- 1Institute of Microbiology and Molecular Genetics, University of Punjab, Lahore, Pakistan
- 2Department of Biology, College of Science, Imam Mohammad Ibn Saud Islamic University (IMSIU), Riyadh, Saudi Arabia
- 3Department of Biology, College of Science, Qassim University, Buraydah, Saudi Arabia
Antibiotic resistance is a critical threat to modern medicine, necessitating new strategies against multi-drug resistant bacteria (MDR). This narrative review evaluates the published evidence on Syzygium aromaticum (clove) and its principal phytochemicals, with particular focus on activity against MDR pathogens. We describe the chemical profile, notably eugenol, quercetin, kaempferol, β-caryophyllene, and α-humulene, and summarize reported in vitro activity and substantial biofilm inhibition (up to about 90%) against clinically relevant MDR bacteria such as Escherichia coli, Staphylococcus aureus, and Klebsiella pneumoniae. Mechanistically, clove phytochemicals act through a hierarchical cascade in which membrane perturbation is the primary initiating event. Lipophilic constituents (notably eugenol) partition into and disorder the phospholipid bilayer, causing rapid loss of membrane potential, K+/ATP efflux and collapse of the proton-motile force (PMF). This primary membrane failure then enables secondary intracellular consequences, impaired electron transport and tricarboxylic acid cycle (TCA-cycle) enzyme activity, increased electron leakage with reactive oxygen species (ROS) generation, macromolecular damage (lipid peroxidation, protein oxidation, DNA strand injury), and functional inhibition of energy-dependent efflux, which together produce bactericidal outcomes and potentiate synergy with conventional antibiotics. Importantly, phytochemicals from clove frequently act synergistically with conventional antibiotics, lowering antibiotic MICs by approximately 4–128-fold and enhancing agents including colistin, imipenem, and amikacin. We evaluate formulation approaches (nano-emulsions, liposomes, solid-lipid nanoparticles) aimed at improving delivery and bioavailability, and we review limited preclinical and early clinical observations that suggest benefits in settings such as ventilator-associated pneumonia and MRSA wound healing. Critical translational gaps remain. Robust in vivo efficacy data, standardized pharmacokinetic and toxicology characterization, stability studies, and rigorous clinical trials are urgently needed. We conclude by proposing a focused research roadmap to validate and responsibly translate clove-derived candidates as adjuncts to existing antibiotic regimens.
1 Introduction
Antimicrobial resistance has emerged as a significant global health concern in the 21st century. Projections indicate a substantial increase in mortality due to drug-Resistant pathogens, with approximately 1.91 million deaths directly attributable to it and 8.22 million associated deaths anticipated by 2050 (Naghavi et al., 2024). The World Health Organization (WHO) has classified antimicrobial resistance as a leading global public health threat (Chawla et al., 2022). The overuse and misuse of antibiotics in humans and animals create selection pressure, leading to the emergence and persistence of resistant bacterial strains (Elhaddadi et al., 2024). Gram-negative bacterial pathogens are responsible for the majority of hard-to-treat illnesses, and they are resistant to almost all antimicrobials (Darwish et al., 2022). The CDC (Centers for Disease Control and Prevention) estimates that antibiotic-resistant infections in the United States affect over 2.8 million individuals annually, resulting in approximately 35,000 deaths (Sulong et al., 2024).
Antimicrobial resistance (AMR) can be defined as the capacity of microorganisms, such as bacteria, fungi, parasites, and viruses to survive exposure to antimicrobial agents such as antibiotics, antifungals, antivirals, and antiparasitic, thereby reducing or nullifying treatment efficacy (Tang et al., 2023). While multi-drug resistant (MDR) bacteria are a subset of AMR; which refers to bacteria that display non-susceptibility to at least one antibiotic in three or more different classes of antibacterial drugs (Alkofide et al., 2020). The increasing burden of AMR and MDR pathogens not only leads to heightened healthcare costs and reduced productivity but is also projected to have severe economic implications, potentially raising global costs to $100 trillion annually by 2050 (Domínguez et al., 2021). MDR bacteria employ various resistance mechanisms, including the production of antibiotic-degrading enzymes and extracellular structures such as biofilms, which inhibit antibiotic penetration and action (Muhammad and Shoge, 2023). Horizontal gene transfer further exacerbates the problem by facilitating the spread of resistance genes, making treatment options increasingly limited (Rao et al., 2023). Horizontal gene transfer, via conjugation, transformation and phage-mediated transduction, rapidly mobilizes resistance determinants across strains, species and ecological niches and creates a shared resistome that accelerates the emergence of MDR pathogens (Lawani et al., 2023). Therefore, multi-target agents or multi-component therapies, which require simultaneous compromise of independent cellular pathways, are a genetically justified alternative to single-target antibiotics.
To address this crisis, the World Health Organization (WHO) has emphasized the ineffectiveness of current antibiotics and underscored the urgent need to develop alternative strategies to combat common pathogens that now threaten medical advancements (Gupta and Bhandary, 2024). The global shortage of novel antibiotics represents a significant research gap that threatens modern medicine in the face of escalating antibacterial resistance and the antibiotic development pipeline remains alarmingly sparse. This crisis demands unified global political action. UN member states must prioritize AMR and MDR nationally while mobilizing sustainable funding and bridging innovation with equitable access. In 2024, the World Health Organization (WHO) released its updated Bacterial Priority Pathogens List (BPPL), identifying 15 families of life-threatening, antibiotic-resistant bacteria stratified into critical, high, and medium priority categories (Figure 1). This list serves as an urgent global alert, underscoring the escalating threat these pathogens pose to human health and healthcare systems. It highlights the pressing need for both the discovery of novel antibacterial agents and innovative strategies, such as synergistic combinations to restore the efficacy of existing antibiotics, to curb the spread of these formidable drug-resistant infections (Jesudason, 2024). Without coordinated investment and policy alignment, scientific breakthroughs will fail to reach those most vulnerable (Balasegaram et al., 2024).
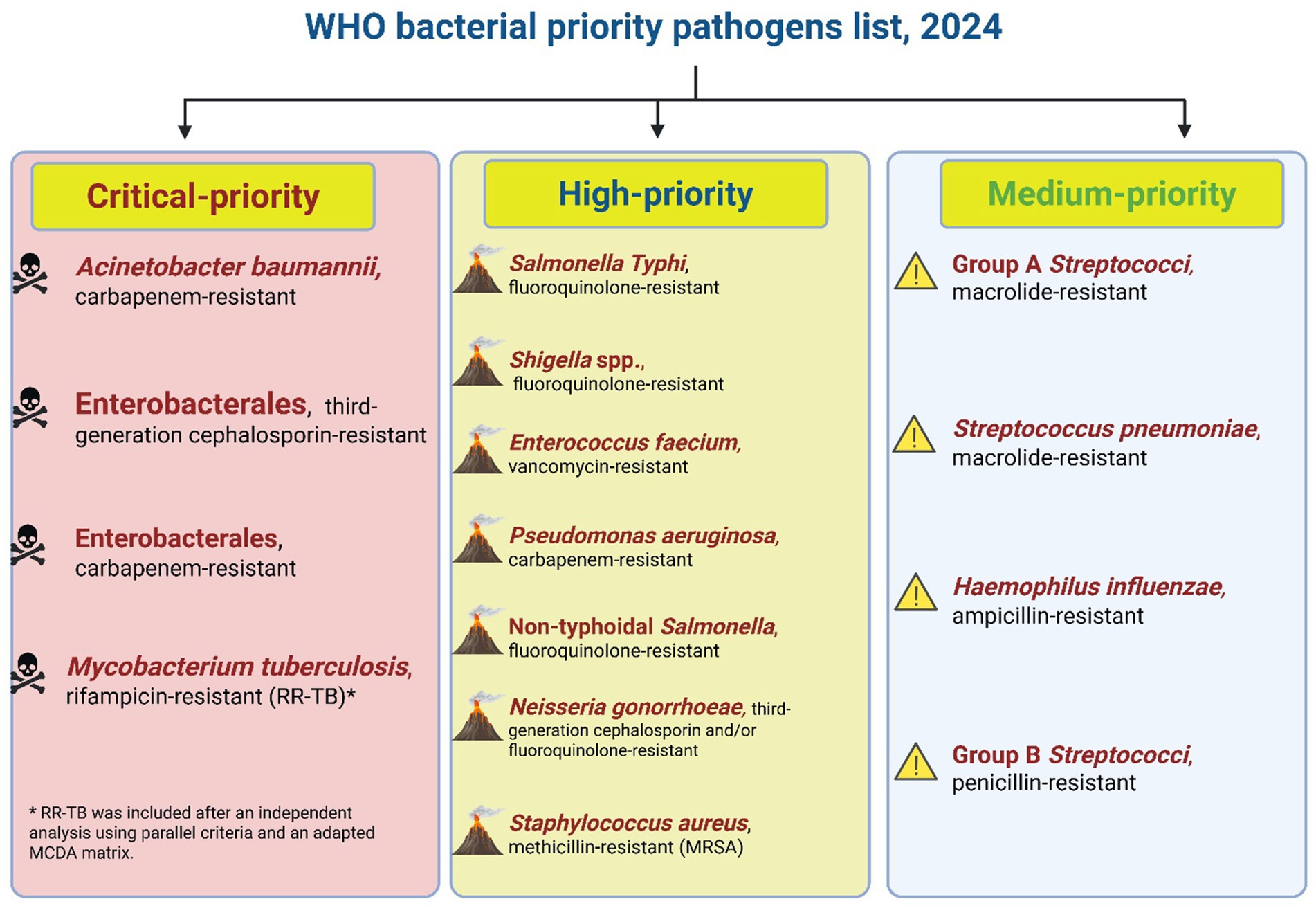
Figure 1. Priority levels for antibiotic-resistant bacteria according to the 2024 WHO report. This figure synthesizes the WHO’s 2024 prioritization of bacterial pathogens into three urgency tiers and is intended as a decision-making framework: it highlights where the greatest unmet clinical need and research investment are required to reduce morbidity and mortality from antimicrobial-resistant infections. Data source: World Health Organization (2024). (This figure was created by the author using BioRender).
Medicinal plants have served as the primary source of therapeutic agents for treating various ailments, including pathogenic infections, since ancient times. Their use and development as anti-infective agents persisted throughout human civilizations. However, with the advent of the modern era, a paradigm shift occurred as scientists increasingly relied on synthetic and semi-synthetic antibiotics for combating microbial infections (Abdallah, 2011). Since the 1960s, antibiotic resistance phenomenon has progressively increased, while the emergence of new antibiotic classes has markedly diminished. This increasing worry poses a renewed danger of epidemics and bacterial diseases, potentially resulting in significant, enduring consequences for human history (Abdallah et al., 2023). A substantial amount of literature has recorded the antibacterial potential of several medicinal plants. A total of 6,083 publications published from 1946 to 2019 examined the antibacterial efficacy of various plant species. The most frequently reported plant families were Lamiaceae, Asteraceae, and Fabaceae, with Cinnamomum verum, Thymus vulgaris, and Rosmarinus officinalis identified as the most extensively studied species (Chassagne et al., 2021). Among different plat extracts, the plant essential oils (EOs) are widely recognized for their biologically active chemical constituents, which exhibit notable therapeutic properties, underscoring their potential as promising candidates in antibacterial agent’s development. Accordingly, considerable scientific attention has been directed toward investigating their antimicrobial efficacy, with studies emphasizing their capacity to serve as sources of novel therapeutic agents for combating infectious pathogens. This growing body of research underscores their viability as alternatives or adjuncts to conventional antimicrobial treatments (Al-Mijalli et al., 2025).
Syzygium aromaticum (L.) Merr. and L. M. Perry, is a significant medicinal plant used in Asia for therapeutic and culinary applications. The blossoming buds are known as cloves, which are dried and widely traded. Its usage dates back to approximately 2000 years, with historical records indicating its employment by the Chinese and Indians for the treatment of numerous illnesses, including respiratory and stomach ailments (Bhowmik et al., 2012). Cloves also cultivated in many countries in tropical regions including Indonesia, Madagascar, Tanzania, Comoros, Sri Lanka, Comoros, Kenya, China, Malaysia, and Grenada (Figure 2). Cloves, originating from Maluku Island in Indonesia, contains eugenol as its primary active ingredient, which is responsible for both its aroma and therapeutic properties (Kamatou et al., 2012). In Ayurvedic medicine, clove has long been used to alleviate pain and inflammation, as well as to combat various microbial infections (Mugnaini et al., 2020). Its traditional applications extend to the treatment of oral and dental infections and periodontal disease, a noteworthy area of exploration (Kaur and Chandrul, 2017). Given that clove oil has emerged as a potential treatment for common oral infections, as supported by Marya et al (Marya et al., 2012), who demonstrated its inhibitory effect on tooth decalcification, its broader therapeutic applications warrant further investigation. Moreover, studies on clove oil suggest that it effectively addresses pain, inflammation, and microbial infections, in line with its traditional medicinal uses. It has also been employed as an analgesic, for treating respiratory tract infections, and to enhance digestion. The antimicrobial properties of clove have garnered increasing attention in recent years, driven by the escalating global problem of antibiotic resistance. Previous research has demonstrated that clove oil is effective against both Gram-positive and Gram-negative bacteria, including Escherichia coli and Staphylococcus spp., and various MDR traits were initially identified in isolates from food animals and later transitioned into clinical environments (Lv et al., 2024). Additionally, clove oil has shown efficacy against the notorious nosocomial pathogen Pseudomonas aeruginosa, which severely affects immunocompromised patients (Costa et al., 2022). The mechanism of action of clove oil is believed to involve disruption of bacterial cell membranes, biofilm breakdown, and interference with metabolic pathways (Bhattacharjee et al., 2023). Combining clove oil with antibiotics has been shown to enhance the efficacy of the latter through synergistic effects (Attaallah Ibrahim and Kadhim Mohammed, 2023). Moreover, cloves phytochemicals exhibit anti-inflammatory and antioxidant properties, with their phytochemical components, such as flavonoids and phenolics, further enhancing their therapeutic potential (AlKhafaji et al., 2024).
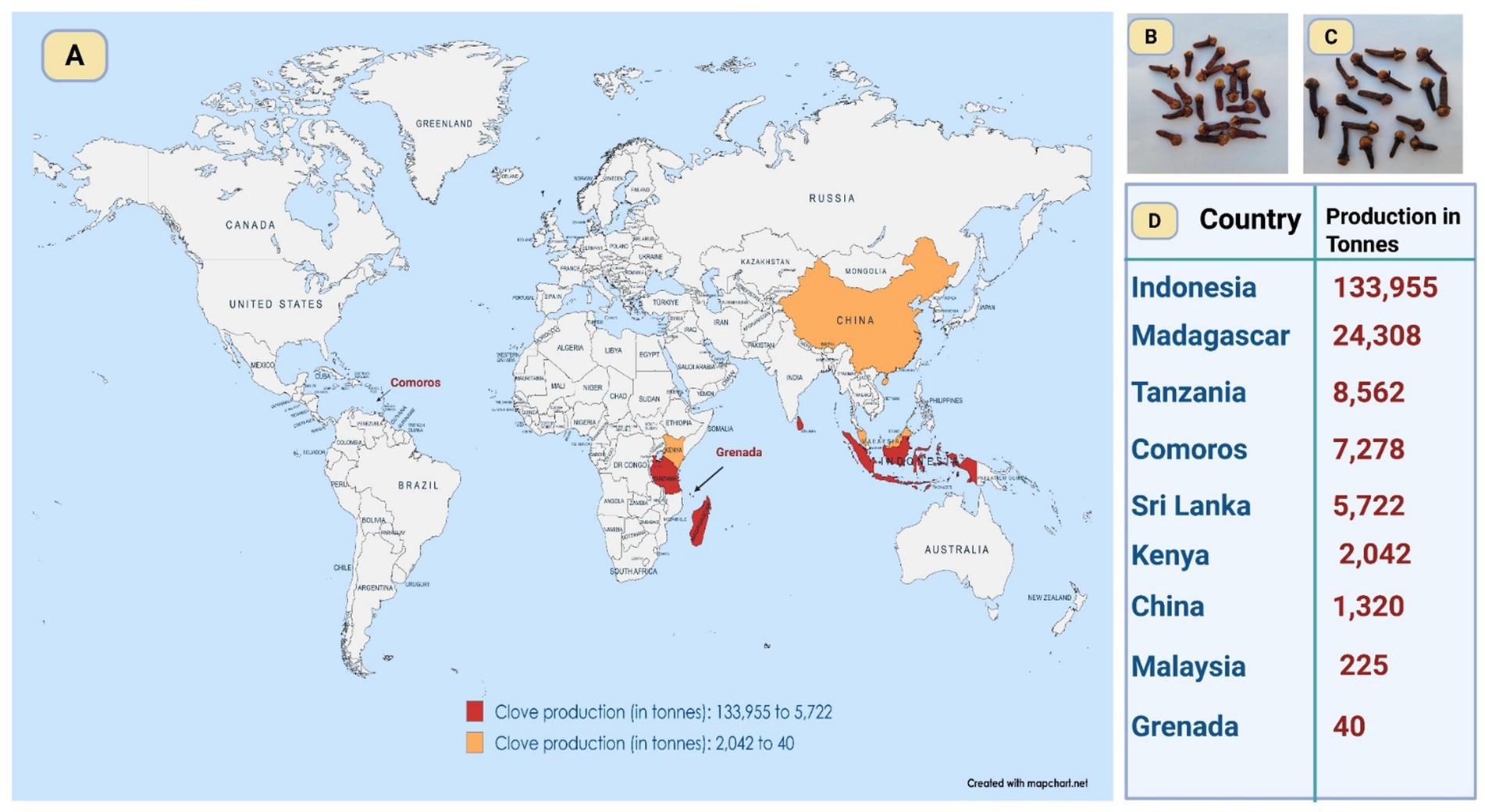
Figure 2. Global clove cultivation at a glance. This map depicts major clove-producing countries (A), linking production hotspots with regional conditions that shape phytochemistry, antimicrobial efficacy, and sourcing potential. It further contrasts Sri Lankan cloves, lighter and milder (B), with Indonesian cloves, darker and more pungent (C), and presents the FAO’s latest (2022) production estimates in tonnes (D). (Source of data: https://www.fao.org/faostat/en/#data/QCL).
The aim of this review is to critically explore the antibacterial potential of extracts of clove buds of Syzygium aromaticum, particularly its essential oilsas a natural alternative to combat MDR bacteria. Given the escalating global health crisis posed by MDR pathogens and the limitations of conventional antibiotics, additionally, it highlights the synergistic effects of clove extracts with existing antibiotics, offering insights into its potential role in mitigating antimicrobial resistance and advancing innovative treatment strategies.
