- 1Department of Cardiology, Heilongjiang Academy of Traditional Chinese Medicine, Harbin, China
- 2Department of Graduate School, Heilongjiang Academy of Traditional Chinese Medicine, Harbin, China
- 3Department of Gastroenterology, Heilongjiang Academy of Traditional Chinese Medicine, Harbin, China
The gut microbiome has emerged as a critical modulator of cardiovascular disease (CVD) risk, offering a novel frontier for therapeutic intervention. This mini-review synthesizes current evidence on how probiotic-like bacteria and their metabolites mediate protective physiological mechanisms against CVD. Drawing from both animal models and human clinical trials, we elucidate the biological pathways, including trimethylamine-N-oxide (TMAO), short-chain fatty acids (SCFAs), and bile acid metabolism, through which the gut microbiota influences hypertension, atherosclerosis, and heart failure. Furthermore, we examine microbiota-based strategies such as dietary modification, fecal microbiota transplantation (FMT), and pharmacological agents aimed at restoring microbial homeostasis. Despite promising mechanistic insights, human trials have yet to consistently demonstrate significant clinical benefits in reversing CVD outcomes via gut microbiota modulation. This review underscores the necessity of moving from correlation to causation, highlighting current limitations and future prospects for leveraging gut microbiome research in the development of personalized, effective therapeutic strategies for cardiovascular diseases.
1 Introduction
In the pursuit of effective and sustainable medicinal intervention, approaches with minimal adverse effects are highly prioritized. While antibiotics, and endocrine drugs, and metabolic therapies aim to promote health, they often face challenges such as pathogen resistance or detrimental side effects on the host. In recent years, the emerging field of gut microbiota medicine has introduced a range of non-pharmacological interventions, offering promising alternatives (Bajinka et al., 2022, 2023). Notably, macromolecules and metabolites derived from dietary supplements are being extensively studied for their potential to reverse life-threatening conditions, including metabolic disorders, cardiovascular disease (CVD), neurodegenerative diseases, and cancer (Yang and Cong, 2021). Alterations in gut microbiota composition can lead to low-grade, systemic, and local inflammation, directly contributing to the development of metabolic disorders such as obesity, diabetes mellitus, CVD, and microvascular complications (Yang L. et al., 2021; Hasani et al., 2024).
The gut microbiota has emerged as a powerful regulator of host physiology, attracting significant scientific interests in its role in reversing metabolic disorders. It mediates host physiology through various mechanisms, including signaling via receptor ligands, serving as substrates for host enzymes, and providing metabolites as energy sources. Through these interactions, gut microbiota plays a direct role in the pathogenesis of CVD. For instance, the breakdown of dietary nutrients such as choline, coupled with lipid metabolism and host immune system modulation, can lead to the development of atherosclerosis (Dovi et al., 2022). Current strategies to mitigate these conditions focus on reducing trimethylamine (TMA) to trimethylamine-N-oxide (TMAO) using compounds like 3,3-dimethyl-1-butanol and archaebiotics. Additionally, converting high fiber diets into TMA precursors to promote beneficial bacterial populations is being explored as an anti-atherogenic approaches (Anbazhagan et al., 2017).
Beyond metabolic pathways, the gut microbiota also interacts with systemic immunity to influence CVD progression. Studies using murine lupus models have revealed connections between gut microbiota dysbiosis, antiphospholipid syndrome (APS), and autoimmune-driven vascular damage. Altered intestinal IgA levels and molecular mimicry between microbial proteins and host autoantigens may exacerbate thrombosis and endothelial dysfunction, while dietary short-chain fatty acids (SCFAs) could mitigate these effects through immunomodulation (van Mourik et al., 2022). Importantly, the integrity of the gut barrier and microbiota homeostasis is closely associated with systemic inflammation-a key driver of both CVD and cerebrovascular conditions. For example, ischemic stroke shares overlapping risk factors and inflammatory pathways with CVD, suggesting a broader role of gut microbiota in circulatory health (Wei et al., 2021). However, the mechanisms by which gut microbiota directly regulates CVD pathogenesis, particularly through metabolites and endothelial interactions, remain a critical research frontier.
Among CVD-related interventions, dietary phytochemicals such as anthocyanins exemplify the therapeutic potential of microbiota-mediated approaches. Anthocyanins confer antioxidant effects by activating nuclear factor erythroid 2-related factor 2 and suppressing pro-inflammatory cytokines, thereby improving endothelial function and nitric oxide bioavailability (Xin et al., 2024). These findings highlight the bidirectional crosstalk between dietary components, gut microbiota, and cardiovascular homeostasis. Giving the growing evidence supporting the role of gut microbiota in mediating CVD, a systematically analysis of the underlying biological mechanisms is crucial. While human clinical trials provide valuable insights, complementary studies using animal models are essential to elucidate these mechanisms.
To comprehensively map the gut microbiota’s role in CVD, a systematic literature search was conducted in PubMed, Web of Science, and Scopus (2013–2023) using keywords including “gut microbiota,” “cardiovascular disease,” “TMAO,” “SCFAs,” and “inflammation” combined with Boolean operators. Included studies investigated the mechanistic role of gut microbiota in cardiovascular diseases in human or animal models and were published in English. Exclusion criteria covered non-peer-reviewed articles and studies lacking mechanistic insights. Data on study design, findings, and mechanisms were extracted independently by two reviewers, with discrepancies resolved through consensus. Reference lists of relevant articles were also screened.
This mini-review aims to provide a comprehensive overview of recent studies on gut microbiota-mediations interventions for CVD, drawing from both human and animal research over the past decade, sourced from various databases. This review innovatively synthesizes a decade of human and animal studies to map the gut microbiota’s role in CVD through specific metabolites and pathways. It critically evaluates the translational gap between mechanistic insights and clinical applications, highlighting the lack of robust human trial data. By proposing advanced future directions, like CRISPR-based microbiome editing and species-specific FMT, it provides a forward-looking framework for moving from correlation to causation, paving the way for targeted, microbiota-based personalized therapeutics in CVD.
2 Gut microbiota-induced risk factors for cardiovascular disease
The risk factors for CVD include hypertension, diabetes, hypercholesterolemia, obesity, chronic inflammation, and genetic factors (Kjeldsen, 2018; Dal Canto et al., 2019; Alfaddagh et al., 2020; Foger et al., 2020; Powell-Wiley et al., 2021; Tada et al., 2022). Among these, gut microbiota diversity, as a key regulator of metabolism and dietary intake, plays a pivotal role in driving the pathological processes of CVD, particularly hypertension, atherosclerosis, and heart failure (Jia et al., 2023).
Hypertension, as a key risk factor for cardio-cerebral vascular diseases, can induce gut microbiota dysbiosis and gut barrier dysfunction. This is mediated by the influx of hydrogen sulfide, lipopolysaccharide (LPS), and pathogenic bacteria, coupled with a reduction in SCFA-producing bacterial populations. These changes lead to increased intestinal permeability and disruption of tight junction proteins (Luqman et al., 2024). Furthermore, reduced alpha diversity and an increased abundance of LPS-producing Gram-negative bacteria contribute to pro-inflammatory responses, resulting in dysregulated blood pressure and hypertension.
Increased gut permeability is strongly associated with LPS translocation into systemic circulation, yet therapeutic targets to mitigate CVD risk remain elusive. Although interventions targeting bile acid receptors show potential in reducing atherosclerosis progression, TMAO has emerged as a pro-atherogenic metabolite, representing one of multiple pathways involved in CVD pathogenesis (Yang et al., 2023). Elevated cholesterol levels are another critical risk factor for CVD. Through gut microbiota modulation, probiotics from the genera Bifidobacterium and Lactobacillus have demonstrated efficacy in controlling cholesterol levels in clinical studies (Fuentes et al., 2013; Costabile et al., 2017; Marras et al., 2021). Moreover, next-generation probiotics, such as Bacteroides spp., and Akkermansia show promise in specifically lowering cholesterol levels (Verhaar et al., 2020). Gut microbiota-derived metabolites, include TMAO, SCFAs, polyphenols, and bile acids, are essential for maintaining the healthy function of cardiovascular organs (Massey et al., 2022).
3 Gut microbiota-induced mechanisms for cardiovascular disease
Distinct gut dysbiosis profiles are closely associated with specific CVD subtypes, reflecting the complex interplay between microbiota composition and disease pathophysiology (Datta et al., 2024). In metabolic disorders such as T2DM, obesity, and hyperlipidemia, the gut microbiota is characterized by a significant reduction in the abundance of Akkermansia and Bifidobacterium, two genera known for their roles in maintaining metabolic homeostasis and gut barrier integrity. In contrast, atherosclerosis is associated with an elevated Firmicutes/Bacteroidetes ratio, a microbial signature often linked to pro-inflammatory states and impaired lipid metabolism. Furthermore, coronary heart disease shows a unique microbial profile marked by increased Collinsella abundance, a genus implicated in promoting inflammation and cholesterol accumulation. Meanwhile, myocardial infarction exhibits a transient microbial shift, featuring enrichment of Lactobacillus and depletion of Bacillus, which may reflect acute inflammatory responses and oxidative stress during the ischemic event.
