- 1Water Research Institute, National Research Council of Italy, CNR-IRSA, Monterotondo, Rome, Italy
- 2National Biodiversity Future Center, Palermo, Italy
- 3Department for Innovation in Agroforestry and Biological Systems, University of Tuscia, Viterbo, Italy
- 4Department of Environmental Sciences, Informatics and Statistics, Ca' Foscari University of Venice, Venice, Italy
The tannery sludge, with its high content of organic and inorganic compounds often hazardous to the environment, represents a major challenge in industrial waste management. Among the strategies to mitigate its impact, the biological production of fatty acids can be considered a cost-effective and sustainable solution. For the first time, this study investigated in depth the microbial community's composition and dynamics during anaerobic fermentation of tannery sludge to produce short-chain fatty acids (SCFAs). Systems with varying hydraulic retention times (HRT) and temperatures were explored, utilizing oxidative (Ox) and thermal (Th) pretreatments to enhance organic matter bioavailability. Mesophilic Ox reactors (Ox_4, Ox_8) achieved stable SCFAs production, primarily acetic acid. Ox_8, with longer HRT than Ox_4, showed higher organic matter conversion (0.32 ± 0.01 and 0.25 ± 0.01 g CODSCFA/g VS, respectively) and bacterial metabolic activity (FISH/CARD-FISH ratio of 0.8 and 0.9, respectively) as highlighted by microbiological analysis. Thermophilic Th reactors (Th_4, Th_8) yielded highest SCFAs production especially at longer HRTs (0.4 ± 0.01 g CODSCFA/g VS). The cutting-edge biomolecular approach herein applied has elucidated microbial community diversity and metabolic functionalities. Ox systems were dominated by Actinobacteria, Bacteroidetes, and Firmicutes, with Proteiniphilum as the key SCFAs producer. In contrast, Th reactors were mainly colonized by Coprothermobacteraeota and Firmicutes, with Coprothermobacter sp. playing a central role in SCFAs synthesis. Despite the compositional differences, both systems exhibited a noteworthy proteolytic functional redundancy, primarily linked to the substrate used. Among the first explorations, this research provides critical insights into the microbial adaptability and resilience of these biological systems.
1 Introduction
The leather tanning industry, a pivotal component of the global fashion and footwear sectors, is inseparably connected to extensive environmental challenges. The generation of substantial quantities of tannery sludge, a complex matrix of organic matter, toxic inorganic compounds, and microorganisms, poses a difficult threat to ecosystems due to its high organic load, heavy metal content, and recalcitrant nature (Abreu and Toffoli, 2009; Ali et al., 2015; Alibardi and Cossu, 2016). Due to the high content of organic and inorganic substances, often hazardous to the environment, this sludge poses a significant challenge for industrial waste management. Traditional waste management practices, such as landfilling and incineration, while seemingly expedient, intensify environmental issues by contributing to soil and water pollution, greenhouse gas emissions, and resource depletion (Alibardi and Cossu, 2016).
In particular, the Italian leather industry generates a significant amount of tannery sludge as a byproduct of leather processing. As outlined in the UNIC sustainability report of 2021, Italy's tannery sector is a global leader, accounting for 23% of the total global leather value (Unic Italian tannery 2021. UNIC Sustainability report). Italian legislation, particularly Legislative Decree 152/2006, strictly regulates the disposal of tannery sludge, requiring companies in the sector to adopt suitable technologies and treatment processes to minimize environmental impact. To reduce environmental impact and valorize these wastes, recent years have seen a growing interest in innovative solutions such as anaerobic digestion, co-composting, and biofuel production (Jaffari et al., 2024). These technologies allow for the transformation of tannery sludge into energy resources and useful bio-based materials, thus promoting a circular economy in the leather sector. In the pursuit of sustainable and circular economy principles, the valorization of tannery sludge emerges as a requiring imperative (Chojnacka et al., 2021; Moktadir et al., 2023). By transforming this waste into valuable products, the leather industry can mitigate its environmental impact while generating economic benefits. Microbial-based technologies represent a promising alternative for achieving this goal. Employing the metabolic capabilities of microorganisms, these technologies can effectively degrade complex organic matter, remove contaminants, and produce high-value products (Angenent et al., 2016; Bai et al., 2017; Ashraf et al., 2018; Saxena et al., 2020; Reyes-Romero et al., 2021; Ameen, 2023; Moktadir et al., 2023).
Fermentation and anaerobic digestion of tannery sludge align with the principles of circular economy. By transforming this waste into valuable bioproducts, these processes contribute to a more sustainable and resource-efficient leather industry. The anaerobic fermentation of tannery sludge produces short-chain fatty acids (SCFAs), which can be further converted into biofuels, bioplastics, or used as a nutrient source for algae cultivation (Han et al., 2019; Wu et al., 2019; Wang and Yin, 2022). This approach not only reroutes waste from landfills but also creates a circular pathway that recovers resources from a previously discarded material, thereby minimizing environmental impact and promoting economic growth. Pre-treatment of sludge prior to anaerobic process is a critical step to enhance microbial accessibility and overall process efficiency (Neyens et al., 2003; Appels et al., 2010; Braguglia et al., 2012; Gianico et al., 2013; Gallipoli et al., 2014; Gagliano et al., 2015; Khadaroo et al., 2019; Tonanzi et al., 2021). Physical, chemical, and biological pre-treatments can disrupt the complex organic matrix of sludge, increasing the surface area available for microbial attack and solubilizing recalcitrant organic compounds (Ariunbaatar et al., 2014; Chang et al., 2020; Gottardo et al., 2022; Wang and Yin, 2022). For instance, thermal hydrolysis has been shown to be effective in disrupting the sludge structure and increasing volatile solids solubilization (Gagliano et al., 2015; Gianico et al., 2015; Gherghel et al., 2019; Jeong et al., 2019). Furthermore, the combination of physical and chemical pre-treatments can synergistically enhance the performance of anaerobic processes (Neyens et al., 2003; Appels et al., 2010; Khadaroo et al., 2019; Li et al., 2023).
Recent improvements in biotechnological applications have shown the potential of microbial communities to address the complexities of tannery sludge (Jaffari et al., 2024). For instance, the application of anaerobic fermentation technology and oxidative or thermal pretreatment has shown high aptitude in minimizing landfilling by producing SCFAs, reducing organic matter content and solubilizing toxic compounds such as chromium, highly abundant in this waste (Zhai et al., 2020; Abate et al., 2021; Tuci et al., 2023, 2024; Zhu et al., 2024). These studies highlighted the potential of microbial-based approaches to transform tannery sludge from waste into a resource opportunity. Despite the key role of microorganisms in such process, the taxonomic diversity and functional potentialities of microbial communities involved in the production of fatty acids from tannery sludge have not been investigated so far. To effectively enhance biotreatment processes and product recovery from tannery sludge, a comprehensive characterization of the microbial community and its performance is necessary. For the first time, this study aims to characterize the taxonomic diversity and dynamics of the microbial communities involved in the anaerobic fermentation of tannery sludge in semi-continuous processes for the production of SCFAs. The insights gained will contribute to the development of innovative bioprocesses for a more sustainable and circular leather industry.
