- 1Department of Gastroenterology, Research Center for Engineering Techniques of Microbiota-Targeted Therapies of Guangdong Province, The First Affiliated Hospital of Guangdong Pharmaceutical University, Guangzhou, China
- 2Guangdong Provincial Key Laboratory for Research and Evaluation of Pharmaceutical Preparations, Guangzhou, China
- 3Guangdong Pharmaceutical University, Guangzhou, China
Autism Spectrum Disorder (ASD) is a severe neurodevelopmental disorder with an increasing global incidence, imposing substantial burdens on both society and affected families. The pathogenesis of ASD is complex, involving genetic, environmental, and other factors. Notably, children with ASD often exhibit gut microbiota dysbiosis, and the relationship between gut microbiota and ASD has garnered growing attention. Current treatments for ASD remain limited and unsatisfactory. As an emerging therapeutic approach, Washed Microbiota Transplantation (WMT) reduces undigested food residues, fungi, parasite eggs, and pro-inflammatory metabolites, thereby lowering the incidence of adverse clinical events. WMT also addresses ethical and aesthetic concerns associated with Fecal Microbiota Transplantation (FMT), enhances treatment safety, and offers new hope for ASD management. This review integrates global literature to analyze the latest findings on ASD epidemiology, societal impacts, existing therapies, and clinical research on WMT, aiming to provide scientific evidence for the clinical application of WMT in ASD treatment.
1 Introduction
Autism Spectrum Disorder (ASD) is a complex neurodevelopmental condition characterized by social communication deficits and restricted, repetitive patterns of behavior (American Psychiatric Association, 2022). Since Kanner’s first clinical description in 1943, ASD has gained global recognition (Kanner, 1943). Recent studies report an ASD prevalence of 0.7% in China, with over 10 million affected individuals and an annual increase of nearly 200,000 cases (Report on the Development Status of China's Autism Education and Rehabilitation Industry, 2024). According to the U.S. Centers for Disease Control and Prevention (2020), the childhood ASD prevalence in the U.S. rose from 1 in 44 in 2018 to 1 in 36 by 2020, while global estimates range between 2.3% and 2.76% (Hirota and King, 2023; Maenner et al., 2021; Maenner et al., 2023). The escalating prevalence of ASD has positioned it as a critical public health challenge worldwide.
Core symptoms of ASD include persistent deficits in social communication across multiple contexts and restricted, repetitive behaviors or interests (Zwaigenbaum and Penner, 2018). Beyond neurological abnormalities, ASD patients frequently present with gastrointestinal (GI) symptoms such as abdominal pain, bloating, diarrhea, constipation, and flatulence. These GI disturbances strongly correlate with ASD severity (Johnson et al., 2020). The gut, a microbial ecosystem, harbors diverse microbiota that interact closely with the host. Emerging evidence highlights the pivotal role of gut microbiota in regulating neurological functions and central nervous system (CNS)-associated behaviors (Johnson et al., 2020). Although the pathogenesis of ASD involves multifactorial interactions, including genetic and environmental triggers (Han et al., 2021), therapeutic options remain limited, underscoring the urgent need for effective interventions. Growing clinical evidence links ASD to gut microbiota dysbiosis, marked by reduced microbial diversity, diminished beneficial bacteria, and altered microbial composition in affected children (Dan et al., 2020; Wang M. et al., 2019). Fecal Microbiota Transplantation (FMT), a strategy to reconstitute gut microbiota, has emerged as a promising therapeutic avenue for ASD (Sharon et al., 2019). Washed Microbiota Transplantation (WMT), an advanced iteration of FMT, employs intelligent fecal microbiota processing systems to isolate, purify, and administer microbiota from healthy donors. By systematically removing harmful components through repeated washing, WMT improves safety, minimizes adverse effects, and ensures quality control compared to conventional FMT (Zhang et al., 2020). This review synthesizes current advancements in WMT for ASD treatment, aiming to inspire novel research directions and clinical applications.
2 The impact of ASD
2.1 Effects on patients
Individuals with ASD typically face challenges in social interaction and communication, which negatively affect their ability to initiate and sustain relationships (Chevallier et al., 2012). Restricted, repetitive behavioral patterns and narrow interests may limit daily functioning and learning capabilities, while impulsive behaviors further compromise quality of life (Wang et al., 2021). Differences in executive functioning and local processing abilities are common, impairing performance in theory of mind tasks (Demetriou et al., 2018). Comorbid conditions such as epilepsy, attention deficits, gastrointestinal disorders, oppositional behaviors, anxiety, depression, sleep disturbances, and eating disorders exacerbate the physical, emotional, and financial burden on patients and families (Kong and Chen, 2023). Additionally, social exclusion and stigmatization negatively impact self-identity, rehabilitation, and mental well-being (Kong and Chen, 2023).
2.2 Effects on families
The long-term costs of specialized education, rehabilitation therapies (e.g., speech training, behavioral interventions), and adaptive equipment impose significant financial strain. In the UK, lifetime costs for caring for a child with ASD exceed £920,000, while in the U. S. and China, these costs reach $1.4 million and ¥116,134.44, respectively (Buescher et al., n.d.; Zhao et al., 2021b). Behavioral challenges and communication barriers often lead to parental frustration, helplessness, and anxiety, with prolonged caregiving contributing to psychological fatigue and emotional burnout (Lee et al., 2024; Wu et al., 2024). Marital conflicts may arise due to disagreements over caregiving approaches and financial pressures, increasing divorce rates and straining sibling relationships (Karst and Van Hecke, 2012; Hartley et al., 2010; Ilias et al., 2019). Social stigma and embarrassment over atypical behaviors in public settings isolate families, reducing social engagement and access to support networks (Wu et al., 2024; Ilias et al., 2019; Li and Wang, 2015). Caregivers frequently sacrifice personal goals, career opportunities, and leisure activities, with one parent often leaving the workforce to provide full-time care, further impacting household income (Wu et al., 2024; Ilias et al., 2019). Persistent guilt and self-blame for their child’s condition also undermine parental mental health (Wu et al., 2024; Wong et al., 2015).
2.3 Societal implications
The rising prevalence of ASD places substantial economic burdens on families and society, including medical expenses, special education costs, and lost productivity due to caregiver absenteeism (Wu et al., 2024; Ilias et al., 2019). Adults with ASD often struggle with employment, perpetuating socioeconomic challenges (Fu and Chen, 2023). Educational systems face pressure to provide tailored environments and interventions to support ASD students’ learning and social integration. Public health infrastructure must prioritize early diagnosis, lifelong intervention services, and equitable access to care. Social participation and acceptance remain hindered by stigma and misconceptions, necessitating societal awareness campaigns and inclusive policies (Wu et al., 2024; Lan and Bai, 2020).
Key Recommendations: Public Awareness: Combat stigma through education to foster societal inclusion. Early Intervention: Strengthen diagnostic and therapeutic services to improve outcomes. Multidisciplinary Collaboration: Integrate medical, psychological, and educational expertise for holistic care. Policy Development: Implement public health strategies addressing prevention, early diagnosis, and lifelong support. Research Investment: Prioritize studies on ASD etiology, mechanisms, and innovative therapies.
3 Current treatment approaches for ASD and their limitations
3.1 Behavioral interventions
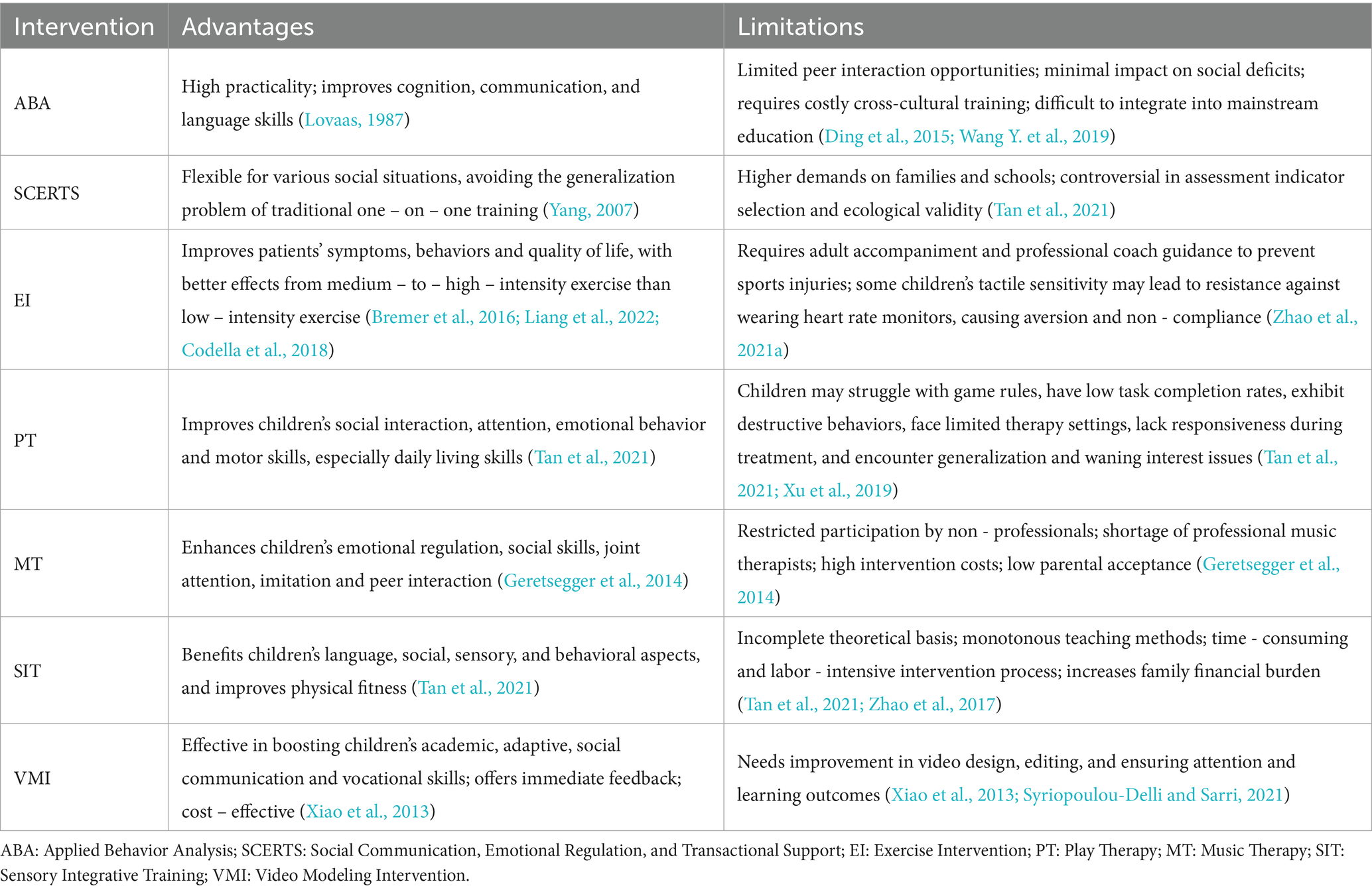
Early intervention is critical for ASD due to its early onset and better outcomes with younger age. Behavioral interventions remain a cornerstone of ASD management, with key methods and their characteristics as follows (see Table 1 for details):
ABA: High practicality in improving cognition and language, but limited in social deficit improvement and high cross-cultural training costs (Lovaas, 1987; Ding et al., 2015; Wang Y. et al., 2019).
SCERTS: Flexible for social scenarios but demands high on families/schools (Yang, 2007; Tan et al., 2021).
EI: Medium-to-high intensity exercise better improves symptoms but requires professional guidance (Bremer et al., 2016; Liang et al., 2022; Zhao et al., 2021a).
PT/MT/SIT/VMI: Each has advantages in specific domains (e.g., MT for emotional regulation, VMI for cost-effectiveness) but faces limitations like limited settings or professional shortages (Tan et al., 2021; Geretsegger et al., 2014; Xiao et al., 2013; Xu et al., 2019; Zhao et al., 2017; Syriopoulou-Delli and Sarri, 2021).
Overall, behavioral interventions require long-term evaluation, and their accessibility is restricted by resource scarcity and cost, highlighting the need for complementary strategies.
