- 1Shandong Provincial Center for Animal Disease Prevention and Control (Shandong Provincial Center for Zoonoses Epidemiology Investigation and Surveillance), Jinan, Shandong, China
- 2Shandong Provincial Key Laboratory of Zoonoses Diseases, Jinan, Shandong, China
- 3Department of Preventive Veterinary Medicine, College of Veterinary Medicine, Shandong Agricultural University, Taian, Shandong, China
- 4Ruminant Diseases Research Center, College of Life Sciences, Shandong Normal University, Jinan, Shandong, China
- 5Key Laboratory of Livestock and Poultry Multi-Omics of MARA, Institute of Animal Science and Veterinary Medicine, Shandong Academy of Agricultural Sciences, Jinan, Shandong, China
- 6College of Veterinary Medicine, Henan Agricultural University, Zhengzhou, Henan, China
- 7Shandong Sinder Technology Co., Ltd., Weifang, Shandong, China
- 8China Animal Health and Epidemiology Center, Qingdao, Shandong, China
Brucellosis is a zoonosis that occurs worldwide, and vaccination is the main strategy for controlling it. In China, the Brucella abortus A19-ΔVirB12 strain is utilized in main vaccines. However, a high-sensitivity nucleic acid detection method to effectively differentiate Brucella infections from immunization with the A19-ΔVirB12 strain is lacking. Therefore, in this study, a duplex droplet digital PCR (ddPCR) assay was established using primers and probes targeting the VirB8 gene and the deleted VirB12 gene in the A19-ΔVirB12 strain. The specificity of the method was tested using genomic DNA of Mycobacterium bovis, Escherichia coli (O:157), Salmonella spp., Streptococcus spp., and A19-ΔVirB12 Brucella. Only A19-ΔVirB12 amplified VirB8 gene. The detection limits of the method for VirB8 and VirB12 were 2.13 × 100 and 2.26 × 100 copies/μL, respectively. In the detection of DNA in epidemic-related samples, the positive rate of ddPCR was much higher than that in the samples analyzed using the commercial fluorescence quantitative reagent kits. Meanwhile, the ddPCR of the A19-ΔVirB12 Brucella vaccine strain was identified in the clinical samples. In summary, the ddPCR method with high sensitivity and specificity was established, which will support the future identification of A19-ΔVirB12 Brucella vaccine strains in immunized and wild-type Brucella.
1 Introduction
Brucellosis is a zoonotic disease that impacts livestock and humans worldwide, leading to chronic conditions such as undulant fever, weakness, myalgia, and arthralgia (Franco et al., 2007; Dean et al., 2012; Esmaeilnejad-Ganji and Esmaeilnejad-Ganji, 2019). Humans contract the disease through different routes, including the ingestion of unpasteurized milk and dairy products; direct contact with infected animal tissues; or accidental ingestion, inhalation, or injection of cultured Brucella (Young, 1983; Corbel et al., 2006; Dadar et al., 2019). More than 500,000 human cases are reported annually worldwide (Pappas et al., 2006; Seleem et al., 2010), although the true number of cases is likely to be much higher due to inaccurate diagnosis, inadequate surveillance, and incomplete reporting (Di Bari et al., 2022; Laine et al., 2023).
Managing brucellosis in livestock is a necessary and cost-effective way to decrease the number of human cases of brucellosis (Golshani and Buozari, 2017). Accurate diagnosis of this bacterial disease is a fundamental challenge, as is finding a suitable medication to eradicate it (Helmy et al., 2025). In China, the main control strategy for brucellosis outbreaks involves vaccination along with quarantine and culling. The Brucella abortus A19 vaccine strain is the most effective and widely used for cattle immunization (He et al., 2022). However, the antibody response induced by the O-side chain of the A19 vaccine interferes with serological diagnosis, making it difficult to distinguish between vaccinated and infected animals (Perkins et al., 2010). Therefore, the development and application of gene-deleted vaccines is needed to overcome this limitation (Weiss et al., 2015; Lin et al., 2020).
The A19-ΔVirB12 gene-deleted vaccine, which carries a diagnostic marker, exhibits a protective efficacy comparable to that of the parental A19 strain but demonstrates lower virulence (Yang et al., 2021). The vaccine strain, which can be distinguished from naturally infected strains using VirB12 gene differential diagnostic methods, was officially launched in 2021 in China. However, no a high-sensitivity nucleic acid detection method for nucleic acid-based differentiation exists.
Droplet digital PCR (ddPCR) is a highly precise nucleic acid quantification technology, with greater sensitivity and absolute quantitative capabilities compared to conventional quantitative PCR (qPCR) (Hindson et al., 2011; Quan et al., 2018; Sancha Dominguez et al., 2024). In this study, primers targeting the VirB8 gene of the type IV secretion system were designed for the genus-level identification of Brucella. The VirB12 gene is deleted in the A19-ΔVirB12 strain and serves as a diagnostic marker. The objective of this study was to establish a duplex ddPCR assay to differentiate between the A19-ΔVirB12 vaccine strain from field strains of Brucella abortus, providing a rapid and reliable diagnostic method for distinguishing vaccinated cattle from those infected with wild-type Brucella. A duplex ddPCR method for identifying A19-ΔVirB12 strain was successfully developed by optimizing parameters, such as primer concentration, probe concentration, and annealing rate, along with the evaluation of sensitivity, specificity and repeatability. This method was successfully applied to both vaccine samples and clinical specimens, indicating its potential a novel and effective technical approach for early detection, accurate diagnosis and scientific control of brucellosis.