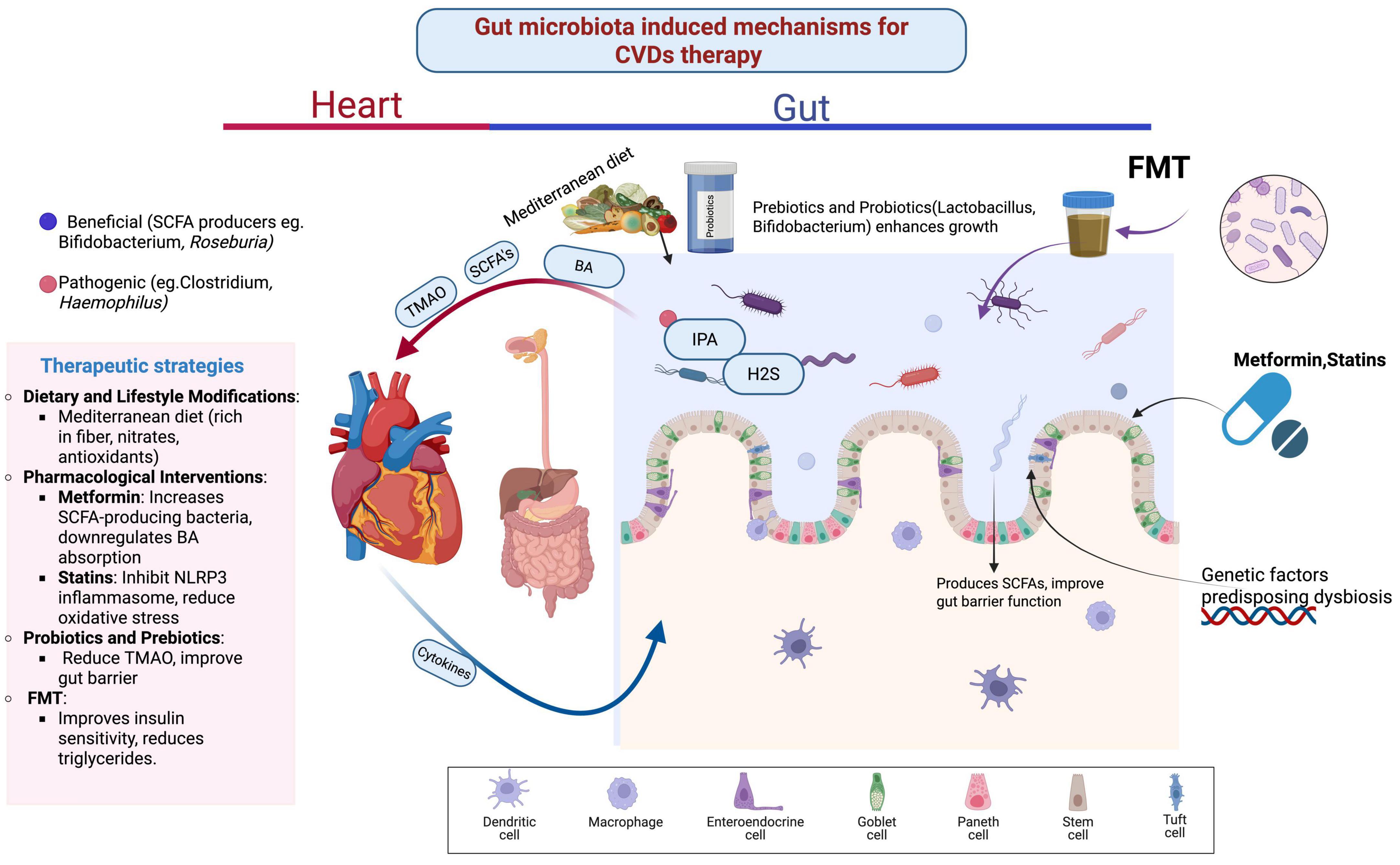
From a metabolic perspective, gut microbiota-derived metabolites play pivotal roles in CVD pathogenesis through multiple mechanisms. Elevated TMAO levels, generated from dietary choline via gut microbial metabolism and hepatic flavin monooxygenase-3 conversion, are associated with CVD risk factors including obesity, type 2 diabetes mellitus (T2DM), hyperlipidemia, as well as specific CVD manifestations such as coronary heart disease and myocardial infarction by promoting platelet hyperreactivity, atherosclerosis, and heart failure (Zhu et al., 2016; Guasti et al., 2021; Zhang et al., 2021). The gut microbiota also influences bile acid metabolism, which activates nuclear receptors such as the farnesoid X receptor (FXR) and pregnane X receptor (PXR). These receptors regulate lipid homeostasis by downregulating triglyceride levels and attenuating pro-inflammatory NF-κB signaling, thereby modulating atherosclerosis progression and coronary heart disease (Wu et al., 2021). Conversely, dysbiosis-driven metabolites like phenylacetylglutamine exacerbate thrombosis and in-stent stenosis (Chassaing et al., 2015; Lahnwong et al., 2018), while hydrogen sulfide exerts protective effects by inhibiting nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome activation via purinoreceptor-7 blockade (Jia et al., 2020). Indole-3-propionic acid (IPA), a microbial metabolite derived from dietary tryptophan by gut microbiota such as Lactobacillus reuteri, Clostridium caloritolerans, Clostridium sporogenes, and Peptostreptococcus. IPA enhances gut–blood barrier function through the expression of tight junction proteins and claudins, activates the aryl hydrocarbon receptor, and protects against lipid peroxidation and oxidative damage (Konopelski and Mogilnicka, 2022). Furthermore, IPA stimulates macrophage reverse cholesterol transport by upregulating ABCA1 expression (Schwartz et al., 2013). As gut microbiota translocates to the aortic artery due to defect in gut barrier integrity, microbial-derived metabolites and inflammation results in renal insufficiency and inflammation-signaling pathway. While gut microbiota modulation of drug efficacy against CVD is harnessed, its combination with physical exercise will lead to healthier cardiovascular organ functions (Jia et al., 2019).
4 Gut microbiota-mediated therapeutic strategies for cardiovascular disease
To prevent CVD, current strategies include evidence-based approaches such as dietary and lifestyle modifications, pharmacological interventions, as well as novel approaches like fecal microbiota transplantation (FMT) and NLRP3 inflammasome inhibition. Mechanistic studies have shown that these interventions may contribute to reduced risks of heart failure and atherosclerosis, though clinical evidence varies across different modalities. Regular physical exercise and supplementation with probiotics, prebiotics, or their combination as synbiotics have been shown to ameliorate cardiac hypertrophy and fibrosis. Furthermore, adopting healthy lifestyle practices enhances microbiome composition and function, thereby augmenting the production of beneficial metabolites essential for gastrointestinal tract homeostasis (Zhao and Wang, 2020; Usman et al., 2024). Through gut microbiota-artery axis, dietary components such as omega-3 polyunsaturated fatty acids, sphingomyelin, and phosphatidylcholine play pivotal roles in lipid metabolism and the progression of atherosclerosis (Shi et al., 2024). Probiotics, in particular, stabilize the gastrointestinal tract dynamics, generating metabolites with cardioprotective properties, thus advancing precision medicine for cardiometabolic complications. Conversely, high-salt dietary supplements reduce the abundance of Lactobacillus, leading to an increase in T helper 17 (Th17) cells, which are biomarkers for salt-sensitive hypertension. The Mediterranean diet, characterized by reduced saturated fatty acids, phosphate, and sodium, along with enriched nitrate, fiber, and antioxidants, promotes gut microbiota diversity. This ultimately mitigates oxidative stress while enhancing antioxidant functions and nitric oxide bioavailability, thereby improving cardiac and vascular function (Barrea et al., 2019).
In addition to dietary and lifestyle interventions, the introduction of healthy gastrointestinal tract microbiome to patient’s FMT is effective for controlling Clostridium difficile. Emerging evidence suggests its potential in improving insulin sensitivity and plasma triglyceride levels, thereby enhancing cardiometabolic health (Miranda et al., 2018). This is evidenced by a downregulation of myocarditis incidence and an increase in Bacteroidetes abundance. Pharmacological interventions targeting gut microbiota composition and dynamics are crucial for attenuating CVD-related dysbiosis. Dipeptidyl peptidase-4 (DPP-4) inhibitors mitigate cardiovascular risks by attenuating high-fat-diet-induced dysbiosis, promoting the abundance of SCFAs-producing microbiota, and reducing the Bacteroidetes to Firmicutes ratio (Zhang et al., 2023). Biguanides, such as metformin, reduce the risk of myocardial infarction in patients with T2DM by regulating the incretin pathway and enhancing peripheral glucose uptake. This is achieved through increased pancreatic and plasma levels of glucagon-like peptide-1, a precursor for SCFAs, which exert cardioprotective effects (Bu et al., 2022). Additionally, metformin downregulates bile acids absorption by inhibiting the apical sodium-dependent bile acid transporter, thereby interfering with primary bile acids-mediated FXR activation. This process is facilitated by SCFA-producing genera such as Bifidobacterium bifidum, Butyrivibrio, and Megasphera. Furthermore, α-glucosidase inhibitors, such as acarbose, upregulate bile salt hydrolase activity, promoting beneficial genera like Bifidobacterium and Lactobacillus while suppressing pathogenic genera such as Clostridium, Alistipes, and Bacteroides, thereby contributing to cardiometabolic homeostasis (Montandon and Jornayvaz, 2017; Hu et al., 2019).
