Abstract
Cyanobacteria, ancient oxygenic photoautotrophs originated in the Precambrian period, exhibit remarkable adaptability to diverse ecological systems. Light, a critical environmental factor, exerts differential pressures on these organisms. The scattering of white light creates dynamic light environments, which poses a significant ecological challenge. To thrive in dynamic light environment, cyanobacteria have developed several light acclimation strategies. This includes chromatic acclimation, which optimize light harvesting by adjusting pigments. Cyanobacteria also employ robust photoprotective mechanisms against quantitative light stress. Under high light, these organisms activate non-photochemical quenching using the proteins such as orange carotenoid protein, iron starvation-induced protein, and high light-induced proteins to safely dissipate excess excitation energy. Additionally, thylakoid-localized respiratory enzymes alleviate electronic pressure arising from over-reduction of the plastoquinone pool. Under low light conditions, cyanobacteria frequently employ state transitions, reversibly associating their phycobilisomes with PSII and PSI to optimize light harvesting. These natural strategies offer a blueprint for engineering cyanobacteria and algae for their application in biomanufacturing and CO2 sequestration. This review synthesizes the key light acclimation and photoprotective mechanisms, underscoring their importance for both the ecological success of cyanobacteria and their implication in biotechnological applications using engineered strains.
Introduction
The groundbreaking oxygen production by cyanobacteria triggered the evolution of aerobic life. The subsequent rise in oxygen levels, a byproduct of their photosynthesis, further catalyzed the formation of the stratospheric ozone layer, which shields Earth from lethal UV-C (100–280 nm) radiation (Sánchez-Baracaldo et al., 2022). Thus, early cyanobacterial oxygenic photosynthesis not only drove the emergence of aerobic organisms but also established a crucial protective ozone shield. While many cyanobacteria are free-living in diverse aquatic and terrestrial environments, they also form symbiotic relationships with lichens, bryophytes, and gymnosperms (Álvarez et al., 2023). Notably, the endosymbiotic integration of cyanobacteria into non-photosynthetic eukaryotes ultimately led to the evolution of chloroplasts (Zimorski et al., 2014).
Cyanobacteria boast remarkable morphological and metabolic diversity, enabling their survival across varied ecological niches. Within these environments, they play crucial roles in food webs and the fixation of both dinitrogen and carbon dioxide (CO2) (Garcia-Pichel and Belnap, 2021). Furthermore, cyanobacteria can be metabolically engineered to redirect fixed carbon and nitrogen into the production of valuable chemicals, including biofuels, bioplastics, and diverse metabolites (Baunach et al., 2024; Rueda et al., 2024; Singh et al., 2017). Nevertheless, the effective use of cyanobacteria for large-scale production of energy-rich molecules and valuable chemicals, as well as their application in CO2 capturing or agriculture as biofertilizers for achieving the United Nations’ sustainable development goals, hinges on fundamental photosynthetic function (Pandey et al., 2025). Cyanobacterial growth, whether in natural settings or large-scale cultivation systems, is susceptible to light fluctuations. These fluctuations encompass diurnal, seasonal, and spatial variations in light quality and quantity, leading to imbalanced photosystem excitation that compromises photochemistry (Maurya et al., 2023; Pfennig et al., 2024; Canonico et al., 2021). Besides suboptimal levels of photosynthetically active radiation (PAR; 400–700 nm), ultraviolet radiation (UVR; 280–400 nm) can directly damage the photosynthetic apparatus or indirectly by inducing the production of reactive oxygen species (ROS) (Kataria et al., 2014). However, cyanobacteria have evolved diverse acclimatization strategies to modulate their development and photosynthetic machinery in response to light signals (Gupta et al., 2023; Konert et al., 2023).
Therefore, solar radiation serves as the primary energy source for photochemistry in all photoautotrophs, including cyanobacteria. Its quality, quantity, direction, and duration also act as key environmental cues that drive developmental and/or morphogenetic programs (Figure 1). This light-driven developmental regulation, termed photomorphogenesis, optimizes organismal fitness by enhancing growth and photon resource utilization (Mondal et al., 2024). Ultimately, biomass production reflects a balance between photosynthetic energy capture and its allocation towards developmental programs and other cellular processes, such as repair mechanisms that promote survival and fitness (Figure 1). Owing to their extensive phenotypic and genotypic diversity, cyanobacteria exhibit a wide array of photoacclimatization strategies. These strategies not only safeguard photosystem (PS) function but also enable efficient photosynthesis under varying light conditions. Notably, these acclimation strategies could significantly improve the biomass production in the large-scale cultivation system. Therefore, these strategies are ideal characteristics for engineering cyanobacteria to enhance photosynthetic performance in fluctuating light conditions (Silkina et al., 2019; Battaglino et al., 2021).
Figure 1

Light energy exerts a dual influence on photoautotrophs, driving both photosynthesis and acting as a signal for developmental processes (photomorphogenesis). The balance between energy gained through photosynthesis and its allocation towards developmental repair and maintenance ultimately determines the organism’s fitness, reflected in biomass accumulation.
Under high light stress, cyanobacteria employ diverse strategies to manage both physical and chemical excitation pressure on photosystem II (PSII). Physical balancing of light energy is achieved through mechanisms involving the orange carotenoid protein (OCP) (Domínguez-Martín et al., 2022), iron-starvation inducible protein (IsiA) (Nagao et al., 2023), high-light inducible proteins (HliPs) (Konert et al., 2023), and state transitions (Calzadilla and Kirilovsky, 2020). The electronic load on PSII is dissipated by respiratory electron transport chain complexes and flavodiiron proteins (Ermakova et al., 2016; He et al., 2025). This interconnected electron transport network helps to alleviate electronic pressure on both PSII and PSI in cyanobacteria. However, when these initial defense systems are overwhelmed by excessive light, various antioxidative systems are activated to shield cells from oxidative damage caused by ROS (Kataria et al., 2014). In addition to protecting PSII, cyanobacteria also exhibit photophobic or phototactic movements, enabling them to move away from or towards light sources, respectively (Gupta et al., 2023).
Light quality also presents a significant ecological challenge, and consequently, cyanobacteria restructure their phycobilisomes (PBSs) by altering phycobiliproteins (PBPs), linker proteins, or chlorophylls (Chl) and carotenoids (Cars). These changes, triggered by light signals, optimize the absorption of available photons for efficient photosynthesis in fluctuating light environments (Gupta et al., 2023). These developmental responses to light color or intensity are termed chromatic acclimation (CA). CA provides an additional advantage to cyanobacteria by modulating their capacity to absorb the prevailing wavelengths of light (Mondal et al., 2024).
This review synthesizes the diverse cellular damages inflicted by high light and UVR stress and comprehensively accounts for the strategies employed by cyanobacteria under varying light conditions. We highlight the superior flexibility and diversity of cyanobacteria compared to plants in tolerating light stress, positioning them as highly efficient photoautotrophs for utilizing natural light. Ultimately, understanding the intricate photoprotective and photoacclimatory mechanisms evolved by these ancient and metabolically versatile prokaryotes—including energy dissipation, antioxidative responses, and CA—is crucial. This knowledge not only elucidates their ecological success and significant role in shaping Earth’s environment but also paves the way for effectively harnessing their remarkable oxygenic photosynthesis for sustainable biotechnological applications to achieve United Nations’ sustainable developmental goals.
Cyanobacterial thylakoid membranes: structure and evolutionary significance
Cyanobacteria, Gram-negative photosynthetic bacteria, exhibit an ultrastructure similar to plant chloroplasts. Their photosynthetic machinery resides in thylakoid membranes (TMs), which are typically arranged as concentric rings towards the periphery of the cytoplasm, enclosing a lumen (Allen and Forsberg, 2001). However, TM distribution can vary with environmental conditions (Huokko et al., 2021). The structural similarities and phylogenetic evidence support the endosymbiotic theory of chloroplast origin from cyanobacteria (Stadnichuk and Kusnetsov, 2021; Whatley et al., 1979).
The endosymbiotic event likely drove TM folding in chloroplast ancestors to accommodate cyanobacteria-like endosymbionts within the host cell’s limited space. Furthermore, the loss of PBSs and an increased PSII cross-section facilitated thylakoid stacking and the spatial segregation of PSII (in grana lamellae) and PSI (in stroma lamellae), minimizing excitation energy spillover. However, far-red light, preferentially absorbed by PSI, can induce PSI migration to grana lamellae, potentially causing spillover (Terashima et al., 2025; Stadnichuk and Kusnetsov, 2021). Thus, endosymbiotic membrane folding resulted in the stacked (grana lamellae) and unstacked (stromal lamellae) thylakoids characteristic of modern plant chloroplasts, contrasting with the appressed thylakoids in cyanobacteria (Huokko et al., 2021). The close homology between cyanobacteria and chloroplasts offers a valuable system to investigate photosynthetic mechanisms and responses to environmental factors, aiding our understanding of photophysiology across cyanobacteria, algae, and plants. Cyanobacteria also serve as a model for studying chloroplast evolution and biogenesis (Nielsen et al., 2016). Notably, cyanobacterial thylakoids house electron carriers for both photosynthesis and respiration (Figure 2). These processes are interconnected in cyanobacteria (Mullineaux, 2014), which is unlike the compartmentalized photosynthesis (in chloroplast) and respiration (in mitochondria) found in algae and higher plants.
Figure 2

Schematic diagram of photosynthetic and respiratory electron transport on the thylakoid membrane (TM). The Z-scheme (black arrows) depicts light absorption by PBSs exciting PSII, followed by water splitting (brown arrow) in the lumen. Cyclic electron transport (red dashed arrow) around PSI generates ATP. Respiratory complexes SDH and NDH-I (blue arrows) reduce the PQ-pool; SDH oxidizes succinate to fumarate, while NDH-I oxidizes NADPH (blue dashed arrow). Orange arrows indicate PSII protection mechanisms involving flavodiiron proteins Flv2/Flv4 (associated with PSII) and Flv1/Flv3 (associated with PSI), which alleviate electronic stress. Complexes Cox, Cyd, and Flv1/Flv3 release water (dark green arrow) by accepting electrons from Pc, PQH2, and NADPH, respectively (Stirbet et al., 2019; Liu, 2016). TM, thylakoid membrane; PSII, photosystem II; NDH-I, NADPH-quinone dehydrogenase; SDH, succinate dehydrogenase; hνPBS, light absorption by phycobilisomes (PBS); hν1, light absorption by PSI; hν2, light absorption by PSII; Flv1/3 and Flv2/4, flavodiiron protein; PQ, plastoquinone; Cyd, cytochrome bd quinol oxidase; Cyt b6f, cytochrome b6f; Pc, plastocyanin; Cyt c6, cytochrome c6; NAD(P)H, nicotinamide-adenine dinucleotide phosphate; Fd, ferredoxin; FNR, ferredoxin-NADP+ oxidoreductase; Cox, cytochrome c oxidase; Pgr5, proton gradient regulation; H+, proton; ATP syn, adenosine triphosphate synthase; pmf, proton motive force; p-side, lumen side; n-side, cytosolic side.
In Synechocystis sp. PCC 6803, PSII resides in the outer TM, while PSI is located in the inner TM (Liu, 2016). Conversely, in Synechococcus elongatus PCC 7942, PSI is peripheral, and PSII is uniformly distributed within the TM (Liu, 2016). Cyanobacterial PSII exhibits a granular structure due to the attachment of its core light-harvesting antenna, PBSs—unlike the peripheral antennae in plants—on the TM’s outer surface. This PBS attachment gives a granular appearance between TMs. Unlike plants, cyanobacteria lack thylakoid stacking; instead, their photosynthetic membranes display diverse peripheral distribution patterns (Mareš et al., 2019). The presence of PBSs prevents membrane appression, but their absence in mutants or specific light conditions can induce thylakoid appression in cyanobacteria (Maurya et al., 2023; Liberton et al., 2013). The branched TMs have been reported in Cyanothece sp. 51,142 where photosynthetic membranes are interconnected through channels and share a common lumen (Liberton et al., 2011).
PBSs function as light-harvesting antennae, analogous to those in higher plants, increasing the absorption cross-section for PSII photochemistry. Cryogenic confocal microscopy indicates that PBSs primarily transfer excitation energy to PSII but can also directly interact with PSI or form PBS-PSII-PSI supercomplexes (Steinbach et al., 2015). The delicate TM, crucial for cellular physiological stability, is susceptible to environmental influences. Light and other factors can alter TM lipid fluidity, leading to topographical changes (Mullineaux and Liu, 2020). While cryoelectron microscopy has been used to study PS topography, its freeze-fracture method can compromise the native structure. Atomic force microscopy (AFM) is emerging as an alternative for studying TM topography in its physiologically active state within aqueous media. AFM offers valuable insights into PS topography, revealing that PSII and PSI can dynamically change their structure in response to environmental conditions (MacGregor-Chatwin et al., 2019; Zhao et al., 2022). Consequently, AFM is a promising tool for exploring the structural and mechanical aspects of photosynthesis that remain largely unknown.
Structural adaptations in photosynthetic machine
Unlike most algae and plants, cyanobacteria primarily utilize PBSs as their main light-harvesting complex (LHC) for PSII. For clarity, PBSs can be divided into a “core,” directly attached to the thylakoid membrane at the photosystem, and “rods,” extending distally from the core (Figure 3A). Linker proteins connect the rods to form a 3D hemispherical PBS (Domínguez-Martín et al., 2022). PBSs are substantial structures, ranging from 300 to 800 Å in diameter, with their size inversely related to light intensity (Tamary et al., 2012; Dou et al., 2025). Under very high light, PBS rods can even dissociate, leaving only the core (Mimuro et al., 2008). Nutrient availability also influences PBS structure and composition (Nagarajan et al., 2019). Cyanobacteria exhibit four main PBS morphologies: hemiellipsoidal, block-shaped, bundle-shaped, and hemidiscoidal (Sui, 2021). Hemidiscoidal PBSs are further classified by the number of core cylinders, i.e., bicylindrical or tricylindrical with six rods or pentacylindrical with eight rods (Singh et al., 2015; Sui, 2021). Notably, rod length, composition, and shape are dynamically adjusted in response to environmental cues, including light (Chenu et al., 2017; Gutu and Kehoe, 2012). This adaptability of PBS rods provides cyanobacteria with their characteristic coloration and the ability to efficiently harvest specific light intensities and wavelengths, granting them a competitive advantage over other photosynthetic organisms in diverse light environments.
