- 1College of Tourism and Landscape Architecture, Guilin University of Science and Technology, Guilin, China
- 2College of Plant and Ecological Engineering, Guilin University of Technology, Guilin, China
- 3Guangxi Key Laboratory of Plant Conservation and Restoration Ecology in Karst Terrain, Guangxi Institute of Botany, Guangxi Zhuang Autonomous Region and Chinese Academy of Sciences, Guilin, China
- 4Nonggang Karst Ecosystem Observation and Research Station of Guangxi, Chongzuo, China
Introduction: Karst ecosystems are highly susceptible to degradation due to their inherent fragility and poor soil conditions. Soil microorganisms play a crucial role in nutrient cycling and ecosystem recovery. While biochar application has been shown to enhance microbial activity, its interaction with mowing–a common grassland management practice-and whether such effects vary with soil type remain unclear.
Methods: A 1-year mesocosm experiment was conducted using red and calcareous soils from southwest China, with four treatments: control (CK), biochar (B), mowing (M), and combined biochar–mowing (BM). Highthroughput sequencing was used to assess microbial abundance, alpha diversity, and community structure.
Results: We found that the individual and combined effects of biochar and mowing on soil microbial communities differed significantly between soil types. Biochar-only treatment consistently increased bacterial and fungal abundance and richness in both soil types. However, significant increases in fungal diversity, evenness and bacterial simpson were observed only in red soil. Mowing enhanced microbial abundance, richness, and diversity in red soil but had no significant effect in calcareous soil. The highest microbial abundance and richness under the combined BM treatment in red soil suggest a potential synergistic effect between biochar and mowing. Biochar significantly increased the relative abundances of dominant bacterial phyla-Proteobacteria and Actinobacteria–while decreasing Chloroflexi in red soil, with minimal changes observed in calcareous soil. Similarly, it elevated the relative abundances of fungal phyla Ascomycota and Basidiomycota, but reduced Chytridiomyota in red soil, whereas calcareous soil showed less pronounced shifts. Strong correlations were observed between soil properties and microbial community structure, particularly in red soil.
Conclusion: These findings indicate that biochar and mowing can jointly improve soil microbial communities, offering potential for restoring degraded karst grasslands. However, their effectiveness is strongly mediated by parent soil type.
1 Introduction
Karst ecosystems are prone to severe degradation (rocky desertification), due to their fragile geological structure and hydrological cycles (Gutiérrez et al., 2014), leading to reduced soil productivity, biodiversity loss, weakened carbon sequestration, and diminished ecosystem services, with significant environmental and socioeconomic impacts (Jiang et al., 2014). Consequently, ecological restoration measures such as forage cultivation and soil amendment are commonly adopted for mitigation (Zhou et al., 2020; Zhang et al., 2021). Biochar is widely utilized as a soil amendment for karst due to its porous structure and surface functional groups (Guo et al., 2024). Besides, mowing represents a prevalent management practice during forage cultivation system (Blüthgen et al., 2012), yet its individual and interactive effects with biochar on soil properties and biota in karst ecosystems remain poorly constrained. Furthermore, given the critical role of soil microorganisms in sustaining ecological functions (Bardgett and van der Putten, 2014), their acute sensitivity to environmental changes makes community structure, composition, and diversity vital indicators of soil health (Fierer et al., 2021). Consequently, there is growing scientific focus on whether and how anthropogenic management practices (e.g., biochar application and mowing) influence soil microbial community structure (Thakur and Geisen, 2019).
It is noteworthy that both biochar application and mowing management can alter soil-related variables, thereby modifying microbial diversity, abundance, and composition (Li et al., 2023). Biochar possesses a high aromatic carbon content, extensive porosity, and substantial surface area, which collectively improve soil aeration, water-holding capacity, and nutrient retention. Simultaneously, it influences microbial communities through mechanisms including provision of microbial habitats, adsorption of toxic substances, and modulation of soil pH and nutrient status (Lehmann et al., 2011). Studies indicate that biochar amendment significantly restructures soil bacterial and fungal community composition while enhancing the relative abundance of dominant microbial taxa (Lei et al., 2023). Mowing is a non-destructive practice that removes surface vegetation, thereby reducing root exudate production and altering litter quantity, which leads to a decline in soil organic matter input (Gilmullina et al., 2020). Concurrently, the reduction in vegetation coverage modifies light penetration, resulting in increased soil evaporation that affects soil temperature and moisture content. These alterations further shift aboveground-belowground carbon allocation patterns (Miao et al., 2020). Consequently, mowing induces structural shifts in soil microbial communities: bacterial communities remain largely unaffected, whereas a significant increase in fungal relative abundance is consistently documented (Li et al., 2020).
Given that both interventions alter the composition and diversity of soil microbial, and considering the crucial role of microbial diversity in maintaining soil functions stability, increasing microbial diversity has become a key strategy for rehabilitating degraded ecosystems (Delgado-Baquerizo et al., 2016). Divergent effects of biochar and mowing on soil microbial communities, in both magnitude and direction, likely arise from variations in background soil conditions (Wang et al., 2025). In acidic soils, biochar application can neutralize soil acidity and provide a stable carbon (Arwenyo et al., 2023), alleviate aluminum toxicity, and thereby likely enhance microbial activity to improve the overall health of acidic soils by promoting microbial metabolic processes (Xia et al., 2023). Conversely, in alkaline soils that naturally contain higher nutrient levels, the effectiveness of biochar may be markedly diminished, with its influence on soil microbial communities being relatively minimal (Zhou et al., 2019). However, the effects of mowing were not directly mediated by soil pH but rather indirectly through alterations in soil moisture, and nutrient availability, which subsequently enhanced specific microbial diversity indices by modifying microbial biomass. It may also be subject to the influences of aboveground biomass and diversity, which could potentially lead to variations in microbial biomass (Song et al., 2020).
The soil types in this karst area are mainly red and calcareous soils, both of which are facing serious degradation problems (Zhong et al., 2022). Red soil is the product of intense biological enrichment and subsequent desilication–aluminization weathering processes under the humid subtropical bioclimatic conditions, characterized by low pH, heavy texture, and high exchangeable ion content (Yang et al., 2017). In contrast, calcareous soil develops on carbonate rock weathering materials in tropical and subtropical regions, featuring neutral pH, strong weathering and leaching effects, and low degrees of calcium depletion (Yan et al., 2022). We report the results of a 1–year mesocosm experiment investigating the effects of biochar application, mowing, and their interactions on soil microbial diversity, abundance, composition, and soil nutrient status across different soil parent materials in a degraded karst region, aiming to enhance soil nutrient availability and improve microbial community structure. We hypothesized that (1) the application of biochar and mowing in karst grassland would change the soil microbial diversity, abundance and composition; (2) different types of parent soil respond differently to relevant management measures and have a certain degree of soil dependence; (3) changes in some soil variables can lead to alterations in microbial diversity, community structure and composition.
2 Materials and methods
2.1 Study site
The experiment was conducted in a greenhouse in Guilin City, located in the karst region of southwest China’s Guangxi Zhuang Autonomous Region (coordinates: 24°15′2′′–26°23′30′′N, 109°36′5′′–111°29′3′′ E). This region experiences a subtropical monsoon climate, with an average annual temperature of approximately 19.1 °C. The lowest monthly mean temperature occurs in January (8 °C), while the highest occurs in July (28 °C). Mean annual precipitation ranges from 1160 to 1378 mm, with a distinct seasonal pattern. The study area is mountainous, interspersed with both karst and non-karst regions. Two parent material soil types were used: iron–rich red soil (pH 5.2–6.6) and calcareous soil (pH 7.8–8.2), which were collected as topsoil from nearby karst grasslands, respectively. The soils were air-dried and homogenized in preparation for potting experiments.
2.2 Experimental design and sample collection
We designed a mesocosm experiment to investigate the effects of biochar application and mowing on the soil microbial community structure in two soil types of karst grasslands on March 1, 2022. The experiment was conducted as a randomized block design, with four blocks (replicates) (n = 4) arranged across the greenhouse space. Each block contained all treatment combinations (2 soil types × 2 biochar levels × 2 mowing regimes = 8 pots per block). Within each block, pot positions were randomly assigned using a random number generator in Excel to ensure unbiased treatment distribution. To minimize the influence of environmental gradients–such as light intensity, temperature, and humidity–blocks were arranged along both east–west and north–south axes, which are known to affect microclimatic conditions in greenhouse settings. Additionally, all pots were rotated weekly within their respective blocks to further homogenize exposure to light and temperature and reduce positional bias.
A total of 32 pots were used, containing two types of soil. The initial physicochemical properties of the soils prior to the experiment are presented in Supplementary Table 1. Each pot has an upper diameter of 30 cm, a bottom diameter of 23 cm, and a height of 23 cm, yielding a volume of approximately 8364 cm3. Each pot was filled with about 8 kg of soil. Biochar was produced by pyrolyzing corn stalks at 500 °C and applied to the soil at a rate of 10% (w/w; 100 g kg−1) prior to planting. This corresponded to an average of 0.8 kg of biochar per pot, based on the dry soil weight. The biochar charaterization are presented in Supplementary Table 2. Each pot was then planted with 20 tall fescue plants (Festuca arundinacea Scherb.), a C4 grass adapted to the climate of Guilin. The plants were watered daily to maintain soil moisture at 70%–80% of the water-holding capacity. Regular manual weeding was carried out to control weeds, prevent resource competition, and maintain consistent experimental conditions. No fertilization or liming was applied to the potted plants in order to isolate the effects of biochar as the controlled variable.
On March 15, 2023 (1–year post–planting), we carried out the mowing treatment, prior to the treatment, foliar height was measured and allometric equations was employed to estimate height–biomass relationships (Chave et al., 2014). The allometric equations are as follow:
B = 0.0993 × H0.9274 (R2 = 0.6628)
Among them:
H = Plant height
B = Aboveground biomass
To achieve 50% removal of aboveground biomass, specific height–based excisions were performed on plants. Mean canopy heights were recorded as 10 cm (non–biochar) versus 20 cm (biochar–amended) in red soils, and 18 cm (non–biochar) versus 25 cm (biochar–amended) in Calcareous soils. Excised plant material was systematically removed from experimental plots.
Full harvest of residual plant material occurred on March 30, 2023, followed by desiccation at 65 °C for 48 h with subsequent quantification of aboveground and belowground biomass. Soil cores (0–15 cm depth) were collected from each pot and sieved (2 mm mesh) to eliminate rock fragments and root residues. The soil samples were bifurcated into two subsamples: Air-dried specimens were homogenized through 0.15 mm mesh sieving; Field–moist specimens were immediately transported to laboratory facilities under refrigerated preservation (4 °C). Prior to analysis, field-moist soils were re-sieved (2 mm mesh) and homogenized through thorough mixing. Air-dried soil specimens were subjected to quantitative analyses for pH, soil organic carbon (SOC), total nitrogen (TN), total phosphorus (TP), cation exchange capacity (CEC), exchangeable calcium (ECa), and exchangeable magnesium (EMg). Concurrently, field-moist soil samples were employed for the determination of microbial biomass carbon (MBC), ammonium (NH4+), and nitrate (NO3–) concentrations, with analyses conducted immediately following sample collection to preserve biochemical integrity.
