- Department of Parasitology, Faculty of Medicine, Universiti Malaya, Kuala Lumpur, Malaysia
Gametocytogenesis is a crucial process in which malaria parasites transition from their asexual stage to the sexual-stage gametocytes. This transformation enables the parasite to infect and multiply within the Anopheles mosquito, the vector responsible for transmitting the disease between hosts. Understanding how gametocytogenesis works and how it can be controlled offer insights into the malaria life cycle and potential strategies for controlling the transmission. Significant efforts have been dedicated to gametocytogenesis induction in the laboratory using in vitro parasite cultures over the past few decades. This mini review aims to summarize the various gametocytogenesis induction methods employed thus far in human Plasmodium species, moving from conventional means of environmental stressors to the cutting-edge technology of genome editing to achieve precise modifications on various sexual conversion-related genes. While massive strides have been made in both domains of gametocytogenesis induction methods, the scalability of gametocytogenesis induction leaves much to be desired, especially for non falciparum species. In conclusion, integrating knowledge from both approaches is crucial to developing highly efficient methods for inducing gametocytogenesis in Plasmodium spp. This integration can enhance our understanding of the processes involved in gametocytogenesis and support the search for novel strategies with potential implications for malaria control and eradication.
1 Introduction
Despite malaria’s first documentation back in 500 B.C.E, humanity has yet to develop a high efficacy vaccine for this disease (Palatnik-de-Sousa and Nico, 2020). In 2023, there were approximately 263 million cases worldwide (World Health Organization, 2024); Malaria parasites are able to infect the female Anopheles mosquito vector after it takes a blood meal from a host infected with malaria, spreading the disease when the infected vector takes a different blood meal from a different host. In humans the five significant species are Plasmodium falciparum, Plasmodium ovale, Plasmodium malariae, Plasmodium vivax and Plasmodium knowlesi (Sato, 2021). Gametocytes are the sole stage of Plasmodium species capable of transmission to mosquitoes, wherein it matures into sporozoites within the infected female mosquitoes thus contributing to infection of the host (Ngotho et al., 2019). As malaria parasites become increasingly resistant to widely used schizonticidal drugs, transmission-blocking strategies have attained prominence in malaria control efforts, with gametocytes emerging as promising targets for new drug development (Kumar et al., 2018; Wadi et al., 2020). Conventional antimalarials are effective against asexual stages and immature gametocytes, but they have limited impact on mature gametocytes, which are crucial for transmission (Baker, 2010; Portugaliza et al., 2020). Looking into vaccine development, P. falciparum sporozoite (PfSPZ) formulations by Sanaria Inc. have demonstrated moderate protection in clinical trials using radiated-, chemo-, or genetically attenuated sporozoites (Bijker et al., 2015; Duffy, 2022). In order to obtain these sporozoites, mosquito infections using gametocytes need to be carried out. A more recent study has also shown to expose a vulnerability in the sporozoite stage of the parasite by targeting a previously cryptic epitope on the malaria parasite’s circumsporozoite protein (PfCSP), allowing the most potent antibody in this group, MAD21-101, provide complete protection against P. falciparum infection in a humanized mouse model (Dacon et al., 2025). All these studies stress the importance of large-scale gametocyte production to acquire sufficient study materials.
2 Gametocytogenesis and its regulation
The decision to become a gametocyte appears to be made during the asexual cycle, prior to the formation of gametocytes. The sexual differentiation necessary for transmission starts when asexual parasites commit to gametocytogenesis (Baker, 2010). This sexually committed stage, which deterministically leads to sexual development subsequently, is identified by the expression of PfAP2-G. Sexual conversion then follows commitment, and present evidence suggests that this can occur through either same-cycle conversion or next-cycle conversion, both of which result in sexual ring stages that eventually develop into gametocytes (Portugaliza et al., 2019). In P. falciparum, low quantities of gametocytes usually appear in peripheral blood around the 10–12th day following the onset of fever and continue to rise during the next 2–3 weeks (Shute and Maryon, 1951; Gautam et al., 2020). On the contrary, P. knowlesi gametocytes mature quickly within 1.5–2 days, and have a remarkably short lifespan in the bloodstream with viability ranging 5–12 h before degenerating (Hawking et al., 1968; Carter et al., 1988; Amir, 2016). P. vivax gametocytes arise early in infection, appearing within 3 days after the emergence of asexual parasites whereas P. malariae gametocyte production is most likely to begin during the early stages of intraerythrocytic asexual replication (Bousema and Drakeley, 2011; Fatmaningsih et al., 2024). In P. ovale, gametocyte formation begins continuously with the early rounds of intraerythrocytic asexual reproduction and appears in the peripheral blood slightly earlier than in benign tertian malaria (Shute and Maryon, 1951). The gametocytes take about 5 days to mature, although their lifespan remains unknown (Amir, 2016).
Zooming down to the molecular mechanism behind gametocytogenesis reveals that at the core of the sexual commitment switch in P. falciparum is the transcription factor AP2-G, a member of the ApiAP2 family, which has been associated with the positive regulation of sexual commitment and regulates over 400 genes involved in the early stages of gametocytogenesis. The expression of AP2-G is tightly linked to gametocyte formation and maturation, and disruption of this gene results in a complete block of gametocytogenesis (Kafsack et al., 2014; Sinha et al., 2014). Among the genes notably upregulated during PfAP2-G overexpression are msrp1, GEXP05, and Pfs16. An experimental parasites line lacking msrp1 (Δmsrp1) exhibit a slightly elevated commitment rate, suggesting that while it serves as a marker of sexually committed parasites, however, it is not essential for inducing or driving gametocyte formation (Josling et al., 2020). Although GEXP05 and Pfs16 are expressed as early as the sexual ring stage, its transcripts are insufficiently specific as markers for sexual parasites (Portugaliza et al., 2019; Singh et al., 2021). In contrast, another gene GEXP02 was identified as a valuable early gametocyte marker and a reliable indicator of sexual conversion (Portugaliza et al., 2019). Under normal asexual conditions, the ap2-g locus is embedded in heterochromatin, marked by H3K9me3 and bound by heterochromatin protein 1 (HP1), maintaining it in a silenced state, and this heterochromatin-based silencing prevents the premature or unnecessary activation of gametocyte development, ensuring that most parasites remain in a proliferative asexual cycle (Brancucci et al., 2014; Bui Hai et al., 2021). In P. falciparum, the GDV1 (Gametocyte Development 1) protein acts upstream in this regulatory hierarchy (Filarsky et al., 2018). GDV1 binds to heterochromatic regions and physically interacts with HP1, triggering HP1 eviction specifically from the ap2-g locus. This chromatin remodeling, which is the ATP-driven reorganization of nucleosomes that opens compacted DNA (Phillips and Shaw, 2008) derepresses ap2-g, allowing its transcription and subsequent expression of PfAP2-G protein. Environmental cues such as nutrient availability influence GDV1 expression, and an antisense RNA regulates its levels, providing a layer of responsiveness to external triggers (Rea et al., 2018). Once expressed, PfAP2-G initiates a positive feedback loop by binding its own promoter and upregulating early gametocyte genes, solidifying sexual commitment. However, while ap2-g is essential for commitment and early gametocytogenesis, its necessity for later stages, such as maturation to stage V, has yet to be clarified (Josling et al., 2020).