Figure 3

Structure of phycobilisome (PBS) and its components. (A) A complete PBS consists of a core and rods. Rods are cylindrical structures composed of PC and/or PE, linked by polypeptides that maintain PBS integrity. (B) A side view of the core reveals cylinders, each containing four discs. These discs are composed of two types of APC: APC660, found in upper and basal cylinders, and APC680, present only in basal cylinders. The antiparallel arrangement of basal cylinders places APC680 adjacent to APC660. Red dots within APC680 indicate variants: one dot represents αAPC680 (replaced by αAP-B), and two dots represent αAPC680βAPC680 (replaced by αLCM and β18.5). (C) A front view of the core clearly shows the arrangement of basal and upper cylinders (Stirbet et al., 2019). PE, phycoerythrin; PC, phycocyanin; APC, allophycocyanin.
PBPs, the chromoprotein building blocks of PBS cores and rods, are pigmented heterodimers of α (~17 kDa) and β (~18 kDa) subunits. These subunits covalently bind bilin chromophores, similar to phytochrome in higher plants (Mancini et al., 2018). Unlike chlorophylls, the linear tetrapyrrole chromophores make PBPs water-soluble. The spectral diversity of PBSs arises from the association of these chromophores with the PBP protein moiety (Stirbet et al., 2019). Four main PBP types exist, characterized by their color and light absorption: allophycocyanin (APC; ~650 nm, dark blue), phycocyanin (PC; 620–638 nm, light blue), phycoerythrin (PE; ~565 nm, pink), and phycoerythrocyanin (PEC; ~568 nm, purple). APC and PC bind phycocyanobilin (PCB), with APC having one PCB per α and β subunit, while PC has one per α and two per β subunit (Szalontai et al., 1989; MacColl, 2004). PE binds phycoerythrobilin (PEB) or phycourobilin (PUB) chromophore, absorbing green or blue light, respectively (Dagnino-Leone et al., 2022; Stirbet et al., 2019). PEC binds both PCB and phycoviobilin (PVB) (Zehetmayer et al., 2004). A single rod cylinder consists of a chromophoric (αβ)6 hexamer. These acidic cylinders stack via basic linker polypeptides to form rods that connect to the PBS core (Anderson and Toole, 1998).
The core of PBS is made up of trimeric units of α and β subunits of APC, i.e., (αAPCβAPC)3 (Sui, 2021). The core is arranged in three piles of cylinders: two at the base and one on top (Figure 3C). The top cylinder connects to the rods via linker proteins. The basal cylinders contain two types of APC, i.e., APC660 and APC680, having emission peaks at 660 nm and 680 nm, respectively (Jallet et al., 2012). The upper cylinder of the core is made up of four discs of APC660 trimers, which transfer energy from the rods to the basal core. Each basal cylinder has four (αAPCβAPC)3 discs—two of APC660 (αAPC660βAPC660)3 and two of APC680 (αAPC680βAPC680)3—which together maintain the structure and function of the PBS (Figure 3B) (Jallet et al., 2012). Out of two discs of APC680, in one APC680 trimer (αAPC680βAPC680)3, the one αAPC680 subunit is replaced by an αAP-B, encoded by the apcD gene while another disc of APC680 trimer (αAPC680βAPC680)3 has β18.5 subunit (encoded by the apcF gene) instead of βAPC, and αLCM subunit (encoded by the apcE gene) instead of αAPC in one monomer (αAPC680βAPC680) of APC680 trimer (Jallet et al., 2012) These variations contribute to the structural complexity and functional specialization of the APC trimers (Figure 3B). ApcE (αLCM) is essential for stabilizing PBSs by linking core to the TM while its α domain interacts with the PBPs (Sui, 2021). ApcE may regulate state transitions based on the plastoquinone pool’s redox state. ApcD, ApcE, and ApcF mediate energy transfer from APC680 to PSII or PSI, with ApcE favoring PSII and ApcD/ApcF primarily exciting PSI during state transitions (Tomar et al., 2024).
Photosynthetic units (photosystems) in cyanobacteria
Photoautotrophs possess natural photocells, termed photosystems (PSs), to perform light absorption and photochemical reactions. These reactions convert light energy into chemical energy, fueling the organism’s survival, maintenance, and reproduction. This energy also forms the base of the food web. A photosystem comprises a light-capturing antenna and a reaction center, orchestrating energy excitation, transfer, and photochemistry. Anoxygenic green and purple sulfur bacteria were the first to evolve strategies for harvesting and converting light energy into chemical bond energy (Blankenship, 2010; Hu et al., 1997; Larkum et al., 2018). These prokaryotes independently developed two distinct types of reaction centers—the site of photochemistry—distinguished by their terminal electron acceptors (Figure 4). These are the FeS-type (type I) reaction center found in green sulfur bacteria and the quinone-type (Q-type or type II) reaction center of purple sulfur bacteria (Blankenship, 2010). Green and purple sulfur bacteria utilize H2, H2S, or S as electron sources for photochemistry (Martin et al., 2018). Given Earth’s early reducing atmosphere, these sulfur bacteria were likely the planet’s first photoautotrophs. Their reaction centers served as the foundation for the evolution of modern oxygenic photosynthesis (Ohashi et al., 2010). The limited availability of H2, H2S, and S, coupled with the abundance of water, likely drove the selection pressure for the evolution of the oxygen-evolving complex (Xiong and Bauer, 2002; Tomitani et al., 2006). Consequently, it is plausible that purple and green sulfur bacteria evolved PSII and PSI, respectively, that eventually leads to evolution of cyanobacteria capable of oxygenic photosynthesis (Sharon et al., 2009; Govindjee and Shevela, 2011).
Figure 4

Evolution of reaction centers in photosynthetic bacteria. The purple rectangle represents the quinone-type reaction center (RC) of purple sulfur bacteria, where quinone is the terminal electron acceptor. The blue rectangle depicts the FeS-type RC of green sulfur bacteria, with an FeS center as the final electron acceptor. An evolutionary event brought these two RC types together in a single organism, forming a complete electron transport chain (ETC) that produces both ATP and NADPH in cyanobacteria (green circle), the first oxygenic photoautotrophs (Shevela et al., 2013). Em, electrode potential in volt; RC2, reaction center 2; RC1, reaction center 1; hν, light; Bchl, bacteriochlorophyll; Chl, chlorophyll; P870/P960, P680, P700, P840, pigment 870/960, 680, 700 and 840 indicates reaction centers; P870*/960*, 680,700 and 840* indicates excited state of all reaction centers; Bpheo, bacteriopheophytin; Pheo, pheophytin; QA/QB, quinone A/quinone B; PSII and PSI, photosystem II and photosystem I; A0, A1, electron acceptors of PSI; 4Fe-4S, iron-sulfur clusters; O2, oxygen; PS, photosystem; hν, light of specific wavelength.
Photosystem II (PSII): structure and function
Crystal structures of PSII solved at various resolutions have provided definitive insights into its physicochemical properties (Shen, 2015; Zouni et al., 2001). Notably, a 1.9 Å resolution structure revealed the dynamic interactions of amino acid side chains with metallic and organic cofactors (pigments) during the light reactions (Umena et al., 2011). The following section will detail the structure of PSII. PSII, a ~ 700 kDa dimeric multisubunit membrane protein supercomplex also known as water/plastoquinone oxidoreductase (Wegener et al., 2015; Shen, 2015), presents challenges for intact isolation, leading to studies on its monomeric (~350 kDa) form. The arrangement of PSII subunits facilitates unidirectional excitation energy and electron transfer from lower to higher redox potentials (Stirbet et al., 2019). Spectrally, PSII comprises two pigment-protein complex regions: the peripheral antenna, which harvests light energy and transfer it to the reaction center or core antenna (Zuber, 1986; Stirbet, 2013).
Cyanobacteria were crucial in elucidating the conserved molecular organization of core antennae, essential for photochemistry in all oxygenic photoautotrophs. Molecular analysis of PSII core antennae reveal that its axial symmetry involve two distinct polypeptides, CP43 and CP47, which bridge the reaction center and antennae for efficient excitation energy transfer (Umena et al., 2011). The core itself consists of a D1 (PsbA) and D2 (PsbD) heterodimer, binding redox-active cofactors near the reaction center for electron transfer. The antennae are composed of Chl-binding proteins CP43 (PsbC) and CP47 (PsbB), binding 13–16 Chls and 4–5 β-carotenes (Stirbet et al., 2019). The 1.9 Å resolution crystal structure of the PSII monomer (Figure 5) showed it comprises 17 integral membrane protein subunits (large and low molecular mass) and three extrinsic protein subunits on the luminal side (Umena et al., 2011). The large molecular mass subunits are integral membrane proteins, while the low molecular mass subunits include the (PsbE) and (PsbF) subunits of cytoplasmic Cytochrome b559 (Cyt b559) (Loll et al., 2007). Although Cyt b559 does not participate in PSII photochemistry, it maintains the structural integrity of the PSII core (Chiu et al., 2022). Three extrinsic membrane proteins, PsbO, PsbV, and PsbU, are attached to the luminal surface. These proteins protect the oxygen-evolving complex from redox-active cofactors of the electron transport chain and maintain the integrity of the large molecular mass subunits (D1, D2, CP43, and CP47) because loops of these integral proteins extend into the thylakoid lumen (Iwai et al., 2010; Shen, 2015). Thus, extrinsic membrane proteins act as a protective cap for these loops.
Figure 5
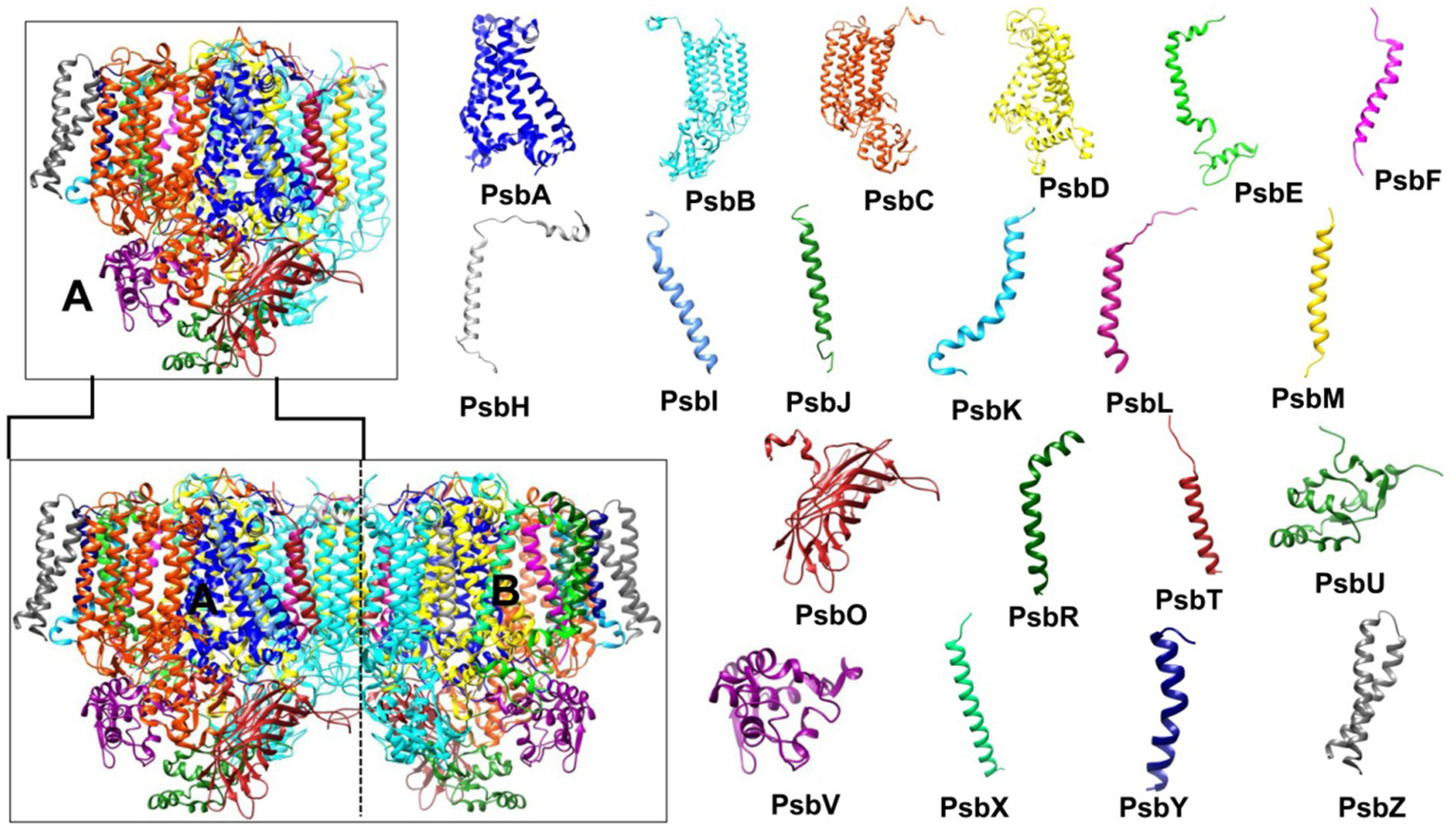
Photosystem II (PSII) structure (PDB ID: 7YQ7, 1.9 Å resolution). The bottom left shows the PSII dimer (subunits A and B), while the top left displays the PSII monomer (subunit A). The right side illustrates the structure of the 20 subunits comprising the PSII monomer. PSII, photosystem II.
The D1 and D2 proteins are symmetrically positioned within the transmembrane region, forming the D1/D2 heterodimer, with two branches binding redox-active cofactors (Umena et al., 2011). These cofactors include six Chl ɑ molecules (PD1, PD2, ChlD1, ChlD2, ChlZD1, ChlZD2, named for their D1 or D2 branch association), two pheophytins (PheoD1 and PheoD2), a tightly bound QA (on D2), and a loosely bound QB (on D1). PQ molecules, QA and QB, are bound to specific amino acids of D2 and D1, respectively. A non-heme iron, equidistant between QA and QB, facilitates sequential electron transfer in the photosynthetic electron transport chain (Umena et al., 2011). Additionally, two β-carotenes, four manganese ions, three or four Ca2+ ions (one bound to Mn4CaO5), three chloride (Cl−) ions, and one carbonate (CO32−) or bicarbonate (HCO3−) ion are bound near the non-heme iron, participating in QB protonation during its reduction by QA (Shevela et al., 2012).
Photosystem I (PSI): structure and function
Jordan et al. (2001) determined the 2.5 Å crystal structure of PSI, revealing its threefold symmetry as a trimer (Figure 6). The PSI monomer comprises 12 subunits (PsaA–F, PsaI–M, and PsaX) and 127 cofactors: 96 Chls, 22 Cars, 2 phylloquinones, PhQ (A1A, A1B), 3 [4Fe-4S] clusters, 1 putative Ca2+, and 4 lipid molecules (Jordan et al., 2001; Nagao et al., 2023; Stirbet et al., 2019). PSI, also known as plastocyanin (Pc)/ferredoxin (Fd)-oxidoreductase, receives electrons from the mobile carrier Pc (transferring electrons from the Cyt b6f complex) and donates them to Fd, the terminal electron acceptor of the Z-scheme, which then reduces Fd-dependent NADPH reductase (FNR) (Stirbet et al., 2019).