2.3 Chemical analysis of the soil samples
The physicochemical properties of the soil samples were analyzed through standardized laboratory procedures. Soil pH was determined via potentiometric analysis using a calibrated pH meter. Gravimetric analysis was performed to quantify soil water content (SWC) by measuring mass loss after oven-drying at 105 °C to constant weight. Soil organic carbon (SOC) content was analyzed using the potassium dichromate oxidation method with external heating in concentrated sulfuric acid medium. Total nitrogen (TN) concentrations were determined through sulfuric acid-catalyzed digestion followed by automated flow injection analysis (AA3 system). For total phosphorus (TP) measurement, samples underwent sulfuric-perchloric acid digestion and subsequent quantification using molybdenum-antimony anti-spectrophotometry. Microbial biomass carbon (MBC) was assessed through chloroform fumigation-extraction methodology with K2SO4 solution. The available N (NH4+ and NO3–) were extracted using KCl solution and analyzed by continuous flow analytical techniques. Cation exchange capacity (CEC) was determined through ammonium acetate saturation followed by flame photometric detection of displaced cations. Complexed iron (CoFe) fractions were extracted using sodium pyrophosphate solution and quantified spectrophotometrically. Exchangeable base cations (Ca2+ and Mg2+) were determined through sequential extraction with ammonium acetate followed by atomic absorption spectroscopic analysis.
2.4 Microbial high-throughput sequencing
Microbial community analysis was conducted using the following standardized high–throughput sequencing pipeline: Bacterial 16S rRNA gene fragments were amplified using primers 338F (5′–ACTCCTACGGGAGGCAGCAG–3′) and 806R (5′–GGACTACHVGGGTWTCTAAT–3′), while fungal ITS regions were amplified with primers ITS1F (5′–CTTGGTCATTTAGAGGAAGTAA–3′) and ITS2R (5′–GCTGCGTTCTTCATCGATGC–3′). Sequencing was performed by Shanghai Majorbio Biomedical Technology Co., Ltd. on an Illumina NovaSeq platform. Sequencing depth is 50,000 reads per sample; the dilution threshold is 40,000 reads per sample. Raw paired-end reads were first quality-controlled using fastp (v0.19.6). Overlapping reads were then assembled using FLASH (v1.2.11). Quality-filtered sequences were clustered into Operational Taxonomic Units (OTUs) at a 97% similarity threshold via UPARSE (v11), with chimeras removed using the UCHIME algorithm. To mitigate sequencing depth bias in α - diversity analyses, all samples were rarefied, maintaining an average Good’s coverage of 99.09%. Taxonomic annotation of OTUs was performed against the SILVA 16S rRNA database (v138) using the RDP Classifier (v2.13) with a 70% confidence threshold, enabling hierarchical compositional profiling from phylum to genus levels. The data presented in the study are deposited in the NCBI Sequence Read Archive (SRA) repository, accession number PRJNA1338870.
2.5 Statistical analysis
Microbial α–diversity was calculated using mother (version v.1.30.1). This includes richness indices (Chao1, ACE), diversity indices (Shannon, Simpson), and evenness index (Pielou).
Chao1 Index Formula:
Among them:
Schao1 = The estimated number of OTUs
Sobs = The actual number of OTUs observed
n1 = The number of OTUs containing only one sequence (such as “singletons”)
n2 = The number of OTUs containing only two sequences (such as “doubletons”)
ACE Index Formula:
Among them:
ni = The number of OTUs containing a sequence
Srare = The number of OTUs containing “abund” sequences or less than “abund”
Sabund = More than the number of OTUs in the “abund” sequence
abund = The threshold for “Advantage “OTU is set to 10 by default
Shannon Index Formula:
Among them:
Sobs = The actual number of OTUs observed
ni = The number of sequences contained in the i-th OTU
N = All the sequence numbers
Simpson Index Formula:
Among them:
Sobs = The actual number of OTUs observed
ni = The number of sequences contained in the i-th OTU
N = All the sequence numbers
Pielou Index Formula:
Among them:
H = Shannon Index
S = The number of species in the ecological community(sobs)
ln = The logarithm to the base “e”
A three-way analysis of variance (ANOVA) was conducted using a general linear model (GLM) to examine the effects of soil type, biochar application, and mowing on soil microbial α-diversity indices (OTUs, Chao1, ACE, Shannon, Simpson and Pielou). Soil type, biochar, and mowing were treated as fixed effects, while block was included as a random effect to account for spatial heterogeneity. A significant interaction indicates that the effects of biochar and/or mowing on microbial communities depend on soil type. To further investigate whether soil type modulates the impacts of biochar and mowing across different microbial community metrics, two-way ANOVAs were performed separately for each soil type. Tukey’s post hoc tests were used to identify significant differences among treatment combinations within each soil type. Each treatment combination was replicated four times (n = 4), with one pot per replicate. All analyses were conducted in SPSS (Version 27.0), with statistical significance set at p < 0.05. The composition of microbial communities, redundancy analysis (RDA), and Pearson correlation analysis were conducted using relevant packages in R (version 4.5.0) and Origin (version 2024), with significance assessed at p < 0.05. All data are presented as mean ± standard error (SE), unless otherwise specified. The assumptions of normality and homogeneity of variances were evaluated using residual plots; data were log-transformed when necessary to meet model assumptions.
3 Results
3.1 Microbial α diversity
Three-way ANOVA indicated that soil type (ST), biochar application (B), and mowing management (M) significantly influenced the diversity index of bacterial and fungal communities (Table 1). Among them, soil type was the most significant driving factor, exerting a highly significant impact on all measured diversity indices, including OTUs, Chao1, ACE, Shannon, Simpson, and Pielou (p < 0.001). Biochar significantly affected OTUs, Chao1, and ACE of both bacteria and fungi (p < 0.001). Mowing significantly influenced the richness of bacteria (OTUs: p < 0.01; Chao1: p = 0.001; ACE: p < 0.01) and strongly affected fungal diversity and evenness (Shannon: p < 0.001; Simpson: p < 0.001; Pielou: p < 0.001), with a marginally significant effect on bacterial Shannon index (p = 0.054). Significant two-way interactions included: ST × B affecting the simpson of both bacterial and fungal communities and fungal richness (p < 0.05), ST × M significantly affecting all diversity indices of both communities (p < 0.01), and B × M significantly shaping bacterial richness (OTUs, Chao1, ACE; p < 0.05) and fungal diversity (Shannon: p = 0.001; Simpson: p = 0.005; Pielou: p < 0.001). Notably, the three-way interaction (ST × B × M) was significant only in terms of fungal diversity and evenness (Shannon: p = 0.004; Simpson: p = 0.006; Pielou: p < 0.001).

Table 1. Summary of three-way ANOVA analysing the effects of soil types (ST), biochar (B), mowing (M) and their interactive effects on soil microbial OTUs, Chao 1, ACE, Shannon, Simpson and Peilou, and using ST, B, M and their interaction as fixed terms.
The two-factor variance analysis conducted within each soil type revealed soil-specific responses to management measures (Table 2). In red soil, biochar significantly affected bacterial OTUs (p < 0.01), Chao1 (p < 0.001), ACE (p < 0.001), and Simpson (p < 0.01), as well as fungal OTUs, Chao1, and ACE (p < 0.001). Mowing significantly enhanced bacterial richness (OTUs, Chao1, ACE; p < 0.01) and diversity (Shannon: p < 0.05), and significantly improved fungal all measured diversity indices, including OTUs, Chao1, ACE, Shannon, Simpson, and Pielou (p < 0.001). The B × M interaction was significant for fungal Shannon (p < 0.01), Simpson (p < 0.05), and Pielou (p = 0.001). In contrast, in calcareous soil, biochar significantly increased bacterial OTUs (p = 0.001), Chao1 (p = 0.001) ACE (p = 0.001), and shannon (p < 0.05), and improved fungal OTUs (p = 0.001), Chao1 (p = 0.001), ACE (p = 0.001) and evenness (Pielou: p < 0.01). Mowing had no significant effect on all bacterial indicators, with only marginally significant improvement in fungal Shannon (p < 0.05).

Table 2. Summary of two-way ANOVA analysing the effects of biochar (B), mowing (M) and their interactive effects on soil microbial OTUs, Chao 1, ACE, Shannon, Simpson and Peilou in red soil (RS) and calcareous soil (CS), and using T, N, G and their interaction as fixed terms.
In red soil, both biochar (B) and mowing (M), either alone or in combination (BM) treatments, significantly increased bacterial richness indices (OTUs, Chao1, ACE), with the greatest enhancement observed under the combined BM treatment. The M and BM treatments also significantly increased the Shannon index, while the Simpson index was significantly elevated by biochar alone. For fungal diversity, all indices–including OTUs, Chao1, ACE, Shannon, Simpson, and Pielou–were significantly increased by each treatment, with B, M, and BM all effective, and the BM treatment showing the strongest effect on richness (OTUs, Chao1, ACE). In contrast, responses in calcareous soil were more limited. Bacterial richness (OTUs, Chao1, ACE) increased significantly only under M and BM treatments, with no significant effect from B alone. For fungi, only the B and BM treatments significantly enhanced richness indices, while mowing alone had no significant impact (Figures 1, 2).

Figure 1. Effect of mowing and biochar on soil bacterial communities OTUs (A), Chao1 (B), ACE (C), Shannon (D), Simpson (E) and Pielou (F) in red soil (RS) and calcareous soil (CS). Values represent mean ± SE (n = 4). Different uppercase letters indicate significant differences among treatments in calcareous soil, different lowercase letters indicate significant differences among treatments in red soil (p≤0.05).