2 Phytochemical composition of clove essential oil
Clove essential oil is characterized by a complex phytochemical profile (up to 30 compounds were identified in cloves), with eugenol (70–95%) as the predominant constituent, followed by eugenol acetate (≤20%) and β-caryophyllene (12–17%), collectively constituting 15–20% of the total oil content (Strižincová et al., 2022). Other minor or trace components comprise less than 10% of the composition, including caryophyllene oxide, diethyl phthalate, cadinene, 4-(2-propenyl)-phenol, α-copaene, α-cubebene and chavicol, among others (Haro-González et al., 2021). The concentrations of the phytochemical constituents of the clove greatly vary based on geographic origin, extraction technique, and environmental conditions. Figure 3 showing the major bioactive components of clove.
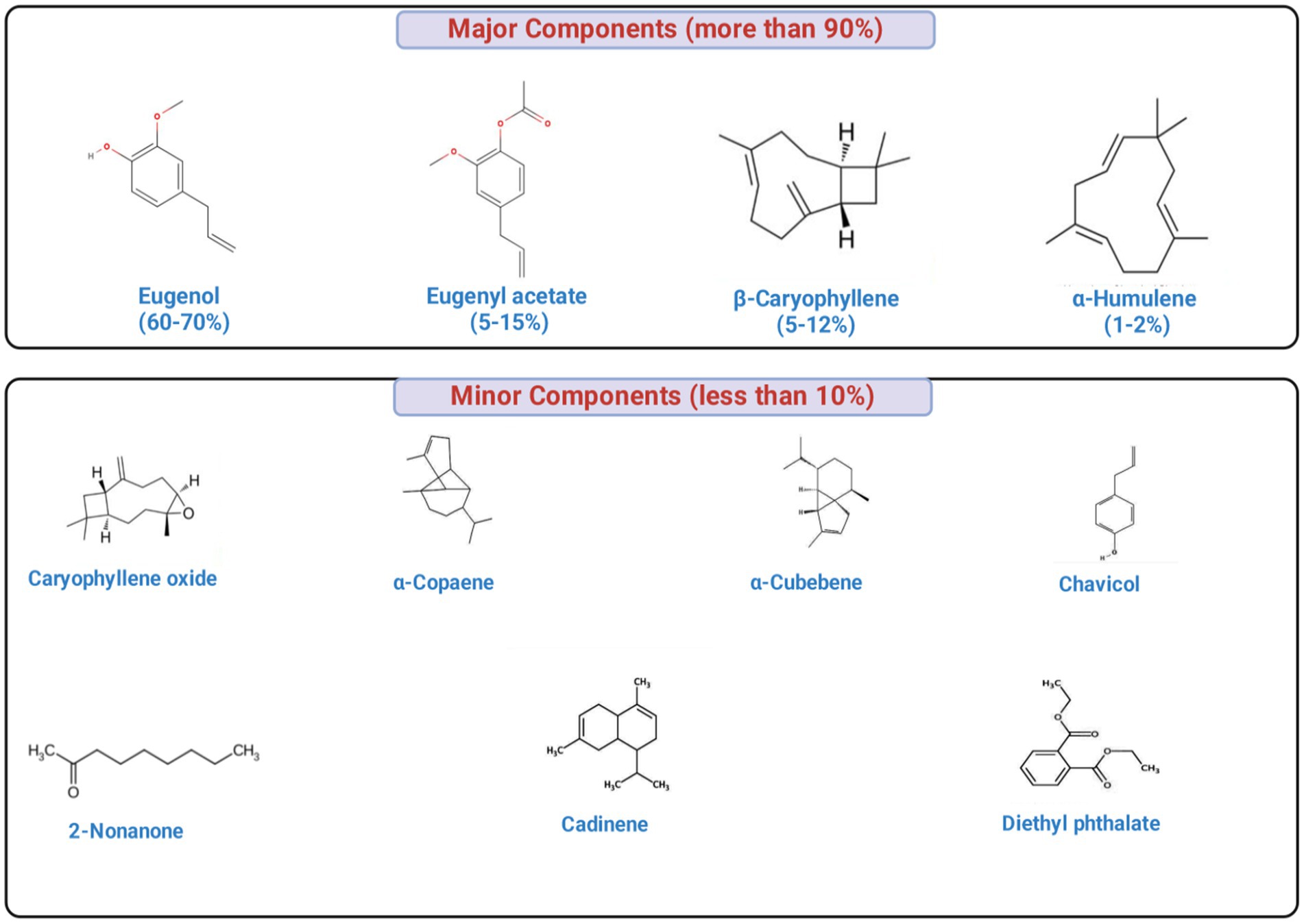
Figure 3. Major and minor bioactive phytochemicals in clove essential oil. The oil is dominated by eugenol (70–95%), with eugenyl acetate (up to 20%), β-caryophyllene (12–17%), and α-humulene (1–2%) as additional key constituents, while minor components (<10%) include caryophyllene oxide, α-copaene, α-cubebene, chavicol, cadinene, 2-nonanone, diethyl phthalate, and related compounds. Notably, eugenol and several terpenoids exhibit strong antibacterial activity through membrane disruption, leakage of intracellular contents, and inhibition of essential cellular processes.
2.1 Eugenol
Eugenol (4-allyl-2-methoxyphenol), an aromatic phenolic compound, is the principal bioactive constituent of clove, with leaf extracts yielding up to 9381.7 mg per 100 g (Nejad et al., 2017). This compound is distinguished by its viscous yellow appearance, pronounced aroma, and lipophilic properties, which facilitate interactions with biological membranes (Ulanowska and Olas, 2021; Zhai et al., 2022). The hydroxyl group in its molecular structure confers antimicrobial activity via disruption of bacterial membrane integrity and inhibition of protease function (Pilong et al., 2023). Additionally, eugenol exhibits potent antioxidant properties, scavenging reactive oxygen species (ROS) and upregulating endogenous antioxidant enzymes (e.g., superoxide dismutase, catalase), thereby mitigating oxidative stress (Ma et al., 2021). Eugenol demonstrates antibacterial activity against Shigella flexneri by inducing oxidative damage. It suppresses superoxide dismutase activity, raising intracellular ROS levels. This oxidizes the cell membrane, compromising its barrier function and causing ATP leakage and depolarization (Khwaza and Aderibigbe, 2025).
In antibacterial applications, eugenol (3 mM) significantly reduces bacterial metabolic activity and biofilm formation, attributed to interference with quorum-sensing pathways (Olszewska et al., 2020). Its anti-inflammatory and analgesic properties underpin its therapeutic use in dentistry for post-procedural care (e.g., root canal therapy, tooth extraction) (Veerapandi et al., 2022). Mechanistic studies in rheumatoid arthritis models demonstrate eugenol-induced apoptosis of fibroblast-like synoviocytes, suppression of pro-inflammatory cytokines (e.g., IL-6, TNF-α), and inhibition of cell migration (Ahmad et al., 2021; Damasceno et al., 2024). Eugenol’s anticancer potential is evidenced by its capacity to induce apoptosis and cell cycle arrest across multiple cancer lineages. In colorectal carcinoma, eugenol modulates Wnt/β-catenin signaling, while in cervical cancer, it synergizes with cisplatin to enhance chemosensitivity through downregulation of anti-apoptotic proteins (Bcl-2) (Fathy et al., 2019; Zari et al., 2021; Islam et al., 2022). Clinically, eugenol is utilized as a topical antiseptic and anesthetic agent (Dable-Tupas et al., 2023).
Eugenol should not be viewed merely as a constituent of clove, but rather as a prototypical phytophenolic derivative that exemplifies the multimodal antimicrobial potential of polyphenols. As demonstrated by Bayode et al. (2024), phytophenols exert broad-spectrum activity through multiple mechanisms, including disruption of microbial membranes, inhibition of efflux pumps, interference with essential enzymes such as DNA gyrase, and modulation of stress responses via antioxidant effects. This multifaceted mode of action confers intrinsic, multi-target efficacy against MDR pathogens. Conceptualizing eugenol within this framework underscores its translational relevance and supports the rationale for developing multi-component phytochemical strategies to combat clinical “superbugs.”
2.2 Eugenol acetate
Eugenol acetate, a phenylpropanoid derivative, constitutes ≤20% of CEO and demonstrates enhanced bioactivity relative to eugenol in prostate and oral squamous carcinoma models (Das et al., 2019). Its antioxidant capacity (90.31% DPPH scavenging at 35 μg/mL) and anti-inflammatory effects (inhibition of TNF-α, IL-1β) are well-documented (Adaramola and Onigbinde, 2016; El Ghallab et al., 2020). Ultrasound-assisted hydrotropic extraction (158 W, 38°C, 30 min) yields 20.04% eugenol acetate, outperforming Soxhlet methods (5.72%) by reducing solvent use and extraction time (Ali et al., 2021; Ahmed et al., 2022). Eugenyl acetate exhibits toxicity against brine shrimp at 0.3 μg/mL and potent larvicidal effects against the yellow fever mosquito (Aedes aegypti) (LC₅₀: 0.1 mg/mL) by targeting octopaminergic receptors. Its diverse properties, including antioxidant, antimicrobial, antitumor, and insecticidal activities, make it valuable for applications in food preservation (Haro-González et al., 2021).
2.3 β-Caryophyllene
β-Caryophyllene (about 13% of cloves essential oils), a bicyclic sesquiterpene, exhibits broad-spectrum antimicrobial activity and anticancer effects via apoptosis induction in ovarian and lung cancers (Santolin et al., 2021; Tarhan, 2021). In neurodegenerative models, it activates NQO1protien, ameliorating oxidative damage in Parkinson’s disease (Nisar et al., 2021). β-Caryophyllene from clove extract shows potent antimicrobial activity against Helicobacter pylori, significantly reducing bacterial load both in vitro and in vivo. Beyond its direct bactericidal effects, it also inhibits gastric mucosal inflammation, highlighting its potential as a promising anti- Helicobacter pylori agent (Jung et al., 2020). Methods of extractions have impact on the quantity extracted from the cloves, hydrodistillation maximizes β-caryophyllene yield (36.94%),
2.4 α-humulene
α-Humulene is a sesquiterpene that constitutes approximately 2.75% of clove buds, although its concentration may vary slightly across different clove varieties. The characteristic woody aroma of clove is largely attributed to this compound (Amelia et al., 2017). Despite being a less abundant constituent, α-humulene demonstrates notable anti-inflammatory and antiproliferative activities in colon, prostate, and breast cancers through inhibition of the NF-κB pathway (Ambrož et al., 2019). In addition, essential oils enriched with α-humulene display antibacterial activity, supporting its therapeutic relevance. Safety assessments reveal that α-humulene is non-toxic to fibroblast and macrophage cell lines at concentrations up to 400 μg/mL, while higher doses induce cytotoxic effects (Jang et al., 2020). Beyond these properties, α-humulene has also been reported to exhibit antifungal, gastroprotective, antiallergic, and antiparasitic activities (Dalavaye et al., 2024).
2.5 Other minor components
Other minor constituents of clove buds essential oil comprise various compounds, each constituting less than 10% of the total composition. These include caryophyllene oxide, α-copaene, α-cubebene, chavicol, diethyl phthalate, 2-nonanone, and several others (Haro-González et al., 2021; Ahamad, 2023; Figure 3). Clove essential oil contains several minor constituents, including 0.91% α-terpineol, 0.40% D-limonene, 0.26% p-cymene, 0.21% γ-muurolene, 0.13% γ-terpinene, 0.12% geranyl acetate, 0.11% 2-nonanone, 0.11% linalool, 0.10% terpinolene, and 0.10% germacrene B (Ahamad, 2023). Therefore, the minor constituents of clove essential oil primarily belong to four chemical classes: monoterpenes (e.g., D-limonene, α-terpineol, linalool), sesquiterpenes (e.g., γ-muurolene, germacrene B), phenylpropanoids (e.g., p-cymene, chavicol), and ketones/esters (e.g., 2-nonanone, geranyl acetate). These compounds contribute to the oil’s aroma and bioactivity.
3 Antibacterial mechanisms and synergy of cloves
3.1 Inhibition of bacterial growth and biofilm disruption
Natural plants containing essential oils have antimicrobial properties and serve as a potential source for addressing antibiotic resistance (Cui et al., 2020). Clove essential oil is a potent antimicrobial agent, primarily due to its major chemical constituent, eugenol. Other components present in lower quantities, such as caryophyllene, eugenol acetate, and benzyl salicylate, may contribute synergistically to its antimicrobial activity (Pateiro et al., 2021).
A previous study evaluated the antibacterial effects of clove essential oil against Gram-negative pathogens, including E. coli, Klebsiella sp., Enterobacter sp., Citrobacter sp., Proteus sp., Pseudomonas sp., and Acinetobacter sp. The inhibition zones at a concentration of 100 mg/mL were reported as 17, 16, 17, 18, 19, 14, and 18 mm, respectively. All tested strains were screened for β-lactamase production (ESBL, MBL, and AmpC enzyme), with Proteus species exhibiting the highest susceptibility. These findings suggest that clove essential oil has varying antibacterial potential against uropathogens and could be explored for the development of novel antimicrobial agents to address the growing issue of antibiotic resistance (Faujdar et al., 2020).
Most natural antibacterial agents exert their activity through the breakdown of the cell wall and membrane (Hu et al., 2019), inhibition of biofilm formation (Latifah-Munirah et al., 2015), and interference with DNA replication processes (Rajkowska et al., 2017). The antibacterial properties of clove essential oils are mainly attributed to eugenol, the major constituent of clove buds. Eugenol has shown strong bactericidal activity against MDR Streptococcus suis. At a 15% oil concentration, the maximum zones of inhibition were observed, and time-kill analysis revealed that S. suis was completely eradicated within 15 min. Scanning electron microscopy (SEM) confirmed atypical cellular morphology and cell membrane lysis (Wongsawan et al., 2019). The chemical composition of clove essential oil contains (–OH) groups at meta and ortho positions, which interact with the cytoplasmic membrane, disrupt phospholipids, and subsequently inhibit protein translocation, phosphorylation, electron transport, and other enzymatic activities, ultimately leading to cell death (Shahbazi, 2019; Figure 4).
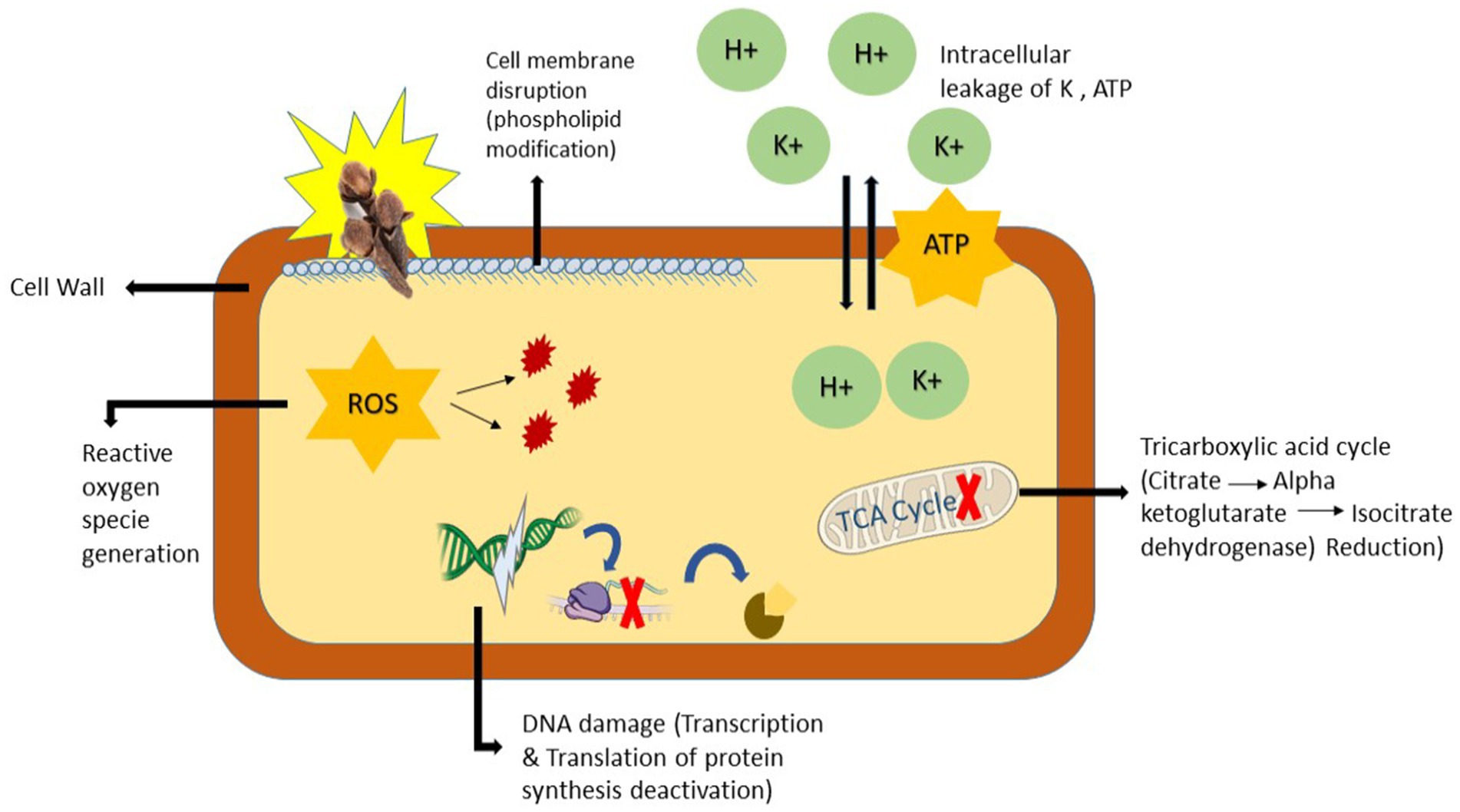
Figure 4. Proposed antibacterial mechanisms of clove essential oil. This figure is a schematic representation of the cascade model of antimicrobial action of clove phytochemical compounds, highlighting eugenol-mediated membrane disruption, ion and ATP leakage, inhibition of TCA-cycle enzymes, ROS overproduction, and consequent macromolecular damage leading to cell death. (This figure was created by the author using power point).
The in vitro minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) assays indicate that cloves essential oils are more effective against Listeria innocua and S. aureus than against Gram-negative bacteria. SEM micrographs of E. coli and L. innocua eradicated with cloves essential oils at MIC values of 6.25 mg/mL and 1.56 mg/mL, respectively, for 12 h revealed increased membrane permeability, disrupted integrity, cytoplasmic leakage, and cell lysis (Behbahani et al., 2019).