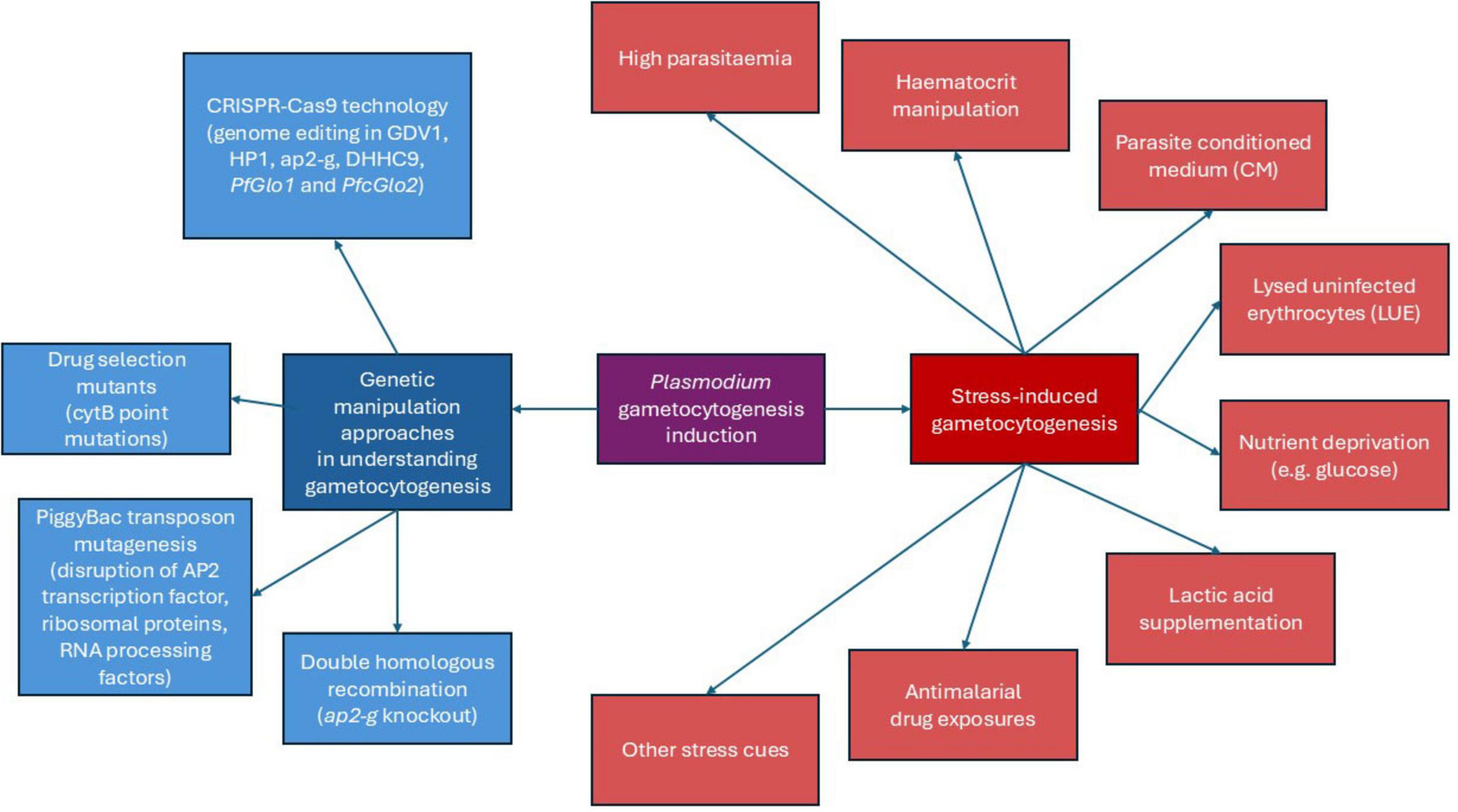
As gametocytogenesis plays a critical role in the transmission of parasites into the mosquito vector, understanding and controlling gametocytogenesis is essential not only for interrupting the transmission cycle but also for developing targeted strategies aimed at reducing the spread of malaria. The next sections summarize the various gametocytogenesis induction methods focusing on stress-based methods and genetic manipulation approaches employed in human Plasmodium species to understand the gametocytogenesis mechanisms thus far (Figure 1).
3 Stress-induced gametocytogenesis
Plasmodium falciparum accounts for over 90% of global malaria cases and is responsible for the majority of malaria-related deaths (World Health Organization, 2024). It is also one of the two only human-infecting Plasmodium species that can be continuously cultured in vitro. P. falciparum was first cultured continuously in vitro by Trager and Jensen (1976), using human red blood cells and serum in RPMI 1640 medium under specific gas conditions (low oxygen and high carbon dioxide) (Trager and Jensen, 1976). Then, P. knowlesi in vitro culture was established in 2010s adopting the similar protocol, thus making it the primary focus of most laboratory-based research.
A basic prerequisite for studying gametocyte biology and developing drugs and vaccines is the availability of healthy, viable, and reproducible gametocytes in vitro (Omorou et al., 2022). Most in vitro research to date has used environmental stressors to trigger gametocytogenesis, mainly targeting P. falciparum. These stressors include nutrient deprivation, sudden drops in haematocrit, conditioned media from parasite cultures that contain metabolites and secreted stress signals, elevated parasitaemia, and adjuvants that mimic the physiological conditions found in the host. Laboratory research involving P. vivax commonly requires the use of infected non-human primates owing to the inability to continuously culture the parasite in vitro (Galinski and Barnwell, 2008; Gunalan et al., 2020). Meanwhile, P. knowlesi’s asexual phases have been successfully modified for in vitro culture in human and macaque erythrocytes (Moon et al., 2013; Armistead et al., 2018). Nevertheless, researchers have yet to report efficient methods for gametocyte production in P. knowlesi. To date, only Armistead et al. (2018) have achieved gametocyte production using a P. knowlesi in vitro culture strain along with sporadic, low-level mosquito infectivity in transmission study.