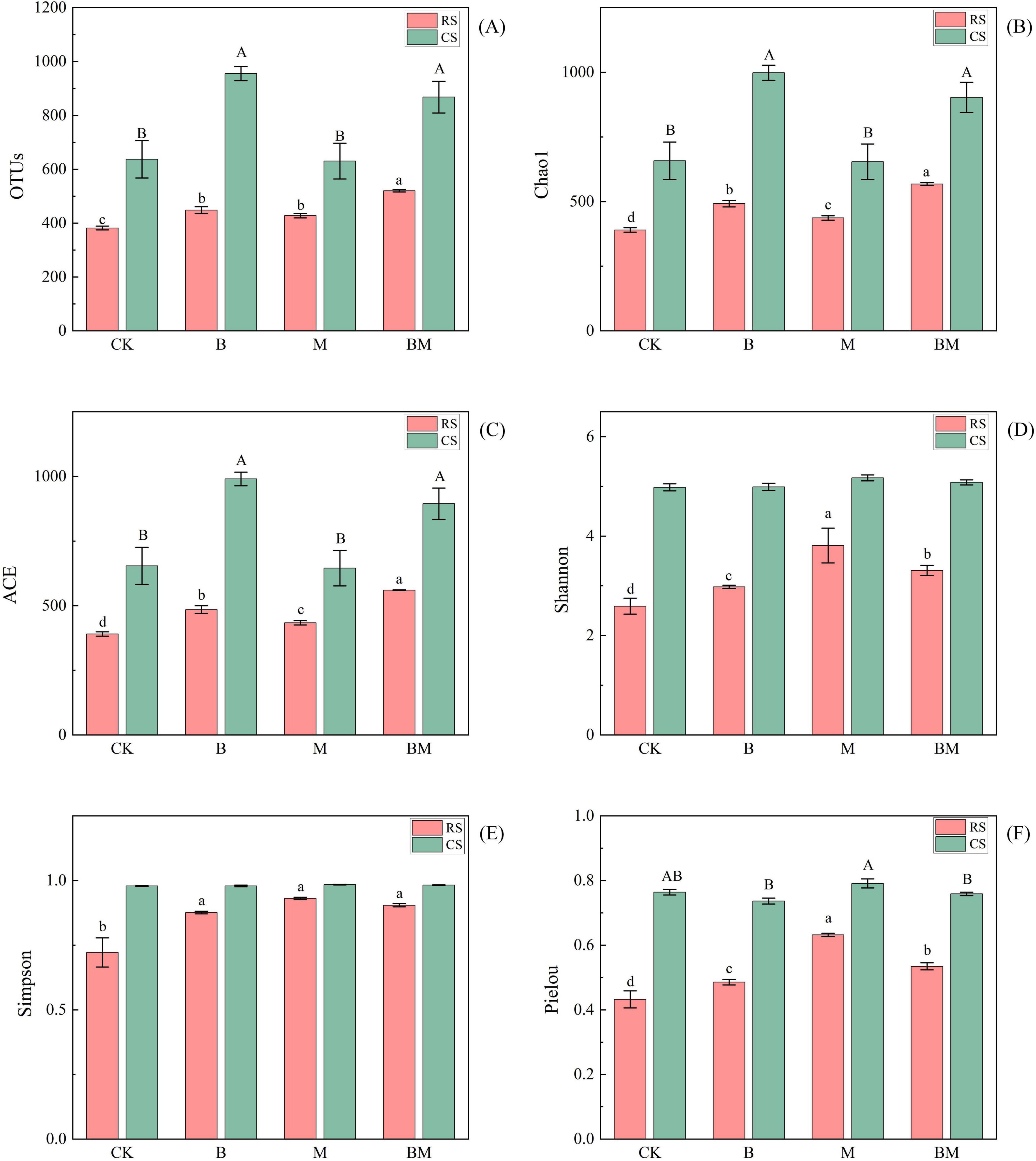
Figure 2. Effect of mowing and biochar on soil fungi communities OTUs (A), Chao1 (B), ACE (C), Shannon (D), Simpson (E) and Pielou (F) in red soil (RS) and calcareous soil (CS). Values represent mean ± SE (n = 4). Different uppercase letters indicate significant differences among treatments in calcareous soil, different lowercase letters indicate significant differences among treatments in red soil (p≤0.05).
3.2 Microbial community structure and composition
At the phylum level of bacterial communities in red soil, Proteobacteria, Actinobacteria, and Chloroflexi were the three most dominant taxa (Figure 3A). Following biochar application, the relative abundances of Proteobacteria and Actinobacteria were significantly increased, while that of Chloroflexi was notably reduced. In contrast, in calcareous soil, Actinobacteria, Proteobacteria, and Acidobacteria exhibited the highest relative abundances (Figure 3B), yet these phyla showed minimal shifts post-biochar treatment. Consequently, biochar exerted more pronounced effects on bacterial community structure in red soil compared to calcareous soil. Under mowing management, neither red nor calcareous soils displayed significant alterations in the relative abundances of dominant bacterial phyla, indicating structural stability. When biochar and mowing were combined, red soil exhibited a marked increase in Proteobacteria abundance alongside a significant decline in Chloroflexi. In calcareous soil, Acidobacteria abundance was moderately reduced under this combined treatment.

Figure 3. The relative abundance at the phylum level of bacterial communities. (A) Red soil (B) Calcareous soil. RS: Red soil; CS: Calcareous soil; CK: Control; B: Biochar treatment; M: Mowing treatment; BM: Biochar and Mowing treatment.
In red soil fungal communities, Ascomycota, Basidiomycota, and Chytridiomycota dominated at the phylum level (Figure 4A). Biochar application significantly elevated the abundances of Ascomycota and Basidiomycota but reduced Chytridiomycota. Mowing, however, induced a contrasting response: Ascomycota decreased, while Basidiomycota and Chytridiomycota increased significantly. In calcareous soil, Ascomycota, unclassified_Fungi, and Basidiomycota were predominant (Figure 4B). These taxa remained largely unresponsive to biochar, underscoring the limited influence of biochar on fungal communities in calcareous soil compared to red soil. Mowing in calcareous soil triggered a modest but significant rise in Basidiomycota, though less pronounced than in red soil. Combined treatment in red soil reduced Chytridiomycota abundance, whereas calcareous soil saw increased Basidiomycota dominance.
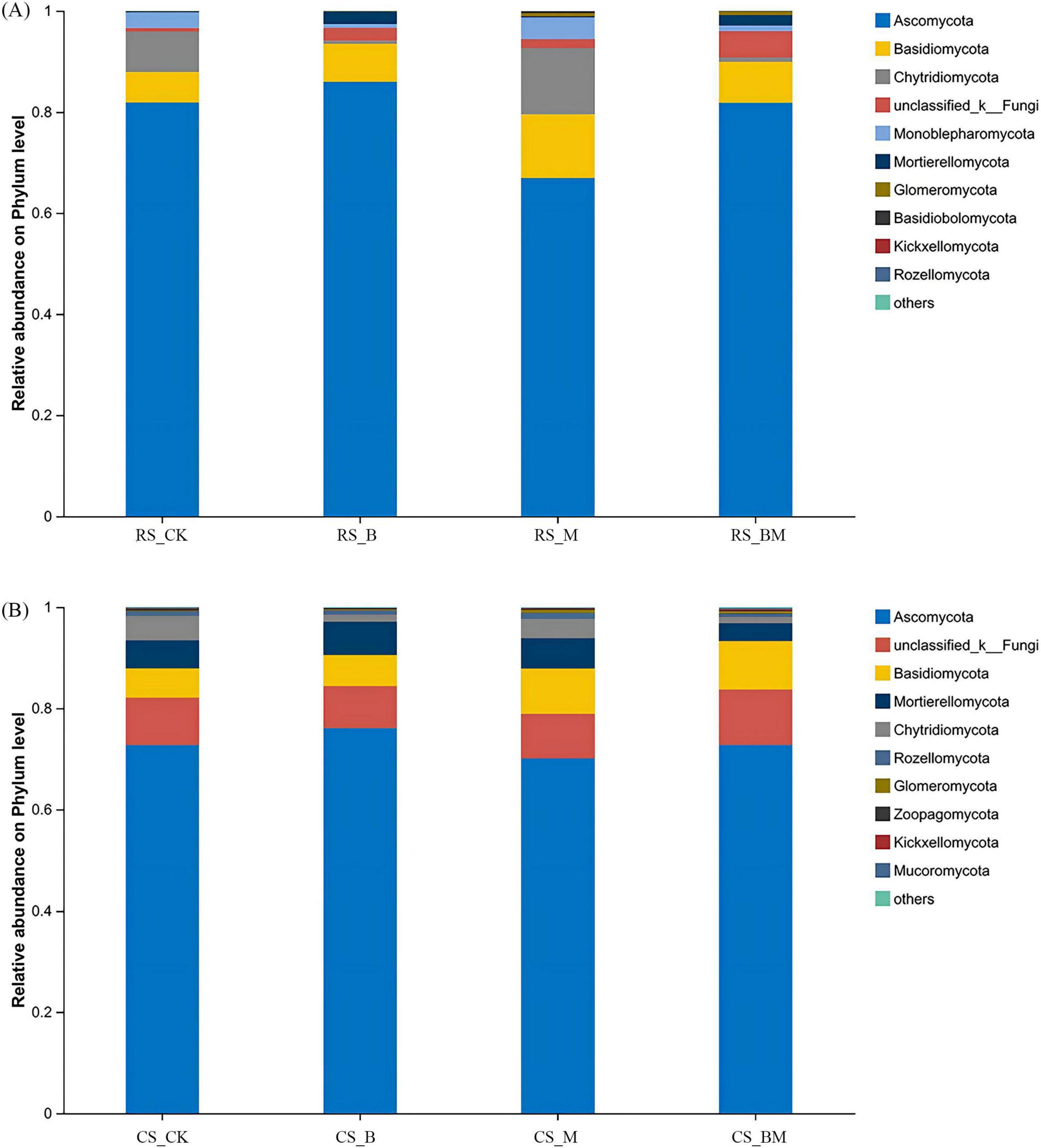
Figure 4. The relative abundance at the phylum level of fungal communities. (A) Red soil (B) Calcareous soil. RS: Red soil; CS: Calcareous soil; CK: Control; B: Biochar treatment; M: Mowing treatment; BM: Biochar and Mowing treatment.
3.3 Relationship between microbial community structure and soil physical and chemical properties
Redundancy analysis (RDA) unveiled the covariation patterns between soil samples and environmental factors regulating bacterial and fungal community assembly, which significantly differed across soil types. The first two RDA axes explained 85.93% (Figure 5A) and 59.87% (Figure 5B) of the total variation in soil bacterial community structure. For fungal communities, the corresponding variations on the primary ordination axes were 67.78% (Figure 6A) and 59.17% (Figure 6B). This variance partitioning highlighted the differential dominance of environmental factors in microbial community assembly, with red soil exhibiting a stronger environmental filtering effect than calcareous soil.
