- 1National Institutes for Food and Drug Control, Beijing, China
- 2Liaoning Institute for Drug Control, Shenyang, China
- 3Henan Institute for Drug and Medical Device Inspection, Zhengzhou, China
- 4Hebei Institute for Drug and Medical Device Control, Shijiazhuang, China
- 5Shandong Institute for Food and Drug Control, Jinan, China
- 6Zibo Institute for Food and Drug Control, Zibo, China
A Correction on
A new selective culture medium for isolation of Burkholderia cepacia complex in pharmaceutical industry
by Yu, M., Wang, S., Zhong, Y., Yuan, L., An, L., Feng, D., Liu, Z., and Ma, S. (2025). Front. Microbiol. 16:1631983. doi: 10.3389/fmicb.2025.1631983
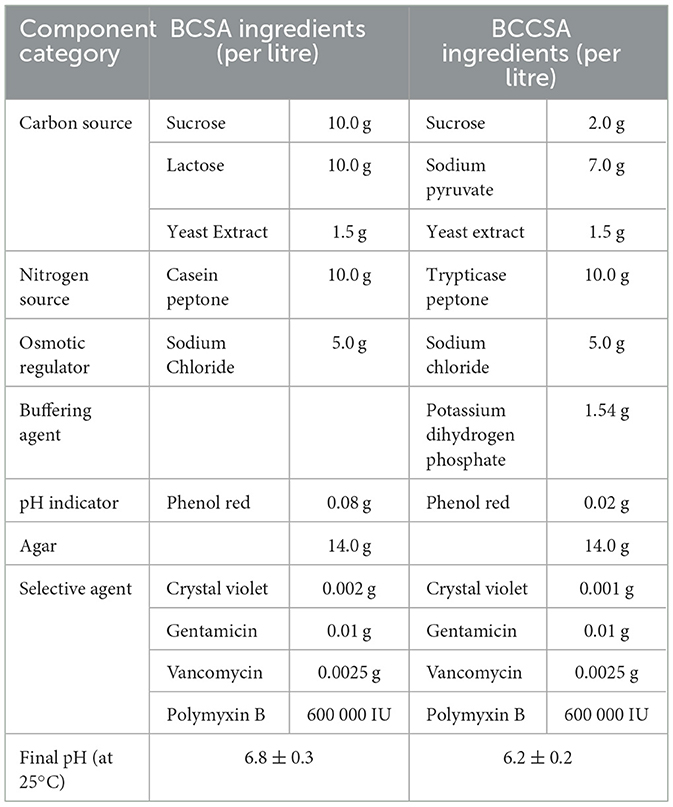
There was a mistake in Table 1 as published. The dosage of vancomycin was mistakenly written as 0.025 g. The correct dosage is 0.0025 g. The corrected Table 1 appears below.
A correction has also been made to the section 2 “Materials and Methods”, sub-section 2.1 “Culture Media”, Paragraph 1: “The components of BCSA and BCCSA are shown in Table 1. BCSA (product ref:11317) and supplement (product ref: 11317a) was obtained from Beijing SanYao Science & Technology Co., Ltd, Beijing, China, and prepared in accordance with manufacturer's instructions. The composition (per liter of distilled water) of BCCSA is as follows: sucrose 2.0 g, sodium pyruvate 7.0 g, trypticase peptone 10.0 g, NaCl 5.0 g, yeast extract 1.5 g, KH2PO4 1.54 g, phenol red 0.02 g, crystal violet 0.001 g, agar 14.0 g, polymyxin B sulphate 600 000 IU, gentamicin 0.01 g, vancomycin 0.0025 g. The phenol red and crystal violet were prepared as 0.2% and 0.01% aqueous solutions, respectively, and 10 ml of each was added per liter. After autoclaving for 15 min at 121 °C, antibiotics were added when the medium is cooled to around 50 °C. Soybean-Casein Digest Agar (TSA, ref: 236950), Soybean-Casein Digest Broth (TSB, ref: 211825) and Sabouraud Dextrose Broth (SDB, ref: 238230) was obtained from Becton-Dickinson, Rungis, France.”
The original version of this article has been updated.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: growth-promoting, selectivity, indicative property, Burkholderia cepacia complex, objectionable microorganisms, selective medium
Citation: Yu M, Wang S, Zhong Y, Yuan L, An L, Feng D, Liu Z and Ma S (2025) Correction: A new selective culture medium for isolation of Burkholderia cepacia complex in pharmaceutical industry. Front. Microbiol. 16:1695214. doi: 10.3389/fmicb.2025.1695214
Received: 29 August 2025; Accepted: 15 September 2025;
Published: 08 October 2025.
Edited and reviewed by: Eric Altermann, Massey University, School of Veterinary Science, New Zealand
Copyright © 2025 Yu, Wang, Zhong, Yuan, An, Feng, Liu and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shihong Ma, bWFzaEBuaWZkYy5vcmcuY24=
 Meng Yu
Meng Yu Sijin Wang1
Sijin Wang1