- 1Institute of Population Medicine, School of Public Health, Fujian Medical University, Fuzhou, China
- 2State Key Laboratory of Medical Proteomics, Beijing Proteome Research Center, National Center for Protein Sciences (Beijing), Beijing Institute of Lifeomics, Beijing, China
- 3Fujian Provincial Key Laboratory of Agroecological Processing and Safety Monitoring, College of Life Sciences, Fujian Agriculture and Forestry University, Fuzhou, China
- 4Key Laboratory of Marine Biotechnology of Fujian Province, Institute of Oceanology, Fujian Agriculture and Forestry University, Fuzhou, China
- 5Changting Branch of Longyan Tobacco Company, Longyan, Fujian, China
Introduction: With the increasing identification of multidrug resistance in Aeromonas hydrophila isolated from diverse food sources, there is an urgent need to investigate its resistance mechanism. Previous studies have demonstrated that OmpR/PhoB-type response regulators (RRs) play critical roles in mediating bacterial tolerance to various environmental stresses.
Methods and results: In this study, we constructed an AHA_4052 gene knockout strain, a member of the OmpR/PhoB family of DNA-binding response regulators, and studied its phenotypic characteristics. Deletion of the AHA_4052 gene rendered the bacteria significantly more sensitive to high temperature, osmotic stress, and aminoglycoside antibiotics compared to the wild-type A. hydrophila. Label-free quantitative proteomic analysis revealed 131 differentially expressed proteins in the ΔAHA_4052 mutant strain. These proteins were predominantly associated with ribosome, butanoate metabolism, and glycerophospholipid metabolism pathways, with ribosome-related proteins accounting for 17.56% of the total. Additionally, seven antibiotic resistance-related proteins exhibited significant expression changes in the mutant strain. Chromatin immunoprecipitation assay–polymerase chain reaction (ChIP-PCR) validation further demonstrated that the transcriptional regulator AHA_4052 directly binds to the promoters of two resistance genes, AHA_2114 and AHA_3488.
Discussion: Collectively, these findings indicate that the transcriptional regulatory factor AHA_4052 plays a critical role in stress tolerance, particularly against aminoglycosides, providing insights into the resistance mechanisms of A. hydrophila and potentially informing the development of new therapeutic strategies.
Introduction
With the continuous increase in global fish consumption, rising from 9.0 kg per capita in 1961 to 20.5 kg in 2018, the food safety of aquatic products has attracted increasing attention (Ibrahim et al., 2020). Aeromonas hydrophila, a Gram-negative pathogen widely distributed in aquatic environments, not only directly causes septicemia in fish but also serves as an emerging opportunistic human pathogen responsible for gastroenteritis, wound infections, and even life-threatening septicemia in immunocompromised individuals (Pessoa et al., 2022; Wang T. et al., 2022). The incidence of A. hydrophila infections peaks during summer, and its incidence has remained high in recent years. For instance, infection rates have been reported to reach 47% in farmed fish in Egypt and exceed 30% in retail fish from the United States, India, and China, highlighting its global distribution and significance as a foodborne hazard (Dahdouh et al., 2016; Yang et al., 2018; Jeamsripong et al., 2025).
In the field of aquaculture, the extensive use of antibiotics to control A. hydrophila has driven increased antibiotic resistance and the emergence of multidrug-resistant strains. The situation is further exacerbated by the fact that A. hydrophila possesses the capability to form biofilms (De Silva and Heo, 2022), which significantly enhances its resilience and persistence in the environment, making its prevention and control problem even more complex and difficult to solve. Previous studies have reported that A. hydrophila was resistant to ampicillin, tetracycline, streptomycin, nalidixic acid, and kanamycin, and multiple studies have reported that the resistance rate of A. hydrophila isolates to ampicillin reached 100% (Yang et al., 2018; Borella et al., 2020; Kaur et al., 2024). However, the potential molecular mechanisms underlying its resistance, particularly the complex interactions between intrinsic resistance genes, metabolic pathways, regulatory networks, and signal transduction-mediated tolerance, have not yet been fully elucidated. Therefore, there is an urgent need not only to elucidate the antibiotic resistance mechanisms of A. hydrophila but also to provide information for developing new and targeted strategies to control the pathogen.
Two-component systems (TCSs) composed of a histidine kinase (HK) and response regulator (RR) are ubiquitous in bacteria. They are essential for maintaining cellular homeostasis and represent a crucial mechanism for many organisms to respond to environmental stress. For example, the EnvZ-OmpR TCS mediates osmotic stress adaptation by regulating porin expression (Cai and Inouye, 2002); the QseB/QseC system acts as a global regulator of quorum sensing (Zhu et al., 2023); and the PhoP-PhoQ TCS is involved in the regulation of bacterial Mg2+ homeostasis, resistance to pH and antimicrobial peptides, and mediates a response to heat, high osmotic pressure, oxide salt or bile salt, and toxicity (Groisman et al., 2021; Cabezudo et al., 2022; Mao et al., 2025). There are also many studies reporting that TCSs are associated with bacterial resistance and directly modulate resistance mechanisms, including cell surface modifications, changes in cell permeability, biofilm formation, and the expression of antibiotic-degrading enzymes (Tierney and Rather, 2019; Li et al., 2023; Long et al., 2025). Notable examples include the Vancomycin resistance-associated Sensor-Regulator (VraSR)-TCS, which regulates genes responsible for vancomycin resistance (Chen et al., 2016), the Polymyxin resistance-associated Sensor-Regulator (PmrAB)-TCS, which confers colistin resistance via QseBC modulation (Fernandez-Ciruelos et al., 2023), and the Biofilm formation-associated Sensor-Regulator (BfmRS)-TCS, which enhances bacterial resistance through biofilm formation (Cao et al., 2020). As TCSs have emerged as a potential target for antibacterial drug design, more than 40 TCSs related to bacterial drug resistance have been found (Tierney and Rather, 2019; De Gaetano et al., 2023).
Response regulators (RRs) are a major family of signaling proteins in prokaryotes that link diverse cellular activities in bacteria to environmental factors (Stock et al., 2000; Zhang et al., 2024). Among these, DNA-binding domains are the main types of RRs, accounting for approximately 60% of RRs, while OmpR/PhoB is the most dominant subfamily of DNA-binding domains, followed by NarL/FixJ and NtrC/DctD (Gao et al., 2007), highlighting their central role in transcriptional regulation. In A. hydrophila, the AHA_4052 gene encodes a two-component system of regulatory proteins belonging to the OmpR/PhoB family of DNA-binding domains; however, its biological function remains clear. In this study, we generated an AHA_4052 knockout strain via homologous recombination and studied its phenotypic characteristics. The mutant strain exhibited increased sensitivity to high temperature, osmotic stress, and aminoglycoside antibiotics. This prompted a comprehensive investigation into the underlying molecular mechanisms. To globally profile the regulatory network governed by AHA_4052, we used a label-free quantitative proteomic method to compare the differential expression of proteins between the ΔAHA_4052 mutant and wild-type A. hydrophila strains. The results of mass spectrometry (MS) identification showed that a total of 131 differentially expressed proteins were detected, and the deletion of the AHA_4052 gene resulted in significant changes in the ribosome, butanoate metabolism, and glycerophospholipid metabolism pathways. Notably, seven known or putative drug resistance-related proteins exhibited significant expression changes. ChIP-PCR validation confirmed direct binding of AHA_4052 to the promoters of two candidate resistance genes, AHA_3488 and AHA_2114. In conclusion, these findings advance our understanding of the AHA_4052 gene’s role in aminoglycoside resistance and highlight its potential as a therapeutic target for combating drug-resistant A. hydrophila.
Materials and methods
Bacterial strains and culture conditions
The bacterial strains and plasmids used in this study are listed in Table 1. All strains were cultured in Luria–Bertani (LB) medium. Antibiotics were added to the culture medium at varying concentrations according to experimental requirements. Aeromonas hydrophila wild-type and gene knockout strains were cultured at 30 °C, while Escherichia coli MC1061 and S17-1 were grown at 37 °C.
Construction of the AHA_4052 mutant and complemented strains
The AHA_4052 gene deletion strain was constructed using the suicide vector pRE112 and the homologous recombination principle (Li et al., 2018). The primers are listed in Supplementary Table 1. Briefly, ~500 bp upstream and downstream regions flanking the AHA_4052 gene were amplified from A. hydrophila genomic DNA and fused to the pRE112 plasmid. The recombinant plasmid was transformed into E. coli MC1061 competent cells. Verified positive plasmids were subsequently transferred to E. coli S17-1 cells. Conjugation between E. coli S17-1 (harboring the pRE112 recombinant vector) and wild-type A. hydrophila was performed at a 4:1 ratio to facilitate the first homologous recombination, integrating the vector into the A. hydrophila genome. This process was screened on an LB agar plate containing ampicillin (Amp) and chloramphenicol (Cm). Then, the second homologous recombination was completed by screening in LB medium supplemented with 20% sucrose. PCR and DNA sequencing were performed on single clones that are sensitive on the Cm plates. Finally, after approximately 20 generations of stable inheritance, the successfully verified ΔAHA_4052 strain was stored at −80 °C for subsequent experiments.
The complemented ΔAHA_4052 strain was constructed using the plasmid pBBRMCS1. The full-length AHA_4052 gene with its promoter region and 6 × His tagged sequence was amplified from A. hydrophila genomic DNA, ligated into the pBBRMCS1 vector, and then transformed into E. coli competent cells. The plasmids that were completely correct in PCR and sequencing, along with the empty vector pBBR1MCS1, were extracted and transferred to ΔAHA_4052 competent cells by electro-transformation, and the complemented strain ΔAHA_4052 + AHA_4052 was verified by PCR and sequencing again.
