- 1Department of Chemical and Biomolecular Engineering, University of Delaware, Newark, DE, United States
- 2Delaware Biotechnology Institute, University of Delaware, Newark, DE, United States
- 3Department of Biological Sciences, University of Delaware, Newark, DE, United States
There is a need for efficient and sustainable production of essential chemicals such as isopropanol and butanol from renewable sugar feedstocks. Microbial fermentations use glycolysis, and as result, a third of the sugar carbon is lost to CO2 through pyruvate decarboxylation to acetyl-CoA, the starting intermediate for the biosynthesis of most microbial metabolites. In nature, microbes exist in syntrophic consortia, allowing for mutually-beneficial interactions, the production of novel products, and the realization of novel benefits—including better carbon conservation—not seen in monocultures. We examined the impact of starting coculture cell densities, the gas atmosphere (N2, H2, or H2/CO2) and coculture species ratios (using a recently developed RNA-FISH flow cytometric assays) on metabolite production, yields and sugar-carbon utilization in serum bottles and bioreactors. Metabolic flux analysis identified the complex patterns by which the two species alter each other’s metabolism in a cell-density and gas-atmosphere dependent manner. For increased acetone production, we transformed Clostridium acetobutylicum with a plasmid (p95ace02a) expressing a synthetic acetone pathway comprising four native genes. This engineered C. acetobutylicum was cocultured with Clostridium ljungdahlii to capture the waste CO2 and H2 generated due to glucose catabolism by C. acetobutylicum, and to convert acetone into isopropanol. C. ljungdahlii activated the dormant acetate uptake in C. acetobutylicum, while coculture density dramatically impacted species ratios, electron management, and the H2 utilization of C. ljungdahlii. We achieved exceptionally-high concentrations of our desired products—246 mM isopropanol and 148 mM butanol—in 64 h, with about 85% of the production occurring before 32 h. We reached maximum productivities of 13.9 mM isopropanol/h and 10.4 mM butanol/h with 0.9 mol alcohol produced per mol of sugar consumed. Total product yields reached 84.7% on a C-mol basis, versus 65.6% that can be reached in a C. acetobutylicum monoculture. Engineered syntrophic cocultures can efficiently and tunably produce target chemicals including isopropanol and butanol for a renewable economy.
1 Introduction
As the world moves toward more sustainable means of chemical manufacture, non-petroleum-based pathways for the production of essential chemicals such as isopropanol, acetone, butanol, and ethanol (IABE) are needed. In the past, microbial fermentation with Clostridium acetobutylicum (Cac) has been used to produce acetone, butanol, and ethanol (ABE) (Gabriel and Crawford, 1930; Gibbs, 1983; Lutke-Eversloh and Bahl, 2011; Adams, 2017). However, these processes were rendered economically uncompetitive after World War II due to the rise of petroleum-based processes and to their own inherent inefficiency: in Cac monoculture fermentations, a third of the sugar carbon is lost as CO2 (Charubin and Papoutsakis, 2019).
These marketplace inefficiencies do not blunt some of the main advantages of microbial fermentations with Clostridia, and new biotechnological advances can engineer greater control over the microbial organisms’ genetics, the operation and analysis of microbial fermentation, and the pairing of organisms to capture waste CO2 and to expand the metabolic space (Lutke-Eversloh and Bahl, 2011; Charubin et al., 2018; Charubin and Papoutsakis, 2019; Jiang et al., 2023). Cac is an appealing model industrial microorganism because it has a broad and powerful substrate utilization system and primary metabolism and because it exhibits high electron and carbon fluxes and distinctive systems for generating and shuffling electrons (Charubin et al., 2018). Metabolic engineering has enabled the design of plasmids that produce butanol, ethanol, and acetone at greater concentrations and purities (Lee et al., 2009; Sillers et al., 2009; Lutke-Eversloh and Bahl, 2011), added isopropanol production capabilities (albeit at low concentration) (Lee et al., 2012; Dusseaux et al., 2013), and used antisense RNA strategies to change the balance of metabolite production (Desai and Papoutsakis, 1999).
Here, we augment a Cac fermentation with in situ carbon-capture via coculture with Clostridium ljungdahlii (Clj), an acetogen that fixes CO2 using H2 as an electron source. Clj, an acetogen, fixes CO2 using H2 as an electron source, making it an ideal coculture partner for Cac, which generates both CO2 and H2 as byproducts of glucose fermentation (Jones et al., 2016; Charubin and Papoutsakis, 2019). Our lab has already demonstrated that, in Cac/Clj cocultures, Clj expands the metabolic space by enabling the production of isopropanol and 2,3-butanediol, which neither Cac nor Clj is capable of on its own. Clj also produces acetate, which can be reassimilated by Cac. Previous research has demonstrated that direct physical contact aids this metabolic coupling (Charubin and Papoutsakis, 2019); TEM tomography shows a distinct physical contact phenotype by which Clj makes contact with Cac at its poles, forming fusion pairs (Charubin et al., 2020). We have also confirmed that cytoplasmic proteins, RNA, and DNA are transferred between cells (Charubin et al., 2024). This study builds on that foundation by incorporating an engineered Cac strain alongside Clj in pH-controlled bioreactors to maximize IABE productivity.
The studies of cocultures across Clostridial species are increasingly relevant. Cocultures of Cac and C. kluyveri (Otten et al., 2022), or Clj and C. kluyveri (Richter et al., 2016), have been used to produce 6–8 carbon compounds such as hexanoate. Clostridium beijerinckii has also been partnered with Propionibacterium freudenreichii (Hocq and Sauer, 2022) and Bacillus subtilis (Cui et al., 2020) to produce BuOH, IPA, and acetone. We aim to produce IABE more quickly and at higher concentrations while utilizing coculture flux analysis (Papoutsakis, 1984; Desai et al., 1999) and RNA-FISH (Hill and Papoutsakis, 2024) to analyze how coculture interactions change the metabolic fluxes and population ratios of the member organisms.
2 Materials and methods
2.1 Strain and plasmid construction
The p95ace02a plasmid was described in our prior publication (Seo et al., 2024). Briefly, it contains the Cac ctfA and ctfB genes under the control of the pta promoter from Clj, and the Cac thl and adc genes under the control of the Pthlsup promoter (Streett et al., 2019), the ColE1 origin of replication, repL, AmpR, and MLSR. It was constructed using the NEBuilder HiFi DNA Assembly kit (New England Biolabs) with necessary primers and gBlocks from IDT. The PCR fragments were amplified with Phusion DNA polymerase (Thermo Fisher Scientific). The plasmid construction was successfully confirmed with colony PCR with Phire DNA polymerase (Thermo Fisher Scientific) and Sanger sequencing.
2.2 Transformation of C. acetobutylicum
Cac was electroporated with the plasmid p95ace02a as previously established (Mermelstein and Papoutsakis, 1993) and recently detailed (Seo et al., 2024).
2.3 Media composition
Luria-Bertani (LB) medium was used for the propagation of Escherichia coli. For plates, 15 g/L Select Agar (MilliporeSigma, MA, USA) was added. Cac cells were grown in T-CGM-G as previously described (Charubin and Papoutsakis, 2019; Otten et al., 2022). 20 mL/L of potassium phosphate buffer consisting of 100 g/L of KH2PO4 and 125 g/L of K2HPO4 adjusted to a pH of 6.8 was used instead of 10 mL/L. Clj cells were grown in this T-CGM-G medium with the glucose removed, termed T-CGM-N. Bioreactors and serum bottles used T-GCM-G with varying levels of glucose. When needed, additional glucose was supplemented with a 600 g/L stock. 1.8 M NaOH and 1.5 M HCl were used for pH adjustment. To prepare the bioreactors, they were autoclaved with T-CGM-G before the phosphate buffer, glucose, fructose, Wolfe’s Vitamins, antibiotics, and antifoam were added. These additions were all injected into the bioreactors after the reactors cooled. The antifoam was a solution of 10% Antifoam 204 (Teknova). The media was deoxygenated inside the anaerobic chamber for at least 24 h or rapidly sparged on a degassing rig with N2. The bioreactors were also sparged with N2 for at least 2 h. When necessary, the following concentrations of antibiotics were used: 100 μg/mL ampicillin, 100 μg/mL erythromycin, 50 μg/mL clarithromycin. All antibiotics were dissolved in pure EtOH, which leads to a starting concentration of ~20 mM EtOH in the media.
2.4 Cell preparation for inoculation
To prepare Cac cells for coculture inoculation, single colonies were picked from an agar plate in the anaerobic chamber and transferred to 15 mL conical tubes containing 10 mL of T-CGM-G. The tubes were sealed and heat-shocked at 80 °C. The tubes were then transferred back to the anaerobic incubator. Once cooled to 40–50 °C, clarithromycin was added and the screwcaps were left loose to allow for gas exchange. Once the cells grew to an OD600 of 3–5, they were passaged at 10×, 100×, and 1,000× dilution to 50 mL conical tubes or GL-45 glass laboratory media bottles to reach the appropriate volume of cells at an OD600 of 3–5. Multiple dilutions were chosen to allow for optimal synchronization of Cac cells at mid-exponential phase to Clj cells at mid-exponential phase. Once the final inoculum OD600 measurements were taken, the cells were concentrated via centrifugation at 8000 × g for 8 min in 50 mL conical tubes and transferred to 30 mL syringes within the anaerobic chamber for inoculation into the bioreactors.
Clj cells were started from glycerol frozen stocks into YTAF-MES media (containing 14 g/L tryptone, 9 g/L yeast extract, 1.4 g/L L-arginine, 10 g/L fructose, and 10 g/L MES) (Cooksley et al., 2012). An entire 1 mL tube of this −80 °C freezer stock is added to a 160 mL serum bottle containing 25 mL YTAF-MES and clarithromycin. The headspace of the bottle is purged with a gas composed of 80% H2 and 20% CO2 and pressurized to 22 psi. These bottles are left to grow on a shaker at 37 °C. Once the OD600 of the Clj reaches 0.7–1.0, the cells are passaged to 500–1,000 mL serum bottles containing 20% of their total volume of T-CGM-N with clarithromycin. The bottles are likewise purged and pressurized to 22 psi with the H2/CO2 blend. Once the cells reach an OD600 of 0.7–1.0, an appropriate volume of cells are concentrated via centrifugation following the same process as Cac. Because Clj grows more slowly, the target OD600 ratio was 4–8 Clj: 1 Cac. Using syringes, Cac and Clj are quickly transferred from the anaerobic chamber to inoculate the external bioreactors.
2.5 Preparation of serum bottles for coculture experiments
To prepare 160 mL serum bottles for coculture experiments, the bottles were autoclaved with foil covering the aperture, passed into the anaerobic chamber, filled with 20 mL of T-CGM-G and concentrated cells (Section 2.4) for an initial target OD600 of 1.0, sealed with a sterile rubber stopper and crimped with an aluminum ring. The bottles were then passaged outside of the chamber where they are purged with gas for several minutes and pressurized to 20 psi. Normally, a blend of 80% H2 and 20% CO2 was used unless the experiment called for manipulation of the headspace composition as required in Section 3.1, where N2, H2, and a blend of 80% H2 and 20% CO2 were used to flush and pressurize the bottles.
2.6 Bioreactor setup and operation
The small-scale bioreactors used in this experiment have been described (Otten et al., 2022). Briefly, spinner flasks (Chemglass CLS-1400-100) were used with their spinner assembly removed. An active volume of 150–200 mL was used. This covers a pH probe that has been inserted through an open GL-32 cap and secured by a grommet. The other GL-32 port hosted a solvent delivery cap with four ports: sampling, base, sparging, and exhaust. The pH was controlled via networked, remotely-accessible pH controllers (Bluelab). They supplied 1.8 M NaOH to each reactor. Each vessel could be adjusted for sparging or headspace gassing through manipulation of the internal length of the PTFE gassing tube. Individual gas flow rates to each reactor were controlled by flowmeters (Dwyer). The reactors can use any nonflammable gas blend, including pure N2, pure CO2, or a blend, including 5% H2, 10% CO2, and 85% N2. Gas is exhausted through a tube that terminates in a water-filled flask to allow for visible bubbling and separation for the aerobic atmosphere. The vessels are placed in a large rectangular tub that sits over a bed of stir plates. The stir plates turn magnetic stir bars within each vessel that allow for gentle mixing. The tub is filled with water that is maintained at 37 °C by sous vide circulators.