Figure 6

Photosystem I (PSI) Structure (PDB ID: 1JB0, 2.5 Å resolution). The bottom left shows the PSI trimer (subunits A, B, and C), while the top left displays the PSI monomer (subunit A). The right side illustrates the structure of the 12 subunits comprising the PSI monomer. PSI, photosystem I.
Of the 12 PSI subunits, PsaL, PsaX, and PsaM are absent in higher plants, with PsaM and PsaL specifically driving PSI trimer or tetramer formation in cyanobacteria (Nagao et al., 2023; Chen et al., 2022). In certain thermophilic and heterocyst-containing cyanobacteria, additional copies of PsaL enable the formation of PSI tetramers (Li et al., 2014). The PSI tetramer is particularly significant for its thermostability, which makes it an ideal target for engineering the PSI. In higher plants, PsaH prevents PSI trimer formation (Li et al., 2014), suggesting that PSI might form trimers under specific environmental conditions, even without PsaL and PsaM, though these two proteins enhance trimer stability.
The PSI core antenna is primarily composed of PsaA and PsaB subunits. Similar to CP47 and CP43 in PSII, the Chl ɑ molecules linked to the six-helix domains in the N-terminus of PsaA and PsaB facilitate light absorption (Barber et al., 2000). Additionally, 10 Chls are associated with subunits PsaG, PsaK–M, PsaX, and a phosphatidylglycerol molecule. Due to trimeric or tetrameric nature of PSI, it has a high number of associated Chls and Cars (60–90 Chls in the inner antenna are associated with Cars, with most β-carotene linked to long-wavelength Chls) (Karapetyan et al., 1999; Karapetyan et al., 2014). In addition to their light absorbing and photoprotective properties, Cars aid in the assembly and stabilization of the pigment-protein complex (Wang et al., 2004).
Photochemistry and photosynthesis-respiration interplay
Photochemistry is a rapid natural chemical reaction fundamental to all photoautotrophs. Photosynthesis results from two sequential chemical reactions that take place in the TM and stroma of chloroplast. However, in cyanobacteria, it takes place in the TM and carboxysome-cum-cytoplasm (Rohnke et al., 2018). The first step involves photochemistry while second step involves enzymatic reduction of CO2 using the byproducts of photochemistry, i.e., NADPH and ATP. Spectral analysis reveals that Chl molecules absorb maximally in the blue and red regions. However, Chl ɑ molecules with red and far-red absorption maxima were evolutionarily favored to form the reaction centers of both PSs due to their capacity to store significant energy for charge separation (Shevela et al., 2013). Thus, Chl molecules in the reaction center are replaced to optimize absorption based on available light wavelengths. Antennae also contain blue-absorbing Chl ɑ molecules. These lower-wavelength absorbing Chls are positioned away from the reaction center to maximize light capture while minimizing excitation pressure (Björn et al., 2009).
Upon absorbing blue light (450–500 nm), Chl molecules reach a highly unstable highest excitation state. They rapidly lose some energy as heat to transition to a lower, more stable excited state, from which they can release energy via fluorescence, heat dissipation, resonance energy transfer, or photochemistry (Björn et al., 2009). In plants, light-harvesting complexes, and in cyanobacteria, the PSII inner core antenna, arrange Chls around the reaction center to facilitate energy transfer through excitation or resonance (Shevela et al., 2013; Romero et al., 2010). The TM houses two PSs where non-cyclic electron flow (Z-scheme) involving both PSII and PSI produces NADPH and ATP (non-cyclic photophosphorylation). Cyclic electron transport, involving only PSI, generates ATP (cyclic photophosphorylation) (Bendall and Manasse, 1995; Petrouleas and Crofts, 2005; Kok et al., 1970; Arnon, 1984; Lea-Smith et al., 2016; Giera et al., 2009; Hill and Rich, 1983; Albertsson, 1995; Tikhonov, 2014). Unlike plants, cyanobacteria lack peripheral antennae but possess PBSs to capture light poorly absorbed by PSs. Chl fluorescence (ChlF) spectra, showing strong emission at 685–695 nm (PSII) and 715–730 nm (PSI), demonstrate PBSs’ role in delivering excitation energy to PSII and PSI via state transition (Rijgersberg and Amesz, 1980). The parallel increase in PSI and PSII fluorescence yield with a decrease in PBS fluorescence over time, evident in time-resolved 77 K fluorescence spectra, confirms PBS-PS interaction (Ueno et al., 2017).
Despite their arrangement, PSs can likely efficiently absorb excitation energy from PBSs (David et al., 2014). Three models explain this transfer: (1) direct PBS-PSI energy transfer (Akhtar et al., 2022), (2) simultaneous PBS interaction with both PSII and PSI forming a PBS-PSII-PSI supercomplex (Liu et al., 2013), and (3) spillover, where a PBS-PSII supercomplex transfers energy to PSI (Ueno et al., 2017; Calzadilla and Kirilovsky, 2020). Cyanobacterial TMs uniquely house both photosynthetic and respiratory electron transport chains (Figure 2), converging at the PQ pool, Pc, and the cytochrome b6f complex. The TM contains succinate dehydrogenase (SDH), NAD(P)H dehydrogenases (NDH), and terminal oxidases like Cyd and Cox. Active in darkness or low light, SDH oxidizes succinate, and NDH oxidizes NADPH (Mullineaux, 2014). The presence of respiratory complexes on the TM means PSI competes with respiratory carriers for electron flow in photochemistry. The PQ pool can be reduced by linear photosynthetic electron transport or by respiratory electron transport from NADPH (via NDH-1) and succinate (via SDH) (Mullineaux, 2014; Willeford et al., 1989). Under high light, Cox competes with P700 for electrons from Pc or Cyt c6 (Ermakova et al., 2016). Changes in PSI stoichiometry under high light can divert electrons from PSII to Cox. Conversely, blocking terminal oxidases, as in darkness, allows respiratory electrons to reduce the acceptor side of PSII (Nikkanen et al., 2021; McDonald et al., 2011). Thus, respiratory electron carriers serve as an electron source in low light and a sink in high light, preventing photoinhibition (Nikkanen et al., 2021; McDonald et al., 2011).
Light regime and cyanobacterial photosynthetic machinery
Photosynthetic organisms, including cyanobacteria, inhabit environments characterized by fluctuating light conditions, encompassing both low and high intensities of PAR and UVR. These variations, inherent in day-night cycles, and seasonal and spatial changes, significantly impact the overall fitness of these organisms by affecting fundamental cellular processes and causing damage to crucial biological molecules (Mondal et al., 2024; Singh et al., 2010). The following sections will summarize the detrimental effects of different light regimes and the photoacclimation strategies cyanobacteria have evolved to thrive in such dynamic environments.
High and low PAR: photosynthetic consequences
Light intensity in natural ecosystems fluctuates seasonally and diurnally, exerting differential effects on photoautotroph fitness. High PAR can damage the D1 protein of PSII, a phenomenon termed photoinhibition (Long et al., 1994). Photoinhibition triggers non-photochemical quenching (NPQ), driven by Cars associated with LHCII in response to low TM pH in higher plants and algae (Li et al., 2000; Calzadilla and Kirilovsky, 2020). This pH decrease also induces state transitions (state 2) of LHCII and the production of ROS (Bennett, 1991; Allen, 2003). To counteract increased ROS, higher plants and algae elevate the levels and activity of antioxidative enzymes, antioxidants, and Cars for ROS mitigation (Middleton and Teramura, 1993).
Despite their long evolutionary history in dynamic light environments, cyanobacteria are negatively impacted by abrupt changes in light intensity. High-intensity blue-green or white light triggers NPQ in these organisms (Gorbunov et al., 2011). Elevated PAR levels induce oxidative stress, or photooxidation, where the over-accumulation of Fd, a strong reductant, readily reduces oxygen molecules, producing superoxide and peroxide radicals that can damage biological molecules (Latifi et al., 2009). Furthermore, the triplet state of Chl ɑ can transfer excitation energy to molecular oxygen, forming singlet oxygen (1O2∗), which can then transfer its energy to lipid molecules. This singlet oxygen can disrupt the integrity of the TM and potentially lead to the disassembly of the oxygen-evolving complex (Asada, 1999). Additionally, increased singlet oxygen levels activate the production of high-light inducible proteins (HLiPs) (Komenda and Sobotka, 2016). Conversely, cyanobacterial cells acclimated to low light conditions are particularly vulnerable to rapid D1 protein damage upon sudden increase in light intensity. Consequently, low-light-grown cultures require extended periods to acclimatize to even slight increases in irradiance (Zhou et al., 2024). Furthermore, low light intensity also promotes state transitions, a phenomenon discussed in detail in a subsequent section.
UVR and photosynthetic impairment
UVR impairs photosynthesis through multiple mechanisms beyond photoinhibition. It negatively impacts the activity of ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO), CO2 intake, and the oxygen-evolving complex (Watanabe et al., 2023). Conversely, low-intensity UV-A (315–400 nm) radiation can enhance photosynthesis and biomass accumulation in certain cyanobacteria (Grettenberger et al., 2024). Furthermore, UVR exposure induces the generation of ROS at various sites within the photosynthetic and respiratory electron transport chains, as well as during diverse metabolic reactions (Napaumpaiporn et al., 2024; Noyma et al., 2015).
UVR exerts numerous damaging effects on cyanobacterial cells, including photobleaching of photosynthetic pigments and a significant decrease in protein content. The reduction in absorption and fluorescence emission spectra of PBPs upon UVR exposure is linked to decreased PBP content and the disassembly of PBSs (Six et al., 2007). UVR also inhibits CO2 fixation and damages DNA by inducing the formation of thymine dimers and other photoproducts (Singh et al., 2023a). Prolonged UVR exposure can impair nitrogenase activity, severely affect heterocyst and akinete differentiation, and reduce the gliding motility of filamentous cyanobacteria (Rastogi et al., 2014). Moreover, UVR inhibits the photophobic and phototactic responses of cyanobacteria, thereby compromising their ability to avoid damaging radiation and ultimately leading to photodamage and cell death (Singh et al., 2023a).
Acclimation strategies to spectral quality
Found in diverse ecological niches—oceans, seas, freshwater, glaciers, and land—cyanobacteria are exposed to temporally, diurnally, and seasonally changing light regimes. Consequently, they have evolved various strategies to adjust their position in the water column and optimize their photosynthetic machinery, including light-harvesting, for efficient photon utilization. Cyanobacteria possess broad range of photosensors that sense different wavelengths/quality/color of light, enabling them to initiate various photoacclimatization and/or developmental processes (Gupta et al., 2023). These photosensors can sense different part of the solar spectrum, including visible light, UVR and far-red light (Bandara et al., 2021; Gupta et al., 2023). Cyanobacterial photosensors belong to the cyanobacteriochrome (CBCR) phytochrome superfamily. Phytochromes (Phys) possess a tripartite N-terminal photosensory domain, comprising PAS, GAF, and PHY domains (Bandara et al., 2021; Gupta et al., 2023), also known as the photosensory core module. The GAF domain (cGMP phosphodiesterase, adenyl cyclase, and FhlA) binds linear tetrapyrrole (bilin) pigments via a thioether bond to detect light signals (Xu et al., 2020). Phytochromobilin bound to the GAF domain can exist in different configurations (5-Z,syn, 10-Z,syn, and 15-Z,anti) in its dark/low-light protonated form (Pr). Conversely, red-light-rich, high-light environments induce rotation around the C15–C16 double bond, resulting in the 15-E,anti configuration (Pfr form). The activated Pfr form of Phy triggers the expression of various genes by entering the nucleus (Bandara et al., 2021).
In contrast to Phys, CBCRs exhibit greater diversity in sensing a wider spectrum of light, from near-UV or visible to far-red, utilizing PCB (Bandara et al., 2021). Like Phys in higher plants, CBCRs possess one or more GAF domains that efficiently bind bilins via thioether linkages (Bandara et al., 2021; Gupta et al., 2023). The molecular evolution of the GAF domain under varying light conditions has resulted in the spectral diversity of CBCRs, enabling cyanobacteria to perceive different light qualities (Bandara et al., 2021; Gupta et al., 2023). The nature of the thioether bond between phycobilin and the GAF domain dictates the spectral properties of CBCRs; for example, a two-thioether linkage allows absorption of near-UV, violet, and blue light (Bandara et al., 2021). The spectral diversity of cyanobacteriochromes (CBCRs) makes them valuable tools in synthetic biology and optogenetics. Their ability to enable light-mediated modulation of gene transcription allows for easy tracking of genetic activity within cells and tissues by combining different CBCRs (Ariyanti et al., 2021; Gupta et al., 2023). Additionally, various CBCRs can be used to promote phototaxis in engineered prokaryotic and eukaryotic strains in response to specific light signals (Ariyanti et al., 2021).
Based on the perceived light color, the corresponding signal is transduced by the two-component signaling system of CBCRs to trigger cellular responses (Gupta et al., 2023). One ecological significant phenomenon in cyanobacteria is CA, which is a wavelength-dependent adjustment in the protein and/or pigment composition of PBSs. CA allows cyanobacteria to optimize their fitness and photosynthetic efficiency in dynamic light environments (Montgomery, 2017; Kumar et al., 2019; Mondal et al., 2024). The following sections will briefly outline different light conditions under which cyanobacteria employ CA to maximize their photosynthetic fitness by modifying their light-harvesting apparatus.
A common type of CA observed in some marine cyanobacteria involves adjustment to blue and green light, which penetrate deeper into clean ocean and sea environments. Under blue light condition, the chromophore phycourobilin (PUB) associates with phycoerythrin (PE), enabling the organism to absorb blue light and appear orange (Carrigee et al., 2020). Conversely, in green light environment, the chromophore phycoerythrobilin (PEB) is incorporated into the PE apoprotein, maximizing green light absorption and giving the organism a brick-red color (Gupta et al., 2023; Mondal et al., 2024). This dynamic chromophore attachment to PE allows these cyanobacteria to efficiently harvest the prevalent wavelengths: PUB under blue light and PEB under green light. While green light is typically not utilized by photosynthetic organisms, PEB allows certain cyanobacteria to perform photosynthesis using green light. Similarly, although cyanobacteria generally rely on PBSs for PSII excitation, limiting blue light utilization (Luimstra et al., 2018), the PUB chromophore provides a unique advantage to some marine species to efficiently use blue light for photochemistry.
