- 1Ministry of Ecology and Environment Peoples Republic of China, Nanjing Institute of Environmental Science, Nanjing, China
- 2Anhui Province Key Lab of Farmland Ecological Conservation and Pollution Prevention, Anhui Province Engineering and Technology Research Center of Intelligent Manufacture and Efficient Utilization of Green Phosphorus Fertilizer, College of Resources and Environment, Anhui Agricultural University, Hefei, China
- 3Key Laboratory of Jianghuai Arable Land Resources Protection and Eco-restoration, Ministry of Natural Resources, College of Resources and Environment, Anhui Agricultural University, Hefei, China
Phosphate-solubilizing fungi (PSF) are commonly employed in the bioremediation of lead contamination through the production of organic acids. However, the secretion of organic acids by PSF is typically affected by various environmental factors. This study investigated the Pb removal process by typical PSF A. niger and P. chrysogenum under different Mn2+ concentrations (0–30 mg/L). The different concentrations of Mn2+ can significantly influence the Pb toxicity tolerance of PSF. PSF A. niger exhibits a stable Pb removal ratio of >99% under different Mn2+ concentrations, much higher than P. chrysogenum <90%. The high concentrations of Mn2+ (15 and 30 mg/L) both inhibited the secretion of organic acids by A. niger and P. chrysogenum. However, 7.5 mg/L Mn2+ can significantly increase the secretion of oxalic acid by A. niger and promote the formation of lead oxalate and pyromorphite. Only 2.25% Pb2+ is released again from the immobilized Pb minerals. Meanwhile, PSF has the highest pyruvate dehydrogenase (PDH) enzyme activities of 31.53 and 17.23 nmol/min/g in 7.5 mg/L Mn2+ treatment. Compared with P. chrysogenum, A. niger is more effective in removing and stabilizing Pb cations. Controlling the appropriate Mn2+ concentration can further improve the Pb bioremediation by PSF.
1 Introduction
Lead (Pb) is widely recognized as a hazardous heavy metal, primarily due to the increased human activities such as mining, battery manufacturing, and paint utilization (Naik et al., 2013). The world’s most severe pollution issues are directly attributed to Pb (Raj and Das, 2023). In recent decades, more than 783,000 tons of Pb contaminants have been produced annually worldwide (Singh et al., 2003). As a persistent environmental contaminant, Pb in the environment has a long-term persistence and can cause high toxicity for organisms (Ozdemir et al., 2004; Naik et al., 2013; Zeng et al., 2017). Therefore, reducing the toxicity of Pb contamination in the environment is necessary and requires great attention in the future.
Bioremediation is an efficient pathway in reducing Pb contamination toxicity in the environment (Liang and Gadd, 2017). Compared with physical and chemical pathway, microbial remediation is more economical and environmentally friendly (Meng et al., 2024). Microorganisms can produce metabolites such as organic acids and extracellular polymeric substances (EPS) to immobilize Pb cantions (Priyadarshanee and Das, 2024). Additionally, the structure of cell waslls in microorganisms can also adsorb Pb cantions (Wang et al., 2019). For example, the fungal of Aspergillus niger (A. niger) can reduce more than 90% Pb cantions via the organic acid secretion and biosorption (Tian et al., 2019; Jalili et al., 2020). Meanwhile, the secretion of EPS from A. niger can also reduce Pb cantions concentration from 1,000 mg/L to 52.4 mg/L via the formation of EPS-Pb (Chen et al., 2018). Therefore, the microbial remediation of Pb exhibits considerable potential and merits further investigation.
Phosphate-solubilizing fungi (PSF) have been widely applied in Pb remediation. On the one hand, PSF exhibit a higher secretion of organic acids (e.g., oxalic acid, malic acid, citric acid, etc.) than other microorganisms (Coutinho et al., 2012; Sturm et al., 2015; Chowdhury et al., 2017). On the other hand, PSF typically has a a higher tolerance to Pb roxicity, which can maintaining organic acid secretion even at more than 1,000 mg/L Pb concentration (Huang et al., 2023). These organic acids can react or chelate with Pb cantions to form insoluble Pb minerals (e.g., lead oxalate), hence reducing the Pb concentration (Rhee et al., 2012). More importantly, these secreted organic acids can also dissolve insoluble phosphate (e.g., fluorapatite, FAp) and release phosphorus (P) (Echeverria et al., 2024). The released P can also react with Pb to form highly insoluble Pb minerals of pyromorphite (Pb10(PO4)6F2) (Park and Bolan, 2013). Hence, the PSF of Aspergillus niger is typically regarded as the leading candidate in Pb remediation, especially combined with FAp (Qiu et al., 2021).
Oxalic acid primarily facilitates the bioremediation of Pb by PSF via the formation of insoluble Pb mineral crystals, both around cell walls and in the external medium (Rhee et al., 2012; Osorio and Habte, 2013). Meanwhile, oxalic acid can also effectively promote the release of P from insoluble phosphate (Kpomblekou-A and Tabatabai, 2003). On the one hand, oxalic acid has the highest acidity constant (pKa1 = 1.25 and pKa2 = 4.27) compared with other organic acids such as citric acid (Palmieri et al., 2019). On the other hand, oxalic acid also has a higher chelating ability with metal cations (e.g., Pb2+, Ca2+, and Mg2+) due to its conjugated structure (Gadd, 1999). Therefore, promoting the secretion of oxalic acid is an effective strategy to enhance the Pb remediation by PSF.
Metal cation is essential to achieve the high secretion of organic acid by PSF (Li et al., 2017; Walaszczyk et al., 2018; Huang et al., 2023). However, the different metal cations concentration would affect the secretion of organic acid by PSF. Supply of divalent cations (e.g., Ca2+, Cu2+, etc.) can favor the secretion of organic acid by PSF, but influenced by the growth-limiting concentration (Palmieri et al., 2019). As a co-factor, the supply of Mn2+ can change the secretion of organic acid via the tricarboxylic acid (TCA) cycle, especially for oxalic acid (Ruijter et al., 1999). Oxalic acid is the primary pathway in Pb remediation by PSF. Therefore, the concentration of Mn2+ could also significantly affect the Pb remediation by PSF theoretically. However, there is insufficient research to confirm the importance and role of Mn2+ in Pb remediation by PSF.
This study aimed to investigate the effect of Mn2+ on Pb remediation by the PSF Aspergillus niger and Penicillium chrysogenum under the addition of fluorapatite (FAp). The capacity of P release from FAp between these two fungi was also investigated. The pH in the medium was measured by a pH meter. The oxalic acid concentration was measured by high-performance liquid chromatography (HPLC). The Pb and P concentrations in the solution were measured by inductively coupled plasma-optical emission spectrometry (ICP-OES). The resulting minerals were characterized by X-ray diffraction (XRD). The Pb in the mycelium was extracted by Toxicity Characteristic Leaching Procedure (TCLP).
2 Materials and methods
2.1 Fungal strains preparation
Aspergillus niger (A. niger) (CGMCC No. 23272) and Penicillium chrysogenum (P. chrysogenum) (CGMCC No. 23271) were isolated from the maize rhizosphere soil located in Suzhou, China (33°41′N, 117°5′E) (Wang et al., 2023; Feng et al., 2025). The Potato Dextrose Agar (PDA) medium was used to collect the spores for these two fungi. After 5 days of incubation at 28 °C, the formed fungal spores on the medium were drenched with sterile water using a fine artist’s brush. Subsequently, the mixture was filtered through a triple-layer sterile cheesecloth to remove mycelial fragments. Finally, a 0.85% sterile saline dilution was used and adjusted to 107 cfu/mL using a hemocytometer.
2.2 Pb remediation by A. niger and P. chrysogenum under different Mn2+ conditions
The Pb(NO3)2 powder (Xilong Scientific Ltd.) was used as the Pb contamination in solution. The initial Pb concentration in the medium was 1,000 mg/L. MnCl2·4H2O (Macklin Inc.) was used as the source of Mn2+. For A. niger (ANG) and P. chrysogenum (PCH), five Mn2+ treatments were performed, i.e., 0 mg/L Mn2+, 3.75 mg/L Mn2+, 7.5 mg/L Mn2+, 15 mg/L Mn2+, and 30 mg/L Mn2+. Prior to the incubation process, 0.16 g of Pb(NO₃)₂ powder and 0.5 g of fluorapatite (FAp) were individually introduced into 150 mL Erlenmeyer flasks containing 100 mL of PDB medium. Then, 1 mL of A. niger and P. chrysogenum suspensions was added to each treatment, respectively. These flasks were incubated at 180 rpm and 28 °C under sterile conditions with the Parafilm (BS-QM-003, Biosharp) seals. After a 7-day incubation period, the PDB medium was collected and filtered through a 0.45 μm polyethersulfone (PES) membrane. The filtrates were collected for organic acids, pH, P concentration, and Pb content analysis. The centrifugal precipitates were collected to detect the enzyme activity. Meanwhile, the precipitates were also dried at 55 °C for 24 h to determine the dry biomass, XRD, and SEM analysis.
2.3 TPLC-Pb extracted from mycelium
The available Pb concentration leached from immobilized Pb minerals was tested by the TCLP method. The formed preceptaties with mycelium were mixed with the extraction solution (1:20) and shaken at 180 rpm for 18 ± 2 h at room temperature. The mixture was centrifuged, and the Pb concentration in the supernatant was measured by ICP-OES (Feng et al., 2025).
2.4 Enzyme activity assay
Using Pyruvate dehydrogenase (PDH) and citrate synthase (CS) activity assay kits (Comin Biotechnology Co., Ltd., Suzhou, China) to determine the enzyme activities from filtered fungal mycelium. Exhaustive methods refer to previous research (Tian et al., 2021).
2.5 Instrumentation
Using a pH meter (FE20, Mettler Toledo, Columbus, OH, United States) to determine the medium pH value. The concentration of P and Pb was analyzed by ICP-OES (PerkinElmer Avio 200, United States). Before the test, the filtrate was diluted 10 times. Calibration curves were prepared at concentrations of 1, 5, 10, 20, 50, and 100 mg/L using P and Pb standards (Feng et al., 2025).