2 Materials and methods
2.1 Experimental set up
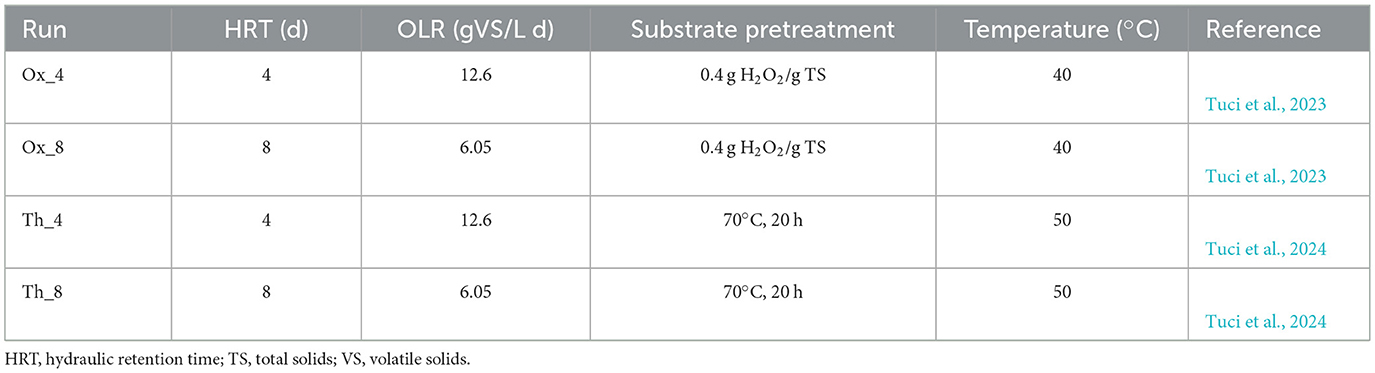
Microbiological analyses were performed on samples coming from SCFAs-producing semi-continuous reactors described in Tuci et al. (2023, 2024) (Table 1). These studies utilized a mixture of primary and secondary tannery sludge obtained from the Montebello Vicentino wastewater treatment plant (WWTP) in northeast Italy. Four different semi-continuous stirred tank reactors (sCSTRs) of 1.5 L working volume were operated to assess anaerobic fermentation of tannery sludge subjected to hydrogen peroxide (H2O2) or thermal pretreatment (Run Ox and Th, respectively). The reactors were inoculated with tannery sludge, maintained at 40°C and 50°C, respectively, and daily fed with pre-treated sludge for 1 week. A hydraulic retention time (HRT) of 4 and 8 days and organic loading rate (OLR) of 12.6 and 6.05 gVS/L d were tested in parallel (Table 1). Reactor performance was evaluated by monitoring SCFAs production, chemical oxygen demand (COD), ammonium, pH, and chromium concentration. The SCFAs composition, COD, volatile solids (VS) and other chemical parameters, including fermentation yield (Y), were analyzed according to the methods outlined in Tuci et al. (2023).
2.2 In situ detection methods (FISH and CARD-FISH)
Samples were collected from the anaerobic systems and promptly fixed in a formaldehyde (2% v/v) and ethanol solution (48% v/v) before storage at −20°C. Prior to analysis, the fixed biomass was disaggregated by vortexing with glass beads. FISH and CARD-FISH analyses were performed using the EUB338mix oligonucleotide probe set (equimolar concentrations of EUB338, EUB338-II, and EUB338-III, http://www.probebase.net; Nielsen et al., 2009; Matturro et al., 2013; Tonanzi et al., 2018). Following hybridization, total cells were detected with DAPI using Vectashield Mounting Medium (Vector Labs, Italy). The abundance of fluorescent cells within the experimental samples was determined through the application of epifluorescence microscopy, a standard procedure consistently applied and documented in previous studies, including Tonanzi et al. (2018). The FISH/CARD-FISH ratio for each sample was determined as previously reported in Tonanzi et al. (2018).
2.3 Sample collection and processing for DNA extraction
Eight anaerobic samples were collected from each reactor throughout the experimental period. For DNA extraction, duplicate 2 mL aliquots were centrifuged as reported in Tonanzi et al. (2018), and the resulting pellets were stored at −20°C. The DNeasy PowerSoil Pro kit (QIAGEN, Hilden, Germany) was used for DNA extraction according to the manufacturer's instructions.
2.4 16S rRNA gene amplicon sequencing and data analysis
To characterize the microbial community structure, the 16S rRNA gene amplicon sequencing was performed using Illumina technology as previously described (Crognale et al., 2019). Briefly, the V1-V3 region of the bacterial 16S rRNA gene was amplified using primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 534R (5′-ATTACCGCGGCTGCTGG-3′). Subsequent sequence data processing and analysis were conducted using QIIME2 (v. 2017.12), including demultiplexing, DADA2 quality filtering and trimming, and taxonomic assignment of amplicon sequence variants (ASVs) (Quast et al., 2013; Callahan et al., 2016). A total of 167,080 reads were obtained by high-throughput sequencing of the V1–V3 region of the bacterial 16S rRNA gene, that resolved into 4701 ASVs. The dataset is available through the Sequence Read Archive (SRA) under accession PRJNA1178871.
2.5 Statistical analysis
The statistical significance of the data was demonstrated through Pearson correlation coefficient, t-tests, and p-values (< 0.01). A non-metric multidimensional scaling (NMDS) ordination plot, based on the Bray-Curtis matrix, was performed to graphically synthesize the dissimilarity between samples. Process parameters and microbial community data were overlaid onto the NMDS plot using a vector-fitting procedure. Process data and values of major bacterial taxa revealed by 16S rRNA gene high-throughput sequencing (only genera ≥1% of total reads were considered) were normalized by log(X + 1). Statistical analyses were performed by using PAST software (Palaeontological STatistics, ver. 4.04) (Hammer et al., 2001).
3 Results and discussion
3.1 Impact of microbial activity on SCFAs production efficiency
As reported in the previous works of (Tuci et al. 2023, 2024), four semi-continuous reactors (Table 1) were operated at different HRT (4 and 8 days), sludge pretreatment (oxidative and thermal), OLR (6.05 and 12.6 gVS/Ld), and temperature (40 e 50°C). Fermentative performance and stability of the process were monitored by evaluating the production and profiles of SCFAs and the fermentation yield (Y), expressed as the ratio between the produced SCFAs over time and the initial VS concentration. In Ox systems, the Y obtained was higher in Ox_8 with respect to Ox_4 (0.32 ± 0.01 and 0.25 ± 0.01 g CODSCFA/g VS respectively) suggesting that a higher HRT associated with a lower OLR promoted a higher conversion rate of organic matter in SCFAs. This evidence was in line with the FISH/CARD-FISH ratio. As previously reported in Tonanzi et al. (2018), this ratio can be used as “gross parameter” to evaluate the active fraction of microorganisms in engineered biological processes. In fact, FISH analysis reveals physiologically active microorganisms in complex populations through the detection of cells with high ribosome content (>103 ribosome per cell), while CARD-FISH analysis can be used to evaluate the total microbial community including the microbial components with low activity (few ribosomes per cell) (Pernthaler A. et al., 2002; Pernthaler J. et al., 2002). This parameter was here utilized to evaluate the fraction of active bacterial cells out of total members belonging to this domain. The FISH/CARD-FISH ratio estimated for bacteria over the operation is reported in Figure 1. In experiment Ox_4, a FISH/CARD-FISH ratio of 0.8 was observed, while in Ox_8, with a longer HRT, this ratio increased to 0.9 (Figure 1). These findings were statistically correlated with the estimated Y in these processes (r = 0.98, p-value < 0.01), supporting the observed similar trends.
