- 1Department of Critical Care Medicine, Affiliated Hospital of Zunyi Medical University, Zunyi, China
- 2Department of Pharmacy, Affiliated Hospital of Zunyi Medical University, Zunyi, China
- 3Department of Respiratory and Critical Care Medicine, Kweichow Moutai Hospital, Zunyi, China
- 4Department of Nephrology, Kweichow Moutai Hospital, Zunyi, China
- 5Guizhou Provincial Key Laboratory of Medicinal Biotechnology in Colleges and Universities, Affiliated Hospital of Zunyi Medical University, Zunyi, China
The mammalian colon lumen exists in a highly anaerobic environment (oxygen partial pressure (PO2) < 1 mmHg), which promotes the growth of beneficial obligate anaerobes (OA) while limiting the expansion of pathogenic facultative anaerobes (FA). Gut dysbiosis is associated with a wide range of human diseases, and is often characterized by an overgrowth of FA, particularly those in the Enterobacteriaceae family. Oxygen (O2) plays a crucial role in bacterial physiology and ecology, and increased O2 availability is a key driver of gut dysbiosis. O2 therapy is commonly used for hypoxic patients, either through inhalation or extracorporeal membrane oxygenation (ECMO), both of which can expose the gut to excess O2, known as hyperoxia. Hyperoxia leads to the overproduction of reactive O2 species, resulting in organ injury and worsening clinical outcomes. Viewing gut dysbiosis from an ecological perspective highlights the disruption of host mechanisms that regulate the gut microbiota, particularly in the context of antibiotic use and a western (low fiber) diet, where physiological hypoxia in the colonic epithelium is compromised. This review extends that perspective to O2 therapy in acute care, discussing the rationale and experimental evidence linking hyperoxia to gut dysbiosis, with a focus on venoarterial (VA)-ECMO support as a potential contributor. Understanding these mechanisms could help clinicians optimize O2 management during therapy.
Introduction
Oxygen (O2) therapy has become one of the most widely prescribed treatments globally since its initial documentation in 1890 (Matthay, 2015; Siemieniuk et al., 2018). However, its toxicity—primarily resulting from the excessive production of reactive oxygen species (ROS)—has always been a major concern. When the inhaled oxygen concentration (FiO₂) exceeds 0.21, it can lead to hyperoxemia (defined as an arterial partial pressure of oxygen PaO₂ > 100 mmHg), causing an excess of oxygen in the blood. This condition triggers hyperoxia, characterized by abnormally elevated tissue oxygen levels, which in turn exacerbates ROS production. Therefore, hyperoxemia is an abnormal indicator in arterial blood, while hyperoxia is its subsequent manifestation at the tissue level, with the latter directly linked to oxidative damage (Singer et al., 2021). Given the potential risks of hyperoxia, the judicious use of oxygen in clinical practice has become a critical issue. In the acute hospital setting, particularly in emergency, respiratory critical care, cardiac, and anesthesiology departments, oxygen therapy is a cornerstone of nursing care (Gelissen et al., 2021; Schjørring et al., 2021). While O2 supplementation is lifesaving for patients experiencing respiratory and/or circulatory failure, super-physiological levels of O2-referred to as hyperoxia- can lead to the overproduction of reactive O2 species (ROS), causing harmful effects both systemically and locally and poor clinical outcomes (Damiani et al., 2018; Nolfi-Donegan et al., 2020). O2 can be delivered to patients by a variety of techniques, ranging from the simple O2 therapy (inhalation) to complex extracorporeal membrane oxygenation (ECMO) (Gu et al., 2017). Traditionally, hyperoxic toxicity during O2 inhalation (e.g., via mechanical ventilators), has been a concern particularly to the lung (Hochberg et al., 2021; Singer et al., 2021). However, the increasing use of venoarterial (VA) -ECMO for patients with severe cardiac or cardiopulmonary failure challenges this view (Winiszewski et al., 2022; Dai et al., 2024). VA-ECMO introduces lower-body hyperoxia during dual circulation, which differs from the typical alveolar hyperoxia seen with O2 inhalation and can also have significant effects on the gastrointestinal tract (Winiszewski et al., 2018; Asija et al., 2023; Winiszewski et al., 2024).
The human gut is home to trillions of microbes, the majority of which reside in the colon, an environment characterized by extremely low O2 levels (with a partial pressure of O2 (PO2) less than 1 mmHg) (Human Microbiome Project Consortium, 2012; Albenberg et al., 2014). The colonic epithelium normally exists in a state of physiological hypoxia that is crucial for maintaining gut homeostasis by supporting the anaerobic conditions (anaerobiosis) in the lumen (Cummins and Crean, 2017; Wang et al., 2024). The predominant microbes in the colon are OA that produce beneficial short fatty chain acid (SCFA), primarily belonging to the Clostridia (Phylum Firmicutes) and Bacteroidia (Phylum Bacteroidetes) classes, which together make up over 90% of the microbial population (Rivera-Chávez et al., 2017). The limited availability of external respiratory electron acceptors, such as O2, restricts the growth of FA, including those from the Bacilli class (Phylum Firmicutes) and the Enterobacteriaceae family (Phylum Proteobacteria), which represent the leading sources of pathogenic microorganisms in clinical practices and only a small faction (<5%) of the overall microbial community in a healthy gut (Eckburg et al., 2005). Dysbiosis, or an imbalance in microbial community, typically refers to an overgrowth of potentially harmful FA (biomarker: Enterobacteriaceae) or a reduction in beneficial OA (Chanderraj et al., 2023). This imbalance has been linked to various chronic and acute diseases in humans and is associated with poorer clinical outcomes (Dickson, 2016; Lynch and Pedersen, 2016; Winter and Bäumler, 2023). Over the past decade, an ecological perspective has highlighted O2 as a critical factor driving gut dysbiosis, particularly in the context of the interactions between gut microbes and the host (Rivera-Chávez et al., 2017; Litvak and Bäumler, 2019; Lee et al., 2022). This review synthesizes current evidence, revisits established concepts, and discusses how hyperoxia, especially from VA-ECMO support, contributes to gut dysbiosis by disrupting the “OA-fiber-butyrate-colonocyte metabolism (oxidative phosphorylation) -epithelial physiological hypoxia-luminal anaerobiosis” axis.
O2 and microbial physiology, ecology and evolution
O2, a diatomic molecule, contains two unpaired electrons in its atomic structure, giving it high reactivity potential (Hara and Kondo, 2015). Owing to this high reactivity, O2 is pivotal in bacterial physiology, ecology and evolution by driving energy metabolism and supporting the synthesis of essential polymers for cell growth and population expansion (Fenchel and Finlay, 2008; Shan et al., 2012).
Earth’s early atmosphere and ocean were extremely anoxic, with O2 levels less than 10−5 of their current concentration. Aerotolerant bacteria are thought to have emerged approximately 3.8 Giga-annum (Ga) ago (Kasting and Siefert, 2002; Lyons et al., 2014). The accumulation of O2 began after the advent of oxygenic photosynthesis in Cyanobacteria around 2.3 Ga (Payne et al., 2011; Fischer and Valentine, 2019). This process led to a significant increase in atmospheric O2 levels, known as the Great Oxygenation Event (GOE), which paved the way for the evolution of complex organisms, including animals, that relied on O2 as a high-potential electron acceptor for producing adenosine triphosphate (ATP) (Olejarz et al., 2021). ATP, known as leading energy currency in cellular processes, drives metabolic activities, protein synthesis and microbial bioproduction (Hara and Kondo, 2015; Nolfi-Donegan et al., 2020). Under selective pressure, the anaerobic respiratory chains of certain bacteria such as FA, adapted to use O2 as a new terminal electron acceptor. Meanwhile, FA rely on a complex antioxidant enzyme network composed of superoxide dismutase (SOD), catalase, peroxidase, and the thioredoxin system. They have evolved crucial antioxidant enzymes to counteract the toxicity of oxygen, enabling successful colonization across ecological niches with varying oxygen conditions (Raymond and Segrè, 2006; Johnson and Hug, 2019; Ślesak et al., 2019). A different group of microorganisms, such as OA, continued their anaerobic metabolic processes and adapted to O2-free habitats, including hot springs, lake sediments and the human gut (Lu and Imlay, 2021). These findings highlight the intertwined evolution of microbial life and earth’s early environments as the planet transitioned from an O2-deficient to an O2-rich atmosphere (Lyons et al., 2024) (Figure 1A).
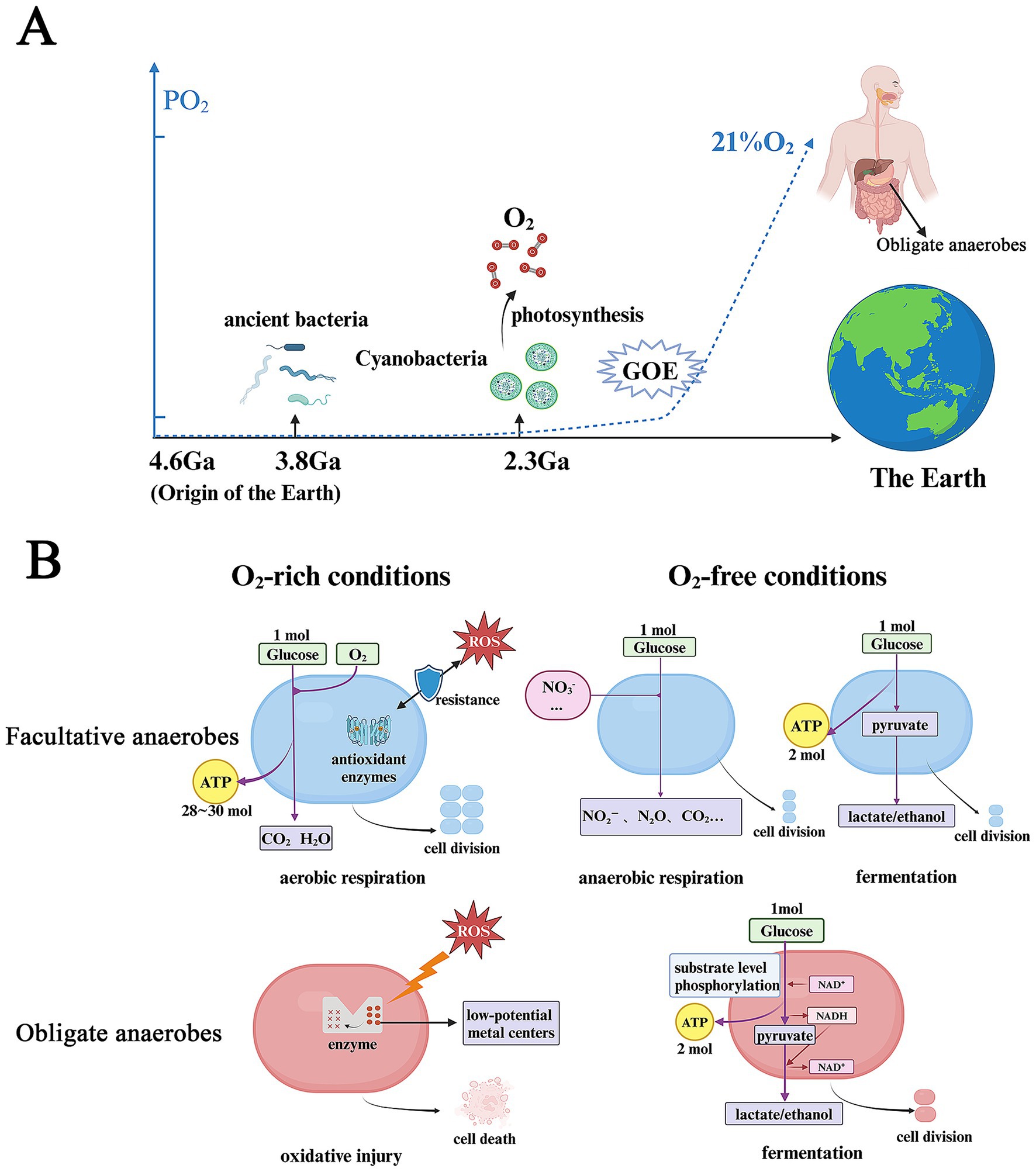
Figure 1. O2 plays a central role in co-evolution of the Earth and microbes and microbial metabolic reprogramming. (A) O2 drives the co-evolution of the Earth and microbes. (B) Bacterial metabolic reprogramming in O2-free and -rich conditions. PO2, partial pressure of oxygen; Ga, giga-annum; GOE, great oxidation event; ROS, reactive oxygen species. Figure created with BioRender.com.