2 Materials and methods
2.1 Vaccines and samples
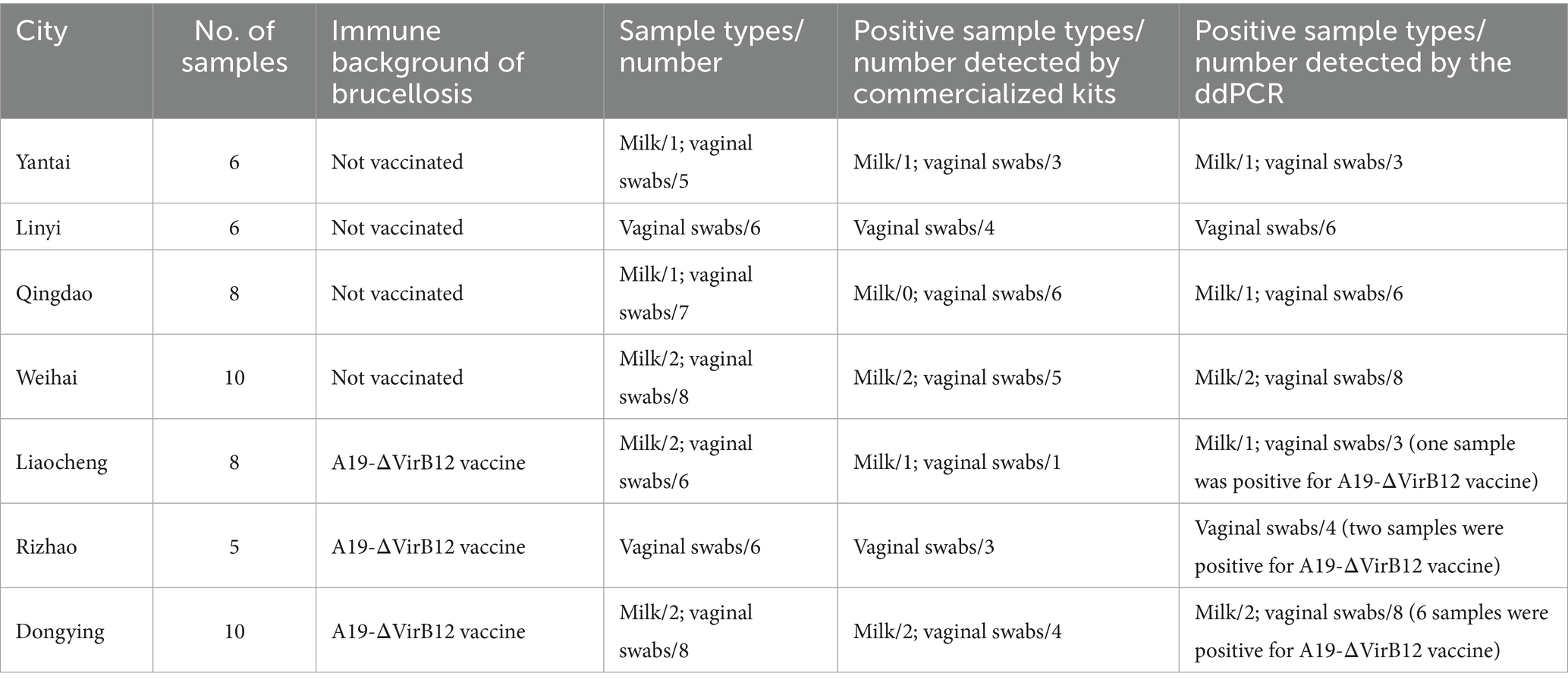
A19-ΔVirB12 Brucella vaccine was purchased from Tecon Biology Co., Ltd. Additionally, 53 clinical samples were collected from some areas’ cattle farms with symptoms of miscarriage from various cattle farms in the cities of Yantai, Zibo, Weifang, Liaocheng, Weihai, and Dongying in the Shandong Province to evaluate the practical performance of the ddPCR assay.
Nucleic acids of Mycobacterium bovis, Escherichia coli (O:157), Salmonella spp., Streptococcus spp. and Bacillus anthracis, preserved at the Shandong Animal Disease Prevention and Control Center, were used to assess assay specificity.
2.2 DNA extraction
Milk samples: 200 μL of milk was taken into a 1.5 mL centrifuge tube containing PBS. The samples were centrifuged at 12,000 × g for 5 min, the supernatant was discarded, and residual milk fat was wiped off with a sterile cotton swab. DNA was extracted from these above samples using a Bacteria DNA Extraction Kit (Vazyme Biotech, Nanjing, China).
Vaginal swabs: the swab heads were immersed in 1 mL of PBS and mixed with shaking. The PBS soaked in the swab was transferred to a 1.5 mL centrifuge tube and centrifuged at 12,000 × g for 5 min, and the upper layer of liquid was discarded. DNA was extracted from these above samples using a Bacteria DNA Extraction Kit (Vazyme Biotech, Nanjing, China).
2.3 Primer and probe design
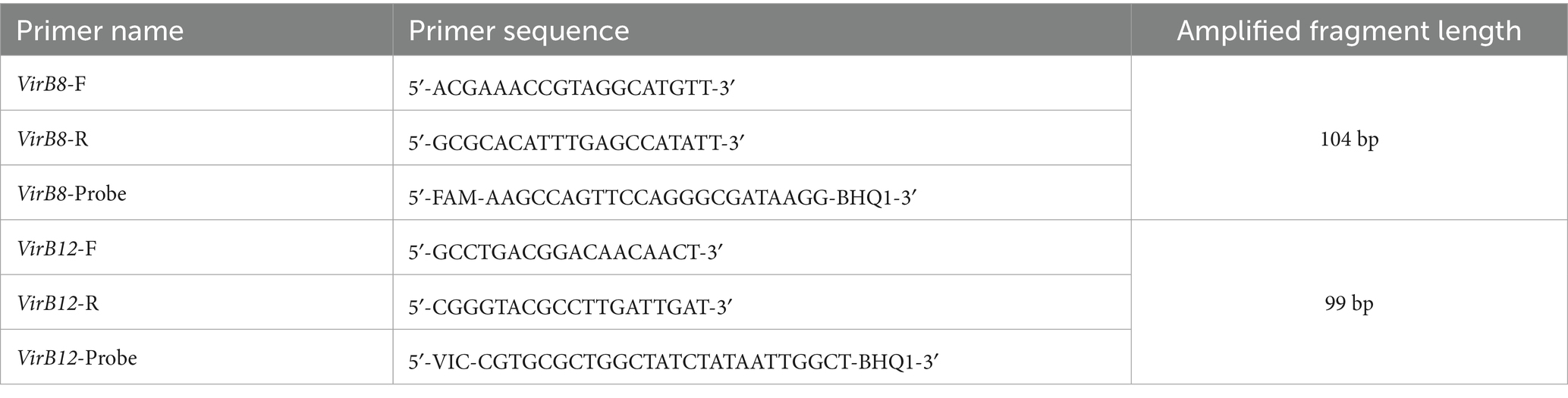
The VirB8 and VirB12 genes sequences were obtained from GenBank (GenBank IDs: CP030752.1 and LT671513.1, respectively). After the consensus sequence was selected, primers and probes were designed using the DNAMAN software (Table 1). Sequence analysis conformed that the primers and probe were situated within highly conserved regions of the VirB8 gene, as illustrated in Figure 1.