Gram-positive bacteria are more susceptible to essential oils due to their single-layered peptidoglycan cell wall, which offers weak resistance to antibacterial agents (Zhao et al., 2021). In contrast, Gram-negative bacteria possess an outer membrane composed of lipopolysaccharides, peptidoglycan, and various proteins, forming a complex structure that hinders the diffusion of lipophilic compounds (Kim et al., 2019). Beyond simple structural differences, a physicochemical explanation explains differential susceptibility: the lipopolysaccharide (LPS)-rich outer membrane of Gram-negative bacteria forms a hydrated, highly anionic and amphipathic barrier that actively limits partitioning of hydrophobic phytochemicals into the inner phospholipid bilayer. As Lawani et al. (2023) emphasize, this LPS layer does not merely act as a passive wall but repels or reduces the local concentration of lipophilic molecules such as eugenol at the membrane surface, thereby diminishing their ability to insert into and disorder bacterial membranes. This physicochemical repulsion helps explain why covle essential oils often shows lower in vitro potency against Gram-negative strains unless delivery (e.g., nano-emulsions) or permeabilizing co-agents are used. Moreover, one study also demonstrated the antibacterial mechanism of eugenol, showing that it alters membrane permeability nonspecifically, interfering with ion and ATP transport. This was evidenced by a significant release of K+ ions in phosphate-buffered saline (PBS) in Listeria monocytogenes, increasing membrane permeability (Marchese et al., 2017).
Several studies indicate that eugenol facilitates ATP and K+ ion efflux from bacterial cells. Due to its hydrophobic nature, eugenol easily penetrates the outer membrane of Gram-negative bacteria, integrates into the phospholipid bilayer, and disrupts membrane integrity. Additionally, its membrane-permeabilizing ability suggests potential synergy with antibiotics (Zhang et al., 2017). Clove oil affects cell permeability by reducing the activity of enzymes such as β-galactosidase and alkaline phosphatase (AKP) and inducing leakage of biological macromolecules like ATP, proteins, and DNA. Furthermore, clove essential oil disrupts the tricarboxylic acid (TCA) cycle by reducing the activity of key enzymes, including citrate synthase, α-ketoglutarate dehydrogenase, and isocitrate dehydrogenase. Most importantly, eugenol alters DNA conformation by forming a eugenol-DNA complex (Cui et al., 2018b).
The molecular mechanism of cloves essential oil has been explored in E. coli and S. aureus. The MIC and MBC values of eugenol were 0.32 mg/mL and 0.64 mg/mL for E. coli and 0.26 mg/mL and 0.52 mg/mL for S. aureus, respectively. Additionally, eugenol exhibited antibiofilm activity at 4 × MIC, with inhibition rates of 88.27% for S. aureus and 73.12% for E. coli, indicating a concentration-dependent disruption of biofilms. Furthermore, incubation of S. aureus with eugenol led to increased production of reactive oxygen species (ROS), hyperactivation of antioxidant enzymes such as superoxide dismutase, glutathione peroxidase, and catalase, ultimately resulting in oxidative stress-induced cell death (Bai et al., 2023). The surge in ROS leads to lipid peroxidation of the cell membrane and subsequent DNA and protein damage (Sun et al., 2018). At the biochemical level these events form a causal chain: hydrophobic eugenol first partitions into lipid bilayers, increasing membrane fluidity and permeability and producing rapid K+ and ATP leakage with PMF collapse. PMF collapse and reduced ATP production immediately compromise energy-dependent repair, active transport and efflux, permitting greater intracellular access of other phytochemicals. Simultaneously, perturbation of the electron transport chain and TCA-cycle enzymes (e.g., citrate synthase, α-ketoglutarate dehydrogenase) increases electron leakage and ROS formation, driving lipid peroxidation and oxidative damage to proteins and nucleic acids. Importantly, Bayode et al. (2024) frame eugenol as a context-dependent oxidant: at low exposures eugenol may show antioxidant activity, but at high local concentrations (as achieved at the disrupted membrane interface or with concentrated formulations) it behaves as a pro-oxidant that overwhelms bacterial redox homeostasis; thus converting membrane perturbation into sustained oxidative injury and bactericidal stress. The combined outcome is multi-target collapse (membrane failure → metabolic/TCA dysfunction → oxidative injury → macromolecular inhibition) that can overcome individual resistance mechanisms.
Additionally, bacterial antioxidant defense mechanisms attempt to neutralize oxidative stress by upregulating intracellular enzyme activity following eugenol exposure (Lin et al., 2019). Moreover, eugenol disrupts DNA synthesis, preventing bacterial replication and leading to cell death (Cui et al., 2018a). In Bacillus cereus, eugenol has also been shown to inhibit the production of enzymes such as amylases and proteases (Liu et al., 2017).
One study detailed the multifaceted antibacterial mechanisms of clove essential oil against S. aureus. These include: (i) inhibition of the tricarboxylic acid cycle via suppression of key respiratory enzymes (ii) disruption of DNA replication and transcription through alterations in DNA conformation, and (iii) modulation of the agr (accessory gene regulator) quorum-sensing system, a central regulator of bacterial virulence. Specifically, cloves essential oils downregulated the expression of agrA and agrC, attenuating pathogenicity. Collectively, these findings underscore the potential of clove essential oil as a natural antimicrobial agent capable of mitigating bacterial resistance (Li et al., 2022).
In general, the primary antibacterial mechanism of clove oil involves disrupting the bacterial membrane, leading to cell death (Dua et al., 2014). In summary, clove essential oil’s antibacterial activity follows a three-step mechanism: (i) interaction with the bacterial cell wall and membrane, causing intracellular leakage; (ii) penetration into the cytoplasm and interference with cellular structures; and (iii) inhibition of essential processes such as DNA and protein synthesis, which are critical for bacterial growth and survival (Figure 4). This multifaceted mechanism confirms its efficacy at the molecular level and its potential application as a natural antimicrobial agent (Xu et al., 2016).
3.2 Clove buds as a quorum sensing inhibitor
Quorum sensing (QS) is a sophisticated cell–cell communication process wherein bacteria collectively monitor their population density by producing, secreting, and detecting the accumulation of specific chemical signal molecules, known as autoinducers, in their extracellular environment. As a bacterial community proliferates, the increasing concentration of these autoinducers serves as a metric for cell density, ultimately triggering a coordinated shift in gene expression that regulates community-wide behaviors (Federle and Bassler, 2003). Biofilm formation arises from bacterial aggregation, where increasing cell density triggers the release of chemical signals that mediate communication through the QS system (Putri et al., 2025). QS interference is an important anti-virulence mechanism by which clove phytochemicals can “disarm” pathogens without applying direct lethal pressure rather than killing cells, QS inhibitors attenuate collective behaviors that mediate virulence, biofilm formation, toxin production, and coordinated resistance mechanisms, and therefore may impose less selective pressure for conventional resistance (Figure 5). Lawani et al. (2023) framed QS inhibition as a physicochemically informed anti-virulence strategy: small lipophilic phytophenols (e.g., eugenol) can perturb membrane microdomains and disrupt signal transduction or ligand–receptor interactions at the cell surface, thereby reducing QS signaling amplitude and downstream virulence gene expression. Several studies on clove and eugenol report downregulation of QS-regulated genes and reduced biofilm biomass at sub-inhibitory concentrations (Husain et al., 2013; Al-Shabib et al., 2017). An interesting study revealed that clove bud extracts (hexane, chloroform, and methanol) exhibit multi-target anti-quorum sensing activity. Hexane and methanol suppressed violacein production in Chromobacterium violaceum, chloroform and methanol reduced E. coli bioluminescence, and in Pseudomonas aeruginosa, the extracts inhibited multiple QS-regulated traits, including lecA:lux expression (hexane), swarming (methanol), and pyocyanin production (hexane) (Krishnan et al., 2012). Therefore, quorum-quenching should be viewed as a complement to bactericidal strategies, a strategic “disarm, then treat” approach that may slow resistance evolution.
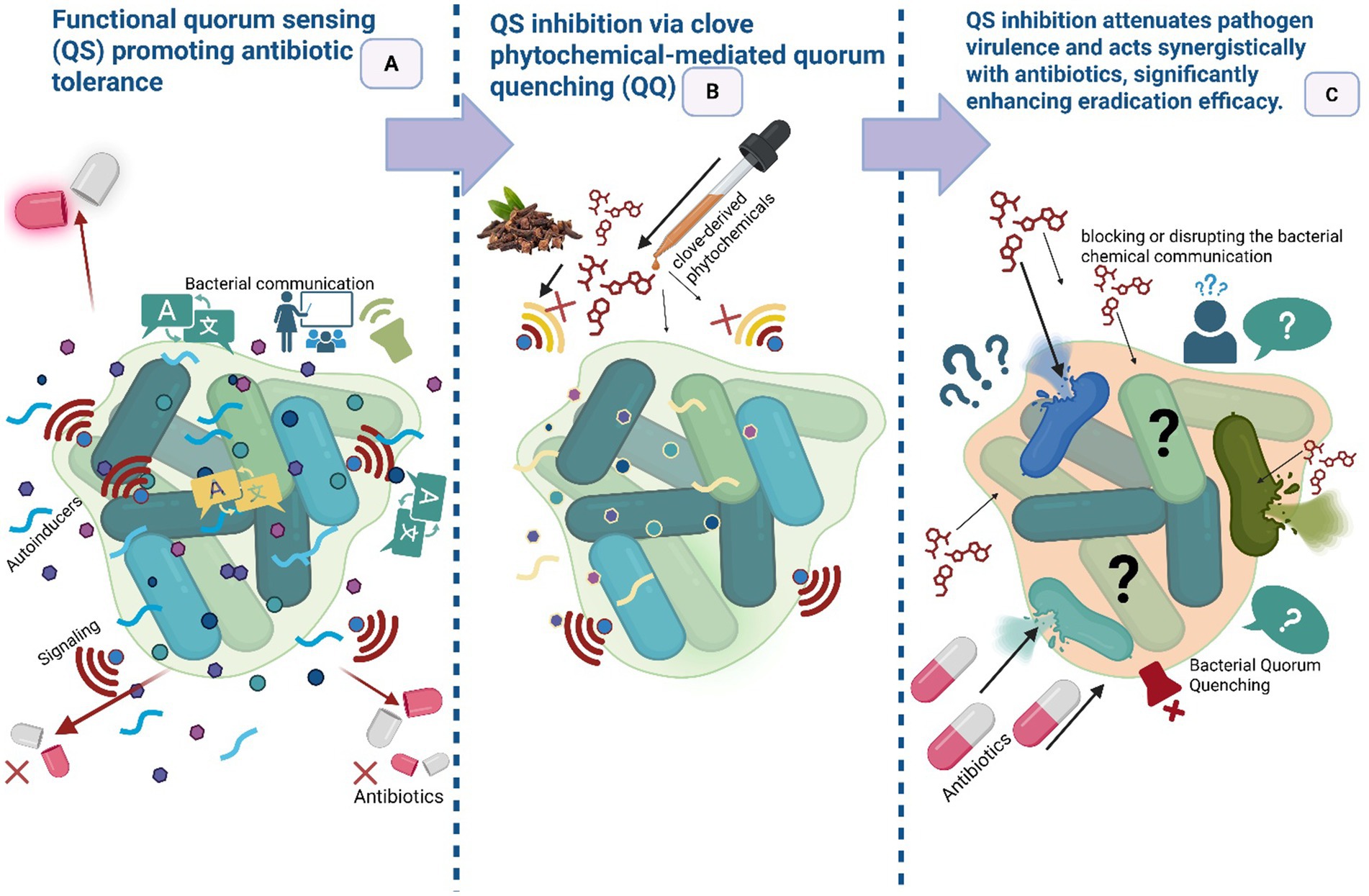
Figure 5. Attenuation of virulence and antibiotic synergy following quorum sensing inhibition (Disarm then treat strategy). (A) Functional quorum sensing (QS) promotes virulence and antibiotic tolerance in a bacterial community. (B) Clove phytochemicals act as quorum quenching (QQ) agents, disrupting QS to disarm pathogens by inhibiting virulence and tolerance mechanisms. (C) This disarming synergizes with conventional antibiotics, leading to significantly enhanced bacterial eradication. (This figure was created by the author using BioRender).
In nature, quorum quenching (QQ) represents a natural competitive strategy in which bacteria and other organisms secrete enzymes such as lactonases and acylases to degrade quorum-sensing signals. By disrupting intercellular communication, these enzymes suppress virulence and biofilm formation in competing species, conferring ecological advantage without exerting direct bactericidal pressure (Sikdar and Elias, 2020). Enzymes involved in quorum quenching, including lactonases and acylases, disrupt conserved features of signaling molecules, thereby interfering at several stages of the quorum-sensing process. Such multi-target activity greatly limits the potential for resistance, as it would demand parallel modifications in signal generation, export, and detection of an evolutionarily demanding outcome (García-Contreras et al., 2013). This “disarm-then-treat” strategy may prove highly beneficial, particularly when quorum-quenching is combined with clove-derived antibacterials such as eugenol. Such synergism holds the potential to restore the efficacy of conventional antibiotics compromised by QS-mediated resistance, highlighting a critical research gap that warrants further investigation.
3.3 Synergistic effects with conventional antibiotics
The concept of synergistic selectivity in plant extracts describes how multiple phytochemicals act cooperatively to produce a stronger antibacterial effect than individual constituents alone, frequently demonstrating enhanced specificity against particular bacterial species while maintaining comparatively low toxicity to patient (Stefanović, 2017). Unlike conventional antibiotics that target single cellular mechanisms, plant-derived antimicrobials act through synergistic interactions among diverse bioactive compounds. Single-target antibiotics exert specific selection pressures that bacteria often avoid by route mutation, while complex phytochemical combinations, such as clove essential oil, simultaneously affect several cellular targets (Burt, 2004). This multi-target strategy disrupts microbial survival by various mechanisms (Figure 4). Inspired by this natural defense system, researchers have enhanced antibiotic efficacy by combining plant phytochemicals with synthetic efflux pump inhibitors, demonstrating the potential of phytochemical-based antimicrobial strategies (Hemaiswarya et al., 2008).
In literature, numerous studies have demonstrated that plant-derived molecules (including cloves) enhance antibiotic efficacy by circumventing resistance mechanisms, even in the absence of intrinsic antimicrobial activity. This synergistic interaction not only potentiates antibacterial effects but also allows for lower drug dosages, thereby reducing toxicity and adverse side effects (Aiyegoro and Okoh, 2009). Scientific literature indicates that the most commonly used methodologies to evaluate the antibacterial activity of cloves against MDR bacteria include disc/well diffusion, MIC/MBC determination, time-kill kinetics, and the checkerboard assay (Figure 6). Although the disk diffusion method is faster, it lacks quantitative precision. In contrast, the broth microdilution technique yields accurate MIC values and is generally preferred, as the results between the two methods often show poor correlation (Liu et al., 2012).
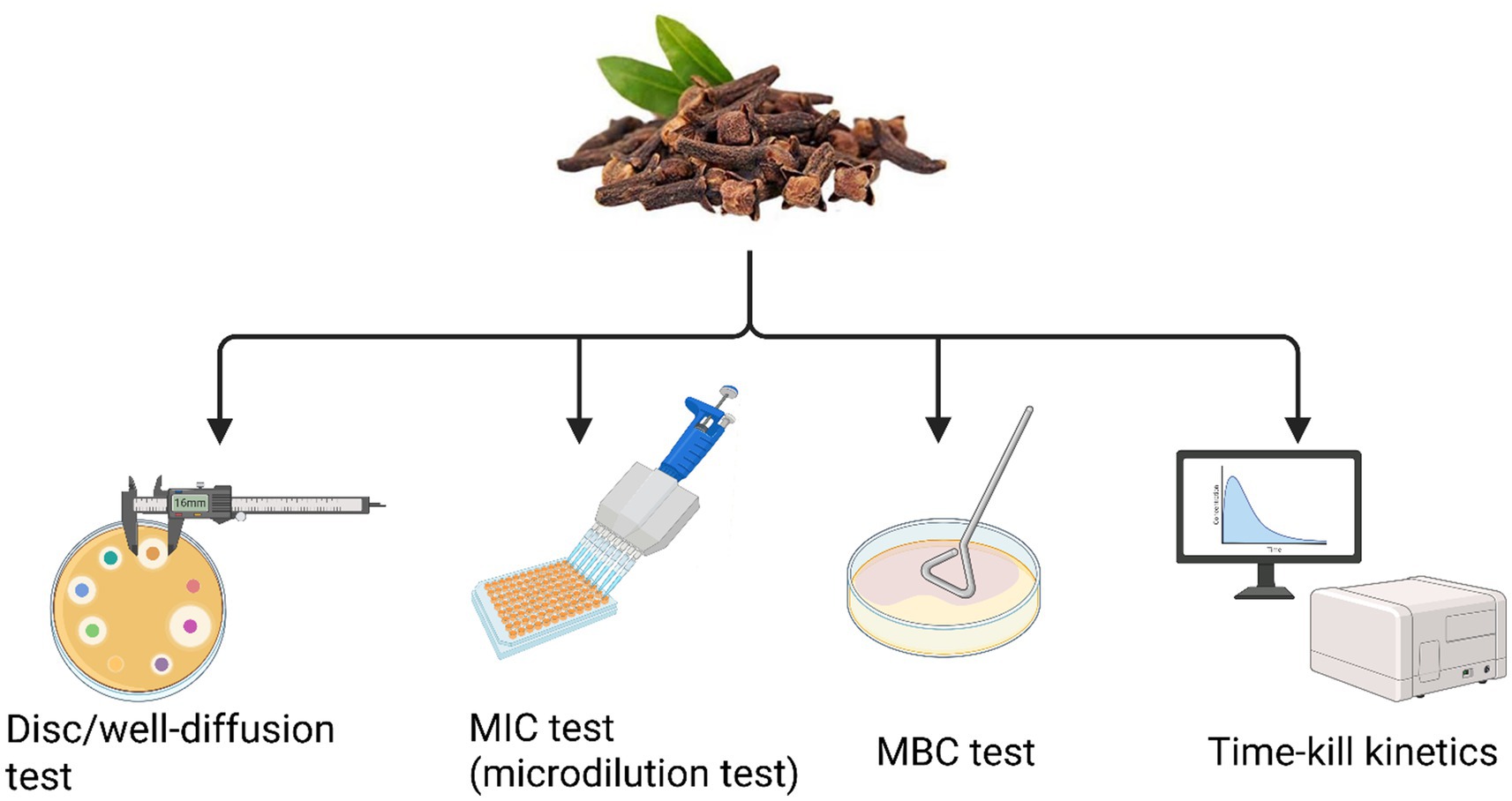
Figure 6. Overview of major in vitro antibacterial assays of clove buds against MDR bacteria. This schematic figure provides a conceptual roadmap of the key methodologies employed to rigorously evaluate the antibacterial efficacy of S. aromaticum extracts against MDR bacterial pathogens. The figure guides the researcher from initial, high-throughput screening assays to more definitive, quantitative measures of bactericidal activity, illustrating a comprehensive workflow for natural product discovery. (This figure was created by the author using BioRender).
