). Pore-forming toxins (PFTs), such as pneumolysin produced by S. pneumoniae, are known factors to induce necroptosis of lung epithelial cells (LECs) and are required for inducing necroptosis during post-influenza S. pneumoniae infection. IFV infection causes residual oxidative stress that enhances susceptibility to bacterial-toxin-mediated necroptosis (Gonzalez-Juarbe et al., 2020). IFV infection can also potentiate S. pneumoniae infiltration in the heart, and induce oxidative stress to enhance bacterial toxin-induced necrotic cell death and cause proteomic remodeling of the heart (Platt et al., 2022). In addition, IFV infection can activate PPARα signaling, mediate RIPK3-dependent necroptosis and result in increased mortality during post-influenza S. aureus infection (Tam et al., 2020). Large quantities of Z-RNAs, type I interferon and dsDNA are produced during post-influenza bacterial infection. All of these factors can induce ZBP1-mediated necroptosis. Whether ZBP1-mediated necroptosis can result in increased mortality during post-influenza bacterial infection remains unknown.
4 Treatment options
As our understanding of the molecular mechanisms underlying virus-bacteria coinfection deepens, these discoveries present opportunities for developing novel therapeutic approaches and preventive strategies. Significant progress has been made in multiple control strategies targeting either pathogens or hosts.
4.1 Vaccination
Influenza vaccination is the most effective strategy for preventing IFV infections (Minozzi et al., 2022). Vaccines against IFV can effectively reduce influenza-associated secondary bacterial infections as well. Live influenza vaccine can protect mice from post-influenza S. pneumoniae infection (Desheva et al., 2022). However, live influenza vaccine could increase the risk of bacterial colonization. Multiple clinical studies indicate that live influenza vaccines can increase nasopharyngeal pneumococcal carriage and density (Peno et al., 2021; Peno et al., 2025; Glennie et al., 2016). Live attenuated influenza vaccines can also enhance colonization of S. pneumoniae and S. aureus in mice (Mina et al., 2014). Vaccines against IFV can impair antibacterial immune response, which is similar to prior IFV infection (Jochems et al., 2018).
Vaccination with the antigen of S. pneumoniae or P. aeruginosa such as PspA or PcrV protein can also exert protective effects against post-influenza bacterial infection in animal models (Majumder et al., 2024; Wu et al., 2023). Vaccination with PspA protein can significantly increase the number of AMs and promote bacterial clearance in the lung (Majumder et al., 2024). Prior S. pneumoniae infection can protect against different serotypes of S. pneumoniae infection following IFV infection by inducing a cross-reactive Th17 response (Li et al., 2023). Vaccination with a conserved NTHi antigen, protein 0529, can protect mice from post-influenza NTHi infection by increasing Th17 response (Zhang et al., 2023). In addition, virus-bacterial vaccines can also provide protection against post-influenza bacterial infection in a mouse model (Desheva et al., 2022; Li et al., 2023). Whole-inactivated influenza A and pneumococcal vaccines can increase IFV-specific CD8+ T cell response in the lung (David et al., 2019). These bacterial vaccines can effectively activate an antibacterial immune response. However, most of these vaccines target single bacterial infections and are not broad-spectrum.
4.2 Antivirals and antibiotics
Antivirals represent an essential strategy for the prevention and treatment of influenza. Antivirals can decrease influenza-associated morbidity, complications and mortality (Arnold et al., 2025; Uyeki et al., 2019). Preceding IFV infection in the lung is a major risk factor for secondary bacterial infection. It destroys the respiratory barriers, increases bacterial adhesion and suppresses antibacterial immune response facilitating bacterial infection to occur. Timely antiviral treatment can effectively prevent secondary bacterial infections and also benefits severe influenza complicated with bacterial infection (Uyeki et al., 2019; Wang et al., 2023). Antivirals, such as peramivir and oseltamivir, can reduce the incidence of secondary bacterial infection, mitigate virus-induced injury and protect mice from post-influenza bacterial infection (Lei et al., 2025; Zhao et al., 2021; Onishi et al., 2015). At present, more and more new antivirals such as suraxavir marboxil and onradivir, are available for the treatment of IFV infection (Wang et al., 2025; Yang et al., 2024). Additionally, neutralizing HA antibodies can also alleviate ALI and increase the survival rate of mice during post-influenza bacterial infection (van Someren Gréve et al., 2018; Robinson et al., 2019). Antivirals can decrease viral load and alleviate virus-induced injury, thus reducing the incidence of secondary bacterial infection. Therefore, timely antiviral treatment is needed to control viral replication.
Secondary bacterial infection can amplify overactivated inflammatory response and further ruin the normal lung tissues. The timely antibiotic treatment against susceptible bacteria benefits flu patients with bacterial infection (Wang et al., 2023). Antibiotic treatment alone can reduce bacterial load and alleviate lung damage caused by post-influenza bacterial infection (Verma et al., 2019; Song et al., 2022). But a study indicates that penicillin G treatment alone cannot increase the survival rate of mice during post-influenza s. aureus infection (Song et al., 2022). Antibiotic treatment can effectively inhibit bacterial load, but it is insufficient to control the replication of IFV and the overactivated inflammatory response.
4.3 Anti-adhesion
Anti-adhesion therapy can prevent microbial adhesion to cells or tissues, and has become a promising strategy in infectious diseases (Asadi et al., 2019). IFV infection can upregulate the expression of multiple adhesion receptors, promoting bacterial adherence. Inhibiting the expression of adhesion receptors can effectively disrupt bacterial adherence (Vimalanathan et al., 2017; Ishikawa et al., 2022). WEB-2086, an antagonist of PAFr, can decrease the adhesion of S. pneumoniae and NTHi to cigarette smoke extract-treated bronchial epithelial cells (Shukla et al., 2016). CV-3988 is also a specific antagonist targeting PAFr. It can inhibit mild steel welding fumes-mediated pneumococcal adhesion in vitro and in vivo. Some traditional Chinese medicines such as Liu Shen Wan and Lianhuaqingwen can reduce S. aureus adherence to IFV-infected respiratory epithelial cells by downregulating the expression of CEACAM1, ICAM-1 and integrin-α5, therefore protecting mice from post-influenza S. aureus infection (Zhao et al., 2021; Song et al., 2022; Du et al., 2021). Targeting adhesion receptors may be a new potential therapy in preventing and treating influenza bacterial coinfection. However, bacteria utilize multiple receptors to infect hosts, rendering single anti-adhesion therapies ineffective. In addition, anti-adhesion agents must be administered during the early stages of infection or preventatively. In clinical practice, this therapeutic window is frequently missed due to delayed presentation.
4.4 Neutralizing antibodies against proinflammatory cytokines
Uncontrolled pathogens can induce the overactivation of inflammatory response and contribute to severe organ injury. In addition to controlling the replication of pathogens, it’s important to inhibit the overactivated inflammatory response during post-influenza bacterial infection (Damjanovic et al., 2013; Tavares et al., 2017). Anti-IL-6 and anti-IL-1 antibodies have been widely used for the treatment of COVID-19 (Batista and Foti, 2021). Some neutralizing antibodies such as tocilizumab and anakinra, can inhibit systemic inflammation and decrease the risk of invasive mechanical ventilation or death in patients with COVID-19 (Guaraldi et al., 2020; Kyriakoulis et al., 2021). Single IFN-γ neutralization can reduce local bacterial load in the lung. Concomitant neutralization of IFN-γ and IL-6 can inhibit bacterial load, reduce the secretion of cytokines and alleviate the degree of pneumonia as well as bacteremia during post-influenza S. pneumoniae infection (Sharma-Chawla et al., 2019). The use of such antibodies carries a risk of immunosuppression, potentially weakening the body’s ability to clear bacteria and increasing the risk of uncontrolled infection. Furthermore, the heterogeneity of patient immune responses complicates distinguishing individuals who may benefit from cytokine blockade versus those who may be harmed by it, thereby complicating clinical trial design and patient stratification.
4.5 Other host-directed therapies
Therapies that modulate the overactivated inflammatory response, can prevent the emergence of severe immunopathology in infectious disease. Anti-C5aR treatment can significantly increase the survival rates of mice and mitigate lung injury by reducing the release of inflammatory mediators such as IFN-γ, TNF-α, IL-6 and IL-8 (Jia et al., 2023). Inhibition the activation of NLRP3 by MCC950 can decrease the secretion of G-CSF, MIP-1α, KC and IL-1β during post-influenza S. aureus infection. However, MCC950 cannot increase the survival rate of mice (Robinson et al., 2018a). DNase I can mitigate lung injury by reducing the expression of MCP-1, IL-1β and ICAM-1 in coinfected animals (Yi et al., 2022).
4.6 Traditional Chinese medicines
Traditional Chinese medicines have been recommended for the treatment of IFV infection since 2009. A Chinese formula contains various active ingredients and plays a versatile role in the treatment of IFV infection. Some of them possess antiviral, antibacterial and anti-inflammatory effects (Zhao et al., 2023; Chen and Wang, 2023; Yang et al., 2024; Zhang J. et al., 2025; Zhang B. et al., 2025). Some Chinese formulas such as Liu Shen Wan, Lianhuaqingwen capsule and Jing-Yin-Gu-Biao formula, have been found to protect mice from post-influenza bacterial infection (Lei et al., 2025; Zhao et al., 2021; Song et al., 2022; Du et al., 2021). Liu Shen Wan and Lianhuaqingwen capsule can inhibit replication of IFV, virus-induced overactivated inflammatory response and the expression of adhesion receptor (Zhao et al., 2021; Du et al., 2021; Ma et al., 2020; Yang et al., 2020). Jing-Yin-Gu-Biao formula can suppress the overactivation of neutrophils and NETosis during post-influenza S. aureus infection (Lei et al., 2025). A homogeneous polysaccharide from Houttuynia cordata can protect mice from post-influenza S. aureus infection, which can reduce excessive intestinal complement activation (C3a and C5a) and block the NLRP3 pathway, helping regulate the Treg/Th17 cell balance in the gut-lung axis (Li et al., 2025). Traditional Chinese medicines are also promising optional agents for the treatment of post-influenza bacterial infection.