Under O2-rich conditions, O2 inhibits the growth of OA that lack the mechanisms to defend against ROS. This vulnerability arises because anaerobic metabolism relies on catalytic sites with low-potential metal centers, which are highly susceptible to damage from ROS (Lu and Imlay, 2021). In contrast, FA utilize O2 as an external terminal electron acceptor during aerobic respiration (oxidative phosphorylation). This process produces approximately 28–30 molecules of ATP per molecule of glucose, supporting a significant increase in biomass production. Under anoxic conditions, OA primarily rely on fermentation, a process in which nicotinamide adenine dinucleotide (NADH) is oxidized by endogenous intermediates (electron acceptors) produced from the decomposition of carbon sources. ATP is generated solely through substrate- level phosphorylation, a process known as glycolysis (2 ATP molecules per glucose molecule metabolized into lactate) (Koropatkin et al., 2012; Shan et al., 2012). In response to O2 scarcity, FA undergo substantial metabolic reprogramming, shifting from the aerobic respiration to anaerobic respiration using alternative exogenous electron acceptors (such as nitrate) or fermentation (Bueno et al., 2012; Shan et al., 2012) (Figure 1B).
Thus, microbes using the redox reaction yielding the greatest ATP production prevail, during which availability to exogenous electron acceptors (especially O2) determines which metabolic groups of bacteria can dominate the microbial communities in an ecological nutrient-niche (Lee et al., 2022).
Coordinated control of multiple factors in the GI microenvironment
The composition and function of the gut microbiota are influenced by oxygen. However, this principle does not fully capture the reality within the specific microenvironment of the human gastrointestinal tract. The gut microbiota is also co-regulated by a variety of biochemical and physiological factors, including pH, antimicrobial peptide (AMP) distribution, bile acids, and intestinal transit time.
When it comes to pH, numerous studies in murine models have demonstrated that the pH gradient along the gastrointestinal tract—from the highly acidic environment of the stomach to the near-neutral conditions in the colon—significantly regulates the structure and function of the gut microbiota. For example, in these models, a lower pH environment (e.g., pH 5.5) generally favors the growth of certain members of the Firmicutes phylum and Bifidobacterium, and promotes butyrate production. In contrast, under higher pH conditions (e.g., pH 6.5–7.0), Bacteroidetes members tend to be more active. pH not only directly influences microbial growth and enzyme activity but also indirectly affects intestinal environmental stability and microbial community structure by modulating the production and absorption of metabolites such as short-chain fatty acids (SCFAs) (Yamamura et al., 2023; Xie et al., 2024). Secondly, bile acids are important molecules synthesized by the liver and metabolized by gut microorganisms, playing a central regulatory role in the intestinal microbiota. Research using mouse models has shown that they disrupt microbial membrane structures through surfactant activity and exert selective antibacterial effects by inhibiting the growth of certain bacteria while promoting the colonization of tolerant species. Meanwhile, microorganisms modify bile acids via enzymes such as bile salt hydrolases, altering their signaling activity and toxicity, thereby modulating the structure of the microbiota and the host’s immune responses (Guzior and Quinn, 2021; Kim et al., 2024). Meanwhile, studies in fish models have demonstrated that dietary AMP supplementation significantly reshaped the gut microbiota structure. Antimicrobial peptides (AMPs) exhibit a dose-dependent “double-edged sword” effect. At appropriate doses, they optimize microbial composition (e.g., promoting Firmicutes while suppressing Bacteroidetes), improve nutrient metabolism, and maintain beneficial microbial stability under pathogenic stress, thereby enhancing ecological resilience. However, excessive supplementation may increase the proportion of Proteobacteria, consequently disrupting microbial homeostasis (Liu et al., 2022). However, in addition to the aforementioned chemical factors, gut transit time—as a core physio-mechanical variable—exhibits a tight bidirectional interaction with the microbiome. Human studies have indicated that it not only serves as a key driver of microbial composition and metabolism (such as the balance between glycolysis and proteolysis), but is itself modulated by microbial metabolites (Procházková et al., 2023).
Therefore, in addition to oxygen, factors such as intestinal pH, the antimicrobial peptide (AMP) gradient, the presence of bile acids, and transit rate are also crucial for the microbiome.
Gastrointestinal O2 gradient shapes microbial composition
At sea level, the PO2 in breathable air is approximately 150 mmHg (21% O2), which leads to a PO2 of 100–110 mmHg (15% O2) within healthy human alveoli, an arterial PO2 (PaO2) of 90–100 mmHg (12% O2) and a PO2 of 60–70 mmHg (8% O2) in the human liver (Schaible et al., 2010; Singhal and Shah, 2020). In contrast, studies in mice show that the intestinal mucosa exists in a relatively low-PO2 environment, showing a steep O2 gradient both along the length of the intestine (longitudinal axis) and from the inner lumen to the outer serosa (radial axis) (Zheng et al., 2015). In the murine gut, O2 levels in the gut lumen remarkably decline as intestinal contents move from the upper to the lower digestive tracts (Friedman et al., 2018). In the stomach and duodenum, O2 levels in tissues and the lumen are similar. However, starting in the ileum, these levels diverge significantly. The luminal O2 concentration drops sharply, reaching its lowest point in the colon (<1 mmHg, 0.2% O2) (Singhal and Shah, 2020). The murine host maintains the colonic epithelium in a physiological hypoxic state (<10 mmHg, 2% O2) due to mitochondrial O2 consumption through oxidative phosphorylation, which is coupled with oxidation of fatty acids (Litvak et al., 2018; Zhang et al., 2022). This mechanism restricts the diffusion of O2 into the intestinal lumen, creating anaerobosis (Pral et al., 2021). Pure O2 inhalation in mouse models results in hyperoxia, leading to a rise in luminal PO2. This increase confirms that O2 diffuses from intestinal tissues and forms a radial gradient extending from the mucosal tissue interface into the lumen (Albenberg et al., 2014). Both the longitudinal and radial O2 gradients within the gut play a critical role in determining the composition of the gut microbiota (Donaldson et al., 2016; Miller et al., 2021) (Figure 2).
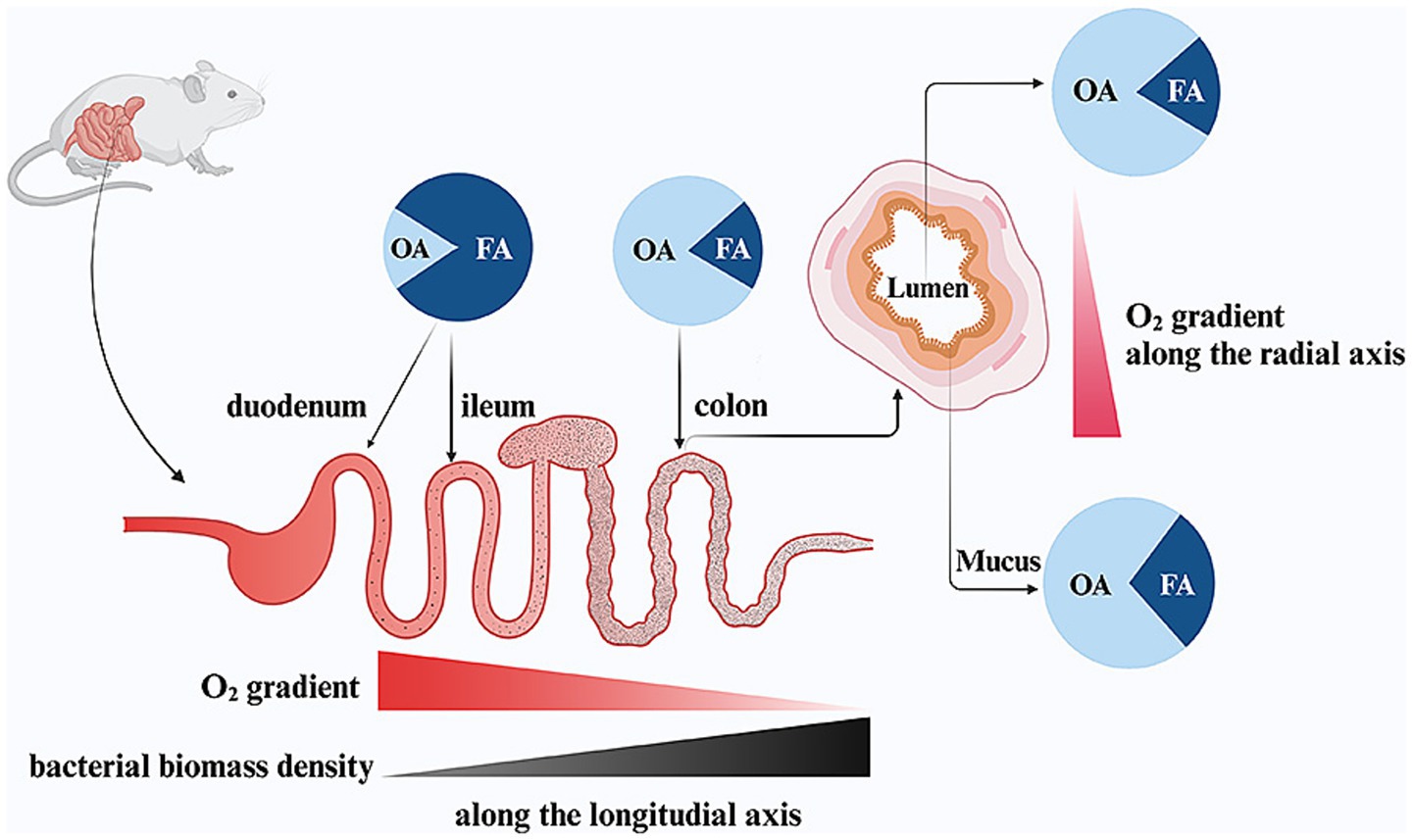
Figure 2. The effect of intestinal oxygen gradients on the gut microbial composition in mice. OA, obligate anaerobes, FA, facultative anaerobes. Figure created with BioRender.com.
Research in mice demonstrates that high O2 tension in the small intestine favors the growth of aerotolerant bacteria (Gu et al., 2013; Sundin et al., 2017). In contrast, the near-zero O2 levels in the colon lumen support the growth of organisms which cannot tolerate O2 (Lee et al., 2022). The luminal O2 concentrations create a distinct environment that shapes the composition of the gut microbiota. As a result, the small intestine in mice is primarily colonized by FA, such as members of the Enterobacteriaceae family (Phylum Proteobacteria) and Lactobacillaceae family (Phylum Firmicutes) (Gu et al., 2013; Sundin et al., 2017). Meanwhile, the colon is dominated by OA, including classes such as Bacteroidia (Phylum Bacteroidetes) and Clostridia (Phylum Firmicutes) (Rivera-Chávez et al., 2017). Similarly, aligning with the radial O2 gradient, FA such as Proteobacteria and Actinobacteria are more prevalent in the mucosal interface (mucus layer) compared to the lumen (Albenberg et al., 2014; Friedman et al., 2018). At the same time, the bacteria biomass undergoes a dramatic increase, rising by more than a million-fold from the small intestine (103–105 cells/ml) to the colon (1011–1012 cells/ml), which corresponds to the decreasing O2 levels in the lumen (Sundin et al., 2017; Friedman et al., 2018; de Vos et al., 2022).
In infancy, there is a sequential colonization by aerotolerant bacteria, followed by a transition to anaerobic bacteria during childhood and adulthood (Orrhage and Nord, 1999). It was once believed that FA were responsible for creating the O2 gradient by progressively consuming O2 as it moves through the small intestine, thus preserving colonic anaerobiosis (Adlerberth and Wold, 2009). However, key experiments comparing conventional and germ-free mice have shown that similar PO2 values are observed in corresponding gut segments of both conventional and germ-free mice (Friedman et al., 2018). Evidence indicates that the steep O2 gradient along the gastrointestinal tract is primarily driven by the host rather than microbial O2 consumption (Lee et al., 2022). Taken together, the host-controlled anaerobiosis in the colon supports the growth of trillions of OA while restricting the expansion of pathogenic FA.