The secretion of oxalic acid was determined by HPLC (Agilent 1200, Agilent Technologies, Santa Clara, CA, United States). The mobile phase included 2.5 wt‰ potassium dihydrogen phosphate (KH2PO4) and methanol (CH3OH) in a ratio of 99:1. The HPLC column temperature was 30 °C. Phosphoric acid was used to adjust the pH of KH2PO4 to 2.8 at a rate of 1 mL/min. Standard solutions of oxalic acid were diluted to concentrations of 1,500, 1,000, 500, 200, 100, 50, and 0 mg/L (Su et al., 2019).
The XRD analysis of precipitates was performed by D/Max-2500 X-ray diffraction (Rigaku Corporation, Tokyo, Japan, Cu-K; 36 kV; 20 mA; scanning from 5 to 60 at a speed of 4/s). Prior to XRD analysis, the filtered dry precipitates were ground in a planetary ball mill (Mitr YXQM, Changsha Mitrcn Instrument Equipment Co., Ltd., Changsha, China). The ground material was then passed through a 100-mesh sieve. Finally, using the MDI Jade 6.5 software to detect the results of XRD precipitation for phase identification.
2.6 Statistical analysis
Each experiment was replicated three times. The mean and standard deviation of each treatment were calculated and reported. Tukey’s honestly significant difference test (p < 0.05) was used to identify the significant differences among the treatments by one-way ANOVA. Before conducting one-way ANOVA, the data were tested for homogeneity of variance and normality using the Shapiro–Wilk and Levene tests. The data were analyzed statistically with SPSS 26.0 software.
3 Results
3.1 Fungal dry biomass and pH value in medium
After 7 days of incubation, the fungal dry biomass in 0 and 3.75 mg/L Mn2+ treatments was 1.07 and 1.12 g (Figure 1A). In 7.5 mg/L Mn2+ treatment, the fungal dry biomass significantly increased to 1.26 g (Figure 1A). In 15 and 30 mg/L Mn2+ treatments, the fungal dry biomass decreased to 1.08 g and 1.10 g, respectively, (Figure 1A). For P. chrysogenum, the fungal dry biomass showed a lower value compared with A. niger, i.e., 0.80, 0.85, 0.78, 0.77, and 0.76 g in each Mn2+ concentration conditions after 7 days of incubation (Figure 1A).
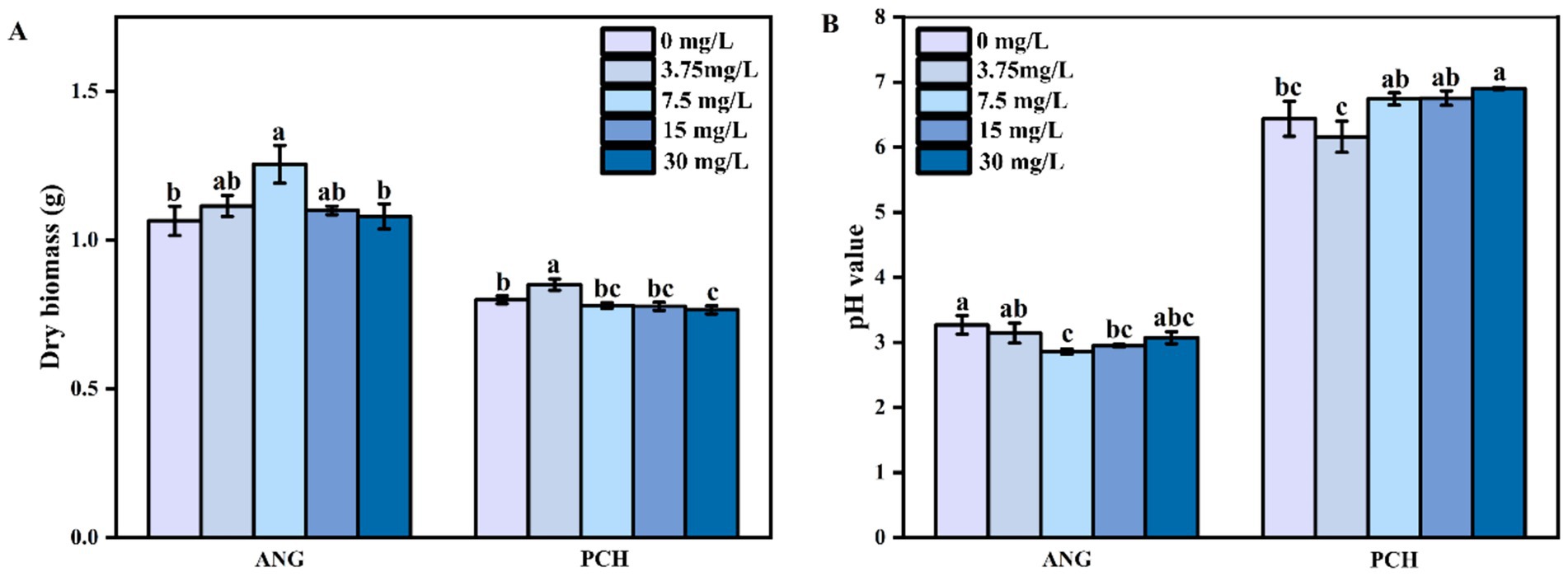
Figure 1. Dry biomass (A) and pH value (B) of Aspergillus niger and Penicillium chrysogenum in different Mn2+ conditions after 7 days of incubation. Standard deviations are shown with N = 3. The significant differences across treatments were carried out using Tukey’s honest significant difference test (p < 0.05) after one-way ANOVA. ANG, Aspergillus niger; PCH, Penicillium chrysogenum.
The initial medium pH value is 6.5. After 7 days of incubation, the medium pH value in A. niger under 0, 3.75, 7.5, 15, and 30 mg/L Mn2+ treatments decreased to 3.27, 3.14, 2.86, 2.95, and 3.07, respectively (Figure 1B). For P. chrysogenum, the medium pH value showed a higher pH value compared to A. niger, i.e., ranged from 6.16 to 6.90 under different Mn2+ concentration, suggesting the lower secretion of organic acids from P. chrysogenum (Figure 1B).
3.2 Secretion of oxalic acid and citric acid by A. niger and P. chrysogenum
In 0 mg/L Mn2+ treatment, the concentration of oxalic acid secreted by A. niger was 436.87 mg/L (Figure 2A). Meanwhile, the oxalic acid concentration gradually increased to 807.01 mg/L in 3.75 mg/L Mn2+ treatment and reached to the highest value of 1310.16 mg/L in 7.5 mg/L Mn2+ treatment (Figure 2A). In 15 and 30 mg/L Mn2+ treatments, the secretion of oxalic acid remained the downward tendency (Figure 2A). For P. chrysogenum, the oxalic acid concentration was 144.12 mg/L in 0 mg/L Mn2+ treatment (Figure 2A). In 3.75, 7.5, 15, and 30 mg/L Mn2+ treatments, the oxalic acid secreted by P. chrysogenum was also shown a low value, ranging from 11.03 to 68.79 mg/L (Figure 2A).
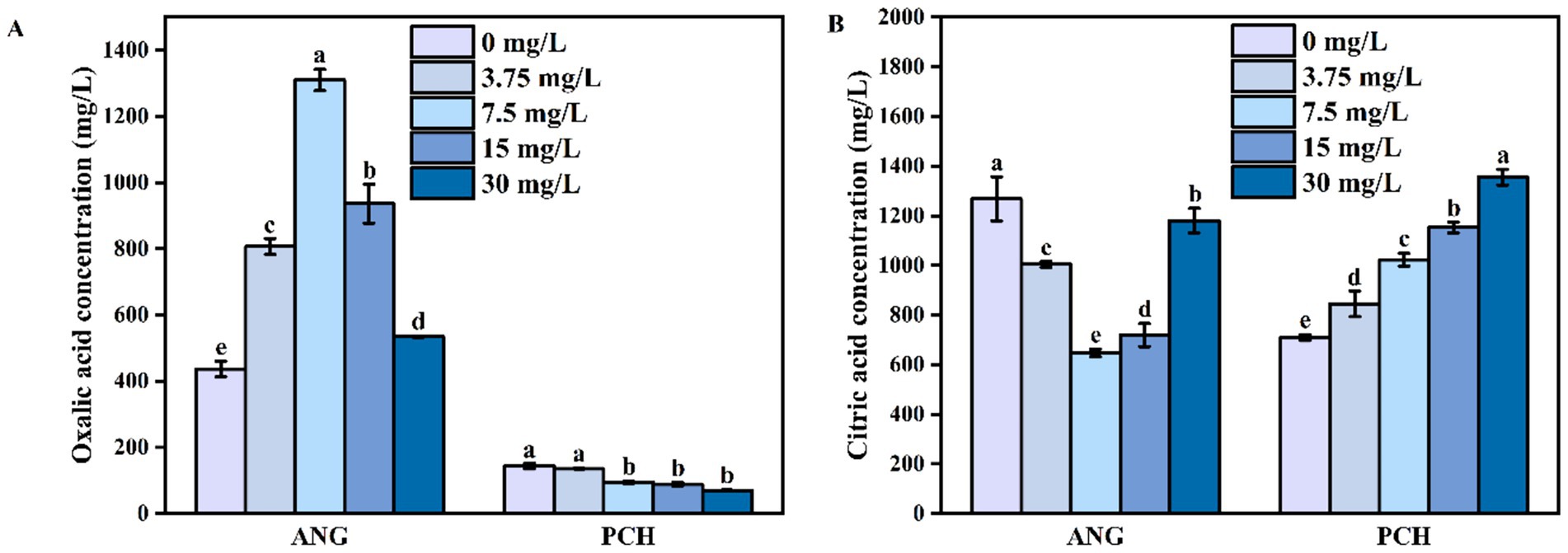
Figure 2. Oxalic acid (A) and citric acid content (B) Aspergillus niger and Penicillium chrysogenum in different Mn2+ conditions after 7 days of incubation. Standard deviations are shown with N = 3. The significant differences across treatments were carried out using Tukey’s honest significant difference test (p < 0.05) after one-way ANOVA. ANG, Aspergillus niger; PCH, Penicillium chrysogenum.