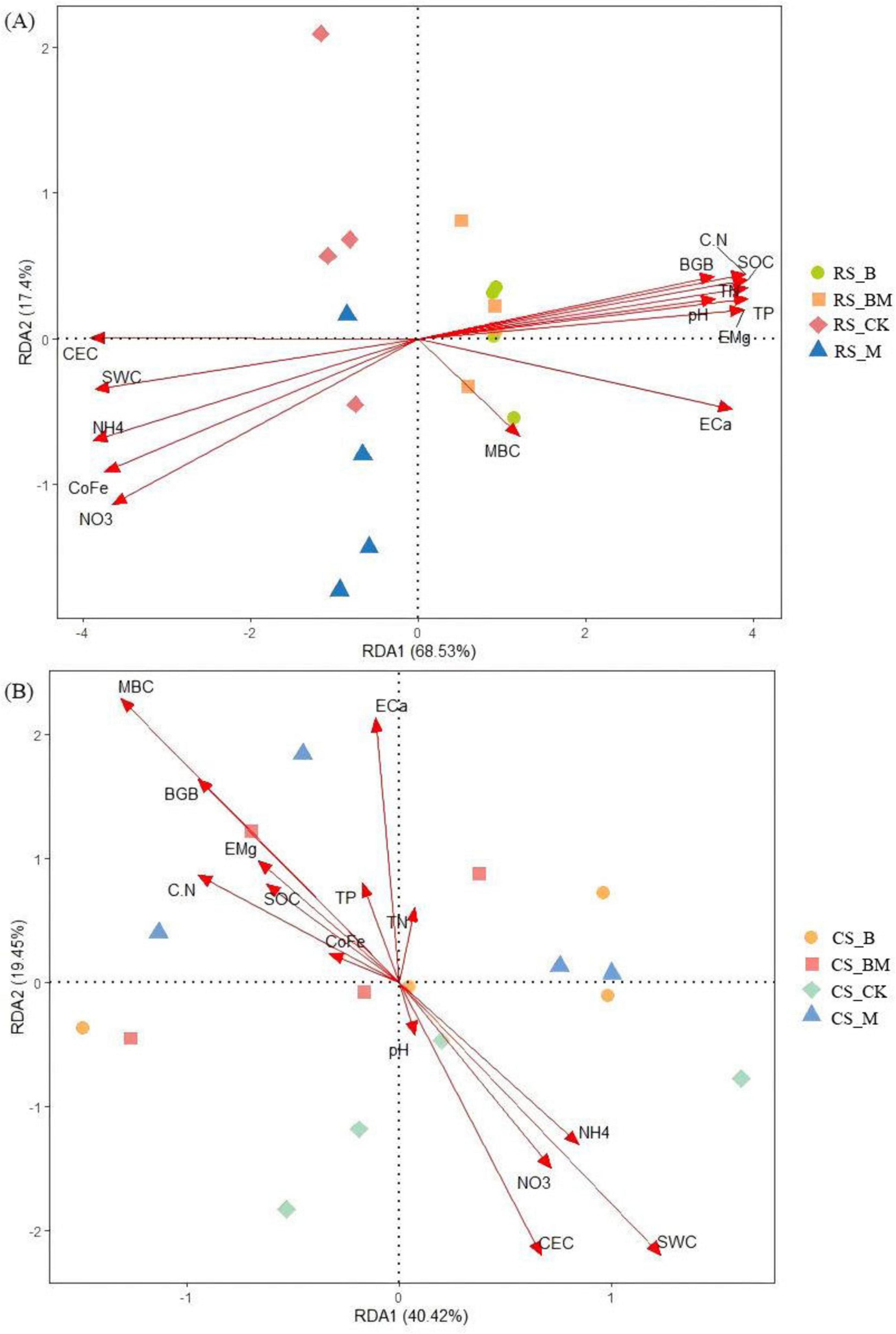
Figure 5. RDA analysis of the relationship between bacteria relative abundance at the phylum level and the soil physicochemical properties. (A) Red soil (B) Calcareous soil. RS: Red soil; CS: Calcareous soil; CK: Control; CK: Control; B: Biochar treatment; M: Mowing treatment; BM: Biochar and Mowing treatment; SWC: soil water content; SOC: soil organic carbon; TN: total nitrogen; TP: total phosphorus; C/N: C/N ratio; MBC: microbial biomass carbon; NH4+: ammonium nitrogen; NO3–: nitrate nitrogen; BGB: belowground biomass; CEC: cation exchange capacity; CoFe: complex iron; ECa: exchangeable calcium; EMg: exchangeable magnesium.

Figure 6. RDA analysis of the relationship between fungi relative abundance at the phylum level and the soil physicochemical properties. (A) Red soil (B) Calcareous soil. RS: Red soil; CS: Calcareous soil; CK: Control; B: Biochar treatment; M: Mowing treatment; BM: Biochar and Mowing treatment; SWC: soil water content; SOC: soil organic carbon; TN: total nitrogen; TP: total phosphorus; C/N: C/N ratio; MBC: microbial biomass carbon; NH4+: ammonium nitrogen; NO3–: nitrate nitrogen; BGB: belowground biomass; CEC: cation exchange capacity; CoFe: complex iron; ECa: exchangeable calcium; EMg: exchangeable magnesium.
Pearson correlation analysis delineated statistically significant, specific associations between microbial communities, α diversity indices, and key environmental factors, uncovering niche differentiation patterns among microbial lineages. The correlations between microbial communities and environmental factors were more pronounced in red soil (p < 0.05). In bacterial communities, Proteobacteria, Chloroflexi, and Firmicutes showed significant positive and negative correlations with most environmental factors. Notably, Chloroflexi and Firmicutes exhibited opposite correlation patterns (Figure 7A). Within fungal communities, Mortierellomycota and Monoblepharomycota demonstrated significant correlations with most environmental factors (Figure 8A). Regarding α diversity, the correlations with environmental factors were more significant in calcareous soil (p < 0.05). The Shannon index of bacteria was significantly positively correlated with SOC, TN, TP, and C/N (Figure 7B). For fungi, ACE, Chao1, and OTUs exhibited significant positive correlations with SOC, TN, TP, and C/N (Figure 8B).
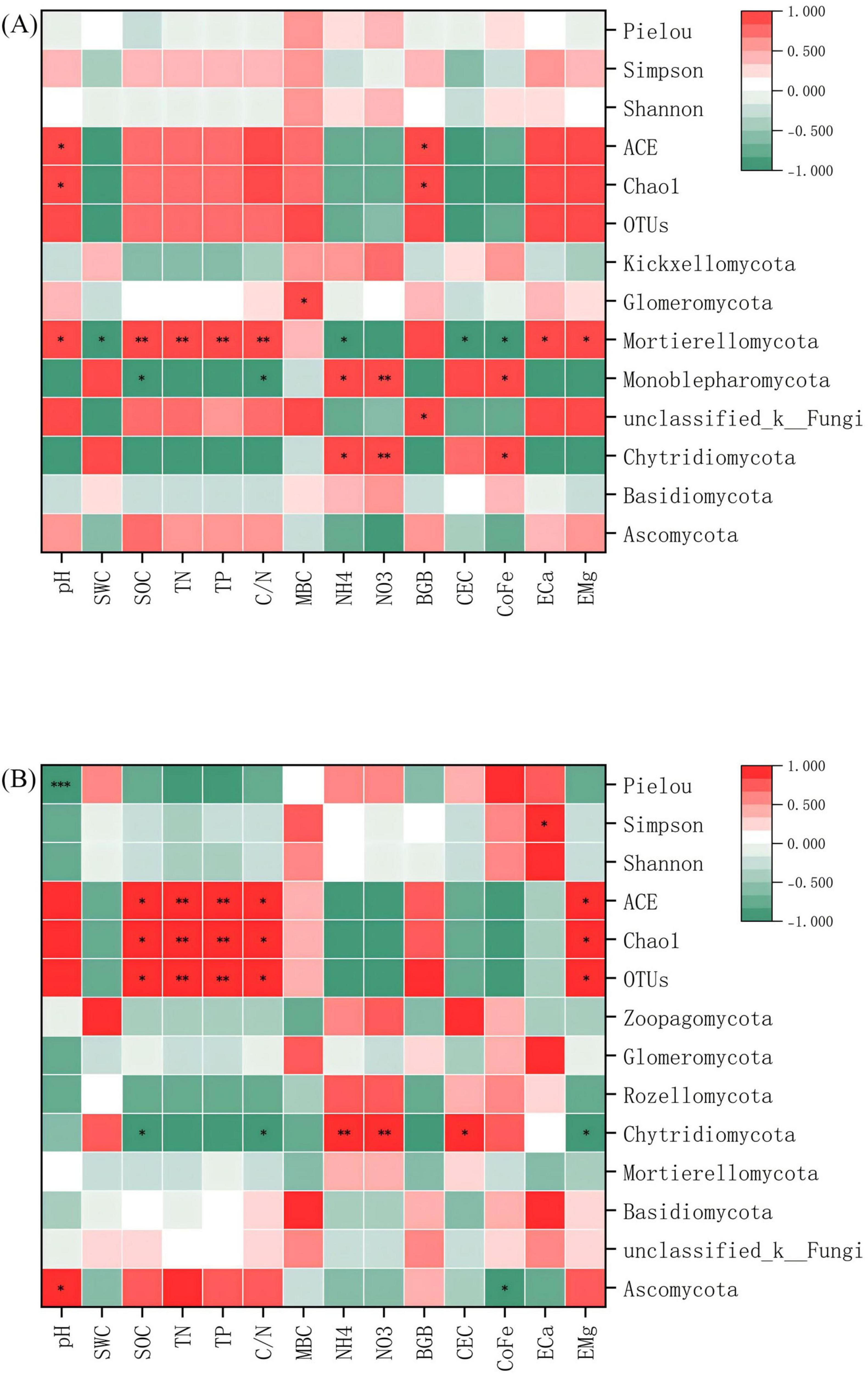
Figure 7. Pearson correlation heat map of bacterial communities, α -diversity and environmental factors at the phylum level. (A) Red soil (B) Calcareous soil. “*”, “**”and “***” are statistically significant at P < 0.05 and P < 0.01 and P < 0.001, respectively. SWC: soil water content; SOC: soil organic carbon; TN: total nitrogen; TP: total phosphorus; C/N: C/N ratio; MBC: microbial biomass carbon; NH4+: ammonium nitrogen; NO3–: nitrate nitrogen; BGB, belowground biomass; CEC, cation exchange capacity; CoFe, complex iron; ECa, exchangeable calcium; EMg, exchangeable magnesium.

Figure 8. Pearson correlation heat map of fungi communities, α -diversity and environmental factors at the phylum level. (A) Red soil (B) Calcareous soil. “*”, “**”and “***” are statistically significant at P < 0.05 and P < 0.01 and P < 0.001, respectively. SWC, soil water content; SOC, soil organic carbon; TN, total nitrogen; TP, total phosphorus; C/N, C/N ratio; MBC, microbial biomass carbon; NH4+, ammonium nitrogen; NO3–, nitrate nitrogen; BGB, belowground biomass; CEC, cation exchange capacity; CoFe, complex iron; ECa, exchangeable calcium; EMg, exchangeable magnesium.