Several studies have reported significant synergistic effects between clove-derived compounds and conventional antibiotics, enhancing their antibacterial efficacy. As shown in (Table 1), these interactions have demonstrated potential in overcoming resistance mechanisms, making clove a promising adjunct in antimicrobial therapy. Table 1 includes Fractional Inhibitory Concentration indices (FICI) derived from checkerboard assays in the original studies. FICI values quantify interaction types (≤0.5 = synergy). Where original papers did not report a full FICI. Therefore, we computed only the antibiotic fractional inhibitory concentration (FIC) contributions: (FIC = MIC of antibiotic in combo ÷ MIC of antibiotic alone) for rows where both MIC values were reported. Several antibiotic contributions are markedly reduced (for example, colistin FICI: 0.0625–0.125 and multiple β-lactam entries ≤0.25), indicating large antibiotic MIC-fold reductions when combined with clove-derived agents, a finding that is consistent with synergy when the partner (phytochemicals of clove) contribution is also small. Therefore, it is recommended that synergy studies should calculate the fractional inhibitory concentration index (FICI), defined as:
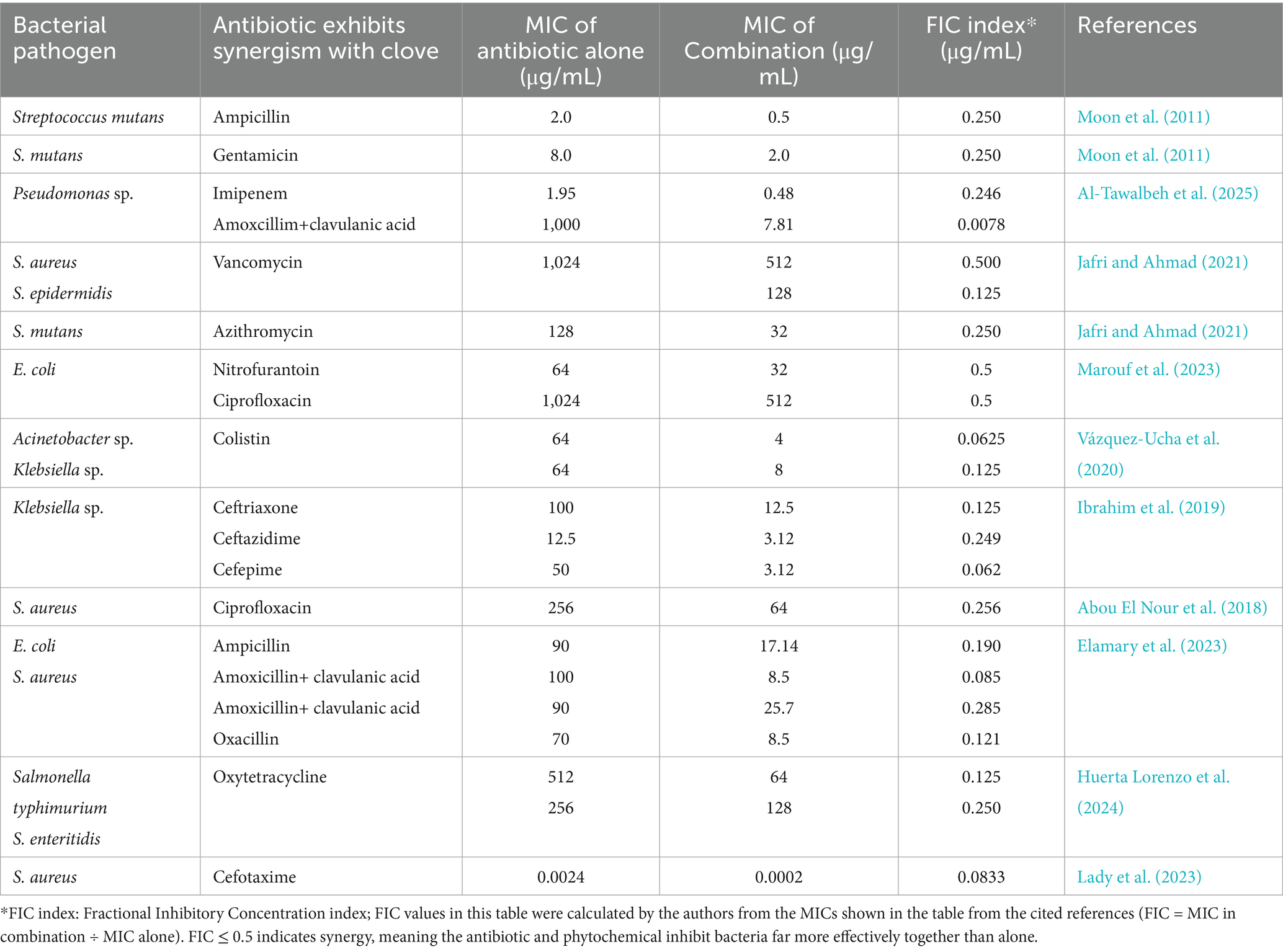
Table 1. Examples of the combined antimicrobial efficacy of clove-derived bioactive compounds and antibiotics.
where “A” is the conventional antibiotic and “B” is the clove-derived phytochemical (or essential oil constituent). FICI values ≤ 0.5 indicate synergy (Wambaugh et al., 2019).
However, these computed antibiotic-only FICs (Table 1) are partial measures: a valid interpretation of synergy requires the phytochemical’s FIC (FIC-B) or the full checkerboard FIC index (FICI = FIC-A + FIC-B). The FICI threshold convention (≤0.5 = synergy) is widely used but has known limitations and can be misleading without standardized methods and confirmation by orthogonal assays (e.g., time-kill) (Odds, 2003). Also, because checkerboard-derived FICIs can vary with method (inoculum, endpoint, extract standardization) and may not fully predict in vivo interactions, we recommend completing FICI calculations by retrieving partner-MICs (or performing two-dimensional checkerboards) and confirming promising combinations with time-kill studies or PK/PD modeling prior to claiming clinical-relevant synergy (Bentley, 2024). Notably, some of the source studies in Table 1 did provide full FICI indices using checkerboard assays (Not mentioned in the table as most studies have no FICI). Jafri and Ahmad (2021) demonstrated strong synergy between eugenol and azithromycin (FICI = 0.141) as well as between clove oil and vancomycin (FICI range 0.25–0.50) against Staphylococcus spp. and S. mutans. Vázquez-Ucha et al. (2020) reported FICI ≤ 0.5 for clove oil–colistin combinations against MDR Acinetobacter and Klebsiella, confirming synergy in clinically challenging strains. Huerta Lorenzo et al. (2024) observed additive to borderline synergistic interactions for clove oil with oxytetracycline against Salmonella (FICI 0.625–1), highlighting strain-specific variability. Similarly, Ibrahim et al. (2019) reported FICI < 0.5 for clove volatile oil combined with β-lactams (ceftriaxone, ceftazidime, cefepime) against Klebsiella pneumoniae. These examples underscore that full FICI determinations are feasible and can robustly confirm synergistic activity, while also illustrating the diversity of interaction outcomes depending on strain, antibiotic partner, and phytochemical preparation.
It was reported that eugenol exhibits antibacterial activity against cariogenic and periodontopathogenic bacteria. Time-kill studies confirmed its synergy with ampicillin and gentamicin, showing a significantly higher reduction in CFU/mL after 60 min of treatment compared to individual agents (Moon et al., 2011). The combination of clove extract with imipenem and amoxicillin-clavulanic acid demonstrated significant synergistic effects, leading to a notable reduction in the minimum inhibitory concentrations (MICs) of these antibiotics against P. aeruginosa (Al-Tawalbeh et al., 2025). The antibacterial and antibiofilm properties of eugenol extracted from clove oil were demonstrated through its ability to enhance antibiotic efficacy and disrupt bacterial biofilms. At sub-inhibitory concentrations, reductions in bacterial surface hydrophobicity and hemolysin production were observed. Unlike conventional antibiotics, which exhibited a 1,000-fold decrease in effectiveness against biofilms, eugenol maintained its potency. Significant synergy with azithromycin was noted, and microscopy confirmed the disruption of mature S. aureus and S. mutans biofilms, highlighting the potential of clove-derived compounds in combating resistant bacterial biofilm (Jafri and Ahmad, 2021). Clove extract exhibited concentration-dependent antibacterial activity against uropathogenic E. coli and E. coli ATCC 25922, with MIC/MBC values of 25 mg/mL and 6.25/25 mg/mL, respectively, confirming its bactericidal effect. Although no formal synergy was detected with nitrofurantoin or ciprofloxacin, MIC reductions were observed in combination treatments. Notably, clove extract induced 96–99% of uropathogenic E. coli to transition into unstable spherical L-forms, indicating potential cell wall disruption (Marouf et al., 2023). Clove and thyme essential oils (EOs) demonstrated potent synergy with colistin against MDR Acinetobacter baumannii and K. pneumoniae. In resistant strains, combining essential oils (EOs) with colistin reduced colistin’s MIC by 8–128-fold, with similar effects in susceptible strains. Time-kill assays confirmed enhanced bactericidal activity. These EO-antibiotic combinations enable lower, safer colistin doses while overcoming resistance, offering a promising strategy to revitalize colistin efficacy against critical pathogens (Vázquez-Ucha et al., 2020). Clove volatile oil exhibited strong antibacterial activity and inhibited AmpC β-lactamase, enhancing susceptibility in K. pneumoniae isolates prevalent in Al Anbar hospitals, Iraq. Its synergy with third- and fourth-generation cephalosporins (ceftazidime, cefepime, ceftriaxone) significantly improved bacterial response, highlighting its potential as a natural adjuvant to restore antibiotic efficacy against AmpC-producing strains. Molecular identification of AmpC genes remains essential for monitoring hospital transmission, reinforcing the role of clove oil in combating MDR infections (Ibrahim et al., 2019). Clove extract and ciprofloxacin exhibited high MICs against MDR S. aureus (1,024 and 256 μg/mL, respectively). However, their synergistic combination significantly enhanced antibacterial efficacy, effectively overcoming resistance. These findings suggest that clove extract could enhance ciprofloxacin’s potency, allowing for reduced antibiotic doses in the treatment of MDR pathogens (Abou El Nour et al., 2018). The combination of oxytetracycline and clove essential oil exhibited additive effects (FIC index = 0.625–1) against Salmonella spp. Clove essential oil reduced the required concentrations of both oxytetracycline (by 2–8×) and itself (by 2–4×), effectively lowering therapeutic doses while maintaining antibacterial efficacy (Huerta Lorenzo et al., 2024). Eugenol purified from clove buds synergized with cefotaxime against S. aureus strains (ATCC 33591, 29213, 25923), including MRSA variants. This combination enhances cefotaxime’s efficacy, suggesting its potential to revitalize β-lactam antibiotics against resistant infections (Lady et al., 2023).
4 Efficacy against MDR pathogens
The U. S. Centers for Disease Control and Prevention (CDC) estimated approximately 2.8 million antibiotic-resistant infections, resulting in about 35,000 deaths annually (Guardabassi et al., 2020). Extensive inappropriate use and misuse of antibiotics further exacerbate this problem, accelerating the selection and spread of antimicrobial resistance (Christaki et al., 2020). On account of this, there is increasing demand to explore alternative strategies to prevent and control bacterial infections. Among the most promising are plant-derived compounds, notably polyphenols, alkaloids, and tannins, which can modulate mechanisms of antimicrobial resistance (AlSheikh et al., 2020). These plant-derived compounds have also been shown to potentiate conventional antibiotics against MDR pathogens (Demgne et al., 2021).
Cloves essential oils in previous studies found to be exhibiting killing effect on both gram positive and gram negative bacteria, cloves essential oil inhibited the growth of two β-lactamase producing bacteria including E.coli and K. pneumonia (Ginting et al., 2021). Additionally also inhibited other gram negative uropathogens which were including E.coli, Klebsiella sp., Enterobacter sp., Citrobacter sp., Proteus sp., P. aeruginosa, A. baumannii all of which are AmpC beta-lactamase, metallo beta-lactamase, and extended spectrum beta-lactamases (Faujdar et al., 2020). Cloves nanoemulsion also impart antimicrobial properties on S. aureus (Nirmala et al., 2019). Cloves antibacterial effect and its contribution on Mmr efflux pump through molecular docking experiments against Mycobacterium tuberculosis was accessed which revealed that its susceptible in range of 10 and 100 μg/mL and its component eugenol bid strongly with Mmr EP proteins (El Ghallab et al., 2023).
It has been reported that the combination of clove extract with certain antibiotics, including imipenem and amoxicillin-clavulanic acid, results in a significant reduction in their respective MICs, suggesting its potential as an antibacterial agent capable of lowering the required dosages of existing antibiotics (Al-Tawalbeh et al., 2025). Another study evaluated the potential of cloves extract against MDR uropathogens, involve in causing UTI, the diethyl ether extract of clove marked big inhibition zones against S. aureus (19 mm) and K. pneumonia (19 mm) and less against Pseudomonas sp. (11 mm) and E. coli (12 mm) with highest concentration at 200 mg/mL and lower at 6.25 mg/mL while the ethanolic extract of clove at concentration of 200 mg/mL impart inhibition zones of 18 mm for both S. aureus and K. pneumonia but no inhibition was observes at 6.25–12.5 mg/mL against E. coli, P. aeruginosa, and Enterobacter spp. (Salisu et al., 2021). Cloves also represent highly effective against WHO priority list pathogens A.baumannii and Klebsiella pneumonia when combined with last resort drug called colistin lower the MIC by 8–64-folds and 8–128-folds, respectively, (Vázquez-Ucha et al., 2020). Antibacterial activity of clove oil at concentration of 10–2 μL/mL observed against MDR Pseudomonas sp. and Burkholderia cepacia complex both of which isolated from burn infections and intensive care unit (Mostafa et al., 2017). A study investigated the activity of clove against zoonotic MDR pathogen Streptococcus suis, at 15% concentration maximum zone of inhibition was observed plus the time kill assay revealed that within 15 min of exposure completely reduced this pathogen urging its use as alternative option for prevention of infectious diseases both in animals and humans (Wongsawan et al., 2019).
Pseudomonas sp. isolated from canine otitis is considered a significant public health concern, having been classified as a critical pathogen by the WHO. Susceptibility of these isolates was preserved using clove essential oil, with MIC and MBC values determined to range from 3.26 to 6.53 mg/mL, and the primary activity being attributed to eugenol (Costa et al., 2022). A study in Egypt conducted on UTI, in which from urine samples most dominant pathogen was Pseudomonas sp. containing antibiotic resistant genes such as blaTEM, blaSHV, etc. against which ethanolic extract of clove showed inhibition zone of 23 mm and MIC and MBC range from 10 to 121.25 mg/mL and 20 to 30 mg/mL, respectively, presenting cloves as potential agent to treat MDR bacteria (Ahmed et al., 2021). The anti-virulence and antibiofilm potential of clove bioactive fraction was evaluated against Pseudomonas sp. from catheter associated UTI, eugenol in CBF lower the expression of mRNA levels of quorum sensing receptor genes at the concentration of 700 μg/mL (Rathinam and Viswanathan, 2018). Previously a study on corneal ulcer showed that main MDR pathogens are commonly involve in this disease and most common one is S. aureus in all cases, for which the activity of cloves found to be effective upon demonstration of its highest sensitivity rate 97.5% along with low MIC value 0.10 μL/mL, explicitly leveling up the usage of cloves treatment of ocular bacterial infections (Mohamed et al., 2018). Clove also exhibit activity against other urinary tract infections caused by E.coli and K. peumoniae, with 16 mm inhibition zone for E. coli and 12 mm for klebsiella sp. while the MIC of them was 0.55 μL/mL (Băicuș et al., 2022). Clove oil also imparts inhibitory effects on plasmid mediated AmpC β-lactamase enzymes producing genes frequently isolated from Klebsiella sp. as an important MDR nosocomial pathogens responsible for major morbidity and mortality, along within also harbor synergistic effect toward β-lactam antibiotics effectively urging the fact that clove oil can helpful in improving their susceptibility profiles (Ibrahim et al., 2019). The potential of clove oil against the virulence of Klebsiella sp. was determined, the main virulence caused by capsule formation, a study in Egypt explored that clove oil can act as anti-virulent agent in destroying the capsule structure or reduce their size and also as promising agent for resistance modification (Edward et al., 2020). The effect of clove oil on MDR isolates of E. coli and K. pneumoniae obtained from patients with chronic hepatic disease was assessed. Treatment with clove oil resulted in a significant reduction in the colony counts of both pathogens compared to pre-treatment levels. The MICs of clove oil were determined to be 0.04 mL for E. coli and 0.05 mL for K. pneumoniae, with corresponding inhibition percentages of 11 and 9%, respectively. These findings suggest that clove oil may serve as a potential adjunct therapy to mitigate secondary bacterial infections in patients with chronic liver disease (Shaaban et al., 2024). Another study explored the antibacterial potential of cloves against four pathogenic bacteria including S. aureus, MRSA, E. coli, and S. typhi; the dichloromethane extract of clove oil form the inhibition zones of 18.20, 17.25, 21.15, and 24.2 mm, respectively, while the MIC of 1 mg/disc for S. aureus, and MRSA and 0.5 mg/disc for E.coli and S. typhi thus maintaining the trend of highest sensitivity of clove oil against gram negative isolates as compare to gram positive isolates (Yassin et al., 2020). The virulence factor quorum sensing and biofilm formation are also been investigated for their control in Pseudomonas sp. and Aeromonas hydrophilia, it has been seen that clove oil at sub inhibitory concentrations lower the virulence agents such as LasB, total proteases, pyocyanin production, chitinase, exoploysaccharides production and swimming motility on the other hand reduce the biofilm formation ability of Aeromonas sp. in concentration dependent manner (Husain et al., 2013). Just like this one more research was conducted on Pseudomonas sp. for quorum sensing and virulence aspect with addition of efflux pump encoding genes (mexA and mexB) all of them reduce to significant level when treated with clove oil at ½ MIC (El-Banna et al., 2023). Clove oil efficacy also being observed against wound pathogen MRSA when it lower the microbial load present in wounds and can to high extent produce good antibacterial effects alone or in combination (Alanazi et al., 2022). One of the recent studies evaluate the clove potential under different conditions in room temperature sunlight and low temperature dark, the extract of cloves prepared in dark temperature exhibit big inhibition zones with diameter of 13, 20, 20, 21, and 15 mm against S. aureus, S. epidermidis, P. aeruginosa, K. pneumonia, and E. coli. Whereas extract of clove in light room temperature exhibited zones of inhibition 17,10, 15, 18, 17 mm, respectively (Mekky et al., 2024). Similarly, one study reported the examination of natural products particularly cloves as potent therapy for threat of MDR bacteria among which K. pneumonia and S. aureus and the calculated MIC for K. pneumonia was 12.5 mg/mL which also emphasize the use of cloves to overcome this main problem (Mohamed et al., 2020). Another study investigated the role of clove essential oil against MDR P. aeruginosa and its virulence genes particularly of biofilm and protease production, upon RT-PCR showed that clove oil at sub inhibitory MIC concentration lower the expression levels of virulence genes suggesting its use for treatment of MDR bacteria (Awad et al., 2024). One of serious problem causing pathogen Enterococcus faecalis isolated frequently from women suffering from urinary tract infections on which the antimicrobial effect of cloves were determined with diameter of inhibition zones obtained in the range of 21.13–7.14 mm suggesting it as novel alternative for UTIs problem of resistance (Saleh et al., 2024). Study from Pakistan for profiling of antibacterial and antibiofilm aspect of clove against multidrug resistant human pathogens was done, in which different extracts of clove were prepared to check the inhibitory effect against S. saprophyticus, K. pneumonia, S. pyogenes, E. coli, S. aureus, and P. aeruginosa, the MIC values of clove oil extract were 40, 80, and 160 μg/mL regarding its antibacterial, antibiofilm and antioxidant properties highlighting the importance of secondary metabolites as source of fight against resistance (Fatima et al., 2023).