2.4 Construction of standard plasmids
Brucella VirB8 and VirB12 genes were amplified via primer pairs (Table 1). The reactions were carried out using 2 × Taq Master Mix (Vazyme Biotech, Nanjing, China). The PCR products were collected and purified using an EasyPure PCR Purification Kit (TransGen Biotech, Beijing, China), and the target sequences were cloned and inserted into the Trans1-T1 vector (TransGen Biotech, Beijing, China). The recombinant plasmids were subsequently purified with a FastPure EndoFree Plasmid Mini Plus Kit (Vazyme Biotech, Nanjing, China). The concentration of the plasmid DNA was measured with a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Delaware, United States). The initial concentrations of the VirB8 and VirB12 plasmids were 2.13 × 109 copies/μL and 2.26 × 109 copies/μL, respectively.
2.5 ddPCR reaction conditions
For the ddPCR reactions, the 30 μL reaction mixture contained 15 μL of ddPCR SuperMix (2×) (TargetingOne Biotech, Beijing, China), 1 μL of DNA template, forward and reverse primers, and probes (which were later optimized). The volume was adjusted to 30 μL with sterile deionized water. Subsequently, 30 μL of reaction mixture and 150 μL of droplet generation oil were loaded into a droplet generator cartridge, sealed with a gasket, and placed into the droplet generator to produce droplets. The generated droplets were transferred to a PCR thermocycler. Following ddPCR amplification, eight-strip tubes were placed in a droplet reader (Targetingone Biotech, Beijing, China) to analyze fluorescence signals.
2.6 Evaluation of the sensitivity of ddPCR
VirB8 and VirB12 standard plasmids were 10-fold serial gradient diluted with ddH2O, respectively. Serial dilutions were prepared as follows: VirB8 plasmids, 2.13 × 108–2.13 × 100 copies/μL and VirB12 plasmids, 2.26 × 108–2.26 × 100 copies/μL. For each dilution, 2 μL of plasmid DNA was used as the template in the established assay system; blank controls and replicates were used to evaluate sensitivity. Standard curves were plotted to display the results.
2.7 Evaluation of the specificity of ddPCR
Different sources of DNA from Mycobacterium bovis, Escherichia coli (O:157), Salmonella spp., Streptococcus spp., Bacillus anthracis, Clostridium perfringens, and A19-ΔVirB12 Brucella vaccine were diluted to 1 ng/μL and then tested to evaluate the specificity of the ddPCR assay.
2.8 Evaluation of ddPCR with clinical samples
Overall, 53 clinical samples were collected from cattle with incidences of miscarriage from farms in the cities of Yantai, Linyi, Qingdao, Liaocheng, Weihai, Rizhao, and Dongying in the Shandong Province from 2023 to 2024. To compare the efficacy of our method, the commercial fluorescence quantitative reagent kits (Lijian Biotech, Qingdao, China) were used in parallel with ddPCR to test clinical samples.
3 Results
3.1 Optimization of ddPCR reaction conditions
In the 30 μL reaction system, the volume of VirB8 and VirB12 probes (10 μmol/L) was fixed at 0.75 μL. Eight final primer concentrations for VirB8 and VirB12 (10 μmol/L) were tested at eight final concentrations: 200 nM, 300 nM, 400 nM, 500 nM, 600 nM, 700 nM, 800 nM, and 900 nM. All the primer concentrations effectively distinguished between positive and negative droplets for both VirB8 and VirB12. A final primer concentration of 600 nM (equivalent to 1.8 μL of primer) was selected for VirB8 and VirB12 to optimize amplification efficiency and minimize “tailing” effects (Figure 2). With the primer volume fixed at 1.8 μL (10 μmol/L) in the 30 μL reaction system, six final probe concentrations for VirB8 and VirB12 (10 μmol/L) were evaluated at six final concentrations: 100 nM, 200 nM, 300 nM, 400 nM, 500 nM, and 600 nM. The results indicated that a probe concentration of 300 nM (equivalent to 0.9 μL of probe) provided the best balance between amplification efficiency, minimization of “tailing” and cost-effectiveness for both VirB8 and VirB12 (Figure 3). Subsequently, with fixed primer (1.8 μL) and probe (0.9 μL) volumes, the annealing temperature was optimized: 53 °C, 54 °C, 55 °C, 56 °C, 57 °C, 58 °C, 59 °C, and 60 °C. The separation between the positive and negative droplets was largest and clearest at 54 °C (Figure 4).
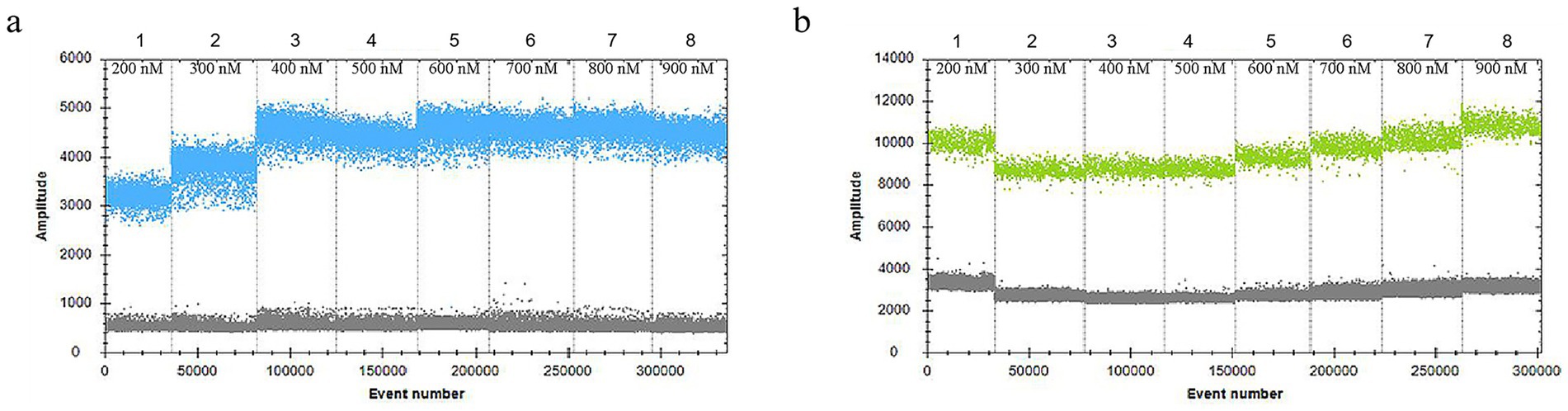
Figure 2. Optimization of primer concentrations of VirB8 and VirB12. (a) Primer concentrations of VirB8; 1–8: 200 nM, 300 nM, 400 nM, 500 nM, 600 nM, 700 nM, 800 nM, and 900 nM, respectively. (b) Primer concentrations of VirB12; 1–8: 200 nM, 300 nM, 400 nM, 500 nM, 600 nM, 700 nM, 800 nM, and 900 nM, respectively.
