- 1College of Life and Health, Nanjing Polytechnic Institute, Nanjing, China
- 2Key Laboratory of Flexible Electronics (KLOFE) and Institute of Advanced Materials (IAM), School of Physical and Mathematical Sciences, Nanjing Tech University (NanjingTech), Nanjing, China
- 3State Key Laboratory of Flexible Electronics (LoFE) & Jiangsu Key Laboratory for Biosensors, Institute of Advanced Materials (IAM), Nanjing University of Posts & Telecommunications, Nanjing, China
- 4A*STAR Infectious Diseases Labs (A*STAR ID Labs), Agency for Science, Technology and Research (A*STAR), Singapore, Singapore
Bacterial persisters represent a metabolically dormant or slow-growing subpopulation within bacterial communities that exhibit resistance to antibiotics. These cells are capable of resuming active proliferation upon the removal of environmental stressors, hence serving as reservoirs for recurrent infections. Extensive clinical evidence links persister cell formation with chronic infection and post-therapeutic recurrence. In this minireview, we highlight the challenges in eradicating persisters and review recent advances in nanomaterial-based antimicrobial strategies that specifically target these resilient cells. We also discuss key translational barriers impeding the clinical application of antibacterial nanoagents. By integrating these insights, we aim to provide a conceptual roadmap for the development of next-generation therapies against persistent bacterial infections.
1 Introduction
Bacterial infections have long posed a significant threat to global public health (Gollan et al., 2019; Zhang et al., 2023). The discovery of antibiotics had been one of the most significant breakthroughs in modern medicine, which dramatically reduced mortality from bacterial diseases. Today, antibiotics remain the cornerstone of bacterial infection treatment (Mo et al., 2025; Zhang et al., 2024b). However, widespread misuse and overuse of antibiotics have led to the growing crisis of antimicrobial resistance (Dai et al., 2012; Hu et al., 2025), rendering many once-reliable antibiotics increasingly ineffective (Zhang et al., 2024a). Alarmingly, drug-resistant infections are responsible for over 1.2 million deaths annually, a number projected to rise to 10 million by 2050, surpassing cancer-related mortality (Xu et al., 2025). Even when initial antibiotic treatment is successful, infection relapse remains a major clinical challenge. Such relapses prolong patient suffering, extend antibiotic treatments, and accelerate the emergence of resistant bacterial strains, fueling a vicious cycle that undermines current therapeutic approaches (Kushwaha et al., 2024).
