- 1Laboratory of Gastrointestinal Microbiology, Jiangsu Key Laboratory of Gastrointestinal Nutrition and Animal Health, College of Animal Science and Technology, Nanjing Agricultural University, Nanjing, China
- 2Department of Laboratory Animal, Jinling Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, China
- 3College of Animal Science, Anhui Science and Technology University, Chuzhou, China
The circadian rhythms of the gut microbiota are biologically significant for the host. However, the association between fluctuations in the relative abundance of the microbiota and nutrient substrates in the gut remains incompletely understood. Using swine as a model, this study employed continuous sampling at 9 time points over 24 h via a colonic T-shaped fistula. It investigated the temporal dynamics of nutrient substrates and Prevotella abundance in the colon of pigs over a 24-h period and further explored dynamic interactions among KEGG level-3 pathways, genes, and Prevotella using metagenomic approaches. Results revealed a significant 24-h periodicity in Prevotella abundance, peaking at T06–T09 and declining to minimal levels at T18–T21, with the nadir at T18. Dynamic correlation network analysis uncovered significant temporal associations between Prevotella rhythms and nutrient substrates: negative correlations with true protein (TP) and ammonia nitrogen (NH₃-N), in contrast to positive correlations with starch and cellulose, exhibiting time lags ranging from −2 to 4 h. Prevotella copri exhibited high relative abundance and pronounced daily fluctuations, while Prevotella sp. MGM2 showed relatively high abundance but lacked daily fluctuations. Furthermore, differences existed in the dynamic correlations of genes and KEGG level-3 metabolic pathways of these two Prevotella species with nutrient substrates. The results revealed that the two Prevotella species in the colon exhibited different response strategies to nutrient substrates: Prevotella copri likely adopted a “rhythmic substrate-responsive strategy,” while Prevotella sp. MGM2 followed a “sustained response strategy,” which may explain their distinct daily fluctuations.
1 Introduction
The mammalian gastrointestinal tract hosts a vast consortium of trillions of microorganisms that engage in symbiotic interactions with the host, critically sustaining intestinal homeostasis (Hooper and Macpherson, 2010). Functioning as a dynamic and intricate ecosystem, the gut microbiota serves crucial roles in modulating host metabolism, orchestrating immune responses, and facilitating nutrient assimilation (Frazier and Chang, 2020). Emerging evidence highlights that microbial communities exhibit intrinsic daily oscillations, which are temporally synchronized with the host’s endogenous circadian clock and feeding fasting cycles. These rhythmic fluctuations are not merely passive adaptations but actively engage in bidirectional crosstalk with host physiology, exerting systemic influences on energy homeostasis, inflammatory signaling, and behavioral outputs (Lotti et al., 2023). Given the high similarity between pigs and humans in genetic information, anatomical structure, dietary patterns, physiological functions, and other aspects, this study uses pigs as a model, aiming for the research findings on porcine gut microbiota to provide an important reference for exploring the mechanisms of human gut microbiota (Xiao et al., 2016).
In rodent models, gut microbial oscillations are modulated by daily behavioral patterns, feeding schedules, and sex-specific factors, exhibiting phylum-level differences, with Bacteroidota (particularly Prevotella) demonstrating more robust rhythmicity in female mice compared to males (Thaiss et al., 2014). Perturbations in microbial rhythms, often induced by erratic feeding regimens or genetic disruptions of circadian regulators, are strongly associated with metabolic dysregulation and compromised gut integrity. Therefore, deciphering the regulatory mechanisms governing microbial daily dynamics is critical for optimizing host-microbe symbiosis and improving health outcomes (Gutierrez Lopez et al., 2021). As the most abundant genus within the Bacteroidota phylum, Prevotella is widely distributed across multiple human body sites (e.g., oral cavity, gut, skin, vagina), animal hosts (e.g., ruminants, swine), and natural environments. Its genome size varies significantly (2.37–4.26 Mb), with high functional gene diversity, particularly in carbohydrate metabolism (Tett et al., 2021). Studies have demonstrated that the abundance of Prevotella in individuals consuming non-Westernized diets (rich in plant-based fibers such as grains, root vegetables, and unprocessed plants) exhibit significantly higher Prevotella abundance than westernized populations, where Bacteroides dominates (Accetto and Avguštin, 2021). Under high-fiber dietary conditions, the gut microbiota exhibits a marked enrichment of Bacteroidota (primarily Prevotella) and a reduction in Firmicutes (De Filippo et al., 2010). Our prior research in swine models demonstrated that nutrient substrates critically influence diurnal oscillations and functional dynamics of colonic microbiota. Notably, these microbial fluctuations correlate closely with oscillations in nutrient substrates and their metabolites, with the most pronounced rhythmic shifts observed in Bacteroidota and Firmicutes (Wang et al., 2023).
Among gut microbiota genera with pivotal functional roles, Prevotella, particularly Prevotella copri, dominates the swine gut, especially in high dietary fiber environments (Abdelsalam et al., 2023). Prevotella copri specializes in degrading non-starch polysaccharides (NSPs), including hemicelluloses (e.g., xylan, arabinoxylan), cellulose, and pectin. By breaking down complex plant polysaccharides, Prevotella copri generates short-chain fatty acids (SCFAs), which provide energy to the host and enhance intestinal barrier function (de Oliveira Melo et al., 2024). Intriguingly, Prevotella copri abundance in the swine gut exhibits significant daily fluctuations. Studies reveal its association with reduced fasting blood glucose, improved glucose tolerance, and lowered insulin resistance indices, suggesting its potential role as a rhythm-sensitive probiotic (Chang et al., 2019; Yang et al., 2024). Notably, the robust oscillations of Prevotella copri align with fluctuations in intestinal nutrient substrates, implying time-dependent functional roles in nutrient processing. However, the drivers of its rhythmic behavior and the functional significance of this temporal regulation remain unclear, warranting in-depth mechanistic exploration.
A key unresolved question is how nutrient substrates temporally shape the circadian programs of microorganisms. In previous studies, we used pigs as a model, analyzed the dynamic correlations between colonic nutrient substrates and gut microbiota over 48 h, and identified daily fluctuations therein (Wang et al., 2023). In this paper, we use Prevotella with strong rhythmicity as the entry point to analyze its nutrient substrate response patterns within 24 h. While feeding-fasting cycles and dietary composition are recognized as key modulators of microbial activity, the precise mechanisms underlying the rhythmic interaction between dietary components and Prevotella remain unclear. This study aimed to analyze the dynamic relationship between dietary components and Prevotella abundance in the colon across circadian timescales and further explore the genetic-level differences among Prevotella species with distinct daily fluctuations.
2 Materials and methods
2.1 Animals, experimental design and sampling
Eight healthy castrated male pigs (Duroc × Landrace × Yorkshire; mean body weight ± SE: 57.03 ± 1.78 kg) were anesthetized preoperatively with 3% phenobarbital sodium solution (30 mg/kg) via ear vein injection. Each pig was surgically implanted with a T-shaped cannula (inner diameter: 15 mm, length: 82 mm, wing: 10 mm) in the proximal colon, followed by intramuscular injection of antibiotics (Sodium Ceftiofur) for 7 days to prevent infection (Rivera-Zavala et al., 2011). Studies have shown that after ceftiofur administration, there are no significant changes in intestinal microbiota at the phylum level, but the community structure (Yue & Clayton index) has recovered to near baseline levels after 14 days (Costa et al., 2015). Pigs were fed under a 12-h light–dark cycle (lights on: 07:00 a.m. to 07:00 p.m.) with free access to commercial diet (Table 1) and water for 15 days. On day 16, five healthy pigs were randomly selected for colonic digesta collection starting at 06:00 a.m., with samples collected every 3 h over a 24-h period: T06 (06:00 a.m.), T09 (09:00 a.m.), T12 (12:00 p.m.), T15 (03:00 p.m.), T18 (06:00 p.m.), T21 (09:00 p.m.), T24 (12:00 a.m.), T27 (03:00 a.m.) and T30 (06:00 a.m.). The digesta samples were immediately flash-frozen in liquid nitrogen and stored at −80 °C for further metagenomic sequencing and nutrient substrate analysis. Under ad libitum feeding, the collected samples at nine time points (n = 5) served as controls for each other.
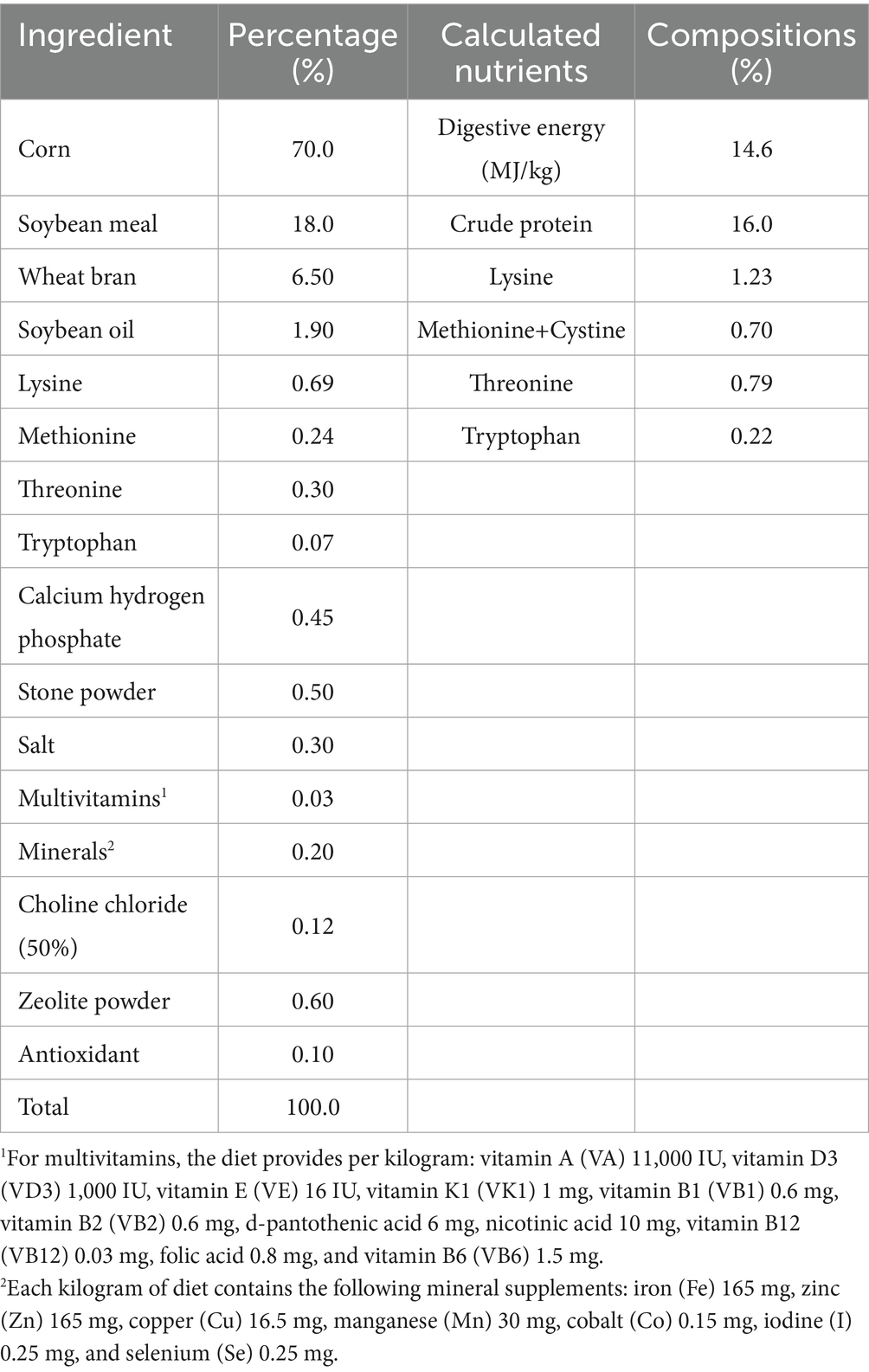
Table 1. Ingredient composition and calculated nutritional level of the experimental diet (as-fed basis).