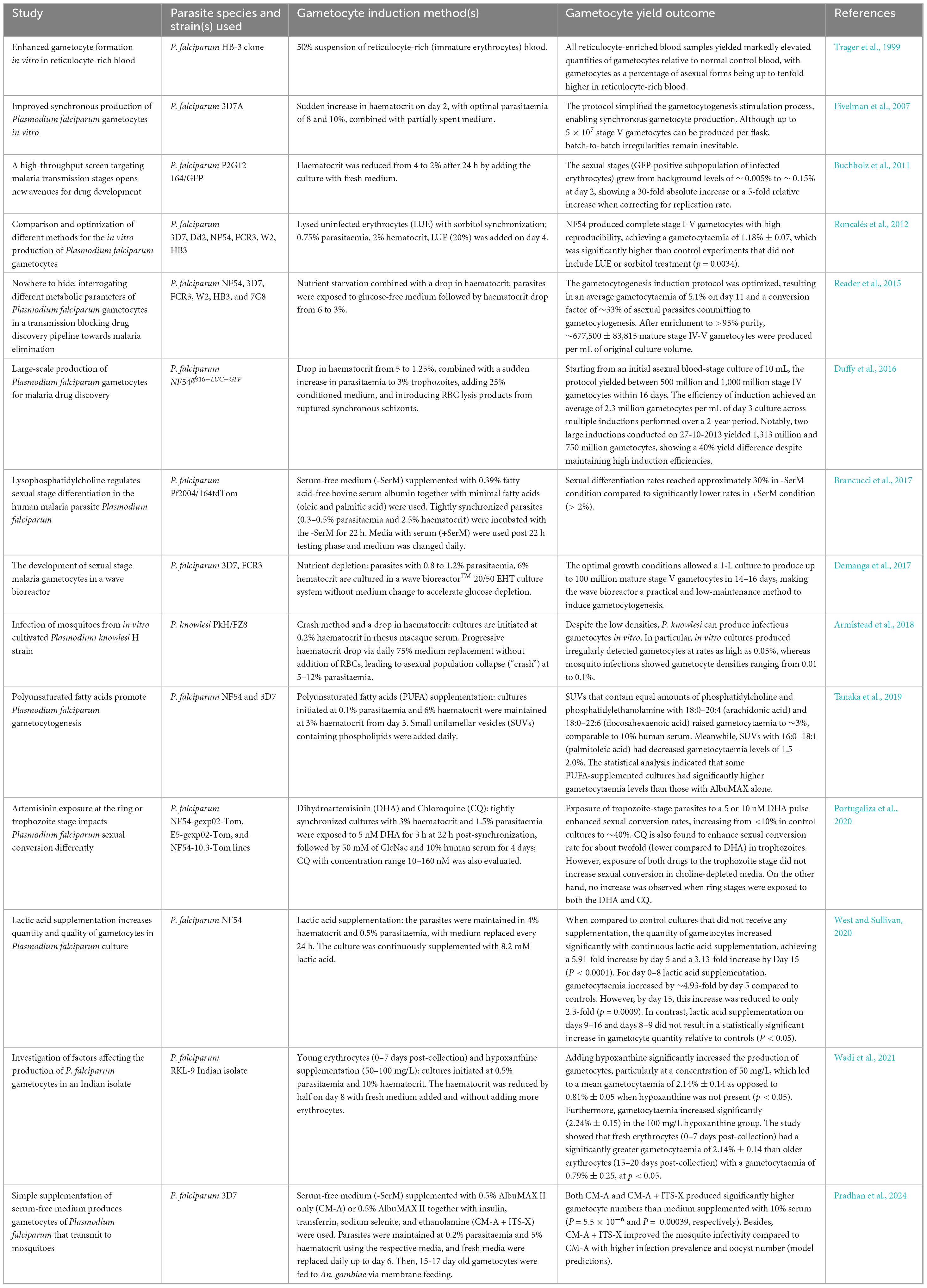
By mimicking the physiological conditions of the host, which are characterized by elevated stress levels, high parasitaemia in vitro (8% or higher) promotes sexual development over asexual forms (Price et al., 1999; Buchholz et al., 2011). When combined with other stressors, such as lysed uninfected erythrocytes (LUE), parasite-conditioned medium, nutrient starvation, and limiting fresh erythrocytes, the impact of elevated parasitaemia on gametocytogenesis is further amplified (Ifediba and Vanderberg, 1981; Omorou et al., 2022). Alternatively, transient reduction of haematocrit during culture has been reported to indirectly facilitate increased parasitaemia. Bounkeua et al. (2010) used high parasitaemia ranging from 8%–15% to initiate gametocyte cultures, with 8%–10% producing the greatest yields of gametocytes. Conversely, Carter and Miller (1979) found that diluting cultures to lower parasitaemia at 0.1% reduced the rate of conversion to gametocytes, which then rose after several days of growth in culture.
As indicated in Table 1, both Buchholz et al. (2011) and Reader et al. (2015) discovered that lowering haematocrit enhances gametocytogenesis, however the outcomes varied with the accompanying stressor. Buchholz et al. (2011) found slight increases in sexual stages (∼ 0.15%) when combined with antimalarial exposure, while Reader et al. (2015) obtained much higher gametocytaemia under nutrient deprivation (∼ 5.1%). This implies that haematocrit drop alone is insufficient, but acts synergistically with other stress cues to promote sexual commitment. Alternatively, high parasitaemia can also be achieved via the crash method, a traditional technique where asexual parasitaemia progressively rises without the addition of fresh erythrocytes. This leads to nutrient stress, which causes the asexual population collapse thereby triggering gametocytogenesis as a survival mechanism (Ifediba and Vanderberg, 1981; Fivelman et al., 2007; Saliba and Jacobs-Lorena, 2013; Tripathi et al., 2020). Tripathi et al. (2020) demonstrated that cultures initiated at 0.5% parasitaemia peaked at ∼15% by days 4–5, followed by a crash with the emergence of early gametocytes on days 6–7. In a culture-adapted strain of P. knowlesi H, Armistead et al. (2018) showed that the crash method did not lead to a marked increase in gametocyte production. However, the highly synchronized in vitro cultures resulted in mosquito infections even when gametocyte concentrations are low or sub-microscopic.