Physiology of O2 therapy
O2 therapy is the standard treatment for hypoxic patients with cardiopulmonary diseases, aiming to ensure adequate tissue oxygenation (Chiumello and Brioni, 2016; Vieira et al., 2020). Tissue hypoxia typically results from an imbalance between O2 delivery (DO2) and O2 consumption (VO2) in circulation. In resting adults, DO2 and VO2 are approximately 600 mL/min/m2 and 120 mL/min/m2, respectively (Pinsky, 2007). Under normal physiological conditions, the DO2: VO2 ratio is maintained at 5:1. When this ratio falls below 2:1, multiple organ dysfunction syndrome (MODS) and lactic acidosis may occur (Lim, 2023). DO2 is determined by the patient’s cardiac output (CO) and the arterial O2 content (CaO2), as described by the following formula (Wemple et al., 2023a,b):
The CaO2 is determined by the product of hemoglobin (Hb), O2 saturation (SaO2), and Hufner’s constant (typically 1.36 mL/g), along with arterial dissolved O2, which is considered negligible due to O2’s low solubility. Therefore, the calculation of CaO2 can be simplified using the following formula (Lim, 2023):
SaO2 depends on arterial PO2 (PaO2) due to the sigmoidal shape of the O2 dissociation curve (Collins et al., 2015). Hence, tissue hypoxia may results from decreased CO and/or decreased PaO2, which are fundamental physiological changes during cardiac failure and/ or pulmonary failure in patients.
O2 delivery systems used in clinical settings can be categorized into O2 inhalation and ECMO, each with distinct indications and physiological mechanisms (Lustbader and Fein, 2000). It is also important to differentiate between hyperoxemia and hyperoxia. Hyperoxemia is defined as PaO2 > 100 mmHg, while hyperoxia refers to excessive O2 at the cellular level (Singer et al., 2021). O2 inhalation, also known as traditional O2 therapy, remains the primary method for treating hypoxic patients with mild to moderate respiratory failure, such as mild acute respiratory distress syndrome (ARDS) (Angus, 2020). However, ECMO is more commonly used for patients with severe pulmonary or cardiopulmonary failure, including severe ARDS, refractory cardiogenic shock, and cardiac arrest (Chiumello and Brioni, 2016; Combes et al., 2020). Inhaled O2 is administered to the patient through the upper respiratory tract using specialized devises (e.g., mechanical ventilators), which increase the fraction of inspired O2 (FiO2) as well as the partial pressure of alveolar O2 (PAO2). The elevated PAO2 drives O2 diffusion across the alveolar-capillary barrier and increases PaO2, thereby increasing DO2 and improving oxygenation (Wemple et al., 2023a,b).
ECMO can be initiated in two distinct forms: venovenous (VV) or venoarterial (VA). VV-ECMO primarily supports the respiratory system, while VA-ECMO supports both the cardiac and respiratory systems simultaneously. Desaturated blood is drawn from the inferior vena cava by a centrifugal pump, and oxygenation occurs in an external membrane oxygenator, and finally the oxygenated blood is directly delivered into either the venous system (VV-ECMO) to increase mixed venous O2 pressure (PVO2) and PaO2 or the arterial system (VA-ECMO) to provide additional CO (Vieira et al., 2020). In this process, O2 input levels are regulated by the fraction of inspired O2 of blender (FbO2) supplied to the oxygenator (Winiszewski et al., 2022) (Figure 3).
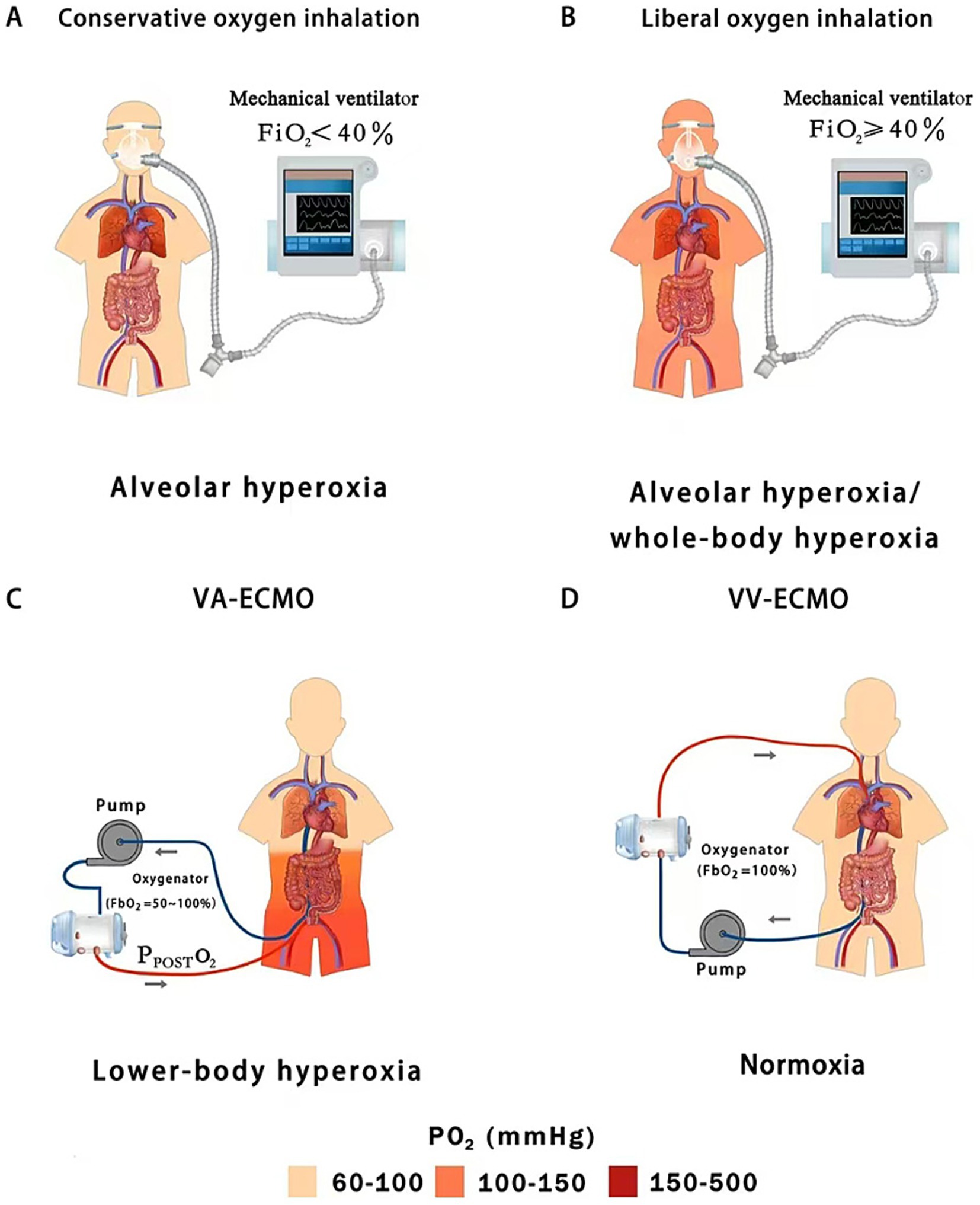
Figure 3. Hyperoxic subtypes of oxygen inhalation (conservative vs. liberal oxygen therapy) and peripheral extracorporeal membrane oxygenation (VA vs. VV); (A) Conservative oxygen therapy can cause slightly high alveolar oxygen concentrations; (B) Liberal oxygen therapy leads to moderate or severe alveolar hyperoxia and mild whole-body hyperoxia; (C) Peripheral VA-ECMO may lead to moderate or severe lower-body hyperoxia; (D) Peripheral VV-ECMO usually does not cause hyperoxemia; FiO2, fraction of inspired oxygen; PPOSTO2, post-oxygenator oxygen partial pressure; FbO2, fraction of inspired O2 of blender; VA-ECMO, venoarterial-extracorporeal membrane oxygenation; VV-ECMO, venovenous-extracorporeal membrane oxygenation.
Intestinal hyperoxia during O2 therapy
O2 inhalation and ECMO expose different organ systems to the risk of hyperoxia (Winiszewski et al., 2022; Dai et al., 2024). O2 inhalation is typically administered using either a liberal or conservative strategy, targeting either a high level (FiO2 ≥ 40%, PaO2 = 100–150 mmHg) or a low level (FiO2 < 40%, PaO2 = 70–100 mmHg) of oxygenation, respectively (Girardis et al., 2016). Conservative O2 inhalation reduces O2 toxicity by preventing arterial hyperoxemia (whole-body hyperoxia) and lowering the risk of alveolar hyperoxia (PAO2 < 300 mmHg). In contrast, patients receiving liberal O2 inhalation face a higher risk of alveolar hyperoxia (PAO2 ≥ 300 mmHg) and whole-body hyperoxia, including the gut (Wemple et al., 2023a,b; Dai et al., 2024) (Figures 3A,B).
VA-ECMO is increasingly used to provide circulatory support in patients with severe pump failure (low CO) by delivering additional oxygenated blood and improving DO2. VA-ECMO is characterized by dual circulation, during which competitive flow develops between blood ejected from the native heart and oxygenated blood traveling retrograde within the aorta from the ECMO reinfusion cannula, which is inserted into the femoral artery (Asija et al., 2023). As a result, oxygenation of the lower body is determined by the fraction of FbO2 and post-oxygenator partial pressure of O2 (PPOSTO2). In current clinical practice, FbO2 is typically set between 50 and 100%, leading to a PPOSTO2 of 300–500 mmHg at the membrane lung outlet. This directly exposes intra-abdominal organs, especially the gastrointestinal tract and its resident microbial communities to severe hyperoxia (Winiszewski et al., 2022; Jentzer et al., 2023; Premraj et al., 2023; Dai et al., 2024) (Figure 3C). Blood flow to the large intestine is mainly supplied by the superior and inferior mesenteric arteries, which are located near the outlet of the VA-ECMO reinfusion cannula (Winiszewski et al., 2018; Shetty et al., 2024). As a result, the colon is directly perfused by hyperoxia blood during VA-ECMO support, raising the levels of O2 diffused from the colonic vasculature into the lumen and disrupting the anaerobic environment.
VV-ECMO oxygenates the venous blood outside the body, increasing PvO2 in the right atrium, thereby increasing PaO2 and DO2. During VV-ECMO support (e.g., using femoro-jugular VV bypass), venous blood (the inferior Vena Cava) is drawn into the ECMO circuit depending on the ECMO flow relative to the total venous return (equivalent to CO), while a proportion of venous deoxygenated blood (the Superior Vena Cava) directly returns to the right heart bypassing the ECMO circuit. The admixture of ECMO-oxygenated blood and deoxygenated venous blood results in an increased PvO2 (e.g., 60–80 mmHg) rather than hyperoxia (PO2 > 100 mmHg) in the pulmonary circulation (Walker et al., 2007; Lim, 2023). In fact, patients with severe ARDS are often still hypoxic or normoxic despite full ECMO support (FbO2 = 100%) (Montisci et al., 2015). Therefore, VV-ECMO rarely leads to hyperoxia on its own due to the shunting of deoxygenated venous blood (Lim, 2023) (Figure 3D). In summary, intestinal hyperoxic injury remains a concern particularly in the settings of VA-ECMO (Winiszewski et al., 2018).
However, studies in rat models have shown that hyperoxia exposure can also significantly impair intestinal barrier function. Hyperoxia induces excessive production of reactive oxygen species (ROS) in the body, leading to the downregulation of key intestinal tight junction proteins such as ZO-1, Occludin, and Claudin-4. This disrupts the intercellular connections in the intestinal epithelium, resulting in the loss of intestinal barrier integrity and increased permeability. Consequently, bacteria and their metabolites, such as D-lactic acid and endotoxins, can translocate into the bloodstream. This further triggers an imbalance in local intestinal inflammatory factors, creating a vicious cycle (Liu et al., 2020).