2.2 DNA extraction and metagenomic sequencing
Total genomic DNA of colonic chyme bacteria was extracted using the cetrimonium bromide (CTAB) method (Dai et al., 2010). A total of 0.5 μg DNA was used for metagenomic sequencing. Raw sequence reads were quality-filtered using Trimmomatic to remove low-quality reads and adapter contaminants (Bolger et al., 2014). Host genome contamination and low-quality data were further eliminated using the BWA-MEM algorithm (parameters: -M -k 32 -t 16)1 after quality control. Clean reads were assembled into contigs using MetaGenome Assembler MEGAHIT (v1.1.3) with the parameter ‘--min-contig-len 500’ (Li et al., 2015). Open reading frames (ORFs) were predicted from the assembled contigs using Prodigal (v2.6.3) (Wu and Singer, 2021). CD-HIT (v4.6) was employed for clustering ORFs into a non-redundant gene catalog with parameters ‘-n 9 -c 0.95 -G 0 -M 0 -d 0 -aS 0.9 -r 1’, where the longest sequence in each cluster was designated as the representative sequence (Fu et al., 2012). Gene abundance across samples was quantified using Salmon (v0.12.0), obtaining read counts per gene. Relative abundance was normalized as transcripts per kilobase per million mapped reads (TPM) (Patro et al., 2017). This non-redundant gene set was then searched against the Kyoto Encyclopedia of Genes and Genomes (KEGG) databases using BLASTX to identify proteins and annotate their functions. Based on the KO (KEGG Orthology) results, the specific functions and metabolic pathways were obtained by mapping the annotated genes to the KEGG Pathway Database. The gene abundance was calculated finally using the following Equations 1–5:
2.3 Measurement of nutrient substrates in the colon
Quantitative analysis of colonic carbon (starch, cellulose) and nitrogen (TP, NH3-N) substrates was performed using standardized biochemical assays. Starch content was determined via enzymatic hydrolysis with the Solarbio BC0700 Detection Kit (Beijing Solarbio Science & Technology Co., Ltd.), while cellulose quantification employed the Solarbio BC4280 Kit, both following the manufacturer’s protocols (Zhuang et al., 2019). TP concentration was assessed through the Coomassie Brilliant Blue G-250 binding method using the Solarbio PC0010 Kit (Li et al., 2019). NH3-N levels were measured colorimetrically according to our established analytical protocol (Wang et al., 2021).
2.4 Metagenomic binning
The gene abundance of the Prevotella genus was investigated using a metagenomic assembly and binning approach. To maximize the recovery of assembled contigs, both individual assemblies for each time point sample and co-assemblies of high-quality reads from porcine colon samples were performed using MEGAHIT (v1.1.1; parameters: --min-contig-len 500 -t 40) (Li et al., 2015, 2016). Contigs (>1.5 kb) from single-sample assemblies and co-assemblies were independently subjected to metagenomic binning based on sequence composition and coverage depth using three tools with default parameters: MaxBin (v2.2.4) (Wu et al., 2016; Wu and Singer, 2021), MetaBAT2 (v2.11.1) (Kang et al., 2015), and CONCOCT (v0.4.0) (Alneberg et al., 2014). The resulting metagenome-assembled genomes (MAGs) were consolidated using DAS Tool (v1.1.1) (Sieber et al., 2018). High-quality MAGs (completeness >90%, contamination <5%) were identified using CheckM (v1.0.7; lineage_wf workflow) (Parks et al., 2015). Dereplication at 99% average nucleotide identity (ANI) was performed with dRep (v2.5.4; parameters: -p 72 --ignore_genome_quality -pa 0.95 -sa 0.99 -cm larger) (Olm et al., 2017).
2.5 Genome annotation
The complete genome sequences of Prevotella copri (accession number: CP085932.1) and Prevotella sp. MGM2 (accession number: NZ_BEWW00000000.1) were retrieved from the National Center for Biotechnology Information (NCBI). To comprehensively investigate gene functions of these two Prevotella species, all coding genes underwent systematic functional annotation using the Clusters of Orthologous Groups (COG) database2 and the Kyoto Encyclopedia of Genes and Genomes (KEGG) database3, where COG focuses on protein functional classification and KEGG emphasizes metabolic pathway annotation.
2.6 Statistical analyses and visualization
Daily fluctuations of Bacteroidota and Prevotella were detected using JTK_cycle (Hughes et al., 2010) over a 24-h period at 3-h intervals. PAdj < 0.05 was considered indicative of significant diurnal oscillation; only microbial taxa with a relative abundance >0.01% and present in >20% of samples were retained for further analysis (Samad et al., 2022). Correlations among Prevotella, colonic nutrient substrates, KEGG genes, contributing taxa, and KEGG pathways were calculated using Extended Local Similarity Analysis (eLSA) (Gálvez et al., 2020). Local Similarity (LS) coefficients were measured pairwise to quantify correlation levels between variables. The delay value (D) represented the time shift between pairwise comparisons.
Temporal similarity was assessed via constrained Principal Coordinate Analysis (cPCoA) using ImageGP4. Visualizations were generated as follows: Pie charts, line graphs, stacked columns, and scatter plots of rhythmic species were generated using Origin 2024. Heatmaps were generated with TBtools-II (v2.154). Network diagrams were plotted using Gephi (v0.10.1).
3 Results
3.1 General overview of Bacteroidota in the colon
In the colonic ecosystem, Bacteroidota occupy the core ecological niche, with Bacteroidales as the dominant lineage. Among these, the predominant bacterial family was Prevotellaceae followed by Bacteroidaceae (Figure 1). At the genus level, the relative abundance of Prevotella significantly surpassed that of other genera, demonstrating clear ecological dominance. Additionally, Bacteroides exhibited relatively notable ecological advantages as a secondary dominant genus.
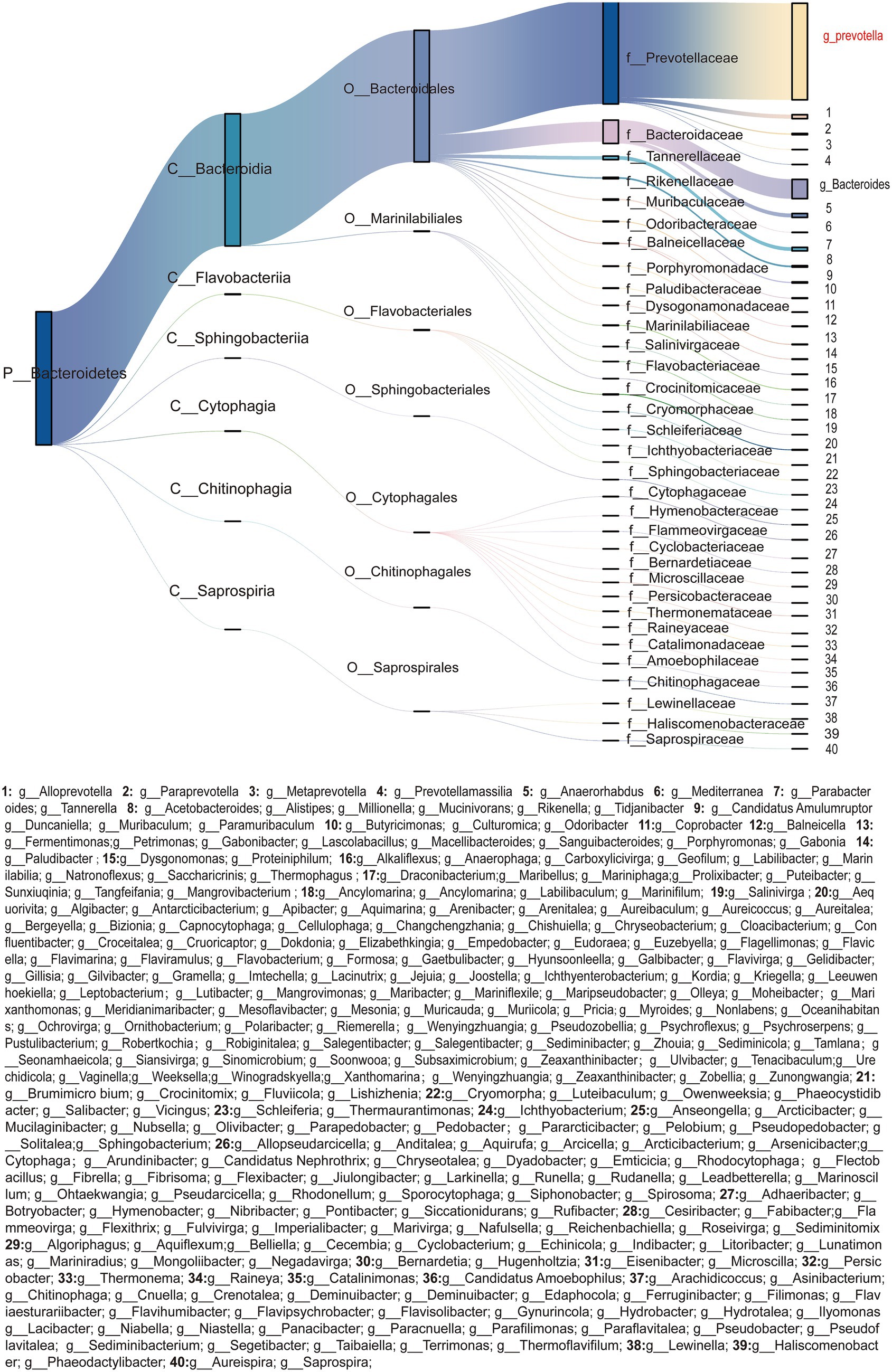
Figure 1. Phylogenetic composition and hierarchical flux of Bacteroidota (n = 5) taxa in the porcine colon. Sankey diagram illustrating the taxonomic composition of Bacteroidota (n = 5) across five hierarchical ranks (Phylum, Class, Order, Family, Genus). Columns represent distinct taxonomic ranks, with node widths proportional to their relative abundances. Streamlines denote the flow of microbial biomass between ranks, Line thickness correlates with taxon-specific contributions to community structure (Bray–Curtis similarity > 0.75, PAdj < 0.05). Nodes are color-coded by phylogenetic affiliation, with insets emphasizing key genus [Prevotella (n = 5) and Bacteroides (n = 5)].