CA in response to red and green light is well-documented in the cyanobacterium Fremyella diplosiphon where change in the levels of PC and PE in PBS take place under red and green light growth conditions, respectively (Montgomery, 2017; Mondal et al., 2024). CA enables cyanobacteria to acclimatize to far-red light environment (Wiltbank and Kehoe, 2019). The far-red shifting of PBS and occurrence of Chl d and Chl f in the PS is the characteristics features of CA in Halomicronema hongdechloris, Chlorogloeopsis frtischii, Leptolyngbya sp. strain JSC-1. Red/far-red CBCRs control the accumulation of proteins that constitute red-shifted PSII and far-red shifted PBSs (Mondal et al., 2024; Montgomery, 2017; Wiltbank and Kehoe, 2019).
Acclimation strategies to light intensity
The detrimental effects of light on photosynthetic organisms are particularly relevant in the context of global climate change. Fluctuating light conditions, coupled with other abiotic stressors like salinity, temperature, nutrient availability, and UVR, can have severe consequences. Consequently, understanding the photoprotective mechanisms evolved by these ancient organisms is crucial for addressing challenges associated with global climate change. The following sections will explore various photoprotective strategies employed by cyanobacteria to mitigate the damaging effects of PAR.
Non-photochemical quenching (NPQ)-based photoprotection
NPQ encompasses processes that dissipate excess excitation energy as heat, rather than through fluorescence or photochemistry. This ensures efficient functioning of PSII under high irradiance. The following are various mechanisms by which cyanobacteria release surplus excitation energy in the form of heat to safeguard their photosynthetic machinery.
Orange carotenoid protein (OCP)
High light intensity induces NPQ in the peripheral antennae of green algae and plants, where a significant portion of energy is released as heat (Asada, 1999). Cars quench excess energy without forming triplet states, thus also acting as ROS scavengers. Cyanobacteria utilize an orange carotenoid protein (OCPO)-based NPQ mechanism. Upon absorbing blue-green light, the OCPO form converts to a red carotenoid protein (OCPR) form. OCPR dissipates excessive high-light energy absorbed by PBSs as heat, ensuring that only an optimal amount of energy is transferred to the reaction centers (Wilson et al., 2022; Domínguez-Martín et al., 2022; Kirilovsky, 2007).
Nearly all cyanobacterial strains possess OCP, and OCP-deficient mutants exhibit increased susceptibility to photodamage. The gene encoding OCP is constitutively expressed and further upregulated under high light conditions (Kirilovsky and Kerfeld, 2012). In Synechocystis, OCP is encoded by the slr1963 gene. However, it is absent in the freshwater Synechococcus elongatus PCC 7942 and the thermophile Thermosynechococcus elongatus (Kirilovsky and Kerfeld, 2012; Tiwari et al., 2024). Consequently, these OCP-lacking cyanobacteria are sensitive to high-light radiation. Nevertheless, they can protect themselves by reducing their PBPs content to mitigate damage from excess light. These organisms can also functionally disconnect their PBSs, a phenomenon more common under iron-limiting conditions (Kirilovsky and Kerfeld, 2012; Tiwari et al., 2024).
OCPs are water-soluble proteins of approximately 35 kDa that non-covalently bind a keto-carotenoid molecule, typically 3′-hydroxyechinenone (3-hECN) or its homologs (Domínguez-Martín et al., 2022). Cyanobacteria possess at least three paralog families of OCPs: OCP1, OCP2, and OCPX, with OCP1 being the most prevalent (Slonimskiy et al., 2022). Localized on the cytoplasmic side of the thylakoid membrane near PBSs, OCPs consist of two domains (Figure 7): an α-helical N-terminal domain (NTD) and an α/β C-terminal domain (CTD). The CTD, belonging to the nuclear transport factor-2 superfamily, is connected to the NTD by a flexible linker of approximately 25 residues, which is part of an unstructured intrinsically disordered protein region (Leverenz et al., 2014; Leverenz et al., 2015).
Figure 7

Conformational states of OCP under light and dark conditions. Blue arrows indicate blue/high light conditions, while black arrows show reversion to the OCPO state in darkness. (A) In the inactive OCPO state, the NTE is attached to the CTD, and the β-ketocarotenoid spans both domains. (B) Absorption of blue/high light induces a conformational change in the β-ketocarotenoid, leading to OCP activation where the NTE detaches from the CTD. The NTD and CTD remain linked by a loop. (C) Complete dissociation of the NTD and CTD occurs, with the β-ketocarotenoid fully translocated into the NTD to bind with APC and regulate excitation energy transfer to the reaction center (Stirbet et al., 2019). CTD, C-terminal domain; NTD, N-terminal domain; NTE, N-terminal extension; OCPO, orange carotenoid protein (orange form); OCPR, orange carotenoid protein (red form).
In darkness or low light, the orange (inactive) form of OCP predominates. However, high-intensity blue-green light induces a conformational change in the bound carotenoid molecule, leading to the conversion of inactive OCPO into a metastable OCPR. This OCPR then interacts with PBSs to perform NPQ by dissipating their excess excitation energy (Domínguez-Martín et al., 2022; Prabha et al., 2025). Recent findings indicate that OCPR forms a dimer to interact with PBSs. However, this interaction is selective, requiring a suitable conformation of the PBSs, meaning not all PBSs can interact with the OCPR dimer (Domínguez-Martín et al., 2022).
In the presence of Cu2+, photoactivation of OCP results in its stabilization in the red form (OCPR). This occurs because Cu2+ binding to a cysteine residue prevent the return of protein to its inactive orange state (Liu et al., 2016). Upon activation, OCP undergoes a structural change where the NTD and CTD separate while remaining connected by a linker loop (Figure 7B). Subsequently, the keto-carotenoid spanning both domains detaches from the CTD and shifts 12 Å into a cavity within the NTD (Figure 7C), forming OCPR (Thurotte et al., 2015; Maksimov et al., 2017a, 2017b). This detachment during photoactivation involves either β-ionylidene ring rotation or a transient keto-enol shift (Maksimov et al., 2015; Bandara et al., 2017). Finally, OCPR interacts with the core hexamer of PBSs, ApcC, becoming buried within the terminal hexamer of the basal core cylinder (Harris et al., 2018; Gwizdala et al., 2018).
Thus, structural changes in PBSs influence excitation energy transfer kinetics. Spectroscopic analysis during OCP docking indicates a temporary decoupling of some PBS rods (Bar Eyal et al., 2017), while another study proposes a PBS conformational change to facilitate OCPR interaction (Domínguez-Martín et al., 2022). Under low light or dark conditions, Fluorescence Recovery Protein (FRP), encoded by slr1964 in Synechocystis sp. PCC 6803, removes OCPR from PBSs (Liu et al., 2018). This action halts the process of NPQ and allows the fluorescence of the PBSs to recover. This ~13 kDa water-soluble protein, lacking a chromophore, primarily exists as a functional dimer, transitioning from an inactive tetramer (Thurotte et al., 2017). FRP specifically interacts with the CTD of OCPR to accelerate its detachment from PBSs and promote its deactivation to OCPO (Thurotte et al., 2017). However, excess Cu2+ inhibits FRP’s interaction with OCPR by locking OCP in the OCPR form, potentially aiding the organism in tolerating high-light conditions in Cu2+ stressed cells (Liu et al., 2018; Lou et al., 2019). In summary, under high light conditions, the OCP cuts off the excitation energy transfer from PBSs to PSII, thereby lowering the photochemical quantum efficiency of PSII. While this strategy protects PSII from photodamage, it also reduces the overall photosynthetic efficiency of cells exposed to high light.
Iron-starvation inducible protein (IsiA)
Fluctuating environments can disrupt the redox balance in cyanobacteria (Jeanjean et al., 2003; Havaux et al., 2005). Under high light and iron depletion, the Chl-binding TM protein IsiA forms photoprotective rings around PSI and its antenna (Zhang et al., 2010). It is homologous to CP43 (PsaC subunit in PSII), and therefore, also called as CP43`. These PSI3-IsiA18 supercomplexes (6 IsiA per PSI monomer) dissipate excess light energy as heat (Reinot et al., 2022). Combined high light and iron starvation also lead to IsiA-PSI-PSII supercomplex formation, a condition where PBSs become functionally disconnected (Wang et al., 2010). However, under iron stress and strong light, free PBSs can couple with IsiA, which prevents damage from dissociated PBSs (Joshua and Mullineaux, 2005). In Synechocystis sp. 6,803 and Synechococcus elongatus PCC 7942, IsiA protects PSI and its antenna under iron-deficient and high-light conditions (Nodop et al., 2008; Sherman and Sherman, 1983). Similarly, in Anabaena sp. PCC 7120, iron depletion causes PSI tetramer disintegration because PsaL, required for tetramer formation, competes with IsiA for binding to the PSI monomer. Increased IsiA production under iron limitation thus favors the formation of PSI monomers with IsiA in place of PsaL (Nagao et al., 2023). Long-term iron deficiency leads to the accumulation of unbound IsiA, which quenches excited Chl molecules. This causes dissipation of energy as heat and results in decreased Chl fluorescence. Excitation energy from Chl a is transferred to a cysteine residue in IsiA for thermal dissipation (Chen et al., 2017; Schoffman and Keren, 2019; Chen et al., 2021). Thus, IsiA protects cyanobacteria from the damaging effects of detached PBSs and Chl a molecules. Considering the function of IsiA protein, caution should be taken when growing cyanobacterial cultures on a large scale under natural light condition. This is because the combined effect of iron limitation and high light intensity can lead to unwanted thermal dissipation of excitation energy, which may reduce biomass production.
High-light-inducible protein (HliP)
HliPs are part of the LHC superfamily and were first identified in Synechococcus elongatus PCC 7942, which lacks OCP-based NPQ and utilizes Hlip for photoprotection under high light. Among various LHCs (Konert et al., 2023), HliPs are the most ancient, characterized by a single-helix structure with the ExxNxR Chl-binding motif, typical of LHCs (Komenda and Sobotka, 2016; Konert et al., 2023). These proteins share homology with one-helix proteins (OHPs) in higher plants and algae, which bind Chl a/b pigments (Konert et al., 2023). Ubiquitous in cyanobacteria, HliPs are involved in Chl biosynthesis, PSII repair, and photoprotection (Konert et al., 2023; Krynická et al., 2023). The expression of hli genes is induced by high light and oxidative stress. Also, the accumulation of HliPs is regulated by FtsH4 protease, which in turn ensures the proper biogenesis and assembly of PSII (Krynická et al., 2023). Furthermore, their interaction with Chl and Cars suggests a role in NPQ, which highlights their importance under high light in OCP-lacking cyanobacteria (Komenda and Sobotka, 2016; Konert et al., 2023). Information on the global importance of HliPs in cyanobacteria is limited, and therefore, further genomic, transcriptomics, proteomics, mutagenesis, and gene overexpression studies are needed to establish their importance across a wider range of organisms.
State transition ensures balanced excitation of PSII and PSI
Fluctuating light can cause an imbalance in the excitation of PSII and PSI, leading to compromised photosynthesis and photooxidative stress (Calzadilla and Kirilovsky, 2020). This imbalance is resolved through state transitions, a reversible process where the peripheral antenna, or light-harvesting complex (LHC), of PSII migrates to PSI (Calzadilla and Kirilovsky, 2020). In higher plants, state transitions are controlled by thylakoid membrane-bound threonine kinase and phosphatase enzymes. These enzymes facilitate the phosphorylation and dephosphorylation of the light-harvesting complex (LHCII), respectively, which triggers its movement between the two PSs (Haldrup et al., 2001; Calzadilla and Kirilovsky, 2020). The signal for LHCII phosphorylation arises from the accumulation of a reduced PQ pool, a consequence of PSII overexcitation. This triggers the transition from state 1 to state 2, leading to the detachment of LHCII from PSII and its subsequent association with PSI. During state 2, PSI is preferentially excited, causing the PQ pool to become oxidized. This oxidation event activates the phosphatase, which then dephosphorylates LHCII, prompting its migration back from PSI to PSII, thus completing the transition from state 2 to state 1 (Calzadilla and Kirilovsky, 2020).
In cyanobacteria, PBSs mediate the distribution of excitation energy between PSII and PSI. The redox state of the PQ pool influences the electronic load on both PSs, triggering the reversible movement of PBSs between state 2 and state 1 (Calzadilla and Kirilovsky, 2020). State transitions can be studied using 77 K fluorescence spectrophotometry, where the relative change in PSII to PSI fluorescence intensity reflects the excitation energy distribution (Kaňa et al., 2012). A preferential transfer of excitation energy from PBSs to PSI results in a decrease in PSII fluorescence yield. Notably, Murata, 1969 first observed that prolonged illumination with blue or far-red light, preferentially absorbed by PSI, induces the state 2 to state 1 transition, characterized by a high PSII:PSI fluorescence ratio.
State transitions occur naturally during illumination but can also be induced by the non-photochemical reduction of the PQ pool through respiration in darkness or low light conditions (Chukhutsina et al., 2015). This can lead to inaccurate measurements of minimum fluorescence (F0) and maximum fluorescence (Fm), resulting in an underestimation of the maximum quantum efficiency of PSII (Fv/Fm) due to the reduced PQ pool (Stirbet et al., 2019). Here, F0 represents the minimum Chl a fluorescence when the reaction center is open (oxidized PQ pool), while Fm represents the maximum fluorescence when the reaction center is closed (reduced PQ pool). To address this issue, dark-adapted cells are briefly illuminated with a weak beam of blue-green or far-red light (preferentially absorbed by the high Chl content in PSI) to oxidize PQH2 (Tiwari et al., 2024). This treatment is commonly employed to induce a state 1 transition, which helps in obtaining a more accurate F0 value in cyanobacteria (Stirbet et al., 2019).
Respiratory cofactors protect cyanobacteria under high light
Respiratory cofactors are crucial in mitigating the damaging effects of high light, which imposes a combined burden of photons and electrons on the photosynthetic machinery. Located in close proximity to PSI, these cofactors protect the system by regulating electron transport (Liu, 2016). When the rate of carbon fixation lags behind electron production, NADPH accumulates in the cytosol. To counteract this, the NDH-1 enzyme, situated in the TM, increases the concentration of NADP+ in the cytosol and protons in the lumen (Lea-Smith et al., 2016). Additionally, the succinate dehydrogenase (SDH), also located in the TM, reduces the PQ pool by oxidizing succinate to fumarate (Lea-Smith et al., 2016). The thylakoidal respiratory electron transport chain also features terminal oxidases, Cyd and Cox. Under high-light conditions, these oxidases compete with PSI for electrons from PQH2 and either Pc or Cyt c6, respectively. This competition helps in preventing the formation of ROS by utilizing protons and oxygen to produce water (Ermakova et al., 2016).