Hyperoxia is associated with poor clinical outcomes
Inhaling O2 (FiO2 > 0.21) can lead to arterial hyperoxemia (PaO2 > 100 mmHg) and, consequently, tissue hyperoxia, which may increase the production of ROS (Singer et al., 2021). Clinical studies have shown that severe hyperoxemia (PaO2 > 300 mmHg) during O2 inhalation is an independent predictor of higher in-hospital mortality in critically ill patients (Kilgannon et al., 2010, 2011; Rincon et al., 2014; Singer et al., 2021). In human patients, administering inhaled O2 to patients without hypoxia has been associated with increased mortality without improving clinical outcomes (Chu et al., 2018). Based on these evidences, more recent clinical guidelines advise a more cautious approach to O2 inhalation to reduce its potential toxic effects (O’Driscoll et al., 2017; Siemieniuk et al., 2018). A target peripheral capillary O2 saturation of ≤ 96% (PaO2 ≤ 100 mmHg) is generally recommended for most acutely ill patients (Siemieniuk et al., 2018). Over the past decade, many clinicians have adjusted O2 levels (FiO2 and PaO2) more carefully to prevent severe hyperoxia in clinical practice (Damiani et al., 2018; Mackle et al., 2020; Hochberg et al., 2021).
Several studies in patients examining hyperoxia in the context of VA-ECMO have reported a link between hyperoxia and adverse clinical outcomes (Rao et al., 2018; Winiszewski et al., 2022; Tigano et al., 2023; Winiszewski et al., 2024). A recent meta-analysis of clinical data revealed that severe hyperoxia following the initiation of VA-ECMO was associated with a twofold increase in rates of poor neurological outcomes and mortality (Tigano et al., 2023). Current guidelines from the Extracorporeal Life Support Organization recommend adjusting FSO2 to achieve mild hyperoxia (PPOSTO2 = 150 mmHg) in order to prevent both excessive hypo- and hyperoxemia (Winiszewski et al., 2022). Despite this, moderate (PaO2 = 150–300 mmHg) and severe hyperoxemia (PaO2 > 300 mmHg) are frequently observed during VA-ECMO support, affecting approximately 30 and 20% of patients, respectively (Rao et al., 2018). PPOSTO2, which serves as an indicator of the lower body and intestinal oxygenation, is rarely measured in clinical settings, yet when it is, it often shows significant hyperoxemia with a median value nearing 200 mmHg (Winiszewski et al., 2024). Additionally, the median FSO2 value was found to be 70%, with a higher FSO2 levels independently associated with increased mortality (Winiszewski et al., 2024). These findings point to a strong connection between hyperoxia, as a result of VA-ECMO blood flow, and poor clinical outcomes.
Gut dysbiosis is associated with poor clinical outcomes
Advancements in culture-independent technologies, such as metagenomics and metabolomics, have led to a deeper understanding of the complex interactions between human health, disease, and the gut microbiota (Lynch and Pedersen, 2016; de Vos et al., 2022). It is increasingly recognized that an imbalance in the gut microbiome, known as dysbiosis, is linked to a wide range of chronic diseases, including obesity (Cani and Jordan, 2018), diabetes (Qin et al., 2012; Ceccarani et al., 2020), chronic kidney disease (Hida et al., 1996), cardiovascular disease (Jie et al., 2017), inflammatory bowel disease (Frank et al., 2007) and colorectal cancer (Louis et al., 2014). Dysbiosis has also been linked to acute conditions like sepsis (Haak and Wiersinga, 2017; Adelman et al., 2020), acute pancreatitis (Ammer-Herrmenau et al., 2024), stroke (Peh et al., 2022), intracerebral hemorrhage (Xu et al., 2019), pneumonia and acute respiratory distress syndrome (Dickson, 2016). In many of these conditions, gut dysbiosis is characterized by a shift in the microbial community OA (health-promoting microbes) to FA (pathobiota) (Corriero et al., 2022; Winter and Bäumler, 2023; Li et al., 2024).
Increased availability of electron acceptors (O2) drives gut dysbiosis
Recently, ecological and evolutionary theories from macroecology have been increasingly used to explain phenomena related to the microbes and their host (Berg et al., 2020; McDonald et al., 2020). The gut microbiome refers to an environment and the community of microbes within it, while gut dysbiosis is characterized not only by abnormal microbial compositions, functions and metabolites but also by disruptions in both microbe-microbe and microbe-host interactions (Tipton et al., 2019; Ashley et al., 2020).
In the healthy gut, the colon lumen is dominated by commensal OA which converts resistant starch and dietary fiber (nonstarch polysaccharides) into SCFA (mainly acetate, propionate, and butyrate) through saccharolytic fermentation (Morrison and Preston, 2016; Louis and Flint, 2017). SCFA contributes to normal colonic function in the humans (Topping and Clifton, 2001). Particularly, butyrate is a preferred substrate for colonocytes which activates nuclear receptor peroxisome proliferator–activated receptor gamma (PPAR-γ) and regulates the energy metabolism by switching to β-oxidation of fatty acids (Byndloss et al., 2017; Fang et al., 2021). The resulting increase in oxidation of fatty acids in the mitochondria leads to high epithelial O2 consumption and maintains the colonic epithelium in a state of physiological hypoxia (Taylor and Colgan, 2017; Singhal and Shah, 2020). Epithelial hypoxia hampers diffusion of O2 from vascular capillaries into the colonic lumen, thereby preserving anaerobiosis suitable for OA (Maslowski, 2019). Meanwhile, the paucity of O2 as exogenous respiratory electron acceptors for cellular respiration limits the expansion of pathogenic FA (Winter et al., 2013; Bäumler and Sperandio, 2016). During gut homeostasis, the host controls the availability of O2 as an important ecological driver governing microbial growth (Byndloss and Bäumler, 2018; Winter and Bäumler, 2023) (Figure 4A).
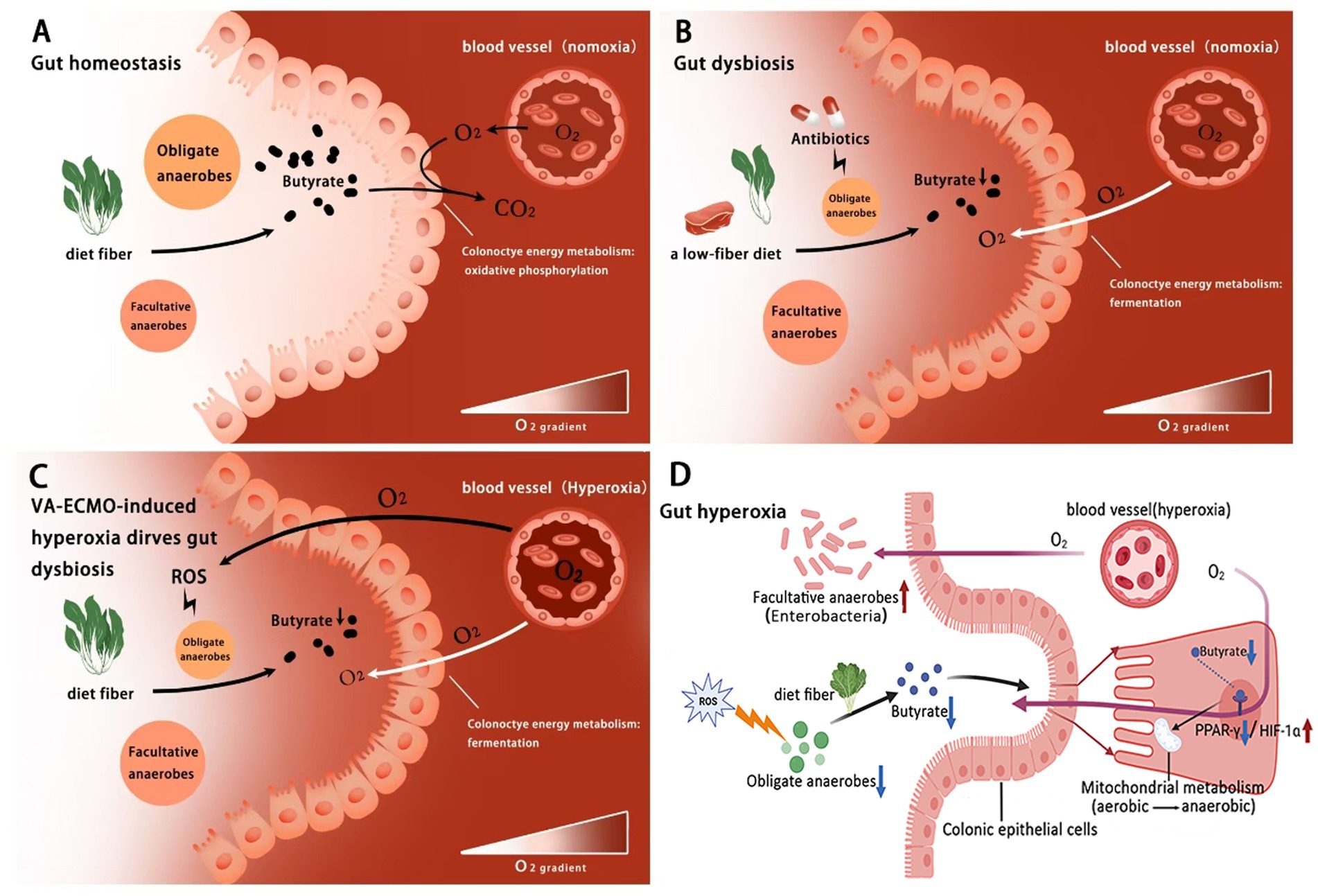
Figure 4. Hyperoxia during VA-ECMO as a driver of gut dysbiosis. (A) Under normal gut homeostasis, the limited availability of external respiratory electron acceptors (O2) in the colon lumen restricts the growth of facultative anaerobes, maintaining a balanced gut microbial community; (B) In cases of gut dysbiosis, factors such as antibiotic use and a low-fiber diet disrupt this balance by increasing O2 availability in the colon lumen, promoting microbial imbalance; (C) VA-ECMO-induced hyperoxia leads to both primary and secondary increases in O2 availability in the colonic lumen, driving gut dysbiosis. (D) Butyric acid may regulate hyperoxia-Induced energy metabolism disorder in colonic epithelium via the PPAR-γ/HIF-1α pathway. ROS, reactive O2 species.
Consequently, any host factors that increase O2 availability in the colonic lumen can disrupt the balance of the gut microbial community (Lee et al., 2024). Studies in murine models have provided key evidence for this mechanism, for instance, antibiotic treatments play a central role in infectious diseases, while they tend to deplete OA from the Clostridia class, which are key producers of SCFA, leading to a decrease in butyrate levels in the colon (Garner et al., 2009; Hays et al., 2024). Diet is another important contributor to gut homeostasis which has a profound impact on the composition, function and diversity of the gut microbes (Trakman et al., 2022; Ross et al., 2024). An unhealthy western diet (high-fat low-fiber and high-calorie) leads to a growing prevalence of obesity, diabetes, cardiovascular diseases and cancer (Swinburn et al., 2011; Song et al., 2015). Lack of dietary fiber reduces the fermentation and expression of butyrate by OA in the colon (O’Keefe, 2016; Amabebe et al., 2020). Crucially, experimental evidence from mice has shown that the depletion of butyrate during antibiotic treatment and low-fiber diet shifts the metabolism of colon epithelium cells from oxidative phosphorylation toward fermentation, lowering mitochondrial O2 consumption and allowing for greater O2 accumulation in the lumen (Kelly et al., 2015; Yoo et al., 2021). As the most efficient terminal electron acceptor in cellular aerobic respiration, O2 plays a critical role in energy conservation whose redox activity is mediated by these unpaired electrons as radicals. O2 can undergo partial reduction by accepting one, two or three electrons, resulting in the production of ROS such as superoxide (O2-) and hydrogen peroxide (H2O2) (Freinbichler et al., 2011). Oxidative injury induced by ROS can lead to a devastating effect on the structure and activity of proteins, and may even lead to bacterial death (Ezraty et al., 2017). This increase in available O2 finally promotes the growth of FA, such as Enterobacteriaceae, leading to gut dysbiosis (Rivera-Chávez et al., 2016; Sun et al., 2023) (Figure 4B).