Targeting the NLRP3 inflammasome, a sensor of deleterious endogenous and exogenous stimuli, is a promising therapeutic strategy for mitigating pro-inflammatory signaling and CVD progression, particularly atherosclerosis. Cholesterol crystals activate the NLRP3 inflammasome, increasing the risk of atherosclerotic plaque formation (Morgan et al., 2014). TMAO, a key trigger of NLRP3 inflammasome activation, induces endothelial barrier dysfunction and hyperpermeability, ultimately leading to cardiac fibrosis. Therefore, interventions targeting NLRP3 inflammasome activation are critical for attenuating atherosclerosis. Statins, analogs of HMG-CoA reductase inhibitors, mitigate oxidative stress, endothelial dysfunction, cardiomyocyte apoptosis, and cardiac hypertrophy (Ning et al., 2010; Viollet et al., 2012). Statins, including rosuvastatin, impede the TLR4/MyD88/NF-κB signaling pathway, a key activator of NLRP3 inflammasome, and are effective in treating diabetic cardiomyopathy (Duewell et al., 2010). Simvastatin enhances endothelial barrier integrity by upregulating tight junction protein expression, reducing vascular endothelial hyperpermeability, and mitigating hyperglycemia-associated endothelial dysfunction (Rajamaki et al., 2010). Mechanistically, simvastatin inhibits HMGB1 release in aortic endothelial cells, thereby obstructing NLRP3 inflammasome activation. Additionally, the natural NLRP3 inflammasome inhibitor, arglabin, exhibits anti-atherogenic effects in high-fat-diet-induced murine models (Zhang et al., 2022).
Acetate-producing bacteria reduce blood pressure and alleviate cardiac fibrosis and hypertrophy, while probiotics modulate oxidative stress, inflammation, renin-angiotensin system overactivity, vascular resistance, and hyperlipidemia. Lactobacilli increase SCFA production and reduce toxin levels, thereby inhibiting atherosclerosis progression. TMAO levels can be reduced by inhibiting TMA production in the gastrointestinal tract, and flavonoids inhibit TMA lyase activity, offering therapeutic potential for coronary heart disease. Oat fiber prevents atherosclerosis by blocking the TLR4 signaling pathway, modifying lipid metabolism, and reducing NF-κB p65 expression (Gao et al., 2022). Furthermore, intestinal mucosal barrier integrity is preserved through alterations in metabolites such as 1-methylguanosine, 2-methylguanosine, isobutyrylcarnitine, and valerylcarnitine. Fish oil-derived long-chain monounsaturated fatty acids (LCMUFAs) have demonstrated cardiovascular risk reduction in murine models, with reduced TMAO levels and improved endothelial function (Tsutsumi et al., 2021). LCMUFAs also increase Akkermansia abundance while reducing Firmicutes and Bacteroidetes, thereby balancing the intestinal microenvironment through SCFA regulation and glucagon-like peptide modulation. Additionally, LCMUFAs attenuate atherosclerosis by reducing macrophage infiltration, regulating inflammation, and lowering serum levels of branched-chain amino acids (Yang et al., 2016; Figure 1 and Table 1).
5 Nutrition, the microbiome, and cardiovascular disease pathogenesis
This section highlights a complex, bidirectional relationship between diet, gut microbiota composition, and host cardiovascular health. The molecular mechanisms underpinning this relationship involve microbial metabolite production, systemic inflammation, oxidative stress, and endothelial function.
5.1 Oxidative stress and microbial metabolites
Oxidative stress is a critical molecular mechanism through which gut dysbiosis contributes to CVD. An imbalanced microbiome can produce metabolites that either exacerbate or ameliorate oxidative damage. Certain dietary patterns foster bacteria that produce metabolites with potent antioxidant properties. For instance, the high-fiber rye intervention in the RyeWeight study led to increased plasma levels of microbial metabolites like indolepropionic acid and enterolactone (Wang et al., 2024). These compounds are known for their antioxidant activities, which can mitigate oxidative damage to lipids and proteins in the vascular endothelium, a key step in atherogenesis. Similarly, the hydroxytyrosol (HT) study demonstrated that responders to this phenolic compound exhibited improved glutathione metabolism, a master regulator of the cellular antioxidant defense system (Li et al., 2025). This suggests that HT’s benefits may be mediated through both direct antioxidant effects and the modulation of microbial communities that support endogenous antioxidant pathways. Direct evidence comes from the trial on postbiotic supplementation in stroke patients, which showed a significant reduction in the oxidative stress marker malondialdehyde (MDA) and an increase in total antioxidant capacity (TAC) (Chavez-Alfaro et al., 2025). This indicates that postbiotics (inanimate microorganisms and/or their components) can confer systemic antioxidant benefits, likely by reducing the production of pro-oxidant molecules by the host’s immune system or by the microbiota itself.
5.2 Inflammation and endothelial dysfunction
Systemic inflammation is a cornerstone of CVD pathogenesis, and the gut microbiome is a primary regulator of this process. Diet directly influences the inflammatory tone via microbial signaling. Dysbiosis can increase gut permeability (“leaky gut”), allowing the translocation of bacterial fragments like LPS into the bloodstream. LPS is a potent endotoxin that triggers systemic inflammation by activating immune cells and promoting the release of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α (Fan et al., 2025). This chronic, low-grade inflammation damages the endothelium and promotes atherosclerosis.
The abstracts show that dietary interventions can reverse this. The CADIMED trial (Ng et al., 2025) and the cycling diet study aim to or demonstrate a reduction in CVD risk factors by promoting anti-inflammatory microbial patterns (Verhaar et al., 2024). The casein peptide study explicitly links its antihypertensive effect to anti-inflammatory and antioxidant effects that improve endothelial function (Gawlik-Kotelnicka et al., 2024). Furthermore, the postbiotic trial confirmed a direct reduction in IL-1β and hs-CRP following intervention, underscoring the gut-inflammatory axis (Spasova et al., 2024).
Butyrate, a SCFA produced by bacterial fermentation of dietary fiber, is generally considered anti-inflammatory. However, the trial by Verhaar et al. (2024) presents a surprising finding: oral butyrate supplementation increased blood pressure in hypertensive patients (San Diego et al., 2025). This critical result indicates that the molecular context (route of administration, baseline health, concomitant diet) drastically alters the effect of a single metabolite. It warns against simplistic supplementation and highlights the need for a holistic, diet-based approach to modulate the entire microbial ecosystem for balanced SCFA production. Conversely, the study by San Diego et al. (2025) found that the blood pressure response to a butyrate enema was correlated with intake of specific food groups (vegetables, whole grains), not just the nutrient itself, emphasizing the importance of the dietary matrix (Rahimi et al., 2024).
5.3 Pro-atherogenic microbial metabolites
The gut microbiome can generate metabolites that directly contribute to CVD pathology. The trial by Spasova et al. (2024) focuses on TMAO, a well-established independent risk marker for CVD (Yang et al., 2025). Gut bacteria metabolize dietary nutrients like L-carnitine (red meat) and choline (eggs, liver) into TMA, which is then oxidized in the liver to TMAO. TMAO promotes atherosclerosis by stimulating foam cell formation, enhancing platelet reactivity, and inducing endothelial dysfunction. Interventions aimed at reducing TMAO-producing bacteria or their activity are a direct molecular strategy for CVD risk reduction.
5.4 Impact of specific nutrients and dietary fibers on microbial ecology
The molecular effects are ultimately driven by diet-induced shifts in the microbial population. Increased fiber intake, as seen with defatted rice bran bread and the high-fiber rye diet, promotes the growth of beneficial bacteria like Faecalibacterium prausnitzii (a major butyrate producer) and Bifidobacterium (Pontes et al., 2025; Union Caballero et al., 2025). These shifts are associated with improved metabolic outcomes, such as increased HDL cholesterol. The physical therapy study also showed that a diet enriched with dietary fibers increased fecal levels of propionic and butyric acid, which was correlated with reduced systemic inflammation and improved vascular stiffness (Noguera-Navarro et al., 2025). The study by Wang et al. (2024) reveals that the molecular and microbial environment is dynamic. Repeatedly adopting and abandoning a healthy dietary pattern led to a cycling pattern in microbial taxa (Collinsella, Mediterraneibacter) that was mirrored by cycling in LDL-C and total cholesterol. This demonstrates that the molecular benefits of a healthy diet are transient and dependent on sustained dietary habits, as the microbiome rapidly reverts to its baseline state.
5.5 Therapeutic targeting
Multi-strain probiotics improved glucose homeostasis (HbA1c) in hypertensive individuals (Noguera-Navarro et al., 2025). More strikingly, probiotic supplementation prior to cardiopulmonary bypass surgery significantly reduced the incidence of acute gastrointestinal injury, likely by preserving gut barrier integrity and preventing bacterial translocation and subsequent systemic inflammation and oxidative stress (Petelina et al., 2025). The study by Gawlik-Kotelnicka et al. (2024) further suggests probiotic efficacy may be enhanced in individuals with a “leaky gut” or specific immunometabolic profiles (Li-Hua and Bajinka, 2025). The FMT trial for hypertension provided proof-of-concept that altering the gut microbiome can affect blood pressure (Bajinka et al., 2025). Although the effect was unsustainable, the study identified specific bacteria (Eggerthella lenta, Erysipelatoclostridium ramosum) whose decreased abundance was correlated with reduced BP, and others whose increase was beneficial. It also linked these microbial shifts to changes in blood pressure-modulating metabolites like tyrosine, glutamine, and phenylalanine, outlining a clear molecular pathway from microbiota to host physiology.
