- 1Department of Dermatology, Affiliated Children's Hospital of Jiangnan University (Wuxi Children's Hospital), Wuxi, Jiangsu, China
- 2Wuxi Key Laboratory of Genetic and Metabolic Diseases in Children, Department of Paediatrics, Affiliated Children's Hospital of Jiangnan University, Wuxi Children's Hospital, Wuxi, Jiangsu, China
- 3The Affiliated Wuxi Center for Disease Control and Prevention of Nanjing Medical University, Wuxi Center for Disease Control and Prevention, Wuxi Medical Center, Nanjing Medical University, Wuxi, Jiangsu, China
Atopic Dermatitis (AD) is a highly prevalent chronic inflammatory disease in children, and its global prevalence is continually rising. However, data from the past decade indicate that this overall trend masks a disparity: while the prevalence has plateaued in high-income countries, it has shown a significant upward trend in low- and middle-income countries. Prenatal exposure to endocrine-disrupting chemicals (EDCs) is an environmental factor of growing scientific concern. Key EDCs of interest include per- and polyfluoroalkyl substances (PFAS), phenolics such as bisphenol A (BPA), parabens, and triclosan (TCS), as well as phthalate esters (PAEs). Although epidemiological studies indicated an association between prenatal EDCs exposure and an increased risk of offspring developing AD, key challenges remain unresolved, including population heterogeneity, methodological variations in exposure assessment, and elucidation of the underlying mechanisms. The review summarized the epidemiological evidence linking prenatal EDCs exposure to childhood AD, aiming to provide a theoretical basis for the early prevention of AD. Furthermore, it highlighted the future need to integrate multi-omics technologies with prospective cohort studies to elucidate the effects of mixed EDCs exposures and identify critical intervention windows.
1 Introduction
Atopic dermatitis (AD) is a chronic, recurrent inflammatory skin disease often accompanied by intense pruritus. The phenotype of AD exhibits significant heterogeneity, with the distribution and morphology of skin lesions varying with age. In infancy, it typically begins on the cheeks and extends to the trunk and extensor surfaces of the limbs, presenting primarily as acute eczema. During childhood, the lesions tend to become more localized, often involving the flexural areas or periarticular regions, with a subacute presentation and less exudation compared to the infantile stage. In adolescence, the eruptions predominantly affect the antecubital and popliteal fossae, neck, and acral regions, characterized by localized latensification and thickening (Guttman-Yassky et al., 2025). Approximately 60% of AD cases are onset in infancy, and 90% manifest before the age of five, often persisting in childhood and adolescence. The severe pruritus associated with AD skin lesions disrupts sleep and impairs growth and development in affected children, diminishing the quality of life for both the patients and their families, while also posing potential risks for behavioral and psychological comorbidities. Children with AD could develop food allergies sequentially or concurrently. Subsequently, about 50% of those with moderate-to-severe AD develop allergic rhinitis, and 34% are comorbid with asthma or other atopic conditions (Guttman-Yassky et al., 2025). Thus, AD is regarded as the “first step” in the atopic march.
The AD affects 15–30% of the global population, with its prevalence continuing to rise. Notably, the incidence of AD in the pediatric population has seen a significant increase over recent decades (Ständer, 2021). Although the development of AD is influenced by multiple factors, substantial evidence suggests that environmental factors play a non-negligible role. Traditional factors (e.g., genetics) cannot fully explain this increasing incidence, suggesting that environmental exposure could be key drivers. Within this context, prenatal exposure to endocrine disrupting chemicals (EDCs) has garnered considerable scientific interest. EDCs are a class of exogenous chemicals capable of interfering with the synthesis, metabolism, or signal transduction of endogenous hormones. They could disrupt immune and metabolic homeostasis by interfering with hormone signaling pathways (Ghassabian et al., 2022). Although traditional EDCs such as polychlorinated biphenyls (PCBs) (Peng et al., 2019; Yu et al., 2020), polybrominated diphenyl ethers (PBDEs) (Kemmlein et al., 2009; Betts, 2008), and organochlorine pesticides (Kabasenche and Skinner, 2014) have been progressively phased out, the research focus in recent years has gradually shifted toward emerging EDCs. These include per- and polyfluoroalkyl substances (PFAS), phenolics [e.g., bisphenol A (BPA), parabens, and triclosan (TCS)], and phthalate esters (PAEs). Due to their widespread use in everyday consumer goods and industrial products, the pregnant women face the risk of low-dose, chronic exposure to EDCs.
However, assessing exposure to persistent (e.g., PFAS) and non-persistent (e.g., BPA, PAEs) EDCs presents distinct challenges. For non-persistent chemicals with short half-lives, their concentrations in the human body could fluctuate significantly over short periods due to rapid metabolism and excretion following exposure. This variability implies that a single biospecimen measurement (e.g., a spot urine sample) could fail to accurately represent an individual’s long-term average exposure level, leading to misclassification of exposure status (Sugeng et al., 2020; Perrier et al., 2016). Therefore, accurate assessment strategies require frequent repeated measurements (e.g., collecting multiple urine samples) to capture temporal exposure patterns. In contrast, persistent chemicals (e.g., PFAS) have very long half-lives and could steadily accumulate in human adipose tissue or blood over years. Their internal concentrations remain relatively stable, allowing a single measurement to reasonably reflect the long-term cumulative exposure burden. The primary challenge in assessing exposure to these chemicals lies in their high persistence: they remain detectable long after exposure has ceased, which complicates the identification of critical time windows during which exposure could influence the development of specific health outcomes (Lenters et al., 2019; Melough et al., 2022).
Pregnancy and early life represent key developmental windows for fetal immune system maturation. Maternal exposure to EDCs could transfer to the fetus and neonate via the placenta or breast milk, and disrupt fetal immune programming by altering the maternal-fetal microenvironment, thereby increasing the child’s risk of developing AD (Bansal et al., 2018). In recent years, a growing body of epidemiological evidence indicated that prenatal EDCs exposure could be significantly associated with the development and progression of AD in children (Spanier et al., 2012). The review was to summarize and critically appraise current evidence on the association between prenatal exposure to the emerging EDCs and AD in children. Furthermore, it could provide a scientific basis for developing effective strategies to mitigate prenatal EDCs exposure and implementing early-life interventions for childhood AD.
2 The relationship between prenatal endocrine disrupting chemical exposure and atopic dermatitis in children
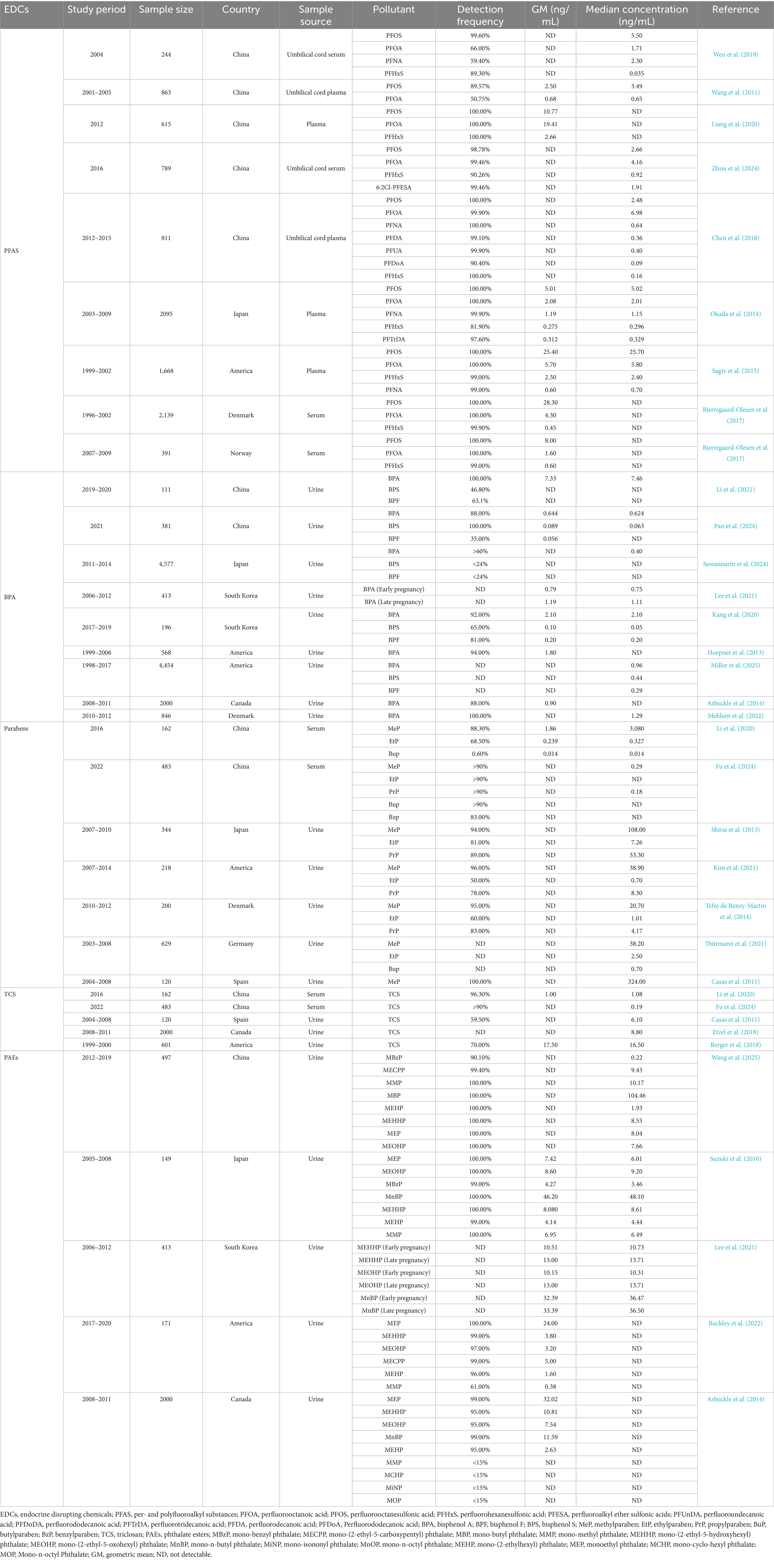
The diversity of EDCs and their widespread environmental distribution contribute to the complexity of exposure pathways (Table 1). In recent years, numerous studies have revealed potential associations between prenatal EDCs exposure and childhood AD. The following section summarized the epidemiological evidence from recent years linking prenatal exposure to PFAS, BPA, parabens, TCS, and PAEs with AD in children.