Hyperoxia alters gut microbial compositions in animals
An early study in mice observed that O2 levels in the colonic lumen increased following pure O2 inhalation, suggesting that O2 diffusion from the host tissue into the intestinal lumen (Albenberg et al., 2014). This finding highlights the potential effects of hyperoxia on gut microbiota and recently spurs the launch of further studies to explore how hyperoxia influences gut microbial compositions (Wang et al., 2023; Dai et al., 2024). In these murine models, a short exposure to hyperoxia (72 h, FiO2 = 80–90%) significantly reduced the relative abundance of obligate anaerobic Ruminococcaceae family (Class: Clostridia) in both the cecum and fecal samples (Ashley et al., 2020; Li Y. et al., 2021). Prolonged exposure to hyperoxia (1–2 weeks, FiO2 = 80–90%) not only reduced beneficial OA such as Ruminococcaceae (Class: Clostridia) and Muribaculaceae (Class: Bacteroidia) (Cai et al., 2023), but also promoted the growth of pathogenic FA, such as Staphylococcus (Abdelgawad et al., 2023) and Enterobacteriaceae (Family: Proteobacteria) (Li H. et al., 2021; Li Y. et al., 2021; Lo et al., 2021; Chen et al., 2023). Both Muribaculaceae and Ruminococcaceae are key producers of SCFAs in the gut (Li H. et al., 2021; Li Y. et al., 2021) and Muribaculaceae is particularly abundant in the healthy mouse gut, often comprising 20–30% of the microbial community (Lagkouvardos et al., 2019; Zhu et al., 2024). Further metagenomic analyzes of these mouse models have shown that the depletion of these OA impairs the gut’s ability to produce SCFA by fermentation of dietary fibers, resulting in low levels of butyrate (Cai et al., 2023). Additionally, gut dysbiosis caused by hyperoxia in mice may also contribute to distant organ injury, such as damage to the lungs and brain through the gut-lung (Ashley et al., 2020; Wedgwood et al., 2020; Shen et al., 2024) and gut–brain axes (Lo et al., 2021; Song and Yang, 2024).
These findings from mouse models indicate that hyperoxia alters the gut microbiome, marked by a reduction in SCFA-producing OA and an increase in FA, particularly Enterobacteriaceae, which is commonly associated with dysbiosis. The depletion of OA appears to precede the expansion of Enterobacteriaceae (Li H. et al., 2021; Li Y. et al., 2021), suggesting that depletion of butyrate plays a crucial role in hyperoxia-induced gut dysbiosis. However, these murine studies have limitations, as they expose healthy animals to nearly pure inhaled O2, a condition rarely encountered in clinical settings, especially in patients without hypoxia (O’Driscoll et al., 2017; Siemieniuk et al., 2018).
Hyperoxia during VA-ECMO disrupts gut homeostasis via metabolic reprogramming and dysbiosis
Under peripheral VA-ECMO support, the typical hemodynamic pattern involves a competitive flow between blood pumped by the heart and blood flowing retrograde through the aorta from the ECMO reinfusion cannula in the femoral artery (Asija et al., 2023). This leads to overproduction of ROS and inhibits the growth of OA, thereby suppressing butyrate expression and shifting colonocyte metabolism from oxidative phosphorylation to fermentation. The metabolic shift reduces the consumption of O2 by colonocytes, leading to further accumulation of luminal O2 and exacerbating the disruption of anaerobiosis. The increased availability of O2 as exogenous electron receptors finally promotes the growth of FA, such as Enterobacteriaceae, causing dysbiosis (Figure 4C). PPAR, a nuclear receptor transcription factor, plays a key role in regulating cellular energy metabolism and mitochondrial function. Among its subtypes, PPAR-γ is highly expressed in colon cells. Studies, primarily in cellular and murine models, have shown that butyrate, as a natural ligand of PPAR-γ, can directly activate this receptor to promote mitochondrial oxidative phosphorylation and suppress anaerobic glycolysis (Byndloss et al., 2017). On the other hand, HIF-1α, as an oxygen-sensitive transcription factor, plays a central role in cellular adaptation to hypoxia and the regulation of glycolysis (Semenza, 2012). High expression of HIF-1α reprograms cellular energy metabolism from aerobic respiration to anaerobic glycolysis (Lin et al., 2023). Notably, PPAR-γ activation inhibits the HIF-1α signaling pathway, creating an important antagonistic relationship in metabolic regulation (Blum et al., 2016) (Figure 4D). Furthermore, research in murine models has established that physiological hypoxia in the gut is essential for maintaining host-microbiota balance. Short-chain fatty acids (such as butyrate) produced by microbial metabolism of dietary fibers enhance mitochondrial respiration in intestinal epithelial cells, thereby consuming oxygen and subsequently stabilizing and activating HIF-1α. Stabilized HIF-1α is critical for reinforcing epithelial barrier function and regulating IL-22 production by ILC3s (Pral et al., 2021). Conversely, hyperoxia disrupts this hypoxic microenvironment, not only inhibiting the HIF-1 signaling pathway and impairing barrier function but also promoting the expansion of FA, thereby serving as a key driver of gut microbiota dysbiosis and inflammation.
Current perspectives and future challenges
From an ecological perspective, the dynamic changes associated with gut dysbiosis are primarily driven by dysfunction of the gut barrier, which allows O2 to leak into the lumen and creates an aerobic nutrient niche that suppresses butyrate-productive OA and favors the growth of pathogenic FA. The central role of O2 in gut dysbiosis has been indirectly demonstrated in various human diseases, where O2 acts as an intermediate factor. Recently, emerging evidence suggests that hyperoxia during O2 therapy can directly drive gut dysbiosis, with O2 serving as the initiating factor. In clinical settings, hyperoxia during VA-ECMO oxygenates the patient from the “bottom up,” potentially raising O2 levels in the intestinal lumen and disrupting the redox balance between obligate and FA—ultimately contributing to dysbiosis. Further clinical and basic research is needed to better understand how hyperoxia during O2 therapy affects redox dynamics in intestinal microbial ecology, and to identify optimal oxygenation strategies for patients undergoing VA-ECMO support.
It is crucial to recognize, however, that gut dysbiosis in critically ill patients, including those on VA-ECMO, is a multifactorial phenomenon. Beyond hyperoxia, factors such as altered enteral nutrition, profound physical inactivity, physiological stress, and the use of broad-spectrum antibiotics are known to independently contribute to microbial imbalance. Nevertheless, this article specifically highlights that hyperoxia remains a potentially critical and underappreciated driver in this context, owing to its direct inhibitory effect on OA. The unique iatrogenic hyperoxemia experienced by VA-ECMO patients may thus represent a major and persistent insult that amplifies the dysbiotic effects of other factors.
Indeed, alterations in the oxygen microenvironment play a critical role in various gut disorders associated with dysbiosis. Taking Clostridium difficile infection as an example, the normal hypoxic gut environment favors the survival of OA, which form a biological barrier inhibiting the colonization of C. difficile. When factors such as antibiotics induce dysbiosis, the hypoxic gut environment is disrupted, weakening the protective role of anaerobic bacteria and potentially promoting the proliferation of C. difficile spores, thereby significantly increasing host susceptibility (Khazaaleh et al., 2022). In summary, disturbances in intestinal oxygen balance—whether hypoxia or hyperoxia—are key mechanisms driving the onset and progression of disease.
Translating findings on oxygen and gut microbiota from animal and cellular studies to humans still poses significant challenges. Oxygen, the host, and the microbial community form a complex multi-factorial structure, making it particularly challenging to establish similar causal relationships in humans. Factors such as ethical constraints in human studies, variations in microbial composition and function, and confounding variables like diet and antibiotic use further complicate research. Nevertheless, animal models have provided strong evidence for hyperoxia-induced dysbiosis (Xing et al., 2020; Chen et al., 2023). However, direct clinical evidence linking hyperoxia to changes in gut microbiota remains relatively scarce. This is particularly pronounced in critically ill patients—where confounding factors such as underlying diseases, antibiotic use, and enteral nutrition are abundant, and longitudinal collection of intestinal samples presents practical challenges. To address this evidence gap, future clinical studies should prioritize specific populations, such as patients receiving veno-arterial extracorporeal membrane oxygenation (VA-ECMO) who are exposed to severe lower-body hyperoxia (Dai et al., 2024). Furthermore, prospective studies should be conducted within this population to analyze the correlation between post-oxygenator arterial oxygen partial pressure (PPOSTO2) and serially measured values of gut injury markers (such as intestinal fatty acid-binding protein), microbial composition, and metabolic profiles. This approach is expected to yield more direct evidence. Therefore, future research should adopt more comprehensive and longitudinal methods, integrating multi-omics data such as metagenomics and transcriptomics, to advance the field from correlation studies toward clinical translation.
It must be acknowledged that this review has certain limitations. First, we have highlighted the challenges in translating findings from animal models to humans, emphasizing that while animal models are indispensable for mechanistic studies, there are significant differences in gut microbiota across species. Therefore, the primary focus of this paper is not to directly predict clinical outcomes but to propose, for the first time, the novel concept that “hyperoxia is a driver of gut microbiota dysbiosis in critically ill patients, “aiming to establish a theoretical foundation for future targeted human studies. Second, we recognize that current clinical evidence is largely derived from observational studies, which are highly susceptible to confounding factors such as antibiotic use and nutritional support, making it difficult to establish a pure causal relationship between hyperoxia and microbiota dysbiosis. Despite these limitations, the conceptual framework proposed in this paper provides a solid foundation for subsequent research. Future work should focus on designing more rigorous prospective studies or mechanistic explorations to isolate and quantify the independent effects of hyperoxia on gut microbiota under controlled conditions.