4.7 Combination therapy
It is uncertain whether the use of antiviral drugs alone can reduce mortality in patients with severe influenza due to the lack of data from clinical trials (Gao et al., 2024). Multiple combination strategies such as antivirals + antivirals, antivirals + monoclonal antibodies, antivirals + anti-inflammatory agents, antivirals + antibiotics, have been considered for the treatment of severe influenza (Koszalka et al., 2022; Zhou et al., 2025; Lim et al., 2020; Lee et al., 2021). Severe influenza especially coinfected with IFV and bacteria is caused by multiple pathogenic factors and should be considered combination therapy. The combination of antibiotics and oseltamivir can inhibit inflammatory response, shorten the length of hospital stay and decrease the incidence of ICU admission and secondary bacterial infections (Ishaqui et al., 2021; Ishaqui et al., 2020; Lee et al., 2017). Clarithromycin-Naproxen-Oseltamivir combination can also decrease the mortality and length of hospitalization in hospitalized patients with IFV infection (Lee et al., 2021; Hung et al., 2017). The combination of antibiotics and clindamycin can increase the survival rate of mice during post-influenza S. pneumoniae infection (Li et al., 2018). Combined oseltamivir and traditional Chinese medicine are superior to oseltamivir treatment alone in inhibiting overactivated inflammatory response and alleviating ALI during post-influenza bacterial infection (Lei et al., 2025). Combination therapy may be the most important strategy for the treatment of coinfection with IFV and bacteria.
5 Future directions
This review summarizes epidemiology, pathogenesis and therapeutic strategies during post-influenza bacterial infection. To advance this field, future research must address several critical topics. First, several mechanisms that may play an important role in the co-pathogenesis of influenza-bacterial infections were summarized in this study. Our findings are constrained by the scarcity of large-scale human transcriptomic datasets. A crucial next step is the generation of transcriptomic data from diverse cohorts to enhance the generalizability of findings from the preclinical study. Second, clinical studies have demonstrated that combination therapy holds significant potential for treating severe influenza. Rational combination therapies concurrently addressing pathogen clearance and host immunomodulation show superior potential for managing coinfection complexity. The promising benefits of combination therapies in influenza-bacterial infection have not been rigorously tested in clinical settings. Large-scale, multicenter clinical trials are required to ultimately evaluate the safety and synergistic potential of combination therapies.
6 Conclusion
IFV infection severely threatens global health, with secondary bacterial coinfection dramatically amplifying morbidity and mortality through synergistic pathogenesis. Crucially, IFV establishes a permissive environment for bacterial invasion by disrupting respiratory epithelial integrity and suppressing innate antimicrobial immunity, underscoring the imperative for prompting viral control to prevent secondary complications. Once coinfection ensues, dysregulated host-pathogen interactions trigger hyperinflammation wherein recruited neutrophils and macrophages paradoxically become key drivers of immunopathology, which fuel tissue damage despite their defensive roles. This mechanistic insight necessitates therapeutic strategies beyond conventional antimicrobials: modulating pathological immune responses, particularly targeting the neutrophil-macrophage axis and associated hyperinflammatory cascades, represents a promising host-directed approach to mitigate organ damage.
Author contributions
BL: Conceptualization, Data curation, Methodology, Investigation, Writing – original draft. SW: Data curation, Conceptualization, Writing – original draft, Investigation, Formal analysis, Validation. LY: Writing – review & editing, Supervision, Validation. QM: Validation, Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Administration of Traditional Chinese Medicine (ZYYCXTD-D-202406), Natural Science Foundation of China (82474155, 82174053), the Young Top Talent of Science and Technology Innovation Department of Guangdong Province (2021TQ060189), the China Postdoctoral Science Foundation (No. 2024M760661), the Science and Technology Project of Guizhou Province (No. ZK [2022]581), and Guangzhou Baiyun Science and Technology Special Project (2024-YL-018).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ardain, A., Marakalala, M. J., and Leslie, A. (2020). Tissue-resident innate immunity in the lung. Immunology 159, 245–256. doi: 10.1111/imm.13143
Arnold, S. M., Klaus, K., Corrado, B., Bin, C., Herman Avner, C., Emily, G., et al. (2025). Efficacy of baloxavir treatment in preventing transmission of influenza. N. Engl. J. Med. 392, 1582–1593. doi: 10.1056/NEJMoa2413156
Arranz-Herrero, J., Presa, J., Rius-Rocabert, S., Utrero-Rico, A., Arranz-Arija, J. Á., Lalueza, A., et al. (2023). Determinants of poor clinical outcome in patients with influenza pneumonia: A systematic review and meta-analysis. Int. J. Infect. Dis. 131, 173–179. doi: 10.1016/j.ijid.2023.04.003
Asadi, A., Razavi, S., Talebi, M., and Gholami, M. (2019). A review on anti-adhesion therapies of bacterial diseases. Infection 47, 13–23. doi: 10.1007/s15010-018-1222-5
Bai, Y., Pan, Y., and Liu, X. (2025). Mechanistic insights into gasdermin-mediated pyroptosis. Nat. Rev. Mol. Cell Biol. 26, 501–521. doi: 10.1038/s41580-025-00837-0
Bai, X., Yang, W., Li, H., Zhao, Y., Fan, W., Zhang, H., et al. (2022). Cyclosporine A regulates influenza A virus-induced macrophages polarization and inflammatory responses by targeting Cyclophilin A. Front. Immunol. 13:861292. doi: 10.3389/fimmu.2022.861292
Bal, A., Casalegno, J. S., Melenotte, C., Daviet, F., Ninove, L., Edouard, S., et al. (2020). Influenza-induced acute respiratory distress syndrome during the 2010-2016 seasons: bacterial co-infections and outcomes by virus type and subtype. Clin. Microbiol. Infect. 26, 947.e1–947.e4. doi: 10.1016/j.cmi.2020.03.010
Bansal, S., Yajjala, V. K., Bauer, C., and Sun, K. (2018). IL-1 signaling prevents alveolar macrophage depletion during influenza and Streptococcus pneumoniae coinfection. J. Immunol. 200, 1425–1433. doi: 10.4049/jimmunol.1700210
Barker, K. A., Etesami, N. S., Shenoy, A. T., Arafa, E. I., Lyon de Ana, C., Smith, N. M., et al. (2021). Lung-resident memory B cells protect against bacterial pneumonia. J. Clin. Invest. 131:e141810. doi: 10.1172/JCI141810
Barthelemy, A., Ivanov, S., Fontaine, J., Soulard, D., Bouabe, H., Paget, C., et al. (2017). Influenza A virus-induced release of interleukin-10 inhibits the anti-microbial activities of invariant natural killer T cells during invasive pneumococcal superinfection. Mucosal Immunol. 10, 460–469. doi: 10.1038/mi.2016.49
Barthelemy, A., Ivanov, S., Hassane, M., Fontaine, J., Heurtault, B., Frisch, B., et al. (2016). Exogenous activation of invariant natural killer T cells by α-Galactosylceramide reduces pneumococcal outgrowth and dissemination Postinfluenza. MBio 7, e01440–e01416. doi: 10.1128/mBio.01440-16
Bartley, P. S., Deshpande, A., Yu, P. C., Klompas, M., Haessler, S. D., Imrey, P. B., et al. (2022). Bacterial coinfection in influenza pneumonia: rates, pathogens, and outcomes. Infect. Control Hosp. Epidemiol. 43, 212–217. doi: 10.1017/ice.2021.96
Batista, C. M., and Foti, L. (2021). Anti-SARS-CoV-2 and anti-cytokine storm neutralizing antibody therapies against COVID-19: update, challenges, and perspectives. Int. Immunopharmacol. 99:108036. doi: 10.1016/j.intimp.2021.108036
Berg, J., Zscheppang, K., Fatykhova, D., Tönnies, M., Bauer, T. T., Schneider, P., et al. (2017). Tyk2 as a target for immune regulation in human viral/bacterial pneumonia. Eur. Respir. J. 50:1601953. doi: 10.1183/13993003.01953-2016
Beshara, R., Sencio, V., Soulard, D., Barthélémy, A., Fontaine, J., Pinteau, T., et al. (2018). Alteration of Flt3-ligand-dependent de novo generation of conventional dendritic cells during influenza infection contributes to respiratory bacterial superinfection. PLoS Pathog. 14:e1007360. doi: 10.1371/journal.ppat.1007360
Braverman, J., Monk, I. R., Ge, C., Westall, G. P., Stinear, T. P., and Wakim, L. M. (2022). Staphylococcus aureus specific lung resident memory CD4(+) Th1 cells attenuate the severity of influenza virus induced secondary bacterial pneumonia. Mucosal Immunol. 15, 783–796. doi: 10.1038/s41385-022-00529-4
Brown, M., Gerrard, J., McDougall, C., MacPhail, J., and Williams, O. (2024). Purpura fulminans in young women with influenza and co-infections. Lancet 403, 2290–2291. doi: 10.1016/S0140-6736(24)00137-5
Broz, P., Pelegrín, P., and Shao, F. (2019). The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 20, 143–157. doi: 10.1038/s41577-019-0228-2
Burn, G. L., Foti, A., Marsman, G., Patel, D. F., and Zychlinsky, A. (2021). The neutrophil. Immunity 54, 1377–1391. doi: 10.1016/j.immuni.2021.06.006
Cao, J., Wang, D., Xu, F., Gong, Y., Wang, H., Song, Z., et al. (2014). Activation of IL-27 signalling promotes development of postinfluenza pneumococcal pneumonia. EMBO Mol. Med. 6, 120–140. doi: 10.1002/emmm.201302890
Casanova, J. L., MacMicking, J. D., and Nathan, C. F. (2024). Interferon-γ and infectious diseases: lessons and prospects. Science (New York, N.Y.) 384:eadl2016. doi: 10.1126/science.adl2016
Centers for Disease Control and Prevention (CDC) (2009). Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1) - United States, May-august 2009. MMWR Morb. Mortal Wkly. Rep. 58, 1071–1074.