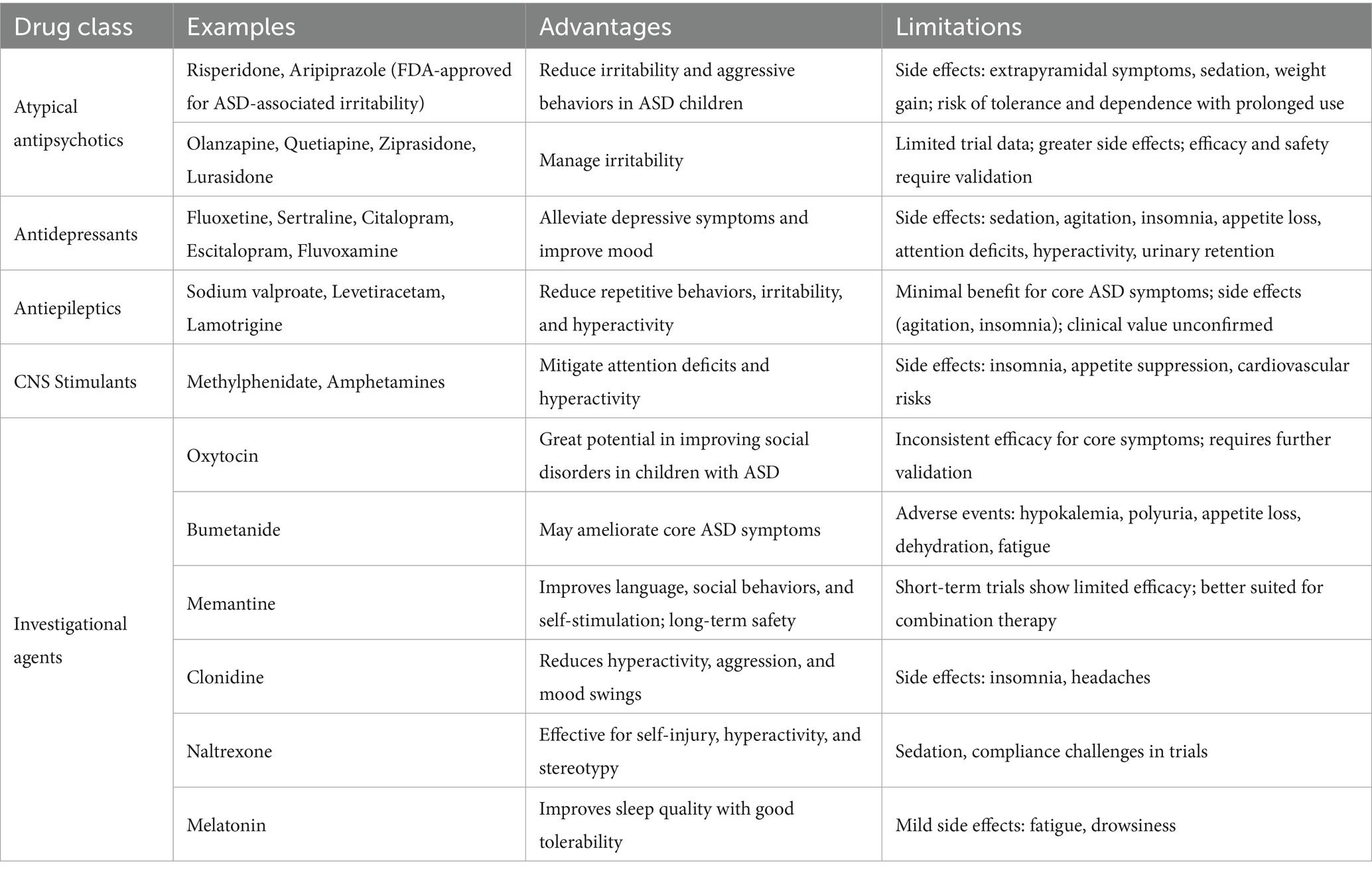
3.2 Pharmacological interventions
Drug treatment mainly targets comorbidiasis symptoms of ASD. For instance, risperidone and aripiprazole have been approved by the FDA for the treatment of irritability symptoms related to ASD (Stepanova et al., 2017; Shafiq and Pringsheim, 2018). However, there is currently no drug that can effectively improve the core symptoms of ASD, and long-term use may cause significant side effects, limiting their clinical application (Sharma et al., 2018; Stepanova et al., 2017; Shafiq and Pringsheim, 2018; Persico et al., 2021; Yan et al., 2022; Kanner and Bicchi, 2022; Alvares et al., 2017; Lemonnier et al., 2017; Howes et al., 2018; Ming et al., 2008; Elchaar et al., 2006; Malow et al., 2021; Xu and Wang, 2018) (see Table 2 for details).
3.3 Traditional Chinese medicine (TCM) approaches
TCM attributes ASD to brain dysfunction linked to kidney/spleen/liver/heart imbalances, with interventions including herbal medicine, acupuncture, and tuina. Limitations include low pediatric compliance (bitter herbs, needle phobia) and insufficient large-scale trials (Yan et al., 2022; Zhang et al., 2023). Notably, TCM’s historical use of “Jin Zhi” (golden juice, a fecal-based medicine) represents an early precursor to modern microbiota transplantation (Shi and Yang, 2017; Zhang et al., 2012; Xu et al., 2017), aligning with the gut-brain axis theory and providing a historical context for WMT’s development.
4 Research progress on WMT for ASD
Autism Spectrum Disorder (ASD) arises from complex interactions between genetic and environmental factors, with gut microbiota dysbiosis emerging as a key environmental contributor. The human gut hosts 10–100 trillion microorganisms, including bacteria, viruses, protozoa, and fungi, collectively termed the gut microbiota. ASD patients exhibit gut dysbiosis characterized by pathogenic overgrowth and metabolic disturbances. Studies report that 9–91% of ASD patients experience gastrointestinal (GI) symptoms such as bloating, diarrhea, constipation, and foul-smelling stools (Moradi et al., 2021; Holingue et al., 2018), often accompanied by esophagitis, gastritis, or lymphocytic infiltration, with approximately half suffering from alternating diarrhea and constipation (Duan et al., 2015). Notably, alleviating GI symptoms correlates with reduced ASD severity (Chen and Chiu, 2022). Recent evidence underscores the role of gut microbiota in ASD pathogenesis, with interventions like probiotics and Fecal Microbiota Transplantation (FMT) showing therapeutic potential (Strati et al., 2017; Kang et al., 2017; Kang et al., 2019). Unlike probiotics, which deliver single bacterial strains, FMT introduces ~1,000 native gut bacterial species, almost full coverage of all strains has been achieved. However, Washed Microbiota Transplantation (WMT), an advanced FMT technique, enhances safety by removing undigested residues, fungi, parasite eggs, and pro-inflammatory metabolites, thereby significantly reducing the incidence of clinical adverse reaction events, while addressing ethical and aesthetic concerns associated with traditional FMT (Zhang et al., 2020; Shi, 2020).
4.1 Principles of WMT
WMT a new stage in the development of FMT involves transferring fecal microbiota from healthy donors into a patient’s gastrointestinal tract through various methods, aiming to reconstruct the patient’s gut microbiota and thereby treat intestinal and systemic diseases. The human microbiota refers to the collective community of microorganisms residing on and within the human body, evolving from the concept of “normal human flora.” These microbial communities are most densely distributed in mucosal organs, such as the oral cavity and intestines (Integrative HMP (iHMP) Research Network Consortium, 2019; Lloyd-Price et al., 2016). The gut microbiota, often termed the “second genome” of the human body, harbors vast numbers of microorganisms, including bacteria, fungi, and others. Studies estimate that approximately 70% of the human microbiota resides in the colon (Ley et al., 2006), with significant individual variations. Research has linked these variations to differences in human health and disease manifestations (Garcia et al., 2022). Gut microbiota plays a critical role in host health, contributing to nutrient absorption, metabolic regulation, intestinal epithelial development, and the induction of innate immunity. Its functions are comparable to those of a vital organ. By transplanting healthy gut microbiota, the intestinal microecology of patients can be modulated to improve immune and neurological functions, thereby achieving therapeutic goals for various diseases.
4.2 Preparation and administration methods of fecal material
The entire process—from the acquisition and preparation of fecal microbiota to its safe delivery and functional integration into the intestines—requires stringent control. Efforts focus on identifying optimal fecal donors, developing preservation methods that maximize therapeutic benefits for patients, and selecting the most suitable administration routes to ensure the microbiota exerts its full effect post-transplantation. For WMT, fecal material is primarily sourced from rigorously screened healthy donors. Donors undergo comprehensive evaluations, including gastrointestinal pathogen screening, blood tests, and mental health assessments, to ensure the fecal material does not transmit infectious diseases to recipients (Shi, 2020). Subsequently, in high-standard laboratory settings, the fecal microbiota is isolated using an intelligent fecal microbiota separation system. This involves steps such as hydration, homogenization, filtration, centrifugation, and sedimentation to obtain purified bacterial preparations (Shi, 2020). Purified microbiota can be preserved via freezing, refrigeration, or specialized storage solutions for sample collection, storage, and transportation under ambient conditions (Shi, 2020). Microcrystalline cellulose particles, a free-flowing material, are employed as adsorbents to concentrate and filter fresh fecal samples. This method maintains microbial viability and diversity even in dry environments. Clinically common WMT administration routes target the upper or lower gastrointestinal tract. Methods include delivery via nasogastric/nasojejunal tubes, oral capsules, esophagogastroduodenoscopy, colonoscopy, enema, or endoscopic techniques (e.g., transendoscopic enteral tubing or percutaneous endoscopic cecostomy). The optimal method is selected based on the patient’s tolerance, disease location, and clinical characteristics.
4.3 Clinical research on WMT for ASD
Currently, FMT is not only used to treat gastrointestinal diseases such as inflammatory bowel disease, irritable bowel syndrome, functional constipation, and cirrhosis but has also been applied to neuropsychiatric disorders (e.g., ASD, anxiety, depression, and Parkinson’s disease), metabolic diseases (e.g., diabetes, obesity, fatty liver, and hyperlipidemia), and immune-related conditions (e.g., cancer immunotherapy, allergic diseases, and chronic fatigue syndrome), demonstrating promising clinical efficacy (Ye et al., 2020). WMT traces its origins to traditional Chinese medicine (TCM). The earliest documented use of human feces as medicine dates back to Wushier Bingfang (Fifty-Two Disease Formulas), a medical text from the Western Zhou or Spring and Autumn period (Shi and Yang, 2017). Ge Hong’s Zhouhou Beiji Fang (Handbook of Prescriptions for Emergencies) from the Eastern Jin Dynasty records the therapeutic use of fecal solutions: “Drinking one liter of fecal juice revives the patient” (Zhang et al., 2012). Li Shizhen’s Compendium of Materia Medica (Ben Cao Gang Mu) from the Ming Dynasty describes over 20 medicinal formulations utilizing human feces. During the Ming and Qing dynasties, fecal-based treatments became widely employed for epidemic febrile diseases. Notably, the physician Ye Tianshi documented cases using fecal preparations to treat such conditions. As febrile diseases often involved heat toxins affecting the gastrointestinal tract, Jin Zhi (golden juice) was frequently used with remarkable efficacy. Its preparation involved collecting feces from healthy boys aged 11–12 during winter, mixing it with mountain spring or well water, filtering, and burying the mixture in a sealed jar for over a year. The resulting clear, water-like liquid was termed Jin Zhi (Xu et al., 2017). In modern times, the use of Jin Zhi declined due to the diversification of TCM practices and the introduction of Western medicine. However, in 2012, Professor Zhang Faming from the Second Affiliated Hospital of Nanjing Medical University pioneered standardized modern fecal microbiota transplantation in China. Using advanced laboratory equipment, his team developed a refined method to isolate highly purified microbiota (now termed WMT), which is regarded as the “new Jin Zhi” (Shi, 2020).