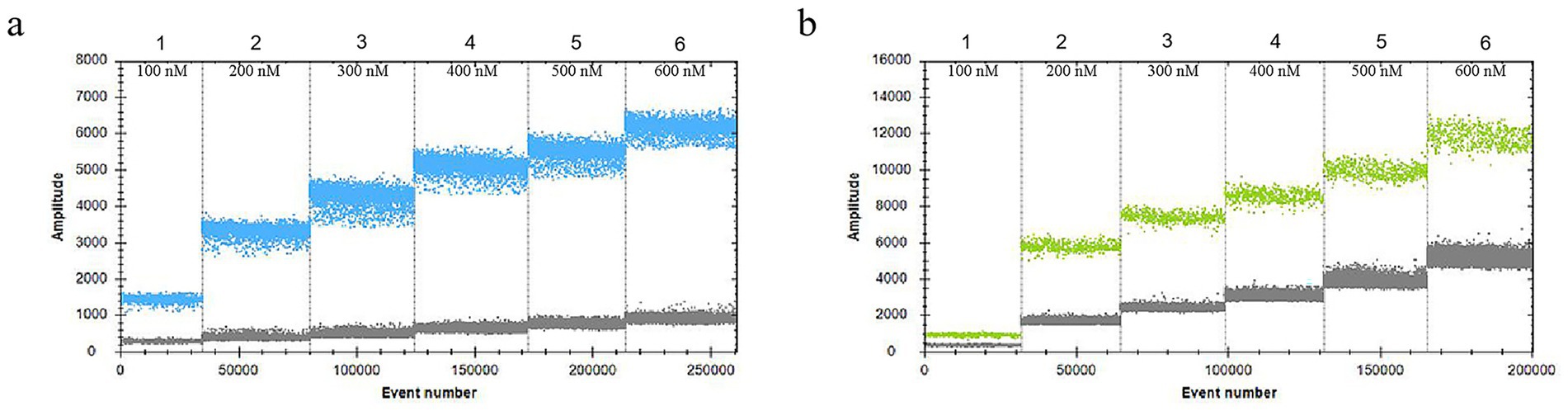
Figure 3. Optimization of probe concentrations of VirB8 and VirB12. (a) Probe concentrations of VirB8 in FAM channel; 1–6: 100 nM, 200 nM, 300 nM, 400 nM, 500 nM, and 600 nM, respectively. (b) Probe concentrations of VirB12 in VIC channel; 1–6: 100 nM, 200 nM, 300 nM, 400 nM, 500 nM, and 600 nM, respectively.
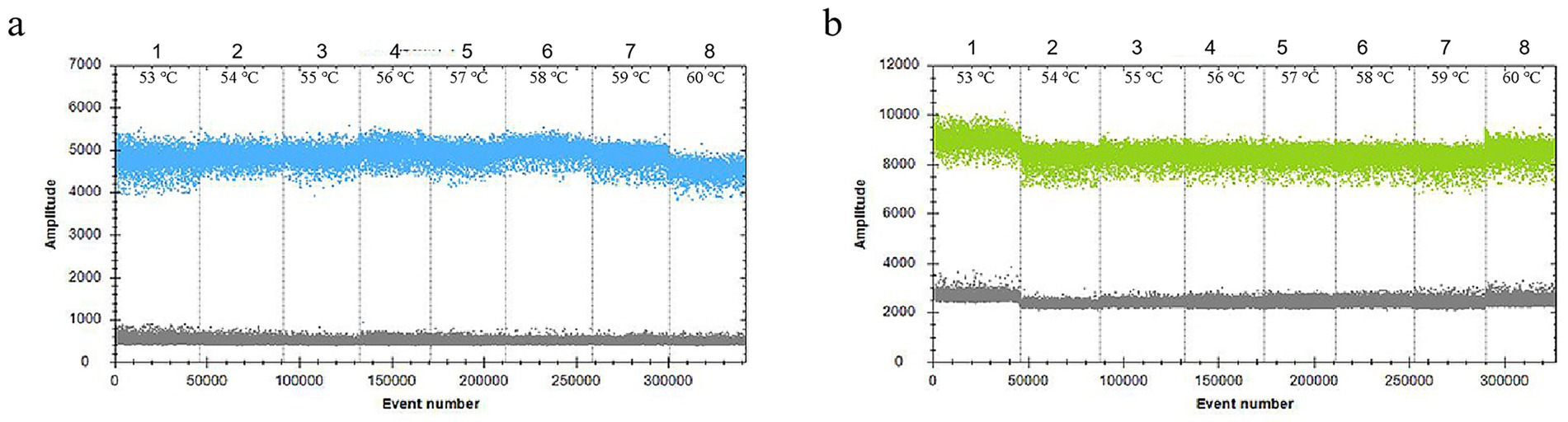
Figure 4. Optimization of the annealing temperature of ddPCR. (a) Annealing temperature of VirB8 in FAM channel; 1–8: 53 °C, 54 °C, 55 °C, 56 °C, 57 °C, 58 °C, 59 °C, and 60 °C, respectively. (b) Annealing temperature of VirB12 in VIC channel; 1–8: 53 °C, 54 °C, 55 °C, 56 °C, 57 °C, 58 °C, 59 °C, and 60 °C, respectively.
Considering the fluorescence signal intensity, stability, minimization of “tailing,” and cost-effectiveness, the optimized reaction mixture was established contained the following: 15 μL of ddPCR SuperMix (2×), 1 μL of DNA template, 1.8 μL of both forward and reverse primers for VirB8 and VirB12 (10 μmol/L), and 0.9 μL of each probe (10 μmol/L). The final reaction volume was adjusted to 30 μL with sterile deionized water. Optimized amplification conditions were as follows: initial denaturation at 95 °C for 10 min; 40 cycles of denaturation at 94 °C for 30 s and annealing at 54 °C for 1 min; followed by a final cooling step at 12 °C for 5 min. Under these conditions, fluorescence signals were concentrated, positive and negative droplets were clearly separated, no “tailing” was observed, and an optimal number of droplets and amplification efficiency were achieved.
3.2 Sensitivity of ddPCR
Standard plasmids containing the VirB8 and VirB12 target sequences were serially diluted to nine concentrations and measured using the established duplex ddPCR assay. VirB8 was consistently detected within the dilution range of 2.13 × 104–2.13 × 100 copies/μL, with a lower detection limit of 2.13 × 100 copies/μL (Figure 5a). Similarly, VirB12 was showed the detectable in the range of 2.26 × 104–2.26 × 100 copies/μL, with a lower detection limit of 2.26 × 100 copies/μL (Figure 5b).