Chegini, Z., Noei, M., Hemmati, J., Arabestani, M. R., and Shariati, A. (2023). The destruction of mucosal barriers, epithelial remodeling, and impaired mucociliary clearance: possible pathogenic mechanisms of Pseudomonas aeruginosa and Staphylococcus aureus in chronic rhinosinusitis. Cell Commun. Signal. 21:306. doi: 10.1186/s12964-023-01347-2
Chen, B., Chen, C., Lu, F., Wang, X., Zhang, X., Wang, Z., et al. (2025). The role of TLR4 in lung epithelial cell injury caused by influenza virus combined with Staphylococcus aureus. Microorganisms 13:1201. doi: 10.3390/microorganisms13061201
Chen, Y. Y., Huang, C. T., Li, S. W., Pan, Y. J., Lin, T. L., Huang, Y. Y., et al. (2021). Bacterial factors required for Streptococcus pneumoniae coinfection with influenza A virus. J. Biomed. Sci. 28:60. doi: 10.1186/s12929-021-00756-0
Chen, Y., Li, F., Hua, M., Liang, M., and Song, C. (2023). Role of GM-CSF in lung balance and disease. Front. Immunol. 14:1158859. doi: 10.3389/fimmu.2023.1158859
Chen, Q., Lin, Y., Wang, K., Li, J., Li, P., and Song, H. (2025). Lactobacillus murinus reduces susceptibility to secondary MRSA infection in IAV-infected mice through promoting a T cell-independent IgA response. Microorganisms 13:1709. doi: 10.3390/microorganisms13071709
Chen, S., Saeed, A., Liu, Q., Jiang, Q., Xu, H., Xiao, G. G., et al. (2023). Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 8:207. doi: 10.1038/s41392-023-01452-1
Chen, X., and Wang, M. (2023). Traditional Chinese medicine during the COVID-19 pandemic: recent successes and future perspectives. Acupunct. Herb. Med. 3, 357–359. doi: 10.1097/HM9.0000000000000084
Cheng, P., Liu, T., Zhou, W. Y., Zhuang, Y., Peng, L. S., Zhang, J. Y., et al. (2012). Role of gamma-delta T cells in host response against Staphylococcus aureus-induced pneumonia. BMC Immunol. 13:38. doi: 10.1186/1471-2172-13-38
Cohn, O., Yankovitz, G., Mandelboim, M., Peshes-Yaloz, N., Brandes, R., Bacharach, E., et al. (2024). The host transcriptional response to superinfection by influenza A virus and Streptococcus pneumoniae. mSystems 9:e0104823. doi: 10.1128/msystems.01048-23
Colicchia, M., Schrottmaier, W. C., Perrella, G., Reyat, J. S., Begum, J., Slater, A., et al. (2022). S100A8/A9 drives the formation of procoagulant platelets through GPIbα. Blood 140, 2626–2643. doi: 10.1182/blood.2021014966
Corry, J., Kettenburg, G., Upadhyay, A. A., Wallace, M., Marti, M. M., Wonderlich, E. R., et al. (2022). Infiltration of inflammatory macrophages and neutrophils and widespread pyroptosis in lung drive influenza lethality in nonhuman primates. PLoS Pathog. 18:e1010395. doi: 10.1371/journal.ppat.1010395
Crosby, C. M., and Kronenberg, M. (2018). Tissue-specific functions of invariant natural killer T cells. Nat. Rev. Immunol. 18, 559–574. doi: 10.1038/s41577-018-0034-2
Damjanovic, D., Lai, R., Jeyanathan, M., Hogaboam, C. M., and Xing, Z. (2013). Marked improvement of severe lung immunopathology by influenza-associated pneumococcal superinfection requires the control of both bacterial replication and host immune responses. Am. J. Pathol. 183, 868–880. doi: 10.1016/j.ajpath.2013.05.016
David, S. C., Norton, T., Tyllis, T., Wilson, J. J., Singleton, E. V., Laan, Z., et al. (2019). Direct interaction of whole-inactivated influenza A and pneumococcal vaccines enhances influenza-specific immunity. Nat. Microbiol. 4, 1316–1327. doi: 10.1038/s41564-019-0443-4
Deinhardt-Emmer, S., Rennert, K., Schicke, E., Cseresnyés, Z., Windolph, M., Nietzsche, S., et al. (2020). Co-infection with Staphylococcus aureus after primary influenza virus infection leads to damage of the endothelium in a human alveolus-on-a-chip model. Biofabrication 12:025012. doi: 10.1088/1758-5090/ab7073
Desheva, Y., Leontieva, G., Kramskaya, T., Losev, I., Petkova, N., Rekstin, A., et al. (2022). Associated virus-bacterial vaccine based on seasonal LAIV and S. pneumoniae chimeric peptide provide protection against post-influenza pneumococcal infection in mouse model. Virulence 13, 558–568. doi: 10.1080/21505594.2022.2049496
Desheva, Y., Leontieva, G., Kramskaya, T., Losev, I., Rekstin, A., Petkova, N., et al. (2022). Live influenza vaccine provides early protection against homologous and heterologous influenza and May prevent post-influenza pneumococcal infections in mice. Microorganisms 10:1150. doi: 10.3390/microorganisms10061150
Du, Q., Huang, W., Zhao, J., Zeng, J., Zhang, W., Huang, X., et al. (2021). Lianhuaqingwen capsule inhibits influenza-induced bacterial adhesion to respiratory epithelial cells through down-regulation of cell adhesion molecules. J. Ethnopharmacol. 280:114128. doi: 10.1016/j.jep.2021.114128
Ellis, G. T., Davidson, S., Crotta, S., Branzk, N., Papayannopoulos, V., and Wack, A. (2015). TRAIL+ monocytes and monocyte-related cells cause lung damage and thereby increase susceptibility to influenza-Streptococcus pneumoniae coinfection. EMBO Rep. 16, 1203–1218. doi: 10.15252/embr.201540473
Estenssoro, E., Ríos, F. G., Apezteguía, C., Reina, R., Neira, J., Ceraso, D. H., et al. (2010). Pandemic 2009 influenza A in Argentina: a study of 337 patients on mechanical ventilation. Am. J. Respir. Crit. Care Med. 182, 41–48. doi: 10.1164/201001-0037OC
Galvão, I., Tavares, L. P., Corrêa, R. O., Fachi, J. L., Rocha, V. M., Rungue, M., et al. (2018). The metabolic sensor GPR43 receptor plays a role in the control of Klebsiella pneumoniae infection in the lung. Front. Immunol. 9:142. doi: 10.3389/fimmu.2018.00142
Gao, Y., Guyatt, G., Uyeki, T. M., Liu, M., Chen, Y., Zhao, Y., et al. (2024). Antivirals for treatment of severe influenza: a systematic review and network meta-analysis of randomised controlled trials. Lancet 404, 753–763. doi: 10.1016/S0140-6736(24)01307-2
Geitani, R., Moubareck, C. A., Xu, Z., Karam Sarkis, D., and Touqui, L. (2020). Expression and roles of antimicrobial peptides in innate defense of airway mucosa: potential implication in cystic fibrosis. Front. Immunol. 11:1198. doi: 10.3389/fimmu.2020.01198
Ghoneim, H. E., Thomas, P. G., and McCullers, J. A. (2013). Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections. J. Immunol. 191, 1250–1259. doi: 10.4049/jimmunol.1300014
Glennie, S., Gritzfeld, J. F., Pennington, S. H., Garner-Jones, M., Coombes, N., Hopkins, M. J., et al. (2016). Modulation of nasopharyngeal innate defenses by viral coinfection predisposes individuals to experimental pneumococcal carriage. Mucosal Immunol. 9, 56–67. doi: 10.1038/mi.2015.35
Golebiewski, L., Liu, H., Javier, R. T., and Rice, A. P. (2011). The avian influenza virus NS1 ESEV PDZ binding motif associates with Dlg1 and scribble to disrupt cellular tight junctions. J. Virol. 85, 10639–10648. doi: 10.1128/JVI.05070-11
Gonzalez-Juarbe, N., Riegler, A. N., Jureka, A. S., Gilley, R. P., Brand, J. D., Trombley, J. E., et al. (2020). Influenza-induced oxidative stress sensitizes lung cells to bacterial-toxin-mediated necroptosis. Cell Rep. 32:108062. doi: 10.1016/j.celrep.2020.108062
Gopal, R., Lee, B., McHugh, K. J., Rich, H. E., Ramanan, K., Mandalapu, S., et al. (2018). STAT2 signaling regulates macrophage phenotype during influenza and bacterial super-infection. Front. Immunol. 9:2151. doi: 10.3389/fimmu.2018.02151