Over the years, many researchers used a parasite-conditioned medium (CM) as the technique for triggering gametocytogenesis. CM is derived from a high-parasitaemia culture (Brancucci et al., 2015), which was hypothesized to be either deprived of particular host components or enriched in particular secreted parasite factors (Brancucci et al., 2017). According to a study led by Brancucci et al. (2015), the addition of CM dramatically boosts the rate of gametocyte yield, with expected conversion rates increasing from ∼ 0.3% to 14%–24%. Based on their protocol, CM collected from cultures achieving parasitaemia levels between 5.5 and 6.5% was most effective for gametocytogenesis when used at a 90% (v/v) working concentration. Meanwhile, the large-scale gametocyte production in P. falciparum has been shown by Duffy et al. (2016) utilizing a combination of fresh media and spent media from the parasite culture. When ring-stage parasitaemia reached 10%–12% on day 2 of gametocyte induction, their procedure recommended resuspending the culture in a medium consisting of 75% fresh asexual culture medium and 25% conditioned (spent) material. In contrast to Brancucci et al. (2015), who primarily relied on CM to obtain high conversion rates of 14%–24%, Duffy et al. (2016) used CM in conjunction with other stressors, including decreased haematocrit levels, elevated parasitaemia, and RBC lysis products, to produce significantly higher absolute gametocyte yields, in the hundreds of millions starting from a 10 mL culture. Whereas Brancucci’s work underscored the efficiency of CM in improving sexual conversion, Duffy’s work showcased its scalability for mass gametocyte production. Human serum has long been used in culture systems, although the use is constrained by its high cost, variability, and ABO compatibility problems (Pradhan et al., 2024). The latest research showed that AlbuMAX™ II (a lipid-rich bovine serum albumin) supplemented with ITS-X (insulin, transferrin, sodium selenite, and ethanolamine) is able to produce P. falciparum 3D7 gametocytes in a serum-free medium (-SerM). This combination not only improved mosquito infectivity in Anopheles gambiae but also increased gametocyte yield, as media containing AlbuMAX™ II as well as AlbuMAX™ II + ITS-X produced significantly higher gametocyte numbers than 10% serum cultures. A number of factors, such as insulin, transferrin, and selenium, are likely responsible for the effect, as they contribute to the growth of asexual parasites (Pollack and Fleming, 1984; Gamain et al., 1996; Balaji et al., 2020; Pradhan et al., 2024). Additionally, ethanolamine, a component of membrane phospholipids, is likely to be essential for the production of transmission-competent gametocytes, which exhibit higher lipid requirements compared to asexual stages (Gulati et al., 2015; Tran et al., 2016; Brancucci et al., 2017; Pradhan et al., 2024). The findings reported by Pradhan et al. (2024) were supported by a similar study by Brancucci et al. (2017), as they unveiled that substituting human serum with fatty acid-free bovine serum albumin together with minimal fatty acids (oleic and palmitic acid) in a serum-free medium led to an increase in gametocyte production across multiple P. falciparum strains, as sexual differentiation rates reached approximately 20%–30% in -SerM conditions, compared to significantly lower rates in +SerM conditions. Therefore, the serum-free approach provides a more affordable and dependable alternative for parasite transmission studies (Pradhan et al., 2024).
Lactic acid supplementation was used in a study by West and Sullivan (2020) to promote gametocytogenesis in low-passage-number NF54. Erythrocytes are able to produce lactic acid independently, and high levels of lactic acid have been detected in the bone marrow of malaria patients (Seheult et al., 2017; Possemiers et al., 2021). Notably, parasites alone can produce millimolar quantities of lactic acid within 24 h in vitro, with the production peaks during asexual phases and decreases as gametocytes mature (Lamour et al., 2014). Lactic acid concentrations above 16 mM may act as a stress signal that enables parasites to detect changes in their environment, preventing asexual parasites from replicating (Zolg et al., 1984; Hikosaka et al., 2015). By adding a final concentration of 8.2 mM D, L-lactic acid to the culture media at various time points and duration, West and Sullivan (2020) discovered that daily culture medium exchange with continuous 8.2 mM lactic acid supplementations (day 0–16) significantly increased gametocytes density after 5 days in comparison to supplementations given during the first half of culturing (days 0–8), the second half (days 9–16), and the short pulse (days 8–9).
LUE suspension mimics the accumulation of erythrocyte debris and hemoglobin-depleted erythrocytes seen in patients after high asexual parasite loads, with free hemoglobin potentially serves as one of the unknown factors triggering sexual differentiation in vitro (Schneweis et al., 1991; Bennett et al., 2005; Roncalés et al., 2012). Building on this, Roncalés et al. (2012) combined both crucial stressors, with 20% (v/v) LUE supplementation and complete elimination of asexual stages via sorbitol treatment to create an optimal protocol using NF54 parasites. This method resulted in the highest, fully staged (I-V) gametocytaemia with remarkable reproducibility. Similarly, Wadi et al. (2021) prepared LUE suspension using a cold-heat shock method and introduced it to RKL-9 P. falciparum culture 48 h post-culture initiation. No significant difference was observed in the final gametocytaemia between the LUE-treated culture compared to the control. This result is contradicted with the previous finding by Schneweis et al. (1991), in which production of gametocytes is enhanced by LUE. Thus, Wadi et al. (2021) proposed that parasites’ ability to produce and develop gametocytes may differ in strains despite the same induction approach being used. They further showed that supplementing RKL-9 gametocyte cultures with hypoxanthine significantly increased gametocyte yield and supported healthy maturation through stage V.
Several studies indicated that exposure to antimalarial drugs may increase the parasite’s commitment to gametocytogenesis, despite the lack of conclusive evidence (Price et al., 1996; Barnes et al., 2008). It has been proposed that stress induced by drugs may cause a “terminal investment” response, in which Plasmodium shifts to gametocyte production when their survival is threatened. This idea is further supported by Portugaliza et al. (2020), who showcased that exposure to dihydroartemisinin (DHA), an important artemisinin derivative, impacts sexual conversion differently depending on the parasite stage. In particular, DHA treatment at 5 nM and 10 nM during trophozoite-stage markedly increased sexual commitment, rising from less than 10% to ∼40% in cultures supplemented with choline. In contrast, DHA treatment applied during the ring stage decreased both gametocytaemia and sexual conversion. Studies on stress induction in gametocytogenesis are summarized in Table 1.
4 Genetic manipulation approaches in understanding gametocytogenesis
While environmental stressors or culture adaptation provides observational understandings on gametocytogenesis, molecular analysis offers critical insights into its fundamental regulation. Gene editing especially, enables precise functional dissection of regulatory genes, thus useful in understanding malaria transmission and targeting important genes. One of the earliest methods applied was double homologous recombination, where plasmid constructs with long homology arms flanking a selectable marker are introduced by electroporation (Janse et al., 2006). This method was key for targeted gene disruption, as demonstrated by Kafsack et al. (2014), who generated ap2-g knockout P. falciparum parasites. The mutants were unable to produce gametocytes, identifying PfAP2-G as a central regulator of sexual commitment. In the FKBP-destabilization domain system, PfAP2-G stability was made dependent on the ligand Shield-1, and its graded depletion led to proportional decrease in gametocyte formation. Despite its precision, this approach has technical limitations, including low transfection efficiency in P. falciparum, the need for laborious cloning and selection, and the inability to scale easily for genome-wide studies.