Unlike oxalic acid, the citric acid secreted by A. niger in 7.5 mg/L Mn2+ treatment had the lowest value of 647.05 mg/L (Figure 2B). In 0 mg/L Mn2+ treatment, the citric acid concentration had the highest value of 1,268 mg/L (Figure 2B). Meanwhile, citric acid gradually decreased to 1004.40 mg/L in 3.75 mg/L Mn2+ treatment (Figure 2B). In addition, the concentrations of citric acid remained the upward tendency in 15 and 30 mg/L Mn2+ treatment (Figure 2B). For P. chrysogenum, the citric acid concentration was 709.52 mg/L without Mn2+ after 7 days of incubation. With the Mn2+ concentration increased to 3.75, 7.5, 15, and 30 mg/L, the secretion of citric acid by P. chrysogenum increased to 845.38, 1022.75, 1152.82, and 1355.10 mg/L, respectively (Figure 2B).
3.3 P concentration in the medium
The P concentration in A. niger medium without Mn2+ addition (0 mg/L) was 421.53 mg/L (Figure 3A). In 3.75 and 7.5 mg/L Mn2+ treatments, the content of P increased to 469.95 and 563.27 mg/L, respectively (Figure 3A). In 15 and 30 mg/L Mn2+ treatments, the P concentration decreased to 483.79 and 436.14 mg/L, respectively (Figure 3A). For P. chrysogenum, the P was hardly released from FAp. The highest P content was 13.38 mg/L in 3.75 mg/ L Mn2+ treatment (Figure 3A). In other Mn2+ treatments, the P content ranged from 6.08 to 6.56 mg/L (Figure 3A). Compared with P. chrysogenum, A. niger is more efficient in the release of P from FAp, i.e., more than 40% P release rate by A. niger vs. less than 5% P release rate by P. chrysogenum (Figure 3B).
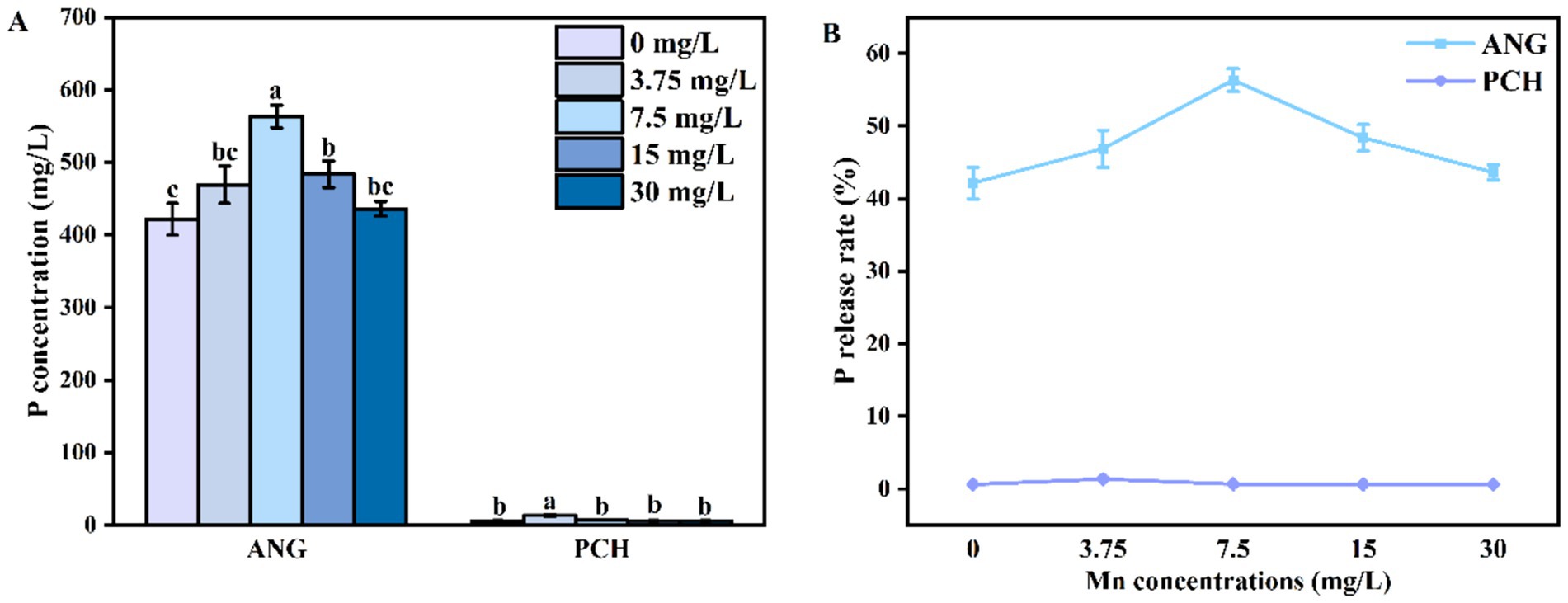
Figure 3. P concentration (A) and P release rate (B) of Aspergillus niger and Penicillium chrysogenum in different Mn2+ conditions after 7 days of incubation. Standard deviations are shown with N = 3. The significant differences across treatments were carried out using Tukey’s honest significant difference test (p < 0.05) after one-way ANOVA. ANG, Aspergillus niger; PCH, Penicillium chrysogenum.
3.4 Pb remediation by A. niger and P. chrysogenum under different Mn2+ conditions
After 7 days of incubation, the Pb concentration in each Mn2+ treatments was significantly decreased from 1,000 mg/L to 8.00, 6.59, 4.94, 5.80, and 6.40 mg/L in A. niger, respectively (Figure 4A). However, the Pb contents in different Mn2+ treatments with P. chrysogenum had about 10 times than A. niger (Figure 4A). The Pb concentration in P. chrysogenum under different Mn2+ treatment ranged from 93.31 to 168.17 mg/L after 7 days of incubation (Figure 4A).
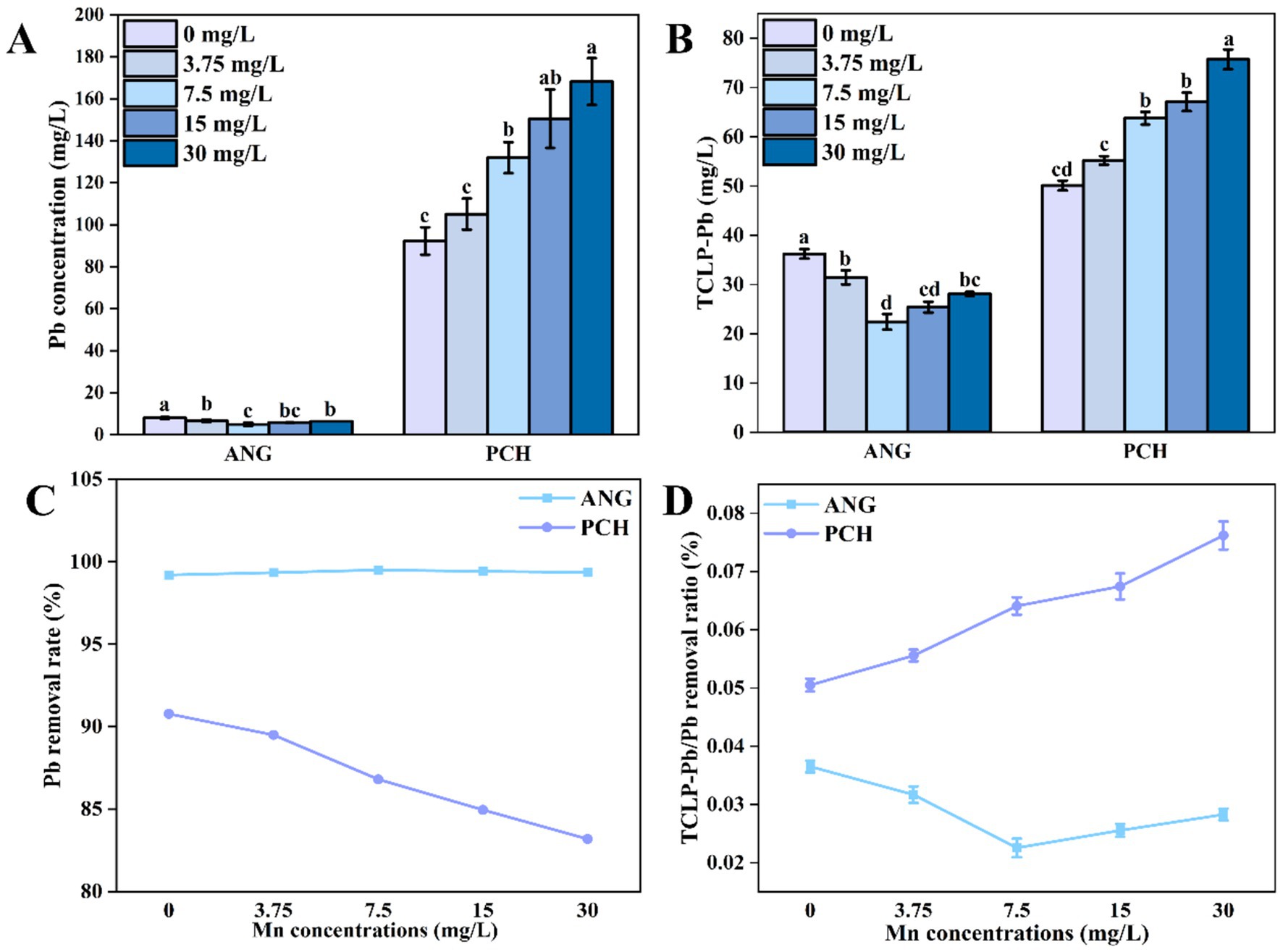
Figure 4. Pb2+ content in solution (A), TCLP-Pb concentration (B), Pb removal ratio (C), and TCLP-Pb/removed Pb ratio (D) of Aspergillus niger and Penicillium chrysogenum in different Mn2+ conditions after 7 days of incubation. Standard deviations are shown with N = 3. The significant differences across treatments were carried out using Tukey’s honest significant difference test (p < 0.05) after one-way ANOVA. ANG, Aspergillus niger; PCH, Penicillium chrysogenum.