Infection recurrence is mainly due to the formation of biofilms and the transition of bacterial cells into a dormant, antibiotic-tolerant state known as persistence (Fisher et al., 2017). Biofilms—structured microbial communities encased in a protective extracellular matrix—impede antibiotic penetration and shield bacteria from host immune defenses (Lv et al., 2023; Xiu et al., 2020; Yang et al., 2023). In response to environmental stress or survival threats, some bacterial subpopulations become metabolically inactive and stop replication (Yin et al., 2025). Unlike genetically acquired antibiotic resistance, persisters exhibit antibiotic tolerance by entering a metabolically quiescent state (Yin et al., 2025). Because conventional antibiotics target actively dividing bacteria by disrupting essential metabolic functions, persister cells evade killing during treatment (Ahmad et al., 2025). When antibiotic concentrations drop below therapeutic thresholds or the host immune response wanes, these dormant cells can resuscitate and proliferate, ultimately causing infection relapse (Sett et al., 2024). While prolonged, high-dose antibiotic regimens may offer temporary relief, such therapeutic approaches pose risks of promoting resistance and disturbing the host microbiota (Szajewska et al., 2025). Thus, devising targeted strategies to eliminate persister cells has become a pressing priority for improving clinical outcomes in bacterial infections (Fisher et al., 2017).
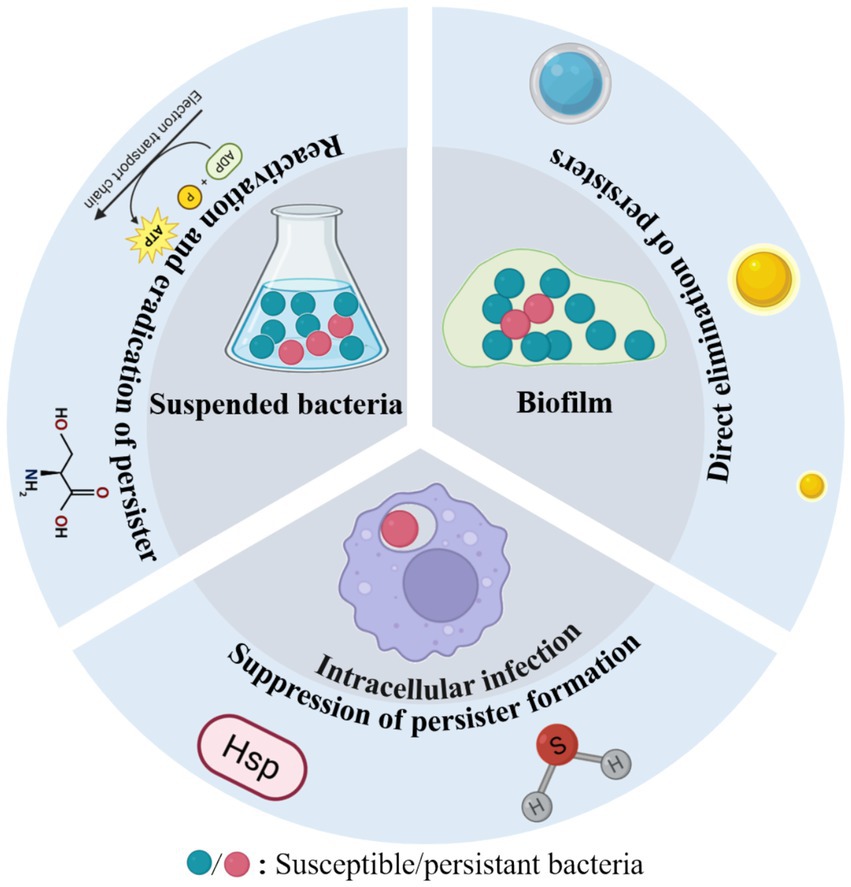
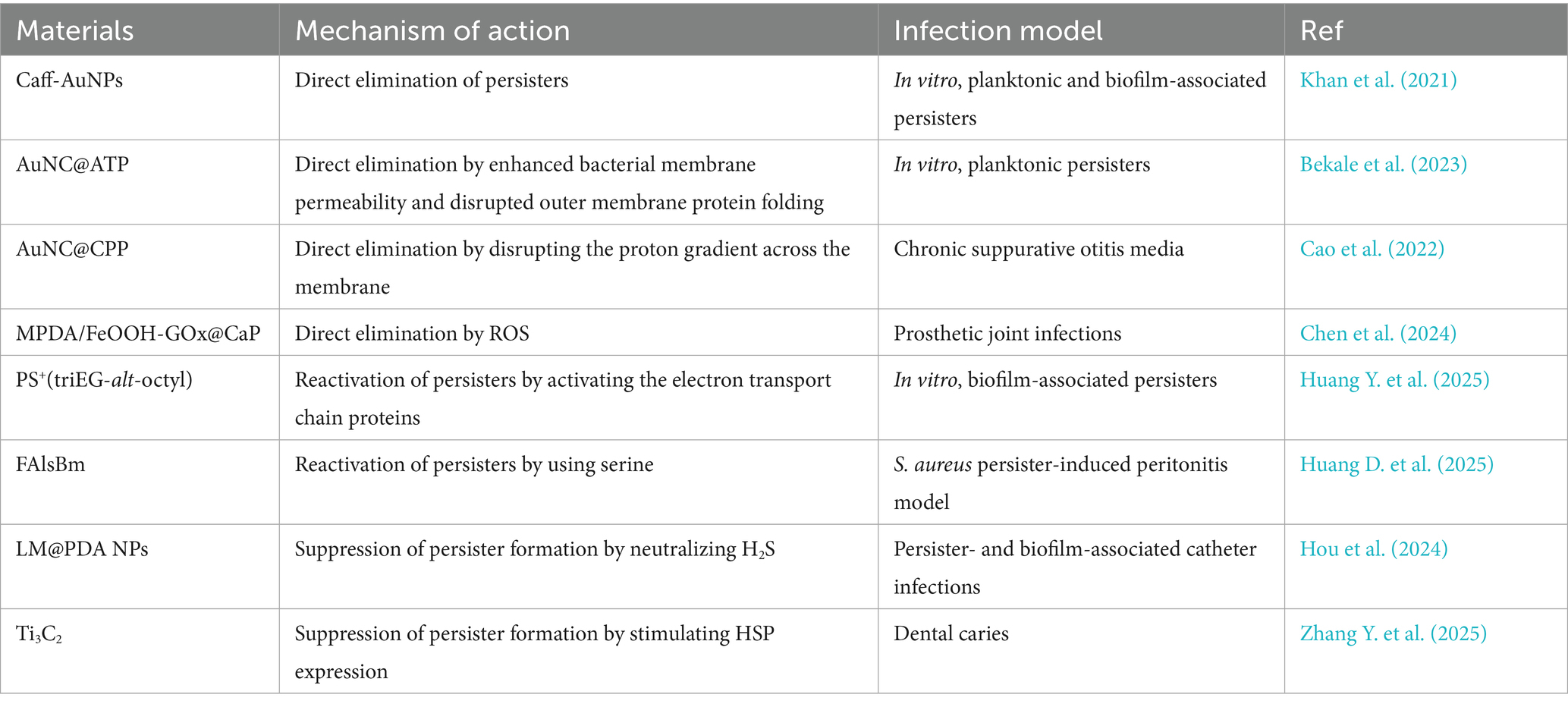
Antimicrobial nanomaterials combat bacterial persisters through diverse mechanisms, including physical disruption of cell structures, chemical interference with metabolic pathways, and biologically mediated effects (Geng et al., 2023; Zhang et al., 2024b). Compared to conventional antibiotics, these nanoagents exhibit superior efficacy against bacterial persisters due to several distinct advantages. First, their nanoscale dimensions and enhanced permeability enable deep penetration through dense extracellular polymeric substances (EPS), facilitating direct interaction with dormant cells embedded within biofilms. Second, their multimodal mechanisms of action—such as membrane perforation, reactive oxygen species (ROS) generation, and synergistic drug delivery—collectively reduce the likelihood of resistance development. Third, advanced surface functionalization enables biofilm matrix degradation, quorum sensing disruption, and targeted, sustained drug delivery and release, prolonging antimicrobial efficacy while minimizing adverse effects (Kalelkar et al., 2022; Makabenta et al., 2021). Notably, many of these materials exhibit excellent biocompatibility, making them promising candidates for clinical applications. In general, antibacterial nanoagents can effectively eliminate bacterial persisters through three principal strategies: (1) direct elimination of persisters, (2) reactivation of dormant bacteria followed by their eradication, and (3) suppression of persistent bacteria formation (Figure 1) (Yin et al., 2025). The following section summarizes the three aspects mentioned above and Table 1.
2 Literature search method
To identify relevant references, we performed a systematic literature search using Google Scholar. The primary keyword pairs used were “bacterial persister” AND “nano” or “persistent bacteria” AND “nano.”