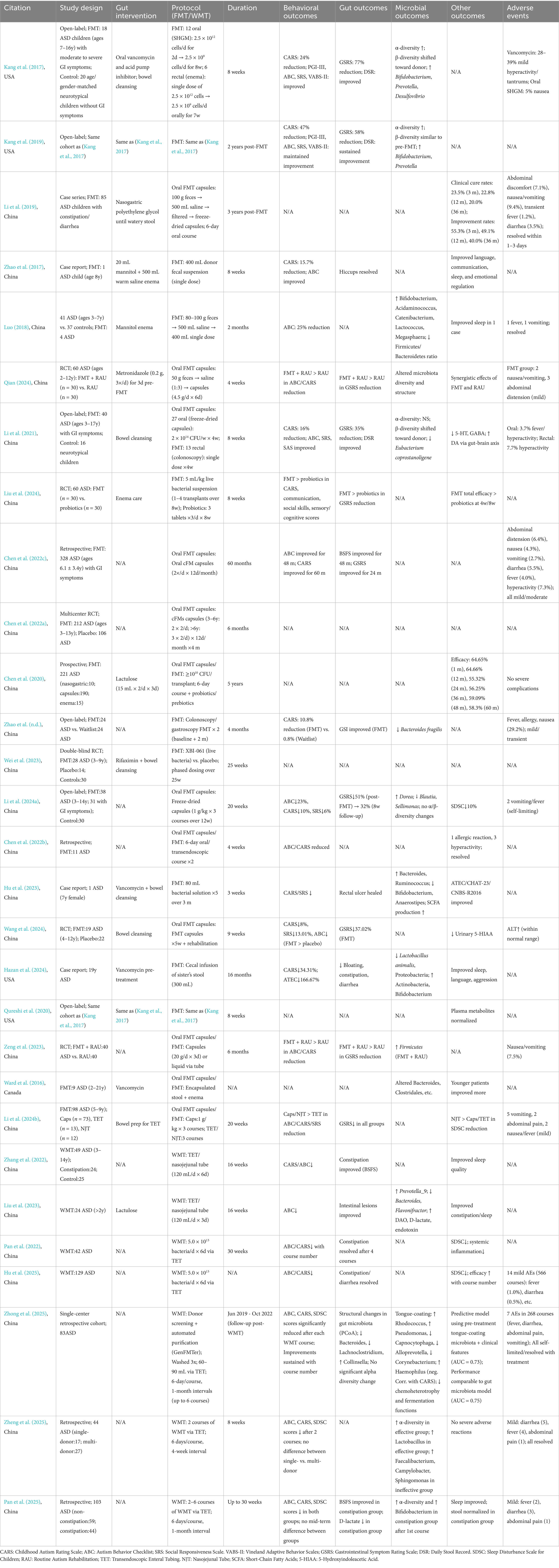
As an emerging therapeutic approach, WMT has garnered significant attention in recent years for treating autism spectrum disorder (ASD), with a growing body of clinical studies specifically investigating its use. These studies, summarized in Table 3, suggest that WMT can alleviate core behavioral symptoms, gastrointestinal distress, and comorbidities in autistic patients (Kang et al., 2017; Kang et al., 2019; Li et al., 2019; Zhao et al., 2017; Luo, 2018; Qian, 2024; Li et al., 2021; Liu et al., 2024; Chen et al., 2022c; Chen et al., 2022a; Chen et al., 2020; Zhao et al., n.d.; Wei et al., 2023; Li et al., 2024a; Chen et al., 2022b; Hu et al., 2023; Wang et al., 2024; Hazan et al., 2024; Qureshi et al., 2020; Zhang et al., 2022; Liu et al., 2023; Pan et al., 2022; Li et al., 2022; Zeng et al., 2023; Ward et al., 2016; Li et al., 2024b; Hu et al., 2025) (see Table 3). Multiple studies on the efficacy and safety of WMT have shown that, multiple studies demonstrate that WMT significantly improves autism-like behaviors, sleep disturbances, stool consistency, gastrointestinal symptoms, and systemic inflammation in ASD patients (Pan et al., 2022). Concurrently, WMT shifts the gut microbiota and metabolic profiles of ASD patients toward beneficial directions. Research methodologies have evolved from open-label designs to multicenter, randomized, double-blind, placebo-controlled trials, enhancing rigor and comprehensiveness. For instance at Peking Union Medical College Hospital, over 100 children with ASD showed marked improvement following FMT-based therapy, with an efficacy rate nearing 80% (Li et al., 2022), and no severe complications were observed during treatment. In terms of the impact of WMT on the Gut Microbiota, Post-WMT, ASD patients exhibit increased gut microbiota diversity, aligning closer to donor profiles (Kang et al., 2017; Kang et al., 2019; Hu et al., 2023). Notably, FMT elevates the relative abundance of Bifidobacterium and Prevotella in the gut, both of which may confer therapeutic benefits for ASD (Kang et al., 2017; Kang et al., 2019). Long-Term Efficacy, studies indicate sustained effects of FMT for up to 2 years. Kang et al. (2019) reported persistent improvements in ASD and gastrointestinal symptoms over a two-year follow-up. Li Ning et al. followed 85 ASD cases for 3 years, observing a 78.8% response rate at 6 months, 71.9% at 1 year, and 60% efficacy at 3 years, with over 20% achieving clinical cure (Li et al., 2019). Chen et al. (2022c) and Chen et al. (2022a) documented maintained therapeutic effects on ABC (Autism Behavior Checklist) and BSFS (Bristol Stool Form Scale) scores for 48 months, with results comparable to initial outcomes at 60 months. Chen et al. (2020) reported efficacy in 7 out of 12 ASD patients during a 60-month follow-up. Among the adverse events of WMT, most adverse events during WMT are mild to moderate, including abdominal distension, nausea, vomiting, diarrhea, fever, and hyperactivity. These resolved with temporary treatment suspension or symptomatic management, with no severe adverse events reported. Intriguingly, Ward et al. (2016) found WMT more effective in younger ASD patients, while older children showed limited responses. Family donor microbiota may play a critical role in outcomes for older patients (Hazan et al., 2024), warranting further exploration. This age-dependent efficacy may stem from the immature gut microbiota structure in younger individuals, which is more susceptible to external modulation. Studies highlight enhanced efficacy when WMT is combined with routine rehabilitation training (RAU), surpassing RAU alone in alleviating clinical and gastrointestinal symptoms (Qian, 2024; Zeng et al., 2023). This underscores WMT’s potential to restore gut microbial balance and ameliorate ASD manifestations. Additionally, WMT demonstrated superior efficacy compared to probiotic treatments in ASD children (Liu et al., 2024). In terms of metabolite changes in WMT, Post-WMT, plasma metabolite profiles in ASD children align more closely with those of neurotypical children (Qureshi et al., 2020). WMT also significantly reduces urinary 5-HIAA (5-hydroxyindoleacetic acid) levels (Wang et al., 2024), regulate the intestinal flora by reducing toxic metabolites and enhancing detoxification effects (Liu et al., 2023), suggesting positive impacts on metabolic health. In terms of the WMT treatment course, it was found that WMT could improve ASD symptoms, gastrointestinal symptoms and sleep disorders, and multiple treatments could bring more significant improvements (Pan et al., 2022). Six treatments could bring significant improvements, and patients with gastrointestinal symptoms before treatment had better therapeutic effects (Hu et al., 2025), indicating that the number of WMT treatment courses also has a positive effect on children with ASD.
In the simple treatment of ASD with WMT, Pan et al. (2022) found that WMT significantly improved the core symptoms of ASD (ABC and CARS scores decreased), sleep disorders (SDSC scores decreased), and gastrointestinal symptoms (the proportion of constipation decreased, and the shape of stool improved). The number of treatment sessions was positively correlated with the therapeutic effect: as the number of WMT sessions increased (especially within 3 sessions), the ABC and SDSC scores further decreased. Systemic inflammatory indicators (WBC, globulin) also significantly decreased. It was concluded that WMT could significantly improve the symptoms, sleep and gastrointestinal problems of ASD, and that multiple treatments were superior to single treatments. Zhang et al. (2022). found that the SDSC score of the constipation group significantly improved after the second WMT (p = 0.026), and sleep quality improved. The improvement in stool shape (BSFS) was synchronous with the improvement in sleep. The non-constipation group did not show a deterioration in sleep, indicating that WMT was safe. It was concluded that WMT had a significant effect on improving sleep in children with ASD accompanied by constipation, and did not cause deterioration of other children’s symptoms. Liu et al. (2023) found that WMT significantly improved sleep disorders and constipation, with decreased ABC and SDSC scores. The bacterial structure changed: Prevotella_9 was upregulated, and Bacteroides/Flavonifractor/Parasutterella were downregulated. Metabolic pathway analysis showed that WMT regulated pathways such as glycolysis, fatty acid β-oxidation, and L-1,2-propanediol degradation to reduce toxic metabolites and enhance detoxification ability. It was concluded that WMT improved the sleep and behavioral symptoms of ASD children through regulating the intestinal microbiota and its metabolites. Hu et al. (2025) found that WMT significantly improved the symptoms, sleep disorders and GI symptoms (constipation, diarrhea, abnormal stool shape) of ASD. The 6-session WMT course was the most effective, and the therapeutic effect improved with the increase in the course. Multiple regression analysis showed that children with diarrhea, abnormal stool shape, long disease course, no other treatment, and severe conditions had a better response to WMT. It was concluded that WMT had a significant effect on ASD children, that GI symptoms could be used as a predictive indicator of therapeutic effect, and that multiple treatments could further enhance the therapeutic effect. The benefits of WMT for ASD children from different perspectives such as therapeutic effect, sleep, bacterial metabolism, and GI symptoms were systematically demonstrated, and it was unanimously concluded that: WMT was safe and effective, could significantly improve the core symptoms, sleep disorders and gastrointestinal problems of ASD; the more treatments, the better the effect; at least 3–6 sessions of treatment were recommended; changes in intestinal microbiota structure and metabolites were the potential mechanism; children with GI symptoms (such as constipation, diarrhea) may benefit more. Zheng et al. (2025) found that among 83 ASD patients, the ABC, CARS, and SDSC scores significantly decreased as the WMT treatment progressed. This indicates that WMT can effectively improve the social impairments, repetitive behaviors, emotional problems, and sleep quality of ASD children. During 268 WMT treatments, only 7 minor adverse reactions (such as fever and diarrhea) occurred, and no serious adverse events were observed. After WMT, the intestinal microbiota structure significantly changed. Bacteroides and Lachnoclostridium (positively correlated with the severity of ASD) significantly decreased. Collinsella (a beneficial bacterium) significantly increased. Changes in tongue coating microbiota: Haemophilus (negatively correlated with the severity of ASD) significantly increased. Capnocytophaga, Alloprevotella, and Corynebacterium (positively correlated with ASD symptoms) decreased. The tongue coating microbiota structure was more similar to that of typical developing (TD) children. Functional prediction: The functions related to chemosynthetic heterotrophy and fermentation in the tongue coating microbiota significantly decreased after WMT. In the WMT-treated germ-free ASD mice: social behaviors improved and repetitive behaviors were reduced. The levels of Th17 cells in the brain and pro-inflammatory cytokines (such as TNF-α, IL-1β, and IL-6) were decreased. The intestinal barrier function was improved (increased villus height and reduced crypt depth). It is indicated that healthy microbiota transplantation can improve ASD behaviors and neuroinflammation by regulating the gut-brain axis. Zheng et al. (2025) found that after two courses of WMT treatment for 44 ASD children, the ABC, CARS, and SDSC scores significantly decreased (p < 0.05), and the core symptoms and sleep quality improved. There was no significant difference in efficacy between the single-donor and multi-donor groups after two courses of treatment, although there were differences in ABC and SDSC scores after the first course (p = 0.049, p = 0.019). The alpha diversity of the intestinal microbiota significantly increased in the effective group, and Lactobacillus was enriched in the effective group, while Faecalibacterium, Campylobacter, and Sphingomonas dominated in the ineffective group. It was concluded that single-donor and multi-donor WMT have equivalent efficacy in ASD treatment, supporting the use of the multi-donor strategy in clinical practice to address the issue of donor availability, and emphasizing the correlation between microbiota diversity and efficacy. Pan et al. (2025) found that WMT significantly improved ABC, CARS, and SDSC scores regardless of whether constipation was present (p < 0.05). In the constipation group, the ABC score improved more slowly in the first two courses of treatment, but there was no difference with the non-constipation group after the third course. After WMT, the diversity of the intestinal microbiota significantly increased in the constipation group, and the relative abundance of Bifidobacterium increased, while the D-lactic acid level decreased, suggesting improvement in intestinal barrier function. It was concluded that constipation does not affect the mid-term efficacy of WMT, and WMT can simultaneously improve ASD core symptoms and constipation problems, especially by promoting the abundance of beneficial bacteria such as Bifidobacterium, promoting intestinal health.
In summary, WMT improves ASD symptoms, enhances gut microbiota diversity, and elevates quality of life, with sustained therapeutic effects. Most studies affirm its safety profile, characterized by mild, reversible adverse events and no severe complications. As a novel strategy, WMT exhibits promising potential for ASD treatment, supported by robust safety and long-term efficacy data. Future research should elucidate WMT’s mechanisms, optimize protocols, and establish standardized workflows to maximize therapeutic outcomes and safety.