All vessels are first autoclaved with DI water after being bleached and cleaned from prior runs. This water is then discarded, and then fresh T-CGM-G media, sans sugar, phosphate buffer, Wolfe’s vitamins, antibiotics, and antifoam, is added to the bioreactors and autoclaved. These supplements are added once the reactors cool from the autoclave. The pH probes are calibrated and sterilized with a solution of hard water and dilute bleach before being rinsed with DI water and ethanol and inserted into the port grommet. Once fully assembled and checked for leaks, the bioreactors are sparged with N2 at 50 cc/min for at least 2 h. The vessels are then ready for inoculation with syringes of concentrated cells that have been prepared in the anaerobic chamber (Section 2.4). To prepare high-starting cell density (SCD) cocultures, three times of the starting volume of the mid-exponential phase monocultures (Section 2.4) were concentrated to form the bioreactor inoculation culture. In monocultures of Cac, the target starting OD600 of the low-SCD cultures was 0.1. For the high-SCD cultures, the target starting OD600 was 0.3. For cocultures, the target starting low-SCD OD600 was 0.9 and the target starting high-SCD OD600 was 2.7 (which is a 1:8 ratio of Cac to Clj).
2.7 High-performance liquid chromatography analysis of metabolites
At each timepoint, the pH, OD600, and metabolic profile of the fermentations were measured. The metabolic composition of the media was analyzed as described (Carlson and Papoutsakis, 2017; Otten et al., 2022; Seo et al., 2024). An HPLC system (Agilent) was used with an Aminex HPX-87H column (Bio-Rad) and a running buffer of 5 mM H2SO4 at 0.5 mL/min. Additionally, to monitor the approximate glucose concentration of the fermentations, a refractometer was used (Milwaukee Instruments). The refractometer values are not reported in this work as the HPLC measurements are more accurate, but the refractometer results informed the supplemental addition of any requisite glucose before the HPLC samples could be processed.
2.8 RNA-FISH sample prep and flow-cytometric analysis
To track the Cac/Clj species ratio in the fermentations, RNA-FISH was employed as described using the ClosAcet and ClosLjun probes (Hill and Papoutsakis, 2024). Briefly, 1 mL of media from each timepoint was frozen at −20 °C. Later, the samples were thawed and combined with an equal volume of ice-cold 1×PBS, washed twice, and resuspended in a blend of 1:1 PBS and ethanol. The OD600 values of these samples were noted. Next, an ODeq of 0.15 of each sample was pelleted and dried at 46 °C to allow any ethanol to evaporate. The pellets were then mixed with 75 μL of a hybridization buffer containing, per 1 mL, 222 μL formamide, 554 μL DI water, 200 μL 5 M NaCl, 22 μL 1 M Tris–HCl, 1.1 μL 10% sodium dodecyl sulfate, and the ClosLjun and ClosAcet probes. The samples were incubated for 5 h at 46 °C.
Next, the cells are pelleted at 10,000 rpm for 10 min. five hundred microlitres of 48 °C washing buffer containing, per mL, 926 μL DI water, 43 μL 5 M NaCl, 20 μL 1 M Tris–HCl, 10 μL 5 M EDTA, and 1 μL 10% sodium dodecyl sulfate, was added and allowed to incubate for 25 min at 48 °C. This washing step was repeated once before the samples were pelleted and resuspended with 500 μL of 1xPBS. The samples were then refrigerated before being analyzed on a flow cytometer (CytoFLEX, Beckman Coulter) as described (Hill and Papoutsakis, 2024).
2.9 Metabolic flux analysis
The methodology of metabolic flux analysis used in this work is similar to that used previously (Papoutsakis, 1984; Desai et al., 1999). The chemical species and metabolic reactions (depicted in Figure 1) for the stochiometric model used to generate the stochiometric coefficient matrix are described in Appendix. In order to apply this method to a co-culture containing two distinct species we adopted a structured, unsegregated modeling approach. In other words, the intracellular metabolites are treated as mathematically unique chemical species despite being chemically identical. For instance, acetyl-CoA generated from glucose in Cac (acetyl-CoA-Cac) and acetyl-CoA generated from the WLP in Clj (acetyl-CoA-Clj) are chemically identical but can only be used in metabolic reactions related to the organism within which it was generated. The particular electron co-factors (i.e., NAD and ferredoxin) used in Cac metabolism is based on what is currently known about electron pathways in Cac (Foulquier et al., 2022). A non-specific electron carrier (i.e., ‘EC’) was used in the Clj reactions, because the electron pathways are less confidently known in Clj. This is an appropriate solution in the case of our model because we are not attempting to calculate ATP generation which is dependent on the interconversion of specific electron carriers (Katsyv and Müller, 2020). Some assumptions were made to prevent the model from being underdefined. First, it was assumed that all fructose is consumed by Clj, despite Cac’s ability to consume fructose. This assumption is sound because we have consistently observed that the rate of fructose consumption in monocultures of Cac is significantly slower than in co-culture. Second, we assumed that Clj’s only metabolic product is acetate. 13C-MFA on mixotrophically grown Clj shows that acetate is the sole product of energy metabolism (Dahle et al., 2022). The code used to solve the matrix equations for each data set was written in Python with Numerical Python and SciPy for matrix solving functions. Spyder was used as the integrated development environment. The code was compiled and tested on a Dell Inspiron 7390 with an Intel Core i7-8565U CPU and 16 GB of RAM.
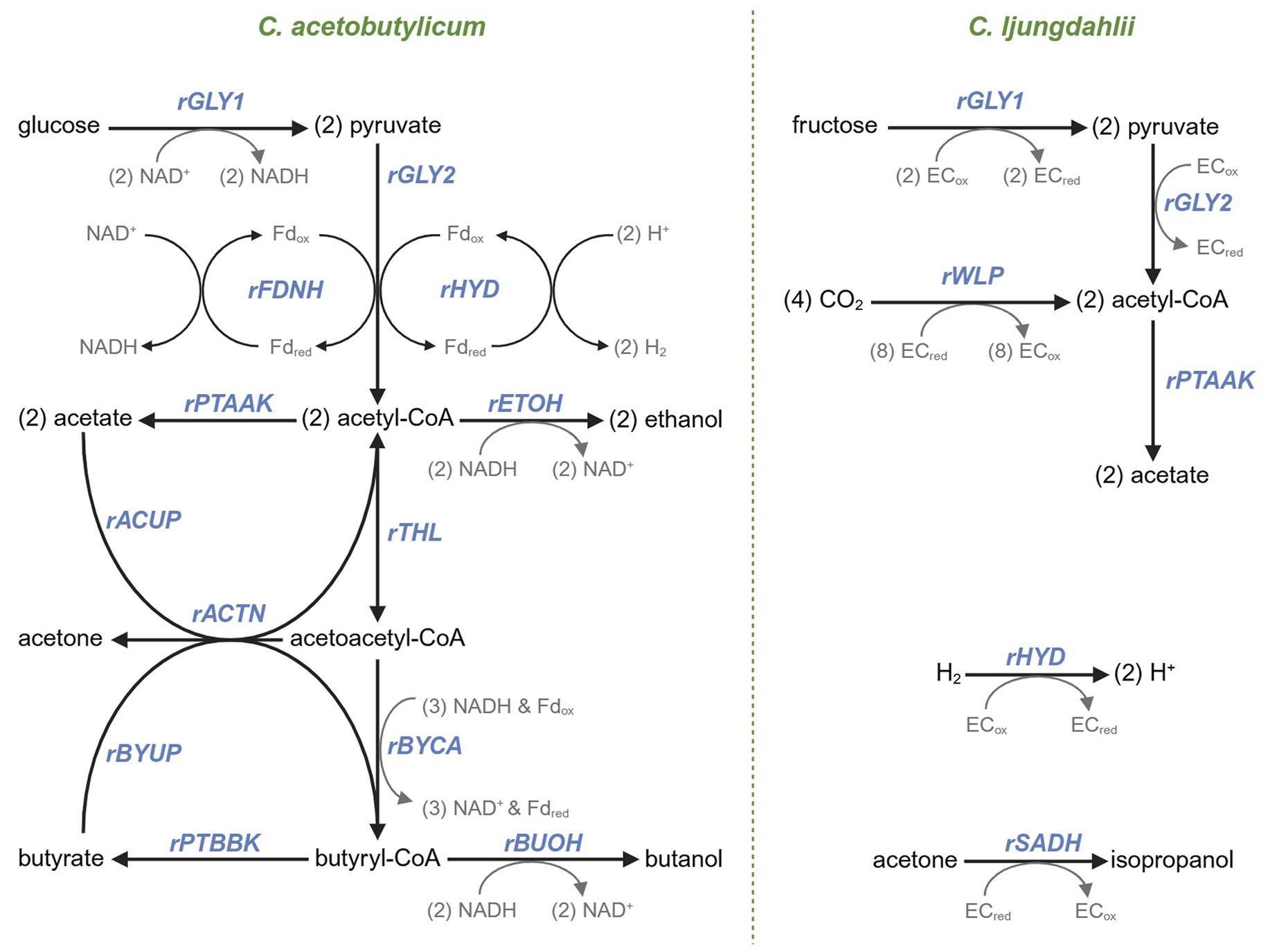
Figure 1. A metabolic map and associated fluxes of the primary metabolism of Cac (left) and Clj (right). In Cac, glycolysis catabolizes glucose to pyruvate (the rGLY1 flux). Pyruvate decarboxylation (rGLY2) forms acetyl-CoA, with electrons being conserved by forming (rFDNH flux) reduced ferredoxin (Fdred) which facilitates the conversion of protons to H2 (rHYD). Acetate (rPTAAK) and ethanol (rETOH) are produced from acetyl-CoA. The thiolase responsible for rTHL flux that catalyzes the formation of acetoacetyl-CoA from acetyl-CoA is expressed from the corresponding chromosomal gene (thl) and also from the copies of the same gene on the p95ace02a plasmid. Acetate (rACUP) and butyrate (rBYUP) reuptake via the CoA transferase is coupled to acetone formation (rACTN) from acetoacetyl-CoA. The three enzymatic step conversion of acetoacetyl-CoA to butyryl-CoA corresponds to the rBYCA flux. From butyryl-CoA, butyrate production is carried by the rPTBBK flux, and butanol production by the rBUOH flux. In Clj, fructose is catabolized to acetyl-CoA through the corresponding rGLY1 and rGLY2 fluxes. Acetyl-CoA is synthesized by the WLP pathway (the rWLP flux) from CO2. Acetate is synthesized through the rPTAAK pathway. Clj takes up (rHYD) H2 through its hydrogenase system and converts acetone to isopropanol (rSADH) using a secondary dehydrogenase. A non-specific electron carrier (‘EC’) is used in the Clj reactions because either several electron carriers exist or the electron carriers are not confidently known.
3 Results and discussion
3.1 Cac-ace02a and Clj cocultures in serum bottles with a H2 and CO2 atmosphere achieve superior product yields
First, we tested the impact of an H2 and H2/CO2 atmosphere on the Cac/Clj coculture. This phenomenon is especially critical in cocultures because of the acetogenic consumption of H2 and CO2 by Clj and the impact of H2 on Cac metabolism, whereby H2 removal at the local level due to utilization by C. ljungdahlii benefits C. acetobutylicum by reducing the feedback inhibition of its hydrogenase (Willis and Papoutsakis, 2025). While pH-controlled bioreactors are preferred, we used 160-mL serum bottles (20 mL liquid volume) because pressure increases the H2 availability to Clj and due to safety concerns with continuous, pressurized H2 feeding into small-scale glass bioreactors at the university setting. Two biological replicates of four conditions (Figure 2) were tested: monocultures with an N2 headspace, cocultures with an N2 headspace, cocultures with an H2 headspace, and cocultures with a headspace of 80% H2 and 20% CO2. The pH in the serum bottles was manually controlled with two base additions at 10 and 27 h (Figure 2H). It mostly remains in the range of 5.0–5.75.