3 The recent treatment strategies for Persistent bacterial infections
3.1 Direct elimination of persisters
Effective elimination of persistent cells requires potent disruption of essential bacterial components, including membranes, proteins, and nucleic acids. Recent researches highlight the potential of novel antibiotics (e.g., polymyxin B, colistin, and aureomycin A), antimicrobial peptides, and metal ions in achieving this goal (Niu et al., 2024). These agents exert strong bactericidal effects even against dormant populations, offering promising avenues for treating persistent infections. However, its potential side effects may prevent its use in living organisms.
Nanomaterials have garnered increasing attention in this context due to their facile functionalization, controllable release kinetics, and low toxicity (Lu et al., 2022; Zuo et al., 2026). For instance, Khan et al. developed caffeine-functionalized gold nanoparticles (Caff-AuNPs), which exhibited potent bactericidal activity against both planktonic and biofilm-associated Gram-positive and Gram-negative bacterial persisters. These nanoparticles not only disrupted mature biofilms but also effectively eradicated embedded dormant cells (Khan et al., 2021).
Bekale and colleagues further advanced this approach by engineering adenosine triphosphate (ATP)-functionalized gold nanoclusters (AuNC@ATP) that selectively enhanced bacterial membrane permeability and disrupted outer membrane protein folding. Remarkably, these nanoclusters achieved a dramatic 7-log reduction in persister cell populations at a concentration of 2.2 μM, while exhibiting minimal toxicity toward exponentially growing bacteria (<1 log reduction) (Bekale et al., 2023). Subsequently, the same group developed nanoclusters modified with a cell-penetrating peptide (CPP, amino acid sequence: YGRKKRRQRRRCPP), termed (AuNC@CPP). These CPP-modified nanoclusters induced membrane hyperpolarization by disrupting the proton gradient across the membrane. When combined with the antibiotic ofloxacin, AuNC@CPP effectively eradicated Pseudomonas aeruginosa PA01 persisters, demonstrating strong therapeutic potential for chronic suppurative otitis media (Cao et al., 2022).
Bacterial persisters are a key pathogenic factor in the recurrence of both chronic suppurative otitis media and prosthetic joint infections. To address this clinical challenge, Chen’s group developed ROS-generating hydrogel microspheres (MPDA/FeOOH-GOx@CaP) through a sophisticated multistep fabrication process (Figure 2) (Chen et al., 2024). First, the hydroxy iron oxide (FeOOH) nanocatalysts were grown in situ on mesoporous polydopamine (MPDA), followed by loading of glucose oxidase (GOx), The resulting nanoparticles were then sealed with a calcium phosphate (CaP) coating. Using microfluidic technology, the team co-encapsulated MPDA/FeOOH-GOx@CaP and glucose within hyaluronic acid methacrylate microspheres to form the HAMA composite gel microspheres. In the acidic microenvironment of infection sites, CaP dissolves, releasing GOx to catalyze the oxidation of glucose into H2O2, which is subsequently converted by FeOOH into membrane-damaging hydroxyl radicals via Fenton-like reactions. These hydrogel microspheres effectively eradicated Staphylococcus aureus and Staphylococcus epidermidis persisters, offering a promising strategy for treating prosthetic joint infections.
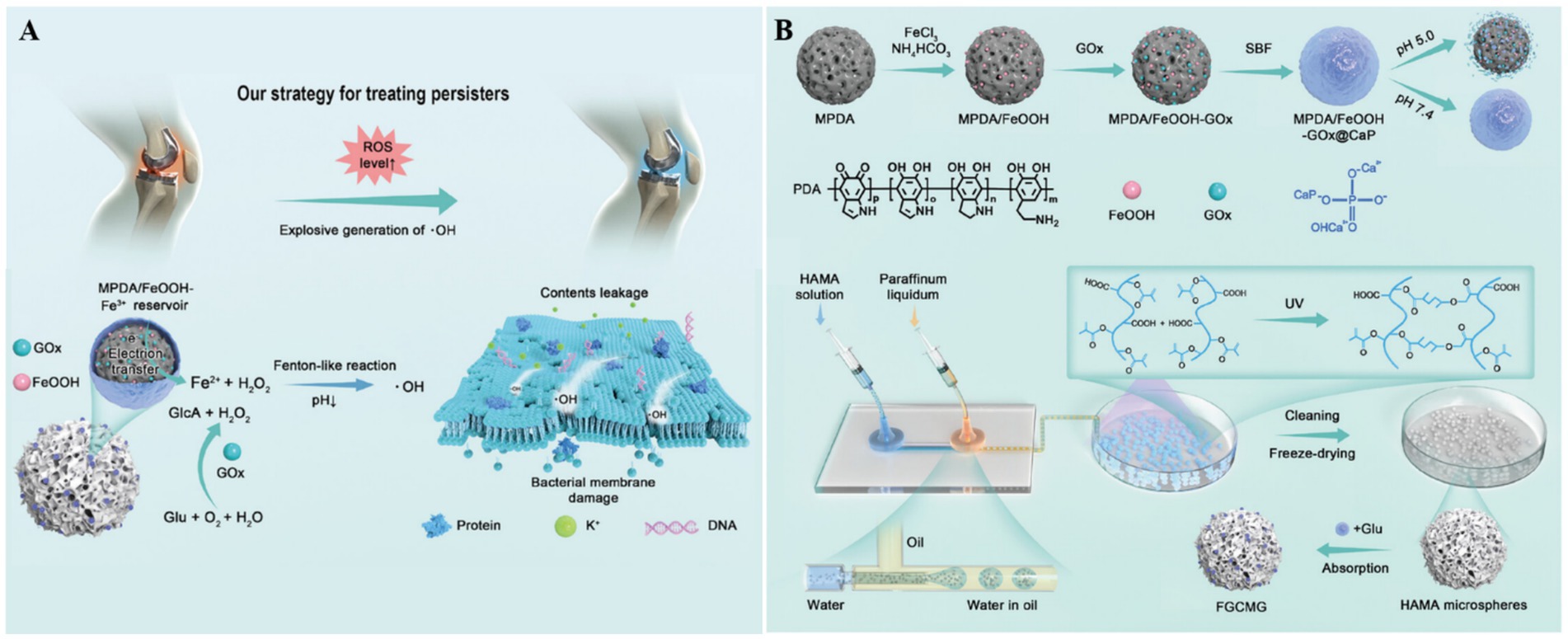
Figure 2. (A) The therapeutic mechanism of HAMA microspheres toward chronic periprosthetic joint infection. (B) The preparation process of HAMA microspheres. Reproduced with permission (Chen et al., 2024). Copyright 2024, Wiley.