PiggyBac transposon-mediated insertional mutagenesis has also been employed in the past (Ikadai et al., 2013). By co-transfecting parasites with a transposon and a transposase-encoding plasmid, they generated random insertional mutants across the genome. Screening 189 drug-resistant clones, they identified 29 mutants that failed to form mature gametocytes. Mapping these insertions revealed disruptions in 16 genes, including an AP2 transcription factor, ribosomal proteins, RNA processing factors, and several genes of unknown function. Complementation of five piggyBac insertional mutants (2A2, 2A11, 2G2, 2G11, and 2F4) restored gametocyte development, confirming causality. Nonetheless, the piggyBac approach has notable drawbacks, which are random insertion making gene targeting unpredictable, essential genes in asexual stages generally absent due to lethality, and multiple insertions in single clones complicating phenotype interpretation.
On the other hand, drug selection has been used to isolate spontaneous resistant mutants (Goodman et al., 2016). By culturing parasites in the presence of atovaquone, which targets mitochondrial cytochrome b (cytB), they obtained parasites with point mutations in cytB. While these mutants grew normally in asexual blood stages and did produce gametocytes, they failed to complete development in mosquitoes, thus blocking transmission. Genetic crossing experiments confirmed that the defect was maternally inherited via the female gamete. This strategy effectively linked mitochondrial function to gametocyte transmission competence. However, selection-based methods are inherently restricted to phenotypes under drug pressure and carry the risk of selecting secondary mutations unrelated to the targeted pathway.
The aforementioned methods were eventually surpassed by CRISPR/Cas9 technology, which enables targeted double-strand breaks, greatly enhances editing efficiency, and allows for more precise and versatile genetic modifications. Most of the works focus on GDV1 as it regulates HP1 and ap2-g expressions. Filarsky et al. (2018) applied CRISPR/Cas9 to tag the endogenous gdv1 gene with a 3 × HA epitope, allowing precise visualization of GDV1 protein dynamics under environmental cues. Their work clearly demonstrated that GDV1 disrupts HP1-mediated silencing at the ap2-g locus, lifting the repression of this master regulator and triggering sexual commitment. Importantly, they linked metabolic triggers like choline depletion to GDV1 upregulation, effectively connecting environmental sensing to genetic control. Next, Tibúrcio et al. (2021) generated a C-terminal truncation mutant, GDV1Δ39 via conditional knockout system. Although GDV1Δ39 retained nuclear localization and could still interact with HP1, it was unable to effectively induce ap2-g expression or drive sexual commitment. The critical reason was that the truncated GDV1 protein was present at insufficient levels to reach the induction threshold required for sexual commitment, despite its preserved targeting functions. Boltryk et al. (2021) engineered inducible gametocyte producer (iGP) lines by inserting a conditional GDV1 overexpression cassette into the cg6 locus. This system, controlled by Shield-1, consistently induced ∼75% sexual conversion and synchronous gametocyte production, generating robust, transmission-competent parasites. The iGP lines provided a powerful platform to study late-stage gametocyte biology and evaluate transmission-blocking interventions.
Bui Hai et al. (2021) shifted focus to HP1, employing CRISPR/Cas9 with DiCre/loxP-mediated conditional deletions to dissect domain-specific functions. Their results showed that loss of any HP1 domain; chromodomain, hinge, or chromoshadow led to collapse of heterochromatin and massive derepression of ap2-g, causing near-total conversion to gametocytogenesis. This firmly positioned HP1 as an indispensable epigenetic gatekeeper in the parasite’s developmental fate. On the other hand, Liang et al. (2022) expanded the toolkit by introducing an inducible CRISPR interference/activation (CRISPRi/a) system, using dCas9 fused to GCN5 or Sir2a to epigenetically modulate gene expression without introducing permanent mutations. Notably, they achieved >10-fold induction of ap2-g and a fourfold increase in gametocyte formation, all under tight rapamycin-inducible control via DiCre/loxP. This system allowed reversible, stage-specific gene regulation, even in gametocytes, providing a highly versatile and fine-tuned approach for dissecting gene function, especially in essential or developmentally regulated loci.
Looking into other target genes, Tay et al. (2016) approached the problem from the angle of post-translational regulation, disrupting the palmitoyl transferase P fDHHC9 via gene knockout. While asexual growth remained unaffected, gametocyte formation was markedly impaired, implicating palmitoylation as a novel regulatory layer in sexual differentiation and opening avenues for exploring lipid modification pathways as potential transmission-blocking targets. Wezena et al. (2017) used CRISPR/Cas9 to disrupt the cytosolic glyoxalase genes PfGlo1 and PfcGlo2. While knockout of either gene did not impair the asexual growth, loss of PfcGlo2 significantly increased gametocyte commitment. This unexpected finding suggests that the accumulation of S-D-lactoylglutathione or related metabolic intermediates may act as stress signals or internal cues tipping the balance toward sexual differentiation, revealing a previously understudied metabolic link in the regulation of commitment.
5 Conclusion
The diverse strategies employed to induce gametocytogenesis highlight the complexity of this process and the need for further mechanistic understanding. Although stress-based methods remain essential, they do not fully replicate the physiological triggers that drive transmission in natural infections, limiting their predictive value for transmission-blocking studies. Molecular approaches, on the other hand, have advanced our understanding of regulatory networks governing sexual commitment, yet this knowledge has yet to yield practical tools for large-scale gametocyte production across Plasmodium species. To achieve large-scale gametocytogenesis, it is imperative to bridge the gap between mechanical and molecular methods, leveraging insights from one domain to refine the other. Such advances have direct implications for global malaria control. Reliable large-scale gametocyte production is not only critical for accelerating the discovery of transmission-blocking drugs, but also indispensable for vaccine development strategies that rely on mosquito infection and sporozoite harvesting. Furthermore, genome editing platforms that fine-tune gametocyte induction provide powerful systems to test candidate interventions under physiologically relevant conditions. Ultimately, translating our understanding of gametocytogenesis into practical laboratory platforms will unlock a pipeline of therapeutic and vaccine innovations, bringing the goal of malaria elimination closer to reality.
Author contributions
RS: Data curation, Writing – original draft, Writing – review & editing. ZC: Writing – original draft, Writing – review & editing. NZ: Writing – original draft, Writing – review & editing. YL: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. FC: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The study was funded by the Wellcome Discovery Award (grant reference number 225254/Z/22/Z and IF006-2023) from the Wellcome Trust Institute, UK.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amir, A. (2016). Establishment of a new line of Plasmodium knowlesi. Kuala Lumpur: University of Malaya (Malaysia).