Author contributions
HW: Conceptualization, Resources, Supervision, Writing – original draft. WZ: Conceptualization, Resources, Writing – original draft, Supervision. ND: Conceptualization, Resources, Visualization, Writing – original draft. JG: Conceptualization, Supervision, Writing – original draft. YH: Formal analysis, Funding acquisition, Investigation, Resources, Visualization, Writing – original draft. HQ: Conceptualization, Resources, Supervision, Visualization, Writing – original draft. LL: Conceptualization, Resources, Supervision, Visualization, Writing – original draft. XF: Conceptualization, Formal analysis, Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing, Writing – original draft. BF: Conceptualization, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing, Writing – original draft. ZX: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (82160370, 82560385); the Science and Technology Program of Guizhou Province [QIANKEHEZHICHEN[2022]YIBAN179]; the Zunyi Science and Technology Planning Project [Zun yi Ke He HZ ZI (2023) 207]; the Kweichow Moutai Hospital (MTyk2022-12); and the Educational Department of Guizhou Province [Qianjiaoji 2023(020)].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelgawad, A., Nicola, T., Martin, I., Halloran, B. A., Tanaka, K., Adegboye, C. Y., et al. (2023). Antimicrobial peptides modulate lung injury by altering the intestinal microbiota. Microbiome 11:226. doi: 10.1186/s40168-023-01673-0
Adelman, M. W., Woodworth, M. H., Langelier, C., Busch, L. M., Kempker, J. A., Kraft, C. S., et al. (2020). The gut microbiome’s role in the development, maintenance, and outcomes of sepsis. Crit. Care 24:278. doi: 10.1186/s13054-020-02989-1
Adlerberth, I., and Wold, A. E. (2009). Establishment of the gut microbiota in western infants. Acta Paediatr. 98, 229–238. doi: 10.1111/j.1651-2227.2008.01060.x
Albenberg, L., Esipova, T. V., Judge, C. P., Bittinger, K., Chen, J., Laughlin, A., et al. (2014). Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology 147, 1055–1063.e8. doi: 10.1053/j.gastro.2014.07.020
Amabebe, E., Robert, F. O., Agbalalah, T., and Orubu, E. (2020). Microbial dysbiosis-induced obesity: role of gut microbiota in homoeostasis of energy metabolism. Br. J. Nutr. 123, 1127–1137. doi: 10.1017/S0007114520000380
Ammer-Herrmenau, C., Antweiler, K. L., Asendorf, T., Beyer, G., Buchholz, S. M., Cameron, S., et al. (2024). Gut microbiota predicts severity and reveals novel metabolic signatures in acute pancreatitis. Gut 73, 485–495. doi: 10.1136/gutjnl-2023-330987
Angus, D. C. (2020). Oxygen therapy for the critically ill. N. Engl. J. Med. 382, 1054–1056. doi: 10.1056/NEJMe2000800
Ashley, S. L., Sjoding, M. W., Popova, A. P., Cui, T. X., Hoostal, M. J., Schmidt, T. M., et al. (2020). Lung and gut microbiota are altered by hyperoxia and contribute to oxygen-induced lung injury in mice. Sci. Transl. Med. 12:eaau9959. doi: 10.1126/scitranslmed.aau9959
Asija, R., Fried, J. A., Siddall, E. C., Mullin, D. A., Agerstrand, C. L., Brodie, D., et al. (2023). How I manage differential gas exchange in peripheral venoarterial extracorporeal membrane oxygenation. Crit. Care 27:408. doi: 10.1186/s13054-023-04703-3
Bäumler, A. J., and Sperandio, V. (2016). Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535, 85–93. doi: 10.1038/nature18849
Berg, G., Rybakova, D., Fischer, D., Cernava, T., Vergès, M. C., Charles, T., et al. (2020). Microbiome definition re-visited: old concepts and new challenges. Microbiome 8:103. doi: 10.1186/s40168-020-00875-0
Blum, J. I., Bijli, K. M., Murphy, T. C., Kleinhenz, J. M., and Hart, C. M. (2016). Time-dependent PPARγ modulation of HIF-1α signaling in hypoxic pulmonary artery smooth muscle cells. Am J Med Sci 352, 71–79. doi: 10.1016/j.amjms.2016.03.019
Bueno, E., Mesa, S., Bedmar, E. J., Richardson, D. J., and Delgado, M. J. (2012). Bacterial adaptation of respiration from oxic to microoxic and anoxic conditions: redox control. Antioxid. Redox Signal. 16, 819–852. doi: 10.1089/ars.2011.4051
Byndloss, M. X., and Bäumler, A. J. (2018). The germ-organ theory of non-communicable diseases. Nat. Rev. Microbiol. 16, 103–110. doi: 10.1038/nrmicro.2017.158
Byndloss, M. X., Olsan, E. E., Rivera-Chávez, F., Tiffany, C. R., Cevallos, S. A., Lokken, K. L., et al. (2017). Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 357, 570–575. doi: 10.1126/science.aam9949
Cai, Y., Luo, Y., Dai, N., Yang, Y., He, Y., Chen, H., et al. (2023). Functional metagenomic and metabolomics analysis of gut dysbiosis induced by hyperoxia. Front. Microbiol. 14:1197970. doi: 10.3389/fmicb.2023.1197970
Cani, P. D., and Jordan, B. F. (2018). Gut microbiota-mediated inflammation in obesity: a link with gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 15, 671–682. doi: 10.1038/s41575-018-0025-6
Ceccarani, C., Bassanini, G., Montanari, C., Casiraghi, M. C., Ottaviano, E., Morace, G., et al. (2020). Proteobacteria overgrowth and butyrate-producing taxa depletion in the gut microbiota of glycogen storage disease type 1 patients. Meta 10:133. doi: 10.3390/metabo10040133
Chanderraj, R., Baker, J. M., Kay, S. G., Brown, C. A., Hinkle, K. J., Fergle, D. J., et al. (2023). In critically ill patients, anti-anaerobic antibiotics increase risk of adverse clinical outcomes. Eur. Respir. J. 61:2200910. doi: 10.1183/13993003.00910-2022
Chen, C. M., Yang, Y., Chou, H. C., and Lin, S. (2023). Intranasal administration of Lactobacillus johnsonii attenuates hyperoxia-induced lung injury by modulating gut microbiota in neonatal mice. J. Biomed. Sci. 30:57. doi: 10.1186/s12929-023-00958-8
Chiumello, D., and Brioni, M. (2016). Severe hypoxemia: which strategy to choose. Crit. Care 20:132. doi: 10.1186/s13054-016-1304-7
Chu, D. K., Kim, L. H., Young, P. J., Zamiri, N., Almenawer, S. A., Jaeschke, R., et al. (2018). Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet 391, 1693–1705. doi: 10.1016/S0140-6736(18)30479-3
Collins, J. A., Rudenski, A., Gibson, J., Howard, L., and O’Driscoll, R. (2015). Relating oxygen partial pressure, saturation and content: the haemoglobin-oxygen dissociation curve. Breathe (Sheff.) 11, 194–201. doi: 10.1183/20734735.001415
Combes, A., Schmidt, M., Hodgson, C. L., Fan, E., Ferguson, N. D., Fraser, J. F., et al. (2020). Extracorporeal life support for adults with acute respiratory distress syndrome. Intensive Care Med. 46, 2464–2476. doi: 10.1007/s00134-020-06290-1
Corriero, A., Gadaleta, R. M., Puntillo, F., Inchingolo, F., Moschetta, A., and Brienza, N. (2022). The central role of the gut in intensive care. Crit. Care 26:379. doi: 10.1186/s13054-022-04259-8
Cummins, E. P., and Crean, D. (2017). Hypoxia and inflammatory bowel disease. Microbes Infect. 19, 210–221. doi: 10.1016/j.micinf.2016.09.004
Dai, N., Gu, J., Luo, Y., Tao, Y., Chou, Y., He, Y., et al. (2024). Impact of hyperoxia on the gut during critical illnesses. Crit. Care 28:66. doi: 10.1186/s13054-024-04848-9
Damiani, E., Donati, A., and Girardis, M. (2018). Oxygen in the critically ill: friend or foe. Curr. Opin. Anaesthesiol. 31, 129–135. doi: 10.1097/ACO.0000000000000559
de Vos, W. M., Tilg, H., Van Hul, M., and Cani, P. D. (2022). Gut microbiome and health: mechanistic insights. Gut 71, 1020–1032. doi: 10.1136/gutjnl-2021-326789
Dickson, R. P. (2016). The microbiome and critical illness. Lancet Respir. Med. 4, 59–72. doi: 10.1016/S2213-2600(15)00427-0
Donaldson, G. P., Lee, S. M., and Mazmanian, S. K. (2016). Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 14, 20–32. doi: 10.1038/nrmicro3552
Eckburg, P. B., Bik, E. M., Bernstein, C. N., Purdom, E., Dethlefsen, L., Sargent, M., et al. (2005). Diversity of the human intestinal microbial flora. Science 308, 1635–1638. doi: 10.1126/science.1110591
Ezraty, B., Gennaris, A., Barras, F., and Collet, J. F. (2017). Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 15, 385–396. doi: 10.1038/nrmicro.2017.26
Fang, J., Wang, H., Xue, Z., Cheng, Y., and Zhang, X. (2021). PPARγ: the central mucus barrier coordinator in ulcerative colitis. Inflamm. Bowel Dis. 27, 732–741. doi: 10.1093/ibd/izaa273
Fenchel, T., and Finlay, B. (2008). Oxygen and the spatial structure of microbial communities. Biol. Rev. Camb. Philos. Soc. 83, 553–569. doi: 10.1111/j.1469-185X.2008.00054.x
Fischer, W. W., and Valentine, J. S. (2019). How did life come to tolerate and thrive in an oxygenated world. Free Radic. Biol. Med. 140, 1–3. doi: 10.1016/j.freeradbiomed.2019.07.021
Frank, D. N., St Amand, A. L., Feldman, R. A., Boedeker, E. C., Harpaz, N., and Pace, N. R. (2007). Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 104, 13780–13785. doi: 10.1073/pnas.0706625104
Freinbichler, W., Colivicchi, M. A., Stefanini, C., Bianchi, L., Ballini, C., Misini, B., et al. (2011). Highly reactive oxygen species: detection, formation, and possible functions. Cell. Mol. Life Sci. 68, 2067–2079. doi: 10.1007/s00018-011-0682-x
Friedman, E. S., Bittinger, K., Esipova, T. V., Hou, L., Chau, L., Jiang, J., et al. (2018). Microbes vs. chemistry in the origin of the anaerobic gut lumen. Proc. Natl. Acad. Sci. USA 115, 4170–4175. doi: 10.1073/pnas.1718635115
Garner, C. D., Antonopoulos, D. A., Wagner, B., Duhamel, G. E., Keresztes, I., Ross, D. A., et al. (2009). Perturbation of the small intestine microbial ecology by streptomycin alters pathology in a Salmonella enterica serovar typhimurium murine model of infection. Infect. Immun. 77, 2691–2702. doi: 10.1128/IAI.01570-08
Gelissen, H., de Grooth, H. J., Smulders, Y., Wils, E. J., de Ruijter, W., Vink, R., et al. (2021). Effect of low-normal vs high-normal oxygenation targets on organ dysfunction in critically ill patients: a randomized clinical trial. JAMA 326, 940–948. doi: 10.1001/jama.2021.13011
Girardis, M., Busani, S., Damiani, E., Donati, A., Rinaldi, L., Marudi, A., et al. (2016). Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA 316, 1583–1589. doi: 10.1001/jama.2016.11993
Gu, S., Chen, D., Zhang, J. N., Lv, X., Wang, K., Duan, L. P., et al. (2013). Bacterial community mapping of the mouse gastrointestinal tract. PLoS One 8:e74957. doi: 10.1371/journal.pone.0074957
Gu, W. J., Zhang, Z., and Van Poucke, S. (2017). Oxygen therapy and Ventilatory support. Can. Respir. J. 2017, 1–2. doi: 10.1155/2017/2462818
Guzior, D. V., and Quinn, R. A. (2021). Review: microbial transformations of human bile acids. Microbiome 9:140. doi: 10.1186/s40168-021-01101-1
Haak, B. W., and Wiersinga, W. J. (2017). The role of the gut microbiota in sepsis. Lancet Gastroenterol. Hepatol. 2, 135–143. doi: 10.1016/S2468-1253(16)30119-4
Hara, K. Y., and Kondo, A. (2015). ATP regulation in bioproduction. Microb. Cell Factories 14:198. doi: 10.1186/s12934-015-0390-6
Hays, K. E., Pfaffinger, J. M., and Ryznar, R. (2024). The interplay between gut microbiota, short-chain fatty acids, and implications for host health and disease. Gut Microbes 16:2393270. doi: 10.1080/19490976.2024.2393270
