- 1School of Medicine, Hunan University of Chinese Medicine, Changsha, Hunan, China
- 2School of Nursing, Hunan University of Chinese Medicine, Changsha, Hunan, China
- 3School of Health, Elderly Care and Nursing, Insurance Professional College, Changsha, Hunan, China
Naegleria fowleri is a rare pathogen responsible for primary amoebic meningoencephalitis (PAM), a fatal central nervous system infection characterized by rapid clinical progression and an extremely high mortality rate. The existing diagnostic methods are insufficiently sensitive, and therapeutic options are minimal, making early recognition and intervention extremely challenging. This review systematically examines the biological characteristics and pathogenic mechanisms of this pathogen, as well as current diagnostic and treatment strategies, with a particular focus on the groundbreaking applications of emerging technologies such as metagenomic next-generation sequencing (mNGS) in the diagnosis of difficult-to-treat infections. The aim is to provide theoretical support and practical guidance for rapid identification, accurate diagnosis, and timely intervention in clinical practice, serving as a reference for the prevention and treatment of N. fowleri infections.
1 Introduction
Primary amoebic meningoencephalitis (PAM) caused by Naegleria fowleri is characterized by acute onset, rapid progression, severe clinical manifestations, and a mortality rate as high as 97% (Güémez and García, 2021), posing a serious threat to human health. In the early stages of infection, N. fowleri primarily causes upper respiratory tract infections, with patients experiencing changes in taste and smell. Subsequently, high fever, nausea, vomiting, and headache, which are signs of meningeal irritation, rapidly develop. Within the next 1 to 2 days, cerebral edema may be induced. Patients often fall into a coma and respiratory failure due to the rapid progression of cerebral edema and increased intracranial pressure, leading to brain herniation. Gharpure et al. (2021) analyzed the case data of 256 patients and found that the median time from symptom onset to death in patients who died from N. fowleri infection was 5 days. Moreover, the early symptoms of N. fowleri infection are extremely similar to those of bacterial meningoencephalitis, which can easily lead to misdiagnosis in clinical practice (Matanock et al., 2018). Given the rapid progression, high mortality rate, and high likelihood of misdiagnosis of PAM, it is particularly important to develop a standardized and rapid diagnostic protocol. Currently, laboratory diagnosis of N. fowleri mainly relies on direct microscopic examination of amoebae in cerebrospinal fluid (CSF), culture, polymerase chain reaction (PCR), and next-generation sequencing (NGS). Direct microscopic examination of CSF is prone to false negatives. PCR can rapidly detect PAM and other types of meningitis, but its appropriate application requires early suspicion of the disease by clinicians. NGS can quickly identify all known infectious diseases with sequenced pathogens, but it is costly. CSF culture is slow and cost-effective, and can be used for postmortem examination to determine the cause of death, but it is not suitable for antemortem diagnosis in infected individuals and may delay treatment; therefore, it is not recommended (Capewell et al., 2015).
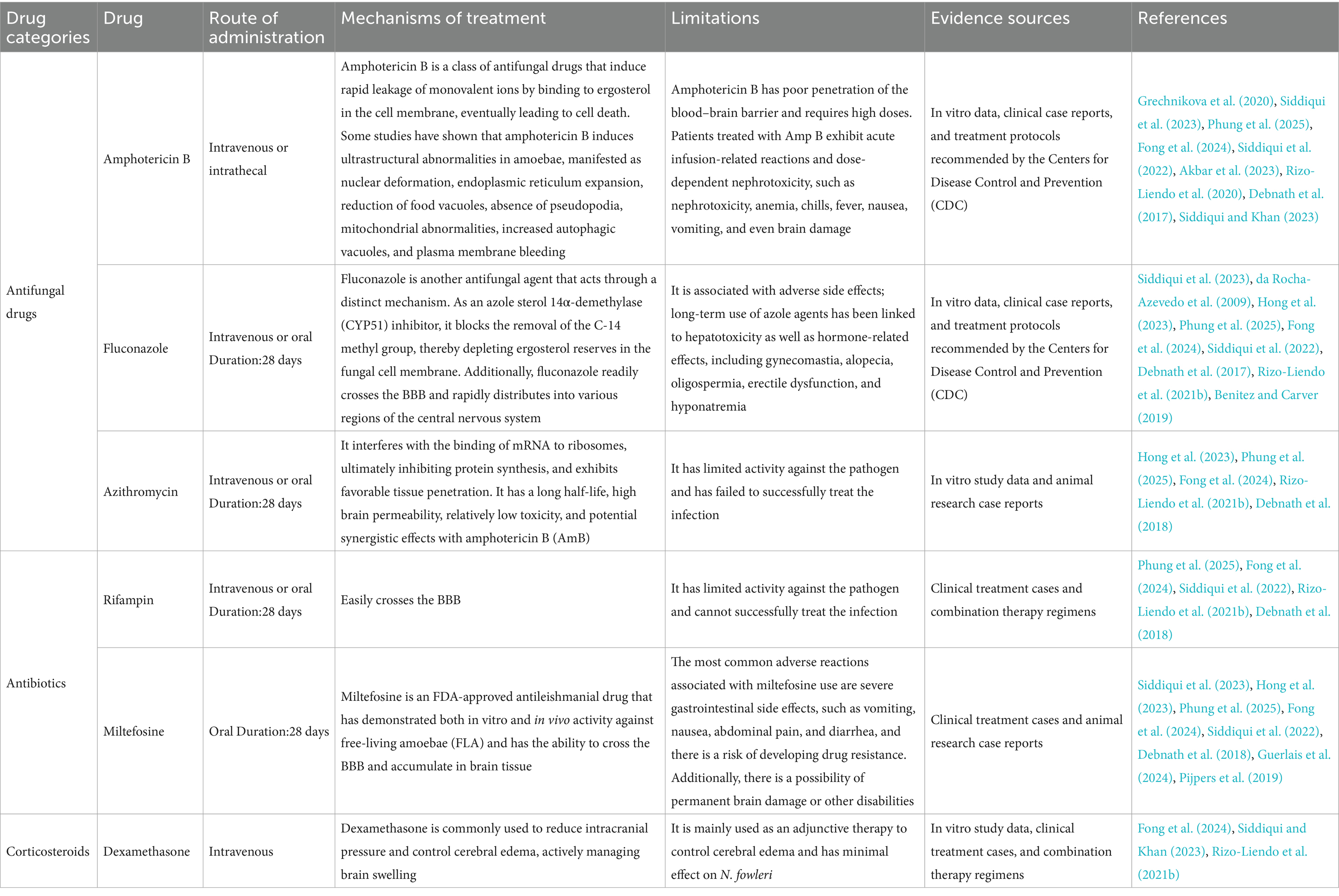
Currently, the clinical treatment of N. fowleri infection lacks specific and effective drugs. Commonly used drugs in clinical practice, such as amphotericin B, miltefosine, fluconazole, and azithromycin, have limited efficacy and are associated with organ toxicity. The concentration of these drugs reaching the brain parenchyma is limited. For recovering patients, higher doses of drugs are required, which can lead to more pronounced side effects (Chang et al., 2024). Therefore, the development of a clinically efficient therapeutic drug and an efficient drug delivery carrier is of utmost importance. In this regard, the development of new targeted drugs, copper metabolism therapy (Grechnikova et al., 2020), and the development of nanomedicines (Siddiqui et al., 2023; Siddiqui et al., 2020) are popular N. fowleri research topics. This article reviews the biological characteristics, infection mechanisms, pathogenic mechanisms, diagnostic methods, and current treatment status of N. fowleri, with the aim of providing references and insights for the prevention and control of N. fowleri.
2 Introduction to N. fowleri
2.1 The life cycle of N. fowleri
Naegleria fowleri is a eukaryotic, free-living, thermophilic microorganism that can live freely in water, soil, or hosts, persisting in warm and humid environments. It typically resides in micro-freshwater habitats and the silt of natural water bodies, feeding primarily on bacteria (Siddiqui et al., 2020). Under different natural environmental conditions, N. fowleri can exhibit three distinct forms: cyst, flagellate trophozoite, and amoeboid trophozoite (Siddiqui et al., 2016). Current scientific research has confirmed that only the amoeboid trophozoite stage of N. fowleri can infect humans and cause PAM. There is no research to date that has proven the other two stages can infect and cause disease in humans. When environmental conditions become adverse, it transforms into a metabolically inactive cyst (Evdokiou et al., 2022). The cyst is highly resistant and can remain dormant at temperatures as low as 4 °C, only commencing reproduction when the temperature increases in the summer (Maciver et al., 2020). However, whether the cysts of N. fowleri can cause human disease remains a matter of debate in the academic community. Maciver et al. (2020) conducted a statistical analysis of 336 cases of N. fowleri infection, and the results showed that 93% of PAM cases (314 cases) were definitively associated with water exposure. However, there were still 22 cases in which the patients were not described as being infected through water contact. Based on this, they speculated that some of these 22 cases might have been caused by the cysts of N. fowleri. Inconsistently, Dorsch et al. (1983) believes that the cases considered to be caused by cysts may have been misjudged due to the failure to fully take into account the presence of N. fowleri in domestic water during the research process. Moreover, Evdokiou et al. (2022) conducted animal experiments. They selected mice aged three to 8 weeks and inoculated the cysts of N. fowleri into their nasal cavities. After culturing for more than 10 days, the mice were euthanized, and their brain tissues were removed for culture; the infection with N. fowleri was negative (Evdokiou et al., 2022). However, the cyst inoculation period in the experiment was relatively short. Some researchers believe that if given enough time to attach to the nasal epithelium, the cysts might be able to complete the infection process (Evdokiou et al., 2022). Whether cysts can infect humans is still unclear. If the cysts of N. fowleri can infect humans, the concentration of airborne N. fowleri cysts that would be required to cause human infection is unknown. Therefore, longer-term animal experiments involving nasal exposure to air containing cysts are required to provide more definitive and convincing evidence for this conclusion.
2.2 The impact of the environment on the survival and distribution of N. fowleri
Naegleria fowleri is a thermophilic microorganism that can survive within a temperature range of 10–46 °C, with an optimal growth temperature of 40 °C (Aurongzeb et al., 2025; Coronado-Velázquez et al., 2018). Berger (2022) found that the concentration of N. fowleri is significantly higher in summer than in winter. With global warming and the increase in human water-based activities, the chances of people coming into contact with N. fowleri have increased (Maciver et al., 2020). Additionally, climate change has also led to an increase in Cyanobacteria and bacteria in water bodies, which are food for N. fowleri, thereby promoting its growth and reproduction (Heilmann et al., 2024). Given the strong survival capacity of N. fowleri in high-temperature environments, against the backdrop of global warming, its range of spread is gradually expanding, which may lead to a northward shift in its distribution areas (Russell et al., 2023). As temperatures increase, regions that were previously relatively cold may gradually reach the suitable survival temperature for N. fowleri, increasing the likelihood of its occurrence in these areas. These studies indicate that N. fowleri exhibits a broad range of temperature adaptability, enabling it to survive in diverse climatic conditions, a characteristic that also makes its spread around the world relatively easy.
Naegleria fowleri grows rapidly in freshwater but has not been found in high-salinity marine environments (Stahl and Olson, 2023; Arberas-Jiménez et al., 2024). This indicates that low salinity is conducive to its growth. N. fowleri can remain viable for at least 96 h at pH values of 4 to 11, whereas it loses activity within 72 h at pH 3 and within 24 h at pH 12 (Berger, 2022). A pH of 6.5 is the optimal pH for the growth of N. fowleri (Stahl and Olson, 2023). Therefore, high concentrations of salt in water, as well as a water pH below 3 or above 11, can all inhibit the survival of N. fowleri and can be used as a means of water disinfection.
2.3 Epidemiological characteristics
The identified global cases are all concentrated in freshwater recreational areas with higher water temperatures (such as the southern United States of America and Pakistan), with over 80% of infections occurring in the summer (Maciver et al., 2020; Sutrave and Richter, 2021). Climate warming may lead to the expansion of the survival space of N. fowleri to temperate regions (Maciver et al., 2020). Among the 11 cases reported in China, 10 were related to outdoor swimming in the hot summer season, indicating that outdoor swimming in summer needs to be a key focus for prevention and control (Chen et al., 2023). Cases of N. fowleri infection were systematically retrieved in various continents from 1937 to 2024 in PubMed and CNKI, and the infection and fatal cases in each continent were summarized (Figure 1).
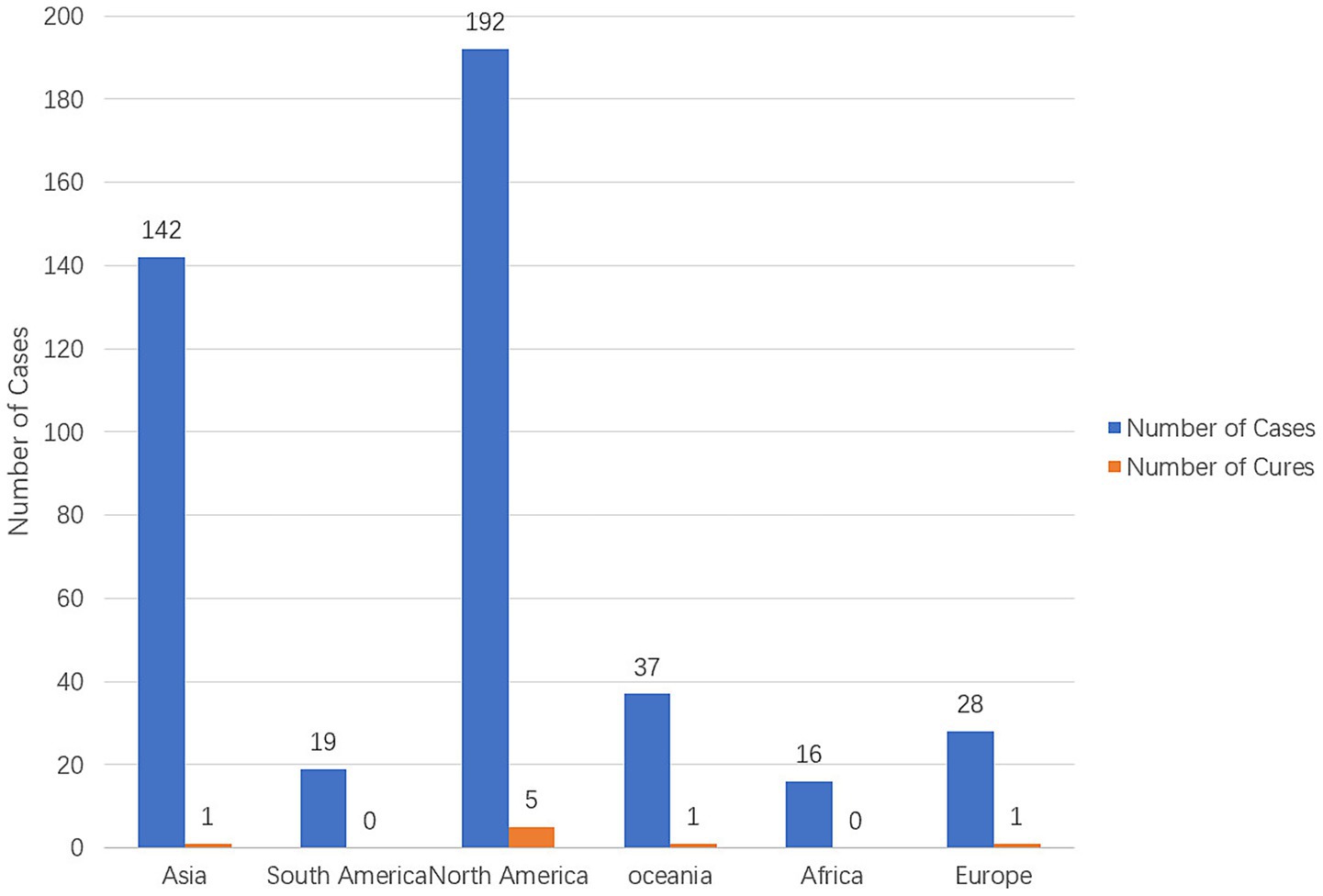
Figure 1. The number of N. fowleri cases and cure status in various continents from 1937 to 2024 (Gharpure et al., 2021; Soontrapa et al., 2022; Nadeem et al., 2023; Hall et al., 2024; Cope et al., 2016).