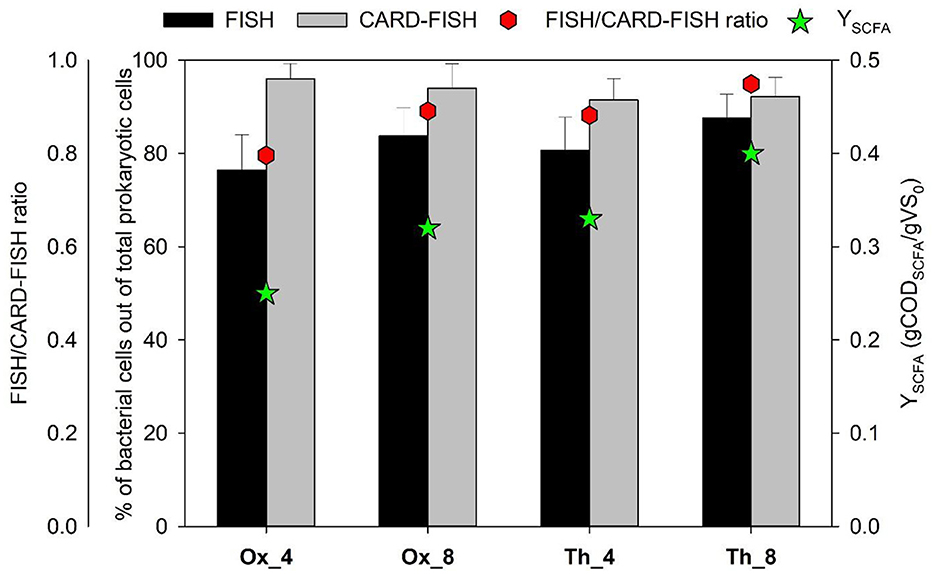
Figure 1. Bacterial abundance estimated by FISH and CARD-FISH analysis, FISH/CARD-FISH ratio and fermentation yield (Y). Values are expressed as: percentage of hybridized cells count out to total counts of DAPI-stained cells; ratio determined by dividing the average bacterial abundance estimated with FISH analysis by that obtained one with CARD-FISH analysis; ratio between the produced SCFAs over time and the initial VS concentration. Error bars indicate standard deviation.
A higher Y was observed in runs performed in Th systems, fed with thermally pretreated tannery sludge and operated under thermophilic conditions, compared to tests Ox_4 and Ox_8 (Figure 1). In particular, when a HRT of 8 days (run Th_8) was imposed, the Y value reached up to 0.4 ± 0.01 g CODSCFA/g VS. In this case as well, the detected FISH/CARD FISH ratio aligned with the trend of the Y, reaching a maximum of 0.95 in the Th_8 test (Figure 1). This high ratio highlighted a bacterial elevated metabolic activity, consistent with the higher yields achieved in this system.
Overall, the integrated evaluation of chemical and microbial metabolic data, in particular those obtained by in situ hybridization techniques, regarding the biological process of anaerobic fermentation of tannery sludge for SCFAs production was applied for the first time in this study. The findings indicated that longer HRTs were correlated with increased biomass activity, leading to improved conversion of organic matter to SCFAs. In addition, thermophilic conditions further enhanced bacterial activity, thereby increasing the resulting yield.
3.2 Bacterial dynamics in Ox and Th systems
The bacterial taxonomic diversity was analyzed via 16S rRNA gene sequencing in samples collected from all experiments both at the beginning of the process and at steady state operation (t0 and tf, respectively). Tannery sludge was used as inoculum in all runs after 1 week of acclimatation. Sequencing analyses showed a massive microbial component attributable to the Firmicutes phylum (96–98% in Ox runs, and 77–81% in Th runs) at the initial time of all trials (t0, Figure 2). In particular, most of the sequences belonged to the Clostridiales order. The almost exclusive presence of these microorganisms can be explained mainly by the nature of the sludge used as inoculum, rich in organic matter and in particular proteins. Several studies have shown that members of the Clostridiales order were able to utilize protein and glucose to produce SCFAs (Zhang et al., 2019; Zhai et al., 2020). Furthermore, the phylum Firmicutes and in particular the class Clostridia, have been often linked to chromium contaminated environments (Branco et al., 2005; Yu et al., 2016). Indeed, the tannery sludge used in this study reported a total chromium (Cr) amount equal to 19.15 ± 0.3 g Cr/kg TS (Tuci et al., 2023, 2024), and this evidence may explain the high presence of this taxon at the beginning of the operations.
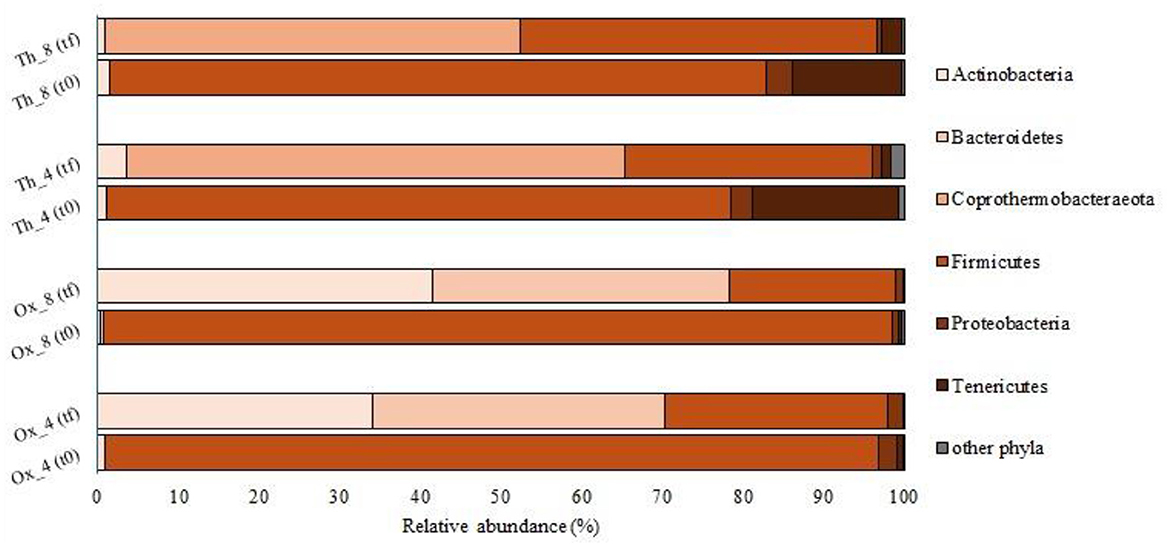
Figure 2. Bacterial community composition (% of total reads) at phylum level at the beginning (t0) and the end (tf) of the tests.