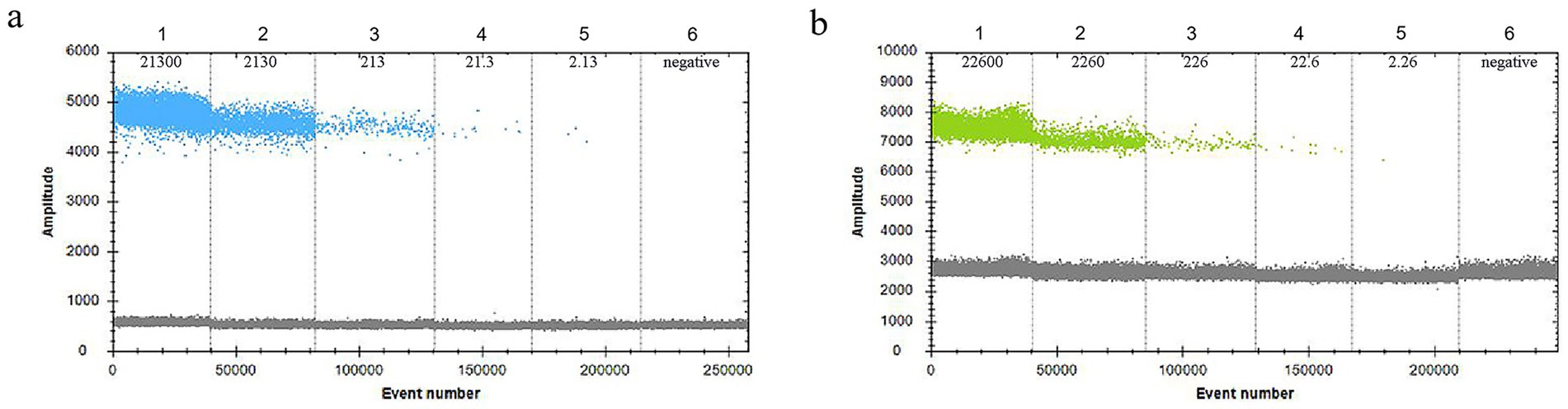
Figure 5. Sensitivity of ddPCR. (a) Sensitivity of VirB8 in FAM channel; 1–5: 2.13 × 104–2.13 × 100 copies/μL dilution standards, 6: negative control. (b) Sensitivity of VirB12 in VIC channel; 1–5: 2.26 × 104–2.26 × 100 copies/μL dilution standards; 6: negative control.
Based on the experimental results, the theoretical copy number was plotted on the x-axis and the measured copy number on the y-axis. For VirB8, the linear regression analysis yielded an R2 value of 0.9991 with the equation y = 1.2652x + 1884. For VirB12, the R2 value = 0.9999 with the equation y = 0.9163x + 371.29. Thus, the copy numbers determined by ddPCR were highly consistent with the theoretical values, demonstrating excellent linearity of the assay (Figure 6).
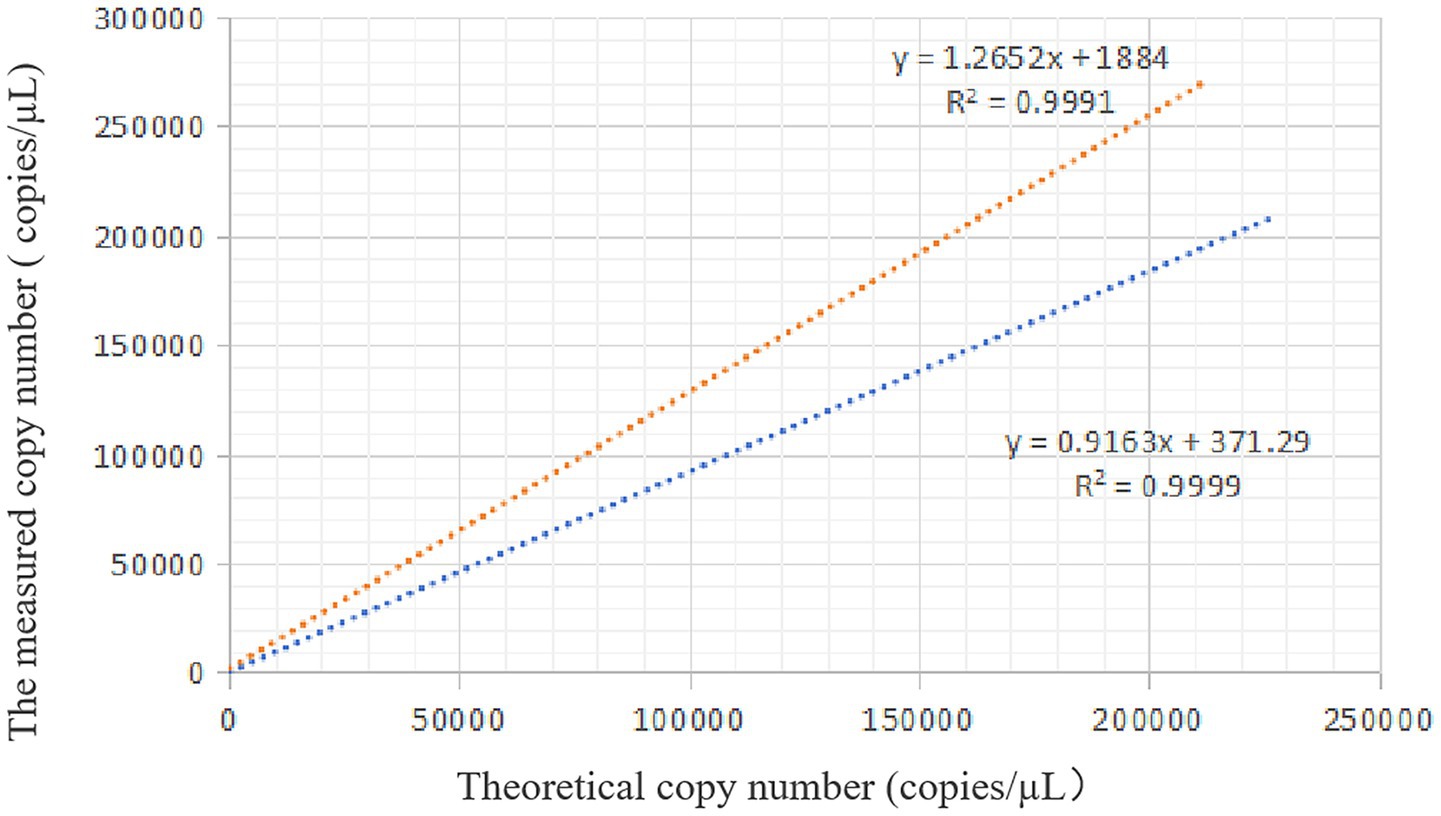
Figure 6. Standard curve of ddPCR. The orange dotted line is the linear regression analysis of VirB8, and the blue dotted line is the linear regression analysis of VirB12.