3.2 Reactivation of dormant bacteria followed by their eradication
Metabolic activation strategies have become a widely used approach for eliminating bacterial persisters. Recent studies have demonstrated that stimulating the electron transport chain can effectively reactivate dormant bacteria, rendering them susceptible to antimicrobial agents (Kim et al., 2016). Building on this principle, Huang Y. et al. (2025) developed a cationic polymer, PS+(triEG-alt-octyl), capable of “waking up and killing” persisters (Figure 3A). This polymer first activates electron transport chain proteins, reawakening dormant bacteria, and subsequently disrupts bacterial membranes, leading to cell lysis and death. To improve delivery efficiency within bacterial biofilms, PS+(triEG-alt-octyl) was loaded onto PDA nanoparticles (triEG referring to triethylene glycol segment). Upon light irradiation, the PS+(triEG-alt-octyl)PDA nanoparticles enable photothermal-triggered polymer release. In addition, PDA enhances the diffusion across the biofilm’s EPS. As a result, the PS+(triEG-alt-octyl)PDA nanoparticles exhibit potent antibiofilm activity, effectively clearing persistent biofilms.
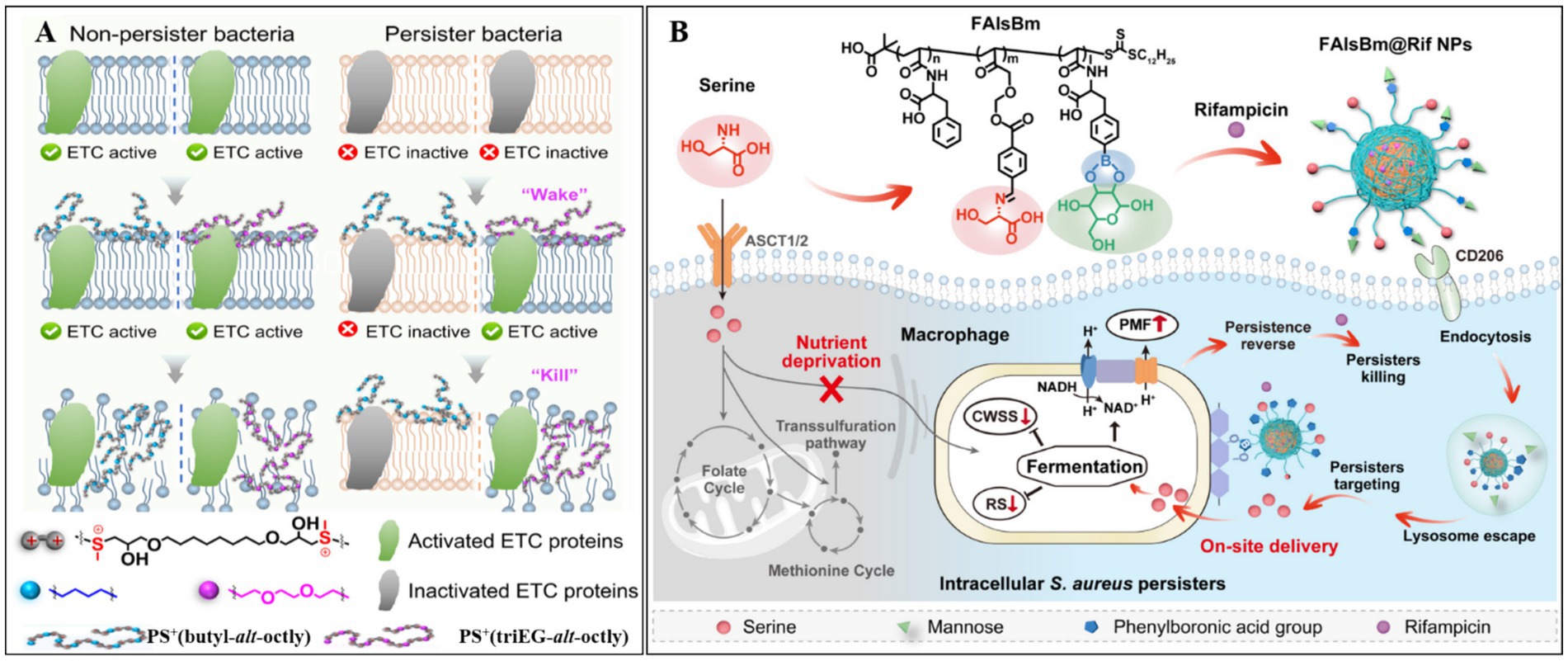
Figure 3. (A) The principle of PS+(triEG-alt-octyl) polymer in removing persister bacteria. Reproduced with permission (Huang Y. et al., 2025). Copyright 2025, American Chemical Society. (B) Mechanism of FalsBm@Rif NPs in eliminating intracellular Staphylococcus aureus persister. CWSS, cell wall stress stimulation; SR, stringent response. Reproduced with permission (Huang D. et al., 2025). Copyright 2025, American Chemical Society.