The bacterial composition observed in the runs using H2O2-pretreated sludge at both short (Ox_4) and long (Ox_8) HRTs during the steady state was similar. Almost all sequences belong to Actinobacteria (34% and 42%, for Ox_4 and Ox_8, respectively), Bacteroidetes (36% and 37%), and Firmicutes (28% and 21%), with a small portion (less than 2% in both experiments) attributable to the Proteobacteria phylum (Figure 2).
Sequences affiliated with genus Proteiniphilum (Bacteroidetes phylum) represented 34 and 35% of the total reads in Ox_4 and Ox_8 tests, respectively (Figure 3). Proteiniphilum sp. is well known for its proteolytic metabolism aimed at producing SCFAs such as mainly acetate and propionate (Hahnke et al., 2016). The selective enrichment of this microorganism during the fermentation process was primarily influenced by the substrate composition. Its ability to efficiently degrade recalcitrant COD and rapidly adapt to nitrogen-rich organic matter, like proteins and collagen found in tannery sludge, facilitated its dominance within the microbial community (Zhu et al., 2024). In addition to the previously identified microorganisms, the reactors contained other microorganisms with similar metabolic functions (Dong et al., 2011; Yin et al., 2021). Notably, sequences belonging to genera Proteiniborus, Proteiniclasticum and Clostridium sensu stricto 13 (Firmicutes, Clostridia) comprised between about 3 and 5% of the total reads obtained. The microbial community selected inside the Ox fermenters also highlighted the presence of genus Corynebacterium 1 (Actinobacteria), which covered specifically 22% and 16% of the total reads in Ox_4 and Ox_8, respectively (Figure 3). Species belonging to Corynebacterium are chemoorganotrophs with a fermentative metabolism (Tauch and Sandbote, 2014) and have been extensively studied in tannery sludge in particular in relation to their resistance to Cr (Viti et al., 2003; Baba et al., 2020; Ameen, 2023).
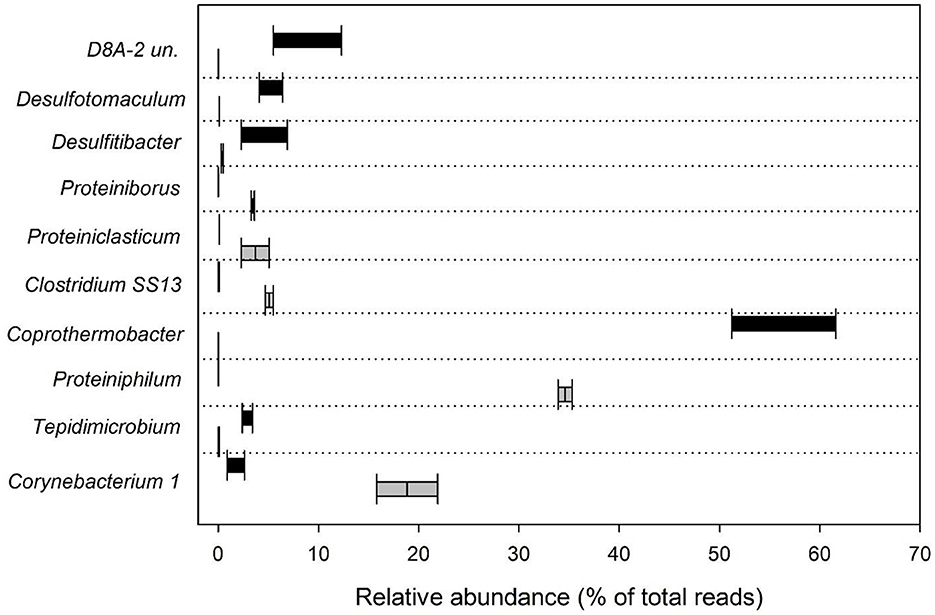
Figure 3. Box plot showing the relative abundance (% of total reads) of the 10 most abundant bacterial genera in Ox and Th runs.
Regarding the experiments conducted under thermophilic conditions with the feed sludge pretreated at high temperature (Th_4 and Th_8), most of the sequences belonged to the phylum Coprothermobacteraeota (62% and 51%, for Th_4 and Th_8, respectively), followed by the phylum Firmicutes (31% and 44%). In these experiments, sequences attributable to Proteobacteria (less than 1%) and to Tenericutes (1% and 3%) were also found (Figure 2). Most of the reads were affiliated with genus Coprothermobacter sp. (61.6% and 51% in Th_4 and 8, respectively). The latter, isolated for the first time from tannery sludge, is a fermentative proteolytic bacterium (Kersters et al., 1994; Gagliano et al., 2015; Pavan et al., 2019). Furthermore, the genus D8A-2 (Firmicutes, Clostridia) was found to comprise between 5.4% and 12.3% of the total sequences in Th_4 and Th_8, respectively (Figure 3). This taxon has been previously reported in thermophilic anaerobic systems characterized by high levels of SCFAs, where it is thought to play a role in syntrophic interactions, especially in the presence of elevated ammonia concentrations (Lee et al., 2019; Li et al., 2022). The prominent presence of Clostridia D8A-2 in these systems can once again be attributed to its ability to metabolize proteins. Indeed, the HRT in Th_8 facilitates the degradation of proteins, leading to a higher production of ammonia compared to Th_4 (Tuci et al., 2024), which creates a more favorable environment for the growth of this microorganism. In addition, the Th reactors contained other fermentative bacteria, such as Desulfitibacter (2.3% and 6.9%, respectively) and Desulfotomaculum (4.1 and 6.4% in Th_4 and 8). Certainly, Desulfotomaculum genus is of particular interest since it is known to employ metals such as Cr as terminal electron acceptors, and is capable of oxidizing H2 while reducing CO2 to acetate (Tebo and Obraztsova, 1998). As reported in previous studies, Desulfitibacter and Desulfotomaculum spp. are associated with the oxidation of sulfur in anaerobic digestion processes (Tebo and Obraztsova, 1998; Nielsen et al., 2006). As a consequence, the presence of this type of metabolic activity in the herein studied systems could also reduce the toxic effects of sulfides and heavy metal (e.g., Cr) on other microorganisms, as previously demonstrated by Zhu et al. (2024). Moreover, Tepidimicrobium genus was retrieved in both Th runs (2.4% and 3.4% of the total reads in Th_4 and 8, respectively). The presence of this microorganism in processes involving the use of tannery sludge is well documented, with studies specifically focusing on microbial analysis in soils contaminated by tannery sludge (Kong et al., 2019). This taxon had a strong adaptability and is usually observed in high organic-containing sewage sludges where it uses both carbohydrates and peptide compounds to produce acetate, ethanol, butyrate, hydrogen and carbon dioxide (Niu et al., 2009; Kong et al., 2019).