2.4 Genomic characteristics and extracellular genetic elements
Naegleria fowleri not only possesses a typical nuclear genome, but its mitochondrial genome (mtDNA) and extracellular circular ribosomal DNA (CERE-rDNA) also have unique structures, which may be closely related to its strong environmental adaptability and pathogenic potential. However, despite the accumulation of hundreds of reported infection cases worldwide (Figure 1), as of 2025, the number of complete or high-quality draft genome sequences available in public databases remains extremely limited, with only eight strains, of which only four are considered pathogenic (Aurongzeb et al., 2022). Studies on the clinical isolate AY27 from Pakistan have revealed that its mitochondrial genome is 49,541 bp in length and contains 69 genes (46 protein-coding genes, 21 tRNAs, and 2 rRNAs). Pan-genome analysis indicates that it is of the “open” type (Bpan = 0.137), suggesting that the species has the potential to continuously acquire new genes and adapt to new environments. This capability may be associated with its global dissemination and the maintenance of its pathogenicity (Aurongzeb et al., 2022). In addition, this strain also carries a circular CERE-rDNA with a full length of approximately 15.8 kb. In addition to encoding 18S-5.8S-28S rRNA, it also contains multiple repeat sequences and open reading frames (ORFs). Although its primary function is to encode rRNA, some ORFs can encode hypothetical proteins. Through structural modeling and virtual screening, researchers have identified small-molecule compounds (such as ZINC77564275 and ZINC15022129) that can target and bind to these proteins, suggesting their potential as drug targets (Aurongzeb et al., 2024b).
In the future, with the reduction of sequencing costs and the strengthening of global cooperation, the establishment of an N. fowleri genome database that includes strains from different regions and genotypes will provide a crucial molecular basis for the development of rapid diagnostic methods, the exploration of new therapeutic targets, and the understanding of its global transmission dynamics.
3 Mechanism of infection
3.1 Infection routes of infection
Naegleria fowleri is a widely distributed unicellular protozoan that commonly inhabits natural water bodies, such as hot springs, ponds, and freshwater lakes. It is also widely found in untreated tap water and water-based recreational venues, such as swimming pools, and has even been detected in environments, such as hospitals and irrigation channels (Bonilla-Lemus et al., 2020). In daily life, most cases of N. fowleri infection in adults and children occur after participation in water-based recreational activities. The trophozoites enter the body through the nasal cavity, causing PAM (Coronado-Velázquez et al., 2018; Flores-Suárez et al., 2024; Jarolim et al., 2000; Cabanes et al., 2001).
3.2 The mechanism of N. fowleri entering the central nervous system (CNS)
Many studies have confirmed that humans contract N. fowleri through contact with contaminated water, leading to PAM and fatality in patients (Nadeem et al., 2023). Despite the body’s multiple defense mechanisms, the mechanism of N. fowleri entry into the nervous system is unclear (Figure 2). Trophozoites of N. fowleri adhere to the nasal mucosa, which is the first step in their invasion of the human body. Compared with non-pathogenic amoebae, N. fowleri has a higher level of adhesion. In addition, there is a structure on the surface of its trophozoites called the feeding cup, which enables N. fowleri to ingest bacteria, fungi, and human tissue (Kim et al., 2024; Jahangeer et al., 2020). Cathepsin B, which is distributed in the feeding cup and pseudopodia, plays an important role in the adhesion process. It is a virulence factor with high immunogenicity (Lê et al., 2022). When it is inhibited, the survival rate increases. The next stage is the invasion into the CNS. The N. fowleri trophozoites adhering to the nasal mucosa travel along the olfactory nerve through the cribriform plate to reach the olfactory bulb of the CNS. This stage involves multiple virulence factors. N. fowleri trophozoites have at least three types of matrix metalloproteinases (MMPs), among which MMP-2 and MMP-9 are used to degrade gelatin and type IV collagen. However, these two enzymes exist in proenzyme form and need to be activated by MMP-14 to function. The MMPs secreted by N. fowleri can degrade the extracellular matrix and destroy the structure of the nasal mucosa and cribriform plate (Que and Reed, 2000). In addition, the cysteine proteases secreted by N. fowleri can also cause dislocation and degradation of tight junction proteins between the brain capillary walls of the blood–brain barrier (BBB) and glial cells. This protease can also modify the actin cytoskeleton, thereby altering the stability of the BBB and allowing the amoeba to successfully enter the CNS (Coronado-Velázquez et al., 2018; Lam et al., 2017). Finally, there is the stage where N. fowleri enters the brain parenchyma. The trophozoites of N. fowleri penetrate the cribriform plate, infiltrate the subarachnoid space, and ultimately reach the brain parenchyma, triggering PAM (Marciano-Cabral and Cabral, 2007).
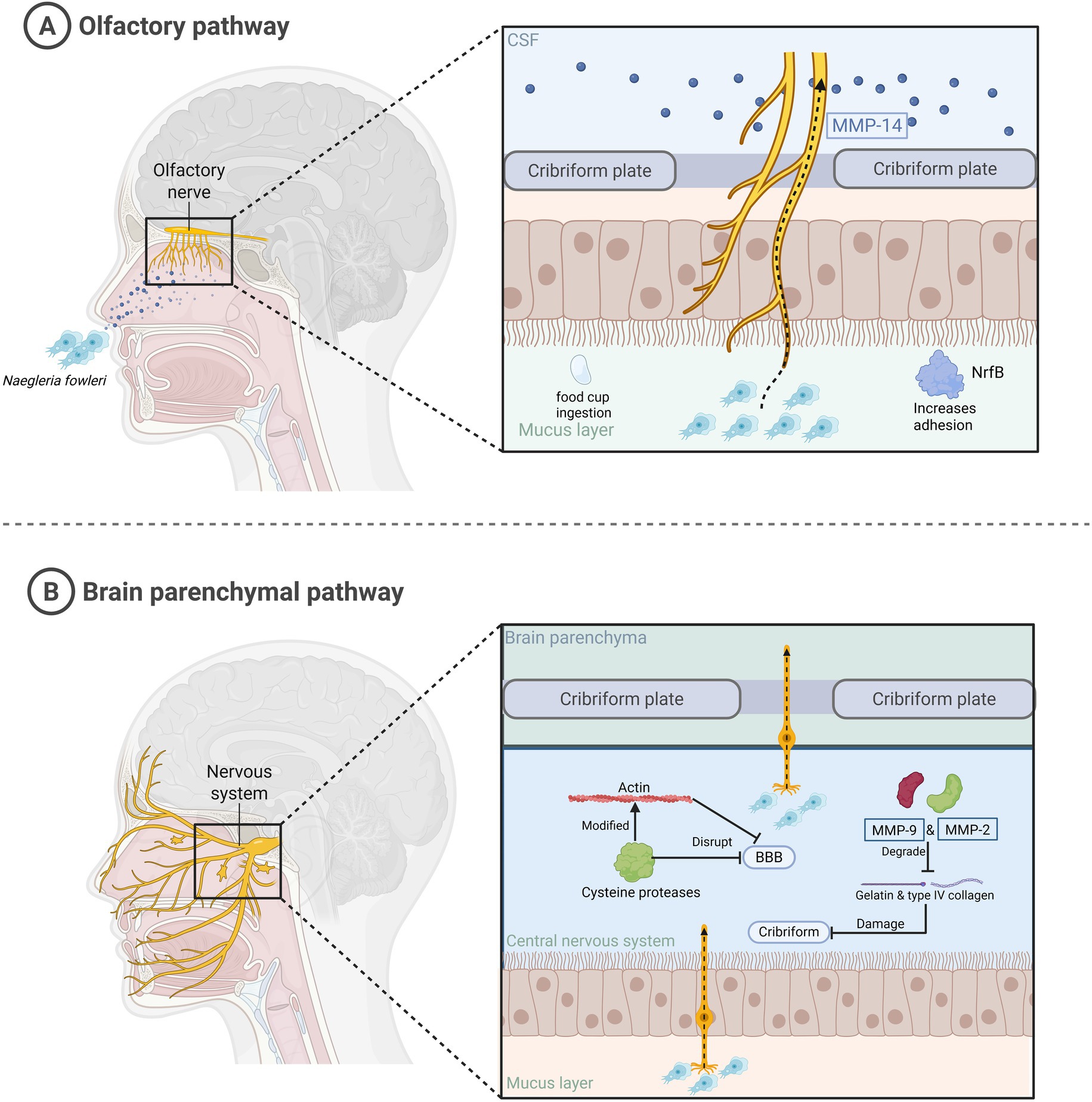
Figure 2. The mechanism by which N. fowleri infects humans and enters the brain parenchyma. This figure summarizes the process of N. fowleri entering the brain parenchyma. (A) N. fowleri enters the central nervous system along the olfactory nerve through the nasal cavity. (B) N. fowleri invades the brain parenchyma.
After summarizing the case data from 1965 to 2018, the majority of infected individuals were male, with a median age of 14 years (Gharpure et al., 2021; Hall et al., 2024). This indicates that patients infected with N. fowleri are generally young. This may be related to the higher permeability of the cribriform plate in children or adolescents, which makes it easier for N. fowleri to invade the CNS, or it may be related to the fact that the immune system of adolescents has not yet fully developed. However, as for the more specific reasons and mechanisms, further experiments and data provided by researchers are needed to confirm them.
3.3 Mechanism of immune evasion
3.3.1 The immune evasion mechanisms of N. fowleri
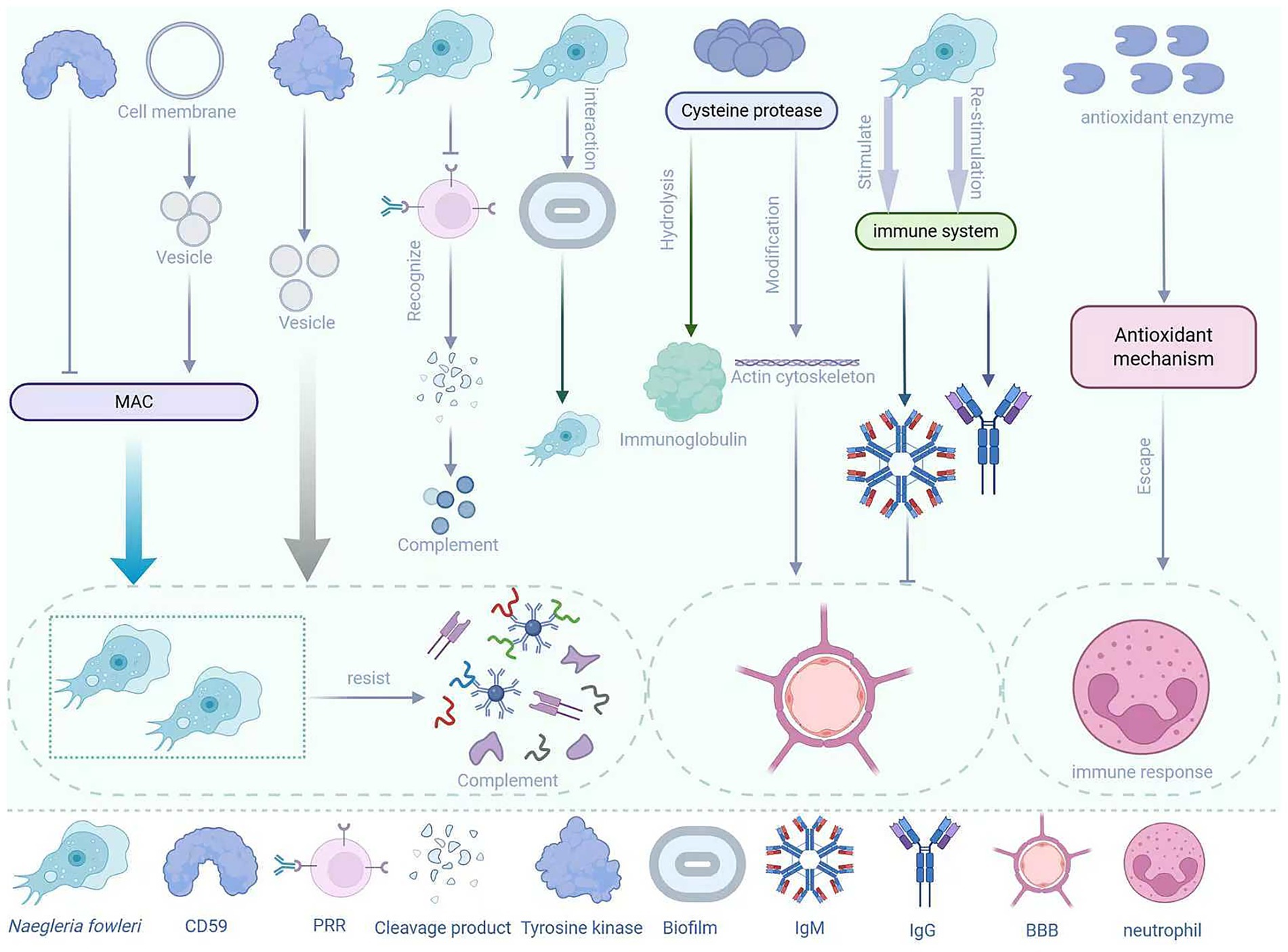
Naegleria fowleri is a pathogen with multiple immune evasion mechanisms, which enable it to survive in the host and cause disease (Figure 3). Under normal circumstances, when the body is first infected with parasites or bacteria and other pathogens, the immune system will rapidly produce IgM antibodies, while IgG antibodies generally only appear during reinfection with the same microorganism (Anam et al., 2024). Therefore, when many people are infected with N. fowleri, the first antibodies to appear are IgM antibodies. However, IgM antibodies have a large molecular weight (approximately 900 kDa) and have difficulty crossing the BBB. This natural barrier limits the immune response of IgM antibodies to the pathogen, allowing N. fowleri to survive and reproduce relatively “safely” in the CNS (Adelman et al., 1980). When the body fails to prevent the parasite from invading through the olfactory route, the innate response within the epithelium is triggered. However, N. fowleri can evade immune detection, penetrate into the lamina propria, and then enter the olfactory nerve bundles (Malych et al., 2025). In addition, Piñero et al. (2019) conducted in vitro studies and found that antioxidant enzymes are overexpressed within the trophozoites of N. fowleri, indicating that its antioxidant mechanisms can be activated under oxidative conditions to help evade immune responses mediated by neutrophils. The pattern recognition receptors of most mammalian immune cells recognize the degradation products of pathogens, which are usually considered chemotactic factors for immune cell recruitment. However, the trophozoites of N. fowleri are resistant to downstream complement-mediated lysis, which is also part of the reason for its immune evasion (Rîpă et al., 2025). Moreover, although the host’s mucin can prevent the trophozoites of N. fowleri from adhering to the host’s mucosa, N. fowleri often releases proteolytic substances to degrade the host’s mucin, thereby escaping capture by mucin (Rîpă et al., 2025). The lethality of N. fowleri infection lies in its ability to rapidly invade the CNS. During this process, the sentinel cells in the respiratory epithelium fail to effectively prevent its invasion and initiate a sufficient immune response (Coronado-Velázquez et al., 2018).