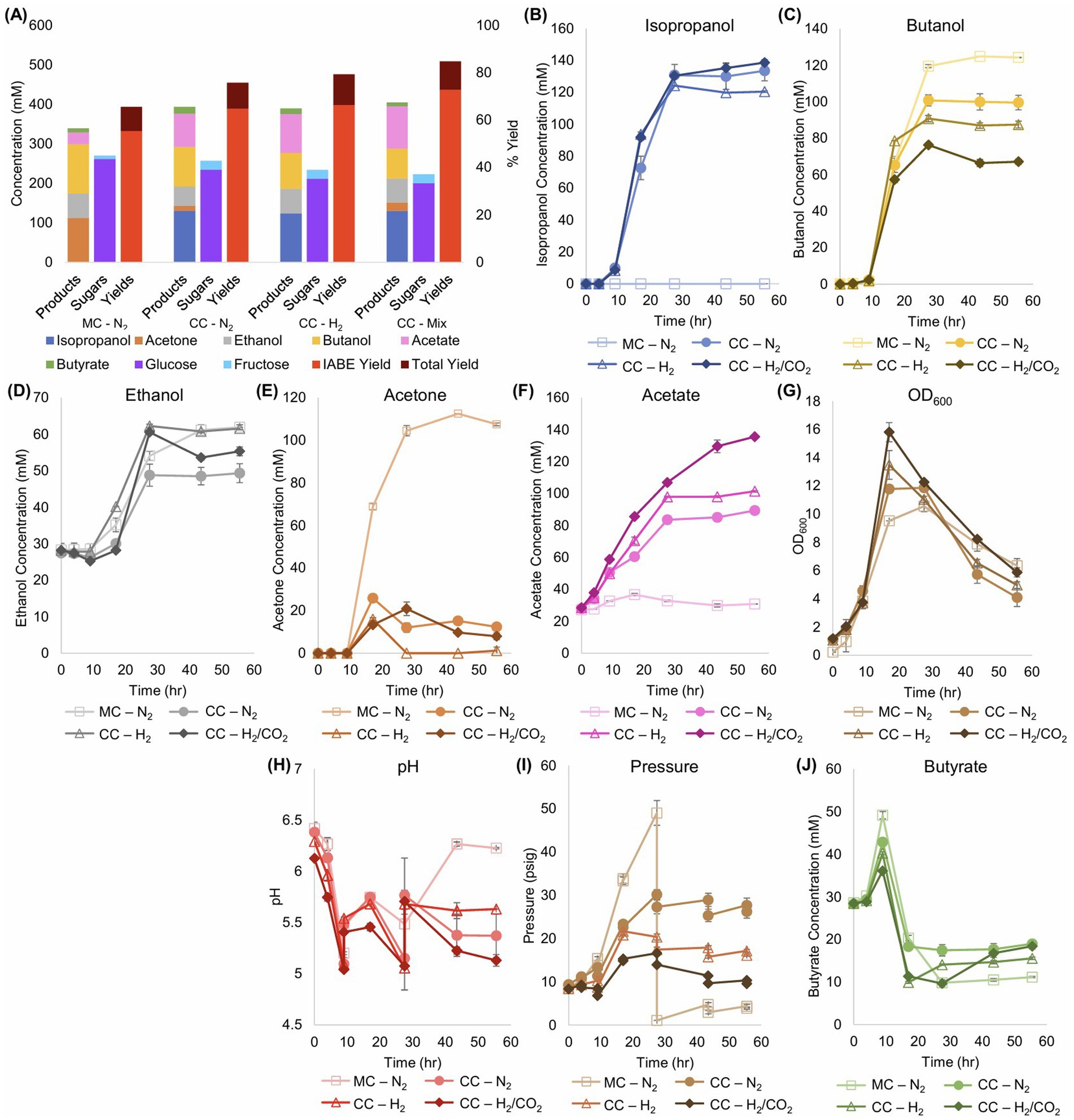
Figure 2. Metabolite production, sugar consumption, and metabolite yields of Cac and Clj cocultures in sealed serum bottles with different headspace gas composition: N2, H2 or Mix [H2/CO2 (80/20)]. MC refers to monocultures and CC refers to cocultures. (A) Summary of product concentrations, sugar consumption, and metabolite yields of the cocultures. The IABE yield refers to IPA, BuOH, EtOH, and acetone. The total yield also includes butyrate and acetate. In all cases, the coculture yields surpassed the monoculture yields, primarily through the production of higher combined concentrations of acetone and IPA versus acetone alone in the monoculture, and through the production of additional acetate. (B) Kinetic profile of IPA formation. The monoculture produces no IPA. (C) Kinetic profile of BuOH formation. The monoculture produced a statistically significantly higher amount of BuOH than any coculture. (D) Kinetic profile of EtOH formation. (E) Kinetic profile of acetone formation. In the cocultures, acetone accumulation is typically less than 20 mM. (F) Kinetic profile of acetate formation. In contrast to the CO2-containing cocultures, the monoculture produced barely any acetate. (G) Biomass (OD600) formation kinetics. The cocultures reached the highest peak OD600. (H) The pH kinetic profile. The pH of the bottles was manually adjusted at 9 and 28 h. (I) The pressure, in psig, of the sealed serum bottles. The monoculture bottles were bled of excess pressure at 28 h to avoid explosion. H2-containing cocultures (including the mixed H2/CO2 gas) displayed the lowest unadjusted gas headspace levels, thus demonstrating the consumption of both gas components. (J) Kinetic profile of butyrate formation and consumption.
Given that glucose utilization generates large quantities of H2 and CO2 (Papoutsakis, 1984) (Figure 1), with a N2 headspace in the coculture, pressures rose, indicating that more gas was produced than consumed by the cells. All bottles were initialized at 10 psig. The N2 monoculture was depressurized at 28 h at 50 psig to prevent bottle rupture, but cumulatively, 40 psi of pressure was produced. With both H2 and H2/CO2, the headspace pressure increased minimally and eventually decreased, demonstrating significant gas reassimilation (Figure 2I). The H2/CO2gas coculture peaked at 16 psig before returning to 10 psig, the starting pressure, thus giving rise to virtually no pressure accumulation.
The presence of Clj resulted in increases in both the IABE (Isopropanol, Acetone, Butanol, Ethanol) and total yields of this fermentation (Figure 2A), whereby the 80% H2 and 20% CO2 atmosphere performed the best. The IABE (ABE correctly as no isopropanol is produced by WT Cac) yield for the monoculture is 55.4% with the total yield at 65.6% right below the theoretical maximum of 66.7% for a monoculture, as approximately a third of all sugar carbon is lost to CO2 (Papoutsakis, 1984). The benefits of Clj in coculture can be seen beginning with the N2 headspace. While the ABE yield is 64.8%, acetate and butyrate production combine for an additional 10.9%, leading to a total yield of 75.7%. Clj, likely through direct cell-to-cell transfer (Charubin and Papoutsakis, 2019; Charubin et al., 2020), is able to assimilate the CO2 produced by Cac and use the H2 electrons for additional energy generation. The IABE yield is determined through Equation 1, with units of mM.
The total yield is determined with Equation 2, with units of mM.
The total yield increased to 79.2% with H2 and 84.7% with 80% H2 and 20% CO2 (Figure 2A). Due to electron cofactor interconversion constraints, the yield for acetone production from glucose by Cac monocultures is described by Equation 3 below, as derived (Willis et al., 2025).
If both glucose and fructose are considered as sugar substrates, the 80% H2 and 20% CO2 bottles produced a yield of 0.68 mol 3C (IPA) per mol of sugar (Supplementary Table S1), exceeding the monoculture yield of 0.42 mol 3C (acetone) per mol of sugar. If only glucose is considered, both the N2 and H2 bottles produced the theoretical maximum, and the 80% H2 and 20% CO2 bottles produced a yield of 0.76 (IPA). This result is consistent with our previous findings which show that Clj enables 3C yields in coculture that cannot be achieved in monoculture Cac fermentations (Willis et al., 2025). While EtOH production is not the focus of this work, a derivation of the yield for acetone and ethanol production from glucose is described by Equation 4 below (Willis et al., 2025).
All cocultures produced at least 130 mM of IPA (Figure 2B) with very little residual acetone (<20 mM), but the Cac monocultures produced about 110 mM of acetone (Figure 2E). In coculture, the presence of Clj led to production of more 3C metabolites (IPA and acetone): all cocultures produced at least 130 mM of IPA (Figure 2B) with very little residual acetone, but the monocultures produced about 110 mM of acetone (Figure 2E). This shows that the presence of Clj “coaxes” more acetone production by Cac, consistent with our previous observations (Charubin and Papoutsakis, 2019; Willis et al., 2025). This was accompanied by reduced BuOH production, from ~125 mM (in monoculture) to ~65 mM in the coculture with H2/CO2 (Figure 2C). Furthermore, the presence of Clj resulted in significant increases in the total cell mass (OD600) in the fermentation, with the H2/CO2 conditions being the highest: an OD600 difference of about 5.5–6 units within about 15 h (Figure 2G). Such OD600 levels cannot be accounted by Clj growth alone as Clj does not grow to high cell densities on H2/CO2, which are typically less than 2.5–3 OD600 units (Willis and Papoutsakis, 2025). This suggests that Clj enhances the growth of Cac likely due to H2 consumption (Willis and Papoutsakis, 2025) and through direct cell-to-cell contact (Charubin and Papoutsakis, 2019; Charubin et al., 2020). Acetate uptake in this coculture is incomplete. Some acetate is being utilized by Cac, which can be seen as the IABE yields exceed theoretical limits. In this experiment, Clj is cultured in its ideal growth condition, and it produces more acetate more quickly than Cac can utilize.
Significant differences in core metabolic fluxes between the culture conditions are depicted in Figure 3, with the remaining fluxes being largely the same. One difference between monoculture and cocultures is the distribution of carbon fluxes (rTHL, rBYCA, rACTN) through the acetoacetyl-CoA node in Cac. Before 10 h, virtually all acetoacetyl-CoA is fed into the rBYCA pathway (Figures 3D,G), and very little acetone (rACTN) is produced (Figure 3H). In the cocultures, about half of carbon is directed through the rACTN reaction (Figure 3H). The early induction of rACTN pathway is probably a response to rising acetate concentration in the cocultures, because the flux through rACUP is high during this time, but rBYUP is nearly zero (Figure 3B). The butyrate uptake pathway becomes active around 12 h. Clj is capable of activating the rACUP reaction since there is negligible flux through rACUP in the monoculture. This is a significant and unanticipated finding. We interpret this to be the result of higher rates of acetate production early in culture due to the growth associated with acetate formation by Clj (Figure 2F). In Clj, higher WLP fluxes in cocultures with H2/CO2 were expected because of the theoretically higher availability of CO2 and H2 to support autotrophic growth (Figure 3L). The resulting higher acetate production may explain why the rACUP flux stayed higher later into the culture in cocultures with H2/CO2, compared to cocultures with N2 or H2. This is more evident between 10 and 20 h. This is also mirrored in the higher rPTAAK and rHYD fluxes (Figures 3J,K).