Protective role of flavodiiron proteins (FDPs) in minimizing ROS production
Flavoproteins (Flvs), also known as FDPs, are prevalent in anaerobic and some aerobic prokaryotes and protozoa (Allahverdiyeva et al., 2015). Despite their diverse classifications, all FDPs share two highly conserved structural domains. The N-terminal domain features a metallo-β-lactamase-like module containing a non-heme diiron center (Allahverdiyeva et al., 2015). This domain is specialized for the reduction of nitric oxide (NO) and O2, thereby preventing the intracellular accumulation of ROS. X-ray crystallographic studies of FDPs from various organisms have elucidated the electron transfer mechanism to O2 and/or NO within the enzyme’s active site (Allahverdiyeva et al., 2015). Connecting the N and C-terminal domains is an intermediate functional domain. The C-terminal domain adopts a flavodoxin-like fold and contains a flavin mononucleotide (FMN) moiety, exhibiting NADPH-flavin oxidoreductase activity (Allahverdiyeva et al., 2015).
FDPs can be classified based on the diverse extensions present in their C-terminal domains (Romão et al., 2016). Class A represents the largest group of the simplest FDPs, possessing only the FMN-binding domain described above. This class is commonly found in bacteria, archaea, and protozoa. Class B FDPs, found in enterobacteria, contain an Fe-S cluster in addition to the FMN-binding domain. Class C FDPs are obligately present in all oxygenic photoautotrophs and are characterized by an NAD(P)H:flavin oxidoreductase-like domain. Class D FDPs are restricted to protozoa and some bacteria and contain a flavin adenine dinucleotide (FAD) domain alongside an Fe-S cluster (Martins et al., 2019; Allahverdiyeva et al., 2015).
FDPs are encoded by flv genes, which exist in multiple copies within the cyanobacterial genome, forming small families of distinct FDPs. In oxygenic photoautotrophs, FDPs are categorized into two clusters: A and B (Allahverdiyeva et al., 2013). Cluster A includes Flv1 and Flv2, whereas Cluster B comprises Flv3 and Flv4. Members from these clusters form heterodimers, such as Flv1/Flv3 and Flv2/Flv4, to execute their functions (Romão et al., 2016; Santana-Sanchez et al., 2019).
Anaerobes exhibit high sensitivity to oxygen, making FDPs essential for their survival. In these organisms, FDPs reduce O2 to water, safeguarding cells from oxidative stress (He et al., 2025). Mehler (1951) and Mehler and Brown (1952) initially described the photoreduction of O2 to H2O2 by the photosynthetic electron transport chain in chloroplasts, a process known as the Mehler reaction. Subsequent research indicated that the primary product of this reaction is the superoxide anion. This anion is then converted to H2O2 by superoxide dismutase, which is further reduced to water by ascorbate peroxidase (Forti and Ehrenheim, 1993). In this cycle, electrons originate from the splitting of water at the PSII side and are ultimately transferred to O2 via PSI (Asada, 2000). Consequently, this process is also termed pseudocyclic electron flow or the water–water cycle. The pseudocyclic electron flow or water–water cycle serves two critical functions: first, it prevents saturation of the Z-scheme by diverting excess electrons from PSI to O2 when CO2 assimilation is limited. Second, it contributes to the generation of a proton gradient across the TM, thereby inducing NPQ and ATP synthesis (Foyer and Noctor, 2000; Allahverdiyeva et al., 2013).
The Flv1/Flv3 heterodimer also plays a role in state transitions by facilitating the oxidation of the PQ pool when carbon fixation by RuBisCO is low (Bersanini et al., 2014; He et al., 2025). Furthermore, under fluctuating light conditions, this heterodimer maintains the redox balance within the electron transport chain, protecting cells from oxidative damage that can arise from an imbalance in electron flow between PSII and PSI, leading to a decrease in photosynthetic rate (Allahverdiyeva et al., 2015; He et al., 2025). Studies on knockout and overexpression strains of flv2/flv4 suggest the existence of an alternative electron transport pathway to an unidentified acceptor near the QB site (Santana-Sanchez et al., 2019). The Flv2/Flv4 heterodimer stabilizes forward electron transfer in PSII, thereby enhancing charge separation and preventing the formation of singlet oxygen, which can occur through energy transfer from excited Chl to O2 (Bersanini et al., 2014). The expression of flv2 and flv4 genes is elevated in the absence of OCP, and their protein products contribute to the stabilization of the PSII dimer and its association with PBSs (Bersanini et al., 2014). In conclusion, the FDPs represent a critical and highly-evolved protective system in cyanobacteria. Their ability to manage electron flow and dissipate excess energy plays fundamental role in maintaining redox homeostasis and preventing photodamage under fluctuating light conditions. Therefore, continued study of these proteins is essential for a complete understanding of their complex roles and leverages their function for biotechnological applications.
Other photoprotective mechanisms in cyanobacteria
As a first line of defense against high-energy PAR and UVR, cyanobacterial cells employ physical barriers. These include the production of a mucilaginous sheath and photoprotective compounds such as mycosporine-like amino acids (MAAs) and scytonemins (Singh et al., 2010; Singh et al., 2023b). Filamentous forms can also create amorphous silica matrices, form mats, and undergo morphological changes to reduce their surface area, providing further protection against intense PAR and UVR (Singh et al., 2010; Singh and Montgomery, 2011). Additionally, these organisms exhibit phototactic and photophobic responses to adjust their position relative to light intensity (Gupta et al., 2023). Beyond these strategies, cyanobacteria possess a range of enzymatic (superoxide dismutase, catalase, glutathione peroxidase, ascorbate peroxidase, and monodehydroascorbate reductase) and non-enzymatic (ascorbate, α-tocopherol, and reduced glutathione) mechanisms to keep ROS at tolerable levels (Latifi et al., 2009). Furthermore, if genomic DNA is directly damaged by UVR absorption or indirectly by ROS production, cyanobacteria can utilize various repair mechanisms to prevent mutations caused by extreme light environments (Pathak et al., 2019; Singh et al., 2023a).
Biotechnological implications and future perspectives
In summary, cyanobacteria, ancient and metabolically versatile prokaryotes, have profoundly influenced on Earth’s environment and remain ecologically significant. Their remarkable oxygenic photosynthesis not only paved the way for aerobic life and the ozone layer but also holds immense promise for sustainable biotechnological applications. However, realizing this potential depends on the resilience of their photosynthetic machinery under fluctuating light environment. Cyanobacteria have evolved photoprotective and photoacclimatory mechanisms, including energy dissipation pathways, antioxidative systems, and chromatic acclimation, to optimize light harvesting and maintain photosynthetic efficiency across diverse and dynamic environments. Understanding these intricate light responses in detail is crucial for both comprehending their ecological success and effectively harnessing their metabolic capabilities for a sustainable future via biomanufacturing.
The extensive adaptive and acclimative strategies of cyanobacteria under fluctuating light conditions make them more suitable than algae and higher plants for open or closed cultivation systems, where light fluctuation is a major concern. Due to the dense cell population in these systems, light intensity is limited and enriched in green-blue wavelengths. This reduces the quantum yield of photosynthesis in algae and higher plants because they lack the photoacclimation strategies found in cyanobacteria. Therefore, the genome bank of cyanobacteria presents an enormous opportunity to engineer photoautotrophs of biotechnological importance for better growth and performance in large-scale cultivation systems. However, the fact that no single cyanobacterial strain possesses the complete repertoire of light stress mitigation mechanisms presents a compelling avenue for future research and bioengineering. Expanding the “umbrella of tolerance” within a single strain by integrating a comprehensive suite of light-adaptive features into a single genome holds significant promise.
For instance, engineering strains to robustly manage excitation pressure through the enhanced expression and coordinated action of proteins like OCP, HliPs, and IsiA could lead to substantial improvements in photochemical efficiency. This would not only conserve cellular energy otherwise invested in mitigating electronic load and repairing cellular machineries under high light but could also intrinsically regulate the rate and content of ROS production. However, constitutive expression of such protective mechanisms can compromise the productivity under light limiting conditions due to loss of excess energy. Considering the growing economic and pharmaceutical significance of cyanobacteria for humankind, the development of such “super-resilient” strains with minimized ROS production presents a highly attractive prospect for various industrial applications. Future efforts should therefore prioritize the genetic engineering and synthetic biology approaches necessary to consolidate these diverse light-acclimation traits within a single, high-performing cyanobacterial strain. The research on how salt stress affects stability of PSI (in trimer or tetramer) and interaction of OCP with PBS and PSs is still very limited This could unlock new frontiers in sustainable biofuel production, carbon capture, bioremediation, and the synthesis of valuable bioactive compounds (biomanufacturing), ultimately leveraging the remarkable photosynthetic capabilities of cyanobacteria for achieving sustainable developmental goals.
Statements
Author contributions
ST: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. AG: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. DP: Conceptualization, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. PP: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. RG: Formal analysis, Writing – original draft, Writing – review & editing. SS: Conceptualization, Formal analysis, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors gratefully acknowledge the financial support from the Science and Engineering Research Board (SERB), New Delhi, to SS (Grant No. SCP/2022/000201). ST is thankful to the University Grants Commission (UGC), New Delhi, India, for a senior research fellowship (NTA Ref. No. 201610202339). AG is thankful to the Indian Council of Medical Research (ICMR), New Delhi, for a senior research fellowship (3/1/3/JRF-2019/HRD-LS), and DP acknowledges to the Science and Engineering Research Board (SERB), New Delhi, for providing a junior research fellowship (Grant No. SCP/2022/000201). PP acknowledges the Council of Scientific and Industrial Research (CSIR), New Delhi, for a senior research fellowship (09/0013(15476)/2022-EMR-I). RG is thankful to University Grants Commission (UGC), New Delhi, India, for a senior research fellowship (NTA Ref. No. 211610075296). SS also acknowledges support from the Institute of Eminence Incentive Grant, Banaras Hindu University (R/Dev/D/IOE/Incentive/2021-2022/32399).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Akhtar P. Biswas A. Balog-Vig F. Domokos I. Kovács L. Lambrev P. H. (2022). Trimeric photosystem I facilitates energy transfer from phycobilisomes in Synechocystis sp. PCC 6803. Plant Physiol.189, 827–838. doi: 10.1093/plphys/kiac130
2
Albertsson P. Å. (1995). The structure and function of the chloroplast photosynthetic membrane—a model for the domain organization. Photosynth. Res.46, 141–149. doi: 10.1007/BF00020424
3
Allahverdiyeva Y. Isojärvi J. Zhang P. Aro E. M. (2015). Cyanobacterial oxygenic photosynthesis is protected by flavodiiron proteins. Life5, 716–743. doi: 10.3390/life5010716
4
Allahverdiyeva Y. Mustila H. Ermakova M. Bersanini L. Richaud P. Ajlani G. et al . (2013). Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light. Proc. Natl. Acad. Sci. U.S.A.110, 4111–4116. doi: 10.1073/pnas.1221194110
5
Allen J. F. (2003). State transitions-a question of balance. Science299, 1530–1532. doi: 10.1126/science.1082833
6
Allen J. F. Forsberg J. (2001). Molecular recognition in thylakoid structure and function. Trends Plant Sci.6, 317–326. doi: 10.1016/S1360-1385(01)02010-6
7
Álvarez C. Jiménez-Ríos L. Iniesta-Pallarés M. Jurado-Flores A. Molina-Heredia F. P. Ng C. K. et al . (2023). Symbiosis between cyanobacteria and plants: from molecular studies to agronomic applications. J. Exp. Bot.74, 6145–6157. doi: 10.1093/jxb/erad261
8
Anderson L. K. Toole C. M. (1998). A model for early events in the assembly pathway of cyanobacterial phycobilisomes. Mol. Microbiol.30, 467–474. doi: 10.1046/j.1365-2958.1998.01081.x
9
Ariyanti D. Ikebukuro K. Sode K. (2021). Artificial complementary chromatic acclimation gene expression system in Escherichia coli. Microb. Cell Fact.20:128. doi: 10.1186/s12934-021-01621-3
10