Bacterial tolerance assay
Aeromonas hydrophila wild-type and ΔAHA_4052 mutant strains were cultured overnight at 30 °C, and then the bacteria were transferred at 1% and sub-packed into a HONEYCOMB® Sterile 100-well plate, with 350 μL per well. The OD600 nm value was determined using a Bioscreen C instrument (Oy Growth Curves AB Ltd., Helsinki, Finland). Parameters were set to record the data every 2 h over 16 h at 30 °C. For high temperature stress, the instrument temperature was set at 42 °C; for osmotic pressure stress, 4% NaCl was added when transferring bacteria, and for acidic stress, the pH of LB medium was adjusted to 4.9. Finally, the results were generated using GraphPad Prism 10 software.
Minimum bactericidal concentration determination
Minimum bactericidal concentrations (MBCs) were determined by the agar dilution method (Li et al., 2021). LB agar plates of antibiotics with two-fold serial dilution gradient concentrations were self-prepared, including the following antibiotics: tobramycin (TOB), apramycin (APR), kanamycin (KAN), gentamicin (GEN), streptomycin (STR), paromomycin (PAR), neomycin (NEO), amikacin (AMK), doxycycline (DOX), oxytetracycline hydrochloride (OXY), methyltetracycline hydrochloride (MT), tetracycline (TET), ciprofloxacin (CIP), levofloxacin (LVX), pefloxacin (PEF), enrofloxacin (ENR), moxifloxacin (MFX), enoxacin (ENO), norfloxacin (NOR), ceftriaxone sodium (CRO), moxalactam (MOX), cefamandole (MAN), cefmetazole sodium (CMZ), meropenem (MEM), aztreonam (AZT), azithromycin (AZM), lincomycin (LIN), roxithromycin (ROX), chloramphenicol (CHL), rifampicin (RIF), vancomycin (VAN), trimethoprim (TMP), and natamycin (NAT). The wild-type and ΔAHA_4052 strains were cultured to an OD600 nm of approximately 1.0 and diluted 100-fold, and then, 2-μl diluted bacterial suspensions were taken and spotted onto the antibiotic-containing LB plate. After 14–16 h incubation at 30 °C, MBC results were recorded. Experiments were repeated independently at least three times. Subsequently, the MBCs for aminoglycoside antibiotics (tobramycin, apramycin, kanamycin, gentamicin, streptomycin, paromomycin, neomycin, and amikacin) were further tested against wild-type, ΔAHA_4052, ΔAHA_4052 + AHA_4052, and ΔAHA_4052 + vector strains.
Preparation and trypsin digestion of bacterial whole protein samples
Aeromonas hydrophila wild-type and ΔAHA_4052 mutant strains were cultured overnight at 30 °C, and the bacteria were diluted 1% into 50 mL of fresh LB medium. After growing to OD600nm ≈ 1.0, the cells were pelleted by centrifugation for 20 min at 8,000 g at 4 °C and washed twice with pre-cooled phosphate-buffered saline (PBS). The pellets were resuspended in 1 mL of lysis buffer (6 M urea, 2 M thiourea, 100 mM Tris–HCl [pH 7.6], and protease inhibitor). Next, the ice was subjected to ultrasonic crushing for 15 minutes at 30% power, in 6-second bursts followed by 9-second breaks, until the sample was clear and transparent. Finally, the supernatants were collected by centrifugation for 15 min at 18,000 g at 4 °C, and protein concentrations were performed using the bicinchoninic acid (BCA) assay. 50 μg mixture sample proteins was prepared for building spectral library using the filter-aided sample preparation (FASP) method and trypsin for digestion (Ni et al., 2017), and then, the peptides were dried in a CentriVap concentrator (Labconco Inc., Kansas City, MO, United States).
Data-independent acquisition (DIA) quantitative proteomics based on spectral library
Peptides from the mixture samples were dissolved in a buffer containing 2% acetonitrile and 0.1% formic acid (pH = 10) and separated using a RIGOL L-3000 high-performance liquid chromatography (HPLC) system (Puyuanjingdian Science and Technology, Ltd., Beijing, China) with a Gemini-NX C18 110A column (size of 250 × 4.6 mm, particle size of 5 μm, Phenomenex, USA) at 1 mL/min using mobile phases A (2% ACN, pH = 10) and B (98% ACN, pH = 10) with a gradient of 5–30% B for 25 min. Ten fractions were obtained and dried under vacuum.
The fractioned and sample peptides were analyzed on the Orbitrap Fusion Lumos system with the same LC condition; a specific process was performed as described previously (Wang et al., 2025a). Solvent A for LC was composed of 0.1% formic acid in water, while solvent B contained 80% acetonitrile and 0.1% formic acid in water. Lyophilized peptides were dissolved in 0.1% formic acid in water and then centrifuged for 15 min at 15,000 g. The resulting supernatant was injected into a C18 analytical column (150 um × 25 cm) using an EASY-nLC 1,200 HPLC (Thermo Fischer Scientific, USA) at a maximum pressure of 300 bar with 12 μL Solvent A. Peptides were eluted from the analytical column at a flow rate of 600 nL/min with a gradient to 6% B at 0 min, 12% B at 18 min, 20% B at 77 min, 32% B at 109 min, and 80% B at 110 min, holding until 120 min.
The fractionated peptides were scanned by the Data-Dependent Acquisition (DDA) model for the spectral library. The eluted peptide was sprayed at a voltage of 2.0 kV with a Nanospray Flex ion source. The ion transfer tube was set at 320 °C. The MS scan resolution was 60,000, the scanning range was 300–1,400 m/z, and the maximum injection time was set to 50 ms. The MS/MS scan resolution was 30,000, using 30 scanning windows, with the first mass set at 120 m/z, the collision energy of 30%, the Automatic gain control (AGC) target of 5e4, and the maximum injection time of 54 ms.
The sample peptides were scanned by the DIA model for sample quantification. The eluted peptide was sprayed at a voltage of 2.0 kV with a Nanospray Flex ion source. The ion transfer tube was set at 320 °C. The MS scan resolution was 60,000, the scanning range was 425–925 m/z, and the maximum injection time was set to 50 ms. The MS/MS scan resolution was 30,000, using a scanning range for MS/MS at 200–1800 m/z, with 45 DIA windows, the collision energy of 33%, the AGC target of 5e5, and the maximum injection time of 60 ms.
DDA raw data were processed using Spectronaut Pulsar 16 software for building a spectral library against the Uniprot A. hydrophila ATCC7966 database with the default parameter. For the DIA data, default parameters were used, and protein identification was performed with a precursor, peptide, and protein false discovery rate (FDR) Q-value cutoff of 0.01 (Wang et al., 2025b). Furthermore, the protein abundance ratio of the ΔAHA_4052 strain was calculated, and a t-test was performed on the raw data with A. hydrophila wild-type as the control. Proteins with the number of peptides greater than 2 and abundance ratio fold change (FC) of ≥2 or ≤0.5 and a p-value of <0.05 were classified as the differentially expressed proteins for further analysis. The mass spectrometry proteomic data have been deposited to the ProteomeXchange Consortium1 via the iProX partner repository with the dataset identifier PXD067330.
Bioinformatics analysis
The differentially expressed proteins were subjected to Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment using OmicsBean software2. The results were visualized using Graphpad Prism 10 software and the R GOplot package. Protein–protein interaction networks were analyzed using STRING software, and visualized graphics were generated using Cytoscape software. Finally, antibiotic resistance-associated proteins were predicted using the Comprehensive Antibiotic Research Database (CARD2).
Chromatin immunoprecipitation assay and PCR validation
Chromatin immunoprecipitation assay (ChIP) is ideally suited for studying protein–DNA interactions in vivo; this study used ChIP to investigate the regulation between transcriptional regulation factor AHA_4052 and candidate genes. When ΔAHA_4052 + AHA_4052 and ΔAHA_4052 + vector strains were cultured to an OD600nm of approximately 1.0, the cells were collected by centrifugation for 10 min at 6,000 g at 4 °C and washed twice with pre-cooled PBS. The cells were cross-linked with 1% formaldehyde for 5 min, sheared to approximately 300–500 bp by sonication on ice for 20 min at 30% power with intervals of 9 s, and subjected to immunoprecipitation with BeaverBeads™ IDA-Nickel (His-tag) for 4–6 h at 4 °C. Elution of DNA from precipitated immunocomplexes was performed using elution buffer (containing 20 mM PBS, 500 mM NaCl, and 500 mM Imidazole, pH = 7.4). Then, 40 μL of 10% SDS and 2 μL of 10 mg/mL protease K were added and incubated overnight at 37 °C. The DNA was extracted with phenol:chloroform:isoamyl alcohol (25:24:1) solution, and then, PCR was performed with specific primers (Supplementary Table 2).