Gou, X., Yuan, J., Wang, H., Wang, X., Xiao, J., Chen, J., et al. (2019). IL-6 during influenza-Streptococcus pneumoniae co-infected pneumonia-a protector. Front. Immunol. 10:3102. doi: 10.3389/fimmu.2019.03102
Grunwell, J. R., Giacalone, V. D., Stephenson, S., Margaroli, C., Dobosh, B. S., Brown, M. R., et al. (2019). Neutrophil dysfunction in the Airways of Children with acute respiratory failure due to lower respiratory tract viral and bacterial coinfections. Sci. Rep. 9:2874. doi: 10.1038/s41598-019-39726-w
Gu, S., Xiao, W., Yu, Z., Xiao, J., Sun, M., Zhang, L., et al. (2025). Single-cell RNA-seq reveals the immune response of co-infection with Streptococcus pneumoniae after influenza A virus by a lung-on-chip: the molecular structure and mechanism of tight junction protein ZO-1. Int. J. Biol. Macromol. 306:141815. doi: 10.1016/j.ijbiomac.2025.141815
Guaraldi, G., Meschiari, M., Cozzi-Lepri, A., Milic, J., Tonelli, R., Menozzi, M., et al. (2020). Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2, e474–e484. doi: 10.1016/S2665-9913(20)30173-9
Gurczynski, S. J., Nathani, N., Warheit-Niemi, H. I., Hult, E. M., Podsiad, A., Deng, J., et al. (2019). CCR2 mediates increased susceptibility to post-H1N1 bacterial pneumonia by limiting dendritic cell induction of IL-17. Mucosal Immunol. 12, 518–530. doi: 10.1038/s41385-018-0106-4
Herrera, A. L., Potts, R., Huber, V. C., and Chaussee, M. S. (2023). Influenza enhances host susceptibility to non-pulmonary invasive Streptococcus pyogenes infections. Virulence 14:2265063. doi: 10.1080/21505594.2023.2265063
Ho, P. L., Loughran, S. T., Power, P. A., Maguire, P. T., McQuaid, S. L., Buchanan, P. J., et al. (2018). Influenza infection directly alters innate IL-23 and IL-12p70 and subsequent IL-17A and IFN-γ responses to pneumococcus in vitro in human monocytes. PLoS One 13:e0203521. doi: 10.1371/journal.pone.0203521
Hu, Y., Jin, Y., Han, D., Zhang, G., Cao, S., Xie, J., et al. (2012). Mast cell-induced lung injury in mice infected with H5N1 influenza virus. J. Virol. 86, 3347–3356. doi: 10.1128/JVI.06053-11
Huang, H., Chen, H. W., Evankovich, J., Yan, W., Rosborough, B. R., Nace, G. W., et al. (2013). Histones activate the NLRP3 inflammasome in Kupffer cells during sterile inflammatory liver injury. J. Immunol. 191, 2665–2679. doi: 10.4049/jimmunol.1202733
Huang, Z. B., Hu, Z., Lu, C. X., Luo, S. D., Chen, Y., Zhou, Z. P., et al. (2022). Gut microbiota-derived indole 3-propionic acid partially activates aryl hydrocarbon receptor to promote macrophage phagocytosis and attenuate septic injury. Front. Cell. Infect. Microbiol. 12:1015386. doi: 10.3389/fcimb.2022.1015386
Hung, I. F. N., To, K. K. W., Chan, J. F. W., Cheng, V. C. C., Liu, K. S. H., Tam, A., et al. (2017). Efficacy of clarithromycin-naproxen-oseltamivir combination in the treatment of patients hospitalized for influenza A(H3N2) infection: an open-label randomized, controlled, phase IIb/III trial. Chest 151, 1069–1080. doi: 10.1016/j.chest.2016.11.012
Huo, C., Li, Y., Tang, Y., Su, R., Xu, J., Dong, H., et al. (2025). Vital role of PINK1/Parkin-mediated Mitophagy of pulmonary epithelial cells in severe pneumonia induced by IAV and secondary Staphylococcus aureus infection. Int. J. Mol. Sci. 26:4162. doi: 10.3390/ijms26094162
Ishaqui, A., Hayat Khan, A., Sulaiman, S. A. S., Taher Alsultan, M., and Khan, I. (2021). Comparative efficacy assessment of antiviral alone and antiviral-antibiotic combination in prevention of influenza-B infection associated complications. Expert Rev. Anti-Infect. Ther. 19, 1165–1173. doi: 10.1080/14787210.2021.1889369
Ishaqui, A. A., Khan, A. H., Sulaiman, S. A. S., Alsultan, M. T., Khan, I., and Naqvi, A. A. (2020). Assessment of efficacy of Oseltamivir-azithromycin combination therapy in prevention of influenza-A (H1N1)pdm09 infection complications and rapidity of symptoms relief. Expert Rev. Respir. Med. 14, 533–541. doi: 10.1080/17476348.2020.1730180
Ishikawa, H., Fukui, T., Ino, S., Sasaki, H., Awano, N., Kohda, C., et al. (2016). Influenza virus infection causes neutrophil dysfunction through reduced G-CSF production and an increased risk of secondary bacteria infection in the lung. Virology 499, 23–29. doi: 10.1016/j.virol.2016.08.025
Ishikawa, H., Kuno, Y., Kohda, C., Sasaki, H., Nagashima, R., and Iyoda, M. (2022). Exopolysaccharides from Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 prevent influenza virus infection and attenuate secondary bacterial infection risk. Lett. Appl. Microbiol. 74, 632–639. doi: 10.1111/lam.13649
Ivanov, S., Renneson, J., Fontaine, J., Barthelemy, A., Paget, C., Fernandez, E. M., et al. (2013). Interleukin-22 reduces lung inflammation during influenza A virus infection and protects against secondary bacterial infection. J. Virol. 87, 6911–6924. doi: 10.1128/JVI.02943-12
Jia, L., Luo, H., Li, L., Wang, M., Liu, J., Liang, Y., et al. (2023). Targeting complement hyperactivation: a novel therapeutic approach for severe pneumonia induced by influenza virus/staphylococcus aureus coinfection. Signal Transduct. Target. Ther. 8:467. doi: 10.1038/s41392-023-01714-y
Jia, L., Zhao, J., Yang, C., Liang, Y., Long, P., Liu, X., et al. (2018). Severe pneumonia caused by coinfection with influenza virus followed by methicillin-resistant Staphylococcus aureus induces higher mortality in mice. Front. Immunol. 9:3189. doi: 10.3389/fimmu.2018.03189
Jie, F., Wu, X., Zhang, F., Li, J., Liu, Z., He, Y., et al. (2023). Influenza virus infection increases host susceptibility to secondary infection with Pseudomonas aeruginosa, and this is attributed to neutrophil dysfunction through reduced myeloperoxidase activity. Microbiol. Spectr. 11:e0365522. doi: 10.1128/spectrum.03655-22
Jochems, S. P., Marcon, F., Carniel, B. F., Holloway, M., Mitsi, E., Smith, E., et al. (2018). Inflammation induced by influenza virus impairs human innate immune control of pneumococcus. Nat. Immunol. 19, 1299–1308. doi: 10.1038/s41590-018-0231-y
Karwelat, D., Schmeck, B., Ringel, M., Benedikter, B. J., Hübner, K., Beinborn, I., et al. (2020). Influenza virus-mediated suppression of bronchial Chitinase-3-like 1 secretion promotes secondary pneumococcal infection. FASEB J. 34, 16432–16448. doi: 10.1096/fj.201902988RR
Katsoulis, O., Toussaint, M., Jackson, M. M., Mallia, P., Footitt, J., Mincham, K. T., et al. (2024). Neutrophil extracellular traps promote immunopathogenesis of virus-induced COPD exacerbations. Nat. Commun. 15:5766. doi: 10.1038/s41467-024-50197-0
Klein, E. Y., Monteforte, B., Gupta, A., Jiang, W., May, L., Hsieh, Y. H., et al. (2016). The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir. Viruses 10, 394–403. doi: 10.1111/irv.12398
Klemm, C., Bruchhagen, C., van Krüchten, A., Niemann, S., Löffler, B., Peters, G., et al. (2017). Mitogen-activated protein kinases (MAPKs) regulate IL-6 over-production during concomitant influenza virus and Staphylococcus aureus infection. Sci. Rep. 7:42473. doi: 10.1038/srep42473
Koszalka, P., Subbarao, K., and Baz, M. (2022). Preclinical and clinical developments for combination treatment of influenza. PLoS Pathog. 18:e1010481. doi: 10.1371/journal.ppat.1010481