Arnon D. I. (1984). The discovery of photosynthetic phosphorylation. Trends Biochem. Sci.9, 258–262. doi: 10.1016/0968-0004(84)90159-2
11
Asada K. (1999). The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Biol.50, 601–639. doi: 10.1146/annurev.arplant.50.1.601
12
Asada K. (2000). The water–water cycle as alternative photon and electron sinks. Philos. Trans. R. Soc. Lond. B355, 1419–1431. doi: 10.1098/rstb.2000.0703
13
Bandara S. Ren Z. Lu L. Zeng X. Shin H. Zhao K. H. et al . (2017). Photoactivation mechanism of a carotenoid-based photoreceptor. Proc. Natl. Acad. Sci. U.S.A.114, 6286–6291. doi: 10.1073/pnas.1700956114
14
Bandara S. Rockwell N. C. Zeng X. Ren Z. Wang C. Shin H. et al . (2021). Crystal structure of a far-red-sensing cyanobacteriochrome reveals an atypical bilin conformation and spectral tuning mechanism. Proc. Natl. Acad. Sci. U.S.A.118:e2025094118. doi: 10.1073/pnas.2025094118
15
Bar Eyal L. Ranjbar Choubeh R. Cohen E. Eisenberg I. Tamburu C. Dorogi M. et al . (2017). Changes in aggregation states of light-harvesting complexes as a mechanism for modulating energy transfer in desert crust cyanobacteria. Proc. Natl. Acad. Sci. U.S.A.114, 9481–9486. doi: 10.1073/pnas.1708206114
16
Barber J. Morris E. Büchel C. (2000). Revealing the structure of the photosystem II chlorophyll binding proteins, CP43 and CP47. Biochim. Biophys. Acta Bioenerg.1459, 239–247. doi: 10.1016/S0005-2728(00)00158-4
17
Battaglino B. Arduino A. Pagliano C. Sforza E. Bertucco A. (2021). Optimization of light and nutrients supply to stabilize long-term industrial cultivation of metabolically engineered cyanobacteria: a model-based analysis. Ind. Eng. Chem. Res.60, 10455–10465. doi: 10.1021/acs.iecr.0c04887
18
Baunach M. Guljamow A. Miguel-Gordo M. Dittmann E. (2024). Harnessing the potential: advances in cyanobacterial natural product research and biotechnology. Nat. Prod. Rep.41, 347–369. doi: 10.1039/D3NP00045A
19
Bendall D. S. Manasse R. S. (1995). Cyclic photophosphorylation and electron transport. Biochim. Biophys. Acta Bioenerg.1229, 23–38. doi: 10.1016/0005-2728(94)00195-B
20
Bennett J. (1991). Protein phosphorylation in green plant chloroplasts. Annu. Rev. Plant Biol.42, 281–311. doi: 10.1146/annurev.pp.42.060191.001433
21
Bersanini L. Battchikova N. Jokel M. Rehman A. Vass I. Allahverdiyeva Y. et al . (2014). Flavodiiron protein Flv2/Flv4-related photoprotective mechanism dissipates excitation pressure of PSII in cooperation with phycobilisomes in cyanobacteria. Plant Physiol.164, 805–818. doi: 10.1104/pp.113.231969
22
Björn L. O. Papageorgiou G. C. Blankenship R. E. Govindjee G. (2009). A viewpoint: why chlorophyll a?Photosynth. Res.99, 85–98. doi: 10.1007/s11120-008-9395-x
23
Blankenship R. E. (2010). Early evolution of photosynthesis. Plant Physiol.154, 434–438. doi: 10.1104/pp.110.161687
24
Calzadilla P. I. Kirilovsky D. (2020). Revisiting cyanobacterial state transitions. Photochem. Photobiol. Sci.19, 585–603. doi: 10.1039/c9pp00451c
25
Canonico M. Konert G. Crepin A. Šedivá B. Kaňa R. (2021). Gradual response of cyanobacterial thylakoids to acute high-light stress—importance of carotenoid accumulation. Cells10:1916. doi: 10.3390/cells10081916
26
Carrigee L. A. Mahmoud R. M. Sanfilippo J. E. Frick J. P. Strnat J. A. Karty J. A. et al . (2020). CpeY is a phycoerythrobilin lyase for cysteine 82 of the phycoerythrin I α-subunit in marine Synechococcus. Biochim. Biophys. Acta Bioenerg.1861:148215. doi: 10.1016/j.bbabio.2020.148215
27
Chen H. Y. S. Liberton M. Pakrasi H. B. Niedzwiedzki D. M. (2017). Reevaluating the mechanism of excitation energy regulation in iron-starved cyanobacteria. Biochim. Biophys. Acta Bioenerg.1858, 249–258. doi: 10.1016/j.bbabio.2017.01.001
28
Chen M. Liu X. He Y. Li N. He J. Zhang Y. (2022). Diversity among cyanobacterial photosystem I oligomers. Front. Microbiol.12:781826. doi: 10.3389/fmicb.2021.781826
29
Chen H. Y. S. Niedzwiedzki D. M. Bandyopadhyay A. Biswas S. Pakrasi H. B. (2021). A novel mode of photoprotection mediated by a cysteine residue in the chlorophyll protein IsiA. mBio12:e03663-20. doi: 10.1128/mBio.03663-20
30
Chenu A. Keren N. Paltiel Y. Nevo R. Reich Z. Cao J. (2017). Light adaptation in phycobilisome antennas: influence on the rod length and structural arrangement. J. Phys. Chem. B121, 9196–9202. doi: 10.1021/acs.jpcb.7b07781
31
Chiu Y. F. Fu H. Y. Skotnicová P. Lin K. M. Komenda J. Chu H. A. (2022). Tandem gene amplification restores photosystem II accumulation in cytochrome b559 mutants of cyanobacteria. New Phytol.233, 766–780. doi: 10.1111/nph.17785
32
Chukhutsina V. Bersanini L. Aro E. M. Van Amerongen H. (2015). Cyanobacterial light-harvesting phycobilisomes uncouple from photosystem I during dark-to-light transitions. Sci. Rep.5:14193. doi: 10.1038/srep14193
33
Dagnino-Leone J. Figueroa C. P. Castañeda M. L. Youlton A. D. Vallejos-Almirall A. Agurto-Muñoz A. et al . (2022). Phycobiliproteins: structural aspects, functional characteristics, and biotechnological perspectives. Comput. Struct. Biotechnol. J.20, 1506–1527. doi: 10.1016/j.csbj.2022.02.016
34
David L. Prado M. Arteni A. A. Elmlund D. A. Blankenship R. E. Adir N. (2014). Structural studies show energy transfer within stabilized phycobilisomes independent of the mode of rod-core assembly. Biochim. Biophys. Acta Bioenerg.1837, 385–395. doi: 10.1016/j.bbabio.2013.12.014
35
Domínguez-Martín M. A. Sauer P. V. Kirst H. Sutter M. Bína D. Greber B. J. et al . (2022). Structures of a phycobilisome in light-harvesting and photoprotected states. Nature609, 835–845. doi: 10.1038/s41586-022-05156-4
36
Dou B. Li Y. Wang F. Chen L. Zhang W. (2025). Chassis engineering for high light tolerance in microalgae and cyanobacteria. Crit. Rev. Biotechnol.45, 257–275. doi: 10.1080/07388551.2024.2357368
37
Ermakova M. Huokko T. Richaud P. Bersanini L. Howe C. J. Lea-Smith D. J. et al . (2016). Distinguishing the roles of thylakoid respiratory terminal oxidases in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol.171, 1307–1319. doi: 10.1104/pp.16.00479
38
Forti G. Ehrenheim A. M. (1993). The role of ascorbic acid in photosynthetic electron transport. Biochim. Biophys. Acta Bioenerg.1183, 408–412. doi: 10.1016/0005-2728(93)90246-C
39
Foyer C. H. Noctor G. (2000). Oxygen processing in photosynthesis: regulation and signalling. New Phytol.146, 359–388. doi: 10.1046/j.1469-8137.2000.00667.x
40
Garcia-Pichel F. Belnap J. (2021). “Cyanobacteria and algae” in Principles and applications of soil microbiology. eds. GentryT. J.JeffryJ. F.ZubererD. A. (Radarweg, NL: Elsevier), 171–189.
41
Giera W. Gibasiewicz K. Ramesh V. M. Lin S. Webber A. (2009). Electron transfer from A0 to A1 in photosystem I from Chlamydomonas reinhardtii occurs in both the a and B branch with 25–30-ps lifetime. Phys. Chem. Chem. Phys.11, 5186–5191. doi: 10.1039/b822938d
42
Gorbunov M. Y. Kuzminov F. I. Fadeev V. V. Kim J. D. Falkowski P. G. (2011). A kinetic model of non-photochemical quenching in cyanobacteria. Biochim. Biophys. Acta Bioenerg.1807, 1591–1599. doi: 10.1016/j.bbabio.2011.08.009
43
Govindjee G. Shevela D. (2011). Adventures with cyanobacteria: a personal perspective. Front. Plant Sci.2:28. doi: 10.3389/fpls.2011.00028
44
Grettenberger C. L. Abou-Shanab R. Hamilton T. L. (2024). Limiting factors in the operation of photosystems I and II in cyanobacteria. Microb. Biotechnol.17:e14519. doi: 10.1111/1751-7915.14519
45
Gupta A. Pandey P. Gupta R. Tiwari S. Singh S. P. (2023). Responding to light signals: a comprehensive update on photomorphogenesis in cyanobacteria. Physiol. Mol. Biol. Plants29, 1915–1930. doi: 10.1007/s12298-023-01386-6
46
Gutu A. Kehoe D. M. (2012). Emerging perspectives on the mechanisms, regulation, and distribution of light color acclimation in cyanobacteria. Mol. Plant5, 1–13. doi: 10.1093/mp/ssr054
47
Gwizdala M. Krüger T. P. Wahadoszamen M. Gruber J. M. Van Grondelle R. (2018). Phycocyanin: one complex, two states, two functions. J. Phys. Chem. Lett.9, 1365–1371. doi: 10.1021/acs.jpclett.8b00621
48
Haldrup A. Jensen P. E. Lunde C. Scheller H. V. (2001). Balance of power: a view of the mechanism of photosynthetic state transitions. Trends Plant Sci.6, 301–305. doi: 10.1016/S1360-1385(01)01953-7
49
Harris D. Bar-Zvi S. Lahav A. Goldshmid I. Adir N. (2018). “The structural basis for the extraordinary energy-transfer capabilities of the phycobilisome” in Membrane protein complexes: structure and function. eds. HarrisJ. R.BoekemaE. J. (Singapore: Springer), 57–82.
50
Havaux M. Guedeney G. Hagemann M. Yeremenko N. Matthijs H. C. Jeanjean R. (2005). The chlorophyll-binding protein IsiA is inducible by high light and protects the cyanobacterium Synechocystis PCC 6803 from photooxidative stress. FEBS Lett.579, 2289–2293. doi: 10.1016/j.febslet.2005.03.021
51
He M. Santana-Sánchez A. Tóth G. S. Ermakova M. Collard D. Kosourov S. et al . (2025). Deletion of Flv3A facilitates long-term H2 photoproduction in diazotrophic Anabaena sp. PCC 7120. Physiol. Plant.177:e70087. doi: 10.1111/ppl.70087
52
Hill R. Rich P. R. (1983). A physical interpretation for the natural photosynthetic process. Proc. Natl. Acad. Sci. U.S.A.80, 978–982. doi: 10.1073/pnas.80.4.978
53
Hu X. Ritz T. Damjanović A. Schulten K. (1997). Pigment organization and transfer of electronic excitation in the photosynthetic unit of purple bacteria. J. Phys. Chem. B101, 3854–3871. doi: 10.1021/jp963777g
54
Huokko T. Ni T. Dykes G. F. Simpson D. M. Brownridge P. Conradi F. D. et al . (2021). Probing the biogenesis pathway and dynamics of thylakoid membranes. Nat. Commun.12:3475. doi: 10.1038/s41467-021-23680-1
55
Iwai M. Suzuki T. Kamiyama A. Sakurai I. Dohmae N. Inoue Y. et al . (2010). The PsbK subunit is required for the stable assembly and stability of other small subunits in the PSII complex in the thermophilic cyanobacterium Thermosynechococcus elongatus BP-1. Plant Cell Physiol.51, 554–560. doi: 10.1093/pcp/pcq020
56
Jallet D. Gwizdala M. Kirilovsky D. (2012). ApcD, ApcF and ApcE are not required for the orange carotenoid protein related phycobilisome fluorescence quenching in the cyanobacterium Synechocystis PCC 6803. Biochim. Biophys. Acta Bioenerg.1817, 1418–1427. doi: 10.1016/j.bbabio.2011.11.020
57
Jeanjean R. Zuther E. Yeremenko N. Havaux M. Matthijs H. C. Hagemann M. (2003). A photosystem 1 psaFJ-null mutant of the cyanobacterium Synechocystis PCC 6803 expresses the isiAB operon under iron replete conditions. FEBS Lett.549, 52–56. doi: 10.1016/s0014-5793(03)00769-5
58
Jordan P. Fromme P. Witt H. T. Klukas O. Saenger W. Krauß N. (2001). Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution. Nature411, 909–917. doi: 10.1038/35082000
59
Joshua S. Mullineaux C. W. (2005). The rpaC gene product regulates phycobilisome–photosystem II interaction in cyanobacteria. Biochim. Biophys. Acta Bioenerg.1709, 58–68. doi: 10.1016/j.bbabio.2005.06.005
60
Kaňa R. Kotabová E. Komárek O. Šedivá B. Papageorgiou G. C. Prášil O. (2012). The slow S to M fluorescence rise in cyanobacteria is due to a state 2 to state 1 transition. Biochim. Biophys. Acta Bioenerg.1817, 1237–1247. doi: 10.1016/j.bbabio.2012.02.024
61
Karapetyan N. V. Bolychevtseva Y. V. Yurina N. P. Terekhova I. V. Shubin V. V. Brecht M. (2014). Long-wavelength chlorophylls in photosystem I of cyanobacteria: origin, localization, and functions. Biochem. Mosc.79, 213–220. doi: 10.1134/S0006297914030067
62
Karapetyan N. V. Holzwarth A. R. Rögner M. (1999). The photosystem I trimer of cyanobacteria: molecular organization, excitation dynamics and physiological significance. FEBS Lett.460, 395–400. doi: 10.1016/S0014-5793(99)01352-6
63
Kataria S. Jajoo A. Guruprasad K. N. (2014). Impact of increasing ultraviolet-B (UV-B) radiation on photosynthetic processes. J. Photochem. Photobiol. B137, 55–66. doi: 10.1016/j.jphotobiol.2014.02.004
64
Kirilovsky D. (2007). Photoprotection in cyanobacteria: the orange carotenoid protein (OCP)-related non-photochemical-quenching mechanism. Photosynth. Res.93, 7–16. doi: 10.1007/s11120-007-9168-y
65
Kirilovsky D. Kerfeld C. A. (2012). The orange carotenoid protein in photoprotection of photosystem II in cyanobacteria. Biochim. Biophys. Acta Bioenerg.1817, 158–166. doi: 10.1016/j.bbabio.2011.04.013
66
Kok B. Forbush B. McGloin M. (1970). Cooperation of charges in photosynthetic O2 evolution–I. A linear four step mechanism. Photochem. Photobiol.11, 457–475. doi: 10.1111/j.1751-1097.1970.tb06017.x
67
Komenda J. Sobotka R. (2016). Cyanobacterial high-light-inducible proteins-protectors of chlorophyll-protein synthesis and assembly. Biochim. Biophys. Acta Bioenerg.1857, 288–295. doi: 10.1016/j.bbabio.2015.08.011
68
Konert M. M. Wysocka A. Koník P. Sobotka R. (2023). Correction: high-light-inducible proteins HliA and HliB: pigment binding and protein–protein interactions. Photosynth. Res.157:53. doi: 10.1007/s11120-023-01016-y
69
Krynická V. Skotnicová P. Jackson P. J. Barnett S. Yu J. Wysocka A. et al . (2023). FtsH4 protease controls biogenesis of the PSII complex by dual regulation of high light-inducible proteins. Plant Commun.4:100502. doi: 10.1016/j.xplc.2022.100502
70
Kumar V. Maurya P. K. Mondal S. Sinha R. P. Singh S. P. (2019). “Photomorphogenesis in the cyanobacterium Fremyella diplosiphon improves photosynthetic efficiency” in Cyanobacteria: from basic science to applications. eds. MishraA. K.TiwariD. N.RaiA. N. (Poole: Elsevier), 79–130.