3.2 Composition and fluctuation of phylum Bacteroidota and genus Prevotella in the colon of growing pigs
At the genus level, 36.73% of taxa exhibited statistically significant oscillations (PAdj < 0.05), with Prevotella, Bacteroides and Lutibacter displaying pronounced rhythmicity (Figure 2A). Within the Bacteroidota phylum, Prevotella, Bacteroides, and Lutibacter exhibited conserved diurnal patterns, peaking between T06-T09 and declining to minimal levels from T18 to T21 (Figures 2C–E). Among the top 20 genera by relative abundance, Prevotella and Bacteroides collectively dominated the microbial landscape, accounting for 83.81% of the total abundance (Figure 2F). Notably, Prevotella maintained the highest relative abundance across all time points, followed by Bacteroides. cPCoA based on Bray–Curtis dissimilarity revealed significant temporal structuring of microbial communities (36.1% variance explained; PERMANOVA, p = 0.0001, Figure 2B). Samples clustered into two temporal clusters: T15, T18, and T21 formed a distinct nocturnal cluster, while T06, T09, and T12 comprised a diurnal cohort.
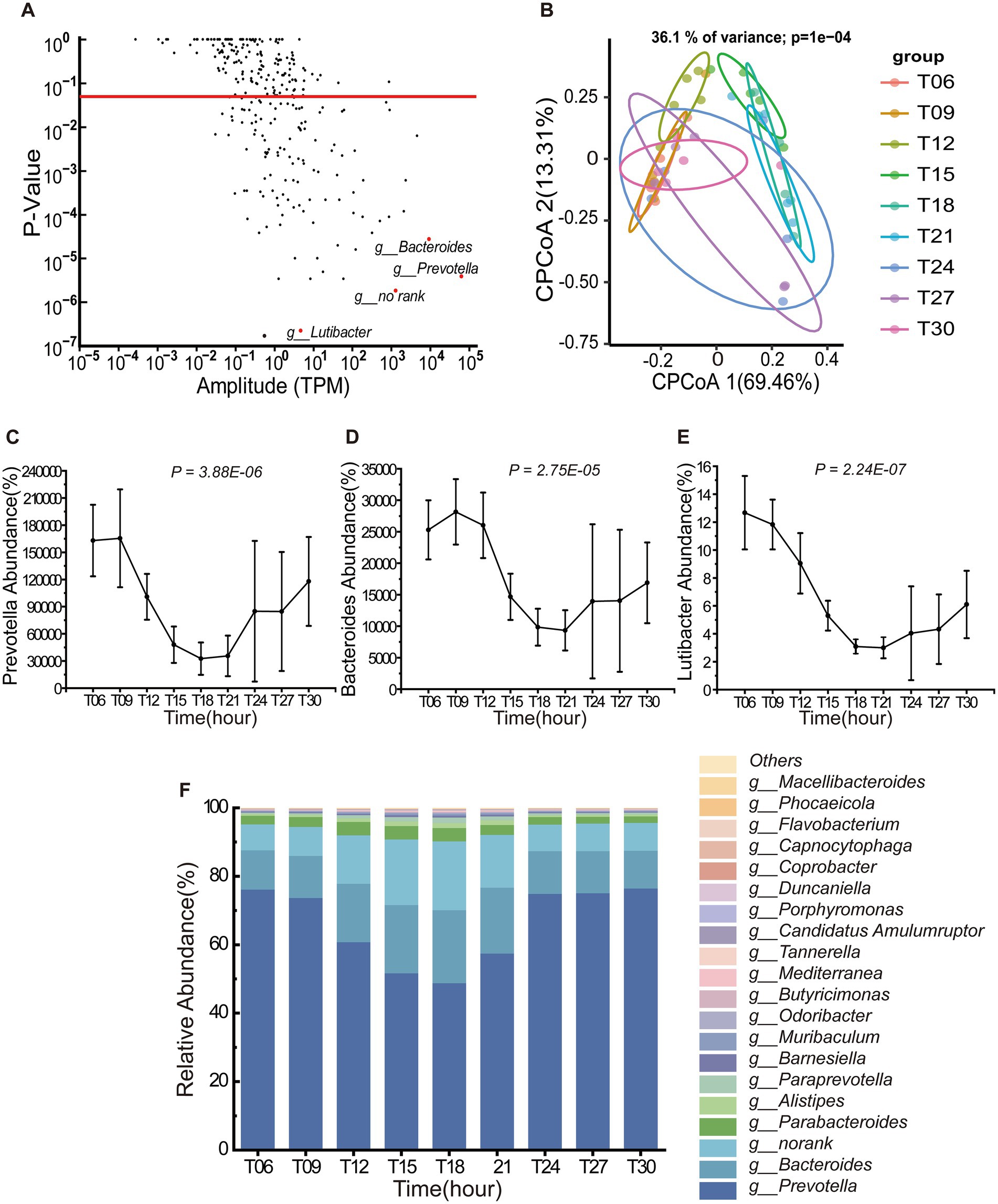
Figure 2. Temporal dynamics of Bacteroidota (n = 5) genera in the porcine colon. (A) Diurnal oscillations of bacterial genera within the phylum Bacteroidota (n = 5) over a 24-h period. The red dashed line denotes the threshold for statistical significance (PAdj < 0.05). (B) Constrained Principal Coordinates Analysis (CPCoA) of Bray–Curtis dissimilarity indices, stratified by Bacteroidota (n = 5) (36.1% variance explained; PERMANOVA, p = 0.0001). Samples are color-coded by different time point, with distinct shapes denoting sampling intervals (T06-T21). Axes labels indicate the proportion of total variance captured by each principal coordinate. (C–E) Rhythmic fluctuations of three representative genera [Prevotella (n = 5), Bacteroides (n = 5) and Lutibacter (n = 5)] across a daily cycle. Data represent mean ± SEM (n = 5 biological replicates). (F) Hierarchical abundance profile of the top 20 Bacteroidota (n = 5) genera in the colonic ecosystem.
At the species level, Prevotella copri dominated among the Prevotella species, representing 20.04% of the total microbial abundance (Figure 3B). Temporally resolved heatmap analysis demonstrated diurnal fluctuations in 156 Prevotella species across nine time points (Figure 3A). These species exhibited conserved biphasic dynamics: relative abundance peaked during early light-phase intervals (T06 and T09, corresponding to postprandial periods) and declined sharply at T18 and T21 (dark-phase troughs), reaching minimal levels at T18 (Figure 3C). Daily fluctuation analysis via the JTK_Cycle algorithm identified 150 rhythmic Prevotella species (PAdj < 0.05). Notably, Prevotella copri exhibited strong amplitude and daily rhythmic fluctuations, whereas Prevotella sp. MGM2 displayed high-amplitude fluctuations without daily fluctuations (PAdj > 0.05) (Figure 3D). Within the Bacteroidota, 75.3% of genera exhibited statistically significant daily oscillations (PAdj < 0.05), with Prevotella and Bacteroides dominating this rhythmic hierarchy. Strikingly, Prevotella emerged as the predominant genus in the porcine colon, constituting 70.04% of the total Bacteroidota population (Figure 3E).
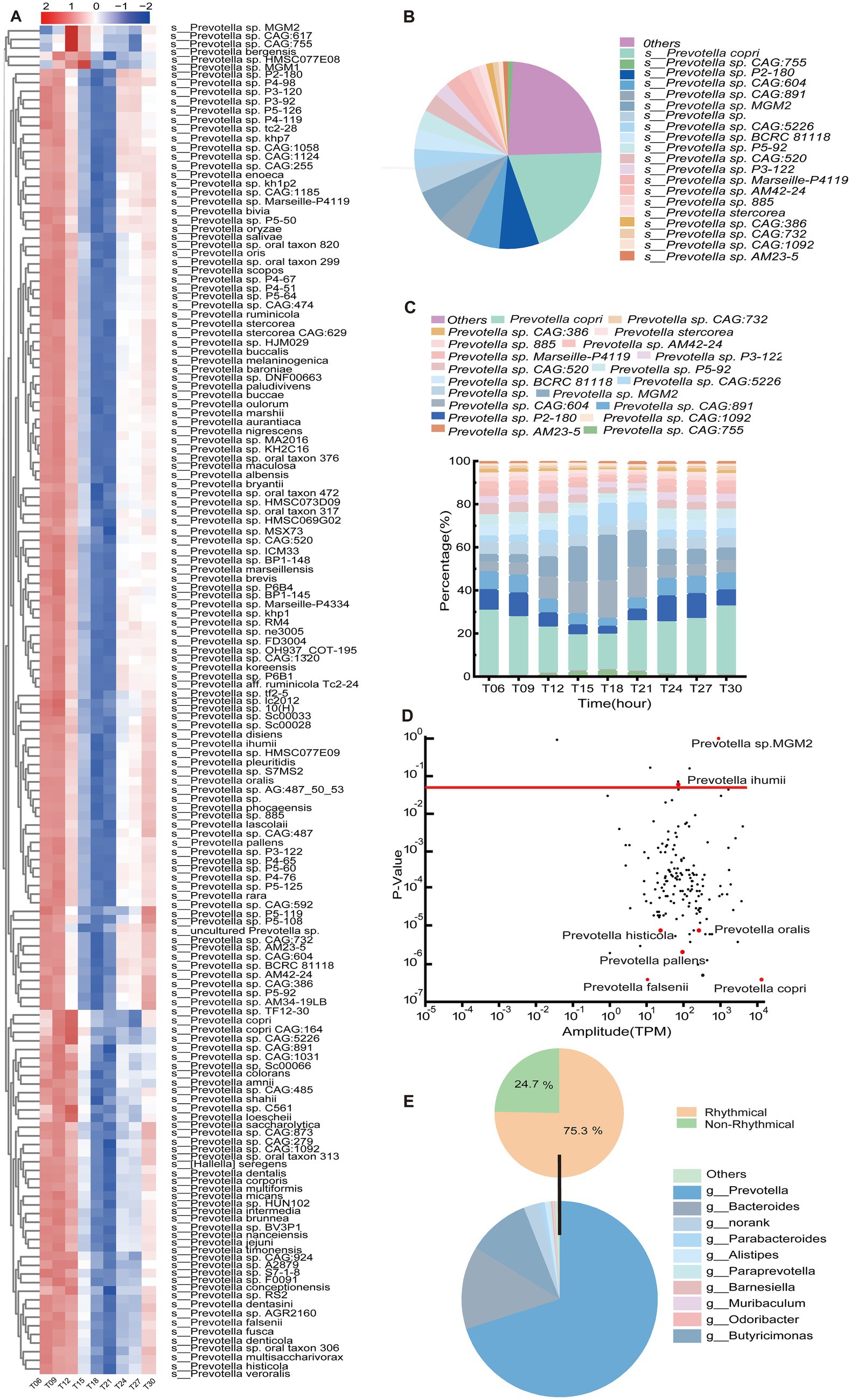
Figure 3. Temporal dynamics and ecological hierarchy of Prevotella (n = 5) in the porcine colon. (A) Heatmap of Z-score-normalized relative abundance of Prevotella (n = 5) species at 9 time points. Row clustering reflects time-dependent aggregation patterns, with row annotations indicating class-level phylogenetic classification. (B) Rank-abundance curve of the top 20 Prevotella (n = 5) species, highlighting Prevotella copri (n = 5) dominance. (C) Composition and fluctuations of the key gut microbial taxa Prevotella (n = 5) genus (top 20) at different time points. (D) Diurnal trajectories of Prevotella (n = 5) species abundance, with the red dashed line marking the significance threshold (PAdj < 0.05). (E) Upper panel: Proportional distribution of rhythmic (PAdj < 0.05) versus non-rhythmic taxa within the phylum Bacteroidota (n = 5). Lower panel: Ranked relative abundance of the top 10 genera in Bacteroidota (n = 5), ordered by daily oscillation amplitude.