The molecular pathogenesis of heart diseases is profoundly influenced by the gut microbiome, which acts as a key interpreter of nutritional intake. Diets rich in fiber and polyphenols (Mediterranean, high-fiber rye) promote a microbial ecosystem that produces beneficial metabolites (SCFAs, antioxidants), reduces inflammation, and protects the endothelium. Conversely, poor dietary patterns lead to dysbiosis, characterized by increased production of pro-inflammatory molecules, pro-oxidant species, and pro-atherogenic metabolites like TMAO, thereby driving CVD progression. The findings caution against the isolated use of microbial metabolites such as butyrate pills and instead advocate for a whole-diet approach to sustainably modulate the complex molecular dialogue between nutrition, the microbiome, and the host cardiovascular system.
6 Limitations to gut microbiota-mediating cardiovascular disease
Despite advancements in targeted preventive and personalized approaches, including FMT, dietary modulation, and pharmacological interventions, significant limitations persist in gut microbiota-mediation strategies for delaying the onset and progression of CVD episodes. A primary challenge lies in the incomplete understanding of the biological mechanisms underlying the pathophysiological state induced by gut microbiota dysbiosis, which contributes to CVD development. While metformin demonstrates effective cardioprotective benefits in diabetic patients, its therapeutic effects in non-diabetic individuals remain mechanistically unexplained. Furthermore, although preliminary evidence suggests improvement in ischemic risk vascular function among angina patients, large-scale randomized controlled trials with extended follow-up periods are required to establish robust clinical significance (Jadhav et al., 2006; Holman et al., 2017; Luo et al., 2019). Notably, metformin intervention has shown no significant impact on carotid intima-media thickness, a well-established CVD biomarker (Chen Y. et al., 2020).
Additional limitations in gut microbiota-mediated CVD pathophysiology include the inconsistent cardioprotective effects of α-glucosidase inhibitors. Specifically, coronary artery disease patients with impaired glucose tolerance demonstrate no significant cardiometabolic improvement following acarbose administration. Moreover, sodium glucose co-transporter 2 inhibitors, despite their demonstrated effects in reducing cellular apoptosis, mitochondrial dysfunction, and cardiovascular inflammation, exhibit no discernible impact on gut microbiota homeostasis (Zeng et al., 2021). The therapeutic application of FMT faces challenges related to donor-recipient incompatibility, particularly concerning extra-intestinal complications such as infections and endotoxin-related adverse events, necessitating rigorous safety evaluations (Cao et al., 2025).
The role of free sulfate concentrations in mitochondrial complex IV function remains unclear, potentially leading to impaired oxygen consumption and reduce butyrate oxidation in the colon following non-digestible carbohydrates administration (Rastall et al., 2022). These prebiotics possess emulsification property that may alter gut microbiota composition and potentially facilitate epithelium bacterial translocation, increasing the risk of septicemia (Allam-Ndoul et al., 2020). While gut microbiota modulation for CVD prevention and treatment is gaining increasing attention, the imperative for well-designed studies adhering to stringent clinical safety and efficacy standards remains paramount.
The field must progress from establishing correlations to demonstrating causality in gut microbiota-mediated CVD mechanisms. This requires the identification of functional metabolites associated with specific molecular pathways and the characterization of microbial species and strains producing bioactive compounds with distinct cardiac phenotypes. Furthermore, the standardization of experimental conditions and optimization of FMT protocols, from sample collection to storage, remain areas requiring resolution due to ongoing procedural controversies.
7 Prospects in gut microbiota-mediated cardiovascular disease
The integration of advanced technologies, such as CRISPR/Cas9, enables precise modulation of gut microbiota metabolic pathways, thereby enhancing the targeted expression of cardioprotective metabolites. This approach will facilitate the identification of specific roles played by gut microbiota-derived metabolites in attenuating CVD pathogenesis, while elucidating the underlying biochemical processes. In addition, to comprehensively understand gut microbiota-derived metabolic changes in CVD, human metabolomics studies should be integrated with bacterial proteomic and metagenomics, providing a holistic view of the onset and progression of CVD episodes. Moreover, FMT strategies focusing on species-specific interventions rather than phyla or genera, along with the use of cytostatic agents instead of cytotoxic drugs, will ensure both target specificity and the maintenance of gut microbiota homeostasis (Ahmad et al., 2024).
Cardiovascular disease-associated conditions, such as hypertension, coronary atherosclerosis and heart failure, represent a significant global health burden, contributing to elevated mortality rates. The bidirectional interplay between these conditions and gut microbiota is mediated by well-defined biological processes, highlighting the functional role of gut microbiota in cardiovascular health. Given that arteriosclerosis is often diagnosed at advanced stages, characterized by elevated blood glucose, lipid, and insulin levels, as well as persistent low-grade inflammation and insulin resistance, there is an urgent need for microbiota-targeted individualized strategies for both the prevention and management of CVD (Hsu et al., 2019). Reduced diversity of SCFAs producing bacteria and the proliferation of pathogenic bacteria in the gut microbiota can lead to increased levels of TMAO, a known risk factor for CVD. Supplementation with probiotics and prebiotics can promote the dominance of beneficial bacterial populations, thereby inhibiting the conversion of dietary lecithin, L-carnitine, and choline into TMA, which is subsequently oxidized to TMAO in the liver. Notably, TMAO serves as a biomarker for stroke, myocardial infarction, and mortality, while SCFAs exert systemic anti-inflammatory effects, serving as energy sources for colonocytes and regulators of blood pressure. Maintaining a consistent supply of SCFAs is crucial for delaying the onset of hypertension, and bile acid biosynthesis, mediated by receptors such as takeda G protein-coupled receptor 5, PXR, and FXR, is significantly influenced by gut microbiota in CVD (Martins et al., 2024; Ronen et al., 2024).
In summary, the three primary gut microbiota-derived metabolites such as SCFAs, TMAO, and bile acids are promising biomarkers for CVD. Their targeted modulation offers novel therapeutic strategies for CVD treatment. Future therapeutic approaches will focus on harnessing molecular mechanistic studies to elucidate the bidirectional biological pathways connecting gut microbiota and CVD risk. Investigating specific microbial taxa, including non-bacterial microorganisms, as well as the shifts in metabolite profiles and their associated metabolic diseases, will provide deeper insights into gut microbiota-mediated prevention and management of CVD.
Hypercholesterolemia, a well-established risk factor for CVD, can be mitigated through gut microbiota modulation via supplementation with prebiotics, probiotics, and statins. These interventions regulate cholesterol homeostasis through metabolites such as TMAO, SCFAs, and bile acids (Vourakis et al., 2021). A meta-omics perspective offers profound implications for understanding the role of gut microbiota in CVD (Xu and Yang, 2021). Beyond dietary interventions, the detection of biomarkers represents another promising strategy for reducing CVD risk (Evans et al., 2020). The interactions between phytochemicals and gut microbiota-mediated histone acetylation, adipose tissue dysfunction, blood pressure regulation, and other bioactive compounds are areas of growing interest (Pieczynska et al., 2020; Yang X. et al., 2021; Yan et al., 2022; Jiang et al., 2024). Additionally, the role of gut microbiota and its metabolites in reverse cholesterol transport, stroke-related risk factors, and exercise-mediated protection in atherosclerotic cardiovascular disease warrants further investigation (Gao C. et al., 2024; Zhong et al., 2024).
With far-reaching clinical applications, gut microbiota research has been significantly advanced through the use of cutting-edge technologies, enabling robust and sensitive analyses. Although mechanistic studies remain incomplete, biochemical and molecular methodologies are yielding increasingly reliable results in this field. The detection of biomarkers via metabolomics analysis holds promise for personalized medicine, offering alternative adjunct therapies through gut microbiota manipulation (Chen L. et al., 2020). Despite clear evidence of the gut-immune-B2 cell axis, including B cell-mediated humoral immunity via TLR signaling pathways, the modulation of atherosclerosis-associated immune responses requires further scientific validation (Forkosh and Ilan, 2019). The heart-gut axis has emerged as a novel therapeutic target for congestive heart failure and atherosclerosis (Singh et al., 2024).