The surface of N. fowleri can express a protein similar to human CD59, which can prevent the insertion and activation of the membrane attack complex (MAC), thereby protecting the amoeba from complement-mediated lysis (Fritzinger and Marciano-Cabral, 2004; Cooke et al., 2025). In addition, N. fowleri can also shed the MAC (C5b-C9) from the cell surface through the process of vesiculation, thereby protecting itself from complement-mediated lysis (Chu et al., 2002). Moreover, upon contact with complement, N. fowleri can activate signaling pathways, such as tyrosine kinase, which are involved in the regulation of vesicle formation and shedding, thereby further enhancing its resistance to complement attacks (Chu et al., 2000). N. fowleri can also secrete a series of cathepsin B family enzymes, among which cysteine proteases can modify the actin cytoskeleton of brain endothelial cells (Coronado-Velázquez et al., 2018). This process alters the stability of the BBB, allowing N. fowleri to enter the CNS. Among them, the 37 KDa cysteine protease with mucinolytic activity is believed to be associated with the degradation of mucin and evasion of the host immune system (Jahangeer et al., 2020). In addition, cysteine proteases can partially hydrolyze IgA, IgG, and IgM, which also indicates that these enzymes have potential roles in host immune evasion (Rodríguez-Mera et al., 2022; Lee et al., 2014).
3.3.2 The protective role of the biofilm
Biofilms are complex microbial communities formed by bacteria, fungi, and other microorganisms, which adhere to various surfaces through the secretion of extracellular polymeric substances (EPS) (Flemming et al., 2025). N. fowleri itself does not form biofilms, but it can interact with biofilms in the environment. First, the bacteria in the biofilm can provide a rich food source for N. fowleri, promoting its growth and reproduction (Flemming et al., 2025). Second, EPS, composed of polysaccharides, proteins, extracellular DNA, and lipids, can promote interactions between biofilms and other cells, as well as the adsorption of organic matter, metals, and chemical pollutants. They also promote cell adhesion at the interface and ensure matrix cohesion (Flemming et al., 2025). The biofilm can provide certain protection for N. fowleri, enhancing its stress resistance and also facilitating its adhesion during the host invasion process. Moreover, Semenzato et al. (2025) pointed out that some bacteria in the biofilm may interact with amoebae, enhancing their pathogenicity. For example, certain bacteria can survive within amoebae after being phagocytized, which may make the amoebae more invasive (Flemming and Wuertz, 2019; Semenzato et al., 2025). Therefore, biofilms are conducive to the survival and dissemination of N. fowleri, which significantly increases the likelihood of its infection in humans.
4 Pathogenic mechanisms
4.1 Virulence factors of N. fowleri
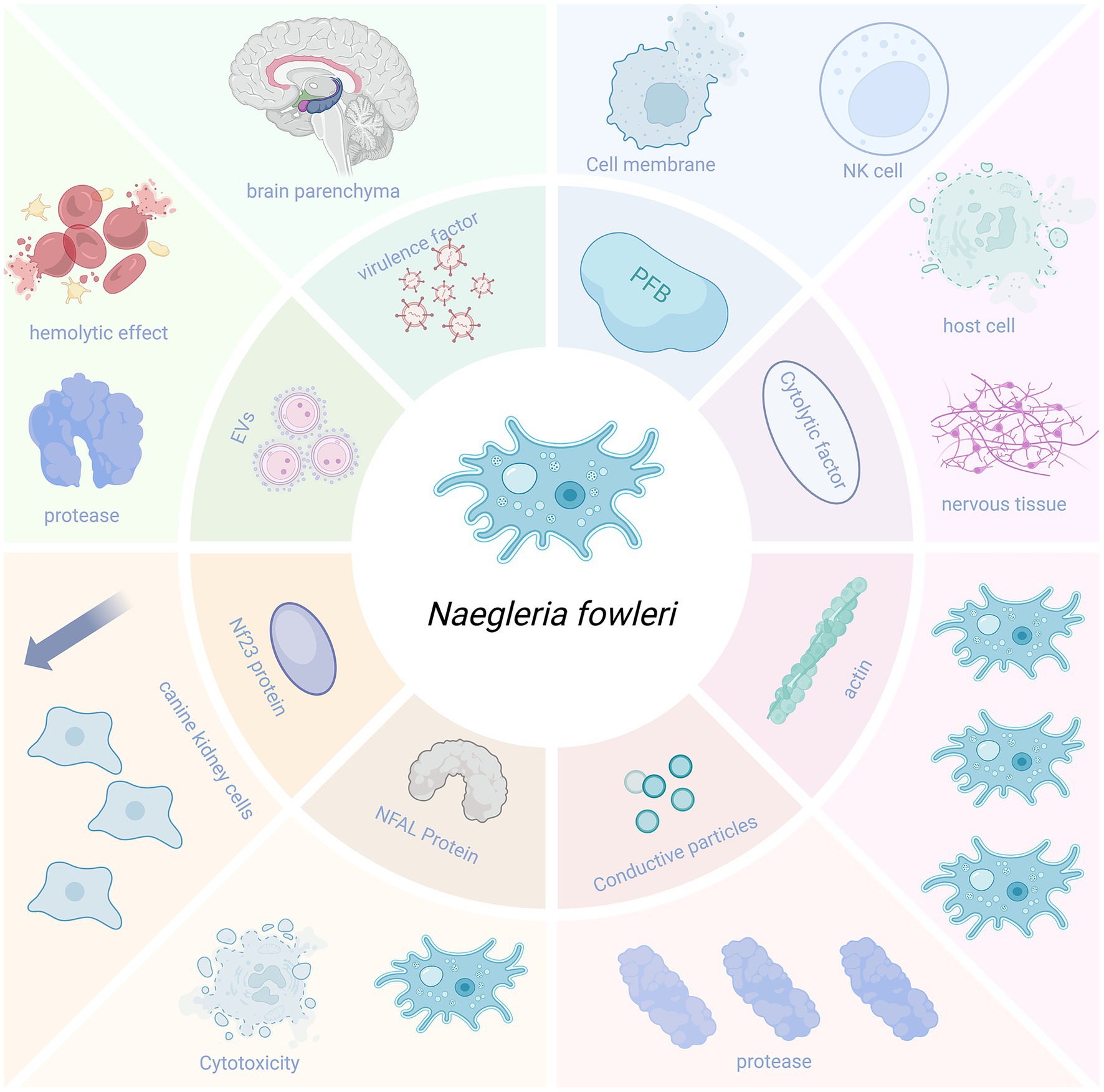
Naegleria fowleri can secrete a variety of virulence factors that enhance its pathogenicity, facilitating its entry into the brain parenchyma and causing damage to it (Figure 4).
4.1.1 Pore-forming proteins
Pore-forming proteins are membrane complex proteins that can lyse nucleated cells and affect membrane integrity by depolarizing the host cell membrane. N. fowleri contains two pore-forming peptides, which have structural similarities to human cytotoxic natural killer (NK) cells and possess strong pore-forming activity, capable of killing both prokaryotic and eukaryotic cells (Young and Lowrey, 1989).
4.1.2 Nfa1 protein
The Nfa1 protein (13.1 kDa) encoded by the Nfa1 gene found in the pseudopodia of N. fowleri is closely related to the amoeba’s formation, adhesion, phagocytosis, and cytotoxicity (Cho et al., 2003). The use of anti-Nfa1 antibodies can reduce the cytotoxicity of N. fowleri (Jeong et al., 2004; Shin et al., 2001).
4.1.3 Cytolytic molecules
The pathogenicity of N. fowleri also relies on the release of cytolytic molecules, including acid hydrolases, phospholipases, neuraminidases, and phosphatases. These enzymes can destroy host cells and neural tissues, causing brain tissue damage. Moreover, they may be associated with the demyelination observed in the white matter of patients with PAM (Coronado-Velázquez et al., 2018).
4.1.4 Conductance granules
Naegleria fowleri contains conductance granules (a cytoplasmic component) with proteolytic activity and lytic function, which may be related to the amoeba’s high levels of collagenase and gelatinase (Coronado-Velázquez et al., 2018).
4.1.5 NF-profilin
NF-profilin is a small actin-binding protein that regulates Nf-actin polymerization and cell motility. NF-profilin is highly expressed in the cell membrane, while Nf-actin is more abundant in pseudopodia, cytoplasm, and food cup structures. This suggests that NF-profilin does not directly participate in the adhesion and phagocytosis of N. fowleri but indirectly regulates Nf-actin (Sohn et al., 2025).
4.1.6 Nf23 protein
Flores-Huerta et al. (2020) detected a 23-kDa protein (Nf23) in N. fowleri. The expression of Nf23 in N. fowleri is higher than that in non-pathogenic microorganisms. Compared with the non-pathogenic species N. lovaniensis and N. gruberi, the mRNA levels of Nf23 in N. fowleri are overexpressed by 4 and 40,000 times, respectively. Flores-Huerta et al. also set up a control experiment, adding anti-Nf23 antibodies and propidium iodide (PI) serum to Madin–Darby canine kidney cells co-incubated with N. fowleri. They found that the cytopathic effect was reduced in the group with anti-Nf23 antibodies, indicating that Nf23 may be associated with the virulence of N. fowleri (Flores-Huerta et al., 2020). This suggests that Nf23 may be a potential virulence factor, and its antibodies can partially neutralize the amoeba’s cytotoxicity in vitro. However, whether it can be developed into a vaccine or therapeutic agent for N. fowleri still needs further validation through in vivo immunoprotection and challenge experiments.
4.1.7 Extracellular vesicles (EV)
The EVs of N. fowleri play an important role in contact-independent pathogenic mechanisms. They exhibit hemolytic activity in red blood cells and can also cause proteolysis, with the proteolytic activity mainly attributed to serine proteases. Additionally, the EVs of N. fowleri can enhance paracellular ion permeability and cause damage to MDCK cells (Castelan-Ramirez et al., 2025).
4.2 Brain tissue damage induced by the host’s immune response
After N. fowleri invades the CNS, it rapidly activates the host’s immune response centered on reactive oxygen species (ROS) burst, NLRP3 inflammasome assembly, and multiple MAPK/NF-κB signaling axes. The excessive recruitment of immune cells, such as microglia, astrocytes, neutrophils, and eosinophils, leads to a cytokine storm, ultimately resulting in the disruption of the BBB, necroptosis, and irreversible brain parenchymal damage (Figure 5).
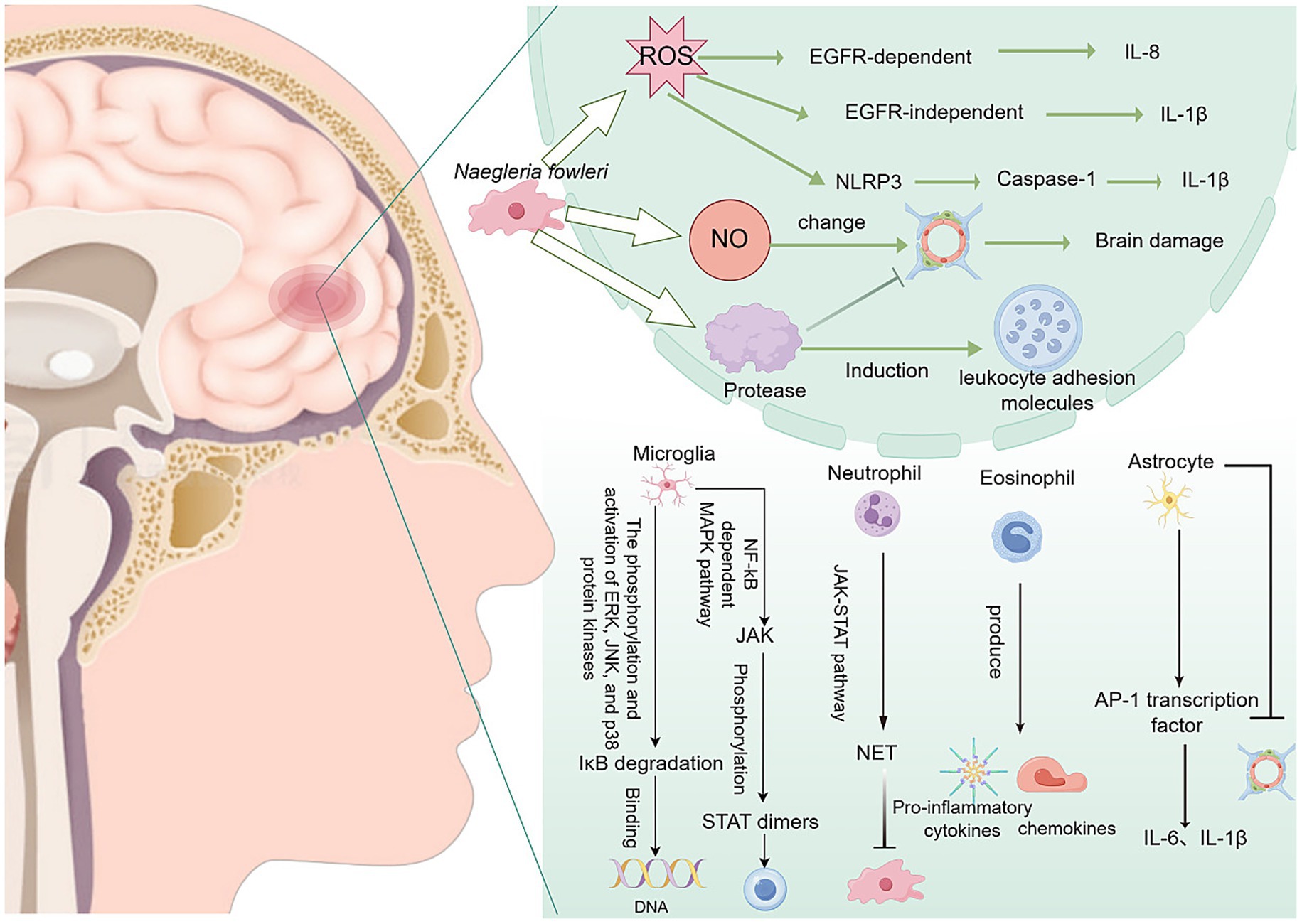
Figure 5. Inflammation and brain damage caused by N. fowleri after entering the central nervous system. NET, neutrophil extracellular trap.