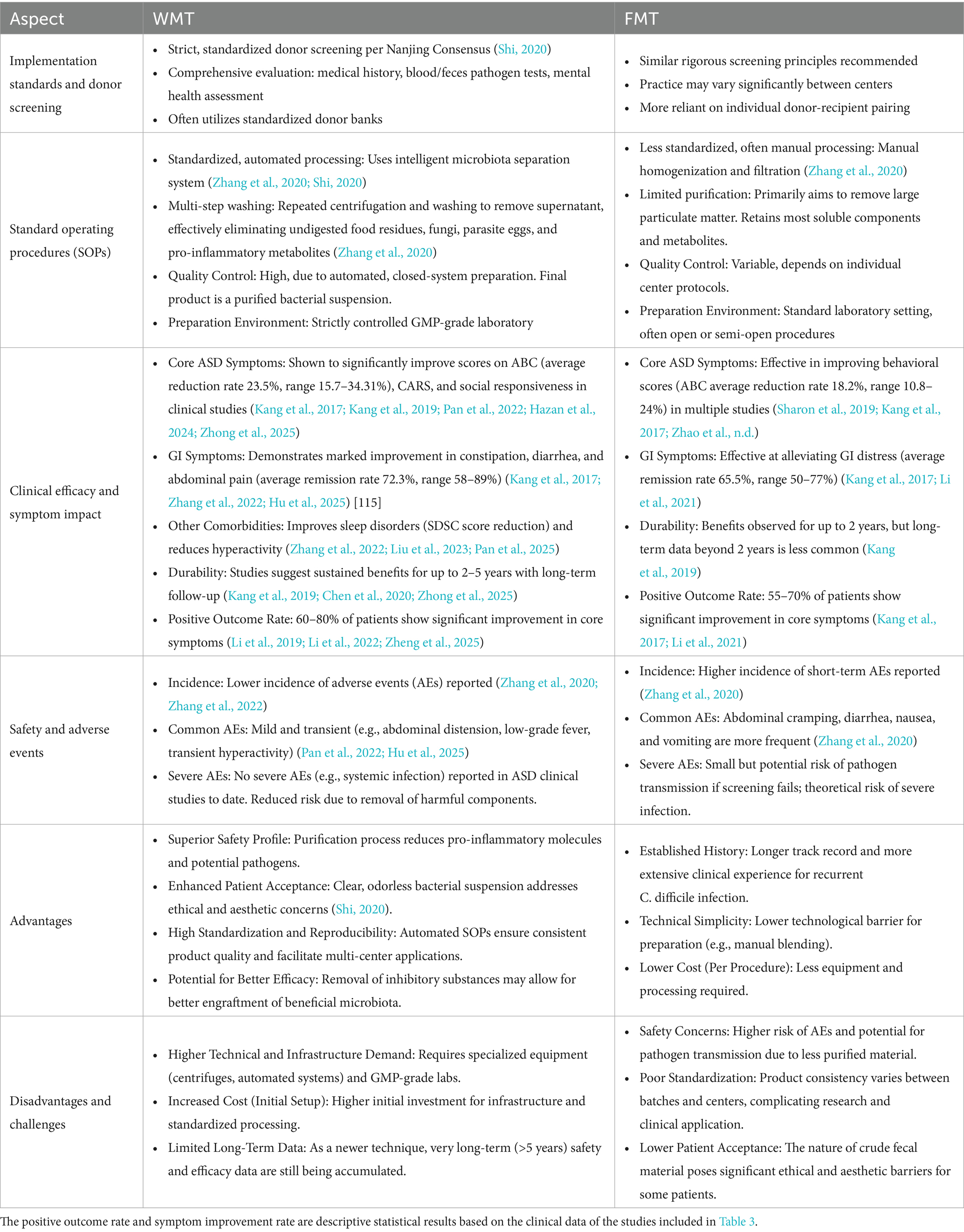
4.3.1 Comparison between WMT and FMT
WMT represents a significant technological advancement over traditional FMT. Table 4 provides a structured comparison between WMT and FMT regarding implementation standards, standard operating procedures (SOPs), clinical efficacy, and advantages and disadvantages.
4.4 Mechanisms of WMT in treating ASD
The precise mechanisms by which WMT alleviates autism spectrum disorder (ASD) remain unclear. As an advanced form of FMT, WMT’s therapeutic effects are primarily attributed to the transplantation of a purified, diverse, and functional microbial community, which may exert more potent and targeted modulation of the “microbiota-gut-brain axis” compared to probiotics or conventional FMT. Current hypotheses suggest that gut microbiota and their metabolites may influence the central nervous system through metabolic pathways, vagus nerve activation, and immune modulation, forming a “microbiota-gut-brain axis” that impacts the onset and progression of ASD (Sharon et al., 2016; Cryan et al., 2019).
ASD patients often exhibit metabolic dysregulation, and gut microbiota can regulate gene expression and neuronal function. Studies indicate that microbial-derived short-chain fatty acids (SCFAs), particularly butyrate and propionate, modulate the expression of genes such as FMR1 and Neurexins, potentially improving ASD symptoms (Kang et al., 2017; Qureshi et al., 2020; Hsiao et al., 2013). Gut microbes also synthesize neurotransmitters, including serotonin (5-HT), dopamine, and γ-aminobutyric acid (GABA), which directly affect central nervous system activity (Yano et al., 2015). For instance, probiotics may enhance the conversion of tryptophan to serotonin, mitigating neuroinflammation (Fattorusso et al., 2019).
The vagus nerve connects the brain with the gastrointestinal tract. The metabolites of the intestine can activate it and affect the central nervous system, thereby participating in the onset of ASD (Cryan et al., 2019). 5-hydroxytryptamine and short-chain fatty acids activate the vagus nerve, regulate oxytocin secretion, and improve the social behaviors of ASD (Sgritta et al., 2019). Notably, Lactobacillus reuteri can improve the social deficits in ASD mice, possibly related to oxytocin (Sgritta et al., 2019). Moreover, the vagus nerve regulates the function of microglia, and abnormalities in this regulation may lead to ASD phenotypes. The gut microbiota and its metabolites also affect brain function and mood (Erny et al., 2015).
Microorganisms form the intestinal barrier, and their imbalance can lead to reduced permeability, triggering immune activation and inflammation, which may be related to ASD (Vuong and Hsiao, 2017). The Th17 cells and IL-17 produced by maternal immune activation during pregnancy may increase the risk of ASD in offspring (Cryan et al., 2019; Vuong and Hsiao, 2017). Short-chain fatty acids regulate microglia and participate in neuroinflammation, which may be one of the causes of ASD (Erny et al., 2015; Silva et al., 2020).
WMT, by providing a purified and diverse consortium of beneficial microbes, may more effectively restore gut microbial balance, reduce neuroinflammation, and improve ASD symptoms by targeting these pathways compared to probiotics or conventional FMT. By modulating the microbiota-gut-brain axis, WMT holds promise as a novel therapeutic strategy to address both gastrointestinal and behavioral manifestations of ASD. Future research is needed to fully elucidate these mechanisms and optimize treatment protocols (Fattorusso et al., 2019; Kang et al., 2017).
4.4.1 WMT treats ASD by regulating gut microbiota homeostasis
Probiotics such as Bifidobacterium and Lactobacillus can inhibit the growth of harmful bacteria by modulating intestinal pH. Their surface protein components specifically bind to intestinal epithelial cells, enhancing gut barrier function. Probiotics also stimulate the intestinal immune system, promoting immune cells to secrete the anti-inflammatory cytokine IL-10 while suppressing the pro-inflammatory factor TNF-α, thereby alleviating intestinal inflammation and improving ASD symptoms (Fattorusso et al., 2019). In terms of neurotransmitter regulation, probiotics modulate microbial metabolism in the gut, influence tryptophan metabolic pathways, increase serotonin synthesis, and ameliorate neurodevelopmental disorder symptoms (Yano et al., 2015). Prebiotics are selectively utilized by beneficial gut bacteria. Through metabolic activities, these bacteria convert prebiotics into short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate (Silva et al., 2020). SCFAs provide energy for intestinal epithelial cells, enhance the expression of tight junction proteins, and strengthen the gut barrier (Silva et al., 2020). Additionally, SCFAs act on the brain via the vagus nerve or bloodstream to regulate neurotransmitter levels, improving behavioral symptoms in ASD patients (Cryan et al., 2019; Silva et al., 2020). Synbiotics, combinations of probiotics and prebiotics, exemplify synergistic effects. For instance, synbiotics containing Bifidobacterium and galactooligosaccharides allow prebiotics to nourish probiotics, promoting their colonization and growth. Probiotics regulate immunity and improve gut barrier function, while prebiotic-derived SCFAs amplify these effects, collectively restoring gut microbiota homeostasis and positively impacting ASD symptoms (Fattorusso et al., 2019).
WMT (Microbiota Transplantation) transfers gut microbiota from healthy donors to ASD patients. After colonization, these microbiota reshape the structure and function of the patient’s microbial community. Beneficial bacteria produce SCFAs to suppress harmful bacteria, reducing pathogens and their toxic metabolites like lipopolysaccharides (LPS) (Vuong and Hsiao, 2017). Decreased LPS levels mitigate systemic inflammation and neurological damage (Vuong and Hsiao, 2017). Concurrently, microbiota alterations influence neurotransmitter synthesis and metabolism, modulating neural signaling to alleviate ASD manifestations (Sharon et al., 2016; Yano et al., 2015). Bacteriophages, with host specificity, enable precise targeting of harmful bacteria. For example, phages targeting Escherichia coli specifically recognize and lyse these pathogens, restoring microbial balance. Reduced harmful bacteria improve intestinal homeostasis, lower inflammation, and subsequently ameliorate ASD-related symptoms (Vuong and Hsiao, 2017).
4.4.2 Mechanisms of WMT in treating ASD through gut microbiota modulation
Gut microbiota regulate the expression of tight junction proteins (e.g., ZO-1, Occludin, Claudin) in intestinal epithelial cells. Beneficial bacteria enhance tight junction protein synthesis and assembly via metabolites such as butyrate, strengthening intercellular connections and preventing harmful substances from entering the bloodstream (Sharon et al., 2016; Vuong and Hsiao, 2017). Additionally, gut microbiota stimulate goblet cells to secrete mucins, forming a protective mucus layer that reinforces the intestinal barrier (Sharon et al., 2016). By maintaining barrier integrity, the impact of harmful substances on the nervous system is reduced, alleviating ASD symptoms (Vuong and Hsiao, 2017). Gut microbiota modulate the activity of antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). Beneficial bacteria activate signaling pathways in host cells to upregulate antioxidant enzyme gene expression and synthesis. These enzymes scavenge excess reactive oxygen species (ROS), mitigate oxidative stress damage, protect neurons from oxidative injury, and improve neural function, thereby ameliorating ASD-related manifestations (Li and Zhou, 2016). Gut microbiota closely interact with the immune system by activating immune cells in gut-associated lymphoid tissue (GALT). Beneficial bacteria bind to pattern recognition receptors (PRRs, e.g., Toll-like receptors [TLRs]) on immune cells, triggering signaling pathways that induce cytokine secretion (Sharon et al., 2016; Vuong and Hsiao, 2017). Th1 cells release IFN-γ to enhance immune defense, while Th2 cells produce IL-4, IL-5, and other cytokines to regulate humoral immunity. This balanced immune response reduces neuroinflammatory disturbances and improves ASD symptoms (Fattorusso et al., 2019; Vuong and Hsiao, 2017). Gut dysbiosis exacerbates inflammation, but microbiota modulation counteracts this. Beneficial bacteria suppress pro-inflammatory factors (e.g., TNF-α, IL-6, IL-1β) and promote anti-inflammatory IL-10 secretion (Fattorusso et al., 2019). In inflammatory signaling pathways, lipopolysaccharides (LPS) from harmful bacteria activate the NF-κB pathway, driving cytokine release. Beneficial bacteria and their metabolites inhibit NF-κB activation, block inflammatory signaling cascades, and attenuate both intestinal and systemic inflammation, leading to symptom improvement in ASD patients (Hsiao et al., 2013; Vuong and Hsiao, 2017) (Figure 1).(This revised figure integrates pathways from the original Figures 1, 2, highlighting key mediators like SCFAs, neurotransmitters, immune factors, and the vagus nerve, and indicating how WMT may restore balance).