2.1 Per- and polyfluoroalkyl substances (PFAS)
2.1.1 Prenatal exposure to PFAS
The PFAS are a group of hydrocarbons in which hydrogen atoms are completely or partially replaced by fluorine atoms (Spyrakis and Dragani, 2023). They exhibit extreme hydrophobicity, chemical stability, and thermal stability, making them highly persistent in the natural environment (Buck et al., 2021). As a result, PFAS are widely distributed in nature, and the population—particularly pregnant women—is easily exposed to these compounds, facing potential health risks (Bangma et al., 2022). PFAS could enter the maternal body through various routes such as the skin, digestive tract, and respiratory system (Schwanz et al., 2016; Sunderland et al., 2019; Guardian et al., 2020; Franko et al., 2012). They could cross the placental barrier and accumulate in the fetus, potentially leading to adverse health outcomes such as allergic or atopic diseases (Liu et al., 2024). Traditional PFAS, including perfluorooctanoic acid (PFOA), perfluorooctanesulfonic acid (PFOS), and perfluorohexanesulfonic acid (PFHxS), have been listed under the Stockholm Convention on Persistent Organic Pollutants due to their persistence in the environment and bioaccumulative toxicity, and are being progressively phased out or restricted (Liang et al., 2022). In response, new substitutes for PFOS, such as perfluoroalkyl ether sulfonic acids (PFESA), continue to emerge (Liu et al., 2021; Xu et al., 2021). The potential toxicity of these emerging PFAS compounds, particularly their long-term effects on the health of pregnant women and fetuses, requires further in-depth research.
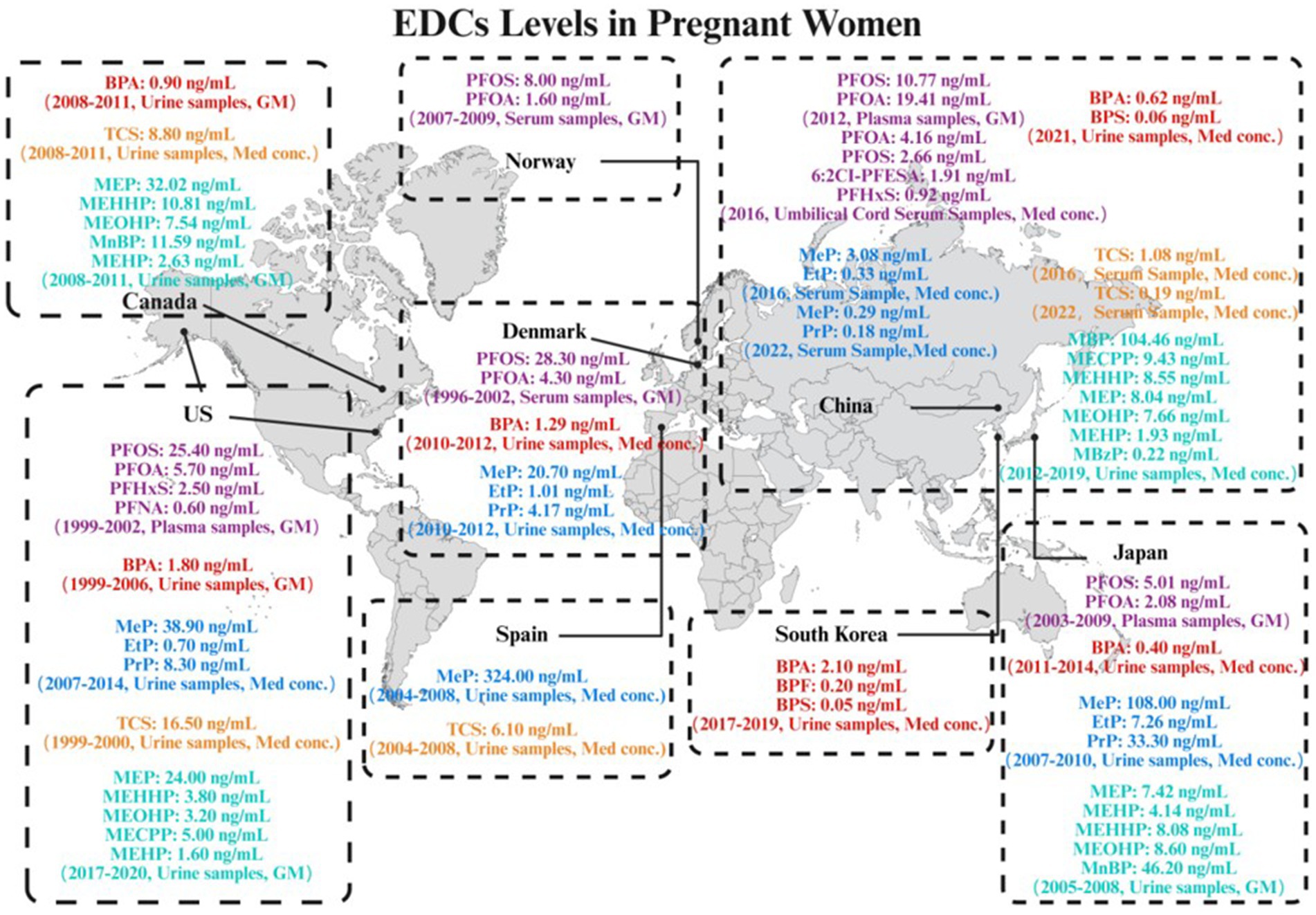
As shown in Figure 1 and Table 1, accumulating evidence indicated ubiquitous exposure to PFAS in pregnant populations. In Asian, PFOA and PFOS were detected in all plasma samples from pregnant women in China and Japan. However, compared to Japan, Chinese pregnant women demonstrated higher plasma exposure levels of PFOA and PFOS, with geometric means (GM) of 19.41 ng/mL and 10.77 ng/mL, respectively (Liang et al., 2020; Okada et al., 2014). PFOA demonstrated the highest median concentration (4.16 ng/mL) and was detectable in over 90% of umbilical cord serum samples, followed by PFOS (2.66 ng/mL) (Zhou et al., 2024). The exposure levels of PFOS and PFOA in umbilical cord plasma were found to be comparable to those in serum (Chen et al., 2018). In addition, the novel alternative 6:2Cl-PFESA was also detected at a relatively high concentration, with a median value intermediate between those of PFOS and PFHxS (Zhou et al., 2024). In a cohort study conducted in North America, PFOS and PFOA were detected in all maternal plasma samples collected from pregnant women, with PFOS exhibiting the highest concentration among all PFAS compounds, having a GM of 25.4 ng/mL (Sagiv et al., 2015). PFOS and PFOA were detected in all serum samples collected from pregnant women in Denmark and Norway. The GM for PFOS concentrations were 28.3 ng/mL and 8.0 ng/mL in Danish and Norwegian pregnant women, respectively, while the GM for PFOA were 4.3 ng/mL and 1.6 ng/mL, respectively (Bjerregaard-Olesen et al., 2017). In Europe, a clear trend of declining exposure to legacy PFAS is evident following their phase-out. Specifically, levels measured in Norwegian pregnant women (2007–2009) were significantly lower than those previously documented in Danish pregnant women (1996–2002). Collectively, global maternal exposure is characterized by dominant long-chain PFAS (PFOA/PFOS) co-occurring with short-chain analogs (e.g., PFHxS) and emerging alternatives (e.g., 6:2Cl-PFESA). These compounds could transgress the placental barrier and bioaccumulation in fetal circulation (Liu et al., 2024; Souza et al., 2020). Given their potential to disrupt offspring immune development—particularly via transplacental transfer-mediated developmental immunotoxicity—investigating associations between prenatal PFAS exposure and childhood AD has emerged as a critical research priority, warranting subsequent synthesis of epidemiological evidence and mechanistic insights.
2.1.2 Prenatal PFAS exposure and childhood atopic dermatitis
Epidemiological studies on prenatal PFAS exposure and childhood AD have primarily emerged from Asian regions yet reveal notably divergent findings.
In the Taiwan, China Birth Cohort, prenatal PFOA and PFOS exposure showed no significant association with the risk of childhood AD (Wang et al., 2011). A recent meta-analysis concluded that prenatal exposure to PFOA, PFOS, perfluoroundecanoic acid (PFUnDA), perfluorododecanoic acid (PFDoDA), and perfluorotridecanoic acid (PFTrDA) showed no significant effect on childhood AD (Hatem et al., 2025).
Conversely, the Hokkaido Study on Environment and Children’s Health suggested that lower prenatal exposure to PFTrDA could reduce the odds of early childhood eczema, but this association was restricted to female infants (Okada et al., 2014). Another cohort study in Taiwan, China indicated that higher prenatal PFOA exposure levels were associated with an increased odds of early-onset AD (Wen et al., 2019). Further analysis within this cohort revealed that prenatal PFOA exposure elevated the risk of AD specifically in children with glutathione S-transferase theta 1 (GSTT1) and glutathione S-transferase mu 1 (GSTM1) null genotypes (Wen et al., 2019). A prospective birth cohort study conducted in Shanghai, China demonstrated that prenatal exposure to PFOA, perfluorodecanoic acid (PFDA), Perfluorododecanoic acid (PFDoA), and PFHxS was significantly associated with an increased odds of AD in female children (Chen et al., 2018) (Figure 2).