Overall, the experimental conditions imposed in this study allowed a clear differentiation between Ox and Th systems both in terms of SCFAs production and bacterial selection, as outlined by NMDS analysis (Figure 4). In particular, Ox systems were characterized by a highest production of acetate and valerate, while Th systems by a highest production of butyrate and propionate and an overall highest Y. This clusterization was reflected also on microbiological dissimilarity among the two systems. In particular, the genera Brevibacterium, Clostridium sensu stricto 13, Proteiniborus, Proteiniphilum and Coriobacteriales unknown (vectors 19, 20, 21, 22, and 23, respectively) were significantly related to Ox runs. Conversely, Tepidimicrobium, Desulfotomaculum, Ruminococcaceae unknown, Iziplasmatales Coprothermobacter and Clostridia D8A-2 (vectors 1, 2, 3, 4, 5, and 6, respectively) were significantly correlated with Th systems (Figure 4).
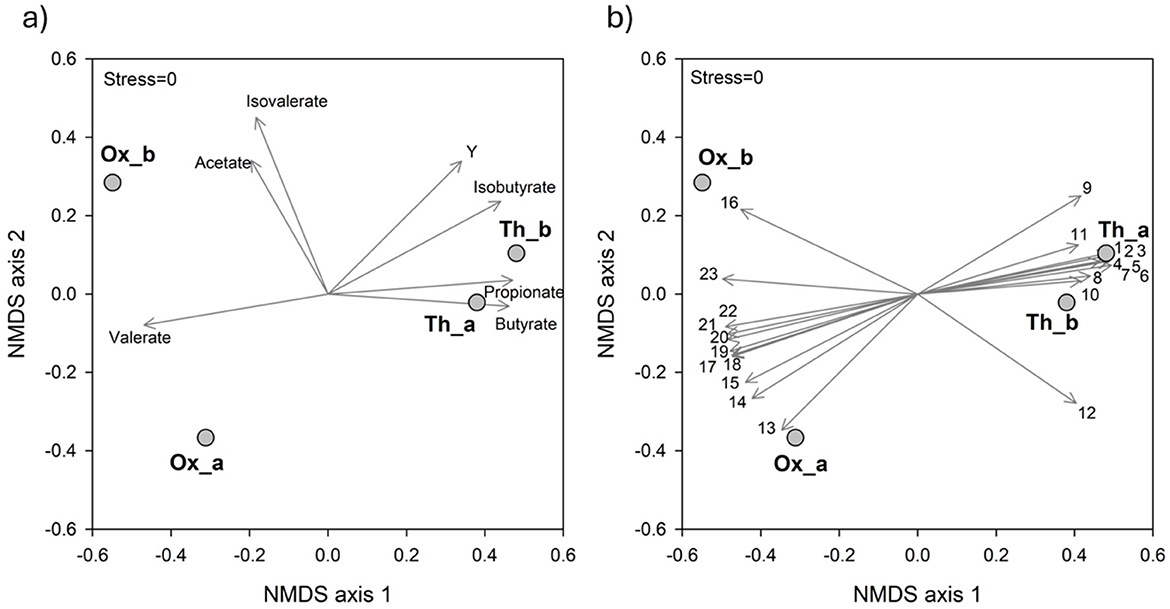
Figure 4. NMDS ordination plots, based on Bray-Curtis distance matrixes of log(X+1) transformed data. The vector length is proportional to the correlation between the NMDS axes and each parameter. The stress value (i.e., <0.2) suggests an accurate representation of the dissimilarity among samples. (a) The variation patterns of process parameters during the operation. (b) The relative abundance of the main genera (≥1% in at least one sample) is projected onto the NMDS ordination synthesizing the microbiological dissimilarity. 1, Tepidimicrobium; 2, Desulfotomaculum; 3, Ruminococcaceae unidentified; 4, Izimaplasmatales unidentified; 5, Coprothermobacter; 6, Clostridia D8A-2 unidentified; 7, Desulfitibacter; 8, Caldicoprobacter; 9, Firmicutes unidentified; 10, Clostridium sensu stricto 5; 11, Haloplasma; 12, Lutispora; 13, Dysgonomonas; 14, Clostridium sensu stricto 7; 15, Proteiniclasticum; 16, Bacillaceae unidentified; 17, Peptostreptococcaceae unidentified; 18, Corynebacterium 1; 19, Brevibacterium; 20, Clostridium sensu stricto 13; 21, Proteiniborus; 22, Proteiniphilum; 23, Coriobacteriales unidentified.
The effect of HRT on SCFAs production has been well documented in literature (Scoma et al., 2012; Jankowska et al., 2018; Aboudi et al., 2023; Chen et al., 2024; Gonçalves et al., 2024; Jimenez-Paez et al., 2025), generally showing that longer HRTs allow microorganisms more time to break down complex substrates, leading to higher SCFAs yields. In line with these evidences, this work highlighted that longer HRT enhanced microbial activity and fermentation yield during tannery sludge hydrolysis, while it did not significantly alter the community composition.
The distinct microbial communities selected in Ox and Th fermenters mainly resulted from the different incubation temperatures acting as the primary stressor. Indeed, temperature can significantly influence microbial growth and activity, affecting enzyme kinetics, membrane fluidity, and cellular stability (Knapp and Huang, 2022). As expected, higher temperature promoted thermophilic or thermotolerant microorganisms, while lower temperature favored mesophilic organisms. These temperature-driven differences can have implications for fermentation, affecting product yields and substrate conversion rates. Nevertheless, while microbial taxa diverged among the different tested systems mainly due to the temperature, the overall functional outcome remained consistent. Interestingly, although different microbial communities were selected under Ox and Th conditions, both systems exhibited the occurrence of various proteolytic microorganisms most likely responsible for the observed SCFAs production, highlighting the occurrence of functional redundancy and suggesting that multiple taxa can fulfill similar metabolic roles.
4 Conclusions
This study marks a significant advance by providing the first in-depth exploration of the microbial community dynamics and metabolic activity responsible for SCFAs production from tannery sludge. Our findings demonstrate that both oxidative and thermal pretreatments of tannery sludge led to high SCFAs production, particularly in systems with a low OLR (6.05 gVS/Ld) and a long HRT (8 d), correlated with the enhancement of microbial metabolic functionalities. Furthermore, process temperature emerged as a critical factor, profoundly influencing the selection of specific microorganisms essential for organic matter conversion to fatty acids. This parameter, coupled with the protein-rich substrate used, strongly drove the microbial community dynamics. In response to the operating conditions tested, a distinct core microbiome was selected among Ox and Th systems, consistently dominated by proteolytic microorganisms such as Proteiniphilum, Proteiniclasticum, and Proteiniborus. The observed presence of taxonomically diverse bacteria exhibiting similar metabolic capabilities strongly suggests a powerful implication: highly performing SCFAs-producing microbial consortia can be strategically selected and enriched to optimize fatty acid production. By exploiting the specific metabolic capabilities of these specialized microorganisms, it is possible to significantly improve the efficiency and yield of SCFAs synthesis processes. This microbiological understanding provides a foundational basis for designing and operating more effective bioreactors for waste valorization.
Data availability statement
The datasets presented in this study are publicly available. This data can be found here: https://www.ncbi.nlm.nih.gov, accession Number PRJNA1178871.