3.3 Dynamic Prevotella-colonic nutrient substrate interactions
A co-occurrence network integrating Prevotella and colonic nutrient substrates revealed potential ecological interactions, comprising 160 nodes (156 Prevotella species and 4 substrates: TP, NH3-N, starch, and cellulose) with 443 edges (Figure 4A). Network topology analysis identified contrasting substrate associations: Prevotella exhibited negative correlations with TP and NH3-N while demonstrating strong positive correlations with starch and cellulose. Time-lagged cross-correlation analysis via the eLSA algorithm, which accounted for temporal offsets of −2 to 4 h, elucidated bidirectional substrate-microbe dynamics. Notably, 64.56% of interactions displayed negative time delays, suggesting that nutrient availability drives Prevotella population dynamics. Conversely, 35.44% exhibited positive time delays, implicating microbial enzymatic activity in substrate degradation (Figure 4C). Prevotella sp. MGM2 exhibited positive correlations with starch and cellulose without a time delay, while Prevotella copri showed negative correlations with NH3-N and TP, with a 4-h delay in its interaction with TP (Figure 4B).
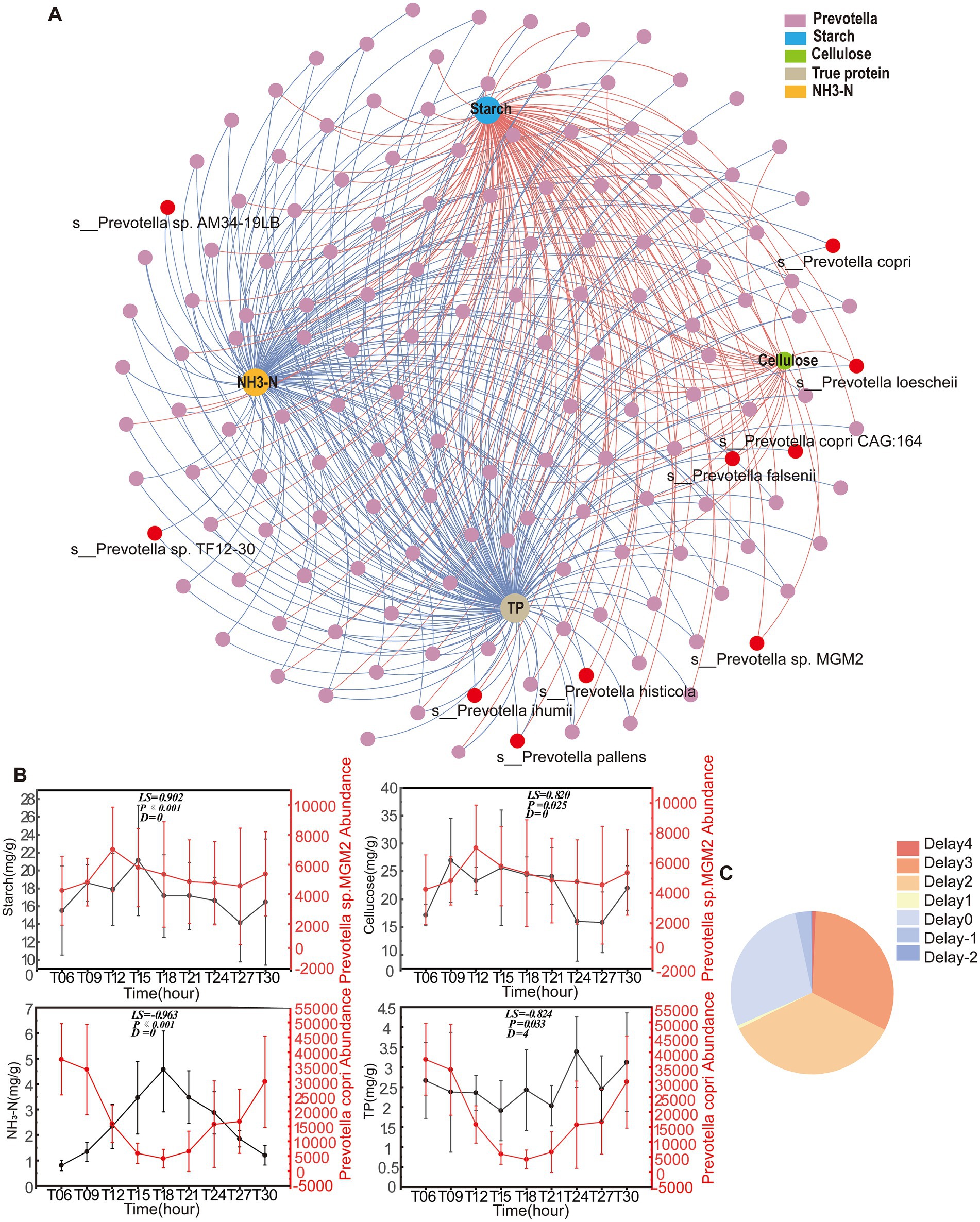
Figure 4. Nutrient-Prevotella interaction network and temporal coupling dynamics. (A) The network analysis revealing the relationship between nutrient substrates and Prevotella (n = 5) genus. The nodes are colored according to the different nutrient substrates and Prevotella (n = 5) genus. The network connection represents significant (PAdj < 0.05) correlation. The size of each node is proportional to the number of connections. (B) Diurnal fluctuations of nutrient substrates and representative Prevotella (n = 5) species over the course of a day. The data are presented as the mean ± SEM. The left vertical axis represents the abundance of nutrient substrates, and the right vertical axis represents the abundance of Prevotella (n = 5) species. (C) Pie chart showing the percentage of the correlation pairwise with different time shifts. Pairwise correlation with a negative delay means the metabolism of nutrient substrates that the was ahead of the Prevotella (n = 5) genus, whereas pairwise correlation with a positive time delay means that the Prevotella (n = 5) genus was ahead of the metabolism of nutrient substrates.
3.4 Functional differences between rhythmic and non-rhythmic species
Through KEGG pathway enrichment analysis of gene IDs identified from two Prevotella species in the colon, we identified metabolic or signaling pathways significantly enriched in these genes. In Prevotella copri, significantly enriched carbohydrate metabolism included glyoxylate and dicarboxylate metabolism, while amino acid metabolism were primarily phenylalanine, tyrosine and tryptophan biosynthesis; valine, leucine and isoleucine biosynthesis; glycine, serine and threonine metabolism; and arginine biosynthesis (Figure 5C). In Prevotella sp. MGM2, significantly enriched carbohydrate metabolism included ascorbate and aldarate metabolism and glycolysis/gluconeogenesis, whereas amino acid metabolism were dominated by valine, leucine and isoleucine biosynthesis (Figure 5D).