71
Larkum A. W. D. Ritchie R. J. Raven J. A. (2018). Living off the sun: chlorophylls, bacteriochlorophylls and rhodopsins. Photosynthetica56, 11–43. doi: 10.1007/s11099-018-0792-x
72
Latifi A. Ruiz M. Zhang C. C. (2009). Oxidative stress in cyanobacteria. FEMS Microbiol. Rev.33, 258–278. doi: 10.1111/j.1574-6976.2008.00134.x
73
Lea-Smith D. J. Bombelli P. Vasudevan R. Howe C. J. (2016). Photosynthetic, respiratory and extracellular electron transport pathways in cyanobacteria. Biochim. Biophys. Acta Bioenerg.1857, 247–255. doi: 10.1016/j.bbabio.2015.10.007
74
Leverenz R. L. Jallet D. Li M. D. Mathies R. A. Kirilovsky D. Kerfeld C. A. (2014). Structural and functional modularity of the orange carotenoid protein: distinct roles for the N-and C-terminal domains in cyanobacterial photoprotection. Plant Cell26, 426–437. doi: 10.1105/tpc.113.118588
75
Leverenz R. L. Sutter M. Wilson A. Gupta S. Thurotte A. de Carbon C. B. et al . (2015). A 12 Å carotenoid translocation in a photoswitch associated with cyanobacterial photoprotection. Science348, 1463–1466. doi: 10.1126/science.aaa7234
76
Li X. P. BjoÈrkman O. Shih C. Grossman A. R. Rosenquist M. Jansson S. et al . (2000). A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature403, 391–395. doi: 10.1038/35000131
77
Li M. Semchonok D. A. Boekema E. J. Bruce B. D. (2014). Characterization and evolution of tetrameric photosystem I from the thermophilic cyanobacterium Chroococcidiopsis sp TS-821. Plant Cell26, 1230–1245. doi: 10.1105/tpc.113.120782
78
Liberton M. Austin J. R. II Berg R. H. Pakrasi H. B. (2011). Insights into the complex 3-D architecture of thylakoid membranes in unicellular cyanobacterium Cyanothece sp. ATCC 51142. Plant Signal. Behav.6, 566–569. doi: 10.4161/psb.6.4.14946
79
Liberton M. Page L. E. O’Dell W. B. O’Neill H. Mamontov E. Urban V. S. et al . (2013). Organization and flexibility of cyanobacterial thylakoid membranes examined by neutron scattering. J. Biol. Chem.288, 3632–3640. doi: 10.1074/jbc.M112.416933
80
Liu L. N. (2016). Distribution and dynamics of electron transport complexes in cyanobacterial thylakoid membranes. Biochim. Biophys. Acta Bioenerg.1857, 256–265. doi: 10.1016/j.bbabio.2015.11.010
81
Liu H. Lu Y. Wolf B. Saer R. King J. D. Blankenship R. E. (2018). Photoactivation and relaxation studies on the cyanobacterial orange carotenoid protein in the presence of copper ion. Photosynth. Res.135, 143–147. doi: 10.1007/s11120-017-0363-1
82
Liu H. Zhang H. Niedzwiedzki D. M. Prado M. He G. Gross M. L. et al . (2013). Phycobilisomes supply excitations to both photosystems in a megacomplex in cyanobacteria. Science342, 1104–1107. doi: 10.1126/science.1242321
83
Liu H. Zhang H. Orf G. S. Lu Y. Jiang J. King J. D. et al . (2016). Dramatic domain rearrangements of the cyanobacterial orange carotenoid protein upon photoactivation. Biochemistry55, 1003–1009. doi: 10.1021/acs.biochem.6b00013
84
Loll B. Kern J. Saenger W. Zouni A. Biesiadka J. (2007). Lipids in photosystem II: interactions with protein and cofactors. Biochim. Biophys. Acta Bioenerg.1767, 509–519. doi: 10.1016/j.bbabio.2006.12.009
85
Long S. P. Humphries S. Falkowski P. G. (1994). Photoinhibition of photosynthesis in nature. Annu. Rev. Plant Physiol. Plant Mol. Biol.45, 633–662. doi: 10.1146/annurev.pp.45.060194.003221
86
Lou W. Wolf B. M. Blankenship R. E. Liu H. (2019). Cu+ contributes to the orange carotenoid protein-related phycobilisome fluorescence quenching and photoprotection in cyanobacteria. Biochemistry58, 3109–3115. doi: 10.1021/acs.biochem.9b00409
87
Luimstra V. M. Schuurmans J. M. Verschoor A. M. Hellingwerf K. J. Huisman J. Matthijs H. C. (2018). Blue light reduces photosynthetic efficiency of cyanobacteria through an imbalance between photosystems I and II. Photosynth. Res.138, 177–189. doi: 10.1007/s11120-018-0561-5
88
MacColl R. (2004). Allophycocyanin and energy transfer. Biochim. Biophys. Acta Bioenerg.1657, 73–81. doi: 10.1016/j.bbabio.2004.04.005
89
MacGregor-Chatwin C. Jackson P. J. Sener M. Chidgey J. W. Hitchcock A. Qian P. et al . (2019). Membrane organization of photosystem I complexes in the most abundant phototroph on earth. Nat. Plants5, 879–889. doi: 10.1038/s41477-019-0475-z
90
Maksimov E. G. Shirshin E. A. Sluchanko N. N. Zlenko D. V. Parshina E. Y. Tsoraev G. V. et al . (2015). The signaling state of orange carotenoid protein. Biophys. J.109, 595–607. doi: 10.1016/j.bpj.2015.06.052
91
Maksimov E. G. Sluchanko N. N. Slonimskiy Y. B. Mironov K. S. Klementiev K. E. Moldenhauer M. et al . (2017a). The unique protein-to-protein carotenoid transfer mechanism. Biophys. J.113, 402–414. doi: 10.1016/j.bpj.2017.06.002
92
Maksimov E. G. Sluchanko N. N. Slonimskiy Y. B. Slutskaya E. A. Stepanov A. V. Argentova-Stevens A. M. et al . (2017b). The photocycle of orange carotenoid protein conceals distinct intermediates and asynchronous changes in the carotenoid and protein components. Sci. Rep.7:15548. doi: 10.1038/s41598-017-15520-4
93
Mancini J. A. Sheehan M. Kodali G. Chow B. Y. Bryant D. A. Dutton P. L. et al . (2018). De novo synthetic biliprotein design, assembly and excitation energy transfer. J. R. Soc. Interface15:20180021. doi: 10.1098/rsif.2018.0021
94
Mareš J. Strunecký O. Bučinská L. Wiedermannová J. (2019). Evolutionary patterns of thylakoid architecture in cyanobacteria. Front. Microbiol.10:277. doi: 10.3389/fmicb.2019.00277
95
Martin W. F. Bryant D. A. Beatty J. T. (2018). A physiological perspective on the origin and evolution of photosynthesis. FEMS Microbiol. Rev.42, 205–231. doi: 10.1093/femsre/fux056
96
Martins M. C. Romão C. V. Folgosa F. Borges P. T. Frazão C. Teixeira M. (2019). How superoxide reductases and flavodiiron proteins combat oxidative stress in anaerobes. Free Radic. Biol. Med.140, 36–60. doi: 10.1016/j.freeradbiomed.2019.01.051
97
Maurya P. K. Mondal S. Kumar V. Singh S. P. (2023). Green and blue light-dependent morphogenesis, decoupling of phycobilisomes and higher accumulation of reactive oxygen species and lipid contents in Synechococcus elongatus PCC 7942. Environ. Exp. Bot.205:105105. doi: 10.1016/j.envexpbot.2022.105105
98
McDonald A. E. Ivanov A. G. Bode R. Maxwell D. P. Rodermel S. R. Hüner N. P. (2011). Flexibility in photosynthetic electron transport: the physiological role of plastoquinol terminal oxidase (PTOX). Biochim. Biophys. Acta Bioenerg.1807, 954–967. doi: 10.1016/j.bbabio.2010.10.024
99
Mehler A. H. (1951). Studies on reactions of illuminated chloroplasts: I. Mechanism of the reduction of oxygen and other hill reagents. Arch. Biochem. Biophys.33, 65–77. doi: 10.1016/0003-9861(51)90082-3
100
Mehler A. H. Brown A. H. (1952). Studies on reactions of illuminated chloroplasts. III. Simultaneous photoproduction and consumption of oxygen studied with oxygen isotopes. Arch. Biochem. Biophys.38, 365–370. doi: 10.1016/0003-9861(52)90042-8
101
Middleton E. M. Teramura A. H. (1993). The role of flavonol glycosides and carotenoids in protecting soybean from ultraviolet-B damage. Plant Physiol.103, 741–752. doi: 10.1104/pp.103.3.741
102
Mimuro M. Tomo T. Tsuchiya T. (2008). Two unique cyanobacteria lead to a traceable approach of the first appearance of oxygenic photosynthesis. Photosynth. Res.97, 167–176. doi: 10.1007/s11120-008-9311-4
103
Mondal S. Pandey D. Singh S. P. (2024). Chromatic acclimation in cyanobacteria renders robust photosynthesis and fitness in dynamic light environment: recent advances and future perspectives. Physiol. Plant.176:e14536. doi: 10.1111/ppl.14536
104
Montgomery B. L. (2017). Seeing new light: recent insights into the occurrence and regulation of chromatic acclimation in cyanobacteria. Curr. Opin. Plant Biol.37, 18–23. doi: 10.1016/j.pbi.2017.03.009
105
Mullineaux C. W. (2014). Co-existence of photosynthetic and respiratory activities in cyanobacterial thylakoid membranes. Biochim. Biophys. Acta Bioenerg.1837, 503–511. doi: 10.1016/j.bbabio.2013.11.017
106
Mullineaux C. W. Liu L. N. (2020). Membrane dynamics in phototrophic bacteria. Ann. Rev. Microbiol.74, 633–654. doi: 10.1146/annurev-micro-020518-120134
107
Murata N. (1969). Control of excitation transfer in photosynthesis I. Light-induced change of chlorophyll a fluoresence in Porphyridium cruentum. Biochim. Biophys. Acta Bioenerg.172, 242–251. doi: 10.1016/0005-2728(69)90067-X
108
Nagao R. Kato K. Hamaguchi T. Ueno Y. Tsuboshita N. Shimizu S. et al . (2023). Structure of a monomeric photosystem I core associated with iron-stress-induced—a proteins from Anabaena sp. PCC 7120. Nat. Commun.14:920. doi: 10.1038/s41467-023-36504-1
109
Nagarajan A. Zhou M. Nguyen A. Y. Liberton M. Kedia K. Shi T. et al . (2019). Proteomic insights into phycobilisome degradation, a selective and tightly controlled process in the fast-growing cyanobacterium Synechococcus elongatus UTEX 2973. Biomolecules9:374. doi: 10.3390/biom9080374
110
Napaumpaiporn P. Ogawa T. Sonoike K. Nishiyama Y. (2024). Improved capacity for the repair of photosystem II via reinforcement of the translational and antioxidation systems in Synechocystis sp. PCC 6803. Plant J.117, 1165–1178. doi: 10.1111/tpj.16551
111
Nielsen A. Z. Mellor S. B. Vavitsas K. Wlodarczyk A. J. Gnanasekaran T. de Jesus M. P. R. H. et al . (2016). Extending the biosynthetic repertoires of cyanobacteria and chloroplasts. Plant J.87, 87–102. doi: 10.1111/tpj.13173
112
Nikkanen L. Solymosi D. Jokel M. Allahverdiyeva Y. (2021). Regulatory electron transport pathways of photosynthesis in cyanobacteria and microalgae: recent advances and biotechnological prospects. Physiol. Plant.173, 514–525. doi: 10.1111/ppl.13404
113
Nodop A. Pietsch D. Hocker R. Becker A. Pistorius E. K. Forchhammer K. et al . (2008). Transcript profiling reveals new insights into the acclimation of the mesophilic fresh-water cyanobacterium Synechococcus elongatus PCC 7942 to iron starvation. Plant Physiol.147, 747–763. doi: 10.1104/pp.107.114058
114
Noyma N. P. Silva T. P. Chiarini-Garcia H. Amado A. M. Roland F. Melo R. C. (2015). Potential effects of UV radiation on photosynthetic structures of the bloom-forming cyanobacterium Cylindrospermopsis raciborskii CYRF-01. Front. Microbiol.6:1202. doi: 10.3389/fmicb.2015.01202
115
Ohashi S. Iemura T. Okada N. Itoh S. Furukawa H. Okuda M. et al . (2010). An overview on chlorophylls and quinones in the photosystem I-type reaction centers. Photosynth. Res.104, 305–319. doi: 10.1007/s11120-010-9530-3
116
Pandey P. Pandey D. Gupta A. Gupta R. Tiwari S. Singh S. P. (2025). Cyanobacterial green chemistry: a blue-green approach for a sustainable environment, energy, and chemical production. RSC Sustain.3, 661–675. doi: 10.1039/D4SU00448E
117
Pathak J. Singh P. R. Häder D. -P. Sinha R. P. (2019). UV-induced DNA damage and repair: a cyanobacterial perspective. Plant Gene19:100194. doi: 10.1016/j.plgene.2019.100194
118
Petrouleas V. Crofts A. R. (2005). “The iron-quinone acceptor complex” in Photosystem II: the light-driven water:plastoquinone oxidoreductase. eds. WydrzynskiT. J.SatohK.FreemanJ. A. (Dordrecht, NL: Springer), 177–206.
119
Pfennig T. Kullmann E. Zavřel T. Nakielski A. Ebenhöh O. Červený J. et al . (2024). Shedding light on blue-green photosynthesis: a wavelength-dependent mathematical model of photosynthesis in Synechocystis sp. PCC 6803. PLoS Comput. Biol.20:e1012445. doi: 10.1371/journal.pcbi.1012445
120
Prabha S. Vijay A. K. Mathew D. E. George B. (2025). Light sensitive orange carotenoid proteins (OCPs) in cyanobacterial photoprotection: evolutionary insights, structural–functional dynamics and biotechnological prospects. Arch. Microbiol.207:32. doi: 10.1007/s00203-024-04215-w
121
Rastogi R. P. Sinha R. P. Moh S. H. Lee T. K. Kottuparambil S. Kim Y. J. et al . (2014). Ultraviolet radiation and cyanobacteria. J. Photochem. Photobiol. B141, 154–169. doi: 10.1016/j.jphotobiol.2014.09.020
122
Reinot T. Khmelnitskiy A. Zazubovich V. Toporik H. Mazor Y. Jankowiak R. (2022). Frequency-domain spectroscopic study of the photosystem I supercomplexes, isolated IsiA monomers, and the intact IsiA ring. J. Phys. Chem. B126, 6891–6910. doi: 10.1021/acs.jpcb.2c04829
123
Rijgersberg C. P. Amesz J. (1980). Fluorescence and energy transfer in phycobiliprotein-containing algae at low temperature. Biochim. Biophys. Acta Bioenerg.593, 261–271. doi: 10.1016/0005-2728(80)90064-X
124
Rohnke B. A. Singh S. P. Pattanaik B. Montgomery B. L. (2018). RcaE-dependent regulation of carboxysome structural proteins has a central role in environmental determination of carboxysome morphology and abundance in Fremyella diplosiphon. mSphere3:e00617-17. doi: 10.1128/mSphere.00617-17
125
Romão C. V. Vicente J. B. Borges P. T. Frazão C. Teixeira M. (2016). The dual function of flavodiiron proteins: oxygen and/or nitric oxide reductases. J. Biol. Inorg. Chem.21, 39–52. doi: 10.1007/s00775-015-1329-4
126
Romero E. Van Stokkum I. H. Novoderezhkin V. I. Dekker J. P. Van Grondelle R. (2010). Two different charge separation pathways in photosystem II. Biochemistry49, 4300–4307. doi: 10.1021/bi1003926
127
Rueda E. Gonzalez-Flo E. Mondal S. Forchhammer K. Arias D. M. Ludwig K. et al . (2024). Challenges, progress, and future perspectives for cyanobacterial polyhydroxyalkanoate production. Rev. Environ. Sci. Biotechnol.23, 321–350. doi: 10.1007/s11157-024-09689-0
128
Sánchez-Baracaldo P. Bianchini G. Wilson J. D. Knoll A. H. (2022). Cyanobacteria and biogeochemical cycles through earth history. Trends Microbiol.30, 143–157. doi: 10.1016/j.tim.2021.05.008
129
Santana-Sanchez A. Solymosi D. Mustila H. Bersanini L. Aro E. M. Allahverdiyeva Y. (2019). Flavodiiron proteins 1-to-4 function in versatile combinations in O2 photoreduction in cyanobacteria. eLife8:e45766. doi: 10.7554/eLife.45766
130
Schoffman H. Keren N. (2019). Function of the IsiA pigment–protein complex in vivo. Photosynth. Res.141, 343–353. doi: 10.1007/s11120-019-00638-5
131
Sharon I. Alperovitch A. Rohwer F. Haynes M. Glaser F. Atamna-Ismaeel N. et al . (2009). Photosystem I gene cassettes are present in marine virus genomes. Nature461, 258–262. doi: 10.1038/nature08284
132
Shen J. R. (2015). The structure of photosystem II and the mechanism of water oxidation in photosynthesis. Annu. Rev. Plant Biol.66, 23–48. doi: 10.1146/annurev-arplant-050312-120129
133
Sherman D. M. Sherman L. A. (1983). Effect of iron deficiency and iron restoration on ultrastructure of Anacystis nidulans. J. Bacteriol.156, 393–401. doi: 10.1128/jb.156.1.393-401.1983
134
Shevela D. Eaton-Rye J. J. Shen J. R. (2012). Photosystem II and the unique role of bicarbonate: a historical perspective. Biochim. Biophys. Acta Bioenerg.1817, 1134–1151. doi: 10.1016/j.bbabio.2012.04.003
135
Shevela D. Pishchalnikov R. Y. Eichacker L. A. Govindjee G. (2013). “Oxygenic photosynthesis in cyanobacteria” in Stress biology of cyanobacteria: molecular mechanisms to cellular responses. eds. ShrivastavaA. K.RaiA. N.NeilanB. A. (Boca Raton, FL: CRC press), 3–40.