In a parallel study, Wang et al. (2021) developed a poly-amino acid-based nanodelivery system (FAlsBm) by covalently conjugating mannose (Man) and serine (Ser) to the side chains of a polymer [i.e., poly(N-acryloyl phenylalanine)-b-poly(2-((4-formylbenzyl)oxy) acrylic acid ethyl ester)-b-poly((S)-2-acrylamido-3-(4-boronphenyl) propionic acid, FAlsB) via ester and imine linkages]. Rifampicin (Rif) was then encapsulated through self-assembly (Figure 3B) (Huang D. et al., 2025). The resulting FAlsBm@Rif system featured a dual-targeting mechanism: first, mannose-mediated macrophage-specific uptake via CD206 mannose receptor binding; second, upon lysosomal internalization, acidic cleavage of ester/imine bonds released Man/Ser while exposing phenylboronic acid groups, which redirected the nanoparticles to Staphylococcus aureus. The liberated serine acted as a nutritional stimulus to revive dormant bacteria, enhancing their susceptibility to rifampicin. In animal experiments, FAlsBm@Rif, achieved 99.78% clearance of intracellular bacteria—a 1.6-fold improvement over conventional rifampicin treatment (63.41% clearance). This intelligent “cell-to-bacteria” targeting strategy offers a promising solution for combating recalcitrant intracellular infections.
3.3 Suppression of persistent bacteria formation
The formation of bacterial persisters is a multifactorial process regulated by several key regulatory mechanisms, including toxin-antitoxin (TA) systems, stress response pathways mediated by (p)ppGpp signaling, SOS repair systems, and quorum sensing (Niu et al., 2024; Yin et al., 2025). Recent studies have suggested that hydrogen sulfide (H2S) and heat shock proteins (HSPs) are also involved in this process. Under environmental stress, bacteria produce H2S, which inhibits the synthesis of adenosine triphosphoric acid, induces a dormant state, and promotes the formation of drug-resistant persister cells. To counteract this mechanism, Hou et al. (2024) developed an innovative H2S-scavenging antibacterial platform. Using ultrasonication, they synthesized PDA-coated liquid metal (LM) gallium nanoparticles (LM@PDA NPs), which were subsequently functionalized with urease and silver nanoparticles (Ag NPs) to produce a multifunctional nanocomposite (LPUA NPs). The PDA coating ensures stable immobilization of LPUA NPs onto urinary catheter surfaces, where they exhibit dual biological functions. First, urease catalyzes the hydrolysis of urea to generate ammonia, which neutralizes bacterial H2S. In parallel, excess H2S reacts with liquid gallium to form Ga2S3, thereby suppressing persister cell formation and enhancing the antibacterial activity of released Ga3+/Ag+. Second, the intrinsic deformability of LM nanoparticles facilitates deep biofilm penetration, enabling efficient bacterial clearance.
Adverse environmental conditions can induce protein misfolding in bacteria, leading to protein aggregation, metabolic activity slowdown, and persister cell formation. HSPs play a critical role in maintaining proteostasis by preventing protein aggregation. Leveraging the temperature-dependent upregulation of HSP expression, Zhang Y. et al. (2025) employed the photothermal and nanoknife properties of Ti3C2 MXene to suppress persister formation and enhance the elimination of cariogenic bacteria. Upon light irradiation, Ti3C2 generates localized heat, stimulating HSP expression to mitigate protein misfolding, thereby reducing persister formation and increasing bacterial membrane permeability. Simultaneously, Ti3C2-mediated photothermal effect disrupts bacterial membranes, causing cytoplasmic leakage and cell death. Compared to traditional antibiotics, this strategy exhibits superior efficacy in eradicating both planktonic and biofilm-embedded persister cells, offering a promising therapeutic avenue for the treatment and prevention of dental caries (Zhang Y. et al., 2025) (see Figure 4).
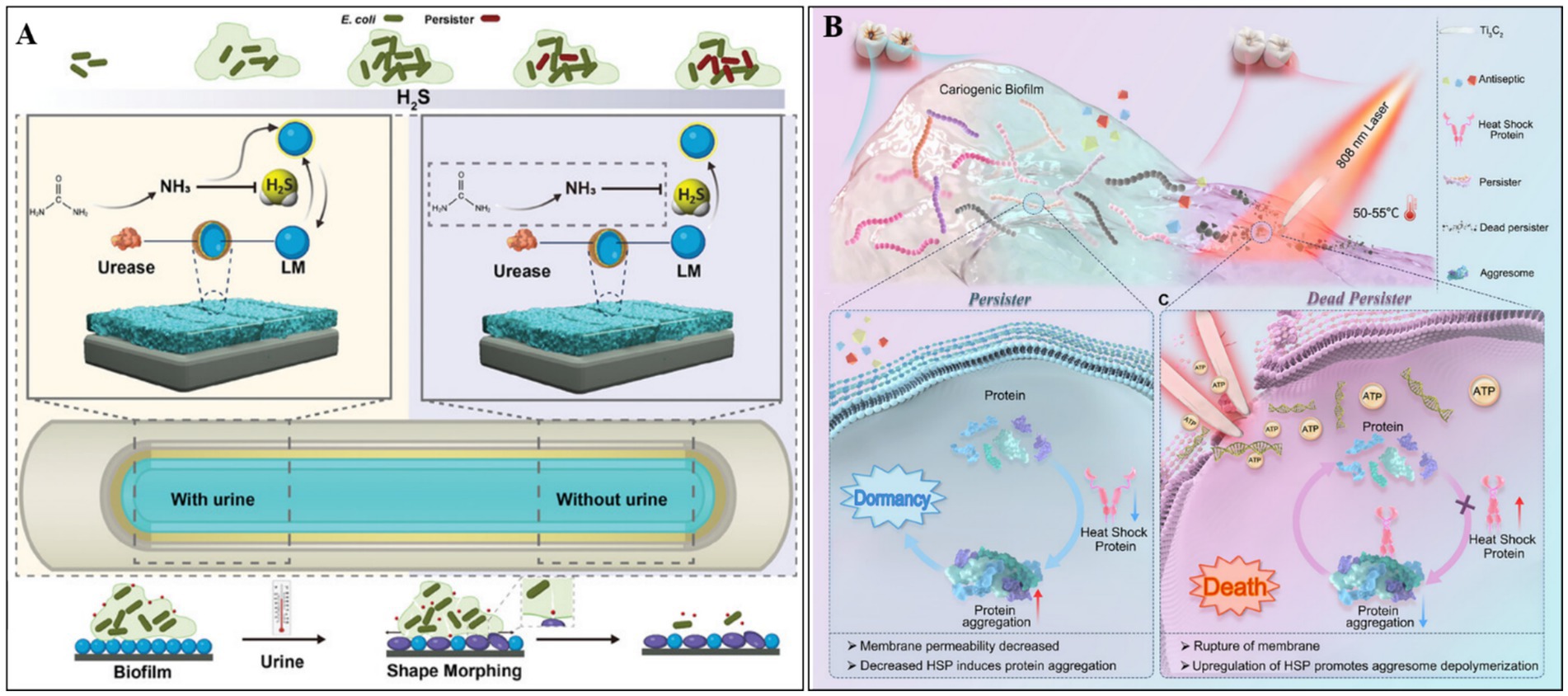
Figure 4. (A) The inhibitory mechanism of LM@PDA@Urease&Ag NPs (LPUA NPs) against persistent bacterial formation. Reproduced with permission (Hou et al., 2024). Copyright 2024, Wiley. (B) Ti3C2-based photothermal therapy suppresses persistent bacterial formation via inducing HSP expression and eradicates persister cells by nanoknife effect and photothermal removal. Reproduced with permission (Zhang Y. et al., 2025). Copyright 2025, Wiley.