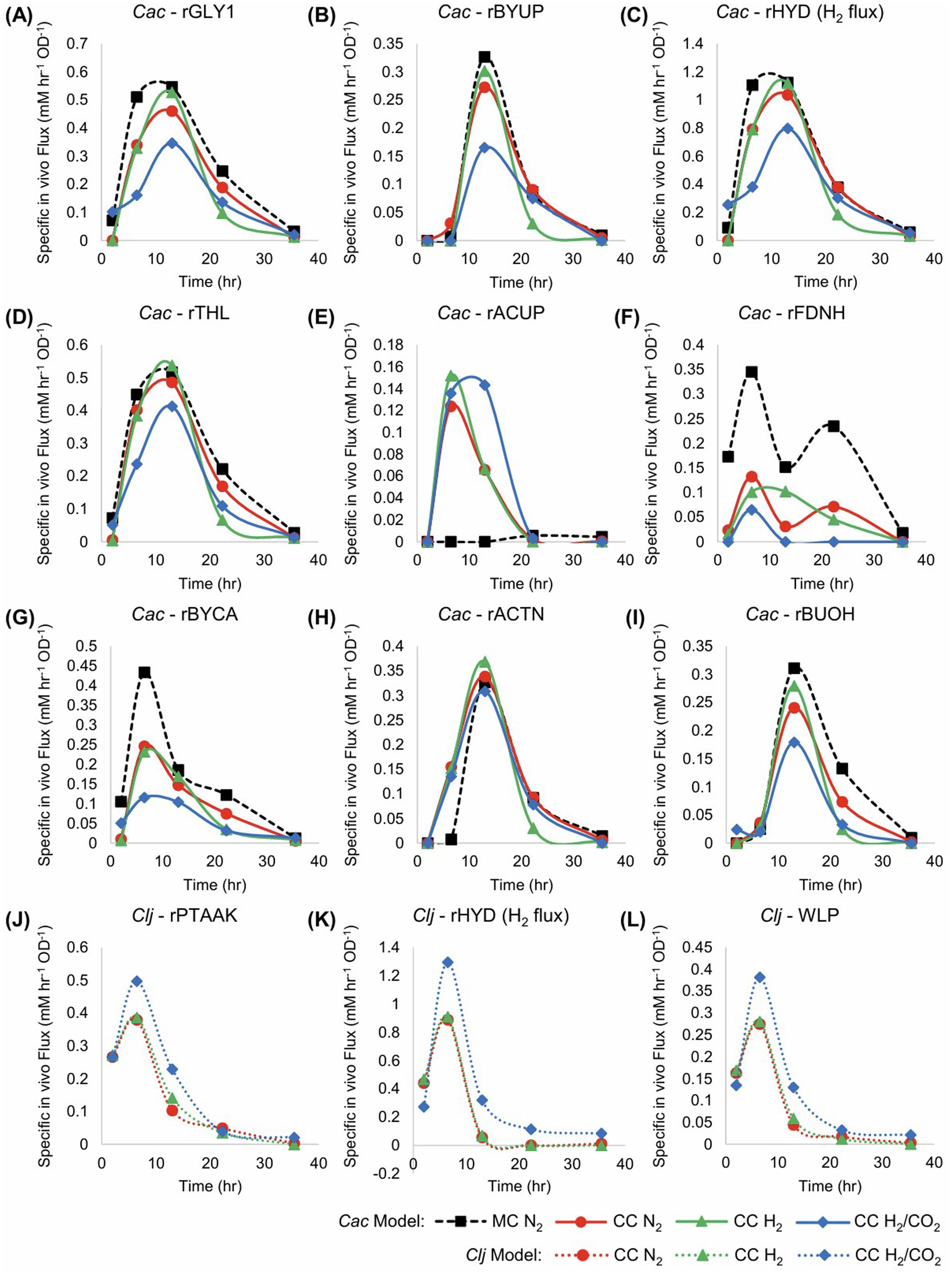
Figure 3. Fluxes of the monocultures (MC) and cocultures (CC) grown in pressurized serum bottles in units of mM/h/OD600, for the data of Figure 2. The flux maps are shown in Figure 1. Cac fluxes are depicted in panels (A–I), and Clj fluxes are depicted in panels (J–L) with dotted lines. The Cac monoculture with a nitrogen headspace is depicted with thick black dashed lines. The Cac monoculture has notably higher rGLY1, rBYUP, rHYD, rFDNH, rBYCA, and rBUOH fluxes. However, unlike the cocultures, it displays to no acetate uptake (E) (rACUP). Compared to the monoculture, in cocultures with the H2/CO2 atmosphere, Cac shows lower fluxes for rGLY1, rBYUP, rHYD, rTHL, rBYCA, and rBUOH, but higher fluxes for acetate uptake (rACUP). Clj clearly has higher fluxes with H2/CO2 atmosphere, suggesting the benefits of CO2 availability in the coculture setting. The pure N2 and pure H2 atmospheres performed similarly, suggesting that the use of CO2 generated by Cac did not require additional, exogenous H2 beyond the H2 produced by Cac.
In the Cac monoculture, a higher percentage of excess Fdred is used to generate NADH than in the cocultures, especially after 10 h (Figure 3F). As expected, this is correlated with butanol production. Butanol production requires NADH, which can be generated via the rFDNH reaction from excess Fdred. At the same time, the higher production of butyryl-CoA in the Cac monoculture, an intermediate in the butanol production pathway, produces additional Fdred (Figure 1). The net result is a higher flux through the rFDNH reaction. In the coculture, butanol production is less strong (Figure 2C), probably resulting in lower Fdred production. In summary, the cocultures result in better carbon management as well as improved solvent yields and selectivity (Figure 2A). In order to address two key deficiencies, lack of good pH control (Figure 2H) and incomplete utilization of glucose (Supplementary Figure S4B), we next utilized controlled-pH bioreactors.
3.2 Exploring core process parameters (pH, intermittent Clj additions, and gassing configurations) for better coculture performance
3.2.1 Evaluating the role of pH setpoints and intermittent Clj additions in coculture performance
With eight bioreactors, we tested a matrix of pH setpoints and intermittent Clj additions to assess their impact on metabolite yields. We considered the following: pH setpoints of 5.5 and 5.9, if a drop to pH 5.0 by 15 h is beneficial, and if additional Clj would be needed for full acetone conversion or additional acetate production. Past research has indicated that a drop in pH to 5.0 may be necessary to facilitate a shift in Cac from acidogenesis to solventogenesis (Grupe and Gottschalk, 1992; Girbal et al., 1995; Grimmler et al., 2011; Haus et al., 2011). To allow for this drop, the one-way pH controllers were set to maintain a floor of 5.0 after the bioreactors were initialized at a pH of 5.5 or 5.9. The acids produced by the cells would lower the pH until the controllers initialized pH control at 5.0. The addition of Clj was proposed both to allow for more complete acetone conversion—if needed—and to replenish the Clj population if it was negatively impacted by the low-pH or pH-recovery period. For the bioreactors that received additional Clj, 160 mL of Clj at an OD600 of 0.7 was concentrated and added to the bioreactors. Our results showed higher yields and increased BuOH and EtOH production with a pH of 5.5 (Supplementary Figures S1A,B,E,F, S2G,H). Isopropanol concentrations (titers) were similar across all conditions (Supplementary Figures S1C,D), and no significant acetone accumulation was observed with intermittent addition of Clj. At a pH of 5.9, higher concentrations of acetate and butyrate were produced than with a pH of 5.5 (Supplementary Figures S2C–F). Based on these results, we thus concluded that both the additional bolus of Clj when using these bioreactors without pressurized H2/CO2 (with the proviso that this may not hold when H2/CO2 are supplied at high pressure) and a pH drop to 5.0 were not needed. Notably, glucose consumption was higher in the bioreactors compared to the serum bottles due to the stable control of pH. We chose to move forward with a pH of 5.5 to produce a greater balance of IABE and lower concentrations of acetate and butyrate.
3.2.2 Evaluating different bioreactor gassing configurations
While the serum bottles produced high yields of our target molecules, they lack online pH control and cannot be practically scaled up. Thus, we utilized biological replicates in bioreactors with three gassing conditions: no gassing, headspace gassing only, or sparging. In this coculture, Cac produces H2 and CO2, and Clj consumes these gases (Charubin and Papoutsakis, 2019). Ideally, we would have liked to gas the bioreactors with exogenous H2/CO2 (80/20), but safety issues in these bioreactors, which lack sufficient exhaust in our labs, prevented that. We still wanted to examine the impact of two key modes of gassing and compare to no gassing as explained below. Thus, for all bioreactors, a blend of 85% N2, 10% CO2, and 5% H2 was selected at a flowrate of 10 mL/min. This allows for some addition of exogenous H2 and CO2 supply, while remaining below flammable limits. Two issues are associated with gassing from the physical point of view. We want to avoid any stripping of the IABE solvents while also avoiding O2 intrusion. Sparging, or direct bubbling of the gas into the fermentation media, would strip more IABE than any other option. Headspace gassing would be expected to lead to less IABE stripping, but it is minimally effective in terms of gas transfer due to a very small interfacial mass transfer rate (kLa). No gassing, which is when the gassing was only enabled during sampling to account for the very minor decrease in media volume, would be associated with the least stripping IABE while potentially allowing O2 intrusion. IABE production and productivity of the coculture was similar across all gassing conditions (Supplementary Figure S3). No negative impact on cell growth or IABE production was observed with no gassing, suggesting that O2 intrusion is not a concern. Concentrations of BuOH and IPA were similar across all gassing conditions after 50 h, indicating that there was no significant stripping of these metabolites. We thus conclude that any gassing configuration is suitable for future fermentation experiments.
3.3 Exploring impact of starting (inoculation) cell densities (SCD) and species population ratios in Cac-Clj cocultures and, for comparison, in Cac monocultures
Industrial fermentations are initiated with high cell densities in order to minimize the lag phase and shorten the duration of the fermentation. In this spirit, we next probed the role of different starting cell densities (SCDs) on metabolite production in cocultures and, for comparative reasons, in Cac monocultures, as Cac is the organism that metabolizes glucose and is responsible for ABE production. We also used RNA-FISH (Hill and Papoutsakis, 2024) to evaluate the changes in cell population ratios over time, which is important for the industrial scaling up of coculture fermentations.
3.3.1 A low starting cell density (SCD) is best for metabolite production by Cac monocultures
To goal of these experiments was to establish a baseline for Cac-ace02a (which has a different metabolite profile compared to the WT Cac) fermentation and examine whether higher SCDs of Cac would improve metabolite yields and titers. For ease of comparison with future coculture experiments, the bioreactors were sparged with 85% N2, 10% CO2, and 5% H2 at 5 CC/min. For the high SCD fermentations, the inocula were 3× as concentrated as the standard (referred to as low below) SCD fermentations, so the high SCD fermentations were inoculated at an OD600 of 0.3 while the low SCD fermentations were inoculated at 0.1. The low-SCD monocultures produced higher titers of BuOH (200 mM) and acetone (152 mM) while consuming less sugar (Figure 4). Thus, the yields in both low-SCD fermentations were higher. Similarly, the ratio of the carbon moles in fermentation products compared to the carbon moles of sugar consumed was 0.5 with a low SCD and 0.4 with a high SCD (Figure 4F).
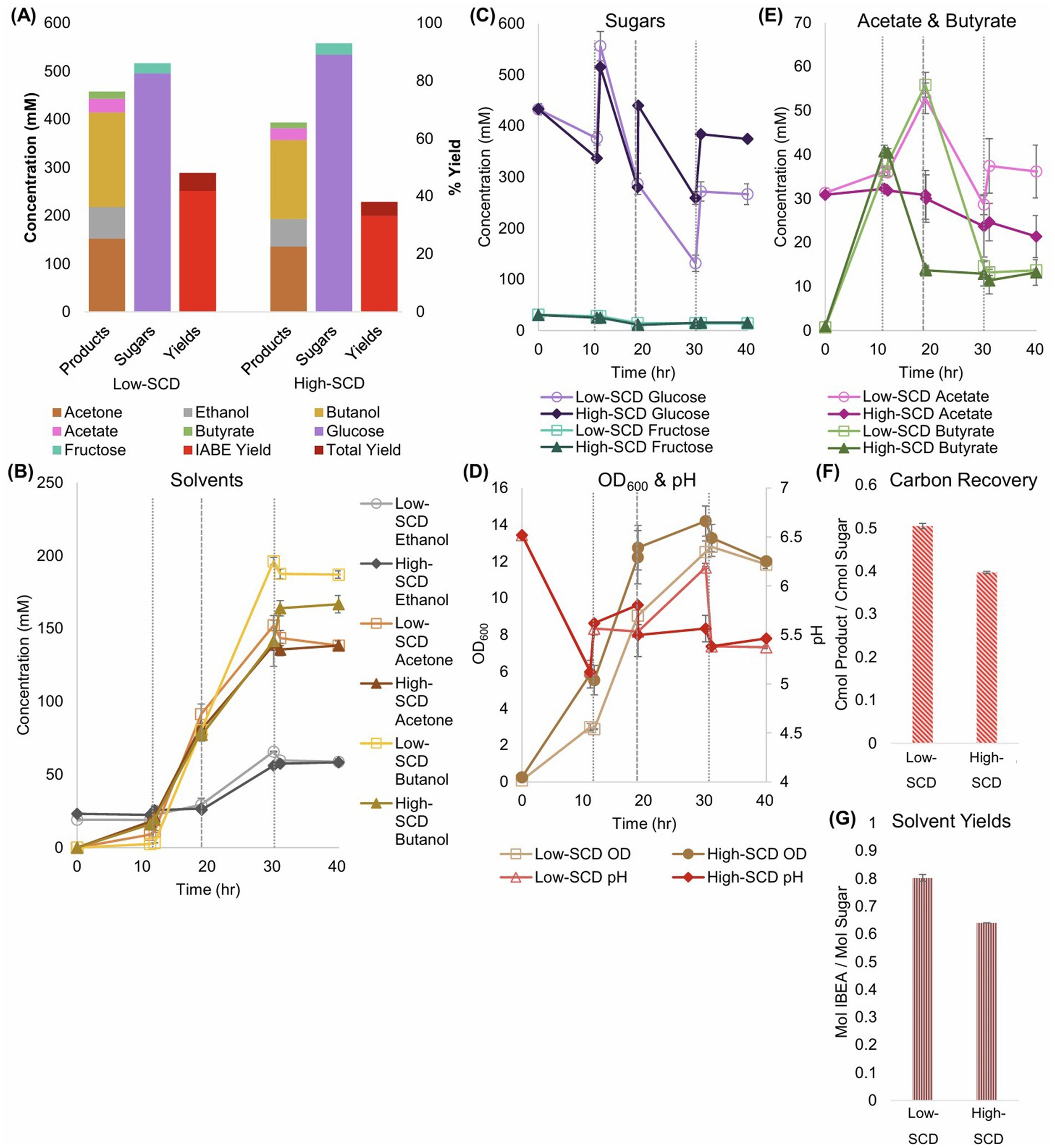
Figure 4. Metabolite production, sugar consumption, metabolite yields and carbon recoveries into metabolites of Cac monocultures in bioreactors at low- and high-starting cell densities (SCDs). Glucose was fed thrice in the high-SCD operation, at 10, 20, and 30 h, and twice in the low-SCD operation, at 10 and 30 h. The vertical, short dashed lines reflect glucose additions to all bioreactors. The vertical, long dashed lines at 20 h reflect glucose additions to only the high-OD bioreactors. (A) Summary of sugar consumption, metabolite concentrations and yields. The IABE yield refers to IPA, BuOH, EtOH, and acetone combined. Without Clj, the monoculture produces only acetone with no IPA. The total yield also includes butyrate and acetate. (B) The EtOH, acetone, and BuOH formation kinetics. (C) The kinetic profile of sugars in the bioreactors. (D) The OD600 (left y-axis) and pH (right y-axis) kinetic profiles. (E) The acetate and butyrate concentration kinetics. (F) Carbon-mol (C-mol) of products produced per C-mol of sugars consumed. (G) Mol of alcohols (IABE) produced per mol of sugar consumed.