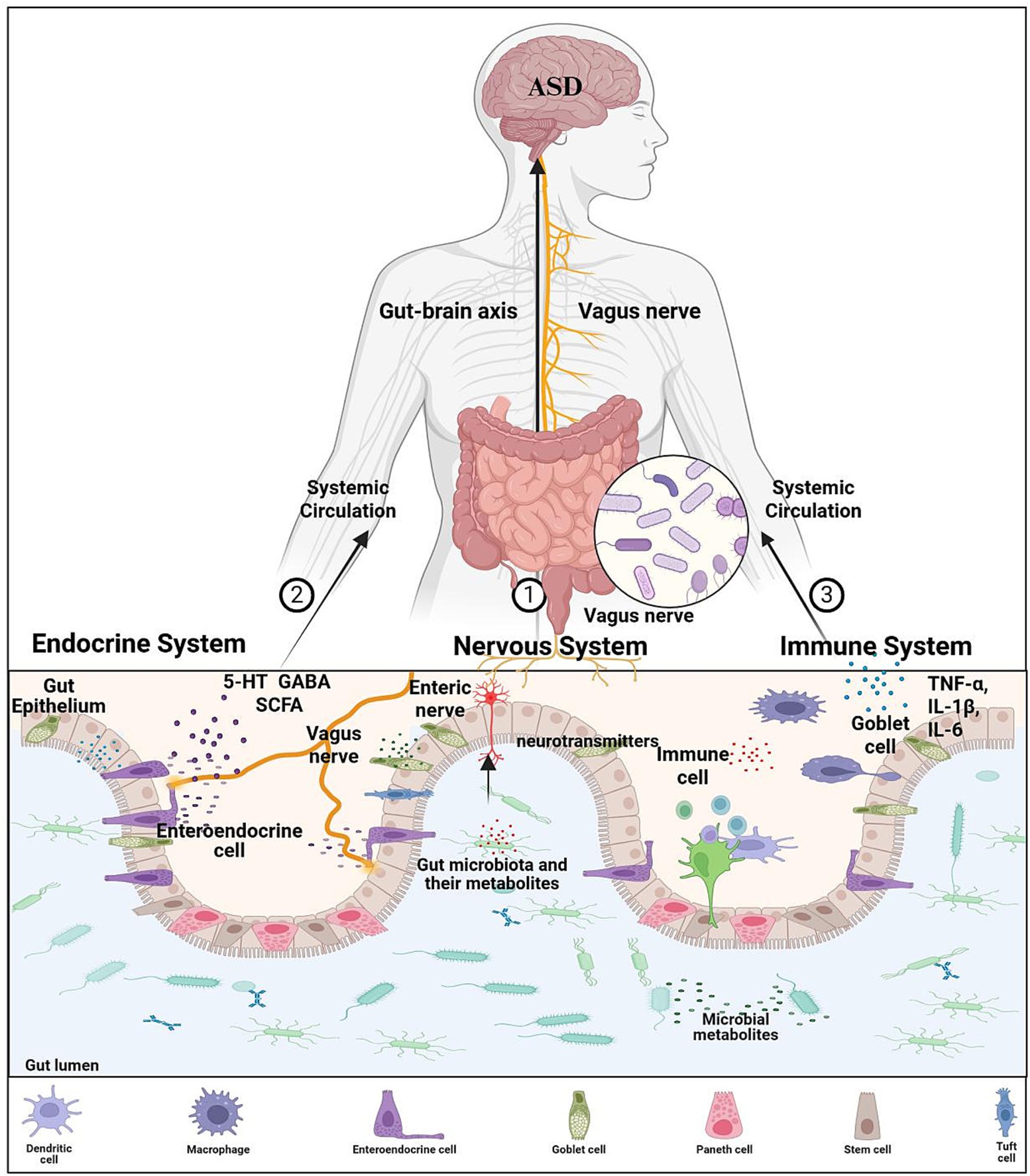
Figure 1. Mechanism of microbiota-gut-brain axis affecting ASD. Gut microbiota and their metabolites can ① regulate the activity of the enteric nervous system, ② promotes production of active substances from intestinal endocrine cells, ③ stimulates the maturation of the intestinal immune system and the release of immune factors. 5-HT, 5-hydroxytryptamine; GABA, gamma-aminobutyric acid; SCFA, short-chain fatty acid; IL-1β, Interleukin-1β; IL-6, Interleukin-6; TNF-α, tumor necrosis factor-α.
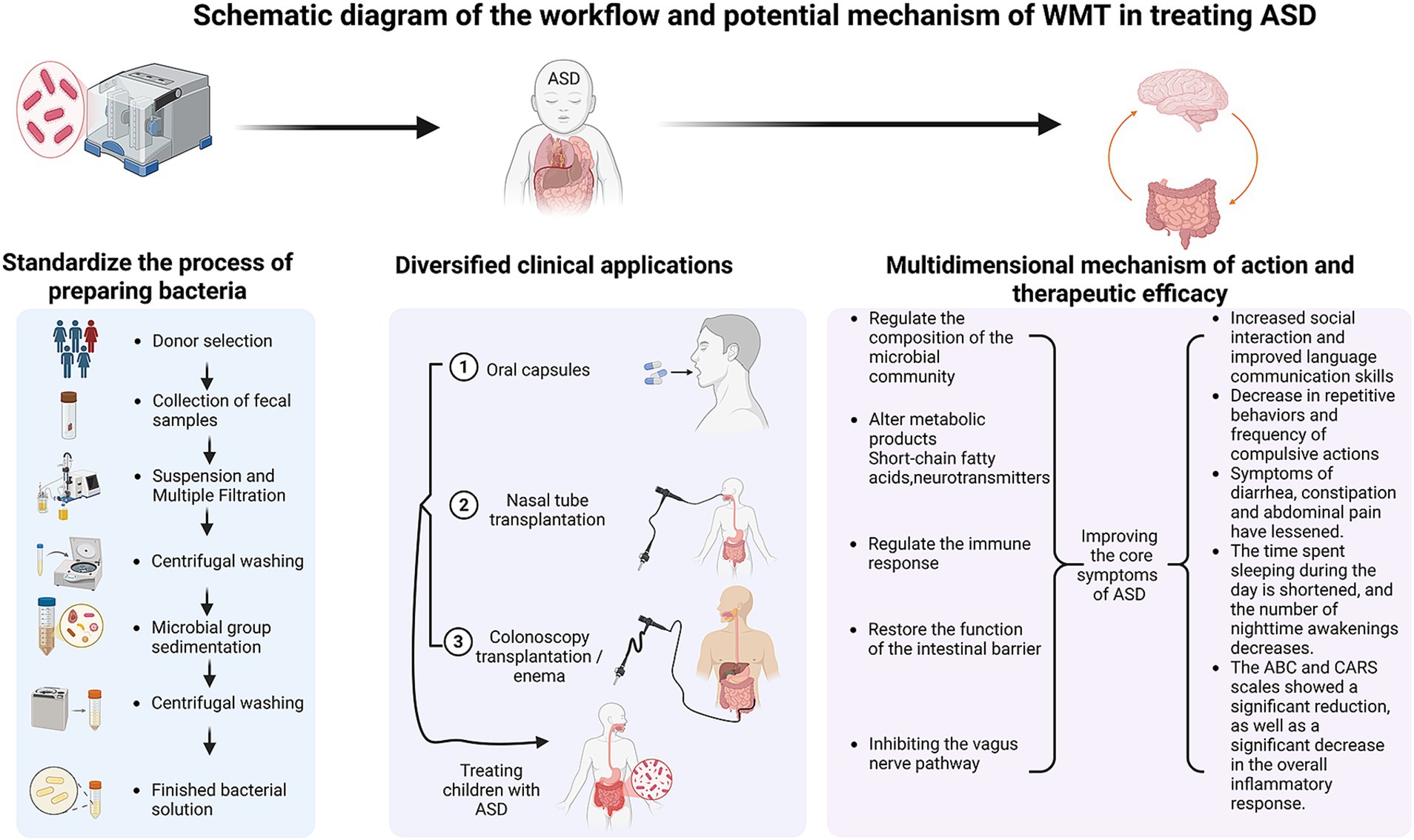
Figure 2. Schematic diagram of the workflow and potential mechanism of WMT in treating ASD. The left panel details the standardized process of preparing the bacterial solution, encompassing critical steps from donor selection and fecal sample collection to suspension, multiple filtration, centrifugal washing, and microbiota sedimentation, culminating in the finished bacterial solution ready for application. The middle panel illustrates the diversified clinical application routes, including oral capsules, nasal tube transplantation, and colonoscopy transplantation/enema, used for treating children with ASD. The right panel summarizes the multidimensional mechanisms of action—including regulating the composition of the microbial community, altering metabolic products (e.g., short-chain fatty acids, neurotransmitters), regulating the immune response, restoring the function of the intestinal barrier, and modulating the vagus nerve pathway—and the consequent therapeutic efficacies observed, such as increased social interaction, decreased repetitive behaviors, alleviated GI symptoms, improved sleep patterns, and significant reductions in ABC/CARS scores and systemic inflammation.
4.4.3 Gut microbiota-derived metabolites regulate ASD via the gut-brain axis
Gut microbiota influence the synthesis and metabolism of neurotransmitters. For example, specific gut bacteria modulate tryptophan metabolism. After intestinal absorption, tryptophan is preferentially channeled into serotonin synthesis pathways under microbial influence (Yano et al., 2015). Serotonin, a critical neurotransmitter, regulates mood, cognition, and behavior. Elevated serotonin levels improve social interactions and emotional dysregulation in ASD patients (Yano et al., 2015; Li and Zhou, 2016). Additionally, gut microbiota modulate dopamine and γ-aminobutyric acid (GABA) metabolism, further impacting neurological function (Sharon et al., 2016; Yano et al., 2015). Fatty acids produced by gut microbiota, such as short-chain fatty acids (SCFAs) and long-chain fatty acids (LCFAs), affect the nervous system through multiple pathways. SCFAs cross the blood–brain barrier via circulation to regulate neurotransmitter levels and neuroplasticity (Silva et al., 2020). Butyrate, a key SCFA, inhibits histone deacetylase (HDAC) activity, altering neuronal gene expression and influencing neurodevelopment (Hsiao et al., 2013; Silva et al., 2020). LCFAs like docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are vital for brain structure and function, improving cognitive and behavioral symptoms in ASD by modulating membrane fluidity and signaling (Li and Zhou, 2016). Specific gut bacteria, such as Bacteroides and Clostridium species, are involved in the synthesis and conversion of LCFAs (Li and Zhou, 2016; Fogelson et al., 2023). Gut microbiota also regulate sphingolipid metabolism, which is critical for neuronal membrane integrity and signaling. Microbial metabolites influence sphingolipid synthesis and degradation pathways. Certain beneficial bacteria enhance sphingolipid production, maintaining neuronal membrane stability and improving synaptic transmission, thereby supporting neurodevelopment and functional recovery in ASD (Li and Zhou, 2016). Certain beneficial bacteria, including Bifidobacterium and Lactobacillus, enhance sphingolipid production, maintaining neuronal membrane stability and improving synaptic transmission, thereby supporting neurodevelopment and functional recovery in ASD (Wu et al., 2023). Beyond these mechanisms, SCFAs activate the vagus nerve to relay signals to the brain (Cryan et al., 2019; Silva et al., 2020). Vagal receptors bind SCFAs, triggering afferent fiber activation that transmits signals to brain regions such as the amygdala and hippocampus, modulating emotion, cognition, and behavior (Cryan et al., 2019; Sgritta et al., 2019). SCFAs also stimulate enteroendocrine cells to secrete hormones like peptide YY (PYY) and glucagon-like peptide-1 (GLP-1), which act systemically to regulate energy metabolism and behavior, alleviating ASD symptoms (Silva et al., 2020). Gut microbiota mediate bile acid metabolism, converting primary bile acids into secondary forms. Beyond aiding fat digestion, bile acids act as signaling molecules via receptors (e.g., FXR, TGR5) to modulate hormone secretion, energy metabolism, and immune responses. Certain bile acids indirectly affect the nervous system by regulating gut barrier function and immune activity, influencing ASD pathogenesis (Vuong and Hsiao, 2017; Li and Zhou, 2016). Gut microbiota modulate branched-chain amino acid (BCAA) metabolism. BCAAs (leucine, isoleucine, valine) play key roles in neuronal metabolism, crossing the blood–brain barrier to support protein synthesis and neurotransmitter pathways. Dysbiosis disrupts BCAA homeostasis, impairing brain function. Restoring microbial balance improves BCAA metabolism, enhancing neurological and behavioral outcomes in ASD (Li and Zhou, 2016). Tryptophan-derived indole compounds, such as indole-3-acetic acid (IAA) and indole-3-propionic acid (IPA), exert anti-inflammatory effects (Yano et al., 2015). IAA modulates gut immunity, reducing neuroinflammatory damage, while IPA activates the aryl hydrocarbon receptor (AhR) to suppress inflammation. Concurrently, serotonin derived from tryptophan metabolism improves ASD behavioral symptoms by optimizing neurotransmitter balance (Yano et al., 2015; Vuong and Hsiao, 2017).