The TCLP-Pb concentration in A. niger with 0, 3.75, 7.5, 15, 30 mg/L Mn2+ treatments were 36.23, 31.47, 22.43, 25.39, and 28.09 mg/L after 7 days of incubation, respectively (Figure 4B). For P. chrysogenum, the TCLP-Pb concentration under different Mn2+ treatments showed a higher value of 50.10, 55.20, 63.77, 67.06, and 75.71 mg/L (Figure 4B). Meanwhile, the Pb removal ratio in A. niger was much higher than P. chrysogenum under different Mn2+ treatments, i.e., 99.1 to 99.5% vs. 83.1 to 90.7% (Figure 4C). In addition, the ratio of TCLP-Pb/immobilized Pb between A. niger and P. chrysogenum in 0, 3.75, 7.5, 15, 30 mg/L Mn2+ treatments was 3.65, 3.17, 2.25, 2.55, 2.83 and 5.27%, 5.84, 6.81, 7.19, 8.19%, respectively (Figure 4D).
3.5 Enzyme activity in A. niger and P. chrysogenum under different Mn2+ conditions
The enzyme activity of pyruvate dehydrogenase (PDH) in both A. niger and P. chrysogenum initially increased and then decreased as the Mn2+ concentration increased from 0 to 30 mg/L (Figure 5A). In 7.5 mg/L Mn2+ treatment, A. niger and P. chrysogenum showed the highest enzyme activity, i.e., 31.53 and 17.23 nmol/min/g, respectively (Figure 5A). In 0, 3.75, 15, and 30 mg/L Mn2+ treatments, the PDH activity in A. niger and P. chrysogenum ranged from 4.13 ~ 19.57 and 3.90 ~ 11.57 nmol/min/g, respectively (Figure 5A). For citrate synthase (CS) enzyme activity, P. chrysogenum showed a similar trend with PDH, i.e., reached the highest value of 66.77 nmol/min/g in 7.5 mg/L Mn2+ treatment and ranged from 39.90 to 60.27 nmol/min/g (Figure 5B). The CS enzyme activity in A. niger under 0, 3.75, 15, and 30 mg/L Mn2+ treatments were 38.40, 39.90, 30.20, 28.10, and 35.60 mg/L, respectively (Figure 5B).
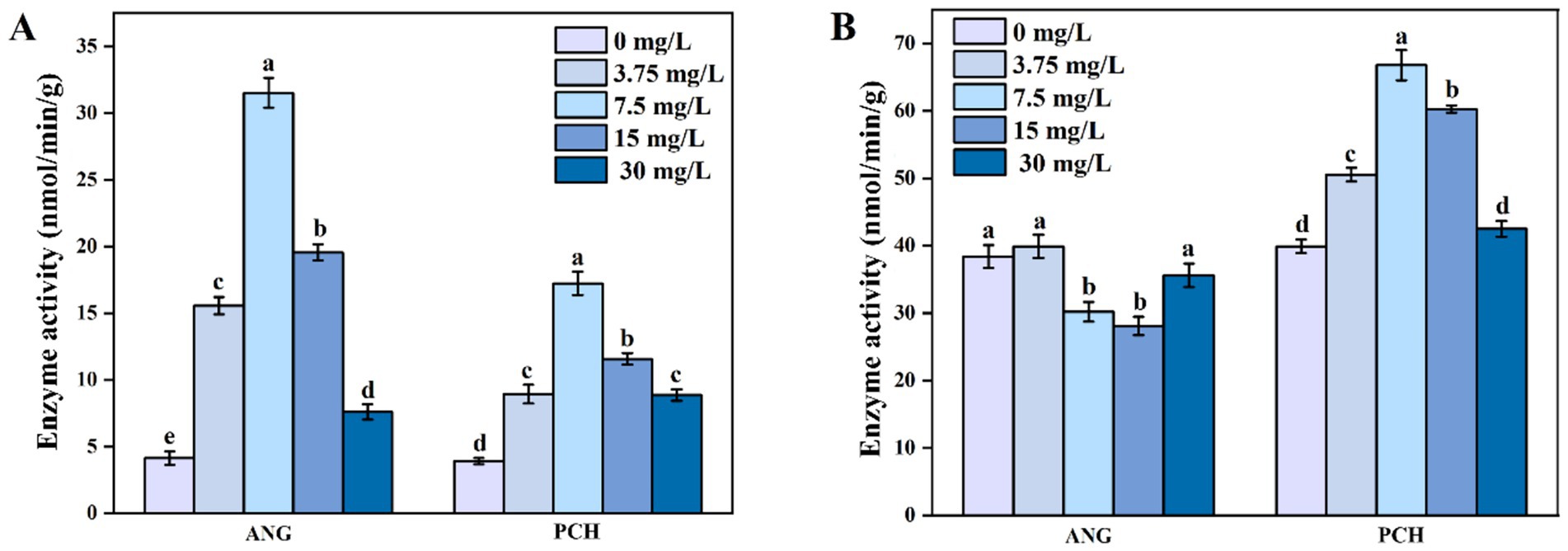
Figure 5. Pyruvate dehydrogenase (PDH) enzyme activities (A) and citrate synthase (CS) enzyme activities (B) of Aspergillus niger and Penicillium chrysogenum in different Mn2+ conditions after 7 days of incubation. Standard deviations are shown with N = 3. The significant differences across treatments were carried out using Tukey’s honest significant difference test (p < 0.05) after one-way ANOVA. ANG, Aspergillus niger; PCH, Penicillium chrysogenum.
3.6 XRD analysis and mineral peak area ratio
XRD patterns showed the Pb mineralization by A. niger and P. chrysogenum at Mn2+ concentrations of 0, 3.75, 7.5, 15, and 30 mg/L (Figure 6). The peaks located at 24.32° and 31.74° represent the minerals of lead oxalate (LO) and fluoropyromorphite (FAL) (Chen et al., 2019). In A. niger treatment, the peaks of LO and FAL were clearly observed at 3.75, 7.5, 15, and 30 mg/L Mn2+ concentrations (Figure 6A). For P. chrysogenum, the peaks of LO and FAL were also observed (Figure 6B). The peak area ratios of minerals between A. niger and P. chrysogenum are shown in Figure 7. For A. niger, the area ratio of LM (LO + FAL)/FAp was much higher than P. chrysogenum, i.e., 3.46 ~ 4.77 vs. 0.09 ~ 0.23 (Figure 7A). Under different Mn2+ treatments, the peak area ratios of LO/FAp were 2.30, 2.31, 2.88, 2.73, and 2.51 in A. niger, much higher than 0.033, 0.054, 0.031, 0.030, and 0.005 in P. chrysogenum (Figure 7B). Meanwhile, the peak area ratios of FAL/FAp in A. niger also higher than P. chrysogenum, i.e., 0.35, 0.49, 0.66, 0.48, and 0.33 vs. 0.04, 0.0550, 0.046, 0.033, and 0.032 (Figure 7C). The peak area ratios of LO/LM were 0.93, 0.82, 0.73, 0.83, and 0.87 in A. niger, while in P. chrysogenum was only 0.35, 0.27, 0.34, 0.38, and 0.44 (Figure 7D). The peak area ratios of FAL/LM were 0.54, 0.65, 0.66, 0.61, 0.55 in A. niger and 0.06, 0.17, 0.26, 0.16, 0.12 in P. chrysogenum (Figure 7E). The XRD peak area ratio of LO and FAL formed in A. niger were 2.64, 3.03, 2.14, 2.18, 1.95 and 8.56, 3.76, 2.48, 3.74, 4.43 times than P. chrysogenum, respectively (Figure 7F).