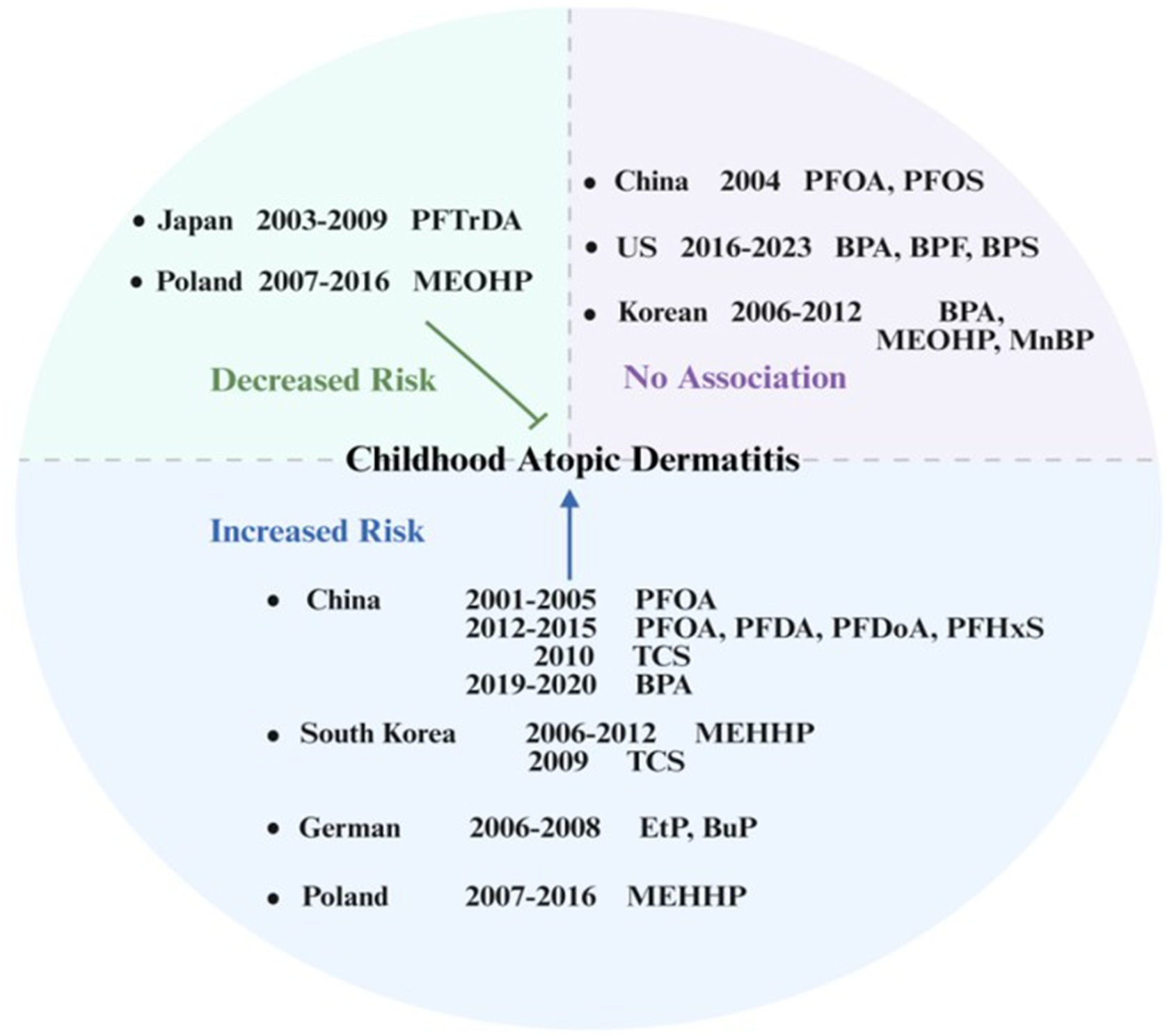
Figure 2. Prenatal exposure to endocrine-disrupting chemicals (EDCs) and risk of atopic dermatitis in offspring: an international perspective.
The heterogeneity in these findings could be partly attributable to the complex nature of real-world chemical exposures, which typically occur as mixtures rather than as isolated compounds (Ribeiro et al., 2017). Health risk assessments based on single substances could overlook potential interactions between co-occurring environmental chemicals. These interactions could be dose-additive, where the combined effect equals the sum of individual effects; synergistic, where the combined effect is greater than the sum; or antagonistic, where one chemical reduces the effect of another (Couleau et al., 2015). For instance, coexposure to certain PFAS could exhibit synergistic immunotoxicity even when individual compound concentrations are below effect thresholds (Du et al., 2025). Therefore, mixture-based exposure assessment approaches are critically needed to better understand the complex etiology of childhood AD (Charles et al., 2002).
Mechanistically, prenatal PFAS exposure could enable these compounds to cross the placental barrier, leading to fetal accumulation (Liu et al., 2024; Souza et al., 2020). In vitro assays combined with RNA-seq analysis have revealed that PFAS could contribute to AD-like skin pathology by impairing vitamin D receptor signaling and antimicrobial defense (Kim et al., 2025). By in vitro assays and RNA-seq analysis PFAS could contribute to AD-like skin pathology by impairing vitamin D receptor signaling and antimicrobial defense.
Collectively, studies indicated that the association between prenatal PFAS exposure and AD exhibited complexity. Certain research suggested specific PFAS compounds (notably PFOA) could increase the risk of childhood AD, with evidence pointing toward gender- or gene-specific susceptibility. However, other studies have failed to establish significant associations. These discrepancies could stem from spatial and temporal heterogeneity in PFAS exposure levels, variations in mixture composition, and modulatory effects of host factors such as sex specificity and genetic background, necessitating further investigation into the integrated effects of chemical mixtures and their biological mechanisms to establish causal relationships.
2.2 Bisphenol A (BPA)
2.2.1 Prenatal exposure to BPA
Bisphenol A, chemically designated as 2,2-bis(4-hydroxyphenyl)propane, is a compound featuring two phenolic hydroxyl groups and ranks among the most widely used chemicals globally (Eladak et al., 2015). It is commonly found in thermal receipt paper, dental sealants, and the linings of numerous food and beverage containers (Thoene et al., 2018). Regarding its biological properties, BPA exposure has been associated with cardiovascular disease, respiratory issues, metabolic disorders, kidney disease, and reproductive health concerns. These health risks have prompted restrictions or bans on its use in certain food or beverage packaging in some countries (Eladak et al., 2015; Lang et al., 2008). Due to these safety concerns, BPA is frequently replaced by structurally similar analogs, bisphenol F (BPF) and bisphenol S (BPS) (Eladak et al., 2015). BPF and BPS are also present in food packaging, beverage containers, and paper products. Additionally, BPF is used in pipe and tank linings to enhance durability and thickness, while BPS finds application in detergents and corrosion inhibitors (Rochester and Bolden, 2015). Significant increases in the use of these alternatives over the past decade have led to higher environmental exposure levels (Eladak et al., 2015; Rochester and Bolden, 2015). The primary route of human exposure to BPA is the ingestion of contaminated food or beverages. Dermal contact (e.g., handling thermal paper) and inhalation of BPA-containing indoor dust represent additional potential exposure pathways (Eladak et al., 2015). Given the widespread human exposure to BPA, its impact as a common EDC on vulnerable populations, particularly pregnant women and their fetuses, is a subject of significant research focus. Prenatal exposure to BPA could pose potential health risks to the developing fetus, making it a critical topic in environmental health research.
As shown in Figure 1 and Table 1, in Asian, BPA and BPS were detected in over 80% of the urine samples from pregnant women in China. BPA exhibited the highest median concentration of 0.624 ng/mL (Pan et al., 2024). In Korean pregnant women, the detection rates of BPA, BPS, and BPF in urine ranged from 65 to 92%. BPA exhibited the highest median concentration at 2.1 ng/mL (Kang et al., 2020). Among Asian countries, pregnant women in Japan exhibit relatively lower exposure levels to BPA compared to those in China and South Korea. BPA was detected in over 60% of the maternal urine samples, with a median concentration of 0.4 ng/mL, while the detection rates of both BPF and BPS were below 24% (Suwannarin et al., 2024). In North America, pregnant women in the United States exhibited higher BPA exposure levels compared to those in Canada. BPA was detectable in 94% of urine samples from U. S. pregnant women, with a GM concentration of 1.8 ng/mL. In contrast, BPA was detected in 88% of Canadian women, at a lower GM concentration of 0.9 ng/mL (Hoepner et al., 2013; Arbuckle et al., 2014). In Europe, BPA was detected in all urine samples collected from pregnant women in Denmark, with a median concentration of 1.29 ng/mL (Mehlsen et al., 2022). Collectively, these large-scale birth cohort studies from diverse global regions consistently demonstrated the high prevalence and ubiquity of BPA exposure among pregnant women. Studies spanning China (Pan et al., 2024), Korea (Kang et al., 2020), Japan (Suwannarin et al., 2024), the United States (Hoepner et al., 2013), Canada (Arbuckle et al., 2014), and Denmark (Mehlsen et al., 2022) revealed very high detection frequencies for urinary BPA, ranging from 59% to over 90%, indicating that most pregnant women were exposed to environmental BPA. Although reported median urinary concentrations exhibited some variability across studies – potentially attributable to geographic differences, population lifestyles, timing of sampling, or analytical methodologies – the widespread presence of BPA in pregnant women and its predominance among bisphenols are unequivocal and consistent findings. Notably, the South China study detected BPA and emerging bisphenols even amidst increasing use of substitutes, suggesting concurrent prenatal exposure to mixtures of BPA and structurally related phenolic compounds (Pan et al., 2024). The cumulative evidence highlighted prenatal exposure to BPA and structural analogs as a pervasive public health burden worldwide. The potential health consequences, specifically the developmental impacts on offspring, demand concerted scientific and regulatory focus.
2.2.2 Prenatal BPA exposure and childhood atopic dermatitis
In this context, investigating the association between prenatal exposure to bisphenol compounds—particularly BPA—and immune-related disorders in children, such as AD, has become a critical research priority. There is limited research on the association between prenatal exposure to bisphenols and AD in children. A prospective cohort study in South Korea showed that BPA was not strongly associated with AD in the single-pollutant model (Lee et al., 2021). A United States cohort study found no association between prenatal exposure to BPA, BPF, and BPS and the risk of childhood AD. Furthermore, in girls, a higher continuous BPS exposure index during pregnancy was inversely associated with AD risk (Miller et al., 2025). A Chinese cohort study revealed that higher prenatal BPA exposure was associated with an increased risk of infantile eczema, suggesting that this could be related to the downregulation of FOXP3 gene expression in cord blood (Li et al., 2021) (Figure 2). Collectively, the association between maternal BPA exposure during pregnancy and the development of AD in children varies across countries. These discrepancies could be attributed to variations in exposure profiles, specific bisphenol analogs or population heterogeneity.