Results
The AHA_4052 mutant affects high temperature and high osmotic pressure in A. hydrophila
To gain a more comprehensive understanding of the biological function of the AHA_4052 gene, we successfully constructed a ΔAHA_4052 mutant in A. hydrophila using suicide vector pRE112 and homologous recombination methods. The PCR and Western blot results showed no bands at the target location (Figures 1A,B), and further sequencing analyses confirmed the successful knockout of the AHA-4052 gene. The growth state and tolerance to environmental stress (high temperature, osmosis, and acid) of ΔAHA_4052 strain were further tested. The results showed that there was no significant difference in the growth rate and acid tolerance between the ΔAHA_4052 mutant and wild-type strain, indicating that the AHA_4052 gene is not essential in regulating the survival and adaptation to acidic environments of A. hydrophila. However, the ΔAHA_4052 mutant exhibited significantly reduced tolerance to high temperature (42 °C) and high osmotic pressure (4% NaCl) compared to the wild-type strain (Figure 1C).
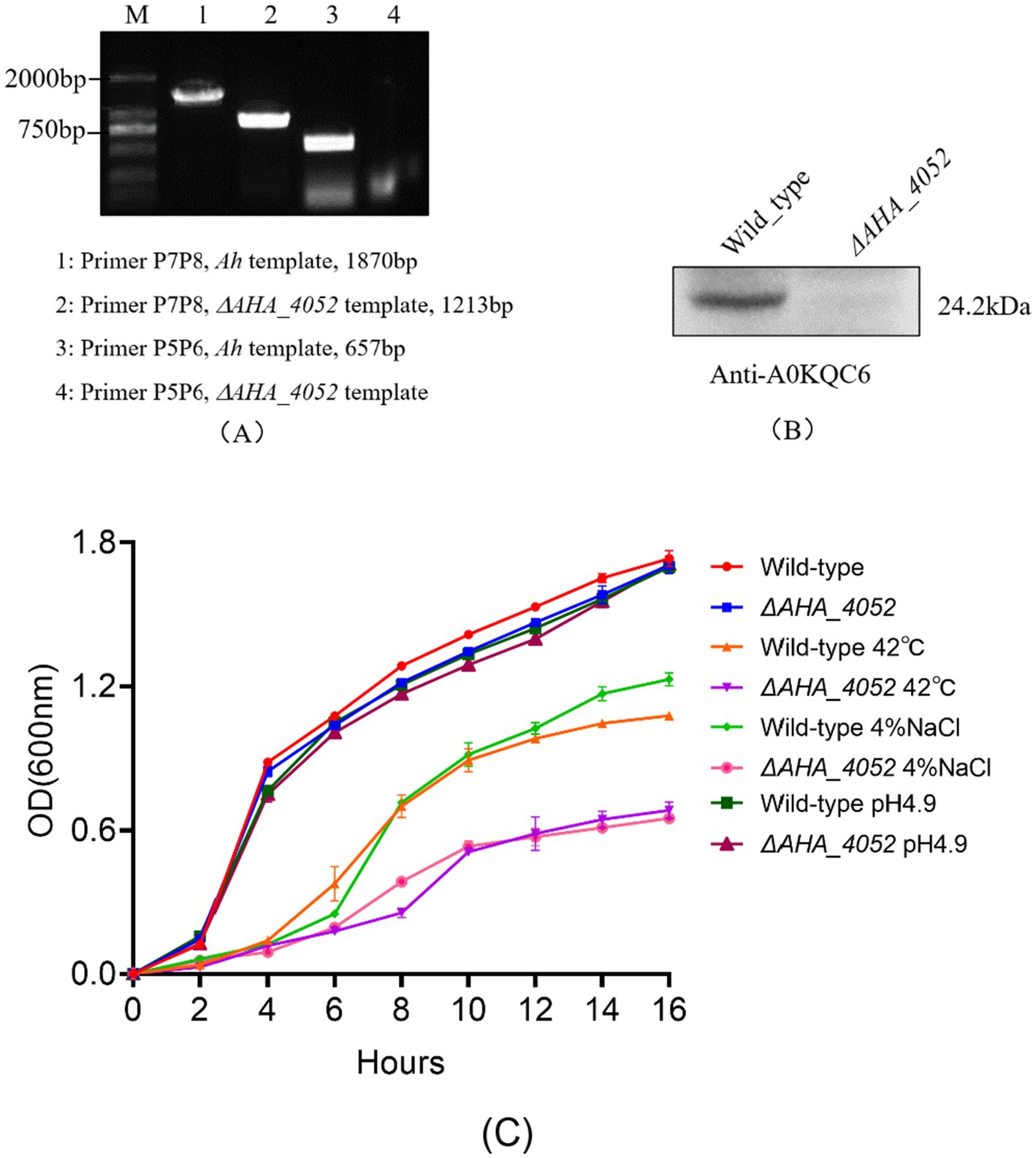
Figure 1. Validation of the ΔAHA_4052 mutant strain and its stress tolerance assays. (A) PCR and (B) Western blotting validation of the ΔAHA_4052 strain. (C) Growth curves of ΔAHA_4052 and wild-type strains under high temperature, high osmotic pressure, and acid (pH 4.9) environmental stress.
AHA_4052 is involved in aminoglycoside antibiotic susceptibility of A. hydrophila
To investigate whether the transcriptional regulation factor AHA_4052 is involved in regulating antibiotic resistance in A. hydrophila, we tested the ΔAHA_4052 mutants against 33 antibiotics spanning six classes: aminoglycosides including TOB, APR, KAN, GEN, STR, PAR, NEO, and AMK; tetracyclines including DOX, OXY, MT, and TET; quinolones including CIP, LVX, PEF, ENR, MFX, ENX, and NOR; cephalosporins including CRO, MOX, MAN, and CMZ; β - lactams including MEM and AZT, macrolides including AZM and ROX, and other antibiotics including LIN, CHL, RIF, VAN, TMP, and NAT (Supplementary Figure 1). The results showed that the ΔAHA_4052 mutant exhibited a 4-fold reduction in MBC values for all aminoglycoside antibiotics compared to the wild-type strain while showing no significant differences in susceptibility to other antibiotic classes. The MBC of its complementary strain against aminoglycoside antibiotics was further determined, and it was found that complementation of the mutant restored aminoglycoside MBCs to wild-type levels (Figure 2), demonstrating that the transcriptional regulation factor AHA_4052 is essential for intrinsic aminoglycoside resistance in A. hydrophila.
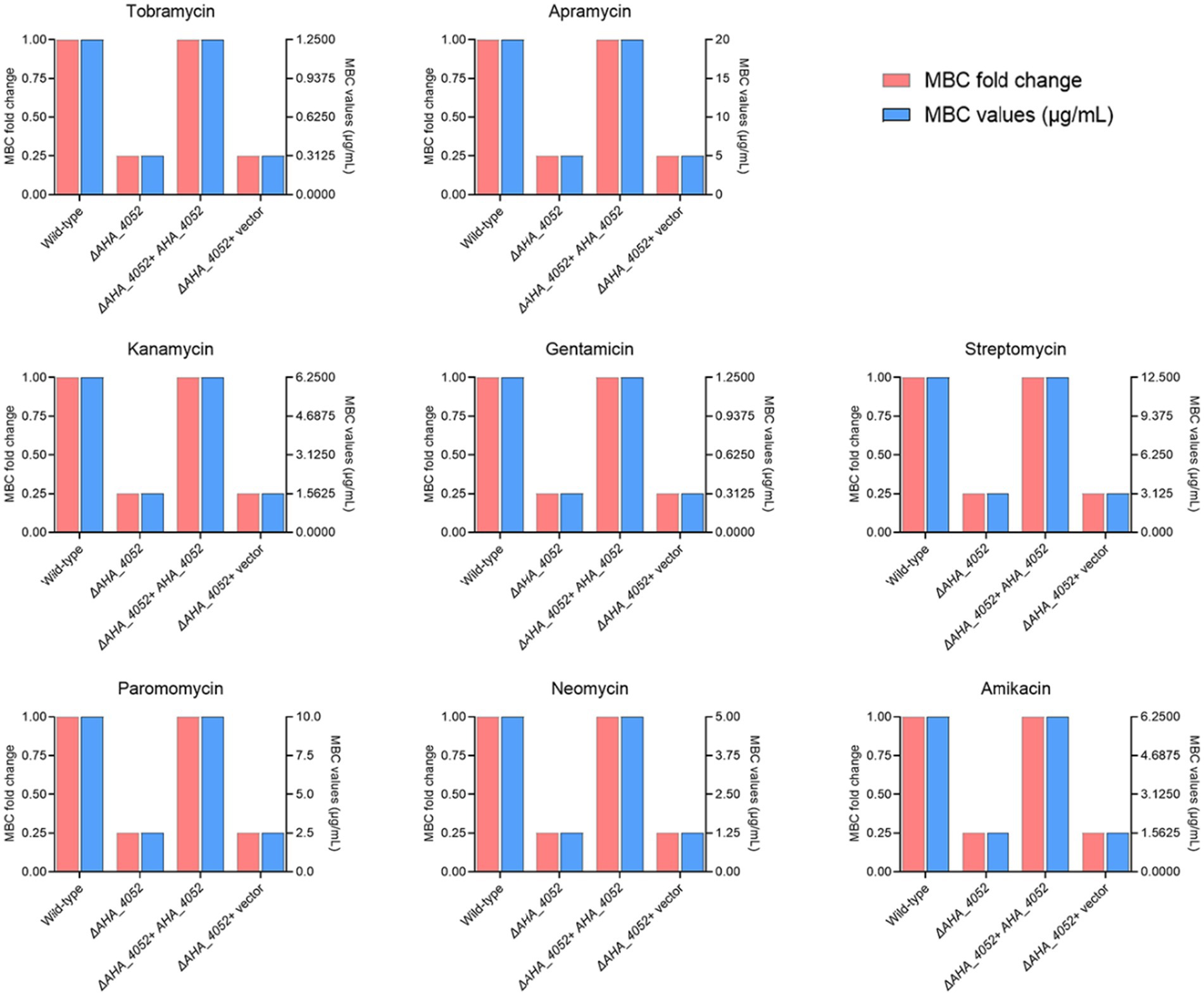
Figure 2. Minimum bactericidal concentrations (MBCs) of eight aminoglycoside antibiotics against wild-type and ΔAHA_4052 mutant strains of A. hydrophila. MBC values of the ΔAHA_4052 strain for each antibiotic are expressed as fold change relative to the wild-type strain (1 × MBC).