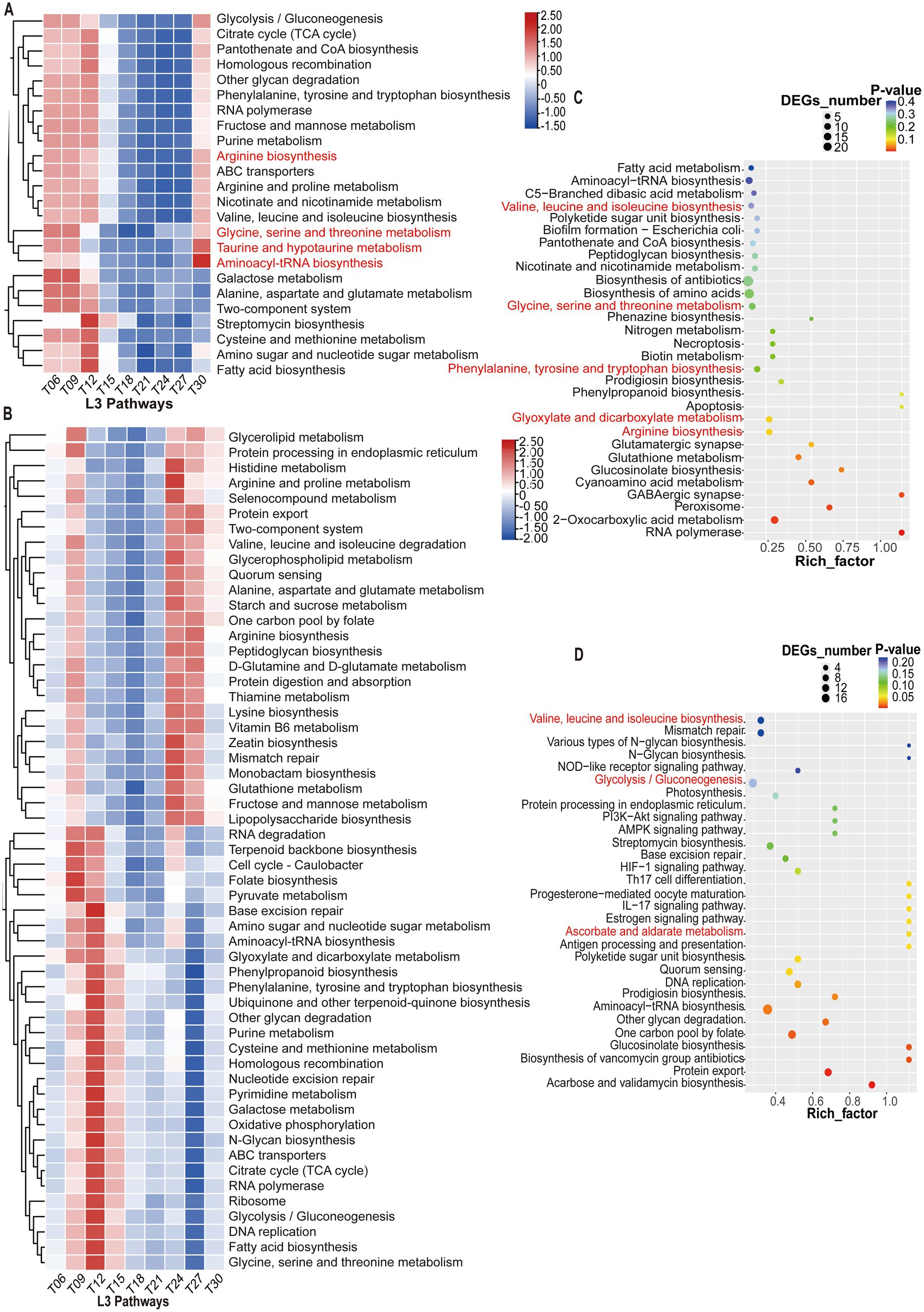
Figure 5. Heatmap of relative abundance of KEGG Level-3 metabolic pathways in two Prevotella (n = 5) species across nine time points. (A) The relative abundances of KEGG level-3 metabolic pathways of Prevotella copri (n = 5) across different time points. (B) The relative abundances of KEGG level-3 metabolic pathways of Prevotella sp. MGM2 (n = 5) across different time points (C) KEGG pathway enrichment bubble plot of Prevotella copri (n = 5). X-axis: Rich factor, representing the ratio of the number of differentially expressed genes to the total annotated genes in the pathway; Y-axis: Significantly enriched KEGG pathway names (sorted in descending order of Rich factor); Bubble size: Number of genes enriched in the pathway; Bubble color: p-value, with deeper red indicating higher enrichment significance. (D) KEGG pathway enrichment bubble plot of Prevotella sp. MGM2 (n = 5).
As the Prevotella with the most pronounced daily fluctuation and highest relative abundance in the genus, Prevotella copri was selected as the rhythmic signature species. In contrast, Prevotella sp. MGM2, which exhibited the highest relative abundance among non-rhythmic species and weakest rhythmicity, served as the representative non-rhythmic species. Following metagenomic sequencing data assembly and binning, metagenome-assembled genomes (MAGs) were taxonomically classified at the species level. Subsequent analysis focused on annotated KEGG metabolic pathways for both species. The relative abundances of KEGG level-3 metabolic pathways of Prevotella copri showed significant temporal fluctuations, with glycine, serine and threonine metabolism; taurine and hypotaurine metabolism; arginine biosynthesis; and aminoacyl-tRNA biosynthesis demonstrating statistically significant rhythmic variations (PAdj < 0.05) (Figure 5A). Conversely, Prevotella sp. MGM2 displayed no consistent temporal trends in its KEGG level-3 pathways. A subset of pathways exhibited increased relative abundance from T09 to T15, reaching minima at T27, while another subset peaked at T09 and T24-T27 (Figure 5B).
This study retrieved the complete genome sequences of both species from the National Center for Biotechnology Information (NCBI) for functional analyses. COG annotation revealed distinct metabolic profiles. Prevotella sp. MGM2 possessed 45 genes in carbohydrate transport and metabolism (G) and 73 genes in amino acid transport and metabolism (E), while Prevotella copri contained 31 and 43 genes in these respective COG categories, demonstrating significantly higher G/E category gene counts in Prevotella sp. MGM2 (Figures 6A,B).
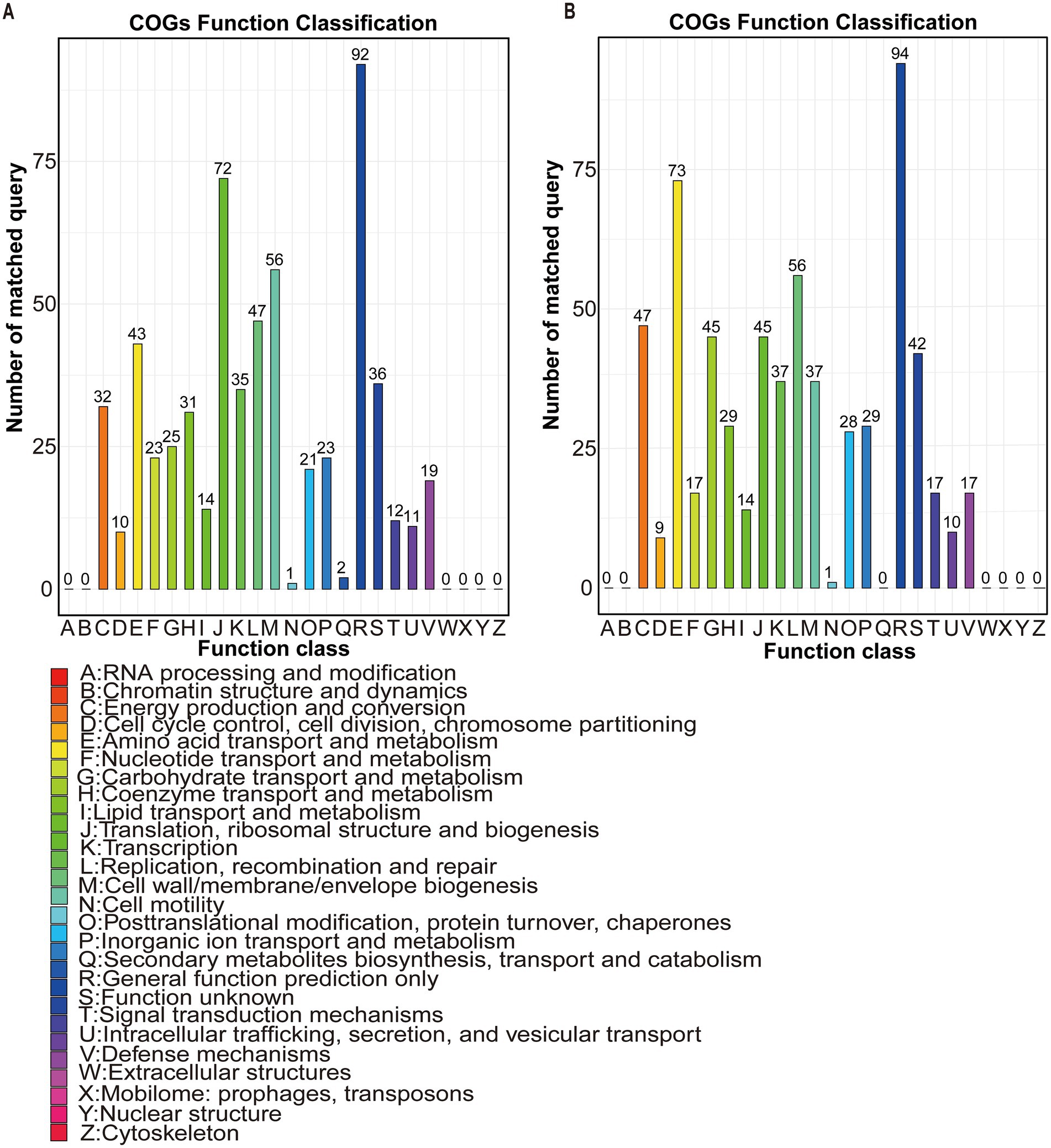
Figure 6. Comparative functional annotation of Prevotella copri and Prevotella sp. MGM2: Integrated KEGG pathway enrichment and COG category profiling reveal strain-specific metabolic divergence. (A) COG functional annotation profile of Prevotella copri. Color: Represents COG functional categories; Bar height: Number of differentially expressed genes annotated to each functional category; X-axis: COG functional categories (sorted in descending order of gene count); Y-axis: Number of annotated genes; (COG classification is based on the NCBI database with BLASTp alignment E-value ≤ 1e-52, 5). (B) COG functional annotation profile of Prevotella sp. MGM2.
3.5 Gene-level correlations between rhythmic and non-rhythmic bacteria and nutrient substrates
Based on the dynamic correlation model eLSA, general relationships were established between KEGG level-3 metabolic pathways, genes, and nutrient substrates for two Prevotella species. In Prevotella copri, Based on the dynamic correlation model eLSA, dynamic relationships were established between KEGG level-3 metabolic pathways, genes, and nutrient substrates for two Prevotella species. In Prevotella copri, three carbohydrate metabolism-related level-3 pathways were identified: the citrate cycle (TCA cycle) and glycolysis/gluconeogenesis showed positive correlations with starch, while fructose and mannose metabolism and glycolysis/gluconeogenesis exhibited positive correlations with cellulose. Additionally, four amino acid metabolism-related pathways—arginine biosynthesis; glycine, serine and threonine metabolism; valine, leucine and isoleucine biosynthesis; and phenylalanine, tyrosine and tryptophan biosynthesis—all showed negative correlations with NH₃-N (Figure 7A). In Prevotella sp. MGM2, carbohydrate metabolism pathways including galactose metabolism and glyoxylate and dicarboxylate metabolism were positively correlated with starch, while in amino acid metabolism, glycine, serine and threonine metabolism and phenylalanine, tyrosine and tryptophan biosynthesis showed negative correlations with NH3-N (Figure 7B).