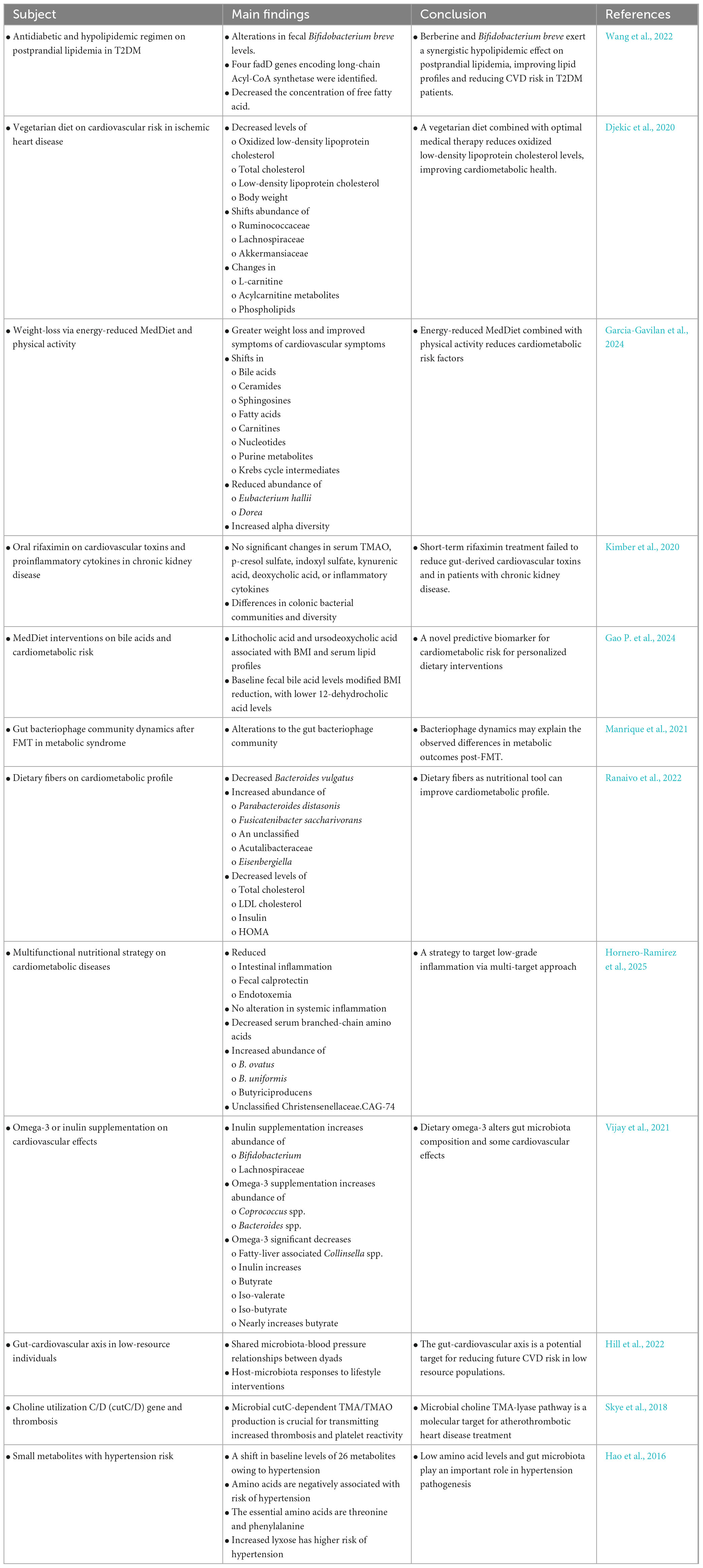
From a clinical perspective, T2DM patients at risk of developing CVD exhibit synergistic hypolipidemic effects following postprandial lipidemia reduction induced by berberine and Bifidobacterium breve. The dynamics of the gut bacteriophage community post-FMT are critical for metabolic syndrome subjects. Vitamin D supplementation has been shown to reduce oxidized low-density lipoprotein cholesterol levels, thereby improving cardiometabolic health (Ostadmohammadi et al., 2019). Weight loss interventions based on energy-reduced Mediterranean diets have demonstrated significant improvements in CVD risk factors. Mediterranean diets interventions targeting bile acids represent a novel approach to biomarker production for cardiometabolic risk, as do dietary fibers in improving cardiometabolic profiles. Microbiota-focused strategies, such as omega-3 fatty acids, inulin, and choline utilization for thrombosis-related genes, can enhance cardiometabolic effects. However, short-term rifaximin treatment has failed to reduce gut-derived cardiovascular toxins in patients with chronic kidney disease.
8 Conclusion
This mini-review underscores the pivotal role of gut microbiota-derived metabolites, particularly TMAO, SCFAs, and bile acids as both biomarkers and mediators in CVD. TMAO is strongly linked to atherosclerosis and thrombosis, while SCFAs confer anti-inflammatory and blood pressure-regulating benefits. Bile acid metabolism, modulated by receptors such as TGR5, FXR, and PXR, further influences lipid homeostasis and vascular health. Therapeutic strategies like FMT, the Mediterranean diet, and supplementation with omega-3 fatty acids or inulin show promise in reshaping microbial communities and reducing CVD risk. However, translating these interventions into sustained clinical benefits remains challenging. Future efforts must focus on elucidating causal mechanisms, standardizing protocols, and advancing personalized microbiota-targeted therapies. Ultimately, harnessing the gut-heart axis offers a transformative approach for the prevention and treatment of cardiovascular diseases.
Author contributions
SZ: Conceptualization, Writing – original draft. JL: Visualization, Writing – original draft. LL: Writing – review & editing. XY: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Youth Talent Cultivation Program of the China Association of Chinese Medicine (202557-011).
Acknowledgments
Figure was created in https://BioRender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmad, A. F., Caparros-Martin, J. A., Gray, N., Lodge, S., Wist, J., Lee, S., et al. (2024). Gut microbiota and metabolomics profiles in patients with chronic stable angina and acute coronary syndrome. Physiol Genom. 56, 48–64. doi: 10.1152/physiolgenomics.00072.2023
Alfaddagh, A., Martin, S. S., Leucker, T. M., Michos, E. D., Blaha, M. J., Lowenstein, C. J., et al. (2020). Inflammation and cardiovascular disease: From mechanisms to therapeutics. Am. J. Prev. Cardiol. 4:100130. doi: 10.1016/j.ajpc.2020.100130
Allam-Ndoul, B., Castonguay-Paradis, S., and Veilleux, A. (2020). Gut microbiota and intestinal trans-epithelial permeability. Int. J. Mol. Sci. 21:6402. doi: 10.3390/ijms21176402
Anbazhagan, A. N., Priyamvada, S., and Priyadarshini, M. (2017). Gut microbiota in vascular disease: Therapeutic target? Curr. Vasc. Pharmacol. 15, 291–295. doi: 10.2174/1570161115666170105095834
Bajinka, O., Kora, M., Sanyang, O., Ouedraogo, S. Y., Bah, M. G., and Jallow, L. (2025). Bio-ethical issues of research in Sub-Saharan Africa. Monash Bioeth. Rev. doi: 10.1007/s40592-025-00264-z [Epub ahead of print].
Bajinka, O., Simbilyabo, L., Tan, Y., Jabang, J., and Saleem, S. A. (2022). Lung-brain axis. Crit. Rev. Microbiol. 48, 257–269. doi: 10.1080/1040841X.2021.1960483
Bajinka, O., Tan, Y., Darboe, A., Ighaede-Edwards, I. G., and Abdelhalim, K. A. (2023). The gut microbiota pathway mechanisms of diabetes. AMB Exp. 13:16. doi: 10.1186/s13568-023-01520-3
Barrea, L., Annunziata, G., Muscogiuri, G., Laudisio, D., Di Somma, C., Maisto, M., et al. (2019). Trimethylamine N-oxide, Mediterranean diet, and nutrition in healthy, normal-weight adults: Also a matter of sex? Nutrition 62, 7–17. doi: 10.1016/j.nut.2018.11.015
Bu, Y., Peng, M., Tang, X., Xu, X., Wu, Y., Chen, A. F., et al. (2022). Protective effects of metformin in various cardiovascular diseases: Clinical evidence and AMPK-dependent mechanisms. J. Cell Mol. Med. 26, 4886–4903. doi: 10.1111/jcmm.17519
Cao, Z., Gao, T., Bajinka, O., Zhang, Y., and Yuan, X. (2025). Fecal microbiota transplantation-current perspective on human health. Front. Med. 12:1523870. doi: 10.3389/fmed.2025.1523870
Chassaing, B., Koren, O., Goodrich, J. K., Poole, A. C., Srinivasan, S., Ley, R. E., et al. (2015). Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 519, 92–96. doi: 10.1038/nature14232
Chavez-Alfaro, L., Tenorio Jimenez, C., Silveira-Sanguino, V., Noguera Gomez, M. J., Fernandez-Moreno, C., Rodriguez Cuesta, A. M., et al. (2025). Intervention design and adherence to mediterranean diet in the cardiovascular risk prevention with a mediterranean dietary pattern reduced in saturated fat (CADIMED) randomized trial. Nutr. Res. 136, 120–132. doi: 10.1016/j.nutres.2025.03.001
Chen, L., Ishigami, T., Doi, H., Arakawa, K., and Tamura, K. (2020). Gut microbiota and atherosclerosis: Role of B cell for atherosclerosis focusing on the gut-immune-B2 cell axis. J. Mol. Med. 98, 1235–1244. doi: 10.1007/s00109-020-01936-5
Chen, Y., Li, H., Ye, Z., Găman, M.-A., Tan, S. C., and Zhu, F. (2020). The effect of metformin on carotid intima-media thickness (CIMT): A systematic review and meta-analysis of randomized clinical trials. Eur. J. Pharmacol. 886:173458. doi: 10.1016/j.ejphar.2020.173458
Costabile, A., Buttarazzi, I., Kolida, S., Quercia, S., Baldini, J., Swann, J. R., et al. (2017). An in vivo assessment of the cholesterol-lowering efficacy of Lactobacillus plantarum ECGC 13110402 in normal to mildly hypercholesterolaemic adults. PLoS One 12:e0187964. doi: 10.1371/journal.pone.0187964
Dal Canto, E., Ceriello, A., Ryden, L., Ferrini, M., Hansen, T. B., Schnell, O., et al. (2019). Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur. J. Prev. Cardiol. 26, 25–32. doi: 10.1177/2047487319878371
Datta, S., Pasham, S., Inavolu, S., Boini, K. M., and Koka, S. (2024). Role of gut microbial metabolites in cardiovascular diseases-current insights and the road ahead. Int. J. Mol. Sci. 25:10208. doi: 10.3390/ijms251810208
Djekic, D., Shi, L., Brolin, H., Carlsson, F., Sarnqvist, C., Savolainen, O., et al. (2020). Effects of a vegetarian diet on cardiometabolic risk factors, gut microbiota, and plasma metabolome in subjects with ischemic heart disease: A randomized, crossover study. J. Am. Heart Assoc. 9:e016518. doi: 10.1161/JAHA.120.016518
Dovi, K. S., Bajinka, O., and Conteh, I. (2022). Evidence and possible mechanisms of probiotics in the management of type 1 diabetes mellitus. J. Diab. Metab. Disord. 21, 1081–1094. doi: 10.1007/s40200-022-01006-2
Duewell, P., Kono, H., Rayner, K. J., Sirois, C. M., Vladimer, G., Bauernfeind, F. G., et al. (2010). NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361. doi: 10.1038/nature08938