To assess the comparative antimicrobial efficacy, several studies have investigated the activity of clove oil and other natural agents against MDR pathogens. One study evaluated the antimicrobial potential of clove and rosemary oils against four MDR bacteria, S. aureus, Enterococcus faecalis, P. aeruginosa, and A. baumannii. The minimum inhibitory concentration (MIC) values of rosemary oil ranged from 0.312 to 5%, whereas clove oil demonstrated MICs between 0.312 and 1.25%, indicating superior inhibitory activity of clove oil over rosemary oil, supporting its use as a natural antimicrobial agent (Abdullah et al., 2015). Another investigation assessed the antibacterial activity of ethanolic and aqueous extracts of clove, cinnamon, and garlic against E. coli isolates from urinary tract infections (UTIs). Clove exhibited the highest antibacterial effect with a mean inhibition zone of 13.33 mm, compared to 11.33 mm for cinnamon. Ethanolic extracts of cinnamon and garlic produced inhibition zones of 14 mm and 16 mm, respectively, whereas clove oil generated the largest zone of inhibition at 27 mm (Noreen et al., 2018). In a comparative study against E. faecalis, clove and thyme were evaluated for their antibacterial potential. The aqueous phase of thyme showed no inhibitory effect, with a mean zone of 0 mm, while clove produced a mean inhibition zone of 13.67 mm (Agarwal and Yeluri, 2023). Additional research on the antibacterial properties of aqueous and ethanolic extracts of clove and garlic against Staphylococcus sp., Streptococcus sp., Pseudomonas sp., Klebsiella sp., and E. coli revealed that ethanolic clove extract had the highest inhibitory effect, followed by the aqueous extract. Garlic extracts exhibited moderate to low antibacterial activity. Clove showed inhibition zones up to 26 mm against Klebsiella sp. and 20 mm against Staphylococcus sp., with MIC values ranging from 64 to 128 μg/mL across pathogens (Liu et al., 2021). In another comparative study, several plant-based agents, including clove, turmeric, cinnamon, nutmeg, and peppermint oils, were assessed for their antimicrobial activity against E. faecalis and S. mutans. Clove oil exhibited the most potent activity, with inhibition zones of 29.8 and 40.33 mm at 100 μL, respectively (Mohapatra et al., 2023). Moreover, a study evaluating the effect of clove oil and licorice on gene expression in S. mutans, a key agent in dental caries, revealed that clove oil significantly downregulated the expression of virulence genes gtfB and gtfD, with fold changes of 0.178, 0.454, and 0.191 compared to licorice extract, based on RT-PCR analysis (Al-Amili and Al-Jobori, 2025). Lastly, the antibacterial and antioxidant activities of clove pollen grain extract were compared with maize extract against Salmonella sp., E. coli, and S. aureus. Clove extract demonstrated higher sensitivity, with inhibition zones of 29, 18, and 22 mm, respectively, and also exhibited stronger antioxidant capacity (Barnawi et al., 2023).
5 Pharmacokinetics and bioavailability of clove compounds
Pharmacokinetics is a dynamic, multidisciplinary field that converts intricate biological processes into quantitative models and expressions. By synthesizing ideas from chemistry, biology, and physiology, it is essential across the pharmaceutical continuum from drug development to the clinical refinement of dosage methods for illness treatment or cure (Figure 7). A thorough comprehension of medication physicochemical properties and human physiological processes is crucial for precisely forecasting and assessing therapeutic and clinical results (Ghazi and Cawley, 2021). In scientific literature. The pharmacokinetic profile of clove oil constituents has been investigated only modestly. It was reported that, eugenol, the major phenolic component, is rapidly absorbed and extensively metabolized after oral intake; in healthy human adults, nearly all of an oral dose is eliminated in urine within 24 h, predominantly (>90%) as glucuronide and sulfate conjugates (Nejad et al., 2017). In rodents, eugenol exhibits a relatively long apparent elimination half-life (~14–18 h) (Guénette et al., 2007), Systemic clearance of Eugenol extracted from cloves in male Sprague–Dawley rats averaged 157 mL/min/kg in plasma and 204 mL/min/kg in whole blood. Analysis of urine revealed both glucuronide and sulfate eugenol conjugates (Guenette et al., 2006), consistent with rapid biotransformation. Other major clove constituents show similarly limited bioavailability: for example, β-caryophyllene extracted from clove essential oil has poor oral absorption (about 2% bioavailability in rats) (Spigarelli et al., 2024). The interaction varied with lipid type, showing greater monolayer expansion for DPPS (1,2-dipalmitoyl-sn-glycero-3-phospho-L-serine) and DODAB (dioctadecyldimethylammonium bromide), and stronger effects on polar headgroups for DPPS and DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine). These results suggest that eugenol’s ability to interact with and penetrate lipid monolayers of cell depends on lipid composition, supporting its potential to cross biological membranes (Goncalves et al., 2015).
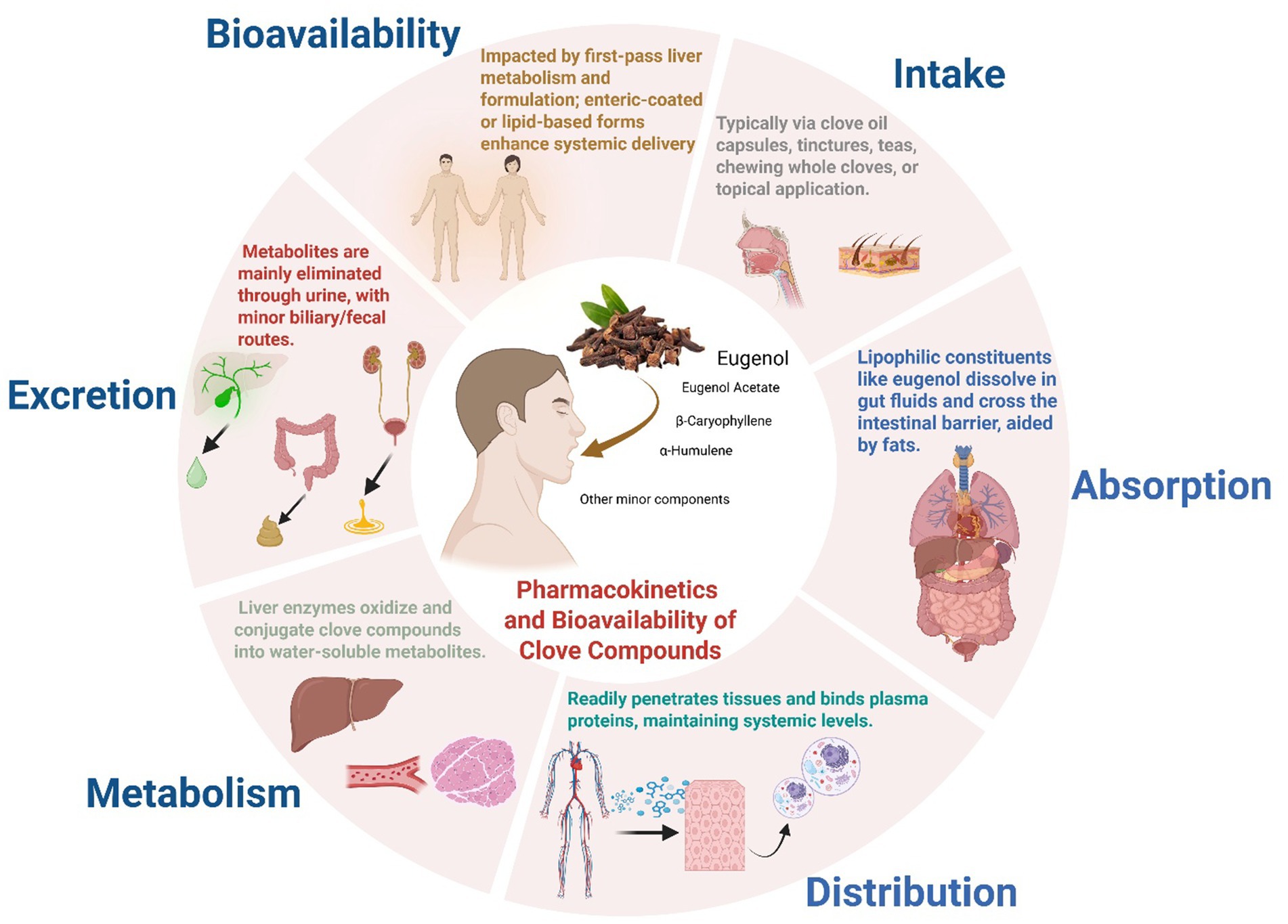
Figure 7. Possible pharmacokinetics and bioavailability of clove compounds. This figure provides schematic overview of the ADME pathway of clove bioactives (e.g., eugenol, β-caryophyllene), illustrating their pharmacokinetic journey from intake through absorption, metabolism, and elimination, and emphasizing how these processes govern systemic bioavailability and in vivo therapeutic potential. (This figure was created by the authors using BioRender).
These results suggest that systemic levels of clove compounds after ingestion are generally low unless delivered by specialized carriers. Safety studies indicate that clove oil/eugenol is well tolerated at low concentrations. The U. S. FDA recognizes clove oil as a safe flavoring and cosmetic additive, it is also widely used as dental analgesic (Nejad et al., 2017). However, eugenol is irritant and potentially toxic at high doses as previously reported using animal models (Singletary, 2014). Human case reports note that therapeutic exposures of eugenol have not caused liver enzyme elevations or frank hepatotoxicity, but overdose can produce severe liver injury (National Institute of Diabetes and Digestive and Kidney Diseases, 2012). Clinically, excessive clove oil (undiluted) is known to cause mucosal irritation and systemic symptoms, and “excessive dose” eugenol exposure is judged toxic (Nejad et al., 2017). Thus, while low-dose use (e.g., flavoring) is generally safe, and beneficial for health (Ullah et al., 2023), targeted antimicrobial applications must respect eugenol’s narrow therapeutic margin and potential for allergic or irritant effects. In summary, the pharmacokinetic data indicate rapid absorption and clearance with extensive conjugation (Marchese et al., 2017; Ulanowska and Olas, 2021). These constraints underscore the need for advanced delivery systems and careful dose optimization in any clinical use of clove-derived antimicrobials. Regretfully, data in human patients are still very limited. Future clinical research; for example, trials of clove-derived formulations in vivo against specific pathogen or for wound care or as adjunctive oral decontamination, will be needed to validate these preliminary findings.
6 Clinical investigations on clove molecules against bacteria
Despite extensive in vitro data, few in vivo studies and human trials of clove oil in infection exist. Clinical studies have mostly examined clove-containing herbal mouthwashes or topical rinses rather than therapeutic antibiotic use. For example, a randomized, triple-blind ICU trial found that nursing care with a clove-oil–based oral rinse halved the incidence of ventilator-associated pneumonia (VAP) compared to control (20.2% vs. 41.7%) (Jahanshir et al., 2023). The clove group’s VAP risk was roughly 2-fold lower, suggesting a real antimicrobial benefit. Similarly, a controlled trial in dental patients showed that a multi-herbal mouth rinse containing clove extract (with tea tree and basil) produced significant reductions in gingival plaque and microbial colony counts, comparable to a commercial essential-oil mouthwash (Kothiwale et al., 2014). These findings illustrate that clove oil can exert measurable antimicrobial effects in vivo when formulated as a rinse or topical antiseptic. The in vivo translational (preclinical) studies further support these clinical hints. In a rat model of MRSA-infected wounds, topical clove bud oil alone or combined with imipenem had accelerated wound closure and markedly reduced bacterial counts relative to untreated controls (Alanazi et al., 2022). Likewise, purified clove fractions rich in eugenol have been shown to inhibit biofilm formation and virulence gene expression in multidrug-resistant uropathogens (Pseudomonas and Enterobacter spp.) recovered from catheter-associated urinary infections (Rathinam and Viswanathan, 2018). These studies confirm that clove compounds can function in complex biological environments at subinhibitory doses, although formal clinical trials are lacking. Notably, clove oil also shows potent in vitro activity against other resistant pathogens (e.g., drug-resistant Helicobacter pylori, foodborne bacteria, etc.) beside its significant anti-inflammatory potential on human erythrocytes (Elbestawy et al., 2023), but preclinical translational studies on humans has not yet been attempted. In summary, the few available clinical and animal studies suggest clove essential oil can reduce pathogenic bacterial load and infection rates when applied topically or orally (Alanazi et al., 2022; Jahanshir et al., 2023). However, clinical data in human patients remain scarce. There is a critical need for future studies, including well-designed clinical trials, to evaluate the efficacy of clove-derived formulations against internal multidrug-resistant (MDR) bacteria and systemic bacterial infections. Addressing this substantial research gap is essential for advancing therapeutic applications of clove bioactive molecules.
7 Formulation strategies for clove-based antibacterials
As previously noted, the clinical application of clove-derived bioactive compounds remains limited. Key challenges including volatility, poor aqueous solubility, and metabolic instability necessitate the use of advanced formulation strategies such as nanoemulsions, lipid-based nanoparticles, targeted delivery systems, and polymeric films to enhance their antibacterial efficacy and stability (Figure 8).
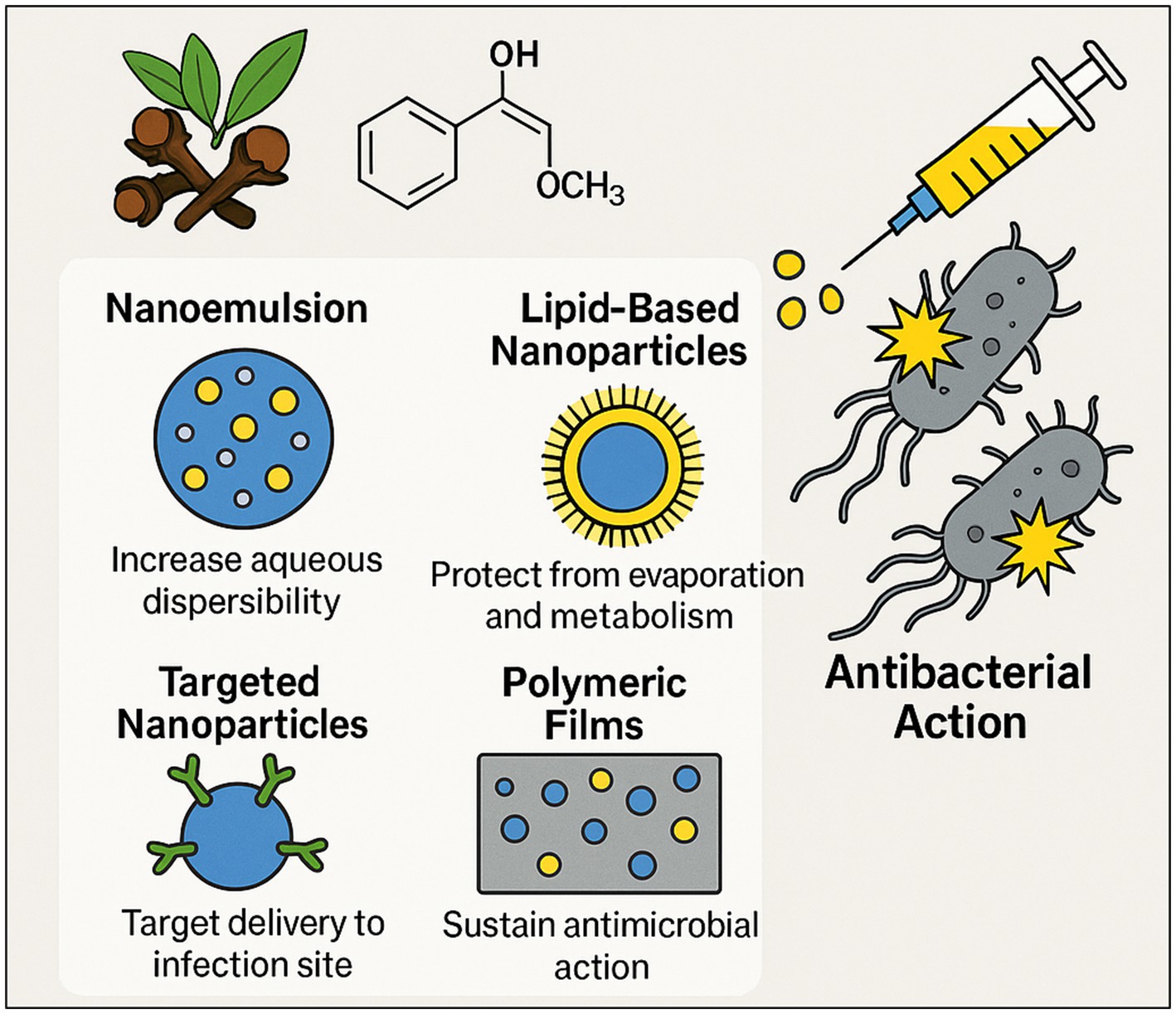
Figure 8. Formulation strategies to optimize antibacterial delivery of clove bioactive molecules. This figure provides a brief conceptual overview of advanced nano- and micro-formulation strategies designed to enhance the stability, bioavailability, and targeted antibacterial delivery of clove bioactives (e.g., eugenol), thereby overcoming physicochemical and pharmacokinetic limitations of raw extracts and improving efficacy against MDR infections. (This figure was created by the authors with the assistance of OpenAI, 2023).