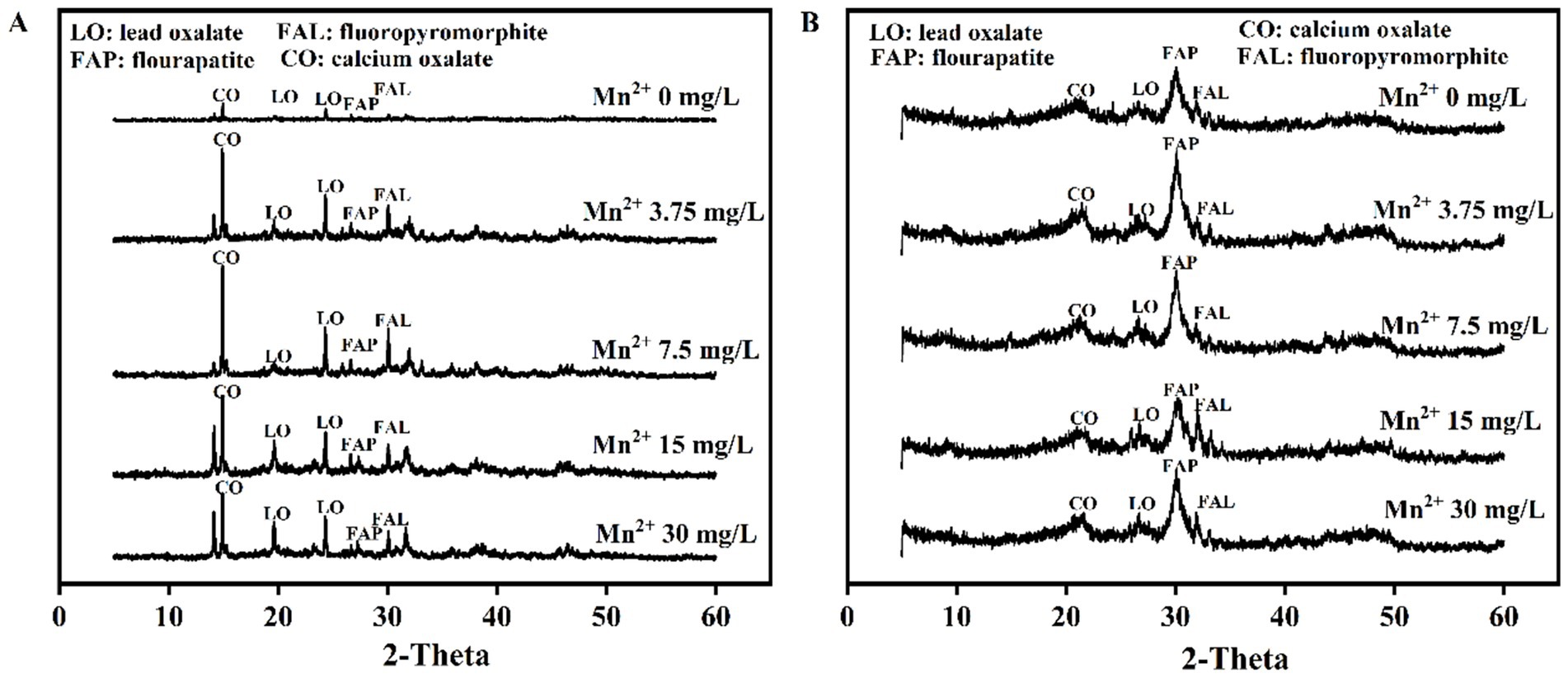
Figure 6. XRD patterns of Aspergillus niger (A) and Penicillium chrysogenum (B) under different Mn2+ concentrations after 7 days of incubation.
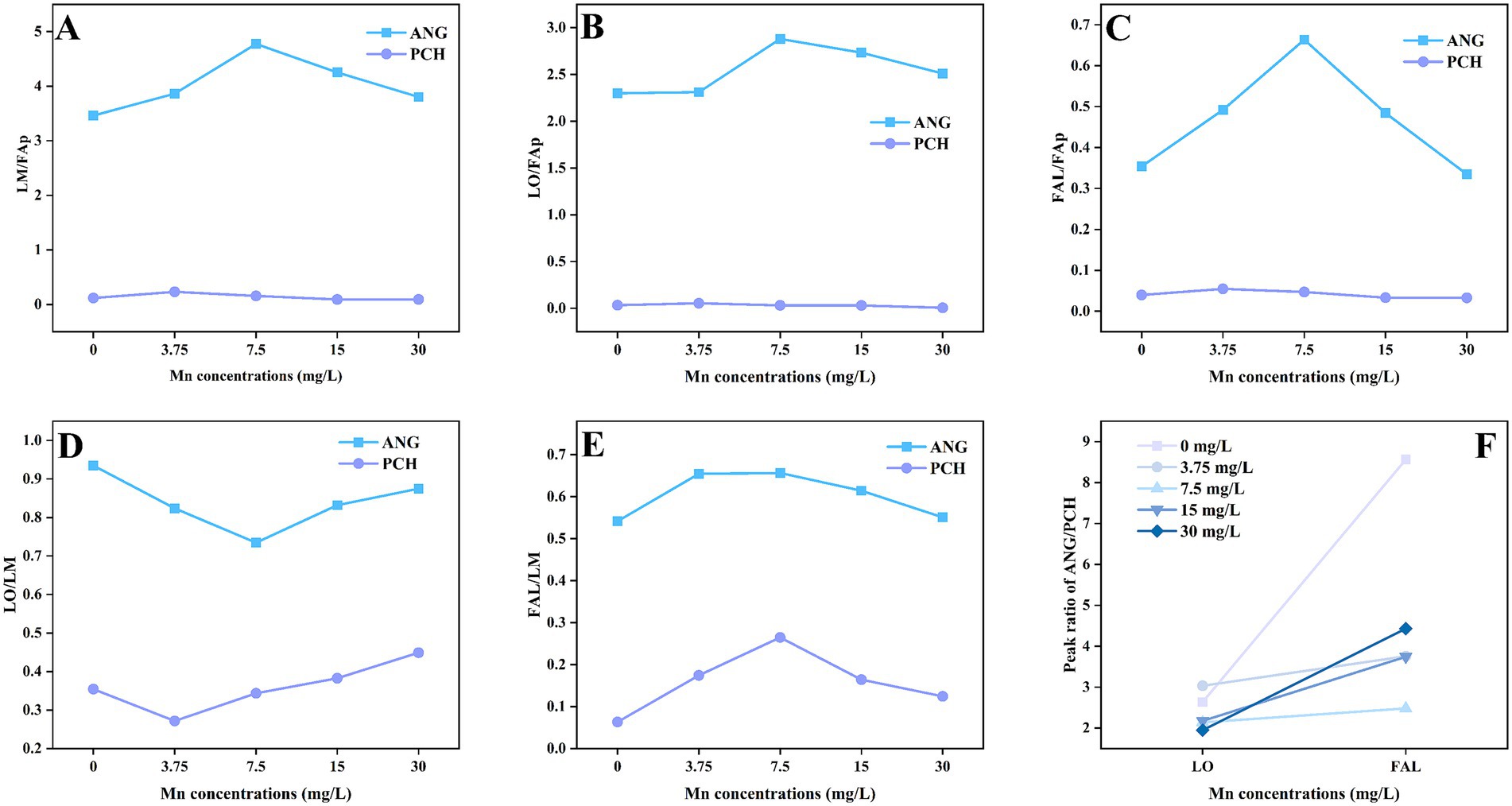
Figure 7. Peak area ratio of Pb minerals of Aspergillus niger and Penicillium chrysogenum in different Mn2+ conditions after 7 days of incubation. (A–E) Peak area ratio of LM/FAp, LO/FAp, FAL/FAp, LO/LM, and FAL/LM between ANG and PCH. (F) The peak area ratio between ANG and PCH in the formed LO and FAL. LO, lead oxalate; FAp, fluorapatite; FAL, fluoropyromorphite; LM, lead minerals (LO + FAL); ANG, Aspergillus niger; PCH, Penicillium chrysogenum.
3.7 SEM analysis
SEM images of Aspergillus niger (7.5 mg/L Mn2+) and Penicillium chrysogenum (3.75 mg/L Mn2+) after 7 days of incubation were shown in Figure 8. Aspergillus niger exhibited robust growth after 7 days, characterized by significant mycelial development encapsulated with calcium oxalate, lead oxalate, and fluoropyromorphite (Figures 8A,B). Similarly, in the presence of Penicillium chrysogenum, the culture displayed the presence of calcium oxalate, lead oxalate, and fluoropyromorphite around the ruptured Penicillium chrysogenum (Figures 8C,D). These findings indicated that the ability of both Aspergillus niger and Penicillium chrysogenum can dissolve FAp and promote Pb immobilization.
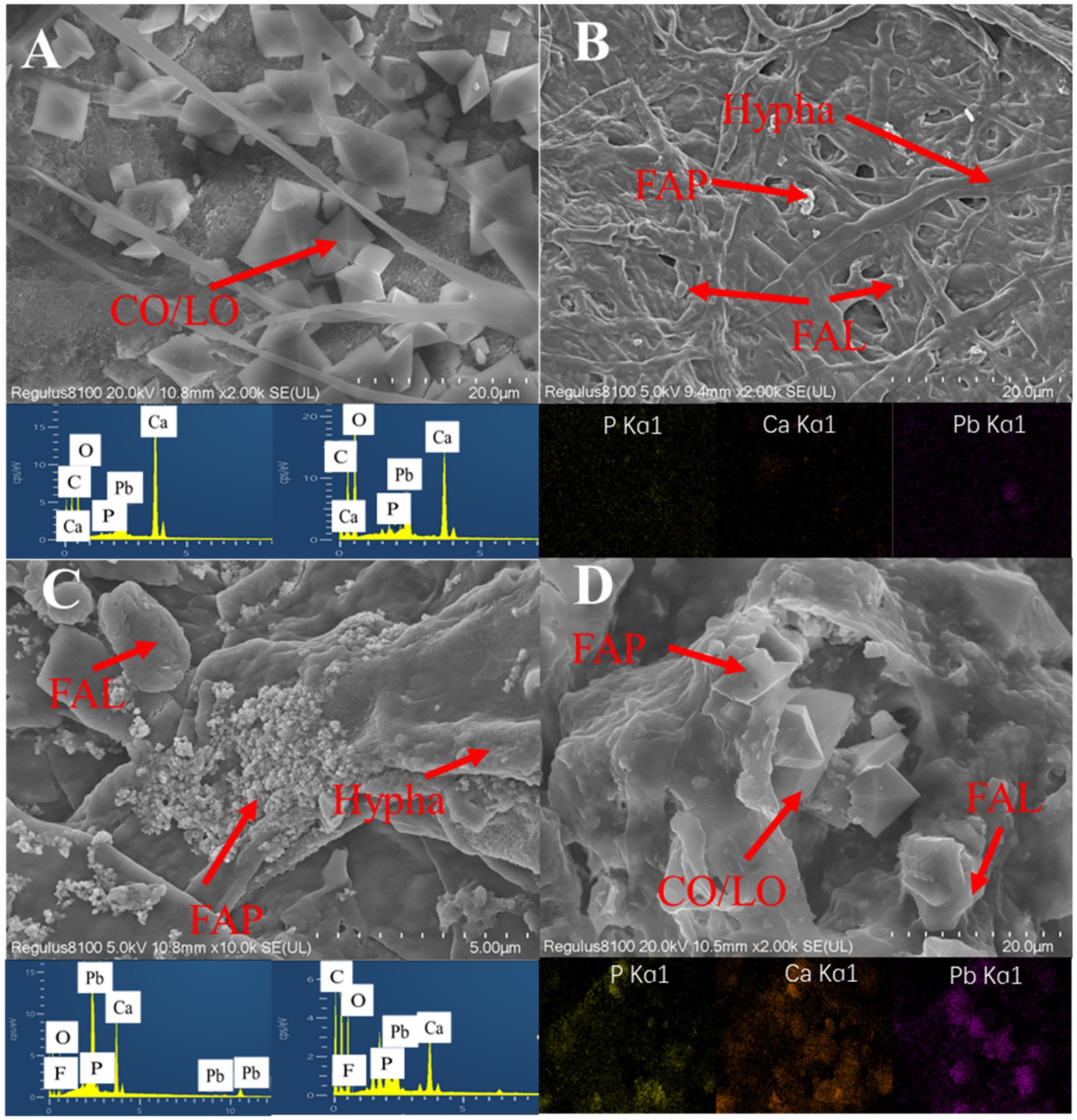
Figure 8. SEM and EDS mapping images of Aspergillus niger and Penicillium chrysogenum after 7 days of incubation. (A,B) A. niger + Pb + FAp. (C,D) P. chrysogenum + Pb + FAp. LO, lead oxalate; CO, calcium oxalate; FAp, fluorapatite; FAL, fluoropyromorphite.