Kyriakoulis, K. G., Kollias, A., Poulakou, G., Kyriakoulis, I. G., Trontzas, I. P., Charpidou, A., et al. (2021). The effect of Anakinra in hospitalized patients with COVID-19: an updated systematic review and Meta-analysis. J. Clin. Med. 10:4462. doi: 10.3390/jcm10194462
Langouët-Astrié, C., Oshima, K., McMurtry, S. A., Yang, Y., Kwiecinski, J. M., LaRivière, W. B., et al. (2022). The influenza-injured lung microenvironment promotes MRSA virulence, contributing to severe secondary bacterial pneumonia. Cell Rep. 41:111721. doi: 10.1016/j.celrep.2022.111721
Le Guern, R., Grandjean, T., Stabler, S., Bauduin, M., Gosset, P., Kipnis, É., et al. (2023). Gut colonisation with multidrug-resistant Klebsiella pneumoniae worsens Pseudomonas aeruginosa lung infection. Nat. Commun. 14:78. doi: 10.1038/s41467-022-35767-4
Lee, B., Gopal, R., Manni, M. L., McHugh, K. J., Mandalapu, S., Robinson, K. M., et al. (2017). STAT1 is required for suppression of type 17 immunity during influenza and bacterial superinfection. Immunohorizons 1, 81–91. doi: 10.4049/immunohorizons.1700030
Lee, W. C., Ho, M. C., Leu, S. W., Chang, C. C., Lin, C. K., Lin, C. M., et al. (2022). The impacts of bacterial co-infections and secondary bacterial infections on patients with severe influenza pneumonitis admitted to the intensive care units. J. Crit. Care 72:154164. doi: 10.1016/j.jcrc.2022.154164
Lee, K. M., Morris-Love, J., Cabral, D. J., Belenky, P., Opal, S. M., and Jamieson, A. M. (2018). Coinfection with influenza A virus and Klebsiella oxytoca: an underrecognized impact on host resistance and tolerance to pulmonary infections. Front. Immunol. 9:2377. doi: 10.3389/fimmu.2018.02377
Lee, B., Robinson, K. M., McHugh, K. J., Scheller, E. V., Mandalapu, S., Chen, C., et al. (2015). Influenza-induced type I interferon enhances susceptibility to gram-negative and gram-positive bacterial pneumonia in mice. Am. J. Phys. Lung Cell. Mol. Phys. 309, L158–L167. doi: 10.1152/ajplung.00338.2014
Lee, C. W., Tai, Y. L., Huang, L. M., Chi, H., Huang, F. Y., Chiu, N. C., et al. (2021). Efficacy of clarithromycin-naproxen-oseltamivir combination therapy versus oseltamivir alone in hospitalized pediatric influenza patients. J. Microbiol. Immunol. Infect. 54, 876–884. doi: 10.1016/j.jmii.2020.08.017
Lee, N., Wong, C. K., Chan, M. C. W., Yeung, E. S. L., Tam, W. W. S., Tsang, O. T. Y., et al. (2017). Anti-inflammatory effects of adjunctive macrolide treatment in adults hospitalized with influenza: A randomized controlled trial. Antivir. Res. 144, 48–56. doi: 10.1016/j.antiviral.2017.05.008
Lei, B., Mu, J., Xu, G., Yang, X., Huang, W., Hu, L., et al. (2025). Jing-yin-gu-biao formula protects mice from postinfluenza Staphylococcus aureus infection by ameliorating acute lung injury and improving hypercoagulable state via inhibiting NETosis. Front. Immunol. 16:1567522. doi: 10.3389/fimmu.2025.1567522
LeMessurier, K. S., Tiwary, M., Morin, N. P., and Samarasinghe, A. E. (2020). Respiratory barrier as a safeguard and regulator of defense against influenza A virus and Streptococcus pneumoniae. Front. Immunol. 11:3. doi: 10.3389/fimmu.2020.00003
Li, H., Chen, X., and Zhou, S. J. (2018). Dauricine combined with clindamycin inhibits severe pneumonia co-infected by influenza virus H5N1 and Streptococcus pneumoniae in vitro and in vivo through NF-κB signaling pathway. J. Pharmacol. Sci. 137, 12–19. doi: 10.1016/j.jphs.2018.01.011
Li, X., Ding, W., Lu, Y., Zhu, H., Bao, W., Liu, Y., et al. (2025). An anti-complement homogeneous polysaccharide from Houttuynia cordata ameliorates acute pneumonia with H1N1 and MRSA coinfection through rectifying Treg/Th17 imbalance in the gut-lung axis and NLRP3 inflammasome activation. Acta Pharm. Sin. B 15, 3073–3091. doi: 10.1016/j.apsb.2025.04.008
Li, L., Guo, T., Yuan, Y., Xiao, J., Yang, R., Wang, H., et al. (2023). ΔA146Ply-HA stem protein immunization protects mice against influenza A virus infection and co-infection with Streptococcus pneumoniae. Mol. Immunol. 161, 91–103. doi: 10.1016/j.molimm.2023.07.011
Li, N., Ren, A., Wang, X., Fan, X., Zhao, Y., Gao, G. F., et al. (2015). Influenza viral neuraminidase primes bacterial coinfection through TGF-β-mediated expression of host cell receptors. Proc. Natl. Acad. Sci. USA 112, 238–243. doi: 10.1073/pnas.1414422112
Li, D., Wei, R., Zhang, X., Gong, S., Wan, M., Wang, F., et al. (2024). Gut commensal metabolite rhamnose promotes macrophages phagocytosis by activating SLC12A4 and protects against sepsis in mice. Acta Pharm. Sin. B 14, 3068–3085. doi: 10.1016/j.apsb.2024.03.025
Li, Y., Yang, Y., Chen, D., Wang, Y., Zhang, X., Li, W., et al. (2023). Memory Th17 cell-mediated protection against lethal secondary pneumococcal pneumonia following influenza infection. MBio 14:e0051923. doi: 10.1128/mbio.00519-23
Li, Z.-J., Zhang, H.-Y., Ren, L.-L., Lu, Q.-B., Ren, X., Zhang, C.-H., et al. (2021). Etiological and epidemiological features of acute respiratory infections in China. Nat. Commun. 12:5026. doi: 10.1038/s41467-021-25120-6
Li-Juan, L., Kang, S., Zhi-Juan, L., Dan, L., Feng, X., Peng, Y., et al. (2022). Klebsiella pneumoniae infection following H9N2 influenza A virus infection contributes to the development of pneumonia in mice. Vet. Microbiol. 264:109303. doi: 10.1016/j.vetmic.2021.109303
Lim, J. J., Nilsson, A. C., Silverman, M., Assy, N., Kulkarni, P., McBride, J. M., et al. (2020). A phase 2 randomized, double-blind, placebo-controlled trial of MHAA4549A, a monoclonal antibody, plus oseltamivir in patients hospitalized with severe influenza A virus infection. Antimicrob. Agents Chemother. 64:e00352-20. doi: 10.1128/AAC.00352-20
Lucarelli, R., Gorrochotegui-Escalante, N., Taddeo, J., Buttaro, B., Beld, J., and Tam, V. (2022). Eicosanoid-activated PPARα inhibits NFκB-dependent bacterial clearance during post-influenza superinfection. Front. Cell. Infect. Microbiol. 12:881462. doi: 10.3389/fcimb.2022.881462
Ma, Q., Huang, W., Zhao, J., and Yang, Z. (2020). Liu Shen Wan inhibits influenza a virus and excessive virus-induced inflammatory response via suppression of TLR4/NF-kappaB signaling pathway in vitro and in vivo. J. Ethnopharmacol. 252:112584. doi: 10.1016/j.jep.2020.112584
Machado, M. G., Patente, T. A., Rouillé, Y., Heumel, S., Melo, E. M., Deruyter, L., et al. (2022). Acetate improves the killing of Streptococcus pneumoniae by alveolar macrophages via NLRP3 Inflammasome and glycolysis-HIF-1α Axis. Front. Immunol. 13:773261. doi: 10.3389/fimmu.2022.773261
MacIntyre, C. R., Chughtai, A. A., Barnes, M., Ridda, I., Seale, H., Toms, R., et al. (2018). The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza A(H1N1)pdm09. BMC Infect. Dis. 18:637. doi: 10.1186/s12879-018-3548-0
Majumder, S., Li, P., Das, S., Nafiz, T. N., Kumar, S., Bai, G., et al. (2024). A bacterial vesicle-based pneumococcal vaccine against influenza-mediated secondary Streptococcus pneumoniae pulmonary infection. Mucosal Immunol. 17, 169–181. doi: 10.1016/j.mucimm.2024.01.002
Malaviya, R., Ikeda, T., Ross, E., and Abraham, S. N. (1996). Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature 381, 77–80.