Armistead, J. S., Moraes Barros, R. R., Gibson, T. J., Kite, W. A., Mershon, J. P., Lambert, L. E., et al. (2018). Infection of mosquitoes from in vitro cultivated Plasmodium knowlesi H strain. Int. J. Parasitol. 48, 601–610. doi: 10.1016/j.ijpara.2018.02.004
Baker, D. A. (2010). Malaria gametocytogenesis. Mol. Biochem. Parasitol. 172, 57–65. doi: 10.1016/j.molbiopara.2010.03.019
Balaji, S. N., Sahasrabuddhe, A. A., and Trivedi, V. (2020). Insulin signalling in RBC is responsible for growth stimulation of malaria parasite in diabetes patients. Biochem. Biophys. Res. Commun. 528, 531–537. doi: 10.1016/j.bbrc.2020.05.1
Barnes, K. I., Little, F., Mabuza, A., Mngomezulu, N., Govere, J., Durrheim, D., et al. (2008). Increased gametocytemia after treatment: An early parasitological indicator of emerging sulfadoxine-pyrimethamine resistance in falciparum malaria. J. Infect. Dis. 11, 1605–1613. doi: 10.1086/587645
Bennett, T. N., Kosar, A. D., and Roepe, P. D. (2005). Plasmodium falciparum strain GC-03 exhibits hyper-gametocytogenesis in partially hemoglobin depleted red blood cells. Mol. Biochem. Parasitol. 139, 261–265. doi: 10.1016/j.molbiopara.2004.11.006
Bijker, E. M., Borrmann, S., Kappe, S. H., Mordmüller, B., Sack, B. K., and Khan, S. M. (2015). Novel approaches to whole sporozoite vaccination against malaria. Vaccine 33, 7462–7468. doi: 10.1016/j.vaccine.2015.09.095
Boltryk, S. D., Passecker, A., Alder, A., Carrington, E., van de Vegte-Bolmer, M., van Gemert, G. J., et al. (2021). CRISPR/Cas9-engineered inducible gametocyte producer lines as a valuable tool for Plasmodium falciparum malaria transmission research. Nat. Commun. 12:4806. doi: 10.1038/s41467-021-24954-4
Bousema, T., and Drakeley, C. (2011). Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin. Microbiol. Rev. 24, 377–410. doi: 10.1128/cmr.00051-10
Bounkeua, V., Li, F., and Vinetz, J. M. (2010). In vitro generation of Plasmodium falciparum ookinetes. Am. J. Trop. Med. 83, 1187–1194. doi: 10.4269/ajtmh.2010.10-0433
Brancucci, N. M. B., Bertschi, N. L., Zhu, L., Niederwieser, I., Chin, W. H., Wampfler, R., et al. (2014). Heterochromatin protein 1 secures survival and transmission of malaria parasites. Cell Host Microbe 16, 165–176. doi: 10.1016/j.chom.2014.07.004
Brancucci, N. M. B., Gerdt, J. P., Wang, C., De Niz, M., Philip, N., Adapa, S. R., et al. (2017). Lysophosphatidylcholine regulates sexual stage differentiation in the human malaria parasite Plasmodium falciparum. Cell 171:1532–1544.e1515. doi: 10.1016/j.cell.2017.10.020
Brancucci, N. M. B., Goldowitz, I., Buchholz, K., Werling, K., and Marti, M. (2015). An assay to probe Plasmodium falciparum growth, transmission stage formation and early gametocyte development. Nat. Protoc. 10, 1131–1142. doi: 10.1038/nprot.2015.072
Buchholz, K., Burke, T. A., Williamson, K. C., Wiegand, R. C., Wirth, D. F., and Marti, M. (2011). A high-throughput screen targeting malaria transmission stages opens new avenues for drug development. J. Infect. Dis. 203, 1445–1453. doi: 10.1093/infdis/jir037
Bui Hai, T. N., Passecker, A., Brancucci Nicolas, M. B., and Voss Till, S. (2021). Investigation of heterochromatin protein 1 function in the malaria parasite Plasmodium falciparum using a conditional domain deletion and swapping approach. mSphere 6:e1220. doi: 10.1128/msphere.01220-20
Carter, R., and Miller, L. H. (1979). Evidence for environmental modulation of gametocytogenesis in Plasmodium falciparum in continuous culture. Bull. World Health Organ. 57, 37–52.
Carter, R., Graves, P., Wernsdorfer, W., and McGregor, I. (1988). “Gametocytes,” in Malaria: Principles and practice of malariology, Vol. 1, eds W. Wernsdorfer and I. McGregor (London: Churchill Livingstone), 233–305.
Dacon, C., Moskovitz, R., Swearingen, K., Da Silva, Pereira, L., Flores-Garcia, Y., et al. (2025). Protective antibodies target cryptic epitope unmasked by cleavage of malaria sporozoite protein. Science 387:eadr0510. doi: 10.1126/science.adr0510
Demanga, C. G., Eng, J. W. L., Gardiner, D. L., Roth, A., Butterworth, A., Adams, J. H., et al. (2017). The development of sexual stage malaria gametocytes in a wave bioreactor. Parasit. Vectors 10:216. doi: 10.1186/s13071-017-2155-z
Duffy, P. E. (2022). Current approaches to malaria vaccines. Curr. Opin. Microbiol. 70:102227. doi: 10.1016/j.mib.2022.102227
Duffy, S., Loganathan, S., Holleran, J. P., and Avery, V. M. (2016). Large-scale production of Plasmodium falciparum gametocytes for malaria drug discovery. Nat. Protoc. 11, 976–992. doi: 10.1038/nprot.2016.056
Fatmaningsih, L., Samasta, N. A., and Octa, L. (2024). Differences in the Life Cycle and Growth of Plasmodium knowlesi, inui, vivax, malariae, falciparum, ovale. J. Biomed. Technol. Nanomater. 1, 59–69. doi: 10.55849/jbtn.v1i1.172
Filarsky, M., Fraschka, S. A., Niederwieser, I., Brancucci, N. M. B., Carrington, E., Carrió, E., et al. (2018). GDV1 induces sexual commitment of malaria parasites by antagonizing HP1-dependent gene silencing. Science 359, 1259–1263. doi: 10.1126/science.aan6042
Fivelman, Q. L., McRobert, L., Sharp, S., Taylor, C. J., Saeed, M., Swales, C. A., et al. (2007). Improved synchronous production of Plasmodium falciparum gametocytes in vitro. Mol. Biochem. Parasitol. 154, 119–123. doi: 10.1016/j.molbiopara.2007.04.008
Galinski, M. R., and Barnwell, J. W. (2008). Plasmodium vivax: Who cares? Malar. J. 7, S9. doi: 10.1186/1475-2875-7-S1-S9
Gamain, B., Arnaud, J., Favier, A., Camus, D., Dive, D., and Slomianny, C. (1996). Increase in glutathione peroxidase activity in malaria parasite after selenium supplementation. Free Radic. Biol. Med. 21, 559–565. doi: 10.1016/0891-5849(96)00120-7
Gautam, P. K., Prajapati, B., and Sujatha, R. (2020). Life cycle, pathogenesis and laboratory diagnosis of malaria parasite, Vol. 105, ed. D. G. Naik (Rohini: AkiNik Publications), 105. doi: 10.22271/ed.book.933
Goodman, C. D., Siregar, J. E., Mollard, V., Vega-Rodríguez, J., Syafruddin, D., Matsuoka, H., et al. (2016). Parasites resistant to the antimalarial atovaquone fail to transmit by mosquitoes. Science 352, 349–353. doi: 10.1126/science.aad9279