4.2.1 Induction of ROS-dependent necrosis
The trophozoites of N. fowleri can induce the production of ROS. ROS can activate the epidermal growth factor receptor (EGFR), thereby promoting the production of interleukin-8 (IL-8). Additionally, ROS can induce the expression of the pro-inflammatory cytokine interleukin-1β (IL-1β) through an EGFR-independent mechanism (Jeong et al., 2005). In addition, ROS can also stimulate the formation of the NLRP3 inflammasome, which is a multiprotein complex that can activate caspase-1 and secrete active IL-1β (Kim et al., 2016). Furthermore, N. fowleri can activate ROS-dependent necrosis, namely necroptosis, in cells. Necroptosis may be a defense mechanism of the body against N. fowleri, but cells are also destroyed, causing irreversible damage to the body itself (Herbst et al., 2002).
4.2.2 Immune cells and inflammatory factors mediate brain tissue injury
After N. fowleri enters the CNS, it induces a strong inflammatory response in the body, leading to bleeding and osteolytic necrosis (Jeong et al., 2005). N. fowleri triggers a strong inflammatory response through multiple pathways and activates various immune cells, thereby releasing many inflammatory mediators, disrupting the BBB, and causing severe brain tissue damage. This is one of the important pathogenic mechanisms of primary amoebic meningoencephalitis.
4.2.2.1 Microglia-mediated brain tissue injury
Microglia are macrophage-like cells that serve as the vanguard of the CNS immune defense. They are primarily located in the brain and other CNS regions, and their main responsibility is to clear pathogens and damaged neuronal cells (Kaur et al., 2025). When microglia are activated by damage, they can perform antigen presentation and pro-inflammatory functions, thereby initiating an immune response (Kaur et al., 2025). The EVs of N. fowleri can induce proinflammatory immune responses in BV-2 microglial cells. This response not only activates the microglial cells themselves but also triggers inflammatory cascades in other brain cells, further infecting host cells (Sarink et al., 2022). In this process, the expression of Toll-like receptor (TLR)-2, TLR-4, and MyD88 is significantly increased. TLRs, as pattern recognition receptors, are important determinants of inflammatory responses and specific downstream intracellular signaling cascades (Sarink et al., 2022). Recombinant N. fowleri cathepsin B (rNfCB) promotes the production of pro-inflammatory cytokines, such as tumor necrosis factor α (TNF-α), IL-1α, IL-1β, and IL-6 through two major signaling pathways: ① NF-κB-dependent MAPK (Lê et al., 2022; Sarink et al., 2022) and ② JAK–STAT (Sarink et al., 2022; Zhang et al., 2013), thereby inducing pro-inflammatory immune responses in BV-2 microglial cells (Lê et al., 2023). These pro-inflammatory responses not only exacerbate the inflammatory damage and tissue injury caused by N. fowleri in the brain but may also trigger a broader immune response. In vitro experiments have shown that microglia can release high doses of cytokines, ROS, and reactive nitrogen species (RNS), which are highly neurotoxic to the CNS and can lead to severe tissue damage (Xin et al., 2020).
4.2.2.2 Astrocyte-mediated brain tissue injury
Astrocytes play a crucial role in the CNS, not only participating in maintaining homeostasis but also regulating the immune system. The lysate of N. fowleri can activate the AP-1 transcription factor, which induces the expression of IL-1β and IL-6 in astrocytes. The release of these pro-inflammatory cytokines not only further disrupts the integrity of the BBB but also induces immune cells from non-neural sites to enter the brain, thereby triggering an excessive inflammatory response (Kim et al., 2013).
After N. fowleri infection, immune cells are recruited to the trophozoites in the olfactory bulb. Eosinophils can produce a variety of pro-inflammatory cytokines and chemokines, such as TNF-α, IL-6, IL-8, and eosinophil chemotactic factors. However, although these responses are somewhat helpful for immune defense, they cannot effectively eliminate N. fowleri. Additionally, they may further increase leukocyte recruitment and enhance the inflammatory response in the later stages of infection (Lê et al., 2023; Chavez-Munguia et al., 2014; Chen and Moseman, 2022).
4.2.2.3 Neutrophil-mediated brain tissue injury
In PAM, the inflammatory response and associated damage of neutrophils are extremely common. The increase in neutrophil numbers is accompanied by the formation of neutrophil extracellular traps (NETs) and secretion of peroxidase (Jahangeer et al., 2020). Notably, the brain damage caused by the inflammation induced by N. fowleri may mainly be due to the host’s excessive immune response, as the degree of damage is relatively mild in brain tissue lacking immune cells (Kim et al., 2016). Neutrophils eliminate pathogens through a variety of mechanisms, including degranulation, proteolytic enzymes, antimicrobial peptides, ROS, and RNS. In the presence of IgA and IgG opsonization and TNF-α, neutrophils can destroy N. fowleri. The massive infiltration of neutrophils into brain tissue produces many immune substances, such as immunoglobulins, tumor necrosis factors, and interleukins, causing a severe inflammatory response (Rodríguez-Mera et al., 2022). In addition, N. fowleri can induce the formation of NETs. NETs are composed of decondensed chromatin and antimicrobial factors, including myeloperoxidase (MPO), an enzyme stored in the azurophilic granules of immature neutrophils. MPO can catalyze the formation of powerful reactive intermediates, such as hypochlorous acid (HOCl), hypobromous acid (HOBr), hypothiocyanous acid (HOSCN), tyrosyl radicals, and RNS, which play an important role in killing pathogens. However, the massive formation of NETs leads to a severe inflammatory response in the body, but this inflammatory response cannot completely eliminate N. fowleri (Carrasco-Yepez et al., 2019). Conversely, it leads to more severe brain tissue damage.
4.2.3 Other factors mediating brain tissue injury
Naegleria fowleri exhibits high tolerance to the toxicity of nitric oxide (NO), which enables it to resist the host’s inflammatory response (Jahangeer et al., 2020). The release of NO not only alters the permeability of the BBB, allowing more white blood cells to enter the CNS and further exacerbate brain tissue damage, but also the nitrite produced by NO metabolism is neurotoxic, causing additional damage to the host (Farias et al., 2025). In addition, N. fowleri may induce the production of NO-like compounds in brain vascular endothelial cells. Through the interaction between cytokine receptors TLRs, especially TLR4, on endothelial cells, it activates endothelial nitric oxide synthase and inducible nitric oxide synthase, thereby further altering the permeability of the BBB (Coronado-Velázquez et al., 2018).
Naegleria fowleri can express a variety of enzymes from the cathepsin B family, including cysteine proteases, which can destroy the structural proteins of host cells, thereby causing tissue damage (Lee et al., 2014). Among them, NfCB (cathepsin B of N. fowleri) plays a key role in inducing pro-inflammatory immune responses in BV-2 microglial cells. This pro-inflammatory response may exacerbate the harmful immune response and tissue damage in brain lesions caused by N. fowleri infection, thereby leading to PAM. Notably, only structurally and functionally intact NfCB can induce an inflammatory response in the body (Lê et al., 2022). In addition, the cysteine proteases released by N. fowleri not only enable it to penetrate the BBB and cause damage but also promote the entry of immune cells into the brain, which is closely related to the extensive production of leukocyte adhesion molecules induced by the trophozoites (Lê et al., 2022).
4.3 Potential role of extracellular genetic elements in pathogenicity
In addition to the aforementioned classical virulence factors, recent studies have revealed that the extracellular genetic elements of N. fowleri, including mtDNA and CERE-rDNA, may play roles in its pathogenicity that have not yet been fully recognized. First, analysis of the mitochondrial genome of the clinical isolate AY27 indicates that it has an “open” pan-genome characteristic (Bpan = 0.137), suggesting that this pathogen has strong genomic plasticity and may enhance its survival and immune evasion capabilities in the host environment by acquiring new genes or regulatory elements (Aurongzeb et al., 2022). This continuous genomic evolutionary capacity may provide the molecular basis for its adaptation to the unique ecological niche of the human central nervous system and the maintenance of high pathogenicity. Second, although the primary function of its CERE-rDNA is to encode rRNA, its non-ribosomal regions contain multiple ORFs that can encode hypothetical proteins. Through structural modeling and virtual screening, researchers have identified small-molecule compounds (such as ZINC77564275 and ZINC15022129) that can target and bind to these proteins, suggesting that these proteins may have important biological functions (Aurongzeb et al., 2024b). Although their specific functions have not yet been experimentally validated, these hypothetical proteins may be involved in key biological processes such as adhesion, invasion, immune modulation, or metabolic adaptation. Future research may reveal their specific roles in the pathogenic mechanism.
Therefore, these extracellular genetic elements may indirectly enhance the pathogenicity of N. fowleri by promoting gene recombination, regulating expression, or encoding proteins with unknown functions. This emerging research direction provides a new molecular perspective for understanding its pathogenic mechanisms and opens up new avenues for developing targeted therapeutic strategies. However, all current hypotheses are based on bioinformatics analysis and urgently need to be confirmed through experimental means such as gene knockout and protein function validation.
5 The diagnostic methods of N. fowleri infection
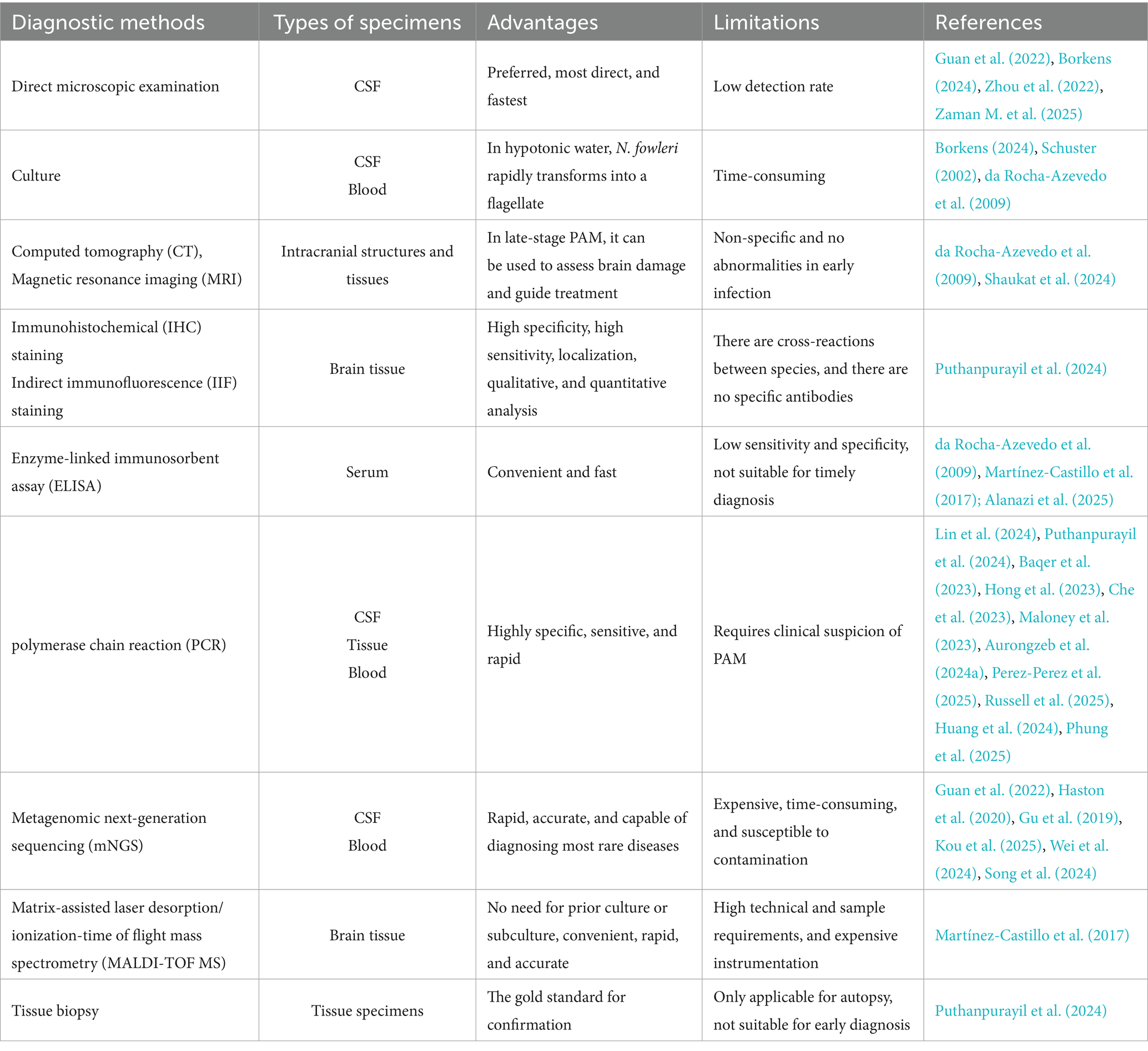
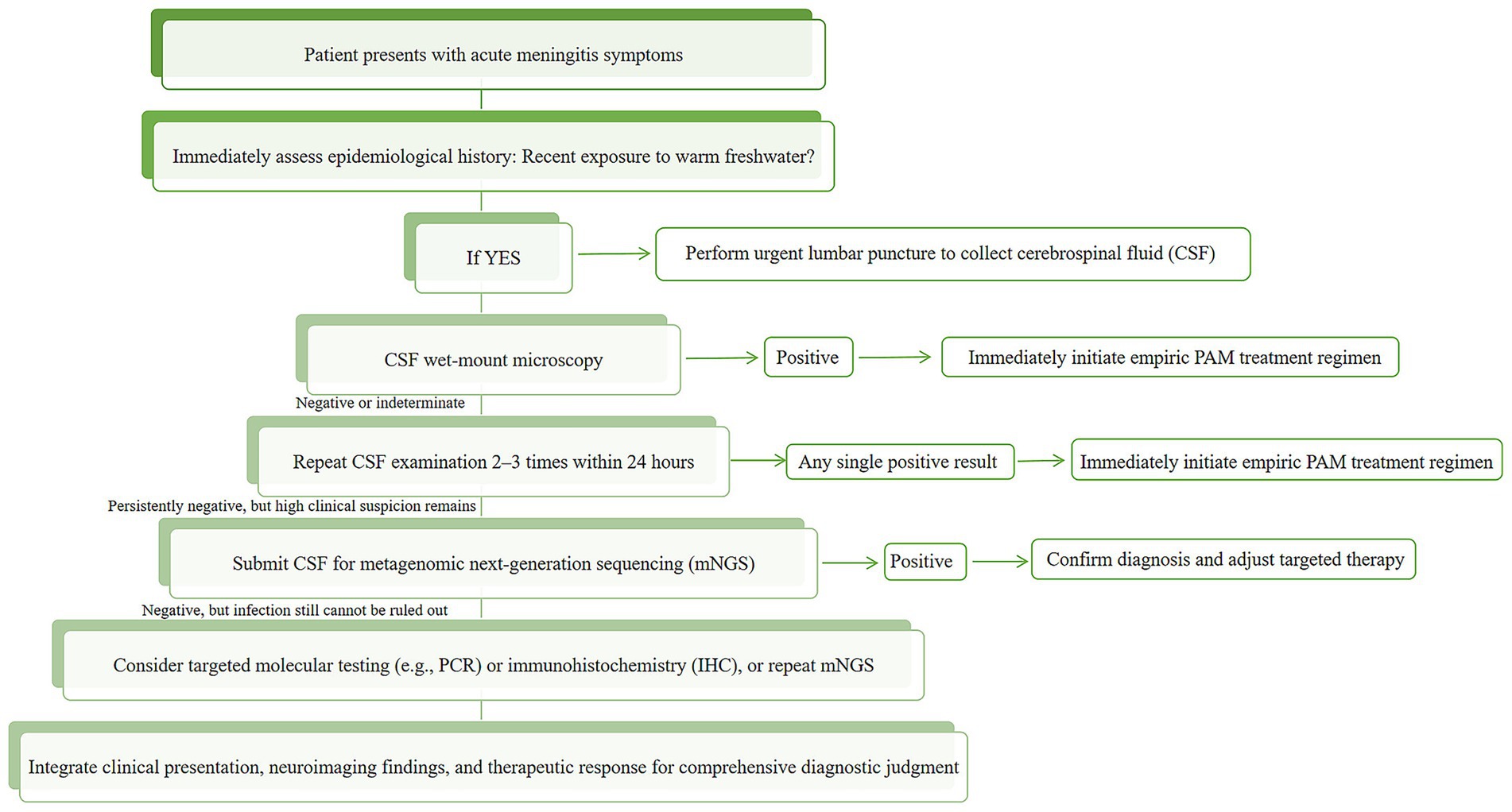
Given the severe clinical challenges posed by N. fowleri infections, rapid and accurate pathogen diagnosis is crucial for saving patients’ lives. Therefore, this review systematically elaborates on the current common diagnostic methods for N. fowleri, analyzes their respective advantages and limitations (Table 1). The analysis reveals that the probability of diagnosing N. fowleri infection using only the current routine clinical diagnostic methods (direct microscopic examination, culture, CT, MRI, etc.) is extremely low. The method with the highest diagnostic yield is mNGS, which can also be used to diagnose other rare infectious diseases, such as Progressive Multifocal Leukoencephalopathy (PML) (Lin et al., 2024). Based on the existing evidence, we have also developed a clinical diagnostic decision pathway for suspected PAM cases (Figure 6). The pathway is designed to guide clinicians to achieve a closed-loop management of “rapid screening-precise confirmation-dynamic evaluation” through a stepwise and progressive diagnostic strategy under the realistic conditions of limited resources and time pressure.