Although epidemiological studies on this topic were still limited, emerging evidence from research on BPA and allergies suggested potential mechanisms through which BPA could contribute to the development of childhood AD. These included the promotion of pro-inflammatory cytokines, increased serum IgE production, eosinophilia, a shift in the Th1/Th2 balance, and alterations in Th17 cell abundance (Weteska et al., 2023).
2.3 Parabens
2.3.1 Prenatal exposure to parabens
Parabens, such as methylparaben (MeP), ethylparaben (EtP), propylparaben (PrP), butylparaben (BuP), and benzylparaben (BzP), are a class of compounds widely used as preservatives in cosmetics, food, and pharmaceutical products (Nowak et al., 2018; Błędzka et al., 2014). The primary routes of human exposure include ingestion of paraben-containing food/pharmaceuticals and dermal absorption from cosmetics. Additionally, their presence in dust and indoor air constitutes a potential secondary exposure source (Błędzka et al., 2014). While human exposure data and health effects remained relatively limited, animal studies demonstrated that parabens exhibited estrogenic properties (Boberg et al., 2010) and could accumulate in amniotic fluid (Frederiksen et al., 2008). Given their extensive use, potential endocrine-disrupting properties, and observed accumulation in amniotic fluid in animal models, assessing actual paraben exposure levels in vulnerable populations—particularly pregnant women—is critically important. Multiple biomonitoring studies across diverse countries and populations provided key evidence, revealing ubiquitous exposure to these compounds among pregnant women.
Biomonitoring studies from multiple countries, including China, Denmark, the United States, Japan, and Spain, consistently confirmed widespread exposure to parabens among pregnant populations (Table 1). In Asian, a Chinese cohort study detected MeP, EtP, PrP, and BuP in over 90% of serum samples, while BzP was detected in 83% of samples. Median levels of MeP (0.29 ng/mL) and PrP (0.18 ng/mL) were higher than those of EtP, BuP, and BzP (Fu et al., 2024). Analysis of maternal serum samples collected from 13 provinces in China in 2016 found the total median concentration of parabens and their metabolites ranged from 0.014 to 3.08 ng/mL. MeP and EtP were the predominant compounds, with median concentration of 3.08 ng/mL and 0.327 ng/mL, respectively. Concentrations of MeP and EtP in maternal serum from Northeastern China were significantly higher than those in other regions (Li et al., 2020). A Japanese cohort study reported that total MeP, EtP, and PrP were detected in over 80% of the participants, while total BuP was detected in 50% of the subjects. The median concentrations of MeP and PrP were 108 ng/mL and 33.3 ng/mL, respectively, whereas those of EtP and BuP were both below 8 ng/mL (Shirai et al., 2013). In North America, a cohort study conducted in California reported that MeP was detectable in 96% of maternal urine samples, while EtP was detectable in 50% of the samples. The median concentration of MeP was substantially higher (approximately 55-fold) than that of EtP. Additionally, PrP was detectable in 78% of the urine samples, with the median MeP concentration being about 4 times higher than that of PrP (Kim et al., 2021). In Europe, The Spanish birth cohort study found that MeP was detected in all maternal urine samples and exhibited the highest median concentration among the parabens analyzed (Casas et al., 2011). A Danish child cohort study revealed median maternal urinary concentrations of MeP and EtP as 20.7 ng/mL and 1.01 ng/mL, respectively (Tefre de Renzy-Martin et al., 2014). MeP was the most frequently detected compound and generally exhibited the highest concentrations. These studies demonstrated extremely high detection frequencies for MeP in maternal serum or urine, and its median or GM concentration was often significantly higher than other parabens (e.g., EtP, PrP, BuP, and BzP). PrP also showed high detection frequencies and concentration levels in maternal serum or urine (Fu et al., 2024; Li et al., 2020; Shirai et al., 2013; Kim et al., 2021; Casas et al., 2011; Tefre de Renzy-Martin et al., 2014). Notably, exposure levels exhibited geographical variation, exemplified by significantly higher serum concentrations of MeP and EtP in pregnant women from Northeastern China compared to other regions (Li et al., 2020). The heterogeneity in paraben exposure levels observed in pregnant women from multinational studies is primarily attributed to divergences in research methods (analytical techniques, target analytes, study period), geographical environments, and population behaviors. These findings collectively demonstrated that, despite existing regulatory measures, pregnant women remained widely exposed to parabens, with MeP and PrP representing the predominant exposure species. Given the ubiquitous presence and notable concentrations of these compounds in pregnant women, combined with their potential association with adverse health outcomes in children, such as AD, the ongoing biomonitoring of paraben exposure levels in this population is of critical importance.
2.3.2 Parabens exposure and childhood atopic dermatitis
A German cohort study evaluated the association between prenatal paraben exposure and childhood AD. The study demonstrated that prenatal exposure to EtP and BuP was associated with an increased risk of very early-onset AD (diagnosed by age two) that persisted beyond this age without remission (Thürmann et al., 2021) (Figure 2).
According to the “hygiene hypothesis,” early-life microbial exposure promotes immune system development, thereby reducing the risk of allergic diseases (Ege, 2017). Conversely, direct exposure to antimicrobial compounds via oral or dermal routes may alter microbial communities in the skin or gut, potentially inducing a hypersensitive state in immune receptors within these organs and increasing the risk of hypersensitivity disorders such as asthma and eczema (Stiemsma and Turvey, 2017). It is proposed that parabens, as antimicrobial compounds, could increase the risk of AD in children prenatally exposed to them through precisely this mechanism: by traversing the placental barrier, these antimicrobial compounds have the potential to disrupt the maternal microbiota and shape the infant’s developing microbiome, thereby influencing a key factor in AD pathogenesis (Jackson-Browne et al., 2019). Beyond their potential effects on the microbiome, long-term exposure to parabens could disrupt keratinocyte differentiation, suggesting a direct interference with skin development processes (Ishiwatari et al., 2007).
Collectively, these findings indicated that gestational exposure to parabens represented a significant environmental risk factor for the development of severe and persistent early-childhood AD.
2.4 Triclosan (TCS)
2.4.1 Prenatal exposure to TCS
Triclosan is a polychlorinated bisphenolic compound characterized by a distinct aromatic odor. As a broad-spectrum lipophilic antimicrobial agent, it is extensively used in products such as hand sanitizers, cosmetics, preservatives, and disinfectants. Consequently, TCS is frequently detected in the environment and has become an almost ubiquitous contaminant (Weatherly and Gosse, 2017; Alfhili and Lee, 2019). The primary routes of TCS exposure in pregnant women include dermal contact (e.g., using TCS-containing personal care products) and direct ingestion of contaminated drinking water, food, or animal-derived products. TCS has been frequently detected in maternal urine, blood, breast milk, and even amniotic fluid (Milanović et al., 2023; Chen et al., 2019; Yueh and Tukey, 2016), indicating that its exposure during pregnancy poses non-negligible risks to fetal health. As a widely used phenolic compound, TCS exhibits endocrine-disrupting properties (Liu et al., 2022), raising particular concern over its potential effects during critical developmental windows, especially in gestation. Assessing TCS exposure levels and associated health risks in pregnant populations is essential for understanding its developmental toxicity mechanisms. To date, multiple biomonitoring studies focusing on pregnant women have provided valuable data for such risk characterization.
Multiple pregnancy cohort studies from China, Spain, Canada, and the United States confirmed widespread TCS exposure in pregnant populations. As shown in Figure 1 and Table 1, in Asian, a 2016 Chinese cohort study detected TCS in 96.3% of maternal serum samples. The highest concentration was observed in North China (GM: 1.184 ng/mL), followed by Northeast China, Southwest China, East China, and Central-South China, in descending order of GM (Li et al., 2020). A 2022 Chinese cohort study detected TCS in over 90% of serum samples, with a median maternal level of 0.19 ng/mL (Fu et al., 2024). In Europe, Spanish birth cohort study detected TCS in only 59.5% of samples, with a median concentration of 6.1 ng/mL (Casas et al., 2011). In North America, a Canadian prospective cohort study reported a higher median concentration of TCS in maternal urine compared to that reported in a Spanish cohort (Etzel et al., 2018). A longitudinal birth cohort study in California detected TCS in 70% of maternal urine samples, with the median concentration being the highest among reported national data at the time (Berger et al., 2018). Although reported detection frequencies and concentration levels vary significantly across studies—likely reflecting differences in geography, population habits, detection methods, or sample type (serum/urine)—the detectability of TCS in maternal biological samples is a clear and prevalent phenomenon. Notably, regional variations in TCS concentrations (e.g., relatively higher levels in North China) were observed even within studies conducted in the same country. In summary, multinational evidence indicated that prenatal TCS exposure was prevalent but exhibited significant geographic heterogeneity. Given this variability in exposure levels and the potential link to childhood AD, further investigation into its determinants and health impacts—particularly regarding its contribution to offspring AD risk—constitutes a critical public health priority.
2.4.2 TCS and childhood atopic dermatitis
Two Asian studies suggested that the association between TCS exposure and childhood AD could exhibit age and sex specificity. A 2009 Korean population-based cross-sectional study demonstrated a positive association between urinary TCS levels and AD in school-aged children (Choi et al., 2024). The Taiwan, China 2010 cohort study found that TCS levels were significantly associated with AD in boys (Lin et al., 2022), which indicated that the immunomodulatory effects of TCS could be regulated by developmental stage and sex factors (Figure 2).
An Animal study further elucidated the underlying mechanisms: TCS exacerbated AD-like skin inflammation in mice by inducing skin inflammation and immune cell infiltration via thymic stromal lymphopoietin (TSLP) (Schuppe et al., 2025).