3.3 Specificity of ddPCR
No copy numbers were detected with qPCR in addition to the DNA from A19-ΔVirB12 Brucella vaccine in the FAM channel (Figure 7), confirming that the primers and probes exhibited high specificity.
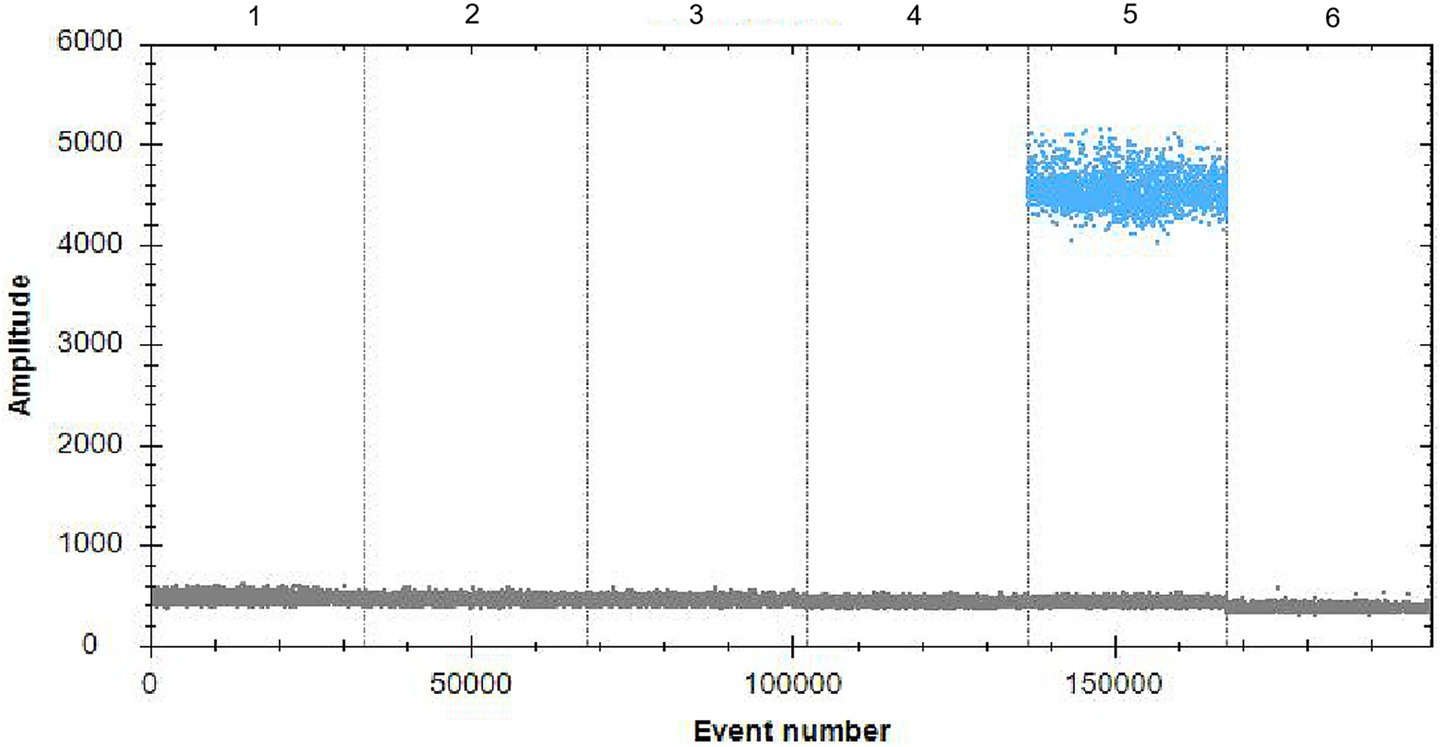
Figure 7. Specificity of ddPCR. 1–6: Mycobacterium bovis, Escherichia coli (O:157), Salmonella spp., Streptococcus spp., A19-ΔVirB12 Brucella vaccine, and Clostridium perfringens, respectively.
3.4 Detection of clinical samples
The commercial fluorescence quantitative reagent kits identified 32 positive samples with the percentage of Brucella-positive samples was 60.38%. In contrast, the ddPCR results showed 45 positive samples including two vaginal swabs positive for the A19-ΔVirB12 vaccines strain, and the percentage of Brucella-positive samples was 84.91% (Table 2). Thus, ddPCR exhibited higher sensitivity for positive samples compared with that of conventional qPCR and distinguish A19-ΔVirB12 vaccine strains and wild-type strains.
4 Discussion
ddPCR is a third-generation nucleic acid quantification technology. It involves separating the fluorescence-based PCR reaction mixture into tens of thousands of nanoliter-sized individual droplets. After PCR amplification, a droplet reader analyzes the fluorescence signal of each droplet, which determines the presence or absence of target nucleic acids (positive fluorescence indicates the presence of target). The fluorescence data are digitized, and analysis software is used to calculate the absolute copy number concentration of the target based on the Poisson distribution and the proportion of positive droplets. This allows for absolute quantification of low-abundance nucleic acid targets without reference standards or internal controls (Gerdes et al., 2016; Mehle and Dreo, 2019). ddPCR has been widely utilized to detect various infectious diseases, including Mycobacterium tuberculosis and HIV (Henrich et al., 2012).
Vaccination against Brucella remains the main strategy for brucellosis prevention and control. In China, the main vaccines available include Brucella suis S2, Brucella melitensis M5/M5-90, Brucella abortus A19, and the Brucella abortus A19-ΔVirB12 strains. The A19 vaccine strain, derived from the S19 strain, was introduced from the Soviet Union in the 1950s. It has been widely used for brucellosis prevention in dairy cattle since the 1930s and is recognized worldwide as the most effective vaccine for bovine brucellosis (Yang et al., 2013). The A19 strain is a smooth-type Brucella that induces a persistent antibody response after vaccination (Cheng et al., 2021). However, since both are specific antibodies, serological tests cannot distinguish vaccine-induced antibodies from those induced by natural infection. The A19-ΔVirB12 strain was developed in China by deleting the VirB12 gene, part of the type IV secretion system in Brucella, from the A19 parent strain using homologous recombination technology. This mutant strain retain exhibits the same protective efficacy as the parental strain but exhibits reduced virulence and simultaneously carries a diagnostic marker (Yi et al., 2013).