4 Conclusion and outlook
In conclusion, this minireview outlines the challenges of eliminating persistent bacteria and the potential of nanoantibacterial agents to address them. Then these nanoagents are systematically categorized by their mechanism of action: direct eradication, awakening persistent bacteria, or inhibiting persistent bacteria formation. Despite their promise, these approaches face several challenges that must be resolved before broader application.
Antibacterial nanomaterials are currently synthesized using a range of methodologies, including physical techniques (e.g., laser ablation, ball milling), chemical approaches (e.g., chemical reduction, sol–gel), and bio-based strategies (e.g., plant- or microorganism-mediated green synthesis) (Rathore et al., 2023; Song et al., 2026; Wei et al., 2023). Their development is increasingly oriented toward green sustainability, precise synthesis control, and scalable industrialization. In clinical contexts, these antibacterial nanoagents demonstrate significant potential across various applications, including anti-biofilm therapies (e.g., medical device coatings, chronic wound management), targeted drug delivery systems (e.g., antibiotic-loaded nano-carriers), and integrated diagnostics-therapeutics (e.g., multimodal imaging-guided antibacterial interventions). However, clinical translation remains limited due to several key challenges: (1) Biological safety concerns, such as nanoparticle-induced cytotoxicity and long-term biodistribution risks; (2) Evolution of antibacterial resistance; (3) Manufacturing challenges, including batch-to-batch variability, nanoparticle aggregation, and issues with the stability of antibacterial activity of material properties during storage (Solanki et al., 2024; Zhang J. et al., 2025); (4) Lack of standardized international regulatory frameworks for nanomedicine evaluation and approval; (5) Current research lacks a deep understanding of how nanomaterials specifically target and eliminate persistent bacteria. Consequently, a key challenge is the precise design of nanoscale antibacterial agents to enhance their targeting and efficacy while minimizing toxicity to host cells.
To overcome these barriers, several innovative strategies are being explored: (1) Toxicity mitigation through surface functionalization (e.g., PEGylation) and the use of biodegradable matrices (e.g., chitosan); (2) Synergistic antibacterial regimens that integrate photothermal or photocatalytic therapies with conventional antibiotics to suppress resistance development (Cui et al., 2024; Wang et al., 2021); (3) Microfluidic-based precision synthesis to improve nanoparticle uniformity; (4) Promotion of harmonized international regulatory frameworks for clinic translation of nanomedicines; (5) Functionalize nanomaterials by targeting specific molecular markers unique to persistent bacteria (Zhang et al., 2021); (6) Design smart nanosystems that remain inert until activated by the unique signals of an infection site. Looking ahead, convergent technological innovations—such as AI-driven material optimization, organ-on-a-chip platforms for toxicity screening, and patient-specific nanotherapeutic designs—are poised to revolutionize the field of nanomedicine. These advancements may establish nanoscale antibacterial agents as pivotal tools in the post-antibiotic era, offering promising solutions to the growing threat of multidrug-resistant pathogens.
In addition, two critical challenges remain in the study of bacterial persisters: (1) The lack of standardized, high-throughput detection methods, and (2) Significant limitations in current screening approaches. Although antibiotic treatment is widely recognized as the primary trigger for persister cell formation, substantial variability in the metabolic profiles of these antibiotic-induced persisters remains—an observation that continues to generate scientific debate. Therefore, the establishment of reproducible induction protocols and the development of reliable, high-sensitivity detection methods are essential prerequisites for the rational design and evaluation of nano-therapeutics targeting persister cells.