4.5 Safety and efficacy of WMT in treating autism
The safety and efficacy of WMT (Microbiota Transplantation) for autism are currently focal points of research. While existing studies on WMT for ASD are limited and involve small sample sizes, preliminary findings suggest its safety and effectiveness, with no significant adverse effects reported. Minor short-term side effects observed post-WMT include abdominal discomfort, diarrhea, constipation, and low-grade fever, which are typically self-limiting or resolve spontaneously without long-term consequences. Some studies hypothesize that these transient reactions may be linked to preexisting immunodeficiency or variations in disease severity among patients (Sharon et al., 2016; Cryan et al., 2019). However, current evidence remains confined to small-scale or case studies. Larger randomized double-blind controlled trials are needed to validate therapeutic outcomes and exclude risks such as fecal microbiota transplantation intolerance, transmission of pathogens, or autoimmune complications. Most ASD patients show no intestinal mucosal damage, indicating relatively high safety for WMT. However, immune dysregulation and microbiota abnormalities in ASD patients suggest potential chronic intestinal inflammation, underscoring the importance of stringent donor screening protocols to mitigate risks (Li et al., 2022). Further research is essential to optimize donor selection, standardize transplantation procedures, and establish long-term safety profiles.
4.6 Integrating workflow and multidimensional evidence: a visual synthesis of WMT’s application in ASD
Because of the highly promising application of WMT in ASD, so we have developed a comprehensive schematic (Figure 2) that synthesizes the standardized operational procedure with the observed multidimensional therapeutic outcomes. This integration moves beyond a purely descriptive account, providing a cohesive visual summary that underscores WMT’s potential as a structured and evidence-supported intervention for ASD.
4.6.1 Standardized workflow: from donor to patient
The efficacy and safety of WMT are fundamentally rooted in its standardized and rigorous workflow, as visualized in the left and middle panels of Figure 2. The process begins with the meticulous selection of healthy donors and collection of fecal samples, ensuring a high-quality source material. Subsequent steps—suspension, multiple filtration, centrifugal washing, and microbial sedimentation—conducted using intelligent microbiota separation systems, are crucial for removing undigested residues, fungi, parasite eggs, and pro-inflammatory metabolites. This yields a purified, functional microbiota suspension, defining the core technical advantage of WMT over traditional FMT (Zhang et al., 2020; Shi, 2020). The purified microbiota can then be administered via flexible clinical routes (Figure 2, middle panel), such as oral capsules, nasal tube, or colonoscopy/enema, allowing for personalized treatment strategies based on patient tolerance and clinical presentation (Shi, 2020).
4.6.2 Linking multidimensional mechanisms to clinical efficacy
The right panel of Figure 2 graphically connects the proposed multidimensional mechanisms of WMT with the spectrum of clinical improvements documented in ASD patients. As detailed in previous sections, WMT facilitates the restoration of a healthy gut microbial community. This restored community, in turn, exerts its therapeutic effects through multiple, often interconnected, pathways: by producing beneficial metabolites like SCFAs and neurotransmitters (Kang et al., 2017; Qureshi et al., 2020; Hsiao et al., 2013; Yano et al., 2015; Silva et al., 2020), modulating systemic and neuro-immune responses (Sharon et al., 2016; Fattorusso et al., 2019; Vuong and Hsiao, 2017), restoring the integrity and function of the intestinal barrier (Sharon et al., 2016; Vuong and Hsiao, 2017), and influencing brain function via the vagus nerve (Cryan et al., 2019; Sgritta et al., 2019). The convergence of these mechanisms is reflected in the significant alleviation of core ASD symptoms (e.g., improved social interaction and language communication, decreased repetitive/compulsive behaviors), co-morbid GI distress, sleep disorders, and a measurable reduction in systemic inflammation and standardized behavioral scores (e.g., ABC, CARS) (Kang et al., 2017; Kang et al., 2019; Li et al., 2019; Zhao et al., 2017; Qian, 2024; Zhang et al., 2022; Liu et al., 2023; Pan et al., 2022; Hu et al., 2025). This visual synthesis (Figure 2) thereby encapsulates the journey of WMT from a meticulously prepared biological product to a multifaceted therapy capable of addressing the complex pathophysiology of ASD, providing a clear and compelling framework that supports its application in this field.
4.6.3 Future directions guided by the workflow
The structured workflow presented in Figure 2 also highlights critical junctures for future research and protocol optimization. Efforts can be directed towards refining each step, such as enhancing donor-recipient matching criteria, optimizing the number and timing of treatments (Pan et al., 2022; Hu et al., 2025), and determining the most effective administration route for specific ASD subtypes. Furthermore, establishing correlations between specific microbial or metabolic changes induced by WMT (as suggested in the mechanisms panel) and particular clinical outcomes will be essential for advancing towards personalized microbiota-based therapy for ASD.
5 Summary and future perspectives
ASD a neurodevelopmental condition with rising global prevalence, imposes significant burdens on families and society. It is imperative to enhance public awareness, reduce stigma, and strengthen social support for individuals with ASD. Early diagnosis and intervention, multidisciplinary collaboration, public health policies, and increased research investment are critical to improving long-term outcomes. Comprehensive intervention strategies should prioritize resource optimization, long-term evaluation, cognitive enhancement, naturalistic environment training, individualized approaches, skill generalization, integration of emerging technologies, parental collaboration, cost reduction, accessibility, cross-disciplinary innovation, and policy support.
Current ASD treatments remain limited, with pharmacological interventions facing challenges such as disputed efficacy, side effects, and insufficient evidence for long-term safety. TCM attributes ASD pathogenesis to dysfunction of the brain, kidneys, spleen, liver, and heart, advocating herbal medicine and acupuncture. However, TCM research is constrained by low pediatric compliance and unclear mechanistic insights. Notably, TCM’s holistic philosophy aligns with the ecosystem theory of ASD, resonating with emerging therapies like WMT (Microbiota Transplantation), which shows promise in preliminary studies. The integration of its standardized workflow with multidimensional mechanisms and efficacy (Figure 2) provides a tangible and structured framework for its clinical application. Existing evidence suggests WMT is safe and effective for ASD children, though rigorous investigation into its mechanisms and long-term outcomes is needed. Current studies on WMT for ASD are predominantly small-scale or case-based, lacking multicenter, large-sample, high-quality evidence. Randomized double-blind controlled trials are essential to validate efficacy and safety, while addressing risks like donor-recipient incompatibility, pathogen transmission, and autoimmune responses. Although gut dysbiosis is widely observed in ASD, its precise role in pathogenesis remains unclear. Metabolomics, bridging microbial and host interactions, may unlock breakthroughs, yet research on gut microbiota-metabolome relationships in ASD is scarce. Future studies must prioritize linking microbial/metabolomic shifts to symptom improvement to advance biomarker discovery and novel therapies. For WMT optimization, donor-recipient age matching is crucial to minimize immune complications. Parameters such as transplantation dosage, duration, administration routes, antibiotic pretreatment, and standardized evaluation methods require refinement. Mechanistic studies on gut microbiota-neurotransmitter-behavioral interactions are also vital. Long-term follow-up is indispensable for assessing sustained efficacy and safety. While WMT is established for gastrointestinal disorders, its broader application demands robust evidence. Enhancing therapeutic outcomes hinges on selecting donor microbiota with optimal diversity and composition, alongside stringent donor screening to mitigate risks. Future efforts to match optimal donors based on ASD patients’ microbial profiles and clinical subtypes may further enhance therapeutic outcomes. As gut microbiota research advances, WMT holds potential to revolutionize ASD treatment and inspire strategies for other complex disorders. Collaborative innovation, policy advocacy, and sustained investment will be key to translating these insights into transformative clinical solutions.
6 Conclusion
This study systematically explores the potential and mechanisms of WMT in treating ASD, with the following key findings: Gut microbiota dysbiosis is a critical pathological feature of ASD; WMT significantly alleviates core ASD symptoms and comorbidities; Multiple mechanisms synergistically regulate the “gut-brain axis”; WMT demonstrates high safety but requires protocol optimization; Future directions focus on precision medicine and multi-omics integration.
WMT offers a safe and effective alternative for ASD patients unresponsive to conventional therapies, reducing familial and societal burdens. It reveals microbiota-metabolite-host interactions, advancing “microbiome-based therapies” for neurological disorders. Standardized protocols and subtype stratification accelerate the translation of laboratory findings into clinical practice, opening new avenues for complex disease treatment. These conclusions deepen the understanding of ASD pathogenesis and provide critical evidence for clinical applications of microbiota-targeted therapies, holding significant scientific and societal value.
Author contributions
SF: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. JW: Conceptualization, Data curation, Writing – original draft. XS: Investigation, Writing – original draft. SL: Investigation, Writing – original draft. CL: Formal analysis, Writing – original draft. ZG: Writing – review & editing. JY: Formal analysis, Writing – original draft. JW: Writing – review & editing. XH: Conceptualization, Funding acquisition, Writing – review & editing. LW: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Key-Area Research and Development Program of Guangdong Province (No. 2022B1111070006), the Basic and Applied Basic Research Fund of Guangdong Province (No. 2025A1515011113), the China Postdoctoral Science Foundation (No. 2023M740782), the Medical Scientific Research Foundation of Guangdong Province (No. B2022209), the Scientific Research Projects of Guangdong Bureau of Traditional Chinese Medicine (No. 20221232).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ASD, Autism Spectrum Disorder; WMT, Washed Microbiota Transplantation; FMT, Fecal Microbiota Transplantation; CNS, Central Nervous System; SCFAs, Short-Chain Fatty Acids; 5-HT, 5-Hydroxytryptamine; LPS, Lipopolysaccharides; IL-6, Interleukin-6; TNF-α, Tumor Necrosis Factor-α; ABC, Autism Behavior Checklist; CARS, Childhood Autism Rating Scale; RCT, Randomized Controlled Trial.
References
Alvares, G. A., Quintana, D. S., and Whitehouse, A. J. (2017). Beyond the hype and hope: critical considerations for intranasal oxytocin research in autism spectrum disorder. Autism Res. 10, 25–41. doi: 10.1002/aur.1692
American Psychiatric Association (2022). Diagnostic and statistical manual of mental disorders, fifth edition, text revision. Washington: American Psychiatric Association.
Bremer, E., Crozier, M., and Lloyd, M. (2016). A systematic review of the behavioural outcomes following exercise interventions for children and youth with autism spectrum disorder. Autism 20, 899–915. doi: 10.1177/1362361315616002
Buescher, A. V., Cidav, Z., Knapp, M., and Mandell, D. S. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatr. 168, 721–728.
Chen, C. C., and Chiu, C. H. (2022). Current and future applications of fecal microbiota transplantation for children. Biom. J. 45, 11–18. doi: 10.1016/j.bj.2021.11.004
Chen, Y., Qiyi, C., Chunlian, M., et al. (2022a). Analysis of the clinical efficacy of intestinal flora transplantation in the treatment of 328 cases of autism spectrum disorder complicated with constipation. Gastrointestinal Surg 25, 798–803. doi: 10.3760/cma.J.c.n441530-20220601-00238
Chen, Y., Qiyi, C., Yinmei, Y., et al. (2022b). Human small intestinal juice transplantation and to establish the system of small intestinal juice capsule preparation and clinical application. Chinese Gastrointestinal Surg 25, 819–825. doi: 10.3760/cma.J.c.n441530-20220601-00239
Chen, Y., Xueying, Z., Jiaqu, C., Qiyi, C., Huanlong, Q., Ning, L., et al. (2022c). FTACMT study protocol: a multicentre, double-blind, randomised, placebo-controlled trial of faecal microbiota transplantation for autism spectrum disorder. BMJ Open 12:e051613. doi: 10.1136/bmjopen-2021-051613
Chen, Q., Yang, B., Tian, H., et al. (2020). Flora transplant 3 932 cases of therapeutic effect and complications of the 5 years follow-up analysis. Chin. Digest Magazine 40, 768–777. doi: 10.3760/cma.J.c.n311367-20200706-00432
Chevallier, C., Kohls, G., Troiani, V., Brodkin, E. S., and Schultz, R. T. (2012). The social motivation theory of autism. Trends Cogn. Sci. 16, 231–239. doi: 10.1016/j.tics.2012.02.007
Codella, R., Luzi, L., and Terruzzi, I. (2018). Exercise has the guts: how physical activity may positively modulate gut microbiota in chronic and immune-based diseases. Dig. Liver Dis. 50, 331–341. doi: 10.1016/j.dld.2017.11.016
Cryan, J. F., O'Riordan, K. J., Cowan, C. S. M., Sandhu, K. V., Bastiaanssen, T. F. S., Boehme, M., et al. (2019). The microbiota-gut-brain Axis. Physiol. Rev. 99, 1877–2013. doi: 10.1152/physrev.00018.2018.