136
Silkina A. Kultschar B. Llewellyn C. A. (2019). Far-red light acclimation for improved mass cultivation of cyanobacteria. Meta9:170. doi: 10.3390/metabo9080170
137
Singh P. R. Gupta A. Pathak J. Sinha R. P. (2023a). Phylogenetic distribution, structural analysis and interaction of nucleotide excision repair proteins in cyanobacteria. DNA Repair126:103487. doi: 10.1016/j.dnarep.2023.103487
138
Singh S. P. Häder D. -P. Sinha R. P. (2010). Cyanobacteria and ultraviolet radiation (UVR) stress: mitigation strategies. Ageing Res. Rev.9, 79–90. doi: 10.1016/j.arr.2009.05.004
139
Singh V. K. Jha S. Rana P. Mishra S. Kumari N. Singh S. C. et al . (2023b). Resilience and mitigation strategies of cyanobacteria under ultraviolet radiation stress. Int. J. Mol. Sci.24:12381. doi: 10.3390/ijms241512381
140
Singh S. P. Montgomery B. L. (2011). Determining cell shape: adaptive regulation of cyanobacterial cellular differentiation and morphology. Trends Microbiol.19, 278–285. doi: 10.1016/j.tim.2011.03.001
141
Singh S. P. Pathak J. Sinha R. P. (2017). Cyanobacterial factories for the production of green energy and value-added products: an integrated approach for economic viability. Renew. Sust. Energ. Rev.69, 578–595. doi: 10.1016/j.rser.2016.11.110
142
Singh N. K. Sonani R. R. Rastogi R. P. Madamwar D. (2015). The phycobilisomes: an early requisite for efficient photosynthesis in cyanobacteria. EXCLI J.14:268. doi: 10.17179/excli2014-723
143
Six C. Joubin L. Partensky F. Holtzendorff J. Garczarek L. (2007). UV-induced phycobilisome dismantling in the marine picocyanobacterium Synechococcus sp. WH8102. Photosynth. Res.92, 75–86. doi: 10.1007/s11120-007-9170-4
144
Slonimskiy Y. B. Zupnik A. O. Varfolomeeva L. A. Boyko K. M. Maksimov E. G. Sluchanko N. N. (2022). A primordial orange carotenoid protein: structure, photoswitching activity and evolutionary aspects. Int. J. Biol. Macromol.222, 167–180. doi: 10.1016/j.ijbiomac.2022.09.131
145
Stadnichuk I. N. Kusnetsov V. V. (2021). Endosymbiotic origin of chloroplasts in plant cells’ evolution. Russ. J. Plant Physiol.68, 1–16. doi: 10.1134/S1021443721010179
146
Steinbach G. Schubert F. Kaňa R. (2015). Cryo-imaging of photosystems and phycobilisomes in Anabaena sp. PCC 7120 cells. J. Photochem. Photobiol. B Biol.152, 395–399. doi: 10.1016/j.jphotobiol.2015.10.003
147
Stirbet A. (2013). Excitonic connectivity between photosystem II units: what is it, and how to measure it?Photosynth. Res.116, 189–214. doi: 10.1007/s11120-013-9863-9
148
Stirbet A. Lazár D. Papageorgiou G. C. (2019). “Chlorophyll a fluorescence in cyanobacteria: relation to photosynthesis” in Cyanobacteria: from basic science to applications. eds. MishraA. K.TiwariD. N.RaiA. N. (Poole: Elsevier), 79–130.
149
Sui S. F. (2021). Structure of phycobilisomes. Annu. Rev. Biophys.50, 53–72. doi: 10.1146/annurev-biophys-062920-063657
150
Szalontai B. Gombos Z. Csizmadia V. Csatorday K. Lutz M. (1989). Chromophore states in allophycocyanin and phycocyanin. A resonance Raman study. Biochemistry28, 6467–6472. doi: 10.1021/bi00441a046
151
Tamary E. Kiss V. Nevo R. Adam Z. Bernat G. Rexroth S. et al . (2012). Structural and functional alterations of cyanobacterial phycobilisomes induced by high-light stress. Biochim. Biophys. Acta Bioenerg.1817, 319–327. doi: 10.1016/j.bbabio.2011.11.008
152
Terashima I. Oguchi R. Atsuzawa K. Kaneko Y. Kono M. (2025). Excitation spillover from PSII to PSI measured in leaves at 77 K. Plant Cell Physiol.66, 358–373. doi: 10.1093/pcp/pcaf002
153
Thurotte A. de Carbon C. B. Wilson A. Talbot L. Cot S. López-Igual R. et al . (2017). The cyanobacterial fluorescence recovery protein has two distinct activities: orange carotenoid protein amino acids involved in FRP interaction. Biochim. Biophys. Acta Bioenerg.1858, 308–317. doi: 10.1016/j.bbabio.2017.02.003
154
Thurotte A. Lopez-Igual R. Wilson A. Comolet L. Bourcier de Carbon C. Xiao F. et al . (2015). Regulation of orange carotenoid protein activity in cyanobacterial photoprotection. Plant Physiol.169, 737–747. doi: 10.1104/pp.15.00843
155
Tikhonov A. N. (2014). The cytochrome b6f complex at the crossroad of photosynthetic electron transport pathways. Plant Physiol. Biochem.81, 163–183. doi: 10.1016/j.plaphy.2013.12.011
156
Tiwari S. Maurya P. K. Mondal S. Gupta A. Häder D.-P. Singh S. P. (2024). “Monitoring photosynthetic performance in cyanobacteria by a modulated fluorometer” in Methods in cyanobacterial research. eds. SinghS. P.SinhaR. P.HäderD.-P. (Boca Raton FL: CRC press), 110–121.
157
Tomar R. S. Niedzwiedzki D. M. Liu H. (2024). Altered excitation energy transfer between phycobilisome and photosystems in the absence of ApcG, a small linker peptide, in Synechocystis sp. PCC 6803, a cyanobacterium. Biochim. Biophys. Acta Bioenerg.1865:149049. doi: 10.1016/j.bbabio.2024.149049
158
Tomitani A. Knoll A. H. Cavanaugh C. M. Ohno T. (2006). The evolutionary diversification of cyanobacteria: molecular–phylogenetic and paleontological perspectives. Proc. Natl. Acad. Sci. U.S.A.103, 5442–5447. doi: 10.1073/pnas.0600999103
159
Ueno Y. Aikawa S. Niwa K. Abe T. Murakami A. Kondo A. et al . (2017). Variety in excitation energy transfer processes from phycobilisomes to photosystems I and II. Photosynth. Res.133, 235–243. doi: 10.1007/s11120-017-0345-3
160
Umena Y. Kawakami K. Shen J. R. Kamiya N. (2011). Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature473, 55–60. doi: 10.1038/nature09913
161
Wang Q. Hall C. L. Al-Adami M. Z. He Q. (2010). IsiA is required for the formation of photosystem I supercomplexes and for efficient state transition in Synechocystis PCC 6803. PLoS One5:e10432. doi: 10.1371/journal.pone.0010432
162
Wang Y. Mao L. Hu X. (2004). Insight into the structural role of carotenoids in the photosystem I: a quantum chemical analysis. Biophys. J.86, 3097–3111. doi: 10.1016/S0006-3495(04)74358-1
163
Watanabe S. Stazic D. Georg J. Ohtake S. Sakamaki Y. Numakura M. et al . (2023). Regulation of RNase E during the UV stress response in the cyanobacterium Synechocystis sp. PCC 6803. mLife2, 43–57. doi: 10.1002/mlf2.12056
164
Wegener K. M. Nagarajan A. Pakrasi H. B. (2015). An atypical psbA gene encodes a sentinel D1 protein to form a physiologically relevant inactive photosystem II complex in cyanobacteria. J. Biol. Chem.290, 3764–3774. doi: 10.1074/jbc.M114.604124
165
Whatley J. M. John P. Whatley F. R. (1979). From extracellular to intracellular: the establishment of mitochondria and chloroplasts. Proc. R. Soc. Lond. B204, 165–187. doi: 10.1098/rspb.1979.0020
166
Willeford K. O. Gombos Z. Gibbs M. (1989). Evidence for chloroplastic succinate dehydrogenase participating in the chloroplastic respiratory and photosynthetic electron transport chains of Chlamydomonas reinhardtii. Plant Physiol.90, 1084–1087. doi: 10.1104/pp.90.3.1084
167
Wilson A. Muzzopappa F. Kirilovsky D. (2022). Elucidation of the essential amino acids involved in the binding of the cyanobacterial orange carotenoid protein to the phycobilisome. Biochim. Biophys. Acta Bioenerg.1863:148504. doi: 10.1016/j.bbabio.2021.148504
168
Wiltbank L. B. Kehoe D. M. (2019). Diverse light responses of cyanobacteria mediated by phytochrome superfamily photoreceptors. Nat. Rev. Microbiol.17, 37–50. doi: 10.1038/s41579-018-0110-4
169
Xiong J. Bauer C. E. (2002). Complex evolution of photosynthesis. Annu. Rev. Plant Biol.53, 503–521. doi: 10.1146/annurev.arplant.53.100301.135212
170
Xu X. Höppner A. Wiebeler C. Zhao K. H. Schapiro I. Gärtner W. (2020). Structural elements regulating the photochromicity in a cyanobacteriochrome. Proc. Natl. Acad. Sci. U.S.A.117, 2432–2440. doi: 10.1073/pnas.1910208117
171
Zehetmayer P. Kupka M. Scheer H. Zumbusch A. (2004). Energy transfer in monomeric phycoerythrocyanin. Biochim. Biophys. Acta Bioenerg.1608, 35–44. doi: 10.1016/j.bbabio.2003.10.003
172
Zhang Y. Chen M. Church W. B. Lau K. W. Larkum A. W. Jermiin L. S. (2010). The molecular structure of the IsiA–photosystem I supercomplex, modelled from high-resolution, crystal structures of photosystem I and the CP43 protein. Biochim. Biophys. Acta Bioenerg.1797, 457–465. doi: 10.1016/j.bbabio.2010.01.002
173
Zhao L. S. Li C. Y. Chen X. L. Wang Q. Zhang Y. Z. Liu L. N. (2022). Native architecture and acclimation of photosynthetic membranes in a fast-growing cyanobacterium. Plant Physiol.190, 1883–1895. doi: 10.1093/plphys/kiac372
174
Zhou H. Wang J. Zhang Z. Lan C. Q. (2024). High cell density culture of microalgae in horizontal thin-layer algal reactor: modeling of light attenuation and cell growth kinetics. Chem. Eng. J.496:154175. doi: 10.1016/j.cej.2024.154175
175
Zimorski V. Ku C. Martin W. F. Gould S. B. (2014). Endosymbiotic theory for organelle origins. Curr. Opin. Microbiol.22, 38–48. doi: 10.1016/j.mib.2014.09.008
176
Zouni A. Witt H. T. Kern J. Fromme P. Krauss N. Saenger W. et al . (2001). Crystal structure of photosystem II from Synechococcus elongatus at 3.8 Å resolution. Nature409, 739–743. doi: 10.1038/35055589
177
Zuber H. (1986). Structure of light-harvesting antenna complexes of photosynthetic bacteria, cyanobacteria and red algae. Trends Biochem. Sci.11, 414–419. doi: 10.1016/0968-0004(86)90175-1
Summary
Keywords
biomanufacturing, cyanobacteriochrome, non-photochemical quenching, photoprotection, synthetic biology
Citation
Tiwari S, Gupta A, Pandey D, Pandey P, Gupta R and Singh SP (2025) Photoacclimation strategies in cyanobacterial photosynthesis under dynamic light environments: implications in growth, fitness, and biotechnological applications. Front. Microbiol. 16:1686386. doi: 10.3389/fmicb.2025.1686386
Received
15 August 2025
Accepted
01 October 2025
Published
20 October 2025
Volume
16 - 2025
Edited by
Kong-Thon Tsen, Arizona State University, United States
Reviewed by
Amit Srivastava, The Czech Academy of Sciences, Czechia
Lirong Tian, Hebei Normal University, China
Updates
Copyright
© 2025 Tiwari, Gupta, Pandey, Pandey, Gupta and Singh.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shailendra Pratap Singh, spsingh@bhu.ac.in
†ORCID: sapna tiwari, orcid.org/0009-0000-5282-0324
Anjali Gupta, orcid.org/0009-0006-4759-9315
Deepa Pandey, orcid.org/0009-0005-0951-6761
Priyul Pandey, orcid.org/0009-0008-7084-2824
Rinkesh Gupta, orcid.org/0009-0006-9779-3618
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.