Author contributions
YH: Conceptualization, Writing – original draft. DY: Conceptualization, Project administration, Writing – review & editing. LY: Data curation, Methodology, Writing – review & editing. GZ: Conceptualization, Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to express my gratitude to my colleagues and the reviewers for their valuable suggestions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmad, M., Aduru, S. V., Smith, R. P., Zhao, Z., and Lopatkin, A. J. (2025). The role of bacterial metabolism in antimicrobial resistance. Nat. Rev. Microbiol. 23, 439–454. doi: 10.1038/s41579-025-01155-0
Bekale, L. A., Sharma, D., Bacacao, B., Chen, J., and Maria, P. L. S. (2023). Eradication of bacterial persister cells by leveraging their low metabolic activity using adenosine triphosphate coated gold nanoclusters. Nano Today 51:101895. doi: 10.1016/j.nantod.2023.101895
Cao, Z., Chen, X., Chen, J., Xia, A., Bacacao, B., Tran, J., et al. (2022). Gold nanocluster adjuvant enables the eradication of persister cells by antibiotics and abolishes the emergence of resistance. Nanoscale 14, 10016–10032. doi: 10.1039/D2NR01003H
Chen, Y., Ding, T., Du, Y., Ling, S., Meng, C., Zhuang, P., et al. (2024). Targeting bacterial Persisters via reactive oxygen species-generating hydrogel microspheres. Adv. Funct. Mater. 34:2405199. doi: 10.1002/adfm.202405199
Cui, L., Fan, L., and Shen, Z. (2024). Application research Progress of nanomaterial graphene and its derivative complexes in tumor diagnosis and therapy. Curr. Med. Chem. 31, 6436–6459. doi: 10.2174/0109298673251648231106112354
Dai, T., Vrahas, M. S., Murray, C. K., and Hamblin, M. R. (2012). Ultraviolet C irradiation: an alternative antimicrobial approach to localized infections? Expert Rev. Anti-Infect. Ther. 10, 185–195. doi: 10.1586/eri.11.166
Fisher, R. A., Gollan, B., and Helaine, S. (2017). Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 15, 453–464. doi: 10.1038/nrmicro.2017.42
Geng, Z., Cao, Z., and Liu, J. (2023). Recent advances in targeted antibacterial therapy basing on nanomaterials. Exploration 3:20210117. doi: 10.1002/EXP.20210117
Gollan, B., Grabe, G., Michaux, C., and Helaine, S. (2019). Bacterial Persisters and infection: past, present, and progressing. Ann. Rev. Microbiol. 73, 359–385. doi: 10.1146/annurev-micro-020518-115650
Hou, Z., Ren, X., Sun, Z., An, R., Huang, M., Gao, C., et al. (2024). Trash into treasure: Nano-coating of catheter utilizes urine to deprive H2S against Persister and rip biofilm matrix. Adv. Healthc. Mater. 13:e2401067. doi: 10.1002/adhm.202401067
Hu, Y., Yang, K., Li, N., Yang, D., and Dong, H. (2025). Gas-generating photocatalytic agents for bacterial infection treatment. Research 8:0672. doi: 10.34133/research.0672
Huang, Y., Cai, S., Shi, L., Zhu, Z., Rao, J., Yu, X., et al. (2025). Hydrophilic–hydrophobic sequence regulation in alternating Polysulfoniums enables “wake-and-kill” eradication of antibiotic-tolerant Persisters and their biofilms. ACS Macro Lett. 14, 878–887. doi: 10.1021/acsmacrolett.5c00253
Huang, D., Kang, X., Yin, Z., Zhao, D., Ning, Y., Liu, H., et al. (2025). On-site serine delivery drives fermentation pathway reprogramming to reverse intracellular Staphylococcus aureus persistence. ACS Nano 19, 26075–26090. doi: 10.1021/acsnano.5c06864
Kalelkar, P. P., Riddick, M., and García, A. J. (2022). Biomaterial-based antimicrobial therapies for the treatment of bacterial infections. Nat. Rev. Mater. 7, 39–54. doi: 10.1038/s41578-021-00362-4
Khan, F., Park, S.-K., Bamunuarachchi, N. I., Oh, D., and Kim, Y.-M. (2021). Caffeine-loaded gold nanoparticles: antibiofilm and anti-persister activities against pathogenic bacteria. Appl. Microbiol. Biotechnol. 105, 3717–3731. doi: 10.1007/s00253-021-11300-3
Kim, J.-S., Cho, D.-H., Heo, P., Jung, S.-C., Park, M., Oh, E.-J., et al. (2016). Fumarate-mediated persistence of Escherichia coli against antibiotics. Antimicrob. Agents Chemother. 60, 2232–2240. doi: 10.1128/AAC.01794-15
Kushwaha, S. O., Sahu, S. K., Yadav, V. K., Rathod, M. C., Patel, D., Sahoo, D. K., et al. (2024). Bacteriophages as a potential substitute for antibiotics: a comprehensive review. Cell Biochem. Funct. 42:e4022. doi: 10.1002/cbf.4022
Lu, P., Zhang, X., Li, F., Xu, K.-F., Li, Y.-H., Liu, X., et al. (2022). Cationic liposomes with different lipid ratios: antibacterial activity, antibacterial mechanism, and cytotoxicity evaluations. Pharmaceuticals 15:1556. doi: 10.3390/ph15121556
Lv, X., Wang, L., Mei, A., Xu, Y., Ruan, X., Wang, W., et al. (2023). Recent nanotechnologies to overcome the bacterial biofilm matrix barriers. Small 19:e2206220. doi: 10.1002/smll.202206220
Makabenta, J. M. V., Nabawy, A., Li, C.-H., Schmidt-Malan, S., Patel, R., and Rotello, V. M. (2021). Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 19, 23–36. doi: 10.1038/s41579-020-0420-1