Proteomic analysis of differential protein expression between WT and ΔAHA_4052 strains
To elucidate the regulatory role of the transcriptional regulation factor AHA_4052 in directly or indirectly modulating bacterial biological functions, we collected whole protein samples of wild-type and ΔAHA_4052 strains for quantitative detection using LC–MS/MS technology. LC–MS/MS analysis identified 2,654 proteins with a considerable conservative threshold, including a confidence level ≥95%, at least two unique peptide matches, and a false discovery rate (FDR) of <1% (Supplementary Table 3). A correlation analysis showed that there was a very high correlation between the three biological repeats of protein quantitative data from wild-type and ΔAHA_4052 samples, and the regression coefficient was greater than 0.99 (Figure 3A). In this study, compared with the wild-type strain, a total of 131 proteins in the ΔAHA_4052 mutant were significantly differentially expressed (fold change ≥2 or ≤0.5 and p-value < 0.05), of which 28 proteins were downregulated and 103 proteins were upregulated (Figure 3B).
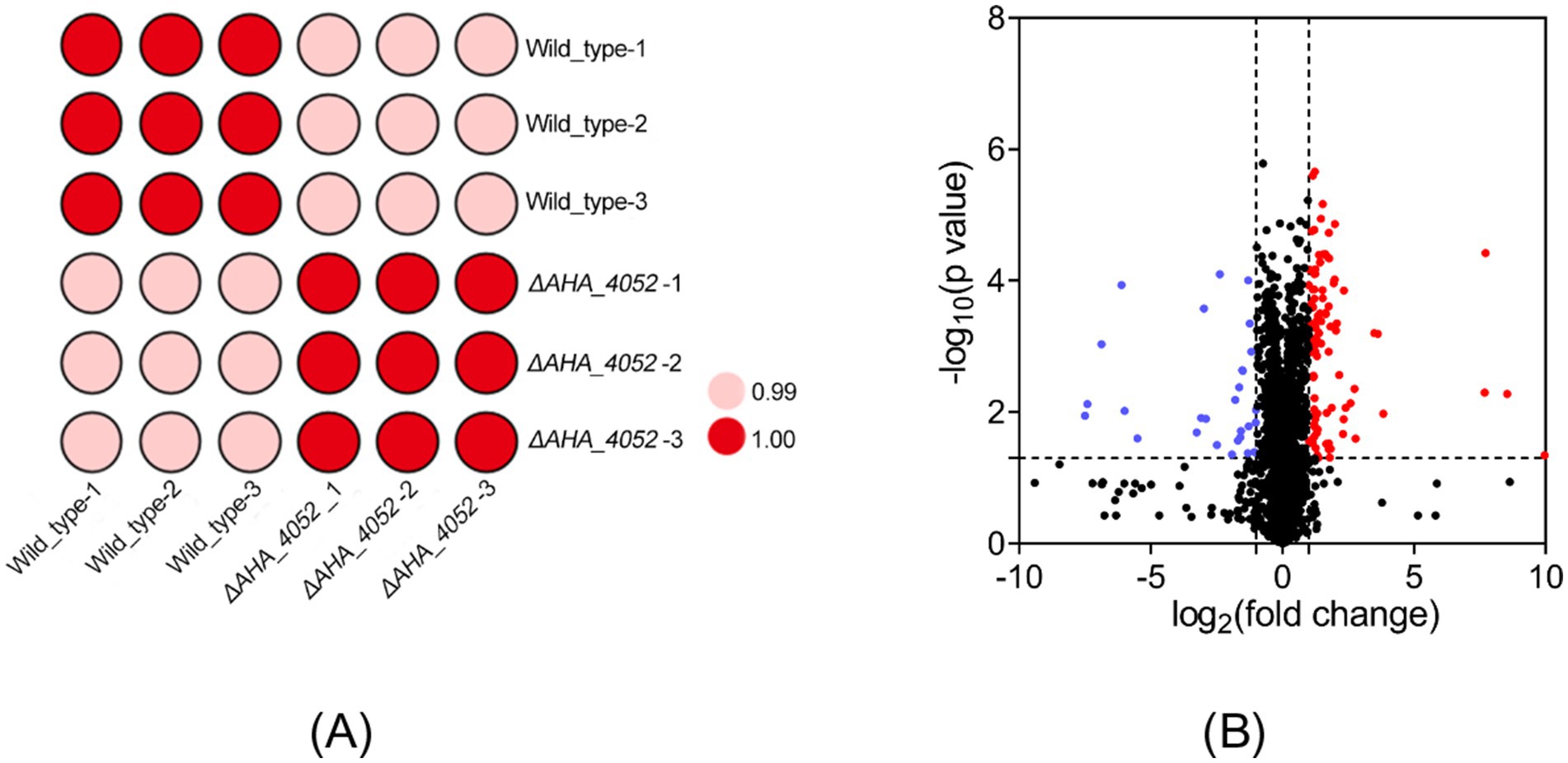
Figure 3. Proteomic profiling of the ΔAHA_4052 mutant. (A) Correlation analysis of protein intensities between A. hydrophila wild-type and ΔAHA_4052 strains. (B) Volcano plots of differentially expressed proteins (|fold change| ≥ 2 and p-value <0.05). Each dot represents one protein; a blue dot represents downregulated proteins, and a red dot represents upregulated proteins.
Bioinformatic analysis of differentially expressed proteins between WT and ΔAHA_4052 strains
We conducted GO functional classification and enrichment analysis of differentially expressed proteins in the ΔAHA_4052 mutant. In the ΔAHA_4052 mutant, the top three biological processes were identified as the peptide metabolic process (19.85%), the organic substance metabolic process (6.87%), and the protein metabolic process (6.11%) (Figure 4A). The molecular function category was predominantly represented by structural constituents of ribosomes (17.56%) (Figure 4B), while the primary cellular components were ribosomes (17.56%), cells (14.5%), and cytoplasm (4.58%) (Figure 4C). Notably, a substantial proportion of proteins remained unclassified across all categories: biological processes (29.77%), molecular functions (32.82%), and cellular components (51.15%), indicating undefined functional roles. KEGG metabolic pathway analysis revealed significant enrichment in ribosome, butanoate metabolism, and glycerophospholipid metabolism, with corresponding proteins showing marked upregulation (Figure 4D).
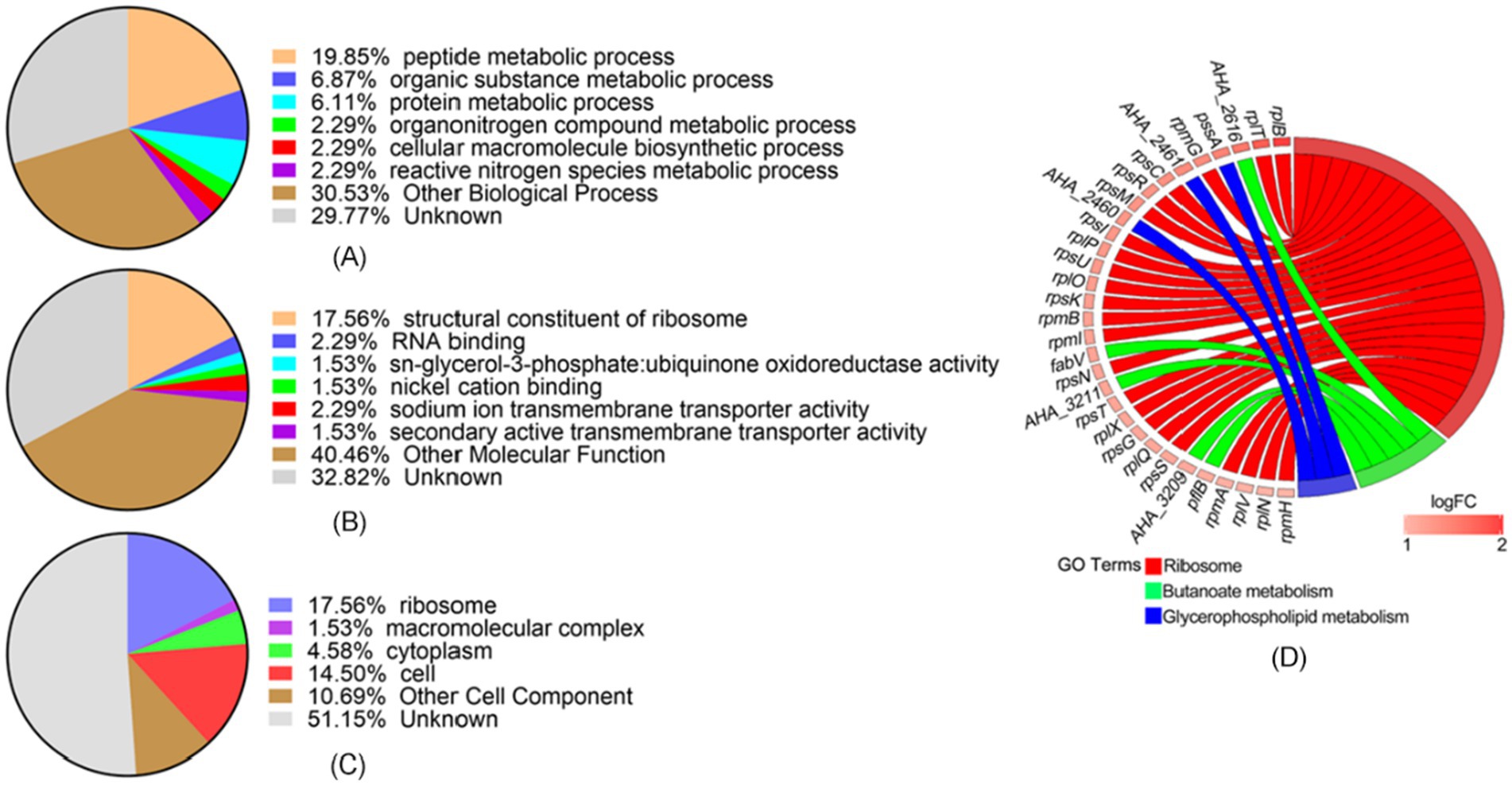
Figure 4. Functional annotation of differentially expressed proteins in the ΔAHA_4052 strain. Gene Ontology analysis of (A) biological processes, (B) molecular functions, and (C) cellular components. (D) The chord plot showed KEGG pathway enrichment analysis in the ΔAHA_4052 strain. The left color bar indicates the trend of protein expression, red represents up-expression genes, and blue represents down-expression. Colored bands link genes to corresponding KEGG terms.