Gulati, S., Ekland, E. H., Ruggles, K. V., Chan, R. B., Jayabalasingham, B., Zhou, B., et al. (2015). Profiling the essential nature of lipid metabolism in asexual blood and gametocyte stages of Plasmodium falciparum. Cell Host Microbe 18, 371–381. doi: 10.1016/j.chom.2015.08.003
Gunalan, K., Rowley, E. H., and Miller, L. H. (2020). A way forward for culturing Plasmodium vivax. Trends Parasitol. 36, 512–519. doi: 10.1016/j.pt.2020.04.002
Hawking, F., Worms, M. J., and Gammage, K. (1968). Host temperature and control of 24-hour and 48-hour cycles in malaria parasites. Lancet 291, 506–509. doi: 10.1016/S0140-6736(68)91469-4
Hikosaka, K., Hirai, M., Komatsuya, K., Ono, Y., and Kita, K. (2015). Lactate retards the development of erythrocytic stages of the human malaria parasite Plasmodium falciparum. Parasitol. Int. 64, 301–303. doi: 10.1016/j.parint.2014.08.003
Ifediba, T., and Vanderberg, J. P. (1981). Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature 294, 364–366. doi: 10.1038/294364a0
Ikadai, H., Shaw Saliba, K., Kanzok, S. M., McLean, K. J., Tanaka, T. Q., Cao, J., et al. (2013). Transposon mutagenesis identifies genes essential for Plasmodium falciparum gametocytogenesis. Proc. Natl. Acad. Sci. U.S.A. 110, E1676–E1684. doi: 10.1073/pnas.1217712110
Janse, C. J., Ramesar, J., and Waters, A. P. (2006). High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat. Protoc. 1, 346–356. doi: 10.1038/nprot.2006.53
Josling, G. A., Russell, T. J., Venezia, J., Orchard, L., van Biljon, R., Painter, H. J., et al. (2020). Dissecting the role of PfAP2-G in malaria gametocytogenesis. Nat. Commun. 11:1503. doi: 10.1038/s41467-020-15026-0
Kafsack, B. F. C., Rovira-Graells, N., Clark, T. G., Bancells, C., Crowley, V. M., Campino, S. G., et al. (2014). A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature 507, 248–252. doi: 10.1038/nature12920
Kumar, S., Bhardwaj, T. R., Prasad, D. N., and Singh, R. K. (2018). Drug targets for resistant malaria: Historic to future perspectives. Biomed. Pharmacother. 104, 8–27. doi: 10.1016/j.biopha.2018.05.009
Lamour, S. D., Straschil, U., Saric, J., and Delves, M. J. (2014). Changes in metabolic phenotypes of Plasmodium falciparum in vitro cultures during gametocyte development. Malar. J. 13, 468. doi: 10.1186/1475-2875-13-468
Liang, X., Boonhok, R., Siddiqui Faiza, A., Xiao, B., Li, X., Qin, J., et al. (2022). A leak-free inducible CRISPRI/a system for gene functional studies in Plasmodium falciparum. Microbiol. Spectr. 10:e02782–21. doi: 10.1128/spectrum.02782-21
Moon, R. W., Hall, J., Rangkuti, F., Ho, Y. S., Almond, N., Mitchell, G. H., et al. (2013). Adaptation of the genetically tractable malaria pathogen Plasmodium knowlesi to continuous culture in human erythrocytes. Proc. Natl. Acad. Sci. U.S.A. 110, 531–536. doi: 10.1073/pnas.1216457110
Ngotho, P., Soares, A. B., Hentzschel, F., Achcar, F., Bertuccini, L., and Marti, M. (2019). Revisiting gametocyte biology in malaria parasites. FEMS Microbiol. Rev. 43, 401–414. doi: 10.1093/femsre/fuz010
Omorou, R., Bin Sa’id, I., Delves, M., Severini, C., Kouakou, Y. I., Bienvenu, A.-L., et al. (2022). Protocols for Plasmodium gametocyte production in vitro: An integrative review and analysis. Parasit. Vectors 15:451. doi: 10.1186/s13071-022-05566-3
Palatnik-de-Sousa, C. B., and Nico, D. (2020). The delay in the licensing of protozoal vaccines: A comparative history. Front. Immunol. 11:204. doi: 10.3389/fimmu.2020.00204
Pollack, S., and Fleming, J. (1984). Plasmodium falciparum takes up iron from transferrin. Br. J. Haematol. 58, 289–293. doi: 10.1111/j.1365-2141.1984.tb06087.x
Portugaliza, H. P., Llorà-Batlle, O., Rosanas-Urgell, A., and Cortés, A. (2019). Reporter lines based on the gexp02 promoter enable early quantification of sexual conversion rates in the malaria parasite Plasmodium falciparum. Sci. Rep. 9:14595. doi: 10.1038/s41598-019-50768-y
Portugaliza, H. P., Miyazaki, S., Geurten, F. J. A., Pell, C., Rosanas-Urgell, A., Janse, C. J., et al. (2020). Artemisinin exposure at the ring or trophozoite stage impacts Plasmodium falciparum sexual conversion differently. eLife 9:e60058. doi: 10.7554/eLife.60058
Possemiers, H., Vandermosten, L., and Van den Steen, P. E. (2021). Etiology of lactic acidosis in malaria. PLoS Pathog. 17:e1009122. doi: 10.1371/journal.ppat.1009122
Pradhan, S., Ubiaru, P. C., and Ranford-Cartwright, L. (2024). Simple supplementation of serum-free medium produces gametocytes of Plasmodium falciparum that transmit to mosquitoes. Malar. J. 23:275. doi: 10.1186/s12936-024-05094-8
Price, R. N., Nosten, F., Luxemburger, C., ter Kuile, F. O., Paiphun, L., Price, R. N., et al. (1996). Effects of artemisinin derivatives on malaria transmissibility. Lancet 347, 1654–1658. doi: 10.1016/S0140-6736(96)91488-9
Price, R., Nosten, F., Simpson, J. A., Luxemburger, C., Phaipun, L., ter Kuile, F., et al. (1999). Risk factors for gametocyte carriage in uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 60, 1019–1023. doi: 10.4269/ajtmh.1999.60.1019
Rea, E., Le Roch, K. G., and Tewari, R. (2018). Sex in Plasmodium falciparum: Silence play between GDV1 and HP1. Trends Parasitol. 34, 450–452. doi: 10.1016/j.pt.2018.04.006
Reader, J., Botha, M., Theron, A., Lauterbach, S. B., Rossouw, C., Engelbrecht, D., et al. (2015). Nowhere to hide: Interrogating different metabolic parameters of Plasmodium falciparum gametocytes in a transmission blocking drug discovery pipeline towards malaria elimination. Malar. J. 14:213. doi: 10.1186/s12936-015-0718-z
Roncalés, M., Vidal-Mas, J., Leroy, D., and Herreros, E. (2012). Comparison and optimization of different methods for the in vitro production of Plasmodium falciparum gametocytes. J. Parasitol. Res. 2012:927148. doi: 10.1155/2012/927148
Saliba, K. S., and Jacobs-Lorena, M. (2013). “Production of Plasmodium falciparum gametocytes in vitro,” in Malaria: Methods and protocols, ed. R. Ménard (Totowa, NJ: Humana Press), 17–25.