Martínez-Colón, G. J., Warheit-Niemi, H., Gurczynski, S. J., Taylor, Q. M., Wilke, C. A., Podsiad, A. B., et al. (2019). Influenza-induced immune suppression to methicillin-resistant Staphylococcus aureus is mediated by TLR9. PLoS Pathog. 15:e1007560. doi: 10.1371/journal.ppat.1007560
Mills, K. H. G. (2023). IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 23, 38–54. doi: 10.1038/s41577-022-00746-9
Min, C., and Shilian, H. (2017). Lung-resident γδ T cells and their roles in lung diseases. Immunology 151, 375–384. doi: 10.1111/imm.12764
Mina, M. J., McCullers, J. A., and Klugman, K. P. (2014). Live attenuated influenza vaccine enhances colonization of Streptococcus pneumoniae and Staphylococcus aureus in mice. MBio 5:e01040-13. doi: 10.1128/mBio.01040-13
Minozzi, S., Lytras, T., Gianola, S., Gonzalez-Lorenzo, M., Castellini, G., Galli, C., et al. (2022). Comparative efficacy and safety of vaccines to prevent seasonal influenza: A systematic review and network meta-analysis. EClinicalMedicine 46:101331. doi: 10.1016/j.eclinm.2022.101331
Moon, S., Han, S., Jang, I. H., Ryu, J., Rha, M. S., Cho, H. J., et al. (2024). Airway epithelial CD47 plays a critical role in inducing influenza virus-mediated bacterial super-infection. Nat. Commun. 15:3666. doi: 10.1038/s41467-024-47963-5
Morens, D. M., Taubenberger, J. K., and Fauci, A. S. (2008). Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J. Infect. Dis. 198, 962–970. doi: 10.1086/591708
Naghavi, M., Ong, K. L., Aali, A., Ababneh, H. S., Abate, Y. H., Abbafati, C., et al. (2024). Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the global burden of disease study 2021. Lancet 403, 2100–2132. doi: 10.1016/S0140-6736(24)00367-2
Narayana Moorthy, A., Narasaraju, T., Rai, P., Perumalsamy, R., Tan, K. B., Wang, S., et al. (2013). In vivo and in vitro studies on the roles of neutrophil extracellular traps during secondary pneumococcal pneumonia after primary pulmonary influenza infection. Front. Immunol. 4:56. doi: 10.3389/fimmu.2013.00056
Neupane, A. S., Willson, M., Chojnacki, A. K., Vargas E Silva Castanheira, F., Morehouse, C., Carestia, A., et al. (2020). Patrolling alveolar macrophages conceal bacteria from the immune system to maintain homeostasis. Cell 183, 110–125.e11. doi: 10.1016/j.cell.2020.08.020
Nickol, M. E., Ciric, J., Falcinelli, S. D., Chertow, D. S., and Kindrachuk, J. (2019). Characterization of host and bacterial contributions to lung barrier dysfunction following co-infection with 2009 pandemic influenza and methicillin resistant Staphylococcus aureus. Viruses 11:116. doi: 10.3390/v11020116
Nickol, M. E., Lyle, S. M., Dennehy, B., and Kindrachuk, J. (2020). Dysregulated host responses underlie 2009 pandemic influenza-methicillin resistant Staphylococcus aureus coinfection pathogenesis at the alveolar-capillary barrier. Cells 9:2472. doi: 10.3390/cells9112472
Nolan, V. G., Arnold, S. R., Bramley, A. M., Ampofo, K., Williams, D. J., Grijalva, C. G., et al. (2018). Etiology and impact of coinfections in children hospitalized with community-acquired pneumonia. J. Infect. Dis. 218, 179–188. doi: 10.1093/infdis/jix641
Novotny, L. A., and Bakaletz, L. O. (2016). Intercellular adhesion molecule 1 serves as a primary cognate receptor for the type IV pilus of nontypeable Haemophilus influenzae. Cell. Microbiol. 18, 1043–1055. doi: 10.1111/cmi.12575
Onishi, M., Kitano, M., Taniguchi, K., Homma, T., Kobayashi, M., Yoshinaga, T., et al. (2015). Intravenous peramivir inhibits viral replication, and leads to bacterial clearance and prevention of mortality during murine bacterial co-infection caused by influenza A(H1N1)pdm09 virus and Streptococcus pneumoniae. Antivir. Res. 117, 52–59. doi: 10.1016/j.antiviral.2015.02.012
Ouyang, W., Liu, C., Pan, Y., Han, Y., Yang, L., Xia, J., et al. (2019). SHP2 deficiency promotes Staphylococcus aureus pneumonia following influenza infection. Cell Prolif. 53:e12721. doi: 10.1111/cpr.12721
Paget, C., Ivanov, S., Fontaine, J., Renneson, J., Blanc, F., Pichavant, M., et al. (2012). Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: potential role in protection against lung epithelial damages. J. Biol. Chem. 287, 8816–8829. doi: 10.1074/jbc.M111.304758
Paget, J., Staadegaard, L., Wang, X., Li, Y., van Pomeren, T., van Summeren, J., et al. (2023). Global and national influenza-associated hospitalisation rates: estimates for 40 countries and administrative regions. J. Glob. Health 13, 962–970. doi: 10.7189/jogh.13.04003
Palani, S., Uddin, M. B., McKelvey, M., Shao, S., and Sun, K. (2023). Immune predisposition drives susceptibility to pneumococcal pneumonia after mild influenza A virus infection in mice. Front. Immunol. 14:1272920. doi: 10.3389/fimmu.2023.1272920
Park, S. S., Gonzalez-Juarbe, N., Riegler, A. N., Im, H., Hale, Y., Platt, M. P., et al. (2021). Streptococcus pneumoniae binds to host GAPDH on dying lung epithelial cells worsening secondary infection following influenza. Cell Rep. 35:109267. doi: 10.1016/j.celrep.2021.109267
Pasparakis, M., and Vandenabeele, P. (2015). Necroptosis and its role in inflammation. Nature 517, 311–320. doi: 10.1038/nature14191
Peng, Y., Wang, X., Wang, H., Xu, W., Wu, K., Go, X., et al. (2021). Interleukin-4 protects mice against lethal influenza and Streptococcus pneumoniae co-infected pneumonia. Clin. Exp. Immunol. 205, 379–390. doi: 10.1111/cei.13628
Peno, C., Armitage, E. P., Clerc, M., Balcazar Lopez, C., Jagne, Y. J., Drammeh, S., et al. (2021). The effect of live attenuated influenza vaccine on pneumococcal colonisation densities among children aged 24-59 months in the Gambia: a phase 4, open label, randomised, controlled trial. Lancet Microbe 2, e656–e665. doi: 10.1016/S2666-5247(21)00179-8
Peno, C., Jagne, Y. J., Clerc, M., Balcazar Lopez, C., Armitage, E. P., Sallah, H., et al. (2025). Interactions between live attenuated influenza vaccine and nasopharyngeal microbiota among children aged 24-59 months in the Gambia: a phase 4, open-label, randomised controlled trial. Lancet Microbe 6:100971. doi: 10.1016/j.lanmic.2024.100971
Platt, M. P., Lin, Y. H., Wiscovitch-Russo, R., Yu, Y., and Gonzalez-Juarbe, N. (2022). Pandemic Influenza Infection Promotes Streptococcus pneumoniae Infiltration, Necrotic Damage, and Proteomic Remodeling in the Heart. mBio 13:e0325721. doi: 10.1128/mbio.03257-21
Pruenster, M., Vogl, T., Roth, J., and Sperandio, M. (2016). S100A8/A9: from basic science to clinical application. Pharmacol. Ther. 167, 120–131. doi: 10.1016/j.pharmthera.2016.07.015
Prystopiuk, V., Feuillie, C., Herman-Bausier, P., Viela, F., Alsteens, D., Pietrocola, G., et al. (2018). Mechanical forces guiding Staphylococcus aureus cellular invasion. ACS Nano 12, 3609–3622. doi: 10.1021/acsnano.8b00716
Qiao, M., Moyes, G., Zhu, F., Li, Y., and Wang, X. (2023). The prevalence of influenza bacterial co-infection and its role in disease severity: A systematic review and meta-analysis. J. Glob. Health 13:04063. doi: 10.7189/jogh.13.04063
Ramasubramanian, B., Kim, J., Ke, Y., Li, Y., Zhang, C. O., Promnares, K., et al. (2022). Mechanisms of pulmonary endothelial permeability and inflammation caused by extracellular histone subunits H3 and H4. FASEB J. 36:e22470. doi: 10.1096/fj.202200303RR
Robinson, K. M., Lee, B., Scheller, E. V., Mandalapu, S., Enelow, R. I., Kolls, J. K., et al. (2015). The role of IL-27 in susceptibility to post-influenza Staphylococcus aureus pneumonia. Respir. Res. 16:10. doi: 10.1186/s12931-015-0168-8
Robinson, K. M., McHugh, K. J., Mandalapu, S., Clay, M. E., Lee, B., Scheller, E. V., et al. (2014). Influenza a virus exacerbates Staphylococcus aureus pneumonia in mice by attenuating antimicrobial peptide production. J. Infect. Dis. 209, 865–875. doi: 10.1093/infdis/jit527
Robinson, K. M., Ramanan, K., Clay, M. E., McHugh, K. J., Pilewski, M. J., Nickolich, K. L., et al. (2018a). The inflammasome potentiates influenza/Staphylococcus aureus superinfection in mice. JCI Insight 3:e97470. doi: 10.1172/jci.insight.97470
Robinson, K. M., Ramanan, K., Clay, M. E., McHugh, K. J., Rich, H. E., and Alcorn, J. F. (2018b). Novel protective mechanism for interleukin-33 at the mucosal barrier during influenza-associated bacterial superinfection. Mucosal Immunol. 11, 199–208. doi: 10.1038/mi.2017.32
Robinson, K. M., Ramanan, K., Tobin, J. M., Nickolich, K. L., Pilewski, M. J., Kallewaard, N. L., et al. (2019). Survival during influenza-associated bacterial superinfection improves following viral- and bacterial-specific monoclonal antibody treatment. JCI Insight 4:e125554. doi: 10.1172/jci.insight.125554
Rodriguez, A. E., Bogart, C., Gilbert, C. M., McCullers, J. A., Smith, A. M., Kanneganti, T. D., et al. (2019). Enhanced IL-1β production is mediated by a TLR2-MYD88-NLRP3 signaling axis during coinfection with influenza A virus and Streptococcus pneumoniae. PLoS One 14:e0212236. doi: 10.1371/journal.pone.0212236
Rojas-Quintero, J., Wang, X., Tipper, J., Burkett, P. R., Zuñiga, J., Ashtekar, A. R., et al. (2018). Matrix metalloproteinase-9 deficiency protects mice from severe influenza A viral infection. JCI Insight 3:e99022. doi: 10.1172/jci.insight.99022
Roquilly, A., McWilliam, H. E. G., Jacqueline, C., Tian, Z., Cinotti, R., Rimbert, M., et al. (2017). Local modulation of antigen-presenting cell development after resolution of pneumonia induces Long-term susceptibility to secondary infections. Immunity 47, 135–47.e5. doi: 10.1016/j.immuni.2017.06.021
Santos, N. B., Vaz da Silva, Z. E., Gomes, C., Reis, C. A., and Amorim, M. J. (2021). Complement decay-accelerating factor is a modulator of influenza A virus lung immunopathology. PLoS Pathog. 17:e1009381. doi: 10.1371/journal.ppat.1009381