Mo, Y., Tan, W. C., and Cooper, B. S. (2025). Antibiotic duration for common bacterial infections—a systematic review. JAC Antimicrob. Resist. 7:dlae215. doi: 10.1093/jacamr/dlae215
Niu, H., Gu, J., and Zhang, Y. (2024). Bacterial persisters: molecular mechanisms and therapeutic development. Signal Transduct. Target. Ther. 9:174. doi: 10.1038/s41392-024-01866-5
Rathore, C., Yadav, V. K., Gacem, A., AbdelRahim, S. K., Verma, R. K., Chundawat, R. S., et al. (2023). Microbial synthesis of titanium dioxide nanoparticles and their importance in wastewater treatment and antimicrobial activities: a review. Front. Microbiol. 14:1270245. doi: 10.3389/fmicb.2023.1270245
Sett, A., Dubey, V., Bhowmik, S., and Pathania, R. (2024). Decoding bacterial persistence: mechanisms and strategies for effective eradication. ACS Infect. Dis. 10, 2525–2539. doi: 10.1021/acsinfecdis.4c00270
Solanki, R., Makwana, N., Kumar, R., Joshi, M., Patel, A., Bhatia, D., et al. (2024). Nanomedicines as a cutting-edge solution to combat antimicrobial resistance. RSC Adv. 14, 33568–33586. doi: 10.1039/D4RA06117A
Song, Y.-N., Wang, Y.-B., Yang, K.-Q., Sun, Y.-R., Wang, Z.-Y., Shi, W., et al. (2026). Natural product-based sonosensitizers for bacterial infection treatment. Tradit. Med. Res. 11, 22–28. doi: 10.53388/TMR20250305001
Szajewska, H., Scott, K. P., de Meij, T., Forslund-Startceva, S. K., Knight, R., Koren, O., et al. (2025). Antibiotic-perturbed microbiota and the role of probiotics. Nat. Rev. Gastroenterol. Hepatol. 22, 155–172. doi: 10.1038/s41575-024-01023-x
Wang, X., Han, X., Li, C., Chen, Z., Huang, H., Chen, J., et al. (2021). 2D materials for bone therapy. Adv. Drug Deliv. Rev. 178:113970. doi: 10.1016/j.addr.2021.113970
Wei, J., Zhu, L., Lu, Q., Li, G., Zhou, Y., Yang, Y., et al. (2023). Recent progress and applications of poly(beta amino esters)-based biomaterials. J. Control. Release 354, 337–353. doi: 10.1016/j.jconrel.2023.01.002
Xiu, W., Gan, S., Wen, Q., Dai, S., Dong, H., Li, Q., et al. (2020). Biofilm microenvironment-responsive nanotheranostics for dual-mode imaging and hypoxia-relief-enhanced photodynamic therapy of bacterial infections. Research 2020:9426453. doi: 10.34133/2020/9426453
Xu, J., Liu, J., Li, X., Zhao, L., Shen, J., and Xia, X. (2025). Burden of bacterial antimicrobial resistance in China: a systematic analysis from 1990 to 2021 and projections to 2050. J. Adv. Res. doi: 10.1016/j.jare.2025.06.021
Yang, D., Ding, M., Song, Y., Hu, Y., Xiu, W., Yuwen, L., et al. (2023). Nanotherapeutics with immunoregulatory functions for the treatment of bacterial infection. Biomater. Res. 27:73. doi: 10.1186/s40824-023-00405-7
Yin, Z., Huang, D., Kuhn, E. M. A., Moriarty, T. F., Li, G., and Wang, X. (2025). Unraveling persistent bacteria: formation, niches, and eradication strategies. Microbiol. Res. 297:128189. doi: 10.1016/j.micres.2025.128189
Zhang, J., Dong, H., Liu, B., and Yang, D. (2024a). Biomimetic materials for antibacterial applications. Small 21:2408543. doi: 10.1002/smll.202408543
Zhang, J., Guo, H., Liu, M., Tang, K., Li, S., Fang, Q., et al. (2023). Recent design strategies for boosting chemodynamic therapy of bacterial infections. Exploration 4:20230087. doi: 10.1002/EXP.20230087
Zhang, W., Hu, E., Wang, Y., Miao, S., Liu, Y., Hu, Y., et al. (2021). Emerging antibacterial strategies with application of targeting drug delivery system and combined treatment. Int. J. Nanomedicine 16, 6141–6156. doi: 10.2147/IJN.S311248
Zhang, J., Liu, M., Guo, H., Gao, S., Hu, Y., Zeng, G., et al. (2024b). Nanotechnology-driven strategies to enhance the treatment of drug-resistant bacterial infections. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 16:e1968. doi: 10.1002/wnan.1968
Zhang, J., Tang, K., Yang, Y., Yang, D., and Fan, W. (2025). Advanced Nanoprobe strategies for imaging macrophage polarization in Cancer immunology. Research 8:0622. doi: 10.34133/research.0622
Zhang, Y., Yang, L., jiao, J., Li, W., Lin, S., Zong, X., et al. (2025). A MXene-based nanothermal knife inhibits aggresome-mediated persister formation for preventing dental caries. Adv. Sci. 12:e01501. doi: 10.1002/advs.202501501
Keywords: bacterial persisters, nanomaterial, recurrent infection, antimicrobial strategies, bacterial infection
Citation: Hu Y, Yang D, Yuwen L and Zeng G (2025) Antibacterial nanoagents: an emerging arsenal against bacterial persisters. Front. Microbiol. 16:1688413. doi: 10.3389/fmicb.2025.1688413
Edited by:
Jess Vergis, Indian Veterinary Research Institute, IndiaReviewed by:
Vajid Nettoor Veettil, IQRAA International Hospital and Research Centre, IndiaCopyright © 2025 Hu, Yang, Yuwen and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongliang Yang, eWFuZ2RsMTAyM0BuanRlY2guZWR1LmNu; Lihui Yuwen, aWFtbGh5dXdlbkBuanVwdC5lZHUuY24=; Guisheng Zeng, emVuZ19ndWlzaGVuZ0BhLXN0YXIuZWR1LnNn
 Yanling Hu1
Yanling Hu1 Dongliang Yang
Dongliang Yang Lihui Yuwen
Lihui Yuwen