Evans, L. W., Athukorala, M., Martinez-Guryn, K., and Ferguson, B. S. (2020). The role of histone acetylation and the microbiome in phytochemical efficacy for cardiovascular diseases. Int. J. Mol. Sci. 21:4006. doi: 10.3390/ijms21114006
Fan, L., Chen, J., Zhang, Q., Ren, J., Chen, Y., Yang, J., et al. (2025). Fecal microbiota transplantation for hypertension: An exploratory, multicenter, randomized, blinded, placebo-controlled trial. Microbiome 13:133. doi: 10.1186/s40168-025-02118-6
Foger, B., Jennings, C., Pirillo, A., Tokgozoglu, L., Pirro, M., and Catapano, A. L. (2020). Hypercholesterolemia and cardiovascular disease: What to do before initiating pharmacological therapy. Atheroscler Suppl. 42, e25–e29. doi: 10.1016/j.atherosclerosissup.2021.01.005
Forkosh, E., and Ilan, Y. (2019). The heart-gut axis: New target for atherosclerosis and congestive heart failure therapy. Open Heart 6:e000993. doi: 10.1136/openhrt-2018-000993
Fuentes, M. C., Lajo, T., Carrion, J. M., and Cune, J. (2013). Cholesterol-lowering efficacy of Lactobacillus plantarum CECT 7527, 7528 and 7529 in hypercholesterolaemic adults. Br. J. Nutr. 109, 1866–1872. doi: 10.1017/S000711451200373X
Gao, C., Wei, J., Lu, C., Wang, L., Dong, D., and Sun, M. (2024). A new perspective in intestinal microecology: Lifting the veil of exercise regulation of cardiometabolic diseases. Gut Microbes 16:2404141. doi: 10.1080/19490976.2024.2404141
Gao, P., Rinott, E., Dong, D., Mei, Z., Wang, F., Liu, Y., et al. (2024). Gut microbial metabolism of bile acids modifies the effect of Mediterranean diet interventions on cardiometabolic risk in a randomized controlled trial. Gut Microbes 16:2426610. doi: 10.1080/19490976.2024.2426610
Gao, H., Song, R. J., Jiang, H., Zhang, W., and Han, S. F. (2022). Oat fiber supplementation alleviates intestinal inflammation and ameliorates intestinal mucosal barrier via acting on gut microbiota-derived metabolites in LDLR(-/-) mice. Nutrition 95:111558. doi: 10.1016/j.nut.2021.111558
Garcia-Gavilan, J. F., Atzeni, A., Babio, N., Liang, L., Belzer, C., Vioque, J., et al. (2024). Effect of 1-year lifestyle intervention with energy-reduced Mediterranean diet and physical activity promotion on the gut metabolome and microbiota: A randomized clinical trial. Am. J. Clin. Nutr. 119, 1143–1154. doi: 10.1016/j.ajcnut.2024.02.021
Gawlik-Kotelnicka, O., Rogalski, J., Czarnecka-Chrebelska, K. H., Burzynski, J., Jakubowska, P., Skowronska, A., et al. (2024). The interplay between depression, probiotics, diet, immunometabolic health, the gut, and the liver-a secondary analysis of the pro-demet randomized clinical trial. Nutrients 16:4024. doi: 10.3390/nu16234024
Guasti, L., Galliazzo, S., Molaro, M., Visconti, E., Pennella, B., Gaudio, G. V., et al. (2021). TMAO as a biomarker of cardiovascular events: A systematic review and meta-analysis. Intern. Emerg. Med. 16, 201–207. doi: 10.1007/s11739-020-02470-5
Hao, Y., Wang, Y., Xi, L., Li, G., Zhao, F., Qi, Y., et al. (2016). A nested case-control study of association between metabolome and hypertension risk. Biomed. Res. Int. 2016:7646979. doi: 10.1155/2016/7646979
Hasani, M., Pilerud, Z. A., Kami, A., Vaezi, A. A., Sobhani, S., Ejtahed, H. S., et al. (2024). Association between gut microbiota compositions with microvascularcomplications in individuals with diabetes: A systematic review. Curr. Diab. Rev. 20:e240124226068. doi: 10.2174/0115733998280396231212114345
Hill, E. B., Chen, L., Bailey, M. T., Singh Khalsa, A., Maltz, R., Kelleher, K., et al. (2022). Facilitating a high-quality dietary pattern induces shared microbial responses linking diet quality, blood pressure, and microbial sterol metabolism in caregiver-child dyads. Gut Microbes 14:2150502. doi: 10.1080/19490976.2022.2150502
Holman, R. R., Coleman, R. L., Chan, J. C. N., Chiasson, J. L., Feng, H., Ge, J., et al. (2017). Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): A randomised, double-blind, placebo-controlled trial. Lancet Diab. Endocrinol. 5, 877–886. doi: 10.1016/S2213-8587(17)30309-1
Hornero-Ramirez, H., Morisette, A., Marcotte, B., Penhoat, A., Lecomte, B., Panthu, B., et al. (2025). Multifunctional dietary approach reduces intestinal inflammation in relation with changes in gut microbiota composition in subjects at cardiometabolic risk: The SINFONI project. Gut Microbes 17:2438823. doi: 10.1080/19490976.2024.2438823
Hsu, B. B., Gibson, T. E., Yeliseyev, V., Liu, Q., Lyon, L., Bry, L., et al. (2019). Dynamic modulation of the gut microbiota and metabolome by bacteriophages in a mouse model. Cell Host Microbe 25, 803–814.e5. doi: 10.1016/j.chom.2019.05.001
Hu, X. F., Zhang, W. Y., Wen, Q., Chen, W. J., Wang, Z. M., Chen, J., et al. (2019). Fecal microbiota transplantation alleviates myocardial damage in myocarditis by restoring the microbiota composition. Pharmacol. Res. 139, 412–421. doi: 10.1016/j.phrs.2018.11.042
Jadhav, S., Ferrell, W., Greer, I. A., Petrie, J. R., Cobbe, S. M., and Sattar, N. (2006). Effects of metformin on microvascular function and exercise tolerance in women with angina and normal coronary arteries: A randomized, double-blind, placebo-controlled study. J. Am. Coll. Cardiol. 48, 956–963. doi: 10.1016/j.jacc.2006.04.088
Jia, B., Zou, Y., Han, X., Bae, J. W., and Jeon, C. O. (2023). Gut microbiome-mediated mechanisms for reducing cholesterol levels: Implications for ameliorating cardiovascular disease. Trends Microbiol. 31, 76–91. doi: 10.1016/j.tim.2022.08.003
Jia, Q., Mehmood, S., Liu, X., Ma, S., and Yang, R. (2020). Hydrogen sulfide mitigates myocardial inflammation by inhibiting nucleotide-binding oligomerization domain-like receptor protein 3 inflammasome activation in diabetic rats. Exp. Biol. Med. 245, 221–230. doi: 10.1177/1535370219899899
Jia, Q., Xie, Y., Lu, C., Zhang, A., Lu, Y., Lv, S., et al. (2019). Endocrine organs of cardiovascular diseases: Gut microbiota. J. Cell Mol. Med. 23, 2314–2323. doi: 10.1111/jcmm.14164
Jiang, Y., Pang, S., Liu, X., Wang, L., and Liu, Y. (2024). The gut microbiome affects atherosclerosis by regulating reverse cholesterol transport. J. Cardiovasc. Transl. Res. 17, 624–637. doi: 10.1007/s12265-024-10480-3
Kimber, C., Zhang, S., Johnson, C., West, R. E. III, Prokopienko, A. J., Mahnken, J. D., et al. (2020). Randomized, placebo-controlled trial of rifaximin therapy for lowering gut-derived cardiovascular toxins and inflammation in CKD. Kidney360 1, 1206–1216. doi: 10.34067/kid.0003942020
Kjeldsen, S. E. (2018). Hypertension and cardiovascular risk: General aspects. Pharmacol. Res. 129, 95–99. doi: 10.1016/j.phrs.2017.11.003
Konopelski, P., and Mogilnicka, I. (2022). Biological effects of Indole-3-Propionic acid, a gut microbiota-derived metabolite, and its precursor tryptophan in mammals’ health and disease. Int. J. Mol. Sci. 23:1222. doi: 10.3390/ijms23031222
Lahnwong, C., Chattipakorn, S. C., and Chattipakorn, N. (2018). Potential mechanisms responsible for cardioprotective effects of sodium-glucose co-transporter 2 inhibitors. Cardiovasc. Diabetol. 17:101. doi: 10.1186/s12933-018-0745-5
Li, K., Jiang, P., Li, S., Sun, J., and Qi, C. (2025). ACE inhibitory casein peptide lowers blood pressure and reshapes gut microbiota in a randomized double blind placebo controlled trial. Sci. Rep. 15:13840. doi: 10.1038/s41598-025-98446-6
Li-Hua, P., and Bajinka, O. (2025). Processed meat health risks: Pathways and dietary solutions. J. Nutr. doi: 10.1016/j.tjnut.2025.08.030 [Epub ahead of print].