Protein–protein interaction network analysis of differentially expressed proteins between WT and ΔAHA_4052 strains
We further investigated the protein–protein interaction (PPI) network of the differentially expressed proteins in the ΔAHA_4052 mutant. As shown in Figure 5, numerous altered proteins formed a complex PPI network, with 24 translation-related proteins demonstrating significant enrichment and substantial upregulation. Other key functional clusters included seven metal ion-binding proteins (AHA_3076, AHA_3827, AHA_4048, AHA_2465, AHA-2466, AHA-1946, and AHA_3209), four proteins involved in protein maturation (hypA, hypC, hypE, and AHA_2515), three components of the protein secretion system (gspH, AHA_0389, and AHA_3953), and two amino acid metabolism-related enzymes (arcC-2 and AHA_3828), and all identified proteins exhibited elevated expression levels. These findings suggest that the transcriptional regulation factor AHA_4052 modulates multiple bacterial metabolic pathways.
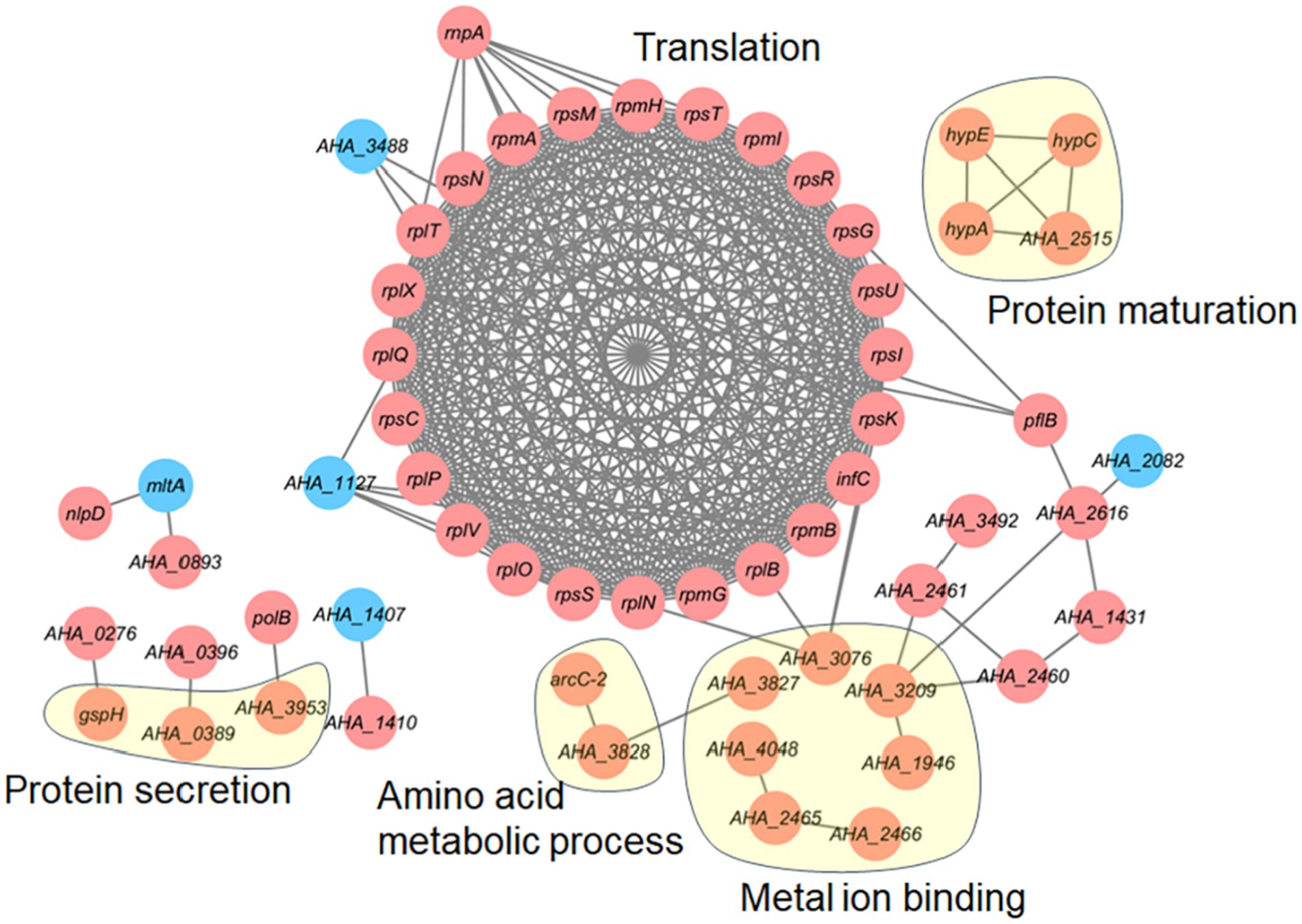
Figure 5. Protein–protein interaction network of differentially expressed proteins in the ΔAHA_4052 strain. The red circle represents upregulated proteins, and the blue circle represents downregulated proteins.
Role of drug resistance gene in the ΔAHA_4052 mutant
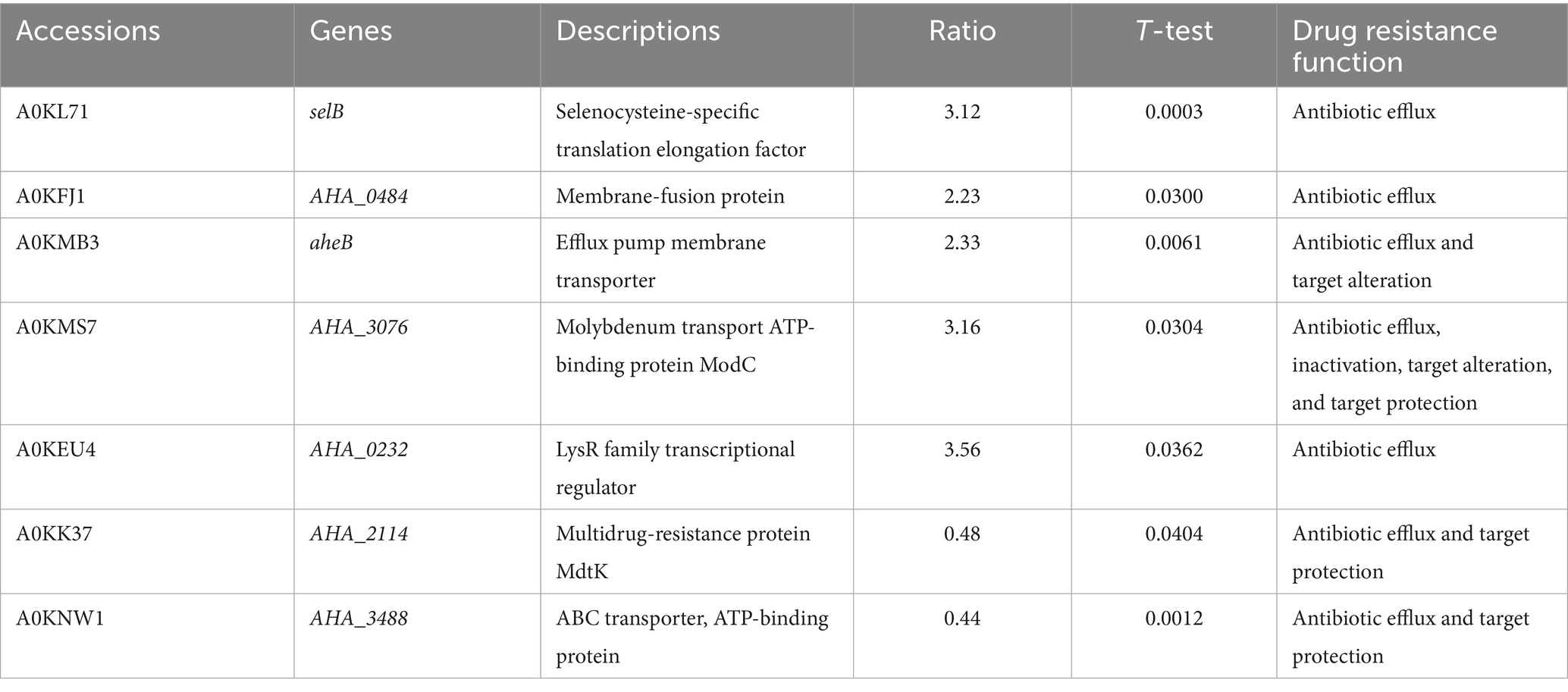
To investigate the antibiotic resistance mechanism of the transcriptional regulation factor AHA_4052, we identified differentially expressed antibiotic resistance genes (ARGs) in the ΔAHA_4052 mutant using the Comprehensive Antibiotic Research Database (CARD). Seven resistance genes were found to be directly or indirectly regulated by the transcriptional regulation factor AHA_4052, with five proteins (aheB, AHA_0484, selB, AHA_3076, and AHA_0232) showing increased abundance and two proteins (AHA_2114 and AHA_3488) exhibiting decreased abundance. These proteins mediate bacterial antibiotic resistance through mechanisms including efflux pumps, antibiotic inactivation, target alteration, and target protection (Table 2).