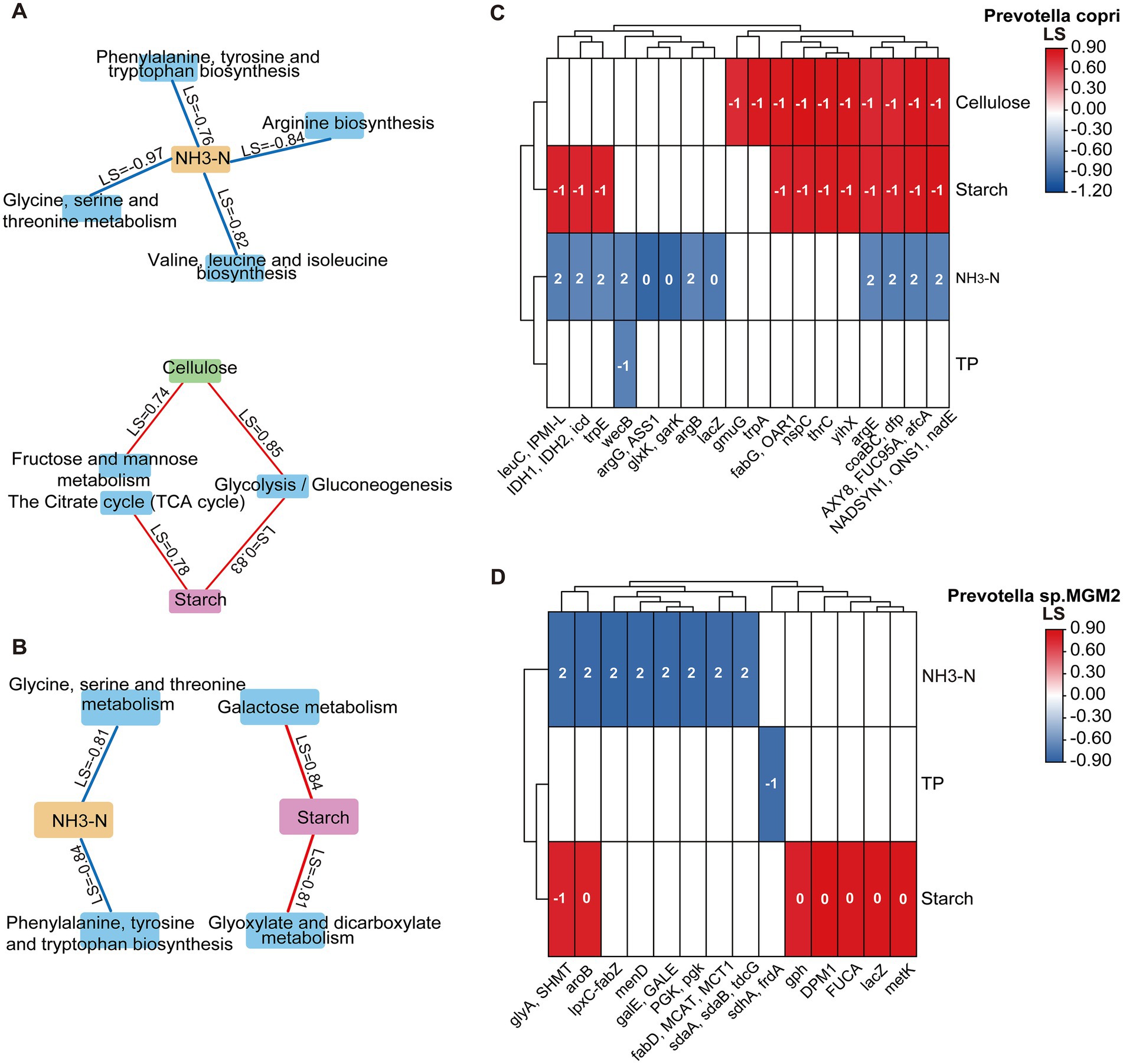
Figure 7. Dynamic correlation network between colonic nutrient substrates and KEGG Level-3 metabolic pathways/gene interactions. (A) Network diagram of Prevotella copri KEGG level-3 metabolic pathways and their associations with nutrient substrates. (B) Network diagram of Prevotella sp. MGM2 KEGG level-3 metabolic pathways and their associations with nutrient substrates. Red lines indicate positive correlations, and blue lines indicate negative correlations, with PAdj representing the LS values between them. (C) Dynamic correlation plot between genes and nutrient substrates in Prevotella copri (n = 5); the number within each cell represents the time delay between them. (D) Dynamic correlation plot between genes and nutrient substrates in Prevotella sp. MGM2 (n = 5); the number within each cell represents the time delay between them.
Genes from both species showed positive correlations with starch and cellulose and negative correlations with NH₃-N and TP, with all interactions exhibiting time delays ranging from −1 to 2 h. In Prevotella copri, carbohydrate metabolism-related genes IDH1 (LS = 0.78), IDH2 (LS = 0.78), icd (LS = 0.78), and yihX (LS = 0.83) correlated positively with starch (delay = −1), while gmuG (LS = 0.74) and yihX (LS = 0.85) showed positive correlations with cellulose (delay = −1). Amino acid metabolism-related genes argB (LS = −0.78), argE (LS = −0.78), argG (LS = −0.97), ASS1 (LS = −0.97), glxK (LS = −0.97), garK (LS = −0.97), trpE (LS = −0.76), leuC (LS = −0.82), and IPMI-L (LS = −0.82) exhibited negative correlations with NH3-N (delay = 0 or 2) (Figure 7C). In Prevotella sp. MGM2, carbohydrate metabolism-related gene gph (LS = 0.78) correlated positively with starch (delay = 0). Amino acid metabolism-related genes glyA (LS = −0.82), SHMT (LS = −0.82), sdaA (LS = −0.79), sdaB (LS = −0.79), tdcG (LS = −0.79), and aroB (LS = −0.84) showed negative correlations with NH3-N (delay = 2) (Figure 7D). Additionally, four amino acid metabolism-related pathways—arginine biosynthesis, glycine, serine and threonine metabolism, valine, leucine and isoleucine biosynthesis, and phenylalanine, tyrosine and tryptophan biosynthesis—all showed negative correlations with NH3-N (Figure 7A). In Prevotella sp. MGM2, carbohydrate metabolism pathways including galactose metabolism and glyoxylate and dicarboxylate metabolism were positively correlated with starch, while in amino acid metabolism, glycine, serine and threonine metabolism and phenylalanine, tyrosine and tryptophan biosynthesis showed negative correlations with NH3-N (Figure 7B).
Genes from both species showed positive correlations with starch and cellulose and negative correlations with NH3-N and TP, with time delays ranging from −1 to 2 h. In Prevotella copri, carbohydrate metabolism-related genes IDH1 (LS = 0.78), IDH2 (LS = 0.78), icd (LS = 0.78), and yihX (LS = 0.83) correlated positively with starch (delay = −1), while gmuG (LS = 0.74) and yihX (LS = 0.85) showed positive correlations with cellulose (delay = −1). Amino acid metabolism-related genes argB (LS = −0.78), argE (LS = −0.78), argG (LS = −0.97), ASS1 (LS = −0.97), glxK (LS = −0.97), garK (LS = −0.97), trpE (LS = −0.76), leuC (LS = −0.82), and IPMI-L (LS = −0.82) exhibited negative correlations with NH3-N (delay = 0 or 2) (Figure 7C). In Prevotella sp. MGM2, carbohydrate metabolism-related gene gph (LS = 0.78) correlated positively with starch (delay = 0). Amino acid metabolism-related genes glyA (LS = −0.82), SHMT (LS = −0.82), sdaA (LS = −0.79), sdaB (LS = −0.79), tdcG (LS = −0.79), and aroB (LS = −0.84) showed negative correlations with NH3-N (delay = 2) (Figure 7D).
4 Discussion
Previous studies in porcine models have demonstrated that fluctuations in colonic microbiota are closely associated with oscillations in nutrient substrates (Wang et al., 2023), but the dynamic correlations between specific bacterial species and nutrient substrates remain unclear. This study focused on Prevotella, which exhibits significant daily fluctuations, to investigate its dynamic interactions with colonic nutrient substrates and elucidate the genetic basis underlying its daily fluctuations. As a dominant genus within the Bacteroidota, Prevotella serves as a key dietary-fiber-fermenting bacterium that degrades complex polysaccharides (e.g., plant fibers) to generate short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate, which provide energy for intestinal epithelial cells and maintain barrier integrity (Kovatcheva Datchary et al., 2015; Gálvez et al., 2020).
This study employed a fistulated pig model to enable continuous sampling within the same individual, with the aim of reducing inter-individual variability. However, under the current experimental conditions, we acknowledge that while the fistulated model offers practical advantages (e.g., enabling long-term repeated non-invasive sampling with reduced animal stress compared to terminal methods), surgical modification may alter local microenvironmental factors (e.g., luminal flow or mucosal barrier function). Consequently, minor discrepancies between sampling data and the true physiological state (e.g., fluctuations in feed intake) may persist. Colonic nutrient substrate content showed high synchronization with feeding rhythms, and microbial abundance fluctuations were strongly correlated with nutrient substrate variations. Previous studies confirmed that in murine models, Bacteroidota and Verrucomicrobia peak during fasting periods, while Firmicutes dominate during feeding periods (Zarrinpar et al., 2014). Human studies revealed diurnal oscillations in 15.2% of microbial operational taxonomic units (OTUs), with Bacteroidota peaking nocturnally and Firmicutes diurnally, accompanied by cyclic fluctuations in alpha-diversity (Reitmeier et al., 2020). Our findings demonstrated that Bacteroidota abundance peaked at T06-T09 and reached trough levels at T18-T21. Within this phylum, 75.3% of species exhibited daily fluctuations, among which Prevotella showed the highest relative abundance and most pronounced rhythmic patterns. Notably, dynamic correlation analysis between Prevotella and colonic nutrient substrates revealed negative correlations with NH3-N (ammonia nitrogen)/TP (total phosphorus) and positive correlations with starch/cellulose, potentially attributable to Prevotella’s metabolic capability to degrade host-indigestible complex fibers for energy acquisition, thereby promoting microbial proliferation.