Author contributions
BT: Data curation, Investigation, Validation, Visualization, Writing – original draft. AM: Data curation, Investigation, Writing – review & editing. FV: Resources, Supervision, Writing – review & editing. GT: Data curation, Investigation, Writing – review & editing. MG: Resources, Writing – review & editing. SR: Resources, Supervision, Writing – review & editing. SC: Conceptualization, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4 - Call for Tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union – NextGenerationEU. Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP B83C22002930006, Project title “National Biodiversity Future Center—NBFC” and the European Union—NextGenerationEU, in the framework of the iNEST—Interconnected Nord-Est Innovation Ecosystem (iNEST ECS_00000043 — CUP H43C22000540006). The views and opinions expressed are solely those of the authors and do not necessarily reflect those of the European Union, nor can the European Union be held responsible for them.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
5 Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abate, T. A., Desta, A. F., and Love, N. G. (2021). Evaluating tannery wastewater treatment performance based on physicochemical and microbiological characteristics: an Ethiopian case study. Water Environ. Res. 93, 658–669. doi: 10.1002/wer.1364
Aboudi, K., Greses, S., and Gonzalez-Fernandez, C. (2023). Hydraulic retention time as an operational tool for the production of short-chain carboxylates via anaerobic fermentation of carbohydrate-rich waste. Molecules 28:6635. doi: 10.3390/molecules28186635
Abreu, M. A., and Toffoli, S. M. (2009). Characterization of a chromium-rich tannery waste and its potential use in ceramics. Ceram. Int. 35, 2225–2234. doi: 10.1016/j.ceramint.2008.12.011
Ali, Z., Malik, R. N., Shinwari, Z. K., and Qadir, A. (2015). Enrichment, risk assessment, and statistical apportionment of heavy metals in tannery-affected areas. Int. J. Environ. Sci. Technol. 12, 537–550. doi: 10.1007/s13762-013-0428-4
Alibardi, L., and Cossu, R. (2016). Pre-treatment of tannery sludge for sustainable landfilling. Waste Manag. 52, 202–211. doi: 10.1016/j.wasman.2016.04.008
Ameen, F. (2023). Improving tannery wastewater treatments using an additional microbial treatment with a bacterial – fungal consortium. Biology 12:1507. doi: 10.3390/biology12121507
Angenent, L. T., Richter, H., Buckel, W., Spirito, C. M., Steinbusch, K. J. J., Plugge, C. M., et al. (2016). Chain elongation with reactor microbiomes: open-culture biotechnology to produce biochemicals. Environ. Sci. Technol. 50, 2796–2810. doi: 10.1021/acs.est.5b04847
Appels, L., Degrève, J., Van Der Bruggen, B., Van Impe, J., and Dewil, R. (2010). Influence of low temperature thermal pre-treatment on sludge solubilisation, heavy metal release and anaerobic digestion. Bioresour. Technol. 101, 5743–5748. doi: 10.1016/j.biortech.2010.02.068
Ariunbaatar, J., Panico, A., Esposito, G., Pirozzi, F., and Lens, P. N. L. (2014). Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl. Energy 123, 143–156. doi: 10.1016/j.apenergy.2014.02.035
Ashraf, S., Naveed, M., Afzal, M., Ashraf, S., Rehman, K., Hussain, A., et al. (2018). Bioremediation of tannery effluent by Cr- and salt-tolerant bacterial strains. Environ. Monit. Assess. 190:716. doi: 10.1007/s10661-018-7098-0
Baba, A., Garba, T. S., and Bello, H. S. (2020). Bioremediation potential of immobilized Corynebacterium kutsceri in the treatment of tannery industry effluent from Challawa Industrial, Kano State, Nigeria. J. Turk. Chem. Soc. Chem. 7, 335–350. doi: 10.18596/jotcsa.643771
Bai, Y., Huo, Y., Liao, K., and Qu, J. (2017). Influence of microbial community diversity and function on pollutant removal in ecological wastewater treatment. Appl. Microbiol. Biotechnol. 101, 7293–7302. doi: 10.1007/s00253-017-8464-5
Braguglia, C. M., Gianico, A., and Mininni, G. (2012). Comparison between ozone and ultrasound disintegration on sludge anaerobic digestion. J. Environ. Manage. 95, S139–S143. doi: 10.1016/j.jenvman.2010.07.030
Branco, R., Chung, A.-P., Verissimo, A., and Morais, P. V. (2005). Impact of chromium-contaminated wastewaters on the microbial community of a river. FEMS Microbiol. Ecol. 54, 35–46. doi: 10.1016/j.femsec.2005.02.014
Callahan, B. J., Mcmurdie, P. J., Rosen, M. J., Han, A. W., Jonson, A. J. A., and Holmes, S. P. (2016). DADA2: high resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. doi: 10.1038/nmeth.3869
Chang, Z., Long, G., Zhou, J. L., and Ma, C. (2020). Valorization of sewage sludge in the fabrication of construction and building materials: a review. Resour. Conserv. Recycl. 154:104606. doi: 10.1016/j.resconrec.2019.104606
Chen, R., Ji, X., Chen, Z., Huang, L., and Zhu, J. (2024). Regulation of hydraulic retention time on caproic acid production via two-phase anaerobic fermentation of Chinese cabbage waste with autopoietic electron donors. J. Biotechnol. 381, 1–10. doi: 10.1016/j.jbiotec.2023.12.012
Chojnacka, K., Skrzypczak, D., Mikula, K., Witek-krowiak, A., Izydorczyk, G., Kuligowsky, K., et al. (2021). Progress in sustainable technologies of leather wastes valorization as solutions for the circular economy. J. Clean. Prod. 313:127902. doi: 10.1016/j.jclepro.2021.127902
Crognale, S., Casentini, B., Amalfitano, S., Fazi, S., Petruccioli, M., and Rossetti, S. (2019). Biological As(III) oxidation in biofilters by using native groundwater microorganisms. Sci. Total Environ. 651, 93–102. doi: 10.1016/j.scitotenv.2018.09.176
Dong, Y., Butler, E. C., Philp, R. P., and Krumholz, L. R. (2011). Impacts of microbial community composition on isotope fractionation during reductive dechlorination of tetrachloroethylene. Biodegradation 22, 431–444. doi: 10.1007/s10532-010-9416-2
Gagliano, M. C., Braguglia, C. M., Gianico, A., Mininni, G., Nakamura, K., and Rossetti, S. (2015). Thermophilic anaerobic digestion of thermal pretreated sludge: role of microbial community structure and correlation with process performances. Water Res. 68, 498–509. doi: 10.1016/j.watres.2014.10.031
Gallipoli, A., Gianico, A., Gagliano, M. C., and Braguglia, C. M. (2014). Potential of high-frequency ultrasounds to improve sludge anaerobic conversion and surfactants removal at different food/inoculum ratio. Bioresour. Technol. 159, 207–214. doi: 10.1016/j.biortech.2014.02.084
Gherghel, A., Teodosiu, C., and De Gisi, S. (2019). A review on wastewater sludge valorisation and its challenges in the context of circular economy. J. Clean. Prod. 228, 244–263. doi: 10.1016/j.jclepro.2019.04.240
Gianico, A., Braguglia, C. M., Cesarini, R., and Mininni, G. (2013). Reduced temperature hydrolysis at 134°C before thermophilic anaerobic digestion of waste activated sludge at increasing organic load. Bioresour. Technol. 143, 96–103. doi: 10.1016/j.biortech.2013.05.069
Gianico, A., Braguglia, C. M., Gallipoli, A., and Mininni, G. (2015). Innovative two-stage mesophilic/thermophilic anaerobic degradation of sonicated sludge: performances and energy balance. Environ. Sci. Pollut. Res. 22, 7248–7256. doi: 10.1007/s11356-014-3123-1
Gonçalves, M. J., Gonzalez-Fernandez, C., and Greses, S. (2024). Long hydraulic retention time mediates stable volatile fatty acids production against slight pH oscillations. Waste Manag. 176, 140–148. doi: 10.1016/j.wasman.2024.01.012
Gottardo, M., Crognale, S., Tonanzi, B., Rossetti, S., Annibale, L. D., Dosta, J., et al. (2022). Volatile fatty acid production from hydrolyzed sewage sludge: effect of hydraulic retention time and insight into thermophilic microbial community. Biomass Convers. Biorefin. 14, 1–12. doi: 10.1007/s13399-022-03659-8
Hahnke, S., Langer, T., Koeck, D. E., and Klocke, M. (2016). Description of Proteiniphilum saccharofermentans sp. nov., Petrimonas mucosa sp. nov. and isolated from mesophilic laboratory-scale biogas reactors, and emended description of the genus Proteiniphilum. Int. J. Syst. Evol. Microbiol. 66, 1466–1475. doi: 10.1099/ijsem.0.000902
Hammer, Ø., Harper, D. A. T., and Ryan, P. D. (2001). PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron. 4, 1–9.