Subsequently, to further verify whether transcriptional regulation factor AHA_4052 directly or indirectly regulates drug resistance genes (AHA_3488, AHA_2114, and aheB), we used chromatin immunoprecipitation assay and PCR technology (ChIP-PCR) to determine the binding capability of candidate genes with AHA_4052. The results showed that three genes could be amplified in the ΔAHA_4052 + AHA_4052 and ΔAHA_4052 + vector input samples. In addition, the predicted promoter regions upstream of the AHA_3488 and AHA_2114 genes (PAHA_3488 and PAHA_2114) could be amplified in the ChIP sample of the ΔAHA_4052 + AHA_4052 strain, while their own gene fragments could not be amplified, and neither fragment could be amplified in the ΔAHA_4052 + vector ChIP sample. At the same time, the predicted promoter region of the aheB gene and its gene fragment were not amplified in ChIP samples (Figure 6). These results demonstrate that the transcriptional regulation factor AHA_4052 may directly regulate drug resistance genes AHA_3488 and AHA_2114 by binding their promoters while indirectly regulating the aheB gene.
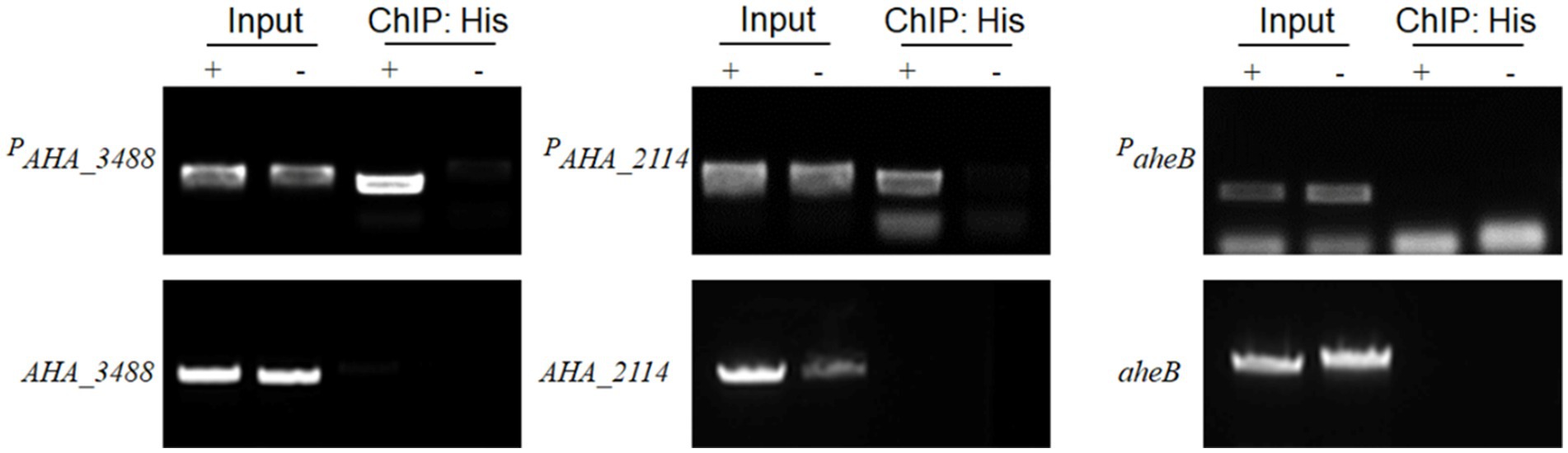
Figure 6. The chromatin immunoprecipitation and PCR assay of transcriptional regulation factor AHA_4052 with the promoters of AHA_3488, AHA_2114, and aheB genes. ΔAHA_4052 + AHA_4052 complementary strain was marked as “+”; ΔAHA_4052 + vector strain was marked as “–.” The chromatin of ΔAHA_4052 + AHA_4052 and ΔAHA_4052 + vector strains was immunoprecipitated with or without anti-His antibody (input or ChIP:His) and validated by PCR. The promoter fragment of the gene is labeled Pgene name.
Discussion
A. hydrophila is a prevalent fish pathogen that has increasingly exhibited multidrug resistance. Studies have detected drug-resistant A. hydrophila strains have been detected across diverse food sources, posing significant public health concerns (Stratev and Odeyemi, 2016). Although numerous studies have explored the drug resistance mechanism of A. hydrophila, the potential drug resistance regulatory network still needs further research.
Many previous studies have established the critical role of the EnvZ/OmpR two-component regulatory system (TCRS) in bacterial adaptation to acid stress, osmotic pressure, and antibiotic resistance through the modulation of outer membrane porins OmpF and OmpC (Ko and Choi, 2022; Wang et al., 2023). Similarly, the PhoP/PhoQ TCRS has been shown to regulate tolerance-related genes and resist various environmental stresses, as well as regulate bacterial resistance to polymyxin B, aminoglycosides, and quinazoline (Macfarlane et al., 2000; Ma et al., 2021; Guo et al., 2022). However, the functional roles of OmpR/PhoB-type proteins in A. hydrophila, particularly the transcriptional regulator A0KQC6 (AHA_4052) containing an OmpR/PhoB-type DNA-binding domain, remain largely unexplored.
In this study, we constructed the AHA_4052 gene mutant (ΔAHA_4052) and complemented strains in A. hydrophila to investigate its biological functions. Subsequently, the growth of the wild-type and ΔAHA_4052 mutant strains under different environmental stresses (high temperature, osmosis, and acid) and the MBCs of different antibiotics were determined. The results revealed that the AHA_4052 gene knockout significantly impaired bacterial growth under high-temperature and osmotic stress conditions. Furthermore, the mutant strain exhibited a four-fold reduction in MBCs against aminoglycoside antibiotics. While the observed osmotic regulation aligns with known OmpR/PhoB-type protein functions (Chakraborty and Kenney, 2018), little is known about the regulation of tolerance to temperature and aminoglycoside antibiotics, suggesting that AHA_4052 may possess novel regulatory functions beyond those previously characterized.
To gain a comprehensive understanding of the mechanisms underlying these phenotypes, we performed label-free quantitative proteomics to analyze the global impact of the ΔAHA_4052 mutant on protein expression. Proteomic analysis revealed that the AHA_4052 gene regulates the expression of a range of proteins involved in a wide range of metabolism pathways, including ribosomes, butanoate metabolism, and glycerophospholipid metabolism. In particular, alterations in glycerophospholipid metabolism may affect membrane fluidity and integrity, potentially explaining the mutant’s increased sensitivity to osmotic and heat stress (Wu et al., 2019). Similarly, butanoate metabolism could influence energy homeostasis and stress response signaling pathways, further leading to phenotypic changes (Abdelhamid and Yousef, 2024). However, a substantial proportion of the differentially expressed proteins are of unknown function, indicating that the transcriptional regulator AHA-4052 may also play a significant role in regulating novel or uncharacterized functions in A. hydrophila.
Aminoglycoside antibiotics are concentration-dependent bactericidal agents known for their rapid and potent killing activity. After knocking out the AHA_4052 gene, the ΔAHA_4052 mutant significantly reduced the minimum bactericidal concentration of aminoglycosides, and 23 ribosomal proteins, including 13 50S ribosomes and 10 30S ribosomal subunit-related proteins (17.56% of differentially expressed proteins), were significantly upregulated. Previous studies have demonstrated that aminoglycoside antibiotics target the bacterial 30S ribosomal subunit, interfering with the normal ribosomal function by misguiding translation to synthesize non-functional or toxic proteins and by blocking translation, leading to a complete halt in protein synthesis, thereby exerting their bactericidal effect (Dunkle et al., 2014; Wang N. Y. et al., 2022). Therefore, we propose a model wherein the upregulation of ribosomal proteins upon AHA_4052 gene deletion increases the number of potential binding sites for aminoglycosides within the cell. This could facilitate more rapid and extensive binding of the antibiotics, thereby potentiating their bactericidal effect by exacerbating translational errors and inhibition. In contrast, the resistance levels to other antibiotic classes such as tetracyclines, quinolones, and cephalosporins remained unchanged in the mutant, which may be attributed to the specificity of drug targets and efflux systems; for instance, aminoglycoside efficacy is closely tied to ribosomal interaction, whereas other antibiotics often depend on distinct mechanisms such as cell wall synthesis or DNA replication, which appear unaffected by AHA_4052 deletion. In summary, these findings imply that the AHA_4052 gene in A. hydrophila may modulate bacterial resistance to aminoglycosides through the regulation of ribosomal protein expression.