6 The current status of treatment for N. fowleri infection
Currently, the clinical treatment mainly relies on “old drugs with new uses” such as antifungal or antibiotic drugs like amphotericin B and miltefosine. Although these drugs have achieved therapeutic effects in individual cases, their therapeutic window is extremely narrow. On the one hand, due to the physiological barrier function of the BBB, it is difficult for drugs to reach an effective bactericidal concentration in the brain parenchyma. On the other hand, the high-dose drug administration schemes that are often used to compensate for the insufficient concentration in the brain frequently lead to nephrotoxicity, hepatotoxicity, and neurological side effects, which further exacerbate the patient’s condition (Zhou et al., 2018). To break through this therapeutic impasse, researchers need to advance from two dimensions simultaneously: one is to optimize drug delivery systems, and the other is to innovate drug discovery strategies. The two are complementary and both point to the therapeutic goal of “precision, efficiency, and low toxicity.”
6.1 Nanomedicine delivery systems
In terms of drug delivery, nanoparticle-based drug delivery systems have emerged as a highly promising research direction. These systems can actively or passively cross the BBB through various mechanisms to achieve targeted drug enrichment and controlled release in brain tissue, thereby enhancing therapeutic efficacy while significantly reducing systemic toxicity. Currently, the main mechanisms by which nanomedicines penetrate the BBB can be summarized into the following five categories: (i) Adsorptive-Mediated Transcytosis (AMT), where cationic nanoparticles bind to the negatively charged proteoglycans on the surface of BBB endothelial cells through electrostatic interactions, triggering clathrin or caveolin-mediated endocytosis to facilitate transmembrane transport. This mechanism does not require specific ligands and is a versatile delivery strategy due to its operational simplicity (Zheng et al., 2025); (ii) Receptor-Mediated Transcytosis (RMT): The surface of the nanocarrier is modified with a specific ligand that binds to receptors highly expressed on the BBB, triggering receptor-mediated endocytosis and transcellular transport. This is the most mature active targeting strategy currently under research and holds the greatest potential for clinical translation (Liu et al., 2025); (iii) Cell-Penetrating Peptide (CPP)-Mediated Transport: CPPs are a class of short peptides that can transport nanocarriers or drugs across the BBB by directly penetrating the cell membrane or inducing endocytosis. The specific mechanisms are not fully understood and may involve membrane perturbation, endocytosis, or interaction with specific membrane receptors. They have the advantages of high efficiency and low immunogenicity (Nithya and Ramanathan, 2025); (iv) Trojan Horse Approach: Utilizing immune cells such as monocytes and macrophages as “carriers,” nanoparticles are phagocytosed by these cells and then transported to sites of inflammation or infection (e.g., PAM lesions) as the cells migrate, thereby “hitchhiking” past the BBB. This strategy is particularly suitable for brain infections, tumors, or neuroinflammatory diseases and has high lesion targeting specificity (Pavlíčková et al., 2023); (v) Passive targeting in pathological states (Enhanced Permeability and Retention, EPR effect): In pathological conditions such as brain infection, tumor, stroke, or neuroinflammation, the tight junctions of the BBB (e.g., claudin-5, occludin) are disrupted, leading to a temporary increase in permeability. Nanomedicines can take advantage of this “window period” to enter brain tissue through passive diffusion or the enhanced permeability and retention (EPR) effect, achieving local drug accumulation at the lesion site (Aggarwal et al., 2024). All five mechanisms mentioned above can serve as the basis for designing nanocarriers to load anti-amebic drugs (such as amphotericin B, miltefosine, ebselen, etc.), to achieve precise, efficient, and low-toxicity treatment of N. fowleri infections. Future research should focus on the synergistic optimization of these mechanisms, the development of intelligent responsive carriers, and preclinical safety evaluations, in order to accelerate their transition from the laboratory to clinical application.
6.2 Targeted rational drug design
It is worth noting that the anti-amebic drugs currently used in the clinic (such as amphotericin B and miltefosine) are mostly “old drugs with new uses,” and their discovery processes mostly rely on empirical screening, lacking a deep understanding of the biological characteristics of the pathogen. With the development of genomics, structural biology, and computational chemistry, target-based rational drug design is becoming the mainstream approach in new drug development (Sohail et al., 2025). Comparative Genomics and Subtractive Genomics have become powerful tools for systematically mining novel drug and vaccine targets (Radusky et al., 2015). The core idea of this strategy is to use bioinformatics methods to screen for candidate targets in the pathogen’s “core genome” that simultaneously meet multiple criteria, including “essential for pathogen survival,” “no homology with the host” (to avoid cross-reactions), “located on the cell surface or secreted proteins” (easily recognized by the immune system or targeted by drugs), and “having suitable pocket structures” (capable of binding small-molecule drugs) (Basharat et al., 2022; Dorella et al., 2006). This strategy has been successful in research on other drug-resistant pathogens. For example, in the study of drug-resistant Salmonella Typhi, scholars such as Muneeba Afzal analyzed the pan-genome of eight strains and ultimately identified four highly conserved, non-host homologous, and structurally defined enzymes (such as ClpP protease and dihydrofolate synthase folP) as ideal targets. They also identified high-affinity inhibitors from tens of thousands of compounds through virtual screening (Afzal et al., 2023). Similarly, for methicillin-resistant Staphylococcus aureus (MRSA), researchers employed a “subtractive” approach, progressively eliminating non-essential and host-homologous proteins from thousands of candidates, ultimately focusing on key targets such as SecY (a protein transport channel). They then screened a library of traditional Chinese medicine compounds, providing new ideas for treating infections caused by drug-resistant bacteria (Khan et al., 2023). Applying this cutting-edge strategy to the study of N. fowleri holds great potential. By systematically identifying and validating key targets that are absolutely essential for its survival and can be intervened by drugs (such as unique enzymes in the sterol synthesis pathway, cysteine proteases, mitochondrial function proteins, etc.), we can hopefully develop a new generation of therapeutic drugs with higher efficacy, lower toxicity, and less likelihood of developing drug resistance. In the future, the integration of “target-based rational drug design” with “nanosmart delivery systems”—that is, combining “precise targeting” with “precise delivery”—holds significant promise for overcoming infections caused by N. fowleri.
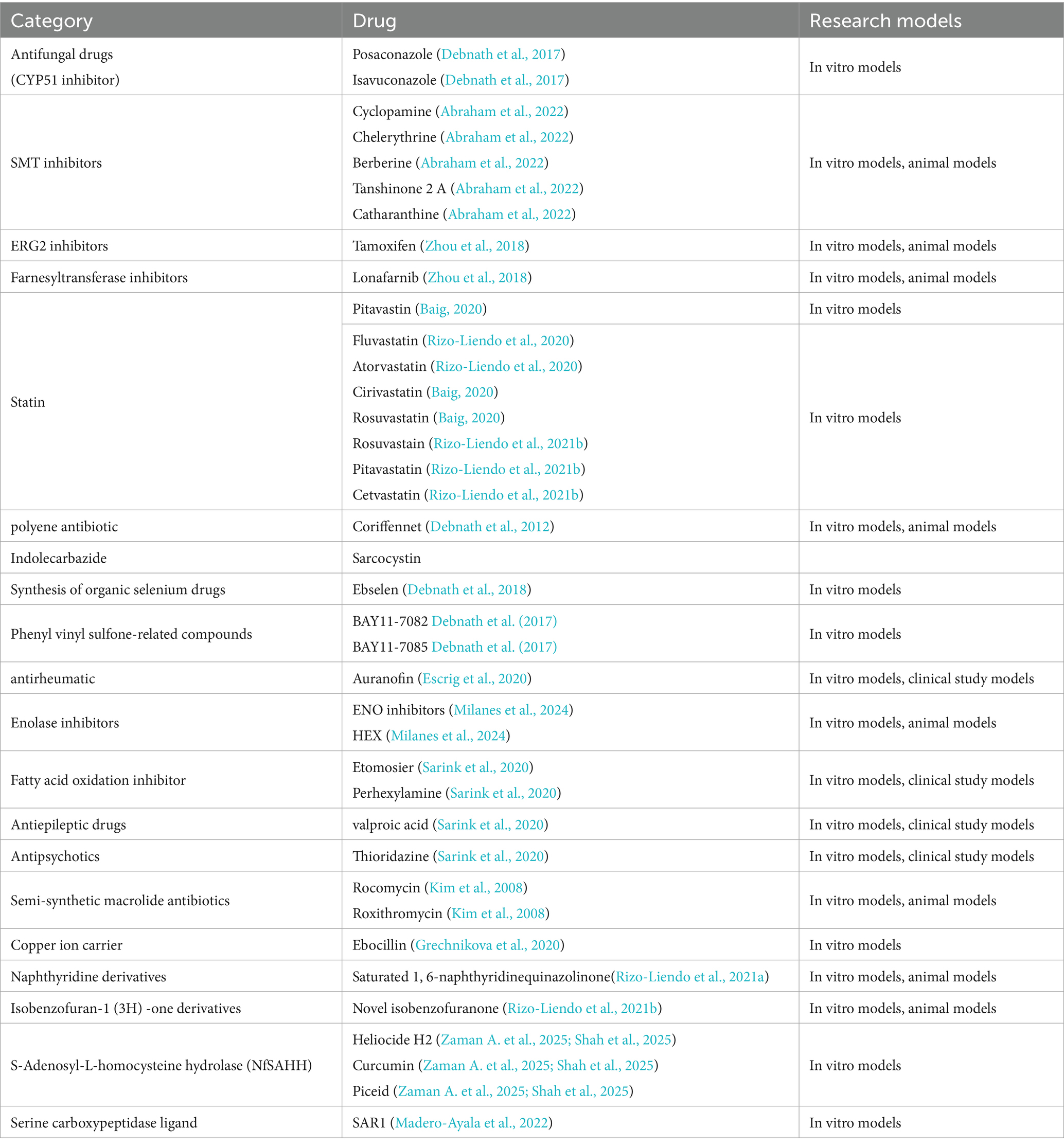
This article systematically reviews the treatment strategies for reported N. fowleri infections and research-level drugs currently under investigation. It analyzes the clinically available drugs (Table 2) and preclinical drugs (Table 3) to provide a scientific reference for clinicians to develop individualized treatment plans.
7 How to prevent infection with N. fowleri
7.1 Water disinfection
Water disinfection is of substantial importance for the elimination of N. fowleri infections. Conventional disinfection methods are divided into physical and chemical disinfection. Presently, the most commonly used method in swimming pools and other recreational places is chemical disinfection, which involves using disinfectants at a certain concentration to achieve the disinfection effect. The use of different types of disinfectants has different inhibitory effects on the activity of N. fowleri (Hu et al., 2025). Hu et al. (2025) compared the disinfection effects of four disinfection methods using chlorine dioxide (ClO₂), free chlorine, chloramine, and ultraviolet (UV) light combined with chlorine agents in drinking water distribution networks. They concluded that using ClO₂ as a disinfectant has the highest inactivation efficiency, with a Ct99% of 1–5 mg·min/L. Using free chlorine as a disinfectant requires a higher concentration to effectively inactivate the trophozoites of N. fowleri, and biofilms can enhance its chlorine resistance. Chloramine has an even lower inactivation efficiency, and the highest number of N. fowleri gene copies was detected in chloraminated distribution networks among the four disinfection methods (Hu et al., 2025). In addition, Sarink et al. (2020) found that in mixed biofilms of Escherichia coli strains cultured under field and laboratory conditions, N. fowleri can survive for 7 days with intermittent dosing of chlorine at 0.6 mg/L. However, when associated with biofilms in drinking water systems, the survival rate of N. fowleri is more than 30 times the recommended chlorine concentration in drinking water (20 mg/L for 3 h). This indicates that compared with biofilms in field and laboratory environments, N. fowleri shows stronger chlorine resistance when forming biofilms in drinking water environments (Sarink et al., 2020). This indicates that different disinfectants have different inactivation rates for N. fowleri, and the differences in biofilms formed under different environmental conditions can also lead to different resistance levels of N. fowleri to the same disinfectant. In large recreational facilities, physical disinfection methods are rarely used alone. Combining UV light with chlorine agents for disinfection can significantly eliminate N. fowleri trophozoites in water, reduce the amount of chlorine agents used, and lower the risk of harm to humans from residual chlorine at high concentrations in the water (Arberas-Jiménez et al., 2022). Additionally, boiling can also eliminate N. fowleri in water.
7.2 Avoiding risky behaviors
In the warmer summer months, when choosing a swimming area, priority should be given to well-maintained, clean, and regularly disinfected sites, such as swimming pools. Avoid swimming or washing your face in stagnant waters or ponds. After swimming, it is recommended to rinse the nasal mucosa and nasal cavity with distilled water or pure bottled water. Additionally, during swimming, try to avoid stirring up sediments in the water, as the bottom sediment layer of water bodies contains a large amount of decayed matter that may harbor N. fowleri. To prevent direct contact of contaminated water with the nasal mucosa, using a nose clip can be a preventive measure (Rizo-Liendo et al., 2021a). When performing certain religious activities that require nasal cleansing, it is recommended to use clean containers and boiled water, or commercially available distilled water or pure water for this activity (Alanazi et al., 2025; Miller et al., 2015).