4 Discussion
Bioremediation is a high-efficiency and low-cost pathway for Pb remediation (Dang et al., 2018; Feng et al., 2022; Guan et al., 2024). This research shows that both A. niger and P. chrysogenum can tolerate a high Pb toxicity (1,000 mg/L) and remove more than 83% Pb2+ in the solution (Figure 4). Especially for A. niger, the combination of FAp removed more than 99.3% Pb2+, not only higher than P. chrysogenum, but also more efficient than other fungi, e.g., red yeast combine calcium phosphate remove 90.64% Pb2+ (Feng et al., 2022; Tian et al., 2022). Meanwhile, the Pb immobilized by A. niger is more stable than P. chrysogenum. In A. niger, less than 3.7% Pb2+ can be re-released from the immobilized Pb minerals, much lower than the 5.3–8.2% observed in P. chrysogenum (Figure 4). Therefore, A. niger is more efficient in Pb remediation compared with P. chrysogenum.
Organic acid plays a key role in Pb remediation by PSF and FAp, determined the efficiency of Pb immobilization (Li et al., 2016; Meng et al., 2022). Our research indicates that the secretion of oxalic acid in A. niger is 5–10 times higher than in P. chrysogenum (Figure 2). Previous research confirmed that A. niger has a stronger ability to secrete oxalic acid compared to other PSF, such as Penicillium oxalicum (Tian et al., 2019; Tian et al., 2024). Therefore, oxalic acid would not be the primary mechanism for P. chrysogenum in Pb immobilization. In addition, the dry biomass of P. chrysogenum was also much lower than A. niger, i.e., 0.76–0.85 g vs. 1.07–1.26 g (Figure 1B). In other words, a 1,000 mg/L Pb concentration inhibits the growth of P. chrysogenum and its secretion of oxalic acid. Hence, the other process like bioadsorption may dominated the Pb immobilization by P. chrysogenum. Compared with other organic acid (e.g., citric acid), oxalic acid has a higher acidity constants (pKa = 1.25) and chelating capacity than other organic acid (Li et al., 2016; Palmieri et al., 2019). Thus, A. niger has a higher Pb removal ratio than P. chrysogenum, i.e., 99% vs. ~90% (Figure 4). Previous research has demonstrated that the secretion of oxalic acid by PSF primarily drives the dissolution of phosphate and the remediation of Pb (Jiang et al., 2020). Especially for PSF, enhancing the immobilization capacity of Pb can be achieved by promoting the secretion of oxalic acid (Tian et al., 2023).
The oxalic acid secretion by PSF would also influence the stability of the removed Pb minerals (Wang et al., 2016; Feng et al., 2025). In Pb bioremediation, the stability of immobilized Pb is usually depended on the formed Pb minerals (Feng et al., 2025). In this research, both XRD patterns and SEM images confirmed the formation of LO and FAL in both A. niger and P. chrysogenum (Figures 6, 8). However, A. niger shows a higher stability of immobilized Pb compared to P. chrysogenum (Figure 4). Due to the high secretion of oxalic acid, A. niger is more effective in forming Pb minerals when combined with FAp (Mendes et al., 2020). The secreted oxalic acid not only forms insoluble LO by chelating with Pb (Equation 1) but also facilitates the release of P from phosphate and reacts with Pb to create highly insoluble fluoropyromorphite (Equations 2, 3) (Ma and Rao, 1999; Tian et al., 2023). The formed Pb minerals of LO and FAL by PSF demonstrated the Pb immobilization and contributed to its stability (Giammar et al., 2008; Ahemad, 2015; Feng et al., 2025).
Meanwhile, the XRD peak area ratio shows that the Pb minerals of LO and FAL formed in A. niger are ~2 and ~4 times than P. chrysogenum, respectively (Figure 7). LO and FAL are the primary pathways in Pb immobilization using the combination of PSF and FAp, which have a low solubility (Li et al., 2016; Tian et al., 2019). Therefore, the different oxalic acid secretion significantly changed the stability of the removed Pb between A. niger and P. chrysogenum (Figure 4).
Metal cations can significantly influence the the secretion of organic acids by fungi (Behera, 2020). The addition of metal cations (Ca2+, Mg2+, and Mn2+, etc.) not only affects the growth of PSF but also influences the secretion of oxalic acid (Kobayashi et al., 2014; Geng et al., 2022). In this research, the addition of Mn2+ significantly changed the secretion of organic acid between A. niger and P. chrysogenum (Figure 2). For A. niger, low Mn2+ concentrations (0 and 3.75 mg/L) and high Mn2+ concentrations (15 and 30 mg/L) both limited oxalic acid secretion but enhanced citric acid secretion (Figure 2). In contrast, the high Mn2+ concentrations stimulated the secretion of citric acid in P. chrysogenum (Figure 2). These differences in organic acid secretion can also alter the Pb immobilization capacity of PSF (Tian et al., 2019). Our research indicates that the addition of Mn2+ enhances the secretion of oxalic acid by A. niger and citric acid by P. chrysogenum, respectively. Compared to P. chrysogenum, A. niger is more effective in Pb remediation across various Mn2+ concentrations.
Mn2+ has been demonstrated to be the co-factor that alters the secretion of organic acids by fungi (Arvieu et al., 2003). Moderate amounts of Mn2+ can promote the growth of fungi and improve their resistance to different environmental stresses (Berovic et al., 2006; Fekete et al., 2022). In contrast, the deficiency or excess Mn2+ would also inhibit fungal growth and alter metabolic pathways (Pinzari et al., 2018). In A. niger and P. chrysogenum, the fungal growth and organic acid secretion are different under different Mn2+ concentrations. This difference could be attributed to the physiological traits and metabolic pathways of the two fungi (Huang et al., 2013). In addition, differences in metabolic pathways and gene expression between these two fungi further influence their response and adaptation mechanisms to Mn2+ (Carrasco et al., 2011; Xie et al., 2018). A. niger has strong environmental adaptability and stress resistance and can survive and reproduce in a variety of environmental conditions (Qiu et al., 2021). P. chrysogenum is sensitive to environmental conditions and vulnerable to external factors, e.g., pH value, phosphate source, etc. (Bodini et al., 2011; Wang et al., 2023). Our previous research has confirmed that the pH > 5.5 condition could decrease the stability of Pb immobilization (Feng et al., 2025). Notably, all of the pH value in P. chrysogenum under different Mn2+ concentrations exceeded 6 (pH > 6), which might explain the lower Pb remove ratio and stability of immobilized Pb.
The tricarboxylic acid (TCA) cycle is the main pathway for organic acid secretion in PSF, determining both the quantity and types of secreted acids (Mäkelä et al., 2010; Beatrice et al., 2022). First, pyruvate dehydrogenase (PDH) catalyzes the conversion of pyruvate to acetyl-CoA, thereby facilitating the production of organic acids in the TCA cycle (Mattevi et al., 1992; Dutton and Evans, 1996). Meanwhile, citrate synthase (CS) catalyzes the synthesis of citric acid in PSF (Alekseev et al., 2017; Behera, 2020). The enzyme activity regulated the secretion of organic acids, as well as the phosphate solubilization and Pb remediation facilitated by PSF (Schmalenberger et al., 2015; Feng et al., 2022). Our research also indicates that the PDH activity in A. niger is higher than P. chrysogenum (Figure 5). Meanwhile, the activity of CS enzyme in P. chrysogenum is much higher than in A. niger, which aligns with the higher secretion of citric acid (Figure 5). In addition, metal cations can influence the organic acid secretion by affecting the enzyme activity. Fe3+ can regulate CS enzyme activity and enhance the secretion of citric acid by PSF (Hao et al., 2021; Wang et al., 2023). Similarly, the 7.5 mg/L Mn2+ can significantly increase the PDH enzyme activity in A. niger and P. chrysogenum (Figure 5A). Meanwhile, the 7.5 mg/L Mn2+ can also significantly increase the CS enzyme activity in P. chrysogenum (Figure 5A). Therefore, controlling the Mn2+ concentration is important for regulating the metabolism of organic acid and Pb remediation in PSF.