Because clove oil components are volatile, hydrophobic, and metabolically labile, many investigators are exploring advanced formulations to enhance their stability, solubility, and antimicrobial efficacy. Nanoemulsions and lipid-based nanoparticles are especially promising (Liñán-Atero et al., 2024; Ye et al., 2024). In one study, a self-emulsifying nanoemulsion of clove oil (droplet size ≈30 nm) exhibited superior antibacterial potency relative to pure oil. The optimal nanoemulsion produced MICs against common pathogens that were comparable to those of a standard antibiotic (amikacin), effectively boosting eugenol’s efficacy (Anwer et al., 2014). Similarly, solid-lipid nanoparticles co-loaded with eugenol and the antibiotic ofloxacin (with chitosan for cationic targeting) achieved striking synergy: the MICs of ofloxacin decreased by 6–16-fold when delivered in these hybrid particles, effectively overcoming resistance in P. aeruginosa and S. aureus (Rodenak-Kladniew et al., 2019). These lipid carriers also enabled sustained antibiotic release and selective bacterial uptake. Targeted nanoparticle systems have been engineered as well. For instance, antibody-modified liposomes encapsulating clove oil were designed to bind Campylobacter jejuni specifically and release eugenol in response to bacterial proteases. This “active” liposome showed greatly enhanced targeting and prolonged antibacterial action against C. jejuni on contaminated foods (Chen et al., 2023). Incorporation into polymeric matrices is another strategy: electrospun nanofiber mats (polycaprolactone/gelatin) loaded with clove oil remained highly bactericidal against S. aureus and E. coli, making them attractive as antibiotic-free wound dressings (Unalan et al., 2019). Likewise, chitosan/PVA films and other biopolymer coatings containing clove oil or eugenol have demonstrated faster and more complete kill kinetics than polymer alone (Kowalewska and Majewska-Smolarek, 2023). Other approaches include solid–solid encapsulation and hybrid nanoparticles. Adsorbing eugenol onto mesoporous silica particles, for example, can “solidify” the oil and accelerate its release without loss of activity (Yao et al., 2024). Inorganic nanomaterials have been employed as well: cloves extract can mediate the green synthesis of silver nanoparticles, and such Ag–eugenol composites show enhanced antimicrobial action. Clove-derived AgNPs exhibited strong antibacterial effects across multiple pathogens and even synergized with clarithromycin (Edis et al., 2025). Finally, all these advanced formulations aim to (1) increase aqueous dispersibility, (2) protect the oil from premature evaporation or metabolism, and (3) target delivery to the infection site. The reported results dramatically lower MICs, sustained release profiles, and improved in vivo efficacy demonstrate that nano/micro-carriers can significantly amplify clove oil’s antibacterial performance. Continued development of such delivery systems is likely crucial to translate clove’s in vitro promise into clinical reality. Finally, a strategic shift in antibiotic discovery is imperative to mitigate the rapid emergence of resistance. Future efforts must transition from the modification of established antibiotic scaffolds and their known bacterial targets toward the identification of novel chemotypes with unprecedented mechanisms of action (Aminov, 2025). In this pursuit, exploiting the poly-pharmacological synergy inherent in medicinal plant molecules could provide a viable pathway to novel therapeutic strategies that circumvent pre-existing resistomes.
8 Limitations of the study
While this review brings together extensive in vitro and preliminary in vivo evidence on clove-derived phytochemicals, several limitations temper the strength of our conclusions. First, the preponderance of data arises from laboratory assays, such as broth microdilution and biofilm inhibition tests, that, although invaluable for elucidating mechanistic insights, cannot fully reproduce the complex interplay of host immunity, tissue distribution, and pathogen virulence encountered in living organisms. Consequently, the actual efficacy and safety profiles of cloves constituents in systemic infections remain uncertain until larger, well-designed animal experiments and human clinical trials are conducted.
The diversity of extraction and formulation techniques reported across studies, ranging from steam distillation and supercritical CO₂ extraction to various nano-emulsion and liposomal encapsulation methods, also introduces significant heterogeneity in phytochemical composition and bioavailability. Without standardized sourcing, processing, and reporting protocols, it is difficult to compare results directly or to establish reproducible dosing regimens for future investigations. In parallel, pharmacokinetic parameters for major clove compounds, especially eugenol, are inadequately characterized; critical information on tissue penetration, metabolic half-lives, and clearance pathways is largely absent from the literature, undermining the ability to predict therapeutic windows or potential toxicities.
Moreover, most synergy studies highlighting dramatic reductions in antibiotic minimum inhibitory concentrations (MICs) may be subject to publication bias: positive interactions are preferentially published, whereas neutral or antagonistic findings receive less attention. This skew could overstate the generalizability of phytochemical–antibiotic combinations. Additionally, antimicrobial testing methods vary widely, such as disk diffusion versus microdilution, differing inoculum sizes, and incubation conditions, which further complicates efforts to synthesize MIC values into clinically meaningful breakpoints. Finally, the current focus on a limited set of pathogens (E. coli, S. aureus, K. pneumoniae, P. aeruginosa) leaves critical gaps regarding emerging multidrug-resistant species and the potential impact of clove derivatives on the broader host microbiome or the evolution of resistance.
Addressing these limitations will require coordinated efforts to standardize extract preparation and assay protocols, to expand in vivo and clinical testing, and to integrate robust pharmacokinetic and toxicological studies. Only then can clove phytochemicals be credibly advanced as adjunctive therapies against MDR bacterial infections.
9 Conclusion
Cloves and its principal phytochemicals present in its essential oils and extracts, mainly eugenol and other minor components like eugenol Acetate, β-Caryophyllene, α-Humulene which could interact synergistically, exhibit broad-spectrum, multi-target antibacterial and anti-virulence activities against a wide array of MDR pathogens. Mechanistically, those clove-derived compounds disrupt bacterial membranes, inhibit efflux pumps, interfere with key metabolic pathways, and attenuate quorum-sensing–mediated virulence, positioning them as promising multi-target agents distinct from conventional antibiotics. Beyond direct antibacterial potency, the concept of “synergistic selectivity” emerges as a compelling clinical strategy: when combined with conventional antibiotics, clove phytochemicals can sensitize pathogens, allowing lower antibiotic doses to achieve equivalent or superior efficacy “disarm, then treat,” thereby expanding the therapeutic window and reducing host toxicity, an especially critical consideration for last-line drugs such as colistin.
Despite robust in vitro evidence, significant translational gaps remain. Key limitations include poorly characterized pharmacokinetics (rapid absorption but extensive metabolism and low systemic bioavailability for eugenol), variable extract standardization, heterogeneous assay methodologies, and limited in vivo and clinical data. Early animal studies and preliminary clinical observations (e.g., improved wound healing, reduced ventilator-associated pneumonia, and biofilm inhibition) are encouraging but insufficient to support routine clinical use. Formulation science is now recognized as a central bottleneck: rational delivery systems, including nanoemulsions, liposomes, and solid lipid nanoparticles, are required to achieve controlled local concentrations, sustained exposure, and acceptable safety profiles in vivo.
To advance clinical translation, we recommend prioritized efforts in three interrelated areas: (i) standardized, reproducible formulation and dose-finding studies integrating pharmacokinetics and pharmacodynamics (PK/PD); (ii) rigorous in vivo efficacy and safety studies that quantify therapeutic index improvements achieved via phytochemical–antibiotic combinations; and (iii) prospective clinical trials targeting high-impact, last-line scenarios where dose reduction could meaningfully mitigate toxicity. By addressing these gaps, future research can bridge the divide between promising bench science and safe, effective clinical applications, establishing clove-derived bioactives as viable adjuncts (or even alternatives) in the management of multidrug-resistant bacterial infections.
Author contributions
HT: Conceptualization, Investigation, Visualization, Writing – original draft. AA: Data curation, Funding acquisition, Project administration, Resources, Validation, Visualization, Writing – review & editing. EA: Conceptualization, Investigation, Supervision, Validation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant no. IMSIU-DDRSP2501).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. Generative AI tools were used solely for language refinement, proofreading, and creating Figure 5. All concepts, analyses, and interpretations in this manuscript remain entirely my original work.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdallah, E. M. (2011). Plants: an alternative source for antimicrobials. J. Appl. Pharm. Sci., 16–20.
Abdallah, E. M., Alhatlani, B. Y., de Paula Menezes, R., and Martins, C. H. G. (2023). Back to nature: medicinal plants as promising sources for antibacterial drugs in the post-antibiotic era. Plants 12:3077. doi: 10.3390/plants12173077
Abdullah, B. H., Hatem, S. F., and Jumaa, W. (2015). A comparative study of the antibacterial activity of clove and rosemary essential oils on multidrug resistant bacteria. Pharmaceutical and Biosciences Journal, 18–22. doi: 10.20510/ukjpb/3/i1/89220
Abou El Nour, M. M., El-Mokadem, M. T., Mohammad, Z. K., and Abdel-Wahab, G. S. (2018). Potential of diethyl ether clove (Syzygium aromaticum) extract against different pathogens and in combination with antibiotic against MDR-resistant Staphylococcus aureus. Egypt. J. Microbiol. 53, 49–68. doi: 10.21608/ejm.2018.2765.1043
Adaramola, B., and Onigbinde, A. (2016). Effect of extraction solvent on the phenolic content, flavonoid content and antioxidant capacity of clove bud. IOSR J. Pharm. Biol. Sci 11, 33–38. doi: 10.9790/3008-1103013338
Agarwal, T., and Yeluri, R. (2023). Comparative evaluation of antimicrobial efficacy of clove and thyme essential oil against Enterococcus faecalis: an in vitro study. J. Adv. Med. Dent. Sci. Res. 11, 23–28. doi: 10.21276/jamdsr
Ahamad, J. (2023). Characterization of essential oil composition of Syzygium aromaticum Linn.(clove) by GC-MS and evaluation of its antioxidant activity. J. Angiother. 7. doi: 10.25163/angiotherapy.719358
Ahmad, A., Elisha, I. L., van Vuuren, S., and Viljoen, A. (2021). Volatile phenolics: a comprehensive review of the anti-infective properties of an important class of essential oil constituents. Phytochemistry 190:112864. doi: 10.1016/j.phytochem.2021.112864
Ahmed, I. A. M., Babiker, E. E., Al-Juhaimi, F. Y., and Bekhit, A. E.-D. A. (2022). Clove polyphenolic compounds improve the microbiological status, lipid stability, and sensory attributes of beef burgers during cold storage. Antioxidants 11:1354. doi: 10.3390/antiox11071354
Ahmed, O., Mohamed, H., Salem, W., Afifi, M., and Song, Y. (2021). Efficacy of Ethanolic extract of Syzygium aromaticum in the treatment of multidrug-resistant Pseudomonas aeruginosa clinical isolates associated with urinary tract infections. Evid. Based Complement. Alternat. Med. 2021:6612058. doi: 10.1155/2021/6612058
Aiyegoro, O., and Okoh, A. (2009). Use of bioactive plant products in combination with standard antibiotics: implications in antimicrobial chemotherapy. J. Med. Plants Res. 3, 1147–1152.
Al-Amili, M. L., and Al-Jobori, K. M. (2025). Evaluation of the effects of Glycyrrhiza glabra and Syzygium aromaticum extracts on gene expression of Streptococcus mutans in patients with dental caries. Biodiv. J. Biol. Divers. 26. doi: 10.13057/biodiv/d260141
Alanazi, A. K., Alqasmi, M. H., Alrouji, M., Kuriri, F. A., Almuhanna, Y., Joseph, B., et al. (2022). Antibacterial activity of Syzygium aromaticum (clove) bud oil and its interaction with imipenem in controlling wound infections in rats caused by methicillin-resistant Staphylococcus aureus. Molecules 27:8551. doi: 10.3390/molecules27238551
Ali, A., Wu, H., Ponnampalam, E. N., Cottrell, J. J., Dunshea, F. R., and Suleria, H. A. (2021). Comprehensive profiling of most widely used spices for their phenolic compounds through LC-ESI-QTOF-MS2 and their antioxidant potential. Antioxidants 10:721. doi: 10.3390/antiox10050721
AlKhafaji, M. H., Mohsin, R. H., and Faqri, A. M. A. (2024). Food additive mediated biosynthesis of AgNPs with antimicrobial activity against hypermucoviscous enterotoxigenic foodborne Klebsiella pneumoniae. Basrah J. Agric. Sci. 37, 278–295. doi: 10.37077/25200860.2024.37.1.21
Alkofide, H., Alhammad, A. M., Alruwaili, A., Aldemerdash, A., Almangour, T. A., Alsuwayegh, A., et al. (2020). Multidrug-resistant and extensively drug-resistant enterobacteriaceae: prevalence, treatments, and outcomes – a retrospective cohort study. Infect. Drug Resist. 13, 4653–4662. doi: 10.2147/IDR.S283488
Al-Mijalli, S. H., Mrabti, H. N., Abdallah, E. M., Assaggaf, H., Qasem, A., Alenazy, R., et al. (2025). Acorus calamus as a promising source of new antibacterial agent against Pseudomonas aeruginosa and Staphylococcus aureus: deciphering volatile compounds and mode of action. Microb. Pathog. 200:107357. doi: 10.1016/j.micpath.2025.107357
Al-Shabib, N. A., Husain, F. M., Ahmad, I., and Baig, M. H. (2017). Eugenol inhibits quorum sensing and biofilm of toxigenic MRSA strains isolated from food handlers employed in Saudi Arabia. Biotechnol. Biotechnol. Equip. 31, 387–396. doi: 10.1080/13102818.2017.1281761
AlSheikh, H. M. A., Sultan, I., Kumar, V., Rather, I. A., Al-Sheikh, H., Tasleem Jan, A., et al. (2020). Plant-based phytochemicals as possible alternative to antibiotics in combating bacterial drug resistance. Antibiotics 9:480. doi: 10.3390/antibiotics9080480
Al-Tawalbeh, D. M., Alawneh, J. M., Momani, W., and Mayyas, A. (2025). Comparative antibacterial activity of clove extract against Pseudomonas aeruginosa. BMC Complement. Med. Ther. 25:7. doi: 10.1186/s12906-024-04740-7
Ambrož, M., Šmatová, M., Šadibolová, M., Pospíšilová, E., Hadravská, P., Kašparová, M., et al. (2019). Sesquiterpenes α-humulene and β-caryophyllene oxide enhance the efficacy of 5-fluorouracil and oxaliplatin in colon cancer cells. Acta Pharma. 69, 121–128. doi: 10.2478/acph-2019-0003
Amelia, B., Saepudin, E., Cahyana, A. H., Rahayu, D., Sulistyoningrum, A., and Haib, J. (2017). “GC-MS analysis of clove (Syzygium aromaticum) bud essential oil from Java and Manado” in AIP conference proceedings (Depok, Jawa Barat, Indonesia: AIP Publishing).
Aminov, R. (2025). Novel antibiotic discovery and the antibiotic resistome. Expert Opin. Drug Discov. 20, 927–941. doi: 10.1080/17460441.2025.2490838
Anwer, M. K., Jamil, S., Ibnouf, E. O., and Shakeel, F. (2014). Enhanced antibacterial effects of clove essential oil by nanoemulsion. J. Oleo Sci. 63, 347–354. doi: 10.5650/jos.ess13213
Attaallah Ibrahim, A., and Kadhim Mohammed, R. (2023). Synergistic antimicrobial activity of eugenol in combination with Fosfomycin to combat Escherichia coli and potential effect on plasmid-mediated Fosfomycin resistance genes. Chem. Biodivers. 20:e202301567. doi: 10.1002/cbdv.202301567
Awad, F. A., Mohaisen, M. R., and Al-Taee, K. T. (2024). The effect of sub-inhibitory concentration of clove essential oil on the expression of Pseudomonas aeruginosa virulence genes. Trop. J. Nat. Prod. Res. 8, 6498–6502. doi: 10.26538/tjnpr/v8i3.4
Bai, J., Li, J., Chen, Z., Bai, X., Yang, Z., Wang, Z., et al. (2023). Antibacterial activity and mechanism of clove essential oil against foodborne pathogens. Lwt 173:114249. doi: 10.1016/j.lwt.2022.114249
Băicuș, A., Mattuzzi, F. C., Paraschiv, A. M., Dinu, R.-S., Dumitrescu, M. C., Marinescu, A. A., et al. (2022). Antibacterial activity of clove, oregano, thyme, eucalyptus, and tea tree essential oils against Escherichia coli and Klebsiella pneumoniae strains. Rev. Rom. Med. Lab. 30, 327–338. doi: 10.2478/rrlm-2022-0023
Balasegaram, M., Outterson, K., and Røttingen, J.-A. (2024). The time to address the antibiotic pipeline and access crisis is now. Lancet 404, 1385–1387. doi: 10.1016/S0140-6736(24)01940-8
Barnawi, H., Qanash, H., Aldarhami, A., Alsaif, G., Alharbi, B., Almashjary, M. N., et al. (2023). Antimicrobial, anticancer, and antioxidant activities of maize and clover pollen grains extracts: a comparative study with phytochemical characterizations. Pharmaceuticals 16:1731. doi: 10.3390/ph16121731
Bayode, M. T., Awodire, E. F., Ojo, E. F., Adenikinju, G. O., Sadibo, M. E., Aro, P. O., et al. (2024). Phytophenolic derivatives as potential ameliorative agents for microbial superbugs: mechanisms of action, cellular pathways and synergistic selectivity with chemotherapeutics. Discov. Appl. Sci. 6:484. doi: 10.1007/s42452-024-06107-6
Behbahani, B. A., Noshad, M., and Falah, F. (2019). Study of chemical structure, m of action of Syzygium aromaticum essential oil on foodborne pathogens. Slovak J. Food Sci. 13. doi: 10.5219/1226
Bentley, D. J. (2024). Revisiting the checkerboard to inform development of β-lactam/β-lactamase inhibitor combinations. Antibiotics 13:337. doi: 10.3390/antibiotics13040337
Bhattacharjee, A., Jamal, T. B., Ahammad, I., Chowdhury, Z. M., Rahman, A., Dewan, G., et al. (2023). Shotgun metagenomics unravels higher antibiotic resistome profile in Bangladeshi gut microbiome. bioRxiv, 2023.2003. 2002.530749.