Beyond the potential indirect effect mediated by ribosomal upregulation, we aimed to identify direct targets of AHA_4052 that are known to be associated with antibiotic resistance. Comprehensive Antibiotic Resistance Database (CARD) analysis identified seven resistance-associated proteins with altered expression in ΔAHA_4052, revealing a complex regulatory network impacting drug resistance. In this study, the downregulation of A0KK37 (AHA_2114, multidrug-resistant protein, MATE family MdtK) and A0KNW1 (AHA_3488, adenosine triphosphate (ATP)-binding cassette (ABC) transporter, ATP-binding protein) in ΔAHA_4052 indicates a direct impairment of critical efflux systems, likely contributing to a significant decrease in antibiotic resistance in the mutants. Studies have reported that the MATE family is a group of multidrug-resistant efflux proteins closely associated with antibiotic resistance. For example, the expression of MATE family proteins in Staphylococcus aureus confers the bacterium resistance to norfloxacin and ciprofloxacin (Kaatz et al., 2005; Claxton et al., 2021). In A. hydrophila, knocking out the AHA_2144 gene (a MATE homolog), the bacteria are sensitive to enoxacin, indicating that this gene plays a certain role in bacterial resistance (Li et al., 2021). ABC transporters are involved in the influx or efflux of various molecules, which is a key part of the mechanism of antibiotic resistance. In bacteria, ABC transporters are found to be involved in the resistance of daunorubicin, doxorubicin, macrolides, colistin, and other antibiotics (Hürlimann et al., 2016; Orelle et al., 2019). Furthermore, chromatin immunoprecipitation-PCR (ChIP-PCR) confirmed direct regulatory interactions between transcriptional regulation factor AHA_4052 and the resistance genes AHA_2114 and AHA_3488. Conversely, the upregulation of proteins A0KFJ1 (AHA_0484, membrane-fusion protein) and A0KMB3 (aheB, efflux pump membrane transporter) may represent a compensatory adaptation to the loss of the primary pumps. Meanwhile, the concurrent upregulation of other resistance factors such as A0KL71 (selB, selenocysteine-specific translation elongation factor), whose increase implies enhanced selenocysteine incorporation potentially boosting antioxidant defenses to mitigate antibiotic-induced oxidative stress (Mukai, 2021), as well as A0KMS7 (AHA_3076, molybdenum transport ATP-binding protein ModC) and A0KEU4 (AHA_0232, LysR family transcriptional regulator), suggests that AHA_4052 influences global stress responses and metabolic pathways linked to antibiotic efficacy. Collectively, these findings indicate that AHA_4052 acts as a master regulator, potentially directly regulating key efflux pumps and indirectly modulating broader resistance that includes oxidative stress management and metabolic adaptation, thereby integratively shaping the antibiotic resistance spectrum of A. hydrophila.
Conclusion
This study demonstrates that the transcriptional regulator AHA_4052 in A. hydrophila plays a critical role in bacterial adaptation to thermal stress, osmotic pressure, and aminoglycoside resistance. Proteomic and molecular validation revealed that AHA_4052 exerts a dual regulatory function: it indirectly modulates aminoglycoside susceptibility through ribosomal protein expression, and directly controls the transcription of efflux pumps (AHA_2114) and ABC transporters (AHA_3488). Therefore, it will be very interesting to further study the mechanism of the transcriptional regulation factor AHA_4052 in aminoglycoside antibiotic resistance of A. hydrophila and to explore whether interfering with its mechanism can effectively kill the bacteria with low-dose antibiotics.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
WL: Formal analysis, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. ZT: Data curation, Methodology, Visualization, Writing – review & editing. HZ: Validation, Writing – review & editing. BL: Formal analysis, Writing – review & editing. FL: Data curation, Methodology, Writing – review & editing. XL: Conceptualization, Investigation, Resources, Supervision, Writing – review & editing. GW: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was sponsored by the National Natural Science Foundation of China (No. 32001045 and 32171435).
Conflict of interest
BL was employed by Changting Branch of Longyan Tobacco Company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1689335/full#supplementary-material
Footnotes
References
Abdelhamid, A. G., and Yousef, A. (2024). Untargeted metabolomics unveiled the role of butanoate metabolism in the development of Pseudomonas aeruginosa hypoxic biofilm. Front. Cell. Infect. Microbiol. 14:1346813. doi: 10.3389/fcimb.2024.1346813
Borella, L., Salogni, C., Vitale, N., Scali, F., Moretti, V. M., Pasquali, P., et al. (2020). Motile aeromonads from farmed and wild freshwater fish in northern Italy: an evaluation of antimicrobial activity and multidrug resistance during 2013 and 2016. Acta Vet. Scand. 62, 6–8. doi: 10.1186/s13028-020-0504-y
Cabezudo, I., Lobertti, C. A., Garcia Vescovi, E., and Furlan, R. L. E. (2022). Effect-directed synthesis of PhoP/PhoQ inhibitors to regulate Salmonella virulence. J. Agric. Food Chem. 70, 6755–6763. doi: 10.1021/acs.jafc.2c01087
Cai, S. J., and Inouye, M. (2002). EnvZ-OmpR interaction and osmoregulation in Escherichia coli. J. Biol. Chem. 277, 24155–24161. doi: 10.1074/jbc.M110715200
Cao, Q., Yang, N., Wang, Y. H., Xu, C. C., Zhang, X., Fan, K., et al. (2020). Mutation-induced remodeling of the BfmRS two-component system in Pseudomonas aeruginosa clinical isolates. Sci. Signal. 13:eaaz1529. doi: 10.1126/scisignal.aaz1529
Chakraborty, S., and Kenney, L. J. (2018). A new role of OmpR in acid and osmotic stress in Salmonella and E. coli. Front. Microbiol. 9:2656. doi: 10.3389/fmicb.2018.02656
Chen, H. B., Xiong, Z. J., Liu, K. Y., Li, S. G., Wang, R. B., Wang, X. J., et al. (2016). Transcriptional profiling of the two-component regulatory system VraSR in Staphylococcus aureus with low-level vancomycin resistance. Int. J. Antimicrob. Agents 47, 362–367. doi: 10.1016/j.ijantimicag.2016.02.003
Claxton, D. P., Jagessar, K. L., and Mchaourab, H. S. (2021). Principles of alternating access in multidrug and toxin extrusion (MTE) transporters. J. Mol. Biol. 433:166959. doi: 10.1016/j.jmb.2021.166959
Dahdouh, B., Basha, O., Khalil, S., and Tanekhy, M. (2016). Molecular characterization, antimicrobial susceptibility and salt tolerance of Aeromonas hydrophila from fresh, brackish and marine fishes. Alex. J. Vet. Sci. 48, 46–53. doi: 10.1128/JCM.01099-08
De Gaetano, G. V., Lentini, G., Famà, A., Coppolino, F., and Beninati, C. (2023). Antimicrobial resistance: two-component regulatory systems and multidrug efflux pumps. Antibiotics (Basel) 12:965. doi: 10.3390/antibiotics12060965
De Silva, L. A. D. S., and Heo, G.-J. (2022). Biofilm formation of pathogenic bacteria isolated from aquatic animals. Arch. Microbiol. 205:36. doi: 10.1007/s00203-022-03332-8
Dunkle, J. A., Vinal, K., Desai, P. M., Zelinskaya, N., Savic, M., West, D. M., et al. (2014). Molecular recognition and modification of the 30S ribosome by the aminoglycoside-resistance methyltransferase NpmA. Proc. Natl. Acad. Sci. U. S. A. 111, 6275–6280. doi: 10.1073/pnas.1402789111
Fernandez-Ciruelos, B., Potmis, T., Solomin, V., and Wells, J. M. (2023). Cross-talk between QseBC and PmrAB two-component systems is crucial for regulation of motility and colistin resistance in enteropathogenic Escherichia coli. PLoS Pathog. 19:e1011345. doi: 10.1371/journal.ppat.1011345
Gao, R., Mack, T. R., and Stock, A. M. (2007). Bacterial response regulators: versatile regulatory strategies from common domains. Trends Biochem. Sci. 32, 225–234. doi: 10.1016/j.tibs.2007.03.002
Groisman, E. A., Duprey, A., and Choi, J. J. (2021). How the PhoP/PhoQ system controls virulence and Mg2+ homeostasis: lessons in signal transduction, pathogenesis, physiology, and evolution. Microbiol. Mol. Biol. Rev. 85:e00176-00120. doi: 10.1128/MMBR.00176-20
Guo, H. R., Zhao, T., Huang, C., and Chen, J. Y. (2022). The role of the two-component system PhoP/PhoQ in intrinsic resistance of Yersinia enterocolitica to polymyxin. Front. Microbiol. 13:758571-758571. doi: 10.3389/fmicb.2022.758571
Hürlimann, L. M., Corradi, V., Hohl, M., Bloemberg, G. V., Tieleman, D. P., and Seeger, M. A. (2016). The heterodimeric ABC transporter EfrCD mediates multidrug efflux in Enterococcus faecalis. Antimicrob. Agents Chemother. 60, 5400–5411. doi: 10.1128/AAC.00661-16
Ibrahim, M., Ahmad, F., Yaqub, B., Ramzan, A., Imran, A., Afzaal, M., et al. (2020). “Chapter 4 -current trends of antimicrobials used in food animals and aquaculture” in Antibiotics and antimicrobial resistance genes in the environment, Ed. M. Lafleur, 39–69. Amsterdam, the Netherlands: Elsevier.