The Brucella VirB8 protein, an essential and highly conserved component of the type IV secretion system, functions as an early-stage secreted protein (Rouot et al., 2003; Patey et al., 2006). In this study, primers and probes were designed based on the Brucella VirB8 fragment, suitable for genus-level identification. The absence of the VirB12 sequence in the Brucella abortus A19-ΔVirB12 vaccine strain prevents its amplification. The optimal reaction system and conditions were determined, leading to the development of a duplex ddPCR assay for differentiating the A19-ΔVirB12 vaccine strain.
The single-digital PCR method for Listeria bacteria and SARS established by Witte et al. (2016) and Paolo et al. (2021), respectively, achieved a sensitivity of single copy per μL. Compared with the above-mentioned detection methods, the duplex ddPCR assay demonstrated detection sensitivities of 2.13 × 100 copies/μL for VirB8 and 2.26 × 100 copies/μL for VirB12, attaining the detection sensitivity of single-digital PCR.
In the analysis of clinical samples, we found that the cattle from farms in four cities (Yantai, Linyi, Qingdao, and Weihai) had not been immunized with the Brucella vaccine. A positive result for Brucella nucleic acid indicated that the animal had been infected with Brucella. To prevent other animals and humans from being infected, the positive animals should be immediately subjected to harmless treatment. The results showed that the number of positive animals detected by ddPCR was significantly higher than that detected by commercial kits, which indicated that ddPCR was more effective in preventing the risk of the spread of Brucella infection in animals. As for cattle that had received the Brucella abortus A19-ΔVirB12 vaccine, the ddPCR assay was able to detect both the vaccine strain and wild-type Brucella infections. Consequently, at the cattle farms in the cities of Liaocheng, Rizhao and Dongying, cattle infected with wild-type Brucella were subjected to harmless disposal, while those immunized with the Brucella abortus A19-ΔVirB12 vaccine were exempted from such disposal-this measure effectively reduced the breeding costs for the farms. The test results showed that the ddPCR method had a higher detection rate and greater sensitivity compared with those of the commercial kits.
These findings suggest that clinical test samples for Brucellosis, such as milk samples and vaginal swabs, contain relatively low levels of bacteria, which requires higher sensitivity detection methods; therefore ddPCR is more suitable for such scenarios. Moreover, the ddPCR method established by us has greater advantages in detecting Brucellosis clinical samples with a Brucella abortus A19-ΔVirB12 vaccine background.
In conclusion, the duplex ddPCR assay established in this study exhibited excellent specificity and sensitivity. It facilitated accurate identification of Brucella in samples while effectively differentiating between strains from natural Brucella infections and immunization with the A19-ΔVirB12 vaccine strain, offering valuable technical support for preventing and controlling brucellosis. Meanwhile, the analysis of clinical samples revealed that Brucella infections existed in coastal cities of the Shandong Province, such as Qingdao, Yantai, and Weihai. Therefore, Brucellosis prevention and control efforts in these regions should be strengthened.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal studies were approved by Shandong Agricultural University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
RX: Conceptualization, Methodology, Writing – original draft. ZC: Supervision, Writing – review & editing. LX: Data curation, Writing – review & editing. ZJ: Software, Writing – review & editing. WJ: Formal analysis, Writing – review & editing. YS: Project administration, Resources, Writing – review & editing. FW: Formal analysis, Writing – review & editing. HW: Resources, Writing – review & editing. YuyZ: Methodology, Writing – review & editing. YW: Visualization, Writing – review & editing. YM: Project administration, Writing – review & editing. XZ: Conceptualization, Writing – review & editing. MS: Validation, Writing – review & editing. ZL: Funding acquisition, Supervision, Writing – review & editing. YueZ: Funding acquisition, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Project of Key Research and Development Program of Shandong Province (Major Scientific and Technological Innovation Project) (2022CXGC020711-1).
Conflict of interest
YM and XZ were employed by Shandong Sinder Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Cheng, Z., Li, Z., Yin, Y., Lian, Z., Abdelgawad, H. A., Hu, H., et al. (2021). Characteristics of Brucella abortus vaccine strain A19 reveals its potential mechanism of attenuated virulence. Vet. Microbiol. 254:109007. doi: 10.1016/j.vetmic.2021.109007
Corbel, M. J.Food and Agriculture Organization of the United Nations; World Health Organization; World Organisation for Animal Health (2006). Brucellosis in humans and animals. Geneva: World Health Organization, 1–89.
Dadar, M., Shahali, Y., and Whatmore, A. M. (2019). Human brucellosis caused by raw dairy products: a review on the occurrence, major risk factors and prevention. Int. J. Food Microbiol. 292, 39–47. doi: 10.1016/j.ijfoodmicro.2018.12.009
Dean, A. S., Crump, L., Greter, H., Hattendorf, J., Schelling, E., and Zinsstag, J. (2012). Clinical manifestations of human brucellosis: a systematic review and meta-analysis. PLoS Negl. Trop. Dis. 6:e1929. doi: 10.1371/journal.pntd.0001929
Di Bari, C., Venkateswaran, N., Bruce, M., Fastl, C., Huntington, B., Patterson, G. T., et al. (2022). Methodological choices in brucellosis burden of disease assessments: a systematic review. PLoS Negl. Trop. Dis. 16:e0010468. doi: 10.1371/journal.pntd.0010468
Esmaeilnejad-Ganji, S. M., and Esmaeilnejad-Ganji, S. M. R. (2019). Osteoarticular manifestations of human brucellosis: a review. World J. Orthop. 10, 54–62. doi: 10.5312/wjo.v10.i2.54