In terms of maximum productivity, the low-SCD condition produced BuOH at 13.7 mM/h, EtOH at 4.4 mM/h, and acetone at 9.1 mM/h. The high-SCD condition produced BuOH at 11.1 mM/h, EtOH at 3.8 mM/h, and acetone at 9.2 mM/h. While the Cac in the high-SCD bioreactors grew more quickly and to a slightly higher OD600, by 30 h, and through the rest of the fermentation, the effects of the higher SCDs disappeared (Figure 4D). The pH values of the bioreactors were well-controlled at 5.5 after an initial drop to the controllers’ baseline and a manual addition of HCl at 30 h. In the end, the low-SCD monocultures produced 0.8 mol of ABE per mol of sugar, and the high-SCD conditions produced 0.64 mol/mol (Figure 4G).
For monoculture fluxes, the primary differences between the metabolism of cells when inoculated at high or low SCDs are the higher specific fluxes seen in the low-SCD condition prior to 24 h (Figure 5). After 24 h, the fluxes are almost indistinguishable, with the exception of the acetate uptake reaction (rACUP), which suggests a second, late phase of acetate uptake (Figure 4E). Overall, at high SCDs, the lower acetate concentrations (Figure 4E) are a reflection of higher rates of acetate uptake (rACUP; Figure 5E). The lower butyrate concentrations (Figure 4E) reflect better flux management around the acetyl-CoA node (Figures 5A,D,G). The lower butyrate formation at higher SCDs results in lower butanol formation and higher acetone selectivity [ratio of acetone to (butanol + ethanol)]. This behavior is also correlated with a lower efficiency of sugar utilization at higher SCDs, as evidenced by the carbon and solvent yields (Figures 4F,G).
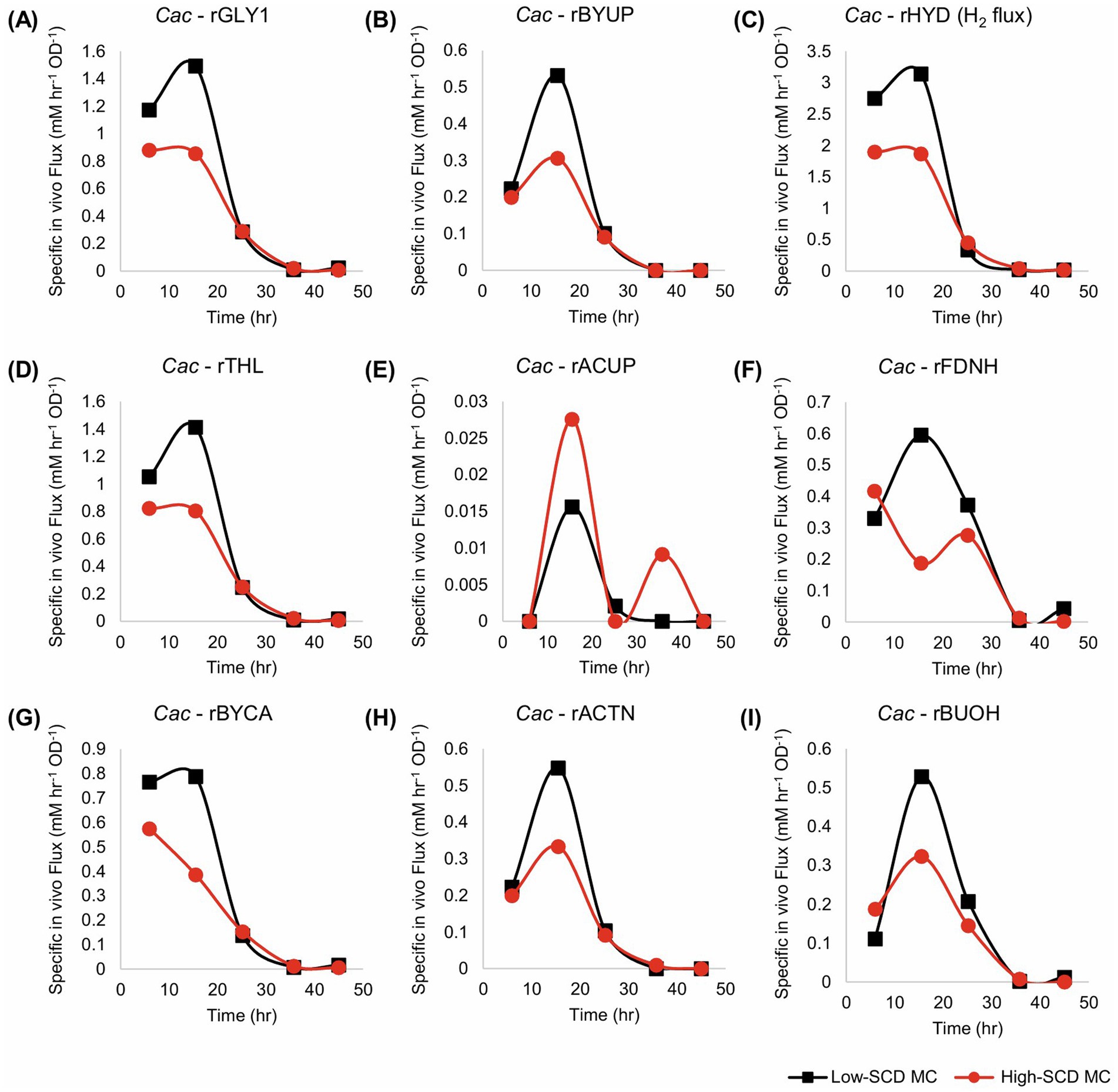
Figure 5. Metabolic fluxes (mM/h/OD600) of the monocultures corresponding to Figure 4. Cac fluxes are depicted with solid lines on panels (A–I). With the exception of rACUP, the cultures with a lower SCD displayed higher fluxes.
3.3.2 Higher coculture starting cell densities (SCDs) result in higher metabolite yields and shift the product balance toward isopropanol
For the experiments to assess the impact of higher SCDs in Cac/Clj cocultures, all experimental conditions were identical to those in Section 3.1 except for the addition of Clj at the beginning of the fermentation. The low SCD fermentations were inoculated with an OD600 of 1 and the high SCD fermentations were inoculated with an OD600 of 3 with the same species ratio of 1 Cac: 8 Clj. To gain clarity about the ratio of each species’ cell population during the fermentation, RNA-FISH was performed across all timepoints (Figures 6D,E; Supplementary Figures S5D,E). Our RNA-FISH method (Hill and Papoutsakis, 2024) identifies the viable population of each species. The non-viable cells are not stained or labeled and are displayed as gray in Figures 6D,E. RNA-FISH analysis showed that Clj predominated, by design, early in the cocultures, but by 10 h, Cac comprised the majority of cells. The high-SCD condition maintained a larger fraction of live Clj cells for longer, although the low-SCD condition ended with more live cells of both species.
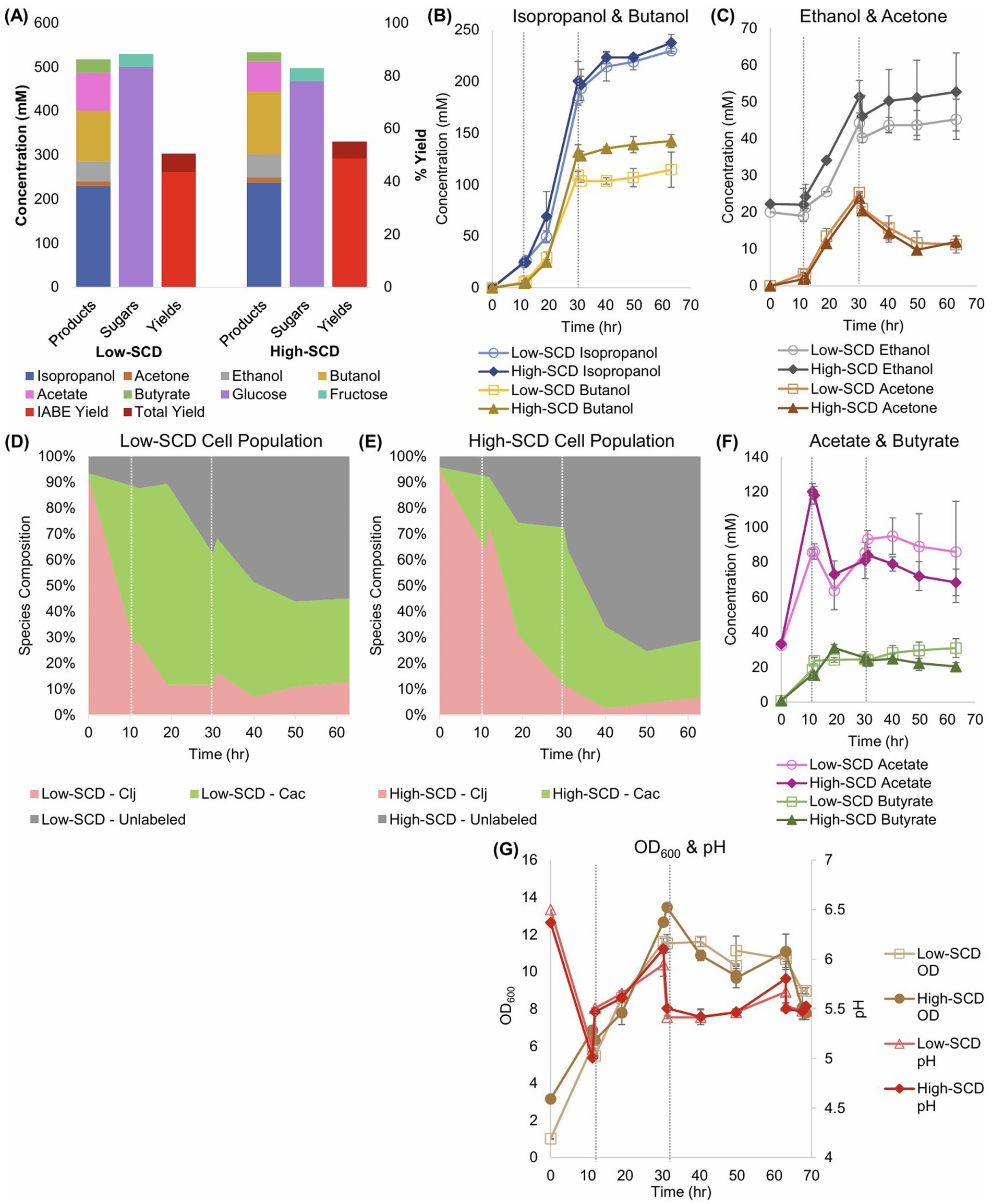
Figure 6. Sugar consumption, metabolite production and yields, and total biomass (OD600) of low- and high-SCD Cac and Clj cocultures. The vertical, short-dashed lines represent glucose additions to the bioreactors. (A) Summary of sugar consumption, metabolite concentrations and yields. IABE yield refers to only IPA, BuOH, EtOH, and acetone. The total yield also includes butyrate and acetate. (B) IPA and BuOH formation kinetics. (C) The EtOH and acetone formation kinetics. (D,E) Species composition over time, as determined by RNA-FISH labeling for (D) the low-SCD and (E) the high-SCD. (F) The acetate and butyrate concentration kinetics. Cac can produce and uptake both acetate and butyrate; acetate is the main product of Clj. (G) The OD600 (left y-axis) and pH (right y-axis) kinetic profiles.