5 Conclusion
The combination of PSF A. niger and P. chrysogenum with FAp can effectively remove Pb cations via the formation of stable Pb minerals. Oxalic acid secreted by A. niger and P. chrysogenum plays a dominate role in Pb removal. Notably, A. niger exhibits a higher oxalic acid secretion compared to P. chrysogenum, which enhances the formation of insoluble lead oxalate and pyromorphite. An optimal Mn2+ concentration (7.5 mg/L) significantly stimulates oxalic acid secretion by A. niger, hence accelerating FAp dissolution and promoting pyromorphite formation. However, suboptimal (≤7.5 mg/L) or excessive (15–30 mg/L) Mn2+ concentrations reduce the stability of Pb immobilization by A. niger. In summary, A. niger is more effective than P. chrysogenum in Pb remediation, and an appropriately regulated Mn2+ concentration further enhances its Pb removal capacity.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
LZ: Data curation, Writing – original draft. QG: Data curation, Writing – original draft. YY: Data curation, Methodology, Writing – original draft. JS: Investigation, Methodology, Writing – original draft. LX: Investigation, Methodology, Writing – original draft. SZ: Methodology, Writing – original draft. DT: Conceptualization, Formal analysis, Investigation, Writing – review & editing. YH: Funding acquisition, Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Key Research and Development Program of China (2020YFF0218301).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahemad, M. (2015). Phosphate-solubilizing bacteria-assisted phytoremediation of metalliferous soils: a review. 3 Biotech 5, 111–121. doi: 10.1007/s13205-014-0206-0
Alekseev, K. V., Dubina, M. V., and Komov, V. P. (2017). Molecular-genetic and biochemical characteristics of citrate synthase from the citric-acid producing fungus Aspergillus niger. Appl. Biochem. Microbiol. 52, 810–817. doi: 10.1134/s0003683816090027
Arvieu, J. C., Leprince, F., and Plassard, C. (2003). Release of oxalate and protons by ectomycorrhizal fungi in response to P-deficiency and calcium carbonate in nutrient solution. Ann. For. Sci. 60, 815–821. doi: 10.1051/forest:2003076
Beatrice, A., Varco, J. J., Dygert, A., Atsar, F. S., Solomon, S., Thirumalai, R., et al. (2022). Lead immobilization in simulated polluted soil by Douglas fir biochar-supported phosphate. Chemosphere 292:133355. doi: 10.1016/j.chemosphere.2021.133355
Behera, B. C. (2020). Citric acid from Aspergillus niger: a comprehensive overview. Crit. Rev. Microbiol. 46, 727–749. doi: 10.1080/1040841X.2020.1828815
Berovic, M., Vodopivec, M., and Milicic, S. (2006). The influence of manganese ions on Aspergillus niger biomass and citric acid biosynthesis in repeated fed batch fermentation. Chem. Biochem. Eng. Q. 20, 281–284.
Bodini, S. F., Cicalini, A. R., and Santori, F. (2011). Rhizosphere dynamics during phytoremediation of olive mill wastewater. Bioresour. Technol. 102, 4383–4389. doi: 10.1016/j.biortech.2010.12.091
Carrasco, L., Azcón, R., Kohler, J., Roldán, A., and Caravaca, F. (2011). Comparative effects of native filamentous and arbuscular mycorrhizal fungi in the establishment of an autochthonous, leguminous shrub growing in a metal-contaminated soil. Sci. Total Environ. 409, 1205–1209. doi: 10.1016/j.scitotenv.2010.12.019
Chen, M., Li, Z., Huang, P., Li, X., Qu, J., Yuan, W., et al. (2018). Mechanochemical transformation of apatite to phosphoric slow-release fertilizer and soluble phosphate. Process. Saf. Environ. Prot. 114, 91–96. doi: 10.1016/j.psep.2017.12.008
Chen, H., Zhang, J., Tang, L., Su, M., Tian, D., Zhang, L., et al. (2019). Enhanced pb immobilization via the combination of biochar and phosphate solubilizing bacteria. Environ. Int. 127, 395–401. doi: 10.1016/j.envint.2019.03.068
Chowdhury, R. B., Moore, G. A., Weatherley, A. J., and Arora, M. (2017). Key sustainability challenges for the global phosphorus resource, their implications for global food security, and options for mitigation. J. Clean. Prod. 140, 945–963. doi: 10.1016/j.jclepro.2016.07.012
Coutinho, F. P., Felix, W. P., and Yano-Melo, A. M. (2012). Solubilization of phosphates in vitro by aspergillus spp. and penicillium spp. Ecol. Eng. 42, 85–89. doi: 10.1016/j.ecoleng.2012.02.002
Dang, C. Y., Yang, Z. X., Liu, W., Du, P. H., Cui, F., and He, K. (2018). Role of extracellular polymeric substances in biosorption of Pb2+ by a high metal ion tolerant fungal strain PTN31. J. Environ. Chem. Eng. 6, 2733–2742. doi: 10.1016/j.jece.2018.04.005
Dutton, M. V., and Evans, C. S. (1996). Oxalate production by fungi: its role in pathogenicity and ecology in the soil environment. Can. J. Microbiol. 42, 881–895. doi: 10.1139/m96-114
Echeverria, M., Izzi, Y. S., Criado, M. V., and Caputo, C. (2024). Isolation and characterization of dematiaceous endophytic fungi isolated from barley (Hordeum vulgare L.) roots and their potential use as phosphate solubilizers. The Microbe 3:100058. doi: 10.1016/j.microb.2024.100058
Fekete, E., Biro, V., Marton, A., Bakondi-Kovacs, I., Nemeth, Z., Sandor, E., et al. (2022). Bioreactor as the root cause of the "manganese effect" during Aspergillus niger citric acid fermentations. Front. Bioeng. Biotechnol. 10:935902. doi: 10.3389/fbioe.2022.935902
Feng, B., Xue, Y., Wang, D., Chen, S., Zhang, S., Zhang, L., et al. (2025). Stability of lead immobilization by aspergillus Niger and fluorapatite under different pH conditions. Ecotoxicol. Environ. Saf. 289:117706. doi: 10.1016/j.ecoenv.2025.117706
Feng, Y., Zhang, L., Li, X., Wang, L., Yusef, K. K., Gao, H., et al. (2022). Remediation of lead contamination by aspergillus Niger and phosphate rocks under different nitrogen sources. Agronomy 12:1639. doi: 10.3390/agronomy12071639
Gadd, G. M. (1999). “Fungal production of citric and oxalic acid: importance in metal speciation, physiology and biogeochemical processes” in Advances in microbial physiology. ed. R. K. Poole (London: Academic Press), 47–92.
Geng, Y., Pan, S., Zhang, L., Qiu, J., He, K., Gao, H., et al. (2022). Phosphorus biogeochemistry regulated by carbonates in soil. Environ. Res. :113894. doi: 10.1016/j.envres.2022.113894
Giammar, D. E., Xie, L. Y., and Pasteris, J. D. (2008). Immobilization of lead with nanocrystalline carbonated apatite present in fish bone. Environ. Eng. Sci. 25, 725–735. doi: 10.1089/ees.2007.0168
Guan, Q., Cheng, X., He, Y., Yan, Y., Zhang, L., Wang, Z., et al. (2024). Lead remediation by geological fluorapatite combined with penicillium Oxalicum and red yeast. Microb. Cell Factories 23:64. doi: 10.1186/s12934-024-02323-2
Hao, S., Tian, J., Liu, X., Wang, P., Liu, Y., Deng, S., et al. (2021). Combined effects of penicillium oxalicum and tricalcium phosphate on lead immobilization: performance, mechanisms and stabilities. Ecotoxicol. Environ. Saf. 227:112880. doi: 10.1016/j.ecoenv.2021.112880
Huang, L.-F., Song, L.-X., Xia, X.-J., Mao, W.-H., Shi, K., Zhou, Y.-H., et al. (2013). Plant-soil feedbacks and soil sickness: from mechanisms to application in agriculture. J. Chem. Ecol. 39, 232–242. doi: 10.1007/s10886-013-0244-9
Huang, Y. J., Zhang, L. L., Yuan, S. J., Liu, W. P., Zhang, C. C., Tian, D., et al. (2023). The production of oxalate by under different lead concentrations. Agronomy-Basel 13:1182. doi: 10.3390/agronomy13041182
Jalili, B., Sadegh Zadeh, F., Jabari Giashi, M., and Emadi, M. (2020). Lead bioimmobilization in contaminated mine soil by Aspergillus niger SANRU. J. Hazard. Mater. 393:122375. doi: 10.1016/j.jhazmat.2020.122375
Jiang, Z., Wang, T., Sun, Y., Nong, Y., Tang, L., Gu, T., et al. (2020). Application of pb(II) to probe the physiological responses of fungal intracellular vesicles. Ecotoxicol. Environ. Saf. 194:110441. doi: 10.1016/j.ecoenv.2020.110441
Kobayashi, K., Hattori, T., Honda, Y., and Kirimura, K. (2014). Oxalic acid production by citric acid-producing Aspergillus niger overexpressing the oxaloacetate hydrolase gene oahA. J. Ind. Microbiol. Biotechnol. 41, 749–756. doi: 10.1007/s10295-014-1419-2
Kpomblekou-A, K., and Tabatabai, M. A. (2003). Effect of low-molecular weight organic acids on phosphorus release and phytoavailabilty of phosphorus in phosphate rocks added to soils. Agric. Ecosyst. Environ. 100, 275–284. doi: 10.1016/s0167-8809(03)00185-3
Li, Z., Su, M., Tian, D., Tang, L., Zhang, L., Zheng, Y., et al. (2017). Effects of elevated atmospheric CO2 on dissolution of geological fluorapatite in water and soil. Sci. Total Environ. 599-600, 1382–1387. doi: 10.1016/j.scitotenv.2017.05.100
Li, Z., Wang, F. W., Bai, T. S., Tao, J. J., Guo, J. Y., Yang, M. Y., et al. (2016). Lead immobilization by geological fluorapatite and fungus Aspergillus niger. J. Hazard. Mater. 320, 386–392. doi: 10.1016/j.jhazmat.2016.08.051
Liang, X. J., and Gadd, G. M. (2017). Metal and metalloid biorecovery using fungi. Microb. Biotechnol. 10, 1199–1205. doi: 10.1111/1751-7915.12767
Ma, L. Q., and Rao, G. N. (1999). Aqueous pb reduction in pb-contaminated soils by Florida phosphate rocks. Water Air Soil Pollut. 110, 1–16. doi: 10.1023/A:1005025708044
Mäkelä, M. R., Hildén, K., and Lundell, T. K. (2010). Oxalate decarboxylase: biotechnological update and prevalence of the enzyme in filamentous fungi. Appl. Microbiol. Biotechnol. 87, 801–814. doi: 10.1007/s00253-010-2650-z
Mattevi, A., Obmolova, G., Schulze, E., Kalk, K. H., Westphal, A. H., de Kok, A., et al. (1992). Atomic structure of the cubic core of the pyruvate dehydrogenase multienzyme complex. Science (New York, N.Y.) 255, 1544–1550. doi: 10.1126/science.1549782
Mendes, G. O., Murta, H. M., Valadares, R. V., Silveira, W. B., Silva, I. R., and Costa, M. D. (2020). Oxalic acid is more efficient than sulfuric acid for rock phosphate solubilization. Miner. Eng. 155:106458. doi: 10.1016/j.mineng.2020.106458
Meng, L., Ding, K., Qiu, Y., Chen, Y., Huo, H., Yu, D., et al. (2024). Application of phosphogypsum and phosphate-solubilizing fungi to pb remediation: from simulation to in vivo incubation. Sci. Total Environ. 933:173171. doi: 10.1016/j.scitotenv.2024.173171
Meng, L., Pan, S., Zhou, L., Santasup, C., Su, M., Tian, D., et al. (2022). Evaluating the survival of Aspergillus niger in a highly polluted red soil with addition of Phosphogypsum and bioorganic fertilizer. Environ. Sci. Pollut. Res. 29, 76446–76455. doi: 10.1007/s11356-022-21243-5
Naik, M. M., Khanolkar, D., and Dubey, S. K. (2013). Lead-resistant Providencia alcalifaciens strain 2EA bioprecipitates pb+2 as lead phosphate. Lett. Appl. Microbiol. 56, 99–104. doi: 10.1111/lam.12026
Osorio, N. W., and Habte, M. (2013). Phosphate desorption from the surface of soil mineral particles by a phosphate-solubilizing fungus. Biol. Fertil. Soils 49, 481–486. doi: 10.1007/s00374-012-0763-5
Ozdemir, G., Ceyhan, N., Ozturk, T., Akirmak, F., and Cosar, T. (2004). Biosorption of chromium(VI), cadmium(II) and copper(II) by Pantoea sp TEM. Chem. Eng. J. 102, 249–253. doi: 10.1016/j.cej.2004.01.032
Palmieri, F., Estoppey, A., House, G. L., Lohberger, A., Bindschedler, S., Chain, P. S. G., et al. (2019). “Chapter two - oxalic acid, a molecule at the crossroads of bacterial-fungal interactions” in Advances in applied microbiology. eds. G. M. Gadd and S. Sariaslani (Cambridge: Academic Press), 49–77.