Han, W., He, P., Shao, L., and Lü, F. (2019). Road to full bioconversion of biowaste to biochemicals centering on chain elongation: a mini review. J. Environ. Sci. 86, 50–64. doi: 10.1016/j.jes.2019.05.018
Jaffari, Z. H., Hong, J., and Park, K. Y. (2024). A systematic review of innovations in tannery solid waste treatment: a viable solution for the circular economy. Sci. Total Environ. 948:174848. doi: 10.1016/j.scitotenv.2024.174848
Jankowska, E., Duber, A., Chwialkowska, J., Stodolny, M., and Oleskowicz-popiel, P. (2018). Conversion of organic waste into volatile fatty acids – the in fl uence of process operating parameters. Chem. Eng. J. 345, 395–403. doi: 10.1016/j.cej.2018.03.180
Jeong, S. Y., Chang, S. W., Ngo, H. H., Guo, W., Nghiem, L. D., Banu, J. R., et al. (2019). Influence of thermal hydrolysis pretreatment on physicochemical properties and anaerobic biodegradability of waste activated sludge with different solids content. Waste Manag. 85, 214–221. doi: 10.1016/j.wasman.2018.12.026
Jimenez-Paez, E., Serrano, A., Purswani, J., Trujillo-Reyes, A., Fernandez-Prior, A., and Fermoso, F. G. (2025). Impact of hydraulic retention time on the production of volatile fatty acids from lignocellulosic feedstock by acidogenic fermentation. Process Saf. Environ. Prot. 200:107405. doi: 10.1016/j.psep.2025.107405
Kersters, I., Maestrojuan, G. M., Torck, U., Vancanneyt, M., Kersters, K., and Verstraete, W. (1994). Isolation of Coprothermobacter proteolyticus from an anaerobic digest and further characterization of the species. Syst. Appl. Microbiol. 295, 289–295. doi: 10.1016/S0723-2020(11)80021-4
Khadaroo, S. N. B. A., Eong, P., Gouwanda, D., and Grassia, P. (2019). Applicability of various pretreatment techniques to enhance the anaerobic digestion of Palm oil Mill e ffl uent (POME): a review. J. Environ. Chem. Eng. 7:103310. doi: 10.1016/j.jece.2019.103310
Knapp, B. D., and Huang, K. C. (2022). The effects of temperature on cellular physiology. Annu. Rev. Biophys. 51, 499–526. doi: 10.1146/annurev-biophys-112221-074832
Kong, X., Li, C., Wang, P., Huang, G., Li, Z., and Han, Z. (2019). Soil pollution characteristics and microbial responses in a vertical profile with long-term tannery sludge contamination in Hebei, China. Int. J. Environ. Res. Public Health 16:563. doi: 10.3390/ijerph16040563
Lee, J., Koo, T., Yulisa, A., and Hwang, S. (2019). Magnetite as an enhancer in methanogenic degradation of volatile fatty acids under ammonia-stressed condition. J. Environ. Manage. 241, 418–426. doi: 10.1016/j.jenvman.2019.04.038
Li, C., Hao, L., Lu, F., Duan, H., Zhang, H., and He, P. (2022). Syntrophic acetate-oxidizing microbial consortia enriched from full-scale mesophilic food waste anaerobic digesters showing high biodiversity and functional redundancy. mSystems 7, 1–17. doi: 10.1128/msystems.00339-22
Li, X., Liu, H., Zhang, Z., Zhou, T., and Wang, Q. (2023). Sulfite pretreatment enhances the medium-chain fatty acids production from waste activated sludge anaerobic fermentation. Sci. Total Environ. 871:162080. doi: 10.1016/j.scitotenv.2023.162080
Matturro, B., Tandoi, V., and Rossetti, S. (2013). Different activity levels of Dehalococcoides mccartyi revealed by FISH and CARD-FISH under non-steady and pseudo-steady state conditions. N. Biotechnol. 30, 756–762. doi: 10.1016/j.nbt.2013.07.003
Moktadir, M. A., Ren, J., and Zhou, J. (2023). A systematic review on tannery sludge to energy route: current practices, impacts, strategies, and future directions. Sci. Total Environ. 901:166244. doi: 10.1016/j.scitotenv.2023.166244
Neyens, E., Baeyens, J., Weemaes, M., and De heyder, B. (2003). Hot acid hydrolysis as a potential treatment of thickened sewage sludge. J. Hazard. Mater. 98, 275–293. doi: 10.1016/S0304-3894(03)00002-5
Nielsen, M. B., Kjeldsen, K. U., and Ingvorsen, K. (2006). Desulfitibacter alkalitolerans gen. nov., sp. nov., anaerobic, alkalitolerant, sulfite-reducing bacterium isolated from a district heating plant. Int. J. Syst. Evol. Microbiol. 56, 2831–2836. doi: 10.1099/ijs.0.64356-0
Nielsen, P. H., Kragelund, C., Seviour, R. J., and Nielsen, J. L. (2009). Identity and ecophysiology of filamentous bacteria in activated sludge. FEMS Microbiol. Rev. 33, 969–998. doi: 10.1111/j.1574-6976.2009.00186.x
Niu, L., Song, L., Liu, X., and Dong, X. (2009). Tepidimicrobium xylanilyticum sp. nov., an anaerobic xylanolytic bacterium, and emended description of the genus Tepidimicrobium. Int. J. Syst. Evol. Microbiol. 2, 2698–2701. doi: 10.1099/ijs.0.005124-0
Pavan, M. E., Pavan, E. E., Kämpfer, P., Pettinari, M. J., and López, N. I. (2019). “Coprothermobacterota,” in Bergey's Manual of Systematics of Archaea and Bacteria (John Wiley & Sons, Inc.).