Schmolke, M., Barman, T. K., Racine, R., Bonin, J. L., Califano, D., Salmon, S. L., et al. (2021). Sequential targeting of interferon pathways for increased host resistance to bacterial superinfection during influenza. PLoS Pathog. 17:e1009405. doi: 10.1371/journal.ppat.1009405
Schuijt, T. J., Lankelma, J. M., Scicluna, B. P., de Sousa e Melo, F., Roelofs, J. J., de Boer, J. D., et al. (2016). The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 65, 575–583. doi: 10.1136/gutjnl-2015-309728
Semeraro, F., Ammollo, C. T., Morrissey, J. H., Dale, G. L., Friese, P., Esmon, N. L., et al. (2011). Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood 118, 1952–1961. doi: 10.1182/blood-2011-03-343061
Sencio, V., Barthelemy, A., Tavares, L. P., Machado, M. G., Soulard, D., Cuinat, C., et al. (2020). Gut Dysbiosis during influenza contributes to pulmonary pneumococcal superinfection through altered Short-chain fatty acid production. Cell Rep. 30, 2934–47.e6. doi: 10.1016/j.celrep.2020.02.013
Sharma-Chawla, N., Stegemann-Koniszewski, S., Christen, H., Boehme, J. D., Kershaw, O., Schreiber, J., et al. (2019). In vivo neutralization of pro-inflammatory cytokines during secondary Streptococcus pneumoniae infection post influenza A virus infection. Front. Immunol. 10:1864. doi: 10.3389/fimmu.2019.01864
Shenoy, A. T., Wasserman, G. A., Arafa, E. I., Wooten, A. K., Smith, N. M. S., Martin, I. M. C., et al. (2020). Lung CD4(+) resident memory T cells remodel epithelial responses to accelerate neutrophil recruitment during pneumonia. Mucosal Immunol. 13, 334–343. doi: 10.1038/s41385-019-0229-2
Shepardson, K. M., Larson, K., Morton, R. V., Prigge, J. R., Schmidt, E. E., Huber, V. C., et al. (2016). Differential type I interferon signaling is a master regulator of susceptibility to Postinfluenza bacterial superinfection. MBio 7, e00506–e00516. doi: 10.1128/mBio.00506-16
Shi, J., Han, S., Zhang, Y., Lu, X., Gan, Y., Tong, N., et al. (2025). Respiratory bacterial and viral pathogen spectrum among influenza-positive and influenza-negative patients. BMC Infect. Dis. 25:866. doi: 10.1186/s12879-025-11232-7
Shi, Y., Shi, X., Liang, J., Luo, J., Ba, J., Chen, J., et al. (2020). Aggravated MRSA pneumonia secondary to influenza A virus infection is derived from decreased expression of IL-1β. J. Med. Virol. 92, 3047–3056. doi: 10.1002/jmv.26329
Shi, J., Zhao, Y., Wang, K., Shi, X., Wang, Y., Huang, H., et al. (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665. doi: 10.1038/nature15514
Shieh, W. J., Blau, D. M., Denison, A. M., Deleon-Carnes, M., Adem, P., Bhatnagar, J., et al. (2010). 2009 pandemic influenza A (H1N1): pathology and pathogenesis of 100 fatal cases in the United States. Am. J. Pathol. 177, 166–175. doi: 10.2353/ajpath.2010.100115
Short, K. R., Kasper, J., van der Aa, S., Andeweg, A. C., Zaaraoui-Boutahar, F., Goeijenbier, M., et al. (2016). Influenza virus damages the alveolar barrier by disrupting epithelial cell tight junctions. Eur. Respir. J. 47, 954–966. doi: 10.1183/13993003.01282-2015
Shukla, S. D., Fairbairn, R. L., Gell, D. A., Latham, R. D., Sohal, S. S., Walters, E. H., et al. (2016). An antagonist of the platelet-activating factor receptor inhibits adherence of both nontypeable Haemophilus influenzae and Streptococcus pneumoniae to cultured human bronchial epithelial cells exposed to cigarette smoke. Int. J. Chron. Obstruct. Pulmon. Dis. 11, 1647–1655. doi: 10.2147/COPD.S108698
Shukla, S. D., Walters, E. H., Simpson, J. L., Keely, S., Wark, P. A. B., O'Toole, R. F., et al. (2020). Hypoxia-inducible factor and bacterial infections in chronic obstructive pulmonary disease. Respirology 25, 53–63. doi: 10.1111/resp.13722
Small, C. L., Shaler, C. R., McCormick, S., Jeyanathan, M., Damjanovic, D., Brown, E. G., et al. (2010). Influenza infection leads to increased susceptibility to subsequent bacterial superinfection by impairing NK cell responses in the lung. J. Immunol. 184, 2048–2056. doi: 10.4049/jimmunol.0902772
Song, J., Zhao, J., Cai, X., Qin, S., Chen, Z., Huang, X., et al. (2022). Lianhuaqingwen capsule inhibits non-lethal doses of influenza virus-induced secondary Staphylococcus aureus infection in mice. J. Ethnopharmacol. 298:115653. doi: 10.1016/j.jep.2022.115653
Sumitomo, T., Nakata, M., Nagase, S., Takahara, Y., Honda-Ogawa, M., Mori, Y., et al. (2021). GP96 drives exacerbation of secondary bacterial pneumonia following influenza A virus infection. MBio 12:e0326920. doi: 10.1128/mBio.03269-20
Sun, K., and Metzger, D. W. (2014). Influenza infection suppresses NADPH oxidase-dependent phagocytic bacterial clearance and enhances susceptibility to secondary methicillin-resistant Staphylococcus aureus infection. J. Immunol. 192, 3301–3307. doi: 10.4049/jimmunol.1303049
Syed, S., Viazmina, L., Mager, R., Meri, S., and Haapasalo, K. (2020). Streptococci and the complement system: interplay during infection, inflammation and autoimmunity. FEBS Lett. 594, 2570–2585. doi: 10.1002/1873-3468.13872
Tam, V. C., Suen, R., Treuting, P. M., Armando, A., Lucarelli, R., Gorrochotegui-Escalante, N., et al. (2020). PPARα exacerbates necroptosis, leading to increased mortality in postinfluenza bacterial superinfection. Proc. Natl. Acad. Sci. USA 117, 15789–15798. doi: 10.1073/pnas.2006343117
Tang, Y., Su, R., Gu, Q., Hu, Y., and Yang, H. (2023). PI3K/AKT-mediated autophagy inhibition facilitates mast cell activation to enhance severe inflammatory lung injury in influenza A virus- and secondary Staphylococcus aureus-infected mice. Antivir. Res. 209:105502. doi: 10.1016/j.antiviral.2022.105502
Tavares, L. P., Brüggemann, T. R., Rezende, R. M., Machado, M. G., Cagnina, R. E., Shay, A. E., et al. (2022). Cysteinyl maresins reprogram macrophages to protect mice from Streptococcus pneumoniae after influenza A virus infection. MBio 13:e0126722. doi: 10.1128/mbio.01267-22
Tavares, L. P., Garcia, C. C., Machado, M. G., Queiroz-Junior, C. M., Barthelemy, A., Trottein, F., et al. (2017). CXCR1/2 antagonism is protective during influenza and post-influenza pneumococcal infection. Front. Immunol. 8:1799. doi: 10.3389/fimmu.2017.01799
Troeger, C. E., Blacker, B. F., Khalil, I. A., Zimsen, S. R. M., Albertson, S. B., Abate, D., et al. (2019). Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the global burden of disease study 2017. Lancet Respir. Med. 7, 69–89. doi: 10.1016/S2213-2600(18)30496-X
Uyeki, T. M., Bernstein, H. H., Bradley, J. S., Englund, J. A., File, T. M., Fry, A. M., et al. (2019). Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenzaa. Clin. Infect. Dis. 68, e1–e47. doi: 10.1093/cid/ciy866
van Someren Gréve, F., van der Sluijs, K. F., Tuip, A. M., Schultz, M. J., de Jong, M. D., and Juffermans, N. P. (2018). Treatment with broadly neutralizing influenza antibodies reduces severity of secondary pneumococcal pneumonia in mice. J. Med. Virol. 90, 1431–1437. doi: 10.1002/jmv.25212
Verma, A. K., Bansal, S., Bauer, C., Muralidharan, A., and Sun, K. (2020). Influenza infection induces alveolar macrophage dysfunction and thereby enables noninvasive Streptococcus pneumoniae to cause deadly pneumonia. J. Immunol. 205, 1601–1607. doi: 10.4049/jimmunol.2000094
Verma, A. K., Bauer, C., Yajjala, V. K., Bansal, S., and Sun, K. (2019). Linezolid attenuates lethal lung damage during Postinfluenza methicillin-resistant Staphylococcus aureus pneumonia. Infect. Immun. 87, e00538–e00519. doi: 10.1128/IAI.00538-19
Verma, A. K., McKelvey, M., Uddin, M. B., Palani, S., Niu, M., Bauer, C., et al. (2022). IFN-γ transforms the transcriptomic landscape and triggers myeloid cell hyperresponsiveness to cause lethal lung injury. Front. Immunol. 13:1011132. doi: 10.3389/fimmu.2022.1011132
Verstraeten, S., Sencio, V., Raise, A., Huillet, E., Layec, S., Deruyter, L., et al. (2022). Description of a newly isolated Blautia faecis strain and its benefit in mouse models of post-influenza secondary enteric and pulmonary infections. Nutrients 14:1478. doi: 10.3390/nu14071478
Villeret, B., Solhonne, B., Straube, M., Lemaire, F., Cazes, A., Garcia-Verdugo, I., et al. (2020). Influenza A virus pre-infection exacerbates Pseudomonas aeruginosa-mediated lung damage through increased MMP-9 expression, decreased Elafin production and tissue resilience. Front. Immunol. 11:117. doi: 10.3389/fimmu.2020.00117
Vimalanathan, S., Schoop, R., Suter, A., and Hudson, J. (2017). Prevention of influenza virus induced bacterial superinfection by standardized Echinacea purpurea, via regulation of surface receptor expression in human bronchial epithelial cells. Virus Res. 233, 51–59. doi: 10.1016/j.virusres.2017.03.006
Walters, K. A., D'Agnillo, F., Sheng, Z. M., Kindrachuk, J., Schwartzman, L. M., Kuestner, R. E., et al. (2015). 1918 pandemic influenza virus and Streptococcus pneumoniae co-infection results in activation of coagulation and widespread pulmonary thrombosis in mice and humans. J. Pathol. 238, 85–97. doi: 10.1002/path.4638
Wang, Y., Wang, H., Zhang, Y., Ma, A., Liu, D., Li, X., et al. (2025). Single-dose suraxavir marboxil for acute uncomplicated influenza in adults and adolescents: a multicenter, randomized, double-blind, placebo-controlled phase 3 trial. Nat. Med. 31, 639–646. doi: 10.1038/s41591-024-03419-3
Wang, Q. Y., Yuan, L., Lin, J. Y., Zhuo, Z. Q., Wang, Y. M., Li, S. S., et al. (2023). Clinical characteristics of severe influenza virus-associated pneumonia complicated with bacterial infection in children: a retrospective analysis. BMC Infect. Dis. 23:545. doi: 10.1186/s12879-023-08536-x
WHO. WHO unveils new worldwide strategy against influenza. (2019). Available online at: https://www.who.int/publications/i/item/9789241515320 (accessed July 26, 2025).