Bhowmik, D., Kumar, K. S., Yadav, A., Srivastava, S., Paswan, S., and Dutta, A. S. (2012). Recent trends in Indian traditional herbs Syzygium aromaticum and its health benefits. J. Pharmacogn. Phytochem. 1, 13–22.
Burt, S. A. (2004). Essential oils: their antibacterial properties and potential applications in foods--a review. Int. J. Food Microbiol. 94, 223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022
Chassagne, F., Samarakoon, T., Porras, G., Lyles, J. T., Dettweiler, M., Marquez, L., et al. (2021). A systematic review of plants with antibacterial activities: a taxonomic and phylogenetic perspective. Front. Pharmacol. 11:586548. doi: 10.3389/fphar.2020.586548
Chawla, M., Verma, J., Gupta, R., and Das, B. (2022). Antibiotic potentiators against multidrug-resistant bacteria: discovery, development, and clinical relevance. Front. Microbiol. 13:887251. doi: 10.3389/fmicb.2022.887251
Chen, X., Yang, H., Li, C., Hu, W., Cui, H., and Lin, L. (2023). Enhancing the targeting performance and prolonging the antibacterial effects of clove essential oil liposomes to Campylobacter jejuni by antibody modification. Food Res. Int. 167:112736. doi: 10.1016/j.foodres.2023.112736
Christaki, E., Marcou, M., and Tofarides, A. (2020). Antimicrobial resistance in bacteria: mechanisms, evolution, and persistence. J. Mol. Evol. 88, 26–40. doi: 10.1007/s00239-019-09914-3
Costa, L. V., Moreira, J. M. A. R., de Godoy Menezes, I., and Dutra, V. (2022). Antibiotic resistance profiles and activity of clove essential oil (Syzygium aromaticum) against Pseudomonas aeruginosa isolated of canine otitis. Vet. World 15:2499. doi: 10.14202/vetworld.2022.2499-2505
Cui, H., Bai, M., Sun, Y., Abdel-Samie, M. A.-S., and Lin, L. (2018a). Antibacterial activity and mechanism of Chuzhou chrysanthemum essential oil. J. Funct. Foods 48, 159–166. doi: 10.1016/j.jff.2018.07.021
Cui, H., Zhang, C., Li, C., and Lin, L. (2018b). Antimicrobial mechanism of clove oil on Listeria monocytogenes. Food Control 94, 140–146. doi: 10.1016/j.foodcont.2018.07.007
Cui, H., Zhang, C., Li, C., and Lin, L. (2020). Inhibition mechanism of cardamom essential oil on methicillin-resistant Staphylococcus aureus biofilm. Lwt 122:109057. doi: 10.1016/j.lwt.2020.109057
Dable-Tupas, G., Tulika, V., Jain, V., Maheshwari, K., Brakad, D. D., Naresh, P. N., et al. (2023). “Bioactive compounds of nutrigenomic importance” in Role of nutrigenomics in modern-day healthcare and drug discovery (Amsterdam, Netherlands: Elsevier), 301–342.
Dalavaye, N., Nicholas, M., Pillai, M., Erridge, S., and Sodergen, M. H. (2024). The clinical translation of α-humulene–A scoping review. Planta Med. 90:664:674. doi: 10.1055/a-2307-8183
Damasceno, R. O. S., Pinheiro, J. L. S., Rodrigues, L. H. M., Gomes, R. C., Duarte, A. B. S., Emídio, J. J., et al. (2024). Anti-inflammatory and antioxidant activities of eugenol: an update. Pharmaceuticals 17:1505. doi: 10.3390/ph17111505
Darwish, R., Almaaytah, A., and Salama, A. (2022). The design and evaluation of the antimicrobial activity of a novel conjugated penta-ultrashort antimicrobial peptide in combination with conventional antibiotics against sensitive and resistant strains of S. aureus and E. coli. Res. Pharmaceut. Sci. 17, 612–620. doi: 10.4103/1735-5362.359429
Das, C., Kothari, S., Muhuri, A., Dutta, A., Ghosh, P., and Chatterjee, S. (2019). Clove based herbal tea: development, phytochemical analysis and evaluation of antimicrobial property. J. Pharm. Sci. Res. 11, 3122–3129.
Demgne, O. M. F., Damen, F., Fankam, A. G., Guefack, M.-G. F., Wamba, B. E., Nayim, P., et al. (2021). Botanicals and phytochemicals from the bark of Hypericum roeperianum (Hypericaceae) had strong antibacterial activity and showed synergistic effects with antibiotics against multidrug-resistant bacteria expressing active efflux pumps. J. Ethnopharmacol. 277:114257. doi: 10.1016/j.jep.2021.114257
Domínguez, D. C., Chacón, L. M., and Wallace, D. J. (2021). Anthropogenic activities and the problem of antibiotic resistance in Latin America: a water issue. Water 13:2693. doi: 10.3390/w13192693
Dua, A., Garg, G., Nagar, S., and Mahajan, R. (2014). Methanol extract of clove (Syzygium aromaticum Linn.) damages cells and inhibits growth of enteropathogens. J. Innov. Biol. 1, 200–205.
Edis, Z., Haj Bloukh, S., Ashames, A. A., Al-Tabakha, M. M., Shahwan, M. J., Abu Sara, H., et al. (2025). Syzygium aromaticum extract mediated, sustainable silver nanoparticle synergetic with heterocyclic antibiotic clarithromycin and their antimicrobial activities. Front. Chem. 12:1513150. doi: 10.3389/fchem.2024.1513150
Edward, E. A., Omar, H. M., and Fathy, H. M. (2020). Evaluation of citric acid and clove oil as safe anti-virulence and resistance-modifying agents against problematic klebsiella clinical isolates in Egypt. J. Microbiol. Biotechnol. Food Sci. 9, 1060–1067. doi: 10.15414/jmbfs.2020.9.6.1060-1067
El Ghallab, Y., Al Jahid, A., Jamal Eddine, J., Ait Haj Said, A., Zarayby, L., and Derfoufi, S. (2020). Syzygium aromaticum L.: phytochemical investigation and comparison of the scavenging activity of essential oil, extracts and eugenol. Adv. Tradit. Med. 20, 153–158. doi: 10.1007/s13596-019-00416-7
El Ghallab, Y., Eddine, J. J., Aainouss, A., El Messaoudi, M. D., Dakir, M., and Derfoufi, S. (2023). Clove buds volatile compounds: inhibitory activity on mycobacterium growth and molecular docking on mmr efflux pump drug resistance. Chem. Biodivers. 20:e202300895. doi: 10.1002/cbdv.202300895
Elamary, R. B., Yassein, A. S., and Dardeer, H. M. (2023). Synergistic profile of Syzygium Aromaticum aqueous extract with standard antibiotics or Rotaxane derivatives against multi-drug resistant, biofilm forming pathogenic bacteria, fungi, and aflatoxin biosynthesis. Egypt. J. Food Sci. 51, 71–90. doi: 10.21608/ejfs.2023.192876.1157
El-Banna, T., Sonbol, F., Sallam, N., and Al-Madboly, L. (2023). Clove oil down regulates the expression of genes encoding the efflux pump, virulence factors and quorum sensing in MDR Pseudomonas aeruginosa. Durham, North Carolina, U.S.: Research Square Platform LLC.
Elbestawy, M. K., El-Sherbiny, G. M., and Moghannem, S. A. (2023). Antibacterial, antibiofilm and anti-inflammatory activities of eugenol clove essential oil against resistant Helicobacter pylori. Molecules 28:2448. doi: 10.3390/molecules28062448
Elhaddadi, H., Hamami, A., Sara, A., Elouali, A., Babakhouya, A., and Rkain, M. (2024). Prevalence and determinants of the use of antibiotics by self-medication in the Pediatric population in Oujda, Morocco. Cureus 16:e60126. doi: 10.7759/cureus.60126
Fathy, M., Fawzy, M. A., Hintzsche, H., Nikaido, T., Dandekar, T., and Othman, E. M. (2019). Eugenol exerts apoptotic effect and modulates the sensitivity of HeLa cells to cisplatin and radiation. Molecules 24:3979. doi: 10.3390/molecules24213979
Fatima, S., Saba, S., Hussain, A., Aslam, L., and Naveed, A. (2023). Antibacterial and anti-biofilm profiling of Syzygium aro-maticum plant extracts against multi-drug resistant human pathogens. Pak. J. Biochem. Biotechnol. 4, 71–82. doi: 10.52700/pjbb.v4i2.236
Faujdar, S. S., Bisht, D., and Sharma, A. (2020). Antibacterial activity of Syzygium aromaticum (clove) against uropathogens producing ESBL, MBL, and AmpC beta-lactamase: are we close to getting a new antibacterial agent? J. Family Med. Prim. Care 9, 180–186. doi: 10.4103/jfmpc.jfmpc_908_19
Federle, M. J., and Bassler, B. L. (2003). Interspecies communication in bacteria. J. Clin. Invest. 112, 1291–1299. doi: 10.1172/JCI20195
García-Contreras, R., Maeda, T., and Wood, T. K. (2013). Resistance to quorum-quenching compounds. Appl. Environ. Microbiol. 79, 6840–6846. doi: 10.1128/AEM.02378-13
Ghazi, I. M., and Cawley, M. J. (2021). The science of pharmacokinetics: an overview and applications. Remington, 207–218. doi: 10.1016/B978-0-12-820007-0.00011-8
Ginting, E. V., Retnaningrum, E., and Widiasih, D. A. (2021). Antibacterial activity of clove (Syzygium aromaticum) and cinnamon (Cinnamomum burmannii) essential oil against extended-spectrum β-lactamase-producing bacteria. Vet. World 14, 2206–2211. doi: 10.14202/vetworld.2021.2206-2211
Goncalves, G. E., de Souza, F. S., Lago, J. H. G., and Caseli, L. (2015). The interaction of eugenol with cell membrane models at the air–water interface is modulated by the lipid monolayer composition. Biophys. Chem. 207, 7–12. doi: 10.1016/j.bpc.2015.07.007
Guardabassi, L., Butaye, P., Dockrell, D. H., Fitzgerald, J. R., and Kuijper, E. J. (2020). One health: a multifaceted concept combining diverse approaches to prevent and control antimicrobial resistance. Clin. Microbiol. Infect. 26, 1604–1605. doi: 10.1016/j.cmi.2020.07.012
Guenette, S., Beaudry, F., Marier, J., and Vachon, P. (2006). Pharmacokinetics and anesthetic activity of eugenol in male Sprague–Dawley rats. J. Vet. Pharmacol. Ther. 29, 265–270. doi: 10.1111/j.1365-2885.2006.00740.x
Guénette, S. A., Ross, A., Marier, J.-F., Beaudry, F., and Vachon, P. (2007). Pharmacokinetics of eugenol and its effects on thermal hypersensitivity in rats. Eur. J. Pharmacol. 562, 60–67. doi: 10.1016/j.ejphar.2007.01.044
Gupta, Y. D., and Bhandary, S. (2024). “Artificial intelligence for understanding mechanisms of antimicrobial resistance and antimicrobial discovery: a new age model for translational research” in Artificial Intelligence and Machine Learning in Drug Design and Development, 117–156. Hoboken, New Jersey, USA.
Haro-González, J. N., Castillo-Herrera, G. A., Martínez-Velázquez, M., and Espinosa-Andrews, H. (2021). Clove essential oil (Syzygium aromaticum L. Myrtaceae): extraction, chemical composition, food applications, and essential bioactivity for human health. Molecules 26:6387. doi: 10.3390/molecules26216387
Hemaiswarya, S., Kruthiventi, A. K., and Doble, M. (2008). Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 15, 639–652. doi: 10.1016/j.phymed.2008.06.008
Hu, W., Li, C., Dai, J., Cui, H., and Lin, L. (2019). Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA). Ind. Crop. Prod. 130, 34–41. doi: 10.1016/j.indcrop.2018.12.078
Huerta Lorenzo, B., Galán-Relaño, Á., Barba-Sánchez, E., Romero-Salmoral, A., Solarte Portilla, A. L., Gómez-Gascón, L., et al. (2024). Potentiation of the antimicrobial effect of oxytetracycline combined with cinnamon, clove, oregano, and red thyme essential oils against MDR Salmonella enterica strains. Animals 14:1347. doi: 10.3390/ani14091347
Husain, F. M., Ahmad, I., Asif, M., and Tahseen, Q. (2013). Influence of clove oil on certain quorum-sensing-regulated functions and biofilm of Pseudomonas aeruginosa and Aeromonas hydrophila. J. Biosci. 38, 835–844. doi: 10.1007/s12038-013-9385-9
Ibrahim, M. O., Meani, A., and Latef, S. A. (2019). Detection of plasmid-mediated AmpC β-lactamase genes and evaluation the synergistic effect of clove volatile oil and antibiotics in clinical isolates of Klebsiella pneumonia in Iraq. Biochem. Cell. Archiv. 19. doi: 10.35124/bca.2019.19.2.4053
Islam, M. R., Akash, S., Rahman, M. M., Nowrin, F. T., Akter, T., Shohag, S., et al. (2022). Colon cancer and colorectal cancer: prevention and treatment by potential natural products. Chemicobiol. Interact. 368:110170. doi: 10.1016/j.cbi.2022.110170
Jafri, H., and Ahmad, I. (2021). In vitro efficacy of clove oil and eugenol against Staphylococcus spp. and Streptococcus mutans on hydrophobicity, hemolysin production and biofilms and their synergy with antibiotics. Adv. Microbiol. 11, 117–143. doi: 10.4236/aim.2021.112009
Jahanshir, M., Nobahar, M., Ghorbani, R., and Malek, F. (2023). Effect of clove mouthwash on the incidence of ventilator-associated pneumonia in intensive care unit patients: a comparative randomized triple-blind clinical trial. Clin. Oral Investig. 27, 3589–3600. doi: 10.1007/s00784-023-04972-w
Jang, H.-I., Rhee, K.-J., and Eom, Y.-B. (2020). Antibacterial and antibiofilm effects of α-humulene against Bacteroides fragilis. Can. J. Microbiol. 66, 389–399. doi: 10.1139/cjm-2020-0004
Jesudason, T. (2024). WHO publishes updated list of bacterial priority pathogens. Lancet 5:100940. doi: 10.1016/j.lanmic.2024.07.003
Jung, D. H., Park, M. H., Kim, C. J., Lee, J. Y., Keum, C. Y., Kim, I. S., et al. (2020). Effect of β-caryophyllene from cloves extract on Helicobacter pylori eradication in mouse model. Nutrients 12:1000. doi: 10.3390/nu12041000
Kamatou, G. P., Vermaak, I., and Viljoen, A. M. (2012). Eugenol—from the remote Maluku Islands to the international market place: a review of a remarkable and versatile molecule. Molecules 17, 6953–6981. doi: 10.3390/molecules17066953
Kaur, D., and Chandrul, K. K. (2017). Syzygium aromaticum L. (clove): a vital herbal drug used in periodontal disease. Indian J. Pharm. Biol. Res. 5, 45–51. doi: 10.30750/ijpbr.5.2.9
Khwaza, V., and Aderibigbe, B. A. (2025). Antibacterial activity of selected essential oil components and their derivatives: a review. Antibiotics 14. doi: 10.3390/antibiotics14010068
Kim, N.-N., Kim, W. J., and Kang, S.-S. (2019). Anti-biofilm effect of crude bacteriocin derived from Lactobacillus brevis DF01 on Escherichia coli and Salmonella Typhimurium. Food Control 98, 274–280. doi: 10.1016/j.foodcont.2018.11.004
Kothiwale, S. V., Patwardhan, V., Gandhi, M., Sohoni, R., and Kumar, A. (2014). A comparative study of antiplaque and antigingivitis effects of herbal mouthrinse containing tea tree oil, clove, and basil with commercially available essential oil mouthrinse. J. Indian Soc. Periodontol. 18, 316–320. doi: 10.4103/0972-124X.134568
Kowalewska, A., and Majewska-Smolarek, K. (2023). Eugenol-based polymeric materials—antibacterial activity and applications. Antibiotics 12:1570. doi: 10.3390/antibiotics12111570
Krishnan, T., Yin, W.-F., and Chan, K.-G. (2012). Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa PAO1 by Ayurveda spice clove (Syzygium Aromaticum) bud extract. Sensors 12, 4016–4030. doi: 10.3390/s120404016
Lady, J., Nurcahyanti, A. D., and Tjoa, E. (2023). Synergistic effect and time-kill evaluation of eugenol combined with cefotaxime against Staphylococcus aureus. Curr. Microbiol. 80:244. doi: 10.1007/s00284-023-03364-3
Latifah-Munirah, B., Himratul-Aznita, W., and Mohd Zain, N. (2015). Eugenol, an essential oil of clove, causes disruption to the cell wall of Candida albicans (ATCC 14053). Front. Life Sci. 8, 231–240. doi: 10.1080/21553769.2015.1045628
Lawani, B. T., Bayode, M. T., Sadibo, M. E., Awodire, E. F., Aro, O. P., and Akindele, A. A. (2023). Antibiotic resistance microbes’ (ARM) mechanisms and management: a phytomedicinal approach. Proc. Natl. Acad. Sci. India Sect B Biol. Sci. 94, 697–704. doi: 10.1007/s40011-023-01525-9
Li, J., Li, C., Shi, C., Aliakbarlu, J., Cui, H., and Lin, L. (2022). Antibacterial mechanisms of clove essential oil against Staphylococcus aureus and its application in pork. Int. J. Food Microbiol. 380:109864. doi: 10.1016/j.ijfoodmicro.2022.109864
Lin, Y., Tang, X., Xu, L., and Wang, S. (2019). Antibacterial properties and possible action mechanism of chelating peptides-zinc nanocomposite against Escherichia coli. Food Control 106:106675. doi: 10.1016/j.foodcont.2019.06.001
Liñán-Atero, R., Aghababaei, F., García, S. R., Hasiri, Z., Ziogkas, D., Moreno, A., et al. (2024). Clove essential oil: chemical profile, biological activities, encapsulation strategies, and food applications. Antioxidants 13:488. doi: 10.3390/antiox13040488
Liu, J.-W., Ko, W.-C., Huang, C.-H., Liao, C.-H., Lu, C.-T., Chuang, Y.-C., et al. (2012). Agreement assessment of tigecycline susceptibilities determined by the disk diffusion and broth microdilution methods among commonly encountered resistant bacterial isolates: results from the Tigecycline in vitro surveillance in Taiwan (TIST) study, 2008 to 2010. Antimicrob. Agents Chemother. 56, 1414–1417. doi: 10.1128/AAC.05879-11
Liu, J., Mahmood, M. S., Abbas, R. Z., Dillawar, A., Nawaz, Z., Luqman, M., et al. (2021). Therapeutic appraisal of ethanolic and aqueous extracts of clove (Syzygium aromaticum) and garlic (Allium sativum) as antimicrobial agent. Pak. J. Agric. Sci. 58, 245–251. doi: 10.21162/PAKJAS/21.650
Liu, Q., Meng, X., Li, Y., Zhao, C.-N., Tang, G.-Y., and Li, H.-B. (2017). Antibacterial and antifungal activities of spices. Int. J. Mol. Sci. 18:1283. doi: 10.3390/ijms18061283
Lv, C., Leng, J., Qian, M., Sun, B., Ye, H., Li, M., et al. (2024). Antimicrobial resistance in Escherichia coli and Staphylococcus aureus at human-animal interfaces on Chongming Island, Shanghai: a one health perspective. One Health 19:100910. doi: 10.1016/j.onehlt.2024.100910
Ma, L., Liu, J., Lin, Q., Gu, Y., and Yu, W. (2021). Eugenol protects cells against oxidative stress via Nrf2. Exp. Ther. Med. 21:1. doi: 10.3892/etm.2020.9539
Marchese, A., Barbieri, R., Coppo, E., Orhan, I. E., Daglia, M., Nabavi, S. F., et al. (2017). Antimicrobial activity of eugenol and essential oils containing eugenol: a mechanistic viewpoint. Crit. Rev. Microbiol. 43, 668–689. doi: 10.1080/1040841X.2017.1295225
Marouf, R., Ermolaev, A. A., Podoprigora, I. V., Senyagin, A. N., and Mbarga, M. J. (2023). Antibacterial activity of clove Syzygium aromaticum L. and synergism with antibiotics against multidrug-resistant uropathogenic E. coli. Вестник Российского университета дружбы народов. Серия: Медицина 27, 379–390. doi: 10.22363/2313-0245-2023-27-3-379-390
Marya, C. M., Satija, G., A, J., Nagpal, R., Kapoor, R., and Ahmad, A. (2012). In vitro inhibitory effect of clove essential oil and its two active principles on tooth decalcification by apple juice. Int. J. Dentist. 2012:759618. doi: 10.1155/2012/759618
Mekky, A. E., Emam, A. E., Selim, M. N., Abdelmouty, E. S., and Khedr, M. (2024). Antibacterial and antineoplastic MCF-7 and HePG-2 characteristics of the methanolic (80%) clove (Syzygium aromaticum L.) extract. Biomass Convers. Biorefinery 14, 16787–16798. doi: 10.1007/s13399-023-03862-1
Mohamed, M. S., Abdallah, A. A., Mahran, M. H., and Shalaby, A. M. (2018). Potential alternative treatment of ocular bacterial infections by oil derived from Syzygium aromaticum flower (clove). Curr. Eye Res. 43, 873–881. doi: 10.1080/02713683.2018.1461907
Mohamed, E. H., Alghamdi, Y. S., Mostafa Abdel-Hafez, S., Soliman, M. M., Alotaibi, S. H., Hassan, M. Y., et al. (2020). Susceptibility assessment of multidrug resistant bacteria to natural products. Dose-Response 18:1559325820936189. doi: 10.1177/1559325820936189
Mohapatra, S., Mohandas, R., and Kumar, R. P. (2023). Comparison of the anti-microbial efficacy of clove, cinnamon, turmeric, nutmeg, and peppermint essential oil against oral pathogens: an in-vitro study. J. Clin. Diagnost. Res. 17. doi: 10.7860/JCDR/2023/61363.17800
Moon, S.-E., Kim, H.-Y., and Cha, J.-D. (2011). Synergistic effect between clove oil and its major compounds and antibiotics against oral bacteria. Arch. Oral Biol. 56, 907–916. doi: 10.1016/j.archoralbio.2011.02.005
Mostafa, O., Hammoda, H., Elsaygh, M., and Abdelwahab, I. (2017). Efficacy of the clove oil, cinnamon oil, thyme oil and origanum oil against multidrug resistant Pseudomonas aeruginosa and Burkholderia cepacia complex. Int. J. Curr. Microbiol. Appl. Sci. 6, 29–35. doi: 10.20546/ijcmas.2017.601.004
Mugnaini, C., Sannio, F., Brizzi, A., Del Prete, R., Simone, T., Ferraro, T., et al. (2020). Screen of unfocused libraries identified compounds with direct or synergistic antibacterial activity. ACS Med. Chem. Lett. 11, 899–905. doi: 10.1021/acsmedchemlett.9b00674
Muhammad, K. M., and Shoge, M. O. (2023). Applications of antimicrobial stewardship and natural product chemistry in tackling antimicrobial resistance. London, UK: IntechOpen.