Franco, M. P., Mulder, M., Gilman, R. H., and Smits, H. L. (2007). Human brucellosis. Lancet Infect. Dis. 7, 775–786. doi: 10.1016/S1473-309(07)70286-4
Gerdes, L., Iwobi, A., Busch, U., and Pecoraro, S. (2016). Optimization of digital droplet polymerase chain reaction for quantification of genetically modified organisms. Biomol. Detect. Quantif. 7, 9–20. doi: 10.1016/j.bdq.2015.12.003
Golshani, M., and Buozari, S. (2017). A review of brucellosis in Iran: epidemiology, risk factors, diagnosis, control, and prevention. Iran. Biomed. J. 21, 349–359. doi: 10.18869/acadpub.ibj.21.6.349
He, C. Y., Zhang, Y. Z., Liu, M. Z., Zhao, H. L., Ren, L. S., Liu, B. S., et al. (2022). Combined immunization with inactivated vaccine reduces the dose of live B. abortus A19 vaccine. BMC Vet. Res. 18:128. doi: 10.1186/s12917-022-03229-0
Helmy, N. M., Zaki, H. M., and Saad, A. (2025). Comparable study of immunological, bacteriological, and molecular techniques for detecting brucellosis in milk of reproductively problematic cows. J. Vet. Sci. 56, 149–157. doi: 10.21608/EJVS.2024.259043.1751
Henrich, T. J., Gallien, S., Li, J. Z., Pereyra, F., and Kuritzkes, D. R. (2012). Low-level detection and quantitation of cellular HIV-1 DNA and 2-LTR circles using droplet digital PCR. J. Virol. Methods 186, 68–72. doi: 10.1016/j.jviromet.2012.08.019
Hindson, B. J., Ness, K. D., Masquelier, D. A., Belgrader, P., Heredia, N. J., Makarewicz, A. J., et al. (2011). High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal. Chem. 83, 8604–8610. doi: 10.1021/ac202028g
Laine, C. G., Johnson, V. E., Scott, H. M., and Arenas-Gamboa, A. M. (2023). Global estimate of human brucellosis incidence. Emerg. Infect. Dis. 29, 1789–1797. doi: 10.3201/eid2909.230052
Lin, Y., Cao, C., Shi, W., Huang, C., Zeng, S., Sun, J., et al. (2020). Development of a triplex real-time PCR assay for detection and differentiation of gene-deleted and wild-type African swine fever virus. J. Virol. Methods 280:113875. doi: 10.1016/j.jviromet.2020.113875
Mehle, N., and Dreo, T. (2019). Quantitative analysis with droplet digital PCR. Methods Mol. Biol. 1875, 171–186. doi: 10.1007/978-1-4939-8837-2_14
Paolo, P., Paola, S., Chiara, V., Veronica, R., Cristina, B., Silvia, S. B., et al. (2021). Digital PCR for high sensitivity viral detection in false-negative SARS-CoV-2 patients. Sci. Rep. 22:4310. doi: 10.1038/s41598-021-83723-x
Pappas, G., Papadimitriou, P., Akritidis, N., Christou, L., and Tsianos, E. V. (2006). The new global map of human brucellosis. Lancet Infect. Dis. 6, 91–99. doi: 10.1016/S1473-3099(06)70382-6
Patey, G., Qi, Z., Bourg, G., Baron, C., and O’Callaghan, D. (2006). Swapping of periplasmic domains between Brucella suis VirB8 and a pSB102 VirB8 homologue allows heterologous complementation. Infect. Immun. 74, 4945–4949. doi: 10.1128/IAI.00584-06
Perkins, S. D., Smither, S. J., and Atkins, H. S. (2010). Towards a Brucella vaccine for humans. FEMS Microbiol. Rev. 34, 379–394. doi: 10.1111/j.1574-6976.2010.00211.x
Quan, P. L., Sauzade, M., and Brouzes, E. (2018). dPCR: a technology review. Sensors 18:1271. doi: 10.3390/s18041271
Rouot, B., Alvarez-Martinez, M. T., Marius, C., Menanteau, P., Guilloteau, L., Boigegrain, R. A., et al. (2003). Production of the type IV secretion system differs among Brucella species as revealed with VirB5- and VirB8-specific antisera. Infect. Immun. 71, 1075–1082. doi: 10.1128/IAI.71.3.1075-1082.2003
Sancha Dominguez, L., Cotos Suárez, A., Sánchez Ledesma, M., and Muñoz Bellido, J. L. (2024). Present and future applications of digital PCR in infectious diseases diagnosis. Diagnostics 14:931. doi: 10.3390/diagnostics14090931
Seleem, M. N., Boyle, S. M., and Sriranganathan, N. (2010). Brucellosis: a re-emerging zoonosis. Vet. Microbiol. 140, 392–398. doi: 10.1016/j.vetmic.2009.06.021
Weiss, M., Brum, M. C., Anziliero, D., Weiblen, R., and Flores, E. F. (2015). A glycoprotein E gene-deleted bovine herpesvirus 1 as a candidate vaccine strain. Braz. J. Med. Biol. Res. 48, 843–851. doi: 10.1590/1414-431X20154243
Witte, A. K., Fister, S., Mester, P., Schoder, D., and Rossmanith, P. (2016). Evaluation of the performance of quantitative detection of the Listeria monocytogenes prfA locus with droplet digital PCR. Anal. Bioanal. Chem. 408, 7583–7593. doi: 10.1007/s00216-016-9861-9
Yang, J., He, C., Zhang, H., Liu, M., Zhao, H., Ren, L., et al. (2021). Evaluation and differential diagnosis of a genetic marked Brucella vaccine A19ΔvirB12 for cattle. Front. Immunol. 12:679560. doi: 10.3389/fimmu.2021.679560
Yang, X., Skyberg, J. A., Cao, L., Clapp, B., Thornburg, T., and Pascual, D. W. (2013). Progress in Brucella vaccine development. Front. Biol. 8, 60–77. doi: 10.1007/s11515-012-1196-0
Yi, X., Ye, F., Yao, G., Gu, W., Ma, X., Wu, D., et al. (2013). Construction of Brucella abortus A19-delta VirB12 mutant and evaluation of its protective efficacy against 2308 strain challenge in BALB/c mice. Wei Sheng Wu Xue Bao 53, 1213–1220.
Keywords: brucellosis, A19-ΔVirB12 strain, VirB8 gene, VirB12 gene, ddPCR
Citation: Xue R, Chu Z, Xing L, Jiang Z, Jiang W, Shang Y, Wang F, Wang H, Zhang Y, Wang Y, Miao Y, Zhang X, Sun M, Lan Z and Zhang Y (2025) Establishment of a dual droplet digital PCR method for detecting the Brucella abortus A19-ΔVirB12 strains. Front. Microbiol. 16:1684156. doi: 10.3389/fmicb.2025.1684156
Edited by:
Xueen Jia, Umeå University, SwedenReviewed by:
Aalaa Samir Saad, Agricultural Research Center, EgyptShubham Mathur, Tel Aviv University, Israel
Copyright © 2025 Xue, Chu, Xing, Jiang, Jiang, Shang, Wang, Wang, Zhang, Wang, Miao, Zhang, Sun, Lan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zouran Lan, bGFuenJqbkAxNjMuY29t; Yue Zhang, c2RjYWRjX3p5QDE2My5jb20=
 Ruixue Xue
Ruixue Xue Zunfeng Chu1,2
Zunfeng Chu1,2 Yingli Shang
Yingli Shang Xinglin Zhang
Xinglin Zhang Mingjun Sun
Mingjun Sun