In summary, exposure to TCS was robustly associated with the development of AD in children. However, more longitudinal cohort studies are warranted to confirm this causal relationship, and further investigation is needed to elucidate the underlying mechanisms.
2.5 Phthalate esters (PAEs)
2.5.1 Prenatal exposure to PAEs
Phthalate esters are a group of compounds used as plasticizers (Zhang et al., 2021). Based on the length of their alcohol side chains, they could be categorized into long-chain PAEs—such as di(2-ethylhexyl) phthalate (DEHP), butyl benzyl phthalate (BBzP), and diisononyl phthalate (DiNP)—and short-chain phthalates, which is phthalate metabolites (mPAEs), including diethyl phthalate (DEP) and di-n-butyl phthalate (DnBP). Long-chain PAEs are predominantly used in polyvinyl chloride (PVC) products such as building materials and food containers, whereas mPAEs are more commonly found in non-PVC applications, including adhesives and personal care products. Diet is considered the primary source of exposure to long-chain PAEs during pregnancy, whereas significant sources of mPAEs, could include personal care products and indoor air (Chang et al., 2021; Mariana et al., 2016). PAEs are clearly classified as EDCs. Upon entering the body, they could disrupt the endocrine system by binding to molecular targets and interfering with hormonal homeostasis (Chang et al., 2021; Mariana et al., 2016). There is growing concern regarding the health impacts of gestational exposure to PAEs on fetal development. This growing concern was substantiated by biomonitoring data from birth cohort studies across the globe, which demonstrated widespread exposure to these toxicants.
As shown in Figure 1 and Table 1, a birth cohort study in China, revealed widespread exposure to mPAEs in pregnant women. Except for mono-benzyl phthalate (MBzP), detected in 90.1% of participants, and mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP), detected in 99.4%, all other metabolites were detected in 100% of the subjects. Among the eight major mPAEs quantitatively analyzed, the concentration distribution showed a distinct hierarchy: mono-butyl phthalate (MBP) was the dominant metabolite by a large margin (median concentration: 104.46 ng/mL), with levels approximately 10 times higher than those of the second most abundant metabolite, mono-methyl phthalate (MMP). The remaining metabolites followed a descending concentration order: MECPP > mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) > monoethyl phthalate (MEP) > mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP) > mono-(2-ethylhexyl) phthalate (MEHP) > MBzP (Wang et al., 2025). MMP, MEP, mono-n-butyl phthalate (MnBP), MBzP, MEOHP, and MEHHP were detected in all urine samples from pregnant women in Japan. Among the monoester metabolites analyzed, urinary MnBP exhibited the highest concentration (median concentration: 48.1 ng/mL), whereas mono-isononyl phthalate (MiNP) and mono-n-octyl phthalate (MnOP) were present at considerably lower levels (Suzuki et al., 2010). In North America, a large US cohort study detected MEP, MEHHP, MECPP, MEHP and MEOHP in over 90% of the study population, with GM ranges of 1.6–24 ng/mL (Buckley et al., 2022). MEP, MEHHP, MnBP, MEHP and MEOHP were widely detected in over 90% among pregnant women in Canada. The highest measured concentrations of mPAEs were MEP (GM: 32.02 ng/mL) and MnBP (GM: 11.59 ng/mL) (Arbuckle et al., 2014). Synthesizing cohort studies from China, the United States, Canada, and Japan revealed that mPAEs exposure was globally prevalent in pregnant populations, but systematic differences existed in the dominant metabolite species and concentration levels across regions. Collectively, these findings demonstrated complex geographic heterogeneity in prenatal PAEs exposure across global populations, with short-chain metabolites such as MnBP dominating the exposure profile in most cohorts. Given that high-abundance metabolites (e.g., MnBP, MEHHP) have been shown to disrupt Th1/Th2 immune homeostasis and elevate AD risk (Lee et al., 2021; Berger et al., 2018), the nexus between their ubiquitous exposure patterns and suspected health implications necessitates further mechanistic investigation.
2.5.2 PAEs and childhood atopic dermatitis
Regarding the association between prenatal PAEs exposure and childhood AD, studies suggested that metabolite type, exposure timing (window), and mixture effects collectively determine the direction of risk. A Polish prospective mother–child cohort study indicated that higher prenatal urinary concentrations of MEOHP were associated with a reduced risk of AD, whereas higher concentrations of MEHHP were associated with an increased AD risk (Podlecka et al., 2020). A Korean prospective birth cohort study, measuring maternal urinary MEHHP, MEOHP, and MnBP concentrations in early and late pregnancy, showed that exposure to MEHHP in late pregnancy was significantly associated with AD incidence. Furthermore, co-exposure to BPA and mPAEs during pregnancy synergistically increased AD risk (Lee et al., 2021) (Figure 2).
Mechanistically, metabolites such as MEHHP acted as co-stimulators that promote IL-4 and IFN-γ secretion, reduced the Th1/Th2 ratio in vitro (Pei et al., 2014), and enhanced allergen-specific IgE production (Han et al., 2014). Animal models confirmed that DEHP activated PPARα, driving Th1/Th2 balance toward Th2 polarization (Larsen and Nielsen, 2007). This mechanistic evidence elucidated a plausible pathway linking PAEs exposure to childhood AD onset.
In summary, PAEs exhibited bidirectional immunomodulatory effects on AD pathogenesis. Their net impact depends on the exposure profile composition, critical developmental windows, and immune microenvironment crosstalk, necessitating future isomer-resolved analyses, which distinguish between individual molecular structures, and mixture-based risk assessments to reconcile conflicting evidence.
3 Prevention and intervention measures
Given that both epidemiological and mechanistic studies suggested an association between prenatal EDCs exposure and childhood AD risk, implementing a multi-level, perinatal integrated intervention strategy is crucial (Wen et al., 2019; Lee et al., 2021; Miller et al., 2025; Kim et al., 2024). Primary measures focus on source avoidance to reduce maternal EDCs exposure levels. Pregnant women should actively modify lifestyles, such as avoiding plastic food containers containing BPA (e.g., packaging, bottles) in favor of safer materials like glass or stainless steel, and choosing personal care and household products (e.g., skincare, detergents) free of PAEs to minimize dermal absorption risk (Vandenberg et al., 2012). Dietarily, prioritizing organic produce helps reduce pesticide residue exposure, while minimizing processed food intake avoids contact with preservatives like parabens (Berni Canani et al., 2024; Çakmakçı and Çakmakçı, 2023). Secondly, nutritional interventions during pregnancy are vital for enhancing maternal-fetal barrier function and immune regulation. Supplementation with omega-3 polyunsaturated fatty acids may exert protective effects by suppressing maternal inflammatory responses and modulating fetal immune development (Gutiérrez et al., 2019). Vitamin D supplementation could reduce offspring AD risk by regulating Treg/Th2 immune balance (Martineau et al., 2017). For pregnant women at high risk of EDCs exposure (e.g., occupational exposure or residing in industrial pollution zones), dynamic monitoring of exposure levels is recommended. Regular testing of serum or urine for EDCs metabolites (e.g., BPA, mPAEs), combined with assessment of clinical biomarkers (e.g., cord blood IgE, IL-4) for early risk stratification, aids in identifying high-risk individuals for targeted interventions. At the medical intervention level, supplementation with specific probiotic strains (e.g., Lactobacillus, Bifidobacterium) shows promise. They could inhibit fetal Th2-type immune skewing by modulating the maternal gut microbiota-immune axis, although evidence-based selection of effective strains is essential (Azad et al., 2013). Simultaneously, household environmental controls, such as reducing indoor dust mites and avoiding tobacco smoke, effectively mitigate ongoing childhood EDCs exposure and allergen trigger risks. Finally, robust policy and regulation form the cornerstone of population health protection. It is critical that such policies evolve to address exposure to chemical mixtures, as humans are consistently exposed to complex combinations of EDCs (e.g., PAEs and PFAS) that could act additively or synergistically to increase AD risk. Regulatory frameworks should therefore incorporate mixture-based risk assessment approaches, establishing exposure limits based on cumulative risk and regulating classes of chemicals—such as PAEs or bisphenols—as groups rather than individual substances. Promoting comprehensive EDC regulatory legislation, such as emulating and expanding upon the EU REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) framework to restrict mixture exposures in toys and mother-infant products, is essential. Strengthening public health education also holds profound significance for enhancing maternal awareness of EDCs and reducing overall population exposure levels.
4 Conclusion and future perspectives
Current research robustly demonstrated that prenatal exposure to multiple EDCs could significantly increase the risk of AD in children. Although epidemiological studies exhibited heterogeneity, they provided evidence for dose-dependent associations between prenatal exposure to specific EDCs and the risk of childhood AD. To effectively address this challenge, preventive strategies must integrate multi-level approaches: ranging from individual-level measures such as exposure avoidance and nutritional optimization to clinical biological monitoring and risk stratification, and further to societal-level policy support and public education. Together, these efforts should establish a comprehensive protection system covering the entire ‘pregnancy to postpartum’ continuum.
Looking ahead, research should focus on several key directions: First, in-depth investigation into the complex synergistic or antagonistic effects of mixed EDCs exposures and their impact on childhood AD, which better reflects real-world exposure scenarios. Second, elucidating the underlying mechanisms through which prenatal EDCs exposure contributes to the pathogenesis of AD in children, utilizing large international birth cohorts (e.g., COPSAC, CHAMACOS) for integrated multi-omics analysis (Maitre et al., 2022). This approach is central to uncovering the mechanisms linking early-life exposure to disease. By conducting multi-omics analyses on biological samples collected from these cohorts and correlating the findings with experimental studies, we could ultimately clarify how prenatal EDC exposure drives the development of AD by regulating gene pathways related to immune programming and skin barrier function. Third, actively exploring the feasibility of novel targeted intervention technologies based on the mechanistic research, such as the development of specific nano-detoxifying agents or receptor antagonists. By fostering deep collaboration across various disciplines—including environmental medicine, immunology, epigenetics, toxicology, and public health—and establishing a comprehensive prevention and control network based on an integrated “exposome-immune programming-clinical phenotype” model, we could potentially develop more precise and effective solutions to reduce the global disease burden of childhood AD.