Luo, F., Das, A., Chen, J., Wu, P., Li, X., and Fang, Z. (2019). Metformin in patients with and without diabetes: A paradigm shift in cardiovascular disease management. Cardiovasc. Diabetol. 18:54. doi: 10.1186/s12933-019-0860-y
Luqman, A., Hassan, A., Ullah, M., Naseem, S., Ullah, M., Zhang, L., et al. (2024). Role of the intestinal microbiome and its therapeutic intervention in cardiovascular disorder. Front. Immunol. 15:1321395. doi: 10.3389/fimmu.2024.1321395
Manrique, P., Zhu, Y., van der Oost, J., Herrema, H., Nieuwdorp, M., de Vos, W. M., et al. (2021). Gut bacteriophage dynamics during fecal microbial transplantation in subjects with metabolic syndrome. Gut Microbes 13, 1–15. doi: 10.1080/19490976.2021.1897217
Marras, L., Caputo, M., Bisicchia, S., Soato, M., Bertolino, G., Vaccaro, S., et al. (2021). The role of bifidobacteria in predictive and preventive medicine: A focus on eczema and hypercholesterolemia. Microorganisms 9:836. doi: 10.3390/microorganisms9040836
Martins, D., Silva, C., Ferreira, A. C., Dourado, S., Albuquerque, A., Saraiva, F., et al. (2024). Unravelling the gut microbiome role in cardiovascular disease: A systematic review and a meta-analysis. Biomolecules 14:731. doi: 10.3390/biom14060731
Massey, W., Osborn, L. J., Banerjee, R., Horak, A., Fung, K. K., Orabi, D., et al. (2022). Flavin-Containing monooxygenase 3 (FMO3) is critical for dioxin-induced reorganization of the gut microbiome and host insulin sensitivity. Metabolites 12:364. doi: 10.3390/metabo12040364
Miranda, P. M., De Palma, G., Serkis, V., Lu, J., Louis-Auguste, M. P., McCarville, J. L., et al. (2018). High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome 6:57. doi: 10.1186/s40168-018-0433-4
Montandon, S. A., and Jornayvaz, F. R. (2017). Effects of antidiabetic drugs on gut microbiota composition. Genes 8:250. doi: 10.3390/genes8100250
Morgan, C. L., Mukherjee, J., Jenkins-Jones, S., Holden, S. E., and Currie, C. J. (2014). Association between first-line monotherapy with sulphonylurea versus metformin and risk of all-cause mortality and cardiovascular events: A retrospective, observational study. Diab. Obes. Metab. 16, 957–962. doi: 10.1111/dom.12302
Ng, H. M., Maggo, J., Wall, C. L., Bayer, S. B., Mullaney, J. A., Cabrera, D., et al. (2025). Effects of defatted rice bran-fortified bread on gut microbiome, cardiovascular risk, gut discomfort, wellbeing and gut physiology in healthy adults with low dietary fibre intake. Clin. Nutr. Espen 67, 362–376. doi: 10.1016/j.clnesp.2025.03.045
Ning, F., Tuomilehto, J., Pyorala, K., Onat, A., Soderberg, S., Qiao, Q., et al. (2010). Cardiovascular disease mortality in Europeans in relation to fasting and 2-h plasma glucose levels within a normoglycemic range. Diab. Care 33, 2211–2216. doi: 10.2337/dc09-2328
Noguera-Navarro, C., Vinten, K. T., Aunon-Calles, D., Carazo-Diaz, C., Janssens, G. E., and Montoro-Garcia, S. (2025). Multi-omic analysis and platelet function distinguish treatment responses to hydroxytyrosol in cardiovascular risk. Food Funct. 16, 5928–5948. doi: 10.1039/d5fo00874c
Ostadmohammadi, V., Milajerdi, A., Ghayour-Mobarhan, M., Ferns, G., Taghizadeh, M., Badehnoosh, B., et al. (2019). The effects of Vitamin D supplementation on glycemic control, lipid profiles and C-Reactive protein among patients with cardiovascular disease: A systematic review and meta-analysis of randomized controlled trials. Curr. Pharm. Des. 25, 201–210. doi: 10.2174/1381612825666190308152943
Petelina, T. I., Avdeeva, K. S., Valeeva, L. L., Bykova, S. G., Zueva, E. V., and Dorodneva, E. F. (2025). [Influence of physical therapy on intestinal microbiota, blood pressure control and systemic inflammation level: A randomized controlled study of 84 patients with arterial hypertension]. Vopr Kurortol Fizioter Lech Fiz Kult 102, 11–20. doi: 10.17116/kurort202510202111
Pieczynska, M. D., Yang, Y., Petrykowski, S., Horbanczuk, O. K., Atanasov, A. G., and Horbanczuk, J. O. (2020). Gut microbiota and its metabolites in atherosclerosis development. Molecules 25:594. doi: 10.3390/molecules25030594
Pontes, K., Guedes, M. R., Souza, P. G., Neves, M. F. T., and Klein, M. (2025). Effects of multi-strain probiotics supplementation on body adiposity and metabolic profile in individuals with hypertension and overweight following an energy-restricted diet: A randomized clinical trial. Clin. Nutr. 50, 117–127. doi: 10.1016/j.clnu.2025.05.005
Powell-Wiley, T. M., Poirier, P., Burke, L. E., Despres, J. P., Gordon-Larsen, P., Lavie, C. J., et al. (2021). Obesity and cardiovascular disease: A scientific statement from the American heart association. Circulation 143, e984–e1010. doi: 10.1161/CIR.0000000000000973
Rahimi, A., Qaisar, S. A., Janeh, T., Karimpour, H., Darbandi, M., and Moludi, J. (2024). Clinical trial of the effects of postbiotic supplementation on inflammation, oxidative stress, and clinical outcomes in patients with CVA. Sci. Rep. 14:24021. doi: 10.1038/s41598-024-76153-y
Rajamaki, K., Lappalainen, J., Oorni, K., Valimaki, E., Matikainen, S., Kovanen, P. T., et al. (2010). Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: A novel link between cholesterol metabolism and inflammation. PLoS One 5:e11765. doi: 10.1371/journal.pone.0011765
Ranaivo, H., Thirion, F., Bera-Maillet, C., Guilly, S., Simon, C., Sothier, M., et al. (2022). Increasing the diversity of dietary fibers in a daily-consumed bread modifies gut microbiota and metabolic profile in subjects at cardiometabolic risk. Gut Microbes 14:2044722. doi: 10.1080/19490976.2022.2044722
Rastall, R. A., Diez-Municio, M., Forssten, S. D., Hamaker, B., Meynier, A., Moreno, F. J., et al. (2022). Structure and function of non-digestible carbohydrates in the gut microbiome. Benef. Microbes 13, 95–168. doi: 10.3920/BM2021.0090
Ronen, D., Rokach, Y., Abedat, S., Qadan, A., Daana, S., Amir, O., et al. (2024). Human gut microbiota in cardiovascular disease. Compr. Physiol. 14, 5449–5490. doi: 10.1002/cphy.c230012
San Diego, L., Hogue, T., Hampton-Marcell, J., Carroll, I. M., Purdom, T., Colleran, H., et al. (2025). Gut butyrate reduction in blood pressure is associated with other vegetables, whole fruit, total grains, and sodium intake. Nutrients 17:1392. doi: 10.3390/nu17081392
Schwartz, M., Gluck, M., and Koon, S. (2013). Norovirus gastroenteritis after fecal microbiota transplantation for treatment of Clostridium difficile infection despite asymptomatic donors and lack of sick contacts. Am. J. Gastroenterol. 108:1367. doi: 10.1038/ajg.2013.164
Shi, B., Li, H., and He, X. (2024). Advancing lifelong precision medicine for cardiovascular diseases through gut microbiota modulation. Gut Microbes 16:2323237. doi: 10.1080/19490976.2024.2323237
Singh, A., Kishore, P. S., and Khan, S. (2024). From microbes to myocardium: A comprehensive review of the impact of the gut-brain axis on cardiovascular disease. Cureus 16:e70877. doi: 10.7759/cureus.70877
Skye, S. M., Zhu, W., Romano, K. A., Guo, C. J., Wang, Z., Jia, X., et al. (2018). Microbial transplantation with human gut commensals containing CutC is sufficient to transmit enhanced platelet reactivity and thrombosis potential. Circ. Res. 123, 1164–1176. doi: 10.1161/CIRCRESAHA.118.313142
Spasova, N., Somleva, D., Krastev, B., Tropcheva, R., Svinarov, D., Kundurzhiev, T., et al. (2024). Effect of Lactobacillus plantarum supplementation on trimethylamine-N-oxide levels in 30 patients with atherosclerotic cardiovascular disease: A double-blind randomized controlled trial. Folia Med. 66, 682–691. doi: 10.3897/folmed.66.e132325