Naghavi, M., Vollset, S. E., Ikuta, K. S., Swetschinski, L. R., Gray, A. P., Wool, E. E., et al. (2024). Global burden of bacterial antimicrobial resistance 1990–2021: a systematic analysis with forecasts to 2050. Lancet 404, 1199–1226. doi: 10.1016/S0140-6736(24)01867-1
National Institute of Diabetes and Digestive and Kidney Diseases (2012). LiverTox: clinical and research information on drug-induced liver injury. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases.
Nejad, S. M., Özgüneş, H., and Başaran, N. (2017). Pharmacological and toxicological properties of eugenol. Turk. J. Pharm. Sci. 14, 201–206. doi: 10.4274/tjps.62207
Nirmala, M. J., Durai, L., Gopakumar, V., and Nagarajan, R. (2019). Anticancer and antibacterial effects of a clove bud essential oil-based nanoscale emulsion system. Int. J. Nanomedicine 14, 6439–6450. doi: 10.2147/IJN.S211047
Nisar, M. F., Khadim, M., Rafiq, M., Chen, J., Yang, Y., and Wan, C. C. (2021). Pharmacological properties and health benefits of eugenol: a comprehensive review. Oxidative Med. Cell. Longev. 2021:2497354. doi: 10.1155/2021/2497354
Noreen, S., Aslam, B., and Alina, Z. (2018). Antibacterial activity of medicinal plants (clove, cinnamon, garlic) extracts and their combined effect with antibiotics in urinary tract infection caused by Escherichia coli. Int. J. Pharm. Pharmacol. 2018:128. doi: 10.31531/25813080(2)
Odds, F. C. (2003). Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. doi: 10.1093/jac/dkg301
Olszewska, M. A., Gędas, A., and Simões, M. (2020). The effects of eugenol, trans-cinnamaldehyde, citronellol, and terpineol on Escherichia coli biofilm control as assessed by culture-dependent and-independent methods. Molecules 25:2641. doi: 10.3390/molecules25112641
Pateiro, M., Munekata, P. E., Sant'Ana, A. S., Domínguez, R., Rodríguez-Lázaro, D., and Lorenzo, J. M. (2021). Application of essential oils as antimicrobial agents against spoilage and pathogenic microorganisms in meat products. Int. J. Food Microbiol. 337:108966. doi: 10.1016/j.ijfoodmicro.2020.108966
Pilong, P., Mishra, D. K., Ruengdech, A., and Siripatrawan, U. (2023). Foam-mat freeze-drying approach for preserving stability and antimicrobial activity of clove essential oil nanoemulsion. Food Biosci. 52:102369. doi: 10.1016/j.fbio.2023.102369
Putri, S. A., Julaeha, E., Kagawa, N., and Kurnia, D. (2025). The potential of secondary metabolites in medicinal plants as anti–quorum sensing in biofilms: a comprehensive review. J. Chem. doi: 10.1155/joch/8838140
Rajkowska, K., Otlewska, A., Kunicka-Styczyńska, A., and Krajewska, A. (2017). Candida albicans impairments induced by peppermint and clove oils at sub-inhibitory concentrations. Int. J. Mol. Sci. 18:1307. doi: 10.3390/ijms18061307
Rao, R. S. P., Ghate, S. D., Shastry, R. P., Kurthkoti, K., Suravajhala, P., Patil, P., et al. (2023). Prevalence and heterogeneity of antibiotic resistance genes in Orientia tsutsugamushi and other rickettsial genomes. Microb. Pathog. 174:105953. doi: 10.1016/j.micpath.2022.105953
Rathinam, P., and Viswanathan, P. (2018). Anti-virulence potential of eugenol-rich fraction of Syzygium aromaticum against multidrug resistant uropathogens isolated from catheterized patients. Avicenna J. Phytomed. 8:416.
Rodenak-Kladniew, B., Montoto, S. S., Sbaraglini, M. L., Di Ianni, M., Ruiz, M. E., Talevi, A., et al. (2019). Hybrid ofloxacin/eugenol co-loaded solid lipid nanoparticles with enhanced and targetable antimicrobial properties. Int. J. Pharm. 569:118575. doi: 10.1016/j.ijpharm.2019.118575
Saleh, R. O., Dheyab, A. S., Hadi, B. H., Hasan, R. N., and Jasim, S. A. (2024). Effect of ethanolic extract of Syzygium aromaticum plant against Enterococcus faecalis isolated from women with urinary tract infections. Iran. J. Clin. Infect. Dis. 19. doi: 10.5812/archcid-134924
Salisu, A., Iliyasu, M., Sahal, M., Titus, I., Isma’il, S., Umar, R., et al. (2021). Antimicrobial potential of Syzygium aromaticium (clove) extracts on multidrug-resistant (MDR) uropathogenic bacteria isolated from clinical specimens in Bauchi, Nigeria. Methodology. 24, 41–57. doi: 10.9734/jamps/2022/v24i11588
Santolin, L., Fiametti, K. G., da Silva Lobo, V., Wancura, J. H., and Oliveira, J. V. (2021). Enzymatic synthesis of eugenyl acetate from essential oil of clove using lipases in liquid formulation as biocatalyst. Appl. Biochem. Biotechnol. 193, 3512–3527. doi: 10.1007/s12010-021-03610-z
Shaaban, M. T., Fahmy, G. M., Saad, E. H., and Salama, H. S. (2024). Clove and peppermint essential oils effect on pathogenic gut micro-biota in chronic hepatic disease patients. Bioactivities 2, 24–29. doi: 10.47352/bioactivities.2963-654X.213
Shahbazi, Y. (2019). Antioxidant, antibacterial, and antifungal properties of nanoemulsion of clove essential oil. Nanomed. Res. J. 4, 204–208. doi: 10.22034/nmrj.2019.04.001
Sikdar, R., and Elias, M. H. (2020). Quorum quenching enzymes and their effects on virulence, biofilm, and microbiomes: a review of recent advances. Expert Rev. Anti-Infect. Ther. 18, 1221–1233. doi: 10.1080/14787210.2020.1794815
Singletary, K. (2014). Clove: overview of potential health benefits. Nutr. Today 49, 207–224. doi: 10.1097/NT.0000000000000036
Spigarelli, R., Spisni, E., Magalhães, M., Cabral, C., Gonçalves, A. C., Saracino, I. M., et al. (2024). Clove essential oil as a source of antitumoral compounds capable of crossing the blood–brain barrier: a focus on the effects of β-caryophyllene and eugenol in a glioblastoma cell line. Int. J. Mol. Sci. 26:238. doi: 10.3390/ijms26010238
Stefanović, O. D. (2017). “Synergistic activity of antibiotics and bioactive plant extracts: a study against gram-positive and gram-negative bacteria” in Bacterial Pathogenesis and Antibacterial Control. London, UK: IntechOpen.
Strižincová, P., Šurina, I., Majová, V., Ház, A., and Hroboňová, K. (2022). Choline chloride: lactic acid as a green solvent for the extraction of eugenol, eugenol acetate, and β-Caryophyllene of clove buds.
Sulong, M. R., Mansor, F. N., Hazman, S. A. H., and Alias, R. (2024). Utilizing Anacardium occidentale leaves extract as an alternative to conventional antibiotics against antimicrobial-resistant microorganisms. J. Halal Sci. Indust. Bus. 2, 79–89. doi: 10.31098/jhasib.v2i1.2326
Sun, X.-h., Zhou, T.-t., Wei, C.-h., Lan, W.-q., Zhao, Y., Pan, Y.-j., et al. (2018). Antibacterial effect and mechanism of anthocyanin rich Chinese wild blueberry extract on various foodborne pathogens. Food Control 94, 155–161. doi: 10.1016/j.foodcont.2018.07.012
Tang, K., Millar, B. C., and Moore, J. E. (2023). Antimicrobial resistance (AMR). Br. J. Biomed. Sci. 80. doi: 10.3389/bjbs.2023.11387
Tarhan, İ. (2021). A robust method for simultaneous quantification of eugenol, eugenyl acetate, and β-caryophyllene in clove essential oil by vibrational spectroscopy. Phytochemistry 191:112928. doi: 10.1016/j.phytochem.2021.112928
Ulanowska, M., and Olas, B. (2021). Biological properties and prospects for the application of eugenol—a review. Int. J. Mol. Sci. 22:3671. doi: 10.3390/ijms22073671
Ullah, M. A., Hassan, A., and Hamza, A. (2023). Role of clove in human medical history. SAR J. Anat. Physiol. 4, 10–19. doi: 10.36346/sarjap.2023.v04i02.001
Unalan, I., Endlein, S. J., Slavik, B., Buettner, A., Goldmann, W. H., Detsch, R., et al. (2019). Evaluation of electrospun poly (ε-caprolactone)/gelatin nanofiber mats containing clove essential oil for antibacterial wound dressing. Pharmaceutics 11:570. doi: 10.3390/pharmaceutics11110570
Vázquez-Ucha, J. C., Martínez-Guitián, M., Lasarte-Monterrubio, C., Conde-Pérez, K., Arca-Suárez, J., Álvarez-Fraga, L., et al. (2020). Syzygium aromaticum (clove) and thymus zygis (thyme) essential oils increase susceptibility to colistin in the nosocomial pathogens Acinetobacter baumannii and Klebsiella pneumoniae. Biomed. Pharmacother. 130:110606. doi: 10.1016/j.biopha.2020.110606
Veerapandi, G., Meenakshi, S., Anitta, S., Arul, C., Ashokkumar, P., and Sekar, C. (2022). Precise and quick detection of ascorbic acid and eugenol in fruits, pharmaceuticals and medicinal herbs using hydroxyapatite-titanium dioxide nanocomposite-based electrode. Food Chem. 382:132251. doi: 10.1016/j.foodchem.2022.132251
Wambaugh, M. A., Denham, S. T., Brammer, B., Stonhill, M., and Brown, J. C. S. (2019). Synergistic and antagonistic drug interactions in the treatment of systemic fungal infections. eLife 9:e54160. doi: 10.7554/eLife.54160
Wongsawan, K., Chaisri, W., Tangtrongsup, S., and Mektrirat, R. (2019). Bactericidal effect of clove oil against multidrug-resistant Streptococcus suis isolated from human patients and slaughtered pigs. Pathogens 9:14. doi: 10.3390/pathogens9010014
World Health Organization. (2024). WHO bacterial priority pathogens list, 2024: bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance. Geneva Switzerland.
Xu, J.-G., Liu, T., Hu, Q.-P., and Cao, X.-M. (2016). Chemical composition, antibacterial properties and mechanism of action of essential oil from clove buds against Staphylococcus aureus. Molecules 21:1194. doi: 10.3390/molecules21091194
Yao, Z., Zhang, W., Hu, Y., An, Z., Fang, Z., Wang, J., et al. (2024). Preparation, characterization, oral bioavailability, and pharmacodynamic study of eugenol-porous silica solidified powder. Drug Deliv. Transl. Res., 1–14. doi: 10.1007/s13346-024-01666-y
Yassin, M. T., Al-Askar, A. A., Mostafa, A. A.-F., and El-Sheikh, M. A. (2020). Bioactivity of Syzygium aromaticum (L.) Merr. & LM Perry extracts as potential antimicrobial and anticancer agents. J. King Saud Univ. Sci. 32, 3273–3278. doi: 10.1016/j.jksus.2020.09.009
Ye, Z.-W., Yang, Q.-Y., Lin, Q.-H., Liu, X.-X., Li, F.-Q., Xuan, H.-D., et al. (2024). Progress of nanopreparation technology applied to volatile oil drug delivery systems. Heliyon 10. doi: 10.1016/j.heliyon.2024.e24302
Zari, A. T., Zari, T. A., and Hakeem, K. R. (2021). Anticancer properties of eugenol: A review. Molecules 26:7407. doi: 10.3390/molecules26237407
Zhai, Z., Zhang, X., Ding, Y., Huang, Z., Li, Q., Zheng, M., et al. (2022). Eugenol restrains abdominal aortic aneurysm progression with down-regulations on NF-κB and COX-2. Phytother. Res. 36, 928–937. doi: 10.1002/ptr.7358
Zhang, Y., Wang, Y., Zhu, X., Cao, P., Wei, S., and Lu, Y. (2017). Antibacterial and antibiofilm activities of eugenol from essential oil of Syzygium aromaticum (L.) Merr. & LM Perry (clove) leaf against periodontal pathogen Porphyromonas gingivalis. Microb. Pathog. 113, 396–402. doi: 10.1016/j.micpath.2017.10.054
Keywords: antibacterial activity, Syzygium aromaticum , multidrug-resistant pathogens, medicinal plants, natural drugs, health
Citation: Tariq H, Alhudhaibi AM and Abdallah EM (2025) Syzygium aromaticum (clove buds) as a natural antibacterial agent: a promising alternative to combat multidrug-resistant bacteria. Front. Microbiol. 16:1674590. doi: 10.3389/fmicb.2025.1674590
Edited by:
Fohad Mabood Husain, King Saud University, Saudi ArabiaReviewed by:
Cristián Quintero, Juan Agustín Maza University, ArgentinaMichael Bayode, Federal University of Technology, Nigeria
Copyright © 2025 Tariq, Alhudhaibi and Abdallah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdulrahman Mohammed Alhudhaibi, YW1hbGh1ZGhhaWJpQGltYW11LmVkdS5zYQ==; Emad M. AbdallahMTQwMjA4QHF1LmVkdS5zYQ==
 Hira Tariq1
Hira Tariq1 Abdulrahman Mohammed Alhudhaibi
Abdulrahman Mohammed Alhudhaibi Emad M. Abdallah
Emad M. Abdallah