3.4 Physical and chemical properties of soil
Compared to the control (CK), biochar application significantly increased soil pH, organic carbon (SOC), total nitrogen (TN), total phosphorus (TP), C/N ratio, grass biomass (BGB), electrical conductivity (ECa), and exchangeable Mg (EMg) in red soil, while decreasing soil water content (SWC), NH4+-N, NO3–-N, cation exchange capacity (CEC), and Co-Fe content (CoFe). In calcareous soil, biochar induced similar changes across most variables, except that ECa remained unchanged. Mowing alone increased NH4+-N, NO3–-N, CoFe, ECa, and EMg in red soil, but reduced SWC and CEC. In contrast, in calcareous soil, mowing increased microbial biomass carbon (MBC), BGB, and ECa, while decreasing pH, NO3–-N, and CEC.
Under the combined biochar and mowing (BM) treatment, red soil showed significant increases in pH, SOC, TN, TP, C/N, MBC, BGB, ECa, and EMg, accompanied by decreases in SWC, NH4+-N, NO3–-N, CEC, and CoFe. In calcareous soil, the BM treatment elicited a largely similar response pattern, with the exception that pH and SWC did not differ significantly from the control (Supplementary Figure 1).
4 Discussion
4.1 The interactive effect of biochar and soil types on microbial diversity
The addition of biochar consistently enhanced microbial abundance and richness across both red and calcareous soils, corroborating numerous previous studies (Wang M. et al., 2023; Figures 1, 2). This effect is widely attributed to biochar’s porous structure and abundant surface functional groups, which provide physical refugia and nutrient sources for microorganisms, thereby promoting their colonization and proliferation (Barnes et al., 2014). Moreover, biochar improves soil physicochemical conditions by neutralizing acidic pH, enhancing soil aggregation, increasing total and capillary porosity, and improving aeration–collectively alleviating abiotic stresses such as drought and oxygen limitation (Murtaza et al., 2023). These modifications create heterogeneous microhabitats that support both aerobic and anaerobic microbial niches, further contributing to increased microbial activity and diversity (Lehmann et al., 2011). However, our study reveals a critical soil-type dependency in biochar’s impact on fungal diversity: significant increases in Shannon and evenness indices were observed only in acidic red soil (initial pH 5.28), not in neutral–alkaline calcareous soil (initial pH 7.96). This divergence highlights that the magnitude and direction of biochar-induced microbial responses are strongly mediated by pre-existing soil conditions.
In red soil, biochar raised the pH from 5.28 to 6.33, effectively alleviating acid stress–a known constraint on fungal growth (Rousk et al., 2010). This shift likely stimulated extracellular enzyme production, particularly those involved in carbon degradation (Luo et al., 2025), and favored the proliferation of decomposer taxa such as Basidiomycota (Tarin et al., 2021). In contrast, calcareous soil exhibited only a marginal pH increase (from 7.96 to 8.13), remaining within a range already favorable for most fungi. Consequently, the microbial community maintained its structural stability, with no significant change in diversity indices–consistent with reports that fungal communities in neutral–alkaline soils are less responsive to pH-modifying amendments (Xu et al., 2014). Notably, this pattern aligns with the “stress alleviation” hypothesis: biochar exerts the greatest positive effect in soils where key environmental constraints (e.g., acidity, nutrient deficiency) are present (Mansoor et al., 2021). In red soil, both pH correction and nutrient enrichment acted synergistically to relieve microbial stress. Conversely, in calcareous soil, the absence of strong limiting factors–combined with high inherent calcium content–may have dampened biochar’s influence. Indeed, calcium ions can bind with organic matter and biochar to form stable micro-aggregates (Jin et al., 2024), potentially reducing organic matter decomposition and slowing carbon release. This stabilization may reduce metabolic stress on microorganisms but also limit resource availability for fast-growing taxa, thereby maintaining community homogeneity.
Supporting this interpretation, biochar amendment in red soil led to substantial increases in soil organic carbon (SOC; from 3.16 to 98.05 g kg−1) and total nitrogen (TN; from 0.43 to 2.42 g kg−1), elevating the C:N ratio (Supplementary Figure 1). These changes alleviated nutrient limitations and favored copiotrophic microbial groups (e.g., Proteobacteria, Actinobacteria), while suppressing oligotrophic taxa (e.g., Chloroflexi)–a classic signature of resource-enriched environments (Fierer et al., 2012; Borny et al., 2024). Similarly, total phosphorus (TP) increased from 0.33 to 1.07 g kg−1, enhancing phosphorus availability and reducing microbial competition for this essential nutrient (Vance et al., 2003). Such nutrient enrichment likely promoted functional diversification and optimized community structure, particularly among fungi adapted to nutrient-rich conditions. In contrast, calcareous soil already possesses relatively high nutrient availability and pH buffering capacity, which may explain the muted microbial response. Furthermore, the formation of Ca–organic–biochar complexes may further stabilize carbon inputs, delaying nutrient mineralization and constraining the proliferation of opportunistic taxa (Abdul et al., 2021). This mechanism may contribute to the observed community stability rather than enhancement in diversity.
Therefore, our findings underscore that biochar does not universally enhance microbial diversity; its efficacy is contingent upon the initial soil environment. In degraded, acidic red soils–common in karst regions–biochar acts as a powerful restorative agent through dual pathways: pH amelioration and nutrient enrichment. In contrast, in already fertile and buffered calcareous soils, biochar’s effects are constrained by minimal environmental change and inherent biogeochemical stability. This context-dependent response reinforces the need for site-specific biochar management strategies rather than blanket application protocols. Our results extend previous work by demonstrating that fungal communities exhibit greater sensitivity to soil-type–mediated changes than bacterial communities (Figure 3), particularly in response to pH shifts. While bacteria are often more resilient to pH fluctuations due to shorter generation times and broader metabolic flexibility (Wang C. et al., 2023), fungi may serve as more sensitive bioindicators of soil restoration progress in red soil systems.
4.2 The interactive effect of mowing and soil types on microbial diversity
Mowing significantly influences soil microbial communities by altering plant residue decomposition dynamics and the quantity and quality of organic matter inputs (Pei et al., 2021). However, our results reveal that these effects are not uniform but are strongly modulated by soil type, highlighting a critical interactive effect between management practice and edaphic context (Figures 1, 2). In acidic red soils, mowing acts as a restorative disturbance, enhancing microbial diversity through multiple synergistic mechanisms. First, mowing stimulates root turnover and exudation (Luo et al., 2021), increasing the input of labile carbon substrates–such as sugars, organic acids, and amino compounds–that serve as readily available energy sources for microbes. This sustained carbon pulse helps alleviate the chronic carbon limitation typical of red soils (Johnson, 1998), thereby promoting microbial growth and species emergence. Second, mowing induces a slight but significant increase in soil pH, partially neutralizing the acidic environment. This pH shift reduces physiological stress on acid-sensitive taxa and activates pH-responsive bacterial groups (Wang et al., 2019), further contributing to community restructuring. Importantly, red soils typically exhibit low initial microbial diversity due to inherent acidity, low nutrient availability, and poor aggregation. This “low-diversity baseline” creates ecological opportunity–microbial communities in such stressed environments are more responsive to resource additions and environmental amelioration (Huston, 2014). Consequently, even modest improvements in carbon supply and pH can trigger substantial increases in diversity, as observed in our study (Liu et al., 2023). Thus, mowing in red soils functions as a facilitative perturbation, shifting the system toward a less stressed, more diverse state.
In contrast, calcareous soils respond to mowing differently–often negatively. These soils have high pH buffering capacity and abundant calcium, which naturally suppress organic matter mineralization due to carbonate stabilization (Gan et al., 2020). Mowing reduces aboveground biomass and photosynthetic return, leading to decreased labile carbon inputs and exacerbating resource scarcity. This constrains nutrient cycling and promotes carbon depletion. Calcareous soils typically have high initial microbial diversity, dominated by copiotrophic taxa adapted to fluctuating resources (Bossolani et al., 2021). Under mowing-induced carbon limitation, competition intensifies, favoring fast-growing specialists–such as Actinobacteria–over slower-growing or sensitive taxa (Hayat et al., 2021). Their high metabolic activity and antibiotic production further accelerate carbon turnover and suppress competitors, reducing evenness and functional redundancy. As a result, microbial communities become more homogeneous, limiting diversity gains. Additionally, mowing exerts minimal influence on soil pH in calcareous systems due to their strong buffering capacity. Without significant environmental amelioration, the potential for mowing to stimulate new microbial niches is limited. Thus, rather than acting as a facilitator, mowing in calcareous soils functions as a competitive stressor, reinforcing existing dominance patterns and constraining diversity gains.
Overall, our findings demonstrate a dichotomous response to mowing: in red soils, mowing alleviates abiotic stress (acidity) and biotic constraints (carbon limitation), thereby enhancing microbial diversity–a pattern consistent with the “stress-gradient hypothesis” (Daebeler et al., 2020; Maestre et al., 2006), where facilitative interactions dominate under harsh conditions. Conversely, in calcareous soils, mowing intensifies resource competition in an already competitive environment, yielding neutral or negative outcomes for diversity. This contrast emphasizes that the same management practice can elicit opposite ecological responses depending on the initial soil context. These results have important implications for sustainable grassland and agroecosystem management. They suggest that mowing may be most beneficial in degraded, acidic soils, where it can promote microbial recovery, but should be applied cautiously in calcareous systems, where it may disrupt established microbial networks and reduce functional resilience.
4.3 The potential synergistic trend of the combined processing
The potential synergistic effect of biochar amendment and mowing on microbial biomass and diversity is more evident in red soil than in calcareous soil, reflecting fundamental differences in soil geochemistry and microbial constraints (Figures 1, 2). In red soil, long-term biochar application alleviates key limitations: it neutralizes soil acidity, provides stable carbon substrates, improves pore structure, and enhances habitat connectivity for microbes (El-Sharkawy et al., 2022). When combined with mowing–which stimulates root exudation and litter inputs–this creates a dual carbon supply: recalcitrant biochar-derived carbon sustains slow-growing, stress-tolerant taxa, while labile plant-derived carbon fuels copiotrophic populations (Wu et al., 2018). This complementary resource input reduces nutrient competition and promotes both bacterial and fungal growth, suggesting a synergistic interaction that enhances overall microbial biomass and diversity. In contrast, such synergy is constrained in calcareous soils. The inherently high pH and carbonate buffering capacity limit biochar’s ability to further modulate soil chemistry, while Ca2 + saturation inhibits the oxidative degradation of biochar surfaces, reducing its bioavailability and slow-release carbon function (Rahmanian and Khadem, 2024). Moreover, calcium ions readily form organo-mineral complexes with mowing-derived organic residues, decreasing substrate accessibility for decomposers and suppressing microbial activity. Mowing alone has minimal impact on microbial diversity in this context, indicating low responsiveness to disturbance. Consequently, the ecological opportunity for synergy is greater in red soils–where abiotic stress and carbon limitation create a “responsive” microbial community–than in calcareous soils, where chemical stability and pre-existing resource constraints dampen the effects of both interventions. Thus, the expression of synergistic trends depends not only on the combination of management practices but critically on the underlying soil context.