Given that the diameter of N. fowleri trophozoites is approximately 10–25 μm and that of cysts is around 7–12 μm, their physical size is much larger than that of common bacteria and viruses (Evdokiou et al., 2022). Therefore, in addition to chemical disinfection, intercepting N. fowleri with physical barriers is an effective supplementary means to control its spread in public places, especially artificial water bodies. This can be achieved through the following methods: (i) Filtering systems: Install micron-level filters in the recirculation systems of recreational facilities; (ii) Sediment management: Regularly clean the sediment at the bottom of swimming pools; (iii) Water flow and recirculation filtration: Ensure that the water in all areas is effectively recirculated and filtered; (iv) Water source treatment: For swimming pools relying on natural sources (rivers, lakes, etc.), enhance physical barriers such as sedimentation and filtration.
8 Future perspectives
PAM caused by N. fowleri poses a severe challenge to global public health due to its extremely high mortality rate and rapid disease progression. Although some progress has been made in diagnosis and treatment in recent years, the current prevention and control system still faces many bottlenecks. In terms of diagnosis, although emerging technologies represented by mNGS have greatly improved the ability to identify this rare disease and have become an important tool for screening encephalitis of unknown etiology, their high cost and dependence on specialized equipment severely limit their widespread application in primary medical institutions and resource-poor areas. At the same time, traditional microscopic examination and culture methods are not sensitive enough or take too long to meet the urgent clinical need for early and rapid diagnosis. Future research urgently needs to focus on developing more economical, convenient, and highly sensitive point-of-care rapid diagnostic techniques, such as optimizing matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS), to move it from laboratory research to clinical practice and thus win valuable golden time for patients’ treatment.
In treatment, current reliance on drugs such as amphotericin B and miltefosine is suboptimal, with significant side effects like anemia, fever, nausea, and nephrotoxicity. Nanomedicine delivery systems, though promising for crossing the blood–brain barrier, are still in preclinical stages. Future drug development must adopt a “dual-track” strategy: accelerating nanocarrier safety evaluation and clinical translation for precise drug delivery, and pushing rational drug design based on targets. Using comparative and subtractive genomics, we aim to find unique, essential drug targets in N. fowleri for developing new, effective, low-toxicity, and less-resistant drugs. In pathogenic mechanism research, while we have identified several virulence factors and host immune responses, current in vitro models cannot fully simulate N. fowleri ‘s behavior in vivo, limiting our understanding. For prevention, we should enhance monitoring and intervention in high-risk areas and raise public awareness of N. fowleri infection prevention.
In summary, effectively addressing PAM caused by N. fowleri hinges on breaking through the bottlenecks in the entire chain of “diagnosis-treatment-prevention.” At present, although mNGS has accelerated diagnostic speed, nanodelivery technology has shown potential for crossing the blood–brain barrier, and physical–chemical combined prevention and control strategies are being advanced, each link still faces the core challenges of “difficult translation, high cost, and unclear mechanisms.” The focus of future research should be on “translational medicine” and “precision prevention and control.” On one hand, it is necessary to transform basic research findings (such as the functional interpretation of CERE-rDNA, mtDNA, etc.), the host-pathogen interaction network, and unique metabolic pathways into practical tools such as high-sensitivity point-of-care diagnostic reagents and smart nanomedicines designed based on targets. On the other hand, there is an urgent need to build a closed-loop research system integrating “laboratory-clinical-field,” and accelerate the process from mechanism exploration to clinical validation and then to policy implementation through interdisciplinary collaboration. Only in this way can we improve the survival rate and quality of life of patients with N. fowleri infections and bring more hope and protection to those threatened by this infection.
Author contributions
LD: Supervision, Visualization, Writing – original draft. X-RG: Visualization, Writing – original draft. X-RC: Writing – original draft. M-HM: Writing – original draft. Z-HL: Writing – original draft. JLa: Writing – original draft. JLu: Writing – original draft. MF: Funding acquisition, Writing – review & editing. X-XL: Funding acquisition, Writing – review & editing. S-HY: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Natural Science Foundation of Hunan Province, China (grant no. 2025JJ90043); Scientific Research Fund of Hunan Provincial Department of Education (grant no. 23C0474); Funded Project of "Basic Medicine" (a First-Class Discipline) at Hunan University of Chinese Medicine; the Provincial Discipline Construction Project of Hunan University of Chinese Medicine (Integrated Traditional Chinese and Western Medicine).
Acknowledgments
We want to thank Editage (www.editage.cn) for the English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abraham, J., Chauhan, N., and Ray, S. (2022). Virtual screening of alkaloid and terpenoid inhibitors of SMT expressed in Naegleria sp. Molecules 27:5727. doi: 10.3390/molecules27175727
Adelman, D. C., Miller, R. A., and Kaplan, H. S. (1980). Humoral immune responses to human lymphoma cell heterotransplants in the central nervous system of athymic, nude mice. Int. J. Cancer 25, 467–473. doi: 10.1002/ijc.2910250408
Afzal, M., Hassan, S. S., Sohail, S., Camps, I., Khan, Y., Basharat, Z., et al. (2023). Genomic landscape of the emerging XDR Salmonella Typhi for mining druggable targets clpP, hisH, folP and gpmI and screening of novel TCM inhibitors, molecular docking and simulation analyses. BMC Microbiol. 23:25. doi: 10.1186/s12866-023-02756-6
Aggarwal, N., Singh, G., Panda, H. S., and Panda, J. J. (2024). Unravelling the potential of L-carnosine analog-based nano-assemblies as pH-responsive therapeutics in treating glioma: an in vitro perspective. J. Mater. Chem. B 12, 10665–10681. doi: 10.1039/D4TB01262C
Akbar, N., Hussain, K., Khalid, M., Siddiqui, R., Shah, M. R., and Khan, N. A. (2023). Azole and 5-nitroimidazole based nanoformulations are potential antiamoebic drug candidates against brain-eating amoebae. J. Appl. Microbiol. 134:72. doi: 10.1093/jambio/lxad072
Alanazi, A., Younas, S., Ejaz, H., Alruwaili, M., Alruwaili, Y., Mazhari, B. B. Z., et al. (2025). Advancing the understanding of N. fowleri: global epidemiology, phylogenetic analysis, and strategies to combat a deadly pathogen. J. Infect. Public Health 18:102690. doi: 10.1016/j.jiph.2025.102690
Anam, V., Guerrero, B. V., Srivastav, A. K., Stollenwerk, N., and Aguiar, M. (2024). Within-host models unravelling the dynamics of dengue reinfections. Infect. Dis. Model. 9, 458–473. doi: 10.1016/j.idm.2024.02.004
Arberas-Jiménez, I., Rodríguez-Expósito, R. L., Sifaoui, I., Chao-Pellicer, J., Sancho, L., Urruticoechea, A., et al. (2024). Influence of salt and temperature in the growth of pathogenic free-living amoebae. Front. Microbiol. 15:1356452. doi: 10.3389/fmicb.2024.1356452
Arberas-Jiménez, I., Sifaoui, I., Reyes-Batlle, M., Rizo-Liendo, A., Sancho, L., Urruticoechea, A., et al. (2022). Ultraviolet-chlorine combined treatment efficiency to eliminate N. fowleri in artificial surf lagoons. Heliyon 8:e11625. doi: 10.1016/j.heliyon.2022.e11625
Aurongzeb, M., Fatima, S. Z., Hussain, S. I., Rashid, Y., Aziz, T., Alhomrani, M., et al. (2025). Detection and identification of Naegleria species along with N. fowleri in the tap water samples. BMC Med. Genet. 18:6. doi: 10.1186/s12920-024-02068-2
Aurongzeb, M., Nazir, M. A., Yasmin, R., Kiran, A., Fatima, R., Ali, R., et al. (2024a). Detection and confirmation of N. fowleri in a primary amebic meningoencephalitis patient using a molecular approach. J. Parasitol. Res. 2024:5514520. doi: 10.1155/2024/5514520
Aurongzeb, M., Rashid, Y., Habib Ahmed Naqvi, S., Muhammad Talha Malik, H., Kamran Azim, M., Hassan, S. S., et al. (2022). Insights into genome evolution, pan-genome, and phylogenetic implication through mitochondrial genome sequence of N. fowleri species. Sci. Rep. 12:13152. doi: 10.1038/s41598-022-17006-4
Aurongzeb, M., Talha Malik, H. M., Jahanzaib, M., Hassan, S. S., Rashid, Y., Aziz, T., et al. (2024b). Exploring the extrachromosomal plasmid rDNA of N. fowleri AY27 genotype II: A human brain-eating amoeba via high-throughput sequencing. BMC Med. Genet. 17:125. doi: 10.1186/s12920-024-01890-y
Baig, A. M. (2020). Can neurotropic free-living Amoeba serve as a model to study SARS-CoV-2 pathogenesis? ACS Chem. Neurosci. 11, 3697–3700. doi: 10.1021/acschemneuro.0c00653
Baqer, N. N., Mohammed, A. S., Aa-A, B., and Ismail, A. M. (2023). Genetic detection of amoebic meningoencephalitis causing by N. fowleri in Iraq: a case report. Iran. J. Parasitol. 18, 408–413. doi: 10.18502/ijpa.v18i3.13765
Basharat, Z., Akhtar, U., Khan, K., Alotaibi, G., Jalal, K., Abbas, M. N., et al. (2022). Differential analysis of Orientia tsutsugamushi genomes for therapeutic target identification and possible intervention through natural product inhibitor screening. Comput. Biol. Med. 141:105165. doi: 10.1016/j.compbiomed.2021.105165
Benitez, L. L., and Carver, P. L. (2019). Adverse effects associated with long-term Administration of Azole Antifungal Agents. Drugs 79, 833–853. doi: 10.1007/s40265-019-01127-8
Berger, J. R. (2022). Amebic infections of the central nervous system. J. Neurovirol. 28, 467–472. doi: 10.1007/s13365-022-01096-x
Bonilla-Lemus, P., Rojas-Hernandez, S., Ramirez-Flores, E., Castillo-Ramirez, D. A., Monsalvo-Reyes, A. C., Ramirez-Flores, M. A., et al. (2020). Isolation and identification of Naegleria species in irrigation channels for recreational use in Mexicali Valley, Mexico. Pathogens. 9:820. doi: 10.3390/pathogens9100820
Borkens, Y. (2024). The pathology of the brain eating Amoeba N. fowleri. Indian. J. Microbiol. 64, 1384–1394. doi: 10.1007/s12088-024-01218-5
Cabanes, P. A., Wallet, F., Pringuez, E., and Pernin, P. (2001). Assessing the risk of primary amoebic meningoencephalitis from swimming in the presence of environmental N. fowleri. Appl. Environ. Microbiol. 67, 2927–2931. doi: 10.1128/AEM.67.7.2927-2931.2001
Capewell, L. G., Harris, A. M., Yoder, J. S., Cope, J. R., Eddy, B. A., Roy, S. L., et al. (2015). Diagnosis, clinical course, and treatment of primary amoebic meningoencephalitis in the United States, 1937-2013. J Pediatric Infect Dis Soc. 4, e68–e75. doi: 10.1093/jpids/piu103
Carrasco-Yepez, M. M., Contis-Montes de Oca, A., Campos-Rodriguez, R., Falcon-Acosta, D., Pacheco-Yepez, J., Rodriguez-Mera, I. B., et al. (2019). Mouse neutrophils release extracellular traps in response to N. fowleri. Parasite Immunol. 41:e12610. doi: 10.1111/pim.12610
Castelan-Ramirez, I., Flores-Maldonado, C., Hernandez-Martinez, D., Salazar-Villatoro, L., Saucedo-Campos, A. D., Segura-Cobos, D., et al. (2025). Advances in the study of extracellular vesicles of N. Fowleri and their role in contact-independent pathogenic mechanisms. Parasit. Vectors 18:164. doi: 10.1186/s13071-025-06786-z
Chang, C. C., Harrison, T. S., Bicanic, T. A., Chayakulkeeree, M., Sorrell, T. C., Warris, A., et al. (2024). Global guideline for the diagnosis and management of cryptococcosis: an initiative of the ECMM and ISHAM in cooperation with the ASM. Lancet Infect. Dis. 24, e495–e512. doi: 10.1016/S1473-3099(23)00731-4
Chavez-Munguia, B., Villatoro, L. S., Omana-Molina, M., Rodriguez-Monroy, M. A., Segovia-Gamboa, N., and Martinez-Palomo, A. (2014). N. fowleri: contact-dependent secretion of electrondense granules (EDG). Exp. Parasitol. 142, 1–6. doi: 10.1016/j.exppara.2014.03.027
Che, X., He, Z., Tung, T. H., Xia, H., and Lu, Z. (2023). Diagnosis of primary amoebic meningoencephalitis by metagenomic next-generation sequencing: A case report. Open Life Sci. 18:20220579. doi: 10.1515/biol-2022-0579
Chen, C. W., and Moseman, E. A. (2022). Pro-inflammatory cytokine responses to N. fowleri infection. Front Trop Dis. 3:1082334. doi: 10.3389/fitd.2022.1082334
Chen, X. T., Zhang, Q., Wen, S. Y., Chen, F. F., and Zhou, C. Q. (2023). Pathogenic free-living amoebic encephalitis from 48 cases in China: A systematic review. Front. Neurol. 14:1100785. doi: 10.3389/fneur.2023.1100785
Cho, M. S., Jung, S. Y., Park, S., Kim, K. H., Kim, H. I., Sohn, S., et al. (2003). Immunological characterizations of a cloned 13.1-kilodalton protein from pathogenic N. fowleri. Clin. Diagn. Lab. Immunol. 10, 954–959. doi: 10.1128/cdli.10.5.954-959.2003
Chu, D. M., Ferguson, T. J., and Marciano-Cabral, F. (2000). Protein kinase activation and protein phosphorylation in N. fowleri amebae in response to normal human serum. J. Eukaryot. Microbiol. 47, 40–47. doi: 10.1111/j.1550-7408.2000.tb00009.x
Chu, D. M., Woodward, J., Fritzinger, A., and Marciano-Cabral, F. (2002). Calcium-dependent protection from complement lysis in N. fowleri amebae. Cell Calcium 31, 105–114. doi: 10.1054/ceca.2001.0256
Cooke, R. S., Spicer, B. A., Harrison, R. A., Dunstone, M. A., Morgan, B. P., and Zelek, W. M. (2025). CD59, disulphide-locked human C9 and horse C9 inhibit human membrane attack complex assembly by similar mechanisms. Immunology 176, 363–372. doi: 10.1111/imm.70008
Cope, J. R., Conrad, D. A., Cohen, N., Cotilla, M., DaSilva, A., Jackson, J., et al. (2016). Use of the novel therapeutic agent miltefosine for the treatment of primary Amebic meningoencephalitis: report of 1 fatal and 1 surviving case. Clin. Infect. Dis. 62, 774–776. doi: 10.1093/cid/civ1021
Coronado-Velázquez, D., Betanzos, A., Serrano-Luna, J., and Shibayama, M. (2018). An in vitro model of the blood-brain barrier: N. fowleri affects the tight junction proteins and activates the microvascular endothelial cells. J. Eukaryot. Microbiol. 65, 804–819. doi: 10.1111/jeu.12522
da Rocha-Azevedo, B., Tanowitz, H. B., and Marciano-Cabral, F. (2009). Diagnosis of infections caused by pathogenic free-living amoebae. Interdiscip. Perspect. Infect. Dis. 2009:251406. doi: 10.1155/2009/251406