Dan, Z., Mao, X., Liu, Q., Guo, M., Zhuang, Y., Liu, Z., et al. (2020). Altered gut microbial profile is associated with abnormal metabolism activity of autism Spectrum disorder. Gut Microbes 11, 1246–1267. doi: 10.1080/19490976.2020.1747329
Demetriou, E. A., Lampit, A., Quintana, D. S., Naismith, S. L., Song, Y. J. C., Pye, J. E., et al. (2018). Autism spectrum disorders: a meta-analysis of executive function. Mol. Psychiatry 23, 1198–1204. doi: 10.1038/mp.2017.75
Ding, D., Zhong, Y., Cheng, X., et al. (2015). Applied behavior analysis training on the quality of the autistic children survive. Chin. J. Clin. Psychol. 23, 564–566. doi: 10.16128/j.carolcarrollnki.1005-3611.2015.03.041
Duan, Y., Wu, X., and Jin, F. (2015). Research progress on the influence of diet on autism. Chin. Sci. Bull. 60, 2845–2861.
Elchaar, G. M., Maisch, N. M., Augusto, L. M., and Wehring, H. J. (2006). Efficacy and safety of naltrexone use in pediatric patients with autistic disorder. Ann. Pharmacother. 40, 1086–1095. doi: 10.1345/aph.1G499
Erny, D., Hrabě de Angelis, A. L., Jaitin, D., Wieghofer, P., Staszewski, O., David, E., et al. (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977. doi: 10.1038/nn.4030
Fattorusso, A., Di Genova, L., Dell'Isola, G. B., Mencaroni, E., and Esposito, S. (2019). Autism spectrum disorders and the gut microbiota. Nutrients 11:521. doi: 10.3390/nu11030521
Fogelson, K. A., Dorrestein, P. C., Zarrinpar, A., and Knight, R. (2023). The gut microbial bile acid modulation and its relevance to digestive health and diseases. Gastroenterology 164, 1069–1085. doi: 10.1053/j.gastro.2023.02.022
Fu, Yunjin, and Chen, Lianjun. Reform trends and implications of vocational rehabilitation services for disabled youth in the United States: based on the interpretation of the workforce innovation and opportunity act special education in China, (2023), 23–30.
Garcia, E. R., Vergara, A., Aziz, F., Narváez, S., Cuesta, G., Hernández, M., et al. (2022). Changes in the gut microbiota and risk of colonization by multidrug-resistant bacteria, infection, and death in critical care patients. Clin. Microbiol. Infect. 28, 975–982. doi: 10.1016/j.cmi.2022.01.004
Geretsegger, M., Elefant, C., Mössler, K. A., and Gold, C. (2014). Music therapy for people with autism spectrum disorder. Cochrane Database Syst. Rev. 2016:CD004381. doi: 10.1002/14651858.CD004381.pub3
Han, V. X., Patel, S., Jones, H. F., and Dale, R. C. (2021). Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 17, 564–579. doi: 10.1038/s41582-021-00530-8
Hartley, S. L., Barker, E. T., Seltzer, M. M., Floyd, F., Greenberg, J., Orsmond, G., et al. (2010). The relative risk and timing of divorce in families of children with an autism spectrum disorder. J. Fam. Psychol. 24, 449–457. doi: 10.1037/a0019847
Hazan, S., Haroon, J., Jordan, S., and Walker, S. J. (2024). Improvements in gut microbiome composition and clinical symptoms following familial fecal microbiota transplantation in a nineteen-year-old adolescent with severe autism. J Med Cases. 15, 82–91. doi: 10.14740/jmc4209
Hirota, T., and King, B. H. (2023). Autism spectrum disorder: a review. JAMA 329, 157–168. doi: 10.1001/jama.2022.23661
Holingue, C., Newill, C., Lee, L. C., Pasricha, P. J., and Daniele Fallin, M. (2018). Gastrointestinal symptoms in autism spectrum disorder: a review of the literature on ascertainment and prevalence. Autism Res. 11, 24–36. doi: 10.1002/aur.1854
Howes, O. D., Rogdaki, M., Findon, J. L., Wichers, R. H., Charman, T., King, B. H., et al. (2018). Autism spectrum disorder: consensus guidelines on assessment, treatment and research from the British Association for Psychopharmacology. J. Psychopharmacol. 32, 3–29. doi: 10.1177/0269881117741766
Hsiao, E. Y., McBride, S. W., Hsien, S., Sharon, G., Hyde, E. R., McCue, T., et al. (2013). Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463. doi: 10.1016/j.cell.2013.11.024
Hu, C., He, T., Zou, B., Li, H., Zhao, J., Cui, J., et al. (2023). Fecal microbiota transplantation in a child with severe ASD comorbidities of gastrointestinal dysfunctions-a case report. Front. Psych. 14:1219104. doi: 10.3389/fpsyt.2023.1219104
Hu, D. X., Lu, C. M., Si, X. Y., Wu, Q. T., Wu, L. H., Zhong, H. J., et al. (2025). Effects of gastrointestinal symptoms on the efficacy of washed microbiota transplantation in patients with autism. Front. Pediatr. 13:1528167. doi: 10.3389/fped.2025.1528167
Ilias, K., Cornish, K., Park, M. S., Park, M. S.-A., Toran, H., and Golden, K. J. (2019). Risk and resilience among mothers and fathers of primary school age children with ASD in Malaysia: a qualitative constructive grounded theory approach. Front. Psychol. 9:2275. doi: 10.3389/fpsyg.2018.02275
Integrative HMP (iHMP) Research Network Consortium (2019). The integrative human microbiome project. Nature 569, 641–648. doi: 10.1038/s41586-019-1238-8
Johnson, D., Letchumanan, V., Thurairajasingam, S., and Lee, L. H. (2020). A revolutionizing approach to autism Spectrum disorder using the microbiome. Nutrients 12:1983. doi: 10.3390/nu12071983
Kang, D. W., Adams, J. B., Coleman, D. M., Pollard, E. L., Maldonado, J., McDonough-Means, S., et al. (2019). Long-term benefit of microbiota transfer therapy on autism symptoms and gut microbiota. Sci. Rep. 9:5821. doi: 10.1038/s41598-019-42183-0
Kang, D. W., Adams, J. B., Gregory, A. C., Borody, T., Chittick, L., Fasano, A., et al. (2017). Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 5:10. doi: 10.1186/s40168-016-0225-7
Kanner, A. M., and Bicchi, M. M. (2022). Antiseizure medications for adults with epilepsy: a review. JAMA 327, 1269–1281. doi: 10.1001/jama.2022.3880
Karst, J. S., and Van Hecke, A. V. (2012). Parent and family impact of autism spectrum disorders: a review and proposed model for intervention evaluation. Clin. Child. Fam. Psychol. Rev. 15, 247–277. doi: 10.1007/s10567-012-0119-6.
Kong, Y., and Chen, Z. (2023). Research trends and implications of identity and its impact in the autism community. Special Educ. China, 54–60.
Lan, J., and Bai, Y. (2020). Autistic children stigma phenomenon and the countermeasures. J. Liaoning Norm. Univ., 75–82. doi: 10.16216/j.carolcarrollnkiLSXBWK.202005075
Lee, J. D., Terol, A. K., Yoon, C. D., and Meadan, H. (2024). Parent-to-parent support among parents of children with autism: a review of the literature. Autism 28, 263–275. doi: 10.1177/13623613221146444
Lemonnier, E., Villeneuve, N., Sonie, S., et al. (2017). Effects of bumetanide on neurobehavioral function in children and adolescents with autism spectrum disorders. Transl. Psychiatry 7:e1056. doi: 10.1038/tp.2017.10
Ley, R. E., Peterson, D. A., and Gordon, J. I. (2006). Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124, 837–848. doi: 10.1016/j.cell.2006.02.017
Li, N., Chen, H., Cheng, Y., Xu, F., Ruan, G., Ying, S., et al. (2021). Fecal microbiota transplantation relieves gastrointestinal and autism symptoms by improving the gut microbiota in an open-label study. Front. Cell. Infect. Microbiol. 11:759435. doi: 10.3389/fcimb.2021.759435
Li, N., Tian, H. L., Chen, Q. Y., Yang, B., Ma, C. L., Lin, Z. L., et al. (2019). Analysis of the therapeutic effect of microbiota transplantation in the treatment of intestinal diseases in 2010 cases. Chin. J. Gastrointest. Surg. 22, 861–868. doi: 10.3760/cma.j.issn.1671-0274.2019.09.01
Li, J., and Wang, Y. (2015). Parental stress of parents of preschool children with disabilities: the role and nature of social support and coping styles. Special Educ. China 5, 3–8+14.
Li, Y., Xiao, P., Cao, R., Le, J., et al. (2024a). Effects and microbiota changes following oral lyophilized fecal microbiota transplantation in children with autism spectrum disorder. Front. Pediatr. 12:1369823. doi: 10.3389/fped.2024.1369823
Li, Y., Xiao, P., Ding, H., Wang, H., Xu, Q., Wang, R., et al. (2024b). Fecal microbiota transplantation in children with autism. Neuropsychiatr. Dis. Treat. 20, 2391–2400. doi: 10.2147/NDT.S488001
Li, X., Xu, X., Luo, X., et al. (2022). Research progress of fecal microbiota transplantation in the treatment of autism spectrum disorder. Peking Union Med. College Hospital J. 13, 753–759.
Li, Q., and Zhou, J. M. (2016). The microbiota-gut-brain axis and its potential therapeutic role in autism spectrum disorder. Neuroscience 324, 131–139. doi: 10.1016/j.neuroscience.2016.03.013
Liang, X., Li, R., Wong, S. H. S., Sum, R. K. W., Wang, P., Yang, B., et al. (2022). The effects of exercise interventions on executive functions in children and adolescents with autism spectrum disorder: a systematic review and meta-analysis. Sports Med. 52, 75–88. doi: 10.1007/s40279-021-01545-3
Liu, N. H., Liu, H. Q., Zheng, J. Y., Zhu, M. L., Wu, L. H., Pan, H. F., et al. (2023). Fresh washed microbiota transplantation alters gut microbiota metabolites to ameliorate sleeping disorder symptom of autistic children. J. Microbiol. 61, 741–753. doi: 10.1007/s12275-023-00069-x
Liu, L., Yang, L., Qin, G., et al. (2024). Fecal bacteria transplantation of intervention in the treatment of children with autism curative effect analysis. Chin. J. Pathog. Biol. 12, 83–87. doi: 10.13350/j.carolcarrollJPB.240117
Lloyd-Price, J., Abu-Ali, G., and Huttenhower, C. (2016). The healthy human microbiome. Genome Med. 8:51. doi: 10.1186/s13073-016-0307-y
Lovaas, O. I. (1987). Behavioral treatment and normal educational and intellectual functioning in young autistic children. J. Consult. Clin. Psychol. 55, 3–9. doi: 10.1037//0022-006x.55.1.3.
Luo, Q. (2018). A preliminary study on the correlation between intestinal microecology and autism Spectrum disorder and fecal microecology transplantation therapy : Medical College of the People's liberation Army of China.
Maenner, M. J., Shaw, K. A., Bakian, A. V., Bilder, D. A., Durkin, M. S., Esler, A., et al. (2021). Prevalence and characteristics of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2018. MMWR Surveill. Summ. 70, 1–16. doi: 10.15585/mmwr.ss7011a1
Maenner, M. J., Warren, Z., Williams, A. R., Amoakohene, E., Bakian, A. V., Bilder, D. A., et al. (2023). Prevalence and characteristics of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2020. MMWR Surveill. Summ. 72, 1–14. doi: 10.15585/mmwr.ss7202a1
Malow, B. A., Findling, R. L., Schroder, C. M., Maras, A., Breddy, J., Nir, T., et al. (2021). Sleep, growth, and puberty after 2 years of prolonged-release melatonin in children with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry 60, 252–261.e3. doi: 10.1016/j.jaac.2019.12.007
Ming, X., Gordon, E., Kang, N., and Wagner, G. C. (2008). Use of clonidine in children with autism spectrum disorders. Brain Dev. 30, 454–460. doi: 10.1016/j.braindev.2007.12.007
Moradi, K., Ashraf-Ganjouei, A., Tavolinejad, H., Bagheri, S., and Akhondzadeh, S. (2021). The interplay between gut microbiota and autism spectrum disorders: a focus on immunological pathways. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 106:110091. doi: 10.1016/j.pnpbp.2020.110091
Pan, Z., Gao, Z., Chen, J., Quan, Y., Xu, J., Liang, X., et al. (2025). Does constipation affect the effectiveness of washed microbiota transplantation in treating autism spectrum disorders? Front. Neurosci. 19:1602681. doi: 10.3389/fnins.2025.1602681
Pan, Z. Y., Zhong, H. J., Huang, D. N., Wu, L. H., and He, X. X. (2022). Beneficial effects of repeated washed microbiota transplantation in children with autism. Front. Pediatr. 10:928785. doi: 10.3389/fped.2022.928785
Persico, A. M., Ricciardello, A., Lamberti, M., Turriziani, L., Cucinotta, F., Brogna, C., et al. (2021). The pediatric psychopharmacology of autism spectrum disorder: a systematic review - part I: the past and the present. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 110:110326. doi: 10.1016/j.pnpbp.2021.110326
Qian, L. (2024). The therapeutic effect of gut microbiota transplantation on patients with autism and its impact on gut microbiota : Hebei University.