In addition to above, our results also demonstrate a significant synergistic effect between biochar application and mowing (BM), which markedly enhanced aboveground biomass in both red soil (RS) and calcareous soil (CS) (Supplementary Figure 1A). This synergy likely arises from biochar-improved soil water retention and nutrient-holding capacity, which facilitate rapid plant regrowth following mowing, while returned plant residues promote organic matter accumulation and microbial activity, accelerating nutrient mineralization and establishing a “soil-plant-management” positive feedback loop (Pan et al., 2025; Hao et al., 2025). This synergistic effect represents a key practical outcome of our research, highlighting a promising integrated management strategy for restoring productivity in degraded grasslands. Surprisingly, biochar application (B and BM) significantly reduced soil cation exchange capacity (CEC) (Supplementary Figure 1L), contrary to the widely reported CEC-enhancing effect (Pidlisnyuk et al., 2025). This anomaly may be attributed to the high-temperature production (>500 °C) of the biochar used, which promotes extensive aromatization and thermal degradation of surface functional groups, thereby reducing negative charge density (Luo et al., 2023). Additionally, abundant Ca2+ and Mg2+ in biochar ash (as shown in Supplementary Figures 1N, O, where B and BM treatments increased calcium and magnesium ion concentrations) may induce “charge shielding” or “ion bridging” effects (Zhang et al., 2025), while biochar-iron/aluminum oxide interactions via ligand exchange or surface complexation could mask variable-charge surfaces (Yuan et al., 2025). Biochar-derived dissolved organic matter (DOM) may also neutralize clay surface charges under certain conditions, further reducing effective CEC (Li et al., 2025). In variable-charge soils, pH elevation from biochar may alter surface charge dynamics near the point of zero charge (PZC), and concurrent base cation inputs could lead to ion competition or precipitation (e.g., calcium carbonate), affecting CEC measurements (Fung et al., 2023).Furthermore, the significant reduction in plant-available nitrogen (NH4+ and NO3–) in biochar-amended pots (Supplementary Figures 1 J, K) is a crucial finding for nutrient management. We postulate that biochar-induced carbon surplus disrupted the soil C:N balance, triggering rapid microbial growth and short-term nitrogen immobilization or “lock-up” (Muhammad et al., 2023). This effect was amplified in the BM treatment due to the co-addition of labile carbon from residues and stable carbon from biochar, creating a synergistic “activation effect” that intensified microbial nitrogen competition (Chamoli et al., 2025). Consequently, early plant growth in nutrient-poor soils may be temporarily limited, necessitating careful nitrogen fertilization. However, in the long term, this mechanism promotes a “slow-release” nitrogen supply by reducing nitrate leaching and N2O emissions, thereby improving nitrogen use efficiency through gradual mineralization. Biochar thus functions not merely as a passive adsorbent but as an active regulator of soil nitrogen dynamics, with important implications for sustainable soil management.
4.4 Correlations between microbial diversity, community structure and soil properties
Microbial community diversity is significantly correlated with soil physicochemical properties (Figures 7, 8), indicating that edaphic factors play a central role in shaping microbial community structure and function. However, the nature and strength of these relationships differ markedly between red and calcareous soils, reflecting divergent ecological constraints.
In red soils, microbial community structure is strongly driven by pH, soil organic carbon (SOC), total nitrogen (TN), total phosphorus (TP), and C/N ratio. The typically low pH of red soils imposes abiotic stress, directly inhibiting microbial enzyme activity and compromising membrane integrity (Wang et al., 2024), while also selecting for acid-tolerant taxa. Svenningsen et al. (2018) have shown that pH is a master driver of microbial biogeography, and even small shifts can restructure communities. Concurrently, low SOC and nutrient availability create strong carbon and nutrient limitation, making microbial communities highly responsive to organic inputs (Goldfarb et al., 2011). This sensitivity is evident in our results: biochar addition increased the relative abundance of Proteobacteria–a typically copiotrophic phylum capable of rapid growth under resource-rich conditions–while decreasing Chloroflexi, which are often oligotrophic and adapted to low-energy environments (Arunrat et al., 2022; Figure 3). Similarly, Firmicutes showed significant positive correlations with SOC, TN, and TP in red soils, consistent with their role in organic matter decomposition and fermentation (Bonanomi et al., 2016), whereas Chloroflexi were negatively correlated, reflecting their competitive disadvantage under elevated nutrient availability.
In contrast, microbial α-diversity in calcareous soils is more strongly influenced by phosphorus dynamics and pH stability. The high calcium content promotes the formation of insoluble calcium-phosphate complexes, limiting phosphorus bioavailability and intensifying competition among microbes–particularly between phosphate-solubilizing bacteria and generalists (Vyas and Gulati, 2009). This resource competition becomes a key regulator of diversity. Meanwhile, the inherent carbonate buffering system maintains a stable, near-neutral to alkaline pH, which falls within the optimal range for many microorganisms. This stability reduces environmental filtering and community turnover, thereby supporting higher baseline α-diversity (Fan et al., 2024). Unlike in red soils, correlations between major microbial taxa (e.g., Firmicutes, Chloroflexi) and nutrient indices were not significant in calcareous soils, suggesting a decoupling of community composition from short-term nutrient fluctuations.
Overall, these contrasting patterns highlight that soil type fundamentally shapes microbial responses: in red soils, abiotic stress (acidity) and resource scarcity (C, N, P limitation) make microbial communities highly sensitive to management-induced changes, with community structure tightly linked to nutrient availability. In calcareous soils, chemical stability and P limitation dominate, shifting the primary response to competition-driven dynamics and stabilizing α-diversity. Thus, the regulation of microbial communities is not solely a function of individual soil properties, but of the integrated physicochemical context that defines stress thresholds and resource strategies.
4.5 Limitations and future Directions
Based on our results and supporting literature (Xiao et al., 2016; Cen et al., 2021; Chen et al., 2021; Guo et al., 2025), we now propose the following field-applicable recommendations. The biochar application rate in this experiment was 10% (w/w), equivalent to approximately 240 t/ha assuming a 20-cm plow layer and a soil bulk density of 1.2 t/mł. While this is higher than typical field applications (usually 5–50 t/ha), it was chosen to clearly detect microbial responses under controlled conditions. Future field studies should test lower, more practical rates (e.g., 20–30 t/ha) to evaluate scalability. Therefore, our findings offer practical insights for restoring degraded karst grasslands. In red soils, where both biochar and mowing significantly enhanced microbial communities, a combined management strategy is recommended: applying biochar at a rate of 20–30 t/ha (e.g., Xiao et al., 2016; Cen et al., 2021), which are economically and logistically feasible while still likely to enhance microbial communities, especially in red soils, and followed by moderate mowing every 6–8 weeks during the growing season, avoiding over-harvesting. This balances biomass removal with plant recovery and root exudation, which likely supports microbial growth. This regime may promote root exudation and organic input while maintaining plant productivity, potentially synergizing with biochar to boost microbial activity. In calcareous soils, where mowing had minimal effects, biochar application alone (at the same rate) may be sufficient to improve microbial conditions. Given the high cost and labor of mowing, this suggests a more targeted, soil-specific approach to restoration. Future field trials should validate these recommendations under real-world conditions, particularly regarding long-term carbon sequestration and plant community recovery.
Although the experiment was carefully controlled, several potential sources of error should be acknowledged. First, despite using a randomized block design and weekly pot rotation, subtle microclimatic gradients–such as variations in light intensity and temperature–within the greenhouse may have introduced residual variability. Second, although soil and plant sampling procedures were standardized, manual collection could lead to minor positional differences within pots. Third, technical variation inherent in high-throughput sequencing and enzyme assays may have influenced the precision of microbial measurements. Nevertheless, the consistency of treatment effects across replicates and the statistical significance of key interactions indicate that these potential errors did not compromise the main conclusions.
Several additional limitations also warrant consideration. First, the study was conducted under controlled mesocosm conditions with a limited number of replicates (n = 4). While sufficient to detect strong treatment effects, higher replication in future field studies would enhance statistical power and better account for micro-scale environmental heterogeneity. Second, mesocosm systems necessarily simplify natural ecosystems. Therefore, our findings should be validated through field-scale restoration experiments in actual degraded karst grasslands, where abiotic and biotic interactions are more complex and dynamic. Finally, the experiment spanned one year; given that biochar effects can evolve over time and microbial communities may undergo succession, long-term monitoring (e.g., 3–5 years) is recommended to evaluate the stability and sustainability of the observed responses. Such studies will be essential for developing effective, science-based restoration strategies for karst ecosystems.
5 Conclusion
This study reveals that the parent soil type is a critical determinant of soil microbial responses to biochar application and mowing–a key insight with broad implications for sustainable soil management in heterogeneous landscapes. While biochar consistently enhanced microbial abundance and richness across both red and calcareous soils, its effects on fungal diversity and community structure were pronounced only in red soil. Similarly, mowing significantly boosted microbial metrics in red soil but had no detectable impact in calcareous soil, underscoring the soil-specific nature of management outcomes. Notably, the combined biochar–mowing (BM) treatment in red soil showed a potential synergistic effect, suggesting that integrated practices may amplify microbial recovery in degraded ecosystems. Stronger correlations between soil physicochemical properties and microbial communities in red soil further indicate a more responsive and manageable microbiome in this substrate. These findings highlight that one-size-fits-all management strategies are unlikely to succeed in regions with diverse soil types, such as karst ecosystems. Instead, interventions should be tailored to local soil conditions. We recommend that future research validate these results through well-replicated field trials, long-term monitoring, and expanded experimental replication. Such studies will be essential to confirm the durability of microbial responses, improve statistical power, and elucidate the mechanisms behind observed interactions under real-world conditions. In the meantime, our results support the targeted use of biochar combined with moderate mowing as a promising strategy for enhancing soil health in red soil systems–with adjustments based on site-specific conditions and validated through robust experimental design.
Data availability statement
The raw sequencing data generated in this study have been deposited in the NCBI Sequence Read Archive (SRA) under BioProject accession number (PRJNA1338870). The data are publicly available at: https://www.ncbi.nlm.nih.gov.