Jeamsripong, S., Odoi, J. O., Shahi, M. K., Anuntawirun, S., Roongrojmongkhon, N., and Thiptara, A. (2025). Global spread and antimicrobial resistance of Aeromonas hydrophila in aquatic food animals: a systematic review and meta-analysis. Sci. Rep. 15:28441. doi: 10.1038/s41598-025-14498-8
Kaatz, G. W., McAleese, F., and Seo, S. M. (2005). Multidrug resistance in Staphylococcus aureus due to overexpression of a novel multidrug and toxin extrusion (MATE) transport protein. Antimicrob. Agents Chemother. 49, 1857–1864. doi: 10.1128/AAC.49.5.1857-1864.2005
Kaur, S., Kaur, H., Kaur, B., Kumar, B. N., Tyagi, A., Singh, P., et al. (2024). Isolating pathogenic multidrug-resistant Aeromonas hydrophila from diseased fish and assessing the effectiveness of a novel lytic Aeromonas veronii bacteriophage (AVP3) for biocontrol. Microb. Pathog. 196:106914. doi: 10.1016/j.micpath.2024.106914
Ko, D., and Choi, S. H. (2022). Mechanistic understanding of antibiotic resistance mediated by EnvZ/OmpR two-component system in Salmonella enterica serovar enteritidis. J. Antimicrob. Chemother. 77, 2419–2428. doi: 10.1093/jac/dkac223
Li, W. X., Ali, F., Cai, Q. L., Yao, Z. J., Sun, L. N., Lin, W. X., et al. (2018). Quantitative proteomic analysis reveals that chemotaxis is involved in chlortetracycline resistance of Aeromonas hydrophila. J. Proteome 172, 143–151. doi: 10.1016/j.jprot.2017.09.011
Li, L. F., Ma, J. Y., Cheng, P., Li, M. C., Yu, Z. Y., Song, X. R., et al. (2023). Roles of two-component regulatory systems in Klebsiella pneumoniae: regulation of virulence, antibiotic resistance, and stress responses. Microbiol. Res. 272:127374. doi: 10.1016/j.micres.2023.127374
Li, Z., Zhang, L. S., Song, Q. L., Wang, G. B., Yang, W. X., Tang, H. M., et al. (2021). Proteomics analysis reveals bacterial antibiotics resistance mechanism mediated by ahslyA against Enoxacin in Aeromonas hydrophila. Front. Microbiol. 12:699415. doi: 10.3389/fmicb.2021.699415
Long, J. F., Xu, H. Z., Qi, X. Y., Yan, C. Y., Sun, X. N., Jin, Y. J., et al. (2025). The deletion of the uvrY in Aeromonas veronii disrupted the BarA/UvrY two-component system, decreasing persister formation and bacterial resistance to multiple antibiotics. Int. J. Food Microbiol. 435:111183. doi: 10.1016/j.ijfoodmicro.2025.111183
Ma, Y., Zhang, Y. Y., Chen, K., Zhang, L. Z., Zhang, Y. B., Wang, X., et al. (2021). The role of PhoP/PhoQ two component system in regulating stress adaptation in Cronobacter sakazakii. Food Microbiol. 100:103851. doi: 10.1016/j.fm.2021.103851
Macfarlane, E. L. A., Kwasnicka, A., and Hancock, R. E. W. (2000). Role of Pseudomonas aeruginosa PhoP-PhoQ in resistance to antimicrobial cationic peptides and aminoglycosides. Microbiology (Reading) 146, 2543–2554. doi: 10.1099/00221287-146-10-2543
Mao, M. Q., He, L., and Yan, Q. P. (2025). An updated overview on the bacterial PhoP/PhoQ two-component signal transduction system. Front. Cell. Infect. Microbiol. 15:1509037. doi: 10.3389/fcimb.2025.1509037
Mukai, T. (2021). Bioinformatic prediction of an tRNASec gene nested inside an elongation factor SelB gene in Alphaproteobacteria. Int. J. Mol. Sci. 22:4605. doi: 10.3390/ijms22094605
Ni, M. W., Wang, L., Chen, W., Mou, H. Z., Zhou, J., and Zheng, Z. G. (2017). Modified filter-aided sample preparation (FASP) method increases peptide and protein identifications for shotgun proteomics. Rapid Commun. Mass Spectrom. 31, 171–178. doi: 10.1002/rcm.7779
Orelle, C., Mathieu, K., and Jault, J. M. (2019). Multidrug ABC transporters in bacteria. Res. Microbiol. 170, 381–391. doi: 10.1016/j.resmic.2019.06.001
Pessoa, R. B. G., Oliveira, W. F. D., Correia, M. T. D. S., Fontes, A., and Coelho, L. C. B. B. (2022). Aeromonas and human health disorders: clinical approaches. Front. Microbiol. 13:1266. doi: 10.3389/fmicb.2022.868890
Stock, A. M., Robinson, V. L., and Goudreau, P. N. (2000). Two-component signal transduction. Annu. Rev. Biochem. 69, 183–215. doi: 10.1146/annurev.biochem.69.1.183
Stratev, D., and Odeyemi, O. A. (2016). Antimicrobial resistance of Aeromonas hydrophila isolated from different food sources: a mini-review. J. Infect. Public Health 9, 535–544. doi: 10.1016/j.jiph.2015.10.006
Tierney, A. R., and Rather, P. N. (2019). Roles of two-component regulatory systems in antibiotic resistance. Future Microbiol. 14, 533–552. doi: 10.2217/fmb-2019-0002
Wang, G. B., Cao, L., Lian, L. L., Wang, Y. Q., Lian, J. Q., Liu, Z. Q., et al. (2025b). Machine learning and DIA proteomics reveal new insights into carbapenem resistance mechanisms in Klebsiella pneumoniae. J. Proteome Res. 24, 4002–4014. doi: 10.1021/acs.jproteome.5c00142
Wang, G. B., Chen, L. X., Lian, J. Q., Gong, L. Q., Tian, F., Wang, Y. Q., et al. (2025a). Proteomic insights into the regulatory role of CobQ deacetylase in Aeromonas hydrophila. J. Proteome Res. 24, 333–343. doi: 10.1021/acs.jproteome.4c00847
Wang, Y.-D., Gong, J.-S., Guan, Y.-C., Zhao, Z.-L., Cai, Y.-N., and Shan, X.-F. (2023). OmpR (TCS response regulator) of Aeromonas veronii plays a major role in drug resistance, stress resistance and virulence by regulating biofilm formation. Microb. Pathog. 181:106176. doi: 10.1016/j.micpath.2023.106176
Wang, N. Y., Luo, J., Deng, F., Huang, Y. S., and Zhou, H. (2022). Antibiotic combination therapy: a strategy to overcome bacterial resistance to aminoglycoside antibiotics. Front. Pharmacol. 13:839808. doi: 10.3389/fphar.2022.839808
Wang, T., Tian, X.-L., Xu, X.-B., Li, H., Tian, Y., Ma, Y.-H., et al. (2022). Dietary supplementation of probiotics fermented Chinese herbal medicine Sanguisorba officinalis cultures enhanced immune response and disease resistance of crucian carp (Carassius auratus) against Aeromonas hydrophila. Fish Shellfish Immunol. 131, 682–696. doi: 10.1016/j.fsi.2022.10.046
Wu, C. J., Zhang, J. L., Zhu, G. X., Yao, R., Chen, X. L., and Liu, L. M. (2019). CgHog1-mediated CgRds2 phosphorylation alters glycerophospholipid composition to coordinate osmotic stress in Candida glabrata. Appl. Environ. Microbiol. 85:e02822-18. doi: 10.1128/AEM.02822-18
Yang, Y. Y., Miao, P. F., Li, H., Tan, S. W., Yu, H. Y., and Yu, H. (2018). Antibiotic susceptibility and molecular characterization of Aeromonas hydrophila from grass carp. J. Food Saf. 38:e12393. doi: 10.1111/jfs.12393
Zhang, B. Y., Zhang, Y., Liu, J. J., Reverter, D., Wang, Q. Y., Choi, S. H., et al. (2024). ChIP-seq and structural analyses delineating the regulatory mechanism of master regulator EsrB in Edwardsiella piscicida. Appl. Environ. Microbiol. 90:e0180524. doi: 10.1128/aem.01805-24
Keywords: Aeromonas hydrophila, OmpR/PhoB family, aminoglycosides, antibiotic resistance, ChIP-PCR
Citation: Li W, Tu Z, Zhang H, Lu B, Liu F, Lin X and Wang G (2025) Transcriptional regulatory factor AHA_4052 regulates aminoglycoside resistance in Aeromonas hydrophila. Front. Microbiol. 16:1689335. doi: 10.3389/fmicb.2025.1689335
Edited by:
Santi M. Mandal, University of California, San Diego, United StatesCopyright © 2025 Li, Tu, Zhang, Lu, Liu, Lin and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guibin Wang, d2FuZ2d1aWJpbjg1QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Wanxin Li
Wanxin Li ZhiWei Tu2†
ZhiWei Tu2† Xiangmin Lin
Xiangmin Lin Guibin Wang
Guibin Wang