Further analysis of different species within Prevotella revealed that Prevotella copri exhibits the highest relative abundance and most pronounced daily fluctuations. Current studies demonstrate its dual roles: improving insulin sensitivity and lipid metabolism (Gong et al., 2024; Ismail et al., 2025). Murine experiments indicate that oral administration of Prevotella copri alleviates traumatic brain injury (TBI)-induced neurological deficits (motor/cognitive dysfunction), anxiety-like behaviors, oxidative stress, blood–brain barrier damage, and neuronal apoptosis (Gu et al., 2024), suggesting its probiotic potential. However, Prevotella copri exacerbates vascular calcification in chronic kidney disease via LPS-mediated gut barrier disruption and NF-κB/NLRP3 inflammasome activation (Hao et al., 2024); meanwhile, it depletes tryptophan (Trp) in breast cancer patients to reduce anticancer indole-3-pyruvate (IPyA) levels and promote tumor progression (Su et al., 2024); furthermore, it correlates with elevated lipopolysaccharide (LPS) levels under high-fat diets, aggravating obesity and systemic inflammation (Yuan et al., 2024; Ismail et al., 2025); and shows no significant colonization changes under high-fiber diets but inhibits fat accumulation by reducing energy intake (Chen et al., 2021). It thrives competitively through the polysaccharide utilization loci (PULs) that efficiently metabolize complex xylans, influencing host health via dietary fiber metabolism (Linares-Pastén et al., 2021). Prevotella copri comprises four genetically distinct clades (Clade A-D) (Tett et al., 2019), with clade-specific metabolic functions (Iljazovic et al., 2021), which may explain the bidirectional effects of prevotella copri in disease contexts.
Prevotella copri demonstrates higher abundance in the gut microbiota of non-Westernized populations (consuming plant-based diets) and harbors carbohydrate-active enzymes (CAZymes) that interact with high-fiber diets to improve glucose homeostasis (Jiang et al., 2022), whereas its abundance is reduced under Westernized diets (high-fat, high-protein) (Tett et al., 2019). Our findings reveal that both Prevotella copri and Prevotella sp. MGM2 show significant enrichment in KEGG pathways related to amino acid metabolism, carbohydrate metabolism, lipid metabolism, and biosynthesis of secondary metabolites. These pathways may mediate host–microbe interactions through amino acid fermentation (e.g., SCFA production), carbohydrate breakdown (e.g., dietary fiber utilization), and lipid metabolism, thereby influencing inflammation and energy homeostasis. Secondary metabolites (e.g., vitamins/neurotransmitter precursors) may further modulate host physiology via the gut-brain axis (Ogata et al., 1999; Kanehisa et al., 2017, 2023). Notably, Prevotella copri exhibited statistically significant rhythmic variations (p < 0.05) in metabolic pathways including glycine/serine/threonine metabolism, taurine and hypotaurine metabolism, arginine biosynthesis, and aminoacyl-tRNA biosynthesis. In contrast, Prevotella sp. MGM2 displayed no consistent temporal trends in the relative abundance of its KEGG level-3 pathways.
Comparative analysis of core gene functions in Prevotella copri and Prevotella sp. MGM2 based on the COG database revealed high gene abundance in functional categories Carbohydrate Transport and Metabolism (G) and Amino Acid Transport and Metabolism (E) for both species, where enrichment of category G genes suggests genetic potential to degrade host-indigestible complex polysaccharides (e.g., cellulose, starch) or utilize diverse monosaccharides (e.g., glucose, fructose), which confers ecological advantages, while category E gene abundance indicates efficient amino acid uptake and metabolism (Tatusov, 2000); notably, Prevotella sp. MGM2 harbored significantly higher gene counts in G (45 genes) and E (73 genes) than Prevotella copri (31 genes in G; 43 genes in E), yet demonstrated fewer nutrient substrates-related KEGG level-3 pathways and annotated genes—specifically, Prevotella sp. MGM2 had only two KEGG level-3 pathways (galactose metabolism and glyoxylate and dicarboxylate metabolism) and one gene (gph) linked to starch, while two pathways (glycine, serine and threonine metabolism and phenylalanine, tyrosine and tryptophan biosynthesis) and six genes (glyA, SHMT, sdaA, sdaB, tdcG, aroB) were associated with NH₃-N; in contrast, Prevotella copri exhibited two pathways (citrate cycle [TCA cycle] and glycolysis/gluconeogenesis) and four genes (IDH1, IDH2, icd, yihX) correlated with starch, two pathways (fructose and mannose metabolism and glycolysis/gluconeogenesis) and two genes (gmuG, yihX) linked to cellulose, and four pathways (arginine biosynthesis, glycine/serine/threonine metabolism, valine/leucine/isoleucine biosynthesis, and phenylalanine/tyrosine/tryptophan biosynthesis) and nine genes (argB, argE, argG, ASS1, glxK, garK, trpE, leuC, IPMI-L) associated with NH₃-N.
These findings highlight that Prevotella sp. MGM2 possesses fewer nutrient-substrates-related KEGG level-3 pathways and functional genes than Prevotella copri. Importantly, the relative abundances of Prevotella copri’s KEGG level-3 pathways exhibited dynamic fluctuations synchronized with colonic substrate concentration variations and host feeding rhythms, whereas Prevotella sp. MGM2’s pathway abundances lacked consistent temporal oscillations. This lack of temporal oscillations suggests that Prevotella sp. MGM2’s metabolic activity is less influenced by substrate concentration fluctuations and exhibits time-independent regulation of pathway activity. Conversely, Prevotella copri likely employs a rhythmic substrate-responsive metabolism, aligning its metabolic efficiency with spatiotemporal variations in substrate availability.
5 Conclusion
In summary, this study investigated the daily fluctuations of the Prevotella and its interaction with colonic nutrient substrates, revealing distinct correlation patterns: NH3-N and TP exhibited significant negative correlations with Prevotella, whereas cellulose and starch showed strong positive correlations. Notably, Prevotella copri displayed the highest abundance and most robust daily fluctuations, whereas Prevotella sp. MGM2 had relatively high abundance but lacked daily fluctuations. Comparative analysis demonstrated that Prevotella sp. MGM2 harbored higher gene abundances in COG categories Carbohydrate Transport and Metabolism and Amino Acid Transport and Metabolism, yet possessed fewer nutrient-substrates-related KEGG level-3 pathways and functionally annotated genes than Prevotella copri. These findings suggest that Prevotella species with different daily fluctuations may adopt different response strategies to nutrient substrates: Prevotella copri may employ a rhythmic substrate-responsive strategy, synchronizing its relative abundance, metabolic activity, and gene expression with temporal variations in colonic substrate concentrations; in contrast, Prevotella sp. MGM2 may adopt a sustained response strategy, characterized by stable metabolic activity independent of fluctuations in substrate concentrations.
Data availability statement
The datasets presented in this study are publicly available. This data can be found here: https://www.ncbi.nlm.nih.gov, accession number PRJNA843783.
Ethics statement
The animal study was approved by Animal Care and Use Committee of Nanjing Agricultural University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YuL: Conceptualization, Writing – review & editing, Methodology, Writing – original draft. JY: Methodology, Writing – original draft, Writing – review & editing. YiL: Writing – original draft, Data curation. DW: Writing – review & editing. HW: Writing – review & editing, Formal analysis, Methodology. YS: Writing – review & editing, Conceptualization, Funding acquisition, Project administration, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Key R&D Program of China (2023YFD1301304).
Acknowledgments
Thank you to Professor Su Yong and all personnel who participated in the experiment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^http://bio-bwa.sourceforge.net/bwa.shtml
2. ^http://www.ncbi.nlm.nih.gov/COG
References
Abdelsalam, N. A., Hegazy, S. M., and Aziz, R. K. (2023). The curious case of Prevotella copri. Gut Microbes 15:9152. doi: 10.1080/19490976.2023.2249152
Accetto, T., and Avguštin, G. (2021). Non-oral Prevotella stepping into the spotlight. Anaerobe 68:102321. doi: 10.1016/j.anaerobe.2021.102321
Alneberg, J., Bjarnason, B. S., de Bruijn, I., Schirmer, M., Quick, J., Ijaz, U. Z., et al. (2014). Binning metagenomic contigs by coverage and composition. Nat. Methods 11, 1144–1146. doi: 10.1038/nmeth.3103
Bolger, A. M., Lohse, M., and Usadel, B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. doi: 10.1093/bioinformatics/btu170
Chang, C. J., Lin, T. L., Tsai, Y. L., Wu, T. R., Lai, W. F., Lu, C. C., et al. (2019). Next generation probiotics in disease amelioration. J. Food Drug Anal. 27, 615–622. doi: 10.1016/j.jfda.2018.12.011
Chen, C., Fang, S., Wei, H., He, M., Fu, H., Xiong, X., et al. (2021). Prevotella copri increases fat accumulation in pigs fed with formula diets. Microbiome 9:175. doi: 10.1186/s40168-021-01110-0
Costa, M. C., Stämpfli, H. R., Arroyo, L. G., AllenVercoe, E., Gomes, R. G., and Weese, J. (2015). Changes in the equine fecal microbiota associated with the use of systemic antimicrobial drugs. BMC Vet. Res. 11:19. doi: 10.1186/s12917-015-0335-7
Dai, Z. L., Zhang, J., Wu, G., and Zhu, W. Y. (2010). Utilization of amino acids by bacteria from the pig small intestine. Amino Acids 39, 1201–1215. doi: 10.1007/s00726-010-0556-9
De Filippo, C., Cavalieri, D., Di Paola, M., Ramazzotti, M., Poullet, J. B., Massart, S., et al. (2010). Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. 107, 14691–14696. doi: 10.1073/pnas.1005963107
de Oliveira Melo, N. C., Cuevas Sierra, A., Souto, V. F., and Martínez, J. A. (2024). Biological rhythms, chrono nutrition, and gut microbiota: epigenomics insights for precision nutrition and metabolic health. Biomolecules 14:559. doi: 10.3390/biom14050559