Pernthaler, A., Pernthaler, J., and Amann, R. (2002). Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Appl. Environ. Microbiol. 68, 3094–3101. doi: 10.1128/AEM.68.6.3094-3101.2002
Pernthaler, J., Glöckner, F. O., Schönhuber, W., and Amann, R. (2002). Fluorescence in situ hybridization with rRNA-targeted oligonucleotide probes. Method. Microbiol. 30, 1–31. doi: 10.1016/S0580-9517(01)30046-6
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Glöckner, F. O., et al. (2013). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, 590–596. doi: 10.1093/nar/gks1219
Reyes-Romero, B., Gutierrez-Lopez, A., Hernandez-Altamirano, R., Mena-Cervantes, V. Y., Ruiz-Baca, E., Neri-Torres, E. E., et al. (2021). Removal of concentrated Cr(III) from real tannery wastewater using abiotic and anaerobic processes with native microbial consortia. J. Environ. Chem. Eng. 9:104626. doi: 10.1016/j.jece.2020.104626
Saxena, G., Purchase, D., Mulla, S. I., and Bharagava, R. N. (2020). Degradation and detoxification of leather tannery effluent by a newly developed bacterial consortium GS-TE1310 for environmental safety. J. Water Process Eng. 38:101592. doi: 10.1016/j.jwpe.2020.101592
Scoma, A., Bertin, L., and Fava, F. (2012). Effect of hydraulic retention time on biohydrogen and volatile fatty acids production during acidogenic digestion of dephenolized olive mill wastewaters. Biomass Bioenergy 48, 51–58. doi: 10.1016/j.biombioe.2012.10.028
Tauch, A., and Sandbote, J. (2014). “The family Corynebacteriaceae,” in The Prokaryotes, eds. E. Rosenberg, E. F. DeLong, S. Lory, E. Stackebrandt, and F. Thompson (Berlin, Heidelberg: Springer).
Tebo, B. M., and Obraztsova, A. Y. (1998). Sulfate-reducing bacterium grows with Cr(VI), U(VI), Mn(IV), and Fe(III) as electron acceptors. FEMS Microbiol. Lett. 162, 193–198. doi: 10.1111/j.1574-6968.1998.tb12998.x
Tonanzi, B., Gallipoli, A., Cristina, M., La, C., Gianico, A., and Maria, C. (2021). Pre-treatments and anaerobic hydrolysis as strategical key steps for resource recovery from sludge: the role of disintegration degree in metals leaching. J. Environ. Chem. Eng. 9:104649. doi: 10.1016/j.jece.2020.104649
Tonanzi, B., Gallipoli, A., Gianico, A., Montecchio, D., Pagliaccia, P., Di Carlo, M., et al. (2018). Long-term anaerobic digestion of food waste at semi-pilot scale: relationship between microbial community structure and process performances. Biomass Bioenergy 118, 55–64. doi: 10.1016/j.biombioe.2018.08.001
Tuci, G. A., Valentino, F., Parmar, A. C., Pavan, P., and Gottardo, M. (2023). Minimizing tannery sludge in landfilling through a mixed microbial culture approach: effect of oxidizing pretreatment, temperature and hydraulic retention time on process performances and chromium fate. Biochem. Eng. J. 200:109073. doi: 10.1016/j.bej.2023.109073
Tuci, G. A., Valentino, F., Pavan, P., and Gottardo, M. (2024). Tannery sludge valorization through zeolite-assisted anaerobic process for short-chain fatty acids (SCFAs) production. Environ. Res. 246:118046. doi: 10.1016/j.envres.2023.118046
Viti, C., Pace, A., and Giovannetti, L. (2003). Characterization of Cr(VI)-resistant bacteria isolated from chromium- contaminated soil by tannery activity. Curr. Microbiol. 46, 1–5. doi: 10.1007/s00284-002-3800-z
Wang, J., and Yin, Y. (2022). Biological production of medium-chain carboxylates through chain elongation: an overview. Biotechnol. Adv. 55:107882. doi: 10.1016/j.biotechadv.2021.107882
Wu, L., Ning, D., Zhang, B., Li, Y., Zhang, P., Shan, X., et al. (2019). Global diversity and biogeography of bacterial communities in wastewater treatment plants. Nat. Microbiol. 4, 1183–1195. doi: 10.1038/s41564-019-0426-5
Yin, Y., Chen, Y., and Wang, J. (2021). Co-fermentation of sewage sludge and algae and Fe2+ addition for enhancing hydrogen production. Int. J. Hydrogen Energy 46, 8950–8960. doi: 10.1016/j.ijhydene.2021.01.009
Yu, Z., He, Z., Tao, X., Zhou, J., Yang, Y., Zhao, M., et al. (2016). The shifts of sediment microbial community phylogenetic and functional structures during chromium (VI) reduction. Ecotoxicology 25, 1759–1770. doi: 10.1007/s10646-016-1719-6
Zhai, S., Li, M., Xiong, Y., Wang, D., and Fu, S. (2020). Dual resource utilization for tannery sludge: effects of sludge biochars (BCs) on volatile fatty acids (VFAs) production from sludge anaerobic digestion. Bioresour. Technol. 316:123903. doi: 10.1016/j.biortech.2020.123903
Zhang, R., Chen, L., Niu, Z., Song, S., and Zhao, Y. (2019). Water stress affects the frequency of Firmicutes, Clostridiales and Lysobacter in rhizosphere soils of greenhouse grape. Agric. Water Manag. 226:105776. doi: 10.1016/j.agwat.2019.105776
Keywords: microbial community analysis, anaerobic sludge fermentation, short-chain fatty acids, tannery sludge, microbiome
Citation: Tonanzi B, Massimi A, Valentino F, Tuci GA, Gottardo M, Rossetti S and Crognale S (2025) Microbial-driven valorization of tannery sludge for fatty acids production: unraveling microbiome activity and functional redundancy. Front. Microbiol. 16:1675618. doi: 10.3389/fmicb.2025.1675618
Received: 31 July 2025; Accepted: 18 September 2025;
Published: 06 October 2025.
Edited by:
Zhiqiang Zuo, University of New South Wales, AustraliaCopyright © 2025 Tonanzi, Massimi, Valentino, Tuci, Gottardo, Rossetti and Crognale. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simona Crognale, c2ltb25hLmNyb2duYWxlQGlyc2EuY25yLml0
 Barbara Tonanzi
Barbara Tonanzi Alessio Massimi
Alessio Massimi Francesco Valentino
Francesco Valentino Giulia Adele Tuci4
Giulia Adele Tuci4 Marco Gottardo
Marco Gottardo Simona Rossetti
Simona Rossetti Simona Crognale
Simona Crognale