IPA (230–237 mM), rather than BuOH (114–142 mM) or acetone (final concentrations of 12 mM), was the dominant product, and the IABE yield exceeded 48% in the high-SCD condition (Figure 6A). The total yield was 55%. Nearly 0.56 carbon moles of metabolites were produced for every carbon mole of sugar consumed in the high-SCD condition (Supplementary Figure S5B). In one high-SCD replicate, 0.93 moles of IABE were produced for every mole of sugar consumed for an average of 0.89 mol/mol (Supplementary Figure S5C). In the low-SCD condition, 0.77 mol IABE/mol sugar was produced. In terms of maximum productivity, both conditions produced IPA at 13.9 mM/h, which is markedly higher than the monoculture acetone production of 9.1–9.2 mM/h. This again shows that the presence of Clj leads to production of more 3C solvents, suggesting that Clj alters the metabolism of Cac to benefit IPA/3C product formation. The low-SCD condition produced BuOH at 8.3 mM/h, EtOH at 3.0 mM/h, and acetone at 1.6 mM/h. The high-SCD condition produced BuOH at 10.4 mM/h, EtOH at 2.8 mM/h, and acetone at 1.5 mM/h. In terms of three-carbon yields, the high-SCD cocultures doubled the yields of the high-SCD monocultures, 0.25–0.53 when only glucose is considered as the sugar substrate (Equation 3; Supplementary Table S1). The advantages of cocultures with CO2-capturing Clj in yields and product ratios are clear, and the shift in the product ratio to IPA from BuOH is notable. Acetate uptake by Cac is seen as the acetate concentrations decline over time (Figure 6F).
Flux calculations corresponding to these cocultures (Figure 7) reveal that the uptake of carboxylic acids for acetone production displays two stages. Before about 20 h, acetate but not butyrate is taken up (Figures 7B,E). After 20 h, mostly butyrate is taken up. This results in a wide peak for the rACTN flux profile (Figure 7H), though the carboxylic acid used to receive the CoA for the CoA-transferase (CtfAB) reaction differs over time. Peak specific H2 production (rHYD) occurred earlier in the low SCD conditions (Figure 7C), and this is likely caused by the increased specific glycolytic rate compared to the high SCD condition. This mirrors the behavior observed in the C. acetobutylicum monocultures where the specific glycolytic rates were higher in the low-SCD condition.
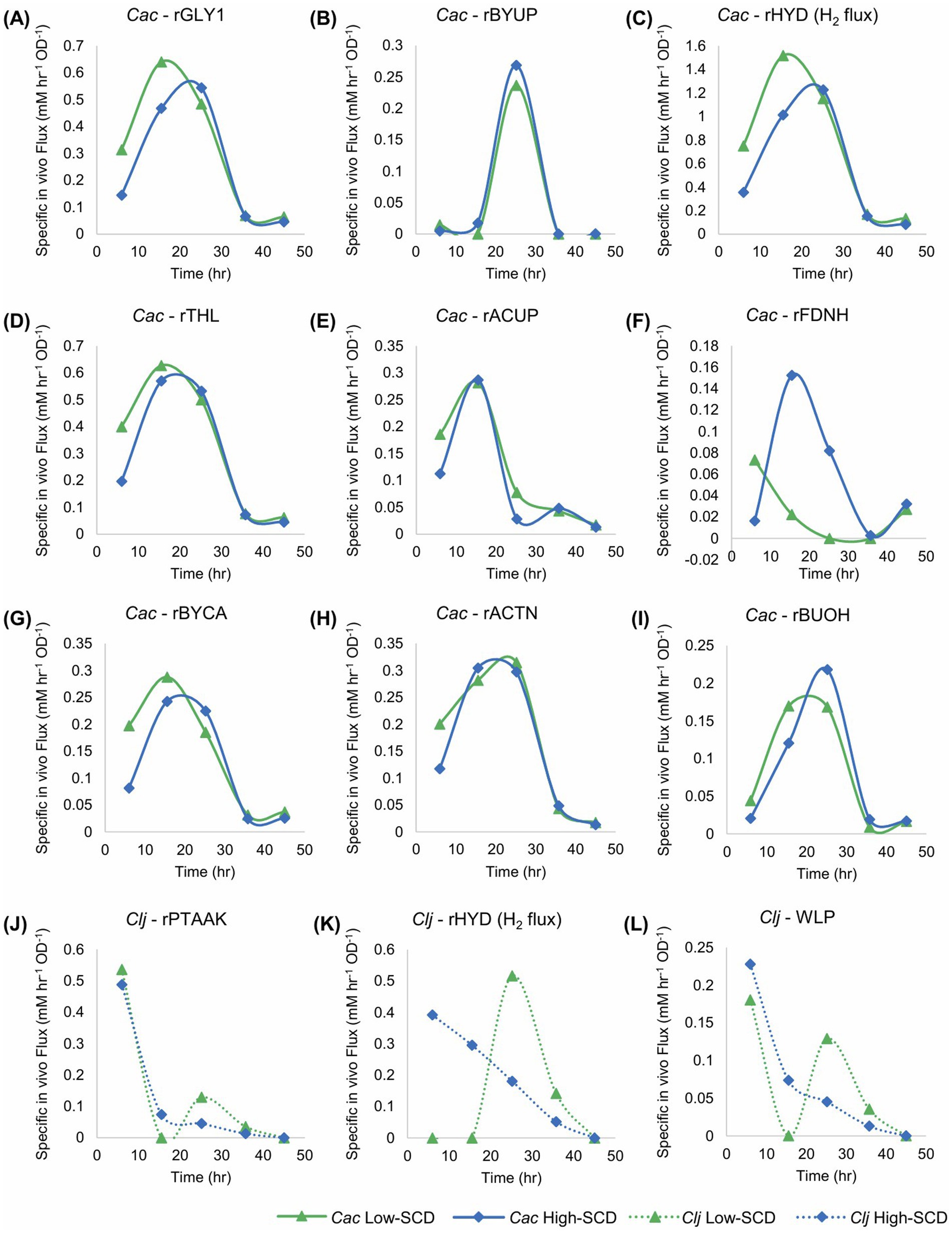
Figure 7. Metabolic fluxes (mM/h/OD600) of the cocultures shown in Figure 6. Cac fluxes are depicted with solid lines on panels (A–I) and Clj fluxes are depicted with dotted lines on panels (J–L).
The difference in the rFDNH flux (NADH generation from Fdred) is very distinct between the two culture conditions: it starts at a modest level and decreases at the low SCD condition to reflect the need for reduced Fd to produce H2 earlier and at a higher specific rate compared to the high SCD culture. In the latter, the rFDNH flux started low but peaked to higher levels around hour 15 [to feed the rBuOH and other alcohol fluxes (not shown)], reflecting a delayed and lower H2 production (compared to the low SCD culture).
The differences between Clj activity in the high and low SCD conditions is stark. Hydrogen uptake (the rHYD flux) is very distinct, as it starts high and decreases monotonically in the high SCD condition, while remaining zero up to 15 h and then increasing dramatically to reach a peak around 25 h. This means that the high SCD cultures are electron-limited until the very end of the active cell growth period. In contrast, the high SCD cultures are electron-sufficient until about 15 h becoming electron limited after that until the end of the active cell growth. What is also interesting is the period between 10 and 30 h where, in Cac, rACTN was very high (Figure 7H), consequently forming more of CO2 and H2 (compared to alcohol production) for Clj, but only at low SCD Clj seems to strongly engage its WLP during this time period as demonstrated by an increasing H2 uptake rate (rHYD) reaching a peak around 30 h (Figure 7K).
These cocultures ultimately represent the highest-performing IB or IABE fermentations to date. With IPA concentrations of nearly 250 mM, while maintaining strong BuOH production of 150 mM, these fermentations provide a strong possibility of sustainable biofuel production. Compared to prior literature, our result of 0.58 cmol product/cmol sugar is near the theoretical maximum of 0.65 (Charubin and Papoutsakis, 2019). Note also that this past work, which only produced 193 mM of IPA, was done in serum bottles, which can be pressurized and do not allow for any solvent evaporation.
3.4 Comparison to prior literature demonstrates superior IABE concentrations and improved productivities
Monocultures and cocultures have long been used to produce IPA and BuOH, often in blends and sometimes with acetone and EtOH as minor components as well. Our lab has discovered the expanded metabolic space that is enabled in cocultures of Cac and Clj (Charubin and Papoutsakis, 2019) and further probed these interactions to discover the exchange of cytoplasmic proteins, RNA, and DNA (Charubin et al., 2020; Charubin et al., 2024). Our work, when contextualized in the literature, demonstrated higher IPA and BuOH titers and productivities (Figure 8) A vs. B than most similar studies. Our process produces mostly IPA on a molar basis. Crucially, our process is fast and efficient, producing most of the IABE by 32 h and finishing by 63 h. Other cell systems reported fermentations lasting from 72 to 158 h, leading to a productivity decline of more than half (Figure 8 and Table 1). Systems based on engineered E. coli (Jojima et al., 2008) and Cupriavidus necator (Boy et al., 2023) monocultures have similar IPA productivities, but they do not produce butanol so the total IABE productivity and titers were much lower compared to our approach. Another study using a different E. coli strain in fed-batch fermentation achieved exceptional IPA productivity and titers (662 mM, 1986 mM-C, 2.05 mM-C per mM glucose) in 71 h, significantly exceeding our IABE titer over a similar timescale (Inokuma et al., 2010). This level of IPA production required nearly twice as much glucose consumption (967 mM, with associated supplementation every ~7 h) compared to our fermentation (496 mM combined glucose and fructose) which produced 237 mM IPA and 142 mM butanol (1,279 mM-C, 2.58 mM-C per mM glucose). This 26% percent increase in mol IABE per mol glucose yield of our fermentations, compared to the results from Inokuma, Liao et al., illustrates the value of our coculture approach which uses Clj to recapture meaningful quantities of CO2 from glycolysis and improve the carbon efficiency of glucose fermentation. Other monocultures and cocultures of C. beijerinckii produced lower IABE titer, lower IABE productivity, or both (Moon et al., 2015; Procentese et al., 2018; Dalal et al., 2019; Rochón et al., 2019; Cui et al., 2020; Carrié et al., 2022; Hocq and Sauer, 2022).
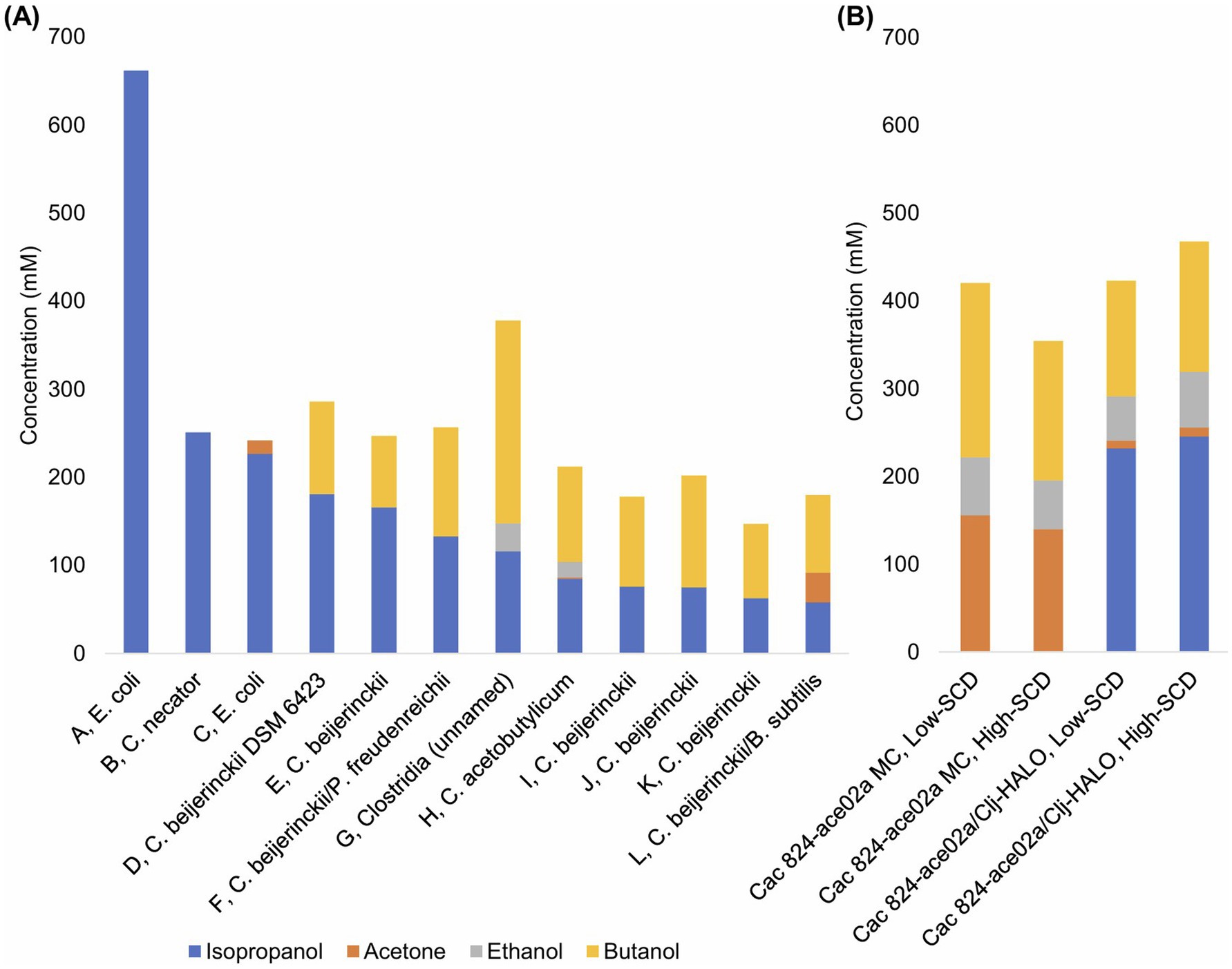
Figure 8. (A) Select metabolite concentrations in the literature for monoculture, coculture, and mixed culture productions of IABE. The letters A–K preceding the commas correspond to the row headings in Table 1. (B) Data from this study for low-SCD and high-SCD Cac 824-ace02a monocultures and Cac 824-ace02a/Clj-HALO cocultures. Note the higher total IABE titer and superior IPA:BuOH ratios.