Frazier, K., and Chang, E. B. (2020). Intersection of the gut microbiome and circadian rhythms in metabolism. Trends Endocrinol. Metab. 31, 25–36. doi: 10.1016/j.tem.2019.08.013
Fu, L., Niu, B., Zhu, Z., Wu, S., and Li, W. (2012). CD-HIT: accelerated for clustering the next generation sequencing data. Bioinformatics 28, 3150–3152. doi: 10.1093/bioinformatics/bts565
Gálvez, E. J. C., Iljazovic, A., Amend, L., Lesker, T. R., Renault, T., Thiemann, S., et al. (2020). Distinct polysaccharide utilization determines interspecies competition between intestinal Prevotella spp. Cell Host Microbe 28, 838–852.e6. doi: 10.1016/j.chom.2020.09.012
Gong, J., Zhang, Q., Hu, R., Yang, X., Fang, C., Yao, L., et al. (2024). Effects of Prevotella copri on insulin, gut microbiota and bile acids. Gut Microbes 16:487. doi: 10.1080/19490976.2024.2340487
Gu, N., Yan, J., Tang, W., Zhang, Z., Wang, L., Li, Z., et al. (2024). Prevotella copri transplantation promotes neurorehabilitation in a mouse model of traumatic brain injury. J. Neuroinflammation 21:147. doi: 10.1186/s12974-024-03116-5
Gutierrez Lopez, D. E., Lashinger, L. M., Weinstock, G. M., and Bray, M. S. (2021). Circadian rhythms and the gut microbiome synchronize the host’s metabolic response to diet. Cell Metab. 33, 873–887. doi: 10.1016/j.cmet.2021.03.015
Hao, Q. Y., Yan, J., Wei, J. T., Zeng, Y. H., Feng, L. Y., Que, D. D., et al. (2024). Prevotella copri promotes vascular calcification via lipopolysaccharide through activation of NF-κB signaling pathway. Gut Microbes 16:1532. doi: 10.1080/19490976.2024.2351532
Hooper, L. V., and Macpherson, A. J. (2010). Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 10, 159–169. doi: 10.1038/nri2710
Hughes, M. E., Hogenesch, J. B., and Kornacker, K. (2010). JTK_CYCLE: an efficient nonparametric algorithm for detecting rhythmic components in genome scale data sets. J. Biol. Rhythm. 25, 372–380. doi: 10.1177/0748730410379711
Iljazovic, A., Amend, L., Galvez, E. J. C., de Oliveira, R., and Strowig, T. (2021). Modulation of inflammatory responses by gastrointestinal Prevotella spp. – from associations to functional studies. Int. J. Med. Microbiol. 311:151472. doi: 10.1016/j.ijmm.2021.151472
Ismail, H. M., Perera, D., Mandal, R., DiMeglio, L. A., Evans Molina, C., Hannon, T., et al. (2025). Gut microbial changes associated with obesity in youth with type 1 diabetes. J. Clin. Endocrinol. Metab. 110, 364–373. doi: 10.1210/clinem/dgae529
Jiang, L., Shang, M., Yu, S., Liu, Y., Zhang, H., Zhou, Y., et al. (2022). A high-fiber diet synergizes with Prevotella copri and exacerbates rheumatoid arthritis. Cell. Mol. Immunol. 19, 1414–1424. doi: 10.1038/s41423-022-00934-6
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M., and Ishiguro Watanabe, M. (2023). KEGG for taxono my based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592. doi: 10.1093/nar/gkac963
Kanehisa, M., Furumichi, M., Tanabe, M., Sato, Y., and Morishima, K. (2017). KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 45, D353–D361. doi: 10.1093/nar/gkw1092
Kang, D. D., Froula, J., Egan, R., and Wang, Z. (2015). MetaBAT, an efficient tool for accurately reconstructing single genomes from complex microbial communities. PeerJ 3:e1165. doi: 10.7717/peerj.1165
Kovatcheva Datchary, P., Nilsson, A., Akrami, R., Lee, Y. S., De Vadder, F., Arora, T., et al. (2015). Dietary fiber induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 22, 971–982. doi: 10.1016/j.cmet.2015.10.001
Li, Z., Li, X., Liu, T., Chen, S., Liu, H., Wang, H., et al. (2019). The critical roles of exposed surface residues for the thermostability and halotolerance of a novel GH11 xylanase from the metagenomic library of a saline alkaline soil. Int. J. Biol. Macromol. 133, 316–323. doi: 10.1016/j.ijbiomac.2019.04.090
Li, D., Liu, C. M., Luo, R., Sadakane, K., and Lam, T. W. (2015). MEGAHIT: an ultra fast single node solution for large and complex metagenomics assembly via succinct de bruijn graph. Bioinformatics 31, 1674–1676. doi: 10.1093/bioinformatics/btv033
Li, D., Luo, R., Liu, C. M., Leung, C. M., Ting, H. F., Sadakane, K., et al. (2016). MEGAHIT v1.0: a fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods 102, 3–11. doi: 10.1016/j.ymeth.2016.02.020
Linares-Pastén, J. A., Hero, J. S., Pisa, J. H., Teixeira, C., Nyman, M., Adlercreutz, P., et al. (2021). Novel xylan degrading enzymes from polysaccharide utilizing loci of Prevotella copri DSM18205. Glycobiology 31:56. doi: 10.1093/glycob/cwab056
Lotti, S., Dinu, M., Colombini, B., Amedei, A., and Sofi, F. (2023). Circadian rhythms, gut microbiota, and diet: possible implications for health. Nutr. Metab. Cardiovasc. Dis. 33, 1490–1500. doi: 10.1016/j.numecd.2023.05.009
Ogata, H., Goto, S., Sato, K., Fujibuchi, W., Bono, H., and Kanehisa, M. (1999). KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 27, 29–34. doi: 10.1093/nar/27.1.29
Olm, M. R., Brown, C. T., Brooks, B., and Banfield, J. F. (2017). dRep: a tool for fast and accurate genomic comparisons that enables improved genome recovery from metagenomes through dereplication. ISME J. 11, 2864–2868. doi: 10.1038/ismej.2017.126
Parks, D. H., Imelfort, M., Skennerton, C. T., Hugenholtz, P., and Tyson, G. W. (2015). CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 25, 1043–1055. doi: 10.1101/gr.186072.114
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A., and Kingsford, C. (2017). Salmon provides fast and biasaware quantification of transcript expression. Nat. Methods 14, 417–419. doi: 10.1038/nmeth.4197
Reitmeier, S., Kiessling, S., Clavel, T., List, M., Almeida, E. L., Ghosh, T. S., et al. (2020). Arrhythmic gut microbiome signatures predict risk of type 2 diabetes. Cell Host Microbe 28, 258–272. doi: 10.1016/j.chom.2020.06.004
Rivera-Zavala, J. B., Báez Ruiz, A., and Díaz Muñoz, M. (2011). Changes in the 24 h rhythmicity of liver PPARs and peroxisomal markers when feeding is restricted to two daytime hours. PPAR Res. 2011, 1–11. doi: 10.1155/2011/261584
Samad, M., Agostinelli, F., Sato, T., Shimaji, K., and Baldi, P. (2022). CircadiOmics: circadian omic web portal. Nucleic Acids Res. 50, W183–W190. doi: 10.1093/nar/gkac419
Sieber, C. M. K., Probst, A. J., Sharrar, A., Thomas, B. C., Hess, M., Tringe, S. G., et al. (2018). Recovery of genomes from metagenomes via a dereplication, aggregation and scoring strategy. Nat. Microbiol. 3, 836–843. doi: 10.1038/s41564-018-0171-1
Su, J., Lin, X., Li, D., Yang, C., Lv, S., Chen, X., et al. (2024). Prevotella copri exhausts intrinsic indole 3-pyruvic acid in the host to promote breast cancer progression: inactivation of AMPK via UHRF1-mediated negative regulation. Gut Microbes 16:7757. doi: 10.1080/19490976.2024.2347757
Tatusov, R. L. (2000). The COG database: a tool for genome scale analysis of protein functions and evolution. Nucleic Acids Res. 28, 33–36. doi: 10.1093/nar/28.1.33
Tett, A., Huang, K. D., Asnicar, F., Fehlner Peach, H., Pasolli, E., Karcher, N., et al. (2019). The Prevotella copri complex comprises four distinct clades underrepresented in westernized populations. Cell Host Microbe 26, 666–679. doi: 10.1016/j.chom.2019.08.018
Tett, A., Pasolli, E., Masetti, G., Ercolini, D., and Segata, N. (2021). Prevotella diversity, niches and interactions with the human host. Nat. Rev. Microbiol. 19, 585–599. doi: 10.1038/s41579-021-00559-y
Thaiss, C. A., Zeevi, D., Levy, M., Zilberman Schapira, G., Suez, J., Tengeler, A. C., et al. (2014). Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159, 514–529. doi: 10.1016/j.cell.2014.09.048
Wang, H., Xia, P., Lu, Z., Su, Y., and Zhu, W. (2021). Metabolome microbiome responses of growing pigs induced by time restricted feeding. Front. Vet. Sci. 8:1202. doi: 10.3389/fvets.2021.681202
Wang, H., Xu, R., Li, Q., Su, Y., and Zhu, W. (2023). Daily fluctuation of colonic microbiome in response to nutrient substrates in a pig model. NPJ Biofilms Microbiomes 9:85. doi: 10.1038/s41522-023-00453-w
Wu, Y. W., Simmons, B. A., and Singer, S. W. (2016). MaxBin 2.0: an automated binning algorithm to recover genomes from multiple metagenomic datasets. Bioinformatics 32, 605–607. doi: 10.1093/bioinformatics/btv638
Wu, Y., and Singer, S. W. (2021). Recovering individual genomes from metagenomes using MaxBin 2.0. Curr. Protoc. 1:128. doi: 10.1002/cpz1.128
Xiao, L., Estellé, J., Kiilerich, P., Ramayo Caldas, Y., Xia, Z., Feng, Q., et al. (2016). A reference gene catalogue of the pig gut microbiome. Nat. Microbiol. 1:16161. doi: 10.1038/nmicrobiol.2016.161
Yang, C., Lan, R., Zhao, L., Pu, J., Hu, D., Yang, J., et al. (2024). Prevotella copri alleviates hyperglycemia and regulates gut microbiota and metabolic profiles in mice. mSystems 9:e0053224. doi: 10.1128/msystems.00532-24
Yuan, H., Wu, X., Wang, X., Zhou, J. Y., and Park, S. (2024). Microbial Dysbiosis linked to metabolic dysfunction associated fatty liver disease in Asians: Prevotella copri promotes lipopolysaccharide biosynthesis and network instability in the Prevotella Enterotype. Int. J. Mol. Sci. 25:2183. doi: 10.3390/ijms25042183
Zarrinpar, A., Chaix, A., Yooseph, S., and Panda, S. (2014). Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 20, 1006–1017. doi: 10.1016/j.cmet.2014.11.008
Keywords: daily fluctuation, nutrient substrate, porcine, Prevotella, gut microbiota
Citation: Li Y, You J, Liao Y, Wang D, Wang H and Su Y (2025) Daily fluctuation of genus Prevotella in porcine colon under ad libitum feeding and its association with nutrient substrates. Front. Microbiol. 16:1688301. doi: 10.3389/fmicb.2025.1688301
Edited by:
Lara Costantini, University of Tuscia, ItalyReviewed by:
Xiaowei Song, Chengdu University of Information Technology, ChinaXiongzhuo Tang, Hunan Agricultural University, China
Copyright © 2025 Li, You, Liao, Wang, Wang and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongyu Wang, MjAxOTIwNTAyOUBuamF1LmVkdS5jbg==; Yong Su, eW9uZy5zdUBuamF1LmVkdS5jbg==
†These authors have contributed equally to this work
 Yue Li
Yue Li Jinwei You
Jinwei You Yiyan Liao1
Yiyan Liao1 Hongyu Wang
Hongyu Wang Yong Su
Yong Su