Debnath, A., Calvet, C. M., Jennings, G., Zhou, W., Aksenov, A., Luth, M. R., et al. (2017). CYP51 is an essential drug target for the treatment of primary amoebic meningoencephalitis (PAM). PLoS Negl. Trop. Dis. 11:e0006104. doi: 10.1371/journal.pntd.0006104
Debnath, A., Nelson, A. T., Silva-Olivares, A., Shibayama, M., Siegel, D., and McKerrow, J. H. (2018). In vitro efficacy of Ebselen and BAY 11-7082 against N. fowleri. Front. Microbiol. 9:414. doi: 10.3389/fmicb.2018.00414
Debnath, A., Tunac, J. B., Galindo-Gomez, S., Silva-Olivares, A., Shibayama, M., and McKerrow, J. H. (2012). Corifungin, a new drug lead against Naegleria, identified from a high-throughput screen. Antimicrob. Agents Chemother. 56, 5450–5457. doi: 10.1128/AAC.00643-12
Dorella, F. A., Pacheco, L. G., Oliveira, S. C., Miyoshi, A., and Azevedo, V. (2006). Corynebacterium pseudotuberculosis: microbiology, biochemical properties, pathogenesis and molecular studies of virulence. Vet. Res. 37, 201–218. doi: 10.1051/vetres:2005056
Dorsch, M. M., Cameron, A. S., and Robinson, B. S. (1983). The epidemiology and control of primary amoebic meningoencephalitis with particular reference to South Australia. Trans. R. Soc. Trop. Med. Hyg. 77, 372–377. doi: 10.1016/0035-9203(83)90167-0
Escrig, J. I., Hahn, H. J., and Debnath, A. (2020). Activity of auranofin against multiple genotypes of N. Fowleri and its synergistic effect with amphotericin B in vitro. ACS Chem. Neurosci. 11, 2464–2471. doi: 10.1021/acschemneuro.0c00165
Evdokiou, A., Marciano-Cabral, F., and Jamerson, M. (2022). Studies on the cyst stage of N. fowleri in vivo and in vitro. J. Eukaryot. Microbiol. 69:e12881. doi: 10.1111/jeu.12881
Farias, H. R., Costa-Beber, L. C., Costa Rodrigues Guma, F. T., and de Oliveira, J. (2025). Hypercholesterolemia, oxidative stress, and low-grade inflammation: a potentially dangerous scenario to blood-brain barrier. Metab. Brain Dis. 40:205. doi: 10.1007/s11011-025-01620-y
Flemming, H. C., van Hullebusch, E. D., Little, B. J., Neu, T. R., Nielsen, P. H., Seviour, T., et al. (2025). Microbial extracellular polymeric substances in the environment, technology and medicine. Nat. Rev. Microbiol. 23, 87–105. doi: 10.1038/s41579-024-01098-y
Flemming, H. C., and Wuertz, S. (2019). Bacteria and archaea on earth and their abundance in biofilms. Nat. Rev. Microbiol. 17, 247–260. doi: 10.1038/s41579-019-0158-9
Flores-Huerta, N., Sanchez-Monroy, V., Rodriguez, M. A., Serrano-Luna, J., and Shibayama, M. (2020). A comparative study of the membrane proteins from Naegleria species: a 23-kDa protein participates in the virulence of N. fowleri. Eur. J. Protistol. 72:125640. doi: 10.1016/j.ejop.2019.125640
Flores-Suárez, B., Bonilla-Lemus, P., Rojas-Hernández, S., Terrazas-Valdés, L. L., and Carrasco-Yépez, M. M. (2024). THE 72-KDA protein of N. fowleri plays an important role in the adhesion of trophozoites to balb/c mice nasal epithelium. J. Parasitol. 110, 360–374. doi: 10.1645/22-86
Fong, H., Leid, Z. H., and Debnath, A. (2024). Approaches for targeting N. fowleri using nanoparticles and artificial peptides. Pathogens 13:695. doi: 10.3390/pathogens13080695
Fritzinger, A. E., and Marciano-Cabral, F. (2004). Modulation of a "CD59-like" protein in N. fowleri amebae by bacteria. J. Eukaryot. Microbiol. 51, 522–528. doi: 10.1111/j.1550-7408.2004.tb00287.x
Gharpure, R., Bliton, J., Goodman, A., Ali, I. K. M., Yoder, J., and Cope, J. R. (2021). Epidemiology and clinical characteristics of primary amebic meningoencephalitis caused by N. fowleri: a global review. Clin. Infect. Dis. 73, e19–e27. doi: 10.1093/cid/ciaa520
Grechnikova, M., Ženíšková, K., Malych, R., Mach, J., and Sutak, R. (2020). Copper detoxification machinery of the brain-eating amoeba N. fowleri involves copper-translocating ATPase and the antioxidant system. Int. J. Parasitol. Drugs Drug Resist. 14, 126–135. doi: 10.1016/j.ijpddr.2020.10.001
Gu, W., Miller, S., and Chiu, C. Y. (2019). Clinical metagenomic next-generation sequencing for pathogen detection. Annu. Rev. Pathol. 14, 319–338. doi: 10.1146/annurev-pathmechdis-012418-012751
Guan, Q., Alhuthali, B., Mfarrej, S., Halim, M. A., Almaghrabi, R. S., and Pain, A. (2022). Metagenomics-driven rapid diagnosis of an imported fatal case of rare amoebic meningoencephalitis. J. Travel Med. 29:172. doi: 10.1093/jtm/taab172
Güémez, A., and García, E.. Primary amoebic meningoencephalitis by N. fowleri: pathogenesis and treatments. Biomolecules. (2021);11:1320. doi: 10.3390/biom11091320
Guerlais, V., Allouch, N., Moseman, E. A., Wojciechowska, A. W., Wojciechowski, J. W., and Marcelino, I. (2024). Transcriptomic profiling of "brain-eating amoeba" N. fowleri infection in mice: the host and the protozoa perspectives. Front. Cell. Infect. Microbiol. 14:1490280. doi: 10.3389/fcimb.2024.1490280
Hall, A. D., Kumar, J. E., Golba, C. E., Luckett, K. M., and Bryant, W. K. (2024). Primary amebic meningoencephalitis: a review of N. Fowleri and analysis of successfully treated cases. Parasitol. Res. 123:84. doi: 10.1007/s00436-023-08094-w
Haston, J. C., Rostad, C. A., Jerris, R. C., Milla, S. S., McCracken, C., Pratt, C., et al. (2020). Prospective cohort study of next-generation sequencing as a diagnostic modality for unexplained encephalitis in children. J. Pediatric. Infect. Dis. Soc. 9, 326–333. doi: 10.1093/jpids/piz032
Heilmann, A., Rueda, Z., Alexander, D., Laupland, K. B., and Keynan, Y. (2024). Impact of climate change on amoeba and the bacteria they host. J. Assoc. Med. Microbiol. Infect. Dis. Can. 9, 1–5. doi: 10.3138/jammi-2023-09-08
Herbst, R., Ott, C., Jacobs, T., Marti, T., Marciano-Cabral, F., and Leippe, M. (2002). Pore-forming polypeptides of the pathogenic protozoon N. fowleri. J. Biol. Chem. 277, 22353–22360. doi: 10.1074/jbc.M201475200
Hong, K. W., Jeong, J. H., Byun, J. H., Hong, S. H., Ju, J. W., and Bae, I. G. (2023). Fatal primary amebic meningoencephalitis due to N. fowleri: the first imported case in Korea. Yonsei Med. J. 64, 641–645. doi: 10.3349/ymj.2023.0189
Hu, Y., Jiang, K., Xia, S., Zhang, W., Guo, J., and Wang, H. (2025). Amoeba community dynamics and assembly mechanisms in full-scale drinking water distribution networks under various disinfectant regimens. Water Res. 271:122861. doi: 10.1016/j.watres.2024.122861
Huang, C. P., Huang, C. L., Yen, M. Y., and Yin, W. H. (2024). A strongly suspected case of concomitant myocarditis in N. fowleri induced primary amoebic meningoencephalitis. Acta Cardiol. Sin. 40, 644–647. doi: 10.6515/ACS.202409_40(5).20240617A
Jahangeer, M., Mahmood, Z., Munir, N., Waraich, U. E., Tahir, I. M., Akram, M., et al. (2020). N. fowleri: sources of infection, pathophysiology, diagnosis, and management; a review. Clin. Exp. Pharmacol. Physiol. 47, 199–212. doi: 10.1111/1440-1681.13192
Jarolim, K. L., McCosh, J. K., Howard, M. J., and John, D. T. (2000). A light microscopy study of the migration of N. fowleri from the nasal submucosa to the central nervous system during the early stage of primary amebic meningoencephalitis in mice. J. Parasitol. 86, 50–55. doi: 10.1645/0022-3395(2000)086[0050:ALMSOT]2.0.CO;2
Jeong, S. R., Kang, S. Y., Lee, S. C., Song, K. J., Im, K. I., and Shin, H. J. (2004). Decreasing effect of an anti-Nfa1 polyclonal antibody on the in vitro cytotoxicity of pathogenic N. fowleri. Korean J. Parasitol. 42, 35–40. doi: 10.3347/kjp.2004.42.1.35
Jeong, S. R., Lee, S. C., Song, K. J., Park, S., Kim, K., Kwon, M. H., et al. (2005). Expression of the nfa1 gene cloned from pathogenic N. fowleri in nonpathogenic N. gruberi enhances cytotoxicity against CHO target cells in vitro. Infect. Immun. 73, 4098–4105. doi: 10.1128/IAI.73.7.4098-4105.2005
Kaur, R., Kumar, S., and Singh, L. (2025). A comprehensive review: neuroinflammation and immune communication between the central nervous system and the periphery. Cytokine 192:156974. doi: 10.1016/j.cyto.2025.156974
Khan, A., Sohail, S., Yaseen, S., Fatima, S., Wisal, A., Ahmed, S., et al. (2023). Exploring and targeting potential druggable antimicrobial resistance targets ArgS, SecY, and MurA in Staphylococcus sciuri with TCM inhibitors through a subtractive genomics strategy. Funct. Integr. Genomics 23:254. doi: 10.1007/s10142-023-01179-w
Kim, J. H., Lee, Y. J., Sohn, H. J., Song, K. J., Kwon, D., Kwon, M. H., et al. (2008). Therapeutic effect of rokitamycin in vitro and on experimental meningoencephalitis due to N. fowleri. Int. J. Antimicrob. Agents 32, 411–417. doi: 10.1016/j.ijantimicag.2008.05.018
Kim, J. H., Sohn, H. J., Shin, H. J., Walz, S. E., and Jung, S. Y. (2024). Understanding the pathogenicity of N. fowleri in association with N. fowleri antigen-1 (Nfa1). Parasites Hosts Dis. 62, 385–398. doi: 10.3347/PHD.24025
Kim, J. H., Sohn, H. J., Yoo, J. K., Kang, H., Seong, G. S., Chwae, Y. J., et al. (2016). Nlrp3 inflammasome activation in THP-1 target cells triggered by pathogenic N. fowleri. Infect. Immun. 84, 2422–2428. doi: 10.1128/IAI.00275-16
Kim, J. H., Song, A. R., Sohn, H. J., Lee, J., Yoo, J. K., Kwon, D., et al. (2013). IL-1β and IL-6 activate inflammatory responses of astrocytes against N. fowleri infection via the modulation of MAPKs and AP-1. Parasite Immunol. 35, 120–128. doi: 10.1111/pim.12021
Kou, Y., Zhang, J., Wang, D., Cui, L., Sun, Q., Lv, Y., et al. (2025). Rare N. fowleri meningoencephalitis diagnosed via combined molecular biology and metagenomic sequencing techniques: a case report. Infect. Dis. Poverty 14:69. doi: 10.1186/s40249-025-01347-z
Lam, C., Jamerson, M., Cabral, G., Carlesso, A. M., and Marciano-Cabral, F. (2017). Expression of matrix metalloproteinases in N. Fowleri and their role in invasion of the central nervous system. Microbiology 163, 1436–1444. doi: 10.1099/mic.0.000537
Lê, H. G., Kang, J. M., Võ, T. C., and Na, B. K. (2022). N. fowleri cathepsin B induces a pro-inflammatory immune response in BV-2 microglial cells via NF-κB and AP-1 dependent-MAPK signaling pathway. Int. J. Mol. Sci. 23:8388. doi: 10.3390/ijms23158388
Lê, H. G., Kang, J. M., Võ, T. C., Yoo, W. G., and Na, B. K. (2023). N. fowleri extracellular vesicles induce proinflammatory immune responses in BV-2 microglial cells. Int. J. Mol. Sci. 24:13623. doi: 10.3390/ijms241713623
Lee, J., Kim, J. H., Sohn, H. J., Yang, H. J., Na, B. K., Chwae, Y. J., et al. (2014). Novel cathepsin B and cathepsin B-like cysteine protease of N. fowleri excretory-secretory proteins and their biochemical properties. Parasitol. Res. 113, 2765–2776. doi: 10.1007/s00436-014-3936-3
Lin, L., Luo, L., Wu, M., Chen, J., Liao, Y., and Zhang, H. (2024). Utilizing metagenomic next-generation sequencing and phylogenetic analysis to identify a rare pediatric case of N. fowleri infection presenting with fulminant myocarditis. Front. Microbiol. 15:1463822. doi: 10.3389/fmicb.2024.1463822
Liu, L., Ma, Z., Jin, L., Chen, M., Han, Q., Mao, Z., et al. (2025). A universal strategy for BBB transport mediated by an inflammatory receptor antagonist for neuroprotection in ischemic stroke. Adv. Mater. :e10035. doi: 10.1002/adma.202510035
Maciver, S. K., Piñero, J. E., and Lorenzo-Morales, J. (2020). Is N. fowleri an emerging parasite? Trends Parasitol. 36, 19–28. doi: 10.1016/j.pt.2019.10.008
Madero-Ayala, P. A., Mares-Alejandre, R. E., and Ramos-Ibarra, M. A. (2022). In silico structural analysis of serine carboxypeptidase Nf314, a potential drug target in N. fowleri infections. Int. J. Mol. Sci. 23:12203. doi: 10.3390/ijms232012203
Maloney, P., Mowrer, C., Jansen, L., Karre, T., Bedrnicek, J., Obaro, S. K., et al. (2023). Fatal primary Amebic meningoencephalitis in Nebraska: case report and environmental investigation, august 2022. Am J Trop Med Hyg. 109, 322–326. doi: 10.4269/ajtmh.23-0211
Malych, R., Folgosa, F., Pilátová, J., Mikeš, L., Dohnálek, V., Mach, J., et al. (2025). Eating the brain-A multidisciplinary study provides new insights into the mechanisms underlying the cytopathogenicity of N. fowleri. PLoS Pathog. 21:e1012995. doi: 10.1371/journal.ppat.1012995
Marciano-Cabral, F., and Cabral, G. A. (2007). The immune response to Naegleria fowleri amebae and pathogenesis of infection. FEMS Immunol Med Microbiol. 51, 243–59. doi: 10.1111/j.1574-695X.2007.00332.x
Martínez-Castillo, M., Cárdenas-Guerra, R. E., Arroyo, R., Debnath, A., Rodríguez, M. A., Sabanero, M., et al. (2017). Nf-GH, a glycosidase secreted by N. fowleri, causes mucin degradation: an in vitro and in vivo study. Future Microbiol. 12, 781–799. doi: 10.2217/fmb-2016-0230
Matanock, A., Mehal, J. M., Liu, L., Blau, D. M., and Cope, J. R. (2018). Estimation of undiagnosed N. fowleri primary amebic meningoencephalitis, United States1. Emerg. Infect. Dis. 24, 162–164. doi: 10.3201/eid2401.170545
Milanes, J. E., Yan, V. C., Pham, C. D., Muller, F., Kwain, S., Rees, K. C., et al. (2024). Enolase inhibitors as therapeutic leads for N. fowleri infection. PLoS Pathog. 20:e1012412. doi: 10.1371/journal.ppat.1012412
Miller, H. C., Wylie, J., Dejean, G., Kaksonen, A. H., Sutton, D., Braun, K., et al. (2015). Reduced efficiency of chlorine disinfection of N. fowleri in a drinking water distribution biofilm. Environ. Sci. Technol. 49, 11125–11131. doi: 10.1021/acs.est.5b02947
Nadeem, A., Malik, I. A., Afridi, E. K., and Shariq, F. (2023). N. fowleri outbreak in Pakistan: unveiling the crisis and path to recovery. Front. Public Health 11:1266400. doi: 10.3389/fpubh.2023.1266400
Nithya, R., and Ramanathan, M. (2025). Advancements in protein-based therapeutic delivery approaches targeting the blood-brain barrier and insights on computational strategies. Crit. Rev. Ther. Drug Carrier Syst. 42, 45–81. doi: 10.1615/CritRevTherDrugCarrierSyst.2025054214
Pavlíčková, V. S., Škubník, J., Ruml, T., and Rimpelová, S. (2023). A trojan horse approach for efficient drug delivery in photodynamic therapy: focus on taxanes. J. Mater. Chem. B 11, 8622–8638. doi: 10.1039/D2TB02147A
Perez-Perez, P., Artigas, P., Reyes-Batlle, M., Cordoba-Lanus, E., Rodriguez-Exposito, R. L., Cuervo, P. F., et al. (2025). Potentially pathogenic free-living amoebae at very high altitude: detection by multiplex qPCR in the northern altiplano fascioliasis hyperendemic area in Bolivia. One Health 20:100985. doi: 10.1016/j.onehlt.2025.100985
Phung, N. T. N., Pham, H. T., Tran, T. T., Dinh, V. H., Tran, N. M., Tran, N. A. N., et al. (2025). N. fowleri: portrait of a cerebral killer. Diagnostics (Basel) 15:10.3390/diagnostics15010089, 89.