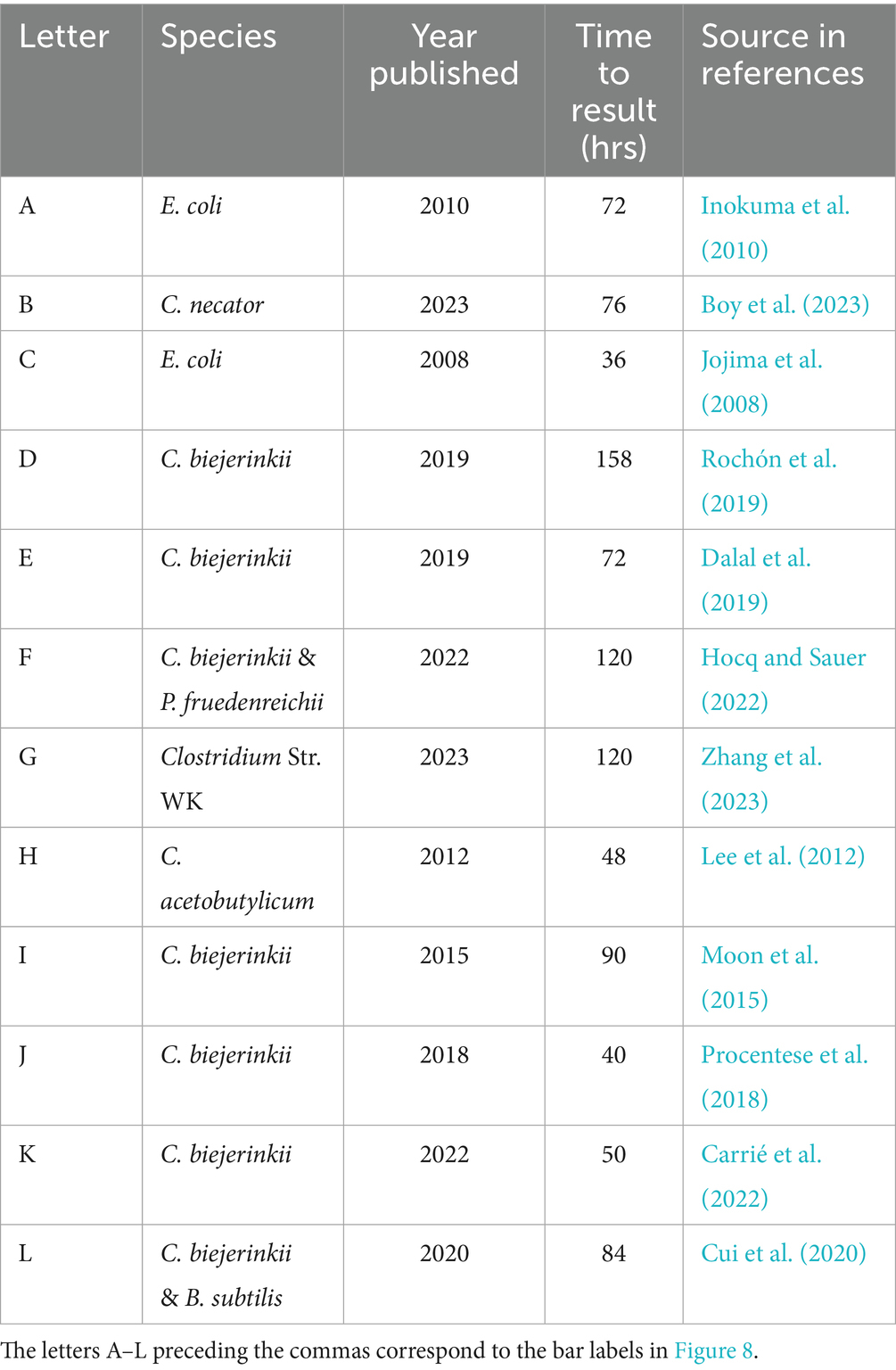
Table 1. Select species used in the literature for monoculture, coculture, and mixed culture productions of IABE.
In this study, the coculture fermentation was most efficient in sealed serum bottles (Figure 2A) as compared to laboratory-scale bioreactors (Figure 6A). This is due to the higher gas transfer rates in small volumes under pressure. Other possibilities for gas transfer exist, including packed beds with sparging of H2 and CO2. Our lab has recently explored this technology for the continuous production of IPA with a separate engineered Cac strain in coculture with Clj, demonstrating the tunability of this approach (Willis et al., 2025). The total volume of these reactors was 15x those of the bioreactors studied here. While H2 addition to bioreactors presents safety and economic considerations, Clj in coculture is also beneficial at many industrial scales, with or without exogenous H2. Cac produces waste CO2 and H2 regardless, and these carbons and electrons should be captured for maximum fermentation efficiency. In current commercial ABE production, including in Brazil and China, these gases are simply lost (Green, 2011). This decreases the profitability of the fermentation. Additionally, recovery of IABE can be improved through increasing the IABE concentrations, as we have here. Through improving the acetate uptake capability of Cac, an area of active research (Willis et al., 2025), product yields could be further increased. While acetate uptake has been shown here (Figures 4; 6, Supplementary Figures S2, S3), when Clj is grown in H2 and CO2 rich conditions (Figure 2A), its rate of acetate production exceeds the rate of acetate uptake by Cac.
4 Conclusion
There is a great need for renewable and sustainable production of essential chemicals from plant-based biomass, but with traditional fermentation techniques, a third of sugar carbon is lost as CO2. An effective way to overcome this limitation is through cocultures with an acetogen such as Clj. Through coculture cell interactions, we can both expand the metabolic space, enabling the production of isopropanol, while also capturing and reassimilating this CO2, bringing our yields to near-theoretical levels. Fortunately, this process is also efficient and scalable, with high isopropanol and butanol productivities that compare well to other proposed biological alternatives. Through RNA-FISH cell population tracking and coculture flux analysis, we have been able to build a superior model of coculture dynamics, observing significant differences in the rHYD and rFDNH fluxes across different experimental conditions. While the goal of this work is to produce IPA and BuOH with a high IPA to total alcohol ratio, this work provides a significant foundation for further improvements. Genetic engineering tools for Cac and Clj are well-understood, so changes to their cell metabolisms and to the balance of fermentation products are possible. For example, further genetic engineering could be employed to increase the acetate uptake of Cac. This will increase the IABE yield of this coculture fermentation. While acetate uptake by Cac is already significant, the production rate of acetate by Clj exceeds the current uptake capability of Cac. We are pursuing several strategies to increase the acetate uptake of Cac. Additionally, if either the acetone or 4-carbon pathways were knocked out, the balance of metabolites would shift to the remaining chemical species as acetate is converted from acetyl-CoA to acetoacetyl-CoA to the desired chemical species. The coculture could also be enhanced with another member species, such as C. kluyveri, or with expression of its genes, to enable chain elongation to produce 6–8 carbon products such as hexanol. From a process engineering perspective, continuous cultures, cell retention, and the feeding of H2 and CO2 could be implemented as this process is scaled to sustainably supply industrial chemical demands. Finally, cocultures should be considered for implementation in existing and planned industrial fermentations of ABE and other solvents. The generation of waste CO2 and H2 is a widespread concern, and the addition of Clj enables the reassimilation of these carbons and electrons into the existing metabolic process. Through these enhancements, the renewable production of essential chemicals can be realized.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
JO: Funding acquisition, Supervision, Project administration, Writing – original draft, Writing – review & editing, Formal analysis, Data curation, Visualization, Investigation, Methodology, Conceptualization, Validation. JH: Methodology, Validation, Investigation, Writing – review & editing, Software, Conceptualization, Visualization, Writing – original draft, Formal analysis. NW: Formal analysis, Data curation, Validation, Investigation, Writing – review & editing, Funding acquisition, Conceptualization. JD: Data curation, Visualization, Formal analysis, Investigation, Writing – review & editing. AD: Visualization, Formal analysis, Writing – review & editing, Data curation, Investigation. EP: Resources, Supervision, Project administration, Writing – review & editing, Conceptualization, Methodology, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by an ARPA-E project under contract AR0001505. NW and JD were supported in part by a U.S. Department of Education GAANN Fellowship under grant P200A210065.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1674318/full#supplementary-material
Abbreviations
Cac, C. acetobutylicum; Clj, C. ljungdahlii; IPA, isopropanol; BuOH, butanol; EtOH, ethanol; ABE, acetone-butanol-ethanol; IABE, isopropanol, acetone, butanol, ethanol; IB, isopropanol, butanol; SCD, starting cell density; WLP, Wood-Ljungdahl Pathway; WT, wild-type.