Author contributions
YC: Writing – original draft, Writing – review & editing. LZ: Data curation, Funding acquisition, Investigation, Writing – review & editing. TY: Data curation, Supervision, Validation, Writing – review & editing. LC: Conceptualization, Data curation, Funding acquisition, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This review was supported by grants from Top medical expert team of Wuxi Taihu Talent Plan (Grant no. DJTD202106, GDTD202105, and YXTD202101), Medical Key Discipline Program of Wuxi Health Commission (Grant no. ZDXK2021007) and Top Talent Support Program for young and middle-aged people of Wuxi Health Committee (Grant no. BJ2023090).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alfhili, M. A., and Lee, M. H. (2019). Triclosan: an update on biochemical and molecular mechanisms. Oxidative Med. Cell. Longev. 2019:1607304. doi: 10.1155/2019/1607304
Arbuckle, T. E., Davis, K., Marro, L., Fisher, M., Legrand, M., LeBlanc, A., et al. (2014). Phthalate and bisphenol a exposure among pregnant women in Canada--results from the MIREC study. Environ. Int. 68, 55–65. doi: 10.1016/j.envint.2014.02.010
Azad, M. B., Coneys, J. G., Kozyrskyj, A. L., Field, C. J., Ramsey, C. D., Becker, A. B., et al. (2013). Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: systematic review and meta-analysis. BMJ 347:f6471. doi: 10.1136/bmj.f6471
Bangma, J., Guillette, T. C., Bommarito, P. A., Ng, C., Reiner, J. L., Lindstrom, A. B., et al. (2022). Understanding the dynamics of physiological changes, protein expression, and PFAS in wildlife. Environ. Int. 159:107037. doi: 10.1016/j.envint.2021.107037
Bansal, A., Henao-Mejia, J., and Simmons, R. A. (2018). Immune system: an emerging player in mediating effects of endocrine disruptors on metabolic health. Endocrinology 159, 32–45. doi: 10.1210/en.2017-00882
Berger, K., Eskenazi, B., Balmes, J., Holland, N., Calafat, A. M., and Harley, K. G. (2018). Associations between prenatal maternal urinary concentrations of personal care product chemical biomarkers and childhood respiratory and allergic outcomes in the CHAMACOS study. Environ. Int. 121, 538–549. doi: 10.1016/j.envint.2018.09.027
Berger, K., Gunier, R. B., Chevrier, J., Calafat, A. M., Ye, X., Eskenazi, B., et al. (2018). Associations of maternal exposure to triclosan, parabens, and other phenols with prenatal maternal and neonatal thyroid hormone levels. Environ. Res. 165, 379–386. doi: 10.1016/j.envres.2018.05.005
Berni Canani, R., Carucci, L., Coppola, S., D’Auria, E., O’Mahony, L., Roth-Walter, F., et al. (2024). Ultra-processed foods, allergy outcomes and underlying mechanisms in children: an EAACI task force report. Pediatr. Allergy Immunol. 35:e14231. doi: 10.1111/pai.14231
Betts, K. S. (2008). Unwelcome guest: PBDEs in indoor dust. Environ. Health Perspect. 116, A202–A208. doi: 10.1289/ehp.116-a202
Bjerregaard-Olesen, C., Bossi, R., Liew, Z., Long, M., Bech, B. H., Olsen, J., et al. (2017). Maternal serum concentrations of perfluoroalkyl acids in five international birth cohorts. Int. J. Hyg. Environ. Health 220, 86–93. doi: 10.1016/j.ijheh.2016.12.005
Błędzka, D., Gromadzińska, J., and Wąsowicz, W. (2014). Parabens. From environmental studies to human health. Environ. Int. 67, 27–42. doi: 10.1016/j.envint.2014.02.007
Boberg, J., Taxvig, C., Christiansen, S., and Hass, U. (2010). Possible endocrine disrupting effects of parabens and their metabolites. Reprod. Toxicol. 30, 301–312. doi: 10.1016/j.reprotox.2010.03.011
Buck, R. C., Korzeniowski, S. H., Laganis, E., and Adamsky, F. (2021). Identification and classification of commercially relevant per- and poly-fluoroalkyl substances (PFAS). Integr. Environ. Assess. Manag. 17, 1045–1055. doi: 10.1002/ieam.4450
Buckley, J. P., Kuiper, J. R., Bennett, D. H., Barrett, E. S., Bastain, T., Breton, C. V., et al. (2022). Exposure to contemporary and emerging Chemicals in Commerce among pregnant women in the United States: the environmental influences on child health outcome (ECHO) program. Environ. Sci. Technol. 56, 6560–6573. doi: 10.1021/acs.est.1c08942
Çakmakçı, S., and Çakmakçı, R. (2023). Quality and nutritional parameters of food in Agri-food production systems. Foods. 12:351. doi: 10.3390/foods12020351
Casas, L., Fernández, M. F., Llop, S., Guxens, M., Ballester, F., Olea, N., et al. (2011). Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ. Int. 37, 858–866. doi: 10.1016/j.envint.2011.02.012
Chang, W. H., Herianto, S., Lee, C. C., Hung, H., and Chen, H. L. (2021). The effects of phthalate ester exposure on human health: a review. Sci. Total Environ. 786:147371. doi: 10.1016/j.scitotenv.2021.147371
Charles, G. D., Gennings, C., Zacharewski, T. R., Gollapudi, B. B., and Carney, E. W. (2002). An approach for assessing estrogen receptor-mediated interactions in mixtures of three chemicals: a pilot study. Toxicol. Sci. 68, 349–360. doi: 10.1093/toxsci/68.2.349
Chen, Q., Huang, R., Hua, L., Guo, Y., Huang, L., Zhao, Y., et al. (2018). Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances and childhood atopic dermatitis: a prospective birth cohort study. Environ. Health 17:8. doi: 10.1186/s12940-018-0352-7
Chen, J., Meng, X. Z., Bergman, A., and Halden, R. U. (2019). Nationwide reconnaissance of five parabens, triclosan, triclocarban and its transformation products in sewage sludge from China. J. Hazard. Mater. 365, 502–510. doi: 10.1016/j.jhazmat.2018.11.021
Choi, Y. H., Huh, D. A., and Moon, K. W. (2024). Exposure to biocides and its association with atopic dermatitis among children and adolescents: a population-based cross-sectional study in South Korea. Ecotoxicol. Environ. Saf. 270:115926. doi: 10.1016/j.ecoenv.2023.115926
Couleau, N., Falla, J., Beillerot, A., Battaglia, E., D’Innocenzo, M., Plançon, S., et al. (2015). Effects of endocrine disruptor compounds, alone or in combination, on human macrophage-like THP-1 cell response. PLoS One 10:e0131428. doi: 10.1371/journal.pone.0131428
Du, X., Xu, X., Dong, X. X., Liang, X., Wu, Y., Du, Z., et al. (2025). Integration of animal, population, and Toxicogenomic evidence on the Hematotoxic and immunosuppressive effects of environmental exposure to PFAS mixtures in adolescents. Environ. Sci. Technol. 59, 10841–10853. doi: 10.1021/acs.est.5c01138
Ege, M. J. (2017). The hygiene hypothesis in the age of the microbiome. Ann. Am. Thorac. Soc. 14, S348–S353. doi: 10.1513/AnnalsATS.201702-139AW
Eladak, S., Grisin, T., Moison, D., Guerquin, M. J., N’Tumba-Byn, T., Pozzi-Gaudin, S., et al. (2015). A new chapter in the bisphenol a story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil. Steril. 103, 11–21. doi: 10.1016/j.fertnstert.2014.11.005
Etzel, T., Muckle, G., Arbuckle, T. E., Fraser, W. D., Ouellet, E., Séguin, J. R., et al. (2018). Prenatal urinary triclosan concentrations and child neurobehavior. Environ. Int. 114, 152–159. doi: 10.1016/j.envint.2018.02.032
Franko, J., Meade, B. J., Frasch, H. F., Barbero, A. M., and Anderson, S. E. (2012). Dermal penetration potential of perfluorooctanoic acid (PFOA) in human and mouse skin. J. Toxicol. Environ. Health A 75, 50–62. doi: 10.1080/15287394.2011.615108
Frederiksen, H., Taxvig, C., Hass, U., Vinggaard, A. M., and Nellemann, C. (2008). Higher levels of ethyl paraben and butyl paraben in rat amniotic fluid than in maternal plasma after subcutaneous administration. Toxicol. Sci. 106, 376–383. doi: 10.1093/toxsci/kfn171
Fu, J., Yao, Y., Huang, Z., Guo, Z., Chen, X., Tang, X., et al. (2024). Sex-specific and trimester-specific associations of prenatal exposure to bisphenols, parabens, and Triclosan with neonatal birth size and gestational age. Environ. Sci. Technol. 58, 13687–13696. doi: 10.1021/acs.est.4c04940
Ghassabian, A., Vandenberg, L., Kannan, K., and Trasande, L. (2022). Endocrine-disrupting chemicals and child health. Annu. Rev. Pharmacol. Toxicol. 62, 573–594. doi: 10.1146/annurev-pharmtox-021921-093352
Guardian, M. G. E., Boongaling, E. G., Bernardo-Boongaling, V. R. R., Gamonchuang, J., Boontongto, T., Burakham, R., et al. (2020). Prevalence of per- and polyfluoroalkyl substances (PFASs) in drinking and source water from two Asian countries. Chemosphere 256:127115. doi: 10.1016/j.chemosphere.2020.127115
Gutiérrez, S., Svahn, S. L., and Johansson, M. E. (2019). Effects of Omega-3 fatty acids on immune cells. Int. J. Mol. Sci. 20:5028. doi: 10.3390/ijms20205028