Hida, M., Aiba, Y., Sawamura, S., Suzuki, N., Satoh, T., and Koga, Y. (1996). Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 74, 349–355. doi: 10.1159/000189334
Hochberg, C. H., Semler, M. W., and Brower, R. G. (2021). Oxygen toxicity in critically ill adults. Am. J. Respir. Crit. Care Med. 204, 632–641. doi: 10.1164/rccm.202102-0417CI
Human Microbiome Project Consortium (2012). Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214. doi: 10.1038/nature11234
Jentzer, J. C., Miller, P. E., Alviar, C., Yalamuri, S., Bohman, J. K., and Tonna, J. E. (2023). Exposure to arterial Hyperoxia during extracorporeal membrane oxygenator support and mortality in patients with cardiogenic shock. Circ. Heart Fail. 16:e010328. doi: 10.1161/CIRCHEARTFAILURE.122.010328
Jie, Z., Xia, H., Zhong, S. L., Feng, Q., Li, S., Liang, S., et al. (2017). The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 8:845. doi: 10.1038/s41467-017-00900-1
Johnson, L. A., and Hug, L. A. (2019). Distribution of reactive oxygen species defense mechanisms across domain bacteria. Free Radic. Biol. Med. 140, 93–102. doi: 10.1016/j.freeradbiomed.2019.03.032
Kasting, J. F., and Siefert, J. L. (2002). Life and the evolution of earth’s atmosphere. Science 296, 1066–1068. doi: 10.1126/science.1071184
Kelly, C. J., Zheng, L., Campbell, E. L., Saeedi, B., Scholz, C. C., Bayless, A. J., et al. (2015). Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17, 662–671. doi: 10.1016/j.chom.2015.03.005
Khazaaleh, S., Gonzalez, A. J., Alomari, M., Wadhwa, V., Shah, B., and Shen, B. (2022). Ischemic colitis is a risk factor for Clostridium difficile infection. Cureus 14:e26076. doi: 10.7759/cureus.26076
Kilgannon, J. H., Jones, A. E., Parrillo, J. E., Dellinger, R. P., Milcarek, B., Hunter, K., et al. (2011). Relationship between supranormal oxygen tension and outcome after resuscitation from cardiac arrest. Circulation 123, 2717–2722. doi: 10.1161/CIRCULATIONAHA.110.001016
Kilgannon, J. H., Jones, A. E., Shapiro, N. I., Angelos, M. G., Milcarek, B., Hunter, K., et al. (2010). Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA 303, 2165–2171. doi: 10.1001/jama.2010.707
Kim, S., Seo, S. U., and Kweon, M. N. (2024). Gut microbiota-derived metabolites tune host homeostasis fate. Semin. Immunopathol. 46:2. doi: 10.1007/s00281-024-01012-x
Koropatkin, N. M., Cameron, E. A., and Martens, E. C. (2012). How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 10, 323–335. doi: 10.1038/nrmicro2746
Lagkouvardos, I., Lesker, T. R., Hitch, T., Gálvez, E., Smit, N., Neuhaus, K., et al. (2019). Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 7:28. doi: 10.1186/s40168-019-0637-2
Lee, J.-Y., Bays, D. J., Savage, H. P., and Bäumler, A. J. (2024). The human gut microbiome in health and disease: time for a new chapter. Infect. Immun. 92:e0030224. doi: 10.1128/iai.00302-24
Lee, J. Y., Tsolis, R. M., and Bäumler, A. J. (2022). The microbiome and gut homeostasis. Science 377:eabp9960. doi: 10.1126/science.abp9960
Li, Y., Tao, Y., Xu, J., He, Y., Zhang, W., Jiang, Z., et al. (2021). Hyperoxia provokes time- and dose-dependent gut injury and Endotoxemia and alters gut microbiome and transcriptome in mice. Front. Med. (Lausanne) 8:732039. doi: 10.3389/fmed.2021.732039
Li, H., Xiang, Y., Zhu, Z., Wang, W., Jiang, Z., Zhao, M., et al. (2021). Rifaximin-mediated gut microbiota regulation modulates the function of microglia and protects against CUMS-induced depression-like behaviors in adolescent rat. J. Neuroinflammation 18:254. doi: 10.1186/s12974-021-02303-y
Li, X., Zheng, P., Zou, Y., Guan, L., Li, N., Liu, J., et al. (2024). Dietary inulin ameliorates obesity-induced severe acute pancreatitis via gut-pancreas axis. Gut Microbes 16:2436949. doi: 10.1080/19490976.2024.2436949
Lim, H. (2023). The physiology of extracorporeal membrane oxygenation: the Fick principle. Perfusion 38, 236–244. doi: 10.1177/02676591211055971
Lin, X., An, T., Fu, D., Duan, S., Jin, H. L., and Wang, H. B. (2023). Optimization of central carbon metabolism by Warburg effect of human cancer cell improves triterpenes biosynthesis in yeast. Adv. Biotechnol. 1:4. doi: 10.1007/s44307-023-00004-6
Litvak, Y., and Bäumler, A. J. (2019). Microbiota-nourishing immunity: a guide to understanding our microbial self. Immunity 51, 214–224. doi: 10.1016/j.immuni.2019.08.003
Litvak, Y., Byndloss, M. X., and Bäumler, A. J. (2018). Colonocyte metabolism shapes the gut microbiota. Science 362:eaat9076. doi: 10.1126/science.aat9076
Liu, D. Y., Lou, W. J., Zhang, D. Y., and Sun, S. Y. (2020). ROS plays a role in the neonatal rat intestinal barrier damages induced by Hyperoxia. Biomed. Res. Int. 2020:8819195. doi: 10.1155/2020/8819195
Liu, S., Wang, S., Liu, X., Wen, L., and Zou, J. (2022). Effects of dietary antimicrobial peptides on intestinal morphology, antioxidant status, immune responses, microbiota and pathogen disease resistance in grass carp Ctenopharyngodon idellus. Microb. Pathog. 165:105386. doi: 10.1016/j.micpath.2021.105386
Lo, Y. C., Chen, K. Y., Chou, H. C., Lin, I. H., and Chen, C. M. (2021). Neonatal hyperoxia induces gut dysbiosis and behavioral changes in adolescent mice. J. Chin. Med. Assoc. 84, 290–298. doi: 10.1097/JCMA.0000000000000488
Louis, P., and Flint, H. J. (2017). Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 19, 29–41. doi: 10.1111/1462-2920.13589
Louis, P., Hold, G. L., and Flint, H. J. (2014). The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 12, 661–672. doi: 10.1038/nrmicro3344
Lu, Z., and Imlay, J. A. (2021). When anaerobes encounter oxygen: mechanisms of oxygen toxicity, tolerance and defence. Nat. Rev. Microbiol. 19, 774–785. doi: 10.1038/s41579-021-00583-y
Lustbader, D., and Fein, A. (2000). Other modalities of oxygen therapy: hyperbaric oxygen, nitric oxide, and ECMO. Respir. Care Clin. N. Am. 6, 659–674. doi: 10.1016/s1078-5337(05)70093-9
Lynch, S. V., and Pedersen, O. (2016). The human intestinal microbiome in health and disease. N. Engl. J. Med. 375, 2369–2379. doi: 10.1056/NEJMra1600266
Lyons, T. W., Reinhard, C. T., and Planavsky, N. J. (2014). The rise of oxygen in earth’s early ocean and atmosphere. Nature 506, 307–315. doi: 10.1038/nature13068
Lyons, T. W., Tino, C. J., Fournier, G. P., Anderson, R. E., Leavitt, W. D., Konhauser, K. O., et al. (2024). Co-evolution of early earth environments and microbial life. Nat. Rev. Microbiol. 22, 572–586. doi: 10.1038/s41579-024-01044-y
Mackle, D., Bellomo, R., Bailey, M., Beasley, R., Deane, A., Eastwood, G., et al. (2020). Conservative oxygen therapy during mechanical ventilation in the ICU. N. Engl. J. Med. 382, 989–998. doi: 10.1056/NEJMoa1903297
Maslowski, K. M. (2019). Metabolism at the Centre of the host-microbe relationship. Clin. Exp. Immunol. 197, 193–204. doi: 10.1111/cei.13329
Matthay, M. A. (2015). Saving lives with high-flow nasal oxygen. N. Engl. J. Med. 372, 2225–2226. doi: 10.1056/NEJMe1504852
McDonald, J. E., Marchesi, J. R., and Koskella, B. (2020). Application of ecological and evolutionary theory to microbiome community dynamics across systems. Proc. Biol. Sci. 287:20202886. doi: 10.1098/rspb.2020.2886
Miller, B. M., Liou, M. J., Lee, J. Y., and Bäumler, A. J. (2021). The longitudinal and cross-sectional heterogeneity of the intestinal microbiota. Curr. Opin. Microbiol. 63, 221–230. doi: 10.1016/j.mib.2021.08.004
Montisci, A., Maj, G., Zangrillo, A., Winterton, D., and Pappalardo, F. (2015). Management of refractory hypoxemia during venovenous extracorporeal membrane oxygenation for ARDS. ASAIO J. 61, 227–236. doi: 10.1097/MAT.0000000000000207
Morrison, D. J., and Preston, T. (2016). Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 7, 189–200. doi: 10.1080/19490976.2015.1134082
Nolfi-Donegan, D., Braganza, A., and Shiva, S. (2020). Mitochondrial electron transport chain: oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 37:101674. doi: 10.1016/j.redox.2020.101674
O’Driscoll, B. R., Howard, L. S., Earis, J., and Mak, V. (2017). BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax 72, ii1–ii90. doi: 10.1136/thoraxjnl-2016-209729
O’Keefe, S. J. (2016). Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 13, 691–706. doi: 10.1038/nrgastro.2016.165
Olejarz, J., Iwasa, Y., Knoll, A. H., and Nowak, M. A. (2021). The great oxygenation event as a consequence of ecological dynamics modulated by planetary change. Nat. Commun. 12:3985. doi: 10.1038/s41467-021-23286-7
Orrhage, K., and Nord, C. E. (1999). Factors controlling the bacterial colonization of the intestine in breastfed infants. Acta Paediatr. Suppl. 88, 47–57. doi: 10.1111/j.1651-2227.1999.tb01300.x
Payne, J. L., McClain, C. R., Boyer, A. G., Brown, J. H., Finnegan, S., Kowalewski, M., et al. (2011). The evolutionary consequences of oxygenic photosynthesis: a body size perspective. Photosynth. Res. 107, 37–57. doi: 10.1007/s11120-010-9593-1
Peh, A., O’Donnell, J. A., Broughton, B., and Marques, F. Z. (2022). Gut microbiota and their metabolites in stroke: a double-edged sword. Stroke 53, 1788–1801. doi: 10.1161/STROKEAHA.121.036800
Pinsky, M. R. (2007). Hemodynamic evaluation and monitoring in the ICU. Chest 132, 2020–2029. doi: 10.1378/chest.07-0073
Pral, L. P., Fachi, J. L., Corrêa, R. O., Colonna, M., and Vinolo, M. (2021). Hypoxia and HIF-1 as key regulators of gut microbiota and host interactions. Trends Immunol. 42, 604–621. doi: 10.1016/j.it.2021.05.004
Premraj, L., Brown, A., Fraser, J. F., Pellegrino, V., Pilcher, D., and Burrell, A. (2023). Oxygenation during venoarterial extracorporeal membrane oxygenation: physiology, current evidence, and a pragmatic approach to oxygen titration. Crit. Care Med. 52, 637–648. doi: 10.1097/CCM.0000000000006134
Procházková, N., Falony, G., Dragsted, L. O., Licht, T. R., Raes, J., and Roager, H. M. (2023). Advancing human gut microbiota research by considering gut transit time. Gut 72, 180–191. doi: 10.1136/gutjnl-2022-328166
Qin, J., Li, Y., Cai, Z., Li, S., Zhu, J., Zhang, F., et al. (2012). A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60. doi: 10.1038/nature11450
Rao, P., Khalpey, Z., Smith, R., Burkhoff, D., and Kociol, R. D. (2018). Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest. Circ. Heart Fail. 11:e004905. doi: 10.1161/CIRCHEARTFAILURE.118.004905
Raymond, J., and Segrè, D. (2006). The effect of oxygen on biochemical networks and the evolution of complex life. Science 311, 1764–1767. doi: 10.1126/science.1118439
Rincon, F., Kang, J., Maltenfort, M., Vibbert, M., Urtecho, J., Athar, M. K., et al. (2014). Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit. Care Med. 42, 387–396. doi: 10.1097/CCM.0b013e3182a27732
Rivera-Chávez, F., Lopez, C. A., and Bäumler, A. J. (2017). Oxygen as a driver of gut dysbiosis. Free Radic. Biol. Med. 105, 93–101. doi: 10.1016/j.freeradbiomed.2016.09.022
Rivera-Chávez, F., Zhang, L. F., Faber, F., Lopez, C. A., Byndloss, M. X., Olsan, E. E., et al. (2016). Depletion of butyrate-producing Clostridia from the gut microbiota drives an aerobic luminal expansion of Salmonella. Cell Host Microbe 19, 443–454. doi: 10.1016/j.chom.2016.03.004