Pijpers, J., den Boer, M. L., Essink, D. R., and Ritmeijer, K. (2019). The safety and efficacy of miltefosine in the long-term treatment of post-kala-azar dermal leishmaniasis in South Asia-a review and meta-analysis. PLoS Negl. Trop. Dis. 13:e0007173. doi: 10.1371/journal.pntd.0007173
Piñero, J. E., Chávez-Munguía, B., Omaña-Molina, M., and Lorenzo-Morales, J. (2019). N. fowleri. Trends Parasitol. 35, 848–849. doi: 10.1016/j.pt.2019.06.011
Puthanpurayil, S. N. T., Mukundan, A., Nair, S. R., John, A. P., Thampi, M. R., John, R., et al. (2024). Free-living amoebic encephalitis-case series. Trop. Parasitol. 14, 108–112. doi: 10.4103/tp.tp_37_23
Que, X., and Reed, S. L. (2000). Cysteine proteinases and the pathogenesis of amebiasis. Clin. Microbiol. Rev. 13, 196–206. doi: 10.1128/CMR.13.2.196
Radusky, L. G., Hassan, S., Lanzarotti, E., Tiwari, S., Jamal, S., Ali, J., et al. (2015). An integrated structural proteomics approach along the druggable genome of Corynebacterium pseudotuberculosis species for putative druggable targets. BMC Genomics 16:S9. doi: 10.1186/1471-2164-16-S5-S9
Rîpă, C., Cobzaru, R. G., Rîpă, M. R., Maștaleru, A., Oancea, A., Cumpăt, C. M., et al. (2025). N. fowleri infections: bridging clinical observations and epidemiological insights. J. Clin. Med. 14:526. doi: 10.3390/jcm14020526
Rizo-Liendo, A., Arberas-Jimenez, I., Martin-Encinas, E., Sifaoui, I., Reyes-Batlle, M., Chao-Pellicer, J., et al. (2021a). Naphthyridine derivatives induce programmed cell death in N. fowleri. Pharmaceuticals 14. doi: 10.3390/ph14101013
Rizo-Liendo, A., Arberas-Jiménez, I., Sifaoui, I., Gkolfi, D., Santana, Y., Cotos, L., et al. (2021b). The therapeutic potential of novel isobenzofuranones against N. fowleri. Int. J. Parasitol. Drugs Drug Resist. 17, 139–149. doi: 10.1016/j.ijpddr.2021.09.004
Rizo-Liendo, A., Sifaoui, I., Arberas-Jimenez, I., Reyes-Batlle, M., Pinero, J. E., and Lorenzo-Morales, J. (2020). Fluvastatin and atorvastatin induce programmed cell death in the brain eating amoeba N. fowleri. Biomed. Pharmacother. 130:110583. doi: 10.1016/j.biopha.2020.110583
Rodríguez-Mera, I. B., Carrasco-Yépez, M. M., Vásquez-Moctezuma, I., Correa-Basurto, J., Salinas, G. R., Castillo-Ramírez, D. A., et al. (2022). Role of cathepsin B of N. fowleri during primary amebic meningoencephalitis. Parasitol. Res. 121, 3287–3303. doi: 10.1007/s00436-022-07660-y
Russell, A. C., Bush, P., Grigorean, G., and Kyle, D. E. (2023). Characterization of the extracellular vesicles, ultrastructural morphology, and intercellular interactions of multiple clinical isolates of the brain-eating amoeba, N. fowleri. Front. Microbiol. 14:1264348. doi: 10.3389/fmicb.2023.1264348
Russell, A. C., Dainis, J., Alexander, J., Ali, I. K. M., and Kyle, D. E.. Secreted small RNAs of N. fowleri are biomarkers for diagnosis of primary amoebic meningoencephalitis. Biorxiv [Preprint]. (2025). doi: 10.1101/2025.01.11.632551
Sarink, M. J., Tielens, A. G. M., Verbon, A., Sutak, R., and van Hellemond, J. J. (2020). Inhibition of fatty acid oxidation as a new target to treat primary amoebic meningoencephalitis. Antimicrob. Agents Chemother. 64:20. doi: 10.1128/AAC.00344-20
Sarink, M. J., van der Meijs, N. L., Denzer, K., Koenderman, L., Tielens, A. G. M., and van Hellemond, J. J. (2022). Three encephalitis-causing amoebae and their distinct interactions with the host. Trends Parasitol. 38, 230–245. doi: 10.1016/j.pt.2021.10.004
Schuster, F. L. (2002). Cultivation of pathogenic and opportunistic free-living amebas. Clin. Microbiol. Rev. 15, 342–354. doi: 10.1128/CMR.15.3.342-354.2002
Semenzato, G., Vitali, F., Frascella, A., Lollini, L., Mocali, S., Papini, A., et al. (2025). Role of metabolism, resistance, and/or antagonism as drivers of endomicrobiomes assemblage in Origanum heracleoticum L. Commun Biol. 8:158. doi: 10.1038/s42003-025-07527-9
Shah, S., Naeem, I., Akram, F., Akhtar, M. T., and Noor, F. (2025). In-silico study of an inhibitor of S-adenosyl-L-homocysteine hydrolase (SAHH) of N. fowleri using molecular docking, density functional theory (DFT), and molecular dynamics (MD) simulation. Mol. Biotechnol. doi: 10.1007/s12033-025-01389-6
Shaukat, A., Khaliq, N., Riaz, R., Munsab, R., Ashraf, T., Raufi, N., et al. (2024). Noninvasive diagnostic biomarkers, genomic profiling, and advanced microscopic imaging in the early detection and characterization of N. fowleri infections leading to primary amebic meningoencephalitis (PAM). Ann. Med. Surg. 86, 2032–2048. doi: 10.1097/MS9.0000000000001843
Shin, H. J., Cho, M. S., Jung, S. U., Kim, H. I., Park, S., Kim, H. J., et al. (2001). Molecular cloning and characterization of a gene encoding a 13.1 kDa antigenic protein of N. fowleri. J. Eukaryot. Microbiol. 48, 713–717. doi: 10.1111/j.1550-7408.2001.tb00211.x
Siddiqui, R., Ali, I. K. M., Cope, J. R., and Khan, N. A. (2016). Biology and pathogenesis of N. fowleri. Acta Trop. 164, 375–394. doi: 10.1016/j.actatropica.2016.09.009
Siddiqui, R., Boghossian, A., Alqassim, S. S., Kawish, M., Gul, J., Jabri, T., et al. (2023). Anti-Balamuthia mandrillaris and anti-N. fowleri effects of drugs conjugated with various nanostructures. Arch. Microbiol. 205:170. doi: 10.1007/s00203-023-03518-8
Siddiqui, R., and Khan, N. A. (2023). Intranasal route for the delivery of antiamebic drugs against brain-eating amoeba. Ther. Deliv. 14, 175–177. doi: 10.4155/tde-2023-0015
Siddiqui, R., Mungroo, M. R., Anuar, T. S., Alharbi, A. M., Alfahemi, H., Elmoselhi, A. B., et al. (2022). Antiamoebic properties of laboratory and clinically used drugs against N. Fowleri and Balamuthia mandrillaris. Antibiotics 11:749. doi: 10.3390/antibiotics11060749
Siddiqui, R., Rajendran, K., Abdella, B., Ayub, Q., Lim, S. Y., and Khan, N. A. (2020). N. fowleri: differential genetic expression following treatment with hesperidin conjugated with silver nanoparticles using RNA-Seq. Parasitol. Res. 119, 2351–2358. doi: 10.1007/s00436-020-06711-6
Sohail, S., Wisal, A., Rana, S., Abid Khan, R. M., Ullah, A., Khan, F. A., et al. (2025). Druggable proteins of Alistipes reveal promising antimicrobial targets against chronic intestinal inflammation. Front. Pharmacol. 16:1555062. doi: 10.3389/fphar.2025.1555062
Sohn, H. J., Ham, A. J., Park, A. Y., Lee, J. H., Park, S., Shin, H. J., et al. (2025). Cloning of nf-profilin and intercellular interaction with nf-actin in N. fowleri cysts. Sci. Rep. 15:7015. doi: 10.1038/s41598-025-90222-w
Song, B., Zheng, J., and Zhao, D. (2024). Primary amebic meningoencephalitis in children: A case report and literature review. IDCases. 37:e02028. doi: 10.1016/j.idcr.2024.e02028
Soontrapa, P., Jitmuang, A., Ruenchit, P., Tiewcharoen, S., Sarasombath, P. T., and Rattanabannakit, C. (2022). The first molecular genotyping of N. fowleri causing primary amebic meningoencephalitis in Thailand with epidemiology and clinical case reviews. Front. Cell. Infect. Microbiol. 12:931546. doi: 10.3389/fcimb.2022.931546
Stahl, L. M., and Olson, J. B. (2023). Investigating the interactive effects of temperature, pH, and salinity on N. fowleri persistence. J. Eukaryot. Microbiol. 70:e12964. doi: 10.1111/jeu.12964
Sutrave, S., and Richter, M. H. (2021). The Truman show for protozoan parasites: A review of in vitro cultivation platforms. PLoS Negl. Trop. Dis. 15:e0009668. doi: 10.1371/journal.pntd.0009668
Wei, H. Y., Lai, Y. W., Li, S. Y., Lee, Y. I., Hu, M. K., Ji, D. D., et al. (2024). Fatal case of N. fowleri primary amebic meningoencephalitis from indoor surfing center, Taiwan, 2023. Emerg. Infect. Dis. 30, 1922–1925. doi: 10.3201/eid3009.231604
Xin, P., Xu, X., Deng, C., Liu, S., Wang, Y., Zhou, X., et al. (2020). The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 80:106210. doi: 10.1016/j.intimp.2020.106210
Young, J. D., and Lowrey, D. M. (1989). Biochemical and functional characterization of a membrane-associated pore-forming protein from the pathogenic ameboflagellate N. fowleri. J. Biol. Chem. 264, 1077–1083.
Zaman, M., Fida, T., Haris, H. M., Naveed, Z., Haider, S., and Khan, T. (2025). Challenges and strategies in Managing N. fowleri-associated primary amoebic meningoencephalitis in Pakistan: a case report. Acta Parasitol. 70:150. doi: 10.1007/s11686-025-01091-2
Zaman, A., Noor, S., Ahmad, I., Shehroz, M., Alhajri, N., Ahmed, S., et al. (2025). Exploring cotton plant compounds for novel treatments against brain-eating N. fowleri: an in-silico approach. PLoS One 20:e0319032. doi: 10.1371/journal.pone.0319032
Zhang, Y., Cardell, L. O., Edvinsson, L., and Xu, C. B. (2013). MAPK/NF-κB-dependent upregulation of kinin receptors mediates airway hyperreactivity: a new perspective for the treatment. Pharmacol. Res. 71, 9–18. doi: 10.1016/j.phrs.2013.02.004
Zheng, H., Zhang, L., Bai, X., Zhu, J., Liu, S., Ke, Y., et al. (2025). GCN5-targeted dual-modal probe across the blood-brain barrier for borders display in invasive glioblastoma. Nat. Commun. 16:2345. doi: 10.1038/s41467-025-57598-9
Zhou, W., Debnath, A., Jennings, G., Hahn, H. J., Vanderloop, B. H., Chaudhuri, M., et al. (2018). Enzymatic chokepoints and synergistic drug targets in the sterol biosynthesis pathway of N. fowleri. PLoS Pathog. 14:e1007245. doi: 10.1371/journal.ppat.1007245
Keywords: Naegleria fowleri , primary amoebic meningoencephalitis, pathogenesis, diagnostics, therapeutics
Citation: Dai L, Guo X-R, Chen X-R, Ma M-H, Liu Z-H, Lai J, Lu J, Feng M, Liu X-X and Yang S-H (2025) A review of the mechanism, diagnosis, and treatment of Naegleria fowleri infection. Front. Microbiol. 16:1686695. doi: 10.3389/fmicb.2025.1686695
Edited by:
Matthew Chidozie Ogwu, Appalachian State University, United StatesReviewed by:
Faham Khamesipour, Ministry of Health and Medical Education, IranSyed S. Hassan, University of Karachi, Pakistan
Copyright © 2025 Dai, Guo, Chen, Ma, Liu, Lai, Lu, Feng, Liu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Feng, NTE0ODY3ODQxQHFxLmNvbQ==; Xi-Xia Liu, NDQxMDc4NTVAcXEuY29t; Sheng-Hui Yang, c2hlbmdodWl5YW5nQDEyNi5jb20=
 Ling Dai
Ling Dai Xin-Ru Guo1
Xin-Ru Guo1 Xu-Rui Chen
Xu-Rui Chen Jun Lu
Jun Lu Sheng-Hui Yang
Sheng-Hui Yang