Park, J. H., and Bolan, N. (2013). Lead immobilization and bioavailability in microbial and root interface. J. Hazard. Mater. 261, 777–783. doi: 10.1016/j.jhazmat.2013.02.010
Pinzari, F., Cuadros, J., Migliore, M., Napoli, R., and Najorka, J. (2018). Manganese translocation and concentration on Quercus cerris decomposing leaf and wood litter by an ascomycetous fungus: an active process with ecosystem consequences? FEMS Microbiol. Ecol. 94. doi: 10.1093/femsec/fiy111
Priyadarshanee, M., and Das, S. (2024). Spectra metrology for interaction of heavy metals with extracellular polymeric substances (EPS) of Pseudomonas aeruginosa OMCS-1 reveals static quenching and complexation dynamics of EPS with heavy metals. J. Hazard. Mater. 466:133617. doi: 10.1016/j.jhazmat.2024.133617
Qiu, J., Song, X., Li, S., Zhu, B., Chen, Y., Zhang, L., et al. (2021). Experimental and modeling studies of competitive pb (II) and cd (II) bioaccumulation by Aspergillus niger. Appl. Microbiol. Biotechnol. 105, 6477–6488. doi: 10.1007/s00253-021-11497-3
Raj, K., and Das, A. P. (2023). Lead pollution: impact on environment and human health and approach for a sustainable solution. Environ. Chem. Ecotoxicol. 5, 79–85. doi: 10.1016/j.enceco.2023.02.001
Rhee, Y. J., Hillier, S., and Gadd, G. M. (2012). Lead transformation to pyromorphite by Fungi. Curr. Biol. 22, 237–241. doi: 10.1016/j.cub.2011.12.017
Ruijter, G. J. G., van de Vondervoort, P. J. I., and Visser, J. (1999). Oxalic acid production by Aspergillus niger: an oxalate-non-producing mutant produces citric acid at pH 5 and in the presence of manganese. Microbiology (Reading) 145, 2569–2576. doi: 10.1099/00221287-145-9-2569
Schmalenberger, A., Duran, A. L., Bray, A. W., Bridge, J., Bonneville, S., Benning, L. G., et al. (2015). Oxalate secretion by ectomycorrhizal Paxillus involutus is mineral-specific and controls calcium weathering from minerals. Sci. Rep. 5:12187. doi: 10.1038/srep12187
Singh, O. V., Labana, S., Pandey, G., Budhiraja, R., and Jain, R. K. (2003). Phytoremediation: an overview of metallic ion decontamination from soil. Appl. Microbiol. Biotechnol. 61, 405–412. doi: 10.1007/s00253-003-1244-4
Sturm, E. V., Frank-Kamenetskaya, O., Vlasov, D., Zelenskaya, M., Sazanova, K., Rusakov, A., et al. (2015). Crystallization of calcium oxalate hydrates by interaction of calcite marble with fungus Aspergillus niger. Am. Mineral. 100, 2559–2565. doi: 10.2138/am-2015-5104
Su, M., Han, F., Wu, Y., Yan, Z., Lv, Z., Tian, D., et al. (2019). Effects of phosphate-solubilizing bacteria on phosphorous release and sorption on montmorillonite. Appl. Clay Sci. 181:105227. doi: 10.1016/j.clay.2019.105227
Tian, D., Cheng, X., Wang, L., Hu, J., Zhou, N., Xia, J., et al. (2022). Remediation of lead-contaminated water by red yeast and different types of phosphate. Front. Bioeng. Biotechnol. 10:775058. doi: 10.3389/fbioe.2022.775058
Tian, D., Gao, H., Zhang, C., and Ye, X. (2024). “Chapter 21 - sustainable release of phosphorus under heavy metal stresses: from microbiology to productivity” in Beneficial microbes for sustainable agriculture under stress conditions. ed. T. Sa (London: Academic Press), 427–443.
Tian, D., Jiang, Z., Jiang, L., Su, M., Feng, Z., Zhang, L., et al. (2019). A new insight into lead (II) tolerance of environmental fungi based on a study of aspergillus Niger and penicillium oxalicum. Environ. Microbiol. 21, 471–479. doi: 10.1111/1462-2920.14478
Tian, D., Wang, L., Hu, J., Zhang, L., Zhou, N., Xia, J., et al. (2021). A study of P release from Fe-P and ca-P via the organic acids secreted by Aspergillus niger. J. Microbiol. 59, 819–826. doi: 10.1007/s12275-021-1178-5
Tian, D., Zhang, X., Wang, L., Han, M., Zhang, C., and Ye, X. (2023). Lead remediation is promoted by phosphate-solubilizing fungi and apatite via the enhanced production of organic acid. Front. Bioeng. Biotechnol. 11:1180431. doi: 10.3389/fbioe.2023.1180431
Walaszczyk, E., Podgórski, W., Janczar-Smuga, M., and Dymarska, E. (2018). Effect of medium pH on chemical selectivity of oxalic acid biosynthesis by Aspergillus niger W78C in submerged batch cultures with sucrose as a carbon source. Chem. Pap. 72, 1089–1093. doi: 10.1007/s11696-017-0354-x
Wang, L., Tian, D., Zhang, X., Han, M., Cheng, X., Ye, X., et al. (2023). The regulation of phosphorus release by penicillium chrysogenum in different phosphate via the TCA cycle and mycelial morphology. J. Microbiol. 61, 765–775. doi: 10.1007/s12275-023-00072-2
Wang, D., Xie, Y., Jaisi, D. P., and Jin, Y. (2016). Effects of low-molecular-weight organic acids on the dissolution of hydroxyapatite nanoparticles. Environ. Sci. Nano 3, 768–779. doi: 10.1039/C6EN00085A
Wang, Y., Yi, B., Sun, X., Yu, L., Wu, L., Liu, W., et al. (2019). Removal and tolerance mechanism of pb by a filamentous fungus: A case study. Chemosphere 225, 200–208. doi: 10.1016/j.chemosphere.2019.03.027
Xie, H., Ma, Q. Y., Wei, D. Z., and Wang, F. Q. (2018). Transcriptomic analysis of Aspergillus niger strains reveals the mechanism underlying high citric acid productivity. Bioresour. Bioprocess. 5:21. doi: 10.1186/s40643-018-0208-6
Keywords: phosphate-solubilizing microorganisms, heavy metals, Pb remediation, organic acid, manganese
Citation: Zhang L, Guan Q, Yan Y, Sun J, Xu L, Zhang S, Tian D and He Y (2025) Pb immobilization by phosphate-solubilizing fungi and fluorapatite under different Mn2+ concentrations. Front. Microbiol. 16:1696000. doi: 10.3389/fmicb.2025.1696000
Edited by:
Chazarenc Florent, Université Paris-Saclay, FranceReviewed by:
Qi Li, Gansu Agricultural University, ChinaSamira Islas Valdez, Texas State University, United States
Copyright © 2025 Zhang, Guan, Yan, Sun, Xu, Zhang, Tian and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Da Tian, dGlhbmRhOTBAaG90bWFpbC5jb20=; Yue He, aGV5dWVAbmllcy5vcmc=
†These authors have contributed equally to this work
 Lei Zhang1†
Lei Zhang1† Shuo Zhang
Shuo Zhang Da Tian
Da Tian