Author contributions
XY: Data curation, Investigation, Methodology, Writing – original draft. W–LT: Formal analysis, Investigation, Writing – review & editing. H–HZ: Data curation, Formal analysis, Writing – review & editing. S–WZ: Investigation, Writing – original draft. X–YW: Investigation, Writing – original draft. Z–LD: Investigation, Writing – original draft. S–YG: Investigation, Writing – original draft. X–KL: Funding acquisition, Resources, Writing – review & editing. X–XS: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. We appreciate the financial support by the Natural Science Foundation of Guangxi Zhuang Autonomous Region (2025GXNSFBA069349), Guangxi Key Technologies R&D Program (Guike AB22080057); National Natural Science Foundation of China (32001141, 32460282) and The Scientific Research Foundation of Guilin University of Technology (GLUTQD2018055).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1680847/full#supplementary-material
References
Abdul, R., Natasha, N. S., Hamid, N. W. A., and Nadarajah, K. (2021). Effects of abiotic stress on soil microbiome. Int. J. Mol. Sci. 22:9036. doi: 10.3390/ijms22169036
Arunrat, N., Sansupa, C., Kongsurakan, P., Sereenonchai, S., and Hatano, R. (2022). Soil microbial diversity and community composition in rice–fish co-culture and rice monoculture farming system. Biology 11:1242. doi: 10.3390/biology11081242
Arwenyo, B., Varco, J. J., Dygert, A., Brown, S., Pittman, C. U., and Mlsna, T. (2023). Contribution of modified P-enriched biochar on pH buffering capacity of acidic soil. J. Environ. Manage 339:117863. doi: 10.1016/j.jenvman.2023.117863
Bardgett, R. D., and van der Putten, W. H. (2014). Belowground biodiversity and ecosystem functioning. Nature 515, 505–511. doi: 10.1038/nature13855
Barnes, R. T., Gallagher, M. E., Masiello, C. A., Liu, Z., and Dugan, B. (2014). Biochar-induced changes in soil hydraulic conductivity and dissolved nutrient fluxes constrained by laboratory experiments. PLoS One 9:e108340. doi: 10.1371/journal.pone.0108340
Blüthgen, N., Dormann, C. F., Prati, D., Klaus, V. H., Kleinebecker, T., Hölzel, N., et al. (2012). A quantitative index of land-use intensity in grasslands: Integrating mowing. Grazing and Fertilization. Basic Appl. Ecol. 13, 207–220. doi: 10.1016/j.baae.2012.04.001
Bonanomi, G., De Filippis, F., Cesarano, G., La Storia, A., Ercolini, D., and Scala, F. (2016). Organic farming induces changes in soil microbiota that affect agro-ecosystem functions. Soil Biol. Biochem. 103, 327–336. doi: 10.1016/j.soilbio.2016.09.005
Borny, N. R., Mostakim, G. M., Raihan, A., and Rahman, Md. S. (2024). Synergistic effects of rice straw return and nitrogen fertilizer on rhizosphere bacterial communities and soil fertility. J. Soil Plant Environ. 3, 41–58. doi: 10.56946/jspae.v3i1.404
Bossolani, J. W., Crusciol, C. A. C., Leite, M. F. A., Merloti, L. F., Moretti, L. G. I., Pascoaloto, M., et al. (2021). Modulation of the soil microbiome by long-term ca-based soil amendments boosts soil organic carbon and physicochemical quality in a tropical no-till crop rotation system. Soil Biol. Biochem. 156:108188. doi: 10.1016/j.soilbio.2021.108188
Cen, R., Feng, W., Yang, F., Wu, W., Liao, H., and Qu, Z. (2021). Effect mechanism of biochar application on soil structure and organic matter in semi-arid areas. J. Environ. Manage 286:112198. doi: 10.1016/j.jenvman.2021.112198
Chamoli, A., Karn, S. K., Kumari, M., and Sivaramasamy, E. (2025). Biochar mediated fixation of nitrogen compounds (ammonia and nitrite) in soil: A review. Biodegradation 36:22. doi: 10.1007/s10532-025-10116-6
Chave, J., Rejou-Mechain, M., Burquez, A., Chidumayo, E., Colgan, M. S., Delitti, W. B., et al. (2014). Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Change Biol. 20, 3177–3190. doi: 10.1111/gcb.12629
Chen, L., Baoyin, T., and Minggagud, H. (2021). Effects of mowing regimes on above- and belowground biota in semi-arid grassland of northern China. J. Environ. Manage 277:111441. doi: 10.1016/j.jenvman.2020.111441
Daebeler, A., Kitzinger, K., Koch, H., Herbold, C. W., Steinfeder, M., Schwarz, J., et al. (2020). Exploring the upper pH limits of nitrite oxidation: Diversity, ecophysiology, and adaptive traits of haloalkalitolerant Nitrospira. ISME J. 14, 2967–2979. doi: 10.1038/s41396-020-0724-1
Delgado-Baquerizo, M., Grinyer, J., Reich, P. B., and Singh, B. K. (2016). Relative importance of soil properties and microbial community for soil functionality: Insights from a microbial swap experiment. Funct. Ecol. 30, 1862–1873. doi: 10.1111/1365-2435.12674
El-Sharkawy, M., El-Naggar, A. H., AL-Huqail, A. A., and Ghoneim, A. M. (2022). Acid-Modified biochar impacts on soil properties and biochemical characteristics of crops grown in saline-sodic soils. Sustainability 14:8190. doi: 10.3390/su14138190
Fan, X., Meng, L., Wang, Y., and Zang, L. (2024). Stochastic process drives the dissimilarity in biodiversity patterns between Pinus kwangtungensis coniferous forest and evergreen deciduous broad-leaved mixed forest in karst area. PeerJ 12:e17899. doi: 10.7717/peerj.17899
Fierer, N., Lauber, C. L., Ramirez, K. S., Zaneveld, J., Bradford, M. A., and Knight, R. (2012). Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 6, 1007–1017. doi: 10.1038/ismej.2011.159
Fierer, N., Wood, S. A., and De Mesquita, C. P. B. (2021). How microbes can, and cannot, be used to assess soil health. Soil Biol. Biochem. 153:108111. doi: 10.1016/j.soilbio.2020.108111
Fung, E., Wang, J., Zhao, X., Farzamian, M., Allred, B., Clevenger, W. B., et al. (2023). Mapping cation exchange capacity and exchangeable potassium using proximal soil sensing data at the multiple-field scale. Soil Tillage Res. 232:105735. doi: 10.1016/j.still.2023.105735
Gan, H. Y., Schöning, I., Schall, P., Ammer, C., and Schrumpf, M. (2020). Soil organic matter mineralization as driven by nutrient stoichiometry in soils under differently managed forest stands. Front. For. Global Change 3:99. doi: 10.3389/ffgc.2020.00099
Gilmullina, A., Rumpel, C., Blagodatskaya, E., and Chabbi, A. (2020). Management of grasslands by mowing versus grazing – impacts on soil organic matter quality and microbial functioning. Appl. Soil Ecol. 156:103701. doi: 10.1016/j.apsoil.2020.103701
Goldfarb, K. C., Karaoz, U., Hanson, C. A., Santee, C. A., Bradford, M. A., Treseder, K. K., et al. (2011). Differential growth responses of soil bacterial taxa to carbon substrates of varying chemical recalcitrance. Front. Microbiol. 2:94. doi: 10.3389/fmicb.2011.00094
Guo, J., Wang, L., Qu, G., Liu, X., Lian, Y., and Hou, D. (2024). Soil health improvement in a karst area with geogenic cd enrichment using biochar and clay-based amendments. J. Soils Sediments 24, 230–243. doi: 10.1007/s11368-023-03645-1
Guo, J., Zhou, H., Jia, L., Wang, Y., Fan, M., Wang, M., et al. (2025). A multi-level fuzzy comprehensive evaluation model to optimize biochar application schemes for potato cultivation in North China. Front. Plant Sci. 16:1571305. doi: 10.3389/fpls.2025.1571305
Gutiérrez, F., Parise, M., De Waele, J., and Jourde, H. (2014). A review on natural and human-induced geohazards and impacts in karst. Earth-Sci. Rev. 138, 61–88. doi: 10.1016/j.earscirev.2014.08.002
Hao, H., Wang, R., Li, S., Duo, P., Ning, P., Ahejiang, S., et al. (2025). Different contributions of microbial and plant residues to soil organic carbon accumulation during planted forest and abandoned farmland restoration, loess plateau, China. Plant Soil 507, 845–862. doi: 10.1007/s11104-024-06772-x
Hayat, S., Ashraf, A., Aslam, B., Asif, R., Muzammil, S., Zahoor, M. A., et al. (2021). “Actinobacteria: Potential candidate as plant growth promoters,” in Plant stress physiology, ed. A. Hossain (London: IntechOpen), doi: 10.5772/intechopen.93272
Huston, M. A. (2014). Disturbance, productivity, and species diversity: Empiricism vs. logic in ecological theory. Ecology 95, 2382–2396. doi: 10.1890/13-1397.1
Jiang, Z., Lian, Y., and Qin, X. (2014). Rocky desertification in southwest china: Impacts, causes, and restoration. Earth-Sci. Rev. 132, 1–12. doi: 10.1016/j.earscirev.2014.01.005
Jin, F., Piao, J., Miao, S., Che, W., Li, X., Li, X., et al. (2024). Long-Term effects of biochar one-off application on soil physicochemical properties, salt concentration, nutrient availability, enzyme activity, and rice yield of highly saline-alkali paddy soils: Based on a 6-year field experiment. Biochar 6:40. doi: 10.1007/s42773-024-00332-3
Johnson, D. B. (1998). Biodiversity and ecology of acidophilic microorganisms. FEMS Microbiol. Ecol. 27, 307–317. doi: 10.1111/j.1574-6941.1998.tb00547.x
Lehmann, J., Rillig, M. C., Thies, J., Masiello, C. A., Hockaday, W. C., and Crowley, D. (2011). Biochar effects on soil biota – a review. Soil Biol. Biochem. 43, 1812–1836. doi: 10.1016/j.soilbio.2011.04.022
Lei, X., Shen, Y., Zhao, J., Huang, J., Wang, H., Yu, Y., et al. (2023). Root exudates mediate the processes of soil organic carbon input and efflux. Plants 12:630. doi: 10.3390/plants12030630
Li, F., Duan, X., Zhou, J., Feng, S., Du, W., He, X., et al. (2025). Inhibited vertical mobility of biochar-derived dissolved organic matter under low-intensity rainfall: Role of mineral retention. Biochar 7:99. doi: 10.1007/s42773-025-00484-w
Li, F., Minggagud, H., Jarvie, S., Wang, Y., Yan, Y., Gong, X., et al. (2023). Mowing mitigates the adverse effects of fertilization on plant diversity and changes soil bacterial and fungal community structure in the inner mongolia grassland. Agricult. Ecosyst. Environ. 346:108358. doi: 10.1016/j.agee.2023.108358