Wilson, A. S., Randall, K. L., Pettitt, J. A., Ellyard, J. I., Blumenthal, A., Enders, A., et al. (2022). Neutrophil extracellular traps and their histones promote Th17 cell differentiation directly via TLR2. Nat. Commun. 13:528. doi: 10.1038/s41467-022-28172-4
Wu, M., Gibbons, J. G., DeLoid, G. M., Bedugnis, A. S., Thimmulappa, R. K., Biswal, S., et al. (2017). Immunomodulators targeting MARCO expression improve resistance to postinfluenza bacterial pneumonia. Am. J. Phys. Lung Cell. Mol. Phys. 313, L138–L153. doi: 10.1152/ajplung.00075.2017
Wu, X., Jie, F., Li, P., Zhuo, C., Pan, W., Zhong, N., et al. (2023). Active immunization with Pseudomonas aeruginosa vaccine protects mice from secondary Pseudomonas aeruginosa challenge post-influenza virus infection. J. Thorac. Dis. 15, 22–32. doi: 10.21037/jtd-22-1012
Wu, X., Li, R., Weng, Y., Zhou, H., Jiang, H., Zhao, J., et al. (2021). Correlation of adhesion molecules and non-typeable haemophilus influenzae growth in a mice coinfected model of acute inflammation. Microbes Infect. 23:104839. doi: 10.1016/j.micinf.2021.104839
Xiao, K., Cao, Y., Han, Z., Zhang, Y., Luu, L. D. W., Chen, L., et al. (2025). A pan-immune panorama of bacterial pneumonia revealed by a large-scale single-cell transcriptome atlas. Signal Transduct. Target. Ther. 10:5. doi: 10.1038/s41392-024-02093-8
Yang, K. J., Bautista, L. Y., Danielle, G., and Tran, D. (2024). The role of ginseng as an anti-asthmatic agent. Acupunct. Herb. Med. 4, 358–366. doi: 10.1097/HM9.0000000000000123
Yang, Z., Li, Z., Zhan, Y., Lin, Z., Fang, Z., Xu, X., et al. (2024). Safety and efficacy of onradivir in adults with acute uncomplicated influenza A infection: a multicentre, double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 24, 535–545. doi: 10.1016/S1473-3099(23)00743-0
Yang, C., Wang, Y., He, J., Yan, W., Jiang, H., Chen, Q., et al. (2020). Lianhua-Qingwen displays antiviral and anti-inflammatory activity and synergistic effects with Oseltamivir against influenza B virus infection in the mouse model. Evid. Based Complement. Alternat. Med. 2020:3196375. doi: 10.1155/2020/3196375
Yang, Z., Zou, X., Feng, P., Zhan, H., Xiong, D., and Lang, J. (2019). Inhibition of the PI3K/AKT signaling pathway or overexpression of Beclin1 blocks reinfection of Streptococcus pneumoniae after infection of influenza A virus in severe community-acquired pneumonia. Inflammation 42, 1741–1753. doi: 10.1007/s10753-019-01035-9
Yi, T., Ding, W., Hao, Y., Cen, L., Li, J., Shi, X., et al. (2022). Neutrophil extracellular traps mediate severe lung injury induced by influenza A virus H1N1 in mice coinfected with Staphylococcus aureus. Microb. Pathog. 166:105558. doi: 10.1016/j.micpath.2022.105558
You, J., Zhou, L., San, X., Li, H., Li, M., and Wang, B. (2023). NEDD4 regulated Pyroptosis occurred from co-infection between influenza A virus and Streptococcus pneumoniae. J. Microbiol. 61, 777–789. doi: 10.1007/s12275-023-00076-y
Yuan, J., and Ofengeim, D. (2023). A guide to cell death pathways. Nat. Rev. Mol. Cell Biol. 25, 379–395. doi: 10.1038/s41580-023-00689-6
Yuki, K., and Koutsogiannaki, S. (2021). Pattern recognition receptors as therapeutic targets for bacterial, viral and fungal sepsis. Int. Immunopharmacol. 98:107909. doi: 10.1016/j.intimp.2021.107909
Zanoni, I., Tan, Y., Di Gioia, M., Broggi, A., Ruan, J., Shi, J., et al. (2016). An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science 352, 1232–1236. doi: 10.1126/science.aaf3036
Zhang, J., Chen, X., Huang, L., Liu, L., Wang, Q., Tian, J., et al. (2025). Traditional Chinese Medicine+ artificial intelligence: Wuzhen consensus. Acupunct. Herb. Med. 5, 134–135.
Zhang, B., Dong, E., Liu, L., Tian, J., Chen, S., Zhu, L., et al. (2025). Ten recommendations for the high-quality development of traditional Chinese medicine in the new era. Acupunct. Herb. Med. 5, 131–133. doi: 10.1097/HM9.0000000000000162
Zhang, X., Yang, Y., Chen, S., Li, W., Li, Y., Akerley, B. J., et al. (2023). Antigen-specific memory Th17 cells promote cross-protection against nontypeable Haemophilus influenzae after mild influenza A virus infection. Mucosal Immunol. 16, 153–166. doi: 10.1016/j.mucimm.2023.01.007
Zhao, J., Wang, Y., Huang, X., Ma, Q., Song, J., Wu, X., et al. (2021). Liu Shen Wan inhibits influenza virus-induced secondary Staphylococcus aureus infection in vivo and in vitro. J. Ethnopharmacol. 277:114066. doi: 10.1016/j.jep.2021.114066
Zhao, Y., Yang, J., Shi, J., Gong, Y. N., Lu, Q., Xu, H., et al. (2011). The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477, 596–600. doi: 10.1038/nature10510
Zhao, Q., Zhao, R., Geng, Z., Bao, L., Guo, S., Wang, Y., et al. (2023). Xuanfei baidu granule alleviates coronavirus-induced pneumonia in low-temperature and high-humidity environments. Acupunct. Herb. Med. 3, 200–206. doi: 10.1097/HM9.0000000000000068
Zheng, Y., Huang, Y., Xu, Y., Sang, L., Liu, X., and Li, Y. (2023). Ferroptosis, pyroptosis and necroptosis in acute respiratory distress syndrome. Cell Death Discov. 9:91. doi: 10.1038/s41420-023-01369-2
Keywords: post-influenza bacterial infection, resident immune cells, respiratory barrier, therapeutic strategies, overactivated inflammatory response
Citation: Lei B, Wang S, Yu L and Ma Q (2025) Post-influenza bacterial infection: mechanisms of pathogenesis and advances in therapeutic strategies. Front. Microbiol. 16:1673643. doi: 10.3389/fmicb.2025.1673643
Edited by:
Sara Louise Cosby, Belfast, United KingdomReviewed by:
Jun Wang, Jiangnan University, ChinaAtefe Panahipoor Javaherdehi, Islamic Azad University, Iran
Copyright © 2025 Lei, Wang, Yu and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qinhai Ma, MTMyNjgyNjgyMTRAMTYzLmNvbQ==; Linzhong Yu, eXVsemhAc211LmVkdS5jbg==
†These authors have contributed equally to this work
 Biao Lei
Biao Lei Shun Wang2†
Shun Wang2† Linzhong Yu
Linzhong Yu Qinhai Ma
Qinhai Ma