Guttman-Yassky, E., Renert-Yuval, Y., and Brunner, P. M. (2025). Atopic dermatitis. Lancet 405, 583–596. doi: 10.1016/S0140-6736(24)02519-4
Han, Y., Wang, X., Chen, G., Xu, G., Liu, X., Zhu, W., et al. (2014). Di-(2-ethylhexyl) phthalate adjuvantly induces imbalanced humoral immunity in ovalbumin-sensitized BALB/c mice ascribing to T follicular helper cells hyperfunction. Toxicology 324, 88–97. doi: 10.1016/j.tox.2014.07.011
Hatem, G., Faria, A. M., Pinto, M. B., Salamova, A., Teixeira, J. P., Costa, C., et al. (2025). Exposure to per-and poly-fluoroalkyl substances and respiratory and skin effects in children and adolescents: a systematic review and meta-analysis. J. Hazard. Mater. 491:137978. doi: 10.1016/j.jhazmat.2025.137978
Hoepner, L. A., Whyatt, R. M., Just, A. C., Calafat, A. M., Perera, F. P., and Rundle, A. G. (2013). Urinary concentrations of bisphenol a in an urban minority birth cohort in new York City, prenatal through age 7 years. Environ. Res. 122, 38–44. doi: 10.1016/j.envres.2012.12.003
Ishiwatari, S., Suzuki, T., Hitomi, T., Yoshino, T., Matsukuma, S., and Tsuji, T. (2007). Effects of methyl paraben on skin keratinocytes. J. Appl. Toxicol. 27, 1–9. doi: 10.1002/jat.1176
Jackson-Browne, M. S., Henderson, N., Patti, M., Spanier, A., and Braun, J. M. (2019). The impact of early-life exposure to antimicrobials on asthma and eczema risk in children. Curr Environ Health Rep. 6, 214–224. doi: 10.1007/s40572-019-00256-2
Kabasenche, W. P., and Skinner, M. K. (2014). DDT, epigenetic harm, and transgenerational environmental justice. Environ. Health 13:62. doi: 10.1186/1476-069X-13-62
Kang, S., Shin, B. H., Kwon, J. A., Lee, C. W., Park, E. K., Park, E. Y., et al. (2020). Urinary bisphenol a and its analogues and haemato-biochemical alterations of pregnant women in Korea. Environ. Res. 182:109104. doi: 10.1016/j.envres.2019.109104
Kemmlein, S., Herzke, D., and Law, R. J. (2009). Brominated flame retardants in the European chemicals policy of REACH-regulation and determination in materials. J. Chromatogr. A 1216, 320–333. doi: 10.1016/j.chroma.2008.05.085
Kim, K., Shin, H. M., Busgang, S. A., Barr, D. B., Panuwet, P., Schmidt, R. J., et al. (2021). Temporal trends of phenol, paraben, and Triclocarban exposure in California pregnant women during 2007-2014. Environ. Sci. Technol. 55, 11155–11165. doi: 10.1021/acs.est.1c01564
Kim, S. H., Yu, S. Y., Choo, J. H., Kim, J., Ahn, K., and Hwang, S. Y. (2024). Epigenetic methylation changes in pregnant women: bisphenol exposure and atopic dermatitis. Int. J. Mol. Sci. 25:1579. doi: 10.3390/ijms25031579
Kim, J., Yu, S., Choo, J., Lee, H., and Hwang, S. Y. (2025). Per- and Polyfluoroalkyl substance-induced skin barrier disruption and the potential role of calcitriol in atopic dermatitis. Int. J. Mol. Sci. 26:7085. doi: 10.3390/ijms26157085
Lang, I. A., Galloway, T. S., Scarlett, A., Henley, W. E., Depledge, M., Wallace, R. B., et al. (2008). Association of urinary bisphenol a concentration with medical disorders and laboratory abnormalities in adults. JAMA 300, 1303–1310. doi: 10.1001/jama.300.11.1303
Larsen, S. T., and Nielsen, G. D. (2007). The adjuvant effect of di-(2-ethylhexyl) phthalate is mediated through a PPARalpha-independent mechanism. Toxicol. Lett. 170, 223–228. doi: 10.1016/j.toxlet.2007.03.009
Lee, S., Park, S. K., Park, H., Lee, W., Lee, J. H., Hong, Y. C., et al. (2021). Joint association of prenatal bisphenol-a and phthalates exposure with risk of atopic dermatitis in 6-month-old infants. Sci. Total Environ. 789:147953. doi: 10.1016/j.scitotenv.2021.147953
Lenters, V., Iszatt, N., Forns, J., Čechová, E., Kočan, A., Legler, J., et al. (2019). Early-life exposure to persistent organic pollutants (OCPs, PBDEs, PCBs, PFASs) and attention-deficit/hyperactivity disorder: a multi-pollutant analysis of a Norwegian birth cohort. Environ. Int. 125, 33–42. doi: 10.1016/j.envint.2019.01.020
Li, X. N., Wu, D., Liu, Y., Zhang, S. S., Tian, F. L., Sun, Q., et al. (2021). Prenatal exposure to bisphenols, immune responses in cord blood and infantile eczema: a nested prospective cohort study in China. Ecotoxicol. Environ. Saf. 228:112987. doi: 10.1016/j.ecoenv.2021.112987
Li, A., Zhuang, T., Zhu, Q., Song, M., Liao, C., and Jiang, G. (2020). Concentration and distribution of parabens, triclosan, and triclocarban in pregnant woman serum in China. Sci. Total Environ. 710:136390. doi: 10.1016/j.scitotenv.2019.136390
Liang, L., Pan, Y., Bin, L., Liu, Y., Huang, W., Li, R., et al. (2022). Immunotoxicity mechanisms of perfluorinated compounds PFOA and PFOS. Chemosphere 291:132892. doi: 10.1016/j.chemosphere.2021.132892
Liang, H., Wang, Z., Miao, M., Tian, Y., Zhou, Y., Wen, S., et al. (2020). Prenatal exposure to perfluoroalkyl substances and thyroid hormone concentrations in cord plasma in a Chinese birth cohort. Environ. Health 19:127. doi: 10.1186/s12940-020-00679-7
Lin, M. H., Chiu, S. Y., Ho, W. C., Chi, K. H., Liu, T. Y., and Wang, I. J. (2022). Effect of triclosan on the pathogenesis of allergic diseases among children. J. Expo. Sci. Environ. Epidemiol. 32, 60–68. doi: 10.1038/s41370-021-00304-w
Liu, J., Gao, X., Wang, Y., Leng, J., Li, J., Zhao, Y., et al. (2021). Profiling of emerging and legacy per−/polyfluoroalkyl substances in serum among pregnant women in China. Environ. Pollut. 271:116376. doi: 10.1016/j.envpol.2020.116376
Liu, L., Yan, P., Liu, X., Zhao, J., Tian, M., Huang, Q., et al. (2024). Profiles and transplacental transfer of per- and polyfluoroalkyl substances in maternal and umbilical cord blood: a birth cohort study in Zhoushan, Zhejiang Province, China. J. Hazard. Mater. 466:133501. doi: 10.1016/j.jhazmat.2024.133501
Liu, F., Zhang, Y., and Wang, F. (2022). Environmental relevant concentrations of triclosan affected developmental toxicity, oxidative stress, and apoptosis in zebrafish embryos. Environ. Toxicol. 37, 848–857. doi: 10.1002/tox.23448
Maitre, L., Bustamante, M., Hernández-Ferrer, C., Thiel, D., Lau, C. H. E., Siskos, A. P., et al. (2022). Multi-omics signatures of the human early life exposome. Nat. Commun. 13:7024. doi: 10.1038/s41467-022-34422-2
Mariana, M., Feiteiro, J., Verde, I., and Cairrao, E. (2016). The effects of phthalates in the cardiovascular and reproductive systems: a review. Environ. Int. 94, 758–776. doi: 10.1016/j.envint.2016.07.004
Martineau, A. R., Jolliffe, D. A., Hooper, R. L., Greenberg, L., Aloia, J. F., Bergman, P., et al. (2017). Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 356:i6583. doi: 10.1136/bmj.i6583
Mehlsen, A., Høllund, L., Boye, H., Frederiksen, H., Andersson, A. M., Bruun, S., et al. (2022). Pregnancy exposure to bisphenol a and duration of breastfeeding. Environ. Res. 206:112471. doi: 10.1016/j.envres.2021.112471
Melough, M. M., Maffini, M. V., Otten, J. J., and Sathyanarayana, S. (2022). Diet quality and exposure to endocrine-disrupting chemicals among US adults. Environ. Res. 211:113049. doi: 10.1016/j.envres.2022.113049
Milanović, M., Đurić, L., Milošević, N., and Milić, N. (2023). Comprehensive insight into triclosan-from widespread occurrence to health outcomes. Environ. Sci. Pollut. Res. Int. 30, 25119–25140. doi: 10.1007/s11356-021-17273-0
Miller, R. L., Wang, Y., Aalborg, J., Alshawabkeh, A. N., Bennett, D. H., Breton, C. V., et al. (2025). Prenatal exposure to environmental bisphenols over time and their association with childhood asthma, allergic rhinitis and atopic dermatitis in the ECHO consortium. Environ. Pollut. 366:125415. doi: 10.1016/j.envpol.2024.125415
Nowak, K., Ratajczak-Wrona, W., Górska, M., and Jabłońska, E. (2018). Parabens and their effects on the endocrine system. Mol. Cell. Endocrinol. 474, 238–251. doi: 10.1016/j.mce.2018.03.014
Okada, E., Sasaki, S., Kashino, I., Matsuura, H., Miyashita, C., Kobayashi, S., et al. (2014). Prenatal exposure to perfluoroalkyl acids and allergic diseases in early childhood. Environ. Int. 65, 127–134. doi: 10.1016/j.envint.2014.01.007
Pan, Y., Jia, C., Zhu, Z., Su, Z., Wei, X., Yin, R., et al. (2024). Occurrence and health risks of multiple emerging bisphenol S analogues in pregnant women from South China. J. Hazard. Mater. 478:135431. doi: 10.1016/j.jhazmat.2024.135431