Li, W., Hodzic, J., Su, J., Zheng, S., and Bai, Y. (2020). A dataset of plant and microbial community structure after long-term grazing and mowing in a semiarid steppe. Sci. Data 7:403. doi: 10.1038/s41597-020-00738-1
Liu, P., Ding, S., Liu, N., Mo, Y., Liang, Y., and Ma, J. (2023). Soil microbial community in relation to soil organic carbon and labile soil organic carbon fractions under detritus treatments in a subtropical karst region during the rainy and dry seasons. Forests 14:2291. doi: 10.3390/f14122291
Luo, M., Jiang, X., Liu, Y., Liu, Y., Yu, H., Niu, Y., et al. (2023). Enhanced adsorption complexation of biochar by nitrogen-containing functional groups. J. Environ. Chem. Eng. 11:111194. doi: 10.1016/j.jece.2023.111194
Luo, W., Petticord, D. F., Zhu, S., Zhu, S., Wu, Y., Yi, X., et al. (2025). Biochar application and mowing independently and interactively influence soil enzyme activity and carbon sequestration in karst and red soils in Southern China. Agronomy 15:252. doi: 10.3390/agronomy15010252
Luo, Z., Xiao, L., Wang, G., Chang, J., Chen, Y., Guo, X., et al. (2021). Depth distribution of belowground net primary production across global biomes. PREPRINT (version 3). Research Square. doi: 10.21203/rs.3.rs-65178/v2
Maestre, F. T., Valladares, J. F., and Reynolds, Md. S. (2006). The stress-gradient hypothesis does not fit all relationships between plant–plant interactions and abiotic stress: Further insights from arid environments. J. Ecol. 94, 17–22. doi: 10.1111/j.1365-2745.2005.01089.x
Mansoor, S., Kour, N., Manhas, S., Zahid, S., Wani, O. A., Sharma, V., et al. (2021). Biochar as a tool for effective management of drought and heavy metal toxicity. Chemosphere 271:129458. doi: 10.1016/j.chemosphere.2020.129458
Miao, R., Guo, M., Ma, J., Gao, B., Miao, Y., Yang, Z., et al. (2020). Shifts in soil seed bank and plant community under nitrogen addition and mowing in an inner mongolian steppe. Ecol. Eng. 153:105900. doi: 10.1016/j.ecoleng.2020.105900
Muhammad, H., Fahad, S., Saud, S., Hassan, S., Nasim, W., Ali, B., et al. (2023). A paradigm shift towards beneficial microbes enhancing the efficiency of organic and inorganic nitrogen sources for a sustainable environment. Land 12:680. doi: 10.3390/land12030680
Murtaza, G., Ahmed, Z., Eldin, S. M., Ali, B., Bawazeer, S., Usman, M., et al. (2023). Biochar-Soil-Plant interactions: A cross talk for sustainable agriculture under changing climate. Front. Environ. Sci. 11:1059449. doi: 10.3389/fenvs.2023.1059449
Pan, Y., Wang, D., Tan, T., An, J., Jin, X., Zou, H., et al. (2025). Effect of organic amendments on soil organic carbon fractions, water retention, and mechanical properties in a Chinese alfisol. Soil Tillage Res. 254:106723. doi: 10.1016/j.still.2025.106723
Pei, G.-T., Sun, J.-F., He, T.-X., and Hu, B.-Q. (2021). Effects of long-term human disturbances on soil microbial diversity and community structure in a karst grassland ecosystem of northwestern Guangxi, China. Chin. J. Plant Ecol. 45, 74–84. doi: 10.17521/cjpe.2020.0316
Pidlisnyuk, V., Herts, A., Kononchuk, O., Khomenchuk, V., Horyn, O., Markiv, V., et al. (2025). Comprehensive study of biochars from different vegetative feedstocks: Influence on soil properties and development of zea mays L. Environ. Sci. Eur. 37:77. doi: 10.1186/s12302-025-01118-5
Rahmanian, M., and Khadem, A. (2024). The effects of biochar on soil extra and intracellular enzymes activity. Biomass Conv. Biorefinery 14, 21993–22005. doi: 10.1007/s13399-023-04330-6
Rousk, J., Bååth, E., Brookes, P. C., Lauber, C. L., Lozupone, C., Caporaso, J. G., et al. (2010). Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 4, 1340–1351. doi: 10.1038/ismej.2010.58
Song, X., Cai, J., Meng, H., Ding, S., Wang, L., Liu, B., et al. (2020). Defoliation and neighbouring legume plants accelerate leaf and root litter decomposition of leymus chinensis dominating grasslands. Agriculture Ecosyst. Environ. 302:107074. doi: 10.1016/j.agee.2020.107074
Svenningsen, N. B., Watts-Williams, S. J., Joner, E. J., Battini, F., Efthymiou, A., Cruz-Paredes, C., et al. (2018). Suppression of the activity of arbuscular mycorrhizal fungi by the soil microbiota. ISME J. 12, 1296–1307. doi: 10.1038/s41396-018-0059-3
Tarin, M. W. K., Fan, L., Xie, D., Tayyab, M., Rong, J., Chen, L., et al. (2021). Response of soil fungal diversity and community composition to varying levels of bamboo biochar in red soils. Microorganisms 9:1385. doi: 10.3390/microorganisms9071385
Thakur, M. P., and Geisen, S. (2019). Trophic regulations of the soil microbiome. Trends Microbiol. 27, 771–780. doi: 10.1016/j.tim.2019.04.008
Vance, C. P., Uhde-Stone, C., and Allan, D. L. (2003). Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 157, 423–447. doi: 10.1046/j.1469-8137.2003.00695.x
Vyas, P., and Gulati, A. (2009). Organic acid production in vitro and plant growth promotion in maize under controlled environment by phosphate-solubilizing fluorescent Pseudomonas. BMC Microbiol. 9:174. doi: 10.1186/1471-2180-9-174
Wang, C., Yu, Q. Y., Ji, N. N., Zheng, Y., Taylor, J. W., Guo, L. D., et al. (2023). Bacterial genome size and gene functional diversity negatively correlate with taxonomic diversity along a pH gradient. Nat. Commun. 14:7437. doi: 10.1038/s41467-023-43297-w
Wang, C., Zhou, X., Guo, D., Zhao, J., Yan, L., Feng, G., et al. (2019). Soil pH is the primary factor driving the distribution and function of microorganisms in farmland soils in Northeastern China. Ann. Microbiol. 69, 1461–1473. doi: 10.1007/s13213-019-01529-9
Wang, J., Wei, K., Li, Z., Wang, Y., Tang, J., and Zhu, B. (2025). Effects of mowing on root biomass, soil properties, microbial biomass, and microbial diversity in grasslands: A meta-analysis. Land Degradation Dev. 36, 1483–1491. doi: 10.1002/ldr.5439
Wang, M., Yu, X., Weng, X., Zeng, X., Li, M., and Sui, X. (2023). Meta-Analysis of the effects of biochar application on the diversity of soil bacteria and fungi. Microorganisms 11:641. doi: 10.3390/microorganisms11030641
Wang, Y., Long, C., Yin, L., Liu, R., Liao, Y., He, G., et al. (2024). Effects of simulated acid rain on hydrochemical factors and microbial community structure in red soil aquifers. RSC Adv. 14, 4482–4491. doi: 10.1039/d3ra08820k
Wu, X., Wu, L., Liu, Y., Zhang, P., Li, Q., Zhou, J., et al. (2018). Microbial interactions with dissolved organic matter drive carbon dynamics and community succession. Front. Microbiol. 9:1234. doi: 10.3389/fmicb.2018.01234
Xia, H., Riaz, M., Babar, S., Yan, L., Li, Y., Wang, X., et al. (2023). Assessing the impact of biochar on microbes in acidic soils: Alleviating the toxicity of aluminum and acidity". J. Environ. Manage 2023:118796. doi: 10.1016/j.jenvman.2023.118796
Xiao, Q., Zhu, L., Shen, Y., and Li, S. (2016). Sensitivity of soil water retention and availability to biochar addition in rainfed semi-arid farmland during a three-year field experiment. Field Crops Res. 196, 284–293. doi: 10.1016/j.fcr.2016.07.014
Xu, H. J., Wang, X. H., Li, H., Yao, H. Y., Su, J. Q., and Zhu, Y. G. (2014). Biochar impacts soil microbial community composition and nitrogen cycling in an acidic soil planted with rape. Environ. Sci. Technol. 48, 9391–9399. doi: 10.1021/es5021058
Yan, J., Li, Q., Hu, L., Wang, J., Zhou, Q., and Zhong, J. (2022). Response of microbial communities and their metabolic functions to calcareous succession process. Sci. Total Environ. 825:154020. doi: 10.1016/j.scitotenv.2022.154020
Yang, Y., Li, X., Liu, J., Zhou, Z., Zhang, T., and Wang, X. (2017). Bacterial diversity as affected by application of manure in red soils of subtropical China. Biol. Fertil. Soils 53, 639–649. doi: 10.1007/s00374-017-1209-x
Yuan, Y., Fang, M., Liu, Y., Zhang, B., Chen, T., Jin, J., et al. (2025). Enhanced soil remediation with iron-synthetic humic-like acid composites: A trade-off between metal immobilization efficacy and micro-ecological health. J. Environ. Manage 389:126045. doi: 10.1016/j.jenvman.2025.126045
Zhang, N., Xing, J., Wei, L., Liu, C., Zhao, W., Liu, Z., et al. (2025). The potential of biochar to mitigate soil acidification: A global meta-analysis. Biochar 7:49. doi: 10.1007/s42773-025-00451-5
Zhang, Z., Huang, X., and Zhou, Y. (2021). Factors influencing the evolution of human-driven rocky desertification in karst areas. Land Degradat. Dev. 32, 817–829. doi: 10.1002/ldr.3731
Zhong, F., Xu, X., Li, Z., Zeng, X., Yi, R., Luo, W., et al. (2022). Relationships between lithology, topography, soil, and vegetation, and their implications for karst vegetation restoration. CATENA 209:105831. doi: 10.1016/j.catena.2021.105831
Zhou, L., Wang, X., Wang, Z., Zhang, X., Chen, C., and Liu, H. (2020). The challenge of soil loss control and vegetation restoration in the karst area of Southwestern China. Int. Soil Water Conserv. Res. 8, 26–34. doi: 10.1016/j.iswcr.2019.12.001
Keywords: calcareous soil, microbial diversity, microbial abundance, red soil, soil properties
Citation: Yi X, Tao W–L, Zhou H–H, Zhu S–W, Wang X–Y, Dong Z–L, Gao S–Y, Li X–K and Song X–X (2025) Parent soil type modulates biochar and mowing effects on soil microbial communities in karst region. Front. Microbiol. 16:1680847. doi: 10.3389/fmicb.2025.1680847
Received: 06 August 2025; Accepted: 26 September 2025;
Published: 28 October 2025.
Edited by:
Kamran Malik, Lanzhou University, ChinaReviewed by:
Shirin Aktar, Wuhan Botanical Garden, Chinese Academy of Sciences (CAS), ChinaMuhammad Usman Ghani, Guangdong Academy of Agricultural Sciences, China
Copyright © 2025 Yi, Tao, Zhou, Zhu, Wang, Dong, Gao, Li and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu–Xin Song, c29uZ3h4QGdsdXQuZWR1LmNu; Xian–Kun Li, eGlhbmt1bmxpQDE2My5jb20=
 Xun Yi1,2
Xun Yi1,2 Xian–Kun Li
Xian–Kun Li Xu–Xin Song
Xu–Xin Song