Tada, H., Fujino, N., Hayashi, K., Kawashiri, M.-A., and Takamura, M. (2022). Human genetics and its impact on cardiovascular disease. J. Cardiol. 79, 233–239. doi: 10.1016/j.jjcc.2021.09.005
Tsutsumi, R., Yamasaki, Y., Takeo, J., Miyahara, H., Sebe, M., Bando, M., et al. (2021). Long-chain monounsaturated fatty acids improve endothelial function with altering microbial flora. Transl. Res. 237, 16–30. doi: 10.1016/j.trsl.2021.03.016
Union Caballero, A., Merono, T., Aberg, S., Nordin, E., Dicksved, J., Sanchez-Pla, A., et al. (2025). Metabolite biomarkers linking a high-fiber rye intervention with cardiometabolic risk factors: The ryeweight study. J. Agric. Food Chem. 73, 21869–21879. doi: 10.1021/acs.jafc.5c01415
Usman, I., Anwar, A., Shukla, S., and Pathak, P. (2024). Mechanistic review on the role of gut microbiota in the pathology of cardiovascular diseases. Cardiovasc. Hematol. Disord. Drug Targets 24, 13–39. doi: 10.2174/011871529X310857240607103028
van Mourik, D. J. M., Salet, D. M., Middeldorp, S., Nieuwdorp, M., and van Mens, T. E. (2022). The role of the intestinal microbiome in antiphospholipid syndrome. Front. Immunol. 13:954764. doi: 10.3389/fimmu.2022.954764
Verhaar, B. J. H., Prodan, A., Nieuwdorp, M., and Muller, M. (2020). Gut microbiota in hypertension and atherosclerosis: A review. Nutrients 12:2982. doi: 10.3390/nu12102982
Verhaar, B. J. H., Wijdeveld, M., Wortelboer, K., Rampanelli, E., Levels, J. H. M., Collard, D., et al. (2024). Effects of oral butyrate on blood pressure in patients with hypertension: A randomized, placebo-controlled trial. Hypertension 81, 2124–2136. doi: 10.1161/HYPERTENSIONAHA.123.22437
Vijay, A., Astbury, S., Le Roy, C., Spector, T. D., and Valdes, A. M. (2021). The prebiotic effects of omega-3 fatty acid supplementation: A six-week randomised intervention trial. Gut Microbes 13, 1–11. doi: 10.1080/19490976.2020.1863133
Viollet, B., Guigas, B., Sanz Garcia, N., Leclerc, J., Foretz, M., and Andreelli, F. (2012). Cellular and molecular mechanisms of metformin: An overview. Clin. Sci. 122, 253–270. doi: 10.1042/CS20110386
Vourakis, M., Mayer, G., and Rousseau, G. (2021). The role of gut microbiota on cholesterol metabolism in atherosclerosis. Int. J. Mol. Sci. 22:8074. doi: 10.3390/ijms22158074
Wang, S., Ren, H., Zhong, H., Zhao, X., Li, C., Ma, J., et al. (2022). Combined berberine and probiotic treatment as an effective regimen for improving postprandial hyperlipidemia in type 2 diabetes patients: A double blinded placebo controlled randomized study. Gut Microbes 14:2003176. doi: 10.1080/19490976.2021.2003176
Wang, Y., Cross, T. L., Lindemann, S. R., Tang, M., and Campbell, W. W. (2024). Healthy dietary pattern cycling affects gut microbiota and cardiovascular disease risk factors: Results from a randomized controlled feeding trial with young, healthy adults. Nutrients 16:3619. doi: 10.3390/nu16213619
Wei, M., Huang, Q., Liu, Z., Luo, Y., and Xia, J. (2021). Intestinal barrier dysfunction participates in the pathophysiology of ischemic stroke. CNS Neurol. Disord. Drug Targets 20, 401–416. doi: 10.2174/1871527320666210322115808
Wu, Q., Sun, L., Hu, X., Wang, X., Xu, F., Chen, B., et al. (2021). Suppressing the intestinal farnesoid X receptor/sphingomyelin phosphodiesterase 3 axis decreases atherosclerosis. J. Clin. Invest. 131:e142865. doi: 10.1172/JCI142865
Xin, M., Xu, A., Tian, J., Wang, L., He, Y., Jiang, H., et al. (2024). Anthocyanins as natural bioactives with anti-hypertensive and atherosclerotic potential: Health benefits and recent advances. Phytomedicine 132:155889. doi: 10.1016/j.phymed.2024.155889
Xu, J., and Yang, Y. (2021). Gut microbiome and its meta-omics perspectives: Profound implications for cardiovascular diseases. Gut Microbes 13:1936379. doi: 10.1080/19490976.2021.1936379
Yan, D., Sun, Y., Zhou, X., Si, W., Liu, J., Li, M., et al. (2022). Regulatory effect of gut microbes on blood pressure. Anim. Model Exp. Med. 5, 513–531. doi: 10.1002/ame2.12233
Yang, L., Bajinka, O., Jarju, P. O., Tan, Y., Taal, A. M., and Ozdemir, G. (2021). The varying effects of antibiotics on gut microbiota. AMB Exp. 11:116. doi: 10.1186/s13568-021-01274-w
Yang, X., Zhang, X., Yang, W., Yu, H., He, Q., Xu, H., et al. (2021). Gut microbiota in adipose tissue dysfunction induced cardiovascular disease: Role as a metabolic organ. Front. Endocrinol. 12:749125. doi: 10.3389/fendo.2021.749125
Yang, W., and Cong, Y. (2021). Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell Mol. Immunol. 18, 866–877. doi: 10.1038/s41423-021-00661-4
Yang, X., Liu, R., An, Z., Li, B., Lin, Y., Li, Y., et al. (2025). Probiotic mitigates gut hypoperfusion-associated acute gastrointestinal injury in patients undergoing cardiopulmonary bypass: A randomized controlled trial. BMC Med. 23:238. doi: 10.1186/s12916-025-04082-2
Yang, Z., Wang, Q., Liu, Y., Wang, L., Ge, Z., Li, Z., et al. (2023). Gut microbiota and hypertension: Association, mechanisms and treatment. Clin. Exp. Hypertens. 45:2195135. doi: 10.1080/10641963.2023.2195135
Yang, Z. H., Bando, M., Sakurai, T., Chen, Y., Emma-Okon, B., Wilhite, B., et al. (2016). Long-chain monounsaturated fatty acid-rich fish oil attenuates the development of atherosclerosis in mouse models. Mol. Nutr. Food Res. 60, 2208–2218. doi: 10.1002/mnfr.201600142
Zeng, Q., Zhou, Q., Liu, W., Wang, Y., Xu, X., and Xu, D. (2021). Mechanisms and perspectives of sodium-glucose co-transporter 2 inhibitors in heart failure. Front. Cardiovasc. Med. 8:636152. doi: 10.3389/fcvm.2021.636152
Zhang, Q., Zhang, L., Chen, C., Li, P., and Lu, B. (2023). The gut microbiota-artery axis: A bridge between dietary lipids and atherosclerosis? Prog. Lipid. Res. 89:101209. doi: 10.1016/j.plipres.2022.101209
Zhang, X. N., Yu, Z. L., Chen, J. Y., Li, X. Y., Wang, Z. P., Wu, M., et al. (2022). The crosstalk between NLRP3 inflammasome and gut microbiome in atherosclerosis. Pharmacol. Res. 181:106289. doi: 10.1016/j.phrs.2022.106289
Zhang, Y., Wang, Y., Ke, B., and Du, J. (2021). TMAO: How gut microbiota contributes to heart failure. Trans. Res. 228, 109–125. doi: 10.1016/j.trsl.2020.08.007
Zhao, Y., and Wang, Z. (2020). Gut microbiome and cardiovascular disease. Curr. Opin. Cardiol. 35, 207–218. doi: 10.1097/HCO.0000000000000720
Zhong, Y., Kang, X., Bai, X., Pu, B., Smerin, D., Zhao, L., et al. (2024). The oral-gut-brain axis: The influence of microbes as a link of periodontitis with ischemic stroke. CNS Neurosci. Ther. 30:e70152. doi: 10.1111/cns.70152
Keywords: gut microbiota, metabolites, cardiovascular disease, biological pathways, therapeutic strategies
Citation: Zhang S, Li J, Li L and Yuan X (2025) Gut microbiota on cardiovascular diseases-a mini review on current evidence. Front. Microbiol. 16:1690411. doi: 10.3389/fmicb.2025.1690411
Received: 21 August 2025; Accepted: 20 October 2025;
Published: 06 November 2025.
Edited by:
Shanshan Hu, Anhui Agricultural University, ChinaReviewed by:
Sylwia Dziegielewska-Gesiak, Medical University of Silesia, PolandCopyright © 2025 Zhang, Li, Li and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liping Li, bGlsaXBpbmcyMDI1QDEyNi5jb20=; Xingxing Yuan, eXVhbnhpbmd4aW5nQGhsanVjbS5lZHUuY24=
 Shouhong Zhang1
Shouhong Zhang1 Xingxing Yuan
Xingxing Yuan