Sato, S. (2021). Plasmodium—a brief introduction to the parasites causing human malaria and their basic biology. J. Physiol. Anthropol. 40:1. doi: 10.1186/s40101-020-00251-9
Schneweis, S., Maier, W. A., and Seitz, H. M. (1991). Haemolysis of infected erythrocytes–a trigger for formation of Plasmodium falciparum gametocytes? Parasitol. Res. 77, 458–460. doi: 10.1007/bf00931646
Seheult, J., Fitzpatrick, G., and Boran, G. (2017). Lactic acidosis: An update. Clin. Chem. Lab. Med. 55, 322–333. doi: 10.1515/cclm-2016-0438
Shute, P. G., and Maryon, M. (1951). A study of gametocytes in a West African strain of Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 44, 421–438. doi: 10.1016/s0035-9203(51)80020-8
Singh, S., Santos, J. M., Orchard, L. M., Yamada, N., van Biljon, R., Painter, H. J., et al. (2021). The PfAP2-G2 transcription factor is a critical regulator of gametocyte maturation. Mol. Microbiol. 115, 1005–1024. doi: 10.1111/mmi.14676
Sinha, A., Hughes, K. R., Modrzynska, K. K., Otto, T. D., Pfander, C., Dickens, N. J., et al. (2014). A cascade of DNA-binding proteins for sexual commitment and development in Plasmodium. Nature 507, 253–257. doi: 10.1038/nature12970
Tanaka, T. Q., Tokuoka, S. M., Nakatani, D., Hamano, F., Kawazu, S.-I., Wellems, T. E., et al. (2019). Polyunsaturated fatty acids promote Plasmodium falciparum gametocytogenesis. Biol. Open. 8:bio042259. doi: 10.1242/bio.042259
Tay, C. L., Jones, M. L., Hodson, N., Theron, M., Choudhary, J. S., and Rayner, J. C. (2016). Study of Plasmodium falciparum DHHC palmitoyl transferases identifies a role for PfDHHC9 in gametocytogenesis. Cell Microbiol. 18, 1596–1610. doi: 10.1111/cmi.12599
Tibúrcio, M., Hitz, E., Niederwieser, I., Kelly, G., Davies, H., Doerig, C., et al. (2021). A 39-amino-Acid C-terminal truncation of GDV1 disrupts sexual commitment in Plasmodium falciparum. mSphere 6:e1093–20. doi: 10.1128/msphere.01093-20
Trager, W., and Jensen, J. (1976). Human malaria parasites in continuous culture. Science 193, 673–675. doi: 10.1126/science.781840
Tran, P. N., Brown, S. H., Rug, M., Ridgway, M. C., Mitchell, T. W., and Maier, A. G. (2016). Changes in lipid composition during sexual development of the malaria parasite Plasmodium falciparum. Malar. J. 15:73. doi: 10.1186/s12936-016-1130-z
Trager, W., Gill, G. S., Lawrence, C., and Nagel, R. L. (1999). Plasmodium falciparum: enhanced gametocyte formation in vitro in reticulocyte-rich blood. Exp. Parasitol. 91, 115–118. doi: 10.1006/expr.1998.4347
Tripathi, A. K., Mlambo, G., Kanatani, S., Sinnis, P., and Dimopoulos, G. (2020). Plasmodium falciparum gametocyte culture and mosquito infection through artificial membrane feeding. J. Vis. Exp. 161:e61426. doi: 10.3791/61426
Wadi, I., Deora, N., Nath, M., and Sinha, A. (2021). Investigation of factors affecting the production of P. falciparum gametocytes in an Indian isolate. 3 Biotech 11:55. doi: 10.1007/s13205-020-02586-7
Wadi, I., Pargat, S., Mahendra, N., and Sinha, A. (2020). Malaria transmission-blocking drugs: Implications and future perspectives. Future Med. Chem. 12, 1071–1101. doi: 10.4155/fmc-2020-0026
West, R., and Sullivan, D. J. (2020). Lactic acid supplementation increases quantity and quality of gametocytes in Plasmodium falciparum culture. Infect. Immun. 89:e635–20. doi: 10.1128/iai.00635-20
Wezena, C. A., Alisch, R., Golzmann, A., Liedgens, L., Staudacher, V., Pradel, G., et al. (2017). The cytosolic glyoxalases of Plasmodium falciparum are dispensable during asexual blood-stage development. Microb. Cell 5, 32–41. doi: 10.15698/mic2018.01.608
Keywords: Plasmodium, gametocytes, malaria, genome engineering, gametocytogenesis induction, genome editing
Citation: Subramaniam R, Chiew ZY, Zuhaidi ND, Lau YL and Cheong FW (2025) Induction of gametocytogenesis in human malaria parasites: from stress to genome editing. Front. Microbiol. 16:1688506. doi: 10.3389/fmicb.2025.1688506
Received: 20 August 2025; Accepted: 07 October 2025;
Published: 22 October 2025.
Edited by:
Letusa Albrecht, Oswaldo Cruz Foundation, BrazilReviewed by:
Glenda Quaresma Ramos, University of the State of Amazonas, BrazilCopyright © 2025 Subramaniam, Chiew, Zuhaidi, Lau and Cheong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Wen Cheong, ZndjaGVvbmcxOEB1bS5lZHUubXk=; Yee Ling Lau, bGF1eWVlbGluZ0B1bS5lZHUubXk=
 Rishitharan Subramaniam
Rishitharan Subramaniam Zi Yan Chiew
Zi Yan Chiew Nabel Darwish binti Zuhaidi
Nabel Darwish binti Zuhaidi Yee Ling Lau
Yee Ling Lau Fei Wen Cheong
Fei Wen Cheong