References
Adams, M. R. (2017). The birth of modern industrial microbiology: the acetone–butanol fermentation. Int. J. Hist. Eng. Technol. 87, 81–95. doi: 10.1080/17581206.2017.1329970
Boy, C., Lesage, J., Alfenore, S., Gorret, N., and Guillouet, S. E. (2023). Comparison of plasmid stabilization systems during heterologous isopropanol production in fed-batch bioreactor. J. Biotechnol. 366, 25–34. doi: 10.1016/j.jbiotec.2023.02.011
Carlson, E. D., and Papoutsakis, E. T. (2017). Heterologous expression of the Clostridium carboxidivorans CO dehydrogenase alone or together with the acetyl coenzyme a synthase enables both reduction of CO2 and oxidation of CO by Clostridium acetobutylicum. Appl. Environ. Microbiol. 83:e00829. doi: 10.1128/AEM.00829-17
Carrié, M., Velly, H., Ben-Chaabane, F., and Gabelle, J.-C. (2022). Modeling fixed bed bioreactors for isopropanol and butanol production using Clostridium beijerinckii DSM 6423 immobilized on polyurethane foams. Biochem. Eng. J. 180:108355. doi: 10.1016/j.bej.2022.108355
Charubin, K., Bennett, R. K., Fast, A. G., and Papoutsakis, E. T. (2018). Engineering Clostridium organisms as microbial cell-factories: challenges & opportunities. Metab. Eng. 50, 173–191. doi: 10.1016/j.ymben.2018.07.012
Charubin, K., Hill, J. D., and Papoutsakis, E. T. (2024). DNA transfer between two different species mediated by heterologous cell fusion in Clostridium coculture. MBio 15, e03133–e03123. doi: 10.1128/mbio.03133-23
Charubin, K., Modla, S., Caplan, J. L., and Papoutsakis, E. T. (2020). Interspecies microbial fusion and large-scale exchange of cytoplasmic proteins and RNA in a syntrophic Clostridium coculture. MBio 11:e02030. doi: 10.1128/mbio.02030-20
Charubin, K., and Papoutsakis, E. T. (2019). Direct cell-to-cell exchange of matter in a synthetic Clostridium syntrophy enables CO2 fixation, superior metabolite yields, and an expanded metabolic space. Metab. Eng. 52, 9–19. doi: 10.1016/j.ymben.2018.10.006
Cooksley, C. M., Zhang, Y., Wang, H., Redl, S., Winzer, K., and Minton, N. P. (2012). Targeted mutagenesis of the Clostridium acetobutylicum acetone-butanol-ethanol fermentation pathway. Metab. Eng. 14, 630–641. doi: 10.1016/j.ymben.2012.09.001
Cui, Y., He, J., Yang, K.-L., and Zhou, K. (2020). Aerobic acetone-butanol-isopropanol (ABI) fermentation through a co-culture of Clostridium beijerinckii G117 and recombinant Bacillus subtilis 1A1. Metabolic Eng. Commun. 11:e00137. doi: 10.1016/j.mec.2020.e00137
Dahle, M. L., Papoutsakis, E. T., and Antoniewicz, M. R. (2022). 13C-metabolic flux analysis of Clostridium ljungdahlii illuminates its core metabolism under mixotrophic culture conditions. Metab. Eng. 72, 161–170. doi: 10.1016/j.ymben.2022.03.011
Dalal, J., Das, M., Joy, S., Yama, M., and Rawat, J. (2019). Efficient isopropanol-butanol (IB) fermentation of rice straw hydrolysate by a newly isolated Clostridium beijerinckii strain C-01. Biomass Bioenergy 127:105292. doi: 10.1016/j.biombioe.2019.105292
Desai, R. P., Nielsen, L. K., and Papoutsakis, E. T. (1999). Stoichiometric modeling of Clostridium acetobutylicum fermentations with non-linear constraints. J. Biotechnol. 71, 191–205. doi: 10.1016/s0168-1656(99)00022-x
Desai, R. P., and Papoutsakis, E. T. (1999). Antisense RNA strategies for metabolic engineering of Clostridium acetobutylicum. Appl. Environ. Microbiol. 65, 936–945. doi: 10.1128/AEM.65.3.936-945.1999
Dusseaux, S., Croux, C., Soucaille, P., and Meynial-Salles, I. (2013). Metabolic engineering of Clostridium acetobutylicum ATCC 824 for the high-yield production of a biofuel composed of an isopropanol/butanol/ethanol mixture. Metab. Eng. 18, 1–8. doi: 10.1016/j.ymben.2013.03.003
Foulquier, C., Rivière, A., Heulot, M., Dos Reis, S., Perdu, C., Girbal, L., et al. (2022). Molecular characterization of the missing electron pathways for butanol synthesis in Clostridium acetobutylicum. Nat. Commun. 13:4691. doi: 10.1038/s41467-022-32269-1
Gabriel, C., and Crawford, F. (1930). Development of the butyl-acetonic fermentation industry. Ind. Eng. Chem. 22, 1163–1165. doi: 10.1021/ie50251a014
Gibbs, D. (1983). The rise and fall (...And rise?) of acetone/butanol fermentations. Trends Biotechnol. 1, 12–15. doi: 10.1016/0167-7799(83)90020-3
Girbal, L., Croux, C., Vasconcelos, I., and Soucaille, P. (1995). Regulation of metabolic shifts in Clostridium acetobutylicum ATCC 824. FEMS Microbiol. Rev. 17, 287–297. doi: 10.1111/j.1574-6976.1995.tb00212.x
Green, E. M. (2011). Fermentative production of butanol—the industrial perspective. Curr. Opin. Biotechnol. 22, 337–343. doi: 10.1016/j.copbio.2011.02.004
Grimmler, C., Janssen, H., Krauβe, D., Fischer, R.-J., Bahl, H., Dürre, P., et al. (2011). Genome-wide gene expression analysis of the switch between Acidogenesis and Solventogenesis in continuous cultures of Clostridium acetobutylicum. Microbial Physiol. 20, 1–15. doi: 10.1159/000320973
Grupe, H., and Gottschalk, G. (1992). Physiological events in Clostridium acetobutylicum during the shift from Acidogenesis to Solventogenesis in continuous culture and presentation of a model for shift induction. Appl. Environ. Microbiol. 58, 3896–3902. doi: 10.1128/aem.58.12.3896-3902.1992
Haus, S., Jabbari, S., Millat, T., Janssen, H., Fischer, R.-J., Bahl, H., et al. (2011). A systems biology approach to investigate the effect of pH-induced gene regulation on solvent production by Clostridium acetobutylicum in continuous culture. BMC Syst. Biol. 5:10. doi: 10.1186/1752-0509-5-10
Hill, J. D., and Papoutsakis, E. T. (2024). Species-specific ribosomal RNA-FISH identifies interspecies cellular-material exchange, active-cell population dynamics and cellular localization of translation machinery in clostridial cultures and co-cultures. mSystems 9, e00572–e00524. doi: 10.1128/msystems.00572-24
Hocq, R., and Sauer, M. (2022). An artificial coculture fermentation system for industrial propanol production. FEMS Microbes 3:xtac013. doi: 10.1093/femsmc/xtac013
Inokuma, K., Liao, J. C., Okamoto, M., and Hanai, T. (2010). Improvement of isopropanol production by metabolically engineered Escherichia coli using gas stripping. J. Biosci. Bioeng. 110, 696–701. doi: 10.1016/j.jbiosc.2010.07.010
Jiang, Y., Wu, R., Zhang, W., Xin, F., and Jiang, M. (2023). Construction of stable microbial consortia for effective biochemical synthesis. Trends Biotechnol. Placeholder Text41: 1430–1441. doi: 10.1016/j.tibtech.2023.05.008
Jojima, T., Inui, M., and Yukawa, H. (2008). Production of isopropanol by metabolically engineered Escherichia coli. Appl. Microbiol. Biotechnol. 77, 1219–1224. doi: 10.1007/s00253-007-1246-8
Jones, S. W., Fast, A. G., Carlson, E. D., Wiedel, C. A., Au, J., Antoniewicz, M. R., et al. (2016). CO2 fixation by anaerobic non-photosynthetic mixotrophy for improved carbon conversion. Nat. Commun. 7:12800. doi: 10.1038/ncomms12800
Katsyv, A., and Müller, V. (2020). Overcoming energetic barriers in acetogenic C1 conversion. Front. Bioeng. Biotechnol. 8:621166. doi: 10.3389/fbioe.2020.621166
Lee, J., Jang, Y.-S., Choi, S. J., Im, J. A., Song, H., Cho, J. H., et al. (2012). Metabolic engineering of Clostridium acetobutylicum ATCC 824 for isopropanol-butanol-ethanol fermentation. Appl. Environ. Microbiol. 78, 1416–1423. doi: 10.1128/AEM.06382-11
Lee, J. Y., Jang, Y.-S., Lee, J., Papoutsakis, E. T., and Lee, S. Y. (2009). Metabolic engineering of Clostridium acetobutylicum M5 for highly selective butanol production. Biotechnol. J. 4, 1432–1440. doi: 10.1002/biot.200900142
Lutke-Eversloh, T., and Bahl, H. (2011). Metabolic engineering of Clostridium acetobutylicum: recent advances to improve butanol production. Curr. Opin. Biotechnol. 22, 634–647. doi: 10.1016/j.copbio.2011.01.011
Mermelstein, L. D., and Papoutsakis, E. T. (1993). In vivo methylation in Escherichia coli by the Bacillus subtilis phage phi 3T I methyltransferase to protect plasmids from restriction upon transformation of Clostridium acetobutylicum ATCC 824. Appl. Environ. Microbiol. 59, 1077–1081. doi: 10.1128/aem.59.4.1077-1081.1993
Moon, Y. H., Han, K. J., Kim, D., and Day, D. F. (2015). Enhanced production of butanol and isopropanol from sugarcane molasses using Clostridium beijerinckii optinoii. Biotechnol. Bioprocess Eng. 20, 871–877. doi: 10.1007/s12257-015-0323-6
Otten, J. K., Zou, Y., and Papoutsakis, E. T. (2022). The potential of caproate (hexanoate) production using Clostridium kluyveri syntrophic cocultures with Clostridium acetobutylicum or Clostridium saccharolyticum. Front. Bioeng. Biotechnol. 10:965614. doi: 10.3389/fbioe.2022.965614
Papoutsakis, E. T. (1984). Equations and calculations for fermentations of butyric acid bacteria. Biotechnol. Bioeng. 26, 174–187. doi: 10.1002/bit.260260210
Procentese, A., Raganati, F., Navarini, L., Olivieri, G., Russo, M. E., and Marzoccchella, A. (2018). Coffee silverskin as a renewable resource to produce butanol and isopropanol. Chem. Eng. Transl. 64, 139–144. doi: 10.3303/CET1864024
Richter, H., Molitor, B., Diender, M., Sousa, D. Z., and Angenent, L. T. (2016). A narrow pH range supports butanol, hexanol, and octanol production from syngas in a continuous co-culture of Clostridium ljungdahlii and Clostridium kluyveri with in-line product extraction. Front. Microbiol. 7:1773. doi: 10.3389/fmicb.2016.01773
Rochón, E., Cebreiros, F., Ferrari, M. D., and Lareo, C. (2019). Isopropanol-butanol production from sugarcane and sugarcane-sweet sorghum juices by Clostridium beijerinckii DSM 6423. Biomass Bioenergy 128:105331. doi: 10.1016/j.biombioe.2019.105331
Seo, H., Capece, S. H., Hill, J. D., Otten, J. K., and Papoutsakis, E. T. (2024). Butyrate as a growth factor of Clostridium acetobutylicum. Metab. Eng. 86, 194–207. doi: 10.1016/j.ymben.2024.10.005
Sillers, R., Al-Hinai, M. A., and Papoutsakis, E. T. (2009). Aldehyde-alcohol dehydrogenase and/or thiolase overexpression coupled with CoA transferase downregulation lead to higher alcohol titers and selectivity in Clostridium acetobutylicum fermentations. Biotechnol. Bioeng. 102, 38–49. doi: 10.1002/bit.22058
Streett, H. E., Kalis, K. M., and Papoutsakis, E. T. (2019). A strongly fluorescing anaerobic reporter and protein-tagging system for Clostridium organisms based on the fluorescence-activating and absorption-shifting tag protein (FAST). Appl. Environ. Microbiol. 85, e00622–e00619. doi: 10.1128/AEM.00622-19
Willis, N. B., Otten, J. K., Seo, H., Munasinghe, P. C., Hill, J. D., and Papoutsakis, E. T. (2025). Enabling supratheoretical isopropanol yields from carbon-negative glucose fermentations with Clostridium acetobutylicum-Clostridium ljungdahlii cocultures. bioRxiv. doi: 10.1101/2025.1107.1114.664808
Willis, N. B., and Papoutsakis, E. T. (2025). Separate, separated, and together: the transcriptional program of the Clostridium acetobutylicum-Clostridium ljungdahlii syntrophy leading to interspecies cell fusion. Msystems 10, e00030–e00025. doi: 10.1128/msystems.00030-25
Zhang, F., Zhang, K., Zhang, Z., Chen, H.-Q., Chen, X.-W., Xian, X.-Y., et al. (2023). Efficient isopropanol-butanol-ethanol (IBE) fermentation by a gene-modified solventogenic Clostridium species under the co-utilization of Fe(III) and butyrate. Bioresour. Technol. 373:128751. doi: 10.1016/j.biortech.2023.128751
Keywords: Clostridium acetobutylicum , Clostridium ljungdahlii , coculture, isopropanol, butanol, consortia, metabolic flux analysis
Citation: Otten JK, Hill JD, Willis NB, Dougherty J, Dalton A and Papoutsakis ET (2025) Cross-talk between engineered Clostridium acetobutylicum and Clostridium ljungdahlii in syntrophic cocultures enhances isopropanol and butanol production. Front. Microbiol. 16:1674318. doi: 10.3389/fmicb.2025.1674318
Edited by:
Jun Liu, Central South University of Forestry and Technology, ChinaReviewed by:
Fu-Li Li, Chinese Academy of Sciences (CAS), ChinaHongzhen Luo, Huaiyin Institute of Technology, China
Copyright © 2025 Otten, Hill, Willis, Dougherty, Dalton and Papoutsakis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eleftherios T. Papoutsakis, ZXBhcHNAdWRlbC5lZHU=
 Jonathan K. Otten
Jonathan K. Otten John D. Hill1,2
John D. Hill1,2 Eleftherios T. Papoutsakis
Eleftherios T. Papoutsakis