Qureshi, F., Adams, J., Hanagan, K., Kang, D.-W., Krajmalnik-Brown, R., and Hahn, J. (2020). Multivariate analysis of fecal metabolites from children with autism Spectrum disorder and gastrointestinal symptoms before and after microbiota transfer therapy. J. Pers. Med. 10:152. doi: 10.3390/jpm10040152
Report on the Development Status of China's Autism Education and Rehabilitation Industry (2024). Available online at: http://cn.chinagate.cn/news/2024-04/03/content_117104748.shtml.
Sgritta, M., Dooling, S. W., Buffington, S. A., Momin, E. N., Francis, M. B., Britton, R. A., et al. (2019). Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron 101, 246–259.e6. doi: 10.1016/j.neuron.2018.11.018
Shafiq, S., and Pringsheim, T. (2018). Using antipsychotics for behavioral problems in children. Expert. Opin. Pharmacother. 19, 1475–1488. doi: 10.1080/14656566.2018.1509069
Sharma, S. R., Gonda, X., and Tarazi, F. I. (2018). Autism Spectrum disorder: classification, diagnosis and therapy. Pharmacol. Ther. 190, 91–104. doi: 10.1016/j.pharmthera.2018.05.007
Sharon, G., Cruz, N. J., Kang, D. W., Gandal, M. J., Wang, B., Kim, Y. M., et al. (2019). Human gut microbiota from autism Spectrum disorder promote behavioral symptoms in mice. Cell 177, 1600–1618.e17. doi: 10.1016/j.cell.2019.05.004
Sharon, G., Sampson, T. R., Geschwind, D. H., and Mazmanian, S. K. (2016). The central nervous system and the gut microbiome. Cell 167, 915–932. doi: 10.1016/j.cell.2016.10.027
Shi, Q. (2020). Nanjing consensus on methodology of washed microbiota transplantation. Chin. Med. J. 133, 2330–2332. doi: 10.1097/CM9.0000000000000954
Shi, X., and Yang, Y. (2017). Fecal drugs in China and their clinical applications. Chin. J. Tradit. Chin. Med. 32, 3417–3420.
Silva, Y. P., Bernardi, A., and Frozza, R. L. (2020). The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne) 11:25. doi: 10.3389/fendo.2020.00025
Stepanova, E., Dowling, S., Phelps, M., and Findling, R. L. (2017). Pharmacotherapy of emotional and behavioral symptoms associated with autism spectrum disorder in children and adolescents. Dialogues Clin. Neurosci. 19, 395–402. doi: 10.31887/DCNS.2017.19.4/rfindling
Strati, F., Cavalieri, D., Albanese, D., De Felice, C., Donati, C., Hayek, J., et al. (2017). New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 5:24. doi: 10.1186/s40168-017-0242-1
Syriopoulou-Delli, C. K., and Sarri, K. (2021). Video-based instruction in enhancing functional living skills of adolescents and young adults with autism spectrum disorder and their transition to independent living: a review. Int. J. Dev. Disabil. 68, 788–799. doi: 10.1080/20473869.2021.1900504
Tan, C., Song, B., Ma, S., et al. (2021). Autism behavior intervention and tendency of the development of research methods. Chin. J. Clin. Psychol. 29, 436–442. doi: 10.16128/j.carolcarrollnki.1005-3611.2021.02.044
Vuong, H. E., and Hsiao, E. Y. (2017). Emerging roles for the gut microbiome in autism spectrum disorder. Biol. Psychiatry 81, 411–423. doi: 10.1016/j.biopsych.2016.08.024
Wang, C., Chen, J., Liu, Y., et al. (2021). A review of self-management strategies for improving repetitive and stereotyped behaviors in individuals with autism spectrum disorder. Special Educ. China, 61–66.
Wang, Y., Kang, S., Ramirez, J., and Tarbox, J. (2019). Multilingual diversity in the field of applied behavior analysis and autism: a brief review and discussion of future directions. Behav. Anal. Pract. 12, 795–804. doi: 10.1007/s40617-019-00382-1
Wang, M., Wan, J., Rong, H., He, F., Wang, H., Zhou, J., et al. (2019). Alterations in gut glutamate metabolism associated with changes in gut microbiota composition in children with autism spectrum disorder. mSystems 4:e00321-18. doi: 10.1128/mSystems.00321-18
Wang, L., Yu, L., Liu, Z., Che, C., Wang, Y., Zhao, Y., et al. (2024). FMT intervention decreases urine 5-HIAA levels: a randomized double-blind controlled study. Front. Med. (Lausanne) 11:1411089. doi: 10.3389/fmed.2024.1411089
Ward, L., O’Grady, H. M., Wu, K., et al. (2016). Combined oral fecal capsules plus fecal enema as treatment of late-onset autism spectrum disorder in children: report of a small case series. Open Forum Infect. Dis. 3:S599.
Wei, J., Chen, J., Fang, X., Liu, T., Yuan, Y., and Zhang, J. (2023). Protocol for the safety and efficacy of fecal microbiota transplantation liquid in children with autism spectrum disorder: a randomized controlled study. Front. Microbiol. 14:1236904. doi: 10.3389/fmicb.2023.1236904
Wong, C., Odom, S. L., Hume, K. A., Cox, A. W., Fettig, A., Kucharczyk, S., et al. (2015). Evidence-based practices for children, youth, and young adults with autism spectrum disorder: a comprehensive review. J. Autism Dev. Disord. 45, 1951–1966. doi: 10.1007/s10803-014-2351-z
Wu, G., Gu, M., Li, X., et al. (2024). Crossing thorns: a review of the parenting stress of parents of children with autism. Modern Special Educ., 36–45.
Wu, Z., Tian, E., Chen, Y., Dong, Z., and Peng, Q. (2023). Gut microbiota and its roles in the pathogenesis and therapy of endocrine system diseases. Microbiol. Res. 268:127291. doi: 10.1016/j.micres.2022.127291
Xiao, X., Qian, L., Yang, N., et al. (2013). Video demonstration method in the teaching of children with autism application. Chin. J. Clin. Psychol. 21, 867–870. doi: 10.16128/j.carolcarrollnki.1005-3611.2013.05.039
Xu, Q., Gao, M., and Chen, J. (2019). Research progress of game therapy applied in the intervention of children with autism. Modern Special Educ., 53–58.
Xu, X., and Wang, K. (2018). Research progress on drug therapy for autism Spectrum disorder. Chin. J. Pharm. 53, 321–3227. doi: 10.16438/J.0513-4870.2017-0912
Xu, J., Wang, Y., Tian, T., et al. (2017). The similarities and differences between traditional Chinese medicine golden juice and fecal microbiota transplantation. Chin. J. Tradit. Chin. Med. 32, 3414–3415.
Yan, H., Zhang, X., Rong, P., et al. (2022). Clinical research progress of traditional Chinese and western medicine in the treatment of autism spectrum disorder in children. Chin. J. Tradit. Chin. Med. 37, 933–938.
Yano, J. M., Yu, K., Donaldson, G. P., Shastri, G. G., Ann, P., Ma, L., et al. (2015). Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276. doi: 10.1016/j.cell.2015.02.047
Ye, C., Chen, Q., Li, N., et al. (2020). The intestinal micro ecology and autism research progress. J. Life Sci. 32, 807–815. doi: 10.13376/j.carolcarrollBLS/2020101
Zeng, K., Wen, X., and Wang, G. (2023). Evaluation of the effect of intestinal flora transplantation in intervening children with autism complicated with gastrointestinal symptoms. Chin. Med. Innov. 20, 1–5.
Zhang, T., Lu, G., Zhao, Z., Liu, Y., Shen, Q., Li, P., et al. (2020). Washed microbiota transplantation vs. manual fecal microbiota transplantation: clinical findings, animal studies and in vitro screening. Protein Cell 11, 251–266. doi: 10.1007/s13238-019-00684-8
Zhang, F., Luo, W., Shi, Y., Fan, Z., and Ji, G. (2012). Should we standardize the 1,700-year-old fecal microbiota transplantation? Am. J. Gastroenterol. 107, 1755–1756. doi: 10.1038/ajg.2012.251
Zhang, Y., Zhang, J., Pan, Z., and He, X. (2022). Effects of washed fecal Bacteria transplantation in sleep quality, stool features and autism symptomatology: a Chinese preliminary observational study. Neuropsychiatr. Dis. Treat. 18, 1165–1173. doi: 10.2147/NDT.S355233
Zhang, K., Zhao, M., and Chen, H. (2023). Clinical research progress of traditional Chinese medicine in the treatment of autism in children. Clin. Res. Tradit. Chin. Med. 15, 50–54.
Zhao, Y., Chen, P., and Wu, Z. (2017). Meta-analysis of the impact of sensory integration training on the rehabilitation of children with autism. Modern Special Educ., 42–47.
Zhao, H., Gao, X., Xi, L., Shi, Y., Peng, L., Wang, C., et al. Mo1667 fecal microbiota transplantation for children with autism spectrum disorder. Gastrointest. Endosc. 89:AB512-AB513. doi: 10.1016/j.gie.2019.03.857
Zhao, F., Lu, F., Tan, C., et al. (2021a). The intervention effect of staged comprehensive motor training on children with autism spectrum disorder: a comparison between comorbidities and non-comorbidities of ADHD. Chin. J. Clin. Psychol. 29, 869–875. doi: 10.16128/j.carolcarrollnki.1005-3611.2021.04.042
Zhao, Y., Luo, Y., Wang, X., et al. (2021b). China 2 ~ 6 years old autistic child family economic burden directly study. Chin. J. Dis. Control 25, 1085–1090. doi: 10.16462/j.carolcarrollnkiZHJBKZ.2021.09.016
Zhao, H., Peng, L., Ren, R., et al. (2017). Dung microecological products transplantation treating autism cases reported. Chin. J. Microecol. 29, 309–312. doi: 10.13381/j.carolcarrollnki.CJM.201703014
Zheng, Y. M., Ye, M. M., Zhang, H. Y., Luo, D. P., Liu, T., He, X. X., et al. (2025). Retrospective review: single- and multidonor washed microbiota transplantation have equivalent efficacy in the treatment of autism. Front. Cell. Infect. Microbiol. 15:1606417. doi: 10.3389/fcimb.2025.1606417
Zhong, H. J., Pan, Z. Y., Wei, Y. F., Yu, Q., Wu, L., Wei, H., et al. (2025). Tongue-coating microbiota as a predictive biomarker of washed microbiota transplantation efficacy in pediatric autism: integration with clinical features. J. Transl. Med. 23:799. doi: 10.1186/s12967-025-06846-z
Keywords: autism spectrum disorder (ASD), gut microbiota, washed microbiota transplantation (WMT), fecal microbiota transplantation (FMT), therapeutic strategies
Citation: Feng S, Wang J, Si X, Lu S, Lu C, Gao Z, Yang J, Wu J, He X and Wu L (2025) Washed microbiota transplantation: candidates for a novel strategy for ameliorating autism spectrum disorder. Front. Microbiol. 16:1688325. doi: 10.3389/fmicb.2025.1688325
Edited by:
Laura Mitrea, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaReviewed by:
Chen Ye, Tongji University, ChinaWu Yuqi, Microbiota I-Center Limited, Hong Kong SAR, China
Copyright © 2025 Feng, Wang, Si, Lu, Lu, Gao, Yang, Wu, He and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingxiang He, aGV4aW5neGlhbmdAZ2RwdS5lZHUuY24=; Lei Wu, d3VsZWlnZGltQDE2My5jb20=
†These authors have contributed equally to this work
 Shuo Feng1,2†
Shuo Feng1,2† Jiangyan Wang
Jiangyan Wang Zheng Gao
Zheng Gao Juan Yang
Juan Yang Jiali Wu
Jiali Wu Xingxiang He
Xingxiang He Lei Wu
Lei Wu