Ross, F. C., Patangia, D., Grimaud, G., Lavelle, A., Dempsey, E. M., Ross, R. P., et al. (2024). The interplay between diet and the gut microbiome: implications for health and disease. Nat. Rev. Microbiol. 22, 671–686. doi: 10.1038/s41579-024-01068-4
Schaible, B., Schaffer, K., and Taylor, C. T. (2010). Hypoxia, innate immunity and infection in the lung. Respir. Physiol. Neurobiol. 174, 235–243. doi: 10.1016/j.resp.2010.08.006
Schjørring, O. L., Klitgaard, T. L., Perner, A., Wetterslev, J., Lange, T., Siegemund, M., et al. (2021). Lower or higher oxygenation targets for acute hypoxemic respiratory failure. N. Engl. J. Med. 384, 1301–1311. doi: 10.1056/NEJMoa2032510
Semenza, G. L. (2012). Hypoxia-inducible factors in physiology and medicine. Cell 148, 399–408. doi: 10.1016/j.cell.2012.01.021
Shan, Y., Lai, Y., and Yan, A. (2012). Metabolic reprogramming under microaerobic and anaerobic conditions in bacteria. Subcell. Biochem. 64, 159–179. doi: 10.1007/978-94-007-5055-5_8
Shen, X., Yang, Z., Wang, Q., Chen, X., Zhu, Q., Liu, Z., et al. (2024). Lactobacillus plantarum L168 improves hyperoxia-induced pulmonary inflammation and hypoalveolarization in a rat model of bronchopulmonary dysplasia. NPJ Biofilms Microbiomes 10:32. doi: 10.1038/s41522-024-00504-w
Shetty, A. S., Fraum, T. J., Ludwig, D. R., Itani, M., Rajput, M. Z., Strnad, B. S., et al. (2024). Imaging of the inferior mesenteric vasculature. Radiographics 44:e240047. doi: 10.1148/rg.240047
Siemieniuk, R., Chu, D. K., Kim, L. H., Güell-Rous, M. R., Alhazzani, W., Soccal, P. M., et al. (2018). Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ (Clinical research ed.) 363:k4169. doi: 10.1136/bmj.k4169
Singer, M., Young, P. J., Laffey, J. G., Asfar, P., Taccone, F. S., Skrifvars, M. B., et al. (2021). Dangers of hyperoxia. Crit. Care 25:440. doi: 10.1186/s13054-021-03815-y
Singhal, R., and Shah, Y. M. (2020). Oxygen battle in the gut: hypoxia and hypoxia-inducible factors in metabolic and inflammatory responses in the intestine. J. Biol. Chem. 295, 10493–10505. doi: 10.1074/jbc.REV120.011188
Ślesak, I., Kula, M., Ślesak, H., Miszalski, Z., and Strzałka, K. (2019). How to define obligatory anaerobiosis? An evolutionary view on the antioxidant response system and the early stages of the evolution of life on earth. Free Radic. Biol. Med. 140, 61–73. doi: 10.1016/j.freeradbiomed.2019.03.004
Song, M., Garrett, W. S., and Chan, A. T. (2015). Nutrients, foods, and colorectal cancer prevention. Gastroenterology 148, 1244–1260.e16. doi: 10.1053/j.gastro.2014.12.035
Song, Y., and Yang, C. (2024). Mechanistic advances of hyperoxia-induced immature brain injury. Heliyon 10:e30005. doi: 10.1016/j.heliyon.2024.e30005
Sun, P., Wang, M., Liu, Y. X., Li, L., Chai, X., Zheng, W., et al. (2023). High-fat diet-disturbed gut microbiota-colonocyte interactions contribute to dysregulating peripheral tryptophan-kynurenine metabolism. Microbiome 11:154. doi: 10.1186/s40168-023-01606-x
Sundin, O. H., Mendoza-Ladd, A., Zeng, M., Diaz-Arévalo, D., Morales, E., Fagan, B. M., et al. (2017). The human jejunum has an endogenous microbiota that differs from those in the oral cavity and colon. BMC Microbiol. 17:160. doi: 10.1186/s12866-017-1059-6
Swinburn, B. A., Sacks, G., Hall, K. D., McPherson, K., Finegood, D. T., Moodie, M. L., et al. (2011). The global obesity pandemic: shaped by global drivers and local environments. Lancet 378, 804–814. doi: 10.1016/S0140-6736(11)60813-1
Taylor, C. T., and Colgan, S. P. (2017). Regulation of immunity and inflammation by hypoxia in immunological niches. Nat. Rev. Immunol. 17, 774–785. doi: 10.1038/nri.2017.103
Tigano, S., Caruso, A., Liotta, C., La Via, L., Vargas, M., Romagnoli, S., et al. (2023). Exposure to severe hyperoxemia worsens survival and neurological outcome in patients supported by veno-arterial extracorporeal membrane oxygenation: a meta-analysis. Resuscitation 194:110071. doi: 10.1016/j.resuscitation.2023.110071
Tipton, L., Darcy, J. L., and Hynson, N. A. (2019). A developing Symbiosis: enabling cross-talk between ecologists and microbiome scientists. Front. Microbiol. 10:292. doi: 10.3389/fmicb.2019.00292
Topping, D. L., and Clifton, P. M. (2001). Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 81, 1031–1064. doi: 10.1152/physrev.2001.81.3.1031
Trakman, G. L., Fehily, S., Basnayake, C., Hamilton, A. L., Russell, E., Wilson-O’Brien, A., et al. (2022). Diet and gut microbiome in gastrointestinal disease. J. Gastroenterol. Hepatol. 37, 237–245. doi: 10.1111/jgh.15728
Vieira, J., Frakes, M., Cohen, J., and Wilcox, S. (2020). Extracorporeal membrane oxygenation in transport part 1: extracorporeal membrane oxygenation configurations and physiology. Air Med. J. 39, 56–63. doi: 10.1016/j.amj.2019.09.008
Walker, J., Primmer, J., Searles, B. E., and Darling, E. M. (2007). The potential of accurate SvO2 monitoring during venovenous extracorporeal membrane oxygenation: an in vitro model using ultrasound dilution. Perfusion 22, 239–244. doi: 10.1177/0267659107083656
Wang, H. C., Chou, H. C., and Chen, C. M. (2023). Molecular mechanisms of Hyperoxia-induced neonatal intestinal injury. Int. J. Mol. Sci. 24:4366. doi: 10.3390/ijms24054366
Wang, T., Wang, R. X., and Colgan, S. P. (2024). Physiologic hypoxia in the intestinal mucosa: a central role for short-chain fatty acids. Am. J. Physiol. Cell Physiol. 327, C1087–C1093. doi: 10.1152/ajpcell.00472.2024
Wedgwood, S., Warford, C., Agvatisiri, S. R., Thai, P. N., Chiamvimonvat, N., Kalanetra, K. M., et al. (2020). The developing gut-lung axis: postnatal growth restriction, intestinal dysbiosis, and pulmonary hypertension in a rodent model. Pediatr. Res. 87, 472–479. doi: 10.1038/s41390-019-0578-2
Wemple, M. L., Swenson, K. E., and Swenson, E. R. (2023a). Oxygen therapy part 1 - history, physiology, and evaluation. NEJM Evid. 2:EVIDra2300005. doi: 10.1056/EVIDra2300005
Wemple, M. L., Swenson, K. E., and Swenson, E. R. (2023b). Oxygen therapy part 2 - indications and toxicity. NEJM Evid. 2:EVIDra2300111. doi: 10.1056/EVIDra2300111
Winiszewski, H., Guinot, P. G., Schmidt, M., Besch, G., Piton, G., Perrotti, A., et al. (2022). Optimizing PO(2) during peripheral veno-arterial ECMO: a narrative review. Crit. Care 26:226. doi: 10.1186/s13054-022-04102-0
Winiszewski, H., Piton, G., Perrotti, A., and Capellier, G. (2018). Hyperoxemia and Veno-arterial extracorporeal membrane oxygenation: do not forget the gut. Crit. Care Med. 46, e98–e99. doi: 10.1097/CCM.0000000000002750
Winiszewski, H., Vieille, T., Guinot, P. G., Nesseler, N., Le Berre, M., Crognier, L., et al. (2024). Oxygenation management during veno-arterial ECMO support for cardiogenic shock: a multicentric retrospective cohort study. Ann. Intensive Care 14:56. doi: 10.1186/s13613-024-01286-2
Winter, S. E., and Bäumler, A. J. (2023). Gut dysbiosis: ecological causes and causative effects on human disease. Proc. Natl. Acad. Sci. USA 120:e2316579120. doi: 10.1073/pnas.2316579120
Winter, S. E., Winter, M. G., Xavier, M. N., Thiennimitr, P., Poon, V., Keestra, A. M., et al. (2013). Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 339, 708–711. doi: 10.1126/science.1232467
Xie, Z., He, W., Gobbi, A., Bertram, H. C., and Nielsen, D. S. (2024). The effect of in vitro simulated colonic pH gradients on microbial activity and metabolite production using common prebiotics as substrates. BMC Microbiol. 24:83. doi: 10.1186/s12866-024-03235-2
Xing, Z., Li, Y., Liu, G., He, Y., Tao, Y., and Chen, M. (2020). Hyperoxia provokes gut dysbiosis in rats. Crit. Care 24:517. doi: 10.1186/s13054-020-03247-0
Xu, R., Tan, C., Zhu, J., Zeng, X., Gao, X., Wu, Q., et al. (2019). Dysbiosis of the intestinal microbiota in neurocritically ill patients and the risk for death. Crit. Care 23:195. doi: 10.1186/s13054-019-2488-4
Yamamura, R., Inoue, K. Y., Nishino, K., and Yamasaki, S. (2023). Intestinal and fecal pH in human health. Front. Microbiol. 2:1192316. doi: 10.3389/frmbi.2023.1192316
Yoo, W., Zieba, J. K., Foegeding, N. J., Torres, T. P., Shelton, C. D., Shealy, N. G., et al. (2021). High-fat diet-induced colonocyte dysfunction escalates microbiota-derived trimethylamine N-oxide. Science 373, 813–818. doi: 10.1126/science.aba3683
Zhang, Y., Zhang, J., and Duan, L. (2022). The role of microbiota-mitochondria crosstalk in pathogenesis and therapy of intestinal diseases. Pharmacol. Res. 186:106530. doi: 10.1016/j.phrs.2022.106530
Zheng, L., Kelly, C. J., and Colgan, S. P. (2015). Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A review in the theme: cellular responses to hypoxia. Am. J. Physiol. Cell Physiol. 309, C350–C360. doi: 10.1152/ajpcell.00191.2015
Zhu, Y., Chen, B., Zhang, X., Akbar, M. T., Wu, T., Zhang, Y., et al. (2024). Exploration of the Muribaculaceae family in the gut microbiota: diversity, metabolism, and function. Nutrients 16:2660. doi: 10.3390/nu16162660
Glossary
VA-ECMO - Venoarterial-extracorporeal membrane oxygenation
VV-ECMO - Venovenous-extracorporeal membrane oxygenation
CS - Cardiogenic shock
CA - Cardiac arrest
ATP - Adenosine triphosphate
PO2 - Oxygen partial pressure
PPOSTO2 - Post-oxygenator oxygen partial pressure
SCFA - Short chain fatty acid
ROS - Reactive oxygen species
SOD - Superoxide dismutase
Ga - Giga-annum
GOE - Great oxygenation event
FA - Facultative anaerobes
OA - Obligate anaerobes
NADH - Nicotinamide adenine dinucleotide
DO2 - O2 delivery
VO2 - O2 consumption
CO - Cardiac output
CaO2 - Arterial O2 content
MODS - Multiple organ dysfunction syndrome
SaO2 - O2 saturation
ARDS - Acute respiratory distress syndrome
FiO2 - Fraction of inspired O2
PAO2 - Partial pressure of alveolar O2
PvO2 - Venous O2 pressure
FbO2 - fraction of inspired O2 of blender
PPAR-γ - Peroxisome proliferator–activated receptor gamma
HIF-1α - Hypoxia-inducible factor 1-Alpha
Keywords: hyperoxia, gut dysbiosis, oxygen therapy, butyrate, reactive oxygen species, electron acceptors
Citation: Wu H, Zeng W, Dai N, Gu J, He Y, Qin H, Lin L, Fu X, Fu B and Xing Z (2025) Hyperoxia as a driver of gut dysbiosis. Front. Microbiol. 16:1675652. doi: 10.3389/fmicb.2025.1675652
Edited by:
Paula Maria Tribelli, National Scientific and Technical Research Council (CONICET), ArgentinaReviewed by:
Jose Luis Fachi, Washington University in St. Louis, United StatesAndrea Millan, University of Buenos Aires, Argentina
María Constanza Pautasso, University of Buenos Aires, Argentina
Copyright © 2025 Wu, Zeng, Dai, Gu, He, Qin, Lin, Fu, Fu and Xing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying He, MTU2NzE3NjA5QHFxLmNvbQ==; Xiaoyun Fu, enl5eHlmeHlAMTYzLmNvbQ==; Bao Fu, ZnViYW8wNjA3QDEyNi5jb20=; Zhouxiong Xing, eGluZ3pob3V4aW9uZzExMUAxMjYuY29t
†These authors have contributed equally to this work
 Hang Wu
Hang Wu Wenxia Zeng1†
Wenxia Zeng1† Ninan Dai
Ninan Dai Juan Gu
Juan Gu Ying He
Ying He Xiaoyun Fu
Xiaoyun Fu Bao Fu
Bao Fu Zhouxiong Xing
Zhouxiong Xing