Pei, X., Duan, Z., Ma, M., Zhang, Y., and Guo, L. (2014). Role of ca/CaN/NFAT signaling in IL-4 expression by splenic lymphocytes exposed to phthalate (2-ethylhexyl) ester in spleen lymphocytes. Mol. Biol. Rep. 41, 2129–2142. doi: 10.1007/s11033-014-3062-4
Peng, Y., Wu, J., Luo, X., Zhang, X., Giesy, J. P., and Mai, B. (2019). Spatial distribution and hazard of halogenated flame retardants and polychlorinated biphenyls to common kingfisher (Alcedo atthis) from a region of South China affected by electronic waste recycling. Environ. Int. 130:104952. doi: 10.1016/j.envint.2019.104952
Perrier, F., Giorgis-Allemand, L., Slama, R., and Philippat, C. (2016). Within-subject pooling of biological samples to reduce exposure misclassification in biomarker-based studies. Epidemiology 27, 378–388. doi: 10.1097/EDE.0000000000000460
Podlecka, D., Gromadzińska, J., Mikołajewska, K., Fijałkowska, B., Stelmach, I., and Jerzynska, J. (2020). Longitudinal effect of phthalates exposure on allergic diseases in children. Ann. Allergy Asthma Immunol. 125, 84–89. doi: 10.1016/j.anai.2020.03.022
Ribeiro, E., Ladeira, C., and Viegas, S. (2017). EDCs mixtures: a stealthy Hazard for human health? Toxics. 5:5. doi: 10.3390/toxics5010005
Rochester, J. R., and Bolden, A. L. (2015). Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol a substitutes. Environ. Health Perspect. 123, 643–650. doi: 10.1289/ehp.1408989
Sagiv, S. K., Rifas-Shiman, S. L., Webster, T. F., Mora, A. M., Harris, M. H., Calafat, A. M., et al. (2015). Sociodemographic and perinatal predictors of early pregnancy per- and Polyfluoroalkyl substance (PFAS) concentrations. Environ. Sci. Technol. 49, 11849–11858. doi: 10.1021/acs.est.5b02489
Schuppe, M. C., Porebski, P., Hahn, K. K., Liao, K., Uhmann, A., Braun, A., et al. (2025). Triclosan exacerbates atopic dermatitis in mouse models via thymic stromal lymphopoietin. J. Dermatol. Sci. 118, 1–8. doi: 10.1016/j.jdermsci.2025.02.007
Schwanz, T. G., Llorca, M., Farré, M., and Barceló, D. (2016). Perfluoroalkyl substances assessment in drinking waters from Brazil, France and Spain. Sci Total Environ. 539, 143–152. doi: 10.1016/j.scitotenv.2015.08.034
Shirai, S., Suzuki, Y., Yoshinaga, J., Shiraishi, H., and Mizumoto, Y. (2013). Urinary excretion of parabens in pregnant Japanese women. Reprod. Toxicol. 35, 96–101. doi: 10.1016/j.reprotox.2012.07.004
Souza, M. C. O., Saraiva, M. C. P., Honda, M., Barbieri, M. A., Bettiol, H., Barbosa, F., et al. (2020). Exposure to per- and polyfluorinated alkyl substances in pregnant Brazilian women and its association with fetal growth. Environ. Res. 187:109585. doi: 10.1016/j.envres.2020.109585
Spanier, A. J., Kahn, R. S., Kunselman, A. R., Hornung, R., Xu, Y., Calafat, A. M., et al. (2012). Prenatal exposure to bisphenol a and child wheeze from birth to 3 years of age. Environ. Health Perspect. 120, 916–920. doi: 10.1289/ehp.1104175
Spyrakis, F., and Dragani, T. A. (2023). The EU’S per- and Polyfluoroalkyl substances (PFAS) ban: a case of policy over science. Toxics. 11:721. doi: 10.3390/toxics11090721
Stiemsma, L. T., and Turvey, S. E. (2017). Asthma and the microbiome: defining the critical window in early life. Allergy Asthma Clin. Immunol. 13:3. doi: 10.1186/s13223-016-0173-6
Sugeng, E. J., Symeonides, C., O’Hely, M., Vuillermin, P., Sly, P. D., Vijayasarathy, S., et al. (2020). Predictors with regard to ingestion, inhalation and dermal absorption of estimated phthalate daily intakes in pregnant women: the Barwon infant study. Environ. Int. 139:105700. doi: 10.1016/j.envint.2020.105700
Sunderland, E. M., Hu, X. C., Dassuncao, C., Tokranov, A. K., Wagner, C. C., and Allen, J. G. (2019). A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 29, 131–147. doi: 10.1038/s41370-018-0094-1
Suwannarin, N., Nishihama, Y., Isobe, T., and Nakayama, S. F.Japan Environment and Children’s Study Group (2024). Urinary concentrations of environmental phenol among pregnant women in the Japan environment and children’s study. Environ. Int. 183:108373. doi: 10.1016/j.envint.2023.108373
Suzuki, Y., Niwa, M., Yoshinaga, J., Mizumoto, Y., Serizawa, S., and Shiraishi, H. (2010). Prenatal exposure to phthalate esters and PAHs and birth outcomes. Environ. Int. 36, 699–704. doi: 10.1016/j.envint.2010.05.003
Tefre de Renzy-Martin, K., Frederiksen, H., Christensen, J. S., Boye Kyhl, H., Andersson, A. M., Husby, S., et al. (2014). Current exposure of 200 pregnant Danish women to phthalates, parabens and phenols. Reproduction 147, 443–453. doi: 10.1530/REP-13-0461
Thoene, M., Rytel, L., Nowicka, N., and Wojtkiewicz, J. (2018). The state of bisphenol research in the lesser developed countries of the EU: a mini-review. Toxicol. Res. 7, 371–380. doi: 10.1039/c8tx00064f
Thürmann, L., Herberth, G., Seiwert, B., Schlittenbauer, L., Rolle-Kampczyk, U., Röder, S., et al. (2021). Prenatal paraben exposure and atopic dermatitis-related outcomes among children. Allergy 76, 3122–3132. doi: 10.1111/all.14890
Vandenberg, L. N., Colborn, T., Hayes, T. B., Heindel, J. J., Jacobs, D. R., Lee, D. H., et al. (2012). Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev. 33, 378–455. doi: 10.1210/er.2011-1050
Wang, I. J., Hsieh, W. S., Chen, C. Y., Fletcher, T., Lien, G. W., Chiang, H. L., et al. (2011). The effect of prenatal perfluorinated chemicals exposures on pediatric atopy. Environ. Res. 111, 785–791. doi: 10.1016/j.envres.2011.04.006
Wang, Z., Zhang, Y., Yang, W., Xu, M., Li, B., Wu, P., et al. (2025). Association between prenatal exposure to phthalate esters and blood pressure in children aged 3-7 years: a prospective cohort study. Ecotoxicol. Environ. Saf. 290:117553. doi: 10.1016/j.ecoenv.2024.117553
Weatherly, L. M., and Gosse, J. A. (2017). Triclosan exposure, transformation, and human health effects. J. Toxicol. Environ. Health B Crit. Rev. 20, 447–469. doi: 10.1080/10937404.2017.1399306
Wen, H. J., Wang, S. L., Chen, P. C., and Guo, Y. L. (2019). Prenatal perfluorooctanoic acid exposure and glutathione s-transferase T1/M1 genotypes and their association with atopic dermatitis at 2 years of age. PLoS One 14:e0210708. doi: 10.1371/journal.pone.0210708
Wen, H. J., Wang, S. L., Chuang, Y. C., Chen, P. C., and Guo, Y. L. (2019). Prenatal perfluorooctanoic acid exposure is associated with early onset atopic dermatitis in 5-year-old children. Chemosphere 231, 25–31. doi: 10.1016/j.chemosphere.2019.05.100
Weteska, M., Zwolińska, A., Pisarska-Troczyńska, K., Janc, M., Polańska, K., Jerzyńska, J., et al. (2023). Relationship between prenatal and postnatal exposure to BPA and its analogues (BPS, BPF) and allergic diseases. Int. J. Occup. Med. Environ. Health 36, 575–586. doi: 10.13075/ijomeh.1896.02184
Xu, B., Liu, S., Zhou, J. L., Zheng, C., Weifeng, J., Chen, B., et al. (2021). PFAS and their substitutes in groundwater: occurrence, transformation and remediation. J. Hazard. Mater. 412:125159. doi: 10.1016/j.jhazmat.2021.125159
Yu, H., Liu, Y., Shu, X., Ma, L., and Pan, Y. (2020). Assessment of the spatial distribution of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in urban soil of China. Chemosphere 243:125392. doi: 10.1016/j.chemosphere.2019.125392
Yueh, M. F., and Tukey, R. H. (2016). Triclosan: a widespread environmental toxicant with many biological effects. Annu. Rev. Pharmacol. Toxicol. 56, 251–272. doi: 10.1146/annurev-pharmtox-010715-103417
Zhang, Y. J., Guo, J. L., Xue, J. C., Bai, C. L., and Guo, Y. (2021). Phthalate metabolites: characterization, toxicities, global distribution, and exposure assessment. Environ. Pollut. 291:118106. doi: 10.1016/j.envpol.2021.118106
Keywords: endocrine disrupting chemicals (EDCs), atopic dermatitis (AD), prenatal exposure, per- and polyfluoroalkyl substances (PFAS), bisphenol A (BPA), parabens, triclosan (TCS), phthalate esters (PAEs)
Citation: Chen Y, Zhang L, Yang T and Chen L (2025) Prenatal exposure to endocrine-disrupting chemicals and childhood atopic dermatitis: epidemiological evidence. Front. Microbiol. 16:1681214. doi: 10.3389/fmicb.2025.1681214
Edited by:
Merih Cetinkaya, University of Health Sciences, TürkiyeReviewed by:
Sergio Gómez-Olarte, Helmholtz Centre for Environmental Research - UFZ, GermanyTing Gan, Queensland University of Technology, Australia
Copyright © 2025 Chen, Zhang, Yang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Yang, MTUxNjE1MTUxMDBAMTYzLmNvbQ==; Limei Chen, Y2hlbmxpbWVpOTE5QDE2My5jb20=
 Yuxin Chen
Yuxin Chen Le Zhang
Le Zhang Ting Yang1*
Ting Yang1*